Abstract
OBJECTIVE:
To evaluate characteristics associated with treatment failure 1 year after midurethral sling in women with mixed urinary incontinence.
METHODS:
Four-hundred three women who participated in a randomized trial that compared midurethral sling and behavioral and pelvic floor muscle therapy (combined group) compared with midurethral sling alone for mixed incontinence with 1-year follow-up data were eligible for this planned secondary analysis. Overall treatment failure was defined as meeting criteria for subjective or objective failure or both. Subjective failure was defined as not meeting the minimal clinical important difference for improvement on the UDI (Urogenital Distress Inventory) total score (26.1 points). Objective failure was defined as not achieving 70% improvement on mean incontinence episodes of any type per day or having undergone any additional treatment for persistent urinary symptoms at 12 months postoperative. Logistic regression models for treatment failure were constructed. Independent variables included site and treatment group, and clinical and demographic variables based on bivariate comparisons (P<.2). Treatment group interaction effects were evaluated.
RESULTS:
One hundred twelve of 379 (29.6%) women had overall treatment failure, with 56 of 379 (14.7%) undergoing additional treatment but only two needing intervention for stress incontinence. Previous overactive bladder (OAB) medication (unadjusted odds ratio [OR] 2.19, adjusted odds ratio [aOR] 1.96, 95% CI 1.17–3.31); detrusor overactivity on cystometrogram (OR 2.25, aOR 2.82, 95% CI 1.60–4.97);and higher volume at first urge (OR 1.03, aOR 1.04, 95% CI 1.01–1.07) were associated with overall failure. Worse UDI-urgency scores were associated with failure, with an added interaction effect in the midurethral sling–alone group.
CONCLUSIONS:
Certain clinical and urodynamic variables are associated with treatment failure after midurethral sling in women with mixed urinary incontinence. Women with more severe urgency symptoms at baseline may benefit from perioperative behavioral and pelvic floor muscle therapy combined with midurethral sling. Overall, the need for additional urinary treatment was low and primarily for OAB.
CLINICAL TRIAL REGISTRATION:
Up to 50% of women with urinary incontinence have mixed urinary incontinence, which includes both stress urinary incontinence (SUI) and urgency urinary incontinence (UUI).1 Mixed urinary incontinence often is considered more severe and more difficult to treat than having either urinary condition alone.2 Clinical guidelines have recommended approaching and treating the conditions separately, cautioning that surgery for the SUI component may worsen UUI.3-5 These recommendations largely are based on limited data for older procedures, including Burch urethropexy and pubovaginal sling. Based on previous observational data that suggest that midurethral sling procedures may have a decreased risk for worse urgency outcomes, the ESTEEM (Effects of Surgical Treatment Enhanced with Exercise for Mixed Urinary Incontinence) trial was designed as a multicenter network trial to test whether combining perioperative behavioral and pelvic floor muscle therapy with midurethral sling would improve urinary symptoms at 12 months, compared with sling alone. The study demonstrated that 85% of women were overall “much better” or “very much better” after midurethral sling, with or without behavioral and pelvic floor muscle therapy, with unexpected significant improvement in UUI symptoms in both groups. Fewer than 5% of women reported worsening urgency at 12 months.6 This would suggest that midurethral sling alone may be an effective treatment for both SUI and UUI in some women.
Understanding risk factors for persistent bothersome urinary symptoms after midurethral sling, either SUI or UUI, in women with mixed urinary incontinence would assist perioperative counseling. Prior studies have mostly focused on populations with pure SUI or stress-predominant mixed incontinence.7,8 Risk factors for sling failure identified in these populations include concurrent prolapse surgery, preoperative anticholinergic medication use, age, obesity, urgency, and baseline mixed incontinence symptoms. The ESTEEM trial presents an opportunity to evaluate characteristics associated with persistent urinary symptoms after midurethral sling in a well-characterized mixed incontinence population. The objective of this study was to identify demographic and clinical variables associated with treatment failure (persistent urinary symptoms) at 12 months in women with mixed incontinence undergoing midurethral sling.
METHODS
The original ESTEEM trial was a multicenter, randomized, superiority trial that compared behavioral and pelvic floor muscle therapy combined with midurethral sling (combined treatment) compared with midurethral sling alone for mixed urinary incontinence conducted by the Pelvic Floor Disorders Network between November 2013 and July 2017. Study methods and results previously have been published.9 Women 21 years of age and older, who reported moderately to severely bothersome SUI and UUI for at least 3 months, and who documented at least one SUI episode and at least one UUI episode on a 3-day bladder diary were eligible. Exclusion criteria included examination findings of or planned concomitant surgery for anterior or apical prolapse, history of prior sling, current overactive bladder (OAB) medication use (participants were eligible after a 3-week washout of OAB medication), or other UUI treatment (neuromodulation, intradetrusor onabotulinumtoxinA). Although the clinical utility of urodynamic testing remains unclear, the team decided that baseline results may help with clinical outcome prediction. Urodynamic testing within the past 18 months could be included, however because eligibility included women already electing surgery, we did not feel repeat testing was clinically justified if certain parameters were missing. The technique of urodynamic testing for sites was consistent with the technique used by Nager et al.10
Surgical technique for midurethral sling and the behavioral and pelvic floor muscle therapy intervention were standardized. Retropubic and transobturator sling approaches were allowed. Patients were not masked and were randomly assigned 1:1 using randomly permuted blocks, stratified by clinical site and UUI severity. Institutional review board approval was obtained at the nine clinical sites, and written informed consent was obtained. An independent data and safety monitoring board reviewed the progress and safety of the study. There was no involvement from industry. Both full length retropubic and transobturator midurethral sling techniques were allowed.
