Abstract
Introduction
Patients with metastatic neuroendocrine tumor (NET) often have an indolent disease course yet the outcomes for patients with metastatic NET undergoing surgery for non-hormonal (NH) symptoms of GI obstruction, bleeding, or pain is not known.
Methods
We identified patients with metastatic gastroenteropancreatic NET who underwent resection from 2000 to 2016 at 8 academic institutions who participated in the US Neuroendocrine Tumor Study Group.
Results
Of 581 patients with metastatic NET to liver (61.3%), lymph nodes (24.1%), lung (2.1%), and bone (2.5%), 332 (57.1%) presented with NH symptoms of pain (n = 223, 67.4%), GI bleeding (n = 54, 16.3%), GI obstruction (n = 49, 14.8%), and biliary obstruction (n = 22, 6.7%). Most patients were undergoing their first operation (85.4%) within 4 weeks of diagnosis. The median overall survival was 110.4 months, and operative intent predicted survival (p < 0.001) with 66.3% undergoing curative resection. Removal of all metastatic disease was associated with the longest median survival (112.5 months) compared to debulking (89.2 months), or palliative resection (50.0 months; p < 0.001). The 1-, 3-, and 12-month mortality was 3.0%, 4.5%, and 9.0%, respectively. Factors associated with 1-year mortality included palliative operations (OR 6.54, p = 0.006), foregut NET (5.62, p = 0.042), major complication (4.91, p = 0.001), and high tumor grade (11.2, p < 0.001). The conditional survival for patients who lived past 1 year was 119 months.
Conclusions
Patients with metastatic NET and NH symptoms that necessitate surgery have long-term survival, and goals of care should focus on both oncologic and quality of life impact. Surgical intervention remains a critical component of multidisciplinary care of symptomatic patients.
Keywords: Neuroendocrine tumor, Metastatic, Non-hormonal, Symptoms, Palliative surgery
Introduction
Neuroendocrine tumors (NETs) of the gastroenteropancreatic (GEP) tract are a heterogeneous group of tumors with often unknown rate of malignant progression and expected indolent nature.1 At the time of diagnosis, metastases to the liver and lymph nodes are found in 40–50% of patients upon presentation and 13% have no primary NET identified.2 Management of patients with metastatic disease is complex as treatment options hinge on a variety of factors including tumor characteristics, resectability, patient symptoms, and prior treatments.3 The timing and use of surgical resection in the metastatic setting has been debated given the proven efficacy of medical therapies and expected prolonged time before tumor progression.4,5 In patients with liver-only metastasis, resection can provide greater than 50% 10-year survival and upon recurrence, subsequent regional therapies can similarly prolong survival.6 Furthermore, in patients with advanced unresectable metastasis, aggressive surgical debulking of tumor volume can achieve 10-year survival rates of 50–70%.7 While surgery can prolong survival for patients with distant metastatic disease, it is rarely curative as 95% of patients recur.8
NETs are classically defined by symptoms of excessive hormonal production, and carcinoid syndrome is a poor prognostic factor and often a sign of more advanced disease.9 Symptomatology, in fact, often drives decision-making and is an impetus for surgical therapy when not controlled by medical therapies. Outcomes based on NET symptomatology alone have not been studied in depth. One study has found symptomatic presentation of NET is associated with more aggressive features and decreased recurrence free survival.10 The benefits of surgery in patients with metastatic NET over other therapies include palliation of symptoms in addition to any oncologic benefit in prolonging progression.11
Advanced metastatic disease from GI cancers is, in general, a contraindication to surgical intervention as these patients have short survival. However, years of survival is expected even in patients with extensive extrahepatic metastatic NET who benefit from a combination of locoregional and systemic therapies to provide meaningful symptom and progression control12 Symptoms of pain, chronic obstruction, and bleeding from an indolent primary and slowly progressing metastatic disease is a significant clinical challenge. This study investigates the presentation and outcomes of metastatic NETs with NH symptoms and examines the utility of palliative operations.
Methods
Patient Population
This study is a retrospective study using a multi-institutional database of all patients who had resected GEP-NET from 2000 to 2014. The eight participating centers in the database are The Ohio State University Wexner Medical Center and James Comprehensive Cancer Center, Columbus, OH; Winship Cancer Institute, Emory University, Atlanta, GA; Stanford University, Palo Alto, CA; Virginia Mason Medical Center, Seattle, WA; University of Wisconsin School of Medicine and Public Health, Madison, WI; Washington University School of Medicine, St. Louis, MI; Vanderbilt University, Nashville, TN; University of Michigan, Ann Arbor, Michigan. The institutional review board from each institution approved the study.
Patients with metastatic disease were identified by pre-operative imaging or if at the time of operation metastatic disease was found and final staging was coded as “M1” or resection status “R2—distant or distant + local”, Fig. 1. Patients with metastatic disease were grouped into subsets based upon presence of pre-operative symptoms and symptoms related to hormonal excess or not. Non-hormonal GI symptoms included GI bleeding, GI obstruction, biliary obstruction, jaundice, pancreatic obstruction, pancreatitis, or pain with other non-specific symptoms such as nausea/vomiting, anorexia, and diarrhea without carcinoid syndrome and hormonal-only symptoms (hypoglycemia, diarrhea with carcinoid symptoms, carcinoid syndrome, carcinoid crisis, carcinoid heart syndrome, known functional tumor). Patients who were at any time symptomatic were included in the symptomatic group even if they had an operation at a later time for no symptoms. The patients who underwent multiple NET operations, the first NH related operation was used as the index time point.
Fig. 1.

Patient flow diagram
Data Collection and Follow-Up
c clinicopathologic data was compiled which included age, sex, ASA class, comorbidities, genetic syndrome, location of operation, location of metastatic NET, location of primary NET, and standard pathologic data of the resected tumor. Tumor location was grouped as foregut if stomach or duodenum without pancreatic resection was performed and hindgut if resection included the appendix, colon, or rectum. Tumor differentiation and grade were compiled from the final pathology and in the case of multiple tumors the highest category was used. Liver regional therapies included bland embolization, chemoembolization, ablation, and selective internal radiotherapy (Y90). Complications were graded by the classification of Clavien-Dindo13 and considered a major complication if the grade was III or more. Curative was defined as any R0/R1 resection with removal of all known tumor, while non-curative involved both “debulking” and “palliative” procedures. Debulking was defined as intent to remove NET volume to increase survival or control functional symptoms and palliative if the operative plan was incomplete resection for symptom control only. Survival was measured as time from first NH operation to the date of last follow-up.
