Abstract
Guillain-Barré syndrome (GBS) is a rare but serious immune-mediated neurological condition characterized by damage to the peripheral nervous system. Two-thirds of cases of GBS are diagnosed following infection; however, vaccination has also been linked to GBS pathogenesis. The aim of this systematic review and meta-analysis was to establish the prevalence of GBS following vaccination against the SARS-CoV-2 virus, which causes COVID-19, describe the clinical and neurophysiological characteristics, and identify potential determinants. A systematic review of the literature regarding post-vaccination GBS was conducted using the PubMed database. Seventy papers were included. The pooled prevalence of GBS after vaccination against COVID-19 per has been established to be 8.1 (95% CI 30-220) per 1,000,000 vaccinations. Vaccination with vector vaccines - but not mRNA - has been associated with an increased risk of GBS. More than 80% of the patients developed GBS within 21 days following the first dose of the vaccination. The interval between the vaccination and GBS was shorter in patients who were vaccinated with mRNA versus vector vaccines (9.7±6.7 days versus 14.2±6.6 days). Epidemiological findings regarding post-vaccination GBS revealed a higher prevalence in males and people between the ages of 40 and 60 years, with a mean age of 56.8±16.1 years. The most common type was the acute inflammatory demyelinating polyneuropathy type. Most cases responded well to treatment. In conclusion, vaccination against COVID-19 with vector vaccines seems to increase the risk of GBS. GBS occurring following vaccination does differ in characteristics from GBS during the pre-COVID-19 era.
Keywords: guillain-barré syndrome, covid-19 vaccination, covid-19, post vaccination complications, immune mediated complications
Introduction and background
From the discovery of a new SARS-CoV-19 single-stranded RNA virus that causes coronavirus disease in 2019 (COVID-19) and the declaration of the COVID-19 pandemic in March 2020 till today, numerous confirmed COVID-19 cases and deaths have been reported. Worldwide research and development took place, resulting in the development of various vaccines against the virus.
There are four main categories of anti-SARS-CoV-2 vaccines, namely the whole virus, protein subunit, nucleic acid piece, and the viral vectors vaccine. These vaccines generally work by initiating immune reactions to the spike proteins on the surface membrane of SAR-CoV-2, thus providing immunity within the community as well as against the severe form of the disease. Various symptoms have been associated with the COVID-19 vaccine and are usually mild and self-limiting. There have been reports, however, of serious reactions, ranging from mild to severe hypersensitivity reactions, as well as numerous cases of neurological complications, including Guillain-Barré syndrome (GBS) [1].
GBS is a rare but serious immune-mediated disease characterized by damage to the peripheral and autonomic nervous system and is one of the known leading causes of flaccid paralysis worldwide [2], with an estimated annual median incidence of 11 persons per 1,000,000 population [3]. Although it is relatively rare, it may be life-threatening and debilitating.
The clinical manifestations of GBS usually reach their peak within four weeks of onset and include progressive ascending limb weakness and profound areflexia, while symptoms, such as paresthesia and/or pain, may occur in a few cases [3]. Autonomic dysfunction, respiratory failure, and, less commonly, cranial neuropathies are other symptoms associated with GBS.
Nerve conduction studies (NCS) are extremely useful in supporting the diagnosis of GBS and in differentiating between the numerous phenotypes. The electrodiagnostic patterns seen in GBS can be classified as Acute Inflammatory Demyelinating Polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN) or motor and sensory axonal neuropathy (AMSAN) or Miller-Fisher syndrome (MFS), according to their respective clinical manifestations and findings derived from NCS [3-6]. GBS is treated with intravenous immunoglobulin infusion or plasma exchange, depending on the severity of the symptoms, and the outcome is generally favorable [5].
The aim of this study is to systematically review the current literature to establish the prevalence, severity, and determinants of GBS in COVID-19 vaccine recipients.
Review
Methods
Literature Search Strategy
A systematic search was conducted using the PubMed database on May 23, 2022 using three Medical Subject Headings terms. Term A was “vaccine OR vaccinated,” Term B was “COVID-19 OR SARS-CoV-2 OR coronavirus,” and Term C was “GBS OR Guillain-Barré syndrome OR acute demyelinating polyneuropathy OR acute motor axonal neuropathy OR acute sensorimotor polyneuropathy OR Miller-Fisher syndrome OR AIDP OR MFS OR AMAN OR AMSAN”. English language filter was applied. For completeness, any results from ongoing or unpublished trials were searched for at http://www.clinicaltrials.gov/. All study data were aggregated and referenced in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.
Inclusion and Exclusion Criteria
Articles eligible for inclusion in this review had to meet the following criteria: papers containing information about human subjects who had received any type of vaccination against COVID-19 and who subsequently developed clinically confirmed GBS.
The exclusion criteria for this review include (i) non-human studies, (ii) duplicate studies or studies referring to the same populations, and (iii) nonoriginal articles (review, medical hypothesis, letter to the editor, etc.).
