Abstract
Synthetic oligodeoxyribonucleotides containing CpG-dinucleotides (CpG DNA) in specific sequence contexts activate the vertebrate immune system. We have examined the effect of 3′-deoxy-2′–5′-ribonucleoside (3′-deoxynucleoside) incorporation into CpG DNA on the immunostimulatory activity. Incorporation of 3′-deoxynucleosides results in the formation of 2′–5′-internucleotide linkages in an otherwise 3′–5′-linked CpG DNA. In studies, both in vitro and in vivo, CpG DNA containing unnatural 3′-deoxynucleoside either within the CpG-dinucleotide or adjacent to the CpG-dinucleotide failed to induce immunostimulatory activity, suggesting that the modification was not recognized by the receptors. Incorporation of the same modification distal to the CpG-dinucleotide in the 5′-flanking sequence potentiated the immunostimulatory activity of the CpG DNA. The same modification when incorporated in the 3′-flanking sequence had an insignificant effect on immunostimulatory activity of CpG DNA. Interestingly, substitution of a 3′-deoxynucleoside in the 5′-flanking sequence distal to the CpG-dinucleotide resulted in increased IL-6 and IL-10 secretion with similar levels of IL-12 compared with parent CpG DNA. The incorporation of the same modification in the 3′-flanking sequence resulted in lower IL-6 and IL-10 secretion with similar levels of IL-12 compared with parent CpG DNA. These results suggest that site-specific incorporation of 3′-deoxynucleotides in CpG DNA modulates immunostimulatory properties.
INTRODUCTION
Bacterial DNA and synthetic oligodeoxyribonucleotides containing unmethylated CpG-dinucleotides (CpG DNA) stimulate B-cell proliferation and activate macrophages, monocytes and dendritic cells (1,2). The activation of immune cells by CpG DNA results in the secretion of a number of cytokines, including IL-6, IL-12, TNF-α and IFN-γ (1,2). The use of CpG DNA as antitumor, antiviral, antibacterial and anti-inflammatory agents and as adjuvants in immunotherapy has been reported (3–13).
Recent studies suggested that a transmembrane protein, TLR9, plays a critical role in the recognition of CpG DNA and initiation of signaling pathways that lead to the upregulation of NF-κB and the subsequent production of cytokines (14). Alternately, CpG DNA triggers DNA protein kinase activation, which phosphorylates IkB-kinaseB, leading to the activation of NF-κB, which further leads to the production of cytokines (15). It is not clear whether the two pathways are activated sequentially or in parallel leading to a common function of activating the NF-κB pathway. The evidence suggests that TLR9 exhibits considerable sequence selectivity for CpG DNA (16,17). The receptor shows a strict preference for CpG-dinucleotides, but it can accommodate a range of flanking sequences (18,19), which may vary from species to species (20,21). It appears that the receptor exhibits considerable specificity for the chemical and conformational nature of the d(CpG) dinucleotide.
Our laboratory has been studying the effects of sequence and structural changes in the flanking sequences that potentiate or neutralize the immunostimulatory activity of CpG DNA in an attempt to understand the molecular recognition events between the receptors and CpG DNA. These studies suggested that the receptor recognized base modifications that mimic natural C and G (22) but with a strict preference for 2′-deoxyribonucleotides (23) and also required a negatively charged internucleotide linkage between C and G of the CpG-dinucleotide (23). However, a number of different modifications at the 2′-position of the sugar and phosphate backbone were tolerated ~3 nt away from the CpG-dinucleotide in both the 5′- and 3′-flanking sequences (24–26). In fact, the incorporation of modifications in the 5′-flanking sequence usually potentiated the immunostimulatory activity of CpG DNA, while the incorporation of the same in the 3′-flanking sequence had minimal effect on the immunostimulatory activity. Recently, we have also shown that deletion of a nucleobase on nucleosides, which is achieved by incorporation of ‘d-spacer’ or abasic linker, in certain positions in the 5′- or the 3′-flanking sequence to the CpG-dinucleotide potentiated immunostimulatory activity (27).
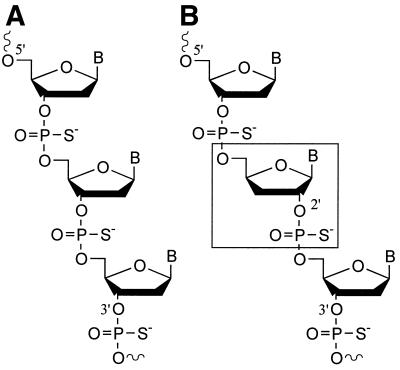
RNA and DNA with 2′–5′-linkages possess significantly different structural and chemical characteristics than the natural 3′–5′-linked nucleic acids (28–31). A number of studies have been reported on the possible application of 2′–5′-linked RNA and DNA for antisense uses (32–34). It has also been shown that 2′–5′-linked RNA does not induce spleen cell proliferation (32), complement activation, and does not prolong aPTT (35) compared with 3′–5′-linked DNA. However, there are no reports on the studies of immunostimulatory properties of 3′-deoxynucleosides in CpG DNA. In this report, we studied the effect of a 3′-deoxy-2′–5′-ribonucleoside (3′-deoxynucleoside) substitution for natural 2′-deoxy-3′–5′-ribonucleosides (2′-deoxynucleosides) within the CpG-dinucleotide or in the flanking sequences to the CpG-dinucleotide on the immunostimulatory activity. The incorporation of 3′-deoxynucleosides also results in an unnatural 2′–5′-internucleoside phosphate linkage in an otherwise 3′–5′-linked DNA chain (Fig. 1).
