Abstract
Background and Aims
The evolution of mating systems from outcrossing to self-fertilization is a common transition in flowering plants. This shift is often associated with the ‘selfing syndrome’, which is characterized by less visible flowers with functional changes to control outcrossing. In most cases, the evolutionary history and demographic dynamics underlying the evolution of the selfing syndrome remain poorly understood.
Methods
Here, we characterize differences in the demographic genetic consequences and associated floral-specific traits between two distinct geographical groups of a wild shrub, Daphne kiusiana, endemic to East Asia; plants in the eastern region (southeastern Korea and Kyushu, Japan) exhibit smaller and fewer flowers compared to those of plants in the western region (southwestern Korea). Genetic analyses were conducted using nuclear microsatellites and chloroplast DNA (multiplexed phylogenetic marker sequencing) datasets.
Key Results
A high selfing rate with significantly increased homozygosity characterized the eastern lineage, associated with lower levels of visibility and herkogamy in the floral traits. The two lineages harboured independent phylogeographical histories. In contrast to the western lineage, the eastern lineage showed a gradual reduction in the effective population size with no signs of a severe bottleneck despite its extreme range contraction during the last glacial period.
Conclusions
Our results suggest that the selfing-associated morphological changes in D. kiusiana are of relatively old origin (at least 100 000 years ago) and were driven by directional selection for efficient self-pollination. We provide evidence that the evolution of the selfing syndrome in D. kiusiana is not strongly associated with a severe population bottleneck.
Keywords: Daphne kiusiana, demographic dynamics, directional selection, evergreen shrub, inflorescence, NGS technique, selfing syndrome
INTRODUCTION
Most flowering plants have perfect flowers in where the anthers and stigmas of a single sporophyte are held in close spatiotemporal proximity (Busch and Delph, 2012). Although seemingly ordinary, these arrangements allow for a flexible mating system [outcrossing, self-fertilization (hereafter ‘selfing’] or a combination of both) that can induce strategic decisions to develop offspring in response to variations in environmental factors (Lloyd, 1979; Barrett and Eckert, 1990; Pannell and Barrett, 1998). Selfing has advantages that may be favourable in two circumstances. First, selfing can provide reproductive assurance when pollinators and/or potential mates are limited (Darwin, 1876; Eckert et al., 2006). Second, plants that undergo selfing also harbour improved colonization ability, which can act when migrating to a new habitat (Baker, 1955; Foxe et al., 2009). In general, selfing is considered a mating strategy that can be substituted for outcrossing when its benefits outweigh the costs of inbreeding depression (Lloyd, 1979, 1992; Busch and Delph, 2012).
The selfing species/lineages derived from outcrossing ancestors are often accompanied by a common set of morphological and functional changes to the flowers, termed the ‘selfing syndrome’ (Ornduff, 1969; Sicard et al., 2011). Compared with their outcrossing sister taxa, selfers generally tend to have smaller flowers that narrow the spatial separation between anthers and stigmas, which appears to be effective for self-pollination (Takebayashi et al., 2006; Slotte et al., 2012). Consequently, a mating system shift toward selfing has occurred independently in many plants (Stebbins, 1974; Grant, 1981; Barrett, 2002), and it has been followed by very similar changes in floral morphology and function (Woźniak et al., 2020). It is therefore conceivable that the concerted flower changes for a given species/lineage have been driven by persistent natural selection, even if such advantages are only in the short term (Sicard et al., 2011, 2016; Slotte et al., 2012; Woźniak et al., 2020). Such floral characteristics are thought to partly reflect diverse ecological scenarios that have driven the evolution of the selfing lineage (Runions and Geber, 2000). Nevertheless, our knowledge of the historical factors leading to these morphological changes remains limited. A better understanding of the evolution of the selfing syndrome requires insight into the population establishment histories associated with Quaternary climatic oscillations, which have played a major role in changing the geographical distribution of plant species (Comes and Kadereit, 1998; Qiu et al., 2011).
A few population-scale genetic analyses have been informative regarding the demographic histories associated with the evolution of the selfing syndrome. Studies have suggested that the transition to selfing of Capsella rubella was driven preliminarily by a severe population bottleneck within the last 50 000 years (Foxe et al., 2009; Guo et al., 2009). Subsequently, its morphological modifications are thought to have evolved relatively rapidly during the geographical expansion after the Last Glacial Maximum (Foxe et al., 2009; Guo et al., 2009). These inferences can lead to the perception that the selfing syndrome is not adaptive since it is associated with random genetic drift on a relatively short evolutionary timescale. However, a strong genetic drift can occur regardless of the evolution of mating system or after such flower changes in selfing lineages (Sicard et al., 2011). The relationship between the evolution of the selfing syndrome and historical demographic change remains ambiguous. To gain keen insights, it is necessary to account for the demographic details of more species associated with evolution of the selfing syndrome.
Daphne kiusiana is an evergreen broad-leaved shrub endemic to East Asia (Murata, 1999; Lee, 2003; Wang et al., 2007) that has perfect flowers and is capable of spontaneous selfing (Huang, 2017). Two varieties of this species have been recognized – var. kiusiana (distributed in Korea and Japan) and var. atrocaulis (found in China and Taiwan). The former has small flowers with calyx tubes 7–8 mm long, whereas the latter has slightly larger flowers with calyx tubes 10–14 mm long (Murata, 1999; Lee, 2003; Wang et al., 2007). However, within var. kiusiana, the floral sizes (calyx tubes 12–22 mm long) in the populations on offshore islands of southwestern Korea (hereafter referred to as western populations) are much larger than those in southeastern Korea and Kyushu, Japan (referred to as eastern populations) (Lee et al., 2013a; Lee and Oh, 2017). These morphological patterns are typical for selfing lineages and their outcrossing ancestors. Indeed, in addition to the characteristics of the selfing syndrome mentioned above, our field observations show that plants in the western populations have many more flowers per inflorescence than those in the eastern populations, with 5–12 flowers per inflorescence (Fig. 1).
Fig. 1.
Comparison of floral morphological characteristics according to the geographical division of Daphne kiusiana. The western populations show more and larger flowers, whereas the eastern populations show fewer and smaller flowers. The longitudinal sections of the flowers illustrate that the levels of herkogamy are significantly different between the western and eastern populations. (A) inflorescences; (B) habitat; (C) longitudinal section of the flower.
Selfing leads to increased homozygosity through the fixation of alleles at gene loci and reduces effective recombination (Nordborg and Donnelly, 1997; Yang et al., 2009; Manzanares et al., 2016). Therefore, we expected to observe a loss of genetic diversity (Wright et al., 2013), an increase in genetic differentiation (Duminil et al., 2009; Voillemot and Pannell, 2017) and differences in the floral traits within the eastern populations (with smaller flowers) compared to the western populations. Genotyping using microsatellite markers offers an ideal technique for testing these expectations, especially when estimating selfing rates (Voillemot and Pannell, 2017; Zhong et al., 2019; Gasca-Pineda et al., 2020) and lineage divergence times (Tamaki et al., 2019). In addition, maternally inherited chloroplast DNA (cpDNA) reflects past seed gene flow (Ennos et al., 1999; Calviño-Cancela et al., 2012). Therefore, using cpDNA as an auxiliary tool, it is possible to address the historical geographical influence of distribution changes. With advances in next-generation sequencing technology, it is now possible to produce large datasets cost-effectively at the population level. Recently, a multiplexed phylogenetic marker sequencing (MPM-seq) technique for large data generation has been developed to obtain a good understanding of plastid genetic information (Suyama et al., 2021). Therefore, here we produced the chloroplast sequences using the MPM-seq method with minor modification and provide the detailed methods.
To our knowledge, the majority of previous population-level studies related to the evolution of the selfing syndrome have been of herbaceous plants. In this study, we aim to elucidate the evolutionary history of a wild shrub, D. kiusiana, with subcapitate inflorescences focusing on genetic characteristics, palaeodistribution, historical population demography and floral morphological traits between the two geographical populations (west vs. east). We address the following specific questions: (1) What differences do the genetic makeups of these two geographical groups exhibit? (2) What factors promoted the evolution of the eastern populations with inconspicuous (visually impoverished) floral traits that have probably been induced by selfing? (3) How are the demographic signatures of the group with these characteristic flowers exhibited? We characterize the genetic composition of D. kiusiana distributed in Korea and Japan using nuclear microsatellites and multilocus-based cpDNA datasets, and examine the associated variations in morphological floral traits. Finally, we employ ecological niche modelling (ENM) and approximate Bayesian computation (ABC) to gain further insights into the historical migration patterns and demographic details of these populations. Our study provides evolutionary insights into how selfing affects the sustainability and adaptive potential of wild plant populations.
MATERIALS AND METHODS
Study species and population sampling
Daphne kiusiana Miq. is commonly used as an ornamental plant because of its graceful inflorescence shape (resembling a bride’s bouquet) and pleasant fragrance (Ro et al., 2010). In Korea, the populations are now extremely restricted to a few islands, and the plants are being managed as an endangered species (Korea National Arboretum, 2008). In comparison, Japanese plants are widely distributed throughout the southern region. Flowering in Korea and Japan occurs from winter to early spring between January and April (Murata, 1999; Lee, 2003). Approximately 90 d after fertilization, the berries mature, and the seeds are then dispersed by birds (Aoki et al., 2004). In garden conditions, seed production usually commences when the plants are 5–6 years old (S. H. Yang, Halla Arboretum, Republic of Korea; pers. comm.). However, because most wild seeds have a dormancy of several years before germination (Alonso and Herrera, 2001; Fang et al., 2016), the life cycle of D. kiusiana is thought to span a period of ~10 years.
Our study was conducted on almost all known populations of D. kiusiana in Korea and a significant part of Japan, which is sufficient to address the proposed issues. We collected 237 leaf samples from five populations in Korea and four populations in Japan. We selected individuals at least 5 m apart to avoid collecting material from relatives, and collected one leaf sample per individual to minimize damage. The collected leaf samples were dried in silica gel and stored at −70 °C in the laboratory of Biological Education, Chonnam National University (BEC), until use. Total genomic DNA was extracted from dried leaf samples using the DNeasy Plant Mini Kit (Qiagen, Seoul, Korea) following the manufacturer’s instructions. The concentration of extracted DNA was determined using a Nano-300 microspectrophotometer (Allsheng, Hangzhou, China) and diluted to 15 ng µL−1 to obtain the same concentration of template DNA in each sample for analysis.
