Abstract
Cipaglucosidase alfa (Pombiliti™) is a recombinant human acid α-glucosidase (GAA) product being developed by Amicus Therapeutics along with the enzyme stabilizer miglustat as a two-component therapy for Pompe disease. Pompe disease is a rare, inherited lysosomal disease caused by a deficiency of the enzyme GAA, which leads to accumulation of glycogen in various tissues. On 27 March 2023, cipaglucosidase alfa was approved in the EU as a long-term enzyme replacement therapy (ERT) used in combination with miglustat for the treatment of adults with late-onset Pompe disease. This article summarizes the milestones in the development of cipaglucosidase alfa leading to this first approval.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-023-01886-5.
| Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.22724324. |
Cipaglucosidase alfa (Pombiliti™): Key Points
| A long-term ERT being developed by Amicus Therapeutics for the treatment of Pompe disease |
| Received its first approval on 27 March 2023 in the EU |
| Approved for use in combination with miglustat for the treatment of adults with late-onset Pompe disease |
Introduction
Pompe disease, also known as acid α-glucosidase (GAA) deficiency or glycogen storage disease type II, is a rare disorder caused by mutations in the gene that encodes GAA [1], the enzyme responsible for breaking down lysosomal glycogen into glucose [2]. A shortage of GAA results in the accumulation of glycogen in various tissues, particularly in skeletal muscle, cardiac muscle, and smooth muscle [1]. Pompe disease is classified into infantile- and late-onset subtypes according to age at symptom onset. The classic infantile form of Pompe disease is characterized by a severe, progressive and rapidly fatal course [1]. Late-onset Pompe disease is characterized by progressive weakness in the axial, limb-girdle, and respiratory muscles, resulting in motor and respiratory problems [3]. The first approved treatment for Pompe disease, the enzyme replacement therapy (ERT) alglucosidase alfa, has been shown to slow disease progression and improve quality of life (QOL) [4]. However, there is a need for more efficient treatments that are able to reach skeletal muscle [4].
Cipaglucosidase alfa (Pombiliti™) is long-term ERT being developed by Amicus Therapeutics for the treatment of Pompe disease. On 27 March 2023, cipaglucosidase alfa received its first approval in the EU for use in combination with miglustat for the treatment of adults with late-onset Pompe disease [5]. The recommended dosage of cipaglucosidase alfa is 20 mg/kg every 2 weeks [2]. Cipaglucosidase alfa is administered as an intravenous infusion, which should be started 1 h after taking oral miglustat supplied as 65 mg capsules. If the cipaglucosidase alfa infusion is delayed, it must be started within 3 h of miglustat administration. The recommended initial infusion rate of cipaglucosidase alfa is 1 mg/kg/h, which can be gradually increased by 2 mg/kg/h every ≈ 30 min if there are no signs of infusion-associated reactions (IARs). The maximum infusion rate is 7 mg/kg/h and the typical infusion duration is 4 h. Treatment should be supervised by a healthcare professional with experience in managing patients with Pompe disease or other inherited metabolic or neuromuscular diseases. Home infusion of cipaglucosidase alfa may be considered for patients who tolerate infusions well and have no recent history of moderate or severe IARs [2]. Cipaglucosidase alfa is under regulatory review in the USA for the treatment of late-onset Pompe disease and is currently in phase III clinical development for this indication in multiple other countries worldwide.
