Abstract
Background
The novel coronavirus disease (COVID‐19) has led to significant mortality and morbidity, including a high incidence of related thrombotic events. There has been concern regarding hormonal contraception use during the COVID‐19 pandemic, as this is an independent risk factor for thrombosis, particularly with estrogen‐containing formulations. However, higher estrogen levels may be protective against severe COVID‐19 disease. Evidence for risks of hormonal contraception use during the COVID‐19 pandemic is sparse. We conducted a living systematic review that will be updated as new data emerge on the risk of thromboembolism with hormonal contraception use in patients with COVID‐19.
Objectives
To determine if use of hormonal contraception increases risk of venous and arterial thromboembolism in women with COVID‐19.
To determine if use of hormonal contraception increases other markers of COVID‐19 severity including hospitalization in the intensive care unit, acute respiratory distress syndrome, intubation, and mortality.
A secondary objective is to maintain the currency of the evidence, using a living systematic review approach.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, Global Index Medicus, Global Health, and Scopus on a monthly basis, with the most recent search conducted in November 2023. We updated the search strategies with new terms and added the database Global Index Medicus in lieu of LILACS as of March 2023.
Selection criteria
We included all published and ongoing studies of patients with COVID‐19 comparing outcomes of those on hormonal contraception versus those not on hormonal contraception. This included case series and non‐randomized studies of interventions (NRSI).
Data collection and analysis
One review author extracted study data and this was checked by a second author. Two authors individually assessed risk of bias for the comparative studies using the ROBINS‐I tool and a third helped reconcile differences. For the living systematic review, we will publish updates to our synthesis every six months. In the event that we identify a study with a more rigorous study design than the current included evidence prior to the planned six‐month update, we will expedite the synthesis publication.
Main results
We included three comparative NRSIs with 314,704 participants total and two case series describing 13 patients. The three NRSIs had serious to critical risk of bias in several domains and low study quality. Only one NRSI ascertained current use of contraceptives based on patient report; the other two used diagnostic codes within medical records to assess hormonal contraception use, but did not confirm current use nor indication for use. None of the NRSIs included thromboembolism as an outcome. Studies were not similar enough in terms of their outcomes, interventions, and study populations to combine with meta‐analyses. We therefore narratively synthesized all included studies.
Based on results from one NRSI, there may be little to no effect of combined hormonal contraception use on odds of mortality for COVID‐19 positive patients (OR 1.00, 95% CI 0.41 to 2.40; 1 study, 18,892 participants; very low‐certainty evidence).
Two NRSIs examined hospitalization rates for hormonal contraception users versus non‐users. Based on results from one NRSI, the odds of hospitalization for COVID‐19 positive combined hormonal contraception users may be slightly decreased compared with non‐users for patients with BMI under 35 kg/m2 (OR 0.79, 95% CI 0.64 to 0.97; 1 study, 295,689 participants; very low‐certainty evidence). According to results of the other NRSI assessing use of any type of hormonal contraception, there may be little to no effect on hospitalization rates for COVID‐19 positive individuals (OR 0.99, 95% CI 0.68 to 1.44; 1 study, 123 participants; very low‐certainty evidence).
We included two case series because no comparative studies directly assessed thromboembolism as an outcome. In a case series of six pediatric COVID‐19 positive patients with pulmonary embolism, one (older than 15 years of age) was using combined hormonal contraception. In a second case series of seven COVID‐19 positive patients with cerebral venous thrombosis, one was using oral contraceptives.
One comparative study and one case series reported on intubation rates, but the evidence for both is very uncertain. In the comparative study of 123 COVID‐19 positive patients (N = 44 using hormonal contraception and N = 79 not using hormonal contraception), no patients in either group required intubation. In the case series of seven individuals with cerebral venous thromboembolism, one oral contraceptive user and one non‐user required intubation.
Authors' conclusions
There are no comparative studies assessing risk of thromboembolism in COVID‐19 patients who use hormonal contraception, which was the primary objective of this review. Very little evidence exists examining the risk of increased COVID‐19 disease severity for combined hormonal contraception users compared to non‐users of hormonal contraception, and the evidence that does exist is of very low certainty.
The odds of hospitalization for COVID‐19 positive users of combined hormonal contraceptives may be slightly decreased compared with those of hormonal contraceptive non‐users, but the evidence is very uncertain as this is based on one study restricted to patients with BMI under 35 kg/m2. There may be little to no effect of combined hormonal contraception use on odds of intubation or mortality among COVID‐19 positive patients, and little to no effect of using any type of hormonal contraception on odds of hospitalization and intubation for COVID‐19 patients. We noted no large effect for risk of increased COVID‐19 disease severity among hormonal contraception users.
We specifically noted gaps in pertinent data collection regarding hormonal contraception use such as formulation, hormone doses, and duration or timing of contraceptive use. Differing estrogens may have different thrombogenic potential given differing potency, so it would be important to know if a formulation contained, for example, ethinyl estradiol versus estradiol valerate. Additionally, we downgraded several studies for risk of bias because information on the timing of contraceptive use relative to COVID‐19 infection and method adherence were not ascertained. No studies reported indication for hormonal contraceptive use, which is important as individuals who use hormonal management for medical conditions like heavy menstrual bleeding might have different risk profiles compared to individuals using hormones for contraception. Future studies should focus on including pertinent confounders like age, obesity, history of prior venous thromboembolism, risk factors for venous thromboembolism, and recent pregnancy.
Keywords: Child, Female, Humans, Pregnancy, Contraceptive Agents, COVID-19, Estrogens, Estrogens/adverse effects, Hormonal Contraception, Pandemics, Thrombosis, Venous Thromboembolism, Venous Thromboembolism/chemically induced, Venous Thromboembolism/epidemiology
Plain language summary
Chances of developing blood clots for people who have COVID‐19 and are taking hormonal birth control compared to people not taking hormonal birth control
Review question
We reviewed the evidence about the effects of hormonal birth control on developing blood clots, including heart attack or stroke, or other severe outcomes for people with COVID‐19 disease. We wanted to look at people using combined forms of birth control (containing both an estrogen and a progestin hormone) compared to people using no hormonal birth control or people using birth control containing only the progestin hormone. We found only five studies to include.
Background
Hormonal birth control, especially birth control with estrogen, can increase chances of developing blood clots in the leg or lung or increase the chance of having a stroke. We have also seen that blood clots in the leg or lung can be a result of developing COVID‐19. We are not sure if people who are taking hormonal birth control have a higher chance of developing blood clots if they contract COVID‐19. We want to study this further so people who use hormonal contraception will know if they should stop or switch their birth control methods if they become COVID‐19 positive.
Study characteristics
We included studies published up to March 2022. We looked for studies that reported on risk of developing blood clots, being hospitalized, needing high levels of care like requiring a breathing tube, or risk of dying from COVID‐19 for people who used birth control, especially birth control with estrogen like combined pills, compared to people who did not use birth control. Because there were very few studies, we also looked at studies that reported on a group of individuals with COVID‐19 using birth control who developed clots who were not compared to another group. We included five studies in total. One study of 18,892 people looked at the risk of dying for people with COVID‐19 using combined birth control methods. Another study of 295,689 people looked at the risk of being hospitalized for people using combined birth control methods who were tracking their COVID symptoms on a smartphone application, but not specifically tested for COVID‐19. A third study of only 123 people looked at the risk of being hospitalized for people with COVID‐19 who were using any type of hormonal birth control. Finally, two studies of 13 people total with COVID‐19 who had developed blood clots looked at the number of those people who had used combined birth control.
Key results
One study reported similar risks of dying from COVID‐19 among people using combined hormonal birth control and those people not using it, but the evidence was very uncertain.
Based on results from one study, there may be a slightly decreased risk of hospitalization with COVID‐19 for people who use combined hormonal birth control, but the evidence was very uncertain. Results from a smaller study found that there may be little to no effect of using any type of hormonal birth control on risk of hospitalization for people with COVID‐19 disease, but the evidence was very uncertain.
Using hormonal birth control may have little to no effect on risk of needing a breathing tube for people with COVID‐19 disease, but the evidence is very uncertain.
Reports describing a series of 13 women and girls with COVID‐19 that had blood clots found that two of the people used combined hormonal birth control.
We did not find any evidence on risk of heart attack or stroke for users of hormonal birth control with COVID‐19 disease.
We did not find any evidence for any outcome for people with COVID‐19 using combined hormonal birth control as compared to those using birth control containing progestin hormone only.
Overall there were few studies we were able to include and they all had serious design issues that made it very difficult to interpret the evidence. The evidence was very uncertain about the risk of clotting‐related harms for people with COVID‐19 who use hormonal birth control. There may be similar or reduced risk of being hospitalized for people who use hormonal contraception.
Certainty of the evidence
We have little to no confidence in the evidence base because the studies did not provide important information we were interested in, such as reasons people may be at risk for developing blood clots. The studies also did not include the exact types of people we were looking for, like people who had COVID‐19 that was confirmed with a test or people who were confirmed to be taking birth control when they had COVID‐19. There are also not enough studies to be certain about the results.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Combined hormonal contraception (estrogen plus progestin) compared to no contraception for COVID‐19 positive patients.
| Combined hormonal contraception (estrogen plus progestin) compared to no contraception for COVID‐19 positive patients | ||||||
| Patient or population: COVID‐19 positive patients Setting: COVID‐19 positive patients in tertiary care setting Intervention: combined hormonal contraception Comparison: no hormonal contraception | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no hormonal contraception | Risk with combined hormonal contraception | |||||
| Mortality | 5 per 1000 | 5 per 1000 (2 to 11) | OR 1.00 (0.41 to 2.40) | 18892 (1 observational study) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Hospitalization | 7 per 1000d | 5 per 1000 (4 to 6)d | OR 0.79 (0.64 to 0.97) | 295689 (1 observational study) | ⊕⊝⊝⊝ Very lowc,e,f | Combined hormonal contraception may reduce hospitalization slightly. COVID‐19 positivity not confirmed through testing; women were using a mobile phone application to track COVID‐19 symptoms. Restricted to patients with BMI < 35 kg/m2. |
| Venous thromboembolism | 1 of 6 pediatric COVID‐19 patients with pulmonary embolism had reportedly been using combined hormonal contraception. 1 of 7 reproductive‐aged female COVID‐19 patients with cerebral venous thromboembolism was using "oral contraceptive pills." This patient also had positive anti‐phospholipid antibodies. | 13 (2 observational studies) | ⊕⊝⊝⊝ Very lowg,h | 2 case series were included with 13 total patients, describing venous thromboembolism in COVID‐19 patients. Neither case study ascertained active use of hormonal contraception at time of the outcome. i | ||
| Intubation | 1 of 6 people who were not using hormonal contraception required intubation compared to 1 of 1 person reportedly using oral contraceptive pills. | 7 (1 observational study) | ⊕⊝⊝⊝ Very lowg,i | Case series of 7 reproductive‐aged women, all of whom were COVID‐19 positive and developed cerebral venous thromboembolism. | ||
| Arterial thromboembolism ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Acute respiratory distress syndrome ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_432099564019182608. | ||||||
a Downgraded for serious risk of bias given no ascertainment of combined hormonal contraception exposure and no information on variables used for propensity score matching, increasing risk of residual confounding. b Downgraded for indirectness given no ascertainment of combined hormonal contraception use during time of outcome. c Downgraded for imprecision given results reported in only 1 study. d Overall 1889 of 295,689 total patients were hospitalized for an absolute risk of 6.4 per 1000 total. Hospitalizations were not reported separately for those using combined hormonal contraception (n = 64,253) versus those not using contraception (n = 231,436). Anticipated absolute effects were estimated by applying the adjusted relative effect estimate to determine expected number of intervention and control patients who were hospitalized. e Downgraded for serious risk of bias due to risk of selection bias and all data are self‐reported by users. f Downgraded for indirectness as users of the application were not confirmed to be COVID‐19 positive, but were tracking symptoms given concern for possible COVID‐19 positivity. g Downgraded 2 levels for risk of bias as case series are likely to be subject to significant bias. h Downgraded for imprecision for small sample sizes. i Downgraded 2 levels for imprecision given small sample size and results reported in only 1 study.
Summary of findings 2. Summary of findings table ‐ Any type of hormonal contraception (estrogen plus progestin or progestin‐only) compared to no contraception for COVID‐19 positive patients.
| Any type of hormonal contraception (estrogen plus progestin or progestin‐only) compared to no contraception for COVID‐19 positive patients | ||||||
| Patient or population: COVID‐19 positive patients Setting: COVID‐19 positive patients in tertiary care setting Intervention: any type of hormonal contraception (estrogen plus progestin or progestin‐only) Comparison: no contraception | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no contraception | Risk with any type of hormonal contraception (estrogen plus progestin or progestin‐only) | |||||
| Hospitalization | 38 per 1000 | 38 per 1000 (26 to 54) | OR 0.99 (0.68 to 1.44) | 123 (1 observational study) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Intubation | 0 of 79 patients who did not use hormonal contraception required intubation compared to 0 of 44 patients who used hormonal contraception. | 123 (1 observational study) | ⊕⊝⊝⊝ Very lowa,b,c | |||
| Mortality ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Venous thromboembolism ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Arterial thromboembolism ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Acute respiratory distress syndrome ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_432122675422248852. | ||||||
a Downgraded for serious risk of bias given no ascertainment of hormonal contraception exposure and no information on variables used for adjustment, increasing risk of residual confounding. b Downgraded for indirectness as the study was not performed in patients confirmed to be using contraception at time of outcome. c Downgraded 2 levels for imprecision due to small sample size with wide confidence interval with results reported in only 1 study.
Background
We acknowledge that individuals who use hormonal contraception may not identify as women, and we have endeavored to use gender‐inclusive language throughout this review. When reporting on individual studies that identified participants as 'women' or 'female', we have kept this language to accurately describe the study as it was reported.
Description of the condition
The novel coronavirus disease (COVID‐19), caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has spread rapidly worldwide. COVID‐19 has affected millions and led to significant mortality and morbidity, including a high incidence of related thrombotic events (Ahmed 2020). The pro‐thrombotic effects of COVID‐19 are thought to be related to increased inflammatory cytokine release, platelet activation, endothelial dysfunction, upregulation of the renin‐angiotensin‐aldosterone system, and blood flow abnormalities (Ahmed 2020; Bikdeli 2020). Data regarding the pathogenicity of COVID‐19 continue to emerge, but it is not yet entirely certain how this may be modulated by various individual‐level characteristics and medications, including the influence of sex hormones.
Description of the intervention
Hormonal contraception includes: combined estrogen and progestin pills, patches, and rings; systemic progestin‐only methods, including pills, injectables, and rings; and progestin‐releasing intrauterine devices. Hormonal contraception is a common medication used by over 250 million people worldwide (UN 2019). It is unclear if hormonal contraception use among COVID‐19 positive women increases or attenuates risk of thromboembolism.
How the intervention might work
Combined hormonal contraception (CHC), which contains estrogen, may exacerbate thrombotic risk in individuals infected with COVID‐19. Use of CHC methods confers a two‐to‐three‐fold increased risk of venous thromboembolism (VTE) compared to non‐use (de Bastos 2014). Ethinyl estradiol (EE) in CHCs leads to increased levels of coagulation factors II, VII, VIII, X and fibrinogen, and decreased plasma levels of anticoagulant factors, including antithrombin and tissue factor pathway inhibitor, as shown in human studies (Abou‐Ismail 2020). This effect is dose dependent, with higher levels of EE affording increased risks of thromboembolism. Coagulation factor levels may not return to normal until several weeks after CHC cessation (Robinson 1991). The use of CHCs containing certain progestins, such as drospirenone, desogestrel, or gestodene, may be associated with one and a half to two times the odds of increased risk of VTE compared to use of levonorgestrel‐containing contraceptives; however, that association is controversial given the data limitations and biases (Dragoman 2018). Progestin‐only contraceptive (POC) methods do not appear to increase risk of VTE in most populations, though some studies have shown increased risk of VTE with depot medroxyprogesterone acetate use (Tepper 2016).
Estrogen and progesterone may play a protective role in the pathogenicity of COVID‐19. There are well‐documented sex differences in COVID‐19 outcomes, with increased mortality seen in males (Jin 2020) and a protective effect from death in post‐menopausal women treated with estrogen (Sund 2022). Among a cohort of hospitalized COVID‐19 positive people in China, the proportion of non‐menopausal women with severe COVID‐19 disease was significantly lower than the proportion with severe COVID‐19 disease among age‐matched men (Ding 2021). Estradiol levels were shown to be negatively correlated with disease severity as well as interleukin (IL) IL‐6 and IL‐8 levels (Ding 2021). In humans and mouse models, estradiol is seen to suppress production of pro‐inflammatory cytokines while stimulating the anti‐inflammatory cytokine response (Mauvais‐Jarvis 2020). Additionally, estradiol may decrease gene expression of angiotensin‐converting enzyme 2 (ACE2) receptors in bronchial epithelial cells (Stelzig 2020), which are the means of cell‐entry for SARS‐CoV‐2. SARS‐CoV‐2 has also been shown to activate platelets by binding ACE2 receptors, leading to increased risk of thrombosis in mouse models (Zhang 2020).
Why it is important to do this review
Synthesizing the evidence regarding the influence of hormonal contraceptive use on thrombosis risk among COVID‐19 positive women will affect national guidelines for contraceptive use, for which there is no current global consensus. At present, the World Health Organization supports the use of all forms of contraception during the COVID‐19 pandemic (WHO 2020). The Society of Family Planning currently recommends that CHCs be discontinued for all hospitalized women with COVID‐19, but progestin‐only and non‐hormonal methods may be continued. CHC use may be continued for non‐hospitalized or asymptomatic women with COVID‐19, but it is recommended to discuss the theoretical increased risk of thromboembolism (Benson 2020). The Board of the Italian Society of Contraception states that CHC can be continued for asymptomatic COVID‐19 positive women or women with mild symptoms, but should be stopped for severe symptoms (including severe pneumonia), immobilization, and in cases of increased thromboembolic risk (Fruzzetti 2020). The Italian guidance states that CHC can be restarted immediately after recovery, but makes no mention of initiating prophylactic anticoagulation (Fruzzetti 2020). The French guideline suggests continuing CHCs in low risk patients with mild COVID‐19 disease, as even after CHC discontinuation there is a delay in return to baseline VTE risk; however, it recommends adding weight‐based prophylactic low‐molecular weight heparin (LMWH) if the woman has symptomatic COVID‐19 or additional risk factors such as age > 35, prolonged immobilization, smoking, or obesity (CNGOF 2020). The Faculty of Sexual and Reproductive Healthcare (FSRH) of the Royal College of Obstetricians and Gynaecologists states that, in the absence of clear evidence regarding thromboembolism risk with COVID‐19, it can make no recommendation to deviate from existing guidance regarding assessment of VTE risk for prescribing CHC (FSRH 2020). However, the FRSH recognizes that CHC will likely be stopped for hospitalized women, and recommends considering providing alternative progestin‐only or other effective contraception prior to discharge. For non‐hospitalized women with COVID‐19, the FSRH recommends considering on a case‐by‐case basis whether women should switch to progestin‐only contraception, taking into account whether the woman will be adherent and able to receive supplies. Current recommendations in Spain for perimenopausal women using CHC as contraception suggest discontinuing CHC and starting prophylactic LMWH for women hospitalized with COVID‐19 (especially for those requiring intensive care), and discontinuing CHC use for non‐hospitalized women with COVID‐19 during the acute illness phase associated with immobilization (Ramírez 2020). For women recovering from COVID‐19 pneumonia with persistent symptoms requiring only outpatient monitoring, the Spanish guidelines recommend discontinuing CHC use and initiating LMWH use (Ramírez 2020). If hormonal therapy is required, Spanish recommendations suggest considering switching to POC use, unless women have mild symptoms and only suspected, not confirmed, COVID‐19 (Ramírez 2020).
