Abstract
Background
A small number of patients can develop stent thrombosis after coronary stent implantation. Diabetes, malignant tumors, anemia, etc. have been identified as risk factors for stent thrombosis. A previous study confirmed that the systemic immune-inflammatory index is associated with venous thrombosis. However, there are no studies investigating the association between the systemic immune-inflammation index and stent thrombosis after coronary stent implantation, and thus, we designed this study.
Methods
A total of 887 myocardial infarction patients admitted to the Wuhan University Hospital from January 2019 to June 2021 were included. All of the patients received coronary stent implantation and were followed up for 1 year by clinic visit. The patients were divided into a stent thrombosis group (n=27) and a control group (n=860) according to whether or not they suffered stent thrombosis. The clinical features of the two groups were observed, and the receiver operator characteristic (ROC) curve was used to analyze the predictive value of the systemic immune-inflammation index for stent thrombosis in patients with myocardial infarction after coronary artery stenting.
Results
Compared with the control group, the proportion of stent number ≥4 in the stent thrombosis group was significantly higher (62.96% vs. 38.72%, P=0.011), and the proportion of patients with a systemic immune-inflammation index ≥636 was markedly increased (55.56% vs. 23.26%, P=0.000). The number of stents and the systemic immune-inflammation index were both valuable in predicting stent thrombosis, and the predictive value of the systemic immune-inflammation index was higher, with an area under the curve of 0.736 (95% confidence interval: 0.647–0.824, P=0.000), the best diagnostic value was 636, and the sensitivity and specificity were 0.556 and 0.767. The systemic immune-inflammation index ≥636 and the number of stents ≥4 were independent risk factors for stent thrombosis after coronary stent implantation (P<0.05). Compared with the control group, the incidence of recurrent myocardial infarction was notably increased in the stent thrombosis group (33.33% vs. 3.26%, P=0.000), and mortality was significantly higher in the stent thrombosis group (14.81% vs. 0.93%, P=0.000).
Conclusions
The systemic immune-inflammation index was associated with the development of stent thrombosis in patients with myocardial infarction after coronary stent implantation.
Keywords: Systemic immune-inflammatory index, coronary stent implantation, myocardial infarction, stent thrombosis
Highlight box.
Key findings
• The systemic immune-inflammation index was valuable in predicting stent thrombosis in myocardial infarction patients after coronary artery stenting.
What is known and what is new?
• At present, diabetes, malignant tumors, anemia, etc. have been identified as risk factors for stent thrombosis.
• This study confirmed the value of the systemic immune-inflammation index in predicting stent thrombosis in myocardial infarction patients after coronary stent implantation.
What is the implication, and what should change now?
• Further exploration of the molecular mechanism of the systemic immune-inflammation index leading to stent thrombosis is needed.
Introduction
Myocardial infarction is one of the main factors leading to death in middle-aged and elderly people, and coronary stent implantation is one of the primary means of treating myocardial infarction. There were up to 900,000 patients undergoing coronary stent implantation for coronary artery disease in China in 2018. Coronary stent implantation effectively reduces the incidence of recurrent myocardial infarction, but stent thrombosis occurs occasionally (incidence rate of about 2%), mostly occurring within 1 year after surgery (1,2). Stent thrombosis is one of the most dangerous complications after coronary artery stenting, with mortality rates ranging from 5% to 45% (3). At present, smoking, diabetes, malignant tumors, anemia, etc. have been identified as risk factors for stent thrombosis (4-9). The key to preventing stent thrombosis is to identify risk factors and target them accordingly. At present, some scholars used white blood cells and C reactive protein to predict the value of stent thrombosis, but their sensitivity and specificity were limited (10). A previous study confirmed that the systemic immune-inflammatory index is associated with venous thrombosis (11). The stent thrombosis is related to inflammation, and the systemic immune-inflammation index is an indicator of the severity of body inflammation. Therefore, the systemic immune-inflammation index may be related to the development of stent thrombosis. However, there are no studies investigating the association between the systemic immune-inflammation index and stent thrombosis after coronary stent implantation, and thus, we designed this study. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-363/rc).