Figure 1.
Comparison of chemical structures of (A) a normal 3′–5′-DNA strand and (B) a DNA strand containing a 3′-deoxy-2′–5′-ribonucleoside (boxed).
MATERIALS AND METHODS
CpG DNA synthesis and purification
CpG DNA was synthesized using β-cyanoethylphosphoramidite chemistry on a PerSeptive Biosystem’s 8900 Expedite DNA synthesizer on a 1–2 µmol scale. The phosphoramidites of dA, dG, dC and T were obtained from PerSeptive Biosystems. The 5′-DMT-3′-deoxy-2′-phosphoramidites of C and G were purchased from ChemGenes. Beaucage reagent was used as an oxidant to obtain the phosphorothioate backbone modification (36). After the synthesis, CpG DNAs were deprotected using standard protocols, purified by HPLC, converted to the sodium form and dialyzed against distilled water. Then the CpG DNAs were lyophilized and redissolved in distilled water and the concentrations were determined by measuring the UV absorbance at 260 nm. All the CpG DNAs synthesized were characterized by CGE and MALDI-TOF mass spectrometry (Brucker Proflex III MALDI-TOF mass spectrometer with 337 nm N2 laser) for purity and molecular mass, respectively. The purity of the CpG DNAs ranged from 87 to 92% as determined by CGE.
Mouse lymphocyte proliferation assay
Lymphocytes obtained from BALB/c mouse (4–8 weeks) spleens were cultured in RPMI complete medium as described earlier (23,37). The cells were plated in 96-well dishes at a density of 106 cells/ml in a final volume of 100 µl. The CpG DNA at a final concentration of 0.1, 0.3, 1.0 and 3.0 µg/ml, lipopolysaccharide (a positive control) (10 µg/ml) or medium were added to the cell culture in 10 µl of TE buffer (10 mM Tris–HCl pH 7.5, 1 mM EDTA). The cells were then incubated at 37°C. After 44 h, 1 µCi 3H-uridine (Amersham) was added to the culture in 20 µl of RPMI medium, and the cells were pulse-labeled for another 4 h. The cells were harvested by automatic cell harvester and the filters were counted by a scintillation counter. The experiments were performed two or three times for each CpG DNA in triplicate at each concentration. The averages were calculated, normalized and presented as a proliferation index (PI).
Assays for IL-12, IL-6 and IL-10 secretion in mouse spleen cell cultures
The secretion of IL-12, IL-6 and IL-10 in BALB/c mouse spleen cell cultures was measured by sandwich ELISA. The required reagents, including cytokine antibodies and cytokine standards, were purchased form PharMingen. ELISA plates (Costar) were incubated with appropriate antibodies at 5 µg/ml in PBSN buffer (PBS/0.05% sodium azide, pH 9.6) overnight at 4°C and then blocked with PBS/10% FBS at 37°C for 30 min. Different concentrations of CpG DNAs were added to the BALB/c mouse spleen cell cultures and incubated for 24 h, as described above, and the supernatants were collected. Cell culture supernatants and cytokine standards were appropriately diluted with PBS/10% FBS, added to the plates in triplicate and incubated at 25°C for 2 h. Plates were overlaid with 1 µg/ml of the appropriate biotinylated antibody and incubated at 25°C for 1.5 h. Then the plates were washed extensively with PBS/0.05% Tween 20 and then further incubated at 25°C for 1.5 h after adding streptavidine-conjugated peroxidase (Sigma). Then the plates were developed with chromatin (Kirkegaard and Perry) and the color change was measured on a Ceres 900 HDI spectrophotometer (Bio-Tek Instruments). The levels of IL-12, IL-6 and IL-10 in the cell culture supernatants were calculated from the standard curve constructed under the same experimental conditions for IL-12, IL-6 and IL-10.
Mouse splenomegaly assay of CpG DNA
Female BALB/c mice (4–6 weeks, 19–21 g) were divided into different groups with four mice in each group. CpG DNAs were dissolved in sterile PBS and administered intraperitoneally to mice at a dose of 5 mg/kg. After 72 h, mice were killed and the spleens were harvested and weighed.