For this planned secondary analysis, women from the original ESTEEM trial were eligible if they had 12 months of data. Women completed questionnaires including the UDI (Urogenital Distress Inventory)11 and 3-day bladder diaries at baseline and at 3, 6, and 12 months postsurgery. The UDI is a 19-item, validated, patient-reported outcome questionnaire that includes three symptom subscales (stress incontinence symptoms, irritative symptoms [includes UUI, frequency, nocturia, and urgency], and obstructive symptoms). Each subscale ranges from 0 to 100 points, with a total score range of 0–300 points; higher scores indicate greater symptom severity. In the primary ESTEEM study, it was previously reported that the minimal clinically important difference for the UDI was estimated in the trial’s study population by using distribution and anchor-based methods.6 The minimal clinically important difference for the UDI-total score was estimated to be 26.1 points, 10.2 for the UDI-irritative subscale score, and 5.4 points for the UDI-stress subscale score based on anchor-based methods using the Patient Global Impression of Improvement. These minimal clinically important difference values were used in this secondary analysis as they are estimated directly from a mixed incontinence population and thus are most relevant to our population of interest. Additional questionnaires included the Incontinence Impact Questionnaire,11 the Overactive Bladder Questionnaire,12 and the Patient Global Impression of Severity.13
Questionnaires were administered in person by research personnel on paper case report forms and completed by participants. Data were keyed into the electronic data capture system by site personnel. Consistency between data recorded on the paper forms and in the electronic data capture system was verified for a subset of study participants during routine site monitoring visits conducted by the data coordinating center. Questionnaires that were completely missing were excluded from analysis. Individual questions that were missing were handled according to the scoring instructions for the particular questionnaire.
For this secondary analysis, we defined urinary treatment failure outcomes at 12 months, subjectively and objectively, based on persistent urinary symptoms or the need for additional treatment after surgery. Women with mixed urinary incontinence are a unique population that can be challenging to treat. Persistent or worsening UUI after surgery often is considered treatment “failure” by patients. Therefore, it was critical that the outcomes we used were meaningful from a patient perspective and were able to also capture SUI, UUI, and OAB improvement and worsening or no change in symptoms. If either treatment (combined or sling alone) was associated with significantly worsening UUI, the team could not consider that a “successful outcome,” even if SUI was improved. This is unique to the mixed urinary incontinence patient population and is different compared with a pure SUI- or SUI-predominant population. Therefore, overall treatment failure was defined as meeting the criteria for subjective, objective, or both, where subjective failure was defined as not meeting the minimal clinically important difference for improvement on the UDI-total score (26.1 points). Objective failure was defined as not achieving at least a 70% decrease in mean incontinence episodes of any type per day, based on bladder diary or having undergone any additional treatment for lower urinary tract symptoms at 12 months after surgery. Additional urinary treatment could include treatment for SUI, OAB, or voiding dysfunction. We included both subjective and objective outcomes in our definition because they often do not correlate and both are important to include when defining a composite definition of treatment failure. Women enrolled in the trial who completed at least 12 months of follow-up or met criteria for treatment failure before the 12-month visit were included in this analysis. For women who underwent additional treatment, the last UDI measure collected before initiating additional treatment was used to assess subjective failure.
This was a secondary, exploratory analysis and a sample size estimate was not performed. Bivariate analyses were performed using χ2 tests, student’ t tests, and Wilcoxon rank-sum tests, as appropriate, to identify potential clinical, demographic, and urodynamic characteristics associated with failure. Logistic regression models were constructed for each outcome (overall, subjective, and objective failure) at 12 months. Initial models included site and assigned treatment group, and clinical and demographic variables significant in bivariate comparisons at the P<.2 level. Final models included site and treatment group, and backward selection was used to determine which other risk factors to retain. Selection was based on a 0.10 significance level to stay in the model, and candidate variables for exclusion were assessed based on changes to Akaike’s Information Criteria. Potential interaction effects that involved treatment group and collinearity were assessed. Unadjusted odds ratios (ORs), adjusted odds ratios (aORs), and 95% CIs described the associations between preoperative patient characteristics and the outcomes. A sensitivity analysis was conducted using imputation to assess the importance of variables that were excluded due to the degree of missingness. A separate sensitivity analysis excluded women who required a 3-week washout of OAB medication to be eligible for ESTEEM. Additional analyses were performed to investigate the association between the type of persistent urinary symptoms (SUI or UUI and OAB) and failure. Demographic and clinical characteristics were compared between ESTEEM participants who were included compared with those who were excluded from this secondary analysis subpopulation to provide additional information about comparability and generalizability of findings. Race was self-reported and included to describe our study population.
