Abstract
Background:
As an adjunct to antidepressant treatment, Tai Chi Chih (TCC) is superior to health education and wellness (HEW) training in improving the general health of patients with geriatric depression (GD). This study investigated the brain connectivity changes associated with TCC and HEW in combination with antidepressant treatment in patients with GD.
Methods:
Forty patients with GD under stable antidepressant treatment underwent TCC training (n = 21) or HEW training (n = 19) for 12 weeks, and completed baseline and 3-month follow-up resting state magnetic resonance imaging scans. Within-group and between-group differences in parcel-to-parcel connectivity changes with intervention were evaluated by general linear modeling. Relationships between significant connectivity changes and symptom/resilience improvement were evaluated by partial least squares correlation analysis.
Results:
Significantly greater increases in connectivity with TCC than with HEW (FDR-corrected p < .05) were observed for 167 pairwise connections, most frequently involving the default mode network (DMN). In both groups, increased connectivity involving largely DMN regions was significantly and positively correlated with improvement in symptoms/resilience.
Limitations:
The sample size was relatively small, mainly due to neuroimaging contraindications (e.g., implants). Additionally, the standard antidepressant treatment varied greatly among patients, adding heterogeneity.
Conclusions:
Non-pharmacological adjuncts, such as TCC, may enhance DMN connectivity changes associated with improved depressive symptoms and psychological resilience in the treatment of GD.
Keywords: Mind-body intervention, Depression, Resting-state networks, Tai chi, Geriatric, Resilience
1. Introduction
Geriatric depression (GD) is associated with significant medical comorbidity, cognitive impairment, and suboptimal treatment response compared to that in younger adults with depression (Lenze et al., 2008; Mitchell and Subramaniam, 2005). More efficacious treatments to improve mood, cognition, and the quality of life in GD are urgently needed. As mind-body interventions, such as Tai Chi, can reduce negative emotions and systemic inflammation, and improve physical and psychological wellbeing (Abbott and Lavretsky, 2013; Bower and Irwin, 2016; Ibanez et al., 2020; Laird et al., 2018; Lavretsky et al., 2011; Solloway et al., 2016; Wang et al., 2010; Zhang et al., 2019), and show beneficial effects on depression, anxiety, and general stress management (Wang et al., 2014), they comprise a promising adjunct treatment to antidepressants to improve treatment efficacy.
We previously reported the results of a 3-month randomized single-blind controlled trial of Tai Chi Chih (TCC), a brief manualized version of Tai Chi practice, versus health education and wellness (HEW) training in older depressed adults stable on standard antidepressant therapies for at least four months. We found that both TCC and HEW, in combination with standard antidepressant treatment, improved depressive symptoms and psychological resilience, with TCC superior to HEW in improving general health (Lavretsky et al., 2022). However, the neural mechanisms underlying these improvements remain unknown.
Numerous studies on major depressive disorder (MDD) have indicated that depression is generally associated with dysconnectivity, especially among brain regions in the frontal cortex, default mode network (DMN), and cerebellum (Abdallah et al., 2017a; Abdallah et al., 2017b; Kraus et al., 2020; Li et al., 2018; Murrough et al., 2016). Additionally, in a recent study on MDD, patients with higher levels of peripheral inflammatory markers (C-reactive protein, interleukin-6, neutrophils) showed greater dysconnectivity mainly involving DMN and ventral attentional network regions (Aruldass et al., 2021). Such dysconnectivity may disrupt interoceptive processes linked to emotional regulation and motivation (Aruldass et al., 2021; Craig, 2002; Critchley and Garfinkel, 2017; Quadt et al., 2018). Thus, reversing brain dysconnectivity may be a treatment target. Based on this information, we hypothesized that TCC may increase connectivity, especially involving DMN regions, and that these increases would be associated with symptom improvement.
Therefore, we evaluated the effect of TCC, as an adjunct to antidepressant treatment, on intrinsic brain connectivity.
2. Materials and methods
2.1. Participants
Participants comprised older adults with MDD under stable antidepressant treatment who participated in a parent randomized controlled trial (RCT; NCT02460666) of (TCC) versus HEW training (as an active control for non-specific attention and social support effects). A subsample of these participants (TCC, n = 21, mean age ± standard deviation [SD] = 67.1 ± 7.4 years, 81 % females, HEW, n = 19, mean age ± SD = 68.2 ± 5.6 years, 79 % females) completed resting state magnetic resonance imaging (rsMRI) at baseline and 3 months of follow up (Table 1). Eligibility criteria for the RCT were as follows: age ≥ 60 years; a diagnosis of MDD according to Diagnostic and Statistical Manual (DSM-5) diagnostic criteria;(American Psychological Association, 2013) a score of ≥15 on Hamilton Depression Rating Scale (HAMD);(Hamilton, 1960) absence of dementia (i.e. a score of ≥25 on the Mini-Mental State Examination and no established diagnosis of dementia). (Folstein et al., 1975) Exclusion criteria were as follows: a history of psychiatric disorders over than MDD, comorbid anxiety, or insomnia; acute, severe, or unstable medical illness; and a diagnosis of moderate to severe cognitive impairment. Additionally, participants who consented to the neuroimaging component could not have any MRI-incompatible implants or other imaging contraindications. Participants were stable on one or more antidepressants for at least 4 months before starting the trial. All participants were TCC naïve and did not have any other ongoing mind-body practices. Additionally, participants were asked not to initiate any new mind-body classes for the duration of the study.
