Abstract
Background
Iron‐deficiency anaemia is highly prevalent among non‐pregnant women of reproductive age (menstruating women) worldwide, although the prevalence is highest in lower‐income settings. Iron‐deficiency anaemia has been associated with a range of adverse health outcomes, which restitution of iron stores using iron supplementation has been considered likely to resolve. Although there have been many trials reporting effects of iron in non‐pregnant women, these trials have never been synthesised in a systematic review.
Objectives
To establish the evidence for effects of daily supplementation with iron on anaemia and iron status, as well as on physical, psychological and neurocognitive health, in menstruating women.
Search methods
In November 2015 we searched CENTRAL, Ovid MEDLINE, EMBASE, and nine other databases, as well as four digital thesis repositories. In addition, we searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and reference lists of relevant reviews.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs comparing daily oral iron supplementation with or without a cointervention (folic acid or vitamin C), for at least five days per week at any dose, to control or placebo using either individual‐ or cluster‐randomisation. Inclusion criteria were menstruating women (or women aged 12 to 50 years) reporting on predefined primary (anaemia, haemoglobin concentration, iron deficiency, iron‐deficiency anaemia, all‐cause mortality, adverse effects, and cognitive function) or secondary (iron status measured by iron indices, physical exercise performance, psychological health, adherence, anthropometric measures, serum/plasma zinc levels, vitamin A status, and red cell folate) outcomes.
Data collection and analysis
We used the standard methodological procedures of Cochrane.
Main results
The search strategy identified 31,767 records; after screening, 90 full‐text reports were assessed for eligibility. We included 67 trials (from 76 reports), recruiting 8506 women; the number of women included in analyses varied greatly between outcomes, with endpoint haemoglobin concentration being the outcome with the largest number of participants analysed (6861 women). Only 10 studies were considered at low overall risk of bias, with most studies presenting insufficient details about trial quality.
Women receiving iron were significantly less likely to be anaemic at the end of intervention compared to women receiving control (risk ratio (RR) 0.39 (95% confidence interval (CI) 0.25 to 0.60, 10 studies, 3273 women, moderate quality evidence). Women receiving iron had a higher haemoglobin concentration at the end of intervention compared to women receiving control (mean difference (MD) 5.30, 95% CI 4.14 to 6.45, 51 studies, 6861 women, high quality evidence). Women receiving iron had a reduced risk of iron deficiency compared to women receiving control (RR 0.62, 95% CI 0.50 to 0.76, 7 studies, 1088 women, moderate quality evidence). Only one study (55 women) specifically reported iron‐deficiency anaemia and no studies reported mortality. Seven trials recruiting 901 women reported on 'any side effect' and did not identify an overall increased prevalence of side effects from iron supplements (RR 2.14, 95% CI 0.94 to 4.86, low quality evidence). Five studies recruiting 521 women identified an increased prevalence of gastrointestinal side effects in women taking iron (RR 1.99, 95% CI 1.26 to 3.12, low quality evidence). Six studies recruiting 604 women identified an increased prevalence of loose stools/diarrhoea (RR 2.13, 95% CI 1.10, 4.11, high quality evidence); eight studies recruiting 1036 women identified an increased prevalence of hard stools/constipation (RR 2.07, 95% CI 1.35 to 3.17, high quality evidence). Seven studies recruiting 1190 women identified evidence of an increased prevalence of abdominal pain among women randomised to iron (RR 1.55, 95% CI 0.99 to 2.41, low quality evidence). Eight studies recruiting 1214 women did not find any evidence of an increased prevalence of nausea among women randomised to iron (RR 1.19, 95% CI 0.78 to 1.82). Evidence that iron supplementation improves cognitive performance in women is uncertain, as studies could not be meta‐analysed and individual studies reported conflicting results. Iron supplementation improved maximal and submaximal exercise performance, and appears to reduce symptomatic fatigue. Although adherence could not be formally meta‐analysed due to differences in reporting, there was no evident difference in adherence between women randomised to iron and control.
Authors' conclusions
Daily iron supplementation effectively reduces the prevalence of anaemia and iron deficiency, raises haemoglobin and iron stores, improves exercise performance and reduces symptomatic fatigue. These benefits come at the expense of increased gastrointestinal symptomatic side effects.
Plain language summary
Iron supplementation taken daily for improving health in menstruating women
Review question
What are the effects of iron, taken orally for at least five days a week, on health outcomes in menstruating women (compared with not giving iron)?
Background
Iron deficiency (a shortage of iron stored in the body) and anaemia (low levels of haemoglobin ‐ healthy red blood cells ‐ in the blood) are common problems globally, especially in women. Low levels of iron can eventually cause anaemia (iron‐deficiency anaemia). Among non‐pregnant women, around one third are anaemic worldwide. The problem is seen most commonly in low‐income countries, but iron deficiency and anaemia are more common in women in all contexts. Iron‐deficiency anaemia is considered to impair health and well‐being in women, and iron supplements ‐ tablets, capsules, syrup or drops containing iron ‐ are a commonly used intervention to prevent and treat this condition. We sought to review the evidence of iron, taken orally for at least five days per week, for improving health outcomes in non‐pregnant women of reproductive age (menstruating women).
Search data
The review is current to November 2015.
Study characteristics
We included studies comparing the effects of iron compared with no iron when given at least five days per week to menstruating women. We identified 67 trials recruiting 8506 women eligible for inclusion in the review. Most trials lasted between one and three months. The most commonly used iron form was ferrous sulphate.
Key results
We found evidence that iron supplements reduce the prevalence of anaemia and iron deficiency, and raise levels of haemoglobin in the blood and in iron stores. Iron supplementation clearly increases the risk of side effects, for example, constipation and abdominal pain.
Quality of the evidence
We found high quality evidence that iron improves haemoglobin and produces changes in bowel function, but moderate quality evidence that iron reduces the prevalence of anaemia and iron deficiency. Evidence of the effects of iron on other outcomes, such as abdominal pain, is of low quality. There are no data on the effects of iron on mortality in this population group.
Further definitive studies are needed to identify whether taking iron supplements orally for at least five days a week has an impact on key, health‐related outcomes.
Summary of findings
for the main comparison.
| Daily oral iron supplementation | ||||
|
Patient or population: menstruating women (non‐pregnant women of reproductive age) Settings: all settings Intervention: daily oral iron supplementation Comparison: no daily iron supplementation | ||||
| Outcomes | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Anaemia at end of therapy (total), as defined by trial authors | RR 0.39 (0.25 to 0.60) | 3273 (10) | ⊕⊕⊕⊝ Moderate1 | Large effect, but downgraded 1 level for risk of bias and 1 level for inconsistency |
| Haemoglobin at end of therapy (total), g/L | MD 5.30 (4.14 to 6.45) | 6861 (51) | ⊕⊕⊕⊕ High | ‐ |
| Iron deficiency at end of therapy (total), as defined by trial authors | RR 0.62 (0.50 to 0.76) | 1088 (7) | ⊕⊕⊕⊝ Moderate1 | Downgraded 1 level for risk of bias |
| Iron‐deficiency anaemia at end of therapy, as defined by trial authors | ‐ | 55 (1) | ‐ | Meta‐analysis not possible |
| All‐cause mortality, over the course of the study | ‐ | ‐ | ‐ | Not measured |
| Any adverse side effects (total), as defined and reported by trial authors | RR 2.14 (0.94 to 4.86) | 901 (7) | ⊕⊕⊝⊝ Low2 | Downgraded 1 level for imprecision, and 1 level for risk of bias |
| GI side effects (total), events during study, as defined and reported by trial authors | RR 1.99 (1.26 to 3.12) | 521 (5) | ⊕⊕⊝⊝ Low2 | Downgraded 1 level for risk of bias, and 1 level for imprecision |
| Loose stools/diarrhoea (total), events during study, as defined and reported by trial authors | RR 2.13, (1.10 to 4.11) | 604 (6) | ⊕⊕⊕⊕ High2 | ‐ |
| Hard stools/constipation (total), events during study, as defined and reported by trial authors | RR 2.07 (1.35 to 3.17) | 1036 (8) | ⊕⊕⊕⊕ High2 | ‐ |
| Abdominal pain (total), events during study, as defined and reported by trial authors | RR 1.55 (0.99 to 2.41) | 1190 (7) | ⊕⊕⊝⊝ Low2 | Downgraded 1 level for risk of bias, and 1 level for imprecision |
| Cognitive function, as measured by trial authors | ‐ | ‐ | ‐ | Unable to combine the data in a meta‐analysis |
| CI: confidence interval; GI: gastrointestinal; GRADE: Grades of Recommendation, Assessment, Development, and Evaluation; MD: mean difference; RR: risk ratio | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1Anaemia and iron deficiency are rated as moderate as, although iron benefits both outcomes, further studies are needed to more accurately quantify benefit. 2The quality of evidence for several adverse outcomes (any adverse effect, GI side effects and abdominal pain) were deemed low due to insufficient numbers to determine the true effect of intervention with wide CIs. Diarrhoea and constipation had similar participant numbers, however, the magnitude of the difference between intervention and control arms were larger.
Background
Description of the condition
Over 1.6 billion people worldwide have anaemia, a condition in which haemoglobin production is diminished. Women of menstruating age account for approximately a third of all cases of anaemia across the globe (WHO/CDC 2008). The most recent estimates suggest that 29% of non‐pregnant women worldwide are anaemic (Stevens 2013). Iron deficiency is believed to contribute to at least half the global burden of anaemia, especially in non‐malaria‐endemic countries (Stoltzfus 2001). Iron deficiency is thus considered the most prevalent nutritional deficiency in the world.
Iron deficiency occurs following negative iron balance. As body iron stores are exhausted, the production of red blood cells is impaired, and finally iron‐deficiency anaemia results (Suominen 1998). The major causes of negative iron balance include inadequate dietary iron intake (due to consumption of a diet with a low overall or bioavailable iron content); increased losses of iron due to chronic blood loss (in women, due to menstruation and exacerbated in cases of heavier menstrual bleeding, and by intestinal hookworm infection in individuals living in endemic settings (Hotez 2005)); and increased iron requirements (e.g. during growth or pregnancy). Low dietary iron intake and bioavailability are considered key contributors to the burden of iron deficiency. This is especially so in populations consuming diets that are low in meat sources and high in cereals such as wheat, rice, maize and millet, which are rich in phytates, compounds that bind to iron in the meal preventing its absorption (Sharpe 1950). Other dietary components such as tannins (found in tea) and calcium (contained in milk products) also inhibit iron absorption.
Women beyond menarche and prior to menopause are at especially high risk of iron deficiency due to menstrual blood losses. The onset of menstrual blood losses accompanied by rapid growth, with an associated expansion of red cell mass and tissue iron requirements, means adolescent girls have a particularly high iron need compared with their male counterparts. If this is compounded by inadequate dietary iron intake, they may be at especially high risk of iron deficiency (Dallman 1992). Other important causes of iron deficiency in women include intestinal malabsorptive conditions such as coeliac disease, chronic blood losses due to menorrhagia from uterine pathologies (such as fibroids), frequent blood donation, and benign and malignant gastrointestinal lesions (Goddard 2011). Iron deficiency, with and without anaemia, has also been noted to be prevalent among female athletes and is thought to be due to diets deficient in iron, increased losses due to gastrointestinal tract bleeding, and reduced iron absorption due to subclinical inflammation (Peeling 2008). The risk of iron deficiency may be modified by genetic factors such as inheritance of genes associated with haemochromatosis and polymorphisms in the TMPRSS6 gene (Chambers 2009).
As well as being critical to the production of haemoglobin, iron has a critical role in many other aspects of human physiology as it is involved in a range of oxidation‐reduction enzymatic reactions in the muscle and nervous tissue (Andrews 1999), as well as other organs. Iron deficiency and iron‐deficiency anaemia have been associated with a range of adverse physical, psychological, and cognitive effects. Animal models suggest a role for iron in brain development and function, with iron depletion being associated with dysregulated neurotransmitter levels (Lozoff 2007), and some, but not all, clinical studies have shown associations between iron supplementation and improvement in cognitive performance (Murray‐Kolb 2007) and mood and well being, with a reduction in fatigue (Verdon 2003). Observational studies have suggested that iron deficiency in the absence of anaemia impairs exercise performance in women (Scholz 1997), while some, but not all, interventional studies of iron supplementation among the same population have shown variable improvements in maximal and submaximal exercise performance (Brownlie 2002; LaManca 1993), endurance (Brownlie 2004; Hinton 2000), and muscle fatigue (Brutsaert 2003). There may also be associations between iron status and haemoglobin concentrations and work productivity (Li 1994: Scholz 1997; Wolgemuth 1982). When anaemia is severe, it may cause lethargy, fatigue, irritability, pallor, breathlessness and reduced tolerance for exertion.
Alleviation of iron‐deficiency anaemia among menstruating women is thus considered a major public health priority, both to improve their existing health status and to enhance their health in preparation for future pregnancies (WHO 2009).
Other causes of anaemia important to distinguish from iron deficiency include anaemia of chronic disease (associated with inflammation, which causes iron to be withheld from erythropoiesis (the process by which red blood cells are produced)), functional iron deficiency (associated with renal impairment), genetic conditions of the red cell (haemoglobin, enzymes and membrane), and infectious diseases (including malaria).
Description of the intervention
Strategies to improve iron intake and alleviate iron‐deficiency anaemia include mass and point‐of‐use fortification of foods with iron; dietary diversification to increase iron intake, absorption and utilisation; iron supplementation; and antihelminthic treatment. Supplementation is probably the most widespread intervention practiced clinically and in public health.
Oral iron supplementation, administered once a day or more frequently, is the standard clinical practice of many physicians in the treatment of iron deficiency in women (Goddard 2011). Daily iron and folic acid supplementation for three months should be considered for the prophylaxis of iron deficiency in populations where the prevalence of anaemia exceeds 40% (WHO/UNICEF/UNU 2001). In addition to its haematological effects, the use of folic acid during the periconceptional period helps prevent the risk of neural tube defects in babies (WHO/UNICEF/UNU 2001).
Iron is generally administered as a salt compound in a tablet, capsule, liquid or dispersible formulation. The most commonly prescribed salts are ferrous sulphate, fumarate, and gluconate (Pasricha 2010). Ferrous sulphate is perhaps the most commonly used of these interventions. Iron formulations are commonly combined with vitamin C to improve absorption, or folic acid to improve child outcomes when used before or during pregnancy. Commonly reported side effects of iron supplements include gastrointestinal disturbances (especially constipation and nausea) and dark stools. In those using liquid formulations, tooth staining can occur. Slow or sustained‐release formulations in which iron is surrounded by a coating, aim to alleviate gastrointestinal side effects by delaying delivery of iron to a more distal point in the gastrointestinal tract, but their efficacy has been questioned. Thus, compliance to daily oral iron interventions due to adverse events can be a critical limiting factor to their effectiveness.
How the intervention might work
Iron is absorbed by intestinal cell luminal and basal transporters, bound to proteins and transported to the bone marrow, muscle and other tissue, where it is taken up by specific receptors and used for biological functions or stored (Andrews 1999). Textbooks advise that in an iron‐deficient anaemic individual, haemoglobin concentrations should rise by 1 g/dL per week, with early evidence of red blood cell formation discernible in the peripheral blood after 72 hours of supplementation (Mahoney 2011).
There is an inverse relationship between iron status and the ability to absorb iron. Iron deficiency induces changes in intestinal iron transport that can double absorption of iron from the diet (Thankachan 2008). Thus, as with dietary sources of iron, absorption from supplements depends on the baseline iron status of the individual and the co‐consumption of iron absorption enhancers (such as vitamin C, other acidic foods, and meat) and inhibitors (calcium, phytates and tannins) (Hurrell 2010; Sharpe 1950).
As mentioned above, the ubiquitous presence of iron in the human body is such that its deficiency impairs a number of physiological functions and iron supplementation may thus benefit physical, psychological and cognitive health. Improvements in haemoglobin and myoglobin concentrations may ensure adequate tissue oxygenation and performance (Umbreit 2005). Iron is also present in the brain in relatively large amounts and is involved in neurotransmitter function (Burhans 2005); an adequate supply may contribute to maintaining normal cognitive and psychological health, although the mechanisms are not completely elucidated as yet.
An additional consideration when providing supplements at population level is the endemicity of malaria in a given region. Approximately 40% of the world's population is exposed to the malaria parasite and it is endemic in over 100 countries, causing more than a million deaths per year (WHO 2010). Provision of iron in malaria‐endemic areas, particularly to children, has been controversial due to concerns that iron therapy may exacerbate infections, in particular malaria (Okebe 2011; Oppenheimer 2001). It is still not completely clear whether iron produces the same effects among older populations or whether subclinical malaria alters the response to iron supplementation.
Why it is important to do this review
Daily oral supplementation in women has been a longstanding intervention in both public health and clinical fields. Many patients and clinicians ascribe adverse health outcomes (including fatigue and lethargy, impaired cognitive performance and psychological dysfunction) to iron deficiency, even in the absence of anaemia, and attribute improvement in these symptoms to iron supplementation. In addition, many sporting authorities (including the International Olympic Committee (IOC 2009)) recommend screening of female athletes for iron deficiency in order to target the use of iron supplementation, with a view to improving performance. Daily iron and folic acid supplementation for three months should be considered for the prophylaxis of iron deficiency in populations where the prevalence of anaemia exceeds 40%. Iron supplementation has been recommended for preventing anaemia in women of childbearing age, and to optimise pre‐conception iron status (WHO 2011).
Several intervention trials have evaluated improvements in haemoglobin and iron status, as well as non‐haematologic outcomes such as physical, cognitive and psychological health, in menstruating women receiving iron supplementation. However, evaluation of this intervention has not been subject to systematic review and thus it is difficult to estimate the benefits and risks associated with the daily use of iron supplements in menstruating women.
This review will complement the findings of other Cochrane systematic reviews assessing the use of iron supplements alone, or in combination with other vitamins and minerals, in different female populations: intermittent supplementation in children (De‐Regil 2011), iron supplementation among children in malaria‐endemic areas (Okebe 2011), intermittent iron supplementation in menstruating women (Fernández‐Gaxiola 2011), daily and intermittent iron and folic acid supplementation in pregnant women (Peña‐Rosas 2009), multiple micronutrient supplementation in pregnancy (Haider 2006), and iron supplementation during the postpartum period (Dodd 2004).
Objectives
To establish the evidence for effects of daily supplementation with iron on anaemia and iron status, as well as on physical, psychological and neurocognitive health, in menstruating women.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs with either individual‐ or cluster‐randomisation. Quasi‐RCTs are trials that use non‐random systematic methods to allocate participants to treatment groups such as alternation, or assignment based on date of birth or case record number (Higgins 2011a).
We did not include observational study designs (e.g. cohort or case‐control studies) in the meta‐analysis but, where relevant, considered such evidence in the discussion.
Types of participants
Inclusion criteria
Menstruating women, that is, women beyond menarche and prior to menopause who were not pregnant or lactating or had any condition that impeded the presence of menstrual periods, regardless of their baseline iron or anaemia status (or both), ethnicity, country of residence or level of endurance.
We included studies for which results for females between 12 years and 50 years of age (plausible age range for menstruation) could be extracted separately, or in which more than half of the participants fulfilled this criterion.
Exclusion criteria
Studies targeting populations with conditions affecting iron metabolism, intestinal malabsorption conditions, ongoing excessive blood loss (including ongoing blood donations), inflammatory bowel disease, cancer, chronic congestive cardiac failure, chronic renal failure, chronic liver failure or chronic infectious disease.
Studies that were purely evaluating kinetics of erythropoiesis or pharmacology of iron supplements or absorption.
Studies in hospitalised or ill people.
Types of interventions
We considered iron supplements to comprise iron formulations that may or may not have also contained folic acid or vitamin C, since these are commonly included in iron preparations. Doses needed to be given no less than five days a week, regardless of dose and duration of the intervention.
We included, in an overall comparison, effects of daily oral supplementation with iron alone, or in combination with folic acid or vitamin C, versus receiving no supplemental iron. In this review, 'iron supplement' refers to compounds containing iron salts such as ferrous sulphate, ferrous fumarate, ferrous gluconate, carbonyl or colloidal iron. Iron may have been delivered as a tablet (including dispersible forms), capsule, or liquid.
We included (and noted) studies in which iron supplements were given along with cointerventions such as other nutrients (e.g. zinc, vitamin A), deworming, education or other approaches but only if the cointerventions were the same in both the intervention and comparison groups. We did not include studies where additional haemopoietic agents were administered such as exogenous erythropoietin.
We undertook a simple overall comparison (iron versus control) and considered use of cointerventions as subgroups. This enabled us to appraise the overall evidence for intervening with iron supplementation, but differed from what we had proposed in our original protocol (Differences between protocol and review).
Interventions excluded from this review include point‐of‐use fortification with micronutrient powders or lipid‐based foods, mass fortification of staple foods such as wheat or maize flours or condiments, and intermittent iron supplementation, which are evaluated in previous or ongoing Cochrane reviews (see Fernández‐Gaxiola 2011; Pasrischa 2012; Peña‐Rosas 2014; Self 2012).
Types of outcome measures
Primary outcomes
Anaemia (haemoglobin concentrations below a cut‐off defined by trial authors).*
Haemoglobin (g/L).*
Iron deficiency (as measured by trial authors using indicators of iron status such as ferritin or transferrin).*
Iron‐deficiency anaemia (defined by the presence of anaemia plus iron deficiency, diagnosed with an indicator of iron status selected by trialists).*
All‐cause mortality.*
Adverse side effects (as measured by trial authors such as abdominal pain, vomiting, nausea, heartburn, diarrhoea, constipation).*
Cognitive function (as defined by trial authors. For example, for adolescents, school grades, test performance, intelligence testing; for adults not in school, formal tests addressing intelligence, memory, attention, and other cognitive domains). We accepted any measure of cognitive function that has been previously validated as an appropriate test in this domain.*
Outcomes marked with an asterisk (*) are included in Table 1.
Secondary outcomes
Iron status (as reported: ferritin, transferrin saturation, soluble transferrin receptor, soluble transferrin receptor‐ferritin index, total iron binding capacity, serum iron).
Physical exercise performance (as defined by trial authors, in particular peak exercise performance (VO2 max/peak ‐ absolute and relative), submaximal exercise performance (heart rate, percentage VO2 max, energy consumption), and endurance (time)).
Psychological health (e.g. depression as defined by the Center for Epidemiological Studies ‐ Depression (CES‐D) scale (Radloff 1977) or visual analogue scales; fatigue as defined by the trial authors, anxiety as defined by trial authors.
Adherence (percentage of women who consumed more than 70% of the expected doses).
Anthropometric measures (Z scores for height and weight by age for adolescents, and body mass index for adults).
Serum/plasma zinc (μmol/L).
Vitamin A status (serum/plasma retinol (mmol/L) or retinol binding protein (mmol/L)).
Red cell folate (mmol/L).
For populations in malaria‐endemic areas, we reported two additional outcomes.
Malaria incidence.
Malaria severity.
If two outcomes evaluated the same construct (e.g. iron status evaluated with either ferritin or soluble transferrin receptors), we treated them separately.
Search methods for identification of studies
Electronic searches
We searched the following databases in March 2012, November 2014 and again on 12 November 2015.
Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 10, part of The Cochrane Library), and which includes the specialised register of the Cochrane Developmental, Psychosocial and Learning Problems Group.
Ovid MEDLINE (1946 to November Week 1 2015).
Embase (1980 to 2015 Week 45; Ovid).
CINAHL (1937 to current; EBSCOHost).
Conference Proceedings Citation Index ‐ Science (CPCI‐S; 1937 to current; Web of Science).
Science Citation Index (SCI; 1970 to 10 November 2015; Web of Science).
POPLINE (popline.org; all available years).
We searched the following regional indexes from the World Health Organization (WHO) Global Health Library on 28 May 2015, and again on 8 December 2015.
Literature in the Health Sciences in Latin America and the Caribbean (LILACS; all available years).
African Index Medicus (AIM; all available years).
Western Pacific Region Index Medicus (WPRIM; all available years).
Index Medicus for the Eastern Mediterranean Region (IMEMR; all available years).
Index Medicus for South‐East Asia Region (IMSEAR; all available years).
We used the following sources to search for theses on 28 May 2015, and again on 8 December 2015.
WorldCat (worldcat.org; all available years).
DART‐Europe E‐theses Portal (dart‐europe.eu; all available years).
Australasian Digital Theses Program (trove.nla.gov.au; all available years).
Proquest Dissertations and Theses Global (all available years).
The search strategies for each database are reported in Appendix 1. We did not apply any date or language restrictions.
Searching other resources
We searched all available years of the WHO International Clinical Trials Registry Platform (ICTRP) on 25 May 2015, and again on 8 December 2015 (apps.who.int/trialsearch). We also screened previously published reviews in order to identify other possible studies.
Data collection and analysis
Selection of studies
We stored all studies identified by our search strategy in Endnote 2015 reference manager software prior to evaluation. Titles and abstracts of obtained studies were screened by two authors (MSYL and SRP) independently. For those studies that were selected as potentially eligible for inclusion, two of the review authors (from CES, JS, MSYL or SRP) assessed whether they met the review's inclusion criteria. We kept records of all eligibility decisions using a digital eligibility form for each study. If study reports contained insufficient information on methods, participants or interventions, we attempted to contact the authors for further information. Disagreements were resolved through discussion between the coauthors.
Data extraction and management
We extracted data from studies using a digital extraction form designed for this review. We first piloted the form on a small number of study reports and modified it as necessary. For eligible studies, two review authors (two from JS, MSYL, CS or SRP) independently extracted data using the form. One author (MSYL) then entered data into Review Manager (RevMan) software (RevMan 2014) and a second author (SRP) checked data entry for accuracy. We resolved discrepancies through discussion between all review authors.
For each study, we collected data on the following domains.
-
Trial methods:
study design;
unit and method of allocation;
masking of participants and outcomes; and
exclusion of participants after randomisation and proportion of losses at follow‐up.
-
Participants:
location of the study;
sample size;
age;
baseline status of anaemia;
baseline status of iron deficiency; and
inclusion and exclusion criteria, as described in Criteria for considering studies for this review.
-
Intervention:
dose of iron;
type of iron compound;
duration of the intervention; and
cointerventions.
-
Comparison group:
use of placebo or no intervention.ppe
-
Outcomes:
primary and secondary outcomes, as outlined in Types of outcome measures.
We recorded outcomes that were both prespecified and not prespecified, although we did not use the latter to underpin the conclusions of the review.
Assessment of risk of bias in included studies
For each study, two of the four review authors (CES, JS, MSYL, SRP) used the standard Cochrane 'Risk of bias' tool to assess the risk of bias of each included study across the following eight domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, other biases, and overall risk of bias (Higgins 2011b). They applied the following criteria. Disagreements were resolved through discussion between review authors.
1. Random sequence generation (checking for possible selection bias)
We assessed whether the method used to generate the allocation sequence was described in sufficient detail to allow an assessment of whether it produced comparable groups and rated it as follows.
Low risk of bias: any truly random process (e.g. random number table, computer random number generator).
High risk of bias: any non‐random process (e.g. odd or even date of birth, hospital or clinic record number).
Unclear risk of bias: random sequence generation not stated or insufficient information to deem whether study was at low or high risk of bias.
2. Allocation concealment (checking for possible selection bias)
We assessed whether the method used to conceal the allocation sequence was described in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment and rated it as follows.
Low risk of bias: telephone or central randomisation, consecutively numbered sealed opaque envelopes used to conceal the allocation sequence.
High risk of bias: open random allocation, unsealed or non‐opaque envelopes used to conceal the allocation sequence.
Unclear risk of bias: not stated or insufficient information to deem whether study was at low or high risk of bias.
3. Blinding of participants and personnel (checking for possible performance bias)
We assessed all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We rated the risk of performance bias associated with blinding as follows.
Low risk of bias: participants and personnel were reported to be blinded in such a manner that they could not determine the groups to which participants belonged.
High risk of bias: participant or personnel were not blinded or blinding was performed in such a manner that it was possible to determine the groups to which participants belonged.
Unclear risk of bias: insufficient information to permit a judgement of low or high risk of bias.
Whilst assessed separately, we combined these assessments into a single evaluation of risk of bias associated with blinding (Higgins 2011b).
4. Blinding of outcome assessment (checking for possible detection bias)
We assessed all measures used, if any, to blind outcome assessors from knowledge as to which intervention a participant received and rated it as follows.
Low risk of bias: blinding of outcome assessment or no blinding of outcome assessment but measurement is unlikely to be influenced by lack of blinding.
High risk of bias: no blinding of outcome assessment, where measurement is likely to be influenced by lack of blinding, or where blinding could have been broken.
Unclear risk of bias: insufficient information to permit a judgement of low or high risk of bias.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed whether incomplete outcome data were adequately addressed and rated it as follows.
Low risk of bias: either there were no missing outcome data, or the missing outcome data were unlikely to bias the results because the study authors provided transparent documentation of participant flow throughout the study, or the proportion of missing data was similar in the intervention and control groups, the reasons for missing data were provided and balanced across intervention and control groups, or the reasons for missing data were not likely to bias the results (e.g. moving house).
High risk of bias: missing outcome data were likely to bias the results. Studies were also considered at high risk of bias if more than 30% of randomised participants were lost to follow‐up and unavailable for final assessment.
Unclear risk of bias: insufficient information was available to permit a judgement of low or high risk of bias.
6. Selective outcome reporting (checking for possible reporting bias)
We evaluated whether reports of the study were free from selective outcome reporting and rated it as follows.
Low risk of bias: where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported.
High risk of bias: where not all of the study’s pre‐specified outcomes were reported, one or more reported primary outcomes were not prespecified, outcomes of interest were reported incompletely and so could not be used, or the study failed to include results of a key outcome that was expected to have been reported.
Unclear risk of bias: insufficient information to deem whether the study was at low or high risk of bias.
7. Other sources of bias
We assessed whether the study was free from other problems that could put it at risk of bias as follows.
Low risk of bias: no other sources of bias appeared relevant to the trial that were not covered in previous categories of bias.
High risk of bias: another source of bias was uncovered.
Unclear risk of bias: insufficient evidence was available to permit a judgement of high or low risk of bias.
8. Overall risk of bias
We summarised the risk of bias at two levels: within studies (across domains) and across studies (for each primary outcome).
For the first, we assessed the likely magnitude and direction of the bias in each of the above mentioned domains and whether we considered them likely to impact on the findings. We considered studies to be at low overall risk of bias if they were not at high risk of bias for any category, and were assessed as having low risk of bias for random sequence generation OR low risk of bias for allocation concealment (selection bias), and were also rated at low risk of bias for either blinding (performance or detection bias) or incomplete outcome data (attrition bias). Studies which failed to provide sufficient information (i.e. unclear risk of bias) to enable categorisation of risk of bias were excluded from categorisation as being at low risk of bias. We explored the impact of including only studies at low risk of bias on primary outcomes through a Sensitivity analysis.
For the assessment across studies, we set out the main findings of the review in Table 1. The primary outcomes for each comparison were listed with estimates of relative effects along with the number of participants and studies contributing data for those outcomes. For each primary outcome, we assessed the quality of the evidence across all trials contributing data using the GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) approach (Balshem 2011), which involves consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias. The results were expressed as one out of four levels of quality (high, moderate, low or very low). This assessment was limited to the trials included in this review only. We produced the tables using GRADEpro GDT 2015.
Measures of treatment effect
We did not combine dichotomous and continuous data for analysis, and instead considered them separately.
Dichotomous data
We presented the results as average risk ratios (RRs) with 95% confidence intervals (CI).
Continuous data
We used the mean difference (MD) with 95% CI if outcomes were measured in the same way between trials. Where some studies reported endpoint data and others reported change from baseline data (with errors), we combined these in the meta‐analysis using the MD providing the outcomes were reported using the same scale.
We used the standardised mean difference (SMD) with 95% CI to combine trials that measured the same outcome but used different methods of measurement.
Unit of analysis issues
Cluster‐randomised trials
We combined the results from both cluster‐randomised and individually‐randomised studies if there was little heterogeneity between these study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered as unlikely.
If the results from cluster trials were not adjusted by trial authors, we calculated the trials' effective sample size to account for the effect of clustering in the data. We used the intracluster correlation coefficient (ICC) derived from the trial (if available), or from another source (e.g. used the ICCs derived from other, similar trials), and then calculated the design effect with the formula provided in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Studies with multiple intervention groups
For studies with more than two intervention groups (multi‐arm studies), we included the directly relevant arms only. Where we identified studies with various relevant arms, we combined the groups into a single pair‐wise comparison (Deeks 2011) and included the disaggregated data in the corresponding subgroup category. If the control group was shared by two or more study arms, we divided the control group (events and total population) over the number of relevant subgroup categories to avoid double counting the participants.
Cross‐over trials
We only included the first period of any randomised cross‐over trial prior to the wash‐out period or to a change in the sequence of treatments, and treated them as parallel trials.
Dealing with missing data
Missing individuals
We noted the dropout rate for each included study, which can be seen in Characteristics of included studies tables. We reported rates of attrition in the 'Risk of bias' tables (beneath the Characteristics of included studies tables) and included them in the 'Risk of bias' summary graph. We conducted analysis on an available case‐analysis basis: data were included from those participants whose results were known. We considered variation in the degree of missing data as a potential source of heterogeneity.
Missing data
Where key data (e.g. standard deviations) were missing from the report, we attempted to contact corresponding authors (or other authors if necessary) of included studies to request unreported data. Two authors were contacted for further information (Pereira 2014; Waldvogel 2012). If we were not able to obtain this information, we used methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c), to attempt to calculate it (performed in one study Zavaleta 2000). If this could not be achieved, we did not impute it and noted that the study did not provide data for that particular outcome.
Assessment of heterogeneity
We assessed methodological heterogeneity by examining the methodological characteristics and risk of bias of the studies, and clinical heterogeneity by examining the similarity between the types of participants, interventions and outcomes (Deeks 2011).
For statistical heterogeneity, we examined the forest plots from meta‐analyses for heterogeneity among studies and used the I² statistic (Higgins 2003), Tau², and Chi² test for heterogeneity to quantify the level of heterogeneity among the trials in each meta‐analysis.
Assessment of reporting biases
Where more than 10 trials contributed data to the primary outcomes, we presented a funnel plot to evaluate asymmetry ‐ a possible indicator of publication bias. Where funnel plot asymmetry was evident, this was formally assessed using Egger's regression test (continuous outcomes) or Peter's or Harbord's test (Sterne 2011); see Differences between protocol and review for more information. This was undertaken using the metan and metabias user‐written modules in Stata 13 (Harbord 2009).
Data synthesis
We conducted a meta‐analysis to obtain an overall estimate of the effect of treatment when more than one study examined similar interventions using similar methods, was conducted in similar populations, and measured similar (comparable) outcomes. We carried out statistical analysis using RevMan 2014.
We used a random‐effects meta‐analysis for combining data, as we anticipated that there was natural heterogeneity between studies attributable to the different doses, durations, populations and implementation/delivery strategies.
Where different studies reported the same outcomes using both continuous and dichotomous measures, we re‐expressed RRs as SMDs or vice versa, and combined the results using the generic inverse‐variance method, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
We performed meta‐analyses of dichotomous outcomes using the Mantel‐Haenszel method.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses on the primary outcomes only.
Age: adolescents (12 to 18 years), older adults (50 to 55 years).
Nutrient: iron alone or iron + other intervention versus intervention alone, iron plus vitamin C versus vitamin C alone, iron + any cointervention versus that same cointervention alone.
Baseline anaemia status (as defined by trial authors): anaemic, non‐anaemic, mixed or unknown.
Baseline iron status (as defined by trial authors): iron deficient, non‐iron deficient, mixed or unknown.
Baseline iron‐deficiency anaemia status (as defined by trial authors): iron deficient with anaemia, iron deficient without anaemia, non‐iron deficient/unknown status of deficiency.
Daily dose of elemental iron supplementation: less than 30 mg, 30 mg to 60 mg, 61 mg to 100 mg, 101 mg or more elemental iron.
Duration of iron supplementation: 30 days (one month) or less, more than one month to three months inclusive, more than three months.
Malaria endemicity of the setting in which the study was performed: endemic, not endemic, not reported/unknown.
We added a further subgroup analysis post‐hoc: types of iron (ferrous sulphate, ferrous fumarate and others). In addition, we decided to undertake subgroup analysis on the following secondary outcome: ferritin (see Differences between protocol and review).
For meta‐analysis including both endpoint and change scores data, we also conducted a subgroup analysis to separate the effects of the two outcome measures.
We did not conduct subgroup analyses in those outcomes with three or less trials. We explored the forest plots visually and identified where CIs did not overlap to assess differences between subgroup categories. We also formally investigated differences between two or more subgroups (Borenstein 2008).
Sensitivity analysis
We conducted sensitivity analyses examining effects of removing studies at high risk of bias (studies with poor or unclear allocation concealment and either inadequate blinding or high/imbalanced loss to follow‐up) from the analysis. Likewise, for cluster studies reporting outcomes where reliable ICCs could not be obtained, we examined the effects of removing these studies from the analysis.
For additional sensitivity analyses archived for future updates of this review, please see our protocol (Pasricha 2012) and Appendix 2.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The search strategy identified 31,767 records for possible inclusion, 9918 of which were duplicates. Three studies were published in languages other than English (Machado 2011; Radjen 2011; Wang 2012) ‐ these were translated to English for extraction. After screening, we assessed 90 full‐text reports for eligibility. We included 67 studies (from 76 reports and one personal communication (see DellaValle 2012)), excluded six studies and classified seven studies as awaiting assessment either because we were unable to access the full text for the trials, despite assistance from an academic library, or determine if they were eligible for inclusion. Our search of WHO ICTRP identified one ongoing study, which may be eligible for inclusion when the results become available, although the findings are unlikely to alter the conclusions of this analysis (IRCT201409082365N9).
