Abstract
Providing universal access to high-cost medications like anticancer drugs is not an easy feat. Although basic medical insurance has covered over 95% of China’s population since 2012, reimbursement for high-priced medicines is limited. In 2015, the Chinese government proposed establishing an open and transparent price negotiation mechanism for some patented and expensive drugs, where oncology was among the prioritized areas. In 2016, three drugs (gefitinib, icotinib, and tenofovir disoprox) underwent negotiation with the government, eventually reducing their prices by over 50% so that they could be prioritized during reimbursement processes. Focusing on anticancer medicines, this study comprehensively summarizes the progress in drug price and national reimbursement negotiation in China. Furthermore, we investigated the changes and development regarding negotiated anticancer medicines from quantity negotiated, classification, indication coverage, utilization, and procurement spending. Our findings could provide a reference for follow-up negotiations and reimbursement policies for high-value anticancer medications in other countries. From 2016 to 2021, 82 anticancer medicines were newly incorporated into the national reimbursement drug list (NRDL) via 6 rounds of negotiation. The majority of these were innovative pharmaceutics (ie, protein kinase inhibitors (28) and monoclonal antibodies (13)). Drug pricing and national reimbursement negotiation led to a marked decrease in prices and a sharp increase in the utilization of negotiated anticancer medicines. Following negotiations, the defined daily doses (DDDs) of innovative anticancer medicines experienced remarkable growth. Their proportion in total anticancer drugs DDDs also increased from 3.4% in 2014 to 20.9% in 2019. However, although drug prices decreased substantially after the negotiations, insurance spending still showed an upward trend owing to the significant increase in utilization. This calls for the government to carefully monitor the rational use of these expensive medicines and explore innovative payment models.
Keywords: antineoplastic agents, neoplasms/drug therapy, drug costs, negotiating, insurance coverage, National Health Programs, health policy, China
What do we already know about this topic?
To effectively provide cancer patients with a timely and affordable supply of life-saving medicines, the Chinese government has implemented a series of policies in recent years, in which drug price and national reimbursement negotiation were considered the most important aspects.
How does your research contribute to the field?
Focusing on anticancer medicines, this review comprehensively summarizes the progress in drug price and national reimbursement negotiations in China, and analyzes the changes and development of negotiated anticancer medicines from various aspects. Further, problems and achievements within the negotiation process are identified, and references for follow-up negotiation are provided.
What are your research’s implications toward theory, practice, or policy?
The negotiation provides an opportunity for innovative anticancer medicines to be subsided promptly, which significantly improves the utilization and affordability of these life-saving medicines. However, despite a substantial price reduction, the boosting utilization still leads to an increase in overall insurance spending, which calls for the government to monitor the rational use of negotiated medicines and explore innovative payment models.
Background
Cancer is one of the most serious health problems globally and has been the leading cause of death in China since 2010. 1 According to the data released by the International Agency for Research on Cancer (IARC), in 2020, there were 19.29 million patients who were newly diagnosed with cancer, and 9.96 million deaths reported worldwide. 2 China accounted for 23.7% (4.57 million) and 30.1% (3 million) of the total global numbers, respectively, partly due to its large population. 3 With a rapid aging population and other risk factors, the global number of new cancer cases is estimated to increase by approximately 60% over the next 2 decades. 4 China is predicted to record approximately 6.85 million new cancer cases and 5.07 million deaths in 2040, which will bring significant challenges to the health system of the country. 4
Based on the national cancer registry data, the age-standardized 5-year relative survival rate of patients with malignant tumors in China was approximately 40.5% between 2012 and 2015, an increase of more than 10% compared with the 2003 to 2005 period, but remaining lower than many developed countries. 5 Taking breast cancer as an example, which is the most frequent cancer type among Chinese women (incidence rate in 2020: 41.4/100 000), 3 despite the 5-year relative survival rate increasing to 83.2% (2012-2015) from 75.9% (2003-2005), it was still 7% lower than the survival estimates calculated using the same method for the USA (90.8%) and Japan (89.4%) between 2010-2014. 6 In addition to inadequate early detection and non-standard treatment, low accessibility and lagging clinical application of novel anticancer medicines are among the important factors leading to this survival gap.7,8 Over the past decade, with advances in drug development and clinical research, targeted therapy and immunotherapy have emerged as novel and essential approaches to cancer treatment, significantly improving the survival outcomes and living conditions of cancer patients.9-11 Trastuzumab, a monoclonal antibody against human epidermal growth receptor 2 (HER2), has successfully increased the 5-year survival rate of patients with HER2+ breast cancers. 12 Following marked clinical efficacy, clinical guidelines currently recommend targeted therapy with trastuzumab as standard treatment for HER2+ breast cancers. 13 However, due to patent protection and technology monopoly, most innovative anticancer medicines are expensive and were not covered by basic medical insurance before 2016 in China. 14 Without insurance coverage, patients who were prescribed trastuzumab had to pay over US$50 000 in that year, almost 24 times the Chinese annual per capita disposable income in the same period. 15 The extremely high out-of-pocket (OOP) expenditure makes these lifesaving medicines not affordable to most patients. Observational studies between 2000 and 2015 in patients with HER2+ metastatic breast cancer showed that nearly 12% in the US, between 27% to 54% in Europe, and 27.1% to 49.2% in China did not receive trastuzumab or any other HER2-targeted agent for treatment. 16 In 2018, the WHO released a report on cancer medicine, which showed that only a few patients used innovative anticancer medicines globally. 17 High treatment expenditures and lack of drug funding are among the key barriers to these innovative medicines.
In an attempt to improve the accessibility and affordability of innovative medicines, China has introduced a series of policies in recent years, in which drug price and national reimbursement negotiation were the key approaches. 18 This new approach enables innovative medicines to be included in the national reimbursement drug list after price negotiation with the Chinese government. In 2016, three drugs, including 2 innovative anticancer medicines, underwent the first negotiation round, eventually reducing their prices by over 50% so that they could be prioritized during reimbursement processes. Focusing on anticancer medicines, this review comprehensively summarizes the progress in drug price and national reimbursement negotiations in China, and analyzes the changes and development of negotiated anticancer medicines from various aspects. Further, problems and achievements within the negotiation process are identified, and references for follow-up negotiation and reimbursement policies for high-value anticancer medications in other countries are provided.
