Abstract
Background and Objectives:
In healthcare settings, hospital water and water-related devices can act as a reservoir for waterborne infections. Potable water, sinks, faucet aerators, showers, tub immersion, toilets, dialysis water, water baths, eyewash stations, and dental-unit water stations have all been linked to nosocomial outbreaks. This study aimed to determine the microbial profile and pattern of antibiotic resistance in the water supply of a tertiary care hospital in Uttarakhand.
Materials and Methods:
This is a 1-year prospective study which was carried out by the Department of Microbiology and Immunology at Sri Mahant Indersh Hospital (SMIH), Dehradun. A total of 154 water samples were collected from the AC outlets, ventilators in the Intensive care unit (ICUs), Operation theatre (OTs), and High dependency unit (HDUs), scrub stations, pantry, and blood bank, patient’s bathroom, private ward, septic ward, labour room, transplant unit, laboratory, scope rinse water, the dialysis unit and tank throughout the hospital, including tap water (pre and post flush [25%]), tap swabs (24%), drinking water (9%), AC outlets (13%), and other areas (3%).
Results:
30 of the 154 (19.5%) water samples tested were culture-positive. The most contaminated water samples were tap swabs (27%, n = 8/30). A total of nine organisms were isolated, of which the most predominant was Pseudomonas aeruginosa (40%; 12/30), followed by Legionella pneumophila (13%; 4/30), Acinetobacter baumanii (10%; 3/30), Klebsiella pneumoniae (10%; 3/30), Escherichia coli (7%; 2/30), Enterococcus faecalis (7%; 2/30), Aspergillus flavus (7%; 2/30), Stenotrophomonas (3%; 1/30), and Fusarium spp. (3%; 1/30). Gram-negative bacilli and non-lactose fermenting (GNB and NLF) showed a high rate of contamination, 53.3% (n = 16/30). P. aeruginosa showed resistance to gentamicin and amikacin (42%), imipenem (50%), levofloxacin (58%), and colistin (25%), while Acinetobacter baumanii showed resistance to gentamicin and amikacin (67%), minocycline (63%), and levofloxacin, imipenem and colistin (33%).
Conclusion:
The study's findings show that a variety of microorganisms are contaminating hospital water supplies and can be a source of hospital-acquired infections. A suitable and robust surveillance program for hospital water supplies, as well as strict adherence to infection control practices, is strongly advised.
Keywords: Hospital water supply, Pre and post flush, Waterborne pathogens, Nosocomial infections
INTRODUCTION
Emerging water-borne pathogens pose a significant health risk in both developed and developing countries because they can spread quickly and affect large populations. Patients with immunocompromised states (e.g., organ transplantation, stem cell transplantation, malignancies) are particularly vulnerable to severe nosocomial infections caused by waterborne pathogens such as fungi, viruses, bacteria, and parasites, which can result in significant morbidity and mortality (1). According to the Global Burden of Disease report, the burden of water-borne disease was the second leading cause of death in 1990, but it will be the ninth leading cause of death in 2020. Waterborne pathogens can infect patients through a variety of routes, including direct aerosol transmission from water to patients, indirect transmission from fomites that came into contact with contaminated water, improper use of non-sterile water for oral/tracheostomy care of ventilated patients, rinsing respiratory therapy or endoscopic equipment in tap water, hand washing with contaminated water, exposure of implanted devices to contaminated water (e.g., bathing with an improperly covered central venous catheter). Water colonisation of healthcare facility waterworks can occur in either the proximal or distal infrastructure, or in both (2–4). Microbial pathogens can be found in hospital tap water, and biofilms in water systems have long been recognised as a favourable environment for Legionella, Mycobacteria, and Pseudomonas growth (5–7). Waterborne bacteria can grow to varying degrees in both hot and cold water. Cold water is delivered directly to the point of use. Hot water is delivered via a recirculation loop that contains nutrients to feed waterborne microbes, maintains optimum temperatures for microbial growth, and promotes the formation of biofilm on the internal surfaces of pipes and fixtures (2, 8, 9). Waterborne epidemics have appeared in hospital settings as a result of the emergence of novel reported reservoirs, such as electronic faucets (Pseudomonas aeruginosa and Legionella), artistic water wall fountains (Legionella), and heater-cooler equipment used in heart surgery (Mycobacterium chimaera) (10, 11). A significant portion of the microbiota in drinking water is made up of opportunistic pathogens, which have grown to be a major public health concern (12). Among the most prevalent pathogens that cause disease are bacteria like Legionella, Pseudomonas, Acinetobacter, Burkhoderia, Roseobacter, Klebsiella, Alcaligenes, Serratia, Stenotrophomonas, Enterobacter, and Nontuberculous Mycobacteria (NTM) (7, 13–16). Plastic surgery, heart infections, and abscesses at dialysis sites have all been linked to NTM. Municipal water systems are a significant environmental reservoir for NTM (17–19). Fungi such as Aspergillus, Mucor, Trichosporon, Fusarium exophialae (13, 20, 21), viruses (Norovirus), and other microorganisms can be found in hospital water distribution systems and cause nosocomial infections (13). A hospital's water distribution system with adequate air filtration is a potential indoor reservoir of fungi, resulting in secondary aerosolization of fungal propagules and patient exposure to the fungus (20). Many recreational water outbreaks, including a large number of swimming pool outbreaks, have been caused by Adenoviruses (21). The presence of Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) in water and wastewater has recently been reported. Enteric virus contamination of water supplies is a significant source of viral infection (22). Given the aforementioned information, it is essential that every hospital creates their own reliable water supply surveillance system to stop these pathogens from infecting patients (9). Numerous precautionary measures have been suggested. These include hospital epidemiology surveillance, administrative measures, and isolation policies. It was emphasised that temperature, flow, and residual oxidant (chlorine) concentration must all be managed at critical control points. The steps taken to prevent hospital infections from waterborne pathogens should be made clear to hospital staff members. Precautions include washing your hands frequently, using aseptic techniques, limiting the use of equipment connected to water sources, and thoroughly sterilising instruments. In the scientific literature, point-of-use filtration studies have frequently discussed the role of the technology in lowering infections caused by waterborne pathogens and saving money for the medical facility (23, 24).
