Abstract
Background:
The adoption of spleen-preserving distal pancreatectomy (SPDP) for malignant disease such as pancreatic neuroendocrine tumors (pNETs) has been controversial. The objective of the current study was to assess the impact of SPDP on outcomes of patients with pNETs.
Methods:
Patients undergoing a distal pancreatectomy for pNET between 2002 and 2016 were identified in the US Neuroendocrine Tumor Study Group database. Propensity score matching (PSM) was used to compare short- and long-term outcomes of patients undergoing SPDP versus distal pancreatectomy with splenectomy (DPS).
Results:
Among 621 patients, 103 patients (16.6%) underwent an SPDP. Patients who underwent SPDP were more likely to have lower BMI (median, 27.5 [IQR 24.0–31.2] vs. 28.7 [IQR 25.7–33.6]; p = 0.005) and have undergone minimally invasive surgery (n = 56, 54.4% vs. n = 185, 35.7%; p < 0.001). After PSM, while the median total number of lymph nodes examined among patients who underwent an SPDP was lower compared with DPS (3 [IQR 1–8] vs. 9 [5–13]; p < 0.001), 5-year overall survival (OS) and recurrence-free survival (RFS) were comparable (OS: 96.8 vs. 92.0%, log-rank p = 0.21, RFS: 91.1 vs. 84.7%, log-rank p = 0.93). In addition, patients undergoing SPDP had less intraoperative blood loss (median, 100 mL [IQR 10–250] vs. 150 mL [IQR 100–400]; p = 0.001), lower incidence of serious complications (n = 13, 12.8% vs. n = 28, 27.5%; p = 0.014), and shorter length of stay (median: 5 days [IQR 4–7] vs. 6 days [IQR 5–13]; p = 0.049) compared with patients undergoing DPS.
Conclusion:
SPDP for pNET was associated with acceptable perioperative and long-term outcomes that were comparable to DPS. SPDP should be considered for patients with pNET.
Keywords: Spleen, Pancreatic neuroendocrine tumor, Distal pancreatectomy, Survival
Introduction
Although pancreatic neuroendocrine tumors (pNETs) are rare neoplasms of the gastrointestinal tract, the incidence of pNETs has been increasing over the last 3 decades [1]. In fact, the incidence of pNET currently is now roughly 0.48 per 100,000 persons [2]. While several non-surgical modalities such as systemic chemotherapy, somatostatin analogs, and liver-directed therapies have been employed for patients with advanced disease [3–8], resection remains the mainstay treatment for patients with resectable pNETs. Specifically, among patients with a body or tail lesion, distal pancreatectomy (DP) is the surgical procedure of choice performed either in an open or, more commonly, via a minimally invasive approach. In spite of improvements in surgical techniques, the incidence of overall morbidity and postoperative pancreatic fistula after DP for pNETs can be as high as 70 and 39%, respectively [9–12]. In turn, there has been interest in approaches to mitigate the risk of postoperative morbidity.
Spleen-preserving DP (SPDP) was first proposed by Mallet-Guy and Vachon in 1943 as a means to reduce the risk of post-splenectomy sepsis [13]. Shoup and colleagues [14] reported that SPDP was associated with a reduction in perioperative infectious complications, severe complications, and length of hospital stay compared with conventional DP. While SPDP may be appropriate for patients with benign or low-grade malignant disease, conventional DP with splenectomy (DPS) is recommended for patients with pancreatic adenocarcinoma to ensure an adequate oncologic operation [15]. The adoption of SPDP for diseases such as pNETs has, however, been a topic of particular controversy. While the European Neuroendocrine Tumor Society (ENETS) has noted that atypical resections are acceptable in the management of non-functional, well-demarcated, small pNETs, the general role of SPDP for a broader range of pNETs has not been defined [16]. Previous data on SPDP included a variety of tumors – not only pNETs [14], and therefore there remains a lack of evidence. In particular, the role of organ-preserving surgery such as SPDP from an oncological aspect among patients with pNETs has not been studied. As such, the objective of the current study was to define the impact of SPDP on long-term survival of patients with pNETs using a large multi-institutional database. In addition, we sought to examine whether the total number of lymph nodes examined (TNLE) varied among patients with SPDP versus DPS after controlling for other clinical and perioperative factors.
Methods
Study Population and Data Collection from the US-NETSG Database
Patients undergoing DP for pNET between 2002 and 2016 were identified in the US Neuroendocrine Tumor Study Group (US-NETSG) database that included data from 8 tertiary institutions (The Ohio State University Comprehensive Cancer Center, Columbus, OH; University of Michigan, Ann Arbor, MI; Stanford University, Palo Alto, CA; Virginia Mason Medical Center, Seattle, WA; Winship Cancer Institute, Emory University, Atlanta, GA; Washington University, School of Medicine, St. Louis, MO; University of Wisconsin, School of Medicine and Public Health, Madison, WI; Vanderbilt University, Nashville, TN, USA). All patients included in the study had a histologically proven pNET and underwent a curative intent DP. Patients with metastatic disease at presentation, as well as individuals who had missing follow-up data, macroscopically positive surgical margins (R2 resection), and underwent an enucleation were excluded from the analysis. The study was approved by the Institutional Review Board of all participating institutions.
Patient demographic and clinicopathologic data were recorded in a prospectively maintained database. Patient demographic and clinicopathologic data included age, sex, race, body mass index (BMI), ASA (American Society of Anesthesiologists) classification, comorbidities, symptomatic status, functional status, surgical approach, tumor size and number, tumor location, number of lymph nodes (LNs) examined, LN metastasis (LNM), tumor grade, lymph-vascular and perineural invasion. Tumor location, body or tail, was defined according to the AJCC 8th guidelines [17]. Specifically, tumors of the body of the pancreas were defined as those arising between the left edge of the superior mesenteric-portal vein confluence and the left edge of the aorta. In contrast, tumors of the tail of the pancreas were those arising to the left of the left edge of the aorta. Functional tumors were defined as lesions with hormone overproduction (i.e., insulinoma, gastrinoma, somatostatinoma, and VIPoma) [18].
