Supplemental Digital Content is Available in the Text.
Keywords: EEG, MEG, Biomarker, Chronic pain, Systematic review
Abstract
Reliable and objective biomarkers promise to improve the assessment and treatment of chronic pain. Resting-state electroencephalography (EEG) is broadly available, easy to use, and cost efficient and, therefore, appealing as a potential biomarker of chronic pain. However, results of EEG studies are heterogeneous. Therefore, we conducted a systematic review (PROSPERO CRD42021272622) of quantitative resting-state EEG and magnetoencephalography (MEG) studies in adult patients with different types of chronic pain. We excluded populations with severe psychiatric or neurologic comorbidity. Risk of bias was assessed using a modified Newcastle–Ottawa Scale. Semiquantitative data synthesis was conducted using modified albatross plots. We included 76 studies after searching MEDLINE, Web of Science Core Collection, Cochrane Central Register of Controlled Trials, and EMBASE. For cross-sectional studies that can serve to develop diagnostic biomarkers, we found higher theta and beta power in patients with chronic pain than in healthy participants. For longitudinal studies, which can yield monitoring and/or predictive biomarkers, we found no clear associations of pain relief with M/EEG measures. Similarly, descriptive studies that can yield diagnostic or monitoring biomarkers showed no clear correlations of pain intensity with M/EEG measures. Risk of bias was high in many studies and domains. Together, this systematic review synthesizes evidence on how resting-state M/EEG might serve as a diagnostic biomarker of chronic pain. Beyond, this review might help to guide future M/EEG studies on the development of pain biomarkers.
1. Introduction
Chronic pain is one of the most prevalent and cost-intensive health conditions worldwide.116 In this context, the inherently subjective nature of experiencing pain has long been recognized as a key issue when dealing with chronic pain in clinical and research settings.119 To improve assessment and guide treatment of chronic pain, the quest for objective measures of acute and chronic pain has gained increasing attention.14 For these purposes, the development of reliable and objective biomarkers of chronic pain has become a key challenge in pain research.64 In line with the complexity of pain, there is an increasing awareness that there may not be a single, uniform biomarker but that different biomarkers subserving different functions, eg, for the diagnosis, monitoring, prognosis, and prediction of treatment responses may be needed to best capture pain.9
Over the past decades, alterations in brain structure and function, ranging from the molecular to the network level, have been increasingly recognized in patients with chronic pain.53 Consequently, neuroimaging methods have been used to develop noninvasive, brain-based biomarkers of chronic pain. Electroencephalography (EEG) has gained attention as an easy-to-use and cost-efficient tool to assess brain function with a high temporal resolution in chronic pain. Electroencephalography is complemented by magnetoencephalography (MEG). Magnetoencephalography measures brain signals closely related to EEG. However, MEG is technically more demanding and less cost-efficient. Both methods have been used to investigate changes in oscillatory brain activity and functional connectivity at different frequencies as potential biomarkers of chronic pain. In particular, slowing of the peak frequency in the alpha band (8-13 Hz)21,96 and increases of oscillatory brain activity at theta frequencies (4-8 Hz)69,76,100 have been reported and discussed. However, M/EEG approaches are not standardized, and results are heterogeneous and inconsistent.
Here, we aimed at synthesizing the available evidence on resting-state M/EEG as biomarkers of chronic pain in adult humans. To this end, we conducted a systematic review of cross-sectional and longitudinal studies of patients with chronic pain using resting-state M/EEG as a measure of brain function. These studies can particularly serve the development of diagnostic, monitoring, and predictive biomarkers of chronic pain.
2. Methods
This review was conducted and is reported in accordance with the most recent Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA77). The project protocol was registered on PROSPERO on September 10, 2021 (CRD42021272622). Deduplication of records, title and abstract screening, full-text review, and data extraction were conducted using Covidence software.12
2.1. Search strategy
The databases MEDLINE (through PubMed), Web of Science Core Collection (through Web of Science), Cochrane Central Register of Controlled Trials (through Ovid), and EMBASE (through Ovid) were searched on September 15, 2021. All searches were repeated before the final analysis on December 29, 2021. No historical limit was applied, and no filters for study type were used. For EMBASE, we excluded conference proceedings. In addition, we screened reference lists of included studies and personal files for further relevant publications.
The search string combined “electroencephalography” or “magnetoencephalography” and related terms with “chronic pain” and specifiers for several relevant chronic pain conditions, which do not entail “chronic pain” in their name (eg, fibromyalgia or postherpetic neuralgia). To this end, we searched the recently published International Association for the Study of Pain classification system of chronic pain conditions108 for relevant diagnostic entities and terms. The complete search strategy is available in the supplementary material (available at http://links.lww.com/PAIN/B751).
2.2. Study selection
Study inclusion and exclusion criteria are presented in Table 1. In summary, we included peer-reviewed studies that used quantitative resting-state M/EEG to analyze brain activity in chronic pain conditions. We excluded studies in patients with primary headache conditions or severe psychiatric/neurologic comorbidity because abnormalities in resting-state M/EEG that are unrelated to chronic pain have been found in migraine,78 severe depression,17 and neurodegenerative disease.99
Table 1.
Inclusion and exclusion criteria.
| Inclusion (included, if all apply) | Exclusion (excluded, if any applies) |
|---|---|
| Published, peer-reviewed study | Review article or case report |
| Chronic pain primary condition studied | Primary headache condition |
| M/EEG during resting state | Severe psychiatric or neurologic comorbidity |
| Reporting of quantitative M/EEG data | |
| Human participants ≥18 y |
M/EEG, magneto-/electroencephalography.
2.3. Record screening, full-text review, and data extraction
Two authors (P.T.Z., M.P.) screened titles and abstracts for eligibility blinded to each other's decisions. In case of disagreement, a third researcher (V.D.H.) was consulted, and conflicts were discussed. The same procedure was followed for full-text review. Data extraction was performed by one author (P.T.Z.) and checked by another author (V.D.H.). Extracted data comprised quantitative M/EEG measures (peak alpha frequency [PAF], frequency-specific power, frequency-specific connectivity), patient characteristics (sample size, sex, age, diagnostic entity, type of pain [nociceptive, neuropathic, nociplastic, mixed], pain intensity), and study design according to the study by Grimes and Schulz.34
2.4. Data synthesis strategy
For data synthesis, we grouped the studies according to study designs. By doing so, we were able to relate study designs to different types of biomarkers according to the Biomarkers, EndpointS, and other Tools-initiative9 (see Table 2 for an overview of study and biomarker types). Cross-sectional studies (comparison of patients and healthy participants) can serve the development of diagnostic biomarkers of chronic pain. Longitudinal studies (longitudinal descriptive, randomized and nonrandomized studies) can help to develop biomarkers for the monitoring of chronic pain and, in few cases, for the prediction of treatment responses. Descriptive studies correlating M/EEG with pain intensity in a single assessment can be useful to establish biomarkers for the diagnosis and monitoring of chronic pain.
Table 2.
Study designs and biomarker types.
| Study design | BEST biomarker(s) |
|---|---|
| Cross-sectional observational | Diagnostic |
| Longitudinal descriptive | Monitoring |
| Longitudinal randomized and nonrandomized controlled trial | Monitoring, predictive (treatment response) |
| Descriptive | Diagnostic/monitoring |
BEST, Biomarkers, EndpointS, and other Tools9
Due to the reported outcome measures and the high level of heterogeneity of study designs, a formal meta-analysis was not feasible. For instance, for cross-sectional comparisons of theta power, 47% of all included studies, 79% of studies with negative results, and 23% of studies with positive results did not report the required parameters for meta-analysis. Hence, formal meta-analysis would have introduced a reporting bias. Therefore, we used vote-counting and modified albatross plots36 for semiquantitative data synthesis. By plotting P values against sample sizes for different directions of effects, albatross plots allow for graphically estimating effect sizes for studies with similar research questions (eg, is there a difference in alpha power between patients with chronic pain and healthy participants?). However, because included studies used heterogeneous statistical methods for hypothesis testing, we did not superimpose effect size estimation contours on the plots. Modified albatross plots were used for comparison of PAF, frequency-specific power, and frequency-specific connectivity at theta (4-8 Hz), alpha (8-13 Hz), beta (13-30 Hz), and gamma (30-80 Hz) frequencies in cross-sectional (diagnostic biomarker), longitudinal (monitoring biomarker), and descriptive studies (monitoring/diagnostic biomarker). For graphical presentation of longitudinal study results, we only included studies that reported pain relief. In case of multiple P-values for different regions of interest in a single study, we reported the lowest P value. P values were displayed as reported in the primary studies, independently of possible adjustment for multiple comparisons. In case of imprecise reporting of P values (eg, “P < 0.05”), we chose the nearest decimal (eg, “P = 0.049”) for graphical representation in albatross plots.
When summarizing results across studies for a certain parameter (eg, theta power in cross-sectional studies), we focused on vote counting and labeled results as positive, if more studies were found for either the “lower” or “higher” category compared with the “nonsignificant” and the respective other category.
For studies reporting quantitative M/EEG measures other than the aforementioned variables (eg, microstate analysis or machine learning algorithms) and/or using M/EEG as a predictive biomarker, narrative data synthesis was performed due to the low overall number of studies and high heterogeneity of methods and outcome measures.
2.5. Risk of bias and quality assessment
Risk of bias and study quality were assessed using a modified version of the Newcastle–Ottawa Scale117 adapted by Pinheiro et al.87 for EEG studies on pain. This tool assesses risk of bias and quality of studies included in systematic reviews and/or meta-analyses across the domains “selection of study participants” (4 items), “comparability/confounders” (2 items), and “outcome data” (3 items). Although in the original version stars are awarded for single domains, we rated items as “high” (negative for study quality) or “low” (positive for study quality) risk of bias because this allows for easier interpretation of scoring results. We did not calculate sum scores for single studies because single items had frequently to be scored “n/a” (not applicable), and comparison of sum scores across studies would have been misleading.
Furthermore, we evaluated all included studies regarding adherence to core open science principles (preregistration, sample size calculation, correction for multiple testing, and sharing of primary data and code).
Risk of bias and quality assessment was performed by one author (P.T.Z.) and checked by another author (V.D.H.).
3. Results
3.1. Study selection
Searching databases resulted in 2430 results after deduplication. We added 2 more studies from citation screening and personal records. Following title and abstract screening, 141 studies were identified for full-text review. Finally, 76 studies were included.1,5–8,10,11,15,16,18–27,30–33,35,37–49,51,56,59,60,63,66–69,74–76,79,80,82,89–91,95–98,100–105,109,110,112–115,120,122,124–126 Figure 1 depicts the PRISMA flow diagram of study selection along with reasons for study exclusion at every stage.
Figure 1.
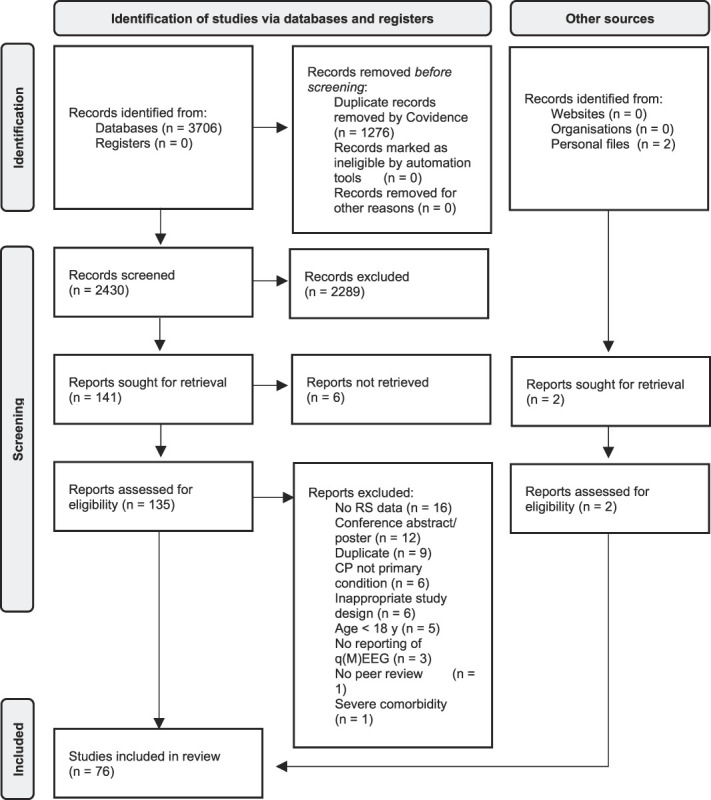
PRISMA flow diagram of study selection. CP, chronic pain; EEG, electroencephalography; PRISMA, preferred reporting items for systematic reviews and meta-analyses; RS, resting state; qM/EEG, quantitative M/EEG.
