Abstract
Background
Blood pressure (BP) variability is involved in the appraisal of threat and safety, and can serve as a potential marker of psychological resilience against stress. The relationship between biological rhythms of BP and resilience was cross-sectionally assessed by 7-day/24-hour chronobiologic screening in a rural Japanese community (Tosa), with focus on the 12-hour component and the “circadian-circasemidian coupling” of systolic (S) BP.
Subjects and Methods
Tosa residents (N = 239, 147 women, 23–74 years), free of anti-hypertensive medication, completed 7-day/24-hour ambulatory BP monitoring. The circadian-circasemidian coupling was determined individually by computing the difference between the circadian phase and the circasemidian morning-phase of SBP. Participants were classified into three groups: those with a short coupling interval of about 4.5 hours (Group A), those with an intermediate coupling interval of about 6.0 hours (Group B), and those with a long coupling interval of about 8.0 hours (Group C).
Results
Residents of Group B who showed optimal circadian-circasemidian coordination had less pronounced morning and evening SBP surges, as compared to residents of Group A (10.82 vs 14.29 mmHg, P < 0.0001) and Group C (11.86 vs 15.21 mmHg, P < 0.0001), respectively. The incidence of morning or evening SBP surge was less in Group B than in Group A (P < 0.0001) or Group C (P < 0.0001). Group B residents showed highest measures of wellbeing and psychological resilience, assessed by good relation with friends (P < 0.05), life satisfaction (P < 0.05), and subjective happiness (P < 0.05). A disturbed circadian-circasemidian coupling was associated with elevated BP, dyslipidemia, arteriosclerosis and a depressive mood.
Conclusion
The circadian-circasemidian coupling of SBP could serve as a new biomarker in clinical practice to guide precision medicine interventions aimed at achieving properly timed rhythms, and thereby resilience and wellbeing.
Keywords: 7-day/24-hour chronobiologic screening, blood pressure, biological 12-hour rhythm, appropriate circadian-circasemidian coupling, circadian acrophase, 12-hour morning acrophase, morning blood pressure surge, evening blood pressure surge, human resilience, wellbeing
Plain Language Summary
Blood pressure measurements obtained every 30 minutes for 7 days from 239 adults, 23 to 74 years of age, are analyzed for their daily variation, with usually lower values during nightly sleep and higher values during the active daytime. The daily blood pressure pattern mostly stems from two components with periods of 24 and 12 hours (changes recurring every day and half a day). We define the concept of “circadian-circasemidian coupling” to capture the timing relation between these two components. We show how this relation affects the development of a morning or evening elevation in blood pressure, and how these features relate to a person’s physical health, resilience and wellbeing.
Introduction
Resilience, the ability to adapt to stress in daily life, is important for wellbeing, life satisfaction and health.1–6 As often reported in previous investigations,7–14 blood pressure (BP) variability is involved in the appraisal of threat and safety, and can serve as a potential marker of psychological resilience against stress. BP variability (BPV), including its circadian rhythm,15–19 reflects the status of one’s continued adjustment to stress from daily life in response to constantly changing environmental demands. Claude Bernard enunciated this bidirectional heart-brain connection, which was confirmed by Thayer and Lane.20
The circadian system plays a key role of resilience in the adaptation to a novel environment.21–31 New evidence suggests that harmonic components of the 24-hour rhythm provided an evolutionary advantage for almost all life forms, from bacteria to humans.32–34 In particular, the 12-hour (circasemidian) rhythm may be important for the rapid adaptation to a novel environment. We reported that the 12-hour response to the space environment appeared faster and was larger than the circadian response to it.35 Prevalent 12-hour gene expression and metabolic rhythms were found by others in mouse liver, coupled with a physiological 12-hour unfolded protein response oscillation. Most 12-hour rhythms are reportedly regulated by a distinct and cell-autonomous pacemaker that includes the unfolded protein response (UPR) transcription factor spliced form of XBP1 (XBP1s) that can be entrained in vitro by metabolic and endoplasmic reticulum (ER) stress cues.36–39 The ER functions to properly fold and process secreted and transmembrane proteins. ER stress refers to the accumulation of misfolded and unfolded proteins in the ER lumen, which occurs when ER function is disrupted by environmental and genetic factors.40 The 12-hour rhythm could thus be first to respond to ER stress when faced to a novel environment to secure wellbeing and psychological resilience in humans. However, it is not clear yet whether coordination between circadian and cirasemidian rhythms plays an important role in the adaptation to a new environment, and, if so, how it contributes to human health. While recognizing the potential role of the circasemidian clock in regulating human diseases,38 how to harness the temporal dynamics between circadian and circasemidian rhythms for chronodiagnosis and/or chronotherapy41 largely remains to be investigated.
Over the past 30 years, we have investigated the merits of long-term BP monitoring to assess human health, including wellbeing and vascular disease risk. We documented the predictive value of mapping chronomes (rhythms, chaos and age trends) based on 7-day/24-hour BP monitoring.42–62 Since functional MRI showed that higher psychological resilience relates to harmonic oscillations with frequency-specific subcomponents of several brain regions,63 harmonic 24-hour and 12-hour oscillations of BP may reflect human resilience and wellbeing, the topic of this investigation.
Subjects and Methods
Study Participants
Initially, 371 citizens were recruited to participate in a 7-day/24-hour chronobiologic BP screening as part of a community-based comprehensive medical assessment. All participants were residents of Tosa, a rural Japanese town in Kochi Prefecture. They took part in free health screening, counseling and educational services offered by the town’s office, from which we obtained their medical history, medications, and latest laboratory data. Among the 371 citizens, 239 (147 women), 23 to 74 years of age, satisfied the inclusion criteria for this study, as they did not take any anti-hypertensive medication and completed 7 days of BP monitoring. This study was approved by the Medical Ethics Committee of the Tokyo Women’s Medical University as Clinical Study #2912, titled “Health assessment of community-dwelling elderly in Japan”. A detailed explanation of the study protocol was given to the study participants before they gave written, informed consent. The study complies with the Declaration of Helsinki Principles.
7-Day/24-Hour Chronobiologic Blood Pressure Screening
Noninvasive ambulatory BP monitoring (ABPM) was performed using an oscillometric device (TM-2431, A&D Co., Tokyo, Japan) to record systolic (S) and diastolic (D) BP and heart rate (HR). As reported earlier,50–52 BP was measured oscillometrically every 30 min from 07:00 to 22:00 and every 60 min from 22:00 to 07:00 for 7 consecutive days. At the town’s office, all participants were fitted with a monitor and asked to revisit the office 7 days later. Participants were taught how to attach and remove the recorder and were instructed to remove the recorder while taking a bath. They were asked to keep a diary noting the times they went to sleep and woke up. Stored data were retrieved on a personal computer using commercially available software (TM-2430-15, A&D Co., Tokyo, Japan). Bracketing the 7-day ABPM, home BP measurements were collected for 30 days, taken twice a day, in the morning upon getting-up and in the evening just before going to bed, in a sitting position after resting for 1 min.
Circadian Parameters of BP and HR
Each record was analyzed by the Maximum Entropy Method (MEM), using the MemCalc/Win software (Suwa Trust GMS, Tokyo, Japan)64 to estimate the circadian period of SBP, DBP, and HR. The period was determined as the inverse of the frequency corresponding to a peak in the MEM spectrum located in the vicinity of 1/24 (h−1). Missing data were linearly interpolated prior to analysis. Next, 24-hour and 12-hour cosine curves were fitted independently to each record by cosinor61,65,66 to estimate the MESOR (Midline Estimating Statistic Of Rhythm, a rhythm-adjusted mean) and the amplitude and acrophase of each cosine curve. The acrophase is defined as the phase of the maximum assumed by the fitted cosine curve in relation to local midnight, used as reference time, as reported earlier.57–61 The morning surge in SBP was defined as the difference between SBP at the time corresponding to the 12-hour morning acrophase and the SBP MESOR (24-hour average of the 7-day/24-hour record). Likewise, the evening surge in SBP was defined as the difference between SBP at the time corresponding to the 12-hour evening acrophase and the SBP MESOR.
