Abstract
The incidence and prevalence of youth-onset type 2 diabetes mellitus (T2DM) and its complications is increasing worldwide. Youth-onset T2DM has been reported in all racial and ethnic groups but Indigenous peoples and people of colour are disproportionately affected. People with youth-onset T2DM often have a more aggressive clinical course than those with adult-onset T2DM and those with type 1 diabetes mellitus. Moreover, the available treatment options for children and adolescents with T2DM are more limited than for adult patients. Intermediate complications of youth-onset T2DM, such as increased albuminuria, often develop in late childhood or early adulthood, and end-stage complications, including kidney failure, develop in mid-life. The increasing frequency, earlier onset, and greater severity of childhood obesity in the past 50 years together with increasingly sedentary lifestyles and an increasing frequency of intrauterine exposure to diabetes are important drivers of the epidemic of youth-onset T2DM. The particularly high risk of the disease in historically disadvantaged populations suggests an important contribution of social and environmental factors, including limited access to high-quality healthcare, healthy food choices and opportunities for physical activity as well as exposure to stressors including systemic racism and environmental pollutants. Understanding the mechanisms that underlie the development and aggressive clinical course of youth-onset T2DM is key to identifying successful prevention and management strategies.
ToC
This Review describes the global epidemiology, clinical course and key complications of youth-onset type 2 diabetes mellitus. The authors also discuss the mechanisms that might underlie the aggressive clinical phenotype of this disease and current management strategies.
Introduction
Type 2 diabetes mellitus (T2DM) was traditionally considered to be a disease that arises in response to long-term insulin resistance and impaired insulin secretion, and therefore typically affects individuals aged ≥50 years. By contrast, type 1 diabetes mellitus (T1DM) is caused by an autoimmune reaction that destroys the insulin-producing β-cells of the pancreas and typically develops in children, adolescents and young adults. However, in the 1960s and 1970s, the first reports of asymptomatic hyperglycaemia and glucose intolerance in children and adults aged <45 years with obesity were published in the US and Europe1–5. These early studies were followed by reports of T2DM in children in populations across the Americas, Europe, Asia, Australasia, and the Middle East6–15.
A review of T2DM among people aged <20 years in North America, where some of the highest prevalences of youth-onset T2DM have been reported, confirmed its presence in all racial and ethnic groups and noted a particularly high prevalence among American Indian and First Nations peoples16. In 2000, a consensus statement from the American Diabetes Association (ADA) concluded that up to 45% of new cases of diabetes among individuals aged <20 years in North America were not immune-mediated and the majority of these non-immune mediated cases were T2DM17. Since 2001, an increasing prevalence and incidence of youth-onset T2DM, particularly among minority racial and ethnic groups, has been reported in large multicenter studies in the US18,19. Importantly, youth-onset diabetes is a global problem and increasing prevalences have also been reported in studies from East Asia, Australasia and the Near and Middle East14. In Japan, for example, a study published in 2005 reported that 80% of new cases of youth-onset diabetes identified by diabetes screening in a large school health system were T2DM14.
Youth-onset T2DM is associated with the development in midlife of major diabetes complications, including diabetic kidney disease (DKD), cardiovascular disease, retinopathy, and neuropathy20,21. The increasing frequency of youth-onset T2DM in populations worldwide together with its unexpectedly rapid and aggressive course compared with later-onset T2DM illustrates the urgent challenge of heightening our awareness of this rapidly evolving disease, establishing the mechanisms underlying its rapid progression, and finding effective therapeutic strategies to ameliorate its impact.
In this Review, we examine the emergence of youth-onset T2DM and its complicated course worldwide, emphasizing populations that are at considerable risk of this disease. We compare the clinical course of youth who develop T2DM with those who develop T1DM and with adults who develop T2DM. We also explore the impact of youth-onset T2DM on the key microvascular and macrovascular complications of diabetes, with a focus on the development and progression of DKD. Lastly, we examine the mechanisms that might explain the aggressive clinical phenotype observed in patients with youth-onset T2DM and discuss current management strategies and the challenges that are associated with their implementation in this population.
Epidemiology of youth-onset T2DM
Studies of adverse outcomes among patients with youth-onset T2DM are challenging because diagnosis of T2DM in youth, although increasing, remains uncommon compared with diagnosis in adulthood. Moreover, T2DM in children and adolescents is often not recognized because of its long subclinical stage and a lack of awareness among patients and providers of the increasing risk. Accordingly, data from national population-based disease registries might not adequately reflect the frequency and evolving trends of youth-onset T2DM.
United States and Canada
Although youth-onset T2DM has been reported in all racial and ethnic groups in the US, some populations are particularly notable for their increased risk. For example, Indigenous peoples in the Americas have an exceptionally high prevalence of T2DM and were among the first populations in which youth-onset T2DM was identified. The high risk of youth-onset T2DM in these historically disadvantaged populations suggests an important contribution of stress, historical trauma, and adverse socioeconomic factors to the development of this disease22,23.
United States.
In the US, the National Health and Nutrition Examination Survey (NHANES) and the National Health Interview Survey (NHIS) monitor the prevalence and incidence of diabetes. Differentiation between T1DM and T2DM in these surveys is based on self-reported age at diabetes onset, insulin use and reported diabetes type. The prevalence of diabetes among people aged 12–19-years was estimated to be 0.41% in NHANES 1988-199424 and 0.84% in NHANES 1999-2010, which also reported a prevalence of T1DM of 0.48% and of T2DM of 0.36% in this age group25. In 2016, NHIS estimated that the prevalence of diabetes among people aged 18-29 years in the US was 0.45% for T1DM and 0.66% for T2DM26. None of the national surveys have a large enough sample size to accurately assess diabetes prevalence and incidence in youth at the state or national level or in race and ethnicity subgroups.
In the US, 574 American Indian and Alaska Native tribes and villages are federally recognized. Much of what is known about the long-term effects of youth-onset T2DM comes from population research conducted in Pima Indians from the Gila River Indian Community in Arizona. Diabetes among Pima Indian children and adolescents was first identified in the mid-1960s owing to the initiation of a longitudinal population study27. This youth-onset diabetes was entirely attributed to T2DM12 as it was characterized by ongoing insulin secretion28, lack of insulin dependence, absent or low levels of islet cell and glutamic acid decarboxylase antibodies29,30, and absence of strong linkage or association with maturity-onset diabetes of youth (MODY) loci31,32. The prevalence of youth-onset T2DM among Pima Indian children increased between 1967 and 199633, due in part to an increasing prevalence and severity of obesity in childhood and adolescence34–36 and to a growing incidence of exposure to intrauterine diabetes33,34. In boys, the prevalence of T2DM increased from 0% in 1967-1976 to 1.40% in 1987-1996 among those aged 10-14 years and from 2.43% to 3.78% among those aged 15-19 years. In the same period, the prevalence of T2DM in girls increased from 0.72% to 2.88% among those aged 10-14-years and from 2.73% to 5.31% among those aged 15-19 years33.
Canada.
A national surveillance study that was conducted in Canada between April 2006 and March 2008 reported a minimum incidence of T2DM among children aged <18 years of 1.54 cases per 100,000 per year37. The study identified substantial regional variation, with the highest minimum incidence of 12.45 cases per 100,000 children per year in Manitoba and no cases reported in the Northwest Territories, Yukon and Nunavut. This regional variation could be explained predominantly by the distribution of race and ethnicity, as 44.1% of children with new-onset T2DM were Indigenous people and most of these children were from Manitoba.
Canada recognizes three distinct groups of Indigenous peoples, First Nations, Inuit, and Métis, of which First Nations peoples are the largest and most diverse group. Diabetes among these populations was increasingly recognized following the Second World War38. The incidence increased dramatically between the 1970s and 1990s, with studies consistently reporting a 2-5-fold increased risk of diabetes among First Nations peoples compared to other populations39,40. A 2016 study estimated that as many as 8 in 10 First Nations people aged 20 years will develop T2DM in their lifetime, compared with 5 in 10 non-First Nations people aged 20 years41.
In the 1980s, the first cases of youth-onset T2DM in Canada were identified among Oji-Cree children in Manitoba, about two decades after cases were first observed in Pima Indian youth from Arizona38. Subsequent studies suggest that the majority of Canadian patients with youth-onset T2DM are located in the provinces of Manitoba and Ontario due to their large populations of First Nations people7,42,43. The prevalence of youth-onset diabetes in Canadian First Nations communities with active surveillance is as high as 1-3%43,44. Health disparities such as a lack of access to high quality health care, particularly for First Nations children living in rural areas, might contribute to the differences in prevalence between First Nations and non-First Nations individuals45.
Europe
The prevalence of youth-onset T2DM is generally lower in Europe than in other developed countries23,46. This lower prevalence is partly due to the lower numbers of youths who belong to high risk racial and ethnic groups in many European countries46. The United Kingdom has the highest reported prevalence of childhood T2DM in Europe and a more racially diverse population than many other European countries47. A study that estimated the incidence of youth-onset T2DM in 11,274,750 children aged <17 years in 2015 found that the incidence in the 1,096,304 Asian and 537,938 Black children (2.92 and 1.67 per 100,000, respectively) was much higher than the incidence in the 8,921,552 white children (0.44 per 100,000)48.
South America
Data on youth-onset T2DM in South America are scarce. However, a Brazilian cross-sectional school-based study that collected data from 37,854 students aged 12-17 years in 2013 and 2014 reported a prevalence of T2DM among adolescents of 3.3%15. Brazil has the fourth largest population of people with T2DM worldwide49. These data show that T2DM is not only an important public health challenge for the adult population, but also for the adolescent population, which has a high prevalence of T2DM15.
Africa
Few studies have examined the prevalence of diabetes in youth in Africa and most studies in sub-Saharan Africa have focused on T1DM. In 2004, a review of population-based studies of childhood T2DM identified 4 studies conducted in the 1980s that reported either no cases or a very low prevalence of youth-onset T2DM in Togo, Tanzania and Mali50. The available data suggest that the prevalence and/or detection of youth-onset T2DM in sub-Saharan Africa remains low relative to high-income countries. However, the proportion of school-aged children who are overweight or obese is rising across sub-Saharan Africa51, suggesting that the prevalence of obesity-related diseases, including T2DM, will increase among African youth.
East Asia
As the incidence of T1DM in East Asian countries is very low52–56, diabetes in these countries is largely attributed to T2DM. Data from the Korean National Health and Nutrition Examination Survey (KNHANES) showed an increasing prevalence of diabetes among adolescents from 0.19% in 2007 to 0.43% in 2018, in parallel with a rising prevalence of obesity52.
In China, the prevalence of youth-onset T2DM, estimated by review of hospital records from 14 medical centres, increased 2.5-fold between 2005 and 201054. A national cross-sectional survey reported a prevalence of diabetes among Chinese children aged 10-17 years of 0.13% in 2013-201453.
The incidence of youth-onset T2DM in India in 2006-2012 was estimated using registry data from centres in New Delhi and Chennai. After correction for under-ascertainment, the incidence of T2DM among people aged <20 years was estimated to be 0.5 per 100,000 per year57.
In Japan, a large number of children aged 6-15 years with T2DM have been identified over the past four decades. A study that used data from urine glucose testing of nearly 12 million primary and junior high school students in Tokyo estimated an incidence of 2.58 per 100,000 children per year during 1974-201555.
Australia and New Zealand
Estimation of the prevalence and incidence of T2DM in Australia and New Zealand is facilitated by the existence of several national population-based government registries. The Australian National Diabetes Service Scheme (NDSS) is a population-based registry that captures 80-90% of people with diabetes in Australia. The NDSS reported that during 2003-2019, the prevalence of T2DM was stable among people aged <20 years and among those aged 20–45 years. However, the stable prevalence of T2DM among those aged <20 years might partly reflect the poor representation of Indigenous Australians in the NDSS.
No national studies of the prevalence of youth-onset diabetes among Indigenous Australians are available and the few regional studies have reported vastly variable data. A hospital-based study from the Top End of the Northern Territory, using data collected from 2007-2011, reported a prevalence of T2DM among people aged <25 years of 0.05%. Most of the youths with T2DM (83%) were Indigenous Australians, who comprised 30% of the Northern Territory population58. A retrospective cross-sectional study conducted in Northern Australia between 2016-2017 reported prevalences of T2DM of 0.14% and 1.41% among Indigenous Australians aged <15 years and 15-24 years, respectively59. In this study, the prevalence of T2DM was higher in girls than in boys (0.944% versus 0.416%) and varied considerably across regions. For example, among 15–24-year-olds, the prevalence of T2DM ranged from a very high 3.11% in Central Australia to 0.83% in Far North Queensland. The reasons for these regional differences are unclear but might be related to variations in socioeconomic factors.
Data from Western Australia suggest that Indigenous children have a 20-fold higher mean incidence of T2DM than non-Indigenous children60. The incidence of T2DM increased among Indigenous and non-Indigenous Australian youth between 1990 and 2012. However, the mean rate of increase in incidence was greater among Indigenous children (12.5% per year) than non-Indigenous children (10.9% per year).
The Virtual Diabetes Registry (VDR) is a robust source of diabetes prevalence data in New Zealand that contains administrative data about people suspected of having diabetes who are identified through their use of diabetes-related health services. Data from the VDR collected between 2015 and 2020 indicate that the prevalence of diabetes among those younger than 20 years in New Zealand is highest among Maori people (0.24%), Pacific Islanders (0.23%) and those of European descent (0.27%)61. However, these data are not stratified by diabetes type so the impact of youth-onset T2DM on these estimates cannot be assessed.
Near and Middle East
The Near and Middle East region encompasses 20 countries62: Algeria, Bahrain, Egypt63, Iran64,65, Iraq66, Israel67,68, Jordan, Kuwait69,70, Lebanon71, Libya13, Morocco, Oman72, Palestinian Authority, Qatar73,74, Saudi Arabia75–77, Syria, Tunisia, Turkey78,79, United Arab Emirates (UAE)80,81 and Yemen82. Regional comparisons reported by the International Diabetes Federation indicate that the prevalence of diabetes in this region is the highest worldwide and is increasing rapidly83. High prevalences of youth-onset T2DM have been documented among children and adolescents in Kuwait70 (0.035%) and Qatar73 (0.053%). In addition, high incidences of youth-onset diabetes have been reported in Kuwait69 (8.0 per 100,000 people aged 10-14 years per year), Libya13 (1.8 per 100,000 people aged 10-14 years per year, 5.9 per 100,000 people aged 15-19 years per year), and Qatar73 (4.2 per 100,000 people aged <18 years per year). A national observational study of Israeli children and adolescents aged 10-18 years with T2DM reported a 10-fold increase in the incidence of youth-onset T2DM among Israeli Arabs between 2008 (0.66 per 100,000) and 2019 (6.4 per 100,000) and a 3.5-fold increase among Israeli Jews during the same period (0.62 per 100,000 in 2008 versus 2.3 per 100,000 in 2019)67.
Similar to western countries, a higher frequency of youth-onset T2DM has been reported among girls than among boys in Iran65, Israel67, Turkey78,79, and Egypt70. However, in the UAE80, Iraq66, and Kuwait70 a higher frequency of youth-onset T2DM was reported among boys. These findings might reflect differences in the frequency and distribution of obesity among male and female children in these countries84. In the UAE, for example, the prevalence of morbid obesity (BMI ≥99th percentile) among children aged 11-14 years was 9.6-fold higher in boys than in girls85. In Kuwait, 41.4% of boys aged 6-18 years had obesity compared to 28.9% of girls in the same age group84. Although data on youth-onset T2DM in the Near and Middle East are sparse, the available data indicate a high and growing risk of T2DM among children and adolescents.
Key studies of youth-onset T2DM
The limitations associated with adequately characterizing youth-onset T2DM using data from national disease registries and regional surveillance studies together with the urgent need to expand understanding of this disease beyond small high-risk populations, necessitates rigorously conducted multicenter studies to provide more detailed and nuanced characterization of this emerging problem. Two such studies, the SEARCH for diabetes in youth study and the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) trial, have provided important insights.
The SEARCH for diabetes in youth study
Data from the SEARCH study has greatly expanded our understanding of the epidemiology of paediatric diabetes, the risk factors that contribute to childhood-onset diabetes and diabetes-related complications86,87. SEARCH was conducted between 2001 and 2020 and included >2,500 participants with youth-onset T1DM and 300 with youth-onset T2DM at five centres across the US. In addition, SEARCH included active surveillance of patients with incident diabetes, enabling direct comparisons of epidemiology and complications by diabetes type and providing insights into the differences between paediatric and adult-onset diabetes86,87.
