Abstract
Background
Up to 17% of pancreatic cancer (PDAC) patients harbor pathogenic (germline or somatic) mutations in a homologous recombination, DNA damage response and repair (HR-DDR) gene, such as BRCA1/2, or PALB2. Platinum-based chemotherapy, or treatment with PARP inhibitors are of particular benefit in these patients. However, there may be even greater benefit when platinums and PARP inhibitors are combined.
Patients and Methods
We performed a single-arm, open-label, phase I/II study of the PARP inhibitor, veliparib, with 5-fluorouracil (no 5FU bolus) and oxaliplatin (FOLFOX) for patients with metastatic PDAC. Thirty-one patients were enrolled in a Phase I dose escalation of veliparib (40mg to 250mg BID, days 1–7 of each 14 day cycle), to identify the recommended phase II dose (RP2D) of veliparib for the combination. Another 33 patients were enrolled in two parallel Phase II trials to assess the objective response rate (ORR) in untreated or in previously treated patients. If available, germline or somatic testing was collected to identify pathogenic HR-DDR mutations.
Results
The combination of veliparib and FOLFOX was tolerable at a RP2D of veliparib of 200mg BID. The primary endpoint for both Phase II cohorts was met, and the ORR overall was 26%. There was greater activity in platinum-naïve patients, and those who harbored a pathogenic HR-DDR mutation. Specifically, the ORR of HR-DDR mutated, platinum-naive patients was 57%.
Conclusions
The combination of veliparib and FOLFOX was safe for patients with metastatic PDAC and showed promising activity particularly in patients with platinum-naive disease that harbors a pathogenic HR-DDR mutation.
ClinicalTrials.gov identifier: NCT01489865; ABT-888 with Modified FOLFOX6 in Patients with Metastatic Pancreatic Cancer
Keywords: veliparib, FOLFOX, pancreatic cancer, BRCA, homologous recombination
INTRODUCTION
Pancreatic cancer (PDAC) is a deadly disease which, with a 5 year survival of only 10%, is poised to become the second leading cause of cancer related death in the United States by 2030(1). Treatment for metastatic PDAC (mPDAC) has improved, but the median overall survival (OS) remains less than 1 year(2,3). However, for the subgroup of ~17% of patients with PDAC whose tumors harbor defects in the homologous recombination-DNA damage response and repair (HR-DDR) pathway(4–7), such as BRCA1/2 and PALB2 mutations, preliminary data suggest that treatment with platinum-based chemotherapy and/or poly(ADP-ribose) polymerase (PARP) inhibitors offers significant improvements in patient outcomes(8–14). In fact, based on the Phase III POLO trial, PARP inhibitors are now an FDA approved standard treatment option as maintenance therapy for mPDAC patients who harbor pathogenic germline BRCA1/2 mutations(15). PARP inhibitors have also been demonstrated to be active in PDAC patients whose tumors harbor pathogenic PALB2 mutations(16). There is also evidence in other cancer types of the efficacy of PARP inhibitors in tumors with other (non-BRCA1/2/PALB2) HR-DDR mutations(17).
Moreover, there exists the potential for enhanced benefit when a PARP inhibitor is combined with DNA damaging chemotherapy. PARP plays a critical role in facilitating the repair of both single- and double-stranded DNA breaks(18–20), and PARP inhibition results in less efficient DNA repair. Thus, PARP inhibitors act as sensitizing agents for DNA-damaging chemotherapies(21). Veliparib (ABT-888, Abbvie) is a PARP inhibitor that has proven in vivo activity(22), and increases tumor cell sensitivity to chemotherapy and radiation(23). In humans, veliparib is safe and demonstrates inhibition of PARP activity in tumor biopsies(22). In Phase III trials, veliparib has been used in combination with platinum-based chemotherapy at a dose of 150mg orally twice a day (BID); and at 400mg BID as maintenance therapy without chemotherapy(24).
We previously demonstrated in PDAC cells that genetically disrupting the DNA binding domain of PARP1, or treating with veliparib synergizes with oxaliplatin(21), particularly in HR-DDR deficient cell lines. Therefore, we designed a trial combining veliparib with oxaliplatin-based chemotherapy for patients with mPDAC. At the time of study design, FOLFIRINOX was not yet standard of care, and may present toxicity challenges in combination with a PARP inhibitor due to overlapping myelosuppression. Thus, herein, we present a Phase I/II clinical trial of veliparib plus 5-fluorouracil (5FU) and oxaliplatin (FOLFOX) for patients with mPDAC.
PATIENTS AND METHODS
Patients
Patients with mPDAC with measurable disease (as per RECIST 1.1(25)) were eligible. Patients were aged ≥18 years, had an Eastern Cooperative Oncology Group performance status score of ≤2, and had adequate organ and bone marrow function (hemoglobin ≥9.5 g/dL, absolute neutrophil count ≥1.5 × 109/L, platelet count ≥75 × 109/L, serum creatinine level <1.5 mg/dL, bilirubin level ≤2.5 × upper limit of normal (ULN), and ALT/AST levels ≤3 × ULN). For the Phase I portion of the study, patients were not selected based on prior therapy, family history (FH), nor germline or tumor HR-DDR mutational status.
For the Phase II portion, there were two cohorts. Patients in the untreated cohort had not had any prior systemic therapy for mPDAC, though adjuvant chemotherapy was allowed if completed >6 months prior; and prior palliative radiation to the primary mass was allowable. Patients in the previously treated cohort may have had any number of prior therapies, including platinum-based regimens such as FOLFIRINOX, FOLFOX, or gemcitabine and cisplatin, but they may not have received a prior PARP inhibitor. Also, for the Phase II portion (both cohorts), we preselected patients who either had a known pathogenic germline or somatic mutation (though testing was not required) in one of the HR-DDR genes (e.g. BRCA1/2, PALB2, ATM (expanded list in the supplemental materials)); and/or patients who had a FH suggestive of a breast or ovarian cancer syndrome, as detailed in the NCCN guidelines (summarized in the supplemental material)(26). For the post-hoc analyses detailed in the Results, outcomes for different patient subgroups were compared. Subgroups included: 1) Prior platinum exposure: Patients were placed into one of three categories as to whether their disease had:
Never been exposed to prior platinum
Been exposed, but not progressed on prior platinum (defined as no disease progression within 3 months of stopping platinum)
Progressed while on prior platinum
2) The presence or absence of a FH (as defined above); 3) The presence of a mutation in an HR-DDR gene; patients who were tested but did not harbor a pathogenic germline or somatic HR-DDR mutation were also identified and highlighted. The study protocol, amendments, and informed consent forms were approved by the Institutional Review Board at Georgetown University. Investigators obtained written informed consent from each participant or participant’s guardian prior to screening. The research was conducted in accordance with recognized ethical guidelines including the Declaration of Helsinki, CIOMS, Belmont Report, and U.S. Common Rule, as described during training in Good Clinical Practice guidelines (CITI Training).
