Abstract
Background: Acupuncture has been shown to be effective in treating cerebral palsy (CP), reducing muscle tension, and improving motor function. However, macro-screening of key gene sets and gene-causal interaction networks for their therapeutic mechanisms have not been studied. Methods: Applying high-throughput sequencing technology, this research discussed differentially expressed mRNAs and differential alternative splicing pre-mRNAs at the transcriptome level in rats with CP treated with acupuncture and moxibustion, and analyzed the regulatory mechanisms of these differentially expressed genes (DEGs) in CP. Changes in the levels of transcripts and alternative splicing in the hippocampi of CP rats after acupuncture treatment were analyzed. Global genes that were differentially expressed and alternative splicing events (ASEs) and regulated ASEs (RASEs) in acupuncture treatment of CP rats were analyzed. Results: The RNA-seq data of acupuncture-treated rat hippocampi revealed 198 DEGs, 125 of which were related to CP, and the transcriptional regulation of RNA polymerase II was up-regulated; moreover, there were 1168 significantly different ASEs associated with CP and transcriptional regulation. There were 14 overlapping gene expression changes in transcription factors (TFs) and DEGs. Conclusions: This study found that 14 TFs were differentially expressed and a large number of TFs underwent differential alternative splicing. It is speculated that these TFs and the translated proteins of the two different transcripts produced by the differential alternative splicing of these TFs may play corresponding functions in acupuncture treatment of young rats with CP by modulating the differential expression of their target mRNAs.
Keywords: Cerebral palsy, genome-wide analysis, RNA-seq, transcription factors
Introduction
Cerebral palsy (CP), the most prevalent impairment that induces motor dysfunction in young children, refers to a group of heterogeneous early-onset, non-progressive neuromotor disorders, seriously affecting the cerebral development of fetuses or infants. Overall, the prevalence of CP is estimated to be 2.11 per 1000 live births [1,2], versus nearly 100 in 1000 surviving babies among extremely low gestational age newborns (i.e., gestational age < 28 weeks), a 100-fold higher risk than infants born at term multidisciplinary teams that consists of physiotherapists, orthopedic surgeons, and physiatrists [3]. Physical therapy, though its effects have not been verified by randomized trials, is commonly used and widely accepted as a component of standard management for CP [4]. While therapies such as Botulinum toxin (BTX) type A, hemiplegia-specific treatments like hand-arm bimanual intensive therapy (HABIT), and constraint-induced movement therapy (CIMT) are effective in the treatment of CP, most CP patients still experience adverse outcome with serious nerve dysfunction resulting from permanent neuronal impairment [5,6]. Further research is warranted, given the considerable uncertainty about the effectiveness and toxicity of these therapies.
Currently, spasticity is mainly intervened by oral drugs (baclofen, tizanidine, dantrolene, etc.), physiotherapy, occupational therapy, and surgical treatment [7-11]. However, these have undesirable side effects and even serious adverse events. Acupuncture-centered rehabilitation, as part of a unified treatment plan that includes routine treatment, has been proposed as an appropriate strategy to address CP-related disability, and is the dominant approach used in first-class hospitals and national research institutions in China [12]. Compared to other conventional interventions, acupuncture is a relatively simple, cheap, and safe intervention that has been shown to improve motor activity, sense, language and other neurologic functions of children with CP [13]. Modern neuroimaging methods (magnetic resonance imaging) have confirmed the activation of subcortical and cortical centers after acupuncture, and that acupuncture can increase cerebral blood flow and cerebral oxygen supply in children with CP [14]. In recent years, acupuncture has been used worldwide. It is increasingly popular in the treatment and care of children’s diseases with validated safety and effectiveness [15].
In CP, the genomic landscape of CNV reveals underlying hotspots on the 2, 22 and X chromosomes and emphasizes considerable genetic heterogeneity that underlies CP heterogeneity [16]. Unfortunately, there is limited research on acupuncture treatment for CP and no macroscopic screening of key genes. Currently, CP remains a global health challenge, highlighting the need for further molecular mechanism research in CP and the identification of feasible therapeutic targets. Thus, finding ideal cerebral biomarkers with high performance in terms of specificity and sensitivity to predict drug response and efficacy may be a promising approach. Also, there have been no reports on the effects of acupuncture on CP from a genomics perspective.
Consequently, this study used RNA sequencing (RNA-seq) to identify changes in gene expression in the prefrontal cortex of CP newborn rats after acupuncture treatment.
Materials and methods
Animals
Timed pregnant Sprague-Dawley (SD) rats (Animal Centre of Xinjiang Medical University) were caged separately. After birth, the baby rats were raised with their dam under a 12:12 light:dark cycle and allowed to eat rat chow and drink water freely throughout the study period. All animal experiments, approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University, were performed following the Regulations for the Administration of Affairs Concerning Experimental Animals (2004 version, Ministry of Science and Technology, China) and the National Institutes of Health Guidelines. Animal care and breeding procedures involved in animal experiments in this study complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Surgical procedures were performed in a strictly maintained aseptic environment.
CP model establishment
Construction of a CP model
A total of 9 seven-day-old SD rats (12 g-15 g) was used for the establishment of CP model by blocking the right common carotid artery (CCA) [17]. After anesthesia using 3% isoflurane, the pup rats were tied supine for surgery. The right CCA was identified and exposed for permanent ligation with the use of an electrocoagulator supplied by Wuhan Spring Medical Beauty Equipment, China. The post-surgical rats were sent back to their dams for 1 h and then to an airtight chamber maintained at 37°C with constant hypoxia (8% O2 + 92% N2 at 4 L/minutes) for 2 hours. The temperature was kept at 37°C throughout the procedure. Five pup rats were selected as a sham group, receiving anesthesia for CCA exposure but with no ligation nor hypoxia.