Statistical Analysis
Descriptive statistics were presented as median (interquartile range [IQR]) and frequency (%) for continuous and categorical variables, respectively. Continuous variables were compared with the Mann-Whitney U or Kruskal-Wallis tests, as appropriate. Categorical variables were compared with the χ2 test or Fisher’s exact test, as appropriate. All the analyses were performed with multivariate normal imputation method for missing data [19]. To overcome selection bias, a 1:1 nearest neighbor propensity score matching (PSM) was performed to compare outcomes among patients undergoing DP with and without splenectomy based on age, sex race, BMI, ASA classification, comorbidity, surgical approach, and adjuvant therapy, as well as tumor behaviors such as symptomatic status, functional status, tumor location, tumor size, tumor differentiation, Ki-67, and lympho-vascular and perineural invasion. Statistical significance was assessed at α = 0.05 (two-tailed). Logistic regression model was performed to identify independent factors that predicted use of SPDP. Cox regression model was used to identify independent predictors of recurrence-free survival (RFS). Variables used in the multivariable analysis were selected from a number of possible perioperative predictors based on a stepwise selection method (forward selection method using the lowest Bayesian information criterion). A sensitivity analysis was also performed only with complete cases. Overall survival (OS) and RFS were calculated by creating Kaplan-Meier curves and differences were evaluated using the log-rank test. OS was defined as the time interval from the date of surgery to the date of death, whereas RFS was defined as time from the date of surgery to tumor recurrence. All statistical analyses were performed using SPSS, version 25 (IBM Corp., Armonk, NY, USA) along with JMP statistical package version 14 (SAS Institute Inc., Cary, NC, USA).
Results
Patient Characteristics with and without Splenectomy
Among 621 patients who met the inclusion criteria and were included in the final analytic cohort, 103 patients (16.6%) underwent an SPDP; the Warshaw method was exclusively utilized for SPDP. Overall, the proportion of patients who underwent an SPDP did not increase over time (p = 0.62) (online suppl. Fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000506399). In particular, the proportion of patients with SPDP was 14.6% during the period 2002–2006 versus 14.8% during 2012–2016. The frequency of SPDP utilization varied from 9.1 to 28.8% across the participating hospitals (p = 0.006). Among patients undergoing an SPDP, median age was 58 (IQR 48–66) and most patients were male (n = 55, 53.4%) and white (n = 89, 86.4%); roughly one-half of patients had ASA of 3–4 (n = 51, 49.5%). Patients who underwent an SPDP were more likely to have a lower BMI (median, 27.5 [IQR 24.0–31.2] vs. 28.7 [IQR 25.7–33.6]; p = 0.005), undergone a minimally invasive approach (n = 56, 54.4% vs. n = 185, 35.7%; p < 0.001), have a smaller tumor size (median, 1.6 [IQR 1.1–2.3] vs. 2.4 [IQR 1.5–4.5]; p < 0.001), and have tumors with Ki-67 <3% (n = 75, 72.8% vs. n = 311, 60.0%; p = 0.019). In contrast, lymph-vascular invasion (n = 12, 11.7% vs. n = 162, 31.3%; p < 0.001) was more common among patients who underwent DPS (Table 1). Additionally, among 83 patients with a functional tumor, 52 patients (62.7%) were diagnosed with insulinoma, while the remaining patients had other functional tumors (n = 31, 37.3%). In multivariable logistic regression analysis, tumor size ≤2 cm (odds ratio [OR] 2.85; 95% confidence interval [CI], 1.78–4.54), minimally invasive approach (OR 1.94; 95% CI, 1.24–3.03), and lower BMI (OR 1.06; 95% CI, 1.02–1.10) were each associated with a higher likelihood of SPDP.
Table 1.
Demographic and patient characteristics in the entire cohort before and after propensity score matching (n = 621)
| Variable | Before PSM |
After PSM |
||||
|---|---|---|---|---|---|---|
