Abstract
Objectives: Ovarian cancer is a frequent malignancy among women. Fucoxanthin has been discovered to exert anti-tumor impacts on numerous tumors. Herein, the current work was carried out to identify the biological function of fucoxanthin on the malignant progression of ovarian cancer and to explore the underlying molecular mechanisms. Methods: In this study, cell counting kit-8 (CCK-8), 5-ethynyl-2’-deoxyuridine (EdU) staining, wound healing, as well as transwell assays were employed to assess the malignant cell phenotypes, including cell proliferation, migration and invasion in ovarian cancer. The expression of related proteins was evaluated using western blot. Additionally, the glucose uptake, intracellular adenosine triphosphate (ATP), extracellular acidifications rates (ECAR) and glycolysis-associated enzymes were measured to evaluate glycolysis level. Results: It was demonstrated that fucoxanthin restrained the proliferative, migratory and invasive capabilities in both A2780 and OVCAR3 cells. Fucoxanthin could inhibit glycolysis and inactivate signal transducers and activators of transcription 3 (STAT3)/c-Myc signaling. In addition, Colivelin, a STAT3 activator, greatly weakened the suppressive effects of fucoxanthin on ovarian cancer cell proliferation, migration, invasion and glycolysis. Conclusion: Fucoxanthin exerts anti-tumor activity in ovarian cancer, possibly via inactivation of the STAT3/c-Myc signaling pathway, and thus provides a novel therapeutic strategy for the treatment of ovarian cancer.
Keywords: Fucoxanthin, ovarian cancer, glycolysis, STAT3, c-Myc
Introduction
Ovarian cancer is one of the most common and deadliest cancers in women worldwide [1]. Metastasis accounts for the lethality of ovarian cancer [2]. Because of the insidious onset and atypical symptoms of ovarian cancer in early stages, the patients are usually diagnosed at advanced stages [3]. Despite the fact that great progresses have been achieved in common therapeutic strategies such as surgery and adjuvant chemotherapy, more than 70% of ovarian cancer patients develop relapse within three years [4,5]. Therefore, searching for natural, safe, and effective therapeutic drugs has become an urgent and important task in the study of ovarian cancer.
Fucoxanthin is an oxygenated carotenoid abundant in brown algae and certain diatoms [6]. Fucoxanthin possesses a variety of biological activities such as anti-tumor, anti-inflammation and anti-obesity dependeing on its special chemical structure [7,8]. Fucoxanthin can repress the proliferation ability of cervical cancer cells [9]. Also, fucoxanthin can suppress cell proliferation, migration, invasion and lymph-angiogenesis in breast cancer [10]. Eid et al [11] reported that fucoxanthin could sensitize multidrug-resistant ovarian cancer cells to doxorubicin by inducing apoptosis. Nevertheless, the impacts of fucoxanthin on ovarian cancer metastasis still need to be elucidated.
Signal transducers and activators of transcription 3 (STAT3) is a transcription factor involved in a variety of biological functions, which transfers extracellular signals to the nucleus, thus activating the transcription of downstream oncogenes, such as MYC [12,13]. Abnormal activation of STAT3 is critical for the occurrence and development of cancers through promoting malignant cell proliferation, migration and metastasis [14]. Emerging studies proposed that high STAT3 activity or phosphorylated (p)-STAT3 expression was markedly associated with poor overall survival (OS) and unfavorable progression free survival (PFS) in ovarian cancer patients [15]. In addition, persistent STAT3 activation could promote tumor progression and metastasis in various cancers [16,17]. As an oncogene, c-Myc is known to contribute to cell proliferation, migration and invasion capabilities of ovarian cancer [18]. Furthermore, Wang et al [19] proved that fucoxanthin could suppress the STAT3/epidermal growth factor receptor (EGFR) signaling in sarcoma 180. However, the effect of fucoxanthin on STAT3/c-Myc signaling in ovarian cancer cells is still unclear.
This study aimed to investigate the potential function as well as the possible mechanism of fucoxanthin in ovarian cancer metastasis and glycolysis. Understanding the anti-tumor activity of fucoxanthin in ovarian cancer is anticipated to provide a novel therapeutic strategy for the treatment of ovarian cancer.
Materials and methods
Cell culture
The ovarian cancer cell lines OVCAR-3 and A2780 were provided by BeNa Culture Collection (Beijing, China). A2780 cells were cultivated in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc., USA) while OVCAR-3 cells were cultivated in Roswell Park Memorial Institute (RPMI)-1640 medium with 15% FBS and 0.01 mg/ml insulin. All cells were placed at 37°C in a humidified incubator with 5% CO2.
Cell counting kit-8 (CCK-8) assay
The OVCAR-3 and A2780 cells were seeded in a 96-well plate at a density of 1 × 104 cells/well. Thereafter, varying concentrations (0, 25, 50, 75, 100 μM) of fucoxanthin were adopted for cell treatment for 48 h. CCK-8 solution (Beijing Solarbio Science & Technology Co., Ltd.) was then added to each well and the cells were cultured for another 2 h. With the application of a microplate reader, the absorbance at 450 nm was assessed.