In consecutive order, title screening, abstract screening, full-text screening, and reference screening, were implemented to filter out non-relevant papers. This left relevant articles suitable for inclusion. The papers, which were eligible for inclusion, were decided upon by two researchers. Reference screening was implemented using Google Scholar for the sole use of identifying papers that may have been missed during the original search; however, no additional literature was yielded.
Data Extraction
Data from the included literature were extracted and recorded using an Excel spreadsheet. Data collected included the title; the name of the author; year of publication; demographics such as the age of subjects (years), gender, the prevalence of GBS after vaccination, time of onset of clinical symptoms of GBS after vaccination, number of vaccines received, type of vaccine administered (AstraZeneca, Moderna, Pfizer, Johnson & Johnson or others) and laboratory data such as covid serological screen, specific antibody titers (such as anti-ganglioside antibody titer, and anti-GMQ1e.t.c), cerebrospinal fluid (CSF) analysis (protein and cell counts), electromyography and NCS’s findings (axonal or demyelinating), treatment received, outcome of treatment and any disability or life-changing events.
Synthesis of Results and Statistical Analyses
Aggregated data were used where possible. Statistical pooled proportion calculations conducted in R language, used default settings of the “meta” package and “metaprop” function (random effects model) [7]. Each meta-analysis presented the I² statistic and forest plots, which evaluated heterogeneity [8]. Variation can be suggested as study heterogeneity or chance using this statistic. Negative I² values are put equal to 0, and values range between 0% and 100% [8]. Heterogeneity can be quantified as low, moderate, and high, with upper limits of 25%, 50%, and 75% for I², respectively [8]. Where data did not lend itself to meta-analysis, a narrative approach was taken.
Frequencies and descriptive statistics were examined for each variable. Comparisons between two groups were made using Student’s t-tests for normally distributed continuous data and chi-square test for categorical data. Analyses between more than two groups were made using one-way analysis of variance for continuous data and chi-square test for categorical data. Bonferroni’s correction for multiple comparisons was applied as appropriate. Level of significance was set at 0.05.
Compliance With Ethical Guidelines
As this is a systematic review based on the existing literature regarding post-covid vaccination GBS, there was no need to conduct an ethics review.
Results
Study Characteristics
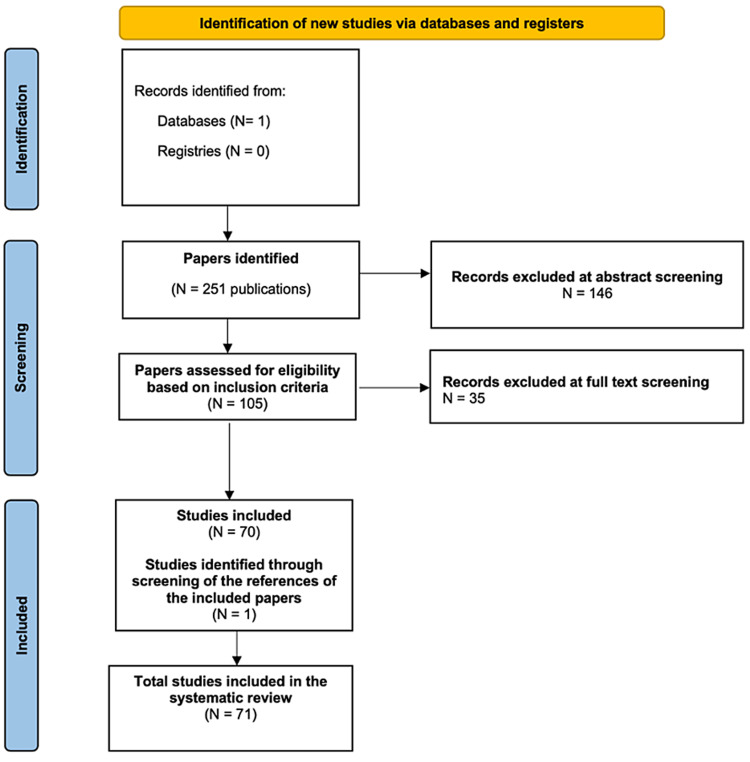
The above-mentioned research strategy yielded 251 studies. Following abstract screening, 146 studies were excluded. After evaluation of the full texts of the remaining 105 reports, 36 more publications were excluded as they did not meet the outlined inclusion criteria. One paper which was eligible for inclusion was identified through the reference lists of the included papers. In total 71 articles have been included in the review [1,2,6,9-76]. Three of those papers provided relevant data regarding the prevalence and incidence of post COVID-19 vaccination GBS [9-11], while the others were mainly case reports or small case series comprising of a total of 138 patients with COVID-19 vaccine-related GBS. The PRISMA chart in Figure 1 shows the outcome of the selection process at each stage of the procedure.
Figure 1. PRISMA flow diagram of the study.