This review will help to determine which types of hormonal contraception increase risk of venous and arterial thrombosis among COVID‐19 positive women, and if this differs by subgroups of COVID‐19 severity or other individual characteristics. Evidence and recommendations are rapidly evolving as this is a novel coronavirus; therefore, we intend to perform a living systematic review. It is likely that the conclusions of this review, including estimates of effect, will change as new evidence is generated.
Objectives
To determine if use of hormonal contraception increases risk of venous and arterial thromboembolism in women with COVID‐19.
To determine if use of hormonal contraception increases other markers of COVID‐19 severity including hospitalization in the intensive care unit, acute respiratory distress syndrome, intubation, and mortality.
A secondary objective is to maintain the currency of the evidence, using a living systematic review approach.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomized controlled trials (RCTs) and non‐randomized studies of interventions (NRSIs). We planned to include parallel RCTs including those randomized at the individual or cluster level, but not to include cross‐over trials because this is not feasible for studies of the intervention evaluated in this review. While RCTs represent the most rigorous type of study for addressing questions of efficacy and safety, we included NRSIs for this topic because we did not expect to find adequate trial evidence to address the review objectives. It is extremely unlikely for the hormonal contraception method to be randomized in this clinical situation. Additionally, the efficacy and safety outcomes of interest are very rare and the number of participants willing to be randomized to hormonal contraceptive methods would likely be limited. This reduces the feasibility and likelihood of adequately powered randomized trials. NRSIs are likely to provide the best available data for observing differences in outcomes associated with different hormonal contraceptive methods among women with COVID‐19. We included studies irrespective of their publication status and language of publication. As expected, we did not find any eligible RCTs for inclusion.
We planned to include cohort studies that compare individuals or clusters exposed to the intervention to a comparable group of unexposed individuals or clusters over the same time period (e.g. comparative cohort, case‐control studies nested in a prospective cohort).
We planned to include cohort studies that compare individuals or clusters exposed to the intervention over one time period to a comparable group of unexposed individuals or clusters from another time period (e.g. before‐after study designs, interrupted time series), or from different geographic sites. As these data are emerging, we also included case series and non‐comparative studies of combined hormonal contraception (CHC) users with COVID‐19. We decided to include case series if they had at least five cases meeting participant inclusion criteria. As the evidence base grows, we plan to exclude studies without a comparison group in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Reeves 2022).
Types of participants
We included studies of women of reproductive age (ages 15 to 51) who were COVID‐19 positive (or presumed COVID‐19 positive). We excluded women who were pregnant or less than three weeks postpartum. According to the Centers for Disease Control Medical Eligibility Criteria for Contraceptive Use, women without underlying risk for venous thromboembolism who are not breastfeeding should wait until three weeks postpartum to initiate CHCs, given the elevated risk of venous thromboembolism in the immediate postpartum time period (Curtis 2016). We intended to include studies of women using hormonal contraception for contraceptive purposes and exclude women using hormonal methods for medical treatment of abnormal uterine bleeding or other conditions unless fewer than 10% of women were using hormonal methods for non‐contraceptive purposes; however, we were not able to ascertain the indication for CHC use in the studies using data from administrative databases. For the current review, we retained these studies given the otherwise very sparse data.
Types of interventions
We included studies comparing COVID‐19 positive women using combined hormonal contraception with similar non‐pregnant individuals not using contraception or using non‐hormonal contraception. We planned to also include studies comparing COVID‐19 positive women using combined hormonal contraception with those using progestin‐only methods of hormonal contraception.
The comparisons for this review were planned as follows.
Combined hormonal contraception versus no contraceptive method.
Combined hormonal contraception versus non‐hormonal contraception.
Combined hormonal contraception versus progestin‐only contraception.
Progestin‐only contraception versus no contraceptive method.
Progestin‐only contraception versus non‐hormonal contraception.
Types of outcome measures
Primary outcomes
Venous thromboembolism during the study period.
Arterial thromboembolism during the study period.
Secondary outcomes
Mortality.
Critical illness requiring intensive care unit hospitalization.
Acute respiratory distress syndrome.
Intubation.
Search methods for identification of studies
The Fertility Regulation Group Information Specialist (IS) conducted a search for all published, unpublished, and ongoing studies, without restrictions on language or publication status. This review is up to date as of the November 2023 search. The IS reviewed and updated the search strategies in February 2023 to include new subject and keyword terms. To improve coverage of international regional journals, the IS substituted the World Health Organization's (WHO) Global Index Medicus (includes 5 regional indices) in lieu of only searching WHO's Latin American and Caribbean Health Science Information (LILACS) database. The updated and initial search strategies for each database are available in Appendix 1 and Appendix 2 respectively.
Electronic searches
We searched the following databases from their inception.
Cochrane Central Register of Controlled Trials (Ovid EBM Reviews) (September 2023).
MEDLINE ALL (Ovid) (1946 to 31 October 2023).
Embase.com (inception to 31 October 2023).
CINAHL (EBSCOHost) (Cumulative Index to Nursing and Allied Health Literature; 1937 to 31 October 2023).
Global Index Medicus (WHO) (1973 to 31 October 2023).
Global Health (Ovid) (1973 to 2023 Week 43).
Scopus (inception to 31 October 2023) conference abstracts only.
We initially intended to search pre‐print servers as well, but determined not to include these given concerns regarding data quality for these manuscripts as the pandemic evolved.
Living systematic review considerations
As this is a living systematic review, we will monitor the literature monthly and will publish updates to our synthesis every six months. We will review the search methods and strategies every six months to ensure they reflect any terminology changes in the topic area, or in the databases. We anticipate that we will maintain the living systematic review for two years.
Searching other resources
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant studies. We will contact experts/organizations in the field to obtain additional information on relevant studies. If necessary, we will contact authors of included studies for data clarification and further information.
Living systematic review considerations
We will note when key conferences are to be held and will search conference proceedings when published. We will contact corresponding authors of ongoing studies as we identify them and will ask them to advise when study results are available, or to share early or unpublished data. We will contact the corresponding authors of any newly included studies for advice regarding other relevant studies. We will manually search the reference lists of all newly included studies.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database and removed duplicates (Covidence 2022). Two review authors (Megan A Cohen (MAC), Alison Edelman (AE)) independently screened titles and abstracts for inclusion. We retrieved the full‐text study reports/publications and two review authors (MAC, AE) independently screened the full text, identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, when required, we consulted a third review author (Jillian Henderson (JH)). We listed studies that initially appeared to meet the inclusion criteria but that we later excluded in the Characteristics of excluded studies table. We collated multiple reports of the same study so that each study rather than each report is the unit of interest in the review. We also provided any information we can obtain about ongoing studies. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009).
Living systematic review considerations
We will immediately screen any new citations retrieved by the monthly searches. We expect initial search yields to be fairly small, so we intend to screen all records manually; however, we may employ automated techniques over time if the volume of retrieved citations increases substantially.
Data extraction and management
We used a standard data collection form for study characteristics and outcome data; we piloted the form on one study in the review. One review author (MAC) independently extracted the study characteristics below from the included studies, and the data were independently checked by a second review author (AE).
Methods: study design, number of study centers and location, study setting, withdrawals, date of study, follow‐up.
Participants: number, mean age, age range, severity of condition, diagnostic criteria, inclusion criteria, exclusion criteria, other relevant characteristics.
Interventions: type of hormonal contraception, comparison, length of hormonal contraception use, timing of hormonal contraception initiation, medication adherence.
Outcomes: main and other outcomes specified and collected, time points reported.
Notes: funding for trial, notable conflicts of interest of trial authors, ethical approval.
One review author (MAC) independently extracted the outcome data from the included studies, and this was checked by a second study author (AE) for accuracy. We noted in the Characteristics of included studies table if a trial reported outcome data in an unusable way. We resolved disagreements by consensus or by involving a third review author (JH).
Assessment of risk of bias in included studies
Two review authors (MAC, AE) independently assessed risk of bias for each study. We resolved any disagreements by discussion or by involving another author (JH).
We assessed the risk of bias for key outcomes from NRSI using the Risk Of Bias In Non‐randomized Studies ‐ of Interventions (ROBINS‐I) instrument (Sterne 2020). We considered the following factors to be possible confounding factors for this topic: age, personal history of venous thromboembolism (VTE), recent pregnancy, obesity, severity of COVID‐19, ethinyl estradiol dose, progestogen type. Using the ROBINS‐I tool, which includes signaling questions for assessing different potential sources of bias, we evaluated the following domains.
-
Pre‐intervention.
Bias due to confounding.
Bias in selection of participants into the study (selection bias).
-
At intervention.
Bias in classification of interventions (information bias).
-
Post‐intervention.
Bias due to deviations from intended interventions (confounding).
Bias due to missing data (selection bias).
Bias in measurement of outcomes (information bias).
Bias in selection of the reported result (reporting bias).
Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table. We did not exclude studies on the grounds of their risk of bias, but clearly reported the risk of bias when presenting the results of the studies. When considering treatment effects, we took into account the risks of bias for the studies that contributed to that outcome.
If included in future updates, we will assess the risk of bias in randomized trials using version two of the Cochrane risk of bias tool (ROB2) (Sterne 2019). Our effect of interest will be the effect of assignment, also known as the intention‐to‐treat effect. We will assess all the outcomes defined in this review for risk of bias. We will answer the signaling questions to assess the following domains.
Bias arising from the randomization process.
Bias due to deviations from intended interventions.
Bias due to missing outcome data.
Bias in measurement of the outcome.
Bias in selection of the reported result.
An additional domain is included for cluster‐randomized trials.
Bias arising from identification or recruitment of individual participants within clusters.
We will use the variant of ROB2 for cluster‐RCTs if we identify eligible trials with this study design.
For each outcome, we will use the signaling questions to categorize each domain as either 'low risk of bias', 'some concerns', or 'high risk of bias'. We will record our answers to the signaling questions on the ROB2 Excel tool and make this available in an online repository. We will summarize the risk of bias judgments across different studies for each of the domains for each pre‐specified outcome. For each study, we will derive an overall judgment from the tool, as follows.
Low risk of bias: the study is considered to show a low risk of bias.
Some concerns: a few concerns are expected to be associated with the study in at least one domain, but it does not warrant categorization as a study with a high risk of bias with regard to any domain.
High risk of bias: the study is considered to be at high risk of bias in at least one domain; or a few concerns with regard to multiple domains are observed in the study such that these concerns significantly lower confidence in the study results.
Applying risk of bias assessments in this review
We took into account the risk of bias for the studies that are used to estimate intervention effects. We provided figures to illustrate the risk of bias. For future reviews, we will conduct sensitivity analyses (see Sensitivity analysis section below) to assess whether estimated effects differ when high risk of bias studies are excluded from analyses as pertinent. The risk of bias assessment informed the GRADE ratings and summary of findings tables.
Measures of treatment effect
For dichotomized outcomes from NRSIs, we reported the adjusted estimate of effect including odds ratio (OR) and 95% confidence interval (95% CI). Where studies reported count data, that is, the number of events rather than the number of people who experienced at least one event, we reported the number of events and the total number of participants in the intervention and control groups, as person‐years of follow‐up were not reported in the included studies and rate ratios could not be calculated.
For case‐series outcomes, we presented data in a narrative format.
Unit of analysis issues
We intended to perform the primary analysis per individual randomized. We abstracted information on the study design and unit of analysis for each study, indicating whether clustering of observations was present due to allocation to the intervention at the group level or clustering of individually randomized observations (e.g. patients within clinics). We planned to abstract available statistical information needed to account for the implications of clustering on the estimation of outcome variances, such as design effects or intra‐cluster correlations, and whether the study adjusted results for the correlations in the data. In cases where the study did not account for clustering, we planned to ensure that appropriate adjustments were made to the effective sample size following Cochrane guidance (Higgins 2020). Where possible, we planned to derive the intra‐cluster correlation (ICC) for these adjustments from the trial itself, or from a similar trial. If an appropriate ICC was unavailable, we planned to conduct sensitivity analyses to investigate the potential effect of clustering by imputing a range of values of ICC.
If any trials had multiple arms that were compared against the same control condition and we needed to include them in the same meta‐analysis, we would have divided the control group numerators and denominators by the number of interventions to be included in the meta‐analysis, to avoid double‐counting observations.
Dealing with missing data
We attempted to contact investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data for those studies identified as abstract only. For this review, we were unable to obtain information from study investigators.
For future reviews, we will calculate missing standard deviations or other necessary data using other data from the trial, such as confidence intervals, based on methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020).
We reported the number of studies that have results missing for the synthesis of each outcome.
We reported all responses and data provided in the Characteristics of included studies table. Where we made any assumptions about missing data, we reported the potential impact in the Discussion section of the review.
Assessment of heterogeneity
We described the clinical diversity and methodological variability of the evidence in the review text and used tables to describe study characteristics, including design features, population characteristics, and intervention details.
To assess statistical heterogeneity, we planned to visually inspect forest plots and describe the direction and magnitude of effects, and the degree of overlap between confidence intervals. We planned to also consider the statistics generated in forest plots that measure statistical heterogeneity. We intended to use the I2 statistic to quantify inconsistency among the trials in each analysis. We planned to also consider the P value from the Chi2 test to assess whether this heterogeneity is significant (P < 0.1). If we had identified substantial heterogeneity, we planned to report the finding and explore possible explanatory factors using pre‐specified subgroup analysis.
We planned to use a rough guideline to interpret the I2 value rather than a simple threshold, and to take into account an understanding that measures of heterogeneity (I2 and Tau2) would be estimated with high uncertainty when the number of studies was small (Deeks 2020). However, we determined that there were not enough data in order to conduct meta‐analyses, and the populations and outcomes reported in the studies included were too dissimilar to pool for reporting.
Assessment of reporting biases
If we had identified enough studies available for meta‐analysis to support a funnel plot (at least 10), we planned to create and visually inspect the funnel plot and run a formal statistical test for asymmetry, as proposed by Egger 1997. In the event that we observed funnel plot asymmetry, we planned to discuss the potential for this to be attributed to small study effects and not just non‐reporting bias. We intended to provide a funnel plot for risk of deep venous thromboembolism, risk of pulmonary embolism, and risk of arterial thromboembolism, data permitting.
Insufficient studies were included in the review to allow construction of a funnel plot and formal testing of asymmetry, which may indicate publication bias. Should enough studies be included in future updates of the review we plan to undertake these analyses.
Data synthesis
The decision to meta‐analyze data or not was based on an assessment of whether the interventions in the included trials were similar enough in terms of participants, settings, intervention, comparison, and outcome measures to ensure meaningful conclusions from a statistically pooled result.
We were unable to pool the data statistically using meta‐analysis given heterogeneity of populations and outcomes, and the very few studies included in the review. We therefore conducted a narrative synthesis of results. We presented the major outcomes and results, organized by intervention categories according to the major types or aims of the identified interventions, or both. Within the data categories we explored the following main comparisons of the review.
Combined hormonal contraception versus no contraception.
Hormonal contraception versus no contraception.
Where studies compared more than one intervention, we compared each separately to no intervention/control.
Subgroup analysis and investigation of heterogeneity
We a priori planned to carry out the following subgroup analyses of factors that may contribute to heterogeneity in the effects of the intervention.
Studies using ethinyl estradiol dosage less than 30 mcg versus studies greater than or equal to 30 mcg.
Types of progestin contraception (i.e. oral progestin‐only contraception, injectable, progestin‐releasing intrauterine device).
Studies investigating hospitalized women versus women treated as outpatients.
Studies from areas with large outbreaks of COVID‐19 versus studies from areas without large outbreaks.
Type of anticoagulation (i.e. prophylactic anticoagulation, intermediate‐dose anticoagulation, therapeutic anticoagulation).
We planned to use the following outcomes in subgroup analyses if there were enough studies reporting the outcome to support valid subgroup comparisons.
Venous thromboembolism.
Arterial thromboembolism.
Mortality.
Ambulatory versus non‐ICU (intensive care unit) hospitalized versus ICU hospitalized women with COVID‐19.
However, there were not enough data included to be able to conduct subgroup analyses.
Sensitivity analysis
In the current review, we did not conduct any sensitivity analyses as there were not enough data to do so.
Summary of findings and assessment of the certainty of the evidence
We evaluated the evidence according to the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence as it related to our pre‐specified outcomes.
We followed the methods and recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2020), and used GRADEpro GDT 2021 software. Given that we used the ROBINS‐I tool to assess risk of bias for included NRSIs, we designated the evidence for each NRSI to start at 'high certainty' and we then downgraded the certainty of the evidence as appropriate.
We planned to provide separate summary of findings tables for the following comparisons.
Combined hormonal contraception versus no contraceptive method.
Combined hormonal contraception versus non‐hormonal contraception.
Combined hormonal contraception versus progestin‐only contraception.
Progestin‐only contraception versus no contraceptive method.
Progestin‐only contraceptive versus non‐hormonal contraception.
We added a table for hormonal contraception versus no contraceptive method as one study did not stratify outcomes by hormonal contraceptive type.
We planned to summarize evidence for a given outcome from RCTs and NRSIs in separate rows; for this review version, only NRSIs were included.
We used footnotes to give justifications for our decisions to downgrade the certainty of evidence and provide comments to aid readers’ understanding of the review where necessary.
Two review authors (MAC, AE) made independent judgments about the certainty of the evidence, with disagreements resolved by discussion or involving a third author (JH). We justified the judgments, documented them and incorporated them into reporting of results for each outcome.
Deciding when to incorporate new evidence
Living systematic review consideration
We will monitor the literature on a monthly basis, as described in the protocol, and will publish updates to our synthesis every six months. In the event that we identify a study with a more rigorous study design than the included evidence to date (e.g. prospective cohort study with concurrent comparison groups or an RCT) in our active monitoring of the literature, we will expedite the synthesis publication to ensure that newer evidence with lower risk of bias is added to the review prior to the planned six‐month time frame.
Results
Description of studies
Results of the search
The PRISMA study flow diagram (Figure 1) shows results of the search process. Our database search as of March 2022 yielded 8220 studies, leaving 2119 studies for title and abstract screening after removal of duplicates. We identified 31 studies for full‐text review. Of these, we excluded 26 studies, most commonly due to incorrect study design (see Excluded studies).
1.
Included studies
We included three comparative non‐randomized studies of interventions (NRSIs) with 314,704 participants total (Costeira 2021; Mujumdar 2020; Seeland 2020) and two case series with 13 participants (Chima 2021; Hameed 2021). Characteristics of the studies can be seen in Table 3.
