Abstract
Purpose:
The final analyses of the INSIGHT phase II study evaluating tepotinib (a selective MET inhibitor) plus gefitinib versus chemotherapy in patients with MET-altered EGFR-mutant NSCLC (data cut-off: September 3, 2021).
Patients and Methods:
Adults with advanced/metastatic EGFR-mutant NSCLC, acquired resistance to first-/second-generation EGFR inhibitors, and MET gene copy number (GCN) ≥5, MET:CEP7 ≥2, or MET IHC 2+/3+ were randomized to tepotinib 500 mg (450 mg active moiety) plus gefitinib 250 mg once daily, or chemotherapy. Primary endpoint was investigator-assessed progression-free survival (PFS). MET-amplified subgroup analysis was preplanned.
Results:
Overall (N = 55), median PFS was 4.9 months versus 4.4 months [stratified HR, 0.67; 90% CI, 0.35–1.28] with tepotinib plus gefitinib versus chemotherapy. In 19 patients with MET amplification (median age 60.4 years; 68.4% never-smokers; median GCN 8.8; median MET/CEP7 2.8; 89.5% with MET IHC 3+), tepotinib plus gefitinib improved PFS (HR, 0.13; 90% CI, 0.04–0.43) and overall survival (OS; HR, 0.10; 90% CI, 0.02–0.36) versus chemotherapy. Objective response rate was 66.7% with tepotinib plus gefitinib versus 42.9% with chemotherapy; median duration of response was 19.9 months versus 2.8 months. Median duration of tepotinib plus gefitinib was 11.3 months (range, 1.1–56.5), with treatment >1 year in six (50.0%) and >4 years in three patients (25.0%). Seven patients (58.3%) had treatment-related grade ≥3 adverse events with tepotinib plus gefitinib and five (71.4%) had chemotherapy.
Conclusions:
Final analysis of INSIGHT suggests improved PFS and OS with tepotinib plus gefitinib versus chemotherapy in a subgroup of patients with MET-amplified EGFR-mutant NSCLC, after progression on EGFR inhibitors.
Translational Relevance.
There is a high unmet need for patients with EGFR-mutant non–small cell lung cancer (NSCLC) that have developed resistance to EGFR inhibitors. We present final analyses from the phase II part of INSIGHT, evaluating tepotinib (a once-daily, highly selective MET inhibitor) plus gefitinib versus chemotherapy in patients with EGFR-mutant NSCLC and MET-driven EGFR inhibitor resistance, with a median follow-up duration of 57.5 months. This long follow-up emphasizes that the greatest benefit from tepotinib plus gefitinib is derived by patients with MET amplification, who showed substantially improved progression-free and overall survival versus chemotherapy in this updated analysis. Furthermore, all patients who received long-term tepotinib plus gefitinib had MET amplification, with 25% of patients with MET amplification receiving combination treatment for >4 years, and 17% for >5 years (including continuing treatment outside the study). Tepotinib plus an EGFR inhibitor is a promising strategy in this disease setting.
Introduction
EGFR tyrosine kinase inhibitors (TKIs) are the standard of care in patients with EGFR-mutant metastatic non–small cell lung cancer (NSCLC), although most patients ultimately develop resistance to EGFR TKI monotherapy (1–3). Across first- to third-generation EGFR TKIs, the main EGFR-independent resistance mechanism is dysregulation of mesenchymal–epithelial transition factor (MET) signaling—for example, due to MET amplification (4, 5)—which has been reported in up to 30% of patients with acquired resistance (6, 7). There is currently no clear-cut treatment strategy in this setting, and these patients have a high unmet need for new treatments. Promising results, however, have been obtained with MET TKIs in combination with EGFR TKIs in EGFR-mutant MET-amplified NSCLC (8–14). These data underscore a need to accurately test for MET amplification to determine who may benefit from such treatment.
Tepotinib, a once-daily and highly selective MET TKI, is approved in multiple countries for the treatment of patients with NSCLC with MET exon 14 (METex14) skipping. In this setting, tepotinib has demonstrated robust and durable clinical activity, with a manageable safety profile (15–17). Tepotinib is also being investigated in combination with EGFR TKI therapy in EGFR-mutant MET-amplified NSCLC (6, 9). The phase Ib/II INSIGHT trial evaluated tepotinib plus gefitinib versus chemotherapy in patients with EGFR-mutant NSCLC who had progressed on an EGFR TKI due to MET amplification or moderate (IHC 2+) or high (IHC 3+) MET overexpression (9). In the 18-month follow-up analysis (data cut-off: December 12, 2018; median follow-up: 21.8 months), no significant difference was observed in the overall population, in which 38% of patients had moderate MET overexpression. However, substantial improvements in progression-free survival (PFS) and overall survival (OS) were seen in the preplanned subgroup analysis of patients with high MET overexpression or MET amplification (9). In patients with MET amplification, median PFS was 16.6 versus 4.2 months [HR, 0.13; 90% confidence interval (CI), 0.04–0.43], median OS was 37.3 months versus 13.1 months (HR, 0.08; 90% CI, 0.01–0.51), objective response rate (ORR) was 67% versus 43%, and median duration of response (DOR) was 19.9 versus 2.8 months with tepotinib plus gefitinib versus chemotherapy, respectively (9). Here, we report the final analyses from phase II of the INSIGHT trial, after an extended duration of follow-up, with a focus on patients with MET amplification and an update on patients with high MET overexpression.
Patients and Methods
Study design
The design of the open-label, phase Ib/II, multicenter, randomized INSIGHT trial has been published previously (9). The phase II part evaluated the efficacy and safety of tepotinib plus gefitinib compared with chemotherapy in patients with EGFR-mutant T790M-negative, MET-dysregulated NSCLC. Following the previous 18-month analysis (data cut-off: December 12, 2018; ref. 9), this final analysis (data cut-off: September 3, 2021) was conducted to assess updated outcomes with more robust estimation of time-dependent efficacy endpoints owing to the longer duration of follow-up.