Methods
This study employed a multifaceted approach that synthesize various data sources. In the first step, a review of China’s medication reimbursement policy and drug price negotiation implementation was performed. We applied content analysis methodology to review the existing literature and the information extracted from government documents, government statistical handbooks, and news sources. Next, we searched English and Chinese databases, viz. PubMed, Embase, and China National Knowledge Infrastructure for published literatures. The publishing dates were set between 2000 and 2022, and clinical and laboratory evaluations were excluded. The search strategy, terms, and results are presented in Supplemental Appendix 1. Second, with an emphasis on negotiated anticancer medicines, all the official documents related to negotiated anticancer medicines issued by the National Healthcare Security Administration and other authorities from 2015 to 2021 were searched systematically with a cut-off date of December 5th, 2021. Furthermore, changes over the years were compared in quantity negotiated, classification and indication coverage. Thirdly, based on the Monitoring Report on Hospital Drug Use [Available from www.cpa.org.cn], published by the Chinese Pharmaceutical Association (CPA) annually between 2015 and 2020, a descriptive secondary analysis was conducted to detect the changes in utilization and procurement spending of negotiated anticancer drugs after policy implementation. The monitoring report is based on the drug procurement and usage data submitted by the Chinese Medicine Economic Information (CMEI) sample hospitals affiliated with the CPA and formed by in-depth mining and scientific analysis using statistical methods. By 2020, the CMEI included more than 1500 sample hospitals in 31 provinces. Most of the sample hospitals are secondary and tertiary hospitals. Defined daily doses (DDDs) and procurement spending were selected as indicators in this descriptive secondary analysis. Recommended by the World Health Organization (WHO) for drug utilization monitoring and research, DDDs are the number of daily doses of each medication based on dosage regimens based on the product labels and approved by the National Medical Products Administration. 19 The greater the DDDs, the higher frequency of medicines used.
Drug Price and National Reimbursement Negotiation
The National Reimbursement Drug List
The National Reimbursement Drug List (NRDL) was first created in 2000 to improve access and reimbursement for hospital-purchased drugs. It was formulated by representatives of 5 government agencies and determined how drugs are covered under basic medical insurance. 20 Since its establishment, NRDL has worked as the primary mechanism for medicine reimbursement in China. 21 After the update in 2009, the list contained 1140 Western and 987 traditional Chinese medications, categorized into class A and class B. 22 Class A medications consist of widely used, relatively cheap drugs and generics that are considered indispensable and fully reimbursed by basic medical insurance. Class B medicines includes drugs considered less indispensable of a higher price, and have different co-payments depending on the province. The reimbursement ratio is generally between 50% and 90%. 23 Furthermore, provincial governments are given autonomy to add or remove about 15% of medicines on their regional class B lists to account for local needs or provincial hospital demand. 24
The NRDL was meant to be updated every 2 years, with thousands of national and provincial experts participating in a complex voting process. Nevertheless, due to the complexity and tedious nature of the adjustment process and a lack of available staff to execute the task, it was only updated twice (2004 and 2009) before 2016.25,26 The lengthy time between NRDL updates meant that new drugs would experience significant delays between approval and reimbursement. From 2003 to 2013, there were 360 new drugs approved in China. However, only 76 (21%) entered the NRDL. 22 Medications not on the NRDL must be paid out-of-pocket. Since NRDL generally prioritizes local brands and generics, some innovative life-saving medicines, especially cancer treatment, are out of the list due to their high price, leading to access barriers for many cancer patients despite their recognized clinical benefits. 8
In 2015, the WHO conducted a comprehensive review of essential medicines for cancer, where 16 new anticancer medicines, including 3 targeted therapy drugs, were added to the WHO’s Model Lists of Essential Medicines. 27 Furthermore, the WHO has also suggested its member countries to re-examine their reimbursement policies for anticancer medicines and update national reimbursable medicines lists in a timely manner. To effectively provide cancer patients with a timely and affordable supply of life-saving medicines, the Chinese government has implemented policies to accelerate approval of medicines and update the NRDL, in which drug price and national reimbursement negotiation were considered the most important aspects. 18
Introduction of Drug Price Negotiation
Drug price negotiation has been practiced in many countries as a policy approach to price and reimburse innovative medicines. 28 As such, medicines are reimbursed by public funding at an agreed lower price following negotiations among stakeholders like the government, pharmaceutical companies, and insurance institutions. 17 In developed countries such as Italy and Australia, negotiations successfully reduced the price of innovative drugs and promoted drug utilization.29,30 In middle-income countries like Mexico, between 2010 and 2016, negotiation led to an approximately 40% to 85% decrease in the price of 8 innovative cancer medicines. Moreover, the volume of these medicines supplied in public hospitals increased over the years, suggesting better access. 31
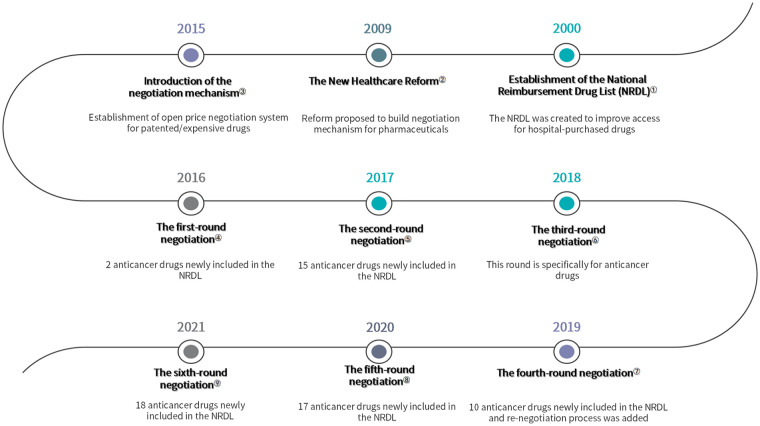
On February 9th, 2015, the State Council of China proposed to establish an open and transparent price negotiation mechanism for some patented and expensive drugs, prioritized areas including oncology, rare diseases, pediatric diseases and public health outbreaks. 32 Three months later, the Opinions on Promoting the Drug Pricing Reform was issued, which further denoted that the drug price negotiation will be generally applied to patented and exclusively manufactured medicines. 33 Prior to the start of national-level negotiations, some economically developed regions, like Zhejiang province, have explored the negotiation mechanism at the local level. 34 The scope of those early negotiations was relatively small in terms of the number of medicines on the negotiation table and most of the process was closed to the pharmaceutical suppliers. However, it offered referential experience and lessons for the national negotiation (Figure 1).
Figure 1.
Timeline of important events.
Notes: ①-②Pharmaceutical policy in China: challenges and opportunities for reform. World Health Organization. Regional Office for Europe, European Observatory on Health Systems and Policies, Mossialos, Elias, Ge, Yanfeng, Hu, Jia. et al. (2016). https://apps.who.int/iris/handle/10665/326313; ③ The State Council. Guiding Opinions of the General Office of the State Council on Improving Centralized Purchasing of Drugs for Public Hospitals. 2015 (In Chinese) http://www.gov.cn/gongbao/content/2015/content_2827191.htm; ④-⑨Available from http://www.nhsa.gov.cn/ (National Healthcare Security Administration).
