Abstract
IMPORTANCE
As the incidence of cancer and metabolic disorders, such as obesity, concurrently rise, there has been increasing awareness of the pervasive effect of nutrition. The whole foods plant-based diet (WFPBD) and ketogenic diet (KD) have gained popularity in oncology, and this topic is increasingly permeating clinical dialogue.
OBSERVATIONS
Dietary intake is associated with multiple pathways involved in carcinogenesis and tumor progression. Consumption of a plant-enriched diet is associated with reduced cancer incidence and is recommended by dietary guidelines for cancer prevention. Despite a starkly different nutrient composition, a WFPBD and KD can be associated with weight loss, decreased inflammation, and decreased insulin levels. In addition, a WFPBD is associated with increased fiber, phytochemicals, and butyrate levels and decreased insulin-like growth factor 1 levels, whereas a KD exerts potential anticancer effects by increasing β hydroxybutyrate levels. A KD may be of interest in select, less common settings, such as tumors treated with phosphatidylinositol 3-kinase inhibitors, which induce hyperinsulinemia and hyperglycemia. Completed interventional trials have focused on increasing fruit and vegetable intake or reducing fat intake but have not specifically tested WFPBD or KD for cancer prevention or treatment. Currently available data support plant-based diets as opposed to KD as part of a lifestyle associated with reduced cancer risk. In the postdiagnosis setting, there are currently no rigorously tested approaches that support the recommendation of any diet to treat cancer.
CONCLUSIONS AND RELEVANCE
The results of this review suggest that the collective evidence supports plant-enriched diets vs KD for the reduction of cancer risk and the improvement of metabolic disorders in survivors. Additional prospective randomized clinical trials are needed to encourage use of dietary modification across the cancer continuum. Rigorous trial designs that adapt classical oncologic end points may identify populations that are likely to benefit from starkly contrasting diets. Current data support prioritization of plant-based diets, and future data could further personalize dietary recommendations in cancer populations.
The link between nutrition and health has long been of interest, dating back to the saying, “Let food be thy medicine and let medicine be thy food.” As human dietary patterns have evolved based on the availability of processed foods, we have seen a rise in chronic diseases, including cancer. A National Health and Nutrition Examination Survey study attributed more than 80 000 new cancer cases to a poor diet in the US.1 Simultaneously, we have seen nutrition research evolve to match new diet trends. Substantial emphasis has recently been placed on 2 popular diets for cancer prevention/treatment that have divergent dietary macronutrient and micronutrient profiles: the whole foods plant-based diet (WFPBD) and the ketogenic diet (KD). The merits are debated, despite the evidence available to support WFPBD for cancer prevention. This topic is increasingly permeating clinical dialogue with patients.
The WFPBD maximizes nutrient-dense plant foods and minimizes processed foods, oils, and animal foods, focusing on micronutrient density rather than a fixed macronutrient proportion. Thus, it is a minimally processed, low-fat, moderate-to-high–carbohydrate (unrefined) diet. The KD challenges the dogma that meat intake should be reduced and reverses the dietary macronutrient profile with fat and protein as major macronutrients instead of carbohydrates. The KD aims to promote the production of ketone bodies from fatty acids, typically through very low carbohydrate (approximately 5% calories), high fat (approximately 75% calories), and moderate protein (approximately 20% calories) intake. Most calories in a KD are obtained from meat, dairy, fish, oils, and eggs, with some nuts, seeds, and nonstarchy vegetables. These calculations typically translate to fewer than 40 g of carbohydrates per day compared with the standard Western-style diet (>250 g/day). In contrast, low-carbohydrate diets (LCDs), although also low in carbohydrates, usually have modest carbohydrate reductions of less than 26% calories or fewer than 100 to 130 g per day.
Epidemiologic Studies
In the prevention setting, plant-based diets are consistently associated with a reduced cancer incidence; seminal large epidemiologic studies include the Adventist Health Study–2 (hazard ratio [HR], 0.84; 95% CI, 0.72–0.99),2 EPIC Oxford and Oxford Vegetarian Cohort (HR, 0.88; 95% CI, 0.82–0.95)3 and NutriNet-Santé (HR, 0.85; 95%CI,0.76–0.97).4To our knowledge, long-term epidemiologic evidence for a KD is not available, but participants with diets low in plant foods had an increased cancer incidence in these studies. Furthermore, 2meta-analyses showed thatLCDs are associated with higher mortality, especially for those whose diets favored animal-derived protein and fat.5,6 Low protein intake (<10% calories) has been associated with a 4-fold reduction in cancer mortality compared with high protein intake (>20% calories) in adults aged 50 to 65 years in the National Health and Nutrition Examination Survey III study. This association was abolished or attenuated if the source of proteinswere plant-based,7 similar to other studies in which plant proteins were associated with lower all-cause mortality.8
Consistent with these data and many other studies, the American Institute of Cancer Research/World Cancer Research Fund and the American Cancer Society recommend a diet comprising primarily whole plant-based foods and limited sugary drinks, highly processed foods, refined grains, and red and processed meats based on cumulative generalizable evidence for cancer.9 The World Cancer Research Fund also summarized the evidence for individual cancers. Few US adults meet these dietary recommendations, irrespective of body mass index.10
Pathophysiology
Nutrition may play a substantial role in cancer prevention and treatment via multiple mechanisms. Although there are considerable differences in the KD and WFPBD, there are some common mechanisms by which they may be associated with a decrease in cancer risk. A summary of potential mechanisms is shown in the Figure.
Figure.
