Abstract
Patients with rheumatoid arthritis (RA) remain at an increased risk for cardiovascular disease (CVD) and mortality. RA CVD results from a combination of traditional risk factors and RA‐related systemic inflammation. One hypothetical means of improving overall RA CVD risk is through reduction of excess body weight and increased physical activity. Together, weight loss and physical activity can improve traditional cardiometabolic health through fat mass loss, while also improving skeletal muscle health. Additionally, disease‐related CVD risk may improve as both fat mass loss and exercise reduce systemic inflammation. To explore this hypothesis, 26 older persons with RA and overweight/obesity will be randomized to 16 weeks of a usual care control arm or to a remotely Supervised Weight Loss Plus Exercise Training (SWET) program. A caloric restriction diet (targeting 7% weight loss) will occur via a dietitian‐led intervention, with weekly weigh‐ins and group support sessions. Exercise training will consist of both aerobic training (150 minutes/week moderate‐to‐vigorous exercise) and resistance training (twice weekly). The SWET remote program will be delivered via a combination of video conference, the study YouTube channel, and study mobile applications. The primary cardiometabolic outcome is the metabolic syndrome Z score, calculated from blood pressure, waist circumference, high‐density lipoprotein cholesterol, triglycerides, and glucose. RA‐specific CVD risk will be assessed with measures of systemic inflammation, disease activity, patient‐reported outcomes, and immune cell function. The SWET‐RA trial will be the first to assess whether a remotely supervised, combined lifestyle intervention improves cardiometabolic health in an at‐risk population of older individuals with RA and overweight/obesity.
Introduction
Despite recent, revolutionary improvements in pharmacologic management (1), rheumatoid arthritis (RA) remains associated with increased rates of cardiovascular disease (CVD) and mortality (2, 3, 4, 5). CVD risk in RA is greatly increased compared with the general population and is at least comparable with that of diabetes mellitus–associated CVD risk (6). CVD risk ensues early in the RA disease course (ie, within 3 to 5 years), and compared with those without RA, patients with RA have over double the risk of CVD hospitalization (4, 7, 8). Most importantly, CVD is the leading cause of death and reduced life expectancy in RA (9, 10, 11).
Current paradigms for the prevention and management of CVD in individuals with RA attempt to address both traditional and inflammatory factors (12, 13). Approximately 50% of RA CVD risk can be explained by the following traditional risks: smoking, hypertension, dyslipidemia, impaired glucose handling, and overweight/obesity (14, 15). Although management of these traditional CVD risk factors is important (16), it is notable that traditional CVD risk calculators greatly underestimate RA CVD risk (17, 18). Thus, recent focus in the management of RA CVD risk has been on disease‐modifying antirheumatic drug (DMARD) therapy to target inflammation (16, 19, 20). However, the primary drawbacks to using currently available DMARD therapy to improve RA CVD risk are the same for using these powerful anti‐inflammatory therapies to manage RA disease activity: immune suppression and infection risk (21). Further, the persistence and progression of CVD in RA—despite biologic DMARD agents and early intensive treatment—implies pharmacologic control of disease activity is insufficient for completely rectifying systemic consequences (10, 11, 22, 23, 24).
One promising means of improving RA CVD risk—for both traditional and inflammatory factors—is through overweight/obesity reduction and increased physical activity. Both overweight/obesity and physical inactivity worsen RA CVD risk, yet interventions targeting these factors to improve RA CVD risk are lacking (25, 26, 27, 28, 29, 30, 31, 32, 33). Here, we outline the rationale for incorporating both overweight/obesity reduction and physical activity as primary intervention strategies for RA CVD. We then discuss the potential benefits of a remotely supervised weight loss and exercise training intervention to improve use of lifestyle management strategies in RA clinical care.
The effect of weight loss on RA CVD risk
In the context of RA, overweight/obesity has multiple negative consequences. Overweight/obesity (body mass index [BMI] ≥ 25 kg/m2) is a risk factor for RA development and associates with greater disease activity, poorer treatment responses, and greater insulin resistance (25, 26, 27, 34, 35, 36). Conversely, when measured by body mass index, overweight/obesity is associated with reduced RA CVD mortality rates compared with patients with RA with normal BMI (37). Although counterintuitive, this apparent reduction in CVD risk (ie, obesity paradox) appears to be a misreport driven by an underestimation of adiposity in patients with RA with normal BMI and low muscle mass (29). Indeed, obesity/adiposity is not protective of RA CVD; rather, there is a relatively greater risk for RA CVD due to loss of skeletal muscle mass (ie, rheumatoid cachexia) driven by inflammatory disease activity and physical disability (38, 39, 40, 41).
Although unintentional weight loss leads to negative outcomes in RA, intentional weight loss provides benefits. Retrospective analyses of a cohort with RA and overweight/obesity showed weight loss of greater than 5 kg is associated with a threefold odds of improved disease activity (42). Weight loss via bariatric surgery in RA improves disease activity, medication use, and inflammatory markers, with effects sustained over 5 years (43). One randomized controlled trial and other observational studies have shown promise in weight loss via caloric restriction for improving RA immune function and disease activity (44, 45, 46, 47). Previous RA dietary interventions have altered macronutrient and micronutrient quality (eg, vegetarian and Mediterranean diets); however, no intervention trial has specifically assessed the effects of weight loss via caloric restriction on RA CVD risk (48, 49).
The effect of exercise training on RA CVD risk
In addition to overweight/obesity‐related threats, physical inactivity also has negative health consequences for those with RA. Patients with RA have a very low aerobic capacity and are typically physically inactive; only 10% of patients engage in more than 30 minutes of moderate‐intensity aerobic exercise per day (50, 51). Physical inactivity associates with an increased risk of RA and more severe disease presentation (52, 53). Physical inactivity in RA is also linked to alterations in skeletal muscle—a central component of cardiometabolic health (54). Critically important, physical inactivity intensifies the CVD risk conferred by RA (30, 32, 55).
Although exercise prescription for RA was traditionally controversial because of fear that excessive exercise would worsen joint disease, more recent research has shown instead that exercise training for RA is overall safe and confers many health benefits (56, 57, 58). Exercise training programs impact traditional CVD risk factors by improving RA aerobic fitness, blood pressure, lipid profiles, and body composition (ie, increased muscle mass, decreased fat mass) (59, 60, 61, 62, 63). Exercise training also improves RA inflammatory CVD risk factors. Both traditional aerobic/cardio training and resistance/strength training individually improve RA disease activity and systemic inflammation (64, 65, 66, 67). Moreover, combined aerobic and resistance exercise training programs may potentially improve RA disease activity to a greater extent than either modality alone (59, 63, 68). Our previous work also shows that high‐intensity interval exercise training improves RA disease activity, markers of inflammation (ie, erythrocyte sedimentation rate [ESR]), as well as neutrophil migration and monocyte phagocytosis (60). Despite the known benefits of exercise training for RA, the optimal dose and prescription to improve RA CVD risk remains uncertain (69).
The combined effect of diet and exercise training in RA and non‐RA populations
In persons without RA, the combination of weight loss with exercise training has greater benefit in improving CVD risk when compared with either intervention alone. This combined benefit was particularly shown in the Studies of a Targeted Risk Reduction Intervention through Defined Exercise (STRRIDE) studies, a series of randomized controlled trials of exercise training with and without weight loss in persons at‐risk for CVD but without RA (70, 71, 72). These studies show that relative to a control group that demonstrated little change in cardiometabolic risk—as measured by the continuous metabolic syndrome Z score (MSSc)—aerobic exercise training improves MSSc by −0.8 (SD = 2.2). The addition of resistance exercise training incrementally improves MSSc by −1.1 (SD = 1.7). Notably, adding weight loss further improves MSSc by −2.5 (SD = 2.1). In a different study of older adults with obesity (BMI ≥30 kg/m2) and without RA, one year of combined weight loss and exercise training significantly reduced the prevalence of cardiometabolic syndrome and improved insulin sensitivity compared with the independent effect of either intervention (73).
