Abstract
Background
Intraoperative goal-directed fluid therapy (GDFT) has been reported to reduce postoperative complications of patients undergoing major abdominal surgery. The clinical benefits of pleth variability index (PVI)-directed fluid management for gastrointestinal (GI) surgical patients remain unclear. Therefore, this study aimed to evaluate the impact of PVI-directed GDFT on GI surgical outcomes in elderly patients.
Methods
This randomised controlled trial was conducted in two university teaching hospitals from November 2017 to December 2020. In total, 220 older adults undergoing GI surgery were randomised to the GDFT or conventional fluid therapy (CFT) group (n = 110 each). The primary outcome was a composite of complications within 30 postoperative days. The secondary outcomes were cardiopulmonary complications, time to first flatus, postoperative nausea and vomiting, and postoperative length of stay.
Results
The total volumes of fluid administered were less in the GDFT group than in the CFT group (2.075 L versus [vs.] 2.5 L, P = 0.008). In intention-to-treat analysis, there was no difference in overall complications between the CFT group (41.3%) and GDFT group (43.0%) (odds ratio [OR] = 0.935; 95% confidence interval [CI], 0.541–1.615; P = 0.809). The proportion of cardiopulmonary complications was higher in the CFT group than in the GDFT group (19.2% vs. 8.4%; OR = 2.593, 95% CI, 1.120–5.999; P = 0.022). No other differences were identified between the two groups.
Conclusions
Among elderly patients undergoing GI surgery, intraoperative GDFT based on the simple and non-invasive PVI did not reduce the occurrence of composite postoperative complications but was associated with a lower cardiopulmonary complication rate than usual fluid management.
Trial registration
This trial was registered with the Chinese Clinical Trial Registry (ChiCTR-TRC-17012220) on 1 August 2017.
Keywords: Gastrointestinal surgery, Goal-directed fluid therapy, Pleth variability index, Elderly patients, Postoperative complications, Dual-centre trial, Randomised controlled trial
Background
The number of elderly patients undergoing surgical procedures is growing drastically with the ageing of society. This population is at a greater risk of mortality and morbidity after gastrointestinal (GI) surgical procedures than younger patients. Despite advances in surgical and perioperative care, postoperative complications occur in approximately 45% of patients, with an in-hospital mortality rate of approximately 3% in elderly patients undergoing GI surgical procedures (Pearse et al. 2014). To minimise these risks, anaesthesiologists try to optimise intraoperative haemodynamics and fluid management in these patients.
Fluid deficits are frequent during GI procedures because of preoperative fasting, bowel preparation, and intraoperative fluid loss, particularly in elderly patients (Holte et al. 2002). Therefore, intraoperative fluid management is critical for anaesthetic practice in these procedures (Lobo et al. 2002; Junghans et al. 2006). Individualised goal-directed fluid therapy (GDFT) has been reported to improve oxygen delivery and overall haemodynamic function, thereby reducing postoperative complications in high-risk patients (Gan et al. 2002; Corcoran et al. 2012; Hamilton et al. 2011). GDFT has been performed with different haemodynamic monitoring devices to guide intravenous fluid administration in high-risk surgical patients (Sandham et al. 2003; Challand et al. 2012; Lopes et al. 2007; Benes et al. 2010; Malbouisson et al. 2017; Bloria et al.2022). However, data on the optimal haemodynamic parameters and devices for elderly patients undergoing GI surgery are limited. Most of these monitoring devices are invasive and not routinely available in daily clinical practice. A non-invasive ‘plug and play’ sensor (Masimo Corporation, Irvine, CA, USA) that uses the pleth variability index (PVI) was developed to assess fluid responsiveness based on plethysmographic variations induced by mechanical ventilation. The PVI has been shown to perform similarly to more invasive and expensive dynamic fluid assessment technologies (such as pulse pressure variation and stroke volume variation) during cardiac surgery (Cannesson et al. 2008; Haas et al. 2012), colorectal surgery (Hood and Wilson 2011), bariatric surgery (Demirel et al. 2018). A small-sized randomised controlled trial (RCT) showed that PVI-directed fluid management reduced the lactate concentrations and improved fluid management in abdominal surgery recipients (Forget et al. 2010). However, its clinical benefits for elderly GI surgical patients remain unclear.
This dual-centre RCT primarily aimed to analyse whether perioperative GDFT based on the PVI resulted in decreased composite postoperative complications compared with conventional fluid management practice in elderly patients who underwent major GI surgery. The secondary objective of this RCT was to evaluate cardiopulmonary major postoperative complications, postoperative nausea and vomiting (PONV), time to first flatus, and postoperative length of stay (PLOS).
