Summary
Background
Refractory out-of-hospital cardiac arrest (OHCA) treated with standard advanced cardiac life support (ACLS) has poor outcomes. Transport to hospital followed by in-hospital extracorporeal cardiopulmonary resuscitation (ECPR) initiation may improve outcomes. We performed a pooled individual patient data analysis of two randomised controlled trials evaluating ECPR based approach in OHCA.
Methods
The individual patient data from two published randomised controlled trials (RCTs) were pooled: ARREST (enrolled Aug 2019–June 2020; NCT03880565) and PRAGUE-OHCA (enrolled March 1, 2013–Oct 25, 2020; NCT01511666). Both trials enrolled patients with refractory OHCA and compared: intra-arrest transport with in-hospital ECPR initiation (invasive approach) versus continued standard ACLS. The primary outcome was 180-day survival with favourable neurological outcome (defined as Cerebral Performance Category 1–2). Secondary outcomes included: cumulative survival at 180 days, 30-day favourable neurological survival, and 30-day cardiac recovery. Risk of bias in each trial was assessed by two independent reviewers using the Cochrane risk-of-bias tool. Heterogeneity was assessed via Forest plots.
Findings
The two RCTs included 286 patients. Of those randomised to the invasive (n = 147) and standard (n = 139) groups, respectively: the median age was 57 (IQR 47–65) and 58 years (IQR 48–66), and the median duration of resuscitation was 58 (IQR 43–69) and 49 (IQR 33–71) minutes (p = 0.17). In a modified intention to treat analysis, 45 (32.4%) in the invasive and 29 (19.7%) patients in the standard arm survived to 180 days with a favourable neurological outcome [absolute difference (AD), 95% CI: 12.7%, 2.6–22.7%, p = 0.015]. Forty-seven (33.8%) and 33 (22.4%) patients survived to 180 days [HR 0.59 (0.43–0.81); log rank test p = 0.0009]. At 30 days, 44 (31.7%) and 24 (16.3%) patients had favourable neurological outcome (AD 15.4%, 5.6–25.1%, p = 0.003), 60 (43.2%), and 46 (31.3%) patients had cardiac recovery (AD: 11.9%, 0.7–23%, p = 0.05), in the invasive and standard arms, respectively. The effect was larger in patients presenting with shockable rhythms (AD 18.8%, 7.6–29.4; p = 0.01; HR 2.26 [1.23–4.15]; p = 0.009) and prolonged CPR (>45 min; HR 3.99 (1.54–10.35); p = 0.005).
Interpretation
In patients with refractory OHCA, the invasive approach significantly improved 30- and 180-day neurologically favourable survival.
Funding
None.
Keywords: Resuscitation, Cardiac arrest, Extracorporeal circulation, Invasive
Research in context.
Evidence before this study
We searched PUBMED, Google Scholar, Web of Science for interventional randomised trials comparing standard ACLS and ECPR based approach in refractory cardiac arrest up to December 31 of 2022. We identified two studies meeting these criteria. The PRAGUE-OHCA and ARREST randomised controlled trials suggested an improvement in neurologically favourable survival with the use of an early invasive ECPR strategy for refractory out-of-hospital cardiac arrest in well organised, experienced centres with preestablished prehospital and hospital protocols of care.
Added value of this study
A number of case reports, matched cohort studies and metanalyses have suggested that ECPR is associated with improved survival from refractory cardiac arrest. Our pooled analysis of the two included trials (The PRAGUE-OHCA and ARREST) show that in the setting of experienced and high-volume centres, ECPR significantly improves 180 days neurologically favourable survival. The main clinical effect is observed in patients presenting with shockable rhythms receiving prolonged CPR.
Implications of all the available evidence
ECPR should be strongly considered as a lifesaving therapy in patients with refractory OHCA (especially those presenting with shockable rhythms) in health care systems that can provide the following: high baseline level of bystander CPR, centralised protocol involving emergency medical service with readily available telephone assisted CPR, high-volume receiving medical centre with experienced operators, targeted and achieved efficient cannulation times, immediate invasive assessment and treatment, established intensive care unit protocols that are appropriate for critically ill patients with prolonged resuscitation durations, and guideline recommended neuroprognostication and overall complex care that these patients need. The recently published INCEPTION trial showed that, in the absence of the above critical elements, ECPR does not offer any benefit.
Introduction
Out-of-hospital cardiac arrest (OHCA) is one of the leading causes of mortality and disability in the world with approximately 750,000 people dying each year in Europe1 and US2 alone. Despite advances in resuscitation science, rates of survival in patients treated with conventional cardiopulmonary resuscitation (CPR) and advanced cardiac life support (ACLS) remain low and a small but significant portion of survivors have persistent neurological damage.3,4 Chances of survival for refractory cardiac arrest dramatically decrease after 30 min of standard CPR strategies,5 to the point that after 40 min, <1% of patients achieve return of spontaneous circulation (ROSC) and survive with a favourable neurological outcome even in the most favourable group of patients that present with shockable rhythms.
The recent publications of the ARREST6 and PRAGUE-OHCA7 trials represent the first attempts to compare invasive, extracorporeal cardiopulmonary resuscitation (ECPR) based strategies to ACLS in a randomised controlled trial setting. Both of these trials were single centre, city wide, structured EMS (emergency medical service), open-label trials, with ECPR initiated at experienced high-volume centres with established ECPR programs.
In the ARREST trial, an ECPR-based approach was compared to ACLS for the management of refractory ventricular tachycardia/ventricular fibrillation OHCA. In this trial, patients were randomised after admission to the emergency department and 43% of the patients treated by the ECPR based strategy achieved neurologically favourable survival to discharge versus only 7% of patients who were randomised to ACLS. At 6 months, survival was 43% and 0%, respectively (p = 0.0063) The ARREST trial was terminated after 30 patients were randomised because of the demonstrated efficacy.
The PRAGUE-OHCA study compared an ECPR-based strategy to conventional ACLS in patients with witnessed refractory OHCA with both shockable and non-shockable rhythms. Patients were randomised during ongoing ACLS at the field. The PRAGUE-OHCA study failed to prove the difference in the primary endpoint of neurological intact survival at 6 months but showed a significant survival benefit at 180 days, and significant improvement in neurological outcome at 30 days. In the same trial, 11 patients who were randomised to the standard ACLS arm crossed over to invasive arm, with five of them surviving with neurological favourable outcome.
Given the limited randomised controlled data, the diversity in the ECPR strategies that are employed and the potentially high impact on survival, there is value in pooling these two similar randomised controlled trials to assess the effect of ECPR based approach on survival and identify optimal patient characteristics for deploying such strategies.8,9
Therefore, we conducted an individual participant level pooled analysis of the two RCTs. We hypothesised that an invasive ECPR based strategy would improve neurologically favourable survival at 180 days when compared to standard ACLS strategies for refractory OHCA.
