Supplemental Digital Content is Available in the Text.
Keywords: Placebo effects, Pain, Itch, Conditioning, Verbal suggestion, Observational learning
Abstract
Placebo effects, positive treatment outcomes that go beyond treatment processes, can alter sensations through learning mechanisms. Understanding how methodological factors contribute to the magnitude of placebo effects will help define the mechanisms by which these effects occur. We conducted a systematic review and meta-analysis of experimental placebo studies in cutaneous pain and itch in healthy samples, focused on how differences in methodology contribute to the resulting placebo effect magnitude. We conducted meta-analyses by learning mechanism and sensation, namely, for classical conditioning with verbal suggestion, verbal suggestion alone, and observational learning, separately for pain and itch. We conducted subgroup analyses and meta-regression on the type of sensory stimuli, placebo treatment, number of acquisition and evocation trials, differences in calibrated intensities for placebo and control stimuli during acquisition, age, and sex. We replicated findings showing that a combination of classical conditioning with verbal suggestion induced larger placebo effects on pain (k = 68, g = 0.59) than verbal suggestion alone (k = 39, g = 0.38) and found a smaller effect for itch with verbal suggestion alone (k = 7, g = 0.14). Using sham electrodes as placebo treatments corresponded with larger placebo effects on pain than when topical gels were used. Other methodological and demographic factors did not significantly affect placebo magnitudes. Placebo effects on pain and itch reliably occur in experimental settings with varied methods, and conditioning with verbal suggestion produced the strongest effects. Although methods may shape the placebo effect to some extent, these effects appear robust overall, and their underlying learning mechanisms may be harnessed for applications outside the laboratory.
1. Introduction
Placebo effects, positive treatment outcomes for sensations such as pain and itch that arise through psychobiological mechanisms independent of an actual treatment,53,56 are routinely observed in clinical trials and practice.85,142 Their prevalence and magnitude likely vary across conditions and contexts, but these effects are thought to occur in many clinical trial participants receiving placebos,46 and magnitudes can vary extensively, from no effect to large effects.141 Placebo effects are routinely studied in healthy participants,15,32 allowing for better-controlled investigation of the underlying mechanisms compared with research in clinical settings. Although these effects are most often studied in pain, itch is a similar but distinct sensation with overlapping neurobiological mechanisms,122 highly susceptible to psychological influence.2,100,126 Placebo effects on itch routinely occur in the treatment of dermatological conditions,139 but their relation to placebo effects on pain is not well understood. A deeper investigation of the factors that shape the magnitude of both placebo effects on pain and itch will further our understanding of when and how these effects occur, and the mechanisms that underlie them.
In mechanistic placebo research, positive treatment expectations are typically induced using classical conditioning, verbal suggestions, observational learning, or a combination of these learning processes.28 Classical conditioning induces placebo effects by forming associations between an (inert) treatment and a decrease in sensation8,13; initially reinforced with a genuine reduction in sensation, the effect of which becomes associated with the inert treatment. For example, if one experiences pain relief every time they take a given medicine, they may come to expect pain relief from this medicine. Those expectations alone may be enough to foment some pain relief, such that if this person ingested a pill that they believed to be their analgesic medicine but was in fact a placebo, they would still experience some degree of pain reduction. Verbal suggestions explicitly provide positive information regarding the pain-relieving or itch-relieving effects of a treatment.140 This could come in the form of a doctor telling you that a new medicine will reduce your itch symptoms, inducing expectations for this outcome, which propagate some degree of itch relief on top of any biological effects of the treatment. Placebo effects can also be formed by observing the effects of a pain-relieving or itch-relieving treatment in another person.10,131 Observational learning could, for example, form expectations for pain relief by seeing a friend's pain symptoms improve after trying a different physical therapy exercise. There seems to be an additive benefit to combining multiple learning processes when inducing these effects,12,28 although this has not been systematically reviewed.
One goal of experimental research into placebo effects on pain and itch has been to identify factors that influence these effects.98,143 Methods used in experimental placebo research are heterogeneous, varying factors such as the type of sensation (eg, thermal pain, electrical pain), the type of placebo intervention (eg, sham electrodes, gels, or pills), the number of acquisition and evocation trials used in a conditioning design,33,124 and the difference in intensity of pain stimulations between placebo and control trials.63 Demographic characteristics of study populations like sex and age may also potentially impact resulting placebo effects. Although some studies investigating sex differences in placebo responses have found that men are more responsive to verbal suggestions for placebo effects on pain,52,138 findings are mixed for classical conditioning and remain unexplored for itch. Age differences across the adult lifespan similarly have not been investigated for placebo effects on pain or itch. Systematic review and meta-analysis allows us to study what influence these methodological and demographic factors may have across studies. Previous meta-analyses of placebo effects on pain and itch have documented their widespread prevalence in clinical trials,139,142 and for pain, they demonstrated that mechanistic research tends to find larger placebo effects than those seen in clinical research. The use of longer pain stimuli was also associated with larger placebo effects.141 Since the most recent meta-analysis of mechanistic research into placebo effects on pain over a decade ago,141 numerous new studies have been published, particularly studies with healthy samples. To date, no meta-analyses have sought to quantify the magnitude of experimentally induced placebo effects on itch, nor have methodological factors been studied systematically as a potential source of heterogeneity in placebo effect magnitudes for pain or itch.
Given the growing body of research into placebo effects in cutaneous sensations, a systematic review and meta-analysis is warranted to provide insights into the distinct contributions of experimental components. Examining placebo effects across the literature may provide a better understanding of how these effects can be enhanced and potentially used in clinical settings, creating research avenues for novel therapies or informing doctor–patient communication. In pursuit of this aim and building on previous meta-analyses of similar scope,110,111,141,142 we conducted a systematic review and meta-analysis on the magnitude of placebo effects for inert treatments, in experiments on pain and itch, in healthy participants. First, we assessed the magnitude of placebo effects (defined as the decrease in pain or itch intensity after an inert treatment compared with a within-subject or between-subject control) by learning process (verbal suggestion, classical conditioning with verbal suggestion, and observational learning). To investigate the role of methodological and demographic factors, we then conducted subgroup analyses assessing the effect of the type of cutaneous sensation and the type of placebo intervention, and meta-regression to assess the impact of the number of learning and evocation trials used in classical conditioning models, the difference in calibrated intensity of placebo and control stimulations, sex distribution, and mean age of the participants on placebo-effect magnitudes.
2. Methods
2.1. Protocol and registration
The protocol for this study was preregistered on ClinicalTrials.gov (ID: NCT04387851) and was conducted following PRISMA guidelines106 (Supplementary digital content, available at http://links.lww.com/PAIN/B752). We registered a single search strategy for placebo and nocebo studies, the results of which have been divided into 2 articles after evaluating the amount of articles yielded by the search, facilitating a more clear and nuanced discussion for each set of findings. Here, we report on the placebo studies.
2.2. Databases and selection criteria
PubMed, PsycINFO, EMBASE, and the Cochrane CENTRAL Methodology Library were searched to identify studies. Languages were limited to English, Dutch, and German, and the publication period was not restricted. Searches were initially conducted on March 18, 2019, and subsequently updated on April 10, 2020, and July 15, 2021. The complete key-worded search strategy for each database is available in the supplementary digital content (available at http://links.lww.com/PAIN/B752).
We searched for original, controlled experimental studies on healthy participants that aimed to experimentally induce placebo or nocebo effects on cutaneous sensations (ie, pain or itch stimulations that were administered on the skin); of which, the results of studies on placebo effects are reported here. Patient samples were not included because the current review focuses on learning mechanisms and methodological factors, which can be studied with better experimental control in healthy samples. For better homogeneity of study designs, we focused only on cutaneous sensations, and excluded, eg, studies on visceral or ischemic sensation. For the purposes of inclusion and exclusion, studies were considered to have induced a placebo effect if a learning mechanism (eg, conditioning, verbal suggestion, observational learning) was used to induce positive expectations about an inert treatment and not to purely ambiguous stimuli (eg, colored shapes). This was done to focus the scope of this review on experimental studies, which induced expectations around treatments as opposed to abstract stimuli, thereby improving the clinical relevance of the meta-analyses. We only included studies that featured some form of control comparator, whether that was within or between subjects, so that the placebo effect could be calculated as the difference between placebo and control. Studies that excluded nonresponders from the analyses were excluded. Studies that did not fulfill one or more of the criteria mentioned above were excluded from further review and meta-analysis. Our search terms did not include words specifically intended to collect observational learning studies because we did not originally plan to investigate this learning mechanism in our preregistration. Still, our search identified observational learning studies, and we decided to include them in our review because we likely identified all relevant observational learning studies with reference list and Web of Science searches.
2.3. Study selection
Titles and abstracts of articles retrieved using the above search strategy were independently screened by 2 authors (J.S.B. and M.M.E.V.S). The full text of articles to be included and articles about which doubts existed were then retrieved and assessed for eligibility by 2 authors independently (J.S.B. and M.A.T.). The reference lists of all included articles were also screened for study inclusion by one author (J.S.B.) and a student assistant, and included articles were also entered in Web of Science to identify articles that have cited them and should potentially be included in the meta-analysis in April, 2020. When full texts were not available online, authors were contracted through email to request access. Disagreements concerning study inclusion decisions were resolved by a third author (K.J.P).
2.4. Data extraction
One author (J.S.B.) used a standardized form to independently extract data from the included studies to derive study characteristics and data for analyses. Another author (M.A.T.) checked 25% of extracted values for accuracy. Extracted information included details of the experimental induction (ie, learning mechanism used), control condition, study population, placebo treatment, sensation type, pain/itch outcome data, how sensations were measured (eg, 0-10 numeric rating scale, visual analogue scale, 0-20 Gracely scale, etc), type of cutaneous stimulation (eg, heat pain, pressure pain, histamine-evoked itch), information for quality and bias assessment, and outcome data for meta-analysis (eg, sample size, pain/itch rating means and standard deviations). Doubts regarding data extraction were resolved through discussion with a third author (K.J.P.). Missing data were requested from the authors of included studies. If the authors did not respond, but data could be extracted from published figures, this was done with the software WebPlotDigitizer version 4.4 (Rohatgi, 2020).
2.5. Risk of bias
2.5.1. Risk of bias assessment within studies
Risk of bias was assessed by a student assistant and one of the authors (M.A.T.), independently from one another, using the method developed by Marcuzzi et al.,91 specifically for quantitative sensory testing studies. This method assesses (1) whether the inclusion criteria were clearly described (3 items), (2) whether the sample is clearly described and representative of the population (5 items), (3) whether the recruitment process was clearly described (3 items) (4) whether the somatosensory assessment methods are standardized, validated, and well described (6 items), (5) adequate blinding if relevant (1 item), and (6) whether potential confounders were considered (2 items). Items were scored as satisfied (0 points), not satisfied (2 points), partially satisfied or unclear (1 point), or not applicable. Studies receive a score ranging from 0 to 40 based on these criteria, with higher scores indicating a greater risk of bias. Meta-regression was used to test for a relationship between risk of bias score and the magnitude of the placebo effect. An example of the risk of bias tool can be found in the supplementary digital content (available at http://links.lww.com/PAIN/B752).
2.5.2. Risk of publication bias across studies
Risk of publication bias across studies was assessed visually with funnel plots. Studies lying outside the funnel of expected results were included in subsequent analyses, but their outlier status was noted in the study characteristics table (Tables 1–4). Publication bias was assessed with Duval and Tweedie's trim and fill method,47 a nonparametric technique for estimating the number of missing studies in a meta-analysis and the impact these studies would likely have on the overall effect size.
Table 1.
