Abstract
The childhood-onset or juvenile idiopathic inflammatory myopathies (JIIMs) are a heterogenous group of rare and serious autoimmune diseases of children and young people that predominantly affect the muscles and skin but can also involve other organs, including the lungs, gut, joints, heart and central nervous system. Different myositis-specific autoantibodies have been identified that are associated with different muscle biopsy features, as well as with different clinical characteristics, prognoses and treatment responses. Thus, myositis-specific autoantibodies can be used to subset JIIMs into sub-phenotypes; some of these sub-phenotypes parallel disease seen in adults, whereas others are distinct from adult-onset idiopathic inflammatory myopathies. Although treatments and management have much improved over the past decade, evidence is still lacking for many of the current treatments and few validated prognostic biomarkers are available with which to predict response to treatment, comorbidities (such as calcinosis) or outcome. Emerging data on the pathogenesis of the JIIMs are leading to proposals for new trials and tools for monitoring disease.
Subject terms: Paediatric rheumatic diseases, Idiopathic inflammatory myopathies
This Review provides an overview of the clinical features and subtypes, pathophysiology and management of juvenile idiopathic inflammatory myopathies, including updates to our understanding of this heterogenous group of diseases that might change clinical practice in the near future.
Key points
Juvenile idiopathic inflammatory myopathies (JIIMs) can differ from adult-onset myopathies in terms of the pathogenesis, autoantibody profile, disease phenotype and treatment response, but these differences need to be further defined.
The myositis-specific autoantibody and myositis-associated autoantibody profile of a patient can help to determine the disease phenotype and likely outcome of the patient, including their risk of having disease complications.
More research is needed to provide an evidence-based approach to the management of refractory JIIM, major organ involvement and myositis-related complications or comorbidities.
New therapeutic targets have been strongly implicated in JIIM by pathogenesis studies, most notably, the type I interferon pathway; clinical trials are urgently needed but innovative designs are required.
Further research is needed to identify specific dysregulated pathways in addition to type I interferon and how these pathways relate to the myositis-specific autoantibody or myositis-associated autoantibody clinical subtypes.
A better understanding is needed of the long-term outcomes of patients with JIIM into adulthood, including the factors that are important to patients and their families.
Introduction
The childhood-onset or juvenile idiopathic inflammatory myopathies (JIIMs) are a group of rare but serious conditions of children and young people that predominantly affect the muscles and skin but can also involve other organs, including the lungs, gut, joints, heart and central nervous system. A newly defined European Alliance of Associations for Rheumatology–American College of Rheumatology (EULAR–ACR) system of classification1 captures the most prevalent group of JIIM, namely juvenile dermatomyositis (JDM). However, further refinement will be required for a classification that accurately captures the subtypes of JDM and delineates other forms of JIIM, including juvenile polymyositis, immune-mediated necrotizing myopathy (IMNM) in children or the overlap myositis syndromes. Unlike previous criteria, one advantage of the new EULAR–ACR criteria, according to an evaluation of these criteria in adult patients, is their ability to capture amyopathic dermatomyositis2. Although the EULAR–ACR classification criteria represent a new and superior standard, the Bohan and Peter criteria proposed in 1975 (ref. 3) have still been used in some recent literature.
An important advancement in the past 10 years is a greater understanding of the disease phenotype on the basis of the myositis-specific autoantibody (MSA) profile. MSAs, present in approximately 60% of children with JIIM4,5, can help to inform the disease course and risk of complications, such as interstitial lung disease (ILD) or calcinosis. MSA testing has helped to identify patients with IMNM, anti-synthetase syndrome or overlap syndromes who previously might have been classified as having juvenile polymyositis.
In terms of JIIM pathophysiology, vasculopathy and endothelial dysfunction are increasingly recognized as important elements, with number of circulating endothelial cells correlating with disease activity and nailfold abnormalities6. Type 1 interferon signature is a known key feature of JIIM but more work is needed to define the key drivers of this signature and the downstream effects that lead to immune dysregulation. Growing evidence supports involvement of mitochondrial dysfunction and endoplasmic reticulum (ER) stress. Greater understanding of pathogenesis might help to identify important therapeutic targets, shown most recently by the promise of JAK–STAT inhibition in the treatment of JIIM-related muscle, skin and lung disease7–11. This approach needs to be explored further by clinical trials.
In this Review, we describe the key features of JDM and its subtypes, as well as juvenile-onset IMNM, juvenile polymyositis and the overlap syndromes. We also discuss the clinical phenotypes of JIIM in relation to the MSA profile, highlighting the main clinical associations, response to treatment and caveats of antibody testing (Table 1). We review advances in our knowledge of the pathogenesis of JIIM and assess how evidence over the last decade has contributed to the understanding and management of these complex conditions, and what evidence is urgently needed to address the unmet needs in JIIM. Where data are available, we compare childhood-onset myositis and adult-onset myositis to highlight parallels or differences in antibody associations, genetics, clinical features, prognosis or outcomes. Detailed comparisons between adult and paediatric myositis have also been reviewed elsewhere12,13.
Table 1.
