Abstract
The protective coating of the electrode materials is a known source of improvement of the cycling performances in battery devices. In the case of the LiMn2O4 cathodes, the coating with a thin alumina layer has been proven to show performance efficiency. However, the precise mechanism of its effect on the performance improvement of the electrodes is still not clear. In this work we investigate alumina-coating-induced effects on the structural dynamics of the active materials in correlation to the modified solid electrolyte interface dynamics. The local structures of coated and uncoated samples at different galvanostatic points are studied by both soft X-ray absorption measurements at the Mn L-edges and O K-edge (in total electron yield mode) and hard X-ray absorption at the Mn K-edge (in transmission mode). The different probing depths of the employed techniques allowed us to study the structural dynamics both at the surface and within the bulk of the active material. We demonstrate that the coating successfully hinders the Mn3+ disproportionation and, hence, the degradation of the active material. Side products (layered Li2MnO3 and MnO) and changes in the local crystal symmetry with formation of Li2Mn2O4 are observed in uncoated electrodes. The role of alumina coating on the stability of the passivation layer and its consequent effect on the structural stability of the bulk active materials is discussed.
Introduction
Li-ion batteries have been among the most used energy storage devices for over a decade. They are one of the first choices as a power supply for consumer electronics and electric vehicles.1 Today, with respect to the high capacity obtainable from anode materials, the limited capacity of the cathode materials represents the bottleneck of the technological advancement for these battery devices.2,3 Recent studies highlighted the critical role of the active material–electrolyte interface in the prevention of the capacity fading of the cathode.4,5 The repeated cycling of the battery electrodes outside the electrolyte’s voltage window of stability provokes the formation of a protective passivating layer on the electrodes, the so-called solid electrolyte interphase (SEI) on the anodes and cathode–electrolyte interphase (CEI) on the cathode.6,7 Such protective layers prevent ulterior oxidation/reduction at the active material–electrolyte interface and stabilize the diffusion of Li+ ions, essential for battery operation. It is known that in the presence of the electrolyte salt the redox process starts almost immediately, while the formation of passivation layers occurs primarily during the first cycle of battery operation.7−9 However, a limited growth of the passivation layers on Li-ion cathode materials can result in a consequent aging of the electrodes and substantial deterioration of the active material. Thus, one of the primary goals of the research on cathode materials is to improve the interfacial process of the reversible ion uptake, while limiting the deterioration of the active material during cycling.9−12 A leading process for the enhancement of interface stability and diffusion has been the introduction of a second oxide coating on the active materials. These kinds of coatings are mostly insulating metal oxides that are expected to maintain the ionic diffusion into the active materials while hindering the interfacial deteriorations. While several studies have indicated performance improvement by the secondary oxide coating of the active materials, particularly at the interface, the bulk structural dynamics of the active materials in the presence of such coatings remain unclear.
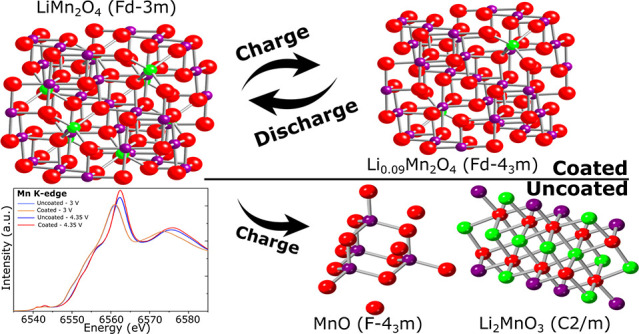
Lithium manganates13 are one of the classes of cathode active materials that have shown superior properties compared with their similar counterparts.13−17 In particular, LiMn2O4 (LMO) is interesting for the low cost and high working potential. Nevertheless, LMO’s thermal and compositional stability must be the object of further studies, which could result in improved stability and efficiency of the cathode material without resorting to Ni or Co doping, detrimental for safety and economical issues, respectively. Stoichiometric LiMn2O4 has a spinel structure (space group Fd3̅m), characterized by the general formula A[B2]O4, with A divalent ions in tetrahedral symmetry (Td) and B trivalent ions in octahedral symmetry (D3d). In particular, Li+ ions occupy the 8a tetrahedral site and Mn3+ or Mn4+ the 16d octahedral site. Oxygen anions occupy the 32e site in the cubic close-packed C3v symmetry.18,19 However, the LMO spinel crystal structure has shown to be unstable during cycling, with a change of the crystal symmetry from cubic to tetragonal due to Jahn–Teller (J–T) distortion in octahedrally coordinated Mn3+, which results in a volumetric expansion.20,21 Moreover, the Mn3+ disproportion (2Mn3+ → Mn2+ + Mn4+) triggers the dissolution of Mn2+ species, which are leached into the electrolyte. Such Mn dissolution is one of the main effects associated with the capacity fading of LMO cathode materials and, hence, needs to be minimized.
Recently it has been shown that the oxide coating (e.g., alumina) of the LMO active material reduces the severe decomposition at the active material–electrolyte interface, resulting in the formation of a more stable CEI.21 However, the bulk propagation of these interfacial interactions and their effect on the bulk structure of the active material remain unknown. In this work we have exploited X-ray absorption spectroscopy (XAS) to investigate the effect of Al2O3 coating on the disproportion mechanism within LiMn2O4 electrodes and study the correlation with the Jahn–Teller distortions and side products suppression within both coated and uncoated bulk structures. The XAS measurements were performed in two soft and one hard X-ray energy ranges in order to compare interface and bulk dynamics at two different galvanostatic points.22,23 The results are organized in two parts. The first part focusses on the soft XAS analysis with which it is shown how the Mn3+ disproportion is hindered via coating, resulting in a reduced Mn2+ dissolution at the electrode/electrolyte interface. In the second part, the preservation of the structural order within the bulk structure of the coated samples is investigated using hard X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) analysis. The improvement of the battery performance based on the consequent bulk structure dynamics and side product formation is discussed in detail.