Table 1.
Demographic and clinical data.
| TCC (n = 21) | HEW (n = 19) | P-value | |
|---|---|---|---|
|
| |||
| Female sex, n (%) | 17 (81) | 15 (79) | 0.59 |
| Age (yrs) | 67.1 ± 7.4 | 68.2 ± 5.4 | 0.29 |
| Education (yrs) | 15.9 ± 2.0 | 16.1 ± 2.1 | 0.69 |
| Race, n (%) | |||
| White | 16 (76) | 17 (90) | 1.00 |
| Asian | 2 (10) | 1 (5) | |
| Black | 2 (10) | 1 (5) | |
| Hispanic | 1 (5) | 0 (0) | |
| Homework (d/wk) | 4.48 ± 2.34 | 4.26 ± 3.05 | 0.92 |
| HAMD | |||
| Pre-intervention | 18.6 ± 3.9 | 18.1 ± 3.3 | 0.64 |
| Post-intervention | 8.7 ± 5.9 | 10.0 ± 4.5 | 0.28 |
| GDS | |||
| Pre-intervention | 15.6 ± 6.1 | 14.2 ± 7.0 | 0.58 |
| Post-intervention | 9.7 ± 6.6 | 12.9 ± 7.3 | 0.02 |
| CDRISC | |||
| Pre-intervention | 61.9 ± 13.4 | 60.6 ± 14.6 | 1.00 |
| Post-intervention | 65.0 ± 14.5 | 61.8 ± 14.9 | 0.51 |
Data are presented as mean ± SD, unless otherwise indicated. Group differ- ences in post-intervention scores were evaluated controlling for pre- intervention values. fIn the TCC group, one participant did not complete the post-intervention language assessment.
Abbreviations: TCC, Tai Chi Chih (TCC); HEW, health education and wellness training; HAMD, Hamilton Depression Rating Scale; GDS, Geriatric Depression Scale; CDRISC, Connor-Davidson Resilience Scale.
The study was approved by the UCLA Institutional Review Board, and written informed consent was obtained from all participants.
2.2. Study design
Participants were randomized (1:1) to undergo TCC or HEW for 12 weeks. Classes (60 min/week) were held in person, with the exception of the last recruited cohort, which received 6 in-person classes and 6 virtual classes due to a COVID-19 quarantine order on March 17, 2020. Participants were required to attend 9 of the 12 total weekly classes.
In the TCC group, participants were informed that TCC constitutes a health management intervention, incorporating meditation and physical activity to promote a sense of well-being and control over negative symptoms associated with depression. The TCC protocol was adapted from “Tai-Chi-Chih! Joy Through Movement” (Stone, 1996). Each class allowed 10 min of warm-up (e.g., stretching, breathing) and 5 min of cool down. Additionally, participants were instructed to practice at home for at least 20 min per day using handouts.
In the HEW group, participants were informed the HEW intervention was designed to help reduce the severity of depressive symptoms. The HEW protocol followed a manual of educational information and learning objectives and patient activities to promote the integration of material. Additionally, participants were instructed to practice at home by performing computer searches on health topics discussed in the session for 20 min per day, which was then discussed at the next class. The HEW condition served as an active control for nonspecific treatment elements such as attention and group support that could pose rival explanations for the effectiveness of TCC. This novel use of a non-exercise control intervention, which matched the TCC intervention in duration, frequency and social contact, represents an important methodological advancement (Lawlor and Hopker, 2001).
Additional details regarding the non-imaging portion of the study design can be found in our previous publication (Lavretsky et al., 2022).
Participants completed rsMRI scanning and behavioral assessments at baseline, i.e., before intervention initiation, and at 3 months, after completing the intervention. Depressive symptom severity was assessed by the HAMD (Hamilton, 1960) and Geriatric Depression Scale (GDS) (Yesavage, 1988). Symptom improvement scores were calculated as follows: (−1 × (3-month score – baseline score)). Resilience was assessed by the Connor-Davidson Resilience Scale (CDRISC) (Connor and Davidson, 2003). Improvement in resilience was calculated as follows: (3-month CDRISC score – baseline CDRISC score).
2.3. Neuroimaging
MRI-eligible participants underwent scanning at baseline and the 3-month follow up. High-resolution T1-weighted and T2-weighted MR images and resting-state BOLD images were collected using a Prisma-fit system (Siemens, Erlangen, Germany) with a 32-channel head coil. The multi-echo MPRAGE scan was collected using the following parameters: isotropic 0.8-mm3 voxels; 208 slices; TR: 2400 ms; TE: 2.24 ms; TI: 1060 ms; FoV read: 256 mm; matrix size: 256 × 240 mm; and flip angle: 8 degrees. The T2-weighted SPC scan was performed with the following parameters: isotropic 0.8-mm3 voxels; 208 slices; TR: 3200 ms; TE: 564 ms; FoV read: 256 mm; matrix size: 256 × 240 mm; and flip angle: 8 degrees. Resting-state scans (eyes open, blinking freely) were collected in both anterior-posterior and posterior-anterior directions (5.2 min each; total scan time, 10.4 min) using the following parameters: isotropic 2.0-mm3 voxels; 72 slices; TR: 720 ms; TE: 37.0 ms; FoV read: 208 mm; matrix size: 208 × 208 mm; and flip angle: 52 degrees.