Assessment of Citizens’ Resilience
Since circadian disruption could impair the health of citizens and lead to cardiovascular, metabolic and psychiatric disorders, cancer and cognitive decline in the aged, we examined whether circadian misalignment of BP, assessed by the circadian-circasemidian coordination of SBP, could account for the morning or evening surge in BP and the psychophysiological health status of the participants. ABPM results are associated with sleep duration and sleep quality, including time of getting-up and its variability over 30 consecutive days, time to fall asleep, and answer to the question “do you feel tired getting up in the morning?”. We also estimated wellbeing based on scores (0 to 15) from the Geriatric Depression Scale 15 (GDS15)50,51,60,61 and on scores (0 to 100) from the Visual Analogue Scale (VAS),50,51,60,61 which includes items such as subjective health, mood, good relation with family and friends, economic satisfaction, life satisfaction, and happiness. Finally, several laboratory examinations provided general health information, including fasting blood glucose, uric acid, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, cardio-ankle vascular index (CAVI)61 and ankle brachial index (ABI).61
Classification of Citizens Based on Circadian-Circasemidian SBP Coupling
Circadian-circasemidian coupling is defined as the time difference between the circadian acrophase and the morning acrophase of the 12-hour component. The circadian-circasemidian coupling was individually determined for SBP by fitting to the 7-day/24-hour SBP data a two-component model consisting of cosine curves with periods of 24 and 12 hours by cosinor.61,65,66 The 239 participants were equally divided into three groups: Group A had a short coupling; Group B an intermediate coupling; and Group C a long coupling.
Statistical Analysis
Data are expressed in terms of the mean ± SD. Between-group differences for continuous variables are assessed using unpaired Student’s t-tests with Bonferroni correction for multiple comparisons. Categorical data are compared using the chi-squared test. Linear regression analysis was performed for assessing changes as a function of age and relations of psychophysiological variables as a function of the circadian-circasemidian coupling of SBP. The Stat Flex (Ver. 6) software (Artec Co., Ltd., Osaka, Japan) was used. Differences with P-values less than 0.05 are considered to indicate statistical significance.
Results
Example of 7-Day/24-Hour ABPM Record
Currently, 24-hour ABPM is considered a gold standard, reserved for special cases of high BP, but its time structure is only interpreted in terms of 24-hour, daytime and nighttime means. General reliance upon a single measurement, or even a single 24-hour profile of BP, however, was referred to as “flying blind”.67 Long-term BP monitoring, analyzed time-structurally (chronomically, from chronome = time structure), detects physiological–physical interactions. Even within the conventionally accepted normal range, vascular variability disorders (VVDs) have been associated with a statistically significant increase in risk. Long-term chronobiologically interpreted ABPM (C-ABPM) records help to “know ourselves”, serving for relief of psychological and other strain once transient VVDs are linked to the source of a load, prompting adjustment of one’s lifestyle for strain reduction.68
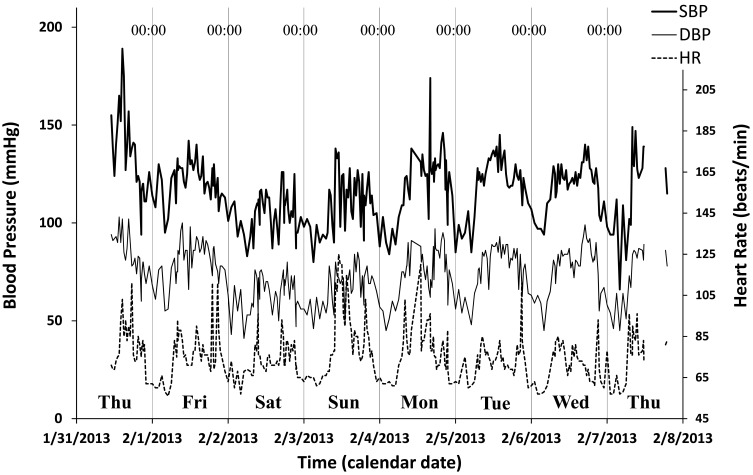
One study participant’s record of SBP, DBP and HR is shown in Figure 1. He did not take any anti-hypertensive medication. His office BP was 155/94 mmHg and his home BP measurements averaged over 30 days were 113.4/70.8 mmHg (morning) and 105.7/58.5 mmHg (evening). The 7-day/24-hour MESOR (midline estimating statistic of rhythm) estimates of SBP, DBP, and HR were 114.6 mmHg, 71.4 mmHg and 74.1 bpm, respectively. The MEM spectrum showed a prominent circadian component with a period of 24.51 hours, together with three smaller peaks at periods of 11.43 (circasemidian), 92.74, and 142.66 hours.
Figure 1.
Example of 7-day/24-hour ABPM. Time plots of the 7-day/24-hour records of systolic blood pressure (SBP, thick continuous line), diastolic blood pressure (DBP, thin continuous line), and heart rate (HR, dashed line) of a 40-year old man with white-coat hypertension. When the 7-day/24-hour ABPM was started at 11:00 in front of a doctor, high SBP on the first day of monitoring can readily be seen. Measurements dropped thereafter within the normal range, where they fluctuated periodically, following a circadian rhythm, suggesting a regular rest-activity schedule.
Changes with Age in Circadian and Circasemidian Rhythm Characteristics Assessed by 7-Day/24-Hour ABPM
The MESOR of SBP increased with age (P = 0.0014), while the MESOR of HR decreased with age (P = 0.0082). While the circadian and circasemidian amplitudes of SBP did not change with age, the circasemidian amplitude of DBP decreased with age (P = 0.0033). The circadian amplitude of HR increased (P = 0.0060) but its circasemidian amplitude decreased (P = 0.0002) with age. The period of the circadian, circasemidian, and circaoctohoran components of SBP, estimated by MEM, averaged 24.03±0.49, 12.08±0.85, and 8.13±0.68 hours, respectively, and did not change statistically significantly with advancing age in either gender.
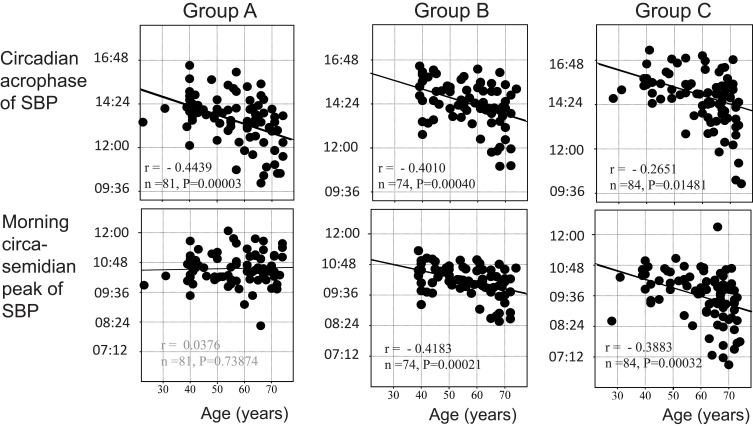
Noteworthy are phase advances of the circadian rhythm in SBP (P < 0.0001), DBP (P = 0.0001) and HR (P = 0.0060) with increasing age. A similar phase advance with age characterizes the circasemidian component of SBP (P = 0.0081) and DBP (P = 0.0138), but not that of HR.
Effects of the Circadian-Circasemidian Coupling on Citizens’ Resilience
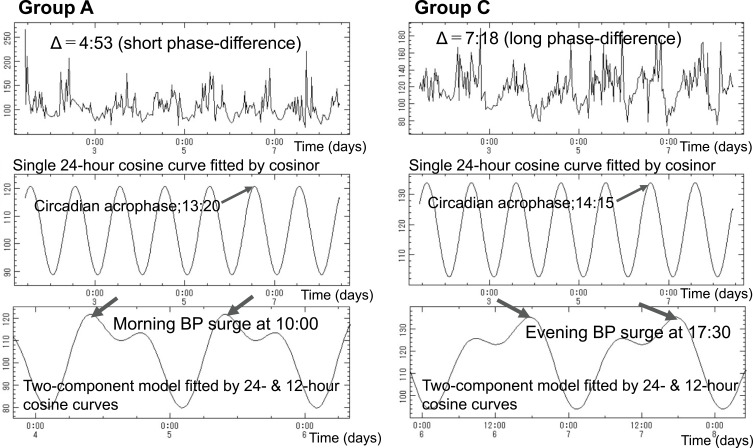
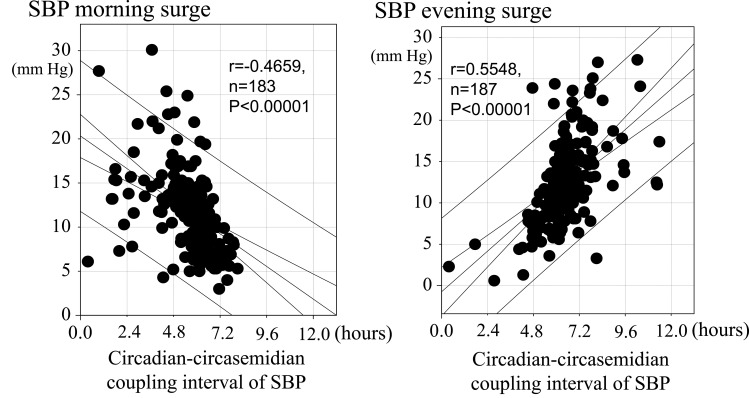
Resilience to daily life stress, wellbeing, and life satisfaction may be reflected in BPV, notably in the coordination between circadian and circasemidian rhythms, akin to the bidirectional heart-vessel-brain connection proposed by Claude Bernard and Thayer and Lane.20 To check this proposition, we evaluated citizens’ resilience by assessing their circadian-circasemidian coupling of SBP. We found that, as compared to citizens of Group B, the average morning SBP surge was larger in citizens of Group A (14.29 vs 10.82 mmHg, P < 0.001), who had a shorter coupling interval (example shown in Figure 2, left). Likewise, as compared to citizens of Group B, the average evening SBP surge was larger in citizens of Group C (15.21 vs 11.86 mmHg, P < 0.001), who had a longer coupling interval (example shown in Figure 2, right) (Table 1). Moreover, a shorter circadian-circasemidian coupling of SBP is associated with a larger morning SBP surge in the 183 participants in Groups A-C for whom a morning surge was detected (Table 1, Figure 3, left). A longer circadian-circasemidian coupling of SBP is also associated with a larger evening SBP surge in 187 participants in Groups A-C for whom an evening BP surge was detected (Table 1, Figure 3, right).
