Abstract
Ocular fungal infections annually affect more than one million individuals worldwide. The management of these infections is problematic, mainly due to the limited availability of effective antifungal agents. Thus, ocular infections are increasingly recognized as important causes of morbidity and blindness, especially keratitis and endophthalmitis. Thus, this review aims to demonstrate the importance of fungal eye infections through the description of the main related aspects, with emphasis on the treatment of these infections. For this purpose, a search for scientific articles was conducted in databases, such as Medline, published from 2000 onwards, addressing important aspects involving fungal eye infections. In addition, this work highlighted the limited therapeutic arsenal available and the severity associated with these infections. Thus, highlighting the importance of constantly updating knowledge about these pathologies, as it contributes to agility in choosing the available and most appropriate therapeutic alternatives, aiming at positive and minimally harmful results for that particular patient.
Keywords: Eye fungal infection, Antifungal, Keratitis, Endophthalmitis
Introduction
Annually, fungal eye infections affect more than one million people worldwide and have shown a significant increase in recent decades (Mehrandish and Mirzaeei 2021). Keratitis (infection of the cornea) is the most frequent form, but the orbit, eyelids, lacrimal apparatus, conjunctiva, sclera, and intraocular structures (endophthalmitis) can also be affected (Thomas 2003a). The distribution of these infections is global and the dominant etiology varies based on geographic origin, socioeconomic level, and climatic conditions (Mahmoudi et al. 2018). These ophthalmic diseases are highly prevalent in developing, lower-middle-income countries with hot and humid climates. (Mills et al. 2020; Mehrandish and Mirzaeei 2021). While filamentous fungi are the most common etiologic agents in tropical and subtropical regions, yeast plays an important role in temperate regions (Mahmoudi et al. 2018; Sahay et al. 2019; Mills et al. 2020).
An overwhelming number of fungal genera and species have been identified as a cause of ophthalmic mycoses and there is still a steady increase in this number (Thomas 2003a, b; Mills et al 2020). In cases of filamentous fungal infections, Fusarium and Aspergillus species, followed by other hyaline fungi, as well as Curvularia species and other dematiaceous fungi, are the most common isolates. When referring to infections caused by yeasts and related fungi, Candida species are the most frequent (Thomas 2003b; Czakó et al 2019). Although contamination can occur from the environment or from the ocular microbiome, inoculation does not occur with intact corneal epithelium, a predisposing factor is necessary. Ocular trauma, whether of plant origin or contaminated foreign body, is commonly involved in the inoculation of the pathogen. However, there are other risk factors such as the use of contact lenses (damage to the corneal epithelium or contamination of the storage solution), ophthalmic surgery, eye disease, corticosteroid therapy, and hematogenous spread in primary infection from another site (Czakó et al. 2019; Mills et al. 2020).
Ocular fungal infections require rapid recognition, as prompt diagnosis and appropriate therapy can minimize the morbidity normally caused by these infections (Thomas 2003b; Srinivasan 2004). Important to aid in a correct diagnosis and appropriate treatment are differences in geographic prevalence, risk factors, pathogenesis, differential signs of fungal infection, and susceptibility to the invading pathogen (Mills et al. 2020).
The diagnosis occurs in the clinical context (corneal trauma, use of contact lenses, history of recent surgery), associated with direct examination for observation of fungal structures and the culture of the isolated fungus, these represent the standard used in the routine of laboratory diagnosis (Mahmoudi et al. 2018; Czakó et al. 2019). Since there is a broad-spectrum of fungi that cause eye infections, differences in susceptibility patterns are not unexpected. Thus, accurate species-level identification and antifungal susceptibility testing represent an issue of great clinical and epidemiological importance and can provide useful data, assisting the ophthalmologist in the selection of the most effective antifungal (Mahmoudi et al. 2018). Despite this, many corneal ulcers are still being treated empirically based on clinical features alone, which contributes to poor prognosis and antimicrobial resistance (Mills et al. 2020). Each case, although there are basic features, may be different from others, depending on the etiologic agent (Thomas 2003b).
Outcomes can be potentially serious and therefore these infections should be treated as soon as possible to prevent such consequences (Mehrandish and Mirzaeei 2021). However, the management of fungal eye infections is constrained by the availability of effective antifungal agents, poor eye penetration, and thus, difficulty in achieving adequate concentration at the site of injury, as well as possible unwanted effects and toxicity profile (Thomas 2003b; Kaur and Kakkar 2010; Sahay et al. 2019). Although topical administration has always been the most common route of ocular administration due to its numerous advantages, its limited ocular bioavailability poses a major challenge (Mehrandish and Mirzaeei 2021). Thus, current therapies are often ineffective, and there have been no new topical treatments approved by the Food and Drug Administration (FDA) since the introduction of natamycin in the 1960s, which is a first-line treatment (Srinivasan 2004; Mahmoudi et al. 2018; Mills et al. 2020).
Associated with the limitation of clinically approved antifungals, the diagnosis is usually late, and the pharmacological treatment is often inadequate, including the use of corticosteroids. Thus, a considerable portion of patients suffer from medical treatment failure and may require surgical intervention, such as therapeutic keratoplasty, at the risk of having a moderate or severe visual impairment (Mills et al. 2020). Thus, according to the World Health Organization (WHO), these infections are one of the main causes of a partial or total loss of vision (Srinivasan 2004; Mehrandish and Mirzaeei 2021).
Thus, the objective of this review is to demonstrate the importance of fungal eye infections related to factors that guide the outcome of cases, usually associated with high rates of morbidity. For this, aspects involving fungal eye infections were addressed concerning their epidemiology associated with their clinical form, routes of infection, the effectiveness of antifungal treatments concerning their spectrum of activity, routes of administration and potential unwanted effects. Evidencing, in addition to the fungal agents commonly involved in eye fungal infections, agents that are less frequent, but no less significant in terms of morbidity. Thus, highlighting the seriousness that these infections represent and how important it is to know all the aspects involved in these pathologies for a positive outcome for the patient.
Search methodology
The search for scientific articles was carried out using the “Pubmed” search engine, in Medline database, using different combinations of terms: “fungal”; “keratitis”; “endophthalmitis”; “epidemiology”; “treatment”; “antifungals”; “clinical case”; “case report”; eye infection”. Refining the search for works published from 2000 onwards. In the end, 71 articles that fit the scope of the work were used (Fig. 1).
Fig. 1.
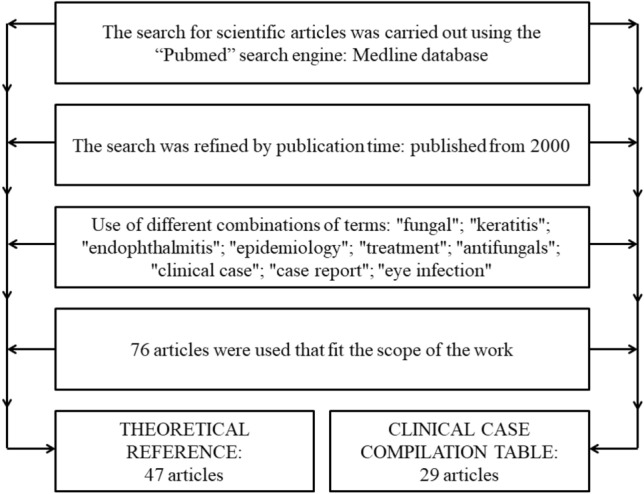
Flowchart of the search methodology used to select the articles to compose the work
Fungal etiological agents and their distribution
In recent decades, the incidence of fungal eye infections has increased substantially due to the increasing number of patients with risk factors such as immunosuppression, use of medical devices, widespread use of antimicrobials, and currently hospitalized patients with COVID-19 (SARS-Cov-2 infection) (Pappas et al. 2018; Mehrandish and Mirzaeei 2021; Musuuza et al. 2021; Spallone and Schwartz 2021). The eye is a complex organ and although fungal infections are less common compared to bacterial or viral infections, they are usually serious and can lead to vision loss (Kaur and Kakkar 2010; Mehrandish and Mirzaeei 2021). However, fungi cannot penetrate intact corneal epithelium; a gateway is required. Hematogenous spread, a penetrating injury, or a previous epithelial alteration can facilitate the establishment of the fungus, and, once inside the cornea, they are able to proliferate rapidly (Kaur and Kakkar 2010).
In fungal keratitis, there is a special occurrence of cases in rural workers, mainly in tropical countries, as a result of the traumatic inoculation of plant material contaminated by fungi. Thus, filamentous and saprophytic species constitute the dominant group of pathogens in these cases (Klotz et al. 2000; Kalkanci and Ozdek 2010). Furthermore, since the 1980s, contact lenses have been increasingly recognized as a risk factor for keratitis, especially for some species (Kalkanci and Ozdek 2010). In cases of exogenous endophthalmitis, eye infection results from inoculation of the pathogen present on the ocular surface or from external sources (Lupia et al. 2021). This type of infection usually occurs after eye surgeries and invasive clinical procedures, as well as the complication of keratitis and in a post-traumatic way (Durand 2017; Lupia et al. 2021). Endogenous endophthalmitis, on the other hand, has its epidemiology intrinsically related to the ability of certain fungal species to spread via the hematogenous route from an infectious focus, which can be transient or continuous (ongoing one) (Chakrabarti et al. 2008; Durand 2017). The risk of developing these infections by pathogenic fungi is higher in immunosuppressed patients, due to their inability to mount an adequate immune response to the offending agent (Klotz et al. 2000; Chakrabarti et al. 2008).
Therefore, failures or pre-existing damage to the eye tissue are most commonly associated with Candida species, while filamentous fungi are the main cause of post-traumatic infection (Kaur and Kakkar 2010). The most common fungal etiologic agents in eye infections are the genera Aspergillus, Candida, and Fusarium, followed by other genera such as Blastomyces, Cryptococcus, and Sporothrix (Kaur and Kakkar 2010; Słowik et al. 2015; Ahmadikia et al. 2021). However, the etiology of these infections, in addition to being often related to the form of inoculation of the agent, is also related to the patient's risk factors, circumstantial socioeconomic level, agricultural activity and extent of urbanization, geographic and climatic conditions of the region, varying widely in different regions of the same country (Klotz et al. 2000; Mahmoudi et al. 2018). The characteristically hot and humid climate of tropical regions, as well as the economic base of countries such as Asia, based on agriculture, are associated with favoring the development of infections caused by filamentous fungi. It is observed that farmers, rural producers, and workers who are exposed to the outdoors are more likely to suffer eye injuries from plant material, metals, and dust. In contrast, in urban areas, with a cold climate, there is a higher prevalence of yeast fungi at the origin of the infection (Liu et al. 2019). There is an apparent relationship between the frequency of isolation of certain fungal species and the seasonal variations in temperature, humidity, and wind of the respective regions (Thomas and Kaliamurthy 2013). Table 1 presents the main fungal pathogens associated with the main clinical forms presented above, as well as some of the less prevalent fungal pathogens, but which can also be causative agents of these infections.
Table 1.