| SPDP (n = 103) | DPS (n = 518) | p | SPDP (n = 102) | DPS (n = 102) | p | |
| Age* | 58 (48–66) | 59 (49–65) | 0.81 | 59 (49–65) | 58 (47–66) | 0.71 |
| Sex (male) | 55 (53.4%) | 283 (54.6%) | 0.83 | 55 (53.9%) | 59 (57.8%) | 0.67 |
| Race | 0.66 | 0.67 | ||||
| White | 89 (86.4%) | 436 (84.2%) | 88 (86.3%) | 91 (89.2%) | ||
| Non-white | 14 (13.6%) | 82 (15.8%) | 14 (13.7%) | 11 (10.8%) | ||
| BMI* | 27.5 (24.0–31.2) | 28.7 (25.7–33.6) | 0.005 | 27.4 (24.0–31.2) | 27.4 (24.5–33.0) | 0.47 |
| ASA classification | 0.13 | 1.00 | ||||
| 1–2 | 52 (50.5%) | 219 (42.3%) | 52 (51.0%) | 52 (51.0%) | ||
| 3–4 | 51 (49.5%) | 299 (57.7%) | 50 (49.0%) | 50 (49.0%) | ||
| Diabetes | 22 (21.4%) | 122 (23.6%) | 22 (21.6%) | 24 (23.5%) | 0.87 | |
| Hypertension | 38 (36.9%) | 245 (45.6%) | 0.065 | 37 (36.3%) | 43 (42.2%) | 0.47 |
| Current smoking | 13 (12.6%) | 76 (14.7%) | 0.65 | 13 (12.8%) | 11 (10.8%) | 0.66 |
| Symptomatic | 44 (42.7%) | 273 (52.7%) | 0.067 | 44 (43.1%) | 40 (39.2%) | 0.67 |
| Functional status | 0.75 | 0.84 | ||||
| Nonfunctional | 88 (85.4%) | 450 (86.9%) | 87 (85.3%) | 89 (87.3%) | ||
| Functional | 15 (14.6%) | 68 (13.1%) | 15 (14.7%) | 13 (12.7%) | ||
| MI approach | 56 (54.4%) | 185 (35.7%) | <0.001 | 56 (54.9%) | 55 (53.9%) | 1.00 |
| Primary location | 0.20 | 1.00 | ||||
| Body | 26 (25.2%) | 165 (31.9%) | 26 (25.5%) | 27 (26.5%) | ||
| Tail | 77 (74.8%) | 353 (68.2%) | 76 (74.5%) | 75 (73.5%) | ||
| Tumor size* | 1.6 (1.1–2.3) | 2.4 (1.5–4.5) | <0.001 | 1.6 (1.1–2.3) | 1.7 (1.2–2.3) | 0.52 |
| Multiple tumor | 11 (10.7%) | 51 (9.9%) | 0.86 | 11 (10.8%) | 14 (13.7%) | 0.67 |
| AJCC T stage | <0.001 | 0.81 | ||||
| T1 | 66 (64.1%) | 211 (40.7%) | 66 (64.7%) | 69 (67.7%) | ||
| T2 | 29 (28.2%) | 189 (36.5%) | 28 (27.5%) | 24 (23.5%) | ||
| T3 | 8 (7.8%) | 117 (22.6%) | 8 (7.8%) | 9 (8.8%) | ||
| T4 | 0 | 1 (2.0%) | 0 | 0 | ||
| Tumor differentiation | 0.78 | 0.77 | ||||
| Well | 97 (94.2%) | 459 (88.6%) | 97 (95.1%) | 95 (93.1%) | ||
| Moderate | 6 (5.8%) | 50 (9.7%) | 5 (4.9%) | 7 (6.9%) | ||
| Poor | 0 | 9 (1.7%) | 0 | 0 | ||
| Ki-67 | 0.019 | 0.88 | ||||
| <3% | 75 (72.8%) | 311 (60.0%) | 75 (73.5%) | 73 (71.6%) | ||
| 3%–20% | 28 (27.2%) | 189 (36.5%) | 27 (26.5%) | 29 (28.4%) | ||
| >20% | 0 | 18 (3.5%) | 0 | 0 | ||
| Lymph-vascular invasion | 12 (11.7%) | 162 (31.3%) | <0.001 | 12 (11.8%) | 8 (7.8%) | 0.48 |
| Perineural invasion | 9 (8.7%) | 83 (16.0%) | 0.068 | 9 (8.8%) | 6 (5.9%) | 0.59 |
| Adjuvant therapy | 8 (7.8%) | 38 (7.3%) | 0.84 | 8 (7.8%) | 8 (7.8%) | 1.00 |
Median (IQR). PSM, propensity score matching; SPDP, spleen-preserving distal pancreatectomy; DPS, distal pancreatectomy with splenectomy; BMI, body mass index; ASA, American Society of Anesthesiologists; MI, minimally invasive; IQR, interquartile range.
Comparison of Long-Term Outcomes with and without Splenectomy after PSM
To minimize potential confounding, PSM was utilized to create two matched cohorts of 102 patients. Following PSM, the patient cohorts were similar in regards to demographics, tumor characteristics, and pathological findings (Table 1). With a median follow-up of 35.7 months (IQR 11.6–67.5), 5-year OS and RFS after DP for pNET were 94.3 and 87.9%, respectively. In assessing the PSM cohort, there was no difference in OS among patients relative to the performance of splenectomy at the time of surgery (5-year OS, SPDP: 96.8% vs. DPS: 92.0%, log-rank p = 0.21) (Fig. 1a). RFS was also comparable among patients who did versus did not undergo splenectomy (5-year OS, SPDP: 91.1% vs. DPS: 84.7%, log-rank p = 0.93) (Fig. 1b). Specifically, 10 patients (9.9%) who underwent an SPDP experienced a recurrence versus 9 patients (8.9%) after DPS (p = 1.0); the incidence of local recurrence was also comparable (n = 4, 40.0% vs. n = 4, 44.4%; p = 0.84). Of note, no patient experienced a local recurrence in the splenic hilum after SPDP.
Fig. 1.
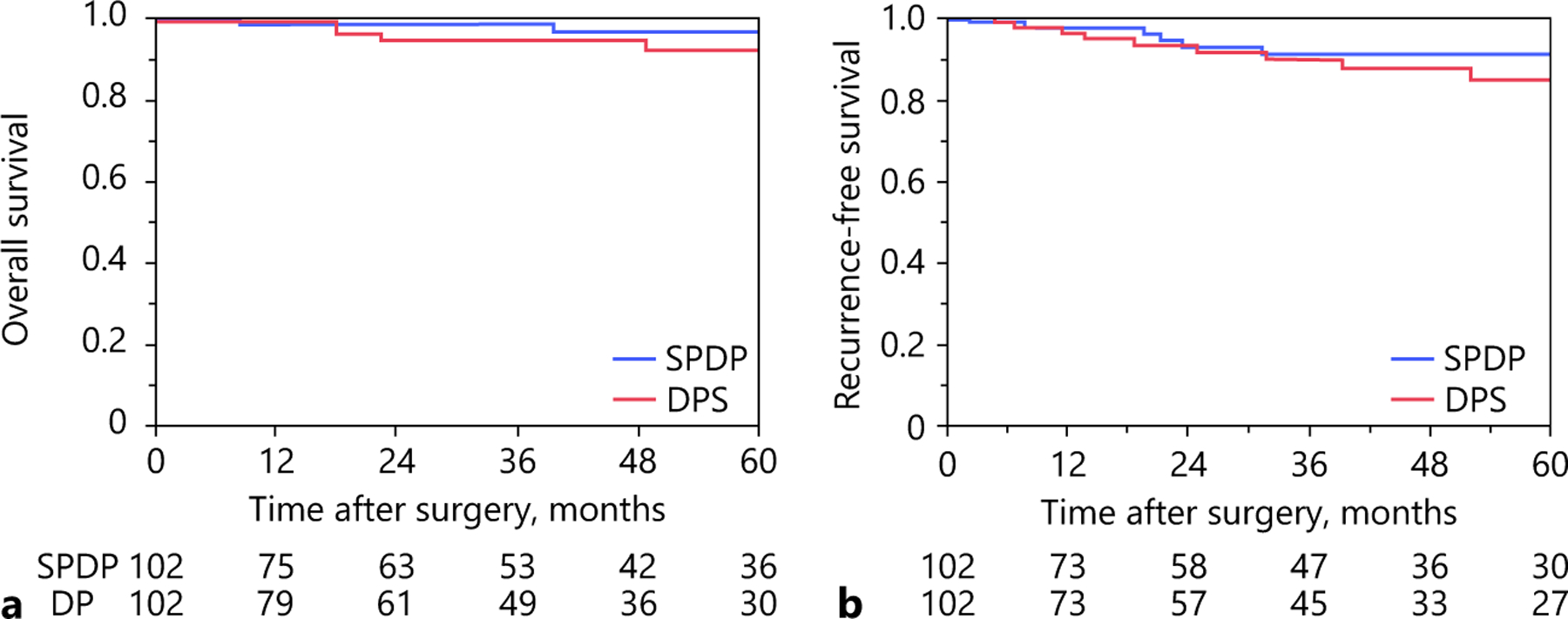
Propensity score-matched overall (a) and recurrence-free survival (b) after distal pancreatectomy with and without splenectomy.
