Abstract
Background:
The incidence, clinical characteristics, and long-term outcomes of patients with gastroenteropancreatic neuroendrocrine tumors and carcinoid syndrome undergoing operative resection have not been well characterized.
Methods:
Patients undergoing resection of primary or metastatic gastroenteropancreatic neuroendrocrine tumors between 2000 and 2016 were identified from an 8-institution collaborative database. Clinicopathologic and postoperative characteristics as well as overall survival and disease-free survival were compared among patients with and without carcinoid syndrome.
Results:
Among 2,182 patients who underwent resection, 139 (6.4%) had preoperative carcinoid syndrome. Patients with carcinoid syndrome were more likely to have midgut primary tumors (44.6% vs 21.4%, P < .001), lymph node metastasis (63.4% vs 44.3%, P < .001), and metastatic disease (62.8% vs 26.7%, P < .001). There was no difference in tumor differentiation, grade, or Ki67 status. Perioperative carcinoid crisis was rare (1.6% vs 0%, P < .01), and the presence of preoperative carcinoid syndrome was not associated with postoperative morbidity (38.8% vs 45.5%, P = .129). Substantial symptom improvement was reported in 59.5% of patients who underwent curative-intent resection, but occurred in only 22.7% who underwent debulking. Despite an association on univariate analysis (P = .04), carcinoid syndrome was not independently associated with disease-free survival after controlling for confounding factors (hazard ratio 0.97, 95% confidence interval 0.64–1.45). Preoperative carcinoid syndrome was not associated with overall survival on univariate or multivariate analysis.
Conclusion:
Among patients undergoing operative resection of gastroenteropancreatic neuroendrocrine tumors, the prevalence of preoperative carcinoid syndrome was low. Although operative intervention with resection or especially debulking in patients with carcinoid syndrome was disappointing and often failed to improve symptoms, after controlling for markers of tumor burden, carcinoid syndrome was not independently associated with worse disease-free survival or overall survival.
Graphical Abstract

“That was a good start. Love your showing six graphs at once. It will totally confuse them.”
Introduction
Neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms with a wide spectrum of clinical behavior. Although rare, their incidence of diagnosis is increasing, with a 6-fold increase from 1.09 per 100,000 persons in 1973 to 6.98 per 100,000 in 2012.1 NETs can present in numerous ways, such as incidental findings on cross-sectional imaging, a mass effect from the primary tumor, or with symptoms from excess production of functionally active hormones. Although some pancreatic NETs produce specific hormones that result in well-defined clinical syndromes (eg, insulinoma, gastrinoma), all gastroenteropancreatic (GEP) NETs in advanced stages have the ability to oversecrete biologically active hormones, resulting in carcinoid syndrome.
The reported incidence of carcinoid syndrome in patients with GEP-NETs ranges from 3.2% to 18.7% but can vary considerably based on the anatomic site of the primary tumor and overall disease burden.2–6 The poor survival outcomes observed in this population are thought to be related primarily to the presence of more advanced disease,7–9 but a recent study demonstrats worse overall survival for patients with carcinoid syndrome, even after controlling for tumor stage, grade, and primary site.2 Whether or not this survival difference results from a difference in tumor burden or tumor biology is debatable.10,11 Nevertheless, the incidence, characteristics, and impact of carcinoid syndrome on the patients with GEP-NETs undergoing operative resection remains unclear. Most previous investigations on carcinoid syndrome have focused on patients with advanced disease not amenable to operative therapy involving either resection or debulking. Studies that included surgical patients have been limited by their single-institution nature, narrow inclusion criteria, or lack of clinical information owing to the administrative nature of the dataset.1–4,12
Therefore, the objective of the current study was to characterize the frequency and clinicopathologic characteristics of carcinoid syndrome among patients with GEP-NETs undergoing operative therapy (resection or debulking) and to assess its impact on long-term outcomes using a large, multi-institutional database.
Methods
Data source and patient characteristics
The U.S. Neuroendocrine Study Group is a multi-institutional collaboration among the following 8 centers: Emory University, The Ohio State University, Stanford University Medical Center, Virginia Mason Medical Center, the University of Wisconsin, Washington University, Vanderbilt University, and the University of Michigan. All centers collected data retrospectively on the clinicopathologic factors and postoperative outcomes of patients with primary or metastatic GEP-NETs who underwent operative therapy (resection or debulking) from 2000 to 2016. Institutional review board approval was obtained at each participating institution.
For purposes of this study, patients with preoperatively diagnosed carcinoid syndrome were compared with patients without evidence of carcinoid syndrome. Patients were identified by each participating institution, and data were collected through retrospective chart review using a universal template. Categorical data fields had a predefined range of responses for standardized coding. Central collation and review of the final dataset was performed by a supervising institution. Data collected included patient demographics; past medical history and comorbidities; symptoms at presentation; baseline laboratory values; preoperative workup; operative factors; final histopathology; any neoadjuvant or adjuvant therapy; and outcomes, including complications, recurrence, and survival.
