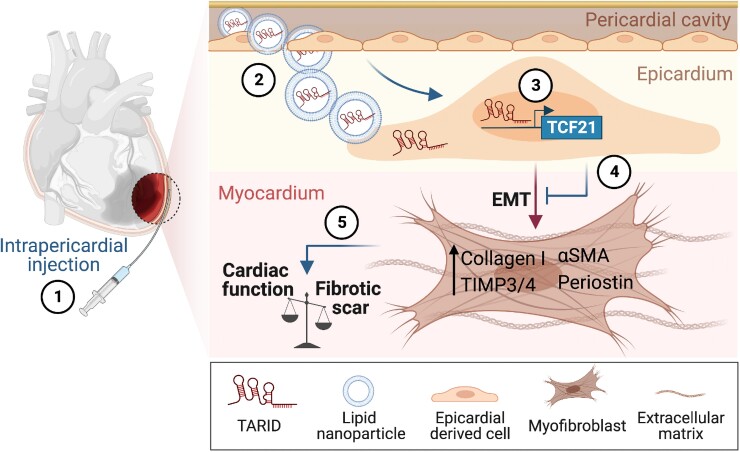
Graphical Abstract
Graphical Abstract.
Intrapericardial lncRNA-TARID therapy mitigates myocardial fibrosis. The figure was designed by using BioRender.com.
This editorial refers to ‘Intrapericardial long non-coding RNA–Tcf21 antisense RNA inducing demethylation administration promotes cardiac repair’, by D. Zhu et al., https://doi.org/10.1093/eurheartj/ehad114.
Acute myocardial infarction (MI) is one of the leading causes of death in the world.1 With advances in interventional therapies, post-MI mortality has decreased over the past decades. However, many MI survivors have massive losses of cardiomyocytes that elicit a series of intertwined cellular events, including immune cell infiltration, epicardial cell expansion, and myofibroblast transdifferentiation, collectively resulting in the formation of a collagen-rich scar.2 Although this fibrous scar can preserve myocardial integrity in the short term, it also drives pathological remodelling and heart failure (HF) in the long term. Due to the lack of drugs that can effectively target cardiac fibrosis, post-MI patients may continue to suffer from repeated HF hospitalizations and premature death.3
Because adult mammalian hearts have negligible regenerative capacity, researchers have proposed the regeneration of damaged heart tissue by various types of stem cells. Among them, mesenchymal stem cells (MSCs) show promise because of their enriched secretory profile.4 Thus far, however, adult cardiac stem cell clinical trials have only shown neutral or marginal benefits, perhaps due to short-lived paracrine release instead of providing new cardiac myocytes.4 Similarly, the systemic application of MSC-derived extracellular vehicles (MSC-EVs) in a pre-clinical study promoted cardiac repair to a moderate extent, probably due to their poor homing efficiency.5 In this regard, a recent study involving intrapericardial delivery of MSC-EVs found augmented effects in the heart after MI, reinforcing the therapeutic benefits of MSC-EVs.6 Therefore, defining the role of specific EV cargo(s) may be a promising therapeutic approach.
In this issue of the European Heart Journal, Zhu et al. further optimized intrapericardial EV therapy by conjugating it with hyaluronic acid (HA) to prolong cardiac retention.7 Using a mouse model of MI, the investigators observed that intrapericardial injection of HA-coupled MSC-EVs led to superior collagen fibre alignment, smaller scar size, and better heart function compared with MSC-EVs alone. This suggests that HA-based modification further boosted the beneficial effect of EV treatment on cardiac remodelling. Mechanistically, they illustrated that EVs within the perivascular cavity were taken up by epicardium-derived cells (EPDCs) and thereafter altered EPDC lineage differentiation. EPDCs are a subset of epicardial cells that delaminate from the epicardial sheet and migrate to the subepicardial space during development.8,9 EPDCs are known to serve as a regeneration hub during heart development and repair due to their differentiation potency to mesenchymal lineages, including smooth muscle cells, cardiac fibroblasts, and pericytes, through epithelial to mesenchymal transition (EMT).8 Zhu and colleagues observed an increased population of EPDCs undergoing EMT after MI, as indicated by reduced epithelial marker expression (pan-Cadherin and pan-Cytokeratin), while generating increased numbers of Vimentin+ mesenchymal cells and α-SMA+ myofibroblasts. Remarkably, they further showed that these changes were reversed after EV injection, supporting the hypothesis that EV treatment impeded EPDCs from differentiating towards myofibroblasts. Of note, recent lineage tracing studies identified pre-existing resident fibroblasts as the main cell population contributing to the myofibroblast pool in the adult injured heart.10,11 Although this does not necessarily argue against the results from this study, it does raise the question as to whether the injected EVs are able to penetrate the epicardial layer and act on interstitial fibroblasts to attenuate fibroblast to myofibroblast transition.
