Abstract
Organoboron acids are stable, organic-soluble Lewis acids with potential application as catalysts for a wide variety of chemical reactions. In this review, we summarize the utility of boronic and borinic acids, as well as boric acid, as catalysts for organic transformations. Typically, the catalytic processes exploit the Lewis acidity of trivalent boron, enabling the reversible formation of a covalent bond with oxygen. Our focus is on recent developments in the catalysis of dehydration, carbonyl condensation, acylation, alkylation, and cycloaddition reactions. We conclude that organoboron acids have a highly favorable prospectus as the source of new catalysts.
Graphical Abstract

Boronic acids have become ubiquitous reagents in organic chemistry due to their application in cross-coupling reactions.1–3 Apart from their roles as synthetic reagents,4,5 boronic acids and other organoboron species can act as catalysts due to the unique electronic properties of boron. Modification of the carbon substituents and complexation to nucleophilic species can dramatically alter the electronics of the boron center. In addition, the metal-like ability of boron to transition between ionization states and to form complexes with ligands allows for various activation modes. Organoboron species are especially attractive as catalysts in accordance with the principles of green chemistry.6,7 They typically have low toxicity, and their unique chemoselectivity can obviate the need for orthogonal protecting group strategies. Moreover, boric acid itself is often effective as a catalyst in an aqueous solution, avoiding the need for organic solvents. In the last decade or so, Hall8–10 and Taylor11,12 have published scholarly reviews on organoboron acids as catalysts. In this Perspective, we focus on recent advances in the context of historical precedents.
Boron has been used as a catalyst for organic reactions in all of its oxidation states (Figure 1). The highest oxidation state, boric acid, can be used as a simple and inexpensive Lewis acid promoter. Boronic acids, which are widely available, electronically tunable, and easily modified by complexation, have found the broadest application in catalysis, both as free boronic acids and in complexes. Borinic acids, with two carbon substituents and a single hydroxy group, have unique complexation behavior relative to other organoboron species and have found particular application as regioselective catalysts in polyol systems. Boranes, which have three carbon substituents on boron, have been used extensively as Lewis acids in organic chemistry13,14 and will not be discussed here. Nor will more exotic boron species, such as borylenes15 and boron cations,16 which display interesting reactivity, especially in their metallomimetic behavior.17 In considering emergent organoboron acid catalysts, we adhere to the IUPAC definition of “catalyst” as “a substance that increases the rate of a reaction without modifying the overall standard Gibbs energy change in the reaction.”18 Thus, reactions in which an organoboron species is present in stoichiometric or even superstoichiometric amounts but lowers the activation barrier and is not consumed are included in our discussion.
Figure 1.
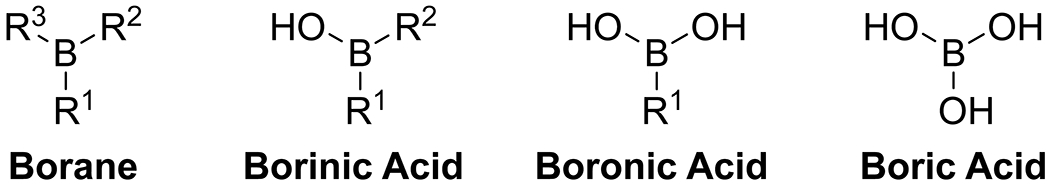
Structures of the various oxidation states of organoboron derivatives. R1, R2, R3 = alkyl, aryl, vinyl, alkynyl.
Lewis acids have found broad utility as catalysts for organic reactions.19,20 Despite the presence of potentially acidic hydroxy groups, organoboron species act almost exclusively as Lewis acids.21 Traditional Brønsted acidity can also be accessible,22 though these instances are rare and have not found application in catalysis. Rather, hydrogen bonding or Lewis acid-assisted Brønsted acidity of water or hydroxy groups can be an active mode of catalysis where more common Lewis acid–base complexes are not favored.23 Catalysis by organoboron species generally relies on the complexation of the substrate to boron as a Lewis acid–base complex. Thus, several general reaction classes are popular targets for organoboron catalysis: dehydration, carbonyl condensation, acylation, alkylation, and cycloaddition (Figure 2). We highlight examples from these five reaction classes, showing the structures of key intermediates when particularly illustrative.
Figure 2.
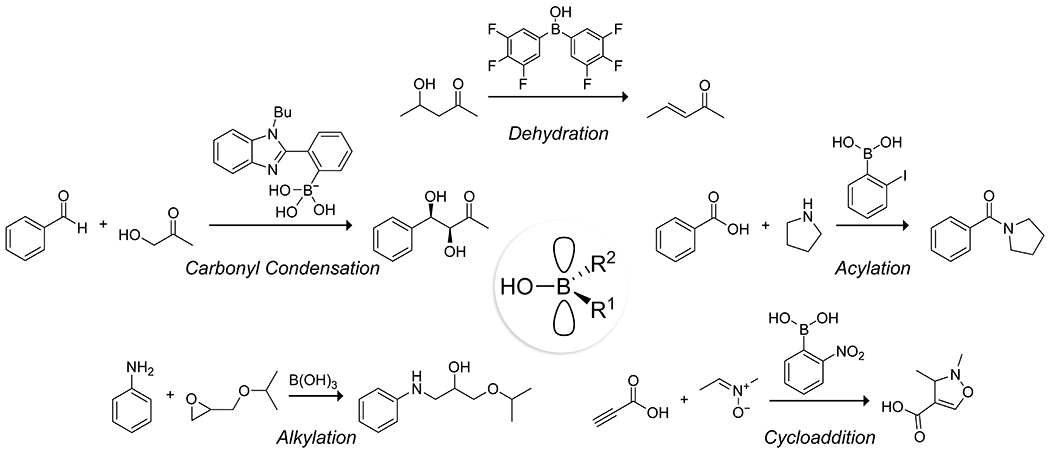
Representative reactions that are catalyzed by organoboron acids: dehydration,24 carbonyl condensation,25 acylation,26 alkylation,27 and cycloaddition.28 R1, R2 = alkyl, aryl, vinyl, alkynyl, hydroxy.
DEHYDRATION
The propensity for organoboron acids to bind to alcohols and diols allows them to activate these compounds for the loss of water, leading to cyclizations and other rearrangements. A practical application is in the dehydration of biomass-derived hexose carbohydrates, typically glucose or fructose, to furanic compounds such as 5-hydroxymethylfurfural (HMF). Several groups have reported on the boric acid-promoted conversion of glucose to HMF. These reactions generally take place in ionic liquid solvents,29,30 which are hygroscopic salts that promote dehydration. The reaction can also occur in an aqueous solution with the assistance of salt additives.31 Higher yields can be obtained by the addition of transition-metal Lewis acids as cocatalysts.32,33 In all cases, however, yields are relatively low and limited by significant side reactions, typically non-specific condensation reactions that lead to polymeric byproducts (e.g., humins). Glucose can also be converted to HMF using electron-poor arylboronic acids, either in ionic liquids34 or in organic solvents;35,36 these reactions typically give higher selectivity (Scheme 1). Boronic acids are, however, more expensive than boric acid.
Scheme 1.
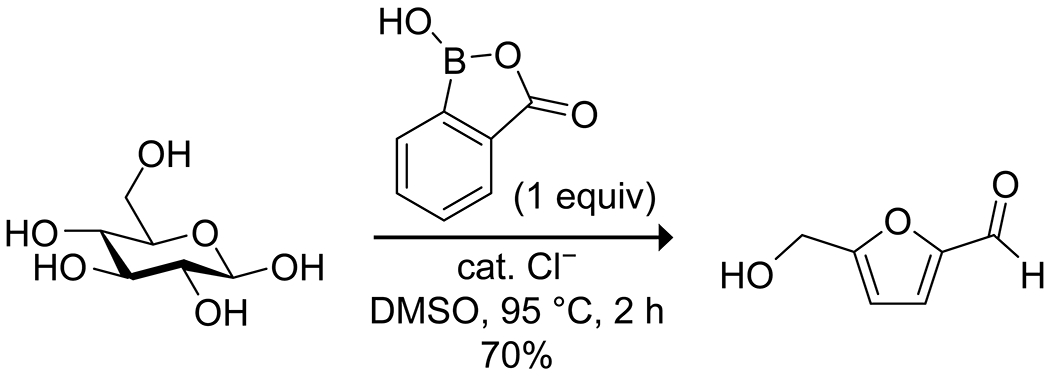
Boronic Acid Catalyzed Dehydration of Biomass-Derived Glucose to 5-Hydroxymethylfurfural36
Arylboronic acids can also be employed to promote the intramolecular condensation of vicinal dicarboxylic acids to form the corresponding anhydride.37,38 Simple arylboronic acids give a low yield in this reaction, but the use of ortho dialkylaminomethyl-substituted arylboronic acids leads to a high yield of the desired anhydride (Scheme 2). The authors attribute the increased efficacy to Lewis acid–Brønsted base catalysis simultaneously activating one carboxylic acid as an electrophilic covalent Lewis acid–base complex while activating the other carboxylic acid as a nucleophile through deprotonation by the amine. Steric bulk prevents the formation of less active N→B chelated species. Boric acid itself catalyzes the direct condensation of benzene-1,2-diamines with carboxylic acids to produce various substituted benzimidazoles (Scheme 3). The reactions are, however, somewhat slow, and the yields are variable.39 Boric acid has also been shown to effectively promote the condensation of ninhydrin with 1,6-diamino-2-oxo-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitriles to form highly substituted 1,2,4-triazolopyridinones with interesting near-infrared emission spectra (Scheme 4).40
Scheme 2.
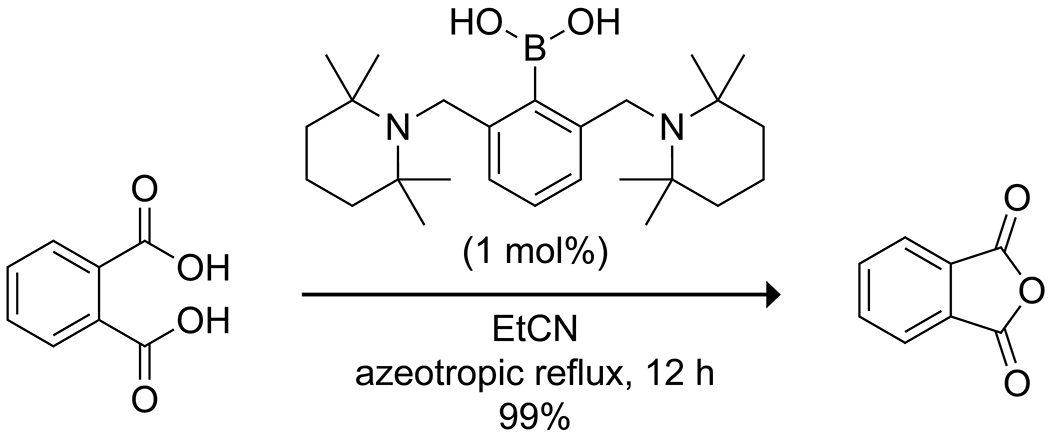
Catalysis of Anhydride Formation by an o-Dialkylaminomethyl-Substituted Phenylboronic Acid.37–38
Scheme 3.
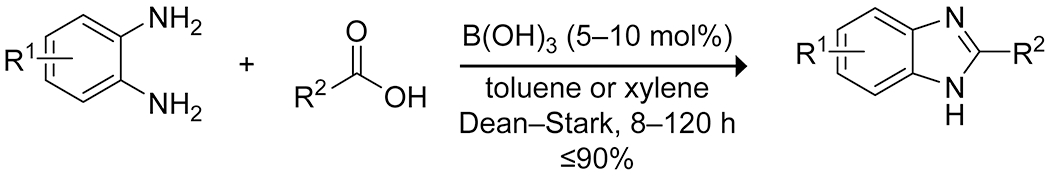
Boric Acid Catalyzed Formation of Benzimidazoles from Diamines and Carboxylic Acids39
Scheme 4.
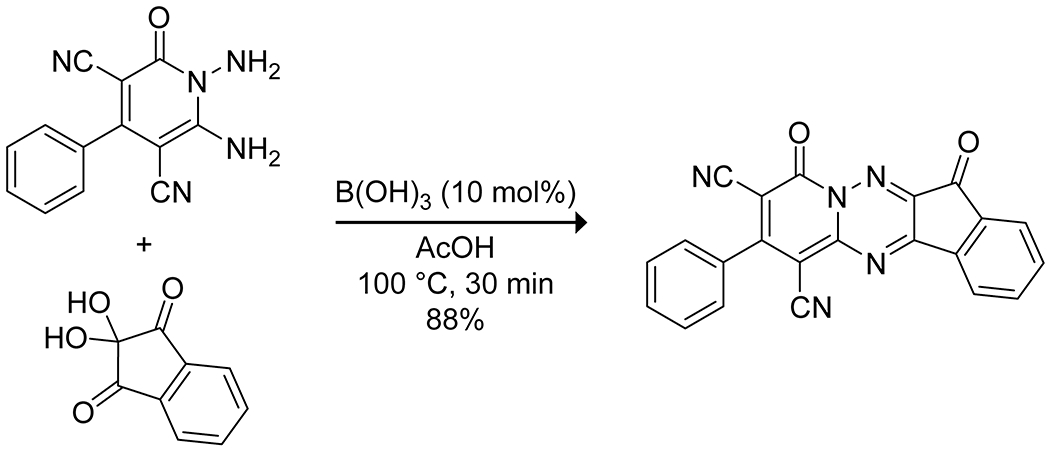
Boric Acid Catalyzed Synthesis of a 1,2,4-Triazolopyridinone40
Diaryl borinic acids bearing strong electron-withdrawing groups, typically fluoro groups, have been found to be effective for the selective dehydration of β-hydroxy carbonyl compounds produced from Mukaiyama aldol reactions.24 The borinic acid proved effective at promoting the dehydration where standard Brønsted acids failed to give the desired product. Borinic acids were also advantageous over more traditional Lewis acids due to their low sensitivity to water. Interestingly, the catalyst selectively promoted dehydration of the anti aldol product to produce the E enone, leaving the syn aldol unreacted (Scheme 5; Figure 2), allowing for separations of a diastereomeric mixture.
Scheme 5.
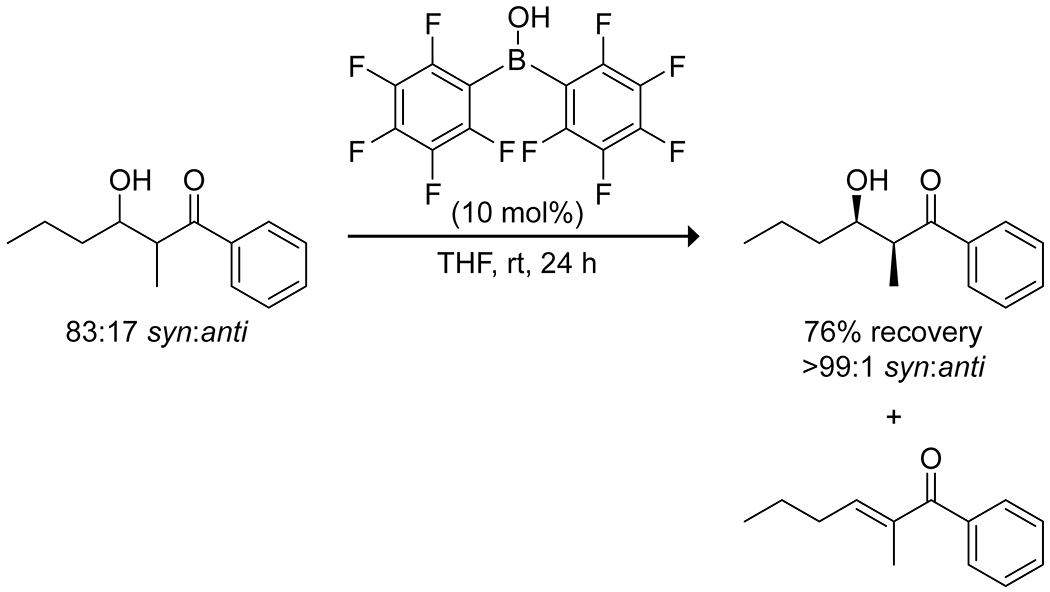
Stereoselective Catalysis of the Dehydration of an anti Aldol Reaction Product by a Borinic Acid24
CARBONYL CONDENSATION
Reactions traditionally accelerated by Lewis acid catalysts are prime targets for boronic acid catalysis. Carbonyl compounds undergo a wide variety of canonical condensation reactions that are promoted by Lewis acids, ranging from simple imine or oxime formation to more complicated multicomponent reactions such as the Mannich reaction.19,20 The dynamic covalent adducts formed by organoboron species can lead to highly organized transition states, affording diastereoselective or enantioselective processes with versatile carbon–carbon bond formation.
Lewis-acid-activation of a carbonyl group by a boronic acid can be assured through intramolecular coordination. Schmidt and Stress reported that in 2-formylphenylboronic acid, formation of an oxime with the aldehyde was dramatically accelerated by the presence of the boronic acid (Scheme 6).41 They applied this reaction as a method for bioorthogonal conjugation that was efficacious even in human serum, though some oxidative instability of the boronic acid was evident. Similarly, Rao and Philipp42 reported the catalytic effect of boric and arylboronic acids on the hydrolysis of salicylaldehyde imines through the formation of a borate ester with the phenol and subsequent stabilization of the hemiaminal intermediate (Scheme 7).
Scheme 6.
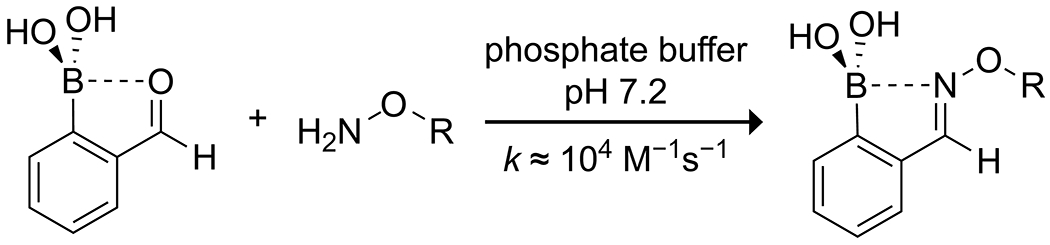
Boronic Acid-Accelerated Formation of Oximes for Bioconjugation41
Scheme 7.
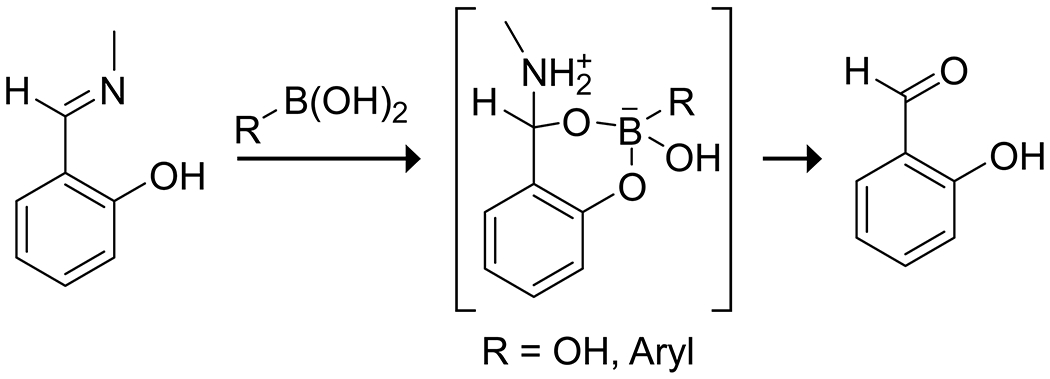
Hydrolysis of a Salicylaldehyde Imine, Catalyzed by Boric Acid or a Boronic Acid through the Stabilization of a Hemiaminal Intermediate42
Boron enolates have a long history in the asymmetric aldol reaction, giving predictable stereochemical outcomes through the stoichiometric reaction of a carbonyl group with a borane Lewis acid to form the boron enolate.43 The use of organoboron acids in catalytic aldol reactions is an obvious extension of the useful reactivity of boron enolates and has seen development, though traditional Lewis acids are significantly more effective. Aldol-type condensations of aldehydes and ketones to form α,β-unsaturated carbonyl groups are catalyzed by boric acid as reported by Offenhauer and Nelsen in 1968.44 Their reaction gave a low yield and required azeotropic removal of water, but did suggest the formation of the expected enol borate ester.
Aminoboronic acids have shown promise as aldol catalysts through a dual-activation mechanism, combining Lewis-acid activation from the boron with base activation or enamine formation from the amine. Whiting and co-workers reported on the ability of the N-butyl-1-benzimidazole-2-phenylboronic acid hydroxide complex to catalyze aldol addition and condensation reactions in water between aldehydes and either acetone or hydroxyacetone.25 They proposed that the reaction proceeds through a boron enolate (or enediol in the case of hydroxyacetone) and activation of the aldehyde by a hydrogen bond to the benzimidazole nitrogen (Scheme 8; Figure 2). The reaction gave syn diastereoselectivity due to the organized transition state and a steric clash between the aldehyde aryl group and the boronate complex. The presence of the benzimidazole was crucial for catalytic activity; phenylboronic acid showed no activity, even with the addition of exogenous benzimidazole. The Whiting group reported that chiral homoboroproline could combine the enamine catalysis of classical proline catalyst systems with the Lewis acid catalysis of boronic acid systems to give high yields of the aldol addition product and significant enantioselectivity (Scheme 9).45 Enantioselectivity was enhanced by forming a boronic ester in situ with a chiral diol, most effectively diisopropyl tartrate, though the stereoselectivity was found to derive solely from the stereochemistry of the homoboroproline with no effect from the stereochemistry of the boronic ester.
Scheme 8.
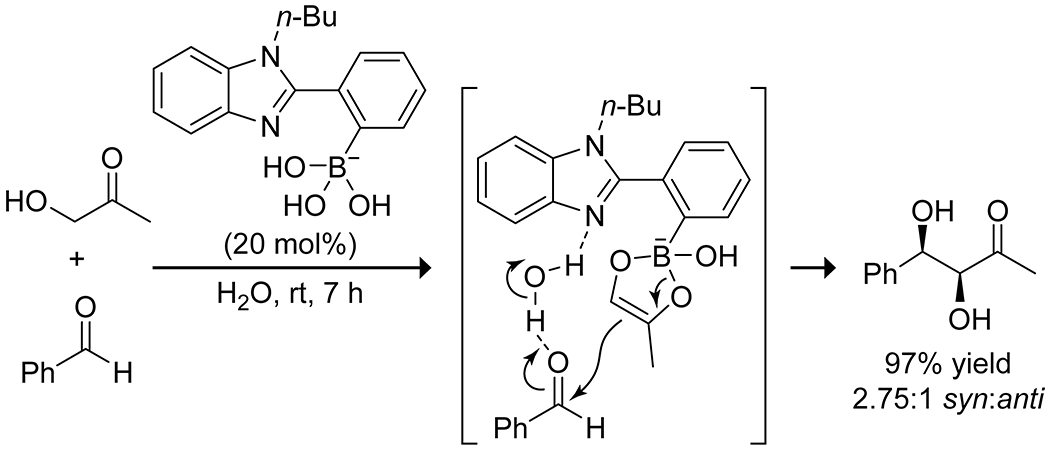
Catalysis of an Aldol Reaction by a Benzimidazole-Substituted Phenylboronic Acid25
Scheme 9.