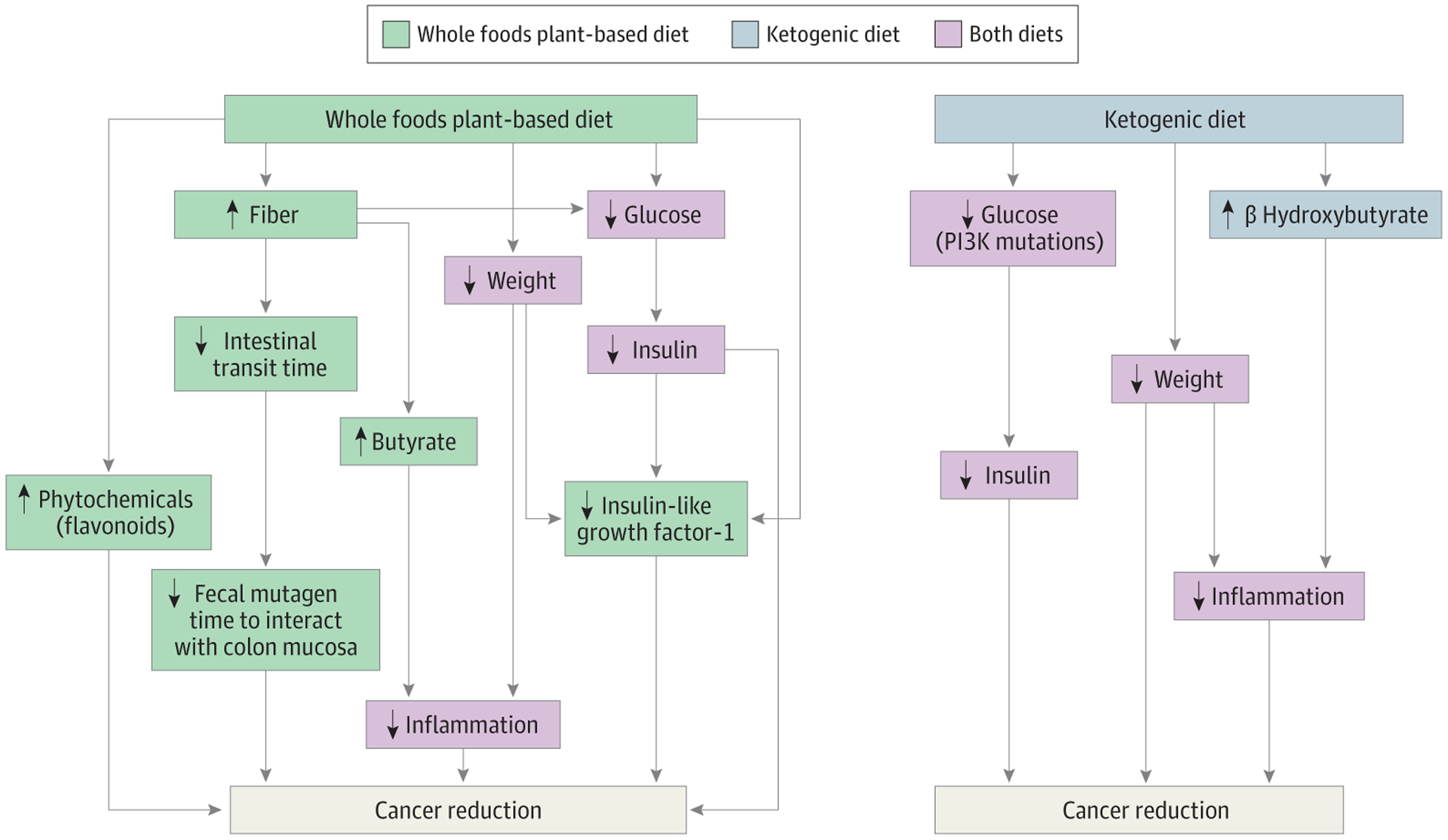
Association of Plant-Based Diets With Multiple Additional Pathways That Suppress Cancer Growth Compared With Ketogenic Diets
Weight Loss
Obesity is a risk factor for several cancers,11 and its incidence is rising in younger adults.12 Both diets eliminate processed foods and refined carbohydrates, dietary components that are associated with increased mortality and weight gain, although there are some differences.13,14 Fiber-rich foods in a WFPBD are low in calories and lead to early satiety,15 whereas KDs can suppress appetite,16 similarly reducing calorie intake.
An inpatient randomized clinical trial (RCT) of 20 healthy patients assessed an ad libitum WFPBD or KD for 2 weeks each. The WFPBD led to significantly less energy intake. Despite this, patients adhering to the KD lost more weight, and most of this weight loss was owing to a decrease in fat-free (lean) mass, whereas the WFPBD had a significant decrease in fat mass.13
In the postdiagnosis setting, in the KETOCOMP study, 29 patients with breast cancer who were adhering to a KD lost total body weight and fat mass after initial water loss compared with patients following a standard diet.17 Similar weight loss was seen in astrocytoma18 and prostate cancer populations.19 Similar significant weight loss with a WFPBD intervention was seen in a randomized study in patients with prostate cancer.20
Despite the KD being an effective weight loss strategy, concerns have been raised about its long-term adverse effects. A nonketogenic LCD compared with a KD was equally effective in reducing body weight and insulin resistance, but the KD was associated with higher low-density lipoprotein (LDL) cholesterol levels and fatigue-inertia scores.21 Studies have shown variable long-term changes in LDL cholesterol levels and evidence of dyslipidemia in those adhering to a KD; thus, the association with long-term cardiovascular outcomes is unclear.22 In contrast, a WFPBD led to significantly lower LDL and total cholesterol levels compared with a standard low-fat diet.23 It is unclear whether loss in lean mass and increase in fatigue occur consistently while following a KD; if so, it is also unknown whether this could represent a long-term risk for patients with cancer cachexia.