Definitions
Carcinoid syndrome was defined clinically as the presence of pathognomonic symptoms (including flushing, diarrhea, tachycardia, shortness of breath, or purpura) attributable to the GEP-NET. Most patients carried a documented diagnosis evident on chart review; the determination of ambiguous cases was at the discretion of the authors at each participating site. Although symptomatic flushing alone was sufficient for the diagnosis of carcinoid syndrome, isolated diarrhea or tachycardia, given their nonspecific nature, were not considered diagnostic of carcinoid syndrome. Tumor markers and biochemical confirmation of carcinoid syndrome through levels of either serum serotonin or urinary 5-hydroxyindoleacetic acid were included whenever available, but given the retrospective and multi-institutional nature of this study, biochemical confirmation of carcinoid syndrome was not available for most patients.
Postoperative morbidity encompassed any complication within 90 days of operation. Tracked complications included superficial surgical site infections, intra-abdominal infections, wound dehiscence, carcinoid crisis (perioperative hypertensive emergency or hemodynamic collapse attributable to hormone release), stroke, cardiac arrest, myocardial infarction, pneumonia, respiratory failure, thromboembolic events, bleeding (requiring transfusion or procedure/operation), pancreatitis, ileus, anastomotic leak, reoperation, pancreatic fistula, acute kidney injury, renal failure, and urinary tract infection. The Clavien-Dindo score for the most severe complication was also reported. After resection, symptom improvement was documented when a patient indicated subjective resolution of functional symptoms (diarrhea, flushing, tachycardia, etc.) attributed to their tumor.
Statistical analysis
Categorical variables were expressed as whole numbers and proportions and compared using the Pearson χ2 test or Fisher exact test where appropriate. Normally distributed continuous data were described as means with standard deviation and compared using Student’s t test. Nonparametric continuous variables were reported as medians with interquartile range and compared using the Wilcoxon rank sum test. On univariate analysis, demographic, clinical, and pathologic factors were compared. Clinically relevant factors identified in the literature and select variables associated with tumor burden on univariate analysis were incorporated into a multivariable logistic regression model to test the hypothesis that carcinoid syndrome was related to tumor location and disease burden.13
Primary endpoints were overall survival (OS), disease-free survival (DFS), and progression-free survival (PFS). OS was measured from the time of resection to death or last follow-up. DFS was measured from time of resection to recurrence, death, or last follow-up in patients who had macroscopically negative (R0/R1) final resection margins. PFS was measured from time of resection to first evidence of progression among patients with macroscopically positive (R2) resection margins. Survival probabilities were estimated using the Kaplan-Meier method and compared using the log-rank test. Prognostic factors for survival were then evaluated using the multivariable Cox proportional hazards regression model. All statistical analysis was performed using SPSS, version 21 (2012).
Results
Patient characteristics and factors associated with carcinoid syndrome
Among 2,182 patients who underwent surgical operative therapy (resection or debulking) for primary or metastatic GEP-NETs, the frequency of carcinoid syndrome was low (6.4%, n = 139). Overall, the median age was 58.0 years (interquartile range 48.3–66.3), most patients were white (76.3%, n = 1,664), and patients were evenly divided between male (50.3%, n = 1,098) and female (49.7%, n = 1,084). The pancreas was the most common site for the primary tumor (57.1%, n = 1,247). Most patients had a good functional status (Eastern Cooperative Oncology Group 0–1; 74.5%, n = 1625), with no difference observed when stratified by the presence of carcinoid syndrome. Additional comparison of clinical and pathologic features among patients with and without carcinoid syndrome is shown in Table 1. In general, patients with carcinoid syndrome had increased tumor burden with a greater proportion of metastatic disease (62.8% vs 26.7%, P < .001), liver metastasis (54.5% vs 18.8%, P < .001), and lymph node involvement (42.4% vs 30.2%, P = .002). No differences were observed across histologic features, including size, differentiation, Ki67 status, or grade (Table 2).
Table 1.
Demographics and preoperative characteristics.