Experiment
Al2O3–LiMn2O4 synthesis and the electrode preparation process followed the solid-state method described in refs (14 and 24). The method consisted of milling and calcination of Li2CO3 at 400 °C for 10 h, followed by 48 h of reground and calcination at 750 °C of MnCO3. A few nanometers of Al2O3 coating (3 wt %) was obtained via the coprecipitation route method.25 The LMO powder, immersed in a 0.02 M solution of Al(NO3)3·9H2O and deionized water, was stirred while adding ammonia solution in order to obtain Al(OH)3 in precipitation. The slurry, separated and washed with deionized water, was dried in a vacuum at 40 °C. After calcination at 450 °C with 10 °C/min heating and cooling rate for 120 min in air, Al2O3–LiMn2O4 is finally obtained. To prepare cathode slurries, the active material was blended with sodium carboxymethyl cellulose (Na-CMC) and SuperC65 conductive carbon (80:10:10 mass ratio) in deionized water. The slurry was then cast onto Al foil collector using the doctor blade technique, making a 200 μm thick laminate, and then dried at 80 °C for 3 h. Before transferring it to a glovebox, the laminate was pressed, and a second drying step was performed at 120 °C under vacuum overnight. The active material within the electrode was 2 mg cm–2. Lithium metal was employed as a counter and reference electrode, separated by glass fiber (Whatman GF/A), in 1 M LiPF6 in EC:DMC (1:1 v/v) electrolyte.
LiMn2O4 uncoated samples as well as Al2O3-coated ones were prepared in two conditions: fully charged electrodes at 4.35 V; fully discharged electrodes after the first cycle, at 3 V. The charge steps were applied at constant current (1 C rate, 1 C assumed to be 148 mAg–1 with respect to the active material mass), followed by a constant-potential equilibration step, in which the current was left to decay below C/20 (i.e., I = 7.5 mA g–1). All potentials are given respect to the Li+/Li redox couple. For X-ray absorption spectroscopy (XAS), electrodes were dried in an Ar atmosphere and protected in sealed packs while moved to the measurement chambers. Then, via an argon-filled load lock chamber, samples were placed in the experimental chambers. Soft XAS measurements were performed in total electron yield mode (TEY), using the radiation at the exit of the 8.1 bending magnet of the ELETTRA synchrotron facility in Trieste (Italy) (BEAR end-station BL8.1L). The incident light was horizontally polarized, fixing the incidence angle of the light with respect to the sample surface at 45° with the s polarization. The spectral energy was calibrated with respect to the carbon π–π* transitions. The data were normalized to the incident photon flux.23 Hard XAS experiments at the Mn K-edge were performed in transmission mode on equivalent samples at the bending-magnet beamline SAMBA in SOLEIL synchrotron. Nine acquisitions were collected for each sample. Each acquisition was normalized to the incident photon flux and calibrated by aligning the first maximum of the first derivative of the reference spectra, acquired from a Mn foil, to the theoretical value of the Mn K-edge absorption threshold (6539 eV).26 The spectra were then merged with the software ATHENA.27 EXAFS analysis was performed using the GNXAS package.28
Results
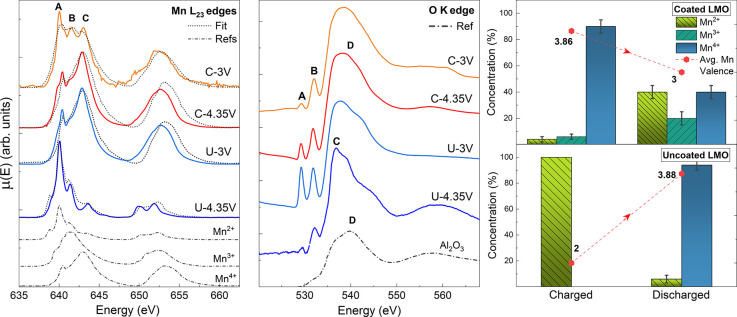
The XAS spectra of Al2O3 coated and uncoated LiMn2O4 cathodes taken at two galvanostatic points by soft X-ray total electron yield (TEY) measurements are reported in Figure 1. TEY measurements with a mean probing depth between 2 and 10 nm21 provide surface information and the structure at the electrode/electrolyte interface. At the Mn L23 edge, the spectra show three distinct peaks at 640.0 eV (A), 641.5 eV (B), and 643.0 eV (C) which can be assigned to the Mn2+, Mn3+, and Mn4+ states, respectively.
Figure 1.
Soft XAS spectra of coated (C) and uncoated (U) samples in fully charged (4.35 V) and discharged conditions (3 V) at the Mn L2,3 and oxygen K-edges. At the bottom of the figures, the reference spectra of manganese oxides29 and alumina30 are plotted with an intensity divided by a factor of 2. The best fits of the Mn L-edge spectra are displayed as dashed lines and confronted with the experimental data (continuous lines). The results of the fits are highlighted in the bar graph (right), with the respective error bars. The average oxidation states of the Mn ions at the two galvanostatic points are indicated by the label of the red points.