Characteristics of studies included in the classical conditioning with verbal suggestion (pain) meta-analysis.
| Author | Year | N | Sample age | Percent female | Sensation induction method | Placebo manipulation | Rating scale | Acquisition trials (Placebo/Control) | Evocation trials (Placebo/Control) | Calibrated stimulus intensity difference (0-100)* | First or mean outcome measure | Risk of bias score | Comparison | Outlier based on funnel plot |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Au Yeung7 | 2014 | 20 | 19.8 | 59% | Electrical | Sham TENS | 0-100 VAS | 32 (16P/16C) | 32 (16P/16C) | NA | First | 3 | W | |
| Barnes11 | 2021 | 62 | 19.4 | 52% | Electrical | Sham TENS | 0-10 pain intensity | 30 (15P/15C) | 20 (10P/10C) | 45 | Mean | 3 | W | |
| Case21 | 2019 | 28 | NR | 53% | Thermal | Inert gel | 0-80 pain intensity | 8 (4P/4C) | 16 (8P/8C) | NA | Mean | 7 | W | − |
| Choi23 | 2011 | 15 | 25.3 | 0% | Electrical | Sham IV | 0-100 NRS | Unknown | 10 (5P/5C) | NA | Mean | 3 | W | |
| Chouchou24 | 2015 | 26 | 23.4 | 46% | Thermal | Inert gel | 0-100 VAS | 16 (8P/8C) | 10 (5P/5C) | 35 | Mean | 4 | W | |
| Colagiuri27 | 2018 | 21 | 20.2 | 71% | Electrical | Sham TENS | 0-100 VAS | 32 (16P/16C) | 32 (16P/16C) | NA | First | 5 | W | |
| Colloca30 | 2006 | 10 | 22.7 | 83% | Electrical | Sham electrode | 0-10 NRS | 36 (18P/18C) | 12 (6P/6C) | NA | Mean | 5 | W | + |
| Colloca34 | 2008 | 15 | 22.5 | 100% | Electrical | Sham electrode | 0-10 VAS | 24 (12P/12C) | 12 (6P/6C) | NA | Mean | 3 | W | |
| Colloca35 | 2008a | 16 | 32.0 | 66% | Laser | Inert gel | 0-10 NRS | 30 (15P/15C) | 30 (15P/15C) | NA | Mean | 5 | W | |
| Colloca31 | 2009 | 16 | 22.6 | 100% | Electrical | Sham electrode | 0-10 NRS | 24 (12P/12C) | 12 (6P/6C) | NA | Mean | 5 | W | |
| Colloca33 | 2010 | 46 | 22.8 | 65% | Electrical | Sham electrode | 0-10 VAS | 20 (10P/10C) | 40 (20P/20C) | 30 | Mean | 3 | W | + |
| Colloca33 | 2010 | 46 | 22.8 | 65% | Electrical | Sham electrode | 0-10 VAS | 80 (40P/40C) | 40 (20P/20C) | 30 | Mean | 3 | W | + |
| Colloca36 | 2019 | 53 | 28.1 | 64% | Electrical | Sham electrode | 0-10 NRS | 18 (9P/9C) | 36 (18P/18C) | 60 | Mean | 3 | W | |
| Colloca29 | 2020 | 400 | 29.4 | 59% | Thermal | Sham electrode | 0-100 VAS | 24 (12P/12C) | 12 (6P/6C) | NA | Mean | 5 | W | |
| Corsi37 | 2017 | 46 | 27.4 | 52% | Thermal | Sham electrode | 0-100 VAS | 12 (6P/6C) | 6 (3P/3C) | NA | Mean | 3 | W | |
| de Jong39 | 1996 | 36 | 21.3 | 100% | Electrical | Inert gel | 0-100 VAS | 20 (10P/10C) | 10 (5P/5C) | 25 | Mean | 5 | B | |
| De Pascalis40 | 2002 | 36 | 25.4 | 65% | Electrical | Inert gel | 0-10 VAS | 12 (6P/6C) | 30 (15P/15C) | NA | Mean | 2 | W | |
| De Pascalis43 | 2021 | 56 | 23.3 | 100% | Cold cup | Inert gel | 0-100 NRS | 2 (1P/1C) | 2 (1P/1C) | NA | Mean | 4 | W | |
| Egorova48 | 2020 | 24 | NR | 50% | Thermal | Inert gel | 0-20 Gracely scale | 48 (24P/24C) | 24 (12P/12C) | 25 | Mean | 5 | W | |
| Eippert49 | 2009 | 19 | 25.0 | 0% | Thermal | Inert gel | 0-100 VAS | 12 (6P/6C) | 30 (15P/15C) | 40 | Mean | 6 | W | |
| Eippert50 | 2009a | 13 | 25.0 | 0% | Thermal | Inert gel | 0-100 VAS | 12 (6P/6C) | 30 (15P/15C) | 40 | Mean | 0 | W | |
| Feldhaus55 | 2021 | 624 | 24.6 | 60% | Thermal | Inert gel | 0-100 VAS | 16 (8P/8C) | 16 (8P/8C) | 40 | Mean | 3 | W | |
| Flaten57 | 2018 | 25 | 21.9 | 56% | Thermal | Inert pill | 0-10 NRS | 3 (2P/1C) | 2 (1P/1C) | NA | Mean | 0 | W | |
| Frangos58 | 2021 | 46 | 39.7 | 85% | Thermal | Inert gel | 0-200 VAS | 24 (12P/12C) | 20 (10P/10C) | 45 | Mean | 3 | W | |
| Freeman59 | 2015 | 24 | NR | 50% | Thermal | Inert gel | 0-20 Gracely scale | 18 (9P/9C) | Unknown | 25 | Mean | 5 | W | |
| Gaab60 | 2019 | 81 | 25.2 | 60% | Thermal | Inert gel | 0-10 VAS | 16 (8P/8C) | 4 (2P/2C) | 30 | Mean | 3 | W | |
| Geisler62 | 2020 | 33 | 27.4 | 0% | Thermal | Inert gel | 0-100 VAS | 16 (8P/8C) | 8 (4P/4C) | 40 | Mean | 3 | W | |
| Geuter63 | 2013 | 40 | 26.0 | 0% | Thermal | Inert gel | 0-100 VAS | 24 (12P/12C) | 30 (15P/15C) | 50 | Mean | 3 | W | |
| Geuter63 | 2013 | 40 | 26.0 | 0% | Thermal | Inert gel | 0-100 VAS | 24 (12P/12C) | 30 (15P/15C) | 30 | Mean | 3 | W | |
| Grahl66 | 2018 | 23 | 24.6 | 0% | Thermal | Sham TENS | 0-100 VAS | 24 (12P/12C) | 24 (12P/12C) | 40 | Mean | 0 | W | |
| Hartmann67 | 2021 | 45 | 23.8 | 51% | Electrical | Inert gel | 0-8 pain intensity | Unknown | 32 (16P/16C) | 30 | Mean | 1 | W | |
| Huneke74 | 2013 | 73 | 37.6 | 66% | Laser | Inert gel | 0-10 NRS | 20 (10P/10C) | 20 (10P/10C) | 40 | Mean | 3 | B | |
| Jarcho77 | 2016 | 15 | 24.3 | 100% | Thermal | Inert gel | 0-100 VAS | 2 (1P/1C) | 2 (1P/1C) | NA | Mean | 4 | W | |
| Kirsch79 | 2014 | 48 | 26.4 | 50% | Thermal | Sham acupuncture | 0-20 Gracely scale | Unknown | Unknown | 40 | Mean | 5 | B | |
| Klinger80 | 2007 | 12 | 26.1 | 50% | Electrical | Inert gel | 0-8 pain intensity | 10 (5P/5C) | 10 (5P/5C) | 25 | Mean | 8 | W | |
| Kong82 | 2006 | 16 | 28.4 | 44% | Thermal | Sham acupuncture | 0-20 Gracely scale | 48 (24P/24C) | 24 (12P/12C) | 40 | Mean | 5 | W | |
| Laverdure-Dupont85 | 2009 | 38 | 23.4 | 58% | Thermal | Inert gel | 0-100 VAS | 16 (8P/8C) | 10 (5P/5C) | 20 | Mean | 3 | W | |
| Lee86 | 2020 | 21 | 23.6 | 43% | Pressure | Inert gel | 0-100 VAS | 12 (6P/6C) | 12 (6P/6C) | NA | Mean | 5 | W | |
| Lui89 | 2010 | 31 | 23.5 | 58% | Laser | Sham electrode | 0-100 VAS | 24 (12P/12C) | 12 (6P/6C) | NA | Mean | 7 | W | |
| Martin95 | 2010 | 40 | 21.2 | 70% | Thermal | Inert gel | 0-10 NRS | 16 (8P/8C) | 2 (1P/1C) | 30 | Mean | 5 | W | |
| Martini96 | 2015 | 28 | 23.5 | 50% | Laser | Inert gel | 0-100 NRS | 24 (12P/12C) | 32 (16P/16C) | NA | Mean | 7 | W | |
| Martin-Pichora94 | 2011 | 15 | 22.8 | 68% | Thermal | Inert gel | 0-10 NRS | 16 (8P/8C) | 2 (1P/1C) | 30 | Mean | 3 | W | |
| Montgomery104 | 1997 | 24 | NR | 50% | Electrical | Inert gel | 0-10 VAS | 20 (10P/10C) | 12 (6P/6C) | 30 | Mean | 9 | B | |
| Morton106 | 2009 | 66 | 25.0 | 64% | Laser | Inert gel | 0-10 pain intensity | 60 (30P/30C) | 60 (30P/30C) | 40 | Mean | 5 | B | |
| Morton105 | 2010 | 56 | 25.0 | 62% | Laser | Inert gel | 0-10 pain intensity | 60 (30P/30C) | 60 (30P/30C) | 40 | Mean | 7 | B | |
| Power117 | 2020 | 57 | 49.0 | 65% | Laser | Inert gel | 0-10 NRS | 20 (10P/10C) | 20 (10P/10C) | 40 | Mean | 2 | W | |
| Price118 | 1999 | 34 | 19.3 | 60% | Thermal | Inert gel | 0-10 VAS | 30 (15P/15C) | 4 (2P/2C) | 40 | Mean | 6 | W | |
| Rhudy120 | 2018 | 33 | 36.4 | 51% | Electrical | Inert gel | 0-100 VAS | 24 (12P/12C) | 24 (12P/12C) | NA | Mean | 6 | W | + |
| Rosén124 | 2016 | 36 | 25.0 | 58% | Thermal | Sham electrode | 0-100 NRS | 3 (1P/2C) | 3 (1P/2C) | NA | Mean | 0 | W | |
| Rütgen126 | 2015 | 102 | 26.2 | 67% | Electrical | Inert pill | 0-7 pain intensity | 4 (2P/2C) | Unknown | 25 | Unknown | 3 | B | |
| Schafer127 | 2015 | 40 | NR | 67% | Thermal | Inert cream | 0-100 VAS | 16 (8P/8C) | 40 (24P/16C) | 40 | Mean | 6 | W | |
| Schafer127 | 2015 | 40 | NR | 67% | Thermal | Inert cream | 0-100 VAS | 112 (56P/56C) | 40 (24P/16C) | 40 | Mean | 6 | W | |
| Schenk129 | 2017 | 24 | 25.4 | 48% | Thermal | Sham TENS | 0-100 VAS | 18 (9P/9C) | 18 (9P/9C) | 40 | Mean | 3 | W | |
| Skvortsova131 | 2020 | 37 | 23.1 | 0% | Thermal | Inert nasal spray | 0-10 NRS | 24 (12P/12C) | 20 (10P/10C) | 30 | Mean | 0 | W | |
| Tang138 | 2019 | 30 | 22.5 | 67% | Electrical | Sham TENS | 0-100 graphic rating scale | 32 (16P/16C) | 4 (2P/2C) | NA | Unknown | 4 | W | |
| Tu139 | 2021 | 27 | 27.4 | 46% | Thermal | Inert cream | 0-20 Gracely scale | 48 (24P/24C) | 24 (12P/12C) | 25 | Mean | 3 | W | |
| Valentini141 | 2014 | 27 | 24.9 | 54% | Laser | Sham electrode | 0-100 VAS | 24 (12P/12C) | 8 (4P/4C) | 42 | Mean | 5 | W | |
| Vambheim142 | 2021 | 59 | 21.5 | 44% | Thermal | Inert gel | 0-10 NRS | 30 (15P/15C) | 30 (15P/15C) | NA | Mean | 0 | W | − |
| Vambheim142 | 2021 | 32 | NR | 54% | Electrical | Inert gel | 0-10 NRS | 22 (11P/11C) | 36 (18P/18C) | 20 | Mean | 0 | W | |
| Wager152 | 2004 | 24 | NR | NR | Thermal | Inert gel | 0-10 VAS | 60 (12P/12C) | 12 (6P/6C) | 60 | Mean | 12 | W | |
| Wager149 | 2006 | 39 | 23.2 | 55% | Laser | Inert gel | −2 to 10 VAS | 10 (5P/5C) | 80 (40P/40C) | NA | Mean | 7 | W | |
| Wager150 | 2007 | 15 | NR | 0% | Thermal | Inert gel | 0-10 VAS | 10 (5P/5C) | 60 (30P/30C) | NA | Mean | 7 | W | |
| Watson154 | 2006 | 24 | 23.8 | 55% | Laser | Inert gel | 0-100 NRS | 20 (10P/10C) | 20 (10P/10C) | NA | Mean | 7 | W | |
| Watson156 | 2007 | 18 | NR | 45% | Laser | Inert gel | 0-10 pain intensity | 80 (40P/40C) | 40 (20P/20C) | 40 | Mean | 7 | B | |
| Watson155 | 2009 | 11 | NR | 54% | Laser | Inert gel | 0-10 NRS | 30 (15P/15C) | 30 (15P/15C) | 50 | Mean | 9 | W | |
| Wei157 | 2018 | 18 | 20.9 | 100% | Electrical | Sham electrode | 0-10 pain intensity | 40 (20P/20C) | 16 (8P/8C) | NA | Mean | 4 | W | |
| Weimer159 | 2019 | 78 | 27.5 | 73% | Thermal | Inert gel | 0-10 VAS | 16 (8P/8C) | 16 (8P/8C) | 30 | Mean | 2 | W | |
| Weng160 | 2021 | 32 | 22.0 | 75% | Thermal | Sham electrode | 0-10 NRS | 30 (15P/15C) | 10 (5P/5C) | 25 | Mean | 1 | W | |
| Wrobel162 | 2014 | 17 | 26.6 | 46% | Thermal | Inert gel | 0-100 NRS | 36 (18P/18C) | 30 (15P/15C) | 40 | Mean | 0 | W | |
| Wrobel161 | 2015 | 23 | 27.5 | 37% | Thermal | Inert gel | 0-100 VAS | 30 (15P/15C) | 24 (12P/12C) | 30 | Mean | 7 | W | |
| Zunhammer163 | 2018 | 33 | 25.0 | 50% | Thermal | Inert gel | 0-100 VAS | 30 (15P/15C) | 30 (15P/15C) | 40 | Mean | 1 | W |
For studies using a within-subject comparison to measure the placebo effect, N is reported as the number of participants from the group in which the comparison was made. For studies using a between-subject comparison, N is reported as the combined number of participants from the placebo and control groups from which the comparison was made, or only the placebo condition in the case of within subjects comparisons.