3.2. Study characteristics
Figure 2 and Table 3 summarize the main characteristics and results of included studies. Thirty-two studies were conducted in patients with mixed pain, 26 in patients with neuropathic pain and 18 studies in patients with nociplastic pain (mainly fibromyalgia). Most studies used EEG (n = 66), whereas only few studies used MEG (n = 10). The most frequent study type was cross sectional (n = 41; comparing patients to healthy participants), followed by longitudinal descriptive studies (n = 17; longitudinal tracking of patients). Five studies comprised different study designs, ie, cross-sectional comparison of patients and healthy participants at baseline and longitudinal tracking of patients. Longitudinal studies included controlled and noncontrolled interventional designs and were heterogeneous regarding the type of intervention (Table 3). The most frequent interventions were noninvasive neuromodulation, cognitive–behavioral therapy, surgery, and pharmacological approaches. Sample sizes varied between 10 and 342 participants (median 38.0, see Fig. S1, http://links.lww.com/PAIN/B751 for a graphical illustration). In cross-sectional studies, these sample sizes included not only patients but also the sum of patients and healthy participants. Studies were published between 1999 and 2021 (Fig. S2, http://links.lww.com/PAIN/B751).
Figure 2.

Pain type, recording modality, and study designs of included studies. The charts show absolute numbers of studies. EEG, electroencephalography; MEG, magnetoencephalography.
Table 3.
Characteristics of included studies.
| Author and year | Study design | Intervention | EEG/MEG | Condition | Type of pain | Total sample size | Pain duration (mo) | Pain intensity (0-10) | Main results (EEG/MEG analysis) |
|---|---|---|---|---|---|---|---|---|---|
| Ahn et al. 20191 | (Randomized) controlled trial | tACS vs sham | EEG | Back pain | Mixed | 20 | 84.8 | 4.4 | Negative correlation between somatosensory alpha power and pain intensity Higher somatosensory alpha power after alpha-tACS compared with sham tACS Negative correlation between stimulation-induced alpha power increase and pain relief |
| Barbosa-Torres and Cubo-Delgado, 20215 | Longitudinal descriptive | NFB | EEG | Fibromyalgia | Nociplastic | 37 | n.rep. | 8.4 | Higher SMR/theta wave ratio after NFB Lower pain after NFB |
| Baroni et al. 20206 | Cross-sectional observational | — | EEG | Orofacial pain | Mixed | 48 | 49.2 | 6.4 | No power differences between patients and controls |
| Bell et al. 20017 | Cross-sectional observational | — | EEG | Fibromyalgia | Nociplastic | 31 | 57.6 | n.rep. | Higher alpha power (posterior midline) in patients compared with controls |
| Bernardi et al. 20218 | Longitudinal descriptive | tACS vs tRNS | EEG | Fibromyalgia | Nociplastic | 36 | n.rep. | n.rep. | Higher alpha power after tACS compared with tRNS Lower pain symptoms after tACS compared with tRNS |
| Camfferman et al. 201710 | Descriptive | — | EEG | Mixed | Mixed | 103 | n.rep. | n.rep. | Correlation between alpha and theta power and pain intensity |
| Choe et al. 201811 | Cross-sectional observational | — | MEG | Fibromyalgia | Nociplastic | 37 | 36.6 | 6.1 | Lower theta global connectivity and connectivity (within the default mode network/between middle/inferior temporal gyrus and visual cortex) in patients compared with controls Correlation between pain duration and reduced connectivity (between inferior temporal gyrus and visual cortex) |
| Day et al. 202116 | Longitudinal descriptive | Different cognitive and/or behavioral interventions | EEG | Low back pain | Mixed | 57 | 168 | 4.6 | Lower theta, alpha, beta power across all interventions, no differences between the groups Positive correlation between beta power reduction (in central ROI) and pain intensity reduction in mindfulness-based cognitive therapy group only Lower pain across all interventions |
| Day et al. 202015 | (Randomized) controlled trial | Different cognitive and/or behavioral interventions | EEG | Low back pain | Mixed | 69 | 173.5 | 4.8 | Moderation analysis: baseline alpha and theta power did not moderate changes in pain |
| De Melo et al. 202019 | (Randomized) controlled trial | tDCS (different protocols) vs sham | EEG | Fibromyalgia | Nociplastic | 31 | 79.2 | 6.7 | Lower frontal and parietal alpha power after 5d tDCS Lower pain across all interventions |
| De Melo et al. 202118 | Descriptive | — | EEG | Fibromyalgia | Nociplastic | 31 | 79.8 | 6.7 | No correlation between alpha power and pain intensity |
| De Ridder and Vanneste, 201720 | Cross-sectional observational | tDCS vs sham | EEG | Fibromyalgia | Nociplastic | 38 | n.rep. | n.rep. | Higher beta power for patints (dorsal anterior cingulate cortex) compared with controls Lower beta lagged phase coherence and granger causality for patients compared with controls Lower pain after tDCS (stronger reduction for active compared with sham tDCS) Higher beta power after tDCS in patients compared with controls (anterior cingulate cortex) Higher beta and gamma connectivity after tDCS in patients compared with controls (dorsal anterior cingulate cortex and pregenual anterior cingulate cortex) |
| De Vries 201321 | Cross-sectional observational | — | EEG | Chronic pancreatitis | Mixed | 32 | 64.8 | n.rep. | Lower PAF in patients compared with controls Correlation between pain duration and PAF |
| Di Pietro et al. 201822 | Cross-sectional observational | — | EEG | Trigeminal neuropathy | Neuropathic | 40 | 66 | 3.7 | Higher power in patients (4-25 Hz) compared with controls, most marked in the theta and low alpha range Correlation between pain intensity and alpha (T4) and beta power (FC6, CP6, O1) for selected electrodes |
| Fallon et al. 201823 | Cross-sectional observational | — | EEG | Fibromyalgia | Nociplastic | 37 | 115.2 | n.rep. | Higher theta power in patients compared with controls Correlation between frontal theta power and measures of tenderness |
| Fauchon et al. 202224 | Cross-sectional observational | — | MEG | Neuropathic pain of various etiologies | Neuropathic | 100 | 161.4 | 6.1 | Higher alpha power in patients compared with controls Lower PAF in male patients compared with male controls Higher PAF for women compared with men with neuropathic pain Negative correlation between PAF and pain intensity in the neuropathic pain group (posterior insula; right TPJ) |
| Feng et al. 202125 | Descriptive | — | EEG | Low back pain | Mixed | 27 | 67.2 | n.rep. | Negative correlation between central alpha power and pain intensity |
| Ferdek et al. 201926 | Cross-sectional observational | — | EEG | Endometriosis | Mixed | 37 | 88.8 | 6.8 | Higher beta connectivity in patients compared with controls (left dorsolateral prefrontal cortex—left somatosensory cortex; left somatosensory cortex—orbitofrontal cortex and right temporal cortex) |
| Freye and Levy 200627 | Longitudinal descriptive | Tramadol | EEG | Osteoarthritis | Mixed | 19 | n.rep. | 7.8 | Higher alpha and beta power after tramadol administration Lower pain after tramadol |
| González-Roldán et al. 201630 | Cross-sectional observational | — | EEG | Fibromyalgia | Nociplastic | 38 | 225.6 | 6.0 | Higher beta power in patients compared with controls Higher theta and beta connectivity (left hemisphere) in patients compared with controls |
| González-Villar 202031 | Cross-sectional observational | — | EEG | Fibromyalgia | Nociplastic | 94 | n.rep. | 7.0 | Higher beta connectivity in patients compared with controls Microstate analysis: shorter microstate 1 occurrence and coverage in patients compared with controls |
| Gram 201732 | Longitudinal descriptive | Surgery | EEG | Chronic hip pain | Mixed | 81 | n.rep. | n.rep. | No post-OP power or connectivity differences between opioid treatment responders and nonresponders Machine learning: No distinction of responders and nonresponders based on EEG features |
| Graversen 201233 | Longitudinal descriptive | Pregabalin vs placebo | EEG | Chronic pancreatitis | Mixed | 28 | 116 | 3.8 | Higher theta power after pregabalin (not after placebo) Lower pain for most patients (individual level data only) |
| Hargrove 201035 | Cross-sectional observational | — | EEG | Fibromyalgia | Nociplastic | 170 | 132 | n.rep. | Higher beta power in patients compared with controls |
| Heitmann 202237 | Longitudinal descriptive | Interdisciplinary multimodal pain therapy | EEG | Mixed | Mixed | 41 | 101.6 | 5.4 | Lower pain after intervention No correlation between pain changes and PAF or power changes Network analysis: correlation between pain changes and increase in theta global network efficiency |
| Hsiao 201738 | Cross-sectional observational | — | MEG | Fibromyalgia | Nociplastic | 56 | 87.6 | 5.3 | Lower theta connectivity (insula—DMN bilaterally) for patients compared with controls No correlation between pain intensity and duration and connectivity measures Correlation between number of tender points and connectivity (right insula—DMN) |
| Hunter 200939 | Longitudinal descriptive | Duloxetine vs placebo | EEG | Fibromyalgia | Nociplastic | 12 | n.rep. | 6.7 | Regression analysis: prediction of pain improvement by theta-cordance change in duloxetine group after 12 wk (also after controlling for depression) |
| Iwatsuki 202140 | Cross-sectional observational | — | MEG | CPRS | Nociplastic | 42 | n.rep. | n.rep. | Correlation between pain intensity and alpha/theta connectivity (alpha: right SII; precuneus/insula, theta: right SII; posterior cingulate cortex) |
| Jensen 201341 | Longitudinal descriptive | NFB (different protocols) | EEG | Spinal cord injury | Neuropathic | 10 | n.rep. | 5.9 | Lower theta and higher alpha power after NFB Lower pain after NFB |
| Jensen 202143 | (Randomized) controlled trial | Different cognitive and/or behavioral interventions | EEG | Mixed | Mixed | 147 | n.rep. | 4.7 | Lower theta power after intervention only for pain education group Lower pain intensity across all interventions No correlation between changes in pain intensity and EEG measures |
| Jensen 201342 | Cross-sectional observational | — | EEG | Spinal cord injury | Mixed | 82 | n.rep. | n.rep. | Lower alpha power for patients compared with controls, no differences in other frequency bands No correlations between pain intensity and global power measures Correlation between pain intensity and frontal alpha power |
| Juel 201744 | (Randomized) controlled trial | Acupuncture vs sham stimulations | EEG | Chronic pancreatitis | Mixed | 15 | 141.6 | n.rep. | No changes in theta, alpha, beta power after intervention Lower pain after intervention |
| Kayiran 201045 | Longitudinal descriptive | NFB vs escitalopram | EEG | Fibromyalgia | Nociplastic | 54 | 55.3 | 8.9 | Lower theta/SMR ratio after NFB No changes in theta, alpha or beta power |
| Kim 202047 | Cross-sectional observational | — | MEG | MS with chronic pain | Mixed | 63 | n.rep. | 3.7 | Abnormalities in theta, alpha, beta, and gamma cross-network functional coupling for patients compared with controls |
| Kim 201946 | Cross-sectional observational | — | MEG | MS with chronic pain | Mixed | 53 | n.rep. | 3.4 | Higher alpha and lower beta power in patients compared with controls (most prominently in thalamus, insula, and right TPJ) Higher alpha power in neuropathic compared with nonneuropathic pain patients Correlation between pain intensity and alpha power |
| Kim 202148 | Longitudinal descriptive | Non-invasive painless signaling therapy | EEG | Failed back surgery syndrome | Mixed | 11 | n.rep. | 4.3 | Higher alpha and beta power after treatment in responder group (pain reduction) |
| Kisler 202049 | Cross-sectional observational | — | MEG | Ankylosing spondylitis | Mixed | 76 | 164.4 | 3.6 | Higher theta and lower gamma in patients compared with controls Higher alpha power in subgroup of neuropathic pain patients compared with other patients and to controls Correlation between pain intensity and alpha power in mixed-pain patients |
| Klug 201151 | Cross-sectional observational | — | EEG | Somatoform pain disorder | Nociplastic | 30 | n.rep. | n.rep. | Lower beta power in patients compared with controls |
| Lee 201856 | Descriptive | — | EEG | Fibromyalgia | Nociplastic | 10 | n.rep. | n.rep. | Network analysis: “Explosive synchronization”—characteristics found in patients, correlation between pain intensity and these characteristics |
| Levitt 202059 | Cross-sectional observational | — | EEG | Chronic lumbar radiculopathy | Neuropathic | 37 | n.rep. | 7.1 | Gamma-band differences between patients and controls for number, power, and frequency span of transient spectral events No differences between patients and controls for power spectral density, coherence, or phase-amplitude coupling Machine learning: binary SVM (patients vs controls) and 3-way-classifier (lumbar pain vs radiculopathy vs controls) based on power spectral density, coherence, and phase-amplitude coupling performed significantly better than chance |
| Lim 201660 | Cross-sectional observational | — | MEG | Fibromyalgia | Nociplastic | 36 | 36.6 | n.rep. | Lower PAF in patients compared with controls Higher theta, beta, gamma power in patients compared with controls (no test statistics provided) |
| Lopez 201563 | Cross-sectional observational | — | EEG | Fibromyalgia | Nociplastic | 26 | n.rep. | n.rep. | Lower theta and alpha in patients compared with controls Higher beta in patients compared with controls |
| Martín-Brufau 202166 | Cross-sectional observational | — | EEG | Fibromyalgia | Nociplastic | 46 | n.rep. | n.rep. | Lower theta and alpha in patients compared with controls Higher beta in patients compared with controls |
| May 202167 | Cross-sectional observational | — | EEG | Mixed | Mixed | 185 | 121.9 | 5.7 | Microstate analysis: less dominant role of microstate D in patients (lower time coverage, lower frequency of occurrence, lower global explained variance, shorter mean duration); lower transition probability from microstates A, B, and E to microstate D in patients Microstate analysis: no correlation between microstate D measures and clinical characteristics |