Methods
Study design and ethics statements
This dual-centre clinical RCT was conducted in two university teaching hospitals from November 2017 to December 2020 (Peking University Shenzhen Hospital and First Hospital of Shanxi Medical University) The study analysed whether perioperative GDFT based on PVI reduced composite postoperative complications in elderly patients who underwent major GI surgery compared to conventional fluid management practice It also evaluated cardiopulmonary major postoperative complications, PONV, time to first flatus, and PLOS. This study was approved by the Clinical Research Ethics Committee of Peking University Shenzhen Hospital (IRB 2017-001-2), and written informed consent was obtained from all participants in the trial. Prior to patient enrolment, this trial was registered with the Chinese Clinical Trial Registry (ChiCTR-TRC-17012220, principal investigator: Xinhai Wu, date of registration: 1 August 2017). This manuscript adheres to the applicable guidelines of the Consolidated Standards of Reporting Trials (CONSORT).
Inclusion criteria
Potential participants were screened on the day before surgery (or on the preceding Friday for those who underwent surgery on a Monday). Adult patients aged ≥ 65 who underwent elective major GI surgery (including gastrectomy, small bowel resection, and colorectal surgery) were considered eligible.
Exclusion criteria
Patients were excluded if they met any of the following criteria: (1) history of severe cardiac disease, including severe arrhythmia, myocardial infarction, and cardiac insufficiency; (2) presence of hepatorenal dysfunction; (3) presence of cardiac failure (New York Heart Association class III or IV); (4) history of asthma; (5) presence of pulmonary infection; and (6) body mass index ≥ 30 kg/m2.
Data collection
Detailed information, including baseline demographic data, preoperative medical history, diagnosis at the time of admission, illness severity and perioperative variables were obtained after recruitment. After obtaining written informed consent, baseline data (including demographic data, surgical diagnosis, and comorbidities) were collected.
Randomisation and blinding
A biostatistician not involved in the data management and statistical analyses generated random numbers (at a 1:1 ratio, stratified by centre) using SAS software version 9.2 (SAS Institute, Cary, NC, USA), with a block size of 4. Results of this randomisation were sealed in sequentially numbered envelopes and kept until the end of the study by a study coordinator (J.Z.) who was not involved in data collection, perioperative care, or postoperative follow-up. During the study period, consecutively recruited patients received either intraoperative GDFT or conventional haemodynamic management, according to the random number allocation by the study coordinator (J.Z.). Intraoperative data of each recruited patient were collected by anaesthesiologists (Y.W, X.D, Z.G, and J.Z). Anaesthesiologists and investigators did not communicate with each other regarding the patient data collected. Both patients and postoperative investigators were blinded to the study group assignment. Unblinding was conducted after the trial was closed and data of all patients were collected. Older adults undergoing GI surgery were randomly assigned to the GDFT or conventional fluid therapy (CFT) group.
Technical information
Interventions, anaesthesia, and perioperative care
No premedication was administered, and solid food and clear fluid intakewere allowed until 8 h and 2 h before surgery, respectively. Patients’ electrocardiographic activity, arterial blood pressure, oxygen saturation, and body temperature were continuously monitored. General anaesthesia was induced with etomidate or propofol and sufentanil; atracurium or rocuronium was used for neuromuscular block. A 20-gauge radial artery catheter and central venous access catheter were inserted at the end of the induction phase. Anaesthesia was maintained with sevoflurane or propofol and remifentanil. The PVI sensor (Rainbow R2-25a, Masimo Corporation, Irvine, CA, USA) was placed on the patient’s index finger to monitor the PVI continuously.. The use of additional regional anaesthesia, choice of anaesthetic drugs, and operative pain management were at the discretion of the attending anaesthesiologist. The investigators indicated that the ventilator patterns should be restricted as follows: controlled ventilation with a tidal volume of 8 mL/kg of ideal body weight; an initial respiratory rate of 12 breaths/min adjusted to achieve end-tidal CO2 between 35 and 45 mmHg; and pulse oxygen saturation > 96%. In all cases, the anaesthetic procedure was chosen by the attending anaesthesiologist. Packed red blood cells were administered at the anaesthesiologist’s discretion (our perioperative care protocol only suggested using haemoglobin levels of 7 g/dL as a threshold for healthy patients and 9 g/dL for patients with pulmonary or cardiac diseases). Lactate concentrations at the beginning of surgery and at discharge from the post-anaesthesia care unit (PACU) were analysed.