RESULTS AND DISCUSSION
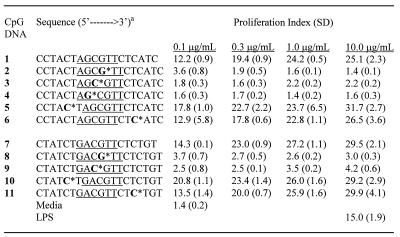
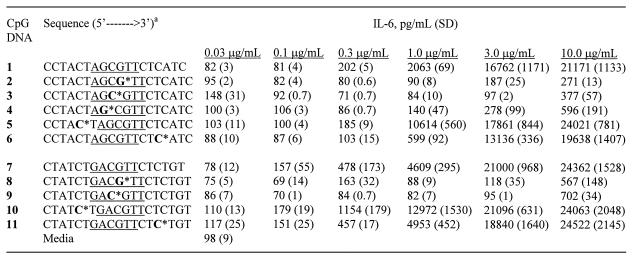
We selected two CpG DNA sequences containing either a ‘GACGTT’ (CpG DNA 1) or an ‘AGCGTT’ (CpG DNA 7) hexameric motif, which have been shown to stimulate immune response in mice. To understand the effect of site-specific incorporation of 3′-deoxynucleosides, we synthesized CpG DNAs 2–6 and 8–11 (Table 1), in which one natural (2′-deoxy) nucleoside was substituted within the CpG-dinucleotide, or in the 3′- or the 5′-flanking sequence to the CpG-dinucleotide with a 3′-deoxynucleoside. The sites of substitutions in each CpG DNA are shown in Table 1. In CpG DNAs 2 and 8, 2′-deoxyguanosine of CpG-dinucleotide was substituted with 3′-deoxyguanosine, which also resulted in a 2′–5′-internucleotide phosphate linkage between the nucleotides as represented with an asterisk (Table 1). In CpG DNAs 3 and 9, 2′-deoxycytidine of CpG-dinucleotide was substituted with 3′-deoxycytidine. In CpG DNAs 4, 5 and 10, a 2′-deoxynucleoside was substituted with a 3′-deoxynucleoside in the 5′-flanking region to the CpG-dinucleotide at different positions. Similarly, in CpG DNAs 6 and 11, a nucleoside was substituted with a 3′-deoxynucleoside in the 3′-flanking region to the CpG-dinucleotide (Table 1). The immunostimulatory activity of the parent and modified CpG DNAs was assessed by their ability to induce cell proliferation and secrete cytokines in mouse spleen cell cultures in vitro and enlargement of spleen in mice in vivo. All the CpG DNAs used in the study are phosphorothioate back-bone modified.
Table 1. Spleen cell proliferation in BALB/c mouse spleen cell cultures by CpG DNAs.
aNucleosides shown in bold represent 3′-deoxyribonucleosides and * indicates the position of the 2′–5′-phosphorothioate internucleoside linkage. The two CpG sequence motifs used in the study are underlined. All CpG DNA is phosphorothioate modified.
Spleen cell proliferation in vitro
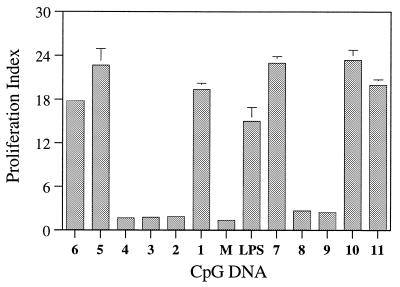
The immunostimulatory activity of the parent and modified CpG DNAs was studied in BALB/c mouse spleen cell cultures and is expressed as the proliferation index. Spleen cells from BALB/c mouse were cultured with CpG DNA at concentrations of 0.1, 0.3, 1.0 and 3.0 µg/ml for 48 h and cell proliferation was determined by 3H-uridine incorporation (37,38). All the CpG DNAs that were active showed a concentration-dependent spleen cell proliferation (Table 1). The parent CpG DNAs 1 and 7, which did not have any modification, showed PIs of 19.4 ± 0.9 and 23.0 ± 0.9, respectively, at a concentration of 0.3 µg/ml (Fig. 2). Under the same conditions, CpG DNAs 2, 3, 8 and 9 that had either C or G in the CpG-dinucleotide substituted with a 3′-deoxynucleoside, failed to induce significant proliferation of spleen cells compared with the media control (Fig. 2). These results suggest that a 3′-deoxynucleoside in either the C or G position of a CpG-dinucleotide is not recognized. Perhaps the receptor specifically recognizes the confirmation of 2′-deoxynucleotides in these positions or the structural changes caused by 3′-deoxynucleosides do not permit recognition by the receptor. CpG DNA 4, which had a 3′-deoxynucleoside substitution adjacent to the CpG-dinucleotide on the 5′-side, also failed to induce significant proliferation of spleen cells, suggesting that a 3′-deoxynucleoside at this position was detrimental to immunostimulatory activity.
Figure 2.
Proliferation indices of CpG DNA 1–11 in BALB/c mouse spleen cell cultures at a concentration of 0.3 µg/ml. M represents the media control and LPS stands for lypopolysaccharide at a concentration of 10.0 µg/ml. The assays were performed in triplicate at least three times.