Implementation and Evolvement of the Negotiation
In 2016, the National Health Commission, National Development and Reform Commission, and the Ministry of Human Resources and Social Security initiated the first-round national-level drug price and NRDL access negotiation. By the new mechanism, the Chinese government can “cherry-pick” drugs and slash drug prices in exchange for reimbursement status (NRDL class B). Finally, 3 drugs (gefitinib, icotinib and tenofovir disoprox) out of 5 candidates successfully reached an agreement with the government after reducing their prices by more than 50% (Table 1). 35 So that they will benefit from priority in the reimbursement processes. After the introduction of negotiation mechanism, the new NRDL can be divided into 2 parts: (1) negotiated drugs within a contracted period and (2) drugs on the routine list with no time limit. The negotiated drugs have a contracted period of usually 2 years. When drug agreements are about to expire, the government evaluates the negotiated medicines’ actual expenditure during the contracted period and analyzes the impacts on insurance funds. Thereafter, the government conducts renewal negotiations with the manufacturer to decide whether to adjust the price and payment range. Negotiated medicines whose price and payment scope have not been adjusted in 2 consecutive contracted periods are applicable to be moved into the routine list.
Table 1.
Overview of Drug Price and National Reimbursement Negotiations in China From 2016 to 2021.
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | |
|---|---|---|---|---|---|---|
| Quantity of drugs to be negotiated | 5 | 44 | 18 | 150 | 162 | 117 |
| Number of successfully negotiated drugs | 3 | 36 | 17 | 97 | 119 | 94 |
| (first negotiated) | 3 | 36 | 17 | 70 | 96 | 67 |
| (renewal negotiation) | 27 | 23 | 27 | |||
| Negotiation success rate (%) | 60 | 82 | 94.3 | 64.7 | 73.5 | 80.3 |
| Average price reduction (%) | 59 | 44 | 57 | 61 | 51 | 62 |
| Number of anticancer drugs newly included in NRDL | 2 | 18 | 17 | 10 | 17 | 18 |
| The proportion of anticancer drugs in newly added drugs (%) | 66.7 | 50 | 100 | 14.3 | 17.7 | 26.9 |
In 2018, the National Healthcare Security Administration (NHSA) was established as a functional department to integrate the responsibility for the basic medical insurance and price management of pharmaceuticals and medical services. 36 Shortly after its establishment, the NSHA initiated the third-round negotiation, particularly for anticancer drugs. After 2 months of negotiation, 17 anticancer drugs entered the NRDL with an average price discount of about 56.7% (highest up to 70%). Osimertinib (Tagrisso) was one of the successfully negotiated medicines in this round, which is a targeted medicine for lung cancer with excellent results. Before the agent was listed in the NRDL, patients could only purchase it at the original price, that is, 51 000 CNY/box (1 month supply). After negotiation, the price dropped to 15 300 CNY/box. Moreover, if a patient was covered by insurance and paid a 30% share, then their monthly expense was 4590 CNY, which was 91% lower than before. 37
The Negotiation Mechanism
After years of practice, the mechanism of drug price negotiation and dynamic adjustment of NRDL were gradually formed. On July 30th, 2020, the NHSA published new administrative measures, which contained a detailed process for negotiation and management of NRDL (Table 2). 38 For the first time, pharmaceutical companies could initiate the process by submitting their applications for review. Previously, only products selected by NHSA experts could be put forward for consideration. This change highly encouraged the industry. In 2020, approximately 700 drugs applied for the expert review, and 119 eventually made the listing, breaking the record for most inclusions in history. Additionally, the guideline also declared that the negotiation and NDRL adjustment would be implemented once a year to optimize the NRDL and enable more urgently needed medicines to be included in the list in a timely manner. 39
Table 2.
Process and Mechanism of Drug Price and National Reimbursement Negotiation in China.
| Stage | Main content | |
|---|---|---|
| 1 | Preparation | • NHSA draws up work plans and principles for the new round of negotiation. • NHSA solicits public opinion, and establishes working groups and expert pools. |
| 2 | Application | • NHSA release guidelines on the application of NRDL negotiation. • Pharma companies submit applications and materials required by NHSA, mainly including basic information on medicines, safety, effectiveness, economic data, and expected price. • NHSA to review applications and release public notices on applications that passed the formal review process. |
| 3 | Expert Review | • NHSA organizes experts in clinical, pharmacy, pharmacoeconomics, and medical insurance to evaluate the candidate medicines from the aspects of effectiveness, safety, economy, and innovation. Furthermore, to determine the list of medicines to enter price negotiation. |
| 4 | Negotiation | • NHSA organizes experts for pharmacoeconomics and budget impact to determine a target price for each medicine to be negotiated. • NHSA representatives negotiate with enterprises on site, to confirm the price and payment restriction. • After the negotiation, both parties shall sign the result confirmation letter on-site, regardless of whether they reach an agreement or not. |
| 5 | Results | • NHSA announces the negotiation results and updated the NRDL. • NHSA Specifies NRDL management and implementation measures. |
Regarding price setting, NHSA mainly adopts the “value-based” pricing concept to explore the price benchmark by comparing the differences in incremental health output and cost between negotiated and reference drugs. 40 In practice, 2 expert panels (pharmacoeconomics evaluation and budget impact analysis panels) conduct parallel calculations to generate a floor price. Pharmacoeconomics experts mainly calculate the floor price through pharmacoeconomic methods with preset economic thresholds and further adjust it considering the international reference price and the price of competitive products. Budget impact experts mainly focus on the affordability of the insurance fund and offer a price that the fund can bear. Finally, the 2 prices are converted into a target price—the highest price NHSA would accept. 41 The calculation process is highly confidential, and pharma companies are unaware of the government’s target price. After establishing the target price, on-site negotiations are conducted between the NHSA and pharma companies. In the negotiation, pharma companies are required to offer their expected price first and have 2 chances to offer and confirm the price. If the final price confirmed by manufacturers exceeds the government’s target price by 15%, the negotiation will cease and fail. On the contrary, if the price offered does not exceed that limit, the negotiation will proceed to decide on a final price. However, the final negotiated price agreed between NHSA and the manufacturers must be, at most, the target price. By putting pressure on manufacturers that an exorbitant price will lead to failure in negotiation, this mechanism tries to force manufacturers to offer the lowest price they can accept.
Analysis on the Changes and Development of Negotiated Anticancer Medicine
Quantity, Classification, and Indication Coverage
Anticancer medicines have been always the critical guarantee scope of the negotiations, accounting for 28.4% of all successfully negotiated drugs. From 2016 to 2021, a total of 82 anticancer medicines were newly incorporated into the NRDL through 6 rounds of negotiation, including 78 Western medicines and 4 Chinese patent medicines. Moreover, 15 of these products have been moved into the routine list, and 34 products participated in renewal negotiations between 2019 and 2021. Regarding renegotiated drugs, 32 renewed successfully. Furthermore, 10 out of theses 32 products had their reimbursement indications expanded after renewal negotiation (See Supplemental Appendix 2 for details).
