Abstract
Objective
Previous laboratory reports implicate heat shock protein (HSP)–specific T-cell responses in glaucoma pathogenesis; here, we aimed to provide direct clinical evidence by correlating systemic HSP-specific T-cell levels with glaucoma severity in patients with primary open-angle glaucoma (POAG).
Design
Cross-sectional case-control study.
Subjects
Thirty-two adult patients with POAG and 38 controls underwent blood draw and optic nerve imaging.
Methods
Peripheral blood monocytes (PBMC) were stimulated in culture with HSP27, α-crystallin, a member of the small HSP family, or HSP60. Both interferon-γ (IFN-γ)+ CD4+ T helper type 1 cells (Th1) and transforming growth factor-β1 (TGF-β1)+ CD4+ regulatory T cells (Treg) were quantified by flow cytometry and presented as a percentage of total PBMC counts. Relevant cytokines were measured using enzyme-linked immunosorbent assays. Retinal nerve fiber layer thickness (RNFLT) was measured with OCT. Pearson’s correlation (r) was used to assess correlations.
Main Outcome Measures
Correlations of HSP-specific T-cell counts, and serum levels of corresponding cytokine levels with RNFLT.
Results
Patients with POAG (visual field mean deviation, -4.7 ± 4.0 dB) and controls were similar in age, gender, and body mass index. Moreover, 46.9% of POAG and 60.0% of control subjects had prior cataract surgery (P = 0.48). Although no significant difference in total nonstimulated CD4+ Th1 or Treg cells was detected, patients with POAG exhibited significantly higher frequencies of Th1 cells specific for HSP27, α-crystallin, or HSP60 than controls (7.3 ± 7.9% vs. 2.6 ± 2.0%, P = 0.004; 5.8 ± 2.7% vs. 1.8 ± 1.3%, P < 0.001; 13.2 ± 13.3 vs. 4.3 ± 5.2, P = 0.01; respectively), but similar Treg specific for the same HSPs compared with controls (P ≥ 0.10 for all). Concordantly, the serum levels of IFN-γ were higher in POAG than in controls (36.2 ± 12.1 pg/ml vs. 10.0 ± 4.3 pg/ml; P < 0.001), but TGF-β1 levels did not differ. Average RNFLT of both eyes negatively correlated with HSP27- and α-crystallin-specific Th1 cell counts, and IFN-γ levels in all subjects after adjusting for age (partial correlation coefficient r = -0.31, P = 0.03; r = -0.52, p = 0.002; r = -0.72, P < 0.001, respectively).
Conclusions
Higher levels of HSP-specific Th1 cells are associated with thinner RNFLT in patients with POAG and control subjects. The significant inverse relationship between systemic HSP-specific Th1 cell count and RNFLT supports the role of these T cells in glaucomatous neurodegeneration.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found after the references.
Keywords: Glaucoma, Heat shock protein, Primary open-angle glaucoma, T-cell count, Retinal nerve fiber layer thickness
Systemic immune response contributes to glaucomatous neurodegeneration.1, 2, 3 Reports suggest that patients with glaucoma have elevated levels of serum autoantibodies against retinal self-antigens, such as heat shock protein 27 (HSP27), HSP60, and α-crystallin.3, 4, 5 Heat shock proteins, which usually act as intracellular protein chaperones to facilitate the proper refolding of damaged proteins,6 can trigger immune responses when they are expressed on the outer surface of cells or released extracellularly under stressful conditions.7, 8, 9, 10, 11 Prior laboratory studies demonstrate that elevated intraocular pressure (IOP) triggers upregulation of HSP27 expression on retinal ganglion cells (RGCs).1 This is associated with retinal infiltration and systemic responses of HSP27-specific T cells. Notably, RGC and axonal loss following IOP elevation occurred in immunocompetent mice, but not in T-cell-deficient mice, suggesting a critical contribution of T-cell responses to glaucomatous neurodegeneration.1 Another study also reported shifted T-cell homeostasis in patients with glaucoma compared with controls.12
The role of autoimmunity and T-cell-mediated response in glaucoma is further supported by our retrospective clinical study. We demonstrated an increased prevalence of predominantly T-cell-mediated autoimmune diseases in patients with primary open-angle glaucoma (POAG) undergoing ophthalmic surgeries compared with controls.13 Furthermore, others have provided indirect evidence by showing a thicker retinal nerve fiber layer in patients with human immunodeficiency virus and low CD4+ T-cell counts.14
In this study, we aimed to provide direct clinical evidence for the role of HSP-specific T-cell responses in glaucoma pathogenesis. Specifically, we examined peripheral blood T-cell levels in patients with POAG and healthy controls to establish a relationship between systemic levels of HSP-specific T cells and glaucomatous optic nerve damage measured by retinal nerve fiber layer thickness (RNFLT) using OCT imaging. T helper type 1 (Th1) cells are a lineage of CD4+ T cells that are often associated with inflammation and tissue injury through cell-mediated immunity.15, 16, 17 Regulatory T cells (Treg) are a subtype of CD4+ T cells that are responsible for maintaining self-tolerance and down-regulating immune response to self-antigens.18 We specifically examined HSP-specific Th1 and Treg cell counts, as well as the serum levels of relevant cytokines associated with these T cells, namely, interferon-γ (IFN- γ) for Th1 and transforming growth factor-β1 (TGF-β1) for Treg.19,20 We also conducted OCT angiography (OCTA) analysis and assessed the association of these T cells and the microvasculature of the circumpapillary region, which is reportedly compromised in POAG.21
Methods
Study Design and Study Population
This cross-sectional case-control study was approved by the Massachusetts Eye and Ear Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. We recruited patients with POAG and control subjects aged 35 to 89 years from the Glaucoma Service and the Comprehensive Ophthalmology Service, respectively, at Massachusetts Eye and Ear (Boston, Massachusetts) between August 2020 and January 2023. Written informed consent was obtained from all subjects.
The inclusion criteria for all participants were visual acuity of at least 20/40 and refractive error between -6 diopters (D) to +6D. Exclusion criteria were significant ocular disease affecting visual acuity or visual field (VF) other than glaucoma, prior ocular surgery within a month of enrollment, any autoimmune or immunodeficiency disease, malignancy, body mass index ≥ 40,22 diabetes mellitus, topical or systemic steroid use at the time of recruitment, and current smokers.23
Additional inclusion criteria for patients with POAG were: open-angles on gonioscopy, reproducible glaucomatous VF loss, and corresponding glaucomatous optic nerve damage evidenced by abnormal quadrant(s) on OCT. Patients with secondary open-angle glaucoma were excluded. Additional inclusion criteria for control subjects were: a negative family history of glaucoma, IOP measurements ≤ 21 mmHg, cup-to-disc ratio ≤ 0.6, and cup-to-disc ratio asymmetry between eyes < 0.2.
All participants underwent OCT and OCTA imaging followed by a peripheral venous blood draw. OCT imaging provided quantitative RNFLT measurements, an indicator of disease severity.24 OCT angiography measurements have been shown to be compromised in patients with POAG and may be an indicator of vascular pathology.21 Baseline demographic information was collected for all participants from the clinic visit nearest to the blood draw. A reliable Humphrey VF test (HVF, Carl Zeiss Meditec), defined as fixation losses ≤ 33% and false-positive and false-negative rates ≤ 20%,25 was used for all patients with POAG if it was dated within 12 months of the time of blood draw.