A 5% two-sided significance level was used for all statistical testing, and no adjustments for multiple testing were made. Analyses were performed using SAS 9.4.
RESULTS
Baseline data were obtained from 480 women; 16 were discovered to be ineligible after randomization. Of the 464 eligible women, 403 (86.9%) had sufficient data at 12 months for this secondary analysis; 348 (75%) women underwent retropubic sling, 83 (17.9%) underwent transobturator sling, and 33 (7%) had missing sling data. Twenty-four women had missing diary and additional treatment data, leaving 379 women for this analysis. Of these, 112 (29.6%) had overall treatment failure, with 17 of 388 (4.4%) having subjective failure and 108 of 379 (28.5%) having objective failure. Thirteen of the 379 (3.4%) women who met criteria for overall failure met criteria for both subjective and objective failure, and 56 of the 379 (14.8%) required any additional urinary treatment, with 51 of 379 (13.5%) requiring UUI and OAB treatment.
Baseline demographic, clinical, and incontinence severity characteristics of women with overall failure compared with those without overall failure are shown in Table 1.
Table 1.
Characteristics of Women With Overall Treatment Failure Compared With Overall Treatment Success
| Characteristics and Baseline Measures | Failure (n=112) | Success (n=267) | Mean or Median Difference or OR (95% CI)* |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age (y) | 56.1±11.2 [34.7–82.0] | 53.9±10.0 [26.2–77.6] | 2.2 (−0.3 to 4.6) |
| Race† | |||
| Black–African American | 11 (9.8) | 17 (6.4) | 1.7 (0.7–3.7) |
| White | 84 (75.0) | 216 (80.9) | Ref |
| Other | 17 (15.2) | 34 (12.7) | 1.3 (0.7–2.4) |
| Ethnicity | |||
| Hispanic, Latina | 29 (25.9) | 64 (24.0) | Ref |
| Not Hispanic, not Latina | 82 (73.2) | 198 (74.2) | 0.9 (0.6–1.5) |
| Unknown or not reported | 1 (0.9) | 5 (1.9) | 0.4 (0.0–3.9) |
| Insurance | |||
| Private or HMO | 67 (59.8) | 204 (76.4) | 0.5 (0.3–0.7) |
| Medicaid or Medicare | 53 (47.3) | 71 (26.6) | 2.5 (1.6–3.9) |
| Self-pay | 0 (0.0) | 1 (0.4) | — |
| BMI (kg/m2) | 33.4±6.6 [22.0–50.0] | 31.1±6.6 [17.0–59.0] | 2.4 (0.9–3.9) |
| Current smoker | 15 (13.4) | 24 (9.0) | 1.6 (0.8–3.1) |
| Functional comorbidity index‡ | 3.5±2.3 [0.0–10.0] | 2.8±2.3 [0.0–18.0] | 0.6 (0.1–1.1) |
| No. of vaginal deliveries | 2 [0–6] | 2 [0–9] | 0 [0–0] |
| Previous OAB medication tried | 57 (51.4) | 91 (34.1) | 2.0 (1.3–3.2) |
| Taking OAB medications and requiring washout | 8 (14.0) | 13 (14.0) | 1.0 (0.39–2.62) |
| Any prior behavioral or pelvic floor muscle training | 79 (71.2) | 186 (69.7) | 1.1 (0.7–1.7) |
| Brinks score | 7.8±2.2 [3.0–12.0] | 8.0±2.4 [3.0–12.0] | −0.1 (−0.6 to 0.4) |
| Menopausal status and estrogen use§ | |||
| Premenopausal | 25 (22.3) | 89 (33.5) | Ref |
| Not sure without HT | 13 (11.6) | 36 (13.5) | 1.3 (0.6–2.8) |
| Postmenopausal with HT | 16 (14.3) | 51 (19.2) | 1.1 (0.5–2.3) |
| Postmenopausal without HT | 58 (51.8) | 90 (33.8) | 2.3 (1.3–4.0) |
| Transobturator midurethral sling initiated | 23 (21.7) | 50 (18.9) | 1.2 (0.7–2.1) |
| Retropubic midurethral sling initiated | 79 (74.5) | 209 (79.2) | 0.8 (0.5–1.3) |
| Bladder diary characteristics | |||
| Average episodes/d | |||
| Total incontinence | 5.6±3.1 [0.7–14.0] | 5.3±3.3 [0.7–24.7] | 0.3 (−0.4 to 1.0) |
| UUI | 3.1±2.6 [0.3–12.7] | 2.4±2.3 [0.3–17.0] | 0.7 (0.1–1.2) |
| SUI | 2.2±1.9 [0.3–11.0] | 2.5±2.0 [0.3–21.0] | −0.3 (−0.8 to 0.1) |
| Patient-reported outcome questionnaires | |||
| PGI-S (dichotomous)‖ | 16 (14.3) | 58 (21.7) | 1.7 (0.9–3.0) |
| IIQ-LF total score | 201.9±93.7 [8.9–394.4] | 171.9±98.8 [0.0–400.0] | 30 (8.8–51.1) |
| OABq-LF HRQL total score | 45.7±23.4 [0.0–100.0] | 54.4±23.8 [0.0–100.0] | −8.8 (−14 to −3.5) |
| UDI-total score | 179.0±44.2 [90.4–279.8] | 174.1±40.5 [75.3–290.9] | 4.9 (−4.7 to 14.5) |
| UDI-stress subscale score | 82.4±20.0 [33.3–100.0] | 86.0±17.0 [33.3–100.0] | −3.6 (−7.8 to 0.7) |
| UDI-irritative subscale score | 71.4±18.9 [22.2–100.0] | 63.5±19.7 [11.1–100.0] | 7.8 (3.6–12.1) |