Prevalence of GBS Following COVID-19 Vaccination
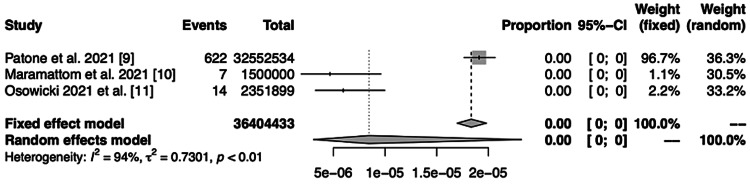
Meta-analysis of the three studies which contained prevalence data was performed. In their study, Patone et al. [9] showed that in England, there was an overall increased risk of GBS with an incidence rate ratio (IRR) of 2.9 (95% confidence interval 2.2-3.9) 15-21 days after vaccination. Interestingly, 3.8 per million excess cases of GBS were estimated in subjects who received the Astrazeneca vaccine in the 1-28-day risk period. However, the authors did not observe an increased risk of GBS in those who received the Pfizer vaccine. Similarly, in Kerala, India, Maramattom et al. reported that following vaccination with the Astrazeneca vaccine the frequency of GBS was 1.4- to 10-fold higher than what expected in the same period [10] and Osowicki et al. reported the frequency of GBS subsequent to vaccination with the Astrazeneca vaccine to be 1.6-fold higher than expected [11].
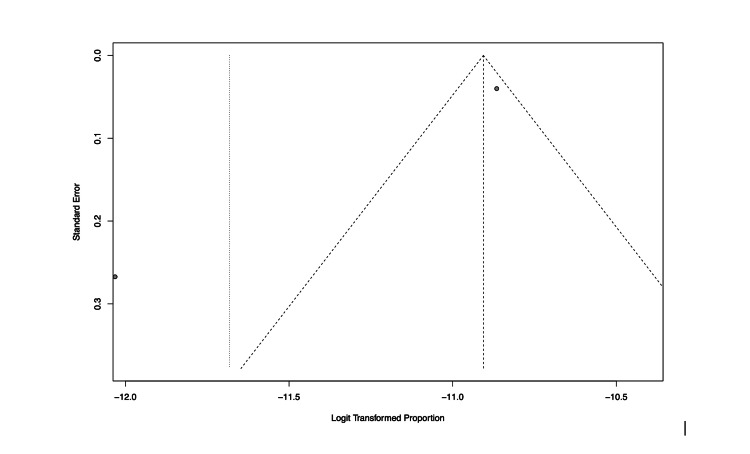
The pooled prevalence of GBS after vaccination against COVID-19 has been established to be 8.1 (95% CI 30-220) per 1,000,000 vaccinations (Figure 2). However, as shown in the respective funnel plot (Figure 3) there was high heterogeneity across these studies (I2 = 93.7%).
Figure 2. Forest plot showing outcome of meta-analysis of prevalence GBS following COVID-19 vaccination.
Figure 3. Funnel plot showing high heterogeneity in the data and outcome of meta- analysis of prevalence of post covid-vaccination GBS.
Characteristics of Gbs Patients Following COVID-19 Vaccination
Type of vaccines: Most cases received the Astrazeneca vaccine (56%), followed by Pfizer (20%), J&J (7%), Sputnik (7%), Moderna (5%), Sinovac (4%) and Novavax (1%). GBS occurred after receiving the first dose of the vaccination (where applicable) in 110 cases (80%).
Demographics: In total, 138 cases of GBS following the COVID-19 vaccination were recorded, of which, 82 (59.4%) occurred in males while 56 (40.6%) were in females. The highest number of cases was reported in the age group 41-60 years and the mean age of patients was found to be 56.8±16.1 years.
Time interval: The mean interval between vaccination and GBS development was 13.0±6.9 days, ranging from one to 37 days. More than 90% of GBS cases developed three weeks post-vaccination. Table 1 summarizes the demographic characteristics and the time interval of GBS development and the CSF findings per type of vaccine.
Table 1. Summary of the demographic characteristics and the time interval of GBS development and the CSF findings per type of vaccine.
| Astrazeneca (n=77) | Pfizer (n=28) | J&J (n=9) | Moderna (n=7) | Novavax (n=1) | Sputnik (n=9) | Sinovac (n=6) | |
| Age, in years (SD) | 57.9 (12.7) | 56.4 (22.1) | 48.1 (11.9) | 68.2 (13.9) | 76 | 50.7 (15.4) | 51.7 (25.0) |
| Interval, in days (SD) | 13.9 (6.3) | 9.8 (6.1) | 14.7 (5.9) | 9.5 (9.5) | 8 | 16.3 (6.0) | 13.7 (12.2) |
CSF findings: There was found to be an elevated CSF protein level in 87 (96%) of the 91 patients on which a lumbar puncture was performed while 4 patients had low to normal CSF protein levels. The mean reported value of CSF protein level was 221mg/dL.