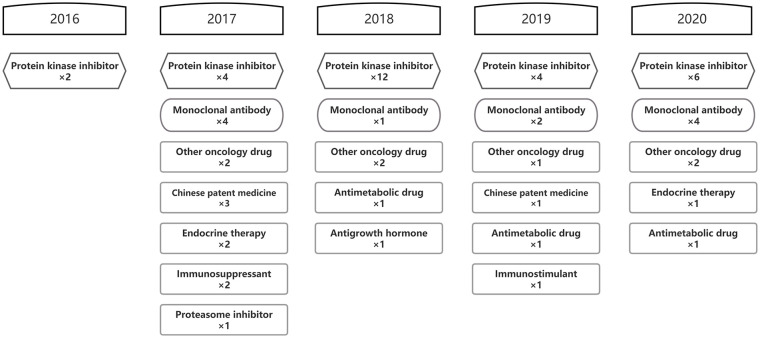
Each round of negotiations includes a variety of new anticancer drugs with high social concern and urgent demand. Innovative medicines, represented by small molecular targeted drugs and macromolecular monoclonal antibodies, are the main components of negotiated anticancer drugs over the years. By 2021, 28 protein kinase inhibitors and 13 monoclonal antibodies obtained spots on NRDL, including 4 PD-1/L1 inhibitors (Figure 2). In addition, blockbuster products from other classifications, like abiraterone, are also included in NRDL, providing more choices for cancer treatment.
Figure 2.
Anatomical therapeutic chemical classification of negotiated anticancer medicines from 2016 to 2021.
Currently, cancers of the lung, gastric, colorectal, liver, and breast are the top 5 cancer types in terms of incidence among Chinese residents. 42 After 6 rounds of negotiations, the reimbursement indications of negotiated anticancer drugs have covered multiple cancer types. Drugs for lung cancer, breast cancer, and lymphoma are the most negotiated, reaching 18, 12, and 12, respectively. In terms of lung cancer, after the inclusion of furmonertinib in 2021, all 3-generations EGFR TKI targeted medicines marketed in China have been listed on NRDL, which provides more treatment options for lung cancer patients. With the progress of negotiations, some drugs targeting rare tumor indications like gastroenteropancreatic neuroendocrine tumors (GEP-NET) also entered the NRDL.
Utilization
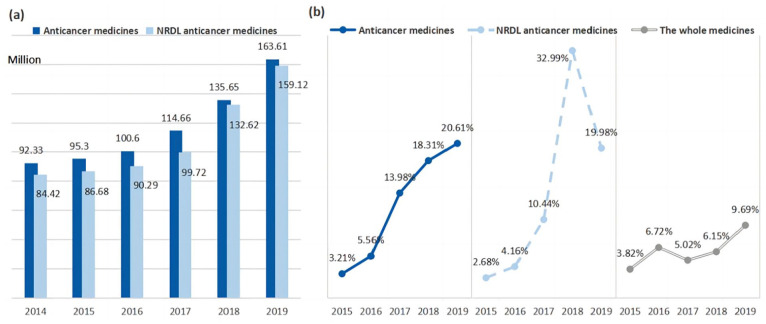
The DDDs of anticancer medicines increased continuously in China from 92.33 to 163.61 million between 2014 and 2019, whereas the growth rate accelerated significantly post NRDL negotiations, as shown in Figure 3. Between 2014 and 2016, the growth rate remained constant at around 5%, slightly lower than all drugs. Following the start of negotiations, in 2016, the growth rate exhibited a rapid increase, reaching 20.61% in 2019, more than twice of the all drugs. Since the successful incorporation of 20 innovative anticancer medicines into NRDL after the first 2 rounds of negotiations, the DDDs of NRDL anticancer medicine increased significantly by 33% in 2018, accounting for 97.77% of the total anticancer medicine utilization, and have shown continuous growth momentum in 2019.
Figure 3.
Defined daily doses and growth rate of anticancer medicines from 2014 to 2019 in China: (a) defined daily doses and (b) growth rate of defined daily doses.
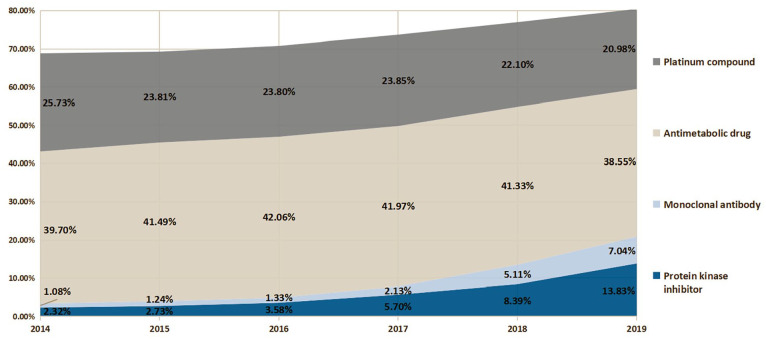
Driven by improved access, innovative medicines, like protein kinase inhibitors and monoclonal antibodies, experienced much higher DDDs growth than other anatomical therapeutic chemical (ATC) categories post NRDL listing, reaching 2.26 and 1.15 million in 2019, respectively (Figure 4). Furthermore, their proportions in total anticancer drugs DDDs have also shown rapid upward trends, which increased from 2.32% and 1.08% in 2014 to 13.83% and 7.04% in 2019, occupying the third and fifth places among all anticancer drugs, only behind the chemotherapy medicines. Although chemotherapy medicines remain dominant in anticancer drug therapy, their volume share began to experience a slowdown with the wide-spreading utilization of innovative drugs.
Figure 4.
Proportion of defined daily doses of anticancer medicines classified by anatomical therapeutic chemical sub-class between 2014 and 2019.
Procurement Spending
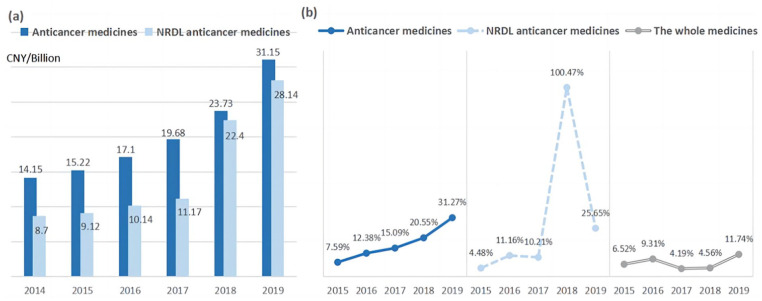
Overall, the procurement spending on total anticancer medicines increased continuously between 2014 and 2019, from 14.15 billion CNY to 31.15 billion CNY, with a compound growth rate of 17.1% (Figure 5). Although negotiations directly reduce drug prices, the boosting utilization still leads to increased overall expenditures on NRDL anticancer medicines. In 2018, the spending on NRDL anticancer medicines experienced notable growth acceleration post NRDL listing, increasing by 11.22 billion CNY with an incredible 100.47% growth rate, 5 times higher than that of total anticancer medicines and nearly 20 times higher than that of all drugs. While in 2015, before the negotiation started, the growth rate of NRDL anticancer medicines was only 4.48%, lower than that of total anticancer medicines and all drugs. It is worth noting that the proportion of NRDL medicines spendings in the total anticancer drugs reached 94.4% in 2018 from 61.5% in 2014, indicating that most spending for anticancer medicines has been covered by basic medical insurance following policy implementation. The economic burden of anticancer drug therapy was greatly improved.