Figure 2.
Examples of 7-day/24-hour ABPM from one citizen in Group A and one in Group C. Left: record from Group A citizen who has a short phase difference of about 4.5 hours between the circasemidian morning acrophase and the circadian acrophase of systolic blood pressure. Right: record from Group C citizen who has a long phase difference of about 7.0 hours between the circasemidian morning acrophase and the circadian acrophase of systolic blood pressure. Corresponding 2-component models show the presence of a morning BP surge in Group A citizen (left bottom), and of an evening BP surge in Group C citizen (right bottom).
Table 1.
Effects of Circadian-Circasemidian Coupling of Systolic Blood Pressure on Results from 7-Day/24-Hour ABPM, Wellbeing and Psychological Resilience
| Group A Short Coupling <5.66 Hours (~ 4.5 Hours) | Group B Intermediate Coupling 5.66–6.5 Hours (~ 6.0 Hours) | Group C Long Coupling >6.5 Hours (~ 8.0 Hours) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | ||
| Citizens’ characteristics | Age (years) | 81 | 56.2 | 12.1 | 74 | 57.1 | 10.7 | 84 | 60.1 | 11.2 |
| BMI (kg/m2) | 81 | 23.2 | 3.2 | 74 | 23.9 | 3.3 | 83 | 23.6 | 3.4 | |
| Circadian-circasemidian coupling interval | SBP 24h-12h-AM phase difference | 81 | 4:24** | 1:15 | 74 | 6:07 | 0:13 | 84 | 8:02** | 2:41 |
| HR 24h-12h-AM phase difference | 81 | 5:07* | 1:56 | 74 | 5:59 | 2:06 | 84 | 6:12 | 2:32 | |
| 7-day/24-hour ABPM | SBP MESOR (mmHg) | 81 | 122.1 | 11.5 | 74 | 125.1 | 14.2 | 84 | 127.1 | 14.4 |
| SBP 24h Amplitude (mmHg) | 81 | 14.1 | 5.1 | 74 | 14.4 | 4.7 | 84 | 14.6 | 5.2 | |
| SBP 24h Acrophase (hh:mm) | 81 | 13:31** | 1:21 | 74 | 14:24 | 1:14 | 84 | 14:53 | 1:57 | |
| SBP 12h Amplitude (mmHg) | 81 | 5.7 | 2.8 | 74 | 6.5 | 2.8 | 84 | 6.1 | 3.3 | |
| SBP 12h-AM Acrophase (hh:mm) | 81 | 9:07** | 1:16 | 74 | 8:16 | 1:13 | 84 | 7:08** | 1:59 | |
| SBP morning surge (mmHg) | 81 | 14.29*** | 4.81 | 63 | 10.82 | 3.53 | 39 | 8.12*** | 3.53 | |
| (number of participants with morning surge; + / -) | (81/0)### | (63/11) | (39/45)### | |||||||
| SBP evening surge (mmHg) | 37 | 7.11*** | 4.42 | 68 | 11.86 | 3.74 | 82 | 15.21*** | 4.78 | |
| (number of participants with evening surge; + / -) | (37/44)### | (68/6) | (82/2) | |||||||
| Sleep | Sleep duration (min) | 81 | 454.7 | 64.1 | 74 | 454.3 | 53.9 | 84 | 467.2 | 68.0 |
| Time of getting-up (hh:mm) | 81 | 6:30 | 0:36 | 74 | 6:23 | 0:38 | 84 | 6:30 | 0:43 | |
| SD of getting-up time (hh:mm) | 78 | 0:35 | 0:15 | 66 | 0:34 | 0:13 | 73 | 0:36 | 0:19 | |
| Time to go to sleep (min) | 76 | 22.8 | 25.4 | 69 | 21.2 | 21.8 | 72 | 25.6 | 24.8 | |
| Do you feel tired getting up in the morning? (yes:1, no:0) | 61 | 0.62 | 0.71 | 55 | 0.65 | 0.70 | 57 | 0.56 | 0.66 | |
| Wellbeing | GDS (score: 0–15) | 77 | 3.61 | 2.99 | 69 | 3.67 | 2.95 | 73 | 3.68 | 2.91 |
| How about your health? (%) | 76 | 74.2 | 18.2 | 68 | 71.7 | 19.4 | 71 | 71.9 | 22.4 | |
| How about your mood? (%) | 68 | 69.7 | 20.0 | 63 | 74.1 | 18.0 | 64 | 70.8 | 19.1 | |
| Good relation with family? (%) | 68 | 76.9 | 21.4 | 63 | 83.9 | 20.3 | 64 | 83.8 | 18.5 | |
| Good relation with friends? (%) | 68 | 74.4* | 23.0 | 63 | 83.9 | 17.4 | 64 | 82.3 | 17.7 | |
| Economical satisfaction? (%) | 68 | 56.4 | 23.8 | 63 | 58.5 | 20.9 | 65 | 54.4 | 21.3 | |
| Life satisfaction? (%) | 68 | 65.6 | 20.7 | 63 | 73.4 | 22.5 | 64 | 62.5* | 21.0 | |
| How about happiness? (%) | 76 | 75.1 | 19.7 | 68 | 77.0 | 19.0 | 71 | 68.4* | 19.9 | |
Notes: Bonferroni correction *P < 0.05, **P < 0.01, ***P < 0.001 (versus Group B). Chi-square test. ###P < 0.0001 (versus Group B). Cells highlighted in gray indicate statistically significant differences (versus Group B).
Figure 3.
Relation between circadian-circasemidian coupling of SBP and morning SBP surge (left), or evening SBP surge (right). Left: Shorter circadian-circasemidian couplings of SBP are associated with larger morning SBP surges. Right: Longer circadian-circasemidian couplings of SBP are associated with larger evening SBP surges.
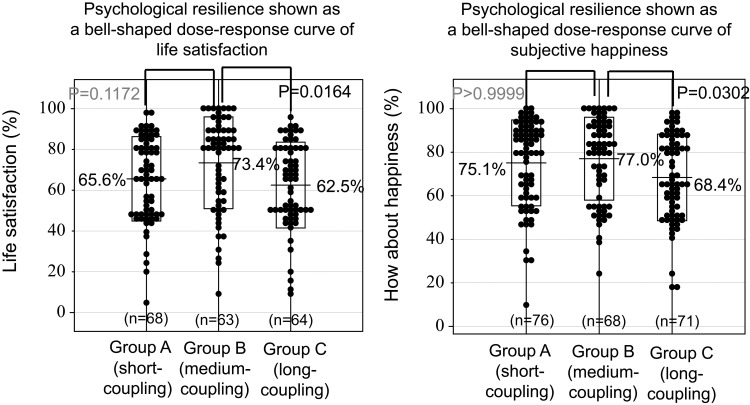
No statistically significant differences were found among Groups A, B and C in terms of sleep duration, time of getting-up and its variability over 30 consecutive days (assessed by the standard deviation, SD), time to fall asleep, or answer to the question “do you feel tired getting up in the morning?”. These results indicate that the circadian-circasemidian coupling of SBP did not affect sleep quality in this population. Comparing all three groups, we found that a short circadian-circasemidian coupling of SBP (Group A) was associated with a lower score of wellbeing on the GDS15 and VAS scales (good relation with friends, P = 0.0278). Moreover, a long circadian-circasemidian coupling of SBP (Group C) was also associated with lower scores of wellbeing on the GDS15 and VAS scales (life satisfaction, P = 0.0164; happiness, P = 0.0302). Considering all eight wellbeing variables listed in Table 1, scores of Group B citizens who had an intermediate coupling are on average higher than scores of Group A (short coupling; paired t = 2.862, P = 0.024) or Group C (long coupling; paired t = 2.377, P = 0.049) citizens. Measures of wellbeing in Groups A-C show a bell-shaped distribution in response to the circadian-circasemidian coupling of SBP, where responses appear only in a certain range of stimuli (so-called “windows”), as proposed by Murase69 (see Figure 4).
