Abstract
People living with HIV (PLWH) are a vulnerable patient population due to their immunosuppressed state and the risks associated with interruptions in treatment. After the unprecedented start of the COVID-19 pandemic, PLWH experienced complications involving interruptions in care and treatment, potentially leading to adverse outcomes including reduced rates of viral suppression, increased hospitalizations, and death. A systematic, comprehensive literature search was completed using PubMed, Google Scholar, and bibliography review to identify relevant articles related to clinical outcomes of HIV and SARS-CoV-2 co-infection. Related keywords were used as search terms: “COVID”, “SARS-CoV-2”, “coronavirus”, “HIV”, “viral load”, “viral suppression”, and “disease severity”. Of the 492 results, 7 systematic reviews and 14 individual studies were included in the current review of literature regarding COVID-19-related outcomes in PLWH. In total, 2 systematic reviews and 8 individual studies found an increased rate of mortality, hospitalizations, and/or severe COVID-19 outcomes in PLWH co-infected with SARS-CoV-2, whereas the other 5 systematic reviews and 6 individual studies concluded PLWH were not at an increased risk compared to patients without HIV. Regarding viral suppression, 4 of 5 studies found viral suppression in PLWH was not impacted by the COVID-19 pandemic. The current literature suggests that the morbidity and mortality associated with SARS-CoV-2 infection in PLWH is complex and involves multiple factors including age and comorbid conditions; however, there is no clear consensus thus far. In contrast, literature consistently demonstrates that viral suppression during the pandemic has remained unchanged, potentially due to increased implementation of telemedicine and multicomponent interventions deployed.
Keywords: PLWH, HIV, COVID-19, SARS-CoV-2, Mortality, Viral Suppression
Introduction
The global prevalence of Human Immunodeficiency Virus (HIV) diagnosis was estimated to be 38 million at the end of 2019 [1]. With the unprecedented start of the COVID-19 pandemic, first declared in Wuhan, China in December 2019, people living with HIV (PLWH) reported difficulties in accessing care, interruption in routine testing and treatment, and economic consequences early on [2, 3]. PLWH are an especially vulnerable population due to risks associated with disruptions in antiretroviral therapy (ART) and complications such as increased substance use and symptoms of depression and anxiety [4]. Achieving viral suppression preserves the health of PLWH and prevents transmission. Delays or interruptions in treatment can cause the development of drug resistance rendering certain regimens or components of a regimen to be ineffective and limiting future treatment options. As the pandemic has progressed, the question of whether PLWH are at an increased risk for morbidity and mortality when co-infected with severe acute respiratory syndrome-related coronavirus (SARS-CoV-2) has been debated. Further, several studies have explored whether the pandemic has impacted viral suppression in PLWH. The objective of this manuscript was to review the most current literature evaluating the morbidity and mortality risk associated with SARS-CoV-2 infection in PLWH and the impact the COVID-19 pandemic had on viral suppression.
Methods
A systematic, comprehensive literature search was performed using PubMed, Google Scholar, and bibliography review to identify relevant articles related to clinical outcomes of HIV and SARS-CoV-2 co-infection. For literature regarding morbidity and mortality, the search included systematic reviews published between December 1, 2019 and April 16, 2022 and articles published between January 1, 2021 and April 16, 2022. Individual articles were not reviewed prior to 2021 since the oldest systematic review included studies published through January 2021. For literature regarding viral suppression, the search included individual articles published between December 1, 2019 and April 16, 2022. Related keywords were used as search terms: “COVID”, “SARS-CoV-2”, “coronavirus”, “HIV”, “viral load”, “viral suppression”, and “disease severity.” Articles were excluded if they included any persons under the age of 18 years. Only articles in English were included in this study. Due to the large difference in HIV prevalence and management in other countries, individual articles with a geographical region of the United States or Europe were included. However, systematic reviews or meta analyses included evaluated multinational outcomes.
Results
Study Selection
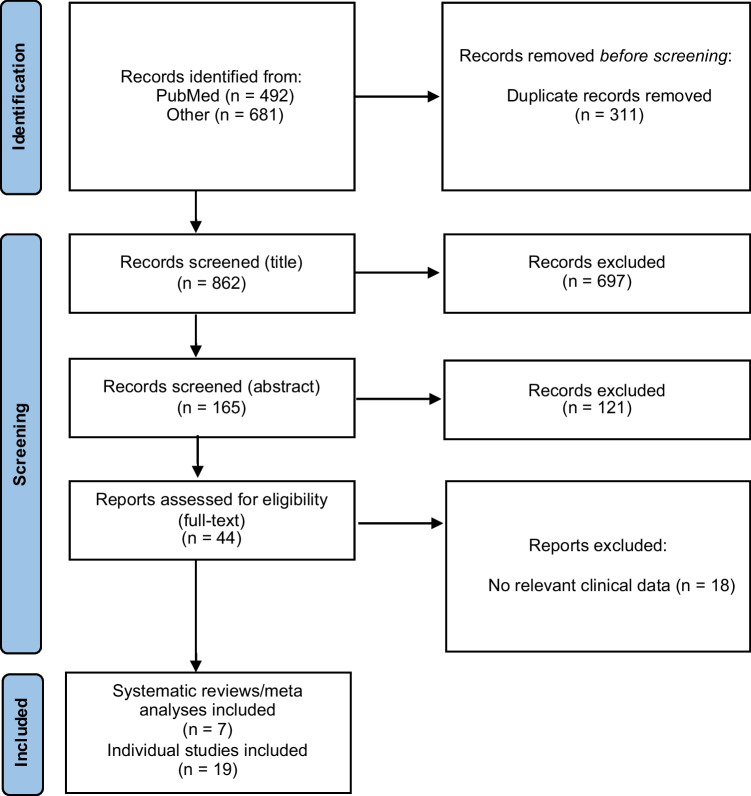
Figure 1 describes the identification process for the inclusion of studies [5]. The systematic search of databases generated an initial total of 1,173 studies. After removing 311 duplicates, 862 remained for initial title review. Initial title and abstract review led to the exclusion of 818 studies. Full-text screening resulted in the exclusion of 18 additional studies due to clinical irrelevance (i.e. did not provide clinically relevant results pertaining to COVID-19-related morbidity or mortality in PLWH or viral suppression during the pandemic). Thus, a total of 26 studies, 7 systematic reviews or meta analyses and 19 individual studies, were included in the current review.
