Abstract
Metabolic syndrome (MS), a chronic and non‐communicable pathological condition, is characterized by a constellation of clinical manifestations including insulin resistance, abdominal adiposity, elevated blood pressure, and perturbations in lipid metabolism. The prevalence of MS has increased dramatically in both developed and developing countries and has now become a truly global problem. Excessive energy intake and concomitant obesity are the main drivers of this syndrome. Mitophagy, in which cells degrade damaged mitochondria through a selective form of autophagy, assumes a crucial position in the regulation of mitochondrial integrity and maintenance. Abnormal mitochondrial quality could result in a spectrum of pathological conditions related to metabolic dysfunction, including metabolic syndrome, cardiovascular ailments, and neoplasms. Recently, there has been a proliferation of research pertaining to the process of mitophagy in the context of MS, and there are various regulatory pathways in MS, including pathways like the ubiquitin‐dependent mechanism and receptor‐mediated mechanisms, among others. Furthermore, studies have uncovered that the process of mitophagy serves a defensive function in the advancement of Metabolic Syndrome, and inhibition of mitophagy exacerbates the advancement of MS. As a result, the regulation of mitophagy holds great promise as a therapeutic approach in the management of Metabolic Syndrome. In this comprehensive analysis, the authors present a synthesis of the diverse regulatory pathways involved in mitophagy in the context of Metabolic Syndrome, as well as its modes of action in metabolic disorders implicated in the development of MS, Including obesity, insulin resistance (IR), and type 2 diabetes mellitus (T2DM), offering novel avenues for the prophylaxis and therapeutic management of MS.
Keywords: insulin resistance, metabolic dysfunction, metabolic syndrome, MS, mitophagy
1. INTRODUCTION
Metabolic syndrome (MS), is a complex medical condition characterized by a cluster of interrelated risk factors, including insulin resistance, high blood pressure, elevated blood sugar levels, excess body fat around the waist, and abnormal cholesterol levels. These factors increase the likelihood of developing serious health problems such as cardiovascular disease and type 2 diabetes. The condition is also commonly referred to as Syndrome X, or insulin resistance syndrome. 1 The development of metabolic syndrome is primarily driven by over‐consumption of calorie‐dense foods and sedentary lifestyles. These factors result in imbalances in energy intake and expenditure, leading to excess weight gain and accumulation of abdominal fat. This, in turn, increases the risk of insulin resistance and the development of the cluster of health problems associated with metabolic syndrome. 2 In the United States, MS is estimated to affect 34.7% of adults, 3 and with the global spread of Western lifestyles, the incidence of MS is increasing dramatically not only in the United States and Europe, but also in Asian countries such as China, Korea, and India, 4 , 5 and MS has now become a truly global problem. It is estimated that by 2040 there will be about 2.568 billion people living with MS worldwide. 1
Autophagy is a cellular degradation pathway that is characterized by its dependence on lysosomes and is widely prevalent in eukaryotic cells as a self‐protective mechanism to maintain a self‐stabilizing intracellular environment. 6 Mitophagy, a form of macroautophagy, is the process the process of autophagy facilitates the selective removal of damaged mitochondria from cells, which are then transported to lysosomes for subsequent degradation, thereby maintaining mitochondrial homeostasis. Mitophagy is widely recognized as a crucial mechanism of mitochondrial quality control (MQC) and involves three main processes: disruption of the mitochondrial membrane potential, triggering mitochondrial depolarization, and promoting the buildup of mitophagy receptors on the outer mitochondrial membrane (OMM). An increasing body of research has demonstrated a correlation between the cellular process of mitophagy and various metabolic disorders, including obesity, 7 insulin resistance (IR), 8 Type 2 diabetes mellitus (T2DM), 9 non‐alcoholic fatty liver disease (NAFLD), 10 atherosclerosis (AS), and heart disease, 11 which are pathologically associated with mitophagy dysfunction and may influence the onset of MS. Therefore, the authors present a comprehensive overview of the underlying mechanisms in the MS and its related metabolic diseases (Figure 1).
FIGURE 1.
