Abstract
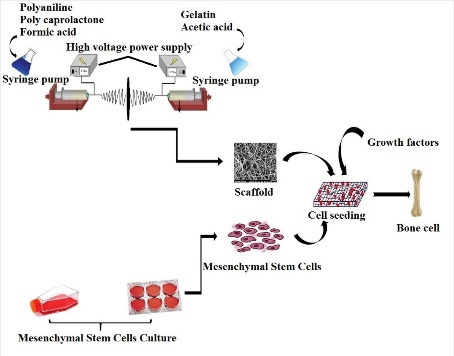
Introduction: Biocompatible and biodegradable scaffolds have gained tremendous attention because of their potential in tissue engineering. In this study, the aim was to reach a feasible setup from a ternary hybrid of polyaniline (PANI), gelatin (GEL), and polycaprolactone (PCL) to fabricate aligned and random nanofibrous scaffolds by electrospinning for tissue engineering purposes.
Methods: Different setups of PANI, PCL, and GEL were electrospun. Then, the best aligned and random scaffolds were chosen. SEM imaging was done to observe nanoscaffolds before and after stem cell differentiation. Mechanical properties of the fibers were tested. Their hydrophilicity was measured using the sessile drop method. SNL Cells were then seeded onto the fiber, and MTT was performed to assess its toxicity. The cells were then differentiated. After osteogenic differentiation, alkaline phosphatase activity, calcium content assay, and alizarin red staining were done to check the validity of osteogenic differentiation.
Results: The two chosen scaffolds had an average diameter of 300 ± 50 (random) and 200 ± 50 (aligned). MTT was performed and its results showed that the scaffolds were non-toxic to cells. After stem cell differentiation, alkaline phosphatase activity was performed, confirming differentiation on both types of scaffolds. Calcium content and alizarin red staining also confirmed stem cell differentiation. Morphological analysis showed no difference regarding differentiation on either type of scaffold. However, unlike on the random fibers, cells followed a specific direction and had a parallel-like growth pattern on aligned fibers.
Conclusion: All in all, PCL-PANI-GEL fibers showed to be capable candidates for cell attachment and growth. Furthermore, they proved to be of excellent use in bone tissue differentiation.
Keywords: Polyaniline, Polycaprolactone, Gelatin, Electrospinning, Osteoblast differentiation, Mesenchymal stem cells
Introduction
What structure a scaffold possesses is one of the concerns of tissue engineers. Ideally, a scaffold should mimic structural and biological functions of extra-cellular matrix (ECM); regarding both chemical components and physical structure.1-3 One highly crucial property of ECM is its nanoscale structure which promotes cell attachment and function.4 A polymeric scaffold should ideally support cell attachment and ingrowth of new tissues, and have a biodegradation rate which matches that of the formation of new tissues.5 To prepare porous scaffolds, numerous methods have been proposed, such as electrospinning, freeze drying, liquid-liquid and liquid-solid phase inversion, thermally induced phase separation, gas foaming, and solvent casting/particulate leaching.6-9 Scientists have found that nanofibrous scaffolds can provide a suitable environment for cell attachment, proliferation, and differentiation and thus, play a vital role in tissue engineering.10-12 Recently, electrospinning, a method for the fabrication of nanofibers, has attracted a lot of attention.13 In this method, a strong electrical field is generated between a polymer solution held in a syringe and a collector. The electrical field converts the polymer solution droplets into Taylor cones. After reaching a specific value, the electrical force overcomes the surface tension of the droplet and a jet of ultra-fine fiber is produced. These fibers are gathered on the collector’s surface. A wide range of polymers have been used in electrospinning for tissue engineering means. Alloying hydrophilic polymers with hydrophilic polymers is one of the new approaches to improve cell adhesion and cell proliferation on scaffolds.14-16 Gelatin (GEL) is a biocompatible, biodegradable, and a natural protein that is derived from collagen which is a main component of ECM. Polycaprolactone (PCL) is a semi-crystalline, biodegradable soluble polymer and as a result, it has stimulated a lot of research on its potential and applications in tissue engineering.17,18 Conductive polymers, due to their direct electrical stimulation capabilities, have received a great deal of attention.19,20 This is solely due to their ability to affect cellular behavior. Based on the literature, conductive polymers such as polyaniline (PANI) enhance the growth and differentiation of neurons, cardiac myoblasts, and skeletal muscles.21-26 In addition to the material parameters in the preparation of tissue engineering scaffolds, the structure of scaffolds also has a direct effect on cellular behavior. Numerous researchers have shown that the arrangement of electrified fibers has a positive effect on cell growth and proliferation.27,28 Yardimci et al29 fabricated random and aligned polyacrylonitrile (PAN)/polypyrrole nanofibrous scaffolds for osteogenic differentiation of mesenchymal stem cells (MSCs). They observed that mineralization occurred in the fiber alignment direction.
In the present study, a variety of aligned and random PCL-PANI-GEL nanofibrous scaffolds were electrospun to determine their potential for bone tissue engineering purposes. A set of characterization methods were used to compare the effects of PANI and GEL on different aspects such as physicochemical, mechanical, morphological, and biocompatibility properties of the scaffolds. To complete the experiment, osteogenic differentiation of MSCs cultured on the fabricated scaffolds was investigated. It was concluded that the electrospun scaffolds were able to provide new insights into bone tissue engineering.
Materials and methods
Materials
Polyaniline (PANI) (100 000 MW, emerladine base), PCL (80 000 MW), GEL type A from porcine skin, penicillin/streptomycin and MTT assay kit were all obtained from Sigma–Aldrich. Fetal bovine serum (FBS), Dulbecco’s modified eagle medium (DMEM) (high glucose), and phosphate-buffered saline (PBS) were all purchased from Gibco, Singapore. Flask (SPL Life Sciences CO, Korea), calcium assay kit (Parsazmun, Tehran, Iran), radio immune precipitation (RIPA), chloroform, paraformaldehyde, acetic acid, hydrochloric acid, formic acid, dimethyl formaldehyde (DMF), acridine orange (AO), Alizarin Red (Sigma), MSCs, and SNL76/7 were obtained from Stem Cells Technology Research Center Cell Bank as a gift (Tehran, Iran).