Main clinical forms of ocular fungal infections and associated fungal agents
| Main clinical forms | Main fungal agents | Fungal agents less prevalent | Additional information | References |
|---|---|---|---|---|
| Keratitis |
Fusarium spp., Aspergillus spp. Curvularia spp.1,2,3 Candida spp.4,2 |
Filamentous species, such as Scedosporium, Paecilomyces, Acremonium, Penicillium, Microsporum and Bipolaris11,1 |
Yeast, especially Candida spp., tends to appear in temperate zones of the globe3,2 Typically, patients in regions with Candida albicans keratitis previously have some involvement in the ocular structure, including systemic conditions such as diabetes and immunosuppression11,1 |
1. Thomas and Kaliamurthy (2013) 2. Mahmoudi et al. (2018) 3. Ong et al. (2016) 4. Klotz et al. (2000) 5. Kalkanci and Ozdek (2010) 6. Wykoff et al. (2008) 7. Buchta et al. (2014) 8. Liu et al. (2019) 9. Lupia et al. (2021) 10. Durand (2013) 11. Thomas (2003a) 12. Vilela et al. (2013) 13. Chakrabarti et al. (2008) 14. Lashof et al. (2011) 15. Kaur and Kakkar (2010) 16. Silva et al. (2015) 17. Gupta et al. (2008) |
| Endogenous endophthalmitis | Candida species, mainly C. albicans4,5 |
Opportunistic pathogens, such as genera: Aspergillus, Fusarium and Cryptococcus Penicillium, Pseudallescheria, dimorphic fungi such as Histoplasma capsulatum, Blastomyces dermatitidis, Coccidioides immitis, Coccidioides posadasii, Paracoccidioides brasiliensis, and Sporothrix schenckii, even in apparently healthy individuals5,12 |
Endophthalmitis can occur as a complication of candidemia, which usually occurs in hospitalized patients, by hematogenous dissemination and with potentially devastating consequences14 The incidence of filamentous, opportunistic, or pathogenic fungal species capable of crossing the blood-ocular barrier has increased in the last 20 years. Much of this growth is related to the aging of the population and the development of invasive and aggressive therapies, in addition to the increase in the number of immunosuppressed patients. Opportunistic pathogens enter the hematogenic system by breaking natural defense barriers and/or weakening the immune response, reaching the eyeball12 The pathogenesis of eye infections is linked to the epidemiology of the disease, in addition, the intrinsic virulence of each fungus must be taken into account. This, in turn, depends on the fungal substances produced (toxins, enzymes) and the response that will be generated by the host (inflammatory response)5,15 |
|
| Exogenous endophthalmitis |
In tropical regions, fungi of the Aspergillus and Fusarium genera6,7,8,9 Genus Candida, mainly C. albicans, followed by C. parapsilosis10 |
Genera Curvularia,13 Acremonium, Paecilomyces, Scedosporium,6 Colletotrichum, Pseudallescheria, Trichosporon, Cryptococcus, Fonsecaea,13 Penicillium, Alternaria, Phialophora, Rhinocladiella,8 Exophiala and Sporothrix5 |
The fungal microorganisms responsible for the infection present a significant variation in their frequency and depend on the different risk factors, climatic and geographic conditions of the region, the inoculation method, and the individual's immunocompetence7 There is a large participation of opportunistic species16 The scope of fungal agents documented to trigger invasive disease is vast, with evidence of varying levels of virulence17 Mainly ubiquitous and/or saprophytic fungi, the source of contamination can often be in the intraocular irrigation solution and in the instruments used during the surgical performance, in intraocular lens prostheses, and in-hospital ventilation systems13 In the case of yeast infections of the genus Candida, although C. albicans is normally more prevalent, Candida parapsilosis is the most common species in post-surgical outbreaks, as it seems to survive well in irrigation fluids and prosthetic materials10 |
Main clinical presentations
The eye is a complex organ that can be affected by numerous diseases (Mehrandish and Mirzaeei 2021). In ophthalmic fungal infections, corneal involvement (keratitis) may occur, the predominant clinical form, however, other ocular structures may also be affected, such as the orbit, eyelids, lacrimal apparatus, conjunctiva, sclera, and intraocular structures. In the latter case, they are classified as endophthalmitis: exogenous when a pathogen is introduced by an external source and endogenous when this pathogen originates from an internal source (Kaur and Kakkar 2010; Słowik et al. 2015).
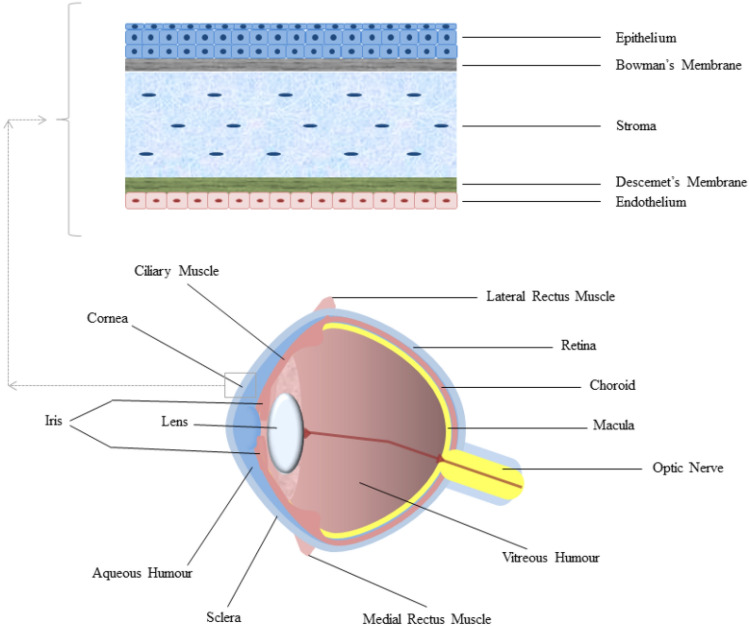
Fungi require a portal of entry, a penetrating lesion, or an anterior epithelial defect because they cannot penetrate the intact corneal epithelium (as exemplified in Fig. 2). Once they have penetrated the tissue, they quickly establish the infectious process and its proliferation (Kaur and Kakkar 2010). The success of this process is closely related to some factors, such as the host’s immune response, the size of the inoculum, and the pathogenicity of the fungus (Durand 2017). Thus, the risk of infection increases when a fungal inoculum with a large number of pathogens is introduced, as it can overload the host’s defense system (Durand 2017). Also, in cases of exogenous endophthalmitis, the clinical findings may vary according to the inoculation mechanism (Chee and Eliott 2017).
Fig. 2.
Anatomy of the eyeball, highlighting the cornea and the cell layers that compose it
Ophthalmic fungal infections, for the most part, are associated with outcomes with severe morbidity, which in turn are closely related to factors such as the physical damage that the very presence of the fungal pathogen can cause, the response developed by the host to the invading agent and the secondary damage from fungal toxins and enzymes (Kaur and Kakkar 2010; Mills et al. 2020). The epidemiology of the disease also correlates with the pathogenesis of fungal eye infections (Kalkanci and Ozdek 2010).
Fungal keratitis presents as severe suppurative, often ulcerative lesions with the presence of hypopium, a deposit of leukocytes and necrotic cells in the anterior chamber forming a lower level by gravity (Thomas 2003b; Thomas and Kaliamurthy 2013; Durand 2013, 2017). The clinical features commonly seen are grayish-white or yellowish-white infiltrates with diffuse borders or irregular, feathered margins. Lines can be seen extending beyond the edge of the ulcer, the base of which often has creamy exudates and raised edges. Cell infiltration is minimal in the adjacent stroma, iritis is mild but has a rough, dry texture, elevated necrotic area, and Descemet's folds (Thomas 2003a; Srinivasan 2004). The absence of eyelid swelling is a common feature (Srinivasan 2004; Thomas and Kaliamurthy 2013). It represents a challenge because it tends to mimic other types of stromal inflammation, that is, other conditions that also present stromal inflammation (Thomas 2003b). Fungal hyphae can be observed within the corneal stroma in some cases (Thomas and Kaliamurthy 2013). These infections require rapid recognition for a positive outcome. However, symptoms are generally nonspecific and more prolonged concerning bacterial corneal infections (Thomas 2003b).
Keratitis can progress to endophthalmitis as the fungus proliferates and spreads through the cornea and into the aqueous humor (Durand 2013). Endophthalmitis has a high potential for devastation, which can lead to irreversible blindness (Durand 2017). In cases where endophthalmitis results from the evolution of keratitis, fungal filaments can often be seen extending from the back of the cornea to the aqueous humor (Durand 2013; Thomas and Kaliamurthy 2013). Diminished vision is the most common symptom of endophthalmitis, present in almost all patients. Pain, eye discomfort, and redness are also common symptoms. Systemic symptoms, such as fever, are frequent in endogenous endophthalmitis, however, usually absent in exogenous endophthalmitis (Chee and Eliott 2017; Durand 2017). In most cases, hypopyon can be observed (Thomas 2003b; Thomas and Kaliamurthy 2013; Durand 2017). Patients with fungal endophthalmitis caused by the progression of a keratitis generally present inflammation in the anterior chamber with possible extension to the vitreous, while fungal endophthalmitis originating from penetrating trauma often present with more significant vitritis and greater involvement of the posterior segment (Chee and Eliott 2017). Initially, the rhythm of symptoms and the type of intraocular inflammation drive the diagnosis of the etiology of the infection, whether bacterial or fungal. While bacterial endophthalmitis usually manifests acutely, days after the triggering event. Fungal endophthalmitis usually has a subacute presentation, worsening symptoms occur over days to weeks, with intraocular inflammation that tends to present in "clumps" within the aqueous and/or vitreous (Durand 2013, 2017). Furthermore, endophthalmitis can spread when it affects more vulnerable populations, such as immunocompromised individuals (Debourgogne et al. 2016; Kauffman 2016; Relhan et al. 2018).
Available treatments and their problems
The ocular morbidity caused by ocular fungal infections results from the relationship between invading fungal pathogens and the host's defense mechanisms. Even fungi of the same species can have different virulence patterns. Furthermore, we have different host defense responses and the adjacent ocular microbiome (Mills et al. 2020). Furthermore, the choice of antifungal therapy for the treatment of these infections should be rational, based on the susceptibility of the isolated fungus (Thomas and Kaliamurthy 2013). Importantly, in determining an appropriate antifungal regimen, it is essential to evaluate some aspects of the proposed antifungal agent within the clinical picture that is presented: ability to penetrate ocular tissues, the spectrum of activity, and toxicity profile (Patil and Majumdar 2017).
The severity and location of fungal eye infections dictate the route of drug administration (Lakhani et al. 2019; Mehrandish and Mirzaeei 2021). In the current context, conventional therapy for cases of fungal keratitis or other infections of the anterior chamber is mostly treated by topically administering drugs (Mahmoudi et al. 2018; Lakhani et al. 2019; Sahay et al. 2019). Considering the benefits of topical forms, such as high patient compliance, ease of use, non-invasiveness, painlessness, reduced side effects, and selective treatment of the anterior chamber, the topical route, through eye drops, is preferred for drug administration in the treatment of these ophthalmic infections (Lakhani et al. 2019; Mehrandish and Mirzaeei 2021). Unfortunately, topical dosage forms face some challenges, such as low drug penetration into the different layers of the eye, and compounds with a molecular mass > 500 daltons (Da) barely penetrate the intact corneal epithelium. In addition, the high frequency of administration, low residence time, and toxicity caused by long-term use are also important challenges (Kaur and Kakkar 2010; Mehrandish and Mirzaeei 2021). To date, however, no new FDA-approved topical antifungal eye delivery system is available (Mehrandish and Mirzaeei 2021). Strategies using contact lenses containing the active, with gradual release to the ocular surface, are occasionally used in the treatment of fungal keratitis (Sahay et al. 2019).
