Abstract
Background:
To define recurrence patterns and time course, as well as risk factors associated with recurrence following curative resection of pNETs.
Method:
Patients who underwent curative-intent resection for pNET between 1997 and 2016 were identified from the US Neuroendocrine Tumor Study Group. Data on baseline and tumor-specific characteristics, overall survival (OS), timing and first-site of recurrence, predictors and recurrence management were analyzed.
Results:
Among 1020 patients, 154 (15.1%) patients developed recurrence. Among patients who experienced recurrence, 76 (49.4%) had liver-only recurrence, while 35 (22.7%) had pancreas-only recurrence. The proportion of liver-only recurrence increased from 54.3% within one-year after surgery to 61.5% from four-to-six years after surgery; whereas the proportion of pancreas-only recurrence decreased from 26.1% to 7.7% over these time periods. While liver-only recurrence was associated with tumor characteristics, pancreas-only recurrence was only associated with surgical margin status. Patients undergoing curative resection of recurrence had comparable OS with patients who had no recurrence (median OS, pancreas-only recurrence, 133.9 months; liver-only recurrence, not attained; no recurrence, 143.0 months, p = 0.499)
Conclusions:
Different recurrence patterns and timing course, as well as risk factors suggest biological heterogeneity of pNET recurrence. A personalized approach to postoperative surveillance and treatment of recurrence disease should be considered.
Introduction
Gastro-entero-pancreatic neuroendocrine tumors (GEP-NET) are a heterogeneous group of tumors that originate from the diffuse neuroendocrine cell system of the gastrointestinal tract and pancreas.1 Pancreatic neuroendocrine tumors (pNETs) are generally the most malignant tumor type among the various GEP-NET.2 Of note, the worldwide incidence of pNET has increased three to seven fold over the past decade.3–5 In fact, the incidence of pNET is now about 1 in 100,000 persons in the United States.6
While surgical resection is the only curative treatment for pNETs,7,8 recurrence is common and can be associated with a worse quality of life and shorter survival among patients with pNET.9,10 Specific data on recurrence after surgical resection of pNETs are, however, relatively ill-defined.11–13 Previous studies have largely focused on factors associated with recurrence such as tumor features and surgical characteristics.10,14–17 In contrast, patterns and timing of recurrence, which may impact patient outcomes, have not been a specific focus of inquiry. Data on timing of recurrence may be important, however. For example, Groot et al. reported distinct predictive factors related to different disease sites and time courses for recurrence among patients undergoing curative resection of pancreatic ductal adenocarcinoma.18 In turn, defining the time course and patterns of pNET after oncological resection may help elucidate the heterogeneity in biological behavior and natural history of pNET, as well as inform prognostic stratification and guide surveillance and treatment. As such, the objective of the current study was to define the patterns and time course of recurrence following curative-intent resection of pNET, and characterize the risk factors associated with different patterns of recurrence, as well as describe outcomes following re-treatment of pNET recurrences.
Methods
Study cohort and data collection
Patients undergoing surgical resection for pNETs between 1997 and 2016 were identified from the US Neuroendocrine Tumor Study Group.10 All patients were diagnosed with pNET by final histologic examination. Patients who underwent R2 resection and who had concomitant liver metastases were excluded from the study cohort. The Institutional Review Boards of each participating institution approved the study.
Standard patient demographic, clinicopathologic, and treatment data were collected. Functional tumor was defined as symptoms associated with hormone overproduction, including insulinoma, glucagonoma, gastrinoma, VIPoma and somatostatinoma.19 Tumor number, size and differentiation, nodal status, Ki-67 category, perieural and vascular invasion were identified from the final pathologic report. An R0 resection was defined as a minimum margin length of >1 mm; an R1 resection was defined as the microscopic presence of tumor at the margin or a minimum margin length of ≤ 1 mm.10 Recurrence-free survival (RFS) was defined as the time duration from the date of initial surgery to tumor recurrence. Overall survival (OS) after recurrence was defined as the time duration from the date of recurrence after surgery to patient death or the end of the study.
Follow up and pattern of recurrence
All patients were followed regularly in each participating institution. The follow-up protocol at each center was once every 3–4 months within the first 3 years after operation and then once every 6 months until year 5, after which screening occurred annually. Serum tumor markers were prospectively monitored with imaging studies. Recurrent pNET was defined as identification of suspicious imaging findings on postoperative surveillance or biopsy proven disease. The initial recurrence site was identified for purposes of classification. The recurrence sites were classified into 3 patterns: pancreas-only recurrence, liver-only recurrence and other site recurrence. Pancreas recurrence was defined as the initial recurrence only if the recurrence occurred in the remnant pancreas or at the cut surface; liver recurrence was defined if the initial recurrence occurred only in the liver. Other site recurrence included multiple site recurrence or recurrence in organs other than pancreas or liver.