Fabrication of electrospun nanofibrous PCL-PANI-GEL scaffolds
First, 1.8 g of PCL was solved in 9 mL of formic acid. Then, 0.1 g of PANI was solved in 1 mL of formic acid and after solving, it was added to the PCL solution. At the same time, 0.8 g of GEL was solved in 5 mL of acetic acid. Each sample then filled a syringe and the fibers were bi-electrospun. To reach a feasible electrospinning setup, a variety of collector rotation rates (rpm) were tested. The experiment was carried out to obtain 6 different nanofibrous scaffolds, three of them as aligned and three as random. The collector rotation rates for the random nanofibers were 300, 400, and 500, whereas they were 700, 800, and 900 for the aligned nanofibers, respectively. Then, based on having fewer beads and their diameter, two candidates were chosen, one aligned and one random. As for the two chosen candidates, the distance of the syringe from the collector was 15 cm and the flow rate was set to 0.4 mL/h. Then, the fibers were bi-electrospun. Moreover, the porosity of the scaffolds was calculated using the formula below:
In which V1, V2, and V3 are initial volume ethanol in container, total volume of the ethanol and the scaffold, and residual ethanol volume after removing the scaffold from the container, respectively.
Scanning electron microscopy
Scanning electron microscopy (SEM) imaging was done to observe nanofibrous scaffolds, prior and after cell culture and differentiation. Cell-polymer constructs were fixed in 2.5% glutaraldehyde. They were then dehydrated through a graded series of ethanol, vacuum dried, mounted onto aluminum stubs, and ultimately sputter coated with gold. Samples were examined using SEM imaging (S-4500; Hitachi, Japan).
Mechanical analysis of electrospun mats
To evaluate mechanical properties of the electrospun scaffolds, tensile tests were considered using an Instron tensile testing apparatus (5566-Applied Science Co., Ithaca, NY) with a 5 kg load cell under 0.5 mm/s test speed. For tensile tests, sample dimensions were kept as 30 mm × 5 mm. Three samples of each composition were tested and averages of Young’s modulus of elasticity (E), ultimate tensile strength, and elongation at break (εb) were evaluated from stress–strain curves.
X-ray diffraction analysis
In order to evaluate the crystalline structure of the fabricated nanoscaffolds, x-ray diffraction (XRD) analysis was performed (D8 Advance Bruker, Germany).
Hydrophilicity of electrospun scaffolds
Using 2 μL water droplets and a CCD camera connected to a computer (Dataphysics, OCA 15plus), static contact angle (CA) of mats was measured by sessile drop method. Five measurements on different parts of the samples were averaged.
Cell culture and seeding
Stem Cell Technology Center (Tehran, Iran) supplied fibroblast cells (SNL) and MSCs. They were maintained in T-75 culture flasks. The cells were cultured in DMEM which contained 10% FBS supplemented with penicillin/ streptomycin (10 units/mL). All of the aforementioned materials were obtained from Gibco BRL (NY, USA). Every 48 hours, culture medium was replaced until the cells reached the confluency of 70%. After 3 passages, cells were used for the study.
Prior to seeding of the cells, scaffolds were cut into 0.5 × 0.5 cm2 pieces and then, put in 24-well culture plates (Orange Science, Belgium). They were divided into three groups: aligned PCL-PANI-Gel, random PCL-PANI-Gel, and a control group. They were then sterilized for 45 minutes in 70% ethanol and washed two times with sterile PBS. With a density of 1×104 cells per well, cells were seeded on different substrates using a serum medium which contained DMEM, 10% FBS, and 1% penicillin/streptomycin. Seeded cells were maintained for 24 hours at 37°C in a humidified incubator with a CO2 concentration of 5%.
Cytotoxicity assay
All biological tests were conducted based on ISO standards 10993-5:1999 (Biological evaluation of medical devices; Part 5: tests for in vitro cytotoxicity). To address cell toxicity, MTT assay was used.30,31 SNL cells were plated (104 cells/well) in a 48-well plate which contained PCL-PANI-GEL scaffold. The samples were sterilized with a combination of ethanol (70% w/w) and UV radiation. Then, the scaffolds were washed twice with D-PBS and pre-incubated overnight with culture media. After trypsin/EDTA treatment, the cells were collected and centrifuged at 1200 rpm and cultured into 48-well plate at a density of 10 000 cells/well. The cells were then transferred into an incubator. After 1, 3, and 5 days, proliferation of cells was determined using MTT assay. Afterwards, the culture solution in wells was removed and washed with DMEM. MTT solution was added to the wells and incubated for three hours. One hundred microliters of DMSO (while the oven lamp was turned off) was added to each well and pipetted several times after removing MTT solution and washing wells with DMEM. The contents of each well was transferred to a 96-well plate to measure optical density (OD) by ELISA plate reader (540-630 nm). Additionally, AO staining was performed to evaluate cell viability. A fluorescent staining solution (1 µ) which contained 100 µg/mL of AO was added to the wells. Prior to this process, the cells had been rinsed with PBS. Afterwards, wells were investigated using a fluorescent microscope one by one (Leica Inc., Foster City, CA).
Osteogenic differentiation
To induce osteogenic differentiation of hMSCs, basal medium was replaced with an osteogenic medium. The osteogenic medium contained DMEM supplemented with 10% FBS, 50 mg/mL ascorbic acid 2-phosphate (Sigma Chemical Co), 10 nM dexamethasone (Sigma Chemical Co.) and 10 mM β-glycerophosphate (Sigma Chemical Co.). The next step was to incubate cultures for two weeks in an incubator at 37°C with 5% CO2.
Alkaline phosphatase activity
ALP activity was measured during osteogenic differentiation of hMSCs since it is an osteogenesis marker. On the 7th and 14th days, the plates were washed with ice-cold PBS three times. By using 200 µL RIPA lysis buffer, total protein of cells on Tissue Culture Polystyrene plates (TCPS) and scaffolds was extracted. Then, the mixture was centrifuged at 15 000 rpm for 15 minutes to sediment cell debris. After supernatant removal, ALP activity was measured with an ALP assay kit (Parsazmun, Tehran, Iran). ALP activity level was determined in cell lysates using p-nitrophenyl phosphate as the substrate. Enzyme activity (IU/L) was normalized against total protein (mg). Using a micro-plate reader (BioTek Instruments, USA), fluorescence intensity was determined at 480 nm excitation and 520 nm emission.