SEARCH reported that in 2002-2012, few cases of T2DM were identified in children under the age of 10 years; however, the incidence of T2DM was greater than that of T1DM among Asian and Pacific Islander, American Indian, and non-Hispanic Black children aged 10-14 years88. For those aged 15-19 years, a higher incidence of T2DM than T1DM was observed for every group except non-Hispanic white people88. The differences between racial and ethnic groups persisted over time, with the most recent SEARCH estimates from 2015 showing a higher incidence of T2DM than T1DM among youths aged 10-19 years in all racial and ethnic groups apart from non-Hispanic white people 89. SEARCH reported that between 2002 and 2015, the age, sex, and race or ethnic group adjusted incidence of T2DM among youths in the US increased by 4.8% annually, compared with an annual increase in the incidence of T1DM in this population of 1.9%88,89.
The SEARCH study estimated that in 2017, 0.067% of youths in the US had T2DM, suggesting that the prevalence of this disease doubled in just 16 years19. This finding parallels the reported increase in childhood obesity in the US from 13.9% in 1999-2000 to 18.5% in 2015-201690. Non-Hispanic Black and Mexican American teenagers experienced the greatest increases in prevalence of obesity and severe obesity between 1999 and 2018, which may contribute to the reported racial and ethnic differences in the incidence and prevalence of T2DM91,92.
Similar to adult T2DM, risk factors for youth-onset T2DM include obesity and inactivity. However, risk factors that are unique to children might also contribute to the risk of T2DM in childhood. The SEARCH Case-Control Study found that exposure to maternal gestational diabetes was associated with a 5.7-fold increase in the odds of youth-onset T2DM93. Exposure to maternal diabetes and/or pre-pregnancy maternal obesity accounted for 47% of youth-onset T2DM in this study. SEARCH also found that breastfeeding of any duration was associated with significantly lower odds of youth-onset T2DM94. However, the odds ratio was attenuated after adjustment for current BMI z-score in the offspring, suggesting that the protective effect of breastfeeding is mediated in part by the weight of the child94. Poor maternal diet95, exposure to environmental chemicals96,97, limited access to healthy foods, limited facilities for physical activity and reductions in the walkability of neighbourhoods might also contribute to the continued rise in youth-onset T2DM98–100. SEARCH found that the incidence of youth-onset T2DM was highest in rural areas with low population density98. The researchers suggested that this increased incidence in rural areas could be attributed to lower access to healthy foods, fewer recreational resources, and greater dependence on automobiles for transportation compared to urban areas. Exposure to pesticides used in agriculture might also be higher in rural communities. Findings from the SEARCH study highlight the importance of surveillance of childhood diabetes within populations and provide insights into the complex and heterogeneous nature of youth-onset T2DM.
The TODAY trial
The TODAY multicenter randomized control trial compared the efficacy of three treatment regimens to achieve durable glycemic control in children and adolescents with T2DM101. Between July 2004 and February 2009, the trial investigators enrolled 699 youths aged 10-18 years with a T2DM duration of <2 years, BMI ≥85th percentile, fasting C-peptide >0.6 mmol/L and negative pancreatic antibodies. The participants were randomly assigned to metformin monotherapy, metformin plus rosiglitazone or metformin plus an intensive lifestyle intervention and followed for an average of 3.9 years. The primary outcome of loss of glycemic control (defined as HbA1c ≥8% for 6 months or sustained metabolic decompensation requiring insulin) was reached by 46% (n=319) of participants, comprising 51.7%, 46.6% and 38.6% of those in the metformin alone, metformin plus lifestyle intervention and metformin plus rosiglitazone groups, respectively101. The finding that metformin monotherapy failed to achieve durable glycaemic control in half of the trial participants is consistent with the rapid β-cell dysfunction that is experienced by young people with T2DM (discussed further below).
In 2011, 572 (82%) of the TODAY trial participants were enrolled in an observational follow-up study, TODAY2, that was conducted in two phases to characterize the burden of vascular complications experienced by youths with T2DM as they transition to adulthood. Between 2011-2014, the participants received all diabetes care from the study and were treated with metformin with or without insulin to maintain glycaemic control. From 2014-2020, 518 participants transitioned to a fully observational study with annual study visits and medical management by their healthcare providers. The average follow-up of the combined studies was 10.2 years. The TODAY2 data demonstrate that diabetes-related complications develop early and accumulate rapidly during the course of youth-onset T2DM, with 15-year cumulative incidences of hypertension, DKD, dyslipidemia and neuropathy of 67.5%, 54.8%, 51.6% and 32.4%, respectively (FIG. 1)20. The 15-year cumulative incidence of any microvascular complication was 80.1% at a mean age of 26.4 years20. Serious cardiovascular events were uncommon (3.73 events per 1000 person-years) but occurred in 17 participants despite their young age. Collectively, these data demonstrate the serious health consequences of youth-onset T2DM as patients transition to young adulthood and the challenges of treating T2DM in this age group20.
Figure 1 |. Long-term complications of youth-onset T2DM.
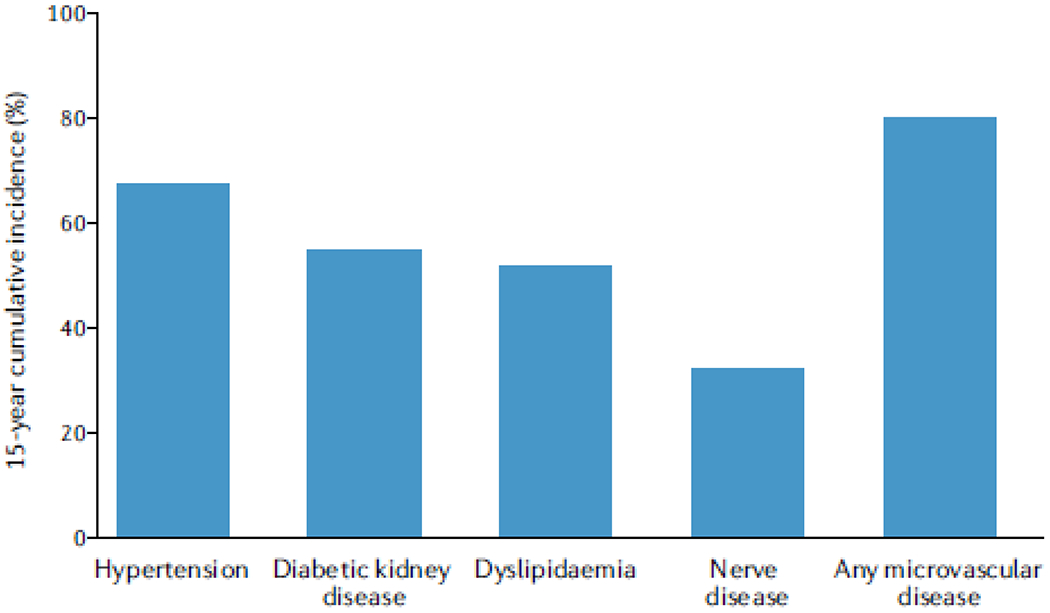
The 15-year cumulative incidences of hypertension, diabetic kidney disease, dyslipidemia (that is, low-density-lipoprotein or triglyceride dyslipidemia), neuropathy and any microvascular disease in participants with youth-onset type 2 diabetes mellitus (T2DM) in the TODAY trial20.
Complications and their risk factors
DKD is the most common and aggressive complication in youths with T2DM, but the incidence of other diabetes-related complications is also increased in these individuals, adversely affecting their health at an early age. In SEARCH, the prevalence of DKD, diabetic retinopathy, peripheral neuropathy, hypertension and arterial stiffness were all higher in young adults with T2DM than in those of similar age and diabetes duration with T1DM (FIG. 2), even after adjustment for sociodemographic factors21. At a mean age of 21 years, three quarters of participants with T2DM and one-third of those with T1DM had at least one microvascular complication or cardiovascular comorbidity21. The only complication that did not have a higher prevalence in patients with T2DM than in those with T1DM was cardiac autonomic neuropathy.
Figure 2 |. Odds of long-term complications in children and adolescents with T2DM versus those with T1DM.
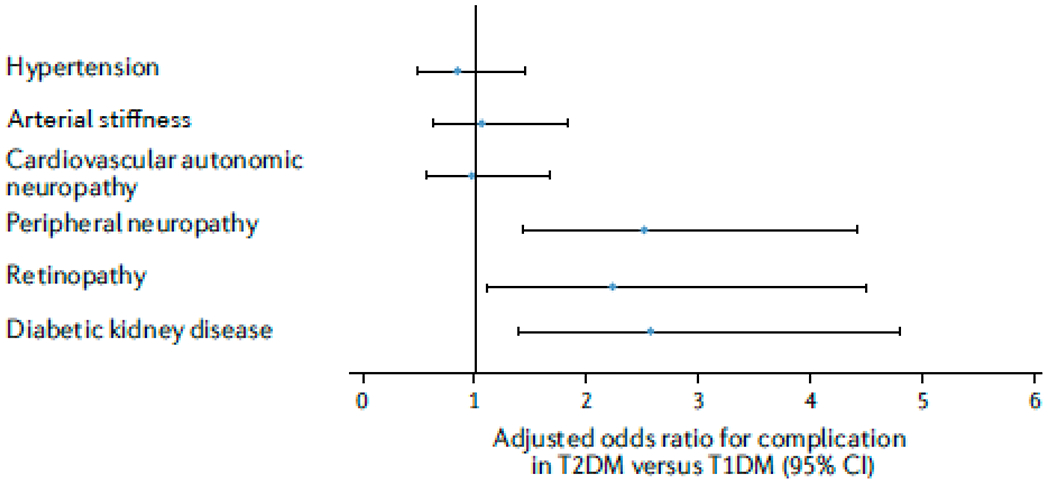
Forest plot showing odds ratios and 95% confidence intervals of diabetes complications in adolescents and young adults with type 2 diabetes mellitus (T2DM) versus those with T1DM in the SEARCH study21,106. After adjustment for established risk factors measured over time, participants with T2DM had significantly higher odds of diabetic kidney disease (DKD; defined as the presence of albuminuria (≥30 μg/mg of creatinine) or reduced kidney function (estimated glomerular filtration rate ≤60 ml/min/1.73m2)), retinopathy (determined using graded digital fundus images), and peripheral neuropathy (defined using the Michigan Neuropathy Screening Instrument) than those with T1DM. The odds of cardiovascular autonomic neuropathy (heart rate variability abnormalities), arterial stiffness (pulse wave velocity ≥90th centile of controls) and hypertension (blood pressure levels ≥95th centile for age) did not differ significantly between the two groups.
Diabetic kidney disease
Albuminuria is the earliest clinical marker of DKD and occurs very early in the disease course of youth-onset T2DM compared with youth-onset T1DM and adult T2DM102,103. In adults with T2DM, albuminuria typically develops after a minimum diabetes duration of 5 years; however, studies of youth-onset diabetes have reported albuminuria in some patients at the time of diagnosis104. In 2021, a systematic review reported that the prevalence of albuminuria among patients of all ethnicities with youth-onset T2DM was 22.2% (95% CI 17.3-27.4%)105. In a multi-ethnic cohort of US patients with diabetes who were diagnosed before the age of 20 years, the odds of elevated albuminuria after a mean diabetes duration of 7.9 years were 2.6-fold higher in those with T2DM than in those with T1DM21,106. The TODAY2 study, which also included a multi-ethnic cohort of participants, reported a 15-year cumulative incidence of albuminuria in patients with youth-onset T2DM of 54.8%20. Although nonalbuminuric DKD has been widely reported in T1DM and in adult T2DM, to our knowledge no data are available on nonalbuminuric DKD in patients with youth-onset T2DM.
Observational studies of Indigenous peoples in the US, Canada and Australia with T2DM and long-term follow-up data, have reported higher rates of progression of DKD to kidney failure among those who developed T2DM in youth compared with those who developed T2DM in adulthood103,107,108. In Canada, the incidence of kidney failure is 4-fold higher in youth with T2DM, most of whom are First Nations people, than in youth with T1DM98. Similarly, studies in Pima Indians from Arizona reported a 5-fold greater risk of kidney failure and a 2-fold greater risk of death between the ages of 25-54 years among those with youth-onset T2DM compared with those with older-onset T2DM109, 110. The aggressive nature of kidney complications in youth-onset T2DM was further confirmed by studies of kidney biopsy samples from Pima Indians with T2DM; those from patients with youth-onset diabetes had more severe structural lesions, including greater glomerular basement membrane width, higher mesangial fractional volume, lower glomerular surface density available for filtration, and lower podocyte density per glomerulus compared with those from patients with adult-onset T2DM irrespective of age at time of biopsy and diabetes duration111. DKD was the leading cause of death among Pima Indians with diabetes until the mid-1980s when it was superseded by cardiovascular disease largely as a result of improved availability of kidney replacement therapies in these communities112. Nevertheless, DKD remains the most prevalent end-stage complication of diabetes and contributes substantially to morbidity and mortality in this population113.
The Australian and New Zealand Dialysis and Transplant Registry (ANZDATA) collects data from all kidney replacement therapy units in Australia with opt-out consent, resulting in almost complete data. ANZDATA is linked to the Australian NDSS and the Australian National Death Index, providing a robust national database that enables characterization of youth-onset T2DM and risk of kidney failure114. A study that analysed linked data for 2,782 people with diabetes who were diagnosed between the ages of 15 and 35 years at a tertiary diabetes center in New South Wales, Australia, showed that the risk of kidney replacement therapy and kidney-related death was 2-fold higher in those with T2DM than in those with T1DM115. This difference remained significant after adjustment for sex, ethnicity, duration of diabetes and the ‘first available’ measurements of haemoglobin A1c (HbA1c), blood pressure and lipids, but was attenuated with the addition of BMI as a covariate, illustrating the important role of obesity in the development and progression of DKD in youth-onset T2DM.
Cardiovascular disease and mortality
Cardiovascular disease is the leading cause of death among adults with T2DM and its contribution to the morbidity and mortality that is associated with youth-onset T2DM is under intense scrutiny. An echocardiogram study that included 479 SEARCH participants with a median duration of diabetes of 11.6 years reported that those with T2DM had greater left ventricular (LV) mass and worse diastolic function, a precursor to heart failure, than those with T1DM116. This difference persisted after adjustment for differences in BMI, blood pressure, and HbA1c. Both patient groups showed abnormal diastolic function compared with healthy individuals, but the percentage of any dysfunction was greater among those with T2DM (58.1%) than among those with T1DM (47.5%).
In the SEARCH study, 133 of 14,721individuals with T1DM and 55 of 4,141 individuals with T2DM died during a median follow-up of 8.5 years117. Diabetes was the underlying cause of 42.1% of deaths among those with T1DM and 9.1% of deaths among those with T2DM. The standardized mortality ratio was greater for T2DM than for T1DM in this cohort117. Similarly, in a study of 24,415 individuals with diabetes who were recruited from a tertiary hospital-based clinic in New South Wales, Australia, 824 had youth-onset diabetes defined as diabetes diagnosed between 15 and 30 years of age: 354 had youth-onset T2DM and 470 had youth-onset T1DM. Those with youth-onset T2DM had 2-fold higher mortality than those with T1DM of similar age of onset and 50% of deaths among those with youth-onset T2DM and 30% of deaths among those with T1DM were due to cardiovascular disease118.
The TODAY trial participants underwent echocardiography a median of 4.5 years after diagnosis of T2DM and at an average age of 18 years. In this cohort of adolescents with T2DM, mean LV mass, LV shortening fraction and left atrial internal dimension were in the high normal range and 16.2% of participants had adverse LV geometry119. Adverse measures of cardiac structure and function were associated with higher BMI and blood pressure and no effect of treatment group on cardiac target organ injury was observed, suggesting a need for more aggressive therapy. Repeat echocardiograms that were performed approximately 5 years after the initial echocardiograms at a mean participant age of 23 years and diabetes duration of approximately 9.5 years demonstrated substantial worsening of cardiac function from adolescence to early adulthood. Of particular concern, ejection fraction was <52% in 11.7% of male participants and diastolic function (E/Em) had declined since the initial echocardiograms were performed120.
The TODAY2 long-term follow-up study of the TODAY cohort, reported an incidence of all adjudicated heart, vascular, and cerebrovascular events of 3.73 per 1,000 person-years during an average follow-up of 10.2 years20. These events comprised 17 serious cardiovascular events (4 myocardial infarctions, 6 congestive heart failure events, 3 coronary artery disease events, 4 stroke events) and one death owing to myocardial infarction. This high rate of cardiovascular disease occurred despite the young age of the participants (mean age at the end of the study period of 26.4±2.8 years and mean diabetes duration of 13.3±1.8 years). Together, the available data indicate that youth-onset T2DM is associated with an increased risk of cardiovascular disease and death in young adulthood.
