Abstract
Hemophagocytic syndrome (HPS) is a proliferative disease of the mononuclear macrophage system involving multiple organs and systems. We report a 50-year-old Asian woman who presented with unexplained fever and proteinuria. Laboratory tests showed cytopenia, considerably elevated serum ferritin and IL-2 receptor concentrations, and evidence of hemophagocytosis in the bone marrow. A renal biopsy showed macrophage infiltration into the glomerulus, resulting in podocyte and endothelial cell damage. We finally diagnosed the patient with extranodal natural killer/T-cell lymphoma, nasal type that induced HPS-related histiocytic glomerulopathy. Proteinuria and inflammation responded to treatment with high-dose pulsed methylprednisolone combined with VP-16 and cyclosporine. To the best of our knowledge, this is the first documented case of HPS-related histiocytic glomerulopathy triggered by a malignant tumor.
Keywords: Histiocytic glomerulopathy, hemophagocytic syndrome, extranodal natural killer/T-cell lymphoma, proteinuria, podocyte damage, renal biopsy
Introduction
Hemophagocytic syndrome (HPS) is a proliferative disease of the mononuclear macrophage system involving multiple organs and systems. 1 This disease is divided into primary and secondary categories according to its cause. Primary HPS is an autosomal recessive inherited disease that usually occurs in children. Secondary HPS (sHPS) can be observed at any age. HPS mostly occurs secondary to a malignant tumor, viral infection, or serious immune disease, among which malignant tumors, especially lymphomas, are the main causes in adults. Extranodal natural killer (NK)/T-cell lymphoma-associated HPS (ENKTL-LAHPS) is most common in Asia. ENKTL-LAHPS progresses rapidly. Most of these patients die in a short period of time; therefore, the prognosis of patients with this disease is poor. 2 We report the first documented case of extranodal NK/T-cell lymphoma, nasal type that induced sHPS-related histiocytic glomerulopathy.
Case report
A 50-year-old Asian woman presented to a doctor with a sustained high fever for 5 months due to an unknown cause (body temperature fluctuated between 38.5°C and 40.0°C), anemia, proteinuria, fatigue, and anorexia. The patient denied a previous medical or family history. No skin rash, cough, night sweats, nasal congestion, sore throat, abdominal pain, diarrhea, or joint/muscle pain was noted. Because the cause of the fever was unclear, only antipyretic and analgesic drugs were administered to reduce the patient’s temperature.
A physical examination at our hospital showed a body temperature of 39.2°C, heart rate of 111 beats/minute, breathing rate of 18 beats/minute, and blood pressure of 119/53 mmHg. The patient weighed 49.5 kg (11 kg of weight loss compared with 5 months previously). Scattered ecchymosis was observed on the skin of the abdomen and lower back. No palpable swelling of superficial lymph nodes throughout the body or second-degree pitting edema of the lower limbs was observed.