Main clinical associations of myositis-specific and myositis-associated autoantibodies in children
| Autoantibody | Frequency in JIIM | Main clinical associations | IIF pattern on HEp-2 cells | Specific issues related to antibody testinga | Key differences between childhood-onset and adult-onset disease | Refs. |
|---|---|---|---|---|---|---|
| Anti-TIF1 antibodies | 17–35%; highest prevalence in white populations and younger age groups. Median age of onset 7 years (3.8–10.4 years) in a North American cohort | Worse cutaneous disease than other JIIM MSA subgroups, including cutaneous ulceration, photosensitivity and lipodystrophy. Some patients can have an amyopathic phenotype, or extensive erythema, including V sign, shawl sign or holster sign, and periungual nailfold changes. Disease is often chronic or polycyclic. Patients are more likely to receive second- or third-line treatment than patients with other JIIM autoantibody subtypes | Nuclear fine speckled | ELISA might be more sensitive than immunoprecipitation for anti-TIF1 antibodies. Poor sensitivity with line immune-assays (LIA) or dot immune-assays (DIA) means that false-negative or false-positive results can occur. Levels of anti-TIF1 antibodies are reported to decrease with rituximab therapy and correlate with disease activity | Association with malignancy in adult-onset IIM is not seen in children. Anti-TIF1 antibodies are more common in childhood-onset disease than in adult-onset disease. Children are less likely to develop the V-sign than adults | 4,5,41,63,64,129–131,138,224 |
| Anti-NXP2 (initially called anti-MJ) antibodies | 15–25%; highest prevalence in white populations and younger age groups. Median age of onset of 5.8 years (3.9–10.2 years) in a North American cohort | Main features include calcinosis, prominent muscle weakness, dysphagia and dysphonia. Some patients have joint contractures. Disease course is often severe, with persistent disease activity and remission at 2 years less likely compared with other JIIM autoantibody sub-types | Nuclear fine speckled or multiple dots. | Sensitivity of LIA might be suboptimal for anti-NXP2 antibodies. If measured by standard radio-labelled immunoprecipitation, additional testing is required, such as western blot (immunoblot), to differentiate between anti-NXP2 and anti-MDA5 antibodies, which produce similar immunoprecipitation patterns (the presence of a 140-kDa band). Commercial ELISA is not yet available for anti-NXP2 antibodies | Association with cancer in adult-onset IIM not seen in children. Anti-NXP2 antibodies are more common in childhood-onset disease than in adult-onset disease | 4,5,41,63,64,129,131,138 |
| Anti-MDA5 antibodies | 6–38%; highest prevalence in Japanese cohorts. Median age of onset of 8.7 years (6–13.2 years) in a North American cohort | Mild muscle disease, including clinically amyopathic phenotype (more common in adult-onset disease than in childhood-onset disease). Patients might have constitutional symptoms and weight loss. Higher risk of cutaneous and oral ulceration, arthritis and ILD than in other JIIM autoantibody subtypes and increased risk of rapidly progressive ILD (particularly in Japanese, Korean and Chinese patients). Disease frequently requires intensive immunosuppressive therapy | Negative or cytoplasmic. | Can be detected by LIA, immunoprecipitation-blot or ELISA. If measured by standard radio-labelled immunoprecipitation, additional testing is required to distinguish between anti-MDA5 and anti-NXP2 antibodies. Levels of anti-MDA5 antibodies quantified by ELISA, reported to correlate with risk of ILD and cutaneous disease in Japanese cohorts, and might be helpful in determining response to treatment | Similar disease phenotype | 4,50,57,63,64,129,131,138 |
| Anti-Mi2 antibodies | 4–10%; highest prevalence in patients of Hispanic ethnicity and older age groups. Median age of onset of 10.7 years (range 6.7–14.9 years) in a North American cohort | Known as ‘classical JDM’. Marked muscle disease in early disease stages that responds well to conventional treatment. Higher chance of being off treatment after 2 years than with other JIIM autoantibody subtypes. Can follow a polycyclic course. Associated with pharyngeal weakness or dysphagia, oedema and cutaneous features. Lower risk of ILD and lower mortality than other JIIM autoantibody subtypes | Nuclear fine speckled | Can be detected by immunoprecipitation or LIA. Anti-Mi2 antibodies reported to decrease following rituximab therapy and correlate with disease activity | Similar phenotype across ages but children less likely to have a V-sign or shawl sign and are at an increased risk of muscle weakness and dysphagia compared with adults. Anti-Mi2 antibodies associated with cancer in adults, but not in children | 4,5,41,63,64,129–131,138,224,225 |
| Anti-SAE antibodies | 0.3–9.1% |
Predominant cutaneous involvement. Amyopathic at onset. Might be associated with dysphagia. ILD has been reported in a single case report of an anti-SAE antibody-positive patient with JDM |
Nuclear coarse speckled with nucleolar sparing | Detected by LIA, dot blot or immunoprecipitation | Anti-SAE antibody is rarely detected in JIIM and hence the clinical phenotype and response to treatment is difficult to define. Anti-SAE antibody association with malignancy reported for adult-onset disease only | 4,41,64,129,131,138 |
| Anti-aminoacyl-tRNA synthetase (anti-ARS) antibodies, also known as anti-synthetase antibodiesb | 2–5%; highest prevalence in patients of Black ethnicity and older age at onset. Median age at onset of 12.3 years (range 7.1–15 years) in a North American cohort |
Anti-ARS antibodies are associated with an increased likelihood of having a juvenile connective tissue myopathy phenotype. Patients with anti-ARS antibodies frequently have a chronic continuous disease course and a need for additional immunosuppressive therapy. Antibody positivity is also associated with high rates of ILD and increased mortality. Some patients might present to respiratory services with isolated ILD. Anti-synthetase syndrome describes a combination of symptoms, including myositis, ILD, Raynaud phenomenon, fever, arthritis and mechanics hands. The presence of anti-ARS antibodies can be associated with lipodystrophy. Different anti-ARS antibodies are associated with muscle-predominant or skin-predominant disease. Non-Jo1 anti-ARS antibodies (such as anti-PL7 and anti-PL12 antibodies) are associated with severe lung involvement |
Negative or cytoplasmic |