Statistical Analysis
Patient-specific demographics were reported as medians and interquartile range for continuous variables and compared using non-parametric Kruskal-Wallis test. Categorical variables were reported as number (percent) and analyzed using chi-square analysis. Survival calculations were performed using Kaplan-Meier curves and logrank tests. Logistic regression analysis was used to evaluate any association among variables with 1-year mortality, with coefficients reported as odds ratios (ORs) and corresponding 95% confidence intervals (CIs). Variables with clinical significance or a p value < 0.1 on univariable analysis were included in the final multivariable model. All statistical calculations and graphing were performed using SPSS (v21.0, IBM).
Results
Demographics and Survival of Metastatic NET by Symptomatology
There were 581 patients identified with metastatic neuroendocrine tumors who underwent resection—332 (57.1%) had NH symptoms, 133 (27.7%) were asymptomatic, and 116 (20.0%) had hormonal symptoms only (Table 1). Patients with hormonal-only symptoms were younger at 54.5 years (IQR 33.3–75.7 years) compared to NH symptoms (59.0 years, 41.8–76.2 years) and asymptomatic (60.0 years, 42.4–77.6 years; p = 0.004). A known genetic syndrome was least likely in NH symptom patients (n = 8, 2.4%) and highest in the hormonal group (n = 21, 18.4%; p < 0.001). Overall metastatic disease was known at the time of resection in almost all patients (n = 518, 89.9%) and most commonly was found in the liver (n = 347, 61.3%) or lymph nodes (n = 140, 24.1%). NH symptoms were more likely to come from primary NETs within the small intestine (34.9%), colon (7.5%), or stomach (4.2%) and less likely to be associated with pancreatic primaries (38.0%, p = 0.006). NH symptoms were associated with nearly all of the palliative (n = 36, 10.8%) operations, and nearly half had the only the primary resected (n = 150, 45.2%). NH symptoms were associated with an increased incidence of high-grade tumors (n = 31, 9.3%).
Table 1.
Clinical characteristics of patients with metastatic neuroendocrine tumors by symptom group
| Patient variables, median (IQR) or n (%) | All patients n = 581 | NH symptoms n = 332 | Hormonal only n =116 | Asymptomatic n =133 | p value |
|---|---|---|---|---|---|
|
| |||||
| Age | 58.1 (49.4–67.0) | 59.0 (41.8–76.2) | 54.5 (33.3–75.7) | 60.0 (42.4–77.6) | 0.004 |
| Sex, male | 306 (52.7) | 167 (50.3) | 59 (50.9) | 80 (60.2) | 0.143 |
| Race | 0.028 | ||||
| White | 458 (80.6) | 244 (74.8) | 97 (87.4) | 117 (89.3) | |
| AA | 65 (11.4) | 50 (15.3) | 9 (8.1) | 6 (4.6) | |
| Other | 45 (7.9) | 32 (9.8) | 5 (4.5) | 8 (6.1) | |
| Comorbidities | |||||
| HTN | 263 (45.6) | 163 (49.2) | 45 (39.8) | 55 (41.4) | 0.119 |
| Diabetes | 126 (21.8) | 76 (22.9) | 22 (19.3) | 28 (21.1) | 0.659 |
| BMI | 27.3 (23.8–31.5) | 26.5 (19.4–33.6) | 27.7 (15.9–39.5) | 27.8 (19.5–36.1) | 0.046 |
| Genetic syndrome | 35 (6.0) | 8 (2.4) | 21 (18.4) | 6 (4.5) | < 0.001 |
| Metastasis | 0.444 | ||||
| Imaging | 518 (89.9) | 294 (88.8) | 106 (93.0) | 118 (90.1) | |
| Operative | 63 (10.1) | 38 (11.1) | 10 (7.0) | 15 (9.9) | |
| Metastasis location | |||||
| Liver | 347 (61.3) | 189 (58.2) | 79 (69.9) | 79 (61.7) | 0.086 |
| Lung | 12 (2.1) | 9 (2.8) | 2 (1.8) | 1 (0.8) | 0.397 |
| Bone | 14 (2.5) | 9 (2.8) | 4 (3.6) | 1 (0.8) | 0.333 |
| Lymph node | 140 (24.1) | 82 (24.7) | 30 (25.9) | 28 (21.1) | 0.626 |
| Other | 47 (8.1) | 27 (8.1) | 9 (7.8) | 11 (8.3) | 0.988 |
| Location of primary NET | 0.006 | ||||
| Pancreas | 250 (43.0) | 126 (38.0) | 53 (45.7) | 71 (53.4) | |
| Small bowel | 180 (31.0) | 116 (34.9) | 33 (28.4) | 31 (23.3) | |
| Unknown | 46 (7.9) | 22 (6.6) | 12 (10.3) | 12 (9.0) | |
| Colon/rectum | 32 (5.5) | 25 (7.5) | 4 (3.4) | 3 (2.3) | |
| Duodenum | 32 (5.5) | 14 (4.2) | 12 (10.3) | 6 (4.5) | |
| Stomach | 15 (2.6) | 14 (4.2) | 0 | 1 (0.8) | |
| Other | 26 (4.5) | 15 (4.5) | 2 (1.7) | 9 (6.8) | |
| Operative intent | < 0.001 | ||||
| Curative | 396 (68.2) | 220 (66.3) | 72 (62.1) | 104 (78.2) | |
| Debulking | 146 (25.1) | 76 (22.9) | 41 (35.3) | 29 (21.8) | |
| Palliative | 39 (6.7) | 36 (10.8) | 3 (2.6) | 0 | |
| Operation location | < 0.001 | ||||
| Primary only | 214 (36.8) | 150 (45.2) | 26 (22.4) | 38 (28.6) | |
| Primary + mets | 222 (38.2) | 113 (34.0) | 62 (53.4) | 47 (35.3) | |
| Mets only | 68 (11.7) | 37 (11.1) | 15 (12.9) | 16 (12.0) | |
| Local recurrence | 14 (2.4) | 4 (1.2) | 6 (5.2) | 4 (3.0) | |
| Mets recurrence | 63 (10.8) | 28 (8.4) | 7 (6.0) | 28 (21.1) | |
| Tumor grade | 0.069 | ||||
| Low | 199 (34.3) | 116 (34.9) | 40 (34.5) | 43 (32.3) | |
| Intermediate | 140 (24.1) | 81 (24.4) | 27 (23.3) | 32 (24.1) | |
| High | 39 (6.7) | 31 (9.3) | 5 (4.3) | 3 (2.3) | |
| Unknown | 203 (34.9) | 104 (31.3) | 44 (37.9) | 55 (41.4) | |
Italics used for significant variables
Symptomatology as an indication for resection was used to categorized patients into three separate groups, and their survival outcomes were examined. All patients with metastatic spread who underwent an operation had a median overall survival (OS) of 132.3 months (95%CI 113.5–151.1). Survival between groups was longest in the asymptomatic patients (median 139.1 months, 95%CI 132.5–142.5 months) compared to hormonal-only (median not reached) and NH symptom groups (median 110.4 months, 95%CI 88.3–136.7 months; p = 0.002) (Fig. 2). There was no difference between groups in recurrence-free survival (asymptomatic median 40.7 months, 95%CI 28.7–52.7 months; hormonal only 47.9 months, 95%CI 30.5–65.3 months; NH symptom 52.6 months, 95%CI 37.2–68.1 months; p = 0.418) or progression-free survival (asymptomatic median 61.1 months, 95%CI 4.5–117.8 months; hormonal only 15.9 months,95% CI 2.0–29.8 months; NH symptom 24.1 months, 95%CI 16.6–31.6 months; p = 0.418).