Microsatellite genotyping
We used 16 nuclear microsatellite markers (DKi020, DKi021, DKi022, DKi023, DKi062, DKi070, DKi072, DKi073, DKi082, DKi091, DKi101, DKi104, DKi117, DKi128, DKi129 and DKi131) previously developed for D. kiusiana (Lee et al., 2017). PCR was performed using a Veriti 96-well thermal cycler (Applied Biosystems, Foster City, CA, USA) in a final volume of 5 µL containing 15 ng of extracted DNA, 2.5 µL Multiplex PCR Master Mix (Qiagen, Valencia, CA, USA), 0.01 µm forward primer, 0.2 µm reverse primer and 0.1 µm of the M13 primer (fluorescently labelled). PCR amplification was performed as follows: initial denaturation at 95 °C for 15 min; 35 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 1.5 min and extension at 72 °C for 1 min; and final extension at 72 °C for 10 min. The PCR products were diluted with ddH2O (1:30), and 1 µL was analysed on an ABI 3730XL sequencer with GeneScanTM-500LIZTM Size Standard (Applied Biosystems). Allele sizes and peaks for each sample were determined in triplicate using Peak Scanner 2 software (Applied Biosystems) to minimize genotyping errors.
Before analysing the microsatellite data, we estimated the null allele frequency using INEst (inbreeding/null allele estimation) software, which calculates the null allele frequency regardless of the effect of inbreeding (Chybicki and Burczyk, 2009) by running a Markov chain Monte Carlo (MCMC) analysis (number of cycles = 200 000; every nth update = 200; burn-in = 2000). This showed that the null allele frequency of all the loci examined ranged from 0.004 % to 0.015 %. Therefore, we used 16 microsatellite markers for statistical analysis.
To ensure that the number of loci was sufficient to analyse the genotype of each individual in the samples, a genotype accumulation curve was applied using the ‘poppr’ (v.2.0.2) package in R (Kamvar et al., 2014). This curve shows the ability to discriminate between unique genotypes using an increasing number of molecular markers (Kamvar et al., 2015; Capador et al., 2018; Inoue et al., 2021). The genotype accumulation curve showed that the full recognition (100 %) of all the studied multilocus genotypes could be achieved using 15 loci, and confirmed that 16 loci were sufficient for the identification of individuals (Supplementary Data Fig. S1). We then determined the number of different multilocus genotypes (MLGs) and sorted the homozygous MLGs (HMLGs) that were homozygous at all loci by referring to the raw data.
Genetic diversity and selfing rate estimation
Genetic diversity parameters were evaluated for each locus based on simple sequence repeat (SSR) allele frequency data using GenAlEx 6.5 (Peakall and Smouse, 2006). These included the number of alleles (NA), number of private alleles (PA), observed heterozygosity (HO), expected heterozygosity (HE) and inbreeding coefficient (FIS). Allele richness (AR) and genetic differentiation among populations (FST) were determined by calculating the overall fixation index according to the method of Weir and Cockerham (1984) using FSTAT 1.2 (Goudet, 1995). The significance of genotypic population differentiation at each locus and over loci was tested using the log-likelihood-based (G)-based exact test (Goudet et al., 1996) in FSTAT. The values of the genetic diversity parameters were then averaged across the population within each group (hereafter ‘lineage’; see details in the Results). Additionally, we calculated genetic diversity parameters at the pooled lineage level.
To estimate selfing rates at the pooled lineage level, we employed ‘RMES’ software (robust multilocus estimation of selfing rates). This technique is relatively insensitive to null alleles and scoring errors, which may otherwise lead to the overestimation of selfing rates (see David et al., 2007). In addition, empirical studies have confirmed that this method is reliable for estimating selfing rates (Bürkli et al., 2017). We estimated the selfing rates using two methods implemented in RMES. First, the g2 method uses two-locus heterozygosity disequilibrium values and, second, the maximum-likelihood (ML) method maximizes the log-likelihood of the multilocus heterozygosity structure of a sample. As a significance test for selfing rate associated with the g2 method, RMES computes the probability that there is no selfing (g2 = s = 0) obtained from 10 000 iterations of a random assortment of single-locus heterozygosities among individuals. A 95 % confidence interval (CI) for selfing rate provided by RMES was used to determine the significance of selfing rate associated with the ML method.
Enrichment and sequencing for multilocus cpDNA
Detailed methods are provided in Supplementary Data Method S1.
We targeted 27 non-coding regions of cpDNA using MPM-seq for all samples. This method can provide noteworthy information faster and more cost-effectively in phylogenetic analyses based on independent sources of genomic data (Suyama et al., 2021). Notably, we made several modifications to this method as described in Supplementary Data Method S1 with Figs S2 and S3. Finally, we sorted 16 high-productivity regions and used these sequences for analysis (Table S1). The raw reads and haplotype sequences were deposited in GenBank under BioProject ID PRJNA794292 and accession numbers SRX13610178–SRX13610414.
Genetic structure analysis and phylogenetic inference
For microsatellite analysis, we used a Bayesian clustering approach implemented in STRUCTURE 2.3 (Pritchard et al., 2000) using 1 000 000 MCMC iterations (100 000 burn-in with admixture). The simulation used 20 iterations with K = 1 to K = 9 clusters. The optimal number of clusters, K, was determined via the K method, using ‘STRUCTURE HARVESTER’ (Earl and vonHoldt, 2012). CLUMPP v.1.1.2 (Jakobsson and Rosenberg, 2007) with a greedy algorithm was used to combine the membership coefficient matrices (Q-matrices) from 1000 iterations for K = 2 using random input orders. We also conducted a principal coordinate analysis (PCoA) to determine the genetic structure using the covariance-standardized approach of pairwise Nei’s genetic distances in GenAlEx 6.5. To identify genetic boundaries between the populations, we performed a barrier analysis (Manni et al., 2004) based on Monmonier’s algorithm (1973) with 1000 bootstrap matrices of pairwise genetic distances (Nei and Tajima, 1983) that were calculated using ‘MICROSATELLITE ANALYZER’ (MSA) v.4.05 (Dieringer and Schlötterer, 2003). A neighbour-joining (N-J) tree for the populations was constructed using PHYLIP 3.68 (Felsenstein, 2004) based on Nei’s chord distance (DA) and the proportion of shared alleles (Dps). Pairwise genetic distances (DA and Dps) were generated with MSA software using a bootstrap analysis of 1000 replicates.
For cpDNA analysis, a parsimony haplotype network was constructed using TCS v.1.21 (Clement et al., 2000) with a 99 % connection limit. All indels and inversions were treated as single-point mutations. To investigate the phylogenetic relationships between D. kiusiana, including var. atrocaulis (MT627481), an ML phylogenetic tree was constructed using Geneious R11.0.5 (Biomatters Ltd, Auckland, New Zealand). We considered two closely related species, Daphne depauperata (MW245833) and D. acutiloba (MW246150), as the outgroups (Kim et al., 2021). Additional data were downloaded from the National Center for Biotechnology Information (NCBI; https://www.ncbi.nlm.nih.gov/). Alignments were performed using ‘MAFFT’ (Katoh and Toh, 2010). The ML analysis was performed using RAxML v.8.2 (Stamatakis, 2014) using default parameters and 1000 bootstrap replicates. For the RAxML tree, the general time-reversible (GTR) model of nucleotide substitution was used along with the gamma model of rate heterogeneity.
Inference of population demography
To clarify the population size change and divergence histories of the two lineages detected by the structure analysis, an ABC approach was used. ABC enabled us to compare population demographic models and estimate their parameters (Beaumont, 2019). First, we constructed three population size change models, namely a standard neutral model (SNM), population growth model (PGM) and size reduction model (SRM; Supplementary Data Fig. S4a). Then, using the information obtained from the population size change analyses (see details in the Results), three population divergence models (DM1–3) were constructed (Fig. S4b). As the two lineages did not share their ancestries estimated by the structure analysis and cpDNA haplotypes, we did not consider migration between lineages in order to simplify the hypotheses. We used 16 SSR loci datasets, which were converted from fragment size to repeat number prior to the analyses, and calculated the following summary statistics: the averages and standard deviations of the number of alleles, expected heterozygosity, and the allele size range for population size change analyses of both lineages. For the population divergence analysis between the two lineages, in addition to these summary statistics, we calculated the averages of the overall number of alleles, expected heterozygosity, allele size range and FST, meaning a total of 16 summary statistics were obtained. The summary statistics were calculated using Arlsumstat v.3.5.2 (Excoffier and Lischer, 2010).
Each model was simulated 104 times with ‘fastsimcoal2’ v.2.6.0.3 (Excoffier and Foll, 2011), and summary statistics were calculated using Arlsumstat. Inbreeding was considered by including the observed fixation index value in the input file of fastsimcoal2. A generalized stepwise mutation model (GSM) was used for the SSR mutation model (Estoup et al., 2002). The average mutation rate among loci was set to 5 × 10−4 considering the range of mutation rates of SSR, i.e. from 5 × 10−5 to 5 × 10−3 (Estoup and Angers, 1998; Estoup et al., 2002; Marriage et al., 2009). GSM was implemented as described by Setsuko et al. (2020). All prior distributions of the parameters were generated using R v.4.1.2 (R Core Team, 2021) and their ranges are summarized in Supplementary Data Table S2. Models were compared using the ABC-random forest (RF) approach with 1000 decision trees implemented in the ‘abcrf’ v.1.8.1 package in R (Pudlo et al., 2016).
Using the best model selected by ABC-RF, 2 × 105 simulations were repeated, and the nearest 1000 summary statistic values to the observed values were used for the parameter estimation. We used a neural net regression implemented in the ‘abc’ v.2.1 package in R (Blum and François, 2010; Csilléry et al., 2012). The logit transformation option was used to maintain the estimated parameter values within their ranges. Posterior mode and 95 % highest posterior density (HPD) were estimated using the ‘density’ function in R and the ‘coda’ v.0.19.4 package in R (Plummer et al., 2006), respectively. To convert the timescale from generations ago to years ago, 10 years per generation was used considering the longevity of the target species. By plotting the mode and 95 % HPD over time, the trajectory of population size change for each lineage was obtained (Lu et al., 2020). Using 1000 randomly drawn posteriors, a predictive simulation was carried out, and predictive values were compared with the observed values to confirm the goodness-of-fit of the model to the observed data (Gelman et al., 2014).
