Abstract
Women show an increased lifetime risk of Alzheimer’s disease (AD) compared with men. Characteristic brain connectivity changes, particularly within the default mode network (DMN), have been associated with both symptomatic and preclinical AD, but the impact of sex on DMN function throughout aging is poorly understood. We investigated sex differences in DMN connectivity over the lifespan in 595 cognitively healthy participants from the Human Connectome Project-Aging cohort. We used the intrinsic connectivity distribution (a robust voxel-based metric of functional connectivity) and a seed connectivity approach to determine sex differences within the DMN and between the DMN and whole brain. Compared with men, women demonstrated higher connectivity with age in posterior DMN nodes and lower connectivity in the medial prefrontal cortex. Differences were most prominent in the decades surrounding menopause. Seed-based analysis revealed higher connectivity in women from the posterior cingulate to angular gyrus, which correlated with neuropsychological measures of declarative memory, and hippocampus. Taken together, we show significant sex differences in DMN subnetworks over the lifespan, including patterns in aging women that resemble changes previously seen in preclinical AD. These findings highlight the importance of considering sex in neuroimaging studies of aging and neurodegeneration.
Keywords: Alzheimer’s disease, functional MRI, human connectome project aging, women
Introduction
More than two-thirds of people with Alzheimer’s disease (AD) are women (2021 Alzheimer’s disease facts and figures 2021). Women’s elevated risk reflects both sex- and gender-based factors, including women’s increased longevity relative to men. Whether female sex represents an independent risk factor for the development of AD is a subject of active debate, but women are clearly at risk for AD “differently” than men are. Women with AD progress more quickly than men do, suffering a faster rate of both cognitive and functional decline (Agüero-Torres et al. 1998; Tschanz et al. 2011); the same burden of AD pathology is more likely to cause AD dementia in women than men (Barnes et al. 2005), and the single greatest genetic risk factor for AD, the APOE-ε4 allele, disproportionately impacts women (Payami et al. 1996; Ungar et al. 2014; Riedel et al. 2016). Amyloid and tau PET studies have revealed that women are more likely than men to harbor entorhinal tau even in the absence of cognitive symptoms (Buckley et al. 2019) and have a greater regional tau burden than men at the same clinical stage (Edwards et al. 2021); women with elevated amyloid as measured by amyloid PET decline faster than do men with the same extent of amyloid (Buckley et al. 2018). The menopausal transition may be a period of particular vulnerability: perimenopausal and postmenopausal women show decreased metabolic and mitochondrial function, as well as increased amyloid deposition, compared with age-matched premenopausal women and to men (Mosconi et al. 2017a, b; Scheyer et al. 2018; Rahman et al. 2020).
While AD has been associated with robust and characteristic changes in brain connectivity, including in preclinical and other at-risk populations, sex differences over the course of aging in the functional brain networks most linked to AD—which might yield significant insights into women’s AD risk—are poorly understood. Amnestic AD specifically targets an intrinsic connectivity network called the default mode network (DMN), anchored in the posterior cingulate cortex (PCC), mesial prefrontal cortex (mPFC), and angular gyrus (AG), which subserves self-referential processing and episodic memory. AD patients show decreased connectivity within this network, and abnormalities in DMN function characterize even adults with preclinical AD (Greicius et al. 2004; Sheline et al. 2010; Mormino et al. 2011; Brier et al. 2012). These network changes are dynamic over the course of disease: vulnerable and early stage individuals (such as APOE-ε4 carriers and those with preclinical AD) may show intra- or internetwork hyperconnectivity from key DMN nodes (Mormino et al. 2011; Schultz et al. 2017), whereas clinical illness is generally associated with progressive DMN hypoconnectivity (Greicius et al. 2004; Sheline et al. 2010; Brier et al. 2012).
Over the course of healthy aging, within-network DMN connectivity (and, for those studies that examined DMN subnetworks, the connectivity of posterior DMN nodes specifically) declines as well (Jones et al. 2011; Geerligs et al. 2015; Huang et al. 2015), though not to the extent seen in illness (Jones et al. 2011). Declines in posterior DMN connectivity correlate with poorer cognitive performance, even in amyloid-negative older individuals (Andrews-Hanna et al. 2007; Hansen et al. 2014; Persson et al. 2014; Bernard et al. 2015). Anterior DMN connectivity, on the other hand, may increase with older age (Persson et al. 2014; Geerligs et al. 2015).
These prior studies of DMN in aging, however, have generally ignored the impact of sex.
Prior work that has considered sex as a variable of interest and assessed functional connectivity within the brain over the course of aging has concluded variously that sex has no significant impact on functional networks of interest, including the DMN, and may safely be ignored (Bluhm et al. 2008; Weissman-Fogel et al. 2010); that both sexes show decreased DMN connectivity with age, albeit at different rates (Scheinost et al. 2015); or that women generally show higher overall DMN connectivity than men do (Biswal et al. 2010; Ritchie et al. 2018). However, these studies have generally been limited by smaller sample sizes or limited age ranges, or have collapsed findings across a predefined set of networks of interest (including whole DMN), rather than assessing connectivity within components of the DMN or across the whole brain.
Given the differences in AD risk between men and women, and a potential impact of the menopausal transition on women’s cognition specifically, we sought to closely interrogate DMN function in a large sample of cognitively normal aging men and women over the lifespan, with the goal of pinpointing sex differences in DMN connectivity both over the larger course of aging and in specific decades.
We therefore pursued a cross-sectional assessment of DMN connectivity in healthy aging in both sexes, leveraging imaging and behavioral data from the unprecedentedly large Human Connectome Project-Aging (HCP-A) data set (n = 689) (Harms et al. 2018). Specifically, we hypothesized that women would show a distinct DMN connectivity pattern across their lifespan when compared with men, with most pronounced differences occurring around the menopausal transition. On an exploratory basis, we also hypothesized that connectivity between key DMN nodes implicated in recollective memory (i.e. PCC, AG, and hippocampus [HIP]) would correlate with delayed memory performance on neuropsychological testing, even in this cognitively healthy sample, and that the relationship between connectivity and memory performance would vary by sex.
Materials and methods
Participants
Data were from the HCP-A data set (Bookheimer et al. 2019); imaging data were from release 1.0, and corresponding behavioral data were made available in release 2.0. Imaging data consisted of 689 healthy subjects ages 36–100 from 4 data collection sites. Exclusion criteria of the original HCP-A study included any history of major psychiatric and neurological conditions, including stroke, severe head injury or brain surgery, or suspected AD. The study aimed to include individuals with common health conditions typical of their age and did not exclude for hypertension or other vascular risk factors. However, those with new or poorly controlled conditions, such as a diagnosis of diabetes within the past 3 years or blood pressure > 170/100, were excluded. In addition, to allow a sample of “typical aging,” the original HCP-A study did not exclude for mild cognitive complaints, and used a tiered cut-off approach for Montreal Cognitive Assessment (MOCA) scores (requiring a minimum score of 19 for those under 80 years of age and 17 for those over 80), as well as for hearing and macular degeneration (specifically, participants under 80 were excluded if hearing loss precluded communication via telephone, whereas participants over 80 were only excluded if they could not communicate using a microphone in the scanner; only participants under 80 were excluded for macular degeneration). See Bookheimer et al. (2019) for full exclusion criteria. We further added strict exclusion criteria based on motion (see below for details), missing data (17 subjects were missing one or more scans), and anatomical abnormalities (5 subjects). After exclusion, the remaining sample size was n = 595 (346 F; 249 M). Distribution of participants across the lifespan is available as Supplementary Fig. 1.
Participants were well-matched in age, ethnicity, race, handedness, and NEO-neuroticism score, but women outnumbered men and outperformed men in global cognitive function (MOCA) and verbal learning (Rey Auditory Verbal Learning Test [RAVLT]) (Table 1).
Table 1.