MATERIALS AND METHODS
This is a prospective study which was carried out by the Department of Microbiology at Shri Guru Ram Rai Institute of Medical and Health sciences, Dehradun between March 2019 to March 2020.
Location of water sample
Water samples were taken from the Air conditioning (AC) outlets, Reverse osmosis (RO) water, ventilators in the Intensive care unit (ICUs), Operation theatre (OTs), and High dependency unit (HDUs), the taps of the scrub stations, pantry and blood bank, patient’s bathroom, private ward, septic ward, labour room, transplant unit, laboratory, scope rinse water, the dialysis unit and tank and swab samples were also taken from the faucet of these respective taps.
Table 1 shows the distribution of all the water samples which were collected. CCU= Cardiac care unit, CHDU= Cardiac high dependency unit, CTVS= Cardio vascular and thoracic surgery, ENT= Ear Nose Throat, GICU= General intensive unit, HDU= High dependency unit, KTU= Kidney transplant unit, MICU= Medical intensive unit, NICU= Neonatal intensive unit, OT= Operation theatre, PICU= Pediatric intensive unit, RICU= Renal intensive unit, SICU= Surgical intensive care unit.
Table 1.
Distribution of water samples
| S. no | Location | Sample type | Total | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Swab | Pre | Post | RO | AC | Others | |||
| 1. | OT-1 (Urology) | 1 | 1 | 1 | 3 | |||
| 2. | OT-2 (Pediatric) | 1 | 1 | 1 | 3 | |||
| 3. | OT-3 (Orthopedic) | 1 | 1 | 1 | 3 | |||
| 4. | OT-4 (Plastic surgery) | 1 | 1 | 1 | 3 | |||
| 5. | OT-5 (Gynaecology) | 1 | 1 | 1 | 3 | |||
| 6. | OT-6 (ENT) | 1 | 1 | 1 | 3 | |||
| 7. | OT-7 (ENT) | 1 | 1 | 1 | 3 | |||
| 8. | OT-8 (Eye) | 1 | 1 | 1 | 3 | |||
| 9. | OT-9 (Neuro) | 1 | 1 | 1 | 3 | |||
| 10. | OT-10 (Orthopedic) | 1 | 1 | 1 | 3 | |||
| 11. | OT-11 (Surgery) | 1 | 1 | 1 | 3 | |||
| 12. | OT-12 (Surgery) | 1 | 1 | 1 | 3 | |||
| 13. | OT-13 (Emergency) | 1 | 1 | 1 | 3 | |||
| 14. | Cardio OT | 1 | 1 | 1 | 3 | |||
| 15. | Dialysis Direct | 1 | 1 | 1 | 3 | |||
| 16. | CHDU | 1 | 1 | 1 | 3 | |||
| 17. | PICU | 1 | 1 | 1 | 3 | |||
| 18. | SICU 1 | 1 | 1 | 1 | 3 | |||
| 19. | SICU 2 | 1 | 1 | 1 | 3 | |||
| 20. | NICU | 1 | 1 | 1 | 3 | |||
| 21. | MICU | 1 | 1 | 1 | 3 | |||
| 22. | KTU | 1 | 1 | 1 | 3 | |||
| 23. | CTVS 1 | 1 | 1 | 1 | 3 | |||
| 24. | CTVS 2 | 1 | 1 | 1 | 3 | |||
| 25. | RICU | 1 | 1 | 1 | 3 | |||
| 26. | GICU | 1 | 1 | 1 | 3 | |||
| 27. | Neuro ICU | 1 | 1 | 1 | 3 | |||
| 28. | Serology Lab | 1 | 1 | 1 | 3 | |||
| 29. | Bacteriology lab | 1 | 1 | 1 | 3 | |||
| 30. | Biochemistry lab | 1 | 1 | 1 | 3 | |||
| 31. | Private ward A | 1 | 1 | 1 | 3 | |||
| 32. | Labour room | 1 | 1 | 1 | 3 | |||
| 33. | Private ward B | 1 | 1 | 1 | 3 | |||
| 34. | Septic ward | 1 | 1 | 1 | 3 | |||
| 35. | CCU | 1 | 1 | 1 | 3 | |||
| 36. | Burn ward | 1 | 1 | 1 | 3 | |||
| 37. | GHDU | 1 | 1 | 1 | 3 | |||
| 38. | Neuro HDU | 1 | 1 | 1 | 3 | |||
| 39. | NABH | 1 | 1 | |||||
| 40. | Plastic surgery | 1 | 1 | |||||
| 41. | Labour room | 1 | 1 | |||||
| 42. | CHDU | 1 | 1 | |||||
| 43. | Skin ward | 1 | 1 | |||||
| 44. | Surgery gallery | 1 | 1 | |||||
| 45. | CCU | 1 | 1 | |||||
| 46. | NICU | 1 | 1 | |||||
| 47. | Neuro ward | 1 | 1 | |||||
| 48. | PICU | 1 | 1 | |||||
| 49. | CTVS | 1 | 1 | |||||
| 50. | Pulmonary med | 1 | 1 | |||||
| 51. | Dialysis ward | 1 | 1 | |||||
| 52. | Gynae ward | 1 | 1 | |||||
| 53. | ENT ward | 1 | 1 | |||||
| 54. | Neuro HDU | 1 | 1 | |||||
| 55. | CHDU | 1 | 1 | |||||
| 56. | Cardio OT | 1 | 1 | |||||
| 57. | CHDU | 1 | 1 | |||||
| 58. | Nephro ward | 1 | 1 | |||||
| 59. | Blood bank | 1 | 1 | |||||
| 60. | Gastro HDU | 1 | 1 | |||||
| 61. | OT-1 | 1 | 1 | |||||
| 62. | OT-2 | 1 | 1 | |||||
| 63. | OT-3 | 1 | 1 | |||||
| 64. | OT-4 | 1 | 1 | |||||
| 65. | OT-5 | 1 | 1 | |||||
| 66. | OT-6 | 1 | 1 | |||||
| 67. | OT-7 | 1 | 1 | |||||
| 68. | OT-8 | 1 | 1 | |||||
| 69. | OT-9 | 1 | 1 | |||||
| 70. | OT-10 | 1 | 1 | |||||
| 71. | OT-11 | 1 | 1 | |||||
| 72. | OT-12 | 1 | 1 | |||||
| 73. | OT-13 | 1 | 1 | |||||
| 74. | Dialysis Tank | 1 | 1 | |||||
| 75. | Eye wash solution | 1 | 1 | |||||
| 76. | Dental wash line | 1 | 1 | |||||
| 77. | Endoscope rinse water | 1 | 1 | |||||
| 78. | Laproscope rinse water | 1 | 1 | |||||
