Abstract
The details of immune molecules' expression in desmoid tumors (DTs) remain unclear. This study aimed to determine the expression status of the programmed death-1/programmed death ligand 1 (PD1/PD-L1) immune checkpoint mechanism in DTs. The study included patients with DTs (n=9) treated at our institution between April 2006 and December 2012. Immunostaining for CD4, CD8, PD-1, PD-L1, interleukin-2 (IL-2), and interferon- gamma (IFN-γ) was performed on pathological specimens harvested during the biopsy. The positivity rate of each immune component was calculated as the number of positive cells/total cells. The positivity rate was quantified and correlations between the positivity rates of each immune molecule were also investigated. Immune molecules other than PD-1 were stained in tumor cells and intra-tumor infiltrating lymphocytes. The mean ± SD expression rates of β-catenin, CD4, CD8, PD-1, PD-L1, IL-2, and IFN-g were 43.9±18.9, 14.6±6.80, 0.75±4.70, 0±0, 5.1±6.73, 8.75±6.38, and 7.03±12.1, respectively. The correlation between β-catenin and CD4 was positively moderate (r=0.49); β-catenin and PD-L1, positively weak (r=0.25); CD4 and PD-L1, positively medium (r=0.36); CD8 and IL-2, positively medium (r=0.38); CD8 and IFN-g, positively weak (r=0.28); and IL-2 and IFN-g, positively medium (r=0.36). Our findings suggest that PD-L1-centered immune checkpoint mechanisms may be involved in the tumor microenvironment of DTs.
Key words: β-catenin, myofibroblasts, mutation, melanoma, cytokines
Introduction
Desmoid tumors (DTs), also known as invasive fibromas, are monoclonal neoplasms of myofibroblasts originating from muscle, tendon, and neural stromata.1 Morphologically, the tumor comprises small elongated spindle-shaped cells without characteristic cytoplasmic borders.2 According to the latest World Health Organization (WHO) classification of soft tissue and bone tumors, a DT is an intermediate-grade neoplasm characterized by an invasive growth pattern into surrounding normal structures and, rarely, metastasis.3,4 DTs are relatively rare, accounting for less than 3% of soft tissue tumors.3 The invasive nature of DTs is evident from the propensity of the tumor cells to invade and engulf surrounding normal structures. Thus, DTs pose an imminent danger when they grow adjacent to major blood vessels and other vital organs.5,6 The pathogenesis of DTs remains largely elusive. Nonetheless, 85% of DTs have somatic mutations in the β-catenin gene CTNNB1.7 Furthermore, DTs have been shown to occur as part of inherited diseases, such as familial adenomatous polyposis (FAP) syndrome.8,9 The CTNNB1 and APC genes are part of the Wnt signaling pathway. Mutations in these genes upregulate β-catenin expression. Consequently, β-catenin accumulates in the nucleus and activates transcription factors in the Wnt pathway.10 Depending on the location of the lesion and the patient’s general condition, various treatment options exist, ranging from surgery to chemotherapy and radiation therapy.11 However, in many cases tumors recur more aggressively after treatment. Thus, the most desirable option for a stable asymptomatic disease is careful monitoring of the patient, also known as the “wait and see policy.”12 Thus, DT treatment remains challenging, and its potentially invasive nature necessitates new treatment strategies. The programmed death-1/programmed death ligand 1 (PD1/PD-L1) immune checkpoint mechanism has recently been shown to be involved in the pathogenesis of various malignancies.13,14 Anti-PD-1/PD-L1 drugs are approved for treating some malignancies, including melanoma and kidney cancer.15,16 Recently, the involvement of PD-1/PD-L1 in aggressive soft tissue sarcomas has been reported.17 However, the literature on the involvement of PD-1/PD-L1 immune checkpoint mechanisms in DTs is scarce and controversial.5,18 Therefore, this study aimed to determine PD-1/PD-L1 expression, including inflammatory cytokines, in DTs.