Determining model success
To confirm the success of the CP model building, we performed local behavioral measurements at 3 weeks after modeling and pathological tests.
(1) Behavioral measurement: If the pup rat was observed to turn or fall to the contralateral side while walking, the CP model was considered successful.
(2) Pathological examination: Three rats were randomly selected for histopathologic examination. They were anesthetized with 2% pentobarbital sodium, and their hearts were exposed after a thoracotomy. The left ventricle was injected with sterile saline, and the right ventricle was cut until the liquid flowing out became clear, followed by injection of 0.1 moL phosphate buffered saline (PBS) with 4% paraformaldehyde. Then, the rats were euthanized with neck dislocation and the brain tissue was fixed, paraffin-embedded and sectioned for hematoxylin and eosin (H&E) staining to observe pathological alterations [18]. Destruction of brain structure was observed in CP model rats [19].
Experimental animals and grouping
The rest 6 CP model rats were randomized into sham, model, and acupuncture groups. Two normal-fed rats without any treatment were selected as a control group. The intervention of each group was as follows:
(1) Control group: No treatment was given.
(2) Sham group: Only neck incisions were performed and CCA was isolated without ligation or hypoxia treatment.
(3) Model group: CP model rats were given intraperitoneal injection of the same amount of normal saline (NS).
(4) Acupuncture group (ZJ group): Acupuncture intervention was started on the 3rd day after modeling. Acupuncture on GV20 (Baihui, located at the midmost point of the parietal bone) [20] was performed for 25 minutes/day. In addition, rats were treated with moxibustion at the GV14 (Dazhui, between the seventh cervical vertebrae and the first thoracic vertebrae, in the middle of the back) [21] and GV9 (Zhiyang, located on the posterior midline and in the depression below the spinous process of the seventh thoracic vertebra in prone position) [22]. The intervention consisted of 4 consecutive courses of acupuncture and moxibustion for 7 days each.
Twenty-eight days after intervention, all rats were euthanized by neck dislocation, and the brain tissue and hippocampus tissue collected and were used for H&E staining and RNA-sequencing, respectively.
RNA extraction and sequencing
Total RNAs were isolated from SD young rat hippocampus by TRIzol (Invitrogen), followed by DNA digestion and the subsequent RNA quality detection (A260/A280) and integrity determination by DNaseI, NanodropTM OneCspectrophotometer (Thermo Fisher Scientific Inc), and 1.5% agarose gel electrophoresis, respectively. This was followed by quantification of qualified RNAs by Qubit3.0 with QubitTM RNA Broad Range Assay kit (Life Technologies, Q10210). Total RNAs with an amount of 2 μg were used for stranded RNA sequencing library preparation as per the Ribooff rRNA Depletion Kit (Human/Mouse/Rat) (MRZG12324, Illumina) and KCTM Stranded mRNA Library Prep Kit for Illumina (DR08402, Wuhan Seqhealth Co., Ltd., China) instructions. After enrichment and quantification, the library products corresponding to 200-500 bps were finally sequenced on NovaSeq 6000 sequencer (PE150, Illumina).
RNA-Seq raw data cleaning and alignment
Raw reads > 2-N bases or < 16nt were discarded first. FastX-Toolkit v0.0.13 was then utilized to trim adaptors and low-quality bases from raw sequencing reads. Next, HISAT2 was used to align the clean reads to the mRatBN7.2 genome, allowing for 4 mismatches [23]. Uniquely mapped reads were used for gene read counts and FPKM calculations (fragments per kilobase of transcript per million mapped fragments) [24].
Differentially expressed gene (DEG) analysis
The DESeq R package (1.10.1) was used to screen out the DEGs, with P < 0.05 and log2|Fold Change| ≥ 1 as the cut-off criteria.
Alternative splicing (AS) analysis
The ABLas software was used to define and quantify the alternative splicing events (ASEs) and regulated alternative splicing events (RASEs) between the samples as described before [25,26]. In order to evaluate RNA-binding protein-RASEs, the Student’s t-test was used to evaluate the significance of changes in the ASE ratio. Those events that were significant at the P-value cutoff, corresponding to a 5% error detection rate cutoff, were considered RBP-RASEs.
Functional enrichment analysis
Gene ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed to sort out DEGs’ functional categories with the help of KOBAS 2.0 server [27], followed by the determination of each term’s enrichment using the hypergeometric test and Benjamini-Hochberg FDR controlling procedure.
Statistical analysis
Statistical analyses were performed using R (version 4.0.4) and its corresponding packages. Principal component analysis (PCA) was performed using the R package to display the clustering pattern of samples based on the top two components. The mean ± standard deviation was used to represent measured data, and the constituent ratio was used to indicate counted data. Student’s t tests were performed to determine the significance of differences between two groups; a one-way ANOVA was used to compare the differences between groups, followed by a Bonferroni post hoc test. P < 0.05 indicated significant differences.