Methods
General information
A total of 887 myocardial infarction patients admitted to the Wuhan University Hospital from January 2019 to June 2021 were included. All patients received coronary stent implantation and were followed up for 1 year. The patients were divided into a stent thrombosis group (n=27) and a control group (n=860) according to whether or not they had stent thrombosis (all stent thrombosis occurred 1 month after surgery). The inclusion criteria were as follows: (I) acute myocardial infarction; (II) coronary stent implantation; (III) age ≥18 years old; and (IV) complete medical records. The exclusion criteria were as follows: (I) receiving thrombolytic therapy; (II) insufficiency of important organs such as the liver and kidneys; (III) complicated by acute heart failure; (IV) combined with malignant tumors; (V) immune system diseases such as primary immune dysfunction; (VI) chronic infectious diseases; (VII) chronic pain diseases; (VIII) mental abnormalities or cognitive impairment; (IX) failure to cooperate with treatment, transfer, or lost to follow-up; and (X) other major diseases. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Wuhan University Hospital (No. 20220042). Individual consent for this retrospective analysis was waived.
Treatment methods
Patients in both groups received coronary stent implantation and were given dual antiplatelet therapy (aspirin enteric-coated tablets, 100 mg/d; Clopidogrel, 75 mg/day) for 1 year postoperatively. Patients with hypertension, diabetes, or hyperlipidemia received antihypertensive, hypoglycemic, or lipid-lowering drugs, respectively. For patients with stent thrombosis, stent catheter thrombolysis was used for treatment, and if necessary, stent thrombectomy was used for treatment.
Observation indicators and follow-up methods
After surgery, monthly follow-up was conducted through outpatient visits or telephone interviews. The follow-up time was up to 1 year postoperatively. The observation indicators included the following: (I) general information: age, gender, hypertension, diabetes, hyperlipidemia; (II) stent situation: number of coronary artery-implanted stents, stent diameter, and stent length; and (III) biological indicators: D-dimer and the systemic immune-inflammatory index, which was calculated as follows: systemic immune-inflammation index = platelet count × neutrophil count/lymphocyte.
Statistical analysis
Data analysis in this study was performed using SPSS 26.0 (IBM, USA, Chicago), and P<0.05 (two-tailed) was considered to indicate a statistically significant difference. The count data were compared and analyzed by the chi-square test between the two groups and expressed in n (%). The predictive value of the systemic immune-inflammation index for stent thrombosis was analyzed using the receiver operator characteristic (ROC) curve [the optimal diagnostic threshold was the value corresponding to the maximum Yoden index (the point closest to the upper left of the ROC curve)]. Single factor analysis was used to analyze the related factors of stent thrombosis. If P<0.100, the factor would be included in the multivariate regression analysis. Multivariate logistics regression analysis was used to explore the risk factors for stent thrombosis.
Results
Comparison of clinical features of the two groups
Compared with the control group, the proportion of stent number ≥4 was significantly higher in the stent thrombosis group (62.96% vs. 38.72%, P=0.011), and the proportion of patients with systemic immune-inflammation index ≥636 was markedly higher in the stent thrombosis group (55.56% vs. 23.26%, P=0.000) (Table 1).
Table 1. The clinical characteristics of the two groups.