Intraoperative haemodynamic protocol
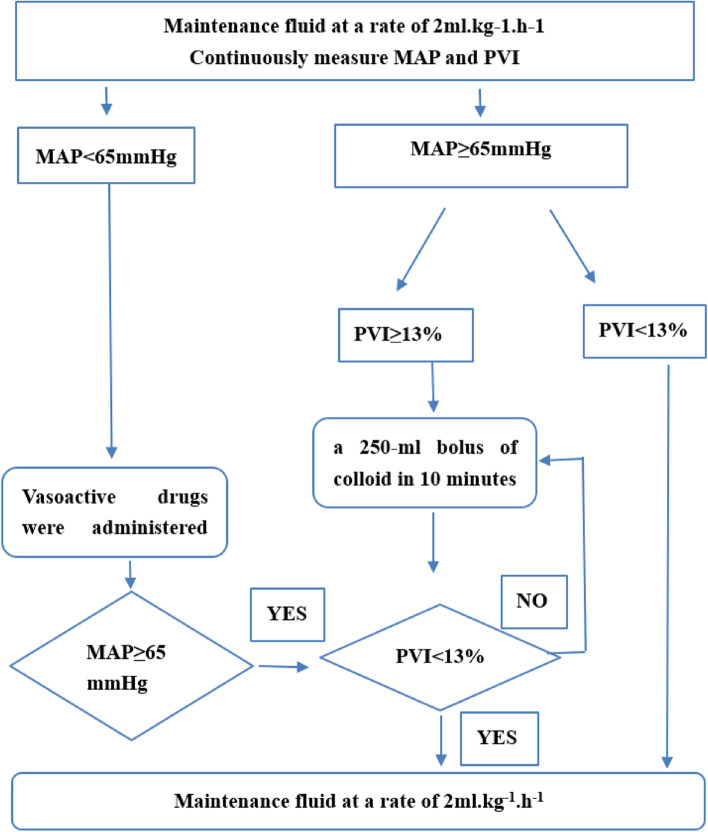
In the GDFT group, 500 mL of crystalloids were infused during induction, followed by continuous infusion at a rate of 2 mL·kg−1.h−1. When MAP is < 65 mmHg, the vasoactive drugs((phenylephrine, ephedrine, or norepinephrine)) were promptly administered to maintain a mean arterial blood pressure of ≥ 65 mmHg regardless of PVI. When MAP is ≥ 65 mmHg, we judge fluid responsiveness is present via PVI > 13%. Whenever the PVI was > 13% for 5 min, we administered a 250-mL bolus of colloid (6% hydroxyethyl starch, Voluven®, Fresenius Kabi, Beijing, China). The dose was repeated every 5 min until the PVI was < 13% (Fig. 1).
Fig. 1.
Intraoperative fluid treatment in the intervention groups. MAP, mean arterial pressure; PVI, pleth variability index
In the CFT group, 500 mL of crystalloid was infused during induction, followed by a continuous infusion of crystalloids (6 mL·kg−1.h−1). A bolus of 250-mL colloids(6% hydroxyethyl starch, Voluven®, Fresenius Kabi, Beijing, China) was administered whenever acute blood loss was > 50 mL, the mean arterial blood pressure decreased to < 65 mmHg, or the central venous pressure decreased to < 5 mmHg. A repeat bolus was administered after waiting for 5 min if any one of these criteria was met. Whenever the mean arterial blood pressure decreased to < 65 mmHg and remained unresponsive to fluids, vasoactive drugs (phenylephrine, ephedrine, or norepinephrine) were administered to maintain a mean arterial blood pressure of > 65 mmHg.
Outcome assessment
The primary outcome assessed was the proportion of patients who experienced clinically detected postoperative complications at 30 postoperative days, including surgical site infection, organ/space infection, anastomotic leakage, new-onset stroke, confusion/delirium, pneumonia, atelectasis, pleural effusion requiring drainage, arrhythmias, acute myocardial infarction, acute kidney injury, reoperation for bleeding, pulmonary embolism, deep vein thrombosis, paralytic ileus, and mortality within 30 days after surgery. The postoperative complications were based on standard definitions for cardiovascular, respiratory, abdominal, renal, and central nervous complications as well as relevant infections similar to previous studies (Pearse et al. 2014; Szturz et al. 2019).
We assessed the following secondary outcomes: cardiopulmonary complications included pneumonia, atelectasis, pulm oedema, arrhythmia and acute myocardial infarction, PONV, time to first flatus, and PLOS.
Data were recorded in a case report file maintained by the principal investigator at each centre and stored in the REDCap database (Vanderbilt University, Nashville, TN, USA). Data were obtained from the clinical files completed by the surgeons responsible for the patient, but who were blinded to the study. Data validation (conformity between the case report file and database, screening for internal coherence of recorded values, and detection of abnormalities and discrepancies according to the plan of controls previously prepared) were performed by the principal investigator (X-H.W.).
Statistical analysis
Based on previous published data (Pearse et al. 2014) and past patient data available in our hospital, we estimated that complications might appear in 45% of patients; therefore, we considered a reduction from 45 to 35% as clinically relevant. Assuming a two-sided type I error rate of 5% and a power of 70%, the ideal sample size was determined to be 196 patients. Considering a sample loss rate of approximately 10%, we were required to enrol 110 patients for each group. The sample size was calculated using Stata 13.1 (StataCorp, College Station, TX, USA).