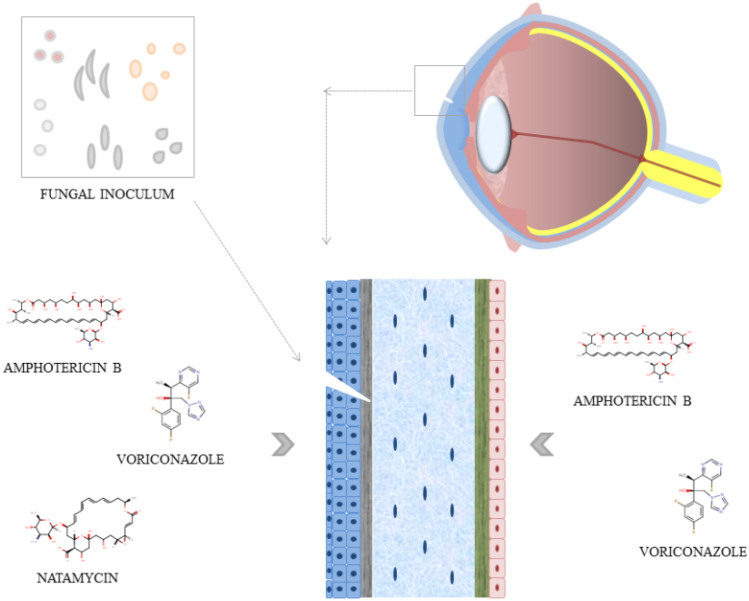
In cases of deeper ocular fungal infections, routes other than the topical one are necessary, such as parenteral or intraocular routes (Lakhani et al. 2019). Systemic or oral antifungal treatment is used in cases with scleral or limbic involvement, endophthalmitis, impending perforation or perforated corneal ulcer, pediatric cases, recalcitrant cases, and post-keratoplasty patient cases (Sahay et al. 2019). The association of oral antifungal agents in cases of refractory ocular fungal infections has been adopted, with some success. They are not routinely recommended as a single treatment, based on the currently available evidence (Sahay et al. 2019). The intrastromal and intracameral routes for antifungal release are used in cases of deep and recalcitrant fungal keratitis (Sahay et al. 2019). Exemplified in Fig. 3.
Fig. 3.
Fungal inoculation by trauma and ways of using the main pharmacological treatments
The most suitable choice for the treatment of ophthalmic mycoses has the ultimate goal of preserving vision, and this depends on a rapid diagnosis and efficient administration of appropriate antifungal therapy (Thomas 2003a). However, currently, the therapy for fungal diseases of the eye is unsatisfactory (Srinivasan 2004).
Polyene and azole antifungals are known to exhibit ocular and systemic toxicities, which manifest as one of the main challenges associated with their therapeutic use (Lakhani et al. 2019). These classes of antifungals come to occupy an important niche in ophthalmic antifungal therapy due to their broad spectrum of activity against a variety of filamentous and yeast fungi (Patil and Majumdar 2017; Lakhani et al. 2019). Thus, they have been used as first-line therapeutic agents to treat ocular fungal infections, with their topical application as the primary approach (Patil and Majumdar 2017; Lakhani et al. 2019). However, their use in ocular antifungal therapy has been a challenge due to their physicochemical properties and the ocular anatomy and physiology, since in many cases, they exhibit low penetration through the cornea, limiting their usefulness in cases of deeper infections (Patil and Majumdar 2017; Lakhani et al. 2019).
Currently, 5% natamycin suspension is the only FDA-approved topical formulation for the treatment of ophthalmic fungal infections (Kaur and Kakkar 2010; Czakó et al. 2019; Lakhani et al. 2019; Sahay et al. 2019). Natamycin is an effective drug in the treatment of fungal infections such as keratitis, being an effective first line of treatment (Patil and Majumdar 2017; Mahmoudi et al. 2018). Its antifungal activity is related to the dose used clinically, at which it is mainly fungicidal (Sahay et al. 2019). This drug has low solubility in water, but is stable in suspension, and in this form, it has good adhesion to the cornea for clinically useful periods (Thomas 2003a; Kaur and Kakkar 2010). Topical 5% ophthalmic suspension, although viscous, has better ocular tolerability compared to other antifungals and does not cause pain or secondary corneal damage (Thomas 2003a; Kaur and Kakkar 2010; Patil and Majumdar 2017). There is poor penetration into the corneal stroma and conjunctiva after topical application, due to its high molecular weight, thus low bioavailability, necessitating frequent administrations (Thomas 2003a; Kaur and Kakkar 2010; Patil and Majumdar 2017; Mahmoudi et al. 2018; Lakhani et al. 2019). Generally, this administration ensures a sufficient concentration of the antifungal within the corneal stroma, but not in the aqueous, and there is no systemic absorption. Thus, corneal epithelial debridement may be an option to increase its penetration (Sahay et al. 2019). It can only be administered topically, as intravenous and subconjunctival administration does not lead to therapeutic concentrations in the cornea, aqueous humor, and vitreous. Thus, it is only useful in the treatment of superficial infection and has no application in deep keratomycosis (Kaur and Kakkar 2010; Patil and Majumdar 2017). Likewise, it cannot be administered intracamerally either (Czakó et al. 2019). It has been used in association with other forms of treatment for the therapy of fungal scleritis, conjunctivitis, and endophthalmitis (Thomas 2003a; Kaur and Kakkar 2010).
Another polyene of great importance is amphotericin B, an antifungal variably fungistatic and occasionally fungicidal, depending on the concentration reached in the tissue and the susceptibility of the pathogen (Thomas 2003a; Sahay et al. 2019). It penetrates poorly into ocular tissues, so it has been used intravenously to treat invasive orbital and intraocular infections, being the antifungal of choice for the treatment of endogenous fungal endophthalmitis (Armstrong 2000; Thomas 2003a; Kalkanci and Ozdek 2010; Kaur and Kakkar 2010). Efficacy in fungal keratitis via the off-label topical route depends on corneal integrity, being reduced in the presence of an intact corneal epithelium (Kalkanci and Ozdek 2010; Patil and Majumdar 2017; Lakhani et al. 2019). This is due to its high molecular weight and low aqueous solubility, which lead to greater difficulty in penetration through the cornea and the blood-retinal barrier (Lakhani et al. 2019). A 0.15% solution of amphotericin B in sterile water used in clinical practice appears to be well tolerated. Topical application of 0.5–2.0% ointment has been reported to be well tolerated, but may cause some conjunctival irritation (Thomas 2003a; Kalkanci and Ozdek 2010). Administration of amphotericin B through a collagen lens can improve compliance and ensure a more constant rate of drug delivery in mycotic keratitis. However, it can make it difficult for the clinician to perform frequent clinical examinations on the affected eye, in addition, improper use can also lead to increased toxicity (Thomas 2003a). Intravitreal injections of amphotericin B have been recommended for the treatment of fungal endophthalmitis (Thomas 2003a). Intracameral administration may be considered in the treatment of deep fungal corneal ulcers, with deep stromal involvement, associated with severe anterior chamber reaction, or resistant cases, with minimal toxicity being reported (Mahmoudi et al. 2018; Sahay et al. 2019). Intrastromal administration leads to higher concentrations of the drug in the corneal stroma and therefore, a shorter healing time (Czakó et al. 2019).
The azoles, such as thiabendazole, itraconazole, clotrimazole, miconazole, ketoconazole, fluconazole, voriconazole, and econazole, are therapeutic options for eye infections (Armstrong 2000). All azoles except fluconazole appear to decrease the function of immune cells, especially lymphocytes; this may decrease the degree of tissue damage that occurs with the inflammatory reaction, but it also affects the effectiveness of azoles in vivo (Thomas 2003a).
Fluconazole is a suitable candidate for the treatment of deep Candida keratitis, but cannot be considered the agent of choice in the treatment of filamentous fungal keratitis (Thomas 2003b; Lakhani et al. 2019). This antifungal drug is a stable and water-soluble, with low molecular weight and high bioavailability, which may be useful as an ocular agent (Kaur and Kakkar 2010). It penetrates ocular barriers, both in off-label topical (1and %), oral and systemic administration, and has shown a therapeutic response in fungal keratitis. Indicated even in deep eye fungal infections (Thomas and Kaliamurthy 2013; Patil and Majumdar 2017; Lakhani et al. 2019). Fluconazole achieves high levels of penetration into ocular tissues hours after a single dose, its oral bioavailability is about 90%, with low plasma protein binding capacity (10–20%) and low lipophilicity, reaching a concentration in the aqueous humor. 64% of the plasma concentration (Armstrong 2000; Thomas 2003a; Sahay et al. 2019). One study evaluated the combined therapy of subconjunctival injection of fluconazole plus topical amphotericin B, compared with topical amphotericin alone. This work demonstrated shorter healing time and lower rates of complications, such as tissue perforation, in the association group, with possible benefits in cases of resistant or more severe pathogens (Nada et al. 2017).
Fluconazole is considered a cost-effective and safe antifungal agent with a low toxicity profile among azole antifungals (Lakhani et al. 2019). However, the use of fluconazole has its challenges, which is the rapid development of resistance and cross-resistance to the fungal species on prolonged exposure or due to an incomplete therapeutic regimen (Patil and Majumdar 2017).
Voriconazole has demonstrated therapeutic efficacy in fungal keratitis and endophthalmitis after intrastromal, intracameral, intravitreal, oral, and topical administration (Thomas and Kaliamurthy 2013; Patil and Majumdar 2017; Mahmoudi et al. 2018; Lakhani et al. 2019). It is available in oral and parenteral formulations, its ophthalmic use in topical form off-label requires reconstitution of the available parenteral formulation to a 1–2% solution (Thomas and Kaliamurthy 2013; Sahay et al. 2019). Voriconazole is the preferred azole to be used topically in the treatment of fungal eye infections (Patil and Majumdar 2017; Mahmoudi et al. 2018). Topical voriconazole has high intraocular penetration and is well tolerated by ocular tissue (Thomas and Kaliamurthy 2013). This drug by the topical route reaches a concentration in the aqueous humor above the minimum inhibitory concentration (MIC). However, it has high variability of its concentration in this tissue, and thus concentrations lower than the MIC required to treat the infection can also be found (Mahmoudi et al. 2018; Sahay et al. 2019).
Generally, voriconazole is administered orally for the treatment of keratitis because of greater patient compliance, high oral bioavailability (approximately 90%), good penetration and bioavailability in ocular tissues (Mahmoudi et al. 2018; Sahay et al. 2018, 2019). Oral administration of voriconazole may be an effective treatment in some cases of fungal keratitis, with an intraocular antifungal concentration above the MIC required for most corneal fungal pathogens (Mahmoudi et al. 2018). Intracameral administration of voriconazole (50 µg/0.1 mL) has been reported to be effective in cases of deep, recalcitrant fungal corneal ulcers (Thomas and Kaliamurthy 2013; Mahmoudi et al. 2018; Sahay et al. 2019). Intrastromal administration leads to higher concentrations of the drug in the corneal stroma, providing a depot of the drug, close to the ulcerated area, from where the antifungal is slowly released into the infected tissue, to envelop the infected tissue (Czakó et al. 2019; Sahay et al. 2019). It leads to a shorter healing time (Czakó et al. 2019). It is an effective approach for recalcitrant deep corneal fungal infections that do not respond to conventional treatment modalities (Sahay et al. 2019). There have been reports of successful use in recalcitrant keratitis by Acremonium and Alternaria, which may have a cure rate above 70% (Sahay et al. 2019).
A randomized study evaluated 323 patients with filamentous fungal keratitis and compared topical treatment with 5% natamycin and 1% voriconazole. The group treated with voriconazole had a significantly worse outcome, showing a higher risk of perforation and need for surgical treatment, in addition to a lower visual acuity after three months of treatment, with the worst response against Fusarium spp. (Prajna et al. 2013). One of the possible explanations for this performance was the widespread use of the azole class in agriculture and the development of cross-resistance (Prajna et al. 2016a). The same group evaluated the use of oral voriconazole as an adjuvant to the use of topical natamycin in the treatment of severe filamentous fungal keratitis and found a greater adverse effect in the oral voriconazole group. However, when stratifying only Fusarium spp. keratitis, it showed a lower rate of perforations and the need for surgical treatment, in addition to fewer scars after healing of the infection in the oral voriconazole group, demonstrating a potential synergistic effect in this fungal species (Prajna et al. 2016b, Prajna et al. 2017). However, voriconazole has a disadvantage concerning its cost, which is high, which makes routine use unfeasible (Prajna et al. 2010).
Ketoconazole was the first oral broad-spectrum azole antifungal and its absorption is strongly dependent on gastric pH, requiring an acidic medium for dissolution and absorption (Thomas 2003a; Sahay et al. 2019). Topical and systemic use of this antifungal is recommended for the treatment of fungal keratitis (Kaur and Kakkar 2010). Topical administration showed penetration only when the corneal epithelium was scraped, as it has poor penetration through the intact cornea and blood barriers. This is due to its high molecular weight, hydrophobic character, and its ability to bind proteins (Patil and Majumdar 2017; Lakhani et al. 2019; Sahay et al. 2019). As such, it is often used only as an adjunct to ocular antifungal therapy (Patil and Majumdar 2017). In oral administration, high concentrations of ketoconazole were achieved in the cornea and anterior chamber, thus it is recommended for deep ocular fungal infections (Patil and Majumdar 2017).