Fig. 2.

Overall survival of patients with metastatic neuroendocrine tumor patients by symptomatology, n = 581. Table shows the number at risk every 2 years
Outcomes of Surgical Resection for Non-Hormonal Symptomatic NETs
In the 332 patients with NH symptoms who underwent operation, the indications included curative resection in 220 (66.3%), debulking in 76 (22.9%), and almost all of the palliative resections in 36 (10.8%) (Table 2). Symptoms were most commonly pain with other non-specific symptoms in 223 (67.4%), diarrhea without carcinoid syndrome in 84 (25.4%), nausea and vomiting in 96 (29.0%), and anorexia in 57 (17.3%). GI bleeding (palliative n = 9, 25.0%; debulking n = 6, 7.9%; curative n = 39, 17.8%; p = 0.043) and obstruction (palliative n = 22, 61.1%; debulking n = 5, 6.6%; curative n = 22, 10.0%; p < 0.001) were common and more likely to be associated with palliative intent procedures. Resection for NH symptoms was commonly performed shortly after diagnosis (median 0.8 months, IQR 0.3–2.3 months) and was the first NET operation in 275 (85.4%). Resection of multiple sites was common (n = 148, 46.1%) and the majority underwent resection of the liver (n = 151, 45.5%), pancreas (n = 136, 41.0%), or small bowel (n = 99, 29.8%).
Table 2.
Characteristics of patients with metastatic neuroendocrine tumors and non-hormonal symptoms by operative intent
| Patient variables, Median (IQR) or n (%) | All NH symptoms n = 332 | Curative n = 220 | Debulking n = 76 | Palliative n = 36 | p value |
|---|---|---|---|---|---|
|
| |||||
| Symptoms | |||||
| GI bleeding | 54 (16.3) | 39 (17.8) | 6 (7.9) | 9 (25.0) | 0.043 |
| GI obstruction | 49 (14.8) | 22 (10.0) | 5 (6.6) | 22 (61.1) | < 0.001 |
| Biliary obstruction | 22 (6.7) | 14 (6.4) | 4 (5.3) | 4 (11.4) | 0.462 |
| Pancreatic obstruction | 11 (3.3) | 9 (4.1) | 1 (1.3) | 1 (2.9) | 0.498 |
| Pancreatitis | 12 (3.6) | 9 (4.1) | 3 (3.9) | 0 | 0.477 |
| Pain | 223 (67.4) | 146 (66.4) | 57 (75.0) | 20 (57.1) | 0.151 |
| Diarrhea | 84 (25.4) | 50 (22.7) | 22 (28.9) | 12 (34.3) | 0.247 |
| Nausea/vomiting | 96 (29.0) | 58 (26.4) | 22 (28.9) | 16 (45.7) | 0.064 |
| Anorexia | 57 (17.3) | 37 (16.8) | 13 (17.1) | 7 (20.6) | 0.863 |
| Metastasis location | |||||
| Liver only | 159 (47.9) | 91 (41.4) | 51 (67.1) | 17 (47.2) | < 0.001 |
| Liver and EHD | 43 (13.0) | 18 (8.2) | 15 (19.7) | 10 (27.8) | |
| EHD only | 92 (27.7) | 80 (36.4) | 7 (9.2) | 5 (13.9) | |
| Unknown | 38 (11.4) | 31 (14.1) | 3 (3.9) | 4 (11.1) | |
| Diagnosis to surgery (months) | 0.8 (0.3–2.3) | 0.7 (0–3.4) | 0.8 (0–3.4) | 0.9 (0–2.6) | 0.659 |
| First NET operation | 275 (85.4) | 191 (87.2) | 60 (84.5) | 24 (75.0) | 0.183 |
| Neoadjuvant treatment | |||||
| Somatostatin analog | 35 (11.3) | 13 (6.2) | 10 (14.3) | 12 (40) | < 0.001 |
| Chemotherapy | 27 (8.4) | 12 (5.5) | 8 (11.3) | 7 (21.9) | 0.005 |
| Radiotherapy | 4 (1.3) | 2 (0.9) | 1 (1.4) | 1 (3.1) | 0.573 |
| Liver regional therapy | 5 (1.6) | 3 (1.4) | 2 (2.8) | 0 | 0.531 |
| Resection site | |||||
| Multiple | 148 (46.1) | 83 (37.9) | 46 (64.8) | 19 (61.3) | < 0.001 |
| Foregut | 19 (5.7) | 15 (6.8) | 2 (2.6) | 2 (5.6) | 0.399 |
| Liver | 151 (45.5) | 84 (38.2) | 51 (67.1) | 16 (44.4) | < 0.001 |
| Pancreas | 136 (41.0) | 87 (39.5) | 37 (48.7) | 12 (33.3) | 0.232 |
| Small bowel | 99 (29.8) | 72 (32.7) | 12 (15.8) | 15 (41.7) | 0.005 |
| Hindgut | 32 (9.6) | 17 (7.7) | 8 (10.5) | 7 (19.4) | 0.083 |
| Lymph node | 16 (4.8) | 9 (4.1) | 5 (6.6) | 2 (5.6) | 0.667 |
| Peritoneum | 12 (3.6) | 6 (2.7) | 3 (3.9) | 3 (8.3) | 0.244 |
Italics used for significant variables
Patients who underwent palliative operations were more likely to have emergent operations (n = 5, 13.9%) and in increase in major complication rates (n = 11, 30.6%) (Table 3). Adjuvant therapies were used more commonly in the non-curative debulking and palliative groups including increased use of chemotherapy, somatostatin analogs, and regional liver therapies. Data regarding recurrence or progression after resection were limited as 47% had missing data and could not be accurately reported. After recurrence or progression subsequent treatment with surgery (n = 34, 21.8%), systemic treatment (n = 97, 61.8%), radiotherapy (n = 15, 9.7%), or regional liver therapy (51, 33.6%) was common. One-year mortality was highest in the palliative cohort at 33.3% (n = 12).
