Abstract
Introduction:
While metastases of malignant thymomas have been shown, type A thymomas are often treated as benign. Type A thymomas often have excellent response to treatment, low recurrence rate, and a small malignant potential. To date, there have been no reports of type A thymomas with spinal metastases.
Case Report:
A 66-year-old female with a type A thymoma metastatic to the T7 and T8 vertebral bodies and brain, with associated pathologic burst fracture, collapse of T7, and significant focal kyphosis . The patient underwent successful T7–T8 posterior corpectomy and T4–T11 posterior spinal fusion. At 2 years of follow-up, she was ambulating without assistive devices and completed spinal radiation and initial chemotherapy.
Conclusion:
Metastatic type A thymoma is a rare phenomenon. While traditionally thought to have low recurrence rates and overall excellent survival rates, our case suggests that the biologic malignant potential of a type A thymoma may not be fully understood.
Keywords: Thymoma, spine, metastatic, cranial, type A
Learning Point of the Article:
Type A thymomas, while rarely metastatic, are capable to spreading to the spine and can be treated with mechanical stabilization if needed while undergoing systemic treatment.
Introduction
Malignant thymoma can have extrathoracic metastases in up to 15% of patients [1]. Approximately one-third of patients with thymoma also have myasthenia gravis, which portends to a poorer prognosis [2]. The World Health Organization (WHO) histologic system classifies thymomas based on the shape of neoplastic epithelial cells from type A to type B3. Prognosis worsens with letter progression [3]. While thymic tumors have a 62–100% response rate to radiation or chemotherapy, surgery is sometimes indicated [4]. We present a rare type A thymoma with spinal and cranial metastases requiring urgent surgical intervention.
Case Report
A 66-year-old female presented with a several weeks history of back pain following mechanical injury. Radiographs (Fig. 1a and b) and follow-up computed tomography (CT)-guided biopsy (Fig. 2a-e) showed a new anterior mediastinal mass and metastatic bony lesions to the T6-T8 vertebrae.
Figure 1.
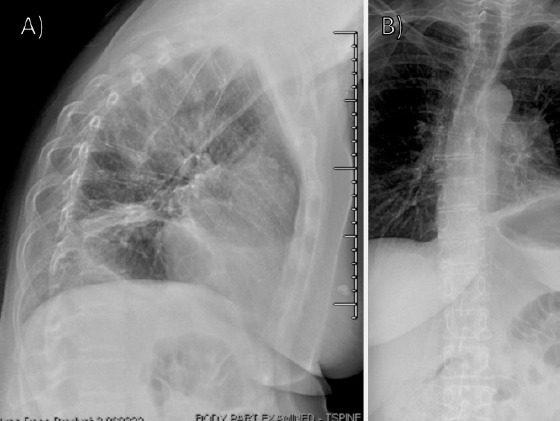
Initial radiographs (a) lateral and (b) anterior posterior radiographs of the thoracic and lumbar spine obtained at initial presentation, concerning for compression fractures involving the mid-thoracic spine.
Figure 2.
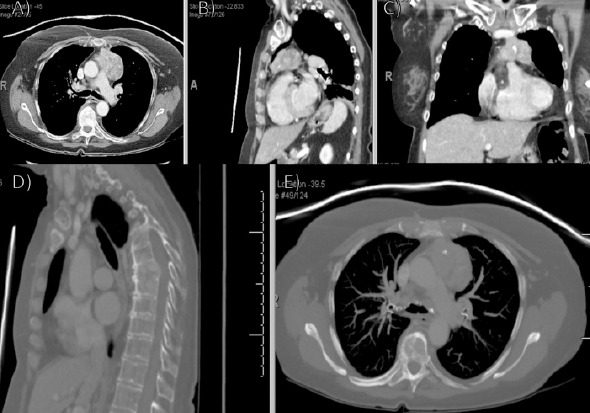
Initial computed tomography (CT) (a) axial, (b) sagittal, and (c) coronal follow-up CT imaging demonstrating new anterior mediastinal mass with lymphadenopathy; (d) sagittal and (e) axial CT imaging demonstrating metastatic bony lesions involving the T6–8 vertebrae.
At her 1-month visit, she endorsed left-sided chest pain with numbness in her left arm and was sent to the emergency department. Imaging revealed an extra-axial mass within the left posterior fossa and a left anterior mediastinal mass measuring 5.7 × 3.5 cm with internal calcifications. Also seen was a T7 compression deformity and associated soft-tissue mass eroding through the T7 body, intervertebral disk, and superior endplate of the T8 vertebral body with significant retropulsion and canal and left neural foraminal stenosis (Fig. 3a and b). Magnetic resonance imaging (MRI) and hospitalization were recommended; however, she left against medical advice.
Figure 3.
