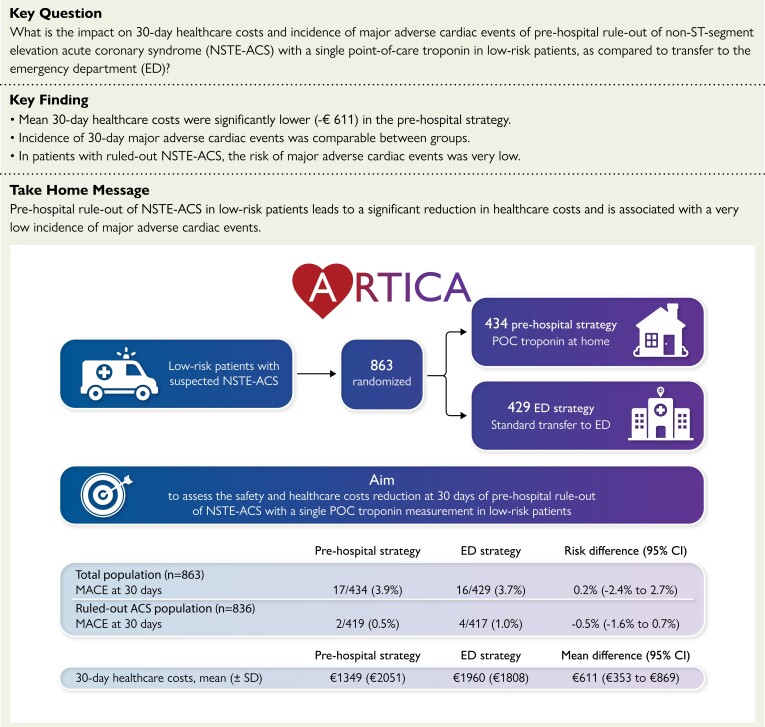
Abstract
Aims
Patients with suspected non-ST-segment elevation acute coronary syndrome (NSTE-ACS) are routinely transferred to the emergency department (ED). A clinical risk score with point-of-care (POC) troponin measurement might enable ambulance paramedics to identify low-risk patients in whom ED evaluation is unnecessary. The aim was to assess safety and healthcare costs of a pre-hospital rule-out strategy using a POC troponin measurement in low-risk suspected NSTE-ACS patients.
Methods and results
This investigator-initiated, randomized clinical trial was conducted in five ambulance regions in the Netherlands. Suspected NSTE-ACS patients with HEAR (History, ECG, Age, Risk factors) score ≤3 were randomized to pre-hospital rule-out with POC troponin measurement or direct transfer to the ED. The sample size calculation was based on the primary outcome of 30-day healthcare costs. Secondary outcome was safety, defined as 30-day major adverse cardiac events (MACE), consisting of ACS, unplanned revascularization or all-cause death. : A total of 863 participants were randomized. Healthcare costs were significantly lower in the pre-hospital strategy (€1349 ± €2051 vs. €1960 ± €1808) with a mean difference of €611 [95% confidence interval (CI): 353–869; P < 0.001]. In the total population, MACE were comparable between groups [3.9% (17/434) in pre-hospital strategy vs. 3.7% (16/429) in ED strategy; P = 0.89]. In the ruled-out ACS population, MACE were very low [0.5% (2/419) vs. 1.0% (4/417)], with a risk difference of −0.5% (95% CI −1.6%–0.7%; P = 0.41) in favour of the pre-hospital strategy.
Conclusion
Pre-hospital rule-out of ACS with a POC troponin measurement in low-risk patients significantly reduces healthcare costs while incidence of MACE was low in both strategies.
Trial registration
Clinicaltrials.gov identifier NCT05466591 and International Clinical Trials Registry Platform id NTR 7346.
Keywords: Point-of-care troponin, Risk stratification, Acute coronary syndrome, HEART score, Pre-hospital triage
Structured Graphical Abstract
Structured Graphical Abstract.
ACS, acute coronary syndrome; CI, confidence interval; ED, emergency department; MACE, major adverse cardiac events; NSTE-ACS, non-ST-elevation acute coronary syndrome; POC, point-of-care
See the editorial comment for this article ‘To be or not to be admitted to the emergency department for chest pain? A costly dilemma’, by B. Gigante, https://doi.org/10.1093/eurheartj/ehad116.
Introduction
Emergency department (ED) overcrowding is a growing phenomenon, which is associated with increased length of stay, worse patient outcomes and high costs.1 According to current guidelines, patients with suspected non-ST-segment elevation acute coronary syndrome (NSTE-ACS) are routinely transported to the ED.2,3 Of all ED visits, about 10% consists of patients with chest pain suspected of NSTE-ACS4, and this number is increasing.5 At the same time, 80%–90% of these patients do not have an actual ACS.6–8 Especially in low-risk patients, ACS is rarely found.9,10 ED visits for chest pain often include costly additional testing and prolonged in-hospital stay, from which low-risk patients are not likely to benefit.5,11–13 Therefore, the current management of chest pain in the ED contributes to ED overcrowding.14 Pre-hospital risk stratification of chest pain patients has been shown to help to reduce ED overcrowding and reduce costs.13 The HEART [History, Electrocardiogram (ECG), Age, Risk factors and Troponin] score is a simple and well validated tool for risk stratification of chest pain patients in the ED, with a very strong overall inter-operator reliability, regardless of the level of education of healthcare professionals.10,15 When a point-of-care (POC) troponin measurement is incorporated in the HEART score, ambulance paramedics can adequately identify low-risk patients by pre-hospital HEART score assessment.16,17 However, rule-out of NSTE-ACS in the pre-hospital setting has not been investigated in a randomized trial and its impact on healthcare costs is currently unknown. Therefore, the ‘Acute rule-out of non-ST-segment elevation acute coronary syndrome in the (pre)hospital setting by HEART score assessment and a single point-of-care troponin’ (ARTICA) randomized trial assessed the 30-day healthcare costs and incidence of major adverse cardiac events (MACE) of a pre-hospital rule-out strategy for patients with suspected NSTE-ACS.