Retinal Nerve Fiber Layer and OCTA Image Acquisition and Analysis
For all participants, the optic nerve head and peripapillary region of both eyes were imaged after pharmacological pupil dilation by utilizing an SS-OCT device (Triton, Topcon) to obtain a 6 × 6–mm 3-dimensional optic disc scan and a 4.5 × 4.5–mm angiogram centered on the optic nerve head. Average peripapillary RNFLT was automatically obtained from the 3-dimensional optic disc scan. OCT angiography images were processed based on a previously published protocol to obtain the superficial circumpapillary vessel density (cpVD) measurements using customized ImageJ (Fiji) software (ImageJ, United States National Institutes of Health) plugins.21,26 OCT or OCTA scans with significant artifacts or image quality scores below 45 and 40, respectively, were excluded.27, 28, 29
T-Cell Assay
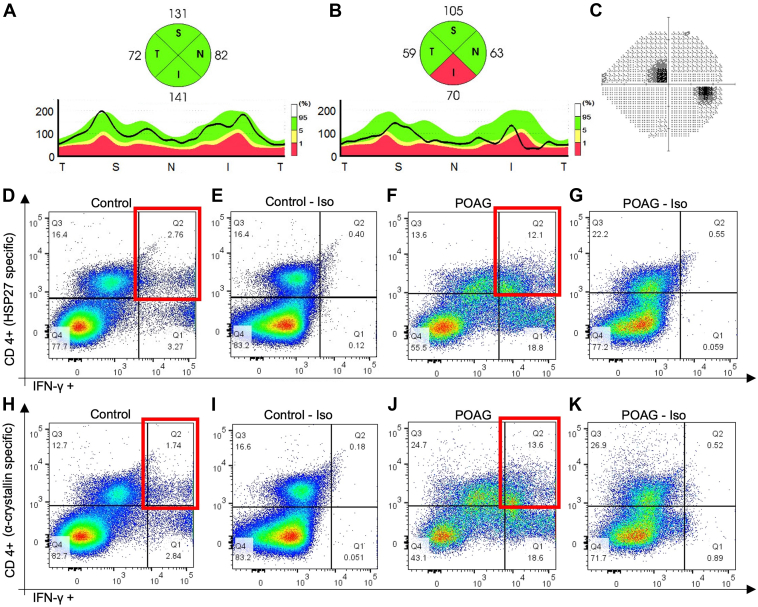
From each participant, 24 ml of venous blood was collected in 3 vacutainer cell preparation tubes with sodium citrate (Becton Dickinson Vacutainer glass cell preparation tube [CPT] molecular diagnostics tube) and processed according to the manufacturer’s instructions to isolate peripheral blood mononuclear cells (PBMCs). The PBMCs were isolated and stimulated in culture as previously described1 with minor modification as described here. In brief, cells were re-suspended in Roswell Park Memorial Institute-1640 medium containing 10% heat-inactivated fetal bovine serum. Peripheral blood mononuclear cells were incubated with or without HSP27 (10 μg/ml). Additional blood samples, when available, were randomly allocated for incubation with or without α-crystallin, a small HSP expressed prominently in the normal and pathological retina that confers stress-induced response,30 or HSP60 (10 μg/ml). After 48 hours of incubation, cells were incubated with a solution containing Brefeldin A (Biolegend) for 4 hours and processed for immunostaining. Peripheral blood mononuclear cells were double-immunolabeled for CD4 (Biolegend) and then for IFN-γ or TGF-β1 (BioLegend). Peripheral blood mononuclear cells reacted with corresponding isotype antibodies were used as controls (Isotype, Fig 1E, G, I, K). Data acquisition was performed with a NovoCyte 3000 Flow Cytometer (ACEA Biosciences, Inc) and analyzed by FlowJo (Tree Star). The percentage of CD4+ T cells positive for specific cytokine markers was assessed (Fig 1).
Figure 1.
Examples of heat shock protein (HSP)-specific helper T cells type 1 (Th1) cell measurements for control and primary open-angle glaucoma (POAG) subjects. Retinal nerve fiber layer (RNFL) thickness profile and quadrant thickness of the right eye from a control subject (A) and patient with POAG (B) are shown. The average RNFL thickness of both eyes for the control was 105 μm. The average RNFL thickness of both eyes was 70 μm for the patient with POAG and 68 μm for the inferior quadrants. On the Humphrey visual field (VF), the POAG eye shows a superior paracentral defect (C). The average VF mean deviation was -6.12 dB for both eyes of the patient with POAG. Flow cytometry plots for quantifying HSP27 specific Th1 cells (D-G) and α-crystallin–specific Th1 cells (H-K) in the peripheral blood of the control subject and patient with POAG are shown, respectively. Peripheral blood mononuclear cells were stimulated by HSP27 or α-crystallin in culture, double-immunolabeled for CD4 and interferon-gamma (IFN-γ), and assayed by flow cytometry. Isotype (Iso) reveals the background control staining in the absence of primary antibody (E, G, I, and K) for the corresponding plot shown to its left (D, F, H, and J, respectively). The percentages of Th1 cells (CD4+IFN-γ+) specific for HSP27 and for α-crystallin are obtained by subtracting the values displayed in the upper right box (Q2) of the Iso plots from the values displayed in the upper right box of the corresponding flow cytometry dot plots (highlighted in red). Therefore, for the control subject, the HSP27-specific Th1 cell frequency was 2.36%, and α-crystallin–specific Th1 cell frequency was 1.56%. For the patient with POAG, the counts were 11.55% and 13.08%, respectively.
Enzyme-Linked Immunosorbent Assay
We measured the serum levels of IFN-γ and TGF-β1, as Th1 and Treg T cell subtypes have been shown to express these cytokines, respectively.19,20 For each subject, 9 ml serum was obtained from the venous blood and stored at -80˚C until assayed. The levels of IFN-γ and TGF-β1 in serum were measured using a standard commercial enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Human IFN-γ ELISA kit, Abcam and Human latency associated peptide [TGF-β1] Quantikine ELISA Kit, B&D). The threshold sensitivity of human IFN-γ and TGF-β1 were 5 pg/ml and 1.31 pg/ml, respectively. A detailed method outlining HSP-specific antibody measurement is provided in Supplemental Appendix 1.
Primary and Secondary Outcome Measures
Primary outcome measures were as follows: (1) HSP-specific Th1 and Treg cell measurements in POAG and controls, presented as a percentage of total PBMC count, as well as the serum levels of relevant cytokines, IFN-γ for Th1 and TGF-β1 for Treg; and (2) correlation of RNFLT with the HSP-specific T-cell counts and the serum levels of related cytokines in all participants or POAG subjects only. Secondary outcome measures were as follows: (1) correlation of VF mean deviation (MD) with T-cell measurements; (2) correlation of OCTA cpVD with T-cell measurements; and (3) serum levels of HSP-specific antibodies in POAG and controls.
Statistical Analysis
Data analysis was performed using the statistical software STATA 16.1 (StataCorp LLC). The normality of continuous variables was assessed using the Shapiro-Wilk test. Continuous variables were compared with an independent sample t-test or Mann-Whitney U test, contingent on the normality of data. Tests of proportion, including the chi-square test or Fisher exact test, were used for categorical variables when appropriate. Pearson’s correlations were performed for HSP-specific peripheral blood T-cell counts or cytokine levels and OCT parameters; partial correlations were used to calculate the correlation between these variables after adjusting for age.31,32 All tests were 2-tailed, and statistical significance was determined at P < 0.05.