Figure 5.
Procurement spending and growth rate of anticancer medicines from 2014 to 2019 in China: (a) procurement spending and (b) growth rate of procurement spending.
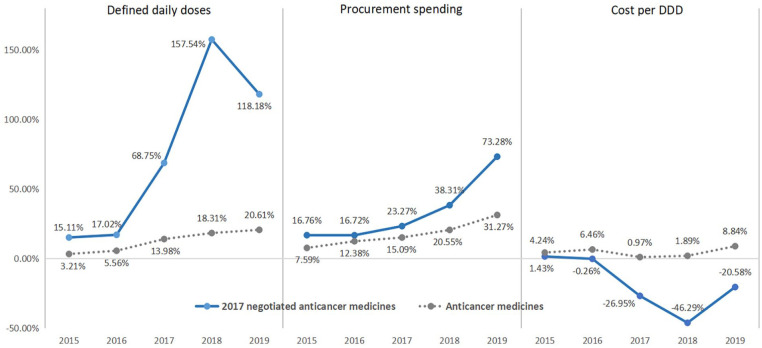
Regarding the 18 anticancer medicines that were successful in the 2017 NRDL negotiation, NRDL listing boosted the DDDs growth rate by 157.54% in 2018, which led to a 38.11% growth in spending despite an average 46.29% price (cost per DDD) cut. In 2019, though the average price (cost per DDD) further decreased by 20.58%, the growth rate of procurement spending increased to 73.28% with growing utilization (Figure 6).
Figure 6.
Trends of DDDs, cost per DDD and procurement spending of 2017 negotiated anticancer drugs.
Discussion
Principal Findings
The above analysis indicates that the drug price and national reimbursement negotiation led to a marked decrease in price (cost per DDD) and a sharp increase in the utilization of negotiated anticancer medicines. Benefiting from price reduction and insurance reimbursement after negotiation, the DDDs of anticancer medicines increased continuously from 92.33 million to 163.61 million between 2014 and 2019. The growth rate accelerated substantially post-NRDL negotiations being 6-fold higher than that before negotiations. Increased utilization volume suggests that patient access to these anticancer medicines may have improved. 31 Driven by improved access, innovative medicines such as protein kinase inhibitors and monoclonal antibodies experienced much higher DDD growth than the other ATC categories post-NRDL listing. Their total anticancer drugs DDD proportion also showed a rapid upward trend, increasing from 2.32 and 1.08% in 2014 to 13.83 and 7.04% in 2019. These findings are consistent with the results of other studies. An interrupted time-series (ITS) study conducted in Nanjing City between January 2016 and December 2018 showed that, following negotiations, the mean availability rate of 15 innovative anticancer medicines increased to 47.33 from 27.44%, and the usage rate of bevacizumab, bortezomib, and apatinib increased significantly (P < .001, P = .009, and P < .001, respectively). 43 Zhang et al 44 used an ITS design to assess the impacts of negotiation on prices, volumes, and spending on targeted anticancer medicines with negotiated prices in 2017. The results showed that the inclusion of expensive targeted anticancer medicines in NRDL led to a notable decrease in cost per DDD (48.9%, P = .000), a significant increase in utilization (143.0%, P = .000), and a 6.9% decrease in hospital medication spending (P = .146). In addition, previous studies also analyzed the increase in the number of patients who gained access to those innovative drugs thanks to the negotiation. Li et al 45 found that the monthly number of patients in Fuzhou City who began treatment with 2 negotiated anti-lung cancer medications increased significantly after NRDL coverage (P < .001). Diao et al 46 found that the average proportion of HER2+ breast cancer patients who initiated target therapy with trastuzumab increased from 37.4% to 69.2% after the agent was included in NRDL by negotiation. The quick growth post-negotiation and NRDL listing demonstrated considerable unmet needs for these drugs previously, and drug price negotiation combined with NRDL listing effectively lifted the accessibility and affordability barrier to these innovative products.
However, the inclusion of innovative anticancer medicines in the NRDL implies an increase in insurance expenditures. Although the drug price decreased considerably after the negotiations, procurement spending still showed an upward trend due to the significant increase in utilization. In 2017, 18 anticancer medicines were negotiated successfully with an average 46.29% price cut. However, the procurement spending of these 18 medicines increased by 38.31% in 2018, owing to a 157.54% growth in utilization. In 2019, spending of these 18 medicines further increased by 73.28% with continuously increasing utilization. Diao et al 47 assessed the financial impact on insurance spending of 6 negotiated anticancer medicines in Hangzhou City. Their study indicated that in the first 2 years after insurance inclusion, insurance spending on these medicines was approximately 121 million CNY when the reimbursement rate was set at a basic 60%, accounting for 46% of the Hangzhou Catastrophic Health Insurance Program funds (a type of “add-on insurance” based on basic medical insurance, aiming to offer extra protection against catastrophic illnesses like cancer) 48 during the same period. Because some areas adopt a higher reimbursement rate at 80% or 90%, the spending and proportion would further increase. Since an increasing amount of medicines enter the NRDL through annual negotiations, the utilization and insurance spending of negotiated medicines are expected to increase continuously. Moreover, Ding et al 49 proposed that there is an induced demand effect after innovative drugs enter NRDL through negotiation. With price reductions and insurance reimbursement, physicians may induce patients to use these drugs initially and ignore some more cost-effective treatment options. The increases in fund expenditure may exceed the authorities’ expectations resulting from the expansion in patient groups and drug utilization. To avoid unnecessary expenditure, it is essential to insist on the rational use of the negotiated medicines following clinical guidelines. Governments ought to monitor the consumption of these medicines and promote the use of clinical guidelines in hospitals. Moreover, innovative payment models like price-volume agreements, outcome-based payment, and spending caps may help address payer concerns and have been practiced by some authorities. 43 China should explore these payment models proactively, especially in light of the accelerated access to innovative drugs in the NRDL and continuously increasing spending.