Patients were enrolled from multiple sites across China, South Korea, Taiwan, Singapore, Japan, and Malaysia. All patients provided written informed consent. The trial was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice, local laws and applicable regulatory requirements, and was approved by the institutional review board or independent ethics committee of each center. INSIGHT is registered with ClinicalTrials.gov (NCT01982955) and the European Union Drug Regulating Authorities Clinical Trials Database, Eudra-CT 2016–001604–28.
Eligibility criteria
Adults with advanced or metastatic NSCLC with an activating EGFR mutation, acquired resistance to prior first- or second-generation EGFR TKI therapy (gefitinib, erlotinib, icotinib, or afatinib), T790M-negative status, and MET amplification or MET overexpression were enrolled. MET amplification was centrally evaluated by FISH [Q2 Solutions Sponsor-Specific MET IQ FISH Kit-111480 Assay (Dako Denmark A/S)] and defined as mean gene copy number (GCN) of ≥5, or MET to centromere of chromosome 7 (CEP7) ratio of ≥2:1. MET overexpression was determined centrally by IHC (D1C1 antibody) and defined as IHC 2+ (moderate) or 3+ (high).
Treatment administration
Patients were randomized to receive tepotinib 500 mg (450 mg active moiety) plus gefitinib 250 mg once daily, or chemotherapy [pemetrexed 500 mg/m², plus cisplatin 75 mg/m² or carboplatin (AUC 5–6), intravenously on day 1 of each 21-day cycle] for up to six 21-day cycles or four cycles plus pemetrexed maintenance. Treatment allocation was initially randomized 1:1 then changed to 2:1 following a protocol amendment, and was stratified by MET status (IHC2+ vs. IHC3 vs. MET amplification) and previous EGFR TKI treatment. Treatment was continued until disease progression, intolerance, or consent withdrawal, or until six combination chemotherapy cycles had been administered.
Study endpoints and assessments
The primary endpoint was PFS by investigator assessment. Secondary endpoints were PFS [assessed by an independent review committee (IRC)], OS, objective response (partial response or complete response as best overall response according to investigator assessment), best overall response, disease control (complete response, partial response or stable disease), DOR (by investigator assessment), and safety. Tumors were assessed at baseline and every 6 weeks by CT scan or MRI (RECIST v1.1). Adverse events (AEs) were assessed using the NCI-CTCAE v4.0.
Statistical analysis
As reported previously (9), this study was prematurely terminated due to difficulties identifying patients meeting eligibility criteria; hence, all analyses should be considered exploratory. The planned sample size of 156 patients was determined by 111 PFS events required to ensure 80% power with a two-sided significance level of 10% for rejecting the null hypothesis of equal treatment effect (assuming a true HR of 0.6, expected median PFS of 8.3 months vs. 5.0 months with tepotinib plus gefitinib versus chemotherapy, an accrual period of 39 months, follow-up of 9 months, dropout rate of 15%, and one nonbinding futility analysis with a boundary of α0 = 0.81 after 50% PFS events). Randomized patients were analyzed on an intent-to-treat basis for baseline characteristics and efficacy, whereas all patients who received at least one dose of any study drug were assessed for safety. Subgroup analyses in patients with MET amplification or high MET overexpression (IHC 3+) were preplanned. Time-dependent endpoints were analyzed by Kaplan–Meier methods. PFS and OS were compared between groups by Cox proportional hazards models, which were used to estimate HRs and their corresponding 90% CIs. Cox models for the overall population were stratified according to type of MET alteration and prior EGFR TKI; Cox models for subgroups were unstratified. Objective response and disease control were summarized as rates with the corresponding 90% Clopper–Pearson CIs.
Ongoing treatment outside the study
Patients with tepotinib plus gefitinib ongoing at the end of the study could continue treatment outside the study through early access. Investigators provided further data on treatment, response, and safety outside the study up to October 2022.
Data availability
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the data-sharing policy of the healthcare business of Merck KGaA, Darmstadt, Germany. All requests should be submitted in writing to the data-sharing portal of the healthcare business of Merck KGaA, Darmstadt, Germany: https://www.emdgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html.
Results
Baseline characteristics
In the overall population, there were 31 (56%) patients who received tepotinib plus gefitinib and 24 (44%) patients who received chemotherapy (Supplementary Table S1). The median age of all patients was 60.4 years (range, 42–82), 58.2% were female, all were Asian, 67.3% had never smoked, and 80% had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 1. The median duration of prior EGFR TKI therapy was 12 months (range, 2.9–54.0), and the most common prior EGFR TKIs were gefitinib (60%) and afatinib (14.5%). Representativeness of study participants is described in Supplementary Table S2.
Of 55 patients randomized, 19 (34.5%) had MET amplification, of whom 12 received tepotinib plus gefitinib and seven received chemotherapy (Table 1). Median GCN was 8.8 (range, 4.8–29.5), median MET/CEP7 ratio was 2.8 (range, 1.0–20.3), and 17 patients (89.5%) also had MET IHC 3+. The median age of patients was 60.4 years (range, 42–74), 68.4% were female, all were Asian, 68.4% had never smoked, and 73.7% had an ECOG PS of 1. The median duration of prior EGFR TKI therapy was 10.0 months (range, 4.0–46.9), and the most common prior EGFR TKIs were gefitinib (57.9%) and afatinib (21.1%).
Table 1.
