Abstract
Background and Objectives:
Perioperative blood transfusion is associated with poor outcomes in several malignancies. Its effect in gallbladder cancer (GBC) is unknown.
Methods:
All patients with GBC who underwent curative-intent resection at 10-institutions from 2000 to 2015 were included. The effect of blood transfusion on overall survival (OS) and recurrence-free (RFS) was evaluated.
Results:
Of 262 patients with curative-intent resection for GBC, 61 patients (23%) received blood transfusions. Radical cholecystectomy was the most common procedure (80%), but major hepatectomy was more frequent in the transfusion versus no-transfusion group (13% vs 4%; P = 0.02). The transfusion group was less likely to have incidentally discovered disease (57% vs 74%) and receive adjuvant therapy (29% vs 48%), but more likely to have preoperative jaundice (23% vs 11%), T3/T4 tumors (60% vs 39%), LVI (71% vs 40%), PNI (71% vs 48%), and major complications (39% vs 12%) (all P < 0.05). Transfusion was associated with lower median OS compared to notransfusion (20 vs 32 mos; P < 0.001), which persisted on multivariable (MV) analysis (HR:1.9; 95%CI 1.1–3.5; P = 0.035), controlling for comorbidities, serum albumin, INR, preoperative jaundice, major hepatectomy, incidental discovery, margin status, T-Stage, LN status, and major complications. Median RFS of transfused patients was 13mo compared to 49mo for non-transfused patients (P = 0.1). Transfusion, however, was an independent predictor of decreased RFS on MV analysis (HR:2.3; 95%CI 1.1–5.1; P = 0.035).
Conclusions:
Perioperative blood transfusion is associated with decreased OS and RFS after resection for GCC, accounting for other adverse factors. Transfusions should thus be administered with well-defined protocols.
Keywords: gallbladder cancer, perioperative blood transfusion, recurrence, survival
1 |. INTRODUCTION
Gallbladder cancer is a rare disease with a reported incidence of less than 5000 new cases per year and approximately 3000 deaths annually.1 It occurs more frequently in women, and up to 70% of cases are found incidentally during elective cholecystectomy.1–3 The only possible cure for gallbladder cancer is via surgical resection, but 5-year survival rates following surgery range from 10% to 100% depending on tumor histology, stage of disease, and the extent of surgical resection.4–6 It is currently recommended by the National Comprehensive Cancer Network (NCCN) that all patients with a T1b or higher staged tumor per the American Joint Committee on Cancer (AJCC) staging system undergo a radical cholecystectomy with en bloc resection of segments IVb and V, as well as regional portal lymph node dissection.7,8 Patients with lymph node metastasis outside the locoregional lymph node basin or with distant metastasis, however, have not been shown to experience a survival advantage following surgery.9,10
There are multiple factors that have been found to be associated with patient morbidity and mortality in gallbladder cancer. These include jaundice, advanced T-stage, and lymph node metastases.11,12 Perioperative blood transfusion is a known predictor of poor prognosis and postoperative morbidity in multiple cancers, including gastric, colorectal, bladder, and pancreatic cancer.13–17 To date, evidence has been conflicting, with differing results based on the type of cancer, number of units transfused, and timing of transfusion.14,18–20 While the mechanism through which allogeneic blood transfusion affects prognosis in patients who undergo curative cancer surgery remains unclear, the predominant theory is via an immunomodulatory effect by transfused allogeneic leukocytes.21 Clinical trials are currently lacking to further delineate this immunosuppressive effect, but patients undergoing surgical resection for cancer continue to be transfused without universally pre-determined transfusion protocols, despite evidence suggesting possible deleterious outcomes.
Given the current and sometimes conflicting data regarding perioperative allogeneic blood transfusion, as well as a lack of data regarding its effect on gallbladder cancer specifically, the aim of this study was to assess the association of perioperative packed red blood cell (pRBC) transfusion with long-term overall and recurrence-free survival among patients with gallbladder cancer from a large, multi-institutional cohort of patients.
2 |. MATERIALS AND METHODS
2.1 |. Study population
All patients with primary gallbladder cancer who underwent surgical resection from January 1, 2000 to December 31, 2015 at the 10 diverse, high-volume academic institutions (Emory University, Johns Hopkins University, New York University, The Ohio State University, Stanford University, University of Louisville, University of Wisconsin, Vanderbilt University, Wake Forest University, and Washington University in St. Louis) that form the US Extrahepatic Biliary Malignancy Consortium (US-EBMC) were identified. Only patients who had curative-intent surgery and information regarding preoperative and postoperative pRBC transfusion were analyzed. Patients with R2 resections and mortality within 30 days of surgery were excluded from analysis. Primary and secondary endpoints were overall survival (OS) and recurrence-free survival (RFS), respectively.
2.2 |. Study variables
Retrospective chart review captured baseline demographic, preoperative, operative, pathologic, and postoperative data. Preoperative comorbidities were defined using the Charlson Comorbidity Scoring System, and staging was assigned per the American Joint Committee on Cancer 7th edition guidelines.8 Transfusion data were collected from operative reports and postoperative medical records. Survival data were also collected and verified according to the Social Security Death Index, when appropriate; and recurrence of disease data was determined by review of patient medical records, surveillance imaging reports, and/or biopsy results. Institutional review board approval was obtained at each institution prior to data retrieval.
2.3 |. Statistical analyses
Descriptive and comparative analyses were used for the entire study cohort. OS was calculated from the date of operation to the date of death, while RFS was calculated from the date of operation to the date of recurrence diagnosis. All statistical analyses were conducted using SPSS version 23.0 (Armonk New York Software, IBM Inc.). Chi-squared analyses and Fisher’s exact tests were used to compare categorical variables, and Student’s t-test was used for continuous variables. Univariable and multivariable Cox regression analyses were performed to assess the association of individual pathologic factors and transfusion with OS and RFS. Kaplan-Meier survival plots were calculated for both OS and RFS, and variables were compared using log-rank tests. Statistical significance was predefined as P < 0.05.