Figure 1 depicts the process by which we assessed and selected studies.
1.
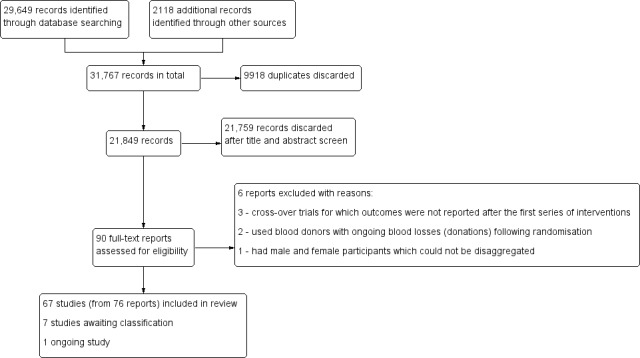
Study flow diagram.
Included studies
Overall, we included 67 trials that recruited a total of 8506 women.
The sample size ranged between 10 and 1390 participants but overall tended to be small: 96% of the studies included fewer than 400 women.
Settings
Studies were conducted in numerous countries of differing cultural and economic background. Included studies in this review were conducted in USA (Binkoski 2004; Bruner 1996; Cooter 1978; DellaValle 2012; Gordeuk 1987; Gordeuk 1990; Hinton 2000; Hinton 2007; Jensen 1991; Kiss 2015; Klingshirn 1992; LaManca 1993; Lyle 1992; McClung 2009; Murray‐Kolb 2007; Rajaram 1995; Rowland 1988; Swain 2007; Viteri 1999; Yadrick 1989; Zhu 1998), Australia (Booth 2014; Leonard 2014; Marks 2014; Walsh 1989; Zaman 2013), United Kingdom (Bryson 1968; Elwood 1966; Elwood 1970; Pereira 2014), Iran (Eftekhari 2006; Kianfar 2000; Maghsudlu 2008), Sri Lanka (Edgerton 1979; Jayatissa 1999; Lanerolle 2000), Sweden (Flink 2006; Hoppe 2013; Rybo 1985), Canada (Larocque 2006; Newhouse 1989), China (Li 1994; Wang 2012), Finland (Fogelholm 1992; Fogelholm 1994), India (Agarwal 2003; Kanani 2000), Israel (Ballin 1992; Magazanik 1991), Japan (Taniguchi 1991; Yoshida 1990), New Zealand (Heath 2001; Prosser 2010), Switzerland (Verdon 2003; Waldvogel 2012), Bolivia (Berger 1997), Brazil (Machado 2011), Chile (Mujica‐Coopman 2015), Korea (Kang 2004), Mexico (Brutsaert 2003), Nepal (Shah 2002), Norway (Røsvik 2010), Peru (Zavaleta 2000), Phillipines (Florencio 1981), Serbia (Radjen 2011), Tanzania (Gunaratna 2015) and Thailand (Charoenlarp 1988).
Only two studies specifically stated being conducted in low socioeconomic settings (Kanani 2000; Zavaleta 2000); however it is likely that other studies were also performed in situations that would include low socioeconomic participants. One study specifically targeted middle‐class participants (as defined by the trial authors) (Agarwal 2003).
Nine studies were performed specifically in an urban setting (Agarwal 2003; Ballin 1992; Bruner 1996; Florencio 1981; Heath 2001; Rybo 1985; Shah 2002; Wang 2012; Zavaleta 2000), four in a rural setting (Berger 1997; Charoenlarp 1988; Edgerton 1979; Gunaratna 2015). One study reports specifically recruiting from both rural and urban settings (Lanerolle 2000). The majority of studies did not specifically state whether the trials were performed in rural or urban settings (Binkoski 2004; Booth 2014; Brutsaert 2003; Bryson 1968; Cooter 1978; DellaValle 2012; Eftekhari 2006; Elwood 1966; Elwood 1970; Flink 2006; Fogelholm 1992; Fogelholm 1994; Gordeuk 1987; Gordeuk 1990; Hinton 2000; Hinton 2007; Jayatissa 1999; Jensen 1991; Hoppe 2013; Kanani 2000; Kang 2004; Kianfar 2000; Kiss 2015; Klingshirn 1992; LaManca 1993; Larocque 2006; Leonard 2014; Li 1994; Lyle 1992; Machado 2011; Magazanik 1991; Maghsudlu 2008; Marks 2014; McClung 2009; Mujica‐Coopman 2015, Murray‐Kolb 2007; Newhouse 1989; Pereira 2014; Prosser 2010; Radjen 2011; Rajaram 1995; Rowland 1988; Røsvik 2010; Swain 2007; Taniguchi 1991; Verdon 2003; Viteri 1999; Waldvogel 2012; Walsh 1989; Yadrick 1989; Yoshida 1990; Zaman 2013; Zhu 1998).
Only two studies specifically reported being performed in a malaria‐endemic area (Charoenlarp 1988; Gunaratna 2015), with the majority not reporting malaria endemicity at the site of the trial.
Participants
Across the included studies a total of 8508 women were included; 4444 in the intervention arm, 4,064 in the control arm. The majority of studies recruited women between the ages of 13 years and 45 years. Three studies included women below 13 years of age: Agarwal 2003: range 10 years to 17 years (mean age not stated); Shah 2002: age range 11 years to 18 years (mean age 15 years); Zavaleta 2000: age range 12 years to 18 years (mean age 15 years). Six studies recruited females older than 45 years (Edgerton 1979: age range 20 years to 60 years (mean age: 35 years); Kiss 2015: age range not reported (mean age: 45.7 years); Machado 2011: age range 20 years to 49 years (mean age: not reported); Røsvik 2010: age range 18 years to 69 years (mean age: 43 years); Swain 2007: age range 21 years to 51 years (mean age: 40 years); Verdon 2003: age range 18 years to 55 years (mean age: 35 years). In these trials, data for participants aged within the target age range could not be extracted separately, although they met our inclusion criteria of comprising more than half of participants within the eligible age range.
Twenty‐six studies recruited women in an educational setting with 12 in secondary education (Agarwal 2003; Ballin 1992; Bruner 1996; Eftekhari 2006; Jayatissa 1999; Kanani 2000; Kianfar 2000; Lanerolle 2000; Larocque 2006; Rowland 1988; Shah 2002; Zavaleta 2000) and 14 in tertiary education (Cooter 1978; DellaValle 2012; Hoppe 2013; Jensen 1991; Klingshirn 1992; Lyle 1992; Murray‐Kolb 2007; Pereira 2014; Rajaram 1995; Taniguchi 1991; Viteri 1999; Yoshida 1990; Zaman 2013; Zhu 1998).
Four studies recruited women through a specific workplace: factory workers (Bryson 1968; Florencio 1981), tea pickers (Edgerton 1979; Li 1994). Ten studies recruited women through sports teams (Cooter 1978; DellaValle 2012; Fogelholm 1992; Kang 2004; Klingshirn 1992; LaManca 1993; Radjen 2011; Rowland 1988; Walsh 1989; Yoshida 1990). Seven studies recruited women through blood donation centres (Gordeuk 1987; Gordeuk 1990; Kiss 2015; Maghsudlu 2008; Marks 2014; Røsvik 2010; Waldvogel 2012); in these studies, women did not undergo further blood donations between enrolment and outcome measurement.
Dose and type of iron interventions
A variety of oral iron formulations were included in this review. The most frequently used was ferrous sulphate (33 studies; Binkoski 2004; Bruner 1996; Brutsaert 2003; DellaValle 2012; Edgerton 1979; Eftekhari 2006; Florencio 1981; Fogelholm 1992; Hinton 2000; Hinton 2007; Jensen 1991; Kianfar 2000; Klingshirn 1992; Lanerolle 2000; Leonard 2014; Li 1994; Lyle 1992; Machado 2011; Magazanik 1991; Maghsudlu 2008; McClung 2009; Mujica‐Coopman 2015; Murray‐Kolb 2007; Newhouse 1989; Pereira 2014; Radjen 2011; Rajaram 1995; Shah 2002; Verdon 2003; Viteri 1999; Waldvogel 2012; Zavaleta 2000; Zhu 1998). One study included two arms: ferrous sulphate and carbonyl iron (Gordeuk 1987). Two studies used carbonyl iron (Gordeuk 1990; Marks 2014). Five studies used ferrous fumarate (Bryson 1968; Cooter 1978; Flink 2006; Fogelholm 1994; Hoppe 2013), and one study used ferric pyrophosphate and ferrous fumurate together (Wang 2012).
Other iron formulations that were used included ferrous carbonate (Elwood 1966; Elwood 1970), ferrous gluconate (Booth 2014; Kiss 2015; Larocque 2006; Zaman 2013), ferric ammonium citrate (Taniguchi 1991), ferrous succinate (Rybo 1985), Niferex ferrous glycine sulphate (Røsvik 2010), amino acid chelate (Heath 2001; Prosser 2010), ferrous sodium citrate (Yoshida 1990), LiquiFer® (Iron polystyrene sulfonate) (Ballin 1992). Twelve studies did not state the specific iron formulation used (Agarwal 2003; Berger 1997; Charoenlarp 1988; Gunaratna 2015; Jayatissa 1999; Kanani 2000; Kang 2004; LaManca 1993; Rowland 1988; Swain 2007; Walsh 1989; Yadrick 1989). Doses of elemental iron varied from 1 mg of elemental iron to approximately 300 mg of elemental iron a day. Duration of iron supplement also varied significantly, ranging from 1 week to 24 weeks.
Excluded studies
We excluded six studies because they did not meet eligibility criteria. Three studies were cross‐over trials that did not report on outcomes at the end of the first parallel intervention period (Brigham 1993; Powell 1991; Schoene 1983). Two studies were undertaken in blood donors in whom further donations during the trial indicated ongoing blood losses (Cable 1988; Simon 1984). In one trial, data from male and female participants could not be disaggregated (Powers 1988).
Risk of bias in included studies
Study methods were generally not well described in many of the studies and thus 'Risk of bias' assessment was difficult (see Figure 2 and Figure 3). Using the criteria defined above, only 11 studies were assessed as being at low risk of bias (Bruner 1996; DellaValle 2012; Flink 2006; Fogelholm 1992; Gunaratna 2015; Machado 2011; Marks 2014; Pereira 2014; Verdon 2003; Waldvogel 2012; Zaman 2013). The remaining studies were either assessed as being at high risk of bias or the methods were unclear and thus could not be rated as being at low risk of bias.
2.
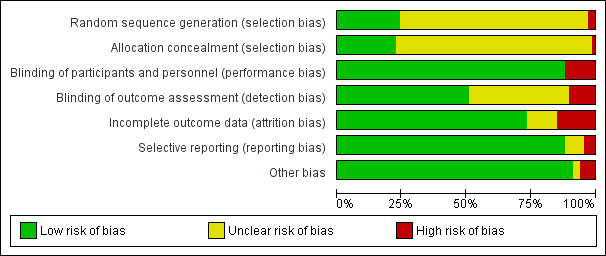
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
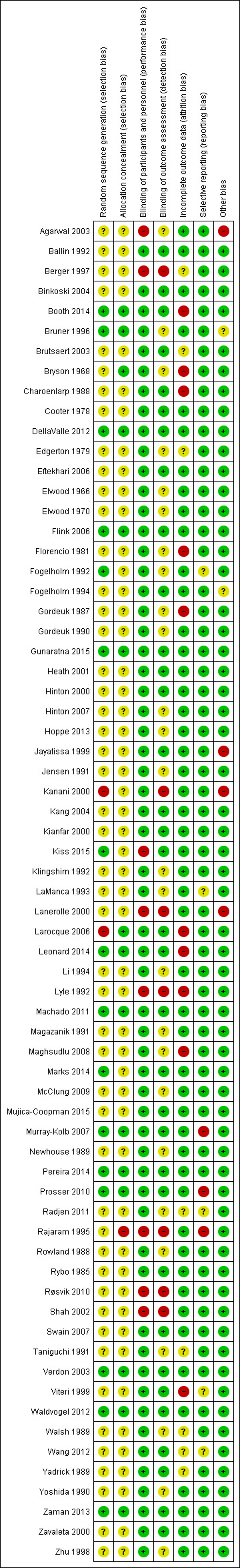
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sixteen studies were considered to have generated the random sequence using a method considered to be at low risk of bias (Booth 2014; Bruner 1996; DellaValle 2012; Flink 2006; Fogelholm 1992; Gunaratna 2015; Kiss 2015; Leonard 2014; Machado 2011; Marks 2014; Murray‐Kolb 2007; Pereira 2014; Prosser 2010; Verdon 2003; Waldvogel 2012; Zaman 2013. Sequence generation was considered at high risk of bias in two studies (Kanani 2000; Larocque 2006). In 49 of the included trials, it was unclear how the randomisation sequence had been generated.
Fifteen of the included studies used methods of concealing group allocation that we judged to be at low risk of bias (Booth 2014; Bruner 1996; Bryson 1968; DellaValle 2012; Flink 2006; Gunaratna 2015; Larocque 2006; Leonard 2014; Machado 2011; Murray‐Kolb 2007; Pereira 2014; Prosser 2010; Verdon 2003; Waldvogel 2012; Zaman 2013 ). In one trial, methods were considered at high risk of bias (Rajaram 1995). In the remaining 51 trials, methods were either not described or were unclear.
Blinding
Most trials administered the placebo to blinded participants and relatively few trials reported on methods for blinding outcome assessors. Overall, 59 studies reported blinding of participants and were deemed at low risk of performance bias (Ballin 1992; Binkoski 2004; Booth 2014; Bruner 1996; Brutsaert 2003; Bryson 1968; Charoenlarp 1988; Cooter 1978; DellaValle 2012; Edgerton 1979; Eftekhari 2006; Elwood 1966; Elwood 1970; Flink 2006; Florencio 1981; Fogelholm 1992; Fogelholm 1994; Gordeuk 1987; Gordeuk 1990; Gunaratna 2015; Heath 2001; Hinton 2000; Hinton 2007; Hoppe 2013; Jayatissa 1999; Jensen 1991; Kanani 2000; Kang 2004; Kianfar 2000; Klingshirn 1992; LaManca 1993; Larocque 2006; Leonard 2014; Li 1994; Machado 2011; Magazanik 1991; Marks 2014; Maghsudlu 2008; McClung 2009; Mujica‐Coopman 2015; Murray‐Kolb 2007; Newhouse 1989; Pereira 2014; Prosser 2010; Radjen 2011; Rowland 1988; Rybo 1985; Swain 2007; Taniguchi 1991; Verdon 2003; Viteri 1999; Waldvogel 2012; Walsh 1989; Wang 2012; Yadrick 1989; Yoshida 1990; Zaman 2013; Zavaleta 2000; Zhu 1998). Eight studies were deemed to be at high risk of performance bias: seven as placebo was not used (Agarwal 2003; Berger 1997; Kiss 2015; Lanerolle 2000; Rajaram 1995; Røsvik 2010; Shah 2002), one as diet intervention was not blinded and would have revealed placebo group from intervention group (Lyle 1992).
Thirty‐four studies were deemed to be at low risk of detection bias (Ballin 1992; Binkoski 2004; Booth 2014; Brutsaert 2003; Charoenlarp 1988; Cooter 1978; DellaValle 2012; Eftekhari 2006; Flink 2006; Fogelholm 1994; Gunaratna 2015; Heath 2001; Hinton 2000; Jayatissa 1999; Kang 2004; Kianfar 2000; Kiss 2015; Larocque 2006; Leonard 2014; Machado 2011; Marks 2014; Mujica‐Coopman 2015; Murray‐Kolb 2007; Pereira 2014; Prosser 2010; Rybo 1985; Swain 2007; Verdon 2003; Viteri 1999; Waldvogel 2012; Wang 2012; Yadrick 1989; Zaman 2013; Zavaleta 2000). Twenty‐six studies were deemed unclear for detection bias as the study failed to state whether personnel were blinded or it remained unclear if outcomes would be affected by lack of blinding (Agarwal 2003; Bruner 1996; Bryson 1968; Edgerton 1979; Elwood 1966; Elwood 1970; Florencio 1981; Fogelholm 1992; Gordeuk 1987; Gordeuk 1990; Hinton 2007; Hoppe 2013; Jensen 1991; Klingshirn 1992; LaManca 1993; Li 1994; Magazanik 1991; Maghsudlu 2008; McClung 2009; Newhouse 1989; Radjen 2011; Rowland 1988; Taniguchi 1991; Walsh 1989; Yoshida 1990; Zhu 1998). Seven studies were deemed at high risk of detection bias as assessors may have known which participants belonged to which group due to a lack of placebo or unblinded personnel, with outcomes that may have been influenced by this lack of blinding (Berger 1997; Kanani 2000; Lanerolle 2000; Lyle 1992; Rajaram 1995; Røsvik 2010; Shah 2002).
Incomplete outcome data
While we assessed that the majority of the included trials had acceptable levels of attrition (with loss to follow‐up and missing data being less than 30% and balanced across groups), in nine trials the levels of attrition were high or not balanced across groups (Booth 2014; Bryson 1968; Charoenlarp 1988; Florencio 1981; Gordeuk 1987; Larocque 2006; Leonard 2014; Lyle 1992; Viteri 1999), while attrition was not reported in a further eight trials (Berger 1997, Brutsaert 2003, Edgerton 1979, Radjen 2011, Taniguchi 1991, Walsh 1989; Wang 2012; Yadrick 1989).
Selective reporting
We were not able to fully assess outcome reporting bias as we only had access to published study reports. We assessed publication bias using funnel plots for haemoglobin, anaemia, iron deficiency, ferritin and adverse effects (any effects, any gastrointestinal effects, constipation, loose stools/diarrhoea, and abdominal pain). While we detected no funnel plot asymmetry for haemoglobin or ferritin, we observed evidence of asymmetry for anaemia and ferritin (both indicating the possibility of missing studies reporting a smaller than observed effect on anaemia prevalence from iron). However, there were few studies reporting these outcomes precluding more detailed statistical analysis of these funnel plots. Nevertheless, the possibility of publication bias exists for these key outcomes. Five studies were deemed to be at an unclear risk of selective reporting (Fogelholm 1992; LaManca 1993; Radjen 2011; Viteri 1999; Wang 2012) and three studies were deemed to be at high risk of selective reporting due to outcomes mentioned being analysed but not presented (Murray‐Kolb 2007; Prosser 2010; Rajaram 1995).
Other potential sources of bias
The majority of trials had no clear other sources of bias. Only four studies used a cluster design but did not report the ICC or other relevant data in the manuscript and were thus deemed to be at high risk of other bias (Agarwal 2003; Jayatissa 1999; Kanani 2000; Lanerolle 2000). These papers reported on the following outcomes: haemoglobin and anaemia (Agarwal 2003); haemoglobin, anaemia and ferritin (Jayatissa 1999); haemoglobin, weight and body mass index (Kanani 2000); haemoglobin, ferritin, iron deficiency, transferrin saturation (Lanerolle 2000). We obtained the ICC from external sources (Gulliford 1999): the ICC for haemoglobin was 0.00059, which is low; for example, for a cluster comprising 30 individuals, the design effect would be only 1.017, which implies adjustment of the sample size would only be minor. Likewise, the ICC for ferritin from this source was only 0.00004, which again is unlikely to result in a large design effect and obviates the need for an adjustment of the sample size (Ukoumunne 1999). For weight and body mass index, reported in Kanani 2000, we undertook a sensitivity analysis to evaluate effects of excluding this study (which can be seen in Analysis 9.5).
9.5. Analysis.
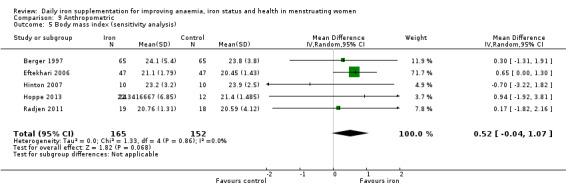
Comparison 9 Anthropometric, Outcome 5 Body mass index (sensitivity analysis).
Of the remaining studies, two were deemed at unclear risk of other bias (Bruner 1996; Fogelholm 1994) as data was only presented in a table making other sources of bias unable to be excluded, and 61 studies (from 71 reports) had no other identifiable potential source of bias and were therefore deemed at low risk (Ballin 1992; Berger 1997; Binkoski 2004; Booth 2014; Brutsaert 2003; Bryson 1968; Charoenlarp 1988; Cooter 1978; DellaValle 2012; Edgerton 1979; Eftekhari 2006; Elwood 1966; Elwood 1970; Flink 2006; Florencio 1981; Fogelholm 1992; Gordeuk 1987; Gordeuk 1990; Gunaratna 2015; Heath 2001; Hinton 2000; Hinton 2007; Hoppe 2013; Jensen 1991; Kang 2004; Kianfar 2000; Kiss 2015; Klingshirn 1992; LaManca 1993; Larocque 2006; Leonard 2014; Li 1994; Lyle 1992; Machado 2011; Magazanik 1991; Maghsudlu 2008; Marks 2014; McClung 2009; Mujica‐Coopman 2015; Murray‐Kolb 2007; Newhouse 1989; Pereira 2014; Prosser 2010; Radjen 2011; Rajaram 1995; Rowland 1988; Rybo 1985; Røsvik 2010; Shah 2002; Swain 2007; Taniguchi 1991; Verdon 2003; Viteri 1999; Waldvogel 2012; Walsh 1989; Wang 2012; Yadrick 1989; Yoshida 1990; Zaman 2013; Zavaleta 2000; Zhu 1998.
Effects of interventions
See: Table 1
All included trials contributed data to the review but some studies randomised participants to intervention arms that were not relevant to the comparisons we assessed. For these studies we did not include data from all groups in the analyses. Furthermore, some studies did not contain data in an extractable form, or did not contain data in a way in which they could be combined in meta‐analyses. For these studies, we provided a narrative description of the results.
For cluster‐randomised trials we extracted the estimated effective sample size by adjusting the data to account for the clustering effect.
Primary Outcomes
Anaemia
Ten studies, comprising 3273 women, measured anaemia prevalence at the end of intervention (Agarwal 2003; Charoenlarp 1988; Florencio 1981; Gordeuk 1990; Gunaratna 2015; Jayatissa 1999; Shah 2002; Viteri 1999; Wang 2012; Zavaleta 2000). Women receiving iron were significantly less likely to be anaemic at the end of intervention compared to women receiving control (RR 0.39, 95% CI 0.25 to 0.60, moderate quality evidence; Analysis 1.1; Table 1). There was variation among trials in terms of the size of the treatment effect (Tau² = 0.37; Chi² = 124.24, df = 9, (P < 0.00001); I² = 93%). Although visual inspection of the funnel plot indicated asymmetry, broadly suggesting missing studies reported smaller effect sizes on anaemia, which may be in keeping with a reporting bias, formal statistical testing using the Harbord and Peters tests did not demonstrate evidence of publication bias (Sterne 2011); see Figure 4.
1.1. Analysis.
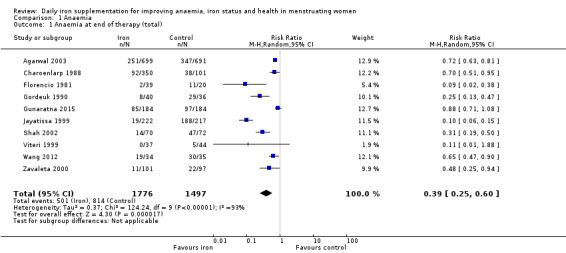
Comparison 1 Anaemia, Outcome 1 Anaemia at end of therapy (total).
4.
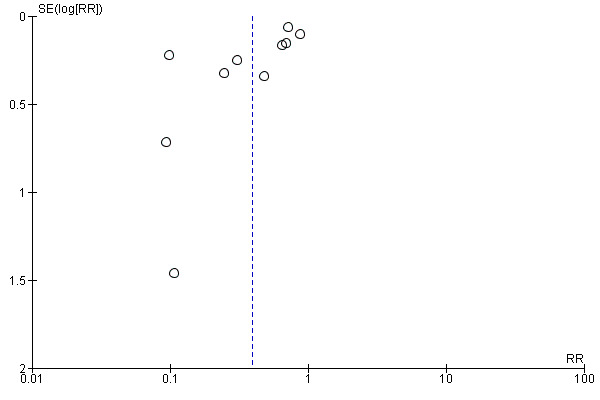
Funnel plot of comparison: 1 Anaemia, outcome: 1.1 Anaemia at end of therapy (total).
Only one study reporting this outcome was considered at low overall risk of bias (Gunaratna 2015). Analysis of this study did not show a difference between iron and control (Analysis 1.2).
1.2. Analysis.
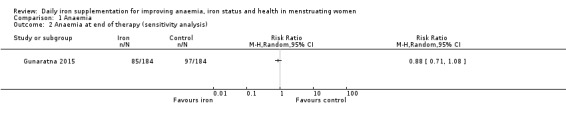
Comparison 1 Anaemia, Outcome 2 Anaemia at end of therapy (sensitivity analysis).
Subgroup analysis
There was evidence of differences between subgroups. Specifically, women in studies comparing iron alone with control experienced a smaller reduction in the prevalence of anaemia (RR 0.57, 95% CI 0.45 to 0.74, 8 studies, 2775 women) compared with women randomised to iron + vitamin C versus vitamin C alone (RR 0.10, 95% CI 0.06 to 0.15, 2 studies, 498 women; test for subgroup differences: Chi² = 51.2, df = 1 (P < 0.00001), I² = 98%; Analysis 1.3). There were no differences observed in effect sizes based on age of participants (Analysis 1.4). Although subgroup differences were observed based on baseline anaemia status (Analysis 1.5), iron status (Analysis 1.6), and iron‐deficiency anaemia status (Analysis 1.7), most studies fell into the 'unclassified' subgroup and thus subgroup analyses were not constructive. Significant differences in effect size on risk of anaemia were seen for different doses and durations of iron supplementation, however these were non‐linear with increasing dose (Analysis 1.8) or duration (Analysis 1.9). No studies in malaria‐endemic settings were included. Limited data indicated that ferrous sulphate is more effective than other formulations in reducing prevalence of anaemia (ferrous sulphate: RR 0.20, 95% CI 0.09 to 0.48, 4 studies, 838 women; ferrous fumarate: RR 0.65, 95% CI 0.47 to 0.90, 1 study, 69 women; other formulations: RR 0.66, 95% CI 0.50 to 0.87, 4 studies, 2285 women; test for subgroup differences: Chi² = 6.85, df = 2 (P value = 0.03), I² = 70.8%; Analysis 1.10).
1.3. Analysis.
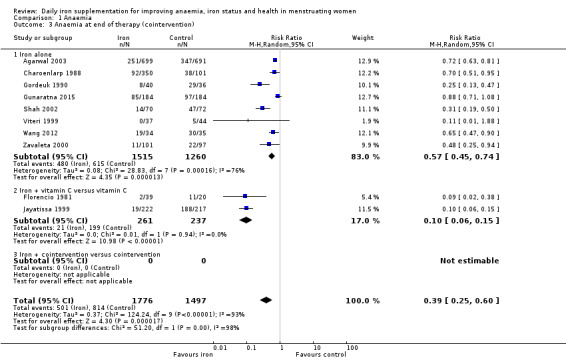
Comparison 1 Anaemia, Outcome 3 Anaemia at end of therapy (cointervention).
1.4. Analysis.
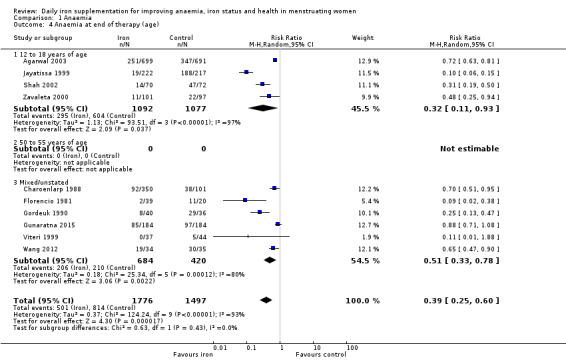
Comparison 1 Anaemia, Outcome 4 Anaemia at end of therapy (age).
1.5. Analysis.
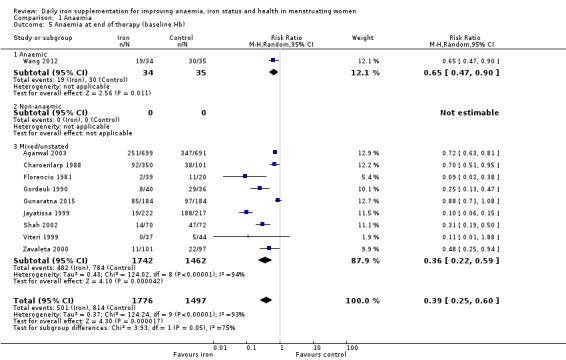
Comparison 1 Anaemia, Outcome 5 Anaemia at end of therapy (baseline Hb).
1.6. Analysis.
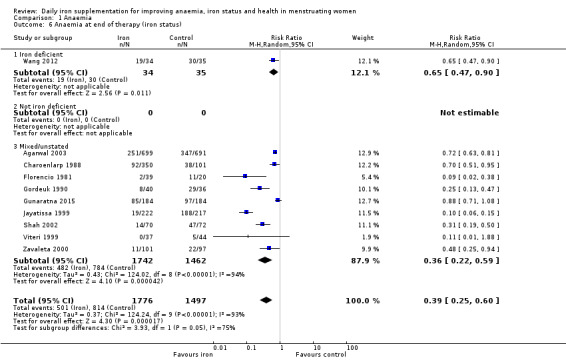
Comparison 1 Anaemia, Outcome 6 Anaemia at end of therapy (iron status).
1.7. Analysis.
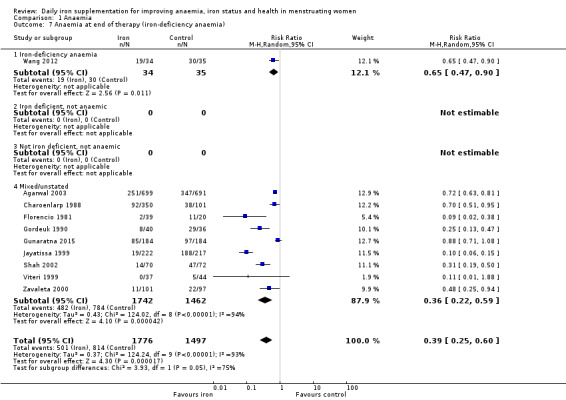
Comparison 1 Anaemia, Outcome 7 Anaemia at end of therapy (iron‐deficiency anaemia).
1.8. Analysis.
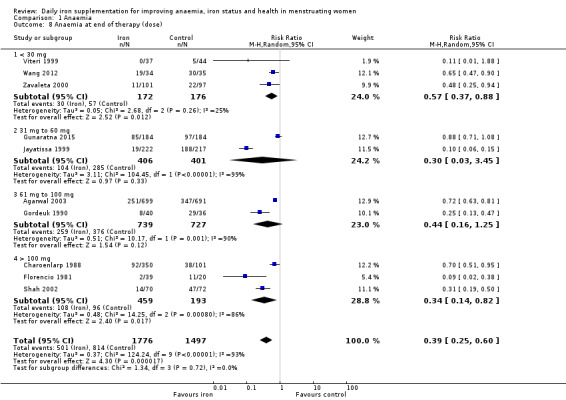
Comparison 1 Anaemia, Outcome 8 Anaemia at end of therapy (dose).
1.9. Analysis.
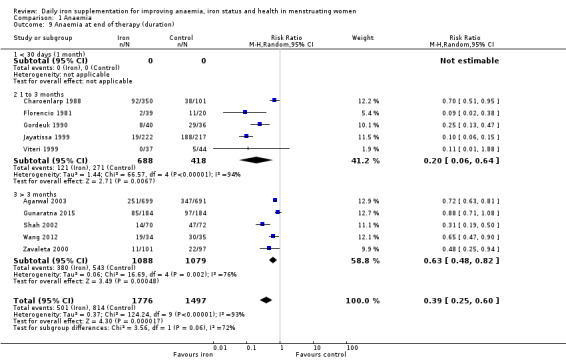
Comparison 1 Anaemia, Outcome 9 Anaemia at end of therapy (duration).
1.10. Analysis.
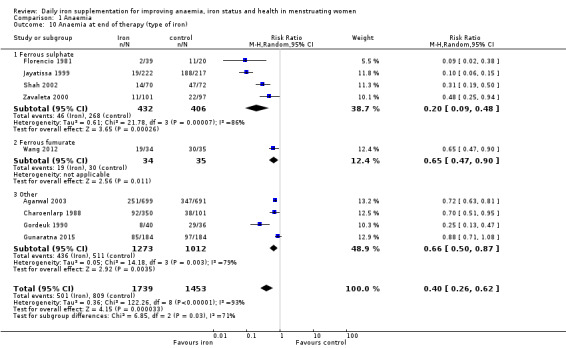
Comparison 1 Anaemia, Outcome 10 Anaemia at end of therapy (type of iron).
High levels of heterogeneity may be explained by variation in the clinical matrix of study designs (i.e. more than one factor could account for heterogeneity, which cannot be adequately captured by each subgroup analysis. For example, studies used different doses and durations, and recruited participants with different underlying iron status.
Haemoglobin
Fifty‐one trials recruiting 6861 women measured haemoglobin concentrations at the end of intervention (Agarwal 2003; Berger 1997; Binkoski 2004; Booth 2014; Bruner 1996; Brutsaert 2003; Charoenlarp 1988; Cooter 1978; DellaValle 2012; Edgerton 1979; Eftekhari 2006; Elwood 1966; Florencio 1981; Fogelholm 1992; Fogelholm 1994; Gordeuk 1987; Gordeuk 1990; Hinton 2000; Hinton 2007; Hoppe 2013; Jayatissa 1999; Jensen 1991; Kanani 2000; Kang 2004; Kianfar 2000; Klingshirn 1992; LaManca 1993; Lanerolle 2000; Larocque 2006; Leonard 2014; Li 1994; Maghsudlu 2008; Marks 2014; McClung 2009; Mujica‐Coopman 2015; Murray‐Kolb 2007; Newhouse 1989; Radjen 2011; Rowland 1988; Rybo 1985; Røsvik 2010; Taniguchi 1991; Viteri 1999; Waldvogel 2012; Walsh 1989; Wang 2012; Yadrick 1989; Yoshida 1990; Zaman 2013; Zavaleta 2000; Zhu 1998). Women receiving iron had a higher haemoglobin concentration at the end of intervention compared with women receiving control (MD 5.30, 95% CI 4.14 to 6.45; heterogeneity: Tau² = 11.74; Chi² = 356.76, df = 50 (P < 0.00001); I² = 86%; high quality evidence; Analysis 2.1; Table 1). There was no obvious funnel plot asymmetry (Figure 5).
2.1. Analysis.
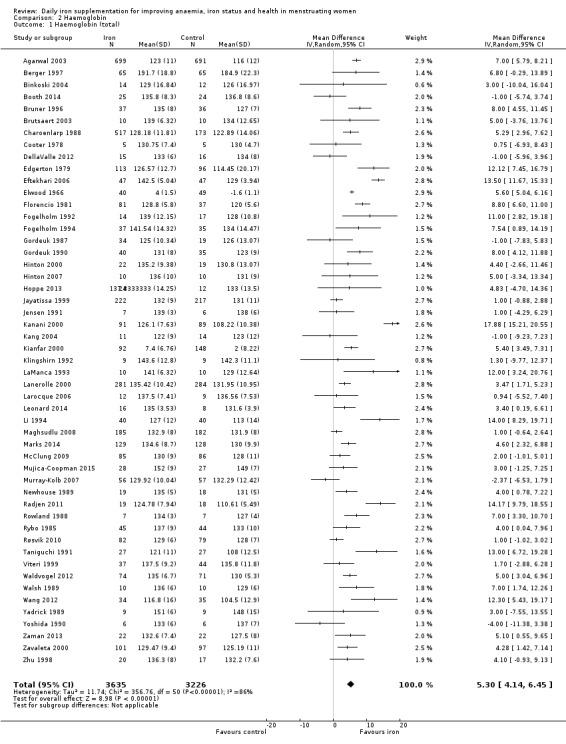
Comparison 2 Haemoglobin, Outcome 1 Haemoglobin (total).
5.
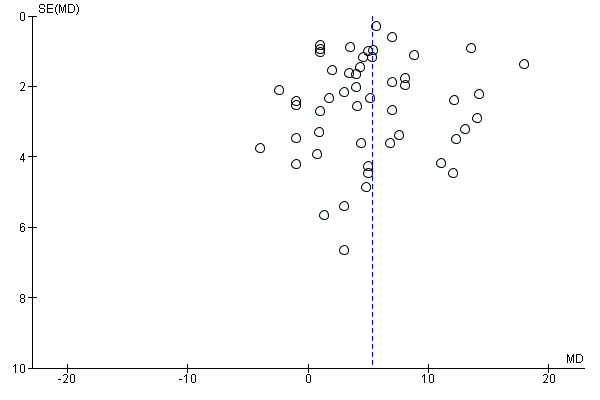
Funnel plot of comparison: 2 Haemoglobin, outcome: 2.1 Haemoglobin (total).
When only studies considered at low overall risk of bias were included in the analysis (six studies; 581 women: Bruner 1996; DellaValle 2012; Fogelholm 1992; Marks 2014; Waldvogel 2012; Zaman 2013), the effect size was similar (MD 5.08, 95% CI 2.99 to 7.17; Analysis 2.2).