| Category | Stent thrombosis group (n=27) | Control group (n=860) | χ2 value | P value |
|---|---|---|---|---|
| Age (years), n (%) | 1.639 | 0.200 | ||
| ≥65 | 12 (44.44) | 281 (32.67) | ||
| <65 | 15 (55.56) | 579 (67.33) | ||
| Gender, n (%) | 0.983 | 0.322 | ||
| Male | 19 (70.37) | 524 (60.93) | ||
| Female | 8 (29.63) | 336 (39.07) | ||
| Hypertensive disease, n (%) | 18 (66.67) | 610 (70.93) | 0.230 | 0.631 |
| Diabetes, n (%) | 12 (44.44) | 259 (30.12) | 2.533 | 0.111 |
| Hyperlipidemia, n (%) | 24 (88.89) | 692 (80.47) | 1.194 | 0.275 |
| Stent number, n (%) | 6.440 | 0.011 | ||
| ≥4 | 17 (62.96) | 333 (38.72) | ||
| <4 | 10 (37.04) | 527 (61.28) | ||
| Stent diameter (mm), n (%) | 0.398 | 0.528 | ||
| ≥3.4 | 17 (62.96) | 489 (56.86) | ||
| <3.4 | 10 (37.04) | 371 (43.14) | ||
| Stent length (cm), n (%) | 2.713 | 0.100 | ||
| ≥15 | 15 (55.56) | 342 (39.77) | ||
| <15 | 12 (44.44) | 518 (60.23) | ||
| D-dimer, n (%) | 1.834 | 0.176 | ||
| ≥0.5 mg/L | 26 (96.30) | 754 (87.67) | ||
| <0.5 mg/L | 1 (3.70) | 106 (12.33) | ||
| Systemic immune inflammatory index, n (%) | 14.872 | 0.000 | ||
| ≥636 | 15 (55.56) | 200 (23.26) | ||
| <636 | 12 (23.26) | 660 (76.74) | ||
| Stent type, n (%) | 0.001 | 0.979 | ||
| Drug coated balloon | 15 (55.56) | 480 (55.81) | ||
| Drug eluting stent | 12 (44.44) | 380 (44.19) | ||
| Platelet count, n (%) | 1.138 | 0.286 | ||
| ≥300 | 16 (59.26) | 420 (48.84) | ||
| <300 | 11 (40.74) | 440 (51.16) | ||
| Neutrophil count, n (%) | 2.160 | 0.142 | ||
| ≥70% | 17 (62.96) | 418 (48.60) | ||
| <70% | 10 (37.04) | 442 (51.40) | ||
| Lymphocyte, n (%) | 0.645 | 0.422 | ||
| ≥40% | 8 (29.63) | 320 (37.21) | ||
| <40% | 19 (70.37) | 540 (62.79) | ||
Predictive value of different indexes for stent thrombosis
The stent number and the systemic immune-inflammation index were valuable in predicting stent thrombosis, among which the systemic immune-inflammation index was the highest, and the area under the curve was 0.736 (95% confidence interval: 0.647–0.824, P=0.000). Age, stent diameter, total stent length, and D-dimer had no significant value in predicting stent thrombosis (P>0.05) (Table 2 and Figure 1).
Table 2. Predictive value of different indicators for stent thrombosis.
| Variables | Area under the curve | P value | 95% confidence interval | Optimal diagnostic cut-off | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Age | 0.525 | 0.660 | 0.414–0.636 | – | – | – |
| Stent diameter | 0.544 | 0.434 | 0.451–0.638 | – | – | – |
| Stent number | 0.625 | 0.026 | 0.551–0.700 | 3.5 | 0.630 | 0.613 |
| Total stent length | 0.595 | 0.092 | 0.495–0.696 | – | – | – |
| D-dimer | 0.561 | 0.283 | 0.447–0.674 | – | – | – |
| Systemic immune-inflammatory index | 0.736 | 0.000 | 0.647–0.824 | 636 | 0.556 | 0.767 |
Figure 1.
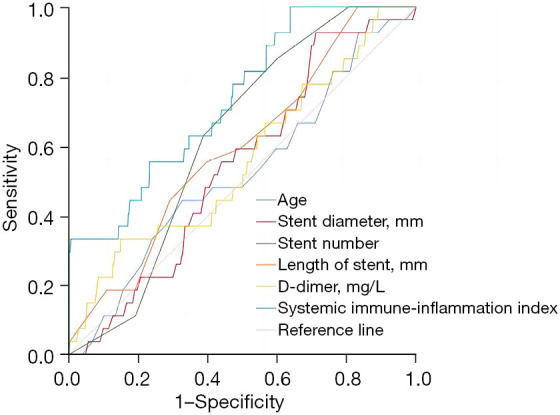
The value of different indicators in predicting stent thrombosis.
Risk factors for stent thrombosis after coronary stent implantation
The systemic immune inflammation index ≥636 and the stent number ≥4 were independent risk factors for stent thrombosis after coronary stenting (P<0.05) (Table 3).
Table 3. Risk factors for stent thrombosis after coronary artery stenting.
| Variables | B | S.E. | Wald | P | Relative risk | 95% confidence interval |
|---|---|---|---|---|---|---|
| Stent number ≥4 | 0.908 | 0.408 | 4.949 | 0.026 | 2.480 | 1.114–5.520 |
| Systemic immune inflammatory index ≥636 | 1.359 | 0.398 | 11.666 | 0.001 | 3.890 | 1.784–8.483 |
| Constant | −0.087 | 0.812 | 0.011 | 0.915 | 0.917 | – |
S.E., standard error.
