Abstract
Background
Evidence from systematic reviews of observational studies suggests that hormone therapy may have beneficial effects in reducing the incidence of cardiovascular disease events in post‐menopausal women, however the results of randomised controlled trials (RCTs) have had mixed results. This is an updated version of a Cochrane review published in 2013.
Objectives
To assess the effects of hormone therapy for the prevention of cardiovascular disease in post‐menopausal women, and whether there are differential effects between use in primary or secondary prevention. Secondary aims were to undertake exploratory analyses to (i) assess the impact of time since menopause that treatment was commenced (≥ 10 years versus < 10 years), and where these data were not available, use age of trial participants at baseline as a proxy (≥ 60 years of age versus < 60 years of age); and (ii) assess the effects of length of time on treatment.
Search methods
We searched the following databases on 25 February 2014: Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE and LILACS. We also searched research and trials registers, and conducted reference checking of relevant studies and related systematic reviews to identify additional studies.
Selection criteria
RCTs of women comparing orally administered hormone therapy with placebo or a no treatment control, with a minimum of six months follow‐up.
Data collection and analysis
Two authors independently assessed study quality and extracted data. We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for each outcome. We combined results using random effects meta‐analyses, and undertook further analyses to assess the effects of treatment as primary or secondary prevention, and whether treatment was commenced more than or less than 10 years after menopause.
Main results
We identified six new trials through this update. Therefore the review includes 19 trials with a total of 40,410 post‐menopausal women. On the whole, study quality was good and generally at low risk of bias; the findings are dominated by the three largest trials. We found high quality evidence that hormone therapy in both primary and secondary prevention conferred no protective effects for all‐cause mortality, cardiovascular death, non‐fatal myocardial infarction, angina, or revascularisation. However, there was an increased risk of stroke in those in the hormone therapy arm for combined primary and secondary prevention (RR 1.24, 95% CI 1.10 to 1.41). Venous thromboembolic events were increased (RR 1.92, 95% CI 1.36 to 2.69), as were pulmonary emboli (RR 1.81, 95% CI 1.32 to 2.48) on hormone therapy relative to placebo.
The absolute risk increase for stroke was 6 per 1000 women (number needed to treat for an additional harmful outcome (NNTH) = 165; mean length of follow‐up: 4.21 years (range: 2.0 to 7.1)); for venous thromboembolism 8 per 1000 women (NNTH = 118; mean length of follow‐up: 5.95 years (range: 1.0 to 7.1)); and for pulmonary embolism 4 per 1000 (NNTH = 242; mean length of follow‐up: 3.13 years (range: 1.0 to 7.1)).
We performed subgroup analyses according to when treatment was started in relation to the menopause. Those who started hormone therapy less than 10 years after the menopause had lower mortality (RR 0.70, 95% CI 0.52 to 0.95, moderate quality evidence) and coronary heart disease (composite of death from cardiovascular causes and non‐fatal myocardial infarction) (RR 0.52, 95% CI 0.29 to 0.96; moderate quality evidence), though they were still at increased risk of venous thromboembolism (RR 1.74, 95% CI 1.11 to 2.73, high quality evidence) compared to placebo or no treatment. There was no strong evidence of effect on risk of stroke in this group. In those who started treatment more than 10 years after the menopause there was high quality evidence that it had little effect on death or coronary heart disease between groups but there was an increased risk of stroke (RR 1.21, 95% CI 1.06 to 1.38, high quality evidence) and venous thromboembolism (RR 1.96, 95% CI 1.37 to 2.80, high quality evidence).
Authors' conclusions
Our review findings provide strong evidence that treatment with hormone therapy in post‐menopausal women overall, for either primary or secondary prevention of cardiovascular disease events has little if any benefit and causes an increase in the risk of stroke and venous thromboembolic events.
Keywords: Adult; Aged; Aged, 80 and over; Female; Humans; Middle Aged; Cardiovascular Diseases; Cardiovascular Diseases/mortality; Cardiovascular Diseases/prevention & control; Cause of Death; Estrogen Replacement Therapy; Estrogen Replacement Therapy/adverse effects; Estrogen Replacement Therapy/methods; Hormone Replacement Therapy; Hormone Replacement Therapy/adverse effects; Hormone Replacement Therapy/methods; Postmenopause; Primary Prevention; Secondary Prevention; Stroke; Stroke/chemically induced; Venous Thromboembolism; Venous Thromboembolism/chemically induced
Plain language summary
Hormone therapy for preventing cardiovascular disease in both healthy post‐menopausal women and post‐menopausal women with pre‐existing cardiovascular disease
Hormone therapy is used for controlling menopausal symptoms. It has also been used for the prevention of cardiovascular disease in post‐menopausal women. The present review assessed the effects of using hormone therapy for six months or more. Nineteen randomised controlled trials (involving 40,410 women) compared oral hormone therapy (oestrogen, with or without progestogen) with placebo. Most participants were from the Unites States (US), and the mean age in most studies was over 60 years. The length of time women were on treatment varied across the trials from 7 months to 10.1 years. The studies were generally well conducted with overall low risk of bias. Overall, results showed no evidence that hormone therapy provides any protective effects against death from any cause, death specifically from cardiovascular disease, non‐fatal heart attack or angina, either in healthy women or women with pre‐existing heart disease. Rather, in post‐menopausal women hormone therapy increased the risk of stroke and obstruction of a vein by a blood clot (venous thromboembolism).
We are confident that the results of are review are close to the true effects for most of the outcomes we looked at. The studies were large, well‐designed and the results were generally consistent across the studies.
Summary of findings
Summary of findings for the main comparison. Hormone therapy compared to placebo for primary prevention of cardiovascular disease in post‐menopausal women.
| Hormone therapy compared to placebo for primary prevention of cardiovascular disease in post‐menopausal women | ||||||
| Patient or population: Post‐menopausal women without prior cardiovascular disease Intervention: Hormone therapy Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Hormone therapy | |||||
| Death (all‐causes) | Study population | RR 1 (0.89 to 1.12) | 34,422 (8 studies) | ⊕⊕⊕⊕ high | ||
| 32 per 1000 | 32 per 1000 (29 to 36) | |||||
| Death (cardiovascular causes) | Study population | RR 0.81 (0.47 to 1.40) | 28,353 (3 studies) | ⊕⊕⊕⊕ high | ||
| 8 per 1000 | 7 per 1000 (4 to 11) | |||||
| Stroke | Study population | RR 1.32 (1.12 to 1.56) | 28,719 (4 studies) | ⊕⊕⊕⊕ high | ||
| 18 per 1000 | 23 per 1000 (20 to 28) | |||||
| Venous thromboembolism | Study population | RR 1.92 (1.24 to 2.99) | 33,477 (6 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 10 per 1000 | 20 per 1000 (13 to 31) | |||||
| Pulmonary embolism | Study population | See comment | 31,732 (3 studies) | ⊕⊕⊕⊝ moderate1 | Risks were calculated from pooled risk differences | |
| 5 per 1000 | 9 per 1000 (5 to 15) | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to inconsistency.
Summary of findings 2. Hormone therapy compared to placebo for secondary prevention of cardiovascular disease in post‐menopausal women.
| Hormone therapy compared to placebo for secondary prevention of cardiovascular disease in post‐menopausal women | ||||||
|
Patient or population: Post‐menopausal women with pre‐exisiting cardiovascular disease Intervention: Hormone therapy Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Hormone therapy | |||||
| Death (all‐causes) | Study population |
RR 1.04 (0.87 to 1.24) |
5445 (7 studies) |
⊕⊕⊕⊕ high | ||
| 84 per 1000 |
88 per 1000 (73 to 105) |
|||||
| Death (cardiovascular causes) | Study population |
RR 1.00 (0.78 to 1.29) |
5259 (6 studies) |
⊕⊕⊕⊕ high | ||
| 45 per 1000 |
45 per 1000 (35 to 58) |
|||||
| Stroke | Study population |
RR 1.09 (0.89 to 1.33) |
5172 (5 studies) |
⊕⊕⊕⊝ moderate1 | ||
| 65 per 1000 |
71 per 1000 (58 to 86) |
|||||
| Venous thromboembolism | Study population |
RR 2.02 (1.13 to 3.62) |
4399 (6 studies) |
⊕⊕⊕⊕ high | ||
| 11 per 1000 |
23 per 1000 (13 to 40) |
|||||
| Pulmonary embolism | Study population |
RR 2.48 (0.92 to 6.70) |
3920 (3 studies) |
⊕⊕⊕⊝ moderate1 | ||
| 4 per 1000 |
10 per 1000 (4 to 27) |
|||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to imprecision: Confidence interval for the absolute effect includes small decrease and large increased risk.
Summary of findings 3. Hormone therapy commenced less than 10 years after the menopause for preventing cardiovascular disease in post‐menopausal women.
| Hormone therapy commenced less than 10 years after the menopause for preventing cardiovascular disease in post‐menopausal women | ||||||
|
Patient or population: Post‐menopausal women
Intervention: Hormone therapy commenced lessthan 10 years after the menopause Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Hormone therapy commenced less than 10 years after the menopause | |||||
| Death (all‐causes) | Study population | RR 0.70 (0.52 to 0.95) | 9088 (5 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 22 per 1000 | 16 per 1000 (12 to 21) | |||||
| Coronary heart disease (death from cardiovascular causes and non‐fatal myocardial infarction) | Study population | RR 0.52 (0.29 to 0.96) | 8311 (4 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 18 per 1000 | 10 per 1000 (5 to 18) | |||||
| Stroke | Study population | RR 1.37 (0.80 to 2.34) | 8143 (3 studies) | ⊕⊕⊕⊕ high | ||
| 9 per 1000 | 13 per 1000 (7 to 21) | |||||
| Venous thromboembolism | Study population | RR 1.74 (1.11 to 2.73) | 9838 (3 studies) | ⊕⊕⊕⊕ high | ||
| 6 per 1000 | 11 per 1000 (7 to 17) | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to risk of bias. The results are significant due to the DOPS 2012 trial, that has high risk of bias. We downgraded our confidence in the results of that metanalysis because, had the DOPS not been there, the metanalysis would not be significant. 2 Composite outcome.
Summary of findings 4. Hormone therapy commenced more than 10 years after the menopause for preventing cardiovascular disease in post‐menopausal women.
| Hormone therapy commenced more than 10 years after the menopause for preventing cardiovascular disease in post‐menopausal women | ||||||
|
Patient or population: Post‐menopausal women Intervention: Hormone therapy commenced less than 10 years after the menopause Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Hormone therapy commenced morethan 10 years after the menopause | |||||
| Death (all‐causes) | Study population |
RR 1.06 (0.95 to 1.18) |
27,750 (12 studies) |
⊕⊕⊕⊕ high | ||
| 45 per 1000 |
47 per 1000 (42 to 53) |
|||||
| Coronary heart disease (death from cardiovascular causes and non‐fatal myocardial infarction) | Study population |
RR 1.07 (0.96 to 1.20) |
23,491 (12 studies) |
⊕⊕⊕⊕ high | ||
| 49 per 1000 |
52 per 1000 (47 to 59) |
|||||
| Stroke | Study population |
RR 1.21 (1.06 to 1.38) |
22,722 (8 studies) |
⊕⊕⊕⊕ high | ||
| 32 per 1000 |
39 per 1000 (34 to 44) |
|||||
| Venous thromboembolism | Study population |
RR 1.96 (1.37 to 2.80) |
27,475 (9 studies) |
⊕⊕⊕⊕ high1 | ||
| 12 per 1000 |
24 per 1000 (16 to 34) |
|||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1We did not downgrade for inconsistency. Although the I square indicated that there was a substantial amount of statistical variation (41%), the direction of effect across the results of the studies was consistent.
Background
Despite falling incidence of coronary heart disease and stroke, which makes up the majority of cardiovascular disease, there is increasing prevalence due to lower case‐fatality. It remains the leading cause of death in both high‐ and middle‐income countries (WHO 2008), and is increasingly the cause in low‐income countries. In 2013, cardiovascular disease caused an estimated 30% of all global deaths, and killed 17.3 million people worldwide (WHO 2011). In the United Kingdom (UK), in 2010, cardiovascular disease caused 32% of deaths, and killed just over 179,000 people. Analysing this data according to gender demonstrates no significant overall difference in numbers between men and women; 31% of women died from cardiovascular disease in 2010 in the UK (British Heart Foundation Statistics Database 2012).
The burden of coronary heart disease is costly, both in terms of reduced patient health‐related quality of life, and healthcare costs in the management of these conditions. Morbidity statistics indicate that cardiovascular disease is the leading single cause of disability in Europe, with a prevalence of 6.0% to 6.5% in men and 4.0% to 4.5% in women within the UK. Cardiovascular disease is therefore costly in terms of both direct and indirect healthcare costs, accounting for 9.8% of total disability‐adjusted years (Townsend 2012). In 2009, it was estimated that cardiovascular disease cost the UK healthcare system approximately GBP 8.6 billion, equating to approximately just under GBP 141 per capita. Overall, the cost from cardiovascular disease in the UK is estimated to be GBP 19 billion, the balance attributed to lost productivity and caring for those with cardiovascular disease (British Heart Foundation Statistics Database 2012).
Description of the condition
Whilst the overall risk of cardiovascular disease is matched in men and women, this risk varies according to age. The risk is higher in men compared to women in younger age groups, with women’s cardiovascular disease incidence rates found to lag approximately ten years behind those of men. Most women experience the menopause (the last menstrual period) in their early fifties, after a phase of changing ovarian function (the peri‐menopause) that may last several years and which is characterised by irregular menstrual cycles (Greendale 1999). Following menopause and loss of endogenous oestradiol (major ovarian oestrogen), these gender‐based differences narrow (Barrett‐Connor 1997; Maxwell 1998). Most women who enter menopause are asymptomatic for cardiovascular disease, and 95% of women who develop cardiovascular disease do so after menopause. Evidence suggests that younger age at natural menopause is associated with cardiovascular disease (Hu 1999) and cardiovascular disease mortality (Jacobsen 1997; van der Schouw 1996). Post‐menopausal women have 2.6 times the rate of cardiovascular events compared to their age‐matched pre‐menopausal peers (Kannel 1976). There are many possible explanations for this associated increase in risk. The menopause has an adverse effect on lipid profile; low‐density lipoprotein and triglyceride levels rise after the menopause, and high‐density lipoprotein falls (Kilim 2013). Weight gain and a change in body fat distribution, increases in blood pressure and a host of other metabolic factors are amongst the other changes seen. The management and prognosis of women with cardiovascular disease is not aided by their being under‐represented in trials (Melloni 2010) and are more likely to present atypically with lower cardiovascular revascularisation rates (Rathore 2003).
Description of the intervention
The term 'hormone replacement therapy' has been replaced by 'hormone therapy', as the older term infers that hormone therapy is replacing the function of a defective organ. Hormone therapy includes either oestrogen alone (oestrogen‐only hormone therapy) or oestrogen in combination with a progestogen (combined hormone therapy). It is used in a variety of formulations and doses which can be taken orally, vaginally, intranasally or as an implant, skin patch, cream or gel. The clinical effects vary according to the type of hormone therapy and the duration of its use. Formulations of oral oestrogen may include oestradiol (an oestrogen derived from wild Mexican yam), oestradiol valerate (a pro‐drug for oestradiol), or conjugated equine oestrogen, a blend of equine oestrogens extracted from horse urine. Historically, larger doses were prescribed but doses have fallen in the past two decades as prescribers aim to minimise side effects associated with larger doses. The progestogens used for hormone therapy include synthetic derivatives of progesterone, synthetic derivatives of testosterone, and natural progesterones derived from plants. These differ in their metabolic action and potential for adverse effects, and the risk‐benefit profile of each type of progestogen for use in hormone therapy is currently unclear. In combined hormone therapy, progestogen can be taken either every day (continuous combined therapy), cyclically with oestrogens taken daily and progestogens taken for part of the month (sequentially combined hormone therapy), or less frequently. The addition of a progestogen to oestrogen reduces the risk of endometrial hyperplasia associated with the use of oestrogen alone in women with a uterus (Furness 2009). However, the addition of progestogens can be problematic as they have adverse effects on blood lipid profiles and may cause symptoms such as headaches, bloating and breast tenderness (McKinney 1998).
How the intervention might work
The finding that cardiovascular disease rates in women rise sharply after the menopause has led to the suggestion that endogenous oestradiol may attenuate age‐related vascular remodelling in pre‐menopausal women. Age‐associated vascular remodelling involves endothelial dysfunction, enhanced growth of intimal smooth muscle cells, and increased prevalence of vascular plaques. The same cellular processes participate in atherosclerosis (Lakatta 2003). The decline in oestradiol levels during menopause leads to a higher androgen‐to‐oestradiol ratio. Androgens induce vasoconstriction and smooth muscle cell growth and exacerbate diet‐induced atherosclerosis, plaque formation, and pro‐atherosclerotic arterial remodelling. This suggests that the increase in the androgen‐to‐oestradiol ratio in post‐menopausal women may be another mechanism which contributes to the observed acceleration of atherosclerosis. The exact mechanism by which cardiovascular disease risk may be reduced by oestrogen is not completely understood, but leading hypotheses involve inhibition of vascular remodelling, lowering cholesterol and improving vascular tone (Dubey 2001; Mendelsohn 1999; PEPI 1995; Walsh 1991). Other factors that may play a role are changes in coagulation factors, blood pressure, insulin, and body fat distribution (Koh 2004; Lieberman 1994; PEPI 1995).
Hormone therapy to treat menopausal oestrogen deficiency has been in widespread use for more than 60 years (Wallach 1959). Long‐term treatment was assumed to prevent atherosclerosis, and the increased cardiovascular disease and mortality risk observed following the menopausal transition (Robinson 1959; Wallach 1959; Wilson 1963), either in the form of primary prevention (prevention of disease before it has first presented) or secondary prevention (preventing the progression or recurrence of disease). Since the early 1980s, several observational studies have consistently shown that hormone therapy users, many of whom started treatment shortly after menopause, had a reduction in total mortality and risk of cardiovascular disease events of approximately 30% to 50% relative to women who did not use hormone therapy (Grady 1992; Grodstein 1999; Grodstein 2000; Mann 1994; Psaty 1994; Rosenberg 1993; Stampfer 1991). However, most observational data sets suggest that the risk reduction in mortality and coronary heart disease events, is coupled with a higher impact of the risk of venous thromboembolic events and an apparent increased incidence of stroke, but lower stroke mortality (Paganini‐Hill 2001). Overall, the accumulated available epidemiological evidence supported the use of hormone therapy to increase longevity in post‐menopausal women (Mishell 1989). Following these observational studies two large RCTs, the Heart and Estrogen/progestin Replacement Study, assessing secondary prevention (HERS I 1998) and the Women’s Health Initiative, assessing primary prevention (WHI I 2002) were carried out and both appeared to contradict the evidence from observational studies.
In light of these trials not confirming a cardioprotective effect of oestrogen, attention was focused on the age of the women enrolled in both HERS I 1998 and WHI I 2002 (mean age: 67 and 63 years, respectively), as non‐significant data trends suggested hormone therapy did not lead to excess coronary risk when started shortly after the menopause and interest alighted upon the timing of initiation of hormone therapy in relation to the time of menopause. This ‘timing hypothesis’, first proposed in 2002, states that there may be a window of opportunity where hormone therapy is beneficial for prevention of cardiovascular disease in women when started in early menopause, with this benefit lost in older women. This hypothesis is supported by the Clarkson primate model, where conjugated equine oestrogen prevented atherosclerosis only in animals treated early after surgically induced menopause (within the calculated equivalent of six human post‐menopausal years) before the onset of diet‐induced atherosclerosis (Mikkola 2002). The reasoning behind the ‘timing hypothesis’ is that oestrogen effects differ with the presence and severity of atherosclerosis and that this is linked to the timing of the menopause and age. This may be due to fewer oestrogen receptors in the artery wall (Losordo 1994) and reduced vasodilatory effects of oestrogen with progressing atherosclerosis (Campisi 2002). When there is minimal or no atherosclerosis, oestrogen leads to reduced platelet and inflammatory activation. It also enables nitric oxide mediated vasodilatation, an important component of healthy endothelial function (Mendelsohn 1999). When there is established atherosclerosis, many of these beneficial physiological changes are attenuated or even reversed, with reduced vasodilatation and increased inflammatory activation (Ouyang 2006). It is also hypothesised that although hormone therapy reduces the risk of plaque formation, it increases plaque instability and the risk of plaque erosion or rupture, through production of matrix metalloproteinases (Phillips 2005). Therefore, as it is known that cardiovascular risk (including atherosclerosis) increases significantly after the menopause (Kannel 1976) the ‘timing hypothesis’ suggests that if hormone therapy is instigated many years after menopause, it is much more likely that there will be established atherosclerosis, and therefore the benefit of reduced plaque formation will be abrogated by the increased risk of plaque erosion or rupture. In support of the ‘timing hypothesis’, reanalysis of the Nurses’ Health Study (Grodstein 2006) demonstrated a benefit to starting treatment less than four years after the menopause compared to more than 10 years after the menopause. A stratified meta‐analysis by Salpeter 2004 also indicated differential treatment effects with hormone therapy relative to placebo, according to the participants' baseline age, favouring use in women under 60 years of age.
Why it is important to do this review
The previous Cochrane Review on hormone therapy for the prevention of cardiovascular disease in post‐menopausal women (Main 2013) identified a total of 13 RCTs which included 38,171 post‐menopausal women (19,302 randomised to hormone therapy and 18,869 to placebo). The review reported no protective cardiovascular effects for hormone therapy observed in either healthy women or women with one or more pre‐existing cardiovascular disease risk factors, but a higher risk of stroke, venous thromboembolic events and pulmonary embolism was observed.
Since the literature search for the previous Cochrane review (Main 2013), the BMJ (October 2012) reported the results of the cardiovascular outcomes of the Danish Osteoporosis Prevention Study (DOPS 2012). This trial was designed to assess the long‐term impact of hormone therapy on bone mineral density and predefined adverse cardiovascular endpoints in healthy women compared to a no treatment control group. The trial had over 10 years of randomised follow‐up and a further 5.7 years of post‐interventional follow‐up, the longest of any comparable trial. The trial had some noticeable differences to the majority of previous studies that used hormone therapy to prevent cardiovascular disease, other than its long follow‐up; the women included were younger and the hormone preparation was different to that used in the majority of previous trials. The results indicated that women receiving hormone therapy early after menopause had a reduced risk of the composite endpoint of mortality, heart failure or myocardial infarction, without any apparent increase in risk of cancer, venous thromboembolism, or stroke. This led to great media interest and a revival of the debate on whether hormone therapy is safe, when it should be prescribed and in whom it should be prescribed.
However, the DOPS trial had many criticisms levelled against it; the low event rate, open‐label design and the higher mean age in the control group. To bring clarity to the subject, we felt it was important to update the review to assess whether this new trial altered the balance of evidence on hormone therapy and its effect on cardiovascular disease.
There are also further data not available to the previous review, which reported events by year of treatment and also by participants’ age from trials. Additionally, it has become increasingly clear that the risk from hormone therapy is not constant for the time for which it is taken. Whether women are taking it to treat symptoms or reduce the risk of other diseases, it is important that they are informed regarding the risks as accurately as possible for them as individuals, and this varies according to duration of therapy, their age and when their menopause started.
Objectives
To assess the effects of hormone therapy for the prevention of cardiovascular disease in post‐menopausal women, and whether there are differential effects between use in primary or secondary prevention. Secondary aims were to undertake exploratory analyses to (i) assess the impact of time since menopause that treatment was commenced (≥ 10 years versus < 10 years), and where these data were not available, use age of trial participants at baseline as a proxy (≥ 60 years of age versus < 60 years of age); and (ii) assess the effects of length of time on treatment.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing oral hormone therapy with either placebo or a no treatment control for a follow‐up duration of six months or longer. We included RCTs which compared two or more different types of oral hormone therapy, provided that they were additionally compared with a placebo or a no treatment control arm.
Types of participants
Post‐menopausal women (with either spontaneous or induced cessation of menstrual bleeding for a continuous period of six months or more), either with or without evidence of existing cardiovascular disease.
Types of interventions
Oral hormone therapy, consisting of either oestrogen alone or in combination with a progestogen, compared with either a placebo or a no treatment control. Combined hormone therapy (oestrogen plus progestogen) could be delivered continuously daily (continuous combined hormone therapy) or sequentially (oestrogen taken daily with progestogens taken for part of the month). In accordance with the inclusion criteria from the previous review (Main 2013), we excluded RCTs in which hormone therapy was delivered to the body via either patches, tablets, creams, troches, an intrauterine device, vaginal ring, gels or injections compared with placebo or no treatment. Likewise, we excluded RCTs assessing the effects of selective oestrogen receptor modulators (e.g. raloxifene) compared to placebo or a no treatment control.
Types of outcome measures
Primary outcomes
Death from any cause
Cardiovascular death
Non‐fatal myocardial infarction
Stroke
Angina
Secondary outcomes
Venous thromboemboli (pulmonary emboli plus deep vein thromboses)
Pulmonary emboli
Revascularisation (coronary artery bypass grafting and angioplasty (with or without a stent))
We reviewed trials which were above a given size (based on number of participants and length of follow‐up) to see if relevant outcomes were reported as adverse events. We reviewed trials which included 1000 or more participants (where the participants were followed up for six months or more) and all other trials of comparable or larger size. For example, we reviewed trials which included 500 participants (where the participants were followed up for one year or more). We also included trials with 250 participants or more (where the participants were followed up for two years or more). All the trials which were reviewed due to their size to assess whether they reported relevant outcomes as adverse events are listed in Table 5. Outcomes with zero events in one or more arms of a trial were not included from the trial in question. This was done to minimise bias.
1. Studies reviewed for relevant adverse events.
| Study | Population size | Duration of follow‐up (years) | Outcomes | Included in Review | Adverse events reported | |||||||
| Death (all‐ causes) | Death (CV causes) | Non‐fatal MI | Stroke | Angina | Revascularisation | VTE | PE | |||||
| CHART 2006 | 1265 | 2 | Bone mineral density Serum lipids (LDL, HDL, TG) Endometrial hyperplasia and proliferation |
No | ||||||||
| de Maat 2007² | 436 | 5 | CRP Fibrinogen |
No | ||||||||
| ERT II 1979 | 168 | 10 | Not specified | Yes | ✓ | ✓ | ✓ | |||||
| Genant 1997 | 406 | 2 | Bone mineral density Serum lipids Endometrial tissue structure |
No (no events in placebo arm) |
✓ | ✓ | ||||||
| Greenspan 2005 | 373 | 3 | Time to rise from a chair Timed walking Balance Instrumental Activities of Daily Living Physical Activity Scale of the Elderly Folstein Mini‐Mental State Examination Falls |
Yes | ✓ | ✓ | ✓ | |||||
| Hart 1984 | 72 | 10 | Bone mineral density Serum lipids |
No | ||||||||
| Heikkinen 1997 | 232 | 3 | Serum lipids | No | ||||||||
| HOPE 2002 | 2673 | 2 | Number and severity of hot flushes
Papanicolaou smear with vaginal maturation index Bone mineral density Serum lipids Lipoproteins Glucose tolerance Coagulation/fibrinolytic factors |
No | ||||||||
| Kim 1996 | 551 | 1 | Serum lipids, Lipoproteins |
No | ||||||||
| Leggate 1984 | 54 | 10 | Bone mineral density | No | ||||||||
| OPAL 2006 | 571 | 3 | Change in carotid intima media thickness | No (no events in one group) |
✓ | ✓* | ✓* | ✓* | ✓* | |||
| PEPI 1995 | 875 | 3 | HDL Systolic blood pressure Serum insulin Serum glucose Fibrinogen |
No (no events in some groups) |
✓ | ✓* | ✓* | ✓* | ✓* | |||
| SMART‐5 2012 | 503 | 1 | Endometrial hyperplasia | No (no events in one group) |
✓✝ | ✓ | ||||||
| Stevenson 2005 | 579 | 2 | Serum lipids | No | ||||||||
| STOP IT 2001 | 369 | 3 | Bone mineral density Serum 25‐hydroxyvitamin D Serum lipids Depression Calcium absorption Serum PTH Osteocalcin Urinary N ‐telopeptides/creatinine ratio |
Yes | ✓ | ✓ | ✓ | ✓ | ||||
| Tofteng 2002² | 429 | 5 | Bone mineral density | No | ||||||||
| WELL‐HART 2003 | 226 | 3.3 | Change in coronary stenosis | Yes | ✓ | ✓* | ✓* | ✓* | ✓* | ✓* | ✓* | ✓* |
| WHISP 2006 | 100 | 0.58 (7 months) |
Serum lipids Antithrombin Factor VII Fibrinogen |
Yes | ✓ | ✓¹ | ✓ | ✓¹ | ✓ | |||
| *Part of a composite endpoint. ✝In a non‐relevant treatment group (bazedoxifene and oestrogen). ¹Not included in analysis as no event in one of the groups. ²Sub‐study of DOPS 2012 | ||||||||||||
Search methods for identification of studies
Electronic searches
We identified randomised controlled trials (RCTs) that assessed the effects of hormone therapy compared to placebo or no treatment with a minimum of six months duration through searching the following electronic databases on 25 February 2014:
Cochrane Central Register of Controlled Trials (CENTRAL; Issue 1, 2014) in The Cochrane Library,
MEDLINE (Ovid; 1946 to week 9 2014),
EMBASE (Ovid; 1980 to week 9 2014),
LILACS (http://lilacs.bvsalud.org/en/) (1982 to 25 February 2014).
Additionally, we searched the following trials and research registers for any ongoing trials on cardiovascular disease on 25 February 2014: ClinicalTrials.gov (www.clinicaltrials.gov), the World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictrp/), the UK Clinical Research Network Portfolio Database (http://public.ukcrn.org.uk) and Centerwatch (www.centerwatch.com). We did not apply any language restrictions. The full search strategies, designed and run in each database, are presented in Appendix 1 (original review), Appendix 2 (2010) and Appendix 3 (2014).
Searching other resources
We searched reference lists of all eligible RCTs and relevant systematic reviews for additional trials.
Data collection and analysis
Selection of studies
We identified relevant studies in two stages. Two review authors (HB and LH) independently screened the titles and abstracts returned by the database searches for relevance. The full text of any references that were considered as potentially relevant by either author were obtained. They then independently assessed the relevance of each paper, according to the review's prespecified eligibility criteria. This assessment was performed unblinded. Any discrepancies between the authors were resolved by recourse to the papers, and if necessary, a third author was consulted.
Data extraction and management
Two review authors (HB and LH) independently extracted data from the included studies using a standardised data extraction form in Microsoft Word. The data extraction was checked for agreement and any discrepancies were resolved through recourse to the papers. We assessed the following study details.
Trial characteristics
Method of randomisation
Method of allocation concealment
Use of stratification
Adequacy of double‐blinding
Means of recruitment
Number of participants screened for eligibility, randomised, analysed, excluded, lost to follow‐up or dropped‐out (i.e. withdrew from the trial but were followed‐up)
Baseline equality of treatment groups
Level of adherence to therapy
Whether analyses were conducted on an intention‐to‐treat basis
Study design (parallel versus multi‐arm, single centre or multi‐centre)
Funding source
Characteristics of the trial participants
Inclusion and exclusion criteria
Age and other recorded prognostic baseline variables
Menopausal status (definition of menopause and how this was defined, surgical or natural menopause) of participants
Interventions
Type of hormone therapy (oestrogen‐only or combination oestrogen and progestogen)
Dosage
Duration of therapy (minimum six months)
Outcomes
Which relevant primary and secondary outcomes were measured
How relevant outcomes were defined and measured
See Description of studies; Risk of bias in included studies
Assessment of risk of bias in included studies
We assessed risk of bias according to the risk of bias assessment criteria detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). These criteria focus upon the quality of random sequence generation and allocation concealment, blinding (participants, trial personnel and outcome assessors), incomplete outcome data, selective outcome reporting, and other sources of bias. Assessment of risk of bias was undertaken by two review authors (HB and LH) independently, with any disagreements resolved by discussion.
Assessment of heterogeneity
We explored heterogeneity between studies qualitatively (by comparing the characteristics of included studies) and quantitatively using the Chi2 test of heterogeneity and the I2 statistic. We considered trials with a Chi2 test resulting in a P value < 0.10 indicative of significant statistical heterogeneity. In order to assess and quantify the possible magnitude of heterogeneity between trials, and the potential impact for undertaking meta‐analyses, we interpreted an I2 statistic of 0% to 40% as potentially not being important; 30% to 60% as representing moderate heterogeneity; 50% to 90% as representing substantial heterogeneity; and 75% to 100% as being considerably heterogeneous and potentially unsuitable for meta‐analyses (Deeks 2011). Published graphs display the results of analysis using the random‐effects model. We assessed reporting bias through the examination of funnel plots.
Data synthesis
We undertook statistical analyses following the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For dichotomous data, we generated two‐by‐two tables for each study and expressed the data as a risk ratio (RR) with 95% confidence intervals (CIs). We grouped the data according to whether the intervention was primary or secondary prevention. We undertook further analyses to assess the effect of both single and combination therapy in the overall patient population (both primary and secondary prevention) and commencement of treatment according to time since the menopause (< 10 years and ≥ 10 years). Where time since menopause data was not available then mean age of the population at baseline was used (< 60 years old and ≥ 60 years old). We combined data for meta‐analysis in Review Manager software (RevMan 2014), using the Peto‐modified Mantel‐Haenszel method with a random‐effects model to provide an overall estimate of treatment effect. We chose the random‐effects model due to the wide variety in sample sizes between studies and the heterogeneity found for some outcomes. For comparisons showing statistically significant differences between treatment groups, we calculated the absolute risk reduction and number needed to treat, or absolute risk increase and number needed to harm using the pooled RR data.
Subgroup analysis and investigation of heterogeneity
To assess the effect of duration of treatment, we compared data in two different ways. Where data were reported by year of follow‐up, we analysed the data both non‐cumulatively (year‐by‐year), as well as cumulatively, incorporating the remainder of the trials where data were not reported by year of follow‐up, according to the total duration of trial follow‐up. We classified these trials a priori as exploratory given the heterogeneity between the different hormone therapy regimens assessed and the patient populations in the different trials.
To assess the timing hypothesis and the potential impact of the time since menopause that treatment was commenced, we stratified trials according to when treatment was started. This was characterised as starting treatment either < 10 or ≥ 10 years after the menopause, or if these data were not available, then we used mean age of participants at baseline (> 60 years of age versus < 60 years of age) as a surrogate.
Summary of Findings tables
We used methods developed by the GRADE working group to rate the quality of the evidence for the following outcomes:
Death (all causes)
Coronary heart disease
Stroke
Venous thromboembolism
Pulmonary embolism
We have presented the quality ratings in Summary of Findings tables for the comparison of HT and placebo in primary and secondary prevention of cardiovascular disease and for the subgroup analysis addressing the timing hypothesis.
Results
Description of studies
See: Included studies; Excluded studies.
Results of the search
For this update, we retrieved 3930 records from the database searches and found 33 additional records through other sources, bringing the total results to 3963. Of these, we excluded 3866 records after assessment of titles and abstracts. We considered the full text of 97 references for inclusion.
We found six new trials (12 papers) for inclusion and additional 25 papers on ten previously included studies. We excluded 58 papers. One study has completed and is awaiting classification (NCT00154180) and one study is ongoing (NCT00114517). The process of study selection for the original review in Figure 1 and for this updated review is presented in Figure 2.
1.
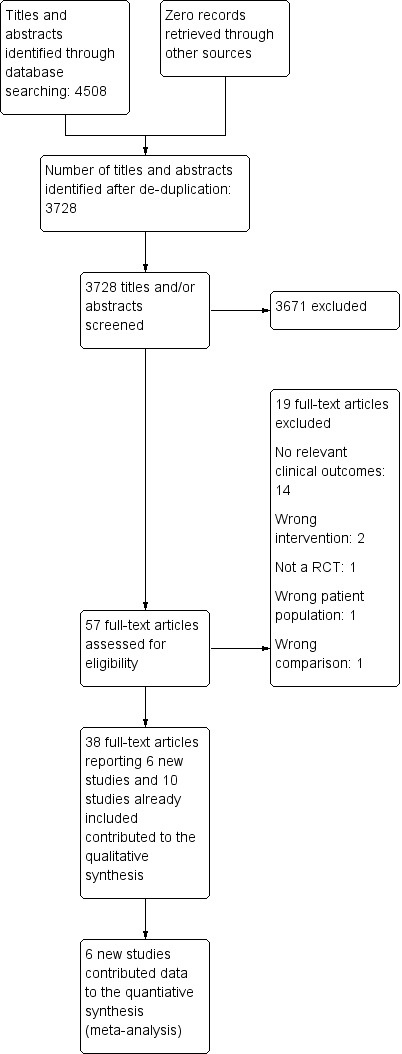
Figure 1: Process of study selection for the review
2.
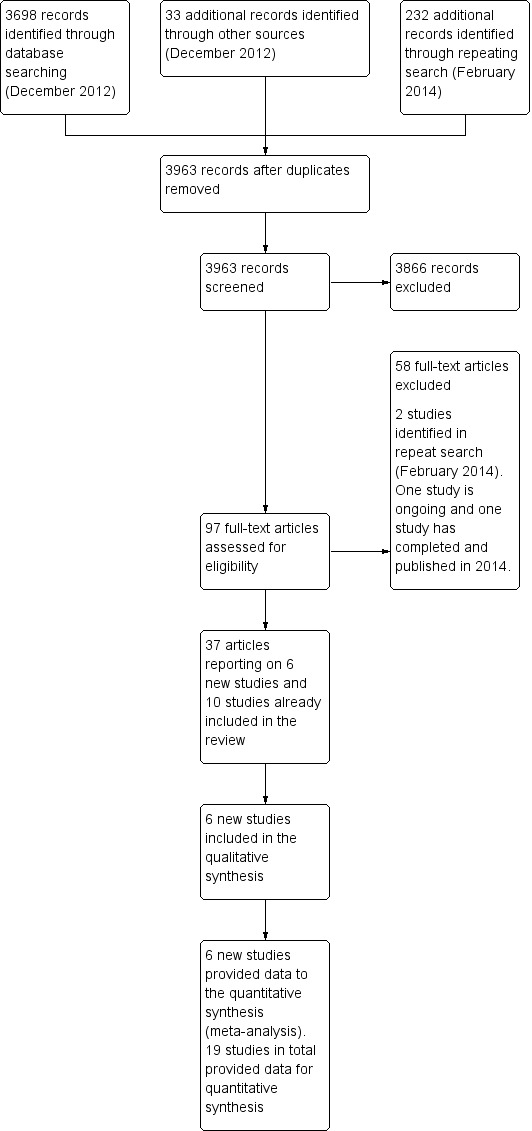
Process of study selection for the updated review
Included studies
In total we identified 19 RCTs with at least six‐months follow‐up that compared hormone therapy to placebo or no treatment published between 1979 and 2012 (DOPS 2012; EAGAR 2006; EPAT 2001; EPHT 2006; ERA 2000; ERT II 1979; ESPRIT 2002; EVTET 2000; Greenspan 2005; HALL 1998; HERS I 1998; STOP IT 2001; WAVE 2002; WELL‐HART 2003; WEST 2001; WHI I 2002; WHI II 2004; WHISP 2006; WISDOM 2007). Thirteen of the identified trials had been included in the previous review. Six new trials were therefore identified for this update (DOPS 2012; ERT II 1979; Greenspan 2005; STOP IT 2001; WELL‐HART 2003; WHISP 2006). The 19 trials included 40,410 post‐menopausal women; 20,517 randomised to receive some form of hormone therapy and 19,893 to receive either placebo or a no treatment control. STOP IT 2001 also included a group randomised to calcitriol who were not included in this review. Likewise, WISDOM 2007 also included a further 1307 women randomised to a comparison of two active hormone therapies, and EPHT 2006, also included 1001 women randomised to either open‐label hormone therapy, or a no treatment control. The data from these further 2306 women randomised into either of these trials (EPHT 2006; WISDOM 2007) were not included in this review. The trials varied considerably in size, ranging from 40 (HALL 1998) to 16,608 (WHI I 2002). Likewise, there was large variation in the length of follow‐up within the trials ranging from seven months (WHISP 2006) to 10.1 years (DOPS 2012). Overall, three large trials (HERS I 1998; WHI I 2002; WHI II 2004) with a mean follow‐up duration of 5.6 years (range: 4.1 to 7.1) randomised 30,110 women to either hormone therapy treatment or placebo, and therefore contributed approximately 75% of the included participants from the 19 trials. The majority of the trials (n = 11) had been conducted in the US, two were conducted in more than one country (one in the US and Canada, and one in England, New Zealand and Australia), two trials were conducted in the UK, with one trial conducted in each of the following countries: Norway, Denmark, Sweden, and Estonia. Seven trials were stopped early (DOPS 2012; EAGAR 2006; EPHT 2006; EVTET 2000; WHI I 2002; WHI II 2004; WISDOM 2007); either as other trial results were published showing no beneficial effect, or a detrimental effect of hormone therapy on cardiovascular disease outcomes (DOPS 2012; EAGAR 2006; EPHT 2006; EVTET 2000; WISDOM 2007), or due to it being established that the overall risks (adverse events) associated with hormone therapy use were unlikely to be outweighed by any potential benefits of hormone therapy use on cardiovascular disease outcomes within the time frame of the trial (WHI I 2002; WHI II 2004). A summary of the main characteristics of the included trials is displayed in Table 6.