3 |. RESULTS
3.1 |. Study population
Of 449 patients with gallbladder cancer, 295 (66%) underwent curative-intent, R0/R1 resection. Thirty-three patients who were missing either preoperative or postoperative pRBC transfusion data were excluded, leaving 262 patients (58%) available for subsequent analysis. Baseline demographics and clinicopathologic factors for the entire cohort are reported in Table 1. Median age was 65 years, 34% (n = 90) were male, and 73% (n = 175) were white. Mean tumor size was 36.6 mm, and the majority of patients (80%) underwent radical cholecystectomy (including partial hepatectomy of segments IVb/V) with portal lymphadenectomy. Average blood loss was 459.7 mL, and 61 patients (23%) underwent perioperative transfusion with 1 or more units of pRBCs. Transfusion was associated with preoperative jaundice (22.6% vs 10.7%; P = 0.046), as well as more extensive operative procedures including: major hepatectomy (13.3% vs 4%; P = 0.021), common bile duct resection (50.8% vs 22.8%; P < 0.001), and portal vein resection (4.9% vs 0%; P = 0.012). Transfusion was also associated with increased blood loss (877.4 mL vs 318.7 mL; P < 0.001), more aggressive pathologic factors, such as advanced AJCC T-stage (T3/T4: 60.3% vs 39.3%; P = 0.007), lymphovascular invasion (LVI) (70.6% vs 39.8%; P = 0.003), and perineural invasion (PNI) (71.4% vs 48.2%; P = 0.027), and increased major postoperative complications (39.2% vs 12.2%; P < 0.001). Patients who were transfused were less likely to have their disease discovered incidentally during initial cholecystectomy (57.4% vs 74.0%; P = 0.020), and they were also less likely to receive adjuvant chemotherapy (28.9% vs 48.1%; P = 0.035) compared to the non-transfused group. Table 1 summarizes the differences between patients who were transfused and those who were not.
TABLE 1.
Clinicopathologic features of all patients stratified by pRBC transfusion
| No. (%) | ||||
|---|---|---|---|---|
|
|
||||
| Variable | All patients (n = 262) | No transfusion (n = 201) | Transfusion (n = 61) | P-value |
| Malea | 90 (34.4) | 68 (33.8) | 22 (36.1) | 0.867 |
| Age (years; median, IQR)b | 66 (17.4) | 65 (17.7) | 67 (14.2) | 0.079 |
| Racea | 0.568 | |||
| White | 175 (72.9) | 133 (71.9) | 42 (76.4) | |
| African American | 27 (11.3) | 23 (12.4) | 4 (7.3) | |
| Other | 38 (15.8) | 29 (15.7) | 9 (16.4) | |
| BMI (kg/m2; mean±SD)c | 28.3 ± 7.1 | 28.7 ±7.1 | 26.4 ±6.8 | 0.064 |
| ASA classa | 0.599 | |||
| 1 | 2 (1.1) | 2 (1.4) | 0 (0.0) | |
| 2 | 72 (40.4) | 58 (41.7) | 14 (35.9) | |
| 3 | 99 (55.6) | 76 (54.7) | 23 (59.0) | |
| 4 | 5 (2.8) | 3 (2.2) | 2 (5.1) | |
| Comorbiditiesa | 0.778 | |||
| 0 | 81 (36.5) | 64 (36.6) | 17 (36.2) | |
| 1 | 91 (41.0) | 70 (40.0) | 21 (44.7) | |
| ≥2 | 50 (22.5) | 41 (23.4) | 9 (19.1) | |
| Preoperative albumin (g/dL; mean ± SD)c | 3.7 ± 0.7 | 3.8 ±0.7 | 3.6 ± 0.7 | 0.050 |
| Preoperative INR (mean ± SD)c | 1.1 ± 0.2 | 1.1 ±0.2 | 1.1 ±0.3 | 0.336 |
| Jaundicea | 31 (13.5) | 19 (10.7) | 12 (22.6) | 0.046 |
| Operative approacha | 0.076 | |||
| Open | 240 (92.3) | 182 (91.0) | 58 (96.7) | |
| Laparoscopic | 13 (5.0) | 13 (6.5) | 0 (0.0) | |
| Laparoscopic hand-assisted | 2 (0.8) | 2 (1.0) | 0 (0.0) | |
| Robotic | 2 (0.8) | 2 (1.0) | 0 (0.0) | |
| Laparoscopic converted to open | 3 (1.2) | 1 (0.5) | 2 (3.3) | |
| Incidental discoverya | 183 (70.1) | 148 (74.0) | 35 (57.4) | 0.020 |
| Extent of resectiona | 0.021 | |||
| Bile duct resection only | 7 (2.7) | 6 (3.0) | 1 (1.7) | |
| Cholecystectomy only | 30 (11.5) | 27 (13.4) | 3 (5.0) | |
| Radical cholecystectomy + portal LNs | 208 (79.7) | 160 (79.6) | 48 (80.0) | |
| Major hepatectomy | 16 (6.1) | 8 (4.0) | 8 (13.3) | |
| Common bile duct resectiona | 76 (29.5) | 45 (22.8) | 31 (50.8) | <0.001 |
| Portal vein resectiond | 43 (1.1) | 0 (0.0) | 3 (4.9) | 0.012 |
| Major arterial resectiond | 2 (0.8) | 1 (0.5) | 1 (1.7) | 0.404 |
| Estimated blood loss (mL; mean ± SD)c | 459.7 ± 538.4 | 318.7 ± 282.5 | 877.4 ± 829.3 | <0.001 |
| Transfusion (total units; mean ± SD)c | 1.2 ±9.8 | - | - | - |
| Transfusion (total units) | - | - | - | |
| 0 | 201 (78.5) | |||
| 1 | 18 (7.0) | |||
| 2 | 19 (7.4) | |||
| ≥3 | 18 (7.2) | |||
| Final margin statusa | 0.058 | |||
| R0 | 211 (85.4) | 175 (87.9) | 47 (77.0) | |
| R1 | 38 (14.6) | 24 (12.1) | 14 (23.0) | |
| Tumor size (mm; mean ±SD)c | 36.6 ±27.2 | 34.4 ± 24.2 | 44.2 ± 34.9 | 0.110 |
| AJCC T stagea | 0.007 | |||
| Tis/Tla/b | 31 (12.4) | 23 (12.0) | 8 (13.8) | |
| T2 | 108 (43.4) | 93 (48.7) | 15 (25.9) | |
| T3/T4 | 110 (44.2) | 75 (39.3) | 35 (60.3) | |
| Gradea | 0.384 | |||
| Well-differentiated | 28 (12.6) | 21 (12.5) | 7 (12.7) | |
| Moderately differentiated | 122 (54.7) | 96 (57.1) | 26 (47.3) | |
| Poorly/undifferentiated | 73 (32.7) | 51 (30.4) | 22 (40.0) | |
| Lymphovascular invasiona | 69 (46.9) | 45 (39.8) | 24 (70.6) | 0.003 |
| Perineural invasiona | 80 (53.7) | 55 (48.2) | 25 (71.4) | 0.027 |
| LN positivea | 91 (41.7) | 70 (43.2) | 21 (37.5) | 0.555 |
| Major complicationa | 42 (18.2) | 22 (12.2) | 20 (39.2) | <0.001 |
| Neoadjuvant chemotherapyd | 10 (3.9) | 8 (4.0) | 2 (3.3) | 1.000 |
| Adjuvant chemotherapya | 87 (43.7) | 74 (48.1) | 13 (28.9) | 0.035 |
pRBC, packed red blood cells; IQR, interquartile range; BMI, body mass index; SD, standard deviation; ASA, American Society of Anesthesiologists; INR, international normalized ratio; AJCC, American Joint Committee on Cancer; LN, lymph nodes.