Baseline characteristics of patients with MET amplification.
| Tepotinib + gefitinib | Chemotherapy | ||
|---|---|---|---|
| Baseline characteristics | (n = 12) | (n = 7) | |
| Median age, years (range) | 59.3 (42–70) | 60.4 (44–74) | |
| Sex, n (%) | Female | 9 (75.0) | 4 (57.1) |
| Male | 3 (25.0) | 3 (42.9) | |
| Smoking history, n (%) | Never smoker | 9 (75.0) | 4 (57.1) |
| Prior smoker | 3 (25.0) | 2 (28.6) | |
| Current smoker | 0 | 1 (14.3) | |
| ECOG PS, n (%) | 0 | 3 (25.0) | 2 (28.6) |
| 1 | 9 (75.0) | 5 (71.4) | |
| EGFR mutation, n (%) | Del19 | 7 (58.3) | 3 (42.9) |
| L858R | 4 (33.3) | 4 (57.1) | |
| G719X | 1 (8.3) | 0 | |
| MET status, n (%) | METamp | 12 (100) | 7 (100) |
| MET GCN ≥5 | 11 (91.7) | 7 (100) | |
| MET:CEP7 ≥2 | 7 (58.3) | 6 (85.7) | |
| MET IHC 3+ | 11 (91.7) | 6 (85.7) | |
| Prior EGFR TKI | TKI, n (%) | ||
| Gefitinib | 6 (50.0) | 5 (71.4) | |
| Afatinib | 2 (16.7) | 2 (28.6) | |
| Erlotinib | 2 (16.7) | 0 | |
| Icotinib | 2 (16.7) | 0 | |
| Median duration, months (range) | 10.6 (4.0–46.9) | 9.5 (6.2–13.0) | |
Abbreviations: CEP7, centromere of chromosome 7; ECOG PS, Eastern Cooperative Oncology Group performance status; GCN, gene copy number; IHC, immunohistochemistry; METamp, MET amplification.
Efficacy in the overall population
Median duration of follow-up in the overall population was 57.5 months. Similarly to previous timepoints (9), efficacy outcomes were similar between the tepotinib plus gefitinib (n = 31) versus the chemotherapy treatment arm (n = 24; Supplementary Fig. S1), respectively. Median PFS (by investigator assessment) was 4.9 months versus 4.4 months (stratified HR, 0.67; 90% CI, 0.35–1.28); median PFS (by IRC) was 10.2 months versus 4.3 months (stratified HR, 0.47; 90% CI, 0.21–1.03); and median OS was 17.3 months versus 19.5 months (stratified HR, 0.67; 90% CI, 0.34–1.32). ORR was 45.2% (90% CI, 29.7–61.3) versus 33.3% (90% CI, 17.8–52.1; stratified OR, 1.99; 90% CI, 0.56–6.87), and median DOR was 7.0 months (90% CI, 4.1–19.9) versus 4.6 months (90% CI, 2.8–5.6) in the tepotinib plus gefitinib arm versus the chemotherapy arm, respectively.
Efficacy in patients with MET amplification
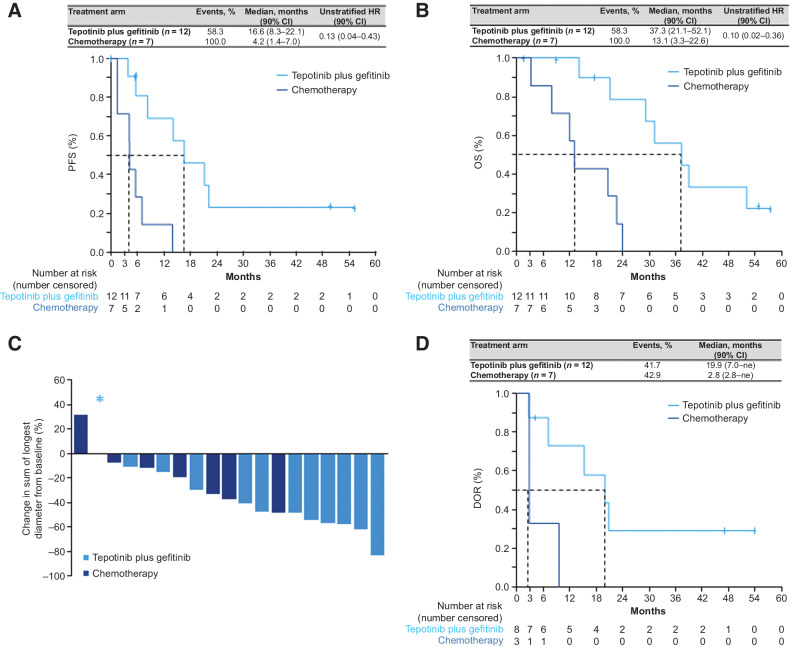
Preplanned subgroup analyses in patients with MET amplification suggested promising results for treatment with tepotinib plus gefitinib. The minimum follow-up duration in patients with MET amplification was 50 months. PFS by investigator assessment was greatly improved in the tepotinib plus gefitinib treatment arm (median 16.6 months; 90% CI, 8.3–22.1) versus the chemotherapy arm (median 4.2 months; 90% CI, 1.4–7.0), with an unstratified HR of 0.13 (90% CI, 0.04–0.43; Fig. 1A). Median PFS by IRC showed similar improvement with tepotinib plus gefitinib versus chemotherapy at 19.3 months (90% CI, 5.6–22.1) versus 4.2 months (90% CI, 1.4–7.0), respectively (unstratified HR, 0.16; 90% CI, 0.05–0.52; Supplementary Fig. S2). Patients also showed a marked improvement in median OS when treated with tepotinib plus gefitinib (37.3 months; 90% CI, 21.1–52.1) versus chemotherapy (13.1 months; 90% CI, 3.3–22.6; unstratified HR, 0.10; 90% CI, 0.02–0.36; Fig. 1B).
Figure 1.
Efficacy outcomes in patients with MET amplification. A, PFS per investigator. B, OS. C, Best relative change in SOLD per investigator. D, DOR per investigator. DOR, duration of response; HR, hazard ratio; ne, not estimable; OS, overall survival; PFS, progression-free survival; SOLD, sum of longest diameters. *, Denotes a patient with tepotinib plus gefitinib with no change in SOLD.