Table 3.
Perioperative treatmentand outcomes of patients withmetastatic neuroendocrine tumorsand non-hormonal symptoms by operative intent
| Patient variables, n (%) | All NH symptoms n = 332 | Curative n = 220 | Debulking n = 76 | Palliative n = 36 | p value |
|---|---|---|---|---|---|
|
| |||||
| Emergency operation | 13 (3.9) | 7 (3.2) | 1 (1.3) | 5 (13.9) | 0.004 |
| Any complication | 164 (49.5) | 111 (50.5) | 36 (47.4) | 17 (48.6) | 0.891 |
| Major (CD ≥ III) | 74 (22.3) | 39 (17.7) | 24 (31.6) | 11 (30.6) | 0.020 |
| Readmission | 68 (20.7) | 41 (18.6) | 15 (20.5) | 12 (34.3) | 0.105 |
| Adjuvant therapy | |||||
| Chemotherapy | 38 (11.8) | 19 (8.8) | 13 (17.1) | 6 (19.4) | 0.060 |
| mTOR inhibitor | 12 (3.7) | 5 (2.3) | 5 (6.6) | 2 (6.7) | 0.162 |
| Somatostatin analog | 68 (21.1) | 30 (13.8) | 27 (35.5) | 11 (36.7) | < 0.001 |
| Radiotherapy | 6 (1.9) | 3 (1.4) | 3 (1.4) | 0 | 0.260 |
| Liver regional therapy | 20 (6.7) | 7 (3.5) | 9 (13.0) | 4 (13.8) | 0.006 |
| Treatment after recurrence/progression | |||||
| Operation | 34 (21.8) | 28 (33.3) | 5 (10.4) | 1 (4.2) | 0.092 |
| Systemic therapy | 97 (61.8) | 50 (58.8) | 32 (66.7) | 15 (62.5) | 0.669 |
| Radiotherapy | 15 (9.7) | 8 (9.6) | 7 (14.6) | 0 | 0.143 |
| Liver regional therapy | 51 (33.6) | 20 (23.8) | 24 (51.1) | 7 (33.3) | 0.007 |
| Mortality | |||||
| 30-day | 10 (3.0) | 4 (1.8) | 3 (3.9) | 3 (8.3) | 0.091 |
| 90-day | 15 (4.5) | 4 (1.8) | 7 (9.2) | 4 (11.1) | 0.004 |
| 1-year | 30 (9.0) | 6 (2.7) | 12 (15.8) | 12 (33.3) | < 0.001 |
Italics used for significant variables
With a median follow-up of 30.4 months (IQR 12.7–66.6 months), curative intent surgery was found to clearly prolong survival (median 112.5 months, 95%CI 86.9–138.1 months) compared to the non-curative options of debulking (89.2 months, 27.9–150.5 months) and palliative surgery (50.0 months, 1.6–98.4; p < 0.001) (Fig. 3a). The 1-, 5-, and 10-year overall survival was 97%, 68%, and 45% in the curative group; 83%, 51%, and 42% in debulking group; and 65%, 38%, and not reached in the palliative group. The survival curves demonstrated a sharp decline in the first 12 months and then remain parallel. The symptomatology indication for operation also drove survival as the patients with biliary and pancreatic obstruction or pancreatitis had improved survival (median NR) compared to pain plus non-specific symptoms of nausea/vomiting, diarrhea, anorexia (median 115.9 months, 95%CI 78.3–153.5 months), and GI obstruction or bleeding (89.2 months, 62.9–115.4 months; p = 0.025) (Fig. 3b).
Fig. 3.