2.2. Analysis.
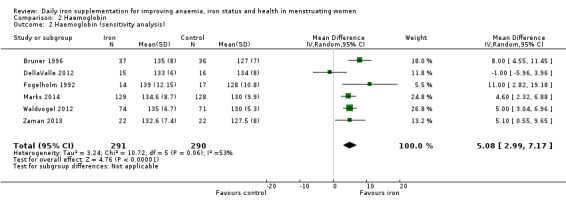
Comparison 2 Haemoglobin, Outcome 2 Haemoglobin (sensitivity analysis).
Subgroup analysis
Subgroup analyses may explain the observed heterogeneity. The large number of studies and participants for this outcome provide a rich data set for evaluation of subgroup differences. There was no evidence of a difference in MD between women receiving iron alone or iron with vitamin C or another cointervention (Analysis 2.3). There was no subgroup difference based on age of women (Analysis 2.4). There was a greater increase in haemoglobin in studies among women with baseline anaemia (MD 8.67, 95% CI 5.16 to 12.18, 8 studies, 558 women) or in whom baseline anaemia status was not defined (MD 6.30, 95% CI 4.52 to 8.08, 25 studies, 4207 women) compared with those who were non‐anaemic at baseline (MD 3.11, 95% CI 1.67 to 4.54, 25 studies, 2120 women; test for subgroup differences: Chi² = 12.73, df = 2 (P value = 0.002), I² = 84.3%; Analysis 2.5). Similarly, iron did not improve haemoglobin in iron replete women (MD 0.84, 95% CI ‐2.26 to 3.95, 5 studies, 421 women), but did increase haemoglobin concentration in women who were either iron deficient (as defined by the trial authors) (MD 6.92, 95% CI 4.76 to 9.09, 21 studies, 1124 women) or in whom iron status had not been defined at baseline (MD 4.92, 95% CI 3.49 to 6.35, 28 studies, 5296 women; test for subgroup differences: Chi² = 9.90, df = 2 (P value = 0.007), I² = 79.8%); see Analysis 2.6. There was no subgroup difference in the effect of iron on haemoglobin between women who were iron‐deficient anaemic, non‐anaemic iron deficient, non‐anaemic non‐iron deficient, and undefined (Analysis 2.7). There was no difference in effect from iron on haemoglobin according to dose of iron given (Analysis 2.8). Haemoglobin levels increased more when iron was given for one to three months (MD 6.14, 95% CI 4.70 to 7.58, 37 studies, 4171 women) when compared to less than one month (MD 2.60, 95% CI 0.28 to 4.91, 6 studies, 765 women) or greater than three months (MD 3.84, 95% CI 0.94 to 6.75, 8 studies, 1925 women; test for subgroup differences: Chi² = 7.15, df = 2 (P value = 0.03), I² = 72%; Analysis 2.9). Only one study had been undertaken in a malaria‐endemic area limiting subgroup analyses by malaria endemicity. There was no evidence of subgroup difference between trials using different formulations of iron (ferrous sulphate, ferrous fumarate, and others) (Analysis 2.10).
2.3. Analysis.
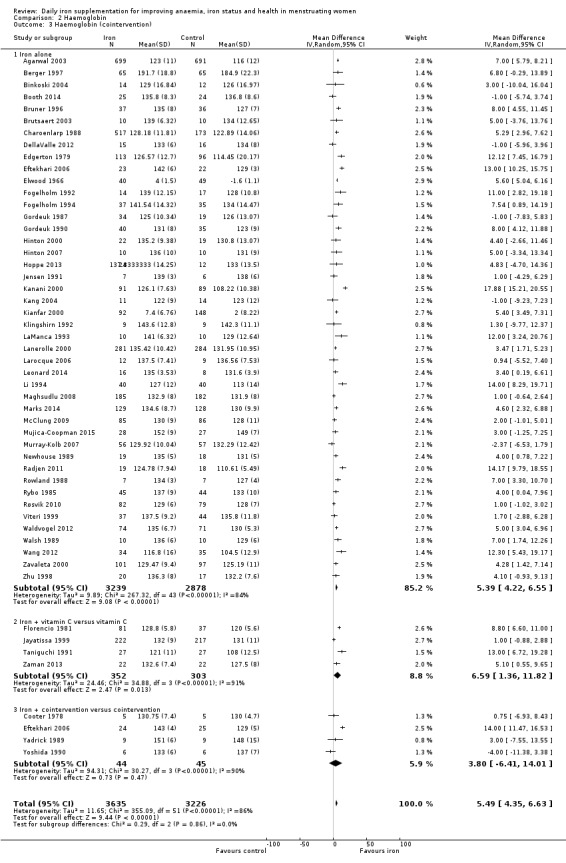
Comparison 2 Haemoglobin, Outcome 3 Haemoglobin (cointervention).
2.4. Analysis.
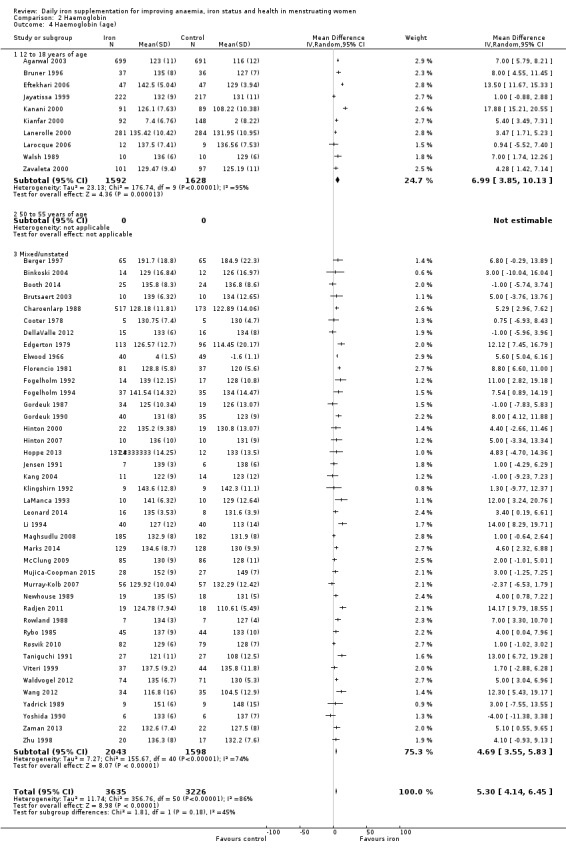
Comparison 2 Haemoglobin, Outcome 4 Haemoglobin (age).
2.5. Analysis.
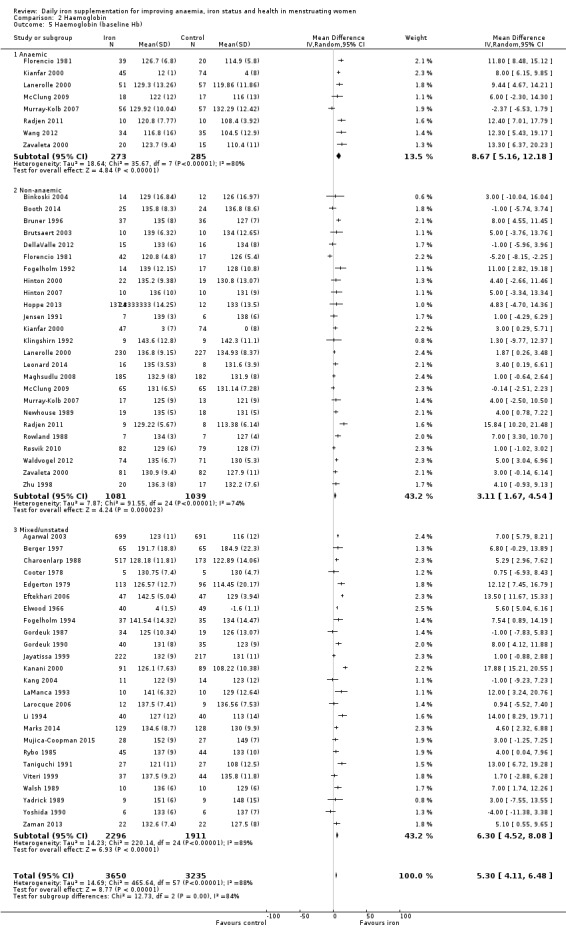
Comparison 2 Haemoglobin, Outcome 5 Haemoglobin (baseline Hb).
2.6. Analysis.
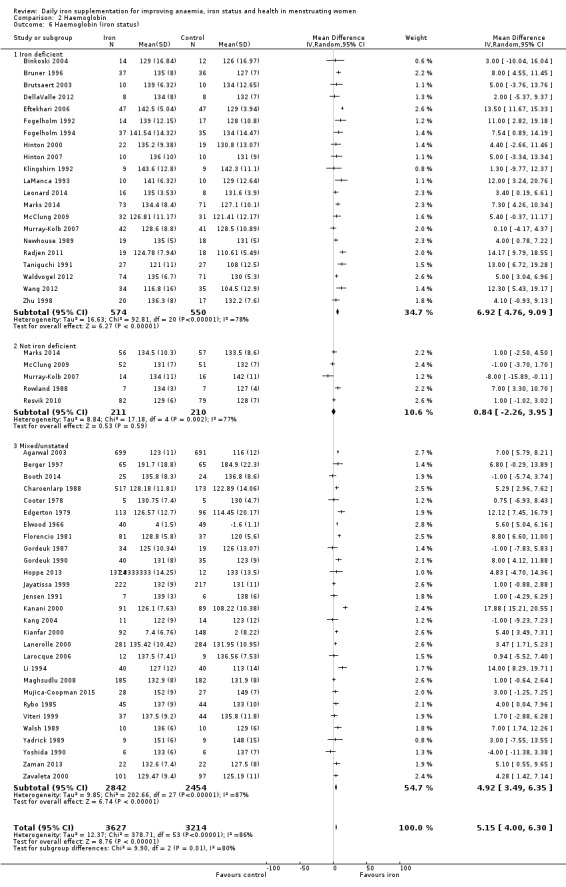
Comparison 2 Haemoglobin, Outcome 6 Haemoglobin (iron status).
2.7. Analysis.
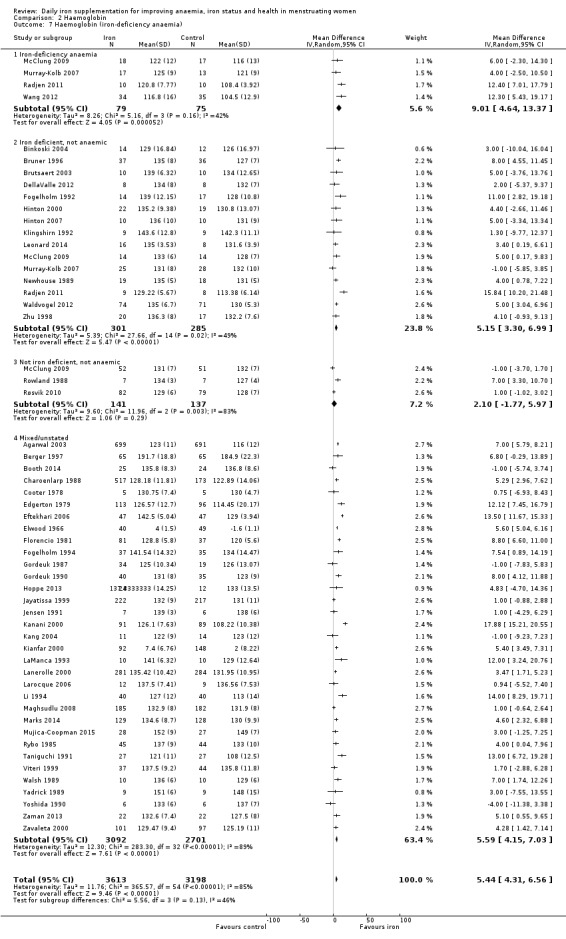
Comparison 2 Haemoglobin, Outcome 7 Haemoglobin (iron‐deficiency anaemia).
2.8. Analysis.
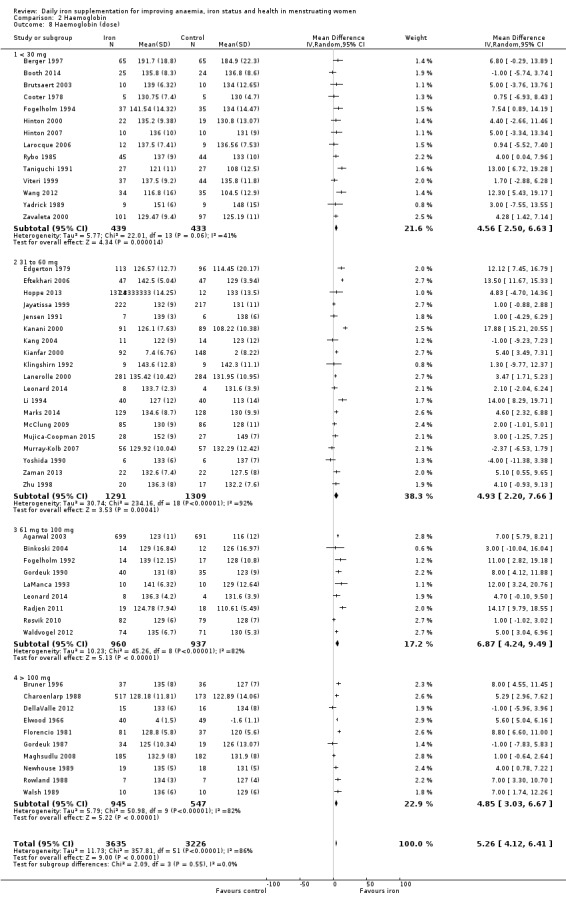
Comparison 2 Haemoglobin, Outcome 8 Haemoglobin (dose).
2.9. Analysis.
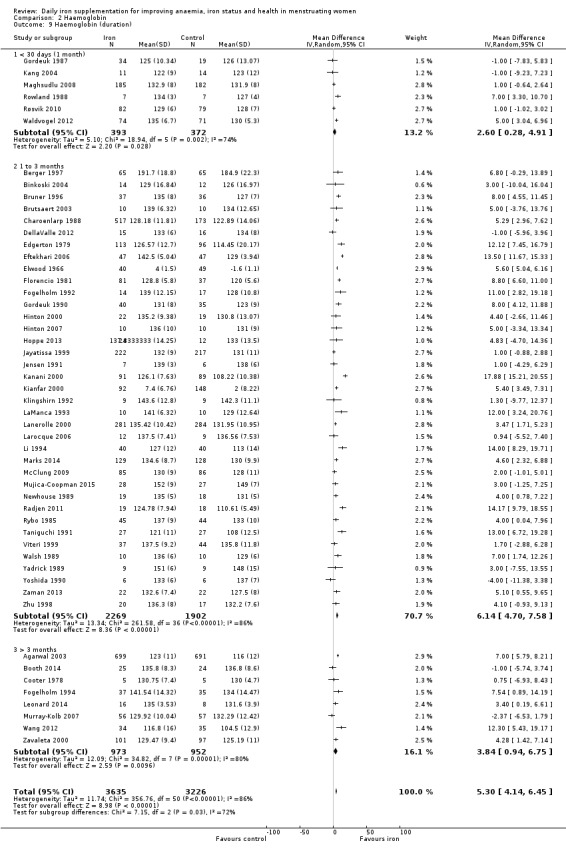
Comparison 2 Haemoglobin, Outcome 9 Haemoglobin (duration).
2.10. Analysis.
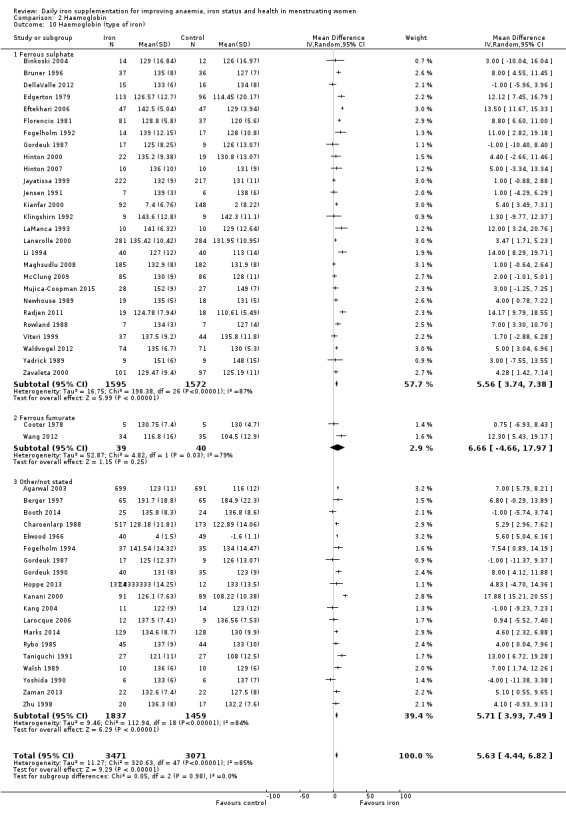
Comparison 2 Haemoglobin, Outcome 10 Haemoglobin (type of iron).
Iron deficiency
Seven studies recruiting 1088 women measured iron deficiency at the end of the intervention (Ballin 1992; Lanerolle 2000; Leonard 2014; Marks 2014; Mujica‐Coopman 2015; Viteri 1999; Wang 2012). Women receiving iron had a reduced risk of iron deficiency compared with women receiving control (RR 00.62, 95% CI 0.50 to 0.76; heterogeneity: Tau² = 0.02; Chi² = 8.37, df = 6 (P value = 0.21); I² = 28%; moderate quality evidence; Analysis 3.1). When only the single study (257 women) at low risk of bias was included (Marks 2014), the effect size was similar (RR 0.65, 95% CI 0.54 to 0.78; Analysis 3.2).
3.1. Analysis.
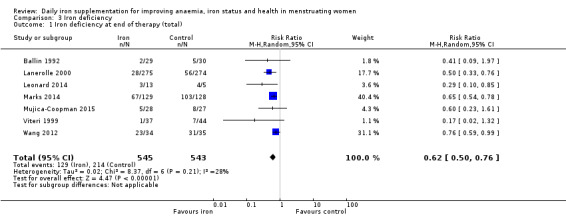
Comparison 3 Iron deficiency, Outcome 1 Iron deficiency at end of therapy (total).
3.2. Analysis.
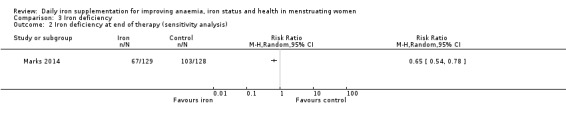
Comparison 3 Iron deficiency, Outcome 2 Iron deficiency at end of therapy (sensitivity analysis).
Subgroup analysis
There were too few studies to enable subgroup analysis.
Iron‐deficiency anaemia
Only one study (Mujica‐Coopman 2015), involving 55 women, specifically reported iron‐deficiency anaemia, with no events in either the iron or control groups (Analysis 4.1). One other study (Gunaratna 2015) reported microcytic anaemia and showed a significant reduction with iron therapy compared to controls (RR 0.51, 95% CI 0.33 to 0.77, 378 women; Analysis 4.2).
4.1. Analysis.
Comparison 4 Iron‐deficiency anaemia, Outcome 1 Iron‐deficiency anaemia (total).
4.2. Analysis.
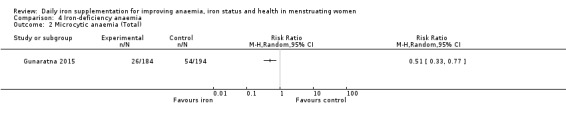
Comparison 4 Iron‐deficiency anaemia, Outcome 2 Microcytic anaemia (Total).
All‐cause mortality
No studies reported data on all‐cause mortality.
Adverse side effects
Data on adverse effects were generally reported as proportions of populations experiencing side effects. Data were amalgamated using terms defined by the trial authors: 'any side effect' (Ballin 1992; Hoppe 2013; Leonard 2014; Maghsudlu 2008; Marks 2014; Pereira 2014; Waldvogel 2012), 'any gastrointestinal side effect' (Gordeuk 1987; Hoppe 2013; Marks 2014; Pereira 2014; Waldvogel 2012), 'loose stools/diarrhoea' (Gordeuk 1987; Leonard 2014; Marks 2014; Pereira 2014; Rybo 1985; Waldvogel 2012), 'hard stools/constipation' (Bruner 1996; Gordeuk 1990; Leonard 2014; Maghsudlu 2008; Marks 2014; Pereira 2014; Rybo 1985; Waldvogel 2012), 'abdominal pain' (Bryson 1968; Gordeuk 1990; Maghsudlu 2008; Marks 2014; Pereira 2014; Rybo 1985; Waldvogel 2012), 'nausea' (Bryson 1968; Gordeuk 1990; Leonard 2014; Maghsudlu 2008; Marks 2014; Pereira 2014; Rybo 1985; Waldvogel 2012), 'change in stool colour' (Bruner 1996; Leonard 2014; Marks 2014; Pereira 2014), 'reflux/heartburn' (Pereira 2014), and 'headache' (Gordeuk 1987; Gordeuk 1990; Maghsudlu 2008; Pereira 2014).
Any side effects
Seven trials recruiting 901 women reported on 'any side effect' and did not identify an overall increased prevalence of side effects from iron supplements (RR 2.14, 95% CI 0.94 to 4.86, P value = 0.07; heterogeneity: Tau² = 0.84; Chi² = 49.95, df = 6 (P < 0.00001); I² = 88%, low quality evidence; Analysis 5.1; Table 1). The funnel plot of this outcome indicates asymmetry (Figure 6), raising the possibility of missing studies with fewer adverse effects. When only the three trials (415 women) considered at low overall risk of bias were included in the analysis (Marks 2014; Pereira 2014; Waldvogel 2012), the effect of iron on 'any adverse effect' was similar (RR 1.59, 95% CI 0.66 to 3.81; Analysis 5.2).
5.1. Analysis.
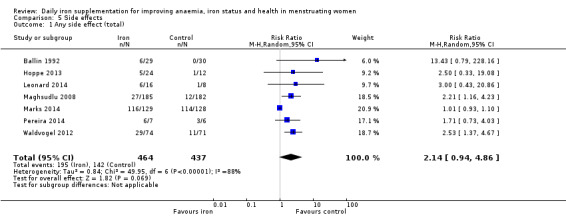
Comparison 5 Side effects, Outcome 1 Any side effect (total).
6.
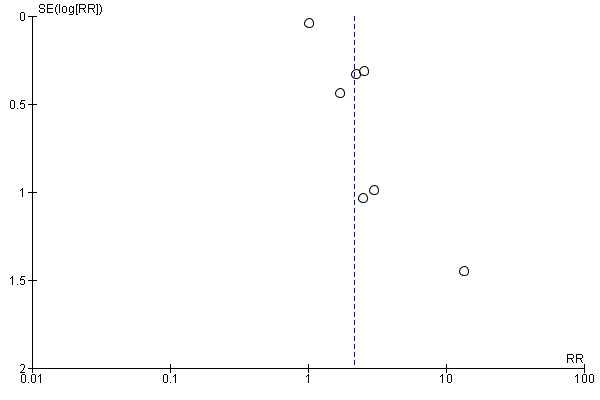
Funnel plot of comparison: 7 Side effects, outcome: 7.1 Any Side effect (Total).
5.2. Analysis.
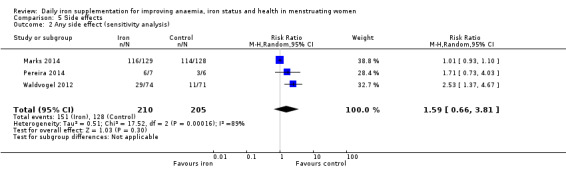
Comparison 5 Side effects, Outcome 2 Any side effect (sensitivity analysis).
Subgroup analysis
There were too few studies in different subgroup categories to enable subgroup analyses by cointervention, age, baseline anaemia/iron deficiency/iron‐deficiency anaemia status, duration of intervention, malaria endemicity, or type of iron utilised. However, there was evidence of a trend towards an increase in risk of any adverse effects as dose of elemental iron was increased, from 30 mg to 60 mg (RR 1.01, 95% CI 0.93 to 1.10, 3 studies, 305 women), to 61 mg to 100 mg (RR 2.61, 95% 1.44 to 4.75, 2 studies, 157 women), to more than 100 mg (2.15, 95% CI 1.24 to 3.73, 3 studies, 439 women; test for subgroup differences: Chi² = 16.30, df = 2 (P value = 0.0003), I² = 87.7%; Analysis 5.3).
5.3. Analysis.
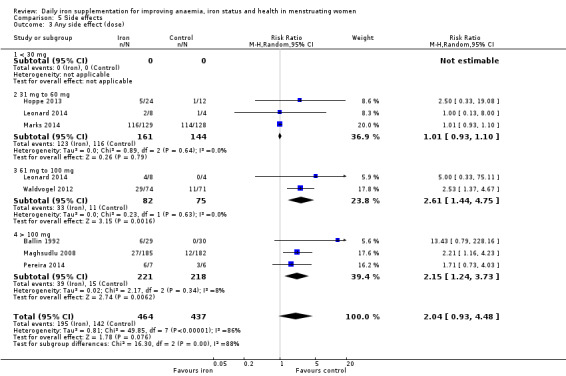
Comparison 5 Side effects, Outcome 3 Any side effect (dose).
Any gastrointestinal side effects
Five studies recruiting 521 women identified an increased prevalence of gastrointestinal side effects in women taking iron (RR 1.99, 95% CI 1.26 to 3.12; heterogeneity: Tau² = 0.11; Chi² = 7.33, df = 4 (P value = 0.12); I² = 45%; low quality evidence; Analysis 5.4). When three studies (415 women) considered at low overall risk of bias were included in the analysis (Marks 2014; Pereira 2014; Waldvogel 2012), the magnitude of effect was similar (RR 1.91, 95% CI 0.96 to 3.80; heterogeneity: Tau² = 0.23; Chi² = 5.56, df = 2 (P value = 0.06); I² = 64%; Analysis 5.5).
5.4. Analysis.
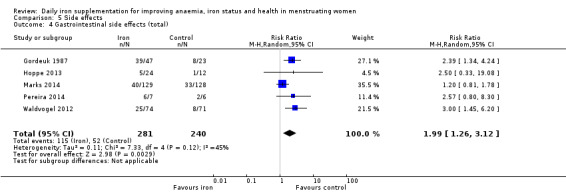
Comparison 5 Side effects, Outcome 4 Gastrointestinal side effects (total).
5.5. Analysis.
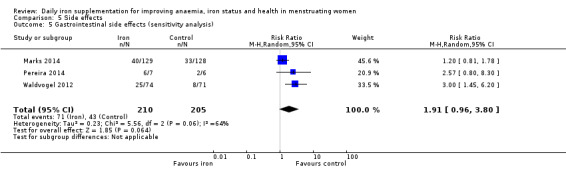
Comparison 5 Side effects, Outcome 5 Gastrointestinal side effects (sensitivity analysis).
Subgroup analysis
There were too few studies in different subgroup categories to enable subgroup analyses by cointervention, age, baseline anaemia/iron deficiency/iron‐deficiency anaemia status, duration of intervention, malaria endemicity, or type of iron utilised. However, there was evidence of a trend towards an increase in risk of gastrointestinal adverse effects as dose of elemental iron was increased: from 31 mg to 60 mg (RR 1.23, 95% CI 0.84 to 1.81, 2 studies, 293 women), to 61 mg to 100 mg (RR 3.00, 95% CI 1.45 to 6.20, 1 study, 145 women), to more than 100 mg (RR 2.42, 95% CI 1.45 to 4.05, 2 studies, 83 women; test for subgroup differences: Chi² = 6.80, df = 2 (P value = 0.03), I² = 70.6%; Analysis 5.6).
5.6. Analysis.
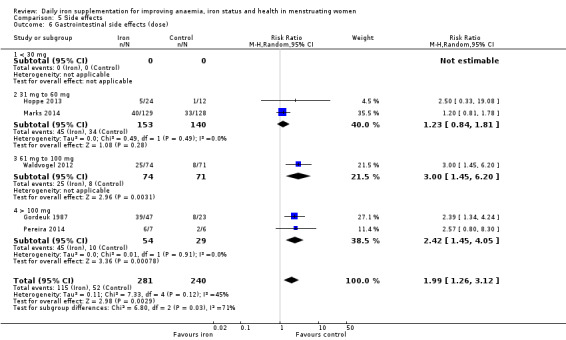
Comparison 5 Side effects, Outcome 6 Gastrointestinal side effects (dose).
Loose stools/diarrhoea
Six studies recruiting 604 women identified an increased prevalence of loose stools/diarrhoea (defined by the trial authors): RR 2.13, 95% CI 1.10 to 4.11; heterogeneity: Tau² = 0.11; Chi² = 5.99, df = 5 (P value = 0.31); I² = 17%; high quality evidence; Analysis 5.7; Table 1.
5.7. Analysis.
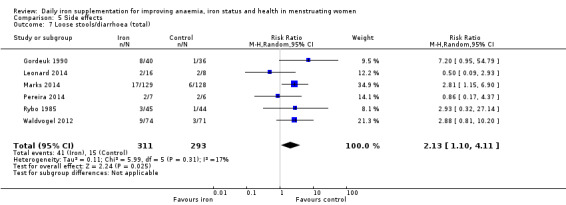
Comparison 5 Side effects, Outcome 7 Loose stools/diarrhoea (total).
Subgroup analysis
Data were inadequate for subgroup analyses for this outcome given the small number of trials in each subgroup category.
Hard stools/constipation
Eight studies recruiting 1036 women demonstrated an increased prevalence of hard stools/constipation (as defined by the authors): RR 2.07, 95% CI 1.35 to 3.17; heterogeneity: Tau² = 0.00; Chi² = 4.10, df = 7 (P value = 0.77); I² = 0%; high quality evidence; Analysis 5.8. When only the four studies (480 women) considered at low overall risk of bias were included (Bruner 1996; Marks 2014; Pereira 2014; Waldvogel 2012), an increased risk for this outcome was still observed (RR 2.14, 95% CI 1.04 to 4.38; Analysis 5.9).
5.8. Analysis.
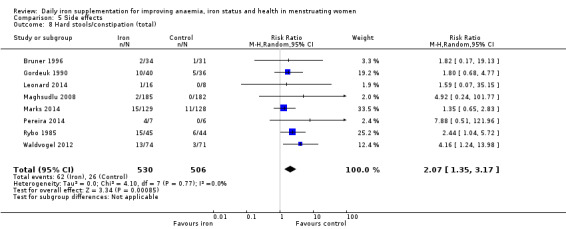
Comparison 5 Side effects, Outcome 8 Hard stools/constipation (total).
5.9. Analysis.
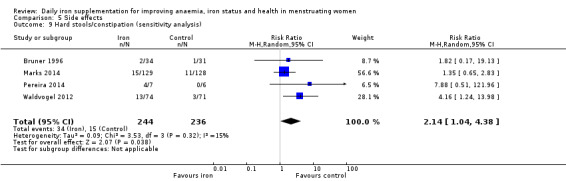
Comparison 5 Side effects, Outcome 9 Hard stools/constipation (sensitivity analysis).
Subgroup analysis
Data were inadequate for subgroup analyses given the small number of trials in each subgroup category.
Abdominal pain
Seven studies recruiting 1190 women showed no definitive increase in abdominal pain (RR 1.55, 95% CI 0.99 to 2.41; heterogeneity: Tau² = 0.00; Chi² = 4.04, df = 6 (P value = 0.67); I² = 0%; low quality evidence; Analysis 5.10).
5.10. Analysis.
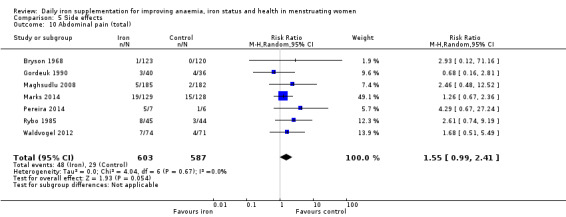
Comparison 5 Side effects, Outcome 10 Abdominal pain (total).
Subgroup analysis
Data were inadequate for subgroup analyses given the small number of trials in each subgroup category.
Nausea
Eight studies recruiting 1214 women did not find any evidence of an increased prevalence of nausea among women randomised to iron (RR 1.19, 95% CI 0.78 to 1.82; heterogeneity: Tau² = 0.00; Chi² = 6.30, df = 7 (P value = 0.51); I² = 0%; Analysis 5.11).
5.11. Analysis.
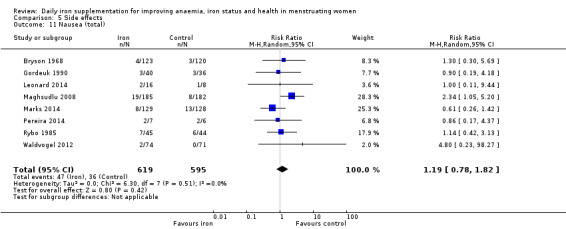
Comparison 5 Side effects, Outcome 11 Nausea (total).
Subgroup analysis
Data were inadequate for subgroup analyses given the small number of trials in each subgroup category.
Change in stool colour
Four studies (359 women) reported a markedly elevated increase in prevalence reporting a change in stool colour among women receiving iron (RR 6.92, 95% CI 3.83 to 12.52; heterogeneity: Tau² = 0.00; Chi² = 0.08, df = 3 (P value = 0.99); I² = 0%; Analysis 5.12).
5.12. Analysis.
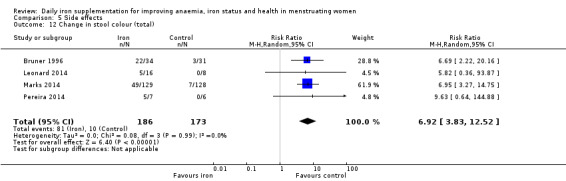
Comparison 5 Side effects, Outcome 12 Change in stool colour (total).
Subgroup analysis
Data were inadequate for subgroup analyses given the small number of trials in each subgroup category.
Reflux/Heartburn
Only one study reported rates of reflux/heartburn (Pereira 2014). Four patients in the iron intervention group reported reflux/heartburn compared to none in the control group.
Headache
Four studies involving 526 women reported on prevalence of headache and found no evidence of an effect on this outcome from iron (RR 0.98, 95% CI 0.58 to 1.66; heterogeneity: Tau² = 0.00; Chi² = 1.11, df = 3 (P value = 0.78); I² = 0%; Analysis 5.13).
5.13. Analysis.
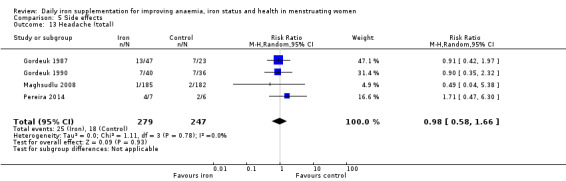
Comparison 5 Side effects, Outcome 13 Headache (total).
Subgroup analysis
Data were inadequate for subgroup analyses given the small number of trials in each subgroup category.
Cognitive function
Five studies reported on cognitive function but reported outcomes using different tools or domains, and thus results could not be meta‐analysed. We present the data from the five studies below.
Bruner 1996 randomised 81 adolescent girls with non‐anaemic iron deficiency to iron supplementation versus placebo and found that girls taking iron had a significant improvement over baseline and end of treatment, compared to those taking placebo, in the total recall score in a test of verbal learning (Hopkins Verbal Learning Test) (P < 0.02), with no differences in any other domains of this test. There were no differences attributable to iron on the Symbol Digit Modalities Test, the Visual Search and Attention Test, or the Brief Test of Attention.
Elwood 1970 randomised women, aged 20 years or older, with anaemia (Hb < 10.5 g/dL) to daily iron supplementation or placebo for eight weeks, and administered several cognitive tests. Data were not reported to directly compare intervention and control, and thus groups (haematologic responders and non‐responders) were merged. Women randomised to iron demonstrated a reduction in the number of errors made while completing a maze (MD ‐9.73, 95% CI ‐17.22 to ‐2.24). However, no effects from iron were seen on other cognitive tests (Serial 7s, E‐test, Card Sorter test, Peg board time).
Larocque 2006 randomised schoolgirls, aged 14 years to 16 years, to iron or placebo and measured a series of cognitive outcomes. There was no effect from iron on the results of any of the cognitive tests performed (Trail Making Test Part A and Part B, Motor‐Free Visual Perception Test, Digit Span, Covert Orienting of Visual Attention Task).
Leonard 2014 randomised 24 women, aged 18 years to 35 years, who were not iron deficient and not currently taking iron, to ferrous sulphate at two doses (60 mg and 80 mg) and compared them to placebo. Participants underwent testing at baseline and the end of intervention with the IntegNeuro Battery of Cognitive Tests (Brainclinics 2015). Women treated with iron (at either dose) had a significant reduction in impulsivity (P value = 0.047), but no difference in memory, response speed, attention, information processing, executive function or emotion identification.
Murray‐Kolb 2007 randomised women, aged 18 to 35 years, of differing iron status (iron replete, non‐anaemic iron deficient, iron‐deficient anaemic) to daily iron supplementation or placebo. Unfortunately, effect sizes for cognitive scores at end of intervention or change from baseline were not reported and hence could not be extracted.
Secondary outcomes
Iron status
Ferritin
Forty‐two studies (3881 women) reported on ferritin concentrations at the end of intervention; iron increased ferritin levels (MD 10.27, 95% CI 8.90 to 11.65; heterogeneity: Tau² = 9.96; Chi² = 475.21, df = 41 (P < 0.00001); I² = 91%; Analysis 6.1).