Neurophysiological findings: Out of the 87 cases with reported NCS and EMG findings, 57 (66%) of the patients presented with demyelinating polyneuropathy while 23 (26%) had axonal phenotype GBS. Other forms included isolated bilateral facial nerve palsy and MFS. There was no significant difference in patient demographics or clinical characteristics when comparing demyelinating and axonal GBS groups.
Comparison of Vector and mRNA Vaccines
Comparison between 101 patients who received a vector vaccine (Astrazeneca, J&J, Sputnik, Sinovac) and 35 patients who received an mRNA vaccine (Moderna, Pfizer) was performed. Novavax is a different technology and thus, was not included in this pooled analysis.
The two groups did not differ significantly regarding age (56.0±14.0 versus 58.5±21.2, p=0.440). A trend of statistical significance was noted regarding gender as more males were found among the patients who developed GBS following a vector vaccine (64% versus 46%, p=0.053). This trend disappeared during the secondary analysis which only included the patients that developed GBS following the first dose of vaccinations requiring two doses (64% versus 52%, p=0.315).
The time interval of GBS manifestation was significantly lower in mRNA vaccine recipients (9.7±6.7 days versus 14.2±6.6 days, p < 0.001). A secondary analysis including only patients that developed GBS following the first dose of vaccinations requiring two doses was performed between the 88 patients who received a vector vaccine and 22 patients who received an mRNA vaccine. The time interval between vaccination and GBS was still significantly lower in mRNA vaccine recipients (11.1±6.8 days versus 14.2±6.4 days, p=0.048). No differences between CSF findings (number of cells, protein levels) where found between the groups.
Treatment Outcome and Severity of Gbs in Vaccine Recipients
Information about treatment and outcome was available for 127 patients. Most patients received intravenous immunoglobulins (79.5%), followed by plasma exchange (11.8%). A small minority (8.7%) presented with mild symptoms only and were managed conservatively. One in five patients (20%) required intensive care admission. The only death that was reported was due to autonomic dysfunction.
Most of the affected patients recovered to varying extents and at different rates. The activity level at final follow-up varies widely in each case, ranging from full recovery to persistent on-going motor and facial weakness, ataxia, quadriplegia, extreme pain, and other debilitating symptoms.
Discussion
One of the key findings of this systematic review and meta-analysis is that the prevalence of GBS related to vaccination against COVID-19 is 8.1 (95% CI 30-220) per 1,000,000 vaccinations. This is significantly higher to the epidemiology of GBS in the general population as previous literature suggests that the annual incidence of GBS follows a rising pattern with increasing age, from six (in children) to 27 (in elderly patients of more than 80 years of age) per million per year [3]. All three studies that provide big data about the epidemiology of GBS following vaccination against COVID-19 show that the majority of cases concern vaccination with a vector vaccine. In fact, Patone et al. [9] did not observe an increased risk of GBS in those who received a mRNA vaccine.
Secondly, our analysis showed that the time interval between vaccination and GBS was much lower for mRNA than for vector vaccines. The different technology and resultant immune-mediated mechanisms may account for this finding. However, these figures should be interpreted with caution given the fact that a true association between development of GBS following vaccination with an mRNA vaccine may not exist.
Thirdly, the most common neurophysiological type of GBS secondary to vaccination against COVID-19 was the demyelinating type (AIDP), a finding which agrees with the literature on GBS [77]. Finally, the outcome of GBS following vaccination against COVID-19 did not differ compared to reported GBS series during the pre-COVID-19 era [78].
Our meta-analysis has some limitations that need to be taken into consideration. Firstly, the heterogeneity of the included prevalence studies was high and therefore our findings should be interpreted with caution and confirmed with a wider meta-analysis should new prevalence studies be published in the future. Excluding the prevalence finding, the remainder of our results are based on isolated published case reports or small case series. This carries a significant publication bias which may undermine the validity of the results. We opted to include those papers in our analyses, however, as they gave valuable information about the neurophysiological type, clinical course, and outcome of the cases. Moreover, although the literature was rich regarding GBS cases after the vector and mRNA vaccines, only a single case of GBS following a protein vaccine was reported. Therefore, our review could not provide a high level of evidential information about protein-vaccine-related GBS.
Conclusions
In conclusion, vaccination against COVID-19 seems to increase slightly the risk of GBS. GBS following vaccination did not differ in characteristics from GBS during the pre-COVID-19 era. The finding that the interval between the vaccination and GBS was shorter in patients who were vaccinated with the mRNA vaccine merits further investigation in future research projects. However, it should be underscored that a possible explanation is that in the published cases of having received an mRNA vaccine, GBS was an incidental diagnosis following and not a complication related to the vaccination.
Acknowledgments
The authors do not have any conflict of interest to declare. All of them are grateful to the Paolo Procacci Foundation for their support on the publication process of this research.
The authors have declared that no competing interests exist.