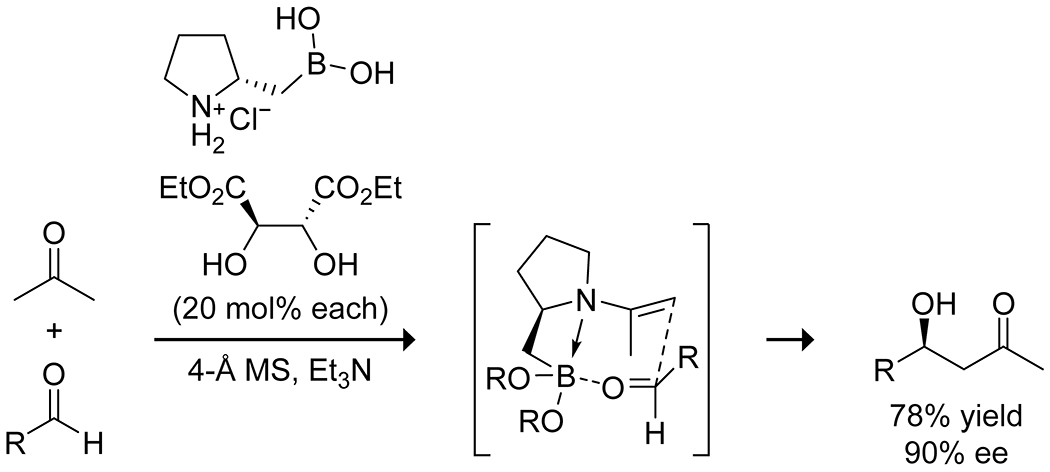
Aldol Addition Through Combined Enamine–Boronic Acid Catalysis by Homoboroproline45
The Taylor group reported a highly efficient boron-catalyzed aldol reaction of pyruvic acids and aldehydes in water to produce isotetronic acids (Scheme 10).46 This reaction takes particular advantage of the bidentate binding of pyruvic acids to the organoboron catalyst, which is known to bind tightly and quickly even in an aqueous solution. Both arylboronic and arylborinic acids proved to be effective at promoting the reaction of substituted pyruvic acids with aldehydes at only 5% catalyst loading, though borinic acids gave a higher yield. Notably, the specific binding of the catalyst to the pyruvic acid allows for the use of enolizable aldehydes without homo-aldol coupling of the aldehyde. Chiral amine boronates showed some potential for enantioselectivity, but further optimization is required to obtain synthetically useful results.
Scheme 10.
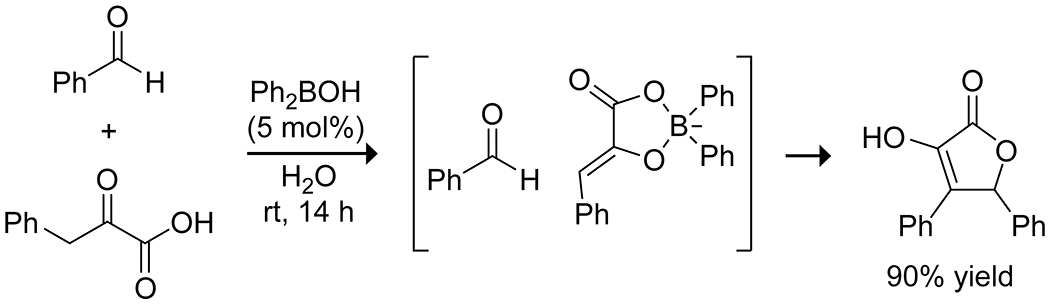
Catalysis of Isotetronic Acid Synthesis by a Borinic Acid46
Similar to aldol reactions, multicomponent condensation reactions are a ripe target for catalysis by organoboron compounds. Boric acid has been shown to be an effective catalyst for several multicomponent reactions. In an ionic liquid, boric acid silica promoted a three-component Mannich reaction under mild conditions, providing a variety of β-amino carbonyl compounds.47 A Biginelli reaction using boric acid in glacial acetic acid gave increased yields and decreased reaction times relative to more traditional acid catalysts, likely reflecting the ability of the boric acid to complex the 1,3-dicarbonyl and promote enolization (Scheme 11).48 Zheng and co-workers used boric acid in PEG-400 to promote a one-pot series of unselective aldol, Michael, and Friedel–Crafts reactions between aromatic aldehydes and ketones, producing a small library of flavonoid and triarylmethane compounds.49 This work highlights the variety of reactions that can be catalyzed by boric acid under mild conditions.
Scheme 11.
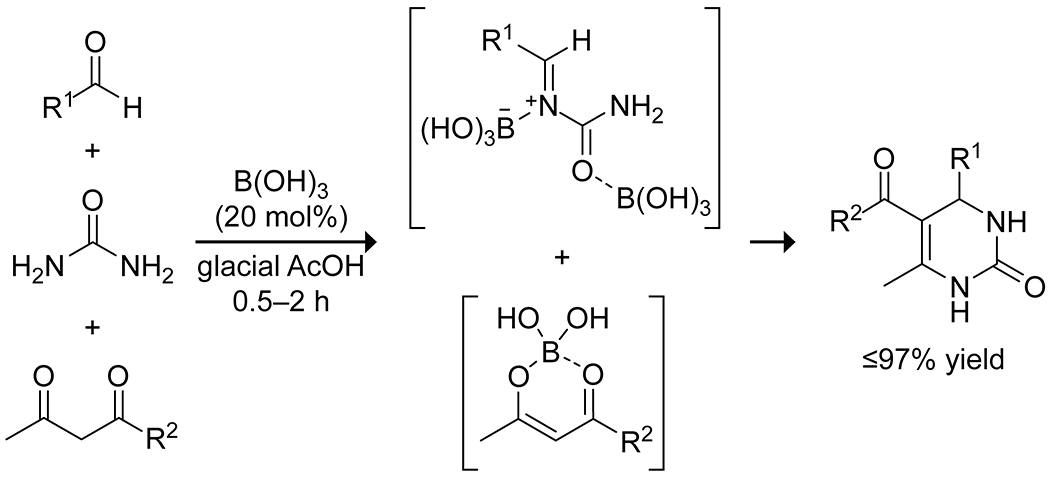
Boric Acid Catalyzed Biginelli Reaction for the Production of 3,4-Dihydropyirimidin-2(1H)-ones48
Boric acid is particularly interesting as a catalyst in aqueous systems and has been applied to several multicomponent reactions in this context to generate molecular complexity. Boric acid in water can provide pronounced increases in reaction rate, apart from its advantages as a green catalyst. In a three-component Ugi reaction, boric acid accelerated the rate more efficiently than did transition metal salts, producing high yields of several 2-arylamino-2-phenylacetamide derivatives under mild conditions, with water proving essential for the catalytic activity (Scheme 12).50 The authors did not provide a rationale for this effect. Similar results were obtained for a three-component synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines51 (Scheme 13) and a four-component synthesis of pyranopyrazole derivatives (Scheme 14).52 In an interesting variation, Mukhopadhyay and co-workers reported that the addition of a small amount of glycerol, thereby forming a boric acid ester, enhanced the activity of boric acid in a three-component Mannich reaction, an effect they attribute to the increased acidity of the complex compared to free boric acid.53
Scheme 12.
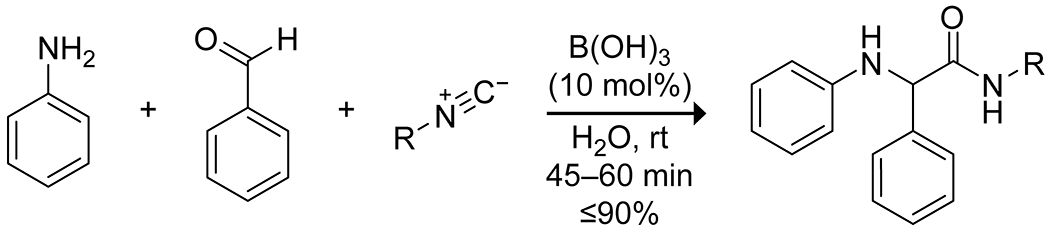
Catalysis of a Three-Component Ugi Reaction by Boric Acid in Water50
Scheme 13.
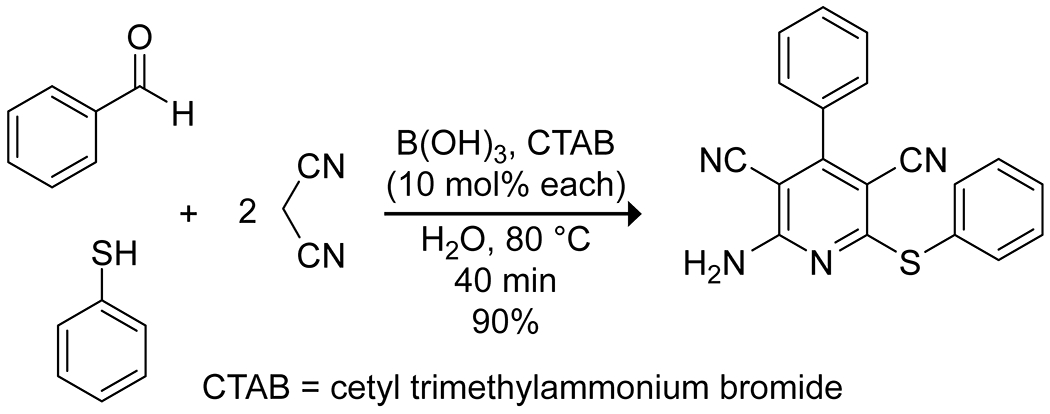
Boric Acid Catalyzed Synthesis of a Thiopyridine via a Three-Component Condensation51
Scheme 14.
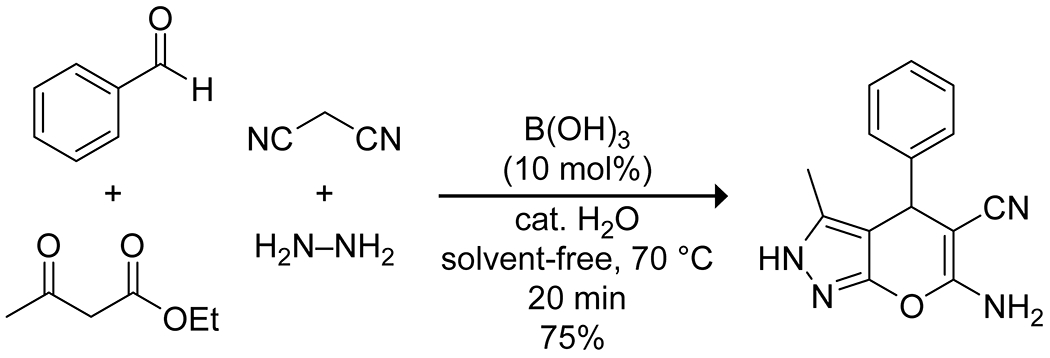
Solvent-Free Four-Component Condensation Catalyzed by Boric Acid and Water52
Boronic acids have also been used to promote multicomponent reactions using the same principles that make boric acid an effective catalyst. Carboni, Debache, and co-workers reported that phenylboronic acid is an effective catalyst for Biginelli54 and Hantzsch55 reactions, and a three-component synthesis of tetrahydrobenzo[b]pyrans (Scheme 15).56 In stoichiometric quantities, boronic acids and esters can participate in Petasis-type Mannich reactions, which result in transfer of a carbon substituent from the boron to the iminium carbon from a boronate ester intermediate.57 The highly Lewis acidic pentafluorophenylboronic acid effectively promotes the three-component reaction of indoles, thiols, and glyoxylic acids to produce α-sulfanyl-substituted indole-3-acetic acids with relatively low catalyst loading (Scheme 16).58 Kinetic analyses indicated that the indole reacted rapidly with the glyoxylic acid to form an α-hydroxyacid intermediate even in the absence of catalyst, but product formation from this intermediate was slow without the boronic acid. Computational investigations suggested that the boronic acid complexed with the hydroxy group and worked in tandem with an intramolecular hydrogen bond from the carboxylic acid to promote loss of the hydroxy group and ultimate substitution with the thiol.
Scheme 15.
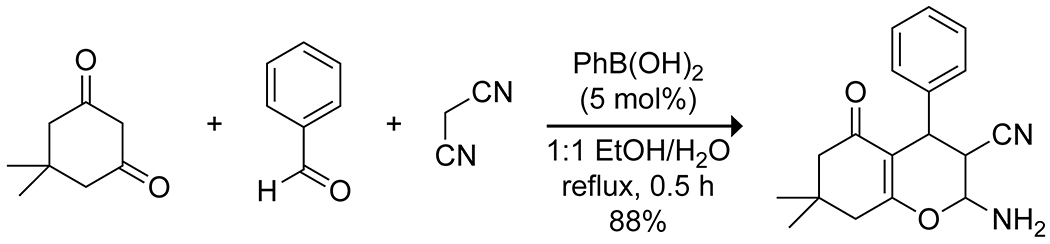
Catalysis of a Three-Component Synthesis of a Tetrahydrobenzo[b]pyran by Phenylboronic Acid56
Scheme 16.
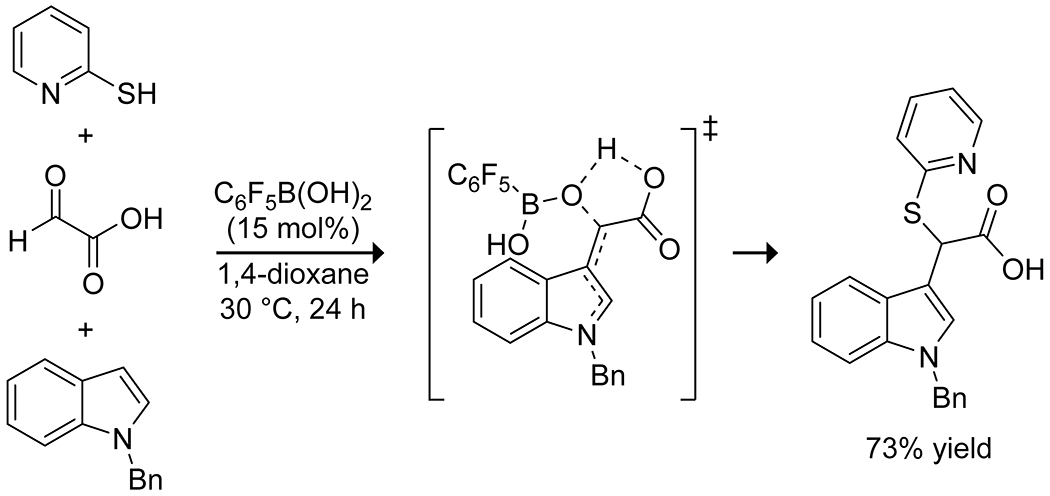
Catalysis of a Three-Component Condensation by Pentafluorophenylboronic Acid58
ACYLATION
Perhaps the most highly developed area of boronic acid catalysis is their use in esterification and amidation reactions. Typically, these reactions proceed through a mixed anhydride between the boronic acid and the carboxylic acid component, activating the carboxylic acid toward nucleophilic attack. The regiospecificity of the complex formation can allow for site-selective acylation reactions under mild conditions, particularly in contexts where formation of a bidentate complex is possible. The use of organoboron compounds is particularly attractive compared to other amidation and esterification protocols, as the boron complex with the carboxylic acid acts as a catalytic activating group, obviating the need for traditional stoichiometric activation chemistry and thereby affording high atom efficiency.
Early work showed that boric acid in combination with sulfuric acid could promote the esterification of phenols59 and that various organoboranes could promote amide formation under mild conditions.60 In 1996, the Yamamoto group published on the use of 3,4,5-trifluorophenylboronic acid as a powerful amidation catalyst, providing near quantitative yields of several sterically bulky amides and lactams using only 1% catalyst combined with azeotropic removal of water.61 The authors also report activity of the system for some esterification reactions, albeit with low yields. Further, they applied their catalyst system to the synthesis of polyamides by direct condensation of diamines and dicarboxylic acids or amino acids, though in some instances with higher catalyst loading.62 In 2001, the Wang group reported that N-methylpyridinium-3-boronic acid salts could also catalyze amidations under the Yamamoto protocol (Scheme 17), and developed an immobilized version of the catalyst, which produced high yields for a number of amidations but resulted in complete racemization upon formation of a simple dipeptide.63
Scheme 17.
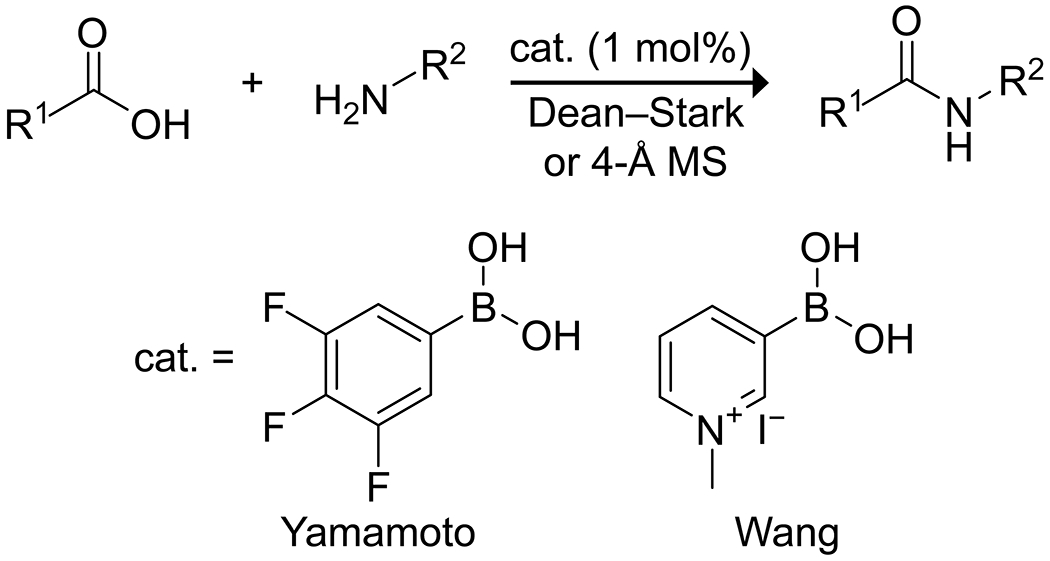
Arylboronic Acid Catalysts Reported by Yamamoto62 and Wang63 for the Formation of Amide Bonds
In 2004, Houston and co-workers showed that α-hydroxycarboxylic acids could be esterified selectively in the presence of other carboxylic acids by using catalytic boric acid in alcoholic solvent (Scheme 18).64 Though limited to small alcohols, the boric acid catalyzed procedure provided a unique and mild way to esterify the α-hydroxy acid in compounds such as citric acid while leaving other carboxylic acids intact.
Scheme 18.
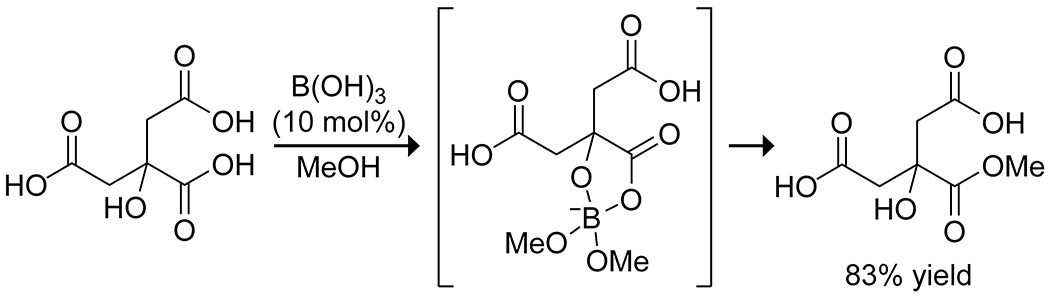
Boric Acid Catalyzed Selective Esterification of an α-Hydroxycarboxylic Acid64
Since these reports, a number of groups have developed the use of boric acid,65–73 borinic acids,74–77 or boronic acids78–93 as catalysts for esterification and amidation reactions, including applications in peptide synthesis,75,84,94 the total synthesis of natural products,92 and the synthesis of active pharmaceutical ingredients.66 A few noteworthy examples are highlighted here, focusing on unique catalyst systems and mechanistic analyses.
The mechanism of acylation reactions catalyzed by boronic acids is generally proposed to proceed through complexation of the carboxylic acid with the boronic acid to form an acyloxyboronic acid intermediate (Figure 3).61 Theoretical investigations by Fu and co-workers agreed with this mechanism, showing that the formation of the acyloxyboronic acid intermediate was facile under mild conditions (Figure 3A).81 Nucleophilic attack on the carbonyl group then proceeds through a relatively low barrier, but the breakdown of the tetrahedral intermediate to form the amide product and release the boronic acid has a high barrier and limits the reaction rate. The removal of water from the reaction by the use of molecular sieves (MS) or azeotropic distillation, as is standard in these reactions, promoted this step. The addition of bulky ortho substituents to the arylboronic acid catalyst reduces conjugation from the phenyl ring to the boron p orbital and increased reaction rates. The reaction might also proceed through a bis(acyloxy)boronic acid intermediate, but its reaction barriers were higher, and Fu and co-workers suggested that the mono(acyloxy) intermediate is the relevant species.81 In contrast, experimental work by the Ishihara group with 2,4-bis(trifluoromethyl)phenylboronic acid suggested that a 2:2 mixed anhydride of the carboxylic acid and the catalyst is the only species active toward amidation and that the ortho trifluoromethyl group is vital to prevent interaction of the amine with the boron rather than the carboxylic acid (Figure 3B).86 Premixing of the carboxylic acid and catalyst under dehydrating conditions proved necessary in this instance, and in situ IR spectra were consistent with the formation of a 2:2 complex. Mechanistic studies by Whiting and co-workers95 had previously highlighted the importance of boronic acid anhydrides and supported the formation of the 2:2 complex (Figure 3C). The relevant mechanism in a given acylation reaction could depend on the particular catalyst used, though all proceed through formation of the acyloxyboronic acid.
Figure 3.
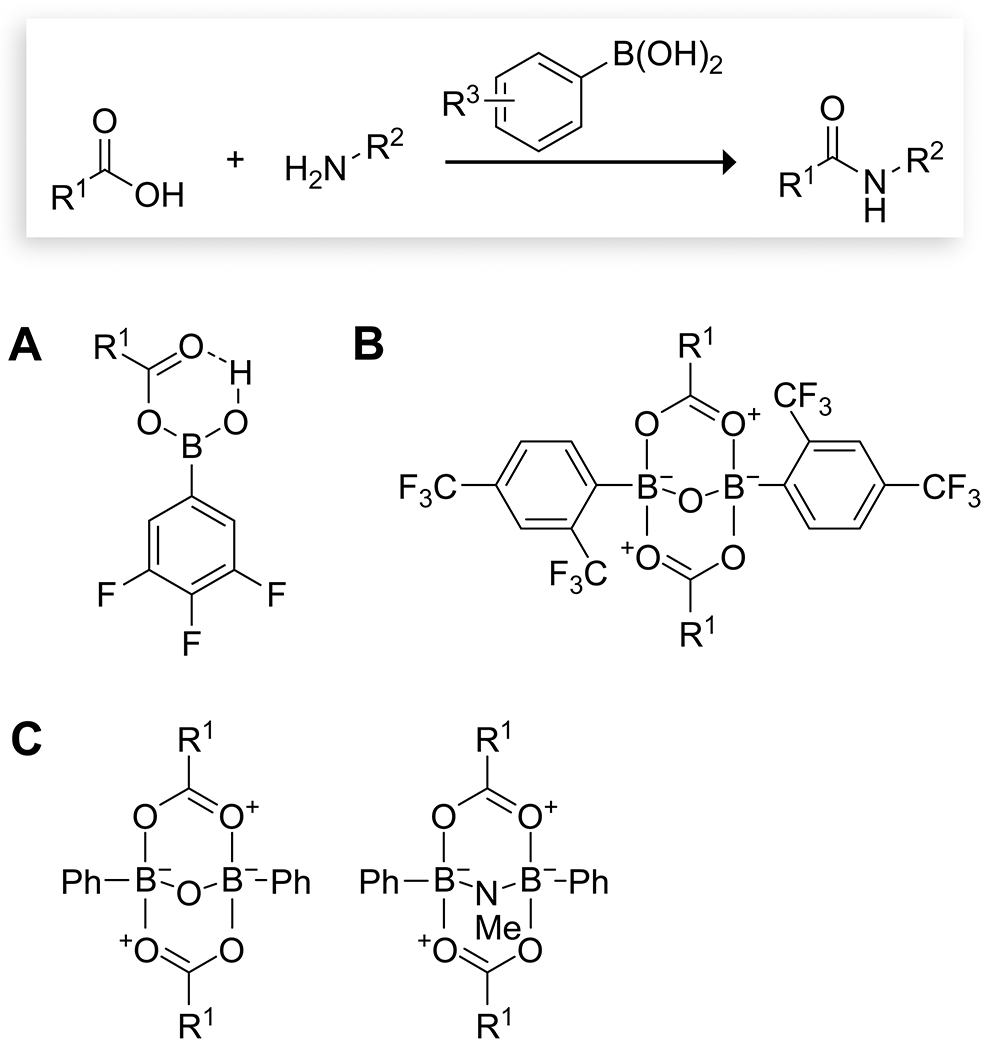
Key intermediates proposed by (A) Ishihara,61 (B) Ishihara,86 and (C) Whiting95 in the activation of carboxylic acids toward nucleophilic attack through complexation with arylboronic acids to form an acyloxy boronic acid.