6.1. Analysis.
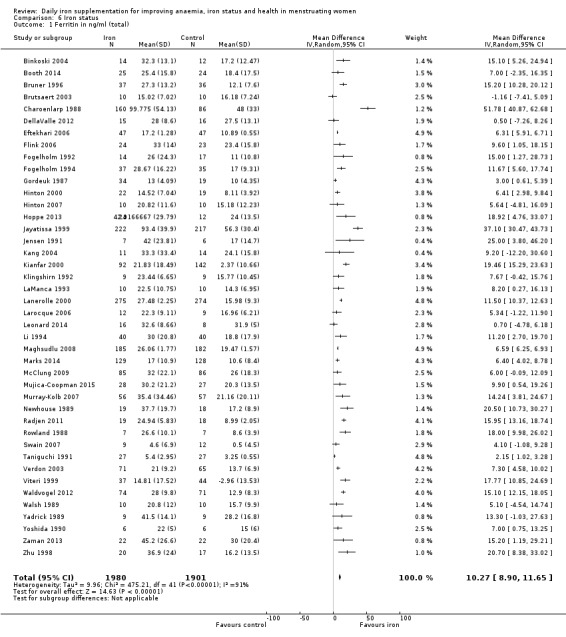
Comparison 6 Iron status, Outcome 1 Ferritin in ng/ml (total).
Subgroup analysis
We further explored potential sources of heterogeneity with subgroup analyses (even though these were not prespecified), as it offered an opportunity to evaluate subgroup effects on effects of iron status changes induced by iron supplementation (see Differences between protocol and review). Subgroup analyses indicated that iron interventions had a lesser effect when coadministered with vitamin C (Analysis 6.2), but had no effect on difference of effect on different age groups (Analysis 6.3). The effect of iron on ferritin was not affected by baseline anaemia status (Analysis 6.4), but women who were iron deficient had a smaller increase in ferritin (MD 8.40, 95% CI 6.31 to 10.49, 20 studies, 1065 women) compared with women who were iron replete (MD 13.38, 95% CI 6.74 to 20.01, 5 studies, 297 women) or in whom iron status had not been characterised (MD 12.88, 95% CI 9.99 to 15.78, 20 studies, 2499 women; test for subgroup differences: Chi² = 7.02, df = 2 (P value = 0.03), I² = 71.5%; Analysis 6.5). No difference in effect was observed by iron‐deficiency anaemia status (Analysis 6.6). Ferritin levels rose less among women given 30 mg or less elemental iron compared with women given higher doses; test for subgroup differences: Chi² = 8.59, df = 3 (P value = 0.04), I² = 65.1%; Analysis 6.7). Giving iron for one to three months (MD 12.17, 95% CI 9.81 to 14.53, 31 studies, 2829 women) showed a larger increase in ferritin than giving iron for less than one month (MD 7.60, 95% CI 4.64 to 10.57, 7 studies, 794 women) or more than three months (MD 7.85, 95% CI 1.31 to 14.38, 4 studies, 258 women; test for subgroup differences: Chi² = 6.12, df = 2 (P value = 0.05), I² = 67.3%; Analysis 6.8). There was no evidence of an effect from different iron formulations (Analysis 6.9). Although examination of the funnel plot suggested asymmetry, Egger’s regression test did not indicate evidence of publication bias (P value = 0.644).
6.2. Analysis.
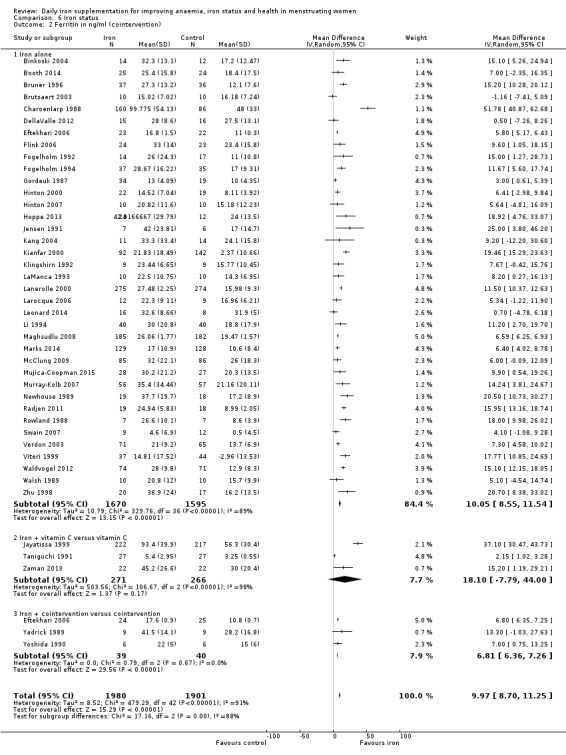
Comparison 6 Iron status, Outcome 2 Ferritin in ng/ml (cointervention).
6.3. Analysis.
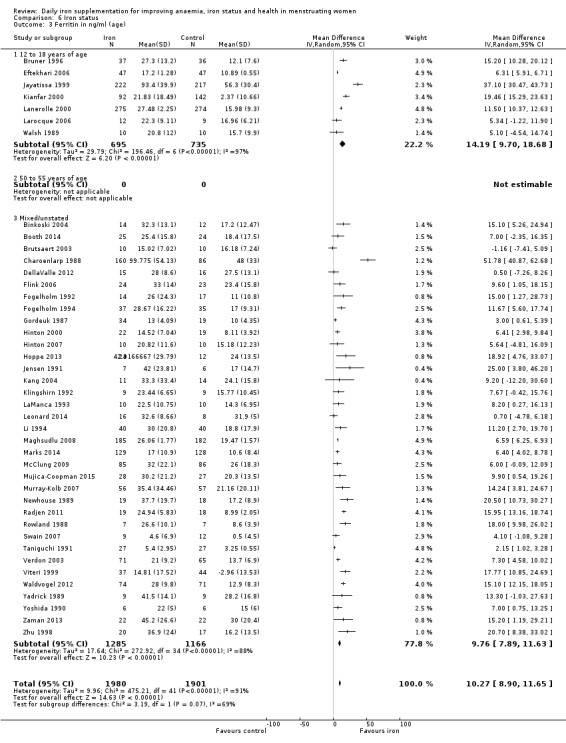
Comparison 6 Iron status, Outcome 3 Ferritin in ng/ml (age).
6.4. Analysis.
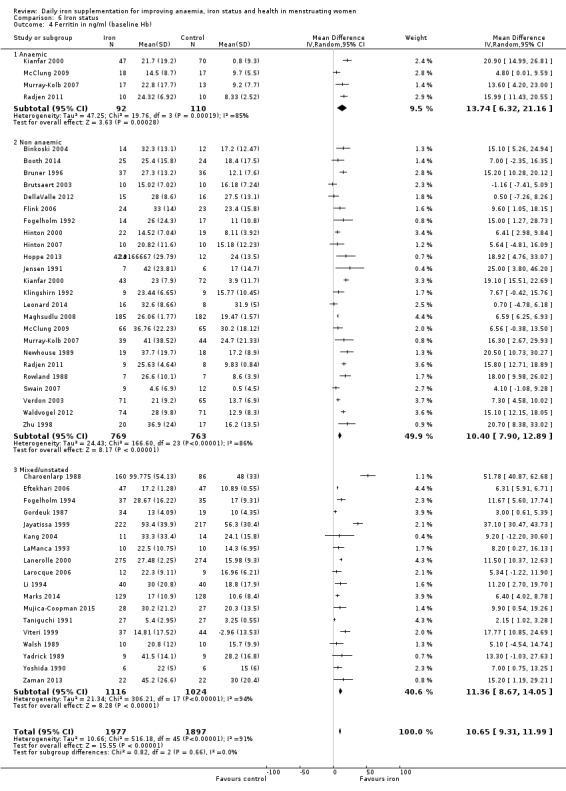
Comparison 6 Iron status, Outcome 4 Ferritin in ng/ml (baseline Hb).
6.5. Analysis.
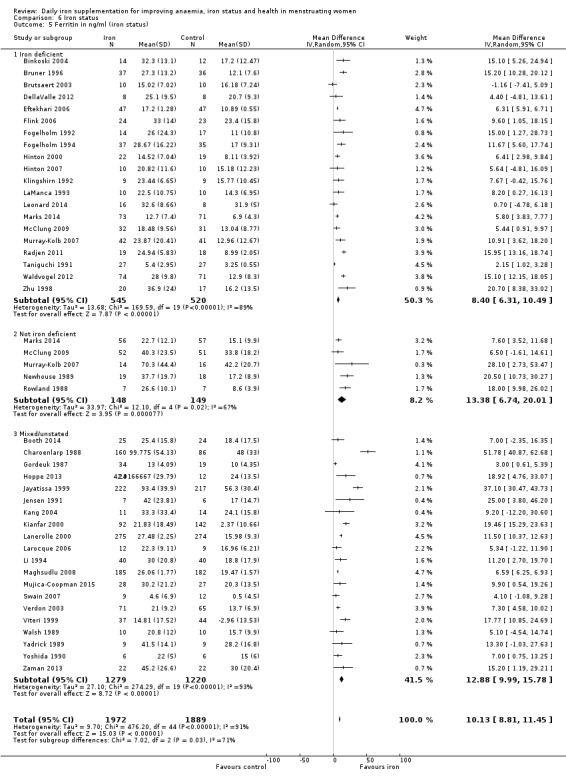
Comparison 6 Iron status, Outcome 5 Ferritin in ng/ml (iron status).
6.6. Analysis.
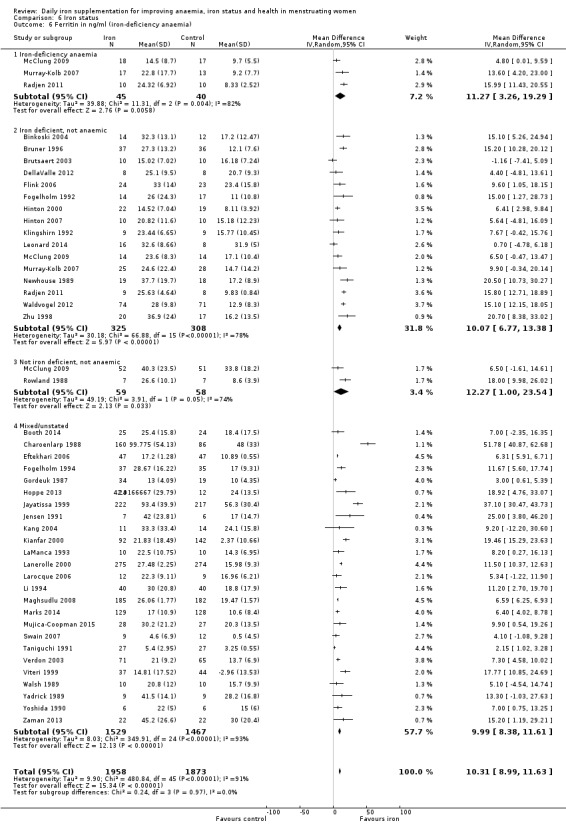
Comparison 6 Iron status, Outcome 6 Ferritin in ng/ml (iron‐deficiency anaemia).
6.7. Analysis.
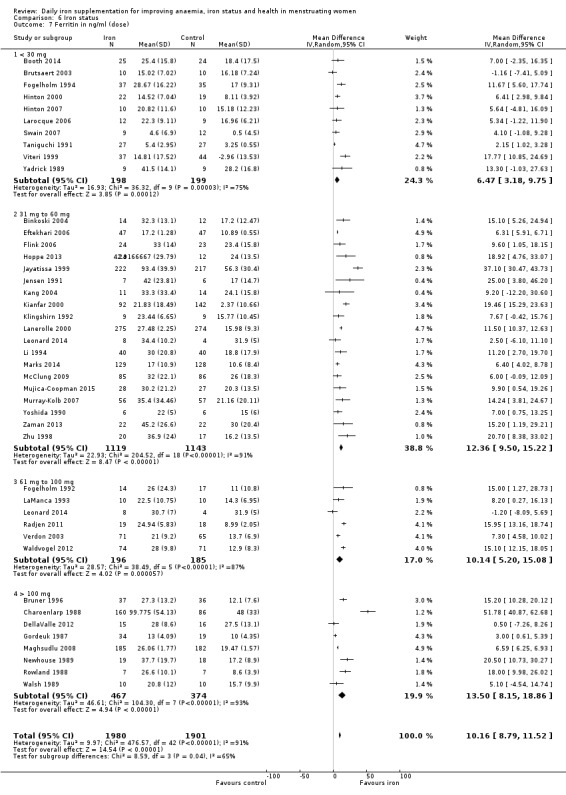
Comparison 6 Iron status, Outcome 7 Ferritin in ng/ml (dose).
6.8. Analysis.
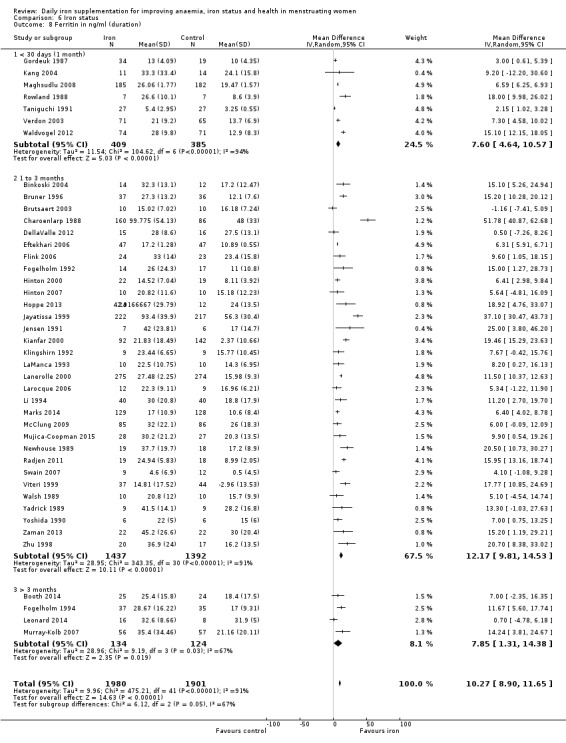
Comparison 6 Iron status, Outcome 8 Ferritin in ng/ml (duration).
6.9. Analysis.
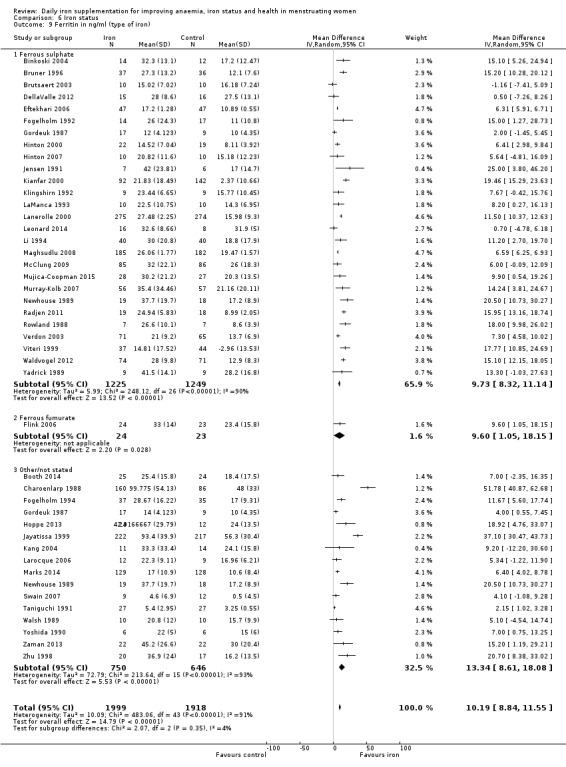
Comparison 6 Iron status, Outcome 9 Ferritin in ng/ml (type of iron).
Transferrin saturation
Twenty‐three studies recruiting 1637 women identified an effect from iron supplementation on transferrin saturation (5.98, 95% CI 3.93 to 8.02; heterogeneity: Tau² = 13.38; Chi² = 142.46, df = 22 (P < 0.00001); I² = 85%; Analysis 6.10).
6.10. Analysis.
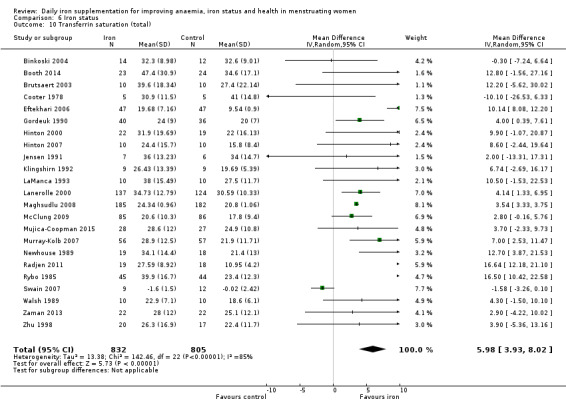
Comparison 6 Iron status, Outcome 10 Transferrin saturation (total).
Soluble transferrin receptor
Eleven studies recruiting 579 women identified an effect from iron supplementation on soluble transferrin receptor (as many assays are available, each with a different scale, we estimated the SMD (‐0.32, 95% CI ‐0.49 to ‐0.16; heterogeneity: Tau² = 0.00; Chi² = 9.34, df = 10 (P value = 0.50); I² = 0%; Analysis 6.11).
6.11. Analysis.
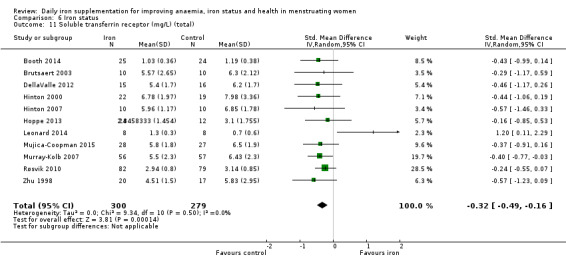
Comparison 6 Iron status, Outcome 11 Soluble transferrin receptor (mg/L) (total).
Total iron binding capacity
Nineteen studies recruiting 960 women identified no effect from iron supplementation on total iron binding capacity at the end of the intervention (SMD ‐0.64, 95% CI ‐1.38 to 0.09; heterogeneity: Tau² = 2.49; Chi² = 390.10, df = 18 (P < 0.00001); I² = 95%; Analysis 6.12).
6.12. Analysis.
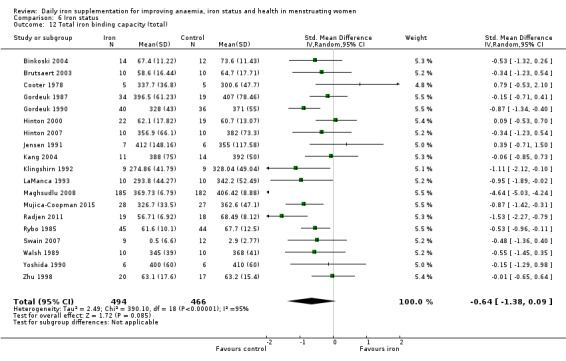
Comparison 6 Iron status, Outcome 12 Total iron binding capacity (total).
Serum iron
Seventeen studies recruiting 902 women identified an increase from iron supplementation on serum iron concentrations (SMD 0.47, 95% CI 0.19 to 0.74; heterogeneity: Tau² = 0.19; Chi² = 48.20, df = 16 (P < 0.00001); I² = 67%; Analysis 6.13).
6.13. Analysis.
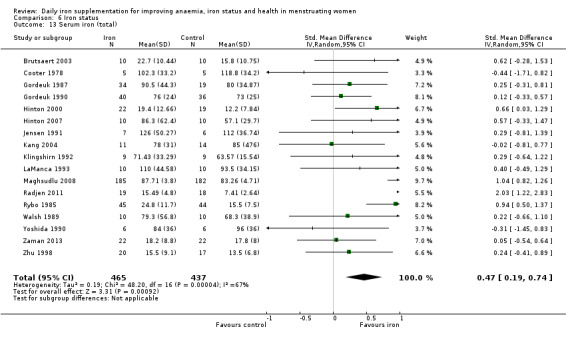
Comparison 6 Iron status, Outcome 13 Serum iron (total).
Erythrocyte protoporphyrin
Only a single study reported on erythrocyte protoporphyrin (Berger 1997), finding that iron supplementation did not significantly affect erythrocyte protoporphyrin. (For illustrative purposes, see Analysis 6.14).
6.14. Analysis.
Comparison 6 Iron status, Outcome 14 Erythrocyte protophyrin (ug/g Hb) (total).
Physical exercise performance
Exercise performance was reported in terms of both peak (maximal) and submaximal performance.
Peak (maximal) exercise performance
A meta‐analysis found that women receiving iron had increased absolute VO2 max score (MD 0.11 L/min, 95% CI 0.02 to 0.20, 8 studies, 276 women ; heterogeneity: Tau² = 0.00; Chi² = 4.96, df = 7 (P value = 0.66); I² = 0%; Analysis 7.1) and relative VO2 max (MD 2.36 mL/kg/min, 95% CI 0.55 to 4.17, 15 studies, 407 women; heterogeneity: Tau² = 8.39; Chi² = 58.26, df = 14 (P < 0.00001), I² = 0.76; Analysis 7.2), indicating that iron supplementation increases peak exercise performance in women. No effects on peak respiratory exchange ratio (RER; Analysis 7.3), heart rate (Analysis 7.4), or lactate at longest point of exercise (Analysis 7.5) were observed from iron. There was no evidence of funnel plot asymmetry.
7.1. Analysis.
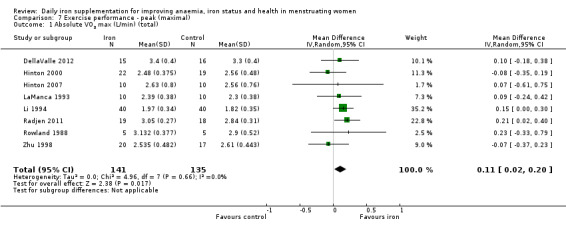
Comparison 7 Exercise performance ‐ peak (maximal), Outcome 1 Absolute VO2 max (L/min) (total).
7.2. Analysis.
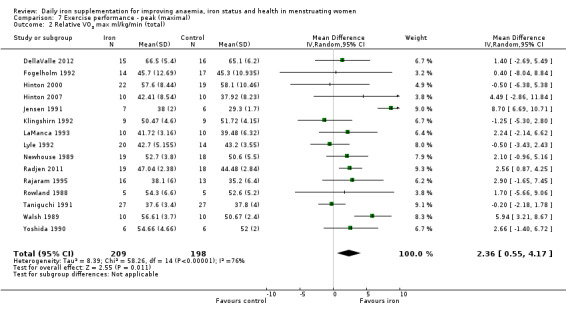
Comparison 7 Exercise performance ‐ peak (maximal), Outcome 2 Relative VO2 max ml/kg/min (total).
7.3. Analysis.
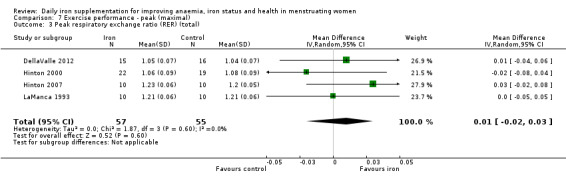
Comparison 7 Exercise performance ‐ peak (maximal), Outcome 3 Peak respiratory exchange ratio (RER) (total).
7.4. Analysis.
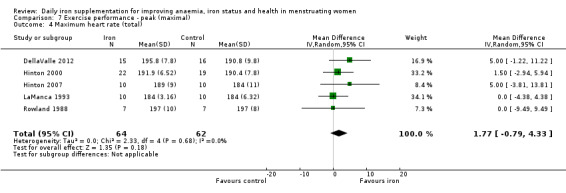
Comparison 7 Exercise performance ‐ peak (maximal), Outcome 4 Maximum heart rate (total).
7.5. Analysis.
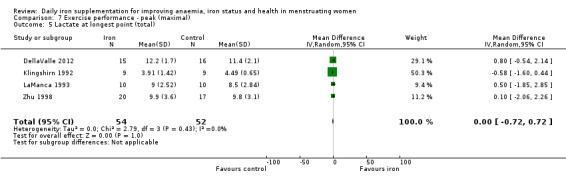
Comparison 7 Exercise performance ‐ peak (maximal), Outcome 5 Lactate at longest point (total).
Submaximal exercise performance
Five studies recruiting 126 women found that women randomised to iron required a lower proportion of their VO2 max to achieve a defined submaximal exercise task (MD ‐3.34%, 95% CI ‐6.17 to ‐0.51; heterogeneity: Tau² = 4.45; Chi² = 7.33, df = 4 (P value = 0.12); I² = 45%; Analysis 8.1). Similarly, six studies recruiting 212 women found that women randomised to iron required a lower heart rate to achieve the same exercise task (MD ‐4.72 beats per minute, 95% CI ‐8.64 to ‐0.80; heterogeneity: Tau² = 0.00; Chi² = 2.27, df = 5 (P value = 0.81); I² = 0%; Analysis 8.2). No effects from iron on energy consumption during exercise (Analysis 8.3), submaximal RER (Analysis 8.4), achieved workload (Analysis 8.5) or time to exhaustion (Analysis 8.6) were observed. There was no evidence of funnel plot asymmetry.
8.1. Analysis.
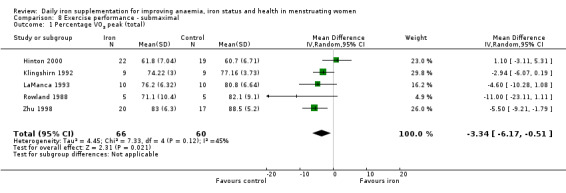
Comparison 8 Exercise performance ‐ submaximal, Outcome 1 Percentage VO2 peak (total).
8.2. Analysis.
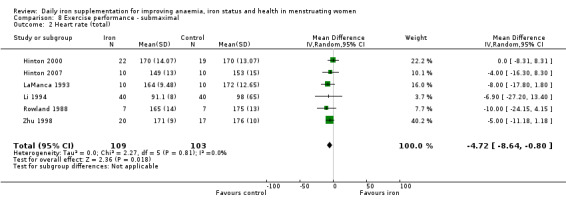
Comparison 8 Exercise performance ‐ submaximal, Outcome 2 Heart rate (total).
8.3. Analysis.
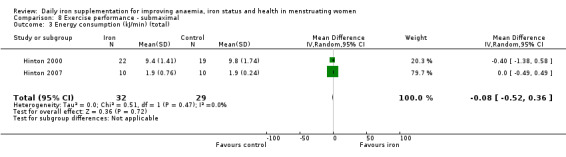
Comparison 8 Exercise performance ‐ submaximal, Outcome 3 Energy consumption (kJ/min) (total).
8.4. Analysis.
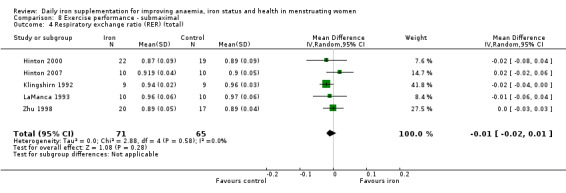
Comparison 8 Exercise performance ‐ submaximal, Outcome 4 Respiratory exchange ratio (RER) (total).
8.5. Analysis.
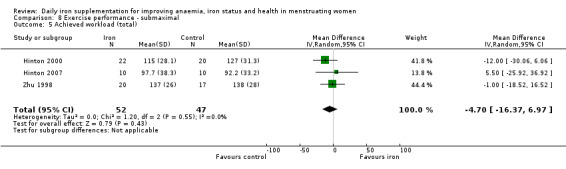
Comparison 8 Exercise performance ‐ submaximal, Outcome 5 Achieved workload (total).
8.6. Analysis.
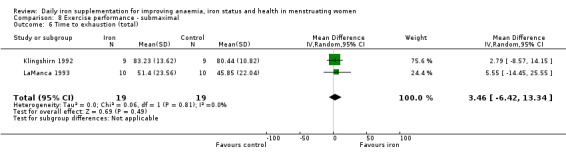
Comparison 8 Exercise performance ‐ submaximal, Outcome 6 Time to exhaustion (total).
Psychological health
Waldvogel 2012 compared four weeks of iron supplementation with placebo following blood donation in females, and observed an improvement in self‐reported physical condition (as assessed by the Short Form 12 (SF‐12) health survey (Gandek 1998)) at the end of intervention, but found no differences in self‐reported fatigue, vitality or mental health scores.
Zaman 2013 compared 12 weeks of iron supplementation as ferrous gluconate with vitamin C to placebo in females recruited through advertisements at the Univeristy of Sydney. Using the Short Form 36 Health Survey (SF‐36) (Ware 1992), participants receiving iron self‐reported improvement in vitality but no difference in other scores.
Adherence
Adherence was not reported in any form in 34 of the studies; other studies reported adherence in heterogenous ways, and hence we could not include data in a meta‐analysis. We have described data in the 'Notes' section of the Characteristics of included studies tables. Participants randomised to iron did not appear to have poorer adherence compared with those randomised to placebo.
Anthropometric measures
Height
Four studies recruiting 302 women did not identify an effect of iron on height (MD ‐0.32, 95% CI ‐2.25 to 1.61; heterogeneity: Tau² = 1.84; Chi² = 5.87, df = 3 (P value = 0.12); I² = 49%; Analysis 9.1).
9.1. Analysis.
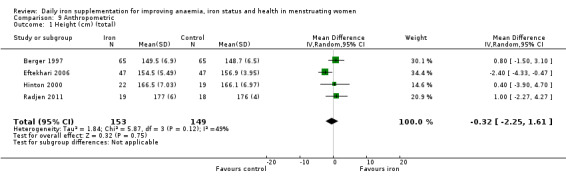
Comparison 9 Anthropometric, Outcome 1 Height (cm) (total).
Weight
Eight studies recruiting 593 women did not identify evidence of an effect from iron supplementation on weight (MD 0.76 kg, 95% CI ‐0.41 to 1.92; heterogeneity: Tau² = 0.00; Chi² = 3.19, df = 7 (P value = 0.87); I² = 0%; Analysis 9.2). A sensitivity analysis excluding the single cluster randomised trial (Kanani 2000) did not meaningfully affect this finding (MD 0.24 kg, 95% CI ‐1.13 to 1.60; Analysis 9.3).
9.2. Analysis.
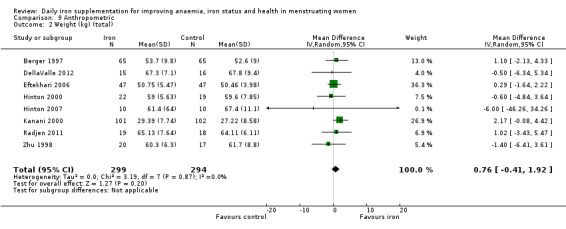
Comparison 9 Anthropometric, Outcome 2 Weight (kg) (total).
9.3. Analysis.
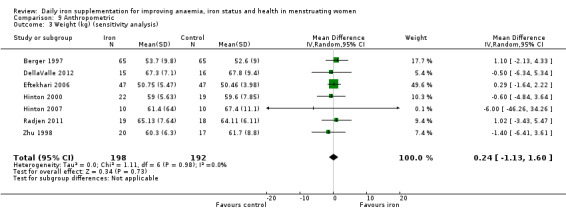
Comparison 9 Anthropometric, Outcome 3 Weight (kg) (sensitivity analysis).
Body mass index
Six studies recruiting 520 women found that iron supplementation increased body mass index in women (MD 0.53, 95% CI 0.10 to 0.96; heterogeneity: Tau² = 0.00; Chi² = 1.33, df = 5 (P value = 0.93); I² = 0%; Analysis 9.4). A sensitivity analysis excluding the single cluster randomised trial (Kanani 2000) resulted in a similar effect size although the statistical significance of this finding was no longer observed (MD 0.52, 95% CI ‐0.04 to 1.07; Analysis 9.5).
9.4. Analysis.
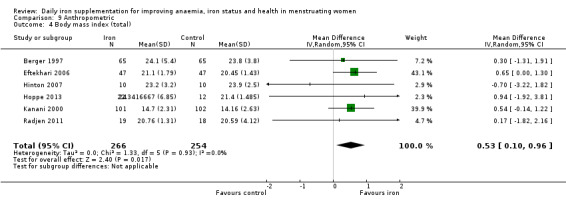
Comparison 9 Anthropometric, Outcome 4 Body mass index (total).
Serum/plasma zinc (μmol/L)
Four studies recruiting 151 women did not identify evidence of an effect from iron supplementation on zinc concentrations (MD ‐0.65, 95% CI ‐2.70 to 1.40; Analysis 10.1).
10.1. Analysis.
Comparison 10 Serum/plasma zinc, Outcome 1 Zinc levels (total).
No studies reported data on the following secondary outcomes: vitamin A status and red cell folate.
Other outcomes
Productivity
Although not a pre‐specified outcome, productivity is an important clinical and economic outcome linked with iron interventions and thus we extracted these data where available (see Differences between protocol and review). We identified three studies (446 women), which reported effects of iron supplementation on productivity, defined as a particular work‐related output per unit time (Edgerton 1979; Florencio 1981; Li 1994). Meta‐analysis of these studies revealed that iron supplementation did not increase productivity (SMD 0.07, 95% CI ‐0.12 to 0.26; Analysis 11.1).
11.1. Analysis.
Comparison 11 Productivity, Outcome 1 Productivity.
Malaria prevalence
Only one study (378 women) reported malaria prevalence (Gunaratna 2015), with no difference between iron and control groups (P value = 0.66; Analysis 12.1).
12.1. Analysis.
Comparison 12 Malaria, Outcome 1 Malaria prevalence at end of therapy (Total).
Fatigue
Although not a pre‐specified outcome, fatigue is considered an important clinical outcome from iron deficiency and anaemia, and thus we extracted data from studies reporting on effects of daily iron supplementation on fatigue.
Ballin 1992 reported that a larger number of adolescent girls randomised to iron (n = 29) compared to placebo (n = 30) experienced an improvement in 'lassitude' (about 25% iron, about 4% control, data reported on graphs).
Booth 2014 recruited 49 women undertaking cadet training in the Australian army and reported no difference in fatigue scores (P > 0.9) with daily oral iron (mean 12.9) compared to controls (mean 15.7).
Bruner 1996 reported that 35.3% (n = 37) of adolescent girls randomised to iron compared with 22.6% (n = 36) randomised to placebo reported an improvement in 'energy' levels after intervention.
Elwood 1966 reported that 40 women with a Hb > 10 g/dL randomised to iron experienced a mean increase in fatigue scores of 0.15 ± 0.78 points (graded along a 16‐point scale), whereas 49 women randomised to placebo experienced a mean increase in fatigue scores of 0.39 ± 0.73 points.
Elwood 1970 randomised anaemic (Hb < 10.5 g/dL), community‐living women to iron (n = 26) or placebo (n = 21) and reported that women receiving iron experienced a mean ‐1.32 point (standard deviation (SD) 1.78) change in fatigue scores, compared with a ‐0.7 point (SD 1.83) change in women receiving placebo.
McClung 2009 randomised female soldiers at the onset of their rigorous basic combat training to iron (n = 86) versus placebo (n = 85), and did not find evidence that iron benefited fatigue (mean: 9.8 ± 7.0 iron, 9.3 ± 6.4 placebo), as measured by the Profile of Mood States (McNair 1971), although there was an increase (P < 0.05 for group interaction) in reported 'vigour' (mean: 13.1 ± 6.3 iron, 11.6 ± 6.5 placebo).
Verdon 2003 specifically recruited women presenting with fatigue for which no other cause (including anaemia) could be identified, and randomised them to iron (n = 75) versus placebo (n = 69): women receiving iron experienced a greater reduction in fatigue scores along a 10‐point scale (mean ‐1.82, SD 1.7) compared with control (mean 0.85, SD 2.1) (difference 0.97, P value = 0.004); interestingly, a benefit was identified exclusively in women with a baseline ferritin < 50 ug/L.
Waldvogel 2012 recruited 154 non‐anaemic iron‐deficient female blood donors and randomised them to iron or placebo; women receiving iron experienced similar endpoint fatigue scores (mean: 3.4 iron, 3.5 control) and fatigue severity scores (mean: 2.5 iron, 2.6 control), as assessed by the Fatigue Severity Scale (Krupp 1989).
The variation in outcome measures and proportion/change from baseline/endpoint data reported precludes meta‐analysis. However, these data appear to indicate that iron may improve symptoms among women who are fatigued, although the effects on asymptomatic women appear less evident.
Discussion
Summary of main results
The findings, together with an assessment of the quality of the evidence for the primary outcomes, are summarised in Table 1. Overall, 67 studies involving 8506 women were included in the review.
Findings suggest that women receiving iron were less likely to be anaemic and iron deficient at the end of intervention, and more likely to experience an increase in haemoglobin concentrations and iron stores (as measured by indices such as ferritin and soluble transferrin receptor). Effects of iron supplementation on haemoglobin did not appear dose related, although increases in ferritin concentration were greater at higher doses. Also, providing iron for one to three months achieved greater increases in haemoglobin and ferritin than either shorter or more prolonged durations.
Although only limited data reported on functional health outcomes associated with iron supplementation, our meta‐analyses indicate that iron supplementation improves maximal and submaximal exercise performance in women, and reduces symptomatic fatigue. No effects on cognitive function or self‐reported psychological health were evident.
Iron supplementation was associated with an increase in gastrointestinal adverse effects, among women receiving doses exceeding 30 mg elemental iron.
Vitamin C appeared to augment the beneficial effect of iron on anaemia prevalence (but not on haemoglobin or ferritin concentration), although only limited data were available for these subgroup analyses.
Overall completeness and applicability of evidence
Despite the overall large number of trials assessing the effects of daily iron supplementation in menstruating women, most of these collected basic haematologic and iron indices data, with surprisingly few studies reporting on key outcomes ‐ anaemia, iron deficiency, iron‐deficiency anaemia; functional outcomes such as cognitive performance and psychological health (e.g. depression, fatigue); and only a very small proportion of the overall number of studies collected data on adverse effects experienced by the participants. This indicates that trials included in this review frequently did not address these clinically relevant endpoints. In particular, even though haemoglobin measurements are commonly performed, the field is limited by the lack of reporting of the effects of iron on anaemia in these key trials. Ultimately, however, the trials which do report on anaemia collectively provide moderate quality evidence to support a substantial benefit from iron on this outcome (RR 0.39, 95% CI 0.25 to 0.60). Likewise, reporting of effects of iron supplementation on iron deficiency and iron‐deficiency anaemia was uncommon (even though ferritin levels were frequently reported). A similar pattern of reporting the continuous rather than dichotomous outcomes has been observed in systematic reviews of daily iron supplementation in children (Low 2013; Pasricha 2013).