| Meneses 201668 | Cross-sectional observational | — | EEG | Rheumatoid arthritis | Mixed | 42 | 107.4 | n.rep. | Higher alpha and theta power in patients compared with controls No correlations between power and pain measures |
| Michels 201169 | Cross-sectional observational, longitudinal descriptive | Thalamotomy | EEG | Neuropathic pain of various etiologies | Neuropathic | 38 | 55.2 | 6.5 | Higher theta and alpha power in all patients compared with controls Higher beta and gamma power in patients with low pain relief (after surgery) compared with controls Correlation between theta and beta power with pain intensity presurgery normalization of power in all frequency bands postsurgery in patients with high pain relief |
| Nomura 199974 | Cross-sectional observational | — | EEG | Inflammatory bowel syndrome | Nociplastic | 48 | n.rep. | n.rep. | Lower alpha and higher beta in patients compared with controls |
| Oga 200275 | Longitudinal descriptive | Ketamine i.v. | EEG | Neuropathic pain of various etiologies | Neuropathic | 10 | n.rep. | n.rep. | Lower absolute theta power and higher relative theta power after ketamine Lower absolute/relative alpha power and higher absolute/relative beta power after ketamine Pain relief after ketamine Correlation between changes in pain intensity and alpha power (right central electrode) |
| Olesen 201176 | Cross-sectional observational | — | EEG | Chronic pancreatitis | Mixed | 46 | 120 | 3.8 | Higher theta and alpha power in patients compared with controls Lower beta power in patients compared with controls |
| Parker 202179 | Cross-sectional observational | — | MEG | Neuropathic pain of various etiologies | Neuropathic | 13 | n.rep. | n.rep. | Higher PAF after DRGS Higher theta power (DRGS-OFF) in patients with severe pain compared with mild pain Lower alpha power (DRGS-OFF) in patients with severe pain compared with moderate pain |
| Parker 202180 | (Randomized) controlled trial | DRGS + (tDCS vs sham) | EEG | Neuropathic pain of various etiologies | Neuropathic | 16 | n.rep. | 6.0 | Higher beta power after DRGS-ON vs OFF and after DRGS-ON/active tDCS vs DRGS-ON/sham stimulation Lower pain across all interventions (highest reduction for DRGS-ON paired with active tDCS) |
| Patel 202082 | Longitudinal “comparative” | NFB | EEG | Mixed | Mixed | 8 | n.rep. | n.rep. | No differences in alpha power between patients and controls (baseline and longitudinally) Alpha power fluctuation analysis: no differences in fractional occupancy, dwell time, distribution and transition probability between patients and controls (baseline and longitudinally) Network analysis: negative correlations of changes in pain with fractional occupancy, mean dwell times, and transition probability from low to high alpha state |
| Prichep 201189 | Longitudinal descriptive | Various treatments for neuropathic pain | EEG | Neuropathic pain of various etiologies | Neuropathic | 5 | 13.6 | 6.4 | Higher alpha power after intervention |
| Prichep 201890 | Cross-sectional observational | — | EEG | Mixed | Mixed | 154 | 100.8 | 5.0 | Higher theta and low alpha power in patients compared with controls Higher theta connectivity in patients compared with controls |
| Prinsloo 201791 | (Randomized) controlled trial | NFB vs waiting list | EEG | Chemotherapy-induced peripheral neuropathy | Neuropathic | 62 | n.rep. | 4.7 | Higher alpha power after NFB Lower beta power after NFB Greater decrease in worst pain in NFB group, but similar outcomes for groups in average pain |
| Santana 202195 | Cross-sectional observational | — | EEG | Sickle cell disease with chronic hip pain | Mixed | 40 | 55.5 | 5.5 | Network analysis: lower full synchronization time in patients compared with controls |
| Sarnthein 200696 | Cross-sectional observational, longitudinal descriptive | Thalamotomy | EEG | Neuropathic pain of various etiologies | Neuropathic | 30 | n.rep. | n.rep. | Lower PAF and theta power in patients compared with controls Lower theta power and lower pain 12 mo after surgery |
| Schmidt 201598 | Longitudinal descriptive | Cognitive and/or behavioral intervention | EEG | Back pain | Mixed | 21 | n.rep. | 5.9 | No EEG differences after intervention Lower pain after intervention No correlation between EEG and pain |
| Schmidt 201297 | Cross-sectional observational | — | EEG | Back pain | Mixed | 74 | n.rep. | n.rep. | No differences in PAF and overall power between patients and controls Correlation between overall power and pain intensity, no correlation with PAF |
| Stern 2006100 | Cross-sectional observational, longitudinal descriptive | Thalamotomy | EEG | Neuropathic pain of various etiologies | Neuropathic | 32 | n.rep. | 7.1 | Higher theta, alpha, beta power in patients compared with controls “Deoveractivation” (lowering of alpha and beta power) for subgroup of patients with high pain relief after surgery |
| Sufianov 2014101 | Cross-sectional observational, longitudinal descriptive | Epidural spinal cord stimulation | EEG | Failed back surgery syndrome | Mixed | 40 | 38.4 | 7.2 | Lower PAF and theta power in patients compared with controls Higher beta power in patients compared with controls Lower theta power and higher PAF after intervention Lower pain after intervention |
| Ta Dinh 2019102 | Cross-sectional observational | — | EEG | Mixed | Mixed | 185 | 121.9 | 5.7 | No difference in power and PAF between patients and controls Higher theta and gamma connectivity in patients compared with controls Network analysis: decrease in global efficiency in gamma frequencies in patients compared with controls |
| Teixeira 2021103 | Cross-sectional observational | — | EEG | Neuropathic pain of various etiologies | Neuropathic | 22 | n.rep. | 4.2 | Lower beta power in patients compared with controls Negative correlation between beta power and pain intensity in subgroup of patients with more severe pain |
| Teixera 2022104 | Descriptive | — | EEG | Low back pain | Mixed | 30 | n.rep. | 4.7 | Regression analysis: prediction of pain intensity by theta power (while controlling for depression) |
| Thibaut 2017105 | Cross-sectional observational | tPCS + tDCS vs tPCS vs tDCS vs sham | EEG | Chronic pancreatitis | Mixed | 54 | n.rep. | n.rep. | Higher theta, alpha, beta power in patients compared with controls Lower alpha/beta ratio in patients compared with controls No pain reduction after intervention |
| Uygur-Kucukseymen 2020109 | Descriptive | — | EEG | Fibromyalgia | Nociplastic | 21 | n.rep. | 5.9 | Negative correlation between pain intensity and alpha and beta power |
| Van den Broeke 2013110 | Cross-sectional observational | — | EEG | Chronic pain after breast cancer treatment | Mixed | 19 | n.rep. | 5.0 | Higher alpha power in patients compared with controls No differences in PAF No correlation between pain intensity and alpha power |
| Vanneste 2021112 | Cross-sectional observational | — | EEG | Neuropathic pain of various etiologies | Neuropathic | 100 | n.rep. | 5.8 | Higher theta, alpha, beta, gamma power in patients compared with controls Correlations between pain intensity and gamma and beta power (not for theta and alpha) Higher theta and lower alpha functional connectivity in patients compared with controls Source analysis: imbalance between ROIs involved in pain input and pain suppression |
| Vanneste 2017113 | Cross-sectional observational | — | EEG | Fibromyalgia | Nociplastic | 88 | 14.9 | n.rep. | Lower alpha power in patients compared with controls Higher beta power in patients compared with controls Differences in alpha connectivity patterns in patients compared with controls for several regions (higher/lower depending on ROI) |
| Vanneste 2018114 | Cross-sectional observational | — | EEG | Neuropathic pain of various etiologies | Neuropathic | 342 | n.rep. | n.rep. | Higher theta, beta, and gamma power in patients compared with controls (different ROIs) Lower alpha power in patients compared with controls Cross-frequency coupling: increased nesting of theta–beta and theta–gamma in patients compared with controls Machine learning (SVM): discrimination of patients and controls based on EEG features |
| Villafaina 2019115 | Cross-sectional observational | — | EEG | Fibromyalgia | Nociplastic | 62 | n.rep. | 5.9 | Lower alpha power in patients compared with controls Negative correlation between pain scores and alpha power (single electrode level) |
| Witjes 2021120 | Cross-sectional observational | — | MEG | Failed back surgery syndrome | Mixed | 46 | 120 | 6.1 | Higher alpha power ratio (low/high alpha) in patients compared with controls No differences in PAF or theta, alpha, beta, gamma power between patients and controls No correlation between pain intensity or duration and alpha power ratio |
| Yüksel 2019122 | Cross-sectional observational, longitudinal descriptive | tENS vs acupuncture | EEG | Fibromyalgia | Nociplastic | 63 | 4,5 | 5.0 | Lower theta power in both patient groups compared with controls Lower alpha and beta power only in acupuncture group compared with controls Lower pain across interventions Correlation between theta power and alpha power with pain in acupuncture group Higher alpha power across interventions, diverging effects for alpha power in TENS group (higher/lower depending on ROI), higher beta power in acupuncture group |
| Zhou 2018124 | Cross-sectional observational | — | EEG | Postherpetic neuralgia | Neuropathic | 28 | 11.9 | 5.0 | Higher gamma power in patients compared with controls (not significant after controlling for anxiety) Correlation between pain intensity and gamma power |
| Zolezzi 2021125 | Descriptive | — | EEG | Neuropathic pain of various etiologies | Neuropathic | 35 | n.rep. | n.rep. | Machine learning (SVM): classification into 3 groups (low, moderate, high pain) with high accuracy based on linear (frequency-specific power) and nonlinear (“approximate entropy”) features; frontal beta power most relevant feature |
| Zortea 2021126 | Cross-sectional observational | — | EEG | Fibromyalgia | Nociplastic | 47 | n.rep. | 8.8 | No differences for theta, alpha, or beta power between opioid users compared with nonopioid users Lower theta and beta peak amplitudes in opioid users compared with nonopioid users No differences in pain between the groups |
DRGS, dorsal root ganglion stimulation; EC, eyes closed; EO, eyes open; MS, multiple sclerosis; n.rep, not reported; NFB, neurofeedback; PAF, peak alpha frequency; ROI, region of interest; SMR, sensorimotor; SVM, support vector machine; tACS, transcranial alternating current stimulation; tDCS, transcranial direct current stimulation; TENS, transcutaneous electrical nerve stimulation; TPJ, temporoparietal junction; tRNS, transcranial random noise stimulation.
3.3. Risk of bias and open science practices
The results of the risk of bias assessment are summarized in Figure 3. Scores of individual studies are presented in Table S1, http://links.lww.com/PAIN/B751.
Figure 3.

Risk of bias of included studies. M/EEG, magneto-/electroencephalography.
In the domain “selection of study participants,” the most significant risk of bias arose from “case representativeness” because most studies provided no description of their sampling/recruiting strategy. For the item “case definition,” risk of bias was low for most studies because pain diagnoses and diagnostic criteria were specified frequently. For the items “matching of controls” and “definition of controls,” mixed results were obtained. In the domain “comparability/confounders,” risk of bias was high for most studies because frequent comorbidities of chronic pain (eg, depression or anxiety) were rarely controlled for. Regarding “outcome data,” risk of bias was “low” for the items “data acquisition/processing” and “appropriate statistical test” for most studies. However, most studies had a high risk of bias regarding the “assessment of outcome” because preprocessing/analysis of M/EEG data was performed manually and blinding to condition, or clinical data were not explicitly stated.
Figure 4 shows the results of the evaluation of open science practices. Only few studies were preregistered, provided sample size calculations/justifications or offered data or code sharing. Almost half of the included studies adjusted P values to correct for multiple comparisons. However, in some studies, correction for multiple comparisons was performed for selected analyses only.
Figure 4.

Open science practices of included studies. The charts show absolute numbers of studies.
3.4. Data synthesis
For semiquantitative analysis of studies, we used modified albatross plots. Figure 5 explains the interpretation of albatross plots based on fictional data. A few studies reported diverging directions of effects for different regions of interest.112,114 These studies were not included in the modified albatross plots, but their results are listed in Table 3. For studies using less common analysis techniques and outcome measures (eg, microstate analysis or machine learning algorithms) and for predictive biomarker studies, narrative data synthesis was performed due to the low overall number of studies and high heterogeneity of methods and outcome measures.
Figure 5.

Explanatory albatross plot based on fictional data. P values on the x-axis are displayed on a logarithmic scale (log10). n.s., not significant.
3.4.1. Semiquantitative data synthesis
3.4.1.1. Cross-sectional studies
Forty-four cross-sectional studies, which can serve the development of diagnostic biomarkers of chronic pain, evaluated differences in PAF, frequency-specific power, and/or connectivity between patients with chronic pain and healthy participants. Sample sizes varied between 13 and 342 participants (median, 44.0).
For PAF, 7 studies (58%) found no statistically significant differences between patients with chronic pain and healthy participants; 4 studies (33%) found lower and one study (8%) found higher peak frequencies in patients (Fig. 6).
Figure 6.

Peak alpha frequency differences between patients and healthy participants in cross-sectional studies. P values on the x-axis are displayed on a logarithmic scale (log10). n.s., not significant.