The Shimada group has developed the use of unique diboronic acid anhydrides as powerful amidation catalysts for β-hydroxyacids (Scheme 19), allowing for exceptionally low catalyst loading relative to other boron species.89,94 The intramolecular diboronic anhydride is thought to allow for bidentate coordination of the carboxylic acid, leading to stronger activation and faster reaction rates, and the catalyst is directed by formation of a covalent complex with the β-hydroxy group, positioning the carboxylic acid for activation. The optimal catalyst proved effective with as little as 0.01% loading and, notably, did not require dehydrative conditions.
Scheme 19.
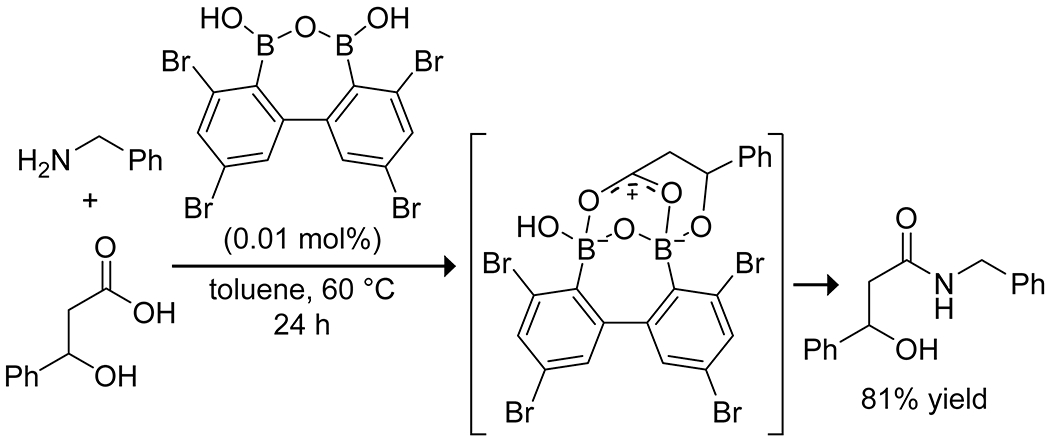
Catalytic Amidation of a β-Hydroxycarboxylic Acid using a Diboronic Acid Anhydride89
Sheppard and co-workers developed the use of borate esters, particularly B(OCH2CF3)3, for the amidation of a wide variety of protected and unprotected amino acid derivatives (Scheme 20).96–98 As with most other boron amidation catalyst systems, the reaction required dehydrative conditions, typically Dean–Stark, to obtain high yields. The reaction conditions proved effective for a variety of carboxylic acids and amines, though some more difficult reactions resulted in racemization, and they were able to perform multiple sequential amidations in a single pot. Further optimization of the solvent showed tert-butyl acetate to be superior to toluene, as it forms a lower temperature azeotrope with high water content, enhancing catalyst stability, and increases the solubility of polar reactants relative to toluene. This solvent allowed for gram-scale reactions with high process efficiency.98
Scheme 20.
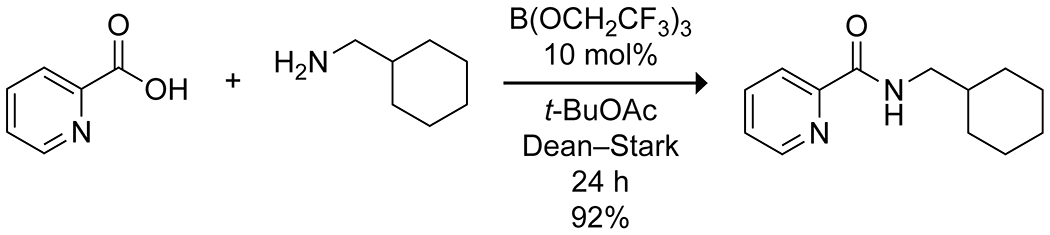
Borate Ester-Catalyzed Amidation.98
Hall and co-workers reported one of the only boron-catalyzed amidation systems that is active at room temperature.26 They found that ortho-halophenylboronic acids, particularly the iodo derivative, efficiently catalyze amide formation with the aid of molecular sieves in dichloromethane (Scheme 21). Notably, the reaction does not require an excess of either amine or carboxylic acid, and the mild conditions generally prevent racemization even for sensitive substrates. Catalysis was enhanced by the addition of electron-donating groups para to the iodo substituent, suggesting a hydrogen bond at the iodo group in the key transition state.80 Al-Zoubi and co-workers developed this scaffold further, adding an electron-rich aryl group ortho to the iodide to increase the reaction rate further.91
Scheme 21.
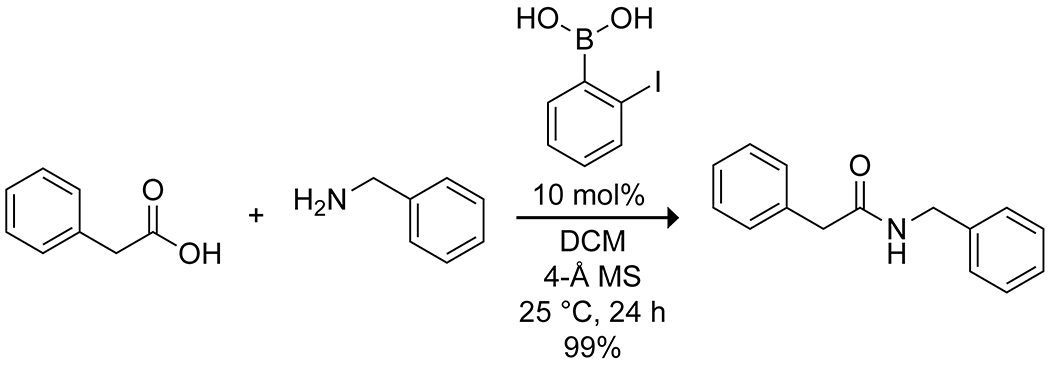
Boronic Acid Catalyzed Amidation26
Historically, the site-selective reactivity of carbohydrates and their derivatives has required a lengthy series of orthogonal protecting group strategies, as the multiple hydroxy groups in these systems are difficult to differentiate.99 Boron compounds have a well-known propensity for binding to cis-1,2 diols,100–102 and this complexation alters the electronics of the bound oxygen atoms due to the low electronegativity of boron (χ = 2.04) compared to hydrogen (χ = 2.20), as well as conjugation of the oxygen lone pairs with the vacant boron p orbital. Boron acids thus have great potential for selective reactions in carbohydrate systems. The Taylor group has developed the use of diarylborinic acids as catalysts for site-selective monoacylation of unprotected carbohydrates with acyl chlorides (Scheme 22).74 Analogous to known reactions using tin reagents, complexation of the borinic acid activated the bound oxygen atoms as nucleophiles (Figure 4), with selectivities based on dipole–dipole interactions along with steric interference to favor one of the two bound oxygen atoms. These reaction conditions have also been applied to polymerization reactions to produce sugar-based polyesters103 and to the site-selective formation of sulfate esters104 and sulfamate esters105 of sugars. Boronic acids displayed significantly reduced catalytic activity and selectivity in these reactions. Borinic acids solely form anionic complexes with diols due to the presence of two carbon substituents on boron (Figure 4); the anionic complex is likely essential for the increased nucleophilicity of the oxygen atoms. Though the formal negative charge is drawn on boron, the oxygen atoms bear that charge due to the low electronegativity of boron. As hydrogen has greater electronegativity than boron, the hydroxy groups in the starting material have less electron density on oxygen than the borinate complex and are thus less nucleophilic. In a neutral boronic acid–diol complex, the boron maintains an empty p orbital that is partially filled by donation from the oxygen lone pairs, resulting in less electron density on oxygen than on the uncomplexed hydroxy groups.
Scheme 22.
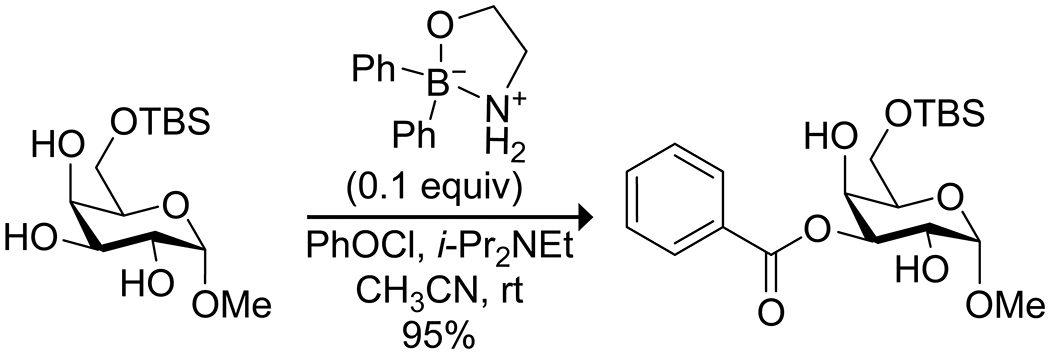
Borinic Acid Catalyzed Site-Selective Monoacylation of a Carbohydrate74
Figure 4.
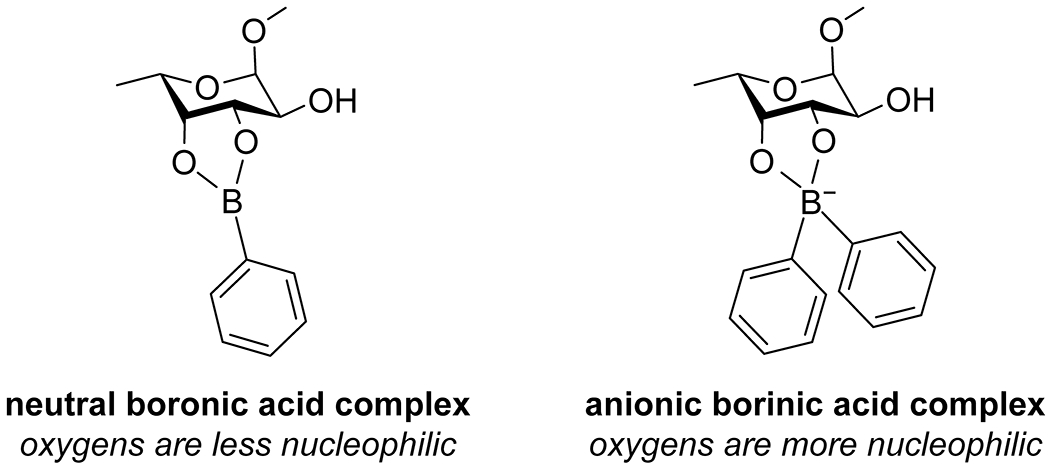
Activation of oxygen atoms as nucleophiles by complexation with a borinic acid. Complexation of a diol with a boronic acid leads to a neutral complex and no additional electron density on the oxygen atoms. The anionic complex formed with a borinic acid leads to an increase in electron density on oxygen and its activation as a nucleophile.
Shimada and co-workers achieved similar selectivity with lower catalyst loading for the selective carbohydrate acylation using 4-methoxy-2-(N-methylimidazol-2-yl)phenylboronic acid as the catalyst (Scheme 23).88 The ortho imidazolyl substituent on the catalyst coordinated internally to the boronic acid to form zwitterionic complexes, resulting in similar activation to the borinic acid systems. The reduced activity of boronic acid complexes was used to advantage by Taylor and co-workers90 to allow for site-selective esterification of sugar alcohols. Formation of diol–boronic acid complexes acted as protecting groups in situ, leaving a single hydroxy group available for reaction. The boronic acid ester also served to increase the solubility of the sugar alcohols in nonpolar solvents.
Scheme 23.
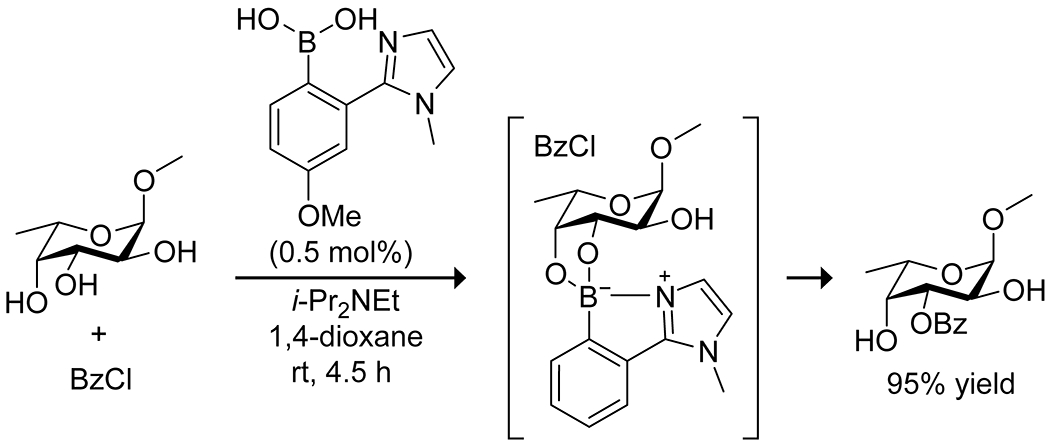
Boronic Acid Catalyzed Site-Selective Monoacylation of a Carbohydrate88
Borinic acids also display interesting reactivity that enables the formylating transamidation of DMF with amines (Scheme 24).76 Combining 2-chlorophenylborinic acid and catalytic acetic acid formed an acylated borinic acid, which could activate DMF or formamide through a Lewis acid–base complex toward attack by an exogenous amine. The reaction proceeded under mild conditions and allowed for formylation of chiral substrates without racemization. The resulting formamides could also be converted in a single pot to isocyanides in reasonable yields.
Scheme 24.
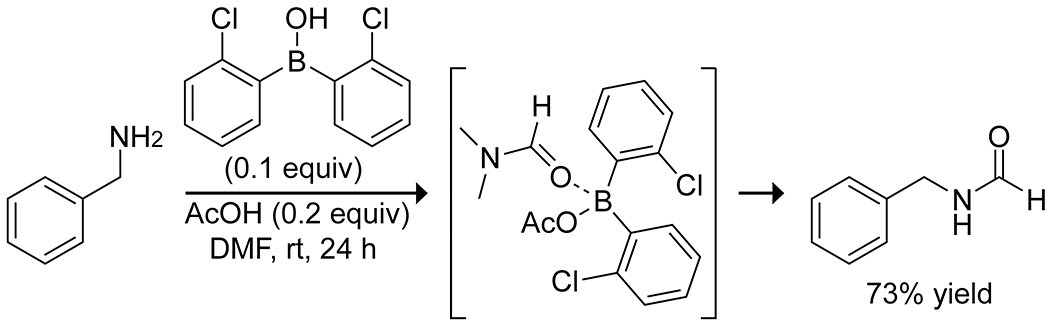
Borinic Acid Catalyzed Formylating Transamidation with DMF76
Hall and co-workers reported high yields of amides through another mode of activation by boronic acids, catalyzing a Beckmann rearrangement.85 In situ formation of the perfluoropinacol boronate ester of 2-(methoxycarbonyl)phenylboronic acid produced the active catalyst, which underwent transesterification of the carboxy ester with an oxime. Coordination of the boron to the carbonyl group promoted the Beckmann rearrangement by stabilizing the carboxylate leaving group in the key rearrangement step, producing an acyl imidate that was hydrolyzed by solvent or another molecule of oxime to release the amide (Scheme 25). The boronic acid played a role in both the transesterification and rearrangement steps by coordination to the carbonyl oxygen as an internal Lewis acid, lowering the barrier for the rate-limiting transesterification and increasing the leaving-group ability of the benzoyl unit. This example is an especially interesting advance in boronic acid catalysis, highlighting the mild conditions and multiple modes of activation available with these catalyst systems.
Scheme 25.
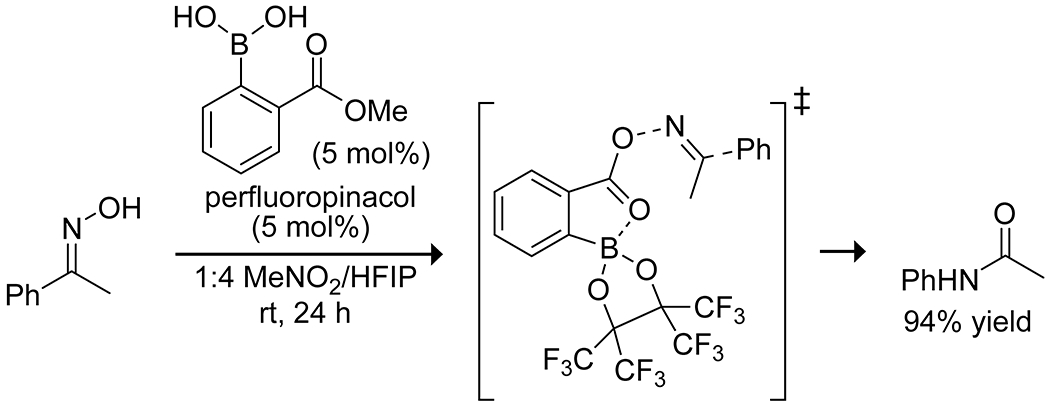
Boronic Acid Catalyzed Beckmann Rearrangement85
ALKYLATION
Activation modes already discussed for acylations and dehydrations have also been applied to alkylation reactions. A popular reaction mode is the boron-mediated deoxygenation of activated alcohols to produce carbocations, which can then undergo Friedel–Crafts alkylations with arenes. McCubbin and co-workers developed the use of pentafluorophenylboronic acid as a catalyst for Friedel–Crafts alkylations of electron-rich arenes using pi-activated alcohols, including allylic,106 benzylic,107 and propargylic108 alcohols (Scheme 26). The reactions proceeded best with alcohols that are most able to stabilize a carbocation, with electron-rich benzylic alcohols being superior electrophiles, indicating the likely formation of an SN1-type intermediate. Notably, many of these reactions proceed well at room temperature, forming water as the only byproduct, and often outperform similar reactions catalyzed by traditional metal Lewis acids. The Hall group has reported several variations on this reaction, all of which rely on highly electron-deficient boronic acids to promote the desired dehydration, allowing for alkylation of unactivated arenes under mild conditions.109–112 Of particular note is their use of ferroceniumboronic acid hexafluoroantimonate as a catalyst for production of unsymmetrical diarylmethanes from benzylic alcohols and electron-rich arenes;111 the ionic nature of the catalyst results in the formation of an ion pair between the carbocation and the hexafluoroantimonate counterion, accelerating the reaction by stabilizing the carbocation intermediate (Scheme 27). The boronic acid catalysts generally allow for a broader substrate scope than do other Lewis acids.
Scheme 26.
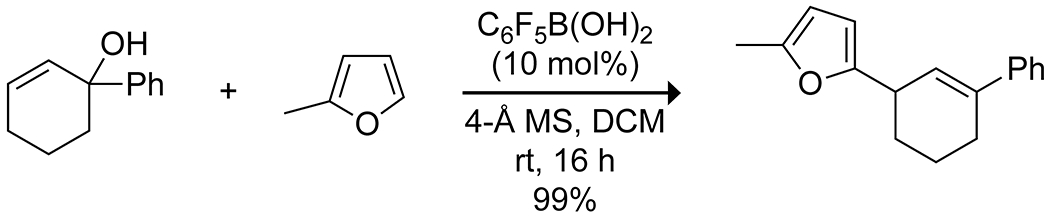
Boronic Acid Catalyzed Friedel–Crafts Alkylation106
Scheme 27.
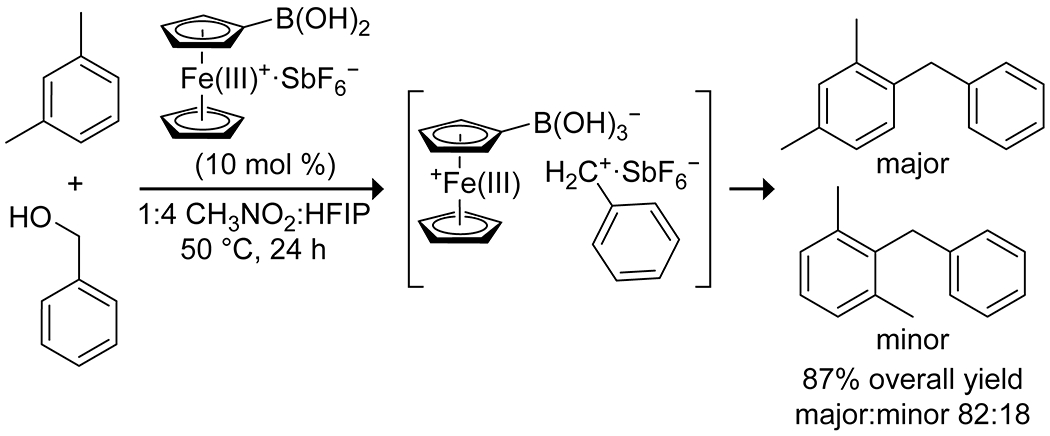
Synthesis of Asymmetric Diarylmethanes, Catalyzed by Ferroceniumboronic Acid111
Apart from conveying Lewis acidity, boronic acids can be applied as strong Brønsted acids through internal coordination of the ortho carboxylic acid in pinacol esters of 2-carboxyphenylboronic acid. This approach was applied to the alkylation of indoles with nitrostyrenes (Scheme 28).113 The same reaction has been reported using boric acid as a catalyst in water, though the authors do not propose a mechanism for the transformation.114
Scheme 28.
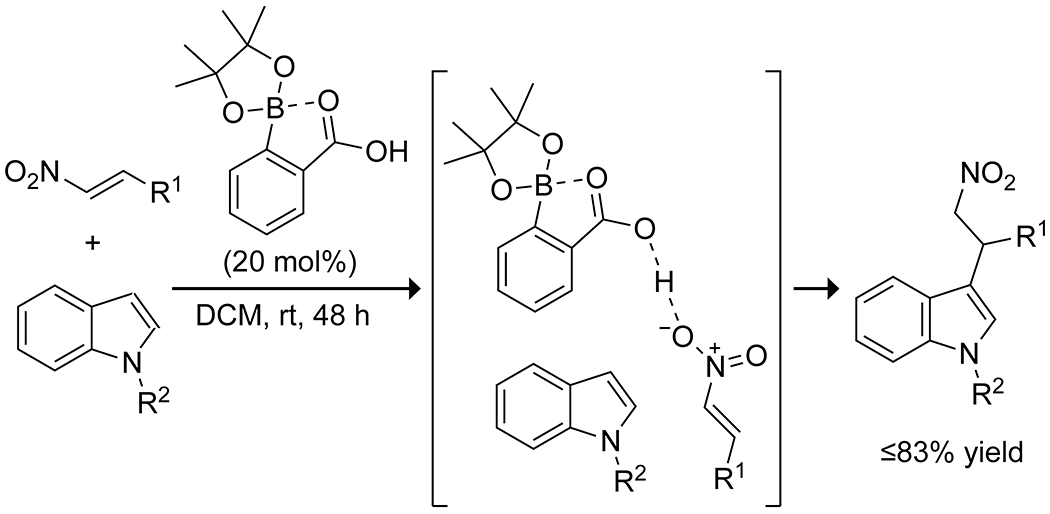
Indole Addition to Nitroolefins Through Boron-Assisted Brønsted Acidity113
Electron-poor arylboronic acids have shown promise as catalysts for dehydrative C–O and C–C bond formation from activated alcohols. Pentafluorophenylboronic acid in conjunction with oxalic acid as a cocatalyst efficiently catalyzes the etherification of secondary benzylic alcohols with alkyl alcohols through an SN1 pathway (Scheme 29).115 The authors proposed that complexation of the boronic acid with oxalic acid in situ produces a strong Brønsted acid, which catalyzes the dehydration of the benzylic alcohol, producing an ion pair between the carbocation and the boronate complex. Reaction with an alkyl alcohol produces the product ester. The same catalyst system also allowed for the formation of C–C bonds from benzylic alcohols and 1,3-dicarbonyl compounds or allyl silanes116 through a similar mechanism.
Scheme 29.