We did not undertake a formal subgroup analysis to compare trials undertaken in low‐ and middle‐income countries and those undertaken in high‐income countries. Many trials in high‐income countries were undertaken in participants who were iron deficient at baseline, and thus, such a subgroup comparison would not have been appropriate. However, the effects of iron in anaemic and iron‐deficient populations, reminiscent of the burden of these conditions in low‐income settings, can be inferred from the subgroup analyses we did perform.
The design of this review could not account for differences in efficacy between iron alone or iron in addition to common cointerventions such as folic acid or vitamin C (compared to no intervention).
Quality of the evidence
Although there have been many RCTs addressing the issue of daily iron supplementation in menstruating women, we considered only few at overall low risk of bias. In particular, only 14 studies reported using a low risk of bias method of random sequence generation (with two being at high risk of bias, and the remainder not reporting on sequence generation), and 15 reported using a low risk method of allocation concealment. Eight studies did not attempt to blind participants; while this is unlikely to affect laboratory‐measured outcomes, such as haemoglobin and iron indices, outcomes relying on more subjective tools (e.g. patient reports of adverse effects, fatigue, exercise performance and self‐reported quality of life) may have been at risk of bias. Attrition was a problem in nine trials. Overall, only 10 studies were assessed as being at low overall risk of bias.
The quality of evidence for haematologic and iron status‐related outcomes was generally moderate or high, but was poor for other outcomes, including the pre‐specified primary outcome of cognitive function. Adherence was frequently not reported, and where it was reported, it was described heterogeneously, preventing detailed analysis of the effects of adherence on outcomes.
Potential biases in the review process
The systematic review encompassed a broad and sensitive search strategy across multiple international databases, and at least two authors independently screened and extracted data. We did not apply language restrictions. We sought to identify published data and data published in the grey literature.
One potential bias in the review, however, is that it is possible that historic studies may have been undertaken that are no longer indexed or available on accessible databases, and hence for which data were not identified. Also, our classification of risk of bias may have been excessively stringent, as many trials were undertaken many years ago, before formal recommendations for trial reporting were released, and thus for which methods used to reduce risk of bias were not included in the manuscript.
Agreements and disagreements with other studies or reviews
The effects of daily iron supplementation on women's health have not been previously subject to a systematic review and meta‐analysis. A systematic review evaluating intermittent iron supplementation in menstruating women found that it was a feasible intervention for reducing anaemia compared with no iron intervention, although in comparison with daily supplementation, intermittent iron was less effective in controlling anaemia (Fernández‐Gaxiola 2011).
Authors' conclusions
Implications for practice.
Daily iron supplementation appears to be an effective clinical and public health strategy for alleviating anaemia and iron deficiency, and for increasing haemoglobin and iron stores. Daily iron supplementation also improves exercise performance (maximal and submaximal) in women. There is evidence, moreover, that iron supplementation improves fatigue scores, particularly among women with baseline fatigue. However, these benefits come at the risk of adverse effects, especially abdominal side effects. Providing iron at lower doses (e.g. up to 30 mg elemental iron) for one to three months may have an optimal benefit and adverse effect profile. There is no evidence of difference in efficacy between different iron salts.
Implications for research.
Studies reporting on haemoglobin and ferritin alone are no longer required. Only limited data exist for a range of key outcomes (both primary and secondary) relating to iron supplementation ‐ for example, effects of iron on cognitive function, psychological health, well‐being, and economic productivity. Lack of these data preclude precise economic and risk‐benefit analyses of this intervention. Further studies are needed to identify whether iron has effects on these outcomes. In the public health setting, further research is needed to understand the benefits of oral iron interventions in the preconception context on future pregnancy outcomes, and again whether iron interventions ultimately have functional benefits on well‐being and health. In low‐ and middle‐income countries, where iron may interact with infection and iron supplementation may coexist with other micronutrient deficiencies, the risk benefit of iron interventions must be more clearly understood.
What's new
| Date | Event | Description |
|---|---|---|
| 27 April 2016 | Amended | In the abstract, we added information on the number of women included in the single analysis on iron‐deficiency anaemia. We also reversed the order in which we present the results from the analyses on hard stools/constipation and loose stools/diarrhoea so these are consistent with the order in which they appear in the ‘Summary of findings’ table. Finally, we corrected the ‘Summary of findings’ table to ensure consistency of contents with the heading of column three (i.e. Number of participants (studies)). |
Acknowledgements
We would like to thank the staff at the editorial office of the Cochrane Developmental, Psychosocial and Learning Problems Group for their support in the preparation of this review.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Studies (CENTRAL)
CENTRAL 2012, Issue 2, searched 6 March 2012 [4417 records] CENTRAL 2014, Issue 8, searched 17 September 2014 [1202 records] CENTRAL 2015, Issue 10, searched 12 November 2015 [487 records]
#1MeSH descriptor: [Iron] this term only #2MeSH descriptor: [Anemia, Iron‐Deficiency] this term only #3MeSH descriptor: [Iron, Dietary] this term only #4MeSH descriptor: [Folic Acid] this term only #5MeSH descriptor: [Micronutrients] this term only #6MeSH descriptor: [Dietary Supplements] this term only #7iron* #8(folic* or folate* or folvite* or folacin* or pteroylglutamic*) #9MeSH descriptor: [Trace Elements] this term only #10(diet* near/3 supplement*) #11micronutrient* or micro next nutrient* or multinutrient* or multi next nutrient* #12MeSH descriptor: [Ferric Compounds] this term only #13MeSH descriptor: [Ferrous Compounds] this term only #14ferrous* or ferric* or fe #15#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 #16MeSH descriptor: [Drug Administration Schedule] this term only #17MeSH descriptor: [Dose‐Response Relationship, Drug] this term only #18MeSH descriptor: [Time Factors] explode all trees #19day or daily or week* or biweek* or bi next week* or intermittent* or alternat* #20#16 or #17 or #18 or #19 #21#15 and #20 #22MeSH descriptor: [Menstruation] this term only #23(menstruat* or menstrual*) #24#22 or #23 #25(teen* or adolescen* or puberty or pubescen* or ADULT or MIDDLE next AGE*) #26(girl* or woman* or women* or female*) #27#25 and #26 #28#24 or #27 #29#21 and #28, in Trials
Ovid MEDLINE(R)
1948 to February Week 4 2012, searched 6 March 2012 [6714 records] 1946 to September Week 1 2014, searched 17 September 2014 [1737 records] 1946 to November Week 1 2015, searched 12 November 2015 [839 records]
1 iron/ 2 anemia, iron deficiency/ 3 iron, Dietary/ 4 Folic acid/ 5 Micronutrients/ 6 Dietary Supplements/ 7 iron$.tw. 8 (folic$ or folate$ or folvite$ or folacin$ or pteroylglutamic$).tw. 9 trace elements/ 10 (diet$ adj3 supplement$).tw. 11 (micronutrient$ or micro‐nutrient$ or multinutrient$ or multi‐nutrient$).tw. 12 Ferric compounds/ 13 Ferrous compounds/ 14 (ferrous$ or ferric$ or fe).tw. 15 or/1‐14 16 Drug Administration Schedule/ 17 Dose‐Response Relationship, Drug/ 18 Time Factors/ 19 (day or daily or week$ or bi‐week$ or biweek$ or intermittent$ or alternate$).tw. 20 or/16‐19 21 15 and 20 22 (iron adj3 (dose$ or dosage or administer$ or administration or frequency or regimen$)).tw. 23 21 or 22 24 adult/ 25 middle aged/ 26 adolescent/ 27 (teen$ or adolescen$ or puberty or pubescen$).tw. 28 or/24‐27 29 (girl$ or wom#n$ or female$).tw. 30 female/ 31 29 or 30 32 28 and 31 33 Menstruation/ 34 (menstruat$ or menstrual$).tw. 35 or/33‐34 36 32 or 35 37 randomized controlled trial.pt. 38 controlled clinical trial.pt. 39 randomi#ed.ab. 40 placebo$.ab. 41 drug therapy.fs. 42 randomly.ab. 43 trial.ab. 44 groups.ab. 45 or/37‐44 46 exp animals/ not humans.sh. 47 45 not 46 48 23 and 36 and 47
EMBASE (Ovid)
1980 to 2012 Week 9, searched 4 March 2012 [7146 records] 1980 to 2014 Week 37, searched 17 September 2014 [1826 records] 1980 to 2015 Week 45, searched 12 November 2015 [918 records]
1 iron/ 2 iron intake/ 3 iron deficiency anemia/ 4 folic acid/ 5 exp trace element/ 6 diet supplementation/ 7 iron$.tw. 8 (folic$ or folate$ or folvite$ or folacin$ or pteroylglutamic$).tw. 9 (diet$ adj3 supplement$).tw. 10 (micro‐nutrient$ or micronutrient$ or multi‐nutrient$ or multinutrient$).tw. 11 ferric ion/ 12 ferrous ion/ 13 (ferric$ or ferrous$ or fe).tw. 14 or/1‐13 15 drug administration/ 16 dose response/ 17 (day or daily or week$ or biweek$ or bi‐week$ or intermittent$ or alternat$).tw. 18 15 or 16 or 17 19 14 and 18 20 (iron adj3 (dose$ or dosage or administer$ or administration or frequency)).tw. 21 19 or 20 22 adult/ 23 middle aged/ 24 adolescent/ 25 (teen$ or adolescen$ or puberty or pubescen$).tw. 26 or/22‐25 27 female/ 28 (girl$ or wom#n or female$).tw. 29 27 or 28 30 26 and 29 31 menstruation/ 32 (menstruat$ or menstrual$).tw. 33 31 or 32 34 30 or 33 35 exp Clinical trial/ 36 Randomized controlled trial/ 37 Randomization/ 38 Single blind procedure/ 39 Double blind procedure/ 40 Crossover procedure/ 41 Placebo/ 42 Randomi#ed.tw. 43 RCT.tw. 44 (random$ adj3 (allocat$ or assign$)).tw. 45 randomly.ab. 46 groups.ab. 47 trial.ab. 48 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 49 Placebo$.tw. 50 Prospective study/ 51 (crossover or cross‐over).tw. 52 prospective.tw. 53 or/35‐52 54 21 and 34 and 53 55 remove duplicates from 54
CINAHL (EBSCOhost)
1937 to current, searched 5 March 2012 [1210 records] 1937 to current, searched 17 September 2014 [456 records] 1937 to current, searched 12 November 2015 [180 records]
S47 S31 and S46 S46 S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 S45 TI (evaluat* study or evaluat* research) or AB (evaluate* study or evaluat* research) or TI (effectiv* study or effectiv* research) or AB (effectiv* study or effectiv* research) OR TI(prospectiv* study or prospectiv* research) or AB(prospectiv* study or prospectiv* research) or TI (follow‐up study or follow‐up research) or AB (follow‐up study or follow‐up research) S44 placebo* S43 crossover* or "cross over*" S42 (MH "Crossover Design") S41 (tripl* N3 mask*) or (tripl* N3 blind*) S40 (trebl* N3 mask*) or (trebl* N3 blind*) S39 (doubl* N3 mask*) or (doubl* N3 blind*) S38 (singl* N3 mask*) or (singl* N3 blind*) S37 (clinic* N3 trial*) or (control* N3 trial*) S36 (random* N3 allocat* ) or (random* N3 assign*) S35 randomis* or randomiz* S34 (MH "Meta Analysis") S33 (MH "Clinical Trials+") S32 MH random assignment S31 S19 and S30 S30 S26 or S29 S29 S27 or S28 S28 menstruat* or menstrual* S27 (MH "Menstruation") S26 S22 and S25 S25 S23 or S24 S24 female* or wom#n or girl* S23 (MH "Female") S22 S20 or S21 S21 (teen* or adolescen* or puberty or pubescen* or adult* or middle age*) S20 (AG adolescent) OR (AG middle aged) OR (AG adult) Limiters ‐ Age Groups: Adult: 19‐44 years, Middle Aged: 45‐64 years S19 S17 or S18 S18 (iron N3 dose*) or (iron N3 dosage) or (iron N3 administer*) or (iron N3 administration) or (iron N3 frequency) S17 S11 and S16 S16 S12 or S13 or S14 or S15 S15 (day or daily or week* or biweek* or bi‐week*or bi week* or intermittent* or alternat*) S14 (MH "Time Factors") S13 (MH "Dose‐Response Relationship, Drug") S12 (MH "Drug Administration Schedule") S11 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 S10 micro‐nutrient* or micronutrient* or micro nutrient* multi‐nutrient* or multinutrient* or multi nutrient* S9 ferrous* or ferric* or "fe" S8 diet* N3 supplement* S7 folic* or folate* or folvite* or folacin* or pteroylglutamic* S6 iron* S5 (MH "Micronutrients") S4 (MH "Trace Elements") S3 (MH "Dietary Supplements") S2 (MH "Folic Acid") S1 (MH "Iron") OR (MH "Anemia, Iron Deficiency") OR (MH "Iron Compounds") OR (MH "Ferric Compounds") OR (MH "Ferrous Compounds")
Conference Proceedings Citation Index ‐ Science (CPCI‐S; Web of Science)
1990 to 2 March 2012, searched 6 March 2012 [154 records] 1990 to 12 September 2014, searched 17 September 2014 [5 records] 1990 to current, searched 12 November 2015 [2 records]
# 8 #7 AND #6 AND #3 # 7 TS=(random* or RCT or trial* or allocat* or assign* or placebo* or cross‐over or crossover or "cross over" or factorial* or "double blind*" or "single blind") # 6 #5 OR #4 # 5 TS=(menstruat* or menstrual*) # 4 TS=(women or woman or female* or girl*) # 3 #1 or #2 # 2 TS= (iron near/3 (dose* or dosage or administer* or administration or frequency or regimen*)) # 1 TS=((iron or ferrous or ferric or micronutrient* or multinutrient* or micro‐nutrient* or multi‐nutrient* or folic* or folate* or folvite* or folacin* or pteroylglutamic*) NEAR/5 (alternate* or week* or intermittent or biweek* or bi‐week* or supplement*
Science Citation Index (SCI; Web of Science)
1970 to 2 March 2012, searched 6 March 2012 [1802 records] 1970 to 12 September 2014, searched 17 September 2014 [301 records] 1970 to 10 November 2015, searched 12 November 2015 [185 records]
# 8 #7 AND #6 AND #3 # 7 TS=(random* or RCT or trial* or allocat* or assign* or placebo* or cross‐over or crossover or "cross over" or factorial* or "double blind*" or "single blind") # 6 #5 OR #4 # 5 TS=(menstruat* or menstrual*) # 4 TS=(women or woman or female* or girl*) # 3 #1 or #2 # 2 TS= (iron near/3 (dose* or dosage or administer* or administration or frequency or regimen*)) # 1 TS=((iron or ferrous or ferric or micronutrient* or multinutrient* or micro‐nutrient* or multi‐nutrient* or folic* or folate* or folvite* or folacin* or pteroylglutamic*) NEAR/5 (alternate* or week* or intermittent or biweek* or bi‐week* or supplement*))
Popline
(popline.org)
All available years, searched 6 March 2012 [33 records] All available years, searched 18 September 2014 [21 records] All available years, searched 12 November 2015 [14 records]
Advanced search: All fields : iron* OR folic* OR folate OR ferrous OR fe AND women OR woman OR menstru* OR girl* OR female* AND day OR daily OR week* OR biweek* OR bi weekly OR intermittent* OR alternat* AND random* OR trial* OR control* OR placebo*
World Health Organization (WHO) Regional Indexes
(globalhealthlibrary.net/php/index.php)
The following WHO regional indexes were searched for all available years on 25 May 2015, and again on 8 December 2015.
Literature in the Health Sciences in Latin America and the Caribbean (LILACS). African Index Medicus (AIM; all available). Western Pacific Region Index Medicus (WPRIM). Index Medicus for the Eastern Mediterranean Region (IMEMR). Index Medicus for South‐East Asia Region ( IMSEAR).
Searched on: Title : Iron AND Women
Worldcat
Searched 25 May 2015, and again on 8 December 2015.
Search on: Iron AND Women
DART‐Europe E‐theses Portal
Searched 25 May 2015, and again on 8 December 2015.
Searched on: Iron AND Women
Australasian Digital Theses Program
Searched 25 May 2015, and again on 8 December 2015.
Searched on: Iron AND Women
ProQuest Dissertations & Theses Global
Searched 25 May 2015, and again on 8 December 2015.
Searched on: Iron AND Women
WHO International Clinical Trials Registry Platform (ICTRP)
(apps.who.int/trialsearch)
Searched 25 May 2015, and again on 8 December 2015.
Searched on: Iron AND Women
Appendix 2. Unused methods archived for future updates of this review
In future updates of this review, we will conduct a sensitivity analysis to examine the following.
The effects of different ICC values for cluster studies.
The risk of publication bias by excluding unpublished studies.
Data and analyses
Comparison 1. Anaemia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anaemia at end of therapy (total) | 10 | 3273 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.60] |
| 2 Anaemia at end of therapy (sensitivity analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Anaemia at end of therapy (cointervention) | 10 | 3273 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.60] |
| 3.1 Iron alone | 8 | 2775 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.45, 0.74] |
| 3.2 Iron + vitamin C versus vitamin C | 2 | 498 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.06, 0.15] |
| 3.3 Iron + cointervention versus cointervention | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Anaemia at end of therapy (age) | 10 | 3273 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.60] |
| 4.1 12 to 18 years of age | 4 | 2169 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.11, 0.93] |
| 4.2 50 to 55 years of age | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Mixed/unstated | 6 | 1104 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.33, 0.78] |
| 5 Anaemia at end of therapy (baseline Hb) | 10 | 3273 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.60] |
| 5.1 Anaemic | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.47, 0.90] |
| 5.2 Non‐anaemic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Mixed/unstated | 9 | 3204 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.22, 0.59] |
| 6 Anaemia at end of therapy (iron status) | 10 | 3273 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.60] |
| 6.1 Iron deficient | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.47, 0.90] |
| 6.2 Not iron deficient | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Mixed/unstated | 9 | 3204 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.22, 0.59] |
| 7 Anaemia at end of therapy (iron‐deficiency anaemia) | 10 | 3273 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.60] |
| 7.1 Iron‐deficiency anaemia | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.47, 0.90] |
| 7.2 Iron deficient, not anaemic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Not iron deficient, not anaemic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Mixed/unstated | 9 | 3204 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.22, 0.59] |
| 8 Anaemia at end of therapy (dose) | 10 | 3273 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.60] |
| 8.1 < 30 mg | 3 | 348 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.37, 0.88] |
| 8.2 31 mg to 60 mg | 2 | 807 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.03, 3.45] |
| 8.3 61 mg to 100 mg | 2 | 1466 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.25] |
| 8.4 > 100 mg | 3 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.14, 0.82] |
| 9 Anaemia at end of therapy (duration) | 10 | 3273 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.60] |
| 9.1 < 30 days (1 month) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 1 to 3 months | 5 | 1106 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.06, 0.64] |
| 9.3 > 3 months | 5 | 2167 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.48, 0.82] |
| 10 Anaemia at end of therapy (type of iron) | 9 | 3192 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.26, 0.62] |
| 10.1 Ferrous sulphate | 4 | 838 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.09, 0.48] |
| 10.2 Ferrous fumurate | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.47, 0.90] |
| 10.3 Other | 4 | 2285 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.50, 0.87] |
Comparison 2. Haemoglobin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin (total) | 51 | 6861 | Mean Difference (IV, Random, 95% CI) | 5.30 [4.14, 6.45] |
| 2 Haemoglobin (sensitivity analysis) | 6 | 581 | Mean Difference (IV, Random, 95% CI) | 5.08 [2.99, 7.17] |
| 3 Haemoglobin (cointervention) | 51 | 6861 | Mean Difference (IV, Random, 95% CI) | 5.49 [4.35, 6.63] |
| 3.1 Iron alone | 44 | 6117 | Mean Difference (IV, Random, 95% CI) | 5.39 [4.22, 6.55] |
| 3.2 Iron + vitamin C versus vitamin C | 4 | 655 | Mean Difference (IV, Random, 95% CI) | 6.59 [1.36, 11.82] |
| 3.3 Iron + cointervention versus cointervention | 4 | 89 | Mean Difference (IV, Random, 95% CI) | 3.80 [‐6.41, 14.01] |
| 4 Haemoglobin (age) | 51 | 6861 | Mean Difference (IV, Random, 95% CI) | 5.30 [4.14, 6.45] |
| 4.1 12 to 18 years of age | 10 | 3220 | Mean Difference (IV, Random, 95% CI) | 6.99 [3.85, 10.13] |
| 4.2 50 to 55 years of age | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Mixed/unstated | 41 | 3641 | Mean Difference (IV, Random, 95% CI) | 4.69 [3.55, 5.83] |
| 5 Haemoglobin (baseline Hb) | 51 | 6885 | Mean Difference (IV, Random, 95% CI) | 5.30 [4.11, 6.48] |
| 5.1 Anaemic | 8 | 558 | Mean Difference (IV, Random, 95% CI) | 8.67 [5.16, 12.18] |
| 5.2 Non‐anaemic | 25 | 2120 | Mean Difference (IV, Random, 95% CI) | 3.11 [1.67, 4.54] |
| 5.3 Mixed/unstated | 25 | 4207 | Mean Difference (IV, Random, 95% CI) | 6.30 [4.52, 8.08] |
| 6 Haemoglobin (iron status) | 51 | 6841 | Mean Difference (IV, Random, 95% CI) | 5.15 [4.00, 6.30] |
| 6.1 Iron deficient | 21 | 1124 | Mean Difference (IV, Random, 95% CI) | 6.92 [4.76, 9.09] |
| 6.2 Not iron deficient | 5 | 421 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐2.26, 3.95] |
| 6.3 Mixed/unstated | 28 | 5296 | Mean Difference (IV, Random, 95% CI) | 4.92 [3.49, 6.35] |
| 7 Haemoglobin (iron‐deficiency anaemia) | 51 | 6811 | Mean Difference (IV, Random, 95% CI) | 5.44 [4.31, 6.56] |
| 7.1 Iron‐deficiency anaemia | 4 | 154 | Mean Difference (IV, Random, 95% CI) | 9.01 [4.64, 13.37] |
| 7.2 Iron deficient, not anaemic | 15 | 586 | Mean Difference (IV, Random, 95% CI) | 5.15 [3.30, 6.99] |
| 7.3 Not iron deficient, not anaemic | 3 | 278 | Mean Difference (IV, Random, 95% CI) | 2.10 [‐1.77, 5.97] |
| 7.4 Mixed/unstated | 33 | 5793 | Mean Difference (IV, Random, 95% CI) | 5.59 [4.15, 7.03] |
| 8 Haemoglobin (dose) | 51 | 6861 | Mean Difference (IV, Random, 95% CI) | 5.26 [4.12, 6.41] |
| 8.1 < 30 mg | 14 | 872 | Mean Difference (IV, Random, 95% CI) | 4.56 [2.50, 6.63] |
| 8.2 31 to 60 mg | 19 | 2600 | Mean Difference (IV, Random, 95% CI) | 4.93 [2.20, 7.66] |
| 8.3 61 mg to 100 mg | 9 | 1897 | Mean Difference (IV, Random, 95% CI) | 6.87 [4.24, 9.49] |
| 8.4 > 100 mg | 10 | 1492 | Mean Difference (IV, Random, 95% CI) | 4.85 [3.03, 6.67] |
| 9 Haemoglobin (duration) | 51 | 6861 | Mean Difference (IV, Random, 95% CI) | 5.30 [4.14, 6.45] |
| 9.1 < 30 days (1 month) | 6 | 765 | Mean Difference (IV, Random, 95% CI) | 2.60 [0.28, 4.91] |
| 9.2 1 to 3 months | 37 | 4171 | Mean Difference (IV, Random, 95% CI) | 6.14 [4.70, 7.58] |
| 9.3 > 3 months | 8 | 1925 | Mean Difference (IV, Random, 95% CI) | 3.84 [0.94, 6.75] |
| 10 Haemoglobin (type of iron) | 47 | 6542 | Mean Difference (IV, Random, 95% CI) | 5.63 [4.44, 6.82] |
| 10.1 Ferrous sulphate | 27 | 3167 | Mean Difference (IV, Random, 95% CI) | 5.56 [3.74, 7.38] |
| 10.2 Ferrous fumurate | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 6.66 [‐4.66, 17.97] |
| 10.3 Other/not stated | 19 | 3296 | Mean Difference (IV, Random, 95% CI) | 5.71 [3.93, 7.49] |
Comparison 3. Iron deficiency.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Iron deficiency at end of therapy (total) | 7 | 1088 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.50, 0.76] |
| 2 Iron deficiency at end of therapy (sensitivity analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Iron‐deficiency anaemia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Iron‐deficiency anaemia (total) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Microcytic anaemia (Total) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. Side effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any side effect (total) | 7 | 901 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [0.94, 4.86] |
| 2 Any side effect (sensitivity analysis) | 3 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.66, 3.81] |
| 3 Any side effect (dose) | 7 | 901 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.93, 4.48] |
| 3.1 < 30 mg | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 31 mg to 60 mg | 3 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.93, 1.10] |
| 3.3 61 mg to 100 mg | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [1.44, 4.75] |
| 3.4 > 100 mg | 3 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.24, 3.73] |
| 4 Gastrointestinal side effects (total) | 5 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.26, 3.12] |
| 5 Gastrointestinal side effects (sensitivity analysis) | 3 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.96, 3.80] |
| 6 Gastrointestinal side effects (dose) | 5 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.26, 3.12] |
| 6.1 < 30 mg | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 31 mg to 60 mg | 2 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.84, 1.81] |
| 6.3 61 mg to 100 mg | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [1.45, 6.20] |
| 6.4 > 100 mg | 2 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [1.45, 4.05] |
| 7 Loose stools/diarrhoea (total) | 6 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.10, 4.11] |
| 8 Hard stools/constipation (total) | 8 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [1.35, 3.17] |
| 9 Hard stools/constipation (sensitivity analysis) | 4 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.04, 4.38] |
| 10 Abdominal pain (total) | 7 | 1190 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.99, 2.41] |
| 11 Nausea (total) | 8 | 1214 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.78, 1.82] |
| 12 Change in stool colour (total) | 4 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 6.92 [3.83, 12.52] |
| 13 Headache (total) | 4 | 526 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.58, 1.66] |
Comparison 6. Iron status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ferritin in ng/ml (total) | 42 | 3881 | Mean Difference (IV, Random, 95% CI) | 10.27 [8.90, 11.65] |
| 2 Ferritin in ng/ml (cointervention) | 42 | 3881 | Mean Difference (IV, Random, 95% CI) | 9.97 [8.70, 11.25] |
| 2.1 Iron alone | 37 | 3265 | Mean Difference (IV, Random, 95% CI) | 10.05 [8.55, 11.54] |
| 2.2 Iron + vitamin C versus vitamin C | 3 | 537 | Mean Difference (IV, Random, 95% CI) | 18.10 [‐7.79, 44.00] |
| 2.3 Iron + cointervention versus cointervention | 3 | 79 | Mean Difference (IV, Random, 95% CI) | 6.81 [6.36, 7.26] |
| 3 Ferritin in ng/ml (age) | 42 | 3881 | Mean Difference (IV, Random, 95% CI) | 10.27 [8.90, 11.65] |
| 3.1 12 to 18 years of age | 7 | 1430 | Mean Difference (IV, Random, 95% CI) | 14.19 [9.70, 18.68] |
| 3.2 50 to 55 years of age | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Mixed/unstated | 35 | 2451 | Mean Difference (IV, Random, 95% CI) | 9.76 [7.89, 11.63] |
| 4 Ferritin in ng/ml (baseline Hb) | 42 | 3874 | Mean Difference (IV, Random, 95% CI) | 10.65 [9.31, 11.99] |
| 4.1 Anaemic | 4 | 202 | Mean Difference (IV, Random, 95% CI) | 13.74 [6.32, 21.16] |
| 4.2 Non anaemic | 24 | 1532 | Mean Difference (IV, Random, 95% CI) | 10.40 [7.90, 12.89] |
| 4.3 Mixed/unstated | 18 | 2140 | Mean Difference (IV, Random, 95% CI) | 11.36 [8.67, 14.05] |
| 5 Ferritin in ng/ml (iron status) | 42 | 3861 | Mean Difference (IV, Random, 95% CI) | 10.13 [8.81, 11.45] |
| 5.1 Iron deficient | 20 | 1065 | Mean Difference (IV, Random, 95% CI) | 8.40 [6.31, 10.49] |
| 5.2 Not iron deficient | 5 | 297 | Mean Difference (IV, Random, 95% CI) | 13.38 [6.74, 20.01] |
| 5.3 Mixed/unstated | 20 | 2499 | Mean Difference (IV, Random, 95% CI) | 12.88 [9.99, 15.78] |
| 6 Ferritin in ng/ml (iron‐deficiency anaemia) | 42 | 3831 | Mean Difference (IV, Random, 95% CI) | 10.31 [8.99, 11.63] |
| 6.1 Iron‐deficiency anaemia | 3 | 85 | Mean Difference (IV, Random, 95% CI) | 11.27 [3.26, 19.29] |
| 6.2 Iron deficient, not anaemic | 16 | 633 | Mean Difference (IV, Random, 95% CI) | 10.07 [6.77, 13.38] |
| 6.3 Not iron deficient, not anaemic | 2 | 117 | Mean Difference (IV, Random, 95% CI) | 12.27 [1.00, 23.54] |
| 6.4 Mixed/unstated | 25 | 2996 | Mean Difference (IV, Random, 95% CI) | 9.99 [8.38, 11.61] |
| 7 Ferritin in ng/ml (dose) | 42 | 3881 | Mean Difference (IV, Random, 95% CI) | 10.16 [8.79, 11.52] |
| 7.1 < 30 mg | 10 | 397 | Mean Difference (IV, Random, 95% CI) | 6.47 [3.18, 9.75] |
| 7.2 31 mg to 60 mg | 19 | 2262 | Mean Difference (IV, Random, 95% CI) | 12.36 [9.50, 15.22] |
| 7.3 61 mg to 100 mg | 6 | 381 | Mean Difference (IV, Random, 95% CI) | 10.14 [5.20, 15.08] |
| 7.4 > 100 mg | 8 | 841 | Mean Difference (IV, Random, 95% CI) | 13.50 [8.15, 18.86] |
| 8 Ferritin in ng/ml (duration) | 42 | 3881 | Mean Difference (IV, Random, 95% CI) | 10.27 [8.90, 11.65] |
| 8.1 < 30 days (1 month) | 7 | 794 | Mean Difference (IV, Random, 95% CI) | 7.60 [4.64, 10.57] |
| 8.2 1 to 3 months | 31 | 2829 | Mean Difference (IV, Random, 95% CI) | 12.17 [9.81, 14.53] |
| 8.3 > 3 months | 4 | 258 | Mean Difference (IV, Random, 95% CI) | 7.85 [1.31, 14.38] |
| 9 Ferritin in ng/ml (type of iron) | 42 | 3917 | Mean Difference (IV, Random, 95% CI) | 10.19 [8.84, 11.55] |
| 9.1 Ferrous sulphate | 27 | 2474 | Mean Difference (IV, Random, 95% CI) | 9.73 [8.32, 11.14] |
| 9.2 Ferrous fumurate | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 9.60 [1.05, 18.15] |
| 9.3 Other/not stated | 16 | 1396 | Mean Difference (IV, Random, 95% CI) | 13.34 [8.61, 18.08] |
| 10 Transferrin saturation (total) | 23 | 1637 | Mean Difference (IV, Random, 95% CI) | 5.98 [3.93, 8.02] |
| 11 Soluble transferrin receptor (mg/L) (total) | 11 | 579 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.49, ‐0.16] |
| 12 Total iron binding capacity (total) | 19 | 960 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.38, 0.09] |
| 13 Serum iron (total) | 17 | 902 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.19, 0.74] |
| 14 Erythrocyte protophyrin (ug/g Hb) (total) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 7. Exercise performance ‐ peak (maximal).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Absolute VO2 max (L/min) (total) | 8 | 276 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.02, 0.20] |
| 2 Relative VO2 max ml/kg/min (total) | 15 | 407 | Mean Difference (IV, Random, 95% CI) | 2.36 [0.55, 4.17] |
| 3 Peak respiratory exchange ratio (RER) (total) | 4 | 112 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| 4 Maximum heart rate (total) | 5 | 126 | Mean Difference (IV, Random, 95% CI) | 1.77 [‐0.79, 4.33] |
| 5 Lactate at longest point (total) | 4 | 106 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.72, 0.72] |
Comparison 8. Exercise performance ‐ submaximal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percentage VO2 peak (total) | 5 | 126 | Mean Difference (IV, Random, 95% CI) | ‐3.34 [‐6.17, ‐0.51] |
| 2 Heart rate (total) | 6 | 212 | Mean Difference (IV, Random, 95% CI) | ‐4.72 [‐8.64, ‐0.80] |
| 3 Energy consumption (kJ/min) (total) | 2 | 61 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.52, 0.36] |
| 4 Respiratory exchange ratio (RER) (total) | 5 | 136 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 5 Achieved workload (total) | 3 | 99 | Mean Difference (IV, Random, 95% CI) | ‐4.70 [‐16.37, 6.97] |
| 6 Time to exhaustion (total) | 2 | 38 | Mean Difference (IV, Random, 95% CI) | 3.46 [‐6.42, 13.34] |
Comparison 9. Anthropometric.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Height (cm) (total) | 4 | 302 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐2.25, 1.61] |
| 2 Weight (kg) (total) | 8 | 593 | Mean Difference (IV, Random, 95% CI) | 0.76 [‐0.41, 1.92] |
| 3 Weight (kg) (sensitivity analysis) | 7 | 390 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐1.13, 1.60] |
| 4 Body mass index (total) | 6 | 520 | Mean Difference (IV, Random, 95% CI) | 0.53 [0.10, 0.96] |
| 5 Body mass index (sensitivity analysis) | 5 | 317 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.04, 1.07] |
Comparison 10. Serum/plasma zinc.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Zinc levels (total) | 4 | 151 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐2.70, 1.40] |
Comparison 11. Productivity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Productivity | 3 | 446 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.12, 0.26] |
Comparison 12. Malaria.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Malaria prevalence at end of therapy (Total) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 2003.
| Methods |
Design: cluster randomised controlled trial Randomisation: by school class Trial: daily iron versus weekly iron versus control. Weekly iron arm not extracted for this review Date of study: not stated |
|
| Participants |
Setting: middle class area of New Delhi, North India Malaria endemicity: not stated Included: adolescent high‐school girls aged 10 years to 17 years (mean age not reported), attending government high schools Excluded: children with haemoglobin < 7 g/dL Dropouts: 7 girls from daily iron group failed to complete trial. No reports in other groups Sample size: total: 1390; intervention: 699, control: 691 |
|
| Interventions |
Intervention: daily iron (100 mg) + folic acid (500 mcg) daily Control: no intervention Duration: 100 days |
|
| Outcomes | Haemoglobin, anaemia, iron status* | |
| Notes |
ICC: not provided Compliance: not reported Conflicts of interest: not reported Funded by: UNICEF *Not included in our analyses as ferritin was not measured at study endpoint in daily iron arm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Class randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Only biochemical measures were reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 girls dropped out |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | High risk | No intracluster correlation coefficient included |
Ballin 1992.
| Methods |
Design: randomised, double‐blind, placebo‐controlled trial Randomisation: individual Trial: daily iron versus placebo Date of study: not stated |
|
| Participants |
Setting: high school in a middle socioeconomic‐level community in urban Israel Malaria endemicity: not stated Included: adolescent girls aged 16 years to 17 years attending high school (mean age not reported) Excluded: if they had a prior gastrointestinal or haematologic illness Dropouts: not reported Sample size: total: 59; intervention: 29, control: 30 |
|
| Interventions |
Intervention: LiquiFer® liquid iron solution (105 mg elemental iron) daily Control: placebo Duration: 2 months |
|
| Outcomes | Haematology, subjective reports of health, physical fitness, side effects | |
| Notes |
Compliance: not reported Conflicts of interest: not stated Funded by: not stated Other notes: physical fitness not reported in extractable manner, subjective reports of health data derived from bar charts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo liquid administered to control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported in paper, outcomes unlikely to be affected. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | No evidence of other bias |
Berger 1997.
| Methods |
Design: randomised, double‐blind, controlled trial Randomisation: individual Trial: daily oral iron versus control Date of study: not stated |
|
| Participants |
Setting: two rural populations of the Bolivian Altiplano in the region of Potosi: Atocha (3600 m) and Santa Barbara (4800 m) Malaria endemicity: not stated Included: women aged 15 years to 40 years (mean age 28 years), non‐pregnant, well‐nourished, not suffering from chronic illness and/or acute infection, residing in the study region for at least the two previous years Excluded: not meeting above criteria or planning to leave the study region during the following 6 months Dropouts: not reported Sample size: total: 130; intervention: 65, control: 65 |
|
| Interventions |
Intervention: 3 mg elemental iron/d (6 days a week) + 20 ug folic acid Control: no intervention Duration: 3 months |
|
| Outcomes | Haemoglobin, height, weight, erythrocyte protoporphyrin | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Binkoski 2004.