Unless otherwise indicated, all results are expressed as mean and standard deviation or as the median and interquartile range (IQR). Normally distributed continuous data were compared using the independent-samples t-test, whereas non-normally distributed continuous data were compared using the independent-samples Mann–Whitney U test. Categorical variables were analysed using the χ2 test, continuity correction χ2 test, or Fisher exact test; two-tailed tests were performed whenever appropriate, and a P-value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, NY, USA).
Results
Participant flow and recruitment
From 5 November 2017 to 29 October 2020, 537 patients were eligible for enrolment, and 220 patients from two hospitals (160 participants from the Peking University Shenzhen Hospital and 60 participants from the First Hospital of Shanxi Medical University) were enrolled and randomised. The final follow-up of the last randomised participants was performed in December 2020. Of 220 patients, 9 were excluded after randomisation based on the protocol-defined exclusion criteria: 6 patients withdrew from the study, and 3 patients were excluded because of a change in the surgical procedure. All evaluable patients were followed up for 30 days postoperatively, and none were lost to follow-up. A total of 211 patients were included in the intention-to-treat analyses (CFT group, 104; GDFT group, 107) (Fig. 2). Results were reported according to the CONSORT guidelines.
Fig. 2.
Consolidated Standards of Reporting Trials (CONSORT) diagram. CFT, conventional fluid therapy; GDFT, goal-directed fluid therapy; ITT, intention to treat
Baseline data
There were no between-group differences in patient characteristics, physical status according to the American Society of Anaesthesiologists guidelines, comorbidities, preoperative haemoglobin level, and creatinine level (Table 1). The overall median age was 70 years (IQR, 68–79 years).
Table 1.
Baseline characteristics
| CFT (n = 104) | GDFT (n = 107) | P-value | |
|---|---|---|---|
| Age (years) | 70.7 ± 5.2 | 70.7 ± 5.4 | 0.859 |
| Male sex (%) | 72 (69.2) | 73 (68.2) | 0.875 |
| BMI, kg/m2 | 22.9 ± 3.2 | 22.4 ± 3.0 | 0.253 |
| ASA physical status, n (%) | |||
| I | 7 (6.7) | 12 (11.2) | |
| II | 79 (56.4) | 80 (74.8) | |
| III | 18 (17.3) | 15 (8.8) | 0.460 |
| Smoker, n (%) | 12 (11.5) | 12 (11.2) | 0.941 |
| Chronic alcohol consumption, n (%) | 5 (4.8) | 4 (3.7) | 0.701 |
| Medical history, n (%) | |||
| Pulmonary disease | 1 (0.9) | 3 (2.8) | 0.634 |
| Cardiovascular disease | 39 (37.5) | 36 (33.6) | 0.929 |
| Diabetes mellitus | 14 (13.5) | 22 (20.6) | 0.171 |
| Cerebral vascular disease | 3 (2.9) | 5 (4.7) | 0.749 |
| Preoperative haemoglobin level, g/dL | 13.5 ± 1.3 | 13.4 ± 1.6 | 0.878 |
| Preoperative creatinine level, mmol/L | 71.3 ± 13.4 | 82.0 ± 21.9 | 0.675 |
Continuous data are expressed as mean ± standard deviation or median (interquartile range). Categorical data are expressed as the number of patients (percentage). Statistical significance was tested by the t-test, Mann–Whitney test, and Fisher exact test
CFT Conventional fluid therapy, GDFT Goal-directed fluid therapy, BMI Body mass index, ASA American Society of Anaesthesiologists
Intraoperative data
The surgery types and surgical methods were similar between the CFT and GDFT groups (P = 0.280 and P = 0.254, respectively). The duration of surgery and anaesthesia was not significantly different between the two groups (235 [190, 290] min versus [vs.] 202 [175, 270] min and 290 [230, 345] min vs. 257 [210, 313] min; P = 0.093 and P = 0.096, respectively). The total volumes of fluid administered and crystalloid infusion were lesser in the GDFT group than in the CFT group (2075 [1900, 2600] mL vs. 2500 [2000, 3100] mL, and 1450 [1200, 2100] mL vs. 1795 [1500, 2187] mL; P = 0.008 and P = 0.009, respectively). Lactate concentrations at the beginning of the surgery (0.9 [0.7, 1.1] mEq/L vs. 1.0 [0.7, 1.4] mEq/L, P = 0.675) and at discharge (0.9 [0.7, 1.1] mEq/L vs. 1.1 [0.8, 1.5] mEq/L; P = 0.733) from the PACU were similar between the CFT and GDFT groups (Table 2).
Table 2.