Bold values indicate P < 0.05.
Chi-squared test.
Non-parametric test.
Independent t-test.
Two-tailed Fisher's Exact test.
3.2 |. Transfusion and predictors of overall survival
Among the 262 patients with pRBC perioperative transfusion data who underwent survival analysis, median follow-up was 15 months (IQR 24.1). On univariable Cox regression, the preoperative factors associated with decreased overall survival were low serum albumin (HR 0.59; 95% CI 0.47–0.75; P < 0.001), elevated INR (HR 2.62; 95% CI 1.13–6.08; P = 0.025), and presence of jaundice at presentation (HR 2.43; 95% CI 1.50–3.92; P < 0.001). Incidental discovery of gallbladder cancer during non-oncologic surgery was associated with improved survival (HR 0.53; 95% CI 0.37–0.76; P = 0.001). The use of more invasive operative procedures, such as major hepatectomy (HR 2.62; 95% CI 1.46–4.70; P = 0.001), as well as postoperative complications (HR 2.99; 95% CI 1.92–4.66; P < 0.001), had an over twofold increase in risk of death. Pathologic factors such as tumor size (HR 1.02; 95% CI 1.01–1.02; P < 0.001), positive resection margins (HR 4.06; 95% CI 2.68–6.15; P < 0.001), advanced T-stage (T3/T4: HR 6.98; 95% CI 3.02–16.12; P < 0.001), LVI (HR 2.89; 95% CI 1.78–4.69; P < 0.001), PNI (HR 2.97; 95% CI 1.76–5.00), and lymph node positivity (HR 2.28; 95% CI 1.55–3.36; P < 0.001) were all associated with worse survival (Table 2).
TABLE 2.
Univariable and multivariable Cox regression analysis of risk factors associated with OS
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Male | 0.95 | 0.66–1.37 | 0.795 | - | - | - |
| Age (yrs) | 1.01 | 1.00–1.03 | 0.101 | - | - | - |
| Race | - | - | - | |||
| White | Ref | - | - | |||
| African American | 0.86 | 0.45–1.66 | 0.654 | |||
| Other | 0.71 | 0.41–1.25 | 0.240 | |||
| BMI (kg/m2) | 0.98 | 0.94–1.01 | 0.170 | - | - | - |
| ASA class | - | - | - | |||
| 1 | Ref | - | - | |||
| 2 | 0.17 | 0.02–1.24 | 0.080 | |||
| 3 | 0.20 | 0.03–1.51 | 0.119 | |||
| 4 | 0.70 | 0.06–7.78 | 0.770 | |||
| Comorbidities (>1) | 1.47 | 0.95–2.26 | 0.084 | - | - | - |
| Preoperative albumin (g/dL) | 0.59 | 0.47–0.75 | <0.001 | 0.54 | 0.36–0.80 | 0.002 |
| Preoperative INR | 2.62 | 1.13–6.08 | 0.025 | 1.11 | 0.39–3.15 | 0.839 |
| Jaundice | 2.43 | 1.50–3.92 | <0.001 | 0.96 | 0.45–2.05 | 0.914 |
| Operative approach | - | - | - | |||
| Open | Ref | - | - | |||
| Laparoscopic | 0.53 | 0.17–1.66 | 0.276 | |||
| Laparoscopic hand-assisted | 0.00 | 0.00–9E187 | 0.961 | |||
| Robotic | 3.04 | 0.42–21.95 | 0.271 | |||
| Laparoscopic converted to open | 1.14 | 0.28–4.61 | 0.857 | |||
| Incidental discovery | 0.53 | 0.37–0.76 | 0.001 | 0.77 | 0.42–1.40 | 0.387 |
| Major hepatectomy | 2.62 | 1.46–4.70 | 0.001 | 0.68 | 0.28–1.64 | 0.394 |
| Common bile duct resection | 1.61 | 1.13–2.30 | 0.008 | - | - | - |
| Portal vein resection | 3.35 | 1.05–10.63 | 0.041 | - | - | - |
| Major arterial resection | 28.33 | 3.54–226.53 | 0.002 | - | - | - |
| Estimated blood loss | 1.00 | 1.00–1.00 | 0.125 | - | - | - |
| Transfusion | 1.90 | 1.32–2.73 | 0.001 | 1.90 | 1.05–3.47 | 0.035 |
| Transfusion >2 unitsa | 2.25 | 1.45–3.48 | <0.001 | - | - | - |
| Tumor size (mm) | 1.02 | 1.01–1.02 | <0.001 | - | - | - |
| Final margin status | 1.13 | 0.48–2.68 | 0.774 | |||
| R0 | Ref | - | - | |||
| R1 | 4.06 | 2.68–6.15 | <0.001 | |||
| AJCC T-stage | ||||||
| Tis/T1a/b | Ref | - | - | Ref | - | - |
| T2 | 2.25 | 0.95–5.35 | 0.066 | 3.74 | 1.03–13.57 | 0.045 |
| T3/T4 | 6.98 | 3.02–16.12 | <0.001 | 10.62 | 3.04–37.17 | <0.001 |
| Grade | - | - | - | |||
| Well-differentiated | Ref | - | - | |||
| Moderately differentiated | 1.84 | 0.91–3.72 | 0.092 | |||
| Poorly/undifferentiated | 3.16 | 1.54–6.49 | 0.002 | |||
| Lymphovascular invasion | 2.89 | 1.78–4.69 | <0.001 | - | - | - |
| Perineural invasión | 2.97 | 1.76–5.00 | <0.001 | - | - | - |
| Lymph node positive | 2.28 | 1.55–3.36 | <0.001 | 2.01 | 1.19–3.39 | 0.009 |
| Major complication | 2.99 | 1.92–4.66 | <0.001 | 1.49 | 0.82–2.71 | 0.191 |
| Neoadjuvant chemotherapy | 1.57 | 0.73–3.37 | 0.249 | - | - | - |
| Adjuvant chemotherapy | 0.97 | 0.63–1.49 | 0.888 | - | - | - |
OS, overall survival; HR, hazard ratio; CI, confidence interval; BMI, body mass index, ASA, American Society of Anesthesiologists; INR, international normalized ratio; AJCC, American Joint Committee on Cancer.
