Dysfunction of GABAergic systems has been implicated in schizophrenia and other psychosis spectrum disorders (Taylor and Tso, 2015). Using a benzodiazepine challenge in combination with fMRI, our group has demonstrated GABAergic dysfunction in schizophrenia both while patients passively viewed emotionally salient images (Taylor et al., 2014) and while performing a face processing task (Tso et al., 2015). Further, the altered blood oxygen level-dependent (BOLD) response to the benzodiazepine in schizophrenia was positively correlated with increased negative affect (NA). These emerging links between GABAergic systems, affect and emotion processing indicate an important line of inquiry.
Here, we aimed to build upon these findings by exploring these links using Magnetic Resonance Spectroscopy (MRS). Thus far, studies of GABA concentrations in schizophrenia using MRS have reported mixed findings (Egerton et al., 2017; Nakahara et al., 2021; Simmonite et al., 2022), likely reflecting the influence of multiple factors upon GABAergic systems, such as illness stage and heterogeneous symptom presentations. To address this issue, we compared GABA+ concentrations in the rostral MFC and midline occipital cortex in patients with first episode psychosis (FEP) and individuals meeting criteria for attenuated psychosis syndrome (APS), with healthy control participants, testing the hypothesis that negative affect (NA), measured by the Psychological Stress Index (PSI9: Tso et al., 2012), would be associated with reduced GABA+ signal in the rostral MFC. Based on prior reports of increased rostral MFC GABA in early schizophrenia (Chen et al., 2017; de la Fuente-Sandoval et al., 2018; Kegeles et al., 2012), we hypothesized that GABA+ concentrations in this voxel would be increased relative to control participants, but a second process, related to affective dysregulation in the face of minor stress, would drive GABA+ levels in the opposite direction.
Study participants were 14 patients with FEP (9 men, 5 women, age = 21.64 ±2.56), 7 individuals meeting criteria for APS (5 men, 2 women, age = 19.86 ±3.63), and 15 demographically matched controls (10 males, 5 women, age = 21.60 ±3.56) who were free from personal histories of psychiatric disorders or first-degree relatives with psychotic illnesses. Exclusion criteria included contraindications to MRI and history of head injury or concussions with unconsciousness lasting > 5 minutes. Participants completed the 9-item PSI9, a validated self-report scale which measures stress sensitivity and the tendency to experience NA as a trait characteristic (Tso et al., 2012). The study and all its procedures were approved by the University of Michigan Medical School IRB.
MRS data from rostral MFC and midline occipital cortex voxels (Figure 1A and 1B) were obtained using a MEGA-PRESS (Mescher et al., 1998) sequence on a 3T Phillips Ingenia system with a 32-channel head coil. Preprocessing and quantification of the edited MRS spectrum were performed using standard Gannet 3.1 processing steps (Edden et al., 2014). As the GABA signal detected at 3.00 ppm using our experimental parameters is expected to contain contributions from macromolecules and homocarnosine, we refer to it as GABA+. GABA+ concentrations were evaluated relative to the unsuppressed water signal and corrected for voxel tissue composition.
Figure 1.
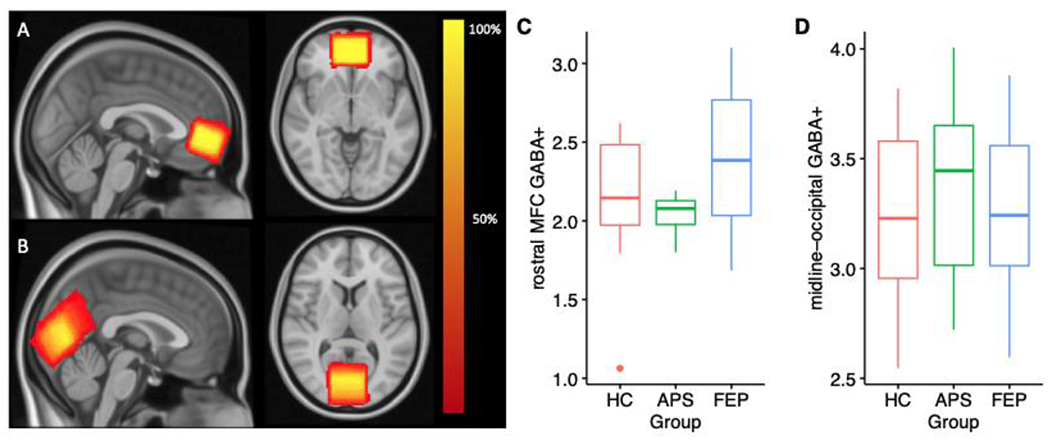
Heat maps describing the spatial overlap of MRS voxels in (A) rostral MFC and (B) midline occipital cortex across all participants. Boxplots demonstrating GABA+ concentrations in (C) rostral MFC and (D) midline occipital cortex in each of the three participant groups. HC: healthy control participants, APS: attenuated psychosis syndrome; FEP: first episode psychosis. These values are uncorrected for PSI9.