| VLPP less than 60 cm H2O | 11 (14.3) | 18 (8.5) | 1.8 (0.8–4.0) |
| Baseline detrusor pressure | −0.7±3.5 [−14.0 to 9.0] | 0.5±5.6 [−22.0 to 42.0] | −1.2 (−2.2 to −0.3) |
| Does CMG demonstrate SUI | 102 (94.4) | 248 (94.3) | 1.0 (0.4–2.7) |
| Volume at 1st urge | 135.8±96.1 [15.0–490.0] | 115.5±79.2 [0.0–479.0] | 20.3 (−0.2 to 40.9) |
| Volume at strong urge | 228.2±121.6 [47.0–625.0] | 222.2±115.0 [0.0–710.0] | 6.0 (−20.9 to 32.9) |
| Volume at maximum cystometric capacity | 330.9±135.1 [114.0–900.0] | 326.3±121.7 [0.0–933.0] | 4.6 (−24.9 to 34.1) |
| Maximum detrusor pressure during filling | 35.9±71.9 [−5.0 to 370.0] | 33.8±78.8 [−15.0 to 536.0] | 2.1 (−14.5 to 18.8) |
| CMG demonstrate detrusor overactivity | 47 (42.7) | 67 (25.4) | 2.2 (1.4–3.5) |
OR, odds ratio; Ref, reference group for OR; HMO, health maintenance organization; —, unable to be calculated; BMI, body mass index; OAB, overactive bladder; HT, hormone therapy; UUI, urgency urinary incontinence; SUI, stress urinary incontinence; PGI-S, Patient Global Impression of Severity; IIQ-LF, Incontinence Impact Questionnaire–Long Form; OABq-LF, Overactive Bladder Questionnaire—Long Form; HRQL, health-related quality of life; UDI, Urogenital Distress Inventory; VLPP, Valsalva leak point pressure; CMG, cystometrogram.
Data are mean±SD [minimum-maximum], n (%), or median [minimum–maximum] unless otherwise specified.
Bold indicates candidate variables to be further assessed as a predictor using backward selection.
Mean difference: age, BMI, functional comorbidity index, Brinks score, total incontinence, UUI, SUI, PGI-S, IIQ-LF total score, OABq-LF HRQL total score, UDI-total, stress subscale, and irritative subscale scores, baseline detrusor pressure, volume at 1st and strong urge, volume at maximum cystometric capacity, and maximum detrusor pressure during filling; median difference: no. of vaginal deliveries; odds ratios: all other categories.
Participants were able to select American Indian/Alaska Native, Asian, or more than one race, as well as an “Other” race category, which was accompanied by a free response field. Due to the small numbers of participants, these categories were combined for the purposes of this analysis.
The functional comorbidity index is a validated instrument measuring general health status. The score ranges from 0 to 18 with higher scores indicating worse overall health.
Participants were able to select premenopausal, postmenopausal, or that they were unsure of menopausal status.
The PGI-S is dichotomized as “Normal” and “Mild” vs all other categories.
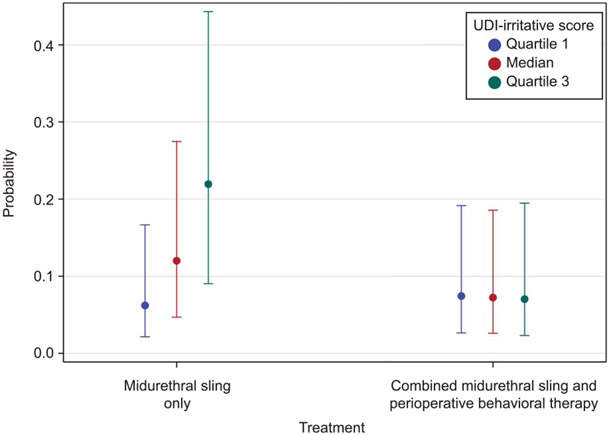
On multivariable logistic regression, factors associated with overall treatment failure included previous use of OAB medication (OR 2.19, aOR 1.96, 95% CI 1.17–3.31, P=.01), detrusor overactivity on cystometrogram (OR 2.25, aOR 2.82, 95% CI 1.60–4.97, P<.001), volume at first urge (OR 1.03, aOR 1.04, 95% CI 1.01–1.07, P=.01 for each 10-mL increase), and UDI stress scores (OR 0.94, aOR 0.92, 95% CI 0.85–1.00, P=.04 for each 5.4-unit increase) (Table 2). There was an interaction effect between baseline UDI-irritative subscale score and treatment group. Women with higher UDI-irritative subscale scores at baseline were at increased risk of failure if randomized to the midurethral sling only group. For each 10.2-point (minimal clinically important difference) increase in UDI-irritative score, the risk of failure after midurethral sling alone increased (OR 1.52, aOR 1.56, 95% CI 1.27–1.91, P<.001). This effect was not seen in the combined group. This is also illustrated in Figure 1. Looked at another way, women with higher UDI-irritative subscale scores were more likely to benefit from combined treatment (OR 1.37, aOR 1.75, 95% CI 1.04–2.96, P=.04 for women at the 50th percentile of UDI-irritative scores [66.7 points]; OR 2.77, aOR 3.72, 95% CI 1.88–7.37, P<.001 for women at the 75th percentile of UDI-irritative scores [83.3 points]).