2. Summary of trial characteristics.
| Trial (year) | Country | Length of follow‐up (years) | No. participants | Mean age of participants (years) | % hysterectomy | Primary or secondary prevention | HT type | Participant previous indication |
| DOPS 2012 | Denmark | 10.1 | 1006 | 49.8 | 18.9 | Primary | Single and combination | None |
| EAGAR 20061 | US | 2.8 | 83 | 64 | NR | Secondary | Single | CAGB |
| EPAT 2001 | US | 2 | 222 | 62 | 38 | Primary | Single | None |
| EPHT 20061 | Estonia | 3.4 | 777 | 59 | 10 | Primary | Combination | None |
| ERA 2000 | US | 3.2 | 310 | 66 | 61 | Secondary | Single and combination | CHD |
| ERT II 1979 | US | 10 | 168 | 55.1 | 0 | Primary | Combination | None |
| ESPRIT 2002 | UK | 2 .0 | 1017 | 63 | 25 | Secondary | Single | MI or TIA |
| EVTET 20002 | Norway | 1.3 | 140 | 56 | NR | Secondary | Combination | DVT or PE |
| STOP IT 2001 | US | 3 | 366 | 71 | 41 | Primary | Single and combination | None |
| Greenspan 2005 | US | 3 | 373 | 71.2 | 35 | Primary | Single and combination | None |
| HALL 1998 | Sweden | 1.0 | 40 | 60 | NR | Secondary | Combination | CHD |
| HERS I 1998 | US | 4.1 | 2763 | 67 | 0 | Secondary | Combination | CHD |
| WAVE 2002 | International | 2.8 | 423 | 66 | NR | Secondary | Combination | CHD |
| WELL‐HART 2003 | US | 3.3 | 226 | 63.5 | 44 | Secondary | Single and combination | CHD |
| WEST 2001 | US | 2.8 | 664 | 72 | 45 | Secondary | Single | MI or TIA |
| WHI I 20023 | US | 5.6 | 16,608 | 63 | 0 | Primary | Combination | None |
| WHI II 20044 | US | 7.1 | 10,739 | 64 | 100 | Primary | Single | None |
| WHISP 2006 | UK | 0.58 (7 months) | 100 | 68.9 | NR | Secondary | Combination | ACS |
| WISDOM 20071 | International | 1 | 4385 | 63 | NR | Primary | Combination | None |
1 Trial stopped early due to publication of WHI I 2002 results 2 Trial stopped early due to publication of HER I 1998 results 3 Trial stopped early as the weighted log‐rank test statistic for breast cancer crossed designated stopping boundary, and global index supportive of finding of overall harm. 4 Trial stopped early as National Institutes of Health (NIH) concluded that conjugated equine oestrogen alone did not appear to affect the risk of heart disease, but was associated with a significant increase in the risk of stroke CABG: coronary artery bypass graft; CHD: Coronary heart disease; DVT: Deep vein thrombosis; MI: myocardial infarction; NR: not reported; PE: Pulmonary embolism; TIA: Transient ischaemic attack
Participants
All the trials included post‐menopausal women, irrespective of whether the absence of menses was natural or an artefact of hysterectomy or oophorectomy, with a mean age of 64 years. In 14 of the 19 trials the mean participant age was over 60 years at baseline. The hysterectomy status of the women in four of the trials was related to the inclusion criteria and therefore in ERT II 1979; HERS I 1998 and WHI I 2002 was 0%, and in WHI II 2004 it was 100%. In the nine trials in which baseline hysterectomy status was reported, this ranged from 10% to 61% (DOPS 2012; EPAT 2001; EPHT 2006; ERA 2000; ESPRIT 2002; Greenspan 2005; STOP IT 2001; WELL‐HART 2003; WEST 2001). Six studies did not report data on hysterectomy status (EAGAR 2006; EVTET 2000; HALL 1998; WAVE 2002; WHISP 2006; WISDOM 2007). The trial inclusion criteria varied according to the primary study objectives. Six of the trials were designed to assess the effects of hormone therapy in the primary prevention of cardiovascular disease and therefore enrolled predominantly healthy patient populations (DOPS 2012; EPAT 2001; EPHT 2006; WHI I 2002; WHI II 2004; WISDOM 2007). Ten of the trials aimed to assess the impact of hormone therapy in secondary prevention, and therefore enrolled women with established cardiovascular disease (ERA 2000; HERS I 1998; WAVE 2002; WELL‐HART 2003) or after a designated specific cardiovascular disease event of interest, such as coronary artery bypass graft (EAGAR 2006), angina (HALL 1998), acute coronary syndrome (WHISP 2006), myocardial infarction or transient ischaemic attack (ESPRIT 2002; WEST 2001), or pulmonary embolism or deep vein thrombosis (EVTET 2000). Three trials were designed to assess non‐cardiovascular endpoints but reported relevant outcomes as adverse events, safety of long‐term hormone therapy (ERT II 1979), bone mineral density (STOP IT 2001), physical performance, functional ability, falls and cognitive function (Greenspan 2005).
Primary prevention trials
Nine studies enrolled relatively healthy women (DOPS 2012; EPAT 2001; EPHT 2006; ERT II 1979; Greenspan 2005; STOP IT 2001; WHI I 2002; WHI II 2004; WISDOM 2007). Although one of the studies enrolled women with one cardiovascular disease risk factor, namely hypercholesterolaemia (EPAT 2001) and a small minority (approximately ≤ 5%) of women within all trials had a history of cardiovascular disease, the trial participants were representative of population samples of fit women in this age group without overt disease. Four of these trials (EPHT 2006; WHI I 2002; WHI II 2004; WISDOM 2007) assessed the impact of hormone therapy on both cardiovascular disease, as well as a wide range of other endpoints, including cancer, osteoporosis and gallbladder disease, and therefore reported detailed lists of participant inclusion and exclusion criteria. One study included only participants who were admitted for the entire duration of the study (ten years) in a hospital for patients with chronic illnesses (ERT II 1979). The illnesses of the participants are not reported. However, it specified that acute heart disease and hypertension were exclusion criteria. Three studies, mentioned above, were designed to assess non‐cardiovascular outcomes but reported relevant outcomes as adverse events (ERT II 1979; Greenspan 2005; STOP IT 2001). The two biggest primary prevention trials (WHI I 2002; WHI II 2004) both set enrolment targets to establish set fractions for baseline age categories and to achieve racial and ethnic group representation within participant groups in the proportions recorded in the US census for the 50 to 79 year old age group. This was achieved, with it being noted that baseline cardiovascular risk factors in the trial participants in both WHI I 2002 and WHI II 2004 were low and consistent with those observed in a generally healthy population of post‐menopausal women (Manson 2003; Stefanick 2003). WISDOM 2007 recruited women with no major health problems from general practice registers in England, Australia and New Zealand, whilst EPHT 2006 included healthy women with no major health problems drawn from population samples in Estonia. In both trials, participant baseline cardiovascular risk factors were low and consistent with those observed in the general population of post‐menopausal women within this age group.
Secondary prevention trials
Ten studies included women with established cardiovascular disease (EAGAR 2006; ERA 2000; ESPRIT 2002; EVTET 2000; HALL 1998; HERS I 1998; WAVE 2002; WELL‐HART 2003; WEST 2001; WHISP 2006). ERA 2000, WAVE 2002 and WELL‐HART 2003 included women who had coronary artery stenosis evidenced by angiogram. HERS I 1998 and EAGAR 2006 both included women who had undergone a revascularisation procedure (coronary artery bypass graft or percutaneous coronary intervention), whilst ESPRIT 2002, WEST 2001 and WHISP 2006 included women who had had a previous acute coronary syndrome or a transient ischaemic attack (TIA). HALL 1998 included women previously hospitalised with angina, and EVTET 2000 included women who had suffered a thromboembolic event, pulmonary embolism or deep vein thrombosis. The largest of the ten trials (HERS I 1998) compared the baseline characteristics of the trial participants with a similar group of women presumed to have coronary heart disease who were participants in a survey designed to produce nationally representative data. The HERS I 1998 participants had significantly fewer smokers, women with hypertension and diabetes than the comparison group but were comparable with respect to blood pressure, body mass index, physical activity and cholesterol levels (Grady 1998).
Interventions
A number of different oestrogen alone or oestrogen and progestogen combinations had been assessed in the different trials. Two trials (ERA 2000; WELL‐HART 2003) were three‐armed trials, and therefore assessed both oestrogen alone and in combination with a progestogen versus placebo. Most of the included comparisons used a moderate dose of oestrogen, for example, oestradiol 1 mg or conjugated equine oestrogen 0.625 mg daily. We assessed the following interventions.
Oestrogen‐alone hormone therapy
1 mg 17‐ß oestradiol (EAGAR 2006; EPAT 2001; WELL‐HART 2003; WEST 2001).
2 mg 17‐ß oestradiol (DOPS 2012).
2 mg oestradiol valerate (ESPRIT 2002).
0.625 mg conjugated equine oestrogen (ERA 2000; Greenspan 2005; STOP IT 2001; WAVE 2002; WHI II 2004).
Combined hormone therapy regimes
Combined hormone therapy regimens included one of the above types of oestrogen in combination with one of the two progestogens:
medroxyprogesterone acetate (MPA);
norethisterone.
The continuous combined regimens were composed of the following.
Conjugated equine oestrogen 0.625 mg with MPA 2.5 mg daily (EPHT 2006; ERA 2000; Greenspan 2005; HERS I 1998; STOP IT 2001; WAVE 2002; WHI I 2002; WISDOM 2007).
Oestradiol 2 mg with 1 mg norethisterone daily (DOPS 2012; EVTET 2000).
Whist the combined sequential regimes included:
oestradiol 1 mg daily with MPA 5 mg for 12 days each month (WELL‐HART 2003);
conjugated equine oestrogen 0.625 mg for 18 days followed by a combination with oral 5 mg MPA (HALL 1998);
conjugated equine oestrogen 2.5 mg with MPA 10 mg for seven days each month (ERT II 1979); and
oestradiol 1 mg daily with 0.5 mg norethisterone daily (WHISP 2006).
The control arm in each of the trials received placebo tablets, except DOPS 2012 which used a no treatment control. The duration of hormone therapy use varied widely across the trials, with follow‐up duration ranging from seven months (WHISP 2006) to 10.1 years (DOPS 2012). Four trials reported outcomes after hormone therapy use for around one year (EVTET 2000; HALL 1998; WHISP 2006; WISDOM 2007); two trials for two years (EPAT 2001; ESPRIT 2002), and eight trials for approximately three years (EAGAR 2006; EPHT 2006; ERA 2000; Greenspan 2005; STOP IT 2001; WAVE 2002; WELL‐HART 2003; WEST 2001). HERS I 1998 measured outcomes after 4.1 years, and continued the study unblinded for a further 2.7 years follow‐up (HERS II). Both the WHI I 2002 and WHI II 2004 trials were planned to continue for 8.5 years, but both trials were terminated early. Outcomes in WHI I 2002 were reported at 5.2 years and subsequently for a further four months of follow‐up (total follow‐up 5.6 years) for primary and selected secondary outcome measures. WHI II 2004 reported outcomes at 6.8 years and for a subsequent further three months of follow‐up (7.1 years) for primary and selected secondary outcomes, with a median time of 5.9 and 5.8 years on treatment for the hormone therapy and placebo groups, respectively. ERT II 1979 measured outcomes at 10 years. DOPS 2012 measured outcomes after 10.1 years, and continued the study unblinded for a further 5.7 years (DOPS 2012).
Outcomes
The outcomes assessed in the individual trials varied according to the trial objectives. One primary prevention trial (EPAT 2001) and five secondary prevention trials (ERA 2000; ESPRIT 2002; WAVE 2002; WELL‐HART 2003; WHISP 2006) aimed to assess the effects of hormone therapy upon intermediate outcomes: carotid artery intima‐media thickness, lipid and coagulation biomarkers and the impact on coronary atherosclerosis as measured by angiographic coronary stenosis. Two trials, including populations free from cardiovascular disease assessed bone mineral density, physical and cognitive parameters (Greenspan 2005; STOP IT 2001), and one study did not state any outcomes but reported a range of adverse events seen in the participants over a ten year follow‐up period (ERT II 1979).
The primary aim in the largest two trials, WHI I 2002 and WHI II 2004, was to assess the potential cardioprotective effect of hormone therapy in relatively healthy post‐menopausal women, and therefore both trials reported cardiovascular clinical endpoints as the primary outcome. Invasive breast cancer was the designated primary adverse outcome in both trials, with the incidence of other cancers, fractures, gallbladder disease and death reported as secondary outcomes. A further two primary prevention trials (EPHT 2006; WISDOM 2007) also measured similar outcomes, with cardiovascular disease outcomes designated as the primary ones of interest. A further primary prevention study (DOPS 2012) had a primary outcome of osteoporotic fractures but had a predefined safety composite endpoint of death, admission to hospital for myocardial infarction and heart failure; it also reported a number of cardiovascular endpoints individually. The remaining five secondary prevention trials aimed to examine the effects of hormone therapy in women with already established clinical disease, with the primary outcome designated according to the underlying patient pathology. Their primary outcomes were myocardial infarction or death (ESPRIT 2002; HERS I 1998), thromboembolism (EVTET 2000), stroke (WEST 2001), and angina (HALL 1998). Adverse events due to hormone therapy were not analysed other than those prespecified as primary or secondary outcomes.
Funding Source
Eighteen out of 19 trials reported the funding source. Only one of the trials, HERS I 1998 was exclusively funded by the pharmaceutical industry (Wyeth‐Ayerst), whilst EVTET 2000 and WHISP 2006 were part funded by a grant from Novo‐Nordisk Pharmaceutical. STOP IT 2001 was mainly funded by the US National Institutes of Health (NIH) but was additionally supported by Wyeth‐Ayerst, Hoffman‐LaRoche, Inc. Pharm. and Pharmacia and Upjohn. The study medication for ERA 2000, WHI I 2002 and WHI II 2004 was provided by Wyeth‐Ayerst Research, for ESPRIT 2002 by Schering AG and for WEST 2001 by Mead Johnson laboratories. DOPS 2012 was supported by study medication from Novo Nordisk, Novartis and Leo Pharma Denmark, Greenspan 2005 by medication from Wyeth‐Ayerst Laboratories and Merck Research Laboratories and WELL‐HART 2003 by medication from Mead Johnson Laboratries and Pharmacia and Upjohn.
Excluded studies
We excluded fifty papers. The primary reasons for the exclusion were:
thirty‐seven studies reported no relevant outcomes of interest to this review;
three assessed a different intervention;
five were not RCTs;
two did not investigate the relevant population;
one reported the open‐label follow‐up of an included study; and
two had insufficient duration of intervention or follow‐up.
Risk of bias in included studies
The design and methods within the trials were generally well reported. The review authors’ judgements about the risk of bias in the included studies are presented in Figure 3 and Figure 4.
3.
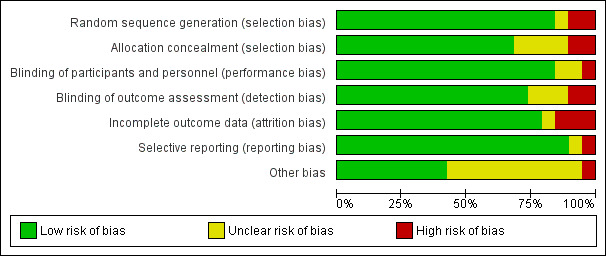
Figure 3: Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
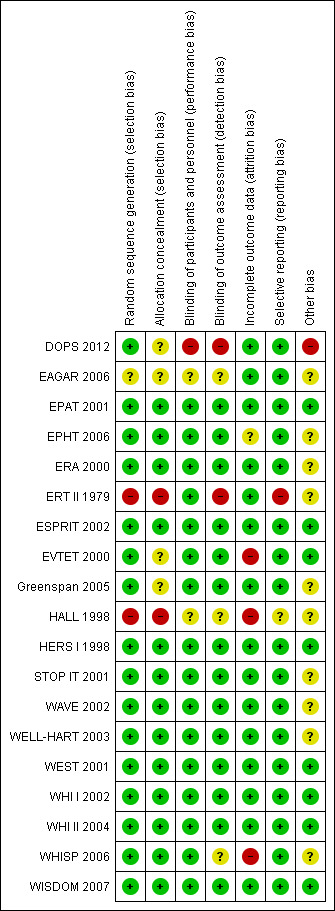
Figure 4: Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The generation of randomised sequence was adequate in 15 out of the 19 trials; in all but three of these 15 trials (Greenspan 2005; STOP IT 2001; WHISP 2006) it was computer‐generated. Three trials (DOPS 2012; EAGAR 2006; HALL 1998) did not report the methods used to generate random allocation, and therefore it is unclear as to whether the method used was satisfactory. One trial used a research nurse to randomly assign matched pairs of participants to treatment or placebo (ERT II 1979).
Thirteen trials described a satisfactory method of allocation concealment. One of these thirteen trials, EPHT 2006 randomised women who expressed an interest in participating, but did not open the randomisation envelope until their eligibility had been checked and they had consented. Five of the trials (DOPS 2012; EAGAR 2006; EVTET 2000; HALL 1998; WELL‐HART 2003) did not report methods of allocation concealment. One trial reported inadequate methods for allocation concealment (ERT II 1979).
Blinding
All the trials except HALL 1998 and DOPS 2012 were described as double‐blind. Twelve of the trials explicitly stated that all participants, clinical staff and outcome assessors were blinded to treatment allocation, and all 19 trials reported ‘hard’ outcomes; the verification of which is unlikely to be affected by blinding. Unblinding of participants occurred in 331 women initially randomised into the active single hormone therapy treatment arm in WHI II 2004, who, after a protocol change, were unblinded and changed arms into the WHI I 2002 combined therapy arm. Eight of the trials additionally described an unblinding mechanism to be used in the management of adverse events (ERA 2000; ERT II 1979; ESPRIT 2002; WAVE 2002; WEST 2001; WHI I 2002; WHI II 2004; WISDOM 2007).
Incomplete outcome data
Thirteen of the trials analysed all participants on an intention‐to‐treat basis at least for the outcomes of interest in the present review, whilst data in WAVE 2002 were analysed on an ITT basis for over 97% of participants. Drop‐out rates (medication non‐compliance) were generally high, particularly in the active treatment groups, and tended to increase over time. In the 15 trials that reported data on adherence, these ranged from greater than 90% compliance rates in EPAT 2001 and WELL‐HART 2003 at two and three years follow‐up, respectively, to less than 40% compliance in EPHT 2006 at four‐years follow‐up. In the two WHI trials with the greatest number of participants, 42% of the active treatment group and 38% of the placebo group were no longer taking their allocated treatment at 5.2 years in WHI I 2002, and 10.7% of the placebo group had initiated active hormone therapy treatment outside of the trial. Whilst in WHI II 2004, 53% of participants overall were no longer taking their allocated treatment at 6.8 years, and a further 5.7% had initiated hormone use outside the study. The two studies with longest follow‐up duration did not report adherence, DOPS 2012 and ERT II 1979. However the latter is likely to have very high, if not complete adherence, as all patients were inpatients for the duration of the study. A summary of medication compliance within the trials is given in Table 7. Losses to follow‐up were low in most of the trials, with no women lost to follow‐up in nine trials (DOPS 2012; EPAT 2001; ERA 2000; ERT II 1979; ESPRIT 2002; EVTET 2000; Greenspan 2005; HALL 1998; WEST 2001) and between 0.1% to 5.2% lost in six other trials (EPHT 2006; HERS I 1998; WAVE 2002; WHI I 2002; WHI II 2004; WISDOM 2007); two trials with significant levels of participants lost to follow‐up were STOP IT 2001 (14.9%) and WHISP 2006 (19%).
3. Medication adherence in the trials.
| Trial | Adherence definition | Assessment method | HR arm | Placebo arm |
| DOPS 2012 | Not reported | |||
| EAGAR 2006 | % study medication taken |
Pill counts | > 80% up to 30 months of treatment | > 80% up to 30 months of treatment |
| EPAT 2001 | % study medication taken |
Pill counts | Level of adherence 95% (87% of participants evaluated) | Level of adherence 92% (92% of participants evaluated) |
| EPHT 2006 | > 80% of prescribed treatment taken |
Number of collected/returned drugs and clinic reports |
< 40% compliant at three years (estimated from graph) | < 30% compliant at three years (estimated from graph) |
| ERA 2000 | % study medication taken |
Pill counts | Level of adherence at 3.2 years: Women on single therapy (measured in 79% of participants): 74%; women on combination therapy (measured in 82% of participants): 84% |
Level of adherence at 3.2 years: (measured in 80% of participants): 86% 5 women initiated treatment outside study |
| ERT II 1979 | Not reported | |||
| ESPRIT 2002 | “Regular tablet use” | Self report to family doctor. Self report to study nurse at six weeks and whenever in contact with trial staff |
Number non‐adherent: 51% at 12 months 57% at 24 months |
Number non‐adherent: 31% at 12 months 37% at 24 months |
| EVTET 2000 | Not reported | |||
| STOP IT 2001 | Not defined | Pill counts | 65% at 36 months in those taking HRT 62% at 36 months in those taking HRT + calcitriol |
78% at 36 months |
| Greenspan 2005 | ≥ 80% of the medication ≥ 80% of the study period | Not reported | 61% | 67% |
| HALL 1998 | Not reported |
|||
| HERS I 1998 | Taking at least 80% of study medication |
Pill counts | 82% adherent at one year; 75% adherent at three years 3% initiated treatment outside study About 50% continued to use open‐label HT during unblinded follow‐up (4.2 to 6.8 years) |
91% adherent at one year; 81% adherent at three years Under 10% used HRT during unblinded follow‐up (4.2 to 6.8 years) |
| WAVE 2002 | % study medication taken |
Pill counts | At 2.8 years: Adherence 67% in the 78% of women analysed |
At 2.8 years: Adherence 70% in the 81% of women analysed |
| WELL‐HART 2003 | % study medication taken |
Pill counts | Oestrogen group: 92.6% with oestrogen and 99.9% with progestin matched placebo Oestrogen‐Progestin group: 94.1% with oestrogen and 96.1% with progestin |
93.6% with oestrogen matched placebo and 98.4% with progestin matched placebo |
| WEST 2001 | % study medication taken |
Self report to study nurse three‐monthly Computer chip in medication bottle records opening date and time Pill counts |
At 2.8 years: Mean adherence including drop‐outs: 70% Mean adherence excluding dropouts: 90% 35% discontinued medication by 2.8 years, of whom 1% initiated treatment outside study |
At 2.8 years: Mean adherence including dropouts: 74% over 2.8 years Mean adherence excluding dropouts: 90% 24% discontinued medication 2% initiated treatment outside study |
| WHI I 2002 | Taking at least 80% of study medication Temporary discontinuation (e.g. during surgery) permitted |
Weighing medication bottles | 42% non‐adherent by 5.2 years Of these 6.2% initiated HRT outside study |
38% non‐adherent by 5.2 years Of these 10.7% initiated HRT outside study |
| WHI II 2004 | Taking at least 80% of study medication Temporary discontinuation (e.g. during surgery) permitted |
Weighing medication bottles | At 6.8 years, about 53.8% of women were non‐adherent In addition 5.7% of women had initiated hormone use through their own physician |
At 6.8 years, about 53.8% of women were non‐adherent In addition 9.1% of women had initiated hormone use through their own physician |
| WHISP 2006 | Not specified | Not specified | 25 out of 49 discontinued study drug (51%) | 14 out of 51 discontinued study drug (27%) |
| WISDOM 2007 | Supply of study medication |
Time at risk minus temporary interruptions and time after withdrawal from treatment | 73% of time | 86% of time |
HRT: hormone replacement therapy
Selective reporting
Two trials (ERT II 1979; HALL 1998) may have been subject to selective reporting. The remaining 17 trials reported all expected outcomes.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Results are reported below. It was not possible to conduct analyses for all outcomes stratified by time of hormone therapy commenced since menopause (< 10 years or ≥ 10 years), where these data were not available, participants' mean age at baseline was used (< 60 years of age or ≥ 60 years of age), as WHI I 2002 and WHI II 2004, which contributed the majority of events to this analysis, only reported some of the outcomes of interest in these subgroups.
Hormone therapy versus placebo in primary prevention
This comparison was assessed in nine trials (DOPS 2012; EPAT 2001; EPHT 2006; ERT II 1979; Greenspan 2005; STOP IT 2001; WHI I 2002; WHI II 2004; WISDOM 2007) with a total of 34,767 participants. A summary of the trials reporting each outcome is presented in Table 8 and a summary of the outcomes and their relative risks are presented in Table 1.
4. Summary of outcomes by trials ‐ primary prevention.
| Outcome | Death | Death (CV causes) | Non‐fatal MI | Stroke | Angina | Venous thromboembolism | Pulmonary embolism | Revascularisation |
| Trial | ||||||||
| DOPS 2012 | ✓ | ✓ | ✓ | ✓ | X | ✓ | X | X |
| EPAT 2001 | X | X | ✓ | X | X | X | X | ✓ |
| EPHT 2006 | ✓ | X | X1 | X | X | X | X | X |
| ERT II 1979 | ✓ | X | ✓ | X | X | X | X | X |
| Greenspan 2005 | ✓ | X | ✓ | X | X | ✓ | X | X |
| STOP IT 2001 | ✓ | X | ✓ | ✓ | X | ✓ | X | X |
| WHI I 2002 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| WHI II 2004 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| WISDOM 2007 | ✓ | X | X | X | X | ✓ | ✓ | X |
| Number of trials | 8 | 3 | 7 | 4 | 2 | 6 | 3 | 3 |
| Total N | 34,422 | 28,353 | 29,482 | 28,719 | 27,347 | 33,477 | 31,732 | 27,569 |
1 No events in control group. CV: cardiovascular; MI: myocardial infarction
There was no strong evidence that hormone therapy has an effect on all‐cause mortality (RR 1.00, 95% CI 0.89 to 1.12; 34,422 participants in 8 studies) (Analysis 1.1), or any cardiovascular disease outcomes, including death by cardiovascular causes (RR 0.81, 95% CI 0.47 to 1.40; 28,353 participants in 3 studies) (Analysis 1.2), non‐fatal myocardial infarction (RR 1.02, 95% CI 0.80 to 1.31; 29,482 participants in 7 studies) (Analysis 1.3), angina (RR 0.90, 95% CI 0.74 to 1.08; 27,347 participants in 2 studies) (Analysis 1.5), or revascularisation (RR 0.96, 95% CI 0.85 to 1.09; 27,569 participants in 3 studies) (Analysis 1.8). However there was an increased risk of stroke (RR 1.32, 95% CI 1.12 to 1.56; 28,719 participants in 4 studies) relative to placebo (Analysis 1.4). The WHI II 2004 authors noted that the excess risk of stroke in the intervention arm was due to an increased risk of ischaemic rather than haemorrhagic stroke, which became apparent after four years of follow‐up (Hendrix 2006). There was also an increased risk of venous thromboembolism (RR 1.92, 95% CI 1.24 to 2.99; 33,477 participants in 6 studies) relative to placebo (Analysis 1.6), and also of pulmonary embolism (RR 1.89, 95% CI 1.17 to 3.04; 31,732 participants in 3 studies) relative to placebo (Analysis 1.7). The absolute risk increase for stroke was 0.006 (number needed to treat to harm (NNTH)) = 165; mean length of follow‐up: 4.21 years (range: 2.0 to 7.1), for venous thromboembolism 0.008 (NNTH = 118; mean length of follow‐up: 5.95 years (range: 1.0 to 7.1), and for pulmonary embolism 0.004 (NNTH = 242; mean length of follow‐up: 3.13 years (range: 1.0 to 7.1)). There was substantial heterogeneity in the studies assessing the outcome of death from cardiovascular causes (I2 = 69%). There was also moderate heterogeneity in the studies assessing the outcome of venous thromboembolism and pulmonary embolism (I2 = 56% and 54%, respectively). There was no significant heterogeneity between studies for the remaining outcomes.
1.1. Analysis.
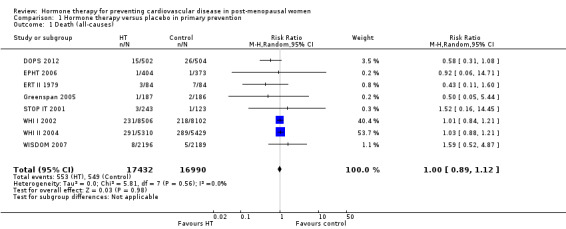
Comparison 1 Hormone therapy versus placebo in primary prevention, Outcome 1 Death (all‐causes).
1.2. Analysis.
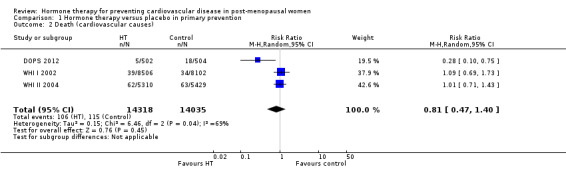
Comparison 1 Hormone therapy versus placebo in primary prevention, Outcome 2 Death (cardiovascular causes).
1.3. Analysis.
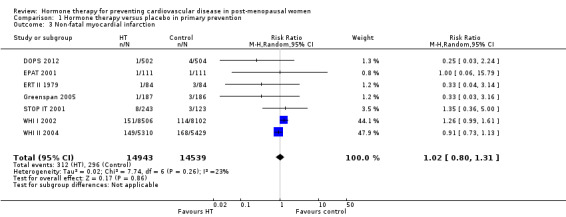
Comparison 1 Hormone therapy versus placebo in primary prevention, Outcome 3 Non‐fatal myocardial infarction.
1.5. Analysis.
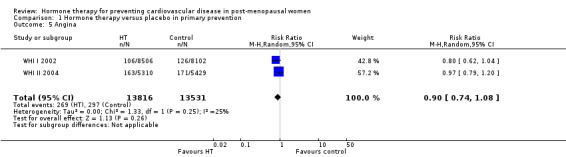
Comparison 1 Hormone therapy versus placebo in primary prevention, Outcome 5 Angina.
1.8. Analysis.
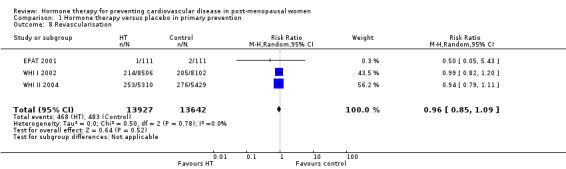
Comparison 1 Hormone therapy versus placebo in primary prevention, Outcome 8 Revascularisation.
1.4. Analysis.
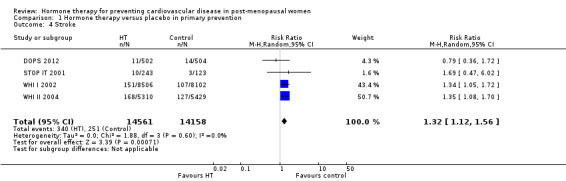
Comparison 1 Hormone therapy versus placebo in primary prevention, Outcome 4 Stroke.
1.6. Analysis.
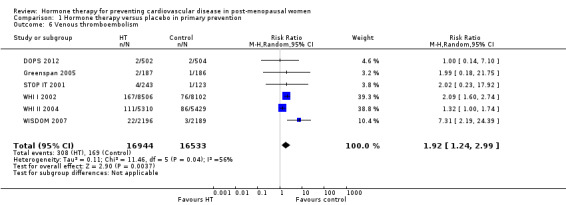
Comparison 1 Hormone therapy versus placebo in primary prevention, Outcome 6 Venous thromboembolism.
1.7. Analysis.
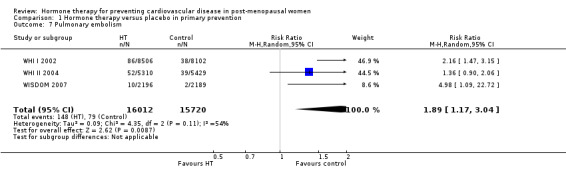
Comparison 1 Hormone therapy versus placebo in primary prevention, Outcome 7 Pulmonary embolism.
Hormone therapy versus placebo in secondary prevention
This comparison was assessed in ten trials (EAGAR 2006; ERA 2000; ESPRIT 2002; EVTET 2000; HALL 1998; HERS I 1998; WAVE 2002; WELL‐HART 2003; WEST 2001; WHISP 2006) with a total of 5766 participants. A summary of the outcomes assessed in each of the trials is presented in Table 9.
5. Summary of outcomes by trials ‐ secondary prevention.
| Outcome | Death | Death (CV causes) | Non‐fatal MI | Stroke | Angina | Venous thromboembolism | Pulmonary embolism | Revascularisation |
| Trial | ||||||||
| EAGAR 2006 | X | ✓ | ✓ | X | ✓ | X | X | ✓ |
| ERA 2000 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X | ✓ |
| ESPRIT 2002 | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | X |
| EVTET 2000 | X | X | X | X | X | ✓ | ✓ | X |
| HALL 1998 | X | X | X | X | X | X | X | X |
| HERS I 1998 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| WAVE 2002 | ✓ | ✓ | ✓ | ✓ | X | ✓ | X | X |
| WELL‐HART 2003 | ✓ | X | X | X | X | X | X | X |
| WEST 2001 | ✓ | ✓ | ✓ | ✓ | X | ✓ | X | X |
| WHISP 2006 | ✓ | X1 | ✓ | X | X | ✓ | X | X |
| Number of trials | 7 | 6 | 7 | 5 | 3 | 6 | 3 | 3 |
| Total N | 5445 | 5259 | 5359 | 5172 | 3155 | 4399 | 3920 | 3155 |
1 No outcomes relevant to this review. CV: cardiovascular; MI: myocardial infarction
There was no strong evidence that hormone therapy has an effect on all‐cause mortality (RR 1.04, 95% CI 0.87 to 1.24; 5445 participants in 7 studies) (Analysis 2.1), death by cardiovascular causes (RR 1.00, 95% CI 0.78 to 1.29; 5259 participants in 6 studies) (Analysis 2.2), non‐fatal myocardial infarction (RR 0.98, 95% CI 0.81 to 1.18; 5359 participants in 7 studies) (Analysis 2.3), angina (RR 0.91, 95% CI 0.74 to 1.12; 3155 participants in 3 studies) (Analysis 2.5), revascularisation (RR 0.98, 95% CI 0.63 to 1.53; 3155 participants in 3 studies) (Analysis 2.8), stroke RR 1.09 (95% CI 0.89 to 1.33; 5172 participants in 5 studies) (Analysis 2.4), or pulmonary embolism (RR 2.48, 95% CI 0.92 to 6.70; 3920 participants in 3 studies) (Analysis 2.7). However there was an increased risk of venous thromboembolism (RR 2.02, 95% CI 1.13 to 3.62; 4399 participants in 6 studies) relative to placebo (Analysis 2.6). The absolute risk increase was 0.014 (NNTH = 71; mean length of follow‐up: 2.46 years (range: 1.3 to 4.1)) for venous thromboembolism. There was substantial heterogeneity in studies reporting revascularisation (I² = 60%). There was no significant heterogeneity between studies for the remaining outcomes.
2.1. Analysis.
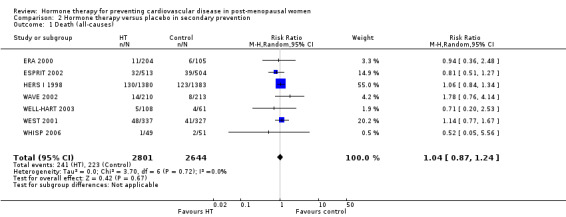
Comparison 2 Hormone therapy versus placebo in secondary prevention, Outcome 1 Death (all‐causes).
2.2. Analysis.
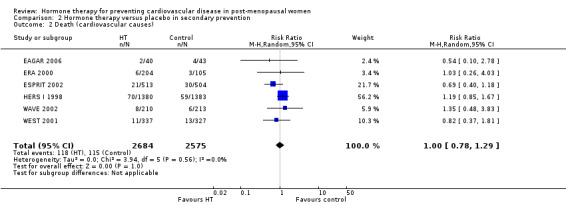
Comparison 2 Hormone therapy versus placebo in secondary prevention, Outcome 2 Death (cardiovascular causes).
2.3. Analysis.
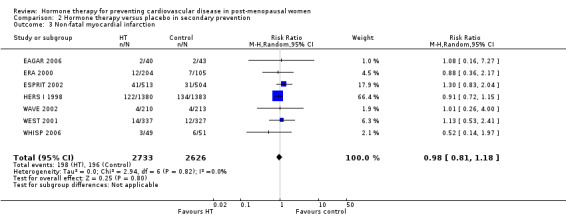
Comparison 2 Hormone therapy versus placebo in secondary prevention, Outcome 3 Non‐fatal myocardial infarction.
2.5. Analysis.
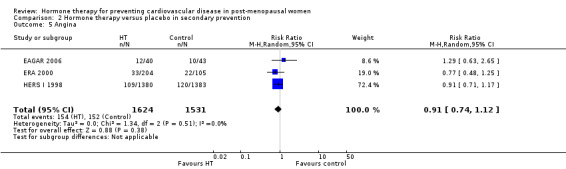
Comparison 2 Hormone therapy versus placebo in secondary prevention, Outcome 5 Angina.
2.8. Analysis.
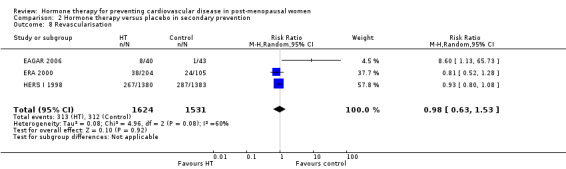
Comparison 2 Hormone therapy versus placebo in secondary prevention, Outcome 8 Revascularisation.
2.4. Analysis.
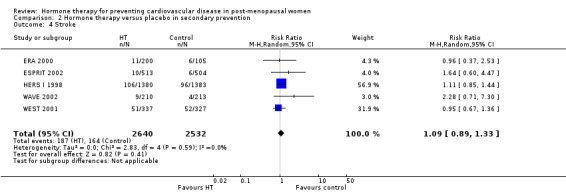
Comparison 2 Hormone therapy versus placebo in secondary prevention, Outcome 4 Stroke.
2.7. Analysis.
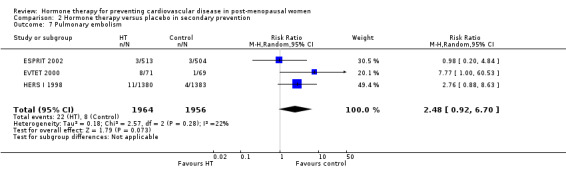
Comparison 2 Hormone therapy versus placebo in secondary prevention, Outcome 7 Pulmonary embolism.