Methods
Study design and ethics
For both RCTs, the corresponding authors provided anonymised individual patient data, coding, and definition of variables. Risk of bias in each trial was assessed by two independent reviewers (BG and RK) using the Cochrane risk-of-bias tool (Supplementary Table S1). This was not applied separately for each outcome.10
Three authors had access and verified the data of the combined patient populations (JB, DY, MH) and two authors were responsible to submit the manuscript (JB, DY). Data consistency, data completeness, and baseline imbalance were verified by JB, DY, and MH.
Ethics approval was not required for this pooled analysis. Both primary studies were approved by the local University hospital Institutional Review Boards and the ARREST trial was further approved and monitored by the Food and Drug Administration (FDA). Individual consent was obtained from all patients included in the primary studies. Pooled analysis combined anonymous data.
Protocols of the original trials used for pooling the data
The study was conducted with a protocol a priori but it was not registered. An intention to treat analysis and an as-treated analysis were the primary analyses prespecified at the inception of this study design. A post-hoc per-protocol analysis was performed as a sensitivity analysis. Out of the data from the primary studies, there was one point missing for the ARREST trial when a patient in the ECPR group refused further participation in the study. Outcomes results were censored for this one patient. For the PRAGUE-OHCA trial, there was no missing data for the primary and secondary outcomes.
The ARREST trial was a single-centre, city-wide, multiple EMS randomised controlled trial that assessed the efficacy of an early, invasive ECPR-based approach to patients arriving to the hospital after presenting with refractory OHCA due to a shockable rhythm.6,11 The ARREST trial enrolled patients between August 8, 2019 to June 14, 2020. Refractory OHCA was defined as a failure to achieve ROSC after three defibrillatory shocks. Upon arrival, patients were randomised to: (1) gain immediate access to the cardiac catheterisation laboratory for initiation of ECPR (if clinically indicated) followed by coronary angiography and intervention, or (2) standard ACLS until the patients were either declared dead or ROSC was achieved. In the latter case, those patients could also gain access to the cardiac catheterisation laboratory for the same interventions as the ECPR group. The primary and safety analyses were both analysed using the modified intention-to-treat principle. The trial qualified for exception from informed consent under emergency circumstances (21 Code of Federal Regulations 50.24), with applicable requirements and oversight by the US Food & Drug Administration (FDA), an investigational device exemption, approval by the Institutional Review Board of the University of Minnesota, and monitoring by an independent NHLBI appointed Data and Safety Monitoring Board (DSMB). After admission to the hospital, patients who were enrolled under exemption of informed consent had to provide written consent upon awakening. Until this was possible, the research team obtained consent to continue participation within 24 h from admission from the legally authorised representative. The representative and the patient had the freedom to withdraw from the study at any time.
The PRAGUE-OHCA study was a randomised controlled trial which was conducted at a single centre in Prague, Czech Republic from March 1, 2013 to October 25, 2020. Adult patients, while receiving ongoing resuscitation for witnessed OHCA of presumed cardiac aetiology after at least 5 min of ACLS were eligible for enrolment in the trial. A web-based secured randomisation system was used to assign patient number and intervention group prehospitally during ongoing CPR in the field. The methodology and results of the intention to treat analysis were published in detail elsewhere.7,12 The original study as well as secondary analyses were approved by the Institutional Review Board of the General University Hospital and First Faculty of Medicine, Charles University in Prague (192/11, S-IV). All patients who regained normal neurological function were asked to provide their written consent regarding the use of their data. Consent requirements were waived for patients who died at the scene and never reached the hospital and for participants without known legal representatives.
Both studies used individual randomisation for group allocation. Both studies also employed 1:1 randomisation as was published in the original trial protocols and primary results papers. The two trials differed mainly by the place of randomisation: at hospital arrival in the ARREST trial and in the prehospital setting in the PRAGUE-OHCA study. The ARREST trial enrolled only patients presenting with shockable rhythm, while the PRAGUE-OHCA study enrolled all rhythms. In both trials the “standard arm” cases were treated with local protocols: in the PRAGUE-OHCA trial standard therapy was primarily on-scene treatment, however, some cases still eventually underwent intra-arrest transport; in the ARREST trial, the standard therapy was intra-arrest transport for those in whom ROSC was not achieved during the initial resuscitation.
Outcome measures
The primary outcome of the present analysis is the 180-day survival with favourable neurological outcome [defined as cerebral performance category (CPC) 1–2]. Secondary outcomes include: cumulative survival at 180 days, 30-day favourable neurological survival (defined as CPC 1–2), and 30-day cardiac recovery, which was defined as no need for pharmacological or mechanical support for at least 24 h. The analysis for these endpoints was performed using the modified intention-to-treat approach (as randomised) in the whole, shockable rhythms and resuscitated ≥45 min populations.
We further repeated these methods also for an “as-treated” and “per protocol” analyses, classifying patients based on the treatment they received, rather than the assigned treatment at randomisation and excluding patients who received other treatment than the assigned at randomisation, respectively.
We also specified the following sub-group analyses for the primary study outcome in the modified intention-to-treat populations based on the original publications: sex (male versus female), age (we dichotomised by the median age, rounded to the nearest low multiple of 5, <55 years or >55 years), duration of resuscitation (<30 min, ≥30 and <45 min or ≥45 min), initial cardiac rhythm (shockable or non-shockable), location of OHCA (public place or home), epinephrine used and study enrolment (ARREST or PRAGUE-OHCA study). The subgroup analyses in this manuscript are considered hypothesis generating. Post-hoc subgroup analyses were performed for the whole and shockable rhythm populations.
Statistical analysis
The cardiac arrest time intervals and other continuous numeric variables are expressed as medians and interquartile range (IQR). The 2-sided Mann–Whitney test was used to compare cardiac arrest intervals and laboratory values between the standard and invasive groups. For categorical values and outcome assessment the Fisher’s exact test (for 2 × 2 table) or chi-square test were used, with absolute difference and 95% confidence intervals calculated and reported.
The survival analysis was performed using Kaplan–Meier analyses and log rank test p value calculation and considered patients alive at day 180 regardless of their neurological status. The results of the subgroup analyses were reported as an absolute difference with 95% confidence intervals. We also fit a series of Mantel–Haenszel models, examining the association of treatment assignment and outcomes, with each separate model including an interaction term between the invasive group strategy and each of the subgroup categories; p values were calculated for the interaction terms. The multivariable analysis was performed by logistic regression (OR; 95% CI; p-value). A two-tailed p value of <0.05 was considered significant for all analyses.
The decision to use non-parametric tests was based on evaluation of the distribution of all continuous variables when tested by the Shapiro–Wilk test. Further, we examined histograms to identify deviations in skewness and/or kurtosis. When identified as significant, we chose to use non-parametric methods. Forest plots for heterogeneity of the enrolled studies were developed and presented.
We included all the enroled patients from both studies for analysis, therefore no sample size calculations were done. We performed per-protocol and as-treated sensitivity analyses. We compared the results of fixed and mixed effect models. Further, we considered and conducted a multilevel model, specifically a generalised mixed-effect model. However, the results of the model were almost identical not only in the odds ratio values but also in Akaike information criterion (AIC). The mixed-effect model had an AIC of 226.2, and the fixed-level model had an AIC of 224.16. This result confirmed our assumptions that heterogeneity has no important impact on the result of the multivariable regression model. Therefore, we report the results of the fixed-effect model.