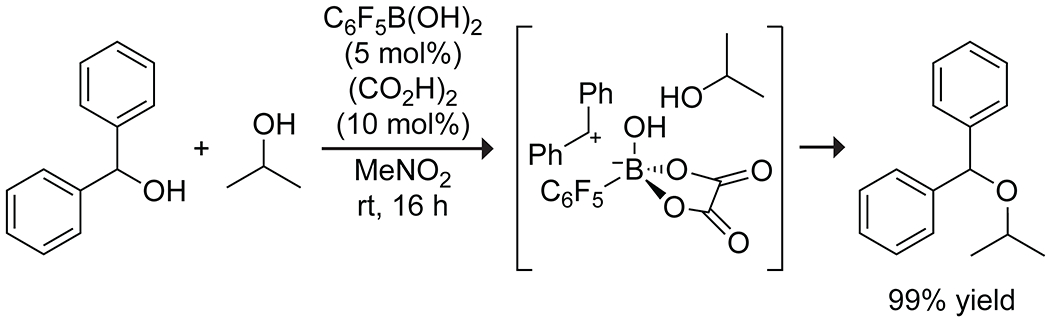
Boronic Acid Catalyzed Dehydrative Etherification.115
Boronic acid-mediated carbocation formation was also reported in the dimerization of allylic alcohols reported by the Michaelis group (Scheme 30).117 In this case, the phenylboronic acid acts as a template to organize the allylic alcohols as boronic esters. Catalytic triflic acid or copper(II) then activates one alcohol in tandem with the boronic acid, inducing formation of a cation that reacts rapidly with the other molecule of allylic alcohol, forming a 1,3-diol with high diastereoselectivity.
Scheme 30.
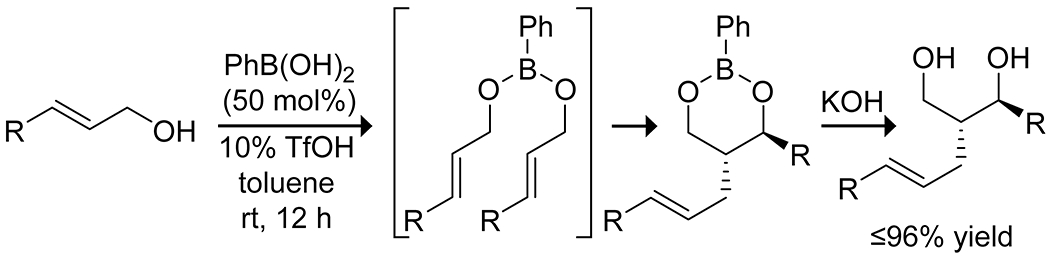
Boron-Templated Acid Catalyzed Dimerization of Allylic Alcohols117
Beyond carbocation reactions, boronic acids have been applied to both C–C and C–O bond formation. The Kürti group118 developed the combination of an electron-poor arylboronic acid catalyst and allylboronic esters as nucleophilic species in the allylation of oximes, though they do not propose a mechanistic role for the catalyst (Scheme 31). Wu, Wu, Zou, and co-workers reported on an ether interchange reaction of methoxynitroarenes catalyzed by various organoboron species.119 They proposed a boron-mediated nucleophilic aromatic substitution mechanism to explain the observed reactivity, resulting in the exchange of an alkoxy group on the boron for the methoxy group on the arene (Scheme 32). Dixon and co-workers reported on a carbocyclization of ketoesters to a pendant terminal alkyne through the boronic acid catalyzed enolization of the ketoester and subsequent ene reaction to produce a variety of five-membered rings (Scheme 33).120
Scheme 31.
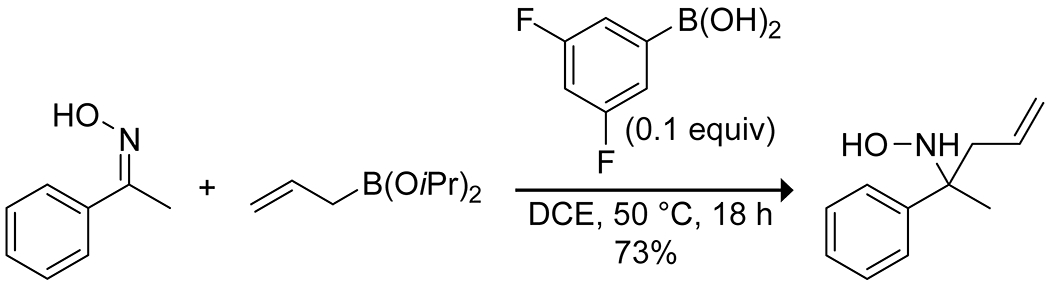
Boronic Acid Catalyzed Allylation of an Oxime118
Scheme 32.
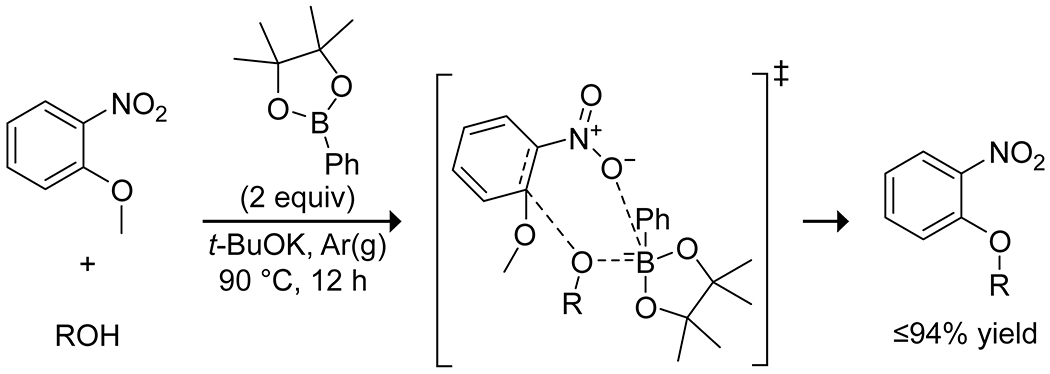
Ether Exchange of Methoxynitroarenes Promoted by Phenylboronic Acid Pinacol Ester119
Scheme 33.
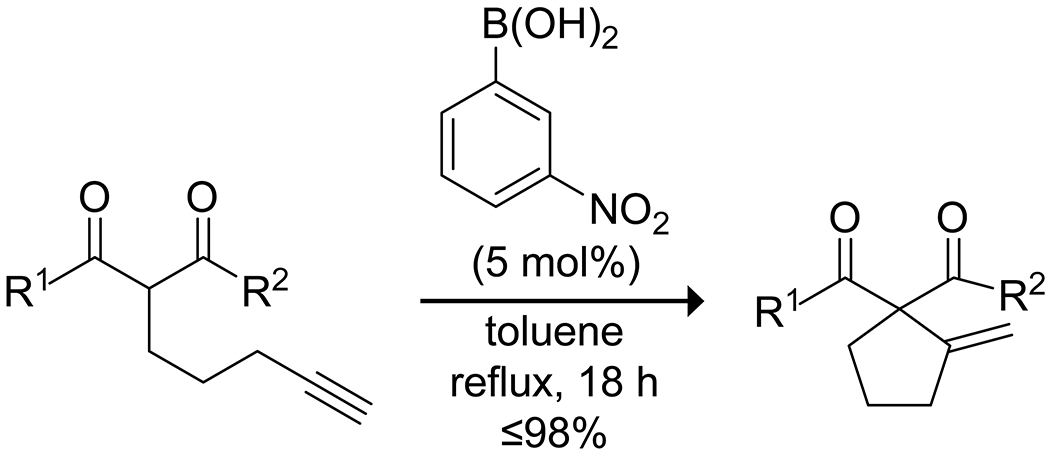
Ene Carbocyclization of Pendant Alkynes, Catalyzed by a Boronic Acid120
Lewis acids have significant application in ring-opening reactions, and boron catalysts have been applied to the selective opening of epoxides and cyclopropanes. Saidi and co-workers found a combination of boric acid and glycerol to be effective for catalyzing the regioselective opening of epoxides with anilines.27 Wang and co-workers reported that 3,4,5-trifluorophenylboronic acid effectively catalyzed the selective C-3 aminolysis of 3,4-epoxy alcohols through a highly organized transition state, involving simultaneous coordination of the alcohol and amine by the boronic acid to form a chair-like structure, resulting in both enantio- and diastereoselectivity (Scheme 34).121 They expanded this reaction to thiol nucleophiles, finding 3-nitrophenylboronic acid to be the optimal catalyst with these nucleophiles but proposing a similar transition state. Taylor and co-workers reported a similar reaction catalyzed by borinic acids to open 3,4-epoxy alcohols with amine and thiol nucleophiles122 and reported more recently a variant with nitrogen-containing heterocycles.123 Mattson and co-workers developed urea–boronic acids as strong hydrogen bond-donating catalysts for the opening of nitrocyclopropane rings, with internal coordination between the boronate and the urea oxygen resulting in stronger hydrogen-bonding capability and higher catalytic activity than more traditional urea catalysts (Scheme 35).124,125
Scheme 34.
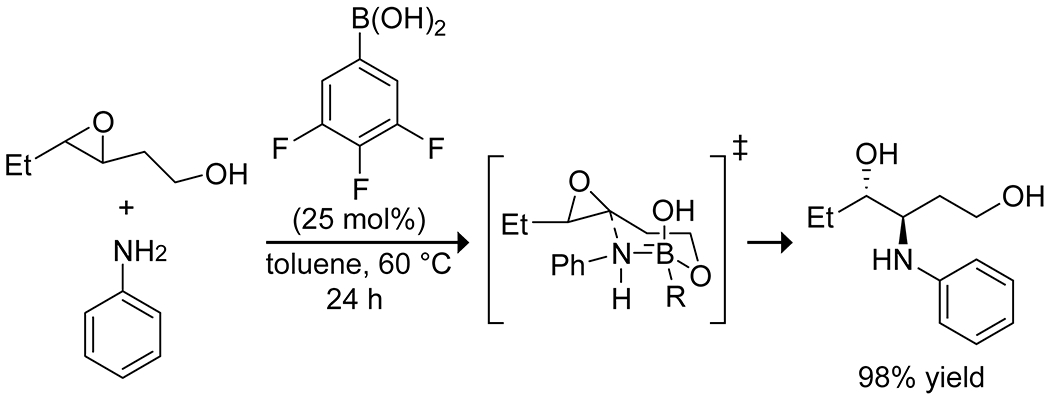
Boronic Acid Catalyzed Site-Selective Epoxide Aminolysis121
Scheme 35.
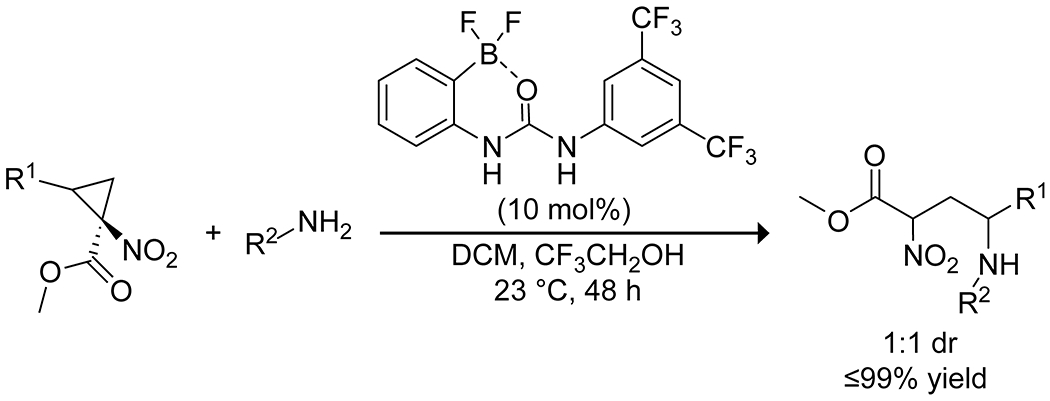
Catalysis of Nitrocyclopropane Ring-Opening by a Urea-Substituted Arylboronic Acid125
Given the ability of boron acids to bind carbohydrates selectively combined with their ability to activate hydroxy groups as both nucleophiles and electrophiles, glycosylation reactions and other selective alkylations of carbohydrates and similar compounds using organoboron acids has seen significant development. As with the acylation of carbohydrates, the phenylborinic acid systems developed by Taylor and co-workers can be applied to the site-selective alkylation of diols126 and carbohydrate derivatives,127 and for regioselective glycosylations.128–130 These reactions rely on the activation of hydroxy groups on the carbohydrate as nucleophiles by coordination with the borinic or boronic acid (Figure 4). Similar borinic acid catalysts were applied by Takemoto and co-workers to the stereoselective synthesis of disaccharides.131,132 The stereochemistry of the glycosidic linkage was controlled by both the stereochemistry of the glycosyl donor and the cis diol activated by the borinic acid. Notably, the more electrophilic borinic acid used in this case was also capable of producing an acidic proton upon complexation with the diol moiety that could activate glycosyl donors. In related polyol systems, Niu and co-workers used the ability of borinic acids to activate alkyl alcohols as nucleophiles combined with copper-catalyzed activation of propargylic alcohols to achieve enantioselective propargylation of polyols along with high enantioselectivities determined by the copper ligand.133 As expected, the borinic acid catalyst selectively activated 1,2- and 1,3-diols with high functional group tolerance, though only relatively simple substrates were reported. Hall and co-workers reported on the optimization of a BINOL-derived boroxarophenanthrene catalyst for the desymmetrization of 2-substituted 1,3-propanediols (Scheme 36).134 Activation of the alcohol oxygen proceeded through the formation of a tetrahedral boronate complex. Stereoselectivity was achieved by the addition of sterically bulky substituents to the boroxarophenanthrene scaffold to block the formation of the undesired stereoisomer.
Scheme 36.
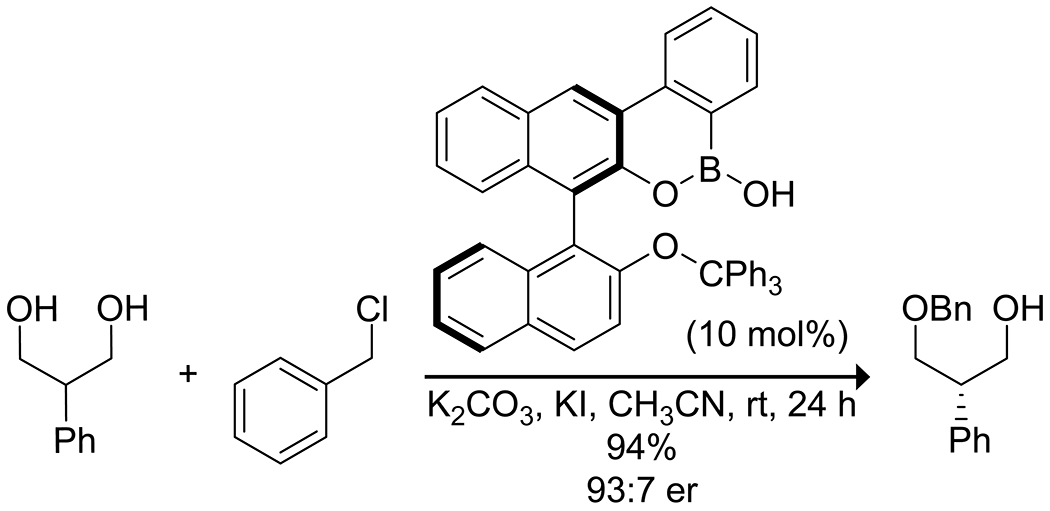
Boronic Acid-Catalyzed Desymmetrization of a 2-Aryl-1,3-propanediol134
The nucleophilic activation of alcohols in sugars can be achieved by combining a boronic acid and a Lewis base, which promotes the formation of a tetrahedral boronate complex.135,136 Intramolecular complexation of a Lewis base in the ortho imidazolylphenylboronic acids applied by Shimada and co-workers to acylations88 of carbohydrates also proved effective for glycosylation (Scheme 37).137 Compared with borinic acids, this system required a lower catalyst loading and worked with unprotected carbohydrates to yield regioselective glycosylation products. The system allows for kinetic resolution of carbohydrate mixtures based on the affinity of the boronic acid for cis diols; carbohydrates with fewer cis diols react more slowly. Boronic acids have also been applied by Toshima138 and by Takahashi139 to effect regio- and stereoselective glycosylations with various glycosyl donors, using 1,2-anhydrosugars as the glycosyl acceptor and 4-nitrophenylboronic acid as the catalyst (Scheme 38). The electron-deficient boronic acid complexes to both the donor and acceptor, activating the acceptor as an oxonium cation and activating the donor as a nucleophile by forming an anionic tetrahedral complex. The stereochemistry of the resulting glycoside is controlled by the stereochemistry of the acceptor at C2, and the regiochemistry is controlled by stereochemistry of the donor. The system can be applied to synthesis of oligosaccharides, as well as synthesis of glycosides of natural products using mono alcohols as the glycosyl donor and a borinic acid catalyst. To achieve C-glycosidation of unprotected carbohydrates, Tanaka utilized a combined catalytic system of boric acid and pyrolidine.140 The coordination of a carbohydrate to boric acid is proposed to promote the opening of the pyranose ring, and the pyrrolidine activates both the aldehyde and the ketone nucleophile to promote the desired C-glycosidation, albeit in modest yields (Scheme 39).
Scheme 37.
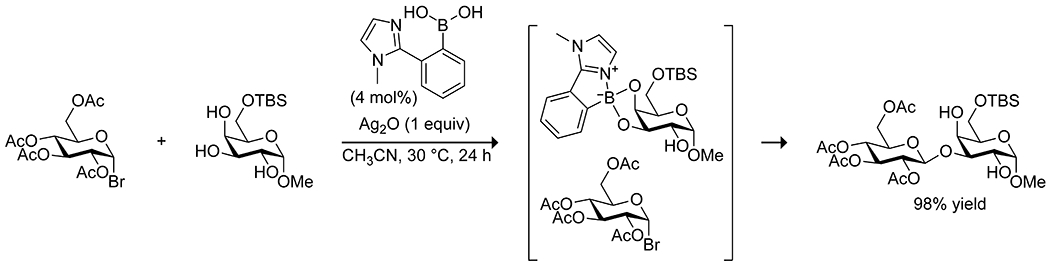
Catalysis of O-Glycosylation by an Imidazole-Substituted Phenylboronic Acid137
Scheme 38.
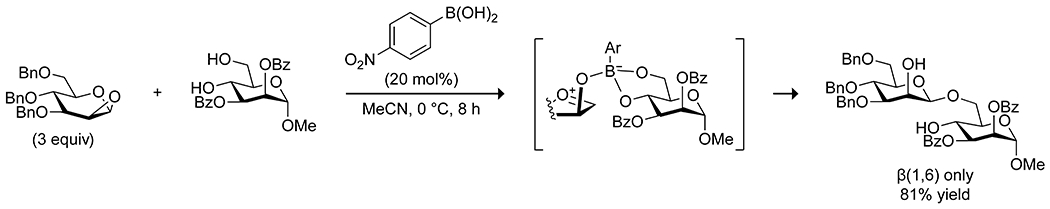
Catalysis of O-Glycosylation by an p-Nitrophenylboronic Acid138
Scheme 39.
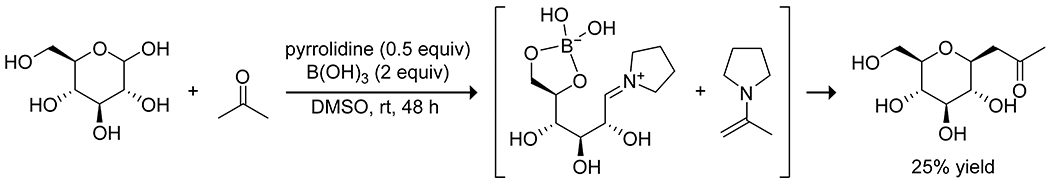
Catalysis of C-Glycosidation by Boric Acid and Pyrrolidine140
The hydrophobicity of organoboron acids can also be leveraged for catalytic effect. Manhas and Taylor90 reported on the utility of boronic acids as phase-transfer catalysts in Fischer glycosidations, allowing the reaction to be run in a nonpolar solvent (Scheme 40). In addition to enhancing the reaction rate, the formation of the boronic acid–sugar complex selectively promoted conversion to either the furanoside or the pyranoside, depending on the sugar structure, and allowed for selective functionalization from mixtures of sugars.
Scheme 40.
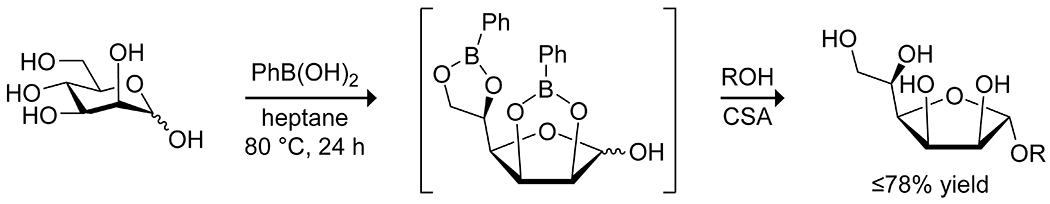
Phase-Transfer Catalysis of O-Glycosidation by Phenylboronic Acid90
Boron acids have been applied to Michael additions to form carbon–heteroatom bonds with α,β-unsaturated carbonyl compounds, with particular attention directed towards the aza-Michael addition. Initial reports demonstrated that boric acid in water could effectively catalyze the aza-Michael addition of aliphatic amines (Scheme 41).141 The reaction produced high yields but was limited to aliphatic amines and relatively unsubstituted α,β-unsaturated acceptors. More recently, 3-borono-BINOL was shown to catalyze an enantioselective aza-Michael addition of hydroxamic acid to quinone imine ketals, producing densely functionalized bridged cyclohexanes (Scheme 42).142 The catalyst in this case appears to form a 2:1 complex with the hydroxamic acid, forming a dioxazaborole and a boronate hemiester. This complex is proposed to activate and direct the quinone by hydrogen bonding, resulting in high enantioselectivity. Takemoto has developed hybrid thiourea–boronic acid catalysts for the aza-Michael addition (Scheme 43).143–145 These bifunctional catalysts form ordered complexes through both hydrogen bonding and complexation of the carboxylic acid substrate to the boronic acid to activate and deliver the amine nucleophile selectively to a single face of the alkene.146 Similar enantioselective Michael addition through hydrogen-bond direction of the nucleophile was reported by Toste and co-workers for desymmetrization of para-quinols using a combination of an arylboronic acid catalyst and a chiral phosphoric acid.147 A combination of hydrogen bonding and Lewis acidity from arylboronic acids has also been applied to the reductive alkylation of quinolines with aldehydes, using a Hantzsch ester as the reductant (Scheme 44).148 The boronic acid was proposed to activate both the quinoline and Hantzsch ester by hydrogen bonding to promote reduction to the tetrahydroquinoline, followed by Lewis-acid activation of the aldehyde to promote reductive amination with the aldehyde, again highlighting the multiple modes of activation available to boronic acids.
Scheme 41.
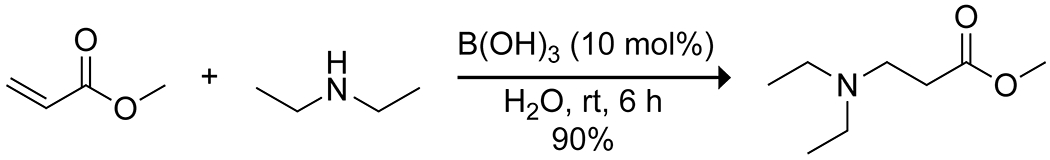
Boric Acid-Catalyzed aza-Michael Addition141
Scheme 42.
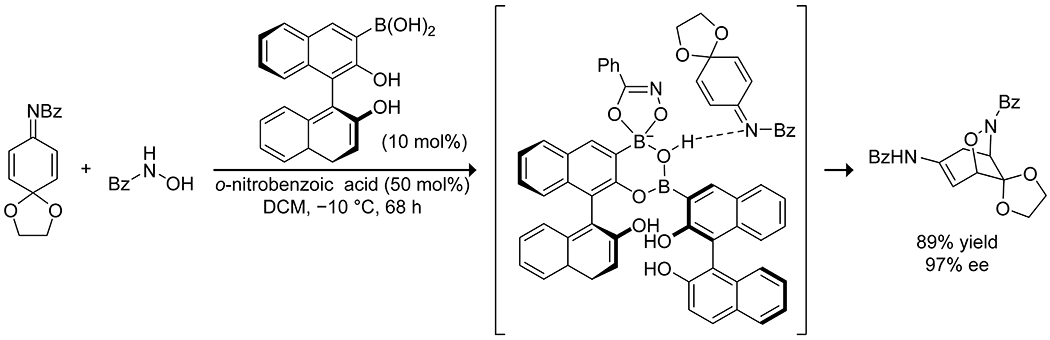
Catalysis of an Enantioselective aza-Michael Addition by a BINOL Boronic Acid142
Scheme 43.