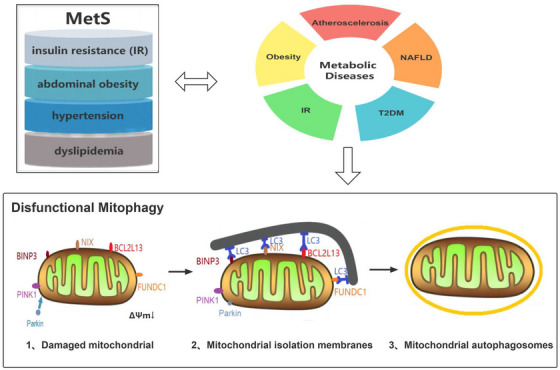
Relationship between mitophagy and MS. MS refers to a conglomeration of metabolic disturbances that encompass overweight, IR, hypertension and abnormal lipid levels. Promotion of mitophagy occurs through specific receptors on the outer mitochondrial membrane or through ubiquitin molecules attached to proteins on the mitochondrial surface, resulting in the formation of autophagosomes surrounding the mitochondria. A growing number of studies have shown that mitophagy has been linked to a range of metabolic disturbances including obesity, IR, T2DM, NAFLD, atherosclerosis (AS), and heart disease, which are pathologically associated with mitophagy dysfunction and may influence the onset of MetS. Source: Ref [8].
2. MITOPHAGY PATHWAYS IN MS
Mitophagy can be triggered through a variety of pathways, such as nutritional deficiency (starvation), disruption of the mitochondrial membrane potential, respiratory depression, hypoxia, and ROS accumulation. 12 Presently, there exist two principal regulatory pathways that regulate the process of mitophagy: The first is mitophagy chiefly overseen by PTEN‐induced putative kinase 1 (PINK1)/cytoplasmic E3 ubiquitin ligase (Parkin) and non‐Parkin‐dependent ubiquitin‐dependent mitophagy; The second refers to mitophagy mediated by mitophagy receptors, that is, non‐ubiquitin‐dependent mitophagy. 13 These mitophagy receptors are usually mitochondrial proteins containing the microtubule‐associated protein 1 light chain 3 (LC3)/GABARAP‐interacting region (LIR) motif, which can regulates the formation of mitochondrial isolation membranes through specific binding of LIR to members of the mitochondrial Atg8 family (LC3A/B/C, GABARAP, GABARAP‐L1/2). Subsequently, autophagic vesicles selectively encapsulate damaged mitochondria, forming mitochondrial autophagosomes. The degradation of damaged mitochondria within autophagosomes occurs through rapid fusion with lysosomes, thereby promoting the clearance of said mitochondria. 12 It has been well documented that impaired mitochondrial function is associated with MS and that mitochondrial autophagy leads to impaired mitochondrial function and is involved in the development of MS. 8 , 14
2.1. Parkin/PINK1
Mitophagy is mainly regulated by the PINK1/Parkin pathway, PINK1/Parkin‐driven mitophagy is the most characteristic pathway and the field of mitophagy is founded on the study of these two proteins. 15 In normal physiological conditions, PINK1 located in the inner mitochondrial membrane (IMM) is subjected to cleavage and degradation. In the event of mitochondrial damage, a loss of membrane potential impairs IMM translocation and subsequent degradation, leading to the accumulation of PINK1 on the OMM surface where it attracts cytosolic Parkin. Subsequently, the phosphorylation of Ser65 in the ubiquitin and ubiquitin‐like structural domains of Parkin facilitates the localization of PINK1 from the cell membrane to the OMM. Furthermore, Parkin initiates mitophagy through the promotion of mitochondrial fission to isolate damaged fragments, and by ubiquitinating mitochondrial proteins, thereby promoting their recognition and association with the surface of autophagosomes. 16 , 17 Hoshino and colleagues demonstrate that PINK1/Parkin pathway‐derived mitophagy protects pancreatic β‐cells and improves glucose intolerance in T2DM patients. 18 Also, Wang and colleagues found that Long non‐coding RNA H19 exerts a restraining effect on excessive mitophagy by restricting the expression of Pink1 protein, which alleviates this cardiac abnormality that arises during the condition of obesity. 19 These studies demonstrated that the development of metabolic syndrome (MS) is associated with the PINK1/Parkin pathway‐intervened process of mitophagy.