| Variable | Carcinoid syndrome (n = 139) | Non-carcinoid syndrome (n = 2043) | P value |
|---|---|---|---|
|
| |||
| Demographics | |||
| Sex | (n = 139) | (n = 2043) | |
| Male | 59 (42.4%) | 1039 (50.9%) | .055 |
| Female | 80 (57.6%) | 1004 (49.1%) | |
| Age | 56.2 ± 12.2 (n = 139) | 57.0 ± 13.6 (n = 2038) | .494 |
| Body mass index | 30.1 ± 7.1 (n = 131) | 29.2 ± 7.0 (n = 1828) | .158 |
| ECOG 0 | 101 (84.2%) | 1280 (82.5%) | .404 |
| 1 | 15 (12.5%) | 229 (14.8%) | |
| 2 | 3 (2.5%) | 34 (2.2%) | |
| 3 | 0 | 6 (0.4%) | |
| 4 | 1 (0.8%) | 2 (0.1%) | |
| ASA class | |||
| 1 | 8 (6.1%) | 90 (4.8%) | .718 |
| 2 | 44 (33.6%) | 715 (37.9%) | |
| 3 | 75 (57.3%) | 1033 (54.8%) | |
| 4 | 4 (3.1%) | 47 (2.5%) | |
| Race | (n = 136) | (n = 1984) | |
| White | 118 (86.8%) | 1546 (77.9%) | .007 |
| Black | 16 (11.8%) | 260 (13.1%) | |
| Other/unknown | 2 (1.4%) | 178 (9.0%) | |
| Preop characteristics | |||
| Preop somatostatin | 31/129 (24.0%) | 65/1742 (3.7%) | <.001 |
| Functional tumor | 16/139 (11.5%) | 246/2012 (12.2%) | .803 |
| Symptomatic at presentation | 127/139 (91.4%) | 1215/2009 (60.5%) | <. 001 |
| Any symptoms | |||
| Flushing | 127/139 (91.4%) | 0/2005 (0%) | <. 001 |
| Diarrhea | 85/139 (61.2%) | 247/2004 (12.3%) | <. 001 |
| Tachycardia | 28/139 (20.1%) | 9/2004 (0.4%) | <. 001 |
| Carcinoid heart | 2/139 (1.4%) | 0/2006 (0%) | .004 |
| Pain | 84/139 (60.4%) | 815/2002 (40.7%) | <. 001 |
| Location of primary | (n = 139) | (n = 2042) | |
| Ampulla | 0 | 37 (1.8%) | <. 001 |
| Appendix | 1 (0.7%) | 64 (3.1%) | |
| Colon (midgut) | 5 (3.6%) | 50 (2.4%) | |
| Colon (hindgut) | 1 (0.7%) | 5 (0.2%) | |
| Duodenum | 9 (6.5%) | 128 (6.3%) | |
| Gallbladder | 0 | 8 (0.4%) | |
| Pancreas | 40 (28.8%) | 1207 (59.1%) | |
| Rectum | 3 (2.2%) | 65 (3.2%) | |
| Small bowel | 56 (40.3%) | 324 (15.9%) | |
| Stomach | 5 (3.6%) | 99 (4.8%) | |
| Unknown | 19 (13.7%) | 56 (2.7%) | |
| Metastatic disease | 86/137 (62.8%) | 516/1929 (26.7%) | <. 001 |
| Liver metastasis | 73/134 ( 54.5%) | 340/1806 (18.8%) | <. 001 |
| Serotonin level (ng/mL) | 1107 ± 949 (n = 9) | 357 ± 472 (n = 74) | .046 |
| Urinary 5-HIAA (mg/day) | 20.5 ± 18.2 (n = 20) | 9.9 ± 12.1 (n = 91) | .021 |
| Chromogranin A (ng/L) | 2059 ± 7635 (n = 48) | 545 ± 1684 (n = 617) | .177 |
5-HIAA, 5-hydroxyindoleacetic acid; ECOG, Eastern Cooperative Oncology Group.
Table 2.
Histopathology and perioperative course.
| Variable | Carcinoid syndrome (n = 139) | Noncarcinoid syndrome (n=2043) | P value |
|---|---|---|---|
|
| |||
| Operation | (n = 138) | (n = 2039) | |
| Curative intent | 95 (68.8%) | 1856 (91.0%) | <.001 |
| Debulking/palliative | 43 (31.2%) | 183 (9.0%) | |
| Histopathology | |||
| Tumor size (largest lesion, cm) | 2.70 ± 2.22 (n = 102) | 2.81 ± 2.65 (n = 1728) | .682 |
| Tumor differentiation | (n = 89) | (n = 1489) | |
| Well dif. | 79 (88.8%) | 1321 (88.7%) | .754 |
| Mod dif. | 9 (10.1%) | 135 (9.1%) | |
| Poorly dif. | 1 (1.1%) | 33 (2.2%) | |
| Tumor grade | (n = 71) | (n = 1252) | |
| G1 | 51 (71.8%) | 814 (65.0%) | .330 |
| G2 | 19 (26.8%) | 383 (30.6%) | |
| G3 | 1 (1.4%) | 55 (4.4%) | |
| Ki67 (%) | 3.6 ± 4.1 (n = 52) | 5.6 ± 10.1 (n = 687) | .149 |
| Lymph node | (n = 139) | (n = 2043) | |
| N0 | 34 (24.5%) | 774 (37.9%) | .002 |
| N1 | 59 (42.4%) | 616 (30.2%) | |
| Nx | 46 (33.1%) | 653 (32.0%) | |
| Margins | ( n = 102) | (n = 1726) | |
| R0 | 70 (68.6%) | 1371 (79.4%) | <. 001 |
| R1 | 10 (9.8%) | 237 (13.7%) | |
| R2 | 22 (21.6%) | 118 (6.8%) | |
| Postop course | |||
| Any complication | 54 (38.8%) | 918 (45.5%) | .129 |
| Highest Clavien-Dindo classification | |||
| I -II | 527 (58.1%) | 31 (56.4%) | .814 |