| TOTAL | 154 | |||||||
Sample collection frequency
The samples were taken twice a week in a sterile 100ml jar. All samples were processed within two hours of collection.
Processing of samples
Initially, direct examination of each sample was done by making a wet mount preparation to detect any waterborne pathogenic protozoans which may be present. Then, swabs of the water samples were taken from the faucets of the appropriate taps and streaked on blood and MacConkey agar. Also, each 100 ml water sample was split into five 20 ml portions, with three parts screened for pathogenic Gram negative bacilli, while the fourth and fifth portions screened for fungal and nontuberculous mycobacterium isolates, respectively.
The first part of the sediment was inoculated in MacConkey agar, and the second part was inoculated in Blood agar. The plates were nurtured at 37°C for 48 hours. Sediment from the third part was then inoculated in differential buffered charcoal yeast extract (BCYE) agar and kept in a candle jar at 37°C for 3–5 days. The sediment from the fourth part was inoculated into Sabouraud dextrose agar (SDA) slants containing 400 mg/L chloramphenicol and 25 mg/L gentamicin. Plates were incubated at 30°C for≥28 days (25).
The sample in the fifth part was then processed for the isolation of nontuberculous mycobacterium. Decontamination of the sample was done to eliminate bacterial contamination. This was achieved by taking 2 ml of sample in a sterile15ml falcon tube to which 1 ml of NaOH and 1 ml of trisodium citrate were added and vortex for 10–15 seconds. Then 15ml of phosphate buffer was added and the sample was centrifuged at 3000rpm for 15 minutes. The supernatant was discarded and to the sediment 2 ml of phosphate buffer was added and vortex. 500 ml of sample was then, inoculated into Lowenstein Jenson (LJ) medium and incubated at 37°C for 6–8 weeks.
The isolated colonies of Legionella were identified by performing Gram staining, motility by the hanging drop method, rapid tests such as catalase, oxidase and biochemical tests hippurate hydrolysis.
For fungal identification, Lactophenol cotton blue (LPCB) staining was performed on the pathogenic isolates (26).
The isolated organism from MacConkey and blood agar was tested for identification and antimicrobial susceptibility testing (AST) using an automated system called VITEK 2. It makes use of a colorimetric reagent card with 64 wells, each containing a different test substrate. For Gram-negative, Gram-positive, fastidious, and yeast bacteria, separate cards are available. The system's ability to read each test every 15 minutes requires kinetic analysis. To record fluorescence, turbidity, and colorimetric signals, the optical system combines multichannel fluorimeter and photometer readings. The suspensions were made by emulsifying bacterial isolates in 0.45% saline, equivalent to the McFarland turbidity standard of 0.5. For the VITEK 2 system, identification and AST were performed using the same suspension. The substrate in a well allows for the measurement of a variety of metabolic processes, including enzyme hydrolysis, acidification, and alkalinization, which results in the identification of the organism. A confidence level of matching is reported for the identification after a database comparison of the reaction pattern obtained from the test organism. AST with VITEK 2: It works on the microbroth dilution principle. The 0.5 McFarland bacterial suspension was diluted to 1.5 × 107 CFU/mL. Cards were automatically filled, sealed, and loaded into the VITEK 2 incubation and reading instrument. The minimum inhibitory concentration (MIC) is the highest dilution of an antimicrobial agent that inhibits organism growth. The criteria for selecting antibiotics for the AST were based on the Clinical and Laboratory Standards Institute (CLSI) M100, 30th edition, which was published in January 2020. The AST-N281 card used for Gram-negative bacteria contained amikacin, aztreonam, cefepime, cefoperazone/sulbactam, ceftazidime, ciprofloxacin, colistin, doripenem, gentamicin, imipenem, levofloxacin, meropenem, minocycline, piperacillin/tazobactam, ticarcillin/clavulanic acid, tigecycline, and trimethoprim/sulfamethoxazole.