Reduction in Insulin-Like Growth Factor 1 Levels
Insulin-like growth factor 1 (IGF-1) is positively associated with the risk of several cancers by stimulating proliferation and inhibiting apoptosis.24,25 Individuals who consume a vegan diet have significantly lower levels of IGF-1 and higher levels of IGF-binding proteins 1 and 2,26 which may be associated with the specific effects of plant proteins and lower overall protein intake compared with a Western diet.27 Ketogenic diets have shown a reduction in insulin levels but the reduction in IGF-1 was not significant.18,28
In vivo studies with a lymphoma mouse model also suggested that a low-protein diet compared with an LCD was associated with a decrease in tumor growth and increase in tumor-infiltrating lymphocytes, which were followed by ananticancer immuneresponse.29 Similar findings were seen in hepatocellular cancer rat models.30
Reduction in Insulin Resistance
Insulin and some of the most prevalent genomic alterations in human cancers (such as PIK3CA variations and PTEN loss) activate the phosphoinositide 3-kinase (PI3K) signaling cascade. This pathway regulates cellular metabolism as well as cell survival and proliferation. Thus, high insulin levels can promote and sustain tumor growth.31 A WFPBD is associated with decreased fasting plasma insulin concentrations and improved insulin sensitivity.32 Decrease d insulin levels have also been reported in KD clinical trials,18,28 although some preclinical studies showed contrary findings, suggesting increased insulin resistance.33Treatment with a KD had variable effects on different tumor mouse models (including accelerated disease progression in acute myeloid leukemia).31 Therapeutic targeting of the PI3K signaling cascade with PI3K inhibitors leads to systemic feedback with acute insulin release and hyperglycemia that impairs the efficacy of these agents. Approaches that reduce insulin exposure might increase the efficacy of these inhibitors. Inmouse models, KDs deplete hepatic glycogen content, which prevents the degree of hyperglycemia and hyperinsulinemia observed following PI3K inhibitor treatment, thus enhancing the drug’s effectiveness.31 It may be possible that a WFPBD may also have some benefit for managing PI3K inhibitor–induced hyperglycemia, althoughtoourknowl-edge this has not been studied.32
Increased Fiber
A large meta-analysis showed a decrease in cancer mortality (relative risk [RR], 0.85; 95% CI, 0.80–0.91) and all-cause mortality (RR, 0.83; 95% CI, 0.77–0.90) per 90-g per day increase in whole grain intake.34 Fiber intake was inversely correlated with the risk of cancers in the EPIC study as well.35
In patients with melanoma who were treated with checkpoint inhibitors, patients with sufficient dietary fiber intake ([H11350]20 g per day) and no probiotic use had higher odds of responding to treatment with programmed cell death 1 (PD-1) inhibitors as well as a longer progression-freesurvival.These findings were confirmed in preclinical animal models, and mice that received a low-fiber diet or probiotics had a lower frequency of interferon γ–positive cytotoxic T cells in the tumor microenvironment and an impaired response to anti–PD-1 therapy.36 A preclinical model with anti–cytotoxic T-lymphocyte associated protein 4 inhibitors showed contrasting results.37 Similar positive effects of fiber have been observed in the setting of colon cancer prevention. Given higher rates of colon cancer in African American individuals compared with African individuals living in rural areas, 20 healthy African American individuals and 20 African individuals from rural areas were administered a 2-week dietswitch(high-fiber, low-fat,AfricanstyledietadministeredtoAfrican American individuals and a high-fat, low-fiber, Western stylediet to African individuals from rural areas). The high-fiber diet was associated with decreases in cell proliferation (Ki67) and CD3+ lymphocytes in colon mucosa, as well as an increase in butyrate in the stool.38 These positive effects of high-fiber diets are typically not achieved in KDs because the recommended dietary fiber intake is rarely met compared with a WFPBD.39
Reduction in Inflammation
When weight loss was achieved via either diet or exercise, parallel reductions in circulating inflammatory molecules were observed.40 Additionally, a WFPBD was associated with lower levels of oxidative stress and inflammation.41,42 While data are limited, some clinical studies of KD suggested proinflammatory effects, such as an increase in Creactive protein and a decrease in fibroblast growthfactor 21 levels, which are regulators with anti-inflammatory properties compared with the average US diet.43
Phytochemicals
Plant foods contain phytochemicals, such as flavonoids, that have anti cancer properties; therefore, they are more abundant in a WFPBD than a KD. Large epidemiologic studies have shown that moderate habitual intake of flavonoids is inversely associated with all-cause and cancer-related mortality.44 Flavonoids have anti-inflammatory effects (via the mitogen-activated protein kinases, nuclear factor-κB (NF-kB), nodlike receptor pyrin domain–containing 3 inflammasome, signal transducer and activator of transcription 3 pathways) and antioxidant effects (via the Warburg effect, nuclear factor erythroid 2–related factor 2, and hypoxia-inducible factor 1 α signaling)and lead to apoptosis and cell cycle arrest, alter cell growth and metabolism (via protein kinase B/mammalian target of rapamycin and renin-angiotensin system/extracellular signal-regulated kinase inhibition), and modulate autophagy.45
Short-Chain Fatty Acids
Some of the beneficial anticancer effects of both diets were associated with an increase in short-chainfattyacids(SCFAs)bydifferent mechanisms. Fiber-rich plant-based diets were associated with increased butyrate producers while animal-based diets were associated with increased bile tolerant organisms in the gut microbiome.46,47The butyrate producers facilitate production of SCFAs (butyrate/acetate), which inhibit the nuclear factor-ΚB pathway and histone deacetylases (HDACs), leading to anticancer and anti-inflammatory effects.48,49 Higher stool butyrate concentrations and the relative abundance of butyrate producers were associated with minimal residualdisease negativity in patients with multiple myeloma who were receiving maintenance therapy in a small single-center study.50
Conversely, KDs were associated with increased blood ketone body levels(mainlyβ-hydroxybutyrate[βHB]), aSCFA.AlthoughβHB also has HDAC inhibitory effects, butyrate is a stronger HDAC inhibitor.51 There is conflicting evidence as to whether βHB has anticancer effects vs proinflammatory and tumor proliferative effects in in vitro and in vivo models.51–53 The discrepant findings may be because of a βHB paradox. In transgenic mice bearing a spontaneous mouse mammary tumor virus NEU-NT model, tumors that were inhibited by βHB preferentially utilized glucose vs βHB and accumulated high βHB levels that inhibited HDAC, whereas some tumors preferentially utilized βHB, which enhanced their growth rate andreducedβHBconcentrationstolessthanthethresholdforHDAC inhibition.53 Therefore, tumor screening for ketone body metabolizing enzyme activation may identify those likely to benefit from a KD; however, concerns remain that the tumor could metabolically adapt and eventually develop tolerance to the KD.