Calcium content assay
Calcium assay kit (Parsazmun, Tehran, Iran) was used in order to measure the amount of calcium minerals deposited on both TCPS and the scaffolds by hMSCs under osteogenic induction. To perform calcium extraction, homogenization of the scaffolds in 0.6 mol/L hydrochloric acid followed by shaking for 4 hours at 4°C was done. Addition of the reagent to calcium solutions was done to measure OD at 570 nm. Calcium content was measured using a standard concentration curve of calcium dilutions versus corresponding OD.
Alizarin red staining
Calcium deposition was determined by Alizarin Red staining on day 14 to evaluate the mineralized matrix. At first, the medium was removed and then, the cells were washed with cold PBS three times and fixed in cold 4% paraformaldehyde for 10 minutes at 4°C. Afterwards, the cells were washed with PBS twice. Fixed samples were then stained with 1% Alizarin Red at pH 7.2 (Sigma Aldrich, Germany). After being maintained for 5–10 minutes at room temperature, the cells were washed again with PBS three times and examined by light microscopy.
Statistical analysis
Analysis of variance (two–way ANOVA) was used in order to evaluate the significance between the incubation days for biological parameters, cell proliferation, and cytotoxicity. The significance of statistics was evaluated at P ≤ 0.05.
Results
PCL-PANI-GEL nanofibrous scaffold morphology
Throughout this study, different aligned and random scaffolds were fabricated. Fig. 1 shows SEM images of the fabricated nanofibers by collector rotation rates A: 300 rpm (Fig. 1A), B: 400 rpm (Fig. 1B), C: 500 rpm (Fig. 1C), whereas it was for the aligned nanofibers D: 700 rpm (Fig. 1D), E: 800 rpm (Fig. 1E), and F: 900 rpm (Fig. 1F), respectively. The characteristics of nanoscaffolds are summarized in Table 1. As can be seen, samples C and F have beads in their structure. The formation of beads in the specimens may be due to interfering fibers before reaching the collector.32 Another point that is evident in Table 1 is that the average diameter of the fibers that decreases with increasing collector speed due to the higher stretching level imposed on them.33 The amount of scaffold porosities has not been uniform, but has generally increased with increasing collector speed and decreasing fiber diameter. The presence of porosity in the scaffolds provides the connection of cellular signals for the vital acts of the cells.
Fig. 1.
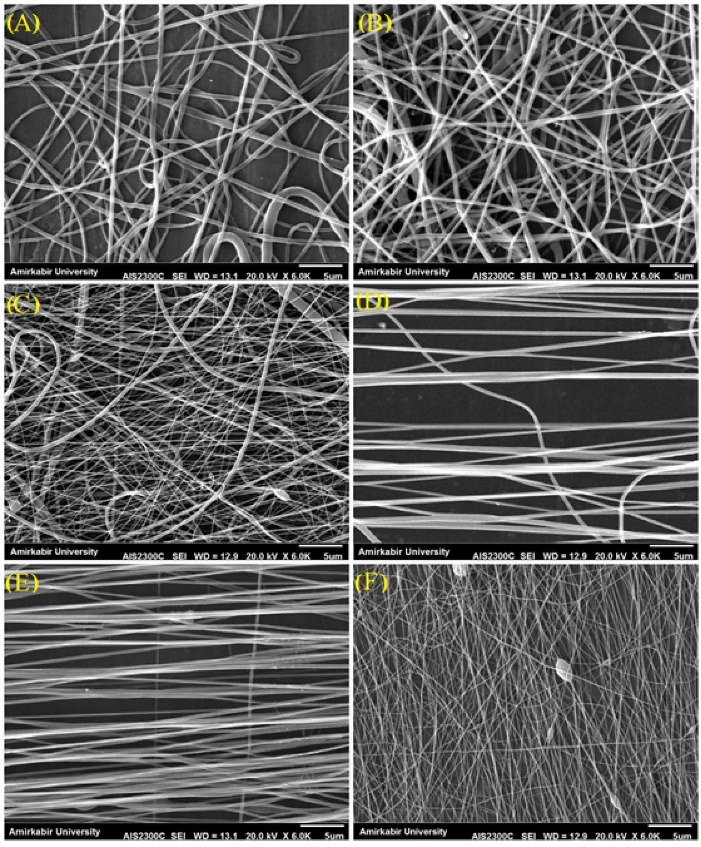
SEM images of PCL-PANI-GEL nanofibrous scaffolds at collector rotation rates of (A) 300 rpm, (B) 400 rpm, (C) 500 rpm, (D) 700 rpm, (E) 800 rpm, and (F) 900 rpm.
Table 1. Fiber diameter, porosity, contact angle, and mechanical measurement of electrospun scaffolds .
| Substrate | Average diameter (nm) | Beads | Orientation | Porosity (%) | Tensile strain (%) | Tensile strength (MPa) | Young's modulus (MPa) | Contact angle (°) |
| Scaffold A | 420 ± 50 | No | Random | 76 | 113 | 1.3 | 2.8 | 0 |
| Scaffold B | 300 ± 50 | No | Random | 74 | 102 | 1.7 | 3.2 | 0 |
| Scaffold C | 200 ± 50 | Yes | Random | 80 | 94 | 1.8 | 3.5 | 0 |
| Scaffold D | 220 ± 50 | No | Aligned | 78 | 86 | 2.6 | 6.9 | 0 |
| Scaffold E | 200 ± 50 | No | Aligned | 81 | 83 | 2.2 | 7.4 | 0 |
| Scaffold F | 80 ± 50 | Yes | Aligned | 83 | 75 | 3.1 | 8.6 | 0 |
Grain-containing specimens (C and F) are not suitable for use as scaffolds due to adverse mechanical and structural properties. Fig. 2 shows the diameter distribution of scaffolds’ fibers. The fiber diameter distribution diagram is for samples A and C with two peaks. Samples B, D, and E are uniform. Uniformity of the fiber diameters has a direct effect on the mechanical properties of the tissue engineering scaffolds.
Fig. 2.