Clinicopathological Factors Associated with Poor RFS
In analyzing RFS, several factors were associated with worse RFS on bivariate analyses (Table 2). Specifically, symptomatic tumor (hazard ratio [HR], 1.83; 95% CI, 1.21–2.77), tumor size >2 cm (HR, 4.61; 95% CI, 2.73–7.79), Ki-67 >3% (HR, 3.77; 95% CI, 2.46–5.80), LNM (HR, 3.19; 95% CI, 2.13–4.79), R1 margin status (HR, 1.86; 95% CI, 1.14–3.02), and lymph-vascular invasion (HR, 4.27; 95% CI, 2.83–6.45) were each associated with a worse RFS. On multivariable Cox regression analysis, tumor size >2 cm (HR, 2.64; 95% CI, 1.50–4.67) and Ki-67 >3% (HR, 1.93; 95% CI, 1.18–3.14) were associated with a higher hazard of recurrence among preoperatively measurable variables. In addition to multiple imputation, to account for possible differences due to missing data, sensitivity analyses were also performed only with complete cases. On multivariate Cox regression analysis that only included complete cases, tumor size >2 cm, Ki-67 >3%, and lymph-vascular invasion remained associated with a higher chance of recurrence (online suppl. Table 1).
Table 2.
Cox regression analysis of factors associated with recurrence-free survival after distal pancreatectomy for pNET in the entire cohort (n = 621)
| Variable | Bivariate |
Multivariable |
||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age >65 years | 1.10 (0.68–1.78) | 0.69 | ||
| Sex | 0.91 | |||
| Female | Ref. | |||
| Male | 1.02 (0.68–1.53) | |||
| Race | 0.16 | |||
| Non-white | Ref. | |||
| White | 1.59 (0.83–3.07) | |||
| Symptomatic | 0.004 | |||
| No | Ref. | |||
| Yes | 1.83 (1.21–2.77) | |||
| Primary location | 0.85 | |||
| Body | Ref. | |||
| Tail | 1.04 (0.67–1.63) | |||
| Tumor size | <0.001 | <0.001 | ||
| ≤2 cm | Ref. | Ref. | ||
| >2 cm | 4.61 (2.73–7.79) | 2.64 (1.50–4.67) | ||
| Multiple tumors | 0.86 (0.45–1.66) | 0.65 | ||
| Tumor differentiation | 0.13 | |||
| Well | Ref. | |||
| Moderate to poor | 1.57 (0.88–2.83) | |||
| Ki-67 | <0.001 | 0.008 | ||
| <3% | Ref. | Ref. | ||
| ≥3% | 3.77 (2.46–5.80) | 1.93 (1.18–3.14) | ||
| LNM | 3.19 (2.13–4.79) | <0.001 | ||
| Margin status | 0.012 | |||
| R0 | Ref. | |||
| R1 | 1.86 (1.14–3.02) | |||
| Lymph-vascular invasion | 4.27 (2.83–6.45) | <0.001 | 2.23 (1.39–3.57) | <0.001 |
| Perineural invasion | 1.55 (0.94–2.57) | 0.087 | ||
Bold values indicate preoperatively measurable variables through imaging or biopsy. HR, hazard ratio; LNM, lymph node metastasis.
Among the 204 patients included in the PSM cohort, 121 patients (59.3%) had neither of these risk factors, while 83 patients (40.7%) had at least one risk factor. There was no difference in RFS whether patients did or did not undergo splenectomy among those patients without a risk factor (5-year RFS, SPDP: 98.2% vs. DPS: 93.2%, log-rank p = 0.58) (Fig. 2a). Similarly, among patients with tumor size >2 cm or Ki-67 >3%, RFS was comparable among patients who underwent SPDP versus DPS (5-year RFS, SPDP: 100.0% vs. DPS: 81.0%, log-rank p = 0.34). In addition, splenectomy did not impact RFS among patients with both tumor size >2 cm and Ki-67 >3% (5-year RFS, SPDP: 62.2% vs. DPS: 60.0%, log-rank p = 0.93) (Fig. 2b, c).
Fig. 2.
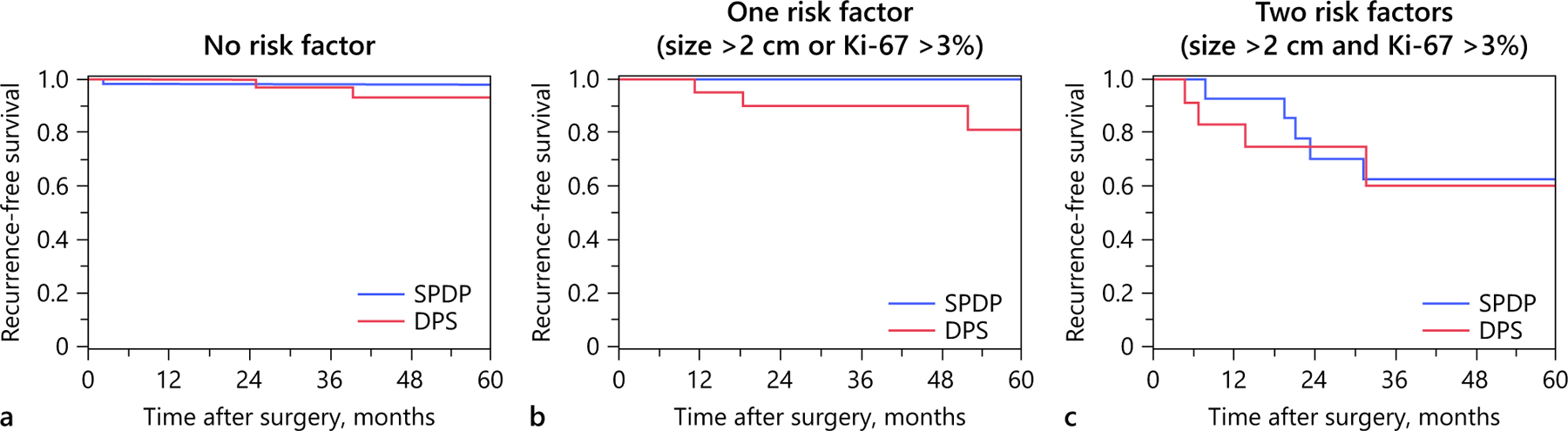
Propensity score matched recurrence-free survival after distal pancreatectomy with and without splenectomy stratified by number of risk factors for recurrence.