Results
We enrolled 32 patients with POAG and 38 control subjects. Primary open-angle glaucoma and control groups did not differ in age (68.8 ± 11.1 years vs. 70.3 ± 8.4 years; P = 0.64) and gender (62.5% vs. 39.5% male; P = 0.055; Table 1). A lower proportion of patients with POAG were White compared with controls (78.1% vs. 97.4% White; P = 0.01). Both groups had similar body mass index and a similar proportion of subjects with systemic hypertension (P ≥ 0.29). The proportion of subjects who had undergone cataract surgery in ≥ 1 eye was similar in the POAG and control groups (46.9% vs. 60.0%; P = 0.48). Phakic patients in both groups had similar average spherical equivalence (0.1 ± 2.3D in patients with POAG vs. -0.01 ± 1.8 D in controls, P = 0.85). Patients with POAG and controls had similar best corrected visual acuity (mean 20/22 vs. 20/23, 0.04 ± 0.07 logarithm of the minimum angle of resolution vs. 0.06 ± 0.08 logarithm of the minimum angle of resolution; P = 0.18). Baseline IOP was lower in POAG (13.3 ± 2.8 mmHg) than in controls (15.3 ± 2.2 mmHg; P = 0.003). Among patients with POAG, 90.6 % were being treated with IOP lowering medications; 18.7% of the patients with POAG had undergone penetrating glaucoma surgery in ≥ 1 eye, and 28.1% had undergone minimally invasive glaucoma surgery in ≥ 1 eye. Patients with POAG had increased cup-to-disc ratio versus controls (0.7 ± 0.1 vs. 0.3 ± 0.1; P < 0.001). OCT images of 2 controls and 1 POAG were excluded due to poor image quality (4.3% of all images). Therefore, 94.7% of controls and 96.9% of patients with POAG had usable OCT images. OCT angiography images of 8 controls and 4 patients with POAG were excluded due to poor image quality (15.7% of images). Therefore, 78.9% of controls and 87.5% of patients with POAG had usable OCT images. The average RNFLT of both eyes was lower for patients with POAG (76.2 ± 13.3 μm) than for controls (99.8 ± 10.0 μm; P < 0.001; Table 1). The mean HVF MD for the POAG group was -4.7 ± 4.0 dB. For OCTA, patients with POAG had lower cpVD compared with control subjects (40.0 ± 2.9% vs. 43.0 ± 2.7%; P < 0.001; Table 1).
Table 1.
Baseline Characteristics of Control Subjects and Patients with POAG
| Control (n = 38) | POAG (n = 32) | P value | |
|---|---|---|---|
| Systemic characteristics | |||
| Age (yrs) | 70.3 ± 8.4 | 68.8 ± 11.1 | 0.64 |
| Gender (% male) | 39.5 | 62.5 | 0.055 |
| Race (% White) | 97.4 | 78.1 | 0.01 |
| Body mass index (kg/m2) | 25.8 ± 4.3 | 25.7 ± 3.8 | 0.89 |
| Systemic hypertension (%) | 51.4 | 37.5 | 0.29 |
| Ophthalmic characteristics | |||
| Visual acuity (Snellen, logMAR) | 20/23, 0.06 ± 0.08 |
20/22, 0.04 ± 0.07 |
0.18 |
| Spherical equivalent (diopters)∗ | -0.01 ± 1.8 | 0.1 ± 2.3 | 0.85 |
| Prior cataract surgery in ≥ 1 eye (%) | 60.0 | 46.9 | 0.48 |
| Prior penetrating glaucoma surgery in ≥ 1 eye (%) | - | 18.7 | - |
| Prior minimally invasive glaucoma surgery in ≥ 1 eye (%) | - | 28.1 | - |
| Intraocular pressure-lowering medications | - | 2.0 ± 1.3 | - |
| Intraocular pressure (mmHg)† | 15.3 ± 2.2 | 13.3 ± 2.8 | 0.003 |
| Cup-to-disc ratio | 0.3 ± 0.1 | 0.7 ± 0.1 | < 0.001 |
| Retinal nerve fiber layer thickness (μm) | 99.8 ± 10.0 | 76.2 ± 13.3 | < 0.001 |
| HVF MD (dB) | - | -4.7 ± 4.0 | - |
| OCTA measurements‡ | |||
| cpVD (%) | 43.0 ± 2.7 | 40.0 ± 2.9 | < 0.001 |
All data are presented as mean ± standard deviation unless specified otherwise. Significant P values are in bold.
cpVD = circumpapillary vessel density; HVF = Humphrey visual field; logMAR = logarithm of the minimum angle of resolution; MD = mean deviation; OCTA = OCT angiography; POAG = primary open-angle glaucoma.
Only calculated for patients who had both eyes phakic as an average of spherical equivalent of both eyes.
Intraocular pressure was measured on the day of blood draw. All patients with POAG received treatment for intraocular pressure.
Available for 78.9% controls (n = 30) and 87.5% patients with POAG (n = 28).
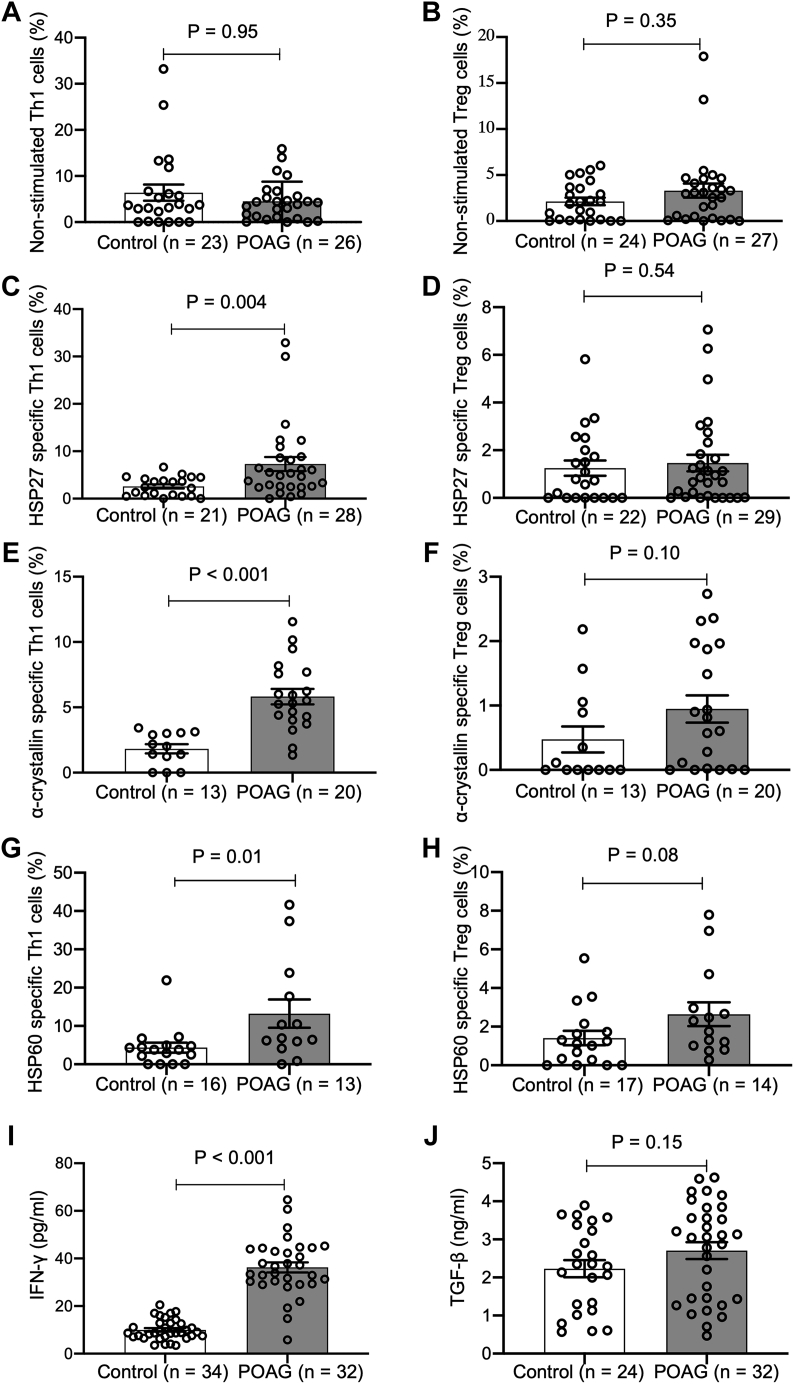