Statistical analysis
EPI Info software (version 6.0, Centers for Disease Control and Prevention, Atlanta, GA, USA) was used to collect, tabulate, and statistically analyse data. Water samples showing positivity were compared to those with negative samples. Cross tabulation, chi square (X2) and Fischer exact test were used to detect the significant differences between the various samples; a p-value less than 0.05 was considered significant.
RESULTS
A total of 154 water samples were collected from different locations of the hospital, of which 30 samples came out to be positive. The total contamination rate of the water supply was 19.5% (30/154).
The predominant organism isolated was P. aeruginosa, contaminating 40% (12/30) of all the sampled water sources followed by L. pneumophila at 13% (4/30).
A total of 154 samples were collected, as shown in Table 2, of which 38 were swabs from different tap faucets, 38 were pre-flush and post-flush water samples, 15 were reverse osmosis (RO) water samples, 20 were air conditioning (AC) outlet samples, and 5 were samples from various locations, such as the dental wash line, dialysis tank, eye wash solution, laparoscope, and endoscope rinse water. The percentage of positive culture was 19.5% (30/154). Tap swabs were contaminated the most (27%, n =8/30), while other water sources (dialysis tanks) contaminated the least (3%, n=1/30). The percentage of positive culture (30/154) was 19.5%.
Table 2.
Type of water samples
| Sample type | Source | Sample number (n=154) | Sample positive 30 (19.5%) |
|---|---|---|---|
| Swab Water | Tap faucet | 38 | 08 |
| Pre flush | 38 | 07 | |
| Post flush | 38 | 07 | |
| RO | 15 | 03 | |
| AC outlets | 20 | 04 | |
| Others | 05 | 01 |
Table 3 shows the distribution of isolates in various water samples. The predominant organism isolated was P. aeruginosa 40% (n =12/30), followed by L. pneumophila 13% (n =4/30), A. baumanii 10% (n =3/30), K. pneumoniae 10% (n=3/30), E. coli 7% (n =2/30), Enterococcus fecalis 7% (n=2/30), Aspergillus flavus 7% (n=2/30), S. maltophilia 3% (n=1/30), Fusarium spp. 3% (n=1/30).
Table 3.
Occurrence of isolates in water samples
| Orgamism isolated | Number (30) | Percentage (%) |
|---|---|---|
| Pseudomonas aeruginosa | 12 | 40% |
| Legionella pneumophila | 4 | 13% |
| Acinetobacter baumanii | 3 | 10% |
| Klebsiella pneumoniae | 3 | 10% |
| Escherichia coli | 2 | 7% |
| Enterococcus fecalis | 2 | 7% |
| Stenotrophomonas maltophilia | 1 | 3% |
| Fusarium spp. | 1 | 3% |
| Aspergillus flavus | 2 | 7% |
Table 4 depicts the distribution of isolated organisms from their respective locations.
Table 4.
Isolate distribution location wise
| S. No | Sample | Location | Isolated organism |
|---|---|---|---|
| 1. | Tap swab | Neuro ICU | P. aeruginosa |
| PICU | Acinetobacter baumanii | ||
| OT-8 | P. aeruginosa | ||
| NICU | P. aeruginosa | ||
| CTVS 2 | Acinetobacter baumanii | ||
| SICU 2 | P. aeruginosa | ||
| OT-3 | Fuarium spp. | ||
| Dialysis direct | P. aeruginosa | ||
| 2. | Pre flush | Private ward B | L. pneumophila |
| Cardio OT | E. faecalis | ||
| NHDU | K. pneumoniae | ||
| GHDU | Aspergillus flavus | ||
| NICU | P. aeruginosa | ||
| SICU 1 | P. aeruginosa | ||
| OT-2 | E. coli | ||
| 3. | Post flush | MICU | S. maltophilia |
| CCU Septic | P. aeruginosa | ||
| ward GICU |
P. aeruginosa
P. aeruginosa |
||
| RICU | Acinetobacter baumanii | ||
| OT-1 | P. aeruginosa | ||
| KTU | E. faecalis | ||
| 4. | R.O water | Surgery gallery | P. aeruginosa |
| Plastic surgery | K. pneumoniae | ||
| CHDU | K. pneumoniae | ||
| 5. | AC outlet | OT-5 | L. pneumophilia |
| GHDU | Aspergillus flavus | ||
| OT-6 | L. pneumophilia | ||
| OT-8 | L. pneumophilia | ||
| 6. | Others | Dialysis tank | E. coli |
Fig. 1 shows the distribution of P. aeruginosa in different water supplies. As shown in the fig above, the maximum contamination with P. aeruginosa was seen in tap swabs at 42% (n = 5/12), followed by post flush water at 33% (n = 4/12), pre flush water at 17% (n = 2/12), and RO water at 8% (n = 1/12). The contamination of water samples with P. aeruginosa as compared to that with other contaminants was not found statistically non-significant (p = 0.154 >0.05 level).