Methods
Trial design
The ARTICA trial is an investigator-initiated, multicentre, open-label, randomized controlled trial in five ambulance regions in the Netherlands, with a total of 112 ambulances and 552 ambulance paramedics. Combined, these regions have a population of ∼2.5 million people. All ambulance paramedics were trained as sub-investigators in order to be able to include patients in the trial. The mandatory training for ambulance paramedics consisted of on-site sessions, online sessions, scheduled online ‘frequently asked questions’ sessions, an instruction video and an e-learning module with a final test. The training was designed to instruct the ambulance paramedics on how to calculate the HEAR score, how to perform informed consent, how to perform randomization and how to perform POC troponin measurements. Examples of the training materials are available in the Supplementary material online, Supplementary Appendix. In the Netherlands, ambulance paramedics are registered nurses with bachelor degrees in nursing and at least two subsequent specializations in critical care nursing. Every participating ambulance region had at least one ambulance paramedic who was appointed to coordinate the ARTICA trial within their respective region. The trial design has been published previously.18 A clinical research organization (Radboud University Technology Centre Clinical Trials) was responsible for maintaining and monitoring the patient data. An independent and blinded Clinical Events Committee (CEC) was installed to adjudicate MACE. A Data and Safety Monitoring Board (DSMB) oversaw the trial and performed interim analyses after 200, 500 and 750 recruited patients. Following each of these board meetings, the DSMB concluded after analysis of the number of adverse events and serious adverse events that there were no safety issues and that the trial could continue as planned. The members of the DSMB and CEC are listed in the Supplementary material online, Supplementary Appendix. The study was conducted according to the principles of the Declaration of Helsinki and in accordance with the Medical Research Involving Human Subjects Act (WMO) and the statements of the Dutch Central Committee on Research Involving Human Subject (CCMO). The trial was approved by the medical ethics committee Oost-Nederland, the Netherlands on 27 November 2018 (NL66755.091.18).
HEAR clinical risk score
The HEAR score includes the history, ECG, age and risk factors components of the total HEART score (see Supplementary material online, Figure S1). The history component was given 2 points for highly suspicious symptoms, 1 point for moderately suspicious symptoms and 0 points for slightly suspicious symptoms. The ECG component was given 2 points in case of significant ST-segment depressions, 1 point in case of non-specific repolarization disturbances, a left bundle branch block or a ventricular paced rhythm and 0 points in case of a normal ECG. The age component was given 2 points if the patient was 65 years or older, 1 point if the patient was 45 to 64 years and 0 points if the patient was below 45 years. The risk factors component was given 2 points if the patient had a history of atherosclerotic disease (coronary revascularization, myocardial infarction, stroke, or peripheral artery disease) or three or more risk factors, 1 point if the patient had one or two risk factors and 0 points in the absence of risk factors. The risk factors that were scored were active or recent (<90 days) smoking, hypertension, diabetes mellitus, obesity [body mass index (BMI) > 30 kg/m2], hypercholesterolaemia and positive family history. Before informed consent was provided, the individual components of the HEAR score were entered into the case report form, resulting in an automatically calculated HEAR score.
Participants
The study population consisted of low-risk chest pain patients with suspected NSTE-ACS, who had an onset of symptoms at least 2 h before ambulance presentation. Low-risk was defined as a HEAR score ≤3. The patients either called the emergency number or were reported by their general practitioner (GP) for emergency evaluation by ambulance personnel. In all patients, an on-site 12-lead ECG was performed and evaluated by ambulance paramedics and if there was any doubt, the ECG could be transmitted for immediate evaluation by an independent cardiologist. The patients were screened by ambulance paramedics, after which the patients were invited to participate in the study by the ambulance paramedics. In order to aid the ambulance paramedics in informing the patients, an animated version of the trial information for patients was available in all ambulances. Patients were not eligible for inclusion if they were suspected of another diagnosis requiring ED presentation [e.g. aortic dissection or pulmonary embolism (PE)] or if they were unable to provide written informed consent. The complete list of inclusion and exclusion criteria is provided in the Supplementary material online, Supplementary Appendix (Supplementary material online, Table S1).
Randomization
After providing written informed consent, patients were randomized to either the pre-hospital rule-out strategy or the ED rule-out strategy using Castor Electronic Data Capture (Castor EDC). The randomization algorithm performed a 1:1 randomization and included a variable block randomization with block sizes of 4, 6 or 8.
Intervention
In the pre-hospital rule-out strategy, patients underwent an on-site POC troponin T measurement. If POC troponin T was low (<40 ng/L), the care for the patient was transferred to the GP, as is the normal procedure in patients who are not transported. If POC troponin T was elevated (≥40 ng/L), the patient was mandatory transported to the ED.
Patients in the ED rule-out strategy were transferred directly to the ED for evaluation without POC troponin measurement and evaluated according to standard practice.
Procedures
In the pre-hospital rule-out strategy, POC troponin T was measured using the Cobas h232 (Roche diagnostics, Basel, Switzerland). The detection limits are 40–2000 ng/L. Concentrations of 40–2000 ng/L on this assay are comparable to high-sensitivity troponin T concentrations in the laboratory.19 The 99th percentile of normal on the laboratory high-sensitivity troponin T assay is 14 ng/L, which is below the detection limit of the Cobas h232. All ambulances were equipped with a refrigerator to store the strips at 2–8°C. Blood (150 µL) was obtained in a heparinized tube by venepuncture or venous line. The results were available after 12 min. Every seven days, the Cobas h232 was automatically locked until functional testing of the optical system was performed with an internal quality control. Every year, all devices were maintained and tested with external quality controls.
In the ED rule-out strategy, high-sensitivity troponin (T or I) was measured at the ED according to the prevailing guidelines. Additional diagnostic tests were at the discretion of the cardiologist.
Follow-up was performed for all patients after 30 days by contacting the patients by telephone and e-mail. If patients visited a hospital, that hospital was contacted to collect all data on procedures and events. In case of non-response of the patient, the GP and hospital were contacted. Data were collected on all healthcare resource use and productivity losses (assessed by the Institute for Medical Technology Assessment Productivity Cost Questionnaire).18,20
Healthcare resource use and cost prices
Healthcare resource use data were collected for all randomized patients. The cost prices were determined according to the 2018 reference list of the Dutch National Healthcare Institute.21 Hospital procedure costs were based on standard 2018 list prices and the diagnosis-treatment combination, which is the current reimbursement system in the Netherlands. This diagnosis–treatment combination is comparable to the diagnosis-related group system in other countries.22 No standard cost prices were available for a POC troponin measurement and an ambulance visit without transporting the patient. Since no formal costing was available for these elements, the current cost analysis did not take into account additional costs for the POC troponin measurement, nor additional savings for not transporting the patient in the pre-hospital rule-out strategy. Hence, in the current analysis, the costs for the index ambulance visit were considered equal in both strategies. Supplementary material online, Table S2 shows a detailed description of the average prices of ambulance transportations, hospital visits, procedures, hospitalizations, GP visits and additional tests.
Outcomes
The primary outcome was costs from a healthcare perspective at 30 days. Healthcare costs consisted of all costs related to healthcare consumption in-hospital, diagnostic tests and GP consultations. MACE at 30 days were a secondary outcome, which was defined as one or more of the following events: ACS, unplanned revascularization or all-cause death. MACE at 30 days were compared between groups in the total population and in the ruled-out ACS population (all patients for whom an ACS was ruled out, either in the pre-hospital setting or in the ED). Costs from a societal perspective were a secondary outcome, which was defined as the sum of the healthcare costs and the productivity loss costs.