Bold values indicate P < 0.05.
A separate multivariable model for OS using this variable was constructed, though not shown here in table form.
The use of perioperative pRBC transfusion was associated with a nearly twofold increase in risk of death on univariable Cox regression (HR 1.90; 95% CI 1.32–2.73; P = 0.001). On Kaplan-Meier analysis, transfused patients had a lower median OS compared to patients who were not transfused (20 vs 32 months; P < 0.001; Figure 1). Even when taking into account other preoperative and clinicopathologic risk factors associated with worse OS, transfusion remained independently significant on multivariable Cox regression analysis (HR 1.90; 95% CI 1.05–3.47; P = 0.035; Table 2). When considering the effect of number of pRBC units given, a worse OS was seen in those patients transfused with 2 or more units of pRBCs (HR 2.25; 95% CI 1.45–3.48; P < 0.001). However, this finding did not persist in a multivariable model. Similarly, timing of transfusion (intraoperative vs postoperative) was also not found to be independently associated with decreased survival.
FIGURE 1.
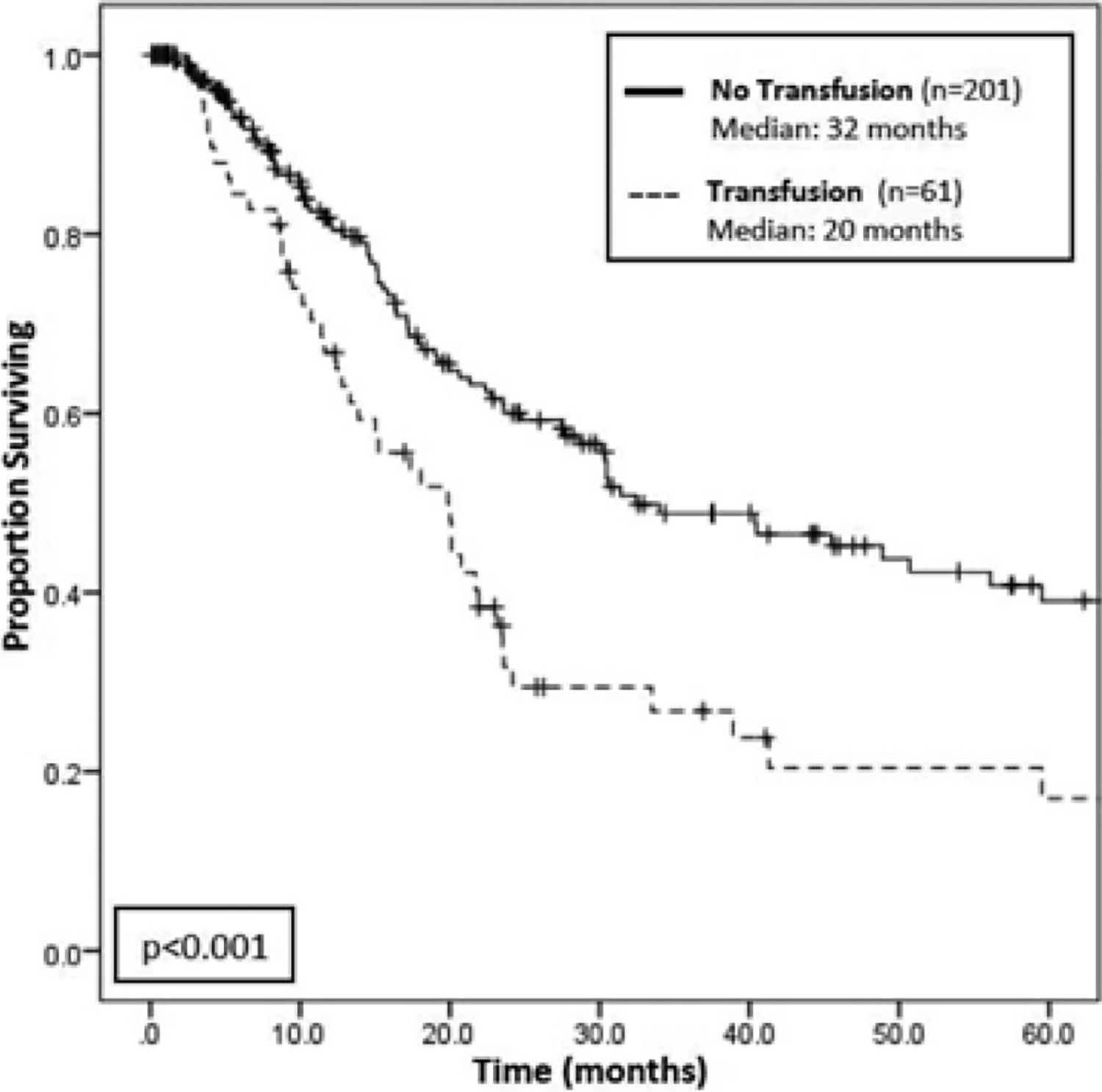
Kaplan-Meier survival analysis for overall survival among transfusion versus no transfusion groups. There is a statistically significant difference in overall survival (OS) between patients who were transfused (n = 61) and patients who were not transfused (n = 201), where those who were transfused had a median survival of 20 months compared to 32 months for those were not transfused (P < 0.001)
3.3 |. Transfusion and predictors of recurrence-free survival
On analysis of RFS, perioperative and clinicopathologic factors found to be associated with increased risk of disease recurrence included preoperative comorbidities (HR 2.01; 95% CI 1.16–3.46; P = 0.012), elevated INR (HR 3.38; 95% CI 1.25–9.18; P = 0.017), need for major hepatectomy (HR 2.25; 95% CI 1.02–4.98; P = 0.044), increased tumor size (HR 1.01; 95% CI 1.00–1.02; P = 0.016), positive resection margins (HR 3.08; 95% CI 1.70–5.58; P < 0.001), advanced T-stage (T3/T4: HR 8.11; 95% CI 2.50–26.30; P < 0.001), LVI (HR 3.85; 95% CI 2.02–7.33; P < 0.001), PNI (HR 2.61; 95% CI 1.42–4.81; P = 0.002), and lymph node positivity (HR 2.26; 95% CI 1.37–3.72; P = 0.001) (Table 3). Major postoperative complications were also associated with worse RFS on univariable analysis.