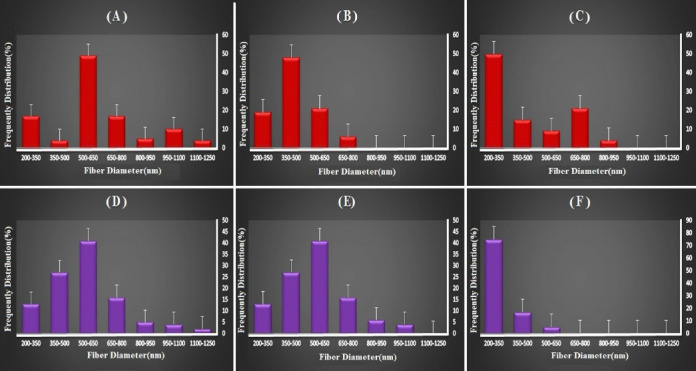
Fiber diameter distribution of electrospun mats prepared at collector rotation rates of (A) 300 rpm, (B) 400 rpm, (C) 500 rpm, (D) 700 rpm, (E) 800 rpm, and (F) 900 rpm.
Tensile strength and Young's modulus of prepared mats increased as collector speed rate increased (Fig. 3). This can be due to induced crystallization resulted by fibers stretch when being formed. There are three ways to increase the intrinsic mechanical properties of a polymer: (I) creating rigid structures in the backbone of polymer; (II) crystallinity; and (III) crosslinking polymer chains.34,35 Therefore, increasing the modulus and strength of the fibers can be due to the arrangement resulting from the increase in the rotational speed of the collector.36 Furthermore, hydrophilicity test showed that scaffolds, random and aligned, were hydrophilic. After analyzing the results and based on having less beads and diameter, we selected one aligned (scaffold E) and one random (scaffold B) scaffold as representatives for other tests of our study.
Fig. 3.
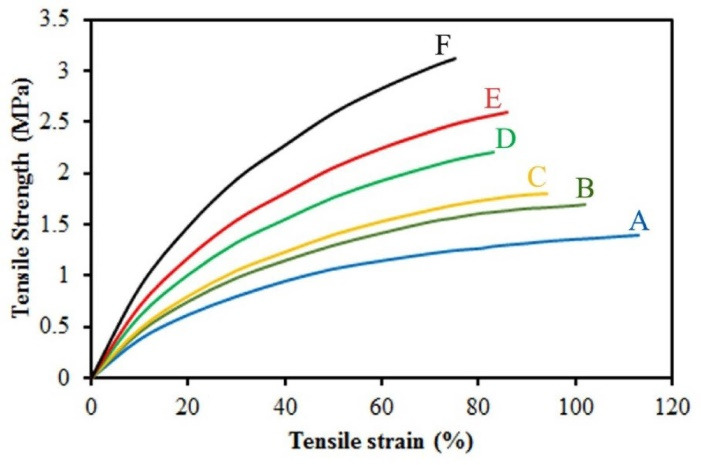
Tensile evaluation of electrospun mats prepared at collector rotation rates (A) 300 rpm, (B) 400 rpm, (C) 500 rpm, (D) 700 rpm, (E) 800 rpm, and (F) 900 rpm.
X-ray diffraction
XRD pattern of PCL-GEL and PCL-PANI-Gel is shown in Fig. 4. As seen, 3 crystalline structures are observed for PCL-GEL sample. Moreover, 110 and 200 structures contribute to the presence of PCL. A high-intensity peak is observed at 32° which is related to GEL crystalline nature. In PCL-PANI-Gel sample, it is observed that peak intensity decreased, and only crystalline peaks related to PCL were noticeable. This indicates that PANi, due to high interaction with GEL, can blend in the structure well and alter its crystalline nature.
Fig. 4.
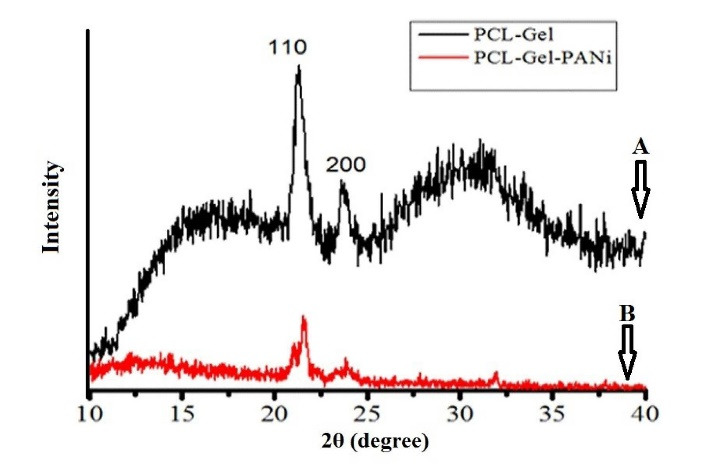
XRD pattern of (A) PCL-GEL and (B) PCL-PANI-Gel.
Cell viability and cytotoxicity of bi-electrospun nanofibers
In order to assess the cytotoxicity of nanofibers, MTT test was performed (Fig. 5). Our MTT assay results showed a significant difference between the control group and nanofibers. It should be noted that on days 1, 3, and 7, the difference between aligned and random nanofibers were not significant. On the contrary, on days 1 and 3, the difference between nanofibers and control group were significant. However, on day 7, our results indicated a rather insignificant difference between the control group, aligned and random nanofibers. The AO cell staining shown in Fig. 5B-D also well confirms the presence of cells in the control and electrospun samples.
Fig. 5.
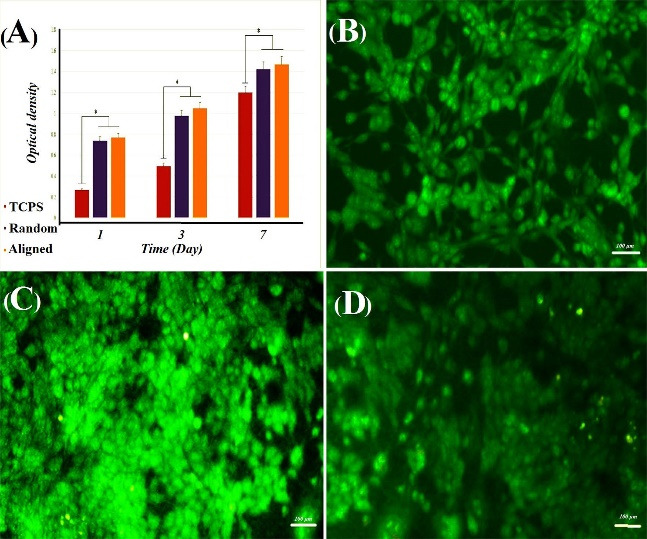
(A) MTT results of SNLs on random and aligned PCL-PANI-GEL nanofiber and TCPS after 1, 3, and 7 days of cell seeding. Results are presented as mean ± SD (P<0.05); cell staining by AO for cell cultured on (B) TCPS as control, (C) random PCL-PANI-GEL nanofiber, and (D) aligned PCL-PANI-GEL nanofiber.