Limited preclinical data suggest that KD may enhance the effects of specific cancer treatments and/or have tumor subtype–specific effects. For example, KDs induced tumor growth retardation in models in which anti–PD-1 treatment alone or in combination with anti–cytotoxic T-lymphocyte associated protein 4 failed to reduce tumor growth via alterations in the gut microbiome.54 Pre-clinical data in BRAF V600E–expressing melanoma suggested that a KD upregulated 3-hydroxy-3-methylglutaryl coenzyme A lyase (a key enzyme in ketogenesis), which raised levels of acetoacetate that then activated MEK1 and fueled tumor growth. Additionally, hypolipidemic agents attenuate tumor growth by reducing serum acetoacetate.55 The βHB promotes anabolic growth of breast cancercells(approximately2.5fold)andmetastaticdissemination.These data led to the study of ketone inhibitors for cancer treatment.56,57
Ketogenic diets have shown antitumorpotentialinsomemouse studies(especiallybraintumormodels18)58and variable effects in other studies.31 To date, definitive human clinical trial data are awaited.59
Interventional Clinical Trials
Plant-Enriched Dietary Interventions
Several trials have demonstrated that weight loss and dietary modification are feasible in cancer populations and have been reviewed elsewhere.60Although most completed interventional studies have not incorporated a whole foods approach or, more specifically, a WFPBD, several trial interventions aimed to increase fruit and vegetable intake or reduce fat intake. In this article, we discuss some of the largest dietary studies in oncology that have tested interventions that are most representative of WFPBD or KD.60
In the postdiagnosis setting, for patients with prostate cancer on observation, aWFPBDcompared with control was associated with a significantly reduced incidence of conventional prostate cancer treatment.61 The growth of prostate cancer cells was significantly inhibited almost 8 times by serum from patients adhering to a WFPBD vs the control group in vitro.20 Favorable changes were also found in tumor gene expression, and an increase in relative telo mere length was observed with a WFPBD.62,63TheMen’sEating and Living (MEAL) Study (CALGB 70807 [Alliance]), a larger RCT promoting 7 or more servings of vegetables daily, showed no difference in the time to cancer progression, possibly owing to low dietary adherence, and points to the challenges of changing dietary habits with counseling alone.64
In breast cancer, several trials have tested plant-enriched diets, although not strictly WFPBD. In the prevention setting, the Women’s Health Initiative (WHI) Study compared the effect of a reduced fat dietary intervention with a usual diet group in 48 835 postmenopausal women and showed that those in the low-fat group had improved breast cancer–specific and overall survival.65 In the postdiagnosis setting, the 2 largest RCTs of dietary interventions are the Women’s Healthy Eating and Living (WHEL) Study and the Women’s Intervention Nutrition Study (WINS). The WHEL Study tested a diet comprising 5 vegetable servings, 3 fruit servings, and 30 g of fiber with a 15% to 20% energy intake from fat vs a control arm diet (usual care). The primary outcome measures were recurrent or new invasive breast cancer or death of any cause. During a 7.3-year follow-up period, the dietary intervention did not reduce breast cancer events or mortality.66 The WINS study tested the effect of reduced fat intake (<15%) in women with resected, early-stage breast cancer who were receiving conventional cancer treatment. In contrast to the WHEL study, participants in the low-fat diet arm of this trial experienced a 24% reduction in breast cancer relapse events compared with the usual diet group, although this effect was attenuated in long-term follow up.67,68 The conflicting results of these seminal dietary trials highlight the need for further research and do not adequately test the efficacy of a WFPBD.
Ketogenic and Similar Dietary Interventions
A systematic review and meta-analysis of 6 RCTs evaluating the efficacy of KD as antitumor therapy concluded that there was inconclusive evidence. Completion rates for KD interventions ranged from 45%to75%, highlighting the challenges of ensuring compliance with KDs.69 To date, to our knowledge, no KD RCTs powered to cancer-specific outcomes have been reported.
An LCD study in patients with prostate cancer with biochemical recurrence, elevated body mass index, and a prostate-specific antigen doubling time of 3 to 36 months showed no difference in the mean prostate-specific antigen doubling time, although there was significant weight loss and improvements in high-density lipoprotein cholesterol, triglyceride, and hemoglobin A1c levels in the interventional arm.19 More recently, a nonrandomized study (KOLBIRI) tested the feasibility of a KD, LCD vs standard diet in survivors of breast cancer and showed significant weight loss and improved quality of life in all arms, although breast cancer–specific end points were not studied.70 Similar findings were reported in non-cancer populations. Currently, there are 46 KD trials (20 active, 10 completed, and 6 terminated [poor accrual/compliance]; 5 with unknown status) and only 8 WFPBD trials (6 active, 2 completed, and 0 terminated) (eTables 1 and 2 in the Supplement).