Results
Histopathological changes of hippocampus in CP rats after acupuncture treatment
H&E staining results showed that the neuron and glial cells were normal in structure in the control group, with abundant cytoplasm, clear nuclei, and obvious nucleoli, and the hippocampus was normal. There was no significant difference in pathologic changes between the sham and control groups. However, in the model group, brain tissue cells were disorderedly arranged, and some neurons were denaturated and atrophied, with pyknotic or disappeared nuclei, and disappeared nucleoli. The glial cells were atrophied and decreased in some regions, showing hyperplasia, and the hippocampal structure was slightly disordered. Compared to the model group, ZJ_1 (acupuncture group 1), ZJ_2 (acupuncture group 2) and ZJ_3 (acupuncture group 3) groups showed less brain cell disorder, neuronal degeneration, and brain tissue atrophy, and reduced glial cell atrophy and proliferation. The pathologic changes of the acupuncture group (ZJ_1, ZJ_2 and ZJ_3 groups) were better than those of model group. No evident differences were identified among acupuncture subgroups. As shown in Figure 1.
Figure 1.
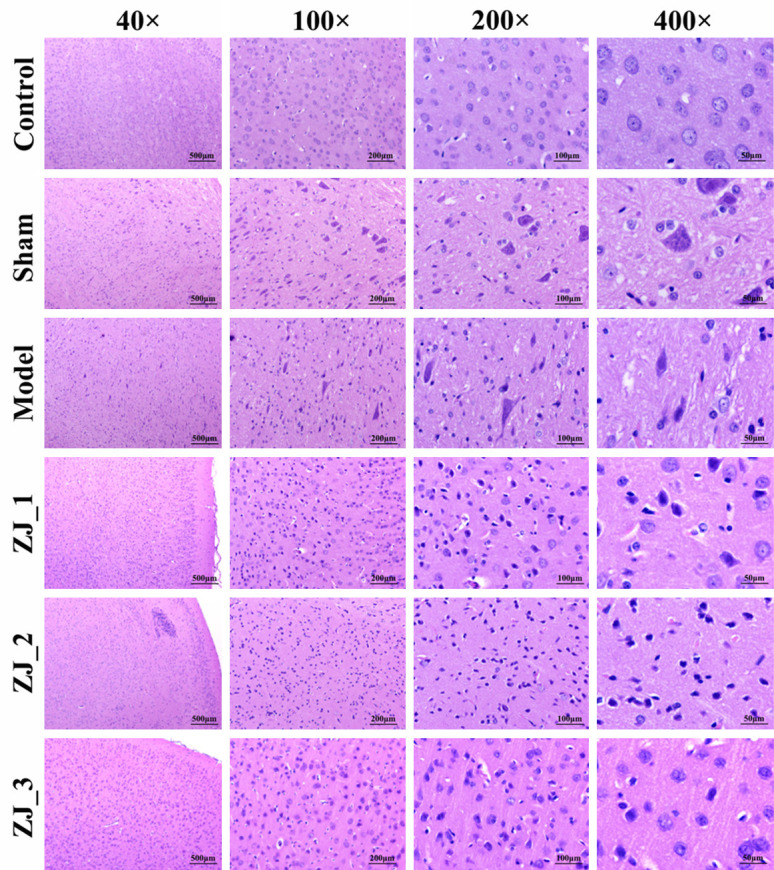
Hematoxylin and eosin (H&E) staining of hippocampal tissue of rats in different groups.
Transcriptome profile analysis of hippocampal tissue in CP rats after acupuncture treatment
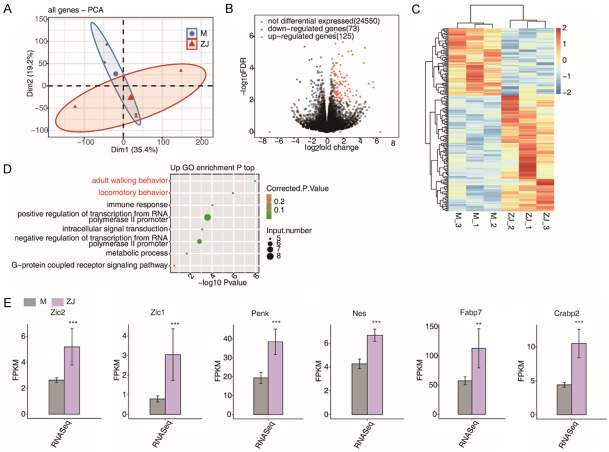
PCA analysis showed that model samples and acupuncture samples were significantly divided into two groups (Figure 2A). In our study, 198 genes with P < 0.01 and |log2FC| > 1 were identified between model and acupuncture samples as DEGs by differential expression analysis, including 73 downregulated genes and 125 upregulated ones (Figure 2B and 2C). GO functional enrichment analysis showed that these DEGs were mainly enriched in walking behavior, and locomotory behavior processes (Figure 2D). Meanwhile, as shown in Figure 2E, the expression of six vital DEGs (Zic1, Zic2, Penk, Fabp7, Crabp2, and Nes) in the acupuncture group were significantly higher than those in the model group.
Figure 2.
Differentially expressed gene expression in model and acupuncture samples. A. Sample correlation principal component analysis (PCA) plot. B. Volcano plot of all differentially expressed genes (DEGs) between acupuncture and model samples. C. Hierarchical clustering heat map displaying the expression patterns of all DEGs between acupuncture and model specimens; D. Bubble plot listing the top ten enriched GO terms (up-regulated DEGs) associated with biological processes (BP); E. Expression of Zic1, Zic2, Penk, Fabp7, Crabp2, Nes and other genes. ***P-value < 0.001; **P-value < 0.01.
