Abstract
Background
The end of smallpox in 1980 and the subsequent stopping of vaccination against smallpox was followed by the emergence of monkeypox (mpox), a viral disease of animal origin, meaning that it is transmitted from animal to human. The symptoms of mpox are similar to smallpox, except that they are less severe in terms of clinical features. In the case of public health, the mpox virus is one of the most important orthopoxviruses (such as variola, cowpox, and vaccinia) that come from the family Poxviridae. Mpox occurs mostly in central Africa and sometimes in tropical rainforests or some urban areas. Also, there are threats other than COVID-19, that must be addressed and prevented from spreading, as there has been an outbreak of mpox cases since May 7, 2022, throughout the USA, Europe, Australia, and part of Africa.
Objectives
In this review, we will discuss mpox between the past, the present and during the COVID-19 pandemic. Also, it offers an updated summary of the taxonomy, etiology, transmission, and epidemiology of mpox illness. In addition, the current review aims to highlight the importance of emerging pandemics in the same era such as mpox and COVID-19.
Methods
A literature search was done for the study using online sources like PubMed and Google Scholar. Publications in English were included. Data for study variables were extracted. After the duplicate articles were eliminated, full-text screening was performed on the papers' titles and abstracts.
Results
The evaluation included a series documenting mpox virus outbreaks, and both prospective and retrospectiveinvestigations.
Conclusions
monkeypox is a viral disease caused by the monkeypox virus (MPXV), which is primarily found in central and western Africa. The disease is transmitted from animals to humans and presents symptoms similar to those of smallpox, including fever, headache, muscle aches, and a rash. Monkeypox can lead to complications such as secondary integument infection, bronchopneumonia, sepsis, and encephalitis, as well as corneal infection that can result in blindness. There is no specific clinically proven treatment for monkeypox, and treatment is primarily supportive. However, antiviral drugs and vaccines are available for cross-protection against the virus, and strict infection control measures and vaccination of close contacts of affected individuals can help prevent and control outbreaks.
Keywords: Mpox, Smallpox, COVID-19, Outbreak, Infectious diseases
Introduction
Since the start of the COVID-19 pandemic at the end of 2019, the world has been trying to control the rapid spread of the SARS-COV-2 virus and developing a vaccination dose against this virus and its variants. At the beginning of 2022, the world started recovering from the COVID-19 pandemic and also began to think about the mistakes that occurred, which did not contain the SARS-COV-2 virus in the global health system, as there are still threats from infectious diseases due to the movement of people worldwide [1], [2]. Unfortunately, the repeated warnings and continuous calls for the prevention of these infectious diseases have gone unheeded, and this was demonstrated by the outbreak of human smallpox cases since May 7, 2022, throughout Europe, the Americas, Australia, and part of Africa [3], [4]. During the ongoing outbreak of mpox in humans, the first mpox case was reported by Health Security in the United Kingdom on May 7, 2022, and was found that they had a travel history to Nigeria, Africa. A week later on May 14, 2022, two other cases were discovered living in the same area, but they have no travel history in or outside of the United Kingdom. At the same time, it was confirmed that they had no contact with the case that was discovered on May 7 that traveled to Africa [5]. Over time, new cases of mpox were continuously reported from 12 World Health Organization (WHO) member countries in three different regions. As of May 21, 2022, there were 92 laboratory-confirmed cases and 28 suspected mpox cases, as these cases were reported to the WHO from the United Kingdom, the United States of America, Canada, Portugal, France, Spain, Germany, Sweden, Belgium, and Australia. Generally, there is an expectation of increasing cases. We urgently need to identify the epidemiological links between these cases that are not known at the moment. While mpox is mostly unknown to medical professionals, especially front-line healthcare staff in prehospital, emergency departments, hospitals, and acute care/sexually transmitted disease clinics, there is an urgent need for fast access to clear, concise, fact-based information [6]. Currently, no deaths have been reported, but at the same time, many unusual and uncomfortable aspects of these outbreaks need to be studied closely [4]. In the weekly report from 13 to 19 February, the number of new cases worldwide decreased by 55.1% when compared to the previous week (111 cases, 06 Feb - 12 Feb). The vast majority of cases were reported in both the Americas (86%) and Africa (6.4%). Brazil (n = 10,808), the United States of America (n = 29,987), Mexico (n = 3828), Peru (n = 3752), Spain (n = 7538), Colombia (n = 4080), Germany (n = 3692), France (n = 4128), The United Kingdom (n = 3735), and Canada (n = 1460) were the top ten globally affected nations, accounting for 84.9% of all reported cases worldwide.[7]. This review summarizes the history, biology, epidemiology, risk factors, pathogenesis, and clinical presentation of the Monkey Pox Virus (MPV). In addition to highlighting the current knowledge about available treatments and vaccines against this virus in the COVID-19 era.
The origin of the mpox virus (history and epidemiology)
MPV, a virus that belongs to the orthopoxvirus genus and comes from the family Poxviridae, was first recognized in infected monkeys in a Danish laboratory in 1958, many years before it became a human-relevant disease [8], [9]. In 1970, during a decline in the incidence of smallpox, the first human mpox case was identified in the Democratic Republic of the Congo (DRC) in a nine-month-old child who was admitted to the hospital. The boy had a smallpox-like disease from which the MPV-like virus was isolated [10]. In the period from October 1970 to May 1971, 6 cases of MPV were detected in Sierra Leone, Liberia, and Nigeria. The first case was recorded in Nigeria in 1971, and time did not pass before 10 more cases were reported from 1971 to 1978. Since that time, the number of people infected with mpox has reached thousands, and it has been distributed in 15 countries worldwide, including 11 African countries. Since the emergence of mpox in 1970; most reported cases have been based in Western and Central Africa, with the highest prevalence being in the Democratic Republic of the Congo [11]. From 1970–1979, 47 cases were reported to have MPV, all originating in Western and Central Africa [12]. These areas were known to be occupied by large rainforests, surrounded by small villages with a population of fewer than 1000 citizens at most. The residents of these rural areas practiced wild hunting as part of their daily lives and cultures, exposing them to different wild species [13], [14], [15]. The causative agent of smallpox would be thought to be the variola virus, as it adapted to the human host over 3000 years ago. Vaccination against smallpox has been practiced in China for over a thousand years, according to some records [16], [17]. Because the vaccine is made from a virus that is transmitted to humans by host animals and may cause mild disease, rational vaccine manufacturing began with Jenner's 1796 cowpox vaccine [18]. The official vaccination journey to eradicate smallpox began in 1980 [19]. It is believed that the occurrence of moderate and intermittent human disease due to orthopoxviruses, including the mpox virus, occurred before the invention of vaccination against smallpox [20]. These viruses remain in host animals, with a periodic appearance in human populations. The variola virus is less common and genetically distinct compared to the smallpox virus [21], and the death rate in smallpox was in the range of 30–50% of the proportion of infected people, while the rate of variola was less than one percent. Vaccination and preventive exposure to smallpox have contributed to the eradication of smallpox, and it is also possible to reduce the number of infections in human orthopoxviruses [22]. Similar to the smallpox virus, which evolved into two different groups, the mpox displays two distinct species: the Congo Basin and West Africa. The Congo Basin bloc has reported a mortality rate of up to 10% from mpox, but it is as high as 1% in the West African clade, and this rate is much higher in HIV patients [23], [24].