When the 3′-deoxynucleoside modification was placed four nucleosides upstream of the CpG-dinucleotide in the 5′-flanking sequence (CpG DNAs 5 and 10), both CpG DNAs induced proliferation of spleen cells (Fig. 2). The PIs measured for CpG DNAs 5 and 10 were 22.7 ± 2.2 and 23.4 ± 1.4, respectively, at a concentration of 0.3 µg/ml. These PI values for CpG DNAs 5 and 10 are higher or equal to those observed with their parent CpG DNAs 1 and 7, respectively. These results provided an initial indication that 3′-deoxynucleosides incorporated at least about four nucleosides away from the CpG-dinucleotide in the 5′-flanking sequence had no neutralizing effect but may potentiate the immunostimulatory activity of CpG DNA.
As shown in Figure 2, when a 3′-deoxynucleoside was placed ~5 nt downstream of the CpG-dinucleotide in the 3′-flanking sequence (CpG DNAs 6 and 11), both CpG DNAs induced proliferation of spleen cells. The PIs measured were 17.8 ± 0.6 and 20.0 ± 0.7 for CpG DNAs 6 and 11, respectively. These PI values are comparable with their respective parent CpG DNAs 1 and 7, suggesting that incorporation of a 3′-deoxynucloside modification in the 3′-flanking sequence has an insignificant effect on the immunostimulatory activity of CpG DNA.
Cytokine secretion in vitro
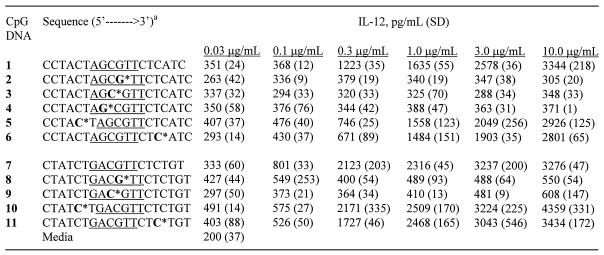
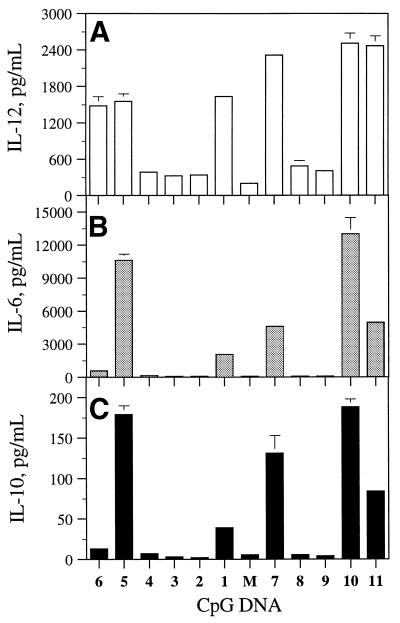
In addition, we measured the induction of cytokines, IL-12 (Table 2), IL-6 (Table 3) and IL-10, by CpG DNAs 1–11, at different concentrations in BALB/c mouse spleen cell cultures. All CpG DNAs that induced cytokine secretion showed a concentration-dependent production of cytokines. For comparison, the levels of IL-12, IL-6 and IL-10 produced at a concentration of 1 µg/ml of CpG DNAs are shown in Figure 3. The parent CpG DNAs 1 and 7 induced 1635 ± 55 and 2316 ± 45 pg/ml of IL-12, 2063 ± 69 and 4609 ± 295 pg/ml of IL-6, and 39 ± 4 and 131 ± 22 pg/ml of IL-10 secretion, respectively. CpG DNAs 2–4, 8 and 9, which had a 3′-deoxynucleoside substitution in or adjacent to the CpG-dinucleotide, showed no or little cytokine production at the same concentration (Fig. 3).
Table 2. Induction of IL-12 secretion in BALB/c mouse spleen cell cultures by CpG DNAs.
aNucleosides shown in bold represent 3′-deoxyribonucleosides and * indicates the position of the 2′–5′-phosphorothioate internucleoside linkage. The two CpG sequence motifs used in the study are underlined. All CpG DNA is phosphorothioate modified.
Table 3. Induction of IL-6 secretion in BALB/c mouse spleen cell cultures by CpG DNAs.
aNucleosides shown in bold represent 3′-deoxyribonucleosides and * indicates the position of the 2′–5′-phosphorothioate internucleoside linkage. The two CpG sequence motifs used in the study are underlined. All CpG DNA is phosphorothioate modified.
Figure 3.
Secretion of IL-12 (A), IL-6 (B) and IL-10 (C) in BALB/c mouse spleen cell cultures in the presence of CpG DNAs 1–11 at a concentration of 1.0 µg/ml. M, media control.
CpG DNAs 5 and 10, which had the 3′-deoxynucleoside in the 5′-flanking sequence, showed similar levels of IL-12 secretion (1558 ± 123 and 2509 ± 170 pg/ml, respectively) compared with their parent CpG DNAs 1 and 7, respectively. Both CpG DNAs induced a higher level of IL-6 (10 614 ± 560 and 12 972 ± 1530 pg/ml, respectively) and IL-10 (179 ± 11 and 188 ± 10 pg/ml, respectively) production at the same concentration than did their respective parent CpG DNAs (Fig. 3).