At 4.35 V the coated sample shows a high Mn4+ concentration, as expected in the delithiation half-cycle, while Mn2+ dominates the spectra in the uncoated case. After the discharging process, the coated sample shows higher intensities of the peaks related to lower oxidation states (A and B). Conversely, the average oxidation state of uncoated samples drastically increases to Mn4+. The qualitative assessments were confirmed by performing a linear combination fit (LCF) of the experimental data using suitable manganese oxide references (see Figure 1 and the Supporting Information). As shown by the bar graph, at 4.35 V the oxidation state of the superficial Mn ions within the coated sample is close to 4+, with a minimum trace of Mn3+ (6%) and Mn2+ (4%) species. The best fit obtained for the uncoated sample at 4.35 V points to the complete reduction of the active material to Mn2+. After the discharge, the increase of the Mn2+ and Mn3+ concentrations within the coated sample results in the expected reduction of the average Mn oxidation state. On the other hand, the drastic increase of the oxidation state in the uncoated case is proven by the decrease of the Mn2+ content (6%) and concurrent increase of the Mn4+ concentration. These results are in good agreement with previous work on similar samples.21
A similar process is also observed in the O K-edge spectra. The alumina coating on the coated samples is pinpointed by a broad feature at ∼540 eV (D), resulting from the Al–O contribution (see reference Al2O3 spectra in Figure 1). All samples show two components at 529 and 531 eV (peaks A and B), attributed to the Mn–O phases in the active material and to the hybridization of O 2p and Mn 3d electrons from the eg state.31 The increase of the Mn oxidation state by charging is confirmed in the O K-edge spectra of coated samples by the raising of component A. The higher Mn valency of the coated sample at 4.35 V compared with the stoichiometric LiMn2O4 is expected via the removal of Li ions from the structure.21 These results are in agreement with the preservation of the initial cubic symmetry of LiMn2O4 and further formation of spinel λ-MnO2.5,32 The uncoated sample shows an opposite behavior, in agreement with the manganese L-edge results. The decrease of the Mn valence upon charging is confirmed by the quenching of component A as well as by the increase of the component at 537 eV (peak C), indicating the formation of Mn2+ species.29 The distinct dynamics at the surface of the uncoated sample indicate the presence of an unstable interface layer at which the disproportionation leaves a high trace of Mn2+. The dissolution of Mn2+ ions results in a drastic increase of the superficial oxidation state at 3 V, highlighted at the O K-edge of uncoated samples by the drastic raising of component A and quenching of C.
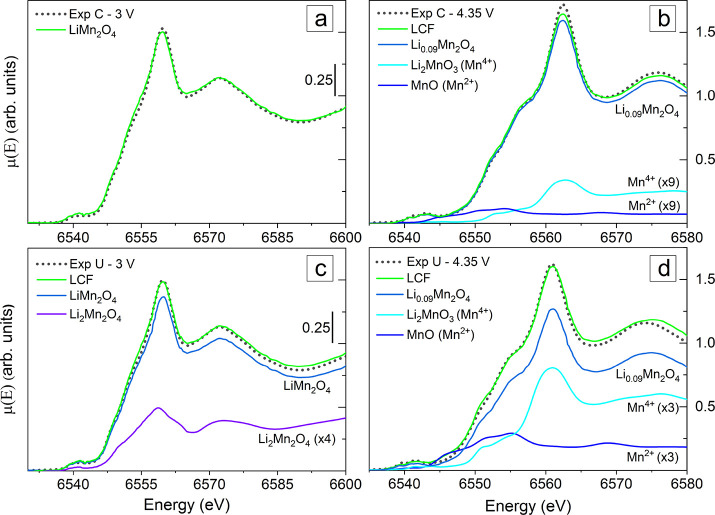
The propagation of the disproportionation and redox, originating at the interface, into the bulk of the active material was investigated using hard X-ray absorption spectroscopy at the Mn K-edge. Figure 2 shows the X-ray absorption near-edge structure (XANES) spectra of coated and uncoated samples at 3 and 4.35 V. Increasing the charging potential results in a shift of the white line from 6561.1 to 6562.4 eV (in uncoated samples) and 6561.1 to 6562.5 eV (in coated samples). The relative intensities of the white lines and pre-edge peaks (inset in Figure 2) are also higher by the increase of the charging potential. These modifications can be attributed to the increase of the Mn oxidation state and overall structural order within the samples.33,34 The white line fluctuations can be correlated to the deintercalation of lithium ions from the structural matrix (i.e., x in LixMn2O4), resulting in the oxidation of the Mn. The shift of the white line and the Mn valence state are inversely proportional to x. If no side products are formed, a complete deintercalation of Li ions (x = 0) leads to the formation of spinel λ-MnO2. Our results, in comparison with ref (34), suggest a Li content of x = 1 at 3 V and 0.25 > x > 0.08 at 4.35 V. In a previous work on similar samples,21 we observed via Raman spectroscopy the formation of a defective layered structure in the uncoated sample. Here, the relatively lower white line intensities displayed by the uncoated samples suggest a lower structural order with respect to the coated case.22 Considering the possible side products formed as a result of the disproportionation mechanism and J–T distortion (discussed later), a LCF of the XANES spectra at different stages was performed using suitable references (see Figure 3 and the Supporting Information).
Figure 2.
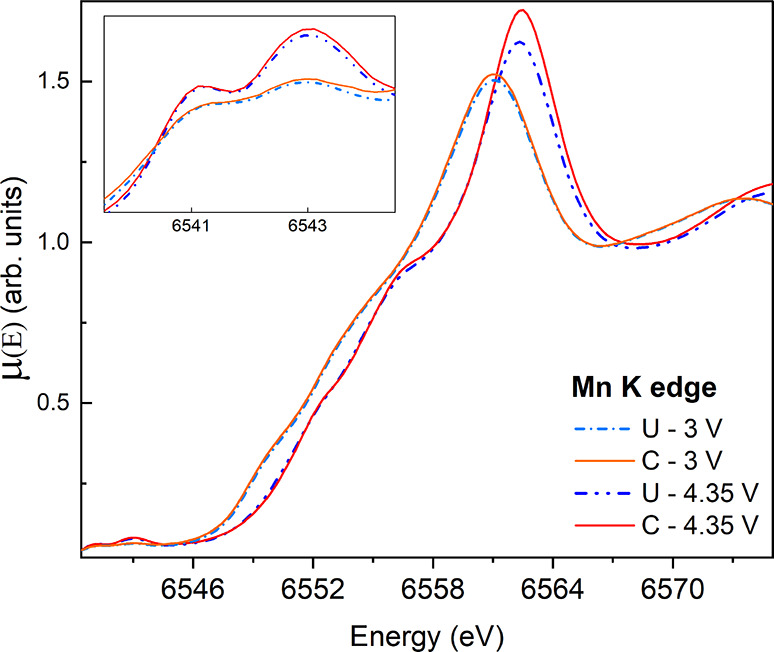
XANES spectra, acquired at the Mn K-edge, of coated (C) and uncoated (U) samples at 3 and 4.35 V. Uncoated samples are represented by dashed lines. Inset: the pre-edge peaks.