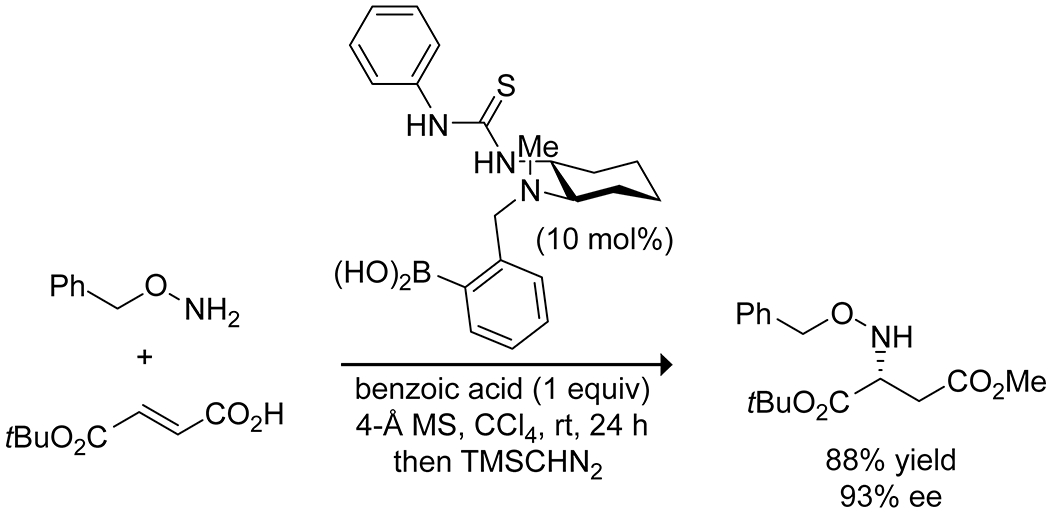
Catalysis of an Asymmetric aza-Michael Addition by a Thiourea-Substituted Phenylboronic Acid143
Scheme 44.
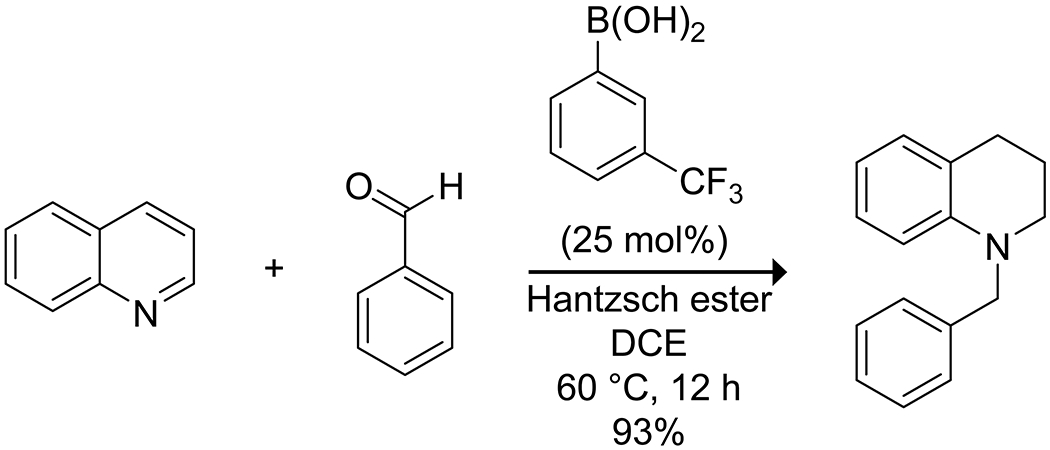
Boronic Acid-Catalyzed Reductive Alkylation of a Quinoline148
Recently, the Ishihara group reported the enantioselective 1,4-addition of cycloalkanones to α,β-unsaturated carboxylic acids using the combination of a catalytic chiral secondary amine and an arylboronic acid (Scheme 45).149 They proposed that the boronic acid activates the carboxylic acid by forming an acyloxy boron complex, and the chiral amine activates the ketone as the enamine, leading to high yields of the alkylated species at relatively high ee, though diastereoselectivity was often poor due to facile racemization. Notably, the complexation of the boronic acid to the carboxylic acid prevents deactivation upon carboxylate formation, and using a sterically hindered amine prevents the formation of the amide with the activated carboxylic acid. Still, the reaction was quite slow.
Scheme 45.
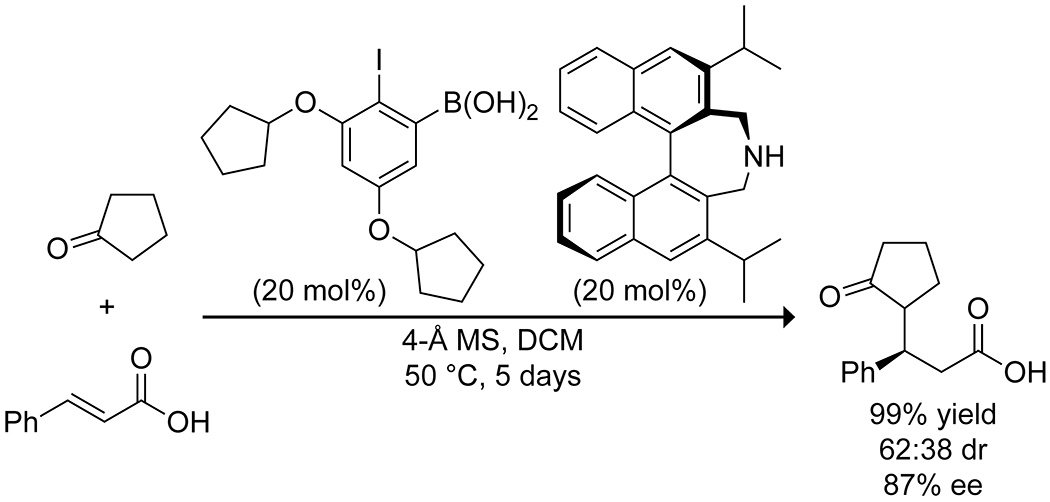
Enantioselective 1,4-Addition Reaction, Catalyzed by a Boronic Acid and Chiral Amine.149
Recently, Taylor and co-workers reported the ability of diphenylborinic acid in tandem with simple amine co-catalysts to promote the regioselective N-alkylation of a variety of azole heterocycles (Scheme 46).150 Interestingly, though the reaction proceeded well with several classes of electrophilic alkenes and epoxides, the use of other electronically similar electrophilic alkenes abolished regioselectivity. Though the origins of the loss of selectivity are unclear, these data indicate that the precise nature of the electrophile can have a profound impact.
Scheme 46.
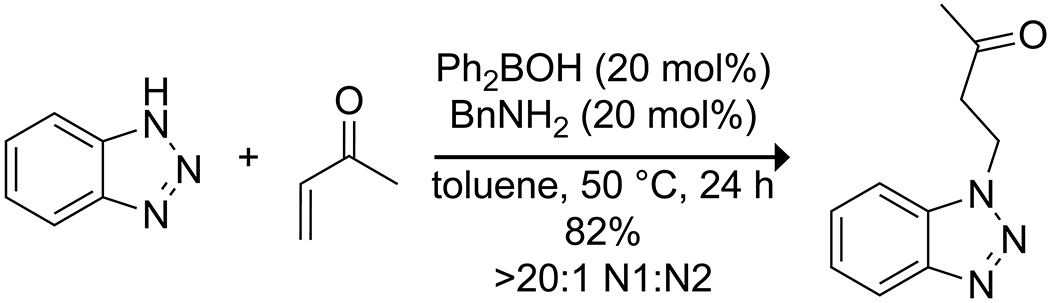
Borinic Acid-Catalyzed Regioselective N-Alkylation of an Azole Heterocycle150
CYCLOADDITION
Though not used extensively, boronic acids have demonstrated efficacy in the catalysis of cycloaddition reactions through both Lewis-acid activation of reactants and hydrogen bond-mediated reaction-templating, which allow for selective reactivity. The Hall group has developed the use of ortho-substituted arylboronic acids to promote the reactivity of unsaturated carboxylic acids (Scheme 47). For the reaction of 2-alkynoic acids as dienophiles in [4 + 2] cycloadditions, 2-bromophenylboronic acid was found to be the optimal catalyst, likely lowering the LUMO energy through formation of a mixed anhydride with the alkynoic acid and the boronic acid.151 This strategy allowed for the formation of cycloadducts in high yields. Similar LUMO-lowering activity was observed using 2-nitrophenylboronic acid to activate 2-alkynoic and 2-alkenoic acids toward [3 + 2] cycloadditions with azides, nitrile oxides, and nitrones (Scheme 48; Figure 2).28 More recently, Zheng and co-workers reported that 3,5-bis(trifluoromethyl)phenylboronic acid promotes the [4 + 3] cycloaddition of 1,3-dienes and hydroxymethyl-substituted benzofurans, benzothiophenes, and benzopyrroles (Scheme 49).152 They proposed that the boronic acid promotes the heterolytic cleavage of the hydroxy C–O bond of the benzofuran through the formation of a borate ester and that the resulting stabilized cation undergoes a cycloaddition with the diene to produce the desired seven-membered ring under mild conditions. In the solid state, boronic acids have been used to template reactants through hydrogen bonding, heteroatom coordination, or the formation of borate esters to allow for selective and high-yielding [2 + 2] cycloadditions upon irradiation.153,154 Thus, boronic acids present a relatively untapped stratagem for cycloaddition catalysis.
Scheme 47.
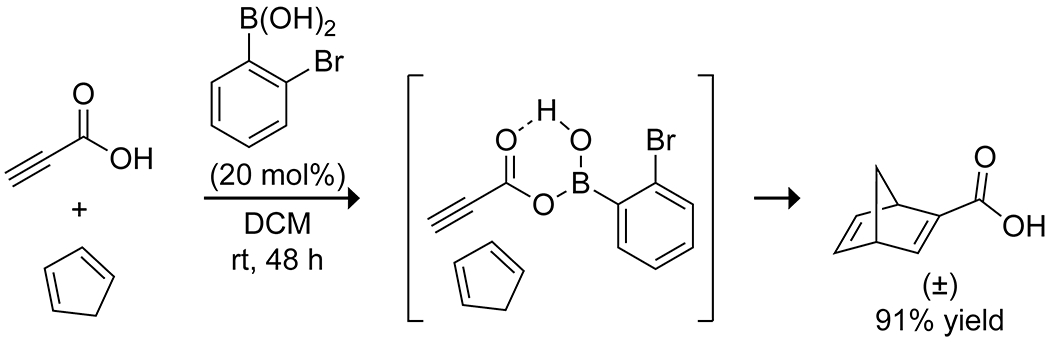
Boronic Acid-Catalyzed [4 + 2] Cycloaddition151
Scheme 48.
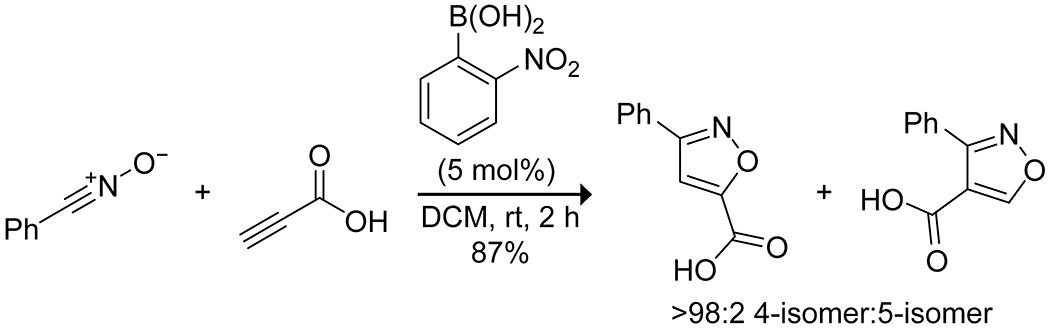
Boronic Acid-Catalyzed [3 + 2] Cycloaddition28
Scheme 49.
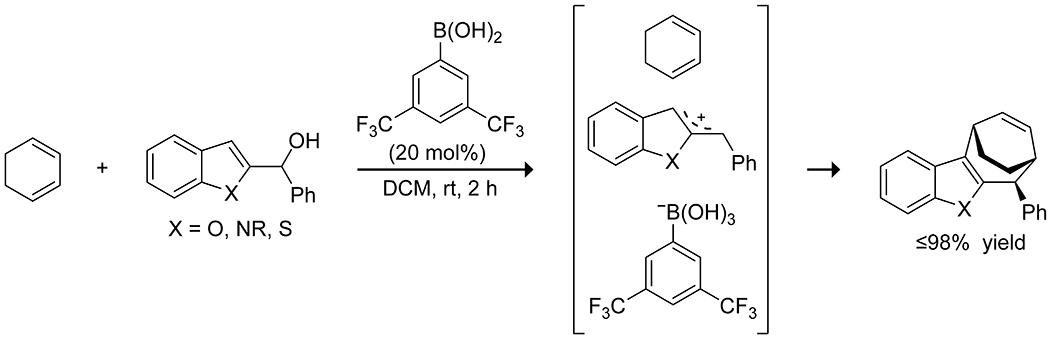
Boronic Acid-Catalyzed [4 + 3] Cycloaddition152
OTHER REACTIVITY
Boronic acids and their derivatives have also found application in other reaction classes through modes of activation similar to those described above. Perhaps the most widely known organoboron derivatives in catalysis are the oxazaborolidine catalysts used in the Corey–Bakshi–Shibata reduction, which can induce asymmetry.155–158 These catalysts, which are easily accessed by the condensation of arylboronic acids and amino alcohols, can provide versatile systems for asymmetric catalysis. Here, we focus on lesser-known examples of catalysis by organoboron acids of other reaction classes, specifically, epoxide ring-opening, aziridination, chemoselective oxidation and reduction, CO2 activation, silylation, reactions with alkenes and alkynes, and C–H activation.
Epoxide Ring-Opening.
Similar to the aminolysis of epoxy alcohols described above, Taylor and co-workers have described the use of arylborinic acids to effect several regioselective epoxide ring-openings in 2,3- and 3,4-epoxy alcohols with halide nucleophiles,159 acyl or sulfonyl chlorides to effect chloroacylations and chlorosulfonations,160,161 and a semipinacol rearrangement through tandem catalysis with an iodide salt, all of which generally proceed with nearly complete diastereoselectivity (Scheme 50).162 Coordination of the borinic acid to the alcohol appears to result in an organized transition state that allows for the regioselective reaction of the nucleophile.
Scheme 50.
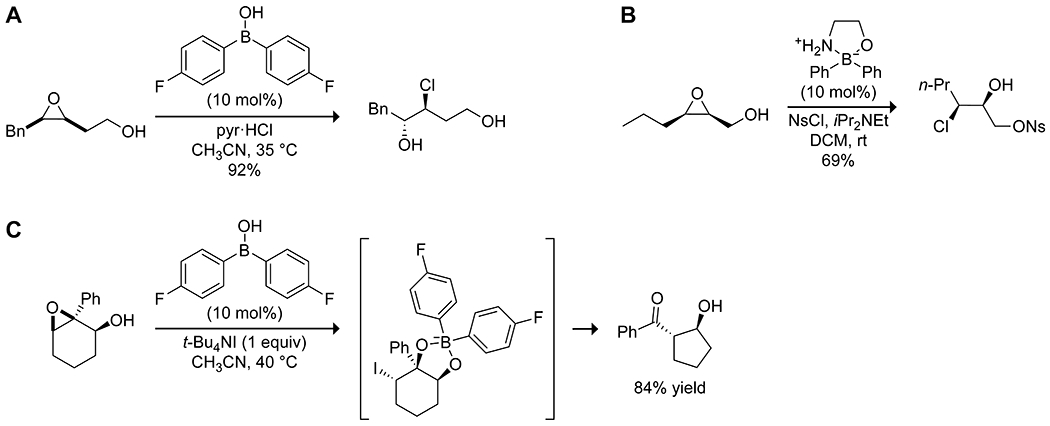
Borinic Acid-Catalyzed Epoxide Ring-Opening with (A) a Halide,159 (B) a Sulfonyl or Acyl Chloride,160,161 and (C) a Semipinacol Rearrangement162
Aziridination.
In an interesting example, Maruoka163 deployed the in situ coordination of 2-carboxyphenylboronic acid with chiral diols to produce a chiral carboxylic acid catalyst for the aziridination of N-benzyl imines with diazoacetamides (Scheme 51). Internal coordination of the boron increased the acidity of the carboxylic acid allowing the reaction to proceed; weaker acids were insufficient. This catalyst system was able to promote the aziridination of several substituted imines in moderate to high yields and generally high enantioselectivity. Notably, this system enables the assembly of a chiral Brønsted acid catalyst based without reliance on axial chirality (Figure 5).
Scheme 51.
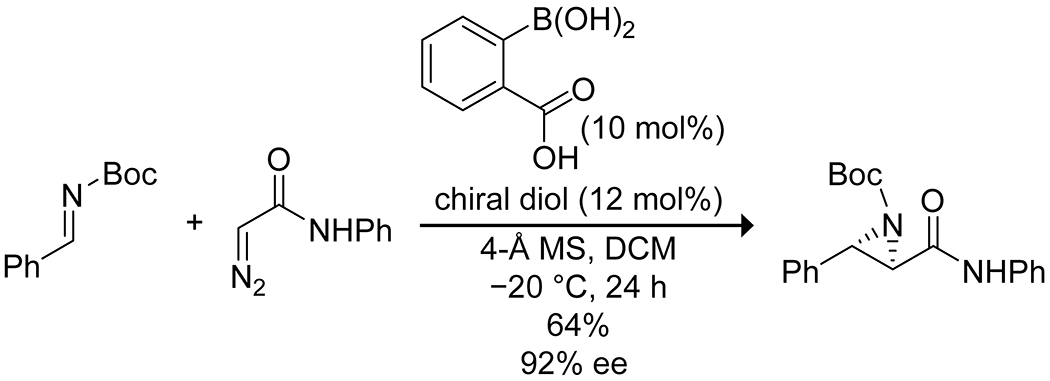
Boronic Acid-Catalyzed Asymmetric Aziridination of an Imine163
Figure 5.
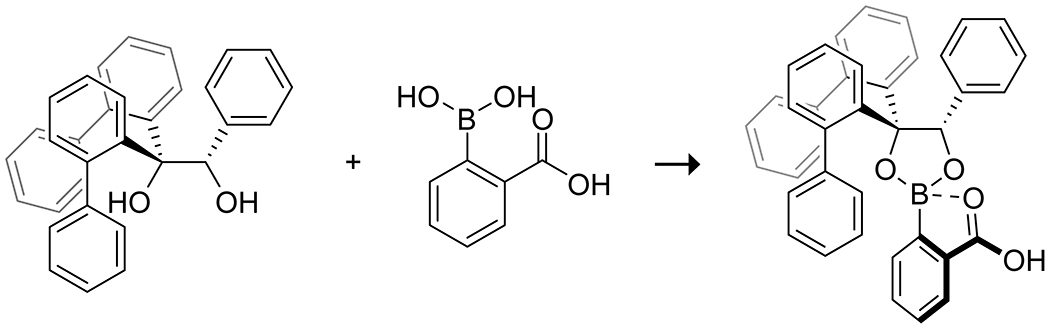
Strategy for the in-situ formation of a chiral Brønsted acid from 2-carboxyphenylboronic acid and a chiral diol.163 Internal coordination of the carboxylic acid to the boronic acid increases the Brønsted acidity.
Chemoselective Oxidation and Reduction.
Organoboron acids have been used to catalyze selective oxidation or reduction reactions. Onomura164 utilized the coordination of boronic acids to 1,2-diols as an activation mode to oxidize a diol selectively to an α-hydroxy ketone without overoxidation, utilizing either electrochemical oxidation or a chemical oxidant (Scheme 52). Yamamoto165 reported that bis(pentafluorophenyl)borinic acid was an effective catalyst for the Oppenauer oxidation of allylic and benzylic alcohols, using pivaldehyde as the hydride acceptor (Scheme 53). The highly electron-deficient catalyst was prone to degradation during the reaction due to hydrolysis of the borinic acid to the corresponding boronic acid, which was catalytically inert. This shortcoming was remedied by the addition of MgSO4(s) to the reaction mixture as a drying agent. Boric acid has been used to activate hydrogen peroxide as an oxidant in the oxidation of sulfides to sulfoxides or sulfones166 and oxidation of arylboronic acids to phenols,167 as well as to promote oxidation of bromide to tribromide for bromination reactions.168 In a particularly notable example, Taylor and co-workers reported on the use of a boronic acid for the site-selective photocatalytic oxidation of carbohydrates to ketosugars (Scheme 54).169 The use of 2-carboxyphenylboronic acid was critical for these transformations due to its high oxidative stability.170 Mannose configured sugars, which were expected to give exclusive C-2 oxidation due to the kinetic preference for oxidation at an axial OH group in the boronate complex, instead yielded C-3 oxidation along with epimerization at C-2. Mechanistic experiments indicated the reaction likely proceeded through oxidation at C-2, followed by isomerization via the enediol. The isomerization could be reduced by replacing quinuclidine with a less basic hydrogen transfer catalyst, though the procedure requires further optimization.
Scheme 52.
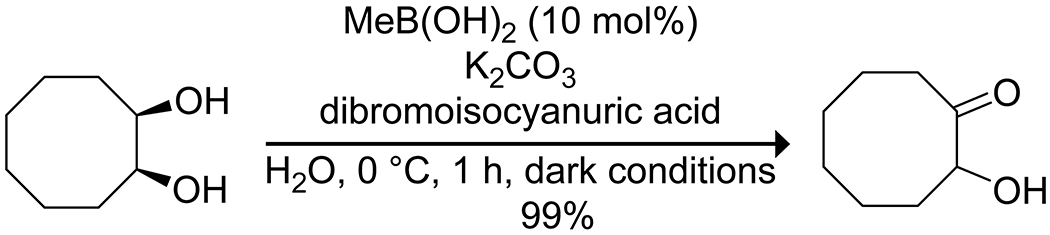
Boronic Acid Catalyzed Oxidation of a 1,2-Diol to an α-Hydroxy Ketone164
Scheme 53.
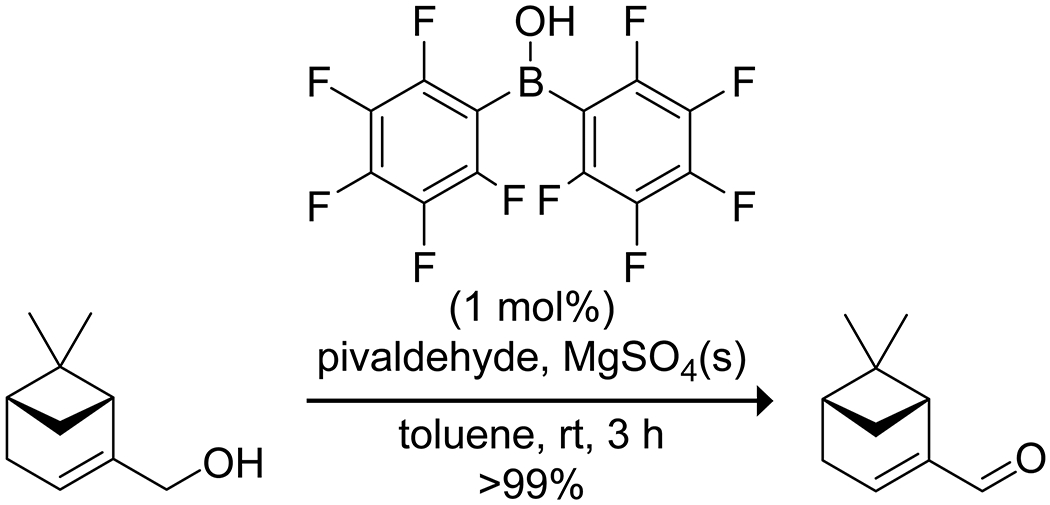
Borinic Acid Catalyzed Oppenauer Oxidation of an Alcohol165
Scheme 54.