| III-V | 382 (41.9%) | 24 (43.6%) | |
| Myocardial infarct | 1 (0.8%) | 7 (0.4%) | .410 |
| Respiratory failure | 3 (2.3%) | 56 (2.9%) | 1.0 0 0 |
| Carcinoid crisis | 2/128 (1.6%) | 0/1910 (0%) | .004 |
| Postoperative symptom improvement | 24/55 (43.6%) | 184/354 (52.0%) | .25 |
| Readmission | 20 (14.4%) | 373 (18.4%) | .231 |
| Recurrence | 28/102 (27.5%) | 355/1808 (19.6%) | .55 |
| Region of recurrence | (n = 28) | (n = 357) | |
| Locoregional | 5 (17.9%) | 88 (24.6%) | .713 |
| Locoregional/distant | 3 (10.7%) | 38 (10.6%) | |
| Distant only | 20 (71.4%) | 231 (64.7%) | |
| Death | 25/139 (18.0%) | 305/2039 (15.0%) | .335 |
| Other therapies | |||
| Chemotherapy | 6 (4.3%) | 104 (5.4%) | .687 |
| Radiotherapy | 1 (0.7%) | 24 (1.2%) | 1.000 |
| Somatostatin analog | 45 (32.8%) | 164 (8.2%) | <. 001 |
| Liver-directed* | 8 (5.8%) | 30 (1.5%) | <. 001 |
Patients with carcinoid syndrome were more likely to report symptoms at presentation compared to patients without carcinoid syndrome (91.4% vs 60.5%, P < .001). Overall, the most common complaints were flushing and diarrhea, followed by tachycardia (Table 1). Although these patients also had greater levels of preoperative serum serotonin and urinary 5-hydroxyindoleacetic acid, these data were available for only a minority of patients. A greater proportion of patients with carcinoid syndrome were on preoperative somatostatin analogs (SSAs; 24.0% vs 3.7%, P < .001). Preoperative SSA use was not associated with decreased symptoms of flushing (86% vs 87%, P = .911), although there was a greater frequency of diarrhea (76% vs 53%, P = .028) and tachycardia (27.6% vs 12%, P = .041) among SSA users.
There was substantial variation in the frequency of carcinoid syndrome according to the site of the primary tumor (Table 3). The majority of patients with carcinoid syndrome had either regional or distant metastatic disease (76.3%, n = 109).The greatest frequency of carcinoid syndrome was observed with tumors of the small bowel (ileum or jejunum, 14.7%), and in those patients with an unknown primary (25.3%). In contrast, pancreatic neuroendocrine tumors (PNETs) had a much lower rate of carcinoid syndrome (3.2%). Similarly, low rates of carcinoid syndrome were observed with rectal, appendiceal, duodenal, and gastric primaries. Among all gastric tumors, most were either type I (61.0%, n = 47) or type III (28.6%, n = 22), and no individual subtype was associated with carcinoid syndrome (P = .147).
Table 3.
Frequency of carcinoid syndrome and association with regional/metastatic disease, by primary site.
| Primary site | Carcinoid syndrome | CS with regional/metastatic disease |
|---|---|---|
|
| ||
| Ampulla | 0/37 (0%) | n/a |
| Appendix | 1/65 (1.5%) | 1/1 (100%) |
| Colon (midgut) | 5/55 (9.1%) | 5/4 (80%) |
| Colon (hindgut) | 1/6 (16.7%) | 0/1 (0%) |
| Duodenum | 9/137 (6.6%) | 4/9 (44.4%) |
| Gallbladder | 0/8 (0%) | n/a |
| Pancreas | 40/1247 (3.2%) | 24/40 (60%) |
| Rectum | 3/68 (4.4%) | 1/3 (33.3%) |
| Small bowel | 56/380 (14.7%) | 51/56 (91.1%) |
| Stomach | 5/104 (4.8%) | 1/5 (20.0%) |
| Unknown | 19/75 (25.3%) | 19/19 (100%) |
On analysis of 1,896 patients with complete data available, several factors were independently associated with carcinoid syndrome on multivariable logistic regression analysis (Table 4). Patients with carcinoid syndrome were more likely to be white and have lymph node or liver metastases. Compared with pancreatic tumors, most gastrointestinal sites had an increased likelihood of carcinoid syndrome, with the greatest odds observed in midgut tumors and patients with an unknown primary.
Table 4.