Intraoperative data
| CFT (n = 104) | GDFT (n = 107) | P-value | |
|---|---|---|---|
| Type of surgery, n (%) | |||
| Colon resection | 49 (47.1) | 40 (37.4) | |
| Rectum resection | 26 (25.0) | 36 (33.6) | |
| Gastric resection | 29 (27.9) | 31 (29.0) | 0.280 |
| Surgical method, n (%) | |||
| Open | 23 (22.1) | 31 (29.0) | |
| Laparoscopic | 81 (77.9) | 76 (71.0) | 0.254 |
| Duration of surgery (min) | 235 (190, 290) | 202 (175, 270) | 0.093 |
| Anaesthesia time (min) | 290 (230, 345) | 257 (210, 313) | 0.096 |
| Total volume of fluid administered (mL) | 2500 (2000, 3100) | 2075 (1900, 2600) | 0.008* |
| Crystalloid infusion (mL) | 1795 (1500, 2187) | 1450 (1200, 2100) | 0.009* |
| Colloid infusion (mL) | 500 (500, 1000) | 500 (500, 500) | 0.308 |
| Estimated blood loss (mL) | 100 (30, 200) | 50 (30, 200) | 0.410 |
| Patients receiving a blood transfusion, n (%) | 19 (18.3) | 13 (12.1) | 0.214 |
| Urine output (mL) | 400 (300, 600) | 400 (200, 500) | 0.350 |
| Patients receiving a vasoactive infusion, n (%) | 50 (48.1) | 44 (41.1) | 0.295 |
| Lactate before skin incision, mEq/L | 0.9 (0.7, 1.1) | 1.0 (0.7, 1.4) | 0.675 |
| Lactate at PACU departure, mEq/L | 0.9 (0.7,1.1) | 1.1 (0.8, 1.5) | 0.733 |
Continuous data are expressed as mean ± standard deviation or median (interquartile range). Categorical data are expressed as the number of patients (percentage). Statistical significance was tested by the t-test, Mann–Whitney test, and Fisher exact test
CFT Conventional fluid therapy, GDFT Goal-directed fluid therapy, PACU Post-anaesthesia care unit
*Statistically significant difference
Outcomes
The number of patients with one or more complications at 30 days was similar between the CFT group (43 [41.3%]) and GDFT group (46 [43.0%]) (odds ratio [OR] = 0.935; 95% confidence interval [CI], 0.541–1.615; P = 0.809). The proportion of cardiopulmonary complication was higher in the CFT group than in the GDFT group (19.2% vs. 8.4%; OR = 2.593, 95% CI, 1.120–5.999; P = 0.022). No significant between-group differences in PONV, the time to first flatus, and PLOS were identified (P = 0.398, P = 0.475, and P = 0.614, respectively) (Table 3).
Table 3.
Postoperative outcomes
| CFT (n = 104) | GDFT (n = 107) | P-value | OR (95% CI) | |
|---|---|---|---|---|
| Primary outcome | ||||
| Number of patients with one or more complications, n (%) | 43 (41.3) | 46 (43.0) | 0.809 | 0.935 (0.541–1.615) |
| Individual elements | ||||
| Surgical site infection, n (%) | 6 (5.8) | 3 (2.8) | 0.468 | |
| Organ/space infection, n (%) | 3 (2.9) | 2 (1.9) | 0.974 | |
| Other infection, n (%) | 3 (2.9) | 3 (2.8) | > 0.999 | |
| Anastomotic leakage, n (%) | 5 (4.8) | 1 (0.9) | 0.201 | |
| New-onset stroke, n (%) | 0 (0) | 1 (0.9) | > 0.999 | |
| Confusion/delirium, n (%) | 3 (2.9) | 3 (2.8) | > 0.999 | |
| Pneumonia, n (%) | 6 (5.8) | 2 (1.9) | 0.262 | |
| Atelectasis, n (%) | 2 (1.9) | 0 (0) | 0.242 | |
| Pulmonary oedema, n (%) | 5 (4.8) | 2 (1.9) | 0.420 | |
| Arrhythmia, n (%) | 5 (4.8) | 2 (1.9) | 0.420 | |
| Acute myocardial infarction, n (%) | 2(1.9) | 3 (2.8) | > 0.999 | |
| Reoperation for bleeding, n (%) | 1 (0.9) | 1 (0.9) | > 0.999 | |
| Acute kidney injury, n (%) | 3 (2.9) | 5 (4.7) | 0.749 | |
| Pulmonary embolism, n (%) | 0 (0) | 0 (0) | > 0.999 | |
| Deep vein thrombosis, n (%) | 2 (1.9) | 1 (0.9) | 0.980 | |
| Paralytic ileus, n (%) | 15 (14.4) | 20 (18.7) | 0.405 | |
| Mortality, n (%) | 2 (1.9) | 1 (0.9) | 0.980 | |
| Secondary outcome | ||||
| Cardiopulmonary complication, n (%) | 20 (19.2) | 9 (8.4) | 0.022* | 2.593 (1.120–5.999) |
| PONV, n (%) | 35 (33.7) | 42 (39.3) | 0.398 | 0.785 (0.447–1.377) |
| Time to first flatus (h) | 52 (34, 81) | 60 (30, 93) | 0.475 | |
| PLOS (days) | 10 (8, 12) | 9(8, 14) | 0.614 | |
Continuous data are expressed as mean ± standard deviation or median (interquartile range). Categorical data are expressed as the number of patients (percentage). Statistical significance was tested by the t-test, Mann–Whitney test, and Fisher exact test
CFT Conventional fluid therapy, GDFT Goal-directed fluid therapy, PONV Postoperative nausea and vomiting, PLOS Postoperative length of stay, OR Odds ratio, CI Confidence interval
*Statistically significant difference
Discussion
This study examined the impact of intraoperative GDFT based on the non-invasive PVI on GI surgical outcomes in elderly patients. The principal finding of our trial was that among elderly patients undergoing major GI surgery, PVI-directed intraoperative GDFT was not associated with a significant reduction in the proportion of patients who died or experienced complications within 30 postoperative days, compared with CFT. The analysis of secondary outcomes revealed that the intervention reduced cardiopulmonary complications but did not provide clinical benefits in terms of PONV, time to first flatus, and PLOS compared with usual fluid management.