Study Design and Treatment Schedule
This was a single center, Phase I/II, open label study. Initially, the dose and schedule for modified FOLFOX6(27) was used (5FU bolus 400mg/m2 Day 1; leucovorin 400mg/m2 Day 1; oxaliplatin 85mg/m2 Day 1; and 5FU 2400mg/m2 continuous infusion over 46 hours, Days 1–3). Each cycle was 14 days. However, the first 6 patients dosed at 40mg of veliparib demonstrated prolonged Grade 2 or 3 myelosuppression. Thus, the 5FU bolus was dropped for all subsequent patients. For the Phase I portion, the dose of veliparib was escalated in a standard 3+3 design from 40 to 60, 80, 100, 150, 200, and 250mg twice a day (BID), days 1–7 (Supplemental Table 1). As the primary endpoint of the Phase I portion was to determine the recommended Phase II dose (RP2D; as well as the maximally tolerated dose), Dose Limiting Toxicities (DLTs) were defined as any of the following that were definitely, possibly or probably related to therapy (veliparib + FOLFOX) that occurred during the first cycle of therapy:
Grade 4 neutropenia lasting greater than 5 days or complicated by fever or infection
Grade 4 anemia or thrombocytopenia
Grade 3 thrombocytopenia associated with bleeding for which a transfusion was required.
Grade 3 or 4 non-hematologic toxicity not manageable with routine supportive care (e.g. over the counter anti-diarrheals for diarrhea).
Any toxicity, regardless of grade, which resulted in withholding of therapy for greater than three weeks.
For the Phase II portion, the RP2D was 200mg of veliparib, though the protocol did allow for stepwise de-escalation to 150mg and then to 100mg for toxicity after the first cycle. Safety assessments were performed every 2 weeks for the first 4 cycles, then every 4 weeks thereafter. Tumor response was assessed radiographically every 8 – 12 weeks using RECIST 1.1(25). Study treatment was continued without interruption in the absence of unacceptable toxicity or progressive disease (PD). The protocol did allow for patients to stop the oxaliplatin for persistent neuropathy, at which time patients were maintained on the RP2D of veliparib plus the standard dose of continuous infusion 5FU.
Correlative Markers of Response to Therapy
For the Phase I portion, plasma samples were obtained according to the schedule in Supplemental Figure 1 for pharmacokinetic (PK) assessment of veliparib performed at Abbvie. Results were compared to historical controls to identify any effect on veliparib PKs by FOLFOX. In addition, when samples were available, next generation sequencing of cancer-related genes was performed commercially by Foundation Medicine, Caris Life Sciences, or Tempus laboratories on patient tumor samples. Several patients also had germline testing through Myriad or Ambry. Patients were defined as harboring HR-DDR mutations if a pathogenic mutation in an HR-DDR gene was identified in a blood sample (germline) or tumor sample (somatic). Pathogenic mutations for each patient are included in Supplemental Table 4. The study team centrally validated pathogenic mutations in the ClinVar and Cosmic databases.
Statistical Analysis
The primary objective of the Phase I portion was to determine the RP2D, with the primary endpoint being adverse events (AEs), as measured by NIH CTC version 4.03. The efficacy assessments included the objective response rate (ORR), disease control rate (DCR; stable disease through at least 4 cycles, partial response, or complete response), progression-free survival (PFS), and OS. For the Phase II portion, the primary endpoint was the ORR, and each Phase II cohort followed a Simon’s two-stage optimal design(28). For each cohort, 9 patients were accrued in the first Stage, and if ≥1 patient demonstrated a response, 15 additional patients were to be accrued in the second Stage for a total of 24 patients. If ≥3 patients among 24 patients demonstrated a response, then the treatment was considered sufficiently promising to warrant further testing. At the time this protocol was designed, the ORR for first-line standard of care gemcitabine was only 7%(29), and there was no standard second-line therapy. Each cohort was designed to differentiate a 5% ORR from a 25% ORR at a 1-sided 10% significance level with 90% power. Patient characteristics, medical features at study entry, and AEs at least possibly related to study therapy were tabulated. Differences in ORR and DCR among subgroups were compared using Fisher’s Exact tests. OS was defined as the number of months from enrollment until death or last contact. Patients who were alive at the time of analysis were censored at their last contact. PFS was defined as the number of months from enrollment to progression or death, whichever occurred first. Patients who were alive and progression-free at the time of analysis were censored at their last tumor assessment time (as of May 3, 2019). The Kaplan-Meier methodology was used for analyzing OS and PFS. Analyses were performed in SAS software Version 9.4 [SAS Institute Inc., Cary, NC, USA.] and figures were created using STATA 12.1 [StatCorp LP, College Station, TX, USA].
The trial opened in January, 2011 and the Phase I portion accrued rapidly. However, when we restricted enrollment in the Phase II portion, as defined above, the accrual rate slowed considerably, and the trial was closed to enrollment in 2019 due to slow accrual, although, as discussed below, the primary endpoint had been met in the two Phase II cohorts.
RESULTS
Patient Characteristics and Treatment Cohorts
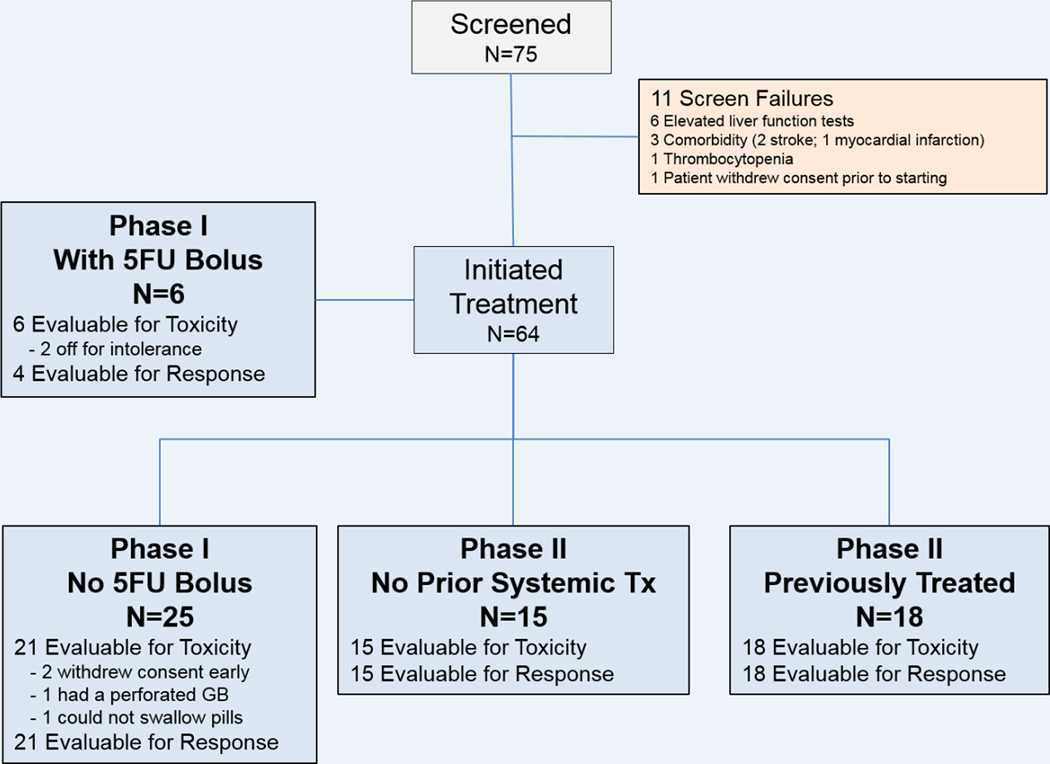
Between January, 2011 and December, 2018, 75 patients were consented, and 64 patients initiated treatment. Figure 1 depicts the screen failure and enrollment into the different cohorts. Thirty one patients initiated treatment in the Phase I portion; 15 patients initiated treatment in the untreated Phase II cohort; and 18 patients initiated treatment in the previously treated Phase II cohort. Patient characteristics are listed in Table 1. The median age for all 64 treated patients was 64 years (range 40 – 84); most patients had an ECOG score of 0 or 1 (95%); and 56% of patients were male. In the Phase I, and previously treated Phase II cohort, patients had a median of 1 and 2 lines of prior therapy, respectively (range, 1–7).