Comparison of Short-Term Outcomes with and without Splenectomy after PSM
After PSM, median TNLE among patients who underwent an SPDP was lower compared with DPS (3 [IQR 1–8] vs. 9 [5–13]; p < 0.001) (Table 3). When stratified by surgical approach and splenectomy, median number of LNs examined was highest among patients undergoing a minimally invasive DPS (9 [IQR 2–13]) followed by open DPS (8 [IQR 3–12]), whereas the median number of LNs examined was equivalent among patients undergoing an SPDP (minimally invasive: 3 [IQR 1–7] vs. open: 3 [IQR 1–9]; p = 0.66) (Fig. 3).
Table 3.
Comparison of operative factors and short-term outcomes among patients undergoing distal pancreatectomy with and without splenectomy after propensity score matching
| Variable | SPDP (n = 102) | DPS (n = 102) | p |
|---|---|---|---|
| Operative factors | |||
| Operative time, min* | 210 (181–249) | 214 (179–282) | 0.51 |
| Blood loss, mL* | 100 (10–250) | 150 (100–400) | 0.001 |
| Blood transfusion, U | 0 | 5 (4.9%) | 0.059 |
| Complication | |||
| Any complications | 46 (45.1%) | 64 (62.8%) | 0.017 |
| Serious complications | 13 (12.8%) | 28 (27.5%) | 0.014 |
| Pulmonary embolism | 2 (2.0%) | 3 (2.9%) | 1.00 |
| Pneumonia | 1 (1.0%) | 8 (7.8%) | 0.035 |
| UTI | 1 (1.0%) | 3 (2.9%) | 0.62 |
| DVT | 1 (1.0%) | 3 (2.9%) | 0.62 |
| Superficial SSI | 3 (2.9%) | 8 (7.8%) | 0.21 |
| Deep SSI | 1 (1.0%) | 9 (8.8%) | 0.019 |
| Organ space SSI | 7 (7.2%) | 13 (13.4%) | 0.24 |
| Wound dehiscence | 0 | 5 (4.9%) | 0.059 |
| Postoperative bleeding | 0 | 5 (4.9%) | 0.059 |
| Pancreatic fistula | 23 (22.6%) | 30 (29.4%) | 0.34 |
| Pancreatic fistula ≥ grade BC | 10 (9.8%) | 13 (12.8%) | 0.66 |
| Sepsis | 1 (1.0%) | 3 (3.1%) | 0.62 |
| Return to OR | 0 | 1 (1.0%) | 1.00 |
| Mortality | 0 | 1 (1.0%) | 1.00 |
| LOS* | 5 (4–7) | 6 (4–8) | 0.049 |
| Readmission | 16 (15.7%) | 24 (23.5%) | 0.22 |
| Number of LNs examined* | 3 (1–8) | 9 (5–13) | <0.001 |
| Patients with LN metastasis | 9 (8.8%) | 5 (4.9%) | 0.41 |
| Number of LN metastasis | 0 (0–0) | 0 (0–0) | 0.28 |
| R1 margin status | 11 (10.8%) | 10 (10.0%) | 0.86 |
Median (IQR). SPDP, spleen-preserving distal pancreatectomy; DPS, distal pancreatectomy with splenectomy; UTI, urinary tract infection; DVT, deep vein thrombosis; SSI: surgical site infection; LN, lymph node; OR, operating room; LOS, length of hospital stay.
Fig. 3.
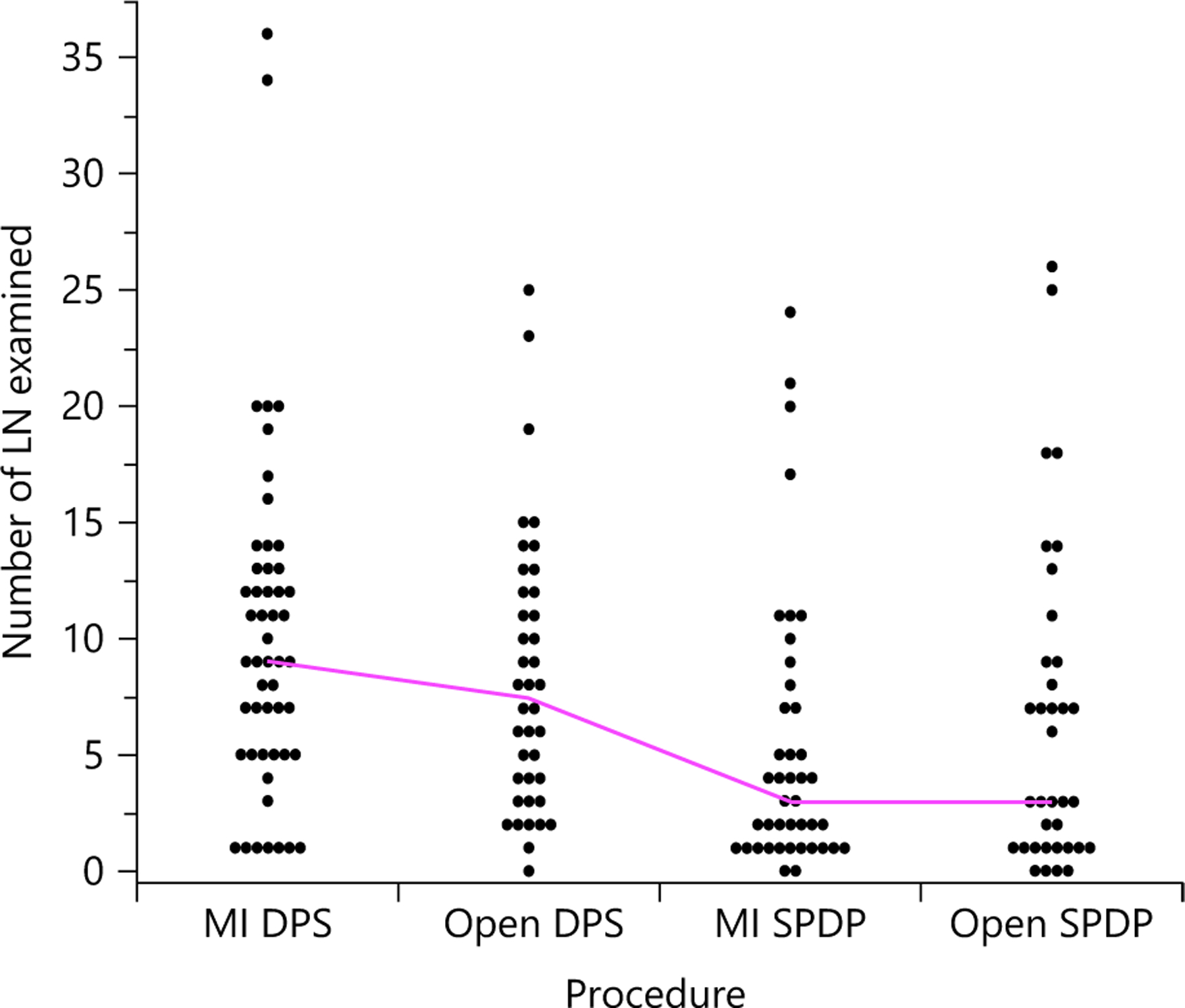
Total number of lymph nodes (LN) examined stratified by surgical approach and splenectomy in the propensity score-matched cohort. Median values displayed, as well as individual patient numbers. MI, minimally invasive.