Figure 3.
(a) Comparison of the coated sample at 3 V with a LiMn2O4 reference.22 (b) LCF of the coated sample at 4.35 V with delithiated LMO, MnO, and layered Li2MnO3 references.33,35 (c) LCF of the discharged uncoated sample with cubic LiMn2O4 and tetragonal Li2Mn2O4 references.22,36 (d) LCF of the uncoated sample at 4.35 V with the delithiated LMO, MnO, and Li2MnO3 references.33,35 To improve visualization, the minor components have been multiplied by the factors indicated.
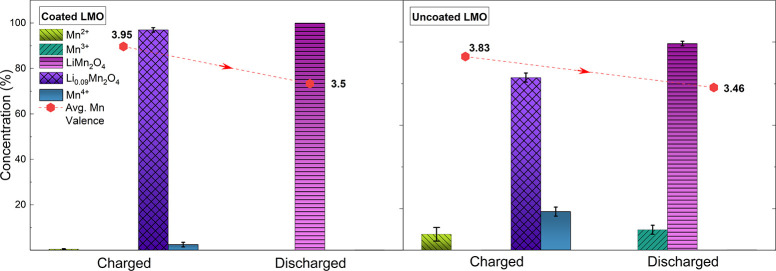
The results of the best fits are summarized in Figure 4 (see also Supporting Information). The coated sample at 3 V shows the initial LiMn2O4 structure (see Figure 3a). At 4.35 V, this sample fits well with a delithiated Li0.09Mn2O4 reference (Figure 3b), in agreement with the expected Li removal for potential over 4 V,33 in addition to a layered Li2MnO3 and a negligible amount of the MnO. On the other hand, the discharged uncoated sample (3 V) shows a cubic LiMn2O4 structure along with a tetragonal Li2Mn2O4 component (see Figure 3c). This sample at 4.35 V shows the expected Li0.09Mn2O4 structure resulting from the Li ions removal, along with a higher content of layered Li2MnO3 and MnO with respect to the coated sample (see Figure 3d). The higher ratio of these side products in uncoated samples confirms a lower structural order in these samples,22 justifying the lower white line intensity in the XAS spectra of these samples as well (see the Supporting Information for further details).
Figure 4.
Species content (%) in coated and uncoated samples, obtained via LCF with reference XANES spectra as shown in Figure 3. In red is shown the resulting average of the Mn oxidation state.
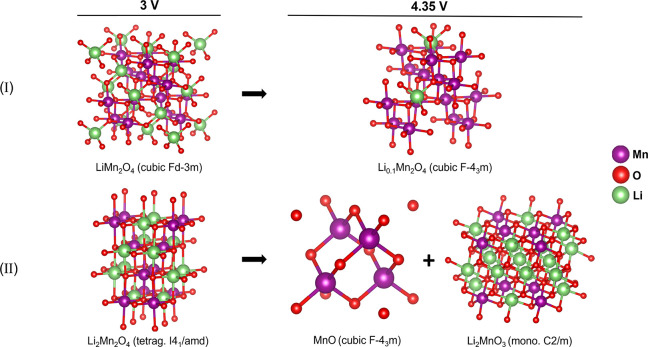
Figure 5.
Proposed models of the LMO at 3 and 4.35 V: (I) the expected lithium intercalation that preserves the cubic symmetry; (II) disproportion of defective phase with Li in octahedral sites formed by J–T distortion.
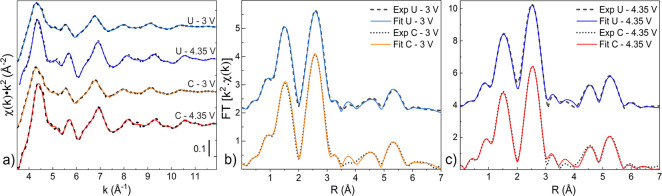
The suggested structural dynamics were also examined via extended X-ray absorption fine structure (EXAFS) analysis. The fit was performed using γ(2) XAS two-body signals, relative to the two-body distribution functions g2(28) associated with different Mn–O and Mn–Mn bonds within several coordination shells. The γ(2) signals were calculated from suggested reference structures (see the Supporting Information). A suitable background was realized with a linear function for the pre-edge region and fifth-order spline functions for the post-edge region. Around 6629 eV a second edge contribution, due to the double-electron excitation channel related to a KM shake-off (1s → ϵp, 3s → ϵs), was included as well in the background function.37 This feature is located 90 eV above the Mn K-edge, near the Fe 3s ionization energy (92 eV), in good agreement with the Z + 1 approximation.
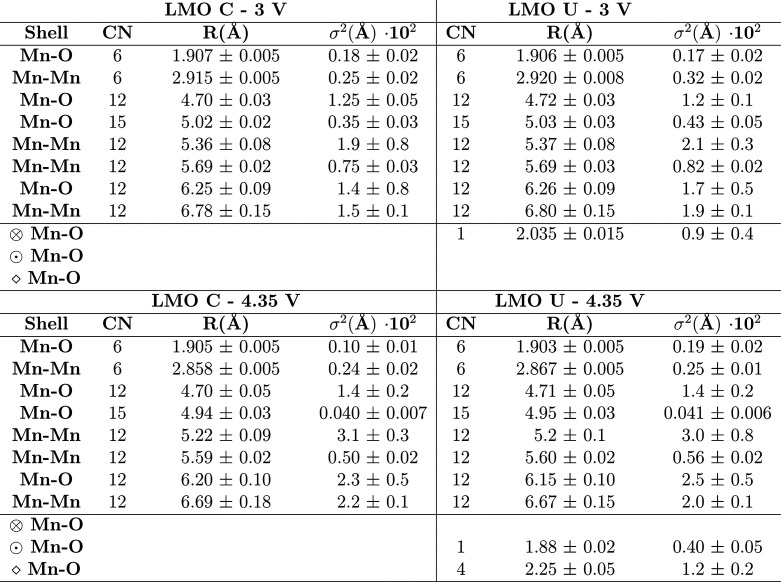
The first refinement was performed on the coated sample at 3 V, being the closest structure to that of the original spinel structure. A cubic spinel LiMn2O4 structure (cubic, Fd3̅m space group) was used as the reference. Fitting was performed, fixing the coordination numbers (CN) to that of the reference values and varying distances (R) and mean-square fluctuations of the interatomic distances (i.e., Debye–Waller factor, σ2).