CpG DNAs 6 and 11, which had a 3′-deoxynucleoside in the 3′-flanking sequence, also induced 1484 ± 151 and 2468 ± 165 pg/ml of IL-12 production similar to those of their parent CpG DNAs 1 and 7, respectively. However, they induced lower or similar levels of IL-6 (599 ± 92, 4953 ± 452 pg/ml, respectively) and IL-10 (13 ± 1 and 84 ± 4 pg/ml, respectively) compared with CpG DNAs 1 and 7, respectively (Fig. 3).
Splenomegaly in vivo
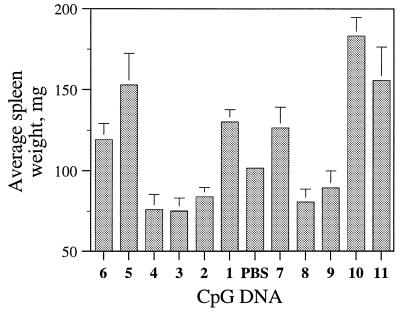
In general, the increase in spleen weight (spelnomegaly) of mice injected with oligonucleotides is considered a result of the immunostimulatory activity of CpG DNA (37–39). To further confirm the effect of 3′-deoxynucleoside modification on immunostimulatory activity of CpG DNA in vivo, we administered CpG DNAs 1–11 intraperitoneally to BALB/c mice at a dose of 5 mg/kg and measured the spleen weights after 72 h of CpG DNA administration (Fig. 4). Mice injected with parent CpG DNAs 1 and 7 had spleen weights of 130.2 ± 7.7 and 126.5 ± 12.8 mg, respectively. These spleen weights of mice treated with CpG DNAs 1 and 7 were ~28 and 24% higher, respectively, than the spleen weights of the control mice that were treated with vehicle (PBS). The administration of CpG DNAs 2–4, 8 and 9 at the same dose did not result in spleen weight increase, suggesting that these CpG DNAs did not induce immune responses in BALB/c mice (Fig. 4). These results further confirmed the results observed with CpG DNAs 2–4, 8 and 9 in spleen cell cultures. In addition, these results suggest that the presence of a CpG-dinucleotide is required for the immunostimulatory activity of CpG DNAs.
Figure 4.
Spleen enlargement in BALB/c mice following intraperitoneal administration of CpG DNA 1–11 at a dose of 5 mg/kg. Control mice received vehicle (PBS). Four animals were used for each CpG DNA treatment and the averages were calculated.
CpG DNAs 5 and 10, which had a 3′-deoxynucleoside in the 5′-flanking sequence, increased spleen weights of BALB/c mice to ~50.3 and 80.1% at the same dose compared with the mice that received vehicle (PBS) (Fig. 4). These data confirm that the modifications incorporated in the 5′-flanking sequence distal to the CpG-dinucleotide potentiated immunostimulatory activity of CpG DNA in vivo as well as in in vitro proliferation assays. At the same dose of 5 mg/kg, CpG DNAs 6 and 11 also increased spleen weights of mice compared with the control mice. The spleen weight increase caused by CpG DNA 6 (17.2%) was smaller than that observed with its parent CpG DNA 1 (28%). CpG DNA 11 produced a greater spleen enlargement (53%) compared with its parent CpG DNA 7 (24.3%) in contrast to the in vitro proliferation data.
Taken together, the present results suggest that the unnatural 3′-deoxy-2′–5′-nucleoside incorporated either in the CpG-dinucleotide or adjacent to the CpG-dinucleotide neutralizes CpG-related immunostimulatory activity. The same modification when incorporated distal to the CpG-dinucleotide in the 5′-flanking sequence potentiates the immunostimulatory activity of CpG DNA. When the modification is incorporated in the 3′-flanking sequence, it has an insignificant effect on the immunostimulatory activity of CpG DNA. These results are consistent with our earlier studies in which 2′-sugar modifications were incorporated and examined for immunostimulatory activity of CpG DNA (19,20).
The incorporation of 3′-deoxynucleosides into CpG DNA results in the increased nuclease stability of the modified CpG DNAs (27,28). The increased nuclease stability could contribute to the increased immunostimulatory activity of the modified CpG DNAs (2–6 and 8–11). In general, the 3′-exonucleases present in the cells are mostly responsible for degradation of oligonucleotides. The modification incorporated towards the 3′ end should impart a relatively higher stability against nucleases than those incorporated towards the 5′ end. It is reasonable to assume that CpG DNAs 6 and 11 should have higher nuclease stability and, therefore, should have relatively higher immunostimulatory activity. In contrast, the results presented here show that CpG DNAs 5 and 10, which have the modifications incorporated towards the 5′ end, are more active than CpG DNAs 6 and 11, suggesting that the observed increase in immunostimulatory activity of CpG DNAs 5 and 10 is not the result of increased nuclease stability, but the result of the structural modifications introduced in the CpG DNA.