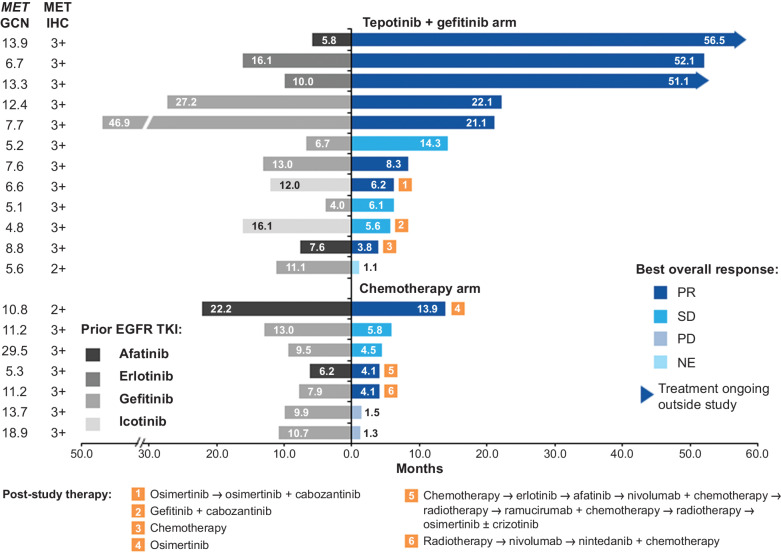
ORR was higher in the tepotinib plus gefitinib arm (66.7%; 90% CI, 39.1–87.7) than in the chemotherapy arm (42.9%; 90% CI, 12.9–77.5), with an OR of 2.67 (90% CI, 0.37–19.6). Tumor stability or shrinkage was attained in over 90% of patients who received tepotinib plus gefitinib and over 85% of patients who received chemotherapy (Fig. 1C). Disease control rate was 91.7% (90% CI, 66.1–99.6) in patients treated with tepotinib plus gefitinib versus 71.4% (90% CI, 34.1–94.7) in patients treated with chemotherapy. Median DOR was substantially longer with tepotinib plus gefitinib [19.9 months; 90% CI, 7.0–not estimable (ne)] than with chemotherapy (2.8 months; 90% CI, 2.8–ne; Fig. 1D). Six patients (31.6%) received poststudy therapies, which included TKIs in two patients (16.7%) in the tepotinib plus gefitinib arm and three patients (42.9%) in the chemotherapy arm (Fig. 2).
Figure 2.
Treatment duration and best overall response in patients with MET amplification. NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Treatment duration in patients with MET amplification
Patients in the tepotinib plus gefitinib arm received treatment for a median duration of 11.3 months (range, 1.1–56.5), whereas patients in the chemotherapy arm received pemetrexed and either cisplatin or carboplatin for median durations of 4.1 months (1.4–13.9), 2.8 months (1.5–5.8), and 2.9 months (1.4–4.5), respectively. The duration of tepotinib plus gefitinib was more than 1 year in six patients (50.0%) and more than 4 years in three patients (25.0%; hereafter referred to as patients 1–3; Fig. 2). The duration of tepotinib plus gefitinib appeared unrelated to the duration of prior EGFR TKI therapy. However, patients with a longer duration of tepotinib plus gefitinib treatment tended to have a higher MET GCN (Fig. 2). At the end of the trial, patients 1 and 2 transitioned to receive treatment through early access; both were still on treatment as of October 2022, each with a total treatment duration of over 5 years.
Efficacy in patients with high MET overexpression (IHC 3+)
Of 55 patients, 34 (61.8%) had high MET overexpression (IHC 3+), of whom 19 were included in the tepotinib plus gefitinib arm and 15 in the chemotherapy arm (Supplementary Table S3). A total of 17 patients (50.0%) with MET IHC 3+ also had MET amplification. The median age of patients with MET IHC 3+ was 55.8 years (range, 42–82 years), 58.8% were female, 73.5% had never smoked, and 76.5% had an ECOG PS of 1. Patients received tepotinib plus gefitinib for a median duration of 6.2 months (range, 2.8–56.5), whereas patients in the chemotherapy arm received pemetrexed and either cisplatin or carboplatin for median durations of 4.2 months (0.7–8.1), 2.8 months (0.7–5.8), and 2.9 months (1.4–4.5), respectively.
In patients with MET IHC 3+, tepotinib plus gefitinib markedly improved PFS by investigator assessment versus chemotherapy (median 8.3 months vs. 4.4 months, respectively; unstratified HR, 0.35; 90% CI, 0.17–0.74), as well as PFS by IRC (median 19.3 months vs. 4.4 months, respectively; unstratified HR, 0.28; 90% CI, 0.11–0.69), and OS (median 29.1 months vs. 17.9 months, respectively; unstratified HR, 0.44; 90% CI, 0.23–0.84; Supplementary Fig. S3). ORR was 68.4% (90% CI, 47.0–85.3) in the tepotinib plus gefitinib arm versus 33.3% (90% CI, 14.2–57.7) in the chemotherapy arm (OR, 4.3; 90% CI, 1.03–18.3). In these patients, median DOR was 8.7 months (90% CI, 4.2, 19.9) versus 2.8 months (90% CI, 2.8–ne) in the tepotinib plus gefitinib arm versus the chemotherapy arm, respectively.
Safety
In the overall population, grade ≥3 treatment-related AEs (TRAEs) were reported in 51.6% versus 52.2% of patients in the tepotinib plus gefitinib arm versus the chemotherapy arm, respectively. The most frequently reported TRAEs in patients treated with tepotinib plus gefitinib were diarrhea (58.1%; grade ≥3: 9.7%), increased alanine aminotransferase (32.3%; grade ≥3: 3.2%), and peripheral edema (29.0%; grade ≥3: 6.5%); in patients treated with chemotherapy, they were nausea (56.5%; grade ≥3: 4.3%), anemia (56.5%; grade ≥3: 30.4%), and decreased white blood cell count (52.5%; grade ≥3: 8.7%; Supplementary Fig. S4). In the tepotinib plus gefitinib arm versus the chemotherapy arm, TEAEs of any cause led to permanent discontinuation of any treatment in 9.7% versus 4.3%, and TEAEs led to dose reduction of any treatment in 16.1% versus 17.4%, respectively. The TEAEs that led to permanent discontinuation of any treatment were periodontal disease, peripheral edema, atypical pneumonia, and decreased weight (all 3.2%) in the tepotinib plus gefitinib arm, and chest discomfort, facial edema, malaise, decreased appetite, and dyspnea (all 4.3%) in the chemotherapy arm. The most common TEAEs (>5%) that led to dose reduction of any treatment were peripheral edema (9.7%) in the tepotinib plus gefitinib arm, and asthenia, increased blood creatinine, decreased neutrophil count, hypochloremia, hyponatremia, hypoproteinemia, and inadequate diet (all 8.7%) in the chemotherapy arm.