Imaging data were pre-processed with the minimal Human Connectome Project (HCP) pipeline, including structural preprocessing of the T1-weighted and T2-weighted images by PreFreeSurfer, FreeSurfer, and PostFreeSurfer and functional preprocessing of the BOLD images by fM-RIVolume and fMRISurface, followed by denoising using multi-run ICAFIX.(Glasser et al., 2018; Glasser et al., 2016a; Glasser et al., 2016b; Glasser et al., 2013; Robinson et al., 2018) The denoised and normalized resting-state images were parcellated according to the Cole-Anticevic Brain Network Parcellation (CAB-NP) atlas (Ji et al., 2019). This atlas extends the HCPMMP atlas by including a fine-grained parcellation of subcortical regions. The CAB-NP atlas comprises 360 cortical and 358 subcortical parcels, with each parcel assigned to one of 12 major networks (specifically, the DMN, auditory network, frontoparietal network [FPN], language network, dorsal attention network, cingulo-opercular network, sensorimotor network, posterior multimodal network, ventral multimodal network, primary visual network, and secondary visual network) (Ji et al., 2019). For each participant, normalized (Fisher’s z-transformed) pairwise correlations between parcel time-series were computed, creating 718 × 718 connectivity matrices for each scan (pre- and post-intervention). Finally, the change in connectivity was calculated by subtracting these matrices (post-intervention minus pre-intervention).
2.4. Statistical analysis
Group differences in clinical characteristics were evaluated using the non-parametric Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables. Group differences in post-intervention HAMD/GDS/CDRISC scores were evaluated in general linear models controlling for pre-intervention values. Statistical analyses were performed using SPSS version 26 (IBM Corp. Albany, NY).
Within-group and between-group differences in parcel-to-parcel connectivity changes with intervention were evaluated by general linear modeling, with correction for multiple comparisons (10,000 permutations; false discovery rate [FDR]) and adjustment for sex and age using GraphVar (Waller et al., 2018). Pairwise connections showing significant group differences in the change in connectivity were further evaluated by partial least squares correlation analysis, a multivariate analytical approach that identifies relationships between patterns in two or more blocks of variables (McIntosh and Lobaugh, 2004). In the present analysis, one block of variables comprised improvements in symptoms (HAMD and GDS) and resilience (CDRISC) and the other block comprised the change in connectivity for pairwise connections of interest. Latent variables (or linear combinations of the original variables), are derived from each block such that they have maximal covariance with each other. Partial least squares correlation analysis was implemented in Matlab using plscmd scripts, with boot-strap estimation (5000 samples) (https://www.rotman-baycrest.on.ca). P-values <.05 were considered significant.
3. Results
3.1. Demographic and clinical data
Baseline demographics and clinical data, including pre-intervention and improvement scores, are summarized in Table 1. There were no significant differences between groups in baseline demographic and clinical characteristics. Additionally, there was no significant difference between groups in homework compliance (average number of days per week). The 3-month improvement in depressive symptoms was significantly greater in the TCC group than in the HEW group for the GDS score (F(1,38) = 7.221; p = .01), and the groups did not differ in their changes in HAMD or CDRISC measures.
3.2. Connectivity changes
In total, 167 pairwise connections showed a significantly greater increase in connectivity in the TCC group than in the HEW group (FDR-corrected p < .05). These significant differences most frequently involved the DMN (Fig. 1). Further, the TCC group showed numerous significant increases in connectivity (262 connections among ~257 K pairwise connections), with very few significant decreases. In contrast, the HEW group showed very few significant increases (5 connections) (Fig. 1).
Fig. 1.