Cell morphology on PCL-PANI-GEL nanofibers
After morphological analysis and selection of the two chosen aligned and random nanofibers as suitable candidates for cell culture and tissue engineering studies, SNL cells were cultured onto the nanofibers. After 7 days of culture, our SEM imaging showed that in both aligned and random nanofibers, SNL cells grew significantly. The observational difference of cell density between aligned and random nanofibers were not noticeable. However, the cells followed a linear formation in aligned nanofibers, whereas on random nanofibers, cell growth did not have a fixed direction and was patternless (Fig. 6).
Fig. 6.
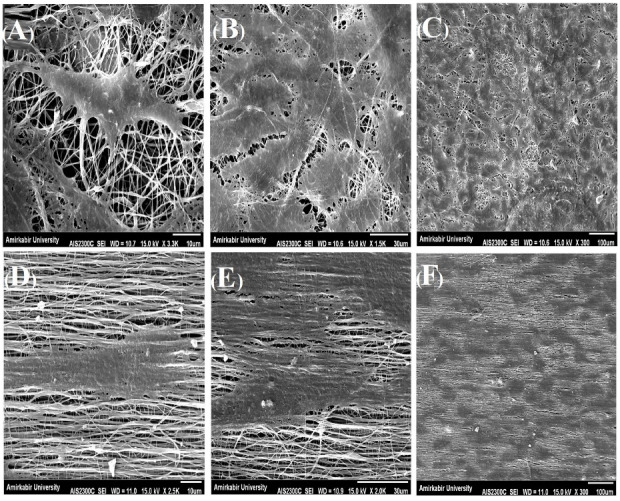
Morphology of SNLs on PCL-PANI-GEL after 7 days. A-C represent the selected random nanoscaffold and D-F represent the selected aligned nanoscaffold.
Osteoblast differentiation analysis
Alkaline phosphatase activity analysis
In order to evaluate PCL-PANI-GEL nanofibrous scaffold’s potential for osteoblastic differentiation of MSCs, Alkaline Phosphatase activity was performed. After one day of culture in standard media, MSCs were seeded on plates that contained osteoblast differentiation media for 14 days. Alkaline Phosphatase activity was measured on days 7 and 14. Fig. 7A represents the results. The result of being in osteoblast differentiation media demonstrated significant enzyme activity of cells on random and aligned PCL-PANI-GEL scaffolds over samples that were differentiated on TCPS (free on nanofibers). However, no significant difference was spotted between stem cell differentiation on random and aligned fibers.
Fig. 7.
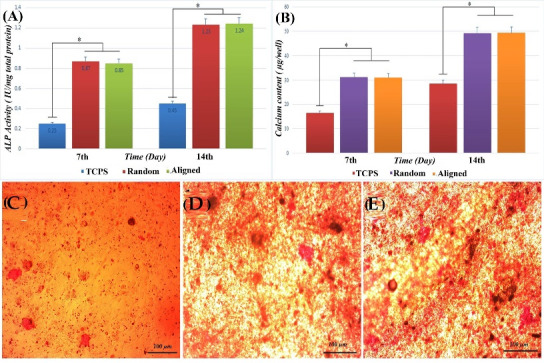
(A)ALP expression of MSCs indicating the osteogenic differentiation after 7 and 14 days of culture (P<0.05); (B) The measured optical density of calcium minerals deposited on TCPS and PCL-PANI-GEL electrospun scaffolds by MSCs under osteogenic induction (at 570 nm). Alizarin Red staining minerals deposited on (C) TCPS, (D) random PCL-PANI-GEL electrospun scaffolds, and (E) aligned PCL-PANI-GEL electrospun scaffolds.
Calcium content evaluation
One of the defining markers of osteoblast differentiation is calcium deposition. Therefore, calcium content is one of the osteoblast differentiation markers. Same as ALP activity, calcium content showed a significant difference between plates that were coated with nanofibers compared to TCPS. Moreover, our results showed no significant difference between aligned and random fibers regarding calcium deposition. Thus, it can be concluded that the alignment of nanofibers might not be of much importance in the amount of calcium deposition (Fig. 7B).
Alizarin Red staining
After 14 days of MSC differentiation on nanofiber coated plates with the presence of osteoblast differentiation factors, calcium deposition was examined by Alizarin Red staining. Darker red-color shows more calcium deposition. All samples, as shown in Fig. 7 C-E, show osteoblast differentiation. The amount of calcium deposition was significant on aligned and random nanofibers, unlike TCPS. This could be due to the fact that hMSCs cultured in induction medium produced higher levels of Alkaline Phosphatase which is a necessary enzyme regarding in vivo bone mineralization, cleaving phosphates from organic phosphates. Induction medium contains organic sources of phosphates, which are substrates for Alkaline Phosphatase and therefore, mineralization is enhanced.
Morphological analysis after differentiation
After 14 days of differentiation, differentiated cells’ morphology was observed by scanning electron microscopy. As seen in the images, cells were attached on nanofibers. There was no significant difference between cell attachment on aligned and random fibers. Interestingly though, the direction and alignment of cells were different between the two types of fibers. On aligned fibers, cells had moved in the same direction as nanofibers, in a rather parallel manner. However, on random fibers, cell formation was random and followed no specific pattern (Fig. 8).
Fig. 8.