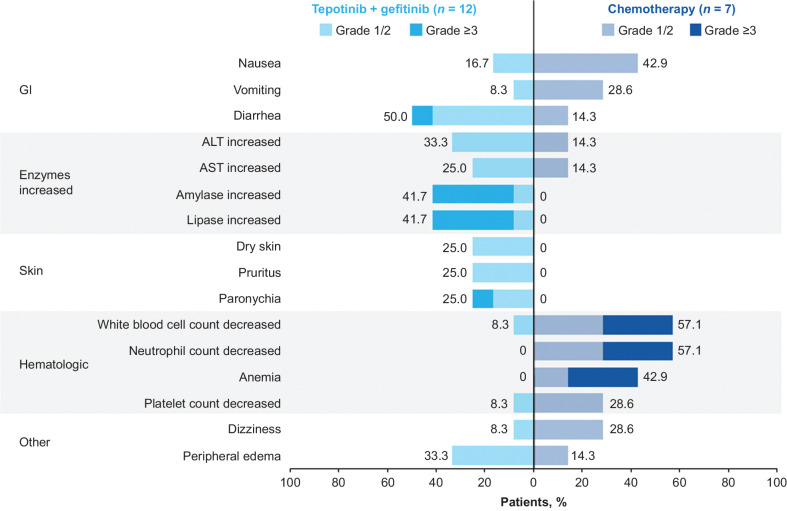
Despite patients with MET amplification having a longer treatment duration with tepotinib plus gefitinib (median 11.3 months versus 4.9 months in the overall population), safety in the overall population and in patients with MET amplification was comparable. Across patients with MET amplification, 58.3% in the tepotinib plus gefitinib arm and 71.4% in the chemotherapy arm had a grade ≥3 TRAE. The most frequently reported TRAEs in the tepotinib plus gefitinib arm were diarrhea (50%; grade ≥3: 8.3%), amylase increase and lipase increase (both 41.7%; grade ≥3: 33.3%), and peripheral edema (33.3%; grade ≥3: 0%; Fig. 3). The most frequently reported TRAEs in the chemotherapy arm were white blood cell count decrease and neutrophil count decrease (both 57.1%; grade ≥3: 28.6%), anemia (42.9%; grade ≥3: 28.6%), and nausea (42.9%; grade ≥3: 0%). TEAEs led to permanent discontinuation of any treatment in 16.7% of patients in the tepotinib plus gefitinib arm and no patients receiving chemotherapy, and to dose reduction of any treatment in 16.7% and 14.3% of patients, respectively.
Figure 3.
TRAEs in ≥20% of patients with MET amplification in either treatment arm. Numbers in black indicate the percentages of patients with TRAEs of any grade. ALT, alanine aminotransferase; AST, aspartate transaminase; GI, gastrointestinal.
Discussion
In this first randomized trial of a combination of a MET TKI plus an EGFR TKI versus chemotherapy, in patients with EGFR-mutant NSCLC who had progressed on an EGFR TKI, tepotinib plus gefitinib did not significantly improve survival outcomes versus chemotherapy in the overall population. However, the preplanned subgroup analysis in patients with MET amplification showed that tepotinib plus gefitinib improved PFS and OS versus chemotherapy. These final analyses confirm previous observations in INSIGHT (9), with an extended duration of follow-up and a more mature dataset, allowing for a more precise estimation of time-dependent endpoints. The median OS of 37.3 months with tepotinib plus gefitinib in patients with MET amplification is in the same range as reported for first-line osimertinib in EGFR-mutant NSCLC (18) and is considerably longer than the duration of prior therapy with first- or second-generation EGFR TKIs in our MET amplification subgroup, which had a median of 10.6 months.
In patients with MET amplification, a substantial number achieved prolonged clinical benefit in the tepotinib plus gefitinib arm; treatment duration was longer than 4 years in three patients. Time on the combination treatment appeared to be unrelated to the duration of prior EGFR TKI. However, of six patients with a treatment duration of longer than 1 year, three patients had a MET GCN >12.
Compared with other MET TKI and EGFR TKI combination therapies in this setting, tepotinib plus gefitinib showed favorable efficacy (10, 11, 19–21). Since the INSIGHT trial was initiated, the third-generation EGFR TKI osimertinib has emerged as a recommended treatment option for EGFR-mutant NSCLC (22). MET amplification is the most common EGFR-independent resistance mechanism to osimertinib, occurring in up to 30% of patients who progress on first-line treatment (23, 24). Three studies (ORCHARD, SAVANNAH, TATTON) investigating the MET TKI savolitinib plus osimertinib in patients with EGFR-mutant NSCLC with progression due to MET alterations, including MET amplification, have shown promising activity (14, 25, 26). Initial results from the phase II INSIGHT 2 study (NCT03940703) have shown promising clinical benefit with tepotinib plus osimertinib in patients with EGFR-mutant NSCLC with osimertinib resistance due to MET amplification, with an ORR of 54.5% in patients with ≥9 months’ follow-up (n = 22; ref. 13). Together with real-world evidence supporting the activity of this combination (12, 27), these data may help inform clinical practice for this population with no clear treatment options. MET amplification has been reported as a potential driver of resistance to drugs other than EGFR TKIs in NSCLC (28–30). Thus, further studies of tepotinib combination strategies may be warranted to tackle resistance due to MET amplification in patients with other oncogene-driven NSCLC subtypes.
Given the prevalence of MET amplification as a driver of resistance to EGFR TKIs, accurate and reliable biomarker testing for this oncogenic alteration is important to identify patients who could benefit from targeted therapy. Despite the advantages of liquid biopsy over tissue biopsy in terms of invasiveness and speed, tissue biopsy analysis using FISH is associated with higher sensitivity and reliability in detecting MET amplification in NSCLC (31, 32). Although MET amplification can be detected using next-generation sequencing (NGS) methods, FISH has been shown to be the most sensitive diagnostic tool, with MET amplification detected in 63% versus 25% of patients by FISH and NGS, respectively (33). Thus, FISH testing of tissue biopsies is the currently recommended method for detecting MET amplification in patients with NSCLC and has been shown to increase detection by two- or 2.7-fold compared with tissue NGS or circulating tumor DNA NGS, respectively (7).