Ecological niche modelling
We developed a model of the current and historical potential distributions of D. kiusiana using Maxent 3.4.4 (Merow et al., 2013). Occurrence data were obtained from the Global Biodiversity Information Facility (GBIF; https://www.gbif.org/), including D. kiusiana var. atrocaulis (Rehder) F. Maek., and the sample localities in our investigation. A total of 452 occurrence points were spatially filtered with 25 km between each point using SDMtoolbox 2.4 (Brown et al., 2017) to decrease bias when modelling the potential distributions. We used 19 bioclimatic variables (Online Resource 2) obtained for two periods [the present and Last Glacial Maximum (LGM)] from the climatologies at high resolution for the Earth’s land surface areas (CHELSA) dataset (http://chelsa-climate.org; Karger et al., 2017). The bioclimatic variables of the LGM are based on three general circulation models (GCMs) – the Community Climate System Model (CCSM4; Gent et al., 2011), the Earth System Model based on the Model for Interdisciplinary Research on Climate (MIROC-ESM; Watanabe et al., 2011) and the Max Planck Institute for Meteorology Earth System model (MPI-ESM-P). The bioclimate data for 20–45°N and 100–145°E (30 arc-second resolution) were extracted using QGIS 3.16.6. To avoid multicollinearity, we excluded one of the bioclimate variables sharing a high Spearman correlation coefficient (>0.8) using SDMtoolbox 2.4. Therefore, seven of the 19 variables were selected and used to develop the model. To reduce the uncertainty in previous climate models, the LGM distributions from the three GCMs were averaged. Prior to the Maxent runs in batch mode, response curves, jackknife tests, 20 replicates, cloglog output, random seeds, 10 000 background points and 2000 iterations were used. After developing the distribution model, this was projected onto the other two periods of the LGM to estimate the past distribution, and we averaged the LGM distributions of the three GCMs.
Morphological measurements and statistical analysis
To evaluate the effectiveness of the mechanism to control variability in levels of outcrossing, we explored the floral morphological traits of D. kiusiana. A total of 117 samples from eight populations (80 in the west and 37 in the east) were collected during the peak flowering season from 2016 to 2022 (mainly during February and March 2022). During the field survey, the number of flowers was counted by randomly selecting one inflorescence on each individual. Then, we cut the branched stems, including the inflorescences, and brought them to the laboratory at Chonnam National University for subsequent measurements. For each sample, we imaged the longitudinal sections of the flowers using a digital camera (MFX1600; Nahwoo, South Korea) connected to a stereomicroscope (Olympus SZ61; Olympus, Japan). The length and width of the calyx lobe, length of the calyx tube, length of the pistil, and minimum distance between the stigma and anthers were measured using digital calipers built into iworks 2.0 software (Nahwoo) (Supplementary Data Fig. S5). Samples were prepared as voucher specimens by press drying or preserving in 70 % ethanol, and then stored in the National Forest Seed Variety Center of Korea Forest Service (Voucher Nos. NFSV-22-Dk001–110) and the Korea Institute of Oriental Medicine (Voucher Nos. KIOM-19-Dk001–007). Measurements for seven of the samples from the Kyushu population were obtained from dried specimens using the same method as described. All the related images are provided in Supplementary Data Morphological dataset S1.
We compared differences in floral traits related to visibility and herkogamy between the two lineages (west and east). The significance of the differences between the two lineages was confirmed by a statistical F test in one-way ANOVA. We also examined the correlations between flower traits and herkogamy in the pooled samples. The degree of herkogamy was estimated as the distance between the shortest stigma and the anther, as their stamens generally consist of two pairs of four. The area of the calyx lobe was roughly estimated by half the length × width. All statistical analyses and significance tests were performed using R v.4.1.2 (R Core Team, 2021).
RESULTS
Genetic diversity and selfing rate
Significant differences in genetic diversity were observed between the western and eastern populations (Table 1).
Table 1.
Genetic variation based on 16 microsatellite loci in nine populations of Daphne kiusiana.
| Pop. ID | Location | N | G type | HOMO MLG (no.) | R Homo | N A | A R | P A | H O (SE) | H E (SE) | F IS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LI | West | ||||||||||
| k-SBI | Sinan-gun, Jeollanam-do | 21 | 21 | – | – | 41 | 2.563 | 7 | 0.235 (0.059) | 0.356 (0.057) | 0.314* |
| k-JSI | Sinpyeong-ri, Jeju | 25 | 25 | – | – | 48 | 2.920 | 3 | 0.358 (0.046) | 0.398 (0.038) | 0.146* |
| k-JCH | Cheongsu-ri, Jeju | 30 | 30 | – | – | 42 | 2.471 | 3 | 0.360 (0.043) | 0.387 (0.038) | 0.062 |
| k-JSE | Seonheul-ri Jeju | 28 | 28 | – | – | 35 | 2.120 | 0 | 0.283 (0.045) | 0.312 (0.046) | 0.093 |
| Population mean | 26.0 | 26.0 | – | – | 41.5 | 2.518 | 3.3 | 0.309 (0.025) | 0.363 (0.022) | 0.147 | |
| Pooled lineage | 104 | 104 | – | – | 65 | 4.063 | 43 | 0.314 (0.036) | 0.484 (0.031) | 0.374 | |
| LII | East | ||||||||||
| k-GWA | Geoje-si, Gyeongsangnam-do | 22 | 1 | H 1 (22) | 1.000 | 16 | 1.000 | 1 | 0.000 (0.000) | 0.000 (0.000) | NA |
| j-SKA | Karatsu-shi, Saga Pref. | 22 | 17 | H 2 (3) | 0.136 | 26 | 1.617 | 3 | 0.071 (0.034) | 0.153 (0.050) | 0.384* |
| j-KYA | Yatsushiro-shi, Kumamoto Pref. | 30 | 8 | H 3 (15), H4 (7), H5 (2), H6 (2) | 0.867 | 22 | 1.363 | 4 | 0.000 (0.000) | 0.067 (0.033) | 1.000* |
| j-MNO | Nobeoka-shi, Miyazaki Pref. | 33 | 17 | H 7 (12), H8 (3), H9 (3), H10 (2) | 0.606 | 26 | 1.570 | 2 | 0.021 (0.008) | 0.137 (0.047) | 0.745* |
| j-MKO | Kobayashi-shi, Miyazaki Pref. | 26 | 16 | H 11 (4), H12 (3), H13 (3), H14 (2), H15(2), H16(2) | 0.615 | 25 | 1.560 | 4 | 0.012 (0.006) | 0.120 (0.054) | 0.901* |
| Population mean | 26.6 | 11.8 | 0.645 | 23.0 | 1.422 | 2.8 | 0.021 (0.007) | 0.095 (0.019) | 0.691 | ||
| Pooled lineage | 133 | 59 | 0.654 | 50 | 3.074 | 28 | 0.019 (0.007) | 0.301 (0.081) | 0.539 | ||
N, number of individuals; Gtype, number of genotypes; HOMOMLG, homozygous multilocus genotypes; RHomo, ratio of homozygous individuals at all loci within a population; NA, number of alleles; AR, allelic richness; PA, number of private alleles; HO, observed heterozygosity; HE, expected heterozygosity; SE, standard error; FIS, inbreeding coefficient; NA, not available.
*Significant deviation of FIS from zero (P < 0.01).
Genetic diversity was lower in the eastern lineage (AR = 1.422, HE = 0.095) than in the western lineage (AR = 2.518, HE = 0.363). Similarly, the genetic diversity of the pooled eastern lineage (AR = 3.074, HE = 0.301) was lower than that of the pooled western lineage (AR = 4.063, HE = 0.484) (Fig. 2A).
Fig. 2.
Comparison of genetic diversity between the two lineages of Daphne kiusiana. (A) Boxplots showing mean allelic richness (AR), expected heterozygosity (HE) and haplotype diversity (Hd). The mean within-population values for each lineage are shown as red lines. Each value of the pooled lineages is marked with an asterisk. (B) Histogram showing the observed heterozygosity (HO) at the level of individuals for each lineage. LI, lineage I (west); LII, lineage II (east).
Based on the genotyping of 237 individuals, 87 were homozygous at all loci and they consisted of 16 MLGs (homozygous multilocus genotypes, hereafter ‘HMLGs’). HMLGs occurred only in the eastern lineage, where one or a few genotypes dominated each population (0.136–1.000), and the observed heterozygosity was extremely low (Fig. 2B). In addition, none of the HMLGs in the eastern lineage were shared across populations. By contrast, in the western lineage, all individuals had different genotypes, and HMLGs did not appear. FST values evaluated between the populations within each lineage were higher in the east (mean = 0.712) than in the west (mean = 0.291), and were significant over all loci (P < 0.001) (Table 2).
Table 2.
Summary statistics of selfing rate and genetic differentiation. The selfing rate was estimated from empirical datasets using the g2 and maximum likelihood (ML) methods. For the g2 method, estimated selfing rate and P-values for the condition Ho:s = 0 are given. For the ML method, the hypothesis Ho:s = 0 was tested by model simplification, comparing deltadev to a chi-square with one degree of freedom.
| Lineage | g 2 method | ML method | F ST | |||
|---|---|---|---|---|---|---|
| Selfing rate | P-value | Selfing rate | δdevs = 0 | 95 % CI | ||
| I | 0.178 | 0.002 | 0.129 | 3.92 | [0.0015–0.2455] | 0.291* |
| II | 0.920 | 0.000 | 0.914 | 32.07 | [0.8559–0.9448] | 0.712* |
Values in bold type indicate that the selfing rate is significantly different from 0. The selfing rate was estimated from each pooled lineage. FST values are the means of genetic differentiation among populations for each lineage.
*Significant differentiation where P < 0.001.
Notable differences in selfing ratios were observed between the western and eastern populations. Selfing rates by the g2 method and ML method at the lineage level were considerably higher in the east, averaging 92.0 and 91.4 % compared to 17.8 and 12.9 % in the west, respectively (Table 2).
