Abstract
A 23-year-old man with no significant medical history was rushed to a hospital due to transient loss of consciousness with incontinence. The patient had developed a fever after his second dose of coronavirus disease 2019 (COVID-19) vaccine, and the patient was found groaning in bed approximately 40 hours after the vaccination in the early morning. The patient was diagnosed with Brugada syndrome (BrS) based on a drug-provocation test. His father had been diagnosed with BrS and died suddenly at 51 years of age. Young adults with a family history of BrS should be cautioned about fever following COVID-19 vaccination.
Keywords: electrocardiography, electrophysiological examination, ventricular fibrillation, fever
Introduction
There have been reports of a relationship between fever and sudden cardiac death events in patients with Brugada syndrome (BrS), and there is also a report of induced ventricular fibrillation (VF) by fever as a side effect of coronavirus disease 2019 (COVID-19) vaccination (1). If patients with BrS develop a fever after COVID-19 vaccination, prompt fever reduction with antipyretics medication is advisable. In cases of BrS patients with fever who are unresponsive to antipyretic medications, hospitalization for monitoring until the fever resolves is recommended (2).
We report the case of a young man with no significant medical history who fainted due to fever as a side effect after he received his second dose of COVID-19 vaccine and was diagnosed with BrS based on a class I antiarrhythmic drug challenge.
Case Report
A 23-year-old man with no significant medical history was rushed to a hospital due to a transient loss of consciousness with incontinence. He had received his first dose of COVID-19 (Moderna) vaccine 3 weeks previously without any major side effects. After his second dose of the Moderna COVID-19 vaccine, he developed a fever (38°C) and was found incontinent and groaning in bed in the early morning approximately 40 hours after receiving the vaccination. He was then transported to a hospital by an ambulance.
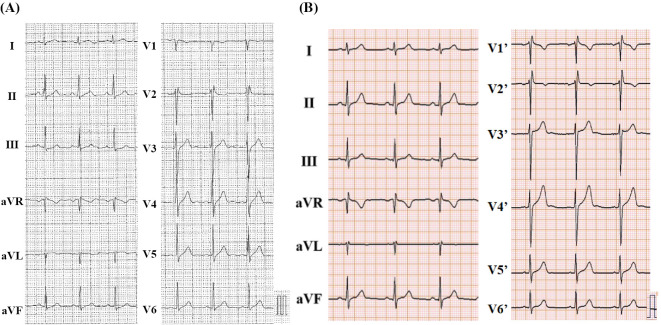
On arrival, the initial assessment revealed the following: blood pressure, 121/84 mm Hg; heart rate, 72 beats/min; and body temperature, 35.6°C. There were no abnormal neurological findings. Blood tests showed mildly elevated troponin I at 0.143 ng/mL, but there were no other noteworthy findings. The 12-lead electrocardiogram (ECG) showed no ST-T changes (Fig. 1A). A transthoracic echocardiogram showed a preserved cardiac function with no anatomic abnormalities.
Figure 1.
(A) The patient’s initial 12-lead electrocardiogram at the standard intercostal space in the emergency department. (B) The patient’s electrocardiogram with the precordial leads placed at two intercostal spaces above standard position (V1’-V6’).
The patient had never been diagnosed with an ECG abnormality and had never fainted during or after a fever before; however, his father had been diagnosed with BrS and had died suddenly at 51 years of age. Based on the patient's medical and family history, BrS was suspected and the patient was transferred to our hospital for further investigation and treatment.
At the time of his admission to our hospital, ECG showed only an rSR' pattern-like incomplete right bundle branch block, and no characteristic type 1 Brugada ECG pattern was observed (Fig. 1B). We administered a challenge with a class I antiarrhythmic drug (pilsicainide 1 mg/kg) to induce a type 1 Brugada ECG pattern, and we observed that the type 1 Brugada ECG pattern was induced 10 minutes after the administration of the drug (Fig. 2). He was diagnosed with BrS based on the drug-induced type 1 Brugada ECG pattern and pertinent clinical features, including his personal history of syncope and family history of sudden cardiac death.
Figure 2.