The international spread of the human mpox virus before the COVID-19 era
The most significant outbreak of MPV was in the US in 2003; when an infected Gambian giant pouched rat was shipped with 800 small animals from Ghana to Texas, and these animals transmitted the virus to adjacent prairie dogs where they were sent [25].
The West African virus implicated in the US outbreak had different characteristics from the African original. All cases in the US had contact with the infected animals, so tracing strategies and detections were clearer and more practical. Moreover, no proven data was found to suggest human-to-human transmission. The incubation period slightly differed from what was known, being 14.5 days on average [26]. No deaths were reported, and no gender preferences were found. What makes the American version distinct is the morphology and number of skin lesions, which have a more haphazard course [25].
In 2005, there was an outbreak in Sudan, which was not in Western or Central Africa. The reason is not quite known, but it is thought to be the expansion of the infection from surrounding endemic regions or from migrating infected animals [27], [28]. The Sudan outbreak did not differ from the west and central African outbreaks in regards to signs and symptoms, but notably showed no case fatality rates similar to the American outbreak [27], [29].
In 2017, a Nigerian outbreak yielded many confirmed cases of MPV [30]. From there, three people transmitted the disease outside of Africa to the UK after being exposed to the virus. One healthcare worker had contact with one of the patients, making four cases in total. This was the second outbreak outside of Africa after its spread in the US [31].
The international spread of the human mpox virus after the COVID-19 era
During COVID-19 years, Mpox cases have been reported to WHO since January 1, 2022, from 42 member states in five WHO regions (the Americas, Africa, Europe, the Eastern Mediterranean, and the Western Pacific). Moreover, 2103 laboratory-confirmed cases and one probable case, which also included one death, had been reported to the WHO as of June 15th, 2022. Men who have intercourse with other men who have recently reported having sex with new partners or multiple partners are still largely affected by the mpox outbreak.
While epidemiological investigations are ongoing, the majority of cases in the recent outbreak have been reported through sexual health or other health services in primary or secondary healthcare facilities, with a history of travel that has been primarily to European, North American, or other countries rather than countries where the virus has not previously been known to exist, and increasingly, recent local travel or no travel at all. An outbreak of mpox is defined as one confirmed case within a nation. The sudden emergence of mpox in several locations, despite an initial lack of epidemiological links to previously reported mpox regions, suggests that there may have been long-term undetected transmission.
Given that, this is the first time that a lot of mpox cases and clusters have been reported at the same time in a lot of different countries in different WHO regions. Despite the fact that mortality has remained low during the current outbreak, WHO rates the risk at the global level as moderate [32].
MPV biology and morphology
MPV has the same shape as other orthopoxviruses, which are made up of oval or sometimes brick-shaped particles covered on the outside by a lipoprotein membrane with geometrically wavy lines. Usually, the size of MPV viruses is approximately 200 × 250 nm [33], [34]. The outer membrane protects the membrane bond as well as the densely packed core containing enzymes, a double-stranded DNA genome, and transcription factors. The core is described as biconcave, with lateral bodies on each side, due to an electron microscopy fixation artifact [35], [36], [37]. The MPV double DNA is bound by covalent bonds at its end through symmetric hairpins and also contains inverted terminal repeats [38]. These inverted terminal repeats consist of a hairpin, tandem repeats, and open reading frames. As we said earlier, the life cycle of the MPV virus occurs inside the cytoplasm of the infected cell, although it is a DNA virus. The MPV virus genome encodes proteins required for viral DNA replication, transcription, and virus assembly. Encodings related to the essential functions of the virus are located in the central region of the MPV genome, and genes related to virus-host interactions are located in the peripheral region of the genome [34], [39], [40].
The mpox virus is an orthopoxvirus, which is one of the Poxviridae family [41]. Poxviridae viruses are double-stranded DNA viruses with very large genomes as they contain more than 150 genes per genome [42]. The group entomopoxvirinae, which infects insects, and the group chordopoxvirinae, which infects vertebrates, constitute the two main groups of poxviridae [43]. The mpox and smallpox viruses prevent the host from using its nuclear enzymes and force it to encode the virus's enzymes to replicate its DNA because the virus replicates in the cytoplasm. Virus-specific virion proteins result from a delayed transcription process that is regulated by virus DNA replication, which provides a DNA template that ultimately expresses virion proteins [44]. The occurrence of one step in the life cycle of the poxvirus group is sufficient to produce a distinct type of virus that has the characteristics of the entire group of eukaryotic viruses with large nucleic acids.
MPV genome
The variola virus (VARV), mpox virus (MPXV), cowpox virus (CPXV), and the vaccinia virus (VACV). are all in the family Poxviridae, genus Orthopoxvirus. All of these can cause infections in humans. The genomes of these viruses are 200 kilobase (kb) or less long, and they have genes involved in pathogenesis and figuring out which hosts to infect. They also have highly conserved core sections that code for replication and assembly machinery. Orthopoxviruses share genetic makeup and antigens. However, they differ in their host range and virulence characteristics. According to comparative genomics research, the evolution of orthopoxviruses is ongoing and can be influenced by selective pressure from a host species. Progressive gene loss, particularly at the ends of the genome, has been theorized to be a major factor in the evolution of these viruses. CPXV has the largest genome of all sequenced orthopoxviruses (220 kb), encodes 223 open reading frames (ORFs), and has a wide host range that includes rodents, humans, felids, bovids, and voles. It only causes a mild infection in humans. The smallpox virus, VARV, has the smallest genome of all naturally occurring orthopoxviruses (186 kb), is predicted to encode 20% fewer functional proteins than CPXV, and only infects humans. In contrast, VARV is highly pathogenic, its case-fatality rate was 30%, and its host range is limited to humans. Similar to VARV, MPXV is a highly virulent orthopoxvirus that has a case-fatality rate of 10% and results in high levels of disease and mortality [44], [45], [46]. Naturally occurring VARV infections, on the other hand, were deemed exterminated in 1979, and naturally occurring MPXV infections occur in areas of Africa where MPXV is endemic, such as in the Democratic Republic of the Congo (DRC) and the Republic of the Congo. Human infection with MPXV is riskier when exposed to animal reservoirs, such as squirrels, of the species Funisciurus and Heliosciurus. According to recent reports, MPXV transmission from person to person is growing. Seven generations of unbroken human transmission were documented in 2003 in the DRC. It is anticipated that the introduction and spread of MPXV among humans would significantly rise with increasing susceptibility and declining herd immunity. A mpox outbreak in the midwestern United States in 2003 demonstrated the likelihood of transmission to MPXV-uninfected populations. The MPXV strain in question belonged to the Western African clade, which has less virulent strains than the Central African strains found in the DRC [26], [47]. MPXV has a 197 kb linear DNA genome with 190 nonoverlapping ORFs that are longer than 180 nucleotides. The central coding region sequence (CRS) of MPXV is highly conserved, like that of all orthopoxviruses, and is flanked by variable ends that include inverted terminal repeats (ITRs). Most VACV homologs of genes in the terminal ends of the MPXV genome are involved in immunomodulation and have either been predicted or are known to affect pathogenicity and host range determination. In contrast to VARV, which lacks ORFs in the ITR region, MPXV has at least 4 ORFs. The development of short-read, high-throughput sequencing technology has made it possible to conduct genomic surveys on enormous populations. It has been proposed that a highly adapted virus that causes significant illness and is capable of effective and quick human-to-human transmission emerged as a result of the gradual loss of genes not required for pathogenesis in humans. Gene copy number variation may be a key element in modifying virus fitness [48], [49], [50].