Figure 4.
Bell-shaped distribution of resilience measures follows grouping by circadian-circasemidian coupling of SBP (Groups A, B, and C). An intermediate circadian-circasemidian coupling interval of systolic blood pressure (SBP) (Group B) corresponds to the distribution peak, suggesting that it may promote psychological resilience, as gauged by life satisfaction (left) and subjective happiness (right).
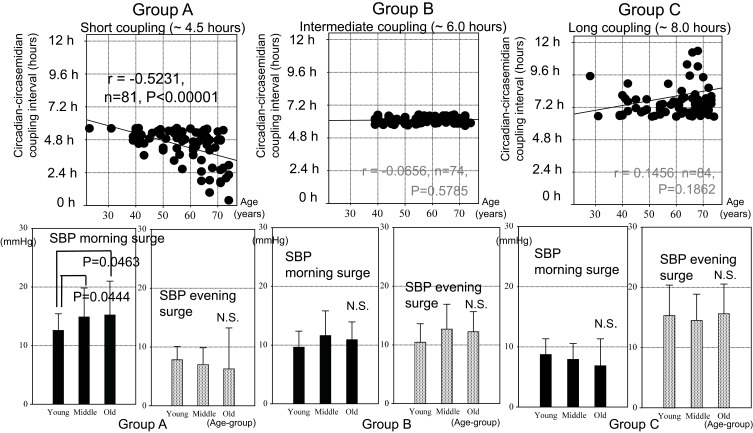
Aging effects on the circadian or circasemidian acrophase of SBP differed among the three groups. As shown in Figure 5 (top), the circadian acrophase of SBP advanced with age statistically significantly in all three groups. The circasemidian acrophase, however, only advanced with age in Group B (P = 0.00021) and even more so in Group C (P = 0.00032) (Figure 5, bottom). As a result, aging effects on the circadian-circasemidian coupling interval of SBP differed among the three groups (Figure 6, top). In Group A, the circadian-circasemidian coupling interval shortened with age (P < 0.00001), whereas in Group B it did not change, and in Group C it tended to lengthen with age (P = 0.18618). When participants of Groups A, B and C were classified into young (<50 years), middle-aged (50~65 years) and elderly (>65 years), the morning SBP surge in Group A was less pronounced in younger participants than in middle-aged (P = 0.0444) and the elderly (P = 0.0463) (Figure 6, bottom left). No difference with age in the extent of the evening SBP surge was found in either group.
Figure 5.
Effect of age on circadian and circasemidian acrophases of systolic blood pressure (SBP) in Groups A, B and C. The circadian acrophase of SBP advances with age statistically significantly in all three groups. The circasemidian morning acrophase of SBP also advances with age statistically significantly in Groups B and C, but not in Group A.
Figure 6.
Effect of age on circadian-circasemidian coupling interval of SBP (top) and extent of morning and evening SBP surge (bottom) in Groups A, B and C. Top: The circadian-circasemidian coupling interval of SBP becomes even shorter with advancing age in Group A, and it tends to become even longer with advancing age in Group C, thereby exacerbating the morning or evening SBP surge. By contrast, there is no change with age in the circadian-circasemidian coupling interval of SBP in Group B. Bottom: Participants of each Group were further subdivided into three age groups: Young (<50 years), Middle-aged (50~65 years) and Old (>65 years). Whereas there was no significant change in evening SBP surge as a function of age in any of the three groups, the morning SBP surge increased in middle-aged (P = 0.0444) and elderly (P = 0.0463) as compared to young participants, observed only in Group A (bottom left).
State of Health Influencing Citizens’ Resilience
Among Group A participants (with a short circadian-circasemidian SBP coupling), those with a longer coupling interval are closer to citizens of Group B and have a less pronounced morning SBP surge (Figure 3, left and Table 1, left), suggesting that they may also have a better resilience. As seen from Table 2 (left), we indeed found by linear regression that Group A citizens with a longer coupling interval have a larger circadian amplitude of SBP (r = 0.2984, P = 0.0068) and DBP (r = 0.4112, P = 0.0001), a lower nighttime SBP (r = −0.4152, P = 0.0003), and a deeper SBP nightly dip (r = 0.4319, P = 0.0001). They also have higher HDL-cholesterol (r = 0.2819, P = 0.0473), lower CAVI (r = −0.5574, P = 0.0001), and lower fasting blood glucose (r = −0.3621, P = 0.0325). Their longer circadian period of SBP (r = 0.2467, P = 0.0274) and larger circadian phase delay of SBP (r = 0.5251, P < 0.0001) and DBP (r = 0.3148, P = 0.0042) may have contributed to promote resilience, as seen from their higher score of happiness (r = 0.2319, P = 0.04380). Increasing age, however, is associated with a shorter coupling interval (r = −0.5231, P < 0.0001).
Table 2.
Difference in Resilience Assessed by 7-Day/24-Hour ABPM, Sleep, Wellbeing and Laboratory Examinations Related to Circadian-Circasemidian Interval of Systolic Blood Pressure (SBP) Between Group A and Group C Participants
| Group A Short Coupling, <5.66 Hours (~ 4.5 Hours) | Group C Long Coupling, >6.5 Hours (~ 8.0 Hours) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | n | Mean | SD | Correlation Coefficient | P-value | n | Mean | SD | Correlation Coefficient | P-value | |
| Citizens’ characteristics | Age (years) | 81 | 56.2 | 12.1 | −0.5231 | <0.00001 | 84 | 60.14 | 11.18 | 0.1450 | 0.18618 |
| BMI (kg/m2) | 81 | 23.2 | 3.2 | 0.1265 | 0.2605 | 83 | 23.58 | 3.36 | 0.0936 | 0.40004 | |
| 7-day/24-h ABPM | SBP 7-day/24-h day average (mmHg) | 73 | 125.6 | 11.9 | −0.1772 | 0.1338 | 84 | 127.12 | 14.44 | 0.1511 | 0.1701 |
| SBP 7-day daytime average (mmHg) | 73 | 131.8 | 12.9 | −0.0940 | 0.4291 | 74 | 136.93 | 15.82 | 0.0604 | 0.6094 | |
| SBP 7-day nighttime average (mmHg) | 73 | 106.9 | 12.7 | −0.4152 | 0.0003 | 74 | 112.38 | 16.47 | 0.3671 | 0.0013 | |
| SBP dip average (mmHg) | 73 | 18.8 | 7.1 | 0.4319 | 0.0001 | 74 | 17.90 | 7.77 | −0.4770 | <0.0001 | |
| SBP circadian period (hours) | 80 | 23.98 | 0.45 | 0.2467 | 0.0274 | 79 | 24.09 | 0.52 | 0.0495 | 0.6646 | |
| SBP 24-h Amplitude (mmHg) | 81 | 14.14 | 5.12 | 0.2984 | 0.0068 | 84 | 14.57 | 5.24 | −0.1586 | 0.1495 | |
| DBP 24-h Amplitude (mmHg) | 81 | 9.50 | 3.45 | 0.4112 | 0.0001 | 84 | 8.85 | 3.87 | −0.2600 | 0.0169 | |
| HR 24-h Amplitude (bpm) | 81 | 8.96 | 3.80 | −0.0990 | 0.3792 | 84 | 7.99 | 3.05 | 0.0876 | 0.4281 | |
| SBP 24-h Acrophase (hh:mm) | 81 | 13:31 | 1:21 | 0.5251 | <0.00001 | 84 | 14:53 | 1:57 | 0.3293 | 0.0022 | |
| DBP 24-h Acrophase (hh:mm) | 81 | 13:38 | 1:27 | 0.3148 | 0.0042 | 84 | 14:24 | 1:48 | 0.2401 | 0.0278 | |
| HR 24-h Acrophase (hh:mm) | 81 | 14:45 | 1:20 | 0.1907 | 0.0882 | 84 | 15:10 | 2:10 | −0.1465 | 0.1835 | |
| Home BP | Morning HBP SBP (mmHg) | 79 | 126.9 | 14.2 | −0.4478 | <0.0001 | 81 | 129.07 | 15.57 | 0.0922 | 0.4129 |
| Morning HBP DBP (mmHg) | 79 | 79.9 | 8.5 | −0.2950 | 0.0083 | 81 | 80.87 | 9.76 | −0.0978 | 0.3852 | |
| Morning HBP Pulse (bpm) | 79 | 68.4 | 9.1 | 0.0219 | 0.8479 | 81 | 66.58 | 8.23 | −0.2950 | 0.0075 | |
| Sleep | Sleep duration (min) | 81 | 454.7 | 64.1 | −0.2172 | 0.0515 | 84 | 467.24 | 68.02 | 0.0912 | 0.4095 |
| Time to fall asleep (min) | 76 | 22.8 | 25.4 | −0.0081 | 0.9447 | 72 | 25.64 | 24.77 | −0.0421 | 0.7257 | |
| Wellbeing | GDS15 | 77 | 3.61 | 2.99 | −0.1464 | 0.2040 | 73 | 3.68 | 2.91 | 0.3340 | 0.0039 |
| How about your health? (%) | 76 | 74.2 | 18.2 | −0.1555 | 0.1799 | 71 | 71.92 | 22.36 | −0.2161 | 0.0703 | |