Statistical analysis
For the primary outcome, the mean cost difference was estimated to be €507 in an intention-to-treat analysis.18 A small effect size [0.2 standard deviation (SD)] and equal SD in both groups were assumed. A sample size of 392 per group was estimated to achieve 80% power to detect a difference of €507 between both groups with an alpha of 0.05 using a two-sample t-test. To compensate for potential loss to follow-up, the sample size was increased to a total of 866 patients. The complete statistical analysis plan is included in the Supplementary material online, Supplementary Appendix.
For the cost analysis, mean differences and 95% confidence intervals (CIs) for differences were calculated (univariable analysis). To take potentially skewed distribution into account as well as possible heteroscedasticity, a generalized linear model (GLM) with gamma distribution was included. Continuous data were summarized as means ± SD or medians [interquartile ranges (IQRs)]. Categorical data were summarized by frequencies and percentages. Categorical variables were reported as (relative) frequencies and compared using the chi-squared test or Fisher’s exact test, whichever was most appropriate. Pre-specified subgroups included sex, diabetes mellitus and age (≤64 years vs. ≥65 years). For the subgroups, mean cost differences and 95% CI for differences were calculated. Data analyses were performed using the software package Stata version 16 (StataCorp LLC, College Station, TX, USA) or SPSS statistics version 25 (IBM, Armonk, NY, USA).
For the MACE endpoint, 30-day incidence was calculated in both groups in the total population and in the ruled-out ACS population. Risk differences were calculated along with 95% CI.
In post hoc analyses, the ambulance on-scene time, transportation time and time to availability were calculated and expressed as medians (IQR). On-scene time was defined as the interval (in minutes) from the time of arrival at the location of the patient to the time of departure. Transportation time was defined as the interval (in minutes) from the time of departure from the location of the patient to the time of arrival at the hospital. Time to availability was defined as the interval (in minutes) from the time of arrival at the location of the patient to the time the ambulance was available for a new call. On-scene times and times to availability were compared between both strategies using the Wilcoxon rank sum test.
Patient and public involvement
A patient member of the Harteraad (a Dutch patient federation for cardiovascular patients) was involved in the development of patient information. During the development of the trial information (i.e. patient information letters, letter to the general practitioner), valuable feedback was given which helped to improve the quality of these documents. During the conduct of the trial, the patient representative provided advice to the ambulance personnel on how to perform informed consent and how to increase communication skills from a patient perspective.
Results
Participants
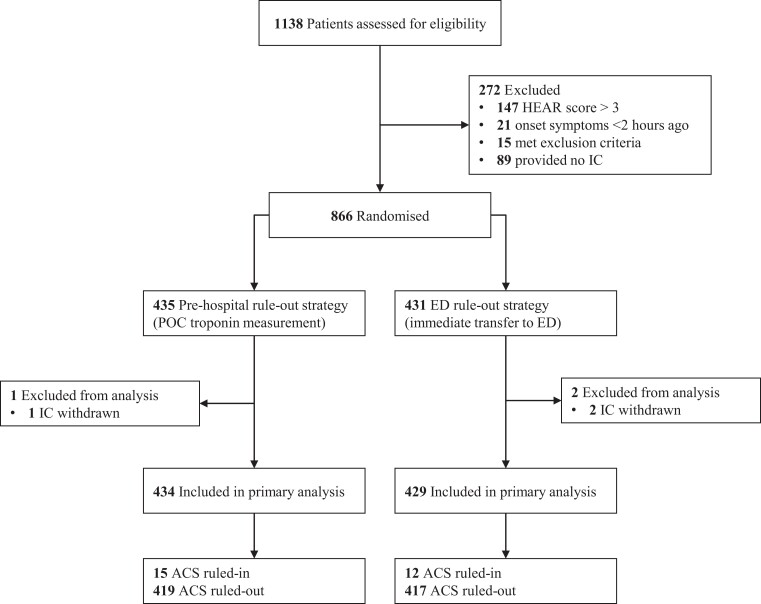
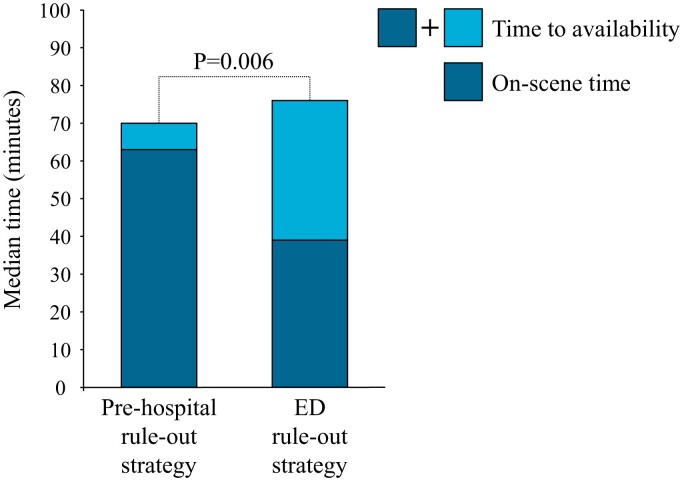
From 1 March 2019 to 4 May 2022, 1138 patients were screened and a total of 866 randomizations were performed. Three patients were excluded from the analyses because of withdrawn informed consent (n = 3). No patients were lost to follow-up, resulting in a total of 863 patients available for analyses. The consort flow chart is depicted in Figure 1. Mean age was 54 ± 13 years and 57% of the patients were female. Median HEAR score was 3 (IQR 2–3). Median on-scene time was 63 min (IQR 52–73) in the pre-hospital rule-out strategy and 39 min (IQR 32–48) in the ED rule-out strategy (P < 0.001). The median ambulance time to availability was 70 min (IQR 58–88) in the pre-hospital rule-out strategy and 76 min (IQR 63–89) in the ED rule-out strategy (P = 0.006). The median on-scene times and times to availability are shown in Figure 2. Median transportation time was 15 min (IQR 8–19). Elevated POC troponin was observed in 4.1% of patients in the pre-hospital rule-out strategy and these patients were all transported to the ED. 8.5% of patients in the pre-hospital rule-out strategy were transferred to the ED because of elevated POC troponin (n = 18), failed POC troponin test (n = 12) or decision of the GP (n = 7). In 836 patients (96.5% of the total population), ACS was ruled-out, either in the pre-hospital setting or at the ED. Baseline characteristics were well balanced and are shown in Table 1.
Figure 1.
Flow chart of recruitment and participants. ED, emergency department; HEAR score, History Electrocardiogram Age Risk factors score; IC, informed consent; POC, point-of-care.
Figure 2.
Median on-scene time and time to availability. ED, emergency department.
Table 1.