Transmission of mpox virus
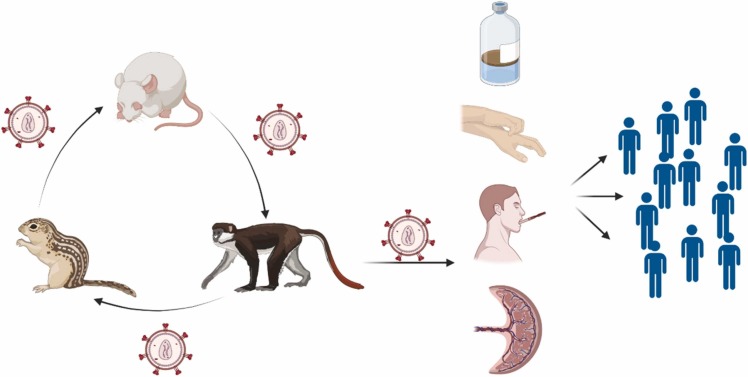
MPV is transmitted in two ways, either from humans to animals or from humans to humans. The spread of infection in humans is associated with the presence of a skin lesion, as the infection is transmitted through respiratory droplets, the patient's contaminated environment, or contact with body fluids ( Fig. 1). It is believed that the Congo Basin clade virus is more severe and powerful than that of West Africa, and that is why the Congo Basin clade spreads more from person to person [29], [51], [52], [53]. The spread from animal to human occurs through direct contact with any of the virus hosts, and this type of transmission is called zoonotic transmission. Animal transmission can also occur through contact with the blood or fluids of an infected animal [54], [55], [56]. The results of thorough investigations and continuous tracing revealed that the family of the 9-month-old was found to eat monkeys periodically, which suggested that monkeys are heavily contributing to the disease. Although the virus was first isolated from captive monkeys in 1958 [9], [28], a quite variable range of animals was postulated to be the main reservoir, examples include rodents, squirrels, and bats [57], [58]. Many studies were carried out to investigate the definite reservoir, but none yielded any relevant results. Since mpox outbreaks occurred in rural villages and near rainforests, the primary method to contract MPV was direct contact with infected animals, either by consumption or hunting [59], [60]. The virus can indeed be transmitted from human to animal, but to this moment, it has not been proven that the virus can be transmitted from human to animal. The reproduction number (R0) in the Congo Basin clade is much higher than that of the West African clade, so the rate of human-to-human transmission, secondary attack rates (SARs), and serial transmission events are much higher. The virus will not only be transmitted quickly between humans, but it will also remain present and persistent among them [61], [62].
Fig. 1.
The route of MPV transmission and spreading. Animal-human transmission and human-human transmission are the two potential MPV transmission pathways. Human-to-human transmission has been linked to respiratory droplets and contact with bodily fluids, contaminated patient settings or objects, and skin lesions from an infected person. As well as inoculation through the mucocutaneous lesions of an infected animal, direct contact with or eating of one of the natural viral hosts are other ways that zoonotic transmission can happen.
Risk factors and highly susceptible patients for MPV infection
In many reports, the highest age group affected was shown to be younger than 15 years old [63]. Since the smallpox vaccine provided cross-protective immunity, or herd immunity, against mpox too, the affected age group has been increasing since the eradication and the ending of smallpox vaccination around the 1980 s [64], [65], [66]. Needless to say, vaccinated people showed fewer severe presentations and minor complications, with lower mortality rates [63]. Adult men accounted for the majority of primary cases because they are more exposed to wild animals and hunting practices. However, females and children represented the majority of secondary cases [59]. Mentioning secondary cases, human–human transmission mainly occurred via large droplets expelled by coughing or sneezing, body fluids, or contaminated household materials [13], [14], [67]. Although they occur rarely, nosocomial infections with the virus have been documented, meaning that prolonged exposure to patients puts healthcare workers at a higher risk of contracting the disease [68], [69]. Also, vertical transmission (through the placenta) and inoculation of the virus through open wounds or scratches, using the same household utensils, and sleeping in the same room with a patient seemed to increase the risk of contracting the disease [28], [67], [70]. Surprisingly, people with light skin were detected earlier because the lesions were easily apparent compared to patients with a darker skin complexion. This risk factor somehow affected mortality and morbidity [25].
Clinical presentation
Recent travel to endemic areas, contact with wild animals brought in from endemic areas, and caring for an infected animal or person are all historical signs of mpox infection, but the symptoms are what really matter.
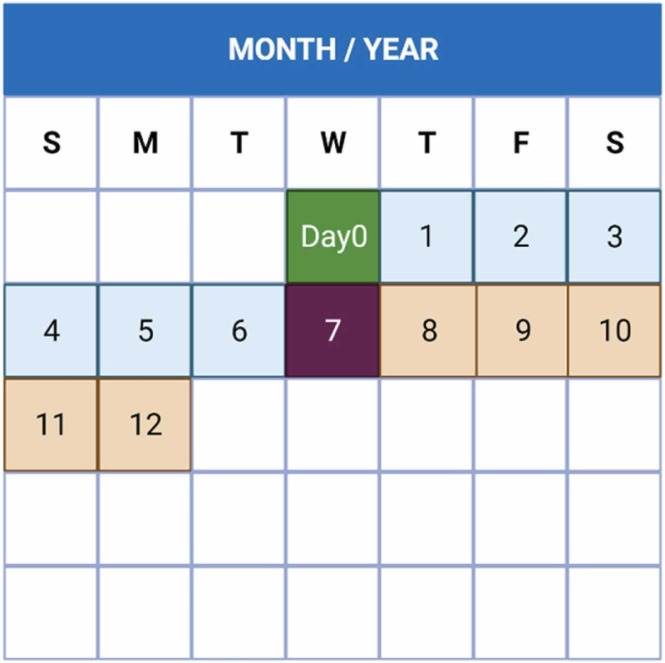
The mpox virus replicates at the inoculation site after entering via any route (oropharynx, nasopharynx, or intradermal). It subsequently travels to local lymph nodes. Following then, viral propagation and seeding of other organs occur as a result of initial viremia. This is the incubation period, which can last anywhere from 7 to 14 days with a maximum of 21 days ( Fig. 2). Symptom onset is linked to secondary viremia, which causes prodromal symptoms including fever and lymphadenopathy for 1–2 days before lesions emerge. At this time, infected patients may be contagious. Lesions begin in the oropharynx and progress to the skin, by the time lesions emerge, serum antibodies are frequently detected [3]. Fever, headache, myalgia, weariness, and lymphadenopathy are among the first signs of mpox, which distinguishes it from smallpox. Mucosal lesions in the mouth appear 1–2 days later, followed by centrifugally concentrated skin lesions on the face and extremities (including the palms and soles). The rash may or may not spread to other parts of the body, and the number of lesions can range from a few to thousands [71].
