Abstract
Background
Data on the association between the development of autoimmune diseases and COVID-19 vaccination are limited.
Objective
To investigate the incidence and risk of autoimmune connective tissue disorders following mRNA-based COVID-19 vaccination.
Methods
This nationwide population-based study was conducted in South Korea. Individuals who received vaccination between September 8, 2020-December 31, 2021, were identified. Historical prepandemic controls were matched for age and sex in 1:1 ratio. The incidence rate and risk of disease outcomes were compared.
Results
A total of 3,838,120 vaccinated individuals and 3,834,804 controls without evidence of COVID-19 were included. The risk of alopecia areata, alopecia totalis, primary cicatricial alopecia, psoriasis, vitiligo, anti-neutrophil cytoplasmic antibody-associated vasculitis, sarcoidosis, Behcet disease, Crohn disease, ulcerative colitis, rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, Sjogren syndrome, ankylosing spondylitis, dermato/polymyositis, and bullous pemphigoid was not significantly higher in vaccinated individuals than in controls. The risk was comparable according to age, sex, type of mRNA-based vaccine, and cross-vaccination status.
Limitations
Possible selection bias and residual confounders.
Conclusion
These findings suggest that most autoimmune connective tissue disorders are not associated with a significant increase in risk. However, caution is necessary when interpreting results for rare outcomes due to limited statistical power.
Key words: autoimmune disease, connective tissue disease, COVID-19, epidemiology, mRNA, risk, skin disease, vaccination
Capsule Summary.
-
•
The risk of autoimmune connective tissue diseases after mRNA-based COVID-19 vaccination is not well elucidated.
-
•
This nationwide study found that most autoimmune connective tissue diseases were not significantly increased after mRNA-based COVID-19 vaccination. However, limited statistical power prevented detection of potential risks for some rare outcomes. Nevertheless, results suggest any existing risk is not large. These findings could aid in the evaluation and management of autoimmune manifestations following vaccination for COVID-19.
Introduction
COVID-19 vaccines have been introduced to reduce the impact of corona virus 2 (SARS-CoV-2) infections worldwide, preventing 90% of hospitalizations and deaths and 40% to 65% of symptomatic illnesses.1 The 2 most common COVID-19 vaccine platforms include mRNA and adenovirus vector vaccines based on whether they deliver the genetic material mRNA or spike protein using adenoviruses as vectors. Multiple clinical trials have shown that mRNA-based COVID-19 vaccines are effective and have a tolerable safety profile2 , 3; however, real-world data regarding the safety of these vaccines are lacking.
As vaccination programs have been conducted worldwide, new-onset autoimmune manifestations following COVID-19 vaccination have been reported in several studies.4, 5, 6, 7 Previous studies have suggested that molecular mimicry between vaccines and their adjuvants with self-antigens can perturb self-tolerance and consequently lead to the production of autoantibodies and autoimmune response.8 However, the association between COVID-19 vaccines and the risk of autoimmune connective tissue disorders remains unclear. Therefore, we investigated the risk of autoimmune connective tissue disorders associated with mRNA-based COVID-19 vaccination.
Methods
Data source
We performed a nationwide population-based cohort study using data from the Korea Disease Control and Prevention Agency-COVID-19-National Health Insurance Service (NHIS) cohort. The COVID-19 vaccination registry is managed by the Korean government and provides information on the date, type, and dose of COVID-19 vaccination for all individuals vaccinated in Korea. Korea has a single health care insurance system that covers over 99% of the entire Korean population; hence, the NHIS database provides comprehensive information on socioeconomic status, inpatient and outpatient care, diagnoses of diseases, procedures, and prescriptions of enrolled patients.9
Study design
Our database included approximately 20% of all individuals in Korea. We first identified all individuals who had received at least 1 dose of the mRNA-based COVID-19 vaccination (BNT162b2, Pfizer-BioNTech; mRNA-1273, Moderna) before December 31, 2021, as the primary cohort. Individuals with any evidence of SARS-CoV-2 infection (confirmed by polymerase chain reaction) before December 31, 2021, were excluded from the study. The vaccination cohort was established by extracting 50% of the individuals from the primary cohort. The date of the first dose of the mRNA-based COVID-19 vaccination served as the study index date for the vaccination cohort. For comparison, as the COVID-19 vaccination was conducted nationwide, utilizing individuals who had never received COVID-19 vaccination as controls would be more likely to have a higher risk of selection bias. Therefore, we used historical controls that were observed over the same period of time, which was shifted back by 1 year. A historical control cohort was established, with the remaining 50% of individuals not selected for the vaccination cohort. To ensure that the 2 cohorts had a similar observational period, we randomly assigned the study index date for the historical controls based on the distribution of the study index date of the vaccination group but with a subtraction of 1 year (365 days). The study population was followed up from the study index date until disease diagnosis, emigration, death, or the end of the study period. Observations for the vaccination group ceased on December 31, 2021, and those for the historical cohorts ceased on December 21, 2020.