All statistical analyses were conducted using MedCalc® Statistical Software version 20.211 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2023).
Role of the funding source
No funding was received for this work.
Results
Baseline, pre-hospital, and procedural characteristics
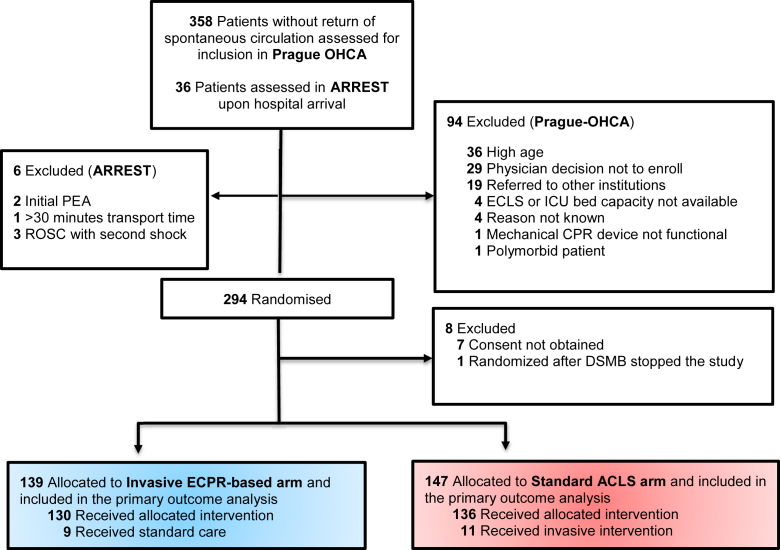
The two studies enrolled a total of 286 patients: 139 patients were randomised to the invasive ECPR-based arm and 147 in the standard ACLS arm. The CONSORT diagram of the pooled populations is shown in Fig. 1.
Fig. 1.
CONSORT diagram for the pooled analysis of the ARREST and Prague-OHCA trials.
Demographics and pooled data of baseline characteristics of the Invasive and Standard groups are shown in Table 1. The characteristics were evenly distributed between groups, except for prehospital dose of epinephrine used. Notably, eleven patients in the standard ACLS group crossed over to receive ECPR based approach, with 10 of them ultimately receiving extracorporeal life support therapy (ECLS) with similar time to initiation as in the invasive strategy group. The risk of bias was judged to be low in both trials (Supplementary Figure S1). The assessors did not have any discordance in the assessment of study quality. No issues were identified in checking the integrity of the individual data.
Table 1.
Demographic and baseline data pooled from the ARREST and PRAGUE-OHCA randomised trials.
| Demographic and baseline data | Invasive (N = 139) | Standard (N = 147) | p value |
|---|---|---|---|
| Age (years), mean (IQR) | 58 (48–66) | 57 (47–65) | 0.58 |
| Sex, n (%) | |||
| Women | 23 (16.5%) | 26 (17.7%) | 0.88 |
| Men | 116 (83.5%) | 121 (82.3%) | |
| Medical history, n/N (%) | |||
| Hypertension | 49/123 (39.8%) | 47/98 (48%) | 0.27 |
| Coronary artery disease | 19/119 (16%) | 21/98 (21.4%) | 0.38 |
| Chronic heart failure | 12/121 (9.9%) | 5/94 (5.3%) | 0.31 |
| Diabetes | 22/119 (18.5%) | 20/98 (20.4%) | 0.73 |
| Chronic kidney disease | 3/119 (2.5%) | 4/94 (4.3%) | 0.7 |
| COPD | 9/120 (7.5%) | 3/94 (3.2%) | 0.24 |
| ICD implanted | 3/121 (2.5%) | 0/89 (0.0%) | 0.26 |
| Location of cardiac arrest, n (%) | N = 138 | N = 147 | |
| Home | 48 (34.8%) | 41 (27.9%) | 0.41 |
| Public place | 52 (37.7%) | 61 (41.5%) | |
| EMS | 19 (13.8%) | 17 (11.6%) | |
| Health facility | 2 (1.4%) | 1 (0.7%) | |
| Car | 8 (5.8%) | 7 (4.8%) | |
| Hotel | 4 (2.9%) | 6 (4.1%) | |
| Workplace | 5 (3.6%) | 14 (9.5%) | |
| Initial rhythm, n (%) | N = 139 | N = 147 | |
| VF | 87 (62.6%) | 99 (67.3%) | 0.44 |
| Asystole | 31 (22.3%) | 24 (16.3%) | |
| PEA | 21 (15.1%) | 24 (16.3%) | |
| Bystander CPR, n (%) | 137 (98.6%) | 143 (97.3%) | 0.68 |
| Collapse-to-EMS arrival interval (min), median (IQR) | 8 (7–10.3) | 9 (6–11) | 0.63 |
| Collapse-to-randomisation interval (Prague) (min), median (IQR) | 24 (21–30) | 26 (20–35) | 0.17 |
| Collapse-to-randomisation interval (ARREST) (min), median (IQR) | 52.5 (42–60.5) | 58 (44–66) | 0.57 |
| Number of epinephrine doses prehospitally, median (IQR) | 4 (2–5) | 5 (2.3–7) | 0.003 |
| Number of defibrillations prehospitally, median (IQR) | 4 (3–6) | 4 (2–7) | 0.97 |
| Intermittent ROSC, n/N (%) | 41/124 (33.1%) | 45/132 (34.1%) | 0.7 |
Denominators in respective groups demonstrated as N.
Bold: p value is less than the level of significance (<0.05).
Legend: COPD, chronic obstructive pulmonary disease; CPR, cardiopulmonary resuscitation; EMS, emergency medical services; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; PEA, pulseless electrical asystole; ROSC, return of spontaneous circulation; VF, ventricular fibrillation.
Procedural characteristics and in hospital care comparisons are shown in Table 2. Patients who were randomised to the invasive strategy group had a longer CPR duration and significantly lower sustained ROSC rates on admission but were more likely to be admitted to the hospital. Blood gas analysis revealed significantly lower pH and higher lactate levels on admission in the invasive strategy group.
Table 2.