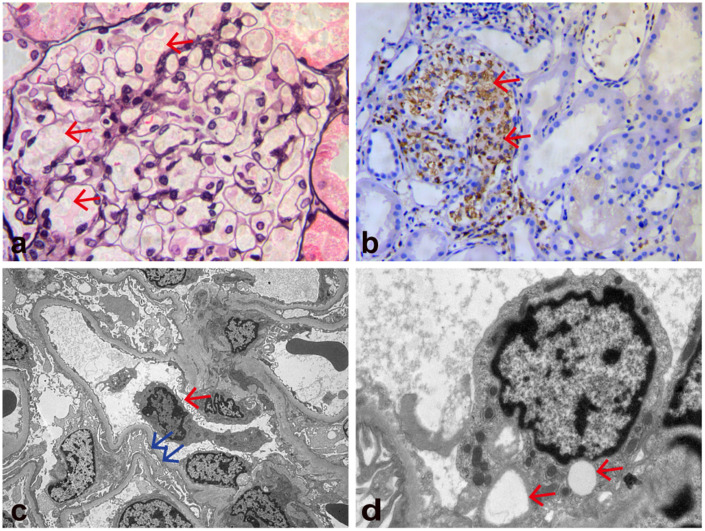
The patient’s initial laboratory data are shown in Table 1. She had obvious anemia, a low white blood cell count, thrombocytopenia, hypoalbuminemia, elevated lactate dehydrogenase, D-dimer, and C-reactive protein concentrations, an elevated erythrocyte sedimentation rate, and considerably elevated soluble interleukin (IL)-2 receptor and ferritin concentrations. Her blood triglyceride concentration, fibrinogen concentration, and kidney function were normal. Her Epstein–Barr virus (EBV) DNA concentration was 8.36 × 106 IU/mL. Other infections were evaluated, and the serology results showed that Mycobacterium tuberculosis, Salmonella typhi, Treponema pallidum, cytomegalovirus, hepatitis virus, and human immunodeficiency virus were negative. Anti-double-stranded DNA antibody, anti-Smith antibody, and anti-neutrophil cytoplasmic antibody were negative. Rheumatoid factor and complement concentrations were normal. A urine test showed protein (3+) and a 24-hour urine protein value of 1.72 g/24 hours. Chest and abdominal computed tomography (CT) showed ascites, pleural effusion, and bilateral renal enlargement (left kidney, 12.7 × 5.4 cm; right kidney, 12.0 × 4.8 cm), and no space-occupying or swelling of the liver, spleen, or lymph nodes. The first bone marrow aspiration showed granulocytic and erythrocytic hyperplasia, without evidence of hemophagocytosis. The patient underwent a kidney biopsy on the fifth day of admission. Light microscopy (Figure 1a, b) showed 21 intact glomeruli, none of which was globally sclerotic. Crescent or necrotizing lesions were not present. The glomeruli were enlarged, and the glomerular capillary loops were filled with a large number of foamy macrophages. The endothelial cells were swollen and showed vacuolar degeneration. All glomeruli had normal cellularity without mesangial, endocapillary, or extracapillary proliferation. No basement membrane thickening, spikes, or double contours were observed under the trichome–periodic acid–Schiff method. Renal tubular epithelial cells showed edema and granular degeneration, and protein casts were visible in the lumen. There was no considerable tubular atrophy, interstitial inflammation, or interstitial fibrosis. Parts of afferent arterioles were considerably expanded, and arcuate arteries and interlobular arteries were normal. Immunofluorescence studies were negative for immune deposits in glomeruli. Immunohistochemistry revealed diffuse CD68-positive cells in the glomerulus, while CD3, CD20, and CD138 were negative. CD68-positive cells were occasionally observed in the renal interstitium. Electron microscopy (Figure 1c, d) showed macrophages in the glomerulus, endothelial swelling, and vacuolar degeneration, as well as effacement of most foot processes. There was no hemophagocytosis or immune-type electron dense deposits.
Table 1.
Initial laboratory findings.