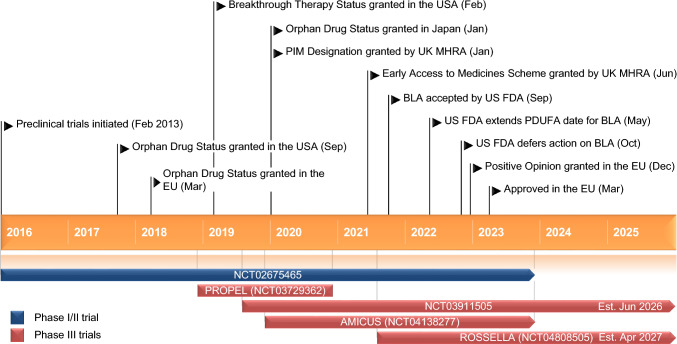
Key milestones in the development of cipaglucosidase alfa (co-administered with miglustat) for the treatment of Pompe disease. BLA Biologic License Application, FDA Food and Drug Administration, MHRA Medicines and Healthcare Products Regulatory Agency, PDUFA Prescription Drug User Fee Act, PIM Promising Innovative Medicine
Company Agreements
Amicus Therapeutics is using differentiated peptide tagging technology acquired from Callidus Biopharma to engineer its next-generation ERT compounds, including cipaglucosidase alfa. Amicus Therapeutics acquired cipaglucosidase alfa as part of its acquisition of Callidus Biopharma in November 2013 [6].
In February 2019, WuXi Biologics and Amicus Therapeutics announced an exclusive commercial manufacturing partnership for cipaglucosidase alfa [7]. Under the terms of the agreement, WuXi Biologics will be the exclusive commercial drug substance manufacturing partner and key commercial drug product supplier. WuXi Biologics will manufacture both the drug substance and drug product at sites across its global commercial supply network in the EU, the USA, and China. The contract, which has an initial 5-year term, will be automatically renewed every 2 years until cancellation [7].
Patent Information
In February 2019, Amicus Therapeutics was issued the first of two US patents for cipaglucosidase alfa [8]. This patent, which is set to expire in 2035, reflects composition of matter for highly potent recombinant human GAA with enhanced carbohydrates. The second patent, issued in March 2019, covers the methods of manufacturing cipaglucosidase alfa, with protection extending through 2037. Amicus Therapeutics is pursuing corresponding patent applications in other regions, including Europe and Japan [8].
Scientific Summary
Pharmacodynamics
Cipaglucosidase alfa is a novel recombinant human GAA enriched with bis-phosphorylated (bis-M6P) N-glycans for high affinity cation-independent mannose-6-phosphate receptor (CI-MPR) binding [2]. It is coadministered with miglustat, which acts as a stabilizer and prevents loss of enzyme activity during cipaglucosidase alfa infusion. After CI-MPR binding, cipaglucosidase alfa is internalized in the lysosome and is then processed (via proteolytic cleavage and N-glycan trimming) into the most mature and active form of the GAA enzyme. The enzymatic activity of cipaglucosidase alfa is then exerted through cleaving glycogen, reducing intramuscular glycogen and repairing tissue damage [2].
Cipaglucosidase alfa plus miglustat reduces biomarkers of muscle damage (creatine kinase) and glycogen accumulation (hexose tetrasaccharide; Hex4) in patients with late-onset Pompe disease. Long-term (48 months) results from a phase I/II trial (NCT02675465) demonstrated that cipaglucosidase alfa plus miglustat was associated with reductions from baseline in levels of serum creatine kinase and urine Hex4; reductions were seen in all cohorts, but were most notable among ERT-naïve patients [9].
Pharmacokinetics
In ambulatory ERT-experienced patients with late-onset Pompe disease, maximum plasma concentrations of cipaglucosidase alfa (20 mg/kg) and miglustat (260 mg) were reached by approximately the end of the 4-h infusion and declined in a biphasic manner to negligible levels 24 h after the start of infusion [2, 10]. Compared with administration of cipaglucosidase alfa alone, coadministration with miglustat increased the plasma total GAA protein area under the concentration-time curve of cipaglucosidase alfa by ≈ 35% [10].
The volume of distribution of cipaglucosidase alfa ranged from 2.0 to 4.7 L [2]. Cipaglucosidase alfa does not cross the blood-brain barrier and is not expected to bind to plasma proteins. Coadministration of cipaglucosidase alfa and miglustat was associated with a 48% increase in the distribution half-life of cipaglucosidase alfa and a 27% reduction in plasma clearance. Cipaglucosidase alfa is mainly eliminated in the liver by proteolytic hydrolysis. The mean terminal half-life of cipaglucosidase alfa ranged from 1.6 to 2.6 h [2].