Analysis of hippocampal tissue in CP rats after acupuncture treatment
We identified 661 high-confidence RASEs by applying a strict cut-off of P ≤ 0.05 and an AS ratio of ≥ 0.2. A5SS (alternative 5’ splice site) and A3SS (alternative 3’ splice site) were the most frequently reported (Figure 3A and 3B). In addition, 13 of the 185 DEGs were involved in ASEs, suggesting that AS of genes may play a role in the treatment process of acupuncture (Figure 3C). GO analysis demonstrated that all the regulated alternative splicing genes (RASGs) were mainly enriched in DNA template transcriptional regulation, DNA Template transcription negative, DNA template transcription, positive transcriptional modulation of RNA polymeraseIIpromoter, and RNA polymerase II promoter negative transcriptional regulation (Figure 3D). Moreover, we found that the acupuncture treatment regulates AS of Thoc7 and Nrcam (Figure 3E and 3F). Acupuncture treatment also regulates AS of genes, including Emc1, Mef2c, Snrpc1, and Hnrnpul1 (Figure 4A-D).
Figure 3.
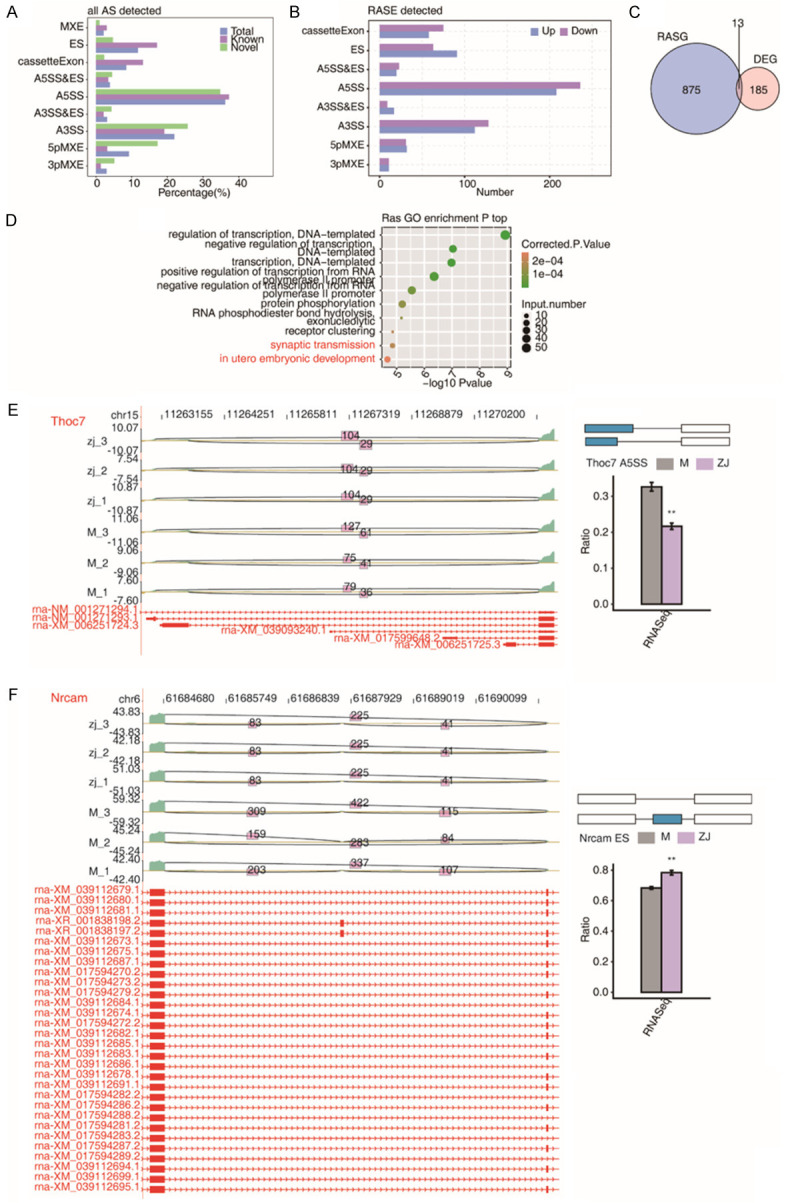
Standard alternative splicing (AS) analysis. A. Alternative splicing event (ASE) distributions in the acupuncture treatment; B. Acupuncture treatment regulated ASEs (RASEs); C. Venn diagram exhibiting overlapped genes between RASGs and DEGs; D. GO analysis of alternative spliced genes (RASGs) shown by bubble plot; E. Acupuncture treatment regulates Thoc7 AS; F. Acupuncture treatment modulates AS of Nrcam. AS: alternative splicing; RASE: regulated alternative splicing event; RASG: regulated alternative splicing gene; DEG: differentially expressed gene.
Figure 4.