Other Diets
Other dietary patterns with limited data for cancer prevention and controlincludeLCD,Mediterranean, andmacrobioticdiets.The macrobiotic and Mediterranean diets are very similar to a WFPBD, as they are enriched for plant foods and fiber. The LCDs are similar to KDs bylimiting carbohydrate intake, although they are less restrictive and typically do not achieve ketosis. Several of these dietary patterns have garnered attention in popular literature, although the evidence for benefit in cancer populations is sparse. Some additional data are anticipated from ongoing trials. For example, the Diet and Androgen-5 (DIANA-5) study is an ongoing RCT in 1344 women aimed at testing whether a Mediterranean-macrobiotic dietary pattern can reduce the incidence of breast cancer–related events.71
Time-restricted eating via fasting or fasting-mimicking diets has also gained increasing popularity inrecent years.Preclinical and preliminary clinical data indicate that these approaches are associated with alterations in metabolites, growth factors, and antitumor immunity that limit the ability of cancer cells to adapt and survive within the host environment.72While fasting approaches have been shown to induce weight loss, it is unclear whether fasting is superior to caloric restriction, and caution is advised in patients with low lean mass.73 The mechanisms and applications of fasting approaches in the setting of cancer have been reviewed elsewhere.74
Challenges
Nutrition trials with cancer-specific primary objectives often require large sample sizes and long intervention periods to see changes, especially given that long-term adherence to dietary changes during clinical trials is difficult. Other challenges with nutrition studies in oncology include differences inintervention duration, lack of standardization of macronutrient content, conflation of weight loss effect vs nutrient intake effect, and suboptimal adherence. For example, if ketosis is not achieved, a KD essentially amounts to carbohydrate restriction. Personal food preferences, psychosocial issues around a cancer diagnosis, adverse effects of cancer and its treatment (such as appetite loss, nausea, and cachexia), and other medical comorbidities can further accentuate these challenges, making it harder to initiate and sustain dietary changes.
Plant-based KDs are a potential alternative, with benefits from both dietary patterns.This approach may be difficult to sustain given the limited number of high-fat plant foods. Limited food variety is also associated with decreased microbial diversity.75
Conclusions and Future Directions
A large body of evidence suggests that dietary intake is associated with cancer outcomes (Box). The preponderance of available data supports plant-enriched diets for cancer prevention and control, although confirmatory prospective trials are required. The WFPBD is part of established, universally recommended healthy lifestyle habits. Adherence to a WFPBD is associated with reduced cancer risk and has been shown to confer additional benefits for cardiovascular disease, diabetes, body weight, and body composition.32,76–78
Box. Summary of Current Evidence, Limitations, and Future Directions in Nutrition for Cancer.
Current Evidence
Epidemiologic studies suggest that there is a reduced cancer incidence with plant-based dietary patterns.
Mechanistic evidence for both diets includes weight loss, decreased inflammation, and decreased insulin levels.
Mechanistic evidence for WFPBD alone includes increased fiber, phytochemicals, and butyrate levels and decreased IGF-1 levels.
Mechanistic evidence for KD alone includes increased β hydroxybutyrate levels.
Large RCTs have tested low-fat and weight loss interventions in cancer populations, although trials with WFPBD or KD interventions with cancer-specific outcomes are not available.
Limitations
Nutrition trials with cancer-specific outcomes require long intervention periods and large sample sizes to be able to identify a meaningful difference.
Long-term adherence to dietary changes on clinical trials is challenging.
Future Directions
Cancer nutrition research is likely to benefit from trial designs adapted from drug development.
Develop clinical trials with prescriptive meal delivery services to improve compliance and be better able to study the outcomes of interest.
Design clinical trials with mechanistic translational correlatives, such as epigenetic, immune, and microbiome changes.
Prioritize studies that evaluate the role of a WFPBD in cancer populations given the limited number of trials that are currently ongoing.
Abbreviations: IGF, insulin-like growth factor; KD, ketogenic diet; RCT, randomized clinical trial; WFPBD, whole food plant-based diet.
Accordingly, several professional organizations recommend dietary patterns that are consistent with WFPBD for primary risk reduction. In contrast, far fewer data support the KD for cancer prevention or survivorship. Exceptions may be supported by data from well-designed prescriptive KD studies in specific tumors that have an underlying mechanistic basis, such as PIK3CA-mutated tumors, if ongoing trials are affirmative.
Data from prospective RCTs are required to change clinical recommendations. Currently, to our knowledge, no such data exist to support the recommendation of specific diets for adjunctive cancer treatment. The National Cancer Institute’s translational framework provides a blueprint for lifestyle intervention development and should guide future WFPBD and KD trials.79 The safety and tolerability of these specific diets could be substantially affected by ongoing cancer therapy, which underscores the need to start with phase 1 safety studies, followed by phase 2 preliminary efficacy trials (biologic and/or cancer-related) that confirm safety, and finally, confirmatory phase 3 trials testing classical oncologic end points.80
Despite the epidemiologic evidence supporting plant-enriched diets, there are currently more ongoing trials testing KDs than WFPBDs in cancer populations. The data reviewed in this article support an urgent call to investigators, clinicians, and funding agencies to prioritize interventional WFPBD trials in oncology.
Supplementary Material
Funding/Support:
Drs Shah and Iyengar are supported by the National Institutes of Health/National Cancer Institute Memorial Sloan Kettering Cancer Center Support grant P30 CA008748 and participated in the TREC Training Workshop (grant R25CA203650). Dr Shah is supported by research grants from International Myeloma Society Career Development Award, Paula and Rodger Riney Foundation, the Allen Foundation Inc, the HealthTree Foundation, the Parker Institute of Cancer Immunotherapy, and the National Cancer Institute MSK Paul Calabresi Career Development Award for Clinical Oncology (grant K12CA184746) to her institution. Dr Iyengar is supported by research grants from National Institutes of Health/National Cancer Institute (R01 CA235711, R01 CA241409), the American Cancer Society (Research Scholar Grant), the Breast Cancer Research Foundation, Novartis, and Kat’s Ribbon of Hope to his institution.
Role of the Funder/Sponsor:
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr Shah reported grants from Celgene/BMS and Janssen to her institution and personal fees from MJH Life Sciences, Association of Community Cancer Centers, MashUp MD, and Janssen Biotech outside the submitted work. Dr Iyengar reported grants from Novartis to his institution and personal fees from Novartis and Seattle Genetics outside the submitted work.