1. Characteristics of included studies.
| Study | Study design | Country/Setting | Data collection methods | Intervention(s) description | COVID‐19 definition | Inclusion criteria | Exclusion criteria | Confounders (variables, how measured) | Confounder adjustment | Analysis method | Outcomes of interest | Total participants (n) | Participants HC user (n) | Participants non‐HC user (n) |
| Seeland 2020 | Retrospetive database cohort | Derived from electronic health records of multiple healthcare organizations across 17 countries | Data collected from electronic health records in the TriNetX RealWorld database. Stratified by age: pre‐menopausal cohort (15 to 49 years) and post‐menopausal (> 50 years). Only pre‐menopausal cohort reported here | Hormone use: estradiol and combined hormonal contraception (unclear how many people were using estradiol only). Hormone use was identified via RxNorm codes 4083 (estradiol), 4124 (ethinyl estradiol), progestins VA:HS800, and systemic contraceptives VA:HS200 | COVID‐19 patients were identified via the ICD‐10 code U07.1 or the presence of a SARS‐CoV‐2‐related RNA diagnosis | Pre‐menopausal women ages 15 to 49 who were COVID positive in the last 7 months (n = 18,892) | Those without gender information | TriNetX analytics tools were used to assess baseline characteristics including "demographics, diagnoses, procedures, and medication" No information on exactly which variables were collected or how they were measured | Cohorts were balanced 1:1 using propensity score matching, using a nearest neighbor greedy matching algorithm with a caliper of 0.25 times the standard deviation | A logistic regression analysis was performed for the combined outcome variable "death" incorporating the propensity score matching | Mortality | n = 18,892 | n = 2078 | n = 16,814 |
| Mujumdar 2020 | Retrospective database cohort | Tertiary medical center in the US | Chart review from tertiary medical center electronic health record from 28 March 28 to 27 April 2020 | Hormonal contraception including LNG IUD, POP, CHC, injectable progestin reported in medical chart (patients not contacted to confirm contraceptive use) | Patients who tested positive for SARS‐CoV‐2 | Reproductive age women ages 12 to 49 who tested COVID positive | Pregnancy | Uncertain | "Multivariable logistic regression was used to control for differences at baseline" | Logistic regression | Hospitalization Intubation |
n = 123 COVID positive patients | n = 44 | n = 79 |
| Costeira 2021 | Prospective cohort study | Users of the application in the UK | Self‐reported data from users input to COVID Symptom Study Smartphone Application Data obtained between 7 May 7 to 15 June 2020 exposures, outcomes, and covariates were ascertained following quality control with purpose‐built scripts | Combined oral contraceptive use (self‐report) | Subjects with predicted COVID‐19 probability of > 50% considered COVID positive; model incorporated age, sex, anosmia, persistent cough, severe fatigue, and skipped meals | Female app users 20 to 45 years with BMI between 18 to 35 kg/m2 85% were pre‐menopausal | Use of estrogen for gender transitioning | Age, BMI, smoking status ‐ self‐reported | Binomial generalized mixed models | Binomial generalized mixed models with with a log‐odds/logit link function used for association Age: continuous fixed effect BMI: continuous fixed effect Smoker: categorical fixed effect ‐ never, ex, and current sensitivity analyses performed to match the mean and median age of cases and controls for the exposure variables in subsets of users in 5‐year age bins | Hospitalization | n = 295,689 | n = 64,253 | n = 231,436 |
| Chima 2021 | Case series | 41 healthcare organizations participating in TriNetX ‐ data for 8 patients in the US | Derived from TriNet X EHR | Combined hormonal contraception Case definition: pulmonary embolism: defined using any ICD‐10 root diagnostic code; medications 30 days before and after acute PE diagnosis and COVID diagnosis |
COVID‐19 patients were identified via the ICD‐10code U07.1. Assumed that the day the diagnostic code was entered for billing was the day the diagnosis was made | Pediatric patients < 18 years old with PE and COVID positive. PE diagnosed concurrently or within 30 days of COVID diagnosis | _ | Age, BMI, race, ethnicity, lab results, medications | None, descriptive only | Descriptive | Pulmonary embolism | n = 6 girls | n = 1 | n = 5 |
| Hameed 2021 | Case series | Multicenter multinational study ‐ 10 tertiary care centers in Pakistan, Egypt, Singapore, and the US | Data collected retrospectively from COVID‐19 registries and medical records | "Oral contraception" CVT case definition: "Diagnosis of CVT was confirmed by at least one of the following imaging studies according to the established criteria: magnetic resonance (MR) imaging, MR venography (MRV), computed tomography (CT), CT venography, or cerebral venography" |
COVID‐19 infection, confirmed either by reverse transcriptase‐polymerase chain reaction assay of a nasopharyngeal swab or serum antibody testing for CO‐VID‐19 | "Patients aged 18 years or above with recent COVID‐19 infection, confirmed either by reverse transcriptase‐polymerase chain reaction assay of a nasopharyngeal swab or serum antibody testing for CO‐VID‐19" | Patients with recent trauma or those on anticoagulation | "Risk factors, clinical features, laboratory findings, imaging findings, COVID‐19‐related information"; of note, OCP user also antiphospholipid antibody + | None, descriptive only | Descriptive: frequencies and corellations | Cerebral venous thrombosis | n = 7 women with CVT | n = 1 patient using "oral contraceptive" | n = 6 |
BMI: body mass index; CVT: cerebral venous thrombosis; CHC: combined hormonal contraception; HC: hormonal contraception; ICD‐10: International Classification of Diseases 10th Revision; LNG IUD: levonorgestrel intrauterine device; OCP: oral contraception pill; PE: pulmonary embolism; POP: progestogen‐only pill.
The three NRSIs (Costeira 2021; Mujumdar 2020; Seeland 2020) were database cohort studies. Costeira 2021 and Seeland 2020 stratified outcomes for premenopausal and postmenopausal patients; we included only information from the premenopausal, reproductive‐aged women groups in line with our inclusion criteria. Only one NRSI (Costeira 2021) ascertained current use of contraceptives based on patient report; the other two comparative studies (Mujumdar 2020; Seeland 2020) used diagnostic codes within medical records to determine hormonal contraception use, but did not attempt to confirm current contraceptive use at time of the outcome. None of the NRSIs included thromboembolism as an outcome.
Of note, Costeira 2021 included self‐reported data from reproductive‐aged women in the United Kingdom who used the COVID Symptom Study Smartphone Application from 7 May to 15 June 2020. Users were not required to be COVID‐19 positive, so this may represent a different population than outlined in our protocol. However, we anticipate that users of the application who were tracking symptoms were doing so due to concern for having COVID‐19. These were additionally data obtained during a time when antibody positivity rates in the UK were approximately 6.8% and there were an estimated 54,000 new cases of COVID‐19 weekly in England (Office for National Statistics 2020). The study also represented some of the best data available to date. Therefore, we determined to include the study for analysis.
Seeland 2020 measured mortality for COVID‐19 positive individuals who were reportedly using combined hormonal contraception versus those who were not.
Two studies (Costeira 2021; Mujumdar 2020) measured hospitalization rates for patients reportedly using hormonal contraception versus those not using hormonal contraception. Mujumdar 2020 included all users of any type of hormonal contraception as their exposure group while Costeira 2021 compared combined hormonal contraceptive users to people without any hormonal therapy.
Chima 2021 and Hameed 2021 reported case series of COVID‐19 positive patients who experienced venous thromboembolism. Chima 2021 reported on adolescent patients with pulmonary embolism. Hameed 2021 reported on patients with cerebral venous thrombosis. No comparative studies directly assessed thromboembolism as an outcome.
Excluded studies
During full‐text review, we excluded 26 studies (see Excluded studies), for the following reasons.
We excluded 19 studies with incorrect study design; of these, nine were case reports (Alyousefi 2021; Badrawi 2021; Fiorini 2021; Grimes 2021; Iguina 2020; Koritala 2021; Kundal 2021; Rebelo 2021; Valenzuela‐Vallejo 2021), five were editorials (Cagnacci 2022; Kow 2021; Kurdoğlu 2021; Mitra 2021; Spratt 2020), four were review articles (Lete 2021; Pires 2020; Ramírez 2020; Traish 2021), and one study was a guideline development article for thromboprophylaxis recommendations for COVID‐19 positive patients (Riera‐Mestre 2021).
We excluded four studies for incorrect study populations. Two studies (Fernández‐Capitán 2021; Vahey 2021) did not stratify analyses by sex. One study (Ostovan 2021) was a case series, but did not include any cases who had been using hormonal contraception. One study analyzed outcomes for postmenopausal patients who either used or did not use menopausal hormonal therapy (Lee 2020).
We excluded Ding 2021 for incorrect intervention as they used physiologic serum estradiol levels as an exposure variable instead of exogenous hormonal contraception. We excluded one study (Limanova 2021) for incorrect outcome as they investigated drug interactions between hormonal contraception and COVID‐19 therapeutic agents.
We identified one study protocol (NCT04865029) for an ongoing study which did not meet inclusion criteria given intervention indications as it planned to use hormonal contraception as treatment for patients with COVID‐19 disease.
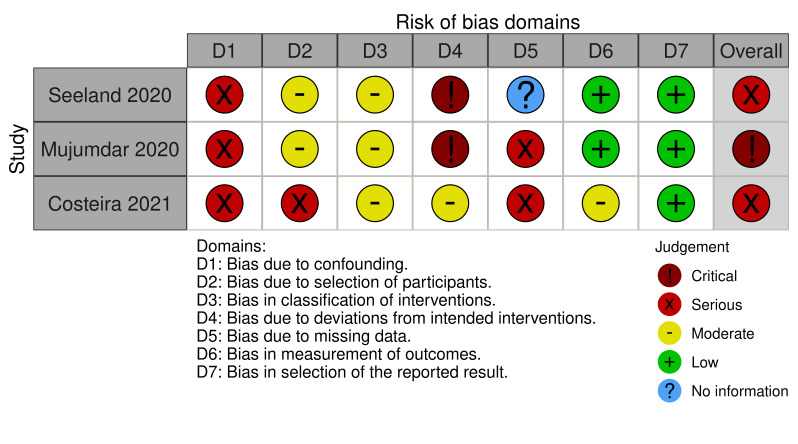
Risk of bias in included studies
We used the ROBINS‐I tool to assess the risk of bias of the three included NRSIs. We judged the overall risk of bias of the three studies to vary between serious to critical (Figure 2). Justifications for our risk of bias judgments are summarized in Table 4. We judged two NSRI studies to be at serious risk of bias (Costeira 2021; Seeland 2020) and Mujumdar 2020 to be at critical risk of bias. The two cases series (Chima 2021; Hameed 2021) we judged to be at high risk of bias given the nature of the study design and there is no applicable risk of bias tool for case series. There was also no information presented in the case series regarding the temporality of contraceptive use in relation to the thromboembolic outcomes.
2.
2. Risk of bias assessments using ROBINS‐I tool.
| Study | D1a | D2b | D3c | D4d | D5e | D6f | D7g | Overall |
| Seeland 2020 | Serious Use propensity score matching but unclear what variables they are matching on; may not have accounted for all confounders as listed in our protocol |
Moderate Selection based on COVID‐19 positivity in electronic health record, any factors affecting positivity are likely to be similar between groups |
Moderate Used clear Rx Norm codes to define combined contraceptives, but no ascertainment of actual patient use of contraception during the timeframe of the outcome | Critical Actual use of contraception is not ascertained | No information | Low Outcome = mortality derived from electronic health record, likely to be accurately reported | Low | Serious Use propensity score matching but we are not certain what the actual variables were included in this; also not sure whether patients are using contraception during time of outcome |
| Mujumdar 2020 | Serious Concern for possible residual confounding especially as there is no information on what was adjusted for and whether this was a valid adjustment | Moderate Selection based on COVID‐19 positivity in electronic health record of 1 tertiary care facility, any factors affecting positivity are likely to be similar between groups | Moderate Contraceptive prescription derived from medical record data, but no ascertainment of actual patient use of contraception during the timeframe of the outcome | Critical Actual use of contraception was not ascertained | Serious Outcome data missing for ~7% of the total sample and those individuals were excluded from analysis | Low Outcome = hospitalization derived from electronic health record, likely to be accurately reported | Low | Critical Unknown confounders adjusted for unknown if patients using contraception during timeframe of outcome |
| Costeira 2021 | Serious Adjusted for age, BMI, and smoking status. No information on personal history of DVT. However, variables were self‐reported and uncertain if they were measured validly. Variables such as smoking status likely subject to social desirability bias in self‐reporting | Serious Participants self‐selected to use application; possible that people who are using contraception are more likely/interested in using app as they are more health savvy | Moderate Use and contraceptive type based on user self‐report | Moderate Reported by patients they were taking the intervention | Serious Participants with missing smoking status were excluded from analyses | Moderate Outcome = hospitalization, but self‐reported so subject to incorrect reporting (e.g. individual reports an emergency room visit as hospitalization) | Low | Serious Has better classification of intervention and likely adherence to intervention, but all self‐report data and subject to bias |
aD1: bias due to confounding. bD2: bias in selection of participants into the study. cD3: bias in classification of interventions. dD4: bias due to deviations from intended interventions. eD5: bias due to missing data. fD6: bias in measurement of outcomes. gD7: bias in selection of the reported result. BMI: body mass index; DVT: deep vein thrombosis.
Bias due to confounding
We noted serious risk of bias due to confounding for all three NRSIs. We judged Seeland 2020 to be at serious risk of bias from confounding as they did not identify which covariates were used for propensity score matching. Similarly, Mujumdar 2020 did not report on variables used for confounding assessment. Costeira 2021 did adjust for body mass index (BMI) and age, but did not include all pre‐specified confounders, and we judged there to be additional risk that variables were not validly measured as they were self‐reported. No study included personal history of thromboembolism, estradiol dose, or progestogen type as confounding variables, which we pre‐specified in our protocol as likely confounders.
Bias due to selection of participants
Seeland 2020 and Mujumdar 2020 derived their data from electronic health records, but they were retrospective cohorts, so we rated them as at moderate risk of bias. As Costeira 2021 relied on patient use of their mobile electronic tracking application, we judged there to be serious risk of selection bias as individuals using contraception may be more conscious of potential health risks and more likely to use a health and symptom mobile tracking application.
Bias in classification of interventions
All three NRSI included had clear definitions for hormonal contraception or combined hormonal contraception use, either derived from electronic health record codes (Mujumdar 2020; Seeland 2020) or based on patient self‐report (Costeira 2021).
Bias due to deviations from intended intervention
We judged Seeland 2020 and Mujumdar 2020 to be at critical risk of bias due to deviations from intended intervention, as they did not ascertain if patients were in fact actively using the forms of contraception documented in the medical record at the time of the outcome. We judged Costeira 2021 as at moderate risk of bias for deviations from intended intervention as they utilized patient self‐report for determining active use of contraception.
Bias due to missing data
Both Mujumdar 2020 and Costeira 2021 performed analyses on a smaller subsample due to missing data. We deemed Mujumdar 2020 at serious risk of bias due to its small sample size which was further reduced due to missing data for the outcome variable (hospitalization). Costeira 2021 restricted analysis to individuals with available data on smoking status. Seeland 2020 reported no information on missing data or how they were handled.
Bias in measurement of outcomes
Hospitalization (Mujumdar 2020) and mortality (Seeland 2020) are definite outcomes unlikely to be measured incorrectly in electronic health record data. We thus judged these at low risk of bias. The outcome of hospitalization for Costeira 2021 was derived from self‐reported data, so we judged this to be at moderate risk of bias as conceivably some people could have misclassified the outcome: for example, if a patient only had an emergency room visit but reported this as a hospitalization.
Bias in selection of the reported results
All three NRSIs were at low risk of bias due to selection of the reported results.
Effects of interventions
Four studies reported on outcomes for use of combined hormonal contraception compared to no use of contraception in COVID‐19 patients (Chima 2021; Hameed 2021; Seeland 2020) or patients who are at risk of having COVID‐19 (Costeira 2021). We summarized these in the Table 1.
Seeland 2020 measured mortality of COVID‐19 positive patients who were users of combined hormonal contraception versus contraception non‐users with data derived from electronic health records from a healthcare organization in 17 countries. Given the limitations of the database, the authors could not ascertain current contraceptive use at the time of the outcome. Based on results from this NRSI, there may be little to no effect of combined hormonal contraception use on odds of mortality for COVID‐19 positive patients (adjusted odds ratio (OR) of 1.00, 95% confidence interval (CI) 0.41 to 2.40; 1 study, 18,892 participants; very low‐certainty evidence).
Costeira 2021 studied hospitalization for COVID‐19 in individuals self‐reporting use or non‐use of combined hormonal contraceptive where COVID‐19 disease status was not confirmed through testing but via symptom reporting through a mobile tracking application; thus we deemed this evidence to be of low certainty, downgraded for serious risk of bias and for indirectness. Adjusting for BMI, age, and smoking status, hormonal contraceptive users compared to non‐users may have a slight decrease in their odds of hospitalization (adjusted OR 0.79, 95% CI 0.64 to 0.97; 1 study, 295,689 participants; very low‐certainty evidence). Notably, this study excluded any individuals with a BMI greater than or equal to 35 kg/m2, so these results may not be generalizable to patients of higher BMI who are known to have an independently higher risk of thromboembolism.
In combination, the two case series (Chima 2021; Hameed 2021) reported on 13 patients with a venous thromboembolism concurrent with COVID‐19 infection. Of these 13 patients, two patients were reportedly using combined hormonal contraception, but actual use was not available. One of the six female pediatric patients (less than 18 years in age) with COVID‐19 who developed pulmonary embolism was taking hormonal contraception (Chima 2021). One of seven reproductive‐aged women with COVID‐19 and cerebral venous thromboembolism (CVT) was taking oral contraceptive pills (Hameed 2021). Notably, the individual with the CVT was also diagnosed with antiphospholipid antibody syndrome, a thrombophilia. This patient, in addition to another patient not using combined hormonal contraception (CHC), required intubation. Thus, there may be little to no effect of CHC use on odds of requiring intubation in patients with COVID‐19, but the evidence is very uncertain.
Only one study reported on outcomes for use of any type of hormonal contraception compared to no use of contraception in COVID‐19 patients (Mujumdar 2020). Results are summarized in Table 2. They obtained their data from electronic health records from one tertiary care organization, but could not ascertain current contraceptive use at the time of the outcome. They found little to no effect on risk of hospitalization for COVID‐19 positive patients based on exposure to hormonal contraception (adjusted OR 0.99, 95% CI 0.68 to 1.44; 1 study, 123 participants; very low‐certainty evidence). No patients in either the exposure nor control arms required intubation.
No comparative studies reported on arterial or venous thromboembolic outcomes. No studies reported data on acute respiratory distress syndrome or need for hospitalization in the intensive care unit.
Discussion
Summary of main results
Our primary objective was to assess the risk of venous or arterial thromboembolism in patients with COVID‐19 disease using combined hormonal contraception. Secondary objectives were to investigate other markers of COVID‐19 severity such as acute respiratory distress syndrome, intubation, need for (intensive care unit) ICU care, and mortality for those using combined hormonal contraception or other forms of hormonal contraception. After our search, we additionally included hospitalization as a secondary outcome as studies commonly reported this outcome, while few to no studies measured our pre‐specified outcomes. We identified only five studies addressing these objectives (Chima 2021; Costeira 2021; Hameed 2021; Mujumdar 2020; Seeland 2020). We included case series with limited interpretable evidence for this review, as they were the only studies that addressed our primary outcome.
We found no comparative studies assessing thromboembolism risk among COVID‐19 positive individuals using hormonal contraception users compared with non‐users. Two case series (Chima 2021; Hameed 2021) reported on a total of 13 individuals with venous thromboembolism who were COVID‐19 positive, of whom only two individuals were taking combined contraception or oral contraceptive pills. The evidence for any effect of combined hormonal contraception on risk of developing venous thromboembolism is very uncertain, and we found no evidence assessing risk of arterial embolism.
We identified two observational studies (Costeira 2021; Seeland 2020) and one case series (Hameed 2021) that assessed markers of COVID‐19 severity for users of combined hormonal contraception versus non‐users. We found little to no effect of combined hormonal contraceptive use on odds of mortality among COVID‐19 patients, but the evidence is very uncertain (Seeland 2020). Combined hormonal contraception use may slightly decrease odds of hospitalization for individuals with a body mass index (BMI) less than 35kg/m2; however, the study population was not confirmed to be COVID‐19 positive and the evidence is very uncertain (Costeira 2021). Use of combined hormonal contraception in COVID‐19 positive patients appears to have little to no effect on the odds of intubation, particularly with a cerebral venous thromboembolism, but this evidence is very uncertain (Hameed 2021).