Statistical analysis
Continuous variables were expressed as medians with interquartile ranges (IQR) and categorical variables were expressed as totals and percentages. Statistical analyses were performed with the Mann–Whitney U test, χ2 test or Fisher exact test as appropriated. OS, RFS and OS after recurrence were estimated using the Kaplan–Meier method and compared by log-rank analysis. Factors associated with RFS for different recurrence patterns were identified via univariate and multivariable analyses using Cox-proportional hazard regression models; results were reported as hazard ratios (HRs) and 95% confidence intervals (95% CI). Statistical analysis was performed using SPSS Version 22.0 (IBM Corporation, Armonk, NY).
Results
Baseline characteristics
A total of 1020 patients undergoing R0/R1 resection for pNET without liver metastases were identified (Table 1). The clinicopathologic characteristics and details of the surgical procedures were summarized in Table 1. The most common surgical procedure was a distal pancreatectomy (n = 576, 56.5%), which was consistent with the tumor primary location (neck/body, n = 234, 22.9%; tail, n = 401, 39.3%). Among patients who underwent distal pancreatectomy, 477 of them (82.8%) had a concomitant splenectomy. Lymphadenectomy was performed in 858 (84.1%) patients; the median number of lymph nodes retrieved was 10 (IQR 5–16). Post-operatively, half of patients experienced a complication (n = 514, 53.3%) with 22.1% having a severe Clavien-Dindo III–V complication (Table 1). Median, 3-, 5-, and 10-year RFS for the entire cohort was 135.1 months (95% CI, 116.6 – 153.6), 85.6%, 78.0% and 57.0%, respectively. Median, 3-, 5-, and 10-year OS was 176.4 months (95% CI, 134.3–218.6), 92.5%, 88.6%, and 71.9%, respectively.
Table 1.
Clinical and pathological characteristics of the study cohort
| Variables | Value |
| Age (years) | 58 (47–66) |
| Sex | |
| Female | 496 (48.6%) |
| Male | 524 (51.4%) |
| Functional status | |
| Nonfunctional | 838 (82.2%) |
| Functional | 164 (16.1%) |
| Genetic syndrome | |
| None | 895 (87.7%) |
| MEN-1 | 85 (8.3%) |
| VHL | 10 (1.0%) |
| Symptomatic | |
| N0 | 442 (43.3%) |
| Yes | 558 (54.7%) |
| Primary location | |
| Head | 284 (27.8%) |
| Uncinated | 44 (4.3%) |
| Neck/body | 234 (22.9%) |
| Tail | 401 (39.3%) |
| Multiple | 51 (5.0%) |
| Multiple tumor nodules | |
| Single | 909 (89.1%) |
| Multiple | 106 (10.4%) |
| Tumor size (cm) 2.1 (1.4–3.5) | |
| Lymph nodes metastasis | |
| No | 623 (61.1%) |
| Yes | 235 (23.0%) |
| Ki-67 category | |
| <3% | 412 (40.4%) |
| 3–20% | 249 (24.4%) |
| >20% | 26 (2.5%) |
| Tumor differentiation | |
| Well | 781 (76.6%) |
| Moderately | 89 (8.7%) |
| Poorly | 20 (2.0%) |
| Surgical technique | |
| Open | 778 (76.3%) |
| Laparoscopic/robotic | 239 (23.4%) |
| Type of resection | |
| Enucleation | 107 (10.5%) |
| Classic PD | 129 (12.6%) |
| Pylorus preserving PD | 159 (15.6%) |
| Central pancreatectomy | 32 (3.1%) |
| Distal pancreatectomy | 576 (56.5%) |
| Total pancreatectomy | 17 (1.7%) |
| Perineural invasion | 169 (16.6%) |
| Major vascular resection | 49 (4.8%) |
| Lymphadenectomy | 858 (84.1%) |
| Number of lymph node retrieved | 10 (5–16) |
| Operation time (min) | 235 (185–315) |
| Blood loss (ml) | 200 (100–400) |
| Margin status | |
| R0 | 866 (84.9%) |
| R1 | 154 (15.1%) |
| Postoperative morbidity | 544 (53.3%) |
| Severe complication (III–V) | 225 (22.1%) |
| Adjuvant chemotherapy | 25 (2.5%) |
Recurrence pattern and timing
Among the 1020 patients who underwent curative resection for pNET, 154 (15.1%) patients developed tumor recurrence after a median follow-up of 34.7 months (IQR 12.0 – 62.7 months). Among patients who recurred, half of all recurrences occurred within two years after surgery (n = 82, 53.3%), while 72 (46.7%) patients experienced a later recurrence. The liver (n = 76, 49.4%) and pancreas (n = 35, 22.7%) were the most common sites of recurrence; other recurrent sites included the lungs (n = 2), distant lymph nodes (n = 2), peritoneum (n = 3), retroperitoneum (n = 1) and multiple organs (n = 34). The proportion of liver-only recurrence gradually increased from 54.3% in the first years after surgery to 61.5% from four to six years after surgery; in contrast, the proportion of pancreas-only recurrence decreased from 26.1% in the first year after surgery to 7.7% from four to six years after surgery (Fig. 1).
Figure 1.