| How about your mood? (%) | 68 | 69.7 | 20.0 | −0.0922 | 0.4546 | 64 | 70.81 | 19.14 | −0.0790 | 0.5351 | |
| Good relation with family? (%) | 68 | 76.9 | 21.4 | −0.1009 | 0.4129 | 64 | 83.81 | 18.49 | 0.0692 | 0.5869 | |
| Good relation with friends? (%) | 68 | 74.4 | 23.0 | −0.0461 | 0.7088 | 64 | 82.33 | 17.73 | −0.0324 | 0.7997 | |
| Economic satisfaction? (%) | 68 | 56.4 | 23.8 | −0.1127 | 0.3604 | 65 | 54.40 | 21.26 | −0.2637 | 0.0338 | |
| Life satisfaction? (%) | 68 | 65.6 | 20.7 | −0.0380 | 0.7585 | 64 | 62.52 | 21.04 | −0.3158 | 0.0110 | |
| How about happiness? (%) | 76 | 75.1 | 19.7 | 0.2319 | 0.0438 | 71 | 68.42 | 19.87 | −0.3234 | 0.0059 | |
| Laboratory Examinations | Fasting blood glucose (mg/dl) | 35 | 97.6 | 11.4 | −0.3621 | 0.0325 | 29 | 97.86 | 18.33 | −0.0619 | >0.05 |
| Uric acid (mg/dl) | 39 | 5.4 | 1.1 | 0.0435 | 0.7924 | 28 | 5.28 | 1.38 | −0.2215 | >0.05 | |
| Total cholesterol (mg/dl) | 39 | 208.2 | 38.4 | 0.1887 | 0.2499 | 31 | 201.55 | 36.94 | −0.0024 | 0.9898 | |
| LDL-cholesterol (mg/dl) | 49 | 125.8 | 35.6 | −0.1074 | 0.4628 | 39 | 119.80 | 30.76 | 0.1165 | 0.4799 | |
| HDL-cholesterol (mg/dl) | 50 | 62.5 | 15.8 | 0.2819 | 0.0473 | 39 | 62.65 | 14.20 | −0.0939 | 0.5696 | |
| Triglycerides (mg/dl) | 50 | 107.7 | 64.2 | −0.0856 | 0.5544 | 38 | 120.30 | 86.52 | −0.0991 | 0.5540 | |
| CAVI | 44 | 8.28 | 1.26 | −0.5574 | 0.0001 | 31 | 8.31 | 1.11 | −0.0619 | 0.7408 | |
| ABI | 46 | 1.09 | 0.09 | −0.1304 | 0.3876 | 38 | 1.09 | 0.10 | 0.0299 | 0.8584 | |
Note: Cells highlighted in gray indicate statistically significant association of corresponding variable as a function of the circadian-circasemidian coupling interval, separately in Group A or C.
Among Group C participants (with a long circadian-circasemidian SBP coupling), those with a shorter coupling interval are closer to citizens of Group B and have a less pronounced evening SBP surge (Figure 3, right and Table 1, right), suggesting that they may also have a better resilience. As seen from Table 2 (right), we indeed found by linear regression that Group C citizens with a shorter coupling interval have a larger circadian amplitude of DBP (r = −0.2600, P = 0.0169), a lower nighttime SBP (r = 0.3671, P = 0.0013) and a deeper SBP nightly dip (r = −0.4770, P < 0.0001). Their shorter circadian phase delay of SBP (r = 0.3293, P = 0.0022) and DBP (r = 0.2401, P = 0.0278) may have contributed to promote resilience, as seen from their higher score of economic satisfaction (r = −0.2637, P = 0.0338), life satisfaction (r = −0.3158, P = 0.0110), and happiness (r = −0.3234, P = 0.0059).
Discussion
We previously found that an amplified 12-hour (circasemidian) rhythm could help consolidate the circadian system and contribute to a rapid adaptation to microgravity in space.35 Namely, we reported that spaceflight upregulated the 12-hour rhythm, closely cross-talking with the circadian clock, contributing to an amplified circadian rhythm, and switching to an “alerted mode” in space, which also amplified 8-hour, 6-hour, 3-hour and 90-min harmonics, extending adaptive reactions up to 12-months in space.35 Most life on Earth is governed by biological rhythms that are defined as self-sustained oscillations, cycling with a specified period.70–75 Circadian disruption can lead to cancer, cardiovascular, metabolic, and psychiatric disorders, and to cognitive decline in the aged. Alteration of the 12-hour component related to cardiovascular dysfunction76 indicates that the 12-hour component may be important for human health.
Following the pioneering study by Hughes et al,32 a series of studies by Pittsburgh’s group identified a cell-autonomous 12-hour oscillator of nuclear speckle liquid–liquid phase separation dynamics in mammals,39,41,77 which regulates 12-hour rhythms of systemic gene expression and metabolism, independently from the 24-hour circadian clock. These studies enabled the formulation of novel hypotheses that the biological 12-hour component could play an important role not only in the adaptation to space,35 but also in the adaptation to everyday physiology by adjusting to recurring daily changes in the external environment.74,77
Herein, we investigated how the biological 12-hour rhythm of SBP can help citizens in a community adapt to recurring daily changes in the external environment with help from the circadian clock. The relationship between BP rhythms and citizens’ resilience was cross-sectionally assessed by a 7-day/24-hour chronobiologic screening of a rural Japanese community, focusing on the 12-hour component and the circadian-circasemidian coupling of SBP. The 239 residents ranging in age from 23 to 74 years who participated in the study were free of anti-hypertensive medication and fully completed their 7-day/24-hour ABPM. We showed that both a morning and an evening SBP surge stemmed from an inappropriate coordination between the 24-hour and 12-hour rhythmic components of SBP. Citizens of Group A who had a short coupling interval had a circasemidian morning acrophase preceding the circadian acrophase by about 4.5 hours, which induced a morning BP surge in all 81 citizens. Moreover, the shorter the circadian-circasemidian coupling of SBP was, the larger was the morning SBP surge in 183 participants from all three groups (Figure 3). These findings may be relevant to residents’ resilience in terms of both health and wellbeing, as suggested from associations summarized in Table 2. These results are in agreement with those of many other investigations. An abrupt and dramatic morning BP surge is a predictor of cardiovascular events, including chronic kidney disease independently of ambulatory BP values and nocturnal BP falls. They also indicate that a larger morning BP surge is associated with an exaggerated risk of cardiovascular diseases and mortality.52,55,78–84
Results herein suggest that a circadian-circasemidian SBP coupling interval of about 6.0 hours (ranging from 5.67 to 6.5 hours) may be optimal, protect from a morning or evening BP surge, and promote resilience and wellbeing. Indeed, when the circadian-circasemidian coordination is appropriate, the biological clock is reinforced and the SBP surge in the morning or evening is less pronounced (morning surge in Group B vs Group A: 10.82 vs 14.29, P < 0.0001; evening surge in Group B vs Group C: 11.86 vs 15.21 mmHg, P < 0.0001). The incidence of a morning or evening SBP surge was also less frequent in Group B than in Group A (P < 0.0001) or in Group C (P < 0.0001) (Table 1). Overall, wellbeing and psychological resilience assessed by good relation with friends (P < 0.05), life satisfaction (P < 0.05), and subjective happiness (P < 0.05) were also highest for citizens of Group B than for citizens in Group A or Group C (Table 1).