Procedural characteristics pooled from the ARREST and PRAGUE-OHCA randomised trials.
| Procedural characteristics | Invasive (N = 139) | Standard (N = 147) | p value |
|---|---|---|---|
| Admitted to hospital | 138 (99.3%) | 102 (69.4%) | <0.0000001 |
| Collapse-to-hospital admission interval (min), median (IQR) | 49 (44–60) | 59 (49–68.3) | <0.0001 |
| Declared dead | 13 (9.4%) | 78 (53.1%) | <0.0001 |
| In the prehospital setting | 1 (7.7%) | 46 (59.0%) | 0.0006 |
| Within 1 h from admission | 12 (92.3%) | 32 (41.0%) | |
| Collapse-to-Resuscitation End interval (death, ROSC, or ECLS) (min), median (IQR) | 58 (43.2–68.5) | 49 (33–71) | 0.17 |
| <30 min | 14 (10.7%) | 26 (18.3%) | 0.02 |
| ≥30 and <45 min | 20 (15.3%) | 33 (23.2%) | |
| ≥45 min | 97 (74.0%) | 83 (58.5%) | |
| Sustained ROSC on admission to hospital | 34 (24.5%) | 59 (40.1%) | 0.005 |
| TTM used at the ICU n/N (%) | 129/138 (93.5%) | 63/102 (61.8%) | <0.0001 |
| ECPR | |||
| ECLS implanted | 94 (67.6%) | 10 (6.8%) | <0.0001 |
| Collapse-to-ECLS (min) | 61 (55–70) | 62 (51–73) | 0.9 |
| Door-to-ECLS interval (min) | 12 (9–15) | 15 (11–17) | 0.055 |
| Invasive assessment n/N (%) | |||
| Coronary angiography | 128/136 (94.1%) | 68/83 (81.9%) | 0.006 |
| Emergency invasive interventions n/N (%) | |||
| PCI (both for ACS and CAD) | |||
| Successful | 62/68 (91.2%) | 26/32 (81.2%) | 0.19 |
| Unsuccessful | 6/68 (8.8%) | 6/32 (18.8%) | |
| Laboratory values on admission n/N (%), median (IQR) | |||
| pH | 6.94 (6.8–7.1) | 7.03 (6.9–7.2) | 0.003 |
| pH ≤ 6.85 and CPC 1 + 2 | 9/138 (6.5%) | 1/92 (1.1%) | 0.05 |
| pH ≤ 6.80 and CPC 1 + 2 | 4/138 (2.9%) | 1/92 (1.1%) | 0.65 |
| Lactate (mmol/L) | 12.4 (9.4–15.9) | 10.2 (7.6–13.5) | 0.006 |
| Cause of cardiac arrest (including autopsy findings) | N = 124 | N = 132 | |
| Acute coronary syndrome | 64 (51.6%) | 63 (47.7%) | 0.19 |
| Coronary artery disease–chronic | 14 (11.3%) | 18 (13.6%) | |
| Pulmonary embolism | 12 (9.7%) | 12 (9.1%) | |
| Chronic heart failure | 8 (6.5%) | 6 (4.5%) | |
| Cardiomyopathy | 3 (2.4%) | 6 (4.5%) | |
| Myocarditis | 6 (4.8%) | 2 (1.5%) | |
| Aortic stenosis | 2 (1.6%) | 6 (4.5%) | |
| Aortic dissection type A | 2 (1.6%) | 2 (1.5%) | |
| Intracranial haemorrhage | 1 (0.8%) | 2 (1.5%) | |
| Bleeding–other | 3 (2.4%) | 0 (0.0%) | |
| Accidental hypothermia | 3 (2.4%) | 1 (0.8%) | |
| Pulmonary hypertension | 2 (1.6%) | 0 (0.0%) | |
| Sepsis | 0 (0.0%) | 1 (0.8%) | |
| Other | 1 (0.8%) | 1 (0.8%) | |
| Unknown | 3 (2.4%) | 12 (9.1%) | |
| Cause of death | N = 89 | N = 116 | |
| Refractory arrest | 13 (14.6%) | 81 (69.8%) | <0.0001 |
| Brain death | 24 (27.0%) | 10 (8.6%) | |
| MODS | 36 (40.4%) | 17 (14.7%) | |
| Cardiogenic shock | 10 (11.2%) | 4 (3.4%) | |
| UNK | 1 (1.1%) | 4 (3.4%) | |
| Bleeding | 4 (4.5%) | 0 (0.0%) | |
| Other | 1 (1.1%) | 0 (0%) | |
| Complications n/N (%) | |||
| Bleeding -any | 43/123 (35.0%) | 10/69 (14.5%) | 0.002 |
| Fatal | 4 (9.3%) | 0 (0.0%) | 0.6 |
| Intracranial haemorrhage | 8 (18.6%) | 2 (20.0%) | |
| Overt | 31 (72.1%) | 8 (80.0%) |
Denominators in respective groups demonstrated as N.
Bold: p value is less than the level of significance (<0.05).
Legend: ACS, acute coronary syndrome; CAD, coronary artery disease; CPC, cerebral performance category; CPR, cardiopulmonary resuscitation; ECLS, extracorporeal life support; EMS, emergency medical services; ICD, implantable cardioverter-defibrillator; ICU, intensive care unit; IQR, interquartile range; PCI, percutaneous coronary intervention; PEA, pulseless electrical asystole; ROSC, return of spontaneous circulation; TTM, targeted temperature management; MODS, multiple organ dysfunction syndrome; UNK, unknown; VF, ventricular fibrillation.
There were significantly more patients who received targeted temperature management therapy in the invasive strategy compared to the standard ACLS group, reflecting the lower admission rate to ICU. Patients with ECPR were more likely to receive angiography than the standard group, but once that was obtained, coronary interventions were similar between the two groups.
Among cases randomised to the invasive group: 34 achieved sustained ROSC during the transport to hospital or at the hospital arrival with conventional resuscitation (25%); 94 were treated with ECPR (69%); and 11 were declared dead (7.9%) prehospitally or on admission to hospital. Among cases randomised to the standard group: 59 (40%) achieved sustained ROSC during the transport to hospital or at the hospital arrival with conventional resuscitation; 10 (6.8%) were treated with ECPR (cross over); and 78 (53%) were declared dead prehospitally or at the hospital arrival.
The cause of arrest, identified in almost 90% of the patients, was similar in both groups with the predominant aetiology being acute coronary syndromes and chronic coronary artery disease. In general, patients randomised to the invasive strategy died of severe neurological injury or multiple organ failure rather than cardiac injury once they were on ECLS support in the hospital. Bleeding complications were more frequent in patients randomised to the invasive strategy. However, intracranial bleeding rates were not different between groups.
Primary outcome
The invasive, ECPR-based strategy significantly improved 180-day survival with favourable neurological outcome compared to standard ACLS: 45/139 (32.4%) patients in the invasive and 29/147 (19.7%) patients in the standard arm survived to 180 days with a good neurological outcome (AD, 95% CI: 12.7%, 2.5%–22.6%, p = 0.015; Table 3, Panel A).
Table 3.