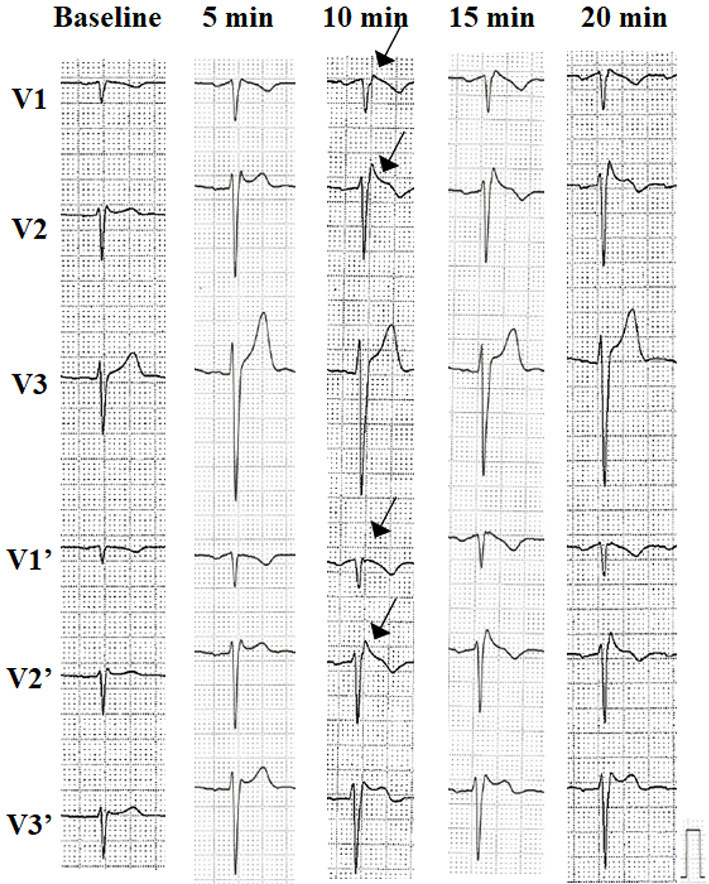
The patient's ECG with a class I antiarrhythmic drug (pilsicainide, 1 mg/kg) challenge test. At 10 minutes after the drug administration, the type 1 Brugada ECG pattern was unmasked. The ECG of V1’-V3’ with the precordial leads was placed at two intercostal spaces above the standard position. ECG: electrocardiogram
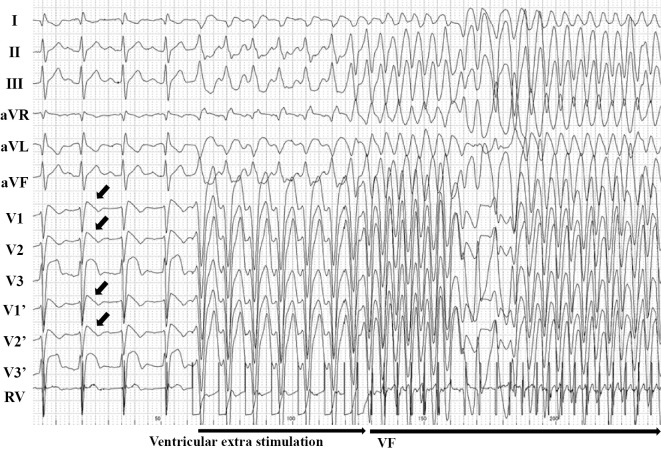
On the 6th day of hospitalization, an electrophysiological examination (EPS) was performed. At baseline, polymorphic ventricular tachycardia (VT)/venticular fibrilation (VF) was not induced by double extra-ventricular stimulation (minimum stimulation interval 200 ms) in the apex of the right ventricle or the outflow tract of the right ventricle. A class I antiarrhythmic drug was administered to induce a type-1 Brugada ECG pattern again. Under this condition, VF was induced by double extra-ventricular stimulation (400-300-200 ms) in the right ventricular outflow tract (Fig. 3). The VF did not spontaneously terminate, and VF was defibrillated promptly after the patient lost consciousness (35 seconds after the onset of VF). At the same time, the patient underwent coronary angiography and an acetylcholine spasm provocation test, and the results confirmed that his coronary arteries were normal and coronary spasm was not induced. Cardiac magnetic resonance imaging (MRI) was also performed; there were no findings suggesting myocardial damage or late gadolinium enhancement.
Figure 3.
The patient’s 12-lead and intracardiac electrocardiogram. VF was induced by double extra-ventricular stimulation (400-300-200 msec) in the right ventricular outflow tract after the administration of a class I antiarrhythmic drug to induce the type 1 Brugada ECG pattern. ECG: electrocardiogram, RV: right ventricular, VF: ventricular fibrillation
The patient's profile met the criteria for a diagnosis of unexplained syncope and inducible VF on EPS, the Japanese guidelines recommend implantable cardioverter defibrillator (ICD) implantation for class IIa, while the international guidelines recommend ICD implantation for class IIb (3,4). ICD implantation was performed with the patient's consent. The patient underwent implantation of a subcutaneous ICD (S-ICD) because he did not require pacing or anti-tachycardia pacing for the primary prevention of sudden death.