LIA, immunoprecipitation or ELISA is commonly used to detect anti-Jo1 antibodies. Line blot might not detect rare anti-ARS antibodies (for example, anti-OJ antibodies). Anti-Jo1 antibodies are reported to decrease following rituximab therapy and correlate with disease activity |
Similar phenotype in juvenile and adult-onset disease, although this subtype is much less frequent in childhood than in adulthood. Important features such as Raynaud phenomenon, mechanics hands and ILD seem to occur at a lower frequency in childhood-onset disease than in adult-onset disease | 4,5,38,64,129,131,138,224,225 |
| Anti-SRP antibodies | 1.6-4%; highest prevalence in Black populations and older age at onset. Median age of onset of 14.6 years (range 11.6–16.1 years) in a North American cohort | IMNM, characterized by necrosis of muscle fibres with no or minimal inflammation on muscle biopsy. Patients can have high serum levels of creatinine kinase. Disease is often chronic (and treatment-resistant) and might benefit from treatment with rituximab in addition to corticosteroids and disease-modifying drugs, as well as physiotherapy. Patients with anti-SRP antibodies are more likely have severe muscle weakness and extra-muscular manifestations than patients with anti-HMGCR antibodies. Anti-SRP antibodies are also associated with risk of dysphagia, joint contractures, ILD, or cardiac involvement. Non-specific cutaneous features can be seen (<10%) | Cytoplasmic | Often screened for by ELISA or LIA. Commercially available kits only test for the 54-kDa subunit of SRP; hence, false-negative results can occur. False-positive results are common with LIA. Anti-SRP antibody levels are unchanged following rituximab therapy but correlate with levels of muscle enzymes | Less common in childhood-onset disease than in adult-onset disease, but a similar phenotype. Children might be less likely to have palpitations and are less likely to die than patients with adult-onset anti-SRP antibody-positive IIM. Children have been reported to have increased distal weakness, muscle atrophy and falling episodes than patients with adult-onset disease. Younger age groups may have a worse prognosis but mortality is lower in childhood-onset than in adult-onset disease | 4,5,41,64,66,68,129,131,138,224 |
| Anti-HMGCR antibodies | 1.1% | IMNM, characterized by severe proximal muscle weakness, joint contractures, high serum creatinine kinase levels and muscle fibre necrosis with no or minimal inflammation on muscle biopsy. Patients with these antibodies often have a poor response to medication and a chronic disease course. Patients are also more likely to receive second- or third-line therapy, including biologics, than other JIIM autoantibody subgroups, but might not benefit from rituximab therapy. IVIG can be beneficial for some patients. Physiotherapy is important as part of the treatment regime. Patients with anti-HMGCR antibodies are less likely to have extra-muscular manifestations than patients with anti-SRP antibodies, but can have cutaneous disease. Anti-HMGCR antibodies are also associated with dysphagia | Cytoplasmic | Usually screened for using ELISAs but false positives can occur with this assay (with a false-positive rate of up to 0.7%). A positive result can be confirmed by immunoprecipitation | Less common in JIIM than in adult-onset IIM but a similar phenotype to adult-onset disease. Unlike disease in adults, disease in children is not associated with previous exposure to statin medication. Children and young adults (typically statin naïve) can have a worse prognosis than older age groups. Cutaneous disease is reported more frequently in childhood-onset disease than in adult-onset disease | 4,5,37,41,64,66,68–70,129,138,226 |
| Myositis-associated autoantibodies | ||||||
| Anti-Ro52 antibodies | 6–14% | The presence of anti-Ro52 antibodies is associated with a myositis overlap phenotype, as well as an increased risk of ILD. The disease course is frequently chronic, with an increased number of medications and a lower chance of remission than with other JIIM autoantibody subtypes | Negative or cytoplasmic | Can be detected by LIA and ELISA; not immunoprecipitation | Less common in JIIM than in adult-onset IIM | 64,74,129,138 |
| Anti-PM/Scl antibodies | 3–5% | Anti-PM/Scl antibodies are associated with overlap syndromes, most commonly overall with scleroderma. These antibodies are also associated with an increased risk of calcinosis and lipoatrophy | Nucleolar, homogenous | Can be screened for using IIF and identified by different immunoassays | Less common in JIIM than in adult-onset IIM | 4,41,64,131,138 |
| Anti-U1RNP antibodies | 4–5.6% | Anti-U1RNP antibodies are associated with polymyositis or a polymyositis overlap phenotype, scleroderma overlap and mixed connective tissue disease. These antibodies are also detected in patients with SLE. Muscle weakness is less likely in patients with anti-U1RNP antibodies than in other JIIM autoantibody subgroups | Nuclear speckled | Might not be included in all myositis panels or myositis LIAs and additional testing might be needed if clinically relevant | Less common in JIIM than in adult-onset IIM | 4,64,131,138 |
| Other myositis-associated autoantibodies | Anti-Ku, anti-Scl70, anti-Ro60, anti-U3RNP and anti-mitochondrial antibodies are more likely to be identified in older patients than in younger patients and are associated with polymyositis, a polymyositis phenotype or scleroderma overlap | Less common in JIIM than in adult-onset IIM | 4,64,131,138 | |||
DIA, dot immune assay; HEp-2, human epithelial type 2; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; IIF, indirect immunofluorescence; IIM, idiopathic inflammatory myopathy; ILD, interstitial lung disease; IMNM, immune-mediated necrotizing myopathy; JIIM, juvenile idiopathic inflammatory myopathy; LIA, line immune-assay; MAA, myositis-associated antibodies; MDA5, melanoma differentiation-associated gene 5; MSA, myositis-specific antibodies; NXP2, nuclear matrix protein 2; SAE, small ubiquitin-like modifier activating enzyme; SLE, systemic lupus erythematosus; SRP, signal recognition particle; TIF1, transcriptional intermediary factor 1. aA clinician’s guide to MSA and MAA testing is available at the Juvenile Dermatomyositis Cohort Biomarker Study and Repository. bAnti-aminoacyl-tRNA synthetase antibodies include anti-Jo1 (anti-histidyl-tRNA synthetase), anti-PL7 (anti-threonyl-tRNA synthetase), anti-PL12 (anti-alanyl-tRNA synthetase), anti-EJ (anti-glycyl tRNA synthetase), anti-KS (anti-asparaginyl-tRNA synthetase), anti-OJ (anti-isoleucyl-tRNA synthetase), anti-Ha (anti-tyrosyl-tRNA synthetase) and anti-Zo (anti-phenylalanyl-tRNA synthetase) antibodies.