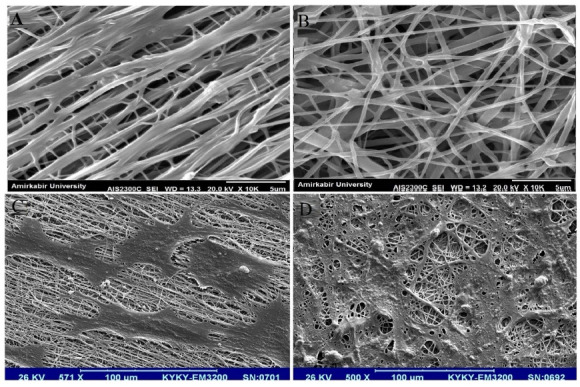
SEM micrograph of (A) aligned electrospun PCL-PANI-GEL, (B) random electrospun PCL-PANI-GEL, (C) attached and differentiated MSCs on aligned electrospun PCL-PANI-GEL, and (D) attached and differentiated MSCs on random electrospun PCL-PANI-GEL
Discussion
In recent years, scientists have been searching to find a feasible polymer to address tissue engineering problems.37 Following this goal, a lot of endeavor has been made in order to find a proper combination of polymers so that they could have low to zero toxicity while being able to provide cells with properties, as close as possible, to ECM.38 In this regard, we tried to make a new blend of polymers with a fabrication setup different from previous similar efforts. PANI is a conductive polymer and has shown to be of help with stem cell differentiation and growth.39 Although its toxicity is concerning when it is used alone, so long as it is combined with biocompatible polymers with the correct proportion, it seems to be rather helpful in tissue engineering. GEL is a biodegradable and biocompatible polymer. A lot of work regarding different aspects of tissue engineering has been done to show the effectiveness of GEL in tissue engineering projects.40 PCL is a biodegradable polymer.41 When used alone, it can have a long degeneration time, but if it is used with assigned portions, it can provide a timed degeneration.
In this study, we tried combining all of the aforementioned polymers and the results were significant. Not only the blend showed close to zero toxicity, but also the diameter and SEM images showed that these fibers were electrospun in the finest way. PCL-PANI-GEL nanofibers possess great properties. As indicated by our results, SNL cells moved in the same direction of aligned nanofibers.
Our results and observations show that cell attachment and growth on PCL-PANI-GEL nanofibrous scaffold are far more efficient and greater than that of TCPS. The reason behind this could be nanofibers’ nanotopography being similar to that of ECM. Cell attachment is one of the important parameters in tissue engineering. Scanning electron microscopy results on day 7 showed that the cells had attached significantly to nanofibers. MTT results on days 1, 3, and 7 showed that the cells had attached significantly to nanofibers. Osteoblasts are known for their response to electrical signals. Thus, a number of different studies have been conducted regarding electrical differentiation of osteoblast mediums on conductive materials. Some results such as significant cell growth, calcium concentration, and collagen I gene expression have been obtained from such studies. Shao et al42 specified a range of electricity currents in which feasible results of osteoblast attachment and growth could be obtained. However, these studies were carried on materials such as nanotube composites. This raises concern regarding the toxicity of nanomaterials. Despite the latter, studies have been done using PANI for tissue engineering purposes. They showed that PANI increases the biocompatibility of electroconductive nanofibers. Other similar studies showed that PANI increases bone formation and mineralization.
We took our experiment one step further and not only produced an innovative PANI containing nanofibrous scaffold, but also studied its capability for osteoblast differentiation of MSCs. There are reports that mention PANI’s role in osteoblast differentiation of stem cells. However, there was no study that had evaluated a nanofirbrous scaffold made of PCL-PANI-GEL for osteoblast differentiation of stem cells prior to our study. Hence, this is the first study that examined PCL-PANI-GEL nanofibers’ potential in osteoblast differentiation of stem cells.
After determining that fabricated nanoscaffolds were non-toxic, hydrophilic and possessed proper attachment potential, they were used for osteoblast differentiation of MSCs. It was observed that both types of scaffolds (aligned and random) were capable candidates for bone tissue engineering purposes.
In addition to feasible physical characteristics of polymers and their biocompatibility, finding a proper mixture of polymers is crucial for tissue engineering studies. To evaluate the aforementioned features, bone tissue engineering tests were performed. It was observed, using Alizarin Red staining, that the differentiated cells had calcium deposition potential. This staining method had been used widely for osteoblast differentiation prior to this study. In this study it was observed that cells, seeded on nanoscaffolds, were well stained and their population was significantly more than the control group. However, no noticeable difference was observed between aligned and random fibers.
Alkaline phosphatase is an enzyme which plays a crucial role in bone formation by detaching phosphate containing substrates. This event usually takes place before mineralization of calcium containing salts. As a result, the activity of this enzyme is an early indicator of osteoblast formation, as reported in previous studies. In this study, it was observed that on days 7 and 14 of differentiation, nanoscaffold-containing samples had increased Alkaline Phosphatase activity compared to the control group.
In addition to alkaline phosphatase, calcium deposition is a major determinant test for osteoblast detection. Our results showed an increased calcium deposition in samples with nanoscaffolds compared to the control group, yet no noticeable difference was observed between aligned and random groups.
Research Highlights
What is the current knowledge?
√ There is a lack of feasible scaffolds for tissue engineering purposes.
√ Polymer blends have not been investigated fully despite showing great potential.
√ Electrospinning might be a promising method to fabricate useful scaffolds for tissue engineering.
What is new here?
√ A blend of polymers that have never been used together has been analyzed.
√ Aligned and random fibers of the same polymer blend were compared with each other regarding tissue engineering.
√ The new polymer blend is suggested to be of use for further investigations.
Conclusion
In conclusion, PCL-PANI-GEL electrospun fibers could be used as an appropriate scaffold for efficient regeneration of bone defects. These synthetic nanofibers show promising applications in bone tissue engineering. Numerous tests such as ALP activity, calcium content, Alizarin Red, mineralization staining, and SEM micrographs showed that the random and aligned electrospun scaffolds are appropriate for MSCs osteogenesis. These scaffolds are possibly of worth in in-vivo analysis, contributing to bone healing in critical-sized bone defects. These results indicate that scaffold structural cues alone can be used to drive cell differentiation and create an osteogenic environment, without the use of exogenous factors.
Acknowledgments
We are grateful to the manager of Stem Cell Technology Research Center.
Funding sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical statement
There is none to be disclosed.
Competing interests
Authors declare no conflict of interests.