Fig. 2.
Timeline for monkeypox infection clarifying the pathogenesis through the first 12 days of infection. The initial replication occurs around the second day at the primary site of inoculation. After day seven of infection, the initial symptoms (secondary viremia) of monkeypox happened and seeding to additional tissues, this includes a 2- to 3-day febrile illness, usually occurring from 10 to 14 days after initial exposure. Also, the characteristic skin lesions developed.
Also, lymphadenopathy in the inguinal, axillary, or cervical areas is common and can happen before or after the rash appears [72]. The skin lesions progress through macular, papular, vesicular, and pustular phases during the next 2–4 weeks in 1- to 2-day increments. Lesions are firm, deep-seated, and 2–10 mm in size, and they alter synchronously. Before crusts form, lesions are in the pustular phase for 5–7 days. Crusts form and desquamate over the next 7–14 days, and in most cases, the condition cures in 3–4 weeks following the symptom's beginning. After all the crusts have fallen off, the patient is no longer considered contagious [73].
Varicella zoster virus (VZV) infection looks a lot like herpes simplex virus (MPV) infection, so the two are often mixed up in places where MPV is common. However, various characteristics of illness distinguish one infection from another. VZV patients, for example, usually have a brief, moderate febrile prodrome, or none at all, followed by a rapidly growing (1–2 day) pleomorphic rash (i.e., a rash in which neighboring lesions may be at different stages of development). Also, VZV lesions frequently have uneven borders and are located on the skin's surface (relative to those of MPV). Varicella lesions also tend to form in a centripetal pattern on the body. Lesions on the palms of the hands and soles of the feet are not typical in VZV infections, however, they have been observed in rare cases. Because VZV patients rarely exhibit severe lymphadenopathy, the presence of lymphadenopathy is one distinguishing feature that can separate MPV from both smallpox and varicella. Other herpetic diseases (including VZV), drug eruptions, syphilis, yaws, scabies, and rickettsia pox are all conditions that might be misdiagnosed for MPV. In MPV-endemic areas, specimen collection and laboratory testing can be challenging to complete for all suspected cases, enabling more accurate and expedient case discovery, the collection of higher-quality surveillance data, and improved patient treatment; and a clinical case definition capable of strengthening the differentiation between MPX and other disorders would be valuable [72].
Diagnosis
During the 2003 outbreak in the United States, the Centers for Disease Control and Prevention (CDC) created case definition criteria for human mpox. However, the same criteria may not be as useful in endemic locations. As the population's potential exposure to infected mammals or people grows, the specificity of epidemiological criteria becomes lower. Furthermore, as the prevalence of identical disorders increases, the specificity of clinical criteria decreases, which is the same with chickenpox in Africa due to the lack of a routine varicella-zoster vaccine [74].
Diagnosis by clinical features
Although the main reservoirs of mpox animals have not yet been identified, it is believed that rodents such as Gambian giant rats and snake squirrels represent a reservoir [75]. Close contact with infected animals and cuts in the skin from handling or eating infected animals are thought to be the sources of infection from animals to humans. Respiratory transmission from an infected animal to a human was discovered in some cases of human mpox in the United States. In addition, respiratory droplets are believed to be involved in some human-to-human transmissions [73].
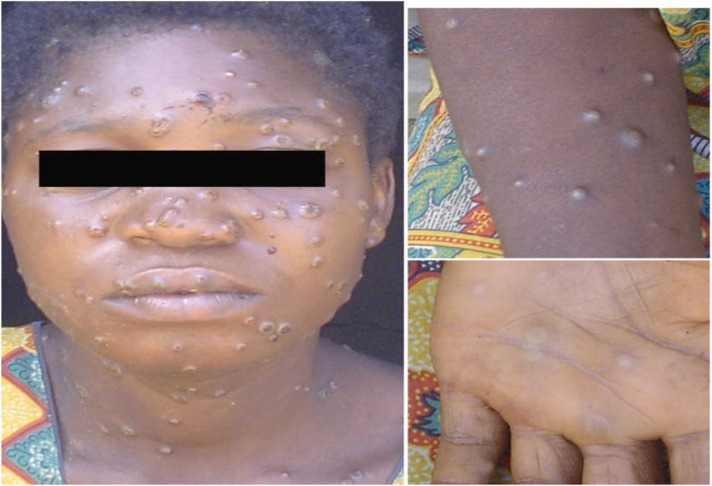
There is an incubation period of 10–14 days after exposure and infection, followed by a prodromal period of 2 days. During this time, infected people may have a fever, chills, malaise, headache, back pain, sore throat, shortness of breath, and swollen lymph nodes (lymphadenopathy). About 90% of all human mpox infections have lymphadenopathy in the submandibular, neck, or groin areas. It is a characteristic feature and can distinguish human MPV infection from smallpox infection [76]. The mpox maculopapular rash has diameters ranging from 0.2 to 1 cm, which develop gradually after the prodrome stage. This period is considered the most contagious and can spread the virus to others. Moreover, these skin lesions begin on the face and trunk and then spread to the extremities including the palms and soles. During a two- to four-week period, lesions advance through multiple and different stages, from macules to papules, vesicles, and pustules ( Fig. 3). Finally, desquamation and scabbing, which were part of the crusting phase, can cause dyspigmented scars in some situations [73], [77].
Fig. 3.
The classical MPV rash with lesions on the face, arms, and palms for African females.
Laboratory diagnosis
The importance of laboratory diagnosis came from the similarity in clinical features of smallpox and mpox. To distinguish between these viruses, reference labs can develop a variety of laboratory tests based on differences in host immune responses to orthopoxviruses, even with the high degree of similarity in the genomes. Molecular techniques like a real-time polymerase chain reaction (PCR) assay can identify two genes that are targeted by the MPV. In one of the PCR assays, it can detect about thirteen different Eurasian orthopoxviruses by targeting the DNA polymerase gene (Copenhagen (COP) E9L gene). Another assay targets the single nucleotide polymorphisms in the MPV (COP-B5R gene), which codes for the virus’ envelope protein, which makes this assay specific and sensitive to MPV [78].