Outcomes
The incidence and risk of autoimmune and autoinflammatory disease outcomes were assessed during follow-up in patients without history of each outcome before the study index date. The occurrence of the outcome disease was ascertained based on at least 3 medical visits using the corresponding International Classification of Diseases, tenth revision (ICD-10) codes as diagnostic codes. To validate our cohort and analyses, the positive control outcomes of certain diseases that have been reported to be strongly associated with COVID-19 vaccination and negative outcome controls, which are less likely to be associated with COVID-19 vaccination, were set and examined.10 The outcomes and the corresponding ICD-10 codes are summarized in Supplementary Table I, available via Mendeley at https://doi.org/10.17632/4kzy8v78bm.1.
Covariates control
Demographics, socioeconomic status, and comorbidity profiles of the study population were obtained from the NHIS database. Although both the vaccination and historical cohorts were constructed from the primary cohort, there could be considerable differences in baseline characteristics that may be associated with the occurrence of disease outcomes. Therefore, 18 covariates, including demographic, socioeconomic, and comorbidity factors, were balanced using inverse probability weighting. The predefined covariates are listed in Table I .
Table I.
Demographic and health characteristics of the COVID-19 vaccination cohort and the historical control cohort before and after the inverse probability treatment weighting
| Preweighting |
Postweighting |
|||||
|---|---|---|---|---|---|---|
| COVID-19 vaccination (N = 3,838,120) | Historical control (N = 3,834,804) | SMD | COVID-19 vaccination, mean or (%) | Historical control, mean or (%) | SMD | |
| Age, mean, ± SD, y | 45.7 ± 18.7 | 44.8 ± 18.7 | 0.054 | 45.3 | 45.3 | 0.000 |
| <20, n (%) | 307,952 (8.0) | 355,898 (9.3) | (8.3) | (8.9) | ||
| 20-39, n (%) | 1,212,206 (31.6) | 1,233,807 (32.2) | (32.4) | (31.4) | ||
| 40-59, n (%) | 1,317,711 (34.3) | 1,312,149 (34.2) | (34.2) | (34.4) | ||
| >60, n (%) | 1,000,251 (26.1) | 932,950 (24.3) | (25.1) | (25.3) | ||
| Sex, n (%) | 0.000 | 0.000 | ||||
| Male | 1,978,161 (51.5) | 1,975,860 (51.5) | (51.4) | (51.4) | ||
| Female | 1,859,959 (48.5) | 1,858,944 (48.5) | (48.6) | (48.6) | ||
| Insurance type, n (%) | 0.000 | 0.000 | ||||
| Standard | 3,686,686 (96.1) | 3,683,382 (96.1) | (96.0) | (96.0) | ||
| Medicaid | 151,434 (3.9) | 151,422 (3.9) | (4.0) | (4.0) | ||
| Income level, n (%) | 0.000 | 0.000 | ||||
| Highest | 1,202,331 (31.3) | 1,203,161 (31.4) | (31.5) | (31.6) | ||
| Higher | 1,066,344 (27.8) | 1,063,166 (27.7) | (28.0) | (27.8) | ||
| Lower | 931,261 (24.3) | 931,833 (24.3) | (24.5) | (24.4) | ||
| Lowest | 614,555 (16.0) | 613,542 (16.0) | (16.0) | (16.2) | ||
| Location of residence, n (%) | 0.000 | 0.000 | ||||