Effect of stent thrombosis on patient prognosis after coronary artery stent implantation
Compared with the control group, the incidence of recurrent myocardial infarction was significantly increased in the stent thrombosis group (33.33% vs. 3.26%, P=0.000), and the mortality was markedly higher in the stent thrombosis group (14.81% vs. 0.93%, P=0.000) (Table 4).
Table 4. Effect of stent thrombosis on prognosis after coronary stent implantation.
| Category | Intra-stent thrombosis group (n=27) | Control group (n=860) | χ2 value | P value |
|---|---|---|---|---|
| Recurrent myocardial infarction, n (%) | 9 (33.33) | 28 (3.26) | 59.245 | 0.000 |
| Mortality, n (%) | 4 (14.81) | 8 (0.93) | 37.815 | 0.000 |
Discussion
Stent thrombosis is one of the most dangerous complications after coronary artery stenting, with a mortality rate of between 5% and 45%. This study showed that patients with stent thrombosis had significantly increased recurrent myocardial infarction and mortality. In recent years, a large number of scholars have studied the risk factors of various diseases to identify high-risk patients early and intervene promptly (12-15). Therefore, determining the risk factors for stent thrombosis after coronary stent implantation is very important. This present study showed that the systemic immune-inflammation index was valuable in predicting stent thrombosis, and the area under the curve was 0.736 (95% confidence interval: 0.647–0.824, P=0.000). The systemic immune-inflammation index ≥636 was an independent risk factor for intra-stent thrombosis after coronary stent implantation (P<0.05).
The systemic immune-inflammation index comprehensively measures three indicators, namely, neutrophils, lymphocytes, and platelets. An increase in the level of the systemic immune-inflammation index indicates that the level of inflammation in the patient’s body is elevated, and it has been confirmed that the systemic immune-inflammation index can better measure the level of inflammation in patients and correlates with disease severity in a variety of diseases (16-20). An increased systemic immune-inflammatory index has been observed in patients with coronary artery disease (21). The systemic immune-inflammation index has also been shown to have a good value for predicting mortality in patients with coronary artery disease (22). An increased systemic immune-inflammation index was also valuable in predicting new-onset atrial fibrillation after coronary artery bypass graft (23). Several other studies have confirmed that the systemic immune-inflammation index correlates with coronary artery disease severity (24-27). The main pathophysiology of myocardial infarction is coronary atherosclerosis, resulting in coronary artery stenosis, which results in blockage and eventually leads to myocardial infarction. Coronary stent implantation is the main means of treating such myocardial infarction patients; it can restore the blood flow of the myocardium to prevent patients from re-myocardial infarction. These patients often require antiplatelet therapy after surgery; double antiplatelet therapy is a commonly used treatment but stent thrombosis still occurs from time to time. The influencing factors of stent thrombosis include surgical methods, stent types, etc. However, inflammation and the hypercoagulability of blood are also important causes of stent thrombosis. When the systemic immune-inflammation index increases, it signifies that the patient is in an inflammatory state, and the elevated level of inflammation can promote the coagulation process, damage vascular endothelial cells, and promote thrombosis. A previous study also confirmed that an increased systemic immune-inflammation index was a risk factor for acute stent thrombosis after coronary artery stenting (28), which is consistent with our findings. However, this study differs from the previous study in that patients were followed up for 1 year after surgery and explored the effect of the systemic immune-inflammation index on stent thrombosis within 1 year.
Limitations
This was a single-center retrospectively clinical study, which is likely to cause some deviations in the results, therefore the results needs to be further confirmed by multi-center clinical trials. Due to the technical progress in recent years, the incidence of stent thrombosis is relatively low, so the number of patients with stent thrombosis included in this study is relatively small. In addition, this study failed to explore the molecular mechanism through which the systemic immune-inflammatory index leads to stent thrombosis. Moreover, we did not study the predictive value of the systemic immune-inflammation index on the main unconscionable vascular events. Finally, we failed to study the influence of timing of stent implantation on the burden of thrombus during the initial coronary stent implantation.