Instead of using calibrated stimulus intensities, some studies used fixed intensities, which were recorded here as NA. In the final column, “+” indicates a potential outlier with an effect size larger than the bounds of a 99% confidence interval, and “−” indicates a potential outlier with an effect size smaller than the bounds of a 99% confidence interval, based on inspection of funnel plots (Supplementary Materials, http://links.lww.com/PAIN/B752).
C, control; IV, intravenous; N, sample size; NA, not applicable; NR, not reported; NRS, numeric rating scale; P, placebo; TENS, transcutaneous electric nerve stimulation; VAS, visual analogue scale.
Table 4.
Characteristics of studies included in the verbal suggestion (itch) meta-analysis.
| Author | Year | N | Sample age | Percent female | Sensation induction method | Placebo manipulation | Rating scale | Risk of bias score | Comparison |
|---|---|---|---|---|---|---|---|---|---|
| Bartels12 | 2014 | 48 | 22.7 | 77% | Electrical | Sham electrode | 0-10 VAS | 4 | B |
| Darragh38 | 2015 | 48 | 22 | 78% | Histamine | Inert gel | 0-10 itch intensity scale | 1 | W |
| Peerdeman112 | 2015 | 59 | 21.8 | 71% | Histamine | Inert pill | 0-10 NRS | 3 | B |
| Skvortsova133 | 2018 | 54 | 22.1 | 100% | Histamine | Inert nasal spray | 0-10 NRS | 0 | B |
| van Laarhoven145 | 2011 | 36 | 21.8 | 100% | Histamine | Inert gel* | 0-10 VAS | 1 | B |
| Meeuwis99 | 2019 | 45 | 23.2 | 83% | Histamine | Inert gel | 0-10 NRS | 4 | W |
| Meeuwis98 | 2021 | 28 | 21.3 | 81% | Histamine | Sham patch | 0-10 NRS | 4 | W |
For studies using a within-subject comparison to measure the placebo effect, N is reported as the number of participants from the group in which the comparison was made. For studies using a between-subject comparison, N is reported as the number of participants from the placebo and control groups from which the comparison was made, or only the placebo condition in the case of within-subject comparisons.
No actual inert gel was applied, instead the histamine gel was used and participants were led to believe an additional medical gel was added. No outliers based on inspection of funnel plots (Supplementary material, http://links.lww.com/PAIN/B752) were detected in this analysis.
N, sample size; NRS, numeric rating scale; VAS, visual analogue scale.
2.6. Statistical analyses
Analyses were conducted with the Comprehensive Meta-Analysis software, version 3.3.070 (Comprehensive Meta-Analysis, 2014) and R for visualizations (R Core Team, 2019). Given the heterogeneity of study designs and methods, a random-effects model was used for all meta-analyses. Effect sizes were calculated with means and SDs for each group (between-subject placebo vs control groups) or trial type (within-subject placebo vs control). If only difference scores with standard deviations for placebo vs control trials were reported, these were used instead. If only standard errors were reported, these were converted to standard deviations. For each included study, an effect size (Hedge g) weighted to the sample size (n) was computed, for which positive values indicate the presence of a placebo effect. Hedge g is a standardized parametric measure of effect size that represents the difference between 2 means in units of pooled standard deviations, commonly used in meta-analysis.67,68 It is similar to Cohen d but provides more accurate estimates of effect sizes for samples of less than n = 20, whereas the 2 perform equally well for samples of n > 20. Both can be interpreted on the same scale, in which values of approximately 0.20 can be considered small, 0.50 medium, and 0.80 large.26
2.6.1. Primary outcome measure
The primary outcome measure was the magnitude of the placebo effect, defined as the difference in reported sensation intensity between placebo and control groups (between subjects) or trials (within subjects), when the intensity of the stimulus was equal across conditions (typically referred to as the test phase, evocation phase, or extinction phase in conditioning studies). The minimum group size for analysis was k = 3, where k denotes the number of included studies, based on a previous meta-analysis on a similar topic.110 Whenever possible, the mean of pain or itch ratings across the entire test phase was used because this was by far the most commonly reported outcome (Tables 1–4). If only values from the first trial(s) were reported, these were used instead. Sensitivity analyses tested for differences in placebo magnitudes between studies reporting the mean pain values for the entire evocation phase vs the first trials, where effects are thought to be strongest with less opportunity for extinction to occur. Similarly, for consistency, we used a within-subject comparison to measure the placebo effect when the necessary data were reported, and when this was not possible, between subjects or mixed within–between comparisons were used; we conducted sensitivity analyses to assess whether the type of comparison affected the placebo-effect magnitude. A Cochran Q test was used to measure the degree to which heterogeneity in placebo effect sizes could be explained by these factors.25 For within-subject comparisons, a prepost correlation value of 0.5 was used, and sensitivity analyses conducted by previous meta-analyses in related topics found that adjusting this value did not impact overall results.110,139 When a single study had more than one arm eligible for inclusion in a single meta-analysis (eg, a study comparing 2 classical conditioning paradigms with different numbers of trials,33 data from both arms were averaged across for the primary outcome but included separately for relevant subgroup analysis). Heterogeneity of resulting effect sizes was measured with I2, the proportional amount of variance in effect sizes attributed to heterogeneity between included studies,70 treating 0% to 40% as negligible, 30% to 60% as moderate, 60% to 90% as substantial, and 75% to 100% as considerable amounts of heterogeneity.44 Statistical significance of heterogeneity was measured with a Cochran Q test.
2.6.2. Additional analyses
Subgroup analyses with random-effects models were used to explore differences between pain induction methods (eg, thermal pain, electrical pain) and placebo treatments (eg, placebo gels, electrodes). A Cochran Q test was used to measure the degree to which heterogeneity in placebo effect sizes could be explained by these subgrouping variables. Meta-regression was used to assess the impact of the number of acquisition trials, or instances in which a pain stimulus and associated placebo or control cue are paired during the acquisition phase of a classical conditioning paradigm. Similarly, meta-regression analysis was conducted for the number of trials in the evocation phase of a classical conditioning paradigm and for the difference in placebo and control stimulus intensity (during the acquisition phase of conditioning paradigms) when these were calibrated on the basis of subjective pain ratings. Meta-regression was also used to assess the potential impact of sample age and sex, measured in years and percentage female-identified participants, respectively. These demographic analyses were not preregistered and were conducted post hoc. All meta-regressions used mixed-effects models, and Q values are reported. For all additional analyses, the magnitude of the placebo effect served as the outcome variable. The minimum group size for subgroup analyses was k = 3.110
3. Results
3.1. Study selection
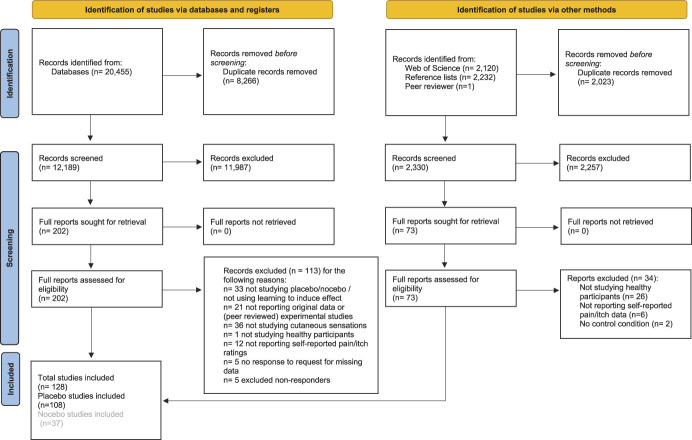
A total of 17,546 articles were identified through the initial database search (Fig. 1). After removal of 6672 duplicate results, 10,874 articles remained for consideration based on title and abstract. Of these, 174 articles remained whose full texts were reviewed, culminating in 80 articles initially included. The reference lists of these included articles were then screened (2232 referenced articles), yielding 17 more articles fit for inclusion. A Web of Science search for articles citing the 80 articles that were initially included then produced another 2120 articles for screening of which 22 articles fit all inclusion criteria. The database search was repeated in April 2020 and June 2021, ultimately yielding an additional 10 and 24 inclusions, respectively. During data extraction, 29 articles were excluded. In all, 24,814 articles were identified in various searches, 24,687 articles were excluded, resulting in 127 articles included; of which, 107 were included in placebo effect meta-analyses. One observational learning article was included during the revision process, bringing the total number of articles to 108. Details on inclusion and exclusions at each stage of the search can be found in Figure 1. Reasons for exclusions were assigned based on the first detected exclusion criteria to be violated.
Figure 1.
Flowchart of study inclusion process. Flow diagram of the inclusion and exclusion of studies for all searches. Nocebo studies are reported in a separate publication.
3.2. Characteristics of included studies
Our search strategy identified 108 unique placebo studies, which met all inclusion criteria. Of these 108 studies, 68 had sufficient data for inclusion in the classical conditioning with verbal suggestions (CC + VS) on pain meta-analysis (Table 1), 39 had sufficient data for inclusion in the verbal suggestions alone (VS) on pain meta-analysis (Table 2), and 7 had sufficient data for inclusion in the observational learning on pain meta-analysis (Table 3). There are more arms than total unique studies because several studies that compared different learning processes had arms included in multiple meta-analyses. For itch, 7 studies had sufficient data for inclusion in the meta-analysis of verbal suggestions alone (Table 4). Only one study, Bartels et al.,12 was identified for classical conditioning with verbal suggestions on itch (the characteristics of this study are reported in Table 3 as a verbal suggestion arm of the study was included), and no studies for observational learning of placebo effects on itch, so no meta-analyses were conducted in these cases. The studies were published between the years 1996 and 2021. Several studies that met all inclusion criteria except the use of actual placebo intervention, and instead induced placebo effects with only abstract stimuli like color or shapes, were not included in the analysis (eg, Carlino et al.,20 Świder and Bąbel, 132 Bąbel et al.,9 Brączyk and Bąbel16). A record of inclusion and exclusion decisions can be found in the supplementary digital content for this article (available at http://links.lww.com/PAIN/B752), along with data extraction materials.
Table 2.
Characteristics of studies included in the verbal suggestion (pain) meta-analysis.
| Author | Year | N | Sample age | Percent female | Sensation induction method | Placebo manipulation | Rating scale | Risk of bias score | Comparison | Outlier based on funnel plot |
|---|---|---|---|---|---|---|---|---|---|---|
| Aslaksen4 | 2008 | 63 | 24.2 | 51% | Thermal | Inert pill | 0-100 VAS | 0 | W | |
| Aslaksen6 | 2015 | 48 | 23.4 | 51% | Thermal | Inert gel | 0-100 VAS | 3 | B | |
| Aslaksen3 | 2016 | 32 | 21.6 | 54% | Thermal | Inert gel | 0-100 VAS | 3 | B | |
| Brown17 | 2013 | 61 | 19.5 | 59% | Cold pressor | Inert gel | 0-10 NRS | 5 | W | |
| Camerone19 | 2021 | 21 | 22.0 | 52% | Electrical | Inert gel | 0-10 NRS | 4 | W | |
| Colloca34 | 2008 | 14 | 22.3 | 100% | Electrical | Sham electrode | 0-10 NRS | 3 | W | |
| Colloca35 | 2008a | 16 | 32.0 | 66% | Laser | Inert gel | 0-10 NRS | 5 | W | |
| Colloca31 | 2009 | 16 | 22.6 | 100% | Electrical | Sham electrode | 0-10 NRS | 5 | W | |
| Colloca36 | 2019 | 107 | 28.1 | 64% | Electrical | Sham electrode | 0-100 NRS | 3 | W | |
| De Pascalis41 | 2017 | 55 | 23.4 | 100% | Cold cup | Inert gel | 0-100 NRS | 3 | W | |
| De Pascalis42 | 2019 | 58 | 24.5 | 100% | Cold cup | Inert gel | 0-100 pain intensity scale | 1 | W | |
| Disley45 | 2021 | 50 | 21.0 | 87% | Cold pressor | Inert nasal spray | −5 to +5 VAS | 1 | W | |
| Ellingsen51 | 2013 | 28 | 25.5 | 33% | Thermal | Inert nasal spray | 0-100 NRS | 4 | W | + |
| Fehse54 | 2015 | 27 | 32.0 | 0% | Thermal | Inert pill | 0-10 pain intensity scale | 5 | B | |
| Geers61 | 2014 | 106 | 19.6 | 67% | Cold pressor | Inert gel | 0-100 VAS | 5 | W | |
| Gniß65 | 2020 | 32 | 21.0 | 50% | Thermal | Inert gel | 0-100 VAS | 2 | W | |
| Horing73 | 2020 | 17 | 19.6 | 54% | Thermal | Inert pill | 0-10 VAS | 2 | W | |
| Hunter75 | 2014 | 15 | 27.0 | 100% | Electrical | Sham electrode | 0-10 VAS | 3 | W | |
| Johnson78 | 1997 | 24 | NR | 50% | Cold pressor | Sham TENS | 0-100 VAS | 2 | W | |
| Kube84 | 2020 | 25 | 23.6 | 44% | Thermal | Inert gel | 0-100 VAS | 2 | W | |
| Locher88 | 2017 | 37 | 26.6 | 62% | Thermal | Inert gel | 0-100 VAS | 6 | W | |
| Lyby90 | 2010 | 63 | NR | 48% | Thermal | Inert pill | 0-100 VAS | 2 | W | |
| Lyby91 | 2011 | 33 | 22.0 | 51% | Thermal | Inert gel | 0-10 NRS | 1 | W | |
| Lyby92 | 2012 | 33 | 22.0 | 30% | Thermal | Inert pill | 0-10 NRS | 2 | W | |
| Matre97 | 2006 | 18 | NR | 41% | Thermal | Sham magnets | 0-100 VAS | 6 | W | |
| Milling101 | 2009 | 41 | NR | 63% | Pressure | Inert gel | 0-30 pain intensity scale | 4 | W | |
| Montgomery103 | 1996 | 56 | NR | 57% | Pressure | Inert gel | 0-10 pain intensity scale | 10 | W | |
| Nemoto107 | 2007 | 10 | NR | 50% | Laser | Inert pill | 0-10 pain intensity scale | 3 | W | |
| Nir108 | 2012 | 24 | 25.8 | 0% | Hot water | Inert pill | 0-100 NRS | 3 | B | |
| Peerdeman112 | 2015 | 59 | 21.8 | 71% | Cold pressor | Inert pill | 0-10 NRS | 3 | B | |
| Petrovic115 | 2002 | 9 | NR | NR | Thermal | Inert pill | 0-100 VAS | 6 | W | |
| Pontén116 | 2019 | 15 | 27 | 60% | Thermal | Sham electrode | 0-100 NRS | 2 | W | |
| Rhudy120 | 2018 | 33 | 35.3 | 51% | Electrical | Inert gel | 0-100 VAS | 6 | W | |
| Roelofs121 | 2000 | 30 | 21.6 | 0% | Electrical | Sham IV | 0-100 VAS | 5 | B | |
| Rose122 | 2012 | 41 | NR | 61% | Cold pressor | Inert gel | 0-10 VAS | 6 | B | |
| Skvortsova133 | 2018 | 54 | 22.1 | 100% | Cold pressor | Inert nasal spray | 0-10 NRS | 0 | B | |
| Valentini140 | 2018 | 39 | 24.9 | 54% | Laser | Inert gel | 0-10 NRS | 2 | B | |
| van Laarhoven145 | 2011 | 33 | 21.8 | 100% | Histamine | Inert gel | 0-100 VAS | 1 | W | |
| Yeung153 | 2020 | 60 | 24.5 | 72% | Cold pressor | Inert gel | 0-10 VAS | 4 | B |
For studies using a within-subject comparison to measure the placebo effect, N is reported as the number of participants from the group in which the comparison was made. For studies using a between-subject comparison, N is reported as the number of participants from the placebo and control groups from which the comparison was made or only the placebo condition in the case of within-subject comparisons.