Another observation that stems from these studies is that the incorporation of a 3′-deoxynucleoside in the 5′-flanking sequence distal (at least 3–5 nt away) to the CpG-dinucleotide increases IL-6 production significantly without affecting IL-12 secretion compared with the parent CpG DNA. This property could be of particular importance in the application of CpG DNA as an adjuvant with prophylactic and therapeutic vaccines, antigens and peptides, where IL-6 production is highly desirable for maturation of B cells and subsequent production of antigen-specific immunoglobulins. When the same modification is incorporated in the 3′-flanking sequence, IL-12 secretion is not significantly altered compared with the parent CpG DNAs, but IL-6 and IL-10 secretion is minimal. This property of modified CpG DNA may be specifically useful for treating infectious diseases and cancer.
Several antisense oligonucleotides that are currently in human clinical trials contain CpG-dinucleotides (6,40,41). The incorporation of a 3′-deoxynucleoside in certain positions of CpG DNA, as in CpG DNAs 2–4, 8 and 9, resulted in the neutralization of CpG-related activity. Though such modifications are not beneficial for CpG DNA therapeutics development, they could be of immense importance for antisense oligonucleotide design, when a CpG-dinucleotide cannot be avoided in them. The incorporation of a single 3′-deoxynucleoside at an appropriate position of an antisense oligonucleotide containing a CpG-dinucleotide is useful to reduce non-specific immune-related activity, provided the modification does not significantly affect the biochemical and biophysical properties of modified oligonucleotide (28–33). However, for neutralization of the immune effects of antisense oligonucleotides containing CpG-dinucleotides, the use of 2′-alkyl-substituted nucleosides or backbone modifications, which do not affect the binding affinity and pharmacokinetic properties, would be more appropriate (23–26).
CONCLUSION
In conclusion, our results suggest that a 3′-deoxynucleoside substitution incorporated ~3–5 nt away from the CpG-dinucleotide either in the 5′- or the 3′-flanking sequence does not interfere with immunostimulatory activity. In fact, substitution in the 5′-flanking sequence potentiates immunostimulatory activity of CpG DNA. The same substitution within or adjacent to the CpG-dinucleotide neutralizes CpG-related immunostimulatory activity. In addition, our studies demonstrate that it may be possible to induce specific cytokines as desired for different applications by site-specific incorporation of 3′-deoxynucleosides in a CpG DNA. The on-going studies of modified CpG DNA with specific immune cell lineages should help with understanding the molecular mechanisms of interactions in detail and enable us to further fine tune the incorporation of modifications, eventually allowing the broad application of CpG DNAs as immunological tools and therapeutic agents.
REFERENCES
- 1.Wagner H. (2000) Immunology of Bacterial CpG-DNA. Springer-Verlag, Heidelberg, Germany.
- 2.Raz E. (2000) Immunostimulatory DNA Sequences. Springer-Verlag, Heidelberg, Germany.
- 3.Hafner M., Zawatzky,R., Hirtreiter,C., Buurman,W.A., Echtenacher,B., Hehlgans,T. and Mannel,D.N. (2001) Antimetastatic effect of CpG DNA mediated by type I IFN. Cancer Res., 61, 5523–5528. [PubMed] [Google Scholar]
- 4.Kawarada Y., Ganss,R., Garbi,N., Sacher,T., Arnold,B. and Hammerling,G.J. (2001) NK- and CD8(+) T cell-mediated eradication of established tumors by peritumoral injection of CpG-containing oligodeoxynucleotides. J. Immunol., 167, 5247–5253. [DOI] [PubMed] [Google Scholar]
- 5.Ballas Z.K., Krieg,A.M., Warren,T., Rasmussen,W., Davis,H.L., Waldschmidt,M. and Weiner,G.J. (2001) Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J. Immunol., 167, 4878–4886. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal S. and Kandimalla,E.R. (2000) Antisense therapeutics: is it as simple as complementary base recognition? Mol. Med. Today, 6, 72–81. [DOI] [PubMed] [Google Scholar]
- 7.Lewis E.J., Agrawal,S., Bishop,J., Chadwick,J., Cristensen,N.D., Cuthill,S., Dunford,P., Field,A.K., Francis,J., Gibson,V. et al. (2000) Non-specific antiviral activity of antisense molecules targeted to the E1 region of human papillomavirus. Antiviral Res., 48, 187–196. [DOI] [PubMed] [Google Scholar]