Fig. 1.
Distribution of P. aeruginosa in different water sources
Fig. 2 shows the distribution of L. pneumophila in different water supply. As shown in the fig above the maximum contamination was seen in AC outlet 75% (n=3/4) followed by pre flush water sample 25% (n=1/4). The contamination of AC water outlets with L. pneumophila was significantly higher than with other contaminants (p<0.05 level).
Fig. 2.
Distribution of L. pneumophila in different water sources
Fig. 3 depicts the distribution of A. baumanii in different hospital water sources. As shown in the figure above, the maximum contamination was seen in tap swabs at 67% (n =2/3), followed by a post-flush water sample at 33% (n =1/3). The contamination of tap swabs with A. baumanii was significantly higher than with other contaminants (p 0.05 level).
Fig. 3.
Distribution of A. baumanii in different water sources
Fig. 4 depicts the distribution of K. pneumoniae in different hospital water sources. As shown in the figure above, the maximum contamination was seen in RO water 67% (n=2/3) followed by pre flush water sample 33% (n=1/3). The contamination of RO water with K. pneumoniae was significantly higher than with other contaminants (p<0.05 level).
Fig. 4.
Distribution of K. pneumoniae in different water sources
As per Fig. 5, P. aeruginosa showed resistance of 58% towards levofloxacin, 50% for imipenem, 42% for amikacin and gentamicin and 25% for colistin.
Fig. 5.
Resistance profile of Pseudomonas aeruginosa
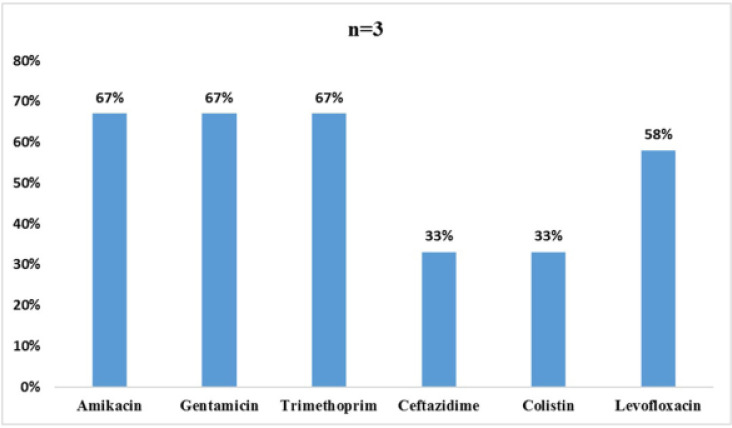
As per Fig. 6, K. pneumoniae showed resistance of 67% towards amikacin, gentamicin, trimethoprim, 58% for levofloxacin and 33 % resistance each to ceftazidime and colistin.
Fig. 6.
Resistance profile of K. pneumoniae
As per Fig. 7, A. baumanii showed showed resistance of 67% towards amikacin, gentamicin and 63% for minocycline and 33% resistance each to levofloxacin, imipenem and colistin
Fig. 7.
Resistance profile of Acinetobacter baumanii
DISCUSSION
The purpose of the microbiological examination of hospital water supplies is to protect the patients as well as the health care workers from nosocomial illnesses and outbreaks brought on by contact with or consumption of water that may be contaminated with pathogens. This study was conducted with the objective of identifying the source of infection.
In the course of our research, we discovered that up to 19.5% (n = 30/154) of samples of different hospital water outlets were contaminated with potential nosocomial pathogens. This is comparable to the findings of Jiun-Ling Wang et al. in 2009 (27), in Japan, and Farzaneh B Asghari et al. in 2013 (28), in Iran, who reported contamination rates ranging from 13.6 to 49.6% in various hospital areas. In 2018, Srivastava et al. conducted a study in India that found that 26.3 percent of hospitals have contaminated drinking water, which is similar to our results (29).
In total, the following three GNB-NLF species were found in hospital water samples: P. aeruginosa, A. baumanii, and S. maltophilia. P. aeruginosa dominated the GNB-NLF strains isolated from water samples, accounting for 40% (12/30), with A. baumanii accounting for 10% (3/30) and S. maltophilia accounting for 3.3% (1/30). On average, they made up 53.3% of the total GNB-NLF count, which is less than findings of Nimfa Maria Stojek et al. in 2008 (30), in Poland, who discovered 71.5 percent of the total GNB-NLF count. This disparity could be attributed to the diverse geographical locations involved.
P. aeruginosa was responsible for 40% of the water contaminants in our study (n = 12/30). The majority of studies have found this, with prevalence rates ranging from 6.8% to 50%. Water samples were collected from various tap water outlets in this study, including the ICU, operating room, surgical ward, and private wards; RO water; AC outlets; and scope rinse water (27).
P. aeruginosa was found in 50% (n = 06/12) of the tap water samples, which is comparable to studies by Matthias Trautmann et al. in 2001 (31), which discovered that 68% (49/72) of water samples taken from water taps in surgical and medical ICUs were positive for P. aeruginosa, and Stefan Reuter et al. in 2002 (32), which discovered a contamination rate of 58% by P. aeruginosa.