| Parameter | Value | Reference range |
|---|---|---|
| Blood analysis | ||
| White blood cell count (×109/L) | 3.79 | 3.5–9.5 |
| Hemoglobin (g/L) | 72.0 | 115–150 |
| Platelets (×109/L) | 79.0 | 94–268 |
| Lymphocytes (×109/L) | 1.06 | 0.8–4.0 |
| Eosinophils (×109/L) | 0.0 | 0.4–8.0 |
| Serum albumin (g/L) | 19.4 | 40.0–55.0 |
| Lactate dehydrogenase (U/L) | 628.3 | 120.0–250.0 |
| Creatinine (μmol/L) | 36.9 | 41–73 |
| Cystatin C (mg/L) | 1.62 | 0.0–1.26 |
| Total cholesterol (mmol/L) | 1.99 | 3.1–5.72 |
| Triglycerides (mmol/L) | 1.45 | 0.3–1.7 |
| C-reactive protein (mg/L) | 60.24 | 0.114–0.282 |
| Procalcitonin (ng/mL) | 0.19 | 0.0–0.25 |
| Soluble interleukin-2 receptor (pg/mL) | 16584 | <6400 |
| Erythrocyte sedimentation rate (mm/hour) | 94.0 | 0.0–20.0 |
| Ferritin (ng/mL) | 2000.0 | 13–150 |
| Antinuclear antibody | Weakly positive (titer 1:100) | Negative |
| Anti-dsDNA antibody | Negative | Negative |
| Anti-Smith antibody | Negative | Negative |
| Complement C3 (g/L) | 0.82 | 0.79–1.52 |
| Complement C4 (g/L) | 0.35 | 0.16–0.38 |
| Rheumatoid factor (KIU/L) | <20 | 0–30 |
| Antineutrophil cytoplasmic antibody | Negative | Negative |
| Anticardiolipin antibody | Negative | Negative |
| Anti-glomerular basement membrane antibody | Negative | Negative |
| Immunofixation electrophoresis | Negative | Negative |
| Fibrinogen (g/L) | 2.71 | 2.0–4.0 |
| D-dimer (µg/L) | 992.25 | 0.0–232.0 |
| T-SPOT.TB, TB-Ab, TB.DNA | Negative | Negative |
| Widal test and Weil–Felix test | Negative | Negative |
| EBV.DNA (IU/mL) | 8.36 × 106 | Negative |
| Anti-EBV nuclear antigen IgM | Negative | Negative |
| Anti-EBV viral capsid antigen IgM | Negative | Negative |
| Anti-EBV nuclear antigen IgG | Positive | Negative |
| Anti-EBV viral capsid antigen IgG | Positive | Negative |
| Cytomegalovirus DNA | Negative | Negative |
| TORCH test | Negative | Negative |
| Hepatitis B DNA, Hepatitis C RNA, Hepatitis E RNA | Negative | Negative |
| TRUST and HIV | Negative | Negative |
| Blood, urine, stool culture | Negative | Negative |
| Urinalysis | ||
| Urine protein | 3+ | Negative |
| Urine RBCs/HPF | Negative | Negative |
| Urine mALB/urine Cr (mg/g of Cr) | 2121.83 | 0.0–30.0 |
| 24-hour urine protein (g/24 hours) | 1.72 | 0.0–0.1 |
T-SPOT.TB, tuberculosis infection T lymphocyte enzyme-linked immunospot assay; TB-Ab: tuberculosis antibody; TB-DNA: Mycobacterium tuberculosis DNA; EBV, Epstein–Barr virus; Ig, immunoglobulin; TORCH, TO (toxoplasmosis), R (rubella virus), C (cytomegalovirus), and H (herpes simplex virus); TRUST, toluidine red unheated serum test; HIV: human immunodeficiency virus; RBCs, red blood cells; HPF, high-power field; mALB, microalbumin; Cr, creatinine.
Figure 1.
Kidney biopsy findings. (a) Light microscopy shows that the glomeruli are enlarged and the glomerular capillary loops are filled with a large number of foamy cells (red arrows). The endothelial cells are swollen and show vacuolar degeneration (periodic acid-methenamine silver staining; original magnification, ×400). (b) Light microscopy shows granular cytoplasmic staining (CD68 immunohistochemical stain) of abundant intracapillary infiltrating histiocytes and a few interstitial histiocytes (red arrows; original magnification, ×200) and (c, d) Electron microscopy shows macrophages in the glomerulus (red arrow in c), effacement of most foot processes (blue arrows in c), endothelial swelling, and vacuolar degeneration (red arrows in d). No hemophagocytosis or immune-type electron dense deposits were observed (original magnification, c: ×4000; d: ×15,000).