Age (18–74 years), sex and race/ethnicity did not affect the pharmacokinetics of cipaglucosidase alfa to a clinically meaningful extent when coadministered with miglustat [2]. Cipaglucosidase alfa plus miglustat has not been studied in patients with kidney dysfunction or hepatic impairment. However, kidney dysfunction is not expected to have an impact on the pharmacokinetics of cipaglucosidase alfa. No drug interaction studies have been conducted with cipaglucosidase alfa alone or in combination with miglustat. However, as a recombinant human protein, cipaglucosidase alfa is not expected to interact with other medicinal products metabolized by cytochrome P450 enzymes or P-glycoprotein [2].
Features and properties of cipaglucosidase alfa
| Alternative names | ATB200; Pombiliti™; recombinant human acid α-glucosidase enzyme-Amicus; rhGAA-Amicus |
| Class | Alpha-glucosidases; enzymes |
| Mechanism of action | Alpha glucosidase replacements |
| Route of administration | Intravenous infusion |
| Pharmacodynamics | Binds to cation-independent mannose-6-phosphate receptor; undergoes rhGAA processing (proteolytic cleavage and N-glycan trimming) into mature and active form of GAA |
| Enzymatic activity: cleaves glycogen, reduces intramuscular glycogen and repairs tissue damage | |
| Reduces biomarkers of muscle damage (CK) and glycogen accumulation (Hex4) in patients with LOPD | |
| Pharmacokinetics | Cmax is reached after ≈ 4 h and declines to negligible levels over 24 h; volume of distribution 2.0–4.7 L; mean terminal t½ 1.6–2.6 h |
| Coadministration with miglustat increases plasma total GAA protein AUC by ≈ 35%, increases distribution t½ by 48% and reduces plasma clearance by 27% | |
| Most frequent adverse events | Chills, dizziness, flushing, somnolence, chest discomfort, cough, infusion site swelling, pain |
| ATC codes | |
| WHO ATC code | A16A-B23 (cipaglucosidase alfa) |
| EphMRA ATC code | A16 (other alimentary tract and metabolism products) |
AUC area under the concentration-time curve, CK creatine kinase, Cmax maximum plasma concentration, (rh)GAA (recombinant human) acid α-glucosidase, Hex4 hexose tetrasaccharide, LOPD late-onset Pompe disease, t½ half-life
Therapeutic Trials
Phase III
Cipaglucosidase alfa plus miglustat did not achieve statistical superiority over alglucosidase alfa plus placebo for improving 6-min walk distance (6MWD) in patients with late-onset Pompe disease participating in the randomized, double-blind, multicentre, phase III PROPEL trial (NCT03729362) [3]. However, cipaglucosidase alfa plus miglustat did provide potentially clinically meaningful improvements in motor and respiratory function compared with alglucosidase alfa plus placebo. Key eligibility criteria were age ≥ 18 years, diagnosis of late-onset Pompe disease based on documented GAA enzyme deficiency or GAA genotyping, body weight ≥ 40 kg and sitting forced vital capacity (FVC) ≥ 30% of the predicted value for healthy adults. Patients were also required to have performed two valid 6-min walk tests, both with screening values of ≥ 75 m and ≤ 90% of the predicted value for healthy adults. They had either received alglucosidase alfa 20 mg/kg once every 2 weeks for ≥ 2 years (ERT-experienced; n = 95) or were ERT-naïve (n = 28). Patients were stratified by 6MWD at baseline (75 to < 150 m, 150 to < 400 m or ≥ 400 m) and previous ERT status (ERT-naïve or ERT-experienced) and randomized to receive intravenous cipaglucosidase alfa 20 mg/kg plus oral miglustat (n = 85) or intravenous alglucosidase alfa 20 mg/kg plus placebo (n = 40) once every 2 weeks for 52 weeks. Miglustat (195 mg in patients weighing 40 to < 50 kg and 260 mg in patients weighing ≥ 50 kg) and placebo were administered ≈ 1 h prior to infusion of cipaglucosidase alfa or alglucosidase alfa, respectively [3].