2.2. FUNDC1
FUNDC1 is a newly discovered OMM protein that serves as a receptor for hypoxia‐induced mitophagy. It possesses a characteristic LIR motif and three TM structural domains near the N‐terminal region. 12 Mutations in the LIR motif of FUNDC1 result in the disruption of FUNDC1‐LC3 interactions and the induction of mitophagy. The function of FUNDC1 is subject to regulation through reversible phosphorylation and ubiquitination, thereby enabling the detachment of damaged mitochondria and the initiation of mitophagy. 20 , 21 FUNDC1 regulation is strongly linked to the onset, evolvement, and prognosis of many ailment, including MS. Several experiments have now found that FUNDC1−/− mice develop more severe obesity and IR, MS, and myocardial remodelling, and that FUNDC1 deficiency leads to the inhibition of mitochondrial biogenesis, MQC dysregulation and even cell death, suggesting an important role for FUNDC1 in the cardiac function of patients with obese MS. 14 , 22
2.3. BINP3/NIX
LIR motifs are usually found in mitochondrial proteins that encode mitophagy receptors that recruits LC3 and growing mitochondrial phagocytes into the mitochondria designated to be removed. 23 Mutations in the N‐terminal region of both BNIP3 and BNIP3L (NIX) block the interaction with LC3 and result in defective mitophagy. 12 Hypoxia is a relevant trigger for mitophagy, and NIX and BNIP3 mediate post‐hypoxia mitophagy through enhanced activity by phosphorylation and dimerization and transcriptional regulation by hypoxia‐inducible factor‐1α (HIF1α). 15 It was also found that knockdown of NIX and FUNDC1 resulted in reduced mitophagy during differentiation. 24 Furthermore, BNIP3 acted upon PINK1 and promotes PINK1 heap up on the OMM and promotes PINK1‐Parkin mitophagy. 15 A study by Li and colleagues 25 demonstrated that the downregulation of Sirtuin 3 (Sirt3) inhibited BINP3/NIX pathway‐mediated mitophagy, and activated Sirt3 further activated the ERK‐CREB‐BNIP3 mitophagy pathway, result in the treatment of NAFLD. Activation of BNIP3L caused mitochondrial depolarization, mitophagy, and impaired insulin signaling via the MTOR‐RPS6KB pathway. Furthermore, exercise or pharmacological activation of PRKA, downstream of adrenergic signaling, has been shown to inhibit the function of BNIP3L via mechanisms and restored insulin signaling could overcome IR in myocytes. 26 These findings suggest the significance of BNIP3 and BNIP3L (NIX) pathways in the regulation of MS‐associated mitophagy, but the exact mechanisms remain to be further investigated.
2.4. Others
Bcl‐2‐like protein 13 (BCL2L13), which was recognized as BCL2‐RAMBO, is another OMM protein that is involved in mitochondrial fission in mammalian cells. Li and colleagues 27 showed the human BCL2L13 protein contains a LIR structural domain, which displays specificity in its binding to three autophagy‐related proteins, namely, LC3C, GABARAP, and GABARAP‐L1 in cells lacking Atg32, which in turn promotes mitophagy. BCL2L13 is significant in controlling apoptosis, mitochondrial fracture, and promoting mitochondrial autophagy. 28 Ju and colleagues 29 were the first to publish the novel finding that BCL2L13 promotes the differentiation and facilitation process of adipocytes. Subsequently, a study by Fujiwara and colleagues 30 found that knockdown of BCL2L13 in 3T3‐L1 adipocytes decreased the number of mitochondria, leading to increased MFN2 protein for mitochondrial fusion, decreased DRP1 protein for mitochondrial division, and enhanced mitophagy, and silencing of BCL2L13 inhibited adipogenic differentiation, mitochondrial biogenesis, and mitochondrial dynamics. Therefore, BCL2L13 plays a role in promoting adipogenesis by enhancing oxidative phosphorylation, preventing apoptosis, and regulating the maintenance of MQC through the process of mitophagy. These fundings suggested that BCL2L13 could be a potential pharmacological therapeutic target for MetS as a promising biomarker.