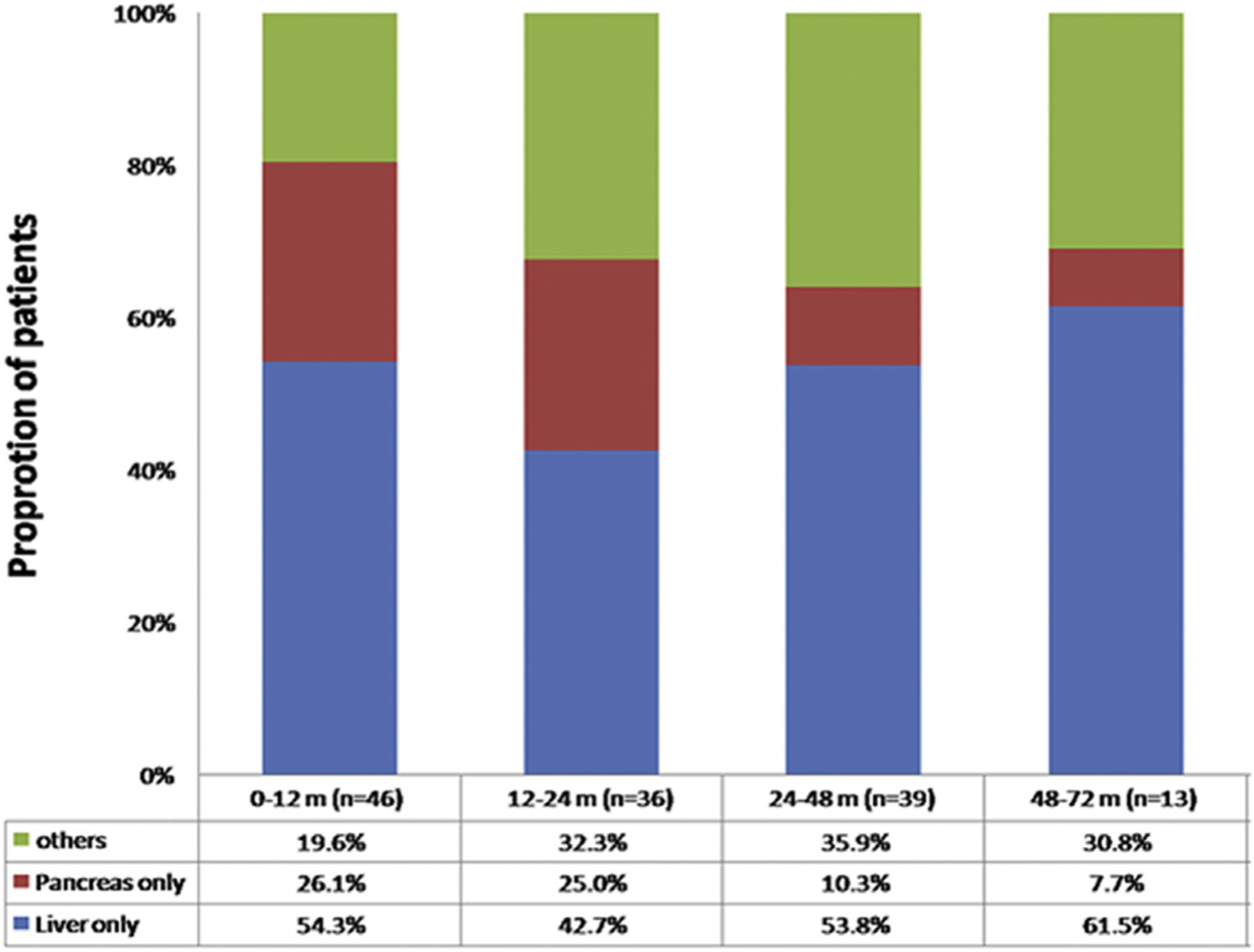
Recurrence patterns of pNET at different time periods
Among the 76 patients who developed liver-only recurrence, half of the patients recurred within two years after surgery (n = 40, 52.6%), whereas 36 (47.3%) recurred at a later time point (Fig. 2a). For patients who experienced pancreas-only recurrence, 21 patients recurred within 2 years after surgery; 14 patients recurred after two years with one patient recurring 10 years following surgery (Fig. 2a). Of note, the patient who experienced recurrent isolated pancreatic disease actually had a second pNET primary tumor that was distinct from the primary resection bed.
Figure 2.