- 8.Fiedler M., Lu,M., Siegel,F., Whipple,J. and Roggendorf,M. (2001) Immunization of woodchucks (Marmota monax) with hepatitis delta virus DNA vaccine. Vaccine, 19, 4618–4626. [DOI] [PubMed] [Google Scholar]
- 9.Davis H.L., Suparto,I.I., Weeratna,R.R., Jumintarto, Iskandriati,D.D., Chamzah,S.S., Ma’ruf,A.A., Nente,C.C., Pawitri,D.D., Krieg,A.M. et al. (2000) CpG DNA overcomes hyporesponsiveness to hepatitis B vaccine in orangutans. Vaccine, 18, 1920–1924. [DOI] [PubMed] [Google Scholar]
- 10.Cafaro A., Titti,F., Fracasso,C., Maggiorella,M.T., Baroncelli,S., Caputo,A., Goletti,D., Borsetti,A., Pace,M., Fanales-Belasio,E. et al. (2001) Vaccination with DNA containing tat coding sequences and unmethylated CpG motifs protects cynomolgus monkeys upon infection with simian/human immunodeficiency virus (SHIV89.6P). Vaccine, 19, 2862–2877. [DOI] [PubMed] [Google Scholar]
- 11.Tighe H., Takabayashi,K., Schwartz,D., Van Nest,G., Tuck,S., Eiden,J.J., Kagey-Sobotka,A., Creticos,P.S., Lichtenstein,L.M., Spiegelberg,H.L. and Raz,E. (2000) Conjugation of immunostimulatory DNA to the short ragweed allergen amb a 1 enhances its immunogenicity and reduces its allergenicity. J. Allergy Clin. Immunol., 106, 124–134. [DOI] [PubMed] [Google Scholar]
- 12.Shirota H., Sano,K., Kikuchi,T., Tamura,G. and Shirato,K. (2000) Regulation of murine airway eosinophilia and Th2 cells by antigen-conjugated CpG oligodeoxynucleotides as a novel antigen-specific immunomodulator. J. Immunol., 164, 5575–5582. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann S., Egeter,O., Hausmann,S., Lipford,G.B., Rocken,M., Wagner,H. and Heeg,K. (1998) CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol., 160, 3627–3630. [PubMed] [Google Scholar]
- 14.Hemmi H., Takeuchi,O., Kawai,T., Kaisho,T., Sato,S., Sanjo,H., Matsumoto,M., Hoshino,K., Wagner,H., Takeda,K. and Akira,S. (2000) A Toll-like receptor recognizes bacterial DNA. Nature, 408, 740–745. [DOI] [PubMed] [Google Scholar]
- 15.Chu W., Gong,X., Li,Z., Takabayashi,K., Ouyang,H., Chen,Y., Lois,A., Chen,D.J., Li,G.C., Karin,M. and Raz,E. (2000) DNA-PKcs is required for activation of innate immunity by immunostimulatory DNA. Cell, 103, 909–918. [DOI] [PubMed] [Google Scholar]
- 16.Takeshita F., Leifer,C.A., Gursel,I., Ishii,K.J., Takeshita,S., Gursel,M. and Klinman,D.M. (2001) Role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J. Immunol., 167, 3555–3558. [DOI] [PubMed] [Google Scholar]
- 17.Bauer S., Kirschning,C.J., Hacker,H., Redecke,V., Hausmann,S., Akira,S., Wagner,H. and Lipford,G.B. (2001) Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl Acad. Sci. USA, 98, 9237–9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokunaga T., Yano,O., Kuramoto,E., Kimura,Y., Yamamoto,T., Kataoka,T. and Yamamoto,S. (1992) Synthetic oligonucleotides with particular base sequences from the cDNA encoding proteins of Mycobacterium bovis BCG induce interferons and activate natural killer cells. Microbiol. Immunol., 36, 55–66. [DOI] [PubMed] [Google Scholar]
- 19.Krieg A.M., Yi,A.K., Matson,S., Waldschmidt,T.J., Bishop,G.A., Teasdale,R., Koretzky,G.A. and Klinman,D.M. (1995) CpG motifs in bacterial DNA trigger direct B-cell activation. Nature, 374, 546–549. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann G., Weeratna,R.D., Ballas,Z.K., Payette,P., Blackwell,S., Suparto,I., Rasmussen,W.L., Waldschmidt,M., Sajuthi,D., Purcell,R.H., Davis,H.L. and Krieg,A.M. (2000) Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol., 164, 1617–1624. [DOI] [PubMed] [Google Scholar]
- 21.Verthelyi D., Ishii,K.J., Gursel,M., Takeshita,F. and Klinman,D.M. (2001) Human peripheral blood cells differentially recognize and respond to two distinct CpG motifs. J. Immunol., 166, 2372–2377. [DOI] [PubMed] [Google Scholar]