One of the reported effects of PVI-directed GDFT was the decreased volume of fluid infusion required intraoperatively, with no effect on lactate levels (Demirel et al. 2018; Fischer et al. 2020). However, the difference in fluid administration between the GDFT and standard care groups was varied in numerous GDFT trials. A meta-analysis of 56 GDFT studies reported that the differences were within 500 mL in 35 (62%) trials, > 500 mL in 10 (18%) trials, and < 500 mL in 11 (20%) trials (Jessen et al. 2022). Greater volume difference was shown in earlier studies because of adoption of liberal fluid therapy in their control group (Sandham et al. 2003; Malbouisson et al. 2017; Lopes et al. 2007; Benes et al. 2010). With enhanced recovery after surgery (ERAS) programmes and laparoscopic procedures being widely implemented, restricted fluid therapy has been mostly used for intraoperative conventional fluid administration, recently. In our study, the difference of total fluid administration between the CFT and GDFT groups was 425 mL, which was consistent with most findings in the literature. Nonetheless, the difference in fluid administration in the CFT and GDFT groups was not as significant as expected. This could partly explain why, in the primary analysis, GDFT based on the PVI was not found to reduce the occurrence of composite postoperative complications.
Previous studies have shown that GDFT based on different invasive haemodynamic parameters can reduce postoperative complication rates and shorten PLOS in high-risk surgery (Sandham et al. 2003; Challand et al. 2012; Lopes et al. 2007; Benes et al. 2010; Szturz et al. 2019). However, the overall certainty in the evidence was low because of the heterogeneity of the studies (Jessen et al. 2022). Our findings are not as conclusive as the results of these previous studies. We believe that the differences in the research findings can be attributed to the heterogeneous populations, varying surgeries, and inconsistencies in the implementation of fluid management protocols. In addition, with the improvement of surgical techniques and the popularisation of ERAS, the incidence of postoperative complications has decreased remarkably. Therefore, the potential effect of GDFT to reduce postoperative complications is inapparent under the current ERAS clinical path (Rollins and Lobo 2016; Gómez-Izquierdo et al. 2017).
Interestingly, in our secondary outcomes assessment, reduction was noted in the incidence of cardiopulmonary complications in the GDFT group compared with the CFT group (8.4% vs. 19.2%). The mechanism of the beneficial effect of GDFT could not be determined in our study. It is possible that elderly patients receiving GDFT benefit from fluid optimisation and avoid excessive fluid intake and, therefore, have a decreased risk of overload of the heart and tissue oedema, which, compared to CFT, could have potentially lowered the risk of postoperative complications such as pulmonary oedema (1.9% vs. 4.8%), arrhythmia (1.9 vs. 4.8%), pneumonia (1.9% vs. 5.8%), surgical site infection (2.8% vs. 5.8%), and anastomotic leakage (0.9% vs. 4.8%). A recent meta-analysis of GDFT trials concluded that GDFT during general anaesthesia might reduce pneumonia, surgical site infection, and anastomotic leakage, and this result reached moderate certainty in the evidence (Jessen et al. 2022). However, RCTs with greater sample sizes are warranted to accurately demonstrate statistical differences because of the low incidence of these postoperative complications.
Recovery of GI function is a significant determinant of in-hospital recovery after GI surgery (Augestad and Delaney 2010). Postoperative GI disturbance is a common complication manifested by delayed intestinal motility and PONV. Individualised GDFT guided by haemodynamic parameters seems to be the logical approach to avoid inappropriate intestinal perfusion, which can lead to postoperative GI dysfunction. In our trial, the occurrence rate of paralytic ileus and time to first flatus were similar between the two groups. GDFT was not found to improve postoperative GI function of elderly patients undergoing GI surgery. There is conflicting evidence on whether optimising fluids management can reduce the risk of PONV. In an earlier study, Gan et al. (2002) reported that GDFT guided by oesophageal Doppler monitoring results in an earlier return to bowel function and a lower incidence of PONV. In a meta-analysis, Jewer et al. (2019) concluded that supplemental intravenous crystalloid administration prevents PONV in patients undergoing surgical procedures under general anaesthesia. In our trial, there were more patients with PONV in the GDFT group (39.3%) than in the CFT group (33.7%), although this difference was not statistically significant. Large sample studies are needed to assess the risk-benefit profile of fluid therapy and PONV.