Total nonstimulated Th1 and Treg cell counts in PBMCs were similar in POAG (4.5 ± 4.3% and 3.3 ± 4.0%) and controls (6.4 ± 8.4% and 2.1 ± 2.0%; P > 0.35 for both; Table 2; Fig 2A, B). After stimulation with HSPs, CD4+ Th1 cells specific for HSP27, α-crystallin, or HSP60 were more abundant in patients with POAG than in controls (7.3 ± 7.9% vs. 2.6 ± 2.0%, P = 0.004; 5.8 ± 2.7% vs. 1.8 ± 1.3%, P < 0.001; 13.2 ± 13.3% vs. 4.3 ± 5.2%, P = 0.01; respectively; Fig 1, Fig 2C–G). In contrast, Treg cells specific for HSP27, α-crystallin, or HSP60 did not differ between POAG and controls (1.5 ± 1.9% vs. 1.2 ± 1.5%, P = 0.54; 0.9 ± 0.9% vs. 0.5 ± 0.7%, P = 0.10; 2.6 ± 2.3% vs. 1.4 ± 1.5%, P = 0.08, respectively; Fig 2D, F,H). Fewer blood samples were available for α-crystallin (n = 13 controls and n = 20 POAG for Th1 and Treg cells) and HSP60 stimulation (n = 16 controls and n = 13 POAG for Th1 cells, and n = 17 controls and n = 14 POAG for Treg cells) compared with those for HSP27 (n = 21 controls and n = 28 POAG for Th1 cells, and n = 22 controls and n = 29 POAG for Treg cells) due to insufficient number of PBMCs from some study subjects.
Table 2.
T-cell Counts and Cytokine Measurements in Patients with POAG and Control Subjects
| Controls | POAG | P value | |
|---|---|---|---|
| Nonstimulated T cell | |||
| Nonstimulated Th1 cells (%, [n = 23 controls and 26 POAG]) | 6.4 ± 8.4 | 4.5 ± 4.3 | 0.95 |
| Nonstimulated Treg cells (%, [n = 24 controls and 27 POAG]) | 2.1 ± 2.0 | 3.3 ± 4.0 | 0.35 |
| HSP27-stimulated T cell | |||
| HSP27 stimulated Th1 cells (%, [n = 21 controls and 28 POAG]) | 2.6 ± 2.0 | 7.3 ± 7.9 | 0.004 |
| HSP27 stimulated Treg cells (%, [n = 22 controls and 29 POAG]) | 1.2 ± 1.5 | 1.5 ± 1.9 | 0.54 |
| α-crystallin–stimulated T cell | |||
| α-crystallin–stimulated Th1 cells (%, [n = 13 controls and 20 POAG]) | 1.8 ± 1.3 | 5.8 ± 2.7 | < 0.001 |
| α-crystallin–stimulated Treg cells (%, [n = 13 controls and 20 POAG]) | 0.5 ± 0.7 | 0.9 ± 0.9 | 0.10 |
| HSP60-stimulated T cell | |||
| HSP60-stimulated Th1 cells (%, [n = 16 controls and 13 POAG]) | 4.3 ± 5.2 | 13.2 ± 13.3 | 0.01 |
| HSP60-stimulated Treg cells (%, [n = 17 controls and 14 POAG]) | 1.4 ± 1.5 | 2.6 ± 2.3 | 0.08 |
| Cytokine measurements | |||
| Interferon-γ (pg/ml, [n = 34 controls and 32 POAG]) | 10.0 ± 4.3 | 36.2 ± 12.1 | < 0.001 |
| TGF-β1(ng/ml, [n = 24 controls and 32 POAG]) | 2.2 ± 1.1 | 2.7 ± 1.3 | 0.15 |
All data are presented as mean ± standard deviation.
Significant P values are in bold.
HSP = heat shock protein; POAG, primary open-angle glaucoma; TGF-β1= transforming growth factor beta; Th1 cells = T helper type 1 cells; Treg cells = regulatory T cells.
Figure 2.
T-cell counts and cytokine measurements in patients with primary open-angle glaucoma (POAG) and control subjects. The average and standard deviation values are shown in Table 2. HSP = heat shock protein; Th1 = helper T cells type 1; Treg = regulatory T cells; IFN-γ = interferon-γ; TGF-β1 = transforming growth factor-β1.
Serum levels of Th1-related cytokine, IFN-γ, were significantly higher in POAG compared with controls (36.2 ± 12.1 pg/ml vs. 10.0 ± 4.3 pg/ml; P < 0.001; Table 2 and Fig 2I), whereas TGF-β1 levels did not significantly differ between the 2 groups (2.7 ± 1.3 ng/ml vs. 2.2 ± 1.1 ng/ml; P = 0.15). Given our controls and POAG populations differed by race, we also conducted a multivariable analysis to compare T-cell frequencies and cytokine levels in patients with POAG compared with controls with adjustment for race (Table S3, available at www.ophthalmologyscience.org). We obtained similar results demonstrating that patients with POAG had higher HSP27-, α-crystallin-, or HSP60-specific Th1 cells and higher serum level of IFN-γ compared with controls, even after adjusting for race (all P ≤ 0.02). Serum levels of anti-HSP antibodies, specifically anti-HSP27, anti-HSP60, and anti-α-crystallin, were similar in the POAG and control groups (all P > 0.45, Table S4).
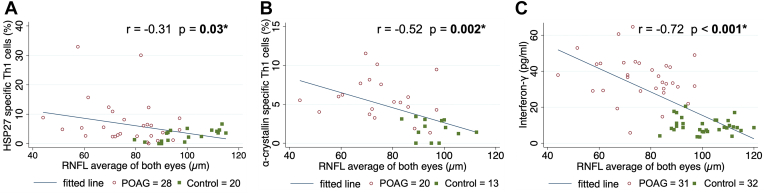
All patients with HSP-specific T-cell counts, cytokine measurements, and good-quality OCT images were used for the correlation analysis (Table 5). Average RNFLT of both eyes adjusted for age negatively correlated with HSP27- and α-crystallin–specific Th1 cell counts in all subjects (age-adjusted partial correlation coefficient r = -0.31, P = 0.03 and r = -0.52, P = 0.002; respectively; Table 5, Fig 3A,B). Similarly, serum levels of IFN-γ also negatively correlated with RNFLT in all subjects (r = -0.72; P < 0.001; Fig 3C). However, OCTA cpVD did not correlate with HSP27- and α-crystallin–specific T-cell counts in all subjects (P ≥ 0.19 for all), while IFN-γ significantly correlated with vessel density average of all subjects (r = -0.40; P = 0.002; Table S6).