Swabs samples show P. aeruginosa colonization rate of 41.6% (n = 05/12) from the inner section of the tap faucets, which is comparable to Dominique Blanc et al.'s findings in 2004 (33), in Switzerland, where they discovered that 42% of the tap swabs were contaminated with P. aeruginosa.
This is because it can cause biofilm to form in water systems, which enables germs to remain in hospital water systems for a long time (4, 34, 35). P. aeruginosa has become well known for its ability to cause nosocomial infection outbreaks with high rates of morbidity and mortality (34, 36, 37). Hospital P. aeruginosa populations are therefore a serious public health problem (33).
Legionella pneumophila was found in 13.3% (n = 4/30) of the positive samples in our study, which is comparable to the 9.6% (5/52) of water samples that Somayeh Yaslianifard et al. found to be positive for Legionella in their study in 2012 (38), in Iran.
However, the mean prevalence of Legionella in the water samples collected in hospitals is only 13.3%, which is lower than 65.7% reported by Nimfa Maria Stojek et al. in 2008 (30), in Poland, and the 63% reported by Pei-Yi Yu et al. in 2008 (39), in Taiwan.
Different sample sizes, a longer study period, and clearly defined geographic borders are most likely the causes of the difference in the isolation rate for Legionella in our study.
Over the course of the study, 10% (n=03/30) of the samples tested positive for filamentous fungus, which is lower than the 50% reported in studies by Marie Pierre Hayette et al. in 2010 (40), in Belgiumand Mesquita-Rocha et al. in 2013 (41), in Brazil.
In our investigation, 6.6% (2/30) of samples were found to be contaminated with Aspergillus flavus, which is similar to Mesquita-Rocha et al. in 2013 (41), in Brazil, who discovered 5.7% (6/106) of infected water samples with Aspergillus flavus.
The 3.3% (1/30) of samples were found to be contaminated with Fusarium spp., which is similar to the study of Mesquita-Rocha et al. in 2013 (41), in Brazil, in which they found 14.1% (15/106) of samples contaminated with Fusarium (39), and Marie Pierre Hayette et al. in 2010 (40) in Belgium, in which they found 11% (11/102) of samples contaminated with Fusarium spp.
We did not find growth of NTM in any of our water samples.
In the current study, P. aeruginosa demonstrated 42% resistance to aminoglycosides, 50% resistance to imipenem, 58% resistance to quinolones, and 25% resistance to colistin, which is comparable to the results observed from a study in 2012 (42), in Italy, that found imipenem resistance to be 36%, quinolone resistance to be 42%, and aminoglycoside resistance to be 14%.
In our study, resistance shown by Acinetobacter baumanii to amikacin was 67%, for imipenem 67%, for quinolones 33%, for colistin 33%, which is similar to a surveillance study in 2009 (43), in Korea, which showed that the resistance rates of A. baumanii were very high: 67% of isolates were resistant toquinolones, 48% to amikacin, 66% to ceftazidime and 51% to imipenem and to another study in 2012 (42), in Italy, which reported resistance to imipenem (80%), ceftazidime and ciprofloxacin (84%), whereas resistance to amikacin was less frequent (18%).
The importance of AST was to learn about the resistance of common waterborne pathogens. It is required for effective decision-making regarding empirical treatment, infection control policies, the rational formulation of public health care policies, effective antibiotic therapy, and appropriate antibiotic usage guidelines.
CONCLUSION
The findings of this study show that the hospital's water supply is a breeding ground for a variety of waterborne pathogens. The identification of these microbes as well as their resistance pattern, would be helpful in preparing strategy for dealing with hospital acquired microbes. In total, 154 samples were collected and tested, and the positivity rate was 19.5% (n = 30). GNB-NLF demonstrated a high rate of contamination (53.3%). Tap swabs contained the highest levels of contamination in water samples (27%) and are a sign of biofilm buildup and contamination close to the point of use. In order to achieve the goal of pathogen-free water stringent application of regulations for water quality are urgently needed and must incorporate more effective microbiological monitoring, pathogen detection, and health risk assessment. To prevent waterborne pathogen infections in hospitals and outpatient settings, hospital staff members and patients should be educated on the necessary precautions. For drinking, brushing teeth, flushing nasogastric tubes, and cleaning nebulizers and other semi-critical respiratory care equipment, the CDC advises using sterile water. Since Legionella species and opportunistic moulds are present in hospital water systems but are not routinely cultured, we advise performing the procedure. The characterization of waterborne pathogens is improved by molecular techniques such as the ability to detect viable microorganisms, which could increase our understanding of waterborne pathogens, our ability to predict pathogen contamination, and our ability to protect public health. Before use, medical equipment, such as nebulizers and endoscopes, should be rinsed with sterile water to prevent the spread of waterborne infections from contaminated tap water and faucet aerators. The accumulation of waterborne pathogens was clearly caused by biofilm deposition within the plastic taps. Because copper and brass have antimicrobial properties, they should be used instead of plastic faucets. They should, however, be replaced on a regular basis to prevent biofilm buildup. It is advised to check for faecal contamination on a regular basis. Tank cleaning must be performed on a regular basis, which is every six months. Thus, surveillance programs and guidelines for safe water supply and infection control practices are highly recommended to limit the spread of infection.