Retinopathy
Diabetic retinopathy is a major cause of vision loss worldwide121. The prevalence and progression of diabetic retinopathy in youth with T1DM is well characterized and intensive glycaemic control has been shown to slow the progression of this complication122. However, the impact of diabetic retinopathy in youth-onset T2DM is less certain. Studies of retinopathy in youth-onset diabetes have reported a similar prevalence in T2DM and T1DM123 and both a higher124 and lower prevalence in T2DM than in T1DM125. SEARCH reported a higher prevalence of diabetic retinopathy in T2DM (42%) than in T1DM (17%) at similar diabetes duration (~7 years)126. The prevalence of retinopathy was lower in non-Hispanic white participants than in other racial and ethnic groups irrespective of diabetes type and this difference was not explained by glycemia, suggesting that socioeconomic factors and disparities related to race and ethnicity might have a role in this variability.
The impact of diabetic retinopathy in youth-onset and older-onset T2DM is also variable. In a study of 2,508 Pima Indians, those with youth-onset T2DM (age at onset <20 years) had less than half the risk of diabetic retinopathy identified by direct ophthalmoscopy than those with adult-onset T2DM (age at onset 20-59 years)109. On the other hand, a study that analysed clinical records from a diabetes centre in south India reported a higher prevalence of diabetic retinopathy in patients with youth-onset T2DM (age at onset <25 years) than in sex and diabetes duration-matched patients with older-onset T2DM (age at onset >50 years), with an odds ratio of 2.1 after adjustment for clinical covariates127.
Estimates from clinical trials suggest that the risk of retinopathy in patients with youth-onset T2DM might be greater than published estimates of the risk in those with older-onset T2DM. The TODAY2 study reported a prevalence of diabetic retinopathy of 51% (identified by digital fundus photography of seven standard stereoscopic fields) at a mean diabetes duration of 12 years with 9% of retinopathy cases being moderate or severe20. By contrast, NHANES reported a prevalence of retinopathy of 28.5% among individuals with T1DM or T2DM aged ≥40 year with a mean diabetes duration of 15 years128.
Neuropathy
Diabetic neuropathy is often subclinical or mild in youth with diabetes. However, early recognition of diabetic neuropathy is important for initiation of treatment to reduce the morbidity and mortality associated with this complication, which often appears in young adulthood among those who developed diabetes in youth129. In TODAY2, 32.4% of the 500 participants with youth-onset T2DM developed diabetic neuropathy (assessed by annual Michigan Neuropathy Screening Instrument [MNSI] and Semmes-Weinstein monofilament examinations) within a mean of 13.3 years following their diagnosis of diabetes20.
The odds of diabetic neuropathy diagnosed using the MNSI was 2.52 times higher among participants in SEARCH with youth-onset T2DM than in those with youth-onset T1DM of similar diabetes duration after adjustment for established risk factors21. Similarly, individuals with T2DM developed neuropathy sooner than those with T1DM in a Canadian cohort identified using a large healthcare administrative database130. By contrast, although cardiovascular autonomic neuropathy was prevalent in youth-onset diabetes in SEARCH, no statistically significant difference was observed between those with youth-onset T2DM (age-adjusted prevalence = 15.7%) and those with T1DM (age-adjusted prevalence = 14.4%)131.
SEARCH was the first study to compare cognitive function in young adults with T1DM and T2DM132. Those with youth-onset T2DM had lower fluid cognitive scores (assessed using the NIH Toolbox Cognition Battery), reflecting reductions in executive function, than those with T1DM. This association was attenuated by adjustment for age, race, ethnicity, adiposity, parental education and depressive symptoms132, suggesting that these differences are mediated by adiposity and mental health and might reflect the impact of social inequities that are associated with race and ethnicity.
Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) is very common in patients with youth-onset T2DM; one study found that 48% of tested individuals had elevated alanine aminotransferase (ALT), a surrogate for NAFLD, and a third had ALT values that were three times the upper limit of normal133. Another study found that 20% of African Americans, 71% of Hispanic and 88% of non-Hispanic White people with youth-onset T2DM had elevated ALT at diagnosis134. In the TODAY study, liver fat was not assessed, and exclusion criteria included aspartate aminotransaminase (AST) or ALT levels >2.5 times the upper limit of normal, preventing enrolment of patients with substantial NAFLD. Nevertheless, after a mean follow-up of 3.8 years, liver transaminase levels had increased to >2.5 times the upper limit of normal in 13% of the 699 study participants.
Data from the TODAY study suggested a lower prevalence of elevated liver transaminases in the metformin plus rosiglitazone group than in the metformin and lifestyle intervention and metformin only groups101. Despite reductions in liver transaminase levels suggesting improvement in NAFLD with rosiglitazone, increases in subcutaneous adipose tissue and abdominal visceral adipose tissue were greater in the metformin plus rosiglitazone group than in the other two groups135. This finding indicates that in contrast to its reported effects on body composition in adults136–139, roglitazone treatment does not lead to fat redistribution from the viscera and liver to subcutaneous fat in youths.
Risk factors
In patients with youth-onset T2DM, hyperglycemia and hypertension are important risk factors for DKD140–143, cardiovascular disease20,120,144,145 and diabetic retinopathy123,124,127,146. Poor glycemic control is a well-established risk factor for diabetic neuropathy in T1DM but its role in T2DM is less clear. SEARCH demonstrated that diabetes duration was significantly associated with diabetic neuropathy in youth with T1DM and T2DM, but glycemic control over time was only associated with diabetic neuropathy in youth with T1DM144. The optimal targets for glycemic and blood pressure control in youth-onset T2DM have not been identified, but mean HbA1c values in youth with T2DM are often above current recommended adult targets due in part to the challenges of chronic disease management during adolescence20,147.
The excess burden of diabetes-related complications in young adults with T2DM compared with those with T1DM might be related to a greater number and/or severity of vascular risk factors early in T2DM. In the TODAY study, the 15-year cumulative incidence of dyslipidemia was 51.6%20 and hypertension (defined as blood pressure ≥95th percentile for age, sex, and height or systolic blood pressure ≥130mmHg and/or diastolic blood pressure ≥80mmHg on three consecutive occasions or elevated blood pressure followed by initiation of anti-hypertensive therapy) was documented in 18.7% of participants at baseline with a 15-year cumulative incidence of 67.5%20,145. Adverse echocardiographic changes in TODAY were associated with hypertension, obesity, female sex, Hispanic ethnicity and non-Hispanic Black race, worse glycemic control, and elevated heart rate120. The SEARCH study reported a higher prevalence of poor glycemic control, adiposity, hypertension, and dysplipidemia in youth with T2DM compared to youth with T1DM of similar disease duration148–151.
Dyslipidaemia is also associated with diabetic neuropathy in youth. SEARCH found that increased LDL-cholesterol was associated with diabetic neuropathy (defined by the MNSI) in youth with T1DM, whereas decreased LDL-cholesterol was associated with diabetic neuropathy in youth with T2DM144. The reasons for the differing association of LDL-cholesterol with diabetic neuropathy by type of diabetes are unknown. Elevated triglycerides were also associated with cardiovascular autonomic neuropathy in youth with T1DM and T2DM152. No ethnic differences in the prevalence of diabetic neuropathy diagnosed by the MNSI were observed in youth-onset T1DM or T2DM in SEARCH, but the prevalence of cardiovascular autonomic neuropathy in youth-onset T2DM was higher in Hispanic and non-Hispanic White people than in Asian and Pacific Islander, non-Hispanic Black people, and other racial and ethnic groups. In SEARCH, tobacco use was reported among youth with T1DM and T2DM153 and was associated with diabetic neuropathy in both types of diabetes144.
Metabolic syndrome is a risk factor for NAFLD. In the TODAY study, the baseline prevalence of metabolic syndrome (according to the NCEP ATP III definition) was 75.8% and this prevalence did not improve over time with diabetes treatment135. Elevated ALT as a surrogate for NAFLD was more frequent in those with metabolic syndrome, as was elevated C-reactive protein, IL-6, and PAI-1135. Thus, the metabolic syndrome and markers of NAFLD and inflammation were frequently abnormal in patients with youth-onset T2DM and did not respond to multiple diabetes treatments, suggesting a need for new therapies.
Mechanisms of the aggressive phenotype
Longer diabetes duration is associated with a greater burden of diabetes complications154–158. However, a more aggressive clinical course is observed in youth-onset than in adult onset T2DM even after controlling for duration of diabetes127,159–163. Several factors might contribute to this aggressive T2DM phenotype (FIG. 3).
Figure 3 |. Potential mechanisms responsible for the aggressive clinical phenotype in youth-onset T2DM.
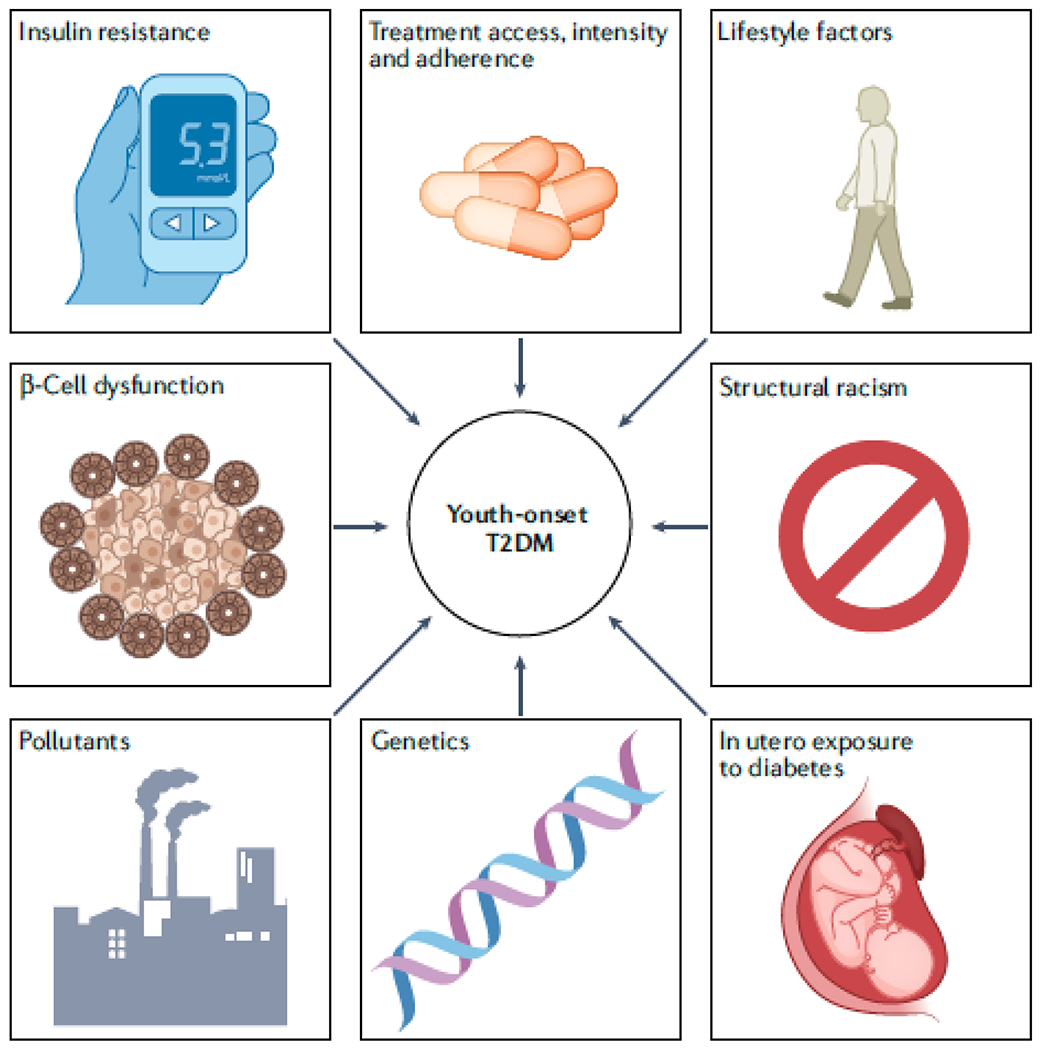
Several factors might contribute to the more aggressive phenotype in youth onset versus adult-onset type 2 diabetes mellitus (T2DM). The more severe glycaemic phenotype in youth-onset than in adult-onset T2DM might be partly explained by worse pancreatic β-cell function, leading to more rapid β-cell failure163–166. Early development of insulin resistance might lead to increased insulin secretion, driving β-cell failure and thereby lowering insulin clearance. Demand on the pancreas might be highest during adolescence owing to the marked insulin resistance of puberty168,172–175. Lower adherence to medical therapy, lifestyle modifications178,179 and self-monitoring168 as well as less aggressive medical treatment and fewer treatment options183–186 might also contribute to poorer glycaemic control and higher rates of complications in youth compared with adults with T2DM. Youth with T2DM are generally a more disadvantaged group than adults with T2DM172 so might have less access to quality healthcare, healthy food choices187 and opportunities for physical activity188. Other factors that are associated with historical disadvantage, including depression, poorer sleep quality197, low birth weight, and childhood exposure to stressors, including systemic racism, and environmental pollutants198–201, might also increase the risk of youth-onset T2DM and the aggressiveness of the disease. Exposure to diabetes in utero is associated with higher BMI in childhood and increased risk of insulin resistance and T2DM in youth202–207. In utero exposure to diabetes might also result in impaired nephron development, which increases the risk of DKD in later life212,214,215,275, as well as other developmental disruptions that could accelerate the appearance and severity of diabetes complications212. Genetic factors might contribute to the more aggressive clinical phenotype that is associated with youth-onset compared with adult-onset T2DM176.
β-cell function and insulin resistance
Inherently worse pancreatic β-cell function, leading to rapid β-cell failure, might be partly responsible for the severe glycemic phenotype in youth with T2DM163–166. This hypothesis is supported by the observation that poor β-cell function, and not insulin sensitivity, was a driving factor for loss of glycemic control in youth in the TODAY167 and Restoring Insulin Secretion (RISE) studies168–170. By contrast, lower insulin sensitivity was associated with loss of glycaemic control among adults in the RISE study (FIG. 4)171.
Figure 4 |. Greater loss of β-cell function in youth-onset versus adult-onset T2DM.