Table 5.
Partial Correlation of Average RNFL Thickness of both Eyes and T-cell Counts or Cytokine Levels in all Participants and in Patients with POAG, Adjusted for Age
| All participants (POAG and controls) | Partial correlation coefficient (r) | P value |
|---|---|---|
| HSP27 stimulated Th1 cells (%, [n = 20 controls and 28 POAG]) | -0.31 | 0.03 |
| HSP27 stimulated Treg cells (%, [n = 21 controls and 29 POAG]) | -0.11 | 0.44 |
| α-crystallin–stimulated Th1 cells (%, [n = 13 controls and 20 POAG]) | -0.52 | 0.002 |
| α-crystallin–stimulated Treg cells (%, [n = 13 controls and 20 POAG]) | -0.26 | 0.14 |
| Interferon-γ (pg/ml, [n = 32 controls and 31 POAG]) | -0.72 | < 0.001 |
| Patients with POAG | Partial correlation coefficient (r) | P value |
|---|---|---|
| HSP27-stimulated Th1 cells (%, [n = 28]) | -0.19 | 0.34 |
| HSP27-stimulated Treg cells (%, [n = 29]) | -0.07 | 0.72 |
| α-crystallin–stimulated Th1 cells (%, [n = 20]) | -0.09 | 0.71 |
| α-crystallin–stimulated Treg cells (%, [n = 20]) | -0.24 | 0.31 |
| Interferon-γ (pg/ml, [n = 31]) | -0.31 | 0.09 |
Significant P values are in bold.
HSP = heat shock protein; POAG = primary open-angle glaucoma; RNFL = retinal nerve fiber layer; Th1 cells = T helper type 1 cells; Treg cells = regulatory T cells
Figure 3.
Scatter plots and correlations of average retinal nerve fiber layer (RNFL) thickness of both eyes and heat shock protein (HSP)-specific counts or cytokine levels in all participants adjusted for age. Note that the fitted line in the plot is not adjusted for age. Partial correlation coefficients (r) and corresponding P values were calculated with age adjustment. Significant P values are indicated with an asterisk and are in bold. POAG = primary open-angle glaucoma; Th1 = T helper type 1 cells.
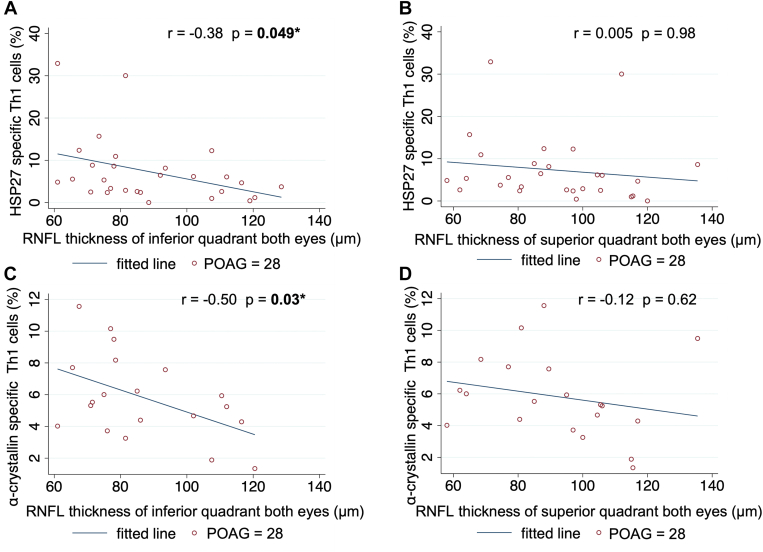
In patients with POAG, the RNFLT average of both eyes adjusted for age did not correlate with HSP-specific T-cell counts or cytokine levels (Table 5). However, average RNFLT of the inferior quadrant of both eyes significantly correlated with HSP27- and α-crystallin-specific Th1 cell counts (age-adjusted partial correlation coefficient, r = -0.38, P = 0.049 and r = -0.50, P = 0.03; respectively; Table 7, Fig 4A, C), while average RNFLT of the superior quadrant of both eyes did not (P ≥ 0.62 for both; Fig 4B, D). In patients with POAG, HSP27- and α-crystallin-specific Treg cells and IFN-γ did not correlate significantly with RNFLT of either the inferior or superior quadrant of both eyes (P ≥ 0.16). Similarly, OCTA cpVD did not correlate with T -cell counts or cytokine levels in the POAG-only group (Table S6).
Table 7.
Partial Correlation of RNFL thickness in the Superior or Inferior Quadrants of both Eyes and T-cell Counts or Cytokine Levels in Patients with POAG, Adjusted for Age
| RNFL thickness of superior quadrant | Partial correlation coefficient (r) | P value |
|---|---|---|
| HSP27-stimulated Th1 cells (%, [n = 28]) | 0.005 | 0.98 |
| HSP27-stimulated Treg cells (%, [n = 29]) | 0.02 | 0.90 |
| α-crystallin–stimulated Th1 cells (%, [n = 20]) | -0.12 | 0.62 |
| α-crystallin–stimulated Treg cells (%, [n = 20]) | -0.05 | 0.86 |
| Interferon-γ (pg/ml, [n = 31]) | -0.26 | 0.16 |
| RNFL thickness of inferior quadrant | Partial correlation coefficient (r) | P value |
|---|---|---|
| HSP27-stimulated Th1 cells (%, [n = 28]) | -0.38 | 0.049 |
| HSP27-stimulated Treg cells (%, [n = 29]) | -0.27 | 0.16 |
| α-crystallin–stimulated Th1 cells (%, [n = 20]) | -0.50 | 0.03 |
| α-crystallin–stimulated Treg cells (%, [n = 20]) | -0.14 | 0.56 |
| Interferon-γ (pg/ml, [n = 31]) | -0.25 | 0.19 |
Significant P values are in bold.
HSP = heat shock protein; POAG = primary open-angle glaucoma; RNFL = retinal nerve fiber layer; Th1 cells = T helper type 1 cells; Treg cells = regulatory T cells.
Figure 4.
Scatter plots and correlations of retinal nerve fiber layer (RNFL) thickness in the inferior or superior quadrant of both eyes and heat shock protein (HSP)27-specific T-cell counts (A, B) and α-crystallin-specific helper T-cells type 1 (Th1) cells (C, D) in patients with primary open-angle glaucoma (POAG). Note that the fitted line in the plot is not adjusted for age. Partial correlation coefficients (r) and corresponding P values were calculated with age adjustment. Significant P values are indicated with an asterisk and are in bold.
Additional analysis of patients with POAG assessed the correlation with visual function. Neither HSP27- nor α-crystallin-specific Th1 cell counts significantly correlated with the average HVF MD of both eyes (P ≥ 0.33; Fig S5A, B). However, in patients with POAG with an HVF MD average better than -12 dB, α-crystallin-specific Th1 cells were significantly inversely correlated with the HVF MD average of both eyes (r = -0.57; P = 0.02; Fig S5D). Among patients with POAG, 90.6% were being treated with 2.0 ± 1.3 IOP-lowering medications at the time of enrollment. Heat shock protein-specific Th1 cell counts and IFN-γ did not correlate with the number of medications when adjusted for HVF MD (Table S8).