The biological 12-hour rhythm reflects both the function of “endogenous endoplasmic reticulum (ER) stress and unfolded protein response (UPRER) cycle” and the reaction of “mitochondrial stress response pathway and unfolded protein response (UPRmt)”, which can protect cells from widespread proteome stress in both central and peripheral tissues.32,37–39,41,77,85–87 An XBP1s-SON axis implies a cell-autonomous 12-hour rhythm of nuclear speckle liquid–liquid phase separation (LLPS) dynamics.41,88 A large protein called Son is essential for appropriate subnuclear organization of pre-mRNA splicing factors and for promoting normal cell cycle progression.89,90 An evolutionarily conserved XBP1s-SON axis coordinates a rapidly functioning feedforward loop connecting nuclear speckle LLPS and proteostasis, resulting in a highly efficient genetic information transfer functioning at multiple temporal scales. At the molecular level, it modulates the rates of chemical reactions; at the mesoscale, it organizes large structures within cells; and at the cellular level, it facilitates the localization of cellular materials and homeostatic responses.41,88,91 Previously, we noted that both ER hormesis and mitohormesis are known to positively associate with adaptation to novel environments, stress resistance, anti-aging and longevity,92–96 and that this may be why the biological 12-hour rhythm was first activated, to consolidate a stronger circadian system in space. Whether a similar mechanism may account for results herein that relate to the adaptation to everyday physiology responding to the challenges of everyday life requires further investigation.
Several factors are associated with a BP surge, such as neurohormonal changes, notably the activation of the sympathetic nervous system,54,61 a depressive mood,50,51,60 acceleration of physical activity, sleep problems, smoking, bathing61 or drinking habits.52,62,81–83 Baross et al84 reported that practical lifestyle interventions are effective in markedly reducing the morning BP surge that might lower the incidence of adverse cardiovascular events, which often occur in the morning. Our results herein also suggest that practical lifestyle interventions to lower BP, dyslipidemia, arteriosclerosis and a depressive mood (Table 2) could counteract adverse effects from disrupted biological rhythms, a sub-optimal circadian-circasemidian coupling in particular.
Conclusion
The 12-hour rhythm may provide an evolutionary advantage34,36,41,67–69 as it may be even more ancient and having evolved earlier than the circadian clock: 12-hour clock genes are conserved in even more divergent species.89 Hence, assessing whether the circadian-circasemidian coupling is optimal or not, ie, dynamically balancing interactions with circadian and ultradian oscillations could play an important role in improving the health of citizens. The idea of Dion et al41 to prepare an atlas at the molecular level of four-dimensional (spatial and temporal) maps of biological processes to improve human health could detect unidentified chronotherapeutic targets for pathologies associated with disrupted biological rhythms. Our results herein support Dion’s idea of four-dimensional integrative medicine. It could be achieved by means of precision medicine based on chronomodecine and chronomics medicine, including an assessment of the circadian-circasemidian coupling in concert with 8-hour, 6-hour, 3-hour, and 90-min harmonics of the circadian rhythm23,27,35,38,41,68,74 to optimize their constellation. Devising a new approach focused at least on circadian-circasemidian coordination could be the first step as it offers great promise for achieving properly timed rhythms and lead to overall resilience as part of a precision chronomics medicine.15,18,19,21,22,31,61,97
Acknowledgments
The authors thank the town’s mayor and the public health nurses from the Health and Welfare Center, Tosa town, Kochi, Japan, for cooperation in our study. The authors also acknowledge all participants in the town.
Data Sharing Statement
Due to the sensitive nature of the questions asked in this study, survey respondents were assured that the raw data would remain confidential and would not be shared.
Ethics
This study was approved by the Medical Ethics Committee of Tokyo Women’s Medical University as Clinical Study #2912, entitled “Health assessment of community-dwelling elderly in Japan”. Written informed consent was obtained from all participants regarding data analysis and the publication of results thereof. A detailed explanation of the study protocol was given to the study participants before they gave written, informed consent. The study complies with the Declaration of Helsinki Principles.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, design or execution of the study, and/or in relation to data acquisition, analysis and interpretation, or in all these areas. They took part in the drafting, revising and/or critically reviewing the article. They gave final approval of the version submitted for publication, and agreed on the journal to which the article has been submitted. They also agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing financial and non-financial interests in relation to the work described. There is no sponsor’s role.
References
- 1.Davydov DM, Stewart R, Ritchie K, Chaudieu I. Resilience and mental health. Clin Psychol Rev. 2010;30:479–495. doi: 10.1016/j.cpr.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 2.Yoshino K, Morimoto T, Itagaki T, Iketani S, Nagata M, Tsujishita M. Relationship between life satisfaction and sympathovagal balance in healthy elderly males at home at night. Health. 2012;4:1068–1072. doi: 10.4236/health.2012.411163 [DOI] [Google Scholar]
- 3.Kong F, Wang X, Hu S, Liu J. Neural correlates of psychological resilience and their relation to life satisfaction in a sample of healthy young adults. Neuroimage. 2015;123:165–172. doi: 10.1016/j.neuroimage.2015.08.020 [DOI] [PubMed] [Google Scholar]
- 4.Satici SA. Psychological vulnerability, resilience, and subjective well-being: the mediating role of hope. Pers Individ Differ. 2016;102:68–73. doi: 10.1016/j.paid.2016.06.057 [DOI] [Google Scholar]
- 5.Kong F, Ma X, You X, Xiang Y. The resilient brain: psychological resilience mediates the effect of amplitude of low-frequency fluctuations in orbitofrontal cortex on subjective well-being in young healthy adults. Soc Cogn Affect Neurosci. 2018;13:755–763. doi: 10.1093/scan/nsy045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King ML. The neural correlates of well-being: a systematic review of the human neuroimaging and neuropsychological literature. Cogn Affect Behav Neurosci. 2019;19:779–796. doi: 10.3758/s13415-019-00720-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamarck TW, Janicki DL, Shiffman S, et al. Psychosocial demands and ambulatory blood pressure: a field assessment approach. Physiol Behav. 2002;77:699–704. doi: 10.1016/s0031-9384(02)00921-6 [DOI] [PubMed] [Google Scholar]
- 8.Zanstra YJ, Johnston DW. Cardiovascular reactivity in real life settings: measurement, mechanisms and meaning. Biol Psychol. 2011;86:98–105. doi: 10.1016/j.biopsycho.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamarck TW, Li X, Wright AGC, Muldoon MF, Manuck SB. Ambulatory blood pressure reactivity as a moderator in the association between daily life psychosocial stress and carotid artery atherosclerosis. Psychosom Med. 2018;80:774–782. doi: 10.1097/PSY.0000000000000627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas MC, Kamarck TW, Li X, Erickson KI, Manuck SB. Physical activity moderates the effects of daily psychosocial stressors on ambulatory blood pressure. Health Psychol. 2019;38:925–935. doi: 10.1037/hea0000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomitani N, Kanegae H, Suzuki Y, Kuwabara M, Kario K. Stress-induced blood pressure elevation self-measured by a wearable watch-type device. Am J Hypertens. 2021;34:377–382. doi: 10.1093/ajh/hpaa139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon AM, Mendes WB. A large-scale study of stress, emotions, and blood pressure in daily life using a digital platform. Proc Natl Acad Sci U S A. 2021;118:e2105573118. doi: 10.1073/pnas.2105573118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber J, Angerer P, Apolinário-Hagen J. Physiological reactions to acute stressors and subjective stress during daily life: a systematic review on ecological momentary assessment (EMA) studies. PLoS One. 2022;17:e0271996. doi: 10.1371/journal.pone.0271996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomitani N, Kanegae H, Kario K. The effect of psychological stress and physical activity on ambulatory blood pressure variability detected by a multisensor ambulatory blood pressure monitoring device. Hypertens Res. 2022;1–6. doi: 10.1038/s41440-022-01123-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jafari Roodbandi A, Choobineh A, Daneshvar S. Relationship between circadian rhythm amplitude and stability with sleep quality and sleepiness among shift nurses and health care workers. Int J Occup Saf Ergon. 2015;21:312–317. doi: 10.1080/10803548.2015.1081770 [DOI] [PubMed] [Google Scholar]