Cipaglucosidase alfa plus miglustat was not superior to alglucosidase alfa plus placebo in terms of the mean change from baseline to week 52 in 6MWD (20.8 vs 7.2 m; between-group difference 13.6 m; primary endpoint) [3]. At week 52, cipaglucosidase alfa plus miglustat was associated with a clinically meaningful improvement in sitting FVC (% predicted) relative to alglucosidase alfa plus placebo (mean change from baseline –0.9 vs –4.0%; nominal p = 0.023). The other key secondary endpoints numerically favoured cipaglucosidase alfa plus miglustat over alglucosidase alfa plus placebo, including change from baseline to week 52 in the lower manual muscle test score (1.6 vs 0.9), Patient-Reported Outcomes Measurement Information System (PROMIS) physical function (1.9 vs 0.2) and fatigue (– 2.0 vs – 1.7) scores and the Gait, Stairs, Gower’s manoeuvre, Chair total score (– 0.5 vs 0.8; nominal p < 0.05) [3].
Prespecified and post hoc subgroup analyses demonstrated that in the overall study population, including ERT-naïve and ERT-experienced patients, cipaglucosidase alfa plus miglustat was associated with positive trends or clinically meaningful improvements in motor and respiratory function compared with alglucosidase alfa plus placebo, regardless of baseline 6MWD and FVC [11]. The change from baseline in 6MWD was significantly associated with improvements in QOL and other patient-reported outcomes, including PROMIS physical function and fatigue scores and the Rasch-built Pompe-specific Activity (R-PAct) score [12].
Long-term treatment with cipaglucosidase alfa plus miglustat for up to 104 weeks was associated with a durable effect in patients with late-onset Pompe disease participating in an ongoing, open-label extension (OLE) of the PROPEL trial (NCT04138277) [13]. The OLE enrolled 117 patients who completed PROPEL and two patients who had been enrolled but did not complete PROPEL. Patients either continued to receive cipaglucosidase alfa plus miglustat (n = 81) or switched from alglucosidase alfa plus placebo to cipaglucosidase alfa plus miglustat (n = 37). One patient did not receive any study treatment in the OLE; therefore, the OLE safety population included 118 patients. Patients treated with cipaglucosidase alfa plus miglustat throughout demonstrated durable improvements in 6MWD that were maintained throughout the OLE, while 6MWD was stable throughout the OLE in patients who switched from alglucosidase alfa plus placebo to cipaglucosidase alfa plus miglustat. In terms of respiratory function, ERT-experienced patients who received alglucosidase alfa plus placebo in PROPEL experienced a decline in sitting FVC that stabilized after switching to cipaglucosidase alfa plus miglustat in the OLE. ERT-naïve patients in both treatment groups experienced some decline in FVC during PROPEL that stabilized during the OLE [13].
Phase I/II
Cipaglucosidase alfa plus miglustat was associated with durable improvements in motor and respiratory function in patients with late-onset Pompe disease participating in an ongoing, open-label, multicentre, phase I/II trial (NCT02675465) [9]. The study enrolled four cohorts of patients at staggered time points. Cohort 1 included ERT-experienced patients with 2–6 years of prior ERT (n = 11) who received single doses of cipaglucosidase alfa (5 mg/kg → 10 mg/kg → 20 mg/kg) for 6 weeks (stage 1) followed by cipaglucosidase alfa 20 mg/kg plus miglustat (130 mg → 260 mg) every 2 weeks for 12 weeks (stage 2). Cohort 2 included non-ambulatory ERT-experienced patients with ≥ 2 years of prior ERT (n = 6), cohort 3 included ERT-naïve patients (n = 6) and cohort 4 included ERT-experienced patients with ≥ 7 years of prior ERT (n = 6). Patients in cohorts 1–3 received cipaglucosidase alfa 20 mg/kg plus miglustat 260 mg every 2 weeks for 2 years (stage 3) [9].