| Metropolitan area | 1,703,487 (44.4) | 1,702,755 (44.4) | (44.5) | (44.5) | ||
| Rural area | 2,134,633 (55.6) | 2,132,049 (55.6) | (55.5) | (55.5) | ||
| Underlying disease, n (%) | ||||||
| Hypertension | 873,910 (22.8) | 809,426 (21.1) | 0.040 | (22.0) | (22.0) | 0.000 |
| Diabetes mellitus | 480,764 (12.5) | 427,834 (11.2) | 0.042 | (11.9) | (11.9) | 0.000 |
| Dyslipidaemia | 1,170,293 (30.5) | 1,041,841 (27.2) | 0.073 | (28.9) | (28.9) | 0.000 |
| Atopic dermatitis | 61,573 (1.6) | 55,219 (1.4) | 0.013 | (1.5) | (1.5) | 0.000 |
| Allergic rhinitis | 201,309 (5.2) | 192,579 (5.0) | 0.010 | (5.1) | (5.1) | 0.000 |
| Asthma | 84,739 (2.2) | 82,440 (2.2) | 0.004 | (2.2) | (2.2) | 0.000 |
| Hypothyroidism | 138,433 (3.6) | 121,613 (3.2) | 0.024 | (3.4) | (3.4) | 0.000 |
| Hyperthyroidism | 54,618 (1.4) | 49,120 (1.3) | 0.012 | (1.4) | (1.4) | 0.000 |
| Hashimoto thyroiditis | 23,205 (0.6) | 20,708 (0.5) | 0.008 | (0.6) | (0.6) | 0.000 |
| Vitamin D deficiency | 113,309 (3.0) | 84,192 (2.2) | 0.048 | (2.6) | (2.6) | 0.000 |
| Hepatitis B | 60,342 (1.6) | 55,555 (1.5) | 0.010 | (1.5) | (1.5) | 0.000 |
| Hepatitis C | 6159 (0.2) | 8597 (0.2) | 0.003 | (0.2) | (0.2) | 0.000 |
| HIV infection | 189 (0.0) | 163 (0.0) | 0.000 | (0.0) | (0.0) | 0.000 |
COVID-19, Coronavirus 2019 disease; HIV, human immunodeficiency virus; SMD, absolute standardized mean difference.
Statistical analyses
The baseline demographic characteristics of the study population are described as frequencies with percentages or means with standard deviations. The propensity score for individuals was estimated as the probability of belonging to the vaccination cohort based on the 18 aforementioned covariates and was used to calculate the inverse probability weight, which is the probability of belonging to the vaccination cohort divided by 1-the probability of being in the vaccination cohort. The covariate balance before and after the application of weights was assessed using standardized mean differences. We estimated the risk of predefined outcomes in the vaccination cohort versus the historical control cohort. Statistical estimates were derived from multivariate Cox proportional hazards analysis after adjusting for all 18 covariates used for inverse probability weighting. For each analysis, individuals who had already been diagnosed with the target outcome at the index date or before were excluded; hence, the analysis included only at-risk individuals. We then conducted subgroup analyses according to age, sex, type of mRNA vaccination (BNT162b2 or mRNA-1273), and history of non-mRNA vaccination prior to mRNA vaccination (AZD1222, AstraZeneca, or Ad26.COV2.S, Janssen). All statistical analyses were performed using the SAS statistical software (version 9.4; SAS Institute, Cary, NC, USA) and R statistical software (version 3.4.1; R Foundation for Statistical Computing, Vienna, Austria) at a significance level of 5%.