Materials and Methods
This study included patients with DTs (n=9) treated at our institution between April 2006 and December 2012. Immunostaining for CD4, CD8, PD-1, PD-L1, IL-2, and IFN-γ was performed on pathological specimens harvested during the biopsy. Immunostaining was performed as previously described.17 Tissue sections were formalin-fixed and paraffin-embedded. Sections of 4 μm thickness were cut and mounted onto slides. The tissues were deparaffinized and rehydrated, and endogenous peroxidase activity was inhibited using 3% hydrogen peroxide. The sections were pre-treated using heat-mediated antigen retrieval with sodium citrate buffer as per the manufacturers’ protocol.17,19 PD-1 was heat-treated (95°C, 5 min) in a pH 6 environment, and the other antigens were heat-treated (95°C, 5 min) in a pH 9 environment. The tissue sections were incubated with the following primary antibodies: CD4 antibody (rabbit monoclonal, SP35/Roche Diagnostics, Risch-Rotkreuz, Switzerland) for 32 min at 37°C after 60 min high-pH (9.0) heat activation; CD8 antibody (rabbit monoclonal, C8/144B; Nichirei Corporation, Tokyo, Japan) for 32 min at 37°C after 60 min high-pH (9.0) heat activation; PD-1 (mouse monoclonal, ab52587; NAT105/Abcam, Cambridge, UK) for 30 min at 37°C after 30 min low-pH (6.0) heat activation; PD-L1 antibody (rabbit monoclonal, ab205921; 28-8/Abcam, Cambridge, UK) for 32 min at 37°C after 60 min high-pH (9.0) heat activation; IL-2 antibody (rabbit monoclonal, ab92381; Abcam) for 32 min at 37°C after 60 min high-pH (9.0) heat activation; and IFN-γ antibody (rabbit polyclonal, ab9657; Abcam) for 32 min at 37°C after 60 min high-pH (9.0) heat activation. Sections were incubated with the corresponding secondary antibodies for 30 min at 37°C. The reaction was visualized using 3,3-diaminobenzidine (DAB) (DAB Substrate Chromogen System; DAKO, Kyoto, Japan) and counterstained with hematoxylin. Tissues from the tonsils were used as positive controls. As negative controls, immunostaining was performed without the primary antibody. The omission of the primary antibody is a usual negative control for the immunohistochemical assays. Slides were observed under a microscope (BIOREVO BZ-9000; Keyence, Osaka, Japan), and brown granules in the cytoplasm, nuclei, or membrane indicated positive staining. The immune marker staining within the tumor was quantified in 4 high-power representative (40x magnification).20 The positivity rate of each immune component was calculated as the number of positive cells/total cell number. The positivity rate was quantified using the software provided with BIOREVO-BZ 9000 (Keyence, Osaka, Japan). In addition, correlations between the positivity rates of each immune molecule were investigated.
The study complied with the ethical guidelines of the Declaration of Helsinki (2013) and was approved by the Kindai University Ethics Committee (approval number: 31-187; approved on 16 January 2020). Comprehensive consent was obtained from the patients.
Statistical analyses
The positivity rate of each molecule was plotted, and a correlation diagram was drawn. The coefficient of determination (R) was calculated by drawing an approximation line to examine the correlation between the positive cell rates of the molecules. Values of positive cell rates are expressed as mean ± SD. Pearson's correlation method was used to confirm the correlations between positive cell rates of each immune component. Correlations were defined as follows: very strong, 1.0≧|R|≧0.7; strong, 0.7≧|R|≧0.5; moderate, 0.5≧|R|≧0.4; medium, 0.4≧|R|≧0.3; weak, 0.3≧|R|≧0.2; and no correlation, 0.2≧|R|≧0.0. The Kaplan–Meier method was used to evaluate the 3-year survival rates. Analyses were performed using Stat Mate 5.05 (ATMS, Tokyo, Japan).
Table 1.
Characteristics of the study population.
| Age (years) | |
| Median | 37 |
| Range | 11-84 |
| Factor | Number of patients |
|---|---|
| Sex | |
| Male | 4 |
| Female | 5 |
| Tumor site | |
| Arms | 3 |
| Trunk | 6 |
| Tumor size (cm) | |
| <5 | 5 |
| 5-10 | 3 |
| >10 | 1 |
| Treatment | |
| Wide resection | 7 |
| Marginal resection | 2 |
Results
Patient characteristics
The clinical characteristics of the patients enrolled in this study are summarized in Table 1. Four male and five female patients were enrolled in the present study. The mean age was 37.0 (range, 11-84) years. The mean longest diameter of the tumor in a 2-dimensional plane was 6.08 cm (range, 1.0-22.3 cm). Three tumors occurred in the extremities and six in the trunk.