Demographics and selected neuropsychological battery scores (Costa and McCrae 1992; Nasreddine et al. 2005; Bean 2011). (*Note that years of education was not part of the HCP-A version 1.0 release.) T-tests or chi square tests were performed as appropriate, excluding unknown/not reported values.
| Female | Male | P-value | |
|---|---|---|---|
| Total number of participants | 346 (58%) | 249 (42%) | N/A |
| Age | 57.59 (14.81) | 58.13 (14.07) | 0.687 |
| Race | American Indian/Alaska Native: 1 Asian: 20 Black/African American: 56 White: 246 More than 1 race: 16 Unknown/not reported: 7 |
American Indian/Alaska Native: 1 Asian: 27 Black/African American: 32 White: 177 More than 1 race: 10 Unknown/not reported: 2 |
0.204 |
| Ethnicity | Hispanic or Latino: 38 Not Hispanic or Latino: 307 Unknown/not reported: 1 |
Hispanic or Latino: 21 Not Hispanic or Latino: 228 Unknown/not reported: 0 |
0.299 |
| Handedness | Right: 301 Left: 22 Ambidextrous: 23 |
Right: 206 Left: 24 Ambidextrous: 19 |
0.283 |
| MOCA total (points out of 30) | 26.86 (2.25) | 26.31 (2.51) | 0.009 |
| RAVLT-L (Sum of Trials 1–5, # words recalled) | 48.22 (9.71) | 44.06 (10.50) | <0.001 |
| RAVLT-IR (Immediate Recall, # words recalled) | 10.13 (2.99) | 9.07 (3.31) | <0.001 |
| NEO-Neuroticism score | 15.23 (7.25) | 14.57 (7.88) | 0.349 |
Imaging parameters
Imaging parameters for HCP-A have been published elsewhere (Harms et al. 2018). In summary, all subjects were scanned in a Prisma 3 T scanner with 80 mT/m gradients and a 32-channel head coil. The following scans were acquired: 1 T1-weighted, T2-weighted, diffusion, perfusion (pseudo-continuous arterial spin labeling), and turbo-spin-echo scan per subject; 4 resting-state fMRI scans; and 3 task-fMRI scans. In this analysis, we focus on the 4 resting-state fMRI scans.
All T1-weighted structural images were acquired using a multi-echo MPRAGE sequence (0.8 × 0.8 × 0.8 mm voxel size; FOV = 256 × 240 × 166 mm; matrix size of 320 × 300 × 208 slices; TR/TI = 2,500/1,000 ms; TE = 1.8/3.6/5.4/7.2 ms; flip angle = 8 degrees; water excitation for fat suppression; up to 30 TRs allowed for motion-induced reacquisition). All fMRI scans were acquired with a 2D multiband (MB) gradient-recalled echo echo-planar imaging sequence (MB8; 2 × 2 × 2 mm voxel size; 72 oblique-axial slices; TR/TE = 800/37 ms; flip angle = 52 degrees).
Each resting-state fMRI scan was acquired over 6.5 min (26 min total for 4 runs) with 488 frames per run, during which participants were told to look at a fixation cross. Two runs were done in session 1, and 2 were done later that same day in session 2. In each session, 1 resting-state fMRI scan had an anterior to posterior phase encoding direction (AP), and the other had a posterior to anterior phase encoding direction (PA).
Image preprocessing
The preprocessing approach has been described elsewhere (Greene et al. 2018; Horien et al. 2019) and was performed in BioImageSuite unless otherwise noted (Joshi et al. 2011). MPRAGE images were skull stripped using optiBET (Lutkenhoff et al. 2014). Nonlinear registration of the MPRAGE to the MNI template was performed in BioImage Suite (Joshi et al. 2011). Linear registration of a participant’s mean functional image to the same participant’s MPRAGE image was also performed using BioImage Suite. All skull-stripped images and registrations were visually inspected; 5 participants had structural abnormalities and were excluded from further analyses. All transformation pairs were calculated independently and then combined into a single transform that warps single participant results into common space. From this, all subjects’ images can be transformed into common space using a single transformation, which reduces interpolation error (Noble et al. 2017). Functional data were motion corrected using SPM8. Exclusion criteria for motion were a maximum mean frame-to-frame displacement above 0.3 mm for any scan, and a mean maximum frame-to-frame displacement over all 4 scans above 0.25 mm. That is, we required all scans to be below 0.3 mm FFD, as well as the average of all 4 resting-state scans to be below 0.25 mm. Such an approach to handle motion has been shown to limit motion artifacts (Greene et al. 2018; Horien et al. 2018, 2019; Ju et al. 2020). To assess for significant effects of sex, age, and their interaction on motion, we implemented the regression model:
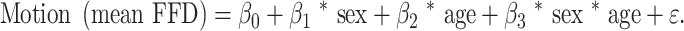 |
There was a significant main effect of age with higher age being associated with greater mean frame-to-frame displacement (z = 2.392, P = 0.017) and no main effect of sex (z = −0.470, P = 0.638) or their interaction (z = 0.363, P = 0.717). There were also no differences in motion between sexes within each age bin (Supplementary Table 1). Covariates of no interest were regressed from the data, including linear, quadratic, and cubic drift, a 24-parameter model of motion (Satterthwaite et al. 2013), mean cerebrospinal fluid signal, mean white matter signal, and the global signal. As global signal regression has been shown to limit the consequences of motion artifact (Power et al. 2017) and strengthen brain-phenotype associations (Li et al. 2019), we have used it in the current preprocessing pipeline.
Intrinsic connectivity distribution
We then applied a DMN mask based on the Yeo parcellation (Yeo et al. 2011; Horien et al. 2019), which was defined on the MNI brain and applied to the subject-space resting-state scan using a series of inverse transformations. To assess within-DMN functional connectivity, we used a voxel-wise assessment, the intrinsic connectivity distribution (ICD) (Scheinost et al. 2012). In this method, the time course for each voxel is correlated with the time course of every other voxel, and using network theory, the entire distribution of degree is modeled without needing to specify a priori thresholds. As in previous work (Scheinost et al. 2015, 2019; Rolison et al. 2022), these connections were then converted to a survival function and fitted with a stretched exponential with unknown variance, alpha. The alpha for each voxel was converted into a parametric image of a single alpha for each subject. Larger alphas represent greater spread of distributions for a single voxel and thus higher global correlation. Because we restricted analysis to the DMN, the ICD alpha value for each individual represents that individual’s overall within-DMN connectedness. As in previous work, we calculated estimates of connectivity across the four resting-state scans and then averaged them within subject (i.e. we averaged the ICD measurements) to ensure that phase encoding differences (AP/PA) did not result in spatial differences in connectivity (Greene et al. 2018; Barron et al. 2021; Rosenblatt et al. 2021). In addition, combining data across scans has been shown to boost reliability of functional connections (Noble et al. 2017).
ICD group analysis
Using the alpha-value parametric maps obtained from ICD, we used the Analysis of Functional Neuroimages (AFNI) 3dLME function (Chen et al. 2013) (https://afni.nimh.nih.gov) to fit the following model: ICD = β0 + β1 * sex + β2 * age + β3 * sex * age + ε. Multiple comparisons correction was conducted using family-wise error correction via Monte Carlo simulation in AFNI 3dClustSim (version 18.0.09). We used the autocorrelation values of the 3dFWHMx using the residuals of the 3dLME as input into 3dClustSim. Our resulting cluster value for an initial threshold of P < 0.001 and alpha correction of P < 0.05 was 13, yielding a 104 mm3 cluster.
We also assessed for effects of sex, age, and the interaction of these on head motion using the following model: motion(FFD) = β0 + β1 * sex + β2 * age + β3 * sex * age + ε. We observed a significant effect of age (z = 2.392, P = 0.017), but no effect of sex (P = 0.638, P = −0.277) or the interaction of sex and age (z = 0.363, P = 0.717) on head motion. To assess for a significant effect of motion on our ICD results, we repeated the ICD analysis incorporating motion as a covariate in the linear model (Supplementary Fig. 2). As the results were unchanged, we present the analysis without the motion covariate here.
ICD analysis by age
Subjects were then binned into six categories by age group (36–39, 40–49, 50–59, 60–69, 70–79, and 80–89). The minimum age in the HCP-A cohort was 36 years old, and the cohort included no subjects in their 90s. The 5 subjects who were 100 years old (from the n = 595 cohort) were excluded from binning because of low sample size. Female-to-male ratios were similar per decade and representative of the ratio of the overall cohort. Separate analyses were done by decade bin using the same design and linear model as for the overall ICD above:
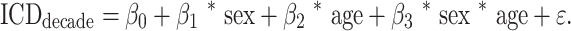 |
We then selected regions of interest (ROIs) based on the ICD analysis above to represent major nodes within the DMN and calculated average voxel values for each of these for each participant. Only some ROIs were chosen in an effort to determine the overall pattern of sex differences by decade while minimizing multiple comparisons. We then performed 2-tailed, independent-samples t-tests between the average connectivity values of females and males by decade for each ROI. The Bonferroni correction was applied to adjust for multiple comparisons. All regression and t-test computations were conducted in Python 3.9.5 (Python Language Reference n.d.). ICD values for each ROI were fit to both linear and quadratic regressions for each sex across decade age ranges (Supplementary Figs. 3 and 4). Since linear regressions were best fit across each ROI, quadratic regressions were discarded and we proceeded with linear regressions alone.
Seed connectivity
Having assessed within-network differences in connectivity by sex, we next wanted to assess sex differences in connectivity between the DMN and the whole brain. To seed the DMN for this purpose, we used an empiric left posterior cingulate (PCC) coordinate derived from a meta-analysis of 825 prior studies which robustly elicits the DMN (Toro et al. 2008; Shehzad et al. 2009), selecting this particular coordinate because it has proven to have the most consistent membership within the DMN in a hierarchical clustering analysis (Shehzad et al. 2009). We created a 5 mm3 cubic seed centered at this coordinate (−6, −58, and 28) in MNI space.