Figure 1: Cohort Flowchart.
Of the 75 patients consented, 64 initiated study treatment. Two of the 6 patients in the Phase I portion with the 5FU bolus come off due to toxicity before response evaluation. In the Phase I portion without the 5FU bolus, 2 patients withdrew consent early, 1 patient came off due to a perforated gall bladder, and one patient could not swallow the pills. In the Phase II cohorts, all patients were evaluable for toxicity and response.
Table 1.
Patient Characteristics
| All | Phase I | Phase II Untreated | Phase II Pre-Treated | ||
|---|---|---|---|---|---|
|
| |||||
| Category | Subgroup | 64 (100%) | 31 (49%) | 15 (23%) | 18 (28%) |
|
| |||||
| Age - median (min,max) | 64 (40,84) | 64 (46,84) | 65 (40,73) | 64 (52,80) | |
|
| |||||
| Gender | |||||
| Female | 28 (44%) | 21 (68%) | 10 (67%) | 8 (44%) | |
| Male | 36 (56%) | 10 (32%) | 5 (33%) | 10 (56%) | |
|
| |||||
| Race/Ethnicity | |||||
| White/Non-Hispanic | 51 (80%) | 26 (84%) | 11 (73%) | 14 (78%) | |
| Black/Non-Hispanic | 10 (16%) | 4 (13%) | 3 (20%) | 3 (16%) | |
| Asian-PI/Non-Hispanic | 2 (3%) | 0 (0%) | 1 (7%) | 1 (6%) | |
| Any/Hispanic | 1 (1%) | 1 (3%) | 0 (0%) | 0 (0%) | |
|
| |||||
| ECOG | |||||
| 0 | 16 (25%) | 7 (23%) | 4 (27%) | 5 (28%) | |
| 1 | 45 (70%) | 23 (74%) | 9 (60%) | 13 (72%) | |
| 2 | 3 (5%) | 1 (3%) | 2 (13%) | 0 (0%) | |
|
| |||||
| Prior Platinum | |||||
| Yes | 17 (27%) | 7 (23%) | 0 (0%) | 10 (56%) | |
| No | 47 (73%) | 24 (77%) | 15 (100%) | 8 (44%) | |
|
| |||||
| Family History | |||||
| Yes | 44 (69%) | 12 (39%) | 15 (100%) | 17 (94%) | |
| No | 20 (31%) | 19 (61%) | 0 (0%) | 1 (6%) | |
|
| |||||
| Known HR-DDR mutation | |||||
| Yes | 19 (30%) | 2 (6%) | 5 (33%) | 12 (67%) | |
| No | 45 (70%) | 29 (94%) | 10 (67%) | 6 (33%) | |
Phase I Portion
For the Phase I portion, 27/31 patients were evaluable for dose limiting toxicities (DLTs), with four patients withdrawing consent after one cycle (not for toxicity). In the 40mg cohort, 3 of 6 patients required significant (>2 week) treatment delays for Grade 2 or 3 myelosuppression, but the only protocol-defined DLT was a treatment delay of >3 weeks. Therefore, the protocol was amended to drop the 5FU bolus. Only one other DLT occurred, which was at the 250mg cohort. Four of the 6 patients at 250mg experienced significant Grade 3 or 4 myelosuppression. Therefore, 200mg was selected as the RP2D, though the protocol allowed for stepwise de-escalation to 150mg and then to 100mg for toxicity after the first cycle.
PK samples were available for 14 patients in 5 dosing cohorts. The veliparib PK data suggested that co-administration of FOLFOX had no apparent impact on veliparib PKs (Supplemental Tables 2 and 3).
Suspected Drug Related Adverse Events (All Cohorts)
All 64 patients who received treatment were evaluable for AEs. Table 2 provides the number of patients experiencing AEs by category and cohort that are at least possibly related to treatment. No Grade 5 events occurred. Overall, the combination of veliparib and FOLFOX was well tolerated, although minor treatment adjustments were required over the course of therapy for most patients. The primary toxicity of concern was myelosuppression, and 16% of patients experienced Grade 3 or 4 neutropenia. One patient experienced Grade 3 thrombocytopenia, and 2 patients had Grade 3 or 4 anemia. Fifty-two and sixty-four percent, respectively of patients experienced mild fatigue and nausea, but only 2% and 6%, respectively were Grade 3 or 4. Other notable Grade 3 or 4 non-hematologic AEs included 1 patient each with a rash, diarrhea, and peripheral neuropathy. All patients required a reduction in the dose of veliparib, 5FU, and/or oxaliplatin at some point in the trial, either due to myelosuppression or nausea prior to the first restaging imaging, or beyond 4 cycles typically for neuropathy.
Table 2.