Immunohistochemical expression
β-catenin showed staining mainly in the cytoplasm of tumor cells (Figure 1A). CD4 (Figure 1B) and CD8 (Figure 1C) showed staining mainly in tumor-infiltrating lymphocytes. PD-1 was not stained in all cases (data not shown), and PD-L1 showed staining mainly in DT cells (Figure 1D). IL-2 and IFN-g were found to be stained in tumor cells and lymphocytes (Figure 1 E,F). The mean expression rates for β-catenin, CD4, CD8, PD-1, PD-L1, IL-2, and IFN-g were 43.9±18.9, 14.6±6.80, 0.75±4.70, 0±0, 5.1±6.73, 8.75±6.38, and 7.03±12.1, respectively (Table 2).
Figure 1.
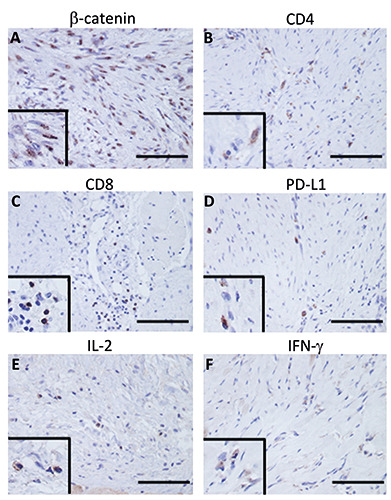
Representative immunostaining for each immune molecule. β-catenin showed staining primarily in the cytoplasm of the tumor cells (A). CD4 (B) and CD8 (C) showed staining primarily in tumor-infiltrating lymphocytes. PD-L1 showed staining mainly in DT cells (D). IL-2 (E) and IFN-g (F) were found to be stained in tumor cells and lymphocytes; scale bar: 100 μm. The wipe screens show a higher magnification field.
Correlation between immune molecules
The correlation between β-catenin and CD4 was positively moderate (r=0.49, Figure 2A); β-catenin and CD8, no correlation (R=0.06, data not shown); β-catenin and PD-L1, positively weak (R=0.25, Figure 2B); CD4 and PD-L1, positively medium (R=0.36, Figure 2C); CD4 and IL-2, no correlation (R=0.07, data not shown); CD8 and IL-2, positively medium (R=0.38, Figure 2D); CD8 and IFN-g, positively weak (R=0.28, Figure 2E); IFN-γ and PD-L1, no correlation (R=0.05, data not shown); and IL-2 and IFN-g, positively medium (R=0.36, Figure 2F).
Discussion
Since the immune mechanisms within DTs remain unclear, the current study used immunostaining to confirm PD-1/PD-L1 involvement in DTs. We identified intratumor expression of PD-L1 and other immune molecules in DTs.
In recent years, significant advances in the characterization of the tumor microenvironment of soft-tissue sarcomas have described “hot tumors” with massive infiltration of immune cells and “cold tumors” with no significant immune infiltration.1 In addition, Petitprez et al. established an immune-based classification based on the composition of the tumor microenvironment and identified five distinct phenotypes: hypoimmune (A and B), hyperimmune (D and E), and hypervascular (C).21 Based on the results of this study, DTs are hyperimmune tumors.
Table 2.
Positive cell rates of immune molecules.
| Immune molecule | β-catenin | CD4 | CD8 | PD-1 | PD-L1 | IL-2 | IFN-g |
|---|---|---|---|---|---|---|---|
| Positive cell rate (mean ± SD) | 43.9±18.9 | 14.6±6.80 | 0.75±4.70 | 0±0 | 5.1±6.73 | 8.75±6.38 | 7.03±12.1 |
PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1; IL-2, interleukin-2; IFN-g, interferon gamma.
Figure 2.