Topical ketoconazole use is not associated with significant corneal toxicity and is generally well tolerated (Thomas 2003a; Patil and Majumdar 2017). However, oral administration of ketoconazole can lead to several reversible side effects. However, since prolonged therapeutic regimens may be necessary, the use of oral ketoconazole has been limited (Thomas 2003a; Patil and Majumdar 2017).
Itraconazole is strongly associated with the emergence of resistance and cross-resistance (Patil and Majumdar 2017). This drug is available in systemic, oral (capsules and oral solution) and topical (ointments and 1% ophthalmic solutions) formulations, these formulations are not universally available (Thomas and Kaliamurthy 2013; Sahay et al. 2019). Topical administration has low penetration, due to molecular weight, binding to proteins in the tear-lipid film, and high hydrophobicity (Lakhani et al. 2019). This antifungal is well absorbed after oral administration, but gastric absorption depends on a low pH. It is very hydrophobic, thus highly concentrated in lipid-rich tissue, and has 90–99% binding to plasma proteins (Thomas 2003a; Sahay et al. 2019). Itraconazole is generally well tolerated after oral administration, but the main disadvantage of using this route for the therapy of fungal eye infections is its low availability in ocular tissues, cornea, aqueous humor, and vitreous (Thomas 2003a; Kaur and Kakkar 2010; Patil and Majumdar 2017). Therefore, it is generally not recommended for the treatment of eye infections due to a lack of consistent clinical efficacy (Patil and Majumdar 2017; Sahay et al. 2019). Even though intravitreal and subconjunctival injections exhibit therapeutic activity, some studies have reported the occurrence of retinal necrosis (Patil and Majumdar 2017). Due to the availability of scarce data in this regard, intravitreal and subconjunctival administration has not been clinically adopted (Patil and Majumdar 2017). Typically, itraconazole is only used as a systemic adjunct to topical or intraocular antifungal therapy (Patil and Majumdar 2017).
Posaconazole has been used successfully to treat mycotic keratitis, either as an oral monotherapy or in combination with a topical formulation of other antifungals, primarily in the treatment of severe Fusarium species keratitis and keratitis resistant to common antifungals (ketoconazole, fluconazole, and voriconazole) (Sahay et al. 2019; Mills et al. 2020). It is available as an oral suspension, delayed-release tablet, and intravenous solution and has off-label topical use. Highly protein bound (98%) and with low penetration into ocular compartments (Sahay et al. 2019).
Some other azole drugs may represent treatment options, although with less frequent and/or more restricted use. Miconazole shows variable activity against filamentous fungal species, and potent activity against Aspergillus species but weak activity against Fusarium species (Lakhani et al. 2019). Miconazole appears to be important in the treatment of Scedosporium apiospermum keratitis (Thomas and Kaliamurthy 2013). The intravenous administration solution can be used for topical ocular (1%) off-label administration or for subconjunctival administration (Thomas 2003a; Kaur and Kakkar 2010; Thomas and Kaliamurthy 2013; Lakhani et al. 2019). However, it has low penetration through the cornea and blood barriers due to its high molecular weight, hydrophobic character, and protein binding (Lakhani et al. 2019).
Clotrimazole is used topically off-label (1%), in the form of drops and/or ointment, however, clotrimazole as monotherapy is not the ideal choice (Srinivasan 2004; Thomas and Kaliamurthy 2013; Lakhani et al. 2019). Econazole 1% is available as an ophthalmic drug with an effect similar to that of natamycin against filamentous fungi (Srinivasan 2004). The drug was recommended in the 1970s, but there are no recent reports on its use as a first-line therapy for mycotic keratitis (Thomas 2003b).
Isavuconazole is an option when first-line treatment has a contraindication. Successfully used orally in case of endophthalmitis due to Candida spp., with improvement of the infection (Sng et al. 2021). In tests of endophthalmitis by Aspergillus fumigatus in mice, isavuconazole showed the ability to considerably reduce the fungal burden and intraocular inflammation (Guest et al. 2018).
Amphotericin B, natamycin, and voriconazole are the only topical antifungals with sufficient evidence regarding their efficacy, safety, and clinical indications (Sahay et al. 2019). However, because of the challenges associated with polyenes and azoles, drugs from the newest class of antifungals, echinocandins, are being extensively investigated as potential therapeutic agents in ophthalmic fungal infections (Mahmoudi et al. 2018).
Echinocandins (caspofungin, micafungin, and anidulafungin) is poorly absorbed by the gastrointestinal system and the drugs are therefore only available in parenteral formulations. They have limited distribution to ocular tissues, leading to a low concentration in these tissues (Sahay et al. 2019). For penetration of echinocandins to occur, it is essential that the corneal barrier is not intact, and corneal scraping is necessary during therapy (Patil and Majumdar 2017).
Topical (0.5%) off-label caspofungin has been reported to be beneficial in refractory mycotic keratitis and the intravenous formulation has been used successfully in cases of recurrent endophthalmitis, as well as reports of use in the treatment of cases of refractory keratitis by species from Alternaria (Thomas and Kaliamurthy 2013; Sahay et al. 2019). In a study of two cases of fungal endophthalmitis, the intravitreal injection of caspofungin was observed as the clinical turning point in the course of the treatment of both cases, as well as no damage to the intraocular structures resulting from the use of the antifungal agent was observed (Von Jagow et al. 2020).
Echinocandins intopical formulations (caspofungin and micafungin), in monotherapy or in combination, are reported successfully in the treatment of keratitis and endophthalmitis caused by Candida, Alternaria, Aspergillus, Trichosporon species (Patil and Majumdar 2017; Mills et al. 2020). According to some case studies, intravenous micafungin can be considered as ocular antifungal therapy in cases of mild endogenous fungal endophthalmitis without vitrectomy. It can also be combined with an intravitreal antifungal agent and vitrectomy for the treatment of severe endogenous fungal endophthalmitis (Mochizuki et al. 2013). However, the most successful clinical outcomes with caspofungin and micafungin were with concomitant therapy with other antifungals such as voriconazole, fluconazole, and amphotericin B (Patil and Majumdar 2017).
Efficacy is greater or similar to commonly used antifungals such as fluconazole. In addition, 0.5% caspofungin has been shown to be as effective as 0.15% amphotericin B in treating fungal keratitis (Patil and Majumdar 2017; Mills et al. 2020). It is imperative to compare the efficacy and safety of echinocandin monotherapies using topical, oral, intraocular, and systemic routes of administration (Patil and Majumdar 2017). In the case of anidulafungin for ophthalmic fungal infections, studies are still scarce, therefore, it is essential to evaluate anidulafungin for its ocular pharmacokinetics, efficacy, safety, and tolerability (Patil and Majumdar 2017). However, the use of a single intravitreal dose of anidulafungin in a model of endophthalmitis by C. albicans in rabbits, demonstrated that this echicandin was non-inferior to voriconazole and amphotericin B. Furthermore, this study demonstrated the non-toxicity for the retina of this antifungal agent (Karagoz et al. 2016).
Flucytosine is used as topical (1.0–1.5%) and systemic formulations, well tolerated as eye drops, but with low eye penetration (Armstrong 2000; Thomas and Kaliamurthy 2013; Sahay et al. 2019). Polyhexamethylene biguanide is a general environmental biocide. It has been used as a preservative in topical ophthalmic products. It is soluble in water and tested in the form of 0.02% eye drops, with success reported in clinical practice in many institutions (Thomas 2003a; Czakó et al. 2019). Corticosteroid-containing eye drops are contraindicated in eye fungal infections, as they are used in an attempt to reduce tissue damage caused by the inflammatory reaction directed against a microorganism and end up worsening the course of an existing fungal infection that has not yet been diagnosed (Thomas 2003b; Czakó et al. 2019). Table 2 presents the main pharmacological therapeutic options available for the treatment of ocular fungal infections, as well as the main related aspects.
Table 2.
Pharmacological therapy available for the treatment of ocular fungal infections, presented by its spectrum of activity, routes of administration (including off-label use) and the associated unwanted effects and/or toxicity
| Antifungal agent | Activity spectrum | Administration routes | Undesirable effects/toxicity | References |
|---|---|---|---|---|
| Anfotericin B | Broad spectrum of activity, is active against most fungi, with potent antifungal activity against Candida and Aspergillus species, but moderate to weak activity against Fusarium and Scedosporium species1,2,3 | Intravenous, topical, intrastromal, intracameral, intravitreal, collagen lens1,5,7,14 |
Intraocular injections (> 1% w/v) are associated with adverse effects such as retinal toxicity, loss of retinal ganglion cells, vitreous inflammation, corneal edema, neovascularization, and corneal inflammation1 Cataract, hyphema, iritis and corneal edema3 Intravenous can lead to dose-dependent nephrotoxicity1,7 Headaches, chills, fever, and anorexia are common, other adverse side effects include mild anemia, nausea, vomiting, gastrointestinal cramps and diarrhea, and local thrombophlebitis at the infusion site7 Intravitreal injections can have highly destructive effects, leading to retinal necrosis and detachment7 Amphotericin B is a safe antifungal for use during pregnancy, labeled as a Category B drug by the FDA3 |
1. Patil and Majumdar (2017) 2. Lakhani et al. (2019) 3. Sahay et al. (2019) 4. Kaur and Kakkar (2010) 5. Thomas and Kaliamurthy (2013) 6. Mahmoudi et al. (2018) 7. Thomas (2003a) 8. Mills et al. (2020) 9. Guest et al. (2018) 10. Sng et al. (2021) 11. Srinivasan (2004) 12. Armstrong (2000) 13. Czakó et al. (2019) 14. Kalkanci and Ozdek (2010) 15. Von Jagow et al. (2020) 16. Mochizuki et al. (2013) |
| Natamycin | Broad spectrum of activity but is limited to filamentous fungi, such as Fusarium and Aspergillus species, Acremonium, Alternaria, Cephalosporium, Colletotrichum, Curvularia, Lasiodiplodia, Scedosporium, Trichophyton and Penicillium. Only moderate to weak activity against yeast fungi (Candida species)2,3,4 | Topical, 5% ophthalmic suspension1,4,5 | Prolonged use may be associated with some local symptoms of discomfort, such as mild irritation, redness, foreign body sensation, stinging, burning sensation, and tearing. Adherence of particles from the natamycin suspension to the area of epithelial ulceration as well as the fornix and eyelid margins is a common problem3 | |
| Voriconazole | Broader spectrum of activity, potent antifungal action against Candida and Aspergillus species (even those known to be resistant to amphotericin B, fluconazole, and itraconazole), Scedosporium, Fusarium, Paecilomyces, Cryptococcus, and dimorphic fungal pathogens. Activity against Mucorales is minimal5,6 | Intrastromal, intracameral, intravitreal, oral, intravenous, topical5,1,6,2 |
Adverse effects associated with the use of oral voriconazole include visual disturbances, changes in color vision, and photophobia. Rarely, transient visual hallucinations or confusion may occur. Rashes, hepatotoxicity, electrocardiographic changes, and fluoride-associated bone toxicity may also be seen. Topical use may cause periocular contact dermatitis. It is an FDA-labeled category D antifungal and is contraindicated during pregnancy as it is teratogenic and results in skeletal and visceral abnormalities3 Intracameral administration it represents the spread of infection (invasiveness), intraocular inflammation, lenticular damage, glaucoma, hyphema, and potential endothelial damage3 |