Results
Study population
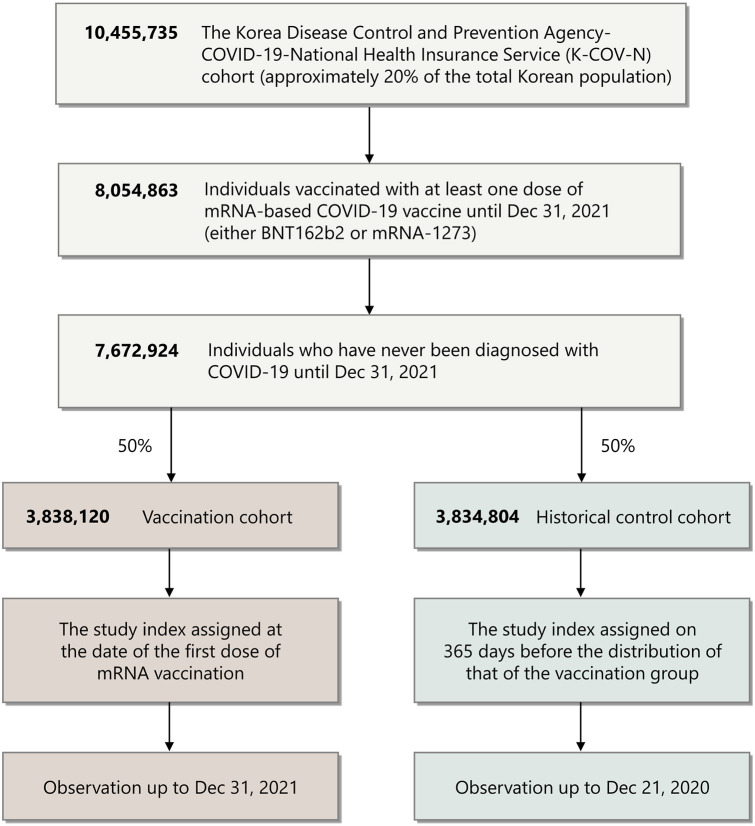
From the entire database, 7,672,924 individuals who were vaccinated with at least 1 dose of the mRNA-based COVID-19 vaccine and had never been diagnosed with COVID-19 were selected (Fig 1 ). Among them, 3,838,120 and 3,834,804 were assigned to the vaccination and historical cohorts, respectively. Baseline demographics of the study population are summarized in Table I. The COVID-19 vaccination profiles of the vaccination cohort are summarized in Supplementary Table II, available via Mendeley at https://doi.org/10.17632/4kzy8v78bm.1. The assessment of covariate balance after the application of inverse probability weighting suggested that the covariates were well-balanced (Supplementary Fig 1, available via Mendeley at https://doi.org/10.17632/4kzy8v78bm.1). The mean follow-up times for the vaccination cohort and the historical cohorts were 100.7 ± 90.3 and 100.7 ± 88.5 days, respectively.
Fig 1.
Flow chart of study population selection. A total of 3,838,120 individuals who received mRNA-based COVID-19 vaccination and 3,834,804 individuals in the historical control cohort were selected from the Korean Disease Control and Prevention Agency-COVID-19-National Health Insurance Service cohort. COVID-19, Coronavirus disease 2019.
Positive and negative outcomes
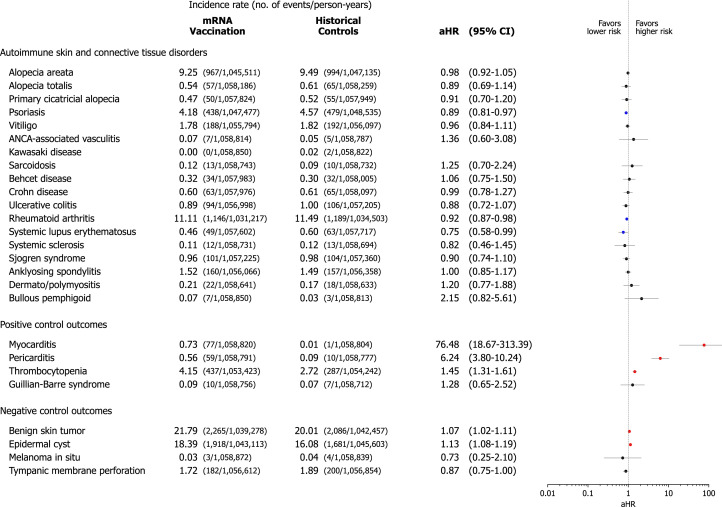
Prior to the main analysis, we investigated the risk of positive and negative control outcomes to validate our cohort and capture overdetection bias (Fig 2 ). Our data showed a considerably increased risk of myocarditis (adjusted hazard ratio [aHR], 76.48; 95% confidence interval (CI), 18.67-313.39), pericarditis (aHR, 6.24; 95% CI, 3.80-10.24), and thrombocytopenia (aHR 1.45; 95% CI, 1.31-1.61) in the vaccination cohort compared with the historical control cohort. Negative control outcomes indicated minimal overdetection bias in the vaccination cohort.
Fig 2.