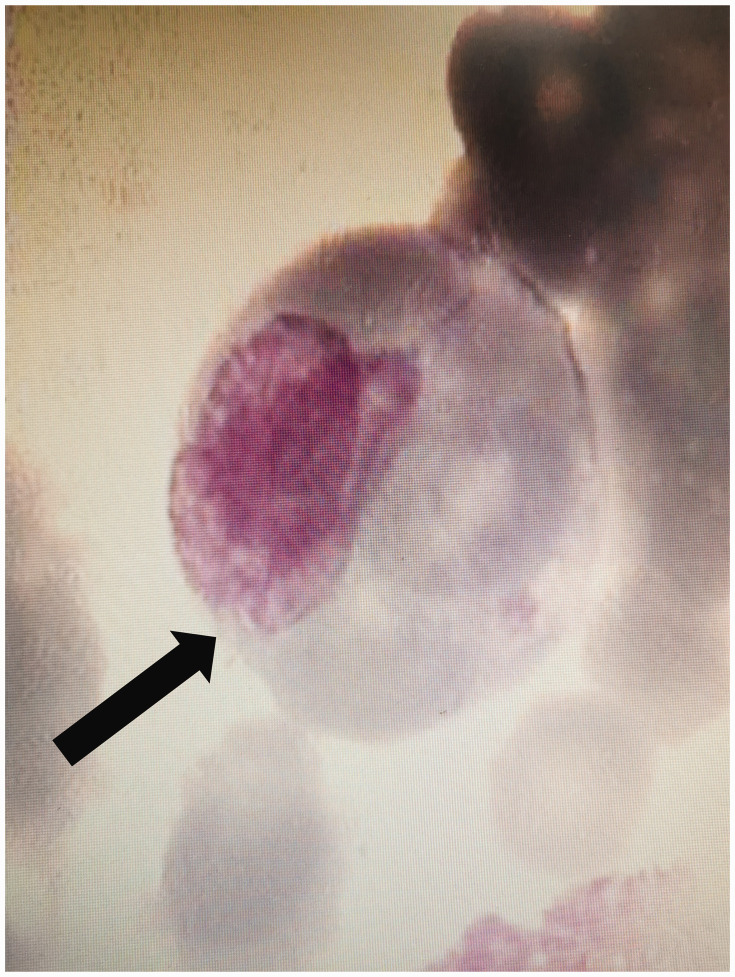
After the patient was admitted to the hospital, her blood cell count progressively decreased. Her white blood cell count was decreased to 2.44 × 109/L, hemoglobin concentration was decreased to 56.0 g/L, and platelet count was decreased to 37.0 × 109/L on the eighth day after admission. At this time, we administered hormone therapy and performed a second bone marrow aspiration. The bone marrow aspirate showed typical hemophagocytic histiocytes that swallowed red blood cells and platelets (Figure 2), but there were no findings of malignancy. She was diagnosed with HPS-related histiocytic glomerulopathy.
Figure 2.
Bone marrow cytology shows hemophagocytes (black arrow).
Further positron emission tomography (PET)/CT showed that the soft tissues of the nasal cavity, and the top and right-sided wall of the nasopharynx were thickened, and fluorodeoxyglucose metabolism was greatly increased. Nasal endoscopy revealed swelling of the nasopharyngeal mucosa with purulent material on the surface. A nasopharyngeal tissue biopsy showed proliferative lesions in lymphoid tissue. Immunophenotyping findings supported a diagnosis of ENKTL, nasal type, stage IV group B. According to the HScore (190) for the diagnosis of hemophagocytic lymphohistiocytosis (HLH) (Table 2) and renal biopsy results, the patient was diagnosed with ENKTL, nasal type-induced sHPS-related histiocytic glomerulopathy.
Table 2.