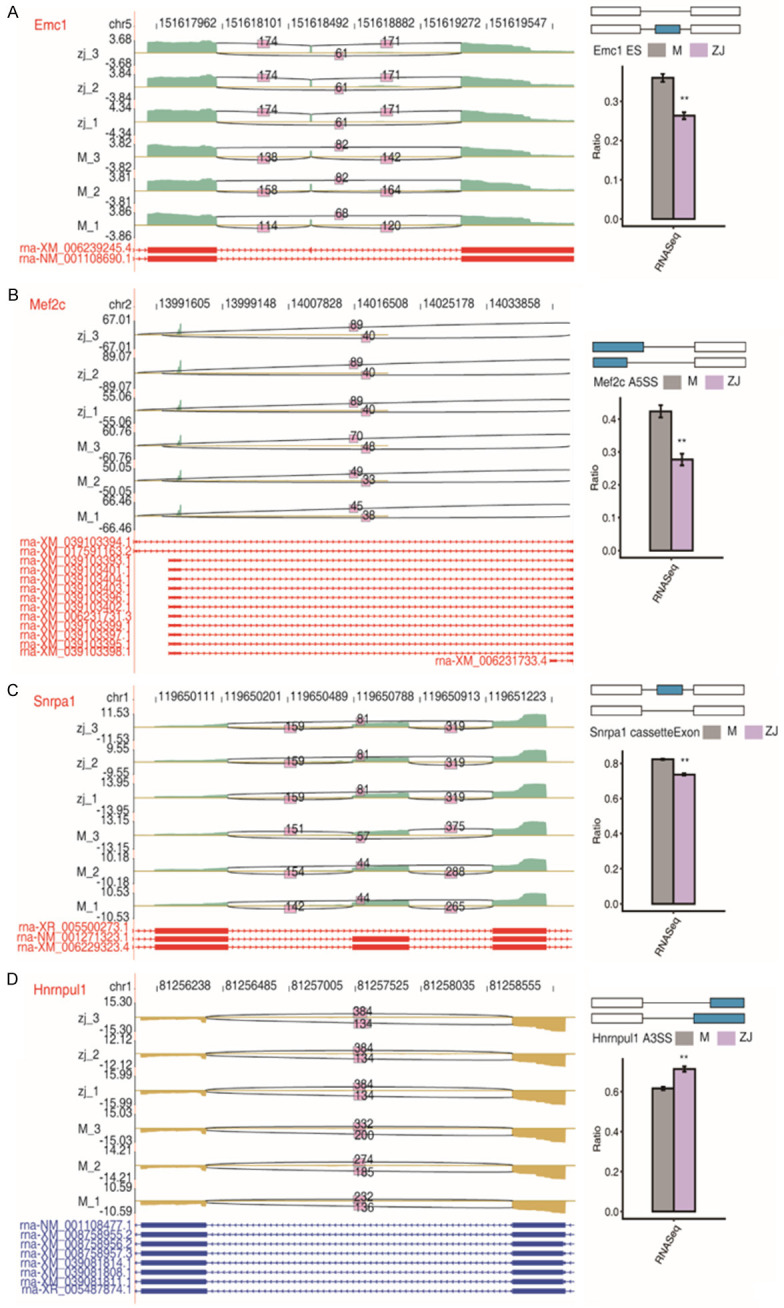
Acupuncture treatment modulates alternative splicing (AS) of genes A. Emc1; B. Mef2c; C. Snrpc1; D. Hnrnpul1.
Co-expression networks between hippocampal tissue-associated transcription factors (TFs) and DEGs in CP rats after acupuncture treatment
Fourteen of the 184 DEGs were TFs (Figure 5A). The heat map of the expression patterns of these 14 TFs in samples are shown in Figure 5B. The number of mRNAs co-expressed with these 14 TFs can be found in Figure 5C. Then, we performed a GO analysis with these differentially expressed TFs, and their enriched functional pathways were found to be signal transduction, immune response, and positive regulation of transcription from RNA polymerase II promoter (Figure 5D).
Figure 5.
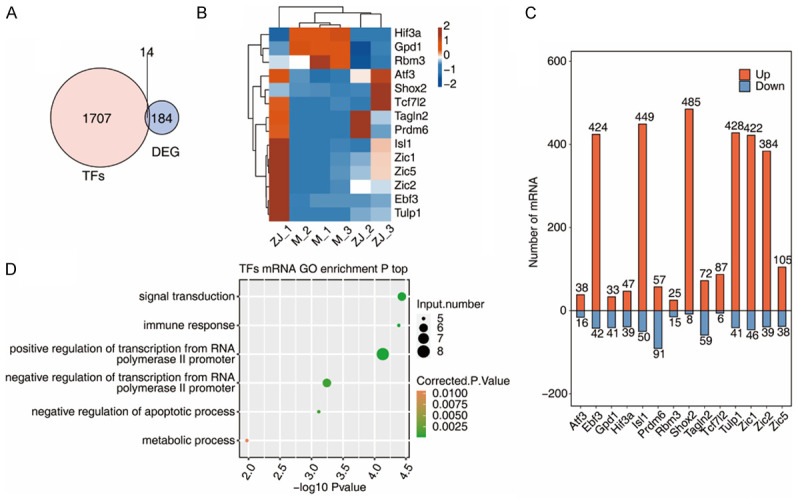
Co-expression networks between transcription factors (TFs) and diffierentially expressed genes (DEGs) associated with hippocampal tissue of CP rats after acupuncture treatment. A. Venn diagram showing the overlapping genes between TFs and DEGs; B. Hierarchical clustering heat map showing the expression levels of differentially expressed TFs; C. Bar plot showing the top ten expression pattern and the number of mRNAs co-expressed with the diffierentially expressed TFs; D. Bubble plot showing enriched GO terms for mRNAs co-expressed with differentially expressed TFs. TF: transcription factor; DEG: differentially expressed gene.
Co-expression networks between hippocampal tissue-associated RASEs of TFs and differentially expressed mRNAs in CP rats after acupuncture treatment
In RASEs of TFs, A5SS and A3SS were the most frequently reported (Figure 6A). The hierarchical clustering heatmap showed the consistent expression patterns of RASEs of TFs in samples (Figure 6B). The network demonstrated a regulatory relationship between the 10 most highly expressed RASEs of TFs and DEGs (Figure 6C). Also, the associated enriched functional pathways were found to be positive regulation of ERK1 and ERK2 cascade and peptidyl-tyrosine phosphorylation by GO analysis (Figure 6D).
Figure 6.