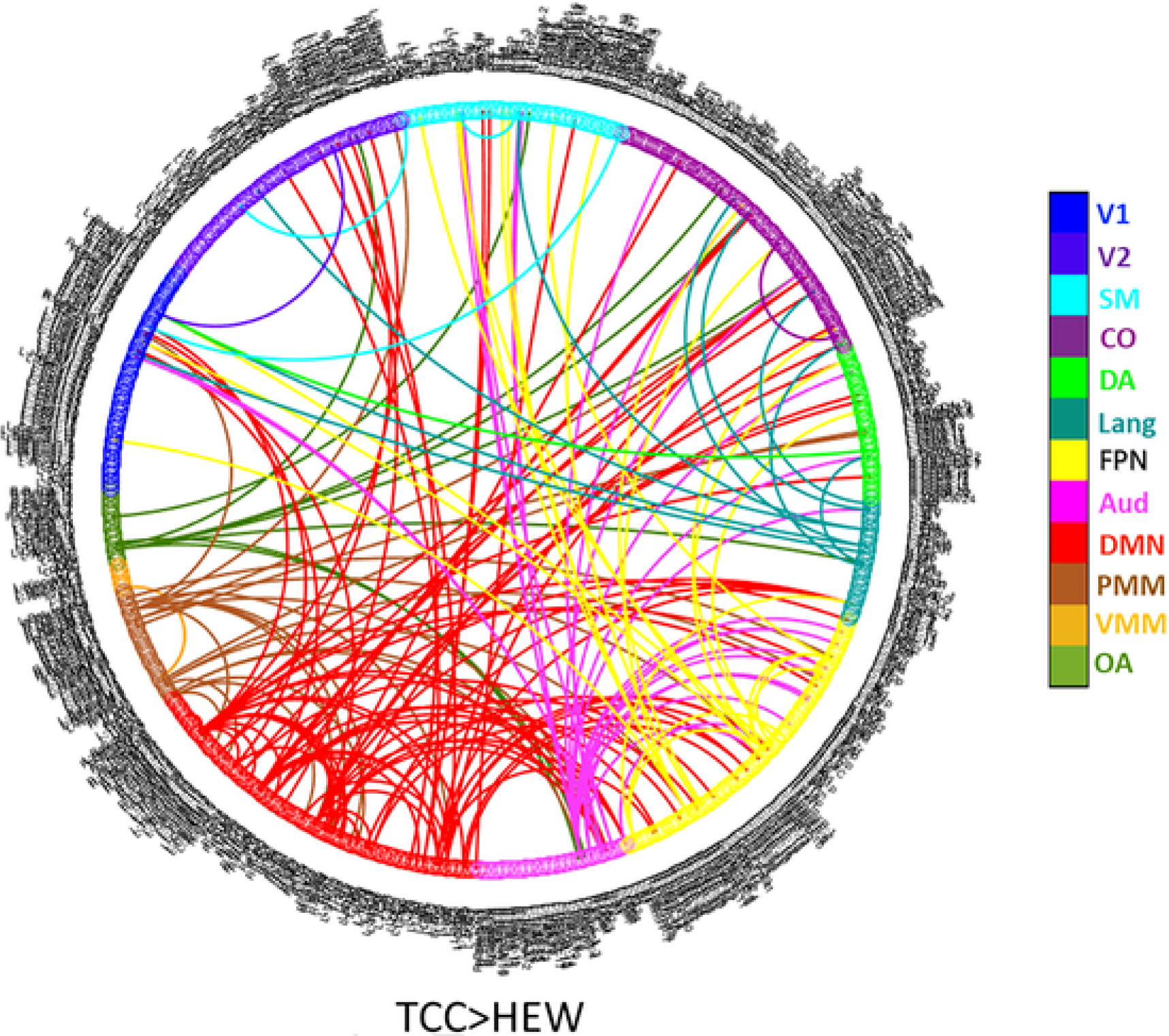
Greater increases in connectivity with T’ai Chi Chih training than with health education wellness training (corrected_p < 0.05). (A) V1, primary visual; V2, secondary visual; CO, cingulo-opercular; DMN, default mode network; FPN, frontoparietal network; PMM, posterior multimodal; SM, sensorimotor; DA, dorsal attention; OA, orbito-affective; VMM, ventral multimodal; Lang, language; Aud, auditory.
3.3. Relationships between improvement in clinical scores and connectivity changes
The 262 pairwise connections that showed significantly increases in the TCC group were submitted to PLSC analysis. In the TCC group, the first latent variable (accounting for 48.2 % of the cross-block correlation) reflected connectivity changes significantly correlated with improvement in HAMD and CDRISC scores, but not GDS scores (correlation between latent variable brain scores and behavioral scores: r = 0.53, 95 % confidence interval [CI]: 0.37–0.82, p = .01; r = 0.61, 95 % CI: 0.48–0.86, p = .003; r = 0.22, 95 % CI: −0.04–0.52, p = .34, respectively). The second latent variable (accounting for 32.0 % of the cross-block correlation) reflected connectivity changes significantly correlated with improvement in GDS scores, but not HAMD/CDRISC scores (r = 0.64, 95 % CI: 0.52–0.86, p = .002; r = 0.14, 95 % CI:−0.20–0.55, p = .38; r = −0.18, 95 % CI: −0.55–0.22, p = .43, respectively). The pairwise connections reliably contributing to each latent variable (p < .05 by bootstrap estimation) are shown in Fig. 2A. In the HEW group, the first latent variable (accounting for 42.3 % of the cross-block correlation) reflected connectivity changes significantly correlated with improvement in GDS and CDRISC scores, but not HAMD scores (r = 0.87, 95 % CI: 0.64–0.93, p < .001; r = 0.83, 95 % CI: 0.64–0.92, p < .001; r = 0.09, 95 % CI: −0.39–0.56, p = .71, respectively). The second latent variable (accounting for 36.6 % of the cross-block correlation) reflected connectivity changes significantly correlated with improvement in HAMD scores, but not GDS/CDRISC scores (r = 0.86, 95 % CI: 0.78–0.95, p < .001; r = −0.02, 95 % CI: −0.42–0.52, p = .94; r = −0.03, 95 % CI: −0.42–0.46, p = .90, respectively). The pairwise connections reliably contributing to the each latent variable (p < .05 by bootstrap estimation) are shown in Fig. 2B.