C, control; IV, intravenous; N, sample size; NR, not reported; NRS, numeric rating scale; P, placebo; TENS, transcutaneous electric nerve stimulation; VAS, visual analogue scale.
Table 3.
Characteristics of studies included in the observational learning (pain) meta-analysis.
| Author | Year | N | Sample age | Percent female | Sensation induction method | Placebo manipulation | Rating scale | Risk of bias score | Comparison | Outlier based on funnel plot |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen22* | 2019 | 24 | NR | 52% | Thermal | Inert gel | 0-100 VAS | 3 | W | |
| Chen22* | 2019 | 43 | NR | 63% | Thermal | Inert gel | 0-100 VAS | 3 | W | − |
| Chen22* | 2019 | 30 | NR | 65% | Thermal | Inert gel | 0-100 VAS | 3 | W | − |
| Colloca31 | 2009 | 16 | 22.6 | 100% | Electrical | Sham electrode | 0-10 NRS | 3 | W | + |
| Hunter75 | 2014 | 30 | 27.0 | 100% | Electrical | Sham electrode | 0-10 VAS | 3 | W | |
| Raghuraman119 | 2019 | 28 | 23.4 | 61% | Thermal | Inert gel | 0-100 VAS | 3 | W | |
| Schenk128 | 2020 | 31 | 28.1 | 48% | Thermal | Inert gel | 0-100 VAS | 2 | W |
For studies using a within-subject comparison to measure the placebo effect, N is reported as the number of participants from the group in which the comparison was made. For studies using a between-subject comparison, N is reported as the number of participants from the placebo and control groups from which the comparison was made, or only the placebo condition in the case of within-subject comparisons. In the final column, “−” indicates a potential outlier with an effect size smaller than the bounds of a 99% confidence interval, based on inspection of funnel plots (Supplementary Materials, http://links.lww.com/PAIN/B752).
Three independent studies reported in a single article.
NRS, Numeric Rating Scale; VAS, visual analogue scale.
3.3. Primary outcome: magnitude of placebo effects
Placebo effects on pain induced with CC + VS paradigms (k = 68) were found to have an average effect size of g = 0.59, SE = 0.04, 95% confidence interval (CI) = 0.50 to 0.67, Q(67) = 310.75, P < 0.001, I2 = 78.44%, indicating a medium positive effect with substantial heterogeneity (Fig. 2). Visual inspection of the funnel plot (Supplementary digital content Fig. S1, available at http://links.lww.com/PAIN/B752) indicated a likely effect of publication bias, and trim-and-fill method of Duval and Tweedie indicated that an estimated 22 studies were missing, resulting in an adjusted effect size of g = 0.41, 95% CI = 0.32 to 0.50. For placebo effects on pain induced with VS alone (k = 39), an average effect size of g = 0.38, SE = 0.04, 95% CI = 0.30 to 0.45, Q(38) = 65.64, P = 0.005, I2 = 40.58%, was found, indicating a small to medium positive effect with moderate heterogeneity (Fig. 3). Inspection of the funnel plot (Supplementary digital content Fig. S2, available at http://links.lww.com/PAIN/B752) indicated no clear risk of publication bias, and no studies were imputed with trim and fill. For placebo effects on pain induced with observational learning (k = 7), an average effect size of g = 0.57, SE = 0.21, 95% CI = 0.16 to 0.99, Q(6) = 26.72, P < 0.001, I2 = 77.50%, was found, indicating a medium positive effect, albeit from a relatively small sample of studies (Fig. 4), with substantial heterogeneity. Inspection of the funnel plot (Supplementary digital content Fig. S3), available at http://links.lww.com/PAIN/B752, indicated a potential risk of publication bias, and although no studies were imputed with trim and fill, this is inconclusive given the small sample size of studies. For placebo effects on itch induced with VS (k = 7), an average effect size of g = 0.14, SE = 0.12, 95% CI = −0.08 to 0.37, Q(6) = 12.16, P = 0.06, I2 = 50.78%, was found, indicating a small positive effect with moderate, marginally significant heterogeneity (Fig. 5). Inspection of the funnel plot (Supplementary digital content Fig. S4, available at http://links.lww.com/PAIN/B752) indicated no clear risks of publication bias, and no studies were imputed with trim and fill.
Figure 2.
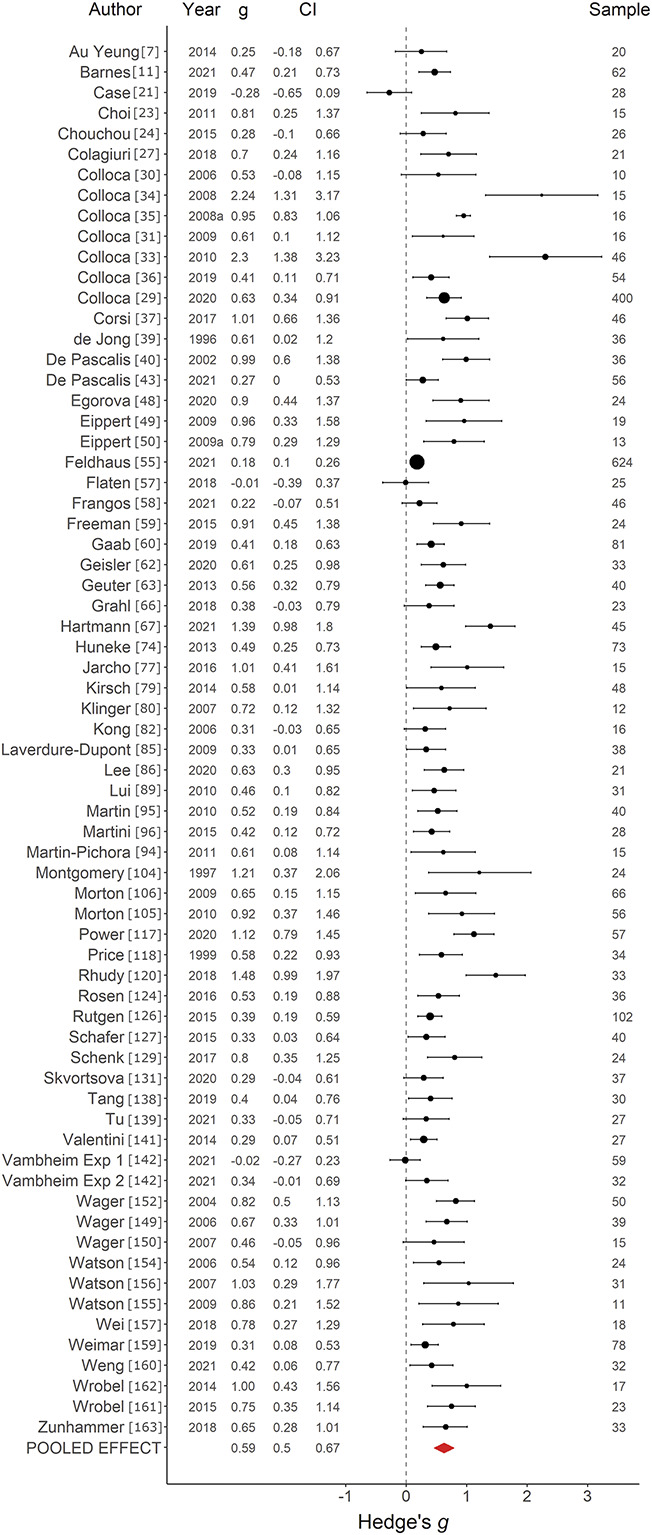
Forest plot of classical conditioning with verbal suggestion on pain studies. Forest plot depicting the magnitude of placebo effects on pain, measured with Hedge g in a random-effects model, in studies using a classical conditioning with verbal suggestion paradigm. CI, confidence interval.
Figure 3.
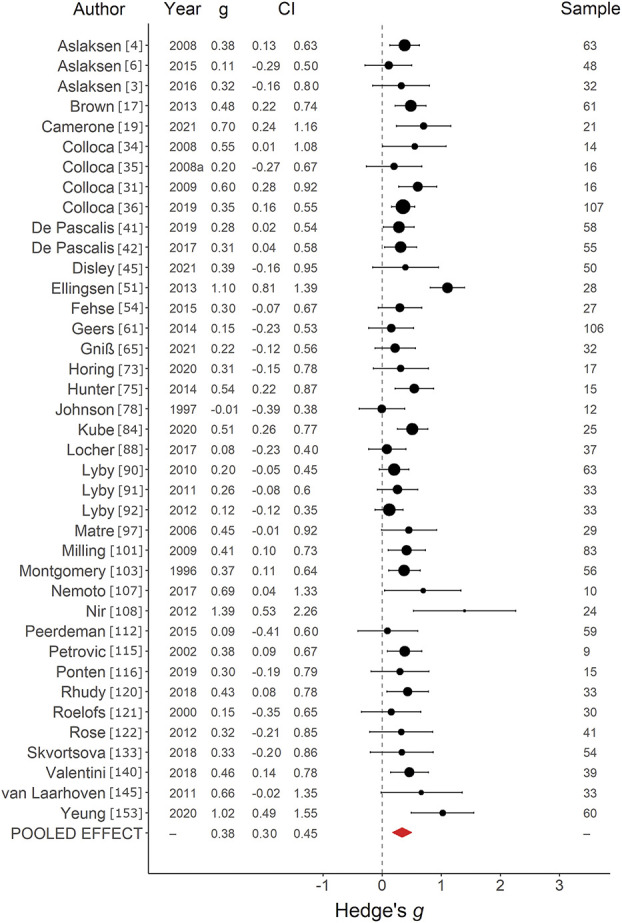
Forest plot of verbal suggestion on pain studies. Forest plot depicting the magnitude of placebo effects on pain, measured with Hedge g in a random-effects model, in studies using a verbal suggestion paradigm. CI, confidence interval.
Figure 4.
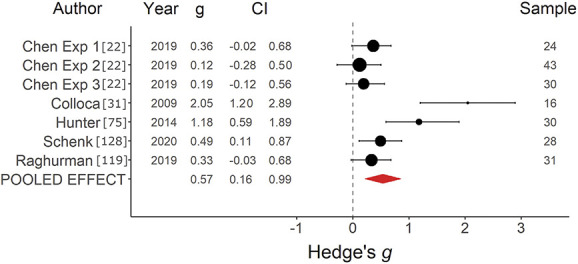
Forest plot of observational learning on pain studies. Forest plot depicting the magnitude of placebo effects on pain, measured with Hedge g in a random-effects model, in studies using an observational learning paradigm. CI, confidence interval.
Figure 5.
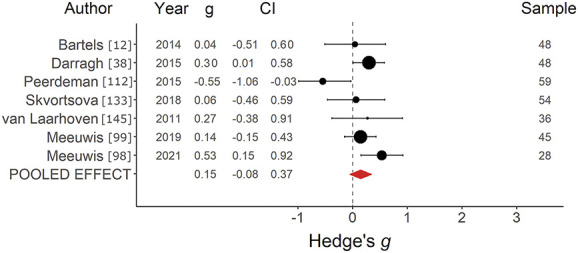
Forest plot of verbal suggestion on itch studies. Forest plot depicting the magnitude of placebo effects on itch, measured with Hedge g in a random-effects model, in studies using a verbal suggestion paradigm. CI, confidence interval.