- 17.Norsk P, Asmar A, Damgaard M, Christensen NJ. Fluid shifts, vasodilatation and ambulatory blood pressure reduction during long duration spaceflight. J Physiol. 2015;593:573–584. doi: 10.1113/jphysiol.2014.284869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Milia L, Folkard S. More than morningness: the effect of circadian rhythm amplitude and stability on resilience, coping, and sleep duration. Front Psychol. 2021;12:782349. doi: 10.3389/fpsyg.2021.782349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roenneberg T, Foster RG, Klerman EB. The circadian system, sleep, and the health/disease balance: a conceptual review. J Sleep Res. 2022;31:e13621. doi: 10.1111/jsr.13621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 21.Smolensky M, Halberg F, Sargent F. Chronobiology of the life sequence. In: Itoh S, Ogata K, Yoshimura H, editors. Advances in Climatic Physiology. Tokyo: Igaku Shoin Ltd.; 1972:281–318. [Google Scholar]
- 22.Cornelissen G, Breus TK, Bingham C, et al. Beyond circadian chronorisk: worldwide circaseptan-circasemiseptan patterns of myocardial infarctions, other vascular events, and emergencies. Chronobiologia. 1993;20:87–115. [PubMed] [Google Scholar]
- 23.Halberg F, Cornelissen G, Otsuka K, Schwartzkopff O, Halberg J, Bakken EE. Chronomics. Biomed Pharmacother. 2001;55(Suppl 1):153s–190s. doi: 10.1016/S0753-3322(01)90022-8 [DOI] [PubMed] [Google Scholar]
- 24.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965 [DOI] [PubMed] [Google Scholar]
- 25.Halberg F, Cornelissen G, Katinas G, et al. Transdisciplinary unifying implications of circadian findings in the 1950s. J Circadian Rhythms. 2003;1:2. doi: 10.1186/1740-3391-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177 [DOI] [PubMed] [Google Scholar]
- 27.Halberg F, Cornelissen G, Sothern RB, Katinas GS, Schwartzkopff O, Otsuka K. Cycles tipping the scale between death and survival (=“Life”). Prog Theor Phys Supp. 2008;173:153–181. doi: 10.1143/PTPS.173.153 [DOI] [Google Scholar]
- 28.Halberg F. Quo vadis basic and clinical chronobiology: promise for health maintenance. Am J Anat. 1983;168:543–594. doi: 10.1002/aja.1001680408 [DOI] [PubMed] [Google Scholar]
- 29.West AC, Bechtold DA. The cost of circadian desynchrony: evidence, insights and open questions. Bioessays. 2015;37:777‐788. doi: 10.1002/bies.201400173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornelissen G, Otsuka K. Chronobiology of aging: a mini-review. Gerontology. 2017;63:118–128. doi: 10.1159/000450945 [DOI] [PubMed] [Google Scholar]
- 31.Panda S. The arrival of circadian medicine. Nat Rev Endocrinol. 2019;15:67–69. doi: 10.1038/s41574-018-0142-x [DOI] [PubMed] [Google Scholar]
- 32.Hughes ME, DiTacchio L, Hayes KR, et al. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5:e1000442. doi: 10.1371/journal.pgen.1000442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes ME, Hong HK, Chong JL, et al. Brain-specific rescue of Clock reveals system-driven transcriptional rhythms in peripheral tissue. PLoS Genet. 2012;8:e1002835. doi: 10.1371/journal.pgen.1002835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balance H, Zhu B. Revealing the hidden reality of the mammalian 12-h ultradian rhythms. Cell Mol Life Sci. 2021;78:3127–3140. doi: 10.1007/s00018-020-03730-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otsuka K, Cornelissen G, Furukawa S, et al. Unconscious mind activates central cardiovascular network and promotes adaptation to microgravity possibly anti-aging during 1-year-long spaceflight. Sci Rep. 2022;12:11862. doi: 10.1038/s41598-022-14858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–634. doi: 10.1016/j.cmet.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 37.Zhu B, Zhang Q, Pan Y, et al. A cell-autonomous mammalian 12 hr clock coordinates metabolic and stress rhythms. Cell Metab. 2017;25:1305–1319.e9. doi: 10.1016/j.cmet.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu B, Dacso CC, O’Malley BW. Unveiling “Musica Universalis” of the cell: a brief history of biological 12-hour rhythms. J Endocr Soc. 2018;2:727–752. doi: 10.1210/js.2018-00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan Y, Ballance H, Meng H, et al. 12-h clock regulation of genetic information flow by XBP1s. PLoS Biol. 2020;18:e3000580. doi: 10.1371/journal.pbio.3000580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71–92. doi: 10.1016/B978-0-12-385114-7.00004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dion W, Ballance H, Lee J, et al. Four-dimensional nuclear speckle phase separation dynamics regulate proteostasis. Sci Adv. 2022;8:eabl4150. doi: 10.1126/sciadv.abl4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otsuka K, Watanabe H, Cornelissen G, et al. Gender, age and circadian blood pressure variation of apparently healthy rural vs. metropolitan Japanese. Chronobiologia. 1990;17:253–265. [PubMed] [Google Scholar]
- 43.Otsuka K, Cornelissen G, Halberg F. Predictive value of blood pressure dipping and swinging with regard to vascular disease risk. Clin Drug Invest. 1996;11:20–31. doi: 10.2165/00044011-199611010-00003 [DOI] [Google Scholar]
- 44.Otsuka K, Cornelissen G, Halberg F, Oehlert G. Excessive circadian amplitude of blood pressure increases risk of ischemic stroke and nephropathy. J Med Eng Technol. 1997;21:23–30. doi: 10.3109/03091909709030299 [DOI] [PubMed] [Google Scholar]
- 45.Otsuka K, Nishimura Y, Kubo Y, Cornelissen G, Halberg F. Chronomes (Rhythms, Chaos and Age Trends) of human heart rate variability in both genders. Comput Cardiol. 1997;24:49–52. [Google Scholar]
- 46.Otsuka K, Cornelissen G, Shinagawa M, et al. Circadian reference values for different endpoints of heart rate variability. Comput Cardiol. 1999;26:587–590. [Google Scholar]
- 47.Cornelissen G, Schwartzkopff O, Halberg F, Otsuka K, Watanabe Y. 7-day ambulatory monitoring for adults with hypertension and diabetes. Am J Kidney Dis. 2001;37:878. doi: 10.1016/s0272-6386(01)80145-1 [DOI] [PubMed] [Google Scholar]
- 48.Halberg F, Cornelissen G, Wall D, et al. Engineering and governmental challenge: 7-day/24-hour chronobiologic blood pressure and heart rate screening: part I. Biomed Instrum Technol. 2002;36:89–122. doi: 10.2345/0899-8205(2002)36[89:EAGCHC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 49.Halberg F, Cornelissen G, Wall D, et al. Engineering and governmental challenge: 7-day/24-hour chronobiologic blood pressure and heart rate screening: part II. Biomed Instrum Technol. 2002;36:183–197. doi: 10.2345/0899-8205(2002)36[183:EAGCHC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 50.Shinagawa M, Otsuka K, Murakami S, et al. Seven-day (24-h) ambulatory blood pressure monitoring, self-reported depression and quality of life scores. Blood Pressure Monit. 2002;7:69–76. doi: 10.1097/00126097-200202000-00015 [DOI] [PubMed] [Google Scholar]
- 51.Otsuka K, Yamanaka G, Shinagawa M, et al. Chronomic community screening reveals about 31% depression, elevated blood pressure and infradian vascular rhythm alteration. Biomed Pharmacother. 2004;58(Suppl 1):48s–55s. doi: 10.1016/s0753-3322(04)80010-6 [DOI] [PubMed] [Google Scholar]
- 52.Murakami S, Otsuka K, Kubo Y, et al. Repeated ambulatory monitoring reveals a Monday morning surge in blood pressure in a community-dwelling population. Am J Hypertens. 2004;17:1179–1183. doi: 10.1016/j.amjhyper.2004.07.016 [DOI] [PubMed] [Google Scholar]
- 53.Cornelissen G, Halberg F, Otsuka K, Singh RB, Chen CH. Chronobiology predicts actual and proxy outcomes when dipping fails. Hypertension. 2007;49:237–239. doi: 10.1161/01.HYP.0000250392.51418.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halberg F, Cornelissen G, Otsuka K, et al. Extended consensus on need and means to detect vascular variability disorders (VVDs) and vascular variability syndromes (VVSs). Int Geronto Geriatr. 2008;11:119–146. [Google Scholar]
- 55.Murakami S, Otsuka K, Kono T, et al. Impact of outdoor temperature on prewaking morning surge and nocturnal decline in blood pressure in a Japanese population. Hypertens Res. 2011;34:70–73. doi: 10.1038/hr.2010.176 [DOI] [PubMed] [Google Scholar]