FK506‐binding protein 8 (FKBP8), which referred to as FKBP38, is another mitochondrial autophagy recognition protein that integrates LC3 independently of Parkin and mediates mitophagy and fission. 31 Recent study have found that the transmembrane structural domain of FKBP8 needed for its role in starvation‐induced autophagy, and that FKBP8 regulates activation of VPS34 lipid kinase by starvation and autophagy and co‐localizes with complexes of VPS34 lipid kinases and interacts with BECN1. 32 FKBP8 was found to interact with uncoupling protein 2 (UCP2), which is anchored to the OMM and dynamically regulates insulin secretion from islet β cells and glucose‐stimulated insulin, 33 and theoretically, FKBP8 is important in the development of IR or T2DM. However, the current research on FKBP8 is limited to cancer and neurological diseases, and the role of FKBP8 in cardiovascular diseases still needs to be further explored.
3. MITOPHAGY IN MS
In general, metabolic diseases such as obesity, IR, T2DM, NAFLD, AS, and heart disease lead to abnormal mitochondrial metabolism, which in turn leads to inadequacy β‐oxidation, oxidative stress, and accumulation of toxic lipid antioxidants and mitochondrial dysfunction, and mitochondrial autophagy can treat such metabolic diseases by eliminating this oxidative stress and mitochondrial damage. 34 In addition, mitochondrial autophagy was found to protect the adipose tissue microenvironment by suppressing obesity‐induced chronic inflammation, excessive oxidative stress, and ER stress, 35 thereby improving MS. Experiments by Tong and colleagues 7 showed that HFD‐induced obese mice had mitochondrial dysfunction and heart failure compared to healthy control case. Also, Xiang and colleagues 36 observed that PINK1/Parkin‐mediated mitochondrial autophagy was activated in submandibular gland cells of diabetic mice. Liu and colleagues 37 on the other hand showed that quercetin alleviated HFD‐induced improving PINK1/Parkin‐dependent mitophagy as a way to treat hepatic steatosis. In conclusion, mitophagy is related to metabolic diseases like obesity, IR, T2DM, NAFLD, AS, and heart disease, and mitophagy dysfunction is correlated to the etiopathogenesis of these diseases and may influence the pathogenesis of MS.
3.1. Mitophagy and obesity
Obesity is a natural consequence of excessive energy intake and sedentary lifestyle, mainly influenced by genetic and environmental factors. Excessive fat deposition in internal organs produces chronic inflammation, which eventually leads to glucolipid abnormalities, hypertension, diabetes, and other complications associated with IR and MS. 38 It was found that in the muscle of FUNDC1−/− mice, LC3‐mediated mitophagy is defective and ATP is reduced, but protective against high‐fat‐diet (HFD) induced obesity, while also improving systemic insulin sensitivity and glucose tolerance. 39 Lin and colleagues 40 suggest that sesamol may promote the browning of white adipocytes. Recent studies have found that binding of the nuclear receptors (NR1D1 and ULK1) by which adipocytes are induced to undergo mitophagy, thereby alleviating overweight. 41 The above studies demonstrate that increased mitophagy may alleviate obesity and that defects in mitophagy are protective against obesity.
3.2. Mitophagy and IR
Typically, IR is considered to be the underlying causative factor not only for the metabolic syndrome but also for its associated NAFLD, obesity‐related T2DM and atherosclerotic cardiovascular disease (ASCVD). 42 Extensive research has shown that mitophagy can improve MQC by increasing ROS production and inhibiting the inflammatory response in adipocytes, thereby inhibiting hepatic IR and steatosis. 8 , 43 A recent study found that ginsenoside CK could activate mitophagy in skeletal muscle through the DRP1/PINK1 pathway to maintain MQC, thereby reducing IR in diabetic mice. 44 In brief, mitophagy can exert its inhibitory effects in IR by maintaining MQC, promoting oxidative stress and inhibiting inflammatory responses, but there are still few studies on the related molecular mechanisms, and a large number of experiments are needed to verify them.