5-ethynyl-2’-deoxyuridine (EdU) staining assay
Following 48 h of administration with different concentrations of fucoxanthin (50, 75, 100 μM), OVCAR-3 and A2780 cells were exposed to EdU solution (Thermo Fisher Scientific, Inc.). Then, cells were subjected to 4% paraformaldehyde and then 0.5% Triton X-100. Finally, cells were incubated with reaction mix for 20 min and DAPI was applied for nuclear staining for 30 min in the darkness. The images were observed under a fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Wound healing assay
The OVCAR-3 and A2780 cells were inoculated into 6-well plates. When 95% cell fusion was achieved, a straight scratch was made using a 100 μl pipette tip. Then, the phosphate buffer saline (PBS)-rinsed cells were subjected to serum-free medium for 48 h of cultivation. Finally, the wounds at 0 and 48 h were captured under an optical microscope (Olympus Corporation).
Transwell invasion assay
The OVCAR-3 and A2780 cells (2 × 104) were resuspended in serum-free medium and seeded onto the upper transwell chambers (Corning, NY, USA) precoated with Matrigel (BD Biosciences, CA, USA). The complete culture medium supplemented with 10% FBS was added to the lower chamber. Following 48 h of cultivation, cotton swabs were utilized for the removal of non-invaded cells and the cells on the lower surface were exposed to 4% paraformaldehyde, and then stained with 0.2% crystal violet. Finally, the invaded cells were observed under an optical microscope (Olympus Corporation).
Western blot analysis
After treatment, the cells were lysed using radioimmunoprecipitation (RIPA) lysis buffer (Beyotime, Shanghai, China), followed by the quantification of protein concentrations by a BCA protein assay kit (Beyotime, Shanghai, China). A total of 30 μg of protein were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (Solarbio, Beijing, China). Subsequently, the membranes were blocked at room temperature using 5% skimmed milk powder for 1 h at room temperature and then incubated with primary antibodies against matrixmetalloproteinase (MMP)2 (ab92536, 1:1000, Abcam), MMP9 (ab283575, 1:1000, Abcam), lactate dehydrogenase A (LDHA; ab52488, 1:5000; Abcam), hexokinase II (HK2, ab209847, 1:1000; Abcam), 3-Phosphoinositide-dependent protein kinase 1 (PDK1; ab202468, 1:2000, Abcam), STAT3 (ab68153, 1:1000, Abcam), phosphorylated (p)-STAT3 (ab267373, 1:1000, Abcam), c-Myc (ab185656, 1:2000, Abcam) and reduced glyceraldehyde-phosphate dehydrogenase (GAPDH; ab9485, 1:2500, Abcam) at 4°C overnight. After rinsing with Tris Buffered Saline with Tween-20 (TBST) for 5 min (3 times), membranes were exposed to a horseradish peroxidase (HRP)-labeled secondary antibody (ab6721, 1:2000, Abcam) at room temperature for 1 h. Immunoblot bands were developed with an enhanced chemiluminescence (ECL) detection kit (Beyotime, Shanghai, China). Protein expression was analyzed using Image J software with GAPDH as an internal reference.
Glucose uptake measurement
To indicate the intracellular glucose uptake levels, the OVCAR-3 and A2780 cells were incubated at 37°C for 30 min with 100 kBq of fluorodeoxyglucose (FDG). After washing twice with cold PBS, the cells were lysed with 0.5 mol/L NaOH. The lysates were then measured for radioactivity on a γ-counter (Waltham, MA, USA). Next, each parallel plate was measured for protein content by a modified Bradford assay (Bio-Rad, CA, USA). Glucose uptake levels were expressed as protein content-corrected counts relative to those of the control cells.
Measurement of adenosine triphosphate (ATP)
After treatment, OVCAR-3 and A2780 cells were seeded in a 6-well plate (4 × 104 cell/well) for 24 h. The cellular ATP content were determined by ATP Assay Kit (Sigma-Aldrich, MO, USA) according to the manufacturer’s instruction.
Extracellular acidifications rates (ECAR) analysis
Basal ECAR were determined using an XF24 Seahorse Bioanalyzer (Seahorse Bioscience, MA, USA). In brief, the OVCAR-3 and A2780 cells were seeded in a 6-well plate (4 × 104 cell/well) overnight. Next, the culture medium was replaced by the medium without glucose and pyruvate. The ECAR components were measured, and then 5 mM glucose, 1 μM oligomycin, and 100 mM 2-Deoxyglucose (2-DG) were injected sequentially, with two measurements after each injection. The fold-changes of glucose-induced ECAR for the OVCAR-3 and A2780 cells were normalized to the control group (no treatment).
Statistical analysis
All data that collected form independent experiments were analyzed by GraphPad Prism 8.0 (GraphPad software, Inc.) and displayed in the form of mean ± standard deviation (SD). Comparisons among multiple groups were carried out by employing one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. P value <0.05 indicated a statistically significant difference.
Results
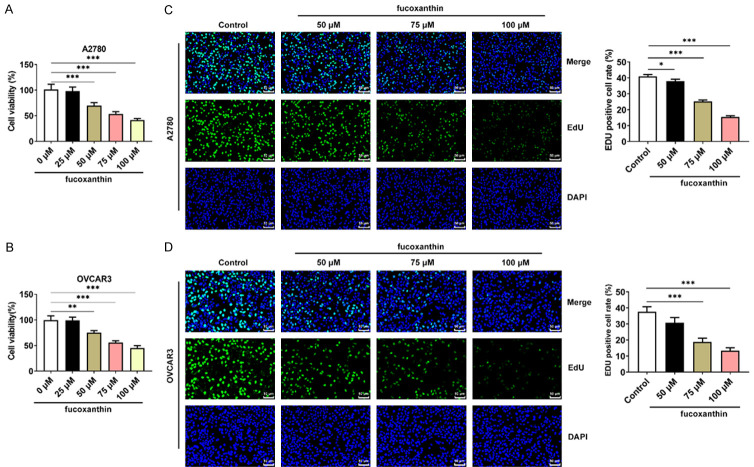
Fucoxanthin treatment restrained the proliferation of ovarian cancer cells
The OVCAR-3 and A2780 cells were treated with different concentrations of fucoxanthin for 48 h. Results of CCK8 assays revealed that fucoxanthin treatment significantly suppressed the viability of ovarian cancer cells in a concentration-dependent manner (Figure 1A, 1B). Additionally, the results of EdU staining assays proved that the proliferation of OVCAR-3 and A2780 cells was reduced following treatment with different concentrations (50, 75, 100 μM) of fucoxanthin (Figure 1C, 1D).