In Figure 7, cross-sectional results for frequency-specific power and connectivity are depicted. In 14 studies (47%), theta power was higher for patients compared with healthy participants, closely followed by nonsignificant results (n = 13; 43%). Only 3 studies (10%) found lower theta power for patients. For alpha power, no clear differences were observed (n = 13; 39% no difference, higher n = 11; 33%, lower n = 9; 27%). Regarding beta power, most studies found higher values for patients (n = 14; 47%), whereas 11 studies (37%) found no differences and 5 (17%) studies found lower values for patients compared with healthy participants. For gamma power, 9 studies (60%) found no group differences, 5 studies (33%) reported higher gamma power, and 1 (7%) study reported lower gamma in patients than in healthy participants.
Figure 7.
Power and connectivity differences between patients and healthy participants in cross-sectional studies. P values on the x-axis are displayed on a logarithmic scale (log10). n.s., not significant.
Compared with frequency-specific power, the number of cross-sectional studies analyzing connectivity differences between patients and healthy participants was lower. No clear differences across studies could be observed for any frequency band. However, more studies reported higher than lower connectivity at theta frequencies for patients (theta: n = 4; 40% higher, n = 4; 40% no difference, n = 2; 20% lower/alpha: n = 8; 80% no difference, n = 2; 20% lower, n = 0 higher/beta: 6; 55% no difference, n = 3; 27% higher, n = 2; 18% lower/gamma: n = 7; 78% no difference, n = 1; 11% higher, n = 1; 11% lower).
In summary, more cross-sectional studies reported higher theta and beta power in patients with chronic pain compared with lower power or nonsignificant results. Although most studies reported nonsignificant results for the remaining parameters, more studies found higher than lower gamma power and theta connectivity and lower than higher peak alpha frequency in patients with chronic pain.
3.4.1.2. Longitudinal studies
Twenty-two (interventional) longitudinal studies, which can aid the development of monitoring and predictive biomarkers, reported pain relief and premeasurements and postmeasurements of frequency-specific power (Fig. 8). No study analyzed connectivity longitudinally. Sample size (patients only) varied between 6 and 147 patients (median, 24.5).
Figure 8.

Power changes with pain relief in longitudinal studies. On the right (“[higher]”) part of each albatross plot, studies with postinterventional power increases are displayed. On the left ([“lower”]) part of each plot, studies with postinterventional power decreases are displayed. Only studies that found a postinterventional pain relief were included. P values on the x-axis are displayed on a logarithmic scale (log10). n.s., not significant.
No trend toward higher or lower power values after intervention could be observed across studies for any frequency band (theta: n = 7; 43.8% no difference, n = 6; 37.5% lower, n = 3; 18.8% higher/alpha: n = 5; 31.3% no difference, n = 5; 31.3% lower, n = 6; 37.5% higher/beta: n = 7; 43.8% no difference, n = 4; 25% lower, n = 5; 31.3% higher/gamma: n = 1; 33.3% no difference, n = 2; 66.7% lower, n = 0 higher).
In summary, included studies did not show consistent longitudinal power changes in patients undergoing interventions. However, more studies reported decreases than increases in theta power.
3.4.1.3. Descriptive studies
Twenty-five descriptive studies, which can aid the development of diagnostic and/or monitoring biomarkers correlated M/EEG measures with pain intensity (Fig. 9). Sample sizes ranged from 12 to 103 patients (median 27.0). For all frequency bands, most studies found nonsignificant correlations with pain intensity, whereas for the alpha band, more studies reported a negative correlation than a positive correlation (theta: n = 10; 91% no correlation, n = 1; 9% positive correlation, n = 0 negative correlation/alpha: n = 9; 50% no correlation, n = 7; 38.9% negative correlation, n = 2; 11.1% positive correlation/beta: n = 9; 75% no correlation, n = 3; 25% positive correlation, n = 0 negative correlation/gamma: n = 3; 60% no correlation, n = 2; 40% positive correlation, n = 0 negative correlation). Similarly, for connectivity, mainly nonsignificant correlations were observed for all frequency bands (theta: n = 4; 80% no correlation, n = 1; 20% negative correlation, n = 0 positive correlation/alpha: n = 2; 50% no correlation, n = 1; 25% negative correlation, n = 1; 25% positive correlation/beta: n = 4; 66.7% no correlation, n = 2; 33.3% negative correlation, n = 0 positive correlation/gamma: n = 3; 75% no correlation, n = 1; 25% negative correlation, n = 0 positive correlation).
Figure 9.
Correlations of power and connectivity with pain intensity in descriptive studies. On the right (“[pos.]”) part of each albatross plot, studies with positive correlations of pain intensity and power/connectivity are displayed. On the left ([“neg.”]), part of each plot, studies with negative correlations of pain intensity and power/connectivity are displayed. P values on the x-axis are displayed on a logarithmic scale (log10). n.s., not significant.
In summary, neither for power nor for connectivity measures, a clear relation with pain intensity was found across studies. However, more negative than positive correlations of pain intensity and alpha power were observed.
3.4.2. Narrative synthesis of studies using microstate analysis, network analysis, and machine learning approaches
In recent years, a variety of advanced analysis methods for M/EEG data have been applied to assess brain function in chronic pain populations. Furthermore, machine learning approaches have been used to differentiate between patients and healthy participants based on M/EEG data. These studies include cross-sectional, longitudinal, and descriptive studies, which can yield diagnostic, monitoring, and predictive biomarkers of chronic pain.
Two cross-sectional studies performed microstate analysis of EEG recordings. Although traditional quantitative resting-state EEG analysis focuses on static measures of brain activity over minutes, it is increasingly recognized that brain activity varies over short time intervals and that corresponding temporal dynamics provide physiologically relevant information. It has been shown that these dynamics can be characterized as sequences of recurrent topographies of EEG activity termed microstates, which are believed to reflect large-scale brain network activity. Microstate analysis describes EEG activity as a sequence of a limited number of microstates that remain stable for tens of milliseconds before abruptly transitioning to another microstate. Electroencephalography resting-state activity is usually described well as a series of 4 to 6 microstates, which are remarkably similar across participants and labeled with letters. One microstate study detected lower occurrence and time coverage of microstate C in patients with chronic widespread pain compared with healthy participants.31 Another study found a less predominant role of microstate D in patients with chronic back pain than in healthy participants.67 In the field of network analysis (an overarching term for analysis of global measures of brain connectivity mainly based on graph theory), one cross-sectional study found a decrease in global efficiency in gamma frequencies in patients with chronic pain compared with healthy participants.102 Another cross-sectional study observed a lower “full synchronization time” (described as the time required for a network to become approximately complete) in sickle cell patients with chronic hip pain.95 In a longitudinal study examining changes in brain activity following multimodal pain therapy, a correlation between pain decrease and theta global efficiency increase was observed.37 A longitudinal neurofeedback study found negative correlations of changes in pain with fractional occupancy, mean dwell times, and transition probability from low to high alpha state.82 A descriptive study described an association of “explosive synchronization” characteristics and pain intensity in patients with fibromyalgia.56
M/EEG studies using machine learning algorithms have addressed diagnostic and predictive biomarker functions. Cross-sectional studies have been shown to differentiate better than chance between chronic pain populations and healthy participants in several studies (114accuracy 92.5%102; accuracy 57%59; accuracy 82.3%). Two studies applied machine learning to differentiate between different patient groups. In one of these studies, a 3-way classifier distinguished between patients with lumbar pain, patients with radiculopathy, and healthy participants with a cross-validation accuracy of 71.9%.59 The other study differentiated with high accuracy between patients with low (97%), moderate (96%), and high (96%) pain.125 Only 2 studies used EEG as predictive biomarkers examining response to treatment. One study in patients with lower back pain found no predictive value of baseline EEG regarding pain changes after cognitive/behavioral interventions.15 In another study, preoperative EEG characteristics could not predict pain-related outcome after surgery in patients with chronic hip pain.32
4. Discussion
4.1. Main findings
In this study, we summarized evidence on resting-state M/EEG as biomarkers of chronic pain. To this end, we conducted a systematic review of cross-sectional, longitudinal, and descriptive studies of patients with chronic pain using resting-state M/EEG as a measure of brain function. Overall, this approach revealed differences between study and, thus, potential biomarker types. For cross-sectional studies that can serve to develop diagnostic biomarkers, we found increased theta and beta power in patients with chronic pain. For longitudinal studies that can yield monitoring and/or predictive biomarkers, we found no clear associations of pain relief with M/EEG measures. Similarly, descriptive studies that can yield diagnostic or monitoring biomarkers showed no clear correlations of pain intensity with M/EEG measures. Notable findings regarding frequently discussed biomarker candidates in pain research included some evidence for lower peak alpha frequency, higher gamma power, and higher theta connectivity in patients with chronic pain in cross-sectional studies. Furthermore, we found some evidence for lower theta power along with pain relief in longitudinal studies and for a negative correlation of alpha power with pain intensity. However, for those parameters, studies reported mainly nonsignificant results.
A few previous systematic reviews of M/EEG findings in chronic pain have been published. However, their scope differed from this systematic review because of the focus on certain pain types and/or the joint examination of different biomarker types. One systematic review focused on patients with neuropathic pain caused by spinal cord injury and found evidence suggesting a reduction of PAF.81 Another review87 summarized resting-state and event-related EEG studies in a variety of patients with chronic pain. Regarding resting-state studies, they found evidence for an increase of theta and alpha power in patients. Recently, a third review focused on resting-state EEG studies in patients with neuropathic pain. They found power increases at theta and—to a lesser degree—at high beta frequencies. In addition, they observed power decreases at high alpha and low beta frequencies.72 No clear correlations with pain intensity were found.
In general, these results are in line with our findings. The most consistent finding across reviews was an increase in theta power in patients. In addition, we found increased beta power and some evidence for increases of gamma power and theta connectivity and toward a slowing of the PAF. These additional findings of this review might be the result of significantly larger number of studies included in this review (76 studies) than in the previous reviews (4-15 studies). Moreover, the semiquantitative approach of this review might be more sensitive than qualitative methods.
Our central finding of increased theta and beta power is compatible with the thalamocortical dysrhythmia concept of neuropsychiatric disorders,61,62 which is supported by animal studies in chronic pain models.55,93 The concept proposes that abnormal thalamocortical theta activity yields abnormal oscillations at beta/gamma frequencies, which eventually result in different neuropsychiatric symptoms, including ongoing pain. However, the concept has been proposed to account not only for chronic pain but also for various neuropsychiatric disorders. Moreover, opioid and antiepileptic treatment, frequently used in patients with chronic pain, can also yield increases of theta activity.65,94 In addition, the included studies in our review reported no consistent correlation between theta or beta power and pain intensity, and pain relief was not associated with power changes in those frequency bands. Thus, the specificity of increased theta and beta power for chronic pain remains unclear.
Besides frequency-specific power and connectivity, PAF has been discussed as a biomarker of chronic21,96 and experimental pain.28 Across the studies included in our review, we found some evidence for a slowing of PAF in patients, which can also be interpreted in the context of the thalamocortical dysrhythmia concept. However, peak alpha frequency has also been related to treatment response in attention deficit hyperactivity disorder2 depression,3 cognitive functioning,50 and age.4 Hence, also the specificity of PAF slowing for chronic pain remains unclear.
4.2. Risk of bias and limitations
Risk of bias assessment revealed limitations of many studies included in this review. First, most studies have small sample sizes (median 38.0) and a priori sample size calculations were rarely reported. Correction for multiple comparisons was performed in only about half of studies. Second, in almost all studies, M/EEG preprocessing and analysis was performed manually and was thus not well standardized. Third, only few studies accounted for potential confounds by medication effects, demographic factors (eg, age or sex/gender106), and neuropsychiatric comorbidities.
Limitations also apply to this systematic review itself. First, most studies included patients with different types and etiologies of chronic pain. Although such mixed pain types and populations reflect clinical reality, we did not take possible differences in brain mechanisms of different pain etiologies or types (eg, neuropathic pain and nociplastic pain) into account. However, color coding of studies with different pain types in the albatross plots did not indicate clear differences in results between pain types. Second, the designs of longitudinal studies were highly heterogeneous. The types of interventions and the timing of postinterventional assessments varied considerably. This heterogeneity might have obscured effects of single treatment methods. Third, for semiquantitative data synthesis by means of albatross plots, we summarized studies regardless of whether they reported global or local M/EEG measures. This might obscure contributions of specific brain regions and/or networks to the neural representation of chronic pain. Fourth, we excluded studies of patients with chronic pain caused by severe neurological comorbidity (eg, severe spinal cord injury) to increase specificity of results. Fifth, a few studies (not represented in the albatross plots) reported diverging effects for M/EEG measures depending on the region of interest. However, graphical or numerical inclusion of these results in our analysis would have led to misrepresentation of results being interpreted as complex network changes in these studies. Sixth, meta-analysis would have further increased the validity of our findings. However, because of reported outcome measures (eg, for cross-sectional comparisons of theta power, 47% of all studies but 79% of studies with negative results did not report the minimally required parameters for meta-analysis) and heterogeneous study designs, we could not perform formal meta-analysis.
4.3. Outlook and recommendations
This systematic review might help to guide future M/EEG studies on the development of pain biomarkers.