|
| Fluconazole | Active against Candida species (except Candida krusei and C. glabrata may have low susceptibility) and Aspergillus, with resistance by other filamentous fungi, such as Fusarium spp., Scedosporium spp., and Mucorales, a narrow spectrum compared to other triazoles3,5,6 | Topical, oral systemic5,2 |
Subconjunctival injection of fluconazole has been investigated3,5,2 Low toxicity2 Common systemic adverse effects include gastritis, headache, and rash. In some cases, thrombocytopenia, Stevens-Johnson Syndrome, and hepatotoxicity may occur. Labeled a Category D drug by the FDA for use during pregnancy and therefore should not be used in these cases3 |
|
| Ketoconazole |
Broader spectrum of activity3,7 Antifungal activity limited to some filamentous, yeast (including Candida spp.), dimorphic fungi, and dermatophytes1,3 |
Oral, topical, systemic subconjunctival1,3,4 |
The main adverse effect of its oral use is hepatotoxicity, gynecomastia, hyperglycemia, menstrual changes, hypertension, decreased libido, oligospermia, anaphylaxis, adrenal insufficiency, anorexia, hyperlipidemia, increased appetite, insomnia, nervousness may also be reported, headache, dizziness, paresthesia, drowsiness, photochemical toxicity, and orthostatic hypotension1,3 Other adverse effects include gastrointestinal disturbances like vomiting, diarrhea, nausea, constipation, abdominal pain, dry mouth, dysgeusia, dyspepsia, flatulence, tongue discoloration, hepatitis, and jaundice. Skin and subcutaneous tissue disorders include erythema multiforme, rash, dermatitis, erythema, urticaria, pruritus, alopecia, xeroderma, malaise, peripheral edema, pyrexia, chills, and decreased platelet counts.1,3 It has been shown to be teratogenic in animal studies at high doses and has been labeled a Category C drug for use in pregnancy by the FDA3 |
|
| Itraconazole | Activity against most Candida species, and several Aspergillus species, minimal or negligible activity against Fusarium and Mucorales species1,3 | Systemic, oral, topical, intravitreal, subconjunctival injections1,3,5 |
Subconjunctival injections, some studies have reported the occurrence of retinal necrosis1 Oral administration, the most common complaints are gastrointestinal disturbances, rash, and headache, less frequently hypertriglyceridemia, hypokalemia, edema, decreased libido, and gynecomastia are observed1,3,7 Hepatotoxicity and liver failure are also among the most common adverse effects and are serious. It has been labeled Category C by the FDA for use in pregnant women as it is teratogenic3 |
|
| Pozaconazole | Potent broad-spectrum activity against most Candida species (even fluconazole-resistant isolates), Fusarium, Aspergillus, and Mucorales3,8 | Oral, intravenous, topical3,8 | Fever, diarrhea, nausea, vomiting, and headache. In addition, hypokalemia, rash, thrombocytopenia, abdominal pain, elevated liver enzyme levels, and hepatocellular damage may be observed. Good long-term tolerability. Classified in Category C by the FDA for use during pregnancy as it is teratogenic3 | |
| Miconazole | Variable activity against filamentous fungal species, potent activity against Aspergillus species, and weak activity against Fusarium species.2 Appears to be important in the treatment of Scedosporium apiospermum keratitis5 | Intravenous, oral, topical, subconjunctival2,4,5,7 | – | |
| Isavuconazole | Broader spectrum of activity, Candida spp., Aspergillus fumigatus9,10 | Oral, intravenous9,10 | – | |
| Equinocandinal (caspofungin, micafungin, anidulafungin) | Narrow antifungal spectrum, fungicidal activity against Candida spp. and fungistatic for Aspergillus spp., are not active against Fusarium species, but are active against other clinically relevant species3,11 | Intravenous, topical, intravitreal injection, topical, intravitreal1,3,8,15,16 | Systemic echinocandins are generally well tolerated with few adverse effects, which include headache, gastrointestinal upset, elevated liver enzyme (aminotransferase) levels, and mild infusion reaction3 | |
| Flucytosine | Narrow spectrum of activity, is more effective against the genus Candida and some filamentous fungi, limited activity against Aspergillus spp., whereas Fusarium species are resistant to this drug3,12 | Topical, systemic3,5,12 | – | |
| Polyhexamethylene biguanide | General environmental biocide, showing good in vitro activity against fungi7,13 | Topical7,13 | – |
Although much progress has been made in the treatment of these fungal eye infections, 15–27% of patients require surgical intervention due to failure of therapy or due to advanced disease at presentation (Thomas 2003b). If the corneal infection progresses despite vigorous antifungal therapy, surgical intervention may be mandatory. One method or a combination of several methods may be contemplated, depending on the nature, extent, and severity of the corneal infection (Thomas 2003a; Czakó et al. 2019). For the treatment of small superficial ulcers, the recommended methods include techniques with greater or lesser invasive potential, such as debridement, the use of conjunctival flaps (for small peripheral ulcers) in association with antifungal therapy, tissue adhesives (n-butyl cyanoacrylate glue) and a therapeutic contact lens have also been advocated (Thomas 2003b). Amniotic membrane transplantation may also help promote healing and reduce inflammation in keratitis (Srinivasan 2004; Kalkanci and Ozdek 2010).
Different pharmacological treatments are described, but surgical interventions may also be necessary. Therapeutic surgery may be necessary for clinical cases of fungal keratitis where there are more severe ulcers. In cases where there is impending perforation, perforation greater than 2 mm, or when there is no response to medical therapy, penetrating keratoplasty is indicated (Thomas 2003b; Kalkanci and Ozdek 2010; Thomas and Kaliamurthy 2013). In cases of mild to moderate keratitis, the need for therapeutic keratoplasty (TPK) is ~ 15%, however, for severe cases, it can reach 40% (Mills et al. 2020). It is very important to try to maintain the therapy as long as possible, to make the fungus unfeasible before surgery, with an improvement in the prognosis. Surgery attempts to remove antigens and infectious elements, as well as necrotic tissue and other debris, which can make it difficult for the lesion to heal completely (Thomas 2003a; Thomas and Kaliamurthy 2013). Surgery can also help by increasing antifungal penetration, bringing irrigation in the form of conjunctival flaps, by stabilizing the corneal epithelial surface, and providing tectonic support to the globe when integrity is threatened, as in corneal thinning or perforation (Thomas 2003a). The objective of this technique is to eliminate the infection and restore tissue integrity. The cure rate of TPK can range from 60 to 90% (structural integrity and eradication of infection), with a recurrence rate of 6–15% and graft transparency of 36–89% (Srinivasan 2004; Kalkanci and Ozdek 2010; Mills et al. 2020).
Vitrectomy is an important therapeutic strategy in many cases of ocular fungal infections, such as endophthalmitis. Although there have been cases of fungal endophthalmitis treated with pharmacological therapy alone, evidence supports the use of vitrectomy during the course of treatment for fungal endophthalmitis. This evidence is largely based on case reports and large case series, describing it as an important component of treatment, especially in cases of fungal endophthalmitis with dense vitreous infiltrates (Durand 2013; Chee and Eliott 2017). This technique has some advantages, as it removes fungi from the vitreous, ends up reducing the infectious load, provides the elimination of vitreous opacities and improves vision, can facilitate the diffusion of antifungal agents in the vitreous cavity, limits vitreoretinal traction and plays an important role in the prevention and treatment of retinal detachment (Chee and Eliott 2017). Also, vitrectomy allows the removal of intraocular structures, from endogenous and exogenous sources and that had their penetration occurred together with the fungal elements, which may be essential for the complete resolution of the infection (Chee and Eliott 2017). Therefore, this technique leads to improved visual results, especially in patients who have significant visual loss (Durand 2013).When there is no response to any of the therapeutic options performed and infection progression, there is aggressive surgical treatment, which may include orbital exenteration (Kalkanci and Ozdek 2010).
The response to therapy depends on numerous factors related to the host, pathogen, and chosen therapy. Cases of superficial eye infections by Fusarium, Aspergillus, and Curvularia species respond well to topical natamycin therapy, without the need for surgical intervention. In cases of deeper infections by these fungi, surgical intervention may be necessary for most patients, especially without the use of natamycin (Thomas 2003a; Thomas and Kaliamurthy 2013). Pheohyphomycete eye infections, except Curvularia spp., mostly respond to antifungal therapy, topical natamycin, oral and/or topical ketoconazole, oral ketoconazole and topical miconazole, topical amphotericin B alone, or oral itraconazole alone (Thomas 2003a; Thomas and Kaliamurthy 2013). In cases of eye infections by Candida spp. the use of topical amphotericin B alone or in combination with systemic azoles generally has a good prognosis (Thomas 2003a). In eye infections by S. apiospermum, the outcome is closely associated with the severity with which the condition is presented, with frequent need for surgical intervention. Meanwhile, cases related to Acremonium spp., require antifungal therapy combined with the surgical intervention of a keratoplasty. Other fungi present variable results, according to the extent of the infection (Thomas 2003a).
Mycotic keratitis usually responds slowly to antifungal therapy over a period of weeks. Clinical signs of improvement should be watched carefully (Thomas 2003b; Thomas and Kaliamurthy 2013). Decreased pain, decreased infiltrate size, the disappearance of satellite lesions, disappearance of the hypopyon, and fibrosis in the healing region of fungal lesions may indicate signs of improvement (Thomas and Kaliamurthy 2013). Despite repeated scrapings made during treatment, growth may not be observed in the culture. This lack of growth does not necessarily indicate that the fungus has been eradicated, as fungi may proliferate deep into the stroma. Therefore, therapy should be continued for at least 6 weeks. A corneal biopsy may be an alternative for material collection in those very deep lesions (Thomas 2003a; Thomas and Kaliamurthy 2013).
In view of the above, Table 3 brings together a compilation of clinical cases of fungal eye infections, highlighting the main clinical information of each case and demonstrating the criticality that these pathologies can present. Through this representation table of real clinical cases, we can visualize the entire context discussed so far in the work: the etiological diversity, lack of standardization of therapy, dynamism of therapy and the different outcomes (always with the presence of some type of sequel after resolution of the infection). Therefore, due to the severity of ocular fungal infections, the increase in the incidence and fungal resistance, as future perspectives, the emergence of new antifungal drugs is expected, in addition to the improvement of pharmacokinetics and biodistribution through the improvement of drug delivery systems, such as liposomal formulations, polymeric micelles and nanoparticles (Garg et al. 2016).
Table 3.