To understand how EVs could orchestrate differentiation fates of EPDCs, Zhu et al. performed RNA-seq analysis of isolated EPDCs and found up-regulated expression of various transcription factors.7 Among them, transcription factor 21 (TCF21) gained special attention, as TCF21 has been shown to be associated with the specification of EPDCs to cardiac fibroblasts during embryogenesis.12 Indeed, the authors were able to manipulate EPDC differentiation by modulating TCF21 expression via gain- and loss-of-function approaches in both cultured EPDCs and an in vivo implantation model. The negative correlation between TCF21 and myofibroblast transdifferentiation was strengthened by the antagonizing role of TCF21 on transforming growth factor β (TGFβ)/Smad3 signalling, which has been widely accepted as a hallmark of myofibroblast activation and fibrosis progression. Taken together, Zhu et al. showed TCF21 as the key regulator of EPDC phenotype and identified it as an appealing target for anti-fibrotic therapy.
A previous report showed that the transcriptional regulation of TCF21 is associated with the expression profile of antisense RNA-inducing demethylation (lncRNA-TARID), a multi-transcript long non-coding RNA (lncRNA) with nuclear localization.13 In particular, one specific transcriptional isoform was shown to demethylate the TCF21 promoter, thereby activating its expression.13 Intriguingly, Zhu et al. found that lncRNA-TARID was substantially enriched in MSCs as well as in MSC-EVs. LncRNA-TARID silencing by small interfering RNA (siRNA) indeed robustly resulted in TCF21 down-regulation in MSCs. Of importance, lncRNA-TARID-loaded lipid nanoparticles (LNPs) significantly induced TCF21 expression and abolished the TGFβ pathway (Smad3, Acta2, and Myocd expression) in EPDCs. Conversely, TARID-depleted EVs were no longer able to inhibit myofibroblast differentiation. Altogether, these observations elegantly confirmed the regulatory function of the TARID–TCF21 axes during EPDC–myofibroblast differentiation that underpinned the therapeutic potential in targeting cardiac remodelling. In fact, although many functional lncRNAs have been described, only a modest number of them have been successfully modulated in vivo. This is probably due to challenges associated with the length and the number of lncRNA transcripts, as well as their cell-specific expression nature.
Recent successful and worldwide applications of LNPs for mRNA-based COVID-19 vaccines have ignited enthusiasm for lncRNA therapies. By harnessing the advantages of LNPs and HA hydrogel, Zhu and colleagues intrapericardially applied lncRNA-TARID in a mouse model of acute MI. Similar to the MSC-EV treatment, the administration of LNP-TARID increased TCF21 expression, attenuated cardiac dilatation, decreased scar size, and improved cardiac function. More interestingly from a translational perspective, intrapericardial LNP-TARID therapy also exhibited promising protective effects in infarct repair and fibrosis resolution in a porcine model of myocardial ischaemia–reperfusion. Collectively, LNP-TARID therapy appears to be a promising antifibrotic therapy that acts by targeting EPDC–myofibroblast differentiation.
Zhu and colleagues also underscored limitations in their work, such as missing evidence of EV-containing soluble proteins and the relatively small sample size of their pig study (n = 3–4). Beyond those factors, several questions remain for further elucidation. First, EPDCs reportedly contributed to cardiac repair by secreting a spectrum of angiocrine factors.10 Thus, further investigation is needed to explore whether EV treatment alters the EPDC secretome, and whether this may affect the angiogenic role played by EPDCs. Meanwhile, EPDCs are made of multiple transcriptionally heterogeneous subgroups.14 Identifying specific EPDC subpopulations responsive to EVs will be equally important for future drug development. Furthermore, LNPs are able to diffuse to the myocardium following hydrogel degradation after implantation.15 Assessing the effect of LNP-TARID in myocardial cells will help define the specificity of this approach for future therapeutic translation. Lastly, considering the cell-specific function of lncRNAs, it will be useful to further study the effect of LNP-TARID uptake from other pericardial cell types.