The best fit was realized with 8 two-body components with an excellent agreement with the experimental data using simple Gaussian approximations (see Table 1 and the Supporting Information). The atomic distances in the first two shells (1.907 and 2.915 Å) are in excellent agreement with the spinel LiMn2O4 structure as observed also in refs (22 and 33). The low Debye–Waller factors confirm the high structural order in this sample. At 4.35 V, using a similar fitting procedure, a reduction of the Debye–Waller factors related to the 1st, 2nd, 4th, and 6th shell was observed. On the other hand, as reported in Table 1, the 3rd, 5th, 7th, and 8th are related to weak scattering peaks, and hence, the σ2 (Å) fluctuations observed are comprised within the large computational error. This reduction, along with the intensity increase of the main scattering peaks (see Figure 6b,c and the Supporting Information), indicates an improvement of the overall structural order which is expected by the lithium extraction and formation of the LixMn2O4.33 This is also confirmed by the Mn–Mn bond distance reduction (2.915 to 2.858 Å) within the second shell due to the increase of the Mn oxidation state.33 The addition of further side components such as MnO and Li2MnO3 did not improve the fitting results due to their negligible content in this sample.
Table 1. Best-Fit Values of the EXAFS Spectraa.
Debye–Waller factors (σ2) have been multiplied by a 102 factor to improve visualization. In the last three rows are reported the first-shell contributions relative to the Mn–O bond of Li2Mn2O4 (⊗), Li2MnO3 (⊙), and MnO (◇).
Figure 6.
K2-weighted EXAFS spectra (a) and corresponding Fourier transform of coated (C) and uncoated (U) samples discharged (b) and fully charged (c). The experimental data are plotted as dashed lines, together with the fit (continuous lines).
The uncoated sample at 3 V, however, does not comply with the spinel structure used for the fitting of the coated samples. The best fit was achieved including the first Mn–O bond of the Li2Mn2O4 into the overall structure, in agreement with the XANES results. The higher Debye–Waller factors associated with the main scattering peaks (2nd, 4th, and 6th shell in Table 1), with respect to the coated case, can be attributed to the J–T distortion. This result confirms the lower structural order within this sample. In particular, the relatively lower intensity of the first Mn–Mn bond component is in agreement with the formation of Li2Mn2O422,36 (see Figure 6b and the Supporting Information) which suggests a partial change of the crystal symmetry from cubic to tetragonal, as expected from the J–T distortions. At 4.35 V, the best fit for the uncoated sample was obtained by the addition of the first shells of Li2MnO3 and MnO. The increase of the scattering peaks intensity, as well as the shortening of the 2nd shell Mn–Mn bond, confirms the oxidation of the Mn due to Li extraction. However, the decrease of the first scattering peak intensity and the broadening around 2 Å (see Figure 6c and the Supporting Information) correlate with the formation of Li2MnO3 and MnO side products, in agreement with the XANES results. The best result was obtained using a coordination number of 4 for the MnO, suggesting that in this side product the Mn atoms are allocated in tetrahedral sites.
Discussion and Conclusions
Our results suggest that the oxide coating of the LMO active material induces the formation of a more stable interface with the electrolyte that reduces the Mn2+ dissolution due to the Mn3+ disproportionation, preserving the expected redox cycle required by the battery operation. Comparison of our LCF of the soft X-ray TEY and hard X-ray transmission mode measurements analysis shows similar superficial and bulk active material dynamics in the alumina-coated samples. Mn ions are oxidized to Mn4+ both in bulk and at the interface, as a consequence to Li+ extraction, while the other Mn valences (indicating formation of the side products) are negligible in bulk compared with the electrolyte interface. Upon discharge, the Mn4+ ions at the interface are reduced to Mn3+ and Mn2+, while the bulk structure goes back to the initial LiMn2O4 structure, suggesting the segregation of the Mn2+ species mainly at the electrode/electrolyte interface. On the other hand, in charged uncoated samples, the interfacial Mn ions are completely reduced to Mn2+, while within the bulk the disproportion of Mn3+ appears more evident. Together with the expected delithiated phase, the bulk structure analysis highlights the presence of several side products (e.g., 7% component of MnO and 17% of Li2MnO3). Upon discharge, at the interface, the Mn2+ species are exchanged by Mn4+ ions while the spinel bulk structure coexists with the reduced Mn3+ species with tetragonal symmetry.
In the noncoated sample,
the aggressive deterioration of the active
material results in a significant inhomogeneity of the lithium distribution,
with the formation of several side products such as the tetragonal
Li-rich phase (Li2Mn2O4), MnO, and
layered Li2MnO3 at distinct charging stages.
The formation of the Li2Mn2O4, resulting
from a nonuniform lithium distribution within the bulk structure of
the uncoated sample, can be explained by J–T distortion. In
such a case, during the intercalation Li ions can occupy octahedral
sites instead of tetrahedral, favoring the local increase of lithium
concentration.38 This transition is accompanied
by the change of the crystal symmetry from cubic to tetragonal. J–T
distortion in LMO cathodes is strongly dependent on the J–T
active Mn3+ ions content. The structural deterioration
that occurs in the absence of the alumina coating can be explained
by a high concentration of the J–T active Mn3+ in
these samples. In the uncoated material, the charge produced by the
oxidation of the electrolyte can be transferred to the Mn4+ ions, which reduce to Mn3+, increasing the concentration
of J–T active sites on the sample surface.39 On the other hand, the alumina coating can suppress the
Mn3+ formation at the electrode–electrolyte interface.