Conclusions
The systemic immune-inflammation index was valuable in predicting stent thrombosis in patients with myocardial infarction after coronary stent implantation. In patients with an elevated systemic immune-inflammatory index, intervention should be intensified to reduce the incidence of stent thrombosis.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Wuhan University Hospital (No. 20220042). Individual consent for this retrospective analysis was waived.
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-363/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-363/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-363/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-363/coif). The authors have no conflicts of interest to declare.
(English Language Editor: A. Kassem)
References
- 1.Zhou Y, Chen S, Huang L, et al. Definite stent thrombosis after drug-eluting stent implantation in coronary bifurcation lesions: A meta-analysis of 3,107 patients from 14 randomized trials. Catheter Cardiovasc Interv 2018;92:680-91. 10.1002/ccd.27443 [DOI] [PubMed] [Google Scholar]
- 2.Kristensen SL, Galløe AM, Thuesen L, et al. Stent thrombosis is the primary cause of ST-segment elevation myocardial infarction following coronary stent implantation: a five year follow-up of the SORT OUT II study. PLoS One 2014;9:e113399. 10.1371/journal.pone.0113399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolak A, Amit G, Cafri C, et al. Increased long term rates of stent thrombosis and mortality in patients given clopidogrel as compared to ticlopidine following coronary stent implantation. Int J Cardiol 2005;103:293-7. 10.1016/j.ijcard.2004.08.061 [DOI] [PubMed] [Google Scholar]
- 4.Honda T, Fujimoto K, Miyao Y, et al. Current cigarette smoking is an independent risk factor for subacute stent thrombosis in acute myocardial infarction patients. J Cardiol 2014;63:358-64. 10.1016/j.jjcc.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 5.Brener SJ, Cristea E, Kirtane AJ, et al. Intra-procedural stent thrombosis: a new risk factor for adverse outcomes in patients undergoing percutaneous coronary intervention for acute coronary syndromes. JACC Cardiovasc Interv 2013;6:36-43. 10.1016/j.jcin.2012.08.018 [DOI] [PubMed] [Google Scholar]
- 6.Jiang Q, Liang C, Wu Z. Myocardial bridging is a potential risk factor of very late stent thrombosis of drug eluting stent. Med Sci Monit 2012;18:HY9-12. 10.12659/MSM.882717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nogic J, Nerlekar N, Soon K, et al. Diabetes mellitus is independently associated with early stent thrombosis in patients undergoing drug eluting stent implantation: Analysis from the Victorian cardiac outcomes registry. Catheter Cardiovasc Interv 2022;99:554-62. 10.1002/ccd.29913 [DOI] [PubMed] [Google Scholar]
- 8.Zhao X, Lan J, Yu X, et al. Primary Percutaneous Coronary Intervention in Patients With Type 2 Diabetes With Late/Very Late Stent Thrombosis and de novo Lesions: A Single-Center Observational Cohort Study of Clinical Outcomes and Influencing Factors. Front Cardiovasc Med 2021;8:653467. 10.3389/fcvm.2021.653467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhary R, Kaushik A, Sharma JB. COVID-19 pandemic and stent thrombosis in a post percutaneous coronary intervention patient-a case report highlighting the selection of P2Y12 inhibitor. Cardiovasc Diagn Ther 2020;10:898-901. 10.21037/cdt-20-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Shao T, Yao L, et al. Effects of tirofiban on stent thrombosis, Hs-CRP, IL-6 and sICAM-1 after PCI of acute myocardial infarction. Exp Ther Med 2018;16:3383-8. 10.3892/etm.2018.6589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Liu K, Gao Y, et al. Prognostic value of systemic immune-inflammation index in acute/subacute patients with cerebral venous sinus thrombosis. Stroke Vasc Neurol 2020;5:368-73. 10.1136/svn-2020-000362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu Y, Chen Y, Zhu L, et al. Differences of Clinicopathological Features between Metaplastic Breast Carcinoma and Nonspecific Invasive Breast Carcinoma and Prognostic Profile of Metaplastic Breast Carcinoma. Breast J 2022;2022:2500594. 10.1155/2022/2500594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. 10.21037/atm-20-7746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi A, Li Y, Yan S, et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg 2021;10:1470-7. 10.21037/gs-21-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi A, Li Y, Sun H, et al. Incidence and risk factors of sexual dysfunction in young breast cancer survivors. Ann Palliat Med 2021;10:4428-34. 10.21037/apm-21-352 [DOI] [PubMed] [Google Scholar]