Numbers (Percentages) of Patients Experiencing Adverse Events at Least Possibly Related to Study Drug
| AE Category | CTCAE Term | All (N=64) Grade, N (%) | Phase I N=31 Grade, N (%) | PhaseII treated N=18 Grade, N (%) | Phase II Untreated N=15 Grade, N (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| All | 3,4 | 1,2 | All | 3,4 | 1,2 | All | 3,4 | 1,2 | All | 3,4 | 1,2 | ||
| Hematologic | Neutropenia | 21 (33) | 10 (16) | 17 (27) | 13 (42) | 5 (16) | 12 (39) | 2 (11) | 1 (6) | 2 (11) | 6 (40) | 4 (27) | 3 (20) |
| Thrombocytopenia | 13 (20) | 1 (2) | 13 (20) | 12 (39) | 1 (3) | 12 (39) | 1 (7) | 1 (7) | |||||
| Leukopenia | 11 (17) | 3 (5) | 10 (16) | 9 (29) | 3 (10) | 8 (26) | 1 (6) | 1 (6) | 1 (7) | 1 (7) | |||
| Anemia | 6 (9) | 2 (3) | 4 (6) | 4 (13) | 1 (3) | 3 (10) | 1 (6) | 1 (6) | 1 (7) | 1 (7) | |||
| Lymphopenia | 5 (8) | 1 (2) | 4 (6) | 4 (13) | 1 (3) | 3 (10) | 1 (7) | 1 (7) | |||||
|
| |||||||||||||
| Cardiovascular | Hot flashes | 1 (2) | 1 (2) | 1 (3) | 1 (3) | ||||||||
| Hypertension | 1 (2) | 1 (2) | 1 (6) | 1 (6) | |||||||||
| Hypotension | 1 (2) | 1 (2) | 1 (7) | 1 (7) | |||||||||
| Sinus tachycardia | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
|
| |||||||||||||
| Constitutional | Fatigue | 33 (52) | 1 (2) | 33 (52) | 12 (39) | 12 (39) | 11 (61) | 11 (61) | 10 (67) | 1 (7) | 10 (67) | ||
| Anorexia | 14 (22) | 14 (22) | 4 (13) | 4 (13) | 6 (33) | 6 (33) | 4 (27) | 4 (27) | |||||
| Pain | 7 (11) | 7 (11) | 5 (16) | 5 (16) | 2 (11) | 2 (11) | |||||||
| Allergic reaction | 5 (8) | 5 (8) | 2 (11) | 2 (11) | 3 (20) | 3 (20) | |||||||
| Weight loss | 5 (8) | 5 (8) | 2 (6) | 2 (6) | 1 (6) | 1 (6) | 2 (13) | 2 (13) | |||||
| Fever | 2 (3) | 2 (3) | 1 (3) | 1 (3) | 1 (6) | 1 (6) | |||||||
| Chills | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
| Febrile neutropenia | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
| Infusion related reaction | 1 (2) | 1 (2) | 1 (6) | 1 (6) | |||||||||
| Insomnia | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
| Malaise | 1 (2) | 1 (2) | 1 (7) | 1 (7) | |||||||||
|
| |||||||||||||
| Dermatologic | Rash maculo-papular | 2 (3) | 1 (2) | 2 (3) | 1 (6) | 1 (6) | 1 (6) | 1 (7) | 1 (7) | ||||
| Alopecia | 1 (2) | 1 (2) | 1 (7) | 1 (7) | |||||||||
| Hand - Foot Syndrome | 1 (2) | 1 (2) | 1 (6) | 1 (6) | |||||||||
| Pruritus | 1 (2) | 1 (2) | 1 (7) | 1 (7) | |||||||||
|
| |||||||||||||
| Gastrointestinal | Nausea | 41 (64) | 4 (6) | 40 (63) | 17 (55) | 17 (55) | 13 (72) | 2 (11) | 13 (72) | 11 (73) | 2 (13) | 10 (67) | |
| Vomiting | 22 (34) | 4 (6) | 21 (33) | 10 (32) | 10 (32) | 6 (33) | 2 (11) | 6 (33) | 6 (40) | 2 (13) | 5 (33) | ||
| Diarrhea | 14 (22) | 1 (2) | 13 (20) | 7 (23) | 1 (3) | 6 (19) | 4 (22) | 4 (22) | 3 (20) | 3 (20) | |||
| Constipation | 11 (17) | 11 (17) | 5 (16) | 5 (16) | 4 (22) | 4 (22) | 2 (13) | 2 (13) | |||||
| Mucositis oral | 7 (11) | 7 (11) | 2 (6) | 2 (6) | 3 (17) | 3 (17) | 2 (13) | 2 (13) | |||||
| Dysgeusia | 4 (6) | 4 (6) | 1 (3) | 1 (3) | 1 (6) | 1 (6) | 2 (13) | 2 (13) | |||||
| ALT increased | 3 (5) | 1 (2) | 2 (3) | 1 (6) | 1 (6) | 2 (13) | 2 (13) | ||||||
| AST increased | 3 (5) | 1 (2) | 2 (3) | 1 (6) | 1 (6) | 2 (13) | 2 (13) | ||||||
| Gastroesophageal reflux | 3 (5) | 3 (5) | 1 (3) | 1 (3) | 2 (13) | 2 (13) | |||||||
| Abdominal pain | 2 (3) | 2 (3) | 1 (6) | 1 (6) | 1 (7) | 1 (7) | |||||||
| Bloating | 2 (3) | 2 (3) | 2 (6) | 2 (6) | |||||||||
| Dehydration | 2 (3) | 2 (3) | 1 (3) | 1 (3) | 1 (7) | 1 (7) | |||||||
| Flatulence | 2 (3) | 2 (3) | 1 (3) | 1 (3) | 1 (7) | 1 (7) | |||||||
| Gastroparesis | 2 (3) | 2 (3) | 2 (6) | 2 (6) | |||||||||
| Dry mouth | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
| Gastrointestinal pain | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
| Heartburn | 1 (2) | 1 (2) | 1 (7) | 1 (7) | |||||||||
| Hiccups | 1 (2) | 1 (2) | 1 (7) | 1 (7) | |||||||||
| Stomatitis | 1 (2) | 1 (2) | 1 (7) | 1 (7) | |||||||||
| Other | 3 (5) | 3 (5) | 2 (6) | 2 (6) | 1 (6) | 1 (6) | |||||||
| Genitourinary | Vaginal discharge | 1 (2) | 1 (2) | 1 (7) | 1 (7) | ||||||||
| Other | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
|
| |||||||||||||
| Infection | Infections | 1 (2) | 1 (2) | 1 (3) | 1 (3) | ||||||||
| Leukocytosis | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
|
| |||||||||||||
| Musculoskeletal | Pain in extremity | 2 (3) | 2 (3) | 1 (6) | 1 (6) | 1 (7) | 1 (7) | ||||||
| Edema limbs | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
| Muscle weakness left-sided | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
| Decrease range of motion | 1 (2) | 1 (2) | 1 (6) | 1 (6) | |||||||||
| Other | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
|
| |||||||||||||
| Neurologic | Paresthesia | 17 (27) | 17 (27) | 8 (26) | 8 (26) | 6 (33) | 6 (33) | 3 (20) | 3 (20) | ||||
| Dysesthesia | 14 (22) | 14 (22) | 8 (26) | 8 (26) | 3 (17) | 3 (17) | 3 (20) | 3 (20) | |||||
| Headache | 5 (8) | 5 (8) | 1 (3) | 1 (3) | 3 (17) | 3 (17) | 1 (7) | 1 (7) | |||||