3.3.1. Within-subject vs between-subject comparisons
Sensitivity analyses to assess whether our measurement of the placebo effect was impacted by the use of within-subject (k = 60, g = 0.59, 95% CI = 0.48-0.70) or between-subject (k = 8, g = 0.58, 95% CI = 0.41-0.75) comparisons revealed no effect in the CC + VS pain meta-analysis (Q = 0.02, P = 0.88). No differences for within-subject (k = 29, g = 0.38, 95% CI = 0.30-0.46) or between-subject (k = 10, g = 0.37, 95% CI = 0.16-0.58) comparisons were found in the VS pain meta-analysis (Q = 0.01, P = 0.92). It was possible to make a within-group comparison for all 7 studies in the observational learning pain meta-analysis. There was a substantial, marginally significant difference for within-subject (k = 3, g = 0.29, 95% CI = 0.10-0.48) and between-subject (k = 4, g = −0.07, 95% CI = −0.42 to 0.28) comparison in the VS itch meta-analysis (Q = 2.04, P = 0.08), although this should be interpreted with caution given the small sample size.
3.3.2. Single or mean outcome measure
Sensitivity analyses assessed whether measuring a placebo effect on pain using the mean of all evocation phase trials or an initial subset of trials in CC + VS paradigms affected the magnitude of the effect. Most studies (k = 63, g = 0.60) reported using the mean of all evocation phase trials, whereas very few studies (k = 2, g = 0.46) reported using the first control and placebo trials, and k = 3 did not specify how the placebo effect was measured (Table 1).
3.4. Secondary outcomes: subgroup analyses
3.4.1. Sensory induction method
From all included pain studies in the CC + VS meta-analysis, thermal stimulation was the most commonly used method of inducing pain (k = 35), with a medium effect size of g = 0.50 (95% CI = 0.38-0.62). This was followed by electrical stimulation with a medium-large effect size (k = 19, g = 0.77, 95% CI = 0.57-0.96) and laser stimulation with a medium effect size (k = 12, g = 0.61, 95% CI = 0.46-0.77). A Q test indicated that sensory induction method accounted for significant heterogeneity in the resulting placebo effect sizes (Q(2) = 7.2, P = 0.027), likely driven by differences between the thermal and electrical stimuli subgroups. For all included pain studies in the VS meta-analysis, thermal stimulation was again the most commonly used method of inducing pain with a medium-small effect size (k = 15, g = 0.34, 95% CI = 0.21-0.47), followed by cold pressor (k = 9, g = 0.33, 95% CI = 0.13-0.54), electrical stimulation (k = 7, g = 0.45, 95% CI = 0.32-0.57), and laser stimulation (k = 3, g = 0.42, 95% CI = 0.17-0.67), all small-to-medium effects. A Q test indicated that sensory induction method did not account for significant heterogeneity in the resulting placebo effect sizes (Q(3) = 3.78, P = 0.28). For studies in the observational learning meta-analysis, thermal stimulation was the most commonly used method for inducing pain with a small effect size (k = 5, g = 0.25, 95% CI = 0.12-0.39) and the only subgroup to meet the k = 3 threshold. In the itch VS meta-analysis, itch was most often induced with histamine (k = 6, g = 0.15, 95% CI = −0.10 to 0.41), yielding a small effect size, whereas one study used electrical stimulation. With only 1 subgroup meeting the k = 3 threshold, no Q test was conducted.
3.4.2. Placebo treatment
From all included pain studies in the CC + VS meta-analysis, inert gels, creams, and lotions applied on the skin where the pain stimuli would later be administered were the most common form of placebo treatment (k = 44). Studies using this form of sham treatment had an average placebo effect size of g = 0.40, 95% CI = 0.36 to 0.45. This treatment was followed by sham electrodes and transcutaneous electric nerve stimulation (TENS) devices (k = 18), which yielded a larger average placebo effect across studies (g = 0.67, 95% CI = 0.59-0.74). Other placebo treatments (pill, injection, nasal spray, acupuncture) did not meet the k ≥ 3 group size threshold. A Q test indicated that the type of placebo intervention accounted for significant heterogeneity in the resulting placebo effect sizes (Q(2) = 14.05, P = 0.007). From pain studies included in the VS-alone meta-analysis, inert gel was again the most common treatment (k = 17) with an average placebo effect size of g = 0.34 (95% CI = 0.26 to 0.41). Placebo pills were the next most commonly used treatment (k = 9, g = 0.29, 95% CI = 0.17-0.41). Sham electrodes and TENS devices (k = 6) again yielded a larger effect than inert gels (g = 0.47, 95% CI = 0.32-0.63) but with overlapping confidence intervals. A Q test indicated that the type of placebo intervention did not account for significant heterogeneity in the resulting placebo effect sizes (Q(3) = 1.35, P = 0.51). From pain studies included in the observational learning meta-analysis, inert gel was the most common treatment (k = 5) with an average placebo effect size of g = 0.25 (95% CI = 0.12 to 0.39), and the only treatment to meet the k = 3 threshold. From itch studies included in the VS-alone meta-analysis, inert gel was the most common treatment (k = 3) with a small effect size of g = 0.22 (95% CI = 0.03 to 0.42) and the only treatment to meet the k = 3 threshold.
3.4.3. Length of acquisition and evocation phases
From the 68 arms included in the pain CC + VS meta-analysis, 65 reported how many pain stimulus trials were used in the acquisition phase. The mean number of pain stimulus trials used in an acquisition phase for a CC + VS paradigm was 26 (SD = 18.70) and ranged from 2 to 112 placebo and control trials summed. Meta-regression indicated that the length of the acquisition phase did not explain the heterogeneity in resulting placebo effect sizes (Q = 1.94, P = 0.16). Similarly, 66 included studies reported how many pain stimulus trials were used in the evocation phase. The mean number of pain stimulus trials used in an evocation phase for a CC + VS paradigm was 21 (SD = 15.40) and ranged from 2 to 60 trials (placebo and control trials summed). In studies that calculated the placebo effect with the mean pain values from the entire evocation phase (k = 62), the length of the evocation phase did not explain the heterogeneity in resulting placebo effect sizes (Q = 1.89, P = 0.17).
3.4.4. Difference in placebo and control stimulus intensity during acquisition
From the 68 arms included in the pain CC + VS meta-analysis, 49 used individually calibrated pain intensities for placebo and control stimuli during the acquisition phase. On a 0 to 100 pain intensity scale, the mean calibrated difference in placebo and control stimuli was 36 (SD = 8.8) with differences ranging from 20 to 60 points. The difference in calibrated pain intensity for placebo and control acquisition trials did not explain the heterogeneity in resulting placebo effect sizes (Q = 0.70, P = 0.40).
3.4.5. Sex of participant samples
From the 68 arms included in the pain CC + VS meta-analysis, 67 reported the sex distribution of their sample. Sex distributions ranged from 0% to 100% female, with a mean of 54% female identified (SD = 25.4). Participant sex was not found to explain the heterogeneity in placebo effect sizes for the pain CC + VS studies (Q = 0.19, P = 0.66). From the 39 arms included in the pain VS-alone meta-analysis, 37 reported the sex distribution of their sample. Sex distributions ranged from 0% to 100% females with a mean of 59.5% females identified (SD = 27.0). Participant sex was not found to explain the heterogeneity in placebo effect sizes for the pain VS-alone studies (Q = 0.00, P = 0.95). From the 7 arms included in the observational learning pain meta-analysis, each study reported sex distribution of the sample. Sex distribution ranged from 48% to 100% females with a mean of 69.8% females identified (SD = 21.4) Meta regression indicated that sex distribution of the sample could explain heterogeneity in placebo effect sizes, with a higher percentage of female participants corresponding to larger placebo effects (Q = 8.34, P = 0.004). This finding should be interpreted with caution given the small sample of studies it was derived from. From the 7 arms included in the itch VS-alone meta-analysis, each study reported sex distribution of the sample. Sex distribution ranged from 71% to 100% females with a mean of 84.3% females identified (SD = 11.4). Participant sex was not found to explain the heterogeneity in placebo effect sizes for the observational learning pain studies (Q = 0.50, P = 0.48).
3.4.6. Age of participant samples
From the 68 arms included in the pain CC + VS meta-analysis, 58 reported the mean age. Mean age ranged from 19.3 to 49.0, with a mean across arms of 25.4 years (SD = 5.0). Participant age was not found to explain the heterogeneity in placebo effect sizes for the pain CC + VS studies (Q = 1.14, P = 0.28). From the 39 arms included in the pain VS-alone meta-analysis, 30 reported the mean age. Mean age ranged from 19.5 to 32.0 years with a mean age across arms of 23.8 years (SD = 3.2). Participant age was not found to explain the heterogeneity in placebo effect sizes for the pain VS-alone studies (Q = 0.14, P = 0.70). From the 7 arms included in the observational learning pain meta-analysis, 4 studies reported mean age of the sample. Mean age ranged from 22.6 to 28.5 with a mean across arms of 25.3 years (SD = 2.7). Participant age was not found to explain heterogeneity in placebo effect sizes for pain observational studies (Q = 0.37, P = 0.55). From the 7 arms included in the itch VS-alone meta-analysis, each study reported the mean age of the sample. Mean age ranged from 21.3 to 23.2 years with a mean across arms of 22.1 years (SD = 0.6). Participant age was not found to explain heterogeneity in placebo effect sizes for itch VS-alone studies (Q = 0.20, P = 0.65).
3.4.7. Risk of bias
Across included studies, risk of methodological bias measured with the Marcuzzi risk of bias tool for quantitative sensory testing91 was found to be low overall (Tables 1–4). In the pain CC + VS meta-analysis, a relationship between risk of bias cores and placebo effect size was detected (Q = 4.37, P = 0.04), such that higher risk of bias scores corresponded with larger placebo effect sizes. In the VS-alone pain meta-analysis, no relation between risk of bias scores and placebo effects was detected (Q = 0.19, P = 0.66). Similarly, no relationship was detected for observational learning pain studies (Q = 0.22, P = 0.64) or for VS-alone itch studies (Q = 0.00, P = 0.98).
4. Discussion
Our systematic review and meta-analysis of experimentally induced placebo effects on cutaneous pain and itch in healthy participants primarily investigated how different learning mechanisms (classical conditioning, verbal suggestion, observational learning) contributed to placebo effect magnitudes. Additionally, we explored whether sensation induction method (thermal pain, electrical pain, etc), type of placebo treatment (inert gel, electrodes, etc), number of acquisition or evocation trials in a classical conditioning paradigm, calibrated intensity between placebo and control stimuli, and sex and age of the sample can impact the magnitude of placebo effects on cutaneous pain and itch. With 108 included studies from 1996 to 2021, this review offers a comprehensive and systematic assessment of 25 years of experimental placebo research in healthy human participants.
The primary meta-analyses indicated that conditioning with verbal suggestion induced placebo effects on pain (k = 68, g = 0.59) of a medium effect size. This is larger than for verbal suggestion alone (k = 39, g = 0.38), underscoring the premise that when attempting to harness positive treatment expectations to yield better treatment outcomes, diverse and recurrent learning processes will be most effective. However, this estimate is notably smaller than the large pooled effect size found in a previous meta-analysis of experimental placebo analgesia studies published between 2002 and 2007 (d = 1.00), in which effects from studies using different learning processes and patient samples were analyzed together. It appears that the studies included in the current meta-analysis reported smaller placebo effects on average. This may be a product of better scientific practices like preregistration or open science reducing the preponderance of biased results or better experimental design yielding more accurate results. Then again, the trim-and-fill results suggested a bias in these findings, with a likelihood of missing or unreported studies with underwhelming results. Taken with the positive correlation between risk of bias scores and effect sizes in this meta-analysis, the potential for biased methodology, analysis, and reporting, at least in some studies, cannot be ruled out. However, risk of bias scores were generally low and with a sample of 68 studies from numerous laboratories, several biased results or unpublished null findings are not likely to have a major impact on the overall placebo effect size.
Our meta-analysis of verbal suggestion alone studies yielded a smaller placebo effect size on pain (k = 39, g = 0.38) than for verbal suggestion with conditioning, replicating previous findings from several individual experiments and a related meta-analysis of nocebo effects.28,34,36,111 Verbal suggestion studies typically measure placebo effects across a much smaller number of stimuli than conditioning studies, making a comparison between the 2 methods difficult when taking into account the potential for extinction over numerous stimuli. It is likely that besides being smaller than a placebo effect induced with a classical conditioning paradigm, effects induced with verbal suggestion alone may also be more rapidly extinguished without reinforcement. Although we found that placebo effects on pain for studies using observational learning (k = 7, g = 0.57) were comparable in magnitude to those induced with classical conditioning combined with verbal suggestion, the somewhat small sample size of included studies and lack of specific search terms for observational learning, indicating possible missing studies, may skew the results.