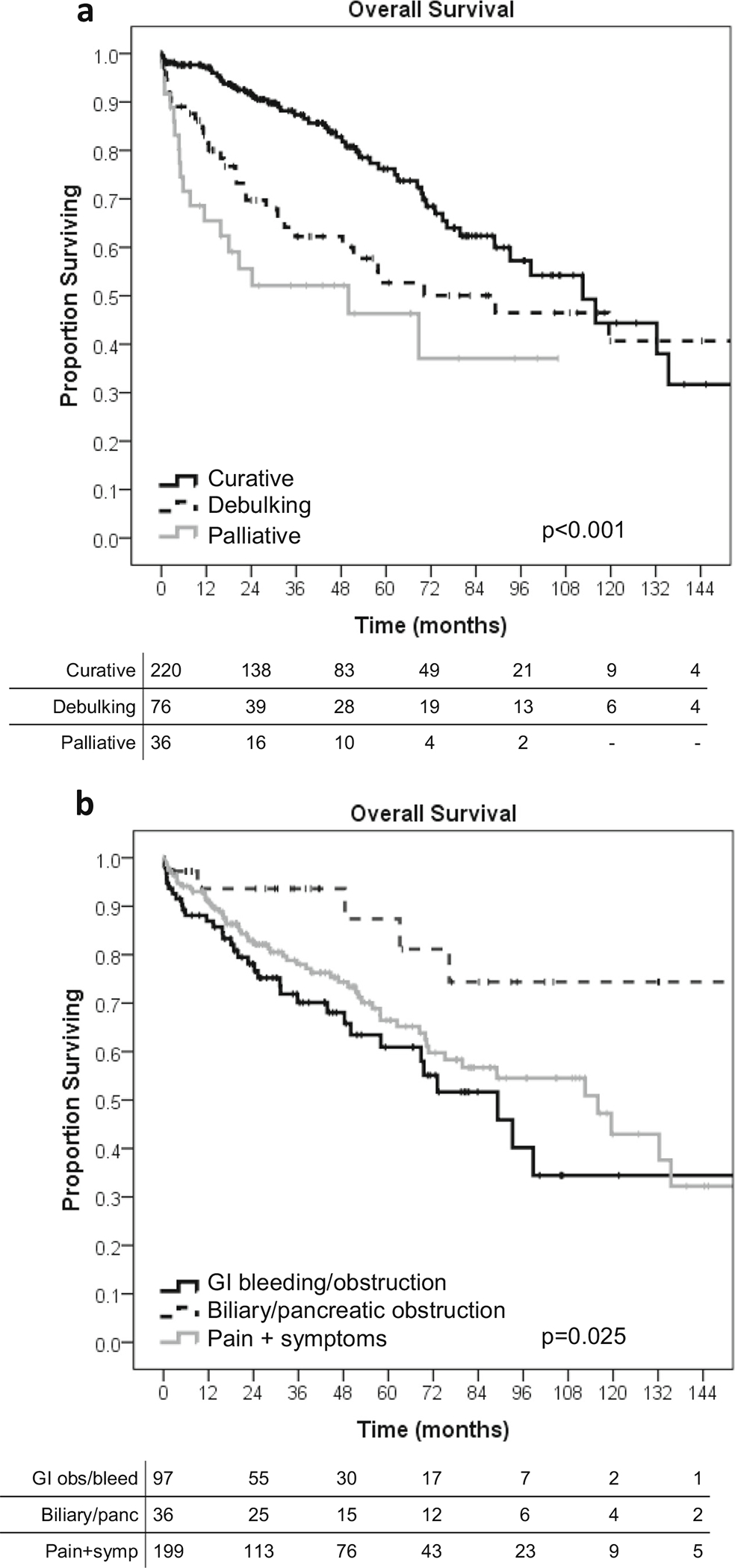
Overall survival of metastatic neuroendocrine tumor patients with non-hormonal symptoms, n = 332. a By operation intent. b By specific non-hormonal symptoms
The tumor differentiation and grade have previously been described as strong prognostic factors in non-metastatic NET, and we examined the effects of tumor biology in metastatic patients with NH symptoms. Grade and differentiation were found to still have prognostic value as those with high-grade disease had poor survival (median 13.5 months, 95%CI 8.3–18.7 months) compared to low (132.3 months, 95% CI 71.2–193.4 months) and intermediate grade (62.3 months, 95%CI 50.0–74,6 months) (Fig. 4a). Differentiation was similarly prognostic as well differentiated (median 115.9 months, 95%CI 77.8–154.0 months), and moderately differentiated tumors (68.3 months, 95%CI 53.0–95.6) had the best survival and poorly differentiated tumors survived only median 4.7 months (95%CI 2.7–6.7 months; p < 0.001) (Fig. 4b).
Fig. 4.

Overall survival of metastatic neuroendocrine tumor patients with non-hormonal symptoms, n = 332. a By tumor grade. b By tumor differentiation
Risk of Early (1-Year) Mortality after Resection in Metastatic NET
To determine if there was a group of patients who had poor survival despite surgical resection, a multivariable logistic regression analysis was performed in all patients with metastatic NET for the risk of dying within the first year (Table 4). After correcting for factors which were significant on univariable analysis, palliative operations (OR 6.54, 95%CI 1.69–25.3; p = 0.006), foregut resection site (5.62, 1.06–29.8; p = 0.042), major complication (4.91, 1.98–12.1; p = 0.001), and high tumor grade (11.2, 3.06–40.9; p < 0.001). Symptom group and specific NH symptoms while prognostic for survival did not predict risk of early mortality. Upon examining conditional survival of surviving for 1 year, the overall median survival was 119.5 months (95%CI 87.5–151.5) and similar between treatment groups (curative median 115.9 months, 95%CI 82.9–148.9 months; debulking 119.5 months, NR; palliative NR; p = 0.676) (Supplemental Fig. 1).
Table 4.