However, we would like to add the following limitations to our study:
Anaerobes were not identified
Follow-up sampling was not done
Molecular characterization was not performed
Time and economics were also our constraints.
In future, we would like to undertake a study with a larger sample size and a longer duration, keeping all these limitations in mind.
REFERENCES
- 1.Sharma S, Sachdeva P, Virdi JS. Emerging water-borne pathogens. Appl Microbiol Biotechnol 2003; 61: 424–428. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang JW, Li Y, Eames I, Chan PKS, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect 2006; 64: 100–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anaissie EJ, Penzak SR, Dignani MC. The hospital water supply as a source of nosocomial infections: a plea for action. Arch Intern Med 2002; 162: 1483–1492. [DOI] [PubMed] [Google Scholar]
- 5.Loveday HP, Wilson JA, Kerr K, Pitchers R, Walker JT, Browne J. Association between healthcare water systems and Pseudomonas aeruginosa infections: a rapid systematic review. J Hosp Infect 2014; 86: 7–15. [DOI] [PubMed] [Google Scholar]
- 6.Cruz AT, Goytia VK, Starke JR. Mycobacterium simiae complex infection in an immunocompetent child. J Clin Microbiol 2007; 45: 2745–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams MM, Armbruster CR, Arduino MJ. Plumbing of hospital premises is a reservoir for opportunistically pathogenic microorganisms: a review. Biofouling 2013; 29: 147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker J, Hoffman P. A pragmatic approach to Pseudomonas. Health Estate 2012; 66: 23–27. [PubMed] [Google Scholar]
- 9.Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis 2001; 7: 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decker BK, Palmore TN. The role of water in healthcare-associated infections. Curr Opin Infect Dis 2013; 26: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar A, Vikas H, Satpathy S, Hakim A. Epidemiological investigation into the source of water contamination at a tertiary care cancer hospital. Int J Res Med Sci 2018; 6: 2796–2800. [Google Scholar]
- 12.Van der Kooij D, Bakker GL, Italiaander R, Veenendaal HR, Wullings BA. Biofilm composition and threshold concentration for growth of Legionella pneumophila on surfaces exposed to flowing warm tap water without disinfectant. Appl Environ Microbiol 2017; 83(5): e02737–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novak Babič M, Gunde-Cimerman N, Vargha M, Tischner Z, Magyar D, Veríssimo C, et al. Fungal contaminants in drinking water regulation? A tale of ecology, exposure, purification and clinical relevance. Int J Environ Res Public Health 2017; 14: 636. [Google Scholar]
- 14.Ferranti G, Marchesi I, Favale M, Borella P, Bargellini A. Aetiology, source and prevention of waterborne healthcare-associated infections: a review. J Med Microbiol 2014; 63: 1247–1259. [DOI] [PubMed] [Google Scholar]
- 15.Wagenlehner FM, MacKenzie FM, Forbes KJ, Gould IM. Molecular epidemiology and antibiotic resistance of Enterobacter spp. from three distinct populations in Grampian, UK. Int J Antimicrob Agents 2002; 20: 419–425. [DOI] [PubMed] [Google Scholar]
- 16.Seara N, Oteo J, Carrillo R, Pérez-Blanco V, Mingorance J, Gómez-Gil R, et al. Interhospital spread of NDM-7-producing Klebsiella pneumoniae belonging to ST437 in Spain. Int J Antimicrob Agents 2015; 46: 169–173. [DOI] [PubMed] [Google Scholar]
- 17.Nagpal A, Wentink JE, Berbari EF, Aronhalt KC, Wright AJ, Krageschmidt DA, et al. A cluster of Mycobacterium wolinskyi surgical site infections at an academic medical center. Infect Control Hosp Epidemiol 2014; 35: 1169–1175. [DOI] [PubMed] [Google Scholar]
- 18.Mulla SA, Revdiwala S. Assessment of biofilm formation in device-associated clinical bacterial isolates in a tertiary level hospital. Indian J Pathol Microbiol 2011; 54: 561–564. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (2015). Non-tuberculous Mycobacterium (NTM) infections and heater-cooler devices interim practical guidance. Atlanta (US) URL: https://stacks.cdc.gov/view/cdc/41693 [Google Scholar]
- 20.Centers for Disease Control and Prevention . Guidelines for environmental infection control in health-care facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003; 52(RR-10):1–48. https://www.cdc.gov/infectioncontrol/guidelines/environmental/index.html [PubMed] [Google Scholar]
- 21.Kumar P, Srivastava S, Banerjee A, Banerjee S. Prevalence and predictors of water-borne diseases among elderly people in India: evidence from Longitudinal Ageing Study in India, 2017–18. BMC Public Health 2022; 22: 993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran HN, Le GT, Nguyen DT, Juang R-S, Rinklebe J, Bhatnagar A, et al. SARS-CoV-2 coronavirus in water and wastewater: A critical review about presence and concern. Environ Res 2021; 193: 110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krageschmidt DA, Kubly AF, Browning MS, Wright AJ, Lonneman JD, Detmer MJ, et al. A comprehensive water management program for multicampus healthcare facilities. Infect Control Hosp Epidemiol 2014; 35: 556–563. [DOI] [PubMed] [Google Scholar]