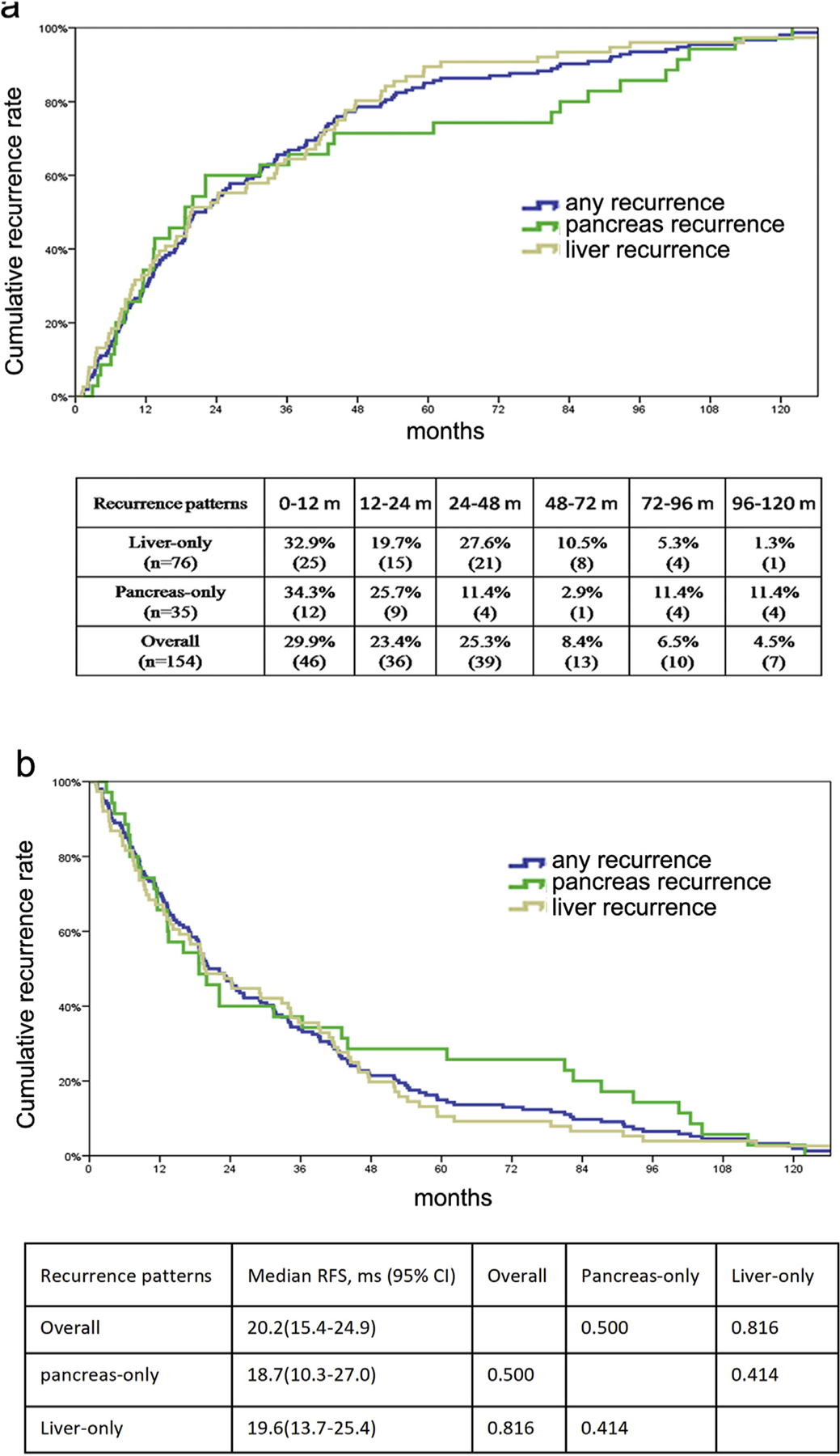
Cumulative recurrence incidence (a) and disease-free survival (b) among patients who experienced different recurrence patterns
Median RFS was 20.2 months among the 154 patients who experienced a recurrence. Among patients who had a liver-only recurrence, the median RFS was 19.6 months, which was comparable to the median RFS of 18.7 months among patients with pancreas-only recurrence (Fig. 2b).
Factors associated with patterns of recurrence
After adjusting for potential confounding factors in the Cox-proportional hazards model, an underlying genetic syndrome (HR 2.49, p = 0.015) and a Ki-67 > 20% (HR 5.29, p = 0.007) were identified as independent risk factors associated with anysite recurrence of pNET after curative resection (Supplementary Table 1). In contrast, multivariable analysis revealed that R1 versus R0 resection (HR 5.12, p = 0.003) was the only risk factor associated with a risk of pancreas-only recurrence after surgery (Table 2). In addition, on multivariable analysis, risk factors associated with increased likelihood of liver-only recurrence included Ki-67 index (both p < 0.05), presence of perineural invasion (HR 3.66, p = 0.003), and major vascular resection (HR 3.23, p = 0.029) (Table 3).
Table 2.