TABLE 3.
Univariable and multivariable Cox regression analysis of risk factors associated with RFS
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Male | 0.96 | 0.60–1.56 | 0.878 | - | - | - |
| Age (yrs) | 1.01 | 0.99–1.03 | 0.506 | - | - | - |
| Race | - | - | - | |||
| White | Ref | - | - | |||
| African American | 0.74 | 0.32–1.73 | 0.490 | |||
| Other | 1.03 | 0.57–1.87 | 0.915 | |||
| BMI (kg/m2) | 1.01 | 0.97–1.04 | 0.802 | - | - | - |
| ASA class | - | - | - | |||
| 1 | Ref | - | - | |||
| 2 | 0.17 | 0.02–1.34 | 0.094 | |||
| 3 | 0.20 | 0.03–1.51 | 0.117 | |||
| 4 | 0.30 | 0.02–4.85 | 0.397 | |||
| Comorbidities (>1) | 2.01 | 1.16–3.46 | 0.012 | 1.63 | 0.80–3.30 | 0.178 |
| Preoperative albumin (g/dL) | 0.78 | 0.55–1.11 | 0.163 | - | - | - |
| Preoperative INR | 3.38 | 1.25–9.18 | 0.017 | 1.30 | 0.41–4.12 | 0.652 |
| Jaundice | 1.71 | 0.90–3.27 | 0.105 | 1.50 | 0.57–3.98 | 0.414 |
| Operative approach | - | - | - | |||
| Open | Ref | - | - | |||
| Laparoscopic | 0.46 | 0.11–1.89 | 0.282 | |||
| Laparoscopic hand-assisted | 0.00 | 0.00–4E208 | 0.965 | |||
| Robotic | 3.04 | 0.42–22.18 | 0.273 | |||
| Laparoscopic converted to open | 2.09 | 0.51–8.58 | 0.308 | |||
| Incidental discovery | 0.66 | 0.40–1.08 | 0.097 | 0.79 | 0.40–1.59 | 0.515 |
| Major hepatectomy | 2.25 | 1.02–4.98 | 0.044 | 1.00 | 0.32–3.07 | 0.994 |
| Common bile duct resection | 1.24 | 0.75–2.04 | 0.400 | - | - | - |
| Portal vein resection | 3.44 | 0.83–14.25 | 0.089 | - | - | - |
| Major arterial resection | 0.05 | 0.00–3.4E35 | 0.945 | - | - | - |
| Estimated blood loss | 1.00 | 1.00–1.00 | <0.001 | - | - | - |
| Transfusion | 1.52 | 0.90–2.57 | 0.116 | 2.32 | 1.06–5.08 | 0.035 |
| Tumor size (mm) | 1.01 | 1.00–1.02 | 0.016 | - | - | - |
| Final margin status | 1.11 | 0.37–3.35 | 0.849 | |||
| R0 | Ref | - | - | |||
| R1 | 3.08 | 1.70–5.58 | <0.001 | |||
| AJCC T-stage | ||||||
| Tis/T1a/b | Ref | - | - | Ref | - | - |
| T2 | 2.91 | 0.87–9.76 | 0.084 | 6.97 | 0.87–55.84 | 0.068 |
| T3/T4 | 8.11 | 2.50–26.30 | <0.001 | 11.97 | 1.51–95.23 | 0.019 |
| Grade | - | - | - | |||
| Well-differentiated | Ref | - | - | |||
| Moderately differentiated | 2.19 | 0.86–5.60 | 0.101 | |||
| Poorly/undifferentiated | 3.72 | 1.42–9.74 | 0.008 | |||
| Lymphovascular invasion | 3.85 | 2.02–7.33 | <0.001 | - | - | - |
| Perineural invasion | 2.61 | 1.42–4.81 | 0.002 | - | - | - |
| Lymph node positive | 2.26 | 1.37–3.72 | 0.001 | 1.71 | 0.92–3.17 | 0.090 |
| Major complication | 2.09 | 1.14–3.82 | 0.017 | 1.30 | 0.59–2.88 | 0.517 |
| Neoadjuvant chemotherapy | 1.78 | 0.71–4.42 | 0.218 | - | - | - |
| Adjuvant chemotherapy | 1.56 | 0.96–2.54 | 0.072 | - | - | - |
RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; BMI, body mass index, ASA, American Society of Anesthesiologists; INR, international normalized ratio; AJCC, American Joint Committee on Cancer.
Bold values indicate P < 0.05.
Unlike OS, perioperative transfusion was not found to be directly associated with worse RFS on univariable Cox regression (HR 1.52; 95% CI 0.90–2.57; 0.116). However, there was a trend toward decreased median RFS on Kaplan-Meier analysis for transfused versus not-transfused patients (13 vs 49 months; P = 0.114; Figure 2). When applying a similar multivariable Cox regression model to RFS, perioperative transfusion was found to be independently associated with worse RFS, even when accounting for other adverse clinicopathologic factors (HR 2.32; 95% CI 1.06–5.08; P = 0.035) (Table 3).