Contributor Information
Urvi A. Shah, Myeloma Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, New York;; Department of Medicine, Weill Cornell Medical College, New York, New York;
Neil M. Iyengar, Department of Medicine, Weill Cornell Medical College, New York, New York;; Breast Medicine Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, New York.
REFERENCES
- 1.Zhang FF, Cudhea F, Shan Z, et al. Preventable cancer burden associated with poor diet in the United States. JNCI Cancer Spectr. 2019;3(2):pkz034. doi: 10.1093/jncics/pkz034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tantamango-Bartley Y, Jaceldo-Siegl K, Fan J, Fraser G. Vegetarian diets and the incidence of cancer in a low-risk population. Cancer Epidemiol Biomarkers Prev. 2013;22(2):286–294. doi: 10.1158/1055-9965.EPI-12-1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Key TJ, Appleby PN, Crowe FL, Bradbury KE, Schmidt JA, Travis RC. Cancer in British vegetarians: updated analyses of 4998 incident cancers in a cohort of 32,491 meat eaters, 8612 fish eaters, 18,298 vegetarians, and 2246 vegans. Am J Clin Nutr. 2014;100(suppl 1):378S–385S. doi: 10.3945/ajcn.113.071266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane-Diallo A, Srour B, Sellem L, et al. Association between a pro plant-based dietary score and cancer risk in the prospective NutriNet-santé cohort. Int J Cancer. 2018;143(9): 2168–2176. doi: 10.1002/ijc.31593 [DOI] [PubMed] [Google Scholar]
- 5.Seidelmann SB, Claggett B, Cheng S, et al. Dietary carbohydrate intake and mortality:a prospective cohort study and meta-analysis. Lancet Public Health. 2018;3(9):e419–e428. doi: 10.1016/S2468-2667(18)30135-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noto H, Goto A, Tsujimoto T, Noda M. Low-carbohydrate diets and all-cause mortality: a systematic review and meta-analysis of observational studies. PLoS One. 2013;8(1):e55030. doi: 10.1371/journal.pone.0055030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine ME, Suarez JA, Brandhorst S, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014; 19(3):407–417. doi: 10.1016/j.cmet.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Y, Liu B, Snetselaar LG, et al. Association of major dietary protein sources with all-cause and cause-specific mortality: prospective cohort study. J Am Heart Assoc. 2021;10(5):e015553. doi: 10.1161/JAHA.119.015553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouvard V, Loomis D, Guyton KZ, et al. ; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599–1600. doi: 10.1016/S1470-2045(15)00444-1 [DOI] [PubMed] [Google Scholar]
- 10.Good M, Braun AC, Taylor CA, Spees CK. US adults fall short of the dietary guidelines for cancer prevention regardless of BMI category. J Acad Nutr Diet. 2021;S2212–2672(21)00120–9. doi: 10.1016/j.jand.2021.02.013 [DOI] [PubMed] [Google Scholar]
- 11.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K; International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–798. doi: 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4(3):e137–e147. doi: 10.1016/S2468-2667(18)30267-6 [DOI] [PubMed] [Google Scholar]
- 13.Hall KD, Guo J, Courville AB, et al. Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat Med. 2021;27(2):344–353. doi: 10.1038/s41591-020-01209-1 [DOI] [PubMed] [Google Scholar]
- 14.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312(9):923–933. doi: 10.1001/jama.2014.10397 [DOI] [PubMed] [Google Scholar]
- 15.Slavin J, Green H. Dietary fibre and satiety. Nutr Bull. 2007;32(s1):32–42. doi: 10.1111/j.1467-3010.2007.00603.x [DOI] [Google Scholar]
- 16.Gibson AA, Seimon RV, Lee CMY, et al. Do ketogenic diets really suppress appetite? a systematic review and meta-analysis. Obes Rev. 2015;16(1):64–76. doi: 10.1111/obr.12230 [DOI] [PubMed] [Google Scholar]
- 17.Klement RJ, Champ CE, Kämmerer U, et al. Impact of a ketogenic diet intervention during radiotherapy on body composition: III-final results of the KETOCOMP study for breast cancer patients. Breast Cancer Res. 2020;22(1):94. doi: 10.1186/s13058-020-01331-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreck KC, Hsu FC, Berrington A, et al. Feasibility and biological activity of a ketogenic/intermittent-fasting diet in patients with glioma. Neurology. 2021;97(9):e953–e963. doi: 10.1212/WNL.0000000000012386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedland SJ, Allen J, Jarman A, et al. A randomized controlled trial of a 6-month low-carbohydrate intervention on disease progression in men with recurrent prostate cancer: Carbohydrate and Prostate Study 2 (CAPS2). Clin Cancer Res. 2020;26(12):3035–3043. doi: 10.1158/1078-0432.CCR-19-3873 [DOI] [PubMed] [Google Scholar]
- 20.Ornish D, Weidner G, Fair WR, et al. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol. 2005;174(3):1065–1069. doi: 10.1097/01.ju.0000169487.49018.73 [DOI] [PubMed] [Google Scholar]
- 21.Johnston CS, Tjonn SL, Swan PD, White A, Hutchins H, Sears B. Ketogenic low-carbohydrate diets have no metabolic advantage over nonketogenic low-carbohydrate diets. Am J Clin Nutr. 2006;83(5):1055–1061. doi: 10.1093/ajcn/83.5.1055 [DOI] [PubMed] [Google Scholar]
- 22.Burén J, Ericsson M, Damasceno NRT, Sjödin A. A ketogenic low-carbohydrate high-fat diet increases LDL cholesterol in healthy, young, normal-weight women: a randomized controlled feeding trial. Nutrients. 2021;13(3):814. doi: 10.3390/nu13030814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardner CD, Coulston A, Chatterjee L, Rigby A, Spiller G, Farquhar JW. The effect of a plant-based diet on plasma lipids in hypercholesterolemic adults: a randomized trial. Ann Intern Med. 2005; 142(9):725–733. doi: 10.7326/0003-4819-142-9-200505030-00007 [DOI] [PubMed] [Google Scholar]
- 24.Knuppel A, Fensom GK, Watts EL, et al. Circulating insulin-like growth factor-I concentrations and risk of 30 cancers: prospective analyses in UK Biobank. Cancer Res. 2020;80(18): 4014–4021. doi: 10.1158/0008-5472.CAN-20-1281 [DOI] [PubMed] [Google Scholar]