| Dizziness | 4 (6) | 4 (6) | 1 (3) | 1 (3) | 1 (6) | 1 (6) | 2 (13) | 2 (13) | |||||
| Cognitive disturbance | 2 (3) | 2 (3) | 2 (6) | 2 (6) | |||||||||
| Peripheral sensory neuropathy | 2 (3) | 1 (2) | 2 (3) | 2 (13) | 1 (7) | 2 (13) | |||||||
| Vertigo | 2 (3) | 2 (3) | 1 (3) | 1 (3) | 1 (7) | 1 (7) | |||||||
| Blurred vision | 1 (2) | 1 (2) | 1 (6) | 1 (6) | |||||||||
| Confusion | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
| Depression | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
| Eye disorders - Other | 1 (2) | 1 (2) | 1 (3) | 1 (3) | |||||||||
| Jaw spasm | 1 (2) | 1 (2) | 1 (7) | 1 (7) | |||||||||
| Laryngopharyngeal dysesthesia | 1 (2) | 1 (2) | 1 (6) | 1 (6) | |||||||||
| Tinnitus | 1 (2) | 1 (2) | 1 (6) | 1 (6) | |||||||||
| Syncope | 1 (2) | 1 (2) | 1 (7) | 1 (7) | |||||||||
| Other | 1 (2) | 1 (2) | 1 (7) | 1 (7) | |||||||||
|
| |||||||||||||
| Pulmonary | Dyspnea | 1 (2) | 1 (2) | 1 (3) | 1 (3) | ||||||||
Clinical Efficacy and Subgroup Assessment
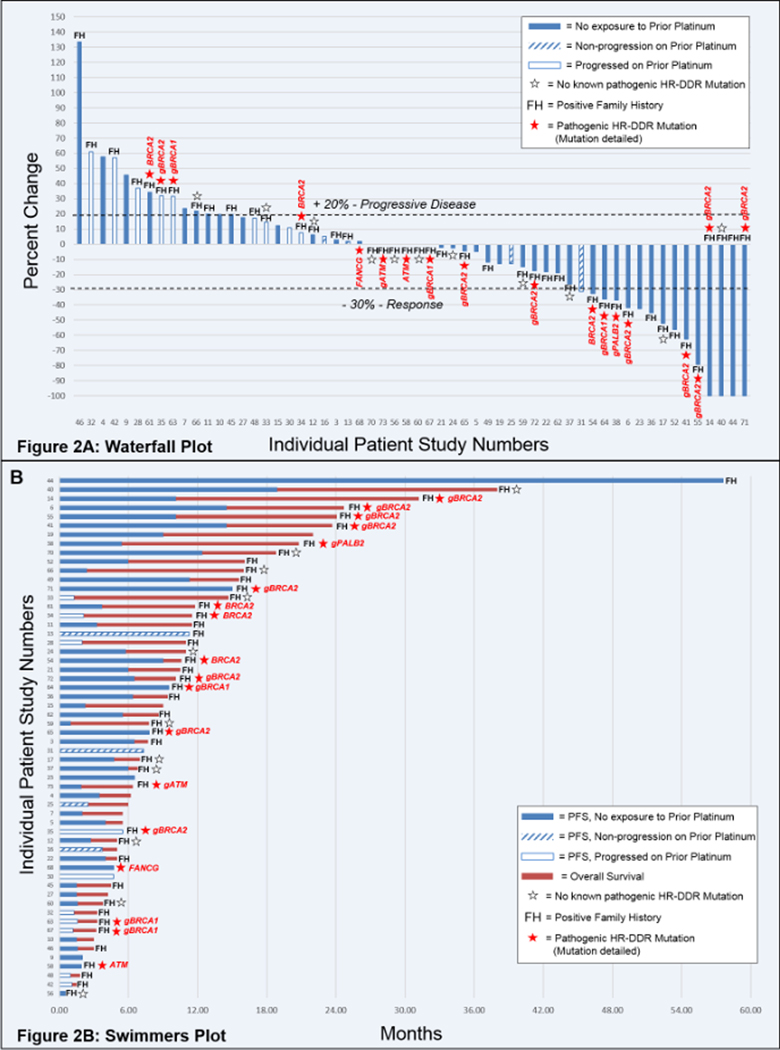
Of the 64 patients who received study treatment, 6 patients in the Phase I portion came off study prior to response evaluation (for reasons other than progression of disease), and thus were not evaluable for response. Table 3 presents the responses and survival times for the 58 response evaluable patients. For the entire group, the ORR was 26%, which included 11 PRs and 4 CRs. The waterfall plot in Figure 2A demonstrates the responses for each patient. The DCR, PFS, and OS were 52%, 4.0 months, and 7.8 months, respectively. The swimmers plot in Figure 2B demonstrates the treatment duration for each patient. The protocol did allow for patients to stop the oxaliplatin for persistent neuropathy, at which time patients were maintained on the RP2D of veliparib plus the standard dose of continuous infusion 5FU (n = 18, none of whom received more than 12 cycles of oxaliplatin). One patient (patient 44) was granted an exception and was allowed to discontinue the 5FU as well, after having remained in a complete response for nearly 4 years.
Table 3. Clinical Outcomes for Cohorts, Family History, and HR-DDR Status.
58 patients total were evaluable for response. Of those 58 patients, several subgroups, as detailed, were compared for the objective response rate (ORR), disease control rate (DCR, defined as partial response or complete response at any time, or stable disease at 2 months), median progression-free survival (PFS), and median overall survival (OS).
| Subgroup (n) | ORR (%) | DCR (%) | PFS (mos) | OS (mos) |
|---|---|---|---|---|
| All Response Evaluable Patients (58) | 26 | 52 | 4.4 | 7.8 |
| Phase I Patients (25) | 20 | 48 | 4.0 | 6.5 |
| Phase II Untreated (15) | 40 | 87 | 6.5 | 15.0 |
| Phase II Previously Treated (18) | 22 | 28 | 1.8 | 4.6 |
| Prior Platinums | ||||
| Exposure to Prior Platinum (14) | 7 | 14 | 2.1 | 5.3 |
| Non-Progression on Prior Platinum (4) | 25 | 50 | 5.5 | 6.7 |
| Progression on Prior Platinum (10) | 0 | 0 | 1.5 | 4.0 |
| No Prior Platinum (44) | 32 | 64 | 5.5 | 8.8 |
| Family History (FH) | ||||
| (+) FH (43) | 30 | 53 | 5.4 | 9.5 |
| No FH (15) | 13 | 47 | 3.8 | 5.5 |
| HR-DDR Mutations | ||||
| HR-DDR mutated (18) | 44 | 61 | 6.0 | 10.4 |
| Non-Mutated/Unknown (40) | 18 | 48 | 3.6 | 6.9 |
| HR-DDR Mutated, No progression on prior platinum (14) | 57 | 79 | 8.4 | 11.2 |
Figure 2: Waterfall and Swimmers Plots.
2A) Waterfall Plot of Patient Responses. Best tumor response is graphed for the 58 evaluable patients. 2B) Swimmers Plot of Patient PFS and OS. For each of the 58 evaluable patients, progression free survival (blue) and overall survival (brown) are graphed. Notations for both figures are indicated are for patients who were never exposed to prior platinum (solid bar); patients who had been treated with, but never progressed on prior platinum (hatched bar); and patients whose disease had progressed on platinum therapy (open bar). Additionally, notations are indication for patients who had a positive FH (as defined in the methods); and patients who had germline or somatic NGS testing. Patients tested, but with no identified HR-DDR mutation are indicated by an open star. Patients found to have a pathogenic HR-DDR mutation are indicated with a solid star, the specific gene mutated is listed, and, in cases where the mutation was germline, the specific mutation is preceded by a “g”.
For the Phase I portion (n=25), patients were not pre-selected based on FH and known HR-DDR mutational status, and the ORR was 20%. For the two Phase II cohorts combined (n=33), with patients selected based on FH or HR-DDR mutational status, the ORR was 31%. The ORR was 40% for patients who received no prior therapy (n=15), with a DCR, PFS, and OS of 87%, 6.5 months, and 13.0 months, respectively. The ORR was 22% for the previously treated patients (N=18), with a DCR, PFS, and OS of 28%, 1.6 months, and 4.5 months, respectively. Although the study was closed early due to slow accrual, the primary endpoint had been met in each of the two Phase II cohorts.