Phylogeographical structure
In the microsatellite analysis, the STRUCTURE, Barrier, PCoA and N-J trees used to infer the population structure of D. kiusiana consistently revealed that the two lineages are genetically distinct, i.e. the western lineage (LI) and the eastern lineage (LII). In the STRUCTURE analysis, while the mean likelihood scores increased progressively with K values of 1–9, ∆K values computed for all K classes indicated a strong signal at K = 2 (Fig. 3A). At K = 2, which was the optimal number of clusters, a strong genetic structure was found among the populations, divided clearly into the two lineages (Fig. 3A). We also made bar plots of individual ancestry from K = 3 to K = 9 (Supplementary Data Fig. S6). Barrier analyses based on pairwise FST identified a strong barrier, which was clearly divided into the west and east lineages (Fig. 3A). The results of the PCoA for individuals also revealed this separation (Fig. S7). The N-J trees based on the DA and Dps genetic distances classified the nine populations into two lineages with a remarkably high bootstrap value of 100 (Fig. 4).
Fig. 3.
Genetic composition of Daphne kiusiana populations. (A) STRUCTURE clustering using 16 microsatellite loci (K = 2) and barrier analysis based on Monmonier’s algorithm and significance, tested using means of 1000 bootstrap matrixes of DA genetic distance (Nei et al., 1983). The rate of change in the log-likelihood probability and ΔK based on the estimated number of genetic clusters (K) is shown in the upper-right inset. (B) Geographical distribution of three chloroplast haplotypes based on 16 non-coding cpDNA regions. The grey shading represents exposed coastal areas and sea basins during times of glacially induced alterations in sea level during the Late Pleistocene.
Fig. 4.
Phylogenetic relationships (neighbour-joining trees) for nine populations of Daphne kiusiana based on (A) Nei’s chord distance (DA) and (B) the proportion of shared alleles (Dps). Values in the tree branches are percentage bootstrap values estimated from 1000 reiterations. Bootstrap support at internodes are shown if values are >50 %.
For the multilocus cpDNA analysis, the cpDNA sequence was aligned with a consensus length of 8904 bp, and polymorphisms were identified in seven non-coding regions (ndhF-rpl32, matK-trnK, rps11-rps8, trnG-atpA, trnE-trnT, ycf4-cemA and atpB-rbcL). Fourteen polymorphisms (11 nucleotide substitutions and three indels) were identified, and three haplotypes (D1–D3) were determined. Each population harboured only a single haplotype. Consistent with the results of the microsatellite analysis, the haplotype network showed a linear topology, indicating that D. kiusiana was clustered into two different lineages (Fig. 3B). LI comprises haplotype D1, and LII harbours haplotypes D2 and D3. The ML tree revealed that haplotype D1 and var. atrocaulis were monophyletic, with a 65 % bootstrap value, whereas D2 and D3 were completely monophyletic. This indicates that, in the phylogeographical history, LI and LII were independent lineages.
Past population size change and population divergence
The average classification errors of the ABC-RF model selection ranged from 0.354 to 0.388 (Table 3). Although these error rate values were not low in all analyses, as votes by RF were concentrated into a single model and the posterior probabilities were relatively high (0.702–0.802), we considered that our ABC-RF model selection was reliable. Based on the goodness-of-fit of the best model, some predicted standard deviation parameters deviated from the observed parameters, although most of the summary statistics were well predicted (Supplementary Data Figs S8 and S9). Therefore, we conclude that our parameter estimation also performed well.
Table 3.
Model comparison using an approximate Bayesian computation random forest (ABC-RF) approach.
| Analysis | Lineage | Proportion of votes by RFa | Posterior probability | Error rate | |||||
|---|---|---|---|---|---|---|---|---|---|
| SNM | PGM | SRM | DM1 | DM2 | DM3 | ||||
| Population size change | I | 0.023 | 0.264 | 0.713 | – | – | – | 0.802 | 0.354 |
| Population size change | II | 0.080 | 0.208 | 0.712 | – | – | – | 0.702 | 0.354 |
| Population divergence | – | – | – | – | 0.086 | 0.157 | 0.757 | 0.731 | 0.388 |
aThe best model is shown in bold type.
SNM, standard neutral model; PGM, population growth model; SRM, size reduction model; DM, divergence model.
In the population size change analyses, the SRM was selected as the best model for both lineages (Table 3). Based on the posterior modes of T (in thousand years ago, kya), a probable bottleneck occurred within LI at 12.1 [4.9–67.1] kya, while within LII at 54.1 [10.4–999.3] kya (Table 4). A severe bottleneck effect is clearly more evident in predominantly outcrossing LI than in predominantly selfing LII (Fig. 5A). The trajectories of population size change showed that although both lineages reduced in size in the Late Pleistocene, the reduction in LI was more rapid and recent than that in LII (Fig. 5A). During the Holocene, both lineages showed similar and very low effective sizes, with posterior mode values of 468 and 439 for LI and LII, respectively (Table 4). In the population divergence analysis, divergence model 3 (DM3) was selected as the best model (Table 3 and Fig. 5B) and the posterior mode (95 % HPD) of the divergence time between the two lineages (TDIV) was estimated to be 21.3 (12.8–70.4) kya (Table 4).
Table 4.
Posterior mode and 95 % highest posterior density (HPD) of parameters of the best model.
| Analysis | Population size change | Population divergence | |
|---|---|---|---|
| Lineage I | Lineage II | ||
| Best model | SRM | SRM | DM3 |
| N CUR | 468 (272–753) | 439 (156–1311) | – |
| N ANC | 10 354 (3137–18 018) | 3364 (2001–19 054) | – |
| T 1 (kya) | 12.1 (4.9–67.1) | 54.1 (10.4–999.3) | – |
| T DIV (kya) | – | – | 21.3 (12.8–70.4) |
| shape | 1.74 (0.84–4.30) | 0.52 (0.50–0.93) | 1.03 (0.53–2.37) |
| P GSM | 0.15 (0.00–0.36) | 0.31 (0.07–0.46) | 0.28 (0.06–0.50) |
SRM, size reduction model; DM3, divergence model 3.
N CUR, current effective population size; NANC, ancestral effective population size; T1, event time for population size change; TDIV, divergence time; shape and PGSM, generalized stepwise mutation model parameters.
Fig. 5.
Inferred demographic history for Daphne kiusiana. (A) Trajectories of effective population size change in lineage I and lineage II predicted by the size reduction model. Lines and coloured areas indicate the posterior mode and 95 % highest posterior density. (B) The best supported population divergence model in ABC analysis of the genetic data sets. NCUR, current effective population size; NANC, ancestral effective population size; T, event time for population size change; TDIV, divergence time.
Ecological niche modelling
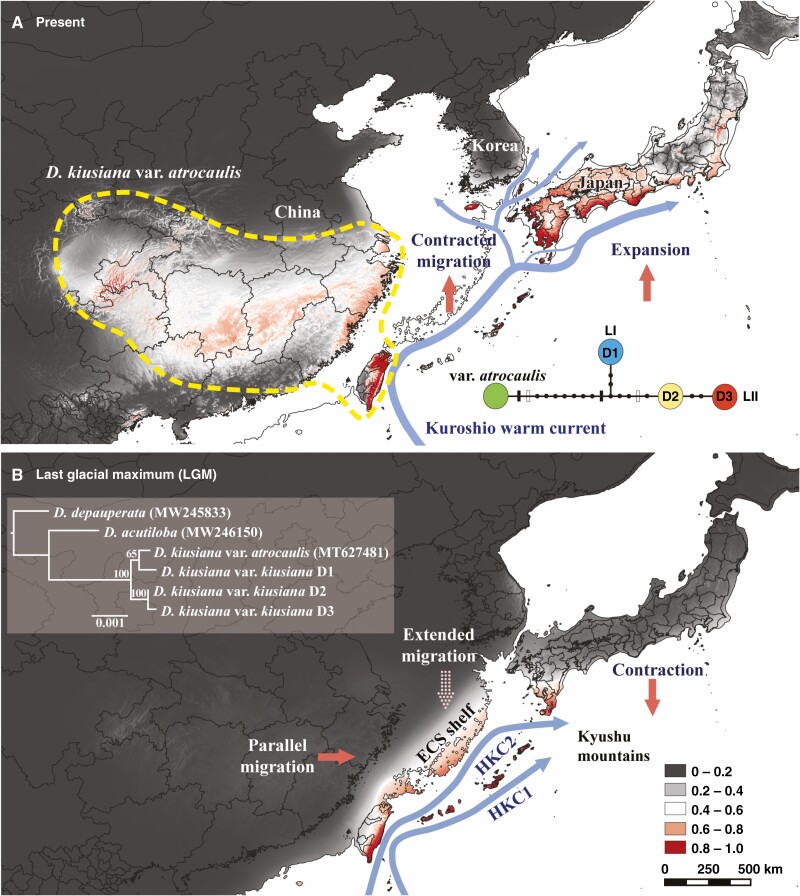
The current distribution model of D. kiusiana (Fig. 6A) showed a high average AUC (area under the ROC curve; 0.906). The highest contributing variable was ‘bio14’ (precipitation of driest month, 57 % contribution) followed by ‘bio04’ (temperature seasonality, 12.0 %) and ‘bio02’ (mean diurnal range, 11.9 %). The current distribution model was significantly consistent with warm-temperate climate zones extending from western Japan to Guizhou in China, including the southern edge of the Korean Peninsula and northern Taiwan. The potential distribution during the LGM was revealed with a high probability (>0.6) along the palaeo-coastline from Taiwan to the southern East China Sea (ECS) shelf, southern Kyushu and the Ryukyu Islands of Japan (Fig. 6B).
Fig. 6.
Potential distributions of Daphne kiusiana predicted by ecological niche modelling. The potential range during the LGM was averaged from three general circulation models. (A) Present; chloroplast haplotype network based on 16 non-coding cpDNA regions of var. kiusiana and var. atrocaulis. (B) LGM; ML tree is boxed in grey. HKC 1 indicates the main track of the Kuroshio Warm Current (proposed by Ujiie et al., 2003; Kao et al., 2006; Zheng et al., 2016). The scale bar in the ML tree represents the average number of nucleotide substitutions per site. HKC 2 is the hypothetical Kuroshio Warm Current (proposed by Vogt-Vincent and Mitarai, 2020).