This study has some limitations. First, GDFT was performed only intraoperatively. Therefore, it is unclear if the intraoperative assessment of fluid administration is adequate to produce a considerable difference in the outcome compared to the perioperative assessment. The use of GDFT throughout the perioperative period may be more effective in improving patient outcomes. Second, the cut-off value for the PVI was a limitation. The threshold value of the PVI in our interventional group was set at 13% based on that reported in previous literature (Cannesson et al. 2008). A recent meta-analysis showed high variability regarding the best threshold value, ranging from 7 to 20% (Perel 2014). One reason for the high variability may be differences in clinical settings and parameters of the study population, including age, vasoactive drug use, position during surgery, and pneumoperitoneum. Therefore, more studies are warranted to verify the optimum threshold value according to the study settings and participant population. Third, given the nature of GDFT, it is practically impossible to blind the clinical team performing the intervention. This may lead to potential research bias. Fourth, our sample size is small, and the power of the trial is low (70%) due to time and financial constraints. Increasing the power may led to different results.
Conclusions
Among elderly patients undergoing GI surgery, intraoperative GDFT based on the PVI showed no superiority to the conventional fluid management regimen in terms of overall complications, PONV, and PLOS, but was associated with a lower cardiopulmonary complication rate than usual fluid management.
Acknowledgements
The authors are grateful to Jian-Zhu, M.M. (Department of Anaesthesiology, First Hospital of Shanxi Medical University) for helping with the postoperative follow-up, XiaoHong-Xieyang (Clinical Research Institute, Shenzhen-Peking University, The Hong Kong University of Science & Technology Medical Center) for helping with data export from the REDCap database, and the surgical teams from the two hospitals involved in the study for their collaboration.
Abbreviations
- CFT
Conventional fluid therapy
- CI
Confidence interval
- GDFT
Goal-directed fluid therapy
- GI
Gastrointestinal
- OR
Odds ratio
- PACU
Post-anaesthesia care unit
- PLOS
Postoperative length of stay
- PONV
Postoperative nausea and vomiting
- PVI
Pleth variability index
- vs.
Versus
Authors’ contributions
Y.W. and X.W. wrote the main manuscript text and Y.Z. analysed and interpreted the data. J.Z., X.D., and Z.G. conducted the study, collected the data, and performed follow-up. X.W. designed the study, conducted the study, and revised the manuscript. All authors read and approved the final manuscript. All authors reviewed the manuscript.
Funding
This study was supported by the Shenzhen Healthcare Clinical Research Project (grant number [no.] SZLY2017001) and General Program for Clinical Research at Peking University Shenzhen Hospital (grant no. LCYJ2020016).
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the REDcap database (http://202.105.127.142:81/).
Declarations
Ethics approval and consent to participate
This study was approved by the Clinical Research Ethics Committee of Peking University Shenzhen Hospital (IRB 2017-001-2), and written informed consent was obtained from all participants in the trial.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Augestad KM, Delaney CP. Postoperative ileus: impact of pharmacological treatment, laparoscopic surgery and enhanced recovery pathways. World J Gastroenterol. 2010;16:2067–2074. doi: 10.3748/wjg.v16.i17.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care. 2010;14:R118. doi: 10.1186/cc9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloria SD, Panda NB, Jangra K, Bhagat H, Mandal B, Kataria K, Singla N, et al. Goal-directed fluid therapy versus conventional fluid therapy during craniotomy and clipping of cerebral aneurysm: a prospective randomized controlled trial. J Neurosurg Anesthesiol. 2022;34:407–414. doi: 10.1097/ANA.0000000000000769. [DOI] [PubMed] [Google Scholar]
- Cannesson M, Desebbe O, Rosamel P, Delannoy B, Robin J, Bastien O, et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101:200–6. doi: 10.1093/bja/aen133. [DOI] [PubMed] [Google Scholar]
- Challand C, Struthers R, Sneyd JR, Erasmus PD, Mellor N, Hosie KB, et al. Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br J Anaesth. 2012;108:53–62. doi: 10.1093/bja/aer273. [DOI] [PubMed] [Google Scholar]
- Corcoran T, Rhodes JE, Clarke S, Myles PS, Ho KM. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg. 2012;114:640–651. doi: 10.1213/ANE.0b013e318240d6eb. [DOI] [PubMed] [Google Scholar]