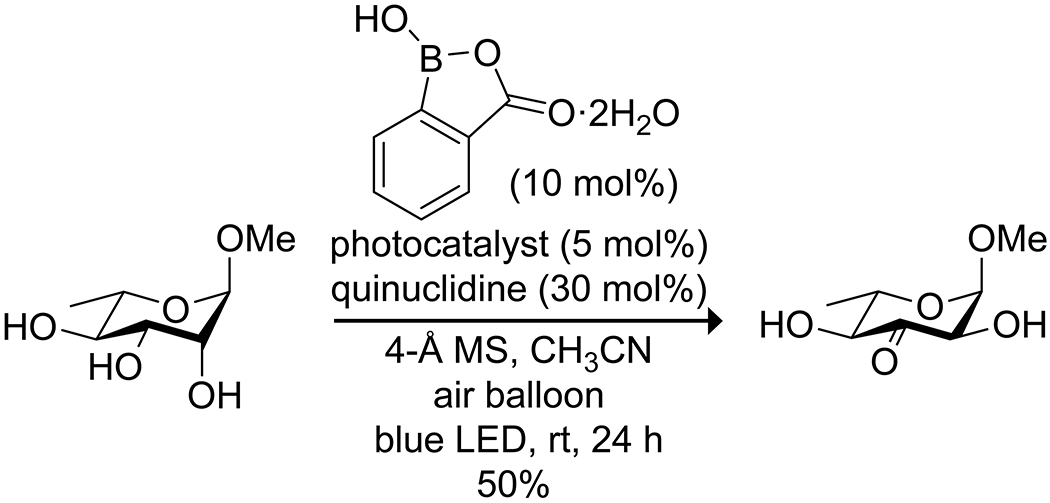
Boronic Acid Catalyzed Site-Selective Photochemical Oxidation of an Unprotected Carbohydrate169
Several interesting reduction reactions have been reported with boronic acids. Beller and co-workers reported that benzothiophene boronic acids were effective catalysts for the chemoselective reduction of tertiary, secondary, and primary amides by hydrosilylation using phenylsilane (Scheme 55).171 Other reducible functional groups, including nitro, cyano, and ester moieties, were untouched by this system. Data from mechanistic studies were inconsistent with direct activation of the amide carbonyl group by complexation to the boron. Instead, the data suggest activation by a hydrogen bond, though that explanation does not explain the observed changes in the boronic acid structure observed by NMR spectroscopy. Yu and Wang showed that arylboronic acids can promote the reduction of aldehydes with tributyltin hydride; however, attempts to use the boronic acid in substoichiometric amounts resulted in poor yields, likely due to preferential complexation with the reaction product, and the reaction failed with ketones (Scheme 56).172 With α,β-unsaturated aldehydes, exclusive 1,2-reduction was apparent. Yu and Wang proposed direct activation of the aldehyde as a Lewis acid–base complex, though they could not explain why boronate esters of the catalyst failed to promote the reaction. Borinic acids were employed by Blanchet and co-workers with silane reductants for the metal-free reduction of phosphine oxides, sulfoxides, and amine N-oxides (Scheme 57).173 Analysis of the mechanism indicated that the active catalyst was a borohydride derived from the borinic acid and the silane. Finally, simple boric acid has been used to promote the reduction of quinolines using Hantzsch esters through coordination of the quinoline nitrogen to the boron.174
Scheme 55.
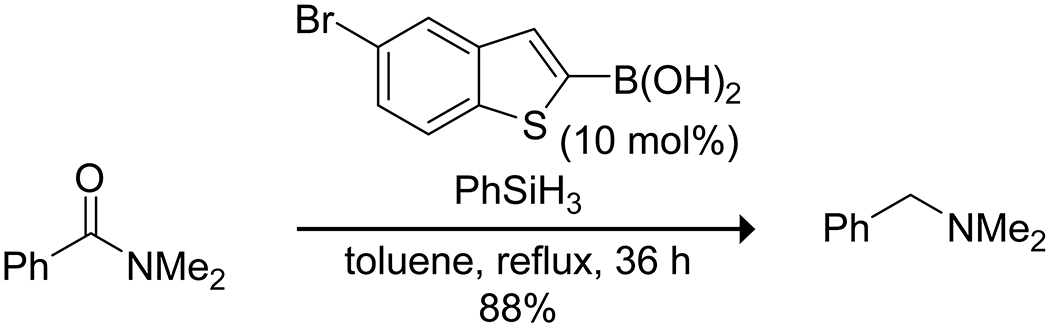
Boronic Acid Catalyzed Reduction of an Amide with a Silane171
Scheme 56.
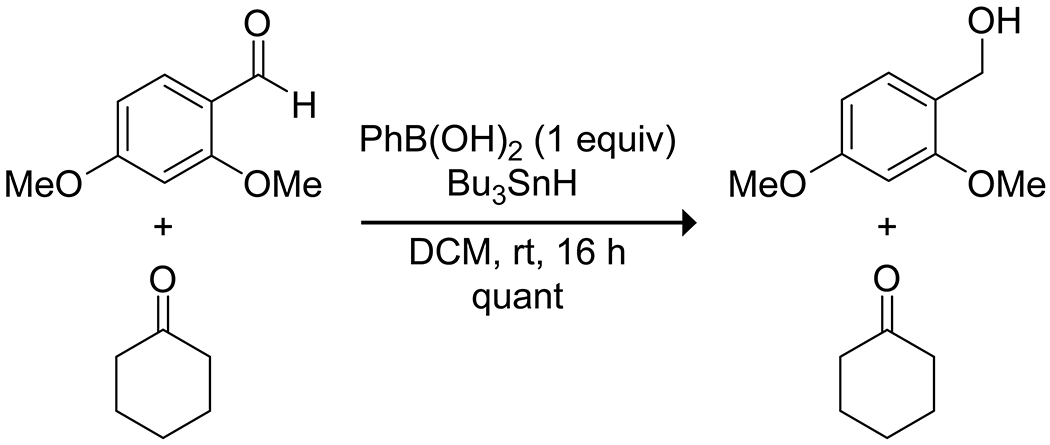
Boronic Acid Promoted Selective Reduction of Aldehydes172
Scheme 57.
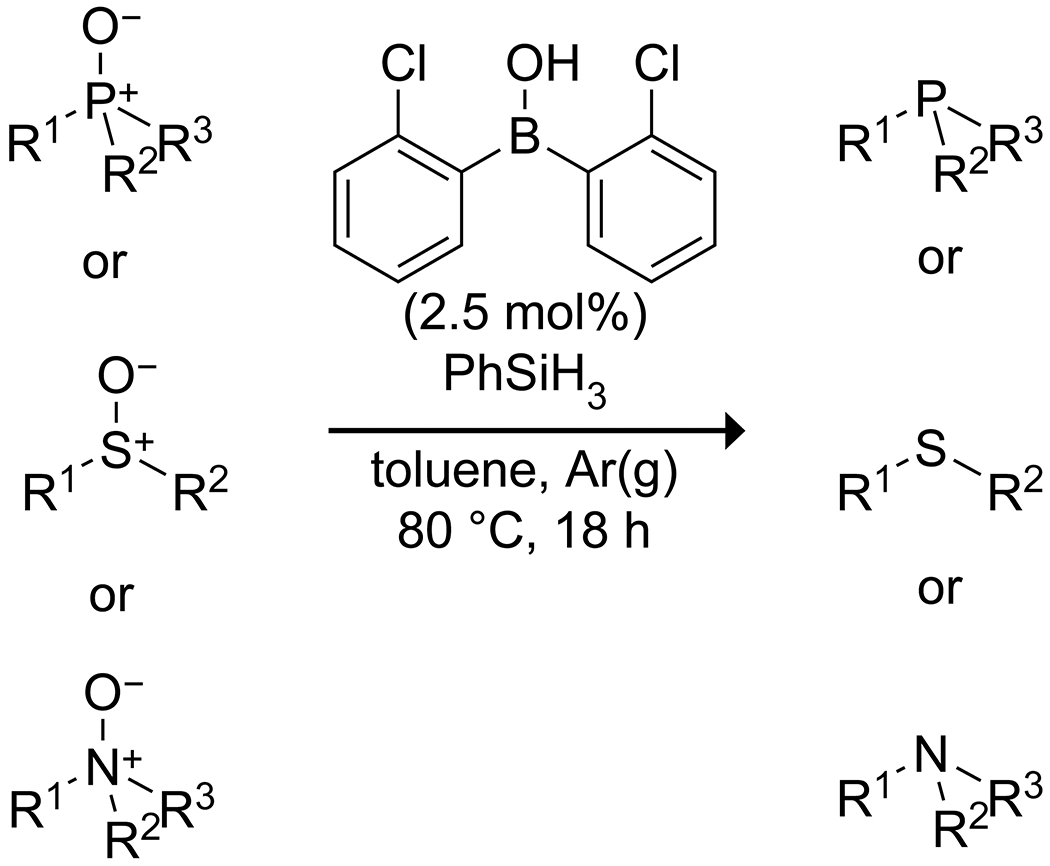
Borinic Acid Catalyzed Reduction of Phosphine Oxides, Sulfoxides, and Amine N-Oxides173
CO2 Activation.
Among the many catalysts developed for the reductive functionalization of CO2, a few boronic acids have shown utility. Li and co-workers reported that sodium phenylboronate salts in combination with a silane can catalyze the reductive formylation of amines with carbon dioxide (Scheme 58).175 The authors suggested that the catalyst acts through electrostatic interactions with the silane to promote the formation of silyl formates with CO2, which then react with amines to produce the formamide product. Wang and Zhang reported that 2,6-dimethylphenylboronic acid in conjunction with tetrabutylammonium iodide could catalyze the reaction of simple epoxides with carbon dioxide to form cyclic carbonates in water (Scheme 59).176 NMR spectroscopy and DFT calculations suggest that the boronic acid activates the epoxide and CO2 by hydrogen bonding through the Brønsted acidity of the boronic acid, allowing for its high catalytic efficiency despite its low Lewis acidity in water.
Scheme 58.
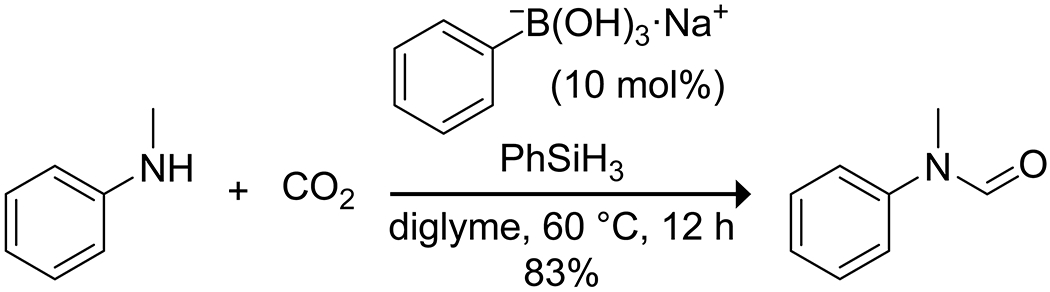
Catalysis of the Reductive Formylation of an Amine with Carbon Dioxide by a Sodium Phenylboronate Salt175
Scheme 59.
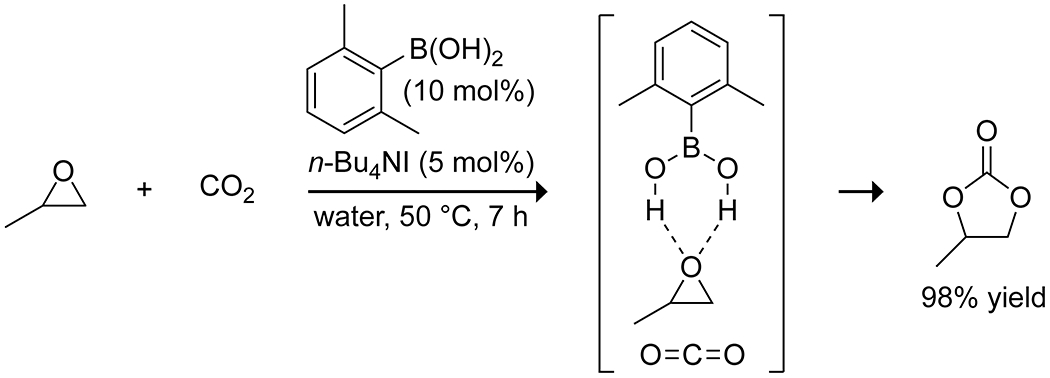
Boronic Acid Catalyzed Synthesis of a Cyclic Carbonate from an Epoxide and CO2176
Silylation.
Building on their work on site-selective functionalization of carbohydrates, the Taylor group developed a methodology that combines a boronic acid and amine to promote site-selective silylation of pyranosides (Scheme 60).177 Here, borinic acids proved to have low activity, and electron-deficient arylboronic acids proved to be most effective. The addition of an amine base and triphenylphosphine oxide promoted the formation of a tetrahedral boronate complex with the pyranoside, activating a hydroxy group as a nucleophile and promoting its reaction with a chlorosilane. Rostami and co-workers reported that boric acid is a useful promoter of the formation and deprotection of trimethylsilyl alcohols (Scheme 61).178 Trimethylsilane-protected alcohols could be obtained with a mixture of hexamethyldisilazane and boric acid in acetonitrile under mild conditions. Catalytic boric acid in water could then hydrolyze the trimethylsilane ethers back to the parent alcohols, likely through simple acid hydrolysis.
Scheme 60.
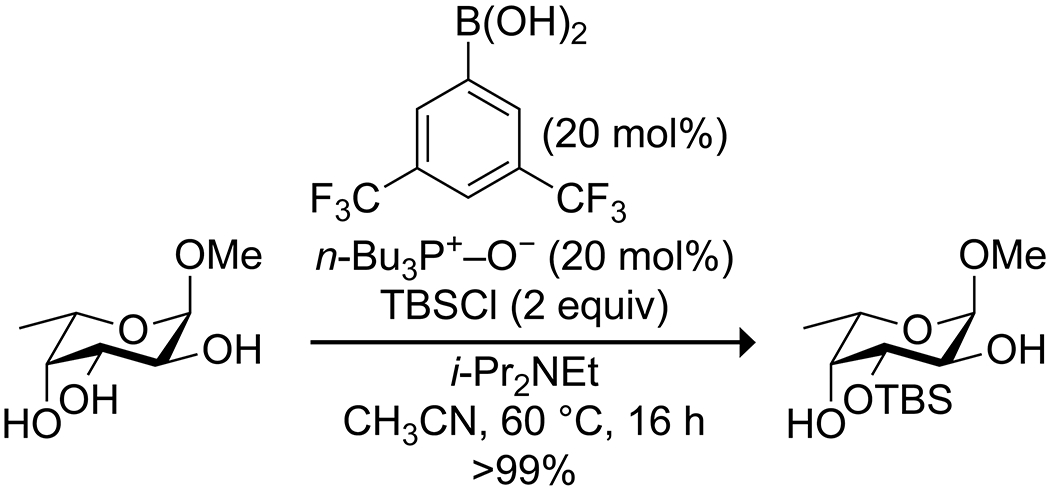
Boronic Acid Catalyzed Site-Selective Silylation of a Pyranoside177
Scheme 61.
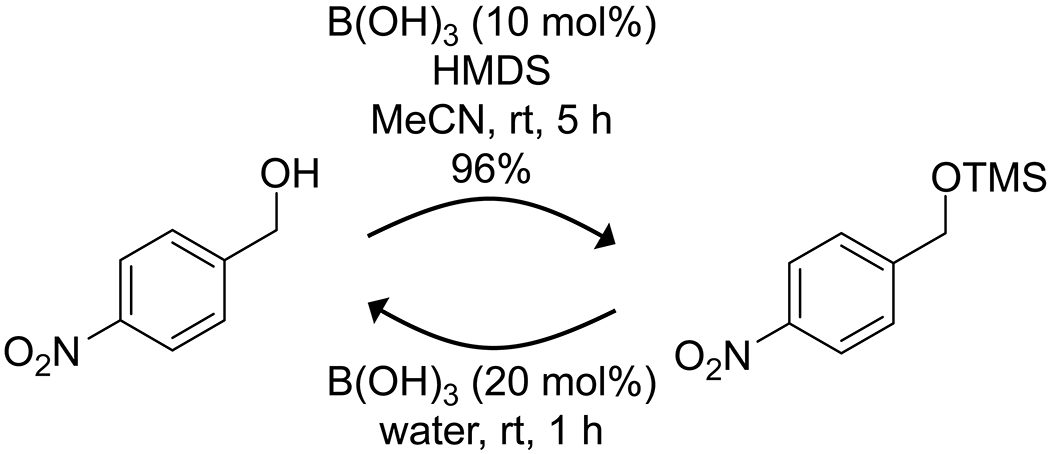
Boric Acid Catalyzed Trimethylsilylation and Deprotection of an Alcohol178
Reactions with Alkenes and Alkynes.
A few reports highlight the promotion of reactions of alkenes and alkynes by organoboron acids. Organ and co-workers showed that the hydrostannylation of internal alkynes promoted by triethylborane does not proceed through a radical mechanism (as was believed previously) but is instead catalyzed by the borinic and boronic acid oxidation products of the borane and can be promoted by boric acid and boronic acids with the involvement of atmospheric oxygen gas (Scheme 62).179 Mechanistic investigations suggested both radical and charged intermediates but are still ambiguous as to the nature of boron catalysis.
Scheme 62.
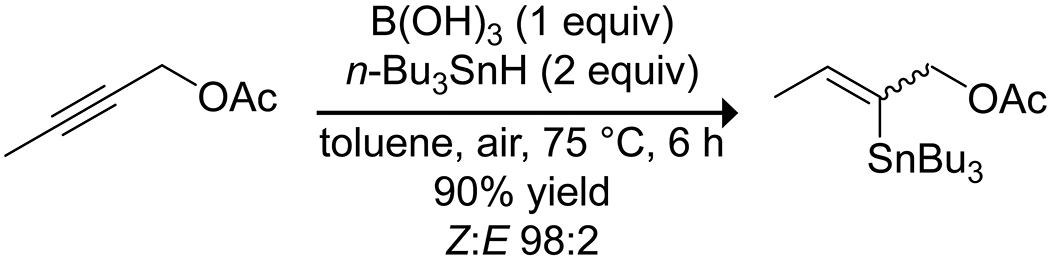
Hydrostannylation of an Alkyne, Promoted by Boric Acid179
C–H Functionalization.
Apart from its effects on the electronics of the oxygen, the complexation of boron acids to diols also affects the bond strength of the C–H bonds of the diol and can lead to stereoselective C–H activation. Taylor and co-workers reported that the combination of a diarylborinic acid and an iridium photocatalyst along with quinuclidine as a hydrogen-transfer agent allowed for the formation of spiro-fused butyrolactones from the reaction of carbohydrates and methyl acrylate (Scheme 63).180 These conditions consistently resulted in reaction at the equatorial C–H bond of cis 1,2-diols, with increased yields and reaction rates relative to the uncatalyzed process and other reported catalysts, though the reaction proved inefficient with other vinyl donors and some carbohydrate scaffolds were relatively unreactive. Density-functional theory calculations indicated that the formation of the borinic acid complex resulted in a small but significant reduction in the bond strength of the C–H bonds alpha to the complexed oxygen atoms (Figure 6), as well as a pronounced kinetic preference for C–H abstraction at the reactive carbon. Using the same photocatalyst and quinuclidine but with an electron-deficient arylboronic acid co-catalyst, Taylor and co-workers were able to convert functionalized furanoside sugars to the corresponding ketodeoxy sugars (Scheme 64).181 This reaction was relatively limited in scope, requiring a cis 2,3-diol, a trans relationship between the anomeric substituent and the C-2 hydroxy group and a limited selection of protecting groups at the 5 position, but showed exquisite site-selectivity for the resulting ketone.
Scheme 63.
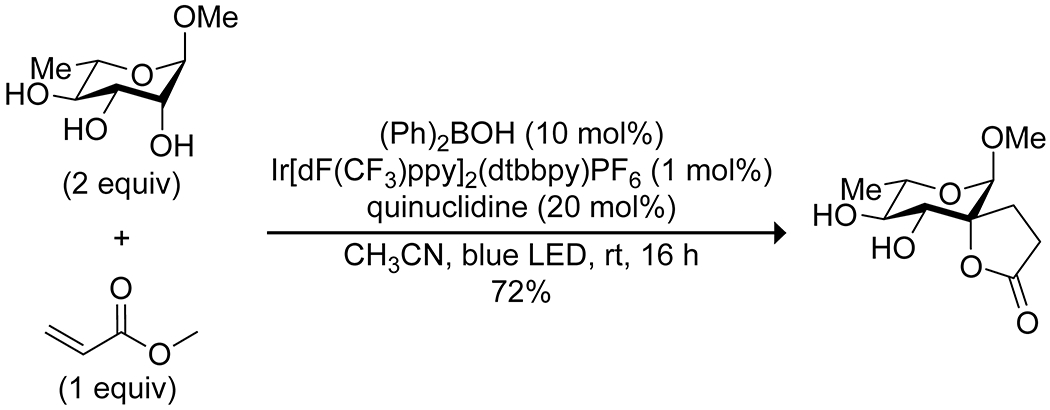
Photocatalytic Alkylation of a Carbohydrate, Directed by a Borinic Acid180
Figure 6.
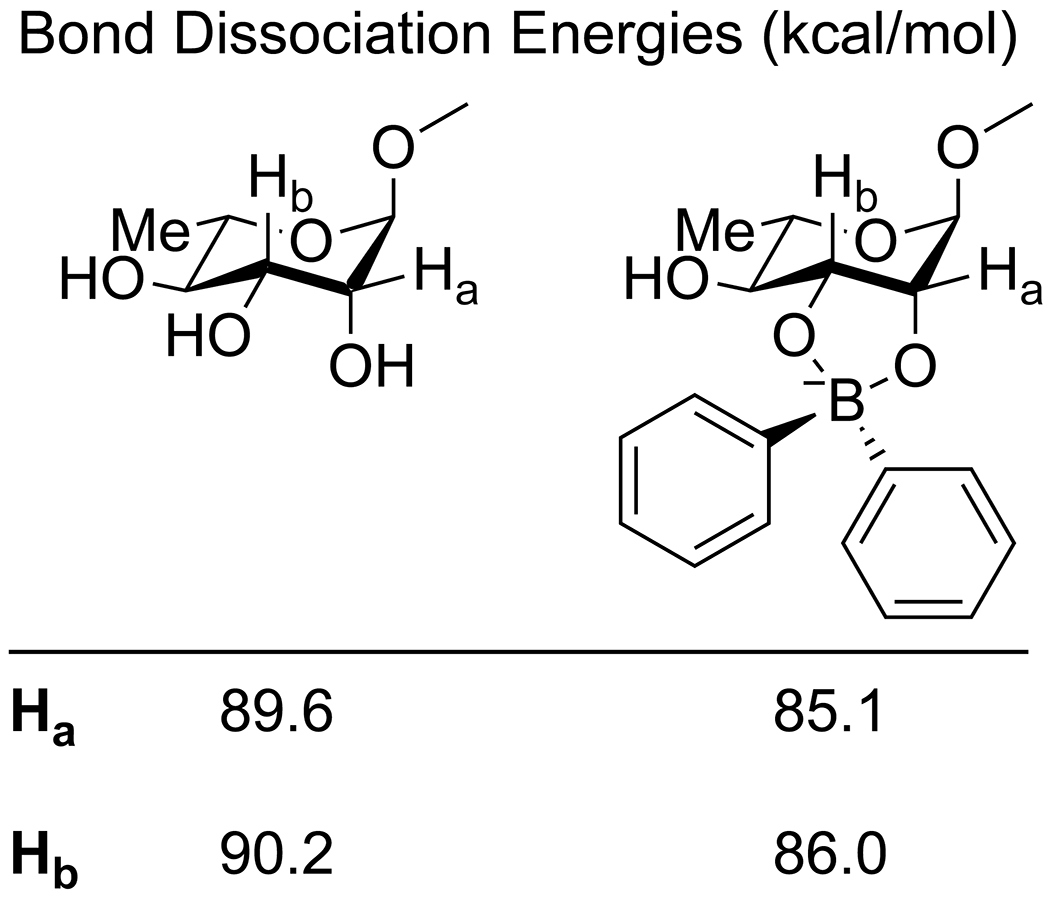
Change in C–H bond energies in rhamnopyranosides upon formation of a complex with diphenylborinic acid.180 Data were calculated in the gas phase with the B97-D3/Def2-TZVP level of theory.