Figure 1.
Fucoxanthin treatment restrained the proliferation of ovarian cancer cells. A, B. The viability of fucoxanthin-treated OVACR3 and A2780 cells was detected by cell counting kit-8 (CCK-8) assay. C, D. The proliferation capacity of fucoxanthin-treated OVACR3 and A2780 cells was measured by 5-ethynyl-2’-deoxyuridine (EdU) staining assays. Magnification × 200. *P<0.05, **P<0.01 and ***P<0.001.
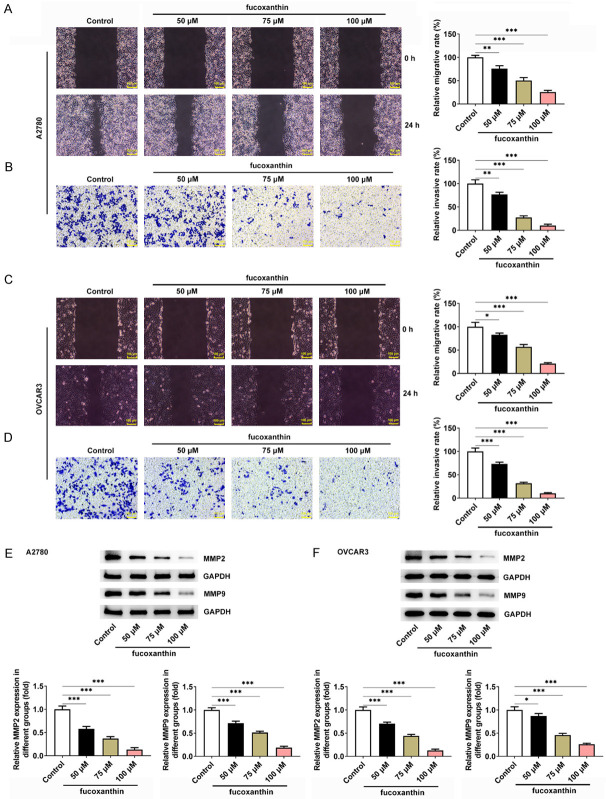
Fucoxanthin treatment suppressed ovarian cancer cell migration and invasion
In addition, we also evaluated the effects of fucoxanthin on ovarian cancer cell migration and invasion. The results from wound healing assay showed that fucoxanthin at 50, 75 or 100 μM could significantly inhibit OVCAR-3 and A2780 cell migration (Figure 2A, 2C). Then, data from transwell invasion assay clearly showed that fucoxanthin could markedly decrease the number of invaded cells in a dose-dependent manner (Figure 2B, 2D). Meanwhile, the reduced expression of MMP2 and MMP9 following fucoxanthin treatment also demonstrated that fucoxanthin repressed the migration and invasion of ovarian cancer cells (Figure 2E, 2F).
Figure 2.
Fucoxanthin treatment suppressed ovarian cancer cell migration and invasion. A, C. The wound healing assay was used to detect the effect of fucoxanthin on the migration abilities of OVACR3 and A2780 cells. Magnification × 100. B, D. The transwell assay was used to detect the effect of fucoxanthin on the invasive abilities of OVACR3 and A2780 cells. Magnification × 100. E, F. Western blot assay was performed to detect the protein expression levels of matrixmetalloproteinase (MMP)2 and MMP9. *P<0.05, **P<0.01 and ***P<0.001.