Table 2.
Multivariable Logistic Regression Model Predicting Overall Treatment Failure
| Effect | Unadjusted OR (95% CI)* | Adjusted OR (95% CI)† |
|---|---|---|
| Site | ||
| Previous OAB medication | 2.19 (1.38–3.47) | 1.96 (1.17–3.31) |
| Detrusor overactivity on cystometrogram | 2.25 (1.40–3.62) | 2.82 (1.60–4.97) |
| Volume at 1st urge (unit=10 mL) | 1.03 (1.00–1.05) | 1.04 (1.01–1.07) |
| Baseline UDI-stress score (unit=5.4) | 0.94 (0.88–1.00) | 0.92 (0.85–1.00) |
| Treatment (combined vs sling only) | 1.56 (0.99–2.46) | |
| Baseline UDI-irritative score (unit=10.2) | 1.24 (1.09–1.40) | |
| Treatment by baseline UDI-irritative score interaction | ||
| Treatment‡ | ||
| At Q1 of UDI-irritative score (50.0) | 0.68 (0.35–1.34) | 0.83 (0.41–1.67) |
| At Q2 of UDI-irritative score (66.7) | 1.37 (0.85–2.22) | 1.75 (1.04–2.96) |
| At Q3 of UDI-irritative score (83.3) | 2.77 (1.51–5.07) | 3.72 (1.88–7.37) |
| UDI-irritative score§ | ||
| Within MUS | 1.52 (1.26–1.84) | 1.56 (1.27–1.91) |
| Within MUS+BPTx | 0.99 (0.83–1.18) | 0.98 (0.80–1.20) |
OR, odds ratio; OAB, overactive bladder; UDI, Urogenital Distress Inventory; Q, quartile; MUS, midurethral sling; BPTx, behavioral–pelvic floor muscle therapy.
Bold indicates statistically significant effect in the model.
Unadjusted ORs were calculated for the subset of women with complete data (no missing values for any of the selected risk factors).
The ORs were calculated from backward-selected model and are adjusted for the selected risk factors. The ORs for risk factors included in interactions were calculated within each level of the other interaction variable.
Odds ratios vs MUS+BPTx.
Odds ratios representing 10.2-unit increase in UDI-irritative score.
Fig. 1.
Interaction effect between UDI (Urinary Distress Inventory)-irritative baseline score and treatment type on probability of failure at 12 months.
Sung. Persistent Symptoms After Sling in Mixed Incontinence. Obstet Gynecol 2021.
Valid observations for the Valsalva leak point pressure were missing from 91 of 379 (24.0%) women. Due to these missing data, Valsalva leak point pressure was excluded as a candidate variable in the main analysis. A sensitivity analysis that used imputation of missing data for Valsalva leak point pressure showed that Valsalva leak point pressure was not a significant predictor of treatment failure, and there were no differences in the selected model.
In the other sensitivity analysis, 32 women who required additional OAB treatment had previously tried OAB medication before enrollment. Of these, six were on active treatment and required washout. Conducting multiple logistic regression that excluded these women resulted in prior OAB medication’s use falling out of the model, but the remaining aORs were consistent with the main analysis (data not shown).
Of the women who met objective failure criteria, 56 of 108 (52%) were due to having additional treatment for urinary symptoms, and 52 of 108 (48%) were due to bladder diary criteria (not meeting more than 70% total urinary incontinence episode reduction). For those women who underwent additional urinary treatment, Table 3 shows the type of treatment and indication. The majority of women (51/56, 91%) who underwent additional treatment within 12 months did so for UUI and OAB, including 48 of 56 (86%) who started OAB medications. Of these 48 women, 32 (67%) had tried an OAB medication at some point before surgery. Only two patients who underwent additional treatment reported persistent SUI symptoms. The 52 women who had diary failures without having had additional urinary treatment had a higher mean number of postoperative total incontinence episodes (SUI and UUI) compared with those without diary failures at 12 months, with a higher number of UUI than SUI episodes (Table 4).
Table 3.