Risk of incident autoimmune connective tissue disorders in mRNA-based COVID-19 vaccinated cohort compared with the historical control cohort. The incidence rate was expressed as the number of events per 10,000 person-years. The forest plot depicts aHRs and 95% confidence intervals (CIs) in individuals with mRNA-based COVID-19 vaccination compared to historical controls. The hazard estimates were adjusted for all 18 covariates used for inverse probability of treatment weighting. Individuals who had already been diagnosed with the target outcome on or before the index date were excluded from each analysis. aHR, Adjusted hazard ratio; CI, confidence interval; COVID-19, coronavirus disease 2019.
The COVID-19 vaccination cohort versus the historical control cohort
The incidence rate and risk of predefined autoimmune and autoinflammatory diseases were estimated in the vaccinated and control cohorts (Fig 2). The vaccinated individuals did not show increased risk of alopecia areata (AA) (aHR, 0.98; 95% CI, 0.92-1.05), alopecia totalis (aHR, 0.89; 95% CI, 0.69-1.14), primary cicatricial alopecia (aHR, 0.91; 95% CI, 0.70-1.20), psoriasis (aHR, 0.89; 95% CI, 0.81-0.97), vitiligo (aHR, 0.96; 95% CI, 0.84-1.11), anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (aHR, 1.36; 95% CI, 0.60-3.08), sarcoidosis (aHR, 1.25; 95% CI, 0.70-2.24), Behcet disease (aHR, 1.06; 95% CI, 0.75-1.50), Crohn disease (aHR, 0.99; 95% CI, 0.78-1.27), ulcerative colitis (aHR, 0.88; 95% CI, 0.72-1.07), rheumatoid arthritis (aHR, 0.92; 95% CI, 0.87-0.98), systemic lupus erythematosus (aHR 0.75; 95% CI, 0.58-0.99), systemic sclerosis (aHR, 0.82; 95% CI, 0.46-1.45), Sjogren syndrome (aHR, 0.90; 95% CI, 0.74-1.10), ankylosing spondylitis (aHR, 1.00; 95% CI, 0.85-1.17), dermato/polymyositis (aHR, 1.20; 95% CI, 0.77-1.88), and bullous pemphigoid (aHR, 2.15; 95% CI, 0.82-5.61) compared with control.
Subgroup analyses
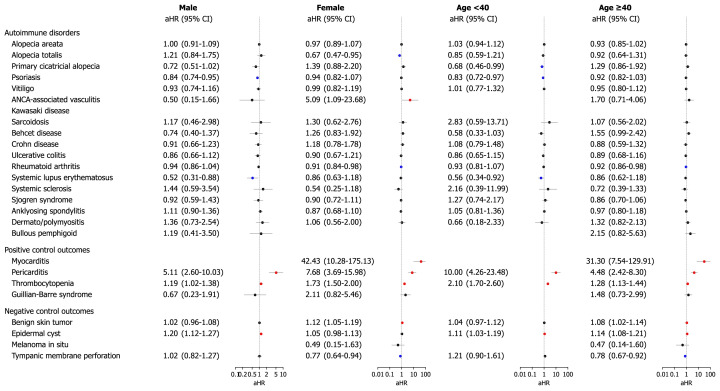
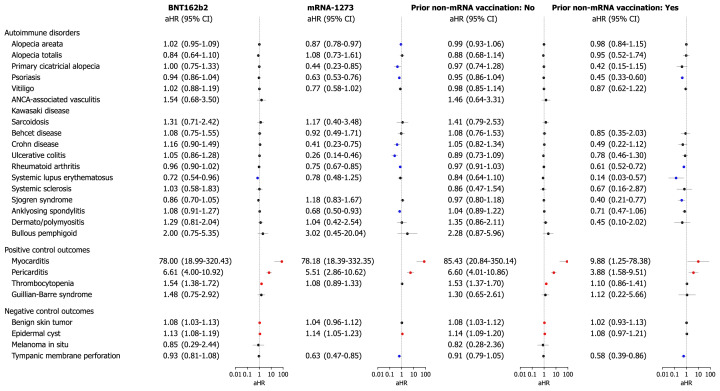
We further examined the risk of outcome diseases in subgroups of the mRNA-based COVID-19 vaccinated cohort in comparison with those in the historical cohort, stratified by age, sex, type of COVID-19 vaccine, and history of non-mRNA vaccination prior to mRNA vaccination. The risks of outcome diseases were not different according to subgroups by age (<40 years or ≥40 years) and sex (Fig 3 ). Anti-neutrophil cytoplasmic antibody-associated vasculitis was increased in female who had vaccination (aHR 5.09; 95% CI, 1.09-23.68); however, this observation requires careful interpretation due to the very small number of events. Regarding the type of mRNA vaccination, the risk of all outcome diseases did not increase, regardless of whether BNT162b2 or mRNA-1273 was used (Fig 4 ). Cross-vaccination status, defined as any history of non-mRNA vaccination (AZD1222, AstraZeneca; or Ad26.COV2.S, Janssen) prior to mRNA vaccination, was not independently associated with an increased risk of disease outcomes (Supplementary Fig 2, available via Mendeley at https://doi.org/10.17632/4kzy8v78bm.1).