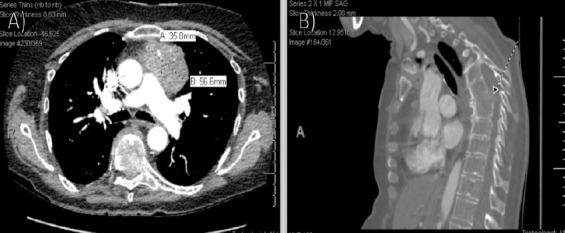
Repeat computed tomography (CT) demonstrating mediastinal mass repeat (a) axial and (b) sagittal CT imaging demonstrating an irregular left anterior mediastinal mass measuring 5.7×3.5 cm with internal calcifications and left basilar compressive atelectasis. Also noted is a wedge compression deformity of the T7 vertebral body with retropulsion and subsequent spinal canal and left neural foraminal stenosis. There is also an associated soft-tissue mass eroding through the vertebral body and involving the intervertebral disk and superior endplate of the T8 vertebral body.
The original anterior mediastinal tumor (Fig. 4) core-needle biopsy showed a sold spindle cell epithelial neoplasm consistent with the WHO type A thymoma. The degree of anaplasia was mild with proliferative index as measured by Ki-67 of only 2%. Lymphocytic infiltration was scant. The lesion stained positive with cytokeratin AE1/3, p63, and PAX8. The lymphocytic component stained positive with LCA and CD3 and negative with TdT. The metastatic lesion to the vertebral body T8 had similar morphology and was composed of similar spindle cells (Fig. 5). Occasional mitosis was noted and the proliferative index was overall low but higher than the limited material on the mediastinal biopsy, near 10% (Fig. 6). The special stains showed positive results with AE1/3 (Fig. 7), EMA, p16, PAX8 (Fig. 8), BCL-2, and vimentin and negative results with actin, desmin, CD117, s100, cdx2, chromogranin, synaptophysin, HMB-45, melan-A, Sox10, MITF, and reticulin.
Figure 4.
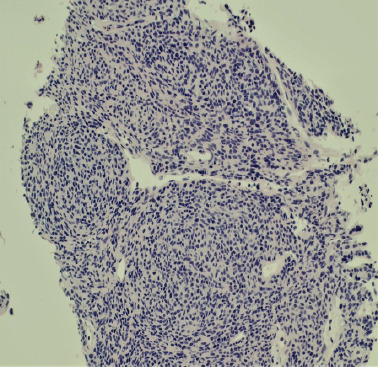
(H&E; ×200): The original anterior mediastinal biopsy showing type A thymoma. The similarity with the metastasis (Fig. 5) is apparent.
Figure 5.
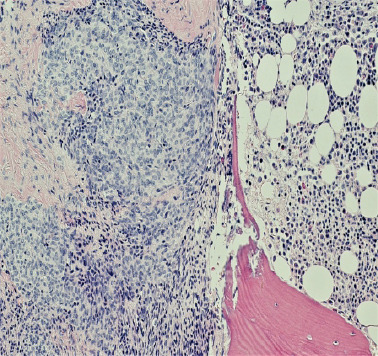
(H&E; ×200): Type A thymic neoplasm/carcinoma with metastasis to the bone at the left side of image with normal bone marrow on the right side.
Figure 6.
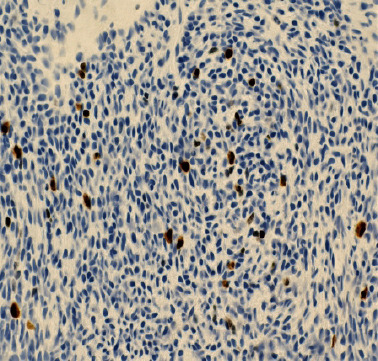
(Mib-1 immunoperoxidase stain; ×400): Shows approximately 1–10% positive nuclei indicating relatively low proliferative activity.
Figure 7.
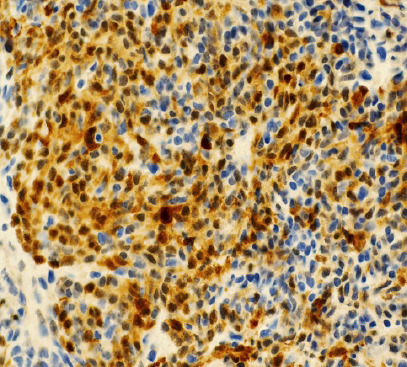
(Cytokeratin AE1/3 immunopeoroxidase stain; ×400): Strong cytoplasmic positive stain indicating epithelial nature of neoplasm.
Figure 8.