- 16.Dincer Rota D, Tanacan E. The utility of systemic-immune inflammation index for predicting the disease activation in patients with psoriasis. Int J Clin Pract 2021;75:e14101. 10.1111/ijcp.14101 [DOI] [PubMed] [Google Scholar]
- 17.Mori K, Resch I, Miura N, et al. Prognostic role of the systemic immune-inflammation index in upper tract urothelial carcinoma treated with radical nephroureterectomy: results from a large multicenter international collaboration. Cancer Immunol Immunother 2021;70:2641-50. 10.1007/s00262-021-02884-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du YN, Chen YJ, Zhang HY, et al. Inverse association between systemic immune-inflammation index and bone mineral density in postmenopausal women. Gynecol Endocrinol 2021;37:650-4. 10.1080/09513590.2021.1885642 [DOI] [PubMed] [Google Scholar]
- 19.Ju Q, Huang T, Zhang Y, et al. Systemic immune-inflammation index predicts prognosis in patients with different EGFR-mutant lung adenocarcinoma. Medicine (Baltimore) 2021;100:e24640. 10.1097/MD.0000000000024640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng Q, Li J, Sun N, et al. Preoperative systemic immune-inflammation index predicts survival and recurrence in patients with resected primary pulmonary sarcomatoid carcinoma. Transl Lung Cancer Res 2021;10:18-31. 10.21037/tlcr-20-960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esenboğa K, Kurtul A, Yamantürk YY, et al. Comparison of systemic immune-inflammation index levels in patients with isolated coronary artery ectasia versus patients with obstructive coronary artery disease and normal coronary angiogram. Scand J Clin Lab Invest 2022;82:132-7. 10.1080/00365513.2022.2034034 [DOI] [PubMed] [Google Scholar]
- 22.Borghini A, Mercuri A, Andreassi MG. Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte Ratios, and Systemic Immune-Inflammation Index as Predictors of Mortality in Coronary Artery Disease. J Cardiovasc Transl Res 2022. [Epub ahead of print]. doi: . 10.1007/s12265-022-10312-2 [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz Y, Kelesoglu S, Elcik D, et al. Predictive Values of Systemic Immune-Inflammation Index in New-Onset Atrial Fibrillation Following Coronary Artery Bypass Grafting. Braz J Cardiovasc Surg 2023;38:96-103. 10.21470/1678-9741-2021-0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Candemir M, Kiziltunç E, Nurkoç S, et al. Relationship Between Systemic Immune-Inflammation Index (SII) and the Severity of Stable Coronary Artery Disease. Angiology 2021;72:575-81. 10.1177/0003319720987743 [DOI] [PubMed] [Google Scholar]
- 25.Ata Y, Abanoz M. Predictive Roles of Right Coronary Artery Disease Severity and Systemic Immune Inflammation Index in Predicting Atrial Fibrillation After Coronary Bypass Operations in Patients with Right Coronary Artery Disease. Heart Surg Forum 2021;24:E977-82. 10.1532/hsf.4279 [DOI] [PubMed] [Google Scholar]
- 26.Yang YL, Wu CH, Hsu PF, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest 2020;50:e13230. 10.1111/eci.13230 [DOI] [PubMed] [Google Scholar]
- 27.Erdoğan M, Erdöl MA, Öztürk S, et al. Systemic immune-inflammation index is a novel marker to predict functionally significant coronary artery stenosis. Biomark Med 2020;14:1553-61. 10.2217/bmm-2020-0274 [DOI] [PubMed] [Google Scholar]
- 28.Akboga MK, Inanc IH, Sabanoglu C, et al. Systemic Immune-Inflammation Index and C-Reactive Protein/Albumin Ratio Could Predict Acute Stent Thrombosis and High SYNTAX Score in Acute Coronary Syndrome. Angiology 2022. [Epub ahead of print]. doi: . 10.1177/00033197221125779 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