We identified one observational study (Mujumdar 2020) that assessed markers of COVID‐19 severity among users of hormonal contraception versus non‐users of contraception. There may be no effect of any hormonal contraception use on odds of hospitalization for COVID‐19 positive patients, but the evidence is very uncertain. We could not measure the relative effect of hormonal contraceptive use on intubation as no intubations occurred in either group.
Although the evidence is of very low certainty and there is heterogeneity amongst studies in exposures, populations, and outcomes, the current available evidence suggests there may be little to no or slightly decreased odds of hospitalization and little to no effect on odds of mortality for hormonal contraception users versus non‐users who are COVID‐19 positive. There is not enough evidence to draw conclusions regarding risk of venous or arterial thromboembolism in patients with COVID‐19 who are using hormonal contraception.
Overall completeness and applicability of evidence
There are very few comparative studies examining outcomes in COVID‐19 patients using combined or other hormonal contraception versus contraceptive non‐users. Given the paucity of the evidence, we were unable to pool results or conduct meta‐analyses. The data that we report have not been shown to be replicable as each included study assessed a different outcome among slightly differing populations. We found no comparative studies directly assessing risk of thromboembolism. This is a large gap in the literature, though it is a topic of paramount importance. Additionally, we found no studies assessing arterial thromboembolism risk; however, this is a rare outcome that needs large cohort or registry studies to address.
The few comparative studies that we did identify have several important flaws. Actual contraceptive use was not ascertained in many studies (Mujumdar 2020; Seeland 2020), nor was the formulation, dose, or duration of use identified. These are known to be important predictors of thromboembolism risk among contraceptive users at baseline. We were therefore unable to conduct subgroup analyses planned, for example, for users of combined contraceptive pills containing less than 30 mcg ethinyl estradiol compared to those containing greater than or equal to 30 mcg ethinyl estradiol, or of hospitalized versus outpatient COVID‐19 positive patients.
There are also no studies assessing risk for users of combined hormonal contraception versus users of progestin‐only contraception, even though considering switching patients to progestin‐only contraception has been a commonly recommended guideline from country obstetric and gynecologic societies. Although there is theoretical biological plausibility to support this recommendation, there is no direct evidence to support progestin‐only contraception being safer for patients with COVID‐19. Additionally, there is reason to suspect that the estrogen component of combined hormonal contraceptives may interact differently or even perhaps mitigate the endothelial, pro‐thrombotic effects of COVID‐19.
Certainty of the evidence
The quality of the evidence is overall low and judged to be at serious or critical risk of bias. All included studies were observational, and are therefore at inherent risk of bias from residual confounding and selection. Additionally, apart from Costeira 2021, the studies included did not identify important confounders used for adjusted estimates of risk, though Seeland 2020 did report using propensity score matching in their analysis.
We used the GRADE process to assess the certainty of the evidence. Studies were of very low certainty. We downgraded all studies for risk of bias, including downgrading the case series two levels for risk of bias. The most direct comparative study assessing risk of COVID‐19 severity for combined hormonal contraception users versus non‐users (Seeland 2020), was downgraded for indirectness given lack of exposure ascertainment.
The quality of the evidence for risk of thrombosis for combined hormonal contraception users versus non‐users who are COVID‐19 positive is extremely poor with no comparative studies identified as of March 2022.
Potential biases in the review process
We conducted our review based on our previously published protocol. However, we decided to include studies measuring a different secondary outcome (hospitalization) given the lack of evidence directly assessing the pre‐specified outcomes. Additionally, we were unable to conduct meta‐analyses given the few studies included. We therefore present primarily narrative results and highlight where the data are lacking.
Data extraction was performed by one author and checked by a second author. While two authors independently performed risk of bias assessments, only one author independently graded the certainty of the evidence, which was then checked by a second author. We reached consensus through discussion.
All studies included in this review are observational which has inherent risk of bias. Where studies were missing important information such as variables included as confounders, we attempted to contact study authors for clarification. Where missing information remained, we downgraded the certainty of the evidence for risk of bias.
Agreements and disagreements with other studies or reviews
This is the first study of its kind investigating thromboembolic and other outcomes for markers of COVID‐19 severity among users and non‐users of contraception. While several country obstetric and gynecological societies have recommended considering switching combined hormonal contraceptive users who are COVID‐19 positive to progestin‐only contraception, we found no evidence to support this. On the contrary, we observed that users of combined hormonal contraception may have a slightly decreased odds of hospitalization compared to non‐users of contraception, though the evidence is very uncertain.
Authors' conclusions
Implications for practice.
Very little evidence exists examining the risk of thromboembolism or increased COVID‐19 severity for users of combined hormonal contraception compared to non‐users of hormonal contraception. The evidence suggests that the use of any hormonal contraceptive method compared to non‐contraceptive use has little to no effect on the risk of hospitalization, intubation, or mortality in patients with COVID‐19. However, all the data are of low to very low certainty. Patients with symptoms but unconfirmed COVID‐19 using combined hormonal contraceptive versus non‐users may be at slightly decreased risk of hospitalization, but this is based on only one study with data only for patients with body mass index (BMI) less than 35 kg/m2. At a minimum, we did not observe any trends towards an increased risk of severe COVID‐19 among contraceptive users.
No comparative studies exist assessing the risk of thromboembolism in COVID‐19 patients who are users of hormonal contraception, which was the primary objective of this review. We cannot make any conclusions regarding the use of hormonal contraception on risk of thromboembolism in COVID‐19 positive individuals given the sparse data of very low certainty.
Implications for research.
This study identified very limited literature to assess whether hormonal contraceptive users with COVID‐19 are at heightened risk for venous or arterial thromboembolism or other complications. Although the results are uncertain, one study showed a possible protective effect of combined hormonal contraception on hospitalization rates. Ongoing investigations could help elucidate whether some COVID‐19 positive hormonal contraceptive users may obtain a protective effect, with important implications for disease management. There is recent evidence for a protective effect of estrogen supplementation on risk of mortality among postmenopausal patients (Sund 2022), but it is uncertain whether a similar effect would be expected for premenopausal patients using hormonal management for contraceptive purposes. It is unlikely that any randomized controlled trials will be performed given the nature of this topic, but there are ways to improve the certainty of the evidence derived from observational studies. Future studies would benefit from collecting pertinent information on confounders. These include patient age, BMI, history of prior thromboembolism, medical comorbidities associated with increased risk of venous thromboembolism, reason for hormonal contraception use (contraception versus treatment of medical condition), recent pregnancy or other thrombophilic state, contraception formulation including type of estrogen and dose of estrogen for combined hormonal contraception, and duration of contraception use. Differing estrogens may have different thrombogenic potential given differing potency, so it would be important to know if a formulation contained, for example, ethinyl estradiol versus estradiol valerate. No studies reported indication for hormonal contraceptive use, which is important as individuals who use hormonal management for medical conditions such as heavy menstrual bleeding may have different risk profiles compared to individuals using hormones for contraceptive purposes. Additionally, several studies included were downgraded due to failure to ascertain actual contraceptive use and adherence at the time of the outcome. Certainty of the evidence would improve if current use or recency of contraception use were regularly ascertained for individuals at the time of the outcome of interest. As COVID‐19 continues to evolve and new variants emerge, reporting of variants as well as therapeutics used for treatment may also be important for analysis, but were not reported in any studies. Additionally, there were no data for populations of differing COVID‐19 severity, i.e. ambulatory versus hospitalized patients, which is needed before evidence‐based recommendations can be provided to hormonal contraceptive users who contract COVID‐19.
Well‐conducted comparative studies on thromboembolic outcomes for COVID‐19 users versus non‐users of contraception will need to be conducted before any conclusions about protective or detrimental associations with thromboembolism and other complications of COVID‐19 infection can be drawn.
What's new
| Date | Event | Description |
|---|---|---|
| 29 November 2023 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Two studies have been found in searches up to 1 September 2023 (DOI: 10.1093/fampra/cmac041 and DOI: 10.1111/ejh.14061) but are unlikely to change review conclusions (as assessed by the authors and editorial team). The conclusions of this review are therefore considered up to date. |
History
Protocol first published: Issue 3, 2021 Review first published: Issue 1, 2023
| Date | Event | Description |
|---|---|---|
| 15 May 2023 | New search has been performed | Literature search updated March 14 2023, conclusions not changed |
| 15 May 2023 | New citation required but conclusions have not changed | This is a Living Systematic Review. Searches are run and screened monthly. One study has been found in searches up to March 14 2023 (DOI 10.1093/fampra/cmac041) but is unlikely to change review conclusions (as assessed by the authors and editorial team). The conclusions of this review are therefore considered up to date. |
Acknowledgements
We acknowledge the help and support of Cochrane Fertility Regulation Review Group, peer reviewers, and copy editors.
Appendices
Appendix 1. Updated Search strategies
Cochrane Central Register of Controlled Trials (Ovid EBM Reviews) September 2023 Date searched: 1 November 2023 1 Coronavirus/ or Coronavirus Infections/ or COVID‐19/ 2 (coronavirus* or corona‐virus* or OC43 or NL63 or 229E or HKU1 or HCoV* or ncov* or covid* or sars‐cov* or sarscov* or Sars‐coronavirus* or "Severe Acute Respiratory Syndrome").ti,ab,kw. 3 or/1‐2 4 3 not (SARS or MERS or MERS‐CoV or "Middle East respiratory syndrome" or camel* or dromedar* or equine or coronary or coronal or covidence* or covidien or "influenza virus" or HIV or bovine or calves or TGEV or feline or porcine or BCoV or PED or PEDV or PDCoV or FIPV or FCoV or SARS‐CoV or canine or CCov or zoonotic or "avian influenza" or H1N1 or H5N1 or H5N6 or IBV or murine).ti,ab,kw. 5 ((pneumonia or covid* or coronavirus* or corona virus* or ncov* or 2019‐ncov or sars*).mp. or exp pneumonia/) and Wuhan.mp. 6 (2019‐ncov or ncov19 or ncov‐19 or 2019‐novel CoV or sars‐cov2 or sars‐cov‐2 or sarscov2 or sarscov‐2 or Sars‐coronavirus2 or Sars‐coronavirus‐2 or SARS‐like coronavirus* or coronavirus‐19 or covid19 or covid‐19 or covid 2019 or ((novel or new or nouveau) adj2 (CoV or nCoV or covid or coronavirus* or corona virus or Pandemi*2)) or ((covid or covid19 or covid‐19) and pandemic*2) or (coronavirus* and pneumonia)).ti,ab,kw. 7 or/4‐6 8 Contraceptive Agents/ or Contraceptive Agents, Female/ or Contraceptives, Oral/ or exp Contraceptives, Oral, Hormonal/ or Contraceptives, Oral, Combined/ or Contraceptives, Oral, Sequential/ or Contraceptives, Oral, Synthetic/ or Hormonal Contraception/ or Contraceptive Agents, Hormonal/ or Intrauterine Devices, Medicated/ or "Long‐Acting Reversible Contraception"/ 9 Cyproterone Acetate/ or Desogestrel/ or Estrogens/ or exp Ethinyl Estradiol/ or Ethinyl Estradiol‐Norgestrel Combination/ or Ethynodiol Diacetate/ or Levonorgestrel/ or Lynestrenol/ or Medroxyprogesterone/ or Norethindrone/ or Norethindrone Acetate/ or Norgestrel/ or Progesterone/ or Progestins/ 10 (contraceptive or contraceptives or contraception or antifertility or anti‐fertility or anticonception or anti‐conception or birth‐control).ti,ab,kw. 11 (CHC or CHCs or COC or COCs or COCP or COCPs or OCP or OCPs or POPs or ((monophasic or mono‐phasic or biphasic or bi‐phasic or triphasic or tri‐phasic or quadriphasic or quadri‐phasic or multiphasic or multi‐phasic or normophasic or normo‐phasic or minidose or mini‐dose or morning‐after or progestin‐only) adj (pill or pills)) or ((first‐generation or 1st‐generation or second‐generation or 2nd‐generation or third‐generation or 3rd‐generation or fourth‐generation or 4th‐generation) adj2 (pill or pills or progest*))).ti. 12 (((progest* or levonorgestrel or LNG) adj2 (ball or balls or coil or coils or device or devices or IUD or IUDs or IUCD or IUCDs or IUS or IUSs or system or systems)) or (medicated adj2 (IUD or IUDs or IUCD or IUCDs or IUS or IUSs)) or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs).ti,ab,kw. 13 ("cyproterone acetate" or desogestrel or drospirenone or dienogest or "estradiol valerate" or "oestradiol valerate" or estradiol or oestradiol or estrogen or oestrogen or EE or ethinylestradiol or ethinyloestradiol or ethinyl‐estradiol or ethinyl‐oestradiol or "ethynodiol diacetate" or ETN or etonogestrel or gestodene or LNG or levonorgestrel or lynestrenol or DMPA or "medroxyprogesterone acetate" or "nomegestrol acetate" or norelgestromin or norethindrone or norgestimate or norgestrel or progesterone or progestin* or progestogen or "segesterone acetate").ti,ab,kw. 14 or/8‐13 15 and/7,14
MEDLINE ALL (Ovid) 1946 to 31 October 2023 Date searched: 1 November 2023 1 exp Coronavirus/ or exp Coronavirus Infections/ or exp COVID‐19/ or "COVID‐19".hw. 2 (coronavirus* or corona‐virus* or OC43 or NL63 or 229E or HKU1 or HCoV* or ncov* or covid* or sars‐cov* or sarscov* or Sars‐coronavirus* or "Severe Acute Respiratory Syndrome").ti,ab,kw,kf. 3 or/1‐2 4 3 not (SARS or MERS or MERS‐CoV or "Middle East respiratory syndrome" or camel* or dromedar* or equine or coronary or coronal or covidence* or covidien or "influenza virus" or HIV or bovine or calves or TGEV or feline or porcine or BCoV or PED or PEDV or PDCoV or FIPV or FCoV or SARS‐CoV or canine or CCov or zoonotic or "avian influenza" or H1N1 or H5N1 or H5N6 or IBV or murine).ti,ab,kw,kf. 5 ((pneumonia or covid* or coronavirus* or corona virus* or ncov* or 2019‐ncov or sars*).mp. or exp pneumonia/) and Wuhan.mp. 6 (2019‐ncov or ncov19 or ncov‐19 or 2019‐novel CoV or sars‐cov2 or sars‐cov‐2 or sarscov2 or sarscov‐2 or Sars‐coronavirus2 or Sars‐coronavirus‐2 or SARS‐like coronavirus* or coronavirus‐19 or covid19 or covid‐19 or covid 2019 or ((novel or new or nouveau) adj2 (CoV or nCoV or covid or coronavirus* or corona virus or Pandemi*2)) or ((covid or covid19 or covid‐19) and pandemic*2) or (coronavirus* and pneumonia)).ti,ab,kw,kf. 7 (COVID‐19 or severe acute respiratory syndrome coronavirus 2).os. 8 or/4‐7 9 Contraceptive Agents/ or Contraceptive Agents, Female/ or Contraceptives, Oral/ or exp Contraceptives, Oral, Hormonal/ or Contraceptives, Oral, Combined/ or Contraceptives, Oral, Sequential/ or Contraceptives, Oral, Synthetic/ or Hormonal Contraception/ or Contraceptive Agents, Hormonal/ or Intrauterine Devices, Medicated/ or "Long‐Acting Reversible Contraception"/ 10 Cyproterone Acetate/ or Desogestrel/ or Estrogens/ or exp Ethinyl Estradiol/ or Ethinyl Estradiol‐Norgestrel Combination/ or Ethynodiol Diacetate/ or Levonorgestrel/ or Lynestrenol/ or Medroxyprogesterone/ or Norethindrone/ or Norethindrone Acetate/ or Norgestrel/ or Progesterone/ or Progestins/ 11 (contraceptive or contraceptives or contraception or antifertility or anti‐fertility or anticonception or anti‐conception or birth‐control).ti,ab,kw,oa,kf. 12 (CHC or CHCs or COC or COCs or COCP or COCPs or OCP or OCPs or POPs or ((monophasic or mono‐phasic or biphasic or bi‐phasic or triphasic or tri‐phasic or quadriphasic or quadri‐phasic or multiphasic or multi‐phasic or normophasic or normo‐phasic or minidose or mini‐dose or morning‐after or progestin‐only) adj (pill or pills)) or ((first‐generation or 1st‐generation or second‐generation or 2nd‐generation or third‐generation or 3rd‐generation or fourth‐generation or 4th‐generation) adj2 (pill or pills or progest*))).ti. 13 (((progest* or levonorgestrel or LNG) adj2 (ball or balls or coil or coils or device or devices or IUD or IUDs or IUCD or IUCDs or IUS or IUSs or system or systems)) or (medicated adj2 (IUD or IUDs or IUCD or IUCDs or IUS or IUSs)) or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs).ti,ab,kw,oa,kf,nm. 14 ("cyproterone acetate" or desogestrel or drospirenone or dienogest or "estradiol valerate" or "oestradiol valerate" or estradiol or oestradiol or estrogen or oestrogen or EE or ethinylestradiol or ethinyloestradiol or ethinyl‐estradiol or ethinyl‐oestradiol or "ethynodiol diacetate" or ETN or etonogestrel or gestodene or LNG or levonorgestrel or lynestrenol or DMPA or "medroxyprogesterone acetate" or "nomegestrol acetate" or norelgestromin or norethindrone or norgestimate or norgestrel or progesterone or progestin* or progestogen or "segesterone acetate").ti,ab,kw,kf,nm,rn. 15 or/9‐14 16 and/8,15