Fig 3.
Subgroup analyses of the risk of incident autoimmune and autoinflammatory disease outcomes in COVID-19 cohort compared with the historical control cohort according to age and sex. The forest plot depicts aHRs and 95% confidence intervals (CIs) in individuals with mRNA-based COVID-19 vaccination compared to historical controls. Subgroup analyses stratified by age and sex are also presented. The hazard estimates were adjusted for all 18 covariates used for inverse probability of treatment weighting. Individuals who had already been diagnosed with the target outcome on or before the index date were excluded from each analysis. aHR, Adjusted hazard ratio; CI, confidence interval; COVID-19, coronavirus disease 2019.
Fig 4.
Subgroup analyses of the risk of incident autoimmune and autoinflammatory disease outcomes in COVID-19 cohort compared with the historical control cohort according to types of mRNA vaccines. The forest plot depicts aHRs and 95% confidence intervals (CIs) in individuals with mRNA-based COVID-19 vaccination compared to historical controls. Subgroup analyses stratified by type of mRNA-based COVID-19 vaccine (BNT162b2 or mRNA-1273) are shown. The hazard estimates were adjusted for all 18 covariates used for inverse probability of treatment weighting. Individuals who had already been diagnosed with the target outcome on or before the index date were excluded from each analysis. aHR, Adjusted hazard ratio; CI, confidence interval; COVID-19, coronavirus disease 2019.
Discussion
This nationwide population-based study extensively examined the risk of developing autoimmune and autoinflammatory diseases in COVID-19 vaccinated individuals in comparison with a historical control cohort. Using real-world data, we found that mRNA-based COVID-19 vaccination was not associated with a significantly increased risk of AA, vitiligo, psoriasis, sarcoidosis, Behcet disease, Crohn disease, ulcerative colitis, rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, ankylosing spondylitis, and dermato/polymyositis; these risks were not increased by age, sex, type of mRNA vaccine, or cross-vaccination status. Albeit statistically not significant, the risk of bullous pemphigoid showed tendency to increase. The female vaccinated group showed an increased risk of ANCA-associated vasculitis, although the number of events was very small.
Clinical trials of mRNA-based COVID-19 vaccines have shown that they are effective and have acceptable safety profiles.11 , 12 Despite the numerous contributions of vaccines to public health, their role in triggering autoimmune diseases has been controversial for decades. Development of postvaccination autoimmune diseases could be explained as molecular mimicry, which is a similarity between specific pathogenic elements contained in the vaccine or vaccine adjuvants and specific human proteins.13 Previous literature reported that the mRNA vaccines stimulate a switch in the immune system to chronic inflammation state through continuous production of particular autoantibodies, including complement products, anti-platelet factor 4, and polyethylene glycols.14 , 15 Moreover, some authors suggested that nucleic acids-based vaccines could induce autoimmune diseases mainly through acting as agonists of toll-like receptor-7/8/9 and stimulate innate immunity.16
With the gradual increase in COVID-19 vaccination, increasing number of studies documented new-onset autoimmune and autoinflammatory diseases including AA, vitiligo, Grave's disease, immune thrombocytopenic purpura, autoimmune hepatic diseases, Guillain-Barre syndrome, rheumatoid arthritis, type-1-diabetes mellitus, and rheumatic diseases following vaccination.17, 18, 19, 20 However, most of these cases were limited to case series and were diagnosed based on the temporal relationship of the administration of vaccination and onset of the disease. Therefore, real-world data with a large sample size are required to confirm the true association between vaccines and the onset of autoimmune diseases and not just the result of an increased number of vaccinated people.