- 25.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3(70):70ra13. doi: 10.1126/scitranslmed.3001845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen NE, Appleby PN, Davey GK, Kaaks R, Rinaldi S, Key TJ. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1441–1448. [PubMed] [Google Scholar]
- 27.Fontana L, Klein S, Holloszy JO. Long-term low-protein, low-calorie diet and endurance exercise modulate metabolic factors associated with cancer risk. Am J Clin Nutr. 2006;84(6):1456–1462. doi: 10.1093/ajcn/84.6.1456 [DOI] [PubMed] [Google Scholar]
- 28.Cohen CW, Fontaine KR, Arend RC, et al. A ketogenic diet reduces central obesity and serum insulin in women with ovarian or endometrial cancer. J Nutr. 2018;148(8):1253–1260. doi: 10.1093/jn/nxy119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubio-Patiño C, Bossowski JP, De Donatis GM, et al. Low-protein diet induces IRE1α-dependent anticancer immunosurveillance. Cell Metab. 2018; 27(4):828–842.e7. doi: 10.1016/j.cmet.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 30.Youngman LD, Campbell TC. Inhibition of aflatoxin B1-induced gamma-glutamyl transpeptidase positive (GGT+) hepatic preneoplastic foci and tumors by low protein diets: evidence that altered GGT+ foci indicate neoplastic potential. Carcinogenesis. 1992;13(9):1607–1613. doi: 10.1093/carcin/13.9.1607 [DOI] [PubMed] [Google Scholar]
- 31.Hopkins BD, Pauli C, Du X, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560(7719):499–503. doi: 10.1038/s41586-018-0343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahleova H, Petersen KF, Shulman GI, et al. Effect of a low-fat vegan diet on body weight, insulin sensitivity, postprandial metabolism, and intramyocellular and hepatocellular lipid levels in overweight adults: a randomized clinical trial. JAMA Netw Open. 2020;3(11):e2025454. doi: 10.1001/jamanetworkopen.2020.25454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grandl G, Straub L, Rudigier C, et al. Short-term feeding of a ketogenic diet induces more severe hepatic insulin resistance than an obesogenic high-fat diet. J Physiol. 2018;596(19):4597–4609. doi: 10.1113/JP275173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aune D, Keum N, Giovannucci E, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016; 353:i2716. doi: 10.1136/bmj.i2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradbury KE, Appleby PN, Key TJ. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Clin Nutr. 2014;100(suppl 1):394S–398S. doi: 10.3945/ajcn.113.071357 [DOI] [PubMed] [Google Scholar]
- 36.Spencer CN, McQuade JL, Gopalakrishnan V, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632–1640. doi: 10.1126/science.aaz7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coutzac C, Jouniaux JM, Paci A, et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat Commun. 2020;11(1):2168. doi: 10.1038/s41467-020-16079-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Keefe SJ, Li JV, Lahti L, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. doi: 10.1038/ncomms7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clemens R, Kranz S, Mobley AR, et al. Filling America’s fiber intake gap: summary of a roundtable to probe realistic solutions with a focus on grain-based foods. J Nutr. 2012;142(7):1390S–1401S. doi: 10.3945/jn.112.160176 [DOI] [PubMed] [Google Scholar]
- 40.Forsythe LK, Wallace JM, Livingstone MB. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21(2):117–133. doi: 10.1017/S0954422408138732 [DOI] [PubMed] [Google Scholar]
- 41.Aleksandrova K, Koelman L, Rodrigues CE. Dietary patterns and biomarkers of oxidative stress and inflammation: a systematic review of observational and intervention studies. Redox Biol. 2021;42:101869. doi: 10.1016/j.redox.2021.101869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–527. doi: 10.1111/nure.12035 [DOI] [PubMed] [Google Scholar]
- 43.Rosenbaum M, Hall KD, Guo J, et al. Glucose and lipid homeostasis and inflammation in humans following an pisocaloric ketogenic diet. Obesity (Silver Spring). 2019;27(6):971–981. doi: 10.1002/oby.22468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bondonno NP, Dalgaard F, Kyrø C, et al. Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat Commun. 2019;10(1):3651. doi: 10.1038/s41467-019-11622-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020;12(2):E457. doi: 10.3390/nu12020457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McOrist AL, Miller RB, Bird AR, et al. Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J Nutr. 2011;141(5):883–889. doi: 10.3945/jn.110.128504 [DOI] [PubMed] [Google Scholar]
- 48.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217(2): 133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x [DOI] [PubMed] [Google Scholar]
- 49.McQuade JL, Daniel CR, Helmink BA, Wargo JA. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019; 20(2):e77–e91. doi: 10.1016/S1470-2045(18)30952-5 [DOI] [PubMed] [Google Scholar]
- 50.Shah U, Derkach A, Adintori P, et al. P-042: sustained minimal residual disease negativity in multiple myeloma is impacted positively by stool butyrate and healthier plant forward diets. Clinical Lymphoma Myeloma and Leukemia. 2021;21:S61. doi: 10.1016/S2152-2650(21)02176-5 [DOI] [Google Scholar]