| Methods |
Design: randomised, cross‐over study Randomisation: individual Trial: daily iron versus placebo Date of study: not stated |
|
| Participants |
Setting: USA, no further information regarding location Malaria endemicity: not stated Included: healthy women aged 19 years to 47 years (mean age 26 years) Excluded: did not have serum low‐density lipoprotein (LDL) cholesterol between the 50th and 90th percentiles and high‐density lipoprotein (HDL) cholesterol and triglycerides between the 5th and 95th percentiles. Must also have had low normal baseline haemoglobin (120 to 140 g/L) and low ferritin (15 to 40 ng/mL) Dropouts: not stated Sample size: total: 26; intervention: 14, control: 12 |
|
| Interventions |
Intervention: 320 mg of ferrous sulphate daily administered as 160 mg ferrous sulphate (50 mg elemental iron) twice a day Control: placebo Duration: 10 weeks |
|
| Outcomes | Haematology, iron status | |
| Notes |
Compliance: not reported Conflicts of interest: reported that no conflict of interest Funded by: donation from "Intelligent Cuisine products" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered to control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Only biochemical outcomes were evaluated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | No evidence of other bias |
Booth 2014.
| Methods |
Design: randomised, double‐blind controlled study Randomisation: individual Trial: daily oral iron versus placebo Date of study: initial recruitment started February 2003 (completion not stated) |
|
| Participants |
Setting: Australian defence force academy, Canberra, Australia Malaria endemicity: not stated Included: first and second year female officer cadets; age range not reported (mean age 20 years) Excluded: current medical problems, recent blood donation, pregnancy in the previous 12 months, breast‐feeding, anaemia (haemoglobin < 120 g/L), iron overload (serum ferritin > 300 μg/L), or a positive Helicobacter pylori antibody test Dropouts: 49 participants completed from 71 initially recruited (69%) Sample size: total: 49, Iron: 25, placebo: 24 |
|
| Interventions |
Intervention: ferrous gluconate containing 18 mg of elemental iron + 0.5 mg of folate daily Control: 0.5 mg of folate daily Duration: 13 weeks |
|
| Outcomes | Haemaglobin, iron status, general fatigue scores | |
| Notes |
Compliance: 85% compliance in both groups (equivalent to 6 tablets a week) Conflicts of interest: not declared Funded by: Defence Science & Technology Organisation’s annual tasking |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | On‐line random number generator |
| Allocation concealment (selection bias) | Low risk | Reported allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported double blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not evident |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 31% dropout rate |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Bruner 1996.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: iron daily versus placebo Date of study: August to September 1993 |
|
| Participants |
Setting: two public high schools and two private Catholic high schools in Baltimore, Maryland, USA Malaria endemicity: not stated Included: girls in grades 9 to 12, aged 13 years to 18 years (mean age 16 years) Excluded: if did not have non‐anaemic iron deficiency (i.e. haemoglobin > 120g/L (> 115g/L for African American girls)); ferritin < 12mg/L) Dropouts: 8 in total. 5 became anaemic and were excluded (3 in intervention, 2 in control group). 3 were lost to follow‐up Sample size: total: 73; intervention: 37, control: 36 |
|
| Interventions |
Intervention: ferrous sulphate 1300 mg daily (420 mg elemental iron daily) Control: placebo Duration: 8 weeks |
|
| Outcomes | Haematology, cognitive function, iron status, side effects | |
| Notes |
Compliance: not reported Conflicts of interest: authors report no conflict of interest Funded by: SmithKline Beecham Consumer Brand Pharmaceuticals Other notes: results of most cognitive tests were presented only in figures (without error bars), not tables, and thus could not be used for meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number lists |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants and investigators were unaware of group assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 dropouts, 5 withdrawn from study. Not stated from which arm losses occurred |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Unclear risk | Outcomes only reported in figures, not in tables or in the text |
Brutsaert 2003.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: Mexico. No further details Malaria endemicity: not stated Included: untrained* women, aged 18 years to 45 years (mean age 29 years), screened and found to have iron depletion (ferritin < 20 ng/mL) and be non‐anaemic (haemoglobin > 120 g/L) Excluded: current pregnancy or pregnancy within the previous year, recent infectious illness or fever, haemolytic anaemia, asthma, musculoskeletal problems, recent history of eating disorders, smoking, excess alcohol consumption, recent use of recreational drugs, or consumption of prescription medications that would interfere with dietary iron absorption Dropouts: unclear. Reports that 20 women were selected for final study from 92 eligible women. Unclear as to how women were selected Sample size: total: 20; intervention: 10, control: 10 |
|
| Interventions |
Intervention: elemental iron 10 mg as ferrous sulphate Control: placebo Duration: 6 weeks |
|
| Outcomes | Haematology, iron indices, exercise performance | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated *The authors did not further define the term "untrained" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported in paper, outcomes unlikely to be affected. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reports that 20 women were selected for final study from 92 eligible women. Unclear as to how women were selected (i.e. if drop outs affected numbers) |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Bryson 1968.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily iron with vitamin C versus vitamin C alone Date of study: not stated |
|
| Participants |
Setting: semi‐skilled female factory workers in Stevenston, Scotland Malaria endemicity: not stated Included: aged 15 years to 19 years (mean age not reported) Excluded: receiving therapy from General Practioner (GP), haemoglobin < 9 g/dL, GP started iron therapy during the trial, those who reported ill effects due to the tablet Dropouts: 94 of 269 failed to take more than 2 months supply (total 34%) Sample size: total: 254; intervention: 134, control: 120 |
|
| Interventions |
Intervention: elemental iron (40 mg/d) as ferrous fumarate + vitamin C Control: vitamin C alone Duration: 3 months |
|
| Outcomes | Haemoglobin, side effects | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated, however Lederle Laboratories provided drugs Other notes: errors not reported for haemoglobin, therefore haemoglobin data not extracted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Manufacturer maintained allocation code |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Side effects being measured, thus blinding of outcome assessment would be important |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 269 enrolled, 175 completed study |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Charoenlarp 1988.
| Methods |
Design: two randomised controlled trials Randomisation: individual Trial: Trial 1: daily iron at 2 different doses with and without supervision versus placebo. Trial 2: daily iron at two different doses with and without folic acid versus placebo Date of study: Trial 1: 1977 to 1979, Trial 2: 1978 to 1980 TWO STUDIES:
|
|
| Participants |
Setting: Trial 1: Rural area of Central Thailand 80 km north of Bangkok near Ayudhya. Trial 2: Northern Thailand; two villages 50 km south and 100 km south west of Chiang Mai Malaria endemicity: malaria is endemic to both trials Included: women of fertile age Trial 1: age range 15 years to 45 years (mean age not stated) Trial 2: age range 16 years to 45 years (mean age not stated) Excluded: Haemoglobin < 80, thalassaemia trait or disease, uncooperative Dropouts: Trial 1: 16% across all groups (reported as similar), Trial 2: reported at 36%, group status unclear Sample size: total: 863; intervention: 690, control: 173 |
|
| Interventions |
Intervention:
Control: placebo Duration: 3 months |
|
| Outcomes | Anaemia, haemoglobin, iron status | |
| Notes |
Compliance: not stated Conflicts of interest: not stated. Funded by: World Health Organization (WHO), Belgian administration of co‐operation to development, Danish International Development Authority, and Swedish International Development Authority |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 16% in trial 1 and 36.6% in trial 2 |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Cooter 1978.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily vitamins including iron versus daily vitamins without iron Date of study: not stated |
|
| Participants |
Setting: university at Georgetown University (USA) Malaria endemicity: not stated Included: female varsity basketball players aged 18 years to 24 years (mean age not reported) Excluded: not stated Dropouts: not stated Sample size: total: 10; intervention: 5, control: 5 |
|
| Interventions |
Intervention: vitamin including iron (18 mg) as ferrous fumarate daily Control: vitamin without iron daily Duration: 4 months |
|
| Outcomes | Haemoglobin, iron indices | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Biochemical indices unlikely to be influenced by assessors' knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent attrition |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
DellaValle 2012.
| Methods |
Design: randomised placebo‐controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: 2008 to 2009 |
|
| Participants |
Setting: university in USA, no further details given Malaria endemicity: not stated Included: female college rowers (varsity and second year novice) > 18 years of age (age range not reported, mean age not reported) Excluded: smokers or anaemic Dropouts: 9; 6 in intervention, 3 in control Sample size: total: 40; intervention: 21, control: 19 |
|
| Interventions |
Intervention: 50 mg ferrous sulphate per capsule twice a day (i.e. 100 mg FeSO4, approximately 30 mg elemental iron daily) Control: placebo Duration: 6 weeks |
|
| Outcomes | Haemoglobin, iron indices, peak exercise performance, perceived exercise quality | |
| Notes |
Compliance: 60.3% of tablets taken iron arm, 75.6% in control arm (mean intake iron arm 64 tablets, control arm 80 tablets) Conflicts of interest: authors report no conflict of interest Funded by: authors report no financial disclosures Other notes: provided both endpoint and change from baseline data for all outcomes. Endpoint data included in meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by assigning each participant a random number, with even and odd numbers being assigned to either treatment group |
| Allocation concealment (selection bias) | Low risk | Each participant was randomly assigned to a treatment group by a research assistant who was not involved in data collection or contact with participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Each participant was randomly assigned to a treatment group by a research assistant who was not involved in data collection or contact with participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 31 of 40 rowers finished the entire study protocol; 22% loss to follow‐up: 6 in iron group, 3 in placebo group |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Edgerton 1979.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: female tea workers of Kandy area, Sri Lanka Malaria endemicity: not stated Included: age range 20 years to 60 years (mean age 35 years). Allocation stratified by economic area and matched by economic productivity and haemoglobin Excluded: not stated Dropouts: not stated Sample size: total: 199; intervention: 103, control: 96 |
|
| Interventions |
Intervention: ferrous sulphate 200 mg/d (elemental iron 67 mg) Control: placebo (calcium lactate) Duration: 7 weeks |
|
| Outcomes | Haematology, productivity, voluntary physical activity | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: B Williams Co., New York Other notes: physical activity only reported in figures without errors: not useable. Change in productivity data not reported with SE, therefore not extractable |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Measurement of productivity may be influenced by knowledge of allocation arm |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Eftekhari 2006.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: two comparisons: daily iron with iodine versus iodine alone, daily iron versus no intervention Date of study: 2002 to 2003 |
|
| Participants |
Setting: Province of Lar in Iran Malaria endemicity: not stated Included: adolescent, grades 1 to 4 in high school; age within the range of 14 years to 18 years (mean age 16 years), who were non‐anaemic iron‐deficient (ferritin < 12 ng/mL & transferrin saturation < 16%, haemoglobin > 120 g/L) Excluded: any systemic disease, abnormal serum albumin (normal range: 3.5 g/dl to 5.5 g/dl), urinary iodine < 4100 mg/L or BMI < 19 kg/m² Dropouts: 9 of 103 girls failed on complete study (groups not described) Sample size: total: 94; iron + iodine 24, iron 23, iodine 25, control 22 |
|
| Interventions |
Intervention: 300 mg of ferrous sulphate (60 mg/day elemental iron) daily (5 days/week), with or without single oral dose of 190 mg of iodine Control: single oral dose of 190 mg of iodine or no intervention Duration: 12 weeks |
|
| Outcomes | Haemoglobin, iron status, weight, height, albumin, TFT (not extracted) | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: Tehran University of Medical Science |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. However, only biochemical indices unlikely to be affected by knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 103 individuals at baseline, on completion of study 9 were excluded (< 9%). No indication as to which arms excluded participants belonged |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Elwood 1966.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: women living in a community near a clinic in Wales Malaria endemicity: not stated Included: women attended for a general checkup: recruited if haemoglobin 100 g/L to 135 g/L, along with a 1:2 ratio of women with haemoglobin > 135 g/L. Age range 15 years to 65 years (mean age not reported) Excluded: not stated Dropouts: 22 of 111 failed to complete study (group not stated) Sample size: total: 89; intervention: 40, control: 49 |
|
| Interventions |
Intervention: ferrous carbonate 200 mg daily Control: placebo Duration: 8 weeks |
|
| Outcomes | Haemoglobin, physical health, symptoms of anaemia (e.g. fatigue, concentration etc.) | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: trial drugs provided by Allen and Hanburys Other notes: not stated whether SD or SE used for error. Assumed to be SD |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported.Recording of symptoms could be influenced by knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 111 enrolled, final data from 89 |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Elwood 1970.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: outpatient women living in a Welsh mining community Malaria endemicity: not stated Included: haemoglobin < 105 g/L; non‐macrocytic anaemia (age range not reported, mean age not reported) Excluded: not stated Dropouts: 2 of 49 women enrolled failed to complete trial (group unstated) Sample size: total: 47; intervention: 26, control: 21 |
|
| Interventions |
Intervention: 150 mg ferrous carbonate daily Control: placebo Duration: 8 weeks |
|
| Outcomes | Haemoglobin, symptoms, cognitive outcomes | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated Other notes: errors presented as SEs (not SDs) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. However, knowledge of allocation could influence subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Flink 2006.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: patients attending public dental clinic in Sala, Sweden Malaria endemicity: not stated. Included: unstimulated salivary flow rate of < 0.2 ml/min; ferritin > 10 ng/mL and < 30 mg/mL (females), < 50 mg/mL (males). Of 50 participants recruited, 46 were female. Age range 16 years to 46 years (mean age 34 years) Excluded: not stated Dropouts: 3 of 50 participants failed to complete trial (group unstated) Sample size: total: 47; intervention: 24, control: 23 |
|
| Interventions |
Intervention: elemental iron (approximately 40 mg) as ferrous fumarate daily Control: placebo Duration: 3 months |
|
| Outcomes | Iron status | |
| Notes |
Compliance: mean compliance during the intervention period was 82% (95% CI 76 to 90) for the placebo group and 71% (95% CI 61 to 82) for the iron group (i.e. resulting in an average daily dose of 85 mg of iron). There was no significant difference in compliance between intervention compared to control groups Conflicts of interest: not stated Funded by: grants from Vastmanland County, Sweden, the Swedish Patent Revenue Research Fund and the Swedish Dental Society |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator |
| Allocation concealment (selection bias) | Low risk | Identical containers, identity in numbered envelopes and not revealed until end of study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. However, biochemical measures unlikely to be affected by knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 1 in iron arm, 2 in placebo arm |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Florencio 1981.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron with vitamin C versus placebo Date of study: not stated |
|
| Participants |
Setting: Manilla, Phillipines Malaria endemicity: not stated Included: garment workers working in a single factory. Minimum age 16 years (age range not reported, mean age not reported) Excluded: not stated Dropouts: 78 of 196 participants failed to complete trial (groups unstated) Sample size: total: 122; intervention: 81, control: 41 |
|
| Interventions |
Intervention: 525 mg ferrous sulphate with vitamin C Control: placebo Duration: 3 months |
|
| Outcomes | Haemoglobin, hematocrit, anaemia, work productivity | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Productivity could be affected by knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 196 participants recruited, 78 dropped out |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not reported |
Fogelholm 1992.
| Methods |
Design: randomised controlled trial Randomisation: individual. Randomisation stratified by menstrual status (regular/irregular) Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: sports teams in Finland (athletics, basketball, handball) Malaria endemicity: not stated Included: women from sport teams aged 17 years to 31 years (mean age not reported, median age 24 years), with subclinical iron depletion (ferritin < 25 mg/mL, haemoglobin > 120 g/L) Excluded: not stated Dropouts: 2 from intervention group, none from control group Sample size: total: 31; intervention: 14, control: 17 |
|
| Interventions |
Intervention: 100 mg elemental iron as ferrous sulphate Control: placebo Duration: 8 weeks |
|
| Outcomes | Haemoglobin, iron status, VO2 max | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: grant from the Ministry of Education (presumed of Finland) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permutated blocks |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Exercise outcomes could be influenced by knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Unclear risk | HR and oxygen consumption data stated to be non significantly different between arms but data not shown. Lactate only shown in a figure |
| Other bias | Low risk | Not evident |
Fogelholm 1994.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron at two doses (9 mg/27 mg per day) versus placebo Date of study: not stated |
|
| Participants |
Setting: Helsinki, Finland Malaria endemicity: not stated Included: premenopausal women who were non‐anaemic, iron depleted (haemoglobin > 120 g/L, ferritin < 20 mg/L). Age range not reported (mean age 38 years) Excluded: not stated Dropouts: 7 in placebo group, 6 in two iron groups Sample size: total: 72; intervention: 37, control: 35 |
|
| Interventions |
Intervention: two iron doses: one and three tablets as 8 mg iron fumarate with 1 mg porcine heme iron (3 mg elemental iron per capsule) Control: placebo Duration: 6 months |
|
| Outcomes | Haemoglobin, iron status | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: Cederroth International |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Biochemical measures only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 (16.7%) loss to follow‐up: 7 placebo group, 2 Fe‐9 group, and 4 Fe‐27 group |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Unclear risk | Outcomes only reported in figures, not in tables or in the text |
Gordeuk 1987.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral carbonyl iron versus ferrous sulphate versus placebo Date of study: not stated |
|
| Participants |
Setting: Ohio red cross blood service (USA) Malaria endemicity: not stated Included: Female, non‐anaemic (haemoglobin > 125 g/L) blood donors aged 18 years to 40 years (mean age not reported); donated at least once previously Excluded: any other medical condition Dropouts: 24 of 75 lost to follow‐up with incomplete results (groups unstated). Partial data available for 70 of 75 enrolled participants Sample size: total: 70; intervention: 47, control: 23 |
|
| Interventions |
Intervention: two intervention arms, extracted separately: carbonyl iron 600 mg three times daily; ferrous sulphate 300 mg three times daily Control: placebo Duration: 2 months |
|
| Outcomes | Haemoglobin, iron status, iron deficiency, side effects | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: Food and Drug Administration orphan drugs development grant Other notes: haematologic outcomes measured 7 weeks following cessation of therapy. Carbonyl iron and ferrous sulphate reported separately: placebo group divided into two because odd number in placebo arm (23) ‐ assumed 11 for carbonyl iron, 12 for ferrous sulphate |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. As side effects were recorded, blinding of outcome assessors is important |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 75 at baseline, 24 lost to follow‐up. Partial data available for 70 of 75 enrolled participants |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Gordeuk 1990.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: Ohio red cross blood service (USA) Malaria endemicity: not stated Included: female, non‐anaemic (haemoglobin > 125 g/L) blood donors aged 18 years to 40 years (mean age not reported); donated at least once previously Excluded: any other medical condition Dropouts: 18 Sample size: total: 76; intervention: 40, control: 36 |
|
| Interventions |
Intervention: carbonyl iron daily (equivalent to 100 mg elemental iron) Control: placebo Duration: 56 days |
|
| Outcomes | Haemoglobin, iron status, side effects, anaemia | |
| Notes |
Compliance: 35% of iron arm consumed all tablets; 44% of placebo arm consumed all tablets Conflicts of interest: not stated Funded by: Food and Drug Administration orphan drugs development grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. As side effects were recorded, blinding of outcome assessors is important |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24% loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Gunaratna 2015.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron with folate versus folate alone versus multivitamin containing iron and folate (multivitamin group not extracted) Date of study: October 2010 to June 2011 |
|
| Participants |
Setting: conducted in Ikwiriri and Kibiti, two rural wards in Rufiji District, Pwani Region, Tanzania Malaria endemicity: endemic Included: women between 15 years and 29 years of age (mean age 21 years), not pregnant, planning to remain in the study area for six months, and willing to provide written informed consent themselves or through a guardian if under 18 years of age Excluded: amenorrhoea, had given birth within the past six months, were already on vitamin supplements, or had any severe illness requiring hospitalisation during screening or enrolment Dropouts: 561 completed of 802 enrolled (70%) Sample size: total: 378, iron: 184, control: 194 (multivitamin and iron: 183 ‐ not included) |
|
| Interventions |
Intervention: 30 mg of elemental iron + 0.4 mg of folate Control: 0.4 mg of folate Duration: 6 months |
|
| Outcomes | Anaemia, malaria infection and microcytosis | |
| Notes |
Compliance: median compliance were 82% in control arm and 84% in iron arm Conflicts of interest: trial authors declare no conflict of interest Funded by: Harvard School of Public Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence using blocks of size 15 created by a scientist |
| Allocation concealment (selection bias) | Low risk | States concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not evident |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 561 completed of 802 enrolled (70%) |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Heath 2001.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus dietary treatment versus placebo. Dietary group not extracted Date of study: not stated |
|
| Participants |
Setting: Dunedin area of New Zealand Malaria endemicity: not stated Included: women aged 18 years to 40 years (mean age 26 years) with mild iron deficiency (ferritin < 20 ng/mL) but haemoglobin > 120 g/L Excluded: pregnancy or lactation, irregular menstruation, health problems likely to influence iron status (for instance, gastrointestinal disease), medication likely to affect iron status, anorexia nervosa or bulimia, and veganism Dropouts: 8 failed to complete trial (groups reported but unclear). 10 excluded for other reasons Sample size: total: 35; intervention: 16, control: 19 |
|
| Interventions |
Intervention: amino acid chelate (bis‐glycino iron II) providing 50 mg of elemental iron with no change to diet Control: maltodextrin with no change in diet Duration: 16 weeks |
|
| Outcomes | Haemoglobin, ferritin (not extractable) | |
| Notes |
Compliance: 97% of tablets taken in iron group; not reported for placebo group Conflicts of interest: not stated Funded by: Health Research Council of New Zealand. Tablets provided by Albion Laboratories, Inc. (Clearfield, Utah) Other notes: no data extractable as no SDs given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Measurement of biochemical outcomes not influenced by knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 75 at baseline, 8 patients excluded during study, further 10 patients withdrew from study (24% attrition), balanced between arms |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Hinton 2000.
| Methods |
Design: randomised placebo controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: local community in USA. No further details given Malaria endemicity: not stated Included: physically active, untrained* women aged between 18 years and 33 years (mean age 21 years) with non‐anaemic iron deficiency (i.e. haemoglobin > 120 g/L and ferritin < 16 mg/L) Excluded: current pregnancy or pregnancy within the previous year, recent infectious illness or fever, haemolytic anaemia, asthma, musculoskeletal problems, recent history of eating disorders, smoking, excess alcohol consumption, recent use of recreational drugs, consumption of prescription medications that may interfere with dietary iron absorption, or participation in competitive athletics Dropouts: 16% groups not stated Sample size: total: 42; intervention: 22, control: 20 |
|
| Interventions |
Intervention: 50 mg ferrous sulphate (8 mg elemental iron) capsules Control: placebo Duration: 6 weeks |
|
| Outcomes | Haemoglobin, iron status, exercise performance, fat mass, height, weight | |
| Notes |
Compliance: 88.6% of all tablets taken in placebo group versus 91.4% in iron group Conflicts of interest: cost of publication defrayed in part of pay charges, thereby marked as advertisement Funded by: in part by Mead Johnson Research Fund and National Institute of Child Health and Human Development Training Grant HD‐07331 *Women were eligible if they were identified as physically active but untrained; further details were not provided by the author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence allocation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators blinded to allocation of participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16% dropped out |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | No evidence of other bias |
Hinton 2007.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: recruited from University of Missouri Colombia (USA) and surrounding community via fliers and newspaper advertisements Malaria endemicity: not stated Included: 17 women and 3 men, aged 18 years to 41 years (mean age 28 years). Participants were iron deficient (serum ferritin < 16 mg/L; serum transferrin receptor > 48.0 mg/L; or transferrin receptor/log ferritin index > 44.5) and non‐anaemic (haemoglobin > 120 g/L for women; > 130 g/L for men) Excluded: current pregnancy or pregnancy within the previous year, recent infectious illness or fever, chronic inflammatory diseases, haemolytic anaemia, musculoskeletal problems, history of eating disorders, smoking, or consumption of iron supplements or medications that may interfere with dietary iron absorption or that have anticoagulant properties Dropouts: no reported dropouts Sample size: total: 20; intervention: 10, control: 10 |
|
| Interventions |
Intervention: ferrous sulphate equivalent to 30 mg elemental iron Control: placebo Duration: 6 weeks |
|
| Outcomes | Haemoglobin, iron status, exercise performance, fat mass, height, weight | |
| Notes |
Compliance: on average, participants in the iron group ingested 98 (±8.2)% and the placebo group 99 (±5.4)% of their supplements. There was no significant difference in compliance between the two groups Conflicts of interest: trial authors report no conflict of interest Funded by: no funding reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Measurement of exercise performance may be influenced by knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | The study appears free of other sources of bias |
Hoppe 2013.
| Methods |
Design: randomised controlled study Randomisation: individual Trial: two doses of oral iron versus folate Date of study: 2010 and 2011 (two stages) |
|
| Participants |
Setting: Swedish universities Malaria endemicity: not stated Included: women of childbearing age who were healthy, non‐smoking without anaemia (haemoglobin < 120 g/L). Not pregnant/lactating and not exercising heavily or had donated blood less than 2 months prior. Age range not reported (mean age 24 years) Excluded: if any medication being taken or dietary supplements or underlying malabsorption or serious illness Dropouts: 3 dropped out (1 in intervention, 2 in control). 3 excluded due to infection Sample size: total: 36; intervention: 24, control: 12 |
|
| Interventions |
Intervention: two doses of iron: 35 mg of elemental iron and 60 mg of elemental iron (ferrous fumarate) Control: folate Duration: 12 weeks |
|
| Outcomes | Haemoglobin, iron status, BMI and side effects | |
| Notes |
Compliance: > 99% of tablets taken in all groups Conflicts of interest: not stated Funded by: Local Research and Development Council of Gothenburg and Southern Bohuslän, Sweden |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo/folate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. Side effects reported and could be influenced. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20% loss to follow‐up (combining exclusion and dropout rates) |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | Not evident |
Jayatissa 1999.
| Methods |
Design: cluster randomised controlled trial Randomisation: by classroom Trial: daily iron with folic acid with vitamin C plus deworming versus weekly iron with folic acid with vitamin C plus deworming versus placebo plus deworming alone (weekly arm not extracted) Date of study: not stated |
|
| Participants |
Setting: randomly selected schools in Columbo, Sri Lanka Malaria endemicity: not stated Included: adolescent girls aged 10 years to 17 years (mean age 13 years), in 3 parallel classes in each school Excluded: chronic infectious diseases or cardiopathies, taken supplements or medications containing iron during the previous month, or had a haemoglobin level less than 10 g/dL with a blood picture showing any other kind of anaemia Dropouts: 4.5% across all groups Sample size: total: 439; intervention: 222, control: 217 |
|
| Interventions |
Intervention: 60 mg elemental iron with 250 mcg folic acid, administered Monday to Friday, plus deworming Control: placebo plus deworming Duration: 8 weeks |
|
| Outcomes | Haemoglobin, iron status, anaemia | |
| Notes |
ICC: not reported Compliance: not stated Conflicts of interest: not stated Funded by: World Health Organization |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Biochemical indices unlikely to be influenced by assessor's knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 690 enrolled, 659 completed |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | High risk | ICC not reported |
Jensen 1991.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: Purdue University, Indiana, USA Malaria endemicity: not stated Included: women aged 18 years to 25 years (mean age 21 years) who were sedentary participants who did not regularly participate in an exercise programme. Willing to participate in an intensive 12‐week exercise programme Excluded: not stated Dropouts: not stated Sample size: total: 13; intervention: 7, control: 6 |
|
| Interventions |
Intervention: 50 mg elemental iron in the form of ferrous sulphate Control: placebo Duration: 12 weeks |
|
| Outcomes | Haemoglobin, iron status, exercise performance, fat mass, height, weight | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: medication provided by SmithKline Consumer Products, Philadelphia, PA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Knowledge of allocation could influence outcome assessment regarding exercise performance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition reported |
| Selective reporting (reporting bias) | Low risk | Not reported |
| Other bias | Low risk | Not evident |
Kanani 2000.
| Methods |
Design: cluster randomised controlled trial Randomisation: by community Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: India (Vadodora) Malaria endemicity: not stated Included: high school students aged 10 years to 18 years (mean age 12.4 years) in 3 low‐income communities Excluded: not stated Dropouts: not reported Sample size: total: 203; intervention: 101, control: 102 |
|
| Interventions |
Intervention: elemental iron (60 mg) + folic acid (0.5 mg) daily Control: placebo Duration: 3 months |
|
| Outcomes | Haemoglobin, BMI, weight, hunger score | |
| Notes |
ICC: not provided Compliance: 90% of the girls consumed > 85 of the 90 tablets provided; not divided by iron/placebo Conflicts of interest: not stated Funded by: Office of Health and Nutrition, USAID, under terms of contract number HRN‐C‐00‐93‐00038‐00, and the MotherCare Project, John Snow, Incorporated (JSI) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Appears to be a cluster randomised trial, randomisation by community Quote: "For feasibility reasons and to ensure similar sample sizes, the two smaller communities were combined with respect to the intervention. Through random allocation, the larger community became the iron group and the two smaller ones became the control group." Sequence generation not presented in paper |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors may have known which group was intervention and which was control |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Baseline 210; follow‐up 180 (loss to follow‐up 14.3%) |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | High risk | ICC not reported |
Kang 2004.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: Korean national women's soccer team Malaria endemicity: not stated Included: members aged 20 years to 28 years (mean age 23 years) Excluded: not stated Dropouts: not stated Sample size: total: 25; intervention: 11, control: 14 |
|
| Interventions |
Intervention: 40 mg elemental iron in liquid daily Control: placebo Duration: 1 month |
|
| Outcomes | Haemoglobin, iron status, antioxidants (not extracted) | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Biochemical indices unlikely to be influenced by knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | The study appears free of other sources of bias |
Kianfar 2000.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus weekly iron (in two forms) versus control. Weekly iron arm not extracted Date of study: 1996 to 1997 |
|
| Participants |
Setting: Iran Malaria endemicity: not stated Included: high school female students. Age range not reported (mean age 16 years) Excluded: cases with suspected thalassaemia (based on red cell indices) Dropouts: no apparent dropouts Sample size: total: 240; intervention: 92, control: 148 |
|
| Interventions |
Intervention: ferrous sulphate: 150 mg (50 mg elemental iron) daily Control: placebo Duration: 3 months |
|
| Outcomes | Haemoglobin, ferritin, anaemia | |
| Notes |
Compliance: "among anaemic and non‐anaemic subjects was 70 to 90% on average" Conflicts of interest: not stated Funded by: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Biochemical indices unlikely to be influenced by knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not reported. Apparently no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | The study appears free of other sources of bias |
Kiss 2015.
| Methods |
Design: randomised control trial Randomisation: individual Trial: daily oral iron versus no intervention Date of study: April to December 2012 |
|
| Participants |
Setting: 4 USA blood centres participating in the National Heart, Lung and Blood Institute (NHLBI) Recipient Epidemiology and Donor Evaluation Study–III (REDS‐III) programme: American Red Cross Blood Services, Farmington, Connecticut; Blood Center of Wisconsin, Milwaukee; Blood Centers of the Pacific, San Francisco, California; and Institute for Transfusion Medicine, Pittsburgh, Pennsylvania Malaria endemicity: not reported Included: successful donation of a full (500 mL) whole blood unit on the day of enrolment and a history of 1 or more previous whole blood donations but no donations in the previous 4 months. Age range not reported (mean age 46 years) Excluded: baseline ferritin level exceeding 300 ng/mL Dropouts: 215 enrolled, 193 included in final analysis. 22 dropouts (10%) Sample size: total: 136, iron: 71, control: 65 |
|
| Interventions |
Intervention: 325 mg of ferrous gluconate (37.5 mg of elemental iron) daily Control: no intervention Duration: 24 weeks |
|
| Outcomes | Time to normalisation of haemoglobin (no extractable data) | |
| Notes |
Compliance: not reported Conflicts of interest: Dr Mask received a grant from Novo Nordisk and honoraria from Siemens. Reports no other conflicts of interests Funded by: National Heart, Lung and Blood Institute (of USA) Other notes: men also included. Female data presented separately |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation to increase those with high risk of iron deficiency in iron group |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not evident |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 215 enrolled, 193 included in final analysis |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Klingshirn 1992.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: Columbia, South Carolina (USA) Malaria endemicity: not stated Included: female endurance runners training at least 3 x per week, attending road races. Age range 22 years to 39 years (mean age 29 years) Excluded: not stated Dropouts: no apparent dropouts Sample size: total: 18; intervention: 9, control: 9 |
|
| Interventions |
Intervention: elemental iron (50 mg) as ferrous sulphate (160 mg) daily Control: placebo Duration: 8 weeks |
|
| Outcomes | Haemoglobin, iron status, exercise performance | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: Society of Sigma Xi and CIBA Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Assessment of exercise outcomes may be influenced by knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant dropped out of study |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
LaManca 1993.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: athletics clubs in Florida, USA Malaria endemicity: not stated Included: healthy women aged 18 years to 35 years (mean age 28 years) with ferritin < 20 ng/mL Excluded: not stated Dropouts: none reported Sample size: total: 20; intervention: 10, control: 10 |
|
| Interventions |
Intervention: 100 mg elemental iron daily Control: placebo Duration: 8 weeks |
|
| Outcomes | Iron indices, haemoglobin, hematocrit, exercise performance | |
| Notes |
Compliance: iron 82% of tablets taken, placebo 85% of tablets taken Conflicts of interest: not stated Funded by: FSU President's Club fund and Sigma Xi. Tablets provided by SmithKline Laboratories |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Assessment of exercise outcomes may be influenced by knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not evident |
| Other bias | Low risk | Not evident |
Lanerolle 2000.