References
- 1. Shahrousvand M, Sadeghi GMM, Salimi A. The superficial mechanical and physical properties of matrix microenvironment as stem cell fate regulator. In: Tiwari A, Garipcan B, Uzun L, eds. Advanced Surfaces for Stem Cell Research. Wiley; 2016; 23. 10.1002/9781119242642. [DOI]
- 2.Hollister SJ, Maddox R, Taboas JM. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials. 2002;23:4095–103. doi: 10.1016/S0142-9612(02)00148-5. [DOI] [PubMed] [Google Scholar]
- 3.Shahrousvand M, Sadeghi GMM, Shahrousvand E, Ghollasi M, Salimi A. Superficial physicochemical properties of polyurethane biomaterials as osteogenic regulators in human mesenchymal stem cells fates. Colloids Surf B Biointerfaces. 2017;156:292–304. doi: 10.1016/j.colsurfb.2017.04.059. [DOI] [PubMed] [Google Scholar]
- 4.Shahrousvand M, Tabar FA, Shahrousvand E, Babaei A, Hasani-Sadrabadi MM, Sadeghi GMM, et al. High aspect ratio phospho-calcified rock candy-like cellulose nanowhiskers of wastepaper applicable in osteogenic differentiation of hMSCs. Carbohydr Polym. 2017;175:293–302. doi: 10.1016/j.carbpol.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Li B, Yoshii T, Hafeman AE, Nyman JS, Wenke JC, Guelcher SA. The effects of rhBMP-2 released from biodegradable polyurethane/microsphere composite scaffolds on new bone formation in rat femora. Biomaterials. 2009;30:6768–79. doi: 10.1016/j.biomaterials.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 6.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–43. doi: 10.1016/S0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 7.O'brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14:88–95. doi: 10.1016/S1369-7021(11)70058-X. [DOI] [Google Scholar]
- 8.Ma PX. Scaffolds for tissue fabrication. Mater Today. 2004;7:30–40. doi: 10.1016/S1369-7021(04)00233-0. [DOI] [Google Scholar]
- 9.Shahrousvand E, Shahrousvand M, Ghollasi M, Seyedjafari E, Jouibari IS, Salimi A. Preparation and evaluation of polyurethane/cellulose nanowhisker bimodal foam nanocomposites for osteogenic differentiation of hMSCs. Carbohydr Polym. 2017;171:281–91. doi: 10.1016/j.carbpol.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Amiri B, Ghollasi M, Shahrousvand M, Kamali M, Salimi A. Osteoblast differentiation of mesenchymal stem cells on modified PES-PEG electrospun fibrous composites loaded with Zn 2 SiO 4 bioceramic nanoparticles. Differentiation. 2016;92:148–58. doi: 10.1016/j.diff.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 11.KarbalaeiMahdi A, Shahrousvand M, Javadi HR, Ghollasi M, Norouz F, Kamali M, et al. Neural differentiation of human induced pluripotent stem cells on polycaprolactone/gelatin bi-electrospun nanofibers. Mater Sci Eng C Mater Biol Appl. 2017;78:1195–202. doi: 10.1016/j.msec.2017.04.083. [DOI] [PubMed] [Google Scholar]
- 12. Ghaffari-Bohlouli P, Shahrousvand M, Zahedi P, Shahrousvand M. Performance evaluation of poly (L-lactide-co-D, L-lactide)/poly (acrylic acid) blends and their nanofibers for tissue engineering applications. Int J Biol Macromol 2019. 122: 1008-16. 10.1016/j.ijbiomac.2018.09.046. [DOI] [PubMed]
- 13.Shamsi M, Karimi M, Ghollasi M, Nezafati N, Shahrousvand M, Kamali M, et al. In vitro proliferation and differentiation of human bone marrow mesenchymal stem cells into osteoblasts on nanocomposite scaffolds based on bioactive glass (64SiO 2-31CaO-5P 2 O 5)-poly-l-lactic acid nanofibers fabricated by electrospinning method. Mater Sci Eng C Mater Biol Appl. 2017;78:114–23. doi: 10.1016/j.msec.2017.02.165. [DOI] [PubMed] [Google Scholar]
- 14.Ghaffari-Bohlouli P, Hamidzadeh F, Zahedi P, Shahrousvand M, Fallah-Darrehchi M. Antibacterial nanofibers based on poly (l-lactide-co-d, l-lactide) and poly (vinyl alcohol) used in wound dressings potentially: A comparison between hybrid and blend properties. J Biomater Sci Polym Ed. 2020;31:219–43. doi: 10.1080/09205063.2019.1683265. [DOI] [PubMed] [Google Scholar]
- 15.Pourbashir S, Shahrousvand M, Ghaffari M. Preparation and characterization of semi-IPNs of polycaprolactone/poly (acrylic acid)/cellulosic nanowhisker as artificial articular cartilage. Int J Biol Macromol. 2020;142:298–310. doi: 10.1016/j.ijbiomac.2019.09.101. [DOI] [PubMed] [Google Scholar]
- 16.Hosseini SM, Shahrousvand M, Shojaei S, Khonakdar HA, Asefnejad A, Goodarzi V. Preparation of superabsorbent eco-friendly semi-interpenetrating network based on cross-linked poly acrylic acid/xanthan gum/graphene oxide (PAA/XG/GO): Characterization and dye removal ability. Int J Biol Macromol. 2020;152:884–93. doi: 10.1016/j.ijbiomac.2020.02.082. [DOI] [PubMed] [Google Scholar]
- 17.Shahrousvand M, Mir Mohamad Sadeghi G, Salimi A, Nourany M. Bulk synthesis of monodisperse and highly biocompatible poly (ε-caprolactone)-diol by transesterification side-reactions. Polym Plast Technol Eng. 2018;57:492–9. doi: 10.1080/03602559.2016.1211690. [DOI] [Google Scholar]
- 18.Jafari H, Shahrousvand M, Kaffashi B. Preparation and characterization of reinforced poly (ε‐caprolactone) nanocomposites by cellulose nanowhiskers. Polym Compos. 2020;41:624–32. doi: 10.1002/pc.25393. [DOI] [Google Scholar]
- 19.Abbasi A, Mir Mohamad Sadeghi G, Ghasemi I, Shahrousvand M. Shape memory performance of green in situ polymerized nanocomposites based on polyurethane/graphene nanoplatelets: Synthesis, properties, and cell behavior. Polym Compos. 2018;39:4020–33. doi: 10.1002/pc.24456. [DOI] [Google Scholar]