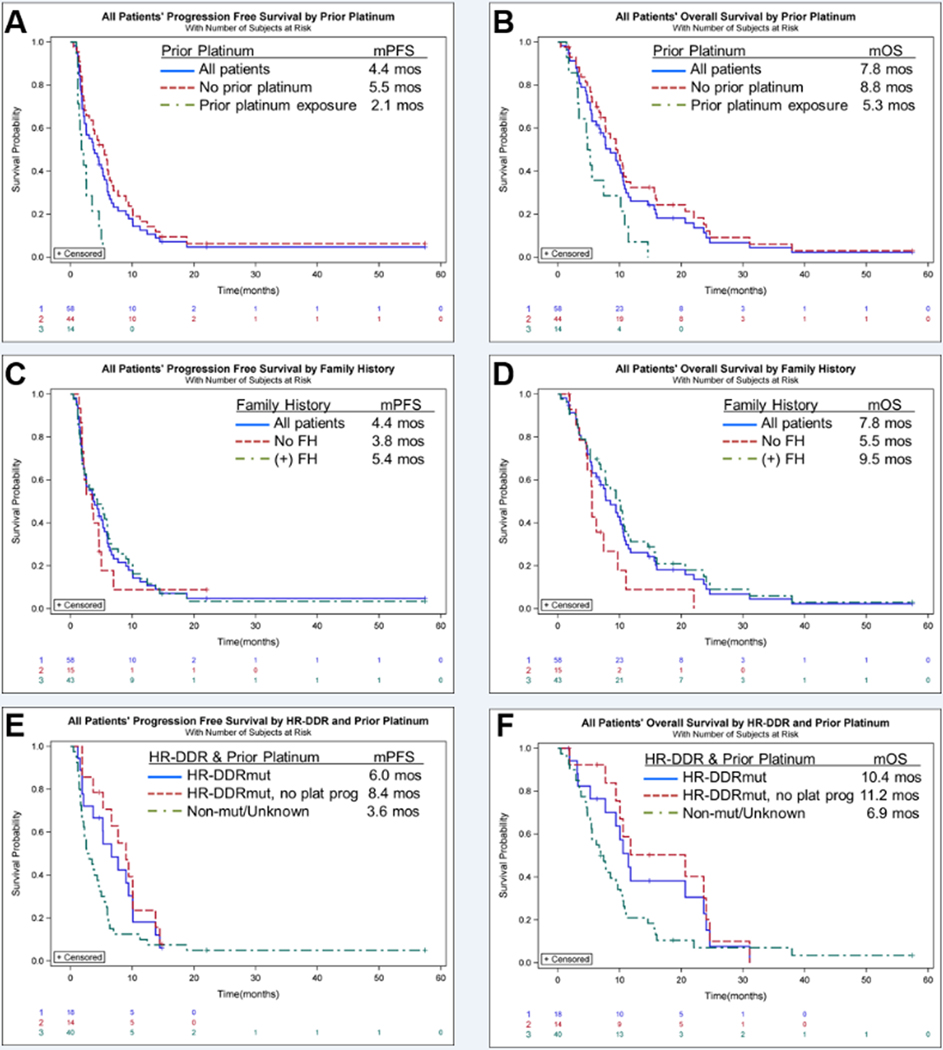
We examined efficacy in several patient subgroups (Table 3, and Figure 3). There is evidence to suggest that the mechanisms of resistance to platinums overlap the mechanisms of resistance to PARP inhibitors. Correspondingly, the ORR for patients who received prior platinum was only 7%, with a DCR of only 14% (N=14). This is compared to an ORR of 32% and a DCR of 64% for those who did not receive prior platinum (N=44) (Table 3, and Figures 3A and 3B). We further assessed outcomes for patients who had been previously exposed to platinums (Table 3 [Patient numbers were too small for corresponding Kalan-Meier curves]. Prior exposure to platinum was broken down into exposure, but non-progression on prior platinum (as defined in the Methods); and frank progression while on prior platinum. The ORR for patients who had been exposed to, but not progressed on prior platinum was 25%, with a DCR of 50% (N=4). However, none of the patients whose disease had progressed on prior platinum had a response, nor any reduction in tumor burden (Figure 2A), nor even disease control with the combination of veliparib and FOLFOX (N=10). Of note, patient 61 received neoadjuvant FOLFIRINOX for non-metastatic disease, and then was treated on study but did not meet the definition of prior platinum exposure in the metastatic setting. Unfortunately, her disease progressed on the combination of veliparib and FOLFOX.
Figure 3: Kaplan-Meier Curves.
Progression-free survival (PFS, A, C, E) and overall survival (OS, B, D, F) are graphed for patient subgroups. Figures 3A and 3B compare the PFS and OS, respectively for all patients, patients who had no prior platinum therapy, and patients who had prior platinum therapy exposure, as indicated in the legend. Figures 3C and 3D compare the PFS and OS, respectively for all patients, patients who had a positive FH (as defined in the methods) and patients who did not have a FH. Figures 3E and 3F compare the PFS and OS, respectively for all patients who harbored a germline or somatic pathogenic homologous recombination, DNA damage repair (HR-DDR) mutation, the HR-DDR mutated patients who had not received prior platinum therapy, and patients who did not (or were not known to) harbor an HR-DDR mutation.
Forty-three patients had a positive FH and the ORR for these patients was 30%, with a DCR of 53% (N=43). This is compared to an ORR of 13% and a DCR of 47% for those with no FH (N=15) (Table 3, and Figures 3C and 3D).
Finally, 29 out of 58 evaluable patients had NGS testing for HR-DDR mutations. Of these, 14 patients had a BRCA1/2 mutation; 2 patients had an ATM mutation; 1 patient had a PALB2 mutation; and 1 patient had a FANCG mutation (Supplementary Table 4). The ORR of the HR-DDR mutated patients was 44%, with a DCR of 61% (N=18). The highest ORR overall was observed in HR-DDR mutated patients who had not previously received platinum at 57% with a DCR of 79% which was irrespective of the line of therapy (N=14) (Table 3, and Figures 3E and 3F). As visualized in Figure 2, all of the responses were observed in patients with germline or somatic BRCA1/2 or PALB2 mutations. The one patient with a FANCG mutation experienced stable disease (PFS of 4.7, and ongoing at the time of data cut-off); but neither of the patients with ATM mutations (both of whom were also platinum-naïve) achieved even stable disease.
DISCUSSION
Patients with mPDAC are in desperate need of additional therapies. The modern chemotherapy regimens of FOLFIRNOX and gemcitabine + nab-paclitaxel have improved outcomes, but response rates are only 31% and 23%, respectively(2,3). However, multiple sequencing efforts have revealed that a significant portion of PDACs harbor HR-DDR gene mutations, most commonly BRCA1/2 and ATM. Emerging data has revealed that patients with HR-DDR-mutated mPDAC, specifically those with BRCA1/2 or PALB2 mutations can respond to PARP inhibitors, and even CRs can be achieved(8–13,15). We and others have published that in pancreatic cancer patients whose tumors harbor HR-DDR mutations (ATM, ATRX, BAP1, BRIP1, FANCG, RAD50/51, etc) have an improved survival when treated with platinum-based therapy(14,30). Additionally, there exists pre-clinical published data suggesting a benefit to PARP inhibitors, or PARP inhibitor combinations in pancreatic cancer models, including cell lines, and patient-derived models, of that tumors with other HR-DDR mutations(31) . While we have had personal communication with colleagues who have shared anecdotal stories of patients with other HR-DDR mutations benefitting from off label PARP inhibitor therapy, there have not as yet been any published case reports of PARP inhibitor activity in such pancreatic cancer patients (PUBMED search, June 1st, 2020).