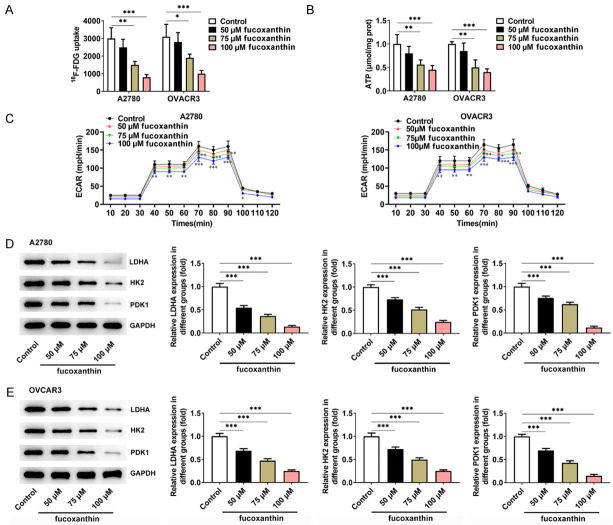
Fucoxanthin treatment inhibited glucose metabolism in ovarian cancer cells
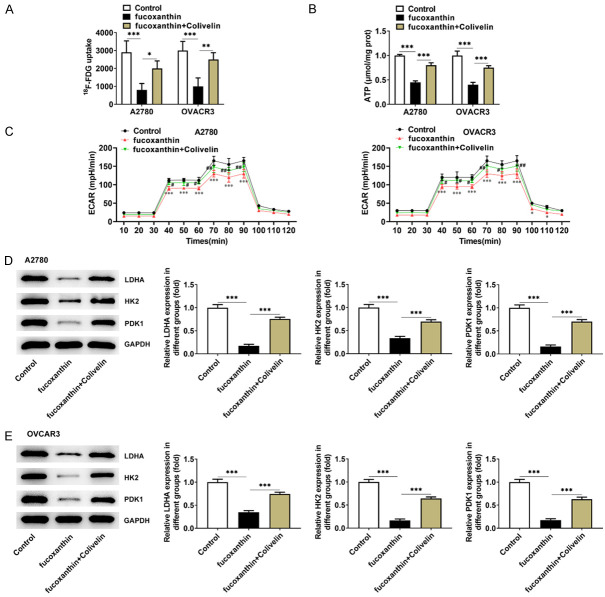
18F-fludeoxyglucose (F-FDG) uptake experiment was used to indicate the intracellular glucose uptake levels of OVCAR-3 and A2780 cells. The FDG uptake in OVCAR-3 and A2780 cells was markedly reduced with the increasing concentrations of fucoxanthin (Figure 3A). Besides, fucoxanthin treatment inhibited ATP levels in OVCAR-3 and A2780 cells, and ATP levels fluctuate based on the fucoxanthin concentration (Figure 3B). ECAR is an indicator of glycolysis. Therefore, the effects of fucoxanthin on ECAR were detected in OVCAR-3 and A2780 cells treated with glucose, oligomycin, or 2-DG. The results showed that fucoxanthin treatment inhibited glycolysis (Figure 3C). Moreover, the expression of glycolysis-related enzymes and proteins such as LDHA, HK2 and PDK1 were discovered to be greatly down-regulated in fucoxanthin-treated OVCAR-3 and A2780 cells (Figure 3D, 3E).
Figure 3.
Fucoxanthin treatment inhibited glucose metabolism in ovarian cancer cells. A. The 18F-fludeoxyglucose (F-FDG) uptake was detected in OVACR3 and A2780 cells after fucoxanthin treatment. B. Adenosine triphosphate (ATP) levels were measured using the ATP Assay Kit in OVCAR-3 and A2780 cells. C. Extracellular acidifications rates (ECAR) of OVCAR-3 and A2780 cells was measured with a seahorse Bioanalyzer. D, E. Western blot assay was performed to detect the protein expression levels of glycolysis-related rate limiting enzymes lactate dehydrogenase A (LDHA), hexokinase II (HK2), 3-Phosphoinositide-dependent protein kinase 1 (PDK1). *P<0.05, **P<0.01 and ***P<0.001 vs control.
Fucoxanthin treatment inhibited STAT3/c-Myc signaling in ovarian cancer cells
To gain mechanistic insights into the effects of fucoxanthin on inhibiting metastasis, the proteins involved in STAT3/c-Myc signaling were measured. The results indicated that fucoxanthin treatment could inhibit the protein expression of p-STAT3 and c-Myc in OVCAR-3 and A2780 cells. In addition, colivelin, a STAT3 activator, could reverse the inhibitory effect of fucoxanthin on STAT3/c-Myc signaling (Figure 4A, 4B).
Figure 4.
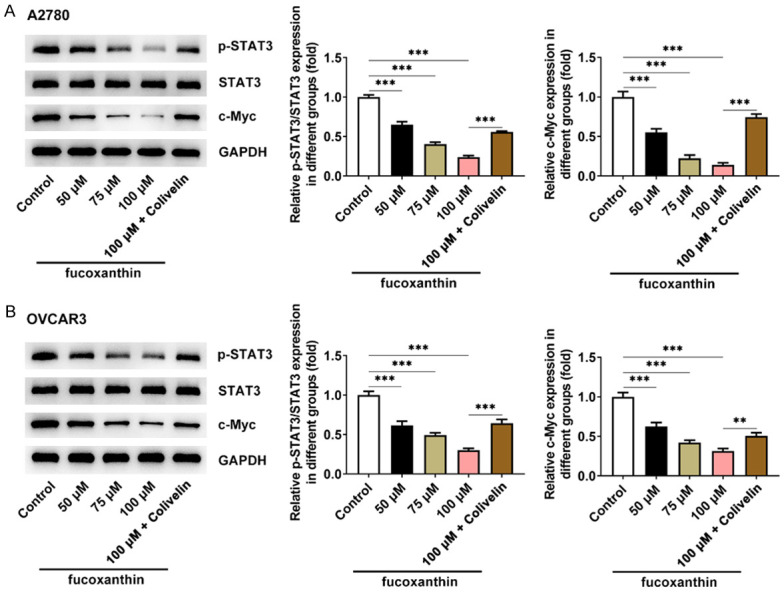
Fucoxanthin treatment inhibited signal transducers and activators of transcription 3 (STAT3)/c-Myc signaling in ovarian cancer cells. A, B. Expression of STAT3, STAT3 and p-STAT3 were detected by western blot in OVACR3 and A2780 cells after fucoxanthin treatment. **P<0.01 and ***P<0.001.