FIGURE 2.
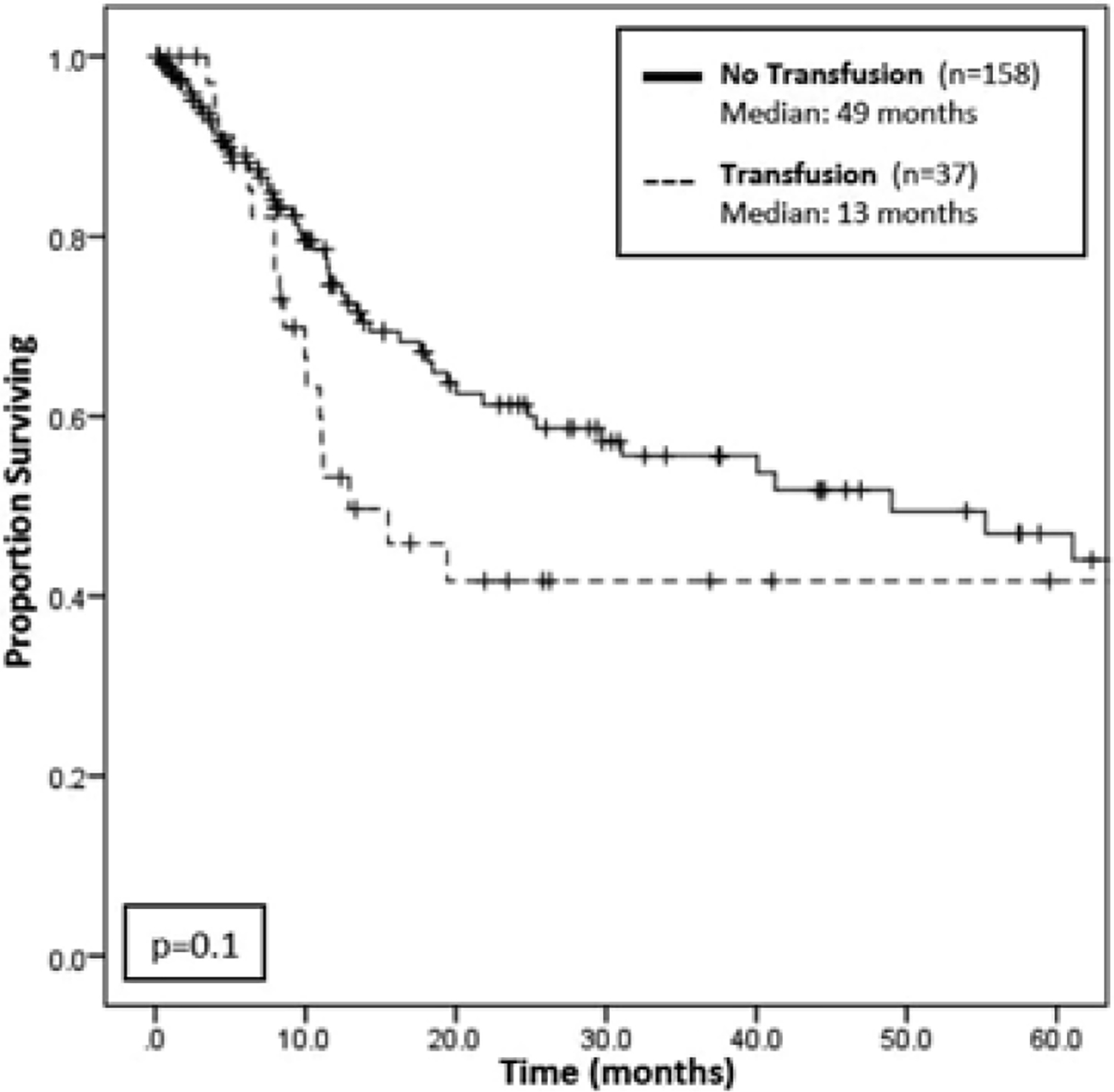
Kaplan-Meier survival analysis for recurrence-free survival among transfusion versus no transfusion groups. Although there is a trend of worse recurrence-free survival (RFS) among patients who were transfused (n = 37) compared to patients who were not transfused (n = 158), this association is not statistically significant, with a P-value of 0.1
4 |. DISCUSSION
Although gallbladder cancer is a known aggressive disease with a grave prognosis, little is understood about the role that pRBC transfusion plays in influencing its outcomes. The current study is the first to date to evaluate the relationship between perioperative allogeneic pRBC transfusion and survival/recurrence data among patients with curative-intent resection for gallbladder cancer. Indeed, our findings suggest that while transfusion is associated with multiple markers of advanced disease and increased major postoperative complications, its negative prognostic influence on OS persists, even when accounting for such factors as jaundice, positive margins, advanced T-stage, positive lymph nodes, and major postoperative complications on multivariable analysis. Perioperative pRBC transfusion was not associated with worse RFS on univariable analysis; however, it was associated with decreased RFS on multivariable analysis even when accounting for other clinicopathologic variables. These findings support current data regarding the deleterious association between pRBC transfusion and outcomes in cancer patients.13,14,18–20,22–24
Gallbladder cancer is often discovered in an advanced stage, which contributes to both its poor prognosis and the need for a substantial resection as part of its management.1,5,25–27 With an overall 5-year survival rate of less than 5% for patients diagnosed with gallbladder cancer, multiple prognostic factors have been implicated in the poor outcomes observed in patients who undergo resection.28 Two key factors are T-stage and positive lymph nodes, as studies have shown that with increasing T-stage, there is a marked rise in the rate of lymph node positivity among gallbladder cancers (15.7% in stage T1b, 46% in stage T2, and 75% in stage T3).27–30 Indeed, our multi-institutional study corroborates such findings, as advanced T-stage (T3/T4 tumors) and lymph node positivity were both found to be associated with worse OS and RFS on Cox regression analysis among our cohort of patients. Several studies have also revealed tumor grade, LVI, and PNI to be associated with poor prognosis.30–33 These findings were similarly supported by the results of the current study, which demonstrated a correlation of these factors with decreased OS and RFS. Evidence of biliary tract disease, such as jaundice, is another known poor prognostic factor.28,34 In a study by Oertli et al, 18 patients with gallbladder cancer who presented with jaundice had a 0% resectability rate.34 Our study found jaundice to be a negative prognostic factor as well, as it was associated with an over twofold increase in risk of mortality (P < 0.001) on univariable analysis for OS. However, this finding did not persist in our multivariable model. Lastly, one positive prognostic indicator that is noted both in the literature and in this study is the incidental discovery of a patient’s gallbladder cancer during routine cholecystectomy.28,35 Per Gourgiotis et al, incidentally diagnosed gallbladder cancers have a 5-year median survival of 26.5 months compared to only 9.2 months for all suspected gallbladder cancer.28 Ethun et al had analogous findings, also showing a higher median OS for incidental versus non-incidental gallbladder cancer (32 vs 17 months; P < 0.001).36 In this study, an incidental diagnosis was similarly associated with improved survival.