Discussion
In this study, we showed that Th1 cells specific for HSP27, α-crystallin, or HSP60 were significantly more abundant in patients with POAG compared with controls, and the higher counts of HSP-specific Th1 cells were associated with thinner RNFLT in all subjects. We included the control subjects in the correlation because retinal nerve fiber layer thinning occurs as a continuum in glaucoma.33 Furthermore, in patients with POAG, the average RNFLT of the inferior quadrant significantly correlated with HSP27- and α-crystallin-specific Th1 cells. Hence, this study provides direct clinical evidence to support previous laboratory findings implicating the role of HSP-specific T cells in glaucomatous neurodegeneration in POAG. Given that POAG is a condition of multiple etiologies, many of which have not been demonstrated, providing clear and detailed information about the autoimmune etiology of this disease carries both clinical and scientific significance.
Previously, our group has shown in mouse models of glaucoma that HSP-specific Th1 cells infiltrate the neuroretina following IOP elevation; HSP-specific Th1 cells become more abundant systemically and mediate RGC and axonal loss.1 In this study, we provide direct clinical evidence that systemic HSP-specific Th1 cells are elevated in patients with POAG compared with controls, and HSP27- and α-crystallin-specific Th1 cell counts are negatively correlated with RNFLT in all participants. The analysis is adjusted for age, a factor known to affect RNFLT.31,32 In patients with POAG, we reported a significant correlation between HSP27 and α-crystallin-specific Th1 cells RNFLT of the inferior quadrant, where initial glaucomatous damage is most likely to occur.34,35 Corresponding to the structural damage we observed, we also showed that visual function measured by average VF MD significantly correlated with α-crystallin-specific Th1 cells in patients with POAG of mild-to-moderate disease severity (MD > -12dB). Additionally, we measured systemic IFN-γ levels, a cytokine expressed by Th1 cells,19,20 and showed a strong negative correlation with RNFLT in all participants. The elevated systemic levels of IFN-γ in patients with POAG support a Th1-mediated autoimmune mechanism underlying the pathogenesis of glaucoma, as IFN-γ is often considered a hallmark of the Th1 type reaction.20,36, 37, 38 We also addressed potential confounders of our results. We excluded any patients with a known diagnosis of autoimmune disease, which is more likely to develop in females than males.39 Despite a higher percentage of females in the control group, we showed that the POAG group had more abundant HSP-specific T-cells than controls. α-crystallin is a lens structural protein and can potentially lead to an increased immunogenic response after cataract surgery.40 However, in the present study, a similar proportion of control subjects and patients with POAG had undergone cataract surgery. In addition, we did not adjust our correlation analyses for axial length, due to inconsistencies in the available data for this variable. However, phakic patients in both the POAG and control groups in our study had similar average spherical equivalence. Finally, we also assessed the potential confounding effect of glaucoma medications on immune response and cytokine expression,41,42 and did not find any significant correlation between the number of glaucoma medications and T-cell counts or IFN-γ levels when adjusted for glaucoma severity. Hence, we are providing convincing clinical evidence that systemic elevation of HSP-specific Th1 cell levels inversely and directly correlates with the loss of RNFLT in POAG.
Our study can be compared with a few previous studies in patients with POAG. Yang et al12 reported that overall Th1 cell counts are not significantly higher in patients with glaucoma versus controls, but patients with glaucoma showed a trend toward the decreased frequency of CD4+/CD25+/FoxP3+ Treg cells within the entire CD4+ cell population. In the present study, we saw no difference in nonstimulated Th1 or TGFβ+ Treg cell counts of patients with POAG compared with controls. Although the results regarding nonstimulated Th1 cell counts are similar to the previous study, we did not observe an increase in Treg cells in our study. This may be explained by different subsets of Tregs evaluated by us, and the fact that CD4+/TGFβ+ Tregs in our study are not identical to CD4+/CD25+/FoxP3+ Treg cells, as the former also includes Th3 and other T cell subsets.43 Moreover, the patient cohort in Yang et al’s12 report had more severe glaucoma with an average VF MD of -10.16 dB, while the POAG group in our study had an average VF MD of -4.7 dB. We went on to show that in addition to an increase in HSP-specific Th1 cells, concurrently, IFN-γ levels were elevated in the POAG group compared with healthy controls. Similar to our findings, Yang et al12 also reported a proinflammatory shift in cytokine balance toward Th1 dominance, with a significant increase in IFN-γ levels in patients with glaucoma. In a different study by Wax et al,3 significantly higher levels of anti-HSP27, anti-HSP60, and anti-α-crystallin antibodies were reported in patients with POAG compared with controls. In their study, the mean VF MD for the POAG population was in the -8.1 to -9.0 dB range. In our prior study, we reported a less than onefold increase in serum titers for anti-HSP antibodies in patients with moderate to severe glaucoma compared with control subjects.1 In the current study, we did not see a significant difference in anti-HSP antibodies in patients with POAG compared with controls, which may be explained by milder disease severity (mean VF MD -4.7 dB) in our patients with POAG. We chose to study patients of this disease severity range as it reflects clinical practice.44
To evaluate the vascular pathophysiology of POAG45 and assess its association with the autoimmune etiology, we correlated cpVD obtained from OCTA with HSP-specific T-cell counts and cytokine levels. We excluded patients with diabetes mellitus as it may be an autoimmune disease and can affect OCTA measurements.46 Although we saw a significant decrease in cpVD in patients with POAG compared with controls, we did not detect significant correlations between cpVD and HSP-specific T-cell counts in all participants. Interferon-γ levels significantly correlated with cpVD in all participants. Prior studies have shown that IFN-γ expression is implicated in vascular pathology;47 hence, the effect of IFN-γ on the microvasculature in the eye may be separate from the autoimmune response mediated by T-cells in glaucoma. Given the lack of correlation between T-cell counts and OCTA parameters, we believe that the systemic HSP-specific T-cell response may directly lead to axonal loss of RGCs, as supported by the laboratory studies,1 and this process is independent of vascular pathology. However, studies with larger sample sizes may be required to confirm this hypothesis.
Primary open-angle glaucoma has previously been associated with T-cell-mediated autoimmune disorders such as psoriasis and rheumatoid arthritis.13 Furthermore, genetic studies have established a relationship between the human leukocyte antigen G, human leukocyte antigen H genes, and POAG.48 Human leukocyte antigen genes encode the major histocompatibility complex proteins which are responsible for immune system regulation.49 Overall, our findings, along with these prior studies, provide more evidence for an immune etiology in POAG, by specifically identifying a significant relationship between systemic HSP-specific Th1 cell counts and RNFLT loss in the eyes. In addition, we established a significant correlation between a Th1-specific cytokine, IFN-γ, with RNFLT loss in all subjects. Heat shock protein–specific Th1 cells or IFN-γ may become systemic biomarkers for assessing the inflammatory status of the patients to better characterize the patients’ glaucoma status and etiology. Interferon-γ directed therapies are already being used for other immune-mediated diseases.50,51 T-cell-mediated immune response, which is antigen or target specific, may have greater therapeutic potential with minimal side effects, especially when locally applied to the eye. Currently, all glaucoma therapies target increased IOP; however, our findings may pave the way for immune-based therapy, partly via selecting for the appropriate patient populations who are likely to respond to HSP- or T-cell-targeted immune therapies.