Although in the general population it is clear that weight loss and physical activity have proven benefits for CVD risk and systemic inflammation, evidence of their impact on RA is currently lacking. In a randomized controlled trial comparing the health benefits of a Mediterranean diet, dynamic exercise program, or a combined diet and exercise program for women with RA, the combined program improved quality of life and disability but did not increase hand grip strength or decrease weight when compared with the individual programs (74, 75). In a secondary analysis, both Mediterranean diet and dynamic exercise groups similarly decreased concentrations of serum pro‐inflammatory cytokines (tumor necrosis factor α and interleukin 6); the effect of the combined program on systemic cytokines was not reported (76). Altogether, given unique immune system alterations and escalating rates of overweight/obesity, physical inactivity, and CVD in RA, there is ongoing need for RA‐specific trials to study the effects of combined lifestyle interventions on cardiometabolic health.
Lifestyle interventions for older adults with RA
Few studies have reported the effects of lifestyle (eg, diet and exercise) interventions on clinical outcomes specifically in older adults with RA. In a randomized controlled trial of older adults (ages 65‐75 years) with RA, a 20‐week patient‐centered supervised combined exercise (resistance and aerobic) training intervention significantly improved physical fitness, fatigue, and symptoms of depression and protected against increased disease activity and pain at four‐year follow‐up (77, 78, 79). Further knowledge of the effects of lifestyle interventions designed for older adults with RA will improve care for this patient demographic at high risk for CVD (80).
The optimal exercise dose to improve CVD in older adults with RA is unknown. Current European League Against Rheumatism guidelines for physical activity include recommendations for older patients with RA; however, these recommendations are based primarily on public health guidelines and evidence from non‐RA populations (81). Public health guidelines for physical activity recommend older adults participate in multicomponent physical activity that includes balance training, aerobic training (150 minutes/week at moderate intensity or 75 minutes/week at vigorous intensity), and resistance training (2 or more days/week) (82). An area for future research is to assess whether or not these public health recommendations apply to older patients with RA.
Remote delivery of a multicomponent lifestyle intervention for older adults with RA has yet to be studied. In non‐RA populations, remote behavioral intervention programs require less cost, time, and resources and are easier to implement into clinical practice compared with in‐person supervised exercise—benefits that have been magnified during the COVID‐19 pandemic (83, 84). Importantly, remotely delivered exercise training programs associate with similar benefits—in terms of physical performance and cardiometabolic parameters—when compared with in‐person programs (85). Thus, in the Supervised Weight Loss Plus Exercise Training (SWET)‐RA randomized controlled trial, we will study the effects of a remotely delivered and supervised multicomponent lifestyle intervention (caloric restriction diet and exercise training based on public health recommendations) on CVD risk in older adults with RA and overweight/obesity.
SWET‐RA trial design
Overview
The objective of the SWET‐RA randomized controlled trial is to determine the effects of a 16‐week remotely delivered weight loss and exercise training intervention on traditional and inflammatory RA CVD risk factors, disease activity, and patient‐reported outcomes in older persons with RA and overweight/obesity. In this study, patients with RA will be randomized to one of two groups: 1) SWET or 2) counseling health as treatment (CHAT), designed to reflect current standard of care (Figure 1).
Figure 1.
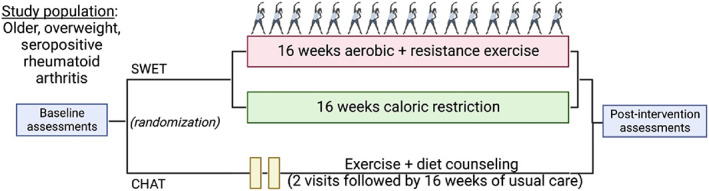
SWET‐RA trial design overview. CHAT, counseling health as treatment; SWET, Supervised Weight Loss Plus Exercise Training.
The premise of the SWET‐RA trial is that 1) in RA, both overweight/obesity and physical inactivity contribute to worse disease activity (30, 32, 55, 86, 87, 88, 89, 90, 91, 92); 2) RA CVD risk is associated with traditional CVD risk, consequences of overweight/obesity and physical inactivity, as well as disease activity and inflammation; 3) in non‐RA populations, physical activity improves immune cell health (93, 94, 95, 96); 4) in our pilot RA investigation, aerobic interval‐based exercise training improved blood pressure, global health, disease activity, and reprogrammed monocyte/macrophage function (97); and 5) these improvements should be enhanced with the addition of resistance training and weight loss.
Study population
The study population will include adults 60 to 80 years of age with overweight/obesity (BMI 28‐40 kg/m2) and seropositive (or erosive) RA, not currently meeting the 2018 Physical Activity Guidelines for Americans (82) and without absolute contraindications to exercise. For the lower end overweight/obesity BMI cutoff, we chose 28 kg/m2 rather than 25 kg/m2 in light of theoretical adverse effects of weight loss reducing muscle mass loss (ie, sarcopenia) in older participants with RA at lower BMI (98). Detailed eligibility criteria are shown in Table 1. Patients will be recruited from outpatient rheumatology clinics serving Duke Health in Durham, NC. Participant recruitment and study program flow are illustrated in Figure 2.
Table 1.
Detailed eligibility criteria for the SWET‐RA trial
| Inclusion criteria |
|---|
| Age 60‐80 y |
| Internet access |
| Body mass index 28‐40 kg/m2 |
| Seropositive (positive rheumatoid factor or anticitrullinated protein antibody) or erosions typical of rheumatoid arthritis on radiographs |
| Exclusion criteria |
| Subject unwilling/unable to use online platforms (eg, ZOOM, REDCap, Pattern Health) for study activities |
| Current use of biologic agents other than those targeting tumor necrosis factor α |
| Current (within the last month) pharmacologic therapy with corticosteroids at doses greater than prednisone 5 mg/d (or equivalent glucocorticoid doses) |
| Other inflammatory arthropathy or myopathy, Paget disease, pigmented villonodular synovitis, joint infection, ochronosis, neuropathic arthropathy, osteochondromatosis, acromegaly, hemochromatosis, or Wilson disease |
| Participating in regular exercise within the past 3 mo (according to 2018 Physical Activity Guidelines for Americans: not more than 150 min/wk of moderate‐intensity exercise or 75 min/wk of vigorous‐intensity exercise) |
| New medications within the last 3 mo |
| Medication‐dose fluctuation within the last 30 d |
| Occurrence of coronary events consisting of occlusive disease (per cardiac catheterization or ST‐elevation myocardial infarction), acute heart failure, or documented dysrhythmia within the last 12 mo |
| Current presence of aortic stenosis rated moderate or worse |
| Diagnosis of type 2 diabetes mellitus |
| Absolute contraindications to exercise: Recent (<6 mo) acute cardiac event, unstable angina, uncontrolled dysrhythmias causing symptoms or hemodynamic compromise, symptomatic aortic stenosis, uncontrolled symptomatic heart failure, acute pulmonary embolus, acute myocarditis or pericarditis, suspected or known dissecting aneurism, or acute systemic infection |
| Relative contraindications to exercise: Left main coronary stenosis, moderate stenotic valvular heart disease, outflow tract obstruction, high degree AV block, ventricular aneurysm, uncontrolled metabolic disease (eg, diabetes, thyrotoxicosis, myxedema), uncontrolled pulmonary disease (eg, severe COPD or pulmonary fibrosis), and mental (including severe depression or anxiety) or physical impairment leading to inability to exercise adequately |
| Significant weight change (gain or loss of >10 lb in 1 mo) within the past 6 mo |
| Unwillingness or inability to adhere to the diet structure of the study |
Abbreviations: AV, Atrioventricular; COPD, chronic obstructive pulmonary disease; ST, segment; SWET‐RA, Supervised Weight Loss Plus Exercise Training‐rheumatoid arthritis.