In summary, Zhu and colleagues identified TCF21 as the underlying key regulator of MSC-EV treatment, helping to create a foundation for a novel potential non-coding lncRNA-TARID therapy to mitigate cardiac remodelling following MI (Graphical Abstract).7 While more work is required to elucidate LNP-TARID therapy targets, this study has enhanced our understanding of the translational role of lncRNAs during cardiac fibrosis and provides a novel therapeutic angle for cardiac repair.
Contributor Information
Xuekun Wu, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA; Department of Medicine, Division of Cardiology, Stanford University School of Medicine, Stanford, CA, USA.
Francesca Vacante, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA; Department of Medicine, Division of Cardiology, Stanford University School of Medicine, Stanford, CA, USA.
Joseph C Wu, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA; Department of Medicine, Division of Cardiology, Stanford University School of Medicine, Stanford, CA, USA; Department of Radiology, Stanford University School of Medicine, Stanford, CA, USA.
Data availability
No new data were generated or analysed in support of this research.
Funding
This publication was supported in part by research grants from the National Institutes of Health (NIH) R01 HL126527, R01 HL130020, R01 HL146690, and R01 HL150693.
References
- 1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang J, Bolli R, Garry DJ, Marbán E, Menasché P, Zimmermann W-H, et al. Basic and translational research in cardiac repair and regeneration: jACC state-of-the-art review. J Am Coll Cardiol 2021;78:2092–2105. 10.1016/j.jacc.2021.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fernandez-Aviles F, Sanz-Ruiz R, Climent AM, Badimon L, Bolli R, Charron D, et al. Global position paper on cardiovascular regenerative medicine. Eur Heart J 2017;38:2532–2546. 10.1093/eurheartj/ehx248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Menasche P. Cell therapy trials for heart regeneratio—lessons learned and future directions. Nat Rev Cardiol 2018;15:659–671. 10.1038/s41569-018-0013-0 [DOI] [PubMed] [Google Scholar]
- 5. Zhang N, Song Y, Huang Z, Chen J, Tan H, Yang H, et al. Monocyte mimics improve mesenchymal stem cell-derived extracellular vesicle homing in a mouse MI/RI model. Biomaterials 2020;255:120168. 10.1016/j.biomaterials.2020.120168 [DOI] [PubMed] [Google Scholar]
- 6. Zhu D, Liu S, Huang K, Wang Z, Hu S, Li J, et al. Intrapericardial exosome therapy dampens cardiac injury via activating Foxo3. Circ Res 2022;131:e135–e150. 10.1161/CIRCRESAHA.122.321384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu D, Liu S, Huang K, Li J, Mei X, Li Z, et al. Intrapericardial long non-coding RNA–Tcf21 antisense RNA inducing demethylation administration promotes cardiac repair. Eur Heart J 2023;44:1748–1760. 10.1093/eurheartj/ehad114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao JL, Poss KD. The epicardium as a hub for heart regeneration. Nat Rev Cardiol 2018;15:631–647. 10.1038/s41569-018-0046-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paik DT, Wu JC. Simply derived epicardial cells. Nat Biomed Eng 2017;1:0015. 10.1038/s41551-016-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou B, Honor LB, He H, Ma Q, Oh J-H, Butterfield C, et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest 2011;121:1894–1904. 10.1172/JCI45529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 2016;7:12260. 10.1038/ncomms12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY., et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 2012;139:2139–2149. 10.1242/dev.079970 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gil N, Ulitsky I. Regulation of gene expression by cis-acting long non-coding RNAs. Nat Rev Genet 2020;21:102–117. 10.1038/s41576-019-0184-5 [DOI] [PubMed] [Google Scholar]
- 14. Hesse J, Owenier C, Lautwein T, Zalfen R, Weber JF, Ding Z, et al. Single-cell transcriptomics defines heterogeneity of epicardial cells and fibroblasts within the infarcted murine heart. Elife 2021;10:e65921. 10.7554/eLife.65921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu DS, Li Z, Huang K, Caranasos TG, Rossi JS, Cheng K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat Commun 2021;12:1412. 10.1038/s41467-021-21682-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.