Discussion
The patient was a young man with a family history of BrS who presented with fever-induced syncope after receiving a COVID-19 vaccine. The type 1 Brugada ECG pattern was induced by a class I antiarrhythmic drug challenge, and VF was induced by EPS. It has been reported that VF was induced by fever as a side effect of COVID-19 vaccination in a patient who had already been diagnosed with BrS (1); however, to the best of our knowledge, there have been no reports of undiagnosed unmasking BrS in a patient who fainted from a dose of COVID-19 vaccine.
BrS is a genetic abnormality of the sodium channels in the myocardium, with a characteristic electrocardiographic pattern. Patients with a type 1 Brugada ECG pattern and pertinent clinical features, such as a history of syncope and ventricular arrhythmia or a family history of sudden cardiac death are diagnosed with BrS; however, patients with pertinent clinical features of BrS but without the identification of the type 1 Brugada ECG pattern require a class I antiarrhythmic drug challenge to induce this ECG pattern in order to meet the diagnosis of BrS (3,4). Inactivated myocardial sodium channels in patients with latent BrS are sensitive to temperature, and an increase in body temperature beyond the physiological range reduces the charge carried by the sodium channel current with the T1620M missense mutation. This mechanism suggests that a febrile state may unmask BrS (5). In our present case, a genetic analysis was not performed as we did not receive the patient's consent.
Moreover, in patients with BrS, a febrile state induces a higher degree of inactivation of the myocardial sodium channels, resulting in a decrease in the sodium channel flow rate, and a febrile state also induces a shortening of the duration of the action potential. This mechanism suggests that a febrile state may trigger reentry arrhythmias as fatal arrhythmias (6). The mechanism of vaccine-induced fever is not known, but it may be related to the mRNA sequence that codes for the spike protein of COVID-19, and/or to the immune response that follows vaccination. It is not known whether COVID-19 infection itself confers a risk of developing VF in patients with BrS.
Our patient had never fainted after a fever before; however, he fainted for the first time after experiencing COVID-19 vaccine-induced fever. This suggests that it may be possible that the COVID-19 vaccine itself can induce fatal arrhythmia in patients with unmasking BrS.
BrS patients with a history of arrhythmia or syncope are currently advised to be followed up with hospitalization after vaccination (2); however, the risk is not clear in cases in which no prior Brugada-type ECG was noted and there is only a family history of sudden death, as in our patient's case. When a person with a family history of BrS is vaccinated with the COVID-19 vaccine, the possibility of unmasking BrS may make it necessary, especially for young people, to be aware of fever due to adverse reactions, to use antipyretics, and to check the patient's ECG if the fever does not resolve.
We experienced the case of a young adult patient with unmasking BrS who had experienced fever-induced syncope after COVID-19 vaccination. Young adults with a family history of BrS should be cautioned about fever following COVID-19 vaccination.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Kokawa T, Yamamoto H, Itoh M, Shimane A, Kawai H, Takaya T. Fever-related ventricular fibrillation potential adverse effect of SARS-CoV-2 vaccination in patients with Brugada syndrome. Circ J 86: 474, 2022. [DOI] [PubMed] [Google Scholar]
- 2. Dendramis G, Brugada P. Intensive care and anesthetic management of patients with Brugada syndrome and COVID-19 infection. Pacing Clin Electrophysiol 43: 1184-1189, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 10: 1932-1963, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Nogami A, Kurita T, Abe H, et al. JCS/JHRS 2019 guideline on non-pharmacotherapy of cardiac arrhythmias. Circ J 85: 1104-1244, 2021. [DOI] [PubMed] [Google Scholar]
- 5. Wang DW, Makita N, Kitabatake A, et al. Enhanced Na+ channel intermediate inactivation in Brugada syndrome. Circ Res 87: e37-e43, 2000. [DOI] [PubMed] [Google Scholar]
- 6. Morita H, Zipes DP, Morita ST, Wu J. Temperature modulation of ventricular arrhythmogenicity in a canine tissue model of Brugada syndrome. Heart Rhythm 4: 188-197, 2007. [DOI] [PubMed] [Google Scholar]