Fig. 2.
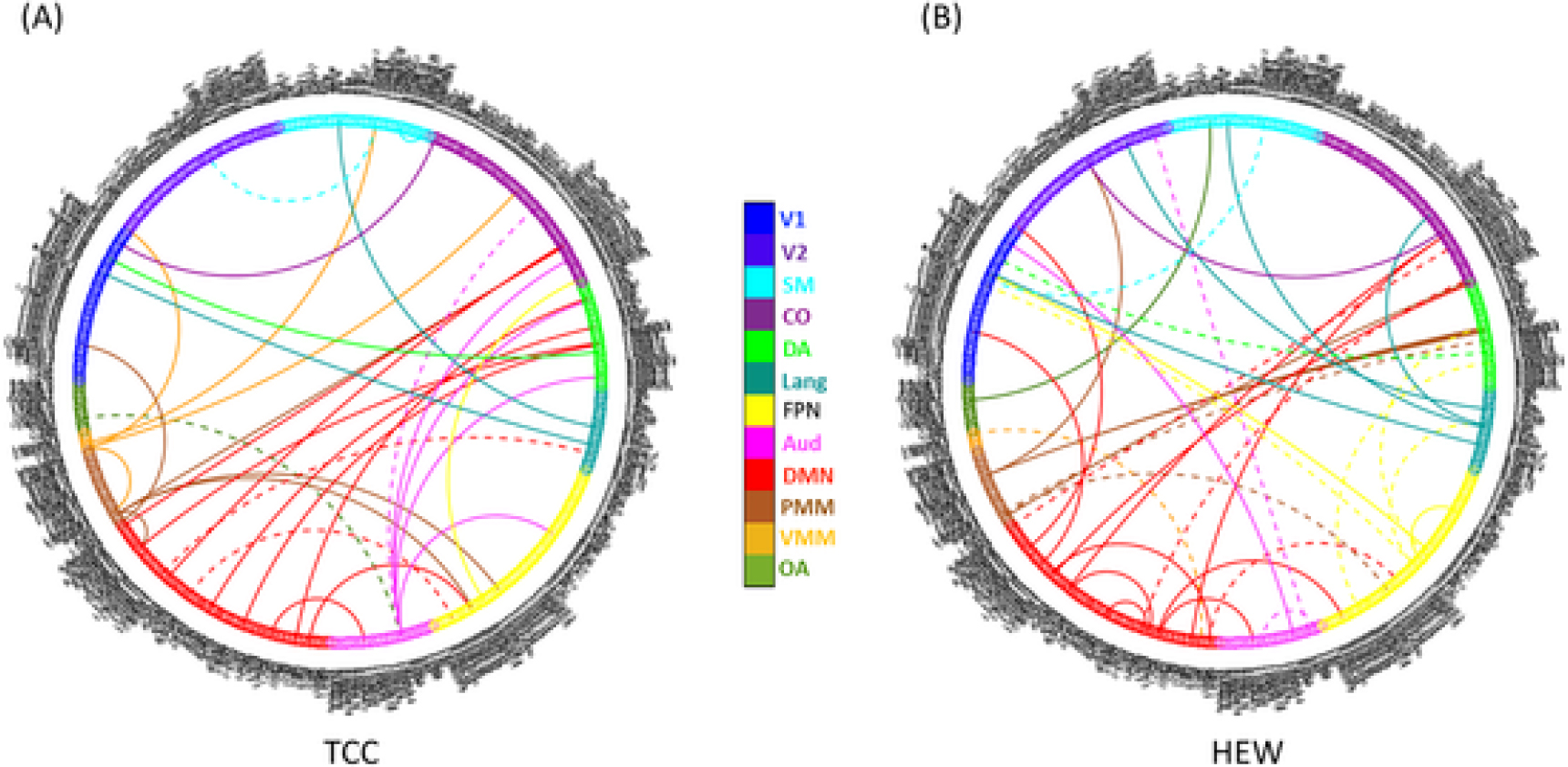
Connectivity changes associated with improvements in symptoms and resilience (bootstrap_p < 0.05). (A) Dashed lines indicate connectivity increases associated with improvement in HAMD and CDRISC (first latent variable) and solid lines indicate connectivity increases associated with improvement in GDS (second latent variable) in the TCC group. (B) Dashed lines indicate connectivity increases associated with improvement in GDS and CDRISC (first latent variable) and solid lines indicate connectivity increases associated with improvement in HAMD (second latent variable) in the HEW group. HAMD, Hamilton Depression Rating Scale; GDS, Geriatric Depression Scale; TCC, Tai Chi Chih; HEW, health education and wellness; V1, primary visual; V2, secondary visual; CO, cingulo-opercular; DMN, default mode network; FPN, frontoparietal network; PMM, posterior multimodal; SM, sensorimotor; DA, dorsal attention; OA, orbito-affective; VMM, ventral multi-modal; Lang, language; Aud, auditory.
4. Discussion
In the present study, we examined the impact of TCC training compared to HEW as an adjunct treatment to antidepressant medication on brain connectivity in older adults with depression. In contrast to HEW, TCC training increased connectivity between numerous regions, especially those of the DMN, and this increased connectivity was associated with improvements in depressive symptoms and resilience.