4.3.1. Differentiating biomarker types
Cross-sectional studies indicated increased theta and beta power in patients with chronic pain, which might serve the development of diagnostic biomarkers. By contrast, longitudinal studies that aim at monitoring rather than diagnostic biomarkers did not show a clear association between changes in pain intensity and changes of brain activity at any frequency. This dissociation suggests that different types of biomarkers might be served by different neuronal phenomena. Hence, different biomarkers should be considered for different functions. Moreover, most studies aimed at diagnostic and monitoring biomarkers. However, other biomarker types, such as predictive or prognostic biomarkers, might be at least equally important in clinical practice. Thus, we might broaden the scope of M/EEG biomarker development toward these biomarker types using longitudinal study designs with baseline or repeated M/EEG measurements. Recent large-scale studies in depression, in which resting-state EEG data predicted response to antidepressant treatment,121,123 could serve as a blueprint for pain research here.
4.3.2. Assessing biomarker specificity
The risk of bias assessment indicated that most included studies did not control for potential neuropsychiatric confounders like depression or anxiety. Thus, the specificity of the observed findings remains unclear. However, specificity is a key criterion in biomarker development.111 Consequently, findings in patients with chronic pain should be related to findings in other neuropsychiatric conditions, especially as chronic pain and affective disorders frequently co-occur.13 A recent review on resting-state EEG activity in neuropsychiatric disorders found increases in delta and theta power in depression, schizophrenia, attention-deficit hyperactivity disorder, and obsessive–compulsive disorder.73 An increase of theta power was also the most consistent finding in our review. Hence, large-scale studies across neuropsychiatric disorders, including chronic pain, are needed to carve out the specific M/EEG signatures of chronic pain. However, it is important to note that for clinical utility, specificity is not mandatory. Clinicians are very much used to make decisions based on patterns of unspecific information.
4.3.3. Investigating connectivity biomarkers
Most studies in this review focused on oscillatory brain activity in chronic pain. Fewer studies assessed connectivity, and we did not find a clear connectivity pattern that could serve as a biomarker of chronic pain. This might at least partly be the result of the complexity and methodological variability of connectivity analyses. However, the complexity of chronic pain and accumulating evidence from neuroimaging studies indicate that changes of connectivity in large-scale brain networks figure prominently in the pathology of chronic pain.52,58,88 For instance, a recent functional magnetic resonance imaging study on long-lasting experimental and clinical pain57 provided convincing evidence for a functional connectivity signature of ongoing pain. Thus, connectivity and network measures are particularly promising for the development of biomarkers of chronic pain.
4.3.4. Enhancing biomarker reproducibility
The sample sizes of studies included in this systematic review is rather small (median n = 38). Moreover, only very few studies have implemented most recent open science measures to enhance the reproducibility and transparency of the findings.71 However, timely approaches for the development of chronic pain biomarkers include machine learning, multivariate models, and multimodal/composite biomarker studies. These approaches essentially depend on large data sets, standardized clinical assessment, and fully transparent and standardized neuroimaging methods.85 In M/EEG biomarker development, this might include performance and reporting of studies according to consensus statements,85 a standardized Data structure (EEG-BIDS),84 automatic preprocessing and analysis pipelines,29,83,86 and sharing of code and data.
4.3.5. Exploring composite biomarkers
We here reviewed M/EEG studies on brain function in chronic pain. However, chronic pain is not only associated with changes of brain function but also associated with brain structure,54 sensory functions,118 psychological and social factors,13 and genetic information.70 Considering these different biopsychosocial determinants of chronic pain, integrating them into multimodal composite biomarkers is an evident next step.107 Such composite biomarkers, which integrate information from M/EEG recordings with other types of information, promise to enhance the sensitivity and specificity of biomarkers and might therefore be particularly worth exploring.
5. Conclusions
The development of biomarkers has been recognized as a crucial step toward optimizing the treatment of chronic pain.14,107 With accumulating evidence for substantial changes of brain structure and function in chronic pain, noninvasive, brain-based measures can likely significantly contribute to the development of pain biomarkers. Because of its scalability and ease of use in clinical settings, M/EEG holds great potential as pain biomarker.14,92,111 In this systematic review, we therefore summarized current evidence on resting-state M/EEG studies as biomarkers of chronic pain. In cross-sectional studies, we found evidence for increased theta and beta power as a diagnostic biomarker in patients with chronic pain. With respect to monitoring or predictive M/EEG biomarkers, evidence from longitudinal and descriptive studies is sparse and inconsistent so far. To proceed and better exploit the potential of M/EEG as biomarkers of pain, large-scale studies that differentiate biomarker types, assess biomarker specificity, investigate brain network connectivity, adhere to highest standards of transparency/reproducibility, and explore the potential of composite biomarkers are most promising.
Conflict of interest statement
The authors have no conflict of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B751.
Acknowledgments
The authors thank the members of the PainLabMunich for helpful discussions and comments on the manuscript. The study was supported by the TUM Innovation Network Neurotechnology in Mental Health (NEUROTECH), the Studienstiftung des Deutschen Volkes, and the TUM School of Medicine Clinician Scientist Program (KKF).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Contributor Information
Paul Theo Zebhauser, Email: paul.zebhauser@tum.de.
Vanessa D. Hohn, Email: vanessa.hohn@tum.de.
References
- [1].Ahn S, Prim JH, Alexander ML, McCulloch KL, Fröhlich F. Identifying and engaging neuronal oscillations by transcranial alternating current stimulation in patients with chronic low back pain: a randomized, crossover, double-blind, sham-controlled pilot study. J Pain 2019;20:277.e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arns M, Gunkelman J, Breteler M, Spronk D. EEG phenotypes predict treatment outcome to stimulants in children with ADHD. J Integr Neurosci 2008;7:421–38. [DOI] [PubMed] [Google Scholar]
- [3].Arns M, Drinkenburg WH, Fitzgerald PB, Kenemans JL. Neurophysiological predictors of non-response to rTMS in depression. Brain Stimul 2012;5:569–76. [DOI] [PubMed] [Google Scholar]
- [4].Aurlien H, Gjerde IO, Aarseth JH, Eldoen G, Karlsen B, Skeidsvoll H, Gilhus NE. EEG background activity described by a large computerized database. Clin Neurophysiol 2004;115:665–73. [DOI] [PubMed] [Google Scholar]
- [5].Barbosa-Torres C, Cubo-Delgado S. Clinical findings in SMR neurofeedback protocol training in women with fibromyalgia syndrome. Brain Sci 2021;11:1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baroni A, Severini G, Straudi S, Buja S, Borsato S, Basaglia N. Hyperalgesia and central sensitization in subjects with chronic orofacial pain: analysis of pain thresholds and EEG biomarkers. Front Neurosci 2020;14:552650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bell IR, Baldwin CM, Stoltz E, Walsh BT, Schwartz GER. Concomitant environmental chemical intolerance modifies the neurobehavioral presentation of women with fibromyalgia. J Chronic Fatigue Syndr 2001;9:3–19. [Google Scholar]
- [8].Bernardi L, Bertuccelli M, Formaggio E, Rubega M, Bosco G, Tenconi E, Cattelan M, Masiero S, Del Felice A. Beyond physiotherapy and pharmacological treatment for fibromyalgia syndrome: tailored tACS as a new therapeutic tool. Eur Arch Psychiatry Clin Neurosci 2021;271:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].BEST (biomarkers, endpoints, and other tools) resource. Silver Spring, MD: Food and Drug Administration (US), 2016. [PubMed] [Google Scholar]
- [10].Camfferman D, Moseley GL, Gertz K, Pettet MW, Jensen MP. Waking EEG cortical markers of chronic pain and sleepiness. Pain Med 2017;18:1921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Choe MK, Lim M, Kim JS, Lee DS, Chung CK. Disrupted resting state network of fibromyalgia in theta frequency. Sci Rep 2018;8:2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, 2021. [Google Scholar]
- [13].Currie SR, Wang J. More data on major depression as an antecedent risk factor for first onset of chronic back pain. Psychol Med 2005;35:1275–82. [DOI] [PubMed] [Google Scholar]
- [14].Davis KD, Aghaeepour N, Ahn AH, Angst MS, Borsook D, Brenton A, Burczynski ME, Crean C, Edwards R, Gaudilliere B, Hergenroeder GW, Iadarola MJ, Iyengar S, Jiang Y, Kong J-T, Mackey S, Saab CY, Sang CN, Scholz J, Segerdahl M, Tracey I, Veasley C, Wang J, Wager TD, Wasan AD, Pelleymounter MA. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nat Rev Neurol 2020;16:381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Day MA, Thorn BE, Ehde DM, Burns JW, Barnier A, Mattingley JB, Matthews N, Jensen MP. Moderators of mindfulness meditation, cognitive therapy, and mindfulness-based cognitive therapy for chronic low back pain: a test of the limit, activate, and enhance model. J Pain 2020;21:161–9. [DOI] [PubMed] [Google Scholar]
- [16].Day MA, Matthews N, Mattingley JB, Ehde DM, Turner AP, Williams RM, Jensen MP. Change in brain oscillations as a mechanism of mindfulness-meditation, cognitive therapy, and mindfulness-based cognitive therapy for chronic low back pain. Pain Med 2021;22:1804–13. [DOI] [PubMed] [Google Scholar]
- [17].de Aguiar Neto FS, Rosa JLG. Depression biomarkers using non-invasive EEG: a review. Neurosci Biobehavioral Rev 2019;105:83–93. [DOI] [PubMed] [Google Scholar]
- [18].de Melo GA, Madruga MLLH, Silva CJdA, Torro N. Association between pain, anxiety, and alpha2 frontal activity in women with fibromyalgia. Acta Sci Health Sci 2021;43; e49846. [Google Scholar]
- [19].de Melo GA, de Oliveira EA, dos Santos Andrade SMM, Fernandez-Calvo B, Torro N. Comparison of two tDCS protocols on pain and EEG alpha-2 oscillations in women with fibromyalgia. Sci Rep 2020;10:18955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].De Ridder D, Vanneste S. Occipital nerve field transcranial direct current stimulation normalizes imbalance between pain detecting and pain inhibitory pathways in fibromyalgia. Neurotherapeutics 2017;14:484–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].de Vries M, Wilder-Smith OH, Jongsma ML, van den Broeke EN, Arns M, van Goor H, van Rijn CM. Altered resting state EEG in chronic pancreatitis patients: toward a marker for chronic pain. J Pain Res 2013;6:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Di Pietro F, Macey PM, Rae CD, Alshelh Z, Macefield VG, Vickers ER, Henderson LA. The relationship between thalamic GABA content and resting cortical rhythm in neuropathic pain. Hum Brain Mapp 2018;39:1945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fallon N, Chiu Y, Nurmikko T, Stancak A. Altered theta oscillations in resting EEG of fibromyalgia syndrome patients. Eur J Pain 2018;22:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fauchon C, Kim JA, El-Sayed R, Osborne NR, Rogachov A, Cheng JC, Hemington KS, Bosma RL, Dunkley BT, Oh J, Bhatia A, Inman RD, Davis KD. Exploring sex differences in alpha brain activity as a potential neuromarker associated with neuropathic pain. PAIN 2022;163:1291–1302. [DOI] [PubMed] [Google Scholar]