Multivariable logistic regression model of early (1-year) mortality in all patients with metastatic NET (n = 581)
| Variable | OR (95%CI) | p value | |
|---|---|---|---|
|
| |||
| Age (years) | 1.00 (0.96–1.04) | 0.875 | |
| Sex | Male | 1.10 (0.46–2.63) | 0.828 |
| ASA class | 1.22 (0.73–2.04) | 0.454 | |
| Genetic syndrome | 0.45 (0.07–2.89) | 0.400 | |
| Symptom group | Asymptomatic | Ref | |
| Functional only | 0.44 (0.08–2.37) | 0.340 | |
| Non-hormonal | 1.61 (0.44–5.87) | 0.473 | |
| Non-hormonal symptoms | Pain + symptoms | Ref | |
| GI bleed/obstruction | 0.90 (0.25–3.21) | 0.875 | |
| Biliary/pancreatic obstruction |
0.55 (0.01–3.13) | 0.496 | |
| Metastasis location | Liver only | Ref | |
| Liver and EHD | 1.36 (0.39–4.71) | 0.633 | |
| EHD only | 0.61 (0.17–2.20) | 0.453 | |
| Neoadjuvant treatment | 1.55 (0.59–4.06) | 0.371 | |
| Operative intent | Curative | Ref | |
| Debulking | 1.65 (0.61–4.48) | 0.327 | |
| Palliative | 6.54 (1.69–25.3) | 0.006 | |
| Resection site | Foregut | 5.62 (1.06–29.8) | 0.042 |
| Liver | 0.65 (0.23–1.79) | 0.400 | |
| Pancreas | 1.44 (0.46–4.52) | 0.535 | |
| Small bowel | 0.47 (0.09–2.33) | 0.355 | |
| Hindgut | 1.59 (0.34–7.40) | 0.552 | |
| Lymph node | 1.80 (0.38–8.60) | 0.462 | |
| Peritoneum | 3.28 (0.49–22.2) | 0.223 | |
| Major complication | 4.91 (1.98–12.1) | 0.001 | |
| Tumor grade | Low (G1) | Ref | |
| Intermediate (G2) | 1.63 (0.47–5.73) | 0.445 | |
| High (G3/G4) | 11.2 (3.06–40.9) | < 0.001 | |
Discussion
Decision-making in patients with metastatic NET is often challenging as there are a variety of medical and less invasive therapeutic options than surgery. Clinical course is often indolent, however can be unpredictable, and the use of surgery in the metastatic setting is rarely curative. Surgery however is the only potentially curative therapy and is also used to reduce tumor bulk for symptom and cancer control.14,15 Survival with NH symptoms from metastatic NET has not been well studied nor has the use of surgery in the palliative setting. The current study found that more than half of patients with metastatic NET who have an operation have NH symptoms. NH symptoms are more likely to come from primary NETs of the GI tract (small bowel, colon, stomach), less likely from pancreatic NETs, and more likely to result in palliative resection. These findings elucidate the natural history of this cancer type and help us understand the presentation and treatment of patient with NH symptoms. Patients with NH symptoms likely face many quality of life issues that may be addressed with surgical interventions. Health-related quality of life (HRQoL) has been assessed in two major trials for medical treatment of NET using EORTC QLQ-C30 and Functional assessment of cancer therapy (FACT-G) questionnaires. These found no overall difference in clinical symptom deterioration with everolimus versus placebo (RADIANT-4) or global HRQoL in sunitinib versus placebo.16,17 In surgical therapies, QoL assessment in treatment of NET liver metastasis found that diarrhea and flushing are the most common symptoms found in 34.1–41.1% and that overall QoL did not change dramatically with medical or surgical treatments.18 Resection of liver metastasis was also found to improve quality adjusted survival in symptomatic patients compared to intra-arterial therapies and subsequently the overall health benefit of treatment.19 Though these validated questionnaires were not used in this study, 77% of patients in our study had symptoms, and understanding how surgical therapy alters symptomatology is important. Questionnaires specific to this disease, such as the EORTC QLQ-GINET21, may be used for defining clinical benefit of surgery outside of oncologic benefit.20,21
Symptoms of gastrointestinal bleeding (16.3%), obstruction (14.8%), and biliary obstruction (6.7%) were found to be clear indications for operation in a smaller group of patients. However, at least half of the patients had non-specific NH symptoms of pain, nausea, vomiting, diarrhea, and anorexia. These symptoms contributed to nearly half receiving only resection of their primary NET without resection of the metastases, while a third received simultaneous resection. Simultaneous resection of the primary NET and liver metastases can safely be performed with low mortality, similar overall survival to staged resection, and 5-year symptom-free survival of 60%.22,23 Small-bowel NETs metastasize to lymph nodes in 80% of patients, and these nodes can be managed with resection of bowel and mesentery until they become unresectable when involving the SMA/SMV at the root of the mesentery.24 These tumors often cause significant mesenteric fibrosis, likely through the same mechanism that results in cardiac valve fibrosis, which can lead to GI symptoms of obstruction and pain.25,26 NH symptoms in combination with bulky lymphadenopathy and fibrosis may be an indication for aggressive resection, though progression to acute bowel ischemia due is rare.27 In the common scenario of incurable but indolent well-differentiated metastatic NET, control of NH symptoms should be a more relevant outcome when considering resection.
Surgery for NH symptoms was most commonly the first treatment and was performed approximately a month after initial diagnosis. This speaks to the natural history of NET in contrast to other GI cancers as they often are not diagnosed until advanced tumor bulk causes symptoms. Curative intent surgery was performed on two thirds of patients, and those receiving non-curative resection had higher rates of adjuvant chemotherapy (17%) and somatostatin analog (34%) use. While data on recurrence and progression rates were limited, a wide variety of multimodal interventions could be used as second-line therapies. Many treatment options are available to NET patients, and though consensus treatment guidelines are available, there is little high-quality data guiding the best therapy.28,29 Similar to quality of life outcomes, survival to receive subsequent therapies may be an important measurement outcome in this population. Ultimately, the biologic NET properties, site of primary, and site of metastasis guide the optimal treatment strategy30 Surgical therapy in this study did not appear to impede subsequent treatments including systemic therapies in two thirds and repeat operation in 20%. In fact, multimodal therapy using surgery and somatostatin analogues has shown to improve disease-specific survival over surgery alone after cytoreduction of metastatic NET.31 Treatment plans for patients with metastatic NET should be made as a multidisciplinary group as surgery can be helpful in patients with symptoms and does not exclude subsequent treatments.
Overall survival of patients with metastatic NET and NH symptoms was 9.2 years, shorter than those with functional symptoms and asymptomatic patients. This is certainly due to the fact there were an increased proportion of patients with high-grade tumors (9.3%) and increase use of non-curative resection (33.7%). Survival in this patient population has not been previously reported, and in contrast to previous studies, we found that survival was different between symptom groups.10 As expected, curative intent surgery provided the longest survival at 112.5 months compared to non-curative debulking and palliative resection. In patients with NH symptoms, the 5- and 10-year survival was 66 and 43%, which is slightly higher than the 35–60% 5-year and 20–40% 10-year survival reported in several large epidemiologic studies that include all patients with metastatic neuroendocrine tumors.32–36 A higher prevalence of midgut small-bowel carcinoids which typically have the best prognosis, and lower prevalence of PNET which have poor prognosis, likely contributed to the differences in overall survival. In general, a long survival is expected in those who present with symptoms and metastatic spread should not exclude an operation for symptom control.