2.6. Analysis.
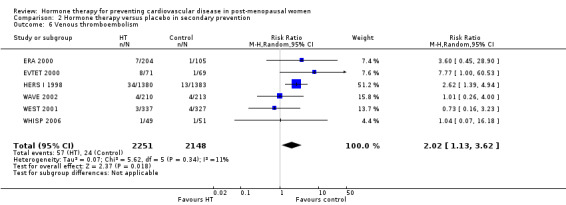
Comparison 2 Hormone therapy versus placebo in secondary prevention, Outcome 6 Venous thromboembolism.
Hormone therapy versus placebo in both primary and secondary prevention
Consistent with effects observed in both primary and secondary prevention, there was no strong evidence that hormone therapy overall had an effect on the outcomes of death (RR 1.01, 95% CI 0.92 to 1.11; 35,483 participants in 14 studies) (Analysis 3.1), death from cardiovascular causes (RR 0.96, 95% CI 0.78 to 1.18; 33,613 participants in 9 studies) (Analysis 3.2), non‐fatal myocardial infarction (RR 1.01, 95% CI 0.89 to 1.14; 34,841 participants in 14 studies) (Analysis 3.3), angina (RR 0.90, 95% CI 0.79 to 1.03; 30,502 participants in 5 studies) (Analysis 3.5), or revascularisation procedures (RR 0.95, 95% CI 0.85 to 1.05; 30,724 participants in 6 studies) (Analysis 3.8) compared to placebo. Again, an increased risk of stroke (RR 1.24, 95% CI 1.10 to 1.41; 34,672 participants in 10 studies), venous thromboembolism (RR 1.92, 95% CI 1.36 to 2.69; 37,313 participants in 10 studies) and pulmonary embolism (RR 1.81, 95% CI 1.32 to 2.48; 36,316 participants in 7 studies) relative to placebo was observed (Analysis 3.4; Analysis 3.6; Analysis 3.7). An absolute risk increase of 0.006 (NNTH = 165; mean length of follow‐up: 4.21 years (range: 2.0 to 7.1)) for stroke, an absolute risk increase of 0.008 (NNTH = 118; mean length of follow‐up: 5.95 years (range: 1.0 to 7.1)) for venous thromboembolism, and an absolute risk increase of 0.004 (NNTH = 242; mean length of follow‐up: 3.13 years (range: 1.0 to 7.1)) for pulmonary embolism were observed. There was moderate statistical heterogeneity present between trials for the outcome of venous thromboembolism (I2 = 40%). There was no significant heterogeneity between studies in the remaining outcomes. There was no evidence of funnel plot asymmetry (Figure 5).
3.1. Analysis.
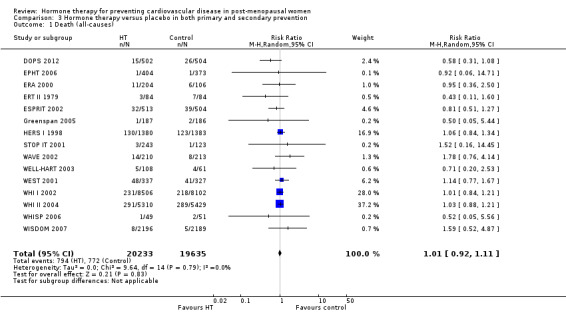
Comparison 3 Hormone therapy versus placebo in both primary and secondary prevention, Outcome 1 Death (all‐causes).
3.2. Analysis.
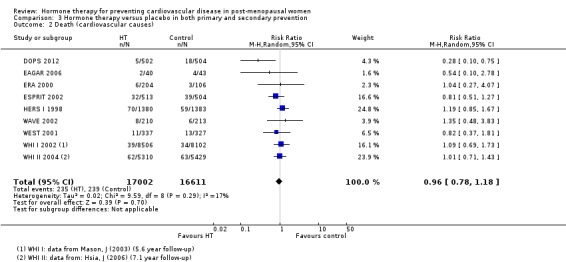
Comparison 3 Hormone therapy versus placebo in both primary and secondary prevention, Outcome 2 Death (cardiovascular causes).
3.3. Analysis.
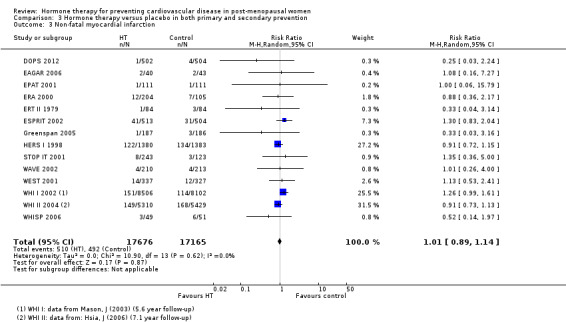
Comparison 3 Hormone therapy versus placebo in both primary and secondary prevention, Outcome 3 Non‐fatal myocardial infarction.
3.5. Analysis.
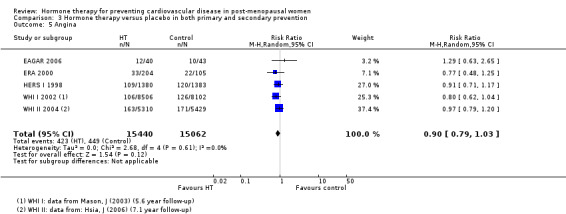
Comparison 3 Hormone therapy versus placebo in both primary and secondary prevention, Outcome 5 Angina.
3.8. Analysis.
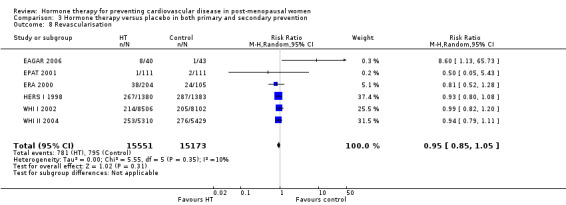
Comparison 3 Hormone therapy versus placebo in both primary and secondary prevention, Outcome 8 Revascularisation.
3.4. Analysis.
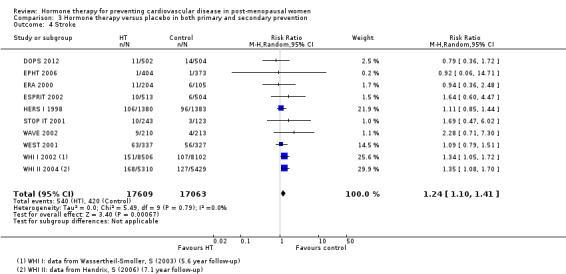
Comparison 3 Hormone therapy versus placebo in both primary and secondary prevention, Outcome 4 Stroke.
3.6. Analysis.
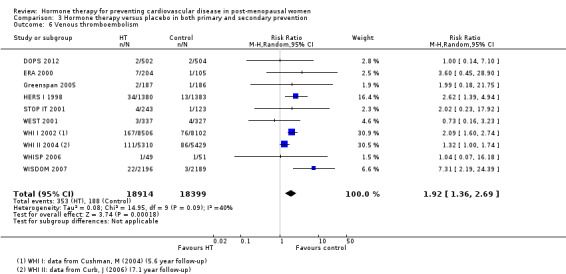
Comparison 3 Hormone therapy versus placebo in both primary and secondary prevention, Outcome 6 Venous thromboembolism.
3.7. Analysis.
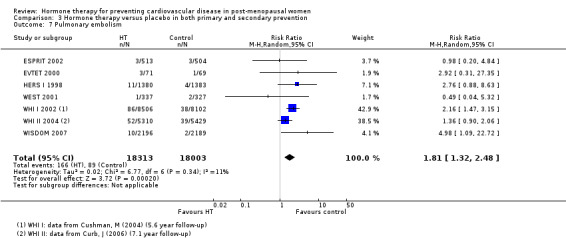
Comparison 3 Hormone therapy versus placebo in both primary and secondary prevention, Outcome 7 Pulmonary embolism.
5.
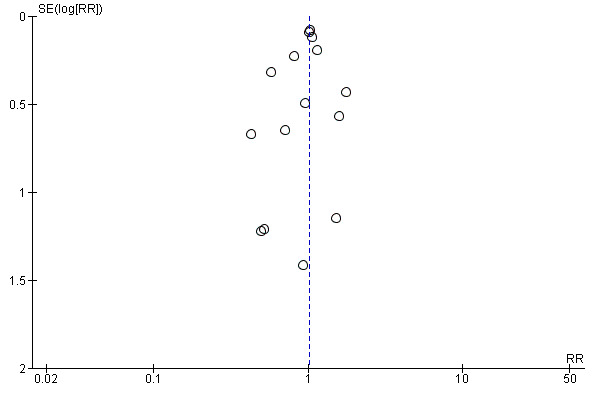
Funnel plot of comparison: Hormone therapy versus placebo in primary and secondary prevention, outcome: 5.1 Death (all‐causes).
Sensitivity Analysis
Five trials assessing hormone therapy in primary prevention were stopped early (DOPS 2012; EPHT 2006; WHI I 2002; WHI II 2004; WISDOM 2007). To assess what effect this may have had, we performed a subgroup analysis on trials which did not stop early to see if the effect was significantly changed. There was no strong evidence of change in the balance of effect for the outcomes of death from any cause, non‐fatal myocardial infarction or revascularisation. It was not possible to perform this analysis for death from cardiovascular causes, angina or pulmonary embolism as there were no studies which reported these outcomes which did not stop early. However, there were changes in the remaining outcomes. For the outcome of stroke, once the studies which stopped early were removed (DOPS 2012; WHI I 2002; WHI II 2004) the outcome changed (from: RR 1.32, 95% CI 1.12 to 1.56 to: RR 1.69, 95% CI 0.47 to 6.02) due to only one study remaining in the analysis (STOP IT 2001). The outcome of venous thromboembolism also changed (from: RR 1.92, 95% CI 1.24 to 2.99 to: RR 2.01, 95% CI 0.40 to 10.06) with only two trials remaining in the analysis (Greenspan 2005; STOP IT 2001).
Two trials assessing hormone therapy in secondary prevention were stopped early (EAGAR 2006; EVTET 2000). To assess what effect this may have had, we performed a subgroup analysis on trials which did not stop early to see if the effect was significantly changed. There was no strong evidence of change in the balance of effect for the outcomes of death from cardiovascular causes, non‐fatal myocardial infarction, angina, venous thromboembolism, pulmonary embolism or revascularisation. It was not possible to perform this analysis for death from any cause or stroke as there were no studies which reported these outcomes which stopped early.
Seven trials assessing hormone therapy in primary and secondary prevention were stopped early (DOPS 2012; EAGAR 2006; EPHT 2006; EVTET 2000; WHI I 2002; WHI II 2004; WISDOM 2007). To assess what effect this may have had, we performed a subgroup analysis on trials which did not stop early to see if the effect was significantly changed. There was no strong evidence of change in the balance of effect for the outcomes of death from any cause, death from cardiovascular causes, non‐fatal myocardial infarction, angina, venous thromboembolism or revascularisation. For the outcome of stroke, once the studies which stopped early were removed (DOPS 2012; EPHT 2006; WHI I 2002; WHI II 2004) the outcome changed (from: RR 1.24, 95% CI 1.10 to 1.41 to: RR 1.14, 95% CI 0.94 to 1.39). The outcome of pulmonary embolism, once the studies which stopped early were removed (EVTET 2000; WHI I 2002; WHI II 2004; WISDOM 2007) changed (from: RR 1.81, 95% CI 1.32 to 2.48 to: RR 1.57, 95% CI 0.62 to 3.95).
Subgroup analyses
Duration of treatment
To assess the effect of duration of treatment, we compared data in two different ways. Where data were reported by year of follow‐up (HERS I 1998; WHI I 2002; WHI II 2004), we analysed them both non‐cumulatively (year‐by‐year), as well as cumulatively, incorporating the remainder of the trials where data were not reported by year of follow‐up, according to the total duration of trial follow‐up. We classified these analyses a priori as exploratory, given the heterogeneity between the different hormone therapy regimens assessed and the patient populations in the different trials. To conduct the analyses, we had to round up or down the time points for the reporting of outcomes in the trials. We rounded data as follows: WHISP 2006 and WISDOM 2007 reported results after a median follow‐up of 7 months and 11.9 months (range 7.1 to 19.6), respectively; results were therefore reported at one‐year follow‐up. EVTET 2000 was conducted for a 1.3 year period; results from this trial were therefore also reported for one‐year follow‐up. EPAT 2001, ESPRIT 2002 and HALL 1998 were all reported for two years of follow‐up. Greenspan 2005 and STOP IT 2001 both reported three years of follow‐up, WELL‐HART 2003 had a median follow‐up of 3.3 years, EPHT 2006 (median length of follow‐up: 3.4 years (range: 2 to 4.9)), EAGAR 2006 (mean follow‐up: 3.5 years (range: 25 to 41)), ERA 2000 (mean follow‐up: 3.2 years (range: 2.8 to 3.8)), WAVE 2002 (mean follow‐up: 2.8 years (range: 2.1 to 3.9)) and WEST 2001 (mean follow‐up: 2.8 years (range: 1.6 to 4.1)) were all classified as having a three‐year follow‐up period. Final outcome results within the blinded part of the HERS I 1998 trial were reported at a mean of 4.1 years and this was classified as having a four‐year follow‐up period. However there were selected clinical outcomes reported for each year of follow‐up which were included in the analysis. Outcome data for the 4 to 6.8 years (unblinded, open‐label) follow‐up period were not included in the standard pair‐wise meta‐analyses. Final outcome results for WHI I 2002 reported results after a mean follow‐up of 5.6 years (range: 3.5 to 8.5), analysed as five years, however selected clinical outcomes were also reported for each year of follow‐up to five years and this was included in the analysis. WHI II 2004 reported results by year of follow‐up for eight years for selected outcomes. ERT II 1979 reported a follow‐up of 10 years. DOPS 2012 reported a mean follow‐up of 10.1 years which was rounded down to 10 years.
We only assessed the outcome of death in this way as with other outcomes there was a risk of counting participants multiple times. By individual year of treatment from year one to eight, there was no strong evidence of difference between hormone therapy and placebo for any year analysed (Analysis 3.9). For the cumulative effect of treatment on the outcome of death, there was no strong evidence of difference between hormone therapy and control for years one up until eight. There were no data for nine years of treatment. Ten years of treatment showed a survival benefit in the hormone therapy group (RR 0.55, 95% CI 0.31 to 0.96) based on the results of two studies (DOPS 2012; ERT II 1979) (Analysis 3.10). The absolute risk reduction for death was 0.003 (NNTH = 333). There was no statistically significant heterogeneity between the trials for any of the outcomes.
3.9. Analysis.
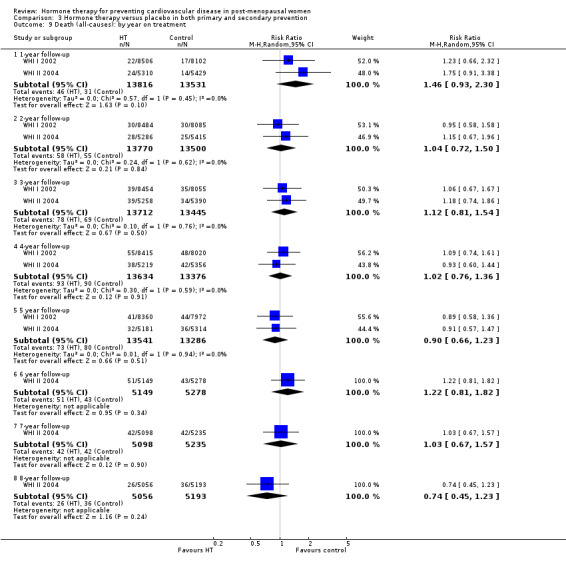
Comparison 3 Hormone therapy versus placebo in both primary and secondary prevention, Outcome 9 Death (all‐causes): by year on treatment.
3.10. Analysis.
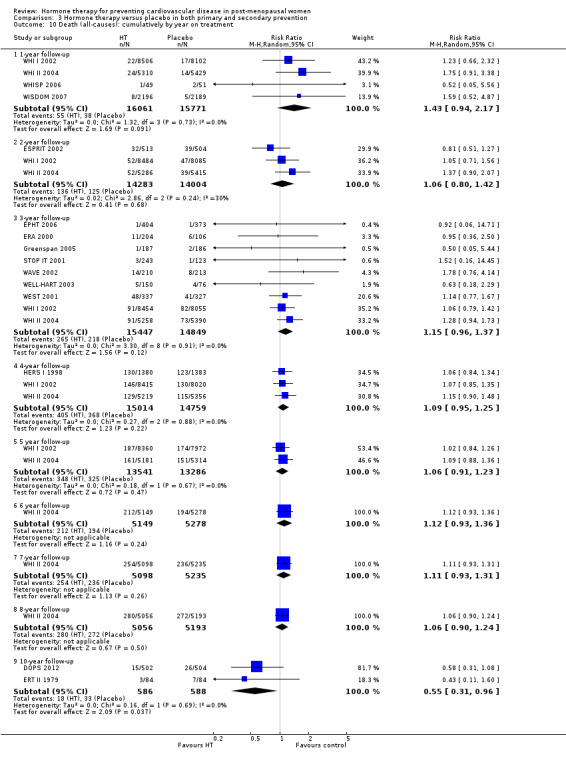
Comparison 3 Hormone therapy versus placebo in both primary and secondary prevention, Outcome 10 Death (all‐causes): cumulatively by year on treatment.
The timing hypothesis
To assess the timing hypothesis and the potential impact of the time since menopause that treatment was commenced, we stratified trials according to when treatment was started. This was characterised as starting treatment either < 10 or ≥10 years after the menopause, or if these data were not available, then we used mean age of participants at baseline (< 60 versus ≥ 60 years of age) as a surrogate. In some cases, studies reported the results in subgroups, according to the time since the menopause that treatment was started, or age that treatment was started (WHI I 2002 and WHI II 2004). For the remaining studies, we used mean time since menopause that treatment commenced or mean age that treatment commenced. It is not possible to say with absolute certainty that all individuals in these studies are correctly attributed to the correct treatment timing subgroup, however, with the reported standard deviations of the mean time since menopause or mean age that treatment was commenced, we can be confident that the vast majority are correctly assigned and this allows us to make an important subgroup analysis, though the limitations of this analysis must be borne in mind.
The reason we used a composite endpoint of coronary heart disease and did not report the remaining prespecified outcomes was due to the largest trials (WHI I 2002; WHI II 2004) reporting this composite endpoint according to number of decades after menopause at which treatment was started, and venous thromboembolism according to age in decades at which treatment was started. We did not include the remaining outcomes in this analysis as there were insufficient data reported according to time that treatment was started in relation to the menopause or age to allow for accurate analysis.
The results of the subgroup analysis provide some supportive evidence for the timing hypothesis for the outcomes of death from all causes and coronary heart disease. All cause death was lower in the subgroup of studies where treatment was started within 10 years of the menopause compared with studies where more than 10 had elapsed since the menopause had started (P = 0.01; Analysis 4.1). The risk of coronary heart disease was also lower in women who had commenced therapy less than 10 since the start of the menopause (P = 0.02; Analysis 4.2). We did not find evidence of a difference between the subgroups for the outcomes of stroke and venous thromboembolism (P = 0.66; Analysis 4.3, and P = 0.7; Analysis 4.4 respectively).
4.1. Analysis.
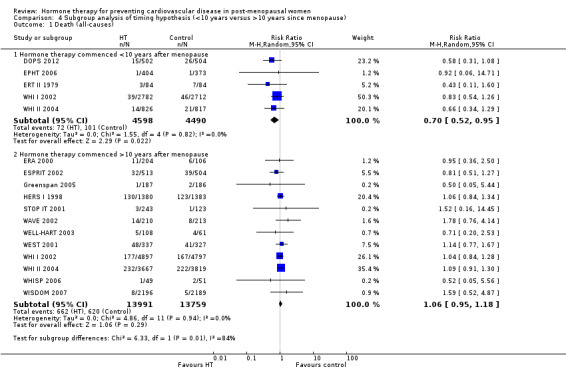
Comparison 4 Subgroup analysis of timing hypothesis (<10 years versus >10 years since menopause), Outcome 1 Death (all‐causes).
4.2. Analysis.
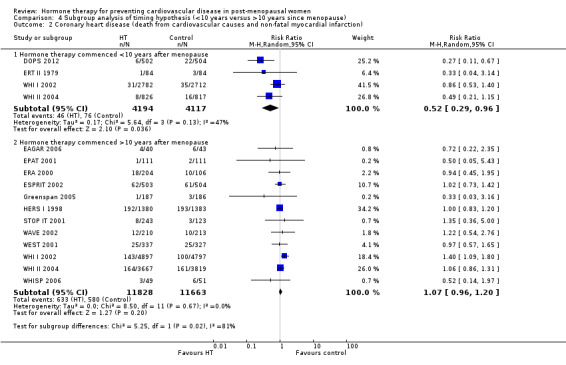
Comparison 4 Subgroup analysis of timing hypothesis (<10 years versus >10 years since menopause), Outcome 2 Coronary heart disease (death from cardiovascular causes and non‐fatal myocardial infarction).
4.3. Analysis.
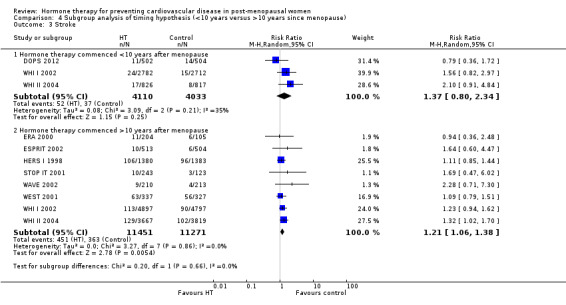
Comparison 4 Subgroup analysis of timing hypothesis (<10 years versus >10 years since menopause), Outcome 3 Stroke.
4.4. Analysis.
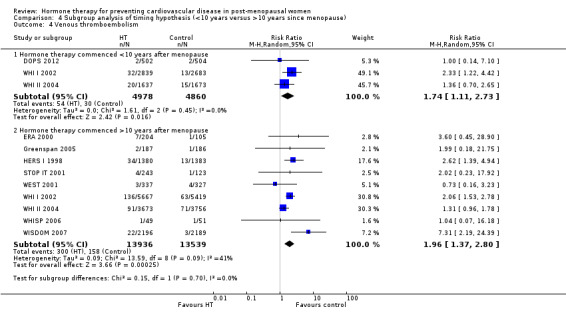
Comparison 4 Subgroup analysis of timing hypothesis (<10 years versus >10 years since menopause), Outcome 4 Venous thromboembolism.
Hormone therapy commenced less than 10 years after the menopause
Six trials and 9629 participants were included in this analysis (DOPS 2012; ERT II 1979; EPHT 2006; EVTET 2000; WHI I 2002; WHI II 2004) (Table 10). Three trials reported a mean time since menopause for the study population at the start of the study of less than 10 years: DOPS 2012 (0.58 years), ERT II 1979 (4.6 years) and EPHT 2006 (8.8 years). One study which did not report number of years since menopause did report a mean age of the study population under 60 years (EVTET 2000 (55 years old)). Two studies reported events stratified by those participants who were less than 10 years since their menopause at the start of the study (WHI I 2002; WHI II 2004).
6. Trials according to number of years since menopause when treatment commenced.
| Study | Population | Time since menopause (years) | Mean age (years) |
| < 10 years since menopause (or mean age < 60) | |||
| DOPS 2012 | 1006 | 0.59 | |
| ERT II 1979 | 168 | 4.6 | |
| EPHT 2006 | 1178 | 8.8 | |
| EVTET 2000 | 140 | NK3 | 55.7 |
| WHI I 2002 | 5494 | < 10 yrs | |
| WHI II 2004 | 1643 | < 10 yrs | |
| TOTAL | 9629 | ||
| ≥ 10 years since menopause (or mean age ≥ 60) | |||
| WHI I 2002 | 9694 | ≥ 10 | |
| WHI II 2004 | 7468 | ≥ 10 | |
| HALL 1998 | 373 | 12.5 | |
| WISDOM 2007 | 4385 | 14.7 | |
| ESPRIT 2002 | 1017 | 16.1 | |
| HERS I 1998 | 2763 | 18 | |
| WELL‐HART 2003 | 226 | 18.2 | |
| WHISP 2006 | 100 | 22.8 | |
| ERA 2000 | 309 | 23.0 | |
| WEST 2001 | 664 | 25 | |
| EPAT 2001 | 222 | NK3 | 62.2 |
| EAGAR 2006 | 83 | NK1 | 64 |
| WAVE 2002 | 423 | NK2 | 65 |
| STOP IT 2001 | 489 | NK1 | 71 |
| Greenspan 2005 | 489 | NK3 | 71.2 |
| TOTAL | 28705 | ||
|
1Author contacted but data was not collected.
2Author contacted; reported data available from National Institutes of Health (NIH) (who funded trial) but data currently pending.
3Author emailed, but no reply. NK: not known | |||
Hormone therapy in a population who, as a mean, started treatment less than 10 years after their menopause or were less than 60 years old, demonstrated an all‐cause mortality benefit (RR 0.70, 95% CI 0.52 to 0.95; 9088 participants in 5 studies) and coronary heart disease benefit (composite of death from cardiovascular causes and non‐fatal myocardial infarction) (RR 0.52, 95% CI 0.29 to 0.96; 8311 participants in 4 studies) compared to placebo (Analysis 4.1; Analysis 4.2). There was a statistically non‐significant trend to increased risk of stroke (RR 1.37, 95% CI 0.80 to 2.34; 8143 participants in 3 studies) (Analysis 4.3). There was, however, strong evidence of increased risk of venous thromboembolism (RR 1.74, 95% CI 1.11 to 2.73; 9838 participants in 3 studies) in the hormone therapy group compared to placebo (Analysis 4.4). The absolute risk reduction for death (all‐causes) was 0.007 (NNTH = 146) and coronary heart disease (death from cardiovascular causes and non‐fatal myocardial infarction) was 0.007 (NNTH = 133). The absolute risk increase for venous thromboembolism was 0.005 (NNTH = 214). A summary of the outcomes and their relative risks are presented in Table 3. There was moderate statistical heterogeneity present between trials for the outcome of coronary heart disease (I2 = 48%). There was no strong evidence of statistically significant heterogeneity for the remaining analyses.
Hormone therapy commenced more than 10 years after the menopause
Fifteen trials and 28,705 participants were included in this analysis (EAGAR 2006; EPAT 2001; ERA 2000; ESPRIT 2002; Greenspan 2005; HALL 1998; HERS I 1998; STOP IT 2001; WAVE 2002; WELL‐HART 2003; WEST 2001; WHI I 2002; WHI II 2004; WHISP 2006; WISDOM 2007) (Table 10). Eight studies reported a mean time since menopause for the study population at the start of the study of more than 10 years: HALL 1998 (12.5 years), WISDOM 2007 (14.7 years), ESPRIT 2002 (16.1 years), HERS I 1998 (18 years), WELL‐HART 2003 (18.2 years), WHISP 2006 (22.8 years), ERA 2000 (23.0 years) and WEST 2001 (25 years). Five studies did not report number of years since menopause, but did report a mean age of the study population > 60 years (EPAT 2001 (62.2 years old), EAGAR 2006 (64 years old), WAVE 2002 (65 years old), STOP IT 2001 (71 years old) and Greenspan 2005 (71.2 years old). Two studies reported events stratified by participants who were ≥ 10 years since their menopause at the start of the study WHI I 2002; WHI II 2004).
Hormone therapy in a population who as a mean started treatment 10 years or more after menopause or were more than 60 years old demonstrated no strong evidence that hormone therapy has an effect on death (RR 1.06, 95% CI 0.95 to 1.18; 27,750 participants in 12 studies) (Analysis 4.1) or coronary heart disease (RR 1.07, 95% CI 0.96 to 1.20; 23,491 participants in 12 studies) (Analysis 4.2). However, there was strong evidence that hormone therapy is associated with an increased risk of stroke (RR 1.21, 95% CI 1.06 to 1.38; 22,722 participants in 8 studies) (Analysis 4.3) and of venous thromboembolism (RR 1.96, 95% CI 1.37 to 2.80; 27,475 participants in 9 studies) (Analysis 4.4). The absolute risk increase for stroke and venous thromboembolism was 0.01 (NNTH = 102) and 0.01 (NNTH = 101), respectively. A summary of the outcomes and their relative risks are presented in Table 4. There was moderate statistical heterogeneity present between trials for the outcome of venous thromboembolism (I2 = 41%) but not for any other outcomes.
Discussion
Summary of main results
The stimulus for updating this review was the publication of DOPS 2012, however this trial only contributed 3.5% and 19.5% weight to the all‐cause mortality and cardiovascular mortality, respectively, in the treatment of a primary prevention population. In the trial population as a whole, there is no evidence that hormone therapy has a role in either the prevention or treatment of cardiovascular disease. There was no strong evidence that treatment with hormone therapy had an effect on overall death rates, cardiovascular disease‐related death, non‐fatal myocardial infarction, angina, or the number of patients undergoing revascularisation procedures. On the contrary, it is associated with an increased risk of stroke, venous thromboembolism and pulmonary embolism.
The excess risk of stroke in our analyses was observed in the primary prevention analysis (which includes those randomised to either oestrogen alone or oestrogen in combination with progestogen compared to placebo). These findings are based on the two largest trials, WHI I 2002 and WHI II 2004, with follow‐up of 5.6 and 7.1 years, respectively. Whilst, no strong evidence of increased risk was observed in any of the secondary prevention trials, including the largest trial HERS I 1998, it is probable that the results from the primary prevention trials are applicable to secondary prevention populations, and that subgroup analyses of these trials were underpowered (due to small trial sizes, low event rates and shorter length of follow‐up) to detect any statistically significant differences in stroke rates between hormone therapy and placebo treatment arms. In both WHI I 2002 and WHI II 2004, the excess risk of stroke observed with hormone therapy use was driven by an excess of ischaemic rather than haemorrhagic stroke, with 79.8% and 80.3% of strokes, respectively, observed within the trials being ischaemic (Hendrix 2006; Wassertheil‐Smoller 2003). In the same two trials, an increased risk of stroke was apparent after two years of treatment in women taking combination hormone therapy, and after four years for women randomised to oestrogen alone (Hendrix 2006). In both trials, the hazard ratios for ischaemic stroke did not differ significantly in subgroups based on age, years since menopause, prior cardiovascular disease, hypertension or diabetes mellitus status, body mass index, or statin or aspirin use at baseline (Hendrix 2006; Wassertheil‐Smoller 2003).
The finding of increased risk for both venous thromboembolism and pulmonary embolism within the overall trial populations appears in our analyses to be driven largely by the excess risk observed in combination hormone therapy (oestrogen combined with progestogen) trials. The greatest risk in primary prevention populations was shown in WISDOM 2007 and WHI I 2002, both testing combination hormone therapy. In secondary prevention populations, the greatest risk was demonstrated in HERS I 1998, also assessing combination hormone therapy. Subgroup analysis of WHI I 2002 for the outcome of venous thromboembolism demonstrated the greatest risk with combination hormone therapy in the first year of treatment (HR 4.01), with lower risk in subsequent years (Cushman 2004). WHI II 2004 also demonstrated a tendency to higher risk early on with only modest increased risk after two years of treatment with oestrogen alone (Curb 2006). When comparing the two studies (combination hormone therapy compared to oestrogen alone), the difference in risk was most apparent after year two when the higher risk in combination hormone therapy was most noticeable (Curb 2006).
Both WHI I 2002 and WHI II 2004 undertook further prespecified subgroup analyses to evaluate the association between participant baseline characteristics and venous thromboembolism and pulmonary embolism risk. Not surprisingly, given the fact that no excess risk was observed within the trial, WHI II 2004 investigators found no strong evidence of interactions between oestrogen alone use and age, body mass index, or most other venous thromboembolism risk factors. The authors did however note, that hazard ratios for combination therapy in WHI II 2004 were significantly higher than those for oestrogen alone, even after adjusting for venous thromboembolism risk factors (Curb 2006). In WHI I 2002, increasing age, being overweight and obese, and having a factor V Leiden mutation (a blood coagulation disorder) were associated with a higher risk of venous thromboembolism compared to placebo (Cushman 2004). Both WHI I 2002 and WHI II 2004 undertook prespecified subgroup analyses to evaluate whether any clinical characteristics of the trial populations may potentially moderate the effects of hormone therapy. The potential predictor variables examined included: age, time since menopause, presence or absence of vasomotor symptoms, prior hormone use, coronary heart disease risk factor status and presence or absence of preexisting cardiovascular disease (Hsia 2006; Manson 2003). None of these variables significantly affected results, although we observed a non‐significant trend for a reduction in coronary heart disease risk for women who initiated hormone therapy use within ten years of menopause.
Subgroup analysis of time of treatment commencement in relation to the menopause, found a benefit in overall survival and coronary heart disease (composite of death from cardiovascular causes and non‐fatal myocardial infarction) in the hormone therapy group in those who started less than 10 years after their menopause (or before the age of 60). This is similar to the trend in coronary heart disease events in WHI II 2004 shown in Hsia 2006. There was no strong evidence of effect on stroke. There was, however, strong evidence of increased risk of venous thromboembolism, whether started before or after the age of 60.
There was no strong evidence of effect on death or coronary heart disease for the group who started treatment at 10 years or more after the menopause, however there was an increased risk of stroke and venous thromboembolism. We did not analyse any other outcomes, as these analyses relied significantly on subgroup reporting from WHI I 2002 and WHI II 2004, which only reported these outcomes according to time since the menopause, or age that treatment was started. There were insufficient data from other trials to make reliable analyses of other outcomes.
It is worth noting that the benefit seen in survival and coronary heart disease for the group starting treatment less than 10 years after the menopause is from combining five trials all performed in primary prevention populations and all with quite long follow‐up, ranging from 3.4 years to 10.1 years. Looking at the event rates in these individual trials it can be seen that the greatest benefit is in those trials with the longest follow‐up. It is possible that this could be due to an interaction with time on treatment, whereby coronary heart disease events occur in predisposed individuals early as opposed to later on with hormone therapy treatment, and therefore any risk reduction is observed in the later stages of treatment. This is consistent with the hazard ratio for coronary heart disease for the one‐year intervals of follow‐up observed in WHI I 2002 (Manson 2003). Therefore, it is not possible to say if short duration hormone therapy is beneficial in this population, only that hormone therapy taken for between 3.4 to 10 years is beneficial in this population.
In analysis of death according to year of treatment, there was no strong evidence of difference between treatment groups by individual year of treatment. There was also no strong evidence of difference in survival comparing cumulative years of treatment, until ten years of treatment, where there was a small survival benefit in the hormone therapy group. However, this was based on two relatively small primary prevention trials, where treatment was started shortly after the menopause (DOPS 2012; ERT II 1979). One of the trials had poor methodology (ERT II 1979). It is possible that there are other explanations for the benefit seen in this analysis, other than the duration of treatment, such as the timing of commencing treatment.
Overall completeness and applicability of evidence
There are a number of limitations to the evidence base reviewed. Firstly, it should be highlighted that the results are based on those obtained in 19 RCTs, with the majority of statistically significant findings derived from the results of the three largest trials, HERS I 1998, WHI I 2002 and WHI II 2004, which dominate the results. These three trials all evaluated oral conjugated equine oestrogen 0.625 mg, with or without continuous medroxyprogesterone (MPA) 2.5 mg. Other trials evaluating different types of hormone therapy tended to be much smaller with a shorter duration of follow‐up, and reported few if any major clinical events. There is some debate regarding the external validity of the findings of WHI I 2002 and WHI II 2004, and the degree to which they apply to any type of hormone therapy, other than continuous combined oral conjugated equine oestrogen 0.625 mg with or without MPA 2.5 mg. The effects of hormone therapy may vary with different oestrogens and progestogens, different doses and routes of administration. However, in order to pool the results of different studies statistically, we had to make assumptions regarding a ‘class effect’ of hormone therapy, which may not be warranted.
The clinical outcomes of interest in the review were secondary outcomes in five of the trials (DOPS 2012; EPAT 2001; ERA 2000; ESPRIT 2002; WAVE 2002) and reported as adverse events in five more (ERT II 1979; Greenspan 2005; STOP IT 2001; WELL‐HART 2003; WHISP 2006). It can therefore be postulated that these trials may not have been sufficiently powered in order to detect differences in clinical treatment effects between the hormone therapy and placebo arms, as this was not the primary aim of these trials. Furthermore, as previously highlighted, seven of the trials were stopped early (DOPS 2012; EAGAR 2006; EPHT 2006; EVTET 2000; WISDOM 2007; WHI I 2002; WHI II 2004), either as other trial results were published showing no beneficial effects on cardiovascular disease outcomes for hormone therapy relative to placebo, or observation of a detrimental effect either on cardiovascular disease outcomes or adverse events was shown. The mean length of trial follow‐up therefore ranged considerably from seven months to 10.1 years, with a mean duration of follow‐up of 3.6 (median of three) years across the trials. The early stopping of the trials has implications both for the power to detect differences in treatment effects between the hormone therapy and placebo arms (as the sample size will have been predicated based on the original proposed length of follow‐up, and assumptions regarding the number of events observed), and also for limiting the availability of evidence on the longer‐term treatment effects of hormone therapy compared to placebo. A further limitation of the evidence base reviewed relates to the impact of patient medication compliance, which ranged considerably between the trials. A high proportion of women in the trials did not receive the treatment to which they were randomised. Overall, the number of women who discontinued their medication or took less than 80% was disproportionately high in the hormone therapy trial arms, presumably due to medication side effects. The authors of WHI I 2002 noted that if discontinuation of treatment and initiation of non‐study treatment occurred independently of risk factors for clinical outcomes, their intention‐to‐treat analysis underestimates both the harms and benefits of hormone therapy among women who adhere to treatment.
Quality of the evidence
A summary of the findings and strength of evidence can be found in Table 1; Table 2; Table 3 and Table 4. In the primary prevention population, the quality of evidence for death and cardiovascular disease was high. We downgraded the quality of the evidence by one level for venous thromboembolism and pulmonary embolism due to inconsistency of effect across the study results. When HT considered as a secondary prevention strategy, the quality of the evidence was also high for death and venous thromboembolism. The confidence intervals for the estimated effect on stroke and pulmonary embolism could not exclude small decreases or large increases in risk. For the subgroup of studies addressing the effects of HT started less than 10 years since the menopause the quality of evidence was downgraded one level for the outcomes of mortality and coronary heart disease as the results of the analysis were dominated by the results of a few large trials. Overall study quality was high (Figure 4). The vast majority of trials had adequate generation of randomised sequences (15 out of 19), 17 out of 19 were double‐blinded and 13 out of 19 were analysed on an intention‐to‐treat basis. Participants lost to follow‐up were generally low, except in two trials: 14.9% in STOP IT 2001 and 19% in WHISP 2006, though these provided relatively low weight to the analysis. Only two out of 19 trials were at risk of selective outcome reporting.
Potential biases in the review process
There are a number of potential biases in the review process, although we made attempts to limit these. The bias of most concern is that of patient selection bias which limits external validity. Nearly all of the included trials had a mean participant age of over 60 years at baseline, and only one trial (DOPS 2012) focused on women who were either peri‐menopausal or around the time of the menopause. Whilst these inclusion criteria reflected the aims of the trials, it does not reflect usual clinical practice, in which hormone therapy is prescribed for the relief of vasomotor symptoms at the time of menopause.
Despite extensive searches it is possible that we failed to identify all relevant studies. However, given the dominance of WHI I 2002 and WHI II 2004 on the results of the review, it is unlikely that we missed any trials large enough to impact substantially on the results. Additionally, as already indicated, assumptions had to be made in the analyses regarding the effects of different HT preparations in order to undertake meta‐analyses. These assumptions may not be warranted, as it is as yet unclear how different preparations and doses may differ.