Type of Additional Urinary Symptom Treatment
| Treatment Type | n (%) (n=56) | Reason for Additional Treatment |
|---|---|---|
| OAB medications* | 48 (86) | OAB or UUI |
| Peripheral tibial nerve stimulation | 1 (2) | OAB or UUI |
| Voiding dysfunction medication | 2 (4) | Voiding dysfunction |
| Pelvic floor physical therapy | 2 (4) | 1 patient had both SUI and UUI |
| 1 patient had urinary urgency, treatment dissatisfaction, and dyspareunia | ||
| Sling revision | 2 (4) | Urinary retention |
| Continence pessary | 1 (2) | SUI |
OAB, overactive bladder; UUI, urgency urinary incontinence; SUI, stress urinary incontinence.
Additional OAB medications included oxybutynin, trospium, solifenacin, tolterodine, mirabegron, and onabotulinumtoxinA.
Table 4.
Bladder Diary Measures and Urogenital Distress Inventory Scores at 12 Months in Women With Treatment Failure Compared With Those Without Treatment Failure*
| Measure | Failure | Success | Difference (95% CI) | P † |
|---|---|---|---|---|
| Bladder diary‡ | n=52 | n=271 | ||
| Average episodes/d | ||||
| SUI | 0.61±1.00 | 0.03±0.14 | 0.58 (0.30–0.85) | <.001 |
| UUI | 2.33±2.56 | 0.16±0.40 | 2.16 (1.45–2.88) | <.001 |
| Total incontinence | 3.60±2.70 | 0.22±0.48 | 3.38 (2.63–4.13) | <.001 |
| UDI score | n=17 | n=371 | ||
| UDI-stress | 79.41±21.67 | 14.20±21.20 | 65.22 (53.91–76.53) | <.001 |
| UDI-irritative | 64.38±23.33 | 15.11±21.44 | 49.27 (37.11–61.42) | <.001 |
| UDI-total | 167.32±50.38 | 34.22±43.01 | 133.10 (106.90–159.30) | <.001 |
SUI, stress urinary incontinence; UUI, urgency urinary incontinence; UDI, Urogenital Distress Inventory.
Data are mean±SD unless otherwise specified.
If women had additional urinary treatment, only data before starting treatment was included.
P-values from Student’s t tests.
Among women who did not receive additional urinary treatment.
To further evaluate subjective failure, a more detailed evaluation of UDI scores was performed (Table 4). Seventeen women met the subjective failure criteria. Postoperatively, the total UDI as well as the stress and irritative subscale scores were higher (worse) in women with treatment failure compared with those without treatment failure. When change from baseline was assessed, women with treatment failure had a mean improvement of 0 points for both UDI-stress and UDI-irritative subscale scores, and a mean UDI-total score change of 3.2±25.7 points. Successes had a mean change in UDI-total score of −143.1±52.8 points.
Eighty-five ESTEEM participants were excluded from this analysis due to missing data. Compared with these women, the 379 participants included were older age (mean difference 3.3 years), less likely to be smokers (10% vs 25%), less likely to demonstrate detrusor overactivity on urodynamics (21% vs 45%), had fewer incontinence episodes on diary (mean difference of one episode per day), and had less severe urgency and overall urinary symptoms on UDI, although neither met the minimal clinically important difference threshold (mean difference of 4.9 and 10.2 points, respectively) (Appendix 2, available online at http://links.lww.com/AOG/C347).
DISCUSSION
Understanding risk factors for treatment failure after midurethral sling in women with mixed urinary incontinence is important for patient counseling and expectations. Although previous studies have evaluated risk factors for treatment failure in SUI and SUI-predominant patient populations, this study focused specifically on women with mixed urinary incontinence, which can be more challenging to treat. Risk factors associated with failure at 12 months identified in this study included patient characteristics as well as urodynamic parameters. Although almost 30% of women met our definition for overall failure, only 4.4% met subjective failure criteria. The majority of women who met failure criteria had UUI and other irritative bladder symptoms, but only 13.5% of women required additional urinary treatment for these symptoms. This information can be helpful in counseling patients with mixed incontinence considering midurethral sling.
In this study, we defined treatment failure as a composite outcome, including subjective and objective measures of both SUI and UUI. The investigators had extensive conversations about how to best define success and failure for a population with mixed urinary incontinence, because there is no accepted standard. Although midurethral sling is aimed at treating SUI and not UUI, patients with mixed urinary incontinence who experience worsening UUI after surgery often do not consider the surgery a “success.” This is reflected in several published guidelines.3-5 The original ESTEEM combined intervention was aimed at treating both SUI and UUI after midurethral sling, and, thus, our primary outcome included a severity measure capturing both conditions.6 We found that the majority of women in both groups actually reported improvement of UUI, with a very small proportion reporting worsening. Because there were only 17 women who did not report large enough urinary improvements on the UDI-total score to be considered clinically meaningful (did not meet minimal clinically important difference criteria), we were not able to analyze these women separately to determine the contributions of SUI compared with UUI severity on subjective failure.