Independent risk factors for death at 1-year were palliative resection, operations involving the stomach and duodenum, major complications, and high-grade tumors, and these factors help us understand which patients may not derive an oncologic benefit from surgery. High-grade neuroendocrine carcinoma (G3/G4) has a known median survival of 5 months in the metastatic setting, and in this study, 31 patients underwent surgery with half undergoing non-curative resection.37 Though there are rare reports of extended survival in localized tumors, surgery is rarely recommended for patients with metastatic high-grade and poorly differentiated NET and should be managed with chemotherapy as the initial treatment.38,39 Major postoperative complications are similarly a known risk factor for decreased OS in resection of NET liver metastasis and other GI cancers such as pancreas and colon.40–42 Gastric and duodenal operations were also at increased risk, possibly related to the nutritional deficits that occur with these operations and re-routing of the GI tract. Interestingly, after correcting for other factors, tumors of the colon and rectum were not at increased risk of early death despite reports of median 6-month survival.43 Ultimately, we can use this information to make decisions on which patients may benefit from other treatment options besides surgery.
Palliative surgery for GI cancers is performed with expected survival of months, however with NETs symptom, management was found to be associated with a 50-month median survival. Surgery was used as a palliative therapy in 39 patients (6.7%) and nearly all for NH symptoms. Palliative resection was more likely to be an emergency (13.9%), and the major complication rate was increased at 30%, resulting in a third of patients dying in the first year. Resection has proven to be effective at relieving mechanical obstruction and bleeding in this population and should be considered in all patients.44, 45 However, in patients with pain and non-specific symptoms, the use of palliative surgery or radiation for symptom control has not been studied. Radiographic response rates of 18% are seen with radiolabeled somatostatin analogue Lutetium-177-Dotate in the NETTER-1 trial, and initial experience has shown improvement in Karnofsky performance score in 58% of patients.46,47 Global quality of life was specifically found to be increased after 6 weeks of treatment with decreased pain scores, and those with disease progression had similar improvement in global quality of life.48,49 As the experience with this therapy grows, understanding how it compares to surgery for control of non-hormonal as well as hormonal symptoms will be important to its utilization as a palliative therapy.
The current retrospective multi-institutional database study has several limitations which includes missing data that may bias the results. There is the potential for selection bias as patients who had symptomatology yet were treated without surgery were not included, and we do not know how their survival compares to those who received surgery. This limits the external validity of our findings to the broad group of patients with NETs and may impose a bias for only the most “fit” patients. We also cannot define the exact indication for the operation, and the extent symptomatology may have played a role in proceeding to surgery. The differentiation between debulking and palliative intent operations therefore may not be clear when reviewing the chart retrospectively. Quality of life was not measured, and we do not have an understanding of the degree of symptoms and whether symptoms resolved after the operation. While the data on survival is robust, we do not have adequate progression or recurrence data on 47% of patients and this limits the ability to accurately determine their respective rates. Similarly, while we measured overall survival as an outcome, death from cancer versus other causes is not known.
In conclusion, patients with NH symptoms make up half of patients with metastasis undergoing an operation, have a median 9-year survival after surgery, and 4-year survival after palliative surgery. The overall clinical benefit of surgical therapy in the metastatic setting should focus not only on expected oncologic benefit but the improvement of symptoms for these patients undergoing surgery. Understanding the impact surgery has in these patients should be studied more thoroughly with a focus on quality of life. Surgery should not be excluded as a management option of symptoms even in the face of widespread metastatic disease and a palliative operation.
Supplementary Material
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
The institutional review board from each institution approved the study
Presented at: SSAT Presidential Plenary Session; Digestive Disease Week 2018; Chicago, Illinois; June 2, 2018
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11605-018-3986-4) contains supplementary material, which is available to authorized users.
References
- 1.Frilling A, Modlin IM, Kidd M, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15(1):e8–21. [DOI] [PubMed] [Google Scholar]
- 2.Pavel M, O’Toole D, Costa F, et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103(2):172–185. [DOI] [PubMed] [Google Scholar]
- 3.Boudreaux JP, Klimstra DS, Hassan MM, et al. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas. 2010;39(6): 753–766. [DOI] [PubMed] [Google Scholar]
- 4.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. [DOI] [PubMed] [Google Scholar]
- 5.Caplin ME, Pavel M, Cwikla JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. [DOI] [PubMed] [Google Scholar]
- 6.Mayo SC, Herman JM, Cosgrove D, et al. Emerging approaches in the management of patients with neuroendocrine liver metastasis: role of liver-directed and systemic therapies. J Am Coll Surg. 2013;216(1):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woltering EA, Voros BA, Beyer DT, et al. Aggressive Surgical Approach to the Management of Neuroendocrine Tumors: A Report of 1,000 Surgical Cytoreductions by a Single Institution. J Am Coll Surg. 2017;224(4):434–447. [DOI] [PubMed] [Google Scholar]
- 8.Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17(12): 3129–3136. [DOI] [PubMed] [Google Scholar]
- 9.Halperin DM, Shen C, Dasari A, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol. 2017;18(4):525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baptiste GG, Postlewait LM, Ethun CG, et al. Symptomatic presentation as a predictor of recurrence in gastroenteropancreatic neuroendocrine tumors: A single institution experience over 15 years. J Surg Oncol. 2016;114(2):163–169. [DOI] [PubMed] [Google Scholar]
- 11.Partelli S, Maurizi A, Tamburrino D, et al. GEP-NETS update: a review on surgery of gastro-entero-pancreatic neuroendocrine tumors. Eur J Endocrinol. 2014;171(4):R153–162. [DOI] [PubMed] [Google Scholar]