- [25].Feng L, Li H, Cui H, Xie X, Xu S, Hu Y. Low back pain assessment based on alpha oscillation changes in spontaneous electroencephalogram (EEG). Neural Plasticity 2021;2021:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ferdek MA, Oosterman JM, Adamczyk AK, van Aken M, Woudsma KJ, Peeters BW, Nap A, Wyczesany M, van Rijn CM. Effective connectivity of beta oscillations in endometriosis-related chronic pain during rest and pain-related mental imagery. J Pain 2019;20:1446–58. [DOI] [PubMed] [Google Scholar]
- [27].Freye E, Levy JV. The effects of tramadol on pain relief, fast EEG-power spectrum and cognitive function in elderly patients with chronic osteoarthritis (OA). Acute Pain 2006;8:55–61. [Google Scholar]
- [28].Furman AJ, Meeker TJ, Rietschel JC, Yoo S, Muthulingam J, Prokhorenko M, Keaser ML, Goodman RN, Mazaheri A, Seminowicz DA. Cerebral peak alpha frequency predicts individual differences in pain sensitivity. Neuroimage 2018;167:203–10. [DOI] [PubMed] [Google Scholar]
- [29].Gabard-Durnam LJ, Mendez Leal AS, Wilkinson CL, Levin AR. The Harvard automated processing pipeline for electroencephalography (HAPPE): standardized processing software for developmental and high-artifact data. Front Neurosci 2018;12:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].González-Roldán AM, Cifre I, Sitges C, Montoya P. Altered dynamic of EEG oscillations in fibromyalgia patients at rest. Pain Med 2016;17:1058–68. [DOI] [PubMed] [Google Scholar]
- [31].Gonzalez-Villar AJ, Trinanes Y, Gomez-Perretta C, Carrillo-de-la-Pena MT. Patients with fibromyalgia show increased beta connectivity across distant networks and microstates alterations in resting-state electroencephalogram. Neuroimage 2020;223:117266. [DOI] [PubMed] [Google Scholar]
- [32].Gram M, Erlenwein J, Petzke F, Falla D, Przemeck M, Emons MI, Reuster M, Olesen SS, Drewes AM. Prediction of postoperative opioid analgesia using clinical-experimental parameters and electroencephalography. Eur J Pain 2017;21:264–77. [DOI] [PubMed] [Google Scholar]
- [33].Graversen C, Olesen SS, Olesen AE, Steimle K, Farina D, Wilder-Smith OHG, Bouwense SAW, van Goor H, Drewes AM. The analgesic effect of pregabalin in patients with chronic pain is reflected by changes in pharmaco-EEG spectral indices. Br J Clin Pharmacol 2012;73:363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Grimes DA, Schulz KF. An overview of clinical research: the lay of the land. Lancet 2002;359:57–61. [DOI] [PubMed] [Google Scholar]
- [35].Hargrove JB, Bennett RM, Simons DG, Smith SJ, Nagpal S, Deering DE. Quantitative electroencephalographic abnormalities in fibromyalgia patients. Clin EEG Neurosci 2010;41:132–9. [DOI] [PubMed] [Google Scholar]
- [36].Harrison S, Jones HE, Martin RM, Lewis SJ, Higgins JPT. The albatross plot: a novel graphical tool for presenting results of diversely reported studies in a systematic review. Res Synth Methods 2017;8:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Heitmann H, Ávila CG, Nickel MM, Dinh ST, May ES, Tiemann L, Hohn VD, Tölle TR, Ploner M. Longitudinal resting-state electroencephalography in chronic pain patients undergoing interdisciplinary multimodal pain therapy. PAIN 2022;163:e997–e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hsiao FJ, Wang SJ, Lin YY, Fuh JL, Ko YC, Wang PN, Chen WT. Altered insula-default mode network connectivity in fibromyalgia: a resting-state magnetoencephalographic study. J Headache Pain 2017;18:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hunter AM, Leuchter AF, Cook IA, Abrams M, Siegman BE, Furst DE, Chappell AS. Brain functional changes and duloxetine treatment response in fibromyalgia: a pilot study. Pain Med 2009;10:730–8. [DOI] [PubMed] [Google Scholar]
- [40].Iwatsuki K, Hoshiyama M, Yoshida A, Uemura JI, Hoshino A, Morikawa I, Nakagawa Y, Hirata H. Chronic pain-related cortical neural activity in patients with complex regional pain syndrome. IBRO Neurosci Rep 2021;10:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jensen MP, Gertz KJ, Kupper AE, Braden AL, Howe JD, Hakimian S, Sherlin LH. Steps toward developing an EEG biofeedback treatment for chronic pain. Appl Psychophysiol Biofeedback 2013;38:101–8. [DOI] [PubMed] [Google Scholar]
- [42].Jensen MP, Sherlin LH, Gertz KJ, Braden AL, Kupper AE, Gianas A, Howe JD, Hakimian S. Brain EEG activity correlates of chronic pain in persons with spinal cord injury: clinical implications. Spinal Cord 2013;51:55–58. [DOI] [PubMed] [Google Scholar]
- [43].Jensen MP, Hakimian S, Ehde DM, Day MA, Pettet MW, Yoshino A, Ciol MA. Pain-related beliefs, cognitive processes, and electroencephalography band power as predictors and mediators of the effects of psychological chronic pain interventions. PAIN 2021;162:2036–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Juel J, Liguori S, Liguori A, Poulsen JL, Valeriani M, Graversen C, Olesen SS, Drewes AM. Acupuncture for pain in chronic pancreatitis: a single-blinded randomized crossover trial. Pancreas 2017;46:170–6. [DOI] [PubMed] [Google Scholar]
- [45].Kayiran S, Dursun E, Dursun N, Ermutlu N, Karamürsel S. Neurofeedback intervention in fibromyalgia syndrome; a randomized, controlled, rater blind clinical trial. Appl Psychophysiol Biofeedback 2010;35:293–302. [DOI] [PubMed] [Google Scholar]
- [46].Kim JA, Bosma RL, Hemington KS, Rogachov A, Osborne NR, Cheng JC, Oh J, Crawley AP, Dunkley BT, Davis KD. Neuropathic pain and pain interference are linked to alpha-band slowing and reduced beta-band magnetoencephalography activity within the dynamic pain connectome in patients with multiple sclerosis. PAIN 2019;160:187–97. [DOI] [PubMed] [Google Scholar]
- [47].Kim JA, Bosma RL, Hemington KS, Rogachov A, Osborne NR, Cheng JC, Oh J, Dunkley BT, Davis KD. Cross-network coupling of neural oscillations in the dynamic pain connectome reflects chronic neuropathic pain in multiple sclerosis. Neuroimage Clin 2020;26:102230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kim YS, Kim HS, Lee CH, Jung S, Yoon CH, Kwon O-Y. Cerebral current-source distribution associated with pain improvement by non-invasive painless signaling therapy in patients with failed back surgery syndrome. Korean J Pain 2021;34:437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kisler LB, Kim JA, Hemington KS, Rogachov A, Cheng JC, Bosma RL, Osborne NR, Dunkley BT, Inman RD, Davis KD. Abnormal alpha band power in the dynamic pain connectome is a marker of chronic pain with a neuropathic component. Neuroimage Clin 2020;26:102241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Klimesch W. alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci 2012;16:606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Klug S, Anderer P, Saletu-Zyhlarz G, Freidl M, Saletu B, Prause W, Aigner M. Dysfunctional pain modulation in somatoform pain disorder patients. Eur Arch Psychiatry Clin Neurosci 2011;261:267–75. [DOI] [PubMed] [Google Scholar]
- [52].Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci 2015;38:86–95. [DOI] [PubMed] [Google Scholar]
- [53].Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci 2017;18:20–30. [DOI] [PubMed] [Google Scholar]
- [54].Kuner R, Kuner T. Cellular circuits in the brain and their modulation in acute and chronic pain. Physiol Rev 2021;101:213–58. [DOI] [PubMed] [Google Scholar]
- [55].LeBlanc BW, Lii TR, Silverman AE, Alleyne RT, Saab CY. Cortical theta is increased while thalamocortical coherence is decreased in rat models of acute and chronic pain. PAIN 2014;155:773–82. [DOI] [PubMed] [Google Scholar]
- [56].Lee U, Kim M, Lee K, Kaplan CM, Clauw DJ, Kim S, Mashour GA, Harris RE. Functional brain network mechanism of hypersensitivity in chronic pain. Sci Rep 2018;8:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lee J-J, Kim HJ, Čeko M, Park By, Lee SA, Park H, Roy M, Kim SG, Wager TD, Woo CW. A neuroimaging biomarker for sustained experimental and clinical pain. Nat Med 2021;27:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lenoir D, Cagnie B, Verhelst H, De Pauw R. Graph measure based connectivity in chronic pain patients: a systematic review. Pain Physician 2021;24:E1037–58. [PubMed] [Google Scholar]
- [59].Levitt J, Edhi MM, Thorpe RV, Leung JW, Michishita M, Koyama S, Yoshikawa S, Scarfo KA, Carayannopoulos AG, Gu W, Srivastava KH, Clark BA, Esteller R, Borton DA, Jones SR, Saab CY. Pain phenotypes classified by machine learning using electroencephalography features. Neuroimage 2020;223:117256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lim M, Kim JS, Kim DJ, Chung CK, Increased low- and high-frequency oscillatory activity in the prefrontal cortex of fibromyalgia patients. Front Hum Neurosci 2016;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci 1999;96:15222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci 2005;28:325–33. [DOI] [PubMed] [Google Scholar]
- [63].Lopez JN, Bergos RD, Marijuan PC. Significant new quantitative EEG patterns in fibromyalgia. Eur J Psychiatr 2015;29:277–92. [Google Scholar]
- [64].Mackey S, Greely HT, Martucci KT. Neuroimaging-based pain biomarkers: definitions, clinical and research applications, and evaluation frameworks to achieve personalized pain medicine. Pain Rep 2019;4:e762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Malver LP, Brokjær A, Staahl C, Graversen C, Andresen T, Drewes AM. Electroencephalography and analgesics. Br J Clin Pharmacol 2014;77:72–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Martín-Brufau R, Gómez MN, Sanchez-Sanchez-Rojas L, Nombela C. Fibromyalgia detection based on EEG connectivity patterns. J Clin Med 2021;10:3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].May ES, Avila CG, Dinh ST, Heitmann H, Hohn VD, Nickel MM, Tiemann L, Tolle TR, Ploner M. Dynamics of brain function in patients with chronic pain assessed by microstate analysis of resting-state electroencephalography. PAIN 2021;162:2894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Meneses FM, Queiros FC, Montoya P, Miranda JGV, Dubois-Mendes SM, Sa KN, Luz-Santos C, Baptista AF. Patients with rheumatoid arthritis and chronic pain display enhanced alpha power density at rest. Front Hum Neurosci 2016;10:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Michels L, Moazami-Goudarzi M, Jeanmonod D. Correlations between EEG and clinical outcome in chronic neuropathic pain: surgical effects and treatment resistance. Brain Imaging Behav 2011;5:329–48. [DOI] [PubMed] [Google Scholar]
- [70].Mogil JS. Pain genetics: past, present and future. Trends Genet 2012;28:258–66. [DOI] [PubMed] [Google Scholar]
- [71].Munafo MR, Nosek BA, Bishop DVM, Button KS, Chambers CD, Percie du Sert N, Simonsohn U, Wagenmakers EJ, Ware JJ, Ioannidis JPA. A manifesto for reproducible science. Nat Hum Behav 2017;1:0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mussigmann T, Bardel B, Lefaucheur J-P. Resting-state electroencephalography (EEG) biomarkers of chronic neuropathic pain. A systematic review. Neuroimage 2022;258:119351. [DOI] [PubMed] [Google Scholar]
- [73].Newson JJ, Thiagarajan TC. EEG frequency bands in psychiatric disorders: a review of resting state studies. Front Hum Neurosci 2018;12:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Nomura T, Fukudo S, Matsuoka H, Hongo M. Abnormal electroencephalogram in irritable bowel syndrome. Scand J Gastroenterol 1999;34:478–84. [DOI] [PubMed] [Google Scholar]