3.3. Mitophagy and T2DM
The metabolic stress triggered by T2DM can lead to pancreatic β‐cell dysfunction and IR, 45 and likewise, these contribute to MS. It has been found that there are many natural products that regulate mitophagy such as curcumin, caffeine, quercetin, berberine, and vitamins that can improve mitochondrial dysfunction related to T2DM. 45 The correlation of T2DM and ischemia‐reperfusion is inextricably linked, and adiponectin was found to attenuate the damage caused by reduced blood flow followed by its restoration, leading to an increase in oxidative stress, inflammation, cell death, and disrupted function of the mitochondria in lung‐injured tissues of T2DM mice by activating SIRT1‐ PINK1 signaling‐mediated mitohagy. 46 Furthermore, a recent study found that myocardial ischemia‐reperfusion (MIR) exacerbates the extent of damage in T2DM by inhibiting mitophagy. MitoQ, a mitochondria‐targeted antioxidant, can exert a protective effect on cardiomyocytes after MIR in T2DM by increasing its expression level through PINK1/Parkin pathway‐mediated mitophagy. 47 These conclusions demonstrate that mitochondrial autophagy participates in the development of T2DM through attenuated mitochondrial dysfunction, inflammation, and oxidative stress.
3.4. Mitophagy and NAFLD
NAFLD is the development of steatosis of the liver, whether had inflammation, fibrosis or not, lack of confounding factors such as excessive alcohol intake that may contribute to secondary liver lipid accumulation. Primary harmful index, for example, obesity, T2DM, and dyslipidemia; therefore, it can be considered a hepatic manifestation of the MS. 48 In recent years, a new concept called “metabolic (dysfunction) associated fatty liver, MAFLD” has even emerged to describe the relationship between NAFLD and metabolic dysfunction, emphasizing the simultaneous presence of lipid deposition in liver and abnormal metabolic function. 49 Zhou and colleagues 50 showed that the NR4A1/DNA‐PKcs/p53 pathway emerged as a novel molecular circadian mechanism leading to NAFLD, NAFLD by regulating mitophagy related to Bnip3 and Drp1, and also confirmed that melatonin stopped mitochondrial fission by interdicting the NR4A1/DNA‐PKcs/p53 pathway and restored mitophagy, finally improving mitochondrial and hepatic feature in NAFLD patients. Furthermore, PINK1‐mediated mitophagy might be responsible for CYANID‐3‐O‐glucoside's facilitation on NAFLD. 51 The latest study found that deletion of selenoprotein M (SELENOM) exacerbated HFD‐induced the presence of steatosis, inflammation, and fibrosis in the liver in obese mice, an increase in fatty acid oxidation and oxidative stress in the liver is associated with this condition; over‐expression of SELENOM activated Parkin‐mediated mitophagy through the AMPKα1‐MFN2 pathway, which cleared HFD‐damaged mitochondria. 52 Mitophagy may combat MS by improving mitochondrial dysfunction, and liver function in patients with NAFLD.
3.5. Miotophagy and AS
In atherosclerosis, lipids and leukocytes heap up in artery due to chronic inflammation, leading to plaque formation. MS was related to metabolic, pro‐inflammatory, and pro‐thrombotic states characterized by a series of interrelated plaque buildup influencing index, including OS, dyslipidemia, hypertension, hyperglycemia, obesity, IR, and living habit, containing diet structure and lack of exercise, that can increase the risk of atherosclerotic injury. 53 A study by Ma and colleagues 54 found that melatonin could scavenge ROS and attenuate the activation of NLRP3 (nucleotide‐binding domain and leucine‐rich repeat pyrin domain containing 3) inflammatory vesicles through the Sirt3/FOXO3a/Parkin mitophagy pathway in macrophages, which in turn significantly attenuated the size and vulnerability of AS plaques for the purpose of treating AS. In addition, Chen and colleagues 55 also summarized the various regulatory mechanisms of mitophagy in AS, including various cytokines, thrombi, and senescence. Thus, it is evident that mitophagy participates in AS pathogenesis and may be a novel therapeutic target.
4. SUMMARY AND OUTLOOK
MS is characterized by the coexistence of obesity, hypertension, dyslipidemia and hyperglycemia in individuals, causing the higher risk of CVDs. This paper focuses on the various cellular pathways of mitophagy in MS, such as the classical PINK1/Parkin pathway, the mitophagy receptor FUNDC1, and the BINP3/NIX pathway, as well as the regulatory mechanisms of mitophagy in MS‐related metabolism‐related illness like obesity, IR, DM, NAFLD, AS, and heart disease. Therefore, mitophagy may serve as a new target for the prevention and arrangement of MS, and an in‐depth investigation of the relationship between mitophagy and MS required fully understand the significance of mitophagy in MS. The current study found that mitophagy basically regulates the development of MS through anti‐inflammation and anti‐oxidative stress, and so on. On this basis, a more in‐depth investigation of the specific mechanisms of FKBP8 and BCL2L13‐related mitophagy pathways in MS‐related metabolic diseases such as IR and T2DM can help to understand the role of mitophagy in MS more comprehensively and thorough.