Several previous studies have identified preoperative urgency and UUI as a risk factor for persistent or recurrent incontinence after midurethral sling for SUI-predominant populations. In the Trial of Midurethral Slings, which included women with pure stress or stress-predominant symptoms, the odds of midurethral sling treatment failure doubled 12 months postsurgery for each 10-point increase in urge score on the Medical, Epidemiological, and Social Aspects of Aging questionnaire, as well as age per 10 years, UDI score per 10 points, and pad weight.14 In another study, Paick et al15 reported that baseline symptoms of UUI was an independent risk factor for persistent SUI at 6 months postsurgery. The findings of our study are consistent with these prior studies in that women with more severe urgency and UUI symptoms as measured by the UDI-irritative subscale score who were randomized to midurethral sling alone were at increased risk for failure. However, more severe urgency was not a risk for failure in the combined treatment group, and combined treatment was associated with success for women with higher UDI-irritative scores. Thus, the subpopulation of women with higher UDI-irritative subscale scores are likely to benefit from combined treatment. Perioperative behavioral and pelvic floor muscle therapy should be considered for these women to help improve postoperative outcomes.
In another large trial that compared retropubic and transobturator midurethral sling, Barber et al16 found that anticholinergic medication use for OAB was an independent risk factor for recurrent incontinence at 12 months; however, the presence of baseline UUI symptoms was not. This study included women with mixed incontinence symptoms but excluded women with detrusor overactivity on urodynamic evaluation. Our current study also identified prior OAB medication use to be a risk factor; approximately 39% of women had previously used an OAB medication, but only 21 women required washout to participate. Our study also identified detrusor overactivity on urodynamic testing as a risk factor for worse urinary outcomes. It is possible that both prior OAB medication use and detrusor overactivity on urodynamic testing may be independent markers of more severe UUI and may represent a more refractory population. In addition, this study found that previous OAB medication use, detrusor overactivity, and higher irritative voiding symptoms independently contributed meaningful information to the model. From a clinical standpoint, although these variables may be related, they may measure different aspects of OAB and UUI, making them independent risk factors.
The clinical usefulness of urodynamic studies in the management of women with urinary incontinence remains controversial and unclear. Our study found that detrusor overactivity was associated with failure, which may be useful to counsel women with mixed incontinence considering midurethral sling. Higher volume at first urge was also a parameter associated with failure, but the actual difference was 20 mL and the clinical significance of this is unclear.
Strengths of the study include a large, well-described mixed incontinence population. Excellent follow up rates and data ascertainment at 12 months increased the robustness of the analysis. Defined, validated patient-reported outcome measures and objective measures were used. Limitations include that the definitions of “objective failure” may not correlate with a patient’s subjective impression of failure. For example, a patient may have met the definition of objective failure based on bladder diary parameters, but still be subjectively improved and satisfied. Also, we included both persistent SUI and UUI symptoms as part of the definition of failure because this is consistent with the primary ESTEEM trial definition, and women with mixed incontinence often view persistent incontinence symptoms as a failure regardless of which type. However, this may overestimate the failure rate of midurethral sling which is a treatment for SUI, and it is important to note that only two patients (4%) required additional treatment for SUI. Women who were excluded from this analysis had less severe UDI scores and incontinence episodes. Their exclusion may plausibly have affected some of the association we found, although overall they were a less-severe population. Another limitation is that, because several baseline and urodynamic parameters were assessed, false-positive results are possible. Also, our logistic regression model was constructed to fit the data from the ESTEEM trial, and the statistical associations we found may or may not be replicated using other data sets. Specific urodynamic parameters were not required for eligibility and, thus, Valsalva leak point pressure could not be included due to missingness. We also did not obtain postoperative urodynamic studies which may have been hypothesis generating for mechanisms on how urgency and UUI are improved after midurethral sling.
Women with mixed incontinence who have previously tried OAB medication, demonstrate detrusor overactivity on urodynamics, and report more severe urgency have an increased risk of failure with persistent lower urinary tract symptoms at 12 months after undergoing midurethral sling with or without behavioral and pelvic floor therapy. The need for additional treatment for persistent urinary symptoms was low, but more commonly due to OAB symptoms and not SUI. Women with more severe urgency symptoms at baseline may benefit from perioperative behavioral and pelvic floor muscle therapy combined with midurethral sling.
Supplementary Material
Authors’ Data Sharing Statement.
Will individual participant data be available (including data dictionaries)? Yes.
What data in particular will be shared? Deidentified participant data, data dictionary through the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Data and Specimen Hub (DASH).
What other documents will be available? Data dictionary, case report forms.
When will data be available (start and end dates)? April 2020
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Request via the DASH website.
Acknowledgments
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health at National Institutes of Health (U10 HD041261, U10 HD069013, U10 HD054214, U10 HD054215, U10 HD041267, U10 HD069025, U10 HD069010, U10 HD069006, and U01 HD069031).
Footnotes
Financial Disclosure
Pamela Moalli received funding from Hologic for serving on the scientific advisory board. Diane K. Newman served on the Steering Committee and was Editor for UroToday Pelvic Health Center of Excellence. Alison C. Weidner was Consultant for Urocure and Dignify Therapeutics. Ariana Smith reports that her institution received funding from the NIDDK and the Pennsylvania Department of Health. Gena Dunivan received Research support from Pelvalon and Viveve. Beri Ridgeway was Consultant for Coloplast, Inc. Marie Gantz reports that money was paid to her institution from the NIDDK, NICHD, and NHLBI. The other authors did not report any potential conflicts of interest.