Ambulatory ERT-experienced patients treated with cipaglucosidase alfa plus miglustat had durable improvements in motor function that were sustained for up to 48 months, with stable respiratory function [9]. In the pooled ERT-experienced cohorts, the mean change from baseline in 6MWD after 6, 12, 24, 36 and 48 months of follow-up was 23.1, 33.5, 25.2, 9.8 and 20.7 m, respectively. Corresponding changes in FVC were − 0.9, − 1.2, 1.0, − 0.3 and 1.0%, respectively. Patients in the ERT-naïve cohort demonstrated durable improvements in motor and respiratory function that were sustained for up to 48 months. In this cohort, the mean change from baseline in 6MWD after 6, 12, 24, 36 and 48 months of follow-up was 36.7, 57.0, 54.4, 43.5 and 52.2 m, respectively. Corresponding changes in FVC were 4.2, 3.2, 4.7, 6.2 and 8.3%, respectively [9].
Non-ambulatory ERT-experienced patients treated with cipaglucosidase alfa plus miglustat had improvements in motor function, respiratory function and patient-reported outcomes [14]. After 6 and 12 months, cipaglucosidase alfa plus miglustat was associated with increased upper extremity muscle strength, as well as improvements from baseline on the R-PAct, the Rotterdam Handicap Scale and the Fatigue Severity Scale. All patients reported improvement in overall physical well-being [14].
Key clinical trials of cipaglucosidase alfa (Amicus Therapeutics)
| Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
| Cipaglucosidase alfa, miglustat | LOPD | I/II | Active, no longer recruiting | Multinational | NCT02675465; EudraCT2015-004798-34 |
| Cipaglucosidase alfa, miglustat | LOPD | III | Completed | Multinational | PROPEL; NCT03729362; EudraCT2018-000755-40; JapicCTI194887 |
| Cipaglucosidase alfa, miglustat | LOPD | III | Active, no longer recruiting | Multinational | AMICUS; NCT04138277; EudraCT2019-000954-67 |
| Cipaglucosidase alfa, miglustat | IOPD | III | Recruiting | USA | ROSSELLA; NCT04808505 |
| Cipaglucosidase alfa, miglustat | LOPD | III | Recruiting | Multinational | NCT03911505 |
| Cipaglucosidase alfa | LOPD | IV | Discontinued | Multinational | STRIDE; EudraCT2017-004370-34 |
| Cipaglucosidase alfa | LOPD | EAP | Recruiting | Unknown | NCT03865836 |
| Cipaglucosidase alfa, miglustat | IOPD | EAP | Recruiting | Italy, Taiwan, USA | NCT04327973 |
EAP expanded access programme, IOPD infantile-onset Pompe disease, LOPD late-onset Pompe disease
Adverse Events
Cipaglucosidase alfa plus miglustat was generally well tolerated in patients with late-onset Pompe disease, according to pooled data from three clinical trials [2]. The most frequent adverse events (AEs) only attributable to cipaglucosidase alfa in the pooled safety population were chills (4%), dizziness (3%), flushing (2%), somnolence (2%), chest discomfort (1%), cough (1%), infusion site swelling (1%) and pain (1%). Serious AEs only attributable to cipaglucosidase alfa were urticaria (2%), anaphylaxis (1%), pyrexia (1%), presyncope (%), dyspnoea (1%), pharyngeal oedema (1%), wheezing (1%) and hypotension (1%) [2].