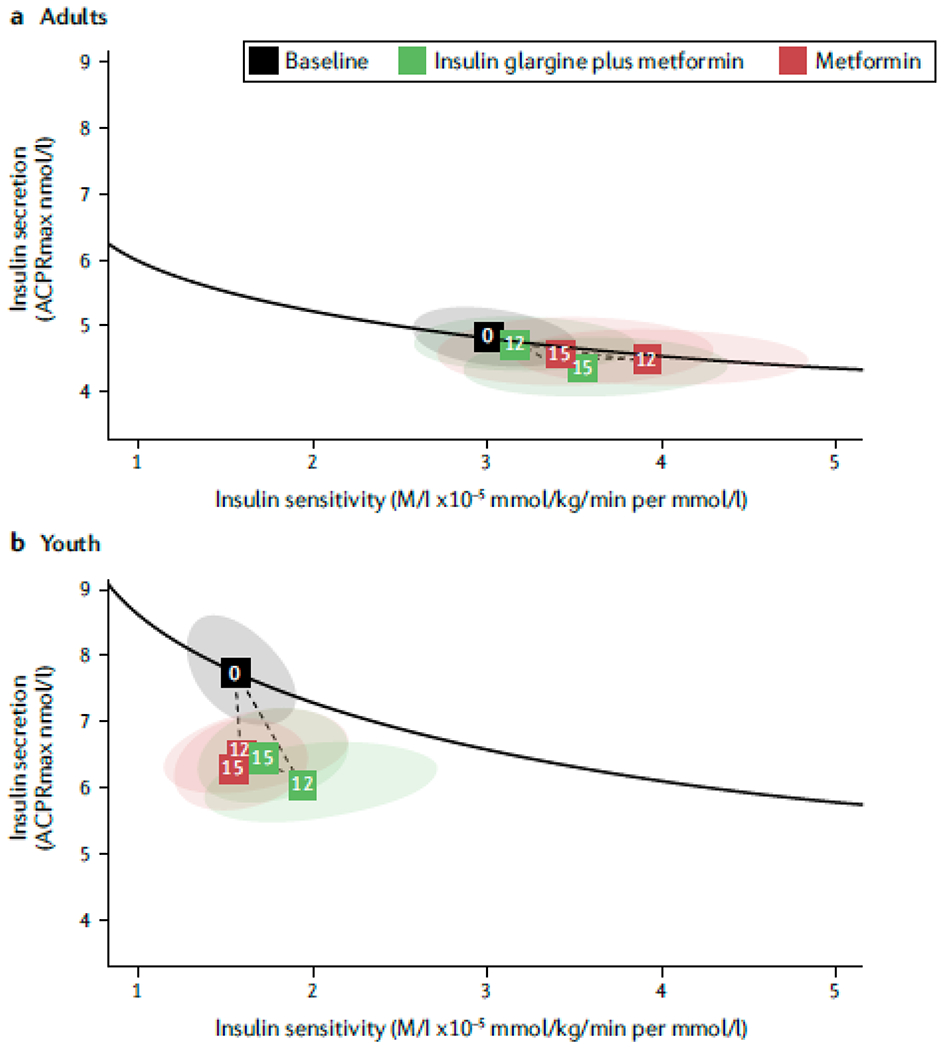
The Restoring Insulin Secretion (RISE) Study used hyperglycemic clamps to examine insulin sensitivity and β-cell function in a | adults and b | youth with impaired glucose tolerance or recently diagnosed T2DM at baseline, after 12 months of treatment for hyperglycemia with either insulin glargine and metformin or metformin alone, and at 15 months of follow-up (3 months after discontinuation of treatment)175. Insulin secretion determined using an arginine stimulation test (acute c-peptide response to arginine at maximal glycemic potentiation [ACPRmax]) is shown on the y axes and insulin sensitivity (glucose infusion rate by steady state insulin concentrations [M/I]) is shown on the x-axes. The solid black lines in the vector plots illustrate the relationship between insulin secretion (that is, β-cell response) and insulin sensitivity at baseline. The numbered boxes represent the mean values for each cohort at baseline (0), 12 months (12) and 15 months (15) and the ellipses around the boxes represent the 95% confidence intervals. The dotted lines show the trajectory of mean values from baseline to 12 months of intervention and to 15 months of follow-up. Values above the solid black line represent improved β-cell function and those below this line represent worse β-cell function compared to baseline. At baseline, youth were markedly more insulin resistant and had much higher insulin secretion than adults despite similar levels of glycemia. After 12 months of treatment, β-cell function had deteriorated in youth but not in adults. The two interventions did not differ substantially in their effects on β-cell function in either age group. The difference in β-cell function outcomes in response to treatment in youths and adults supports a more adverse trajectory of β-cell deterioration in youth. Reprinted with permission from ref 175, American Diabetes Association.
Alternatively, a higher degree of obesity and insulin resistance might be required to develop T2DM at a young age compared to in adulthood when the pancreas might be less healthy. More severe insulin resistance might cause β-cells to secrete more insulin, driving earlier β-cell failure, and lowering insulin clearance. Data from RISE clearly show worse insulin resistance, a higher requirement for insulin secretion, and lower insulin clearance in youth-onset versus adult-onset T2DM168,172–175. As puberty is associated with a marked increase in insulin resistance, the demand on the pancreas might be greatest during adolescence.168,172–175
Genetic factors
The role of genetic factors in the aggressive phenotype of youth-onset T2DM must also be considered. The Progress in Diabetes Genetics in Youth (ProDiGY) study, which analysed data from a multi-ethnic cohort of participants in the TODAY, SEARCH and Type 2 Diabetes Genetics Exploration by Next-generation sequencing in multi-Ethnic Samples (T2DM-GENES) studies, identified seven genome-wide significant loci in or near PHF2 TCF7L2, MC4R, CDC123, KCNQ1, IGF2BP2, and SLC16A11, that were associated with this phenotype176. The variant in PHF2 was novel, whereas the other variants have previously been associated with T2DM in adults. MC4R is a gene that strongly influences obesity. The effect size of the variant in MC4R, rs72982988, for T2DM in youth was greater than that reported in adults, suggesting that the role of obesity-related genes might differ between youth-onset and adult-onset T2DM. Notably, a unique polymorphism in the gene that encodes hepatocyte nuclear factor 1-alpha (HNF-1α G319S) that is found in many Canadian First Nations youth has been associated with a form of maturity-onset diabetes of the young that mimics T2DM177.
Treatment intensity and adherence
Other factors that might contribute to the poor glycemic control and high rates of complications in youth-onset T2DM include lower adherence to medication, lifestyle interventions178,179 and self-monitoring168 compared with adult patients. A study in 212 young adults (mean age 26 years) with T2DM reported that 69.8% were low adherent to oral hypoglycaemia medications. In this study, low adherence was most common among women, non-Hispanic Black people and those who did not have healthcare coverage180. The factors that are associated with low adherence among patients with youth-onset T2DM are incompletely understood but likely include high rates of depression181 and poverty182. In the TODAY cohort, 70% of youth with T2DM lived in single-parent households and 42% lived in households with an annual income of less than US$25,000182.
Youth with T2DM might also receive less aggressive treatment than adults owing to a lack of regulatory approval of newer T2DM therapies for this population as well as a lack of provider comfort with prescribing traditionally adult medications for these patients183–186. A study that compared individuals with youth-onset versus adult-onset T2DM who were matched for sex and diabetes duration, reported that those with younger onset had significantly higher HbA1c, cholesterol, triglycerides, and LDL cholesterol and lower HDL cholesterol127 than those with older onset of T2DM.
Socioeconomic and environmental factors
Youth with T2DM are historically a more disadvantaged group than adults with T2DM172, and therefore are likely to have less access to quality healthcare, healthy food choices187 and physical activity as well as lower activity levels188. Studies published in the past 20 years report that US youth 189,190, particularly girls and African Americans191,192, have very low levels of habitual physical activity.
Poor sleep has also been implicated in youth-onset T2DM owing to its association with insulin resistance. As adolescents currently obtain well below the recommended amount of sleep and have markedly shifted circadian rhythms, which are likely worsened by environmental exposures such as evening use of computers and phones, their circadian patterns are out of sync with typical school schedules193–196. Lower socioeconomic status is also associated with poor sleep quality in the general population197. However, further studies of sleep quality in patients with youth-onset T2DM are needed.
Exposure to other stressors that are more common in populations that are historically disadvantaged, medically underserved and at increased risk of youth-onset T2DM might also increase the aggressiveness of the disease. These stressors include depression, low birth weight and adverse childhood exposures, including systemic racism and environmental pollutants (for example, dichlorodiphenyl-dichloroethylene, polyfluroalkyl and perfluoroalkyl substances)198–201.
In utero exposure to diabetes
Exposure to diabetes in utero is associated with higher BMI in children and accelerates progression to diabetes by various mechanisms, potentially including epigenetic modifications. This in utero exposure can therefore result in a vicious cycle of increasing T2DM prevalence in successive generations202–211.
Exposure to diabetes in utero might preferentially harm poorly replicating, terminally differentiated cells such as those in the brain, pancreas, and nephron, leading to impaired development that can accelerate the appearance and severity of diabetes complications212,213. In the kidneys, impaired nephron development can lead to reduced nephron mass and/or functional capacity, thereby increasing the risk of DKD later in life212,214,215. In utero exposure to diabetes has been associated with elevated albuminuria in Pima Indians with T2DM214 and with single-nephron hyperfiltration in the children of French women who had T1DM during pregnancy.
Management of youth-onset T2DM
Given the aggressive clinical course of youth-onset T2DM, the challenges associated with managing chronic disease in the young, and the frequent development of end-stage diabetes complications in mid-life in these patients, effective diabetes management strategies are important. Current management strategies include lifestyle interventions, pharmacological therapies and metabolic bariatric surgery (MBS).
Lifestyle interventions
The ADA, International Society of Pediatric and Adolescent Diabetes (ISPAD), American Heart Association (AHA) and American Society of Nephrology (ASN) guidelines recommend a combination of lifestyle modifications and drug therapy in youth with T2DM216–218. Their guidelines emphasize the importance of regular moderate-intensity aerobic exercise as a first-line standard-of-care intervention for hypertension. This recommendation is supported by meta-analyses showing that aerobic exercise training in adults reduces casual systolic blood pressure by 2-8 mm Hg on average, with the largest systolic blood pressure-lowering effects observed in those with the highest baseline systolic blood pressure219. Regular aerobic exercise also improves vascular endothelial function in nondiabetic middle aged and older adults220,221 and may mitigate risk of DKD in middle aged and older adults with T2DM222, in large part by decreasing oxidative stress and increasing shear stress-mediated nitric oxide production223,224. Further studies are needed to evaluate the effects of aerobic exercise on vascular endothelial function and risk of DKD in youth with T2DM.
Despite the encouraging findings in adults, the TODAY trial showed that an aerobic exercise intervention did not attenuate hypertension or microvascular complications in youth with T2DM20. The physical activity target of the TODAY trial was 200 minutes per week of moderate to vigorous intensity exercise for most participants and up to 300 minutes per week for those who already engaged in regular, physical activity at the time of study enrollment20,101. Only 53.6% of participants in the TODAY trial met the physical activity target and this failure might be a major contributor to the suboptimal response in the metformin plus lifestyle intervention group in this trial20,101. Notably, youth-onset T2DM has a female predominance, but the lifestyle intervention in TODAY was significantly less effective and associated with worse adherence in girls than in boys20,101. An observational study in Canadian First Nations youth with T2DM identified improved glycemic control and blood pressure in those who engaged in vigorous-intensity physical activity225.
The most commonly reported barrier to regular aerobic exercise is lack of time226–231. Other frequently cited barriers include logistical barriers such as lack of facility access (including as a result of lack of transportation, prohibitive cost, and schedule constraints) and mobility issues228,232–234. Psychological barriers are also common and include low motivation, depression, and poor exercise self-efficacy181,235. Poor physical function is an important contributor to failure to meet physical activity targets and is associated with comorbid conditions such as obesity, neuropathic pain, fatigue, and cardiovascular disease236. Lifestyle strategies to lower risk of microvascular and macrovascular complications in youth-onset T2DM should consider approaches to overcome these barriers to adherence.
Dietary modification targeting a daily caloric deficit and enhanced nutrient intake is an important part of the management of youth-onset T2DM237. Dietary interventions might be more effective in lowering BMI in these patients when coupled with increased physical activity237. Although diet and physical activity can lower BMI and mitigate risk of diabetes complications, these lifestyle modifications are not recommended as monotherapy and should always be used in conjunction with pharmacotherapy for youth-onset T2DM owing to the aggressive nature of the disease.
Pharmacological management
As hyperglycemia is rarely sufficiently controlled by diet and exercise in youth-onset T2DM, pharmacological interventions are typically required. A major limitation, however, is the paucity of trial data regarding optimal pharmacological management of youth-onset T2DM. This limitation is unnecessarily perpetuated by the frequent exclusion of adolescents from clinical trials of T2DM medicines. Thus, many treatment recommendations for youth with T2DM are based on data extrapolated from trials conducted in adults rather than on evidence of efficacy in youths. In addition, several drugs that are available for adults with T2DM are not approved for the treatment of youths owing to limited efficacy and safety data in this population, reducing the options that are available to normalize glycemia in youth-onset T2DM.
Metformin.
The biguanide metformin is the first-choice agent to treat hyperglycemia in youth-onset T2DM238. Metformin activates adenosine monophosphate-activated protein kinase in insulin-sensitive tissues (predominantly liver and skeletal muscle) and therefore reduces hepatic glucose production and increases insulin-stimulated glucose-uptake in skeletal muscle239.
Insulin.
Insulin is a safe and effective treatment to lower hyperglycemia in youth-onset T2DM and is the first choice of therapy when HbA1c is high at diagnosis (e.g. >8.5% or 69 mmol/mol) or if ketoacidosis is present238. Data from the TODAY study show that insulin therapy can be successfully stopped when glycemic targets are met in up to 90% of youth with T2DM240. The RISE study showed that 3 months of insulin glargine treatment followed by 9 months of metformin compared with 12 months of metformin treatment alone did not prevent deterioration of β-cell function during treatment or following treatment cessation. This finding suggests that alternate approaches are necessary to halt the progressive disease course seen in youth with T2DM241.
GLP-1 receptor agonists.
Injectable glucagon-like peptide-1 (GLP-1) receptor agonists harness the beneficial metabolic effects of the gut hormone GLP-1. In the Evaluation of Liraglutide in Pediatric with Diabetes (Ellipse) trial61, liraglutide (1.8 mg daily) plus metformin with or without concomitant insulin treatment, reduced HbA1c levels by 1% at week 26 and induced small reductions in weight in adolescents (aged 10-17 years) with T2DM242. Likewise, in the Assessment of Weekly Administration of LY2189265 in Diabetes – Pediatric Study (AWARDS-PEDS) in youth (aged 10-18 years) with T2DM who were being treated with lifestyle modifications with or without metformin or insulin therapy, weekly administration of dulaglutide 0.75 mg or 1.5 mg reduced HbA1c at 26 weeks by 0.6% or 0.9%, respectively, without an effect on BMI243. Another trial in youth (aged 10-17 years) with T2DM who received a stable dose of oral therapy (metformin and/or sulfonylurea) and/or insulin for at least two months prior to enrollment, reported that exenatide long-acting release (LAR) 2 mg weekly induced a 0.7% reduction in HbA1c levels compared with placebo244. The adverse effects observed in these studies were similar to those observed in adults and included gastrointestinal effects such as nausea and diarrhea245. The long-term safety and cardiovascular and kidney effects of GLP-1 receptor agonists have not been studied in youth with T2DM.
Liraglutide and exenatide LAR have both been approved for the treatment of youth-onset T2DM in the US. Off-label use of GLP-1 receptor agonists is required for patients aged <18 years in many countries.
Thiazolidinediones.
Thiazolidinediones (TZDs) are peroxisome proliferator-activated receptor (PPAR)-gamma receptor activators that increase peripheral insulin sensitivity and improve β-cell function, resulting in durable glycemic control in adults246,247. In youth with T2DM in the TODAY study, glycemic failure rates decreased by 13% when rosiglitazone added to metformin was compared to metformin alone101. However, TZD therapy is associated with increased risk of fractures, fluid retention and heart failure in adults248. Although these adverse effects were not observed in the TODAY study, TZDs have not been approved for youth-onset T2DM despite their beneficial effects on insulin sensitivity that could benefit insulin-resistant adolescents.
Sulfonylureas.
Binding of sulfonylureas to ATP-dependent K+ channels on the cell membrane of pancreatic β-cells causes them to close, inducing insulin secretion. A study in patients with youth-onset T2DM reported similar reductions in Hba1c levels with the sulfonyluera glimepiride compared to metformin; however, patients in the glimepiride group experienced greater weight gain and more episodes of hypoglycemia249. Sulfonylureas are currently not recommended for treatment of hyperglycemia in adolescents in many countries due to limited safety data.
SGLT2 inhibitors.
Oral sodium-glucose cotransporter 2 (SGLT2) inhibitors block glucose uptake in the proximal tubule of the kidneys, thereby inducing glycosuria and lowering plasma glucose concentration. Several studies have evaluated the glucose-lowering effects of these agents in youth with T2DM; however, the results of some of these studies are not yet available. A clinical trial that enrolled participants aged 10-24 years with T2DM, reported that 10 mg of dapagliflozin in addition to standard care had no effect on HbA1c levels compared with placebo in the intention-to-treat analysis, but reduced HbA1c by 1.13% relative to placebo among protocol-compliant participants after 24 weeks250. Another study in 27 youth with T2DM reported that empagliflozin acutely lowered estimated glomerular filtration rate (eGFR) and increased fractional sodium excretion251. This finding is of interest because natriuresis is thought to contribute to the beneficial cardiovascular effects of SGLT2 inhibition and an acute reduction in eGFR is thought to reflect a reduction of intraglomerular pressure that likely underlies the kidney protective effects of SGLT2 inhibitors reported in adults. Given the paucity of published data, SGLT2 inhibitors are not yet approved for treatment of youth onset-T2DM.
Metabolic bariatric surgery
MBS (sometimes called bariatric surgery) can promote weight loss and improve metabolic health. This intervention seems to be the most effective treatment for T2DM in adults252. Over 25 years of data from the Swedish Obese Subjects (SOS) study have shown that in adults with obesity (7.4% of whom had T2DM), MBS, irrespective of operation type, induces durable reductions of 25% in body weight, 30% in mortality, and 50% in microvascular complications compared with usual obesity care253. Furthermore, this study reported T2DM remission at 15 years of follow-up in 30% of patients in the MBS group compared with 6.5% of those in the nonsurgical treatment group254. The Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) study reported T2DM remission in 23% of adults 5 years after vertical sleeve gastrectomy compared to 5% of adults following intensive medical treatment255,256. Extrapolating these data to youth is challenging given the numerous differences between youth-onset and adult-onset T2DM.