EMBASE.com (from inception to 31 October 2023) Date searched: 1 November 2023 #1 'coronavirinae'/exp OR 'coronaviridae infection'/exp OR 'coronavirus disease 2019'/de OR 'covid‐19‐associated coagulopathy'/de #2 coronavirus*:ti,ab,kw OR 'corona virus*':ti,ab,kw OR oc43:ti,ab,kw OR nl63:ti,ab,kw OR 229e:ti,ab,kw OR hku1:ti,ab,kw OR hcov*:ti,ab,kw OR ncov*:ti,ab,kw OR covid*:ti,ab,kw OR 'sars cov*':ti,ab,kw OR sarscov*:ti,ab,kw OR 'sars coronavirus*':ti,ab,kw OR 'severe acute respiratory syndrome':ti,ab,kw #3 #1 OR #2 #4 #3 NOT (sars:ti,ab,kw OR mers:ti,ab,kw OR 'mers cov':ti,ab,kw OR 'middle east respiratory syndrome':ti,ab,kw OR camel*:ti,ab,kw OR dromedar*:ti,ab,kw OR equine:ti,ab,kw OR coronary:ti,ab,kw OR coronal:ti,ab,kw OR covidence*:ti,ab,kw OR covidien:ti,ab,kw OR 'influenza virus':ti,ab,kw OR hiv:ti,ab,kw OR bovine:ti,ab,kw OR calves:ti,ab,kw OR tgev:ti,ab,kw OR feline:ti,ab,kw OR porcine:ti,ab,kw OR bcov:ti,ab,kw OR ped:ti,ab,kw OR pedv:ti,ab,kw OR pdcov:ti,ab,kw OR fipv:ti,ab,kw OR fcov:ti,ab,kw OR 'sars cov':ti,ab,kw OR canine:ti,ab,kw OR ccov:ti,ab,kw OR zoonotic:ti,ab,kw OR 'avian influenza':ti,ab,kw OR h1n1:ti,ab,kw OR h5n1:ti,ab,kw OR h5n6:ti,ab,kw OR ibv:ti,ab,kw OR murine:ti,ab,kw) #5 (pneumonia OR covid* OR coronavirus* OR 'corona virus*' OR ncov* OR '2019 ncov' OR sars* OR 'pneumonia'/exp) AND wuhan #6 '2019 ncov':ti,ab,kw OR ncov19:ti,ab,kw OR 'ncov 19':ti,ab,kw OR '2019‐novel cov':ti,ab,kw OR 'sars cov2':ti,ab,kw OR 'sars cov 2':ti,ab,kw OR sarscov2:ti,ab,kw OR 'sarscov 2':ti,ab,kw OR 'sars coronavirus2':ti,ab,kw OR 'sars coronavirus 2':ti,ab,kw OR 'sars‐like coronavirus*':ti,ab,kw OR 'coronavirus 19':ti,ab,kw OR covid19:ti,ab,kw OR 'covid 19':ti,ab,kw OR 'covid 2019':ti,ab,kw OR (((novel OR new OR nouveau) NEAR/2 (cov OR ncov OR covid OR coronavirus* OR 'corona virus' OR pandemi*2)):ti,ab,kw) OR ((covid:ti,ab,kw OR covid19:ti,ab,kw OR 'covid 19':ti,ab,kw) AND pandemic*2:ti,ab,kw) OR (coronavirus*:ti,ab,kw AND pneumonia:ti,ab,kw) #7 #4 OR #5 OR #6 #8 'contraceptive agent'/de OR 'hormonal contraceptive agent'/exp OR 'injectable contraceptive agent'/exp OR 'oral contraceptive agent'/exp OR 'hormonal contraception'/de OR 'long‐acting reversible contraception'/de OR 'oral contraception'/de OR 'ovulation inhibition'/de #9 'cyproterone acetate'/de OR 'cyproterone acetate plus ethinylestradiol'/de OR 'desogestrel'/de OR 'desogestrel plus ethinylestradiol'/de OR 'estrogen'/de OR 'ethinylestradiol'/de OR 'ethinylestradiol plus norgestrel'/de OR 'etynodiol diacetate'/de OR 'levonorgestrel'/de OR 'lynestrenol'/de OR 'medroxyprogesterone acetate'/de OR 'norethisterone'/de OR 'norethisterone acetate'/de OR 'norgestrel'/de OR 'progesterone'/de OR 'gestagen'/exp #10 contraceptive:ti,ab,kw OR contraceptives:ti,ab,kw OR contraception:ti,ab,kw OR antifertility:ti,ab,kw OR 'anti fertility':ti,ab,kw OR anticonception:ti,ab,kw OR 'anti conception':ti,ab,kw OR 'birth control':ti,ab,kw (99,475) #11 chc:ti OR chcs:ti OR coc:ti OR cocs:ti OR cocp:ti OR cocps:ti OR ocp:ti OR ocps:ti OR pops:ti OR (((monophasic OR 'mono phasic' OR biphasic OR 'bi phasic' OR triphasic OR 'tri phasic' OR quadriphasic OR 'quadri phasic' OR multiphasic OR 'multi phasic' OR normophasic OR 'normo phasic' OR minidose OR 'mini dose' OR 'morning after' OR 'progestin only') NEAR/1 (pill OR pills)):ti) OR ((('first generation' OR '1st generation' OR 'second generation' OR '2nd generation' OR 'third generation' OR '3rd generation' OR 'fourth generation' OR '4th generation') NEAR/2 (pill OR pills OR progest*)):ti) #12 (((progest* OR levonorgestrel OR lng) NEAR/2 (ball OR balls OR coil OR coils OR device OR devices OR iud OR iuds OR iucd OR iucds OR ius OR iuss OR system OR systems)):ti,ab,kw) OR ((medicated NEAR/2 (iud OR iuds OR iucd OR iucds OR ius OR iuss)):ti,ab,kw) OR lngiucd:ti,ab,kw OR lngiucds:ti,ab,kw OR lngiud:ti,ab,kw OR lngiuds:ti,ab,kw OR lngius:ti,ab,kw OR lngiuss:ti,ab,kw #13 'cyproterone acetate':ti,ab,kw OR desogestrel:ti,ab,kw OR drospirenone:ti,ab,kw OR dienogest:ti,ab,kw OR 'estradiol valerate':ti,ab,kw OR 'oestradiol valerate':ti,ab,kw OR estradiol:ti,ab,kw OR oestradiol:ti,ab,kw OR estrogen:ti,ab,kw OR oestrogen:ti,ab,kw OR ee:ti,ab,kw OR ethinylestradiol:ti,ab,kw OR ethinyloestradiol:ti,ab,kw OR 'ethinyl estradiol':ti,ab,kw OR 'ethinyl oestradiol':ti,ab,kw OR 'ethynodiol diacetate':ti,ab,kw OR etn:ti,ab,kw OR etonogestrel:ti,ab,kw OR gestodene:ti,ab,kw OR lng:ti,ab,kw OR levonorgestrel:ti,ab,kw OR lynestrenol:ti,ab,kw OR dmpa:ti,ab,kw OR 'medroxyprogesterone acetate':ti,ab,kw OR 'nomegestrol acetate':ti,ab,kw OR norelgestromin:ti,ab,kw OR norethindrone:ti,ab,kw OR norgestimate:ti,ab,kw OR norgestrel:ti,ab,kw OR progesterone:ti,ab,kw OR progestin*:ti,ab,kw OR progestogen:ti,ab,kw OR 'segesterone acetate':ti,ab,kw #14 #8 OR #9 OR #10 OR #11 OR #12 OR #13 #15 #7 AND #14
CINAHL Plus with Full Text (EBSCOHost) inception to 31 October 2023 Date searched: 1 November 2023 S1 (MH "COVID‐19+") OR (MH "SARS‐CoV‐2") OR (MW "COVID‐19") S2 TI ( (coronavirus* or corona‐virus* or OC43 or NL63 or 229E or HKU1 or HCoV* or ncov* or covid* or sars‐cov* or sarscov* or Sars‐coronavirus* or "Severe Acute Respiratory Syndrome") ) OR AB ( (coronavirus* or corona‐virus* or OC43 or NL63 or 229E or HKU1 or HCoV* or ncov* or covid* or sars‐cov* or sarscov* or Sars‐coronavirus* or "Severe Acute Respiratory Syndrome") ) S3 S1 OR S2 S4 TI ( S3 NOT not (SARS or MERS or MERS‐CoV or "Middle East respiratory syndrome" or camel* or dromedar* or equine or coronary or coronal or covidence* or covidien or "influenza virus" or HIV or bovine or calves or TGEV or feline or porcine or BCoV or PED or PEDV or PDCoV or FIPV or FCoV or SARS‐CoV or canine or CCov or zoonotic or "avian influenza" or H1N1 or H5N1 or H5N6 or IBV or murine) ) OR AB ( S3 NOT not (SARS or MERS or MERS‐CoV or "Middle East respiratory syndrome" or camel* or dromedar* or equine or coronary or coronal or covidence* or covidien or "influenza virus" or HIV or bovine or calves or TGEV or feline or porcine or BCoV or PED or PEDV or PDCoV or FIPV or FCoV or SARS‐CoV or canine or CCov or zoonotic or "avian influenza" or H1N1 or H5N1 or H5N6 or IBV or murine) ) S5 ((pneumonia or covid* or coronavirus* or corona virus* or ncov* or 2019‐ncov or sars*) or (MH "Pneumonia")) AND wuhan) S6 TI ( (2019‐ncov or ncov19 or ncov‐19 or 2019‐novel CoV or sars‐cov2 or sars‐cov‐2 or sarscov2 or sarscov‐2 or Sars‐coronavirus2 or Sars‐coronavirus‐2 or SARS‐like coronavirus* or coronavirus‐19 or covid19 or covid‐19 or covid 2019 or ((novel or new or nouveau) N2 (CoV or nCoV or covid or coronavirus* or corona virus or pandemic*)) or ((covid or covid19 or covid‐19) and pandemic*) or (coronavirus* and pneumonia)) ) OR AB ( (2019‐ncov or ncov19 or ncov‐19 or 2019‐novel CoV or sars‐cov2 or sars‐cov‐2 or sarscov2 or sarscov‐2 or Sars‐coronavirus2 or Sars‐coronavirus‐2 or SARS‐like coronavirus* or coronavirus‐19 or covid19 or covid‐19 or covid 2019 or ((novel or new or nouveau) N2 (CoV or nCoV or covid or coronavirus* or corona virus or pandemic*)) or ((covid or covid19 or covid‐19) and pandemic*) or (coronavirus* and pneumonia)) ) S7 S4 OR S5 OR S6 S8 (MH "Contraceptives, Oral Combined") OR (MH "Hormonal Contraception") OR (MH "Contraceptive Agents, Hormonal") OR (MH "Long‐Acting Reversible Contraceptives") OR (MH "Intrauterine Devices") OR (MH "Contraceptives, Oral") OR (MH "Contraceptive Agents") S9 (MH "Estrogens") OR (MH "Levonorgestrel") OR (MH "Medroxyprogesterone Acetate") OR (MH "Progestational Hormones") OR (MH "Progesterone") S10 TI ( (contraceptive or contraceptives or contraception or antifertility or anti‐fertility or anticonception or anti‐conception or birth‐control) ) OR AB ( (contraceptive or contraceptives or contraception or antifertility or anti‐fertility or anticonception or anti‐conception or birth‐control) ) S11 TI (CHC or CHCs or COC or COCs or COCP or COCPs or OCP or OCPs or POPs or ((monophasic or mono‐phasic or biphasic or bi‐phasic or triphasic or tri‐phasic or quadriphasic or quadri‐phasic or multiphasic or multi‐phasic or normophasic or normo‐phasic or minidose or mini‐dose or morning‐after or progestin‐only) N1 (pill or pills)) or ((first‐generation or 1st‐generation or second‐generation or 2nd‐generation or third‐generation or 3rd‐generation or fourth‐generation or 4th‐generation) N2 (pill or pills or progest*))) S12 TI ( (((progest* or levonorgestrel or LNG) N2 (ball or balls or coil or coils or device or devices or IUD or IUDs or IUCD or IUCDs or IUS or IUSs or system or systems)) or (medicated N2 (IUD or IUDs or IUCD or IUCDs or IUS or IUSs)) or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs) ) OR AB ( (((progest* or levonorgestrel or LNG) N2 (ball or balls or coil or coils or device or devices or IUD or IUDs or IUCD or IUCDs or IUS or IUSs or system or systems)) or (medicated N2 (IUD or IUDs or IUCD or IUCDs or IUS or IUSs)) or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs) ) S13 TI ( ("cyproterone acetate" or desogestrel or drospirenone or dienogest or "estradiol valerate" or "oestradiol valerate" or estradiol or oestradiol or estrogen or oestrogen or EE or ethinylestradiol or ethinyloestradiol or ethinyl‐estradiol or ethinyl‐oestradiol or "ethynodiol diacetate" or ETN or etonogestrel or gestodene or LNG or levonorgestrel or lynestrenol or DMPA or "medroxyprogesterone acetate" or "nomegestrol acetate" or norelgestromin or norethindrone or norgestimate or norgestrel or progesterone or progestin* or progestogen or "segesterone acetate") ) OR AB ( ("cyproterone acetate" or desogestrel or drospirenone or dienogest or "estradiol valerate" or "oestradiol valerate" or estradiol or oestradiol or estrogen or oestrogen or EE or ethinylestradiol or ethinyloestradiol or ethinyl‐estradiol or ethinyl‐oestradiol or "ethynodiol diacetate" or ETN or etonogestrel or gestodene or LNG or levonorgestrel or lynestrenol or DMPA or "medroxyprogesterone acetate" or "nomegestrol acetate" or norelgestromin or norethindrone or norgestimate or norgestrel or progesterone or progestin* or progestogen or "segesterone acetate") ) S14 S8 OR S9 OR S10 OR S11 OR S12 OR S13 S15 S7 AND S14
Global Index Medicus (World Health Organization) Inception to 31 October 2023 Date searched: 1 November 2023 tw:((tw:(covid OR covid19 OR covid‐19 OR 2019‐ncov OR ncov19 OR ncov‐19 OR cov OR ncov OR covid OR 2019‐novel cov OR sars‐cov2 OR sars‐cov‐2 OR sarscov2 OR sarscov‐2 OR sars‐coronavirus2 OR sars‐coronavirus‐2 OR sars‐like coronavirus* OR coronavirus‐19 OR covid19 OR covid‐19 OR covid 2019 OR coronavirus*)) AND (ab:(contraceptive* OR contraception OR antifertility OR anti‐fertility OR anticonception OR anti‐conception OR birth‐control OR chc OR chcs OR coc OR cocs OR cocp OR cocps OR ocp OR ocps OR pops OR "cyproterone acetate" OR desogestrel OR drospirenone OR dienogest OR "estradiol valerate" OR "oestradiol valerate" OR estradiol OR oestradiol OR estrogen OR oestrogen OR ethinylestradiol OR ethinyloestradiol OR ethinyl‐estradiol OR ethinyl‐oestradiol OR "ethynodiol diacetate" OR etn OR etonogestrel OR gestodene OR levonorgestrel OR lynestrenol OR dmpa OR "medroxyprogesterone acetate" OR "nomegestrol acetate" OR norelgestromin OR norethindrone OR norgestimate OR norgestrel OR progesterone OR progestin* OR progestogen OR "segesterone acetate" OR progestin‐only OR progest* OR levonorgestrel OR lng OR iud OR iuds OR iucd OR iucds OR ius OR iuss OR lngiucd OR lngiucds OR lngiud OR lngiuds OR lngius OR lngiuss)))
Global Health (Ovid) 1973 to 2023 Week 43 Date searched: 1 November 2023 1 (coronavirus* or corona‐virus* or OC43 or NL63 or 229E or HKU1 or HCoV* or ncov* or covid* or sars‐cov* or sarscov* or Sars‐coronavirus* or "Severe Acute Respiratory Syndrome").ti,ab. or COVID*.hw. 2 1 not (SARS or MERS or MERS‐CoV or "Middle East respiratory syndrome" or camel* or dromedar* or equine or coronary or coronal or covidence* or covidien or "influenza virus" or HIV or bovine or calves or TGEV or feline or porcine or BCoV or PED or PEDV or PDCoV or FIPV or FCoV or SARS‐CoV or canine or CCov or zoonotic or "avian influenza" or H1N1 or H5N1 or H5N6 or IBV or murine).ti,ab. 3 ((pneumonia or covid* or coronavirus* or corona virus* or ncov* or 2019‐ncov or sars*) and Wuhan).mp. 4 (2019‐ncov or ncov19 or ncov‐19 or 2019‐novel CoV or sars‐cov2 or sars‐cov‐2 or sarscov2 or sarscov‐2 or Sars‐coronavirus2 or Sars‐coronavirus‐2 or SARS‐like coronavirus* or coronavirus‐19 or covid19 or covid‐19 or covid 2019 or ((novel or new or nouveau) adj2 (CoV or nCoV or covid or coronavirus* or corona virus or Pandemi*2)) or ((covid or covid19 or covid‐19) and pandemic*2) or (coronavirus* and pneumonia)).ti,ab. 5 or/2‐4 6 (contraceptive or contraceptives or contraception or antifertility or anti‐fertility or anticonception or anti‐conception or birth‐control).ti,ab. 7 (CHC or CHCs or COC or COCs or COCP or COCPs or OCP or OCPs or POPs or ((monophasic or mono‐phasic or biphasic or bi‐phasic or triphasic or tri‐phasic or quadriphasic or quadri‐phasic or multiphasic or multi‐phasic or normophasic or normo‐phasic or minidose or mini‐dose or morning‐after or progestin‐only) adj (pill or pills)) or ((first‐generation or 1st‐generation or second‐generation or 2nd‐generation or third‐generation or 3rd‐generation or fourth‐generation or 4th‐generation) adj2 (pill or pills or progest*))).ti. 8 (((progest* or levonorgestrel or LNG) adj2 (ball or balls or coil or coils or device or devices or IUD or IUDs or IUCD or IUCDs or IUS or IUSs or system or systems)) or (medicated adj2 (IUD or IUDs or IUCD or IUCDs or IUS or IUSs)) or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs).ti,ab. 9 ("cyproterone acetate" or desogestrel or drospirenone or dienogest or "estradiol valerate" or "oestradiol valerate" or estradiol or oestradiol or estrogen or oestrogen or EE or ethinylestradiol or ethinyloestradiol or ethinyl‐estradiol or ethinyl‐oestradiol or "ethynodiol diacetate" or ETN or etonogestrel or gestodene or LNG or levonorgestrel or lynestrenol or DMPA or "medroxyprogesterone acetate" or "nomegestrol acetate" or norelgestromin or norethindrone or norgestimate or norgestrel or progesterone or progestin* or progestogen or "segesterone acetate").ti,ab. 10 or/6‐9 11 and/5,10
Scopus Inception to 31 October 2023 Date searched: 1 November 2023 ( ( ( TITLE‐ABS‐KEY ( ( coronavirus* OR corona‐virus* OR oc43 OR nl63 OR 229e OR hku1 OR hcov* OR ncov* OR covid* OR sars‐cov* OR sarscov* OR sars‐coronavirus* OR "Severe Acute Respiratory Syndrome" ) ) AND NOT TITLE‐ABS‐KEY ( ( sars OR mers OR mers‐cov OR "Middle East respiratory syndrome" OR camel* OR dromedar* OR equine OR coronary OR coronal OR covidence* OR covidien OR "influenza virus" OR hiv OR bovine OR calves OR tgev OR feline OR porcine OR bcov OR ped OR pedv OR pdcov OR fipv OR fcov OR sars‐cov OR canine OR ccov OR zoonotic OR "avian influenza" OR h1n1 OR h5n1 OR h5n6 OR ibv OR murine ) ) ) ) OR ( TITLE‐ABS‐KEY ( ( ( pneumonia OR covid* OR coronavirus* OR corona AND virus* OR ncov* OR 2019‐ncov OR sars* ) AND wuhan ) ) ) OR ( TITLE‐ABS‐KEY ( 2019‐ncov OR ncov19 OR ncov‐19 OR 2019‐novel AND cov OR sars‐cov2 OR sars‐cov‐2 OR sarscov2 OR sarscov‐2 OR sars‐coronavirus2 OR sars‐coronavirus‐2 OR sars‐like AND coronavirus* OR coronavirus‐19 OR covid19 OR covid‐19 OR covid 2019 ) ) OR ( TITLE‐ABS‐KEY ( ( ( novel OR new OR nouveau ) W/2 ( cov OR ncov OR covid OR coronavirus OR pandemic? ) ) ) ) OR ( TITLE‐ABS‐KEY ( ( ( covid OR covid19 OR covid‐19 ) AND pandemic? ) OR ( coronavirus* AND pneumonia ) ) ) ) AND ( ( TITLE‐ABS‐KEY ( contraceptive OR contraceptives OR contraception OR antifertility OR anti‐fertility OR anticonception OR anti‐conception OR birth‐control ) ) OR ( TITLE‐ABS‐KEY ( chc OR chcs OR coc OR cocs OR cocp OR cocps OR ocp OR ocps OR pops OR ( ( monophasic OR mono‐phasic OR biphasic OR bi‐phasic OR triphasic OR tri‐phasic OR quadriphasic OR quadri‐phasic OR multiphasic OR multi‐phasic OR normophasic OR normo‐phasic OR minidose OR mini‐dose OR morning‐after OR progestin‐only ) W/1 ( pill OR pills ) ) OR ( ( first‐generation OR 1st‐generation OR second‐generation OR 2nd‐generation OR third‐generation OR 3rd‐generation OR fourth‐generation OR 4th‐generation ) W/2 ( pill OR pills OR progest* ) ) ) ) OR ( TITLE‐ABS‐KEY ( ( ( progest* OR levonorgestrel OR lng ) W/2 ( ball OR balls OR coil OR coils OR device OR devices OR iud OR iuds OR iucd OR iucds OR ius OR iuss OR system OR systems ) ) OR ( medicated W/2 ( iud OR iuds OR iucd OR iucds OR ius OR iuss ) ) OR lngiucd OR lngiucds OR lngiud OR lngiuds OR lngius OR lngiuss ) ) OR ( TITLE‐ABS‐KEY ( {cyproterone acetate} OR desogestrel OR drospirenone OR dienogest OR {estradiol valerate} OR {oestradiol valerate} OR estradiol OR oestradiol OR estrogen OR oestrogen OR ee OR ethinylestradiol OR ethinyloestradiol OR ethinyl‐estradiol OR ethinyl‐oestradiol OR {ethynodiol diacetate} OR etn OR etonogestrel OR gestodene OR lng OR levonorgestrel OR lynestrenol OR dmpa OR {medroxyprogesterone acetate} OR {nomegestrol acetate} OR norelgestromin OR norethindrone OR norgestimate OR norgestrel OR progesterone OR progestin* OR progestogen OR {segesterone acetate} ) ) ) AND ( LIMIT‐TO ( DOCTYPE, "cp" ) )