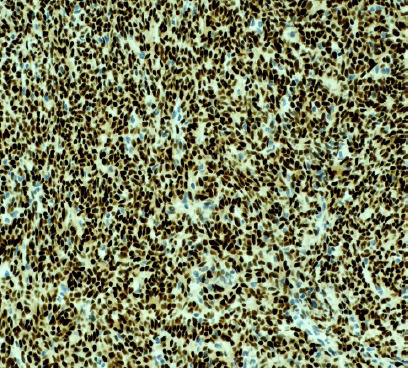
(PAX8 immuoperoxidase; ×200): Strong uniform positive nuclear staining characteristic of thymic epithelial neoplasms.
She endorsed persistent symptoms and was again sent to the emergency department. Repeat CT imaging showed a lytic lesion within the T7 and T8 vertebral bodies, with a pathologic burst fracture and near collapse of T7. There was approximately 40° of focal kyphosis, bony retropulsion, and soft-tissue tumor within the canal. Repeat MRI found no other areas of cord compression (Fig. 9a and b). Brain MRI showed two intramedullary osseous metastatic lesions (Fig. 9c and e). T7 and T8 corpectomy with posterior instrumentation and fusion from T4-T11 was performed (Fig. 10a and b). She was discharged on post-operative day 7.
Figure 9.
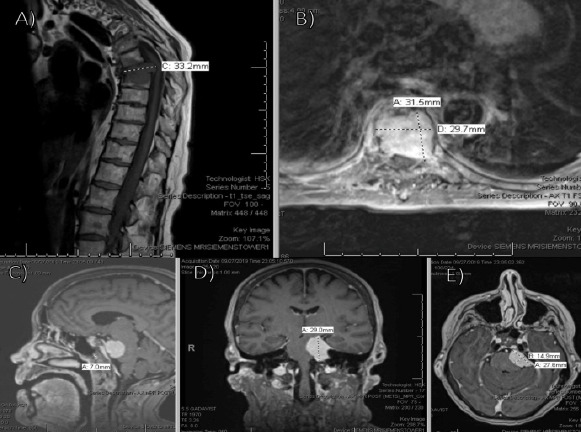
Pre-operative magnetic resonance imaging (MRI) with metastatic lesions (a) sagittal and (b) axial MRI of the thoracic spine demonstrating no additional areas of significant cord compression; (c) sagittal, (d) coronal, and (e) axial MRI of the brain demonstrating enhancing intramedullary subcentimeter lesions within the clivus, concerning for intramedullary osseous metastatic lesions.
Figure 10.
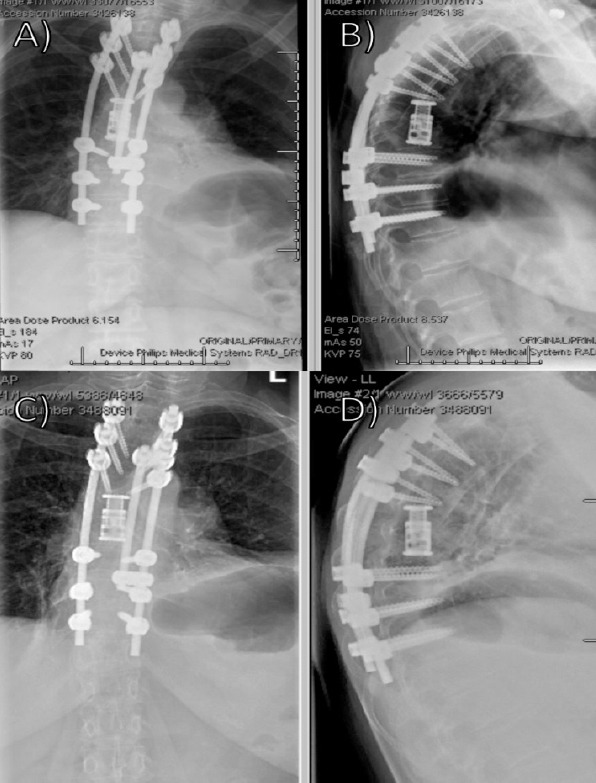
Post-operative radiographs following thoracic corpectomy and fusion (a) Anterior posterior (AP) and (b) lateral initial post-operative radiographs status post T4–T11 posterior thoracic fusion with T7 and T8 corpectomy with cage placement; (c) AP and (d) lateral radiographs 3.5 months post-operative demonstrating intact hardware without evidence of loosening, failure, subsidence, or worsening kyphosis.
Plans were made for surveillance of her brain lesions over the next 4 months. Intraoperative pathology was consistent with metastatic thymic carcinoma and small spindle cell type. At 3.5 months post-operative, she was ambulating without any assistive devices, completed radiation therapy, and had plans for chemotherapy (Fig. 10 and d). At 2 year, she was ambulating without issue and had completed chemotherapy without signs of recurrent metastases.