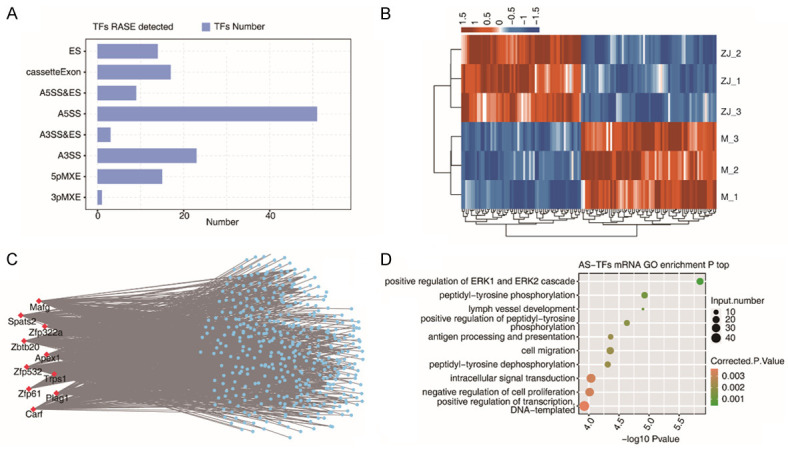
Co-expression networks between RASEs of TFs and differentially expressed mRNAs associated with hippocampal tissue of CP rats after acupuncture treatment. A. Bar plot presenting the RASEs of TFs in the acupuncture treatment; B. Expression levels of RASEs of TFs exhibited by hierarchical clustering heatmap. C. Co-expression networks between RASEs of TFs and DEGs. D. Bubble plot exhibiting enriched GO BP terms for mRNAs co-expressed with RASEs of TFs. TF: transcription factor; AS: alternative splicing; RASE: regulated alternative splicing event; DEG: diffierentially expressed gene.
Discussion
Cerebral palsy (CP), either a congenital or a perinatal disease, is a common brain dysfunction syndrome in children [28]. CP is a lifelong condition attributed to a non-progressive disturbance that occurs in the developing fetal or infant brain. It is the main cause of spasms in children, accounting for 80% [29]. Spasms can lead to muscle longitudinal growth and muscle fiber length problems, muscle volume reduction, size change of motor units, and changes in muscle fiber and neuromotor connection type [30,31]. Because there is no cure, children with CP and their families seek various forms of treatment, especially those that may improve children’s functioning or sensory style [32]. Clinical practice shows that acupuncture and moxibustion have certain advantages in treating CP, and is still widely used due to its unique characteristics and favorable efficacy [33]. With the in-depth and lasting understanding of the pathophysiology of spastic CP, we found that due to damage to the cerebral cortex and related structures, the negative feedback inhibition at the spinal level was basically absent, resulting in an imbalance of the inhibition circuit and the excitation circuit that regulates skeletal muscle contraction, resulting in spasms [14]. However, the mechanism of acupuncture in the treatment of CP is not fully understood.
There is research elucidating gene networks and pathways contributing to CP and assisting prioritisation of genetic variants by examining transcriptomes of clinically heterogeneous CP cases [34]. Meanwhile, studies have used RNA-sequencing technology to identify changes in lncRNAs, mRNAs and circRNAs, in the prefrontal cortex (PFC) of hypoxic-ischemic brain damaged newborn rats after treatment with acupuncture [35]. However, transcriptome analysis of CP models after acupuncture has not been reported. Thus, we investigated genome-wide changes in CP rats after acupuncture treatment by RNA-seq. We identified 198 DEGs between model rats and acupuncture rats, and GO functional enrichment analysis showed that these DEGs were mainly enriched in walking behavior and locomotion behavior processes. This suggests that acupuncture can alter the expression of genes in the genome that mainly regulate motor function. In addition, it indicates that acupuncture may affect the motor function of CP rats.
Alternative splicing (AS) is a mechanism to produce multiple proteins with different functions in a single cell [36]. The significance of selective splicing is evident in highly specialized cells such as neurons. For example, all major neurotransmitter receptors contain alternating splicing subunits, which affects their localization, as well as their ligand binding, signal transduction, and electrophysiological properties [37]. In addition, abnormal splicing of messenger RNAs that encode proteins crucial to the normal function of neurons has been linked to several nervous system diseases [38]. Thus, we also analyzed the ASEs in CP after acupuncture. We found that A5SS and A3SS were the most prevalent in differential ASEs, with 13 overlapped genes identified between RASGs and DEGs. Compared to non-variable splicing genes, variable splicing genes have longer average length, more exons, longer exons, and shorter introns. Non-variable splicing genes are mainly involved in DNA integration, DNA metabolism, nucleic acid metabolism, nervous system, and other biologic processes, while multi-variable splicing genes primarily participate in processes such as biological stress response and external stimulus response [39].
In the present study, 14 TFs were found to be differentially expressed. Through co-expression analysis of the mRNAs co-expressed by these differentially expressed TFs, it was speculated that these TFs could be targeted to modulate the differential expression of their target mRNAs, so as to play a corresponding role in the treatment of CP in young mice by acupuncture.
Furthermore, a large number of TFs were observed to undergo differential variable splicing in this study. By co-expression analysis of the mRNAs of these TFs, it was conjectured that these TFs could produce two different transcripts, and the differences between the translated proteins may regulate the differential expression of the target mRNAs, thus playing a corresponding function in the acupuncture treatment of young CP rats.
Conclusion
The transcription factor-regulated alternative splicing of mRNAs is implicated in the acupuncture treatment of CP, providing profound insight into unveiling the possible pathophysiologic mechanisms of CP with exon skipping, and helping to identify new therapeutic targets.
Acknowledgements
This study was supported by grant from National Natural Science Foundation of China (Grant No. 81960898).