HScore for the diagnosis of HLH.
| HScore | Value |
|---|---|
| Presence of immunosuppression (no: 0 points; yes: 18 points) | 18 |
| Fever (<38.4°C: 0 points; 38.4–39.4°C: 33 points; >39.4°C: 49 points) | 49 |
| Organomegaly (no: 0 points; splenomegaly or hepatomegaly: 23 points; splenomegaly and hepatomegaly: 38 points) | 0 |
| Elevation in triglyceride concentrations (g/L) (<1.5: 0 points; 1.5–4: 44 points; >4: 64 points) | 0 |
| Elevation in ferritin concentrations (µg/L) (<2000: 0 points; 2000–6000: 35 points; > 6000: 50 points) | 35 |
| Elevation in aspartate aminotransferase concentrations (U/L) (<30: 0 points; ≥30: 19 points) | 19 |
| Low fibrinogen concentrations (g/L) (>2.5: 0 points; ≤2.5: 30 points) | 0 |
| Presence of cytopenia (1 lineage: 0 points; 2 lineages: 24 points; 3 lineages: 34 points) | 34 |
| Hemophagocytosis in a bone marrow aspirate (no: 0 points; yes: 35 points) | 35 |
We administered VP-16 combined with methylprednisolone (0.5 g/day, intravenous pulse for 3 days, followed by 40 mg/day) and cyclosporine (75 mg orally for 1 day) for the initial induction therapy. During treatment, the patient’s body temperature briefly returned to normal. A re-examination of proteinuria showed that it had dropped to a classification of (+), the white blood cell count was increased to 5.07 × 109/L, and the platelet count was increased to 59.0 × 109/L at 14 days after admission. However, the patient developed a high fever again, the platelet count decreased again, and coagulation disorders appeared simultaneously. Changes in the main parameters before and after treatment are shown in Table 3. The patient discontinued treatment owing to financial reasons and died at home 7 days after being discharged from the hospital.
Table 3.
Changes in the main parameters before and after treatment.
| Before treatment |
After treatment |
|||
|---|---|---|---|---|
| Parameters | First day of admission | 8 days after admission | 14 days after admission | Reference range |
| Temperature (°C) | 38.5–40.0 | 36.0–38.0 | 36.0–37.3 | |
| Urine protein | 3+ | 3+ | 1+ | Negative |
| Urine RBCs/HPF | 43 | 45 | 58 | Negative |
| Creatinine (μmol/L) | 36.9 | 38.7 | 40.5 | 41–73 |
| eGFR (mL/min/L) | 120.15 | 118.28 | 116.52 | ≥90 |
| White blood cell count (×109/L) | 3.79 | 2.44 | 5.07 | 3.5–9.5 |
| Hemoglobin (g/L) | 72.0 | 56.0 | 69.0 | 115–150 |
| Platelets (×109/L) | 79.0 | 37.0 | 59.0 | 94–268 |
| Serum albumin (g/L) | 19.4 | 16.8 | 23.7 | 40.0-55.0 |
| Aspartate aminotransferase (U/L) | 39.8 | 166.7 | 20.5 | 15–35 |
| Lactate dehydrogenase (U/L) | 628.3 | 662.2 | 458.3 | 120.0–250.0 |
| C-reactive protein (mg/L) | 60.24 | 51.96 | 26.5 | 0.114–0.282 |
| fibrinogen (g/L) | 2.71 | 2.49 | 2.24 | 2.0–4.0 |
| D-dimer (µg/L) | 992.25 | 743.87 | 251 | 0.0–232.0 |
RBCs, red blood cells; HPF, high-power field; eGFR, estimated glomerular filtration rate.
Discussion
HPS is a relatively rare and life-threatening immune system disease caused by excessive T lymphocyte and macrophage activation. Macrophages engulf various blood cells, while activated T cells secrete excessive inflammatory cytokines, leading to a cytokine storm, which attacks normal tissues and cells and causes multiple organ damage and severe systemic inflammation. In our patient, the HScore was 190 (≥169), which confirmed the diagnosis of HLH. Notably, no hemophagocytes were found in the first bone marrow sampling, but a large number of CD68-positive macrophages had infiltrated renal tissue, which indicated the activation and proliferation of well-differentiated macrophages. We performed continuous bone marrow sampling and observed signs of hemophagocytosis.