Figure 2.
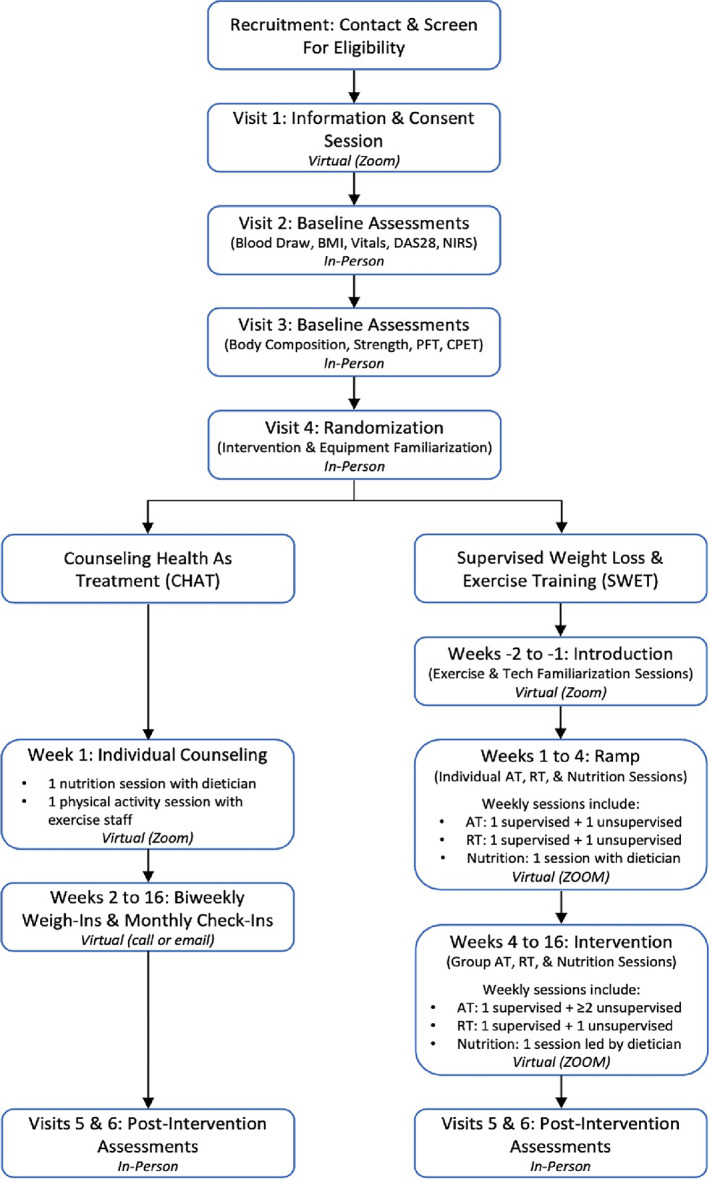
SWET‐RA trial participant recruitment and study program flow diagram. AT, aerobic training; BMI, body mass index; CPET, cardiopulmonary exercise testing; DAS28, disease activity score in 28 joints; NIRS, near‐infrared spectroscopy muscle oxidative capacity testing; PFT, pulmonary function testing; RT, resistance training.
Randomization
Following informed consent, examination by the study physician (Table 2), and baseline assessments, participants will be randomly allocated in a 1:1 ratio blocked by age decile with a variable block size known only to the statistician. Participants and the interventionists leading the exercise and nutrition sessions will not be blinded to randomization group. The trial is single blinded with respect to the study physician performing the 28‐joint disease activity examination.
Table 2.
Physician screening assessment for the SWET‐RA trial
| Study physician screening assessment |
|---|
| Functional capacity assessment |
| “What limits you when you exercise or exert yourself? Breathing, fatigue, pain?” |
| Observation, physical examination |
| Cardiorespiratory fitness assessment |
| “How far are you able to walk/run/bike/swim?” |
| Observation, physical examination |
| Muscle strength assessment |
| “How much are you able to lift/carry?” |
| Observation, physical examination |
| Dynamic balance/flexibility assessment |
| “What kind of activities do you feel comfortable performing?” |
| Observation, physical examination |
Abbreviation: SWET‐RA, Supervised Weight Loss Plus Exercise Training‐rheumatoid arthritis.
Equipment
Regardless of intervention group, all participants will receive the following equipment: 1) a commercial activity monitor (Garmin Forerunner 45)—study staff have access to participant physical activity data via an online platform in order to monitor intervention compliance; 2) a set of exercise resistance bands (Bodylastics Max Tension System) containing five bands with varying levels of resistance—study staff provide guidance on proper technique and use of the bands; 3) a wireless, Bluetooth‐enabled digital scale (A&D Medical PLUSCONNECT wireless weight scale)—study staff monitor body weight throughout the study via the Pattern Health application (app); and 4) a tablet (Dragon Touch Notepad K10 tablet) to allow participants to use the required mobile apps.
Remote intervention
The SWET‐RA trial uses a remotely delivered program for the implementation of a lifestyle intervention for patients with RA. Aside from preintervention and postintervention assessment visits and program introduction visits (Figure 2), all intervention delivery and application are completed via video conference (ie, Zoom), the study YouTube channel, and the study mobile apps (Pattern Health: https://pattern.health; MyFitnessPal: https://www.myfitnesspal.com) (Figure 3). The study mobile apps connect to and obtain data from study equipment (eg, heart rate and step counts from activity monitor, weight from digital scale). Via the study mobile apps on the provided tablet, study participants 1) view all individualized intervention related information, 2) record completion of intervention goals, and 3) receive feedback and reminder notifications regarding weekly task completion.
Figure 3.
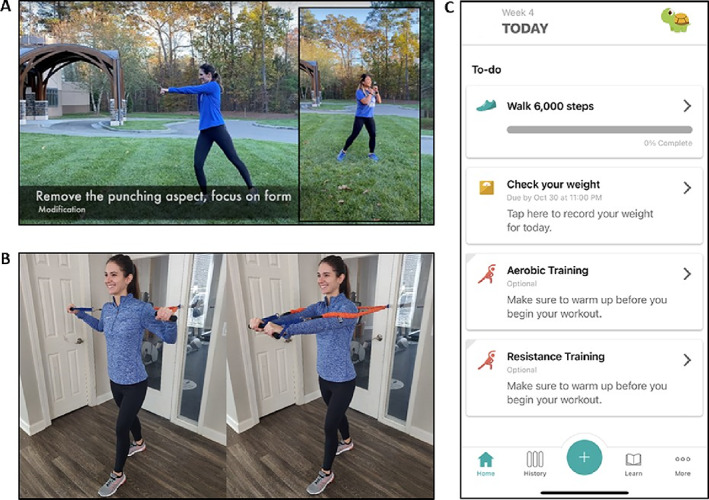
SWET‐RA remote lifestyle intervention. A, Screenshot example of aerobic training video. B, Screenshot example of resistance training video. C, Screenshot of mobile app (Pattern Health). SWET, SWET, Supervised Weight Loss Plus Exercise Training.