- 20.Jun I, Jeong S, Shin H. The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. Biomaterials. 2009;30:2038–47. doi: 10.1016/j.biomaterials.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 21.Garrudo FF, Chapman CA, Hoffman PR, Udangawa RW, Silva JC, Mikael PE, et al. Polyaniline-polycaprolactone blended nanofibers for neural cell culture. Eur Polym J. 2019;117:28–37. doi: 10.1016/j.eurpolymj.2019.04.048. [DOI] [Google Scholar]
- 22.Li M, Guo Y, Wei Y, MacDiarmid AG, Lelkes PI. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials. 2006;27:2705–15. doi: 10.1016/j.biomaterials.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 23. Castagna R, Tunesi M, Saglio B, Della Pina C, Sironi A, Albani D, et al. Ultrathin electrospun PANI nanofibers for neuronal tissue engineering. J Appl Polym Sci 2016; 133. 10.1002/app.43885. [DOI]
- 24.Bidez PR, Li S, MacDiarmid AG, Venancio EC, Wei Y, Lelkes PI. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J Biomater Sci Polym Ed. 2006;17:199–212. doi: 10.1163/156856206774879180. [DOI] [PubMed] [Google Scholar]
- 25.Qazi TH, Rai R, Dippold D, Roether JE, Schubert DW, Rosellini E, et al. Development and characterization of novel electrically conductive PANI–PGS composites for cardiac tissue engineering applications. Acta Biomater. 2014;10:2434–45. doi: 10.1016/j.actbio.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Hosseinzadeh S, Mahmoudifard M, Mohamadyar-Toupkanlou F, Dodel M, Hajarizadeh A, Adabi M, et al. The nanofibrous PAN-PANi scaffold as an efficient substrate for skeletal muscle differentiation using satellite cells. Bioprocess Biosyst Eng. 2016;39:1163–72. doi: 10.1007/s00449-016-1592-y. [DOI] [PubMed] [Google Scholar]
- 27.Ma J, He X, Jabbari E. Osteogenic differentiation of marrow stromal cells on random and aligned electrospun poly (L-lactide) nanofibers. Ann Biomed Eng. 2011;39:14–25. doi: 10.1007/s10439-010-0106-3. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Gittens RA, Song R, Tannenbaum R, Olivares-Navarrete R, Schwartz Z, et al. Effects of structural properties of electrospun TiO2 nanofiber meshes on their osteogenic potential. Acta Biomater. 2012;8:878–85. doi: 10.1016/j.actbio.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ince Yardimci A, Baskan O, Yilmaz S, Mese G, Ozcivici E, Selamet Y. Osteogenic differentiation of mesenchymal stem cells on random and aligned PAN/PPy nanofibrous scaffolds. J Biomater Appl. 2019;34:640–50. doi: 10.1177/0885328219865068. [DOI] [PubMed] [Google Scholar]
- 30. Shahrousvand M, Hoseinian MS, Ghollasi M, Karbalaeimahdi A, Salimi A, Tabar FA. Flexible magnetic polyurethane/Fe 2 O 3 nanoparticles as organic-inorganic nanocomposites for biomedical applications: Properties and cell behavior. Mater Sci Eng C Mater Biol Appl 2016. 10.1016/j.msec.2016.12.117. [DOI] [PubMed]
- 31.Hajikhani M, Khanghahi MM, Shahrousvand M, Mohammadi-Rovshandeh J, Babaei A, Khademi SMH. Intelligent superabsorbents based on a xanthan gum/poly (acrylic acid) semi-interpenetrating polymer network for application in drug delivery systems. Int J Biol Macromol. 2019;139:509–20. doi: 10.1016/j.ijbiomac.2019.07.221. [DOI] [PubMed] [Google Scholar]
- 32.Hanumantharao SN, Que C, Rao S. Self-assembly of 3D nanostructures in electrospun polycaprolactone-polyaniline fibers and their application as scaffolds for tissue engineering. Materialia. 2019;6:100296. doi: 10.1016/j.mtla.2019.100296. [DOI] [Google Scholar]
- 33.Medeiros ES, Mattoso LH, Ito EN, Gregorski KS, Robertson GH, Offeman RD, et al. Electrospun nanofibers of poly (vinyl alcohol) reinforced with cellulose nanofibrils. J Biobased Mater Bioenergy. 2008;2:231–42. doi: 10.1166/jbmb.2008.411. [DOI] [Google Scholar]
- 34.Shahrousvand M, Mir Mohamad Sadeghi G, Salimi A. Artificial extracellular matrix for biomedical applications: biocompatible and biodegradable poly (tetramethylene ether) glycol/poly (ε-caprolactone diol)-based polyurethanes. J Biomater Sci Polym Ed. 2016;27:1712–28. doi: 10.1080/09205063.2016.1231436. [DOI] [PubMed] [Google Scholar]
- 35. Landel RF, Nielsen LE. Mechanical properties of polymers and composites: CRC press; 1993.
- 36.Yee WA, Nguyen AC, Lee PS, Kotaki M, Liu Y, Tan BT, et al. Stress-induced structural changes in electrospun polyvinylidene difluoride nanofibers collected using a modified rotating disk. Polymer. 2008;49:4196–203. doi: 10.1016/j.polymer.2008.07.032. [DOI] [Google Scholar]
- 37.Ghasemi‐Mobarakeh L, Prabhakaran MP, Morshed M, Nasr‐Esfahani MH, Baharvand H, Kiani S, et al. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J Tissue Eng Regen Med. 2011;5:e17–e35. doi: 10.1002/term.383. [DOI] [PubMed] [Google Scholar]
- 38.Sabir MI, Xu X, Li L. A review on biodegradable polymeric materials for bone tissue engineering applications. J Mater Sci. 2009;44:5713–24. doi: 10.1007/s10853-009-3770-7. [DOI] [Google Scholar]
- 39.Qazi TH, Rai R, Boccaccini AR. Tissue engineering of electrically responsive tissues using polyaniline based polymers: A review. Biomaterials. 2014;35:9068–86. doi: 10.1016/j.biomaterials.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 40.Asghari F, Samiei M, Adibkia K, Akbarzadeh A, Davaran S. Biodegradable and biocompatible polymers for tissue engineering application: a review. Artif Cells Nanomed Biotechnol. 2017;45:185–92. doi: 10.3109/21691401.2016.1146731. [DOI] [PubMed] [Google Scholar]
- 41.Bartnikowski M, Dargaville TR, Ivanovski S, Hutmacher DW. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog Polym Sci. 2019;96:1–20. doi: 10.1016/j.progpolymsci.2019.05.004. [DOI] [Google Scholar]
- 42.Shao S, Zhou S, Li L, Li J, Luo C, Wang J, et al. Osteoblast function on electrically conductive electrospun PLA/MWCNTs nanofibers. Biomaterials. 2011;32:2821–33. doi: 10.1016/j.biomaterials.2011.01.051. [DOI] [PubMed] [Google Scholar]