Appendix 2. Initial Search Strategies
Cochrane Central Register of Controlled Trials (CENTRAL) (Ovid EBM Reviews) 1 exp Coronavirus/ (12) 2 exp Coronavirus Infections/ (625) 3 (coronavirus* or corona‐virus* or OC43 or NL63 or 229E or HKU1 or HCoV* or ncov* or covid* or sars‐cov* or sarscov* or Sars‐coronavirus* or "Severe Acute Respiratory Syndrome").ti,ab,kw. (6506) 4 or/1‐3 (6532) 5 4 not (SARS or MERS or MERS‐CoV or "Middle East respiratory syndrome" or camel* or dromedar* or equine or coronary or coronal or covidence* or covidien or "influenza virus" or HIV or bovine or calves or TGEV or feline or porcine or BCoV or PED or PEDV or PDCoV or FIPV or FCoV or SARS‐CoV or canine or CCov or zoonotic or "avian influenza" or H1N1 or H5N1 or H5N6 or IBV or murine).ti,ab,kw. (4097) 6 ((pneumonia or covid* or coronavirus* or corona virus* or ncov* or 2019‐ncov or sars*).mp. or exp pneumonia/) and Wuhan.mp. (181) 7 (2019‐ncov or ncov19 or ncov‐19 or 2019‐novel CoV or sars‐cov2 or sars‐cov‐2 or sarscov2 or sarscov‐2 or Sars‐coronavirus2 or Sars‐coronavirus‐2 or SARS‐like coronavirus* or coronavirus‐19 or covid19 or covid‐19 or covid 2019 or ((novel or new or nouveau) adj2 (CoV or nCoV or covid or coronavirus* or corona virus or Pandemi*2)) or ((covid or covid19 or covid‐19) and pandemic*2) or (coronavirus* and pneumonia)).ti,ab,kw. (5103) 8 or/5‐7 (6185) 9 Contraceptive Agents/ or Contraceptive Agents, Female/ or Contraceptives, Oral/ or exp Contraceptives, Oral, Hormonal/ or Contraceptives, Oral, Combined/ or Contraceptives, Oral, Sequential/ or Contraceptives, Oral, Synthetic/ or Hormonal Contraception/ or Contraceptive Agents, Hormonal/ or Intrauterine Devices, Medicated/ or "Long‐Acting Reversible Contraception"/ (2556) 10 Cyproterone Acetate/ or Desogestrel/ or Estrogens/ or exp Ethinyl Estradiol/ or Ethinyl Estradiol‐Norgestrel Combination/ or Ethynodiol Diacetate/ or Levonorgestrel/ or Lynestrenol/ or Medroxyprogesterone/ or Norethindrone/ or Norethindrone Acetate/ or Norgestrel/ or Progesterone/ or Progestins/ (6345) 11 (contraceptive or contraceptives or contraception or antifertility or anti‐fertility or anticonception or anti‐conception or birth‐control).ti,ab,kw. (14569) 12 (CHC or CHCs or COC or COCs or COCP or COCPs or OCP or OCPs or POPs or ((monophasic or mono‐phasic or biphasic or bi‐phasic or triphasic or tri‐phasic or quadriphasic or quadri‐phasic or multiphasic or multi‐phasic or normophasic or normo‐phasic or minidose or mini‐dose or morning‐after or progestin‐only) adj (pill or pills)) or ((first‐generation or 1st‐generation or second‐generation or 2nd‐generation or third‐generation or 3rd‐generation or fourth‐generation or 4th‐generation) adj2 (pill or pills or progest*))).ti. (246) 13 (((progest* or levonorgestrel or LNG) adj2 (ball or balls or coil or coils or device or devices or IUD or IUDs or IUCD or IUCDs or IUS or IUSs or system or systems)) or (medicated adj2 (IUD or IUDs or IUCD or IUCDs or IUS or IUSs)) or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs).ti,ab,kw. (758) 14 ("cyproterone acetate" or desogestrel or drospirenone or dienogest or "estradiol valerate" or "oestradiol valerate" or estradiol or oestradiol or estrogen or oestrogen or EE or ethinylestradiol or ethinyloestradiol or ethinyl‐estradiol or ethinyl‐oestradiol or "ethynodiol diacetate" or ETN or etonogestrel or gestodene or LNG or levonorgestrel or lynestrenol or DMPA or "medroxyprogesterone acetate" or "nomegestrol acetate" or norelgestromin or norethindrone or norgestimate or norgestrel or progesterone or progestin* or progestogen or "segesterone acetate").ti,ab,kw. (25859) 15 or/9‐14 (37566) 16 and/8,15 (196)
MEDLINE ALL (Ovid) 1 exp Coronavirus/ (71150) 2 exp Coronavirus Infections/ (86638) 3 (coronavirus* or corona‐virus* or OC43 or NL63 or 229E or HKU1 or HCoV* or ncov* or covid* or sars‐cov* or sarscov* or Sars‐coronavirus* or "Severe Acute Respiratory Syndrome").ti,ab,kw,kf. (145234) 4 or/1‐3 (153975) 5 4 not (SARS or MERS or MERS‐CoV or "Middle East respiratory syndrome" or camel* or dromedar* or equine or coronary or coronal or covidence* or covidien or "influenza virus" or HIV or bovine or calves or TGEV or feline or porcine or BCoV or PED or PEDV or PDCoV or FIPV or FCoV or SARS‐CoV or canine or CCov or zoonotic or "avian influenza" or H1N1 or H5N1 or H5N6 or IBV or murine).ti,ab,kw,kf. (92442) 6 ((pneumonia or covid* or coronavirus* or corona virus* or ncov* or 2019‐ncov or sars*).mp. or exp pneumonia/) and Wuhan.mp. (5099) 7 (2019‐ncov or ncov19 or ncov‐19 or 2019‐novel CoV or sars‐cov2 or sars‐cov‐2 or sarscov2 or sarscov‐2 or Sars‐coronavirus2 or Sars‐coronavirus‐2 or SARS‐like coronavirus* or coronavirus‐19 or covid19 or covid‐19 or covid 2019 or ((novel or new or nouveau) adj2 (CoV or nCoV or covid or coronavirus* or corona virus or Pandemi*2)) or ((covid or covid19 or covid‐19) and pandemic*2) or (coronavirus* and pneumonia)).ti,ab,kw,kf. (128397) 8 COVID‐19.rx,px,ox. or severe acute respiratory syndrome coronavirus 2.os. (3879) 9 or/5‐8 (140957) 10 Contraceptive Agents/ or Contraceptive Agents, Female/ or Contraceptives, Oral/ or exp Contraceptives, Oral, Hormonal/ or Contraceptives, Oral, Combined/ or Contraceptives, Oral, Sequential/ or Contraceptives, Oral, Synthetic/ or Hormonal Contraception/ or Contraceptive Agents, Hormonal/ or Intrauterine Devices, Medicated/ or "Long‐Acting Reversible Contraception"/ (52137) 11 Cyproterone Acetate/ or Desogestrel/ or Estrogens/ or exp Ethinyl Estradiol/ or Ethinyl Estradiol‐Norgestrel Combination/ or Ethynodiol Diacetate/ or Levonorgestrel/ or Lynestrenol/ or Medroxyprogesterone/ or Norethindrone/ or Norethindrone Acetate/ or Norgestrel/ or Progesterone/ or Progestins/ (132069) 12 (contraceptive or contraceptives or contraception or antifertility or anti‐fertility or anticonception or anti‐conception or birth‐control).ti,ab,kw,oa,kf. (78952) 13 (CHC or CHCs or COC or COCs or COCP or COCPs or OCP or OCPs or POPs or ((monophasic or mono‐phasic or biphasic or bi‐phasic or triphasic or tri‐phasic or quadriphasic or quadri‐phasic or multiphasic or multi‐phasic or normophasic or normo‐phasic or minidose or mini‐dose or morning‐after or progestin‐only) adj (pill or pills)) or ((first‐generation or 1st‐generation or second‐generation or 2nd‐generation or third‐generation or 3rd‐generation or fourth‐generation or 4th‐generation) adj2 (pill or pills or progest*))).ti. (1256) 14 (((progest* or levonorgestrel or LNG) adj2 (ball or balls or coil or coils or device or devices or IUD or IUDs or IUCD or IUCDs or IUS or IUSs or system or systems)) or (medicated adj2 (IUD or IUDs or IUCD or IUCDs or IUS or IUSs)) or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs).ti,ab,kw,oa,kf,nm. (2509) 15 ("cyproterone acetate" or desogestrel or drospirenone or dienogest or "estradiol valerate" or "oestradiol valerate" or estradiol or oestradiol or estrogen or oestrogen or EE or ethinylestradiol or ethinyloestradiol or ethinyl‐estradiol or ethinyl‐oestradiol or "ethynodiol diacetate" or ETN or etonogestrel or gestodene or LNG or levonorgestrel or lynestrenol or DMPA or "medroxyprogesterone acetate" or "nomegestrol acetate" or norelgestromin or norethindrone or norgestimate or norgestrel or progesterone or progestin* or progestogen or "segesterone acetate").ti,ab,kw,kf,nm,rn. (326323) 16 or/10‐15 (405978) 17 and/9,16 (337)
Embase.com #1 'coronavirinae'/exp OR 'coronaviridae infection'/exp (143,882) #2 coronavirus*:ti,ab,kw OR 'corona virus*':ti,ab,kw OR oc43:ti,ab,kw OR nl63:ti,ab,kw OR 229e:ti,ab,kw OR hku1:ti,ab,kw OR hcov*:ti,ab,kw OR ncov*:ti,ab,kw OR covid*:ti,ab,kw OR 'sars cov*':ti,ab,kw OR sarscov*:ti,ab,kw OR 'sars coronavirus*':ti,ab,kw OR 'severe acute respiratory syndrome':ti,ab,kw (141,040) #3 #1 OR #2 (161,360) #4 #3 NOT (sars:ti,ab,kw OR mers:ti,ab,kw OR 'mers cov':ti,ab,kw OR 'middle east respiratory syndrome':ti,ab,kw OR camel*:ti,ab,kw OR dromedar*:ti,ab,kw OR equine:ti,ab,kw OR coronary:ti,ab,kw OR coronal:ti,ab,kw OR covidence*:ti,ab,kw OR covidien:ti,ab,kw OR 'influenza virus':ti,ab,kw OR hiv:ti,ab,kw OR bovine:ti,ab,kw OR calves:ti,ab,kw OR tgev:ti,ab,kw OR feline:ti,ab,kw OR porcine:ti,ab,kw OR bcov:ti,ab,kw OR ped:ti,ab,kw OR pedv:ti,ab,kw OR pdcov:ti,ab,kw OR fipv:ti,ab,kw OR fcov:ti,ab,kw OR 'sars cov':ti,ab,kw OR canine:ti,ab,kw OR ccov:ti,ab,kw OR zoonotic:ti,ab,kw OR 'avian influenza':ti,ab,kw OR h1n1:ti,ab,kw OR h5n1:ti,ab,kw OR h5n6:ti,ab,kw OR ibv:ti,ab,kw OR murine:ti,ab,kw) (98,821) #5 (pneumonia OR covid* OR coronavirus* OR 'corona virus*' OR ncov* OR '2019 ncov' OR sars* OR 'pneumonia'/exp) AND wuhan (8,322) #6 '2019 ncov':ti,ab,kw OR ncov19:ti,ab,kw OR 'ncov 19':ti,ab,kw OR '2019‐novel cov':ti,ab,kw OR 'sars cov2':ti,ab,kw OR 'sars cov 2':ti,ab,kw OR sarscov2:ti,ab,kw OR 'sarscov 2':ti,ab,kw OR 'sars coronavirus2':ti,ab,kw OR 'sars coronavirus 2':ti,ab,kw OR 'sars‐like coronavirus*':ti,ab,kw OR 'coronavirus 19':ti,ab,kw OR covid19:ti,ab,kw OR 'covid 19':ti,ab,kw OR 'covid 2019':ti,ab,kw OR (((novel OR new OR nouveau) NEAR/2 (cov OR ncov OR covid OR coronavirus* OR 'corona virus' OR pandemi*2)):ti,ab,kw) OR ((covid:ti,ab,kw OR covid19:ti,ab,kw OR 'covid 19':ti,ab,kw) AND pandemic*2:ti,ab,kw) OR (coronavirus*:ti,ab,kw AND pneumonia:ti,ab,kw) (120,653 #7 #4 OR #5 OR #6 (144,625) #8 'contraceptive agent'/de OR 'hormonal contraceptive agent'/exp OR 'injectable contraceptive agent'/exp OR 'oral contraceptive agent'/exp OR 'hormonal contraception'/de OR 'long‐acting reversible contraception'/de OR 'oral contraception'/de OR 'ovulation inhibition'/de (134,285) #9 'cyproterone acetate'/de OR 'cyproterone acetate plus ethinylestradiol'/de OR 'desogestrel'/de OR 'desogestrel plus ethinylestradiol'/de OR 'estrogen'/de OR 'ethinylestradiol'/de OR 'ethinylestradiol plus norgestrel'/de OR 'etynodiol diacetate'/de OR 'levonorgestrel'/de OR 'lynestrenol'/de OR 'medroxyprogesterone acetate'/de OR 'norethisterone'/de OR 'norethisterone acetate'/de OR 'norgestrel'/de OR 'progesterone'/de OR 'gestagen'/exp (276,747) #10 contraceptive:ti,ab,kw OR contraceptives:ti,ab,kw OR contraception:ti,ab,kw OR antifertility:ti,ab,kw OR 'anti fertility':ti,ab,kw OR anticonception:ti,ab,kw OR 'anti conception':ti,ab,kw OR 'birth control':ti,ab,kw (92,788 #11 chc:ti OR chcs:ti OR coc:ti OR cocs:ti OR cocp:ti OR cocps:ti OR ocp:ti OR ocps:ti OR pops:ti OR (((monophasic OR 'mono phasic' OR biphasic OR 'bi phasic' OR triphasic OR 'tri phasic' OR quadriphasic OR 'quadri phasic' OR multiphasic OR 'multi phasic' OR normophasic OR 'normo phasic' OR minidose OR 'mini dose' OR 'morning after' OR 'progestin only') NEAR/1 (pill OR pills)):ti) OR ((('first generation' OR '1st generation' OR 'second generation' OR '2nd generation' OR 'third generation' OR '3rd generation' OR 'fourth generation' OR '4th generation') NEAR/2 (pill OR pills OR progest*)):ti) (2,115) #12 (((progest* OR levonorgestrel OR lng) NEAR/2 (ball OR balls OR coil OR coils OR device OR devices OR iud OR iuds OR iucd OR iucds OR ius OR iuss OR system OR systems)):ti,ab,kw) OR ((medicated NEAR/2 (iud OR iuds OR iucd OR iucds OR ius OR iuss)):ti,ab,kw) OR lngiucd:ti,ab,kw OR lngiucds:ti,ab,kw OR lngiud:ti,ab,kw OR lngiuds:ti,ab,kw OR lngius:ti,ab,kw OR lngiuss:ti,ab,kw (3,439) #13 'cyproterone acetate':ti,ab,kw OR desogestrel:ti,ab,kw OR drospirenone:ti,ab,kw OR dienogest:ti,ab,kw OR 'estradiol valerate':ti,ab,kw OR 'oestradiol valerate':ti,ab,kw OR estradiol:ti,ab,kw OR oestradiol:ti,ab,kw OR estrogen:ti,ab,kw OR oestrogen:ti,ab,kw OR ee:ti,ab,kw OR ethinylestradiol:ti,ab,kw OR ethinyloestradiol:ti,ab,kw OR 'ethinyl estradiol':ti,ab,kw OR 'ethinyl oestradiol':ti,ab,kw OR 'ethynodiol diacetate':ti,ab,kw OR etn:ti,ab,kw OR etonogestrel:ti,ab,kw OR gestodene:ti,ab,kw OR lng:ti,ab,kw OR levonorgestrel:ti,ab,kw OR lynestrenol:ti,ab,kw OR dmpa:ti,ab,kw OR 'medroxyprogesterone acetate':ti,ab,kw OR 'nomegestrol acetate':ti,ab,kw OR norelgestromin:ti,ab,kw OR norethindrone:ti,ab,kw OR norgestimate:ti,ab,kw OR norgestrel:ti,ab,kw OR progesterone:ti,ab,kw OR progestin*:ti,ab,kw OR progestogen:ti,ab,kw OR 'segesterone acetate':ti,ab,kw (349,169) #14 #8 OR #9 OR #10 OR #11 OR #12 OR #13 (559,193) #15 #7 AND #14 (463)
CINAHL (EBSCOHost) S1 (MH "COVID‐19") OR (MH "SARS‐CoV‐2") (15,483) S2 TI ( (coronavirus* or corona‐virus* or OC43 or NL63 or 229E or HKU1 or HCoV* or ncov* or covid* or sars‐cov* or sarscov* or Sars‐coronavirus* or "Severe Acute Respiratory Syndrome") ) OR AB ( (coronavirus* or corona‐virus* or OC43 or NL63 or 229E or HKU1 or HCoV* or ncov* or covid* or sars‐cov* or sarscov* or Sars‐coronavirus* or "Severe Acute Respiratory Syndrome") ) (45,813) S3 S1 OR S2 (48,832) S4 TI ( S3 NOT not (SARS or MERS or MERS‐CoV or "Middle East respiratory syndrome" or camel* or dromedar* or equine or coronary or coronal or covidence* or covidien or "influenza virus" or HIV or bovine or calves or TGEV or feline or porcine or BCoV or PED or PEDV or PDCoV or FIPV or FCoV or SARS‐CoV or canine or CCov or zoonotic or "avian influenza" or H1N1 or H5N1 or H5N6 or IBV or murine) ) OR AB ( S3 NOT not (SARS or MERS or MERS‐CoV or "Middle East respiratory syndrome" or camel* or dromedar* or equine or coronary or coronal or covidence* or covidien or "influenza virus" or HIV or bovine or calves or TGEV or feline or porcine or BCoV or PED or PEDV or PDCoV or FIPV or FCoV or SARS‐CoV or canine or CCov or zoonotic or "avian influenza" or H1N1 or H5N1 or H5N6 or IBV or murine) ) (3,129) S5 ((pneumonia or covid* or coronavirus* or corona virus* or ncov* or 2019‐ncov or sars*) or (MH "Pneumonia")) AND wuhan) (1,130) S6 TI ( (2019‐ncov or ncov19 or ncov‐19 or 2019‐novel CoV or sars‐cov2 or sars‐cov‐2 or sarscov2 or sarscov‐2 or Sars‐coronavirus2 or Sars‐coronavirus‐2 or SARS‐like coronavirus* or coronavirus‐19 or covid19 or covid‐19 or covid 2019 or ((novel or new or nouveau) N2 (CoV or nCoV or covid or coronavirus* or corona virus or pandemic*)) or ((covid or covid19 or covid‐19) and pandemic*) or (coronavirus* and pneumonia)) ) OR AB ( (2019‐ncov or ncov19 or ncov‐19 or 2019‐novel CoV or sars‐cov2 or sars‐cov‐2 or sarscov2 or sarscov‐2 or Sars‐coronavirus2 or Sars‐coronavirus‐2 or SARS‐like coronavirus* or coronavirus‐19 or covid19 or covid‐19 or covid 2019 or ((novel or new or nouveau) N2 (CoV or nCoV or covid or coronavirus* or corona virus or pandemic*)) or ((covid or covid19 or covid‐19) and pandemic*) or (coronavirus* and pneumonia)) ) (42,233) S7 S4 OR S5 OR S6 (43,877) S8 (MH "Contraceptives, Oral Combined") OR (MH "Hormonal Contraception") OR (MH "Contraceptive Agents, Hormonal") OR (MH "Long‐Acting Reversible Contraceptives") OR (MH "Intrauterine Devices") OR (MH "Contraceptives, Oral") OR (MH "Contraceptive Agents") (12,731) S9 (MH "Estrogens") OR (MH "Levonorgestrel") OR (MH "Medroxyprogesterone Acetate") OR (MH "Progestational Hormones") OR (MH "Progesterone") (14,717) S10 TI ( (contraceptive or contraceptives or contraception or antifertility or anti‐fertility or anticonception or anti‐conception or birth‐control) ) OR AB ( (contraceptive or contraceptives or contraception or antifertility or anti‐fertility or anticonception or anti‐conception or birth‐control) ) (22,022) S11 TI (CHC or CHCs or COC or COCs or COCP or COCPs or OCP or OCPs or POPs or ((monophasic or mono‐phasic or biphasic or bi‐phasic or triphasic or tri‐phasic or quadriphasic or quadri‐phasic or multiphasic or multi‐phasic or normophasic or normo‐phasic or minidose or mini‐dose or morning‐after or progestin‐only) N1 (pill or pills)) or ((first‐generation or 1st‐generation or second‐generation or 2nd‐generation or third‐generation or 3rd‐generation or fourth‐generation or 4th‐generation) N2 (pill or pills or progest*))) (351) S12 TI ( (((progest* or levonorgestrel or LNG) N2 (ball or balls or coil or coils or device or devices or IUD or IUDs or IUCD or IUCDs or IUS or IUSs or system or systems)) or (medicated N2 (IUD or IUDs or IUCD or IUCDs or IUS or IUSs)) or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs) ) OR AB ( (((progest* or levonorgestrel or LNG) N2 (ball or balls or coil or coils or device or devices or IUD or IUDs or IUCD or IUCDs or IUS or IUSs or system or systems)) or (medicated N2 (IUD or IUDs or IUCD or IUCDs or IUS or IUSs)) or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs) ) (958) S13 TI ( ("cyproterone acetate" or desogestrel or drospirenone or dienogest or "estradiol valerate" or "oestradiol valerate" or estradiol or oestradiol or estrogen or oestrogen or EE or ethinylestradiol or ethinyloestradiol or ethinyl‐estradiol or ethinyl‐oestradiol or "ethynodiol diacetate" or ETN or etonogestrel or gestodene or LNG or levonorgestrel or lynestrenol or DMPA or "medroxyprogesterone acetate" or "nomegestrol acetate" or norelgestromin or norethindrone or norgestimate or norgestrel or progesterone or progestin* or progestogen or "segesterone acetate") ) OR AB ( ("cyproterone acetate" or desogestrel or drospirenone or dienogest or "estradiol valerate" or "oestradiol valerate" or estradiol or oestradiol or estrogen or oestrogen or EE or ethinylestradiol or ethinyloestradiol or ethinyl‐estradiol or ethinyl‐oestradiol or "ethynodiol diacetate" or ETN or etonogestrel or gestodene or LNG or levonorgestrel or lynestrenol or DMPA or "medroxyprogesterone acetate" or "nomegestrol acetate" or norelgestromin or norethindrone or norgestimate or norgestrel or progesterone or progestin* or progestogen or "segesterone acetate") ) (28,468) S14 S8 OR S9 OR S10 OR S11 OR S12 OR S13 (56,296) S15 S7 AND S14 (95)