The Mn 3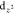 states coupling with the 2p orbitals
of
the oxygen atoms of the alumina coating favors the oxidation of the
Mn3+ to a non-J–T active Mn4+.40 Hence, the suppression of the J–T active
ions inhibits the cubic-to-tetragonal transition, preventing the formation
of Li2Mn2O4 in the coated sample.
states coupling with the 2p orbitals
of
the oxygen atoms of the alumina coating favors the oxidation of the
Mn3+ to a non-J–T active Mn4+.40 Hence, the suppression of the J–T active
ions inhibits the cubic-to-tetragonal transition, preventing the formation
of Li2Mn2O4 in the coated sample.
During the lithium extraction, the oxidation of the electrolyte and the reduction of Mn4+ to Mn3+ result also in oxygen loss. As described by Ben et al.,41 the oxygen loss at the surface of the LMO cathode allows octahedrally coordinated Mn ions to migrate to the now empty Li tetrahedral sites. The higher mobility of Mn3+ in the spinel lattice results in the formation of the J–T distorted structure, such as Li2Mn2O4. At higher potentials, the defective spinel decomposes due to the Mn3+ disproportion. The Mn2+ can remain in the Li tetrahedral sites, forming MnO, while Mn4+ moves to octahedral sites, forming layered Li2MnO3, energetically favorable with respect to the delithiated spinel structure LixMnO2.41 This disproportion mechanism can be described by the following equation:
| 1 |
This reaction is also enhanced by the dissolution of the Mn2+ and the consequent increase of the Li/Mn and O/Mn ratios, which promotes a transition from spinel LiMn2O4 to layered Li2Mn2O4 or Li2MnO3 phases, forming heterostructure within the initial cubic spinel structure.42 Higher charging potentials favor further formation of the layered Li2MnO3, which is expected to leave regions with weakly bounded Mn atoms, favoring the delamination of the surface layer into the electrolyte and, hence, aggravating the Mn dissolution.1,41 The higher concentration of the Li2MnO3, compared with the MnO, is due to the solubility of the Mn2+ species in the electrolyte, in agreement with previous work.5
In conclusion, the oxide coating mitigates the Mn2+ dissolution into the electrolyte and hinders the significant distortions, otherwise propagated through the bulk structure. This shielding effect reduces the loss of active material, while also preventing the formation of side products that can reduce the mobility of the Li ions. These effects lead to improved cyclability and final performance of the electrode.
Acknowledgments
We thank Synchrotron SOLEIL and Elettra Sincrotrone for provision of synchrotron radiation facilities at the SAMBA beamline (proposal number 20201687) and BEAR beamline (proposal number 20215834). We thank A. Giglia and S. Nannarone for their assistance in the soft X-ray absorption measurements. This work has been partially funded by the European Union - NextGenerationEU under the Italian Ministry of University and Research (MUR) National Innovation Ecosystem grant ECS00000041 - VITALITY - Spoke 9. We acknowledge Università degli Studi di Perugia and MUR for support within the project Vitality.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpcc.3c00342.
Linear combination fits results (Tables S1 and S2); supplementary LCF (Figure S1); comparison of the FT of the EXAFS signals with side products first shell signal (Figure S2); structure diagram of the Li extraction (Figure S3) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kaur G.; Gates B. D. Review - Surface Coatings for Cathodes in Lithium Ion Batteries: From Crystal Structures to Electrochemical Performance. J. Electrochem. Soc. 2022, 169, 043504. 10.1149/1945-7111/ac60f3. [DOI] [Google Scholar]
- Zhang J.; Jin Y.; Liu J.; Zhang Q.; Wang H. Recent advances in understanding and relieving capacity decay of lithium ion batteries with layered ternary cathodes. Sustainable Energy Fuels 2021, 5, 5114–5138. 10.1039/D1SE01137E. [DOI] [Google Scholar]
- Darjazi H.; Madinabeitia I.; Zarrabeitia M.; Gonzalo E.; Acebedo B.; Javad Rezvani S.; Fernández-Carretero F. J.; Nobili F.; García-Luis A.; Muñoz-Márquez M. A. LiNi0.5Mn1.5O4 Thin Films Grown by Magnetron Sputtering under Inert Gas Flow Mixtures as High-Voltage Cathode Materials for Lithium-Ion Batteries. ChemElectroChem. 2023, 10, e202201004 10.1002/celc.202201004. [DOI] [Google Scholar]
- Blyr A.; Sigala C.; Amatucci G.; Guyomard D.; Chabre Y.; Tarascon J. Self-Discharge of LiMn2O4/C Li-Ion Cells in Their Discharged State: Understanding by Means of Three-Electrode Measurements. J. Electrochem. Soc. 1998, 145, 194. 10.1149/1.1838235. [DOI] [Google Scholar]
- Rezvani S. J.; Parmar R.; Maroni F.; Nobili F.; Di Cicco A.; Gunnella R. Does Alumina Coating Alter the Solid Permeable Interphase Dynamics in LiMn2O4 Cathodes?. J. Phys. Chem. C 2020, 124, 26670–26677. 10.1021/acs.jpcc.0c08449. [DOI] [Google Scholar]
- Peled E. The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems—The Solid Electrolyte Interphase Model. J. Electrochem. Soc. 1979, 126, 2047–2051. 10.1149/1.2128859. [DOI] [Google Scholar]
- Peled E.; Golodnitsky D.; Ardel G. Advanced model for solid electrolyte interphase [SEI] electrodes in liquid and polymer electrolytes. Journal of The Electrochemical Society 1997, 144, L208. 10.1149/1.1837858. [DOI] [Google Scholar]