- [75].Oga K, Kojima T, Matsuura M, Nagashima M, Kato J, Saeki S, Ogawa S. Effects of low-dose ketamine on neuropathic pain: an electroencephalogram-electrooculogram/behavioral study. Psychiatry Clin Neurosci 2002;56:355–63. [DOI] [PubMed] [Google Scholar]
- [76].Olesen SS, Hansen TM, Graversen C, Steimle K, Wilder-Smith OH, Drewes AM. Slowed EEG rhythmicity in patients with chronic pancreatitis: evidence of abnormal cerebral pain processing? Eur J Gastroenterol Hepatol 2011;23:418–24. [DOI] [PubMed] [Google Scholar]
- [77].Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pan L-LH, Hsiao F-J, Chen W-T, Wang S-J. Resting state electrophysiological cortical activity: a brain signature candidate for patients with migraine. Curr Pain Headache Rep 2022;26:289–97. [DOI] [PubMed] [Google Scholar]
- [79].Parker T, Huang Y, Raghu ALB, FitzGerald J, Aziz TZ, Green AL. Supraspinal effects of dorsal root ganglion stimulation in chronic pain patients. Neuromodulation 2021;24:646–54. [DOI] [PubMed] [Google Scholar]
- [80].Parker T, Raghu A, Huang Y, Gillies MJ, FitzGerald JJ, Aziz T, Green AL. Paired acute invasive/non-invasive stimulation (PAINS) study: a phase I/II randomized, sham-controlled crossover trial in chronic neuropathic pain. Brain Stimul 2021;14:1576–85. [DOI] [PubMed] [Google Scholar]
- [81].Pascoal-Faria P, Yalcin N, Fregni F. Neural markers of neuropathic pain associated with maladaptive plasticity in spinal cord injury. Pain Pract 2015;15:371–7. [DOI] [PubMed] [Google Scholar]
- [82].Patel K, Henshaw J, Sutherland H, Taylor JR, Casson AJ, Lopez-Diaz K, Brown CA, Jones AKP, Sivan M, Trujillo-Barreto NJ. Using EEG alpha states to understand learning during alpha neurofeedback training for chronic pain. Front Neurosci 2020;14:620666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Pedroni A, Bahreini A, Langer N. Automagic: standardized preprocessing of big EEG data. Neuroimage 2019;200:460–73. [DOI] [PubMed] [Google Scholar]
- [84].Pernet CR, Appelhoff S, Gorgolewski KJ, Flandin G, Phillips C, Delorme A, Oostenveld R. EEG-BIDS, an extension to the brain imaging data structure for electroencephalography. Sci Data 2019;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pernet C, Garrido MI, Gramfort A, Maurits N, Michel CM, Pang E, Salmelin R, Schoffelen JM, Valdes-Sosa PA, Puce A. Issues and recommendations from the OHBM COBIDAS MEEG committee for reproducible EEG and MEG research. Nat Neurosci 2020;23:1473–83. [DOI] [PubMed] [Google Scholar]
- [86].Pernet CR, Martinez-Cancino R, Truong D, Makeig S, Delorme A. From BIDS-formatted EEG data to sensor-space group results: a fully reproducible workflow with EEGLAB and LIMO EEG. Front Neurosci 2020;14:610388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Pinheiro ESdS, Queirós FCd, Montoya P, Santos CL, Nascimento MAd, Ito CH, Silva M, Nunes Santos DB, Benevides S, Miranda JGV, Sa KN, Baptista AF. Electroencephalographic patterns in chronic pain: a systematic review of the literature. PLoS One 2016;11:e0149085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ploner M, Sorg C, Gross J. Brain rhythms of pain. Trends Cogn Sci 2017;21:100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Prichep LS, John ER, Howard B, Merkin H, Hiesiger EM. Evaluation of the pain matrix using EEG source localization: a feasibility study. Pain Med 2011;12:1241–8. [DOI] [PubMed] [Google Scholar]
- [90].Prichep LS, Shah J, Merkin H, Hiesiger EM. Exploration of the pathophysiology of chronic pain using quantitative EEG source localization. Clin EEG Neurosci 2018;49:103–13. [DOI] [PubMed] [Google Scholar]
- [91].Prinsloo S, Novy D, Driver L, Lyle R, Ramondetta L, Eng C, McQuade J, Lopez G, Cohen L. Randomized controlled trial of neurofeedback on chemotherapy-induced peripheral neuropathy: a pilot study. Cancer 2017;123:1989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Reckziegel D, Vachon-Presseau E, Petre B, Schnitzer TJ, Baliki MN, Apkarian AV. Deconstructing biomarkers for chronic pain: context- and hypothesis-dependent biomarker types in relation to chronic pain. PAIN 2019;160(suppl 1):S37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Saab CY, Barrett LF. Thalamic bursts and the epic pain model. Front Comput Neurosci 2016;10:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Salinsky MC, Binder LM, Oken BS, Storzbach D, Aron CR, Dodrill CB. Effects of gabapentin and carbamazepine on the EEG and cognition in healthy volunteers. Epilepsia 2002;43:482–90. [DOI] [PubMed] [Google Scholar]
- [95].Santana JERS, Baptista AF, Lucena R, Lopes TDS, do Rosario RS, Xavier MR, Fonseca A, Miranda JGV. Altered dynamic brain connectivity in individuals with sickle cell disease and chronic pain secondary to hip osteonecrosis. Clin EEG Neurosci 2021. doi: 10.1177/15500594211054297. [DOI] [PubMed] [Google Scholar]
- [96].Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain 2006;129:55–64. [DOI] [PubMed] [Google Scholar]
- [97].Schmidt S, Naranjo JR, Brenneisen C, Gundlach J, Schultz C, Kaube H, Hinterberger T, Jeanmonod D. Pain ratings, psychological functioning and quantitative EEG in a controlled study of chronic back pain patients. PLoS One 2012;7:e31138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Schmidt S, Gmeiner S, Schultz C, Lower M, Kuhn K, Naranjo JR, Brenneisen C, Hinterberger T. Mindfulness-based stress reduction (MBSR) as treatment for chronic back pain—an observational study with assessment of thalamocortical dysrhythmia. Complement Med Res 2015;22:298–303. [DOI] [PubMed] [Google Scholar]
- [99].Smailovic U, Jelic V. Neurophysiological markers of alzheimer's disease: quantitative EEG approach. Neurol Ther 2019;8:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Stern J, Jeanmonod D, Sarnthein J. Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. Neuroimage 2006;31:721–31. [DOI] [PubMed] [Google Scholar]
- [101].Sufianov AA, Shapkin AG, Sufianova GZ, Elishev VG, Barashin DA, Berdichevskii VB, Churkin SV. Functional and metabolic changes in the brain in neuropathic pain syndrome against the background of chronic epidural electrostimulation of the spinal cord. Bull Exp Biol Med 2014;157:462–5. [DOI] [PubMed] [Google Scholar]
- [102].Ta Dinh S, Nickel MM, Tiemann L, May ES, Heitmann H, Hohn VD, Edenharter G, Utpadel-Fischler D, Tolle TR, Sauseng P, Gross J, Ploner M. Brain dysfunction in chronic pain patients assessed by resting-state electroencephalography. PAIN 2019;160:2751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Teixeira M, Mancini C, Wicht CA, Maestretti G, Kuntzer T, Cazzoli D, Mouthon M, Annoni JM, Chabwine JN. Beta electroencephalographic oscillation is a potential GABAergic biomarker of chronic peripheral neuropathic pain. Front Neurosci 2021;15:594536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Teixeira PEP, Pacheco-Barrios K, Uygur-Kucukseymen E, Machado RM, Balbuena-Pareja A, Giannoni-Luza S, Luna-Cuadros MA, Cardenas-Rojas A, Gonzalez-Mego P, Mejia-Pando PF, Wagner T, Dipietro L, Fregni F. Electroencephalography signatures for conditioned pain modulation and pain perception in nonspecific chronic low back pain-an exploratory study. Pain Med 2022;23:558–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Thibaut A, Russo C, Hurtado-Puerto AM, Morales-Quezada JL, Deitos A, Petrozza JC, Freedman S, Fregni F. Effects of transcranial direct current stimulation, transcranial pulsed current stimulation, and their combination on brain oscillations in patients with chronic visceral pain: a pilot crossover randomized controlled study. Front Neurol 2017;8:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Tomescu MI, Rihs TA, Rochas V, Hardmeier M, Britz J, Allali G, Fuhr P, Eliez S, Michel CM. From swing to cane: sex differences of EEG resting-state temporal patterns during maturation and aging. Develop Cogn Neurosci 2018;31:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Tracey I, Woolf CJ, Andrews NA. Composite pain biomarker signatures for objective assessment and effective treatment. Neuron 2019;101:783–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang S-J. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). PAIN 2019;160:19–27. [DOI] [PubMed] [Google Scholar]
- [109].Uygur-Kucukseymen E, Castelo-Branco L, Pacheco-Barrios K, Luna-Cuadros MA, Cardenas-Rojas A, Giannoni-Luza S, Zeng H, Gianlorenco AC, Gnoatto-Medeiros M, Shaikh ES, Caumo W, Fregni F. Decreased neural inhibitory state in fibromyalgia pain: a cross-sectional study. Neurophysiol Clin 2020;50:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].van den Broeke EN, Wilder-Smith OHG, van Goor H, Vissers KCP, van Rijn CM. Patients with persistent pain after breast cancer treatment show enhanced alpha activity in spontaneous EEG. Pain Med 2013;14:1893–9. [DOI] [PubMed] [Google Scholar]
- [111].van der Miesen MM, Lindquist MA, Wager TD. Neuroimaging-based biomarkers for pain: state of the field and current directions. Pain Rep 2019;4:e751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Vanneste S, De Ridder D. Chronic pain as a brain imbalance between pain input and pain suppression. Brain Commun 2021;3:fcab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Vanneste S, Ost J, Van Havenbergh T, De Ridder D. Resting state electrical brain activity and connectivity in fibromyalgia. PLoS One 2017;12:e0178516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Vanneste S, Song J-J, De Ridder D. Thalamocortical dysrhythmia detected by machine learning. Nat Commun 2018;9:1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Villafaina S, Collado-Mateo D, Fuentes-García JP, Cano-Plasencia R, Gusi N. Impact of fibromyalgia on alpha-2 EEG power spectrum in the resting condition: a descriptive correlational study. Biomed Res Int 2019;2019:7851047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Vos T Lim SS Abbafati C Abbas KM Abbasi M Abbasifard M Abbasi-Kangevari M Abbastabar H Abd-Allah F Abdelalim A Abdollahi M Abdollahpour I Abolhassani H Aboyans V Abrams EM Abreu LG Abrigo MRM Abu-Raddad LJ Abushouk AI Acebedo A Ackerman IN Adabi M Adamu AA Adebayo OM Adekanmbi V Adelson JD Adetokunboh OO Adham D Afshari M Afshin A Agardh EE Agarwal G Agesa KM Aghaali M Aghamir SMK Agrawal A Ahmad T Ahmadi A Ahmadi M Ahmadieh H Ahmadpour E Akalu TY Akinyemi RO Akinyemiju T Akombi B Al-Aly Z Alam K Alam N Alam S Alam T Alanzi TM Albertson SB Alcalde-Rabanal JE Alema NM Ali M Ali S Alicandro G Alijanzadeh M Alinia C Alipour V Aljunid SM Alla F Allebeck P Almasi-Hashiani A Alonso J Al-Raddadi RM Altirkawi KA Alvis-Guzman N Alvis-Zakzuk NJ. , Amini S Amini-Rarani M Aminorroaya A Amiri F Amit AML Amugsi DA Amul GGH Anderlini D Andrei CL Andrei T Anjomshoa M Ansari F Ansari I Ansari-Moghaddam A Antonio CAT Antony CM Antriyandarti E Anvari D Anwer R Arabloo J Arab-Zozani M Aravkin AY Ariani F Arnlov J Aryal KK Arzani A Asadi-Aliabadi M Asadi-Pooya AA Asghari B Ashbaugh C Atnafu DD Atre SR Ausloos F Ausloos M Ayala Quintanilla BP Ayano G Ayanore MA Aynalem YA Azari S Azarian G Azene ZN Babaee E Badawi A Bagherzadeh M Bakhshaei MH Bakhtiari A Balakrishnan S Balalla S Balassyano S Banach M Banik PC Bannick MS Bante AB Baraki AG Barboza MA Barker-Collo SL Barthelemy CM Barua L Barzegar A Basu S Baune BT Bayati M Bazmandegan G Bedi N Beghi E Bejot Y Bello AK Bender RG Bennett DA Bennitt FB Bensenor IM Benziger CP Berhe K Bernabe E Bertolacci GJ Bhageerathy R Bhala N Bhandari D Bhardwaj P Bhattacharyya K Bhutta ZA Bibi S Biehl MH Bikbov B Bin Sayeed MS Biondi A Birihane BM Bisanzio D Bisignano C Biswas RK Bohlouli S Bohluli M Bolla SRR Boloor A Boon-Dooley AS Borges G Borzi AM Bourne R Brady OJ Brauer M Brayne C Breitborde NJK Brenner H Briant PS Briggs AM Briko NI Britton GB Bryazka D Buchbinder R Bumgarner BR Busse R Butt ZA Caetano dos Santos FL Camera LLA Campos-Nonato IR Car J Cardenas R Carreras G Carrero JJ Carvalho F Castaldelli-Maia