- 12.Arrese D, McNally ME, Chokshi R, et al. Extrahepatic disease should not preclude transarterial chemoembolization for metastatic neuroendocrine carcinoma. Ann Surg Oncol. 2013;20(4):1114–1120. [DOI] [PubMed] [Google Scholar]
- 13.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. [DOI] [PubMed] [Google Scholar]
- 14.Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25(3):458–511. [DOI] [PubMed] [Google Scholar]
- 15.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197(1):29–37. [DOI] [PubMed] [Google Scholar]
- 16.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. [DOI] [PubMed] [Google Scholar]
- 17.Pavel ME, Singh S, Strosberg JR, et al. Health-related quality of life for everolimus versus placebo in patients with advanced, non-functional, well-differentiated gastrointestinal or lung neuroendocrine tumours (RADIANT-4): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(10):1411–1422. [DOI] [PubMed] [Google Scholar]
- 18.Spolverato G, Bagante F, Wagner D, et al. Quality of life after treatment of neuroendocrine liver metastasis. J Surg Res. 2015;198(1):155–164. [DOI] [PubMed] [Google Scholar]
- 19.Spolverato G, Vitale A, Ejaz A, et al. Net health benefit of hepatic resection versus intraarterial therapies for neuroendocrine liver metastases: A Markov decision model. Surgery. 2015;158(2):339–348. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez-Fonseca P, Carmona-Bayonas A, Martin-Perez E, et al. Health-related quality of life in well-differentiated metastatic gastroenteropancreatic neuroendocrine tumors. Cancer Metastasis Rev. 2015;34(3):381–400. [DOI] [PubMed] [Google Scholar]
- 21.Yadegarfar G, Friend L, Jones L, et al. Validation of the EORTC QLQ-GINET21 questionnaire for assessing quality of life of patients with gastrointestinal neuroendocrine tumours. Br J Cancer. 2013;108(2):301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaujoux S, Gonen M, Tang L, et al. Synchronous resection of primary and liver metastases for neuroendocrine tumors. Ann Surg Oncol. 2012;19(13):4270–4277. [DOI] [PubMed] [Google Scholar]
- 23.Birnbaum DJ, Turrini O, Vigano L, et al. Surgical management of advanced pancreatic neuroendocrine tumors: short-term and long-term results from an international multi-institutional study. Ann Surg Oncol. 2015;22(3):1000–1007. [DOI] [PubMed] [Google Scholar]
- 24.Howe JR, Cardona K, Fraker DL, et al. The Surgical Management of Small Bowel Neuroendocrine Tumors: Consensus Guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46(6):715–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daskalakis K, Karakatsanis A, Stalberg P, Norlen O, Hellman P. Clinical signs of fibrosis in small intestinal neuroendocrine tumours. Br J Surg. 2017;104(1):69–75. [DOI] [PubMed] [Google Scholar]
- 26.Laskaratos FM, Rombouts K, Caplin M, Toumpanakis C, Thirlwell C, Mandair D. Neuroendocrine tumors and fibrosis: An unsolved mystery?. Cancer. 2017;123(24):4770–4790. [DOI] [PubMed] [Google Scholar]
- 27.Ohrvall U, Eriksson B, Juhlin C, et al. Method for dissection of mesenteric metastases in mid-gut carcinoid tumors. World J Surg. 2000;24(11):1402–1408. [DOI] [PubMed] [Google Scholar]
- 28.Kulke MH, Shah MH, Benson AB 3rd, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. 2015;13(1): 78–108. [DOI] [PubMed] [Google Scholar]
- 29.Strosberg JR, Fisher GA, Benson AB, et al. Appropriateness of systemic treatments in unresectable metastatic well-differentiated pancreatic neuroendocrine tumors. World J Gastroenterol. 2015;21(8):2450–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SJ, Kim JW, Han SW, et al. Biological characteristics and treatment outcomes of metastatic or recurrent neuroendocrine tumors: tumor grade and metastatic site are important for treatment strategy. BMC Cancer. 2010;10:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deutsch GB, Lee JH, Bilchik AJ. Long-Term Survival with Long-Acting Somatostatin Analogues Plus Aggressive Cytoreductive Surgery in Patients with Metastatic Neuroendocrine Carcinoma. J Am Coll Surg. 2015;221(1):26–36. [DOI] [PubMed] [Google Scholar]
- 32.Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the mid-gut. Neuroendocrinology. 2009;89(4):471–476. [DOI] [PubMed] [Google Scholar]
- 33.Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121(4):589–597. [DOI] [PubMed] [Google Scholar]
- 34.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE). Ann Oncol. 2010;21(9):1794–1803. [DOI] [PubMed] [Google Scholar]
- 36.Mocellin S, Nitti D. Gastrointestinal carcinoid: epidemiological and survival evidence from a large population-based study (n = 25 531). Ann Oncol. 2013;24(12):3040–3044. [DOI] [PubMed] [Google Scholar]
- 37.Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120(18):2814–2823. [DOI] [PubMed] [Google Scholar]
- 38.Ilett EE, Langer SW, Olsen IH, Federspiel B, Kjaer A, Knigge U. Neuroendocrine Carcinomas of the Gastroenteropancreatic System: A Comprehensive Review. Diagnostics (Basel). 2015;5(2):119–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brennan SM, Gregory DL, Stillie A, Herschtal A, Mac Manus M, Ball DL. Should extrapulmonary small cell cancer be managed like small cell lung cancer?. Cancer. 2010;116(4):888–895. [DOI] [PubMed] [Google Scholar]
- 40.Glazer ES, Tseng JF, Al-Refaie W, et al. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford). 2010;12(6):427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg. 2015;261(3):497–505. [DOI] [PubMed] [Google Scholar]
- 42.Scaife CL, Hartz A, Pappas L, et al. Association between postoperative complications and clinical cancer outcomes. Ann Surg Oncol. 2013;20(13):4063–4066. [DOI] [PubMed] [Google Scholar]
- 43.Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chambers AJ, Pasieka JL, Dixon E, Rorstad O. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery. 2008;144(4):645–651; discussion 651–643. [DOI] [PubMed] [Google Scholar]
- 45.Gulec SA, Mountcastle TS, Frey D, et al. Cytoreductive surgery in patients with advanced-stage carcinoid tumors. Am Surg. 2002;68(8):667–671; discussion 671–662. [PubMed] [Google Scholar]
- 46.Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of (177)LuDotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376(2):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delpassand ES, Samarghandi A, Zamanian S, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas. 2014;43(4):518–525. [DOI] [PubMed] [Google Scholar]
- 48.Teunissen JJ, Kwekkeboom DJ, Krenning EP. Quality of life in patients with gastroenteropancreatic tumors treated with [177Lu-DOTA0,Tyr3]octreotate. J Clin Oncol. 2004;22(13):2724–2729. [DOI] [PubMed] [Google Scholar]
- 49.Khan S, Krenning EP, van Essen M, Kam BL, Teunissen JJ, Kwekkeboom DJ. Quality of life in 265 patients with gastroenteropancreatic or bronchial neuroendocrine tumors treated with [177Lu-DOTA0,Tyr3]octreotate. J Nucl Med. 2011;52(9): 1361–1368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