The time course of the seed in each participant was then computed as the average time course across all voxels in the seed region. This time course was correlated with the time course for every other voxel in gray matter to create a map of r-values, reflecting seed-to-whole-brain connectivity. These r-values were transformed to z-values using Fisher transform, yielding 1 map for each seed and representing the strength of correlation with the seed for each participant. Connectivity maps were again averaged across all resting-state runs per participant.
We used 3dttest to compute 2-sample, 2-tailed t-tests, resulting in parametric maps that were visualized in BioImageSuite. For cluster correction, we used the autocorrelation values of 3dFWHMx using the residuals of the 3dttest as input into 3dClustSim. Our resulting cluster value for an initial threshold of P < 0.001 and alpha correction of P < 0.05 was 9, yielding a 72 mm3 cluster.
Neuropsychological measures
We conducted exploratory post-hoc analyses assessing the relationship between selected clusters identified in the seed-based analysis above and neuropsychological task performance using standard neuropsychological measures of declarative memory (RAVLT; Bean 2011) and neuroticism (NEO-N subscale of the Neuroticism-Extraversion-Openness (NEO) Personality Inventory; Costa and McCrae 1992). Clusters were selected on the basis of prior literature suggesting a relationship between those areas and memory or neuroticism score. We assessed both the immediate recall (IR) and sum-of-trials learning (L) metrics from the RAVLT and used the total NEO-N subscale for the neuroticism metric. Mean connectivity values for each selected cluster were derived for each participant. We then used regression models to assess the effects of specific connectivities of interest, sex, age, and the interactions of sex and age (denoted as “sex * age”) and connectivity and age (denoted as “connectivity * age”) on the neuropsychological measures (RAVLT-L, RAVLT-IR, and NEO-N) using regression models. For the RAVLT-L and RAVLT-IR models, we specifically assessed for the effects of the left PCC:left AG and left PCC:left parahippocampal gyrus (PHG) connectivities, whereas for the NEO-N model, we explore the left PCC:right superior temporal sulcus (STS) and left PCC:left insula connectivity effects. We initially fit the following model:
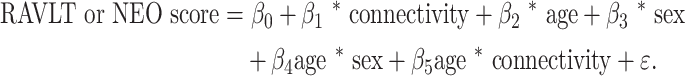 |
(1) |
Where the higher-order interaction terms were significant, we report the results of this model. Where it was not, we removed the nonsignificant interaction term (age * connectivity) and report the results of the following simplified model:
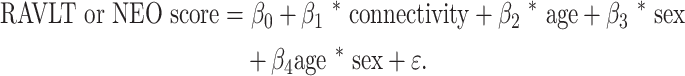 |
(2) |
We report the results of our models in Connectivity between PCC and AG is associated with declarative memory performance; connectivity between PCC and STS is negatively associated with neuroticism of Results, including which model (1) and (2) above was implemented for each.
Given the exploratory nature of these analyses, we do not correct for multiple comparisons.
Regression analyses were conducted in R version 1.1.463 (R Core Team 2019).
Data and code availability
The HCP-A data that support the findings of this study are publicly available through the NIMH Data Archive (https://nda.nih.gov/edit_collection.html?id=2847). The preprocessing software can be freely accessed at https://bioimagesuiteweb.github.io/webapp/. R code to run correlation analyses between neuropsychological measures and seed connectivity values and Python code to conduct the connectivity by age analyses (linear regressions, boxplots, and t-test computation) can be found at https://github.com/frederickslab/connectivity_analysis.
Results
Within-DMN ICD: sex differences over the course of aging
ICD analysis to assess within-network DMN connectivity showed that women had relatively higher within-network connectivity in major posterior nodes of the DMN including posterior cingulate (left t = 3.9; right t = 4.4) and AG (right t = 5.4; left t = 5.0), as well as PHG/subiculum (left t = 4.4; right t = 4.6), whereas men had relatively higher connectivity in anterior DMN nodes, including mPFC (left frontal pole t = −4.3; right t = −3.7) (Fig. 1; see Supplementary Table 2 for a complete list of clusters).
Fig. 1.
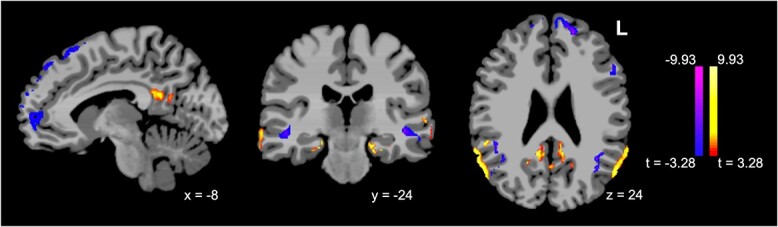
Sex differences in DMN ICD over age (females minus males, P < 0.001/P < 0.05). Relative to men, women show higher within-network connectivity in major DMN nodes including PCC and bilateral AG, as well as PHG, and lower within-network connectivity in mesial frontal structures.
Within-DMN ICD: sex differences by decade
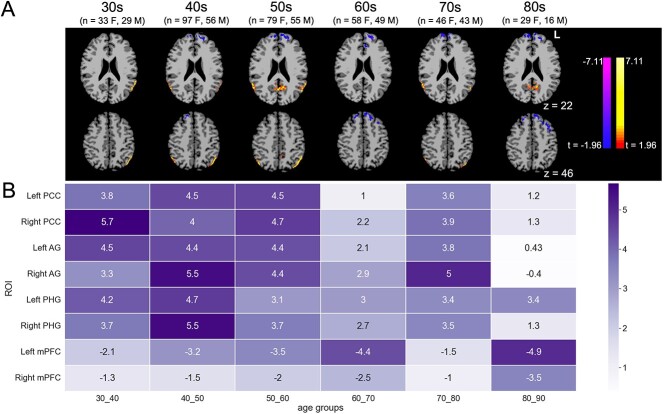
To visualize and quantitatively examine connectivity differences between sexes by age, subjects were divided into age groups by decade as delineated in the methods, and voxel-based 2-tailed t-tests were performed to compare male and female subjects within each age bin. Sex differences in posterior DMN nodes including bilateral posterior cingulate and AG were most prominent for subjects in their 40s and 50s, with women having the highest relative within-network connectivity compared with men during these decades. Peak sex differences in PHG connectivity occurred earlier, with women having the greatest relatively increased within-network connectivity in the 30s and 40s. Mesial prefrontal clusters with the highest relative within-network connectivity for men, on the other hand, showed the most prominent differences in connectivity by sex in later decades (60s and 80s) (Fig. 2). T-scores of ICD values at peak clusters for female minus male contrasts are shown in Fig. 2B.
Fig. 2.
A) Sex differences in DMN ICD, by decade (females minus males, P < 0.05/P < 0.05) and B) heatmap of scores representing the results of 2-tailed, independent samples t-tests between each sex’s average connectivity values for each ROI (color gradient labeling denotes absolute values of t-values; negative scores indicate higher ICD values in male subjects). Sex differences in PHG peak in the 40s, whereas sex differences in PCC and AG are generally highest in the 5th and 6th decades; men’s higher within-network connectivity from mPFC peaks in the 7th and 9th decades (F, female; M, male).
As an alternative means of quantifying sex differences in clusters of interest by decade, we derived ICD values for key ROIs from our whole-group analysis (Fig. 1) for each participant, then performed 2-tailed t-tests to compare male and female participants’ average ICD values within age groups for each ROI (Fig. 2B). As a means of visualizing trends over time for each sex in each key ROI, we also present results of these t-tests as boxplots (Supplementary Fig. 5): women show higher connectivity than do men for the PCC and AG clusters (most notably in the 40s and 50s) as well as PHG (most notably in the 30s and 40s), but lower connectivity in the mPFC (most notably in the 60s and 80s).
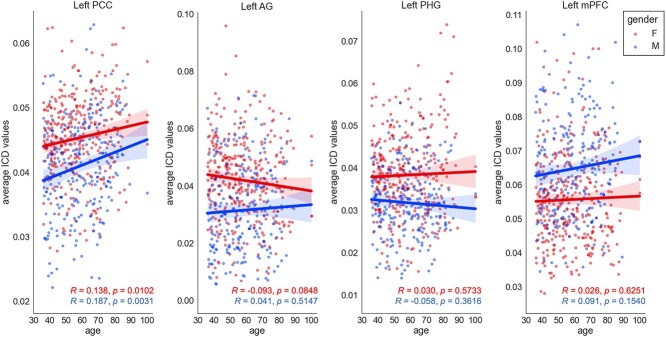
Finally, in order to assess trends over time for each key ROI, we performed linear regressions of average ICD values by sex across bilateral PCC, AG, PHG, and mPFC clusters (Fig. 3).
Fig. 3.
Linear regressions of ICD values by sex for key ROIs. Red dots indicate ICD values for individual female subjects; blue indicates ICD values for individual male subjects.