Differences in floral traits: visibility and herkogamy
We found significant differences in floral visibility and herkogamy between the two lineages. The floral visibility of LI was significantly lower than that of LII. This pattern was evidenced by flower number (F1,115 = 160.3, P < 0.001; Fig. 7A), calyx lobe length (F1,115 = 107.6, P < 0.001; Fig. 7B), calyx lobe width (F1,115 = 25.1, P < 0.001; Fig. 7C) and calyx tube length (F1,115 = 39.03, P < 0.001; Fig. 7E), which affected the increase in the surface area of the inflorescence. A significant difference in anther–stigma distance was also observed between the two lineages (F1,115 = 180.5, P < 0.001; Fig. 7D). Significant differences were also found in the lengths of the calyx tube and pistil (F1,115 = 50.38, P < 0.001; Fig. 7F), the floral organs that make up the herkogamy trait (Fig. 7). Significant positive correlations were found between anther–stigma distance and flower number (R2 = 0.319, P < 0.001; Fig. 8A), calyx lobe area (R2 = 0.211, P < 0.001; Fig. 8B) and calyx tube length (R2 = 0.417, P < 0.001; Fig. 8C). However, a negative relationship between anther–stigma distance and pistil length was observed (R2 = 0.272, P < 0.001; Fig. 8D), indicating that a reduction in the distance between the stigma and anthers occurred via an increase in the length of the pistils.
Fig. 7.
Boxplots expressing morphological variations in two Daphne kiusiana lineages (LI and LII). The box signifies the distribution of the 25–75 % quartiles, and the median is represented by a horizontal line within the box. The ends of the vertical lines indicate minimum and maximum data values, respectively. Mean values are represented by a yellow line, with the value shown next to the line. The differences between the two lineages were significant for each respective trait where P < 0.001 (F test).
Fig. 8.
Scatter plots showing correlations between herkogamy and floral morphological traits of Daphne kiusiana. Blue and red circles represent the data of lineage I (LI) and lineage II (LII), respectively. Grey straight line, fitted values; shaded area, 95 % confidence interval; ***, P < 0.001.
DISCUSSION
To our knowledge, this is the first study to characterize in detail the evolutionary history and demographic dynamics associated with the selfing syndrome in a wild shrub with subcapitate inflorescences. The eastern lineage (LII) of D. kiusiana, which has smaller and fewer flowers, was characterized by a predominantly unique occurrence of HMLGs, lower genetic diversity and higher genetic differentiation compared to those of the western outcrossing lineage (LI). Our results suggest that the selfing lineage in D. kiusiana has been largely driven by gradual directional selection towards lower levels of floral visibility and herkogamy for efficient self-pollination in response to historical environmental changes. The transition to selfing accompanied by morphological modifications may have been triggered by severe competitive interactions among/within species. Intrinsic factors of D. kiusiana, such as its life-history traits and niche in the ecological succession, may also have been important in facilitating its evolution. Thus, the selfing syndrome lineage or species may exhibit a demographic signature of a gradually reduced effective population size, as evidence of adaptation.
Demographic genetic consequences: selfing vs. outcrossing
We found that the eastern populations (LII) had significantly higher homozygosity than the western populations (LI). This pattern is typical of the genetic characteristic observed in selfing species/populations (Duncan and Rausher, 2013; Voillemot and Pannell, 2017) and is consistent with theoretical predictions (Busch and Delph, 2012; Wright et al., 2013). Indeed, RMES allowed the estimation of selfing rates in our study species and revealed that the relative ratio of LII was significantly higher. We also found that HMLGs at all loci occurred only in LII. One or a few HMLGs dominate each population and coexist with several rare genotypes. This is consistent with the genetic patterns in selfing populations in which a few HMLGs dominate specific habitats (Shibayama and Kadono, 2008; Triest et al., 2021). Therefore, we suggest that the genetic characteristics of the LII populations are the result of selfing. This suggestion is strongly supported by our results that show genetically less diversity and higher differences among the LII populations, which are typical indications of selfing populations (Charlesworth and Pannell, 2001; Duminil et al., 2009; Voillemot and Pannell, 2017).
On the other hand, although it is highly unlikely, as many flowering plants flexibly combine sexual and asexual reproduction systems, our results related to HMLGs may open the possibility for asexual reproduction, such as vegetative propagation and apomixis. However, these plants are inherently heterozygous and consistently maintain their original genetic diversities. Moreover, heterozygosity is expected to increase under clonal propagation owing to the accumulation of mutations over long-term evolutionary progress (Birky, 1996; Balloux et al., 2003; and also see the Japanese populations discussed by Zhu et al., 2020). To the best of our knowledge, there have been no reports of asexual reproduction in this species, and its impact on genetic consequences is therefore considered limited.
Evolutionary history of selfing lineage
Our STRUCTURE, Barrier analysis, PCoA, N-J tree, cpDNA ML tree and ENM analyses all point to the fact that LI and LII have independently evolved. In addition, considering previous studies on other evergreen trees belonging to similar forest biomes (Cao et al., 2018; Han et al., 2020), we have reasonably inferred the geographical location of their ancestral populations during the LGM. These populations would have been distributed in separate regions along the palaeo-coastline, including (1) the ECS shelf (LI) and (2) the southern tip of Kyushu (LII). Consequently, the two extant lineages may have resulted from high-latitude expansion from different source populations in response to dynamic changes in land configuration and the contemporary flow of the Kuroshio Warm Current (Fig. 6). Therefore, to gain insight into what boosted the selfing rates sufficiently enough to cause the floral changes, we inferred historical and ecological events likely to have occurred during the last glacial period.
It is worth noting that progressively exposed ECS shelves could have provided new habitat for LI during the last glacial period. Historical circumstances would have been favourable for the ancestors of western populations during the last glacial period. An extensive shelf, such as the ECS basin exposed to climate cooling, would have provided new open habitats for LI. The migration process appears to have progressed along with the development of ecological succession (probably primary succession); plants would have progressively colonized the ECS shelf and predominated the shrub layers of the forests. Indeed, the abundance of congeneric evergreen shrubs often increases in open areas (Sakata and Nakahama, 2018). Climate cooling would not be beneficial but the relative evolutionary selection pressures for the transition to selfing would have been small because of the reward of colonizing the ECS shelf. Subsequently, a severe population bottleneck of LI arose during the high-latitude migrations in the Holocene with rapid sea-level increases.
In contrast, the Japanese islands would not have allowed the populations to expand further south because there was relatively little Pacific coastline retreat (Lee et al., 2013b; Cao et al., 2018). This may imply conflicting selection between the two lineages with respect to their mating systems. For the eastern populations, the climate cooling would have restricted them to the southern tip of Kyushu, and probably forced them to compete with numerous cold-tolerant species, presumably migrated downwards from the Kyushu Mountains (i.e. competitive interaction; Cheptou and Dieckmann, 2002). Furthermore, mating competition in early flowering D. kiusiana may have been accelerated by limited pollinators (Barrett, 1992). Under these conditions (i.e. rare mates and/or rare pollinators), selfing can be favoured because it offers reproductive assurance (Darwin, 1876; Pannell and Barrett, 1998; Busch and Delph, 2012) and boosts seed production (Davis and Delph, 2005). The selfing syndrome may evolve under ecological conditions that demanded relatively strong competitive interactions among and within species. Intrinsic factors of D. kiusiana may also have played an important role in facilitating this evolution. Notably, the life-history traits of this shrub are characterized by early flowering (i.e. lack of resources for pollination; Kalisz and Vogler, 2003), and bird-mediated seed dispersal.
Demographic dynamics and adaptive significance of selfing syndrome
Similar findings showing that independently evolved selfing species/lineages frequently show lower levels of floral visibility (Sicard and Lenhard, 2011; Goodwillie and Weber, 2018) and herkogamy (Takebayashi et al., 2006; Busch and Delph, 2012) compared to their outcrossing ancestors suggest that these alterations can have adaptive significance. Moreover, selfing can promote trait divergence for adaptation (Hodgins and Yeaman, 2019). As a result of morphological analysis for D. kiusiana, quantitative values such as flower number, calyx tube length and calyx lobe area decreased in predominant selfing LII. This suggests that many traits associated with the reduced surface area of the inflorescence are responsible for the reduced visibility. Moreover, the pistil length in plants of LII derived from LI was notably increased. This result is remarkable because it implies that the energy used to improve floral visibility was reallocated to increase pistil length, resulting in a low level of herkogamy. In addition, these plants have flowers in which the filaments of the stamens and calyx tube are fused, which can be effective by simultaneously lowering visibility and herkogamy levels (Figs 7E and 8C). Therefore, by combining resource consumption, structural complexity and functional efficiency, the flowers of LII may represent a gradual by-product that required sufficient time to evolve. The evolution of the selfing syndrome may be limited by genetic drift alone, without gradual adaptation.
In Capsella, the short timescales of speciation suggest that the extensive phenotypic evolution was driven rapidly by genetic drift over about the last 20 000 years (Foxe et al., 2009) or last 50 000 years (Guo et al., 2009). However, these inferences raise the question of when the flower changes began in relation to the species divergence time. Similar to previous studies, our estimate of the divergence time of the two D. kiusiana lineages was 21 300 years ago, and signs of a bottleneck event were found in both lineages. However, we found that the pattern of effective population size reduction shown in the ABC-derived trend lines was different between the two morphologically contrasting lineages. The selfing eastern lineage (LII) shows demographic stability, whereas the predominantly outcrossing western lineage (LI) is associated with a severe population bottleneck. In detail, the effective population size of LI remained stable for a relatively long period but showed a dramatic decline during the Holocene. In contrast, the effective population size of LII decreased in a gradual manner during the last glacial period and then remained stable from the LGM to the present (Fig. 6). As the rate of selfing increased, many of these populations may have maintained a size that can slightly offset the negative effects of genetic drift. This implies that the transition to selfing accompanied by the morphological modifications can evolutionarily stabilize reduction of the effective population size.
The SRM in LII estimated the event time of the size reduction to be ~54 100 years ago, during the last glacial period (ca. 110–10 kya; Huang and Schaal, 2012; Alvarez-Solas et al., 2019). The effective population size reached a minimum during the LGM. These results suggest that the evolution of characteristic selfing flowers in D. kiusiana may have taken place from the onset of the last glacial that could have triggered intense competitive interactions. In this context, functional and morphological changes to the flowers for effective self-pollination may have been almost completed at the LGM. These inferences are consistent with the hypothesis that flower size decreased in the Capsella selfing lineage before its extensive geographical spread (Sicard et al., 2011). It is therefore reasonable that the evolutionary timescale includes the starting point at which the morphological changes begin, slightly further back in time than estimated by previous studies (i.e. Foxe et al., 2009; Guo et al., 2009). The last glacial period may have provided ample time for purifying energetically expensive traits that attract or reward pollinators, as well as for reallocating energies for effective self-pollination.