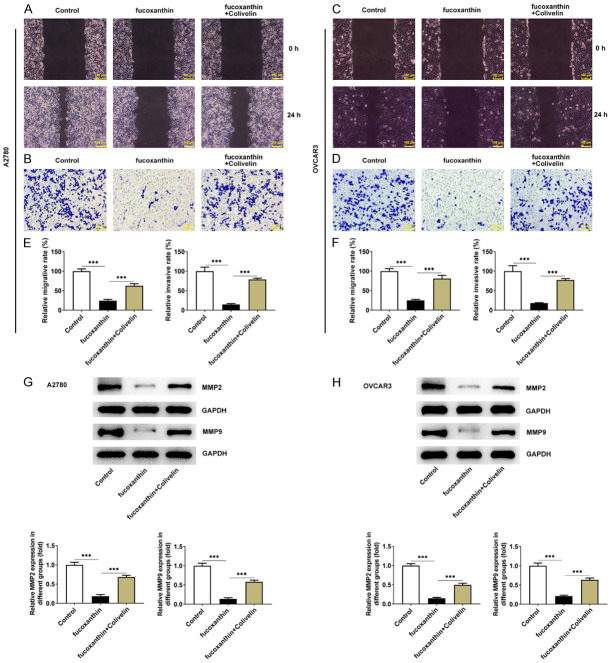
Fucoxanthin treatment restrained the proliferation, migration and invasion of ovarian cancer cells by inactivating STAT3/c-Myc signaling
In order to confirm the proposed mechanisms of fucoxanthin on cell proliferation, migration and invasion in ovarian cancer through the STAT3/c-Myc signaling, OVCAR-3 and A2780 cells were treated with 100 μM of fucoxanthin, with or without colivelin treatment. The CCK8 and EDU staining assays revealed that the proliferation of OVCAR-3 and A2780 cells was suppressed by fucoxanthin treatment, which was restored by colivelin treatment (Figure 5A-C). The results of wound healing assay and transwell invasion assay proved that fucoxanthin greatly inhibited the migration and invasion of OVCAR-3 and A2780 cells, which was partly abolished by additional treatment of colivelin (Figure 6A-F). Furthermore, the downregulated protein expression of MMP2 and MMP9 by fucoxanthin treatment was upregulated by the additional treatment of colivelin (Figure 6G, 6H). The above results suggested that fucoxanthin might repress ovarian cancer cell proliferation, migration and invasion through inactivating STAT3/c-Myc signaling.
Figure 5.
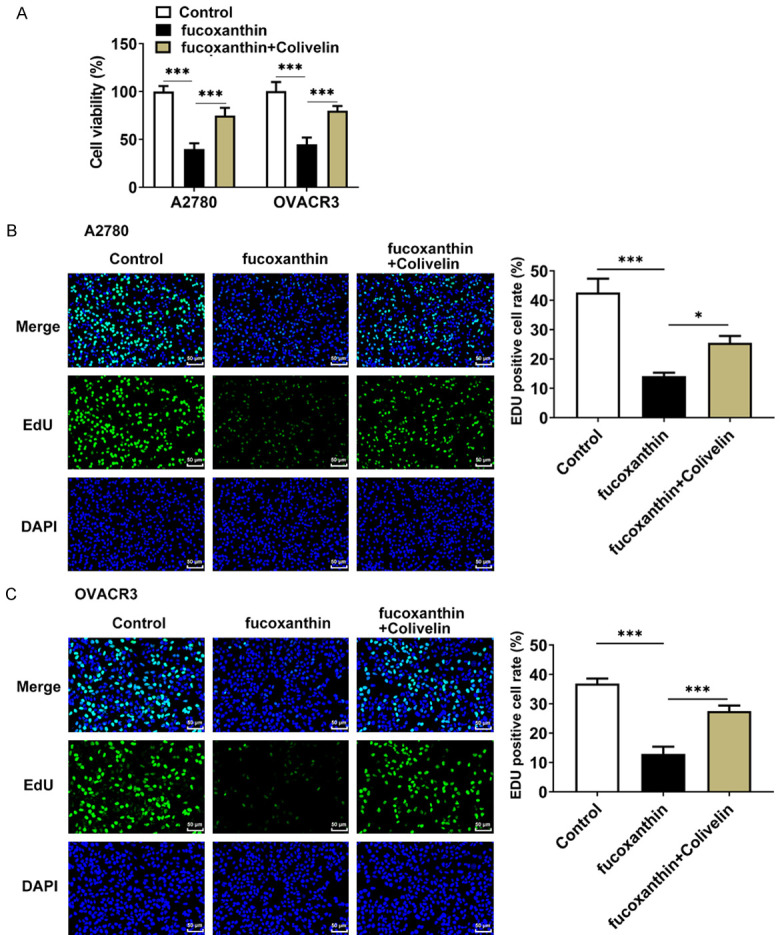
Fucoxanthin treatment restrained the proliferation of ovarian cancer cells by inactivating signal transducers and activators of transcription 3 (STAT3)/c-Myc signaling. A. The viability of OVACR3 and A2780 cells was detected by cell counting kit 8 (CCK8) assay. B, C. The proliferation capacity of OVACR3 and A2780 cells was measured by 5-ethynyl-2’-deoxyuridine (EdU) staining assays. Magnification × 200. *P<0.05 and ***P<0.001.
Figure 6.
Fucoxanthin treatment suppressed ovarian cancer cell migration and invasion by inactivating signal transducers and activators of transcription 3 (STAT3)/c-Myc signaling. A, C. The wound healing assay was used to detect the effects of fucoxanthin and STAT3 on the migration abilities of OVACR3 and A2780 cells. Magnification × 100. B, D. The transwell assay was used to detect the effects of fucoxanthin and STAT3 on the invasive abilities of OVACR3 and A2780 cells. Magnification × 100. E. The quantification of cell migration rate and invasion rate in A2780 cells. F. The quantification of cell migration rate and invasion rate in OVCAR3 cells. G, H. Western blot assay was performed to detect the protein expression levels of matrixmetalloproteinase (MMP)2 and MMP9. ***P<0.001.