In the final section of this Review, we summarize current evidence in terms of JIIM treatment and highlight the need for head-to-head comparison studies to determine the best second-line treatment, options for recalcitrant disease and JIIM-related complications. A treatment algorithm for JIIM based on current available consensus is also presented, including the use of exercise therapy and psychological support, as well as medications. Finally, we discuss some of the key challenges in the management of JIIM and how international collaboration helps to overcome these challenges and improve our understanding of this rare but important group of diseases.
Epidemiology
JIIM has a reported incidence of between 1.6 and 4 cases per million children per year14 and an estimated prevalence of 2.5 cases per 100,000 children14, but limited data are available. Although the mortality in JIIM has decreased considerably since the pre-steroid era and is often reported as being below 4% worldwide15–17, mortality remains as high as 5–8% in some cohorts18–20. In a North American study, the mortality associated with juvenile connective tissue disease (CTD) overlap phenotypes was higher (standardized mortality ratio (SMR) 66.9) than that associated with juvenile polymyositis (SMR 30.7) or JDM (SMR 8.3)21. Risk factors, identified by multivariate analyses, included older age or illness severity at disease onset, weight loss and delay to diagnosis21.
As mortality rates have decreased over the years, more emphasis has been placed on evaluating long-term functional outcomes, morbidity and health-related quality of life. Notably, the risk of disease damage increases almost linearly for each year of disease22, highlighting the importance of early disease control. Damage, usually mild, is most common in the cutaneous, endocrine, muscular or skeletal domains, with identified predictors of damage, including high disease activity or severity of disease at onset, duration of active disease, the presence of early organ damage (within 6 months of diagnosis) and functional disability19,23,24. Functional impairment is usually mild, but reported in up to 41% of patients and can be associated with increased pain and decreased quality of life16,17,19,20,23–25. Children can be affected by impaired growth or delayed puberty, particularly if there is preceding growth failure or if the active phase of disease occurs during early puberty26. In a report of adults who had JDM and were surveyed at an average age of 20 years, 59% perceived that their myositis was still active and 65% were still taking immunosuppressive medication27. JIIM is also associated with long-term risks relating to cardiovascular, pulmonary or cerebrovascular disease6,28–31.
Clinical phenotypes
On the basis of clinical and histopathological findings, JIIM can be separated into various subtypes. JDM, the most common JIIM subtype, represents more than 80% of patients, followed by overlap myositis14,15,19,32. In this section, we first review the clinico-serological subtypes of JDM before discussing the features of amyopathic JDM, anti-synthetase syndrome, IMNM and overlap syndromes. In the absence of myositis, patients with characteristic skin rashes are considered to have amyopathic or clinically amyopathic JDM, but this phenotype is rare in children33–35. Juvenile polymyositis is a very rare subtype, characterized by severe muscle inflammation and characteristic but not pathognomonic histological, radiological and electromyographic findings36. Emerging data suggest that some patients previously diagnosed as having JDM or juvenile polymyositis instead fall within the IMNM37, overlap myositis or anti-synthetase syndrome category38, on the basis of their autoantibody profile (Table 1).
Juvenile dermatomyositis
JDM is defined by the presence of proximal symmetric myositis and characteristic cutaneous features and has a median age at diagnosis of 7.4 years32. Calcinosis has been reported in 20–47% of patients with JDM in different cohorts16,39. Approximately 60% of patients with JDM are positive for a myositis-specific antibody (MSA) (Table 1). Increasingly, expert consensus is that JDM can be divided into the following subtypes defined by the presence of a specific MSA: anti-Mi2 antibody-positive JDM, anti-nuclear matrix protein 2 (NXP2) antibody-positive JDM, anti-transcriptional intermediary factor 1 (TIF1) antibody-positive JDM, anti-melanoma differentiation-associated gene 5 (MDA5) antibody-positive JDM, anti-small ubiquitin-like modifier activating enzyme (SAE) antibody-positive JDM, and MSA-negative JDM38 (Table 1). Two MSAs can coexist in the same patient, but this is extremely rare, although some patients do have both an MSA and one or more myositis-associated autoantibodies (MAAs)5.
Anti-TIF1 antibody-positive JDM
Anti-TIF1 antibodies are the most common MSA in JIIM, with a reported frequency of between 17% and 35% (refs. 4,5,40) (Table 1). These antibodies are most common in white children and those children with a younger age at disease onset5 (median age of 7 years at disease onset in one North American study). The clinical phenotype of anti-TIF1 antibody-positive JDM includes mild muscle disease with relatively low creatinine kinase serum levels but with severe skin involvement, including an increased risk of ulceration and lipodystrophy4,5,41. Other frequent skin manifestations include Gottron papules, malar rash, erythema, ‘shawl-sign’ rash, photosensitivity and cuticular overgrowth5. Dysphagia can also occur in these patients4. Some patients have a chronic severe disease course, requiring the use of second-line or third-line treatment regimes, including cyclophosphamide or biologic drugs4. Although anti-TIF1 antibodies confer an increased risk of malignancy in patients with adult-onset myositis42,43, this association has not been reported in individuals with childhood-onset myositis.