SWET intervention
Participants randomized to the SWET group will complete three supervised components: weight loss, aerobic training, and resistance training.
Weight loss component
Following a randomization and a virtual equipment familiarization session, participants will meet (virtually, in real time) with a registered dietitian to receive an individualized caloric restriction diet prescription. Prescriptions are calculated as below using participant data and estimated total energy expenditure (TEE) (99):
The prescribed calorie amount for a weight loss of approximately 1 to 2 pounds (average of 0.6 kg) per week is based on a reduction of 500 to 1000 kcal/day below TEE; the 16‐week target is 7% weight loss with an expected achievement of 5%. The macronutrient distribution of the SWET diet is 40% carbohydrate, 30% fat, and 30% protein.
Each week, participants will attend one live, virtual group nutrition session led by a registered dietitian. The nutrition curriculum is designed to enhance diet compliance, including group support, goal setting, self‐monitoring, stress management, problem solving, relapse prevention, daily diet journaling, email/phone reminders, and tutorials for online tools. To reduce the difficulty with imposing two behavioral changes simultaneously (ie, diet and exercise training), the curriculum is individualized with focus on use of dietary exchanges and understanding of the diet prescription during the initial weeks with progression toward more complex topics and more aggressive weight loss goals. Diet compliance is monitored by weekly weigh‐ins and weekly collection of daily food records captured via the A&D scale and the study mobile apps, respectively. Additionally, participants will use a weight loss graph to track their weight loss throughout the 16 weeks. As a part of baseline and postintervention assessment visits, participant 3‐day food diaries will be collected to determine diet intake (calories, macronutrients, and micronutrients) using Food Processor Nutrition Analysis Software (version 11.0.3, ESHA Research, Salem, OR).
Aerobic exercise training component
The remotely supervised aerobic exercise component is based on US physical activity guidelines (82) and consists of two weekly goals: 150 minutes of moderate‐to‐vigorous exercise and an average of at least 6000 steps per day. Because moderate‐ to vigorous‐intensity exercise varies according to fitness level, participants will receive individualized prescriptions based on results from a cardiopulmonary exercise test with gas exchange. Exercise intensity is prescribed as the participant's heart rate range that corresponds to moderate‐to‐vigorous intensity (ie, 45%‐65% VO2 reserve), calculated as 45% to 65% of the participant's heart rate reserve (ie, maximum minus resting heart rate).
Participants will undergo an initial ramp period in which study staff exercise physiologists virtually counsel participants on understanding device use, heart rate ranges, and exercise safety while exercises are performed at lower intensities. Over 1 to 2 weeks, intensities progressively increase.
Each participant's exercise prescription will include required attendance at a live, remotely supervised online aerobics class once per week. Conducted via Zoom, an exercise physiologist conducts a 45‐ to 60‐minute class, comprising a warm‐up, one to four sets of moderate aerobic activities that can be done without equipment, and a cool down. During these sessions, participants will monitor heart rates with their Garmin devices (with staff assistance, as needed) to modify activities to maintain heart rates within the prescribed range. Participants will also report an overall rating of perceived exertion (RPE) for each training session (Borg scale 6‐20). Aerobic exercises are individualized by study staff exercise physiologists and physicians to ensure pain‐free range of motion (eg, adapting movements for individuals with chronic joint deformities related to RA).
In addition to the live virtual exercise class, participants will be instructed to perform aerobic exercises on their own to meet both their step count and aerobic minutes goals. Aerobic exercises include (but are not limited to) aerobics videos (recorded on study YouTube channel by study staff exercise physiologists), walking, running, and dancing; diversity of aerobic exercise modalities was chosen to accommodate patient preference and increase exercise prescription compliance.
Resistance exercise training component
Remotely supervised resistance training exercises will be primarily performed with resistance bands; resistance bands were the chosen modality because of practicality (ie, ease of use at home), low cost, similar strength benefits compared with conventional resistance training devices (eg, weight machines and dumbbells), and functional benefits for patients with arthritis (eg, resistance does not rely on gravity, less stress on hand/wrist joints compared with conventional devices) (100). The resistance training goal includes twice weekly sessions, on nonconsecutive days. Resistance training sessions consist of 10 to 11 exercises targeting major muscle groups (1‐3 sets of 8‐15 repetitions with a rest period of at least 60 seconds between sets). A virtual equipment familiarization session with an exercise physiologist will introduce participants to the exercise bands, targeted muscle groups, and proper technique. After a warm‐up period, the exercise physiologist and the participant will work together to decide on a comfortable resistance band (ie, resistance level) to use for each of the 10 to 11 exercises to determine an individualized exercise prescription. The starting band resistance will be set when the participant can perform one set of 8 to 10 repetitions comfortably using proper technique.
Safe and effective resistance exercise technique will be emphasized to promote full range of motion, activation of targeted muscle groups, and proper breathing. Over the 16‐week intervention, the amount of resistance lifted and/or repetitions or sets performed will be slowly increased such that progression occurs while minimizing musculoskeletal injuries. After two back‐to‐back training sessions during which 3 sets of 15 repetitions are performed with proper technique, resistance will be increased (typically by 5%‐10% or one band resistance level) such that 1 to 3 sets of 8 to 10 (but not 15) repetitions can be performed using proper technique. Similar to aerobic exercise modifications, resistance exercises are individualized by study staff exercise physiologists and physicians to ensure pain‐free range of motion.
As with weekly aerobic exercise sessions, participants will be required to attend one live virtual resistance exercise class (supervised by an exercise physiologist) and complete one session on their own. Recordings of both aerobic and resistance exercise routines by study staff exercise physiologists are available on the study YouTube page for participants to view at any time.
CHAT intervention
Participants randomized to the CHAT group will receive initial clinical counseling on healthy diets and physical activity.
Diet counseling
Participants will meet virtually one‐on‐one for approximately 60 minutes with a registered dietitian for dietary recommendations to improve overall health (101). Recommendations are to 1) follow healthy eating patterns; 2) focus on variety, nutrient density, and amount; 3) limit calories from added sugars and saturated fats and reduce sodium intake; 4) shift to healthier food and beverage choices; and 5) support healthy eating patterns. Participants will be given written summaries of the recommendations. In addition, participants will be asked to check their weight using the study‐provided A&D scale every 2 weeks. As part of baseline and postintervention assessment visits, 3‐day food diaries will be analyzed for total calories, macronutrient, and micronutrient consumption using the Food Processor software.
Physical activity counseling
Participants will also meet virtually one on one for approximately 60 minutes with an exercise physiologist for recommendations of physical activity to improve overall health. Aerobic activity recommendations target at least 6000 steps per day and five 30‐ to 35‐minute aerobic exercise sessions (eg, walking) per week in accordance with public health recommendations for adults (82). Discussions include how to assess daily steps and how to increase daily steps each week until the 6000 step per day target is reached. Aerobic exercise is recommended at a moderate intensity and described as a 12 to 14 on the Borg RPE scale. Participants will be provided with written summaries of these recommendations. Resistance training recommendations are two sessions per week, each separated by at least 48 hours. Written instructions for 8 to 10 upper‐ and lower‐body resistance exercises using resistance bands will be provided. In addition to the commercial activity monitors, participants will be provided with an exercise journal to record daily steps and exercise sessions. Each week, activity monitor data will be downloaded for computerized analysis. To minimize attention bias, study staff will contact CHAT participants once monthly through phone or email to ensure they do not have any questions or concerns.