Disclosure of conflict of interest
None.
References
- 1.Gulati S, Sondhi V. Cerebral palsy: an overview. Indian J Pediatr. 2018;85:1006–1016. doi: 10.1007/s12098-017-2475-1. [DOI] [PubMed] [Google Scholar]
- 2.Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, Cioni G, Damiano D, Darrah J, Eliasson AC, de Vries LS, Einspieler C, Fahey M, Fehlings D, Ferriero DM, Fetters L, Fiori S, Forssberg H, Gordon AM, Greaves S, Guzzetta A, Hadders-Algra M, Harbourne R, Kakooza-Mwesige A, Karlsson P, Krumlinde-Sundholm L, Latal B, Loughran-Fowlds A, Maitre N, McIntyre S, Noritz G, Pennington L, Romeo DM, Shepherd R, Spittle AJ, Thornton M, Valentine J, Walker K, White R, Badawi N. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. 2017;171:897–907. doi: 10.1001/jamapediatrics.2017.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel DR, Neelakantan M, Pandher K, Merrick J. Cerebral palsy in children: a clinical overview. Transl Pediatr. 2020;9:S125–S135. doi: 10.21037/tp.2020.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inamdar K, Molinini RM, Panibatla ST, Chow JC, Dusing SC. Physical therapy interventions to improve sitting ability in children with or at-risk for cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2021;63:396–406. doi: 10.1111/dmcn.14772. [DOI] [PubMed] [Google Scholar]
- 5.Michael-Asalu A, Taylor G, Campbell H, Lelea LL, Kirby RS. Cerebral palsy: diagnosis, epidemiology, genetics, and clinical update. Adv Pediatr. 2019;66:189–208. doi: 10.1016/j.yapd.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz Ramírez J, Pérez de la Cruz S. Therapeutic effects of kinesio taping in children with cerebral palsy: a systematic review. Arch Argent Pediatr. 2017;115:e356–e361. doi: 10.5546/aap.2017.eng.e356. [DOI] [PubMed] [Google Scholar]
- 7.Nicolini-Panisson RD, Tedesco AP, Folle MR, Donadio MVF. Selective dorsal rhizotomy in cerebral palsy: selection criteria and postoperative physical therapy protocols. Rev Paul Pediatr. 2018;36:9. doi: 10.1590/1984-0462/;2018;36;1;00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan C, Fahey M, Roy B, Novak I. Diagnosing cerebral palsy in full-term infants. J Paediatr Child Health. 2018;54:1159–1164. doi: 10.1111/jpc.14177. [DOI] [PubMed] [Google Scholar]
- 9.Cho JW, Jung SY, Kim DY, Chung YR, Choi HH, Jeon JW, Han JH. PI3K-Akt-Wnt pathway is implicated in exercise-induced improvement of short-term memory in cerebral palsy rats. Int Neurourol J. 2018;22:S156–164. doi: 10.5213/inj.1836224.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu C, Zheng Y, Lin K, Wang H, Chen T, Li L, Huang J, Lin W, Zhu J, Li P, Fu X, Lin Z. Neuroprotective effect of apigenin against hypoxic-ischemic brain injury in neonatal rats via activation of the PI3K/Akt/Nrf2 signaling pathway. Food Funct. 2021;12:2270–2281. doi: 10.1039/d0fo02555k. [DOI] [PubMed] [Google Scholar]
- 11.Li EY, Zhao PJ, Jian J, Yin BQ, Sun ZY, Xu CX, Tang YC, Wu H. Vitamin B1 and B12 mitigates neuron apoptosis in cerebral palsy by augmenting BDNF expression through MALAT1/miR-1 axis. Cell Cycle. 2019;18:2849–2859. doi: 10.1080/15384101.2019.1638190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao HH, Yen HR, Muo CH, Lee YC, Wu MY, Chou LW, Sun MF, Chang TT. Complementary traditional Chinese medicine use in Children with cerebral palsy: a nationwide retrospective cohort study in Taiwan. BMC Complement Altern Med. 2017;17:155. doi: 10.1186/s12906-017-1668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu L, Zhang XG, Zhong LL, Chen ZX, Li Y, Zheng GQ, Bian ZX. Acupuncture for neurogenesis in experimental ischemic stroke: a systematic review and meta-analysis. Sci Rep. 2016;6:19521. doi: 10.1038/srep19521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Jin Z, Li K, Lu ZL, Wong V, Han TL, Zheng H, Caspi O, Liu G, Zeng YW, Zou LP. Effect of acupuncture on the brain in children with spastic cerebral palsy using functional neuroimaging (FMRI) J Child Neurol. 2008;23:1267–1274. doi: 10.1177/0883073808318049. [DOI] [PubMed] [Google Scholar]
- 15.Yang C, Hao Z, Zhang LL, Guo Q. Efficacy and safety of acupuncture in children: an overview of systematic reviews. Pediatr Res. 2015;78:112–119. doi: 10.1038/pr.2015.91. [DOI] [PubMed] [Google Scholar]
- 16.van Eyk CL, Corbett MA, Maclennan AH. The emerging genetic landscape of cerebral palsy. Handb Clin Neurol. 2018;147:331–342. doi: 10.1016/B978-0-444-63233-3.00022-1. [DOI] [PubMed] [Google Scholar]