Teen-LABS, a prospective, observational cohort study of youth with obesity undergoing MBS, reported a T2DM remission rate of 94% at 3 years following Roux-en-Y gastric bypass (RYGB) in 29 youth with T2DM and no cases of incident T2DM among 153 youth without T2DM at enrollment257. A retrospective comparison of data from Teen-LABS participants with T2DM (n=30) and a matched subset of participants from the TODAY study (n=63), suggested that youth with T2DM who undergo RYGB surgery have better outcomes, including reductions in hypertension, dyslipidemia and risk of DKD and cardiovascular disease258–260. The Teen-LABS and TODAY studies were not designed to be directly compared and have important differences in study design, outcomes collected, and cohort characteristics. Nonetheless, they offer a first suggestion of a management strategy that might provide an efficacious treatment in this challenging high-risk patient population.
The Surgical or Medical Treatment for Pediatric Type 2 Diabetes (ST2OMP) study is intended to address the limitations of the Teen-LABS and TODAY comparison and fill gaps in our understanding of MBS in youth261,262. ST2OMP is prospectively comparing teenagers choosing medical therapy to those choosing vertical sleeve gastrectomy. The medical therapy incorporates an aggressive multi-drug, treat-to-target intervention with the goal of achieving an HbA1c <6.5% and includes off-label use of SGLT-2 inhibitors and on-label use of GLP-1 agonists. The primary outcome of the study is glycemic control at 1 year and the secondary outcomes will compare diabetes related comorbidities. Outcome data from ST2OMP are expected in 2024-2025.
Conclusions
The incidence and prevalence of youth-onset T2DM is increasing worldwide. Children and adolescents who are overweight or obese, the majority of whom live in developing countries, are at increased risk of this disease19,159,263. The prevalence of overweight or obesity among children and adolescents aged 5-19 years increased more than four-fold between 1975 and 2016, reaching 18% globally264. In the US, where nearly 14 million youth are obese, the rising prevalence and severity of obesity and other metabolic conditions are projected to increase the incidence of youth-onset T2DM by 600% between 2017 and 206018,265. The Assessing the Burden of Diabetes by Type in Children, Adolescents, and Young Adults (DiCAYA) Network is monitoring this expected increase266.
Youth-onset T2DM has a more adverse clinical course than youth-onset T1DM and a more extreme metabolic phenotype than adult-onset T2DM, including greater insulin resistance and more rapid deterioration of β-cell function241,267. These factors increase the risk of vascular complications among patients who develop youth-onset T2DM20,21,159,241,268,269. However, the available therapeutic options to manage T2DM in youth and prevent the development of these complications are limited. The current mainstays of therapy to mitigate risk of DKD in patients with T2DM include renin-angiotensin-aldosterone system blockade, SGLT2 inhibitors and GLP1 receptor agonists270–273. However, the only medications that are approved by the US FDA, European Medicines Agency and Health Canada for the treatment of youth with T2DM are metformin, insulin and GLP-1 receptor agonists.
Data from the TODAY and RISE studies show that the physiology underlying the development of T2DM in youth differs from that of adult-onset T2DM, suggesting that different treatments might be needed. Establishing a fuller understanding of the unique pathophysiology of youth-onset T2DM is a necessary first step to enable the design of clinical trials to identify efficacious management strategies for patients241,267,274. Prevention of T2DM in youth is also a top priority. The identification of children and adolescents who are at greatest risk of developing youth-onset T2DM will facilitate the development of more effective interventions to prevent or delay the onset of the disease and to decrease the risk of complications in those who do develop T2DM in youth. The development of effective strategies to overcome barriers to adherence will also be an essential component of these efforts.
Key points.
The incidence and prevalence of youth-onset type 2 diabetes mellitus (T2DM) is increasing worldwide; the disease has been reported in all racial and ethnic groups but Indigenous peoples and people of colour are disproportionately affected.
Important drivers of the epidemic of youth-onset T2DM epidemic include the increasing frequency, severity and earlier onset of childhood obesity, the increasing frequency of intrauterine exposure to diabetes, sedentary lifestyles, structural racism and other psychosocial factors
Individuals who develop T2DM during childhood or adolescence often have a more aggressive clinical course than those with type 1 diabetes mellitus or those who develop T2DM in adulthood.
Youth-onset T2DM has a more extreme metabolic phenotype than adult-onset T2DM with greater insulin resistance and more rapid deterioration of β-cell function; intermediate complications often develop in late childhood or early adulthood, and end-stage complications, including kidney failure, develop in mid-life.
Due to limited efficacy and safety data, several drugs that are available for the treatment of adults with T2DM have not been approved for the treatment of youth, reducing the options that are available to normalize glycemia in these patients.
Managing youth-onset T2DM and mitigating the risk of microvascular and macrovascular complications requires the development of more effective interventions as well as strategies to overcome barriers to adherence that are not typically encountered in adult patients.
Acknowledgements
The authors’ work was supported in part by the Intramural Research Program of the NIDDK. P.B. receives salary and research support from NIDDK (R01 DK129211, R01 DK132399, R21 DK129720, K23 DK116720, UC DK114886 and P30 DK116073), NHLBI (R01 HL165433), JDRF (3-SRA-2022-1097-M-B, 3-SRA-2022-1243-M-B, 2-SRA-2019-845-S-B), Boettcher Foundation, American Heart Association (20IPA35260142), Center for Women’s Health Research at University of Colorado, the Department of Pediatrics, Section of Endocrinology and Barbara Davis Center for Diabetes at University of Colorado School of Medicine. D.H.v.R. is supported by a senior fellowship of the Dutch Diabetes Foundation. K.J.N. receives salary and research support from NIDDK (R01 DK119450) and NHLBI (K24 HL145076, R01 HL165433). A.S.S. receives salary and research support from NIDDK (R01 DK119450), NHLBI (R01 HL157260) and NINDS (R01 NS125316). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Competing interests
P.B. has acted as a consultant for AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eli-Lilly, LG Chem, Sanofi, Novo Nordisk, Horizon Pharma, XORTX. P.B. serves on the advisory boards for AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk and XORTX. D.H.v.R. has acted as a consultant and received honoraria from Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Merck and Sanofi, and received research operating funding from AstraZeneca, Boehringer Ingelheim, Eli Lilly Diabetes Alliance and Merck. The other authors declare no competing interests.
Glossary
- Standardized mortality ratio
The ratio of the number of deaths observed in a population over a given period to the number that would be expected over the same period in the general population.
References
- 1.Fajans SS & Conn JW Tolbutamide-induced improvement in carbohydrate tolerance of young people with mild diabetes mellitus. Diabetes 9, 83–88 (1960). [DOI] [PubMed] [Google Scholar]
- 2.Burkeholder JN, Pickens JM & Womack WN Oral glucose tolerance test in siblings of children with diabetes mellitus. Diabetes 16, 156–160 (1967). [DOI] [PubMed] [Google Scholar]
- 3.Martin MM & Martin AL Obesity, hyperinsulinism, and diabetes mellitus in childhood. J Pediatr 82, 192–201 (1973). [DOI] [PubMed] [Google Scholar]
- 4.Drash A Relationship between diabetes mellitus and obesity in the child. Metabolism 22, 337–344 (1973). [DOI] [PubMed] [Google Scholar]
- 5.Deschamps I, Giron BJ & Lestradet H Blood glucose, insulin, and free fatty acid levels during oral glucose tolerance tests in 158 obese children. Diabetes 26, 89–93 (1977). [DOI] [PubMed] [Google Scholar]
- 6.Pinhas-Hamiel O et al. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. The Journal of pediatrics 128, 608–615 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Dean HJ, Mundy RL & Moffatt M Non-insulin-dependent diabetes mellitus in Indian children in Manitoba. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne 147, 52–57 (1992). [PMC free article] [PubMed] [Google Scholar]
- 8.Kitagawa T, Owada M, Urakami T & Yamauchi K Increased incidence of non-insulin dependent diabetes mellitus among Japanese schoolchildren correlates with an increased intake of animal protein and fat. Clin Pediatr (Phila) 37, 111–115, doi: 10.1177/000992289803700208 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Pihoker C, Scott CR, Lensing SY, Cradock MM & Smith J Non-insulin dependent diabetes mellitus in African-American youths of Arkansas. Clin Pediatr (Phila) 37, 97–102, doi: 10.1177/000992289803700206 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Harris SB, Perkins BA & Whalen-Brough E Non-insulin-dependent diabetes mellitus among First Nations children. New entity among First Nations people of north western Ontario. Can Fam Physician 42, 869–876 (1996). [PMC free article] [PubMed] [Google Scholar]
- 11.Neufeld ND, Raffel LJ, Landon C, Chen YD & Vadheim CM Early presentation of type 2 diabetes in Mexican-American youth. Diabetes care 21, 80–86 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Savage PJ, Bennett PH, Senter RG & Miller M High prevalence of diabetes in young Pima Indians: evidence of phenotypic variation in a genetically isolated population. Diabetes 28, 937–942 (1979). [DOI] [PubMed] [Google Scholar]
- 13.Kadiki OA, Reddy MR & Marzouk AA Incidence of insulin-dependent diabetes (IDDM) and non-insulin-dependent diabetes (NIDDM) (0-34 years at onset) in Benghazi, Libya. Diabetes Res Clin Pract 32, 165–173, doi: 10.1016/0168-8227(96)01262-4 (1996). [DOI] [PubMed] [Google Scholar]
- 14.Pinhas-Hamiel O & Zeitler P The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr 146, 693–700, doi: 10.1016/j.jpeds.2004.12.042 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Telo GH, Cureau FV, Szklo M, Bloch KV & Schaan BD Prevalence of type 2 diabetes among adolescents in Brazil: Findings from Study of Cardiovascular Risk in Adolescents (ERICA). Pediatr Diabetes 20, 389–396, doi: 10.1111/pedi.12828 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Fagot-Campagna A et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. The Journal of pediatrics 136, 664–672 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Type 2 diabetes in children and adolescents. American Diabetes Association. Diabetes care 23, 381–389 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Mayer-Davis EJ et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med 376, 1419–1429, doi: 10.1056/NEJMoa1610187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; The incidence of youth-onset T1DM and T2DM increased significantly between 2002-2012, particularly among minority racial and ethnic groups.
- 19.Lawrence JM et al. Trends in Prevalence of Type 1 and Type 2 Diabetes in Children and Adolescents in the US, 2001-2017. JAMA 326, 717–727, doi: 10.1001/jama.2021.11165 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.TODAY Study Group et al. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med 385, 416–426, doi: 10.1056/NEJMoa2100165 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported a high burden of micro and macrovascular complications in youth with T2DM transitioning to adulthood.
- 21.Dabelea D et al. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. Jama 317, 825–835, doi: 10.1001/jama.2017.0686 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diabetes Canada Clinical Practice Guidelines Expert, C. et al. Type 2 Diabetes and Indigenous Peoples. Can J Diabetes 42 Suppl 1, S296–S306, doi: 10.1016/j.jcjd.2017.10.022 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Nadeau KJ et al. Youth-Onset Type 2 Diabetes Consensus Report: Current Status, Challenges, and Priorities. Diabetes Care 39, 1635–1642, doi: 10.2337/dc16-1066 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagot-Campagna A et al. Diabetes, impaired fasting glucose, and elevated HbA1c in U.S. adolescents: the Third National Health and Nutrition Examination Survey. Diabetes Care 24, 834–837, doi: 10.2337/diacare.24.5.834 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Demmer RT, Zuk AM, Rosenbaum M & Desvarieux M Prevalence of diagnosed and undiagnosed type 2 diabetes mellitus among US adolescents: results from the continuous NHANES, 1999-2010. Am J Epidemiol 178, 1106–1113, doi: 10.1093/aje/kwt088 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bullard KM et al. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type - United States, 2016. MMWR Morb Mortal Wkly Rep 67, 359–361, doi: 10.15585/mmwr.mm6712a2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett PH, Burch TA & Miller M Diabetes mellitus in American (Pima) Indians. Lancet 2, 125–128, doi: 10.1016/s0140-6736(71)92303-8 (1971). [DOI] [PubMed] [Google Scholar]
- 28.Katzeff HL, Savage PJ, Barclay-White B, Nagulesparan M & Bennett PH C-peptide measurement in the differentiation of type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 28, 264–268, doi: 10.1007/BF00271682 (1985). [DOI] [PubMed] [Google Scholar]
- 29.Knowler WC, Bennett PH, Bottazzo GF & Doniach D Islet cell antibodies and diabetes mellitus in Pima Indians. Diabetologia 17, 161–164, doi: 10.1007/BF01219743 (1979). [DOI] [PubMed] [Google Scholar]
- 30.Dabelea D, Palmer JP, Bennett PH, Pettitt DJ & Knowler WC Absence of glutamic acid decarboxylase antibodies in Pima Indian children with diabetes mellitus. Diabetologia 42, 1265–1266, doi: 10.1007/s001250051303 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Janssen RC, Bogardus C, Takeda J, Knowler WC & Thompson DB Linkage analysis of acute insulin secretion with GLUT2 and glucokinase in Pima Indians and the identification of a missense mutation in GLUT2. Diabetes 43, 558–563, doi: 10.2337/diab.43.4.558 (1994). [DOI] [PubMed] [Google Scholar]
- 32.Muller YL et al. Variants in hepatocyte nuclear factor 4alpha are modestly associated with type 2 diabetes in Pima Indians. Diabetes 54, 3035–3039, doi: 10.2337/diabetes.54.10.3035 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabelea D et al. Increasing prevalence of Type II diabetes in American Indian children. Diabetologia 41, 904–910, doi: 10.1007/s001250051006 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Pettitt DJ, Baird HR, Aleck KA, Bennett PH & Knowler WC Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med 308, 242–245, doi: 10.1056/NEJM198302033080502 (1983). [DOI] [PubMed] [Google Scholar]
- 35.Vijayakumar P et al. Secular changes in physical growth and obesity among southwestern American Indian children over four decades. Pediatr Obes 13, 94–102, doi: 10.1111/ijpo.12199 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanamas SK et al. Effect of severe obesity in childhood and adolescence on risk of type 2 diabetes in youth and early adulthood in an American Indian population. Pediatr Diabetes 19, 622–629, doi: 10.1111/pedi.12627 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Amed S et al. Type 2 diabetes, medication-induced diabetes, and monogenic diabetes in Canadian children: a prospective national surveillance study. Diabetes Care 33, 786–791, doi: 10.2337/dc09-1013 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young TK, McIntyre LL, Dooley J & Rodriguez J Epidemiologic features of diabetes mellitus among Indians in northwestern Ontario and northeastern Manitoba. Can Med Assoc J 132, 793–797 (1985). [PMC free article] [PubMed] [Google Scholar]