Outcomes in pooled analysis of the ARREST and PRAGUE-OHCA randomised trials.
|
Panel A. Modified intention to treat analysis in the whole population of both trials | ||||
|---|---|---|---|---|
| Outcomes | Invasive (N = 139) | Standard (N = 147) | Absolute difference (CI), % | p value |
| Primary outcome | ||||
| Survival with minimal or no neurologic impairment at 180 days | 45 (32.4%) | 29 (19.7%) | 12.7 (2.6–22.7) | 0.015 |
| Secondary outcomes | ||||
| Survival with minimal or no neurologic impairment at 30 days | 44 (31.7%) | 24 (16.3%) | 15.4 (5.6–25.1) | 0.003 |
| Cardiac recovery at 30 days | 60 (43.2%) | 46 (31.3%) | 11.9 (0.7–23) | 0.05 |
|
Panel B. Modified intention to treat analysis in patients presenting with shockable rhythm | ||||
|---|---|---|---|---|
| Outcomes | Invasive (N = 87) | Standard (N = 99) | Absolute difference (CI), % | p value |
| Primary outcome | ||||
| Survival with minimal or noneurologic impairment at 180 days | 41 (47.1) | 28 (28.3) | 18.8 (5.1–32.6) | 0.01 |
| Secondary outcomes | ||||
| Survival with minimal or no neurologic impairment at 30 days | 40 (46) | 24 (24.2) | 21.8 (8.3–35.2) | 0.002 |
| Cardiac recovery at 30 days | 49 (56.3) | 42 (42.4) | 13.9 (−0.4 to 28.2) | 0.08 |
|
Panel C. Modified intention to treat for patients with time of cardiopulmonary resuscitation ≥45 min | ||||
|---|---|---|---|---|
| Outcomes | Invasive (N = 97) | Standard (N = 83) | Absolute difference (CI), % | p value |
| Primary outcome | ||||
| Survival with minimal or no neurologic impairment at 180 days | 23 (23.7%) | 6 (7.2%) | 16.5 (6.3–26.6) | 0.004 |
| Secondary outcomes | ||||
| Survival with minimal or no neurologic impairment at 30 days | 23 (23.7%) | 6 (7.2%) | 16.5 (6.3–26.6) | 0.004 |
| Cardiac recovery at 30 days | 32 (33.0%) | 12 (14.5%) | 18.5 (6.5–30.6) | 0.005 |
Bold: p value is less than the level of significance (<0.05).
Legend: CI, confidence interval.
Secondary outcomes
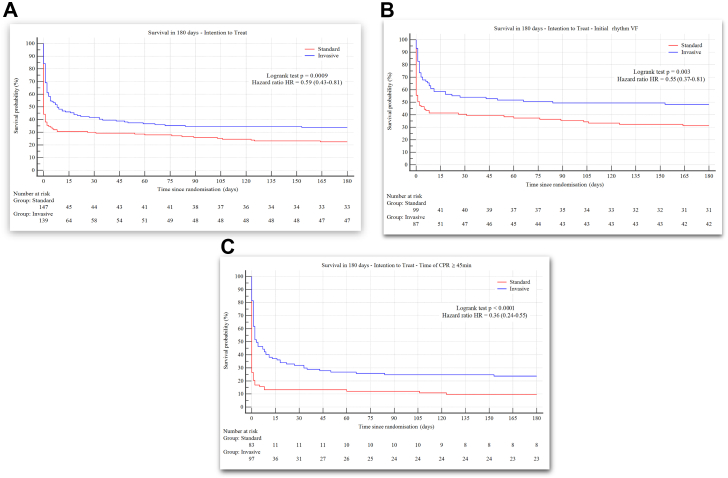
For cumulative survival at 180 days, the invasive ECPR based strategy significantly improved survival over a 6-month follow up period compared to standard ACLS in the whole study population (Fig. 2, Panel A): 47 (33.8%) patients in the invasive arm and 33 (22.4%) in the standard ACLS group survived to 180 days [HR 0.59 (0.43–0.81); log rank test p = 0.0009].
Fig. 2.
Cumulative survival at 180 days in out-of-hospital cardiac arrest patients of the ARREST and PRAGUE-OHCA randomised trials. Panel A: in the whole study population. Panel B: in patients presenting with shockable rhythms. Panel C: in patients with the time of cardiopulmonary resuscitation ≥45 min. Legend: CPR, cardiopulmonary resuscitation; VF, ventricular fibrillation.
For favourable neurological outcome at 30 days, the invasive strategy significantly improved neurological outcomes compared to standard ACLS: 44 (31.7%) and 24 (16.3%) patients reached favourable neurological status in the invasive and standard arms, respectively (AD, 95% CI: 15.4%, 5.5–25, p = 0.003).
For 30-day cardiac function recovery, the invasive strategy appeared to provide better cardiac recovery compared to standard ACLS where 60 (43.2%) and 46 (31.3%) patients (AD, 95% CI: 11.9%, 0.7–22.7, p = 0.05) had cardiac recovery, in the invasive and standard arms, respectively. Table 4 shows multivariable logistic regression analysis on the primary endpoint of the whole study population.
Table 4.
Multivariable logistic regression analysis for the primary outcome in the whole population of pooled ARREST and PRAGUE-OHCA randomised trials (OR > 1: unfavourable outcome, OR < 1: favourable outcome).
| Variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Sex male | 1.61 | 0.57–4.59 | 0.37 |
| Age >55 | 1.83 | 0.89–3.77 | 0.1 |
| Initial rhythm “Shockable” | 0.05 | 0.02–0.16 | <0.0001 |
| Public place Yes | 0.8 | 0.4–1.62 | 0.54 |
| Time of CPR <30 | 0.07 | 0.02–0.21 | <0.0001 |
| Time of CPR ≥30 & <45 | 0.27 | 0.11–0.66 | 0.004 |
| Epinephrine >4 mg | 1.82 | 0.81–4.07 | 0.15 |
| Invasive approach | 0.25 | 0.11–0.54 | 0.0004 |
| Study–ARREST | 1.92 | 0.45–8.16 | 0.38 |
Bold: p value is less than the level of significance (<0.05).
Legend: CI, confidence interval; CPR, cardiopulmonary resuscitation.
Subgroups defined by initial cardiac rhythm
Although patients presenting with shockable rhythms (ventricular tachycardia/ventricular fibrillation) represented only 65% (186/286) of the enrolled subjects, they accounted for 93% (69/74) of neurologically favourably surviving patients at 180 days. Only 5% (5/100) patients presenting with asystole and pulseless electrical activity survived with a favourable neurological outcome in 180 days in both groups.
Among initial shockable rhythms, the invasive strategy significantly improved 180-day survival with favourable neurological outcomes compared to standard ACLS (41 [47.1%] vs. 28 [28.3%] respectively; AD, 95% CI: 18.8%, [5.1–32.6], p = 0.01; Table 3, Panel B). Neurologically favourable survival at 30 days was consistent. All but one (41/42) patient presenting with shockable rhythms and treated with invasive ECPR based strategy who survived 180 days had favourable neurological outcomes (CPC 1–2), Fig. 2, Panel B.
Among cases treated by invasive approach with initial non-shockable rhythms, we did not detect an improvement in 180-day survival with favourable neurological outcomes compared to ACLS (4 [7.7%] vs. 1 [2.1%] respectively; AD, 95% CI: 5.6%, −2.7 to 13.9).
Additional subgroup analyses
Among patients resuscitated ≥45 min, the invasive strategy significantly improved 180-day survival with favourable neurological outcomes compared to standard ACLS (23 [23.7%] vs. 6 [7.2%], respectively; AD, 95% CI: 16.5%, 5.9–26.6, p = 0.004), Table 3, Panel C. The Kaplan–Meier survival comparisons, are shown in Fig. 2, Panel C.