Multivariable model: Factors associated with carcinoid syndrome.
| Factor | OR | 95% Cl | P value |
|---|---|---|---|
|
| |||
| Age | 0.988 | 0.975–1.002 | .105 |
| Sex | |||
| Female | Reference | ||
| Male | 0.751 | 0.509–1.106 | .147 |
| Race | |||
| Nonwhite | Reference | ||
| White | 1.803 | 1.031–3.153 | .039 |
| Location | |||
| Pancreas | Reference | ||
| Foregut | 2.297 | 1.061–4.973 | .035 |
| Midgut | 3.454 | 2.195–5.436 | <. 001 |
| Hindgut | 2.594 | 0.735 –9.153 | .138 |
| Unknown primary | 7.438 | 3.795–14.578 | <. 001 |
| Liver metastasis | |||
| No | Reference | ||
| Yes | 3.984 | 2.686–5.910 | <. 001 |
| Lymph node | |||
| N0/Nx | Reference | ||
| N1 | 1.613 | 1.063–2.449 | .025 |
Perioperative and long-term outcomes
A greater proportion of the 139 patients with carcinoid syndrome underwent a debulking or palliative operation (31.2% vs 9%, P < .001), with more R2 resections (21.6% vs 6.8%, P < .001). There was no difference in postoperative morbidity based on the presence of preoperative carcinoid syndrome (38.8% vs 45.5%, P = .129; Table 2). Only 2 patients (1.6%) in the carcinoid syndrome group developed carcinoid crisis. Overall, there was no difference in symptom improvement between carcinoid and noncarcinoid syndrome patients with and without after operative treatment (43.6% vs 52.0%, P = .25). Patients with carcinoid syndrome were less likely to show symptom improvement after debulking compared with curative-intent operations (22.7% vs 59.4%, P = .012). For patients without carcinoid syndrome, there was a similar rate of symptom improvement after either curative or debulking operations (53.1% vs 43.2%, P = .22).
Across the entire cohort, median OS was 144 months. Patients with carcinoid syndrome experienced lower median DFS (76 vs 155 months, log rank = 0.035; Fig 1, A), but had a similar median OS (144 months vs not reached, log rank = 0.561; Fig 1, B) compared to patients without carcinoid syndrome. On Cox multivariable analysis controlling for age, race, primary location, liver metastases, and lymph node involvement, carcinoid syndrome was not associated with DFS (hazard ratio 0.97, 95% confidence interval [CI] 0.64–1.45). Factors associated with worse DFS included positive lymph nodes (odds ratio [OR] 1.80, 95% CI 1.43–2.28), liver metastases (OR 4.34, 95% CI 3.43–5.56), and white race (OR 1.57, 95% 1.18–2.10).
Fig 1.
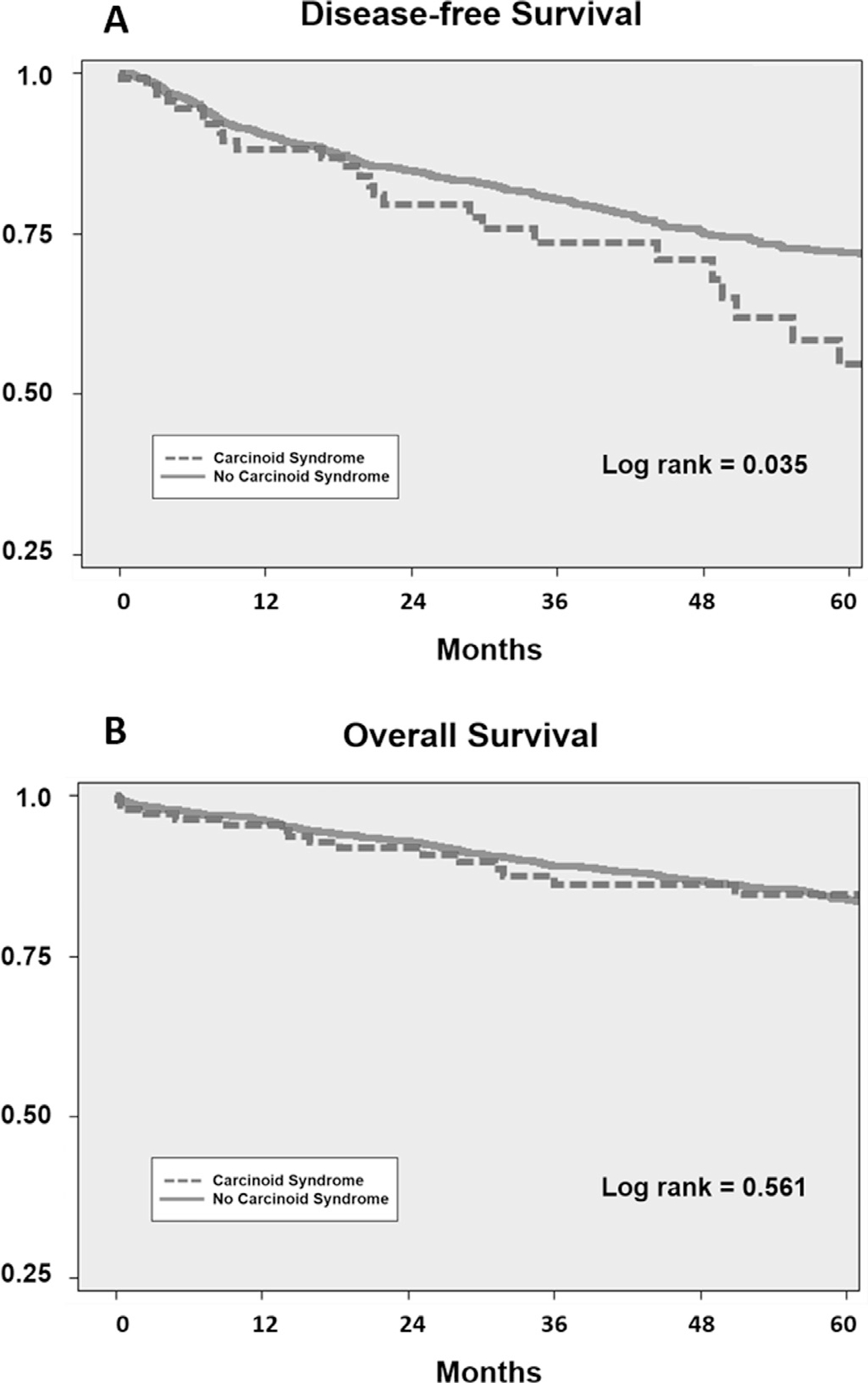
Kaplan-Meier analysis of (A) disease-free survival after complete macroscopic resection (R0/R1 patients) and (B) overall survival of all patients with and without carcinoid syndrome.
On a subset analysis of patients with documented metastatic disease to the liver, the presence of carcinoid syndrome was not associated with decreased DFS (P = .191; Supplemental Fig 1, A) or OS (P = .302; Supplemental Fig 1, B). Patients with liver metastases that underwent debulking operations had worse OS compared to those with curative-intent resection (median OS 89.0 vs 137.5 months, p < 0.001). After a R2 resections, there was no difference in PFS between patients either with or without carcinoid syndrome (P = .547; Supplemental Fig 2).