The main disadvantage of these DNA tests is the necessity of being active in infection while the virus is still present to collect the specimen. This means it cannot be used in mpox diagnosis after the infection has cleared. This made scientists develop other techniques based on the host’s immune response to the MPV. However, cross-reactive immune responses that resulted from previous smallpox vaccination complicate the development of a standard antibody test for mpox. Notwithstanding, the previous smallpox vaccination cannot induce anti-vaccinia IgM antibodies, instead, use an immunoglobulin M (IgM) antibody assay to diagnose MPV infection [79]. A whole-virus enzyme-linked immunosorbent assay (ELISA), is another approach that investigates diffused antigenic variations among the viruses, in which the antibody titers to the mpox and vaccinia viruses are measured and their ratios determined. ELISA was used to distinguish between prior smallpox vaccination and recent mpox infection using a peptide from the protein encoded by the mpox ortholog of the cowpox virus strain Brighton Red (BR) 219 gene. On the other hand, cellular immune responses that depend on antibody responses to specific pathogens could be a new diagnostic target that is better than the previous assays. The measurement of several orthopoxvirus-specific T cells is one such technique in development [80].
Complications
Patients are at risk for a variety of complications, including secondary integument infection, bronchopneumonia (development of cellular necrosis along with the lung tissues that cause pulmonary consolidation), sepsis, and encephalitis. Also, corneal infection is one of the most significant complications of mpox, even if it’s not a common complication, maybe it can lead to permanent corneal scarring and blindness even with the use of eye lubricants. Likewise, it can cause hyperpigmentation, hypopigmentation, a skin rash, dehydration, and sometimes death [81], [82], [83], [84].
Treatment
Mpox infection currently has no specific clinically proven treatments. The treatment is supportive symptom management, as with most viral illnesses. However, some measures can be taken to help prevent an outbreak. Until all lesion crusts have naturally fallen off and a new skin layer has formed, the infected individual should be kept in isolation, wearing a surgical mask, and keeping lesions covered as much as possible [85].
Role of antiviral treatment
Several compounds have shown promise as antiviral therapeutics against orthopoxvirus species, and three of the most promising are listed in. Cidofovir inhibits viral DNA polymerase and thus has antiviral activity against a variety of viruses. It is administered intravenously with hydration and probenecid. Cidofovir may cause nephrotoxicity, but CMX-001, the modified compound of cidofovir, does not cause nephrotoxicity. Also, it has antiviral activity against different species of orthopoxvirus. The intracellular virus released is inhibited by ST-246, this compound is administered orally and shows promising results against different types of orthopoxvirus, including the Variola virus. These compounds have been used in various combinations, including with vaccinia immune globulin, which treats severe vaccine-related adverse events in clinical trials. The development of strategies for using these drugs to treat diseases in endemic areas is needed [86].
Vaccines against MPV
Smallpox vaccines are made up of fully replicative vaccinia viruses and are not currently used in mpox-endemic areas due to concerns about severe adverse events in a population with an unknown immunocompromised status. The risk of pathogenic mpox disease must be measured against the possibility of side effects from replicative vaccines like the live virus vaccine (ACAM 2000). A vaccine that does not contain this risk and can be easily administered to children would be ideal for use in mpox-endemic areas. There is no single vaccine that meets all of these requirements, but some next-generation vaccines get us closer to that goal [86].
Live smallpox virus vaccine (ACAM2000)
The main advantage of this vaccine is that it can be administered as a single dose. However, as with all live vaccines, it is contraindicated to be given to immune-compromised people due to its ability to replicate in the cells of those high-risk patients. Also, it should be avoided by people with a history of eczema, atopic dermatitis, or a current pregnancy. Otherwise, in a minority of people, these vaccines can cause cardiac complications after administration. A raised lesion at the vaccination site is common and can be an indication of successful vaccine administration [86]. The ACAM2000 vaccine produced by Sanofi Pasteur Biologics Co. and the JYNNEOS vaccine produced by Bavarian Nordic A/S and marketed as Jynneos in the United States and Imvanex in Europe both demonstrated efficacy for cross-protection against the MPXV. Both vaccines were licensed for use against smallpox by the Food and Drug Administration (FDA) authorities. If infectious cases are quickly identified, ring vaccinations, or immunizing close contacts of confirmed cases, can be effective. The size and length of controlled outbreaks can vary greatly since epidemic outbreaks are fundamentally unpredictable in nature. The Ebola virus had previously been eradicated using a ring vaccination method. The spread of the MPX infection can be stopped by immunizing close contacts of an affected person. The United States of America (USA) and other European nations have put the plan into practice. To limit infectious illnesses, the Union Ministry of Health and Family Welfare in India has decided to use the ring vaccination technique [87].
Attenuated smallpox virus vaccine
LC16m8 vaccine
This Japanese vaccine showed fewer side effects and a safer profile than the live vaccine ACAM2000, also with a single dose administration. Although this virus is attenuated, it still can replicate inside human cells [86].
Modified vaccine (IMVAMUNE or IMVANEX)
This live attenuated vaccine, which is administrated in two doses, is known as IMVAMUNE in the US and IMVANEX in European countries. Interestingly, the main advantage of this vaccine is that the attenuated virus has a limited ability to replicate inside cells, which makes it appropriate for all adults, including immune-compromised persons. Unlike the live ACAM2000 vaccine, there is no skin lesion at the vaccination site even if it is injected in two doses [86].
Infection control and prevention
A group of scientists from all over the world worked hard on their research, which led to a number of important findings that helped ease these worries to a large extent. Based on historical information and lab research, scholars found that the variola virus would make people less likely to get sick and less likely to spread the disease. Furthermore, they discovered that the protective effects of smallpox vaccination generally transferred to mpox, implying that well-vaccinated populations would be disease-free for the most part. A detailed epidemiologic analysis of the virus's inter-human, In addition to the evidence, they compared it with smallpox and found that the efficiency of transmission was lower by 10-fold among household members. Indeed, stochastic mathematical simulations examining worst-case scenarios of probable mpox epidemics unmistakably pointed to the need for continual zoonotic introductions to feed ongoing outbreaks. Based on what was known at the time about the disease's epidemiologic parameters inter-human contact rates, virus transmissibility, and vaccine effectiveness mpox was believed to be unable to cause sustained epidemics in human populations in the absence of a proximate source of the virus in animal populations [88].
Conclusion
MPV may have been able to come back in Nigeria and other countries in the tropical rainforest belt because smallpox vaccinations were stopped, people had more contact with animals that could be reservoir hosts for MPV because of climate change and deforestation, people ate more bush meat, and health and research infrastructure were not as good as they could have been. Since travelers recently brought MPX from Nigeria and Ghana to the United States, the United Kingdom, and Singapore, MPX is no longer only found in endemic areas. Due to its global reach, MPV is a very dangerous re-emerging pathogen, especially in the COVID-19 era. National and international research efforts should be increased to identify virulence markers of disease, host and viral factors that modulate MPV evolution, human behaviors that support zoonotic spillover events, surrogates for asymptomatic infection, as well as virus and host determinants of immunity, to stop MPV from occupying the ecological niche vacated by Variola major Virus (VARV) and potentially evolving into a much deadlier pathogen than it is at present. Preventive epidemiological surveillance for the MPV virus should be carried out in endemic and high-risk areas, including Nigeria in particular. This means that it should be done regularly rather than only in response to an epidemic. We support the integration of routine, periodic epidemiological surveillance for MPV in humans and animals into the Surveillance Outbreak Response Management and Analysis System (SORMAS) for the MPX outbreak.