Fig. 1.
Identification of studies included
Mortality and Morbidity
Table 1 reviews studies evaluating morbidity and mortality associated with COVID-19 infection in PLWH. Since the start of the pandemic, various reviews have sought to examine co-infection of HIV and SARS-CoV-2 and their related factors [6, 7, 8, 9, 10, 11, 12]. One such study reviewed 18 case series and reports from China including 76 cases of SARS-CoV-2/HIV co-infection which found earlier use of ART to be associated with improved COVID-19 prognosis [6]. Another review of 36 studies including nearly 4 million patients with COVID-19, in which 89,343 were co-infected with HIV, reported that in most of the included studies, all PLWH completely recovered [7]. Authors also reported that patients with stage 3 (advanced HIV disease or CD4 count between 200 and 349 cells/mm3) or 4 (Acquired Immunodeficiency Syndrome (AIDS) where the CD4 count is < 200 cells/mm3), or with low CD4 counts (i.e., < 350 cells/mm3) showed less severe symptoms and lower mortality than patients with less severe disease, possibly due to an inability to provoke the cytokine storm that causes severe COVID-19. Additionally, another study including 63 reports of co-infection, indicated a favorable prognosis for PLWH who remained adherent to ART [8]. Though, patients with more comorbidities (i.e., diabetes, hypertension, chronic obstructive pulmonary disease, etc.) had a poorer prognosis, despite ART and viral suppression. Both of these early reviews had limitations including limited statistical reporting, lack of CD4 counts or viral loads reported, and small sample sizes.
Table 1.
Studies evaluating morbidity and mortality associated with COVID-19 infection in PLWH
| Authors, Publication Date | Timeframe | Design/Methods | Outcomes | Conclusions |
|---|---|---|---|---|
| Systematic Reviews/Meta Analyses | ||||
| Huang D, 2022[6] | 12/1/2019—12/10/2021 |
Literature review of PubMed, Web of Sciences, and 3 Chinese databases (Wanfang, CNKI, and SinoMed) 18 studies (case series/reports, China) 76 cases of SARS-CoV-2/HIV co-infection (69 in Wuhan) |
Late antiretroviral therapy (ART) was reported by 30.4% (7/23) among whom 57.1% (4/7) were confirmed as severe COVID-19 Case fatality rate was 9.1% (3/33) Severe disease and death were less common among persons who took ART prior to the COVID-19 diagnosis |
Earlier use of ART was likely associated with a better COVID- 19 prognosis with SARS-CoV-2/HIV co-infection reported from China through 2021 |
| Oyelade T, 2022[11] | Pandemic start—7/2/2021 |
Literature review of Embase, MEDLINE 43 studies (including 8 US, 10 Europe, 9 Africa) 692,032 COVID-19 cases (9,097 PLWH) |
Mortality: RR 1.5 (95% CI, 1.45–2.03), significant globally Severe COVID-19: RR 1.14, (95% CI, 1.05–1.24), significant only in Africa |
Although there is a low prevalence of PLWH among COVID-19 cases, HIV infection may increase the severity of COVID-19 in Africa and increase the risk of death globally |
| Danwang C, 2022[12] | Pandemic start—10/25/21 |
Literature review of PubMed, Embase, Web of Science, Scopus 44 studies (28 cross-sectional, 8 cross-sectional analyses of cohort, 3 case series, 5 case control), only studies published in peer reviewed journals 38,971,065 COVID-19 cases (26.9% PLWH) |
Mortality: unadjusted OR 0.81 (95% CI, 0.47–1.41, 23 studies); adjusted for age/sex HR 1.76 (95% CI, 1.31–2.35, 2 studies) Hospital admission: OR 1.49 (95% CI, 1.01–2.21) Severe COVID-19: OR 1.28 (95% CI, 0.77–2.13) |
PLWH have an increased risk of hospital admission for COVID-19. HIV seems to be independently associated with increased risk of mortality in COVID-19 patients in adjusted analysis; however, this evidence was derived from only two studies |
| Liang M, 2021[10] | Pandemic start—3/9/21 |
Literature review of PubMed, Embase, BioRXiv, medRxiv 14 studies (9 cohort, 5 case series) 203,761 COVID-19 cases (7,718 PLWH) |
Mortality: RR 0.96 (95% CI, 0.88–1.06) Comorbidities associated with increased mortality in PLWH infected with COVID-19: - DM: RR 5.2 (95% CI, 4.25–6.36) - HTN and chronic cardiac disease: RR 4.2 (95% CI, 1.09–16.10) - CKD: RR 8.42 (95% CI, 5.49–12.93) |
Comorbidities such as CKD, DM, and HTN and chronic cardiac disease are responsible for poor outcomes in PLWH co-infected with COVID-19 |
| Wang Y, 2021[9] | 12/15/19—5/12/21 |
Literature review of Embase, PubMed, Web of Science 84 studies (46 Americas, 25 Europe, 3 Asia, 5 Africa, 5 other) 816,678 COVID-19 cases |
Mortality: pooled RR 1.23 (95% CI, 1.02–1.48) | HIV infection was significantly associated with an increased risk for COVID-19 mortality, which might be modulated by age, regions and study design |
| Patel RH, 2021[8] | 12/2019—1/22/21 |
Literature review of PubMed, MEDLINE, manual search 63 reports of co-infection (28 case reports, 45 case series) |