- 17.Feather-Schussler DN, Ferguson TS. A battery of motor tests in a neonatal mouse model of cerebral palsy. J Vis Exp. 2016;3:53569. doi: 10.3791/53569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He X, Liu Z, Pang Y, Xu W, Zhao L, Li H. Downregulation of transcription factor TCTP elevates microRNA-200a expression to restrain Myt1L expression, thereby improving neurobehavior and oxidative stress injury in cerebral palsy rats. Cell Cycle. 2020;19:855–869. doi: 10.1080/15384101.2020.1717044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng W, Fan C, Fang Y, Zhao Y, Wei Y, Li M, Teng J. Role of XIAP gene overexpressed bone marrow mesenchymal stem cells in the treatment of cerebral injury in rats with cerebral palsy. Cancer Cell Int. 2019;19:273. doi: 10.1186/s12935-019-0988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang IK, Chung JY, Yoo DY, Yi SS, Youn HY, Seong JK, Yoon YS. Comparing the effects of acupuncture and electroacupuncture at Zusanli and Baihui on cell proliferation and neuroblast differentiation in the rat hippocampus. J Vet Med Sci. 2010;72:279–284. doi: 10.1292/jvms.09-0374. [DOI] [PubMed] [Google Scholar]
- 21.Hou X, Zhang R, Lv H, Cai X, Xie G, Song X. Acupuncture at Baihui and Dazhui reduces brain cell apoptosis in heroin readdicts. Neural Regen Res. 2014;9:164–170. doi: 10.4103/1673-5374.125345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding Y, Yan Q, Ruan JW, Zhang YQ, Li WJ, Zhang YJ, Li Y, Dong H, Zeng YS. Electro-acupuncture promotes survival, differentiation of the bone marrow mesenchymal stem cells as well as functional recovery in the spinal cord-transected rats. BMC Neurosci. 2009;10:35. doi: 10.1186/1471-2202-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin L, Li G, Yu D, Huang W, Cheng C, Liao S, Wu Q, Zhang Y. Transcriptome analysis reveals the complexity of alternative splicing regulation in the fungus Verticillium dahliae. BMC Genomics. 2017;18:130. doi: 10.1186/s12864-017-3507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia H, Chen D, Wu Q, Wu G, Zhou Y, Zhang Y, Zhang L. CELF1 preferentially binds to exon-intron boundary and regulates alternative splicing in HeLa cells. Biochim Biophys Acta Gene Regul Mech. 2017;1860:911–921. doi: 10.1016/j.bbagrm.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, Kong L, Gao G, Li CY, Wei L. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39:W316–322. doi: 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appleton RE, Gupta R. Cerebral palsy: not always what it seems. Arch Dis Child. 2019;104:809–814. doi: 10.1136/archdischild-2018-315633. [DOI] [PubMed] [Google Scholar]
- 29.Dabbous OA, Mostafa YM, El Noamany HA, El Shennawy SA, El Bagoury MA. Laser acupuncture as an adjunctive therapy for spastic cerebral palsy in children. Lasers Med Sci. 2016;31:1061–1067. doi: 10.1007/s10103-016-1951-6. [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, Park JW, Nam K. Effect of extracorporeal shockwave therapy on muscle spasticity in patients with cerebral palsy: meta-analysis and systematic review. Eur J Phys Rehabil Med. 2019;55:761–771. doi: 10.23736/S1973-9087.19.05888-X. [DOI] [PubMed] [Google Scholar]
- 31.Pierce SR, Prosser LA, Lee SC, Lauer RT. The relationship between spasticity and muscle volume of the knee extensors in children with cerebral palsy. Pediatr Phys Ther. 2012;24:177–181. doi: 10.1097/PEP.0b013e31824cc0a9. discussion 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi YC, Xiao XJ, Duan RS, Yue YH, Zhang XL, Li JT, Li YZ. Effect of acupuncture on inflammatory cytokines expression of spastic cerebral palsy rats. Asian Pac J Trop Med. 2014;7:492–495. doi: 10.1016/S1995-7645(14)60081-X. [DOI] [PubMed] [Google Scholar]
- 33.Sun JG, Ko CH, Wong V, Sun XR. Randomised control trial of tongue acupuncture versus sham acupuncture in improving functional outcome in cerebral palsy. J Neurol Neurosurg Psychiatry. 2004;75:1054–1057. doi: 10.1136/jnnp.2003.021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Eyk CL, Corbett MA, Gardner A, van Bon BW, Broadbent JL, Harper K, MacLennan AH, Gecz J. Analysis of 182 cerebral palsy transcriptomes points to dysregulation of trophic signalling pathways and overlap with autism. Transl Psychiatry. 2018;8:88. doi: 10.1038/s41398-018-0136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang X, Qin W, Wu J, Xiao J, Yuan Q. Transcriptome analysis and differentially expressed gene screening for hypoxic-ischemic brain damage in rats treated with acupuncture. 2021 [Google Scholar]
- 36.Kelemen O, Convertini P, Zhang Z, Wen Y, Shen M, Falaleeva M, Stamm S. Function of alternative splicing. Gene. 2013;514:1–30. doi: 10.1016/j.gene.2012.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dredge BK, Polydorides AD, Darnell RB. The splice of life: alternative splicing and neurological disease. Nat Rev Neurosci. 2001;2:43–50. doi: 10.1038/35049061. [DOI] [PubMed] [Google Scholar]
- 38.Abbott JA, Francklyn CS, Robey-Bond SM. Transfer RNA and human disease. Front Genet. 2014;5:158. doi: 10.3389/fgene.2014.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Yuan J, Zhang X, Liu C, Xiang J, Li F. Genome-wide analysis of alternative splicing provides insights into stress response of the pacific white shrimp litopenaeus vanname. Front Genet. 2019;10:845. doi: 10.3389/fgene.2019.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]