Consistent with the previously reported data from INSIGHT (9), tepotinib plus gefitinib was generally well tolerated. Despite substantially longer durations of treatment in the present compared with the previous analysis (9) and in patients with MET amplification versus the overall population, the safety profile was similar, underscoring the good tolerability of the combination. The incidence of peripheral edema with tepotinib plus gefitinib (33.3%) was lower than reported with tepotinib monotherapy in patients with METex14 skipping NSCLC (66.5%; ref. 34). This finding may require further investigation but is likely linked to the younger age of the population with MET amplification, given that advanced age is an independent risk factor for this AE (35).
In our study, marked efficacy was also observed in the high MET overexpression (IHC 3+) population treated with tepotinib plus gefitinib, with a PFS of 8.3 months versus 4.4 months with chemotherapy, but this may be driven by the 50% of patients who had concomitant MET amplification. Although amplification of the MET gene can lead to MET protein overexpression, the latter can also occur as a response to tumor growth rather than as a result of oncogenic genomic alterations (36). Therefore, MET amplification may be a more effective predictive marker for response to MET TKIs in EGFR-resistant NSCLC than MET overexpression. Despite this, we observed responses in patients with IHC 3+ MET overexpression who were negative for MET amplification. A possible explanation for this could be alternative MET alterations, such as MET fusions or METex14 skipping, which have also been shown to mediate resistance to EGFR TKIs (37, 38) but were not analyzed in this study.
Despite the positive results of tepotinib plus gefitinib in patients with MET amplification as part of the INSIGHT trial, as previously reported (9), a major limitation of this study was the poor recruitment, resulting in early termination of the study with a lower enrollment than planned. The results should be considered exploratory, considering the small patient sample size treated in phase II. Although protocol amendments were made to enhance recruitment, tests such as the invasive tissue biopsy for detection of MET alterations may have contributed to low enrollment.
This final analysis of the INSIGHT trial shows promising results that tepotinib in combination with an EGFR TKI, such as gefitinib, may be able to overcome resistance due to MET amplification in patients with EGFR-mutant NSCLC who have progressed on prior EGFR TKI therapy, potentially providing a much-needed treatment strategy for this patient population. Importantly, these exploratory yet promising results supported the rationale for a larger study, the ongoing INSIGHT 2 trial, designed to assess efficacy and safety of tepotinib combined with osimertinib, in patients with advanced EGFR-mutant NSCLC and acquired resistance to first-line osimertinib and MET amplification (6, 13).
A plain language summary of this publication can be found in the Supplementary materials (Supplementary Fig. S5).
Supplementary Material
Outcomes of treatment for all individuals enrolled
Duration of PFS in the patient subgroup with MET amplification
Outcomes of treatment for the patient subgroup with MET overexpression
Treatment-related adverse events in treated patients with MET amplification and/or MET IHC 2+/3+
Demographic data of all individuals enrolled
Representativeness of study population
Demographic data in the patient subgroup with MET overexpression
Plain language summary
Acknowledgments
This work was funded by the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945). The authors would like to thank patients and their families, investigators, coinvestigators, and the study teams at all participating centers, and the healthcare business of Merck KGaA, Darmstadt, Germany. Medical writing and editorial assistance were provided by Rebecca Hurst of Syneos Health, and funded by the healthcare business of Merck KGaA, Darmstadt, Germany.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Highlights of This Issue, p. 1833
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
C. Liam reports personal fees from AstraZeneca, Janssen, the healthcare business of Merck KGaA, Darmstadt, Germany, Merck & Co., Novartis, Pfizer, Roche, and Zuellig Pharma; and grants from Boehringer Ingelheim outside the submitted work. T. Hsia reports grants from the healthcare business of Merck KGaA, Darmstadt, Germany, AZ, Merck & Co., BI, Novartis, Lilly, and Roche during the conduct of the study. D. Kim reports grants from the healthcare business of Merck KGaA, Darmstadt, Germany during the conduct of the study; grants from Alpha Biopharma, Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Hanmi, Janssen, Merus, Mirati Therapeutics, Novartis, ONO Pharamceutical, Pfizer, Roche/Genentech, Takeda, TP Therapeutics, Xcovery, Yuhan, Chong Keun Dang, BridgeBioTherapeutics, GSK, and Merck & Co. outside the submitted work; and medical writing assistance from Amgen, AstraZeneca, Boehringer Ingelheim, Bridge BioTherapeutics, Chong Keun Dang, Daiichi-Sankyo, GSK, Janssen, Merus, Merck & Co., the healthcare business of Merck KGaA, Darmstadt, Germany, Novartis, Pfizer, Roche, Takeda, Yuhan. R.A. Soo reports grants and personal fees from AstraZeneca and Boehringer Ingelheim; personal fees from Amgen, Bayer, BMS, Janssen, Lily, the healthcare business of Merck KGaA, Darmstadt, Germany, EMD Serono, Novartis, Pfizer, Puma Biotechnology, Roche, Taiho, Takeda, Thermo Fisher Scientific, and Yuhan outside the submitted work. S. Lu reports grants from AstraZeneca, Hutchison, BMS, Heng Rui, Beigene, Roche, and Hansoh during the conduct of the study; grants from AstraZeneca, Pfizer, Boehringer Ingelheim, Simcere, ZaiLab, Yuhan, Menarini, InventiBio, Hutchison, MediPharma, and Roche outside the submitted work. J. Yang reports personal fees and other support from Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, the healthcare business of Merck KGaA, Darmstadt, Germany, Merck & Co., Novartis, Pfizer, Roche/Genentech, Takeda Oncology, Yuhan Pharmaceuticals; personal fees from Ono Pharmaceuticals; grants, personal fees, and other support from AstraZeneca; other support from Eli Lilly, Janssen Puma Technolgy, Gilead, and GSK outside the submitted work. A. Johne reports other support from the healthcare business of Merck KGaA, Darmstadt, Germany during the conduct of the study. Y. Wu reports grants from AstraZeneca, Boehringer Ingelheim, Novartis, and Takeda; and personal fees from AstraZeneca, Beigen, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly and Company, Merck & Co., Pfizer, Roche, and Sanofi outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
C. Liam: Writing–original draft, writing–review, investigation, and editing. A. Ahmad: Writing–original draft, writing–review, investigation, and editing. T. Hsia: Writing–original draft, writing–review, investigation, and editing. J. Zhou: Writing–original draft, writing–review, investigation, and editing. D. Kim: Writing–original draft, writing–review, investigation, and editing. R.A. Soo: Writing–original draft, writing–review, investigation, and editing. Y. Cheng: Writing–original draft, writing–review, investigation, and editing. S. Lu: Writing–original draft, writing–review, investigation, and editing. S. Shin: Writing–original draft, writing–review, investigation, and editing. J. Yang: Writing–original draft, writing–review, investigation, and editing. Y. Zhang: Writing–original draft, writing–review, investigation, and editing. J. Zhao: Writing–original draft, writing–review, investigation, and editing. K. Berghoff: Writing–original draft, writing–review, formal analysis, and editing. R. Bruns: Writing–original draft, writing–review, formal analysis, and editing. A. Johne: Writing–original draft, writing–review, formal analysis, and editing. Y. Wu: Conceptualization, data curation, formal analysis, writing–original draft, writing–review, investigation, and editing.