Discussion
Metastatic spinal thymoma is a rare phenomenon, with only approximately 30 documented cases in the literature [4]. In their review of 100 thymic epithelial tumors over a 28-year period at a single center, Kondo et al., showed no cases of type A thymomas invading neighboring organs or any association between type A thymomas and myasthenia gravis [3]. All patients with type A thymomas underwent total resection without adjuvant therapy, and no recurrence was seen at latest follow-up. The 5- and 10-year survival rates of type A pathology were 100%. The authors concluded that type A tumors could be treated as benign tumors. In their review of a worldwide database, however, Weis et al. found that approximately 1% of 443 patients with type A thymoma had stage IVb disease (Masaoka’s et al., staging), suggesting biologically malignant potential [5, 6]. Achey et al. found three cases with thymic spinal metastases in their review of patients presenting to their institution over a 17-year period [4]. Metastasis presented with cord compression/cauda equina symptoms at 2–8 years after initial cancer diagnosis. Their systematic review identified an additional 25 cases of spinal metastatic thymic cancer from 2000 to 2017 [4]. The median age at presentation was 51 years with a median time to spinal metastases of 5 years after initial presentation. Most published cases report on type B2/B3 thymomas and type C thymic carcinomas, with the thoracic spine as the most common site for spinal metastases.
In the aforementioned review, most patients underwent surgery followed by adjuvant chemotherapy, radiation, or both [4]. Completeness of primary tumor resection and WHO histologic classification influences survival rates [7, 8, 9]. The current treatment trends favor managing spinal metastases through surgical decompression rather than medical therapy alone, due to improved post-operative ambulatory status and significant reductions in steroid and opioid use in those treated surgically and with radiation over radiation alone [10].
Conclusion
The present study reports on a patient with type A thymoma metastatic to the thoracic spine and cranium who underwent T7 and T8 corpectomy with T4-11 posterior spinal fusion. These findings highlight the malignant potential of type A thymomas.
Clinical Message.
While the malignant potential of thymomas has been previously shown, type A thymomas are often thought to be benign and are managed as such. In addition, although other types of thymomas have been shown to metastasize to the spine, type A thymomas have not been shown previously to have this potential. Our case demonstrates that type A thymomas are capable of spinal and cranial metastases. Clinicians should be aware of the potential for type A thymomas to metastasize in this manner while treating patients with this pathology.
Biography




Footnotes
Conflict of Interest: Nil
Source of Support: Nil
Consent: The authors confirm that informed consent was obtained from the patient for publication of this case report
References
- 1.McLennan MK. Case report 657:Malignant epithelial thymoma with osteoplastic metastases. Skeletal Radiol. 1991;20:141–4. doi: 10.1007/BF00193830. [DOI] [PubMed] [Google Scholar]
- 2.Akamatsu H, Tsukuura T, Matsunaga H, Suzuki A. Study of combination therapy for thymoma:A case of stage IV which presented as total spinal block caused by epidural metastasis and which preoperative combination therapy was effective for minimizing the tumor. Kyobu Geka. 1993;46:1156–60. [PubMed] [Google Scholar]
- 3.Kondo K, Yoshizawa K, Tsuyuguchi M, Kimura S, Sumitomo M, Morita J, et al. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg. 2004;77:1183–8. doi: 10.1016/j.athoracsur.2003.07.042. [DOI] [PubMed] [Google Scholar]
- 4.Achey RL, Lee BS, Sundar S, Benzel EC, Krishnaney AA. Rare thymoma metastases to the Spine:Case reports and review of the literature. World Neurosurg. 2018;110:423–31. doi: 10.1016/j.wneu.2017.11.161. [DOI] [PubMed] [Google Scholar]
- 5.Weis CA, Yao X, Deng Y, Detterbeck FC, Marino M, Nicholson AG, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol. 2015;10:367–72. doi: 10.1097/JTO.0000000000000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–92. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 7.Regnard JF, Magdeleinat P, Dromer C, Dulmet E, De Montpreville V, Levi JF, et al. Prognostic factors and long-term results after thymoma resection:A series of 307 patients. J Thorac Cardiovasc Surg. 1996;112:376–84. doi: 10.1016/S0022-5223(96)70265-9. [DOI] [PubMed] [Google Scholar]
- 8.Eng TY, Fuller CD, Jagirdar J, Bains Y, Thomas CR., Jr Thymic carcinoma:State of the art review. Int J Radiat Oncol Biol Phys. 2004;59:654–4. doi: 10.1016/j.ijrobp.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Sperling B, Marschall J, Kennedy R, Pahwa P, Chibbar R. Thymoma:A review of the clinical and pathological findings in 65 cases. Can J Surg. 2003;46:37–46. [PMC free article] [PubMed] [Google Scholar]
- 10.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer:A randomised trial. Lancet. 2005;366:643–8. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]