Outcome measures
Assessing CVD risk in RA is controversial. Despite a general consensus that RA disease activity contributes to CVD risk, RA‐specific risk calculators perform similar to calculators designed for general populations (102, 103). Additionally, most calculators use presence or absence of risk factors, which (especially for type 2 diabetes or coronary artery disease) are unlikely to be reversed in a 16‐week nonpharmacologic intervention; thus, patients with these conditions are excluded from the study.
The primary outcome chosen to assess change in cardiometabolic risk following the intervention period is the MSSc (70, 71, 104). The MSSc is a continuous weighted score of the five metabolic syndrome variables—fasting high‐density lipoprotein cholesterol, triglycerides, glucose, waist circumference, and mean blood pressure. A modified Z score will be calculated for each participant using continuous differences between the Adult Treatment Panel (ATP) III guideline values and participant values with normalization to the cohort's standard deviations. To account for variations in ATP III criteria between men and women, we will use sex‐specific MSSc equations (105, 106). Secondary outcomes include markers of inflammatory disease activity, immune cell function, body composition, cardiorespiratory and muscle fitness, pulmonary function, and self‐reported health.
Anthropometrics and vital signs
With the participant wearing lightweight clothing and no shoes, height and body weight will be measured with a stadiometer and digital scale, respectively. Although the participant stands with legs parallel and shoulder‐width apart, waist circumference will be measured with a flexible tape measure at the minimal waist (ie, smallest horizontal circumference above the umbilicus and below the xiphoid process) (107). Resting blood pressure and heart rate will be measured after the participant has relaxed in a seated position for approximately 5 minutes.
Body composition
Fat mass and fat‐free mass will be assessed under similar conditions of hydration among participants via air displacement plethysmography (BOD POD® Life Measurement, Concord, CA).
Blood‐based biomarkers
Following an overnight fast, blood samples will be obtained and analyzed for fasting glucose, insulin, and lipid/lipoprotein profiles; complete blood count with differential and platelet count; ESR; and high‐sensitivity C‐reactive protein (CRP). In addition, peripheral blood mononuclear cells will be immediately isolated and stored frozen in liquid nitrogen for future assessment of immune cell function measures, including monocyte/macrophage function, differentiation, and phenotype distributions.
Disease activity
Overall RA disease activity will be assessed with the disease activity score in 28 joints—a composite measure including ESR (or CRP), a self‐reported overall health assessment on a 100 mm visual analog scale, and the number of tender and swollen joints determined from a 28‐joint examination by the blinded study physician (108).
Cardiorespiratory fitness
Under study physician supervision, cardiorespiratory fitness (ie, peak VO2) will be determined by graded maximal cardiopulmonary exercise tests performed with 12‐lead electrocardiography and continuous expired gas analysis (TrueMax Parvomedics, Provo, UT). Blood pressure and RPE (Borg scale 6‐20) will be monitored throughout the test. The graded treadmill protocol consists of 2‐minute stages and begins at 1 mph and 0% grade, then increases speed and/or grade until the subject reaches volitional exhaustion (109).
Muscle fitness
Muscle strength assessments include tests of quadriceps strength via isometric knee extension (CYBEX, Stoughton, MA) and bilateral grip strength (hand‐held dynamometer). Forearm (flexor digitorum profundus) and calf (medial gastrocnemius) muscle oxidative capacities will be estimated using near‐infrared spectroscopy, which measures oxygen consumption (m𝑉̇O2 as the recovery rate constant k) recovery kinetics and intramuscular oxyhemoglobin and deoxyhemoglobin concentrations following a sequence of brief, rapid arterial cuff occlusions (110).
Pulmonary function
Pulmonary function will be assessed by spirometry (TrueMax Parvomedics).
Questionnaires
Medical history, current medication use, and demographics will be assessed at baseline. Baseline and postintervention self‐reported measures of global health, pain, fatigue, physical function, sleep, and self‐efficacy for managing disease are derived from the Patient‐Reported Outcomes Measurement Information System (PROMIS) bank (https://commonfund.nih.gov/promis/index).
Sample size
Sample size calculations are based on data from the STRRIDE studies. We expect MSSc improvements to be comparable to those observed for aerobic training and weight loss in the SWET that includes aerobic training, weight loss, and resistance training. Thus, to detect a STRRIDE‐like significant difference between SWET and the CHAT of −2.5, assuming a SD for change of 2.1, 20 persons total (10 per group) will deliver 82% power at a one‐tailed α of 0.05 and group difference of 2.33. We chose a one‐tailed test because a result in the opposite direction (i.e., metabolic syndrome score reduction in CHAT exceeding SWET) would not change clinical practice (111). Based on our prior experience, we expect an attrition rate of 20% to 25% during the intervention period. Thus, 26 participants with RA will be enrolled and randomized.
Statistical analysis plan
For study analyses, the key questions are what is the impact of the intervention on primary and secondary outcomes? The primary outcome, MSSc, will be measured at two timepoints, preintervention (ie, week 0) and postintervention (ie, week 16). To analyze, we will use regression and calculate change (post‐pre). Thus, at a simple level our model is ΔMSSci = β0 + β1baselineMSSci + β 2SWETGroup + εi, where β 2 is the group effect for the primary outcome. We will control for baseline MSSc (112) to assess group differences while controlling for between‐group differences at baseline. Other important covariates measured at baseline can be incorporated into the model. All enrolled participants will be analyzed in an intention‐to‐treat fashion. Statistical significance will be established as P < 0.05 for the primary outcome, MSSc. Secondary outcomes will be assessed similarly, yet without control for type I error.
Conclusions
The SWET‐RA trial will be the first randomized controlled trial to assess the effects of a remotely delivered weight loss and exercise training lifestyle intervention on health parameters in patients with RA. The primary outcome is change in CVD risk in older patients with RA and overweight/obesity who are at high risk for cardiometabolic disease. The SWET arm of the study will pioneer a remotely delivered, behavioral intervention as a means to implement lifestyle medicine to larger groups of patients with rheumatic disease. The scalability of the SWET intervention and platform will need to be further assessed in future larger studies. Identification of the effects of exercise plus weight loss in persons with RA on CVD risk, disease activity, as well as self‐reported outcomes, will have broad implications for clinical practice and patient uptake of clinician recommendations.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
Supporting information
Disclosure Form
Acknowledgments
Original figure art was created with BioRender.com. The authors appreciate the support of the Duke University Division of Rheumatology and Immunology. They acknowledge the Duke Center for Living research staff members for their help with participant recruitment, intervention implementation and with recording of data. They acknowledge and appreciate greatly all participants in the study.
ClinicalTrials.gov identifier: NCT04356183
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The trial reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH (R21‐AR‐076663). Additional funding was provided by the Duke Pepper Center REC Career Development Award (P30‐AG‐028716; to Dr. Andonian) and the National Institute on Aging of the NIH (R03‐AG‐067949; to Dr. Andonian).
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr2.11536&file=acr211536‐sup‐0001‐Disclosureform.pdf.