The results from the present study are of significance, given the importance of the DMN in the treatment of GD. The key cortical nodes of the DMN include the precuneus and posterior cingulate cortex, medial prefrontal cortex, inferior parietal lobe and lateral temporal cortex. Furthermore, recent research supports the involvement of portions of the cerebellum and other subcortical structures in the DMN (Ji et al., 2019). The DMN plays a role in a variety of functions, including social cognition, episodic memory, and self-referential processes (Greicius and Menon, 2004; Gusnard et al., 2001; Iacoboni et al., 2004). A recent large-scale study reported decreased DMN connectivity as associated with recurrent major depression and medication usage (Yan et al., 2019). Furthermore, previous GD treatment studies have reported increased DMN connectivity in posterior and lateral DMN regions and decreased DMN connectivity in frontal regions following antidepressant treatment, especially in remitters (Andreescu et al., 2013; Karim et al., 2017). Additionally, in our previous pharmacological study, greater symptom improvement following escitalopram treatment in adults with GD and subjective memory complaints was associated with increased DMN connectivity in posterior and lateral DMN regions, and this relationship was strengthened with the addition of memantine. Thus, numerous studies suggest that increased DMN connectivity in posterior/lateral DMN nodes plays a role in ameliorating depressive symptoms. Consistent with this, the present study found that increased connectivity involving multiple DMN regions (particularly prefrontal and cerebellar DMN regions) was associated with improvement in symptoms and resilience in the TCC group, and, to a more limited extent, in the HEW group.
In the present study, patients with GD who underwent TCC training showed increased connectivity among regions in the DMN and cingulo-opercular network (also known as the salience/ventral attention network regions in other atlases), which has previously been shown to have reduced connectivity in patients with MDD with heightened peripheral inflammation (Aruldass et al., 2021). Tai Chi interventions, as well as other mind-body interventions, are reported to affect inflammatory pathways (Bower and Irwin, 2016). For example, a previous RCT evaluating TCC on circulating inflammatory markers in healthy older adults showed a greater drop in circulating interleukin-6 with 16 weeks of TCC than with HEW among those with elevated levels at baseline (Irwin and Olmstead, 2012). Additionally, in a previous study, we showed a greater decline in C-reactive protein levels in patients with GD for escitalopram combined with TCC than for escitalopram combined with HEW (Lavretsky et al., 2011). As the DMN and cingulo-opercular network comprise an important community in the autonomic connectome (Ruffle et al., 2021), increased connectivity among DMN and cingulo-opercular network regions with TCC training could result in changes in inflammatory activity (Bower and Irwin, 2016; Irwin and Cole, 2011). Future studies are needed to examine the coordination among brain connectivity, symptom, and inflammatory marker changes with TCC training.
The present study has several limitations to acknowledge. The sample size was relatively small, as some patients who enrolled in the RCT were unable to participate in the neuroimaging component due to contraindications (e.g., implants); additionally, some participants dropped out of the parent study and did not complete follow-up scanning. Given this small sample size, statistical thresholds were relatively liberal; thus, the results should be considered as preliminary. Furthermore, although participants were asked to maintain their usual medications and not start new treatment until the completion of the study, some participants may have changed medications during the study. In addition, the standard antidepressant treatment varied greatly among patients, adding heterogeneity. Furthermore, although both groups were informed that their respective training program was designed to help with depression symptoms, differences in the expectations may have existed, potentially influencing the results. Finally, these results may not be representative of patients with acute medical illness, severe depression, more severe cognitive impairment, or more demographically diverse samples than the present sample.
5. Conclusion
The present study provides preliminary evidence that non-pharmacological adjuncts, such as TCC, may enhance DMN connectivity changes in the treatment of GD, which was associated with improved depressive symptoms and psychological resilience. Future studies should examine the role of these neural changes in symptomatic improvement over a longer period of follow up, in a larger number of patients.
Acknowledgements
This work was funded by NIH grants AT008383 and AT009198 awarded to HL, and was further supported by the National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
Role of the funding source
The funders had no role in the study design; collection, analysis and interpretation of data; writing of the report; and decision to submit the article for publication.
Abbreviations
- CAB-NP
Cole-Anticevic brain network parcellation
- CDRISC
Connor-Davidson Resilience Scale
- DMN
default mode network
- FDR
false discovery rate
- FPN
frontoparietal network
- GD
geriatric depression
- GDS
Geriatric Depression Scale
- HAMD
Hamilton Depression Rating Scale
- HEW
health education and wellness
- MDD
major depressive disorder
- RCT
randomized controlled trial
- rsMRI
resting state magnetic resonance imaging
- TCC
Tai Chi Chih
Biography
Dr. Helen Lavretsky is a Professor In-Residence in the Department of Psychiatry at UCLA and a geriatric integrative psychiatrist with federally funded research program in geriatric depression and integrative mental health (NIMH, PCORI, and NCCIH) using mind-body interventions. She is a recipient of the Career Development award from NIMH and the NCCIH, and other prestigious research awards. Her current research studies include investigations of psychopharmacological treatment of geriatric depression, mild cognitive impairment and the use of Tai Chi and yoga for treatment and prevention of late-life mood and cognitive disorders. She is the Distinguished Life Fellow of the American Psychiatric Association, the American Association for Geriatric Psychiatry, and the Fellow of the American College of Neuropsychopharmacology, and the recipient of the Distinguished Investigator awards for research in geriatric psychiatry from the American College of Psychiatrists and the American Association for Geriatric Psychiatry. She is the Semel Scholar in Integrative Mental Health and the Director of the Late-life mood, stress and wellness Research program, the Integrative Psychiatry and the Post-COVID Psychiatry programs. She is the President Elect of the American Association for Geriatric Psychiatry.