Baseline characteristics
| Pre-hospital rule-out strategy n = 434 |
ED rule-out strategy n = 429 |
|
|---|---|---|
| Age (years), mean ± SD | 53.7 (13.1) | 53.2 (12.5) |
| Female sex, n (%) | 247 (56.9) | 248 (57.8) |
| HEAR score, median (IQR) | 3 (2–3) | 3 (2–3) |
| History score*, median (IQR) | 0 (0–1) | 1 (0–1) |
| ECG score*, median (IQR) | 0 (0–0) | 0 (0–0) |
| History of atherosclerotic disease, n (%) | 34 (7.8) | 23 (5.4) |
| Hypertension, n (%) | 85 (19.6) | 71 (16.6) |
| Diabetes mellitus, n (%) | 22 (5.1) | 14 (3.3) |
| Current smoker, n (%) | 112 (25.8) | 110 (25.6) |
| Hypercholesterolaemia, n (%) | 37 (8.5) | 35 (8.2) |
| Family history, n (%) | 155 (35.7) | 152 (35.4) |
| BMI ≥30 kg/m2, n (%) | 86 (19.8) | 80 (18.6) |
| Onset of symptoms >24 h, n (%) | 130 (30.0) | 123 (28.7) |
| Onset of symptoms <24 h, n (%) | 304 (70.0) | 306 (71.3) |
| Onset (hours) of symptoms (if onset <24 h), mean ± SD | 7.1 (5.2) | 6.8 (5.3) |
| Elevated troponin, n (%) | 18 (4.1) | 24 (5.6) |
| Heart rate (bpm), mean ± SD | 77.9 (13.2) | 77.4 (13.4) |
| Systolic blood pressure (mm Hg), mean ± SD | 146.0 (20.5) | 145.1 (21.2) |
BMI, body mass index; ECG, electrocardiogram; ED, emergency department; HEAR, History, ECG, Age and Risk factors; IQR, interquartile range; SD, standard deviation. *According to the HEAR score.
MACE in the total population
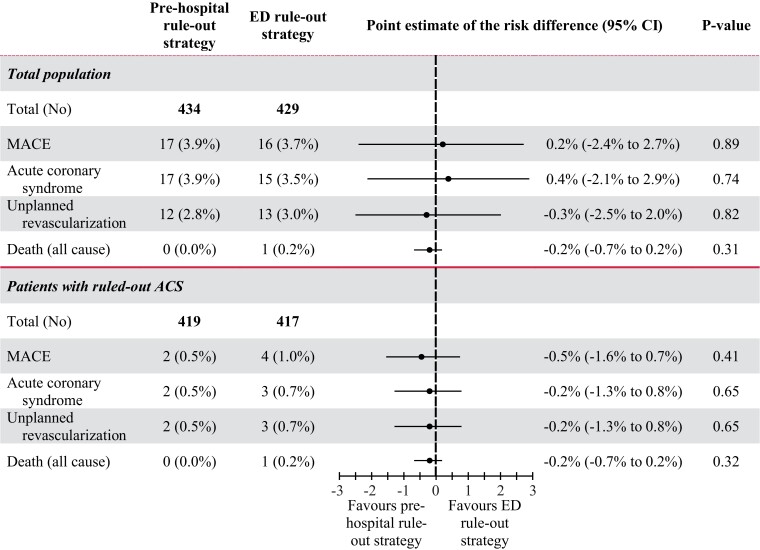
In the total population, MACE within 30 days occurred in 17/434 (3.9%) patients in the pre-hospital rule-out strategy vs. 16/429 (3.7%) patients in the ED rule-out strategy, with a point estimate for the risk difference of 0.2% (95% CI −2.4% to 2.7%, P = 0.89) (Figure 3). In the pre-hospital rule-out strategy, 17 (3.9%) patients had an ACS, of which four patients (0.9%) had an ST-segment elevation myocardial infarction (STEMI), 12 (2.8%) patients received unplanned revascularization and no patients died. A total of 15 of these 17 patients were directly transferred to the ED because they did not have low POC troponin.
Figure 3.
MACE at 30 days. MACE, major adverse cardiac events; ED, emergency department; ACS, acute coronary syndrome.
Safety of the pre-hospital rule-out strategy
In the ruled-out ACS population, MACE within 30 days occurred in 2/419 (0.5%) patients in the pre-hospital rule-out strategy vs. 4/417 (1.0%) patients in the ED rule-out strategy, with a point estimate for the risk difference of −0.5% (95% CI: −1.6% to 0.7%, P = 0.41). In the pre-hospital rule-out strategy, two (0.5%) patients had an ACS, two (0.5%) patients received unplanned revascularization and no patients died after an ACS was ruled-out in the pre-hospital setting. In the ED rule-out strategy, three (0.7%) patients had an ACS, three (0.7%) patients received unplanned revascularization and one (0.2%) patient died after an ACS was ruled-out at the ED. Of the four patients with a STEMI, three patients had normal ECGs and elevated POC troponin concentrations at home, after which ST-segment elevations were observed in the ED. In one patient, the GP decided not to refer the patient to the ED because the ECG and POC troponin measurement were normal. Two days later, the patient called the emergency number because of recurrence of chest pain, after which the ECG showed ST-segment elevations. In the ED rule-out strategy, 15 (3.5%) patients had an ACS, of which zero patients had a STEMI, 13 (3.0%) patients received unplanned revascularization, and one (0.2%) patient died after an out-of-hospital cardiac arrest 1 week after visiting the ED.
PE occurred in 2/434 (0.5%) patients in the pre-hospital rule-out strategy and in 1/429 (0.2%) patient in the ED rule-out strategy. No aortic dissections occurred in the total study population. Final diagnoses of all patients are provided in Supplementary material online, Table S3.
Healthcare costs
Costs from a healthcare perspective at 30 days were significantly lower in the pre-hospital rule-out strategy (€1349 ± €2051 vs. €1960 ± €1808), with a mean difference of €611 (95% CI: €353 to €869), P < 0.001) (Table 2). This difference was mainly driven by the costs of ED visits and their associated diagnostic tests (ECGs, screening blood tests, second troponin measurements and chest X-rays). Of all patients in the pre-hospital rule-out strategy, 13.6% had one ED visit (n = 59), 1.2% had two or more ED visits (n = 5), 7.4% were hospitalized (n = 32) and 4.8% were hospitalized directly after the initial transport to the hospital (n = 21). Of all patients in the ED rule-out strategy, 95.8% had one ED visit (n = 411), 4.2% had two or more ED visits (n = 18), 9.8% were hospitalized (n = 42), and 8.4% were hospitalized directly after the initial transport to the hospital (n = 36). The individual components of the healthcare costs are listed in Table 3. The GLM showed similar results to the T-tests: costs difference of €611 (standard error €131, 95% CI: €353–€869).