References
- 1. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010;376:1094–108. [DOI] [PubMed] [Google Scholar]
- 2. Argnani L, Zanetti A, Carrara G, et al. Rheumatoid arthritis and cardiovascular risk: retrospective matched‐cohort analysis based on the RECORD Study of the Italian Society for Rheumatology. Front Med (Lausanne) 2021;8:745601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiu YM, Lu YP, Lan JL, et al. Lifetime risks, life expectancy, and health care expenditures for rheumatoid arthritis: a nationwide cohort followed up from 2003 to 2016. Arthritis Rheumatol 2021;73:750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Plein S, Erhayiem B, Fent G, et al. Cardiovascular effects of biological versus conventional synthetic disease‐modifying antirheumatic drug therapy in treatment‐naive, early rheumatoid arthritis. Ann Rheum Dis 2020;79:1414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruscitti P, Cipriani P, Liakouli V, et al. Subclinical and clinical atherosclerosis in rheumatoid arthritis: results from the 3‐year, multicentre, prospective, observational GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Arthritis Res Ther 2019;21:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Halm VP, Peters MJ, Voskuyl AE, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: a cross‐sectional study, the CARRE Investigation. Ann Rheum Dis 2009;68:1395–400. [DOI] [PubMed] [Google Scholar]
- 7. Innala L, Moller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011;13:R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zöller B, Li X, Sundquist J, et al. Risk of subsequent coronary heart disease in patients hospitalized for immune‐mediated diseases: a nationwide follow‐up study from Sweden. PLoS One 2012;7:e33442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. England BR, Sayles H, Michaud K, et al. Cause‐specific mortality in male US Veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:36–45. [DOI] [PubMed] [Google Scholar]
- 10. Sparks JA, Chang SC, Liao KP, et al. Rheumatoid arthritis and mortality among women during 36 years of prospective follow‐up: results from the Nurses' Health Study. Arthritis Care Res (Hoboken) 2016;68:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pinheiro FA, Souza DC, Sato EI. A study of multiple causes of death in rheumatoid arthritis. J Rheumatol 2015;42:2221–8. [DOI] [PubMed] [Google Scholar]
- 12. Kitas GD, Dimitroulas T. Cardiovascular comorbidity in rheumatic and musculoskeletal diseases: where we are and how can we move forward? [Editorial] Int J Rheum Dis 2021;24:473–6. [DOI] [PubMed] [Google Scholar]
- 13. DeMizio DJ, Geraldino‐Pardilla LB. Autoimmunity and inflammation link to cardiovascular disease risk in rheumatoid arthritis. Rheumatol Ther 2020;7:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crowson CS, Rollefstad S, Ikdahl E, et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2018;77:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baghdadi LR, Woodman RJ, Shanahan EM, et al. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta‐analysis. PLoS One 2015;10:e0117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Semb AG, Ikdahl E, Wibetoe G, et al. Atherosclerotic cardiovascular disease prevention in rheumatoid arthritis. Nat Rev Rheumatol 2020;16:361–79. [DOI] [PubMed] [Google Scholar]
- 17. Arts EE, Popa CD, Den Broeder AA, et al. Prediction of cardiovascular risk in rheumatoid arthritis: performance of original and adapted SCORE algorithms. Ann Rheum Dis 2016;75:674–80. [DOI] [PubMed] [Google Scholar]
- 18. Crowson CS, Matteson EL, Roger VL, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol 2012;110:420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giles JT, Rist PM, Liao KP, et al. Testing the effects of disease‐modifying antirheumatic drugs on vascular inflammation in rheumatoid arthritis: rationale and design of the TARGET Trial. ACR Open Rheumatol 2021;3:371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karpouzas GA, Ormseth SR, Hernandez E, et al. Biologics may prevent cardiovascular events in rheumatoid arthritis by inhibiting coronary plaque formation and stabilizing high‐risk lesions. Arthritis Rheumatol 2020;72:1467–75. [DOI] [PubMed] [Google Scholar]
- 21. Ozen G, Pedro S, England BR, et al. Risk of serious infection in patients with rheumatoid arthritis treated with biologic versus nonbiologic disease‐modifying antirheumatic drugs. ACR Open Rheumatol 2019;1:424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maradit‐Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population‐based cohort study. Arthritis Rheum 2005;52:402–11. [DOI] [PubMed] [Google Scholar]
- 23. Karpouzas GA, Ormseth SR, Hernandez E, et al. Impact of cumulative inflammation, cardiac risk factors, and medication exposure on coronary atherosclerosis progression in rheumatoid arthritis. Arthritis Rheumatol 2020;72:400–8. [DOI] [PubMed] [Google Scholar]
- 24. Matthijssen XM, Huizinga TW, Niemantsverdriet E, et al. Early intensive treatment normalises excess mortality in ACPA‐negative RA but not in ACPA‐positive RA. Ann Rheum Dis 2020;79:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. AbouAssi H, Tune KN, Gilmore B, et al. Adipose depots, not disease‐related factors, account for skeletal muscle insulin sensitivity in established and treated rheumatoid arthritis. J Rheumatol 2014;41:1974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crowson CS, Matteson EL, Davis JM, 3rd , et al. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu B, Hiraki LT, Sparks JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Ann Rheum Dis 2014;73:1914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schulman E, Bartlett SJ, Schieir O, et al. Overweight, obesity, and the likelihood of achieving sustained remission in early rheumatoid arthritis: results from a multicenter prospective cohort study. Arthritis Care Res (Hoboken) 2018;70:1185–91. [DOI] [PubMed] [Google Scholar]
- 29. Katz PP, Yazdany J, Trupin L, et al. Sex differences in assessment of obesity in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. AbouAssi H, Connelly MA, Bateman LA, et al. Does a lack of physical activity explain the rheumatoid arthritis lipid profile? [Research article] Lipids Health Dis 2017;16:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huffman KM, Jessee R, Andonian B, et al. Molecular alterations in skeletal muscle in rheumatoid arthritis are related to disease activity, physical inactivity, and disability. Arthritis Res Ther 2017;19:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fenton SA, Veldhuijzen van Zanten J, Kitas GD, et al. Sedentary behaviour is associated with increased long‐term cardiovascular risk in patients with rheumatoid arthritis independently of moderate‐to‐vigorous physical activity. BMC Musculoskelet Disord 2017;18:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benatti FB, Pedersen BK. Exercise as an anti‐inflammatory therapy for rheumatic diseases‐myokine regulation. Nat Rev Rheumatol 2015;11:86–97. [DOI] [PubMed] [Google Scholar]
- 34. de Hair MJ, Landewe RB, van de Sande MG, et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis 2013;72:1654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Voigt LF, Koepsell TD, Nelson JL, et al. Smoking, obesity, alcohol consumption, and the risk of rheumatoid arthritis. Epidemiology 1994;5:525–32. [PubMed] [Google Scholar]
- 36. Singh S, Facciorusso A, Singh AG, et al. Obesity and response to anti‐tumor necrosis factor‐alpha agents in patients with select immune‐mediated inflammatory diseases: a systematic review and meta‐analysis. PLoS One 2018;13:e0195123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause‐specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2018;70:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Engvall IL, Elkan AC, Tengstrand B, et al. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin‐like growth factor. Scand J Rheumatol 2008;37:321–8. [DOI] [PubMed] [Google Scholar]