AUTHOR CONTRIBUTIONS
Mei‐Qi Miao contributed to the conception of the article, and wrote the manuscript. Yu‐Bo Han contributed significantly to manuscript preparation, and the review and revision of the original draft. Liu Li provided funding for this review.
CONFLICT OF INTEREST STATEMENT
The authors affirm the absence of any competing interests.
ACKNOWLEDGMENTS
We thank all authors for their outstanding contributions to this paper, especially Dr. Yubo Han, who was responsible for the revision and review of this paper, and Dr. Li Liu, who provided financial support for this paper. National Natural Science Foundation of China, Grant/Award Numbers: 82074346.
Miao M‐Q, Han Y‐B, Liu L. Mitophagy in metabolic syndrome. J Clin Hypertens. 2023;25:397–403. 10.1111/jch.14650
DATA AVAILABILITY STATEMENT
None.
REFERENCES
- 1. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Niekerk G, du Toit A, Loos B, et al. Nutrient excess and autophagic deficiency: explaining metabolic diseases in obesity. Metabolism. 2018;82:14‐21. [DOI] [PubMed] [Google Scholar]
- 3. Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA. 2020;323(24):2526‐2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang HH, Lee DK, Liu M, et al. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr Gastroenterol Hepatol Nutr. 2020;23(3):189‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ge H, Yang Z, Li X, et al. The prevalence and associated factors of metabolic syndrome in Chinese aging population. Sci Rep. 2020;10(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garza‐Lombó C, Pappa A, Panayiotidis MI, et al. Redox homeostasis, oxidative stress and mitophagy. Mitochondrion. 2020;51:105‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tong M, Saito T, Zhai P, et al. Alternative mitophagy protects the heart against obesity‐associated cardiomyopathy. Circ Res. 2021;129(12):1105‐1121. [DOI] [PubMed] [Google Scholar]
- 8. Su Z, Nie Y, Huang X, et al. Mitophagy in hepatic insulin resistance: therapeutic potential and concerns. Front Pharmacol. 2019;10:1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shan Z, Fa WH, Tian CR, et al. Mitophagy and mitochondrial dynamics in type 2 diabetes mellitus treatment. Aging (Albany NY). 2022;14(6):2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aryapour E, Kietzmann T. Mitochondria, mitophagy, and the role of deubiquitinases as novel therapeutic targets in liver pathology. J Cell Biochem. 2022;123(10):1634‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poznyak AV, Nikiforov NG, Wu WK, et al. Autophagy and mitophagy as essential components of atherosclerosis. Cells. 2021;10(2):443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Onishi M, Yamano K, Sato M, et al. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021;40(3):e104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Zheng N, Ding X. Mitophagy disequilibrium, a prominent pathological mechanism in metabolic heart diseases. Diabetes Metab Syndr Obes. 2021;14:4631‐4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu H, Wang Y, Li W, et al. Deficiency of mitophagy receptor FUNDC1 impairs mitochondrial quality and aggravates dietary‐induced obesity and metabolic syndrome. Autophagy. 2019;15(11):1882‐1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Killackey SA, Philpott DJ, Girardin SE. Mitophagy pathways in health and disease. J Cell Biol. 2020;219(11):e202004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Su Z, Guo Y, Huang X, et al. Phytochemicals: targeting mitophagy to treat metabolic disorders. Front Cell Dev Biol. 2021;9:686820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu W, Xu H, Wang Z, et al. PINK1‐Parkin‐mediated mitophagy protects mitochondrial integrity and prevents metabolic stress‐induced endothelial injury. PLoS One. 2015;10(7):e0132499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoshino A, Ariyoshi M, Okawa Y, et al. Inhibition of p53 preserves Parkin‐mediated mitophagy and pancreatic β‐cell function in diabetes. Proc Natl Acad Sci USA. 2014;111(8):3116‐3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang SH, Zhu XL, Wang F, et al. LncRNA H19 governs mitophagy and restores mitochondrial respiration in the heart through Pink1/Parkin signaling during obesity. Cell Death Dis. 2021;12(6):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qiu Z, Wei Y, Song Q, et al. The role of myocardial mitochondrial quality control in heart failure. Front Pharmacol. 