Patients undergoing an SPDP also had less intraoperative blood loss (median, 100 mL [IQR 10–250] vs. 150 mL [IQR 100–400]; p = 0.001) and a lower incidence of any complications (n = 46, 45.1% vs. n = 64, 62.8%; p = 0.017), serious complications (n = 13, 12.8% vs. n = 28, 27.5%; p = 0.014), as well as deep surgical site infection (n = 1, 1.0% vs. n = 9, 8.8%; p = 0.019) (Table 3). In addition, median length of stay (LOS) was shorter among patients who underwent SPDP (5 days [IQR 4–7] vs. 6 days [IQR 4–8]; p = 0.049). The proportion of patients with an R1 resection was similar regardless of splenectomy (n = 11, 10.8% vs. n = 10, 10.0%; p = 0.86). Among patients undergoing a DPS, no post-splenectomy sepsis was observed during the follow-up period.
Discussion
DP can be performed with splenectomy or with splenic preservation. Several studies have suggested the feasibility and potential benefit of splenic preservation during DP including the preservation of immune function and reduction of the risk of overwhelming post-splenectomy infection, intraoperative blood loss, infections, and other complications related to the splenectomy procedure itself [14, 20, 21]. A splenic preserving technique has not been endorsed for pancreatic adenocarcinoma to avoid an inadequate oncologic operation [15]. In contrast, the adoption of SPDP for premalignant and small malignant tumors remains controversial. Specifically, a systematic review by Jain et al. [11] noted the use of SPDP for pNET varied considerably, ranging from 9 to 16%. Most previous studies have only investigated the association of SPDP with perioperative outcomes (e.g., intraoperative blood loss, postoperative pancreatic fistula, and LOS), yet have failed to examine oncologic outcomes such as TNLE or recurrence. As such, the current study was important because we specifically evaluated the effect of SPDP on oncologic metrics, including long-term survival, using a large multi-institutional database consisting exclusively of patients with pNET. Interestingly, 1 in 6 patients (16.6%) who underwent DP had a splenic preservation, with the proportion of patients undergoing SPDP did not change over time. Of note, although median TNLE was lower among patients who underwent an SPDP (3 [IQR 1–8] vs. 9 [5–13]; p < 0.001), OS and RFS were similar regardless of splenic preservation (log-rank p > 0.05). In addition, SPDP was associated with comparable RFS and DFS even among patients who had tumors with more aggressive features such as tumor size >2 cm and Ki-67 >3%. In contrast, SPDP was associated with less operative blood loss, as well as a lower incidence of postoperative morbidity including any or serious complications and a shorter LOS.
While organ-preserving surgical procedures may be associated with better perioperative outcomes, the impact on oncologic outcomes such as R0 margin status, as well as RFS has not been examined. In a study investigating patients with gastric cancer, Harrison et al. [22] reported that proximal versus total gastrectomy did not affect long-term survival. Other studies have noted that patients with pNET who were treated with organ/parenchymal preserving procedures such as enucleation had a similar long-term survival compared with patients who underwent conventional pancreatectomy for pNET [23, 24]. The impact of SPDP relative to oncologic outcomes among patients with pNET has, however, not been previously examined. As such, to the best of our knowledge, the current study is the first to evaluate the effect of SPDP on oncologic and long-term outcomes of patients with pNET. After PSM to mitigate possible confounding, patients who underwent SPDP versus DPS had a comparable likelihood of an R0 resection (10.8 vs. 10.0%), yet TNLE was lower among patients who had an SPDP versus DPS (3 vs. 9). Interestingly, in a single center series reported by Parekh et al. [25], the investigators noted that patients who underwent DP with or without splenectomy had a comparable median TNLE (median: 4; range, 0–17 and 0–7, respectively). The reason for the disparate results is undoubtedly multifactorial, yet may be related to the unexpectedly low TNLE among patients who underwent DPS in the Parekh et al. report [25]. Of note, in the study by Parekh et al. [25], the proportion of patients who had at least one LN examined did, however, differ (DPS: 79.6% vs. SPDP: 54.5%). The TNLE may be important to stage patients adequately. Our group previously reported that a TNLE of at least 8 LN was necessary in order to adequately assess the nodal basin and avoid “missing” LNM [26]. As with other pancreatic and gastrointestinal tumors, the odds of LNM likely depends not only on TNLE but also tumor size and tumor grade/Ki-67 index [27–29]. That said, the data suggest that SPDP may be associated with a lower TNLE and, perhaps, not as comprehensive a nodal assessment of the regional nodal basin as DPS.
Both RFS and OS were comparable among patients with pNET independent of surgical approach. Specifically, patients who underwent SPDP had a similar long-term prognosis and a comparable very low incidence of local or distant recurrence versus patients who had DPS. Specifically, 5-year OS was very favorable regardless of the performance of splenectomy (SPDP: 96.8% vs. DPS: 92.0%), suggesting that surgical approach did not impact long-term outcomes of patients with pNET. The indolent nature of the disease was reflected in an excellent 5-year RFS regardless of splenic preservation (SPDP: 91.1% vs. DPS: 84.7%). Interestingly, the comparable long-term outcomes were attained despite differences in TNLE among patients undergoing SPDP versus DPS. While LN involvement has been demonstrated to be prognostic in some studies [26, 29, 30], other data have suggested that TNLE and LNM may not be as prognostically important among patients with pNET that have other favorable features such as ≤2 cm, Ki-67 <3% [31]. To this point, Mao et al. [32] reported that lymphadenectomy had no additional therapeutic benefit among patients undergoing resection for pNETs of the pancreatic body or tail. In addition, similar to the current study, Shoup et al. [14] had reported comparable OS among patients with pNET undergoing splenectomy versus splenic preservation after adjustment for tumor characteristics. Of note, in the current study, the comparable long-term outcomes for splenic preservation persisted even among patients with pNET >2 cm and Ki-67 >3%. As such, SPDP can be considered to have comparable RFS and OS outcomes as DPS for patients with pNET and, in turn, splenic preservation should be considered when technically feasible without a concern for adverse oncologic outcomes.