The pooled effect size for placebo effects on itch induced with verbal suggestion (k = 7, g = 0.14) was notably smaller than the pooled effect size for placebo effects on pain induced with verbal suggestion (k = 39, g = 0.38), although with overlapping confidence intervals. Although there were not enough studies to make meaningful comparisons between learning methods for itch across studies, within one study that compared classical conditioning with verbal suggestion to verbal suggestion alone for placebo effects on itch found the same pattern of results as seen in pain.12 In contrast to a study in which placebo effects on pain and itch were directly compared,140 verbal suggestion for placebo effects on itch appears to yield smaller effects relative to those on pain. Itch induction methods are generally less precise than those for pain,15 which may make it more challenging for itch studies to reliably induce sensations at a consistent intensity, but it is not clear if that explains the smaller effect size. The underlying biological mechanisms of these effects may also contribute to this difference. Although placebo effects on pain appear to be substantiated in part by endogenous opioids,129 demonstrated by the blockade of placebo effects by opioid antagonists,1,14 itch appears to increase following administration of opioid agonists.75 If placebo effects for other sensations like itch also cooccur with endogenous opioid release, this could explain their smaller effect size relative to placebo effects on pain, as the opioid release blunts the antipruritic placebo effect. However, it remains an open question whether placebo effects on itch are substantiated by endogenous opioids in the same way that placebo effects on pain are. Pathways specific to the itch induction method (eg, histaminergic) could instead play the role that opioid pathways play for pain.
For sensation induction methods in pain, across both classical conditioning with verbal suggestion and verbal suggestion–alone analyses, studies using laser and electrical pain induced larger placebo effects than the more commonly used thermal pain; however, the 95% confidence intervals for these effect size estimates do overlap. Laser and electrical pain stimuli are both generally much shorter in duration than thermal stimuli, with durations typically less than 300-millisecond long103,117 and thermal stimuli typically 5- to 15-second long.48,55 It is possible that the intensity of such short stimuli are harder for the participant to assess, allowing more room for the expectancies thought to underlie placebo effects to shape the pain experience. According to predictive coding models, these short and less precisely experienced stimuli could give way to a larger effect of prior expectations on the sensory experience.18 Alternatively, thermal pain may be more familiar to participants, and familiar sensations could be easier to assess and less influenced by expectancies.61 Cold pressor, another long duration and potentially familiar pain stimulus, typically endured for 1 to 3 minutes109,128 also yielded smaller placebo effects than laser and electrical pain, in line with this supposition.
Regarding the type of placebo treatment, studies that made use of sham electrodes induced larger placebo effects than those using topical gels or creams in the classical conditioning with verbal suggestion pain meta-analysis. A similar pattern was observed in the VS-alone pain meta-analysis, albeit with overlapping 95% confidence intervals. Speculatively, electrodes may have been seen as a more convincing treatment for pain than gels and ointments in the eyes of participants, contributing to greater perceived pain reductions. One study that investigated expectations for pain relief from various forms of treatment found that topical treatments like gels and creams were viewed as less effective than oral or injected treatments, but electrical stimulation was not included as a treatment in this survey.108 Some studies (eg, Colagiuri and Quinn,27 Barnes et al.11) allowed participants to feel several electrical or tactile stimuli through the electrodes before surreptitiously deactivating them, which may enhance the believability of the placebo treatment.
The other planned subgroup analyses on methodological factors did not explain differences in the magnitudes of placebo effects between the studies. We expected the number of trials used in either the acquisition or evocation phases of a classical conditioning paradigm to play a role in shaping the magnitude of placebo effects. More trials during acquisition could be expected to produce a stronger effect and one that is more robust to extinction.33,124 More trials during evocation, inversely, could dilute the effect when taking a mean across all trials because this would allow more time for extinction to take place. Instead, we found no evidence that either metric was related to placebo magnitudes. However, studies that had longer acquisition phases often had longer evocation phases as well, so the two may have cancelled each other out. If future studies were to report or make available data at a trial-by-trial level instead of only the mean of the acquisition or evocation phase, a more nuanced understanding of acquisition and extinction process for placebo effects could be realized. Although a larger calibrated difference in placebo and control stimulus intensities during acquisition could be expected to induce a larger placebo effect at evocation,63 our results did not support this notion. In a predictive coding framework, larger differences between placebo and control stimuli during acquisition should induce expectations for greater reductions in pain, allowing for larger placebo effects, within a certain limit.18 Too large of a difference between the expected and actual intensity of a pain stimulus could diminish the effect of expectation on pain perception.71,107 However, we saw no evidence for this in our results.
Regarding our post hoc investigation of possible moderating effects of sample sex and age, we did not observe any significant relationships between either demographic variable and placebo effects on pain or itch. Two recent systematic reviews of sex differences in experimental placebo and nocebo studies that compared placebo responses by females with those of males as part of their planned analyses found evidence that men show stronger placebo effects on pain than women, particularly when only verbal suggestions are used.52,138 However, this effect was not replicated by our meta-regression, which was not restricted to studies that reported on tests of sex differences in placebo effects. Although sex differences have not been explored for placebo effects on itch, one study to investigate differences for nocebo effects on itch found no effect of sex.130 The relationship between participant age and placebo effects on pain or itch has not been explored across the adult lifespan. Some recent studies have compared placebo effects between child and adult samples152,155 but not between different stages of adulthood. The samples included in these meta-analyses skewed strongly toward young adults in their late teens to mid-20s, limiting an exploration of placebo effects in older adult and geriatric populations. Given the elderly's increased utilization of health care, a better understanding of placebo effects and treatment expectancies in this population could be especially useful, yielding clinically relevant outcomes like improved treatment efficacy through psychological interventions on top of routine care.
Finally, risk of bias measured with the Marcuzzi risk of bias tool was checked as a possible predictor of placebo magnitudes. Generally, studies in this review gave more than sufficient detail on their samples, methods, and results, with low risk of bias overall. Although there was a relationship between larger effect sizes reported in studies with a greater risk of bias in the CC + VS pain meta-analysis, there was a skew toward nearly all studies having a low risk of bias. Furthermore, it should be noted that the Marcuzzi risk of bias tool, whereas specifically designed to assess risk of bias in quantitative sensory testing research, did not measure potential sources of bias such as random allocation of participants into conditions, and it may be useful to augment the tool with the Cochrane risk of bias tool in future work.69
Although this review advances our grasp of the mechanisms underlying placebo effects, considerable heterogeneity in the results remains unexplained. Other variables that may explain some of the heterogeneity, but were not investigated in the current meta-analysis, include demographic characteristics, the inclusion and exclusion criteria used to screen participants, individual differences, and methods unique to individual laboratory results. Regarding demographic characteristics, samples may vary in what proportion of participants are university students, particularly psychology or medical students, and such participants may be more skeptical of placebo manipulations than nonuniversity populations. Considering inclusion and exclusion criteria, and methods specific to individual laboratory results, these potential effects are harder to ascertain because they are not always clearly reported. For example, listing current physical or mental illness as an exclusion criterion is common practice in placebo research, but how these illnesses are screened for (eg, self-report, diagnostic interview or tests) may vary considerably between laboratory results and experiments. Similarly, other laboratory and experimenter effects (eg, demeanor of the experimenter, if the laboratory is set in a hospital vs an academic building, setup of the laboratory, etc.) are often not clearly documented or reported and may contribute to some of the heterogeneity in the placebo effect magnitudes reported here.
Future research, both systematic and at the level of individual studies, would do well to measure the variables described in the previous section, when possible. Similarly, future experimental work on placebo effects for cutaneous sensations could strengthen the field by attempting to replicate and expand on the few findings related to how variables such as gender, number of acquisition or evocation trials in classical conditioning paradigms, and calibrated difference between placebo and control stimuli can moderate placebo effects. In addition, methodological factors that were not investigated in this review, such as the intensity of pain or itch stimuli during the evocation phase, and differences between other pain modalities (eg, ischemic, visceral) could also be explored systematically and in individual experiments to build a more complete understanding of the factors that shape placebo effects. Much heterogeneity in placebo effect magnitudes remains unexplained, and investigating nonlinear interactions between experimental manipulations and individual differences may yield new insights, as indicated by the work of Hird et al.71 on boundary effects. Greater adoption of open science practices like the sharing of datasets, protocols, and other materials would also strengthen the field as a whole, allowing, amongst others, for more in-depth meta-analyses and reviews.
To conclude, in this systematic review and meta-analysis, we demonstrate the robust occurrence of substantial placebo effects on cutaneous pain and itch following various learning processes. We replicated previous findings showing that the size of these effects depends on the learning process used to induce them, with a combination of classical conditioning with verbal suggestion for placebo effects on pain induced a medium-sized effect, larger than the small effect seen from verbal suggestion alone. Verbal suggestions for placebo effects on itch appeared to induce smaller effects than those seen in pain, and more research is needed to understand this difference. Although differences in methodology and sample demographics generally did not seem to impact the placebo effect, consideration related to the believability of the experimental procedure, such as the type of sensation induction and placebo treatment used, may boost the efficacy of learning mechanisms used to induce these effects. The lack of influence from other methodological and demographic factors highlights how robust placebo effects are, suggesting that the learning mechanisms underlying these effects may be applied in settings outside the laboratoery to enhance our sensory experience.
Conflict of interest statement
The authors have no conflict of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B752.
Acknowledgements
The authors thank Barclay Wohlstetter and Elif Sena Uyanik for their help during the search and selection of articles. This work was supported by a VICI grant from the Netherlands Organization for Scientific Research awarded to A.W.M. Evers (NWO; 45316004).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Contributor Information
Mia A. Thomaidou, Email: a.m.thomaidou@fsw.leidenuniv.nl.
Kaya J. Peerdeman, Email: k.j.peerdeman@fsw.leidenuniv.nl.
Antoinette I.M. van Laarhoven, Email: a.vanlaarhoven@fsw.leidenuniv.nl.
Myrthe M.E. van Schothorst, Email: myrthevanschothorst@gmail.com.
Dieuwke S. Veldhuijzen, Email: d.s.veldhuijzen@fsw.leidenuniv.nl.