Our assessment of the timing hypothesis could be considered a post‐hoc change to the original protocol for this review. The data for events since menopause (stratified in decades) were only available for two studies (WHI I 2002; WHI II 2004) and for the remaining studies baseline characteristics for eleven studies (DOPS 2012; EPHT 2006; ERA 2000; ERT II 1979; ESPRIT 2002; HALL 1998; HERS I 1998; WELL‐HART 2003; WEST 2001; WHISP 2006; WISDOM 2007) were available for us to allocate the study population as a whole to either commencing treatment less than 10 years since the menopause or 10 years or more since the menopause. For six studies (EAGAR 2006; EPAT 2001; EVTET 2000; Greenspan 2005; STOP IT 2001; WAVE 2002) these data were not available. However, age was reported and therefore whole study populations were allocated accordingly to those less than 60 years of age or those 60 years of age or older when they commenced treatment. It is highly likely that trial populations were distributed across a range of ages and time since menopause, and it is therefore likely that a proportion of study populations were incorrectly allocated. This will be more of a problem in study samples with large standard deviations for time since menopause, or age, and also in those who have a mean or median age or time since menopause close to the cut‐off (10 years since the menopause and 60 years of age). Although we remain confident that the subgroups are broadly representative of the study populations of interest, subgroup level data for each study or individual participant data would represent more robust approaches to testing the timing hypothesis.
Agreements and disagreements with other studies or reviews
Magliano 2006 pooled results from seven of the trials included in the current review (ERA 2000; ESPRIT 2002; HERS I 1998; WAVE 2002; WEST 2001; WHI I 2002; WHI II 2004), and concluded that there was no impact of hormone therapy compared to placebo on total mortality or non‐fatal myocardial infarction, but strong evidence of an increased risk in the number of strokes (RR 1.29, 95% CI 1.13 to 1.48) observed with hormone therapy use. Likewise, a meta‐analysis by Bath 2005, pooling 28 RCTs, reported hormone therapy was associated with an increase in the risk of stroke, particularly ischaemic stroke. Furthermore, those participants who had a stroke in the hormone therapy groups appeared to have a worse outcome. However, it is unclear to what degree the results of this review are applicable to post‐menopausal women, as the review had very broad inclusion criteria, and pooled a wide range of trials which used different types of hormone therapy for a range of indications, some of which included male participants. Salpeter 2006, in a meta‐analysis aimed to examine the effect of hormone therapy on coronary heart disease events in younger and older post‐menopausal women (defined as participants with a mean time from menopause of less than or greater than ten years, or mean age less than or greater than 60 years). The analyses of 23 trials (ten trials with younger women and 11 trials with older women), included the relevant Women's Health Initiative age‐specific subgroup data in one or the other group as though they had originated from separate RCTs. The results showed that hormone therapy reduced coronary heart disease events in younger women, but not in older women. This is comparable with our findings in this review.
Miller 2002 performed a meta‐analysis of venous thromboembolic outcomes in post‐menopausal women using oestrogen replacement. The review included both oestrogen alone and combination therapy and RCTs, case‐control studies and a cohort study. They found an increased risk with hormone therapy (RR 2.14, 95% CI 1.64 to 2.81). The analysis was published in 2002 and did not include either Women's Health Initiative studies, which contributed the largest portion of our included population, but the risk is comparable with our findings. They also found that the risk was highest in the first year, but in the majority of studies remained elevated for the duration of follow‐up.
Authors' conclusions
Implications for practice.
Our review findings provide strong evidence that treatment with hormone therapy in post‐menopausal women for either primary or secondary prevention of cardiovascular disease events has little if any benefit overall, and causes an increase in the risk of stroke, or venous thromboembolic events.
Implications for research.
Currently there is a lack of evidence regarding factors that may modulate the risks involved in hormone therapy treatment, such as different oestrogen and progestogen preparations, different durations, doses and routes of administration (for example, skin patches and creams). There is one recently published study (NCT00154180) and one ongoing study (NCT00114517) that assess the timing hypothesis through surrogate endpoints. They should lay the foundation for future research in this area, especially as supportive evidence for the timing hypothesis in this review comes from post‐protocol subgroup analyses. Future updates of this review will look to incorporate their findings fully, but it may be that analysis of data at the level of individual participants from the existing trials is needed to evaluate the credibility of the timing hypothesis on mortality and coronary heart disease. Due to low event rates, definitive studies assessing the timing hypothesis through hard endpoints would likely need a very large study population, estimated at 30,000 with 10 to 15 years follow‐up, and hence be very costly (Rossouw 2013).
Feedback
Jim Thornton, 18 April 2015
Summary
I have two concerns with your analysis of the HRT “timing hypothesis” in this review. Firstly, I fear you underestimate the problem of publication bias and selective reporting, especially of small trials performed by pharmaceutical companies in younger women. You mention that “two trials (ERT II 1979; HALL 1998) may have been subject to selective reporting. The remaining 17 trials reported all expected outcomes.” This is not an adequate description of the problems of selective publication and selective reporting of adverse outcomes by pharmaceutical companies in this area prior to 2000. I refer you to the following two papers, not cited in your reference list. Hemminki E and McPherson K (1997) Impact of postmenopausal hormone therapy on cardiovascular events and cancer: pooled data from clinical trials BMJ 1997; 315 doi: http://dx.doi.org/10.1136/bmj.315.7101.149 (Published 19 July 1997) Hemminki E and McPherson K (2000) Value of drug‐licensing documents in studying the effect of postmenopausal hormone therapy on cardiovascular disease The Lancet 355: 566–569 Both studies identified selective reporting and publication bias of both cardiac events and cancer. This is potentially a big problem. I note that your “Characteristics of excluded studies” table includes, by my count, 45 papers reporting randomised trials of HRT in postmenopausal women which were excluded because “no outcomes relevant to the review” were reported. I don’t know what efforts you have made to find cardiac events in those trials, or how many were sponsored by individuals or organisations with a vested interest in minimising harms. I would suggest that until that has been done and publication bias ruled out, it would be safer to conclude that there is a high risk of publication and selective reporting of adverse outcomes in this area. Secondly, I fear, by missing the above to papers you have also missed some relevant trials with informative cardiac endpoints. The relevant studies are reported in detail in the above two papers, and you have included some. I would particularly note: Writing group for PEPI trial JAMA 1995. This is included in the list of included studies in your review but I could not find the cardiac events reported by Hemminki and McPherson (1997) namely 5/875 HRT v 0/174 placebo included in any analyses. Forgive me if I missed them. Derman et al, 1995 is not mentioned at all in your review. According to Hemminki and McPherson (1997) cardiac events were reported, albeit there were none. Derman RJ, Dawood MY, Stone, S. Quality of life during sequential hormone replacement therapy. A placebo‐controlled study. Int J Fertil 1995;40:73‐8. Svendsen et al, 1992 is excluded for “no relevant outcomes” in your review. According to Hemminki and McPherson (1997) cardiac events were reported, albeit there were none. Svendsen OL, Hassager C, Marslew U, Christiansen C. Changes in calcanean bone mineral occurring spontaneously and during hormone replacement therapy in early postmenopausal women. Scand J Clin Lab Invest 1992;52:831‐6. Resch et al, 1990 is not mentioned at all in your review. Hemminki and McPherson (1997) reported cardiac events 1/16 placebo, 1/15 estradiol 2mg/NETA 1mg Resch H, Pietschmann P, Krexner E, Woloszczak W, Willvonseder R. Effects of one‐year hormone replacement therapy on peripheral bone mineral content in patients with osteoporotic spine fractures. Acta Endocrinol 1990;123:14‐8. Christiansen et al, 1990 is excluded for no placebo group in your review. Hemminki and McPherson (1997) say there was a placebo group. Christiansen C, Riis BJ. 170‐estradiol and continuous norethisterone: a unique treatment for established osteoporosis in elderly women. J Clin Endocrinol Metab 1990;71:836‐41. Coope, 1981 is excluded from your review because of “not post‐menopausal and most suffered from depression”. Could you check this? I don’t have a copy of the paper but the title “Is oestrogen therapy effective in the treatment of menopausal depression?” suggests that it might be relevant. Hemminki and McPherson (1997) report cardiac events 0/26 placebo v. 1/29 oestrone Nachtigall et al, 1979 is not mentioned at all in your review. Hemminki and McPherson (1998) state cardiac events 3/84 placebo versus 1/84 CEE 2.5 mg+MPA 10 mg/7 days Lindsay et al, 1984 is excluded for “no relevant outcomes”. According to Hemminki and McPherson (1997) cardiac events were reported, albeit there were none. Aitken et al, 1973 is excluded for “no relevant outcomes”. But Hemminki and McPherson (1997) report 3 cardiac events mestranol v 0 placebo In total Hemminki and McPherson (1997) reported total cardiovascular events 12/1818 HRT versus 5/1041. Can you check that all of these which should have been included in your review were? In their second paper Hemminki and McPherson (2000) described a search of unpublished drug company reports of early phase trials of HRT. 17 reports were found. They included 198 trials, of which nine were placebo controlled trials in which cardiovascular events were reported. Six had not been included in their previous BMJ paper. These six trials were all randomised. Participants were “healthy, recently menopausal women”. Their ages ranged from 35‐66. The investigational drugs were oestradiol (2 trials), tibolone (3 trials) and conjugated equine estrogens (1 trial). Among these six new trials there were 5 cardiovascular events out of 348 in the hormone groups and 1 out of 297 in the control groups. Again, can you check that all of these which should have been included in your review were?
Reply
We thank Prof Thornton for his letter regarding our recent publication on Hormone therapy for preventing cardiovascular disease in post‐menopausal women1. We appreciate his concerns regarding publication bias that affects the accuracy of reviews, particularly those which have been funded by or include studies funded by pharmaceutical companies. Our strategies for identifying and minimizing publication bias are designed to be as robust as possible and a full description of our methods is described in our review1. In brief we carried out extensive searches of healthcare databases (CENTRAL, MEDLINE, EMBASE and LILACS), trial registers and checked references of relevant systematic reviews not limiting our searches by language. Funnel plots show symmetrical distributions of study effects across the range of outcomes. Specifically within the subgroup analysis of the effect of timing of initiation of hormone therapy we acknowledge one limitation of our analysis is that we cannot be confident that the Estonian Postmenopausal Hormone Therapy Trial2 (1178 participants out of 9629 of the subgroup analysis) are all attributed correctly to starting treatment within 10 years of the menopause due to the trial population starting hormone therapy or placebo a mean of 8.8 years after the menopause. It is likely that this underestimates the associations with treatment, a reduction in mortality and coronary heart disease, and an increase in venous thromboembolism. We acknowledge that there may be data on trials which are not published which we have not found in our search and are grateful for information about any that might be relevant so that we can assess them for the next update of the review. We also accept that it is more likely that these types of studies might show no association or an association with harmful outcomes. However the majority of the weight of our analyses comes from 3 large well conducted trials (WHI I3, WHI II4 and HERS5, total number of participants 30 110 out of a total of 40 410). We do not think that we have missed any studies of comparative size or quality and feel that the addition of small unpublished studies is unlikely to change markedly the overall balance of effect that we have found. However in future updates of this review we will provide a more detailed description of this limitation as well as an assessment of any eligible unpublished data we are able to include. We welcome the assistance of those who have an interest in the field to identify any difficult to locate, unpublished studies which we can include in future updates of our review to give us greater confidence in the results of future analyses. Prof Thornton identifies several studies reported in Hemminki and McPherson (1997)6 and PEPI7 which he queries as to whether they should be included in our review. Our pre‐specified method states that trials greater than a pre‐specified size (see paper1 for details) were reviewed to see if relevant outcomes were reported as adverse events. Also outcomes with zero events in one or more arms of a trial were not included from the trial in question. These methodology designs were chosen to limit the inclusion of small trials with limited power to detect relevant outcomes and therefore minimise bias. The following studies identified do not meet these inclusion criteria and are therefore not included in our analysis: Derman et al8; Svendsen et al9; Lindsay et al10 and Aitken et al11. Christiansen et al12 was excluded as there was no true placebo or control group. The paper reports “After an initial examination the women were randomly allocated to one of three main treatment groups: 1) estrogen/gestogen; 2) anabolic steroids; 3) nasal calcitonin”. Coope et al13 was excluded as it used a cross over design using piperazine oestrone sulphate and placebo with a washout period in the middle. Although there were 2 deaths reported in the study population, due to the cross over design, it is not possible to know how these were associated with the intervention or placebo. Nachtigall et al14 is included in the review (ERT II). Resch et al15 reports a participant randomised to estrogen who had a transient ischaemic attack, this is not a pre‐specified outcome in our review. The control group had no reported cardiovascular outcomes. Prof Thornton identifies a second review by Hemminki and McPherson (2000)16 which describes a search of unpublished drug company reports and includes a few additional cardiovascular events attributed to hormone therapy which are unpublished. This paper was not found in our search and we thank Prof Thornton for drawing it to our attention. Some of the papers assessed synthetic oestrogens which were excluded in our review. In addition cardiovascular events were not described in any detail. Without further detail of the cases and interventions in the 3‐5 events (“conservative – best estimates”) in the hormone group and 1‐2 events (“conservative – best estimates”) in the control group we are at present unable to assimilate them into our meta‐analysis. However, due to the relatively small number of events compared to those already included, not including these data at present seems unlikely to make the current overall findings misleading. 1.Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post‐menopausal women. The Cochrane database of systematic reviews. 2015;3:CD002229. 2.Veerus P, Hovi SL, Fischer K, Rahu M, Hakama M, Hemminki E. Results from the Estonian postmenopausal hormone therapy trial [ISRCTN35338757]. Maturitas. Sep 20 2006;55(2):162‐173. 3.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. Jul 17 2002;288(3):321‐333. 4.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. Jama. Apr 14 2004;291(14):1701‐1712. 5.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. Jama. Aug 19 1998;280(7):605‐613. 6.Hemminki E, McPherson K. Impact of postmenopausal hormone therapy on cardiovascular events and cancer: pooled data from clinical trials. Bmj. Jul 19 1997;315(7101):149‐153. 7.Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. Jama. Jan 18 1995;273(3):199‐208. 8.Derman RJ, Dawood MY, Stone S. Quality of life during sequential hormone replacement therapy ‐‐ a placebo‐controlled study. International journal of fertility and menopausal studies. Mar‐Apr 1995;40(2):73‐78. 9.Svendsen OL, Hassager C, Marslew U, Christiansen C. Changes in calcanean bone mineral occurring spontaneously and during hormone replacement therapy in early post‐menopausal women. Scandinavian journal of clinical and laboratory investigation. Dec 1992;52(8):831‐836. 10.Lindsay R, Hart DM, Clark DM. The minimum effective dose of estrogen for prevention of postmenopausal bone loss. Obstetrics and gynecology. Jun 1984;63(6):759‐763. 11.Aitken JM, Hart DM, Lindsay R. Oestrogen replacement therapy for prevention of osteoporosis after oophorectomy. British medical journal. Sep 8 1973;3(5879):515‐518. 12.Christiansen C, Riis BJ. 17 Beta‐estradiol and continuous norethisterone: a unique treatment for established osteoporosis in elderly women. The Journal of clinical endocrinology and metabolism. Oct 1990;71(4):836‐841. 13.Coope J. Is oestrogen therapy effective in the treatment of menopausal depression? The Journal of the Royal College of General Practitioners. Mar 1981;31(224):134‐140. 14.Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM. Estrogen replacement therapy II: a prospective study in the relationship to carcinoma and cardiovascular and metabolic problems. Obstetrics and gynecology. Jul 1979;54(1):74‐79. 15.Resch H, Pietschmann P, Krexner E, Woloszczuk W, Willvonseder R. Effects of one‐year hormone replacement therapy on peripheral bone mineral content in patients with osteoporotic spine fractures. Acta endocrinologica. Jul 1990;123(1):14‐18. 16.Hemminki E, McPherson K. Value of drug‐licensing documents in studying the effect of postmenopausal hormone therapy on cardiovascular disease. Lancet. Feb 12 2000;355(9203):566‐569.
Contributors
Henry MP Boardman, Louise Hartley, Anne Eisinga, Caroline Main, Marta Roqué i Figuls, Xavier Bonfill Cosp, Beatrice Knight
What's new
| Date | Event | Description |
|---|---|---|
| 19 August 2015 | Feedback has been incorporated | Feedback and response included |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 18 May 2015 | Amended | Minor edit in footnotes of Summary of Findings table. |
| 25 February 2014 | New citation required but conclusions have not changed | We included six new trials, with a total of 2239 participants in this review update. The review now includes 40,410 women randomised to either hormone therapy or no treatment/placebo. Nine trials were primary prevention and ten were secondary prevention trials. We updated the searches to February 2014 and combined the cardiovascular outcomes of coronary artery bypass graft and angioplasty under "Revascularisation". We excluded health‐related quality of life from the outcomes. We combined single and combination therapy for analysis. We have presented subgroup analysis results for the timing hypothesis. |
| 25 February 2014 | New search has been performed | Date of search February 2014. |
| 20 June 2012 | New citation required but conclusions have not changed | We updated the searches to April 2010, and included the cardiovascular outcomes of angina, coronary artery bypass graft, angioplasty, and health‐related quality of life as further relevant additional outcomes. |
| 20 June 2012 | New search has been performed | We included four new trials, with a total of 15,984 participants in the review update. We included one trial in the previous review and excluded the open‐label long‐term follow‐up of another trial. The review update therefore included 13 randomised controlled trials with a total of 38,171 women randomised to either hormone therapy or placebo. Five trials were primary prevention and eight were secondary prevention trials. |
| 20 February 2012 | Amended | The results and conclusion of the update review have not changed from those of the original review. However, there is further substantive evidence that treatment with hormone therapy in post‐menopausal women for either primary or secondary prevention of cardiovascular disease events is not effective, and causes an increase in the risk of stroke, and venous thromboembolic events. |
Acknowledgements
We should like to acknowledge the contributions by Alma Adler, Diane Horsley, Fiona Taylor, Juan‐Pablo Casas, Karen Rees and Shah Ebrahim who all gave methodological assistance and support.
Appendices
Appendix 1. Search strategies original review
#1 CARDIOVASCULAR‐DISEASES*:ME #2 CEREBROVASCULAR‐DISORDERS*:ME #3 CHOLESTEROL*:ME #4 BLOOD‐COAGULATION‐FACTORS*:ME #5 CARDIOVASCULAR #6 CORONARY #7 ANGINA* #8 MYOCARDIAL #9 STROKE #10 HYPERTENSION #11 CHOLESTEROL #12 EMBOLI* #13 THROMBO* #14 CEREBROVASCULAR #15 ATHEROSCLERO* #16 ARTERIOSCLERO* #17 LIPIDS*:ME #18 LIPID* #19 HYPERLIPIDEMIA*:ME #20 (HYPERLIPIDEMIA or HYPERLIPIDAEMIA) #21 FIBRIN* #22 ((((((((#1 or #2) or #3) or #4) or #5) or #6) or #7) or #8) or #9) #23 ((((((((((#10 or #11 or #12) or #13) or #14) or #15) or #16) or #17) or #18) or #19) or #20) or #21) #24 (#22 or #23) #25 ESTROGEN‐REPLACEMENT‐THERAPY*:ME #26 HRT #27 (HORMONE near REPLAC*) #28 (OESTROGEN near REPLAC*) #29 (ESTROGEN near REPLAC*) #30 ((MENOPAUS* or POSTMENOPAUS*) or POSTMENOPAUS*) #31 OESTROGEN #32 ESTROGEN #33 (#31 or #32) #34 (#30 and #33) #35 ((((#25 or #26) or #27) or #28) or #29) #36 (#34 or #35) #37 (#24 and #36)
Appendix 2. Search strategies 2010
Cochrane Controlled Trial Register, Issue 1, April 2010 (search date: 20/04/2010)
#1. MeSH descriptor Cardiovascular Diseases explode all trees #2. MeSH descriptor Cerebrovascular Disorders explode all trees #3. CARDIOVASCULAR* #4. CORONARY #5. ANGINA* #6. MYOCARD* #7. HEART NEAR/3 ATTACK #8. STROKE* #9. MeSH descriptor Embolism and Thrombosis explode all trees #10. EMBOL* #11. THROMBO* #12. CEREBROVASCULAR #13. MeSH descriptor Hypertension explode all trees #14. HYPERTENSION #15. MeSH descriptor Arteriosclerosis explode all trees #16. ARTERIOSCLER* OR ARTHEROSCLER* #17. ISCHAEMIC OR ISCHEMIC #18. MeSH descriptor Hyperlipidemias explode all trees #19. HYPERLIPIDEMIA* OR HYPERLIPIDAEMIA* #20. (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) #21. MeSH descriptor Hormone Replacement Therapy explode all trees #22. HRT OR ERT OR ORT #23. HORMONE NEAR/4 (REPLAC* OR THERAP* OR SUPPLEMENT*) #24. ESTROGEN NEAR/4 (REPLAC* OR THERAP* OR SUPPLEMENT*) #25. OESTROGEN NEAR/4 (REPLAC* OR THERAP* OR SUPPLEMENT*) #26. (#21 OR #22 OR #23 OR #24 OR #25) #27. (menopaus* OR postmenopaus* OR post‐menopaus*) #28. MeSH descriptor Postmenopause, this term only #29. (#27 OR #28) #30. oestrogen OR estrogen #31. (#29 AND #30) #32. (#26 OR #31) #33. (#20 AND #32)
MEDLINE search 20/04/2010
1. CARDIOVASCULAR‐DISEASES#.DE. 2. CEREBROVASCULAR‐DISORDERS#.DE. 3. CARDIOVASCULAR.TI,AB. 4. CORONARY.TI,AB. 5. ANGINA$2.TI,AB. 6. (MYOCARDIAL OR HEART NEAR ATTACK).TI,AB. 7. STROKE$4.TI,AB. 8. EMBOLISM‐AND‐THROMBOSIS#.DE. 9. EMBOL$5.TI,AB. 10. THROMBO$6.TI,AB. 11. CEREBROVASCULAR.TI,AB. 12. HYPERTENSION.W..DE. 13. HYPERTENSION.TI,AB. 14. ARTERIOSCLEROSIS#.W..DE. 15. (ARTERIOSCLERO$5 OR ARTHEROSCLERO$5).TI,AB. 16. (ISCHAEMIC OR ISCHEMIC).TI,AB. 17. HYPERLIPIDEMIAS#.W..DE. 18. (HYPERLIPIDEMIA$4 OR HYPERLIPIDAEMIA$4).TI,AB. 19. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 20. 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 21. 19 OR 20 22. HORMONE‐REPLACEMENT‐THERAPY#.DE. 23. (HRT OR ERT OR ORT).TI,AB. 24. HORMONE NEAR (REPLAC$6 OR THERAP$4 OR SUPPLEMENT$6) 25. ESTROGEN NEAR (REPLAC$6 OR THERAP$4 OR SUPPLEMENT$6) 26. OESTROGEN NEAR (REPLAC$6 OR THERAP$4 OR SUPPLEMENT$6) 27. 22 OR 23 OR 24 OR 25 OR 26 28. MENOPAUS$4 OR POSTMENOPAUS$4 OR POST‐MENOPAUS$4 29. POSTMENOPAUSE.W..DE. 30. 28 OR 29 31. (ESTROGEN OR OESTROGEN).TI,AB. 32. 30 AND 31 33. 27 OR 32 34. PT=RANDOMIZED‐CONTROLLED‐TRIAL 35. PT=CONTROLLED‐CLINICAL‐TRIAL 36. (SINGL$4 OR DOUBLE$4 OR TRIPLE$4 OR TREBLE$4) AND (BLIND$4 OR MASK$4) 37. RANDOM$5 OR PLACEBO$2 38. RANDOM‐ALLOCATION.DE. 39. DOUBLE‐BLIND‐METHOD.DE. 40. SINGLE‐BLIND‐METHOD.DE. 41. (CLINIC$3 NEAR TRIAL$2).TI,AB. 42. RETRACT$5 NEAR PUBLICATION 43. 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 44. ANIMAL=YES NOT HUMAN=YES 45. 21 AND 33 AND 43 46. 45 NOT 44
EMBASE search 20/04/2010
1. CARDIOVASCULAR‐DISEASE#.DE. 2. CEREBROVASCULAR‐DISEASE#.DE. 3. CARDIOVASCULAR.TI,AB. 4. CORONARY.TI,AB. 5. ANGINA$2.TI,AB. 6. MYOCARDIAL.TI,AB. OR (HEART NEAR ATTACK).TI,AB. 7. STROKE$4.TI,AB. 8. EMBOL$5.TI,AB. 9. THROMBO$6.TI,AB. 10. CEREBROVASCULAR.TI,AB. 11. HYPERTENSION.W..DE. 12. HYPERTENSION.TI,AB. 13. ARTERIOSCLEROSIS#.W..DE. 14. (ARTERIOSCLERO$5 OR ARTHEROSCLERO$5).TI,AB. 15. (ISCHAEMIC OR ISCHEMIC).TI,AB. 16. (HYPERLIPIDEMIA$4 OR HYPERLIPIDAEMIA$4).TI,AB. 17. HYPERLIPIDEMIA#.W..DE. 18. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 19. Hormone‐Substitution#.DE. 20. (HRT OR ERT OR ORT).TI,AB. 21. HORMONE NEAR (REPLAC$6 OR THERAP$4 OR SUPPLEMENT$6).TI,AB. 22. ESTROGEN NEAR (REPLAC$6 OR THERAP$4 OR SUPPLEMENT$6).TI,AB. 23. OESTROGEN NEAR (REPLAC$6 OR THERAP$4 OR SUPPLEMENT$6).TI,AB. 24. (menopaus$4 OR postmenopaus$4 OR post‐menopaus$4).TI,AB. 25. Postmenopause.W..DE. 26. 39 OR 40 27. (oestrogen OR estrogen).TI,AB. 28. 41 AND 42 29. 19 OR 20 OR 34 OR 37 OR 38 OR 43 30. 44 AND 18 31. factorial$ 32. crossover$2 OR cross ADJ over$2 34. (RANDOM$ OR PLACEBO$).DE,TI,AB.
LILACS search conducted 20/04/2010
Search 1:
"HORMONE REPLACEMENT THERAPY" OR
((hormone OR oestrogen OR oestrogen) AND (replac$ or therap$ or supplement$)) or (hrt OR ert OR ort) AND ("clinical trials, RANDOMIZED" or "controlled clinical trials, RANDOMIZED" OR (( trial$ or ensa$ or estud$) AND (clin$)) OR ((singl$ or doubl$ or doble$ or duplo$ or trebl$ or trip$) AND (blind$ or cego$ or ciego$ or mask$ or mascar$)) OR (random$ or randon$ or casual$ or acaso$ or azar or aleator$)) = 318
Search 2:
(("POSTMENOPAUSE" OR menopaus$ or postmenopaus$ or post‐menopause) AND (oestrogen or estrogen))
Appendix 3. Search strategies 2014
CENTRAL
#1 MeSH descriptor: [Cardiovascular Diseases] explode all trees #2 MeSH descriptor: [Cerebrovascular Disorders] explode all trees #3 MeSH descriptor: [Hypertension] this term only #4 (cardiovascular or coronary or angina* or myocardial or stroke* or embol* or thrombo* or cerebrovascular or hypertension or arteriosclero* or atherosclero* or ischaemi* or ischemi*):ti,ab #5 (heart near/2 attack):ti,ab #6 MeSH descriptor: [Hyperlipidemias] explode all trees #7 MeSH descriptor: [Cholesterol] explode all trees #8 MeSH descriptor: [Blood Coagulation Factors] explode all trees #9 MeSH descriptor: [Lipids] explode all trees #10 (hyperlipidemia* or hyperlipidaemia* or cholesterol or lipid* or fibrin*):ti,ab #11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #12 MeSH descriptor: [Progestins] explode all trees #13 (progestogen or medroxyprogesterone acetate or MPA or dydrogesterone or norethisterone or norethindrone or oestrogen or estrogen or estradiol or CEE or premarin or estriol or oestradiol):ti,ab #14 (hormone* near/3 therap*):ti,ab #15 (hormone* near/3 supplement*):ti,ab #16 #12 or #13 or #14 or #15 #17 MeSH descriptor: [Climacteric] this term only #18 MeSH descriptor: [Menopause] this term only #19 MeSH descriptor: [Perimenopause] this term only #20 MeSH descriptor: [Postmenopause] this term only #21 MeSH descriptor: [Menopause, Premature] this term only #22 (postmenopaus* or post‐menopaus* or perimenopaus* or peri‐menopaus* or climacter* or menopaus*):ti,ab #23 #17 or #18 or #19 or #20 or #21 or #22 #24 #16 and #23 #25 #11 and #24 #26 MeSH descriptor: [Hormone Replacement Therapy] explode all trees #27 (HRT or ERT or ORT):ti,ab #28 (hormone near/3 replac*):ti,ab #29 (estrogen near/3 replac*):ti,ab #30 (oestrogen near/3 replac*):ti,ab #31 #26 or #27 or #28 or #29 or #30 #32 #11 and #31 #33 #25 or #32
MEDLINE
1 exp Cardiovascular Diseases/ 2 exp Cerebrovascular Disorders/ 3 cardiovascular.ti,ab. 4 coronary.ti,ab. 5 angina$.ti,ab. 6 (myocardial or (heart adj2 attack)).ti,ab. 7 stroke$.ti,ab. 8 embol$.ti,ab. 9 thrombo$.ti,ab. 10 cerebrovascular.ti,ab. 11 hypertension.ti,ab. 12 Hypertension/ 13 (arteriosclero$ or artherosclero$).ti,ab. 14 (ischaemi$ or ischemi$).ti,ab. 15 exp Hyperlipidemias/ 16 (hyperlipidemia$ or hyperlipidaemia$).ti,ab. 17 exp Cholesterol/ 18 cholesterol.ti,ab. 19 exp Blood Coagulation Factors/ 20 exp Lipids/ 21 lipid$.ti,ab. 22 fibrin$.ti,ab. 23 or/1‐22 24 exp Progestins/ 25 progestogen.tw. 26 (medroxyprogesterone acetate or MPA).tw. 27 dydrogesterone.tw. 28 (norethisterone or norethindrone).tw. 29 (oestrogen or estrogen).ti,ab. 30 estradiol$.ti,ab. 31 CEE.ti,ab. 32 premarin.ti,ab. 33 estriol.ti,ab. 34 oestradiol.ti,ab. 35 (hormone$ adj3 (therap* or supplement*)).ti,ab 36 or/24‐35 37 climacteric/ or menopause/ or perimenopause/ or postmenopause/ or menopause, premature/ 38 (postmenopaus$ or post‐menopaus$ or post menopaus$).tw. 39 (perimenopaus$ or peri‐menopaus$ or peri menopaus$).tw. 40 (climacter$ or menopaus$).tw. 41 or/37‐40 42 36 and 41 43 23 and 42 44 exp hormone replacement therapy/ 45 (HRT or ERT or ORT).ti,ab. 46 ((hormone or estrogen or oestrogen) adj3 replac$).ti,ab. 47 or/44‐46 48 23 and 47 49 43 or 48 50 randomized controlled trial.pt. 51 controlled clinical trial.pt. 52 randomized.ab. 53 placebo.ab. 54 clinical trials as topic.sh. 55 randomly.ab. 56 trial.ti. 57 or/50‐56 58 49 and 57 59 exp animals/ not humans.sh. 60 58 not 59
Embase
1 exp cardiovascular disease/ 2 exp cerebrovascular disease/ 3 cardiovascular.ti,ab. 4 coronary.ti,ab. 5 angina$.ti,ab. 6 (myocardial or (heart adj2 attack)).ti,ab. 7 stroke$.ti,ab. 8 embol$.ti,ab. 9 thrombo$.ti,ab. 10 cerebrovascular.ti,ab. 11 hypertension.hw. 12 hypertensi$.ti,ab. 13 exp arteriosclerosis/ 14 arteriosclerosis.hw. 15 (arteriosclero$ or atherosclero$).ti,ab. 16 (ischaemi$ or ischemi$).ti,ab. 17 (hyperlipidemia$ or hyperlipidaemia$).ti,ab. 18 exp hyperlipidemia/ 19 hyperlipidemia.hw. 20 exp Cholesterol/ 21 cholesterol.ti,ab. 22 exp blood clotting factor/ 23 exp lipid/ 24 lipid$.ti,ab. 25 fibrin$.ti,ab. 26 or/1‐25 27 exp hormone substitution/ 28 (HRT or ERT or ORT).ti,ab. 29 ((hormone or oestrogen or estrogen) adj3 replac$).ti,ab. 30 or/27‐29 31 26 and 30 32 exp gestagen/ 33 progestin$.ti,ab. 34 progestogen$.ti,ab. 35 (medroxyprogesterone acetate or MPA).tw. 36 dydrogesterone.tw. 37 (norethisterone or norethindrone).ti,ab. 38 (oestrogen or estrogen).ti,ab. 39 estradiol$.ti,ab. 40 CEE.ti,ab. 41 premarin.ti,ab. 42 estriol.ti,ab. 43 oestradiol.ti,ab. 44 progesterone$.ti,ab. 45 (hormone adj3 (therap$ or supplement$)).ti,ab. 46 or/32‐45 47 exp "menopause and climacterium"/ 48 (postmenopaus$ or "post menopaus$").ti,ab. 49 (perimenopaus$ or "peri menopaus$").ti,ab. 50 (climacter$ or menopaus$).ti,ab. 51 or/46‐49 52 26 and 46 and 51 53 crossover procedure/ 54 double blind procedure/ 55 single blind procedure/ 56 (cross over$ or crossover$).ti,ab. 57 placebo$.ti,ab. 58 (doubl$ adj blind$).ti,ab. 59 allocat$.ti,ab. 60 trial.ti. 61 randomized controlled trial/ 62 random$.ti,ab. 63 or/53‐62 64 63 and (31 or 52) 65 exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 66 human/ or normal human/ 67 65 and 66 68 65 not 67 69 64 not 68
LILACS
Search A
((MH:"Estrogen Replacement Therapy" OR "MH:"Hormone Replacement Therapy") OR (terapeutica AND hormonal) OR (terapia AND hormona$) OR (terapia AND reposicao) OR (terapia AND estrogen$) OR (tratamiento AND hormonal) OR (terapia AND reemplazo) OR (hormone AND replac$) OR (hormone AND therap$) OR (hormone AND supplement$) OR (estrogen AND replac$) OR (estrogen AND therap$) OR (estrogen AND supplement$) OR (oestrogen AND replac$) OR (oestrogen AND therap$) or (oestrogen AND supplement$) OR (HRT OR ERT OR ORT OR THS)) limited to Controlled Clinical Trials Topic List
Search B
(MH:Climacteric OR MH:Menopause OR MH:"Menopause, Premature" OR MH:Perimenopause OR MH:Postmenopause OR pos‐menopaus$ OR posmenopaus$ OR postmenopaus$ OR post‐menopaus$ OR climacteric OR climaterio OR menopaus$ OR perimenopaus$) limited to Controlled Clinical Trials Topic List
Data and analyses
Comparison 1. Hormone therapy versus placebo in primary prevention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (all‐causes) | 8 | 34422 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.12] |
| 2 Death (cardiovascular causes) | 3 | 28353 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.47, 1.40] |
| 3 Non‐fatal myocardial infarction | 7 | 29482 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.80, 1.31] |
| 4 Stroke | 4 | 28719 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.12, 1.56] |
| 5 Angina | 2 | 27347 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.74, 1.08] |
| 6 Venous thromboembolism | 6 | 33477 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [1.24, 2.99] |
| 7 Pulmonary embolism | 3 | 31732 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.17, 3.04] |
| 8 Revascularisation | 3 | 27569 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.09] |
Comparison 2. Hormone therapy versus placebo in secondary prevention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (all‐causes) | 7 | 5445 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.87, 1.24] |
| 2 Death (cardiovascular causes) | 6 | 5259 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.78, 1.29] |
| 3 Non‐fatal myocardial infarction | 7 | 5359 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.18] |
| 4 Stroke | 5 | 5172 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.89, 1.33] |
| 5 Angina | 3 | 3155 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.12] |
| 6 Venous thromboembolism | 6 | 4399 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.13, 3.62] |
| 7 Pulmonary embolism | 3 | 3920 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [0.92, 6.70] |
| 8 Revascularisation | 3 | 3155 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.63, 1.53] |
Comparison 3. Hormone therapy versus placebo in both primary and secondary prevention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (all‐causes) | 15 | 39868 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.11] |
| 2 Death (cardiovascular causes) | 9 | 33613 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.78, 1.18] |
| 3 Non‐fatal myocardial infarction | 14 | 34841 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.89, 1.14] |
| 4 Stroke | 10 | 34672 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.10, 1.41] |
| 5 Angina | 5 | 30502 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.79, 1.03] |
| 6 Venous thromboembolism | 10 | 37313 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [1.36, 2.69] |
| 7 Pulmonary embolism | 7 | 36316 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [1.32, 2.48] |
| 8 Revascularisation | 6 | 30724 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.05] |
| 9 Death (all‐causes): by year on treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 1‐year follow‐up | 2 | 27347 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.93, 2.30] |
| 9.2 2‐year follow‐up | 2 | 27270 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.72, 1.50] |
| 9.3 3‐year follow‐up | 2 | 27157 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.81, 1.54] |
| 9.4 4‐year follow‐up | 2 | 27010 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.76, 1.36] |
| 9.5 5 year follow‐up | 2 | 26827 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.23] |
| 9.6 6 year follow‐up | 1 | 10427 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.81, 1.82] |
| 9.7 7‐year follow‐up | 1 | 10333 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.67, 1.57] |
| 9.8 8‐year follow‐up | 1 | 10249 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.45, 1.23] |
| 10 Death (all‐causes): cumulatively by year on treatment | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 1‐year follow‐up | 4 | 31832 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.94, 2.17] |
| 10.2 2‐year follow‐up | 3 | 28287 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.80, 1.42] |
| 10.3 3‐year follow‐up | 9 | 30296 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.96, 1.37] |
| 10.4 4‐year follow‐up | 3 | 29773 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.95, 1.25] |
| 10.5 5 year follow‐up | 2 | 26827 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.91, 1.23] |
| 10.6 6 year follow‐up | 1 | 10427 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.93, 1.36] |
| 10.7 7‐year follow‐up | 1 | 10333 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.93, 1.31] |
| 10.8 8‐year follow‐up | 1 | 10249 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.90, 1.24] |
| 10.9 10‐year follow‐up | 2 | 1174 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.31, 0.96] |
Comparison 4. Subgroup analysis of timing hypothesis (<10 years versus >10 years since menopause).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (all‐causes) | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Hormone therapy commenced <10 years after menopause | 5 | 9088 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.52, 0.95] |
| 1.2 Hormone therapy commenced >10 years after menopause | 12 | 27750 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.95, 1.18] |
| 2 Coronary heart disease (death from cardiovascular causes and non‐fatal myocardial infarction) | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Hormone therapy commenced <10 years after menopause | 4 | 8311 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.29, 0.96] |
| 2.2 Hormone therapy commenced >10 years after menopause | 12 | 23491 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.96, 1.20] |
| 3 Stroke | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Hormone therapy commenced <10 years after menopause | 3 | 8143 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.80, 2.34] |
| 3.2 Hormone therapy commenced >10 years after menopause | 8 | 22722 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.06, 1.38] |
| 4 Venous thromboembolism | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Hormone therapy commenced <10 years after menopause | 3 | 9838 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.11, 2.73] |
| 4.2 Hormone therapy commenced >10 years after menopause | 9 | 27475 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.37, 2.80] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
DOPS 2012.