JM Castaneda-Orjuela CA Castelpietra G Castle CD Castro F Catala-Lopez F Causey K Cederroth CR Cercy KM Cerin E Chandan JS Chang AR Charlson FJ Chattu VK Chaturvedi S Chimed-Ochir O Chin KL Cho DY Christensen H Chu DT Chung MT Cicuttini FM Ciobanu LG Cirillo M Collins EL Compton K Conti S Cortesi PA Costa VM Cousin E Cowden RG Cowie BC Cromwell EA Cross DH Crowe CS Cruz JA Cunningham M Dahlawi SMA Damiani G Dandona L Dandona R Darwesh AM Daryani A Das JK Das Gupta R das Neves J Davila-Cervantes CA Davletov K De Leo D Dean FE DeCleene NK Deen A Degenhardt L Dellavalle RP Demeke FM Demsie DG Denova-Gutierrez E Dereje ND Dervenis N Desai R Desalew A Dessie GA Dharmaratne SD Dhungana GP Dianatinasab M Diaz D Dibaji Forooshani ZS Dingels ZV Dirac MA Djalalinia S Do HT Dokova K Dorostkar F Doshi CP Doshmangir L Douiri A Doxey MC Driscoll TR Dunachie SJ Duncan BB Duraes AR Eagan AW Ebrahimi Kalan M Edvardsson D Ehrlich JR El Nahas N El Sayed I El Tantawi M Elbarazi I Elgendy IY Elhabashy HR El-Jaafary SI Elyazar IR Emamian MH Emmons-Bell S Erskine HE Eshrati B Eskandarieh S Esmaeilnejad S Esmaeilzadeh F Esteghamati A Estep K Etemadi A Etisso AE Farahmand M Faraj A Fareed M Faridnia R Farinha CSeS Farioli A Faro A Faruque M Farzadfar F Fattahi N Fazlzadeh M Feigin VL Feldman R Fereshtehnejad SM Fernandes E Ferrari AJ Ferreira ML Filip I Fischer F Fisher JL Fitzgerald R Flohr C Flor LS Foigt NA Folayan MO Force LM Fornari C Foroutan M Fox JT Freitas M Fu W Fukumoto T Furtado JM Gad MM Gakidou E Galles NC Gallus S Gamkrelidze A Garcia-Basteiro AL Gardner WM Geberemariyam BS Gebrehiwot AM Gebremedhin KB Gebreslassie AAAA Gershberg Hayoon A Gething PW Ghadimi M Ghadiri K Ghafourifard M Ghajar A Ghamari F Ghashghaee A Ghiasvand H Ghith N Gholamian A Gilani SA Gill PS Gitimoghaddam M Giussani G Goli S Gomez RS Gopalani SV Gorini G Gorman TM Gottlich HC Goudarzi H Goulart AC Goulart BNG Grada A Grivna M Grosso G Gubari MIM Gugnani HC Guimaraes ALS Guimaraes RA Guled RA Guo G Guo Y Gupta R Haagsma JA Haddock B Hafezi-Nejad N Hafiz A Hagins H Haile LM Hall BJ Halvaei I Hamadeh RR Hamagharib Abdullah K Hamilton EB Han C Han H Hankey GJ Haro JM Harvey JD Hasaballah AI Hasanzadeh A Hashemian M Hassanipour S Hassankhani H Havmoeller RJ Hay RJ Hay SI Hayat K Heidari B Heidari G Heidari-Soureshjani R Hendrie D Henrikson HJ Henry NJ Herteliu C Heydarpour F Hird TR Hoek HW Hole MK Holla R Hoogar P Hosgood HD Hosseinzadeh M Hostiuc M Hostiuc S Househ M Hoy DG Hsairi M Hsieh VCr Hu G Huda TM Hugo FN Huynh CK Hwang BF Iannucci VC Ibitoye SE Ikuta KS Ilesanmi OS Ilic IM Ilic MD Inbaraj LR Ippolito H Irvani SSN Islam MM Islam M Islam SMS Islami F Iso H Ivers RQ Iwu CCD Iyamu IO Jaafari J Jacobsen KH Jadidi-Niaragh F Jafari H Jafarinia M Jahagirdar D Jahani MA Jahanmehr N Jakovljevic M Jalali A Jalilian F James SL Janjani H Janodia MD Jayatilleke AU Jeemon P Jenabi E Jha RP Jha V Ji JS Jia P John O John-Akinola YO Johnson CO Johnson SC Jonas JB Joo T Joshi A Jozwiak JJ Jurisson M Kabir A Kabir Z Kalani H Kalani R Kalankesh LR Kalhor R Kamiab Z Kanchan T Karami Matin B Karch A Karim MA Karimi SE Kassa GM Kassebaum NJ Katikireddi SV Kawakami N Kayode GA Keddie SH Keller C Kereselidze M Khafaie MA Khalid N Khan M Khatab K Khater MM Khatib MN Khayamzadeh M Khodayari MT Khundkar R Kianipour N Kieling C Kim D Kim YE Kim YJ Kimokoti RW Kisa A Kisa S Kissimova-Skarbek K Kivimaki M Kneib CJ Knudsen AKS Kocarnik JM Kolola T Kopec JA Kosen S Koul PA Koyanagi A Kravchenko MA Krishan K Krohn KJ Kuate Defo B Kucuk Bicer B Kumar GA Kumar M Kumar P Kumar V Kumaresh G Kurmi OP Kusuma D Kyu HH La Vecchia C Lacey B Lal DK Lalloo R Lam JO Lami FH Landires I Lang JJ Lansingh VC Larson SL Larsson AO Lasrado S Lassi ZS Lau KMM Lavados PM Lazarus JV Ledesma JR Lee PH Lee SWH LeGrand KE Leigh J Leonardi M Lescinsky H Leung J Levi M Lewington S Li S Lim LL Lin C Lin RT Linehan C Linn S Liu HC Liu S Liu Z Looker KJ Lopez AD Lopukhov PD Lorkowski S Lotufo PA Lucas TCD Lugo A Lunevicius R Lyons RA Ma J MacLachlan JH Maddison ER Maddison R Madotto F Mahasha PW Mai HT Majeed A Maled V Maleki S Malekzadeh R Malta DC Mamun AA Manafi A Manafi N Manguerra H Mansouri B Mansournia MA Mantilla Herrera AM Maravilla JC Marks A Martins-Melo FR Martopullo I Masoumi SZ Massano J Massenburg BB Mathur MR Maulik PK McAlinden C McGrath JJ McKee M Mehndiratta MM Mehri F Mehta KM Meitei WB Memiah PTN Mendoza W Menezes RG Mengesha EW Mengesha MB Mereke A Meretoja A Meretoja TJ Mestrovic T Miazgowski B Miazgowski T Michalek IM Mihretie KM Miller TR Mills EJ Mirica A Mirrakhimov EM Mirzaei H Mirzaei M Mirzaei-Alavijeh M Misganaw AT Mithra P Moazen B Moghadaszadeh M Mohamadi E Mohammad DK Mohammad Y Mohammad Gholi Mezerji N Mohammadian-Hafshejani A Mohammadifard N Mohammadpourhodki R Mohammed S Mokdad AH Molokhia M Momen NC Monasta L Mondello S Mooney MD Moosazadeh M Moradi G Moradi M Moradi-Lakeh M Moradzadeh R Moraga P Morales L Morawska L Moreno Velasquez I Morgado-da-Costa J Morrison SD Mosser JF Mouodi S Mousavi SM Mousavi Khaneghah A Mueller UO Munro SB Muriithi MK Musa KI Muthupandian S Naderi M Nagarajan AJ Nagel G Naghshtabrizi B Nair S Nandi AK Nangia V Nansseu JR Nayak VC Nazari J Negoi I Negoi RI Netsere HBN Ngunjiri JW Nguyen CT Nguyen J Nguyen M Nguyen M Nichols E Nigatu D Nigatu YT Nikbakhsh R Nixon MR Nnaji CA Nomura S Norrving B Noubiap JJ Nowak C Nunez-Samudio V Otoiu A Oancea B Odell CM Ogbo FA Oh IH Okunga EW Oladnabi M Olagunju AT Olusanya BO Olusanya JO Oluwasanu MM Omar Bali A Omer MO Ong KL Onwujekwe OE Orji AU Orpana HM Ortiz A Ostroff SM Otstavnov N Otstavnov SS Overland S Owolabi MO P A M Padubidri JR Pakhare AP Palladino R Pana A Panda-Jonas S Pandey A Park EK Parmar PGK Pasupula DK Patel SK Paternina-Caicedo AJ Pathak A Pathak M Patten SB Patton GC Paudel D Pazoki Toroudi H Peden AE Pennini A Pepito VCF Peprah EK Pereira A Pereira DM Perico N Pham HQ Phillips MR Pigott DM Pilgrim T Pilz TM Pirsaheb M Plana-Ripoll O Plass D Pokhrel KN Polibin RV Polinder S Polkinghorne KR Postma MJ Pourjafar H Pourmalek F Pourmirza Kalhori R Pourshams A Poznanska A Prada SI Prakash V Pribadi DRA Pupillo E Quazi Syed Z Rabiee M Rabiee N Radfar A Rafiee A Rafiei A Raggi A Rahimi-Movaghar A Rahman MA Rajabpour-Sanati A Rajati F Ramezanzadeh K Ranabhat CL Rao PC Rao SJ Rasella D Rastogi P Rathi P Rawaf DL Rawaf S Rawal L Razo C Redford SB Reiner RC Reinig N Reitsma MB Remuzzi G Renjith V Renzaho AMN Resnikoff S Rezaei N Rezai Ms Rezapour A Rhinehart PA Riahi SM Ribeiro ALP Ribeiro DC Ribeiro D Rickard J Roberts NLS Roberts S Robinson SR Roever L Rolfe S Ronfani L Roshandel G Roth GA Rubagotti E Rumisha SF Sabour S Sachdev PS Saddik B Sadeghi E Sadeghi M Saeidi S Safi S Safiri S Sagar R Sahebkar A Sahraian MA Sajadi SM Salahshoor MR Salamati P Salehi Zahabi S Salem H Salem MRR Salimzadeh H Salomon JA Salz I Samad Z Samy AM Sanabria J Santomauro DF Santos IS Santos JV Santric-Milicevic MM Saraswathy SYI Sarmiento-Suarez R Sarrafzadegan N Sartorius B Sarveazad A Sathian B Sathish T Sattin D Sbarra AN Schaeffer LE Schiavolin S Schmidt MI Schutte AE Schwebel DC Schwendicke F Senbeta AM Senthilkumaran S Sepanlou SG Shackelford KA Shadid J Shahabi S Shaheen AA Shaikh MA Shalash AS Shams-Beyranvand M Shamsizadeh M Shannawaz M Sharafi K Sharara F Sheena BS Sheikhtaheri A Shetty RS Shibuya K Shiferaw WS Shigematsu M Shin JI Shiri R Shirkoohi R Shrime MG Shuval K Siabani S Sigfusdottir ID Sigurvinsdottir R Silva JP Simpson KE Singh A Singh JA Skiadaresi E Skou ST Skryabin VY Sobngwi E Sokhan A Soltani S Sorensen RJD Soriano JB Sorrie MB Soyiri IN Sreeramareddy CT Stanaway JD Stark BA Stefan SC Stein C Steiner C Steiner TJ Stokes MA Stovner LJ Stubbs JL Sudaryanto A Sufiyan MB Sulo G Sultan I Sykes BL Sylte DO Szocska M Tabares-Seisdedos R Tabb KM Tadakamadla SK Taherkhani A Tajdini M Takahashi K Taveira N Teagle WL Teame H Tehrani-Banihashemi A Teklehaimanot BF Terrason S Tessema ZT Thankappan KR Thomson AM Tohidinik HR Tonelli M Topor-Madry R Torre AE Touvier M Tovani-Palone MRR Tran BX Travillian R Troeger CE Truelsen TC Tsai AC Tsatsakis A Tudor Car L Tyrovolas S Uddin R Ullah S Undurraga EA Unnikrishnan B Vacante M Vakilian A Valdez PR Varughese S Vasankari TJ Vasseghian Y Venketasubramanian N Violante FS Vlassov V Vollset SE Vongpradith A Vukovic A Vukovic R Waheed Y Walters MK Wang J Wang Y Wang YP Ward JL Watson A Wei J Weintraub RG Weiss DJ Weiss J Westerman R Whisnant JL Whiteford HA Wiangkham T Wiens KE Wijeratne T Wilner LB Wilson S Wojtyniak B Wolfe CDA Wool EE Wu AM Wulf Hanson S Wunrow HY Xu G Xu R Yadgir S Yahyazadeh Jabbari SH Yamagishi K Yaminfirooz M Yano Y Yaya S Yazdi-Feyzabadi V Yearwood JA Yeheyis TY Yeshitila YG Yip P Yonemoto N Yoon SJ Yoosefi Lebni J Younis MZ Younker TP Yousefi Z Yousefifard M Yousefinezhadi T Yousuf AY Yu C Yusefzadeh H Zahirian Moghadam T Zaki L Zaman SB Zamani M Zamanian M Zandian H Zangeneh A Zastrozhin MS Zewdie KA Zhang Y Zhang ZJ Zhao JT Zhao Y Zheng P Zhou M Ziapour A Zimsen SRM Naghavi M Murray CJL. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 29 Nov 2022. [Google Scholar]
- [118].Westermann A, Rönnau A-K, Krumova E, Regeniter S, Schwenkreis P, Rolke R, Treede R-D, Richter H, Maier C. Pain-associated mild sensory deficits without hyperalgesia in chronic non-neuropathic pain. Clin J Pain 2011;27:782–9. [DOI] [PubMed] [Google Scholar]
- [119].Wideman TH, Edwards RR, Walton DM, Martel MO, Hudon A, Seminowicz DA. The multimodal assessment model of pain. Clin J Pain 2019;35:212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Witjes B, Baillet S, Roy M, Oostenveld R, Huygen FJPM, de Vos CC. Magnetoencephalography reveals increased slow-to-fast alpha power ratios in patients with chronic pain. Pain Rep 2021;6:e928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Wu W, Zhang Y, Jiang J, Lucas MV, Fonzo GA, Rolle CE, Cooper C, Chin-Fatt C, Krepel N, Cornelssen CA, Wright R, Toll RT, Trivedi HM, Monuszko K, Caudle TL, Sarhadi K, Jha MK, Trombello JM, Deckersbach T, Adams P, McGrath PJ, Weissman MM, Fava M, Pizzagalli DA, Arns M, Trivedi MH, Etkin A. An electroencephalographic signature predicts antidepressant response in major depression. Nat Biotechnol 2020;38:439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Yüksel M, Ayas S, Cabioglu MT, Yilmaz D, Cabioglu C. Quantitative data for transcutaneous electrical nerve stimulation and acupuncture effectiveness in treatment of fibromyalgia syndrome. Evid Based Compl Altern Med 2019;2019:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Zhdanov A, Atluri S, Wong W, Vaghei Y, Daskalakis ZJ, Blumberger DM, Frey BN, Giacobbe P, Lam RW, Milev R, Mueller DJ, Turecki G, Parikh SV, Rotzinger S, Soares CN, Brenner CA, Vila-Rodriguez F, McAndrews MP, Kleffner K, Alonso-Prieto E, Arnott SR, Foster JA, Strother SC, Uher R, Kennedy SH, Farzan F. Use of machine learning for predicting escitalopram treatment outcome from electroencephalography recordings in adult patients with depression. JAMA Netw Open 2020;3:e1918377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zhou R, Wang J, Qi W, Liu FY, Yi M, Guo H, Wan Y. Elevated resting state gamma oscillatory activities in electroencephalogram of patients with post-herpetic neuralgia. Front Neurosci 2018;12:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zolezzi DM, Maria Alonso-Valerdi L, Naal-Ruiz NE, Ibarra-Zarate DI. Identification of neuropathic pain severity based on linear and non-linear EEG features. Annu Int Conf IEEE Eng Med Biol Soc 2021;2021:169–73. [DOI] [PubMed] [Google Scholar]
- [126].Zortea M, Beltran G, Alves RL, Vicuña P, Torres ILS, Fregni F, Caumo W. Spectral Power Density analysis of the resting-state as a marker of the central effects of opioid use in fibromyalgia. Sci Rep 2021;11:22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B751.