Supplementary Material
ACKNOWLEDGEMENTS
We are very grateful to the following researchers for their comments, which greatly improved the previous version of the manuscript: M. H. Kwak (National Institute of Biological Resources) and H. T. Lim (Chonnam National University). We thank D. H. Lee of the Korea National Arboretum for providing habitat information on Isl. Geoje and photographs of D. kiusiana. We also thank H. S. Kim, a researcher at the Korea National Arboretum, for providing the illustration in Fig. 2. We would like to thank Prof. Y. Suyama of Tohoku University for advice on the technical methods of MPM-seq. The authors have no conflicts of interest to declare. JHL and BYK planned and designed the study; EKH, WBC, SGY and JHL conducted fieldwork and collected the materials; EKH contributed to the experimental management; EKH, IT, JSP and DPJ conducted data analysis; AG conducted morphological measurements; EKH and SGY produced images; EKH and JHL wrote the manuscript; HJC, SHO, DCS and YI revised the manuscript, provided advice on the experiments and finalized the manuscript. MPM-seq raw data are available at the GenBank database (BioProject ID PRJNA794292; BioSample accession numbers SAMN24619490–SAMN24619726). Haplotype sequences of cpDNA are available in GenBank under accession numbers SRX13610178–SRX13610414. Additional tables and figures are presented as Supplementary Data.
Contributor Information
Eun-Kyeong Han, Department of Biology Education, Chonnam National University, Gwangju 61186, Republic of Korea.
Ichiro Tamaki, Gifu Academy of Forest Science and Culture, 88 Sodai, Mino, Gifu 501-3714, Japan.
Sang-Hun Oh, Department of Biology, Daejeon University, Daejeon 34520, Republic of Korea.
Jong-Soo Park, Department of Botany, Honam National Institute of Biological Resources, Mokpo 58762, Republic of Korea.
Won-Bum Cho, Department of Plant Variety Protection, National Forest Seed and Variety Center, Chungju 27495, Republic of Korea.
Dong-Pil Jin, Urban Biodiversity Research Division, Sejong National Arboretum, Sejong 30106, Republic of Korea.
Bo-Yun Kim, Plant Resources Division, National Institute of Biological Resources, Incheon 22689, Republic of Korea.
Sungyu Yang, Herbal Medicine Resources Research Center, Korea Institute of Oriental Medicine, Naju 58245, Republic of Korea.
Dong Chan Son, Division of Forest Biodiversity and Herbarium, Korea National Arboretum, Pocheon 11186, Republic of Korea.
Hyeok-Jae Choi, Department of Biology and Chemistry, Changwon National University, Changwon 51140, Republic of Korea.
Amarsanaa Gantsetseg, Department of Biology Education, Chonnam National University, Gwangju 61186, Republic of Korea.
Yuji Isagi, Division of Forest and Biomaterials Science, Graduate School of Agriculture, Kyoto University, Kyoto 606-8502, Japan.
Jung-Hyun Lee, Department of Biology Education, Chonnam National University, Gwangju 61186, Republic of Korea.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: The developed 16 cpDNA markers of Daphne kiusiana used in the analysis. Table S2: Prior distribution of compared population size change and population divergence models. Figure S1: Genotype accumulation curve for 16 microsatellite loci in Daphne kiusiana. Figure S2: Schematic diagram of the construction of the multiplexed sequencing library of cpDNA. Figure S3: Schematic diagram of the identification of variations in the cpDNA non-coding regions. Figure S4: Comparison of population size change and population divergence models. Figure S5: All floral measurements made to characterize variations in floral morphology. Figure S6: Bayesian individual-based clustering of Daphne kiusiana populations at K = 3 to K = 9. Figure S7: Principal coordinate analysis based on Nei’s genetic distance. Figure S8: Goodness-of-fit of the size reduction model. Figure S9: Goodness-of-fit of the population divergence model 3. Method S1: Multiplex sequencing for cpDNA: MPM-seq with modification. Dataset S1: Measurement images of floral morphological characters of Daphne kiusiana.
FUNDING
This research was funded by the National Institute of Biological Resources of Korea (No. NIBR202206101) and the National Research Foundation of Korea (No. 2022R1I1A1A01064250).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
LITERATURE CITED
- Alonso C, Herrera CM.. 2001. Neither vegetative nor reproductive advantages account for high frequency of male-steriles in southern Spanish gynodioecious Daphne laureola (Thymelaeaceae). American Journal of Botany 88: 1016–1024. doi: 10.2307/2657083. [DOI] [PubMed] [Google Scholar]
- Alvarez-Solas J, Banderas R, Robinson A, Montoya M.. 2019. Ocean-driven millennial-scale variability of the Eurasian ice sheet during the last glacial period simulated with a hybrid ice-sheet–shelf model. Climate of the Past 15: 957–979. doi: 10.5194/cp-15-957-2019. [DOI] [Google Scholar]
- Aoki K, Suzuki T, Hsu TW, Murakami N.. 2004. Phylogeography of the component species of broad-leaved evergreen forests in Japan, based on chloroplast DNA variation. Journal of Plant Research 117: 77–94. doi: 10.1007/s10265-003-0132-4. [DOI] [PubMed] [Google Scholar]
- Baker HG. 1955. Self-compatibility and establishment after ‘long-distance’ dispersal. Evolution 9: 347–349. doi: 10.2307/2405656. [DOI] [Google Scholar]
- Balloux F, Lehmann L, de Meeûs T.. 2003. The population genetics of clonal and partially clonal diploids. Genetics 164: 1635–1644. doi: 10.1093/genetics/164.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SC. 1992. Gender variation and the evolution of dioecy in Wurmbea dioica (Liliaceae). Journal of Evolutionary Biology 5: 423–444. doi: 10.1046/j.1420-9101.1992.5030423.x. [DOI] [Google Scholar]
- Barrett SC. 2002. The evolution of plant sexual diversity. Nature Reviews Genetics 3: 274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- Barrett SC, Eckert CG.. 1990. Variation and evolution of mating systems in seed plants. In: Kawano S, ed. Biological approaches and evolutionary trends in plants. London: Academic Press, 229–254. [Google Scholar]
- Beaumont MA. 2019. Approximate Bayesian computation. Annual Review of Statistics and Its Application 6: 379–403. doi: 10.1146/annurev-statistics-030718-105212. [DOI] [Google Scholar]
- Birky, CWJr. 1996. Heterozygosity, heteromorphy, and phylogenetic trees in asexual eukaryotes. Genetics 144: 427–437. doi: 10.1093/genetics/144.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum MG, François O.. 2010. Non-linear regression models for Approximate Bayesian Computation. Statistics and Computing 20: 63–73. doi: 10.1007/s11222-009-9116-0. [DOI] [Google Scholar]
- Brown JL, Bennett JR, French CM.. 2017. SDMtoolbox 2.0: the next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 5: e4095. doi: 10.7717/peerj.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkli A, Sieber N, Seppälä K, Jokela J.. 2017. Comparing direct and indirect selfing rate estimates: when are population-structure estimates reliable? Heredity 118: 525–533. doi: 10.1038/hdy.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch JW, Delph LF.. 2012. The relative importance of reproductive assurance and automatic selection as hypotheses for the evolution of self-fertilization. Annals of Botany 109: 553–562. doi: 10.1093/aob/mcr219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calviño-Cancela M, Escudero M, Rodríguez-Pérez J, et al. 2012. The role of seed dispersal, pollination and historical effects on genetic patterns of an insular plant that has lost its only seed disperser. Journal of Biogeography 39: 1996–2006. doi: 10.1111/j.1365-2699.2012.02732.x. [DOI] [Google Scholar]
- Cao YN, Wang IJ, Chen LY, Ding YQ, Liu LX, Qiu YX.. 2018. Inferring spatial patterns and drivers of population divergence of Neolitsea sericea (Lauraceae), based on molecular phylogeography and landscape genomics. Molecular Phylogenetics and Evolution 126: 162–172. doi: 10.1016/j.ympev.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Capador H, Samils B, Olson A.. 2018. Development of microsatellite markers for Thekopsora areolata, the causal agent of cherry spruce rust. Forest Pathology 48: e12413. doi: 10.1111/efp.12413. [DOI] [Google Scholar]
- Charlesworth D, Pannell JR.. 2001. Mating systems and population genetic structure in the light of coalescent theory. In: Silvertown J, Antonovics J, eds. Integrating ecology and evolution in a spatial context. Oxford: Blackwell Science, 73–96. [Google Scholar]
- Cheptou PO, Dieckmann U.. 2002. The evolution of self-fertilization in density-regulated populations. Proceedings of the Royal Society of London. Series B: Biological Sciences 269: 1177–1186. doi: 10.1098/rspb.2002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chybicki IJ, Burczyk J.. 2009. Simultaneous estimation of null alleles and inbreeding coefficients. Journal of Heredity 100: 106–113. doi: 10.1093/jhered/esn088. [DOI] [PubMed] [Google Scholar]
- Clement M, Posada D, Crandall KA.. 2000. TCS: a computer program to estimate gene genealogies. Molecular Ecology 9: 1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- Comes HP, Kadereit JW.. 1998. The effect of Quaternary climatic changes on plant distribution and evolution. Trends in Plant Science 3: 432–438. doi: 10.1016/s1360-1385(98)01327-2. [DOI] [Google Scholar]
- Csilléry K, François O, Blum MG.. 2012. abc: an R package for approximate Bayesian computation (ABC). Methods in Ecology and Evolution 3: 475–479. doi: 10.1111/j.2041-210X.2011.00179.x. [DOI] [PubMed] [Google Scholar]
- Darwin C. 1876. The effects of cross and self-fertilization in the vegetable kingdom. London: John Murray. [Google Scholar]