| Methods |
Design: cluster randomised controlled trial Randomisation: by school Trial: daily oral iron plus education versus education alone Date of study: not stated |
|
| Participants |
Setting: rural and urban schools in Sri Lanka with low socioeconomic status Malaria endemicity: not stated Included: adolescent girls. Age range not reported (mean age 16 years) Excluded: not stated Dropouts: 15.3%, matched between arms Sample size: total: 565; intervention: 281, control: 284 |
|
| Interventions |
Intervention: elemental iron 60 mg (as ferrous sulphate) plus education Control: education alone Duration: 10 weeks |
|
| Outcomes | Haemoglobin, iron status, iron deficiency | |
| Notes |
ICC: not provided Compliance: in the urban area, 71% of participants in the iron‐supplemented group and 77% of girls in the placebo group took more than 50% of the tablets provided; in the rural area, the percentages were 90% and 93%, respectively Conflicts of interest: not stated Funded by: financial support from UNICEF for the study in the urban area and from the OMNI (Opportunities for Micronutrient Interventions) project of the US Agency for International Development for the study in the rural area |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo given (education alone) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo given. It would be possible for assessors to know which intervention arm participants belong to (although measurement of biochemical indices unlikely to be influenced) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15.3% loss to follow‐up, matched between arms |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | High risk | No ICC's reported |
Larocque 2006.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: Thunder Bay area, Canada Malaria endemicity: not stated Included: grade 10 schoolgirls aged 14 years to 16 years (mean age not reported). Iron depleted (i.e. ferritin < 20 ng/mL), non‐anaemic (i.e. haemoglobin > 120g/L) Excluded: not stated Dropouts: 10 out of 31 (32.2%) Sample size: total: 21; intervention: 12, control: 9 |
|
| Interventions |
Intervention: ferrous gluconate 100 mg daily (approximately 12 mg elemental iron) Control: placebo Duration: 8 weeks |
|
| Outcomes | Haemoglobin, ferritin, cognitive scales (Motor Free Visual Perception Test, Digit span, Covert Orienting of Visual Attention Task: Facilitation and Inhibition, Trail Making Test Parts A and B) | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: intervention provided by Jamieson Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants lined up in random order and were allocated to therapy/placebo according to order in queue |
| Allocation concealment (selection bias) | Low risk | Code to numbered bottles kept in sealed envelopes, unknown until conclusion of study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No knowledge of allocation due to allocation being kept in sealed envelope until conclusion of study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 31 enrolled, 21 at final analysis. 32.2 % attrition |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | The study appears free of other sources of bias |
Leonard 2014.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron (two doses) versus placebo Date of study: 2010 to 2013 |
|
| Participants |
Setting: women recruited via flyer through the Hunter Medical Research Institute (Australia) Malaria endemicity: not stated Included: women aged 18 years to 35 years (mean age 26 years) with BMI between 18 kg/m² and 30 kg/m² and English speaking Excluded: iron deficient in last 12 months, taking iron, chronic medical condition or pregnant Dropouts: 12 out of 36 lost to follow‐up Sample size: total: 24; intervention: 16, control: 8 |
|
| Interventions |
Intervention: 60 mg or 80 mg elemental iron as ferrous sulphate Control: placebo Duration: 16 weeks |
|
| Outcomes | Haemoglobin, iron status, side effects and cognitive outcomes | |
| Notes |
Compliance: on average 90.4% of capsules taken Conflicts of interest: authors declare no conflict of interest Funded by: Australian Post‐Graduate Award, Meat and Livestock Australia and the School of Health Sciences at the University of Newcastle |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Low risk | Reports concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No evidence |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 30% loss in many groups |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Li 1994.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: 1989 to 1991 |
|
| Participants |
Setting: cotton workers in Beijing, China Malaria endemicity: not stated Included: women aged 19 years to 44 years (mean age 30 years) with iron deficiency (haemoglobin > 120 g/L, ferritin < 12 ng/mL, FEP > 0.62), or iron‐deficiency anaemia (haemoglobin 120 g/L + iron deficiency) Excluded: not stated Dropouts: 3 of 83 participants failed to complete study Sample size: total: 80; intervention: 40, control: 40 |
|
| Interventions |
Intervention: variable dosage of iron depending on anaemia status. Used pills containing 60 mg ferrous sulphate. Mild Iron‐deficiency anaemia or iron deficiency without anaemia given one pill per day; moderate iron‐deficiency anaemia given 2 pills per day (i.e. 60 mg and 120 mg doses, i.e. elemental iron 20 mg and 40 mg respectively) Control: placebo Duration: 12 weeks |
|
| Outcomes | Haematology, iron indices, productivity/production efficiency, exercise performance | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: Nestlé foundation, Laussanne, Switzerland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Blinding of outcome assessment could influence evaluation of work productivity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Lyle 1992.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron (two groups) plus exercise programme versus placebo plus exercise programme versus no intervention versus low fat muscle plus exercise programme. Only iron and placebo groups extracted Date of study: not stated |
|
| Participants |
Setting: Purdue University College students (USA) Malaria endemicity: not stated Included: Caucasian females who had not participated in exercise programme. Age range not reported (mean age 19 years) Excluded: smokers, on oral contraceptive pill, taking iron supplements or who had irregular menstrual periods Dropouts: 28% dropout rate (groups unstated) Sample size: total: 34; intervention: 20, control: 14 |
|
| Interventions |
Intervention: two doses: 50 mg elemental iron as ferrous sulphate or 10 mg elemental iron as ferrous sulphate. Also received low iron diet and exercise programme Control: exercise alone Duration: 4 weeks |
|
| Outcomes | Exercise performance | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: iron supplements donated by SmithKline Beecham, Parsippany, NJ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Diets not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Diets not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 72% completed protocol but not stated from which group dropouts occurred |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Machado 2011.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: 2005 to 2006 |
|
| Participants |
Setting: clinic in Brazil Malaria endemicity: not stated Included: non‐pregnant women, aged between 20 years and 49 years (mean age not reported), attending a clinic (Centro Integrado de Saúde Amaury de Medeiros; CISAM). Must have had a telephone for follow‐up contact Excluded: excluded if had gastrointestinal disorders or haemoglobin > 15 g/dL or < 11 g/dL Dropouts: 26% dropout rate Sample size: total: 539; intervention: unclear, control: unclear |
|
| Interventions |
Intervention: 60 mg of elemental iron as ferrous sulphate Control: placebo Duration: 8 weeks |
|
| Outcomes | Side effects | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated Other notes: unable to extract data as also had iron tablets twice a week group and results were combined |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers handed out |
| Allocation concealment (selection bias) | Low risk | Reports concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not evident |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26% loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Magazanik 1991.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: Israel physical training programme Malaria endemicity: not stated Included: women aged 19 years who were non‐smokers, menstruating regularly. Age range not reported (mean age 19 years) Excluded: not stated Dropouts: no loss to follow‐up reported Sample size: total: 28; intervention: 13, control: 15 |
|
| Interventions |
Intervention: ferrous sulphate 160 mg (elemental iron about 50 mg) daily Control: placebo Duration: 7 weeks |
|
| Outcomes | Haematology, iron indices, VO2 max | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: Israeli Sports Authority |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Evaluation of exercise performance could be influenced by knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Maghsudlu 2008.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: oral iron three times a day versus placebo Date of study: not stated |
|
| Participants |
Setting: Kermanshah and Golestan blood transfusion services, Iran Malaria endemicity: not stated Included: women attending blood donation. Age range not reported (mean age 28.7 years) Excluded: pregnancy, medical condition such as hereditary haemochromatosis chronic gastrointestinal disorder or intestinal cancer or polyps Dropouts: 207 out of 417 (50%) failed to return for follow‐up visit Sample size: total: 367, iron: 185, control: 182 |
|
| Interventions |
Intervention: 150 mg of ferrous sulphate three times a day Control: placebo Duration: 1 week |
|
| Outcomes | Haemoglobin, iron status, side effects | |
| Notes |
Compliance: 75.2% of tablets taken across all groups Conflicts of interest: not stated Funded by: Iranian blood transfusion organisation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unclear if double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if double blinded. Side effects reported and may be influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 50% dropout rate |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | No other source of bias identified |
Marks 2014.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: study performed between March 2009 and October 2010 |
|
| Participants |
Setting: Australian Red Cross Blood Service Malaria endemicity: not stated Included: premenopausal female blood donors with one successful whole blood donation in the past 2 years, eligibility to donate in accordance with Australian Red Cross Blood Service guidelines (including haemoglobin ≥ 120 g/L), willingness to use an agreed method of contraception for the duration of the study, ability to attend a second visit at 12 weeks, ability to provide written informed consent and a successful whole blood donation on the day of enrolment. Age range not reported (mean age 30 years) Excluded: participants with red blood cell abnormalities or potential allergies to constituents of the placebo or carbonyl iron. Participants with medications that potentially interact with iron or mask or exacerbate gastrointestinal abnormalities by iron supplementation Dropouts: 12/141 in intervention group, 13/141 in control group (8.8% total) Sample size: total: 257; intervention: 129, control: 128 |
|
| Interventions |
Intervention: carbonyl iron containing 45 mg elemental iron Control: placebo Duration: 12 weeks |
|
| Outcomes | Haemoglobin, ferritin, side effects and eligibility to donate blood | |
| Notes |
Compliance: in the carbonyl iron group, 84.4% of the participants were treatment compliant compared to 88.7% of the participants in the placebo group (compliance as per authors) Conflicts of interest: authors report no conflict of interest Funded by: authors report no funding sources Other notes: trial authors contacted regarding breakdown of side effects and responded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator, block randomisation with fixed block lengths |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8% loss to follow‐up, balanced between arms |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
McClung 2009.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: 2007 |
|
| Participants |
Setting: military recruits in the USA, undergoing 8 to 9 weeks of basic combat training Malaria endemicity: not stated Included: female, age range not reported (mean age 20 years) Excluded: iron‐deficiency anaemia Dropouts: 22% loss of follow‐up (groups unstated) Sample size: total: 171; intervention: 86, control: 85 |
|
| Interventions |
Intervention: 100 mg ferrous sulphate, found to have a mean elemental iron content of 15 mg Control: placebo Duration: 8 weeks |
|
| Outcomes | Haemoglobin, iron status, fatigue, exercise performance, mood | |
| Notes |
Compliance: overall compliance in the placebo group was 94% (4378 of 4675 total capsules); compliance in the iron‐treated group was 93% (4391 of 4730 total capsules) Conflicts of interest: authors declare no conflict of interest Funded by: United States Army Medical Research and Materiel Command |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Assessments of mood and exercise performance could be influenced by knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22% loss of follow‐up; from 219 participants at baseline to 171 participants at follow‐up |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | The study appears free of other sources of bias |
Mujica‐Coopman 2015.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo versus daily oral iron with zinc. (Zinc arm not extracted) Date of study: not stated |
|
| Participants |
Setting: Chile, no further details Malaria endemicity: not stated Included: healthy women aged 18 years to 45 years (mean age 32 years) Excluded: consumed vitamin or mineral supplements for 6 months, or were pregnant or breast feeding at time Dropouts: 7 women dropped out from a total of 87 across all groups. 1 control, 0 iron, 6 iron and zinc arm Sample size: total: 55; iron: 28, control: 27 |
|
| Interventions |
Intervention: 30 mg of elemental iron daily as ferrous sulphate Control: placebo Duration: 88 days |
|
| Outcomes | Haemoglogin, iron status and zinc status, anaemia, zinc deficiency | |
| Notes |
Compliance: reports no difference in compliance across groups (no further details) Conflicts of interest: not stated Funded by: Fondo Nacional de Desarrollo Cientifico y Tecnologico Chile Grant number 1130075 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reports being double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not evident |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 out of 87 dropped out |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | No other source of bias identified |
Murray‐Kolb 2007.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: 1999 to 2002 |
|
| Participants |
Setting: communities around University Park campus of The Pennsylvania State University in State College in the USA Malaria endemicity: not stated Included: women aged 18 years to 35 years (mean age 21 years) Excluded: chronic illnesses or serious health problems, not speaking English as the primary language at home Dropouts: 39 out of 152 (26% total loss to follow‐up) Sample size: total: 113; intervention: 56, control: 57 |
|
| Interventions |
Intervention: 160 mg ferrous sulphate containing 60 mg elemental iron Control: placebo Duration: 16 weeks |
|
| Outcomes | Haemoglobin, iron status, anxiety and psychological scores | |
| Notes |
Compliance: 95% (determine by pill count) not divided by iron/placebo group Conflicts of interest: authors declare no conflict of interest Funded by: USDA NRICGP 99‐35200‐7610 and GCRC MO1RR10732 Other notes: cognitive endpoint data reported in figures and without errors, not extractable |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation performed by using random permuted blocks |
| Allocation concealment (selection bias) | Low risk | Bottles coded preventing disclosure of allocated arm |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded as intervention and control could not be discerned; bottles coded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26% loss to follow‐up; 152 enrolled, 113 completed study |
| Selective reporting (reporting bias) | High risk | Endpoint data for several key cognitive outcomes not reported in study. Only shown on figures without SE/SD/CIs to enable extraction |
| Other bias | Low risk | The study appears free of other sources of bias |
Newhouse 1989.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: Canada Malaria endemicity: not stated Included: recreational runners undertaking at least 120 minutes (and at least three times per week) of exercise. Participants had latent iron deficiency: ferritin < 20 ng/mL, haemoglobin > 120 g/L. Age range 15 years to 40 years (mean age not reported) Excluded: Aspirin, PR blood loss, blood donation, urinary blood loss, recent fever, use of oral contraceptive pill Dropouts: 10 out of 47 failed to complete study (groups unstated) Sample size: total: 37; intervention: 19, control: 18 |
|
| Interventions |
Intervention: 200 mg elemental iron daily as ferrous sulphate Control: placebo Duration: 8 weeks |
|
| Outcomes | Haemoglobin, iron status, exercise performance | |
| Notes |
Compliance: authors state "same in both groups and over 75% as obtained by pill counts" Conflicts of interest: not stated Funded by: Ciga‐Geigy Pharmaceuticals of Canada Other notes: reports 40 completed study but only data for 37 available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Knowledge of allocation could influence assessment of exercise performance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 47 enrolled. 37 completed study |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Pereira 2014.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: King's College, London, United Kingdom. Malaria endemicity: not stated Included: 20 healthy participants, 7 men and 13 women aged 18 to 65 years (mean age 32 years) Excluded: chronic disease, pregnancy or lactation Dropouts: reports no loss to follow‐up Sample size: total: 13; intervention: 7, control: 6 |
|
| Interventions |
Intervention: ferrous sulphate 200 mg (65 mg of elemental iron), twice a day Control: placebo Duration: 7 days |
|
| Outcomes | Side effects | |
| Notes |
Compliance: not stated Conflicts of interest: authors declare no conflict of interest Funded by: United Kingdom Medical Research Council Other notes: data for women only, provided by authors via email |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation |
| Allocation concealment (selection bias) | Low risk | Reported allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | SEs unlikely to be affected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Prosser 2010.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo versus dietary advice. Dietary advice group not extracted Date of study: 1997 to 1998 |
|
| Participants |
Setting: Greater Dunedin area, New Zealand Malaria endemicity: not stated Included: women aged 18 years to 40 years (mean age not reported) with mild iron deficiency (serum ferritin < 20 mg/L; haemoglobin > 120 g/L in the absence of infection) and consumption of a non‐vegan Western‐style diet Excluded: anaemia, pregnancy or lactation, and health problems (for example, eating disorders) or medication Dropouts: 30.6% attrition reported (6/23 iron group, 9/26 control) Sample size: total: 34; intervention: 17, control: 17 |
|
| Interventions |
Intervention: 50 mg elemental iron in the form of an amino acid chelate (‘FerroChel’) Control: placebo Duration: 16 weeks |
|
| Outcomes | Zinc levels | |
| Notes |
Compliance: 97% in iron group, 94% in placebo group Conflicts of interest: authors report no conflict of interest Funded by: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Throwing dice |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed envelopes containing allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinding of all the other research staff was maintained until completion of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20.4% attrition reported |
| Selective reporting (reporting bias) | High risk | Data only provided in tables |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Radjen 2011.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: Belgrade (Serbia) Malaria endemicity: not stated Included: female elite volleyball players aged 16 years to 25 years (mean age not reported), otherwise healthy, normal menstrual periods Excluded: not stated Dropouts: dropout rates not reported Sample size: total: 37; intervention: 19, control: 18 |
|
| Interventions |
Intervention: ferrous sulphate 200 mg daily (approximately 50 mg elemental iron) Control: placebo Duration: 8 weeks |
|
| Outcomes | Haemoglobin, iron status, exercise performance, height, body fat, weight | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Knowledge of allocation could influence assessment of exercise performance |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Low risk | Not evident |
Rajaram 1995.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo versus meat and exercise versus control. Only iron and placebo extracted Date of study: not stated |
|
| Participants |
Setting: Purdue University, Indiana (USA) Malaria endemicity: not stated Included: female college students with sedentary life style, not smoking, not on contraceptive pill or iron tablets. Age range not reported (mean age 19 years) Excluded: not stated Dropouts: 62 of 78 completed trial (20% total loss to follow‐up ‐ groups not stated) Sample size: total: 29; intervention: 16, control: 13 |
|
| Interventions |
Intervention: ferrous sulphate 50 mg plus low iron diet plus exercise Control: placebo plus exercise and normal diet Duration: 24 weeks |
|
| Outcomes | Exercise performance | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: National Livestock and Meat Board |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Based on haemoglobin |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20% loss to follow‐up; 78 enrolled, 62 at follow‐up |
| Selective reporting (reporting bias) | High risk | Haemoglobin and transferrin saturation reported in text but not table. No data provided for these outcomes |
| Other bias | Low risk | Not evident |
Rowland 1988.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: high school cross country teams (USA) Malaria endemicity: not stated Included: female, adolescent, iron deficient (ferritin < 20 ng/mL), non‐anaemic (haemoglobin > 120 g/L). Age range not reported (mean age not reported) Excluded: not stated Dropouts: reports no dropouts Sample size: total: 14; intervention: 7, control: 7 |
|
| Interventions |
Intervention: 325 mg elemental iron plus 4 weeks' exercise training Control: placebo plus exercise training Duration: 4 weeks |
|
| Outcomes | Haematology, iron indices, exercise performance | |
| Notes |
Compliance: 75% of iron and 83% of control pills taken Conflicts of interest: not stated Funded by: grant from Sports Therapy for Athletic Rehabilitation and Treatment, Springfield, Mass |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Knowledge of allocation could influence assessment of exercise performance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | Not reported |
| Other bias | Low risk | Not evident |
Rybo 1985.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: 1968 to 1969 |
|
| Participants |
Setting: Gothenberg, Sweden Malaria endemicity: not stated Included: 38‐year‐old women identified on a previous cross‐sectional study. Must have had iron deficiency based on absence of stainable iron on sternal bone marrow aspirate. All participants aged 38 years Excluded: not stated Dropouts: 24 of 113 failed to complete trial (21% total loss to follow‐up ‐ groups unstated) Sample size: total: 89; intervention: 45, control: 44 |
|
| Interventions |
Intervention: ferrous succinate, 37 mg three times daily for a median of 68 days Control: placebo three times daily Duration: variable |
|
| Outcomes | Haemoglobin, iron status, side effects | |
| Notes |
Compliance: median intake was 155 tablets in 68 days Conflicts of interest: not stated Funded by: not stated Other notes: variable follow‐up and duration of treatment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Measurement of biochemical indices unlikely to be influenced by knowledge of allocation by assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 89 of 113 women completed study; 21.4% attrition |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Røsvik 2010.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus no intervention Date of study: not stated |
|
| Participants |
Setting: blood donors in Norway Malaria endemicity: not stated Included: at least one previous blood donation, haemoglobin > 12.5 g/dl (women), serum ferritin > 20mg ⁄ L. Age range 18 years to 69 years (mean age 43 years) Excluded: not stated Dropouts: 20% in intervention group, 18% in control group Sample size: total: 161; intervention: 82, control: 79 |
|
| Interventions |
Intervention: 100 mg standard Niferex ferroglycin sulphate complex tablet daily, following donation Control: no intervention Duration: 8 days |
|
| Outcomes | Haemoglobin, iron status | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: grant from Western Norway Regional Health Authority Other notes: both males and females recruited, analysis presented separately for each sex. Male data not extracted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in study |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described in study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo given to control participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo given to control participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20% loss to follow‐up among female participants |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | The study appears to be free of other source of bias |
Shah 2002.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus weekly iron versus control. Weekly iron group not extracted Date of study: 1998 to 1999 |
|
| Participants |
Setting: government girls' school in Dharan, Nepal, an urban foothill town 305 m above sea level Malaria endemicity: not stated Included: healthy adolescent girls attending a girls' school, matched for age, anthropometry and demography. Age range 11 years to 18 years (mean age 15 years) Excluded: any chronic illnesses (e.g. asthma, rheumatic heart disease), receiving any long‐term allopathic or indigenous drug treatments, those with recent hospitalisation Dropouts: 6 of 148; 4 iron, 2 control Sample size: total: 142; intervention: 70, control: 72 |
|
| Interventions |
Intervention: 350 mg of ferrous sulphate and 1.5 mg of folic acid once a day for 90 to 100 days Control: no intervention Duration: 14 weeks |
|
| Outcomes | Haematocrit, anaemia | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: the Research Committee of B.P. Koirala Institute of Health Sciences, Dharan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in study |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not described in study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo in control arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo in control arm |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition: 4 iron, 2 control |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | The study appears to be free of other source of bias |
Swain 2007.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus electrolytic iron versus reduced iron versus bakery‐grade ferrous sulphate versus placebo. Only daily oral iron and placebo groups extracted Date of study: not stated |
|
| Participants |
Setting: USA community. No further details given Malaria endemicity: not stated Included: healthy women of child‐bearing age. All women were healthy, menstruating, neither pregnant nor breast‐feeding, and were not using medication (except possibly hormonal contraceptives used for > 6 months). Age range 21 years to 51 years (mean 40 years) Excluded: any other medication Dropouts: 3 of 24; 3 intervention, 0 control Sample size: total: 21; intervention: 9, control: 12 |
|
| Interventions |
Intervention: 5 mg iron as heme iron supplement Control: placebo Duration: 12 weeks |
|
| Outcomes | Iron status | |
| Notes |
Compliance: 97% of capsules consumed Conflicts of interest: not stated Funded by: Sharing Science and Technology to aid in the improvement of Nutrition, Washington DC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in study |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described in study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants dropped out from study, not clear from which arm |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Taniguchi 1991.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron with vitamin C plus exercise versus placebo with vitamin C and exercise versus daily oral iron with vitamin C (no exercise) versus vitamin C (no exercise) Date of study: not stated |
|
| Participants |
Setting: colleges in Japan Malaria endemicity: not stated Included: female college students aged 18 years to 22 years (mean age not reported). Iron deficiency (ferritin < 6 ng/mL) not anaemic (haemoglobin > 120 g/L) Excluded: not stated Dropouts: not stated Sample size: total: 54; intervention: 27, control: 27 |
|
| Interventions |
Intervention: ferric ammonium citrate: 6 mg (approximately 1 mg of elemental iron ) + vitamin C ± exercise Control: vitamin C ± exercise Duration: 9 weeks |
|
| Outcomes | Haemoglobin, iron status, exercise performance | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo (iron‐free vitamin C) administered to control participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. May have influenced measurement of exercise outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not indicated in report |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Verdon 2003.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: 1997 to 2000 |
|
| Participants |
Setting: primary care practices in Switzerland Malaria endemicity: not stated Included: women aged 18 years to 55 years (mean age 35 years) presenting with fatigue without anaemia (haemoglobin > 117) or other obvious physical or psychiatric cause for fatigue or chronic fatigue syndrome Excluded: not stated Dropouts: 4 in each arm Sample size: total: 144; intervention: 75, control: 69 |
|
| Interventions |
Intervention: ferrous sulphate (80 mg/day of elemental iron) Control: placebo Duration: one month |
|
| Outcomes | Iron status, fatigue, anxiety, depression | |
| Notes |
Compliance: 95% iron arm versus 98% placebo arm, P value = 0.25 Conflicts of interest: FV and BF received financial support from Robapharm for producing a preliminary report of the study Funded by: Robapharm. The sponsor was not involved in the analysis of the results or in writing or correcting the manuscript |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation took place at an independent pharmacy, according to a pre‐established list |
| Allocation concealment (selection bias) | Low risk | Drug package was coded with a unique number according to the randomisation schedule |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Codes were held by the pharmacist and remained unbroken until the analyses were completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 4 in each arm |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | The study appears to be free of other source of bias |
Viteri 1999.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus weekly iron versus placebo. Weekly arm not extracted Date of study: not stated |
|
| Participants |
Setting: University of California, Berkeley USA Malaria endemicity: not stated Included: healthy, menstruating women > 18 years of age who responded to public notices. Age range 18 years to 44 years (mean age 22 years) Excluded: blood donation during the previous 6 months, pregnancy, pregnancy terminated during the previous year, lactation, menorrhagia, having a chronic condition interfering with normal iron metabolism, currently taking or having taken therapeutic iron in the previous 6 months, and predicted impossibility to comply with the iron protocol Dropouts: 39% dropout across all groups with losses equal across all groups Sample size: Total: 81; intervention: 37, control: 44 |
|
| Interventions |
Intervention: iron (60 mg as ferrous sulphate; 20 mg elemental iron) + folate (250 mcg) Control: folate alone Duration: 3 months |
|
| Outcomes | Iron status, haemoglobin, anaemia | |
| Notes |
Compliance: 88% or more ingested over 90% of all tablets; not reported by intervention group Conflicts of interest: not stated Funded by: partially supported by a grant from the International Nutrition Foundation for Developing Countries (INFDC) and by a Research Grant from the Agricultural Research Station, University of California |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in the study |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebos administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Biochemical indices unlikely to be influenced by assessor knowledge of allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 39% attrition |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting bias |
| Other bias | Low risk | The study appears free of other bias |
Waldvogel 2012.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: November 2008 to September 2011 |
|
| Participants |
Setting: Switzerland Red Cross Blood Service Malaria endemicity: not stated Included: female blood donors aged 18 years to 50 years (mean age 31 years), 1 week post‐donation, with haemoglobin > 120 g/L, ferritin < 30ng/mL Excluded: psychiatric conditions or diseases that rendered the participant unable to give consent; thyroid, hepatic, rheumatic, kidney, cardiopulmonary, or intestinal disease; acute or chronic inflammation; diabetes; haemochromatosis; pregnancy; medical treatment that could alter iron absorption and any iron supplementation Dropouts: 4 in each group Sample size: total: 145; intervention: 74, control: 71 |
|
| Interventions |
Intervention: iron 80 mg/day as ferrous sulphate (FeSO4; Tardyferon, Robapharm, Boulogne, France) Control: placebo Duration: 4 weeks |
|
| Outcomes | Haemoglobin, iron status, quality of life, exercise performance, side effects | |
| Notes |
Compliance: intervention arm took tablets for a mean 26.3 (of 28) days; control arm took tablets for a mean 26.5 (of 28) days Conflicts of interest: one author (BF) gave lectures to both Pierre Fabre Medicament and Vifor Pharma companies that may have interest in work. All other authors had no conflict of interest Funded by: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple random allocation sequence without restriction was generated by an independent pharmacy according to a pre‐established computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Each drug package was identified with a unique number according to the randomisation schedule and given to the nurse in charge of the participant |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The code was held by the pharmacist and remained unbroken until the end of the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 20% attrition, similar in both arms |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Walsh 1989.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: unclear although researchers from Launceston, Tasmania Malaria endemicity: not stated Included: female competitive swimmers. Age range not reported (mean age 15 years) Excluded: not stated Dropouts: no apparent loss to follow‐up Sample size: total: 20; intervention: 10, control: 10 |
|
| Interventions |
Intervention: iron supplementation (150 mg) daily Control: placebo (gelatin) Duration: 12 weeks |
|
| Outcomes | Haemoglobin, iron status, exercise performance | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated Other notes: VO2 max recorded but not reported in the paper for the iron arm (i.e. placebo arm reported, iron arm not reported). Thus, VO2 max data not extractable. Author not contactable |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Exercise performance may have been influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Not evident |
Wang 2012.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: Shanghai Malaria endemicity: not stated Included: women of childbearing age, aged 21 years to 45 years (mean age not reported) with anaemia Excluded: pregnancy Dropouts: dropout rates not stated Sample size: total: 69; intervention: 34, control: 35 |
|
| Interventions |
Intervention: ferric pyrophosphate and ferrous fumarate (8 mg elemental iron) daily Control: placebo Duration: 6 months |
|
| Outcomes | Haemoglobin, iron status, anaemia | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated Other notes: written In Mandarin |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated. Unlikely that biochemical outcomes affected |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Other bias | Low risk | Not stated |
Yadrick 1989.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: Oklahoma (USA) Malaria endemicity: not stated Included: female volunteers aged 25 years to 40 years (mean age not reported). Participants in good health, not using medications, including the oral contraceptive pill Excluded: not stated Dropouts: dropout rates not stated Sample size: total: 18; intervention: 9, control: 9 |
|
| Interventions |
Intervention: 25 mg iron + 25 mg zinc Control: 25 mg zinc alone Duration: 10 weeks |
|
| Outcomes | Haemoglobin, iron status, zinc, ceruloplasmin | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated Other notes: sample sizes for each arm not specifically provided: stated that half the participants allocated to each arm; assume 9 participants per arm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation matched by baseline ferritin and erythrocyte superoxide dismutase. Random sequence generation not described in study |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described in study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo (zinc alone) provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Should not influence biochemical outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | The study appears free of other bias |
Yoshida 1990.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: Japanese institution Malaria endemicity: not stated Included: female endurance (distance) athletes, undergoing a training programme. Age range not stated (mean age 19 years) Excluded: not stated Dropouts: study reports no dropouts Sample size: total: 12; intervention: 6, control: 6 |
|
| Interventions |
Intervention: ferrous sodium citrate 200 mg + multivitamin (containing vitamin C, B6 and folic acid) thrice daily Control: multivitamin alone without iron Duration: 8 weeks |
|
| Outcomes | Haemoglobin, iron status, exercise performance | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: not stated Other notes: data not presented in a table ‐ data extracted from hand‐drawn bar graphs (including SDs) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Detection bias could influence measurement of exercise performance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Zaman 2013.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus diet versus placebo. Diet group not extracted Date of study: not stated |
|
| Participants |
Setting: women recruited through advertisements at the Univeristy of Sydney, Australia Malaria endemicity: not stated Included: women aged 18 years to 35 years (mean age 25 years) and not vegetarian, pregnant, lactating, long‐term illness, hypertension, diabetes, or who consumed nutritional supplements Excluded: not stated Dropouts: 10 of 54 withdrew (4 intervention, 6 in control) Sample size: total: 44; intervention: 22, control: 22 |
|
| Interventions |
Intervention: ferrous gluconate containing 37.4 mg of elemental iron and vitamin C Control: cellulose placebo Duration: 12 weeks |
|
| Outcomes | Iron status, haemoglobin, quality of life scores, zinc, B12 levels | |
| Notes |
Compliance: not stated Conflicts of interest: not stated Funded by: a grant‐in‐aid from the Pork CRC and University of Sydney internal research funds |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number generation |
| Allocation concealment (selection bias) | Low risk | Reports blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reports blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Describes losses as 6 in control and 4 in treatment group |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
Zavaleta 2000.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus weekly iron versus placebo Date of study: August to December 1996 |
|
| Participants |
Setting: school located in a shanty town of Lima, Peru Malaria endemicity: not stated Included: high school students aged 12 years to 18 years (mean age 15 years), living in community for 6 months before the study, healthy, nulliparous, menstruating regularly in the last 3 months, had not taken any multivitamin‐mineral supplement in the last 6 months and a haemoglobin > 80 g/L Excluded: not stated Dropouts: 16 out of 312 lost to follow‐up Sample size: total: 198; intervention: 101, control: 97 |
|
| Interventions |
Intervention: ferrous sulphate 60 mg/d (20 mg elemental iron) administered Monday to Friday (i.e. 5 days per week) Control: placebo Duration: 17 weeks |
|
| Outcomes | Haemoglobin, anaemia | |
| Notes |
Compliance: girls took 94% of the expected dose of 85 pills, and the median consumption was 80 tablets in the three groups Conflicts of interest: not stated Funded by: partially by Office of Health and Nutrition, USAID, under the terms of contract number (HRN‐C‐00–93‐00038–00), and the MotherCare Project, John Snow, Incorporated (JSI) Other notes: change in prevalence reported as % (thus actual n/N calculated from sample sizes). No SDs provided for follow‐up haemoglobin: imputed based on SDs of overall haemoglobin |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described in study |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described in study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Unlikely to affect laboratory outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 312 participants at baseline. 16 dropped out of study |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | Low risk | The study appears free of other bias |
Zhu 1998.
| Methods |
Design: randomised controlled trial Randomisation: individual Trial: daily oral iron versus placebo Date of study: not stated |
|
| Participants |
Setting: Ithaca (Cornell University, USA) Malaria endemicity: not stated Included: women aged 19 years to 36 years (mean age 36 years) with haemoglobin > 120 g/L and ferritin < 16 ng/mL Excluded: current pregnancy or pregnancy within the past year, infectious illness in the past month, fever in the past week, haemolytic anaemia, asthma, musculoskeletal problems, smoking, excess alcohol consumption (more than seven glasses of an alcoholic beverage per week), recent history of eating disorders, and use of prescription medications that potentially interfere with dietary iron absorption Dropouts: 2 of 39 (1 in each arm) Sample Size: total: 37; intervention: 20, control: 17 |
|
| Interventions |
Intervention: 135 mg elemental iron daily (45 mg thrice daily) as ferrous sulphate Control: placebo Duration: 8 weeks |
|
| Outcomes | Haemoglobin, iron status, exercise performance, fat mass, weight, lactate | |
| Notes |
Compliance: on average, the placebo group consumed 144 ± 23 capsules (87.3 ± 9.5% of the total prescription) and the iron‐supplemented group consumed 145 ± 29 capsules (87.5 ± 16.5% of the total prescription); no significant difference between these arms Conflicts of interest: not stated Funded by: United States Department of Agriculture Grant (9500850) and by a Graduate Research Grant from the Division of Nutritional Sciences, Cornell University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo administered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Unlikely to affect biochemical/laboratory indices but could affect assessor's measurement of exercise performance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 39 enrolled. 2 lost to follow‐up, 1 in each arm |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Not evident |
BMI ‐ body mass index CI(s) ‐ confidence interval(s) GP ‐ general practitioner Fe ‐ iron FEP ‐ free erythrocyte protoporphyrin HR ‐ heart rate ICC ‐ intraclass correlation coefficient PR blood loss ‐ bleeding in any part of the gastrointestinal tract SD ‐ standard deviation SE ‐ standard error TFT ‐ thyroid function test UNICEF ‐ United Nations International Children's Emergency Fund VO₂ max ‐ maximal oxygen consumption
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brigham 1993 | Randomised controlled cross‐over trial; data not presented for outcomes at the end of the first parallel comparison |
| Cable 1988 | Study in blood donors; ongoing donations (blood losses) during study |
| Powell 1991 | Randomised controlled cross‐over trial; data not presented for outcomes at the end of the first parallel comparison |
| Powers 1988 | Data for men and women not disaggregated |
| Schoene 1983 | Randomised controlled cross‐over trial; data not presented for outcomes at the end of the first parallel comparison |
| Simon 1984 | Study in blood donors; ongoing donations (blood losses) during study |
Characteristics of studies awaiting assessment [ordered by study ID]
Blot 1980.
| Methods | Randomised controlled trial |
| Participants | Blood donors |
| Interventions | Iron supplementation ‐ women and men |
| Outcomes | Unclear |
| Notes | Unable to obtain text |
Böttiger 1971.
| Methods | No abstract, no details available |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Charoenlarp 1981.
| Methods | No abstract, no details available |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Greene 1995.
| Methods | Apparently a randomised controlled trial |
| Participants | Male and female adolescents aged 11 years to 16 years |
| Interventions | Iron versus placebo |
| Outcomes | Raven's Progressive Matrices (RPM): iron supplementation did not significantly improve RPM compared with placebo in females. IQ measured and not reported |
| Notes |
Isager 1974.
| Methods | No abstract, no details available |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Izak 1973.
| Methods | No abstract, no details available |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Parkinson 1981.
| Methods | No abstract, no details available |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
IQ ‐ intelligence quotient.
Characteristics of ongoing studies [ordered by study ID]
IRCT201409082365N9.
| Trial name or title | The effects of vitamin D or iron‐vitamin supplementation on bone metabolism and inflammation in 18‐year to 40‐year women |
| Methods |
Randomisation: randomised Blinding: double blinded Placebo: used Assignment: parallel Purpose: prevention |
| Participants |
Sample size: 90 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention 1:
Intervention 2:
|
| Outcomes | Haemoglobin, ferritin, serum iron |
| Starting date | 2011 |
| Contact information | Dr Mohammadreza Vafa Nutrition and Health Group, Faculty of Health, Iran University of Medical Sciences, Hemmat highway, Tehran, Iran |
| Notes | Recruitment closed late 2014. Data not published or publicly available at time of closing of data extraction for this review. Pre‐specified outcomes listed do not include any of the primary outcomes of this review for which few data are presently available. Author not contacted as given haematologic and iron outcomes only and relatively small sample size compared with sample size in the meta‐analyses. This study was judged unlikely to produce major alterations to the findings |
IRCT: Iranian Registry of Clinical Trials.
Differences between protocol and review
There were several differences between the pre‐planned protocol (Pasricha 2012) and the review. These are as follows.
In the protocol, we planned three different comparisons: iron alone versus control/placebo alone; iron with a cointervention versus a cointervention alone; and overall iron versus control (combining these two comparisons). In the review, we opted to perform analysis on a single overall comparison (iron with or without a cointervention overall versus control/placebo with or without the same cointervention), and to treat the comparisons above as subgroups. This enabled a subgroup analysis to explore heterogeneity in effect sizes and outcomes, and simplifies the analysis for the reader. In addition, it increased the number of studies considered overall, especially for less commonly reported outcomes, and enabled us to provide an overall effect size of this intervention.
We formally assessed publication bias evident on funnel plots using statistical tests suggested by Egger, Peters and Harbord.
We added a subgroup of 'type of iron' (ferrous sulphate, ferrous fumarate, and others), as we have become aware through conversations with colleagues in the field that many potential users of our review were interested to discover whether different iron formulations could explain differences in efficacy and safety.
Given the rich data set of studies reporting on ferritin and the paucity of trials reporting on iron deficiency, we opted to undertake subgroup analysis reporting on this outcome in order to explore whether heterogeneity in this outcome could be explained by any of the pre‐specified subgroups.
Finally, because of the availability of data and interest in the outcomes, we attempted to analyse the effects of iron on fatigue and on productivity.
Contributions of authors
LMD‐R and SRP conceived and designed the review.
CES, JS, MSYL SRP contributed to screening of studies and extraction of data.
MSYL and SRP entered data into RevMan and undertook the analysis.
LMD‐R, MSYL and SRP wrote the manuscript.
All authors read and approved the final manuscript.
SRP has overall responsibility for this review.
Sources of support
Internal sources
-
Evidence and Programme Guidance, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
Provided training and mentorship for the project
External sources
-
Victoria Fellowship, Government of Victoria, Australia.
Financial support for Dr Sant‐Rayn Pasricha
-
Royal Australasian College of Physician/National Health and Medical Research Council (NHMRC), Australia.
Financial support for Dr Michael Low
-
Monash Health, Australia.
Salary for Dr Michael Low
-
CRB Blackburn Travelling Scholarship, The Royal Australasian College of Physicians, Australia.
Financial support for Dr Sant‐Rayn Pasricha
-
University of Melbourne Overseas Research Experience Scholarship, Australia.
Financial support for Dr Sant‐Rayn Pasricha
-
New Investigator Fellowship, Haematology Society of Australia and New Zealand, Australia.
Financial support for Dr Sant‐Rayn Prasricha
-
NHMRC CJ Martin Early Career Fellowship, Australia.
Financial support for Dr Sant‐Rayn Pasricha
-
Bill and Melinda Gates Foundation, UK.
Financial support for Dr Sant‐Rayn Pasricha
-
CRB Blackburn Scholarship (National Health and Medical Research Council Gustav Nossal Award), Australia.
Financial support for Dr Michael Low
Declarations of interest
The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Michael Sze Yuan Low is employed by Monash Health, an Australian government funded public hospital. MSYL has a PhD scholarship from the Royal Australasian College of Physicians/National Health and Medical Research Council Australia, which was used to fund research outside of this review. Joanna Speedy is currently employed by the Australian Red Cross Blood Service, who sponsored and conducted one of the studies included in the review (Marks 2014), and was involved in conducting the study. Due to this potential conflict of interest Joanna Speedy was not involved in the decision to include this trial, extract data from this trial, or assess the risk of bias of this trial. Claire E Styles is currently employed by the Australian Red Cross Blood Service, who sponsored and conducted one of the studies included in the review (Marks 2014), but was not involved in any aspect of this study. Luz Maria De‐Regil is a staff member of the Micronutrient Initiative (MI), an international not‐for‐profit organisation that delivers, with support of Global Affairs Canada, iron and folic acid through different programmes to children, women of reproductive age and pregnant women. None of these programmes met the inclusion criteria of this review and were not captured by the search process. Sant‐Rayn Pasricha's former institution received an unrestricted research grant in 2012, from Vifor Pharma Ltd for his work as a co‐investigator on a phase II trial of IV iron carboxymaltose in patients with iron‐deficiency anaemia. The work is unrelated to this review and is not included in the review.
Disclaimer: Luz Maria De‐Regil is a full‐time staff member of the Micronutrient Initiative. Jo Speedy, Claire Styles and Sant‐Rayn Pasricha are staff of the Australian Red Cross Blood Service. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the official position, decisions, policy or views of these organisations.