DMN to whole brain seed-based analysis
Having established significant within-network DMN connectivity differences by sex over the course of aging, we turned our attention to the effect of sex on inter-network connectivity, focusing specifically on connectivity between a canonical DMN seed and the whole brain. We performed a whole-brain seed-based analysis using a meta-analysis-derived coordinate for the posterior cingulate, a central hub of the DMN (Andrews-Hanna et al. 2010), and compared female and male participants. Relative to men, women showed significantly increased connectivity to a set of regions both within and beyond the DMN, including bilateral hippocampi, bilateral angular gyri, insula, and ventral prefrontal cortex/anterior cingulate. Relative to women, men showed relatively increased connectivity to the STS and left premotor cortex, among other regions (Fig. 4; see Supplementary Table 3 for a complete list of clusters).
Fig. 4.
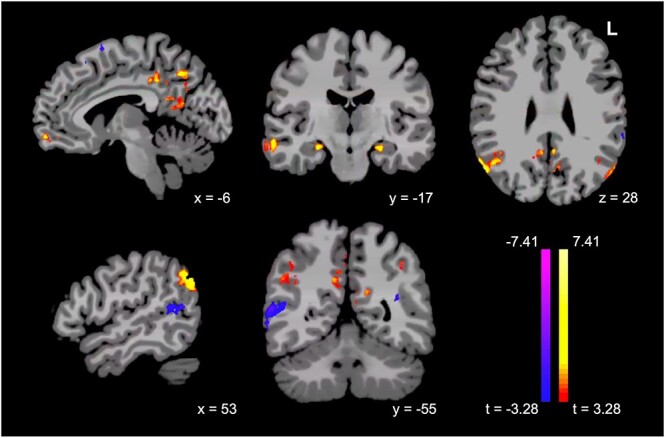
Women show significantly greater connectivity from PCC to critical regions both within and beyond the DMN, including bilateral hippocampi, bilateral angular gyri, insula, and ventral prefrontal cortex/anterior cingulate, but lower connectivity to STS, in a seed-based analysis (females minus males, P < 0.001/P < 0.05).
Connectivity between PCC and AG is associated with declarative memory performance; connectivity between PCC and STS is negatively associated with neuroticism
The clusters highlighted in our seed-based analysis include regions that are critical for memory (including HIP and AG) and social function (including insula and STS). We therefore conducted exploratory analyses to examine the relationship between posterior cingulate connectivity to HIP and AG and declarative memory scores, and the relationship between posterior cingulate connectivity to insula and STS and neuroticism. We also sought to assess the relationship between neuropsychological task measures and sex, age, and the interactions of both sex and age and connectivity and age. We assessed these relationships using general linear models (as outlined in Materials and Methods and in Table 2).
Table 2.
We used generalized linear models to assess the relationship between declarative memory and neuroticism scores and connectivity from the PCC seed to prespecified clusters. We first considered the complete model (1), RAVLT or NEO score = β0 + β1 * connectivity + β2 * age + β3 * sex + β4age * sex + β5age * connectivity + ε. Left AG connectivity significantly impacts both RAVLT-L and RAVLT-IR scores, whereas the interaction of left AG connectivity and age negatively impacts both. The age * connectivity interaction term was not significant for the remaining scenarios, so we ran a simplified model (2) eliminating this interaction term (RAVLT or NEO score = β0 + β1 * connectivity + β2 * age + β3 * sex + β4age * sex + ε). Right STS connectivity has a statistically significant effect on NEO-N under model 2. At the 0.05 level, the significant P-values are labeled with a. (Abbreviations: PCC, posterior cingulate cortex; AG, angular gyrus; HIP, hippocampus; STS, superior temporal sulcus.)
| Model 1 | ||||||
|---|---|---|---|---|---|---|
| Response | Connectivity |
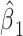
|
P-value |
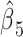
|
P-value | Adjusted 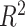
|
| RAVLT-L | Left_PCC_Left_AG_Connectivity | 0.4874 | 0.0068a | −0.4896 | 0.0084a | 0.1351 |
| RAVLT-IR | Left_PCC_Left_AG_Connectivity | 0.5804 | 0.0013a | −0.5879 | 0.0016a | 0.1141 |
| Model 2 | ||||||
| Response | Connectivity |
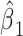
|
P-value | Adjusted 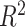
|
||
| RAVLT-L | Left_PCC_Left_HIP_Connectivity | 0.0202 | 0.6464 | 0.1348 | ||
| RAVLT-IR | Left_PCC_Left_HIP_Connectivity | 0.05 | 0.2573 | 0.1275 | ||
| NEO-N | Left_PCC_Right_STS_Connectivity | −0.1072 | 0.0198a | 0.0718 | ||
| NEO-N | Left_PCC_Left_Insula_Connectivity | −0.0465 | 0.3307 | 0.0629 | ||
These general linear models revealed significant effects on short-term memory performance of connectivity between the PCC seed and AG, with an additional negative interactive effect of PCC–AG connectivity and age. Connectivity between PCC and STS significantly impacted NEO-N score, but there was no relationship between PCC–insula connectivity and NEO-N, or PCC–HIP connectivity and RAVLT scores.
Discussion
In contrast to prior research, we show highly significant sex differences in DMN connectivity over the course of aging in a very large, cross-sectional sample of cognitively healthy individuals aged 36–100, drawn from the HCP-A cohort. Some of these differences are most prominent in the decades surrounding menopause; they relate both to memory performance and neuroticism. Specifically, we show that women demonstrate increased connectivity with age in parietal nodes of the DMN, including posterior cingulate and bilateral AG, and decreased connectivity in the medial prefrontal cortex, relative to men; in whole-brain seed-based analyses, women also show relatively higher connectivity from the DMN to other regions critical for memory and socioemotional functioning, including bilateral hippocampi, insula, and ventral prefrontal cortex, and lower connectivity to STS. Furthermore, connectivity between posterior cingulate and AG correlates with memory performance, whereas connectivity between posterior cingulate and STS correlates inversely with neuroticism.
Making sense of sex differences in connectivity and neuropsychological test performance
Prior studies of sex differences in atrophy and glucose metabolism in AD dementia have demonstrated a similar pattern of preserved parietal regions and compromised frontal regions in women compared with men (Herholz et al. 2002; Callen et al. 2004). This preservation may fall off during more advanced stages of illness: while male HCs and men with amnestic MCI and AD dementia show progressively lower cortical thickness of the cingulate and precuneus with more advanced clinical stage, women’s cortical thickness remains relatively stable in these regions between HCs and individuals with MCI, but drops off more steeply in the setting of dementia (Cieri et al. 2022).
Work in healthy older adults has also hinted at relatively higher DMN connectivity (usually assessing the network as a whole) in women compared with men. Two studies taking advantage of large publicly available data sets (UKBB, >5,000 participants aged 44–77 (Ritchie et al. 2018); 1000 Functional Connectomes, 1,093 participants aged 18–71 (Biswal et al. 2010)) both found higher connectivity (by weighted degree, a metric similar to our ICD approach) in the DMN in women as compared with men; Cavedo et al. (2018) found higher DMN connectivity (based on seed-to-seed analysis specifically between mPFC–PCC, precuneus–HIP, and PCC– HIP), as well as higher glucose metabolism in PCC, precuneus, and inferior parietal cortex in healthy older women compared with men. Using an ICD approach in a sample of men and women aged 18–65 (n = 103), Scheinost et al. (2015) concluded that DMN connectivity decreased with age in both sexes, but more steeply in men. None of these studies, however, assessed intra- and inter-network DMN connectivity, which our results, and recent work showing longitudinal increases and decreases within specific DMN components despite an overall pattern of age-related decreases (Malagurski et al. 2022), suggest is an important consideration.
Prior work has also assessed for sex differences in inter-network connectivity from major DMN nodes, generally showing higher connectivity in women. In a large study, Tomasi and Volkow (2012) showed increased short- and long-range functional connectivity in the DMN (PCC, precuneus, angular gurus, and ventral PFC) and PHG in women relative to men (but lower functional connectivity in somatosensory and motor cortex), but found no interaction effects of age and sex, whereas Lopez-Larson et al. (2011) showed increased local connectivity within the mesial temporal lobe specifically in women. Smaller previous studies have also hinted that women show relative hyperconnectivity in specific edges or nodes of the DMN; Bluhm et al. (2008), in their study of 40 men and women, noted that women showed tighter correlation from a PCC/precuneus seed to the medial prefrontal cortex, and relatively greater involvement of right AG using an ICA-based approach.
Our results build upon these prior findings and also add nuance, showing that aging women’s relative DMN hyperconnectivity may be specific to specific nodes, including the PCC/precuneus, AG, and perihippocampal gyrus, with lower connectivity in the medial prefrontal cortex, and that sex differences in these regions’ connectivity to the rest of the DMN vary in magnitude over the course of aging.