Risk factors of pancreas-only recurrence
| Variable | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Gender (F/M) | 1.06 (0.54–2.05) | 0.871 | ||
| Age (<65/≥65) | 1.17 (0.56–2.45) | 0.673 | ||
| Functional status | 1.20 (0.16–8.82) | 0.859 | ||
| Genetic syndrome | 3.59 (1.76–7.32) | 0.000 | 4.41 (1.34–14.52) | 0.015 |
| Symptomatic | 1.25 (0.63–2.46) | 0.524 | ||
| Ki-67 category | ||||
| <3% | Ref. | Ref. | ||
| 3–20% | 1.41 (0.54–3.65) | 0.484 | 2.12 (0.68–6.61) | 0.197 |
| >20% | 4.95 (1.06–23.01) | 0.042 | 1.00 | 0.992 |
| Tumor differentiation | ||||
| Well | Ref. | Ref. | ||
| Moderately | 0.90 (0.21–3.85) | 0.886 | 0.32 (0.04–2.77) | 0.302 |
| Poorly | 6.25 (1.45–26.90) | 0.014 | 1.18 | 1.000 |
| Largest tumor size (<3 cm/≥3 cm) | 2.36 (1.22–4.60) | 0.011 | 1.15 (0.33–3.99) | 0.825 |
| Lymph nodes metastasis | 1.61 (0.76–3.41) | 0.215 | ||
| Perineural invasion | 0.24 (0.03–1.79) | 0.163 | ||
| Lymph-vascular invasion | 2.34 (1.08–5.08) | 0.031 | 1.16 (0.33–4.13) | 0.818 |
| Surgical technique | 0.268 | |||
| Open | Ref. | |||
| Laparoscopic/robotic | 0.55 (0.19–1.58) | |||
| Major vascular resection | 3.14 (1.10–9.01) | 0.033 | 1.12 (0.13–9.45) | 0.919 |
| Final resection status | 0.002 | 0.003 | ||
| R0 | Ref. | Ref. | ||
| R1 | 3.16 (1.55–6.46) | 5.12 (1.74–15.40) | ||
| Adjuvant chemotherapy | 6.23 (1.87–20.72) | 0.003 | 2.16 (0.24–19.53) | 0.495 |
Table 3.
Risk factors of liver-only recurrence
| Variable | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Gender (F/M) | 0.96 (0.61–1.50) | 0.842 | ||
| Age (<65/≥65) | 0.77 (0.44–1.34) | 0.351 | ||
| Functional status | 0.05 (0.00–23.24) | 0.335 | ||
| Genetic syndrome | 0.52 (0.21–1.28) | 0.153 | ||
| Symptomatic | 2.24 (1.37–3.66) | 0.001 | 2.08 (0.77–5.63) | 0.149 |
| Ki-67 category | ||||
| <3% | Ref. | Ref. | ||
| 3–20% | 6.24 (2.89–13.58) | 0.000 | 2.85 (1.05–7.73) | 0.040 |
| >20% | 19.15 (6.92–53.02) | 0.000 | 6.98 (1.21–40.35) | 0.030 |
| Tumor differentiation | ||||
| Well | Ref. | Ref. | ||
| Moderately | 2.83 (1.55–5.18) | 0.001 | 2.34 (0.45–12.28) | 0.314 |
| Poorly | 5.51 (1.98–15.39) | 0.001 | 0.43 (0.05–3.73) | 0.443 |
| Largest tumor size (<3 cm/≥3 cm) | 6.72 (3.92–11.53) | 0.000 | 1.79 (0.67–4.76) | 0.245 |
| Lymph nodes metastasis | 3.40 (2.14–5.38) | 0.000 | 2.01 (0.88–4.60) | 0.098 |
| Perineural invasion | 5.83 (3.37–10.10) | 0.000 | 3.66 (1.53–8.72) | 0.003 |
| Lymphvascular invasion | 6.32 (3.53–11.30) | 0.000 | 0.50 (0.18–1.36) | 0.174 |
| Surgical technique | 0.002 | 0.287 | ||
| Open | Ref. | Ref. | ||
| Laparoscopic/robotic | 0.21 (0.08–0.56) | 0.45 (0.10–1.98) | ||
| Major vascular resection | 5.01 (2.80–9.00) | 0.000 | 3.23 (1.13–9.23) | 0.029 |
| Final resection status | 0.541 | |||
| R0 | Ref. | |||
| R1 | 1.21 (0.66–2.30) | |||
| Adjuvant chemotherapy | 6.49 (3.10–13.57) | 0.000 | 4.62 (1.41–15.08) | 0.011 |
Survival after recurrence
Perhaps not surprisingly, OS among the 154 patients who experienced a recurrence was shorter than OS among the 866 patients with no recurrence (10-year survival, recurrence 57.2% versus no recurrence 81.2%) (HR 1.67, 95% CI 1.12–2.52, p = 0.014) (Fig. 3a). Among the 154 patients who recurred, 48 patients underwent further surgical resection, while 106 patients received non-surgical treatment. Patients who underwent resection of the recurrence had a longer OS versus patients who were treated with non-surgical therapy (OS after recurrence, 49.3 versus 22.3 months, p = 0.026) (Fig. 3b). Of note, the OS of patients undergoing curative resection for pancreas-only recurrence or liver-only recurrence was comparable to the long-term outcomes of patients who experienced no recurrence (median OS, pancreas-only recurrence, 133.9 months; liver-only recurrence, not attained; no recurrence, 143.0 months, p = 0.499) (Fig. 3c).
Figure 3.