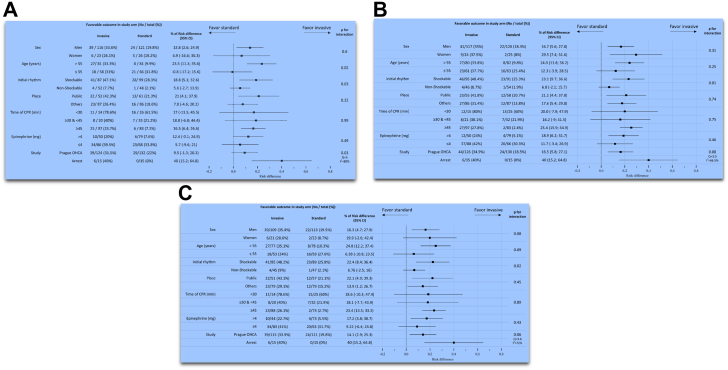
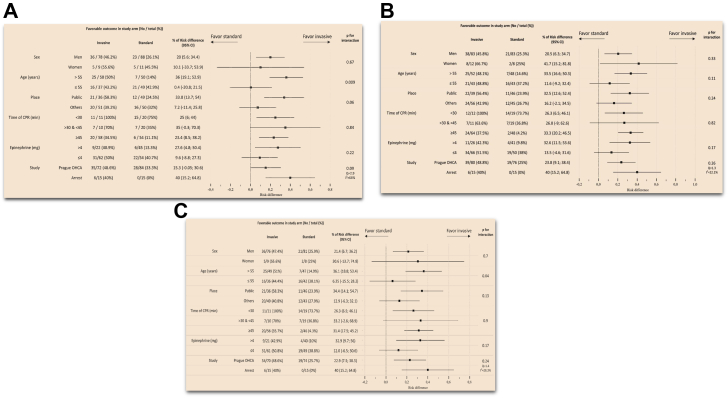
Subgroup analyses from the whole study population and for patients presenting with shockable rhythms in the ARREST and PRAGUE-OHCA randomised trials are shown in Fig. 3, Fig. 4 (Panels A, B, C are based on modified intention to treat, as treated and per protocol analyses). The invasive strategy led to improved 180-day survival among subgroups of: men, age > 55 years, initial shockable rhythms, public location, and those who were treated with ≥45 min of CPR. Interaction terms were statistically significant for: age category and initial cardiac rhythm.
Fig. 3.
Subgroup analyses from the whole study population of the ARREST and PRAGUE-OHCA randomised trials. Panel A: modified intention-to-treat analysis. Panel B: as treated analysis. Panel C: per protocol analysis. Legend: CPR, cardiopulmonary resuscitation; OHCA, out-of-hospital cardiac arrest.
Fig. 4.
Subgroup analyses from the shockable rhythms population of the ARREST and PRAGUE-OHCA randomised trials. Panel A: modified intention to treat analysis. Panel B: as-treated analysis. Panel C: per protocol analysis. Legend: CPR, cardiopulmonary resuscitation; OHCA, out-of-hospital cardiac arrest.
Sensitivity analysis: as-treated
Fig. 2, Fig. 3, show subgroup analyses of the whole and shockable populations using as treated analysis. Supplementary Table S2 includes primary and secondary outcomes for the whole (Panel A), shockable rhythms (Panel B) and resuscitated ≥45 min populations (Panel C). Supplementary Figure S2 shows Kaplan–Meier survival comparisons for the whole (Panel A), shockable rhythms (Panel B) and resuscitated ≥45 min (Panel C) populations.
Sensitivity analysis: per-protocol
Fig. 3, Fig. 4, show subgroup analyses of the whole and shockable populations using per protocol analysis. Supplementary Table S3 includes primary and secondary outcomes for the whole (Panel A), shockable rhythms (Panel B) and resuscitated ≥45 min populations (Panel C). Supplementary Figure S3 delineates Kaplan–Meier survival comparisons for the whole (Panel A), shockable rhythms (Panel B) and resuscitated ≥45 min (Panel C) populations.
Discussion
In this individual-patient pooled data analysis of the ARREST and PRAGUE-OHCA randomised controlled trials, we found that an invasive, ECPR-based resuscitation strategy that included early transport, ECLS, immediate invasive assessment, mainly coronary angiography, with cause identification, and eventual reversal in patients with refractory OHCA, significantly improved survival with favourable neurological outcomes at 180 days when compared to standard ACLS. The analysis further suggests that the clinically relevant effect for such an invasive, resource intensive therapy, was observed in patients presenting with initial shockable rhythms. For this shockable rhythm subgroup, ∼5 patients will have to be treated to save an additional life with favourable neurological outcome at 6 months.
Clinical evidence suggests that survival after 45 min of CPR with standard ACLS is grim.5,13 Universally, survival drops below 10% in refractory arrest after 30 min in all presenting rhythms and survival after 40 min is based on anecdotes and represents rare events. On the contrary, our data suggest that an invasive approach offers a distinctive survival advantage when applied within a mature, centralised system of care.
Our subgroup analyses also point to the phenotypes that demonstrate improved outcomes with an early invasive, ECPR-based approach: male, >55 years of age, initial shockable rhythm, a public place, and refractory to initial defibrillatory shocks and concomitant standard ACLS. These patients are very likely to have significant coronary artery disease as the underlying aetiology. Interestingly, cases over 55 years of age appeared to benefit more from invasive techniques, which may be related to the higher prevalence of acute coronary syndromes within this subgroup. This finding is consistent with a previous analysis that identified ECPR-treated cases aged 60 with the highest probability of survival, in comparison to both older and younger patients.14
In our pooled subgroup analysis, there was no interaction of time to ECPR on the overall effect on survival. The absolute magnitude of survival difference with ECPR for patients presenting with a shockable rhythm varied between 23% and 35%. Despite the fact there was no interaction between time intervals, our subgroup analyses suggest that the primary clinical benefit of ECPR is found among patients treated with ≥45 min of CPR in our trials. This group is the largest group of patients and represented almost 60% of the patient population studied. It is in those patients that standard CPR offers no significant chance of survival, as it can be seen in the as-treated analysis where only 2 patients survived with standard ACLS without ECPR cross over (Supplementary Table S2, Panel C). The major limitation of the time to ECPR effects are system based. Our systems were not capable of receiving patients consistently early enough to have significant numbers for comparison. The effect of expediting ECPR initiation to impact survival further needs to be evaluated in separate system implementation trials.
Our subgroup analysis did not detect an improvement in outcomes from ECPR among those with initial non-shockable rhythms, and our interaction analysis showed that the effect of ECPR on this phenotype is significantly different from its effect on those with initial shockable rhythms. The very high baseline mortality (∼95%) renders this population problematic for wide use of this strategy. Accordingly, given current health care system limitations and our poor prognostication capabilities before we initiate ECPR, these patients should only be considered on a case-by-case basis and not systemically. Originating from the PRAGUE-OHCA study, 4 patients with non-shockable presenting rhythms survived to 180 days neurologically intact in the invasive strategy arm and only a single patient survived in the standard ACLS arm.