Discussion
In this large, multi-institutional study investigating the influence of carcinoid syndrome on the clinicopathologic characteristics and outcomes of patients undergoing operative therapy for GEP-NETs, we found several notable observations. First, carcinoid syndrome occurred infrequently and was associated typically with markers of increased tumor burden, such as nodal or metastatic disease, but not with tumor size, grade, or mitotic rate. Second, the incidence of perioperative carcinoid crisis was low, and the presence of preoperative carcinoid syndrome did not affect perioperative morbidity or mortality. Third, although carcinoid syndrome was a predictor of worse DFS, after controlling for markers of disease burden, the presence of carcinoid syndrome was not independently associated with DFS or OS. Fourth, most patients with carcinoid syndrome do not experience substantial symptom improvement after operative therapy, particularly after debulking operations. These findings have important implications for the selection and prognosis of patients with GEP-NETs being considered for operative therapy.
The accurate identification of patients with carcinoid syndrome is challenging, both in clinical practice and when performing high-quality research. Regarding the latter, previous studies have relied on a number of definitions, including clinical diagnosis, biochemical testing, or administrative coding.2,3,5,14 In our study, we relied on a clinical definition, but our classification of carcinoid syndrome agrees with previously published results. The overall frequency of carcinoid syndrome of 6.4% in our study falls within the 1.7% to 18.7% range previously reported in the literature.15 Given that carcinoid syndrome is associated typically with advanced disease, it may not be surprising that the overall frequency of carcinoid syndrome in this cohort of patients undergoing operative therapy was toward the lower end of this range. In a review of 11,057 cases from a large clinical registry, Soga reported a similar overall incidence of carcinoid syndrome of 7.7%.5 Recent estimates from a survey of the Surveillance, Epidemiology, and End Results-Medicare database determined a rate of 19%, although pancreatic tumors (which typically are less likely to result in carcinoid syndrome) were excluded.2 In a review of 336 patients with gastrointestinal NETs who underwent biochemical testing, Onaitis et al reported 44 patients (13.1%) who presented with carcinoid syndrome. Excluding pancreatic tumors, the incidence of carcinoid syndrome among patients with gastrointestinal NETs in our study was 10.6%.