| Methods |
Objective: To assess the effects of hormone therapy for the prevention of osteoporotic fractures in healthy young post‐ and peri‐menopausal women. The primary outcome was therefore incidence of osteoporotic fracture. Prespecified clinical cardiovascular and cancer outcomes were assessed as secondary outcomes. Multicentre randomised open‐label controlled trial (RCT) conducted in Denmark. The trial was conducted from 1990‐2008, randomised follow‐up period with mean duration of 10.1 years (trial terminated early due to results of WHI I), non‐randomised follow‐up continued for a further 5.7 years. The analysis in this review includes the data reported from the randomised 10.1 years of follow‐up. This was chosen over the extended follow‐up to reduce bias Recruitment: direct mailing to a random selection of the general population Screening: physical examination and biochemical screening at baseline Randomisation: sequence generation was undertaken using stratified (study centre) blocked (n = 10) computer‐generated randomisation Stratification: not reported Allocation: paper states "open label" suggesting that allocation was not concealed, however, later in the paper it is stated that sealed envelopes were used Baseline equality of treatment groups: similar apart from the treatment group who were on average six months younger than the control group Blinding: open‐label Analysis: intention to treat (ITT) Funding Source: Funded by the University of Aarhus, Karen Elise Jensen’s Foundation, Novo Nordic, Novartis, and LEO Pharma. Novo Nordisk, Novartis, and Leo Pharma Denmark provided the study drug free of charge |
|
| Participants | 1006 healthy white peri‐ or post‐menopausal women aged 45‐58 years, with a mean age of 49.5 years (SD ± 2.7 years), underwent treatment with HT or no treatment. The participants had a mean age of 0.58 years (SD ± 0.63 years) since menopause, 95 of 502 (18.9%) participants had undergone a hysterectomy, and 2% had used HT previously, for a median duration of 1 year. In terms of risk factors for cardiovascular disease: Mean body mass index (kg/m2) = 25.3 (SD ± 4.3); hip: waist ratio = 1.27 (SD ± 0.11); total cholesterol concentration (mmol/L) = 6.26 (SD ± 1.10); LDL concentration (mmol/L) = 3.85 (SD ± 1.04); HDL concentration (mmol/L) = 1.71 (SD ± 0.42); triglyceride concentration (mmol/L) = 1.14 (SD ± 0.53) and fasting glucose concentration (mmol/L) = 4.7 (SD ± 0.7). In the treatment arm mean systolic blood pressure (mm Hg) = 129 (SD ± 18); diastolic blood pressure (mm Hg) = 81 (SD ±11); 169 (33.6%) with vitamin D deficiency and 212 (42.3%) were current smokers There were no statistically significant differences in baseline characteristics between the HT treatment group and the no treatment controls for any of the reported variables apart from age. Participants in the HT group were slightly younger with a mean age of 49.5 (SD ± 2.7) years compared to a mean age of 50.0 (SD ± 2.8) years in the control group (P = 0.007) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
HT regimen: 1) Sequentially combined HT: 2 mg synthetic 17‐β‐oestradiol for 12 days, 2 mg 17‐β‐oestradiol plus 1 mg norethisterone acetate for 10 days, and 1 mg 17‐β‐oestradiol for six days (women with intact uterus) 2) HT Monotherapy: 2 mg 17‐β‐oestradiol a day (women whom had had a hysterectomy) Comparator: No treatment control Other treatment modalities were offered to those who experienced side effects of insufficient relief of symptoms. Participants were classified as medication compliant if they took ≥80% of their medication throughout the trial. However, rates of medication compliance were not reported Follow‐up times: All participants underwent a physical examination and biochemical screening at baseline. They were subsequently followed‐up at six months, one year, two, three, five and 10 years. Study drugs were posted to the women randomised to HT, and they were offered an annual visit. Women were advised to visit their own GP or gynaecologist if they had any health concerns. No participants were lost to follow‐up, but two women were censored at time of emigration (one in each treatment group) |
|
| Outcomes |
Primary outcomes: Composite of all‐cause mortality and hospitalisation for MI or heart failure Secondary outcomes: Stroke Pulmonary embolism Venous thromboembolism Adverse events: Cancer |
|
| Notes | Methods for verifying medication compliance, and actual compliance rates were not reported. Follow‐up was annual within the first five years of the trial and then only on a five‐year basis thereafter, therefore it is likely that this was patient self report rather than ascertained by pill count. Patient medication compliance rates (if self reported) may therefore be overestimated, which would potentially underestimate the treatment effect in the HT group relative to the no treatment control Attrition rates were only reported for year 10 follow‐up visit: HT group: 15%; no treatment control group: 17.7% At 10.1 year follow‐up 266/502 (53%) of the women randomised to HT therapy had stopped treatment, whilst 236/502 (47%) continued taking some form of HT treatment. Amongst the 236 women continuing treatment, 104/236 (44%) continued to take the study medication to which they were randomised whilst 132/236 (56%) were taking ‘other’ HT. In the no treatment control group, 290/506 (77%) never used HT, whilst 114/506 (23%) had started using some form of HT treatment during the 10.1 year follow‐up period. Of these, 61/114 (53.5%) were still taking this at the 10.1 year follow‐up whilst 53/114 (46.5%) had stopped taking any form of HT treatment Although data were analysed on an intention‐to‐treat basis, the treatment cross‐over between the two groups makes interpretation of the data more complex. However, overall it is likely that cross‐ over between treatment groups would potentially underestimate the relative treatment effect of HT compared to no treatment control The control group population started treatment on average 0.61 years (SD 0.65) after their menopause with the hormone group starting on average 0.58 years (SD 0.63) after. Events were not reported individually or according to starting treatment < 10 or > 10 years since the menopause. For the subgroup analysis of when treatment was started, the entirety of events in this study were analysed as if all participants had started treatment within 10 years of their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was undertaken using stratified (study centre) blocked (n = 10) computer generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | States open‐label meaning that allocation was not concealed but later in the paper states used sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial in which participants and study personnel knew the treatment arm to which patients were randomised |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | PROBE (prospectively randomised, open with blinded endpoint evaluation) design was used to assess endpoints but not say who endpoint assessors were |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates very low, with censoring unlikely to be related to patient outcome status. Study used intention‐to‐treat analysis. No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | The trial methods paper is available, and all clinical outcomes specified in the paper are reported |
| Other bias | High risk | The number of women randomised to HT treatment who discontinued treatment prior to 10.1 year follow‐up was high (53%), whilst in the no treatment control group 23% of women started using some form of HT during this follow‐up period. This ‘cross‐over’ between treatment arms potentially confounds the interpretation of the HT treatment effect relative to no treatment, and may potentially underestimate the true HT treatment effect |
EAGAR 2006.
| Methods |
Objective: To assess the effects in post‐menopausal women of HT started after coronary artery bypass surgery (CABG) on saphenous vein graft (SVG) disease Multicentre randomised controlled (RCT) trial involving eight hospital sites in the United States. The trial was conducted from 1998‐2002 over a 3.5 year follow‐up (mean duration 33 ± 8 months). The trial was stopped early after the Women’s Health Initiative (WHI 2002) reported an increased risk of breast cancer and no additional benefits for women on HT in terms of CVD risk on combined oestrogen and progestin combination therapy relative to placebo The primary outcome measure was SVG progression assessed by angiography and intravascular ultrasound (IVUS) on percent stenosis, minimal lumen diameter, and total plaque volume. Secondary outcomes (not specified a priori included death from CV disease, MI, angina and angioplasty Recruitment: Not reported Screening: Not reported Randomisation: Not reported Stratification: Not reported Allocation: Not reported Baseline equality of treatment groups: No substantive differences between study groups at baseline Blinding: Not reported Analysis: ITT for secondary clinical outcomes Funding Source: Research Council funded |
|
| Participants | Eighty‐three post‐menopausal women (HT: 40; placebo: 43) with a mean age of 64 (SD: ± 8.5 years) underwent treatment with either HT or placebo within six months following coronary artery bypass surgery. Post‐menopausal status was defined as > 55 years of age and amenorrhoea for ≥ 1 year or follicle stimulating hormone > 50 IU. The number of women who had previously undergone a hysterectomy was not reported. Included women were 78% white, and 22% from an ethnic minority group. 40% had a history of diabetes, 69% hypertension, 81% hyperlipidaemia and 40% MI. In terms of smoking status: 16.5% were current smokers; 59.5% past smokers and 24.5% never smokers. 35% had prior HT use. Mean BMI among the women was 30 kg/m2 (SD: 30 ± 6). Mean systolic blood pressure at baseline was 135 (SD: 6) mm Hg and diastolic blood pressure was 72.5 (SD: 10.5) mm Hg. There were no statistically significant differences between the two groups in terms of baseline demographics Exclusion criteria:
|
|
| Interventions |
HT regimen: 1 mg unopposed 17ß‐oestradiol daily with or without daily 2.5 mg medroxyprogesterone depending on hysterectomy status (continuous dosage regimen) Comparator: identical placebo capsule daily The overall compliance with study intervention assessed by pill count at each visit exceeded 80% in both arms up to 30 months of treatment Follow‐up times: Six‐months: angiogram (n = 83) (actual mean time of angiogram assessment 10.7 months post CABG); intravascular ultrasound assessment (IVUS) (n = 63); 42‐months: angiogram (n = 45), IVUS (n = 20). Actual mean time of participant follow‐up was 33 (SD: 8) months before the study drug was stopped |
|
| Outcomes | Per cent stenosis Minimal lumen diameter Total plaque volume Death from CV disease (definition not provided) Angina (definition not provided) MI (definition not provided) Angioplasty (definition not provided) |
|
| Notes | Sample size calculation not reported, and therefore it is unclear whether the trial was powered adequately to detect significant differences in clinical event rates between the HT and placebo group. It was unclear how the CVD events were defined, and whether definitions may have varied between centres. Additionally, it was unclear how these were corroborated, locally or centrally and whether outcome assessors were blinded to patient treatment status Patient attrition rates were high for both angiographic and intravascular ultrasound (IVUS), with only 24% and 54% of patients undergoing each investigation respectively at trial termination [mean length of follow‐up: 33 months (SD: eight months)]. However, follow‐up for all clinical events was completed for all patients. Data were not published for this study on the time since menopause that treatment was started. The study author was contacted but we were informed that these data were not collected. The mean age of the control group population was 64 years (SD 9). The mean age of the treatment group population was 64 years (SD 8). Events were not reported individually or according to age < 60 or > 60 years. For the subgroup analysis of when treatment was started, the entirety of events in this study were analysed as if all participants had started treatment more than 10 years of their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and study personnel not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data complete for clinical outcomes/events |
| Selective reporting (reporting bias) | Low risk | Paper reports main outcome measures of angiographic and intravascular ultrasound (IVUS) as well as all CVD events that occurred in the trial |
| Other bias | Unclear risk | It is unlikely that the trial was powered to detect differences in clinical events between the HRT and placebo treatment groups. Therefore the lack of significant differences in event rates between the two groups should be treated with caution |
EPAT 2001.
| Methods |
Objective: To determine the effect of oestrogen‐alone HT on the progression of sub‐clinical atherosclerosis in healthy post‐menopausal women without pre‐existing cardiovascular disease, as measured by changes in thickness of carotid artery wall. University‐based clinic randomised controlled (RCT) trial conducted in the United States over a two‐year follow‐up period (1994 to 1998). The primary outcome measure was carotid intima‐media thickness to assess the rate of progression of sub‐clinical atherosclerosis; clinical outcomes were reported as secondary outcomes Recruitment: Not reported Screening: Interested women screened by phone for eligibility, then attended three screening visits two to four weeks apart to determine final study eligibility. 1161 pre‐screened by phone, 422 screened on site, of whom 52% randomised Randomisation: Computer‐generated random numbers Stratification: By LDL cholesterol level (threshold < 4.15 mmol/L), previous duration of HRT, (threshold < 5 years), and diabetes mellitus status Allocation: Blinded medication packets assigned sequentially and remotely after eligibility confirmed Baseline equality of treatment groups: No substantive differences between study groups at baseline apart from a significantly higher proportion of HT patients than placebo patients had undergone a complete or partial oophorectomy at baseline (P = 0.03) Blinding: Participants, gynaecologists, clinical staff, and image analysts. The data monitor and data analyst were blinded to treatment assignment until analyses were completed Analysis: ITT Funding Source: National Institute on Aging |
|
| Participants | 222 post‐menopausal women (HT: 111; placebo: 111) with a mean age of 62.2 years (range: 46 to 80 years) underwent treatment with either HT or placebo. Post‐menopausal status was not defined in the trial. The ethnic origins of the women included in the trial were: 57% White, 11% Black, 21% Hispanic, 10% Asian and 1% Other. 38% of women had undergone a hysterectomy, and 18% an oophorectomy‡. In terms of smoking status: 53% were former smokers and 47% non‐smokers. Mean BMI among the women was 29.4 kg/m2. Systolic blood pressure at baseline was 128 mm Hg and diastolic blood pressure was 76.1 mm Hg Inclusion criteria: Women were eligible if they were:
Women with diabetes were eligible for inclusion provided their fasting blood glucose level was less than 11.1 mmol/L (< 200 mg/dL) Exclusion criteria:
‡a significantly higher proportion of HT patients than placebo patients had undergone a complete or partial oophorectomy at baseline (P = 0.03) |
|
| Interventions |
HT regimen: 1 mg unopposed micronised 17ß‐oestradiol daily (continuous dosage regimen) Comparator: identical placebo capsule daily Overall pill adherence in the trial was 95% in the HT group and 92% in the placebo group (P = 0.08). This was maintained throughout the two year follow‐up trial period Follow‐up times: Patients were followed up every month for the first six months and then every other month for two years. Carotid artery ultrasonography in patients with a uterus was performed at baseline and then every six months. Pelvic examination, papanicolaou smear, and mammography were performed annually During the trial, mean pill adherence was 95% in the oestradiol group and 92% in the placebo group (P = 0.08) Losses to follow‐up: 33 women were not evaluable for primary study endpoints, but clinical endpoints were reported for all outcomes |
|
| Outcomes | Carotid intima‐media thickness All causes of death Death from CV disease MI Coronary artery bypass Angioplasty |
|
| Notes | The sample size power calculation was based on potential differences in change in the intima‐media thickness of the right distal common carotid artery far wall between the HT and placebo groups, and therefore it is unclear whether the trial was powered adequately to detect significant differences in clinical event rates between the HT and placebo group. It was unclear how the CVD events were defined, and whether these were corroborated locally or centrally. Data were not published for this study on the time since menopause that treatment was started. The study author was contacted but we did not receive a reply. The mean age of the control group population was 62.1 years (SD 7.1). The mean age of the treatment group population was 60.9 years (SD 6.7). Events were not reported individually or according to age < 60 or > 60 years. For the subgroup analysis of when treatment was started, the entirety of events in this study were analysed as if all participants had started treatment more than 10 years after their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Blinded medication packets assigned sequentially and remotely after eligibility confirmed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, gynaecologists, clinical staff, image analysts, the data monitor and data analyst were blinded to treatment assignment until analyses were completed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adverse events and bleeding were assessed by the study gynaecologist who was blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 33 women were not able to be evaluated for primary (physiological) study endpoints, but clinical endpoints were reported for all by intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No obvious source of other bias |
EPHT 2006.
| Methods |
Objective: To ascertain harms and benefits of combined HT among healthy post‐menopausal Estonian women Multicentre four‐armed randomised, placebo and non‐treatment controlled trial (RCT) involving three primary care sites in Estonia. The trial was conducted from 1999 to 2001, with a mean follow‐up of 3.4 years (range: 2 to 4.9). The trial was originally planned to be part of the Women’s International Study of Long Duration Oestrogen After Menopause (WISDOM) trial based primarily in the United Kingdom, and therefore no individual sample size was undertaken for the Estonian component of the trial. The trial was planned for five‐year duration, but was stopped early after the reports from WHI I 2002 were published. The primary aim of the trial was to assess the effects of combined oestrogen and progestin HT among healthy post‐menopausal women. The trial also assessed the impact of blinding versus no blinding to treatment allocation on recruitment rates through including four trial arms: (1) blinded HT combination therapy; (2) blinded placebo therapy; (3) unblinded HT combination therapy; and (4) unblinded no treatment control groups. After adjustment of participants’ age at recruitment and former oral contraceptive use between the blinded and non‐blinded groups, the results were then combined, with HT therapy groups combined and placebo and no treatment control group combined, and the outcome data presented for both of the two groups Recruitment: Invitation sent to whole female population aged 50 to 64 of two areas of Estonia Screening: Number of women screened for eligibility: 39,713 (whole female pop aged 50 to 64 of two areas of Estonia) Randomisation: Remotely randomised in permuted block algorithm Stratification: By clinical centre Allocation: Non‐transparent sealed envelopes Baseline equality of treatment groups: More prior use of oral contraceptive in HT group 9.2% versus 6.4%; HT group older (59 versus 58.5) Blinding: Participants and investigators blinded Analysis: ITT Funding Source: Academic and government grants |
|
| Participants | 1778 healthy post‐menopausal women were randomised to HT, placebo or a no treatment control group. The definition of post‐menopausal was at least 12 months since last menses. (1) 404 women were randomised to blinded combination HT treatment; (2) 373 to blinded placebo; (3) 494 to unblinded combination HT therapy, and (4) 507 to no treatment control. Only the results reported for the blinded combination HT and placebo arms (n=777) included in the analyses. The mean age of the women was 58.8 years (SD: ± 4.0), with a mean age of menopause of 50 years (SD: ± 3.9) years. 10% of the women had previously undergone a hysterectomy. Mean BMI was 27 kg/m2. In terms of risk factors for CVD: 15% were current smokers; 13.2% were being treated for hypertension; 8.5% had a history of angina; and 1.3% had a previous MI. Mean systolic blood pressure was 137 mm Hg and mean diastolic blood pressure 86.2 mm Hg Inclusion criteria: Aged between 50 to 64 years and menopausal as defined above Exclusion criteria:
|
|
| Interventions |
HT regimens : 1) 0.625 mg conjugated equine oestrogen and 2.5 mg medroxyprogesterone acetate daily (continuous dosage regimen). For women (n = 251) within three years of their last period 5.0 mg medroxyprogesterone acetate daily along with the standard dose of 0.625 mg conjugated equine oestrogen was prescribed Comparator: 2) Placebo 3) No treatment control Rates of medication compliance in the trial varied considerably with adherence < 40% in HT group and < 30% in placebo group by three years (estimated from graph) Follow‐up times: baseline, seven months, and then annually. Patients underwent a Papanicolaou smear at baseline, and measurement of weight, arterial blood pressure, pelvic and breast examination annually. A Papanicolaou smear was taken every second year. Thirteen patients were lost to follow‐up, so the clinical status of all participants at trial exit was known for 97% of the women |
|
| Outcomes |
Coronary heart disease (angina, acute MI, subsequent MI, current complications following acute MI, other acute ischaemic heart disease) Cerebrovascular disease (subarachnoid haemorrhage, intracerebral haemorrhage, other non‐traumatic intracranial haemorrhage cerebral infarction, stroke, occlusion and stenosis of vertebral arteries, occlusion and stenosis of cerebral arteries, other cerebrovascular diseases, cerebrovascular disorders, sequelae of cerebrovascular disease) Death from any cause Non‐fatal MI Stroke HRQoL |
|
| Notes | No sample size calculation was performed so it is unclear whether the trial was powered to detect differences between the four treatment arms. Given the lack of patient treatment adherence which fell dramatically in the HT blinded, HT unblinded, and placebo groups from 70‐76% at baseline, to 36‐46% by one‐year follow‐up and 4‐5% at four‐year follow‐up it is unlikely that enough clinical events associated with the use of HT relative to placebo would occur for the trial to have the power to detect any excess risks/benefits for the use of HT compared to placebo or no treatment The overall study population started treatment 8.8 years after the menopause. The mean age of the menopause was 50.3 (SD 3.9) in the control group and 50.4 (SD 3.8) in the hormone group. The baseline age of participants was reported according to assigned treatment group, 54.1% of the control group and 62.6% of the hormone treatment group were < 60 years. Events were not reported individually or according to starting treatment < 10 or > 10 years since the menopause. For the subgroup analysis of when treatment was started, the entirety of events in this study were analysed as if all participants had started treatment within 10 years of their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Remotely randomised in permuted blocks |
| Allocation concealment (selection bias) | Low risk | Non‐transparent sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment blinded apart from for cancer outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analysed by intention to treat. However, stated participation rates differ across trial publications |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | EQ‐5D not measured at baseline, and therefore it is unclear whether there is between group baseline imbalance. Follow‐up data for EQ‐5D only reported at 2‐ and 3.6‐years follow‐up |
ERA 2000.
| Methods |
Objective: To evaluate the effects of HT on the progression of coronary atherosclerosis Multicentered three‐armed randomised controlled (RCT) trial involving six hospital sites in the United States. The trial was conducted from January 1996 to December 1997, with a mean follow‐up of 3.2 ± 0.6 years. The primary aim of the trial was to assess the effects of oestrogen replacement therapy with or without low‐dose progestin on angiographic progression or regression of coronary atherosclerosis in post‐menopausal women. The primary outcome was therefore change in the minimum diameter of the major epicardial segments, as assessed by quantitative coronary angiography. Clinical CVD events were all assessed as secondary outcomes Recruitment: Media announcements, contact through hospital records and admissions, screening logs from other studies Screening: Not stated Randomisation: Computerised in random blocks Stratification: According to lipid lowering therapy at baseline and hospital where angiogram was performed Allocation: Computer displayed treatment assignment after eligible participant details entered Baseline equality of treatment groups: No substantive differences between study groups at baseline Blinding: Participants, clinic staff and all outcomes assessment blinded. Treatment assignment available to designated member of data management staff. Questions relating to adverse effects directed to gynaecology physician and nurse not connected with study Analysis: ITT Funding Source: Grants from National Heart, Lung and Blood Institute and NationalCenter for Research Resources General Clinical Research Center, study medications from Wyeth‐Ayerst Research |
|
| Participants | Three hundred and nine post‐menopausal women with angiographically verified coronary disease were randomised to receive either (1) daily conjugated oestrogen alone (n = 100), (2) daily conjugated oestrogen in combination with medroxyprogesterone acetate (n = 105), or daily placebo (n = 105). Coronary artery disease was defined as at least one stenosis of 30% in any single coronary artery. The mean age of the women was 65.8 years (range: 41.8 ‐ 79.9), with a mean number of years since menopause of 22.5. Post‐menopausal status was defined as the presence of one of the following conditions: (1) an age of at least 55 without natural menses for at least five years; (2) no natural menses for at least one year and a serum follicle‐stimulating hormone level of more than 40 IU per litre; (3) documented bilateral oophorectomy; or self reported bilateral oophorectomy, a follicle‐stimulating hormone level of more than 40 IU per litre, and a serum oestradiol level of less than 25 pg per mm (91.1 pmol/L). 61% of the women had undergone a hysterectomy and 30.4% an oophorectomy At baseline 9% of women were taking oestrogen, and therefore underwent a three‐month ‘wash out ‘period prior to randomisation Included women were 82% White, 14% Black, and 4% of other racial origin. 49% had a history of MI and 47% a history of having undergone an angioplasty In terms of risk factors for CVD: 28% had diabetes; 67% had hypertension; 18% were current smokers; and 57% had a BMI > 27.5 kg/m2. The mean systolic blood pressure of the women was 130 mm Hg and the mean diastolic blood pressure 71.8 mm Hg. There were no statistically significant differences between the three treatment groups at baseline Inclusion criteria: Stated above, but only women who were 80% or more medication compliant in the one month prior to randomisation were eligible for participation in the trial Exclusion criteria:
|
|
| Interventions |
HT regimen: 1) 0.625 mg conjugated equine oestrogen daily and a placebo tablet daily (continuous dosage regimen) 2) 0.625 mg conjugated equine oestrogen plus medroxyprogesterone acetate and placebo tablet daily (continuous dosage regimen) Comparator: two placebo tablets daily (continuous dosage regimen) Participants were classified as medication compliant if they took ≥ 80% of their medication throughout the trial. Medication adherences in the 248 participants evaluated was: 74% in the oestrogen alone group (measured in 79% of participants); 84% in the combination therapy group (measured in the 84% of participants) and 86% in the placebo group (measured in 80% of participants). Additionally, five women in the placebo group initiated HT treatment outside the trial Follow‐up times: three months, six months and then every six months thereafter. Pre‐treatment investigations included serum electrolytes, haemoglobin levels, hematocrit, platelet count and prothrombin, a 12‐lead electrocardiogram and angiogram (if needed). Other investigations included annual mammography and gynaecological examinations, including Papanicolaou smears and endometrial aspiration or vaginal ultrasound, to detect sub‐clinical hyperplasia |
|
| Outcomes |
Primary outcomes: Death from any cause Death from CVD disease Non‐fatal MI Fatal MI Stroke Angina (hospitalisation) Any CVD event Secondary outcomes: Venous thromboembolism |
|
| Notes | The sample size calculation was predicated on the ability to detect differences between groups in the primary outcome measure, change in the minimum diameter of the major epicardial segments, as assessed by quantitative coronary angiography. It is therefore possible that the trial was not powered to detect differences between the three treatment groups on clinical events. It is therefore not possible to state whether there is any excess risk/benefit for the use of either oestrogen alone or in combination with medroxyprogesterone acetate compared to placebo on the basis of the results reported from the trial. The control group population started treatment on average 23.1 years after their menopause with the oestrogen group starting on average 22.1 years and the combination oestrogen and medroxyprogesterone group starting 23.8 years after. Events were not reported individually or according to starting treatment < 10 or > 10 years since the menopause. For the subgroup analysis of when treatment was started the entirety of events in this study were analysed as if all participants had started treatment more than 10 years after their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised in random blocks |
| Allocation concealment (selection bias) | Low risk | Computer displayed treatment assignment after eligible participant details entered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinicians blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up for clinical adverse events. Analysed by ITT |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | More in unopposed oestrogen group using nitrates at baseline, otherwise prognostic balance between groups |
ERT II 1979.
| Methods | Single centre 10 year double‐blind randomised control trial with matched pair design to evaluate the effects of oestrogen replacement therapy. Run at a hospital for chronic diseases where the participants were inpatients for the duration of the study. The study ran at Goldwater Memorial Hospital NYC from: 1965 to 1976. Follow‐up duration was 10 years Recruitment: from female hospitalised patients at Goldwater Memorial Hospital in New York City (a hospital for chronic diseases) Screening: history, physical examination and medical record review Randomisation: a research nurse was given matched pairs of participants and randomly selected which member of the pair would be assigned to the treatment group Stratification: not reported Allocation: by research nurse's discretion Baseline equality of treatment groups: all the participants were inpatients with chronic diseases, the matched pairs were identical for their primary diagnosis. The number of years since menopause were matched but gravidity and parity were not matched. Blood pressure, age and weight were not significantly different between groups Blinding: “a serious attempt was made to prevent the research physicians from knowing” however the code was broken in major medical complications 13 times (HRT) and 17 times (control). However the research nurse who did the allocating was not blinded Analysis: all participants included Funding Source: not reported |
|
| Participants | 168 women with long‐term chronic disease who were hospitalised for the entire study duration Inclusion criteria:
Exclusion criteria:
84 matched pairs selected from pool of 329 eligible participants Mean age was 55.3 years in the HRT group and 54.9 in the control group (P = 0.001) Number of years since last menstrual period was 4.7 years in the HT group and 4.5 years in the control group (P = 0.001) Mean blood pressure was 122/79 in the HT group and 122/80 in the control group (P = 0.498) The number of participants with diabetes mellitus was 14 (16.6%) in each group |
|
| Interventions |
HT regimen: conjugated oestrogen (Premarin) 2.5 mg daily and medroxyprogesterone acetate (Provera) 10 mg daily for 7 days in each month Comparator: matching placebo |
|
| Outcomes | Unspecified | |
| Notes | The chronic diseases which the participants were admitted for were unspecified as was any specialty that the hospital focused upon Medication compliance: not reported but as all were inpatients for duration, likely to be high How often assessed: formally assessed on annual basis The code for blinding was broken 13 times in the treated group and 17 times in the control group. When the code was broken the complication was noted and the patient was evaluated to that point of the study. No events were considered if they were recorded after the code was broken. The control group population started treatment on average 4.5 years after their menopause with the hormone group starting on average 4.7 years after. Events were not reported individually or according to starting treatment < 10 or > 10 years since the menopause. For the subgroup analysis of when treatment was started the entirety of events in this study were analysed as if all participants had started treatment within 10 years of their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pairs of participants were matched for age and diagnosis. Research nurse randomly selected which of the pair would be assigned to the treatment group |
| Allocation concealment (selection bias) | High risk | “a serious attempt was made to prevent the research physicians from knowing” however the code was broken in major medical complications 13 times (HRT) and 17 times (control). However the research nurse who did the allocating was not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matched placebo used, so participants likely blinded. No prespecified outcomes but physicians unblinded if participants became ill and therefore adverse event diagnosis and reporting is likely performed by unblinded assessors |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No prespecified outcomes but physicians unblinded if participants became ill and therefore adverse event diagnosis and reporting is likely performed by unblinded assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included |
| Selective reporting (reporting bias) | High risk | No prespecified outcomes |
| Other bias | Unclear risk | Death and cardiovascular outcomes were not prespecified as outcomes for this study but were reported afterwards as adverse events |
ESPRIT 2002.
| Methods |
Objective: To assess whether unopposed oestrogen reduces the risk of further cardiac events in post‐menopausal women who survive a first myocardial infarction Multicentre RCT involving 35 hospital sites in England and Wales. The trial was conducted over a two‐year follow‐up period (with recruitment beginning in July 1996 and ending in February 2000). All participants had suffered a first MI and were recruited within 31 days of the index event. MI was defined as two or more of: typical chest pain; ST elevation of 0.1 mV or more in at least one standard, or two precordial, leads of a 12‐lead ECG; or biochemical makers indicative of MI (serum concentrations of creatinine kinase or aspartate transaminase greater than twice the normal laboratory value, or serum troponin concentration greater than the locally defined threshold for MI. The primary outcome measures were non‐fatal reinfarction or cardiac death, and all‐cause mortality Recruitment: Research nurses checked hospital case notes, approached potentially eligible women if their family doctor agreed to collaborate Screening: Not reported Randomisation: List of random numbers generated by trial statistician in blocks of four Stratification: By clinical centre site Allocation: Women assigned consecutively to numbers kept on list accessible to statistician only Baseline equality of treatment groups: No substantive differences between study groups at baseline Blinding: Participants, clinicians, outcome assessors. Pharmaceutical company dispensed medication/placebo in identical numbered packages Unblinding occurred on request of family doctor or if participant withdrew from treatment (in later stages of study, only if withdrawing participant had not had a hysterectomy). Outcome assessors remained blinded throughout Analysis: ITT Funding Source: Schering AG provided medication |
|
| Participants | 1017 post‐menopausal women (HT: 513; placebo: 504) after a first MI with a mean age of 62.6 years (range: 50 ‐ 69) underwent treatment with either HT or placebo. Post‐menopausal status was defined as no vaginal bleeding in the previous 12 months. The mean age at last menstrual period was 46.5 years of age. 24% of women (n = 245) had undergone a hysterectomy. In terms of ethnic origin and risk factors for a further CVD event: 97% of the women were White; 53% were smokers at the time of admission; mean BMI was 40 kg/m2; 27% had angina; 44% had high blood pressure (not defined); 7.5% had suffered a previous stroke; 15% had diabetes, and 11% had used HT> 12 months before admission to the trial Inclusion criteria:
Exclusion criteria:
Both HT and placebo groups had similar baseline characteristics, including those identified a priori (and listed above) as potential confounders |
|
| Interventions |
HT regimen: 2 mg daily tablet of oestradiol valerate (continuous dosage regimen) Comparator: identical placebo capsule daily Treatment compliance was not formally assessed, but patient reported to the treating physician at follow‐up times. Medication compliance rates were poor, and were lower in the HT group than in the placebo group. At one year 51% of participants on the HT arm and 31% on the placebo arm were not taking their allocated tablets regularly. At two years, 57% of participants on the HT arm and 37% on the placebo arm were not taking their allocated tablets regularly Drop‐outs included 43 women in the HT group (8%) and 57 in the placebo group (11%) who did not take any of the trial medication Follow‐up times: patients were followed‐up at 3, 6, 12, 18 months and at study exit at 24 months |
|
| Outcomes | Death from CVD All causes of death Death from CVD MI (non‐fatal) Stoke Deep vein thrombosis Pulmonary embolism |
|
| Notes | The sample size power calculation was originally based on recruiting 1700 patients to achieve 80% power with a two‐sided test and a 5% significance rate predicated on HT reducing the rate of non‐fatal reinforcing or cardiac death by 13%. Due to financial constraints the trial was based on a total of 1017 women being randomised, and the power to detect a difference between treatment groups with this number was calculated as 56% assuming full treatment compliance in both of the treatment groups. Due to poor treatment compliance it is likely that the trial was underpowered to detect differences between treatment groups for some outcomes, and therefore the point estimates of differences between the groups are likely to be conservative. The overall study population started treatment on average 16.1 years after their menopause. Events were not reported individually or according to starting treatment < 10 or > 10 years since the menopause. For the subgroup analysis of when treatment was started the entirety of events in this study were analysed as if all participants had started treatment within 10 years of their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of random numbers generated by trial statistician in blocks of four |
| Allocation concealment (selection bias) | Low risk | Women assigned consecutively to numbers kept on list accessible to statistician only |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinicians blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up, analysed by ITT |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent source of other bias |
EVTET 2000.
| Methods |
Objective: To determine if HT alters the risk of venous thromboembolism in high risk women Multicentre RCT with a double triangular sequential design involving four hospital sites in Norway. The trial was conducted over a 1.3 year period between February 1996 and March 1998, but stopped early as other published trial results (HERS I 1998) indicated an increased risk of venous thromboembolism (VTE) with use of HT. The primary outcome measure was VTE and the secondary outcome measure pulmonary embolisms. VTE was verified by objective tests (i.e. venography or ultrasound in the case of DVT, and lung‐scan, angiography, or helical computed tomography, in the case of pulmonary embolism). At baseline, all participants underwent a clinical examination including breast and pelvic examinations with cytological smear test and evaluation of the endometrium with transvaginal ultrasound. A screening mammogram was also performed, as were routine haematological and clinical chemistry screening including blood lipids Recruitment: letters to family doctors, gynaecologists and hospitals, health bulletins and media Screening: Not reported Randomisation: computer‐generated 1:1 block randomisation with fixed block sizes of ten Stratification: By age < 60 years or > 60 years 37 (23 HT and 14 placebo) women did not attend all visits due to premature termination of the study Allocation: Not reported Baseline equality of treatment groups: No substantive differences between study groups at baseline Blinding: Double‐blind Analysis: ITT Funding Source: Novo‐Nordisk Pharmaceutical and research forum Ulleval University Hospital |
|
| Participants | 140 post‐menopausal women who had previously had either VTE or PE (HT: 71; placebo: 69) with a mean age of 55.8 years (range: 42 to 69 years) underwent treatment with either HT or placebo. Post‐menopausal status was defined as no natural menstruation for at least one year. The ethnic origin of the women included in the trial was not reported. In terms of risk factors for CVD 0.7% of women had previous/concomitant MI; 3% had angina; 1.4% had thromboembolic stroke; 3% had a transient ischaemic attack; 17% had hypertension and 2% had diabetes. The time since last DVT was four years (range: 0 ‐ 37 years) and last PE five years (range: 0 ‐ 34 years). There were no statistically significant differences between the two groups in terms of risk factors for CVD. In terms of smoking status: 39% were never smokers; 36% were previous smokers; 14% smoked between one to 10 cigarettes daily, whilst 10% smoked > 10 cigarettes per day. Mean BMI among the women was 27.1 kg/m2. Systolic blood pressure at baseline was 138 mm Hg and diastolic blood pressure was 83 mm Hg. Inclusion criteria:
Twenty‐eight women were also enrolled into the trial without objective testing as they had a typical history and had subsequently been treated for VTE. Exclusion criteria:
|
|
| Interventions |
HT regimen: 2 mg oestradiol plus 1 mg norethisterone acetate (1 tablet) daily (continuous dosage regimen) Comparator: identical placebo capsule daily Medication compliance in terms of pill counts was conducted at each follow‐up visit Follow‐up times: patients were followed up at three months, 12 months and 24 months Treatment adherence was not reported Loss to follow‐up: zero, but 37 (23 HT and 14 placebo) women did not attend all visits due to premature termination of the study. There were 33 dropouts, 10 in HT group (two wanted to be sure of being treated with oestrogen for post‐menopausal symptoms, eight had adverse effects), and 23 in the placebo group (11 wanted to be sure of being treated with oestrogen for post‐menopausal symptoms, 10 had adverse effects, two no reason stated) |
|
| Outcomes | Venous thrombosis Myocardial infarction Stroke | |
| Notes | Study terminated early, only 140 women enrolled of 240 planned due to the results from HERS I (1998) being made available. Power calculation: At a significance level of 5% and a power of 90% the sample size was estimated to a maximum of 240 women Data on timing of the menopause or starting treatment in relation to the menopause was not reported. The study author was emailed but no response was received. Baseline age was reported for each treatment group, control group 55.7 (SD 5.9) and 55.8 (SD 7.0) years. Events were not reported individually or according to starting treatment < 10 or > 10 years since the menopause, or age. For the subgroup analysis of when treatment was started, the entirety of events in this study were analysed as if all participants had started treatment within 10 years of their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated 1:1 block randomisation with fixed block sizes of ten |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and study personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The main findings were not reported by ITT, as drop‐outs from the placebo group were not included in the denominator for the rate of recurrent thromboembolism |
| Selective reporting (reporting bias) | Low risk | Reported all expected outcomes |
| Other bias | Low risk | No apparent source of other bias |
Greenspan 2005.
| Methods | Randomised, double‐blind, placebo‐controlled trial using 2 X 2 factorial design to compare HT and alendronate on physical performance, functional ability, physical activity, falls and cognitive function in elderly women. The study ran from January 1996 to May 2001. Randomised follow‐up duration was three years and run at a single centre. Recruitment: Newspaper advertisements, targeted mailings, presentations to senior groups and physician referrals of community‐dwelling women aged 65 and older from the greater Boston Area Screening: Medical history, physical examination, bone densitometry, mammography and laboratory evaluation Randomisation: All participants took part in a three‐month run‐in phase, where they all took hormone therapy alendronate, calcium and vitamin D. Once completed, they were randomised according to randomised lists prepared by the study statistician Stratification: By prior hysterectomy and three levels of total hip bone mineral density Allocation: By research pharmacist Baseline equality of treatment groups: No substantive differences between study groups at baseline Blinding: Block sizes (four, eight or 12) were randomly determined to enhance blinding. Those who administered the intervention and measured the outcomes were blinded to treatment group assignment Analysis: ITT Funding Source: NIH, Wyeth‐Ayerst Laboratories provided the Premarin and Preprom matching placebo and the Os‐Cal plus D. Merck Research laboratories provided the alendronate and matching placebo |
|
| Participants | 485 women entered three‐month open‐label run‐in phase with HT, alendronate, placebo, calcium and vitamin D, 373 completed the run‐in phase and were randomised Inclusion criteria:
Exclusion criteria:
The mean age of the women were: 71.2 years (SD ± 5.6) receiving HT, 71.3 years (SD ± 4.8) in the control group (P = 0.43). The number of participants who had had a hysterectomy were 66 (35%) and 64 (34%) in those receiving HT and the control group, respectively (P = 0.91). The body mass indices were comparable 27.5 (SD ± 4.8) in those receiving HT and 27.7 (SD ± 6.5) in the control group (P = 0.76) |
|
| Interventions | All women in the trial received a calcium and vitamin D supplement (OsCal Plus D) and a multivitamin tablet to ensure their calcium intake was greater than 1000 mg/d and their vitamin D was between 400 and 800 IU/day HT regimen: 1. Conjugated equine oestrogen 0.625 mg/d (Premarin) (Medroxyprogesterone 2.5 mg/d was given to those women with an intact uterus) (n = 93) 2. Conjugated equine oestrogen 0.625 mg/d (Premarin) (Medroxyprogesterone 2.5 mg/d was given to those women with an intact uterus) Plus alendronate (dose not specified) (n = 94) Comparators: 1. Alendronate alone (dose not specified) (n = 93) 2. Placebo alone (n = 93) Follow‐up time: three years |
|
| Outcomes |
Outcomes:
Adverse events: Menstrual spotting Menstrual cramping Endometrial biopsy Endometrial cancer Breast tenderness Breast cancer Bloating Peripheral oedema DVT Weight gain High blood pressure Hospitalisations MI Chest pain Clinical fractures Colon cancer Diabetes Gallstones Depression Stress incontinence |
|
| Notes | Methods for verifying medication compliance, not specified. Medication adherence was defined as women taking at least 80% of the medication at least 80% of the entire study period and present in 115 (61%) of those in the HT group and 124 (67%) in the control group (P = 0.33). Retention was defined as a participant who had a bone mineral density scan at least 33 months after randomisation, this took place in 169 (90%) in the HT allocated group and 168 (90%) of the control group (P = 1.00). Participant visits occurred every six months. Data on timing of the menopause or starting treatment in relation to the menopause was not reported. The study author was emailed but no response was received. Baseline age was reported for each treatment group, control group 71.3 (SD 4.8) and 71.2 (SD 5.6) years. Events were not reported individually or according to starting treatment < 10 or > 10 years since the menopause, or age. For the subgroup analysis of when treatment was started the entirety of events in this study were analysed as if all participants had started treatment more than 10 years after their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised lists were prepared by the study statistician. Randomisation was stratified by prior hysterectomy and three levels of total hip bone mineral density to ensure balance |
| Allocation concealment (selection bias) | Unclear risk | Block sizes (four, eight or 12) were randomly determined to enhance blinding of study staff. Further methods of allocation concealment are not reported. Also randomisation methods were prepared by the study statistician with participants randomised by the research pharmacist. Both were involved later in the study so may introduce bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded but not further specified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded, including those who assessed outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No prior published design paper. All outcomes specified in current paper were reported. Analyses were performed on intention‐to‐treat basis. No drop outs were reported |
| Selective reporting (reporting bias) | Low risk | All patients randomised were included in analysis. Limited data, on events according to alendronate status, most results reported according to HT status only. All outcomes specified were reported |
| Other bias | Unclear risk | Death and cardiovascular outcomes were not prespecified as outcomes for this study but were reported afterwards as adverse events |
HALL 1998.