It is helpful to review the current landscape of OHCA treatment in order to contextualise the results of this pooled analysis. The average survival rate for OHCA presenting with shockable rhythms in the US is 28%–30% and approximately 95% of these patients have CPR <20 min before achieving ROSC.11 In contrast, our combined populations received more than 3× the duration of CPR before circulation was re-established, whether it was by initiation of ECPR or, in a smaller subgroup, by achieving ROSC. Our analysis proved that ∼1/3 of the patients treated with an invasive ECPR based strategy after ∼60 min of CPR can survive 180 days with favourable neurological function in spite of the fact that the populations enrolled in the PRAGUE-OHCA and ARREST trials represent one of the sickest resuscitated populations ever evaluated or treated in modern intensive care units. All of the data presented here are consistent with Bartos et al.5 and Mork et al.15 investigations that suggest that the largest effect of an invasive strategy compared to standard ACLS is seen in patients after 40 min of CPR. It is also true that survival in the invasive approach is mostly correlated with time to cannulation and initiation of ECPR, as it has been shown by the two studies above and between 40 and 60 min, survival can reach 47% in refractory shockable rhythm presenting patients. These numbers would have sounded unimaginable 10 years ago.
Our data are also concordant with previously published analyses and retrospective cohorts assessing the effect size in different presenting rhythms.16 Consequently, it appears that refractory OHCA presenting with a shockable rhythm is the primary target population for an early invasive, ECPR based resuscitation strategy.
Furthermore, an invasive approach significantly improved overall survival and tended to positively affect cardiac recovery at 180 days. The positive effect was documented despite the intention to treat analysis, in which: (1) the standard group included 11 patients treated with ECPR based approach and of whom 5 survived with favourable neurological outcome at 180 days and represented 14% of the survivors in the standard group and (2) the invasive group included 11 patients who were denied ECPR, despite being assigned by the protocol. As such, an intention to treat analysis offers a rather conservative interpretation of the results. The presented as-treated analysis is further informative of the treatment effect when ECLS is actually used and showed that a total of ∼4 patients presenting with refractory shockable rhythm have to be treated to save an extra life. In a population that has 75% mortality with standard ACLS, that is a very large survival benefit, essentially doubling survival rate. The per protocol analysis also reinforced and corroborated the significant clinical benefit that an invasive approach offers especially in shockable rhythms.
Our data are derived from two very organised and experienced centres with well-orchestrated prehospital protocols and high-volume operators. This has created a culture where refractory OHCA patients are aggressively treated and supported in a consistent manner. Thus, replication of these results may be cumbersome in other settings. Reported outcomes require experienced teams, with commitment across disciplines, system-wide collaboration between prehospital services and hospitals and avoidance of competitive fragmentation that might limit the number of patients treated by large centres.17, 18, 19 Simply having ECLS initiation capabilities does not provide the systemic expertise to have a mature ECPR program that delivers survival rates close to 30%.16 In other words, a 30% survival rate is the lowest benchmark for invasive ECPR based programs need to target while further improvements in the systems of care delivery can be investigated. Notably, a recent multicentre randomised study has shown that in systems not organised as outlined above, with low case volumes, long procedural times to initiate ECPR, reduced ICU length of stay, extremely short ECMO support times, and premature neuroprognostication, overall survival may be markedly decreased.20 In contrary, the ARREST trial was stopped early due to benefit. Trials that have been stopped early for benefit tend to overestimate treatment effects. However, the neurologically favourable survival outcomes (in the invasive arm), are nearly identical to previously published data from the group.5,6,21,22 No other case series ever published has demonstrated similar survival rates in any community, for patients treated with ACLS alone due to OHCA refractory cardiac arrest (as defined by the ARREST or PRAGUE-OHCA trials).
Between-study heterogeneity of the effect size point estimates (risk difference) exists in the full cohort, mainly because of the inclusion of non-shockable rhythms, cross-over and a much larger number of included patients in the PRAGUE-OHCA trial. Despite that, the effect in both studies shows a similar direction and degree of benefit. The effect of including all rhythms is clearly shown as the heterogeneity disappears in the per protocol analysis in the shockable rhythms, since, as expected, the survival risk differences were similar between studies. The effect of cross over in the overall population for heterogeneity is further documented by the almost absolute homogeneity of the two studies when tested in the as-treated analysis. This is also evident in the homogeneity documented in the per protocol analysis and is especially true in the shockable rhythms populations.
We have limited our pooled analysis to ARREST and PRAGUE-OHCA trials, as our main target was to combine data from RCTs performed by high volume cardiac arrest and ECPR cardiovascular centres which use similar and well-established protocols for ECPR, with immediate percutaneous invasive strategies. For these reasons, the EROCA and INCEPTION trials were not considered for data pooling.20,23
Our results suggest that OHCA with shockable rhythms are the primary target population but studies need to identify other potential populations for this approach. Further, our studies required patients to be moved to the hospital for cannulation, a protocol process that may have compromised optimal delivery of ACLS in the field as demonstrated by PRAGUE-OHCA results. Intra-arrest transport has been previously associated with decreased OHCA survival.24 This may have adversely impacted outcomes in the ARREST trial. This strategy may also be partially responsible for the variation in survivors in the standard CPR arm between the ARREST and PRAGUE-OHCA trials. In both trials, patients were receiving ACLS based on local protocols: in the PRAGUE-OHCA trial standard therapy was primarily on-scene treatment achieving rather high ROSC rate (44%) and notably even patients in the invasive strategy arm still achieved ROSC in 27% before ECPR might have been implemented. Although our data do not address the effect of time to cannulation, future studies will need to address systematic changes that will facilitate even earlier cannulation for patients that fail to have ROSC within a certain time frame. Future studies should further evaluate the effect of prehospital invasive resuscitation and the complexity of the system changes in order to achieve those time limits to ultimately improve overall survival.
An early invasive, ECPR based resuscitation strategy, significantly improved favourable neurological survival at 180 days compared to standard ACLS for patients with refractory OHCA, absolute difference (CI) 12.7% (2.5–22.6), p = 0.015. The effect was larger in patients presenting with shockable rhythms, absolute difference (CI) 18.8% (7.6–29.4), p = 0.01 and prolonged CPR, absolute difference (CI) 16.5% (5.9–26.6), p = 0.004. Early invasive ECPR based resuscitation should be strongly considered as a first-line therapy for these patients within large, well-organised systems of care if resources are available.
Contributors
Specific author contributions are as follows. Jan Belohlavek: study design, data analysis, writing the manuscript, editing, and response to reviewer and main scientific message. Demetris Yannopoulos: study design, data analysis, writing the manuscript, editing, and response to reviewer and main scientific message. Jana Smalcova, Daniel Rob, and Jason Bartos: data analysis, writing the manuscript, editing, and critical review. Michal Huptych and Petra Kavalkova: statistical analyses, figure and tables, responses to reviewers statistical comments, primary writing of the statistical section. Rajat Kalra, Brian Grunau, and Fabio Silvio Taccone: data analysis, writing the manuscript, editing and critical review, statistical evaluation and analysis, editing manuscript for all revisions. Tom P. Aufderheide: editing manuscript and critical editing manuscript for all revisions. All authors read and approved the final version of the manuscript. Jan Belohlavek, Demetris Yannopoulos, and Michal Huptych accessed and verified the underlying data.