- 39. Challal S, Minichiello E, Boissier MC, et al. Cachexia and adiposity in rheumatoid arthritis. Relevance for disease management and clinical outcomes. Joint Bone Spine 2016;83:127–33. [DOI] [PubMed] [Google Scholar]
- 40. Baker JF, Billig E, Michaud K, et al. Weight loss, the obesity paradox, and the risk of death in rheumatoid arthritis. Arthritis Rheumatol 2015;67:1711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sattar N, McInnes IB. Rheumatoid arthritis: debunking the obesity‐mortality paradox in RA. Nat Rev Rheumatol 2015;11:445–6. [DOI] [PubMed] [Google Scholar]
- 42. Kreps DJ, Halperin F, Desai SP, et al. Association of weight loss with improved disease activity in patients with rheumatoid arthritis: a retrospective analysis using electronic medical record data. Int J Clin Rheumtol 2018;13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2015;67:1619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ranganath VK, La Cava A, Vangala S, et al. Improved outcomes in rheumatoid arthritis with obesity after a weight loss intervention: randomized trial. Rheumatology (Oxford) 2023;62:565–74. [DOI] [PubMed] [Google Scholar]
- 45. Fraser DA, Thoen J, Reseland JE, et al. Decreased CD4+ lymphocyte activation and increased interleukin‐4 production in peripheral blood of rheumatoid arthritis patients after acute starvation. Clin Rheumatol 1999;18:394–401. [DOI] [PubMed] [Google Scholar]
- 46. Iwashige K, Kouda K, Kouda M, et al. Calorie restricted diet and urinary pentosidine in patients with rheumatoid arthritis. J Physiol Anthropol Appl Human Sci 2004;23:19–24. [DOI] [PubMed] [Google Scholar]
- 47. Michalsen A, Riegert M, Ludtke R, et al. Mediterranean diet or extended fasting's influence on changing the intestinal microflora, immunoglobulin A secretion and clinical outcome in patients with rheumatoid arthritis and fibromyalgia: an observational study. BMC Complement Altern Med 2005;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hagen KB, Byfuglien MG, Falzon L, et al. Dietary interventions for rheumatoid arthritis. Cochrane Database Syst Rev 2009:CD006400. [DOI] [PubMed] [Google Scholar]
- 49. Philippou E, Petersson SD, Rodomar C, et al. Rheumatoid arthritis and dietary interventions: systematic review of clinical trials. Nutr Rev 2021;79:410–28. [DOI] [PubMed] [Google Scholar]
- 50. Huffman KM, Pieper CF, Hall KS, et al. Self‐efficacy for exercise, more than disease‐related factors, is associated with objectively assessed exercise time and sedentary behaviour in rheumatoid arthritis. Scand J Rheumatol 2015;44:106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Munsterman T, Takken T, Wittink H. Are persons with rheumatoid arthritis deconditioned? A review of physical activity and aerobic capacity. BMC Musculoskelet Disord 2012;13:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sandberg ME, Wedren S, Klareskog L, et al. Patients with regular physical activity before onset of rheumatoid arthritis present with milder disease. Ann Rheum Dis 2014;73:1541–4. [DOI] [PubMed] [Google Scholar]
- 53. Liu X, Tedeschi SK, Lu B, et al. Long‐term physical activity and subsequent risk for rheumatoid arthritis among women: a prospective cohort study. Arthritis Rheumatol 2019;71:1460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Andonian BJ, Huffman KM. Skeletal muscle disease in rheumatoid arthritis: the center of cardiometabolic comorbidities? [Review] Curr Opin Rheumatol 2020;32:297–306. [DOI] [PubMed] [Google Scholar]
- 55. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol 2018;24:417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long‐term high‐intensity exercise program effective and safe in patients with rheumatoid arthritis? Results of a randomized controlled trial. Arthritis Rheum 2003;48:2415–24. [DOI] [PubMed] [Google Scholar]
- 57. Munneke M, de Jong Z, Zwinderman AH, et al. Effect of a high‐intensity weight‐bearing exercise program on radiologic damage progression of the large joints in subgroups of patients with rheumatoid arthritis. Arthritis Rheum 2005;53:410–7. [DOI] [PubMed] [Google Scholar]
- 58. Katz P, Andonian BJ, Huffman KM. Benefits and promotion of physical activity in rheumatoid arthritis. Curr Opin Rheumatol 2020;32:307–14. [DOI] [PubMed] [Google Scholar]
- 59. Stavropoulos‐Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis 2013;72:1819–25. [DOI] [PubMed] [Google Scholar]
- 60. Bartlett DB, Willis LH, Slentz CA, et al. Ten weeks of high‐intensity interval walk training is associated with reduced disease activity and improved innate immune function in older adults with rheumatoid arthritis: a pilot study. Arthritis Res Ther 2018;20:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Häkkinen A, Pakarinen A, Hannonen P, et al. Effects of prolonged combined strength and endurance training on physical fitness, body composition and serum hormones in women with rheumatoid arthritis and in healthy controls. Clin Exp Rheumatol 2005;23:505–12. [PubMed] [Google Scholar]
- 62. Lemmey AB, Marcora SM, Chester K, et al. Effects of high‐intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum 2009;61:1726–34. [DOI] [PubMed] [Google Scholar]
- 63. Sobejana M, van den Hoek J, Metsios GS, et al. Exercise intervention on cardiorespiratory fitness in rheumatoid arthritis patients with high cardiovascular disease risk: a single‐arm pilot study. Clin Rheumatol 2022;42:3725–34. [DOI] [PubMed] [Google Scholar]
- 64. Wadley AJ, Veldhuijzen van Zanten JJ, Stavropoulos‐Kalinoglou A, et al. Three months of moderate‐intensity exercise reduced plasma 3‐nitrotyrosine in rheumatoid arthritis patients. Eur J Appl Physiol 2014;114:1483–92. [DOI] [PubMed] [Google Scholar]
- 65. Baillet A, Zeboulon N, Gossec L, et al. Efficacy of cardiorespiratory aerobic exercise in rheumatoid arthritis: meta‐analysis of randomized controlled trials. Arthritis Care Res (Hoboken) 2010;62:984–92. [DOI] [PubMed] [Google Scholar]
- 66. Hakkinen A, Sokka T, Kotaniemi A, et al. A randomized two‐year study of the effects of dynamic strength training on muscle strength, disease activity, functional capacity, and bone mineral density in early rheumatoid arthritis. Arthritis Rheum 2001;44:515–22. [DOI] [PubMed] [Google Scholar]
- 67. Wen Z, Chai Y. Effectiveness of resistance exercises in the treatment of rheumatoid arthritis: a meta‐analysis. Medicine (Baltimore) 2021;100:e25019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van den Ende CH, Breedveld FC, le Cessie S, et al. Effect of intensive exercise on patients with active rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2000;59:615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Metsios GS, Brodin N, Vlieland T, et al. Position statement on exercise dosage in rheumatic and musculoskeletal diseases: the fole of the IMPACT‐RMD toolkit. Mediterr J Rheumatol 2021;32:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bateman LA, Slentz CA, Willis LH, et al. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise ‐ STRRIDE‐AT/RT). Am J Cardiol 2011;108:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Johnson JL, Slentz CA, Houmard JA, et al. Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise). Am J Cardiol 2007;100:1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Slentz CA, Bateman LA, Willis LH, et al. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: a randomised controlled trial. Diabetologia 2016;59:2088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bouchonville M, Armamento‐Villareal R, Shah K, et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes (Lond) 2014;38:423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pineda‐Juarez JA, Lozada‐Mellado M, Hinojosa‐Azaola A, et al. Changes in hand grip strength and body weight after a dynamic exercise program and Mediterranean diet in women with rheumatoid arthritis: a randomized clinical trial. Physiother Theory Pract 2022;38:504–12. [DOI] [PubMed] [Google Scholar]