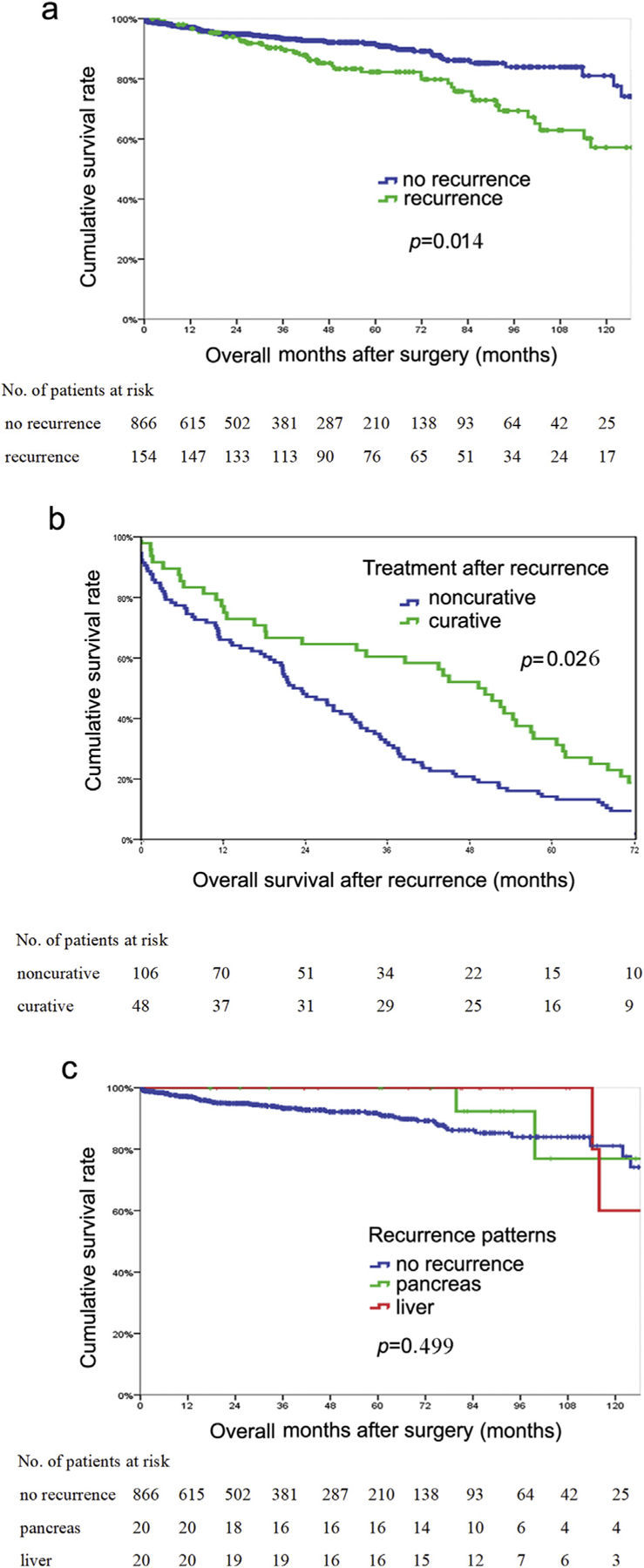
a, Overall survival of patients who experienced recurrence versus patients who had no recurrence; b, Survival among patients who underwent curative treatment for recurrence versus patients who received noncurative treatment for recurrence; c, Overall survival of patients who underwent curative treatment for liver-only recurrence or pancreatic recurrence versus patients who had no recurrence
Discussion
While resection is the cornerstone therapeutic approach for patients with pNET, recurrence can adversely impact quality of life and long-term outcomes.9,10,12 In fact, in the current study, 15% of the patients experienced a recurrence following curative-intent resection of pNET. Given the high incidence of recurrence, data on actual patterns and timing of pNET recurrence after curative resection may be important to plan the most cost-effective follow-up strategy, as well as guide appropriate subsequent therapy.11 In the current study, among patients who experienced a recurrence, more than half developed recurrence within two years after surgery with the risk of recurrence decreasing markedly over time. The most common sites of recurrence were the liver and pancreas, with the incidence of pancreatic recurrence gradually decreasing over time as the proportion of patients with liver recurrence increased. Interestingly, the risk of liver recurrence was largely associated with biological tumor characteristics, while pancreas recurrence was only associated with surgical margin status. In addition, in a subset of select patients who experienced a recurrence, repeat curative-intent re-resection of either liver or pancreas recurrence was associated with a reasonable long-term OS.