- David P, Pujol B, Viard F, Castella V, Goudet J.. 2007. Reliable selfing rate estimates from imperfect population genetic data. Molecular Ecology 16: 2474–2487. doi: 10.1111/j.1365-294X.2007.03330.x. [DOI] [PubMed] [Google Scholar]
- Davis SL, Delph LF.. 2005. Prior selfing and gynomonoecy in Silene noctiflora L. (Caryophyllaceae): opportunities for enhanced outcrossing and reproductive assurance. International Journal of Plant Sciences 166: 475–480. doi: 10.1086/428630. [DOI] [Google Scholar]
- Dieringer D, Schlötterer C.. 2003. Two distinct modes of microsatellite mutation processes: evidence from the complete genomic sequences of nine species. Genome Research 13: 2242–2251. doi: 10.1101/gr.1416703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duminil J, Hardy OJ, Petit RJ.. 2009. Plant traits correlated with generation time directly affect inbreeding depression and mating system and indirectly genetic structure. BMC Evolutionary Biology 9: 1771–1714. doi: 10.1186/1471-2148-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan TM, Rausher MD.. 2013. Evolution of the selfing syndrome in Ipomoea. Frontiers in Plant Science 4: 301. doi: 10.3389/fpls.2013.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl DA, VonHoldt BM.. 2012. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources 4: 359–361. doi: 10.1007/s12686-011-9548-7. [DOI] [Google Scholar]
- Eckert CG, Samis KE, Dart S.. 2006. Reproductive assurance and the evolution of uniparental reproduction in flowering plants. In: Harder LD, Barrett CH, eds. Ecology and evolution of flowers. New York: Oxford University Press, 183–203. [Google Scholar]
- Ennos RA, Sinclair WT, Hu XS, Langdon A.. 1999. Using organelle markers to elucidate the history, ecology and evolution of plant populations. In Hollingsworth PM, Bateman RM, Gornall RJ, ed. Molecular systematics and plant evolution. London: Taylor and Francis Ltd., 1–19. [Google Scholar]
- Estoup A, Angers B.. 1998. Microsatellites and minisatellites for molecular ecology: theoretical and empirical consideration. In: Carvalho G, ed. Advances in molecular ecology. Amsterdam: NATO Press, 55–86. [Google Scholar]
- Estoup A, Jarne P, Cornuet JM.. 2002. Homoplasy and mutation model at microsatellite loci and their consequences for population genetics analysis. Molecular Ecology 11: 1591–1604. doi: 10.1046/j.1365-294x.2002.01576.x. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Foll M.. 2011. Fastsimcoal: a continuous-time coalescent simulator of genomic diversity under arbitrarily complex evolutionary scenarios. Bioinformatics 27: 1332–1334. doi: 10.1093/bioinformatics/btr124. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Lischer HE.. 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10: 564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Fang Y, Enhe Z, Qinli W, Zhuhong M.. 2016. Germination and dormancy-breaking of Daphne giraldii Nitsche (Thymelaeaceae) seeds from northwestern China. Revista Chapingo serie ciencias forestales y del ambiente 22: 99–113. doi: 10.5154/r.rchscfa.2015.04.015. [DOI] [Google Scholar]
- Felsenstein J. 2004. PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author. https://evolution.genetics.washington.edu/phylip.html
- Foxe JP, Slotte T, Stahl EA, Neuffer B, Hurka H, Wright SI.. 2009. Recent speciation associated with the evolution of selfing in Capsella. Proceedings of the National Academy of Sciences 106: 5241–5245. doi: 10.1073/pnas.0807679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasca-Pineda J, Gutiérrez-Guerrero YT, Aguirre-Planter E, Eguiarte LE.. 2020. The role of environment, local adaptation, and past climate fluctuation on the amount and distribution of genetic diversity in two subspecies of Mexican wild Zea mays. American Journal of Botany 107: 1542–1554. doi: 10.1002/ajb2.1561. [DOI] [PubMed] [Google Scholar]
- Gelman A, Hwang J, Vehtari A.. 2014. Understanding predictive information criteria for Bayesian models. Statistics and Computing 24: 997–1016. doi: 10.1007/s11222-013-9416-2. [DOI] [Google Scholar]
- Gent PR, Danabasoglu G, Donner LJ, et al. 2011. The community climate system model version 4. Journal of Climate 24: 4973–4991. doi: 10.1175/2011JCLI4083.1. [DOI] [Google Scholar]
- Goodwillie C, Weber JJ.. 2018. The best of both worlds? A review of delayed selfing in flowering plants. American Journal of Botany 105: 641–655. doi: 10.1002/ajb2.1045. [DOI] [PubMed] [Google Scholar]
- Goudet J. 1995. FSTAT (version 1.2): a computer program to calculate F-statistics. Journal of Heredity 86: 485–486. doi: 10.1093/oxfordjournals.jhered.a111627. [DOI] [Google Scholar]
- Goudet J, Raymond M, de Meeüs T, Rousset F.. 1996. Testing differentiation in diploid populations. Genetics 144: 1933–1940. doi: 10.1093/genetics/144.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant V. 1981. Plant speciation. New York: Columbia University Press. [Google Scholar]
- Guo YL, Bechsgaard JS, Slotte T, et al. 2009. Recent speciation of Capsella rubella from Capsella grandiflora, associated with loss of self-incompatibility and an extreme bottleneck. Proceedings of the National Academy of Sciences 106: 5246–5251. doi: 10.1073/pnas.0808012106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han EK, Cho WB, Park JS, et al. 2020. A disjunctive marginal edge of evergreen broad-leaved oak (Quercus gilva) in East Asia: the high genetic distinctiveness and unusual diversity of Jeju island populations and insight into a massive, independent postglacial colonization. Genes 11: 1114. doi: 10.3390/genes11101114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins KA, Yeaman S.. 2019. Mating system impacts the genetic architecture of adaptation to heterogeneous environments. New Phytologist 224: 1201–1214. doi: 10.1111/nph.16186. [DOI] [PubMed] [Google Scholar]
- Huang WJ. 2017. Sexual system of Daphne arisanensis Hayata (Thymelaeaceae). MrS Thesis, Taiwan National University, Taiwan. [Google Scholar]
- Huang P, Schaal BA.. 2012. Association between the geographic distribution during the last glacial maximum of Asian wild rice, Oryza rufipogon (Poaceae), and its current genetic variation. American Journal of Botany 99: 1866–1874. doi: 10.3732/ajb.1200348. [DOI] [PubMed] [Google Scholar]
- Inoue K, Sietman BE, McMurray SE, Faiman JS, Zanatta DT.. 2021. New microsatellite markers for Ellipse, Venustaconcha ellipsiformis (Bivalvia: Unionidae), with notes on optimal sample size and cross-species amplification. Molecular Biology Reports 48: 3037–3045. doi: 10.1007/s11033-021-06292-2. [DOI] [PubMed] [Google Scholar]
- Jakobsson M, Rosenberg NA.. 2007. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23: 1801–1806. doi: 10.1093/bioinformatics/btm233. [DOI] [PubMed] [Google Scholar]
- Kalisz S, Vogler DW.. 2003. Benefits of autonomous selfing under unpredictable pollinator environments. Ecology 84: 2928–2942. doi: 10.1890/02-0519. [DOI] [Google Scholar]
- Kamvar ZN, Brooks JC, Grünwald NJ.. 2015. Novel R tools for analysis of genome-wide population genetic data with emphasis on clonality. Frontiers in Genetics 208: 20150610. doi: 10.3389/fgene.2015.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamvar ZN, Tabima JF, Grünwald NJ.. 2014. Poppr: an R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2: e281. doi: 10.7717/peerj.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao SJ, Wu CR, Hsin YC, Dai M.. 2006. Effects of sea level change on the upstream Kuroshio Current through the Okinawa Trough. Geophysical Research Letters 33. doi: 10.1029/2006GL026822. [DOI] [Google Scholar]
- Karger DN, Conrad O, Böhner J, et al. 2017. Climatologies at high resolution for the Earth’s land surface areas. Scientific Data 4: 1–20. doi: 10.1038/sdata.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Toh H.. 2010. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 26: 1899–1900. doi: 10.1093/bioinformatics/btq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ST, Oh SH, Park J.. 2021. The complete chloroplast genome of Diarthron linifolium (Thymelaeaceae), a species found on a limestone outcrop in eastern Asia. Korean Journal of Plant Taxonomy 51: 345–352. doi: 10.11110/kjpt.2021.51.4.345. [DOI] [Google Scholar]
- Korea National Arboretum. 2008. Rare plants data book in Korea. Pocheon: Korea National Arboretum; (in Korean). [Google Scholar]
- Lee TB. 2003. Coloured flora of Korea. Seoul: Hayangmunsa. [Google Scholar]
- Lee JH, Cho WB, Yang S, et al. 2017. Development and characterization of 21 microsatellite markers in Daphne kiusiana, an evergreen broad-leaved shrub endemic to Korea and Japan. Korean Journal of Plant Taxonomy 47: 6–10. doi: 10.11110/kjpt.2017.47.1.6. [DOI] [Google Scholar]
- Lee JH, Lee DH, Choi BH.. 2013b. Phylogeography and genetic diversity of East Asian Neolitsea sericea (Lauraceae) based on variations in chloroplast DNA sequences. Journal of Plant Research 126: 193–202. doi: 10.1007/s10265-012-0519-1. [DOI] [PubMed] [Google Scholar]
- Lee J, Lee KH, So S, Choi C, Kim M.. 2013a. A new species of Daphne (Thymelaeaceae): D. jejudoensis M. Kim. Korean Journal of Plant Taxonomy 43: 94–98. doi: 10.11110/kjpt.2013.43.2.94. [DOI] [Google Scholar]
- Lee JJ, Oh SH.. 2017. A comparative morphological study of Thymelaeaceae in Korea. Korean Journal of Plant Taxonomy 47: 207–221. doi: 10.11110/kjpt.2017.47.3.207. [DOI] [Google Scholar]
- Lloyd DG. 1979. Some reproductive factors affecting the selection of self-fertilization in plants. The American Naturalist 113: 67–79. doi: 10.1086/283365. [DOI] [Google Scholar]
- Lloyd DG. 1992. Self-and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Sciences 153: 370–380. doi: 10.1086/297041. [DOI] [Google Scholar]