Case reports: 18/28 reports with favorable outcomes (mild symptoms, recovery), 6/28 (moderate symptoms, recovery), (severe symptoms, recovery), 1 death Case series: no statistics reported |
Despite scattered evidence, reports indicate a favorable prognosis for HIV patients with strict adherence to ART. However, the presence of comorbidities, such as HTN, respiratory disease, CVD, DM, and CKD, was associated with a poorer prognosis in HIV/SARS-CoV-2 patients, despite ART and viral suppression |
| SeyedAhmad S, 2021[7] |
Literature review of PubMed, Scopus, Science Direct, Web of Science 36 studies (including 40% USA, 20% China, 11.4% Italy, 11.4% Spain) 3,993,400 COVID-19 cases (89,343 PLWH) |
In 8/36 studies, mortality was reported ranging from 1 to 36% | In majority of the studies, all HIV patients completely recovered from the COVID‐19 infection. PLWH stage 3 or 4 with low CD4 count/weak immune systems showed less severe symptoms and less mortality, possibly due to inability of HIV patients’ immune systems to provoke the cytokine storm that causes severe COVID-19 | |
| Individual Studies—HIV status Did Not Impact COVID-19-related Outcomes | ||||
| Rial-Crestelo D, 2022[13] | Pandemic start—2/28/21 |
Observational, single-center study (Spain) 2344 PLWH (158 HIV/SARS-CoV-2 co-infection) |
Age was the only independent factor for moderate-severe disease: OR 1.09 (95% CI, 1.04–1.14; p < 0.001) Virologic control was not impacted pre-and post-COVID-19 infection (p = 0.16) |
In PLWH with COVID-19 infection, most with good immunovirological control of the HIV infection, a lower percentage of patients presenting moderate or severe disease, need for mechanical ventilation, and death was observed compared with previous studies. Age was the only variable with an independent association with moderate-severe COVID-19, after adjusting by comorbidities and other factors |
| Durstenfeld MS, 2022[14] | 3/2020—12/2020 |
Prospective cohort study (US) 21,528 patients (220 PLWH) |
In-hospital mortality: RR 1.06 (95% CI, 0.79–1.43, p = 0.71) MACE: aOR 0.99 (95% CI, 0.69–1.44, p = 0.91) COVID-19 severity: aOR 0.96 (95% CI 0.62–1.50, p = 0.86) LOS: aOR 1.03 (95% CI 0.76–1.66, p = 0.21) |
HIV positive status is not associated with an increase in in-hospital mortality, MACE, or severity of illness |
| Verburgh ML, 2021[15] | 2/27/2020—4/30/2021 |
Prospective, observational, cohort study (Amsterdam) 568 adult patients (241 PLWH) |
COVID-19 incidence: p = 0.61 | PLWH with suppressed viremia and adequate CD4 cell counts had similar risk of SARS-CoV-2 acquisition and similar SARS-CoV-2 N antibody levels after infection compared with a comparable HIV-negative cohort |
| Diez C, 2021[16] | Until 6/2020 |
Retrospective matched cohort study (Spain) 10,922 PLWH (45 with HIV/SARS-CoV-2 co-infection) |
Well-controlled HIV doesn't worsen clinical outcomes including presenting signs/symptoms, laboratory parameters, radiology findings, severity scores, or death (p = 0.8) in hospitalized patients with COVID-19 Hospitalized PLWH were older (p = 0.001), had arterial hypertension (p = 0.007) or chronic lung disease excluding asthma (p = 0.025) |
Well-controlled HIV infection does not modify the clinical presentation or worsen clinical outcomes of COVID-19 hospitalization |
| Friedman EE, 2021[17] | 4/10/2020—9/30/2020 |
Cross sectional study (US) 69,763 total patients, 431 PLWH (31 HIV/SARS-CoV-2 co-infection) |
PLWH were not significantly more likely SARS-CoV-2 than people without HIV—7.2% (31/431) vs. 8.4% (5820/69763), p = 0.35 PLWH more likely to be younger, Black, and male (p < 0.0001) |
PLWH appear to have similar rates of SARS-CoV-2 percent positivity as HIV-negative people within the general population, and there were no obvious risk factors among PLWH that increase their chances of testing positive for SARS-CoV-2 in this sample |
| Calza L, 2021[18] | 3/1/2020—6/30/2020 |
Retrospective Analysis (Italy) 31 HIV/SARS-CoV-2 co-infected patients (9 uncontrolled HIV) |
No statistics—descriptive No patients were admitted to the ICU or required mechanical ventilation, and all subjects recovered after a median of 9 days |
Uncontrolled HIV infection did not seem to be associated with greater severity or worse SARS-CoV-2 infection outcomes |
| Individual Studies—HIV Status Impacted COVID-19-related Outcomes | ||||
| Sun J, 2022[19] | 12/10/2020—9/16/2021 [observation ended 10/14/2021] |
Retrospective cohort study (US) 664,722 patients (8,536 PLWH) |
Breakthrough infection post-vaccination: aIRR 1.33 (95% CI, 1.18–1.49); higher in PLWH | Despite full vaccination, persons with immune dysfunction, such as PLWH, had substantially higher risk for COVID-19 breakthrough infection than those without such a condition |
| Nomah DK, 2021[20] | 3/1/2020—12/15/2020 |
Retrospective cohort study (Spain) 13,142 PLWH (749 HIV/SARS-CoV-2 co-infection) |
CD4 count < 200 cells/μL was significantly associated with severe COVID-19 outcomes (p = 0.0010) Detectable HIV RNA viral load was also associated with severe COVID-19 outcomes (p = 0.029) |