In the phase III PROPEL trial (NCT03729362), the safety profile of cipaglucosidase alfa plus miglustat was similar to that of alglucosidase alfa plus placebo [3]. Treatment-emergent AEs (TEAEs) potentially related to treatment occurred in 31% of patients in the cipaglucosidase alfa plus miglustat group and 37% of patients in the alglucosidase alfa plus placebo group. The most frequent (incidence ≥ 15% with cipaglucosidase alfa plus miglustat) TEAEs were fall (29 vs 39%), headache (24 vs 24%), nasopharyngitis (22 vs 8%), myalgia (16 vs 13%) and arthralgia (15 vs 13%). Serious TEAEs occurred in 9% of cipaglucosidase alfa plus miglustat recipients and 3% of alglucosidase alfa plus placebo recipients; one event (anaphylaxis in the cipaglucosidase alfa plus miglustat group) was potentially related to treatment. TEAEs led to treatment discontinuation in three cipaglucosidase alfa plus miglustat recipients (severe chills, severe anaphylaxis, moderate COVID-19-related pneumonia) and one alglucosidase alfa plus placebo recipient (stroke; unrelated to treatment). The incidence of IARs was similar both treatment groups (25% with cipaglucosidase alfa plus miglustat and 26% with alglucosidase alfa plus placebo) [3]. Most IARs were transient and of mild or moderate severity [2].
No new safety signals were identified during the OLE of the PROPEL trial (NCT04138277) [13]. The safety profile in patients who switched from alglucosidase alfa plus placebo to cipaglucosidase alfa plus miglustat was similar to that in patients who continued cipaglucosidase alfa plus miglustat from the start of PROPEL. The majority of TEAEs reported during the OLE were mild or moderate in severity [13].
There have been reports of immune complex-related reactions (including severe cutaneous reactions and nephrotic syndrome) with other ERTs in patients with high immunoglobulin G titres [2]. A potential class effect cannot be excluded. Therefore, patients should be monitored for clinical signs and symptoms of systemic immune complex-related reactions during treatment with cipaglucosidase alfa plus miglustat [2].
As with all therapeutic proteins, there is potential for immunogenicity with cipaglucosidase alfa. In the PROPEL trial, the proportion of ERT-naïve patients treated with cipaglucosidase alfa plus miglustat with detectable anti-drug antibody (ADA) titres increased from 0% at baseline to 88% at the final visit, while the proportion of ERT-experienced patients with detectable ADA titres remained stable (83% at baseline and 74% at the final visit) [15]. The incidence of enzyme-activity neutralizing antibodies was low. The presence of ADAs had no impact on the efficacy (6MWD and FVC), pharmacodynamics (CK and Hex4), pharmacokinetics, or safety of cipaglucosidase alfa plus miglustat [15].
Ongoing Clinical Trials
In addition to the ongoing OLE (NCT04138277) and phase I/II trial (NCT02675465) discussed in Sect. 2.3, recruitment is underway for two open-label, multicentre, phase III trials assessing the safety, efficacy, pharmacokinetics, pharmacodynamics, and immunogenicity of cipaglucosidase alfa plus miglustat in ERT-experienced and ERT-naïve paediatric patients (aged 0 to < 18 years) with infantile-onset Pompe disease (NCT04808505; ROSSELLA) or late-onset Pompe disease (NCT03911505). Two expanded access programmes are also ongoing in patients with Pompe disease (NCT03865836 and NCT04327973).
Current Status
Cipaglucosidase alfa received its first approval on 27 March 2023 as a long-term ERT used in combination with miglustat for the treatment of adults with late-onset Pompe disease in the EU [5].
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Hannah Blair is a salaried employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Footnotes
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
References
- 1.National Organization for Rare Disorders (NORD). Pompe disease. 2021. https://rarediseases.org/rare-diseases/pompe-disease/. Accessed 1 May 2023.
- 2.Amicus Therapeutics Europe Limited. Pombiliti (cipaglucosidase alfa) 105 mg powder for concentrate for solution for infusion: EU summary of product characteristics. 2023. https://ec.europa.eu/health/documents/community-register/2023/20230320158375/anx_158375_en.pdf. Accessed 1 May 2023.