Compilation of clinical cases obtained through a literature review and main aspects involved in each case: reference; description; diagnosis; treatment; outcome
| Author | Description | Diagnosis | Treatment | Outcome |
|---|---|---|---|---|
| Amphornphruet et al. (2017) | Bilateral cryptococcal endogenous endophthalmitis |
Culture Cryptococcus neoformans |
Oral prednisolone Amphotericin B IV Pars plana vitrectomy Intravitreal injections of amphotericin B Timolol maleate and ophthalmic brimonidine tartrate Amphotericin B Fluconazole |
The patient was discharged from the hospital and did not return for follow-up |
| Arinelli et al. (2020) |
21 cases of primary bulbar conjunctival sporotrichosis 5 cases of bulbar conjunctival sporotrichosis associated with skin disease |
Culture Sporotrix sp. |
Initial: 11 patients treated with steroid drops and 9 with antibacterial, alone or in combination Oral itraconazole 1 patient: surgical excision of the conjunctival granuloma 1 patient: itraconazole replaced by posaconazole |
23 patients completely cured 8 patients had ocular sequelae after treatment |
| Baharani et al. (2019) | Postoperative necrotizing fungal scleritis (corneal surgery in the left eye with insertion of an intraocular lens implant) |
Direct exam Culture Aspergillus flavus |
Pars plana vitrectomy Fluorometholone eye drops Oral non-steroidal anti-inflammatory drugs Voriconazole eye drops Oral itraconazole |
Her condition improved significantly after one week with complete resolution of her symptoms. The scleritis had completely resolved within 3 months 1 year after completion of treatment without recurrences |
| Chacon-Cruz et al. (2018) |
Deep and severe keratoconjunctivitis Trauma with plant material of the right cornea |
Culture Fusarium sp. |
Viscoelastic (hyaluronic acid) Voriconazole IV Topical natamycin Keratoplasty Topical natamycin |
90% vision recovery at 1 month follow-up |
| Cheng et al. (2015) | Keratitis |
Direct exam Culture Cladosporium sp. |
Natamycin Amphotericin B Levofloxacin Topical fluconazole Oral ketoconazole Topical and oral voriconazole Immersion in the corneal ulcer area with voriconazole Topical voriconazole |
Symptoms improved 10 days after admission and the patient was discharged 14 days later Controlled keratitis |
| Eddy et al. (2012) | Focal purulent keratitis with severe, extensively ulcerative abscess formation associated with contact lenses |
Culture Candida dubliniensis |
Keratoplasty Propamidine isothionate Polyhexamethylene biguanide Systemic gentamicin Systemic levofloxacin Systemic ciprofloxacin Imipenem + systemic cilastatin sodium Topical voriconazole Amniotic membrane transplantation combined with a dressing and a cefotaxime rinse Amphotericin B IV Nystatin Oral voriconazole Caspofungin IV Cataract surgery HLA transplant not compatible |
5 years after the first presentation, stable clinical conditions and a visual acuity of 0.8 |
| Espinosa-Heidmann et al. (2012) |
Redness, photophobia, pain, and decreased visual acuity in the left eye Onychomycosis of the toes |
Culture Candida dubliniensis |
Topical and oral steroids Ceftazidime IV Vancomycin Fluconazole IV Intravitreal injection of amphotericin B, ceftazidime and vancomycin Oral fluconazole |
The patient's final visual acuity improved to 20/80 with resolution of fungal endophthalmitis in the left eye |
| Fieb et al. (2015) | Endogenous endophthalmitis with lens abscess |
Culture Candida albicans |
Surgery Cefuroxime IV Fluconazole |
Successful case resolution |
| Hayashi et al. (2014) | Escleroceratite |
Culture Molecular biology Aspergillus cibarius |
Topical antibacterials Oral prednisolone Micafungin sodium IV Itraconazole Voriconazole drops Natamycin ophthalmic ointment Intrastromal voriconazole injections Scleral transplant Caspofungin acetate IV |
7 months after starting antifungal therapy, sclerokeratitis had caused bullous keratopathy with the patient's corrected visual acuity limited to light perception |
| Hwang et al. (2020) |
Traumatic corneal laceration Infection in the anterior segment |
Culture Aspergillus sp. |
Bandage contact lens Topical moxifloxacin Topical tomycin Broad spectrum systemic antibiotics Topical prednisolone acetate Intracameral and subconjunctival amphotericin B 1st surgery (removal of infection) 2nd surgery Topical voriconazole Systemic fluconazole Intracameral amphotericin B 3rd surgery Intracameral amphotericin B 4th surgery Topical natamycin 5th surgery (insertion of intraocular lens) |
Visual acuity 0.9 |
| Iwahashi et al. (2020) | Macular edema in the right eye secondary to branch retinal vein occlusion |
Culture Molecular biology Exophiala dermatitidis |
Triamcinolone acetonide Topical levofloxacin Local administration of antibiotics 2nd surgery, irrigation with amphotericin B Oral voriconazole Topical and systemic voriconazole |
Effective therapeutic strategy |
| Lee et al. (2016) | Keratitis and blepharitis associated with insect bite |
Culture Aspergillus flavus |
Topical cefazolin Topical amikacin Topical natamycin Tetracycline ointment Topical natamycin |
Patient recovered without further symptoms. Adequate eyelid hygiene, chronic blepharitis was much less active with no recurrence of keratitis |
| Lever et al. (2021) | Invasive orbital aspergillosis |
Culture PCR Aspergillus fumigatus |
Hydrocortisone ointment Oral prednisolone Oral voriconazole |
In the following 2 years, under treatment with oral voriconazole, he showed a clear clinical and radiological improvement. Best corrected visual acuity left eye 0.7 |
| Lübke et al. (2016) | Keratitis—fulminant inflammation and melting of the left cornea |
Culture Fusarium solanii/keratoplasticum |
Ofloxacin and voriconazole Keratoplasty Amphotericin B Polyhexanide Keratoplasty Anterior chamber washes with voriconazole, amphotericin B, and posaconazole Amphotericin B, voriconazole, posaconazole and ofloxacin eye drops Natamycin ophthalmic ointment Topical polyhexanide Systemic voriconazole and posaconazole |
Due to the severity of the case, the eye was enucleated in combination with washing the eye socket/wound with hydrogen peroxide Subsequently, an ocular prosthesis was placed |
| Maitray et al. (2019) |
Septic shock secondary to dental abscesses Invasive pansinusitis, with progression involving the base of the skull (both orbits) and increasing intracranial extension Almost total ophthalmoplegia |
Culture Staphylococcus aureus methicillin resistant Scedosporium apiospermum |
Amoxicillin-clavulanate IV Metronidazole IV 1st surgery Ceftriaxone IV and fluconazole IV Vancomycin and Metronidazole IV 2nd surgery Amphotericin B IV Oral voriconazole 3rd surgery Amphotericin B IV Oral voriconazole |
The patient decided to be discharged at his own risk, his general condition was worse, and later he did not attend the consultation at the ophthalmology clinic |
| Massa et al. (2020) | Keratitis associated with contact lens wear |
direct exam Culture MALDI-TOF Molecular biology Phaeoacremonium parasiticum |
Gentamicin drops Moxifloxacin drops Chlorhexidine drops associated with desomedine Liposomal Amphotericin B Voriconazole drops Debridement Topical voriconazole Topical amphotericin B |
No recurrence of infection after 2 weeks, at 1-year follow-up visit, visual acuity was 1.0 |
| Mitsuoka et al. (2021) | Recurrent rheumatic keratitis—endophthalmitis |
Direct exam Culture PCR Fusarium sp. |
Betamethasone and tacrolimus Oral prednisolone Subconjunctival dexamethasone Topical moxifloxacin Systemic tobramycin and ceftriaxone Topical voriconazole Natamycin ointment Amphotericin b Fluorometholone Penetrating keratoplasty Vitrectomy Intraocular voriconazole Amphotericin B |
6 months after surgery visual acuity 20/100. No recurrence of infection or vitreous opacity was observed Surgery for intraocular lens placement 1 year later Most recent visual acuity 30/100 |
| Naha et al. (2013) | Swelling and inflammation of the right eye |
Histological Aspergillus fumigatus |
Orbitotomy Antibiotic therapy Systemic voriconazole Oral voriconazole Cefotaxime Phosphomycin |
Spectacular evolution with disappearance of fever after two days of treatment, as well as secretions after the fourth day No recurrence after 18 months of follow-up |
| Puah et al. (2016) | Endogenous endophthalmitis |
Direct exam Culture Aspergillus terreus |
Oral voriconazole Intravitreal voriconazole injections Pars plana vitrectomy Endolaser Buffering with silicone oil Intraocular lens implant |
6 months after surgery, corrected visual acuity was 6/120, with thick posterior capsular plate and attached retina and silicone oil in situ Occasional cough, but no sputum production or shortness of breath |
| Reddy et al. (2013) |
Pigmented mycetoma Conjunctival injury Pigmented mycetoma |
Histopathological examination Dematiaceous fungus |
Surgery Subconjunctival cefazolin and gentamicin Systemic voriconazole Topical voriconazole Bacitracin/polymyxin B eye ointment Tobramycin/dexamethasone ophthalmic ointment Prednisolone acetate |
At the final 10-week follow-up after surgery without all medications, eyeglass-corrected visual acuity was 20/25 and there was no clinical evidence of infection 6 weeks after surgery, eyeglass-corrected visual acuity was 20/20 and the patient was cured |
| Reinoso et al. (2013) | Secondary acute myeloblastic leukemia + orbital cellulitis |
Histopathological examination Culture Molecular biology Scedosporium prolificans |
Meropenem Linezolid Amikacin Amphotericin B Oral voriconazole Oral terbinafine |
Patient outcome was death due to disseminated infection, with endogenous fungal cellulitis and endophthalmitis |
| Roy et al. (2014) | Corneal ulcer associated with plant trauma |
Direct exam Culture Scedosporium apiospermum |
Oral fluconazole Topical natamycin Moxifloxacin Atropine eye drops Topical natamycin Amphotericin B Oral ketoconazole Therapeutic penetrating keratoplasty Intracameral amphotericin B Pars plana vitrectomy Intravitreal vancomycin Intravitreal amphotericin B |
Final follow-up, 6 weeks after vitrectomy, corrected visual acuity was no light perception and the eye became physical |
| Schrecker et al. (2021) | Corneal infiltrate refractory to outpatient treatment |
Histological examination PCR Culture Molecular biology Fusarium tonkinense |
Ofloxacin and dexamethasone Acyclovir ointment Topic: azithromycin, ganciclovir, voriconazole Therapeutic penetrating keratoplasty Topical natamycin and polyhexamethylene biguanide Systemic voriconazole and terbinafine Continuous intracameral and intravitreal injections of voriconazole and amphotericin B Intrastromal voriconazole Large diameter repeated keratoplasty Enucleation |
Enucleation and the subsequent course went without further complications and placement of an esthetically pleasing prosthesis |
| Chiu et al. (2021) | Acute endogenous endophthalmitis |
Culture Serum galactomannan antigen Aspergillus flavus |
Cephalosporin IV Intravitreal injection of vancomycin, ceftazidime, dexamethasone and amikacin Intravitreal injection of voriconazole, ceftazidime, amikacin Voriconazole IV |
The patient died due to severe sepsis |
| Singh et al. (2018) | Scleral buckle infection |
Culture Curvularia sp. |
Surgery (washout using gentamicin and voriconazole) Oral ciprofloxacin Oral fluconazole Moxifloxacin eye drops Voriconazole eye drops Atropine eye drops Carboxymethylcellulose eye drops |
After a month of buckle removal, he had no conjunctival congestion, the superotemporal sclera appeared thin. Subsequent inferior retinal detachment at 2 months had stabilized and no tears were found |
| Ting et al. (2019) | Keratitis |
Culture Molecular biology Histopathological examination Stenotrophomonas maltophilia e Candida parapsilosis Cryptococcus curvatus Cryptococcus spp. C. parapsilosis |
Penetrating keratoplasty Silicone hydrogel Topical miconazole Chloramphenicol Oral fluconazole Topical dexamethasone Penetrating keratoplasty Topical miconazole Amphotericin b drops Oral fluconazole Topical amphotericin B Oral fluconazole Topical voriconazole Intrastromal voriconazole injections Penetrating keratoplasty Oral flucytosine Topical amphotericin Oral fluconazole Intracorneal and intravitreal amphotericin B Intravitreal vancomycin Ceftazidime Focal cryotherapy Topical amphotericin B, voriconazole drops Chlorhexidine drops Oral voriconazole Topical amphotericin B Oral fluconazole |
At her last follow-up visit, corrected visual acuity remained at 20/200 Numerous recurrences of infections by the different agents, 4 years for remission of Cryptococcus curvatus keratitis |
| Todokoro et al. (2018) |
Retinal macular edema resulting from branch retinal vein occlusion Infectious fungal scleritis induced by triamcinolone acetonide injection |
Molecular biology Culture Scedosporium apiospermum |
Oral antibiotic Topical voriconazole Levofloxacin eye drops Povidone iodine Natamycin ointment Atropine eye drops Voriconazole IV Oral voriconazole Topical voriconazole |
No recurrence was observed after the final treatment, the final corrected visual acuity was 0.2 |
| Vicente et al. (2018) | Persistent corneal erosion in the right eye |
Anatomopathological examination Culture Molecular biology Exophiala phaeomuriformis |
Cyclodiode laser ablation Amniotic membrane transplant Topical amphotericin B Oral fluconazole |
Erosion regression and total corneal re-epithelialization after 4 months Corneal stromal scar improved to stabilization with a mild scar after 6 months |
| Yeşiltaş et al. (2019) |
Endogenous endophthalmitis Signs of toenail infection |
Culture Candida albicans |
Oral methylprednisone and co-trimoxazole Amphotericin B IV Systemic steroids Topical dexamethasone Cyclopentolate hydrochloride Intravitreal voriconazole injection Oral fluconazole 2nd intravitreal amphotericin B injection Pars plana vitrectomy Intravitreal amphotericin B Amphotericin B IV Fluconazole IV |
One month after surgery, the abscess regressed and left scar tissue in the macula and the intraocular inflammation gradually subsided. There was no recurrence after discontinuation of antifungal treatments. Low visual acuity. Your nail infection has been successfully treated |
IV intravenous
Conclusion
Despite the high morbidity related to eye fungal infections, there is still a succession of important problems associated with them. Slow, faulty or non-existent identification of the etiologic agent, especially when it has a lower prevalence and, thus, more empirical management, can result in more serious outcomes. Despite the predominance of some species/genera, there is etiological diversity in the cause of these infections. In addition, the slow and complex process of defining the most appropriate pharmacological and/or non-pharmacological treatment is closely related to the patient's clinical history and the correct diagnosis. Still, it is very important to emphasize the limited therapeutic arsenal available for the management of these pathologies, the absence of standard pharmacological therapy and the wide use of natamycin, amphotericin B and voriconazole. The therapy presents intense dynamism of the pharmacological scheme and extensive use of polytherapy. All these aspects about ocular fungal infections were corroborated through the compilation of clinical cases presented. Therefore, for the patient's outcome to be positive and minimally harmful, it is necessary to have a broad knowledge of the aspects that are involved in ocular fungal infections in their entire context.