Data sharing statement
All reasonable requests for data sharing will be considered and should be emailed to Prof Yannopoulos at yanno001@umn.edu.
Declaration of interests
Jan Belohlavek has received research grant from the Internal Grant Agency Ministry of Health, Czech Republic (NT 13225-4/2012) for the PRAGUE OHCA study and is supported by Charles University Research program “Cooperatio – Intensive Care Medicine” and by a research grant from the Ministry of Health, Czech Republic – conceptual development of research organisation, General University Hospital in Prague, MH CZ-DRO-VFN64165. JB also served as a consultant to Abiomed, Getinge, Xenios, and Resuscitec companies. Demetris Yannopoulos has no financial conflicts of interest. Has received NIH funding for the ARREST trial and research in the field of resuscitation. Further has received a grants from Helmsley Charitable Foundation to implement ECPR in the state of MN and deliver 8500 AEDs to first responders. Jason Bartos has no financial conflicts of interest. Has received NIH funding for research in the field of resuscitation. Has received grant funding from the Helmsley Charitable Foundation to implement ECPR in the state of MN and a second grant to distribute AEDs to first responders. Has unpaid positions on the American Heart Association Emergency Care Committee, Science Subcommittee. Served as the President of the Minnesota Mobile Resuscitation Consortium. Fabio Silvio Taccone is a scientific advisor for EUROSETS. Tom P. Aufderheide has no financial conflicts of interest. Has received NIH funding for the ARREST trial and research in the field of resuscitation. He is a consultant for Medtronic, Inc., and has ongoing clinical trials with Cytovale, Inflammatix, Inc., Abbott Laboratories, and ZOLL Medical Corp. All other authors declare no competing interests.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101988.
Appendix B. Supplementary data
References
- 1.Soar J., Böttiger B.W., Carli P., et al. European resuscitation council guidelines 2021: adult advanced life support. Resuscitation. 2021;161:115–151. doi: 10.1016/j.resuscitation.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Panchal A.R., Bartos J.A., Cabañas J.G., et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(16_suppl_2):S366–S468. doi: 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 3.Empana J.-P., Lerner I., Valentin E., et al. Incidence of sudden cardiac death in the European Union. J Am Coll Cardiol. 2022;79(18):1818–1827. doi: 10.1016/j.jacc.2022.02.041. [DOI] [PubMed] [Google Scholar]
- 4.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 5.Bartos J.A., Grunau B., Carlson C., et al. Improved survival with extracorporeal cardiopulmonary resuscitation despite progressive metabolic derangement associated with prolonged resuscitation. Circulation. 2020;141(11):877–886. doi: 10.1161/CIRCULATIONAHA.119.042173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yannopoulos D., Bartos J., Raveendran G., et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020;396(10265):1807–1816. doi: 10.1016/S0140-6736(20)32338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belohlavek J., Smalcova J., Rob D., et al. Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2022;327(8):737–747. doi: 10.1001/jama.2022.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demets D.L. Methods for combining randomized clinical trials: strengths and limitations. Stat Med. 1987;6(3):341–348. doi: 10.1002/sim.4780060325. [DOI] [PubMed] [Google Scholar]
- 9.Bangdiwala S.I., Bhargava A., O'Connor D.P., et al. Statistical methodologies to pool across multiple intervention studies. Transl Behav Med. 2016;6(2):228–235. doi: 10.1007/s13142-016-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 11.Yannopoulos D., Kalra R., Kosmopoulos M., et al. Rationale and methods of the advanced R2Eperfusion STrategies for refractory cardiac arrest (ARREST) trial. Am Heart J. 2020;229:29–39. doi: 10.1016/j.ahj.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Belohlavek J., Kucera K., Jarkovsky J., et al. Hyperinvasive approach to out-of hospital cardiac arrest using mechanical chest compression device, prehospital intraarrest cooling, extracorporeal life support and early invasive assessment compared to standard of care. A randomized parallel groups comparative study proposal. “Prague OHCA study”. J Transl Med. 2012;10:163. doi: 10.1186/1479-5876-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakamoto T., Morimura N., Nagao K., et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85(6):762–768. doi: 10.1016/j.resuscitation.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Goto T., Morita S., Kitamura T., et al. Impact of extracorporeal cardiopulmonary resuscitation on outcomes of elderly patients who had out-of-hospital cardiac arrests: a single-centre retrospective analysis. BMJ Open. 2018;8(5) doi: 10.1136/bmjopen-2017-019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mørk S.R., Bøtker M.T., Christensen S., Tang M., Terkelsen C.J. Survival and neurological outcome after out-of-hospital cardiac arrest treated with and without mechanical circulatory support. Resusc Plus. 2022;10 doi: 10.1016/j.resplu.2022.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yannopoulos D., Bartos J.A., Aufderheide T.P., et al. The evolving role of the cardiac catheterization laboratory in the management of patients with out-of-hospital cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2019;139(12):e530–e552. doi: 10.1161/CIR.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 17.Bartos J.A., Carlson K., Carlson C., et al. Surviving refractory out-of-hospital ventricular fibrillation cardiac arrest: critical care and extracorporeal membrane oxygenation management. Resuscitation. 2018;132:47–55. doi: 10.1016/j.resuscitation.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Tonna J.E., Selzman C.H., Bartos J.A., et al. The association of modifiable postresuscitation management and annual case volume with survival after extracorporeal cardiopulmonary resuscitation. Crit Care Explor. 2022;4(7) doi: 10.1097/CCE.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grunau B., Singh G., Bělohlávek J., et al. A second chance for survival: clinical trial evidence, eligibility, and barriers to implementation of ECPR for out-of-hospital cardiac arrest. Can J Cardiol. 2022;39(4):381–384. doi: 10.1016/j.cjca.2022.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Suverein M.M., Delnoij T.S.R., Lorusso R., et al. Early extracorporeal CPR for refractory out-of-hospital cardiac arrest. N Engl J Med. 2023;388(4):299–309. doi: 10.1056/NEJMoa2204511. [DOI] [PubMed] [Google Scholar]
- 21.Yannopoulos D., Bartos J.A., Raveendran G., et al. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2017;70(9):1109–1117. doi: 10.1016/j.jacc.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 22.Yannopoulos D., Bartos J.A., Martin C., et al. Minnesota resuscitation consortium’s advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J Am Heart Assoc. 2016;5(6) doi: 10.1161/JAHA.116.003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu C.H., Meurer W.J., Domeier R., et al. Extracorporeal cardiopulmonary resuscitation for refractory out-of-hospital cardiac arrest (EROCA): results of a randomized feasibility trial of expedited out-of-hospital transport. Ann Emerg Med. 2021;78(1):92–101. doi: 10.1016/j.annemergmed.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grunau B., Kime N., Leroux B., et al. Association of intra-arrest transport vs continued on-scene resuscitation with survival to hospital discharge among patients with out-of-hospital cardiac arrest. JAMA. 2020;324(11):1058–1067. doi: 10.1001/jama.2020.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.