Anti-NXP2 antibody-positive JDM
Anti-NXP2 antibodies (initially known as anti-MJ antibodies) are present in approximately 15–25% of patients with JDM (Table 1) and are one of the most common MSAs in white populations5,44,45. Anti-NXP2 antibody-positive patients with JDM typically present at a young age, and have the highest incidence of calcinosis among the various JDM antibody subtypes, with age at onset itself found to be linearly associated with risk of calcinosis in a UK cohort46. Calcinosis is also associated with the presence of anti-NXP2 antibodies in adult-onset idiopathic inflammatory myopathy (IIM)47. Muscle disease can be severe in childhood-onset anti-NXP2 antibody-positive disease and can include muscle contractures, muscle atrophy and functional compromise45. Other features of anti-NXP2 antibody-positive disease include gastrointestinal involvement, risk of dysphagia, dysphonia and skin ulceration4. The disease can be difficult to treat, has a low probability of treatment discontinuation, does not always respond well to conventional treatment and can result in a poor long-term prognosis48,49.
Anti-MDA5 antibody-positive JDM
Patients with anti-MDA5 antibody-positive JDM typically have minimal or no muscle involvement4,50,51. The characteristic clinical phenotype includes frequent skin rashes, cutaneous ulceration and arthritis (affecting mainly the small joints of the hand and feet), in addition to constitutional symptoms (such as weight loss), oral ulceration and increased risk of ILD4,50,52–54. Patients with early ILD detected by computerized tomography or pulmonary function tests are frequently asymptomatic55. Rapidly progressive ILD is a rare but potentially fatal complication of IIM in both children and adults with anti-MDA5 antibodies and reports suggest that this complication occurs more often in East Asian populations than in other populations55–57. Anti-MDA5 antibody-positive patients are more likely to receive short-term treatment with glucocorticoids than patients with other JIIM autoantibody subtypes, although overall treatment duration and frequency of clinical remission in anti-MDA5 antibody-positive JDM is similar to that of other JDM subtypes50.
Anti-Mi2 antibody-positive JDM
Anti-Mi2 antibodies are present in 4–10% of patients with JDM4,5. Anti-Mi2 antibody-positive JDM is more common in Hispanic patients with an older disease onset (median age of disease onset of 11 years) than other JIIM autoantibody subtypes5. Children with anti-Mi2 antibody-positive JDM typically present with severe muscle disease and notable skin involvement, frequently referred to as ‘classic JDM’4,5. Common skin rashes include those pathognomonic for JDM (such as heliotrope rash and Gottron papules), along with malar rash and periungual nailfold capillary abnormalities5. The severity of the myositis is reflected by the high muscle biopsy scores of the patients58. Children with anti-Mi2 antibody-positive JDM are less likely to have ILD than patients with other JDM subtypes, but are at a greater risk of dysphagia and oedema4,5. Despite the severe presentation, anti-Mi2 antibody-positive patients respond well to conventional treatment and have a good chance of being off treatment at 2 years48.
Amyopathic juvenile dermatomyositis
Amyopathic JDM can occur in some children but it is rare (<5% of patients with JIIM)35,59. Anti-TIF1 antibodies, followed by anti-MDA5 antibodies, are the most common MSAs associated with this JIIM subtype34. Patients with amyopathic JDM tend to have a young age of disease onset and have less myalgia, arthritis, calcinosis, dysphagia or abdominal pain than other patients with JDM34. Skin manifestations include Gottron papules, heliotrope rash, malar rash, periungual capillary abnormalities and photosensitivity34. Some patients with anti-SAE antibodies can present initially with skin disease, with muscle involvement occurring at a later stage4,60. In a case report, one patient had anti-SAE antibody-positive amyopathic JDM complicated by ILD61. In the absence of myositis, some experts believe that the presence of an MSA can support a diagnosis of JIIM62,63.
Anti-synthetase syndrome
Anti-synthetase syndrome is characterized by the presence of antibodies against aminoacyl tRNA synthetases (anti-ARS antibodies; also known as anti-synthetase antibodies) and a broad spectrum of clinical features. Eight anti-synthetase antibodies have so far been described in IIM: anti-Jo1 (anti-histidyl-tRNA synthetase), anti-PL12 (anti-alanyl-tRNA synthetase), anti-PL7 (anti-threonyl-tRNA synthetase), anti-EJ (anti-glycyl tRNA synthetase), anti-KS (anti-asparaginyl-tRNA synthetase), anti-OJ (anti-isoleucyl-tRNA synthetase), anti-Ha (anti-tyrosyl-tRNA synthetase) and anti-Zo (anti-phenylalanyl-tRNA synthetase) antibodies. Clinical manifestations of anti-synthetase syndrome, as documented in a North American study, include proximal muscle weakness (100%), arthritis (74%), mechanic’s hand (32%), fever (63%), Raynaud phenomenon (32%) and ILD (63%)5. Anti-synthetase syndrome is rare in children and much knowledge is extrapolated from the disease in adults. Among adults with anti-synthetase syndrome, patients positive for anti-Jo1 antibodies are more likely to have myositis, whereas other patients, especially those with anti-PL12 antibodies, are more likely to have isolated ILD and therefore might present initially to a respiratory physician64,65.
Immune mediated necrotizing myopathy
IMNM is a rare and recently characterized subtype of JIIM that includes anti-signal recognition particle (SRP) antibody-positive myopathy, anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) antibody-positive myopathy and antibody-negative IMNM38,66. The hallmark muscle biopsy finding of IMNM is muscle fibre necrosis with the absence or minimal presence of lymphocytic infiltrate66. Children with IMNM characteristically present with severe muscle weakness and notably elevated serum levels of muscle enzymes. Anti-SRP antibody-positive patients can have dysphagia67, and in rare instances can have cardiac involvement66,68. Some patients can also present with skin and other extra-muscular manifestations, which can include arthralgia or Raynaud phenomenon, as well as ILD66. Children with anti-HMGCR antibodies typically present with severe proximal muscle weakness, and can have muscle atrophy, contractures and arthralgia37,66. Although in adults the development of anti-HMGCR antibodies is frequently associated with exposure to statins, this association is absent in children with anti-HMGCR antibodies37,69. Autoantibody negative IMNM remains poorly characterized.