Fucoxanthin treatment inhibited glucose metabolism in ovarian cancer cells by inactivating STAT3/c-Myc signaling
Subsequently, it was observed that the suppressive effects of fucoxanthin on the glucose consumption, ATP levels and ECAR of ovarian cancer cells were counteracted by colivelin (Figure 7A-C). Besides, fucoxanthin treatment reduced the expression levels of the glycolysis-associated enzymes LDHA, HK2 and PDK1 in ovarian cancer cells, and colivelin recovered the expression of LDHA, HK2 and PDK1 (Figure 7D, 7E). Collectively, these results demonstrated that activation of STAT3 could partially abrogate the suppressive effects of fucoxanthin on glucose metabolism in ovarian cancer cells.
Figure 7.
Fucoxanthin treatment inhibited glucose metabolism in ovarian cancer cells by inactivating signal transducers and activators of transcription 3 (STAT3)/c-Myc signaling. A. The 18F-fludeoxyglucose (F-FDG) uptake was detected in OVACR3 and A2780 cells after fucoxanthin and STAT3 activator treatment. B. Adenosine triphosphate (ATP) levels were measured using the ATP Assay Kit in OVCAR-3 and A2780 cells. *P<0.05, **P<0.01 and ***P<0.001. C. Extracellular acidifications rates (ECAR) of OVCAR-3 and A2780 cells was measured with a seahorse Bioanalyzer. *P<0.05 and ***P<0.001 vs Control; #P<0.05 and ##P<0.01 vs fucoxanthin. D, E. Western blot assay was performed to detect the protein expression levels of glycolysis-related rate limiting enzymes lactate dehydrogenase A (LDHA), hexokinase II (HK2), 3-Phosphoinositide-dependent protein kinase 1 (PDK1). ***P<0.001.
Discussion
Due to the asymptomatic development of ovarian cancer and the lack of early diagnostic markers, most patients are diagnosed at advanced stages [3]. Metastasis is the most frequent clinical problem during the treatment of ovarian cancer, making it a deadly gynecological tumor [2]. Li et al [20] have suggested that fucoxanthin could repress allergic rhinitis via STAT3 signaling. Fucoxanthin could also downregulate the levels of STAT3 and p-STAT3 in gastric cancer cell lines [21]. In the present study, the potential function of fucoxanthin in ovarian cancer was characterized and it was determined that fucoxanthin treatment could mitigate metastasis and glycolysis via the STAT3/c-Myc signaling, and thus affected ovarian cancer progression.
Recently, fucoxanthin exhibits a wide range of anti-tumor biological activities [22]. For example, fucoxanthin could suppress cell proliferation and induce cell apoptosis in endometrial cancer [23]. Long et al [24] also proved that fucoxanthin inhibited nasopharyngeal carcinoma cell growth by inducing autophagy and apoptosis. Furthermore, fucoxanthin could inhibit the invasion and migration of glioblastoma cells by suppressing the p38-MMP2/9 pathway [25]. Consistent with previous researches, our results demonstrated that fucoxanthin exhibited inhibitory effects on cell proliferation, migration and invasion capabilities of ovarian cancer. One of the most prominent characteristics of malignant tumors is aerobic glycolysis, also known as glycolysis or Warburg effect [26]. The tumor microenvironment causes activation of a large number of signaling pathways, key proteins, glycolysis-related enzymes and various genes that may initiate and regulate aerobic glycolysis, and ultimately promote activation of aerobic glycolysis [27]. Aerobic glycolysis could directly or indirectly promote various malignant phenotypes of tumor tissues [28,29]. For instance, Pimozide could suppress the growth of breast cancer cells by alleviating the aerobic glycolysis [30]. In this study, fucoxanthin treatment inhibited ATP and the protein levels of important enzymes relating to glycolysis such as LDHA, HK2 and PDK1 in ovarian cancer cell lines. FDG uptake was treated as a marker of tumor aggressiveness as the activation of glucose uptake is a presentation of cancers [31]. Our results indicated that fucoxanthin treatment markedly reduced the FDG uptake in OVCAR-3 and A2780 cells. Therefore, it was suggested that fucoxanthin could inhibit the development of ovarian cancer.
Being a critical member of STAT protein family, STAT3 has been widely recognized to be able to regulate various cellular processes, including cell proliferation, differentiation and apoptosis [12]. Inhibition of STAT3 signaling can effectively reduce the activity of cancer cells, promote their apoptosis process, and provide a therapeutic strategy to improve the anti-tumor effect [14]. C-Myc is a downstream oncogenic gene of STAT3. A study has demonstrated that c-Myc could enhance Warburg effect and promote the occurrence and progression of colorectal cancer [32]. Besides, Li et al [33] reported that STAT3/c-Myc signaling axis could promote cell proliferation, metastasis, and chemoresistance in ovarian cancer. Sun et al [34] proved that Ginsenoside Rh2 could inhibit glycolysis through controlling STAT3/c-Myc signaling in non-small cell lung cancer. In the present study, STAT3 activator could eliminate the impacts of fucoxanthin on cell proliferation, migration and invasion as well as glycolysis in ovarian cancer.