A list of Pelvic Floor Disorders Network Team Members can be found in Appendix 1 online at http://links.lww.com/AOG/C347.
Presented at the American Urogynecologic Society and International Urogynecological Association joint meeting, March 24–28, 2019, Nashville, Tennessee.
Each author has confirmed compliance with the journal’s requirements for authorship.
REFERENCES
- 1.Minassian VA, Bazi T, Stewart WF. Clinical epidemiological insights into urinary incontinence. Int Urogynecol J 2017;28:687–96. doi: 10.1007/s00192-017-3314-7 [DOI] [PubMed] [Google Scholar]
- 2.Dooley Y, Lowenstein L, Kenton K, FitzGerald M, Brubaker L. Mixed incontinence is more bothersome than pure incontinence subtypes. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:1359–62. doi: 10.1007/s00192-008-0637-4 [DOI] [PubMed] [Google Scholar]
- 3.Kobashi KC, Albo ME, Dmochowski RR, Ginsberg DA, Goldman HB, Gomelsky A, et al. Surgical treatment of female stress urinary incontinence: AUA/SUFU guideline. J Urol 2017;198:875–83. doi: 10.1016/j.juro.2017.06.061 [DOI] [PubMed] [Google Scholar]
- 4.Abrams P, Andersson KE, Birder L, Brubaker L, Cardozo L, Chapple C, et al. Fourth international consultation on incontinence recommendations of the international scientific committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn 2010;29:213–40. doi: 10.1002/nau.20870 [DOI] [PubMed] [Google Scholar]
- 5.Kammerer-Doak D, Rizk DE, Sorinola O, Agur W, Ismail S, Bazi T. Mixed urinary incontinence: international urogynecological association research and development committee opinion. Int Urogynecol J 2014;25:1303–12. doi: 10.1007/s00192-014-2485-8 [DOI] [PubMed] [Google Scholar]
- 6.Sung VW, Borello-France D, Newman DK, Richter HE, Lukacz ES, Moalli P, et al. Effect of behavioral and pelvic floor muscle therapy combined with surgery vs surgery alone on incontinence symptoms Among women with mixed urinary incontinence: the ESTEEM randomized clinical trial. JAMA 2019;322:1066–76. doi: 10.1001/jama.2019.12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richter HE, Albo ME, Zyczynski HM, Kenton K, Norton PA, Sirls LT, et al. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med 2010;362:2066–76. doi: 10.1056/NEJMoa0912658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber MD, Kleeman S, Karram MM, Paraiso MF, Walters MD, Vasavada S, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol 2008;111:611–21. doi: 10.1097/AOG.0b013e318162f22e [DOI] [PubMed] [Google Scholar]
- 9.Sung VW, Borello-France D, Dunivan G, Gantz M, Lukacz ES, Moalli P, et al. Methods for a multicenter randomized trial for mixed urinary incontinence: rationale and patient-centeredness of the ESTEEM trial. Int Urogynecol J 2016;27:1479–90. doi: 10.1007/s00192-016-3031-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nager CW, Brubaker L, Litman HJ, Zyczynski HM, Varner RE, Amundsen C, et al. A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med 2012;366:1987–97. doi: 10.1056/NEJMoa1113595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fantl JA. Health-related quality of life measures for women with urinary incontinence: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program in Women (CPW) research group. Qual Life Res 1994;3:291–306. doi: 10.1007/BF00451721 [DOI] [PubMed] [Google Scholar]
- 12.Coyne KS, Matza LS, Thompson CL, Kopp ZS, Khullar V. Determining the importance of change in the Overactive Bladder Questionnaire. J Urol 2006;176:627–32. doi: 10.1016/j.juro.2006.03.088 [DOI] [PubMed] [Google Scholar]
- 13.Yalcin I, Bump RC. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynecol 2003;189:98–101. doi: 10.1067/mob.2003.379 [DOI] [PubMed] [Google Scholar]
- 14.Richter HE, Litman HJ, Lukacz ES, Sirls LT, Rickey L, Norton P, et al. Demographic and clinical predictors of treatment failure one year after midurethral sling surgery. Obstet Gynecol 2011;117:913–21. doi: 10.1097/AOG.0b013e31820f3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paick JS, Ku JH, Shin JW, Son H, Oh SJ, Kim SW. Tension-free vaginal tape procedure for urinary incontinence with low Valsalva leak point pressure. J Urol 2004;172:1370–3. doi: 10.1097/01.ju.0000139882.57216.45 [DOI] [PubMed] [Google Scholar]
- 16.Barber MD, Kleeman S, Karram MM, Paraiso MF, Ellerkmann M, Vasavada S, et al. Risk factors associated with failure 1 year after retropubic or transobturator midurethral slings. Am J Obstet Gynecol 2008;199:666–7. doi: 10.1016/j.ajog.2008.07.050 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.