References
- [1].Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 1999;19:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anzelc M, Burkhart CG. Pain and pruritus: a study of their similarities and differences. Int J Dermatol 2020;59:159–64. [DOI] [PubMed] [Google Scholar]
- [3].Aslaksen PM, Åsli O, Øvervoll M, Bjørkedal E. Nocebo hyperalgesia and the startle response. Neuroscience 2016;339:599–607. [DOI] [PubMed] [Google Scholar]
- [4].Aslaksen PM, Flaten MA. The roles of physiological and subjective stress in the effectiveness of a placebo on experimentally induced pain. Psychosomatic Med 2008;70:811–8. [DOI] [PubMed] [Google Scholar]
- [5].Aslaksen PM, Lyby PS. Fear of pain potentiates nocebo hyperalgesia. J Pain Res 2015;8:703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aslaksen PM, Zwarg ML, Eilertsen H-IH, Gorecka MM, Bjørkedal E. Opposite effects of the same drug: reversal of topical analgesia by nocebo information. PAIN 2015;156:39–46. [DOI] [PubMed] [Google Scholar]
- [7].Au Yeung ST, Colagiuri B, Lovibond PF, Colloca L. Partial reinforcement, extinction, and placebo analgesia. PAIN 2014;155:1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bąbel P. Classical conditioning as a distinct mechanism of placebo effects. Front Psychiatry 2019;10:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bąbel P, Adamczyk W, Świder K, Bajcar EA, Kicman P, Lisińska N. How classical conditioning shapes placebo analgesia: hidden versus open conditioning. Pain Med 2018;19:1156–69. [DOI] [PubMed] [Google Scholar]
- [10].Bajcar EA, Bąbel P. How does observational learning produce placebo effects? A model integrating research findings. Front Psychol 2018;9:2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barnes K, Rottman BM, Colagiuri B. The placebo effect: to explore or to exploit? Cognition 2021;214:104753. [DOI] [PubMed] [Google Scholar]
- [12].Bartels DJP, van Laarhoven AIM, Haverkamp EA, Wilder-Smith OH, Donders ART, van Middendorp H, van de Kerkhof PCM, Evers AWM. Role of conditioning and verbal suggestion in placebo and nocebo effects on itch. PLoS One 2014;9:e91727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bartels DJP, van Laarhoven AIM, van de Kerkhof PCM, Evers AWM. Placebo and nocebo effects on itch: effects, mechanisms, and predictors. Eur J Pain 2016;20:8–13. [DOI] [PubMed] [Google Scholar]
- [14].Benedetti F. The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. PAIN 1996;64:535–43. [DOI] [PubMed] [Google Scholar]
- [15].Blythe JS, Peerdeman KJ, Veldhuijzen DS, van Laarhoven AIM, Evers AWM. Placebo and nocebo effects on itch: a review of experimental methods. Itch 2019;4:e27. [Google Scholar]
- [16].Brączyk J, Bąbel P. The role of the observers' perception of a model's self-confidence in observationally induced placebo analgesia. J Pain 2021;22:1672–80. [DOI] [PubMed] [Google Scholar]
- [17].Brown JA, Fowler SL, Rasinski HM, Rose JP, Geers AL. Choice as a moderator of placebo expectation effects: additional support from two experiments. Basic Appl Soc Psychol 2013;35:436–44. [Google Scholar]
- [18].Büchel C, Geuter S, Sprenger C, Eippert F. Placebo analgesia: a predictive coding perspective. Neuron 2014;81:1223–39. [DOI] [PubMed] [Google Scholar]
- [19].Camerone EM, Piedimonte A, Testa M, Wiech K, Vase L, Zamfira DA, Benedetti F, Carlino E. The effect of temporal information on placebo analgesia and nocebo hyperalgesia. Psychosom Med 2021;83:43–50. [DOI] [PubMed] [Google Scholar]
- [20].Carlino E, Torta DME, Piedimonte A, Frisaldi E, Vighetti S, Benedetti F. Role of explicit verbal information in conditioned analgesia. Eur J Pain 2015;19:546–53. [DOI] [PubMed] [Google Scholar]
- [21].Case LK, Laubacher CM, Richards EA, Grossman M, Atlas LY, Parker S, Bushnell MC. Is placebo analgesia for heat pain a sensory effect? An exploratory study on minimizing the influence of response bias. Neurobiol Pain 2019;5:100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen P-HA, Cheong JH, Jolly E, Elhence H, Wager TD, Chang LJ. Socially transmitted placebo effects. Nat Hum Behav 2019;3:1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Choi JC, Yi D-J, Han BS, Lee PH, Kim JH, Kim B-H. Placebo effects on analgesia related to testosterone and premotor activation. NeuroReport 2011;22:419–23. [DOI] [PubMed] [Google Scholar]
- [24].Chouchou F, Chauny J-M, Rainville P, Lavigne GJ. Selective REM sleep deprivation improves expectation-related placebo analgesia. PLoS One 2015;10:e0144992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–29. [Google Scholar]
- [26].Cohen J. Statistical power analysis for the behavioral sciences. New York, NY: Routledge, 2013. [Google Scholar]
- [27].Colagiuri B, Quinn VF. Autonomic arousal as a mechanism of the persistence of nocebo hyperalgesia. J Pain 2018;19:476–86. [DOI] [PubMed] [Google Scholar]
- [28].Colloca L. Placebo, nocebo, and learning mechanisms. In: Benedetti F, Enck P, Frisaldi E, Schedlowski M, editors. Placebo. Berlin, Heidelberg: Springer Berlin Heidelberg, 2014. p. 17–35. [Google Scholar]
- [29].Colloca L, Akintola T, Haycock NR, Blasini M, Thomas S, Phillips J, Corsi N, Schenk LA, Wang Y. Prior therapeutic experiences, not expectation ratings, predict placebo effects: an experimental study in chronic pain and healthy participants. Psychother Psychosom 2020;89:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Colloca L, Benedetti F. How prior experience shapes placebo analgesia. PAIN 2006;124:126–33. [DOI] [PubMed] [Google Scholar]
- [31].Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. PAIN 2009;144:28–34. [DOI] [PubMed] [Google Scholar]
- [32].Colloca L, Miller FG. How placebo responses are formed: a learning perspective. Philos Trans R Soc B Biol Sci 2011;366:1859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. How the number of learning trials affects placebo and nocebo responses. PAIN 2010;151:430–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. PAIN 2008;136:211–18. [DOI] [PubMed] [Google Scholar]
- [35].Colloca L, Tinazzi M, Recchia S, Le Pera D, Fiaschi A, Benedetti F, Valeriani M. Learning potentiates neurophysiological and behavioral placebo analgesic responses. PAIN 2008;139:306–14. [DOI] [PubMed] [Google Scholar]
- [36].Colloca L, Wang Y, Martinez PE, Chang Y-PC, Ryan KA, Hodgkinson C, Goldman D, Dorsey SG. OPRM1 rs1799971, COMT rs4680, and FAAH rs324420 genes interact with placebo procedures to induce hypoalgesia. PAIN 2019;160:1824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Corsi N, Colloca L. Placebo and nocebo effects: the advantage of measuring expectations and psychological factors. Front Psychol 2017;8:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Darragh M, Chang JWH, Booth RJ, Consedine NS. The placebo effect in inflammatory skin reactions: the influence of verbal suggestion on itch and weal size. J Psychosom Res 2015;78:489–94. [DOI] [PubMed] [Google Scholar]
- [39].de Jong PJ, Baast R, Arntz A, Merckelbach H. The placebo effect in pain reduction: the influence of conditioning experiences and response expectancies. Int J Behav Med 1996;3:14–29. [DOI] [PubMed] [Google Scholar]
- [40].De Pascalis V, Chiaradia C, Carotenuto E. The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. PAIN 2002;96:393–402. [DOI] [PubMed] [Google Scholar]
- [41].De Pascalis V, Scacchia P. Personality and placebo analgesia during cold stimulation in women: a Low-Resolution Brain Electromagnetic Tomography (LORETA) analysis of startle ERPs. Personal Individual Differences 2017;118:64–70. [Google Scholar]
- [42].De Pascalis V, Scacchia P. The influence of reward sensitivity, heart rate dynamics and EEG-delta activity on placebo analgesia. Behav Brain Res 2019;359:320–32. [DOI] [PubMed] [Google Scholar]
- [43].De Pascalis V, Scacchia P, Vecchio A. Influences of hypnotic suggestibility, contextual factors, and EEG alpha on placebo analgesia. Am J Clin Hypnosis 2021;63:302–28. [DOI] [PubMed] [Google Scholar]
- [44].Deeks JJ, Higgins JPT, Altman DG; on behalf of the Cochrane Statistical Methods Group. Analysing data and undertaking meta-analyses. Cochrane Handbook Syst Rev Interv 2019:241–84. [Google Scholar]
- [45].Disley N, Kola-Palmer S, Retzler C. A comparison of open-label and deceptive placebo analgesia in a healthy sample. J Psychosom Res 2021;140:110298. [DOI] [PubMed] [Google Scholar]
- [46].Dolgin E. Fluctuating baseline pain implicated in failure of clinical trials. Nat Med 2010;16:1053. [DOI] [PubMed] [Google Scholar]
- [47].Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [48].Egorova N, Benedetti F, Gollub RL, Kong J. Between placebo and nocebo: response to control treatment is mediated by amygdala activity and connectivity. Eur J Pain 2020;24:580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 2009;63:533–43. [DOI] [PubMed] [Google Scholar]
- [50].Eippert F, Finsterbusch J, Bingel U, Büchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science 2009;326:404. [DOI] [PubMed] [Google Scholar]
- [51].Ellingsen D-M, Wessberg J, Eikemo M, Liljencrantz J, Endestad T, Olausson H, Leknes S. Placebo improves pleasure and pain through opposite modulation of sensory processing. Proc Natl Acad Sci 2013;110:17993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Enck P, Klosterhalfen S. Does sex/gender play a role in placebo and nocebo effects? Conflicting evidence from clinical trials and experimental studies. Front Neurosci 2019;13:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Evers AWM, Bartels DJP, van Laarhoven AIM. Placebo and nocebo effects in itch and pain. In: Benedetti F, Enck P, Frisaldi E, Schedlowski M, editors. Placebo. Berlin, Heidelberg: Springer Berlin Heidelberg, 2014. p. 205–14. [Google Scholar]
- [54].Fehse K, Maikowski L, Simmank F, Gutyrchik E, Meissner K. Placebo responses to original vs generic ASA brands during exposure to noxious heat: a pilot fMRI study of neurofunctional correlates. Pain Med 2015;16:1967–74. [DOI] [PubMed] [Google Scholar]
- [55].Feldhaus MH, Horing B, Sprenger C, Büchel C. Association of nocebo hyperalgesia and basic somatosensory characteristics in a large cohort. Sci Rep 2021;11:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet 2010;375:686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Flaten MA, Bjørkedal E, Lyby PS, Figenschau Y, Aslaksen PM. Failure to find a conditioned placebo analgesic response. Front Psychol 2018;9:1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Frangos E, Čeko M, Wang B, Richards EA, Gracely JL, Colloca L, Schweinhardt P, Bushnell MC. Neural effects of placebo analgesia in fibromyalgia patients and healthy individuals. PAIN 2021;162:641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Freeman S, Yu R, Egorova N, Chen X, Kirsch I, Claggett B, Kaptchuk TJ, Gollub RL, Kong J. Distinct neural representations of placebo and nocebo effects. NeuroImage 2015;112:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gaab J, Bürgin D, Locher C, Werner C, Urech S, Bratschi C, Garcia LB, Hauke M, Bitter S, Bohny M, Bentz D. Endogenous cortisol and conditioned placebo effects on pain—a randomized trial. J Psychosom Res 2019;123:109739. [DOI] [PubMed] [Google Scholar]
- [61].Geers AL, Rose JP, Fowler SL, Brown JA. Patient involvement in treatment decision making can help or hinder placebo analgesia. Z für Psychol 2014;222:165–70. [Google Scholar]
- [62].Geisler M, Herbsleb M, Bär K-J, Weiss T. Dissociation of endogenous pain inhibition due to conditioned pain modulation and placebo in male athletes versus nonathletes. Front Psychol 2020;11:553530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Geuter S, Eippert F, Hindi Attar C, Büchel C. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage 2013;67:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gniß S, Kappesser J, Hermann C. Placebo effect in children: the role of expectation and learning. PAIN 2020;161:1191–1201. [DOI] [PubMed] [Google Scholar]
- [65].Grahl A, Onat S, Büchel C. The periaqueductal gray and Bayesian integration in placebo analgesia. eLife 2018;7:e32930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hartmann H, Rütgen M, Riva F, Lamm C. Another's pain in my brain: no evidence that placebo analgesia affects the sensory-discriminative component in empathy for pain. Neuroimage 2021;224:117397. [DOI] [PubMed] [Google Scholar]
- [67].Hedges LV. Distribution theory for glass's estimator of effect size and related estimators. J Educ Stat 1981;6:107–28. [Google Scholar]
- [68].Hedges LV. Estimation of effect size from a series of independent experiments. Psychol Bull 1982;92:490–99. [Google Scholar]
- [69].Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [71].Hird EJ, Charalambous C, El-Deredy W, Jones AKP, Talmi D. Boundary effects of expectation in human pain perception. Sci Rep 2019;9:9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Horing B, Beadle SC, Inks Z, Robb A, Muth ER, Babu SV. A virtual experimenter does not increase placebo hypoalgesia when delivering an interactive expectancy manipulation. Sci Rep 2020;10:20353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Huneke NTM, Brown CA, Burford E, Watson A, Trujillo-Barreto NJ, El-Deredy W, Jones AKP. Experimental placebo analgesia changes resting-state alpha oscillations. PLoS One 2013;8:e78278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hunter T, Siess F, Colloca L. Socially induced placebo analgesia: a comparison of a pre-recorded versus live face-to-face observation. Eur J Pain 2014;18:914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci 2006;7:535–47. [DOI] [PubMed] [Google Scholar]
- [76].Jarcho JM, Feier NA, Labus JS, Naliboff B, Smith SR, Hong J-Y, Colloca L, Tillisch K, Mandelkern MA, Mayer EA, London ED. Placebo analgesia: self-report measures and preliminary evidence of cortical dopamine release associated with placebo response. Neuroimage Clin 2016;10:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Johnson M, Din A. Ethnocultural differences in the analgesic effects of placebo transcutaneous electrical nerve stimulation on cold-induced pain in healthy subjects: a preliminary study. Compl Therap Med 1997;5:74–79. [Google Scholar]
- [78].Kirsch I, Kong J, Sadler P, Spaeth R, Cook A, Kaptchuk TJ, Gollub R. Expectancy and conditioning in placebo analgesia: separate or connected processes? Psychol Conscious Theor Res Pract 2014;1:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Klinger R, Soost S, Flor H, Worm M. Classical conditioning and expectancy in placebo hypoalgesia: a randomized controlled study in patients with atopic dermatitis and persons with healthy skin. PAIN 2007;128:31–9. [DOI] [PubMed] [Google Scholar]
- [80].Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci 2006;26:381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kong J, Spaeth R, Cook A, Kirsch I, Claggett B, Vangel M, Gollub RL, Smoller JW, Kaptchuk TJ. Are all placebo effects equal? Placebo pills, sham acupuncture, cue conditioning and their association. PLoS One 2013;8:e67485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kube T, Rief W, Vivell M-B, Schäfer NL, Vermillion T, Körfer K, Glombiewski JA. Deceptive and nondeceptive placebos to reduce pain: an experimental study in healthy individuals. Clin J Pain 2020;36:68–79. [DOI] [PubMed] [Google Scholar]