2019;10:1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu L, Li Y, Chen Q. The emerging role of FUNDC1‐mediated mitophagy in cardiovascular diseases. Front Physiol. 2021;12:807654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ren J, Sun M, Zhou H, et al. FUNDC1 interacts with FBXL2 to govern mitochondrial integrity and cardiac function through an IP3R3‐dependent manner in obesity. Sci Adv. 2020;6(38):eabc8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abudureyimu M, Yu W, Cao RY, et al. Berberine promotes cardiac function by upregulating PINK1/Parkin‐mediated mitophagy in heart failure. Front Physiol. 2020;11:565751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lampert MA, Orogo AM, Najor RH, et al. BNIP3L/NIX and FUNDC1‐mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation. Autophagy. 2019;15(7):1182‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li R, Xin T, Li D, et al. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: the role of the ERK‐CREB pathway and Bnip3‐mediated mitophagy. Redox Biol. 2018;18:229‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. da Silva Rosa SC, Martens MD, Field JT, et al. BNIP3L/Nix‐induced mitochondrial fission, mitophagy, and impaired myocyte glucose uptake are abrogated by PRKA/PKA phosphorylation. Autophagy. 2021;17(9):2257‐2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li M, Jia J, Zhang X, et al. Selective binding of mitophagy receptor protein Bcl‐rambo to LC3/GABARAP family proteins. Biochem Biophys Res Commun. 2020;530(1):292‐300. [DOI] [PubMed] [Google Scholar]
- 28. Meng F, Sun N, Liu D, et al. BCL2L13: physiological and pathological meanings. Cell Mol Life Sci. 2021;78(6):2419‐2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ju L, Chen S, Alimujiang M, et al. A novel role for Bcl2l13 in promoting beige adipocyte biogenesis. Biochem Biophys Res Commun. 2018;506(3):485‐491. [DOI] [PubMed] [Google Scholar]
- 30. Fujiwara M, Tian L, Le PT, et al. The mitophagy receptor Bcl‐2‐like protein 13 stimulates adipogenesis by regulating mitochondrial oxidative phosphorylation and apoptosis in mice. J Biol Chem. 2019;294(34):12683‐12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhujabal Z, Birgisdottir ÅB, Sjøttem E, et al. FKBP8 recruits LC3A to mediate Parkin‐independent mitophagy. EMBO Rep. 2017;18(6):947‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aguilera MO, Robledo E, Melani M, et al. FKBP8 is a novel molecule that participates in the regulation of the autophagic pathway. Biochim Biophys Acta Mol Cell Res. 2022;1869(5):119212. [DOI] [PubMed] [Google Scholar]
- 33. Brand MD, Parker N, Affourtit C, et al. Mitochondrial uncoupling protein 2 in pancreatic β‐cells. Diabetes Obes Metab. 2010;12(suppl 2):134‐140. [DOI] [PubMed] [Google Scholar]
- 34. Liu L, Liao X, Wu H, et al. Mitophagy and its contribution to metabolic and aging‐associated disorders. Antioxid Redox Signal. 2020;32(12):906‐927. [DOI] [PubMed] [Google Scholar]
- 35. Marycz K, Weiss C, Śmieszek A, et al. Evaluation of oxidative stress and mitophagy during adipogenic differentiation of adipose‐derived stem cells isolated from equine metabolic syndrome (EMS) horses. Stem Cells Int. 2018;2018:5340756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xiang RL, Huang Y, Zhang Y, et al. Type 2 diabetes‐induced hyposalivation of the submandibular gland through PINK1/Parkin‐mediated mitophagy. J Cell Physiol. 2020;235(1):232‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu P, Lin H, Xu Y, et al. Frataxin‐mediated PINK1‐Parkin‐dependent mitophagy in hepatic steatosis: the protective effects of quercetin. Mol Nutr Food Res. 2018;62(16):e1800164. [DOI] [PubMed] [Google Scholar]