- 24.Ramírez-Castillo FY, Loera-Muro A, Jacques M, Garneau P, Avelar-González FJ, Harel J, et al. Waterborne pathogens: detection methods and challenges. Pathogens 2015; 4: 307–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tille PM. (2021). Bailey & Scott's Diagnostic Microbiology. 15th ed. St. Louis, Missouri. Elsevier. [Google Scholar]
- 26.Collee JG, Mackie TJ, McCartney JE. (1996). Mackie and McCartney Practical Medical Microbiology. International student edition. 14th ed. Churchill Livingstone publication. University of Michigan. [Google Scholar]
- 27.Wang J-L, Chen M-L, Lin YE, Chang S-C, Chen Y-C. Association between contaminated faucets and colonization or infection by nonfermenting gram-negative bacteria in intensive care units in Taiwan. J Clin Microbiol 2009; 47: 3226–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baghal Asghari F, Nikaeen M, Mirhendi H. Rapid monitoring of Pseudomonas aeruginosa in hospital water systems: a key priority in prevention of nosocomial infection. FEMS Microbiol Lett 2013; 343: 77–81. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava S, Singh A, Ahmed A. Bacteriological analysis of water quality in hospital and residential water supply of a tertiary care hospital of northern India. Int J Curr Microbiol App Sci 2018; 7: 2207–2214. [Google Scholar]
- 30.Stojek NM, Szymanska J, Dutkiewicz J. Gram-negative bacteria in water distribution systems of hospitals. Ann Agric Environ Med 2008; 15: 135–142. [PubMed] [Google Scholar]
- 31.Trautmann M, Michalsky T, Wiedeck H, Radosavljevic V, Ruhnke M. Tap water colonization with Pseudomonas aeruginosa in a surgical intensive care unit (ICU) and relation to Pseudomonas infections of ICU patients. Infect Control Hosp Epidemiol 2001; 22: 49–52. [DOI] [PubMed] [Google Scholar]
- 32.Reuter S, Sigge A, Wiedeck H, Trautmann M. Analysis of transmission pathways of Pseudomonas aeruginosa between patients and tap water outlets. Crit Care Med 2002; 30: 2222–2228. [DOI] [PubMed] [Google Scholar]
- 33.Blanc DS, Nahimana I, Petignat C, Wenger A, Bille J, Francioli P. Faucets as a reservoir of endemic Pseudomonas aeruginosa colonization/infections in intensive care units. Intensive Care Med 2004; 30: 1964–1968. [DOI] [PubMed] [Google Scholar]
- 34.Lavenir R, Jocktane D, Laurent F, Nazaret S, Cournoyer B. Improved reliability of Pseudomonas aeruginosa PCR detection by the use of the species-specific ecfX gene target. J Microbiol Methods 2007; 70: 20–29. [DOI] [PubMed] [Google Scholar]
- 35.Mena KD, Gerba CP. Risk assessment of Pseudomonas aeruginosa in water. Rev Environ Contam Toxicol 2009; 201: 71–115. [DOI] [PubMed] [Google Scholar]
- 36.Hota S, Hirji Z, Stockton K, Lemieux C, Dedier H, Wolfaardt G, et al. Outbreak of multidrug-resistant Pseudomonas aeruginosa colonization and infection secondary to imperfect intensive care unit room design. Infect Control Hosp Epidemiol 2009; 30: 25–33. [DOI] [PubMed] [Google Scholar]
- 37.Kerr KG, Snelling AM. Pseudomonas aeruginosa: a formidable and ever-present adversary. J Hosp Infect 2009; 73: 338–344. [DOI] [PubMed] [Google Scholar]
- 38.Yaslianifard S, Mobarez AM, Fatolahzadeh B, Feizabadi MM. Colonization of hospital water systems by Legionella pneumophila, Pseudomonas aeroginosa, and Acinetobacter in ICU wards of Tehran hospitals. Indian J Pathol Microbiol 2012; 55: 352–356. [DOI] [PubMed] [Google Scholar]
- 39.Yu P-Y, Lin Y-E, Lin W-R, Shih H-Y, Chuang Y-C, Ben R-J, et al. The high prevalence of Legionella pneumophila contamination in hospital potable water systems in Taiwan: implications for hospital infection control in Asia. Int J Infect Dis 2008; 12: 416–420. [DOI] [PubMed] [Google Scholar]
- 40.Hayette MP, Christiaens G, Mutsers J, Barbier C, Huynen P, Melin P, et al. Filamentous fungi recovered from the water distribution system of a Belgian university hospital. Med Mycol 2010; 48: 969–974. [DOI] [PubMed] [Google Scholar]
- 41.Mesquita-Rocha S, Godoy-Martinez PC, Gonçalves SS, Urrutia MD, Carlesse F, Seber A, et al. The water supply system as a potential source of fungal infection in paediatric haematopoietic stem cell units. BMC Infect Dis 2013; 13: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Francesco MA, Ravizzola G, Peroni L, Bonfanti C, Manca N. Prevalence of multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa in an Italian hospital. J Infect Public Health 2013; 6: 179–185. [DOI] [PubMed] [Google Scholar]
- 43.Lee K, Lee MA, Lee CH, Lee J, Roh KH, Kim S, et al. Increase of ceftazidime- and fluoroquinolone-resistant Klebsiella pneumoniae and imipenem-resistant Acinetobacter spp. in Korea: analysis of KONSAR study data from 2005 and 2007. Yonsei Med J 2010; 51: 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]