Acknowledgements
This study was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Paula Reginatto and Giovanna de Jesus Agostinetto. The first draft of the manuscript was written by Paula Reginatto and Giovanna de Jesus Agostinetto and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
This study was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) through the granting of a scholarship. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmadikia K, Aghaei GS, Fallah B, Naeimi EM, Malekifar P, Rahsepar S, Getso MI, Sharma S, Mahmoudi S. Distribution, prevalence, and causative agents of fungal keratitis: a systematic review and meta-analysis (1990 to 2020) Front Cell Infect Microbiol. 2021 doi: 10.3389/fcimb.2021.698780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amphornphruet A, Silpa-archa S, Preble JM, Foster CS. Endogenous cryptococcal endophthalmitis in immunocompetent host: case report and review of multimodal imaging findings and treatment. Ocular Immunol Inflam. 2017 doi: 10.1080/09273948.2017.1298820. [DOI] [PubMed] [Google Scholar]
- Arinelli A, Aleixo ALQC, Freitas DFS, do Valle ACF, Almeida-Paes R, Gutierrez-Galhardo MC, Curi ALL. Ocular sporotrichosis: 26 cases with bulbar involvement in a hyperendemic area of zoonotic transmission. Ocular Immunol Inflam. 2019 doi: 10.1080/09273948.2019.1624779. [DOI] [PubMed] [Google Scholar]
- Armstrong RA. The microbiology of the eye. Ophthalmic Physiol Opt. 2000;20(6):429–441. doi: 10.1111/j.1475-1313.2000.tb01121.x. [DOI] [PubMed] [Google Scholar]
- Baharani A, Reddy AK, Reddy PRR. Aspergillus flavus necrotising scleritis following Pars Plana vitrectomy. Ocular Immunol Inflam. 2019 doi: 10.1080/09273948.2019.1625414. [DOI] [PubMed] [Google Scholar]
- Buchta V, Feuermannová A, Váša M, Bašková L, Kutová R, Kubátová A, Vejsová M. Outbreak of fungal endophthalmitis due to Fusarium oxysporum following cataract surgery. Mycopathologia. 2014;177(1–2):115–121. doi: 10.1007/s11046-013-9721-5. [DOI] [PubMed] [Google Scholar]
- Chacon-Cruz E, Male-Valle F, Rivas-Landeros RM, Lopatynsky-Reyes EZ, Almada-Salazar LA, Becka CM. Severe mycotic keratoconjunctivitis caused by Fusarium sp. in an immunocompetent child successfully treated with intravenous voriconazole and keratoplasty: case report and short review of the literature. Therap Adv Infect Disease. 2018;12(6):2049936118811213. doi: 10.1177/2049936118811213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A, Shivaprakash MR, Singh R, Tarai B, George VK, Fomda BA, Gupta A. Fungal endophthalmitis. Retina. 2008;28(10):1400–1407. doi: 10.1097/iae.0b013e318185e943. [DOI] [PubMed] [Google Scholar]
- Chee YE, Eliott D. The role of vitrectomy in the management of fungal endophthalmitis. Semin Ophthalmol. 2017;32(1):29–35. doi: 10.1080/08820538.2016.1228396. [DOI] [PubMed] [Google Scholar]
- Cheng SC-H, Lin Y-Y, Kuo C-N, Lai L-J. Cladosporium keratitis—a case report and literature review. BMC Ophthalmol. 2015 doi: 10.1186/s12886-015-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YY, Chen YH, Hsiao HH, Du JS. A rare patient with primary endogenous Aspergillusendophthalmitis. J Microbiol Immunol Infect. 2021;54(3):538–539. doi: 10.1016/j.jmii.2020.12.014. [DOI] [PubMed] [Google Scholar]
- Czakó C, Sándor G, Popper-Sachetti A, Horváth H, Kovács I, Imre L, Jeannette T, Péter B, Zsolt NZ, Gyula S, Szentmáry N. Fusarium és Sarocladium okozta fertőzések szemészeti vonatkozásai és azok kezelése. Orv Hetil. 2019;160(1):2–11. doi: 10.1556/650.2019.31259. [DOI] [PubMed] [Google Scholar]
- Debourgogne A, Dorin J, Machouart M. Emerging infections due to filamentous fungi in humans and animals: only the tip of the iceberg? Enviro Microbiol Rep. 2016;8(3):332–342. doi: 10.1111/1758-2229.12404. [DOI] [PubMed] [Google Scholar]
- Durand ML. Endophthalmitis. Clin Microbiol Infect. 2013;19(3):227–234. doi: 10.1111/1469-0691.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand ML. Bacterial and fungal endophthalmitis. Clin Microbiol Rev. 2017;30(3):597–613. doi: 10.1128/cmr.00113-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy M-T, Steinberg J, Richard G, Hassenstein A. Schwerste kontaktlinsenassoziierte mykotische Keratitis. Ophthalmologe. 2012;109(11):1106–1111. doi: 10.1007/s00347-012-2570-7. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, McMillan BD, Lasala PR, Stanley J, Larzo CR. Candida dubliniensis endophthalmitis: first case in North America. Int Ophthalmol. 2012;32(1):41–45. doi: 10.1007/s10792-011-9499-8. [DOI] [PubMed] [Google Scholar]
- Fieß A, Bauer J, Schindel C, Knuf M, Dithmar S. Endogener Candida-Linsenabszess bei Frühgeborenem. Ophthalmologe. 2015;113(1):71–74. doi: 10.1007/s00347-015-0068-9. [DOI] [PubMed] [Google Scholar]
- Garg P, Roy A, Roy S. Update on fungal keratitis. Curr Opin Ophthalmol. 2016;27(4):333–339. doi: 10.1097/icu.0000000000000272. [DOI] [PubMed] [Google Scholar]
- Guest JM, Singh PK, Revankar SG, Chandrasekar PH, Kumar A. Isavuconazole for treatment of experimental fungal endophthalmitis caused by Aspergillus fumigatus. Antimicrob Agents Chemother. 2018;62:e01537–e1618. doi: 10.1038/sj.eye.6702463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Srinivasan R, Kaliaperumal S, Saha I. Post-traumatic fungal endophthalmitis—a prospective study. Eye. 2008;22(1):13–17. doi: 10.1038/sj.eye.6702463. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Eguchi H, Toibana T, Mitamura Y, Yaguchi T. Polymicrobial sclerokeratitis caused by Scedosporium apiospermum and Aspergillus cibarius. Cornea. 2014;33(8):875–877. doi: 10.1097/ico.0000000000000172. [DOI] [PubMed] [Google Scholar]
- Hwang HJ, Lee YW, Koh KM, Hwang KY, Kwon YA, Song SW, Kim BY, Kim KY. Lenticular fungal infection caused by Aspergillus in a patient with traumatic corneal laceration: a case report. BMC Ophthalmol. 2020 doi: 10.1186/s12886-020-01441-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahashi C, Eguchi H, Hotta F, Uezumi M, Sawa M, Kimura M, Yaguchi T, Kusaka S. Orbital abscess caused by Exophiala dermatitidis following posterior subtenon injection of triamcinolone acetonide: a case report and a review of literature related to Exophiala eye infections. BMC Infect Diseases. 2020 doi: 10.1186/s12879-020-05294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkanci A, Ozdek S. Ocular fungal infections. Curr Eye Res. 2010;36(3):179–189. doi: 10.3109/02713683.2010.533810. [DOI] [PubMed] [Google Scholar]
- Karagoz E, Ugan RA, Duzgun E, Cadirci E, Keles S, Uyanik MH, Yavan I, Turhan V. A comparative study of the effects of intravitreal anidulafungin, voriconazole, and amphotericin B in an experimental Candida endophthalmitis model. Curr Eye Res. 2016;42(2):225–232. doi: 10.3109/02713683.2016.1170857. [DOI] [PubMed] [Google Scholar]
- Kauffman CA. Endoftalmite Fúngica Exógena. In: Durand M, Miller J, Young L, editors. Endoftalmite. Cham: Springer; 2016. [Google Scholar]
- Kaur IP, Kakkar S. Topical delivery of antifungal agents. Expert Opin Drug Deliv. 2010;7(11):1303–1327. doi: 10.1517/17425247.2010.525230. [DOI] [PubMed] [Google Scholar]
- Klotz SA, Penn CC, Negvesky GJ, Butrus SI. Fungal and parasitic infections of the eye. Clin Microbiol Rev. 2000;13(4):662–685. doi: 10.1128/cmr.13.4.662-685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani P, Patil A, Majumdar S. Challenges in the polyene- and azole-based pharmacotherapy of ocular fungal infections. J Ocul Pharmacol Ther. 2019;35(6):6–22. doi: 10.1089/jop.2018.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashof AML, Rothova A, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Schlamm HT, Oborska IT, Rex JH, Kullberg BJ. Ocular manifestations of candidemia. Clin Infect Dis. 2011;53(3):262–268. doi: 10.1093/cid/cir355. [DOI] [PubMed] [Google Scholar]
- Lee C-Y, Chen H-C, Hsiao C-H, Lin H-C, Sun C-C, Lai C-C, Ma DH-K. Recurrent fungal keratitis and blepharitis caused by Aspergillus flavus. Am J Trop Med Hyg. 2016;95(5):1216–1218. doi: 10.4269/ajtmh.16-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M, Wilde B, Pförtner R, Deuschl C, Witzke O, Bertram S, Eckstein A, Rath P-M. Orbital aspergillosis: a case report and review of the literature. BMC Ophthalmol. 2021 doi: 10.1186/s12886-020-01773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M-Y, Zhang L, Yin X-L, Sun S-Y. Endophthalmitis associated with fungal keratitis and penetrating injuries in North China. Eur J Ophthalmol. 2019 doi: 10.1177/1120672119833896. [DOI] [PubMed] [Google Scholar]
- Lübke J, Auw-Hädrich C, Meyer-ter-Vehn T, Emrani E, Reinhard T. Fusarien keratitis mit dramatischem Ausgang. Ophthalmologe. 2016;114(5):462–465. doi: 10.1007/s00347-016-0303-z. [DOI] [PubMed] [Google Scholar]
- Lupia T, Corcione S, Fea AM, Reibaldi M, Fallico M, Petrillo F, Galdiero M, Scabini S, Polito MS, Ciabatti U, De Rosa FG. Exogenous fungal endophthalmitis: clues to Aspergillus Aetiology with a pharmacological perspective. Microorganisms. 2020;9(1):74. doi: 10.3390/microorganisms9010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi S, Masoomi A, Ahmadikia K, Tabatabaei SA, Soleimani M, Rezaie S, Ghahvechian H, Banafsheafshan A. Fungal keratitis: an overview of clinical and laboratory aspects. Mycoses. 2018;61(12):916–930. doi: 10.1111/myc.12822. [DOI] [PubMed] [Google Scholar]
- Maitray A, Rishi E, Rishi P, Gopal L, Bhende P, Ray R, Therese KL. Endogenous endophthalmitis in children and adolescents. Indian J Ophthalmol. 2019;67(6):795–800. doi: 10.4103/ijo.IJO_710_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa H, Riat A, Panos GD. First report of a new corneal pathogen: Phaeoacremonium parasiticum. Eur J Clin Microbiol Infect Dis. 2020;39:2477–2480. doi: 10.1007/s10096-020-03980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrandish S, Mirzaeei S. A review on ocular novel drug delivery systems of antifungal drugs: functional evaluation and comparison of conventional and novel dosage forms. Adv Pharm Bull. 2021;11(1):28–38. doi: 10.34172/apb.2021.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills B, Radhakrishnan N, Rajapandian SGK, Rameshkumar G, Lalitha P, Prajna NV. The role of fungi in fungal keratitis. Exp Eye Res. 2020;202:108372. doi: 10.1016/j.exer.2020.108372. [DOI] [PubMed] [Google Scholar]