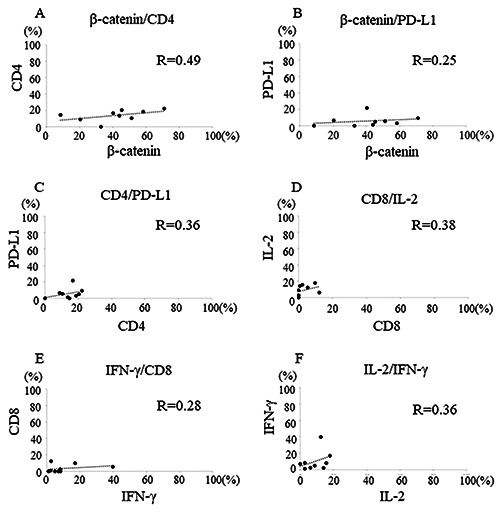
Graphs show correlations between immune molecules. The correlation between β-catenin and CD4 was positively moderate (R=0.49, A); β-catenin and PD-L1, positively weak (R=0.25, B); CD4 and PD-L1, positively medium (R=0.36, C); CD8 and IL-2, positively medium (R=0.38, D); CD8 and IFN-g, positively weak (R=0.28, E); and IL-2 and IFN-g, positively medium (R=0.36, F).
Previous reports have reported no or little involvement of PD- 1/PD-L1 in DTs.5,18 Also, PD-L1 expression was found only at the tumor margins of DTs.5 In the current study, all patients were negative for PD-1, but positive for PD-L1, including inflammatory cytokines. In addition, PD-L1 expression was found inside the tumor, not at the tumor margins. Therefore, within the tumor microenvironment of DT, an immune mechanism by PD-L1 may be involved.
PD-1 receptors are expressed on the surface of CD8+ T cells when activated by the T-cell receptors (TCRs). Conversely, PD-1 ligand (PD-L1) may be expressed constitutively by tumor cells or in response to interferon-induced signals via the interferon receptor. 22 Taken together, similar constitutive and inducible expression of PD-1/PD-L1 may be involved within the tumor microenvironment of DT.
Mutations in the β-catenin gene are the primary driving force behind the formation of DTs.23 In cases of sporadic desmoid fibromatosis, β-catenin is more highly expressed in recurrent tumors, and β-catenin overexpression is considered a prognostic factor that helps shorten disease-free survival.24 The present study observed a positive correlation between β-catenin and CD4 and between β- catenin and PD-L1. Thus, CD4 and PD-L1 may be upregulated in DT with poor prognosis.
There were some limitations to this study. First, the small sample size may have affected the significance of the results and the power of the statistical analysis. Second, this was a retrospective study; therefore, there may have been selection bias in enrolling the patients. Third, PD-1 and PD-L1 expression at the gene level has not been confirmed. Despite these limitations, this study provides evidence for the involvement of inducible PD-L1 in the tumor microenvironment of DTs. However, further studies with multivariate analyses, larger sample sizes, and longer clinical follow- up periods are needed.
This study provides evidence for the involvement of the inflammatory cytokine-induced PD-L1 in the pathogenesis of DTs.
Acknowledgments
We would like to thank Chikoto Tanaka for providing technical assistance.