In children, anti-SRP or anti-HMGCR myopathy presenting with slowly progressive muscle weakness could be mistaken for muscular dystrophy70. If there is a high index of suspicion with muscle biopsy, immunohistochemical or genetic testing might be appropriate. Patients with muscular dystrophy can share the same pattern of muscle weakness (with more proximal than distal involvement), elevation of muscle enzymes, oedema on MRI and myopathic features on biopsy, but can be distinguished from JIIM by a tendency to have a more insidious disease onset, weakness in other muscle groups, calf muscle of generalized muscle hypertrophy, joint contractures, scapular winging, scoliosis, spinal rigidity, cardiomyopathy or macroglossia and the absence of an MSA70.
Overlap myositis
Currently, no unifying internationally accepted definition of overlap myositis exists as different CTDs can have similar clinical features. An international survey of clinical opinion on criteria for JDM–scleroderma overlap, which occurs in 15–20% of patients with JDM according to some reports71, proposed the use of the presence of two or more of the following criteria: Raynaud phenomenon, sclerodactyly and sclerodermatous skin changes in a child fulfilling criteria for JDM72. In a large US study of 1,718 patients with systemic lupus erythematosus (SLE) (451 paediatric and 1,267 adult patients), 6.3% of the patients had concurrent myositis73, whereas in a UK cohort of patients with JIIM, 2.5% of the patients were given a diagnosis of JDM–SLE overlap15.
The most commonly detected autoantibodies in overlap syndromes are MAAs (Table 1), although these antibodies can also be found in other JIIM subtypes. One or more MAAs might co-occur with MSAs in the same patient4,12. MAAs include anti-Ro52, anti-PM/Scl and anti-U1RNP antibodies5,74. For example, in one cohort, MSAs were detected in 6/49 (12%) of patients with overlap CTD or mixed CTD, whereas MAAs were present in 25/49 (51%) of the patients4. Overlap syndromes are associated with an increased risk of extra-muscular manifestations and a higher risk of mortality, in particular because of the higher risk of ILD, compared with other JIIM autoantibody subtypes, highlighting the importance of a correct diagnosis and early treatment32.
Myositis in other paediatric conditions
Other than primary myositis, myopathy or myositis can be a presenting feature in a number of different inflammatory conditions seen in childhood. Clinical presentation of myositis in childhood sarcoidosis is a rare but reported manifestation75. Thus, sarcoidosis or granulomatous myositis should be considered in patients presenting with myositis and hypercalcaemia75. Myositis can also be present in childhood vasculitides, with reports of polyarteritis nodosa presenting as polymyositis76 and deficiency of adenosine deaminase 2 (DADA2), a monogenic autoinflammatory disease, presenting with inflammatory myositis77.
Advances in genetic testing have resulted in an increasing recognition of monogenic autoinflammatory diseases and testing for such diseases should be included in the differential diagnosis of patients with myositis78. Characteristic features of monogenic autoinflammatory diseases include onset at an early age, fever and systemic inflammation affecting the eyes, joints, skin and serosa, but any system can be involved. Monogenic interferonopathies, such as proteasome-associated autoinflammatory syndromes and stimulator of interferon genes (STING)-associated vasculopathy with onset in infancy syndrome, can mimic JDM78,79. Protracted febrile myalgia is a rare manifestation of familial Mediterranean fever characterized by prolonged severe and symmetric muscle pain, fever and elevated inflammatory markers that can also mimic JIIM80.
Pathophysiology
Although the triggers of disease in JIIM remain elusive, several studies over the past few years have implicated new or interconnected mechanisms in the skin, blood vessels and muscle (Fig. 1), as discussed in this section.
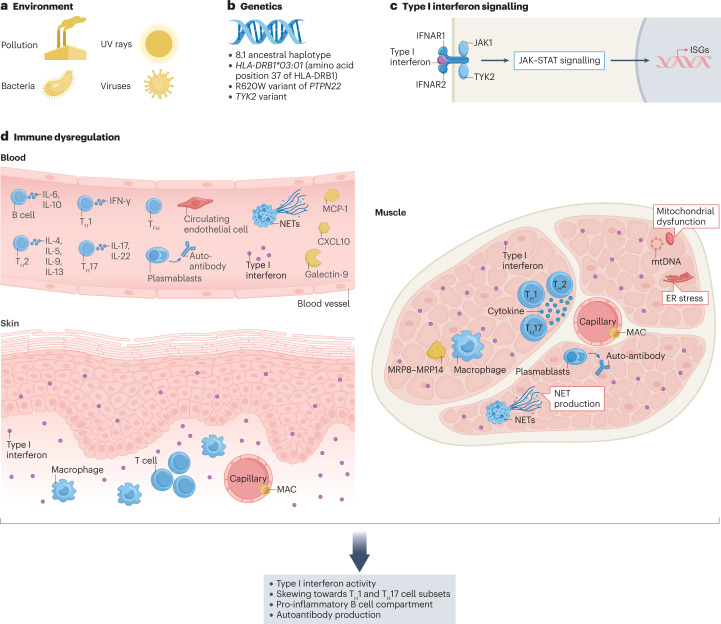
Fig. 1. Factors implicated in the pathogenesis of juvenile myositis.