- Lu RS, Chen Y, Tamaki I, et al. 2020. Pre-quaternary diversification and glacial demographic expansions of Cardiocrinum (Liliaceae) in temperate forest biomes of Sino-Japanese Floristic Region. Molecular Phylogenetics and Evolution 143: 106693. doi: 10.1016/j.ympev.2019.106693. [DOI] [PubMed] [Google Scholar]
- Manni F, Guérard E, Heyer E.. 2004. Geographic patterns of (genetic, morphologic, linguistic) variation: how barriers can be detected by using Monmonier’s algorithm. Human Biology 76: 173–190. doi: 10.1353/hub.2004.0034. [DOI] [PubMed] [Google Scholar]
- Manzanares C, Barth S, Thorogood D, et al. 2016. A gene encoding a DUF247 domain protein cosegregates with the S self-incompatibility locus in perennial ryegrass. Molecular Biology and Evolution 33: 870–884. doi: 10.1093/molbev/msv335. [DOI] [PubMed] [Google Scholar]
- Marriage TN, Hudman S, Mort ME, Orive ME, Shaw RG, Kelly JK.. 2009. Direct estimation of the mutation rate at dinucleotide microsatellite loci in Arabidopsis thaliana (Brassicaceae). Heredity 103: 310–317. doi: 10.1038/hdy.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merow C, Smith MJ, Silander JA Jr. 2013. A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography 36: 1058–1069. doi: 10.1111/j.1600-0587.2013.07872.x. [DOI] [Google Scholar]
- Monmonier MS. 1973. Maximum-difference barriers: An alternative numerical regionalization method. Geographical analysis 5: 245–261. [Google Scholar]
- Murata J. 1999. Daphne. In: Iwatsuki K, Boufford DE, Ohba H, eds. Flora of Japan IIc. Tokyo: Kodansha, 147–149. [Google Scholar]
- Nei M, Tajima F.. 1983. Maximum likelihood estimation of the number of nucleotide substitutions from restriction sites data. Genetics 105: 207–217. doi: 10.1093/genetics/105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Tajima F, Tateno Y.. 1983. Accuracy of estimated phylogenetic trees from molecular data. Journal of Molecular Evolution 19: 153–170. doi: 10.1007/BF02300753. [DOI] [PubMed] [Google Scholar]
- Nordborg M, Donnelly P.. 1997. The coalescent process with selfing. Genetics 146: 1185–1195. doi: 10.1093/genetics/146.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornduff R. 1969. Reproductive biology in relation to systematics. Taxon 18: 121–133. doi: 10.2307/1218671. [DOI] [Google Scholar]
- Pannell JR, Barrett SC.. 1998. Baker’s law revisited: reproductive assurance in a metapopulation. Evolution 52: 657–668. doi: 10.1111/j.1558-5646.1998.tb03691.x. [DOI] [PubMed] [Google Scholar]
- Peakall ROD, Smouse PE.. 2006. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes 6: 288–295. doi: 10.1111/j.1471-8286.2005.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M, Best N, Cowles K, Vines K.. 2006. CODA: convergence diagnosis and output analysis for MCMC. R News 6: 7–11. [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P.. 2000. Inference of population structure using multilocus genotype data. Genetics 155: 945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudlo P, Marin JM, Estoup A, Cornuet JM, Gautier M, Robert CP.. 2016. Reliable ABC model choice via random forests. Bioinformatics 32: 859–866. doi: 10.1093/bioinformatics/btv684. [DOI] [PubMed] [Google Scholar]
- Qiu YX, Fu CX, Comes HP.. 2011. Plant molecular phylogeography in China and adjacent regions: tracing the genetic imprints of Quaternary climate and environmental change in the world’s most diverse temperate flora. Molecular Phylogenetics and Evolution 59: 225–244. doi: 10.1016/j.ympev.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Ro NY, Song EY, Kim SC, Ko HC, Lee SY.. 2010. Propagation of a rare variety Daphne kiusiana Miq. in Korea through softwood cuttings. Journal of Bio-Environment Control 19: 246–250. [Google Scholar]
- Runions CJ, Geber MA.. 2000. Evolution of the self-pollinating flower in Clarkia xantiana (Onagraceae). I. Size and development of floral organs. American Journal of Botany 87: 1439–1451. doi: 10.2307/2656870. [DOI] [PubMed] [Google Scholar]
- Sakata Y, Nakahama N.. 2018. Flexible pollination system in an unpalatable shrub Daphne miyabeana (Thymelaeaceae). Plant Species Biology 33: 239–247. doi: 10.1111/1442-1984.12212. [DOI] [Google Scholar]
- Setsuko S, Sugai K, Tamaki I, Takayama K, Kato H, Yoshimaru H.. 2020. Genetic diversity, structure, and demography of Pandanus boninensis (Pandanaceae) with sea drifted seeds, endemic to the Ogasawara Islands of Japan: Comparison between young and old islands. Molecular Ecology 29: 1050–1068. doi: 10.1111/mec.15383. [DOI] [PubMed] [Google Scholar]
- Shibayama Y, Kadono Y.. 2008. Genetic variations in the endangered aquatic plant Nymphoides coreana (Menyanthaceae) in south-western Japan. Plant Species Biology 23: 212–216. doi: 10.1111/j.1442-1984.2008.00218.x. [DOI] [Google Scholar]
- Sicard A, Kappel C, Lee YW, et al. 2016. Standing genetic variation in a tissue-specific enhancer underlies selfing-syndrome evolution in Capsella. Proceedings of the National Academy of Sciences 113: 13911–13916. doi: 10.1073/pnas.1613394113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard A, Lenhard M.. 2011. The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants. Annals of Botany 107: 1433–1443. doi: 10.1093/aob/mcr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard A, Stacey N, Hermann K, et al. 2011. Genetics, evolution, and adaptive significance of the selfing syndrome in the genus Capsella. The Plant Cell 23: 3156–3171. doi: 10.1105/tpc.111.088237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotte T, Hazzouri KM, Stern D, Andolfatto P, Wright SI.. 2012. Genetic architecture and adaptive significance of the selfing syndrome in Capsella. Evolution; International Journal of Organic Evolution 66: 13606–11374. doi: 10.1111/j.1558-5646.2011.01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. 1974. Flowering plants. Evolution above the species level. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- Suyama Y, Hirota SK, Matsuo A, et al. 2021. Complementary combination of multiplex high-throughput DNA sequencing for molecular phylogeny. Ecological Research 37: 171–181. doi: 10.1111/1440-1703.12270. [DOI] [Google Scholar]
- Takebayashi N, Wolf DE, Delph LF.. 2006. Effect of variation in herkogamy on outcrossing within a population of Gilia achilleifolia. Heredity 96: 159–165. doi: 10.1038/sj.hdy.6800780. [DOI] [PubMed] [Google Scholar]
- Tamaki I, Kawashima N, Setsuko S, et al. 2019. Population genetic structure and demography of Magnolia kobus: variety borealis is not supported genetically. Journal of Plant Research 132: 741–758. doi: 10.1007/s10265-019-01134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2021. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Triest L, Dierick J, Phan TTH, Luong QD, Huy NQ, Sierens T.. 2021. Low genetic connectivity of strongly inbred Ruppia brevipedunculata in aquaculture dominated lagoons (Viet Nam). Frontiers in Conservation Science 54: 6868. doi: 10.3389/fcosc.2021.723376. [DOI] [Google Scholar]
- Ujiié Y, Ujiié H, Taira A, Nakamura T, Oguri K.. 2003. Spatial and temporal variability of surface water in the Kuroshio source region, Pacific Ocean, over the past 21,000 years: evidence from planktonic foraminifera. Marine Micropaleontology 49: 335–364. doi: 10.1016/s0377-8398(03)00062-8. [DOI] [Google Scholar]
- Vogt-Vincent NS, Mitarai S.. 2020. A persistent Kuroshio in the glacial East China Sea and implications for coral paleobiogeography. Paleoceanography and Paleoclimatology 35: e2020–PA003902. doi: 10.1029/2020PA003902. [DOI] [Google Scholar]
- Voillemot M, Pannell JR.. 2017. Maintenance of mixed mating after the loss of self-incompatibility in a long-lived perennial herb. Annals of Botany 119: 177–190. doi: 10.1093/aob/mcw203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YZ, Gilbert MG, Mathew B, Brickell CD.. 2007. Daphne Linnaeus. Flora of China, Vol. 13. Beijing: Science Press, 230–245. [Google Scholar]
- Watanabe S, Hajima T, Sudo K, et al. 2011. MIROC-ESM 2010: Model description and basic results of CMIP5-20c3m experiments. Geoscientific Model Development 4: 845–872. doi: 10.5194/gmd-4-845-2011. [DOI] [Google Scholar]
- Weir BS, Cockerham CC.. 1984. Estimating F-statistics for the analysis of population structure. Evolution 38: 1358–1370. doi: 10.2307/2408641. [DOI] [PubMed] [Google Scholar]
- Woźniak NJ, Kappel C, Marona C, Altschmied L, Neuffer B, Sicard A.. 2020. A similar genetic architecture underlies the convergent evolution of the selfing syndrome in Capsella. The Plant Cell 32: 935–949. doi: 10.1105/tpc.19.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SI, Kalisz S, Slotte T.. 2013. Evolutionary consequences of self-fertilization in plants. Proceedings of the Royal Society B: Biological Sciences 280: 20130133. doi: 10.1098/rspb.2013.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Thorogood D, Armstead IP, Franklin FCH, Barth S.. 2009. Identification of genes expressed during the self-incompatibility response in perennial ryegrass (Lolium perenne L.). Plant Molecular Biology 70: 709–723. doi: 10.1007/s11103-009-9501-2. [DOI] [PubMed] [Google Scholar]
- Zheng X, Li A, Kao S, et al. 2016. Synchronicity of Kuroshio Current and climate system variability since the Last Glacial Maximum. Earth and Planetary Science Letters 452: 247–257. doi: 10.1016/j.epsl.2016.07.028. [DOI] [Google Scholar]
- Zhong L, Barrett SC, Wang XJ, et al. 2019. Phylogenomic analysis reveals multiple evolutionary origins of selfing from outcrossing in a lineage of heterostylous plants. New Phytologist 224: 1290–1303. doi: 10.1111/nph.15905. [DOI] [PubMed] [Google Scholar]
- Zhu SS, Comes HP, Tamaki I, et al. 2020. Patterns of genotype variation and demographic history in Lindera glauca (Lauraceae), an apomict-containing dioecious forest tree. Journal of Biogeography 47: 2002–2016. doi: 10.1111/jbi.13874. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.