Table 2.
Costs at 30 days
| Pre-hospital rule-out strategy n = 434 |
ED rule-out strategy n = 429 |
Mean difference (95% CI) | P-value | |
|---|---|---|---|---|
| Healthcare costs (€), mean ± SD | 1349.42 (2050.83) | 1960.39 (1807.63) | 610.97 (352.57–869.37) | <0.001 |
| Productivity loss costs (€), mean ± SD | 784.66 (1646.18) | 892.61 (1757.02) | 107.95 (−127.67–343.56) | 0.37 |
| Societal costs (€), mean ± SD | 2157.08 (2899.75) | 2842.27 (2815.61) | 685.19 (289.30–1081.09) | 0.001 |
CI, confidence interval; ED, emergency department; SD, standard deviation.
Table 3.
Healthcare resource use at 30 days
| Pre-hospital rule-out strategy n = 434 | ED rule-out strategy n = 429 | P-value | |
|---|---|---|---|
| One additional ambulance transport, n (%) | 24 (5.5%) | 10 (2.3%) | 0.02 |
| Two or more additional ambulance transports, n (%) | 2 (0.5%) | 0 (0.0%) | 0.16 |
| One ED visit, n (%) | 59 (13.6%) | 411 (95.8%) | <0.001 |
| Two or more ED visits, n (%) | 5 (1.2%) | 18 (4.2%) | 0.006 |
| No. of additional tests at ED, mean ± SD | |||
| ȃECGs | 0.56 (1.82) | 1.84 (1.87) | <0.001 |
| ȃScreening blood tests | 0.16 (0.43) | 1.04 (0.22) | <0.001 |
| ȃSecond high-sensitivity troponin measurements | 0.08 (0.31) | 0.44 (0.51) | <0.001 |
| ȃD-dimer measurements | 0.04 (0.21) | 0.27 (0.44) | <0.001 |
| ȃChest X-rays | 0.17 (0.43) | 0.62 (0.56) | <0.001 |
| One GP consultation, n (%) | 338 (77.9%) | 132 (30.8%) | <0.001 |
| Two or more GP consultations, n (%) | 96 (22.1%) | 31 (7.2%) | <0.001 |
| One outpatient clinic visit, n (%) | 54 (12.4%) | 46 (10.7%) | 0.43 |
| Two or more outpatient clinic visits, n (%) | 5 (1.2%) | 3 (0.7%) | 0.49 |
| CT, n (%) | 12 (2.8%) | 17 (4.0%) | 0.33 |
| MRI, n (%) | 1 (0.2%) | 0 (0.0%) | 0.32 |
| Echocardiography, n (%) | 54 (12.4%) | 44 (10.3%) | 0.31 |
| Treadmill, n (%) | 41 (9.4%) | 52 (12.1%) | 0.21 |
| Other additional tests, n (%) | 61 (14.1%) | 59 (13.8%) | 0.90 |
| CAG, n (%) | 20 (4.6%) | 20 (4.7%) | 0.97 |
| PCI, n (%) | 13 (3.0%) | 12 (2.8%) | 0.86 |
| CABG, n (%) | 1 (0.2%) | 1 (0.2%) | 0.99 |
| Hospitalization, n (%) | 32 (7.4%) | 42 (9.8%) | 0.21 |
| Length of hospitalization (days), median (IQR) | 0 (0–0) | 0 (0–0) | 0.24 |
CABG, coronary artery bypass grafting; CAG, coronary angiography; CT, computed tomography; ED, emergency department; GP, general practitioner; IQR, interquartile range; MRI, magnetic resonance imaging; PCI, percutaneous coronary intervention.
Costs of productivity losses showed no significant difference between the groups (€785 ± €1646 vs. 893€ ± €1757), with a mean difference of €108 (95% CI: €−128–€344, P = 0.37). Costs from a societal perspective were significantly lower in the pre-hospital rule-out strategy (€2157 ± €2900 vs. €2842 ± €2819), with a mean difference of €685 (95% CI: €289–€1081, P = 0.001).
The pre-specified subgroup analyses were underpowered but are available in Supplementary material online, Table S4.
Prescribed medications (in the hospital or by the GP) are shown in Supplementary material online, Table S5. Proton pump inhibitors, analgesics and other medications (e.g. antiemetics and inhaled medications) were significantly more frequently prescribed in the ED rule-out strategy, whereas aspirin, P2Y12-inhibitors, statins, betablockers and antihypertensive medication did not differ between both strategies.
Discussion
Principal findings
Our trial shows that pre-hospital rule-out of NSTE-ACS in low-risk patients by a single POC troponin measurement results in a significant reduction of healthcare costs in the first 30 days and that incidence of MACE is low in both strategies (Structured Graphical Abstract). In patients for whom an ACS was ruled out (either at home or at the ED), MACE at 30 days was very low and occurred in only 0.5% of the patients in the pre-hospital rule-out strategy, vs. 1.0% of the patients in the ED rule-out strategy. In this study, 8.5% of the patients in the pre-hospital rule-out strategy were directly referred to the ED of whom only seven patients (1.6%) with a low POC troponin were referred to the ED by the GP.
Safety
Previous observational studies have shown that HEAR score assessment in combination with a POC troponin measurement by ambulance paramedics is a feasible strategy.16,17 The HEART score has been shown to have a very strong overall inter-operator reliability between physicians and nurses and compared with physicians at the ED, ambulance paramedics tend to overestimate the history and ECG components of the HEART score.15,23 Also, the risk of mortality and myocardial infarction is very low in patients with a HEART score ≤3.10,16,24 To prevent missing very early onset myocardial infarction with a risk of a low troponin result, patients with symptom onset shorter than two hours before presentation were excluded from our trial.
MACE occurred in 3.8% of the patients with similar percentages between groups. However, in the pre-hospital rule-out strategy, most of the MACE were diagnosed by POC troponin measurement and therefore not missed. Hence, the safety of pre-hospital rule-out of NSTE-ACS is best demonstrated by the comparison of the incidence of MACE after an ACS is ruled-out in both groups. In the ruled-out ACS population (96.5% of the total population), MACE occurred in 0.5% of the patients in the pre-hospital strategy vs. 1.0% of the patients in the ED rule-out strategy, with a risk difference of −0.5% (95% CI: −1.6% to 0.7%) in favour of the pre-hospital rule-out strategy. MACE were a secondary outcome measure and our trial was not formally powered to conclude that the pre-hospital rule-out strategy is non-inferior for safety. However, since the incidence of MACE in ruled-out ACS patients was very low and the risk difference is at most 0.7% higher in the pre-hospital rule-out strategy, the results of our trial demonstrate that implementation of the pre-hospital rule-out strategy in routine practice is likely to be safe. Moreover, when designing a trial powered to show non-inferiority for MACE in low-risk patients with ruled-out ACS by POC troponin measurement, the sample size should be based on the incidence of MACE in the ruled-out ACS population. Such a trial would require a total of 17 820 patients, based on a mean incidence of MACE of 0.7% (observed in our ruled-out ACS population) and upper limit of a two-sided 95% CI to exclude a difference of more than 0.35% (50% of the incidence). Although a large trial like that is possible with a large number of international sites involved, it will require significant funding and time. Another strategy that could be considered is the implementation of a pre-hospital rule-out strategy in a large multinational registry with very strict monitoring of clinical outcomes.