References
- 1.Guillain-Barre syndrome after two COVID-19 vaccinations: two case reports with follow-up electrodiagnostic study. Kim JW, Kim YG, Park YC, Choi S, Lee S, Min HJ, Kim MJ. J Korean Med Sci. 2022;37:0. doi: 10.3346/jkms.2022.37.e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Could Guillain-Barré syndrome be triggered by COVID-19 vaccination? Aldeeb M, Okar L, Mahmud SS, Adeli GA. Clin Case Rep. 2022;10:0. doi: 10.1002/ccr3.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillain-Barré syndrome. Willison HJ, Jacobs BC, van Doorn PA. Lancet. 2016;13:717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 4.An update in guillain-barré syndrome. Winer JB. Autoimmune Dis. 2014;2014:793024. doi: 10.1155/2014/793024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diagnosis and management of Guillain-Barré syndrome in ten steps. Leonhard SE, Mandarakas MR, Gondim FA, et al. Nat Rev Neurol. 2019;15:671–683. doi: 10.1038/s41582-019-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A rare variant of Guillain-Barre syndrome following Ad26.COV2.S vaccination. Morehouse ZP, Paulus A, Jasti SA, Bing X. Cureus. 2021;13:0. doi: 10.7759/cureus.18153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WGCNA: an R package for weighted correlation network analysis. Langfelder P, Horvath S. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quantifying heterogeneity in a meta-analysis. Higgins JP, Thompson SG. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 9.Publisher correction: Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Patone M, Handunnetthi L, Saatci D, et al. Nat Med. 2021;27:2249. doi: 10.1038/s41591-021-01644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillain-Barré syndrome following ChAdOx1-S/nCoV-19 vaccine. Maramattom BV, Krishnan P, Paul R, Padmanabhan S, Nampoothiri SCV, Syed AA, Mangat HS. Ann Neurol. 2021;90:312–314. doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- 11.Guillain-Barré syndrome in an Australian state using both mRNA and adenovirus-vector SARS-CoV-2 vaccines. Osowicki J, Morgan H, Harris A, Crawford NW, Buttery JP, Kiers L. Ann Neurol. 2021;90:856–858. doi: 10.1002/ana.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller Fisher syndrome following Pfizer COVID-19 vaccine. Abičić A, Adamec I, Habek M. Neurol Sci. 2022;43:1495–1497. doi: 10.1007/s10072-021-05776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guillain-Barré syndrome variant occurring after SARS-CoV-2 vaccination. Allen CM, Ramsamy S, Tarr AW, Tighe PJ, Irving WL, Tanasescu R, Evans JR. Ann Neurol. 2021;90:315–318. doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- 14.Bilateral facial palsy after COVID-19 vaccination. Andreozzi V, D'arco B, Pagliano P, Toriello A, Barone P. Neurol Sci. 2022;43:4069–4079. doi: 10.1007/s10072-022-05982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillain-Barré syndrome after mRNA-1273 (Moderna) COVID-19 vaccination: a case report. Anjum Z, Iyer C, Naz S, et al. Clin Case Rep. 2022;10:0. doi: 10.1002/ccr3.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillain-Barré syndrome in a 67-year-old male post COVID-19 vaccination (Astra Zeneca) Azam S, Khalil A, Taha A. Am J Med Case Reports. 2021;9:424–427. [Google Scholar]
- 17.Clinical variant of Guillain-Barre syndrome with prominent facial diplegia after AstraZeneca coronavirus disease 2019 vaccine. Badoiu A, Moranne O, Coudray S, Ion IM. J Clin Neuromuscul Dis. 2021;23:115–116. doi: 10.1097/CND.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 18.Guillain-Barré syndrome following Covid-19 immunization: a report of two cases. Bax F, Gigli GL, Belgrado E, Brunelli L, Valente M. Acta Neurol Belg. 2022;122:1365–1367. doi: 10.1007/s13760-021-01798-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfizer mRNA COVID-19 vaccination and acute inflammatory demyelinating polyneuropathy. Bernardo KA, Misra A. J Clin Neuromuscul Dis. 2022;23:230–231. doi: 10.1097/CND.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 20.Rare occurrence of Guillain-Barré syndrome after Moderna vaccine. Bijoy George T, Kainat A, Pachika PS, Arnold J. BMJ Case Rep. 2022;15:249749. doi: 10.1136/bcr-2022-249749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilateral facial weakness with paresthesia variant of Guillain-Barré syndrome following Vaxzevria COVID-19 vaccine. Bonifacio GB, Patel D, Cook S, et al. J Neurol Neurosurg Psychiatry. 2022;93:341–342. doi: 10.1136/jnnp-2021-327027. [DOI] [PubMed] [Google Scholar]