Kidney disease with HPS is polymorphic and can affect almost every structure of the kidney, 3 but hemophagocytosis in the kidney has rarely been described.4–7 Acute tubular necrosis with interstitial inflammation is the most common renal histopathological manifestation, and it is found in 45% of patients with HPS. Compared with renal tubular necrosis, HPS-related glomerular involvement confirmed by biopsy is uncommon, including collapsing glomerulopathy, minimal change disease, and thrombotic microangiopathy, especially in African patients with a susceptible genetic background. 4 Eirin et al. introduced the term histiocytic glomerulopathy to describe a case of macrophage activation syndrome and considerable deposition of macrophages in the glomeruli of patients with acute kidney injury. 8 We focused on the existence of macrophages, which are a cell type closely related to the hyperinflammatory state, because they are the central cells of HPS. Macrophages can define the cellular characteristics of glomerulopathy to distinguish HPS-induced glomerulopathy from other causes. We speculate that endothelial and podocyte damage in the present case may have been caused by the release of overexpressed pro-inflammatory cytokines from activated macrophages during HPS. The main clinical manifestations of our patient were bilateral kidney enlargement and moderate proteinuria (1.72 g/24 hours), which suggested glomerular damage. A kidney biopsy showed diffuse macrophage infiltration in the glomerulus, and electron microscopy showed damage to endothelial cells and podocytes (swelling, vacuolar degeneration, and foot process effacement). Because of the limited electron microscopic field of view, although macrophages were observed in the glomerulus, no typical hemophagocytosis was observed. Deposition of tissue cells in the glomeruli in kidney biopsy specimens has also rarely been described in patients with HPS.5,8
After the diagnosis of HPS is established, searching for triggers that may require specific treatment is important. Our patient experienced onset of HPS at an age of 50 years. She had no family history of HPS. Therefore, primary HPS was excluded. An examination excluded rheumatism and solid tumor factors, and bone marrow sampling did not identify a malignant tumor. EBV DNA was greatly elevated, which may have been related to EBV infection, but the possibility of lymphoma, especially extranodal lymphoma, could not be ruled out. We continued a follow-up and then detected swelling of the nasopharyngeal mucosa through PET/CT. After a nasopharyngeal biopsy, the final diagnosis was established. To the best of our knowledge, this is the first documented case of HPS-associated histiocytic glomerulopathy triggered by a malignant tumor. Malignant lymphoma is often difficult to identify as a factor involved in concealing HPS, especially malignant lymphoma with extranodal lesions as the first or main manifestation. PET/CT can help to determine the possible triggers and extent of sHPS. Therefore, an examination of suspicious lesions using both PET/CT and a biopsy to reveal occult disease is recommended. 9
In patients with HPS caused by lymphoma, EBV infection may be a common trigger because viral infection and lymphoma can drive HPS. EBV is a DNA virus of B lymphocytes. EBV can infect T cells or NK cells at high viral loads. Additionally, EBV can evade host immunity, interfere with immune function, cause cellular immune dysfunction, and induce carcinogenesis. EBV can also induce cell proliferation, inhibit cell differentiation and apoptosis, and induce cell immortalization and transformation, as well as other types of carcinogenesis. 10 Moreover, EBV infection causes abnormal activation of CD8+ T lymphocytes and macrophages, resulting in a cytokine storm, involving an increase in IL-1, IL-6, IL-18, tumor necrosis factor-α, chemokines, and other substances. This process causes tissue cell proliferation and phagocytosis of blood cells. 11 Our patient continued to experience fever for 5 months, and the EBV DNA load was greatly elevated, which suggested the presence of chronic active EBV infection. Therefore, in addition to lymphoma, repetition and progression of the patient’s condition were closely related to the important mechanism of EBV in the pathogenesis of LAHPS.