PLWH with detectable HIV viraemia, chronic comorbidities, and some subpopulations could be at increased risk of severe outcomes from COVID-19 |
| Dandachi D, 2021[21] | 4/1/2020—7/1/2020 |
Multicenter Analysis (US) 286 patients with HIV/SARS-CoV-2 co-infection |
Higher age (p < 0.01), lower CD4 count (p = 0.02), CKD (p < 0.01), and chronic lung disease (p < 0.01) predicted likelihood of hospitalization | Severe clinical outcomes occurred commonly in PLWH with COVID-19. The risks for poor outcomes were higher in those with comorbidities and lower CD4 cell counts, despite HIV viral suppression |
| Geretti AM, 2021[22] | 1/17/2020—6/4/2020 |
Prospective, observational study (UK) 47,592 patients (122 PLWH) |
Cumulative 28-day mortality: p = 0.16 < 60 co-infe/o PLWH increased risk of mortality (p < 0.001) Mortality is higher in PLWH after adjusted for age: adjusted HR 1.47 (95% CI, 1.01–2.14, p = 0.05) Restricted analysis to only < 60 years: aHR 2.87 (95% CI 1.70–4.84, p < 0.001) |
Patients hospitalized with COVID-19 were at an increased risk of day-28 mortality due to HIV-positive status |
| Yendewa GA, 2021[23] | 1/1/2020- 2/1/2020 |
Retrospective study (US) 297,194 patients (1,638 PLWH) |
Mortality: p = 0.123 Hospitalization: p < 0.001 ICU admissions p < 0.005 |
PLWH had higher rates of poor COVID-19 outcomes but were not more at risk of death than their non-HIV-infected counterparts. Older age and low CD4 count predicted adverse outcomes |
| Nasreddine R, 2021[24] | 2/15/2020—5/31/2020 |
Retrospective, multicenter, cohort (Belgium) 101 patients co-infected with HIV/COVID-19 |
Factors associated with hospitalization: -- Age ≥ 50 years: OR 7.46 (95% CI 1.94–28.78; p = 0.004) -- Black Sub-Saharan African ethnicity: OR 5.89 (95% CI 1.39–25.05; p = 0.016) -- Having an INSTI-based ART regimen: OR 6.72 (95% CI 1.83–24.66; p = 0.004) |
PLWH with COVID‐19 experienced a high degree of hospitalization despite having elevated CD4 + cell counts and a high rate of virologic suppression |
| Patel VV, 2021[25] | 3/10/2020—5/11/2020 |
Retrospective cohort study (US) 4613 patients (100 PLWH) |
In-hospital death: p = 0.73 Hospitalization: p = 0.92 Intubation: HR 1.73 (95% CI 1.12–2.67; p = 0.01) |
PLWH had increased risk of intubation but no difference in in-hospital death as those without HIV |
| Bhaskaran K, 2021[26] | until 2/1/2020 |
Retrospective Cohort study (UK) 17,282,905 adults (27,480 PLWH) |
COVID-19 death: HR 2.90 (95% CI 1.96–4.30; p < 0.0001) | People with HIV in the UK seem to be at increased risk of COVID-19 mortality |
Abbreviations: aOR = adjusted odds ratio; CI = confidence interval; CVD = cardiovascular disease; CKD = chronic kidney disease; DM = diabetes mellitus; HIV = human immunodeficiency virus; HR = hazard ratio; HTN = hypertension; MACE = major adverse cardiac event; LOS = length of stay; OR = odds ratio; PLWH = people living with HIV; RR = risk ratio
A meta-analysis examined 84 studies and reported a pooled relative risk [RR] of mortality of 1.23 (95% CI, 1.02–1.48) when comparing PLWH to patients without HIV [9]. However, the analysis did not include data to address whether ART impacted the results. A systematic review and meta-analysis published shortly after included 14 additional studies and reported a RR of mortality of 0.96 (95% CI, 0.88–1.06) for PLWH [10]. Similarly, authors identified an increased risk of mortality in patients diagnosed with COVID-19 and co-infected with HIV if comorbidities such as diabetes, hypertension, or chronic kidney disease were present. These reviews were limited in that most studies included unmatched populations. Thus, comparisons between PLWH and patients without HIV must be interpreted with caution.
When assessing the global impact of COVID-19 in PLWH, the most recent systematic reviews and meta-analyses included 43 studies of 692,032 COVID-19 positive cases (9,097 in PLWH) and reported a [RR] of mortality as 1.5 (95% CI, 1.45–2.03) when comparing PLWH to non-PLWH [11]. Though the studies included geographic regions outside of the United States and Europe, the increased risk of mortality was significant globally. Authors also reported an increased risk of severe COVID-19 (RR 1.14, 95% CI, 1.05–1.24), but the risk was only significant in Africa. The other review included 44 studies and reported an unadjusted odds of mortality of 0.81 (95% CI, 0.47–1.41) in 23 studies when comparing PLWH to non-PLWH [12]. After adjusting for age and sex, the hazard ratio was 1.76 (95% CI, 1.31–2.35). Authors also reported an increased risk for hospital admission (OR 1.49, 95% CI, 1.01–2.21). There was no significant difference in COVID-19 severity (OR 1.28, 95% CI, 0.77–2.13). These reviews also had limitations in that there was a high between-study heterogeneity and there was no stratification of analysis according to ART regimen or CD4 count.