- 22.Guillain-Barré syndrome following the first dose of Pfizer-BioNTech COVID-19 vaccine: case report and review of reported cases. Bouattour N, Hdiji O, Sakka S, et al. Neurol Sci. 2022;43:755–761. doi: 10.1007/s10072-021-05733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neurologic sequela of COVID-19: Guillain-Barré syndrome following Johnson & Johnson COVID-19 vaccination. Carranza O, Babici D, Waheed S, Yousuf F. Cureus. 2022;14:0. doi: 10.7759/cureus.24252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guillian Barré syndromeafter mRNA-1273 vaccination against COVID-19 (Article in Danish) Christensen SK, Ballegaard M, Boesen MS. https://pubmed.ncbi.nlm.nih.gov/34477091/ Ugeskr Laeger. 2021;183:0. [PubMed] [Google Scholar]
- 25.Guillain-Barré syndrome after vaccination against COVID-19. Chun JY, Park S, Jung J, et al. Lancet Neurol. 2022;21:117–119. doi: 10.1016/S1474-4422(21)00416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Variant Guillain-Barre syndrome following SARS-CoV-2 vaccination: case report and review of the literature. Donaldson L, Margolin E. Can J Neurol Sci. 2023;50:138–140. doi: 10.1017/cjn.2021.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Axonal-variant Guillian-Barre syndrome temporally associated with mRNA-based Moderna SARS-CoV-2 vaccine. Dalwadi V, Hancock D, Ballout AA, Geraci A. Cureus. 2021;13:0. doi: 10.7759/cureus.18291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller-Fisher syndrome and Guillain-Barre syndrome overlap syndrome in a patient post Oxford-AstraZeneca SARS-CoV-2 vaccination. Dang YL, Bryson A. BMJ Case Rep. 2021;14:246701. doi: 10.1136/bcr-2021-246701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inflammatory demyelinating polyneuropathy after the ChAdOx1 nCoV-19 vaccine may follow a chronic course. de Souza A, Oo WM, Giri P. J Neurol Sci. 2022;436:120231. doi: 10.1016/j.jns.2022.120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Exacerbating Guillain-Barré syndrome eight days after vector-based COVID-19 vaccination. Finsterer J. Case Rep Infect Dis. 2021;2021:3619131. doi: 10.1155/2021/3619131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Consider differentials before diagnosing COVID-19 associated polyradiculitis. Finsterer J, Scorza FA, Scorza CA, Fiorini AC. Eur J Transl Myol. 2022;32 doi: 10.4081/ejtm.2022.10111. [DOI] [PubMed] [Google Scholar]
- 32.A case of sensory ataxic Guillain-Barré syndrome with immunoglobulin G anti-GM1 antibodies following the first dose of mRNA COVID-19 vaccine BNT162b2 (Pfizer) Fukushima T, Tomita M, Ikeda S, Hattori N. QJM. 2022;115:25–27. doi: 10.1093/qjmed/hcab296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Case of Guillain-Barré syndrome following COVID-19 vaccine. Hasan T, Khan M, Khan F, Hamza G. BMJ Case Rep. 2021;14:243629. doi: 10.1136/bcr-2021-243629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guillain-Barré syndrome after COVID-19 mRNA vaccination in a liver transplantation recipient with favorable treatment response. Hughes DL, Brunn JA, Jacobs J, Todd PK, Askari FK, Fontana RJ. Liver Transpl. 2022;28:134–137. doi: 10.1002/lt.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guillain-Barré syndrome after AstraZeneca COVID-19-vaccination: a causal or casual association? Introna A, Caputo F, Santoro C, Guerra T, Ucci M, Mezzapesa DM, Trojano M. Clin Neurol Neurosurg. 2021;208:106887. doi: 10.1016/j.clineuro.2021.106887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Facial diplegia: a rare, atypical variant of Guillain-Barré syndrome and Ad26.COV2.S vaccine. Jain E, Pandav K, Regmi P, Michel G, Altshuler I. Cureus. 2021;13:0. doi: 10.7759/cureus.16612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guillain-Barré syndrome following ChAdOx1 nCoV-19 COVID-19 vaccination: a case series. James J, Jose J, Gafoor VA, Smita B, Balaram N. Neurol Clin Neurosci. 2021;9:402–405. doi: 10.1111/ncn3.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guillain-Barré syndrome presenting with facial diplegia following COVID-19 vaccination in two patients. Kanabar G, Wilkinson P. BMJ Case Rep. 2021;14:244527. doi: 10.1136/bcr-2021-244527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guillain-Barre syndrome and COVID-19 vaccine: a report of nine patients. Karimi N, Boostani R, Fatehi F, et al. Basic Clin Neurosci. 2021;12:703–710. doi: 10.32598/bcn.2021.3565.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Post COVID-19 vaccine Guillain-Barré syndrome. Khadka B, Khanal K. J Nepal Health Res Counc. 2022;19:852–854. doi: 10.33314/jnhrc.v19i04.3803. [DOI] [PubMed] [Google Scholar]