Healthcare costs and overcrowding
Mean healthcare costs were significantly lower in the pre-hospital rule-out strategy. This difference was mainly driven by the costs of the ED visits and their associated diagnostic tests. Implementation of this pre-hospital rule-out strategy in low-risk patients could achieve a significant reduction in healthcare costs by more efficient use of ambulance services and less ED visits.13,25 A recent study has shown that rule-out of NSTE-ACS in emergency primary care is cost-effective, which is in line with our results. In that study, the European Society of Cardiology 0/1 h algorithm with a high-sensitivity troponin assay in a hospital laboratory was used to identify low-risk patients, whereas our study offers an algorithm that does not require a laboratory and can be performed on-site.13 A total of 91.5% of the patients in the pre-hospital rule-out strategy were not transferred to the ED, while all of these patients would have been transferred to the ED in the absence of a pre-hospital strategy. Hence, the pre-hospital rule-out strategy reduces the number unnecessary ED visits, which contributes to a reduction in ED overcrowding. Moreover, no significant differences were seen in downstream outpatient clinic visits, additional tests outside of the ED and hospitalizations. Although the POC troponin test requires the ambulance to stay longer on-site, the ambulance time to availability was actually shorter in the pre-hospital rule-out strategy. Since the study procedures (informed consent, case report form, and randomization) were performed by ambulance paramedics, the on-scene times in both strategies were longer than can be expected of routine ambulance visits. In countries with longer transportation distances and therefore longer transportation times, the time saved by pre-hospital rule-out of ACS is expected to be even more. If the pre-hospital rule-out strategy is implemented, considerable cost savings for transporting the patient will apply to the majority of cases, whereas the time to availability is also reduced. According to the Dutch Healthcare Authority, in the year 2018 almost 200 000 patients in the Netherlands were evaluated at the ED for chest pain without a cardiac diagnosis.26 Given that 39.3% of all chest pain patients visiting the ED are low-risk patients, that the Netherlands has 714 ambulances and that the POC devices can be used for at least five years, the estimated POC troponin costs are €42,50 per measurement (all-in, including purchase of 714 POC devices, materials, time for the measurement and time for the maintenance of the device).10 Therefore, we believe that the reduction in ambulance costs will outweigh the additional costs for the POC troponin test, which might further increase the cost savings of the pre-hospital rule-out strategy. However, according to the current analysis of the reduced healthcare costs (€611 per patient), the added value of our pre-hospital rule-out strategy on the Dutch population already would amount to an estimated €48 000 000 per year in the Netherlands alone.
Generalizability
We believe the results of our trial on pre-hospital rule-out of ACS in low-risk patients are, in general, applicable on a global level, given a well-functioning healthcare system is available. As mentioned, the HEART score has a very strong overall inter-operator reliability, regardless of the level of education.15 HEART score assessment has already been shown to be applicable in the ED worldwide.10 Pre-hospital rule-out of ACS has recently been shown to reduce healthcare costs in a trial in Norway.13 Implementation of routine pre-hospital POC troponin measurement has been shown to effectively identify high-risk patients in Denmark, whereas pre-hospital HEART score assessment including a POC troponin measurement has been shown to adequately identify low-risk patients in the Netherlands and the United States.16,17,27
The Netherlands is a country with highly developed ambulatory care and short distances. However, despite the short distances, the pre-hospital rule-out strategy showed significantly shorter ambulance time to availability. In countries with less ambulance capacity and longer distances, the benefit of pre-hospital rule-out of ACS could be larger. In the Netherlands, GPs cooperate to provide urgent primary care on rotation basis in so-called GP-posts, ensuring a gatekeeping function 24/7.28 Implementation of the pre-hospital strategy therefore also depends on the accessibility of primary care. Furthermore, the average level of education of ambulance paramedics could be different in other countries. Therefore, the local healthcare infrastructure should be taken into account when extrapolating the results of this trial to other countries.
Limitations
The POC troponin assay is less sensitive than high-sensitivity assays in the hospital laboratory. Therefore, patients with a low POC troponin concentration could have mildly increased high-sensitivity troponin concentrations, which is associated with an increased mortality risk for which further investigation is necessary.29 However, the combination of this POC assay and the HEAR score has been shown to identify low-risk chest pain patients with low MACE incidence. Moreover, all patients with elevated POC troponin were directly transferred to the hospital. The study algorithm was primarily focused on ACS rule-out. However, the incidence of MACE and other emergent cardiovascular events in ruled-out ACS patients in our trial is comparable with previous observational studies.10,16 The current costs analysis did not include the additional costs for the POC troponin measurement in the pre-hospital rule-out strategy. However, additional cost savings by not transporting the majority of patients were also not included and we believe that this reduction in ambulance costs will outweigh the costs of the POC troponin test, which might further increase the cost savings of the pre-hospital rule-out strategy. Ambulance on-scene times seem very long, however one should realize that all study procedures (including data entry in Castor EDC) were performed on site by the ambulance paramedics.
Conclusion
Pre-hospital rule-out of NSTE-ACS in low-risk patients with the use of POC troponin testing in combination with a clinical risk score leads to large healthcare cost reductions and is associated with a very low MACE occurrence.
Supplementary Material
Acknowledgements
P. van Grunsven, MD, PhD, medical lead ambulance RAV Gelderland Zuid (2018–2020)
M. Verbakel, Dutch patient federation for cardiovascular patients, ‘Harteraad’.
Contributor Information
Cyril Camaro, Department of Cardiology, Radboud University Medical Centre, P.O. Box 9101, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, Gelderland, The Netherlands.
Goaris W A Aarts, Department of Cardiology, Radboud University Medical Centre, P.O. Box 9101, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, Gelderland, The Netherlands.
Eddy M M Adang, Department of Health Evidence, Radboud Institute for Health Sciences, Geert Grooteplein 21, 6525 EZ Nijmegen, Gelderland, The Netherlands.
Roger van Hout, Ambulance Service, Safety region Gelderland-Zuid, Professor Bellefroidstraat 11, 6525 AG Nijmegen, Gelderland, The Netherlands.
Gijs Brok, Ambulance Service, Safety region Gelderland-Zuid, Professor Bellefroidstraat 11, 6525 AG Nijmegen, Gelderland, The Netherlands.