The pathogenesis of juvenile idiopathic inflammatory myopathy (JIIM) involves a complex interplay between genetic and environmental factors, leading to immunological, vascular and metabolic dysfunction. a, Environmental triggers of JIIM might include ultraviolet (UV) radiation, pollution and microbial infections. b, Genetic loci in the MHC and non-MHC regions are implicated in disease susceptibility and development. c, Type I interferon signalling is thought to have a central role in the pathological changes seen in various tissues. d, Immune dysregulation within the skin, muscle and blood vessels, as well as in other tissues (not shown), is thought to contribute to disease. Within the muscle, the overexpression of MHC proteins, a hallmark feature thought to be driven by interferons, contributes to endoplasmic reticulum (ER) stress leading to an inflammatory cascade via the nuclear factor kappa B (NF-κB) pathway. Autoreactive B cells are present, as demonstrated by the production of myositis-specific antibodies (MSAs), and regulatory B (Breg) cells have a pro-inflammatory phenotype (including producing elevated levels of IL-6). Circulating inflammatory mediators include Galectin-9 and CXCL10, which correlate with disease activity. Abnormalities in the small blood vessels are reflected by a high number of circulating endothelial cells, which correlates with disease activity; muscle capillary loss and complement deposition on capillaries also frequently occur. T cell dysfunction includes a skewing of the T cell compartment towards a T helper 17 (TH17) cell phenotype, including within the follicular helper T (TFH) cell population. Both neutrophil extracellular trap (NET) formation and mitochondria dysfunction occur in JDM and might be part of a pathological loop that drives interferon production. Overall, the pathogenesis of JIIM involves a complex interplay between innate and adaptive immunity that affects muscle, skin and vascular tissues to drive ongoing inflammation and tissue damage. ISGs, interferon-stimulated genes.
Environmental risk factors
Although the exact cause of this heterogeneous group of diseases remain largely unknown, complex interactions between genetic and environmental factors, as well as immune and non-immune mechanisms, have a role in JIIM pathogenesis81. The contributions of several bacterial and viral pathogens have been studied, including streptococcal infections, picornavirus, enterovirus, mycoplasma, with inconclusive results82–85. Some patients have presented with myositis following SARS-CoV-2 infection or vaccination against SARS-CoV-2 (refs. 86,87), but such case reports await confirmation by larger epidemiological studies. Ultraviolet light intensity and exposure have been associated with both disease aetiology and severity in JIIM88–90. Such exposures might be associated with specific clinico-serological subtypes: for example, in a large North American study, previous high exposure to ultraviolet light was associated with increased odds of having anti-TIF1 antibodies89. Other studied risk factors in JIIM include air pollution, maternal smoking and maternal occupation91. Some evidence suggests that certain immunizations, stressor events, heavy exercise prior to diagnosis and prolonged breastfeeding increase the risk of specific JIIM phenotypes92 but the results need to be confirmed in larger multinational studies.
Genetics
In both adult and paediatric IIM, the strongest genetic association in white populations is within the 8.1 ancestral haplotype (AH8.1; also known as the HLA A1-B8-DR3-DQ2 haplotype) of the major histocompatibility complex (MHC), first detected by GWAS analyses93. Subsequent studies using Immunochip data in well-characterized, larger cohorts have confirmed this association94. More recently, in 2022, in a large international genetics study of JDM that used dense exome SNP genotyping, researchers revealed that the allele HLA-DRB1*03:01 and amino acid position 37 within HLA-DRB1 are both strongly associated with JDM, and that this association was independent of position 74, a position associated with adult-onset dermatomyositis, enabling differentiation between juvenile and adult-onset disease95. Further analyses suggested that position 37 of HLA-DRB1 was independent of the AH8.1 ancestral haplotype and confirmed previous associations with AH8.1 and HLA-DRB1*03:01. Specific associations of the HLA-DQB1*02 allele with disease differ between adult-onset and childhood-onset anti-TIF1 antibody-positive dermatomyostis96. Similarly, paediatric-onset anti-HMGCR antibody-positive myositis has a specific association with HLA-DRB1*07:01, whereas adult-onset anti-HMGCR antibody-positive myositis is associated with HLA-DRB1*11:01 (ref. 37). Other non-MHC genetic loci, including the R620W variant of PTPN22 and a non-synonymous SNP (rs2304256) in TYK2, have also been associated with both adult and juvenile IIM, as well as other autoimmune conditions97,98.
Vasculopathy of JIIM
Vasculopathy and endothelial dysfunction are thought to have an important role in JDM and have been associated with systemic disease99. In a flow cytometry analysis, the number of circulating endothelial cells but not circulating endothelial progenitor cells were increased in the peripheral blood of patients with JDM compared with healthy individuals100; a study of 90 patients with JDM found that the number of circulating endothelial cells correlated with disease activity and nailfold abnormalities, and were increased in both patients with active JDM and patients with inactive JDM compared with healthy individuals6. In a separate study, patients with JDM who were positive for anti-TIF1 antibodies had lower nail fold end row loop counts (indicative of vasculopathy) at diagnosis and a prolonged duration of untreated disease, compared with other patients with JDM101. Endothelial soluble adhesion molecules, including soluble intercellular adhesion molecule 1 (sICAM1) and sICAM3, soluble vascular cell adhesion molecule 1 (sVCAM1), VCAM1 and E-selectin, are key players in the adhesion and migration of leukocytes through the endothelium towards inflamed sites and are under investigation as biomarkers of vasculopathy in JIIM6,99,102. These molecules are mainly secreted by activated endothelial cells, highlighting the association of these molecules with vasculopathy in JDM. The soluble forms of these molecules maintain many of the functions and the structure of the cell-bound adhesion molecules and are therefore of interest as potential therapeutic targets.
The role of interferon and immune cells
A strong interferon type I signature has been extensively implicated as a characteristic feature of JIIM, including studies of patient blood, muscle and skin79,103–105. Type II interferon has also been associated with JIIM106. Both type I and II interferons originate as viral interfering proteins; several type I interferons exist (including IFNα and IFNβ), all of which bind to the type I interferon receptor107, whereas IFNγ is the only type II interferon and binds to the separate type II interferon receptor. Several different assays exist that assess the levels of interferon types I and II, the downstream targets and related biomarkers (Table 2).
Table 2.