The liver was the site of roughly half of all recurrences after curative resection for pNET. In addition, the proportion of hepatic recurrence increased gradually over time following resection of pNET. Studies from our group and others had previously reported that the liver is the most common site for the development of NET metastasis.10,11,20–23 Specifically, patients with GEP-NET often develop liver metastasis during the course of the disease.21,23–25 While roughly half of patients will present with synchronous disease, it is estimated that close to 50% of patients will present with metachronous liver disease during the course of their life.22,26 Data from the current study demonstrated that patients who developed metachronous disease most commonly experienced recurrence within the first two years following resection of the primary pNET. Patients who develop NET liver metastasis can have a diverse in their clinical presentation.27 As such, appropriate follow-up and knowledge of the risk factors associated with recurrence are important to ensure timely identification of recurrent disease. The current report builds on previous research in that the data specifically linked biological factors, such as Ki-67, with a systemic pattern of recurrent disease (e.g. liver), as well as a shorter time to recurrence.15,28 Taken together, patients who underwent curative resection for pNET with certain clinicopathologic features, such as advanced tumor stage, higher Ki-67 index, perineural and vascular invasion, should be closely followed up, especially during the first two years after surgery.
A subset of patients recurred with pancreas-only disease after curative resection of pNET. Of note, locoregional pancreatic recurrence accounted for roughly one third of all early recurrences within two years of surgery. Rather than biological tumor characteristics, surgical margin status was the only factor associated with the risk factor of pancreatic only recurrent disease. The role of margin status on recurrence and OS has been somewhat controversial.5,10 The reason for these disparate results are likely multifactorial and may be related to most previous studies only examining OS – rather than RFS – as well as including other colinear variables in the prognostic models. Collectively, the data strongly suggest that achieving a free/negative surgical margin is important to improve the outcome of patients with pNET and a microscopically positive pNET surgical margin may warrant closer local surveillance for local recurrence.
In examining the time of recurrent disease following resection of pNET, the incidence of pancreatic recurrence gradually decreased over time as the proportion of patients with liver recurrence increased. These findings suggest that while most recurrences occur early within the first 1–2 years following resection of pNET, late recurrences can occur even 10 years following surgery. In addition, the fact that liver and systemic recurrences occurred later in the natural time course of the disease was consistent with their association with adverse biological factors. In contrast, the earlier pancreatic-only recurrences may have been more related to recurrent disease due to suboptimal margin clearance at the time of initial surgery. Another important finding of the current study was that re-resection of recurrent NET was demonstrated to be beneficial in some patients. These data are consistent with those reported by Bagante et al. who noted that statistical cure after surgery for NELM was possible and therefore hepatic resection should be strongly considered for patients with recurrent NET disease.29
The current study had several limitations. Although data were derived from a large multi-institutional database, which increased the sample size and generalizability of the results, there were possible inconsistency in patient selection, surgical procedures, postoperative surveillance and re-treatment of recurrences among the different centers. In addition, Moreover, the exact surveillance interval of each patient and each center varied, which might impact diagnosis and treatment of patients who developed recurrence.
In conclusion, recurrence after primary resection of pNETwas common. Most recurrences occurred early within the first two years following surgery, however a smaller subset of patients did experience a recurrence much later. Among patients who recurred, half had liver-only recurrence and a smaller number of patients had pancreas-only recurrence. Of note, while biological tumor factors were associated with liver recurrence, technical factors such as margin status were associated with pancreatic recurrence. Whereas the proportion of patients with liver recurrence gradually increased, the proportion of pancreas-only recurrence decreased over time following surgery. Data from the current study should serve to inform patient discussions regarding risk and timing of recurrence, as well as help tailor surveillance strategies following resection of pNET.
Supplementary Material
Footnotes
Conflicts of interest
None declared.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.hpb.2019.05.020.
References
- 1.Sandvik OM, Soreide K, Gudlaugsson E, Kvaloy JT, Soreide JA. (2016) Epidemiology and classification of gastroenteropancreatic neuroendocrine neoplasms using current coding criteria. Br J Surg 103:226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chi W, Warner RRP, Chan DL, Singh S, Segelov E, Strosberg J et al. (2018) Long-term outcomes of gastroenteropancreatic neuroendocrine tumors. Pancreas 47:321–325. [DOI] [PubMed] [Google Scholar]
- 3.Franko J, Feng W, Yip L, Genovese E, Moser AJ. (2010) Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg 14:541–548. [DOI] [PubMed] [Google Scholar]
- 4.Kuo EJ, Salem RR. (2013) Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann Surg Oncol 20: 2815–2821. [DOI] [PubMed] [Google Scholar]
- 5.Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY et al. (2008) Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg 247:490–500. [DOI] [PubMed] [Google Scholar]