Paper context
In the last few decades, there have been a lot newer and re-emerging viral and zoonotic diseases. Epidemics like these can teach us how to better track and watch for diseases, which will stop them from spreading. In the tropical rainforests of Central and West Africa, mpox may no longer be endemic. The present mpox outbreak in nonendemic nations is most likely the result of decades of persistent outbreaks making it unable to stop the disease's spread in endemic African areas. This review paper offers an updated summary of the taxonomy, etiology, transmission, and epidemiology of mpox illness.
Ethics for approval and consent to participate
Not applicable. This is due to the nature of this article (Review).
Consent for publication
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Almu’atasim Khamees: Conceptualization, Methodology, Software, Investigation,alidation, Investigation, Data curation, Writing – original draft, Writing – review & editing, Project administration. Sajeda Awadi: Conceptualization, Methodology, Software, Data curation, Writing – original draft. Khayry Al-Shami: Conceptualization, Methodology, Software, Validation, Data curation, Writing – original draft. Sharaf F. Al-Eitan: Software, Writing – original draft. Ahmad Malek Alsheikh: Software, Writing – original draft. Ahmad Saeed: Software, Writing – original draft. Raed M. Al-Zoubi: Software, Validation, Data curation, Writing – review & editing. Mazhar Salim Al Zoubi: Conceptualization, Methodology, Software, Validation, Investigation, Data curation, Writing – review & editing, Supervision, Project administration. Hayat Abu Alkhoun: Software, Writing – original draft All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
The APC of this article was generously funded by Qatar National Library (QNL).
Data Availability
The data that supports the findings in this study are available from the corresponding author upon reasonable request.
References
- 1.Ntoumi F., Zumla A. Advancing accurate metrics for future pandemic preparedness. Lancet. 2022;399(10334):1443–1445. doi: 10.1016/S0140-6736(22)00425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khamees A., Bani-Issa J., Zoubi M.S.A., Qasem T., AbuAlArjah M.I., Alawadin S.A., et al. SARS-CoV-2 and coronavirus disease mitigation: treatment options, vaccinations and variants. Pathogens. 2022;11:2. doi: 10.3390/pathogens11020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen E., Abubakar I., Ihekweazu C., Heymann D., Ntoumi F., Blumberg L., et al. Monkeypox - Enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int J Infect Dis: IJID: Publ Int Soc Infect Dis. 2019;78:78–84. doi: 10.1016/j.ijid.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Multi-country monkeypox outbreak in non-endemic countries [Internet], 2022. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385.
- 5.Mahase E. Seven monkeypox cases are confirmed in England. Br Med J Publ Group. 2022 doi: 10.1136/bmj.o1239. [DOI] [PubMed] [Google Scholar]
- 6.Saied A.A., Dhawan M., Metwally A.A., Fahrni M.L., Choudhary P., Choudhary O.P. Disease history, pathogenesis, diagnostics, and therapeutics for human monkeypox disease: a comprehensive review. Vaccines. 2022;10(12):2091. doi: 10.3390/vaccines10122091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization WH 2022–23 Mpox (Monkeypox) Outbreak:https://worldhealthorg.shinyapps.io/mpx_global/#section-fns2%E2%80%982022%20Monkeypox%20Outbreak:%20Global%20Trends%E2%80%99.
- 8.Parker S., Nuara A., Buller R.M.L., Schultz D.A. , 2007. Human monkeypox: an emerging zoonotic disease. 2007. [DOI] [PubMed]
- 9.Arita I., Henderson D. Smallpox and monkeypox in non-human primates. Bull World Health Organ. 1968;39(2):277. [PMC free article] [PubMed] [Google Scholar]
- 10.Control CfD, Prevention, 1997. Human monkeypox--Kasai Oriental, Zaire, 1996–1997. MMWR Morbidity and mortality weekly report. 1997;46(14):304–307. [PubMed]
- 11.Giulio D.B.D., Eckburg P.B. , 2004. Reviews Human monkeypox: an emerging zoonosis. 2004:15–25. [DOI] [PMC free article] [PubMed]
- 12.Breman J.G., Steniowski M., Zanotto E., Gromyko A., Arita I. Human monkeypox, 1970-79. Bull World Health Organ. 1980;58(2):165. [PMC free article] [PubMed] [Google Scholar]
- 13.Hutin Y.J.F., Williams R.J., Malfait P., Pebody R., Loparev V.N., Ropp S.L., et al., 2001. Outbreak of Human Monkeypox, Democratic Republic of Congo, 1996 – 1997. 2001:1996–7. [DOI] [PMC free article] [PubMed]
- 14.Khodakevich L., Jezek Z., Messinger D. Monkeypox virus: ecology and public health significance. Bull World Health Organ. 1988:747–752. [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer H., Perrichot M., Stemmler M., Emmerich P., Schmitz H., Varaine F., et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J Clin Microbiol. 2002;40(8):2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes A.L., Irausquin S., Friedman R. The evolutionary biology of poxviruses. Infect, Genet Evol. 2010;10(1):50–59. doi: 10.1016/j.meegid.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Needham J. , 2000. Science and Civilisation in China: Volume 6, Biology and Biological Technology, Part 6, Medicine: Cambridge University Press; 2000.
- 18.Riedel S., editor Edward Jenner and the history of smallpox and vaccination. Baylor University Medical Center Proceedings; 2005: Taylor & Francis. [DOI] [PMC free article] [PubMed]
- 19.Organization W.H. Smallpox 2022 [Available from: https://www.who.int/health-topics/smallpox#tab=tab_1.