Scheme 64.
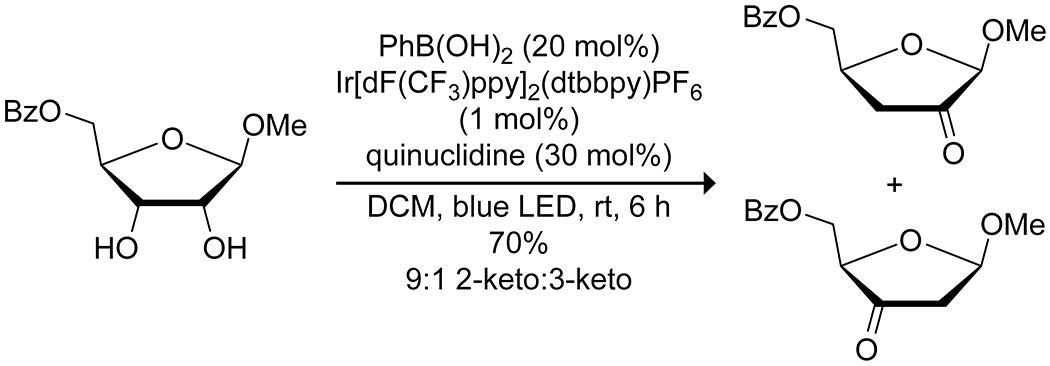
Photocatalytic Oxidation of a Furanoside, Directed by a Boronic Acid181
CONCLUSIONS AND PERSPECTIVES
Boric, boronic, and borinic acids provide useful and highly versatile catalytic platforms. They benefit from mild but tunable Lewis acidity, reversible complexation with Lewis bases such as alcohols, the ability to transition between neutral and anionic states, and strong hydrogen-bonding capabilities. As complements to transition metal catalysts, organoboron acids present a multitude of opportunities for further development.
Often, organoboron acid catalysis entails the use of boron as a simple Lewis acid, enabling reactivity that can also be catalyzed by more traditional Lewis acids. Here, organoboron acids can provide a clear advantage in the “greenness” of the reaction, producing limited byproducts with low toxicity. Access to unique or enhanced reactivity relative to more traditional catalysts is, however, less certain.
Many of the reaction types discussed herein merit further development. The same reactivity provided by organoboron acids can often be achievable by other means and with broader substrate scope. Moreover, boron-catalyzed reactions can require high, sometimes superstoichiometric, catalyst loading, indicating poor catalyst turnover and making scale-up problematic. Catalyst deactivation by the binding of the reaction product can be a substantial challenge. Those reactions that allow for the lowest catalyst loadings, such as amidation reactions, convert a starting material with high affinity for boron (e.g., a carboxylic acid) into a product with low affinity (e.g., an amide), deterring catalyst deactivation.
Organoboron acids are most promising as catalysts of reactions that take advantage of their propensity to form reversible complexes with oxygen atoms. These complexes can lead to both electrophilic activation (in mono alcohols, carboxylic acids, and other carbonyl compounds) and nucleophilic activation (in anionic complex with mono- and diols). Particularly promising is the trend toward combining organoboron acids with other catalysts, taking advantage of the specific complexes formed by boron to provide regio- and stereoselectivity for other catalytic systems. The further development of organoboron acids to increase known reactivity, allowing for lower catalyst loading and milder reaction conditions, as well as the discovery of reactivity unique to organoboron acids will increase the prospects of organoboron acid catalysts for widespread adoption.
ACKNOWLEDGEMENTS
We are grateful to F. G. FitzGerald for comments on the manuscript. Work in our laboratory on organoboron acids is supported by National Institutes of Health (NIH) grant R01 GM044783.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Suzuki A Cross-coupling reactions of organoboranes: An easy way to construct C–C bonds (Nobel lecture). Angew. Chem., Int. Ed 2011, 50, 6722–6737. [DOI] [PubMed] [Google Scholar]
- (2).Brown DG; Boström J Analysis of past and present synthetic methodologies on medicinal chemistry: Where have all the new reactions gone? J. Med. Chem 2016, 59, 4443–4458. [DOI] [PubMed] [Google Scholar]
- (3).Beletskaya IP; Alonso F; Tyurin V The Suzuki–Miyaura reaction after the Nobel prize. Coord. Chem. Rev 2019, 385, 137–173. [Google Scholar]
- (4).Fernández E; Whiting A, Eds. Synthesis and Application of Organoboron Compounds. Springer International: Cham, Switzerland, 2015. [Google Scholar]
- (5).Fernández E, Ed. Advances in Organoboron Chemistry towards Organic Synthesis. Thieme: Stuttgart, Germany, 2020. [Google Scholar]
- (6).Anastas PT; Warner JC, Green Chemistry: Theory and Practice. Oxford University Press: New York, NY, 1998. [Google Scholar]
- (7).Etzkorn FA, Green Chemistry: Principles and Case Studies. Royal Society of Chemistry: London, UK, 2020. [Google Scholar]
- (8).Hall DG, Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, 2nd ed. Wiley–VCH: Weinheim, Germany, 2011. [Google Scholar]
- (9).Zheng H; Hall DG Boronic acid catalysis: An atom-economical platform for direct activation and functionalization of carboxylic acids and alcohols. Aldrichimica Acta 2014, 47, 41–51. [Google Scholar]
- (10).Hall DG Boronic acid catalysis. Chem. Soc. Rev 2019, 48, 3475–3496. [DOI] [PubMed] [Google Scholar]
- (11).Dimitrijević E; Taylor MS Organoboron acids and their derivatives as catalysts for organic synthesis. ACS Catal. 2013, 3, 945–962. [Google Scholar]
- (12).Taylor MS Catalysis based on reversible covalent interactions of organoboron compounds. Acc. Chem. Res 2015, 48, 295–305. [DOI] [PubMed] [Google Scholar]
- (13).Coca A, Ed. Boron Reagents in Synthesis. American Chemical Society: Washington, DC, 2016. [Google Scholar]
- (14).Nori V; Pesciaioli F; Sinibaldi A; Giorgianni G; Carlone A Boron-based Lewis acid catalysis: Challenges and perspectives. Catalysts 2022, 12, 10.3390/catal12010005. [DOI] [Google Scholar]
- (15).Soleilhavoup M; Bertrand G Borylenes: An emerging class of compounds. Angew. Chem., Int. Ed 2017, 56, 10282–10292. [DOI] [PubMed] [Google Scholar]
- (16).Piers WE; Bourke SC; Conroy KD Borinium, borenium, and boronium ions: Synthesis, reactivity, and applications. Angew. Chem., Int. Ed 2005, 44, 5016–5036. [DOI] [PubMed] [Google Scholar]
- (17).Légaré M-A; Pranckevicius C; Braunschweig H Metallomimetic chemistry of boron. Chem. Rev 2019, 8231–8261. [DOI] [PubMed] [Google Scholar]
- (18).McNaught AD; Wilkinson A, Eds. IUPAC. Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”). Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- (19).Yamamoto H, Ed. Lewis Acids in Organic Synthesis. Wiley–VCH: Weinheim, Germany, 2000. [Google Scholar]
- (20).Sandes OM, Ed. The Essential Guide to Lewis Acids. Nova Science: Hauppauge, NY, 2019. [Google Scholar]
- (21).Kazmi MZH; Rygus JPG; Ang HT; Paladino M; Johnson MA; Ferguson MJ; Hall DG Lewis or Brønsted? A rectification of the acidic and aromatic nature of boranol-containing naphthoid heterocycles. J. Am. Chem. Soc 2021, 143, 10143–10156. [DOI] [PubMed] [Google Scholar]
- (22).Dewar MJS; Jones R New heteroaromatic compounds. XXV. Studies of salt formation in boron oxyacids by 11B nuclear magnetic resonance. J. Am. Chem. Soc 1967, 89, 2408–2410. [Google Scholar]
- (23).Zhang S; Lebœuf D; Moran J Brønsted acid and H-bond activation in boronic acid catalysis. Chem.—Eur. J 2020, 26, 9883–9888. [DOI] [PubMed] [Google Scholar]
- (24).Ishihara K; Kurihara H; Yamamoto H Diarylborinic acids as efficient catalysts for selective dehydration of aldols. Synlett 1997, 5, 597–599. [Google Scholar]
- (25).Aelvoet K; Batsanov AS; Blatch AJ; Grosjean C; Patrick LGF; Smethurst CA; Whiting A A catalytic aldol reaction and condensation through in situ boron “ate” complex enolate generation in water. Angew. Chem., Int. Ed 2008, 47, 768–770. [DOI] [PubMed] [Google Scholar]
- (26).Al-Zoubi RM; Marion O; Hall DG Direct and waste-free amidations and cycloadditions by organocatalytic activation of carboxylic acids at room temperature. Angew. Chem., Int. Ed 2008, 47, 2876–2879. [DOI] [PubMed] [Google Scholar]
- (27).Halimehjani AZ; Gholami H; Saidi MR Boric acid/glycerol as an efficient catalyst for regioselective epoxide ring opening by aromatic amines in water. Green Chem. Lett. Rev 2012, 5, 1–5. [Google Scholar]
- (28).Zheng H; McDonald R; Hall DG Boronic acid catalysis for mild and selective [3 + 2] dipolar cycloadditions to unsaturated carboxylic acids. Chem.—Eur. J 2010, 16, 5454–5460. [DOI] [PubMed] [Google Scholar]
- (29).Ståhlberg T; Rodriguez-Rodriguez S; Fristrup P; Riisager A Metal-free dehydration of glucose to 5-(hydroxymethyl)furfural in ionic liquids with boric acid as a promoter. Chem.—Eur. J 2011, 17, 1456–1464. [DOI] [PubMed] [Google Scholar]
- (30).Matsumiya H; Hara T Conversion of glucose into 5-hydroxymethylfurfural with boric acid in molten mixtures of choline salts and carboxylic acids. Biomass Bioenergy 2014, 72, 227–232. [Google Scholar]
- (31).Hansen TS; Mielby J; Riisager A Synergy of boric acid and added salts in the catalytic dehydration of hexoses to 5-hydroxymethylfurfural in water. Green Chem. 2011, 109–114. [Google Scholar]
- (32).Hu L; Sun Y; Lin L; Liu S Catalytic conversion of glucose into 5-hydroxymethylfurfural using double catalysts in ionic liquid. J. Taiwan Inst. Chem. Eng 2012, 43, 718–723. [Google Scholar]
- (33).Xu Z-L; Wang X-Y; Shen M-Y; Du C-H Synthesis of 5-hydroxymethylfurfural from glucose in a biphasic medium with AlCl3 and boric acid as the catalyst. Chem. Pap 2016, 70, 1649–1657. [Google Scholar]
- (34).Lukamto DH; Wang P; Loh TP Catalytic conversion of inert carbohydrates into platform chemical 5-hydroxymethylfurfural using arylboronic acids. Asian J. Org. Chem 2013, 2, 947–951. [Google Scholar]
- (35).Caes BR; Palte MJ; Raines RT Organocatalytic conversion of cellulose into a platform chemical. Chem. Sci 2013, 4, 196–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Graham BJ; Raines RT Efficient metal-free conversion of glucose to 5-hydroxymethylfurfural using a boronic acid. Biomass Convers. Biorefin 2019, 9, 471–477. [Google Scholar]
- (37).Sakakura A; Ohkubo T; Yamashita R; Akakura M; Ishihara K Brønsted base-assisted boronic acid catalysis for the dehydrative intramolecular condensation of dicarboxylic acids. Org. Lett 2011, 13, 892–895. [DOI] [PubMed] [Google Scholar]
- (38).Sakakura A; Yamashita R; Ohkubo T; Akakura M; Ishihara K Intramolecular dehydrative condensation of dicarboxylic acids with Brønsted base-assisted boronic acid catalysts. Aust. J. Chem 2011, 64, 1458–1465. [DOI] [PubMed] [Google Scholar]
- (39).Maraš N; Kočevar M Boric acid-catalyzed direct condensation of carboxylic acids with benzene-1,2-diamine into benzimidazoles. Helv. Chim. Acta 2011, 94, 1860–1874. [Google Scholar]
- (40).Darehkordi A; Hosseini M; Rahmani F Convenient synthesis of new boric acid catalyzed 1,2,4-triazolopyridinone derivatives and an investigation of their optical properties. J. Heterocycl. Chem 2019, 56, 1306–1311. [Google Scholar]
- (41).Schmidt P; Stress C Boronic acids facilitate rapid oxime condensations at neutral pH. Chem. Sci 2015, 6, 3329–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Rao G; Philipp M Boronic acid catalyzed hydrolyses of salicylaldehyde imines. J. Org. Chem 1991, 56, 1505–1512. [Google Scholar]
- (43).Abiko A, Boron enolate chemistry. In Boron Reagents in Synthesis, Coca A, Ed. American Chemical Society: Washington, DC, 2016; pp 123–171. [Google Scholar]
- (44).Offenhauer RD; Nelsen SF Aldehyde and ketone condensation reactions catalyzed by boric acid. J. Org. Chem 1968, 33, 775–777. [Google Scholar]
- (45).Arnold K; Batsanov AS; Davies B; Grosjean C; Schütz T; Whiting A; Zawatzky K The first example of enamine–Lewis acid cooperative bifunctional catalysis: Application to the asymmetric aldol reaction. Chem. Commun 2008, 3879–3881. [DOI] [PubMed] [Google Scholar]
- (46).Lee D; Newman SG; Taylor MS Boron-catalyzed direct aldol reactions of pyruvic acids. Org. Lett 2009, 11, 5486–5489. [DOI] [PubMed] [Google Scholar]
- (47).Kumar V; Sharma U; Verma P; Kumar N; Singh B Silica-supported boric acid with ionic liquid: A novel recyclable catalytic system for one-pot three-component Mannich reaction. Chem. Pharm. Bull 2011, 59, 639–645. [DOI] [PubMed] [Google Scholar]
- (48).Tu S; Fang F; Miao C; Jiang H; Feng Y; Shi D; Wang X One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using boric acid as catalyst. Tetrahedron Lett. 2003, 44, 6153–6155. [Google Scholar]
- (49).Zheng Z-P; Zhang Y-N; Zhang S; Chen J One-pot green synthesis of 1,3,5-triarylpentane-1,5-dione and triarylmethane derivatives as a new class of tyrosinase inhibitors. Bioorg. Med. Chem. Lett 2016, 26, 795–798. [DOI] [PubMed] [Google Scholar]
- (50).Kumar A; Saxena D; Gupta MK Boric acid catalyzed Ugi three-component reaction in aqueous media. RSC Adv. 2013, 3, 4610–4612. [Google Scholar]
- (51).Shinde PV; Sonar SS; Shingate BB; Shingare MS Boric acid catalyzed convenient synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines in aqueous media. Tetrahedron Lett. 2010, 51, 1309–1312. [Google Scholar]
- (52).Moosavi-Zare AR; Afshar-Hezarkhani H; Rezaei MM Tandem four component condensation reaction of aryl aldehydes with ethyl acetoacetate, malononitrile, and hydrazine hydrate using boric acid in water as an efficient and green catalytic system. Polycycl. Aromat. Comp 2020, 40, 150–158. [Google Scholar]
- (53).Mukhopadhyay C; Datta A; Butcher RJ Highly efficient one-pot, three-component Mannich reaction catalysed by boric acid and glycerol in water with major ‘syn’ diastereoselectivity. Tetrahedron Lett. 2009, 50, 4246–4250. [Google Scholar]
- (54).Debache A; Boumoud B; Amimour M; Belfaitah A; Rhouati S; Carboni B Phenylboronic acid as a mild and efficient catalyst for Biginelli reaction. Tetrahedron Lett. 2006, 47. [Google Scholar]
- (55).Debache A; Boulcina R; Belfaitah A; Rhouati S; Carboni B One-pot synthesis of 1,4-dihydropyridines via a phenylboronic acid catalyzed Hantzsch three-component reaction. Synlett 2008, 509–512. [Google Scholar]
- (56).Nemouchi S; Boulcina R; Carboni B; Debache A Phenylboronic acid as an efficient and convenient catalyst for a three-component synthesis of tetrahydrobenzo[b]pyrans. C. R. Chim 2012, 15, 394–397. [Google Scholar]
- (57).Candeias NR; Montalbano F; Cal PMSD; Gois PMP Boronic acids and esters in the Petasis-borono Mannich multicomponent reaction. Chem. Rev 2010, 110, 6169–6193. [DOI] [PubMed] [Google Scholar]
- (58).Das A; Watanabe K; Morimoto H; Ohshima T Boronic acid accelerated three-component reaction for the synthesis of α-sulfanyl-substituted indole-3-acetic acids. Org. Lett 2017, 19, 5794–5797. [DOI] [PubMed] [Google Scholar]
- (59).Lawrence WW Boric acid-catalyzed esterification of phenols. Tetrahedron Lett. 1971, 12, 3453–3454. [Google Scholar]
- (60).Pelter A; Levitt TE; Nelsoni P Some amide forming reactions involving boron reagents. Tetrahedron 1970, 26, 1539–1544. [Google Scholar]
- (61).Ishihara K; Ohara S; Yamamoto H 3,4,5-Trifluorobenzeneboronic acid as an extremely active amidation catalyst. J. Org. Chem 1996, 61, 4196–4197. [DOI] [PubMed] [Google Scholar]
- (62).Ishihara K; Ohara S; Yamamoto H Direct polycondensation of carboxylic acids and amines catalyzed by 3,4,5-trifluorophenylboronic acid. Macromolecules 2000, 33, 3511–3513. [Google Scholar]
- (63).Latta R; Springsteen G; Wang B Development and synthesis of an arylboronic acid-based solid-phase amidation catalyst. Synthesis 2001, 11, 1611–1613. [Google Scholar]
- (64).Houston TA; Wilkinson BL; Blanchfield JT Boric acid catalyzed chemoselective esterification of α-hydroxycarboxylic acids. Org. Lett 2004, 6, 679–681. [DOI] [PubMed] [Google Scholar]
- (65).Levonis SM; Bornaghi LF; Houston TA Selective monoesterification of malonic acid catalyzed by boric acid. Aust. J. Chem 2007, 60, 821–823. [Google Scholar]
- (66).Mylavarapu RK; Kondaiah GCM; Kolla N; Veeramalla R; Koilkonda P; Bhattacharya A; Bandichhor R Boric acid catalyzed amidation in the synthesis of active pharmaceutical ingredients. Org. Process Res. Dev 2007, 11, 1065–1068. [Google Scholar]
- (67).Kondaiah GCM; Reddy AL; Babu KS; Gurav VM; Huge KG; Bandichhor R; Reddy PP; Bhattacharya A; Anand VR Boric acid: An efficient and environmentally benign catalyst for transesterification of ethyl acetoacetate. Tetrahedron Lett. 2008, 49, 106–109. [Google Scholar]
- (68).Barajas JGH; Méndez LYV; Kouznetsov VV; Stashenko EE Efficient synthesis of new N-benzyl- or N-(2-furylmethyl)cinnamamides promoted by the ‘green’ catalyst boric acid, and their spectral analysis. Synthesis 2008, 3, 377–382. [Google Scholar]
- (69).Alemdar N; Erciyes TA; Bicak N Preparation of unsaturated polyesters using boric acid as mild catalyst and their sulfonated derivatives as new family of degradable polymer surfactants. Polymer 2010, 51, 5044–5050. [Google Scholar]
- (70).Nguyen TB; Sorres J; Tran MQ; Ermolenko L; Al-Mourabit A Boric acid: A highly efficient catalyst for transamidation of carboxamides with amines. Org. Lett 2012, 14, 3202–3205. [DOI] [PubMed] [Google Scholar]
- (71).Levonis SM; Pappin BB; Sharp A; Kiefel MJ; Houston TA Boric acid catalyzed methyl esterification of sugar acids. Aust. J. Chem 2014, 67, 528–530. [Google Scholar]
- (72).Ge C; Li L; Zhang R; Wang R; Zhang X Amidation of aromatic amine and benzoic acid under boric acid catalysis. Asian J. Chem 2014, 26, 6805–6807. [Google Scholar]
- (73).Yun F; Cheng C; Zhang J; Li J; Liu X; Xie R; Tang P; Yuan Q Boric acid catalyzed direct amidation between amino-azaarenes and carboxylic acids. Synthesis 2016, 49, 1583–1596. [Google Scholar]
- (74).Lee D; Taylor MS Borinic acid-catalyzed regioselective acylation of carbohydrate derivatives. J. Am. Chem. Soc 2011, 133, 3724–3727. [DOI] [PubMed] [Google Scholar]
- (75).El Dine TM; Rouden J; Blanchet J Borinic acid catalysed peptide synthesis. Chem. Commun 2015, 51, 16084–16087. [DOI] [PubMed] [Google Scholar]
- (76).El Dine TM; Evans D; Rouden J; Blanchet J Formamide synthesis through borinic acid catalysed transamidation under mild conditions. Chem.—Eur. J 2016, 22, 5894–5898. [DOI] [PubMed] [Google Scholar]
- (77).Baraniak MK; Lalancette RA; Jäkle F Electron-deficient borinic acid polymers: Synthesis, supramolecular assembly, and examination as catalysts in amide bond formation. Chem.—Eur. J 2019, 25, 13799–13810. [DOI] [PubMed] [Google Scholar]
- (78).Maki T; Ishihara K; Yamamoto H N-Alkyl-4-boronopyridinium halides versus boric acid as catalysts for the esterification of α-hydroxycarboxylic acids. Org. Lett 2005, 7, 5047–5050. [DOI] [PubMed] [Google Scholar]
- (79).Maki T; Ishihara K; Yamamoto H New boron(III)-catalyzed amide and ester condensation reactions. Tetrahedron 2007, 63, 8645–8657. [Google Scholar]
- (80).Gernigon N; Al-Zoubi RM; Hall DG Direct amidation of carboxylic acids catalyzed by ortho-iodo arylboronic acids: Catalyst optimization, scope, and preliminary mechanistic study supporting a peculiar halogen acceleration effect. J. Org. Chem 2012, 77, 8386–8400. [DOI] [PubMed] [Google Scholar]
- (81).Wang C; Yu H-Z; Fu Y; Guo Q-X Mechanism of arylboronic acid-catalyzed amidation reaction between carboxylic acids and amines. Org. Biomol. Chem 2013, 11, 2140–2146. [DOI] [PubMed] [Google Scholar]
- (82).Yamashita R; Sakakura A; Ishihara K Primary alkylboronic acids as highly active catalysts for the dehydrative amide condensation of α-hydroxycarboxylic acids. Org. Lett 2013, 15, 3654–3657. [DOI] [PubMed] [Google Scholar]
- (83).Mafaune AT; Arnott GE A calix[4]arene based boronic acid catalyst for amide bond formation: Proof of principle study. Arkivoc 2018, 221–229. [Google Scholar]
- (84).Tsuji H; Yamamoto H Synthesis of dipeptides by boronic acid catalysis. Synlett 2018, 29, 318–321. [Google Scholar]
- (85).Mo X; Morgan TDR; Ang HT; Hall DG Scope and mechanism of a true organocatalytic Beckmann rearrangement with a boronic acid/perfluoropinacol system under ambient conditions. J. Am. Chem. Soc 2018, 140, 5264–5271. [DOI] [PubMed] [Google Scholar]
- (86).Wang K; Lu Y; Ishihara K The ortho-substituent on 2,4-bis(trifluoromethyl)phenylboronic acid catalyzed dehydrative condensation between carboxylic acids and amines. Chem. Commun 2018, 54, 5410–5413. [DOI] [PubMed] [Google Scholar]
- (87).Pappin BB; Garget TA; Healy PC; Simone MI; Kiefel MJ; Houston TA Facile amidinations of 2-aminophenylboronic acid promoted by boronate ester formation. Org. Biomol. Chem 2019, 17, 803–806. [DOI] [PubMed] [Google Scholar]
- (88).Shimada N; Nakamura Y; Ochiai T; Makino K Catalytic activation of cis-vicinal diols by boronic acids: Site-selective acylation of carbohydrates. Org. Lett 2019, 21, 3789–3794. [DOI] [PubMed] [Google Scholar]