Patients with carcinoid syndrome in our study also demonstrated a similar pattern of anatomic distribution as reported previously reported. Specifically, small-bowel NETs (ileum and jejunum) or from unknown primary sites were most likely to exhibit carcinoid syndrome, consistent with prior studies.4,14,16 Often these unknown primaries represent small, occult ileal tumors.17–19 In contrast, although pancreatic tumors represented a large proportion of the carcinoid syndrome patients in this series (28.8%), this likely reflects the large number of PNETs in our study, as the development of carcinoid syndrome in PNET patients alone was relatively rare (3.2%). The biologic reasons underlying the differing incidence of carcinoid syndrome at different sites of the primary tumor is not well understood. Carcinoid syndrome has been linked to the excess production of serotonin, which may differ based on anatomic location.14,15,20 For instance, midgut carcinoids have been shown to be greater producers of serotonin than foregut or hindgut tumors.16
Although carcinoid syndrome is associated with increased tumor burden (ie, increased rates of metastatic disease, liver metastases, and positive lymph nodes), a substantial number of patients with carcinoid syndrome in our study had no signs of regional or distant disease (25.9%, n = 36). This finding is consistent with recent findings from the Surveillance, Epidemiology, and End Results-Medicare database, where 27% of patients with carcinoid syndrome presented with localized disease.2 Although the exact reasons for this observation are unclear, one possibility is that these patients had undiagnosed occult metastatic disease,2,21,22 which emphasizes the importance of thoroughly evaluating patients with carcinoid syndrome for metastatic disease, including the use of molecular imaging or intraoperative evaluation of the liver.
One unexpected finding was that only a minority (43.6%) of patients with carcinoid syndrome reported substantial symptom improvement after operative therapy. In fact, the low rate of symptom improvement observed after debulking operations suggests that reliable control of carcinoid syndrome symptoms should no longer be the sole indication for operative intervention with a noncurative (palliative) intent. The difficulty observed in achieving complete or even substantial relief of symptoms underscores the importance of careful patient selection before considering resection and the role of surgical therapy within broader multimodality strategies.23 Future studies should aim to identify preoperative factors that predict symptom improvement with operative therapy to aid in optimal patient selection.
Although estimates of perioperative carcinoid crisis vary from 3.4% to 24% in the literature,24–26 only 2 patients with carcinoid syndrome experienced a perioperative carcinoid crisis in this study (1.4%). Similarly, the presence of carcinoid syndrome did not affect the frequency or severity of postoperative complications, including cardiovascular events. Although our study did not address the role for intraoperative octreotide, the consequences of carcinoid crisis and the minimal downsides to perioperative octreotide have led to its increasing use before operative intervention. Overall, the presence of preoperative carcinoid syndrome does not appear to influence the risk of perioperative complications and, therefore, should not independently represent a contraindication to operative therapy (resection or debulking).
The impact of carcinoid syndrome on the long-term outcomes of patients with GEP-NETs has been the subject of debate. The increased hormone production by carcinoid tumors presumably serves as a marker of increased tumor burden, and therefore, not surprisingly, carcinoid syndrome has been linked to worse survival. In our study of patients with clinically resectable disease, the presence of carcinoid syndrome was not independently associated with either DFS or OS after controlling for other markers of disease burden. Furthermore, there was also no difference in tumor size, differentiation, Ki67 status, or grade to suggest that patients with carcinoid syndrome have more aggressive tumor biology on final pathology. These findings suggest that the presence of carcinoid syndrome, in and of itself, is not independently associated with worse oncologic outcomes and thus should not be an absolute contraindication to operation for otherwise appropriate patients.
There were several limitations to this study. First, despite a large multi-institutional dataset, the sample size of 139 patients with the carcinoid syndrome was limited given the relative low prevalence of patients with carcinoid syndrome. Although we chose to study a diverse population with both primary and secondary disease, subset analyses of patients with only liver metastases as well as those who solely underwent debulking were congruent with our primary findings. Second, as a retrospective study over a 17-year period, complete records were not available for all patients, and therefore it is possible that missing data may have obscured unmeasured differences between the groups. Third, although all operations were performed by experienced surgical oncologists, there was no standardization in the evaluation, multidisciplinary management, histopathologic review, or selection for operative therapy. Fourth, carcinoid syndrome was defined clinically and identified through retrospective chart review, but biochemical confirmation was not available for all patients. Given that over 25% of patients labeled with carcinoid syndrome in this study had no signs of metastatic disease, clinical diagnosis may have overestimated the true prevalence of carcinoid syndrome in this surgical population.
In conclusion, among patients undergoing operative resection of GEP-NETs, the incidence of carcinoid syndrome was low and typically associated with markers of increased tumor burden. Preoperative carcinoid syndrome was not associated with increased perioperative morbidity or mortality, but most patients with carcinoid syndrome do not experience substantial improvement of their symptoms after operative therapy, particularly those who undergo debulking operations. After controlling for measures of tumor burden, carcinoid syndrome was not independently associated with worse DFS or OS. These findings may have important implications for the selection and prognosis of patients with GEP-NETs being considered for operative therapy.