Our study found that the risk of AA, vitiligo, psoriasis, sarcoidosis, Behcet disease, Crohn disease, ulcerative colitis, rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, ankylosing spondylitis, and dermato/polymyositis was not significantly higher in vaccinated individuals than in controls. These results suggest that the post-vaccination onset of autoimmune diseases could be overestimated due to the intensive observation of patients and physicians regarding the possible side effects after vaccination. In addition, vaccination could act as one of the triggering environmental factors only in certain populations with genetic susceptibility and not in a healthy population. The findings of our study may address some of the public's excessive concerns about vaccination through a real-world population-based study.
We observed an increasing trend in the risk of ANCA vasculitis in female population after the COVID-19 vaccination. Multiple case reports have described that COVID-19 vaccination can be associated with development of ANCA-associated vasculitis, both relapsing and de novo cases.21, 22, 23 ANCA-associated vasculitis refers to a multi-system inflammation of small vessels characterized by the presence of anti-neutrophil cytoplasmic antibodies, and environmental factors such as vaccination or infection can lead to the loss of immune tolerance, formation of neutrophil extracellular traps and production of ANCAs.24 T-lymphocyte responses shifting towards specific subsets and production of cytokines, such as interferon-β, following vaccination could also trigger ANCA-associated vasculitis.25 Checking serum creatinine or urinalysis may be considered in susceptible populations who present with systemic symptoms after vaccination. Further studies are needed to clarify the relationship between ANCA-associated vasculitis and vaccination.
In addition, albeit not significant, the risk of bullous pemphigoid tended to increase following vaccination. The development of bullous pemphigoid-like disease following COVID-19 vaccination has been reported in several studies, potentially related to off-target immune activation.26 The exact mechanism of whether bullous pemphigoid-like disease occurred coincidentally in individuals with pre-existing subclinical autoreactivity or whether the vaccine itself increases the disease risk remains to be elucidated.26 In our study, the number of events was too small to detect the statistical significance which requires careful interpretation. Therefore, further studies would be needed to investigate the potential association between COVID-19 vaccination and those conditions.
First, the strength of our study is the use of nationwide real-world data from a single insurance system covering more than 99% of the population. Second, we selected a historical control cohort to minimize selection bias and validated the data using positive and negative outcomes to address detection bias. Finally, we performed a comprehensive subgroup analysis based on age, sex, type of mRNA vaccine, and cross-vaccination status.
This study has several limitations. The demographic composition comprised a single ethnicity. Although we extracted data from the same primary cohort, a possible selection bias exists because of the historical cohort study design. We aimed to maximize our sample size by using Korean national population data, but even with this approach, we were unable to achieve sufficient statistical power for some rare outcomes. Our data lacked detailed information on individual factors such as genetic susceptibility or underlying diseases. Lastly, the follow-up period was not long enough to assess the long-term side effects of the mRNA vaccines.
Conclusion
This study comprehensively investigated the incidence and risk of autoimmune and autoinflammatory outcomes following mRNA-based COVID-19 vaccination. Overall, we did not observe evidence of a significantly increased risk of most autoimmune or autoinflammatory diseases in the vaccinated group compared to controls, although some of these conditions had small number of events, which should be interpreted with caution. Nonetheless, our findings suggest that any potential risk is likely to be not large. Sex-stratified analysis revealed an increasing trend of ANCA-associated vasculitis in female-vaccinated individuals. Our data should relieve excessive public concern about vaccinations and not discourage clinicians from prescribing COVID-19 vaccines; however, long-term follow-up is necessary.
Conflicts of interest
None disclosed.
Acknowledgments
This study used the database of the KDCA and the NHIS for policy and academic research. The research number of this study is KDCA-NHIS-2022-1-496. The KDCA is the Korea Disease Control and Prevention Agency, Republic of Korea. The NHIS is the National Health Insurance Service, Republic of Korea.
Footnotes
Funding sources: This research was supported by a fund from the research program of the Korea Medical Institute and a National Research Foundation (NRF) of Korea grant funded by the Korea government (MSIT) (no. 2017R1A5A2015369).