- Rezvani S. J.; Ciambezi M.; Gunnella R.; Minicucci M.; Muñoz M. A.; Nobili F.; Pasqualini M.; Passerini S.; Schreiner C.; Trapananti A.; Witkowska A.; Di Cicco A. Local Structure and Stability of SEI in Graphite and ZFO Electrodes Probed by As K-Edge Absorption Spectroscopy. J. Phys. Chem. C 2016, 120, 4287–4295. 10.1021/acs.jpcc.5b11798. [DOI] [Google Scholar]
- Rezvani S. J.; Nobili F.; Gunnella R.; Ali M.; Tossici R.; Passerini S.; Di Cicco A. SEI Dynamics in Metal Oxide Conversion Electrodes of Li-Ion Batteries. J. Phys. Chem. C 2017, 121, 26379–26388. 10.1021/acs.jpcc.7b08259. [DOI] [Google Scholar]
- Song J.; Xiao B.; Lin Y.; Xu K.; Li X. Interphases in Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703082. 10.1002/aenm.201703082. [DOI] [Google Scholar]
- Attia P. M.; Das S.; Harris S. J.; Bazant M. Z.; Chueh W. C. Electrochemical Kinetics of SEI Growth on Carbon Black: Part I. Experiments. J. Electrochem. Soc. 2019, 166, E97. 10.1149/2.0231904jes. [DOI] [Google Scholar]
- Raguette L.; Jorn R. Ion Solvation and Dynamics at Solid Electrolyte Interphases: A Long Way from Bulk?. J. Phys. Chem. C 2018, 122, 3219–3232. 10.1021/acs.jpcc.7b11472. [DOI] [Google Scholar]
- Liu C.; Neale Z. G.; Cao G. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today 2016, 19, 109–123. 10.1016/j.mattod.2015.10.009. [DOI] [Google Scholar]
- Pasqualini M.; Calcaterra S.; Maroni F.; Rezvani S.; Di Cicco A.; Alexander S.; Rajantie H.; Tossici R.; Nobili F. Electrochemical and spectroscopic characterization of an alumina-coated LiMn2O4 cathode with enhanced interfacial stability. Electrochim. Acta 2017, 258, 175–181. 10.1016/j.electacta.2017.10.115. [DOI] [Google Scholar]
- Secchiaroli M.; Calcaterra S.; Tran H. Y.; Rezvani S. J.; Nobili F.; Marassi R.; Wohlfahrt-Mehrens M.; Dsoke S. Development of Non-Fluorinated Cathodes Based on Li3V1.95Ni0.05(PO4)3/C with Prolonged Cycle Life: A Comparison among Na-Alginate, Na-Carboxymethyl Cellulose and Poly(acrylic acid) Binders. J. Electrochem. Soc. 2017, 164, A672–A683. 10.1149/2.0781704jes. [DOI] [Google Scholar]
- Minicucci M.; Tabassam L.; Natali R.; Mancini G.; Rezvani S.; Di Cicco A. Double-edge X-ray absorption study of LiFe1–xNixPO4 cathode materials. J. Mater. Sci. 2017, 52, 4886–4893. 10.1007/s10853-016-0723-9. [DOI] [Google Scholar]
- Rezvani S.J.; Mijit E.; Gunnella R.; Nobili F.; Trapananti A.; Minicucci M.; Ciambezi M.; Bresser D.; Nannarone S.; Passerini S.; Di Cicco A. Structure rearrangements induced by lithium insertion in metal alloying oxide mixed spinel structure studied by x-ray absorption near-edge spectroscopy. J. Phys. Chem. Solids 2020, 136, 109172. 10.1016/j.jpcs.2019.109172. [DOI] [Google Scholar]
- Ramana C. V.; Massot M.; Julien C. M. XPS and Raman spectroscopic characterization of LiMn2O4 spinels. Surf. Interface Anal. 2005, 37, 412–416. 10.1002/sia.2022. [DOI] [Google Scholar]
- Julien C. M.; Massot M. Raman spectroscopic studies of lithium manganates with spinel structure. J. Phys.: Condens. Matter 2003, 15, 3151–3162. 10.1088/0953-8984/15/19/315. [DOI] [Google Scholar]
- Julien C. M.; Mauger A.; Zaghib K.; Groult H. Comparative Issues of Cathode Materials for Li-Ion Batteries. Inorganics 2014, 2, 132–154. 10.3390/inorganics2010132. [DOI] [Google Scholar]
- Parmar R.; Rezvani S.; Nobili F.; Di Cicco A.; Trapananti A.; Minicucci M.; Nannarone S.; Giglia A.; Maroni F.; Gunnella R. Electrochemical Response and Structural Stability of the Li+ Ion Battery Cathode with Coated LiMn2O4 Nanoparticles. ACS Appl. Energy Mater. 2020, 3, 8356–8365. 10.1021/acsaem.0c00933. [DOI] [Google Scholar]
- Lee J.-F.; Tsai Y.-W.; Santhanam R.; Hwang B. J.; Yang M.-H.; Liu D.-G. Local structure transformation of nano-sized Al-doped LiMn2O4 sintered at different temperatures. J. Power Sources 2003, 119–121, 721–726. 10.1016/S0378-7753(03)00210-6. [DOI] [Google Scholar]
- Di Cicco A.; Giglia A.; Gunnella R.; Koch S. L.; Mueller F.; Nobili F.; Pasqualini M.; Passerini S.; Tossici R.; Witkowska A. SEI Growth and Depth Profiling on ZFO Electrodes by Soft X-Ray Absorption Spectroscopy. Adv. Energy Mater. 2015, 5, 1500642. 10.1002/aenm.201500642. [DOI] [Google Scholar]
- Kim J.-H.; Pieczonka N. P.; Li Z.; Wu Y.; Harris S.; Powell B. R. Understanding the capacity fading mechanism in LiNi0.5Mn1.5O4/graphite Li-ion batteries. Electrochim. Acta 2013, 90, 556–562. 10.1016/j.electacta.2012.12.069. [DOI] [Google Scholar]
- Liu J.; Manthiram A. Functional surface modifications of a high capacity layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode. J. Mater. Chem. 2010, 20, 3961–3967. 10.1039/b925711j. [DOI] [Google Scholar]
- BEARDEN J. A.; BURR A. F. Reevaluation of X-Ray Atomic Energy Levels. Rev. Mod. Phys. 1967, 39, 125–142. 10.1103/RevModPhys.39.125. [DOI] [Google Scholar]
- Ravel B.; Newville M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. Journal of Synchrotron Radiation 2005, 12, 537–541. 10.1107/S0909049505012719. [DOI] [PubMed] [Google Scholar]
- Filipponi A.; Cicco A. D.. GNXAS: a Software Package for Advanced Exafs Multiple-Scattering Calculations and Data-Analysis, 2000.