- 39.Green C, Blanchard JF, Young TK & Griffith J The epidemiology of diabetes in the Manitoba-registered First Nation population: current patterns and comparative trends. Diabetes Care 26, 1993–1998, doi: 10.2337/diacare.26.7.1993 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Young TK, Reading J, Elias B & O’Neil JD Type 2 diabetes mellitus in Canada’s first nations: status of an epidemic in progress. CMAJ 163, 561–566 (2000). [PMC free article] [PubMed] [Google Scholar]
- 41.Turin TC et al. Lifetime risk of diabetes among First Nations and non-First Nations people. CMAJ 188, 1147–1153, doi: 10.1503/cmaj.150787 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albisser AM, Harris RI, Sakkal S, Parson ID & Chao SC Diabetes intervention in the information age. Med Inform (Lond) 21, 297–316, doi: 10.3109/14639239608999291 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Shulman R et al. Prevalence, incidence and outcomes of diabetes in Ontario First Nations children: a longitudinal population-based cohort study. CMAJ Open 8, E48–E55, doi: 10.9778/cmajo.20190226 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dart A et al. Screening for kidney disease in Indigenous Canadian children: The FINISHED screen, triage and treat program. Paediatr Child Health 23, e134–e142, doi: 10.1093/pch/pxy013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shulman R, Miller FA, Stukel TA, Daneman D & Guttmann A Resources and population served: a description of the Ontario Paediatric Diabetes Network. CMAJ Open 4, E141–146, doi: 10.9778/cmajo.20150006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lynch JL et al. Country-Specific Prevalence and Incidence of Youth-Onset Type 2 Diabetes: A Narrative Literature Review. Ann Nutr Metab 76, 289–296, doi: 10.1159/000510499 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Haines L, Wan KC, Lynn R, Barrett TG & Shield JP Rising incidence of type 2 diabetes in children in the U.K. Diabetes Care 30, 1097–1101, doi: 10.2337/dc06-1813 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Candler TP et al. Continuing rise of Type 2 diabetes incidence in children and young people in the UK. Diabet Med 35, 737–744, doi: 10.1111/dme.13609 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Almeida-Pititto B et al. Type 2 diabetes in Brazil: epidemiology and management. Diabetes Metab Syndr Obes 8, 17–28, doi: 10.2147/DMSO.S72542 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh R, Shaw J & Zimmet P Epidemiology of childhood type 2 diabetes in the developing world. Pediatr Diabetes 5, 154–168, doi: 10.1111/j.1399-543X.2004.00060.x (2004). [DOI] [PubMed] [Google Scholar]
- 51.Muthuri SK et al. Evidence of an overweight/obesity transition among school-aged children and youth in Sub-Saharan Africa: a systematic review. PLoS One 9, e92846, doi: 10.1371/journal.pone.0092846 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim JH & Lim JS Trends of Diabetes and Prediabetes Prevalence among Korean Adolescents From 2007 to 2018. J Korean Med Sci 36, e112, doi: 10.3346/jkms.2021.36.e112 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z et al. Prevalence and risk factors of impaired fasting glucose and diabetes among Chinese children and adolescents: a national observational study. Br J Nutr 120, 813–819, doi: 10.1017/S0007114518002040 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Fu JF et al. Status and trends of diabetes in Chinese children: analysis of data from 14 medical centers. World J Pediatr 9, 127–134, doi: 10.1007/s12519-013-0414-4 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Urakami T Screening of childhood type 2 diabetes in Japan. Diabetes Research and Clinical Practice 120, doi: 10.1016/s0168-8227(16)30891-9 (2016). [DOI] [Google Scholar]
- 56.Wei JN et al. National surveillance for type 2 diabetes mellitus in Taiwanese children. JAMA 290, 1345–1350, doi: 10.1001/jama.290.10.1345 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Jensen ET et al. Comparison of the incidence of diabetes in United States and Indian youth: An international harmonization of youth diabetes registries. Pediatr Diabetes 22, 8–14, doi: 10.1111/pedi.13009 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stone M, Baker A & Maple Brown L Diabetes in young people in the Top End of the Northern Territory. J Paediatr Child Health 49, 976–979, doi: 10.1111/jpc.12265 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Titmuss A et al. Youth-onset type 2 diabetes among First Nations young people in northern Australia: a retrospective, cross-sectional study. Lancet Diabetes Endocrinol 10, 11–13, doi: 10.1016/S2213-8587(21)00286-2 (2022). [DOI] [PubMed] [Google Scholar]
- 60.Haynes A, Kalic R, Cooper M, Hewitt JK & Davis EA Increasing incidence of type 2 diabetes in Indigenous and non-Indigenous children in Western Australia, 1990-2012. Med J Aust 204, 303, doi: 10.5694/mja15.00958 (2016). [DOI] [PubMed] [Google Scholar]
- 61.<https://www.tewhatuora.govt.nz/our-health-system/digital-health/virtual-diabetes-tool/> (
- 62.Assady S RR, Frishberg Y in Brenner and Rector’s The Kidney (Tenth Edition). Vol. 78 (ed Rector Brenner FC, Skorecki K, Chertow GM, Marsden PA, Taal MW, Yu ASL, & Scott RP) 2517–2545 (Elsevier., 2018). [Google Scholar]
- 63.Ali BA, Abdallah ST, Abdallah AM & Hussein MM The Frequency of Type 2 Diabetes Mellitus among Diabetic Children in El Minia Governorate, Egypt. Sultan Qaboos University medical journal 13, 399–403, doi: 10.12816/0003262 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moadab MH, Kelishadi R, Hashemipour M, Amini M & Poursafa P The prevalence of impaired fasting glucose and type 2 diabetes in a population-based sample of overweight/obese children in the Middle East. Pediatric diabetes 11, 101–106, doi: 10.1111/j.1399-5448.2009.00534.x (2010). [DOI] [PubMed] [Google Scholar]
- 65.Mirbolouk M et al. Incidence and predictors of early adulthood pre-diabetes/type 2 diabetes, among Iranian adolescents: the Tehran Lipid and Glucose Study. Pediatric diabetes 17, 608–616, doi: 10.1111/pedi.12343 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Alsaffar Y, Hussain AM & Selman N. A. J. A. V. d. F. y. T. Prevalence of Type 2 Diabetes in pediatrics and adolescents newly diagnosed with diabetes in Babylon Governorate, Iraq. 39, 839–845 (2020). [Google Scholar]
- 67.Zuckerman Levin N et al. Youth-onset type 2 diabetes in Israel: A national cohort. Pediatr Diabetes 23, 649–659, doi: 10.1111/pedi.13351 (2022). [DOI] [PubMed] [Google Scholar]
- 68.Meyerovitch J, Zlotnik M, Yackobovitch-Gavan M, Phillip M & Shalitin S Real-Life Glycemic Control in Children with Type 2 Diabetes: A Population-Based Study. The Journal of pediatrics 188, 173–180.e171, doi: 10.1016/j.jpeds.2017.05.074 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Al-Kandari H et al. Incidence of Type 2 Diabetes in Kuwaiti Children and Adolescents: Results From the Childhood-Onset Diabetes Electronic Registry (CODeR). Front Endocrinol (Lausanne) 10, 836, doi: 10.3389/fendo.2019.00836 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moussa MA et al. Prevalence of type 2 diabetes mellitus among Kuwaiti children and adolescents. Medical principles and practice : international journal of the Kuwait University, Health Science Centre 17, 270–275, doi: 10.1159/000129604 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Salameh P & Barbour BJE-EMHJ, 17 , 226–230,. Pattern of obesity and associated diabetes in Lebanese adolescents: a pilot study. (2011). [PubMed] [Google Scholar]
- 72.Agroiya P & Alrawahi AH Pediatric Diabetic Retinopathy: Experience of a Tertiary Hospital in Oman. Middle East Afr J Ophthalmol 26, 189–195, doi: 10.4103/meajo.MEAJO_208_19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haris B et al. Epidemiology, genetic landscape and classification of childhood diabetes mellitus in the State of Qatar. J Diabetes Investig, doi: 10.1111/jdi.13610 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alyafei F et al. Prevalence of beta-cell antibodies and associated autoimmune diseases in children and adolescents with type 1 diabetes (T1DM) versus type 2 diabetes (T2DM) in Qatar. Acta Biomed 89, 32–39, doi: 10.23750/abm.v89iS4.7359 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al-Agha A, Ocheltree A & Shata N Prevalence of hyperinsulinism, type 2 diabetes mellitus and metabolic syndrome among Saudi overweight and obese pediatric patients. Minerva pediatrica 64, 623–631 (2012). [PubMed] [Google Scholar]
- 76.Al-Rubeaan K National surveillance for type 1, type 2 diabetes and prediabetes among children and adolescents: a population-based study (SAUDI-DM). J Epidemiol Community Health 69, 1045–1051, doi: 10.1136/jech-2015-205710 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Braham R et al. Double diabetes in Saudi Arabia: A new entity or an underestimated condition. World J Diabetes 7, 621–626, doi: 10.4239/wjd.v7.i20.621 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haliloglu B et al. The Distribution of Different Types of Diabetes in Childhood: A Single Center Experience. J Clin Res Pediatr Endocrinol 10, 125–130, doi: 10.4274/jcrpe.5204 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hatun S et al. Characteristics of Turkish children with Type 2 diabetes at onset: a multicentre, cross-sectional study. Diabet Med 36, 1243–1250, doi: 10.1111/dme.14038 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Al Amiri E et al. The prevalence, risk factors, and screening measure for prediabetes and diabetes among Emirati overweight/obese children and adolescents. BMC Public Health 15, 1298–1298, doi: 10.1186/s12889-015-2649-6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Punnose J, Agarwal MM & Bin-Uthman S Type 2 diabetes mellitus among children and adolescents in Al-Ain: a case series. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit 11, 788–797 (2005). [PubMed] [Google Scholar]
- 82.Atef Z, Al-Ghazaly J & M B prediabetes and type 2 diabetes among yemeni adolescents. (2018).
- 83.Federation ID IDF Diabetes Atlas. 10th Edition edn, (International Diabetes Federation, 2021). [Google Scholar]
- 84.Elkum N, Al-Arouj M, Sharifi M, Shaltout A & Bennakhi A Prevalence of childhood obesity in the state of Kuwait. Pediatr Obes 11, e30–e34, doi: 10.1111/ijpo.12090 (2016). [DOI] [PubMed] [Google Scholar]
- 85.AlBlooshi A et al. Increasing obesity rates in school children in United Arab Emirates. Obesity Science & Practice 2, 196–202, doi: 10.1002/osp4.37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hamman RF et al. The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care 37, 3336–3344, doi: 10.2337/dc14-0574 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 25, 458–471, doi: 10.1016/j.cct.2004.08.002 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Mayer-Davis EJ et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med 376, 1419–1429, doi: 10.1056/NEJMoa1610187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Divers J et al. Trends in Incidence of Type 1 and Type 2 Diabetes Among Youths - Selected Counties and Indian Reservations, United States, 2002-2015. MMWR Morb Mortal Wkly Rep 69, 161–165, doi: 10.15585/mmwr.mm6906a3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hales CM, Carroll MD, Fryar CD & Ogden CL Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. NCHS Data Brief, 1–8 (2017). [PubMed] [Google Scholar]
- 91.Ogden CL et al. Trends in Obesity Prevalence by Race and Hispanic Origin-1999-2000 to 2017-2018. Jama 324, 1208–1210, doi: 10.1001/jama.2020.14590 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.CDC. <https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-diabetes.html> (
- 93.Dabelea D et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care 31, 1422–1426, doi: 10.2337/dc07-2417 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mayer-Davis EJ et al. Breast-feeding and type 2 diabetes in the youth of three ethnic groups: the SEARCh for diabetes in youth case-control study. Diabetes Care 31, 470–475, doi: 10.2337/dc07-1321 (2008). [DOI] [PubMed] [Google Scholar]
- 95.Fernandez-Twinn DS, Hjort L, Novakovic B, Ozanne SE & Saffery R Intrauterine programming of obesity and type 2 diabetes. Diabetologia 62, 1789–1801, doi: 10.1007/s00125-019-4951-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R & Guallar E Arsenic exposure and prevalence of type 2 diabetes in US adults. Jama 300, 814–822, doi: 10.1001/jama.300.7.814 (2008). [DOI] [PubMed] [Google Scholar]
- 97.Maull EA et al. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect 120, 1658–1670, doi: 10.1289/ehp.1104579 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liese AD et al. Neighborhood characteristics, food deserts, rurality, and type 2 diabetes in youth: Findings from a case-control study. Health Place 50, 81–88, doi: 10.1016/j.healthplace.2018.01.004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Findholt NE, Michael YL, Davis MM & Brogoitti VW ENVIRONMENTAL INFLUENCES ON CHILDREN’S PHYSICAL ACTIVITY AND DIETS IN RURAL OREGON: RESULTS OF A YOUTH PHOTOVOICE PROJECT. Online J Rural Nurs Health Care 10, 11–20, doi: 10.14574/ojrnhc.v10i2.33 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang X et al. Neighborhood commuting environment and obesity in the United States: an urban-rural stratified multilevel analysis. Prev Med 59, 31–36, doi: 10.1016/j.ypmed.2013.11.004 (2014). [DOI] [PubMed] [Google Scholar]
- 101.Today Study Group et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 366, 2247–2256, doi: 10.1056/NEJMoa1109333 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; This trial showed high rates of glycemic failure irrespective of treatment group (metformin versus metformin plus lifestyle changes versus metformin plus rosiglitazone).
- 102.Maahs DM et al. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes care 30, 2593–2598, doi: 10.2337/dc07-0450 (2007). [DOI] [PubMed] [Google Scholar]
- 103.Pavkov ME et al. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA 296, 421–426, doi: 10.1001/jama.296.4.421 (2006). [DOI] [PubMed] [Google Scholar]; This study reports a 5-fold greater risk of diabetic kidney failure and a 2-fold greater risk of death in mid-life (aged 25-54 years) among Pima Indians with youth-onset compared with older-onset T2DM.
- 104.Sellers EAC et al. Persistent Albuminuria in Children with Type 2 Diabetes: A Canadian Paediatric Surveillance Program Study. J Pediatr 168, 112–117, doi: 10.1016/j.jpeds.2015.09.042 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Cioana M et al. Prevalence of Hypertension and Albuminuria in Pediatric Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Netw Open 4, e216069, doi: 10.1001/jamanetworkopen.2021.6069 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dabelea D et al. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. Jama 317, 825–835, doi: 10.1001/jama.2017.0686 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dart AB, Sellers EA & Dean HJ Kidney disease and youth onset type 2 diabetes: considerations for the general practitioner. Int J Pediatr 2012, 237360, doi: 10.1155/2012/237360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Z & Hoy WE Remaining lifetime risk for developing end stage renal disease among Australian Aboriginal people with diabetes. Diabetes Res Clin Pract 103, e24–26, doi: 10.1016/j.diabres.2013.12.048 (2014). [DOI] [PubMed] [Google Scholar]
- 109.Krakoff J et al. Incidence of retinopathy and nephropathy in youth-onset compared with adult-onset type 2 diabetes. Diabetes Care 26, 76–81, doi: 10.2337/diacare.26.1.76 (2003). [DOI] [PubMed] [Google Scholar]
- 110.Pavkov ME et al. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. Jama-Journal of the American Medical Association 296, 421–426, doi: 10.1001/jama.296.4.421 (2006). [DOI] [PubMed] [Google Scholar]
- 111.Looker HC et al. Structural Lesions on Kidney Biopsy in Youth-Onset and Adult-Onset Type 2 Diabetes. Diabetes Care 45, 436–443, doi: 10.2337/dc21-1688 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; More severe structural lesions are observed in kidney biopsy samples from Pima Indians with youth-onset T2DM than in those with adult-onset T2DM irrespective of age and diabetes duration.