- 51.Chriett S, Dąbek A, Wojtala M, Vidal H, Balcerczyk A, Pirola L. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci Rep. 2019;9(1):742. doi: 10.1038/s41598-018-36941-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ang QY, Alexander M, Newman JC, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181(6): 1263–1275.e16. doi: 10.1016/j.cell.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodrigues LM, Uribe-Lewis S, Madhu B, Honess DJ, Stubbs M, Griffiths JR. The action of β-hydroxybutyrate on the growth, metabolism and global histone H3 acetylation of spontaneous mouse mammary tumours: evidence of a β-hydroxybutyrate paradox. Cancer Metab. 2017;5:4. doi: 10.1186/s40170-017-0166-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6(2): 145207. doi: 10.1172/jci.insight.145207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia S, Lin R, Jin L, et al. Prevention of dietary-fat-fueled ketogenesis attenuates BRAF V600E tumor growth. Cell Metab. 2017;25(2):358–373. doi: 10.1016/j.cmet.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, et al. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9(17):3506–3514. doi: 10.4161/cc.9.17.12731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Ketone body utilization drives tumor growth and metastasis. Cell Cycle. 2012;11(21):3964–3971. doi: 10.4161/cc.22137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klement RJ, Champ CE, Otto C, Kämmerer U. Anti-tumor effects of ketogenic diets in mice: a meta-analysis. PLoS One. 2016;11(5):e0155050. doi: 10.1371/journal.pone.0155050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klement RJ, Brehm N, Sweeney RA. Ketogenic diets in medical oncology: a systematic review with focus on clinical outcomes. Med Oncol. 2020;37(2):14. doi: 10.1007/s12032-020-1337-2 [DOI] [PubMed] [Google Scholar]
- 60.Chlebowski RT, Reeves MM. Weight loss randomized intervention trials in female cancer survivors. J Clin Oncol. 2016;34(35):4238–4248. doi: 10.1200/JCO.2016.69.4026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frattaroli J, Weidner G, Dnistrian AM, et al. Clinical events in prostate cancer lifestyle trial: results from two years of follow-up. Urology. 2008; 72(6):1319–1323. doi: 10.1016/j.urology.2008.04.050 [DOI] [PubMed] [Google Scholar]
- 62.Ornish D, Magbanua MJ, Weidner G, et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc Natl Acad Sci U S A. 2008;105(24):8369–8374. doi: 10.1073/pnas.0803080105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14(11):1112–1120. doi: 10.1016/S1470-2045(13)70366-8 [DOI] [PubMed] [Google Scholar]
- 64.Parsons JK, Zahrieh D, Mohler JL, et al. Effect of a behavioral intervention to increase vegetable consumption on cancer progression among men with early-stage prostate cancer: the MEAL randomized clinical trial. JAMA. 2020;323(2):140–148. doi: 10.1001/jama.2019.20207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chlebowski RT, Aragaki AK, Anderson GL, et al. Association of low-fat dietary pattern with breast cancer overall survival: a secondary analysis of the Women’s Health Initiative Randomized Clinical Trial. JAMA Oncol. 2018;4(10):e181212–e181212. doi: 10.1001/jamaoncol.2018.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298 (3):289–298. doi: 10.1001/jama.298.3.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–1776. doi: 10.1093/jnci/djj494 [DOI] [PubMed] [Google Scholar]
- 68.Chlebowski RT, Blackburn GL, Hoy MK, et al. Survival analyses from the Women’s Intervention Nutrition Study (WINS) evaluating dietary fat reduction and breast cancer outcome. J Clin Oncol. 2008;26(15)(suppl):522–522. doi: 10.1200/jco.2008.26.15_suppl.522 [DOI] [Google Scholar]
- 69.Yang YF, Mattamel PB, Joseph T, et al. Efficacy of low-carbohydrate ketogenic diet as an adjuvant cancer therapy: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2021;13(5):1388. doi: 10.3390/nu13051388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kämmerer U, Klement RJ, Joos FT, Sütterlin M, Reuss-Borst M. Low carb and ketogenic diets increase quality of life, physical performance, body composition, and metabolic health of women with breast cancer. Nutrients. 2021;13(3):1029. doi: 10.3390/nu13031029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bruno E, Krogh V, Gargano G, et al. Adherence to dietary recommendations after one year of intervention in breast cancer women: the DIANA-5 Trial. Nutrients. 2021;13(9):2990. doi: 10.3390/nu13092990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vernieri C, Fucà G, Ligorio F, et al. Fasting-mimicking diet is safe and reshapes metabolism and antitumor immunity in patients with cancer. Cancer Discov. 2022;12(1):90–107. doi: 10.1158/2159-8290.CD-21-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lowe DA, Wu N, Rohdin-Bibby L, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180(11):1491–1499. doi: 10.1001/jamainternmed.2020.4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nencioni A, Caffa I, Cortellino S, Longo VD. Fasting and cancer: molecular mechanisms and clinical application. Nat Rev Cancer. 2018;18(11): 707–719. doi: 10.1038/s41568-018-0061-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McDonald D, Hyde E, Debelius JW, et al. ; American Gut Consortium. American gut: an open platform for citizen science microbiome research. mSystems. 2018;3(3):e00031–18. doi: 10.1128/mSystems.00031-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: A randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256–e256. doi: 10.1038/nutd.2017.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280(23):2001–2007. doi: 10.1001/jama.280.23.2001 [DOI] [PubMed] [Google Scholar]
- 78.Kim H, Caulfield LE, Garcia-Larsen V, Steffen LM, Coresh J, Rebholz CM. Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J Am Heart Assoc. 2019;8(16):e012865. doi: 10.1161/JAHA.119.012865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hawk ET, Greenwood A, Gritz ER, et al. ; Translational Research Working Group. The Translational Research Working Group developmental pathway for lifestyle alterations. Clin Cancer Res. 2008;14(18):5707–5713. doi: 10.1158/1078-0432.CCR-08-1262 [DOI] [PubMed] [Google Scholar]
- 80.Rodgers GP, Collins FS. Precision nutrition—the answer to “what to eat to stay healthy”. JAMA. 2020;324(8):735–736. doi: 10.1001/jama.2020.13601 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