Our study is limited by its cross-sectional nature; thus, no temporal relation between immune pathology and glaucomatous damage can be assessed. In addition, our sample size is small, and the analysis in the POAG-only group is limited. Although a trained phlebotomist obtained blood samples, some participants had only enough sample to conduct HSP27-specific T-cell assays, indicating a need to refine our T-cell analysis techniques. This also limited our ability to analyze for T cells specific to other types of HSPs. Furthermore, some patients were excluded due to artifacts in the OCTA imaging, thus limiting our conclusions regarding vascular pathology due to the smaller sample size. Finally, the average VF MD of our POAG population was -4.7 dB, indicating that most of the patients had mild-to-moderate glaucoma, and thus our results may not be generalizable to all patients with POAG. Longitudinal studies with larger numbers of patients with POAG representative of various glaucoma severities are underway.
In conclusion, we provide direct clinical evidence to support prior laboratory reports that HSP-specific T-cell response plays a role in glaucomatous neurodegeneration by demonstrating higher levels of these cells in the peripheral blood of patients with POAG compared with controls and showing significant negative correlations of HSP-specific Th1 cells with measure of disease severity, namely RNFLT. These findings implicate the role of these HSP-specific T cells in mediating glaucomatous neurodegeneration in patients and may lead to the identification of novel treatment targets for POAG.
Acknowledgments
The authors thank Dr Michael Boland, Dr Stacey C. Brauner, Dr Sherleen Chen, Dr James Chodosh, Dr Joseph Ciolino, Dr David S. Friedman, Dr Matthew F. Gardiner, Dr Scott H. Greenstein, Dr Michael Lin, Dr Milica Margeta, Dr Courtney Ondeck, Dr Roberto Pineda, Dr David Sola-Del Valle, and Dr Silas Wang for their academic and technical contributions and the Massachusetts Eye and Ear fluorescein laboratory photographers.
Manuscript no. XOPS-D-22-00264R2.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Presented at the American Glaucoma Society Annual Meeting, March 2022, Nashville, Tennessee.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): K.S.C.: Consultant – FireCyte Therapeutics, Inc.
L.R.P.: Consultant – Skye Biosciences; Character Biosciences, Eyenovia, and Twenty Twenty
D.F.C.: Consultant – Sichuan PriMed; Advisory board – FireCyte, i-Lumen, and Biovics; Support for attending meetings and/or travel – Stanford University.
L.Q.S.: Consultant – FireCyte Therapeutics, Inc. and AbbVie Inc.
The other authors have no proprietary or commercial interest in any materials discussed in this article.
Supported by the Boston Keratoprosthesis Fund, NEI EY031696, Harvard NeuroDiscovery Center grant, and the American Glaucoma Society Mid-Career Physician Scientist Grant. K.S.C. was supported by the Bright Focus Foundation. L.R.P. is supported by NIH EY015473, an unrestricted challenge grant from Research to Prevent Blindness (NYC), and The Glaucoma Foundation (NYC). The sponsor or funding organization had no role in the design or conduct of this research.
HUMAN SUBJECTS: Human subjects were included in this study. This case-control study was approved by the Massachusetts Eye and Ear (MEE) Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. Patients with primary open-angle glaucoma and control subjects aged 35 to 89 years were recruited from the Glaucoma Service and the Comprehensive Ophthalmology Service, respectively, at MEE (Boston, Massachusetts) between August 2020 and January 2023. Written informed consent was obtained from all subjects.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Saini, Jiang, Devlin, Pan, Tang Y, Tang J, Sun, Lorenzo, Wang, Pasquale, Cho, Chen, Shen
Data collection: Saini, Jiang, Devlin, Pan, Tang Y, Tang J, Sun, Lorenzo, Wang, Pasquale, Cho, Chen, Shen
Analysis and interpretation: Saini, Jiang, Devlin, Pan, Tang Y, Tang J, Sun, Lorenzo, Wang, Pasquale, Cho, Chen, Shen
Obtained funding: Cho, Chen, Shen
Overall responsibility: Saini, Jiang, Devlin, Pan, Tang Y, Tang J, Sun, Lorenzo, Wang, Pasquale, Cho, Chen, Shen
Supplementary Data
References
- 1.Chen H., Cho K.S., Vu T.H.K., et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat Commun. 2018;9 doi: 10.1038/s41467-018-05681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tezel G., Hernandez M.R., Wax M.B. Immunostaining of heat shock proteins in the retina and optic nerve head of normal and glaucomatous eyes. Arch Opthalmol. 2000;118:511–518. doi: 10.1001/archopht.118.4.511. [DOI] [PubMed] [Google Scholar]
- 3.Wax M.B., Tezel G., Kawase K., Kitazawa Y. Serum autoantibodies to heat shock proteins in glaucoma patients from Japan and the United States. Ophthalmology. 2001;108:296–302. doi: 10.1016/s0161-6420(00)00525-x. [DOI] [PubMed] [Google Scholar]
- 4.Tezel G., Seigel G.M., Wax M.B. Autoantibodies to small heat shock proteins in glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2277–2287. [PubMed] [Google Scholar]
- 5.Wax M.B., Tezel G., Saito I., et al. Anti-Ro/SS-A positivity and heat shock protein antibodies in patients with normal-pressure glaucoma. Am J Ophthalmol. 1998;125:145–157. doi: 10.1016/s0002-9394(99)80084-1. [DOI] [PubMed] [Google Scholar]
- 6.Jakob U., Gaestel M., Engel K., Buchner J. Small heat shock proteins are molecular chaperones. Journal of Biological Chemistry. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 7.van Eden W., Jansen M.A.A., Ludwig I., et al. The enigma of heat shock proteins in immune tolerance. Front Immunol. 2017;8:1599. doi: 10.3389/fimmu.2017.01599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dulin E., García-Barreno P., Guisasola M.C. Extracellular heat shock protein 70 (HSPA1A) and classical vascular risk factors in a general population. Cell Stress Chaperones. 2010;15:929–937. doi: 10.1007/s12192-010-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stangl S., Gehrmann M., Riegger J., et al. Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc Natl Acad Sci U S A. 2011;108:733–738. doi: 10.1073/pnas.1016065108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broere F., van der Zee R., van Eden W. Heat shock proteins are no DAMPs, rather “DAMPERs. Nat Rev Immunol. 2011;11 doi: 10.1038/nri2873-c1. [DOI] [PubMed] [Google Scholar]
- 11.Chen W., Syldath U., Bellmann K., et al. Human 60-kDa heat-shock protein: A danger signal to the innate immune system. J Immunol. 1999;162:3212–3219. [PubMed] [Google Scholar]
- 12.Yang X., Zeng Q., Göktaş E., et al. T-Lymphocyte subset distribution and activity in patients with glaucoma. Invest Ophthalmol Vis Sci. 2019;60:877–888. doi: 10.1167/iovs.18-26129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenzo M.M., Devlin J., Saini C., et al. The prevalence of autoimmune diseases in patients with primary open-angle glaucoma undergoing opthalmic surgeries. Ophthalmol Glaucoma. 2022;5:128–136. doi: 10.1016/j.ogla.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Tassel S.H., Petrakos P., Marlow E., et al. Retinal nerve fiber layer changes based on historic CD4 nadir among HIV positive patients undergoing glaucoma evaluation. Int J Ophthalmol. 2019;12:789–794. doi: 10.18240/ijo.2019.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann T.R., Coffman R.L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 17.Tada T., Takemori T., Okumura K., et al. Two distinct types of helper T cells involved in the secondary antibody response: independent and synergistic effects of Ia- and Ia+ helper T cells. J Exp Med. 1978;147:446–458. doi: 10.1084/jem.147.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaguchi S., Sakaguchi N., Asano M., et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 19.Mosmann T.R., Cherwinski H., Bond M.W., et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 20.Raphael I., Nalawade S., Eagar T.N., Forsthuber T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74:5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nascimento E Silva R., Chiou C.A., Wang M., et al. Microvasculature of the optic nerve head and peripapillary region in patients with primarily open-angle glaucoma. J Glaucoma. 2019;28:281–288. doi: 10.1097/IJG.0000000000001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park C.S., Shastri N. The role of T cells in obesity-associated inflammation and metabolic disease. Immune Netw. 2022;22:e13. doi: 10.4110/in.2022.22.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagiwara E., Takahashi K.I., Okubo T., et al. Cigarette smoking depletes cells spontaneously secreting Th(1) cytokines in the human airway. Cytokine. 2001;14:121–126. doi: 10.1006/cyto.2001.0860. [DOI] [PubMed] [Google Scholar]