Challenges and Recommendations
Patient access remains delayed
With the initial establishment of the annual update mechanism, innovative anticancer medicines can enter the NRDL promptly. However, being on the NRDL does not immediately translate to hospital use—the only channel for negotiated anticancer drug provision. Drugs need to be included in the hospital drug formulary through a long and complex selection process performed internally. Therefore, even with faster NRDL inclusion, many patients continued to report that some negotiated drugs could not be prescribed in hospitals, resulting in delayed patient access. 50 Besides, hospitals are unwilling to equip negotiated anticancer drugs due to policies like “zero mark-up rate of medicine” and control of medical costs. 51 Most negotiated drugs are high-value drugs with strict storage requirements and short shelf life, leading to high management, capital occupation, and damage costs. 14 Under DRG/DIP payment reform, medical institutions must bear expenses exceeding the payment benchmark settled with medical insurance. 52 Therefore, the generally high prices and management costs of negotiated medicines will pressure medical institutions to control costs, weakening hospitals’ motivation for acquiring negotiated medicines. To promote the supply willingness of hospitals, the government should release supporting policies to separate the costs of negotiated medicines from the DRG/DIP payment standards or allow adjustment of payment caps according to the utilization of negotiated medicines. Besides, at the end of 2021, some provinces began to pilot a new model that includes designated direct-to-patient (DTP) pharmacies in the supply of negotiated drugs and implemented a unified payment policy with hospitals. 53 This attempt is expected to improve supply capability and give patients more access to negotiated medicines. However, when negotiated drug prescriptions are dispensed, supplied, used, and monitored outside the hospital’s treatment system, it may be challenging to ensure medication safety, especially when many anticancer drugs have considerable side effects. 54 To ensure the welfare of patients, it is necessary to implement “DTP pharmacy qualification management” to strictly control the storage and delivery capacity of designated DTP pharmacies and strengthen pharmaceutical care and specialty in DTP pharmacies. Moreover, the cooperation and coordination of hospitals, designated DTP pharmacies, and medical insurance agencies should be promoted to realize the whole process of monitoring patients’ medication behavior.
The improvements in low-income populations are limited
Following the negotiations, the out-of-pocket expenses of cancer patients decreased substantially, suggesting an improvement in the accessibility of negotiated innovative medicines. However, due to the original high price of negotiated drugs, even if the amount is reduced and reimbursed by basic medical insurance, the burden of out-of-pocket expenses remains massive for the low-income population. 47 An analysis from 2017 based on the income data of urban and rural residents in Hubei province showed that the poverty rate caused by using trastuzumab (Herceptin) did not change significantly in low-income populations after negotiation, and only decreased from 46.00% to 45.42%. As for icotinib (Conmana), a better change after negotiation (45.85%-33.40%) was observed. 55 However, this result is still far from satisfactory. Tian et al 56 evaluate the catastrophic expenditures of gefitinib (Iressa) and sunitinib (Sutent) after negotiations. The results indicate even if gefitinib and sunitinib reduced their prices by 50% and received insurance reimbursement, using of these drugs would still result in catastrophic expenditures of 50.63% and 75.93% patients in rural area. To ensure equity in medicine and healthcare utilization, the government must take strides to prevent catastrophic healthcare expenditures in low-income populations. Cooperating with charity organizations to provide additional medical assistance or promoting critical illness insurance coverage in this group by subsidizing part of insurance premiums could be potential approaches. Currently, available studies have evaluated the impact of drug price negotiation on the utilization of negotiated medicines. However, most only studied the overall changes in drug utilization in a specific region, without evaluating the effects on specific subpopulations. To evaluate policy impacts more comprehensively, future research should study the improvements in drug utilization and financial burdens among different populations, such as different income or insurance groups.
Incomplete data collection and evaluation system
Drug price and insurance access negotiation is one kind of strategic purchase. The internationally accepted model usually requires a comprehensive evaluation of the purchased drugs based on real-world evidence. 57 Although health technology assessment was applied in the negotiation, China has not established an effective patient information collection and tracking system for negotiated medicines. 58 Furthermore, identifying good local data is challenging as electronic medical records and registries are still in the early stage of development in some hospitals. 59 Even when data are available from electronic medical records, they are often not detailed enough to inform economic models. Therefore, it is difficult to conduct a complete assessment of the patient’s condition and drug usage. The effect of negotiations and strategic purchases cannot be fully evaluated, and scientific data support cannot be formed for subsequent national negotiations. To promote the development of effective negotiation and local adaptation of global health economic models, establishing a negotiated drug data collection and evaluation system are necessary. This system should allow health authorities to collect and track the entire process information, including data regarding each patient’s diagnosis, treatment plan, disease progression, treatment response, long-term follow-up, and medical insurance cost. With this system and the collected data, policymakers and researchers can further carry out a multi-dimensional analysis of cost burden, evaluate whether the negotiated medicines result in the expected clinical results, and assess the opportunity costs of innovative medicines spending in cancer patients.
Lessons for Other Countries
As recommended by the WHO, negotiation and tendering are pricing approaches to determine the price that is mutually acceptable for both the sellers and payers, which have been adopted by some authorities in the pricing and reimbursement of patent medicines. 17 Through negotiation mechanisms, governments can use their bargaining power to reduce the high price of innovative medicines, making them more affordable for most patients. Our study showed that the drug price and reimbursement negotiation in China successfully encouraged the utilization of innovative anticancer medicine and improved access and patient affordability. With the increasing cancer incidence worldwide, future demand and expenditures for these innovative medicines are expected to grow rapidly, which will pressure the governments considerably. To effectively provide cancer patients with a timely and affordable supply of life-saving medicines, drug price and reimbursement negotiation can be a strategy to promote drug access and alleviate the economic burden on patients. However, according to past experience in China, although negotiations directly reduce drug prices, the increase in fund expenditure may exceed the expectations of authorities owing to rapid growth in treatment numbers and medicine utilization. In China, insurance spending on negotiated anticancer medicines has shown a continuous upward trend, which calls for the authorities to carefully monitor the rational use of these medicines and explore innovative payment models. In addition, boosting utilization may bring challenges to the supply system. Manufacturers and governments should prepare for a possible increase in medicine demand after negotiation and adjust the supply system to avoid a shortage of medicine. Moreover, from China’s experience, some advice can be considered for the price negotiation to be effective: (1) Collaboration and communication among management sectors, insurance institutions, pharmaceutical companies, and medical institutions are critical for the effectiveness of negotiations. Establishing clear mechanisms of communication, supervision and notification among these stakeholders is necessary. (2) Emphasize the application of pharmacoeconomic evaluation in negotiation, especially the use of real-world data and evidence to evaluate the clinical value and cost-effectiveness of candidate medicines. (3) Establish a follow-up system to evaluate negotiation effects comprehensively. An independent third-party agency can run the system. It should focus on the effects of clinical benefit, health economics, medical service and public policy to provide evidence for subsequent decisions of the negotiation, such as re-negotiation or termination of reimbursement. (4) Pay attention to the low-income population, as affordability problems may remain in the group even after drug price reduction and coverage inclusion by the insurance. Additional medical assistance can be provided to this group to prevent catastrophic healthcare expenditures. Finally, as economic development and health systems vary among various countries, the negotiation and pricing mechanism should also be adjusted according to the local conditions.