- 41.Guillain-Barré syndrome and variants following COVID-19 vaccination: report of 13 cases. Kim JE, Min YG, Shin JY, Kwon YN, Bae JS, Sung JJ, Hong YH. Front Neurol. 2021;12:820723. doi: 10.3389/fneur.2021.820723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guillain-Barré syndrome associated with BNT162b2 COVID vaccination: a first case report from South Korea. Kim N, Kim JH, Park JS. Neurol Sci. 2022;43:1491–1493. doi: 10.1007/s10072-021-05849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.A rare case of Guillain-Barré syndrome following COVID-19 vaccination. Kripalani Y, Lakkappan V, Parulekar L, Shaikh A, Singh R, Vyas P. Eur J Case Rep Intern Med. 2021;8:2707. doi: 10.12890/2021_002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guillain-Barré syndrome with rapid onset and autonomic dysfunction following first dose of Pfizer-BioNTech COVID-19 vaccine: a case report. Lanman TA, Wu C, Cheung H, Goyal N, Greene M. Neurohospitalist. 2022;12:388–390. doi: 10.1177/19418744211065242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guillain-Barré syndrome after SARS-CoV-2 vaccination in a patient with previous vaccine-associated Guillain-Barré syndrome. Ling L, Bagshaw SM, Villeneuve PM. CMAJ. 2021;193:0–9. doi: 10.1503/cmaj.210947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guillain-Barré syndrome following coronavirus disease vaccine: first report from Nepal. Luitel P, Poudel B, Upadhyay D, et al. SAGE Open Med Case Rep. 2022;10:2050313. doi: 10.1177/2050313X221100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guillain-Barrè syndrome following COVID-19 vaccine mRNA-1273: a case report. Masuccio FG, Comi C, Solaro C. Acta Neurol Belg. 2022;122:1369–1371. doi: 10.1007/s13760-021-01838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.COVID-19 vaccine causing Guillain-Barre syndrome, a rare potential side effect. Matarneh AS, Al-Battah AH, Farooqui K, Ghamoodi M, Alhatou M. Clin Case Rep. 2021;9:0. doi: 10.1002/ccr3.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guillain-Barré syndrome after COVID-19 vaccination. McKean N, Chircop C. BMJ Case Rep. 2021;14:244125. doi: 10.1136/bcr-2021-244125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine: report of two cases and review of literature. Min YG, Ju W, Ha YE, Ban JJ, Lee SA, Sung JJ, Shin JY. J Neuroimmunol. 2021;359:577691. doi: 10.1016/j.jneuroim.2021.577691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guillain- Barré syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: temporal associations do not imply causality [PREPRINT] Márquez Loza AM, Holroyd KB, Johnson SA, Pilgrim DM, Amato AA. Neurology. 2021;10 doi: 10.1212/WNL.0000000000011881. [DOI] [PubMed] [Google Scholar]
- 52.Sub-acute Onset of Guillain-Barré Syndrome post-mRNA-1273 vaccination: a case report. Nagalli S, Shankar Kikkeri N. SN Compr Clin Med. 2022;4:41. doi: 10.1007/s42399-022-01124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.A case of acute demyelinating polyradiculoneuropathy with bilateral facial palsy after ChAdOx1 nCoV-19 vaccine. Nasuelli NA, De Marchi F, Cecchin M, et al. Neurol Sci. 2021;42:4747–4749. doi: 10.1007/s10072-021-05467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller Fisher syndrome following BNT162b2 mRNA coronavirus 2019 vaccination. Nishiguchi Y, Matsuyama H, Maeda K, Shindo A, Tomimoto H. BMC Neurol. 2021;21:452. doi: 10.1186/s12883-021-02489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sensory ataxic Guillain-Barré syndrome with dysgeusia after mRNA COVID-19 vaccination. Ogata S, Ishii Y, Asano K, et al. Intern Med. 2022;61:1757–1760. doi: 10.2169/internalmedicine.8967-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guillain-Barré syndrome following the first dose of SARS-CoV-2 vaccine: a temporal occurrence, not a causal association. Ogbebor O, Seth H, Min Z, Bhanot N. IDCases. 2021;24:0. doi: 10.1016/j.idcr.2021.e01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Post coronavirus disease-2019 vaccination Guillain-Barré syndrome. Biswas A, Pandey SK, Kumar D, Vardhan H. Indian J Public Health. 2021;65:422–424. doi: 10.4103/ijph.ijph_1716_21. [DOI] [PubMed] [Google Scholar]