In pancreatic cancer patients with germline or somatic BRCA1/2/PALB2 mutations, the activity of single agent PARP inhibitors has been limited, ranging from 16 – 21%(8,13). Shroff, et al, detailed that, similar to our findings, the majority of the responders were patients whose disease had not progressed on prior platinum(13). Yet, there may be enhanced benefit with the combination of a PARP inhibitor and a platinum, and preliminary results in a Phase Ib trial from O’Reilly, et al demonstrated a remarkable 78% ORR, and 23 month OS with the combination of gemcitabine, cisplatin, and veliparib in PDAC patients harboring germline BRCA1/2 mutations(11).
We present here the results of our Phase I/II trial of FOLFOX + veliparib, and we demonstrate that patients with mPDAC can respond to this combination (ORR = 26%). For the two Phase II cohorts, we chose to close the study early due to slow accrual, but we did meet our protocol-defined primary endpoint, with at least 3 responders in each cohort. In fact, with 40% of untreated, and 22% of previously treated patients achieving a PR or CR, this combination is worthy of further investigation. When patients were selected based on FH and/or the presence of HR-DDR mutations, the ORR increased to 45%, and the highest responses were observed in patients whose tumors harbored HR-DDR mutations, and who had not been exposed to prior platinum (ORR = 57%, N = 14). Our results also highlight the fact that patients whose disease has progressed on prior platinum are very unlikely to benefit from a PARP inhibitor combination. In our study, none of the 10 patients who had experienced disease progression on prior platinum had even a minor reduction in tumor burden (Figure 2), and the ORR and DCR for these patients was 0% (Table 3). Our results are in line with the detailed results provided in the rucaparib study by Shroff, et al (13), and emphasize the notion that future trials of PARP inhibitors for PDAC patients should exclude patients whose disease has progressed while on prior platinum. The combination was well tolerated, and, for the patients with long-term (>4–6 month) disease control, a strategy of “maintenance” therapy without the oxaliplatin was able to maintain disease control for a prolonged period, as demonstrated by the swimmer’s plot (Figure 2B).
What is uncertain is the contribution of the veliparib to FOLFOX. It is possible that patients with HR-DDR mutations would respond to FOLFOX alone. However, two patients whose disease was only stabilized with prior FOLFIRINOX (i.e., no reduction in tumor burden), did have a response to FOLFOX + veliparib. Similarly, Shroff, et al highlighted two patients who did not respond to platinum, but did respond to rucaparib(13). Yet, the results of the recently reported randomized Phase II trial of gemcitabine + cisplatin +/− veliparib in patients with BRCA1/2 or PALB2 mutations failed to demonstrate any benefit to the addition of veliparib(32). In this trial, patients treated with gemcitabine + cisplatin alone had an ORR of 65%, and a median overall survival of 16.4 months, which was not different in the group of patients who received veliparib as well. While veliparib was not of any benefit when added to gemcitabine and cisplatin, the PARP inhibitor may play a different role when combined with oxaliplatin-based chemotherapy, wherein patients can only remain on the platinum for ~4–6 months. In our study, 18 patients remained on therapy for >6 months, all of whom had gone off the oxaliplatin, due to neuropathy. Thus, there may be a role for transitioning patients off oxaliplatin while maintaining the PARP inhibitor as long term maintenance therapy, as was done in the POLO trial with olaparib(33). Such a strategy appeared effective in the Phase III trial of carboplatin and paclitaxel +/− veliparib in ovarian cancer (24). A similar trial of induction FOLFOX or even FOLFIRINOX, followed by maintenance veliparib versus, for example, maintenance 5FU (or oral capecitabine), would be an important study, and would address an important concern (the placebo control arm) of the POLO trial.
Furthermore, there is an ongoing debate on the functional role of PARP inhibition in the treatment of HR-DDR mutated cancers. Originally, PARP inhibitors were demonstrated to inhibit the catalytic activity of the PARP-1 enzyme, thus inhibiting single strand repair, particularly after co-treatment with a DNA-damaging chemotherapy(34). This mechanism was the foundation for exploring the combination of veliparib and chemotherapy(23). However, a second critical role of some PARP inhibitors involves the trapping of the PARP enzyme at the site of DNA damage(35). The trapped PARP enzyme complex results in replication fork arrest, leading to mitotic catastrophe and apoptotic cell death. Several PARP inhibitors such as olaparib, niraparib, rucaparib, and talazoparib can achieve PARP trapping, and thus are active as single agents(8,13). Veliparib at tolerable doses can only achieve catalytic inhibition of the PARP enzyme. While veliparib may not be effective as a single agent, the tradeoff may be that the limited spectrum of activity of veliparib may also allow for the safe combination with DNA damaging agents, such as radiation and chemotherapy. By contrast, many of the PARP trapping inhibitors have been too toxic to use in combination with DNA damaging chemotherapies (36–38). Ultimately, a trial that compares, for example FOLFOX + veliparib to a PARP trapping inhibitor would have to be done to compare outcomes of the two DNA damage targeting strategies.
Finally, our study emphasizes the need to identify patients whose disease harbors HR-DDR mutations. The NCCN has recently recommended that all patients with PDAC undergo germline testing for the presence of an inherited mutation. In addition, there has been more widespread testing for somatic mutations. Thus, we are optimistic that more patients will be identified, and a strategy to steer such patients towards PARP inhibitor-based clinical trials will be critical, and may lead to the proof that we can improve outcomes for this subgroup of patients.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Platinum-based chemotherapies and PARP inhibitors have demonstrated promising activity in patients with metastatic pancreatic cancer who harbor pathogenic germline or somatic mutations in the homologous recombination, DNA damage response and repair (HR-DDR) genes, BRCA1/2, or PALB2. There is evidence of benefit of PARP inhibitors for patients whose tumors harbor other HR-DDR gene mutations as well. However, there may be even greater benefit when platinums and PARP inhibitors are combined. To assess the safety and efficacy of such a combination, we performed a Phase I/II clinical trial of 64 patients, with the combination of the PARP inhibitor, veliparib, with chemotherapy, FOLFOX. The combination was safe and effective for patients with metastatic pancreatic cancer. The greatest activity, with an ORR of 57%, was seen in patients whose disease had not progressed on prior platinum, and who harbored germline or somatic HR-DDR mutations.
Acknowledgements:
We would like to thank Meeta Jaiswal, PhD for scientific guidance throughout protocol development and implementation.
Funding Support:
This work was funded by the Otto J. Ruesch Center for the Cure of GI Cancers, Lombardi Comprehensive Cancer Center.
Abbvie, Inc. has provided veliparib and partial research funding for the correlative science MJP and JRB are supported by 1R01CA212600-01 (NCI, NIH); a 2015 Pancreatic Cancer Action Network American Association for Cancer Research Acceleration Network Grant (15-90-25-BROD); and MJP and JRB are also supported in part by U01CA224012 (NCI, NIH). Dr. Brody was supported in part by the NCI of the NIH Award Number P30CA056036 SKCC Core Grant (Thomas Jefferson University). We also would like to acknowledge The Sarah Parvin Foundation for their generous support.