- 3.Schoser B, Roberts M, Byrne BJ, et al. Safety and efficacy of cipaglucosidase alfa plus miglustat versus alglucosidase alfa plus placebo in late-onset Pompe disease (PROPEL): an international, randomised, double-blind, parallel-group, phase 3 trial. Lancet Neurol. 2021;20(12):1027–1037. doi: 10.1016/S1474-4422(21)00331-8. [DOI] [PubMed] [Google Scholar]
- 4.Meena NK, Raben N. Pompe disease: new developments in an old lysosomal storage disorder. Biomolecules. 2020;10(9):1339. doi: 10.3390/biom10091339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amicus Therapeutics. Amicus Therapeutics announces European Commission approval for Pombiliti™ in patients with late-onset Pompe disease [media release]. 27 Mar 2023. https://ir.amicusrx.com/news-releases/news-release-details/amicus-therapeutics-announces-european-commission-approval-1.
- 6.Amicus Therapeutics. Amicus Therapeutics strengthens biologics business strategy [media release]. 20 Nov 2013. http://www.amicusrx.com.
- 7.WuXi Biologics. WuXi Biologics and Amicus sign exclusive manufacturing partnership [media release]. 11 Feb 2019. http://www.wuxibiologics.com.
- 8.Amicus Therapeutics. Amicus Therapeutics issued two U.S. patents for Pompe enzyme ATB200 [media release]. 20 Mar 2019. http://www.amicusrx.com.
- 9.Byrne B, Schoser B, Kishnani P, et al. Long-term follow-up of cipaglucosidase alfa/miglustat in ambulatory patients with Pompe disease: an open-label phase I/II study (ATB200-02) [abstract no. 59 plus poster] Mol Genet Metab. 2023;138(2):21–22. doi: 10.1016/j.ymgme.2022.107042. [DOI] [Google Scholar]
- 10.Johnson FK, Kang J, Mondick J, et al. Mechanism of action, pharmacokinetic profiles, and pharmacokinetic/pharmacodynamic relationships differ between cipaglucosidase alfa/miglustat and alglucosidase alfa in late-onset Pompe disease [abstract no. 166 plus poster]. In: American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) Annual Meeting. 2022.
- 11.Byrne B, Bratkovic D, Díaz-Manera J, et al. Cipaglucosidase alfa/miglustat versus alglucosidase alfa/placebo in late-onset Pompe disease (LOPD): PROPEL study subgroup analyses [abstract no. 39 plus poster] Mol Genet Metab. 2022;135(2):S27–S28. doi: 10.1016/j.ymgme.2021.11.054. [DOI] [Google Scholar]
- 12.Raza S, Keyzor I, Shohet S, et al. Association of walking distance with quality of life and other patient-reported outcomes in Pompe disease [abstract no. PCR67] Value Health. 2022;25(7 Suppl):S553. doi: 10.1016/j.jval.2022.04.1412. [DOI] [Google Scholar]
- 13.Schoser B, Bratkovic D, Byrne B, et al. Long-term efficacy and safety of cipaglucosidase alfa/miglustat in ambulatory patients with Pompe disease: a phase III open-label extension study (ATB200-07) [poster no. LB-59 plus oral presentation]. In: 19th Annual WORLDSymposiumTM. 2023.
- 14.Clemens PR, Mozaffar T, Schoser B, et al. Safety and efficacy of advanced and targeted acid α-glucosidase (AT-GAA) (ATB200/AT2221) in ERT-switch nonambulatory patients with Pompe disease: preliminary results from the ATB200-02 trial [abstract no. 68] Mol Genet Metab. 2019;126:S40–S41. doi: 10.1016/j.ymgme.2018.12.084. [DOI] [Google Scholar]
- 15.Benjamin E, Schoser B, Kishnani P, et al. Immunogenicity of cipaglucosidase alfa/miglustat versus alglucosidase alfa/placebo in late-onset Pompe disease: a phase III, randomized study (PROPEL) [abstract no. 185 plus poster]. In: American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) Annual Meeting. 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.