Anouk Hoare, Ambulance Service, Witte Kruis, Ringveste 7A, 3992 DD Houten, Utrecht, The Netherlands.
Laura Rodwell, Department of Health Evidence, Radboud Institute for Health Sciences, Geert Grooteplein 21, 6525 EZ Nijmegen, Gelderland, The Netherlands.
Frank de Pooter, Ambulance Service, Witte Kruis, Ringveste 7A, 3992 DD Houten, Utrecht, The Netherlands.
Walter de Wit, Ambulance Service, Witte Kruis, Ringveste 7A, 3992 DD Houten, Utrecht, The Netherlands.
Gilbert E Cramer, Department of Cardiology, Radboud University Medical Centre, P.O. Box 9101, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, Gelderland, The Netherlands.
Roland R J van Kimmenade, Department of Cardiology, Radboud University Medical Centre, P.O. Box 9101, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, Gelderland, The Netherlands.
Peter Damman, Department of Cardiology, Radboud University Medical Centre, P.O. Box 9101, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, Gelderland, The Netherlands.
Eva Ouwendijk, General Practitioner Centre Nijmegen and Boxmeer, Weg door Jonkerbos 108, 6532 SZ Nijmegen, Gelderland, The Netherlands.
Martijn Rutten, Scientific Centre for Quality of Healthcare (IQ Healthcare), Radboud University Medical Centre, Kapittelweg 54, 6525 EP Nijmegen, Gelderland, The Netherlands.
Erwin Zegers, Department of Cardiology, Canisius Wilhelmina Hospital, Weg door Jonkerbos 100, 6532 SZ Nijmegen, Gelderland, The Netherlands.
Robert-Jan M van Geuns, Department of Cardiology, Radboud University Medical Centre, P.O. Box 9101, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, Gelderland, The Netherlands.
Marc E R Gomes, Department of Cardiology, Canisius Wilhelmina Hospital, Weg door Jonkerbos 100, 6532 SZ Nijmegen, Gelderland, The Netherlands.
Niels van Royen, Department of Cardiology, Radboud University Medical Centre, P.O. Box 9101, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, Gelderland, The Netherlands.
Supplementary data
Supplementary data is available at European Heart Journal online.
Data availability
Data will be made available 1 year after publication of the primary manuscript. Data requests should be submitted to the corresponding author.
Funding
The trial was supported by a grant from The Netherlands Organisation for Health Research and Development (ZonMw); 2018 grant number 852001942. ZonMw had no role in the design or monitoring of the trial, the enrolment of participants, the collection, recording, storage, retention, or analysis of the data, the writing of the manuscript or the decision to submit the manuscript for publication.
Contributors
C.C. conceived the idea. G.W.A.A., C.C., R.-J.M.v.G., E.C., R.R.J.v.K., P.D., and N.v.R. designed the study methodology. E.A. designed the economical and statistical analyses. C.C., G.W.A.A. and N.v.R. drafted the manuscript. G.W.A.A., C.C., E.A. and L.R. designed the statistical analysis plan. The first draft of the manuscript was written by C.C,. G.W.A.A., and N.v.R. All authors reviewed and revised the final manuscript. All authors agreed with the final version of the manuscript.
Author contributions
P.D., MD, PhD (conceptualization: supporting; writing – original draft: supporting; writing – review & editing: supporting); E.O., MD (investigation: supporting; writing – review & editing: supporting); G.E.C., MD, PhD (conceptualization: supporting; methodology: supporting; writing – original draft: supporting; writing – review & editing: supporting); R.R.J.v.K., MD, PhD (conceptualization: supporting; writing – original draft: supporting; writing – review & editing: supporting); M.R., MD, PhD (conceptualization: supporting; writing – review & editing: supporting); M.E.R.G., MD, PhD (writing – review & editing: supporting); N.v.R., MD, PhD (conceptualization: equal; formal analysis: equal; methodology: equal; supervision: lead; writing – original draft: lead; writing – review & editing: lead); E.S.Z., MD (investigation: supporting; writing – review & editing: supporting); R.-J.v.G., MD, PhD (conceptualization: equal; funding acquisition: supporting; methodology: supporting; supervision: supporting; writing – original draft: supporting; writing – review & editing: supporting), E.M.M.A., PhD (conceptualization: equal; data curation: equal; formal analysis: supporting; methodology: equal; writing – review & editing: supporting); R.v.H., RN (data curation: equal; investigation: equal; project administration: equal; writing – review & editing: supporting); C.C., M.D. (conceptualization: lead; data curation: equal; formal analysis: equal; funding acquisition: lead; investigation: supporting; methodology: equal; project administration: supporting; supervision: lead; validation: lead; writing – original draft: equal; writing – review & editing: equal; principal investigator of the trial: lead); G.W.A.A., MD (conceptualization: supporting; data curation: equal; formal analysis: equal; investigation: equal; methodology: supporting; project administration: lead; software: equal; writing – original draft: lead; writing – review & editing: lead); G.B., RN (data curation: equal; investigation: equal; project administration: equal; writing – review & editing: supporting); F.d.P., RN (investigation: equal; project administration: equal; writing – review & editing: supporting); W.d.W., RN (investigation: equal; project administration: equal; writing – review & editing: supporting); A.H., MD (data curation: supporting; funding acquisition: equal; investigation: supporting; supervision: supporting; writing – review & editing: supporting); L.R., PhD (formal analysis: equal; methodology: equal; writing – review & editing: supporting).