- (89).Shimada N; Hirata M; Koshizuka M; Ohse N; Kaito R; Makino K Diboronic acid anhydrides as effective catalysts for the hydroxy-directed dehydrative amidation of carboxylic acids. Org. Lett 2019, 21, 4303–4308. [DOI] [PubMed] [Google Scholar]
- (90).Manhas S; Lin Y; Wang G; Kyne LT; Taylor MS Boronic acid-promoted site-selective Fischer esterifications of sugar alcohols. Green Chem. 2019, 21, 5363–5367. [Google Scholar]
- (91).Al-Zoubi RM; Al-Jammal WK; McDonald R Regioselective synthesis of ortho-iodobiphenylboronic acid derivatives: A superior catalyst for carboxylic acid activation. New J. Chem 2020, 44, 3612–3623. [Google Scholar]
- (92).Nakamura Y; Ochiai T; Makino K; Shimada N Boronic acid-catalyzed final-stage site-selective acylation for the total syntheses of O-3′-acyl bisabolol β-D-fucopyranoside natural products and their analogues. Chem. Pharm. Bull 2021, 69, 281–285. [DOI] [PubMed] [Google Scholar]
- (93).Wang M; Zhu G; Li Y; Gu L Arylboronic acids in self-condensation of glucosamines forming deoxyfructosazine, catalysts or reagents? ChemRxiv 2022, DOI: 10.26434/chemrxiv-2021-jmk6d-v4. [DOI] [Google Scholar]
- (94).Koshizuka M; Makino K; Shimada N Diboronic acid anhydride-catalyzed direct peptide bond Formation enabled by hydroxy-directed dehydrative condensation. Org. Lett 2020, 22, 8658–8664. [DOI] [PubMed] [Google Scholar]
- (95).Arkhipenko S; Sabatini MT; Batsanov AS; Karaluka V; Sheppard TD; Rzepa HS; Whiting A Mechanistic insights into boron-catalysed direct amidation reactions. Chem. Sci 2018, 9, 1058–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Sabatini MT; Boulton LT; Sheppard TD Borate esters: Simple catalysts for the sustainable synthesis of complex amides. Sci. Adv 2017, 3, e1701028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Sabatini MT; Karaluka V; Lanigan RM; Boulton LT; Badland M; Sheppard TD Protecting-group–free amidation of amino acids using Lewis acid catalysts. Chem.—Eur. J 2018, 24, 7033–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Coomber CE; Laserna V; Martin LT; Smith PD; Hailes HC; Porter MJ; Sheppard TD Catalytic direct amidations in tert-butyl acetate using B(OCH2CF3)3. Org. Biomol. Chem 2019, 17, 6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Dimakos V; Taylor MS Site-selective functionalization of hydroxyl groups in carbohydrate derivatives. Chem. Rev 2018, 118, 11457–11517. [DOI] [PubMed] [Google Scholar]
- (100).Lorand JP; Edwards JO Polyol complexes and structure of the benzeneboronate ion. J. Org. Chem 1959, 24, 769–774. [Google Scholar]
- (101).Springsteen G; Wang B A detailed examination of boronic acid–diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar]
- (102).Furikado Y; Nagahata T; Okamoto T Universal reaction mechanism of boronic acids with diols in aqueous solution: Kinetics and the basic concept of a conditional formation constant. Chem.—Euro. J 2014, 20, 13194–13202. [DOI] [PubMed] [Google Scholar]
- (103).Slavko E; Taylor MS Site-selective, organoboron-catalyzed polymerization of pyranosides: Access to sugar-derived polyesters with tunable properties. Macromolecules 2020, 53, 8192–8201. [Google Scholar]
- (104).Gorelik D; Lin Y; Briceno-Strocchia AI; Taylor MS Diarylborinic acid-catalyzed, site-selective sulfation of carbohydrate derivatives. J. Org. Chem 2019, 84, 900–908. [DOI] [PubMed] [Google Scholar]
- (105).Gorelik DJ; Turner JA; Turner MS Catalyst-controlled, site-selective sulfamoylation of carbohydrate derivatives. Org. Lett 2022, 24, 5249–5253. [DOI] [PubMed] [Google Scholar]
- (106).McCubbin JA; Hosseini H; Krokhin OV Boronic acid catalyzed Friedel–Crafts reactions of allylic alcohols with electron-rich arenes and heteroarenes. J. Org. Chem 2010, 75, 959–962. [DOI] [PubMed] [Google Scholar]
- (107).McCubbin JA; Krokhin OV Organocatalyzed Friedel–Crafts arylation of benzylic alcohols. Tetrahedron Lett. 2010, 51, 2447–2449. [Google Scholar]
- (108).McCubbin JA; Nassar C; Krokhin OV Waste-free catalytic propargylation/allenylation of aryl and heteroaryl nucleophiles and synthesis of naphthopyrans. Synthesis 2011, 3152–3160. [Google Scholar]
- (109).Zheng H; Ghanbari S; Nakamura S; Hall DG Boronic acid catalysis as a mild and versatile strategy for direct carbo- and heterocyclizations of free allylic alcohols. Angew. Chem., Int. Ed 2012, 51, 6187–6190. [DOI] [PubMed] [Google Scholar]
- (110).Ricardo CL; Mo X; McCubbin AJ; Hall DG A surprising substituent effect provides a superior boronic acid catalyst for mild and metal-free direct Friedel–Crafts alkylations and prenylations of neutral arenes. Chem.—Eur. J 2015, 21, 4218–4223. [DOI] [PubMed] [Google Scholar]
- (111).Mo X; Yakiwchuk J; Dansereau J; McCubbin AJ; Hall DG Unsymmetrical diarylmethanes by ferroceniumboronic acid catalyzed direct Friedel–Crafts reactions with deactivated benzylic alcohols: Enhanced reactivity due to ion-pairing effects. J. Am. Chem. Soc 2015, 137, 9694–9703. [DOI] [PubMed] [Google Scholar]
- (112).Ang HT; Rygus JPG; Hall DG Two-component boronic acid catalysis for increased reactivity in challenging Friedel–Crafts alkylations with deactivated benzylic alcohols. Org. Biomol. Chem 2019, 17, 6007–6014. [DOI] [PubMed] [Google Scholar]
- (113).Auvil TJ; Mattson AE Internal Lewis acid assisted benzoic acid catalysis. Synthesis 2012, 44, 2173–2180. [Google Scholar]
- (114).Meshram HM; Rao NN; Kumar SG Boric acid-mediated mild and efficient Friedel–Crafts alkylation of indoles with nitro styrenes. Synth. Commun 2010, 40, 3496–3500. [Google Scholar]
- (115).Estopiñá-Durán S; Donnelly LJ; McLean EB; Hockin BM; Slawin AMZ; Taylor JE Aryl boronic acid catalysed dehydrative substitution of benzylic alcohols for C−O bond formation. Chem.—Eur. J 2019, 25, 3950–3956. [DOI] [PubMed] [Google Scholar]
- (116).Estopiñá-Durán S; Mclean EB; Donnelly LJ; Hockin BM; Taylor JE Arylboronic acid catalyzed C-alkylation and allylation reactions using benzylic alcohols. Org. Lett 2020, 22, 7547–7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Nazari HS; Forson KG; Martinez EE; Hansen NJ; Gassaway KJ; Lyons NM; Kenney KC; Valdivia-Berroeta GA; Smith SJ; Michaelis DJ Boron-templated dimerization of allylic alcohols to form protected 1,3-diols via acid catalysis. Org. Lett 2019, 21, 9589–9593. [DOI] [PubMed] [Google Scholar]
- (118).Siitonen JH; Kattamuri PV; Yousufuddin M; Kürti L Arylboronic acid-catalyzed C-allylation of unprotected oximes: Total synthesis of N-Me-euphococcine. Org. Lett 2020, 22, 2486–2489. [DOI] [PubMed] [Google Scholar]
- (119).Liu Z; Luan N; Lu H; Liang A; Li J; Zou D; Wu Y; Wu Y Boron-promoted ether interchange reaction: Synthesis of alkyl nitroaromatic ethers from methoxynitroarenes. Eur. J. Org. Chem 2020, 2020, 702–707. [Google Scholar]
- (120).Li M; Yang T; Dixon DJ Boronic acid catalyzed ene carbocyclization of acetylenic dicarbonyl compounds. Chem. Commun 2010, 46, 2191–2193. [DOI] [PubMed] [Google Scholar]
- (121).Liu J; Yao H; Wang C Boronic acid-catalyzed regioselective aminolysis of 3,4-epoxy alcohols. ACS Catal. 2018, 8, 9376–9381. [Google Scholar]
- (122).Wang G; Garrett GE; Taylor MS Borinic acid-catalyzed, regioselective ring opening of 3,4-epoxy alcohols. Org. Lett 2018, 20, 5375–5379. [DOI] [PubMed] [Google Scholar]
- (123).Desai SP; Taylor MS Diarylborinic acid-catalyzed regioselective ring openings of epoxy alcohols with pyrazoles, imidazoles, triazoles, and other nitrogen heterocycles. Org. Lett 2021, 23, 7049–7054. [DOI] [PubMed] [Google Scholar]
- (124).So SS; Burkett JA; Mattson AE Internal Lewis acid assisted hydrogen bond donor catalysis. Org. Lett 2011, 13, 716–719. [DOI] [PubMed] [Google Scholar]
- (125).So SS; Auvil TJ; Garza VJ; Mattson AE Boronate urea activation of nitrocyclopropane carboxylates. Org. Lett 2012, 14, 444–447. [DOI] [PubMed] [Google Scholar]
- (126).Lee D; Williamson CL; Chan L; Taylor MS Regioselective, borinic acid-catalyzed monoacylation, sulfonylation and alkylation of diols and carbohydrates: Expansion of substrate scope and mechanistic studies. J. Am. Chem. Soc 2012, 134, 8260–8267. [DOI] [PubMed] [Google Scholar]
- (127).Chan L; Taylor MS Regioselective alkylation of carbohydrate derivatives catalyzed by a diarylborinic acid derivative. Org. Lett 2011, 13, 3090–3093. [DOI] [PubMed] [Google Scholar]
- (128).Gouliaras C; Lee D; Chan L; Taylor MS Regioselective activation of glycosyl acceptors by a diarylborinic acid-derived catalyst. J. Am. Chem. Soc 2011, 133, 13926–13929. [DOI] [PubMed] [Google Scholar]
- (129).Beale TM; Moon PJ; Taylor MS Organoboron-catalyzed regio- and stereoselective formation of β−2-deoxyglycosidic linkages. Org. Lett 2014, 16, 3604–3607. [DOI] [PubMed] [Google Scholar]
- (130).D’Angelo KA; Taylor MS Borinic acid catalyzed stereo- and regioselective couplings of glycosyl methanesulfonates. J. Am. Chem. Soc 2016, 138, 11058–11066. [DOI] [PubMed] [Google Scholar]
- (131).Izumi S; Kobayashi Y; Takemoto Y Stereoselective synthesis of 1,1′-disaccharides by organoboron catalysis. Angew. Chem., Int. Ed 2020, 59, 14054–14059. [DOI] [PubMed] [Google Scholar]
- (132).Kobayashi Y; Takemoto Y Regio- and stereoselective glycosylation of 1,2-O-unprotected sugars using organoboron catalysts. Tetrahedron 2020, 76, 131328. [Google Scholar]
- (133).Li R-Z; Tang H; Yang KR; Wan L-Q; Zhang X; Liu J; Fu Z; Niu D Enantioselective propargylation of polyols and desymmetrization of meso 1,2-diols by copper/borinic acid dual catalysis. Angew. Chem., Int. Ed 2017, 56, 7213–7217. [DOI] [PubMed] [Google Scholar]
- (134).Estrada CD; Ang HT; Vetter K-M; Ponich AA; Hall DG Enantioselective desymmetrization of 2-aryl-1,3-propanediols by direct O-alkylation with a rationally designed chiral hemiboronic acid catalyst that mitigates substrate conformational poisoning. J. Am. Chem. Soc 2021, 143, 4162–4167. [DOI] [PubMed] [Google Scholar]
- (135).Oshima K; Kitazono E.-i.; Aoyama Y Complexation-induced activation of sugar OH groups. Regioselective alkylation of methyl fucopyranoside via cyclic phenylboronate in the presence of amine. Tetrahedron Lett. 1997, 38, 5001–5004. [Google Scholar]
- (136).Oshima K; Aoyama Y Regiospecific glycosidation of unprotected sugars via arylboronic activation. J. Am. Chem. Soc 1999, 121, 2315–2316. [Google Scholar]
- (137).Shimada N; Sugimoto T; Noguchi M; Ohira C; Kuwashima Y; Takahashi N; Sato N; Makino K Boronic acid-catalyzed regioselective Koenigs–Knorr-type glycosylation. J. Org. Chem 2021, 86, 5973–5982. [DOI] [PubMed] [Google Scholar]
- (138).Nishi N; Nashida J; Kaji E; Takahashi D; Toshima K Regio- and stereoselective β-mannosylation using a boronic acid catalyst and its application in the synthesis of a tetrasaccharide repeating unit of lipopolysaccharide derived from E. coli O75. Chem. Commun 2017, 53, 3018–3021. [DOI] [PubMed] [Google Scholar]
- (139).Takahashi D Boronic-acid-catalyzed regioselective and stereoselective glycosylations via SNi-type mechanism. Trends Glycosci. Glycotechnol 2019, 31, SE93–SE94. [Google Scholar]
- (140).Johnson S; Bagdi AK; Tanaka F C-Glycosidation of unprotected di- and trisaccharide aldopyranoses with ketones using pyrrolidine–boric acid catalysis. J. Org. Chem 2018, 83, 4581–4597. [DOI] [PubMed] [Google Scholar]
- (141).Chaudhuri MK; Hussain S; Kantam ML; Neelima B Boric acid: A novel and safe catalyst for aza-Michael reactions in water. Tetrahedron Lett. 2005, 46, 8329–8331. [Google Scholar]
- (142).Hashimoto T; Gálvez AO; Maruoka K Boronic acid-catalyzed, highly enantioselective aza-Michael additions of hydroxamic acid to quinone imine ketals. J. Am. Chem. Soc 2015, 137, 16016–16019. [DOI] [PubMed] [Google Scholar]
- (143).Michigami K; Murakami H; Nakamura T; Hayama N; Takemoto Y Catalytic asymmetric aza-Michael addition of fumaric monoacids with multifunctional thiourea/boronic acids. Org. Biomol. Chem 2019, 17, 2331–2335. [DOI] [PubMed] [Google Scholar]
- (144).Murakami H; Yamada A; Michigami K; Takemoto Y Novel aza-Michael addition—Asymmetric protonation to α,β-unsaturated carboxylic acids with chiral thiourea–boronic acid hybrid catalysts. Asian J. Org. Chem 2021, 10, 1097–1101. [Google Scholar]
- (145).Hayama N; Kobayashi Y; Takemoto Y Asymmetric hetero-Michael addition to α,β-unsaturated carboxylic acids using thiourea–boronic acid hybrid catalysts. Tetrahedron 2021, 89, 132089. [Google Scholar]
- (146).Li DR; Murugan A; Falck JR Enantioselective, organocatalytic oxy-Michael addition to γ/δ-hydroxy-α,β-enones: Boronate–amine complexes as chiral hydroxide synthons. J. Am. Chem. Soc 2008, 130, 46–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Huang B; He Y; Levin MD; Coelho JAS; Bergman RG; Toste DF Enantioselective kinetic resolution/desymmetrization of para-quinols: A case study in boronic-acid-directed phosphoric acid catalysis. Adv. Synth. Catal 2019, 362, 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Adhikari P; Bhattacharyya D; Nandi S; Kancharla PK; Das A Reductive alkylation of quinolines to N-alkyl tetrahydroquinolines catalyzed by arylboronic acid. Org. Lett 2021, 23, 2437–2442. [DOI] [PubMed] [Google Scholar]
- (149).Horibe T; Hazeyama T; Nakata Y; Takeda K; Ishihara K Enantioselective 1,4-addition reaction of α,β-unsaturated carboxylic acids with cycloalkanones using cooperative chiral amine–boronic acid catalysts. Angew. Chem., Int. Ed 2020, 59, 17256–7260. [DOI] [PubMed] [Google Scholar]
- (150).Desai SP; Zambri MT; Taylor MS Borinic acid catalyzed regioselective N-alkylation of azoles. J. Org. Chem 2022, 87, 5385–5394. [DOI] [PubMed] [Google Scholar]
- (151).Zheng H; Hall DG Mild and efficient boronic acid catalysis of Diels–Alder cycloadditions to 2-alkynoic acids. Tetrahedron Lett. 2010, 51, 3561–3564. [Google Scholar]
- (152).Cao K-S; Bian H-X; Zheng W-H Mild arylboronic acid catalyzed selective [4 + 3] cycloadditions: Access to cyclohepta[b]benzofurans and cyclohepta[b]indoles. Org. Biomol. Chem 2015, 13, 6449–6452. [DOI] [PubMed] [Google Scholar]
- (153).Campillo-Alvarado G; Li C; Feng Z; Hutchins KM; Swenson DC; Höpfl H; Morales-Rojas H; MacGillivray LR Single-crystal-to-single-crystal [2 + 2] photodimerization involving B←N coordination with generation of a thiophene host. Organometallics 2020, 39, 2197–2201. [Google Scholar]
- (154).Campillo-Alvarado G; MacGillivray LR Opportunities using boron to direct reactivity in the organic solid state. Synlett 2021, 32, 655–662. [Google Scholar]
- (155).Corey EJ; Bakshi RK; Shibata S Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines. Mechanism and synthetic implications. J. Am. Chem. Soc 1987, 109, 5551–5553. [Google Scholar]
- (156).Corey EJ; Helal CJ Reduction of carbonyl compounds with chiral oxazaborolidine catalysts: A new paradigm for enantioselective catalysis and a powerful new synthetic method. Angew. Chem., Int. Ed 1998, 37, 1986–2012. [DOI] [PubMed] [Google Scholar]
- (157).Stemmler RT CBS Oxazaborolidines—Versatile catalysts for asymmetric synthesis. Synlett 2007, 6, 997–998. [Google Scholar]
- (158).Corey EJ Enantioselective catalysis based on cationic oxazaborolidines. Angew. Chem., Int. Ed 2009, 48, 2100–2117. [DOI] [PubMed] [Google Scholar]
- (159).Wang G; Taylor MS Borinic acid-catalyzed regioselective ring-opening of 3,4- and 2,3-epoxy alcohols with halides. Adv. Synth. Catal 2020, 362, 398–403. [Google Scholar]
- (160).Tanveer K; Jarrah K; Taylor MS Borinic acid catalyzed, regioselective chloroacylations and chlorosulfonylations of 2,3-epoxy alcohols. Org. Lett 2015, 17, 3482–3485. [DOI] [PubMed] [Google Scholar]
- (161).Garrett GE; Tanveer K; Taylor MS Mechanism of an organoboron-catalyzed domino reaction: Kinetic and computational studies of borinic acid-catalyzed regioselective chloroacylation of 2,3-epoxy alcohols. J. Org. Chem 2017, 82, 1085–1095. [DOI] [PubMed] [Google Scholar]
- (162).Tanveer K; Kim S-J; Taylor MS Borinic acid/halide co-catalyzed semipinacol rearrangements of 2,3-epoxy alcohols. Org. Lett 2018, 20, 5327–5331. [DOI] [PubMed] [Google Scholar]
- (163).Hashimoto T; O. G. l. A.; Maruoka K In situ assembled boronate ester assisted chiral carboxylic acid catalyzed asymmetric trans-aziridinations. J. Am. Chem. Soc 2013, 135, 17667–17670. [DOI] [PubMed] [Google Scholar]
- (164).William JM; Kuriyama M; Onomura O Boronic acid-catalyzed selective oxidation of 1,2-diols to α-hydroxy ketones in water. Adv. Synth. Catal 2014, 356, 934–940. [Google Scholar]
- (165).Ishihara K; Kurihara H; Yamamoto H Bis(pentafluorophenyl)borinic acid as a highly effective Oppenauer oxidation catalyst for allylic and benzylic alcohols. J. Org. Chem 1997, 62, 5664–5665. [Google Scholar]
- (166).Rostami A; Akradi J A highly efficient, green, rapid, and chemoselective oxidation of sulfides using hydrogen peroxide and boric acid as the catalyst under solvent-free conditions. Tetrahedron Lett. 2010, 51, 3501–3503. [Google Scholar]
- (167).Gogoi K; Dewan A; Gogoi A; Borah G; Bora U Boric acid as highly efficient catalyst for the synthesis of phenols from arylboronic acids. Heteroat. Chem 2014, 25, 127–130. [Google Scholar]
- (168).Nath J; Chaudhuri MK Boric acid catalyzed bromination of a variety of organic substrates: An eco-friendly and practical protocol. Green Chem. Lett. Rev 2008, 1, 223–230. [Google Scholar]
- (169).Gorelik DJ; Dimakos V; Adrianov T; Taylor MS Photocatalytic, site-selective oxidations of carbohydrates. Chem. Commun 2021, 57, 12135–12138. [DOI] [PubMed] [Google Scholar]
- (170).Graham BJ; Windsor IW; Gold B; Raines RT Boronic acid with high oxidative stability and utility in biological contexts. Proc. Natl. Acad. Sci. USA 2021, 118, e2013691118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Li Y; de Torre JA; Grabow K; Bentrup U; Junge K; Zhou S; Brückner A; Beller M Selective reduction of amides to amines by boronic acid catalyzed hydrosilylation. Angew. Chem., Int. Ed 2013, 52, 11577–11580. [DOI] [PubMed] [Google Scholar]
- (172).Yu H; Wang B Arylboronic acid-facilitated selective reduction of aldehydes by tributyltin hydride. Synth. Commun 2001, 31, 2719–2725. [Google Scholar]
- (173).Chardon A; Maubert O; Rouden J; Blanchet J Metal-free reduction of phosphine oxides, sulfoxides, and N-oxides with hydrosilanes using a borinic acid precatalyst. ChemCatChem 2017, 9, 4460–4464. [Google Scholar]
- (174).Bhattacharyya D; Nandi S; Adhikari P; Sarmah BK; Konwar M; Das A Boric acid catalyzed chemoselective reduction of quinolines. Org. Biomol. Chem 2020, 18, 1214–1220. [DOI] [PubMed] [Google Scholar]
- (175).Jiang X; Huang Z; Makha M; Du C-X; Zhao D; Wang F; Li Y Tetracoordinate borates as catalysts for reductive formylation of amines with carbon dioxide. Green Chem. 2020, 22, 5317–5324. [Google Scholar]
- (176).Wang J; Zhang Y Boronic acids as hydrogen bond donor catalysts for efficient conversion of CO2 into organic carbonate in water. ACS Catal. 2016, 6, 4871–4876. [Google Scholar]
- (177).Lee D; Taylor MS Regioselective silylation of pyranosides using a boronic acid/Lewis base co-catalyst system. Org. Biomol. Chem 2013, 11, 5409–5412. [DOI] [PubMed] [Google Scholar]
- (178).Rostami A; Akradi J; Ahmad-Jangi F Boric acid as cost-effective and recyclable catalyst for trimethylsilyl protection and deprotection of alcohols and phenols. J. Brazil Chem. Soc 2010, 21, 1587–1592. [Google Scholar]
- (179).Oderinde MS; Hunter HN; Froese RDJ; Organ MG Highly stereo- and regioselective hydrostannylation of internal alkynes promoted by simple boric acid in air. Chem.—Eur. J 2012, 18, 10821–10824. [DOI] [PubMed] [Google Scholar]
- (180).Dimakos V; Su HY; Garrett GE; Taylor MS Site-selective and stereoselective C–H alkylations of carbohydrates via combined diarylborinic acid and photoredox catalysis. J. Am. Chem. Soc 2019, 141, 5149–5153. [DOI] [PubMed] [Google Scholar]
- (181).Dimakos V; Gorelik D; Su HY; Garrett GE; Hughes G; Shibayama H; Taylor MS Site-selective redox isomerizations of furanosides using a combined arylboronic acid/photoredox catalyst system. Chem. Sci 2020, 11, 1531–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]