SPDP is perhaps not utilized as much because surgeons may consider the approach more difficult and time-consuming than conventional DP due to the additional dissection to the left of the pancreas to preserve the splenic vessels [33]. In fact, some surgeons have argued that SPDP is a more tedious procedure with a greater chance of adjacent vascular/organ injury [34, 35]. Data from the current study suggested, however, that SPDP was, in fact, safe – as SPDP was associated with less operative blood loss compared with DPS even after PSM. In addition, patients who underwent SPDP generally had improved postoperative outcomes such as a lower incidence of postoperative serious complications, deep surgical site infection, as well as shorter LOS. These findings were consistent with data in a meta-analysis reported by Pendola and colleagues [21], which included a wide range of pancreatic tumors. In this study, SPDP was associated with less operative blood loss, as well as a lower incidence of fluid collection/abscess and postoperative splenic and portal vein thrombosis [21]. Taken together, the data would strongly suggest that splenic preservation as part of DP for pNETs was not only feasible, but also associated with comparable or even better perioperative outcomes.
The current study had several limitations that should be considered when interpreting the results. Similar to other studies, while PSM reduced bias secondary to confounding, PSM cannot fully adjust for all potential differences in SPDP versus DPS groups. In addition, while the multi-center collaboration improved sample size and generalizability of the results, potential inconsistencies in patient selection, pathologic assessment, use of non-surgical therapies, as well as postoperative surveillance may have existed. Although the present study demonstrated acceptable perioperative and long-term outcomes among patients undergoing SPDP for pNETs, there might be pNET patients where SPDP may not be appropriate. Specifically, SPDP should not be considered for patients with tumors invading the splenic vessels or tumors with suspicious LNs in the splenic hilum. In addition, SPDP may not be recommended for patients with large tumors in the tip of the pancreatic tail since this approach may increase the likelihood of positive resection margins. Finally, given that only the Warshaw method was utilized at the centers comprising the US Neuroendocrine Study Group, it was not feasible to assess the impact of splenic vessel preservation (i.e., Kimura method) on long-term outcomes [36, 37]. Future studies should compare the outcomes of patients undergoing SPDP with or without splenic vessel preservation (i.e., Kimura vs. Warshaw methods).
In conclusion, splenic preservation for pNET was relatively uncommon even at major tertiary hepatopancreatic centers as only 1 in 6 patients underwent SPDP. SPDP was more common among patients with pNET ≤2 cm, as well as among patients with a lower BMI. On PSM, while TNLE was lower among patients who had SPDP, other oncologic outcomes such as RFS and OS were comparable among patients who underwent SPDP versus DPS. SPDP was demonstrated to be feasible and safe, as patients who underwent SPDP had lower intraoperative blood loss and a lower incidence of postoperative complications compared with patients who had DPS. These data support consideration of SPDP for patients with pNET as this organ-preserving approach had similar perioperative and long-term outcomes compared with pancreatectomy associated with spleen removal.
Supplementary Material
Funding Sources
None.
Footnotes
Statement of Ethics
Patients have given their informed consent and the study protocol has been approved by the committees on human research of all participating institutions. The research was performed in accordance with the Helsinki declaration.
Disclosure Statement
There are no conflicts of interest.
References
- 1.Panzuto F, Boninsegna L, Fazio N, Campana D, Pia Brizzi M, Capurso G, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol 2011. Jun;29(17):2372–7. [DOI] [PubMed] [Google Scholar]
- 2.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 2017. Oct;3(10):1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, Chen DT, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011. Jan;117(2):268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aparicio T, Ducreux M, Baudin E, Sabourin JC, De Baere T, Mitry E, et al. Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur J Cancer 2001. May;37(8):1014–9. [DOI] [PubMed] [Google Scholar]
- 5.Öberg KE, Reubi JC, Kwekkeboom DJ, Krenning EP. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology 2010. Sep;139(3):742–53. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, Igarashi H, Jensen RT. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): recent insights and advances. J Gastroenterol 2012. Sep;47(9):941–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valle JW, Eatock M, Clueit B, Gabriel Z, Ferdinand R, Mitchell S. A systematic review of non-surgical treatments for pancreatic neuroendocrine tumours. Cancer Treat Rev 2014. Apr;40(3):376–89. [DOI] [PubMed] [Google Scholar]
- 8.Kouvaraki MA, Ajani JA, Hoff P, Wolff R, Evans DB, Lozano R, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004. Dec;22(23):4762–71. [DOI] [PubMed] [Google Scholar]
- 9.Xourafas D, Tavakkoli A, Clancy TE, Ashley SW. Distal pancreatic resection for neuroendocrine tumors: is laparoscopic really better than open? J Gastrointest Surg 2015. May; 19(5):831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang XF, Lopez-Aguiar AG, Poultsides G, Makris E, Rocha F, Kanji Z, et al. ; United States Neuroendocrine Tumor Study Group. Minimally invasive versus open distal pancreatectomy for pancreatic neuroendocrine tumors: an analysis from the U.S. neuroendocrine tumor study group. J Surg Oncol 2019. Aug;120(2):231–40. [DOI] [PubMed] [Google Scholar]