Of fourteen individual studies identified, the aim was to determine morbidity and mortality associated with HIV and COVID-19 co-infection [13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26]. Several studies of co-infected patients found PLWH were at an increased risk of severe COVID-19 outcomes, including hospitalization and ICU admission [19, 20, 21, 22, 23, 24, 25, 26]. Further, patients with CD4 cell counts less than 200 cells/mm3 and non-suppressed RNA viral load were at an increased risk of severe COVID-19 outcomes, including hospital admission or death (p = 0.039) [20].
In regard to vaccination status, a retrospective cohort study published in February 2022 highlighted the difference in breakthrough infection risks in vaccinated patients with and without HIV [19]. Authors concluded that PLWH had a higher rate of breakthrough infection post-vaccination compared to HIV-negative patients (aIRR 1.33, 95% CI, 1.18–1.4). The study also included patients with other disease states known to cause immune dysfunction such as rheumatoid arthritis and solid organ transplant. The overall conclusion was that despite receiving vaccination, those with these immunocompromising conditions experienced a higher rate of COVID-19 breakthrough infections.
On the contrary, there were several studies that found HIV status did not impact COVID-19 outcomes [13, 14, 15, 16, 17, 18]. In an observational study conducted in Spain published in April 2022, authors concluded that age, not HIV status, was the only factor that was associated with moderate-severe COVID-19 [13]. Of the 2,344 PLWH, 158 were co-infected with COVID-19. The severity of COVID-19 was more pronounced in patients who were older (median age 56.5 versus 43.8 years) (p < 0.001), had a longer duration of HIV infection (p = 0.006), and a history of hepatitis C (p = 0.001). In a recent prospective cohort study, HIV-positive status was not associated with mortality (p = 0.71), major adverse cardiac events (p = 0.91), COVID-19 severity (p = 0.86), or length of hospital stay (p = 1.0) [14]. Though COVID-19-related outcomes were not impacted by HIV-status, authors found PLWH who were hospitalized for COVID-19 were older (p = 0.001) and more likely to have hypertension (p = 0.007) or chronic lung disease (p = 0.025).
One prospective, observational study found 28-day mortality to be similar between PLWH and HIV-negative patients co-infected with COVID-19 (p = 0.16) [22]. However, interestingly, when statistical analysis was restricted to patients younger than 60 years of age, there was an increase in 28-day mortality (aHR of 2.87, 95% CI 1.70–4.84). An additional study examining 30-day mortality concluded that PLWH did not experience increased COVID-19 30-day mortality risk compared to non-PLWH with COVID-19 (p = 0.123) [23].
Viral Suppression
Currently, there is limited data available to answer the question of whether the COVID-19 pandemic has impacted viral suppression in PLWH. The pandemic was associated with interference of accessing HIV care, including the attainment of necessary laboratory values (i.e., HIV RNA) to assess maintenance of viral suppression. In the current review, 5 studies were identified that aimed to describe the impact [27, 28, 29, 30, 31]. Studies are described in Table 2.
Table 2.
Studies evaluating viral suppression in PLWH during the COVID-19 pandemic
| Authors, Publication Date | Timeframe | Design/Methods | Outcomes | Conclusions |
|---|---|---|---|---|
| Spinelli MA, 2022[27] | 24 months before SIP vs. 13 months after SIP |
Mixed-effects logistic regression followed by ITS analysis comparing before and after SIP Multi-component strategies employed during SIP (proactive outreach, expansion of housing programs, etc.) 1,816 PLWH receiving care at HIV clinic in San Francisco, California |
Rate of VS: adjusted OR 1.34 (95%, CI 1.21–1.46) | The VS rate increased following the institution of the multicomponent strategies during SIP. Maintaining in-person care for underserved patients, with flexible telemedicine options, along with provision of social services and permanent expansion of housing programs, will be needed to support VS among underserved populations during the COVID-19 pandemic |
| Giacomelli A, 2021[28] | 1/1/2016—2/20/2020 (before) vs. 2/21/2020—12/31/2020 (COVID-19 period) |
Quasi-experimental, ITS analysis 70,349 viral load determinations during study period (patient overlap since comparing years) HIV outpatient clinic in Milan, Italy |
HIV VL of < 50 copies/mL increased from 88.4% (2016) to 93.2% (2020) Significant trend toward decrease in VL ≥ 50 copies/mL before pandemic (p < 0.001), and this did not significantly change after pandemic began (p = 0.811) |
A high prevalence of viral suppression was maintained among the PLWH, despite the structural barriers raised by the COVID-19 pandemic, potentially due to simplified methods of delivering care (teleconsultations and multiple antiretroviral treatment prescriptions) |
| Hickey MD, 2021[29] | 10/17/2019—3/16/2020 (pre-SIP) vs. 3/17/2020—8/16/2020 (post-SIP) |
ITS analysis comparing care engagement (clinic visits per month), VS, and retention-in-care before and after SIP 85 patients receiving care at Ward 86 (large HIV clinic in San Francisco, California) |
VS: OR 1.19 (95% CI, 0.78–1.82) Proportion of patients with visits each month: OR 0.66 (95% CI, 0.39–1.11) |
Care engagement and VS did not decrease in the five months following implementation of SIP; however, in-person HIV care for homeless individuals may be important for maintaining HIV outcomes during COVID-19 |
| Spinelli MA, 2020[30] | 12/1/2019—2/29/2020 (pre-SIP) vs. 4/1/2020—4/30/2020 (post-SIP) |
ITS analysis comparing retention-in-care and VS before and after SIP Ward 86 (large HIV clinic in San Francisco, California); 16% of patients included homeless |
Viral non-suppression: aOR 1.31 (95% CI, 1.08–1.53) Viral non-suppression in homeless individuals during pandemic: aOR 3.36 (95% CI, 2.74–4.12) |
The odds of viral non-suppression were higher post-SIP, in spite of stable retention-in-care and visit volume, with disproportionate impact on homeless individuals |
| Calza L, 2020[31] | 3/1/2020—4/15/2020 |
Observational, prospective study of 14 adult PLWH (all on stable ART, 13/14 with HIV RNA < 50 copies/mL) with SARS-CoV-2 co-infection Bologna, Italy |
Median changes (IQR) in CD4 + T-lymphocyte count: p = 0.149 IQR in CD8 + T-lymphocyte count: p = 0.469 IQR in CD4/CD8 ratio: p = 0.818 Patients with plasma HIV RNA < 50 copies/mL were 13 (93%), so no patients had virological failure |
COVID-19 did not produce a significant effect on immunological status and plasma HIV viral load after a median follow-up of 8 weeks in 14 PLWH on stable ART |
Abbreviations: aOR = adjusted odds ratio; ART = antiretroviral therapy; CI = confidence interval; HIV = human immunodeficiency virus; IQR = interquartile range; ITS = interrupted time series; OR = odds ratio; PLWH = people living with HIV; SIP = shelter-in-place; VL = viral load; VS = viral suppression
A small, observational, prospective study conducted in Bologna, Italy, examined 14 adult PLWH on stable ART who were co-infected with SARS-CoV-2 between March 1, 2020 and April 15, 2020 [31]. Authors concluded that COVID-19 infection did not produce a significant effect on immunologic status or plasma HIV viral load after 8 weeks.