Edited (no change to conclusions)
References
References to studies included in this review
Agarwal 2003 {published data only}
- Agarwal KN, Gomber S, Bisht H, Som M. Anemia prophylaxis in adolescent school girls by weekly or daily iron‐folate supplementation. Indian Pediatrics 2003;40(4):296‐301. [PUBMED: 12736400] [PubMed] [Google Scholar]
Ballin 1992 {published data only}
- Ballin A, Berar M, Rubinstein U, Kleter Y, Hershkovitz A, Meytes D. Iron state in female adolescents. American Journal of Diseases of Children 1992;146(7):803‐5. [DOI] [PubMed] [Google Scholar]
Berger 1997 {published data only}
- Berger J, Aguayo VM, San Miguel JL, Lujan C, Tellez W, Traissac P. Definition and prevalence of anemia in Bolivian women of childbearing age living at high altitudes: the effect of iron‐folate supplementation. Nutrition Reviews 1997;55(6):247‐56. [DOI: 10.1111/j.1753-4887.1997.tb01612.x] [DOI] [PubMed] [Google Scholar]
Binkoski 2004 {published data only}
Booth 2014 {published data only}
- Booth CK, Carins JE, Robertson IK. Randomised double‐blind, placebo‐controlled trial of iron supplementation attenuates fatigue and declining iron stores for female officers‐in‐training. Journal of Military & Veterans' Health 2014;22(3):13‐24. [Google Scholar]
Bruner 1996 {published data only}
- Bruner AB, Joffe A, Duggan AK, Casella JF, Brandt J. Randomised study of cognitive effects of iron supplementation in non‐anaemic iron‐deficient adolescent girls. Lancet 1996;348(9033):992‐6. [PUBMED: 8855856] [DOI] [PubMed] [Google Scholar]
Brutsaert 2003 {published data only}
- Brutsaert TD, Hernandez‐Cordero S, Rivera J, Viola T, Hughes G, Haas JD. Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron‐depleted, nonanemic women. American Journal of Clinical Nutrition 2003;77(2):441‐8. [DOI] [PubMed] [Google Scholar]
Bryson 1968 {published data only}
- Bryson DD. Iron and anaemia in adolescent girls: a double‐blind trial. Practitioner 1968;200(199):694‐7. [PubMed] [Google Scholar]
Charoenlarp 1988 {published data only}
- Charoenlarp P, Dhanamitta S, Kaewvichit R, Silprasert A, Suwanaradd C, Na‐Nakorn S, et al. A WHO collaborative study on iron supplementation in Burma and in Thailand. American Journal of Clinical Nutrition 1988;47(2):280‐97. [DOI] [PubMed] [Google Scholar]
Cooter 1978 {published data only}
- Cooter RG, Mowbray KW. Effects of iron supplementation and activity on serum iron depletion and hemoglobin levels in female athletes. Research Quarterly 1978;49(2):114‐8. [PubMed] [Google Scholar]
DellaValle 2012 {unpublished data only}
- DellaValle DM [pers comm]. The effects of iron depletion without anemia on training and performance in female collegiate rowers [personal communication]. Email to: SR Pasrischa 10 May 2012.
- DellaValle DM, Haas JD. Iron supplementation improves energetic efficiency in iron‐depleted female rowers. Medicine and Science in Sports and Exercise 2014;46(6):1204‐15. [PUBMED: 24195864 ] [DOI] [PubMed] [Google Scholar]
Edgerton 1979 {published data only}
- Edgerton VR, Gardner GW, Ohira Y, Gunawardena KA, Senewiratne B. Iron‐deficiency anaemia and its effect on worker productivity and activity patterns. British Medical Journal 1979;2(6204):1546‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eftekhari 2006 {published data only}
- Eftekhari MH, Simondon KB, Jalali M, Keshavarz SA, Elguero E, Eshraghian MR, et al. Effects of administration of iron, iodine and simultaneous iron‐plus‐iodine on the thyroid hormone profile in iron‐deficient adolescent Iranian girls. European Journal of Clinical Nutrition 2006;60(4):545‐52. [DOI] [PubMed] [Google Scholar]
Elwood 1966 {published data only}
- Elwood PC, Wood MM. Effect of oral iron therapy on the symptoms of anaemia. British Journal of Preventive & Social Medicine 1966;20(4):172‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwood 1970 {published data only}
- Elwood PC, Hughes D. Clinical trial of iron therapy of psychomotor function in anaemic women. British Medical Journal 1970;3(5717):254‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flink 2006 {published data only}
- Flink H, Tegelberg A, Thörn M, Lagerlöf F. Effect of oral iron supplementation on unstimulated salivary flow rate: a randomized, double‐blind, placebo‐controlled trial. Journal of Oral Pathology & Medicine 2006;35(9):540‐7. [PUBMED: 16968234] [DOI] [PubMed] [Google Scholar]
Florencio 1981 {published data only}
- Florencio CA. Effects of iron and ascorbic acid supplementation on hemoglobin level and work efficiency of anemic women. Journal of Occupational and Environmental Medicine 1981;23(10):699‐704. [DOI] [PubMed] [Google Scholar]
Fogelholm 1992 {published data only}
- Fogelholm M, Jaakkola L, Lampisjärvi T. Effects of iron supplementation in female athletes with low serum ferritin concentration. International Journal of Sports Medicine 1992;13(2):158‐62. [PUBMED: 1555906 ] [DOI] [PubMed] [Google Scholar]
Fogelholm 1994 {published data only}
- Fogelholm M, Suominen M, Rita H. Effects of low‐dose iron supplementation in women with low serum ferritin concentration. European Journal of Clinical Nutrition 1994;48(10):753‐6. [PUBMED: 7835330] [PubMed] [Google Scholar]
Gordeuk 1987 {published data only}
- Gordeuk VR, Brittenham GM, Hughes MA, Keating LJ. Carbonyl iron for short‐term supplementation in female blood donors. Transfusion 1987;27(1):80‐5. [DOI] [PubMed] [Google Scholar]
Gordeuk 1990 {published data only}
- Gordeuk VR, Brittenham GM, Bravo J, Hughes MA, Keating LJ. Prevention of iron deficiency with carbonyl iron in female blood donors. Transfusion 1990;30(3):239‐45. [PUBMED: 2180144] [DOI] [PubMed] [Google Scholar]
Gunaratna 2015 {published data only}
- Gunaratna NS, Masanja H, Mrema S, Levira F, Spiegelman D, Hertzmark E, et al. Multivitamin and iron supplementation to prevent periconceptional anemia in rural Tanzanian women: a randomized, controlled trial. PloS one 2015;10(4):e0121552. [DOI: 10.1371/journal.pone.0121552] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heath 2001 {published data only}
- Heath AL, Skeaff CM, O'Brien SM, Williams SM, Gibson RS. Can dietary treatment of non‐anemic iron deficiency improve iron status?. Journal of the American College of Nutrition 2001;20(5):477‐84. [PUBMED: 11601562] [DOI] [PubMed] [Google Scholar]
Hinton 2000 {published data only}
- Brownlie T, Utermohlen V, Hinton PS, Giordano C, Haas JD. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. American Journal of Clinical Nutrition 2002;75(4):734‐42. [PUBMED: 11916761] [DOI] [PubMed] [Google Scholar]
- Brownlie T, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. American Journal of Clinical Nutrition 2004;79(3):437‐43. [PUBMED: 14985219] [DOI] [PubMed] [Google Scholar]
- Hinton PS, Giordano C, Brownlie T, Haas JD. Iron supplementation improves endurance after training in iron‐depleted, nonanemic women. Journal of Applied Physiology 2000;88(3):1103‐11. [PUBMED: 10710409] [DOI] [PubMed] [Google Scholar]
Hinton 2007 {published data only}
- Hinton PS, Sinclair LM. Iron supplementation maintains ventilatory threshold and improves energetic efficiency in iron‐deficient nonanemic athletes. European Journal of Clinical Nutrition 2007;61:30‐9. [DOI: 10.1038/sj.ejcn.1602479] [DOI] [PubMed] [Google Scholar]
Hoppe 2013 {published data only}
- Hoppe M, Brün B, Larsson MP, Moraeus L, Hulthén L. Heme iron‐based dietary intervention for improvement of iron status in young women. Nutrition 2013;29(1):89‐95. [PUBMED: 22951158] [DOI] [PubMed] [Google Scholar]
Jayatissa 1999 {published data only}
- Jayatissa R, Piyasena C. Adolescent schoolgirls: daily or weekly iron supplementation?. Food and Nutrition Bulletin 1999;20(4):429‐34. [Google Scholar]
Jensen 1991 {published data only}
- Jensen CA, Weaver CM, Sedlock DA. Iron supplementation and iron status in exercising young women. Journal of Nutritional Biochemistry 1991;2(7):368‐73. [DOI: 10.1016/0955-2863(91)90093-K] [DOI] [Google Scholar]
Kanani 2000 {published data only}
- Kanani SJ, Poojara RH. Supplementation with iron and folic acid enhances growth in adolescent Indian girls. Journal of Nutrition 2000;130(2S Suppl):452S‐5S. [DOI] [PubMed] [Google Scholar]
Kang 2004 {published data only}
- Kang HS, Matsuo T. Effects of 4 weeks iron supplementation on haematological and immunological status in elite female soccer players. Asia Pacific Journal of Clinical Nutrition 2004;13(4):353‐8. [PUBMED: 15563440] [PubMed] [Google Scholar]
Kianfar 2000 {published data only}
- Kianfar H, Kimiagar M, Ghaffarpour M. Effect of daily and intermittent iron supplementation on iron status of high school girls. International Journal for Vitamin and Nutrition Research 2000;70(4):172‐7. [PUBMED: 10989766] [DOI] [PubMed] [Google Scholar]
Kiss 2015 {published data only}
- Cable G, Brambilla D, Glynn S, Mast AE, Kiss JE. Quantification of iron stores, body iron, and iron absorption in blood donors: effect of iron supplementation. Transfusion. Abstract Presentations from the AABB Annual Meeting; 2014 October 25‐28; Philadelphia, (PA). 2014; Vol. 54 Suppl S2:37A.
- Kiss JE, Brambilla D, Glynn SA, Mast AE, Spencer BR, Stone M, et al. Oral iron supplementation after blood donation: a randomized clinical trial. JAMA 2015;313(6):575‐83. [DOI: 10.1001/jama.2015.119] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klingshirn 1992 {published data only}
- Klingshirn LA, Pate RR, Bourque SP, Davis JM, Sargent RG. Effect of iron supplementation on endurance capacity in iron‐depleted female runners. Medicine and Science in Sports and Exercise 1992;24(7):819‐24. [PubMed] [Google Scholar]
LaManca 1993 {published data only}
- LaManca JJ. Effects of Iron Supplementation on Aerobic Power, Endurance Performance, Blood Lactate, and Body Iron Stores in Women [Post doctoral thesis]. Florida: Florida State University, 1989. [Google Scholar]
- LaManca JJ, Haymes EM. Effects of iron repletion on VO2max, endurance, and blood lactate in women. Medicine and Science in Sports and Exercise 1993;25(12):1386‐92. [PUBMED: 8107547] [PubMed] [Google Scholar]
Lanerolle 2000 {published data only}
- Lanerolle P, Atukorala S, Silva G, Samarasinghe S, Dharmawardena L. Evaluation of nutrition education for improving iron status in combination with daily iron supplementation. Food and Nutrition Bulletin 2000;21(3):259‐69. [DOI: 10.1177/156482650002100302] [DOI] [Google Scholar]
Larocque 2006 {published data only}
- Larocque T. Iron Status and Cognitive Performance in Adolescent Females [thesis]. Lakehead University, 2006. [Google Scholar]
Leonard 2014 {published data only}
- Leonard AJ. Iron Deficiency in Young Australian Women: Role of Iron Knowledge, Dietary Intake and Supplementation, and the Effects on Cognition [Thesis]. Newcastle Australia: University of Newcastle, 2014. [Google Scholar]
- Leonard AJ, Chalmers KA, Collins CE, Patterson AJ. A study of the effects of latent iron deficiency on measures of cognition: a pilot randomised controlled trial of iron supplementation in young women. Nutrients 2014;6(6):2419‐35. [PUBMED: 24959952] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AJ, Chalmers KA, Collins CE, Patterson AJ. Comparison of two doses of elemental iron in the treatment of latent iron deficiency: efficacy, side effects and blinding capabilities. Nutrients 2014;6(4):1394‐405. [DOI: 10.3390/nu6041394] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 1994 {published data only}
- Li R, Chen X, Yan H, Deurenberg P, Garby L, Hautvast JG. Functional consequences of iron supplementation in iron‐deficient female cotton mill workers in Beijing, China. American Journal of Clinical Nutrition 1994;59(4):908‐13. [DOI] [PubMed] [Google Scholar]
Lyle 1992 {published data only}
- Lyle RM, Weaver CM, Sedlock DA, Rajaram S, Martin B, Melby CL. Iron status in exercising women: the effect of oral iron therapy vs increased consumption of muscle foods. American Journal of Clinical Nutrition 1992;56(6):1049‐55. [DOI] [PubMed] [Google Scholar]
Machado 2011 {published data only}
- Machado KMM, Ferreira LOC, Souza AI, Diniz AS. The side‐effects of different doses of iron sulfate on women of reproductive age: a randomized double‐blind, placebo controlled study [Efeitos colaterais do sulfato ferroso administrado em diferentes posologias em mulheres em idade reprodutiva: estudo randomizado, duplo cego e controlado por placebo]. Revista Brasileira de Saúde Materno Infantil 2011;11(3):275‐81. [DOI: 10.1590/S1519-38292011000300008] [DOI] [Google Scholar]
Magazanik 1991 {published data only}
- Magazanik A, Weinstein Y, Abarbanel J, Lewinski U, Shapiro Y, Inbar O, et al. Effect of an iron supplement on body iron status and aerobic capacity of young training women. European Journal of Applied Physiology and Occupational Physiology 1991;62(5):317‐23. [DOI] [PubMed] [Google Scholar]
Maghsudlu 2008 {published data only}
- Maghsudlu M, Nasizadeh S, Toogeh GR, Zandieh T, Parandoush S, Rezayani M. Short‐term ferrous sulfate supplementation in female blood donors. Transfusion 2008;48(6):1192‐7. [DOI: 10.1111/j.1537-2995.2007.01671.x] [DOI] [PubMed] [Google Scholar]
Marks 2014 {published and unpublished data}
- Marks DC, Speedy J, Robinson KL, Brama T, Capper HR, Mondy P, et al. An 8‐week course of 45 mg of carbonyl iron daily reduces iron deficiency in female whole blood donors aged 18 to 45 years: results of a prospective randomized controlled trial. Transfusion 2014;54(3 Pt 2):780‐8. [DOI: 10.1111/trf.12464] [DOI] [PubMed] [Google Scholar]
McClung 2009 {published data only}
- McClung JP, Karl JP, Cable SJ, Williams KW, Nindl BC, Young AJ, et al. Randomized, double‐blind, placebo‐controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. American Journal of Clinical Nutrition 2009;90(1):124‐31. [PUBMED: 19474138] [DOI] [PubMed] [Google Scholar]
Mujica‐Coopman 2015 {published data only}
- Mujica‐Coopman MF, Borja A, Pizarro F, Olivares M. Effect of daily supplementation with iron and zinc on iron status of childbearing age women. Biological Trace Element Research 2015;165(1):10‐7. [DOI] [PubMed] [Google Scholar]
Murray‐Kolb 2007 {published data only (unpublished sought but not used)}
- Murray‐Kolb LE. Iron Status, Cognitive Performance, and Behavioral Affect in Young Women [Doctoral thesis]. Pennsylvania State University, 2003. [Google Scholar]
- Murray‐Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. American Journal of Clinical Nutrition 2007;85(3):778‐87. [PUBMED: 17344500] [DOI] [PubMed] [Google Scholar]
Newhouse 1989 {published data only}
- Newhouse IJ, Clement DB, Taunton JE, McKenzie DC. The effects of prelatent/latent iron deficiency on physical work capacity. Medicine and Science in Sports and Exercise 1989;21(3):263‐8. [DOI: 10.1249/00005768-198906000-00006] [DOI] [PubMed] [Google Scholar]
Pereira 2014 {published and unpublished data}
- Pereira D, Couto Irving SS, Lomer MC, Powell JJ. A rapid, simple questionnaire to assess gastrointestinal symptoms after oral ferrous sulphate supplementation. BMC Gastroenterology 2014;14:103. [DOI: 10.1186/1471-230X-14-103] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, D. Question re your paper in BMC Gastroenterology 2014 [personal communication]. Email to: SR Pasricha 3 November 2014.
Prosser 2010 {published data only}
- Prosser NR, Heath AL, Williams SM, Gibson RS. Influence of an iron intervention on the zinc status of young adult New Zealand women with mild iron deficiency. British Journal of Nutrition 2010;104(5):742‐50. [DOI: 10.1017/S0007114510001091] [DOI] [PubMed] [Google Scholar]
Radjen 2011 {published data only}
- Radjen S, Radjen G, Životić‐Vanović M, Radaković S, Vasiljević N, Stojanovic D. Effect of iron supplementation on maximal oxygen uptake in female athletes [Uticaj suplementacije gvožđa na maksimalnu potrošnju kiseonika kod sportistkinja]. Military‐Medical and Pharmaceutical Review 2011;68(2):130‐5. [DOI: 10.2298/VSP1102130R] [DOI] [PubMed] [Google Scholar]
Rajaram 1995 {published data only}
- Rajaram S, Weaver CM, Lyle RM, Sedlock DA, Martin B, Templin TJ, et al. Effects of long‐term moderate exercise on iron status in young women. Medicine and Science in Sports and Exercise 1995;27(8):1105‐10. [PubMed] [Google Scholar]
Rowland 1988 {published data only}
- Rowland TW, Deisroth MB, Green GM, Kelleher JF. The effect of iron therapy on the exercise capacity of nonanemic iron‐deficient adolescent runners. American Journal of Diseases of Children 1988;142(24):165‐9. [DOI: 10.1001/archpedi.1988.02150020067030] [DOI] [PubMed] [Google Scholar]
Rybo 1985 {published data only}
- Rybo E, Bengtsson C, Hallberg L, Oden A. Effect of iron supplementation to women with iron deficiency. Scandinavian Journal of Haematology 1985;34(S43):103‐14. [DOI: 10.1111/j.1600-0609.1985.tb00790.x] [DOI] [PubMed] [Google Scholar]
Røsvik 2010 {published data only}
Shah 2002 {published data only}
Swain 2007 {published data only}
- Swain JH, Johnson LK, Hunt JR. Electrolytic iron or ferrous sulfate increase body iron in women with moderate to low iron stores. Journal of Nutrition 2007;137(3):620‐7. [PUBMED: 17311950] [DOI] [PubMed] [Google Scholar]
Taniguchi 1991 {published data only}
- Taniguchi M, Imamura H, Shirota T, Okamatsu H, Fujii Y, Toba M, et al. Improvement in iron deficiency anemia through therapy with ferric ammonium citrate and vitamin C and the effects of aerobic exercise. Journal of Nutritional Science and Vitaminology 1991; Vol. 37, issue 2:161‐71. [PUBMED: 1919803] [DOI] [PubMed]
Verdon 2003 {published data only}
- Verdon F, Burnand B, Stubi CL, Bonard C, Graff M, Michaud A, et al. Iron supplementation for unexplained fatigue in non‐anaemic women: double blind randomised placebo controlled trial. BMJ 2003; Vol. 326, issue 7399:1124. [DOI] [PMC free article] [PubMed]
Viteri 1999 {published data only}
- Viteri FE, Ali F, Tujague J. Long‐term weekly iron supplementation improves and sustains nonpregnant women's iron status as well or better than currently recommended short‐term daily supplementation. Journal of Nutrition 1999;129(11):2013‐20. [DOI] [PubMed] [Google Scholar]
Waldvogel 2012 {published data only}
- Waldvogel S, Pedrazzini B, Vaucher P, Bize R, Cornuz J, Tissot J‐D, et al. Clinical evaluation of iron treatment efficiency among non‐anemic but iron‐deficient female blood donors: a randomized controlled trial. BMC Medicine 2012;10(8):8. [DOI: 10.1186/1741-7015-10-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 1989 {published data only (unpublished sought but not used)}
- Walsh R, McNaughton L. Effects of iron supplementation on iron status of young female swimmers during pre‐season phase of competition. Journal of Swimming Research 1989;5(2):13‐8. [Google Scholar]
Wang 2012 {published data only}
- Wang Z, Sun J, Wang L, Zong M, Chen Y, Lin Y, et al. Effect of iron supplementation on iron deficiency anemia of childbearing age women in Shanghai. Journal of Hygiene Research 2012;41(1):51‐5. [PUBMED: 22443058] [PubMed] [Google Scholar]
Yadrick 1989 {published data only}
- Yadrick MK, Kenney MA, Winterfeldt EA. Iron, copper, and zinc status: response to supplementation with zinc or zinc and iron in adult females. American Journal of Clinical Nutrition 1989;49(1):145‐50. [PUBMED: 2912000] [DOI] [PubMed] [Google Scholar]
Yoshida 1990 {published data only}
- Yoshida T, Udo M, Chida M, Ichioka M, Makiguchi K. Dietary iron supplement during severe physical training in competitive female distance runners. Sports Training, Medicine and Rehabilitation 1990;1(4):279‐85. [DOI: 10.1080/15438629009511885] [DOI] [Google Scholar]
Zaman 2013 {published data only}
- McArthur JO, Petocz P, Caterson ID, Samman S. A randomized controlled trial in young women of the effects of consuming pork meat or iron supplements on nutritional status and feeling of well‐being. Journal of the American College of Nutrition 2012;31(3):175‐84. [DOI: 10.1080/07315724.2012.10720025] [DOI] [PubMed] [Google Scholar]
- Zaman K, McArthur JO, Abboud MN, Ahmad ZI, Garg ML, Petocz P, et al. Iron supplementation decreases plasma zinc but has no effect on plasma fatty acids in non‐anemic women. Nutrition Research 2013;33(4):272‐8. [DOI: 10.1016/j.nutres.2013.02.001] [DOI] [PubMed] [Google Scholar]
Zavaleta 2000 {published data only}
- Zavaleta N, Respicio G, Garcia T. Efficacy and acceptability of two iron supplementation schedules in adolescent school girls in Lima, Peru. Journal of Nutrition 2000;130(2):462S‐4S. [DOI] [PubMed] [Google Scholar]
Zhu 1998 {published data only}
- Zhu YI, Haas JD. Altered metabolic response of iron‐depleted nonanemic women during a 15‐km time trial. Journal of Applied Physiology 1998 May;84(5):1768‐75. [DOI] [PubMed] [Google Scholar]
- Zhu YI, Haas JD. Response of serum transferrin receptor to iron supplementation in iron‐depleted, nonanemic women. American Journal of Clinical Nutrition 1988;67(2):271‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brigham 1993 {published data only}
- Brigham DE, Beard JL, Krimmel RS, Kenney WL. Changes in iron status during competitive season in female collegiate swimmers. Nutrition 1993;9(5):418‐22. [PUBMED: 8286880] [PubMed] [Google Scholar]
Cable 1988 {published data only}
- Cable RG, Morse EE, Keltonic J, Kakaiya R, Kiraly T. Iron supplementation in female blood donors deferred by copper sulfate screening. Transfusion 1988;28(5):422‐6. [DOI: 10.1046/j.1537-2995.1988.28588337328.x] [DOI] [PubMed] [Google Scholar]
Powell 1991 {published data only}
- Powell PD, Tucker A. Iron supplementation and running performance in female cross‐country runners. International Journal of Sports Medicine 1991;12(5):462‐7. [DOI] [PubMed] [Google Scholar]
Powers 1988 {published data only}
- Powers HJ, Bates CJ, Downes R, Brubacher D, Sutcliffe V, Thurnhill A. Running performance in Gambian children: effects of water‐soluble vitamins on iron. European Journal of Clinical Nutrition 1988;42(11):895‐902. [PubMed] [Google Scholar]
Schoene 1983 {published data only}
- Schoene RB, Escourrou P, Robertson HT, Nilson KL, Parsons JR, Smith NJ. Iron repletion decreases maximal exercise lactate concentrations in female athletes with minimal iron‐deficiency anemia. Journal of Laboratory and Clinical Medicine 1983;102(2):306‐12. [PUBMED: 6864076] [PubMed] [Google Scholar]
Simon 1984 {published data only}
- Simon TL, Hunt WC, Garry PJ. Iron supplementation for menstruating female blood donors. Transfusion 1984;24(6):469‐72. [PUBMED: 6506175] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Blot 1980 {published data only}
- Blot I, Chenayer M, Diakhate L, Leluc R, Gross E, Tchernia G. Iron reserves in blood donors. Should we prescribe systematic iron supplementation?. Revue Francaise de Transfusion et Immuno‐hematologie 1980;23(2):119‐29. [DOI] [PubMed] [Google Scholar]
Böttiger 1971 {published data only}
- Böttiger LE, Nyberg A, Astrand I, Astrand PO. Iron administration to healthy, physically very active students. Nordisk Medicin 1971;85(13):396‐8. [PUBMED: 4928926] [PubMed] [Google Scholar]
Charoenlarp 1981 {published data only}
- Charoenlarp P. Iron supplementation studies. Experimental design and interpretation of results. Progress in Clinical and Biological Research 1981;77:355‐61. [PubMed] [Google Scholar]
Greene 1995 {published data only}
- Greene LM. Effect of Iron Supplementation on Measures of Cognitive Functioning During Adolescence. [Thesis]. University of Ulster, 1995. [Google Scholar]
Isager 1974 {published data only}
- Isager H. Iron deficiency, growth, and stimulated erythropoiesis. Scandinavian Journal of Haematology. Supplementum 1974;21:1‐176. [PubMed] [Google Scholar]
Izak 1973 {published data only}
- Izak G, Levy S, Rachmilewitz M, Grossowicz N. The effect of iron and folic acid therapy on combined iron and folate deficiency anaemia: the results of a clinical trial. Scandinavian Journal of Haematology 1973;11(3):236‐40. [DOI] [PubMed] [Google Scholar]
Parkinson 1981 {published data only}
- Parkinson CF, Parkinson SB. Effect of vitamin‐iron preparation on tooth staining studied. Journal of the Missouri Dental Association 1981;61(4):34‐7. [PubMed] [Google Scholar]
References to ongoing studies
IRCT201409082365N9 {published data only}
- IRCT201409082365N9. The effects of vitamin D or iron‐vitamin supplementation on bone metabolism and inflammation in 18‐40‐year women. http://apps.who.int/trialsearch/Trial2.aspx?trialid=IRCT201409082365N9 (accessed 15 November 2015).
Additional references
Andrews 1999
- Andrews NC. Disorders of iron metabolism. New England Journal of Medicine 1999;341(26):1986‐95. [DOI: 10.1056/NEJM199912233412607] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI: 10.1016/j.jclinepi.2010.07.015] [DOI] [PubMed] [Google Scholar]
Borenstein 2008
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis (Statistics in Practice). Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Brainclinics 2015 [Computer program]
- Brainclinics Products. Neuropsychological tests: IntegNeuro. Nijmegen, The Netherlands: Brainclinics Product, 2015.
Brownlie 2002
- Brownlie T, Utermohlen V, Hinton PS, Giordano C, Haas JD. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. American Journal of Clinical Nutrition 2002;75(4):734‐42. [DOI] [PubMed] [Google Scholar]
Brownlie 2004
- Brownlie T 4th, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. American Journal of Clinical Nutrition 2004;79(3):437‐43. [DOI] [PubMed] [Google Scholar]
Burhans 2005
- Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray‐Kolb LJ, Byron C, et al. Iron deficiency: differential effects on monoamine transporters. Nutritional Neuroscience 2005;8(1):31‐8. [DOI] [PubMed] [Google Scholar]
Chambers 2009
- Chambers JC, Zhang W, Li Y, Sehmi J, Wass MN, Zabaneh D, et al. Genome‐wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nature Genetics 2009;41(11):1170‐2. [DOI: 10.1038/ng.462] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dallman 1992
- Dallman PR. Changing iron needs from birth through adolescence. In: Fomon SJ, Zlotkin S editor(s). Nutritional Anemias. Vol. 30, New York: Nestle Nutrition Workshop Series, Nestec Ltd. Vevey/Raven Press Ltd, 1992:34. [Google Scholar]
De‐Regil 2011
- De‐Regil LM, Jefferds MED, Sylvetsky AC, Dowswell T. Intermittent iron supplementation for improving nutrition and development in children under 12 years of age. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009085.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks J, Higgins JPT, Altman D. Chapter 9: Analysing data and undertaking meta‐analyses. In Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dodd 2004
- Dodd J, Dare MR, Middleton P. Treatment for women with postpartum iron deficiency anaemia. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004222] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endnote 2015 [Computer program]
- McCracken MO. Endnote X7. Thomson Reuters, 2015.
Fernández‐Gaxiola 2011
- Fernández‐Gaxiola AC, De‐Regil LM. Intermittent iron supplementation for reducing anaemia and its associated impairments in menstruating women. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009218.pub2] [DOI] [PubMed] [Google Scholar]
Gandek 1998
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross‐validation of item selection and scoring for the SF‐12 Health Survey in nine countries: results from the IQOLA Project. Journal of Clinical Epidemiology 1998;51(11):1171‐8. [DOI: 10.1016/S0895-4356(98)00109-7] [DOI] [PubMed] [Google Scholar]
Goddard 2011
- Goddard AF, James MW, McIntyre AS, Scott BB, on behalf of the British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut 2011;60(10):1309‐16. [DOI: 10.1136/gut.2010.228874] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University. GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University, 2015 (developed by Evidence Prime, Inc.) Available from www.gradepro.org.
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149(9):876‐83. [PUBMED: 10221325] [DOI] [PubMed] [Google Scholar]
Haider 2006
- Haider BA, Bhutta ZA. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004905.pub2] [DOI] [PubMed] [Google Scholar]
Harbord 2009
- Harbord RM, Harris RJ, Sterne JAC. Updated tests for small–study effects in meta–analyses. The Stata Journal 2009;9(2):197‐210. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hotez 2005
- Hotez PJ, Bethony J, Bottazzi ME, Brooker S, Buss P. Hookworm: "the great infection of mankind". PLoS Medicine 2005;2(3):e67. [DOI: 10.1371/journal.pmed.0020067] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurrell 2010
- Hurrell R, Egli I. Iron bioavailability and dietary reference values. American Journal of Clinical Nutrition 2010;91(5):1461S‐7S. [DOI: 10.3945/%E2%80%8Bajcn.2010.28674F] [DOI] [PubMed] [Google Scholar]
IOC 2009
- International Olympic Committee. The International Olympic Committee (IOC) consensus statement on periodic health evaluation of elite athletes: March 2009. http://bit.ly/1Wn3aJX (accessed 25 January 2015).
Krupp 1989
- Krupp LB, LaRocca NG, Muir‐Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology 1989;46(10):1121‐3. [PUBMED: 2803071] [DOI] [PubMed] [Google Scholar]
Low 2013
- Low M, Farrell A, Biggs BA, Pasricha SR. Effects of daily iron supplementation in primary‐school‐aged children: systematic review and meta‐analysis of randomized controlled trials. Canadian Medical Association Journal 2013;185(17):E791‐802. [PUBMED: 24130243] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lozoff 2007
- Lozoff B. Iron deficiency and child development. Food and Nutrition Bulletin 2007;28(4 Suppl):S560‐71. [DOI] [PubMed] [Google Scholar]
Mahoney 2011
- Mahoney DH. Iron deficiency in infants and young children: treatment. www.uptodate.com/contents/iron‐deficiency‐in‐infants‐and‐young‐children‐treatmenthttp (accessed 31 August 2011).
McNair 1971
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service, 1971. [Google Scholar]
Okebe 2011
- Okebe JU, Yahav D, Paul M. Oral iron supplements for children in malaria‐endemic areas. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD006589.pub3] [DOI] [PubMed] [Google Scholar]
Oppenheimer 2001
- Oppenheimer SJ. Iron and its relation to immunity and infectious disease. Journal of Nutrition 2001;131(2):S616‐35S. [DOI] [PubMed] [Google Scholar]
Pasricha 2010
- Pasricha SR, Flecknoe‐Brown SC, Allen KJ, Gibson PR, McMahon LP, Olynyk JK, et al. Diagnosis and management of iron deficiency anaemia: a clinical update. Medical Journal of Australia 2010;193(9):525‐32. [DOI] [PubMed] [Google Scholar]
Pasricha 2013
- Pasricha SR, Hayes E, Kalumba K, Biggs BA. Effect of daily iron supplementation on health in children aged 4‐23 months: a systematic review and meta‐analysis of randomised controlled trials. Lancet Global Health 2013;1(2):e77‐86. [PUBMED: 25104162 ] [DOI] [PubMed] [Google Scholar]
Pasrischa 2012
- Pasricha S‐R, De‐Regil LM, Garcia‐Casal MN, Burford BJ, Gwirtz JA, Peña‐Rosas JP. Fortification of maize flour with iron for preventing anaemia and iron deficiency in populations. Cochrane Database of Systematic Reviews 14 November 2012;11:Art. No.: CD010187. DOI: 10.1002/14651858.CD010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peeling 2008
- Peeling P, Dawson B, Goodman C, Landers G, Trinder D. Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones. European Journal of Applied Physiology 2008;103(4):381‐94. [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2009
- Peña‐Rosas JP, Viteri FE. Effects and safety of preventive oral iron or iron+folic acid supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004736.pub3] [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2014
- Peña‐Rosas J, Field MS, Burford BJ, De‐Regil L. Wheat flour fortification with iron for reducing anaemia and improving iron status in populations. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011302] [DOI] [PMC free article] [PubMed] [Google Scholar]
Radloff 1977
- Radloff LS. The CES‐D Scale. A self‐report depression scale for research in the general population. Applied Psychological Measurement 1977;1(3):385‐401. [DOI: 10.1177/014662167700100306] [DOI] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scholz 1997
- Scholz BD, Gross R, Schultink W, Sastroamidjojo S. Anaemia is associated with reduced productivity of women workers even in less‐physically‐strenuous tasks. British Journal of Nutrition 1997;77(1):47‐57. [DOI: 10.1017/S0007114500002877] [DOI] [PubMed] [Google Scholar]
Self 2012
- Self JL, Serdula M, Dowswell T, De‐Regil LM. Fortification of condiments and seasonings with iron for preventing anaemia and improving health. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009604] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharpe 1950
- Sharpe LM, Peacock WC, Cooke R, Harris RS. The effect of phytate and other food factors on iron absorption. Journal of Nutrition 1950;41(3):433‐46. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, on behalf of the Cochrane Bias Methods Group (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stevens 2013
- Stevens GA, Finucane MM, De‐Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non‐pregnant women for 1995–2011: a systematic analysis of population‐representative data. Lancet Global Health 2013;1(1):e16‐25. [DOI: 10.1016/S2214-109X(13)70001-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoltzfus 2001
- Stoltzfus R. Defining iron‐deficiency anemia in public health terms: a time for reflection. Journal of Nutrition 2001;131(2S‐2):565S‐7S. [DOI] [PubMed] [Google Scholar]
Suominen 1998
- Suominen P, Punnonen K, Rajamäki A, Irjala K. Serum transferrin receptor and transferrin receptor‐ferritin index identify healthy subjects with subclinical iron deficits. Blood 1998;92(8):2934. [PubMed] [Google Scholar]
Thankachan 2008
- Thankachan P, Walczyk T, Muthayya S, Kurpad AV, Hurrell RF. Iron absorption in young Indian women: the interaction of iron status with the influence of tea and ascorbic acid. American Journal of Clinical Nutrition 2008;87(4):881‐6. [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):i‐97. [PUBMED: 10982317] [PubMed] [Google Scholar]
Umbreit 2005
- Umbreit J. Iron deficiency: a concise review. American Journal of Hematology 2005;78(3):225‐31. [DOI: 10.1002/ajh.20249] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PUBMED: 1593914] [PubMed] [Google Scholar]
WHO 2009
- World Health Organization. Weekly iron–folic acid supplementation (WIFS) in women of reproductive age: its role in promoting optimal maternal and child health. www.who.int/nutrition/publications/micronutrients/weekly_iron_folicacid/en/index.html (accessed 31 August 2011). [PubMed]
WHO 2010
- World Health Organization. World Malaria Report 2010. http://whqlibdoc.who.int/publications/2010/9789241564106_eng.pdf (accessed 31 August 2011).
WHO 2011
- World Health Organization. Prevention of Iron Deficiency Anaemia in Adolescents. Role of Weekly Iron and Folic Acid Supplementation. 1st Edition. Vol. 1, New Delhi: SEARO, World Health Organization, 2011. [Google Scholar]
WHO/CDC 2008
- World Health Organization/Centers for Disease Control and Prevention. Worldwide prevalence of anaemia 1993‐2005: WHO global database on anaemia. http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf (accessed 31 August 2011).
WHO/UNICEF/UNU 2001
- World Health Organization/United Nations Children's Fund/United Nations University. Iron Deficiency Anaemia: Assessment, Prevention, and Control. A Guide for Programme Managers. Geneva: World Health Organization, 2001. [Google Scholar]
Wolgemuth 1982
- Wolgemuth JC, Latham MC, Hall A, Chesher A, Crompton DW. Worker productivity and the nutritional status of Kenyan road construction laborers. American Journal of Clinical Nutrition 1982;36(1):68‐78. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Pasricha 2012
- Pasricha SRS, De‐Regil LM. Daily iron supplementation for improving iron status and health among menstruating women. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009747] [DOI] [PMC free article] [PubMed] [Google Scholar]