- 112.Pavkov ME, Sievers ML, Knowler WC, Bennett PH & Nelson RG An explanation for the increase in heart disease mortality rates in diabetic Pima Indians: effect of renal replacement therapy. Diabetes Care 27, 1132–1136, doi: 10.2337/diacare.27.5.1132 (2004). [DOI] [PubMed] [Google Scholar]
- 113.Nelson RG et al. Pima Indian Contributions to Our Understanding of Diabetic Kidney Disease. Diabetes 70, 1603–1616, doi: 10.2337/dbi20-0043 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koye DN et al. Trends in Incidence of ESKD in People With Type 1 and Type 2 Diabetes in Australia, 2002-2013. Am J Kidney Dis 73, 300–308, doi: 10.1053/j.ajkd.2018.10.005 (2019). [DOI] [PubMed] [Google Scholar]
- 115.Middleton TL et al. Young adult onset type 2 diabetes versus type 1 diabetes: Progression to and survival on renal replacement therapy. J Diabetes Complications 35, 108023, doi: 10.1016/j.jdiacomp.2021.108023 (2021). [DOI] [PubMed] [Google Scholar]
- 116.Shah AS et al. A cross sectional study to compare cardiac structure and diastolic function in adolescents and young adults with youth-onset type 1 and type 2 diabetes: The SEARCH for Diabetes in Youth Study. Cardiovasc Diabetol 20, 136, doi: 10.1186/s12933-021-01328-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lawrence JM et al. Demographic Correlates of Short-Term Mortality Among Youth and Young Adults With Youth-Onset Diabetes Diagnosed From 2002 to 2015: The SEARCH for Diabetes in Youth Study. Diabetes Care, doi: 10.2337/dc21-0728 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Constantino MI et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care 36, 3863–3869, doi: 10.2337/dc12-2455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levitt Katz L et al. Alterations in left ventricular, left atrial, and right ventricular structure and function to cardiovascular risk factors in adolescents with type 2 diabetes participating in the TODAY clinical trial. Pediatr Diabetes 16, 39–47, doi: 10.1111/pedi.12119 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Group, T. S. Longitudinal Changes in Cardiac Structure and Function From Adolescence to Young Adulthood in Participants With Type 2 Diabetes Mellitus: The TODAY Follow-Up Study. Circ Heart Fail 13, e006685, doi: 10.1161/CIRCHEARTFAILURE.119.006685 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lynch SK & Abramoff MD Diabetic retinopathy is a neurodegenerative disorder. Vision Res 139, 101–107, doi: 10.1016/j.visres.2017.03.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.White NH et al. Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr 139, 804–812, doi: 10.1067/mpd.2001.118887 (2001). [DOI] [PubMed] [Google Scholar]
- 123.Rajalakshmi R et al. Prevalence and risk factors for diabetic retinopathy in Asian Indians with young onset type 1 and type 2 diabetes. J Diabetes Complications 28, 291–297, doi: 10.1016/j.jdiacomp.2013.12.008 (2014). [DOI] [PubMed] [Google Scholar]
- 124.Bai P, Barkmeier AJ, Hodge DO & Mohney BG Ocular Sequelae in a Population-Based Cohort of Youth Diagnosed With Diabetes During a 50-Year Period. JAMA Ophthalmol 140, 51–57, doi: 10.1001/jamaophthalmol.2021.5052 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang SY, Andrews CA, Herman WH, Gardner TW & Stein JD Incidence and Risk Factors for Developing Diabetic Retinopathy among Youths with Type 1 or Type 2 Diabetes throughout the United States. Ophthalmology 124, 424–430, doi: 10.1016/j.ophtha.2016.10.031 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mayer-Davis EJ et al. Diabetic retinopathy in the SEARCH for Diabetes in Youth Cohort: a pilot study. Diabet Med 29, 1148–1152, doi: 10.1111/j.1464-5491.2012.03591.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Unnikrishnan R et al. Younger-onset versus older-onset type 2 diabetes: Clinical profile and complications. J Diabetes Complications 31, 971–975, doi: 10.1016/j.jdiacomp.2017.03.007 (2017). [DOI] [PubMed] [Google Scholar]
- 128.Zhang X et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 304, 649–656, doi: 10.1001/jama.2010.1111 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Akinci G, Savelieff MG, Gallagher G, Callaghan BC & Feldman EL Diabetic neuropathy in children and youth: New and emerging risk factors. Pediatr Diabetes 22, 132–147, doi: 10.1111/pedi.13153 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dart AB et al. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care 37, 436–443, doi: 10.2337/dc13-0954 (2014). [DOI] [PubMed] [Google Scholar]
- 131.He J, Ryder AG, Li S, Liu W & Zhu X Glycemic extremes are related to cognitive dysfunction in children with type 1 diabetes: A meta-analysis. J Diabetes Investig 9, 1342–1353, doi: 10.1111/jdi.12840 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shapiro ALB et al. Cognitive Function in Adolescents and Young Adults With Youth-Onset Type 1 Versus Type 2 Diabetes: The SEARCH for Diabetes in Youth Study. Diabetes Care 44, 1273–1280, doi: 10.2337/dc20-2308 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nadeau KJ, Klingensmith G & Zeitler P Type 2 diabetes in children is frequently associated with elevated alanine aminotransferase. J Pediatr Gastroenterol Nutr 41, 94–98, doi: 10.1097/01.mpg.0000164698.03164.e5 (2005). [DOI] [PubMed] [Google Scholar]
- 134.Hudson OD, Nunez M & Shaibi GQ Ethnicity and elevated liver transaminases among newly diagnosed children with type 2 diabetes. BMC Pediatr 12, 174, doi: 10.1186/1471-2431-12-174 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weinstock RS et al. Metabolic syndrome is common and persistent in youth-onset type 2 diabetes: Results from the TODAY clinical trial. Obesity (Silver Spring) 23, 1357–1361, doi: 10.1002/oby.21120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Punthakee Z et al. Impact of rosiglitazone on body composition, hepatic fat, fatty acids, adipokines and glucose in persons with impaired fasting glucose or impaired glucose tolerance: a sub-study of the DREAM trial. Diabet Med 31, 1086–1092, doi: 10.1111/dme.12512 (2014). [DOI] [PubMed] [Google Scholar]
- 137.Virtanen KA et al. Differential effects of rosiglitazone and metformin on adipose tissue distribution and glucose uptake in type 2 diabetic subjects. Diabetes 52, 283–290, doi: 10.2337/diabetes.52.2.283 (2003). [DOI] [PubMed] [Google Scholar]
- 138.Iozzo P et al. Effects of metformin and rosiglitazone monotherapy on insulin-mediated hepatic glucose uptake and their relation to visceral fat in type 2 diabetes. Diabetes Care 26, 2069–2074, doi: 10.2337/diacare.26.7.2069 (2003). [DOI] [PubMed] [Google Scholar]
- 139.Carey DG et al. Effect of rosiglitazone on insulin sensitivity and body composition in type 2 diabetic patients [corrected]. Obes Res 10, 1008–1015, doi: 10.1038/oby.2002.137 (2002). [DOI] [PubMed] [Google Scholar]
- 140.Yokoyama H et al. High incidence of diabetic nephropathy in early-onset Japanese NIDDM patients. Risk analysis. Diabetes care 21, 1080–1085, doi: 10.2337/diacare.21.7.1080 (1998). [DOI] [PubMed] [Google Scholar]
- 141.Eppens MC et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 29, 1300–1306, doi: 10.2337/dc05-2470 (2006). [DOI] [PubMed] [Google Scholar]
- 142.Eppens MC et al. Type 2 diabetes in youth from the Western Pacific region: glycaemic control, diabetes care and complications. Curr Med Res Opin 22, 1013–1020, doi: 10.1185/030079906X104795 (2006). [DOI] [PubMed] [Google Scholar]
- 143.Dart AB et al. A Holistic Approach to Risk for Early Kidney Injury in Indigenous Youth With Type 2 Diabetes: A Proof of Concept Paper From the iCARE Cohort. Can J Kidney Health Dis 6, 2054358119838836, doi: 10.1177/2054358119838836 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jaiswal M et al. Prevalence of and Risk Factors for Diabetic Peripheral Neuropathy in Youth With Type 1 and Type 2 Diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care 40, 1226–1232, doi: 10.2337/dc17-0179 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bjornstad P et al. Elevated Serum Uric Acid Is Associated With Greater Risk for Hypertension and Diabetic Kidney Diseases in Obese Adolescents With Type 2 Diabetes: An Observational Analysis From the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study. Diabetes Care 42, 1120–1128, doi: 10.2337/dc18-2147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Group, T. S. Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care 36, 1772–1774, doi: 10.2337/dc12-2387 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Carino M et al. Comparison of Clinical and Social Characteristics of Canadian Youth Living With Type 1 and Type 2 Diabetes. Canadian journal of diabetes 45, 428–435, doi: 10.1016/j.jcjd.2021.01.008 (2021). [DOI] [PubMed] [Google Scholar]
- 148.Kershnar AK et al. Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr 149, 314–319, doi: 10.1016/j.jpeds.2006.04.065 (2006). [DOI] [PubMed] [Google Scholar]
- 149.Rodriguez BL et al. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes Care 29, 1891–1896, doi: 10.2337/dc06-0310 (2006). [DOI] [PubMed] [Google Scholar]
- 150.Rodriguez BL et al. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr 157, 245–251.e241, doi: 10.1016/j.jpeds.2010.02.021 (2010). [DOI] [PubMed] [Google Scholar]
- 151.Albers JJ et al. Prevalence and determinants of elevated apolipoprotein B and dense low-density lipoprotein in youths with type 1 and type 2 diabetes. J Clin Endocrinol Metab 93, 735–742, doi: 10.1210/jc.2007-2176 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jaiswal M et al. Cardiovascular autonomic neuropathy in adolescents and young adults with type 1 and type 2 diabetes: The SEARCH for Diabetes in Youth Cohort Study. Pediatr Diabetes 19, 680–689, doi: 10.1111/pedi.12633 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Reynolds K et al. Prevalence of tobacco use and association between cardiometabolic risk factors and cigarette smoking in youth with type 1 or type 2 diabetes mellitus. J Pediatr 158, 594–601 e591, doi: 10.1016/j.jpeds.2010.10.011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nanayakkara N et al. Age, age at diagnosis and diabetes duration are all associated with vascular complications in type 2 diabetes. J Diabetes Complications 32, 279–290, doi: 10.1016/j.jdiacomp.2017.11.009 (2018). [DOI] [PubMed] [Google Scholar]
- 155.Zoungas S et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 57, 2465–2474, doi: 10.1007/s00125-014-3369-7 (2014). [DOI] [PubMed] [Google Scholar]
- 156.Huo L et al. Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997-2011. Diabetologia 61, 1055–1063, doi: 10.1007/s00125-018-4544-z (2018). [DOI] [PubMed] [Google Scholar]
- 157.Yeap BB et al. Diabetes, myocardial infarction and stroke are distinct and duration-dependent predictors of subsequent cardiovascular events and all-cause mortality in older men. J Clin Endocrinol Metab 100, 1038–1047, doi: 10.1210/jc.2014-3339 (2015). [DOI] [PubMed] [Google Scholar]
- 158.Noh M et al. Impact of diabetes duration and degree of carotid artery stenosis on major adverse cardiovascular events: a single-center, retrospective, observational cohort study. Cardiovasc Diabetol 16, 74, doi: 10.1186/s12933-017-0556-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Al-Saeed AH et al. An Inverse Relationship Between Age of Type 2 Diabetes Onset and Complication Risk and Mortality: The Impact of Youth-Onset Type 2 Diabetes. Diabetes Care 39, 823–829, doi: 10.2337/dc15-0991 (2016). [DOI] [PubMed] [Google Scholar]
- 160.Hu C et al. Association Between Age at Diagnosis of Type 2 Diabetes and Cardiovascular Diseases: A Nationwide, Population-Based, Cohort Study. Front Endocrinol (Lausanne) 12, 717069, doi: 10.3389/fendo.2021.717069 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Visaria J et al. Incidence and Prevalence of Microvascular and Macrovascular Diseases and All-cause Mortality in Type 2 Diabetes Mellitus: A 10-year Study in a US Commercially Insured and Medicare Advantage Population. Clin Ther 41, 1522–1536 e1521, doi: 10.1016/j.clinthera.2019.05.012 (2019). [DOI] [PubMed] [Google Scholar]
- 162.Hillier TA & Pedula KL Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care 26, 2999–3005, doi: 10.2337/diacare.26.11.2999 (2003). [DOI] [PubMed] [Google Scholar]
- 163.Magliano DJ et al. Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol 16, 321–331, doi: 10.1038/s41574-020-0334-z (2020). [DOI] [PubMed] [Google Scholar]
- 164.Lascar N et al. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol 6, 69–80, doi: 10.1016/S2213-8587(17)30186-9 (2018). [DOI] [PubMed] [Google Scholar]
- 165.Druet C et al. Characterization of insulin secretion and resistance in type 2 diabetes of adolescents. J Clin Endocrinol Metab 91, 401–404, doi: 10.1210/jc.2005-1672 (2006). [DOI] [PubMed] [Google Scholar]
- 166.Mohan V et al. Associations of beta-cell function and insulin resistance with youth-onset type 2 diabetes and prediabetes among Asian Indians. Diabetes Technol Ther 15, 315–322, doi: 10.1089/dia.2012.0259 (2013). [DOI] [PubMed] [Google Scholar]
- 167.Bacha F et al. Determinants of glycemic control in youth with type 2 diabetes at randomization in the TODAY study. Pediatr Diabetes 13, 376–383, doi: 10.1111/j.1399-5448.2011.00841.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Utzschneider KM et al. Differential loss of beta-cell function in youth vs. adults following treatment withdrawal in the Restoring Insulin Secretion (RISE) study. Diabetes Res Clin Pract 178, 108948, doi: 10.1016/j.diabres.2021.108948 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Consortium R Restoring Insulin Secretion (RISE): design of studies of beta-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 37, 780–788, doi: 10.2337/dc13-1879 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kahn SE et al. Hyperglucagonemia Does Not Explain the beta-Cell Hyperresponsiveness and Insulin Resistance in Dysglycemic Youth Compared With Adults: Lessons From the RISE Study. Diabetes Care 44, 1961–1969, doi: 10.2337/dc21-0460 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sam S et al. Baseline Predictors of Glycemic Worsening in Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes in the Restoring Insulin Secretion (RISE) Study. Diabetes Care 44, 1938–1947, doi: 10.2337/dc21-0027 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.RISE Consortium. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: I. Observations Using the Hyperglycemic Clamp. Diabetes Care 41, 1696–1706, doi: 10.2337/dc18-0244 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Compared to adults with pre-diabetes and T2DM, youth with pre-diabetes and T2DM were more insulin resistant, had lower insulin clearance and despite similar glycemia, had much higher insulin secretion assessed by hyperglycemic clamp.
- 173.RISE Consortium. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: II. Observations Using the Oral Glucose Tolerance Test. Diabetes Care 41, 1707–1716, doi: 10.2337/dc18-0243 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Utzschneider KM et al. beta-cells in youth with impaired glucose tolerance or early type 2 diabetes secrete more insulin and are more responsive than in adults. Pediatr Diabetes 21, 1421–1429, doi: 10.1111/pedi.13113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.RISE Consortium. Effects of Treatment of Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes With Metformin Alone or in Combination With Insulin Glargine on beta-Cell Function: Comparison of Responses In Youth And Adults. Diabetes 68, 1670–1680, doi: 10.2337/db19-0299 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; More youth than adults had glycemic worsening on treatment at month 12 and in adults, β-cell function improved during treatment whereas β-cell function deteriorated during treatment in youth.