Chronic active EBV (CAEBV) is a rare syndrome of unknown etiology, and is characterized by long-term infectious mononucleosis-like symptoms and the proliferation of B lymphocytes, T lymphocytes, and/or NK cells infected with EBV. CAEBV is common in Asian populations. The clinical course of CAEBV is heterogeneous. 10 Some of these patients have an indolent course that lasts for several years in a stable state, while others have an aggressive course, resulting in a fatal outcome owing to increased hemophagocytic lymphohistiocytosis, multiple organ failure, or progression to leukemia/lymphoma. The extreme heterogeneity in the clinical manifestations and prognosis of CAEBV is a perplexing characteristic. Such heterogeneity may be partly explained by differences in the diagnostic stages of this disease because CAEBV is a progressive disease with various complications throughout its clinical course. If diagnosed in the early stages, CAEBV may appear “lazy” and benign, while if diagnosed at a later stage, a driver mutation may have accumulated in EBV-infected cells, and this disease may appear more malignant and aggressive.
ENKTL-LAHPS treatment includes two aspects of specific treatment for HPS and specific treatment for ENKTL. Generally, treatment for ENKTL-LAHPS has a poor efficacy and a poor prognosis. After the emergence of HPS, the median survival time is only 26 days. 12 In this case, after the diagnosis of HPS was established, we immediately administered hormones, VP-16, and cyclosporine to control the inflammatory cytokine storm according to the HLH-2004 protocol. 9 The patient’s condition became under control, but unfortunately, her condition then worsened again. The reason for her deterioration was not only related to the rapid spread of lymphoma (unproven), but also to HPS. In addition, the prognosis of ENKTL-LAHPS is highly dependent on an early diagnosis and timely treatment. In this case, the diagnosis was not established within 5 months after the disease onset, which led to a delay in treatment. There is no consensus regarding whether lymphoma-directed or HLH-directed regimens should be adopted first for lymphoma-associated HLH. The HLH-94 regimen is still the mainstay of treatment, but >30% of the patients remain unresponsive. A review on malignancy-HLH in adults suggested that once HLH-triggered organ damage occurs, the application of lympholytic agents, such as etoposide, corticosteroids, and polyvalent immunoglobulins, must be considered.13,14 These agents target the cytokine storm and T-cell proliferation. Additionally, the doxorubicin, etoposide, and methylprednisolone regimen as salvage therapy should be considered. The risk factors for mortality of HLH are age, male sex, malignancy, and disseminated intravascular coagulation. High ferritin concentrations, low albumin and hemoglobin concentrations, and a low platelet count have also been described as risk factors for mortality, but the best cut-off value still needs to be determined. 15 A cytokine storm is the main reason of multiorgan failure leading to death in lymphoma-related HPS, and EBV infection itself is an independent prognostic factor. Whether the type of lymphoma (B or T/NK) affects the prognosis is controversial. Some researchers have suggested that the overall prognosis of B-cell lymphoma-associated HLH is better than that of NK/T-cell lymphoma-associated HLH, but studies have shown that the type of lymphoma does not have a significant effect in Cox analysis.15,16
In summary, we present a rare case of histiocytic glomerulopathy caused by ENKTL-LAHPS, which was characterized by a large number of activated macrophages infiltrating the glomeruli, and there was podocyte and endothelial cell damage. ENKTL-LAHPS mostly occurs during the progression or recurrence of ENKTL. This condition is associated with a lack of typical clinical features, low early diagnosis rate, rapid disease progression, and high mortality. In the future, further improvement of the recognition of renal biopsy manifestations to ensure an early diagnosis and treatment of this disease is necessary.
Acknowledgements
We are grateful to the patient and medical staff who participated in this project.
The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Chongqing Municipal Natural Science Foundation (No. cstc2020jcyj-msxmX0013 to Dr Dai).
ORCID iD: Shihui Hou https://orcid.org/0000-0003-4240-0275
Author contributions
The individual contributions of each co-author are listed as follows. Fei Xiao and Huanzi Dai diagnosed and treated the patient. Fei Xiao, Kaizhen Kui, and Shihui Hou collected the data. Xiaoyue Wang and Lihua Bai prepared and stained the kidney tissue. Fei Xiao performed the pathological diagnosis. Huanzi Dai and Shihui Hou edited and submitted the manuscript. All authors read and approved the final manuscript.
Data availability statement
Data are openly available in a public repository.