References
- 1. Passaro A, Jänne PA, Mok T, Peters S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat Cancer 2021;2:377–91. [DOI] [PubMed] [Google Scholar]
- 2. Lim SM, Syn NL, Cho BC, Soo RA. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: mechanisms and therapeutic strategies. Cancer Treat Rev 2018;65:1–10. [DOI] [PubMed] [Google Scholar]
- 3. Shah MP, Neal JW. Targeting acquired and intrinsic resistance mechanisms in epidermal growth factor receptor mutant non-small-cell lung cancer. Drugs 2022;82:649–62. [DOI] [PubMed] [Google Scholar]
- 4. Wu YL, Soo RA, Locatelli G, Stammberger U, Scagliotti G, Park K. Does c-Met remain a rational target for therapy in patients with EGFR TKI-resistant non-small cell lung cancer? Cancer Treat Rev 2017;61:70–81. [DOI] [PubMed] [Google Scholar]
- 5. Drilon A, Cappuzzo F, Ou S-HI, Camidge DR. Targeting MET in lung cancer: will expectations finally Be MET? J Thorac Oncol 2017;12:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smit EF, Dooms C, Raskin J, Nadal E, Tho LM, Le X, et al. INSIGHT 2: a phase II study of tepotinib plus osimertinib in MET-amplified NSCLC and first-line osimertinib resistance. Futur Oncol 2022;18:1039–54. [DOI] [PubMed] [Google Scholar]
- 7. Hartmaier RJ, Han J-Y, Cho BC, Frigault MM, Markovets A, L'Hernault A, et al. Abstract 4897: detection of MET-mediated EGFR tyrosine kinase inhibitor (TKI) resistance in advanced non-small cell lung cancer (NSCLC): biomarker analysis of the TATTON study. Cancer Res 2019;79:4897. [Google Scholar]
- 8. Friese-Hamim M, Bladt F, Locatelli G, Stammberger U, Blaukat A. The selective c-Met inhibitor tepotinib can overcome epidermal growth factor receptor inhibitor resistance mediated by aberrant c-Met activation in NSCLC models. Am J Cancer Res 2017;7:962–72. [PMC free article] [PubMed] [Google Scholar]
- 9. Wu YL, Cheng Y, Zhou J, Lu S, Zhang Y, Zhao J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med 2020;8:1132–43. [DOI] [PubMed] [Google Scholar]
- 10. McCoach CE, Yu A, Gandara DR, Riess JW, Vang DP, Li T, et al. Phase I/II study of capmatinib plus erlotinib in patients with MET-positive non–small-cell lung cancer. JCO Precis Oncol 2021;5:177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sequist LV, Han JY, Ahn MJ, Cho BC, Yu H, Kim SW, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol 2020;21:373–86. [DOI] [PubMed] [Google Scholar]
- 12. Le X, Eisert A, Himpe U, De Bondt C, Mazieres J, Petrini I, et al. EP08.02–162 tepotinib with an EGFR-tyrosine kinase inhibitor (TKI) in patients with EGFR-mutant MET-amplified NSCLC: a case series. J Thorac Oncol 2022;17(9_Suppl):S483–4. [Google Scholar]
- 13. Mazieres J, Kim T-M, Lim B, Wislez M, Dooms C, Finocchiaro G, et al. LBA52 - tepotinib + osimertinib for EGFRm NSCLC with MET amplification (METamp) after progression on first-line (1L) osimertinib: initial results from the INSIGHT 2 study. Ann Oncol 2022;33:S808–69. [Google Scholar]
- 14. Ahn M-J, de Marinis F, Bonanno L, Cho BC, Kim T-M, Cheng S, et al. EP08.02–140 MET biomarker-based preliminary efficacy analysis in SAVANNAH: savolitinib+osimertinib in EGFRm NSCLC post-osimertinib. WCLC 2022.