Transparency
The lead authors (C.C., G.W.A.A., N.v.R.) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1. Sun BC, Hsia RY, Weiss RE, Zingmond D, Liang LJ, Han W, et al. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med 2013;61:605–611.e6. 10.1016/j.annemergmed.2012.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Writing Committee Members, Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol 2021;78:e187–e285. 10.1016/j.jacc.2021.07.053 [DOI] [PubMed] [Google Scholar]
- 3. Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367. 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 4. Pitts SR, Niska RW, Xu J, Burt CW. National hospital ambulatory medical care survey: 2006 emergency department summary. Natl Health Stat Report 2008;7:1–38. [PubMed] [Google Scholar]
- 5. Lee TH, Goldman L. Evaluation of the patient with acute chest pain. N Engl J Med 2000;342:1187–1195. 10.1056/NEJM200004203421607 [DOI] [PubMed] [Google Scholar]
- 6. Poldervaart JM, Reitsma JB, Backus BE, Koffijberg H, Veldkamp RF, Ten Haaf ME, et al. Effect of using the HEART score in patients with chest pain in the emergency department: a stepped-wedge, cluster randomized trial. Ann Intern Med 2017;166:689–697. 10.7326/M16-1600 [DOI] [PubMed] [Google Scholar]
- 7. Chase M, Robey JL, Zogby KE, Sease KL, Shofer FS, Hollander JE. Prospective validation of the thrombolysis in myocardial infarction risk score in the emergency department chest pain population. Ann Emerg Med 2006;48:252–259. 10.1016/j.annemergmed.2006.01.032 [DOI] [PubMed] [Google Scholar]
- 8. Sagel D, Vlaar PJ, van Roosmalen R, Waardenburg I, Nieuwland W, Lettinga R, et al. Prehospital risk stratification in patients with chest pain. Emerg Med J 2021;38:814–819. 10.1136/emermed-2020-210212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nasrallah N, Steiner H, Hasin Y. The challenge of chest pain in the emergency room: now and the future. Eur Heart J 2011;32:656. [PubMed] [Google Scholar]
- 10. Laureano-Phillips J, Robinson RD, Aryal S, Blair S, Wilson D, Boyd K, et al. HEART Score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med 2019;74:187–203. 10.1016/j.annemergmed.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 11. Amsterdam EA, Kirk JD, Bluemke DA, Diercks D, Farkouh ME, Garvey JL, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation 2010;122:1756–1776. 10.1161/CIR.0b013e3181ec61df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fanaroff AC, Rymer JA, Goldstein SA, Simel DL, Newby LK. Does this patient with chest pain have acute coronary syndrome?: the rational clinical examination systematic review. JAMA 2015;314:1955–191965. 10.1001/jama.2015.12735 [DOI] [PubMed] [Google Scholar]
- 13. Johannessen TR, Halvorsen S, Atar D, Munkhaugen J, Nore AK, Wisloff T, et al. Cost-effectiveness of a rule-out algorithm of acute myocardial infarction in low-risk patients: emergency primary care versus hospital setting. BMC Health Serv Res 2022;22:1274. 10.1186/s12913-022-08697-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Than M, Aldous S, Lord SJ, Goodacre S, Frampton CM, Troughton R, et al. A 2-hour diagnostic protocol for possible cardiac chest pain in the emergency department: a randomized clinical trial. JAMA Intern Med 2014;174:51–58. 10.1001/jamainternmed.2013.11362 [DOI] [PubMed] [Google Scholar]
- 15. Niven WGP, Wilson D, Goodacre S, Robertson A, Green SJ, Harris T. Do all HEART scores beat the same: evaluating the interoperator reliability of the HEART score. Emerg Med J 2018;35:732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tolsma RT, Fokkert MJ, van Dongen DN, Badings EA, van der Sluis A, Slingerland RJ, et al. Referral decisions based on a pre-hospital HEART score in suspected non-ST-elevation acute coronary syndrome: final results of the FamouS Triage study. Eur Heart J Acute Cardiovasc Care 2022;11:160–169. 10.1093/ehjacc/zuab109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stopyra JP, Snavely AC, Smith LM, Harris RD, Nelson RD, Winslow JE, et al. Prehospital use of a modified HEART pathway and point-of-care troponin to predict cardiovascular events. PLoS One 2020;15:e0239460. 10.1371/journal.pone.0239460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aarts GWA, Camaro C, van Geuns RJ, Cramer E, van Kimmenade RRJ, Damman P, et al. Acute rule-out of non-ST-segment elevation acute coronary syndrome in the (pre)hospital setting by HEART score assessment and a single point-of-care troponin: rationale and design of the ARTICA randomised trial. BMJ Open 2020;10:e034403. 10.1136/bmjopen-2019-034403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jungbauer C, Hupf J, Giannitsis E, Frick J, Slagman A, Ehret C, et al. Analytical and clinical validation of a point-of-care cardiac troponin T test with an improved detection limit. Clin Lab 2017;63:633–645. 10.7754/Clin.Lab.2016.160814 [DOI] [PubMed] [Google Scholar]
- 20. Bouwmans C, Krol M, Severens H, Koopmanschap M, Brouwer W, Hakkaart-van Roijen L. The iMTA productivity cost questionnaire: a standardized instrument for measuring and valuing health-related productivity losses. Value Health 2015;18:753–758. 10.1016/j.jval.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 21. Kanters TA, Bouwmans CAM, van der Linden N, Tan SS, Hakkaart-van Roijen L. Update of the Dutch manual for costing studies in health care. PLoS One 2017;12:e0187477. 10.1371/journal.pone.0187477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Busse R, Geissler A, Aaviksoo A, Cots F, Hakkinen U, Kobel C, et al. Diagnosis related groups in Europe: moving towards transparency, efficiency, and quality in hospitals? BMJ 2013;346:f3197. 10.1136/bmj.f3197 [DOI] [PubMed] [Google Scholar]
- 23. van Dongen DN, Badings EA, Fokkert MJ, Tolsma RT, van der Sluis A, Slingerland RJ, et al. Pre-hospital versus hospital acquired HEART score for risk classification of suspected non ST-elevation acute coronary syndrome. Eur J Cardiovasc Nurs 2021;20:40–47. 10.1177/1474515120927867 [DOI] [PubMed] [Google Scholar]
- 24. Mahler SA, Lenoir KM, Wells BJ, Burke GL, Duncan PW, Case LD, et al. Safely identifying emergency department patients with acute chest pain for early discharge. Circulation 2018;138:2456–2468. 10.1161/CIRCULATIONAHA.118.036528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Dongen DN, Ottervanger JP, Tolsma R, Fokkert M, van der Sluis A, van ‘t Hof AWJ, et al. In-Hospital healthcare utilization, outcomes, and costs in Pre-hospital-adjudicated low-risk chest-pain patients. Appl Health Econ Health Policy 2019;17:875–882. 10.1007/s40258-019-00502-6 [DOI] [PubMed] [Google Scholar]
- 26. Dutch Healthcare Authority, Open data DBC Information System (DBC). http://www.opendisdata.nl.
- 27. Rasmussen MB, Stengaard C, Sorensen JT, Riddervold IS, Hansen TM, Giebner M, et al. Predictive value of routine point-of-care cardiac troponin T measurement for prehospital diagnosis and risk-stratification in patients with suspected acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 2019;8:299–308. 10.1177/2048872617745893 [DOI] [PubMed] [Google Scholar]
- 28. Kremers MNT, Nanayakkara PWB, Levi M, Bell D, Haak HR. Strengths and weaknesses of the acute care systems in the United Kingdom and The Netherlands: what can we learn from each other? BMC Emerg Med 2019;19:40. 10.1186/s12873-019-0257-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meune C, Reichlin T, Irfan A, Schaub N, Twerenbold R, Meissner J, et al. How safe is the outpatient management of patients with acute chest pain and mildly increased cardiac troponin concentrations? Clin Chem 2012;58:916–924. 10.1373/clinchem.2011.178053 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available 1 year after publication of the primary manuscript. Data requests should be submitted to the corresponding author.