- 24.Strouthidis N.G., Grimm J., Williams G.A., et al. A comparison of optic nerve head morphology viewed by spectral domain optical coherence tomography and by serial histology. Invest Opthalmol Vis Sci. 2010;51:1464–1474. doi: 10.1167/iovs.09-3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bickler-Bluth M., Trick G.L., Kolker A.E., Cooper D.G. Assessing the utility of reliability indices for automated visual fields: testing ocular hypertensives. Ophthalmology. 1989;96:616–619. doi: 10.1016/s0161-6420(89)32840-5. [DOI] [PubMed] [Google Scholar]
- 26.Shoji M.K., Cousins C.C., Saini C., et al. Paired optic nerve microvasculature and nailfold capillary measurements in primary open-angle glaucoma. Transl Vis Sci Technol. 2021;10:1–13. doi: 10.1167/tvst.10.7.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamalipour A., Moghimi S., Hou H., et al. OCT Angiography artifacts in glaucoma. Ophthalmology. 2021;128:1426–1437. doi: 10.1016/j.ophtha.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Z., Tang F., Wong R., et al. OCT Angiography metrics predict progression of diabetic retinopathy and development of diabetic macular edema. Ophthalmology. 2019;126:1675–1684. doi: 10.1016/j.ophtha.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Lee S.Y., Kwon H.J., Bae H.W., et al. Frequency, type and cause of artifacts in swept-source and cirrus HD optical coherence tomography in cases of glaucoma and suspected glaucoma. Curr Eye Res. 2016;41:957–964. doi: 10.3109/02713683.2015.1075219. [DOI] [PubMed] [Google Scholar]
- 30.Liu J.P., Schlosser R., Ma W.Y., et al. Human αA- and αB-crystallins prevent UVA-induced apoptosis through regulation of PKCα, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004;79:393–403. doi: 10.1016/j.exer.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Budenz D.L., Anderson D.R., Varma R., et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007;114:1046–1052. doi: 10.1016/j.ophtha.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riza A., Celebi C., Mirza G.E., Cel-Ebi C. Age-Related change in retinal nerve fiber layer thickness measured with spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:8095–8103. doi: 10.1167/iovs.13-12634. [DOI] [PubMed] [Google Scholar]
- 33.Bourne R.R.A., Medeiros F.A., Bowd C., et al. Comparability of retinal nerve fiber layer thickness measurements of optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2005;46:1280–1285. doi: 10.1167/iovs.04-1000. [DOI] [PubMed] [Google Scholar]
- 34.Gardiner S.K., Kinast R.M., Chen T.C., et al. Clinicians’ use of quantitative information when assessing the rate of structural progression in glaucoma. Ophthalmol Glaucoma. 2022;5:507–515. doi: 10.1016/j.ogla.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatham A.J., Weinreb R.N., Zangwill L.M., et al. Estimated retinal ganglion cell counts in glaucomatous eyes with localized retinal nerve fiber layer defects. Am J Ophthalmol. 2013;156:578–587. doi: 10.1016/j.ajo.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preble O.T., Black R.J., Friedman R.M., et al. Systemic lupus erythematosus: presence in human serum of an unusual acid-labile leukocyte interferon. Science. 1982;216:429–431. doi: 10.1126/science.6176024. [DOI] [PubMed] [Google Scholar]
- 37.Barrat F.J., Crow M.K., Ivashkiv L.B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat Immunol. 2019;20:1574–1583. doi: 10.1038/s41590-019-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Billiau A., Heremans H., Vermeire K., Matthys P. Immunomodulatory properties of interferon-γ: an update. Ann N Y Acad Sci. 1998;856:22–32. doi: 10.1111/j.1749-6632.1998.tb08309.x. [DOI] [PubMed] [Google Scholar]
- 39.Invernizzi P., Pasini S., Selmi C., Gershwin M.E., Podda M. Female predominance and X chromosome defects in autoimmune diseases. J Autoimmun. 2009;33:12–16. doi: 10.1016/j.jaut.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohammed I., Kulkarni B., Faraj L.A., et al. Profiling ocular surface responses to preserved and non-preserved topical glaucoma medications: A 2-year randomized evaluation study. Clin Exp Ophthalmol. 2020;48:973–982. doi: 10.1111/ceo.13814. [DOI] [PubMed] [Google Scholar]
- 42.Sherwood M.B., Grierson I., Milgar L., Hitchings R.A. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon’s capsule in glaucomatous patients. Ophthalmology. 1989;96:327–335. doi: 10.1016/s0161-6420(89)32888-0. [DOI] [PubMed] [Google Scholar]
- 43.Sumitomo S., Fujio K., Okamura T., et al. Transcription factor early growth response 3 is associated with the TGF-β1 expression and the regulatory activity of CD4-positive T cells in vivo. J Immunol. 2013;191:2351–2359. doi: 10.4049/jimmunol.1202106. [DOI] [PubMed] [Google Scholar]
- 44.Wang M., Shen L.Q., Pasquale L.R., et al. An artificial intelligence approach to detect visual field progression in glaucoma based on spatial pattern analysis. Invest Ophthalmol Vis Sci. 2019;60:365–375. doi: 10.1167/iovs.18-25568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L., Jia Y., Takusagawa H.L., et al. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol. 2015;133:1045–1052. doi: 10.1001/jamaophthalmol.2015.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan M., Wang W., Kang S., et al. Peripapillary microvasculature predicts the incidence and development of diabetic retinopathy: an SS-OCTA study. Am J Ophthalmol. 2022;243:19–27. doi: 10.1016/j.ajo.2022.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Langer V., Vivi E., Regensburger D., et al. IFN-γ drives inflammatory bowel disease pathogenesis through VE-cadherin-directed vascular barrier disruption. J Clin Invest. 2019;129:4691–4707. doi: 10.1172/JCI124884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gharahkhani P., Jorgenson E., Hysi P., et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat Commun. 2021;12:1258. doi: 10.1038/s41467-020-20851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trowsdale J., Knight J.C. Major Histocompatibility complex genomics and human disease. Annu Rev Genomics Hum Genet. 2013;14:301–323. doi: 10.1146/annurev-genom-091212-153455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Locatelli F., Jordan M.B., Allen C., et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. 2020;382:1811–1822. doi: 10.1056/NEJMoa1911326. [DOI] [PubMed] [Google Scholar]
- 51.Skurkovich S., Skurkovich B. Anticytokine therapy, especially anti-interferon-gamma, as a pathogenetic treatment in TH-1 autoimmune diseases. Ann N Y Acad Sci. 2005;1051:684–700. doi: 10.1196/annals.1361.113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.