Supplementary Material
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.surg.2018.09.008.
References
- 1.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halperin DM, Shen C, Dasari A, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol. 2017;18:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai B, Broder MS, Chang E, et al. Predictive factors associated with carcinoid syndrome in patients with gastrointestinal neuroendocrine tumors. World J Gastroenterol. 2017;23:7283–7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58–64. [DOI] [PubMed] [Google Scholar]
- 5.Soga J. Carcinoids and their variant endocrinomas. An analysis of 11842 reported cases. J Exp Clin Cancer Res. 2003;22:517–530. [PubMed] [Google Scholar]
- 6.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. [DOI] [PubMed] [Google Scholar]
- 7.Janson ET, Holmberg L, Stridsberg M, et al. Carcinoid tumors: analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol. 1997;8:685–690. [DOI] [PubMed] [Google Scholar]
- 8.Jann H, Roll S, Couvelard A, et al. Neuroendocrine tumors of midgut and hindgut origin: tumor-node-metastasis classification determines clinical outcome. Cancer. 2011;117:3332–3341. [DOI] [PubMed] [Google Scholar]
- 9.Formica V, Wotherspoon A, Cunningham D, et al. The prognostic role of WHO classification, urinary 5-hydroxyindoleacetic acid and liver function tests in metastatic neuroendocrine carcinomas of the gastroenteropancreatic tract. Br J Cancer. 2007;96:1178–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zandee WT, de Herder WW, Jann H. Incidence and prognosis of carcinoid syndrome: hormones or tumour burden? Lancet Oncol. 2017;18:e299. [DOI] [PubMed] [Google Scholar]
- 11.Ducreux M Carcinoid syndrome in neuroendocrine tumors: a prognostic effect? Lancet Oncol. 2017;18:426–428. [DOI] [PubMed] [Google Scholar]
- 12.Soga J, Yakuwa Y, Osaka M. Carcinoid syndrome: a statistical evaluation of 748 reported cases. J Exp Clin Cancer Res. 1999;18:133–141. [PubMed] [Google Scholar]
- 13.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66:411–421. [DOI] [PubMed] [Google Scholar]
- 14.Onaitis MW, Kirshbom PM, Hayward TZ, et al. Gastrointestinal carcinoids: characterization by site of origin and hormone production. Ann Surg. 2000;232:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito T, Lee L, Jensen RT. Carcinoid-syndrome: recent advances, current status and controversies. Curr Opin Endocrinol Diabetes Obes. 2018;25:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirshbom PM, Kherani AR, Onaitis MW, et al. Carcinoids of unknown origin: comparative analysis with foregut, midgut, and hindgut carcinoids. Surgery. 1998;124:1063–1070. [DOI] [PubMed] [Google Scholar]
- 17.Alexandraki K, Angelousi A, Boutzios G, et al. Management of neuroendocrine tumors of unknown primary. Rev Endocr Metab Disord. 2017. [DOI] [PubMed] [Google Scholar]
- 18.Liang PS, Shaffer K. Metastatic gastrointestinal carcinoid tumor with unknown primary site. Radiol Case Rep. 2007;2:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartlett EK, Roses RE, Gupta M, et al. Surgery for metastatic neuroendocrine tumors with occult primaries. J Surg Res. 2013;184:221–227. [DOI] [PubMed] [Google Scholar]
- 20.La Rosa S, Franzi F, Albarello L, et al. Serotonin-producing enterochromaffin cell tumors of the pancreas: clinicopathologic study of 15 cases and comparison with intestinal enterochromaffin cell tumors. Pancreas. 2011;40:883–895. [DOI] [PubMed] [Google Scholar]
- 21.Zavras N, Schizas D, Machairas N, et al. Carcinoid syndrome from a carcinoid tumor of the pancreas without liver metastases: A case report and literature review. Oncol Lett. 2017;13:2373–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman JM, Jones RS. Carcinoid syndrome from gastrointestinal carcinoids without liver metastasis. Ann Surg. 1982;196:33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riechelmann RP, Pereira AA, Rego JF, et al. Refractory carcinoid syndrome: a review of treatment options. Ther Adv Med Oncol. 2017;9:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massimino K, Harrskog O, Pommier S, et al. Octreotide LAR and bolus octreotide are insufficient for preventing intraoperative complications in carcinoid patients. J Surg Oncol. 2013;107:842–846. [DOI] [PubMed] [Google Scholar]
- 25.Woltering EA, Wright AE, Stevens MA, et al. Development of effective prophylaxis against intraoperative carcinoid crisis. J Clin Anesth. 2016;32:189–193. [DOI] [PubMed] [Google Scholar]
- 26.Kinney MA, Warner ME, Nagorney DM, et al. Perianaesthetic risks and outcomes of abdominal surgery for metastatic carcinoid tumours. Br J Anaesth. 2001;87:447–452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