| Methods |
Objective: to assess the effects of HT on angina and HRQoL in women with ischaemic heart disease Single‐centre, randomised, controlled (RCT) trial involving one hospital site in Sweden. The trial was a three‐arm trial comprising: one group who received 50 µg transdermal 17ß‐oestradiol daily for 18 days followed by 5 mg of combined treatment with medroxyprogesterone acetate orally; the second group who received 0.625 mg conjugated oestrogens (CEE) orally for 18 days followed by a combination with oral 5 mg medroxyprogesterone acetate daily, and the third group who received placebo. Due to not confounding the results of other trials in which oestrogens/progestins have been provided orally, the data presented in this trial are from the groups that received only oral medication (i.e. groups two and three). The length of follow‐up of the trial was one year. The primary outcome was angina, with death from CVD causes, MI, and the number of angioplasties and CABG performed reported as secondary outcomes Recruitment: Not reported Screening: Not reported Randomisation: Not reported Stratification: Not reported Allocation: Not reported Baseline equality of treatment groups: Only limited baseline characteristics reported for the treatment groups, and no statistical comparisons made between groups. Probable baseline imbalance between treatment groups for age (placebo group older than HT group); weight (placebo group heavier than HT group); number of years since menopause (placebo group higher number than HT group) Blinding: Not reported Analysis: Unclear. No statistical tests for between group differences conducted Funding Source: Hospital grant funded |
|
| Participants | Forty post‐menopausal women with existing coronary artery disease (HT: 20; placebo: 20) with a mean age of 60 years (range: 44 ‐ 75) underwent treatment with either HT or placebo for a year. No definition of what constituted post‐menopausal status and whether the trial included patients with a hysterectomy was reported. The mean BMI among the 40 women included in the trial was 30 kg/m2 (range: 20.0 to 40.7 years); the mean time of menopause was 12.5 years (range: 2 to 26); 9.5% were former smokers, 5.5% were never smokers and 5.5% were present smokers. In terms of diagnosis of CVD: 55% had a previous MI; 27.5% previous bypass surgery; 22.5% previous percutaneous transluminal coronary angioplasty (PTCA) (balloon dilation); 0% had type I diabetes; 10% had type II diabetes; 32.5% had hypertension, and 12.5% had claudication Inclusion criteria: No inclusion criteria were reported Exclusion criteria: No inclusion criteria were reported |
|
| Interventions |
HT regimen: 0.625 mg conjugated oestrogens (CEE) orally for 18 days followed by a combination with oral 5 mg medroxyprogesterone acetate daily (sequential dosage regimen) Comparator: identical placebo capsule daily The overall compliance with study intervention was not reported Follow‐up times: Baseline, three, six, 12 months and four to six weeks after completion of the trial. Pre‐treatment investigations included gynaecological history and occurrence of climacteric symptoms, Pap smear and mammography (if not performed within two years prior to recruitment). Blood samples were analysed for oestradiol, estrone, estrone sulphate and follicle stimulating hormone at baseline, three, six, 12 months and four to six weeks after trial completion. Additionally, a cardiac history, physical examination, and symptoms of angina pectoris were performed using the Canadian Heart Association protocol before trial entry. Minimal Health Related Quality of Life (HRQoL) data were measured at baseline and at one‐year follow‐up. The domains covered were: (1) well being; (2) mucous membrane changes; (3) climacteric symptoms; (4) breast tenderness; (5) negative mood changes; (6) headache, and (7) bleeding irregularities. The outcomes of these were not reported in the paper Withdrawals: 20%; 10% HT and 30% placebo |
|
| Outcomes | Death from CVD cause Angina Fatal MI Angioplasty Coronary artery bypass |
|
| Notes | No sample size calculation was performed and it is unlikely that the trial was powered adequately to detect significant differences in clinical event rates between the HT and placebo groups. No definition of how clinical events were defined or ascertained was reported. Additionally, no statistical analyses to assess differences in clinical event rates between the groups were performed. It is therefore unclear, whether the groups differed significantly in the number and types of events experienced. The length of trial follow‐up (one year) was unlikely to be long enough to ascertain either the longer‐term effects of HT use compared to placebo, or for other important CVD events to be assessed. The control group population started treatment on average 13.3 (SD 7.8) years after their menopause with the hormone group starting on average 11.6 (SD 6.7) years after. Events were not reported individually or according to starting treatment < 10 or > 10 years since the menopause. For the subgroup analysis of when treatment was started the entirety of events in this study were analysed as if all participants had started treatment more than 10 years after their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods of randomisation not reported; imbalance in baseline participant characteristics between groups |
| Allocation concealment (selection bias) | High risk | Methods of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and study personnel not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not reported, but may not influence ascertainment of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analyses do not appear to be undertaken on an ITT basis, it is unclear whether withdrawals were included in the analyses, and no statistical tests for between group differences conducted |
| Selective reporting (reporting bias) | Unclear risk | The paper reports the results for the main outcome of interest, angina, but it is unclear whether any other outcomes were prespecified but not reported. It appears that just the events that occurred in the trial were reported, rather than these being defined a priori for consideration in the trial. Additionally, HRQoL was measured within the trial, but the results of the assessments were not reported |
| Other bias | Unclear risk | It is unlikely that the trial was powered to detect differences in clinical events between the HT and placebo treatment groups. Furthermore, no statistical analyses were undertaken to assess differences in clinical event rates between the trial arms. Therefore, the lack of significant differences in event rates between the two groups should be treated with caution |
HERS I 1998.
| Methods |
Objective: To assess whether combined HT alters the risk for CHD events in post‐menopausal women with established coronary disease. Multicentre, randomised, placebo‐controlled, secondary prevention trial (RCT) involving 20 primary care sites in the United States. The trial recruitment was conducted from January 1993 to September 1994, with a mean follow‐up of 4.1 years. The primary aim of the trial was to assess the effects of combined oestrogen and progestin therapy compared to placebo for the prevention of recurrent coronary heart disease (CHD) events in post‐menopausal women with CHD. Coronary heart disease was defined as evidenced by prior MI, coronary artery bypass graft surgery (CABG), percutaneous transluminal coronary angioplasty, or other mechanical revascularisation, or at least 50% occlusion of a major coronary artery. The primary outcome was the occurrence of CHD events (CHD death or non‐fatal MI). Secondary outcomes included stroke, venous thromboembolic events, angina, and breast and endometrial cancers Recruitment: Lists of cardiac patients, mass mailing, direct advertising Screening: 3463 of whom 43% were excluded (ineligible, declined to participate, did not return for appointment or did not comply with placebo run‐in period) Randomisation: Computer‐generated random numbers in blocks of four Stratification: By clinical centre Allocation: Computer displayed after participant details entered Baseline equality of treatment groups: More women in control arm on statins at randomisation (67% versus 54%). When adjusted in analyses ‐ made no statistically significant difference Blinding: Participants, clinical centre staff, outcome assessors, data analysts, funders. Unblinding could occur when required for safety or symptom control, participants reported directly to gynaecology staff who were located separately from clinical staff, did not communicate with them about breast or gynaecological problems and were not involved in outcome ascertainment Analysis: ITT and also analysed by treatment received with inclusion limited to women with > 80% compliance Funding Source: Pharmaceutical (Wyeth‐Ayerst) HERS II An unblinded, open‐label observational continuation of HERS I in which 2321 women (93% of 2510 surviving HERS participants) followed up for a further 2.7 years (originally planned for additional four years but executive committee decided no further useful information likely to emerge). Number analysed: 2311 for vital status. Losses to follow‐up: ten women (1%) not contacted at final follow‐up (two in HT arm; eight in control arm) of these, vital status known for five. Adherence to treatment: among women originally assigned to the HT group, 45% reported at least 80% compliance during the sixth year of follow‐up. Among women originally assigned to placebo, 8% reported taking HT at six years |
|
| Participants | 2763 post‐menopausal women with verified CHD were randomised to receive either daily conjugated oestrogen in combination with medroxyprogesterone acetate (= 1380) or placebo (n = 1383). Post‐menopausal status was defined as age at least 55 years and no natural menses for at least five years, or no natural menses for at least one year and serum follicle‐stimulating hormone (FSH) level more than 40 IU/L, or documented bilateral oophorectomy, or reported bilateral oophorectomy with FSH level more than 40 IU/L and oestradiol level less than 92 pmol/L (25 pg/mL). The mean age of the women was 67 years (range: 44 ‐ 79), with a mean time of 18 years (SD: ± 8) since last menses. Included women were 89% White, 8% African‐American, 2% Hispanic, < 1% Asian, and < 1% other. In terms of risk factors for CVD: 13% were current smokers, 49% were past smokers and 38% had never smoked; 18.5% had diabetes and were on oral medication or insulin; mean systolic blood pressure was 135 (SD: ± 19) mm Hg and mean diastolic blood pressure was 73 (SD: ± 10). 56% of the women had a BMI > 27 kg/m2 and 23.5% had previous post‐menopausal oestrogen use (after menopause but not within three‐months of initial screenings for HERS trial). The CHD manifestations within the groups were: 9.5% had signs of congestive heart failure (presence of jugular venous distention more than 8 cm H20, S3 heart sound, rales, or pitting peripheral oedema); 17% had Q‐wave MI; 45% had undergone percutaneous coronary revascularisation, and 41.5% had undergone coronary artery bypass graft surgery. There were no statistically significant differences between treatment groups at baseline. Inclusion criteria: Stated above, plus ≤ 79 years old with uterus present Exclusion criteria:
|
|
| Interventions |
HT regimens: 0.625 mg conjugated oestrogen plus 2.5 mg medroxyprogesterone acetate daily (continuous dosage regimen) Comparator: identical placebo tablet daily. Rates of medication compliance in the trial were reasonably high. At the end of year‐one, 82% of women in the HT group and 91% in the placebo group reported taking study medication. At three years: 75% HT arm; 81% control arm. By pill count in HT arm: at one year: 79%; at three years: 70% HT arm Losses to follow‐up: Vital status known for all women at end of trial. 59 women did not complete follow‐up (32 in experimental arm, 27 in placebo arm) Follow‐up times: Baseline, and then every four months. At baseline, participants had a clinical examination, including breast and pelvic examination with Papanicolaou test and endometrial evaluation, a screening mammogram and standardised 12‐lead electrocardiogram (ECG). Fasting total cholesterol, low‐density lipoprotein (LDL) cholesterol, high‐density lipoprotein (HDL) cholesterol, and triglyceride levels were also measured. Annual examinations included cardiac examination and ECG. Separate annual follow‐up visits to the study gynaecologist included repeat breast and pelvic examinations with Papanicolaou smears and screening mammograms Health Related Quality of Life (HRQoL) was measured on four scales that assessed functional capacity, emotional health, vitality and depression. These were assessed at baseline, four months, and then follow‐up at years one, two, and three. Physical function was assessed using the Duke Activity Status Index, energy/fatigue using a four‐item RAND scale, mental health was measured by the RAND Mental Health Inventory, and depressive symptoms were assessed using an eight‐item scale developed by Burnam 1988 to screen for depression in the National Study of Medical Outcomes |
|
| Outcomes | Primary outcomes: Death from CVD Non‐fatal MI Secondary outcomes:‐ Death from any cause Fatal MI Stroke Angina (necessitating hospitalisation) Pulmonary embolism Venous thrombosis Coronary artery bypass surgery |
|
| Notes | Power calculation: 90% power to observe 24% reduction in coronary events at an average of 4.2 years (P = 0.05) follow‐up.
Further unblinded follow up 2.7 years (HERS II) [included in original Sanchez review]. Data were not reported as events stratified to years since menopause. However, the mean age since menopause was 18 years and 84% of the study participants were > 60 years old when enrolled. For the subgroup analysis of when treatment was started, the entirety of events in this study were analysed as if all participants had started treatment more than 10 years after their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers in blocks of four |
| Allocation concealment (selection bias) | Low risk | Computer displayed after participant details entered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, clinical centre staff, data analysts and funders blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Vital status known for all women at end of trial. 59 women did not complete follow‐up (32 in experimental arm, 27 in placebo arm). Analysed by intention to treat |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | More women in control arm on statins at randomisation (67% versus 54%). When adjusted in analyses ‐ made no statistically significant difference |
STOP IT 2001.
| Methods | Single‐centre, three‐year, double‐blinded, placebo‐controlled trial run in North America. The primary aim of the trial was to examine the effect of HRT and calcitriol, separately or combined, on bone loss in healthy elderly women Recruitment: Mailout sent to women in the Omaha area, followed by a phone call Screening: Medical and medication history Randomisation: Not reported Stratification: Not reported Allocation: Not reported Baseline equality of treatment groups: No substantive differences between groups Blinding: Staff, investigators and participants were blinded throughout the treatment period Analysis: ITT Funding Source: NIH, Wyeth‐Ayerst Laboratories, Inc. Pharm., Hoffman‐LaRoche Inc. and Pharmacia and Upjohn |
|
| Participants | 489 healthy women were recruited Inclusion criteria:
Exclusion criteria:
The mean age of the participants was 71 years (SD 4), range 65 to 77 Age in the placebo group 71 years (SD 4), calcitriol 72 years (SD 3), HRT 72 years (SD 4), HRT + calcitriol 71 years (SD 4) Years since menopause: not specified Previous HRT use: not specified but not allowed within six months prior to recruitment CVS Risk Factors Weight: placebo 69.4 kg (SD 13.0), calcitriol 68.3 kg (SD 13.0), HRT 69.5 kg (SD 12.4), HRT + calcitriol 67.3 kg (SD 12.0) Hysterectomy numbers were comparable in each of the four groups; overall 199 (41%) had undergone a hysterectomy. Those women with an intact uterus assigned to HRT received medroxyprogesterone (MPA) in addition to conjugated equine oestrogen (CEE), those without a uterus received just CEE. |
|
| Interventions |
HT regimen: conjugated oestrogen (Premarin) 0.625mg/d + Medroxyprogesterone (Provera) 2.5mg/d Hysterectomised women not given progestin Comparator 1: Calcitriol (Rocaltrol) 0.25 µg twice daily Comparator 2: HT + calcitriol Comparator 3: Matching placebo |
|
| Outcomes | Bone mineral density Fractures Falls Biochemistry details |
|
| Notes | After randomisation participants were assessed at six weeks, 12 weeks, six months and then every six months for three years. They had bone mineral density assessed, blood tests and a questionnaire at six‐monthly intervals. Medication adherence assessed at 36 months was 78% in the placebo group, 70% in the calcitriol group, 65% in the HRT group and 62% in the HRT + calcitriol group. Out of those still adherent, compliance at 36 months was: 92% in the placebo group, 93% in the calcitriol group, 92% in the oestrogen group and 94% taking MPA. Major reasons for discontinuation were bleeding problems (n = 21), breast tenderness (n = 13), other significant health problems (n = 21), lost interest in the study (n = 19), cerebrovascular incident: cerebral thrombosis, cerebral haemorrhage or TIA (n = 15) and gastrointestinal problems (n = 14). Five participants died during study from causes unrelated to study medication. Subjects were dispensed medication every six months and all returned pills were counted at that time to estimate compliance. The calcitriol group was omitted from analysis. the HRT and HRT + calcitriol were combined for analysis. Data were not collected on when treatment was started in relation to the menopause or when the menopause occurred. The study author was contacted but these data were not collected. The mean age in each treatment group was reported as follows, 72 years (SD 4) HRT, 71 years (SD 4) HRT and calcitriol and 71 years (SD 4). For the subgroup analysis of when treatment was started the entirety of events in this study were analysed as if all participants had started treatment more than 10 years after their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a simple randomisation strategy stratified on hysterectomy status |
| Allocation concealment (selection bias) | Low risk | An independent statistical group performed blinding and randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double‐blind, staff and investigators blinded throughout treatment period. Also used placebo but no description is provided. An independent statistical group performed blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Double‐blinded” but unspecified how |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants stopping their study medication was modest with similar rates in both the intervention and placebo groups. The primary outcome was assessed on an intention‐to‐treat analysis basis. 73 participants did not complete the study (11 in placebo group, 22 in calcitriol group, 20 in HT group and 20 in HT + calcitriol group); the reasons for not completing were not specified |
| Selective reporting (reporting bias) | Low risk | All outcomes stated were reported |
| Other bias | Unclear risk | Death and cardiovascular outcomes were not prespecified as outcomes for this study but were reported afterwards as adverse events |
WAVE 2002.
| Methods |
Objective: To determine whether HT or antioxidant vitamin supplements, alone or in combination, influence the progress of coronary artery disease in post‐menopausal women as measured by angiography. Multicentre, 2 x 2, randomised, factorial, placebo‐controlled (RCT) trial involving seven hospital sites; five in the United States and two in Canada. The trial recruitment was conducted from July 1997 to July 1999), with a mean follow‐up of 2.8 (SD: ± 0.9 years. The primary aim of the trial was to assess the effects of oestrogen and/or progestin with or without antioxidant vitamins for preventing angiographic progression of coronary artery disease. Coronary artery disease was defined as having al least one coronary segment with stenosis of ≥ 15% and 75% in a vessel ≥ 2 mm in diameter at baseline, with the angiograph conducted within four months of trial recruitment. The primary outcome was therefore change in the minimum lumen diameter (MLD) of the vessels from baseline, as assessed by quantitative coronary angiography at follow‐up. Clinical CVD events and health related quality of life were all assessed as secondary outcomes Recruitment: Recruited at clinical sites in USA and Canada Screening: Not reported Randomisation: Computer‐randomised, permuted block design with random blocks of two and four Stratification: Clinical centre, hysterectomy status Allocation: Remotely by phone call to study co‐ordinating centre Baseline equality of treatment groups: Higher prevalence of diabetes and higher fasting blood glucose levels in the HT group Blinding: Participants, investigators and staff at clinical centres blinded except (when necessary) the study gynaecologist. Adverse effects managed by gynaecologist not involved in outcome assessment who had access to treatment assignment if necessary, with permission of coordinating centre (unblinding). Analysis: No (98% of women analysed by ITT). Funding Source: National Heart, Lung and Blood Institute contract, General Clinical Research Center grant, USA. |
|
| Participants | Four hundred and twenty‐three post‐menopausal women with angiographically verified coronary disease were randomised to receive either (1) daily conjugated oestrogen alone for participants who had undergone a hysterectomy; (2) daily conjugated oestrogen in combination with medroxyprogesterone acetate; (3) vitamins E and C, or (4) placebo. Post‐menopausal status was defined as having bilateral oophorectomy at any age, being younger than 55 years old with a follicle‐stimulating hormone level of 40 IU/ml or higher, or being older than 55 years. Included women were 66% White and 34% non‐White (Black or other; specific origins not reported). In terms of risk factors for CVD: 37% had diabetes; 76% had hypertension; 39% were current smokers; 43% had suffered a previous MI, and 37.5% were current HT users. The mean BMI was 30.7 kg/m2; mean systolic blood pressure was 139 (SD: 21) mm Hg and the mean diastolic blood pressure 76 (SD: 10.5) mm Hg. The HT and placebo HT groups were well‐balanced in terms of baseline characteristics, apart from the exception of the active HT group having a statistically significantly higher prevalence of diabetes and higher fasting blood glucose levels Exclusion criteria:
|
|
| Interventions |
HT regimens: 1) 0.625 mg conjugated equine oestrogen daily plus placebo for women who had undergone a hysterectomy (continuous dosage regimen). 2) 0.625 mg conjugated equine oestrogen plus 2.5 mg medroxyprogesterone acetate daily plus placebo for women who had not undergone a hysterectomy (continuous dosage regimen). 3) 400 IU vitamin E twice daily (800 IU) plus 500 mg vitamin C twice daily (1 g) Comparator: two placebo tablets daily. Adherence to treatment: Evaluated for 159/211 who had angiographic follow‐up: HT group took 67% of medication, placebo group took 70%; 9/108 women in placebo group crossed to open‐label oestrogen Losses to follow‐up: Five (three in HT group, two in placebo group) Follow‐up times: Baseline, three months, and then every six months. Patients underwent a coronary angiography at baseline and trial exit. Other investigations performed at baseline were: 12‐lead electrocardiogram; breast and pelvic examinations, mammography, Papanicolaou smears and fulfilment of the five health‐related quality of life questionnaires (HRQoL). Baseline assays included: fasting glucose, insulin, HbA1c, fibrinogen, lipid profile, vitamins C and E and estrone. HRQoL questionnaires: Five HRQoL questionnaires were completed at baseline and at 18 months by participants. The specific questionnaires completed were: (1) the Medical Outcome Study Short Form (SF‐36); (2) Centre for Epidemiological Studies‐Depression Scale; (3) Seattle Angina Questionnaire; (4) Duke Activity Scale Index, and (5) The Medical Outcomes Study Sleep Questionnaire |
|
| Outcomes | Mean change from baseline in MLD of all qualifying angiographic segments Death from any cause Death from CVD Non‐fatal MI Stroke Secondary outcomes: Deep vein thrombosis Health‐related quality of life |
|
| Notes | The sample size calculation was predicated on the ability to detect differences between groups in the primary outcome measure, change in the minimum lumen diameter of all qualifying angiographic segments, as assessed by quantitative coronary angiography. The trial was therefore not powered to detect differences in CVD clinical events between the treatment groups. Additionally, as the factorial design revealed no interactions between treatment groups, results for the two HT versus placebo treatment groups (i.e. oestrogen alone or oestrogen in combination with progestin) were pooled and presented as aggregate numbers of events. It is therefore not possible to state whether there is any excess risk/benefit for the use of either oestrogen alone or in combination with medroxyprogesterone acetate compared to placebo on the basis of the results reported from the trial. Data were not published regarding the time that treatment was started in relation to the menopause or what age the menopause took place. The study author was contacted and advised that these data would be available from NIH. NIH were contacted but as yet these data have not been released. The mean age of study participants was reported, 65 years (SD 9) in the active group and 66 years (SD 9) in the control group. For the subgroup analysis of when treatment was started the entirety of events in this study were analysed as if all participants had started treatment more than 10 years after their menopause. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomised, permuted block design with random blocks of two and four |
| Allocation concealment (selection bias) | Low risk | Remotely by phone call to study coordinating centre |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators and staff at clinical centres blinded except (when necessary) the study gynaecologist |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators and staff at clinical centres blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up five (three in HT group, two in placebo group), 98% of women analysed by intention to treat |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Groups balanced at baseline, apart from HT group had a higher prevalence of diabetes and higher fasting blood glucose levels |
WELL‐HART 2003.
| Methods | Multicentre randomised, double‐blinded, placebo‐controlled trial run at 5 centres in North America. It was run from June 1995 to October 2000 and was designed to test the effects of oral micronised 17β‐oestradiol with or without sequential administered medroxyprogesterone acetate, compared to control, on the progression of atherosclerosis in postmenopausal women with angiographically documented coronary artery disease. Participants were stratified according to the presence or absence of diabetes Recruitment: Not reported Screening: Not reported Randomisation: Computerised random number generator in the data coordinating centre used and adaptive randomisation was used to correct for imbalances between treatment groups in total cholesterol Stratification: According to diabetes status Allocation: not reported Baseline equality of treatment groups: The age in the oestrogen treatment group was significantly lower than the other two treatment groups (P=0.02). Otherwise there were no substantive differences between groups Blinding: The participants, gynaecologists, clinical staff and image analysts were all blinded to treatment assignment Analysis:Only those who could be quantified by quantitative coronary angiography were included Funding Source: NHLBI, Mead Johnson laboratories and Pharmacia and Upjohn |
|
| Participants | 226 postmenopausal women who had at least one coronary artery lesion. The mean age of the participants were: 64.2 years in the control group (SD 6.2), 61.8 years in the oestrogen group (SD 6.7) [E] and 64.4 years in the oestrogen‐progestin group (SD 6.4) [EP] P = 0.02 Years since menopause: 18.3 years in the control group (SD 10.5), 16.7 years in the oestrogen group (SD 10.3) [E] and 19.7 years in the oestrogen‐progestin group (SD 10.5) [EP] P = 0.23 CVS Risk Factors Smoking status: P = 0.55 Current smoker:7 (9%) [control], 11 (14%) [E], 8 (11%) [EP] Former smoker: 28 (37%) [control], 27 (36%) [E], 34 (46%) [EP] Never smoked: 41 (54%) [control], 38 (50%) [E], 32 (43%) [EP] Diabetes: 40 (53%) [control], 38 (50%) [E], 37 (50%) [EP] P = 0.93 Blood pressure – systolic: 141.6 (SD 22.4) [control], 138.1 (SD 21.7) [E], 142.3 (SD 24.6) [EP] P = 0.49 Blood pressure – diastolic: 75.9 (SD10.5) [control], 76.5 (SD 11.1) [E], 75.3 (SD 12.5) [EP] P = 0.82 BMI: 30.0 (SD 5.4) [control], 30.6 (SD 5.6) [E], 30.2 (SD 5.6) [EP] P = 0.83 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
HT regimen: 1 mg of oral micronised 17β‐oestradiol [E] (Estrace, Mea Johnson) daily, plus a placebo tablet matching medroxyprogesterone acetate for 12 consecutive days of every month HT regimen 2: 1 mg of oral micronised 17β‐oestradiol (Estrace, Mea Johnson) daily, plus 5 mg medroxyprogesterone acetate [P] (Provera, Upjohn) for 12 consecutive days of every month Comparator: two placebo tablets, one matching each of the active drugs for 12 consecutive days of every month |
|
| Outcomes |
Primary outcomes: Average (per‐participant) change from baseline in the percent stenosis in all lesions evaluated by quantitative coronary angiography Secondary outcomes: Average (per‐participant) change in minimal luminal diameter (on quantitative angiography) Global change score |
|
| Notes | LDL was reduced to a target of less than 130 mg per decilitre (3.36 mmol/L) by means of dietary intervention and lipid lowering therapy (usually with a statin) Follow‐up times: median duration 3.3 years Medication compliance: P = 0.28 Control group: 93.6% with oestrogen matched placebo and 98.4% with progestin matched placebo Oestrogen group: 92.6% with oestrogen and 99.9% with progestin matched placebo Oestrogen‐Progestin group: 94.1% with oestrogen and 96.1% with progestin Participants were assessed with visits every month for the first six months and then every other month up until 36 months Methods for verifying medication compliance by pill counts at study visits The number who dropped out or swapped medication: not clear, though four participants used open‐label oestrogen The mean time since menopause that treatment was started for the study population overall was 18.2 years. For the subgroup analysis of when treatment was started, the entirety of events in this study were analysed as if all participants had started treatment more than 10 years after their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratification according to the presence or absence of diabetes Randomisation with the use of a computerised random‐number generator Adaptive randomisation was used for imbalances among the treatment groups in the total cholesterol |
| Allocation concealment (selection bias) | Low risk | Data co‐ordinating centre performed randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, gynaecologists and clinical staff were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Image analysts were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 44 participants dropped out between different treatment groups but in comparable numbers between groups (11 in the control group, 17 in the oestrogen group and 16 in the oestrogen‐progestin group). The reasons for dropping out included: death (n = 5), medical problems (n = 7), open‐label use of oestrogen therapy (n = 4), loss of follow‐up (n = 5) and personal reasons (n = 23) |
| Selective reporting (reporting bias) | Low risk | No prior published design paper All outcomes specified in current paper were reported |
| Other bias | Unclear risk | Death and cardiovascular outcomes were not prespecified as outcomes for this study but were reported afterwards as adverse events |
WEST 2001.
| Methods |
Objective: To determine whether 17ß‐oestradiol reduces the risk of recurrent stroke or death among post‐menopausal women who have experienced a transient ischaemic attack or non‐disabling ischaemic stroke. Multicentre, randomised, controlled (RCT) trial involving 21 hospital sites in the United States. The trial was conducted from December 1993 to May1998, with a mean follow‐up duration of 2.8 years ± 17 months. The primary outcome measures were the number of strokes or deaths that occurred, with further clinical events of MI, and TIA also reported. Medical testing for patients at baseline included computerised tomography (CT) scan, clinical breast examination, electrocardiogram, and a pelvic examination including a Papanicolaou smear (in women who had not undergone a hysterectomy). Additionally, neurological examination was performed by a trained nurse using the National Institutes of Health (NIH) Stroke Scale, and tests on physical and cognitive performance, including the Boston Naming Test, a test of digit span recall, category work list generation, a depression screen, the Mini‐Mental Status Examination and a test of delayed spatial recognition were undertaken. Recruitment: Admissions to 20 largest regional hospitals in Connecticut and Massachusetts; also via contact with selected neurology groups and direct referrals from physicians Screening: 5296 screened for eligibility (2772 ineligible, 1843 declined to participate, 17 unable to be randomised within protocol time frame) Randomisation: Computer generated at pharmacy, in blocks of four Stratification: By trial centre and risk level (three levels) Allocation: By remote contact with trial pharmacy Baseline equality of treatment groups: No substantive differences between study groups at baseline Blinding: Participants, investigators and endpoint assessors blinded. Study internist unblinded in the case of overriding concern about a woman’s clinical care Analysis: ITT Funding Source: National Institute of Neurological Disorders and Stroke grant, Medical Research Council of Canada grant. Mead Johnson laboratories provided support and study drug |
|
| Participants | 664 post‐menopausal women (HT: n = 337; placebo: n = 327) with a mean age of 71.5 (SD: ± 10 years) who had undergone either a non‐disabling ischaemic stroke or a transient ischaemic attack in the previous 90 days prior to recruitment. Post‐menopausal status was defined as amenorrhoea for at least 12 months or, for women who had undergone a hysterectomy without oophorectomy, an oestradiol level less than 40 pg/mL and a follicle‐stimulating hormone level over 40 mIU/mL. The number of women who had previously undergone a hysterectomy was 44.5%. 29.5% of the women had previously used oestrogen‐replacement therapy. In terms of ethnic background included women were: 83.5% White; 13% Black; and 3.5% other (unspecified). 24% had a previous MI; 14.5% congestive heart failure; 7% atrial fibrillation; 73.5% hypertension; and 28% diabetes. 12.5% were current cigarette smokers, and the mean BMI among the women was 28 kg/m2 (SD: ± 6). In terms of neurological characteristics: 18.5% had a history of stroke before the index (ischaemic or TIA) event, and 75% had a stroke as the index event. In relation to summary risk stratum of the occurrence of another event [based on a validated instrument that included the five clinical features of age, blood pressure, diabetes, cardiac disease, and index event (stroke versus TIA)] 12.5% of women were classified as low risk, 67% as medium risk, and 20.5% as high risk Inclusion criteria:
Exclusion criteria:
Temporary exclusion criteria that had to be resolved by the time of randomisation were moderate to severe neurological disability, or clinical suspicion of breast or uterine cancer |
|
| Interventions |
HT regimen: 1 mg 17ß‐oestradiol daily (plus a course of 5 mg medroxyprogesterone acetate once a year for 12 days or annual transvaginal ultrasound [to screen for endometrial hyperplasia] for women with a uterus) plus standard care (continuous dosage regimen) Comparator: identical placebo capsule daily plus standard care The overall compliance with study intervention assessed by pill count at each visit (including women who discontinued treatment) was 60% (56% in the HT group and 64% in the placebo group). Compliance among women who did not discontinue the study drug was 90% in both treatment groups. Dropouts: 34% of the HT group and 24% of the placebo group Losses to follow‐up: Zero Follow‐up times: Baseline and then every three‐months |
|
| Outcomes | Death Stroke Death from CVD cause Non‐fatal MI Secondary outcomes:‐ Venous thromboembolism Pulmonary embolism |
|
| Notes | Sample size calculations and recruitment of participants were adequate to allow for drop‐outs, and able to provide the power to detect any statistically significant differences between the HT and placebo group in terms of the primary outcomes of interest. The clinical events of interest were defined according to standard criteria and verified by a neurologist blinded to treatment allocation, or by objective measures of disease such as positive results on a duplex ultrasonogram or venogram for the diagnosis of VTE. All events were centrally corroborated, and sensitivity analyses undertaken to examine the effect of including only medication compliant patients in the analyses. Study publication pools results for women on unopposed and combined therapies. Vital status was confirmed for all women at the conclusion of the trial. Data were not stratified according to time since menopause that treatment was started, however the mean time since menopause for the study population as a whole was 25 years. The age of each treatment group was 71 years (SD 10) for the control group and 72 years (SD 10) for the hormone group. For the subgroup analysis of when treatment was started the entirety of events in this study were analysed as if all participants had started treatment more than 10 years after their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated at pharmacy, in blocks of four |
| Allocation concealment (selection bias) | Low risk | By remote contact with trial pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Endpoint assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up, analysed by ITT |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent source of other bias |
WHI I 2002.
| Methods |
Objective: To assess the major health benefits and risks of the most commonly used combined hormone preparation in the United States Multicentre, randomised, placebo‐controlled, primary prevention trial (RCT) involving 40 primary care sites in the United States. The trial recruitment was conducted from January 1993 to September 1998, with a mean follow‐up of 5.2 years (range: 3.5 to 8.5); planned duration 8.5 years. The primary aim of the trial was to assess the effects of oestrogen in combination with progestin compared to placebo on disease incident rates of CHD, hip fractures and deaths from all causes. The primary outcome measure was CHD events (defined as non‐fatal MI and CHD death), with invasive breast cancer as the primary adverse outcome. Secondary outcomes included stroke (both fatal and non‐fatal), pulmonary embolism, DVT, angina (both hospitalisation due to and confirmed), revascularisation (CABG or percutaneous coronary intervention (PCI) combined), death from all causes, as well as a global index of risks and benefits defined as time to the first event among CHD, invasive breast cancer, stroke, pulmonary embolism, endometrial cancer, colorectal cancer, hip fracture or death due to other causes to summarise overall effects. Late in 1999, the National Institutes of Health Data and Safety Monitoring Board (DSMB) observed small but consistent early adverse effects in cardiovascular outcomes and in the global index. However, none of the disease specific monitoring boundaries had been crossed. These adverse CV effects continued throughout 2000 and 2001, but the trial continued because the balance of risks and benefits remained uncertain. The trial was finally stopped early after a mean follow‐up of 5.2 years in May 2002, when the DSMB found that the adverse effects in CVD persisted, although these remained within the monitoring boundaries, but the weighted log‐rank test statistic for breast cancer had crossed the designated stopping boundary, and the global index was supportive of a finding of overall harm. The trial was therefore terminated at the end of May 2002 Recruitment: Letter of invitation in conjunction with media awareness programme. Sampling method gave women from minority groups six‐fold higher odds for selection than Caucasian women and resulted in sample with 84% racially/ethnically designated “white”, 16% non‐“white” Screening: Interested women screened by phone or mail for eligibility, then attended three screening visits for history, clinical examination and tests. Three‐month washout period before baseline evaluation of women using post‐menopausal hormones at baseline screening. Lead‐in placebo pills given for at least four weeks during screening process to establish compliance with pill taking Randomisation: Centrally randomised by permuted block algorithm Stratification: By clinical centre site and age group Allocation: By local access to remote study database Baseline equality of treatment groups: No substantive differences between study groups at baseline Blinding: All participants, clinic staff, and outcome assessors blinded, with the exception of 331 participants who were unblinded from the unopposed oestrogen arm and reassigned to combined HT arm due to change in protocol Analysis: ITT Funding Source: The National Heart, Lung, and Blood Institute. Wyeth‐Ayerst Research provided the study medication |
|
| Participants | 16,608 healthy post‐menopausal women were randomised to receive either daily conjugated equine oestrogen in combination with progestin (n = 8506) or placebo (n = 8102). Post‐menopausal was defined as no vaginal bleeding for six months (12 months for 50 to 54 years), or having ever used post‐menopausal hormones. The mean age of the women was 63.25 years [(SD: 7.1) (range: 50 to 79]. Age ratio of 33%: 45%: 21% for the baseline age categories of 50 to 59, 60 to 69, 70 to 79, respectively (enrolment targeted to achieve ratio of 30: 45: 25) Included women were 84% White, 7% Black, 5% Hispanic, 0.4% American Indian, 2.2% Asian/Pacific Islander, and 1.4 % unknown. In terms of previous hormone use: 74% were ‘never’ HT users, 20% were past users and 6% were current users (therefore requiring a three‐month washout period prior to randomisation). 70% of women had used HT < five years, 18% for five to < ten years, and 12% for ≥ 10 years In terms of risk factors for CVD: 50% were never smokers, 39.5% were past smokers, and 10.5% were current smokers. The mean BMI among the women was 28.5 kg/m2; mean systolic blood pressure was 128 (SD: ± 17.5) mm Hg and mean diastolic blood pressure was 75.7 (SD: ± 9.1) The CHD manifestations within the groups were: 4.4% were being treated for diabetes, 36% for hypertension or BP ≥ 140/90 mm Hg, 1.8% had a previous MI, 2.9% had angina, 1.3% had undergone either CABG/PTCA surgery, 0.9% had suffered a previous stroke, and 0.9% had DVT or PE.†; 12.7% had elevated cholesterol levels requiring medication, 6.7% were using statins at baseline, and 19.6% aspirin Inclusion criteria:
Exclusion criteria:
† Prior to the publication of the results of HERS I in 1997, (which led to a change in the inclusion criteria) women with a history of venous thromboembolism (VTE) were eligible for inclusion. From this point onwards women with indicated prior VTE were excluded. At this point 171 women with a history of VTE had been enrolled into the trial |
|
| Interventions |
HRT regimens: 0.625 mg conjugated equine oestrogen plus 2.5 mg medroxyprogesterone acetate (MPA) daily Comparator: identical placebo tablet daily Medication adherence was defined as participants taking > 80 study pills, and was monitored by weighing medication bottles at each clinic visit. Medication adherence data for each trial year were not reported, but by the time of study termination 40% of women had stopped taking study medication (HRT: 42%; placebo: 38%). Therefore only 60% of women remained medication compliant. At 5.2 years follow‐up 6.2% of women in the HT arm had initiated hormone use through their own physician and 10.7% of women in the placebo arm had also initiated hormone use (drop‐in) Follow‐up times: baseline, and then every six months. At baseline participants had a clinical examination, including breast and pelvic examination with Papanicolaou test and endometrial evaluation, a screening mammogram and standardised 12‐lead electrocardiogram (ECG). Fasting total cholesterol, low‐density lipoprotein (LDL) cholesterol, high‐density lipoprotein (HDL) cholesterol, and triglyceride levels were measured in a subsample of participants. Annual examinations included mammograms and clinical breast examinations. ECG results were collected at three‐ and six‐year follow‐up. Participant attrition rates were low. Over the 5.2 year follow‐up 3.5% [total n = 583; (HRT: n = 307; placebo: n = 276)] women withdrew, were considered lost to follow‐up, or stopped providing outcome data for more than 18‐months. Vital status at the end of the trial was therefore known for 15,576 (96.5%) of randomised participants, including 580 (2.7%) known to be deceased |
|
| Outcomes |
Primary outcomes: CHD (defined as acute MI requiring overnight hospitalisation, silent MI, or CHD death) Death from CVD Non‐fatal MI (defined as acute MI requiring overnight hospitalisation, silent MI) Secondary outcomes: Death from any cause Stroke (fatal and non‐fatal combined) Angina (confirmed) Revascularisation (CABG or PCI combined) Pulmonary embolisms Venous thrombosis (pulmonary embolism plus DVT combined) HRQoL not included in the analyses; length of follow‐up: 5.6 years |
|
| Notes | The sample size calculation was adequate so the trial was powered to detect differences between the HT and placebo groups in terms of CVD events, and adverse events. All outcomes were prespecified and defined a prior, and reported in the trial results. All study personnel, except the study gynaecologist were ‘blinded’. The gynaecologist was ‘unblinded’ if necessary to treatment group, but separate from the rest of the trial team, and therefore ‘blinding’ is likely to have been maintained. Participant attrition rates were very low at 3.5%. However, medication compliance rates were low, with only 60% of women still medication compliant at 5.2 year follow‐up. This is likely to have ‘diluted’ the true effects, both positive and negative, of the HT combination therapy compared to placebo relative to what might be observed with full medication adherence. Additionally the trial was stopped early which would have further decreased the power to detect differences between the two trial arms, and reduced the precision of the estimated effects for the outcomes assessed. Events for this trial were reported stratified according to the time since menopause that treatment was started. This allowed accurate allocation of events, specifically: death, coronary heart disease (death from cardiovascular causes and non‐fatal myocardial infarction), stroke and venous thromboembolism, to subgroup analysis according to whether treatment was started < 10 years or > 10 years after the menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally randomised by permuted block algorithm |
| Allocation concealment (selection bias) | Low risk | By local access to remote study database |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and clinic staff blinded, with the exception of 331 participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 583 participants (3.5%) withdrew, were lost to follow‐up, or stopped providing outcome information for more than 18 months Analysis conducted on ITT basis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent source of other bias |
WHI II 2004.