Ethics statement
This case report was approved by the Ethics Committee of Daping Hospital (ratification number: 2021(111)). A copy of the medical research ethics approval document is available for review by the Editor of this journal and can be provided on request. Written informed consent pertaining to the patient’s treatment was obtained. The patient was deceased before we wrote this article. Therefore, written informed consent for publication was obtained from her husband. The reporting of this study conforms to the CARE guidelines. 17
References
- 1.Yildiz H, Bailly S, Van Den Neste E, et al. Clinical Management of Relapsed/Refractory Hemophagocytic Lymphohistiocytosis in Adult Patients: A Review of Current Strategies and Emerging Therapies. Ther Clin Risk Manag 2021; 17: 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu YZ, Bi LQ, Chang GL, et al. Clinical characteristics of extranodal NK/T-cell lymphoma-associated hemophagocytic lymphohistiocytosis. Cancer Manag Res 2019; 11: 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karras A.What nephrologists need to know about hemophagocytic syndrome. Nat Rev Nephrol 2009; 5: 329–336. [DOI] [PubMed] [Google Scholar]
- 4.Thaunat O, Delahousse M, Fakhouri F, et al. Nephrotic syndrome associated with hemophagocytic syndrome. Kidney Int 2006; 69: 1892–1898. [DOI] [PubMed] [Google Scholar]
- 5.Santoriello D, Hogan J, D'Agati VD.Hemophagocytic Syndrome With Histiocytic Glomerulopathy and Intraglomerular Hemophagocytosis. Am J Kidney Dis 2016; 67: 978–983. [DOI] [PubMed] [Google Scholar]
- 6.Hiser W, Landgarten M, Zhou XJ, et al. Hemophagocytic syndrome with histiocytic glomerulopathy associated with ovarian serous carcinoma. Proc (Bayl Univ Med Cent) 2020; 34: 153–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dokouhaki P, Van der Merwe DE, Vats K, et al. Histiocytic Glomerulopathy Associated With Hemophagocytic Lymphohistiocytosis. Kidney Med 2021; 4: 100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eirin A, Irazabal MV, Fervenza FC, et al. Histiocytic glomerulopathy associated with macrophage activation syndrome. Clin Kidney J 2015; 8: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019; 133: 2465–2477. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara S, Nakamura H.Chronic Active Epstein-Barr Virus Infection: Is It Immunodeficiency, Malignancy, or Both? Cancers (Basel) 2020; 12: 3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsh RA.Epstein-Barr Virus and Hemophagocytic Lymphohistiocytosis. Front Immunol 2018; 8: 1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia J, Song Y, Lin N, et al. Clinical features and survival of extranodal natural killer/T cell lymphoma with and without hemophagocytic syndrome. Ann Hematol 2016; 95: 2023–2031. [DOI] [PubMed] [Google Scholar]
- 13.Pi Y, Wang J, Zhou H, et al. Modified DEP regimen as induction therapy for lymphoma-associated hemophagocytic lymphohistiocytosis: a prospective, multicenter study. J Cancer Res Clin Oncol 2022. doi: 10.1007/s00432-022-04157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng G, Wang Y, Wang J, et al. The DEP regimen is superior to the HLH-1994 regimen as first-line therapy for lymphoma-associated haemophagocytic lymphohistiocytosis. Leuk Lymphoma 2021; 62: 854–860. [DOI] [PubMed] [Google Scholar]
- 15.Yildiz H, Castanares-Zapatero D, D'Abadie P, et al. Hemophagocytic Lymphohistiocytosis in Adults: A Retrospective Study in a Belgian Teaching Hospital. Int J Gen Med 2022; 15: 8111–8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Y, Wang J, Wang Y, et al. Requirement for containing etoposide in the initial treatment of lymphoma associated hemophagocytic lymphohistiocytosis. Cancer Biol Ther 2021; 22: 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagnier JJ, Kienle G, Altman DG, et al. ; CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are openly available in a public repository.