Although various clinical and pathologic factors have been implicated in poor prognosis in gallbladder cancer, the variable of particular interest in this study was the relationship between perioperative pRBC transfusion with OS and RFS. The mechanism postulated to account for the negative association between transfusion and oncologic outcomes is that transfusion may be linked to the expression and activity of cytokines, the Fas ligand, and HLA class I antigens.37,38 The subsequent alteration in the immune response may predispose patients toward increased tumor recurrence and decreased survival, though further research is necessary to better understand and confirm this theory.18 Although unclear in the exact mechanism, the association between perioperative transfusion and worse oncologic outcomes has been established in many malignancies, including gastric, pancreatic, lung, liver, adrenal, and colorectal cancers.13,14,16,18–20,22,23 However, results have been conflicting depending on the cancer considered. For example, in a study by Postlewait et al which examined the effect of transfusion on metastatic colorectal cancer, although a negative relationship between transfusion and major postoperative complications was established, transfusion was not found to be independently associated with worse disease-specific survival.18 When researching the relationship between transfusion and adrenal cancer, on the other hand, Poorman et al found that transfusion was associated with both a decreased median OS (22.8 vs 91 months; P < 0.001) and RFS(8.9 vs24.7 months; P = 0.006).20 Likewise, a study of gastric cancer by Squires et al had similar results, also noting a decrease in OS (18.6 vs 49.8 months; P < 0.001) and RFS (13.5 vs 37.2 months; P < 0.001) among patients who were transfused.14 The current study supports findings reported by both Poorman et al and Squires et al, as it demonstrates that perioperative transfusion, after resection of gallbladder cancer, is independently associated with worse OS (HR 1.90; P = 0.035) and RFS (HR 2.32; P = 0.035) on multivariable analyses.
Although transfusion protocols for hepatobiliary surgery do not yet exist, many academic centers encourage their surgeons to follow the findings of the TRICC trial from 1999 when considering transfusing patients. This multicenter, randomized, controlled clinical trial showed that a restrictive strategy of red-cell transfusion for hemoglobin <7.0 g per deciliter was at least as effective as, and possibly superior to, a liberal transfusion strategy in critically ill patients.39 As a result, many surgeons, including most surgeons from our multi-institutional collaboration, now use a threshold of 7 g/dL for deciding when to transfuse. However, the TRICC trial was based exclusively on subjects treated in the intensive care unit, and hence is limited in its applicability to the ever-changing environment of the operating room. To address this, the Transfusion Requirements in Cardiac Surgery III (TRICS III) trial is currently underway to establish the right balance for blood transfusion among cardiac surgical patients. There remain no trials to date evaluating transfusion in surgical cancer patients; thus, while retrospective studies suggest that a restrictive transfusion strategy of 7–8 g/dL does not impact mortality compared to a liberal strategy, there is insufficient data to conclusively inform cancer-specific transfusion policies.40
This study is limited by its retrospective design, which precludes the ability to establish a causal relationship between perioperative blood transfusion and survival or disease recurrence, and instead necessitates an interpretation of results to identify associations between transfusion and measured outcomes. Moreover, while transfusion data was available for each patient, specific hemoglobin levels, whether intra- or post-operative, were not collected at the time of collaboration. The RFS analysis was also limited by the availability of recurrence data, and given the current lack of standardized transfusion protocols, the decision to transfuse is surgeon-dependent and determined on a case-by-case basis. This may lead to a selection bias, as patients with worse disease may be favored for liberal transfusion. Nonetheless, this is the first study to date that examines outcomes for transfusion in gallbladder cancer specifically, and its multi-institutional design with data from 10 geographically diverse academic centers enables its results to be generalizable on a national scale.
5 |. CONCLUSIONS
In conclusion, allogeneic red blood cell transfusion is independently associated with decreased OS and RFS in patients undergoing curative-intent resection of primary gallbladder cancer, even after taking into account other adverse clinicopathologic factors. The role of timing and number of transfused units, however, remains unclear, with no independent association with survival determined in this study. Nonetheless, due to the already poor prognosis of gallbladder cancer, judicious perioperative use of red blood cell products and the creation and implementation of transfusion protocols is warranted and necessary.