References
- 1.Tsukamoto S, Takahama T, Mavrogenis AF, Tanaka Y, Tanaka Y, Errani C. Clinical outcomes of medical treatments for progressive desmoid tumors following active surveillance: a systematic review. Musculoskelet Surg 2023;107:7-18. [DOI] [PubMed] [Google Scholar]
- 2.Prete F, Rotelli M, Stella A, Calculli G, Sgaramella LI, Amati A, et al. Intraabdominal sporadic desmoid tumors and inflammation: an updated literature review and presentation and insights on pathogenesis of synchronous sporadic mesenteric desmoid tumors occurring after surgery for necrotizing pancreatitis. Clin Exp Med 2022. Online Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal A, Goyal S, Goyal A, Jana M. WHO classification of soft tissue tumours 2020: an update and simplified approach for radiologists. Eur J Radiol 2021;143:109937. [DOI] [PubMed] [Google Scholar]
- 4.Raveendranadh A, Goutham M, Gowda C, Hegde K, Monappa V, Rodrigues G. Anterior abdominal wall metastasis following curative resection and chemoradiation of rectal cancer masquerading as a desmoid tumour: a clinical conundrum. J Taibah Univ Med Sci 2022;17:146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siozopoulou V, Marcq E, Jacobs J, Zwaenepoel K, Hermans C, Brauns J, et al. Desmoid tumors display a strong immune infiltration at the tumor margins and no PD-L1-driven immune suppression. Cancer Immunol Immunother 2019;68:1573-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J, Cheng F, Yao Z, Zheng B, Niu Z, He W. Surgical management of a giant desmoid fibromatosis of abdominal wall with vessels invasion in a young man: a case report and review of the literature. Front Surg 2022;9:851164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazar AJF, Tuvin D, Hajibashi S, Habeeb S, Bolshakov S, Mayordomo-Aranda E, et al. Specific mutations in the betacatenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol 2008;173:1518-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Mete L, Ferraresi V, Caterino M, Martayan A, Terrenato I, Mannisi E, et al. Desmoid tumors characteristics, clinical management, active surveillance, and description of our FAP case series. J Clin Med 2020;9:4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aelvoet AS, Struik D, Bastiaansen BAJ, Bemelman WA, Hompes R, Bossuyt PMM, et al. Colectomy and desmoid tumours in familial adenomatous polyposis: a systematic review and meta-analysis. Fam Cancer 2022;21:429-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escobar C, Munker R, Thomas JO, Li BD, Burton GV. Update on desmoid tumors. Ann Oncol 2012;23:562-9. [DOI] [PubMed] [Google Scholar]
- 11.Al-Jazrawe M, Au M, Alman B. Optimal therapy for desmoid tumors: current options and challenges for the future. Expert Rev Anticancer Ther 2015;15:1443-58. [DOI] [PubMed] [Google Scholar]
- 12.Tsukamoto S, Tanzi P, Mavrogenis AF, Akahane M, Kido A, Tanaka Y, et al. Upfront surgery is not advantageous compared to more conservative treatments such as observation or medical treatment for patients with desmoid tumors. BMC Musculoskelet Disord 2021;22:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [DOI] [PubMed] [Google Scholar]
- 14.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al- Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 2006;8:190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther 2015;37:764-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer 2018;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto K, Nishimura S, Ito T, Akagi M. Characterization of PD-1/PD-L1 immune checkpoint expression in soft tissue sarcomas. Eur J Histochem 2021;65:3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombo C, Belfiore A, Paielli N, De Cecco L, Canevari S, Laurini E, et al. β-Catenin in desmoid-type fibromatosis: deep insights into the role of T41A and S45F mutations on protein structure and gene expression. Mol Oncol 2017;11:1495-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaewkangsadan V, Verma C, Eremin JM, Cowley G, Ilyas M, Eremin O. Crucial contributions by T lymphocytes (effector, regulatory, and checkpoint inhibitor) and cytokines (TH1, TH2, and TH17) to a pathological complete response induced by neoadjuvant chemotherapy in women with breast cancer. J Immunol Res 2016;2016:4757405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakavand H, Wilmott JS, Long GV, Scolyer RA. Targeted therapies and immune checkpoint inhibitors in the treatment of metastatic melanoma patients: a guide and update for pathologists. Pathology 2016;48:194-202. [DOI] [PubMed] [Google Scholar]
- 21.Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020;577:556-60. [DOI] [PubMed] [Google Scholar]
- 22.Hu-Lieskovan S, Ribas A. New combination strategies using programmed cell death 1/programmed cell death ligand 1 checkpoint inhibitors as a backbone. Cancer J 2017;23:10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tejpar S, Nollet F, Li C, Wunder JS, Michils G, dal Cin P, et al. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene 1999;18:6615-20. [DOI] [PubMed] [Google Scholar]
- 24.Gebert C, Hardes J, Kersting C, August C, Supper H, Winkelmann W, et al. Expression of beta-catenin and p53 are prognostic factors in deep aggressive fibromatosis. Histopathology 2007;50:491-7. [DOI] [PubMed] [Google Scholar]