| Methods |
Objective: To assess the effects on major disease incidence rates of the most commonly used post‐menopausal HT in the United States Trial type: Multicentre randomised placebo controlled primary prevention trial (RCT) involving 40 primary care sites in the United States. The trial recruitment was conducted from January 1993 to September 1998, with a mean follow‐up of 6.8 years (range: 5.7 to 10.7). The primary aim of the trial was to assess the effects of oestrogen therapy compared to placebo on disease incident rates of CHD, hip fractures and deaths from all causes. The primary outcome measure was CHD events (defined as acute MI requiring overnight hospitalisation, silent MI, or CHD death), with invasive breast cancer as the primary adverse outcome. Secondary outcomes included stroke (both fatal and non‐fatal), pulmonary embolism, DVT, angina (both hospitalisation due to and confirmed), revascularisation (CABG or PCI combined), death from all causes, as well as a global index of risks and benefits defined as time to the first event among CHD, stroke, pulmonary embolism, breast cancer, colorectal cancer, hip fracture or death due to other causes to summarise overall effects. The trial was stopped early after a mean follow‐up of 6.8 years when the National Institutes of Health (NIH) concluded that CEE alone did not appear to affect the risk of heart disease, but was associated with a significant increase in the risk of stroke, and given the likelihood that neither cardio‐protection or breast cancer risk would be demonstrated in the remaining intervention period terminated the trial on March 1, 2004 Recruitment: Letter of invitation in conjunction with media awareness programme. Sampling method gave women from minority groups six‐fold higher odds for selection than Caucasian women and resulted in sample with 84% racially/ethnically designated “white”, 16% non‐“white” Screening: Interested women screened by phone or mail for eligibility, then attended three screening visits for history, clinical examination and tests. Three‐month washout period before baseline evaluation of women using post‐menopausal hormones at baseline screening. Lead‐in placebo pills given for at least four weeks during screening process to establish compliance with pill taking Randomisation: Centrally randomised by permuted block algorithm Stratification: By clinical centre site and age group Allocation: By local access to remote study database Baseline equality of treatment groups: No substantive differences between study groups at baseline Blinding: All participants, clinic staff, and outcome assessors blinded, with the exception of 331 participants who were unblinded from the unopposed oestrogen arm and reassigned to combined HT arm due to change in protocol Analysis: ITT Funding Source: The National Heart, Lung, and Blood Institute. Wyeth‐Ayerst Research provided the study medication |
|
| Participants | 10,739 healthy post‐menopausal women who had previously undergone hysterectomy with or without an oophorectomy (including 248 in experimental arm, 183 in placebo arm who joined this study after randomisation to corresponding arms in WHI 2002 having subsequently had a hysterectomy for reasons other than cancer) were randomised to receive either daily conjugated equine oestrogen (n = 5310) or placebo (n = 5429). The mean age of the women was 63.6 years [(SD: ± 7.3; range: 50 to 79)] (Age ratio of 33%: 45%: 21% for the baseline age categories of 50 to 59, 60 to 69, 70 to 79, respectively). Included women were 75% White, 15% Black, 6% Hispanic, 1% American Indian, 1.5% Asian/Pacific Islander, and 1.5% unknown. In terms of previous hormone use: 74% were ‘never’ HT users, 20% were past users and 6% were current users (therefore requiring a three‐month washout period prior to randomisation). 53% of women had used HRT < five years, 19% for five to < 10 years, and 18% for ≥ 10 years. In terms of risk factors for CVD: 51% were never smokers, 38.5% were past smokers, and 10.5% were current smokers. The mean BMI among the women was 28.5 kg/m2 (SD: ± 5.85); mean systolic blood pressure was 127.5 (SD: ±17.55) mm Hg and mean diastolic blood pressure was 75.7 (SD: ± 9.1). The CHD manifestations within the groups were: 4.4% were being treated for diabetes, 36% for hypertension or BP ≥ 140/90 mm Hg, 1.6% had a previous MI, 2.9% had a history of angina, 1.3% had undergone either CABG/PTCA surgery, 0.85% had suffered a previous stroke, and 0.85% had a history of DVT or PE Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
HT regimens: 0.625 mg conjugated equine oestrogen daily (CEE) (continuous dosage regimen) Comparator: identical placebo tablet daily Medication adherence was defined as participants taking > 80 study pills, and was monitored by weighing medication bottles at each clinic visit. Medication adherence data for each trial year were not reported, but by the time of study termination 53.8% of women had stopped taking study medication. Therefore only 46.2% of women remained medication compliant. Compliance rates did not differ significantly between the two trial arms. At 6.8 years follow‐up 5.7% of women in the HT arm had initiated hormone use through their own physician and 9.1% of women in the placebo arm had also initiated hormone use (drop‐in) Follow‐up times: Baseline, and then every six‐month, with an annual clinic visit. At baseline participants completed a medical, reproductive history and psychosocial questionnaire; ECG, and underwent breast examination and gynaecological examination. Mammograms and breast examinations were repeated annually and ECGs were repeated at visit years three and six. Participant attrition rates were low. Over the 6.8 year follow‐up 5.2% [total n = 563; (HT: n = 262; placebo: n = 301)] women withdrew [n = 321 (HT: n = 136; placebo: n = 185)] were considered lost to follow‐up [n = 142 (HT: n = 126; placebo: n = 116)], or stopped providing outcome data for more than 18‐months. Vital status at the end of the trial was therefore known for 10,176 (94.8%) of randomised participants, including 580 (5.4%) known to be deceased |
|
| Outcomes |
Primary outcomes: CHD (defined as acute MI requiring overnight hospitalisation, silent MI, or CHD death) Death from CVD Non‐fatal MI (defined as acute MI requiring overnight hospitalisation, silent MI) Secondary outcomes: Death from any cause Stroke (fatal and non‐fatal combined) Angina (confirmed) Revascularisation (CABG or PCI combined) Pulmonary embolisms Venous thrombosis (pulmonary embolism plus DVT combined) HRQoL not included in the analyses; length of follow‐up: 7.1 years |
|
| Notes | The sample size calculation was adequate so the trial was powered to detect differences between the HT and placebo groups in terms of CVD events. All outcomes were prespecified and defined a prior, and reported in the trial results. All study personnel, except the study gynaecologist were ‘blinded’. The gynaecologist was ‘unblinded’ if necessary to treatment group, but separate from the rest of the trial team, and therefore ‘blinding’ is likely to have been maintained. Participant attrition rates were low at 5.2%. However, medication compliance rates were low, with only 46.2% of women still medication compliant at 6.8 years follow‐up. This is likely to have ‘diluted’ the true effects, both positive and negative, of oestrogens relative to placebo relative to what might be observed with full medication adherence. Additionally, the trial was stopped early which would have further decreased the power to detect differences between the two trial arms, and reduced the precision of the estimated effects for the outcomes assessed. Events for this trial were reported stratified according to the time since menopause that treatment was started. This allowed accurate allocation of events, specifically: death, coronary heart disease (death from cardiovascular causes and non‐fatal myocardial infarction), stroke and venous thromboembolism, to subgroup analysis according to whether treatment was started < 10 years or > 10 years after the menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally randomised by permuted block algorithm |
| Allocation concealment (selection bias) | Low risk | By local access to remote study database |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and clinic staff blinded, with the exception of 331 participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 563 participants (5.2%) withdrew, were lost to follow‐up, or stopped providing outcome information for more than 18 months Analysis conducted on ITT basis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent source of other bias |
WHISP 2006.
| Methods | Multicentre, randomised, controlled, pilot study involving 17 centres in UK. The trial was run from October 1999 to October 2002 and was designed to test effect of oral oestradiol‐17β 1 mg plus norethisterone acetate 0.5 mg daily, or matching placebo, on lipids, lipoproteins and haemostasis markers in post‐menopausal women who had recently had an acute coronary syndrome [ACS] Recruitment: Not reported Screening: Not reported Randomisation: Randomised by telephone call to the co‐ordinating centre Stratification: By centre Allocation: Blinded treatment pack allocated to participant Baseline equality of treatment groups: No substantive differences between treatment groups Blinding: Staff and participants were blinded Analysis: Primary outcome of lipid level analysed according to last recorded level carried through for participants who were lost to follow‐up. For the outcomes of this review reported as adverse events these were analysed in an ITT manner Funding Source: UK MRC and Novo Nordisk |
|
| Participants | 100 postmenopausal women > 55 years who were enrolled 2‐28 days post‐ACS The mean age of participants was: 69.4 years in the HT group (SD 8.6), 68.3 years in the placebo group (SD 9.0) Years since menopause: 21.6 years in the HT group (interquartile range 15.8to 29.9) [HT], 23.9 years in the placebo group(interquartile range 13.8 to 30.5) CVS Risk Factors BMI: 26.0 (SD 3.9) [HT], 26.4 (SD 4.7) [placebo] Smoking (current): 17 (34.7%) [HT], 13 (25.5%) [placebo] Smoking (ex): 14 (28.6%) [HT], 19 (37.2%) [placebo] Smoking (never): 18 (36.7%) [HT], 19 (37.2%) [placebo] Diabetes: 37 (75.5%) [HT], 40 (78.4%) [placebo] Inclusion criteria:
elevated cardiac enzymes (CK or AST twice upper limit or CKMB or troponin above the threshold considered diagnostic for myocardial damage in that centre), changes on the electrocardiogram (ECG) supportive of a diagnosis of acute myocardial ischaemia, prior history of CHD documented by history of prior MI or prior revascularisation or angiography showing > 50% stenosis in at least one major epicardial coronary artery
Exclusion criteria:
|
|
| Interventions |
HT regimen: continuous combined oral oestradiol‐17β 1 mg plus norethisterone acetate 0.5 mg daily Comparator: placebo |
|
| Outcomes |
Primary outcomes: lipid profile, markers of coagulation and fibrinolysis Secondary outcomes: feasibility of enrolment and safety and tolerability of HRT |
|
| Notes | Methods for verifying medication compliance: checked every three months in clinic, not specified how. Follow‐up duration was planned for 12 months, but was reported to be a median of seven months. Events were not stratified according to time since menopause. However the median time since menopause that treatment was started was reported for each group, 23.9 years (interquartile range 13.8 to 30.5) for the control group and 21.6 years (interquartile range 15.8 to 29.9) for the hormone group. For the subgroup analysis of when treatment was started, the entirety of events in this study were analysed as if all participants had started treatment more than 10 years after their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Telephone call to co‐ordinating centre and stratified randomisation (blocks of four) |
| Allocation concealment (selection bias) | Low risk | Telephone call to separate centre |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States double‐blind and placebo‐controlled. A blinded treatment pack was allocated to patients. Not stated if personnel assessing outcome were blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Staff were blinded. Not stated if personnel assessing outcome were blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant difference between numbers of participants lost to follow‐up between different treatment groups, 13 in the treatment group compared to 6 in the control group, the reasons are not stated. Intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | Low risk | No prior published design paper. All outcomes specified in current paper were reported |
| Other bias | Unclear risk | Death and cardiovascular outcomes were not prespecified as outcomes for this study but were reported afterwards as adverse events |
WISDOM 2007.
| Methods |
Objective: To assess the long‐term benefits and risks of HT Multicentre three‐armed randomised controlled (RCT) trial involving 499 general practices; (n = 385 UK); (n = 91 Australia) and (n = 24) New Zealand. The trial was conducted between 1999 ‐ 2002, with an intended follow‐up period of ten‐years. The trial was halted early after the publication of the results from WHI I 2002, which showed no statistically significant benefit for treatment with HT compared with placebo. The median follow‐up time was 11.9 months (interquartile range: 7.1 to 19.6) for the entire trial participants and 12.8 months (range: 7.5 to 20.4 ) months for participants randomised to combination therapy. The trial was composed of three different strata: Strata 1: Women with an intact uterus or sub‐total hysterectomy not taking HT randomised to combined oestrogen and progesterone therapy or placebo Strata 2: Hysterectomised women taking HT and randomised to oestrogen only HT or combined HT Stratum 3: Hysterectomised women not taking HT randomised to oestrogen only HT or combined HT or placebo The design therefore allowed for two main comparisons to be made: (1) combined oestrogen and progestogen therapy versus placebo, and in women who had a hysterectomy, (2) oestrogen alone versus combination oestrogen and progestogen therapy Only the baseline demographic data and results from strata 1 are reported within this report, as this was the only comparison of HT versus placebo within the trial Recruitment: Practice registries Screening: 14,203 screened for eligibility All women took placebo medication during run in: those who achieved 80% compliance were randomised Randomisation: Remote computer‐generated Stratification: By hysterectomy status and intended use of HT: women with no uterus and unwilling to take placebo randomised to CEE or combined HT. Equal probability of any treatment within each stratum Allocation: Remote computer‐generated Baseline equality of treatment groups: No substantive differences at baseline Blinding: All participants, clinic staff, and outcome assessors blinded except when vaginal bleeding triggered a code break Analysis: ITT Funding Source: Non‐commercial medical research funding |
|
| Participants | 4385 healthy women in strata one (out of a total of 5692 women randomised) were randomised to either combined HT or placebo The mean age of the women was 63.3 years (SD: 4.7), with a mean of 14.7 years (SD: 7.1) since menopause. Post‐menopausal status was defined as the presence of no menses in the past 12 months or having undergone a hysterectomy. Women taking HT at baseline screening, who were prepared to enter the placebo controlled strata of the study, ceased therapy for three months before the run‐in phase. During run‐in they took placebo so that at randomisation they had not taken HT for six months At baseline, 9% of women were taking oestrogen, and therefore underwent a three‐month ‘wash out ‘period prior to randomisation. 2% of the included women were of non‐white ethnic status; 18% were using HT at screening and 86% had previously used HT. In terms of risk factors for CVD: mean BMI was 28.0 kg/m2; mean systolic blood pressure was 136.5 mm Hg and mean diastolic blood pressure 73 mm Hg; 24% were current smokers; 55% were former smokers; 10% had previous angina; 3% had a previous MI; 3% had a previous stroke and 7% had diabetes. Inclusion criteria: Stated above, but only women who were 80% or more medication compliant in the run‐in period were eligible for participation in the trial Exclusion criteria:
|
|
| Interventions |
HT regimen: 0.625 mg conjugated equine oestrogen in combination with 2.5 mg medroxyprogesterone acetate (MPA) daily (continuous dosage regimen) Comparator: placebo tablet daily Participants were classified as medication compliant if they took ≥ 80% of their medication throughout the trial. Trial treatment delivered 73% of time to women in combined HT arm and 86% of time to women on placebo Follow‐up times: four, 14, 27, 40 and 52 weeks and then at six‐month intervals. At baseline recent cervical screening and mammography were checked and then at each follow‐up visit information was collected on all outcomes (none of the outcomes were defined), adverse events and patients other medical history Losses to follow‐up: five Dropouts: 615 (14%) had withdrawn from randomised treatment by trial closure |
|
| Outcomes | Primary outcomes: Death from CVD Angina Non‐fatal MI Fatal MI Secondary outcomes: Pulmonary embolism Venous thromboembolism Health‐related quality of life |
|
| Notes | Powered in protocol to detect 25% reduction in CHD over ten years. This assumed an 18,000 sample size but trial stopped early with 26% of target
A further 1307 women were in comparison of combined therapy vs oestrogen only and not included in this review. Events were not stratified according to time since menopause. However the mean time since menopause that treatment was started was reported for each group, 14.7 years (SD 7.1) for the control group and 14.8 years (SD 7.2) for the hormone group. For the subgroup analysis of when treatment was started, the entirety of events in this study were analysed as if all participants had started treatment more than 10 years after their menopause |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Remote computer‐generated |
| Allocation concealment (selection bias) | Low risk | Remote computer‐generated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and clinic staff blinded except when vaginal bleeding triggered a code break |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcome assessors blinded except when vaginal bleeding triggered a code break |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 615 (14%) had withdrawn from randomised treatment by trial closure. Analysed by ITT |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent source of other bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aitken 1973 | No outcomes relevant to this review |
| Aloia 1995 | No outcomes relevant to this review |
| Angerer 2001 | No outcomes relevant to this review |
| Angerer 2002 | No outcomes relevant to this review |
| Bui Minh 2002 | Insufficient follow‐up duration and no relevant outcomes |
| CHART 2006 | No outcomes relevant to this review |
| Christensen 1982 | No outcomes relevant to this review |
| Christiansen 1981 | No outcomes relevant to this review |
| Christiansen 1984 | No outcomes relevant to this review |
| Christiansen 1990 | No control group |
| Clarke 2002 | Wrong intervention. Transdermal patches used |
| Coope 1981 | Study population not post‐menopausal and not generalisable population as majority recruited suffered from depression |
| Davidson 1997 | Abstract only; did not report relevant clinical outcomes |
| Davidson 2000 | No outcomes relevant to this review |
| Enderle 1999 | No outcomes relevant to this review and insufficient follow‐up duration |
| Gallagher 1991 | No outcomes relevant to this review |
| Genant 1990 | No outcomes relevant to this review |
| Genant 1997 | No outcomes relevant to this review |
| Grady 1997 | Letter commenting on historical studies, no new data |
| Hart 1984 | No outcomes relevant to this review |
| Hassager 1987 | No outcomes relevant to this review |
| Heikkinen 1997 | No outcomes relevant to this review |
| Herrington 1996 | Review article, no new data |
| HERS II | This trial was the long‐term open label follow‐up phase of HERS I 1998, and therefore not included as a separate trial, as done in the original review |
| Holmberg 2004 | Wrong patient population: trial is of women with breast cancer |
| HOPE 2002 | No outcomes relevant to this review |
| Hsia 2003 | No outcomes relevant to this review |
| Hsia 2004 | WHI: combination trial with 5.6 year follow‐up; reports peripheral arterial disease outcomes only. No relevant clinical outcomes |
| Huang 2009 | No clinically relevant data reported. Reports number of hot flushes by year and treatment group in the HERS 1 trial |
| Itoi 2000 | No outcomes relevant to this review and no control group |
| Jensen 1989 | No outcomes relevant to this review |
| Karim 2008 | No outcomes relevant to this review |
| Kim 1996 | No outcomes relevant to this review |
| Komulainen 1999 | No clear placebo comparison |
| Lamon‐Fava 2009 | No outcomes relevant to this review |
| Lamon‐Fava 2010 | No outcomes relevant to this review |
| Leggate 1984 | No outcomes relevant to this review |
| Lindsay 1984 | No outcomes relevant to this review |
| Madsen 2003 | No outcomes relevant to this review |
| Marsden 2002 | Abstract for a trial to be undertaken |
| Molander 1990 | Length of follow‐up insufficient |
| Moriyama 2008 | Wrong comparison. HT compared with either being physically active or sedentary, and being active or sedentary compared to placebo |
| Mosca 2009 | Wrong intervention (Raloxifene) and outside the scope of the review |
| Nair 2005 | No relevant clinical outcomes reported. Examination of relationship between baseline brachial pulse pressure and CV outcomes in HERS I |
| Neuhouser 2009 | Does not report results for HT or placebo users separately from all women randomised to take vitamins |
| Nikolov 1999 | Review article no new data |
| Nordin 1980 | Wrong population (included pre‐menopausal women), insufficient duration of treatment and non‐relevant outcomes |
| Obel 1993 | No outcomes relevant to this review |
| OPAL 2006 | No outcomes relevant to this review |
| Pagliaro 1999 | Wrong intervention. Transdermal patches used |
| PEPI 1995 | No outcomes relevant to this review |
| Pinkerton 2009 | No outcomes relevant to this review |
| Post 2003 | No outcomes relevant to this review |
| Prentice 2008 | WHI: outcomes for breast cancer for both the oestrogen alone and combination trials. No relevant outcomes reported |
| Prentice 2009 | No relevant clinical outcomes reported. WHI: outcomes for breast cancer for both the oestrogen alone and combination trials |
| Riggs 1982 | No outcomes relevant to this review |
| SMART‐5 2012 | No outcomes relevant to this review |
| Soma 1991 | No outcomes relevant to this review and not a randomised control trial |
| Steiner 2007 | No outcomes relevant to this review |
| Stevenson 2005 | No outcomes relevant to this review |
| Svendsen 1992 | No outcomes relevant to this review |
| Toh 2010 | Effect of HT in WHI compared with The Nurses Health Study. The outcome is not clear and defined, just stated as CHD risk |
| Tuppurainen 1995 | No outcomes relevant to this review |
| Ulloa 2000 | No relevant clinical outcomes and insufficient follow‐up duration |
| Walter 1977 | No outcomes relevant to this review |
| WHIMS 2009 | No outcomes relevant to this review |
| Wimalawansa 1995 | Percutaneous HRT, no oral HRT intervention |
| Yeboah 2008 | No outcomes relevant to this review |
| Ylikorkala 1999 | No outcomes relevant to this review and no control group |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00154180.
| Methods | Randomised placebo controlled trial |
| Participants | Recently menopausal healthy women (within 36 months of menses) |
| Interventions | 0.45 mg oral oestrogen weekly with 200 mg cyclic oral, micronised progesterone for 12 days each month |
| Outcomes | Rate of change of carotid intimal medial thickness by ultrasound; Cognitive and Affective scores; health related quality of life |
| Notes | Sponsor: Kronos Longevity Research Institute |
Characteristics of ongoing studies [ordered by study ID]
NCT00114517.
| Trial name or title | Early versus Late Intervention Trial with Estradiol (ELITE) |
| Methods | Randomised placebo controlled trial |
| Participants | Post‐menopausal healthy women |
| Interventions | 17β‐oestradiol versus placebo |
| Outcomes | Atherosclerotic progression and cognition |
| Starting date | 2005 to 2013 |
| Contact information | Principal Investigator: Howard N. Hodis, MD; University of Southern California, Atherosclerosis Research Unit, Division of Cardiovascular Medicine, Department of Medicine |
| Notes | Additional information: USC Atherosclerosis Research Unit ELITE Trial |
Differences between protocol and review
In this updated review the outcomes of coronary artery bypass graft (CABG) and angioplasty were combined into a single outcome measure "revascularisation". This treatment options are closely related and their combination gives increased power to assessing the effect of HT upon them.
Health‐related quality of life (HRQoL) was excluded from outcomes to focus on cardiovascular endpoints and relevant subgroup analysis on time of commencing of HT in relation to the menopause.
We have presented subgroup analysis results based on the timing hypothesis in this version of the review.
Contributions of authors
Anne Eisinga developed and ran the search strategies. Study selection, quality assessment and data extraction were performed by Henry Boardman and Louise Hartley. Caroline Main calculated the absolute risk increases/decreases and the numbers needed to treat/harm. Henry Boardman wrote the first draft of the review, and all co‐authors commented on this and contributed to the final draft of the review. All authors have approved the manuscript.
Sources of support
Internal sources
-
UK Cochrane Centre, UK.
Cochrane Fellowship Grant
External sources
-
NIHR, UK.
Cochrane Programme Grant
Declarations of interest
No potential conflicts of interest.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
DOPS 2012 {published data only}
- Bladbjerg E, Madsen J, Kristensen S, Abrahamsen B, Brixen K, Mosekilde L, et al. Effect of long‐term hormone replacement therapy on tissue factor pathway inhibitor and thrombin activatable fibrinolysis inhibitor in healthy postmenopausal women: a randomized controlled study. Journal of Thrombosis and Haemostasis 2003;1(6):1208‐14. [DOI] [PubMed] [Google Scholar]
- Kristensen S, Abrahamsen B, Madsen J, Gram J, Rejnmark L, Rud B, et al. Venous thrombosis is not increased in younger women on genuine oestrogen postmenopausal hormonal replacement therapy: results from the Danish Osteoporosis Prevention Study (DOPS). Thrombosis and Haemostasis 2006;95:915‐6. [PubMed] [Google Scholar]
- Madsen J, Kristensen S, Gram J, Bladbjerg E, Henriksen F, Christensen K, et al. Positive impact of hormone replacement therapy on the fibrinolytic system: a long‐term randomized controlled study in healthy postmenopausal women. Journal of Thrombosis and Haemostasis 2003;1(9):1984‐91. [DOI] [PubMed] [Google Scholar]
- Schierbeck L, Rejnmark L, Tofteng C, Stilgren L, Eiken P, Mosekilde L, et al. Hormone replacement treatment in early postmenopausal women reduces cardiovascular events ‐ A randomized controlled study. Circulation 2011;124:A11380. [Google Scholar]
- Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012;345:e6409. [DOI] [PubMed] [Google Scholar]
- Tofteng C, Jensen J, Abrahamsen B, Odum L, Brot C. Two polymorphisms in the vitamin D receptor gene‐association with bone mass and 5‐year change in bone mass with or without hormone‐replacement therapy in postmenopausal women: the Danish Osteoporosis Prevention Study. Journal of Bone and Mineral Research 2002;17(8):1535‐44. [DOI] [PubMed] [Google Scholar]
- Maat M, Madsen J, Langdal B, Bladbjerg E, Tofteng C, Abrahamsen L, et al. Genetic variation in estrogen receptor, C‐reactive protein and fibrinogen does not predict the plasma levels of inflammation markers after longterm hormone replacement therapy. Thrombosis and Haemostasis 2007;97(2):234‐9. [PubMed] [Google Scholar]
EAGAR 2006 {published data only}
- Ouyang P, Tardif J, Herrington D, Stewart K, Thompson P, Walsh M, et al. Randomized trial of hormone therapy in women after coronary bypass surgery: Evidence of differential effect of hormone therapy on angiographic progression of disease in saphenous vein grafts and native coronary arteries. Atherosclerosis 2006;189(2):375‐86. [DOI] [PubMed] [Google Scholar]
EPAT 2001 {published data only}
- Hodis H, Mack W, Lobo R, Mahrer P, Liu C R, Liu C H. Estrogen replacement therapy effects on the progression of subclinical atherosclerosis are mediated by lipids but not by carbohydrate metabolic factors: results from the Estrogen in the Prevention of Athersclerosis Trial (EPAT). Circulation 2002;106(19 Suppl II):II‐744. [Google Scholar]
- Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. Estrogen in the prevention of atherosclerosis. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 2001;135(11):939‐53. [DOI] [PubMed] [Google Scholar]
EPHT 2006 {published data only}
- Hemminki E, Hovi S, Veerus P, Sevón T, Tuimala R, Rahu M, et al. Blinding decreased recruitment in a prevention trial of postmenopausal women. Journal of Clinical Epidemiology 2004;57:1237‐43. [DOI] [PubMed] [Google Scholar]
- Veerus P, Fischer K, Hovi S‐L, Karro H, Rahu M, Hemminki E. Symptom reporting and quality of life in the Estonian Postmenopausal Hormone Therapy Trial. BMC Women’s Health 2008;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerus P, Hovi S, Fischer K, Rahu M, Hakama M, Hemminki E. Results for the Estonian postmenopausal hormone therapy trial [ISRCTN35338757]. Maturitas 2006;55(2):162‐73. [DOI] [PubMed] [Google Scholar]
ERA 2000 {published data only}
- Herrington DM, Reboussin DM, Bridget Brosnihan K, Sharp PC, Shumaker SA, Snyder TE, et al. Effects of estrogen replacement on the progression of coronary‐artery atherosclerosis. New England Journal of Medicine 2000;343(8):522‐9. [DOI] [PubMed] [Google Scholar]
- Herrington DM, Reboussin DM, Klein KP, Sharp PC, Shumaker SA, Snyder TE, et al. The estrogen replacement and atherosclerosis (ERA) study: study design and baseline characteristics of the cohort. Controlled Clinical Trials 2000;21(3):257‐85. [DOI] [PubMed] [Google Scholar]
- Nair G, Herrington D. The ERA trial: findings and implications for the future. Climacteric 2000;3:227‐32. [DOI] [PubMed] [Google Scholar]
ERT II 1979 {published data only}
- Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM. Estrogen replacement therapy II: A prospective study in the relationship to carcinoma and cardiovascular and metabolic problems. Obstetrics Gynecology 1979;54(1):74‐9. [DOI] [PubMed] [Google Scholar]
ESPRIT 2002 {published data only}
- The ESPRIT Team. Oestrogen therapy for prevention of reinfarction in postmenopausal women: a randomised placebo controlled trial. Lancet 2002;360:2001‐8. [DOI] [PubMed] [Google Scholar]
EVTET 2000 {published data only}
- Hoibraaten E, Arnesen H, Larsen S, Wickstrom E, Sandset PM. Increased risk of recurrent venous thromboembolism during hormone replacement therapy: results of the randomized, double blind, placebo‐controlled estrogen in venous thromboembolism trial (EVTET). Thrombosis and Haemostasis 2000;84:961‐7. [PubMed] [Google Scholar]
- Qvigtad, Hoibraaten H, Arnesen H, Larsen S, Wickstrom E, Sandset P. Recurrent venous thromboembolism during hormone replacement therapy ‐ results of the estrogen in venous thromboembolism trial. XVI FIGO World Congress of O and G 2000;Abstract book 1:58. [PubMed] [Google Scholar]
Greenspan 2005 {published data only}
- Greenspan SL, Resnick NM, Parker RA. The effect of hormone replacement on physical performance in community‐dwelling elderly women. The American Journal of Medicine 2005;118:1232‐9. [DOI] [PubMed] [Google Scholar]
HALL 1998 {published data only}
- Hall G, Pripp U, Schenk‐Gustafsson K, Landgren BM. Long‐term effects of hormone replacement therapy on symptoms of angina pectoris, quality of life and compliance in women with coronary artery disease. Maturitas 1998 ;28 :235‐42 . [DOI] [PubMed] [Google Scholar]
HERS I 1998 {published data only}
- Bibbins‐Domingo K, Lin F, Vittinghoff E, Barrett‐Connor E, Hulley S, Grady D, et al. Effect of hormone therapy on mortality rates among women with heart failure and coronary artery disease. American Journal of Cardiology 2005;95(2):289‐91. [DOI] [PubMed] [Google Scholar]
- Giddings L. Oestrogen plus progestin increased venous thromboembolic disease in postmenopausal women with coronary artery disease. Evidence Based Nursing 2000;3(4):123. [Google Scholar]
- Grady D, Applegate W, Bush T, Furberg C, Riggs B, Hulley S, et al. Heart and Estrogen/progestin Replacement Study (HERS): design, methods, and baseline characteristics. Controlled Clinical Trials 1998;19(4):314‐35. [DOI] [PubMed] [Google Scholar]
- Grady D, Herrington D, Bittner V, Davidson M, Hlatky M, Hsia J, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy. Heart and Estrogen/Progestin Replacement Study Follow‐up (HERS II). JAMA 2002;288(1):49‐57. [DOI] [PubMed] [Google Scholar]
- Grady D, Wenger N, Herrington D, Khan S, Furberg C, Hunninghake, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. Annals of Internal Medicine 2000;132:689‐96. [DOI] [PubMed] [Google Scholar]
- Hlatky M, Boothroyd D, Vittinghoff E, Sharp P, Whooley MA. Quality‐of life and depressive symptoms in postmenopausal women after receiving hormone therapy. Results from the Heart and Estrogen/Progestin Replacement Study (HERS) Trial. JAMA 2002;287(5):591‐7. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 1998;280(7):605‐13. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women Heart and Estrogen/progestin Replacement Study (HERS) Research Group. Obstetrical and Gynecological Survey 1999;54(1):41‐2. [DOI] [PubMed] [Google Scholar]
- Khan M, Hlatky M, Liu M, Lin F, Rogers W, Shlipak M. Effect of postmenopausal hormone therapy on coronary heart disease events after percutaneous transluminal coronary angioplasty. The American Journal of Cardiology 2003;91(8):989‐91, A7. [DOI] [PubMed] [Google Scholar]
- Shlipak M, Simon J, Vittinghoff E, Lin F, Barrett‐Connor E, Knopp R. Estrogen and progestin, lipoprotein(a), and the risk of recurrent coronary heart disease events after menopause. JAMA 2000;283(14):1845‐52. [DOI] [PubMed] [Google Scholar]
- Simon JA, Hsia J, Cauley JA, Richards C, Harris F, Fong J, et al. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen‐progestin Replacement Study (HERS). Circulation 2001;103(5):638‐42. [DOI] [PubMed] [Google Scholar]
- Simon JA, Lin F, Vittinghoff E, Bittner V, Heart and Estrogen‐Progestin Replacement Study (HERS) Research Group. The relation of postmenopausal hormone therapy to serum uric acid and the risk of coronary heart disease events: The heart and estrogen‐progestin replacement study (HERS). Annals of Epidemiology 2006;16(2):138‐45. [DOI] [PubMed] [Google Scholar]
- Speroff L. The heart and estrogen/progestin replacement study (HERS). Maturitas 1998;31(1):9‐14. [DOI] [PubMed] [Google Scholar]
STOP IT 2001 {published data only}
- Gallagher J, Rapuri B, Haynatzki G, Detter R. Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. The Journal of Clinical Endocrinology and Metabolism 2002;87(11):4914‐23. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age‐related bone loss. The Journal of Clinical Endocrinology and Metabolism 2001;86(8):3618‐28. [DOI] [PubMed] [Google Scholar]
- Rapuri P, Gallagher J, Knezetic J, Haynatzka V. Estrogen receptor alpha gene polymorphisms are associated with changes in bone remodeling markers and treatment response to estrogen. Maturitas 2006;53(4):371‐9. [DOI] [PubMed] [Google Scholar]
- Sai A, Gallagher J, Fang X. Effect of hormone therapy and calcitriol on serum lipid profile in postmenopausal older women: association with estrogen receptor‐alpha genotypes. Menopause 2011;18(10):1101‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalamanchili V, Gallagher J. Treatment with hormone therapy and calcitriol did not affect depression in older postmenopausal women: no interaction with estrogen and vitamin D receptor genotype polymorphisms. Menopause 2012;19(6):697‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
WAVE 2002 {published data only}
- Hsia J, Alderman EL, Verter JI, Rogers WJ, Thompson P, Howard BV, et al. Women’s angiographic vitamin and estrogen trial: design and methods. Controlled Clinical Trials 2002;23:708‐27. [DOI] [PubMed] [Google Scholar]
- Waters D, Alderman E, Hsia J, Howard B, Cobb F, Rogers W, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women. JAMA 2001;288(19):2432‐40. [DOI] [PubMed] [Google Scholar]
WELL‐HART 2003 {published data only}
- Hodis HN, Mack WJ, Azen SP, Lobo RA, Shoupe D, Mahrer PR, et al. Hormone therapy and the progression of coronary‐artery atherosclerosis in postmenopausal women. New England Journal of Medicine 2003;349(6):535‐45. [DOI] [PubMed] [Google Scholar]
WEST 2001 {published data only}
- Kernan WN, Brass LM, Viscoli CM, Sarrell PM, Makuch R, Horwitz RI. Estrogen after ischemic stroke: clinical basis and design of the Womens Estrogen for Stroke Trial. Journal of Stroke and Cerebrovascular Diseases 1998;7(1):85‐95. [DOI] [PubMed] [Google Scholar]
- Viscoli C, Brass L, Kernan, Sarrel P, Suissa S, Horwitz R. Estrogen therapy and risk of cognitive decline: Results from the Women's Estrogen for Stroke Trial (WEST). American Journal of Obstetrics and Gynecology 2005;192(2):387‐93. [DOI] [PubMed] [Google Scholar]
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen‐replacement therapy after ischemic stroke. New England Journal of Medicine 2001;345(17):1243‐9. [DOI] [PubMed] [Google Scholar]
WHI I 2002 {published data only}
- Anderson GI, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the womens health initiative study design. Annals of Epidemiology 2003;13:S5‐17. [DOI] [PubMed] [Google Scholar]
- Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292(13):1573‐80. [DOI] [PubMed] [Google Scholar]
- Hays J, Hunt JR, Hubbell, FA, Anderson GL, Limacher M, Allen C, et al. The Women's Health Initiative recruitment methods and results. Annals of Epidemiology 2003;13(Suppl 9):S18‐77. [DOI] [PubMed] [Google Scholar]
- Hays J, Ockene JK, Brunner RL, Kotchen JM, Manson JE, Patterson RE, et al. Effects of estrogen plus progestin on health‐related quality of life. New England Journal of Medicine 2003;348(104):1839‐54. [DOI] [PubMed] [Google Scholar]
- Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SAA, Brzyski R, et al. Health risks and benefit 3 years after stopping randomized treatment with estrogen and progestin. JAMA 2008;299(9):1036–45. [DOI] [PubMed] [Google Scholar]
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf A, Lasser N, et al. Women's Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. New England Journal of Medicine 2003;349(6):523‐34. [DOI] [PubMed] [Google Scholar]
- Rossouw J, Anderson G, Prentice R, LaCroix A, Kooperberg C, Stefanick M. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women's health initiative randomized controlled trial. JAMA 2002;288(3):321‐33. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007;297(13):1465–77. [DOI] [PubMed] [Google Scholar]
- Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR, et al. The Women's Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Annals of Epidemiology 2003;13(Suppl 9):S78‐86. [DOI] [PubMed] [Google Scholar]
- The Womens Health Initiative Study Group. Design of the Womens Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Controlled Clinical Trials 1998;19(1):61‐109. [DOI] [PubMed] [Google Scholar]
- Wassertheil‐Smoller S, Hendrix S, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women. JAMA 2003;289(20):2673‐84. [DOI] [PubMed] [Google Scholar]
- Writing Group for the Women's Health Initiative Investigators. Risk and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the women's health initiative randomized controlled trial. JAMA 2002;288(3):321‐33. [DOI] [PubMed] [Google Scholar]
WHI II 2004 {published data only}
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Women's Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 2004;291(14):1701‐12. [DOI] [PubMed] [Google Scholar]
- Brunner RL, Gass M, Aragaki A, Hays J, Granek I, Woods N, et al. Women's Health Initiative Investigators. Effects of conjugated equine estrogen on health‐related quality of life in postmenopausal women with hysterectomy. Archives of Internal Medicine 2005;165(17):1976‐86. [DOI] [PubMed] [Google Scholar]
- Curb JD, Prentice RL, Bray PF, Langer RD, Horn K, Barnabei VM, et al. Venous thrombosis and conjugated equine estrogen in women without a uterus. Archives of Internal Medicine 2006;166(7):772‐80. [DOI] [PubMed] [Google Scholar]
- Hendrix SL, Wassertheil‐Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, et al. WHI Investigators. Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation 2006;113(20):2425‐34. [DOI] [PubMed] [Google Scholar]
- Hsia J, Langer R, Manson J, Kuller L, Johnson K, Hendrix S, et al. Women's Health Initiative Investigators. Conjugated equine estrogens and coronary heart disease: the Women's Health Initiative. Archives of Internal Medicine 2006;166(3):357‐65. [DOI] [PubMed] [Google Scholar]
- Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, et al. Women's Health Initiative Investigators. Conjugated equine estrogens and coronary heart disease. Archives of Internal Medicine 2006;291:1701‐12. [DOI] [PubMed] [Google Scholar]
WHISP 2006 {published data only}
- Collins P, Flather M, Lees B, Mister R, Proudler A, Stevenson JC, WHISP (Women's Hormone Intervention Secondary Prevention Study) Pilot Study Investigators. Randomized trial of effects of continuous combined HRT on markers of lipids and coagulation in women with acute coronary syndromes: WHISP Pilot Study. European Heart Journal 2006;27(17):2046‐53. [DOI] [PubMed] [Google Scholar]
WISDOM 2007 {published data only}
- Vickers MR, MacLacLennan A, Lawton B, Ford D, Martin J, Meredith S, et al. WISDOM group. Main morbidities recorded in the women's international study of long duration estrogen after menopause (WISDOM): a randomised controlled trial of hormone replacement therapy in postmenopausal women. BMJ 2007;335(7613):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers MR, Martin J, Meade TW, WISDOM study team. The Women’s international study of long‐duration of estrogen after menopause (WISDOM): a randomised controlled trial. BMC Women's Health 2007;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton AJ, Vickers MR, Kim J, Ford D, Lawton BA, MacLennan AH, et al. WISDOM team. Health related quality of life after combined hormone replacement therapy: randomised controlled trial. BMJ 2008;337:a1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aitken 1973 {published data only}
- Aitken JM, Hart DM, Lindsay R. Oestrogen replacement therapy for prevention of osteoporosis after oophorectomy. British Medical Journal 1973;3:515‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aloia 1995 {published data only}
- Aloia JF, Vaswani A, Russo L, Sheehan M, Flaster E. The influence of menopause and hormonal replacement therapy on body cell mass and body fat mass. American Journal of Obstetrics and Gynecology 1995;172(3):896‐900. [DOI] [PubMed] [Google Scholar]
Angerer 2001 {published data only}
- Angerer P, Störk S, Kothny W, Scmitt P, Schacky C. Effect of oral postmenopausal hormone replacement on progression of atherosclerosis. Arteriosclerosis, Thrombosis and Vascular Biology 2001;21:262‐8. [DOI] [PubMed] [Google Scholar]
Angerer 2002 {published data only}
- Angerer P, Stork S, Kothny W, Schacky C. Effect of postmenopausal hormone replacement on atherosclerosis in femoral arteries. Maturitas 2002;41(1):51‐60. [DOI] [PubMed] [Google Scholar]
Bui Minh 2002 {published data only}
- Bui Minh N, Arai A, Hathaway L, Waclawiw M, Csako G, Cannon R. Effect of hormone replacement therapy on carotid arterial compliance in healthy postmenopausal women. The American Journal of Cardiology 2002;90(1):82‐5. [DOI] [PubMed] [Google Scholar]
CHART 2006 {published data only}
- Rowan J, Simon J, Speroff L, Ellman H. Effects of low‐dose norethindrone acetate plus ethinyl estradiol (0.5 mg/2.5 mug) in women with postmenopausal symptoms: Updated analysis of three randomized, controlled trials. Clinical Therapeutics 2006;28(6):921‐32. [DOI] [PubMed] [Google Scholar]
- Speroff L, Rowan J, Symons J, Genant H, Wilborn W. The comparative effect on bone density, endometrium, and lipids of continuous hormones as replacement therapy (CHART study). A randomized controlled trial. JAMA 1996;276(17):1397‐403. [PubMed] [Google Scholar]
Christensen 1982 {published data only}
- Chrsitensen MS, Hagen C, Christiansen C, Transbøl IB. Dose‐response evaluation of cyclic estrogen/gestagen in postmenopausal women: Placebo‐controlled trial of its gynecologic and metabolic actions. Gynecology 1982;144(8):873‐9. [DOI] [PubMed] [Google Scholar]
Christiansen 1981 {published data only}
- Christiansen C, Christiansen MD, Rødbro P, Hagen C, Transbøl I. Effect of 1,25‐dihydroxy‐vitamin D3 in itself or combined with hormone treatment in preventing postmenopausal osteoporosis. European Journal of Clinical Investigation 1981;11(4):305‐9. [DOI] [PubMed] [Google Scholar]
Christiansen 1984 {published data only}
- Christiansen C, Christensen M, Grande P, Transbøl I. Low‐risk lipoprotein pattern in post‐menopausal women on sequential oestrogen/progestogen treatment. Maturitas 1984;5(3):193‐9. [DOI] [PubMed] [Google Scholar]
Christiansen 1990 {published data only}
- Christiansen C, Riis BJ. 17ß‐Estradiol and continuous norethistrone: a unique treatment for established osteoporosis in elderly women. Journal of Clinical Endocrinology and Metabolism 1990;71:836‐41. [DOI] [PubMed] [Google Scholar]
Clarke 2002 {published data only}
- Clarke SC, Kelleher J, Lloyd‐Jones H, Slack M, Schofiel PM. A study of hormone replacement therapy in postmenopausal women with ischaemic heart disease: the Papworth HRT atherosclerosis study. BJOG 2001;109(9):1056‐62. [DOI] [PubMed] [Google Scholar]
Coope 1981 {published data only}
- Coope J. Is oestrogen therapy effective in the treatment of menopausal depression?. The Menopause 1981;31:134‐40. [PMC free article] [PubMed] [Google Scholar]
Davidson 1997 {published data only}
- Davidson MH, Maki KC, Cyrwski MS, Maki AC. Rationale and design of a trial to evaluate the impact of continuous combined hormone replacement therapy on the cardiovascular disease risk profile. 11th International Symposium on Atherosclerosis; 1997 Oct; Paris. 1997.