- Mitsuoka Y, Soma T, Maruyama K, Nishida K. Fusarium infection complicating rheumatic keratitis that acutely progressed to endophthalmitis during regular infusion of tocilizumab: a case report. BMC Ophthalmol. 2021 doi: 10.1186/s12886-021-01981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki K, Sawada A, Suemori S, Kawakami H, Niwa Y, Kondo Y, Ohkusu K, Yamada N, Ogura S, Yaguchi T, Nishimura K, Kishino S. Intraocular penetration of intravenous micafungin in inflamed human eyes. Antimicrob Agents Chemother. 2013;57(8):4027–4030. doi: 10.1128/aac.02300-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS ONE. 2021;16(5):e0251170. doi: 10.1371/journal.pone.0251170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada WM, Al Aswad MA, El-Haig WM. Combined intrastromal injection of amphotericin B and topical fluconazole in the treatment of resistant cases of keratomycosis: a retrospective study. Clin Ophthalmol. 2017;5(11):871–874. doi: 10.2147/OPTH.S135112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naha L, Elakhiri M, Hemmaoui B, Tagajdid R, Nadour K, Lmimouni B. Ethmoïdite aspergillaire compliquée d’un abcès orbitaire. J Mycol Méd. 2013;23(2):136–139. doi: 10.1016/j.mycmed.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Ong HS, Fung SSM, Macleod D, Dart JKG, Tuft SJ, Burton MJ. Altered patterns of fungal keratitis at a london ophthalmic referral hospital: an eight-year retrospective observational study. Am J Ophthalmol. 2016;168:227–236. doi: 10.1016/j.ajo.2016.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas PG, Lionakis MS, Arendrup MC, Zeichner LO, Kullberg BJ. Invasive candidiasis . Nat Rev Disease Primers. 2018 doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- Patil A, Majumdar S. Echinocandins in ocular therapeutics. J Ocul Pharmacol Ther. 2017;33(5):340–352. doi: 10.1089/jop.2016.0186. [DOI] [PubMed] [Google Scholar]
- Prajna NV, Mascarenhas J, Krishnan T, Reddy PR, Prajna L, Srinivasan M, Vaitilingam CM, Hong KC, Lee SM, McLeod SD, Zegans ME, Porco TC, Lietman TM, Acharya NR. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Arch Ophthalmol. 2010;128(6):672–678. doi: 10.1001/archophthalmol.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajna NV, Krishnan T, Mascarenhas J, Rajaraman R, Prajna L, Srinivasan M, Raghavan A, Oldenburg CE, Ray KJ, Zegans ME, McLeod SD, Porco TC, Acharya NR, Lietman TM, Mycotic Ulcer Treatment Trial Group The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131(4):422–429. doi: 10.1001/jamaophthalmol.2013.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajna NV, Lalitha P, Rajaraman R, Krishnan T, Raghavan A, Srinivasan M, O’Brien KS, Zegans M, McLeod SD, Acharya NR, Keenan JD, Lietman TM, Nussbaumer JR, the Mycotic Ulcer Treatment Trial Group Changing azole resistance: a secondary analysis of the MUTT I randomized clinical trial. JAMA Ophthalmol. 2016;134(6):693–696. doi: 10.1001/jamaophthalmol.2016.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajna NV, Krishnan T, Rajaraman R, Patel S, Srinivasan M, Das M, Ray KJ, O'Brien KS, Oldenburg CE, McLeod SD, Zegans ME, Porco TC, Acharya NR, Lietman TM, Rose-Nussbaumer J, Mycotic Ulcer Treatment Trial II Group Effect of oral voriconazole on fungal keratitis in the mycotic ulcer treatment trial II (MUTT II): a randomized clinical trial. JAMA Ophthalmol. 2016;134(12):1365–1372. doi: 10.1001/jamaophthalmol.2016.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajna NV, Krishnan T, Rajaraman R, Patel S, Shah R, Srinivasan M, Devi L, Das M, Ray KJ, O'Brien KS, Oldenburg CE, McLeod SD, Zegans ME, Acharya NR, Lietman TM, Rose-Nussbaumer J, Mycotic Ulcer Treatment Trial Group Adjunctive oral voriconazole treatment of fusarium keratitis: a secondary analysis from the mycotic ulcer treatment trial II. JAMA Ophthalmol. 2017;135(6):520–525. doi: 10.1001/jamaophthalmol.2017.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puah SH, Ng J, Ang B, Xu H, Agrawal R, Ho S-L. Recurrent Aspergillus terreus endophthalmitis from focal bronchiectasis. Ocul Immunol Inflamm. 2016;26(3):358–361. doi: 10.1080/09273948.2016.1199716. [DOI] [PubMed] [Google Scholar]
- Reddy JC, Rapuano CJ, Eagle RC, Jr, Shields CL, Shields JA. Late-onset necrotizing scleritis due to pigmented mycetoma (dematiaceous fungi) in 2 cases. Cornea. 2013;32(4):450–453. doi: 10.1097/ICO.0b013e318254a4c6. [DOI] [PubMed] [Google Scholar]
- Reinoso R, Carreño E, Hileeto D, Corell A, Pastor JC, Cabrero M, Vázquez L, Calonge M. Fatal disseminated Scedosporium prolificans infection initiated by ophthalmic involvement in a patient with acute myeloblastic leukemia. Diagn Microbiol Infect Dis. 2013;76(3):375–378. doi: 10.1016/j.diagmicrobio.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Relhan N, Forster RK, Flynn HW., Jr Endophthalmitis: then and now. Am J Ophthalmol. 2018;187:xx–xxvii. doi: 10.1016/j.ajo.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, Panigrahi PK, Pal SS, Mukherjee A, Bhargava M. Post-traumatic endophthalmitis secondary to keratomycosis caused by Scedosporium apiospermum. Ocular Immunol Inflam. 2014 doi: 10.3109/09273948.2014.902078. [DOI] [PubMed] [Google Scholar]
- Sahay P, Singhal D, Nagpal R, Maharana PK, Farid M, Gelman R, Sinha R, Agarwal T, Titiyal JS, Sharma N. Pharmacologic therapy of mycotic keratitis. Surv Ophthalmol. 2019;64(3):380–400. doi: 10.1016/j.survophthal.2019.02.007. [DOI] [PubMed] [Google Scholar]
- Schrecker J, Seitz B, Berger T, Daas L, Behrens-Baumann W, Auw-Hädrich C, Schütt S, Kerl S, Rentner-Andres S, Hof H. Malignant keratitis caused by a highly-resistant strain of Fusarium tonkinense from the Fusarium solani complex. J Fungi. 2021;7(12):1093. doi: 10.3390/jof7121093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RA, Sridhar J, Miller D, Wykoff CC, Flynn HW. Exogenous fungal endophthalmitis: an analysis of isolates and susceptibilities to antifungal agents over a 20-year period (1990–2010) Am J Ophthalmol. 2015;159(2):257–264. doi: 10.1016/j.ajo.2014.10.027. [DOI] [PubMed] [Google Scholar]
- Sng ECY, Tan AL, Zhou PY, Tan TJ, Waduthantri S, Chee S-P, Tan BH. Candida Endophthalmitis Treated Successfully With Isavuconazole: A Case Report. Open Forum. Infectious Diseases. 2021;8(12):ofab516. doi: 10.1093/ofid/ofab516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Shrivastav A, Agarwal M, Gandhi A, Mayor R, Paul L. A rare case of scleral buckle infection with Curvularia species. BMC Ophthalmol. 2018 doi: 10.1186/s12886-018-0695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Słowik M, Biernat MM, Urbaniak-Kujda D, Kapelko-Słowik K, Misiuk-Hojło M. Mycotic Infections of the Eye. Adv Clin Exp Med. 2015;24(6):1113–1117. doi: 10.17219/acem/50572. [DOI] [PubMed] [Google Scholar]
- Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15:321–327. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- Spallone A, Schwartz IS. Emerging Fungal Infections. Infectious Disease Clinics of North America. 2021;35(2):261–277. doi: 10.1016/j.idc.2021.03.014. [DOI] [PubMed] [Google Scholar]
- Thomas PA. Current perspectives on ophthalmic mycoses. Clin Microbiol Rev. 2003;16(4):730–797. doi: 10.1128/cmr.16.4.730-797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PA. Fungal infections of the cornea. Eye. 2003;17(8):852–862. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19(3):210–220. doi: 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- Ting DSJ, Bignardi G, Koerner R, Irion LD, Johnson E, Morgan SJ, Ghosh S. Polymicrobial keratitis with Cryptococcus curvatus, Candida parapsilosis, and Stenotrophomonas maltophilia after penetrating keratoplasty: a rare case report with literature review. Eye Contact Lens. 2019;45(2):e5–e10. doi: 10.1097/ICL.0000000000000517. [DOI] [PubMed] [Google Scholar]
- Todokoro D, Hoshino J, Yo A, Makimura K, Hirato J, Akiyama H. Scedosporium apiospermum infectious scleritis following posterior subtenon triamcinolone acetonide injection: a case report and literature review. BMC Ophthalmol. 2018 doi: 10.1186/s12886-018-0707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente A, Pedrosa Domellöf F, Byström B. Exophiala phaeomuriformiskeratitis in a subarctic climate region: a case report. Acta Ophthalmol. 2017;96(4):425–428. doi: 10.1111/aos.13624. [DOI] [PubMed] [Google Scholar]
- Vilela RC, Vilela L, Vilela P, Vilela R, Motta R, Pôssa AP, de Almeida C, Mendoza L. Etiological agents of fungal endophthalmitis: diagnosis and management. Int Ophthalmol. 2013 doi: 10.1007/s10792-013-9854-z. [DOI] [PubMed] [Google Scholar]
- Von Jagow B, Kurzai O, Kakkassery V. Case report: beyond the blood-retina barrier: intravitreal caspofungin for fungal endophthalmitis. Optom vis Sci. 2020;97(7):473–476. doi: 10.1097/opx.0000000000001532. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Flynn HW, Miller D, Scott IU, Alfonso EC. Exogenous fungal endophthalmitis: microbiology and clinical outcomes. Ophthalmology. 2008;115(9):1501–1507. doi: 10.1016/j.ophtha.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Yeşiltaş YS, Özcan G, Demirel S, Yalçındağ N. Culture-proven Candida albicans endogenous endophthalmitis in a patient with onychomycosis. Ocular Immunol Inflam. 2019 doi: 10.1080/09273948.2019.1568503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.