Conclusion
Generally, the drug price and national reimbursement negotiation provide an opportunity for innovative anticancer medicines to be included in the NRDL promptly, which considerably improves the accessibility and affordability of these life-saving medicines in China. Owing to price reduction and insurance reimbursement after negotiation, negotiated medicines like protein kinase inhibitors and monoclonal antibodies experienced sharp increase in utilization, contributing to treatment of cancer patients. However, despite a substantial price reduction, the boosting utilization still leads to an increase in overall insurance spending, which calls for the government to monitor the rational use of these expensive medicines and explore innovative payment models. Moreover, problems like delayed medicine access in hospitals and limited improvement in low-income populations may bring challenges to the effectiveness of negotiations, which need to be improved in the future.
Supplemental Material
Supplemental material, sj-pdf-1-inq-10.1177_00469580231170729 for Promoting Access to Innovative Anticancer Medicines: A Review of Drug Price and National Reimbursement Negotiation in China by Xia Mingge, Wen Jingyu, Liu Qi, Zheng Zhe and Ran Qing in INQUIRY: The Journal of Health Care Organization, Provision, and Financing
Supplemental material, sj-pdf-2-inq-10.1177_00469580231170729 for Promoting Access to Innovative Anticancer Medicines: A Review of Drug Price and National Reimbursement Negotiation in China by Xia Mingge, Wen Jingyu, Liu Qi, Zheng Zhe and Ran Qing in INQUIRY: The Journal of Health Care Organization, Provision, and Financing
Footnotes
Author Contributions: Each author has contributed significantly to the work and agreed to the submission.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the China and Key Research Project of Sichuan Science and Technology Department [grant number 2016SZ0077].
ORCID iD: Xia Mingge  https://orcid.org/0000-0002-0368-0692
https://orcid.org/0000-0002-0368-0692
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778-789. [DOI] [PubMed] [Google Scholar]
- 3.Cao W, Chen HD, Yu YW, Li N, Chen WQ.Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134(7):783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 5.Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6(5):e555-e567. [DOI] [PubMed] [Google Scholar]
- 6.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan L, Xu L.Global efforts in conquering lung cancer in China. Chin J Cancer. 2015;34(7):320-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diao Y, Li M, Huang Z, Sun J, Chee YL, Liu Y.Unlocking access to novel medicines in China - a review from a health system perspective. Risk Manag Healthc Policy. 2019;12:357-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151-172. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Chen Z, Li J.The current status of treatment for colorectal cancer in China: a systematic review. Medicine. 2017;96(40):e8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. LBA51 KEYNOTE-024 5-year OS update: first-line (1L) pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ≥50%. Ann Oncol. 2020;31:S1181-S1182. [Google Scholar]
- 12.Cardoso F, Spence D, Mertz S, et al. Global analysis of advanced/metastatic breast cancer: Decade report (2005-2015). Breast. 2018;39:131-138. [DOI] [PubMed] [Google Scholar]
- 13.Giordano SH, Temin S, Kirshner JJ, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2–positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(19):2078-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Wang Y, Sun X, Li X.Availability, Price and affordability of Anticancer Medicines: Evidence from two cross-sectional surveys in the Jiangsu Province, China. Int J Environ Res Public Health. 2019;16(19):3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Jiang Z, Shao Z, Sun Q, Shen K.An economic evaluation of adjuvant trastuzumab therapy in HER2-Positive early breast cancer. Value Health. 2009;12(s3):S82-S84. [DOI] [PubMed] [Google Scholar]
- 16.Blackwell K, Gligorov J, Jacobs I, Twelves C.The global need for a trastuzumab biosimilar for patients with HER2-Positive breast cancer. Clin Breast Cancer. 2018;18(2):95-113. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Technical Report: Pricing of Cancer Medicines and Its Impacts: A Comprehensive Technical Report for the World Health Assembly Resolution. World Health Organization; 2018. [Google Scholar]
- 18.Lanting L, Liujie Y.Comparative study of Sino-British cancer drug policies. Chin J Health Policy. 2019;12(2):15-21. [Google Scholar]
- 19.Teng L, Xin HW, Blix HS, Tsutani K.Review of the use of defined daily dose concept in drug utilisation research in China. Pharmacoepidemiol Drug Saf. 2012;21(10):1118-1124. [DOI] [PubMed] [Google Scholar]
- 20.Guan X, Liang H, Xue Y, Shi L.An analysis of China's national essential medicines policy. J Public Health Policy. 2011;32(3):305-319. [DOI] [PubMed] [Google Scholar]
- 21.Barber SL, Huang B, Santoso B, Laing R, Paris V, Wu C.The reform of the essential medicines system in China: a comprehensive approach to universal coverage. J Glob Health. 2013;3(1):010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mossialos WHO, Ge E, Hu Y, Wang J, L.Pharmaceutical Policy in China: Challenges and Opportunities for Reform. World Health Organization. Regional Office for Europe; 2016. [Google Scholar]
- 23.Meng Q, Fang H, Liu X, Yuan B, Xu J.Consolidating the social health insurance schemes in China: towards an equitable and efficient health system. Lancet. 2015;386(10002):1484-1492. [DOI] [PubMed] [Google Scholar]
- 24.Guan X, Zhang Y, Wushouer H, Shi L, Ross-Degnan D, Wagner AK.Differences in reimbursement listing of anticancer therapies in China: an observational study. BMJ Open. 2020;10(1):e031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J.Application and approval of cancer drugs in China: acceleration should be kept in progress. AME Med J. 2018;3:57-57. [Google Scholar]
- 26.Zhou Q, Chen X-Y, Yang Z-M, Wu YL.The changing landscape of clinical trial and approval processes in China. Nat Rev Clin Oncol. 2017;14(9):577-583. [DOI] [PubMed] [Google Scholar]
- 27.Robertson J, Barr R, Shulman LN, Forte GB, Magrini N.Essential medicines for cancer: WHO recommendations and national priorities. Bull World Health Organ. 2016;94(10):735-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gómez-Dantés O, Wirtz VJ, Reich MR, Terrazas P, Ortiz M.A new entity for the negotiation of public procurement prices for patented medicines in Mexico. Bull World Health Organ. 2012;90(10):788-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke PM.Challenges and opportunities for the Pharmaceutical Benefits Scheme. Med J Aust. 2012;196(3):153-154. [DOI] [PubMed] [Google Scholar]
- 30.Villa F, Tutone M, Altamura G, et al. Determinants of price negotiations for new drugs. The experience of the Italian Medicines Agency. Health Policy. 2019;123(6):595-600. [DOI] [PubMed] [Google Scholar]