Davidson 2000 {published data only}
- Davidson M, Maki K, Marx P, Maki A, Cyrowski M, Nanavati N, et al. Effects of continuous estrogen and estrogen‐progestin replacement regimens on cardiovascular risk markers in postmenopausal women. Archives of Internal Medicine 2000;160(21):3315‐25. [DOI] [PubMed] [Google Scholar]
Enderle 1999 {published data only}
- Enderle M, Sayer R, Balletshofer B, Meisner C, Mueck A, Haasis R, et al. Improved peripheral endothelial function in postmenopausal women with coronary artery disease after acute oral intake of 17 ß‐Estradiol Valerate. Atherosclerosis 1999;144(1):33. [DOI] [PubMed] [Google Scholar]
Gallagher 1991 {published data only}
- Gallagher JC, Kable WT, Goldgar D. Effect of progestin therapy on cortical and trabecular bone: comparison with estrogen. The American Journal of Medicine 1991;90:171‐8. [PubMed] [Google Scholar]
Genant 1990 {published data only}
- Genant HK, Baylink DJ, Gallagher JC, Harris ST, Steiger P, Herber M. Effect of estrone sulphate on postmenopausal bone loss. Obstetrics and Gynecology 1990;76(4):579‐84. [PubMed] [Google Scholar]
Genant 1997 {published data only}
- Genant HK, Lucas J, Weiss S, Akin M, Emkey R, McNaney‐Flint H, et al. Low‐dose esterified estrogen therapy. Archives of Internal Medicine 1997;157:2609‐15. [DOI] [PubMed] [Google Scholar]
Grady 1997 {published data only}
- Grady D, Hulley S, Furberg C. Venous thromboembolic events associated with hormone replacement therapy. JAMA 1997;278(6):477. [PubMed] [Google Scholar]
Hart 1984 {published data only}
- Hart D, Farish E, Fletcher C, Howie C, Kitchener H. Ten years post‐menopausal hormone replacement therapy‐effect on lipoproteins. Maturitas 1984;5(4):271‐6. [DOI] [PubMed] [Google Scholar]
Hassager 1987 {published data only}
- Hassager C, Riis BJ, Strøm V, Guyene TT, Christiansen C. The long‐term effect of oral and percutaneous estradiol on plasma renin substrate and blood pressure. Circulation 1987;76(4):753‐8. [DOI] [PubMed] [Google Scholar]
Heikkinen 1997 {published data only}
- Heikkinen A, Tuppurainen M, Niskanen L, Komulainen M, Penttila I, Saarikoski S. Long‐term vitamin D3 supplementation may have adverse effects on serum lipids during postmenopausal hormone replacement therapy. European Journal of Endocrinology 1997;137(5):495‐502. [DOI] [PubMed] [Google Scholar]
Herrington 1996 {published data only}
- Herrington D. Estrogen Replacement and Progression or Regression of Coronary Atherosclerosis. Proceedings of the 8th International Congress on the Menopause; 1996 Nov 3‐7; Sydney. 1996:1.
HERS II {published data only}
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow‐up (HERS II). JAMA 2002;288(1):49‐57. [DOI] [PubMed] [Google Scholar]
Holmberg 2004 {published data only}
- Holmberg L, Anderson H. HABITS (hormonal replacement therapy after breast cancer ‐ is it safe?): a randomised comparison: trial stopped. Lancet 2004;363(9407):453‐5. [DOI] [PubMed] [Google Scholar]
HOPE 2002 {published data only}
- Lindsay R, Gallagher J, Kleerekoper M, Pickar H. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA 2002;287(20):2668‐76. [DOI] [PubMed] [Google Scholar]
- Lobo R, Bush T, Carr B, Pickar J. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on plasma lipids and lipoproteins, coagulation factors, and carbohydrate metabolism. Fertility and Sterility 2001;76(1):13‐24. [DOI] [PubMed] [Google Scholar]
- Utian W, Shoupe D, Bachmann G, Pinkerton J, Pickar J. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertility and Sterility 2001;75(6):1065‐79. [DOI] [PubMed] [Google Scholar]
Hsia 2003 {published data only}
- Hsia J, Bittner V, Tripputi M, Howard BV. Metabolic syndrome and coronary angiographic disease progression: the Women's Angiographic Vitamin & Estrogen trial. American Heart Journal 2003;146(3):439‐45. [DOI] [PubMed] [Google Scholar]
Hsia 2004 {published data only}
- Hsia JM, Criqui MH, Rodabough RJ, Langer RD, Resnick HE, Phillips LS, et al. Estrogen plus progestin and the risk of peripheral arterial disease: the Women's Health Initiative. Circulation 2003;109(5):620‐6. [DOI] [PubMed] [Google Scholar]
Huang 2009 {published data only}
- Huang AJ, Sawaya GF, Vittinghoff E, Lin F, Grady D. Hot flushes, coronary heart disease, and hormone therapy in postmenopausal women. Menopause 2009;16(4):639‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Itoi 2000 {published data only}
- Itoi H, Minakami H, Iwasaki R, Watanabe T, Araki S, Sato I. Comparison of the long‐term effects of oral estriol with the effects of conjugated estrogen on serum lipids in postmenopausal women. Proceedings of the XVI FIGO World Congress of Gynecology and Obstetrics; 2000 Sept 3‐8; Washington 2000;Abstract book 2:149. [Google Scholar]
Jensen 1989 {published data only}
- Jensen J, Riis BJ, Strøm, Christiansen C. Long‐term and withdrawal effects of two different osetrogen‐progestogen combinations on lipid and lipoprotein profiles in post‐menopausal women.. Maturitas 1989;11:117‐28. [DOI] [PubMed] [Google Scholar]
Karim 2008 {published data only}
- Karim R, Hodis HN, Stanczyk FZ, Lobo RA, Mack WJ. Relationship between serum levels of sex hormones and progression of subclinical atherosclerosis in postmenopausal women. Journal of Clinical Endocrinology and Metabolism 2008;93(1):131‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 1996 {published data only}
- Kim C, Min Y, Ryu W, Kwak J, Ryoo U. Effect of hormone replacement therapy on lipoprotein(a) and lipid levels in postmenopausal women: Influence of various progestogens and duration of therapy. Archives of Internal Medicine 1996;156(15):1693‐1700. [PubMed] [Google Scholar]
Komulainen 1999 {published data only}
- Komulainen M, Kroger H, Tuppurainen M, Heikkinen A, Alhava E, Honkanen R. Prevention of femoral and lumbar bone loss with hormone replacement therapy and vitamin D3 in early postmenopausal women: a population‐based 5‐year randomized trial. The Journal of Clinical Endocrinology and Metabolism 1999;84(2):546‐52. [DOI] [PubMed] [Google Scholar]
Lamon‐Fava 2009 {published data only}
- Lamon‐Fava S, Herrington DSM, Reboussin DM, Sherman M, Horvath K, Schaefer EJ, et al. Changes in remnant and high‐density lipoproteins associated with hormone therapy and progression of coronary artery disease in postmenopausal women. Atherosclerosis 2009;205(1):325‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamon‐Fava 2010 {published data only}
- Lamon‐Fava S, Asztalos B, Howard T, Reboussin D, Horvath K, Schaefer E, et al. Association of polymorphisms in genes involved in lipoprotein metabolism with plasma concentrations of remnant lipoproteins and HDL subpopulations before and after hormone therapy in postmenopausal women. Clinical Endocrinology 2010;72(2):169‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leggate 1984 {published data only}
- Leggate J, Farish E, Fletcher C, McIntosh W, Hart D, Sommerville JM. Calcitonin and postmenopausal osteoporosis. Clinical Endocrinology 1984;20(1):85‐92. [DOI] [PubMed] [Google Scholar]
Lindsay 1984 {published data only}
- Lindsay R, Hart DM, Clark DM. The minimum effective dose of estrogen for prevention of postmenopausal bone loss. Journal of the American College of Obstetricians and Gynecologists 1984;63(6):759‐63. [PubMed] [Google Scholar]
Madsen 2003 {published data only}
- Madsen J, Kristensen S, Gram J, Bladbjerg E, Henriksen F, Christensen K, et al. Positive impact of hormone replacement therapy on the fibrinolytic system: a long‐term randomized controlled study in healthy postmenopausal women. Journal of Thrombosis and Haemostasis 2003;1(9):1984‐91. [DOI] [PubMed] [Google Scholar]
Marsden 2002 {published data only}
- Marsden J, Sacks N. The national randomised trial of hormone replacement therapy in women with a history of early stage breast cancer: an update. Journal of the British Menopause Society 2002;8(4):129. [DOI] [PubMed] [Google Scholar]
Molander 1990 {published data only}
- Molander U, Milsom I, Ekelund P, Mellstrom, Eriksson O. Effect of oral oestriol on vaginal flora and cytology and urogenital symptoms in the post‐menopause. Maturitas 1990;12:113‐120. [DOI] [PubMed] [Google Scholar]
Moriyama 2008 {published data only}
- Moriyama CK, Oneda B, Bernardo FR, Cardoso CG, Forjaz CL, Abrahao SB. A randomized, placebo‐controlled trial of the effects of physical exercises and estrogen therapy on health‐related quality of life in postmenopausal women. Menopause 2008;15(4):613‐8. [DOI] [PubMed] [Google Scholar]
Mosca 2009 {published data only}
- Mosca L, Grady D, Barrettt‐Connor E, Collins P, Wenger N, Amramson B, et al. Effect of raloxifene on stroke and venous thromboembolism according to subgroups in postmenopausal women at increased risk of coronary heart disease. Stroke 2009;40(1):147‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nair 2005 {published data only}
- Nair G, Waters D, Rogers W, Kowalchuk G, Stuckey T, Herrington D. Pulse pressure and coronary atherosclerosis progression in postmenopausal women. Hypertension 2005;45(1):53‐7. [DOI] [PubMed] [Google Scholar]
- Nair GV, Chaput LA, Vittinghoff E, Herrington DM, Heart and Estrogen/Progestin Replacement Study Investigators. Pulse pressure and cardiovascular events in postmenopausal women with coronary heart disease. Chest 2005;127(5):1498‐506. [DOI] [PubMed] [Google Scholar]
Neuhouser 2009 {published data only}
- Neuhouser ML, Wassertheil‐Smoller S, Thomson C, Aragaki A, Anderson GL, Manson JE, et al. Multivitamin use and risk of cancer and cardiovascular disease in the Women's Health Initiative cohorts. Archives of Internal Medicine 2009;169(3):294‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nikolov 1999 {published data only}
- Nikolov R, Golbs S, Schneider H. Estrogen replacement therapy (ERT) and hormone replacement therapy (HRT) in the prevention of cardiovascular diseases. Zentralblatt fur Gynakologie 1999;121(2):101‐4. [PubMed] [Google Scholar]
Nordin 1980 {published data only}
- Nordin B, Jones M, Crilly R, Marshall D, Brooke R. A placebo‐controlled trial of ethinyl oestradiol and norethisterone in climacteric women. Maturitas 1980;2(3):247‐51. [DOI] [PubMed] [Google Scholar]
Obel 1993 {published data only}
- Obel EB, Munk‐Jensen N, Svenstrup B, Bennett P, Micic S, Henrik‐Nielsen R, et al. A two‐year double‐blind controlled study of the clinical effect of combined and sequential postmenopausal replacement therapy and steroid metabolism during treatment. Maturitas 1993;16(1):13‐21. [DOI] [PubMed] [Google Scholar]
OPAL 2006 {published data only (unpublished sought but not used)}
- Bots M, Evans G, Riley W, McBride K, Paskett E, Helmond FA, et al. OPAL Investigators. The effect of tibolone and continuous combined conjugated equine oestrogens plus medroxyprogesterone acetate on progression of carotid intima‐media thickness: The Osteoporosis Prevention and Arterial effects of tiboLone (OPAL) study. European Heart Journal 2006;27(6):746‐55. [DOI] [PubMed] [Google Scholar]
- Meijer R, Störk S, Evans GW, Grobbee DE, Bots ML. Striking increases in carotid artery wall thickness in healthy subjects. Cerebrovascular Diseases 2010;30(5):448‐55. [DOI] [PubMed] [Google Scholar]
Pagliaro 1999 {published data only}
- Pagliaro D, Tangalakis K, Kingwell B, Craven R, Carey M, Stojanovska L. The effects of hormone replacement therapy (HRT) on performance, cardiovascular parameters and bone resorption in post menopausal 'masters' athletes. Proceedings of the 3rd Congress of the Australasian Menopause Society; 1999; Cairns. 1999.
PEPI 1995 {published data only}
- Barrett‐Connor E, Slone S, Greendale G, Kritz‐Silverstein D, Espeland M, Johnson S, et al. The Postmenopausal Estrogen/Progestin Interventions Study: primary outcomes in adherent women. Maturitas 1997;27(3):261‐74. [DOI] [PubMed] [Google Scholar]
- Espeland M, Hogan P, Fineberg S, Howard G, Schrott H, Waclawiw M, et al. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. PEPI Investigators. Postmenopausal Estrogen/Progestin Interventions. Diabetes Care 1998;21(10):1589‐95. [DOI] [PubMed] [Google Scholar]
- Espeland M, Marcovina S, Miller V, Wood P, Wasilauskas C, Sherwin R, et al. Effect of postmenopausal hormone therapy on lipoprotein(a) concentration. PEPI Investigators. Postmenopausal Estrogen/Progestin Interventions. Circulation 1998;97(10):979‐86. [DOI] [PubMed] [Google Scholar]
- Johnson S, Mebane‐Sims I, Hogan P, Soy D. Recruitment of postmenopausal women in the PEPI Trial. Postmenopausal Estrogen/Progestin Interventions. Controlled Clinical Trials 1995;16(Suppl 4):S20‐35. [DOI] [PubMed] [Google Scholar]
- Smith N, Wiley J, Legault C, Rice K, Heckbert S, Psaty B, et al. Effect of progestogen and progestogen type on hemostasis measures in postmenopausal women: the Postmenopausal Estrogen/Progestin Intervention (PEPI) Study. Menopause 2008;15(6):1145‐50. [DOI] [PubMed] [Google Scholar]
- The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. JAMA 1995;273(3):199‐208. [PubMed] [Google Scholar]
Pinkerton 2009 {published data only}
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue‐selective estrogen complex containing bazedoxifene/conjugated estrogens: A randomized, controlled trial. Menopause 2009;16(6):1116‐24. [DOI] [PubMed] [Google Scholar]
Post 2003 {published data only}
- Post M, Thomassen M, Mooren M, Baal W, Rosing J, Kenemans P, et al. Route of HRT administration and risk factors for venous thrombosis: A randomized, placebo‐controlled study in healthy postmenopausal women. International Journal of Obstetrics and Gynecology 2003;83(Suppl 3):35. [Google Scholar]
Prentice 2008 {published data only}
- Prentice R. Women's health initiative studies of postmenopausal breast cancer. Advances in Experimental Medicine and Biology 2009;617:151‐60. [DOI] [PubMed] [Google Scholar]
Prentice 2009 {published data only}
- Prentice RL, Manson JE, Langer RD, Anderson GL, Pettinger M, Jackson RD, et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. American Journal of Epidemiology 2009;170(1):2‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riggs 1982 {published data only}
- Riggs BL, Seeman E, Hodgson SF, Taves DR, O'Fallon WM. Effect of the fluoride/calcium regimen on vertebral fracture occurence in postmenopausal osteoporosis. The New England Journal of Medicine 1982;306(8):446‐50. [DOI] [PubMed] [Google Scholar]
SMART‐5 2012 {published data only}
- Gallagher JC, Lindsay R, Pan K, Ryan KA, Mirkin S, Chines AA. Bazedoxifene/conjugated estrogens: Effects on bone mineral density and bone turnover markers in a double‐blind, randomised, placebo‐ and active‐controlled phase 3 study. Osteoporosis International 2012;23(S2):S63‐S64. [Google Scholar]
- Mirkin S, Archer D, Lobo R, Pan K, Chines A. Safety and tolerability of bazedoxifene/conjugated estrogens in postmenopausal women: Findings from a 1‐year, randomized, placebo‐ and active‐controlled, phase 3 TRIAL. Maturitas 2012;71:S43‐S44. [Google Scholar]
- Mirkin S, Harvey J, Pinkerton J, Pan K, Thompson J, Chines A. The effects of bazedoxifene/conjugated estrogens on breast density in postmenopausal women. Maturitas 2012;71:S44. [Google Scholar]
- Mirkin S, Ryan K, Thompson J, Chines A. Effects of bazedoxifene/conjugated estrogens on coagulation parameters in a 1‐year, randomized, placebo‐and active‐controlled, phase 3 trial of postmenopausal women. Endocrine Reviews 2012;33:3 Meeting Abstracts. [Google Scholar]
Soma 1991 {published data only}
- Soma M, Fumagalli R, Paoletti R, Meschia M, Maini M, Crosignani P, et al. Plasma Lp(a) concentration after oestrogen and progestagen in postmenopausal women. Lancet 1991;337(8741):612. [DOI] [PubMed] [Google Scholar]
Steiner 2007 {published data only}
- Steiner A, Xiang M, Mack W, Shoupe D, Felix J, Lobo R, et al. Unopposed estradiol therapy in postmenopausal women: results from two randomized trials. Obstetrics and Gynecology 2007;109:581‐7. [DOI] [PubMed] [Google Scholar]
Stevenson 2005 {published data only}
- Stevenson J, Rioux J, Komer L, Gelfand M. 1 and 2 mg 17beta‐estradiol combined with sequential dydrogesterone have similar effects on the serum lipid profile of postmenopausal women. Climacteric 2005;8(4):352‐9. [DOI] [PubMed] [Google Scholar]
Svendsen 1992 {published data only}
- Svendsen OL, Hassager C, Marslew U, Christiansen C. Changes in calcanean bone mineral occurring spontaneously and during hormone replacement therapy in early post‐menopausal women. Scandinavian Journal of Clinical and Laboratory Investigation 1992;52:831‐836. [DOI] [PubMed] [Google Scholar]
Toh 2010 {published data only}
- Toh S, Hernandez‐Diaz S, Logan R, Rossouw JE, Hernan MA. Coronary heart disease in postmenopausal recipients of estrogen plus progestin therapy: does the increased risk ever disappear? A randomized trial. Annals of Internal Medicine 2010;152(4):I‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tuppurainen 1995 {published data only}
- Tuppurainen M, Heikkinen A, Penttila I, Saarikoski S. Does vitamin D3 have negative effects on serum levels of lipids? A follow‐up study with a sequential combination of estradiol valerate and cyproterone acetate and/or vitamin D3. Maturitas 1995;22(1):55‐61. [DOI] [PubMed] [Google Scholar]
Ulloa 2000 {published data only}
- Ulloa N, Bustos P, Arteaga E, Duran D, Castro G, Fruchart J, et al. Estrogen‐progestin replacement therapy raises Lp A‐i particles, HDL lipids and cholesterol efflux from Fu5AH cells. Atherosclerosis 2000;151(1):204. [Google Scholar]
Walter 1977 {published data only}
- Walter S, Jensen H. The effect of treatment with oestradiol and oestriol on fasting serum cholesterol and triglyceride levels in postmenopausal women. British journal of Obstetrics and Gynaecology 1977;84(11):869‐72. [DOI] [PubMed] [Google Scholar]
WHIMS 2009 {published data only}
- Casanova R, Espeland M, Goveas J, Davatzikos C, Gaussoin S, Maldjian J, et al. Application of machine learning methods to describe the effects of conjugated equine estrogens therapy on region‐specific brain volumes. Magnetic Resonance Imaging 2011;29:546‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker L, Hogan P, Bryan N, Kuller L, Margolis K, Betterman K, et al. Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS‐MRI Study. Neurology 2009;72:125‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland M, Rapp S, Shumaker S, Brunner R, Manson J, Sherwin B, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA 2004;291(24):2959‐68. [DOI] [PubMed] [Google Scholar]
- Espeland M, Tindle H, Bushnell C, Jaramillo S, Kuller L, Margolis K. Brain volumes, cognitive impairment, and conjugated equine estrogens. The Journals of Gerontology 2009;64:1243‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wimalawansa 1995 {published data only}
- Wimalawansa SJ. Combined therapy with estrogen and etidronate has an additive effect on bone mineral density in the hip and vertebrae: four‐year randomised study. The American Journal of Medicine 1995;99:36‐42. [DOI] [PubMed] [Google Scholar]
Yeboah 2008 {published data only}
- Yeboah J, Klein K, Brosniham B, Reboussin D, Herrington D. Effects of hormone therapy on soluble cell adhesion molecules in postmenopausal women with coronary artery disease. Menopause 2008 2008;15(6):1060‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ylikorkala 1999 {published data only}
- Ylikorkala O, Caubel P, Lim P. Effects of a 1‐year pulsed progestogen, constant estrogen hormone replacement therapy regimen, Prefest, on serum lipid levels in postmenopausal women. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1999;86(Suppl):S18. [Google Scholar]
References to studies awaiting assessment
NCT00154180 {published data only}
- NCT00154180. Kronos Early Estrogen Prevention Study (KEEPS). www.clinicaltrials.gov/ct2/show/NCT00154180 (accessed 15 March 2013). [NCT00154180]
- Naftolin F. The kronos early estrogen prevention study (KEEPS). Climacteric 2011;14:35‐6. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Harman SM, Black D, Brinton E, Budoff M, et al. Adverse events during four years of daily oral conjugated estrogen or transdermal estradiol plus cyclic oral progesterone treatment in recently menopausal women: The KRONOS early estrogen prevention study (KEEPS). Menopause 2012;19(12):1402‐3. [Google Scholar]
References to ongoing studies
NCT00114517 {published data only}
- Howard H. The early versus late intervention trial with estradiol (ELITE). Climacteric 2011;14:35. [Google Scholar]
- NCT00114517. ELITE: early versus late intervention trial with estradiol. www.clinicaltrials.gov/ct2/show/NCT00114517 (accessed 15 March 2013). [NCT00114517]
Additional references
Barrett‐Connor 1997
- Barrett‐Connor E. Sex differences in coronary heart disease. Why are women so superior?. Circulation 1997;95:252–64. [DOI] [PubMed] [Google Scholar]
Bath 2005
- Bath PMW, Gray LJ. Association between hormone replacement therapy and subsequent stroke: a meta‐analysis. BMJ 2005;330:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
British Heart Foundation Statistics Database 2012
- British Heart Foundation Statistics Database 2012. Number dying from CVD and CHD. www.bhf.org.uk/publications/view‐publication.aspx?ps=1002097 (accessed 14 May 2013).
Burnam 1988
- Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Medical Care 1998;26:775‐89. [DOI] [PubMed] [Google Scholar]
Campisi 2002
- Campisi R, Nathan L, Pampaloni MH, Schöder H, Sayre JW, Chaudhuri G, et al. Noninvasive assessment of coronary microcirculatory function in postmenopausal women and effects of short‐term and long‐term estrogen administration. Circulation 2002;105(4):425‐30. [DOI] [PubMed] [Google Scholar]
Curb 2006
- Curb JD, Prentice RL, Bray PF, Langer RD, Horn K, Barnabei VM, et al. Venous thrombosis and conjugated equine estrogen in women without a uterus. Archives of Internal Medicine 2006;166:772‐80. [DOI] [PubMed] [Google Scholar]
Cushman 2004
- Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292(13):1573‐80. [DOI] [PubMed] [Google Scholar]
de Maat 2007
- Maat M, Madsen J, Langdahl B, Bladberg E, Tofteng C, et al. Genetic variation in estrogen receptor, C‐reactive protein and fibrinogen does not predict the plasma levels of inflammation markers after long term hormone replacement therapy. Thrombosis and Haemostasis 2007;97(2):234‐9. [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dubey 2001
- Dubey RK, Jackson EK. Estrogen‐induced cardiorenal protection:potential cellular, biochemical, and molecular mechanisms. American Journal of Physiology. Renal Physiology 2001;280:365–88. [DOI] [PubMed] [Google Scholar]
Furness 2009
- Furness S, Roberts H, Marjoribanks J, Lethaby A, HickeyM, Farquhar C. Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD000402.pub4] [DOI] [PubMed] [Google Scholar]
Grady 1992
- Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Annals of Internal Medicine 1992;117(12):1016‐37. [DOI] [PubMed] [Google Scholar]
Grady 1998
- Grady D, Applegate W, Bush T, Furberg C, Riggs B, Hulley S, et al. Heart and Estrogen/progestin Replacement Study (HERS): design, methods, and baseline characteristics. Controlled Clinical Trials 1998;19(4):314‐35. [DOI] [PubMed] [Google Scholar]
Greendale 1999
- Greendale GA, Lee NP, Arriola ER. The menopause. Lancet 1999;353:571–80. [DOI] [PubMed] [Google Scholar]
Grodstein 1999
- Grodstein F, Stampfer MJ, Falkeborn M, Naessen T, Persson I. Postmenopausal hormone therapy and risk of cardiovascular disease and hip fracture in a cohort of Swedish women. Epidemiology 1999;10:476‐80. [PubMed] [Google Scholar]
Grodstein 2000
- Grodstein F, Manson JE, Colditz GA, Willet WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Annals of Internal Medicine 2000;133:933‐41. [DOI] [PubMed] [Google Scholar]
Grodstein 2006
- Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. Journal of Womens Health 2006;15(1):35‐44. [DOI] [PubMed] [Google Scholar]
Hendrix 2006
- Hendrix SL, Wassertheil‐Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, et al. Effects of conjugated equine estrogen on stroke in the women's health initiative. Circulation 2006;113:2425‐34. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hsia 2006
- Hsia J, Langer R, Manson J, Kuller L, Johnson K, Hendrix S, et al. Conjugated equine estrogens and coronary heart disease: The Women's Health Initiative. Archives of Internal Medicine 2006;166(3):357‐65. [DOI] [PubMed] [Google Scholar]
Hu 1999
- Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, et al. Age at natural menopause and risk of cardiovascular disease. Archives of Internal Medicine 1999;159:1061–6. [DOI] [PubMed] [Google Scholar]
Jacobsen 1997
- Jacobsen BK, Nilssen S, Heuch I, Kvale G. Does age at natural menopause affect mortality from ischemic heart disease?. Journal of Clinical Epidemiology 1997;50:475–9. [DOI] [PubMed] [Google Scholar]
Kannel 1976
- Kannel W, Hjortland M, McNamara P, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Annals of Internal Medicine 1976;85:447‐52. [DOI] [PubMed] [Google Scholar]
Kilim 2013
- Kilim SR, Chandala SR. A comparative study of lipid profile and oestradiol in pre‐ and post‐menopausal women. Journal of Clinical and Diagnostic Research 2013;7(8):1596‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koh 2004
- Koh KK, Shin MS, Sakuma I, Ahn JY, Jin DK, Kim HS, et al. Effects of conventional or lower doses of hormone replacement therapy in postmenopausal women. Arteriosclerosis, Thrombosis and Vascualr Biology 2004;24(8):1516‐21. [DOI] [PubMed] [Google Scholar]
Lakatta 2003
- Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III. Cellular and molecular clues to heart and arterial aging. Circulation 2003;107:490–7. [DOI] [PubMed] [Google Scholar]
Lieberman 1994
- Lieberman EH, Gerhard MD, Uehata A, Walsh BW, Selwyn AP, Ganz P, et al. Estrogen improves endothelium‐dependent, flow mediated vasodilation in postmenopausal women. Annals of Internal Medicine 1994;121(12):936‐41. [DOI] [PubMed] [Google Scholar]
Losordo 1994
- Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation 1994;89:1501‐10. [DOI] [PubMed] [Google Scholar]
Magliano 2006
- Magliano DJ, Rogers SL, Abramson MJ, Tonkin AM. Hormone therapy and cardiovascular disease: a systematic review and meta‐analysis. BJOG 2006;113:5‐15. [DOI] [PubMed] [Google Scholar]
Main 2013
- Main C, Knight B, Moxham T, Gabriel Sanchez R, Sanchez Gomez LM, Roqué i Figuls M, et al. Hormone therapy for preventing cardiovascular disease in post‐menopausal women. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD002229.pub3] [DOI] [PubMed] [Google Scholar]
Mann 1994
- Mann RD, Lis Y, Chukwujindu J, Chanter DO. A study of the association between hormone replacement therapy, smoking and the occurrence of myocardial infarction in women. Journal of Clinical Epidemiology 1994;47:307‐12. [DOI] [PubMed] [Google Scholar]
Manson 2003
- Manson, JE, Hsia J, Johnson KC, Rossouw JE, Assaf A, Lasser N, et al. Estrogen plus progestin and the risk of coronary heart disease. New England Journal of Medicine 2003;349:523‐34. [DOI] [PubMed] [Google Scholar]
Maxwell 1998
- Maxwell SR. Women and heart disease. Basic Research in Cardiology 1998;934(93 (Suppl 2)):79–84. [DOI] [PubMed] [Google Scholar]
McKinney 1998
- McKinney KA, Thompson W. A practical guide to prescribing hormone replacement therapy. Drugs 2009;56(1):49‐56. [DOI] [PubMed] [Google Scholar]
Melloni 2010
- Melloni C, Berger J, Wang T, Gunes F, Stebbins A, Pieper K, et al. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circulation 2010;3(2):135‐42. [DOI] [PubMed] [Google Scholar]
Mendelsohn 1999
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. New England Journal of Medicine 1999;340:1801–11. [DOI] [PubMed] [Google Scholar]
Mikkola 2002
- Mikkola TS, Clarkson TB. Estrogen replacement therapy,atherosclerosis, and vascular function. Cardiovascular Research 2002;53:605–19. [DOI] [PubMed] [Google Scholar]
Miller 2002
- Miller J, Chan B, Nelson H. Postmenopausal Estrogen Replacement and Risk for VenousThromboembolism: A Systematic Review and Meta‐Analysis for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2002;136:680‐90. [DOI] [PubMed] [Google Scholar]
Mishell 1989
- Mishell DR Jr. Estrogen replacement therapy: an overview. American Journal of Obstetrics and Gynecology 1989;161(6 Pt 2):1825‐7. [DOI] [PubMed] [Google Scholar]
Ouyang 2006
- Ouyang P, Michos E, Karas R. Hormone replacement therapy and the cardiovascular system: lessons learned and unanswered questions. Journal of the American College of Cardiology 2006;47(9):1741‐53. [DOI] [PubMed] [Google Scholar]
Paganini‐Hill 2001
- Paganini‐Hill A. Hormone replacement therapy and stroke: risk protection or no effect?. Maturitas 2001;38:243‐61. [DOI] [PubMed] [Google Scholar]
Phillips 2005
- Phillips LS, Langer RD. Postmenopausal hormone therapy: critical reappraisal and a unified hypothesis. Fertility and Sterility 2005;83:558‐66. [DOI] [PubMed] [Google Scholar]
Psaty 1994
- Psaty BM, Heckbert SR, Atkins D, Lemaitre R, Koepsell TD, Wahl PW, et al. The risk of myocardial infarction associated with the combined use of estrogen and progestins in postmenopausal women. Archives of Internal Medicine 1994;154(12):1333‐9. [PubMed] [Google Scholar]
Rathore 2003
- Rathore S, Foody J, Radford M, Krumholz H. Sex differences in use of coronary revascularization in elderly patients after acute myocardial infarction: a tale of two therapies. Chest 2003;124(6):2079‐86. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 1959
- Robinson RW, Higano N, Cohen WD. Increased incidence of coronary heart disease in women castrated prior to the menopause. Archives of Internal Medicine 1959;104:908‐13. [DOI] [PubMed] [Google Scholar]
Rosenberg 1993
- Rosenberg L, Palmer JR, Shapiro S. A case‐control study of myocardial infarction in relation to use of estrogen supplements. American Journal of Epidemiology 1993;137:54‐63. [DOI] [PubMed] [Google Scholar]
Rossouw 2013
- Rossouw JE, Manson JE, Kaunitz AM, Anderson GL. Lessons learned from the Women's Health Initiative trials of menopausal hormone therapy. Obstetrics and Gynecology 2013;121:172‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salpeter 2004
- Salpeter SR, Walsh JME, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women. Journal of General Internal Medicine 2004;19:791‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salpeter 2006
- Salpeter SR, Walsh JME, Greyber E, Salpeter EE. Coronary heart disease events associated with hormone therapy in younger and older women. Journal of General Internal Medicine 2006;21:363‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stampfer 1991
- Stampfer M, Colditz G. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Preventive Medicine 1991;20:47‐63. [DOI] [PubMed] [Google Scholar]
Stefanick 2003
- Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR, et al. The Women's Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Annals of Epidemiology 2003;13(Suppl 9):S78‐86. [DOI] [PubMed] [Google Scholar]
Tofteng 2002
- Tofteng C, Jensen J, Abrahamsen B, Odum L, Brot C. Two Polymorphisms in the Vitamin D Receptor Gene—Association With Bone Mass and 5‐Year Change in BoneMass With or Without Hormone‐Replacement Therapy inPostmenopausal Women: The Danish OsteoporosisPrevention Study. Journal of Bone and Mineral Research 2002;17(8):1535‐44. [DOI] [PubMed] [Google Scholar]
Townsend 2012
- Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Leal J, et al. Coronary Heart Disease Statistics. London: British Heart Foundation, 2012. [Google Scholar]
van der Schouw 1996
- Schouw YT, Graaf GY, Steyerberg EW, Eijkemans JD, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet 1996;347:714–8. [DOI] [PubMed] [Google Scholar]
Wallach 1959
- Wallach S, Henneman PH. Prolonged estrogen therapy in postmenopausal women. Journal of the American Medical Association 1959;171:1637‐42. [PubMed] [Google Scholar]
Walsh 1991
- Walsh B, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks F. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. New England Journal of Medicine 1991;325:1196‐204. [DOI] [PubMed] [Google Scholar]
Wassertheil‐Smoller 2003
- Wassertheil‐Smoller S, Hendrix S, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women. JAMA 2003;289(20):2673‐84. [DOI] [PubMed] [Google Scholar]
WHO 2008
- World Health Organization. Leading causes of death according to income group. http://who.int/mediacentre/factsheets/fs310/en/index.html (accessed 20 May 2013).
WHO 2011
- World Health Organization. Global status report on noncommunicable diseases 2010. www.who.int/nmh/publications/ncd_report2010/en/ 2011.
Wilson 1963
- Wilson RA, Brevetti RE, Wilson TA. Specific procedures for the elimination of the menopause. Western Journal of Surgery, Obstetrics, and Gynecology 1963;71:110‐21. [PubMed] [Google Scholar]