The pattern of relative hyperconnectivity in posterior DMN in women over the course of aging, most significant in the fifth and sixth decades, is strikingly similar to previous findings of relative posterior DMN hyperconnectivity in both preclinical AD (i.e. amyloid-positive but cognitively asymptomatic individuals) and those at increased risk of AD (e.g. APOE-ε4 carriers) (Bookheimer et al. 2000; Filippini et al. 2009; Sperling et al. 2009; Mormino et al. 2011; Schultz et al. 2017). Posterior DMN hyperconnectivity in this context is usually interpreted as the compensatory response of a network under stress in which the brain must “try harder” to achieve the same degree of memory performance (Bondi et al. 2005; Filippini et al. 2009; Qi et al. 2010; Mormino et al. 2011). Whether hyperconnectivity comes first, leaving certain nodes and edges within a network more vulnerable to amyloid spread (Jones et al. 2016), or whether excess amyloid accumulation leads to hyperconnectivity in the first place (as suggested in a mouse model where DMN-like hyperconnectivity was reduced by administering antibodies against amyloid; Shah et al. 2016), is currently under active investigation.
Our finding that connectivity from PCC to AG correlates with delayed memory task performance is in line with findings of a similar relationship in studies of symptomatic individuals with AD (Fredericks et al. 2019; Kang et al. 2021; Vanneste et al. 2021) and under direct brain stimulation in non-amnestic individuals (Natu et al. 2019). It is also consistent with the idea that posterior DMN hyperconnectivity is an effective (albeit potentially harmful in the long-term) strategy for maintaining mnemonic function. The finding that women perform better on tests of verbal memory across the lifespan is well-documented in the literature; interestingly, the gap between men’s and women’s performance widens in later decades (Bleecker et al. 1988). Could compensatory hyperconnectivity in the posterior DMN relate to women’s relatively milder age-related decline in verbal memory?
Our finding that increased connectivity between posterior cingulate and the STS correlates inversely with NEO neuroticism score echoes prior findings in preclinical AD, where global STS connectivity correlated with an emotional reactivity score derived from a subset of NEO-neuroticism (Fredericks et al. 2018). Although this was not the case in our sample, most literature suggests that women score higher than men on the NEO-N both across adulthood and into their older decades (Chapman et al. 2007). Changes in neuroticism and emotional reactivity presage and may in fact represent very early symptoms of AD, potentially preceding cognitive symptoms (Johansson et al. 2014, 2020; Fredericks et al. 2018). The finding that relatively higher neuroticism scores are associated with decreased posterior DMN-STS connectivity, in the setting of women’s relatively lower DMN-STS connectivity compared with men, could thus have implications for early neuropsychiatric effects of incipient AD.
The perimenopausal decades and the mesial temporal lobe
Menopause appears to be a time of emerging vulnerability to AD for women: postmenopausal women show a greater burden of tau and amyloid, as well as decreased metabolic and mitochondrial function, compared with age-matched premenopausal women, as well as men (Mosconi et al. 2017a, b; Scheyer et al. 2018; Rahman et al. 2020; Buckley et al. 2022), and changes in metabolic and mitochondrial function correlate with decreased memory scores (Mosconi et al. 2017a). There is a strong moderating impact of menopause on tau accumulation in inferior parietal cortex that participate in the DMN, among several cortical regions, but not in the entorhinal cortex (Buckley et al. 2022); the reasons for cortical tau accumulation independent of entorhinal tau in postmenopausal women are not understood.
Prior research in mouse models points to multiple potential pathways by which large fluctuations in hormone levels (including both estrogen and FSH) in the setting of perimenopause and postmenopause might increase women’s vulnerability to AD. Lower estrogen levels lead to decreased regulation of glucose metabolism and ultimately to decreased synaptic plasticity, impaired mitochondrial function, and increased amyloid deposition (Brinton 2009; Mosconi et al. 2017a), and estrogen plays a critical role in sustaining LTP in the HIP and in hippocampal-dependent memory task performance (Liu et al. 2008; Bean et al. 2015). Female rodents show marked declines in cognitive function as well as wheel-running following estropause (Febo et al. 2020), and higher estradiol as well as estrogen replacement therapy have also been linked to higher short-term memory function in adult women (Yonker et al. 2006; Rentz et al. 2017). An emerging line of research suggests that the FSH surge that accompanies perimenopause might have independent effects on the HIP that contribute to chronic inflammation, amyloid spread, and abnormal phosphorylation of tau (Xiong et al. 2022).
Whether posterior DMN hyperconnectivity in women in their 40s and 50s relates directly to the effects of perimenopausal changes in sex hormone levels, or to indirect effects of perimenopause (e.g. effects of menopause-related sleep dysregulation on amyloid and tau; Winer et al. 2019), and how posterior DMN hyperconnectivity might predispose perimenopausal and postmenopausal women to cortical tau deposition, warrants future study.
Sex- and age-related differences in PHG and anterior DMN connectivity
Prior literature on aging and DMN subnetworks, which has largely ignored the impact of sex, has generally demonstrated declines in overall DMN or posterior DMN connectivity (Jones et al. 2011; Geerligs et al. 2015; Huang et al. 2015), but increases in anterior DMN connectivity with older age (Jones et al. 2011; Persson et al. 2014; Geerligs et al. 2015). This pattern is strikingly similar to the one we observe in our male participants, who show increased in-network connectivity in medial prefrontal clusters, but decreased connectivity in posterior clusters, compared with women. Staffaroni et al. (2018) interestingly show an inverted U-shaped pattern of DMN connectivity with age, where connectivity increased across DMN in ages 50–66 and declined after age 74, with DMN connectivity correlating with episodic memory performance, findings which parallel our results in posterior nodes of the DMN for women, though the authors do not report effects of sex.
It is also notable that the cluster within DMN where sex differences emerged earliest, in bilateral PHG (where women showed increased in-network connectivity relative to men, particularly in their 30s and 40s), is an important node in the hippocampal complex, with bidirectional connections both to entorhinal cortex, the earliest site of pathologic tau deposition (Braak and Braak 1991), and posterior cingulate (Eichenbaum and Lipton 2008). Prior work has shown relatively increased long-range connectivity from the PHG in aging women as compared with men (Tomasi and Volkow 2012). Might early hyperconnectivity in women from this area relate to early tau pathology in the entorhinal cortex? Over time, could it precipitate posterior DMN hyperconnectivity?
Limitations and future directions
We show significant differences in DMN regions by sex in a large healthy aging population and highlight the importance of considering sex as an independent predictor rather than a nuisance covariate in neuroimaging studies of aging. The HCP-A data set has many advantages, including a broad age range (with later decades well-represented, particularly compared with other healthy aging data sets), an extended imaging protocol (including 24 min of resting state time for each individual), and detailed clinical and neuropsychological phenotyping. This data set, combined with the strengths of ICD, the robust voxel-based technique we used to assess within-network connectivity in DMN, allows us to overcome many of the challenges that faced prior studies evaluating DMN connectivity over the course of aging.
However, our study has several limitations. First, the HCP-A data set is cross-sectional, so we cannot draw conclusions about longitudinal changes in DMN connectivity for a given individual. Relatedly, the nature of a cognitively healthy aging data set is that individuals who have developed cognitive symptoms in later decades are excluded, whereas younger individuals who will go on to develop cognitive symptoms in later decades are not, meaning that the older decades included in this data set are enriched for less vulnerable individuals; we therefore view our findings in the oldest decades as less representative of the general population.
APOE genotype was not available for the participants in our sample. The APOE-ε4 allele is common (Corbo and Scacchi 1999), and is the single greatest genetic risk factor for AD. It also impacts women disproportionately (Payami et al. 1996; Ungar et al. 2014; Riedel et al. 2016). When APOE genotype becomes available for the HCP-A data set, it will be important to incorporate APOE genotype into future analyses to assess for its direct effects and interactions with sex on DMN connectivity. Prior studies assessing the impact of APOE genotype on DMN connectivity (which did not consider the impact of sex) show increased connectivity from the HIP, including specifically between HIP and DMN, which correlates with poorer memory performance, even in cognitively healthy individuals (Westlye et al. 2011; Badhwar et al. 2017; Wang et al. 2017; on behalf of Alzheimer’s Disease Neuroimaging Initiative et al. 2019; Zhu et al. 2019; Shafer et al. 2021). The interaction between sex and APOE genotype on functional connectivity across the brain has rarely been studied, though pioneering work by Damoiseaux et al. (2012) found decreased connectivity from the posterior DMN in ε4-positive women (but not men) relative to ε3 homozygotes; more recent work has begun to uncover the ways in which vascular risk factors and hippocampal volume relate to entorhinal tau and hippocampal connectivity in female ε4 carriers (Shafer et al. 2021; Petersen et al. 2022; Tsiknia et al. 2022). The HCP-A study does plan to release APOE genotypes for its participants (as well as 7 other SNP regions with strong associations to neurodegenerative disease and to AD in particular) (Bookheimer et al. 2019), and future analyses which incorporate these data will be critical.