- 38. Achike FI, To NH, Wang H, et al. Obesity, metabolic syndrome, adipocytes and vascular function: a holistic viewpoint. Clin Exp Pharmacol Physiol. 2011;38(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 39. Fu T, Xu Z, Liu L, et al. Mitophagy directs muscle‐adipose crosstalk to alleviate dietary obesity. Cell Rep. 2018;23(5):1357‐1372. [DOI] [PubMed] [Google Scholar]
- 40. Lin C, Chen J, Hu M, et al. Sesamol promotes browning of white adipocytes to ameliorate obesity by inducing mitochondrial biogenesis and inhibition mitophagy via β3‐AR/PKA signaling pathway. Food Nutr Res. 2021;65:7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu B, Pan JB, Yu FY. The combination of nuclear receptor NR1D1 and ULK1 promotes mitophagy in adipocytes to ameliorate obesity. Adipocyte. 2022;11(1):202‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nolan CJ, Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: time for a conceptual framework shift. Diab Vasc Dis Res. 2019;16(2):118‐127. [DOI] [PubMed] [Google Scholar]
- 43. He F, Huang Y, Song Z, et al. Mitophagy‐mediated adipose inflammation contributes to type 2 diabetes with hepatic insulin resistance. J Exp Med. 2021;218(3):e20201416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li W, Li H, Zheng L, et al. Ginsenoside CK improves skeletal muscle insulin resistance by activating DRP1/PINK1‐mediated mitophagy. Food Funct. 2023;14(2):1024‐1036. [DOI] [PubMed] [Google Scholar]
- 45. Shan Z, Fa WH, Tian CR, et al. Mitophagy and mitochondrial dynamics in type 2 diabetes mellitus treatment. Aging (Albany NY). 2022;14(6):2902‐2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jiang T, Liu T, Deng X, et al. Adiponectin ameliorates lung ischemia–reperfusion injury through SIRT1‐PINK1 signaling‐mediated mitophagy in type 2 diabetic rats. Respir Res. 2021;22(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ji Y, Leng Y, Lei S, et al. The mitochondria‐targeted antioxidant MitoQ ameliorates myocardial ischemia‐reperfusion injury by enhancing PINK1/Parkin‐mediated mitophagy in type 2 diabetic rats. Cell Stress Chaperones. 2022;27(4):353‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Michelotti A, de Scordilli M, Palmero L, et al. NAFLD‐related hepatocarcinoma: the malignant side of metabolic syndrome. Cells. 2021;10(8):2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eslam M, Sanyal AJ, George J, et al. MAFLD: a consensus‐driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999‐2014. e1. [DOI] [PubMed] [Google Scholar]
- 50. Zhou H, Du W, Li YE, et al. Effects of melatonin on fatty liver disease: the role of NR 4A1/DNA‐PK cs/p53 pathway, mitochondrial fission, and mitophagy. J Pineal Res. 2018;64(1). 10.1111/jpi.12450 [DOI] [PubMed] [Google Scholar]
- 51. Li X, Shi Z, Zhu Y, et al. Cyanidin‐3‐O‐glucoside improves non‐alcoholic fatty liver disease by promoting PINK1‐mediated mitophagy in mice. Br J Pharmacol. 2020;177(15):3591‐3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cai J, Huang J, Yang J, et al. The protective effect of selenoprotein M on non‐alcoholic fatty liver disease: the role of the AMPKα1–MFN2 pathway and Parkin mitophagy. Cell Mol Life Sci. 2022;79(7):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aboonabi A, Meyer RR, Singh I. The association between metabolic syndrome components and the development of atherosclerosis. J Hum Hypertens. 2019;33(12):844‐855. [DOI] [PubMed] [Google Scholar]
- 54. Ma S, Chen J, Feng J, et al. Melatonin ameliorates the progression of atherosclerosis via mitophagy activation and NLRP3 inflammasome inhibition. Oxid Med Cell Longev. 2018;2018:9286458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen Y, Qin W, Li L, et al. Mitophagy: critical role in atherosclerosis progression. DNA Cell Biol. 2022;41(10):851‐860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.