- 11.Jain G, Chakravartty S, Patel AG. Spleen-preserving distal pancreatectomy with and without splenic vessel ligation: a systematic review. HPB (Oxford) 2013. Jun;15(6):403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Jin J, Chen S, Gu J, Zhu Y, Qin K, et al. Minimally invasive distal pancreatectomy for PNETs: laparoscopic or robotic approach? Oncotarget 2017. May;8(20):33872–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellemkjoer L, Olsen JH, Linet MS, Gridley G, McLaughlin JK. Cancer risk after splenectomy. Cancer 1995. Jan;75(2):577–83. [DOI] [PubMed] [Google Scholar]
- 14.Shoup M, Brennan MF, McWhite K, Leung DH, Klimstra D, Conlon KC. The value of splenic preservation with distal pancreatectomy. Arch Surg 2002. Feb;137(2):164–8. [DOI] [PubMed] [Google Scholar]
- 15.Beane JD, Pitt HA, Nakeeb A, Schmidt CM, House MG, Zyromski NJ, et al. Splenic preserving distal pancreatectomy: does vessel preservation matter? J Am Coll Surg 2011. Apr;212(4):651–7. [DOI] [PubMed] [Google Scholar]
- 16.Falconi M, Bartsch DK, Eriksson B, Klöppel G, Lopes JM, O’Connor JM, et al. ; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology 2012;95(2):120–34. [DOI] [PubMed] [Google Scholar]
- 17.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017. Mar;67(2):93–9. [DOI] [PubMed] [Google Scholar]
- 18.Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. ; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016;103(2):153–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KJ, Carlin JB. Multiple imputation for missing data: fully conditional specification versus multivariate normal imputation. Am J Epidemiol 2010. Mar;171(5):624–32. [DOI] [PubMed] [Google Scholar]
- 20.Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011. Jul;378(9785):86–97. [DOI] [PubMed] [Google Scholar]
- 21.Pendola F, Gadde R, Ripat C, Sharma R, Picado O, Lobo L, et al. Distal pancreatectomy for benign and low grade malignant tumors: short-term postoperative outcomes of spleen preservation-A systematic review and update meta-analysis. J Surg Oncol 2017. Feb;115(2): 137–43. [DOI] [PubMed] [Google Scholar]
- 22.Harrison LE, Karpeh MS, Brennan MF. Total gastrectomy is not necessary for proximal gastric cancer. Surgery 1998. Feb;123(2):127–30. [PubMed] [Google Scholar]
- 23.Casadei R, Ricci C, Rega D, D’Ambra M, Pezzilli R, Tomassetti P, et al. Pancreatic endocrine tumors less than 4 cm in diameter: resect or enucleate? a single-center experience. Pancreas 2010. Aug;39(6):825–8. [DOI] [PubMed] [Google Scholar]
- 24.Cauley CE, Pitt HA, Ziegler KM, Nakeeb A, Schmidt CM, Zyromski NJ, et al. Pancreatic enucleation: improved outcomes compared to resection. J Gastrointest Surg 2012. Jul; 16(7):1347–53. [DOI] [PubMed] [Google Scholar]
- 25.Parekh JR, Wang SC, Bergsland EK, Venook AP, Warren RS, Kim GE, et al. Lymph node sampling rates and predictors of nodal metastasis in pancreatic neuroendocrine tumor resections: the UCSF experience with 149 patients. Pancreas 2012. Aug;41(6):840–4. [DOI] [PubMed] [Google Scholar]
- 26.Zhang XF, Xue F, Dong DH, Lopez-Aguiar AG, Poultsides G, Makris E, et al. New Nodal Staging for Primary Pancreatic Neuroendocrine Tumors: A Multi-institutional and National Data Analysis. Ann Surg 2019, DOI: 10.1097/SLA.0000000000003478. [DOI] [PMC free article] [PubMed]
- 27.Lopez-Aguiar AG, Ethun CG, Zaidi MY, Rocha FG, Poultsides GA, Dillhoff M, et al. The conundrum of [{LT}] 2-cm pancreatic neuroendocrine tumors: A preoperative risk score to predict lymph node metastases and guide surgical management. Surgery 2019. Jul; 166(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Partelli S, Gaujoux S, Boninsegna L, Cherif R, Crippa S, Couvelard A, et al. Pattern and clinical predictors of lymph node involvement in nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs). JAMA Surg 2013. Oct;148(10):932–9. [DOI] [PubMed] [Google Scholar]
- 29.Hashim YM, Trinkaus KM, Linehan DC, Strasberg SS, Fields RC, Cao D, et al. Regional lymphadenectomy is indicated in the surgical treatment of pancreatic neuroendocrine tumors (PNETs). Ann Surg 2014. Feb;259(2): 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Partelli S, Bartsch DK, Capdevila J, Chen J, Knigge U, Niederle B, et al. ; Antibes Consensus Conference participants. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2017;105(3): 255–65. [DOI] [PubMed] [Google Scholar]
- 31.Wu L, Sahara K, Tsilimigras DI, Maithel SK, Poultsides GA, Rocha FG, et al. ; and other members of the U.S. Neuroendocrine Tumor Study Group. Therapeutic index of lymphadenectomy among patients with pancreatic neuroendocrine tumors: A multi-institutional analysis. J Surg Oncol 2019. Dec;120(7): 1080–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao R, Zhao H, Li K, Luo S, Turner M, Cai JQ, et al. Outcomes of Lymph Node Dissection for Non-metastatic Pancreatic Neuroendocrine Tumors: A Propensity Score-Weighted Analysis of the National Cancer Database. Ann Surg Oncol 2019. Sep;26(9):2722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai MH, Shi N, Xing C, Liao Q, Zhang TP, Chen G, et al. Splenic preservation in laparoscopic distal pancreatectomy. Br J Surg 2017. Mar;104(4):452–62. [DOI] [PubMed] [Google Scholar]
- 34.Tang Y, Tang S, Hu S. The efficacy of spleen-preserving distal pancreatectomy with or without splenic vessel preservation: a meta-analysis. Int J Clin Exp Med 2015. Oct;8(10): 17128–39. [PMC free article] [PubMed] [Google Scholar]
- 35.Boselli C, Barberini F, Listorti C, Castellani E, Renzi C, Corsi A, et al. Distal pancreatectomy with splenic preservation: A short-term outcome analysis of the Warshaw technique. Int J Surg 2015. Sep;21 Suppl 1:S40–3. [DOI] [PubMed] [Google Scholar]
- 36.Ferrone CR, Konstantinidis IT, Sahani DV, Wargo JA, Fernandez-del Castillo C, Warshaw AL. Twenty-three years of the Warshaw operation for distal pancreatectomy with preservation of the spleen. Ann Surg 2011. Jun;253(6):1136–9. [DOI] [PubMed] [Google Scholar]
- 37.Kimura W, Inoue T, Futakawa N, Shinkai H, Han I, Muto T. Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein. Surgery 1996. Nov;120(5): 885–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