- 58.Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. Patel SU, Khurram R, Lakhani A, Quirk B. BMJ Case Rep. 2021;14:242956. doi: 10.1136/bcr-2021-242956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.A novel case of bifacial diplegia variant of Guillain-Barré syndrome following Janssen COVID-19 vaccination. Prasad A, Hurlburt G, Podury S, Tandon M, Kingree S, Sriwastava S. Neurol Int. 2021;13:404–409. doi: 10.3390/neurolint13030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.A case of Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Rao SJ, Khurana S, Murthy G, Dawson ET, Jazebi N, Haas CJ. J Community Hosp Intern Med Perspect. 2021;11:597–600. doi: 10.1080/20009666.2021.1954284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Post-COVID-19 vaccine Guillain-Barré syndrome; first reported case from Qatar. Razok A, Shams A, Almeer A, Zahid M. Ann Med Surg (Lond) 2021;67:102540. doi: 10.1016/j.amsu.2021.102540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guillain-Barré syndrome presenting as facial diplegia after Covid-19 vaccination: a case report. Rossetti A, Gheihman G, O'Hare M, Kosowsky JM. J Emerg Med. 2021;61:0–5. doi: 10.1016/j.jemermed.2021.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Electromyoneurography and laboratory findings in a case of Guillain-Barré syndrome after second dose of Pfizer COVID-19 vaccine. Scendoni R, Petrelli C, Scaloni G, Logullo FO. Hum Vaccin Immunother. 2021;17:4093–4096. doi: 10.1080/21645515.2021.1954826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller Fisher syndrome after COVID-19 vaccination: case report and review of literature. Siddiqi AR, Khan T, Tahir MJ, Asghar MS, Islam MS, Yousaf Z. Medicine (Baltimore) 2022;101:0. doi: 10.1097/MD.0000000000029333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guillain-Barré syndrome after coronavirus disease 2019 vaccine: a temporal association. da Silva GF, da Silva CF, Oliveira RE, et al. Clin Exp Neuroimmunol. 2022;13:92–94. doi: 10.1111/cen3.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Case report of Guillain-Barré Syndrome after COVID BNT162b2 mRNA vaccine (Article in Spanish) Sosa-Hernández O, Sánchez-Cardoza S. Vacunas. 2022;23:68–70. doi: 10.1016/j.vacun.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guillain-Barré syndrome and fulminant encephalomyelitis following Ad26.COV2.S vaccination: double jeopardy. Stefanou MI, Karachaliou E, Chondrogianni M, et al. Neurol Res Pract. 2022;4:6. doi: 10.1186/s42466-022-00172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.The first Guillain-Barr? Syndrome after SARS-CoV-2 vaccination in Taiwan. Su SC, Lyu RK, Chang CW, Tseng WJ. https://pubmed.ncbi.nlm.nih.gov/34988954/ Acta Neurol Taiwan. 2022;31(1):46–51. [PubMed] [Google Scholar]
- 69.Post COVID-19 vaccination Guillain-Barre syndrome: three cases. Tabatabaee S, Rezania F, Alwedaie SM, Malekdar E, Badi Z, Tabatabaei SM, Mirzaasgari Z. Hum Vaccin Immunother. 2022;18:2045153. doi: 10.1080/21645515.2022.2045153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guillain-Barré syndrome after Ad26.COV2.S vaccination. Thant HL, Morgan R, Paese MM, Persaud T, Diaz J, Hurtado L. Am J Case Rep. 2022;23:0. doi: 10.12659/AJCR.935275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guillain-Barré syndrome following first injection of ChAdOx1 nCoV-19 vaccine: first report. Theuriet J, Richard C, Becker J, Pegat A, Bernard E, Vukusic S. Rev Neurol (Paris) 2021;177:1305–1307. doi: 10.1016/j.neurol.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 72.Guillain-Barré syndrome following BNT162b2 COVID-19 vaccine. Trimboli M, Zoleo P, Arabia G, Gambardella A. Neurol Sci. 2021;42:4401–4402. doi: 10.1007/s10072-021-05523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.A variant of Guillain-Barre syndrome after SARS-CoV-2 vaccination: AMSAN. Tutar NK, Eyigürbüz T, Yildirim Z, Kale N. Ideggyogy Sz. 2021;74:286–288. doi: 10.18071/isz.74.0286. [DOI] [PubMed] [Google Scholar]
- 74.Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Cureus. 2021;13:0. doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller Fisher syndrome following vaccination against SARS-CoV-2. Yamakawa M, Nakahara K, Nakanishi T, Nomura T, Ueda M. Intern Med. 2022;61:1067–1069. doi: 10.2169/internalmedicine.8851-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Facial diplegia variant of Guillain-Barré syndrome in pregnancy following COVID-19 vaccination: a case report. Zubair AS, Bae JY, Desai K. Cureus. 2022;14:0. doi: 10.7759/cureus.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guillain-Barré syndrome and COVID-19: a 1-year observational multicenter study. Filosto M, Cotti Piccinelli S, Gazzina S, et al. Eur J Neurol. 2022;29:3358–3367. doi: 10.1111/ene.15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.International Guillain-Barré Syndrome Outcome Study: protocol of a prospective observational cohort study on clinical and biological predictors of disease course and outcome in Guillain-Barré syndrome. Jacobs BC, van den Berg B, Verboon C, et al. J Peripher Nerv Syst. 2017;22:68–76. doi: 10.1111/jns.12209. [DOI] [PubMed] [Google Scholar]