- 15. Schadt O, Blaukat A. Tepotinib. In:Martinez A, Gil C, editors. Comprehensive Medicinal Chemistry III. Oxford: Elsevier; 2017. p. 178–203. [Google Scholar]
- 16. Falchook GS, Kurzrock R, Amin HM, Xiong W, Fu S, Piha-Paul SA, et al. First-in-man phase I trial of the selective MET inhibitor tepotinib in patients with advanced solid tumors. Clin Cancer Res 2020;26:1237–46. [DOI] [PubMed] [Google Scholar]
- 17. Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med 2020;383:931–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2019;382:41–50. [DOI] [PubMed] [Google Scholar]
- 19. Yang JJ, Fang J, Shu YQ, Chang JH, Chen GY, He JX, et al. A phase Ib study of the highly selective MET-TKI savolitinib plus gefitinib in patients with EGFR-mutated, MET-amplified advanced non-small-cell lung cancer. Invest New Drugs 2021;39:477–87. [DOI] [PubMed] [Google Scholar]
- 20. Hayashi H, Sugawara S, Fukuda Y, Fujimoto D, Miura S, Ota K, et al. A randomized Phase II study comparing nivolumab with carboplatin–pemetrexed for EGFR-mutated NSCLC with resistance to EGFR tyrosine kinase inhibitors (WJOG8515L). Clin Cancer Res 2022;28:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu YL, Zhang L, Kim DW, Liu X, Lee DH, Yang JCH, et al. Phase Ib/II study of capmatinib (INC280) plus gefitinib after failure of epidermal growth factor receptor (EGFR) inhibitor therapy in patients with EGFR-mutated, MET factor-dysregulated non-small-cell lung cancer. J Clin Oncol 2018;36:3101–9. [DOI] [PubMed] [Google Scholar]
- 22. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.5.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed October 26, 2022. Referenced with permission. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. [Google Scholar]
- 23. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 2019;121:725–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y, Li L, Han R, Jiao L, Zheng J, He Y. Clinical analysis by next-generation sequencing for NSCLC patients with MET amplification resistant to osimertinib. Lung Cancer 2018;118:105–10. [DOI] [PubMed] [Google Scholar]
- 25. Yu HA, Ambrose H, Baik C, Cho BC, Cocco E, Goldberg SB, et al. 1239P ORCHARD osimertinib + savolitinib interim analysis: a biomarker-directed phase II platform study in patients (pts) with advanced non-small cell lung cancer (NSCLC) whose disease has progressed on first-line (1L) osimertinib. Ann Oncol 2021;32:S978–9. [Google Scholar]
- 26. Hartmaier RJ, Han J-Y, Cho BC, Markovets A, Kurian N, Cantarini M, et al. Tumor response and MET-detection methods: exploratory biomarker analysis of part B of the phase 1b TATTON study. AACR 2021. Poster CT127. [Google Scholar]
- 27. Wang K, Du R, Roy S, Vokes N, Elamin YY, Bueno Hume C, et al. 1012P resistance mechanisms to dual EGFR and MET inhibition in patients with EGFR-mutant MET-amplified non-small cell lung cancer. Ann Oncol 2022;33(Suppl. 7):S1016. [Google Scholar]
- 28. Coleman N, Hong L, Zhang J, Heymach J, Hong D, Le X. Beyond epidermal growth factor receptor: MET amplification as a general resistance driver to targeted therapy in oncogene-driven non-small-cell lung cancer. ESMO Open 2021;6:100319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gouji T, Takashi S, Mitsuhiro T, Yukito I. Crizotinib can overcome acquired resistance to CH5424802: is amplification of the MET gene a key factor? J Thorac Oncol 2014;9:e27–28. [DOI] [PubMed] [Google Scholar]
- 30. Sakakibara-Konishi J, Kitai H, Ikezawa Y, Hatanaka Y, Sasaki T, Yoshida R, et al. Response to crizotinib re-administration after progression on lorlatinib in a patient with ALK-rearranged non–small-cell lung cancer. Clin Lung Cancer 2019;20:e555–9. [DOI] [PubMed] [Google Scholar]
- 31. Felip E, Garassino MC, Sakai H, Le X, Veillon R, Smit E, et al. P45.03 tepotinib in patients with MET exon 14 (METex14) skipping NSCLC as identified by liquid (LBx) or Tissue (TBx) biopsy. WCLC 2021.
- 32. Bauml JM, Mick R, Mccoach C, Weiss J, Marrone K, Nieva J, et al. FP14.06 multicenter analysis of mechanisms of resistance to osimertinib (O) in EGFR mutated NSCLC: an ATOMIC registry study. J Thorac Oncol 2021;16:S229–30. [Google Scholar]
- 33. Peng L-X, Jie G-L, Li A-N, Liu S-Y, Sun H, Zheng M-M, et al. MET amplification identified by next-generation sequencing and its clinical relevance for MET inhibitors. Exp Hematol Oncol 2021;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomas M, Garassino MC, Felip E, Sakai H, Le X, Veillon R, et al. OA03.05 tepotinib in patients with MET Exon 14 (METex14) skipping NSCLC: primary analysis of the confirmatory VISION cohort C. WCLC 2022.
- 35. Xiong W, Hietala SF, Nyberg J, Papasouliotis O, Johne A, Berghoff K, et al. Exposure-response analyses for the MET inhibitor tepotinib including patients in the pivotal VISION trial: support for dosage recommendations. Cancer Chemother Pharmacol 2022;90:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Comoglio PM, Trusolino L, Boccaccio C. Known and novel roles of the MET oncogene in cancer: a coherent approach to targeted therapy. Nat Rev Cancer 2018;18:341–58. [DOI] [PubMed] [Google Scholar]
- 37. Li Y, Wang K, Tian P, Li W. Acquired MET-DSTN fusion mediated resistance to EGFR-TKIs in lung adenocarcinoma and responded to crizotinib plus gefitinib: a case report. Clin Lung Cancer 2022;23:e83–6. [DOI] [PubMed] [Google Scholar]
- 38. Suzawa K, Offin M, Schoenfeld AJ, Plodkowski AJ, Odintsov I, Lu D, et al. Acquired MET Exon 14 alteration drives secondary resistance to epidermal growth factor receptor tyrosine kinase inhibitor in EGFR-mutated lung cancer. JCO Precis Oncol 2019;3:PO.19.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Outcomes of treatment for all individuals enrolled
Duration of PFS in the patient subgroup with MET amplification
Outcomes of treatment for the patient subgroup with MET overexpression
Treatment-related adverse events in treated patients with MET amplification and/or MET IHC 2+/3+
Demographic data of all individuals enrolled
Representativeness of study population
Demographic data in the patient subgroup with MET overexpression
Plain language summary
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the data-sharing policy of the healthcare business of Merck KGaA, Darmstadt, Germany. All requests should be submitted in writing to the data-sharing portal of the healthcare business of Merck KGaA, Darmstadt, Germany: https://www.emdgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html.