Next, we chose to focus our analysis on sex differences specifically in within- and between-network DMN connectivity rather than assessing sex differences in the integrity of intrinsic functional connectivity networks across the brain. We opted to focus on the DMN because of the strong evidence that it is specifically implicated in amnestic AD and our interest in understanding women’s increased vulnerability to AD. However, this focus makes it challenging to situate our work, and prior work focusing on the DMN in aging, in the larger context of the literature around connectivity changes in healthy aging. Healthy aging cohorts typically show lower within-network connectivity across networks with older age, accompanied by increasing between-network connectivity, a process that has been termed dedifferentiation (Ferreira and Busatto 2013; Dennis and Thompson 2014; Grady et al. 2016; Spreng et al. 2016). Dedifferentiation is associated with decreased performance on tests of attention and short-term memory, regardless of age (Chan et al. 2014; Geerligs et al. 2015; Ng et al. 2016), and high levels of dedifferentiation, in turn, correlate with the presence of Alzheimer’s pathology (Cassady et al. 2021). Park and colleagues have hypothesized that, in the setting of dedifferentiation, increased recruitment of structures from other networks during tasks, which they term “scaffolding,” is a compensatory strategy (Park and Reuter-Lorenz 2009; Persson et al. 2014; Reuter-Lorenz and Park 2014; Belleville et al. 2021). In the context of this theory, individuals with higher dedifferentiation, whether at baseline or related to a pathologic process, might rely upon scaffolding in order to perform cognitive tasks successfully. In general, the literature suggests that women rely more upon between-network connections and have a greater degree of dedifferentiation over the course of aging, whereas men have a higher degree of modularity (Ingalhalikar et al. 2014; Rabipour et al. 2021; Subramaniapillai et al. 2022). While our work suggests that women do have relatively greater connectivity from PCC to certain regions outside of the DMN (as well as relatively greater within-network connectivity in posterior nodes), the design of our study precludes our being able to assess sex differences in dedifferentiation across intrinsic connectivity networks. With regard to scaffolding, while we do not assess directly for a scaffolding effect on memory performance, we do show that women’s higher performance on verbal memory testing is associated with higher connectivity between the PCC and AG, suggesting that women may be relying on within-network circuitry—rather than defaulting to between-network connections—to achieve their higher scores.
Finally, the work conducted here did not consider the effects of physiological noise affects connectivity estimates. Future studies could determine how factors like heart rate and respiratory rate collected during the scanning session impact sex differences in DMN connectivity.
Conclusion
While much remains to be learned, the present findings shed much-needed light on sex differences over the lifespan in DMN connectivity. The DMN has been well-characterized in the context of healthy aging, Alzheimer’s risk, and disease, but remarkably little work has focused on the impact of sex on DMN function, despite striking sex differences in AD risk and disease course. Here we highlight that there are meaningful sex differences in DMN connectivity over the lifespan, with relative hyperconnectivity in women in PHG in the 30s and 40s, followed by posterior cingulate and AG in the 40s and 50s, and later relative hypoconnectivity in medial prefrontal cortex. These findings have real-world implications in terms of memory performance and neuropsychiatric symptoms: we find that DMN connectivity metrics and cognitive and psychiatric outcomes are correlated even in healthy aging adults without overt cognitive or psychiatric symptoms.
Funding
This work was supported by awards to C.F. from the National Institutes of Health (5K23AG059919-04); the Alzheimer’s Association (2019-AACSF-644153); and the McCance Foundation. C.H. is supported by a Medical Scientist Training Program training grant (NIH/NIGMS T32GM007205). Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number U01AG052564 and by funds provided by the McDonnell Center for Systems Neuroscience at Washington University in St. Louis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement
None declared.
Supplementary Material
Contributor Information
Bronte Ficek-Tani, Department of Neurology, Yale School of Medicine, New Haven, CT 06520, United States.
Corey Horien, Interdepartmental Neuroscience Program, Yale School of Medicine, New Haven, CT 06520, United States.
Suyeon Ju, Department of Neurology, Yale School of Medicine, New Haven, CT 06520, United States.
Wanwan Xu, Department of Biostatistics, Yale School of Medicine, New Haven, CT 06520, United States.
Nancy Li, Department of Neurology, Yale School of Medicine, New Haven, CT 06520, United States.
Cheryl Lacadie, Department of Radiology and Biomedical Imaging, Yale School of Medicine, New Haven, CT 06520, United States.
Xilin Shen, Department of Radiology and Biomedical Imaging, Yale School of Medicine, New Haven, CT 06520, United States.
Dustin Scheinost, Department of Radiology and Biomedical Imaging, Yale School of Medicine, New Haven, CT 06520, United States.
Todd Constable, Department of Radiology and Biomedical Imaging, Yale School of Medicine, New Haven, CT 06520, United States.
Carolyn Fredericks, Department of Neurology, Yale School of Medicine, New Haven, CT 06520, United States.
References
- 2021 Alzheimer’s disease facts and figures . Alzheimers Dement. 2021:17:327–406. https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- Agüero-Torres H, Fratiglioni L, Guo Z, Viitanen M, Winblad B. Prognostic factors in very old demented adults: a seven-year follow-up from a population-based survey in Stockholm. J Am Geriatr Soc. 1998:46:444–452. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010:65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007:56:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badhwar A, Tam A, Dansereau C, Orban P, Hoffstaedter F, Bellec P. Resting-state network dysfunction in Alzheimer’s disease: a systematic review and meta-analysis. Alzheimers Dement. 2017:8:73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005:62:685–691. [DOI] [PubMed] [Google Scholar]
- Barron DS, Gao S, Dadashkarimi J, Greene AS, Spann MN, Noble S, Lake EMR, Krystal JH, Constable RT, Scheinost D. Transdiagnostic, Connectome-Based Prediction of Memory Constructs Across Psychiatric Disorders. Cerebral Cortex. 2021:31:2523–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean J. Rey auditory verbal learning test, Rey AVLT. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of clinical neuropsychology. New York (NY): Springer; 2011. pp. 2174–2175 [Google Scholar]
- Bean LA, Kumar A, Rani A, Guidi M, Rosario AM, Cruz PE, Golde TE, Foster TC. Re-opening the critical window for Estrogen therapy. J Neurosci. 2015:35:16077–16093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleville S, Mellah S, Cloutier S, Dang-Vu TT, Duchesne S, Maltezos S, Phillips N, Hudon C. Neural correlates of resilience to the effects of hippocampal atrophy on memory. NeuroImage: Clinical. 2021:29:102526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Dilharreguy B, Helmer C, Chanraud S, Amieva H, Dartigues J-F, Allard M, Catheline G. PCC characteristics at rest in 10-year memory decliners. Neurobiol Aging. 2015:36:2812–2820. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010:107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker ML, Bolla-Wilson K, Agnew J, Meyers DA. Age-related sex differences in verbal memory. J Clin Psychol. 1988:44:403–411. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Osuch EA, Lanius RA, Boksman K, Neufeld RWJ, Théberge J, Williamson P. Default mode network connectivity: effects of age, sex, and analytic approach. Neuroreport. 2008:19:887–891. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005:64:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Salat DH, Terpstra M, Ances BM, Barch DM, Buckner RL, Burgess GC, Curtiss SW, Diaz-Santos M, Elam JS, et al. The lifespan human connectome project in aging: an overview. NeuroImage. 2019:185:335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000:343:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991:82:239–259. [DOI] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, Holtzman DM, Morris JC, Ances BM. Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. J Neurosci. 2012:32:8890–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009:30:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RF, Mormino EC, Amariglio RE, Properzi MJ, Rabin JS, Lim YY, Papp KV, Jacobs HIL, Burnham S, Hanseeuw BJ, et al. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer’s disease: findings from three well-characterized cohorts. Alzheimers Dement. 2018:14:1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RF, Mormino EC, Rabin JS, Hohman TJ, Landau S, Hanseeuw BJ, Jacobs HIL, Papp KV, Amariglio RE, Properzi MJ, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 2019:76:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RF, O’Donnell A, McGrath ER, Jacobs HIL, Lois C, Satizabal CL, Ghosh S, Rubinstein ZB, Murabito JM, Sperling RA, et al. Menopause status moderates sex differences in tau burden: a Framingham PET study. Ann Neurol. 2022:92:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callen DJA, Black SE, Caldwell CB, Grady CL. The influence of sex on limbic volume and perfusion in AD. Neurobiol Aging. 2004:25:761–770. [DOI] [PubMed] [Google Scholar]
- Cassady KE, Adams JN, Chen X, Maass A, Harrison TM, Landau S, Baker S, Jagust W. Alzheimer’s pathology is associated with dedifferentiation of intrinsic functional memory networks in aging. Cereb Cortex. 2021:31:4781–4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavedo E, Chiesa PA, Houot M, Ferretti MT, Grothe MJ, Teipel SJ, Lista S, Habert M-O, Potier M-C, Dubois B, et al. Sex differences in functional and molecular neuroimaging biomarkers of Alzheimer’s disease in cognitively normal older adults with subjective memory complaints. Alzheimers Dement. 2018:14:1204–1215. [DOI] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U S A. 2014:111:E4997–E5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Duberstein PR, Sörensen S, Lyness JM. Gender differences in five factor model personality traits in an elderly cohort: extension of robust and surprising findings to an older generation. Personal Individ Differ. 2007:43:1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Britton JC, Pine DS, Cox RW. Linear mixed-effects modeling approach to FMRI group analysis. NeuroImage. 2013:73:176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieri F, Zhuang X, Cordes D, Kaplan N, Cummings J, Caldwell J, for the Alzheimer’s Disease Neuroimaging Initiative (ADNI) . Relationship of sex differences in cortical thickness and memory among cognitively healthy subjects and individuals with mild cognitive impairment and Alzheimer disease. Alzheimers Res Ther. 2022:14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a “thrifty” allele? Ann Hum Genet. 1999:63:301–310. [DOI] [PubMed] [Google Scholar]
- Costa PT Jr, McCrae RR. Revised NEO personality inventory (NEO-PI-R) and NEO five-factor inventory (NEO-FFI) professional manual. Odessa, Florida: Psychological Assessment Resources; 1992.
- Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, Rosen HJ, Miller BL, Kramer JH, Greicius MD. Gender modulates the APOE ε4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012:32:8254–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM. Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol Rev. 2014:24:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards L, La Joie R, Iaccarino L, Strom A, Baker SL, Casaletto KB, Cobigo Y, Grant H, Kim M, Kramer JH, et al. Multimodal neuroimaging of sex differences in cognitively impaired patients on the Alzheimer’s continuum: greater tau-PET retention in females. Neurobiol Aging. 2021:105:86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Lipton PA. Towards a functional organization of the medial temporal lobe memory system: role of the parahippocampal and medial entorhinal cortical areas. Hippocampus. 2008:18:1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Rani A, Yegla B, Barter J, Kumar A, Wolff CA, Esser K, Foster TC. Longitudinal characterization and biomarkers of age and sex differences in the decline of spatial memory. Front Aging Neurosci. 2020:12. https://www.frontiersin.org/articles/10.3389/fnagi.2020.00034/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev. 2013:37:384–400. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. PNAS. 2009:106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericks CA, Brown JA, Deng J, Kramer A, Ossenkoppele R, Rankin K, Kramer JH, Miller BL, Rabinovici GD, Seeley WW. Intrinsic connectivity networks in posterior cortical atrophy: a role for the pulvinar? Neuroimage Clin. 2019:21:101628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericks CA, Sturm VE, Brown JA, Hua AY, Bilgel M, Wong DF, Resnick SM, Seeley WW. Early affective changes and increased connectivity in preclinical Alzheimer’s disease. Alzheimers Dement. 2018:10:471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. A brain-wide study of age-related changes in functional connectivity. Cereb Cortex. 2015:25:1987–1999. [DOI] [PubMed] [Google Scholar]
- Grady C, Sarraf S, Saverino C, Campbell K. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging. 2016:41:159–172. [DOI] [PubMed] [Google Scholar]
- Greene AS, Gao S, Scheinost D, Constable RT. Task-induced brain state manipulation improves prediction of individual traits. Nat Commun. 2018:9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004:101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen NL, Lauritzen M, Mortensen EL, Osler M, Avlund K, Fagerlund B, Rostrup E. Subclinical cognitive decline in middle-age is associated with reduced task-induced deactivation of the brain’s default mode network. Hum Brain Mapp. 2014:35:4488–4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, Bookheimer SY, Brown TB, Buckner RL, Burgess GC, et al. Extending the human connectome project across ages: imaging protocols for the lifespan development and aging projects. NeuroImage. 2018:183:972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frölich L, Schönknecht P, Ito K, Mielke R, Kalbe E, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. NeuroImage. 2002:17:302–316. [DOI] [PubMed] [Google Scholar]
- Horien C, Noble S, Finn ES, Shen X, Scheinost D, Constable RT. Considering factors affecting the connectome-based identification process: comment on Waller et al. NeuroImage. 2018:169:172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horien C, Shen X, Scheinost D, Constable RT. The individual functional connectome is unique and stable over months to years. NeuroImage. 2019:189:676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-C, Hsieh W-J, Lee P-L, Peng L-N, Liu L-K, Lee W-J, Huang J-K, Chen L-K, Lin C-P. Age-related changes in resting-state networks of a large sample size of healthy elderly. CNS Neurosci Ther. 2015:21:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci. 2014:111:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L, Guo X, Duberstein PR, Hallstrom T, Waern M, Ostling S, Skoog I. Midlife personality and risk of Alzheimer disease and distress: a 38-year follow-up. Neurology. 2014:83:1538–1544. [DOI] [PubMed] [Google Scholar]
- Johansson M, Stomrud E, Lindberg O, Westman E, Johansson PM, van Westen D, Mattsson N, Hansson O. Apathy and anxiety are early markers of Alzheimer’s disease. Neurobiol Aging. 2020:85:74–82. [DOI] [PubMed] [Google Scholar]
- Jones DT, Knopman DS, Gunter JL, Graff-Radford J, Vemuri P, Boeve BF, Petersen RC, Weiner MW, Jack CR Jr, Alzheimer’s Disease Neuroimaging I . Cascading network failure across the Alzheimer’s disease spectrum. Brain. 2016:139:547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, MacHulda MM, Vemuri P, McDade EM, Zeng G, Senjem ML, Gunter JL, Przybelski SA, Avula RT, Knopman DS, et al. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology. 2011:77:1524–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Scheinost D, Okuda H, Belhachemi D, Murphy I, Staib LH, Papademetris X. Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics. 2011:9:69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y, Horien C, Chen W, Guo W, Lu X, Sun J, Dong Q, Liu B, Liu J, Yan D, et al. Connectome-based models can predict early symptom improvement in major depressive disorder. J Affect Disord. 2020:273:442–452. [DOI] [PubMed] [Google Scholar]
- Kang DW, Wang S-M, Um YH, Na H-R, Kim N-Y, Lee CU, Lim HK. Distinctive association of the functional connectivity of the posterior cingulate cortex on memory performances in early and late amnestic mild cognitive impairment patients. Front Aging Neurosci. 2021:13. https://www.frontiersin.org/articles/10.3389/fnagi.2021.696735/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kong R, Liégeois R, Orban C, Tan Y, Sun N, Holmes AJ, Sabuncu MR, Ge T, Thomas Yeo BT. Global signal regression strengthens association between resting-state functional connectivity and behavior. NeuroImage. 2019:196:126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muñiz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, et al. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008:11:334–343. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, Anderson JS, Ferguson MA, Yurgelun-Todd D. Local brain connectivity and associations with gender and age. Dev Cogn Neurosci. 2011:1:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhoff ES, Rosenberg M, Chiang J, Zhang K, Pickard JD, Owen AM, Monti MM. Optimized brain extraction for pathological brains (optiBET). PLoS One. 2014:9:e115551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagurski B, Deschwanden PF, Jäncke L, Mérillat S. Longitudinal functional connectivity patterns of the default mode network in healthy older adults. NeuroImage. 2022:259:119414. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Smiljic A, Hayenga AO, Onami SH, Greicius MD, Rabinovici GD, Janabi M, Baker SL, Yen IV, Madison CM, et al. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex. 2011:21:2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Guyara-Quinn C, McHugh P, Petrongolo G, Osorio RS, Connaughty C, Pupi A, Vallabhajosula S, Isaacson RS, et al. Perimenopause and emergence of an Alzheimer’s bioenergetic phenotype in brain and periphery. PLoS One. 2017a:12:e0185926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Varsavsky I, Osorio RS, Pupi A, Vallabhajosula S, Isaacson RS, et al. Sex differences in Alzheimer risk: brain imaging of endocrine vs chronologic aging. Neurology. 2017b:89:1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005:53:695–699. [DOI] [PubMed] [Google Scholar]
- Natu VS, Lin J-J, Burks A, Arora A, Rugg MD, Lega B. Stimulation of the posterior cingulate cortex impairs episodic memory encoding. J Neurosci. 2019:39:7173–7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KK, Lo JC, Lim JKW, Chee MWL, Zhou J. Reduced functional segregation between the default mode network and the executive control network in healthy older adults: a longitudinal study. NeuroImage. 2016:133:321–330. [DOI] [PubMed] [Google Scholar]
- Noble S, Spann MN, Tokoglu F, Shen X, Constable RT, Scheinost D. Influences on the test–retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cereb Cortex. 2017:27:5415–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- on behalf of Alzheimer’s Disease Neuroimaging Initiative, Zhu Y, Gong L, He C, Wang Q, Ren Q, Xie C. Default mode network connectivity moderates the relationship between the APOE genotype and cognition and individualizes identification across the Alzheimer’s disease spectrum. JADA. 2019:70:843–860. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009:60:173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, Yu CE, Wijsman EM, Heston LL, Litt M, et al. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet. 1996:58:803–811. [PMC free article] [PubMed] [Google Scholar]
- Persson J, Pudas S, Nilsson L-G, Nyberg L. Longitudinal assessment of default-mode brain function in aging. Neurobiol Aging. 2014:35:2107–2117. [DOI] [PubMed] [Google Scholar]
- Petersen KK, Grober E, Lipton RB, Sperling RA, Buckley RF, Aisen PS, Ezzati A. Impact of sex and APOE ε4 on the association of cognition and hippocampal volume in clinically normal, amyloid positive adults. Alzheimers Dement. 2022:14:e12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Plitt M, Laumann TO, Martin A. Sources and implications of whole-brain fMRI signals in humans. NeuroImage. 2017:146:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Python Language Reference . n.d.