Groups differed significantly on PSI9 scores (HCmean = 9.87 ±4.09; APSmean = 15.14 ±6.07; FEPmean = 19.29 ±5.70; F2,33 = 12.14, p<.001). Post-hoc Tukey tests revealed PSI9 scores were significantly higher in FEP compared with HC (p <.0001), but there were no differences between the APS and HC groups (p = .08), or the FEP and APS groups (p = .21). ANOVAs conducted to examine GABA+ concentrations revealed a trend toward a significant group effect in the rostral MFC (Figure 1C; HCmean = 2.16 ±0.42; APSmean = 2.04 ±0.14; FEPmean = 2.50 ±0.53; F2,28 = 3.04, p = .06). but no group differences in the midline occipital cortex (Figure 1D; HCmean = 3.24 ±0.40; APSmean = 3.43 ±0.51; FEPmean = 3.24 ±0.41; F2,32 = 0.49, p = .62). When PSI9 was included in the model to adjust for group differences in negative affect, we found a significant effect of group on rostral MFC GABA+ concentrations (F2,28 = 4.26, p = .02). Planned comparisons revealed elevated rostral MFC GABA+ in the FEP group in comparison with the HC and APS groups (t27= 2.90, p = .01). There was a trend towards elevated rostral MFC GABA+ in the APS group when compared with healthy controls (t27= 2.00, p = .0548). We did not find significant group effects on occipital GABA+ (F2,31 = 0.48, p = .62) when PSI9 was included in the model.
While limited by a modest sample size and the confound of medication, we found evidence of significantly elevated rostral MFC GABA+ in psychosis when controlling for levels of NA. When considered alongside previously published demonstrations of altered BOLD signal in response to a benzodiazepine challenge which correlate with levels of NA, we suggest the presence of at least two pathological processes involving GABA in psychosis. The first is distinguished by an increase in rostral MFC GABA which occurs early in the illness and may be driven down by treatment or other factors, which has led to contradictory findings in the literature. This process may reflect a primary pathophysiological process or a compensatory response – for example, to environmental stressors. In addition, we propose a second process reflected by reduced GABA which is associated with increased negative affect and an altered BOLD response to benzodiazepine. As we note, the edited GABA signal – which we refer to as GABA+ - includes significant contributions from macromolecules and homocarnosine, a limitation which means the effect we observed could potentially be unrelated to GABA, and instead driven by other components of the signal. While more data is required to untangle these processes, and clarify GABAergic dysfunction in the psychosis spectrum, NA is a strong, transdiagnostic predictor of functional outcome, and these findings establish potential therapeutic leverage points linking GABAergic treatments, with NA as the clinical outcome.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen T, Wang Y, Zhang J, Wang Z, Xu J, Li Y, Yang Z, Liu D, 2017. Abnormal Concentration of GABA and Glutamate in The Prefrontal Cortex in Schizophrenia.-An in Vivo 1H-MRS Study. Shanghai Arch. Psychiatry 29, 277–286. 10.11919/j.issn.1002-0829.217004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, León-Ortiz P, Rodríguez-Mayoral O, Jung-Cook H, Solís-Vivanco R, Graff-Guerrero A, Shungu DC, 2018. Prefrontal and Striatal Gamma-Aminobutyric Acid Levels and the Effect of Antipsychotic Treatment in First-Episode Psychosis Patients. Biol. Psychiatry 83, 475–483. 10.1016/j.biopsych.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ, 2014. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging 40, 1445–1452. 10.1002/jmri.24478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Modinos G, Ferrera D, McGuire P, 2017. Neuroimaging studies of GABA in schizophrenia: A systematic review with meta-analysis. Transl. Psychiatry 10.1038/tp.2017.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, Gil R, Slifstein M, Abi-Dargham A, Lisanby SH, Shungu DC, 2012. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 69, 449–459. 10.1001/archgenpsychiatry.2011.1519 [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R, 1998. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 11, 266–272. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Tsugawa S, Noda Y, Ueno F, Honda S, Kinjo M, Segawa H, Hondo N, Mori Y, Watanabe H, Nakahara K, Yoshida K, Wada M, Tarumi R, Iwata Y, Plitman E, Moriguchi S, de la Fuente-Sandoval C, Uchida H, Mimura M, Graff-Guerrero A, Nakajima S, 2021. Glutamatergic and GABAergic metabolite levels in schizophrenia-spectrum disorders: a meta-analysis of 1H-magnetic resonance spectroscopy studies. Mol. Psychiatry 10.1038/s41380-021-01297-6 [DOI] [PubMed] [Google Scholar]
- Simmonite M, Steeby CJ, Taylor SF, 2022. MEDIAL FRONTAL CORTEX GAMMA-AMINOBUTYRIC ACID CONCENTRATIONS IN PSYCHOSIS SPECTRUM AND MOOD DISORDERS: A META-ANALYSIS OF PROTON MAGNETIC RESONANCE SPECTROSCOPY STUDIES. Biol. Psychiatry 10.1016/j.biopsych.2022.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Demeter E, Phan KL, Tso IF, Welsh RC, 2014. Abnormal GABAergic Function and Negative Affect in Schizophrenia. Neuropsychopharmacology 39, 1000–1008. 10.1038/npp.2013.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Tso IF, 2015. GABA abnormalities in schizophrenia: A methodological review of in vivo studies. Schizophr. Res 10.1016/j.schres.2014.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso IF, Fang Y, Phan KL, Welsh RC, Taylor SF, 2015. Abnormal GABAergic function and face processing in schizophrenia: A pharmacologic-fMRI study. Schizophr. Res 168, 338–344. 10.1016/j.schres.2015.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso IF, Grove TB, Taylor SF, 2012. Self-assessment of psychological stress in schizophrenia: Preliminary evidence of reliability and validity. Psychiatry Res. 195, 39–44. 10.1016/j.psychres.2011.07.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