However, there are still some limitations in the present study. This study was conducted only in cells, while these findings are needed to be confirmed in further animal and clinical studies. In addition, the further in-depth regulatory mechanism of fucoxanthin in ovarian cancer is needed to be explored in our future work.
Conclusion
In conclusion, our results confirmed that fucoxanthin suppressed cell proliferation, metastasis and glycolysis in ovarian cancer by inhibiting the STAT3/c-Myc signaling. Fucoxanthin might be a promising therapeutic approach for patients with ovarian cancer.
Disclosure of conflict of interest
None.
References
- 1.Maiorano BA, Lorusso D, Maiorano MFP, Ciardiello D, Parrella P, Petracca A, Cormio G, Maiello E. The interplay between PARP inhibitors and immunotherapy in ovarian cancer: the rationale behind a new combination therapy. Int J Mol Sci. 2022;23:3871. doi: 10.3390/ijms23073871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng X, Wang J, Liu C, Jiang T, Yang N, Liu D, Zhao H, Xu Z. Zinc transporter SLC39A13/ZIP13 facilitates the metastasis of human ovarian cancer cells via activating Src/FAK signaling pathway. J Exp Clin Cancer Res. 2021;40:199. doi: 10.1186/s13046-021-01999-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assidi M. High N-cadherin protein expression in ovarian cancer predicts poor survival and triggers cell invasion. Front Oncol. 2022;12:870820. doi: 10.3389/fonc.2022.870820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goff BA. Advanced ovarian cancer: what should be the standard of care? J Gynecol Oncol. 2013;24:83–91. doi: 10.3802/jgo.2013.24.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24–32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 6.Jung HA, Ali MY, Choi RJ, Jeong HO, Chung HY, Choi JS. Kinetics and molecular docking studies of fucosterol and fucoxanthin, BACE1 inhibitors from brown algae Undaria pinnatifida and Ecklonia stolonifera. Food Chem Toxicol. 2016;89:104–111. doi: 10.1016/j.fct.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Muradian K, Vaiserman A, Min KJ, Fraifeld VE. Fucoxanthin and lipid metabolism: a minireview. Nutr Metab Cardiovasc Dis. 2015;25:891–897. doi: 10.1016/j.numecd.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Kim KN, Heo SJ, Kang SM, Ahn G, Jeon YJ. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol In Vitro. 2010;24:1648–1654. doi: 10.1016/j.tiv.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Ye G, Wang L, Yang K, Wang C. Fucoxanthin may inhibit cervical cancer cell proliferation via downregulation of HIST1H3D. J Int Med Res. 2020;48:300060520964011. doi: 10.1177/0300060520964011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Ma Y, Yang J, Jin L, Gao Z, Xue L, Hou L, Sui L, Liu J, Zou X. Fucoxanthin inhibits tumour-related lymphangiogenesis and growth of breast cancer. J Cell Mol Med. 2019;23:2219–2229. doi: 10.1111/jcmm.14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eid SY, Althubiti MA, Abdallah ME, Wink M, El-Readi MZ. The carotenoid fucoxanthin can sensitize multidrug resistant cancer cells to doxorubicin via induction of apoptosis, inhibition of multidrug resistance proteins and metabolic enzymes. Phytomedicine. 2020;77:153280. doi: 10.1016/j.phymed.2020.153280. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Wang L, Li X. The roles of HOXB8 through activating Wnt/beta-catenin and STAT3 signaling pathways in the growth, migration and invasion of ovarian cancer cells. Cytotechnology. 2022;74:77–87. doi: 10.1007/s10616-021-00508-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang QY, Lin ZL, Su ZW, Li S, Li J, Guan S, Ling Y, Zhang L. Peptide identification of hepatocyte growth-promoting factor and its function in cytoprotection and promotion of liver cell proliferation through the JAK2/STAT3/c-MYC pathway. Eur J Pharmacol. 2022;920:174832. doi: 10.1016/j.ejphar.2022.174832. [DOI] [PubMed] [Google Scholar]
- 14.Jiang N, Liao Y, Wang M, Wang Y, Wang K, Guo J, Wu P, Zhong B, Guo T, Wu C. BUB1 drives the occurrence and development of bladder cancer by mediating the STAT3 signaling pathway. J Exp Clin Cancer Res. 2021;40:378. doi: 10.1186/s13046-021-02179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao S, Zhang W, Yan N, Li M, Mu X, Yin H, Wang J. The impact of STAT3 and phospho-STAT3 expression on the prognosis and clinicopathology of ovarian cancer: a systematic review and meta-analysis. J Ovarian Res. 2021;14:164. doi: 10.1186/s13048-021-00918-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YL, Zhang MM, Wu LW, Liu YH, Zhang ZY, Zeng LH, Lin NM, Zhang C. DYRK1A reinforces epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma via cooperatively activating STAT3 and SMAD. J Biomed Sci. 2022;29:34. doi: 10.1186/s12929-022-00817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo DD, Zhao F. KLF4 suppresses the proliferation and metastasis of NSCLC cells via inhibition of MSI2 and regulation of the JAK/STAT3 signaling pathway. Transl Oncol. 