- Qi Z, Wu X, Wang Z, Zhang N, Dong H, Yao L, Li K. Impairment and compensation coexist in amnestic MCI default mode network. NeuroImage. 2010:50:48–55. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019.
- Rabipour S, Rajagopal S, Pasvanis S, Rajah MN. Generalization of memory-related brain function in asymptomatic older women with a family history of late onset Alzheimer’s disease: results from the PREVENT-AD cohort. Neurobiol Aging. 2021:104:42–56. [DOI] [PubMed] [Google Scholar]
- Rahman A, Schelbaum E, Hoffman K, Diaz I, Hristov H, Andrews R, Jett S, Jackson H, Lee A, Sarva H, et al. Sex-driven modifiers of Alzheimer risk: a multimodality brain imaging study. Neurology. 2020:95:e166–e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Weiss BK, Jacobs EG, Cherkerzian S, Klibanski A, Remington A, Aizley H, Goldstein JM. Sex differences in episodic memory in early midlife: impact of reproductive aging. Menopause. 2017:24:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Park DC. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol Rev. 2014:24:355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol. 2016:160:134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, Harris MA, Alderson HL, Hunter S, Neilson E, et al. Sex differences in the adult human brain: evidence from 5216 UK biobank participants. Cereb Cortex. 2018:28:2959–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolison M, Lacadie C, Chawarska K, Spann M, Scheinost D. Atypical intrinsic hemispheric interaction associated with autism spectrum disorder is present within the first year of life. Cereb Cortex. 2022:32:1212–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt M, Rodriguez R, Westwater ML, Horien C, Greene AS, Constable RT, Noble S, Scheinost D. Connectome-based machine learning models are vulnerable to subtle data manipulations. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Benjamin J, Lacadie C, Vohr B, Schneider K, Ment L, Papademetris X, Constable R. The intrinsic connectivity distribution: a novel contrast measure reflecting voxel level functional connectivity. NeuroImage. 2012:62:1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013:64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Finn ES, Tokoglu F, Shen X, Papademetris X, Hampson M, Constable RT. Sex differences in normal age trajectories of functional brain networks. Hum Brain Mapp. 2015:36:1524–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Tokoglu F, Hampson M, Hoffman R, Constable RT. Data-driven analysis of functional connectivity reveals a potential auditory verbal hallucination network. Schizophr Bull. 2019:45:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer O, Rahman A, Hristov H, Berkowitz C, Isaacson RS, Diaz Brinton R, Mosconi L. Female sex and Alzheimer’s risk: the menopause connection. J Prev Alzheimers Dis. 2018:5:225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz AP, Chhatwal JP, Hedden T, Mormino EC, Hanseeuw BJ, Sepulcre J, Huijbers W, LaPoint M, Buckley RF, Johnson KA, et al. Phases of hyperconnectivity and hypoconnectivity in the default mode and salience networks track with amyloid and tau in clinically normal individuals. J Neurosci. 2017:37:4323–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer AT, Lori B-H, An Y, Williams OA, Huo Y, Landman BA, Caffo BS, Resnick SM. Default mode network connectivity and cognition in the aging brain: the effects of age, sex, and APOE genotype. Neurobiol Aging. 2021:104:10–23. [DOI] [PubMed] [Google Scholar]
- Shah D, Praet J, Latif Hernandez A, Hofling C, Anckaerts C, Bard F, Morawski M, Detrez JR, Prinsen E, Villa A, et al. Early pathologic amyloid induces hypersynchrony of BOLD resting-state networks in transgenic mice and provides an early therapeutic window before amyloid plaque deposition. Alzheimers Dement. 2016:12:964–976. [DOI] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AMC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009:19:2209–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010:67:584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, LaViolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009:63:178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Viviano JD, Schacter DL. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol Aging. 2016:45:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffaroni AM, Brown JA, Casaletto KB, Elahi FM, Deng J, Neuhaus J, Cobigo Y, Mumford PS, Walters S, Saloner R, et al. The longitudinal trajectory of default mode network connectivity in healthy older adults varies as a function of age and is associated with changes in episodic memory and processing speed. J Neurosci. 2018:38:2809–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniapillai S, Rajagopal S, Ankudowich E, Pasvanis S, Misic B, Rajah MN. Age- and episodic memory-related differences in task-based functional connectivity in women and men. J Cogn Neurosci. 2022:34:1500–1520. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Aging and functional brain networks. Mol Psychiatry. 2012:17:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008:18:2553–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschanz JT, Corcoran CD, Schwartz S, Treiber K, Green RC, Norton MC, Mielke MM, Piercy K, Steinberg M, Rabins PV, et al. Progression of cognitive, functional and neuropsychiatric symptom domains in a population cohort with Alzheimer’s dementia the Cache County dementia progression study. Am J Geriatr Psychiatry. 2011:19:532–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiknia AA, Reas E, Bangen KJ, Sundermann EE, McEvoy L, Brewer JB, Edland SD, Banks SJ, Alzheimer’s Disease Neuroimaging Initiative . Sex and APOE ɛ4 modify the effect of cardiovascular risk on tau in cognitively normal older adults. Brain Commun. 2022:4:fcac035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar L, Altmann A, Greicius MD. Apolipoprotein E, gender, and Alzheimer’s disease: an overlooked, but potent and promising interaction. Brain Imaging Behav. 2014:8:262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Luckey A, McLeod SL, Robertson IH, To WT. Impaired posterior cingulate cortex–parahippocampus connectivity is associated with episodic memory retrieval problems in amnestic mild cognitive impairment. Eur J Neurosci. 2021:53:3125–3141. [DOI] [PubMed] [Google Scholar]
- Wang Z, Dai Z, Shu H, Liao X, Yue C, Liu D, Guo Q, He Y, Zhang Z. APOE genotype effects on intrinsic brain network connectivity in patients with amnestic mild cognitive impairment. Sci Rep. 2017:7:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman-Fogel I, Moayedi M, Taylor KS, Pope G, Davis KD. Cognitive and default-mode resting state networks: do male and female brains “rest” differently? Hum Brain Mapp. 2010:31:1713–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye ET, Lundervold A, Rootwelt H, Lundervold AJ, Westlye LT. Increased hippocampal default mode synchronization during rest in middle-aged and elderly APOE ε4 carriers: relationships with memory performance. J Neurosci. 2011:31:7775–7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JR, Mander BA, Helfrich RF, Maass A, Harrison TM, Baker SL, Knight RT, Jagust WJ, Walker MP. Sleep as a potential biomarker of tau and β-amyloid burden in the human brain. J Neurosci. 2019:39:6315–6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Kang SS, Wang Z, Liu X, Kuo T-C, Korkmaz F, Padilla A, Miyashita S, Chan P, Zhang Z, et al. FSH blockade improves cognition in mice with Alzheimer’s disease. Nature. 2022:603:470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011:106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonker JE, Adolfsson R, Eriksson E, Hellstrand M, Nilsson L-G, Herlitz A. Verified hormone therapy improves episodic memory performance in healthy postmenopausal women. Aging Neuropsychol Cognit. 2006:13:291–307. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Gong L, He C, Wang Q, Ren Q, Xie C, Initiative on behalf of ADN. Default mode network connectivity moderates the relationship between the APOE genotype and cognition and individualizes identification across the Alzheimer’s disease spectrum. J Alzheimers Dis. 2019:70:843–860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The HCP-A data that support the findings of this study are publicly available through the NIMH Data Archive (https://nda.nih.gov/edit_collection.html?id=2847). The preprocessing software can be freely accessed at https://bioimagesuiteweb.github.io/webapp/. R code to run correlation analyses between neuropsychological measures and seed connectivity values and Python code to conduct the connectivity by age analyses (linear regressions, boxplots, and t-test computation) can be found at https://github.com/frederickslab/connectivity_analysis.