- [83].Laverdure-Dupont D, Rainville P, Montplaisir J, Lavigne G. Changes in rapid eye movement sleep associated with placebo-induced expectations and analgesia. J Neurosci 2009;29:11745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lee Y-S, Jung W-M, Bingel U, Chae Y. The context of values in pain control: understanding the price effect in placebo analgesia. J Pain 2020;21:781–9. [DOI] [PubMed] [Google Scholar]
- [85].Linde K, Fässler M, Meissner K. Placebo interventions, placebo effects and clinical practice. Philos Trans R Soc B Biol Sci 2011;366:1905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Locher C, Frey Nascimento A, Kirsch I, Kossowsky J, Meyer A, Gaab J. Is the rationale more important than deception? A randomized controlled trial of open-label placebo analgesia. PAIN 2017;158:2320–28. [DOI] [PubMed] [Google Scholar]
- [87].Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro CA. Neural bases of conditioned placebo analgesia. PAIN 2010;151:816–24. [DOI] [PubMed] [Google Scholar]
- [88].Lyby PS, Aslaksen PM, Flaten MA. Is fear of pain related to placebo analgesia? J Psychosom Res 2010;68:369–77. [DOI] [PubMed] [Google Scholar]
- [89].Lyby PS, Aslaksen PM, Flaten MA. Variability in placebo analgesia and the role of fear of pain—an ERP study. PAIN 2011;152:2405–412. [DOI] [PubMed] [Google Scholar]
- [90].Lyby PS, Forsberg JT, Åsli O, Flaten MA. Induced fear reduces the effectiveness of a placebo intervention on pain. PAIN 2012;153:1114–121. [DOI] [PubMed] [Google Scholar]
- [91].Marcuzzi A, Dean CM, Wrigley PJ, Hush JM. Early changes in somatosensory function in spinal pain: a systematic review and meta-analysis. PAIN 2015;156:203–14. [DOI] [PubMed] [Google Scholar]
- [92].Martin-Pichora AL, Mankovsky-Arnold TD, Katz J. Implicit versus explicit associative learning and experimentally induced placebo hypoalgesia. J Pain Res 2011;4:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Martin AL, Katz J. Inclusion of authorized deception in the informed consent process does not affect the magnitude of the placebo effect for experimentally induced pain. PAIN 2010;149:208–15. [DOI] [PubMed] [Google Scholar]
- [94].Martini M, Lee MCH, Valentini E, Iannetti GD. Intracortical modulation, and not spinal inhibition, mediates placebo analgesia. Eur J Neurosci 2015;41:498–504. [DOI] [PubMed] [Google Scholar]
- [95].Matre D, Casey KL, Knardahl S. Placebo-induced changes in spinal cord pain processing. J Neurosci 2006;26:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Meeuwis SH, van Middendorp H, Lavrijsen APM, Veldhuijzen DS, Evers AWM. Open- and closed-label placebo and nocebo suggestions about a sham transdermal patch. Psychosom Med 2021;83:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Meeuwis SH, van Middendorp H, van Laarhoven AIM, Veldhuijzen DS, Lavrijsen APM, Evers AWM. Effects of open- and closed-label nocebo and placebo suggestions on itch and itch expectations. Front Psychiatry 2019;10:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Meissner K, Bingel U, Colloca L, Wager TD, Watson A, Flaten MA. The placebo effect: advances from different methodological approaches. J Neurosci 2011;31:16117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Milling LS. Response expectancies: a psychological mechanism of suggested and placebo analgesia. Contemp Hypnosis 2009;26:93–110. [Google Scholar]
- [100].Misery L, Dutray S, Chastaing M, Schollhammer M, Consoli SG, Consoli SM. Psychogenic itch. Translat Psychiatr 2018;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Montgomery G, Kirsch I. Mechanisms of placebo pain reduction: an empirical investigation. Psychol Sci 1996;7:174–6. [Google Scholar]
- [102].Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. PAIN 1997;72:107–113. [DOI] [PubMed] [Google Scholar]
- [103].Morton DL, El-Deredy W, Watson A, Jones AKP. Placebo analgesia as a case of a cognitive style driven by prior expectation. Brain Res 2010;1359:137–41. [DOI] [PubMed] [Google Scholar]
- [104].Nemoto H, Nemoto Y, Toda H, Mikuni M, Fukuyama H. Placebo analgesia: a PET study. Exp Brain Res 2007;179:655–64. [DOI] [PubMed] [Google Scholar]
- [105].Nir R-R, Yarnitsky D, Honigman L, Granot M. Cognitive manipulation targeted at decreasing the conditioning pain perception reduces the efficacy of conditioned pain modulation. PAIN 2012;153:170–76. [DOI] [PubMed] [Google Scholar]
- [106].Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- [107].Peerdeman KJ, Geers AL, Della Porta D, Veldhuijzen DS, Kirsch I. Underpredicting pain: an experimental investigation into the benefits and risks. PAIN 2021;162:2024–35. [DOI] [PubMed] [Google Scholar]
- [108].Peerdeman KJ, Tekampe J, van Laarhoven AIM, van Middendorp H, Rippe RCA, Peters ML, Evers AWM. Expectations about the effectiveness of pain- and itch-relieving medication administered via different routes. Eur J Pain 2018;22:774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Peerdeman KJ, van Laarhoven AIM, Donders ART, Hopman MTE, Peters ML, Evers AWM. Inducing expectations for health: effects of verbal suggestion and imagery on pain, itch, and fatigue as indicators of physical sensitivity. PLoS ONE 2015;10:e0139563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Peerdeman KJ, van Laarhoven AIM, Keij SM, Vase L, Rovers MM, Peters ML, Evers AWM. Relieving patients' pain with expectation interventions: a meta-analysis. PAIN 2016;157:1179–91. [DOI] [PubMed] [Google Scholar]
- [111].Petersen GL, Finnerup NB, Colloca L, Amanzio M, Price DD, Jensen TS, Vase L. The magnitude of nocebo effects in pain: a meta-analysis. PAIN 2014;155:1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia—imaging a shared neuronal network. Science 2002;295:1737–40. [DOI] [PubMed] [Google Scholar]
- [113].Pontén M, Ljótsson B, Jensen K. Shaping placebo analgesic responses on the Internet: a randomized experimental trial. Pain Rep 2019;4:e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Power A, Brown CA, Sivan M, Lenton A, Rainey T, El-Deredy W, Jones AKP, Watson A. Individuals with chronic pain have the same response to placebo analgesia as healthy controls in terms of magnitude and reproducibility. PAIN 2020;161:2720–30. [DOI] [PubMed] [Google Scholar]
- [115].Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. PAIN 1999;83:147–56. [DOI] [PubMed] [Google Scholar]
- [116].Raghuraman N, Wang Y, Schenk LA, Furman AJ, Tricou C, Seminowicz DA, Colloca L. Neural and behavioral changes driven by observationally-induced hypoalgesia. Sci Rep 2019;9:19760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Rhudy JL, Güereca YM, Kuhn BL, Palit S, Flaten MA. The influence of placebo analgesia manipulations on pain report, the nociceptive flexion reflex, and autonomic responses to pain. J Pain 2018;19:1257–74. [DOI] [PubMed] [Google Scholar]
- [118].Roelofs J, ter Riet G, Peters ML, Kessels AGH, Reulen JPH, Menheere PPCA. Expectations of analgesia do not affect spinal nociceptive R-III reflex activity: an experimental study into the mechanism of placebo-induced analgesia. PAIN 2000;89:75–80. [DOI] [PubMed] [Google Scholar]
- [119].Rose JP, Geers AL, Rasinski HM, Fowler SL. Choice and placebo expectation effects in the context of pain analgesia. J Behav Med 2012;35:462–70. [DOI] [PubMed] [Google Scholar]
- [120].Rose JP, Geers AL, Rasinski HM, Fowler SL. Erratum to: choice and placebo expectation effects in the context of pain analgesia. J Behav Med 2012;35:674. [DOI] [PubMed] [Google Scholar]
- [121].Rosén A, Yi J, Kirsch I, Kaptchuk TJ, Ingvar M, Jensen KB. Effects of subtle cognitive manipulations on placebo analgesia—an implicit priming study. Eur J Pain 2017;21:594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol 2011;21:880–7. [DOI] [PubMed] [Google Scholar]
- [123].Rütgen M, Seidel E-M, Silani G, Riečanský I, Hummer A, Windischberger C, Petrovic P, Lamm C. Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self pain. Proc Natl Acad Sci U S A 2015;112:E5638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Schafer SM, Colloca L, Wager TD. Conditioned placebo analgesia persists when subjects know they are receiving a placebo. J Pain 2015;16:412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Schenk LA, Sprenger C, Onat S, Colloca L, Büchel C. Suppression of striatal prediction errors by the prefrontal cortex in placebo hypoalgesia. J Neurosci 2017;37:9715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Schut C, Grossman S, Gieler U, Kupfer J, Yosipovitch G. Contagious itch: what we know and what we would like to know. Front Hum Neurosci 2015;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Skvortsova A, Veldhuijzen DS, van Middendorp H, Colloca L, Evers AWM. Effects of oxytocin on placebo and nocebo effects in a pain conditioning paradigm: a randomized controlled trial. J Pain 2020;21:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Skvortsova A, Veldhuijzen DS, Van Middendorp H, Van den Bergh O, Evers AWM. Enhancing placebo effects in somatic symptoms through oxytocin. Psychosom Med 2018;80:353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Skyt I, Lunde SJ, Baastrup C, Svensson P, Jensen TS, Vase L. Neurotransmitter systems involved in placebo and nocebo effects in healthy participants and patients with chronic pain: a systematic review. PAIN 2020;161:11–23. [DOI] [PubMed] [Google Scholar]
- [130].Stumpf A, Zerey V, Heuft G, Ständer S, Pfleiderer B, Schneider G. Itch perception and skin reactions as modulated by verbal suggestions: role of participant's and investigator's sex. Acta Dermato Venereol 2016;96:619–23. [DOI] [PubMed] [Google Scholar]
- [131].Świder K, Bąbel P. The effect of the sex of a model on nocebo hyperalgesia induced by social observational learning. PAIN 2013;154:1312–17. [DOI] [PubMed] [Google Scholar]
- [132].Świder K, Bąbel P. The effect of the type and colour of placebo stimuli on placebo effects induced by observational learning. PLoS ONE 2016;11:e0158363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Tang B, Geers A, Barnes K, Colagiuri B. Instrumental control enhances placebo analgesia. J Pain 2019;20:1486–97. [DOI] [PubMed] [Google Scholar]
- [134].Tu Y, Wilson G, Camprodon J, Dougherty DD, Vangel M, Benedetti F, Kaptchuk TJ, Gollub RL, Kong J. Manipulating placebo analgesia and nocebo hyperalgesia by changing brain excitability. Proc Natl Acad Sci U S A 2021;118:e2101273118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Valentini E, Aglioti SM, Chakrabarti B. The true size of placebo analgesia: concordant neural and behavioural measures of placebo analgesia during experimental acute pain. bioRxiv 2018:412296. doi: 10.1101/412296. [DOI] [Google Scholar]
- [136].Valentini E, Martini M, Lee M, Aglioti SM, Iannetti G. Seeing facial expressions enhances placebo analgesia. PAIN 2014;155:666–73. [DOI] [PubMed] [Google Scholar]
- [137].Vambheim SM, Daniali H, Flaten MA. Placebo effects on stress, but not on pain reports. A multi-experiment study. Front Psychol 2021;12:639236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Vambheim SM, Flaten MA. A systematic review of sex differences in the placebo and the nocebo effect. J Pain Res 2017;10:1831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].van Laarhoven AIM, van der Sman-Mauriks IM, Donders ART, Pronk MC, van de Kerkhof PCM, Evers AWM. Placebo effects on itch: a meta-analysis of clinical trials of patients with dermatological conditions. J Invest Dermatol 2015;135:1234–43. [DOI] [PubMed] [Google Scholar]
- [140].van Laarhoven AIM, Vogelaar ML, Wilder-Smith OH, van Riel PLCM, van de Kerkhof PCM, Kraaimaat FW, Evers AWM. Induction of nocebo and placebo effects on itch and pain by verbal suggestions. PAIN 2011;152:1486–94. [DOI] [PubMed] [Google Scholar]
- [141].Vase L, Petersen GL, Riley JL, Price DD. Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007. PAIN 2009;145:36–44. [DOI] [PubMed] [Google Scholar]
- [142].Vase L, Riley JL, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. PAIN 2002;99:443–52. [DOI] [PubMed] [Google Scholar]
- [143].Wager TD, Atlas LY. How is pain influenced by cognition? Neuroimaging weighs in. Perspect Psychol Sci 2013;8:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Wager TD, Matre D, Casey KL. Placebo effects in laser-evoked pain potentials. Brain Behav Immun 2006;20:219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Wager TD, Scott DJ, Zubieta J-K. Placebo effects on human μ-opioid activity during pain. Proc Natl Acad Sci 2007;104:11056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage 2009;47:821–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science 2004;303:1162–67. [DOI] [PubMed] [Google Scholar]
- [148].Watson A, El-Deredy W, Bentley DE, Vogt BA, Jones AKP. Categories of placebo response in the absence of site-specific expectation of analgesia. PAIN 2006;126:115–22. [DOI] [PubMed] [Google Scholar]
- [149].Watson A, El-Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt BA, Nadeau V, Jones AKP. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. PAIN 2009;145:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Watson A, El-Deredy W, Vogt BA, Jones AKP. Placebo analgesia is not due to compliance or habituation: EEG and behavioural evidence. Neuroreport 2007;18:771–5. [DOI] [PubMed] [Google Scholar]
- [151].Wei H, Zhou L, Zhang H, Chen J, Lu X, Hu L. The influence of expectation on nondeceptive placebo and nocebo effects. Pain Res Manag 2018;2018:8459429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Weimer K, Gulewitsch MD, Schlarb AA, Schwille-Kiuntke J, Klosterhalfen S, Enck P. Placebo effects in children: a review. Pediatr Res 2013;74:96–102. [DOI] [PubMed] [Google Scholar]
- [153].Weimer K, Hahn E, Mönnikes N, Herr A-K, Stengel A, Enck P. Are individual learning experiences more important than heritable tendencies? A pilot twin study on placebo analgesia. Front Psychiatry 2019;10:679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Weng L, Peerdeman KJ, Della Porta D, van Laarhoven AIM, Evers AWM. Can placebo and nocebo effects generalize within pain modalities and across somatosensory sensations? PAIN 2022;163:548–59. [DOI] [PubMed] [Google Scholar]
- [155].Wrobel N, Fadai T, Sprenger C, Hebebrand J, Wiech K, Bingel U. Are children the better placebo analgesia responders? An experimental approach. J Pain 2015;16:1005–11. [DOI] [PubMed] [Google Scholar]
- [156].Wrobel N, Wiech K, Forkmann K, Ritter C, Bingel U. Haloperidol blocks dorsal striatum activity but not analgesia in a placebo paradigm. Cortex 2014;57:60–73. [DOI] [PubMed] [Google Scholar]
- [157].Zunhammer M, Gerardi M, Bingel U. The effect of dopamine on conditioned placebo analgesia in healthy individuals: a double-blind randomized trial. Psychopharmacology 2018;235:2587–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B752.