2022;22:101396. doi: 10.1016/j.tranon.2022.101396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang H, Zhang X, Li B, Meng X. MAGI2-AS3 suppresses MYC signaling to inhibit cell proliferation and migration in ovarian cancer through targeting miR-525-5p/MXD1 axis. Cancer Med. 2020;9:6377–6386. doi: 10.1002/cam4.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Chen S, Xu S, Yu X, Ma D, Hu X, Cao X. In vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR signaling in sarcoma 180 (S180) xenografts-bearing mice. Mar Drugs. 2012;10:2055–2068. doi: 10.3390/md10092055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Zhang Y, Veeraraghavan VP, Mohan SK, Ma Y. Restorative effect of fucoxanthin in an ovalbumin-induced allergic rhinitis animal model through NF-kappaB p65 and STAT3 signaling. J Environ Pathol Toxicol Oncol. 2019;38:365–375. doi: 10.1615/JEnvironPatholToxicolOncol.2019030997. [DOI] [PubMed] [Google Scholar]
- 21.Yu RX, Yu RT, Liu Z. Inhibition of two gastric cancer cell lines induced by fucoxanthin involves downregulation of Mcl-1 and STAT3. Hum Cell. 2018;31:50–63. doi: 10.1007/s13577-017-0188-4. [DOI] [PubMed] [Google Scholar]
- 22.Xiao H, Zhao J, Fang C, Cao Q, Xing M, Li X, Hou J, Ji A, Song S. Advances in studies on the pharmacological activities of fucoxanthin. Mar Drugs. 2020;18:634. doi: 10.3390/md18120634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu J, Sun Y, Yang L, Niu X, Li L. Fucoxanthin prevents cell growth and induces apoptosis in endometrial cancer HEC-1A cells by the inhibition of the PI3K/Akt/mTOR pathway. J Biochem Mol Toxicol. 2022;36:e23027. doi: 10.1002/jbt.23027. [DOI] [PubMed] [Google Scholar]
- 24.Long Y, Cao X, Zhao R, Gong S, Jin L, Feng C. Fucoxanthin treatment inhibits nasopharyngeal carcinoma cell proliferation through induction of autophagy mechanism. Environ Toxicol. 2020;35:1082–1090. doi: 10.1002/tox.22944. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Zheng J, Zhang Y, Wang Z, Yang Y, Bai M, Dai Y. Fucoxanthin activates apoptosis via inhibition of PI3K/Akt/mTOR pathway and suppresses invasion and migration by restriction of p38-MMP-2/9 pathway in human glioblastoma cells. Neurochem Res. 2016;41:2728–2751. doi: 10.1007/s11064-016-1989-7. [DOI] [PubMed] [Google Scholar]
- 26.Jiang X, Chen Z, Zhu J, Han J, You G, Li Y, Liu T, Ye H. E2F1 promotes Warburg effect and cancer progression via upregulating ENO2 expression in Ewing sarcoma. Mol Med Rep. 2022;26:237. doi: 10.3892/mmr.2022.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin M, Cao W, Chen B, Xiong M, Cao G. Tumor-derived lactate creates a favorable niche for tumor via supplying energy source for tumor and modulating the tumor microenvironment. Front Cell Dev Biol. 2022;10:808859. doi: 10.3389/fcell.2022.808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gahler A, Trufa DI, Chiriac MT, Tausche P, Hohenberger K, Brunst AK, Rauh M, Geppert CI, Rieker RJ, Krammer S, Leberle A, Neurath MF, Sirbu H, Hartmann A, Finotto S. Glucose-restricted diet regulates the tumor immune microenvironment and prevents tumor growth in lung adenocarcinoma. Front Oncol. 2022;12:873293. doi: 10.3389/fonc.2022.873293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Liu DJ, Zheng JH, He RZ, Xu DP, Yang MW, Yao HF, Fu XL, Yang JY, Huo YM, Tao LY, Hua R, Sun YW, Kong XM, Jiang SH, Liu W. IRAK2-NF-kappaB signaling promotes glycolysis-dependent tumor growth in pancreatic cancer. Cell Oncol (Dordr) 2022;45:367–379. doi: 10.1007/s13402-022-00670-z. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Qu P, Zhou XZ, Ji YX, Yuan S, Liu SP, Zhang QG. Pimozide inhibits the growth of breast cancer cells by alleviating the Warburg effect through the P53 signaling pathway. Biomed Pharmacother. 2022;150:113063. doi: 10.1016/j.biopha.2022.113063. [DOI] [PubMed] [Google Scholar]
- 31.Shangguan C, Gan G, Zhang J, Wu J, Miao Y, Zhang M, Li B, Mi J. Cancer-associated fibroblasts enhance tumor (18)F-FDG uptake and contribute to the intratumor heterogeneity of PET-CT. Theranostics. 2018;8:1376–1388. doi: 10.7150/thno.22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jing Z, Liu Q, He X, Jia Z, Xu Z, Yang B, Liu P. NCAPD3 enhances Warburg effect through c-myc and E2F1 and promotes the occurrence and progression of colorectal cancer. J Exp Clin Cancer Res. 2022;41:198. doi: 10.1186/s13046-022-02412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mei W, Zhu B, Shu Y, Liang Y, Lin M, He M, Luo H, Ye J. GDF11 protects against glucotoxicity-induced mice retinal microvascular endothelial cell dysfunction and diabetic retinopathy disease. Mol Cell Endocrinol. 2021;537:111422. doi: 10.1016/j.mce.2021.111422. [DOI] [PubMed] [Google Scholar]
- 34.Sun X, Zhao P, Li H, Liu Y, Wang T, Cheng Y. Ginsenoside Rh2 inhibits glycolysis through the STAT3/c-MYC axis in non-small-cell lung cancer. J Oncol. 2021;2021:9715154. doi: 10.1155/2021/9715154. [DOI] [PMC free article] [PubMed] [Google Scholar]