Authors working in a large, urban HIV clinic in San Francisco conducted two separate analyses to examine the impact of the pandemic on viral suppression. The first was an interrupted time series analysis examining viral suppression and retention-in-care before and after telemedicine was instituted in response to shelter-in-place (SIP) mandates [30]. The adjusted odds of non-viral suppression, defined as viral load ≥ 200 copies/mL, after telemedicine was instituted was 1.31 (95% CI, 1.08–1.53). Viral non-suppression was also found to be higher in the 16% of homeless individuals, regardless of when telemedicine was instituted. Authors concluded that measures to counteract the effect of the pandemic on HIV outcomes were urgently needed. The second study was another interrupted time series examining care engagement (defined as 1 clinic visit per month) and viral suppression and retention-in-care before and after telemedicine was implemented [29]. However, authors extended the time period to 5 months before and after telemedicine implementation rather than 1–2 months. In a total of 85 patients, there was not a significant difference in viral suppression or the proportion of patients with clinic visits each month before and after telemedicine was implemented. Results drew attention to the need for alternative methods to in-person HIV care in the setting of the COVID-19 pandemic.
In a quasi-experimental study designed to examine the impact of the pandemic on viral suppression in PLWH at a large HIV clinic in Italy, authors also conducted an interrupted time series analysis [28]. The percentage of viral loads < 50 copies/mL was compared before (January 1, 2016 to February 20, 2020) and after (February 21, 2020 to December 31, 2020) the pandemic. HIV viral loads < 50 copies/mL increased from 88.4% before the pandemic to 93.2% after the pandemic. There was also a significant decrease in the number of patients who had a viral load ≥ 50 copies/mL (p < 0.0001), which did not change significantly after the pandemic started (p = 0.811). Authors concluded a high prevalence of viral suppression was maintained, despite the COVID-19 pandemic.
Finally, in another interrupted time series analysis, viral suppression 24 months before SIP was compared to 13 months after [27]. The rate of viral suppression increased from 81.4% at the beginning of SIP to 89.8% after multi-component strategies, including proactive patient outreach and expansion of housing programs, were implemented as a result of the pandemic (aOR 1.34, 95%, CI 1.21–1.46). This recent study highlighted the importance of interdisciplinary collaboration to support underserved patient populations and maintain improvement in clinical outcomes during public health crises.
Discussion
Despite the multitude of published studies, there was no association established between COVID-19 and HIV. Although there seems to be more studies indicating increased morbidity and mortality associated with SARS-CoV-2 infection in PLWH, the studies that published the contrary cannot be disregarded. There is likely more than the HIV positivity that determines a patient’s risk. Many of the earlier studies evaluated comorbidities in addition to HIV status as a determining factor for negative COVID-19 outcomes. Additionally, PLWH have a multitude of other factors that may impact how they respond to an infection with other viruses, such as SARS-CoV-2. One such factor is the type of ART they are taking. Much of the available literature fails to stratify patients based on the type of ART regimen taken, which is an important factor to consider when drawing conclusions regarding patients’ response to COVID-19. Another important factor is CD4 count. Some studies began to explore this factor, but there was no concrete data that CD4 count directly impacted a patient's risk for morbidity or mortality after infection with COVID-19. The few studies that acknowledged CD4 counts concluded that lower CD4 counts led to an increase in COVID-19-related hospital or ICU admission or death [20, 21]. As CD4 counts directly reflect immune function in PLWH, this is an area where more evidence is needed. It is possible the inconsistency in evidence was related to differing ART and rates of viral suppression in PLWH included in the studies.
While existing data is uncertain for how HIV status impacts morbidity and mortality associated with co-infection with SARS-CoV-2, studies examining how the pandemic impacted viral suppression reported similar findings. The earliest study suggested COVID-19 infection had no significant impact on HIV viral suppression [31]. Further, authors working at a large clinic managing HIV in an urban setting found that after telemedicine was implemented for eligible, stably housed PLWH, there was no significant difference in viral suppression [29]. Two additional studies had results that confirmed previous findings [27, 28]. Since previous literature suggested the pandemic negatively impacted patients’ access to care and medications, it is imperative to question why PLWH did not experience significant reductions in viral suppression. During the pandemic, telehealth became crucial in bridging the gap for management of PLWH allowing continued access to comprehensive care. Furthermore, the institution of multicomponent strategies including proactive outreach for linkage to social services and programs targeted toward the needs of PLWH with unstable or unpredictable housing was associated with increases in viral suppression [27]. Perhaps implementation of telehealth, in combination with continued in-person visits for specific patients such as those who are homeless or unstably housed, and avenues for increased collaboration between healthcare practitioners during this time allowed for optimal patient care of PLWH to continue, despite the pandemic. Alternatively, it is possible that viral suppression was, in fact, reduced in PLWH during the pandemic, but long-term data is not yet available.