- 31.Moye-Holz D, van Dijk JP, Reijneveld SA, Hogerzeil HV.The impact of price negotiations on public procurement prices and access to 8 innovative cancer medicines in a middle-income country: the Case of Mexico. Value Health Reg Issues. 2019;20:129-135. [DOI] [PubMed] [Google Scholar]
- 32.The State Council. Guiding opinions of the General Office of the State Council on improving centralized purchasing of drugs for public hospitals. 2015. Accessed June 9, 2022. http://www.gov.cn/gongbao/content/2015/content_2827191.htm
- 33.The National Development and Reform Commission of China. Opinions on promoting drug price reform. 2015. Accessed June 9, 2022. https://www.ndrc.gov.cn/xxgk/zcfb/tz/201505/t20150505_963815.html?code=&state=123
- 34.Li H, Liu GG, Wu J, Wu J-H, Dong C-H, Hu S-L.Recent pricing negotiations on innovative medicines pilot in China: experiences, implications, and suggestions. Value Health Reg Issues. 2018;15:133-137. [DOI] [PubMed] [Google Scholar]
- 35.The State Council. China to cut prices of expensive patent drugs. 2016. Accessed June 14, 2022. http://english.www.gov.cn/news/top_news/2016/05/20/content_281475353689066.htm
- 36.Xu J, Jian W, Zhu K, Kwon S, Fang H.Reforming public hospital financing in China: progress and challenges. BMJ. 2019;365:l4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The State Council. 17 cancer drugs added to insurance program list. 2018. Accessed June 14, 2022. http://english.www.gov.cn/state_council/ministries/2018/10/11/content_281476340430956.htm
- 38.The National Healthcare Security Administration. Interim measures for the administration of use of drugs covered by the basic medical insurance. 2020. Accessed June 14, 2022. http://www.gov.cn/zhengce/zhengceku/2020-08/04/content_5532409.htm
- 39.The State Council. China adds 119 drugs to Medicare reimbursement list. 2020. Accessed June 14, 2022. http://english.www.gov.cn/statecouncil/ministries/202012/28/content_WS5fe9a255c6d0f72576942985.html
- 40.Fan C, Zhao M, Xie Y, Jun L.Discussion on the formation of drug value evaluation and payment standard in healthcare security negotiation. China Health Insur. 2020;2020:11. [Google Scholar]
- 41.Tang M, Song P, He J.Progress on drug pricing negotiations in China. Biosci Trends. 2020;13(6):464-468. [DOI] [PubMed] [Google Scholar]
- 42.Zheng RS, Sun KX, Zhang SW, et al. [Report of cancer epidemiology in China, 2015]. Chin J Oncol. 2019;41(1):19-28. [DOI] [PubMed] [Google Scholar]
- 43.Fang W, Xu X, Zhu Y, Dai H, Shang L, Li X.Impact of the National Health Insurance Coverage Policy on the utilisation and accessibility of innovative anti-cancer medicines in China: an interrupted time-series study. Front Public Health. 2021;9:714127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Wushouer H, Han S, et al. The impacts of government reimbursement negotiation on targeted anticancer medication price, volume and spending in China. BMJ Glob Health. 2021;6(7):e006196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Diao Y, Ye J, Sun J, Jiang Y.The public health insurance coverage of novel targeted anticancer medicines in China-in favor of whom? A retrospective analysis of the insurance claim data. Front Pharmacol. 2021;12:778940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diao Y, Lin M, Xu K, et al. How government health insurance coverage of novel anti-cancer medicines benefited patients in China – a retrospective analysis of hospital clinical data. BMC Health Serv Res. 2021;21(1):856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diao Y, Qian J, Liu Y, et al. How government insurance coverage changed the utilization and affordability of expensive targeted anti-cancer medicines in China: an interrupted time-series study. J Glob Health. 2019;9(2):020702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Vanneste J, Xu J, Liu X.Critical illness insurance to alleviate catastrophic health expenditures: new evidence from China. Int J Health Econ Manag. 2019;19(2):193-212. [DOI] [PubMed] [Google Scholar]
- 49.Ding J, Li J, Yuqing R.New medical insurance access pattern of high-value innovative drugs under multi-level healthcare security system. China Health Insur. 2021;2021:2. [Google Scholar]
- 50.Peng Yunjia. “Difficult to enter the hospital” for medical insurance drugs? “Dual channel” Liangjian enters the hospital for the “last mile”. Xinhua News Agency. 2021. http://www.gov.cn/xinwen/2021-05/10/content_5605635.htm, Accessed June 20, 2022. [Google Scholar]
- 51.Cheng W, Fang Y, Fan D, Sun J, Shi X, Li J.The effect of implementing “medicines zero mark-up policy” in Beijing community health facilities. South Med Rev. 2012;5(1):53-56. [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang B, Ding J, Chen Y, Wei L.Research on the policy motivation and implementation model of “dual channel” management mechanism for national reimbursement negotiation drugs. World Clin Drugs. 2021;42:9. [Google Scholar]
- 53.The National Healthcare Secruity Adminstration. Guidelines on establishing and improving the ‘double channel’ management mechanism for medicines on the NDRL negotiation list. 2021. Accessed June 18, 2022. http://www.nhsa.gov.cn/art/2021/5/10/art_37_5023.html
- 54.Zhang Y, Tan Z, Yajuan L.Implementation status and optimized suggestions of the “Dual-Channel” model for national negotiation drugs——taking S city as an example. Res Health Econ. 2022;39(4):15-18,23. [Google Scholar]
- 55.Fang X, Tian M, Zhang Y, Yin X, Hu J, Dan C.Drugs administration and individual affordability under different medical insurance entry price: example of anti-tumor targeted Medicare drugs. Chin J Health Policy. 2016;9:11. [Google Scholar]
- 56.Tian M, Cui D, Zhang Y, Yin X, Fang X, Jianglin H.Affordability evaluation for 3 kinds of anti-tumor targeted drugs: taking Hubei Province as an example. China Pharm. 2017;12:2746-2749. [Google Scholar]
- 57.Bastani P, Ghanbarzadegan A, Vatankhah S, Samadbeik M.Components affecting pharmaceutical strategic purchasing: a scoping review. Health Serv Insights. 2019;12:1178632919837629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Si L, Xu L, Chen M, Jan S.Using strategic price negotiations to contain costs and expand access to medicines in China. BMJ Glob Health. 2020;5(1):e002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang J, Li Y, Zhang Z, et al. Adoption of Electronic Health Records (EHRs) in China during the past 10 years: consecutive survey data analysis and comparison of Sino-American challenges and experiences. J Med Internet Res. 2021;23(2):e24813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-inq-10.1177_00469580231170729 for Promoting Access to Innovative Anticancer Medicines: A Review of Drug Price and National Reimbursement Negotiation in China by Xia Mingge, Wen Jingyu, Liu Qi, Zheng Zhe and Ran Qing in INQUIRY: The Journal of Health Care Organization, Provision, and Financing
Supplemental material, sj-pdf-2-inq-10.1177_00469580231170729 for Promoting Access to Innovative Anticancer Medicines: A Review of Drug Price and National Reimbursement Negotiation in China by Xia Mingge, Wen Jingyu, Liu Qi, Zheng Zhe and Ran Qing in INQUIRY: The Journal of Health Care Organization, Provision, and Financing