LILACS (covid OR covid19 OR covid‐19 OR 2019‐ncov OR ncov19 OR ncov‐19 OR CoV OR nCoV OR covid OR 2019‐novel CoV OR sars‐cov2 OR sars‐cov‐2 OR sarscov2 OR sarscov‐2 OR Sars‐coronavirus2 OR Sars‐coronavirus‐2 OR SARS‐like coronavirus* OR coronavirus‐19 OR covid19 OR covid‐19 OR covid 2019 OR coronavirus*) AND (contraceptive* OR contraception OR antifertility OR anti‐fertility OR anticonception OR anti‐conception OR birth‐control OR CHC OR CHCs OR COC OR COCs OR COCP OR COCPs OR OCP OR OCPs OR POPs OR monophasic OR mono‐phasic OR biphasic OR bi‐phasic OR triphasic OR tri‐phasic OR quadriphasic OR quadri‐phasic OR multiphasic OR multi‐phasic OR normophasic OR normo‐phasic OR minidose OR mini‐dose OR morning‐after OR progestin‐only OR progest* OR levonorgestrel OR LNG OR IUD OR IUDs OR IUCD OR IUCDs OR IUS OR IUSs OR LNGIUCD OR LNGIUCDs OR LNGIUD OR LNGIUDs OR LNGIUS OR LNGIUSs) (114)
Global Health (Ovid) 1 (coronavirus* or corona‐virus* or OC43 or NL63 or 229E or HKU1 or HCoV* or ncov* or covid* or sars‐cov* or sarscov* or Sars‐coronavirus* or "Severe Acute Respiratory Syndrome").ti,ab. (34139) 2 1 not (SARS or MERS or MERS‐CoV or "Middle East respiratory syndrome" or camel* or dromedar* or equine or coronary or coronal or covidence* or covidien or "influenza virus" or HIV or bovine or calves or TGEV or feline or porcine or BCoV or PED or PEDV or PDCoV or FIPV or FCoV or SARS‐CoV or canine or CCov or zoonotic or "avian influenza" or H1N1 or H5N1 or H5N6 or IBV or murine).ti,ab. (17624) 3 ((pneumonia or covid* or coronavirus* or corona virus* or ncov* or 2019‐ncov or sars*) and Wuhan).mp. (2046) 4 (2019‐ncov or ncov19 or ncov‐19 or 2019‐novel CoV or sars‐cov2 or sars‐cov‐2 or sarscov2 or sarscov‐2 or Sars‐coronavirus2 or Sars‐coronavirus‐2 or SARS‐like coronavirus* or coronavirus‐19 or covid19 or covid‐19 or covid 2019 or ((novel or new or nouveau) adj2 (CoV or nCoV or covid or coronavirus* or corona virus or Pandemi*2)) or ((covid or covid19 or covid‐19) and pandemic*2) or (coronavirus* and pneumonia)).ti,ab. (29518) 5 or/2‐4 (31049) 6 (contraceptive or contraceptives or contraception or antifertility or anti‐fertility or anticonception or anti‐conception or birth‐control).ti,ab. (15312 7 (CHC or CHCs or COC or COCs or COCP or COCPs or OCP or OCPs or POPs or ((monophasic or mono‐phasic or biphasic or bi‐phasic or triphasic or tri‐phasic or quadriphasic or quadri‐phasic or multiphasic or multi‐phasic or normophasic or normo‐phasic or minidose or mini‐dose or morning‐after or progestin‐only) adj (pill or pills)) or ((first‐generation or 1st‐generation or second‐generation or 2nd‐generation or third‐generation or 3rd‐generation or fourth‐generation or 4th‐generation) adj2 (pill or pills or progest*))).ti. (316) 8 (((progest* or levonorgestrel or LNG) adj2 (ball or balls or coil or coils or device or devices or IUD or IUDs or IUCD or IUCDs or IUS or IUSs or system or systems)) or (medicated adj2 (IUD or IUDs or IUCD or IUCDs or IUS or IUSs)) or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs).ti,ab. (146) 9 ("cyproterone acetate" or desogestrel or drospirenone or dienogest or "estradiol valerate" or "oestradiol valerate" or estradiol or oestradiol or estrogen or oestrogen or EE or ethinylestradiol or ethinyloestradiol or ethinyl‐estradiol or ethinyl‐oestradiol or "ethynodiol diacetate" or ETN or etonogestrel or gestodene or LNG or levonorgestrel or lynestrenol or DMPA or "medroxyprogesterone acetate" or "nomegestrol acetate" or norelgestromin or norethindrone or norgestimate or norgestrel or progesterone or progestin* or progestogen or "segesterone acetate").ti,ab. (24358) 10 or/6‐9 (38210) 11 and/5,10 (70)
Scopus ( ( ( TITLE‐ABS‐KEY ( ( coronavirus* OR corona‐virus* OR oc43 OR nl63 OR 229e OR hku1 OR hcov* OR ncov* OR covid* OR sars‐cov* OR sarscov* OR sars‐coronavirus* OR "Severe Acute Respiratory Syndrome" ) ) AND NOT TITLE‐ABS‐KEY ( ( sars OR mers OR mers‐cov OR "Middle East respiratory syndrome" OR camel* OR dromedar* OR equine OR coronary OR coronal OR covidence* OR covidien OR "influenza virus" OR hiv OR bovine OR calves OR tgev OR feline OR porcine OR bcov OR ped OR pedv OR pdcov OR fipv OR fcov OR sars‐cov OR canine OR ccov OR zoonotic OR "avian influenza" OR h1n1 OR h5n1 OR h5n6 OR ibv OR murine ) ) ) ) OR ( TITLE‐ABS‐KEY ( ( ( pneumonia OR covid* OR coronavirus* OR corona AND virus* OR ncov* OR 2019‐ncov OR sars* ) AND wuhan ) ) ) OR ( TITLE‐ABS‐KEY ( 2019‐ncov OR ncov19 OR ncov‐19 OR 2019‐novel AND cov OR sars‐cov2 OR sars‐cov‐2 OR sarscov2 OR sarscov‐2 OR sars‐coronavirus2 OR sars‐coronavirus‐2 OR sars‐like AND coronavirus* OR coronavirus‐19 OR covid19 OR covid‐19 OR covid 2019 ) ) OR ( TITLE‐ABS‐KEY ( ( ( novel OR new OR nouveau ) W/2 ( cov OR ncov OR covid OR coronavirus OR pandemic? ) ) ) ) OR ( TITLE‐ABS‐KEY ( ( ( covid OR covid19 OR covid‐19 ) AND pandemic? ) OR ( coronavirus* AND pneumonia ) ) ) ) AND ( ( TITLE‐ABS‐KEY ( contraceptive OR contraceptives OR contraception OR antifertility OR anti‐fertility OR anticonception OR anti‐conception OR birth‐control ) ) OR ( TITLE‐ABS‐KEY ( chc OR chcs OR coc OR cocs OR cocp OR cocps OR ocp OR ocps OR pops OR ( ( monophasic OR mono‐phasic OR biphasic OR bi‐phasic OR triphasic OR tri‐phasic OR quadriphasic OR quadri‐phasic OR multiphasic OR multi‐phasic OR normophasic OR normo‐phasic OR minidose OR mini‐dose OR morning‐after OR progestin‐only ) W/1 ( pill OR pills ) ) OR ( ( first‐generation OR 1st‐generation OR second‐generation OR 2nd‐generation OR third‐generation OR 3rd‐generation OR fourth‐generation OR 4th‐generation ) W/2 ( pill OR pills OR progest* ) ) ) ) OR ( TITLE‐ABS‐KEY ( ( ( progest* OR levonorgestrel OR lng ) W/2 ( ball OR balls OR coil OR coils OR device OR devices OR iud OR iuds OR iucd OR iucds OR ius OR iuss OR system OR systems ) ) OR ( medicated W/2 ( iud OR iuds OR iucd OR iucds OR ius OR iuss ) ) OR lngiucd OR lngiucds OR lngiud OR lngiuds OR lngius OR lngiuss ) ) OR ( TITLE‐ABS‐KEY ( {cyproterone acetate} OR desogestrel OR drospirenone OR dienogest OR {estradiol valerate} OR {oestradiol valerate} OR estradiol OR oestradiol OR estrogen OR oestrogen OR ee OR ethinylestradiol OR ethinyloestradiol OR ethinyl‐estradiol OR ethinyl‐oestradiol OR {ethynodiol diacetate} OR etn OR etonogestrel OR gestodene OR lng OR levonorgestrel OR lynestrenol OR dmpa OR {medroxyprogesterone acetate} OR {nomegestrol acetate} OR norelgestromin OR norethindrone OR norgestimate OR norgestrel OR progesterone OR progestin* OR progestogen OR {segesterone acetate} ) ) ) (457)
Data and analyses
Comparison 1. Combined hormonal contraception (estrogen plus progestin) versus no hormonal contraception.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality | 0 | Other data | No numeric data | |
| 1.2 Hospitalization ‐ indirect | 0 | Other data | No numeric data | |
| 1.3 Venous thromboembolism | 0 | Other data | No numeric data | |
| 1.4 Intubation (patients with cerebral venous thrombosis) | 0 | Other data | No numeric data |
1.1. Analysis.
Comparison 1: Combined hormonal contraception (estrogen plus progestin) versus no hormonal contraception, Outcome 1: Mortality
| Mortality | ||||
| Study | Combined hormonal contraception users (total N) | No hormonal contraception users (total N) | Total reported events | Adjusted odds ratio (95% CI) |
| Seeland 2020 | 2078 | 16814 | 98 | 1.0 (0.41‐2.4) |
1.2. Analysis.
Comparison 1: Combined hormonal contraception (estrogen plus progestin) versus no hormonal contraception, Outcome 2: Hospitalization ‐ indirect
| Hospitalization ‐ indirect | ||||
| Study | Combined hormonal contraceptive users (total n) | Non‐contraceptive users (total n) | Total reported events | Adjusted odds ratio (95% CI) |
| Costeira 2021 | 64,253 | 231,436 | 1,889 | OR 0.79 (0.64‐0.97) |
1.3. Analysis.
Comparison 1: Combined hormonal contraception (estrogen plus progestin) versus no hormonal contraception, Outcome 3: Venous thromboembolism
| Venous thromboembolism | |||
| Study | Event type | Total events | # using combined hormonal contraception |
| Chima 2021 | Pulmonary embolism | 6 | 1 |
| Hameed 2021 | Cerebral venous thromboembolism | 7 | 1 |
1.4. Analysis.
Comparison 1: Combined hormonal contraception (estrogen plus progestin) versus no hormonal contraception, Outcome 4: Intubation (patients with cerebral venous thrombosis)
| Intubation (patients with cerebral venous thrombosis) | ||||
| Study | Combined hormonal contraception user events | Combined hormonal contraception user total | No hormonal contraception user events | No hormonal contraception user total |
| Hameed 2021 | 1 | 1 | 1 | 6 |
Comparison 2. Any hormonal contraception (estrogen plus progestin or progestin‐only) versus no contraception.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Hospitalization | 0 | Other data | No numeric data | |
| 2.2 Intubation | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
2.1. Analysis.
Comparison 2: Any hormonal contraception (estrogen plus progestin or progestin‐only) versus no contraception, Outcome 1: Hospitalization
| Hospitalization | |||||
| Study | Any hormonal contraception events | Any hormonal contraception total | No contraception events | No contraception total | Adjusted odds ratio (95% CI) |
| Mujumdar 2020 | 1 | 44 | 3 | 79 | 0.99 (0.68‐1.44) |
2.2. Analysis.

Comparison 2: Any hormonal contraception (estrogen plus progestin or progestin‐only) versus no contraception, Outcome 2: Intubation
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chima 2021.
| Study characteristics | ||
| Methods | Retrospective case series review from 41 healthcare organizations participating in TriNetX | |
| Participants | Pediatric patients < 18 years old with pulmonary embolism and COVID‐19 positive. Pulmonary embolism diagnosed concurrently or within 30 days of COVID‐19 diagnosis | |
| Interventions | Combined hormonal contraception | |
| Outcomes | Pulmonary embolism (venous thromboembolism) | |
| Notes | ||
Costeira 2021.
| Study characteristics | ||
| Methods | Self‐reported data from users input to COVID Symptom Study Smartphone Application; data obtained between 7 May to 15 June 2020 | |
| Participants | Women in the UK using the Smartphone application, subgroup of n = 295,689 female app users 20 to 45 years old with BMI between 18 to 35 kg/m2 | |
| Interventions | Combined oral contraceptive use | |
| Outcomes | Hospitalization | |
| Notes | Users were not known to be COVID‐19 positive, but were recording COVID‐19 symptoms, so were likely to be concerned that they were positive | |
Hameed 2021.
| Study characteristics | ||
| Methods | Retrospective case series from multicenter multinational study ‐ 10 tertiary care centers in Pakistan, Egypt, Singapore, and US | |
| Participants | Patients aged 18 years or above with recent COVID‐19 infection, confirmed either by reverse transcriptase‐polymerase chain reaction assay of a nasopharyngeal swab or serum antibody testing for COVID‐19, were included. Individuals on anticoagulation or with recent trauma were excluded | |
| Interventions | Oral contraceptive | |
| Outcomes | Cerebral venous thrombosis (venous thromboembolism) | |
| Notes | ||
Mujumdar 2020.
| Study characteristics | ||
| Methods | Retrospective chart review from tertiary medical center electronic health record from 28 March to 27 April 2020 | |
| Participants | Reproductive aged women ages 12 to 49 who tested COVID‐19 positive | |
| Interventions | Hormonal contraception LNG IUD = 9 Injectable progestin = 2 Oral progestin = 3 COC = 24 Patch = 4 Vaginal ring = 4 |
|
| Outcomes | Hospitalization, intubation | |
| Notes | Did not confirm patients were using the contraceptive method at time of COVID‐19 diagnosis | |
Seeland 2020.
| Study characteristics | ||
| Methods | Retrospective cohort analysis. Cohorts were balanced using propensity score matching, using a nearest neighbor greedy matching algorithm with a caliper of 0.25 times the standard deviation | |
| Participants | Pre‐menopausal women aged 15 to 49 in TriNetX who were COVID positive in the last 7 months (n = 18,892) | |
| Interventions | Use of hormones or hormonal contraception, identified via RxNorm codes for 'estradiol,' 'ethinyl estradiol,' 'progestins,' and 'systemic contraception.' Participants must have taken the drug within the past 1 year | |
| Outcomes | Mortality | |
| Notes | The study does not have information for reason of use of hormones (contraception versus therapeutic). Also does not provide information on current use | |
BMI = body mass index, COC = combined oral contraceptive, LNG IUD = levonorgestrel intrauterine device.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alyousefi 2021 | Incorrect study design ‐ case report; wrong outcome ‐ D‐dimer |
| Badrawi 2021 | Incorrect study design ‐ case report, no recent h/o contraceptive use |
| Cagnacci 2022 | Incorrect study design ‐ editorial |
| Ding 2021 | Incorrect intervention and study population |
| Fernández‐Capitán 2021 | Incorrect study design / study population ‐ does not stratify by sex |
| Fiorini 2021 | Incorrect study design ‐ case report |
| Grimes 2021 | Incorrect study design ‐ case report, no use of contraceptives |
| Iguina 2020 | Incorrect study design ‐ case report |
| Koritala 2021 | Incorrect study design ‐ case report |
| Kow 2021 | Incorrect study design ‐ editorial |
| Kundal 2021 | Incorrect study design ‐ case report |
| Kurdoğlu 2021 | Incorrect study design ‐ editorial |
| Lee 2020 | Incorrect intervention ‐ menopausal hormonal therapy |
| Lete 2021 | Incorrect study design ‐ review |
| Limanova 2021 | Incorrect outcome ‐ drug interactions between contraception and therapeutics for COVID‐19 |
| Martin 2022 | Incorrect study design ‐ wrong population |
| Mitra 2021 | Incorrect study design ‐ editorial |
| NCT04865029 | Incorrect study design and intervention indictation ‐ study protocol for using estrogen and progesterone as treatment for COVID‐19 |
| Ostovan 2021 | Incorrect study population ‐ case series but individual with prior not current oral contraceptive pills use |
| Pires 2020 | Incorrect study design ‐ review |
| Ramírez 2020 | Duplicate and incorrect study design ‐ response to review |
| Rebelo 2021 | Incorrect study design ‐ case report (but does have data on time of initiation of oral contraceptive pills) |
| Riera‐Mestre 2021 | Incorrect study design ‐ guideline development using Delphi method for thromboprophylaxis recommendations |
| Sapkota 2022 | Wrong study design ‐ case report |
| Spratt 2020 | Incorrect study design ‐ editorial |
| Traish 2021 | Incorrect study design ‐ review |
| Vahey 2021 | Incorrect study population ‐ did not stratify analysis by sex |
| Valenzuela‐Vallejo 2021 | Incorrect study design ‐ case report |
Differences between protocol and review
As data regarding contraceptive use for patients with COVID‐19 are quite sparse at this time, we made several changes from the original protocol. First, none of the included studies specified whether patients were using hormonal contraception for contraceptive purposes or for treatment purposes, such as for management of heavy menstrual bleeding. Given that women using hormonal management for treatment purposes may have different underlying risk factors for thrombosis, we intended to exclude them from our analyses, but they could not be differentiated from contraceptive users. Second, we included hospitalization as a secondary outcome as it was one of the few outcomes associated with contraceptive used that was assessed currently in the literature. Third, we determined to include case series in this review only if they included five or more cases otherwise meeting participant inclusion criteria to ensure there was adequate meaningful information provided. As the literature surrounding this topic continues to emerge, we anticipate no longer including case series once there are sufficient comparative studies investigating the primary thromboembolic outcomes.