- 176.Srinivasan S et al. The First Genome-Wide Association Study for Type 2 Diabetes in Youth: The Progress in Diabetes Genetics in Youth (ProDiGY) Consortium. Diabetes 70, 996–1005, doi: 10.2337/db20-0443 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jabar F, Colatruglio S, Sellers E, Kroeker K & Wicklow B The next generation cohort: a description of a cohort at high risk for childhood onset type 2 diabetes. J Dev Orig Health Dis 10, 24–30, doi: 10.1017/S204017441800048X (2019). [DOI] [PubMed] [Google Scholar]
- 178.Kriska A et al. Impact of lifestyle behavior change on glycemic control in youth with type 2 diabetes. Pediatr Diabetes 19, 36–44, doi: 10.1111/pedi.12526 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Berkowitz RI et al. Adherence to a lifestyle program for youth with type 2 diabetes and its association with treatment outcome in the TODAY clinical trial. Pediatr Diabetes 19, 191–198, doi: 10.1111/pedi.12555 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Trief PM et al. Medication adherence in young adults with youth-onset type 2 diabetes: iCount, an observational study. Diabetes Res Clin Pract 184, 109216, doi: 10.1016/j.diabres.2022.109216 (2022). [DOI] [PubMed] [Google Scholar]
- 181.Anderson BJ et al. Depressive symptoms and quality of life in adolescents with type 2 diabetes: baseline data from the TODAY study. Diabetes Care 34, 2205–2207, doi: 10.2337/dc11-0431 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Copeland KC et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 96, 159–167, doi: 10.1210/jc.2010-1642 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Miller JL & Silverstein JH The treatment of type 2 diabetes mellitus in youth : which therapies? Treat Endocrinol 5, 201–210, doi: 10.2165/00024677-200605040-00001 (2006). [DOI] [PubMed] [Google Scholar]
- 184.Yeung RO et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol 2, 935–943, doi: 10.1016/S2213-8587(14)70137-8 (2014). [DOI] [PubMed] [Google Scholar]
- 185.Ke C et al. Age at diagnosis, glycemic trajectories, and responses to oral glucose-lowering drugs in type 2 diabetes in Hong Kong: A population-based observational study. PLoS Med 17, e1003316, doi: 10.1371/journal.pmed.1003316 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Group, T. S. Health Care Coverage and Glycemic Control in Young Adults With Youth-Onset Type 2 Diabetes: Results From the TODAY2 Study. Diabetes Care 43, 2469–2477, doi: 10.2337/dc20-0760 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Duke NN Food Insecurity and Prediabetes among Adolescents Taking a School-based Survey. Am J Health Behav 45, 384–396, doi: 10.5993/AJHB.45.2.16 (2021). [DOI] [PubMed] [Google Scholar]
- 188.Pulling Kuhn A et al. Home and Neighborhood Physical Activity Location Availability among African American Adolescent Girls Living in Low-Income, Urban Communities: Associations with Objectively Measured Physical Activity. Int J Environ Res Public Health 18, doi: 10.3390/ijerph18095003 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kornides ML et al. US adolescents at risk for not meeting physical activity recommendations by season. Pediatr Res 84, 50–56, doi: 10.1038/s41390-018-0024-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kahn JA et al. Patterns and determinants of physical activity in U.S. adolescents. J Adolesc Health 42, 369–377, doi: 10.1016/j.jadohealth.2007.11.143 (2008). [DOI] [PubMed] [Google Scholar]
- 191.Gordon-Larsen P, Adair LS & Popkin BM Ethnic differences in physical activity and inactivity patterns and overweight status. Obes Res 10, 141–149, doi: 10.1038/oby.2002.23 (2002). [DOI] [PubMed] [Google Scholar]
- 192.Hannon JC Physical activity levels of overweight and nonoverweight high school students during physical education classes. J Sch Health 78, 425–431, doi: 10.1111/j.1746-1561.2008.00325.x (2008). [DOI] [PubMed] [Google Scholar]
- 193.Simon S et al. Poor Sleep Is Related to Metabolic Syndrome Severity in Adolescents With PCOS and Obesity. J Clin Endocrinol Metab 105, doi: 10.1210/clinem/dgz285 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Simon SL, Higgins J, Melanson E, Wright KP Jr. & Nadeau KJ A Model of Adolescent Sleep Health and Risk for Type 2 Diabetes. Curr Diab Rep 21, 4, doi: 10.1007/s11892-020-01373-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Simon SL et al. Too Late and Not Enough: School Year Sleep Duration, Timing, and Circadian Misalignment Are Associated with Reduced Insulin Sensitivity in Adolescents with Overweight/Obesity. J Pediatr 205, 257–264 e251, doi: 10.1016/j.jpeds.2018.10.027 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mokhlesi B et al. The association of sleep disturbances with glycemia and obesity in youth at risk for or with recently diagnosed type 2 diabetes. Pediatr Diabetes 20, 1056–1063, doi: 10.1111/pedi.12917 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Grandner MA et al. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep Med 11, 470–478, doi: 10.1016/j.sleep.2009.10.006 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Qureshi F et al. Childhood Assets and Cardiometabolic Health in Adolescence. Pediatrics 143, doi: 10.1542/peds.2018-2004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Downs CA & Faulkner MS Toxic stress, inflammation and symptomatology of chronic complications in diabetes. World J Diabetes 6, 554–565, doi: 10.4239/wjd.v6.i4.554 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shomaker LB et al. Design of a randomized controlled trial to decrease depression and improve insulin sensitivity in adolescents: Mood and INsulin sensitivity to prevent Diabetes (MIND). Contemp Clin Trials 75, 19–28, doi: 10.1016/j.cct.2018.10.007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Brenner BM & Chertow GM Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis 23, 171–175 (1994). [PubMed] [Google Scholar]
- 202.Crume TL et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia 54, 87–92, doi: 10.1007/s00125-010-1925-3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fetita LS, Sobngwi E, Serradas P, Calvo F & Gautier JF Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endocrinol Metab 91, 3718–3724, doi: 10.1210/jc.2006-0624 (2006). [DOI] [PubMed] [Google Scholar]
- 204.Dong MZ et al. Diabetic Uterine Environment Leads to Disorders in Metabolism of Offspring. Front Cell Dev Biol 9, 706879, doi: 10.3389/fcell.2021.706879 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hockett CW et al. Persistent effects of in utero overnutrition on offspring adiposity: the Exploring Perinatal Outcomes among Children (EPOCH) study. Diabetologia 62, 2017–2024, doi: 10.1007/s00125-019-04981-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sauder KA, Hockett CW, Ringham BM, Glueck DH & Dabelea D Fetal overnutrition and offspring insulin resistance and beta-cell function: the Exploring Perinatal Outcomes among Children (EPOCH) study. Diabet Med 34, 1392–1399, doi: 10.1111/dme.13417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Crume TL et al. The influence of exposure to maternal diabetes in utero on the rate of decline in beta-cell function among youth with diabetes. J Pediatr Endocrinol Metab 26, 721–727, doi: 10.1515/jpem-2012-0385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wicklow BA et al. Association of Gestational Diabetes and Type 2 Diabetes Exposure In Utero With the Development of Type 2 Diabetes in First Nations and Non-First Nations Offspring. JAMA Pediatr 172, 724–731, doi: 10.1001/jamapediatrics.2018.1201 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Agarwal P et al. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit Rev Clin Lab Sci 55, 71–101, doi: 10.1080/10408363.2017.1422109 (2018). [DOI] [PubMed] [Google Scholar]
- 210.Wicklow BA & Sellers EA Maternal health issues and cardio-metabolic outcomes in the offspring: a focus on Indigenous populations. Best Pract Res Clin Obstet Gynaecol 29, 43–53, doi: 10.1016/j.bpobgyn.2014.04.017 (2015). [DOI] [PubMed] [Google Scholar]
- 211.Dabelea D, Knowler WC & Pettitt DJ Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med 9, 83–88, doi: (2000). [DOI] [PubMed] [Google Scholar]
- 212.Freinkel N Banting Lecture 1980. Of pregnancy and progeny. Diabetes 29, 1023–1035, doi: 10.2337/diab.29.12.1023 (1980). [DOI] [PubMed] [Google Scholar]
- 213.Damm P et al. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia 59, 1396–1399, doi: 10.1007/s00125-016-3985-5 (2016). [DOI] [PubMed] [Google Scholar]
- 214.Nelson RG, Morgenstern H & Bennett PH Intrauterine diabetes exposure and the risk of renal disease in diabetic Pima Indians. Diabetes 47, 1489–1493, doi: 10.2337/diabetes.47.9.1489 (1998). [DOI] [PubMed] [Google Scholar]
- 215.Cerqueira DM et al. In utero exposure to maternal diabetes impairs nephron progenitor differentiation. Am J Physiol Renal Physiol 317, F1318–F1330, doi: 10.1152/ajprenal.00204.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.American Diabetes, A. 13. Children and Adolescents: Standards of Medical Care in Diabetes-2021. Diabetes Care 44, S180–S199, doi: 10.2337/dc21-S013 (2021). [DOI] [PubMed] [Google Scholar]
- 217.American Diabetes, A. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2021. Diabetes Care 44, S125–S150, doi: 10.2337/dc21-S010 (2021). [DOI] [PubMed] [Google Scholar]
- 218.Arnett DK et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140, e596–e646, doi: 10.1161/CIR.0000000000000678 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Whelton SP, Chin A, Xin X & He J Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Annals of internal medicine 136, 493–503, doi: 10.7326/0003-4819-136-7-200204020-00006 (2002). [DOI] [PubMed] [Google Scholar]
- 220.Pierce GL, Eskurza I, Walker AE, Fay TN & Seals DR Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 120, 13–23, doi: 10.1042/CS20100174 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.DeSouza CA et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102, 1351–1357 (2000). [DOI] [PubMed] [Google Scholar]
- 222.Look ARG Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2, 801–809, doi: 10.1016/S2213-8587(14)70156-1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Eskurza I, Monahan KD, Robinson JA & Seals DR Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. The Journal of physiology 556, 315–324 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Martens CR, Kirkman DL & Edwards DG The Vascular Endothelium in Chronic Kidney Disease: A Novel Target for Aerobic Exercise. Exercise and sport sciences reviews 44, 12–19, doi: 10.1249/JES.0000000000000065 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Slaght JL et al. Physical activity and cardiometabolic health in adolescents with type 2 diabetes: a cross-sectional study. BMJ Open Diabetes Res Care 9, doi: 10.1136/bmjdrc-2021-002134 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stutts WC Physical activity determinants in adults. Perceived benefits, barriers, and self efficacy. AAOHN J 50, 499–507 (2002). [PubMed] [Google Scholar]
- 227.El Ansari W & Lovell G Barriers to exercise in younger and older non-exercising adult women: a cross sectional study in London, United Kingdom. Int J Environ Res Public Health 6, 1443–1455, doi: 10.3390/ijerph6041443 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kelly S et al. Barriers and Facilitators to the Uptake and Maintenance of Healthy Behaviours by People at Mid-Life: A Rapid Systematic Review. PloS one 11, e0145074, doi: 10.1371/journal.pone.0145074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Faulkner MS & Michaliszyn SF Exercise Adherence in Hispanic Adolescents with Obesity or Type 2 Diabetes. J Pediatr Nurs 56, 7–12, doi: 10.1016/j.pedn.2020.09.012 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Egan AM et al. Barriers to exercise in obese patients with type 2 diabetes. QJM 106, 635–638, doi: 10.1093/qjmed/hct075 (2013). [DOI] [PubMed] [Google Scholar]
- 231.Thomas N, Alder E & Leese GP Barriers to physical activity in patients with diabetes. Postgrad Med J 80, 287–291, doi: 10.1136/pgmj.2003.010553 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Siddiqi Z, Tiro JA & Shuval K Understanding impediments and enablers to physical activity among African American adults: a systematic review of qualitative studies. Health Educ Res 26, 1010–1024, doi: 10.1093/her/cyr068 (2011). [DOI] [PubMed] [Google Scholar]
- 233.Yarwood J, Carryer J & Gagan MJ Women maintaining physical activity at midlife: contextual complexities. Nurs Prax N Z 21, 24–37 (2005). [PubMed] [Google Scholar]
- 234.Babakus WS & Thompson JL Physical activity among South Asian women: a systematic, mixed-methods review. Int J Behav Nutr Phys Act 9, 150, doi: 10.1186/1479-5868-9-150 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jaser SS, Holl MG, Jefferson V & Grey M Correlates of depressive symptoms in urban youth at risk for type 2 diabetes mellitus. J Sch Health 79, 286–292, doi: 10.1111/j.1746-1561.2009.00411.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Roshanravan B, Gamboa J & Wilund K Exercise and CKD: Skeletal Muscle Dysfunction and Practical Application of Exercise to Prevent and Treat Physical Impairments in CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation 69, 837–852, doi: 10.1053/j.ajkd.2017.01.051 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.McGavock J, Dart A & Wicklow B Lifestyle therapy for the treatment of youth with type 2 diabetes. Curr Diab Rep 15, 568, doi: 10.1007/s11892-014-0568-z (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zeitler P et al. ISPAD Clinical Practice Consensus Guidelines 2018: Type 2 diabetes mellitus in youth. Pediatr Diabetes 19 Suppl 27, 28–46, doi: 10.1111/pedi.12719 (2018). [DOI] [PubMed] [Google Scholar]
- 239.Madiraju AK et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510, 542–546, doi: 10.1038/nature13270 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kelsey MM et al. Presentation and effectiveness of early treatment of type 2 diabetes in youth: lessons from the TODAY study. Pediatr Diabetes 17, 212–221, doi: 10.1111/pedi.12264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Consortium, R. Impact of Insulin and Metformin Versus Metformin Alone on beta-Cell Function in Youth With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care 41, 1717–1725, doi: 10.2337/dc18-0787 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tamborlane WV et al. Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med 381, 637–646, doi: 10.1056/NEJMoa1903822 (2019). [DOI] [PubMed] [Google Scholar]
- 243.Arslanian SA et al. Once-Weekly Dulaglutide for the Treatment of Youths with Type 2 Diabetes. N Engl J Med, doi: 10.1056/NEJMoa2204601 (2022). [DOI] [PubMed] [Google Scholar]
- 244.Tamborlane WV et al. Once-Weekly Exenatide in Youth With Type 2 Diabetes. Diabetes Care 45, 1833–1840, doi: 10.2337/dc21-2275 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nauck MA, Quast DR, Wefers J & Meier JJ GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab 46, 101102, doi: 10.1016/j.molmet.2020.101102 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lebovitz HE Thiazolidinediones: the Forgotten Diabetes Medications. Curr Diab Rep 19, 151, doi: 10.1007/s11892-019-1270-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.DeFronzo RA, Eldor R & Abdul-Ghani M Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 36 Suppl 2, S127–138, doi: 10.2337/dcS13-2011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Singh S, Loke YK & Furberg CD Thiazolidinediones and heart failure: a teleo-analysis. Diabetes Care 30, 2148–2153, doi: 10.2337/dc07-0141 (2007). [DOI] [PubMed] [Google Scholar]
- 249.Gottschalk M, Danne T, Vlajnic A & Cara JF Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes: a randomized, single-blind comparative study. Diabetes Care 30, 790–794, doi: 10.2337/dc06-1554 (2007). [DOI] [PubMed] [Google Scholar]
- 250.Tamborlane WV et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol 10, 341–350, doi: 10.1016/S2213-8587(22)00052-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bjornstad P et al. Acute Effect of Empagliflozin on Fractional Excretion of Sodium and eGFR in Youth With Type 2 Diabetes. Diabetes Care 41, e129–e130, doi: 10.2337/dc18-0394 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Schauer PR et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. The New England journal of medicine 366, 1567–1576, doi: 10.1056/NEJMoa1200225 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sjostrom L Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. Journal of internal medicine 273, 219–234, doi: 10.1111/joim.12012 (2013). [DOI] [PubMed] [Google Scholar]
- 254.Sjostrom L et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. Jama 311, 2297–2304, doi: 10.1001/jama.2014.5988 (2014). [DOI] [PubMed] [Google Scholar]
- 255.Schauer PR et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. The New England journal of medicine 376, 641–651, doi: 10.1056/NEJMoa1600869 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Schauer PR et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. The New England journal of medicine 370, 2002–2013, doi: 10.1056/NEJMoa1401329 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Inge TH et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med 374, 113–123, doi: 10.1056/NEJMoa1506699 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Inge TH et al. Comparison of Surgical and Medical Therapy for Type 2 Diabetes in Severely Obese Adolescents. JAMA Pediatr 172, 452–460, doi: 10.1001/jamapediatrics.2017.5763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ryder JR et al. Effect of surgical versus medical therapy on estimated cardiovascular event risk among adolescents with type 2 diabetes and severe obesity. Surg Obes Relat Dis 17, 23–33, doi: 10.1016/j.soard.2020.09.002 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bjornstad P et al. Effect of Surgical Versus Medical Therapy on Diabetic Kidney Disease Over 5 Years in Severely Obese Adolescents With Type 2 Diabetes. Diabetes Care 43, 187–195, doi: 10.2337/dc19-0708 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Shah AS et al. Study protocol: a prospective controlled clinical trial to assess surgical or medical treatment for paediatric type 2 diabetes (ST(2)OMP). BMJ Open 11, e047766, doi: 10.1136/bmjopen-2020-047766 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Shah AS et al. Metabolic outcomes of surgery in youth with type 2 diabetes. Semin Pediatr Surg 29, 150893, doi: 10.1016/j.sempedsurg.2020.150893 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Dabelea D et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 311, 1778–1786, doi: 10.1001/jama.2014.3201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; Among teenagers and young adults who were diagnosed with diabetes during childhood or adolescence, the prevalence of complications and comorbidities was higher in those with T2DM than T1DM.
- 264.WHO. Obesity, <https://www.who.int/health-topics/obesity> (2022).
- 265.TÖNnies T et al. 156-OR: Projections of Type 1 and Type 2 Diabetes Burden in the U.S. Population Aged <20 Years through 2060. Diabetes 70, doi: 10.2337/db21-156-OR (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.DiCAYA. <https://www.dicaya.org> (
- 267.Consortium, R. & Investigators, R. C. Effects of Treatment of Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes With Metformin Alone or in Combination With Insulin Glargine on beta-Cell Function: Comparison of Responses In Youth And Adults. Diabetes, doi: 10.2337/db19-0299 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care 36, 1735–1741, doi: 10.2337/dc12-2420 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Consortium, R. Lack of Durable Improvements in beta-Cell Function Following Withdrawal of Pharmacological Interventions in Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care 42, 1742–1751, doi: 10.2337/dc19-0556 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bhatt DL et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med, doi: 10.1056/NEJMoa2030186 (2020). [DOI] [PubMed] [Google Scholar]
- 271.Perkovic V et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 380, 2295–2306, doi: 10.1056/NEJMoa1811744 (2019). [DOI] [PubMed] [Google Scholar]
- 272.Mann JFE et al. Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med 377, 839–848, doi: 10.1056/NEJMoa1616011 (2017). [DOI] [PubMed] [Google Scholar]
- 273.Wanner C et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med 375, 323–334, doi: 10.1056/NEJMoa1515920 (2016). [DOI] [PubMed] [Google Scholar]
- 274.A Clinical Trial to Maintain Glycemic Control in Youth with Type 2 Diabetes. New England Journal of Medicine 366, 2247–2256, doi: 10.1056/NEJMoa1109333 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Nelson RG, Morgenstern H & Bennett PH Intrauterine diabetes exposure and the risk of renal disease in diabetic Pima Indians. Diabetes 47, 1489–1493, doi: 10.2337/diabetes.47.9.1489 (1998). [DOI] [PubMed] [Google Scholar]