IRB approval status: This study was approved by the Korean National Institute for Bioethics Policy (NHIS-2022-1-496) and a waiver of informed consent was granted owing to the deidentified data used.
References
- 1.Hajjo R., Sabbah D.A., Tropsha A. Analyzing the systems biology effects of COVID-19 mRNA vaccines to assess their safety and putative side effects. Pathogens. 2022;11(7):743. doi: 10.3390/pathogens11070743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall V.J., Foulkes S., Saei A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akinosoglou K., Tzivaki I., Marangos M. Covid-19 vaccine and autoimmunity: awakening the sleeping dragon. Clin Immunol. 2021;226 doi: 10.1016/j.clim.2021.108721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrido I., Lopes S., Simões M.S., et al. Autoimmune hepatitis after COVID-19 vaccine - more than a coincidence. J Autoimmun. 2021;125 doi: 10.1016/j.jaut.2021.102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jara L.J., Vera-Lastra O., Mahroum N., et al. Autoimmune post-COVID vaccine syndromes: does the spectrum of autoimmune/inflammatory syndrome expand? Clin Rheumatol. 2022;41:1603–1609. doi: 10.1007/s10067-022-06149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok F.A., Pai M., Huisman M.V., et al. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022;9:e73–e80. doi: 10.1016/S2352-3026(21)00306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blank M., Israeli E., Gertel S., et al. In: Autoimmunity: from bench to bedside [internet]. Bogota (Colombia) Anaya J.M., Shoenfeld Y., Rojas-Villarraga A., et al., editors. El Rosario University Press; 2013. Molecular mimicry in autoimmunity and vaccinations. Chapter 21. [PubMed] [Google Scholar]
- 9.Cheol Seong S., Kim Y.Y., Khang Y.H., et al. Data resource profile: the national health information database of the national health insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y., Xu E., Bowe B., et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb Y.N. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81:495–501. doi: 10.1007/s40265-021-01480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segal Y., Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15:586–594. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y., Xu Z., Wang P., et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165:386–401. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 15.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.May Lee M., Bertolani M., Pierobon E., et al. Alopecia areata following COVID-19 vaccination: vaccine-induced autoimmunity? Int J Dermatol. 2022;61:634–635. doi: 10.1111/ijd.16113. [DOI] [PubMed] [Google Scholar]
- 18.Birkett L., Singh P., Mosahebi A., et al. Possible associations between alopecia areata and COVID-19 vaccination and infection. Aesthet Surg J. 2022;42:Np699–Np702. doi: 10.1093/asj/sjac165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vera-Lastra O., Ordinola Navarro A., Cruz Domiguez M.P., et al. Two cases of Graves' disease following SARS-CoV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021;31:1436–1439. doi: 10.1089/thy.2021.0142. [DOI] [PubMed] [Google Scholar]
- 20.Furer V., Eviatar T., Zisman D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 21.El Hasbani G., Uthman I. ANCA-associated vasculitis following the first dose of pfizer-BioNTech COVID-19 vaccine. Nephron. 2022;147(2):103–107. doi: 10.1159/000525562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mai A.S., Tan E.K. COVID-19 vaccination precipitating de novo ANCA-associated vasculitis: clinical implications. Clin Kidney J. 2022;15:1010–1011. doi: 10.1093/ckj/sfac026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feghali E.J., Zafar M., Abid S., et al. De-novo antineutrophil cytoplasmic antibody-associated vasculitis following the mRNA-1273 (Moderna) vaccine for COVID-19. Cureus. 2021;13 doi: 10.7759/cureus.19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitching A.R., Anders H.J., Basu N., et al. ANCA-associated vasculitis. Nat Rev Dis Primers. 2020;6:71. doi: 10.1038/s41572-020-0204-y. [DOI] [PubMed] [Google Scholar]
- 25.Shimagami H., Yamaguchi Y., Kato Y., et al. Marked increase of interferon-β after BNT162b2 mRNA vaccination: a case of polyarthritis with pleurisy. BMJ Case Rep. 2022;15(3) doi: 10.1136/bcr-2021-246533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomayko M.M., Damsky W., Fathy R., et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148(3):750–751. doi: 10.1016/j.jaci.2021.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]