- 56.Halberg F, Powell D, Otsuka K, et al. Diagnosing vascular variability anomalies, not only MESR-hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H279–H294. doi: 10.1152/ajpheart.00212.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Otsuka K, Okajima K, Yamanaka T, et al. Aging and the novelty pressor effect in men on the first day of 7-day/24-hour ambulatory blood pressure monitoring. J Am Geriatric Soc. 2014;62:1602–1605. doi: 10.1111/jgs.12963 [DOI] [PubMed] [Google Scholar]
- 58.Otsuka K, Okajima K, Oinuma S, et al. Aging and circadian disruption of blood pressure observed using 7-day/24-hour ambulatory blood pressure monitoring. J Am Geriatrics Soc. 2014;62:2213–2215. doi: 10.1111/jgs.13115 [DOI] [PubMed] [Google Scholar]
- 59.Okajima K, Otsuka K, Oinuma S, Sasaki J, Yamanaka T, Cornelissen G. Aging and within- and between-day variability assessed using 7-day/24-hour ambulatory blood pressure monitoring. J Am Geriatrics Soc. 2014;62:2440–2442. doi: 10.1111/jgs.13166 [DOI] [PubMed] [Google Scholar]
- 60.Okajima K, Yamanaka G, Oinuma S, et al. Even mild depression is associated with among-day blood pressure variability, including masked non-dipping assessed by 7-d/24-h ambulatory blood pressure monitoring. Clin ExpHypertens. 2015;37:426–432. doi: 10.3109/10641963.2015.1013114 [DOI] [PubMed] [Google Scholar]
- 61.Otsuka K, Cornelissen G, Halberg F. Chronomics and Continuous Ambulatory Blood Pressure Monitoring – Vascular Chronomics: From 7-Day/24-Hour to Lifelong Monitoring. Tokyo: Springer Japan; 2016:870 + lxxv pp. doi: 10.1007/978-4-431-54631-3 [DOI] [Google Scholar]
- 62.Murakami S, Otsuka K, Kono T. Repeated ambulatory monitoring reveals an evening rise in blood pressure in a Japanese population. J Clin Hypertens. 2019;21:1675–1681. doi: 10.1111/jch.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage. 2008;42:169–177. doi: 10.1016/j.neuroimage.2008.04.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saito K, Koyama A, Yoneyama K, et al. A Recent Advances in Time Series Analysis by Maximum Entropy Method. Sapporo: Hokkaido University Press; 1994. [Google Scholar]
- 65.Bingham C, Arbogast B, Cornelissen GG, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- 66.Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16. doi: 10.1186/1742-4682-11-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fossel M. Editor’s Note. J Anti Aging Med. 1998;1:239. [Google Scholar]
- 68.Halberg F, Cornelissen G, Otsuka K, et al. Chronomics detects altered vascular variabilities constituting risks greater than hypertension: with an illustrative case report. In: Mitro P, Pella D, Rybar R, Valocik G, editors. Proceedings, 2nd Congress on Cardiovascular Diseases, Kosice, Slovakia, 25–27 April 2002. Bologna: Monduzzi Editore; 2002:223–258. [Google Scholar]
- 69.Murase M. Environmental pollution and health: an interdisciplinary study of the bioeffects of electromagnetic fields. SANSAI. 2008;3:1–35. [Google Scholar]
- 70.Halberg F, Visscher MB. Effect of light and of availability of food upon the 24-hour rhythm in number of circulating eosinophils in mice. Am J Physiol. 1952;171:732. [Google Scholar]
- 71.Pittendrigh CS. On temperature Independence in the clock system controlling emergence time in drosophila. Proc Natl Acad Sci USA. 1954;40:1018–1029. doi: 10.1073/pnas.40.10.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yagita K, Tamanini F, van Der Horst GT, Okamura H. Molecular mechanisms of the biological clock in cultured fibroblasts. Science. 2001;292:278–281. doi: 10.1126/science.1059542 [DOI] [PubMed] [Google Scholar]
- 74.Halberg F, Cornelissen G, Wilson D, et al. Chronobiology and chronomics: detecting and applying the cycles of nature. Biologist. 2009;56:209–214. [PMC free article] [PubMed] [Google Scholar]
- 75.Aviram R, Adamovich Y, Asher G. Circadian organelles: rhythms at all scales. Cells. 2021;10:2447. doi: 10.3390/cells10092447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Otsuka K, Cornelissen G, Halberg F. Circadian rhythmic fractal scaling of heart rate variability in health and coronary artery disease. Clin Cardiol. 1997;20:631–638. doi: 10.1002/clc.4960200710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meng H, Gonzales NM, Lonard DM, et al. XBP1 links the 12-hour clock to NAFLD and regulation of membrane fluidity and lipid homeostasis. Nat Commun. 2020;11:6215. doi: 10.1038/s41467-020-20028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa [DOI] [PubMed] [Google Scholar]
- 79.Li Y, Thijs L, Hansen TW, et al; International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension. 2010;55:1040–1048. doi: 10.1161/HYPERTENSIONAHA.109.137273 [DOI] [PubMed] [Google Scholar]
- 80.Booth JN, Jaeger BC, Huang L, et al. Morning blood pressure surge and cardiovascular disease events and all-cause mortality in blacks: the Jackson Heart Study. Hypertension. 2020;75:835–843. doi: 10.1161/HYPERTENSIONAHA.119.14233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bilo G, Grillo A, Guida V, Parati G. Morning blood pressure surge: pathophysiology, clinical relevance and therapeutic aspects. Integr Blood Press Control. 2018;11:47–56. doi: 10.2147/IBPC.S130277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shin J. Evening blood pressure rise, from myth to reality. J Clin Hypertens. 2019;21:1682–1683. doi: 10.1111/jch.13708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gapon LI, Shurkevich NP, Vetoshkin AS, Gubin DG. The rhythms of arterial pressure and heart rate in individuals with arterial hypertension under the conditions of Far North. Klin Med (Mosk). 2006;84:39–44. [PubMed] [Google Scholar]
- 84.Baross AW, Brook RD, Kay AD, et al. Effects of isometric leg training on ambulatory blood pressure and morning blood pressure surge in young normotensive men and women. Sci Rep. 2022;12:356. doi: 10.1038/s41598-021-04092-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu B, Wu J. Decoding the function and regulation of the mammalian 12-h clock. J Mol Cell Biol. 2020;12:752–758. doi: 10.1093/jmcb/mjaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cretenet G, Le Clech M, Gachon F. Circadian clock-coordinated 12 Hr period rhythmic activation of the IRE1alpha pathway controls lipid metabolism in mouse liver. Cell Metab. 2010;11:47–57. doi: 10.1016/j.cmet.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 87.Meng H, Gonzales NM, Jung SY, et al. Defining the mammalian coactivation of hepatic 12-h clock and lipid metabolism. Cell Rep. 2022;38:110491. doi: 10.1016/j.celrep.2022.110491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lyon AS, Peeples WB, Rosen MK. A framework for understanding the functions of biomolecular condensates across scales. Nat Rev Mol Cell Biol. 2021;22:215–235. doi: 10.1038/s41580-020-00303-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma A, Takata H, Shibahara K, Bubulya A, Bubulya PA. Son is essential for nuclear speckle organization and cell cycle progression. Mol Biol Cell. 2010;21:650–663. doi: 10.1091/mbc.e09-02-0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu X, Ng HH, Bubulya PA. The role of SON in splicing, development, and disease. Wiley Interdiscip Rev RNA. 2014;5:637–646. doi: 10.1002/wrna.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mofatteh M, Echegaray-Iturra F, Alamban A, Dalla Ricca F, Bakshi A, Aydogan MG. Autonomous clocks that regulate organelle biogenesis, cytoskeletal organization, and intracellular dynamics. Elife. 2021;10:e72104. doi: 10.7554/eLife.72104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alexander KA, Coté A, Nguyen SC, et al. p53 mediates target gene association with nuclear speckles for amplified RNA expression. Mol Cell. 2021;81:1666–1681.e6. doi: 10.1016/j.molcel.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taylor RC, Dillin A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell. 2013;153:1435–1447. doi: 10.1016/j.cell.2013.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Houtkooper RH, Mouchiroud L, Ryu D, et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497(7450):451–457. doi: 10.1038/nature12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun N, Youle RJ, Finkel T. The mitochondrial basis of aging. Mol Cell. 2016;61:654–666. doi: 10.1016/j.molcel.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gubin D, Weinert D, Cornelissen G. Chronotheranostics and chronotherapy – frontiers for personalized medicine. J Chronomed. 2020;22:3–23. doi: 10.36361/2307-4698-2020-22-1-3-23 [DOI] [Google Scholar]