- 20.Simpson K., Heymann D., Brown C.S., Edmunds W.J., Elsgaard J., Fine P., et al. Human monkeypox–After 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38(33):5077–5081. doi: 10.1016/j.vaccine.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shchelkunov S.N., Totmenin A.V., Loparev V.N., Safronov P.F., Gutorov V.V., Chizhikov V.E., et al. Alastrim smallpox variola minor virus genome DNA sequences. Virology. 2000;266(2):361–386. doi: 10.1006/viro.1999.0086. [DOI] [PubMed] [Google Scholar]
- 22.Petersen B.W., Damon I.K., Pertowski C.A., Meaney-Delman D., Guarnizo J.T., Beigi R.H., et al. Clinical guidance for smallpox vaccine use in a postevent vaccination program. Morb Mortal Wkly Rep: Recomm Rep. 2015;64(2):1–26. [PubMed] [Google Scholar]
- 23.Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86(10):2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 24.Doshi R.H., Guagliardo S.A.J., Doty J.B., Babeaux A.D., Matheny A., Burgado J., et al. Epidemiologic and ecologic investigations of monkeypox, Likouala Department, Republic of the Congo, 2017. Emerg Infect Dis. 2019;25(2):273. doi: 10.3201/eid2502.181222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sale T.A., Melski J.W., Stratman E.J., Paul S., 2006. Comparison of African and US disease. 2006:478–81. [DOI] [PMC free article] [PubMed]
- 26.Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Sargent E.K., et al., 2004. The Detection of Monkeypox in Humans in the Western Hemisphere. 2004. [DOI] [PubMed]
- 27.Clade B., Formenty P., Muntasir M.O., Damon I., Chowdhary V., Opoka M.L., et al. Human monkeypox outbreak caused by novel virus belonging to congo basin clade. Sudan, 2005. 2010:1539–1545. doi: 10.3201/eid1610.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker S., Nuara A., Schultz D.A. , 2007. Human monkeypox: an emerging zoonotic disease. 2007:17–34. [DOI] [PubMed]
- 29.Brown K., Leggat P.A. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1(1):8. doi: 10.3390/tropicalmed1010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sklenovska N., Van, Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front Public Health. 2018;6:241. doi: 10.3389/fpubh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughan A., Aarons E., Astbury J., Balasegaram S., Beadsworth M., Beck C.R., et al., 2018. Two cases of monkeypox imported to the United Kingdom, September 2018. Eurosurveillance. 2018;23(38):1800509. [DOI] [PMC free article] [PubMed]
- 32.organization W.H. , 2022. Multi-country monkeypox outbreak: situation update 2022 [Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON393.
- 33.Canada PHAo. Monkeypox virus , 2017. [Available from: https://www.ehs.com/resources/sds-resources/free-safety-data-sheet-index/Monkeypox-virus/?fbclid=IwAR21i4xNp2tqMm5lAnCPnuB84qW3Z6TWxOEMG0dR5rocz2S_QBi-aQPp3BA.
- 34.Cho C.T., Wenner H.A. Monkeypox virus. Bacteriol Rev. 1973;37(1):1–18. doi: 10.1128/br.37.1.1-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fenner F. Elsevier; 2012. The orthopoxviruses. [Google Scholar]
- 36.Martin J., Christopher G., Eitzen E., Dembek Z. History of biological weapons: from poisoned darts to intentional epidemics. Medical aspects of biological warfare. ZF Dembek, Borden Institute. Walter Reed Army Med Cent. 2007:20–353. [Google Scholar]
- 37.Odom M.R., Hendrickson R.C., Lefkowitz E.J. Poxvirus protein evolution: family wide assessment of possible horizontal gene transfer events. Virus Res. 2009;144(1–2):233–249. doi: 10.1016/j.virusres.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kugelman J.R., Johnston S.C., Mulembakani P.M., Kisalu N., Lee M.S., Koroleva G., et al. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg Infect Dis. 2014;20(2):232. doi: 10.3201/eid2002.130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrett J.W., McFadden G. Elsevier; 2008. Origin and evolution of poxviruses. Origin and Evolution of Viruses; pp. 431–446. [Google Scholar]
- 40.Moss B. The Molecular Basis of Viral Replication. Springer; 1987. The molecular biology of poxviruses; pp. 499–516. [Google Scholar]
- 41.Iyer L.M., Aravind L., Koonin E.V. Common origin of four diverse families of large eukaryotic DNA viruses. J Virol. 2001;75(23):11720–11734. doi: 10.1128/JVI.75.23.11720-11734.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lefkowitz E.J., Wang C., Upton C. Poxviruses: past, present and future. Virus Res. 2006;117(1):105–118. doi: 10.1016/j.virusres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Koev G., Liu S., Beckett R., Miller W.A. The 3′-terminal structure required for replication of barley yellow dwarf virus RNA contains an embedded 3′ end. Virology. 2002;292(1):114–126. doi: 10.1006/viro.2001.1268. [DOI] [PubMed] [Google Scholar]
- 44.Hendrickson R.C., Wang C., Hatcher E.L., Lefkowitz E.J. Orthopoxvirus genome evolution: the role of gene loss. Viruses. 2010;2(9):1933–1967. doi: 10.3390/v2091933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werden S.J., Rahman M.M., McFadden G. Poxvirus host range genes. Adv Virus Res. 2008;71:135–171. doi: 10.1016/S0065-3527(08)00003-1. [DOI] [PubMed] [Google Scholar]
- 46.Essbauer S., Pfeffer M., Meyer H. Zoonotic poxviruses. Vet Microbiol. 2010;140(3–4):229–236. doi: 10.1016/j.vetmic.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rimoin A.W., Mulembakani P.M., Johnston S.C., Lloyd Smith J.O., Kisalu N.K., Kinkela T.L., et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci USA. 2010;107(37):16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bahar M.W., Graham S.C., Chen R.A., Cooray S., Smith G.L., Stuart D.I., et al. How vaccinia virus has evolved to subvert the host immune response. J Struct Biol. 2011;175(2):127–134. doi: 10.1016/j.jsb.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elde N.C., Child S.J., Eickbush M.T., Kitzman J.O., Rogers K.S., Shendure J., et al. Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses. Cell. 2012;150(4):831–841. doi: 10.1016/j.cell.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hess M., Sczyrba A., Egan R., Kim T.W., Chokhawala H., Schroth G., et al. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science. 2011;331(6016):463–467. doi: 10.1126/science.1200387. [DOI] [PubMed] [Google Scholar]
- 51.Earl P.L., Americo J.L., Moss B. Natural killer cells expanded in vivo or ex vivo with IL-15 overcomes the inherent susceptibility of CAST mice to lethal infection with orthopoxviruses. PLoS Pathog. 2020;16(4) doi: 10.1371/journal.ppat.1008505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kabuga A.I., El, Zowalaty M.E. A review of the monkeypox virus and a recent outbreak of skin rash disease in Nigeria. J Med Virol. 2019;91(4):533–540. doi: 10.1002/jmv.25348. [DOI] [PubMed] [Google Scholar]
- 53.Sadeuh-Mba S.A., Yonga M.G., Els M., Batejat C., Eyangoh S., Caro V., et al., 2019. Monkeypox virus phylogenetic similarities between a human case detected in Cameroon in 2018 and the 2017–2018 outbreak in Nigeria. Infection, Genetics and Evolution. 2019;69:8–11. [DOI] [PMC free article] [PubMed]
- 54.Chieloka O.S., Amao L.K., Akinrogbe J.T., Iniobong J.-I., Burga J. Outbreak investigation of monkeypox in akwa ibom state: a matched case control study 14th-24th october 2019. East Afr J Health Sci. 2019;1(1):37–44. [Google Scholar]
- 55.Ellis C.K., Carroll D.S., Lash R.R., Peterson A.T., Damon I.K., Malekani J., et al. Ecology and geography of human monkeypox case occurrences across Africa. J Wildl Dis. 2012;48(2):335–347. doi: 10.7589/0090-3558-48.2.335. [DOI] [PubMed] [Google Scholar]
- 56.Ihekweazu C., Yinka-Ogunleye A., Lule S., Ibrahim A. Importance of epidemiological research of monkeypox: is incidence increasing? Expert Rev Anti-Infect Ther. 2020;18(5) doi: 10.1080/14787210.2020.1735361. 389-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khodakevich L., Jezek Z., Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet (Lond, Engl) 1986:98–99. doi: 10.1016/S0140-6736(86)90748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sale T.A., Melski J.W., Stratman E.J. Monkeypox: an epidemiologic and clinical comparison of African and US disease. J Am Acad Dermatol. 2006;55(3):478–481. doi: 10.1016/j.jaad.2006.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeiek Z., Grab B., Szczeniowski M., Paluku K.M., Mutombo M. , 1988. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. 1988:459–464. [PMC free article] [PubMed]
- 60.Nolen L.D., Osadebe L., Katomba J., Likofata J., Mukadi D., Monroe B., et al., 2015. Introduction of Monkeypox into a Community and Household: Risk Factors and Zoonotic Reservoirs in the Democratic Republic of the Congo. 2015. [DOI] [PMC free article] [PubMed]
- 61.McMullen C.L., Mulembekani P., Hoff N.A., Doshi R.H., Mukadi P., Shongo R., et al., 2015. editors. Human monkeypox transmission dynamics thirty years after smallpox eradication in the Sankuru district, democratic republic of Congo. American Journal of Tropical Medicine and Hygiene; 2015: AMER SOC TROP MED & HYGIENE 8000 WESTPARK DR, STE 130, MCLEAN, VA 22101 USA.