REFERENCES
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74(11):2913–21 doi 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364(19):1817–25 doi 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369(18):1691–703 doi 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pishvaian MJ, Bender RJ, Halverson D, Rahib L, Hendifar AE, Mikhail S, et al. Molecular Profiling of Patients with Pancreatic Cancer: Initial Results from the Know Your Tumor Initiative. Clin Cancer Res 2018;24(20):5018–27 doi 10.1158/1078-0432.CCR-18-0531. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre AJ, Nowak JA, Camarda ND, Moffitt RA, Ghazani AA, Hazar-Rethinam M, et al. Real-time Genomic Characterization of Advanced Pancreatic Cancer to Enable Precision Medicine. Cancer Discov 2018;8(9):1096–111 doi 10.1158/2159-8290.CD-18-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowery MA, Jordan EJ, Basturk O, Ptashkin RN, Zehir A, Berger MF, et al. Real-Time Genomic Profiling of Pancreatic Ductal Adenocarcinoma: Potential Actionability and Correlation with Clinical Phenotype. Clin Cancer Res 2017;23(20):6094–100 doi 10.1158/1078-0432.CCR-17-0899. [DOI] [PubMed] [Google Scholar]
- 7.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531(7592):47–52 doi 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33(3):244–50 doi 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golan T, Sella T, O’Reilly EM, Katz MH, Epelbaum R, Kelsen DP, et al. Overall survival and clinical characteristics of BRCA mutation carriers with stage I/II pancreatic cancer. Br J Cancer 2017;116(6):697–702 doi 10.1038/bjc.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowery MA, Kelsen DP, Stadler ZK, Yu KH, Janjigian YY, Ludwig E, et al. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist 2011;16(10):1397–402 doi 10.1634/theoncologist.2011-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Reilly EM, Lee JW, Lowery MA, Capanu M, Stadler ZK, Moore MJ, et al. Phase 1 trial evaluating cisplatin, gemcitabine, and veliparib in 2 patient cohorts: Germline BRCA mutation carriers and wild-type BRCA pancreatic ductal adenocarcinoma. Cancer 2018. doi 10.1002/cncr.31218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Reilly EM LM, Segal MF, Smith SC, Moore MJ, Kindler HL, Golan T, Segal A, Salo-Mullen EE, Hollywood E, Epstein AS, Capanu M, Moynahan ME, Fusco A, Stadler ZK, Do RKG, Chen AP, Yu KH, Tang LH, Kelsen DP . Phase IB trial of cisplatin (C), gemcitabine (G), and veliparib (V) in patients with known or potential BRCA or PALB2-mutated pancreas adenocarcinoma (PC). JCO 2014;32:5s, (suppl; abstr 4023). [Google Scholar]
- 13.Shroff RT, Hendifar A, McWilliams RR, Geva R, Epelbaum R, Rolfe L, et al. Rucaparib Monotherapy in Patients With Pancreatic Cancer and a Known Deleterious BRCA Mutation. JCO Precis Oncol 2018;2018 doi 10.1200/PO.17.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pishvaian MJ BE, Brody JR, Rahib L, Lyons E, De Arbeloa P, Hendifar A, Mikhail S, Chung V, Sohal DPS, Leslie S, Mason K, Tibbets L, Madhavan S, Matrisian LM, Petricoin III E. Outcomes in Patients With Pancreatic Adenocarcinoma With Genetic Mutations in DNA Damage Response Pathways: Results From the Know Your Tumor Program. JCO Precision Oncology 2019. doi DOI: 10.1200/PO.19.00115. [DOI] [PubMed] [Google Scholar]
- 15.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med 2019. doi 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maintenance Rucaparib Controls Some Pancreatic Cancers. Cancer Discov 2019;9(6):OF4 doi 10.1158/2159-8290.CD-NB2019-043. [DOI] [PubMed] [Google Scholar]
- 17.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med 2015;373(18):1697–708 doi 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connor MJ. Targeting the DNA Damage Response in Cancer. Mol Cell 2015;60(4):547–60 doi 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Golan T, Javle M. DNA Repair Dysfunction in Pancreatic Cancer: A Clinically Relevant Subtype for Drug Development. J Natl Compr Canc Netw 2017;15(8):1063–9 doi 10.6004/jnccn.2017.0133. [DOI] [PubMed] [Google Scholar]
- 20.Lord CJ, Ashworth A. Targeted therapy for cancer using PARP inhibitors. Curr Opin Pharmacol 2008;8(4):363–9 doi 10.1016/j.coph.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Steffen JD, Tholey RM, Langelier MF, Planck JL, Schiewer MJ, Lal S, et al. Targeting PARP-1 allosteric regulation offers therapeutic potential against cancer. Cancer Res 2014;74(1):31–7 doi 10.1158/0008-5472.CAN-13-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kummar S, Kinders R, Gutierrez ME, Rubinstein L, Parchment RE, Phillips LR, et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol 2009;27(16):2705–11 doi 10.1200/JCO.2008.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res 2007;13(9):2728–37 doi 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 24.Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N Engl J Med 2019;381(25):2403–15 doi 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–47 doi 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Daly MB, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High Risk Assessment: Breast and Ovarian. 2019;Version 2.2019. [Google Scholar]
- 27.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18(16):2938–47 doi 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 28.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10(1):1–10. [DOI] [PubMed] [Google Scholar]
- 29.Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15(6):2403–13 doi 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 30.Wattenberg MM, Asch D, Yu S, O’Dwyer PJ, Domchek SM, Nathanson KL, et al. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br J Cancer 2020;122(3):333–9 doi 10.1038/s41416-019-0582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res 2006;66(16):8109–15 doi 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 32.O’Reilly EM, Lee JW, Zalupski M, Capanu M, Park J, Golan T, et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin With or Without Veliparib in Patients With Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J Clin Oncol 2020:JCO1902931 doi 10.1200/JCO.19.02931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golan T, Locker GY, Kindler HL. Maintenance Olaparib for Metastatic Pancreatic Cancer. Reply. N Engl J Med 2019;381(15):1492–3 doi 10.1056/NEJMc1911185. [DOI] [PubMed] [Google Scholar]
- 34.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer 2008;8(3):193–204 doi 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 35.Shen Y, Aoyagi-Scharber M, Wang B. Trapping Poly(ADP-Ribose) Polymerase. J Pharmacol Exp Ther 2015;353(3):446–57 doi 10.1124/jpet.114.222448. [DOI] [PubMed] [Google Scholar]
- 36.Rajan A, Carter CA, Kelly RJ, Gutierrez M, Kummar S, Szabo E, et al. A phase I combination study of olaparib with cisplatin and gemcitabine in adults with solid tumors. Clin Cancer Res 2012;18(8):2344–51 doi 10.1158/1078-0432.CCR-11-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samol J, Ranson M, Scott E, Macpherson E, Carmichael J, Thomas A, et al. Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: a phase I study. Invest New Drugs 2012;30(4):1493–500 doi 10.1007/s10637-011-9682-9. [DOI] [PubMed] [Google Scholar]
- 38.Dhawan MS, Bartelink IH, Aggarwal RR, Leng J, Zhang JZ, Pawlowska N, et al. Differential Toxicity in Patients with and without DNA Repair Mutations: Phase I Study of Carboplatin and Talazoparib in Advanced Solid Tumors. Clin Cancer Res 2017;23(21):6400–10 doi 10.1158/1078-0432.CCR-17-0703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.