The current review has several limitations, including overlapping data. Articles published during the window of time included in the systemic reviews and meta-analyses were included. However, this was minimized by excluding studies published prior to January 2021. Systematic reviews that included studies published outside of the United States and Europe were also included in order to produce the most comprehensive review. However, this limits generalizability since regional differences affect prevalence, prevention techniques, and management of HIV. An additional consideration is that a large majority of studies included patients through early 2021, prior to the time period when COVID-19 vaccination was widely available and administered. It is unknown whether studies that found an increased risk in COVID-19-related morbidity or mortality would have found the same results if conducted in a later time period when a majority of patients were vaccinated.
This study adds to the available body of evidence and includes the most recent literature examining the outcomes of COVID-19 infection in PLWH and viral suppression during the pandemic. The risk of morbidity and mortality associated with COVID-19 infection in PLWH is impacted by additional factors, such as age and comorbid conditions [9, 13, 14, 22, 25]. ART and HIV viral suppression potentially play a role as well. Future studies controlling for these factors need to be performed, especially since newer ART regimens have higher genetic barriers to resistance and may reduce the development of AIDS, and thus, immunosuppression which can impact COVID-19 associated morbidity and mortality. An additional area lacking data was the rate of breakthrough infections and associated morbidity and mortality following infection with SARS-CoV-2 in PLWH that have been vaccinated. More studies on this topic would provide valuable information regarding what steps must be taken to prevent severe complications in PLWH.
Conclusion
Conflicting literature on the morbidity and mortality of COVID-19 in PLWH suggests a more complex interplay between the viruses, where factors such as age and other comorbidities have more of an impact on outcomes than HIV. In contrast, literature regarding viral suppression in PLWH during the pandemic consistently demonstrated a high prevalence of viral suppression was maintained among PLWH, despite the COVID-19 pandemic, which may have been due, in part, to the implementation of telehealth and other interdisciplinary and multicomponent interventions deployed during this time of uncertainty. The long-term effects of COVID-19 remain unknown in PLWH along with those who are newly diagnosed. Additional studies should focus on long-term morbidity, mortality, and viral suppression in these patients.
Authors' Contributions
• Hali Hanson: Design, review, writing, and final review.
• Eunice Kim: Design, review, writing, and final review
• Melissa Badowski: Design, review, writing, and final review
Data Availability
None.
Code Availability
None.
Declarations
Ethics Approval
None.
Consent to Participate
None.
Consent for Publication
None.
Conflicts of Interest
None.
Footnotes
This article is part of the Topical Collection on COVID-19
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.HIV Statistics Center. Centers for Disease Control and Prevention. Published 2019. https://www.cdc.gov/hiv/statistics/overview/index.html#:~:text=Worldwide%2C%20there%20were%20about%201.7,AIDS%2Drelated%20illnesses%20in%202019. Accessed 6 Apr 2022.
- 2.Santos GM, Ackerman B, Rao A, et al. Economic, mental health, HIV prevention and HIV treatment impacts of COVID-19 and the COVID-19 response on a global sample of cisgender gay men and other men who have sex with men. AIDS Behav. 2021;25(2):311–321. doi: 10.1007/s10461-020-02969-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown LB, Spinelli MA, Gandhi M. The interplay between HIV and COVID-19: summary of the data and responses to date. Curr Opin HIV AIDS. 2021;16(1):63–73. doi: 10.1097/COH.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooley SA, Nelson B, Doyle J, Rosenow A, Ances BM. Collateral damage: Impact of SARS-CoV-2 pandemic in people living with HIV. J Neurovirol. 2021;27(1):168–170. doi: 10.1007/s13365-020-00928-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021;372.10.1136/bmj.n160. [DOI] [PMC free article] [PubMed]
- 6.Huang D, Zunong J, Li M, et al. COVID-19 Clinical Presentation Among HIV-Infected Persons in China: A Systematic Review. Curr HIV/AIDS Rep. 2022;19(3):167–176. doi: 10.1007/s11904-022-00606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SeyedAlinaghi S, Karimi A, MohsseniPour M, et al. The clinical outcomes of COVID-19 in HIV-positive patients: a systematic review of current evidence. Immun Inflamm Dis. 2021;9(4):1160–1185. doi: 10.1002/iid3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel RH, Acharya A, Chand HS, Mohan M, Byrareddy SN. Human immunodeficiency virus and severe acute respiratory syndrome coronavirus 2 coinfection: a systematic review of the literature and challenges. AIDS Res Hum Retroviruses. 2021;37(4):266–282. doi: 10.1089/aid.2020.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Feng R, Xu J, Shi L, Feng H, Yang H. An updated meta-analysis on the association between HIV infection and COVID-19 mortality. AIDS. 2021;35(11):1875–1878. doi: 10.1097/QAD.0000000000002968. [DOI] [PubMed] [Google Scholar]