Footnotes
Declaration of competing interest
All authors declare no conflicts of interest.
CRediT authorship contribution statement
HL, KLN, PS, MMM, BK-S, and LE, designed the study and wrote the protocol. MMM, PS, and LE collected the data. LAK and MMM performed the analysis. LAK and MMM wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
References
- Abbott R, Lavretsky H, 2013. Tai Chi and Qigong for the treatment and prevention of mental disorders. Psychiatr Clin. North Am. 36, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill CL, Salas R, Averill LA, Baldwin PR, Krystal JH, Mathew SJ, Mathalon DH, 2017a. Prefrontal connectivity and glutamate transmission: relevance to depression pathophysiology and ketamine treatment. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, DeWilde KE, Wong E, Anticevic A, Tang CY, Iosifescu DV, Charney DS, Murrough JW, 2017b. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 42, 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association, 2013. Diagnostic And Statistical Manual of Mental Disorders, 5th ed. Author, Arlington, VA. [Google Scholar]
- Andreescu C, Tudorascu DL, Butters MA, Tamburo E, Patel M, Price J, Karp JF, Reynolds CF 3rd, Aizenstein H, 2013. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. 214, 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruldass AR, Kitzbichler MG, Morgan SE, Lim S, Lynall ME, Turner L, Vertes P, Wellcome Trust Consortium for Neuroimmunology of Mood, D., Alzheimer’s, D., Cavanagh J, Cowen P, Pariante CM, Harrison NA, Bullmore ET, Wellcome Trust Consortium for Neuroimmunology of Mood, 2021. Dysconnectivity of a brain functional network was associated with blood inflammatory markers in depression. Brain Behav. Immunol. 98, 299–309. [DOI] [PubMed] [Google Scholar]
- Bower JE, Irwin MR, 2016. Mind-body therapies and control of inflammatory biology: a descriptive review. Brain Behav. Immun. 51, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, Davidson JR, 2003. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety 18, 76–82. [DOI] [PubMed] [Google Scholar]
- Craig AD, 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Garfinkel SN, 2017. Interoception and emotion. Curr. Opin. Psychol. 17, 7–14. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Bijsterbosch JD, Harrison SJ, Harms MP, Anticevic A, Van Essen DC, Smith SM, 2018. Using temporal ICA to selectively remove global noise while preserving global signal in functional MRI data. NeuroImage 181, 692–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC, 2016a. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Smith SM, Marcus DS, Andersson JL, Auerbach EJ, Behrens TE, Coalson TS, Harms MP, Jenkinson M, Moeller S, Robinson EC, Sotiropoulos SN, Xu J, Yacoub E, Ugurbil K, Van Essen DC, 2016b. The Human Connectome Project’s neuroimaging approach. Nat. Neurosci. 19, 1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M, Consortium WU-MH, 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V, 2004. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J. Cogn. Neurosci. 16, 1484–1492. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME, 2001. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 98, 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, Molnar-Szakacs I, Moritz M, Throop CJ, Fiske AP, 2004. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. NeuroImage 21, 1167–1173. [DOI] [PubMed] [Google Scholar]
- Ibanez GE, Fennie K, Larkey L, Hu N, Algarin AB, Valdivia C, Lavretsky H, 2020. A tai chi/qigong intervention for older adults living with HIV: a study protocol of an exploratory clinical trial. Trials 21, 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Cole SW, 2011. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 11, 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, 2012. Mitigating cellular inflammation in older adults: a randomized controlled trial of Tai Chi Chih. Am. J. Geriatr. Psychiatry 20, 764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji JL, Spronk M, Kulkarni K, Repovs G, Anticevic A, Cole MW, 2019. Mapping the human brain’s cortical-subcortical functional network organization. NeuroImage 185, 35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim HT, Andreescu C, Tudorascu D, Smagula SF, Butters MA, Karp JF, Reynolds C, Aizenstein HJ, 2017. Intrinsic functional connectivity in late-life depression: trajectories over the course of pharmacotherapy in remitters and non-remitters. Mol. Psychiatry 22, 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus C, Mkrtchian A, Kadriu B, Nugent AC, Zarate CA Jr, Evans JW, 2020. Evaluating global brain connectivity as an imaging marker for depression: influence of preprocessing strategies and placebo-controlled ketamine treatment. Neuropsychopharmacology 45, 982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird KT, Paholpak P, Roman M, Rahi B, Lavretsky H, 2018. Mind-body therapies for late-life mental and cognitive health. Curr. Psychiatry Rep. 20, 2. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Alstein LL, Olmstead RE, Ercoli LM, Riparetti-Brown M, Cyr NS, Irwin MR, 2011. Complementary use of tai chi chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. Am. J. Geriatr. Psychiatry 19, 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Milillo MM, Kilpatrick L, Grzenda A, Wu P, Nguyen SA, Ercoli LM, Siddarth P, 2022. A randomized controlled trial of tai chi chih or health education for geriatric depression. Am. J. Geriatr. Psychiatry 30 (3), 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Hopker SW, 2001. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ 322, 763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze EJ, Sheffrin M, Driscoll HC, Mulsant BH, Pollock BG, Dew MA, Lotrich F, Devlin B, Bies R, Reynolds CF 3rd, 2008. Incomplete response in late-life depression: getting to remission. Dialogues Clin. Neurosci. 10, 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BJ, Friston K, Mody M, Wang HN, Lu HB, Hu DW, 2018. A brain network model for depression: from symptom understanding to disease intervention. CNS Neurosci. Ther. 24, 1004–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ, 2004. Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage 23 (Suppl. 1), S250–S263. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Subramaniam H, 2005. Prognosis of depression in old age compared to middle age: a systematic review of comparative studies. Am. J. Psychiatry 162, 1588–1601. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Abdallah CG, Anticevic A, Collins KA, Geha P, Averill LA, Schwartz J, DeWilde KE, Averill C, Jia-Wei Yang G, Wong E, Tang CY, Krystal JH, Iosifescu DV, Charney DS, 2016. Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum. Brain Mapp. 37, 3214–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadt L, Critchley HD, Garfinkel SN, 2018. The neurobiology of interoception in health and disease. Ann. N. Y. Acad. Sci. 1428, 112–128. [DOI] [PubMed] [Google Scholar]
- Robinson EC, Garcia K, Glasser MF, Chen Z, Coalson TS, Makropoulos A, Bozek J, Wright R, Schuh A, Webster M, Hutter J, Price A, Cordero Grande L, Hughes E, Tusor N, Bayly PV, Van Essen DC, Smith SM, Edwards AD, Hajnal J, Jenkinson M, Glocker B, Rueckert D, 2018. Multimodal surface matching with higher-order smoothness constraints. NeuroImage 167, 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffle JK, Hyare H, Howard MA, Farmer AD, Apkarian AV, Williams SCR, Aziz Q, Nachev P, 2021. The autonomic brain: multi-dimensional generative hierarchical modelling of the autonomic connectome. Cortex 143, 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solloway MR, Taylor SL, Shekelle PG, Miake-Lye IM, Beroes JM, Shanman RM, Hempel S, 2016. An evidence map of the effect of Tai Chi on health outcomes. Syst. Rev. 5, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JF, 1996. Tai-Chi-Chih! Joy Through Movement. Good Karma Publishing, Boston, MA. [Google Scholar]
- Waller L, Brovkin A, Dorfschmidt L, Bzdok D, Walter H, Kruschwitz JD, 2018. GraphVar 2.0: a user-friendly toolbox for machine learning on functional connectivity measures. J. Neurosci. Methods 308, 21–33. [DOI] [PubMed] [Google Scholar]
- Wang C, Bannuru R, Ramel J, Kupelnick B, Scott T, Schmid CH, 2010. Tai Chi on psychological well-being: systematic review and meta-analysis. BMC Complement. Altern. Med. 10, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Lee EK, Wu T, Benson H, Fricchione G, Wang W, Yeung AS, 2014. The effects of tai chi on depression, anxiety, and psychological well-being: a systematic review and meta-analysis. Int. J. Behav. Med. 21, 605–617. [DOI] [PubMed] [Google Scholar]
- Yan CG, Chen X, Li L, Castellanos FX, Bai TJ, Bo QJ, Cao J, Chen GM, Chen NX, Chen W, Cheng C, Cheng YQ, Cui XL, Duan J, Fang YR, Gong QY, Guo WB, Hou ZH, Hu L, Kuang L, Li F, Li KM, Li T, Liu YS, Liu ZN, Long YC, Luo QH, Meng HQ, Peng DH, Qiu HT, Qiu J, Shen YD, Shi YS, Wang CY, Wang F, Wang K, Wang L, Wang X, Wang Y, Wu XP, Wu XR, Xie CM, Xie GR, Xie HY, Xie P, Xu XF, Yang H, Yang J, Yao JS, Yao SQ, Yin YY, Yuan YG, Zhang AX, Zhang H, Zhang KR, Zhang L, Zhang ZJ, Zhou RB, Zhou YT, Zhu JJ, Zou CJ, Si TM, Zuo XN, Zhao JP, Zang YF, 2019. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc. Natl. Acad. Sci. U. S. A. 116, 9078–9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, 1988. Geriatric depression scale. Psychopharmacol. Bull. 24, 709–711. [PubMed] [Google Scholar]
- Zhang S, Zou L, Chen LZ, Yao Y, Loprinzi PD, Siu PM, Wei GX, 2019. The effect of tai chi chuan on negative emotions in non-clinical populations: a meta-analysis and systematic review. Int. J. Environ. Res. Public Health 16. [DOI] [PMC free article] [PubMed] [Google Scholar]