- Gilbert B.; Frazer B. H.; Belz A.; Conrad P. G.; Nealson K. H.; Haskel D.; Lang J. C.; Srajer G.; De Stasio G. Multiple Scattering Calculations of Bonding and X-ray Absorption Spectroscopy of Manganese Oxides. J. Phys. Chem. A 2003, 107, 2839–2847. 10.1021/jp021493s. [DOI] [Google Scholar]
- Guglieri C.; Chaboy J. O K-Edge X-ray Absorption Spectroscopy in Al-Doped ZnO Materials: Structural vs Electronic Effects. J. Phys. Chem. C 2014, 118, 25779–25785. 10.1021/jp508461d. [DOI] [Google Scholar]
- Noh H.-J.; Yeo S.; Kang J.-S.; Zhang C. L.; Cheong S.-W.; Oh S.-J.; Johnson P. D. Jahn-Teller effect in spinel manganites probed by soft x-ray absorption spectroscopy. Appl. Phys. Lett. 2006, 88, 081911. 10.1063/1.2178474. [DOI] [Google Scholar]
- Hunter J. C. Preparation of a new crystal form of manganese dioxide: λ-MnO2. J. Solid State Chem. 1981, 39, 142–147. 10.1016/0022-4596(81)90323-6. [DOI] [Google Scholar]
- Shiraishi Y.; Nakai I.; Tsubata T.; Himeda T.; Nishikawa F. In situ Transmission X-Ray Absorption Fine Structure Analysis of the Charge–Discharge Process in LiMn2O4, a Rechargeable Lithium Battery Material. J. Solid State Chem. 1997, 133, 587–590. 10.1006/jssc.1997.7615. [DOI] [Google Scholar]
- Okumura T.; Yamaguchi Y.; Shikano M.; Kobayashi H. Further findings of X-ray absorption near-edge structure in lithium manganese spinel oxide using first-principles calculations. J. Mater. Chem. A 2014, 2, 8017–8025. 10.1039/C3TA15412B. [DOI] [Google Scholar]
- Johnson C. S.; Kim J.-S.; Kropf A. J.; Kahaian A. J.; Vaughey J. T.; Fransson L. M. L.; Edström K.; Thackeray M. M. Structural Characterization of Layered LixNi0.5Mn0.5O2 (0 < x < 2) Oxide Electrodes for Li Batteries. Chem. Mater. 2003, 15, 2313–2322. 10.1021/cm0204728. [DOI] [Google Scholar]
- Hwang S.-J.; Park H.-S.; Choy J.-H. Variation of chemical bonding nature of layered LiMnO2 upon delithiation/relithiation and Cr substitution. Solid State Ionics 2002, 151, 275–283. 10.1016/S0167-2738(02)00724-5. [DOI] [Google Scholar]; Proceedings of the Second International Symposium on Soft Solution Processing, 2000.
- Gunnella R; Morresi L; Pinto N; Di Cicco A; Ottaviano L; Passacantando M; Verna A; Impellizzeri G; Irrera A; d’Acapito F Localization of the dopant in Ge:Mn diluted magnetic semiconductors by x-ray absorption at the Mn K edge. J. Phys.: Condens. Matter 2010, 22, 216006. 10.1088/0953-8984/22/21/216006. [DOI] [PubMed] [Google Scholar]
- Erichsen T.; Pfeiffer B.; Roddatis V.; Volkert C. A. Tracking the Diffusion-Controlled Lithiation Reaction of LiMn2O4 by In Situ TEM. ACS Appl. Energy Mater. 2020, 3, 5405–5414. 10.1021/acsaem.0c00380. [DOI] [Google Scholar]
- Somo T. R.; Mabokela T. E.; Teffu D. M.; Sekgobela T. K.; Ramogayana B.; Hato M. J.; Modibane K. D. A Comparative Review of Metal Oxide Surface Coatings on Three Families of Cathode Materials for Lithium Ion Batteries. Coatings 2021, 11, 744. 10.3390/coatings11070744. [DOI] [Google Scholar]
- Ouyang C. Y.; Zeng X. M.; Sljivancanin Z.; Baldereschi A. Oxidation States of Mn Atoms at Clean and Al2O3-Covered LiMn2O4(001) Surfaces. J. Phys. Chem. C 2010, 114, 4756–4759. 10.1021/jp911746g. [DOI] [Google Scholar]
- Ben L.; Yu H.; Chen B.; Chen Y.; Gong Y.; Yang X.; Gu L.; Huang X. Unusual Spinel-to-Layered Transformation in LiMn2O4 Cathode Explained by Electrochemical and Thermal Stability Investigation. ACS Appl. Mater. Interfaces 2017, 9, 35463–35475. 10.1021/acsami.7b11303. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Meng F.; Zhang Q.; Xue L.; Zhu H.; Lan S.; Liu Q.; Zhao J.; Zhuang Y.; Guo Q.; Liu B.; Gu L.; Lu X.; Ren Y.; Xia H. LiMnO2 cathode stabilized by interfacial orbital ordering for sustainable lithium-ion batteries. Nature Sustainability 2021, 4, 392–401. 10.1038/s41893-020-00660-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.