ACKNOWLEDGMENTS
Supported, in part, by the Katz Foundation. Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 and TL1TR000456. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Choi KS, Choi SB, Park P, et al. Clinical characteristics of incidental or unsuspected gallbladder cancers diagnosed during or after cholecystectomy: a systematic review and meta-analysis. World J Gastroenterol. 2015;21:1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuks D, Regimbeau JM, Le Treut YP, et al. Incidental gallbladder cancer by the AFC-GBC-2009 study group. WorldJ Surg. 2011;35:1887–1897. [DOI] [PubMed] [Google Scholar]
- 4.Ethun CG, Postlewait LM, Le N, et al. Association of optimal time interval to re-resection for incidental gallbladder cancer with overall survival: a multi-institution analysis from the US extrahepatic biliary malignancy consortium. JAMA Surg. 2017;152:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg. 1994;219:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benoist S, Panis Y, Fagniez PL. Long-term results after curative resection for carcinoma of the gallbladder. French University Association for Surgical Research. Am J Surg. 1998;175:118–122. [DOI] [PubMed] [Google Scholar]
- 7.Benson AB 3rd, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 9.Fairweather M, Balachandran VP, D’Angelica MI. Surgical management of biliary tract cancers. Chin Clin Oncol. 2016;5:63. [DOI] [PubMed] [Google Scholar]
- 10.D’Angelica M, Dalal KM, DeMatteo RP, et al. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16:806–816. [DOI] [PubMed] [Google Scholar]
- 11.Wernberg JA, Lucarelli DD. Gallbladder cancer. Surg Clin North Am. 2014;94:343–360. [DOI] [PubMed] [Google Scholar]
- 12.Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton JM, Kooby DA, Wilson GC, et al. Perioperative blood transfusion is associated with decreased survival in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma: a multi-institutional study. J Gastrointest Surg. 2014;18:1575–1587. [DOI] [PubMed] [Google Scholar]
- 14.Squires MH 3rd, Kooby, Poultsides GA et al. Effect of perioperative transfusion on recurrence and survival after gastric cancer resection: a 7-institution analysis of 765 patients from the US gastric cancer collaborative. J Am Coll Surg. 2015;221:767–777. [DOI] [PubMed] [Google Scholar]
- 15.Gierth M, Aziz A, Fritsche HM, et al. The effect of intra- and postoperative allogenic blood transfusion on patients’ survival undergoing radical cystectomy for urothelial carcinoma of the bladder. World J Urol. 2014;32:1447–1453. [DOI] [PubMed] [Google Scholar]
- 16.Amato A, Pescatori M. Perioperative blood transfusions and recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006; Article No. CD005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869; discussion 869–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postlewait LM, Squires MH, Kooby DA, et al. The relationship of blood transfusion with peri-operative and long-term outcomes after major hepatectomy for metastatic colorectal cancer: a multi-institutional study of 456 patients. HPB. 2015;18:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.KneuertzPJ PatelSH, ChuCK, et al. Effects of perioperativered blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol. 2011;18:1327–1334. [DOI] [PubMed] [Google Scholar]
- 20.Poorman CE, Postlewait LM, Ethun CG, et al. Blood transfusion and survival for resected adrenocortical carcinoma: a study from the United States adrenocortical carcinoma group. Am Surg. 2017;83:761–768. [PMC free article] [PubMed] [Google Scholar]
- 21.Bordin JO, Blajchman MA. Immunosuppressive effects of allogeneic blood transfusions: implications for the patient with a malignancy. Hematol Oncol Clin North Am. 1995;9:205–218. [PubMed] [Google Scholar]
- 22.Asahara T, Katayama K, Itamoto T, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg. 1999;23:676–680. [DOI] [PubMed] [Google Scholar]
- 23.Wang T, Luo L, Huang H, et al. Perioperative blood transfusion is associated with worse clinical outcomes in resected lung cancer. Ann Thorac Surg. 2014;97:1827–1837. [DOI] [PubMed] [Google Scholar]
- 24.Linder BJ, Thompson RH, Leibovich BC, et al. The impact of perioperative blood transfusion on survival after nephrectomy for non-metastatic renal cell carcinoma (RCC). BJU Int. 2014;114:368–374. [DOI] [PubMed] [Google Scholar]
- 25.Shukla SK, Singh G, Shahi KS, et al. Staging, treatment, and future approaches of gallbladder carcinoma. J Gastrointest Cancer. 2017;49:9–15. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson DS. Carcinoma of the gall-bladder: an experience and review of the literature. Aust N Z J Surg. 1995;65:724–727. [DOI] [PubMed] [Google Scholar]
- 27.Goetze TO. Gallbladder carcinoma: prognostic factors and therapeutic options. World J Gastroenterol. 2015;21:12211–12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gourgiotis S, Kocher HM, Solaini L, et al. Gallbladder cancer. Am J Surg. 2008;196:252–264. [DOI] [PubMed] [Google Scholar]
- 29.Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167–176. [DOI] [PubMed] [Google Scholar]
- 30.Ethun CG, Postlewait LM, Le N, et al. A novel pathology-based preoperative risk score to predict locoregional residual and distant disease and survival for incidental gallbladder cancer: a 10-institution study from the U.S. extrahepatic biliary malignancy consortium. Ann Surg Oncol. 2016;24:1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butte JM, Kingham TP, Gonen M, et al. Residual disease predicts outcomes after definitive resection for incidental gallbladder cancer. J Am Coll Surg. 2014;219:416–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirai Y, Yoshida K, Tsukada K, et al. Early carcinoma of the gallbladder. Eur J Surg. 1992;158:545–548. [PubMed] [Google Scholar]
- 33.Ouchi K, Suzuki M, Tominaga T, et al. Survival after surgery for cancer of the gallbladder. Br J Surg. 1994;81:1655–1657. [DOI] [PubMed] [Google Scholar]
- 34.Oertli D, Herzog U, Tondelli P. Primary carcinoma of the gallbladder: operative experience during a 16 year period. Eur J Surg. 1993;159:415–420. [PubMed] [Google Scholar]
- 35.Wullstein C, Woeste G, Barkhausen S, et al. Do complications related to laparoscopic cholecystectomy influence the prognosis of gallbladder cancer? Surg Endosc. 2002;16:828–832. [DOI] [PubMed] [Google Scholar]
- 36.Ethun CG, Le N, Lopez-Aguiar AG, et al. Pathologic and prognostic implications of incidental versus nonincidental gallbladder cancer: a 10-institution study from the United States extrahepatic biliary malignancy consortium. Am Surg. 2017;83:679–686. [PMC free article] [PubMed] [Google Scholar]
- 37.Miki C, Hiro J, Ojima E, et al. Perioperative allogeneic blood transfusion, the related cytokine response and long-term survival after potentially curative resection of colorectal cancer. Clin Oncol (R Coll Radiol). 2006;18:60–66. [DOI] [PubMed] [Google Scholar]
- 38.Ghio M, Contini P, Mazzei C, et al. In vitro immunosuppressive activity of soluble HLA class I and Fas ligand molecules: do they play a role in autologous blood transfusion? Transfusion. 2001;41:988–996. [DOI] [PubMed] [Google Scholar]
- 39.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. [DOI] [PubMed] [Google Scholar]
- 40.Carson JL, Stanworth SJ, Roubinian N, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016;10:CD002042. [DOI] [PMC free article] [PubMed] [Google Scholar]