We also more clearly specified the timing of updating syntheses to be published for this living systematic review. We will monitor the literature on a monthly basis, as described in the protocol, and will publish updates to our synthesis every six months. In the event that we identify a study with a more rigorous study design than the included evidence to date (e.g. prospective cohort study with concurrent comparison groups or a randomized controlled trial) in our active monitoring of the literature, we will expedite the synthesis publication to ensure that newer evidence with lower risk of bias is added to the review prior to the planned six‐month time frame.
Contributions of authors
Conceiving and designing the review: Megan A Cohen (MAC), Alison Edelman (AE), Jillian Henderson (JH).
Co‐ordinating the review: MAC.
Designing search strategies: Robin Paynter (RP), MAC, AE.
Searching and selecting studies for inclusion in the review: MAC, AE, JH.
Collecting data for the review: MAC, AE.
Assessing the risk of bias in the included studies: MAC, AE, JH.
Analyzing the data: MAC, AE, JH.
Assessing the certainty in the body of evidence: MAC, AE, JH.
Interpreting the data: MAC, AE, JH.
Writing the review: MAC, AE, JH.
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
Megan A Cohen: has declared that they have no conflict of interest.
Alison Edelman: reports Principal Investigator (PI) of an implant study for Merck, paid to institution; PI of an oral contraceptive study for HRA pharma, paid to institution; reports royalties or licenses from Up to Date, personal payment. These are not COVID‐19 related, but are hormonal contraception related.
Jillian Henderson: has declared that they have no conflict of interest.
Robin Paynter: has declared that they have no conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Chima 2021 {published data only}
- Chima M, Williams D, Thomas NJ, Krawiec C. COVID-19–associated pulmonary embolism in pediatric patients. Hospital Pediatrics 2021;11(6):e90-4. [DOI: 10.1542/hpeds.2021-005866] [DOI] [PMC free article] [PubMed] [Google Scholar]
Costeira 2021 {published data only}
- Costeira R, Lee KA, Murray B, Christiansen C, Castillo-Fernandez J, Ni Lochlainn M, et al. Estrogen and COVID-19 symptoms: associations in women from the COVID symptom study. PLOS One 2021;16(9):e0257051. [DOI: 10.1371/journal.pone.0257051] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hameed 2021 {published data only}
- Hameed S, Wasay M, Soomro BA, Mansour O, Abd-allah F, Tu T, et al. Cerebral venous thrombosis associated with COVID-19 infection: an observational, multicenter study. Cerebrovascular Diseases Extra 2021;11(2):55-60. [DOI: 10.1159/000516641] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mujumdar 2020 {published data only}
- Mujumdar V, Levy AT, Madding R, Berghella V, Quist-Nelson J, Schlaff WD, et al. Effect of hormonal contraception on illness severity in women with positive SARS-CoV2 tests. Fertility and Sterility 2020;114(3):e540–1. [DOI: 10.1016/j.fertnstert.2020.09.060] [DOI] [Google Scholar]
Seeland 2020 {published data only}
- Seeland U, Coluzzi F, Simmaco M, Mura C, Bourne P, Heiland M, et al. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Medicine 2020;18(1):369. [DOI: 10.1186/s12916-020-01851-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alyousefi 2021 {published data only}
- Alyousefi NA. An oral combined contraceptive user with elevated D-dimer post COVID-19: a case report. BMC Women's Health 2021;21:320. [DOI: 10.1186/s12905-021-01456-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Badrawi 2021 {published data only}
- Badrawi N, Abdulghaffar S. Ovarian vein thrombosis as a first manifestation of COVID-19 infection. Radiology Case Report 2021;16(11):3491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cagnacci 2022 {published data only}
- Cagnacci A, Pietro Londero A, Xholli A. COVID-19 and hormonal contraception. Case Reports in Women's Health 2022;34:e00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ding 2021 {published data only}
- Ding T, Zhang J, Wang T, Cui P, Chen Z, Jiang J, et al. Potential influence of menstrual status and sex hormones on female severe acute respiratory syndrome Coronavirus 2 infection: a cross-sectional multicenter study in Wuhan, China. Clinical Infectious Diseases 2021;72(9):e240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernández‐Capitán 2021 {published data only}
- Fernández-Capitán C, Barba R, Díaz-Pedroche MDC, Sigüenza P, Demelo-Rodriguez P, Siniscalchi C, et al. Presenting characteristics, treatment patterns, and outcomes among patients with venous thromboembolism during hospitalization for COVID-19. Seminars in Thrombosis and Hemostasis 2021;47(4):351-61. [DOI] [PubMed] [Google Scholar]
Fiorini 2021 {published data only}
- Fiorini NB, Garagoli F, Bustamante RC, Pizarro R. Acute pulmonary embolism in a patient with mild COVID-19 symptoms: a case report. European Heart Journal 2021;5(1):ytaa563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grimes 2021 {published data only}
- Grimes KM, Somani S, Dutta TM, Chaturvedi S. Cerebral venous sinus thrombosis following mild SARS-CoV-2 infection: infection related hypercoagulability or adverse effect of novel therapy? Annals of Neurology 2021;90(Suppl 27):S54-5. [Google Scholar]
Iguina 2020 {published data only}
- Iguina M, Saleh A, Sayeedi I, Danckers M. Recurrent ischemic strokes in a patient with severe COVID-19 infection and phosphatidylserine antibodies. Chest 2020;158(4):A776. [Google Scholar]
Koritala 2021 {published data only}
- Koritala T, Johnson DW. Internal jugular vein thrombus in coronavirus disease (COVID-19). American Journal of Roentgenology 2021;217(1):257. [DOI] [PubMed] [Google Scholar]
Kow 2021 {published data only}
- Kow CS, Mustafa ZU, Hasan SS. The use of combined hormonal contraceptives amid the COVID-19 pandemic. European Journal of Contraception & Reproductive Health Care 2021;26(1):85-6. [DOI] [PubMed] [Google Scholar]
Kundal 2021 {published data only}
- Kundal SV, Emeasoba EU, Harris C, Randhawa G, Astashkevich M. Aortic thrombosis and renal infarction in a young female with patent foramen ovale and COVID-19 antibody. Clinical Case Reports 2021;9(1):345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurdoğlu 2021 {published data only}
- Kurdoğlu Z. Combined hormonal contraceptive usage in women with Covid-19. International Journal of Women's Health and Reproduction Sciences 2021;9(2):84-5. [Google Scholar]
Lee 2020 {published data only}
- Lee JH, Kim YC, Cho SH, Lee J, You SC, Song YG, et al. Effect of sex hormones on coronavirus disease 2019: an analysis of 5,061 laboratory-confirmed cases in South Korea. Menopause 2020;27(12):1376-81. [DOI: 10.1097/GME.0000000000001657] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lete 2021 {published data only}
- Lete I. Combined hormonal contraception and COVID-19. European Journal of Contraception & Reproductive Health Care 2021;26(2):128-31. [DOI: 10.1080/13625187.2020.1867845] [DOI] [PubMed] [Google Scholar]
Limanova 2021 {published data only}
- Limanova OA, Fedotova LE, Gromova OA. Combined oral contraceptives and treatment of new coronavirus infection (COVID-19): aspects of drug interactions. Infektsionnye Bolezni 2021;19(1):149-58. [Google Scholar]
Martin 2022 {published data only}
- Martin LH, Sainz-Gil M, Navarro-Garcia E, Salado-Valdivieso I, Sanz-Fadrique R. Thromboembolism and Oral Contraceptives During the COVID-19 Pandemic: A Disproportionality Analysis Within the Spanish Pharmacovigilance Database. Drugs - real world outcomes 2022;9:211-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitra 2021 {published data only}
- Mitra A. Blood clots, COVID-19 vaccines and the contraceptive pill: are we heading for a repeat of the 1995 pill scare? BMJ Sexual & Reproductive Health 2021;47:303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04865029 {published data only}
- NCT04865029. Estradiol and progesterone in hospitalized COVID-19 patients [Acute estradiol and progesterone therapy in hospitalized adults to reduce coronavirus disease (COVID-19) severity: a randomized control trial]. clinicaltrials.gov/ct2/show/NCT04865029 (first received 29 April 2021).
Ostovan 2021 {published data only}
- Ostovan VR, Foroughi R, Rostami M, Almasi-Dooghaee M, Esmaili M, Bidaki AA, et al. Cerebral venous sinus thrombosis associated with COVID-19: a case series and literature review. Journal of Neurology 2021;268(10):3549-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pires 2020 {published data only}
- Ribeiro Pires AL, Gomes Batista J, Mendes Aldrighi J, Silva Massaia IFD, Medeiros Delgado D, Ferreira-Filho ES, et al. Risk of venous thromboembolism in users of contraception and menopausal hormone therapy during the COVID-19 pandemic. Revista da Associação Médica Brasileira 2020;66(Suppl 2):22-6. [DOI] [PubMed] [Google Scholar]
Ramírez 2020 {published data only}
- Ramírez I, De la Viuda E, Baquedano L, Jesús Cancelo M, Antonio Páramo J, Cano A. The thromboembolic risk in Covid-19 women under hormonal treatment group. Maturitas 2020;138:78-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rebelo 2021 {published data only}
- Rebelo AT, Rodrigues S, Fontoura-Matias J, Azevedo I, Fernandes S. Massive pulmonary embolism in an adolescent. In: 2021 EIP abstract book, Cogent Medicine. 2021. [DOI: 10.1080/2331205X.2021.2002558] [DOI]
Riera‐Mestre 2021 {published data only}
- Riera-Mestre A, Jara-Palomares L, Lecumberri R, Trujillo-Santos J, Grau E, Blanco-Molina A, on behalf of the COVILAX Project. PICO questions and DELPHI methodology for the management of venous thromboembolism associated with COVID-19. Viruses 2021;13(11):2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sapkota 2022 {published data only}
- Sapkota B, Bou-Karroum S, Dillawn S, Daines B, Zaid-Kaylani S, Bhaskaran, S. COVID-19 infection increases the risk of hypercoagulability in individuals with MTHFR genre mutation. Pediatric Blood and Cancer;69(SUUPL 2):S211. [Google Scholar]
Spratt 2020 {published data only}
- Spratt DI, Buchsbaum RJ. COVID-19 and hypercoagulability: potential impact on management with oral contraceptives, estrogen therapy and pregnancy. Endocrinology 2020;161(12):bqaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Traish 2021 {published data only}
- Traish AM. Sex steroids and COVID-19 mortality in women. Trends in Endocrinology and Metabolism 2021;32(8):533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vahey 2021 {published data only}
- Vahey GM, McDonald E, Marshall K, Martin SW, Chun H, Herlihy R, on behalf of the Colorado Investigation Team. Risk factors for hospitalization among persons with COVID-19 - Colorado. PLOS One 2021;16(9):e0256917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valenzuela‐Vallejo 2021 {published data only}
- Valenzuela-Vallejo L, Corredor-Orlandelli D, Alzate-Ricaurte S, Hernández-Santamaría V, Aguirre-Ruiz JF, Peña-Peña A. Hormonal contraception and massive pulmonary embolism in a COVID-19 ambulatory patient: a case report. Clinics and Practice 2021;11(4):914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abou‐Ismail 2020
- Abou-Ismail MY, Sridhar DC, Nayak L. Estrogen and thrombosis: a bench to bedside review. Thrombosis Research 2020;192:40-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2020
- Ahmed S, Zimba O, Gasparyan AY. Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow’s triad. Clinical Rheumatology 2020;39:2529-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benson 2020
- Benson L, Madden T, Tarleton J, Micks E. Society of Family Planning interim clinical recommendations: contraceptive provision when healthcare access is restricted due to pandemic response; May 2020. Available at societyfp.org/wp-content/uploads/2020/05/SFP-Interim-Recommendations-Contraception-and-COVID-19_05.28.2020.pdf.
Bikdeli 2020
- Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al, Global COVID-19 Thrombosis Collaborative Group, endorsed by the ISTH NATF ESVM and the IUA, supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. Journals of the American College of Cardiology 2020;75(23):2950-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
CNGOF 2020
- Prévention du risque thromboembolique veineux chez les femmes COVID + non hospitalisées utilisant un traitement hormonal (Contraception, Traitement hormonal de ménopause, Tamoxifène); 2020. Available at www.cngof.fr/coronavirus-go-cngof/apercu?path=HEMOSTASE%2B-%2BRISQUE%2BDE%2BMTE%252FHemostase-PreventionMVTECOVIDHormones.pdf&i=36239.
Covidence 2022 [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, 2022. Available at covidence.org.
Curtis 2016
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recommendations and Reports 2016;65(3):1-103. [DOI] [PubMed] [Google Scholar]
de Bastos 2014
- Bastos M, Stegeman BH, Rosendaal FR, Vlieg AV, Helmerhorst FM, Stijnen T, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD010813. [DOI: 10.1002/14651858.CD010813.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Ding 2021
- Ding T, Zhang J, Wang T, Cui P, Chen Z, Jiang J, et al. Potential influence of menstrual status and sex hormones on female severe acute respiratory syndrome Coronavirus 2 infection: a cross-sectional multicenter study in Wuhan, China. Clinical Infectious Diseases 2021;72(9):e240-8. [DOI: 10.1093/cid/ciaa1022] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dragoman 2018
- Dragoman MV, Tepper NK, Fu R, Curtis KM, Chou R, Gaffield ME. A systematic review and meta-analysis of venous thrombosis risk among users of combined oral contraception. International Journal of Gynaecology and Obstetrics 2018;141(3):287-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fruzzetti 2020
- Fruzzetti F, Cagnacci A, Primiero F, Leo VD, Bastianelli C, Bruni V, et al. Contraception during Coronavirus-Covid 19 pandemia. Recommendations of the Board of the Italian Society of Contraception. European Journal of Contraception & Reproductive Health Care 2020;25(3):231-2. [DOI] [PubMed] [Google Scholar]
FSRH 2020
- The Faculty of Sexual & Reproductive Healthcare. FSRH Clinical Effectiveness Unit Statement: Use of combined hormonal contraception during the Covid-19 pandemic; December 2020. Available at www.fsrh.org/documents/fsrh-clinical-effectiveness-unit-statement-use-of-combined/.
GRADEpro GDT 2021 [Computer program]
- GRADEpro GDT. Version accessed 25 February 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2021. Available at gradepro.org.
Higgins 2020
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Jin 2020
- Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health 2020;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mauvais‐Jarvis 2020
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology 2020;161(9):bqaa127. [DOI: 10.1210/endocr/bqaa127] [DOI] [PMC free article] [PubMed] [Google Scholar]
Office for National Statistics 2020
- Office for National Statistics. Coronavirus (COVID-19) Infection Survey pilot: 28 May 2020. Available at www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/28may2020.
Ramírez 2020
- Ramírez I, De la Viuda E, Baquedano L, Coronado P, Llaneza P, Mendoza N, et al. Managing thromboembolic risk with menopausal hormone therapy and hormonal contraception in the COVID-19 pandemic: recommendations from the Spanish Menopause Society, Sociedad Española de Ginecología y Obstetricia and Sociedad Española de Trombosis y Hemostasia. Maturitas 2020;137:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves 2022
- Reeves BC, Deeks JJ, Higgins JP, Shea B, Tugwell P, Wells GA. Chapter 24: Including non-randomized studies on intervention effects. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Robinson 1991
- Robinson GE, Burren T, Mackie IJ, Bounds W, Walshe K, Faint R, et al. Changes in haemostasis after stopping the combined contraceptive pill: implications for major surgery. BMJ 1991;302(6771):269-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Stelzig 2020
- Stelzig KE, Canepa-Escaro F, Schiliro M, Berdnikovs S, Prakash YS, Chiarella SE. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology 2020;318(6):L1280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Sterne 2020
- Sterne JA, Hernán MA, McAleenan A, Reeves BC, Higgins JP. Chapter 25: Assessing risk of bias in a non-randomized study. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Sund 2022
- Sund M, Fonseca-Rodríguez O, Josefsson A, Welen K, Fors Connolly A-M. Association between pharmaceutical modulation of oestrogen in postmenopausal women in Sweden and death due to COVID-19: a cohort study. BMJ Open 2022;12(2):e053032. [DOI: 10.1136/bmjopen-2021-053032] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tepper 2016
- Tepper NK, Whiteman MK, Marchbanks PA, James AH, Curtis KM. Progestin-only contraception and thromboembolism: a systematic review. Contraception 2016;94(6):678-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
UN 2019
- United Nations. Contraceptive Use by Method 2019: Data Booklet. United Nations, 2019. [DOI: 10.18356/1bd58a10-en] [DOI] [Google Scholar]
WHO 2020
- World Health Organization. Coronavirus disease (COVID-19): contraception and family planning; April 2020. Available at www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-contraception-and-family-planning.
Zhang 2020
- Zhang S, Liu YY, Wang XF, Yang L, Li H, Wang Y, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. Journal of Hematology & Oncology 2020;13:161. [DOI: 10.1186/s13045-020-01003-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Cohen 2021
- Cohen MA, Stewart F, Paynter R, Edelman A, Henderson J. Risk of thromboembolism in patients with COVID‐19 who are using hormonal contraception. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD014908. [DOI: 10.1002/14651858.CD014908] [DOI] [PMC free article] [PubMed] [Google Scholar]