- 22.Kandimalla E.R., Yu,D., Zhao,Q. and Agrawal,S. (2001) Effect of chemical modifications of cytosine and guanine in a CpG-motif of oligonucleotides: structure–immunostimulatory activity relationships. Bioorg. Med. Chem., 9, 807–813. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Q., Temsamani,J., Iadarola,P.L., Jiang,Z. and Agrawal,S. (1996) Effect of different chemically modified oligodeoxynucleotides on immune stimulation. Biochem. Pharmacol., 51, 173–182. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Q., Yu,D. and Agrawal,S. (1999) Site of chemical modifications in CpG containing phosphorothioate oligodeoxynucleotide modulates its immunostimulatory activity. Bioorg. Med. Chem. Lett., 9, 3453–3458. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Q., Yu,D. and Agrawal,S. (2000) Immunostimulatory activity of CpG containing phosphorothioate oligodeoxynucleotide is modulated by modification of a single deoxynucleoside. Bioorg. Med. Chem. Lett., 10, 1051–1054. [DOI] [PubMed] [Google Scholar]
- 26.Yu D., Kandimalla,E.R., Zhao,Q., Cong,Y. and Agrawal,S. (2001) Immunostimulatory activity of CpG oligonucleotides containing non-ionic methylphosphonate linkages. Bioorg. Med. Chem., 9, 2803–2808. [DOI] [PubMed] [Google Scholar]
- 27.Yu D., Kandimalla,E.R., Zhao,Q., Cong,Y. and Agrawal,S. (2001) Modulation of immunostimulatory activity of CpG oligonucleotides by site-specific deletion of nucleobases. Bioorg. Med. Chem. Lett., 11, 2263–2267. [DOI] [PubMed] [Google Scholar]
- 28.Giannaris P.A. and Damha,M.J. (1993) Oligoribonucleotides containing 2′,5′-phosphodiester linkages exhibit binding selectivity for 3′,5′-RNA over 3′,5′-ssDNA. Nucleic Acids Res., 21, 4742–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alul R. and Hoke,G.D. (1995) (2′–5′)-Oligo-3′-deoxynucleotides: selective binding to single-stranded RNA but not DNA. Antisense Res. Dev., 5, 3–11. [DOI] [PubMed] [Google Scholar]
- 30.Premraj B.J. and Yathindra,N. (1998) Stereochemistry of 2′,5′ nucleic acids and their constituents. J. Biomol. Struct. Dyn., 16, 313–328. [DOI] [PubMed] [Google Scholar]
- 31.Premraj B.J., Patel,P.K., Kandimalla,E.R., Agrawal,S., Hosur,R.V. and Yathindra,N. (2001) NMR structure of a 2′,5′ RNA favors A type duplex with compact C2′ endo nucleotide repeat. Biochem. Biophys. Res. Commun., 283, 537–543. [DOI] [PubMed] [Google Scholar]
- 32.Kandimalla E.R., Manning,A., Zhao,Q., Shaw,D.R., Byrn,R.A., Sasisekharan,V. and Agrawal.S. (1997) Mixed backbone antisense oligonucleotides: design, biochemical and biological properties of oligonucleotides containing 2′–5′-ribo- and 3′–5′-deoxyribonucleotide segments. Nucleic Acids Res., 25, 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhan P., Bhan,A., Hong,M., Hartwell,J.G., Saunders,J.M. and Hoke,G.D. (1997) 2′,5′-linked oligo-3′-deoxyribonucleoside phosphorothioate chimeras: thermal stability and antisense inhibition of gene expression. Nucleic Acids Res., 25, 3310–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adah S.A., Bayly,S.F., Cramer,H., Silverman,R.H. and Torrence,P.F. (2001) Chemistry and biochemistry of 2′,5′-oligoadenylate-based antisense strategy. Curr. Med. Chem., 8, 1189–1212. [DOI] [PubMed] [Google Scholar]
- 35.Kandimalla E.R., Shaw,D.R. and Agrawal,S. (1998) Effects of phosphorothioate oligodeoxyribonucleotide and oligoribonucleotides on human complement and coagulation. Bioorg. Med. Chem. Lett., 8, 2103–2108. [DOI] [PubMed] [Google Scholar]
- 36.Iyer R.P., Eagan,W., Regan,J.B. and Beaucage,S.L. (1990) 3H-1,2-benzodithiole-3-one 1,1-dioxide as an improved sulfurizing reagent in the solid-phase synthesis of oligodeoxyrionucleoside phosphorothioates. J. Am. Chem. Soc., 112, 1253–1254. [Google Scholar]
- 37.Zhao Q., Temsamani,J., Zhou,R.Z. and Agrawal,S. (1997) Pattern and kinetics of cytokine production following administration of phosphorothioate oligonucleotides in mice. Antisense Nucleic Acid Drug Dev., 7, 495–502. [DOI] [PubMed] [Google Scholar]
- 38.Branda R.F., Moore,A.L., Mathews,L., McCormack,J.J. and Zon,G. (1993) Immune stimulation by an antisense oligomer complementary to the rev gene of HIV-1. Biochem. Pharmacol., 45, 2037–2043. [DOI] [PubMed] [Google Scholar]
- 39.Lippford J.B. and Sparwasser,T. (2000) Hematopoietic remodeling triggered by CpG DNA. In Wagner,H. (ed.), Immunobiology of Bacterial CpG-DNA. Springer-Verlag, Berlin, pp. 119–129. [DOI] [PubMed]
- 40.Krieg A.M. (1998) Leukocyte stimulation by oligodeoxynucleotides. In Stein,C.A. and Krieg,A.M. (eds), Applied Antisense Oligonucleotide Technology. Wiley-Liss, New York, pp. 431–448.
- 41.Agrawal S. and Kandimalla,E.R. (2001) Antisense and/or immunostimulatory oligonucleotide therapeutics. Curr. Cancer Drug Targets, 1, 197–209. [DOI] [PubMed] [Google Scholar]