- 6.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y et al. (2017) Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 3: 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Partelli S, Tamburrino D, Lopez C, Albers M, Milanetto AC, Pasquali C et al. (2016) Active surveillance versus surgery of nonfunctioning pancreatic neuroendocrine neoplasms </=2 cm in MEN1 patients. Neuroendocrinology 103:779–786. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Dey C, Kennecke H, Kocha W, Maroun J, Metrakos P et al. (2015) Consensus recommendations for the diagnosis and management of pancreatic neuroendocrine tumors: guidelines from a Canadian National Expert group. Ann Surg Oncol 22:2685–2699. [DOI] [PubMed] [Google Scholar]
- 9.Merath K, Bagante F, Beal EW, Lopez-Aguiar AG, Poultsides G, Makris E et al. (2018) Nomogram predicting the risk of recurrence after curative-intent resection of primary non-metastatic gastrointestinal neuroendocrine tumors: an analysis of the U.S. Neuroendocrine Tumor Study Group. J Surg Oncol 117:868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang XF, Wu Z, Cloyd J, Lopez-Aguiar AG, Poultsides G, Makris E et al. (2019) Margin status and long-term prognosis of primary pancreatic neuroendocrine tumor after curative resection: results from the US Neuroendocrine Tumor Study Group. Surgery 165:548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchegiani G, Landoni L, Andrianello S, Masini G, Cingarlini S, D’Onofrio M et al. (2018) Patterns of recurrence after resection for pancreatic neuroendocrine tumors: who, when, and where? Neuroendocrinology 108:161–171. [DOI] [PubMed] [Google Scholar]
- 12.Genc CG, Jilesen AP, Partelli S, Falconi M, Muffatti F, van Kemenade FJ et al. (2018) A new scoring system to predict recurrent disease in grade 1 and 2 nonfunctional pancreatic neuroendocrine tumors. Ann Surg 267:1148–1154. [DOI] [PubMed] [Google Scholar]
- 13.Partelli S, Javed AA, Andreasi V, He J, Muffatti F, Weiss MJ et al. (2018) The number of positive nodes accurately predicts recurrence after pancreaticoduodenectomy for nonfunctioning neuroendocrine neoplasms. Eur J Surg Oncol 44:778–783. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z, Tang LH, Klimstra DS. (2011) Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol 35:853–860. [DOI] [PubMed] [Google Scholar]
- 15.Boninsegna L, Panzuto F, Partelli S, Capelli P, Delle Fave G, Bettini R et al. (2012) Malignant pancreatic neuroendocrine tumour: lymph node ratio and Ki67 are predictors of recurrence after curative resections. Eur J Cancer 48:1608–1615. [DOI] [PubMed] [Google Scholar]
- 16.Toste PA, Kadera BE, Tatishchev SF, Dawson DW, Clerkin BM, Muthusamy R et al. (2013) Nonfunctional pancreatic neuroendocrine tumors <2 cm on preoperative imaging are associated with a low incidence of nodal metastasis and an excellent overall survival. J Gastrointest Surg 17:2105–2113. [DOI] [PubMed] [Google Scholar]
- 17.Sho S, Court CM, Winograd P, Toste PA, Pisegna JR, Lewis M et al. (2018. Oct 23) A prognostic scoring system for the prediction of metastatic recurrence following curative resection of pancreatic neuroendocrine tumors. J Gastrointest Surg 10.1007/s11605-018-4011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groot VP, Gemenetzis G, Blair AB, Ding D, Javed AA, Burkhart RA et al. (2018) Implications of the pattern of disease recurrence on survival following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg Oncol 25:2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M et al. (2016) ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 103: 153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaltsas GA, Besser GM, Grossman AB. (2004) The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev 25:458–511. [DOI] [PubMed] [Google Scholar]
- 21.Spolverato G, Bagante F, Aldrighetti L, Poultsides G, Bauer TW, Field RC et al. (2017) Neuroendocrine liver metastasis: prognostic implications of primary tumor site on patients undergoing curative intent liver surgery. J Gastrointest Surg 21:2039–2047. [DOI] [PubMed] [Google Scholar]
- 22.Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC et al. (2010) Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol 17:3129–3136. [DOI] [PubMed] [Google Scholar]
- 23.Spolverato G, Vitale A, Ejaz A, Kim Y, Cosgrove D, Schlacter T et al. (2015) Net health benefit of hepatic resection versus intraarterial therapies for neuroendocrine liver metastases: a Markov decision model. Surgery 158:339–348. [DOI] [PubMed] [Google Scholar]
- 24.Spolverato G, Bagante F, Aldrighetti L, Poultsides GA, Bauer TW, Fields RC et al. (2017) Management and outcomes of patients with recurrent neuroendocrine liver metastasis after curative surgery: an international multi-institutional analysis. J Surg Oncol 116:298–306. [DOI] [PubMed] [Google Scholar]
- 25.Spolverato G, Bagante F, Wagner D, Buettner S, Gupta R, Kim Y et al. (2015) Quality of life after treatment of neuroendocrine liver metastasis. J Surg Res 198:155–164. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y et al. (2000) Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 190:432–445. [DOI] [PubMed] [Google Scholar]
- 27.Mayo SC, Herman JM, Cosgrove D, Bhagat N, Kamel I, Geschwind JF et al. (2013) Emerging approaches in the management of patients with neuroendocrine liver metastasis: role of liver-directed and systemic therapies. J Am Coll Surg 216:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton NA, Liu TC, Cavatiao A, Mawad K, Chen L, Strasberg SS et al. (2012) Ki-67 predicts disease recurrence and poor prognosis in pancreatic neuroendocrine neoplasms. Surgery 152:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagante F, Spolverato G, Merath K, Postlewait LM, Poultsides GA, Mullen MG et al. (2017) Neuroendocrine liver metastasis: the chance to be cured after liver surgery. J Surg Oncol 115:687–695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