- 75. Garcia‐Morales JM, Lozada‐Mellado M, Hinojosa‐Azaola A, et al. Effect of a dynamic exercise program in combination with Mediterranean diet on quality of life in women with rheumatoid arthritis. J Clin Rheumatol 2020;26:S116–22. [DOI] [PubMed] [Google Scholar]
- 76. Lozada‐Mellado M, Llorente L, Hinojosa‐Azaola A, et al. Comparison of the impacts of a dynamic exercise program vs. a Mediterranean diet on serum cytokine concentrations in women with rheumatoid arthritis. A secondary analysis of a randomized clinical trial. Front Nutr 2022;9:834824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lange E, Kucharski D, Svedlund S, et al. Effects of aerobic and resistance exercise in older adults with rheumatoid arthritis: a randomized controlled trial. Arthritis Care Res (Hoboken) 2019;71:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kucharski D, Lange E, Ross AB, et al. Moderate‐to‐high intensity exercise with person‐centered guidance influences fatigue in older adults with rheumatoid arthritis. Rheumatol Int 2019;39:1585–94. [DOI] [PubMed] [Google Scholar]
- 79. Lange E, Gjertsson I, Mannerkorpi K. Long‐time follow up of physical activity level among older adults with rheumatoid arthritis. Eur Rev Aging Phys Act 2020;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Day AL, Singh JA. Cardiovascular disease risk in older adults and elderly patients with rheumatoid arthritis: what role can disease‐modifying antirheumatic drugs play in cardiovascular risk reduction? [Review] Drugs Aging 2019;36:493–510. [DOI] [PubMed] [Google Scholar]
- 81. Rausch Osthoff AK, Niedermann K, Braun J, et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis 2018;77:1251–60. [DOI] [PubMed] [Google Scholar]
- 82. Piercy KL, Troiano RP. Physical activity guidelines for americans from the US Department of Health and Human Services. Circ Cardiovasc Qual Outcomes 2018;11:e005263. [DOI] [PubMed] [Google Scholar]
- 83. Krukowski RA, Tilford JM, Harvey‐Berino J, et al. Comparing behavioral weight loss modalities: incremental cost‐effectiveness of an internet‐based versus an in‐person condition. Obesity (Silver Spring) 2011;19:1629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Perez Garcia de Sevilla G, Barcelo Guido O, De la Cruz MP, et al. Remotely supervised exercise during the COVID‐19 Pandemic versus in‐person‐supervised exercise in achieving long‐term adherence to a healthy lifestyle. Int J Environ Res Public Health 2021;18:12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ganeshan S, Jackson H, Grandis DJ, et al. Clinical outcomes and qualitative perceptions of in‐person, hybrid, and virtual cardiac rehabilitation. J Cardiopulm Rehabil Prev 2022;42:338–46. [DOI] [PubMed] [Google Scholar]
- 86. AbouAssi H, Tune KN, Gilmore B, et al. Adipose depots, not disease‐related factors, account for skeletal muscle insulin sensitivity in established and treated rheumatoid arthritis. J Rheumatol 2014;41:1974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Crowson CS, Matteson EL, Davis JM, 3rd , et al. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. de Hair MJ, Landewe RB, van de Sande MG, et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis 2013;72:1654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lu B, Hiraki LT, Sparks JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Ann Rheum Dis 2014;73:1914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Voigt LF, Koepsell TD, Nelson JL, et al. Smoking, obesity, alcohol consumption, and the risk of rheumatoid arthritis. Epidemiology 1994;5:525–32. [PubMed] [Google Scholar]
- 91. Singh S, Facciorusso A, Singh AG, et al. Obesity and response to anti‐tumor necrosis factor‐alpha agents in patients with select immune‐mediated inflammatory diseases: a systematic review and meta‐analysis. PLoS One 2018;13:e0195123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Diaz BB, Gonzalez DA, Gannar F, et al. Myokines, physical activity, insulin resistance and autoimmune diseases. Immunol Lett 2018;203:1–5. [DOI] [PubMed] [Google Scholar]
- 93. Simpson RJ. Aging, persistent viral infections, and immunosenescence: can exercise "make space"? [Review] Exerc Sport Sci Rev. 2011;39:23–33. [DOI] [PubMed] [Google Scholar]
- 94. Kruger K, Mooren FC. T cell homing and exercise. Exerc Immunol Rev 2007;13:37–54. [PubMed] [Google Scholar]
- 95. Duggal NA. Reversing the immune ageing clock: lifestyle modifications and pharmacological interventions. Biogerontology 2018;29:481–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Duggal NA, Pollock RD, Lazarus NR, et al. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell 2018;17:e12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bartlett DB, Willis LH, Slentz CA, et al. Ten weeks of high‐intensity interval walk training is associated with reduced disease activity and improved innate immune function in older adults with rheumatoid arthritis: a pilot study. Arthritis Res Ther 2018;20:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gill LE, Bartels SJ, Batsis JA. Weight management in older adults. Curr Obes Rep 2015;4:379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Human energy requirements : report of a joint FAO/WHO/UNU Expert Consultation. Food Nutr Bull 2005;26:166. [PubMed] [Google Scholar]
- 100. Lopes JS, Machado AF, Micheletti JK, et al. Effects of training with elastic resistance versus conventional resistance on muscular strength: a systematic review and meta‐analysis. SAGE Open Med 2019;7:2050312119831116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. U.S. Department of Health and Human Services and U.S. Department of Agriculture . 2015‐2020 Dietary Guidelines for Americans. 8th ed. Washington DC: Office of Disease Prevention and Health Promotion; 2015.
- 102. Crowson CS, Gabriel SE, Semb AG, et al. Rheumatoid arthritis‐specific cardiovascular risk scores are not superior to general risk scores: a validation analysis of patients from seven countries. Rheumatology (Oxford) 2017;56:1102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gualtierotti R, Ughi N, Marfia G, et al. Practical management of cardiovascular comorbidities in rheumatoid arthritis. Rheumatol Ther 2017;4:293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Carroll SJ, Paquet C, Howard NJ, et al. Validation of continuous clinical indices of cardiometabolic risk in a cohort of Australian adults. BMC Cardiovasc Disord 2014;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Johnson JL, Slentz CA, Houmard JA, et al. Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise). Am J Cardiol 2007;100:1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bateman LA, Slentz CA, Willis LH, et al. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise ‐ STRRIDE‐AT/RT). Am J Cardiol 2011;108:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Willis LH, Slentz CA, Houmard JA, et al. Minimal versus umbilical waist circumference measures as indicators of cardiovascular disease risk. Obesity (Silver Spring) 2007;15:753–9. [DOI] [PubMed] [Google Scholar]
- 108. Prevoo ML, van 't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 109. Liguori G. ACSM's guidelines for exercise testing and prescription. 11th ed. Wolters Kluwer Health; 2022. [Google Scholar]
- 110. Adami A, Rossiter HB. Principles, insights, and potential pitfalls of the noninvasive determination of muscle oxidative capacity by near‐infrared spectroscopy. J Appl Physiol (1985) 2018;124:245–8. [DOI] [PubMed] [Google Scholar]
- 111. Fleiss JL. The design and analysis of clinical experiments. New York, NY: Wiley; 1986. [Google Scholar]
- 112. Committee for Proprietary Medicinal Products (CPMP) . Committee for Proprietary Medicinal Products (CPMP): points to consider on adjustment for baseline covariates. Stat Med 2004;23:701–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form