- Demirel İ, Bolat E, Altun AY, Özdemir M, Beştaş A. Efficacy of goal-directed fluid therapy via Pleth variability index during laparoscopic Roux-en-Y gastric bypass surgery in morbidly obese patients. Obes Surg. 2018;28:358–363. doi: 10.1007/s11695-017-2840-1. [DOI] [PubMed] [Google Scholar]
- Fischer MO, Lemoine S, Tavernier B, Bouchakour CE, Colas V, Houard M, et al. Individualized fluid management using the Pleth variability index: a randomized clinical trial. Anesthesiology. 2020;133:31–40. doi: 10.1097/ALN.0000000000003260. [DOI] [PubMed] [Google Scholar]
- Forget P, Lois F, de Kock M. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg. 2010;111:910–914. doi: 10.1213/ANE.0b013e3181eb624f. [DOI] [PubMed] [Google Scholar]
- Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97:820–6. doi: 10.1097/00000542-200210000-00012. [DOI] [PubMed] [Google Scholar]
- Gómez-Izquierdo JC, Trainito A, Mirzakandov D, Stein BL, Liberman S, Charlebois P, et al. Goal-directed fluid therapy does not reduce primary postoperative ileus after elective laparoscopic colorectal surgery: a randomized controlled trial. Anesthesiology. 2017;127(1):36–49. doi: 10.1097/ALN.0000000000001663. [DOI] [PubMed] [Google Scholar]
- Haas S, Trepte C, Hinteregger M, Fahje R, Sill B, Herich L, et al. Prediction of volume responsiveness using pleth variability index in patients undergoing cardiac surgery after cardiopulmonary bypass. J Anesth. 2012;26:696–701. doi: 10.1007/s00540-012-1410-x. [DOI] [PubMed] [Google Scholar]
- Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392–1402. doi: 10.1213/ANE.0b013e3181eeaae5. [DOI] [PubMed] [Google Scholar]
- Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89:622–632. doi: 10.1093/bja/aef220. [DOI] [PubMed] [Google Scholar]
- Hood JA, Wilson RJ. Pleth variability index to predict fluid responsiveness in colorectal surgery. Anesth Analg. 2011;113:1058–1063. doi: 10.1213/ANE.0b013e31822c10cd. [DOI] [PubMed] [Google Scholar]
- Jessen MK, Vallentin MF, Holmberg MJ, Bolther M, Hansen FB, Holst JM, et al. Goal-directed haemodynamic therapy during general anaesthesia for noncardiac surgery: a systematic review and meta-analysis. Br J Anaesth. 2022;128:416–433. doi: 10.1016/j.bja.2021.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewer JK, Wong MJ, Bird SJ, Habib AS, Parker R, George RB. Supplemental perioperative intravenous crystalloids for postoperative nausea and vomiting. Cochrane Database Syst Rev. 2019;3:CD012212. doi: 10.1002/14651858.CD012212.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans T, Neuss H, Strohauer M, Raue W, Haase O, Schink T, et al. Hypovolemia after traditional preoperative care in patients undergoing colonic surgery is underrepresented in conventional hemodynamic monitoring. Int J Colorectal Dis. 2006;21:693–697. doi: 10.1007/s00384-005-0065-6. [DOI] [PubMed] [Google Scholar]
- Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, Allison SP. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002;359:1812–1818. doi: 10.1016/S0140-6736(02)08711-1. [DOI] [PubMed] [Google Scholar]
- Lopes MR, Oliveira MA, Pereira VO, Lemos IP, Auler JO, Michard F. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care. 2007;11:R100. doi: 10.1186/cc6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbouisson LMS, Silva JM, Jr, Carmona MJC, Lopes MR, Assunção MS, Valiatti JLDS, et al. A pragmatic multi-center trial of goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery. BMC Anesthesiol. 2017;17(1):70. doi: 10.1186/s12871-017-0356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311:2181–2190. doi: 10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
- Perel A. Excessive variations in the plethysmographic waveform during spontaneous ventilation: an important sign of upper airway obstruction. Anesth Analg. 2014;119:1288–92. doi: 10.1213/ANE.0000000000000378. [DOI] [PubMed] [Google Scholar]
- Rollins KE, Lobo DN. Intraoperative goal-directed fluid therapy in elective major abdominal surgery: a meta-analysis of randomized controlled trials. Ann Surg. 2016;263:465–476. doi: 10.1097/SLA.0000000000001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5–14. doi: 10.1056/NEJMoa021108. [DOI] [PubMed] [Google Scholar]
- Szturz P, Folwarczny P, Kula R, Neiser J, Ševčík P, Benes J. Multi-parametric functional hemodynamic optimization improves postsurgical outcome after intermediate risk open gastrointestinal surgery: a randomized controlled trial. Minerva Anestesiol. 2019;85:244–54. doi: 10.23736/S0375-9393.18.12467-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the REDcap database (http://202.105.127.142:81/).