- 10.Liang M, Luo N, Chen M, et al. Prevalence and mortality due to COVID-19 in HIV co-infected population: a systematic review and meta-analysis. Infect Dis Ther. 2021;10(3):1267–1285. doi: 10.1007/s40121-021-00447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oyelade T, Alqahtani JS, Hjazi AM, Li A, Kamila A, Raya RP. Global and regional prevalence and outcomes of COVID-19 in people living with HIV: a systematic review and meta-analysis. TropicalMed. 2022;7(2):22. doi: 10.3390/tropicalmed7020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danwang C, Noubiap JJ, Robert A, Yombi JC. Outcomes of patients with HIV and COVID-19 co-infection: a systematic review and meta-analysis. AIDS Res Ther. 2022;19(1):3. doi: 10.1186/s12981-021-00427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rial-Crestelo D, Bisbal O, Font R, et al. Incidence and severity of SARS-CoV-2 infection in HIV-infected individuals during the first year of the pandemic. JAIDS J Acquir Immune Defic Syndr. 2022;89(5):511–518. doi: 10.1097/QAI.0000000000002896. [DOI] [PubMed] [Google Scholar]
- 14.Durstenfeld MS, Sun K, Ma Y, et al. Association of HIV infection with outcomes among adults hospitalized with COVID-19. AIDS. 2022;36(3):391–398. doi: 10.1097/QAD.0000000000003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verburgh ML, Boyd A, Wit FWNM, et al. Similar Risk of Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Similar Nucleocapsid Antibody Levels in People With Well-Controlled Human Immunodeficiency Virus (HIV) and a Comparable Cohort of People Without HIV. J Infect Dis. 2021:jiab616. 10.1093/infdis/jiab616 [DOI] [PMC free article] [PubMed]
- 16.Díez C, Del Romero-Raposo J, Mican R, et al. COVID-19 in hospitalized HIV-positive and HIV-negative patients: a matched study. HIV Med. 2021;22(9):867–876. doi: 10.1111/hiv.13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman EE, Devlin SA, McNulty MC, Ridgway JP. SARS-CoV-2 percent positivity and risk factors among people with HIV at an urban academic medical center. Landay A, ed. PLoS ONE. 2021;16(7):e0254994. doi: 10.1371/journal.pone.0254994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calza L, Bon I, Borderi M, et al. COVID-19 outcomes in patients with uncontrolled HIV-1 infection. JAIDS J Acquir Immune Defic Syndr. 2021;86(1):e15–e17. doi: 10.1097/QAI.0000000000002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Zheng Q, Madhira V, et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 2022;182(2):153. doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomah DK, Reyes-Urueña J, Díaz Y, et al. Sociodemographic, clinical, and immunological factors associated with SARS-CoV-2 diagnosis and severe COVID-19 outcomes in people living with HIV: a retrospective cohort study. The Lancet HIV. 2021;8(11):e701–e710. doi: 10.1016/S2352-3018(21)00240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dandachi D, Geiger G, Montgomery MW, et al. Characteristics, comorbidities, and outcomes in a multicenter registry of patients with human immunodeficiency virus and coronavirus disease 2019. Clin Infect Dis. 2021;73(7):e1964–e1972. doi: 10.1093/cid/ciaa1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geretti AM, Stockdale AJ, Kelly SH, et al. Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) clinical characterization protocol (UK): a prospective observational study. Clin Infect Dis. 2021;73(7):e2095–e2106. doi: 10.1093/cid/ciaa1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yendewa GA, Perez JA, Schlick K, Tribout H, McComsey GA. Clinical features and outcomes of coronavirus disease 2019 among people with human immunodeficiency virus in the United States: a multicenter study from a large global health research network (TriNetX). Open Forum Infect Dis. 2021;8(7):ofab272. 10.1093/ofid/ofab272 [DOI] [PMC free article] [PubMed]
- 24.Nasreddine R, Florence E, Moutschen M, et al. Clinical characteristics and outcomes of COVID-19 in people living with HIV in Belgium: a multicenter, retrospective cohort. J Med Virol. 2021;93(5):2971–2978. doi: 10.1002/jmv.26828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel VV, Felsen UR, Fisher M, et al. Clinical outcomes and inflammatory markers by HIV serostatus and viral suppression in a large cohort of patients hospitalized with COVID-19. JAIDS J Acquir Immune Defic Syndr. 2021;86(2):224–230. doi: 10.1097/QAI.0000000000002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. The Lancet HIV. 2021;8(1):e24–e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spinelli MA, Le Tourneau N, Glidden DV, et al. Impact of Multicomponent Support Strategies on Human Immunodeficiency Virus Virologic Suppression Rates During Coronavirus Disease 2019: An Interrupted Time Series Analysis. Clin Infect Dis. 2022:ciac179. 10.1093/cid/ciac179 [DOI] [PMC free article] [PubMed]
- 28.Giacomelli A, Bonazzetti C, Conti F, et al. Impact of the COVID-19 pandemic on virological suppression in people living with HIV attending a large Italian HIV clinic. J Acquir Immune Defic Syndr. 2021;88(3):6. doi: 10.1097/QAI.0000000000002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hickey MD, Imbert E, Glidden DV, et al. Viral suppression during COVID-19 among people with HIV experiencing homelessness in a low-barrier clinic-based program. AIDS. 2021;35(3):517–519. doi: 10.1097/QAD.0000000000002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spinelli MA, Hickey MD, Glidden DV, et al. Viral suppression rates in a safety-net HIV clinic in San Francisco destabilized during COVID-19. AIDS. 2020;34(15):2328–2331. doi: 10.1097/QAD.0000000000002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calza L, Bon I, Borderi M, Colangeli V, Viale P. No significant effect of COVID-19 on immunological and virological parameters in patients with HIV-1 infection. JAIDS J Acquir Immune Defic Syndr. 2020;85(1):e6–e8. doi: 10.1097/QAI.0000000000002427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.
None.