- 62.Fowotade A., Fasuyi T., Bakare R. Re-emergence of monkeypox in Nigeria: a cause for concern and public enlightenment. Afr J Clin Exp Microbiol. 2018;19(4):307–313. [Google Scholar]
- 63.Jezek Z., Szczeniowski M., Paluku K.M. Human Monkeypox. Clin Features 282 Patients. 1987:293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 64.Petersen E., Kantele A., Koopmans M., Asogun D., Yinka-Ogunleye A., Ihekweazu C., et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infectious Disease. Clinics. 2019;33(4):1027–1043. doi: 10.1016/j.idc.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gispen R. , Verlinde J.D. , Zwart P., 1967. Histopathologieal and Virological Studies on Monkeypox By. 1967. [DOI] [PubMed]
- 66.Gispen R., Verlinde J., Zwart P. Histo-palhological and virological studies on monkey-pox. Arch fur die Gesamt Virusforsch. 1967;21(2):205–216. doi: 10.1007/BF01241445. [DOI] [PubMed] [Google Scholar]
- 67.Jeiek Z., Grab B., Szczeniowski M.V., Palju K.M., Mutombo M. Hum Monkey: Second attack rates. 1988:465–470. [PMC free article] [PubMed] [Google Scholar]
- 68.Learned L.A., Reynolds M.G., Wassa D.W., Li Y.U., Olson V.A., Kevin K., et al. EXTENDED INTERHUMAN TRANSMISSION OF MONKEYPOX IN A HOSPITAL. Community Repub Congo. 2005;2003:428–434. [PubMed] [Google Scholar]
- 69.Fleischauer A.T., Kile J.C., Davidson M., Fischer M., Karem K.L., Teclaw R., et al. Evaluation of human-to-human transmission of monkeypox from infected patients to health care workers. Clin Infect Dis. 2005;40(5):689–694. doi: 10.1086/427805. [DOI] [PubMed] [Google Scholar]
- 70.Jezek Z., Grab B., Paluku K., Szczeniowski M. Human monkeypox: disease pattern, incidence and attack rates in a rural area of northern Zaire. Trop Geogr Med. 1988;40(2):73–83. [PubMed] [Google Scholar]
- 71.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58(2):260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osadebe L., Hughes C.M., Shongo Lushima R., Kabamba J., Nguete B., Malekani J., et al. Enhancing case definitions for surveillance of human monkeypox in the Democratic Republic of Congo. PLoS Negl Trop Dis. 2017;11(9) doi: 10.1371/journal.pntd.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weaver J.R., Isaacs S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. 2008;225(1):96–113. doi: 10.1111/j.1600-065X.2008.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hussey H.S., Abdullahi L.H., Collins J.E., Muloiwa R., Hussey G.D., Kagina B.M. Varicella zoster virus-associated morbidity and mortality in Africa: a systematic review protocol. BMJ Open. 2016;6(4) doi: 10.1136/bmjopen-2015-010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khodakevich L., Szczeniowski M., et al. Disu Manbu ma, Jezek Z., Marennikova S., Nakano J. The role of squirrels in sustaining monkeypox virus transmission. Trop Geogr Med. 1987;39(2):115–122. [PubMed] [Google Scholar]
- 76.Nalca A., Rimoin A.W., Bavari S., Whitehouse C.A. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis: Publ Infect Dis Soc Am. 2005;41(12):1765–1771. doi: 10.1086/498155. [DOI] [PubMed] [Google Scholar]
- 77.Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4(1):15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y., Olson V.A., Laue T., Laker M.T., Damon I.K. Detection of monkeypox virus with real-time PCR assays. J Clin Virol: Publ Pan Am Soc Clin Virol. 2006;36(3):194–203. doi: 10.1016/j.jcv.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karem K.L., Reynolds M., Braden Z., Lou G., Bernard N., Patton J., et al. characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005;12(7):867–872. doi: 10.1128/CDLI.12.7.867-872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hammarlund E., Lewis M.W., Carter S.V., Amanna I., Hansen S.G., Strelow L.I., et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11(9):1005–1011. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- 81.Reynolds M.G., McCollum A.M., Nguete B., Shongo Lushima R., Petersen B.W. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:12. doi: 10.3390/v9120380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nalca A., Livingston V.A., Garza N.L., Zumbrun E.E., Frick O.M., Chapman J.L., et al. Experimental infection of cynomolgus macaques (Macaca fascicularis) with aerosolized monkeypox virus. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stagles M., Watson A., Boyd J., More I., McSeveney D. The histopathology and electron microscopy of a human monkeypox lesion. Trans R Soc Trop Med Hyg. 1985;79(2):192–202. doi: 10.1016/0035-9203(85)90333-5. [DOI] [PubMed] [Google Scholar]
- 84.Semba R.D. The ocular complications of smallpox and smallpox immunization. Arch Ophthalmol. 2003;121(5):715–719. doi: 10.1001/archopht.121.5.715. [DOI] [PubMed] [Google Scholar]
- 85.Moore M., Zahra F.Monkeypox. StatPearls. Treasure Island (FL): StatPearls Publishing.
- 86.Copyright © 2022, StatPearls Publishing LLC.; 2022.
- 87.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58(2):260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choudhary O.P., Priyanka, Fahrni M.L., Saied A.A., Chopra H. Ring vaccination for monkeypox containment: Strategic implementation and challenges. Int J Surg (Lond, Engl) 2022;105 doi: 10.1016/j.ijsu.2022.106873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings in this study are available from the corresponding author upon reasonable request.