Studies demonstrating the type I interferon signature in JIIM
| Methods | Sample | Findings | Ref. |
|---|---|---|---|
| Type I interferon protein | |||
| Single-molecule array (Simoa) IFNα assay (digital ELISA technology) | Plasma and serum | Higher IFNα levels in patients with JDM (n = 43) than in healthy individuals (n = 20) | 104 |
| Interferon-stimulated gene transcripts and interferon scores | |||
| qPCR | Whole blood (PAX gene tubes) | 75% of 101 measurements in 59 patients with JIIM showed upregulation of ISG transcripts (IFI27, IFI44L, IFIT1, ISG15, RSAD2 and SIGLEC1) above a pre-determined cut-off point | 227 |
| Muscle | IFNα and/or IFNβ-inducible genes, IFNγ and IFNγ-inducible gene expression were higher in patients with JDM (n = 27) than in patients with muscular dystrophy (n = 24) or healthy individuals (n = 4) | 106 | |
| NanoString Technologies™ | Whole blood (PAXgene tubes) | A 28-gene ISG score in patients with active JDM (n = 57) correlated with muscle and joint disease | 79 |
| Microarray and qPCR | Skin | Skin lesions in patients with JDM had a strong interferon signature (including the expression of CXCL10, CXCL9 and IFI44L) and the interferon signalling pathway was identified as an important canonical pathway | 115 |
| RNAseq | PBMCs | Patients with new-onset JDM (n = 21) had a higher ISG score (5 gene score: MX1, IFI44, IFI44L, LY6E, IFIT3) than patients with muscular dystrophy (n = 7), healthy individuals (n = 6 children, n = 9 adults), or patients with JDM in remission (n = 10) | 228 |
| PBMCs | PBMCs from untreated patients with JDM (n = 11) had a strong type I interferon signature that was associated with disease activity scores | 105 | |
| Muscle and skin | The transcriptomic profile of the muscle and skin of patients with JDM (n = 4) included enrichment in the type I interferon signature | ||
| B cells | Enrichment of the IFNα response pathway. Upregulation of TLR7 and IRF7 expression in patients with JDM prior to treatment (n = 10) compared with in patients with JDM following treatment (n = 9) | 114 | |
| Gene expression meta-analysis | Muscle and skin | Meta-analyses was performed on six publicly available microarray data sets for muscle (that included data from 71 patients with dermatomyositis and 36 controls) and skin (from 77 patients with dermatomyositis and 22 controls). 94 genes were upregulated in JDM across both tissues, which included genes involved in type I and II interferon signalling and MHC class I pathways | 229 |
| Interferon-driven proteins | |||
| Multiplex immunoassay | Plasma | The expression of galectin-9, CXCL10 (also known as IP-10) and TNF receptor 2 (TNFR2) were increased in patients with active JDM (n = 25) compared with healthy children (n = 14) or children with non-autoimmune muscle disease (n = 8) | 230 |
| Serum | Galectin-9 and CXCL10 outperformed creatinine kinase in distinguishing between patients with active JDM with patients with JDM in remission, and these markers were sensitive and reliable markers for disease activity in JDM in three cohorts (n = 120) | 231 | |
| Serum | Analysis of two independent JDM cohorts (n = 30, n = 29) showed that patients with JDM who had high serum levels of CXCL9, CXCL10, TNFR2 and galectin-9 might be more likely to respond poorly to standard treatment than those patients who had low levels of these markers and these chemokines correlated with disease activity and measures of vasculopathy | 232 | |
| Multiarray detection system and ELISA | Serum | The expression of IFNα, IFNλ1 and IFNγ, MCP1, CXCL10 (IP10), TNFR2 and Galectin-9 were higher in patients with JDM (n = 90) than in healthy controls (n = 70). The expression of IFNλ1, MCP1, CXCL10 and galectin were increased in active disease compared with disease in remission, and these markers correlated with disease activity and measures of vasculopathy | 6 |
| Flow cytometry | Blood monocytes | Patients with new-onset JDM (n = 21) and a high expression of Siglec-1 were at an increased risk of intensification therapy 3 months after diagnosis compared with healthy individuals (n = 6 children, n = 9 adults) and patients with JDM during follow-up (n = 10) | 228 |
| Immunohistochemistry | Muscle | The expression of MxA was identified in >50% of samples from patients with JDM and was associated with greater muscle weakness | 103 |
ELISA, enzyme-linked immunosorbent assay; ISG, interferon-stimulated gene; JDM, juvenile dermatomyositis; JIIM, juvenile idiopathic inflammatory myopathy; MSA, myositis-specific autoantibodies; MxA, myxovirus-resistance protein; PBMCs, peripheral blood mononuclear cells; qPCR, quantitative polymerase chain reaction.
In parallel to the interferon pathway, both innate and adaptive immune dysregulation are thought to contribute to JIIM. The presence of MSAs and their association with distinct clinical phenotypes (which differ between juvenile and adult-onset disease52) strongly implicate a role for B cells in disease. Notably, in an international trial of adult and juvenile IIM, B cell depletion appeared to have clinical benefit in patients with JDM, according to a sub-analysis108. In additional to clinical phenotypes, specific MSAs are also associated with pathological conditions and patterns of inflammatory infiltrate in muscle biopsy samples58. In a study of CXCR5+ follicular helper T (TFH) cells in patients with JDM, the cells were skewed towards T helper 2 (TH2) and T helper 17 (TH17) cell phenotypes109, which might drive B cells towards autoantibody production and a pro-inflammatory phenotype. A separate study confirmed skewing of the T cell compartment towards a TH17 phenotype in juvenile, adolescent and adult patients with dermatomyositis110. Inflammatory T cells, B cells and tissue macrophages are all present in the inflamed muscle of patients with JDM111–113. An analysis of peripheral blood B cells in patients with JDM showed that a population of immature transitional B cells (CD19+CD24hiCD38hi cells) is expanded during active disease and correlates with disease activity114. Transcriptional and functional analyses have confirmed that these immature transitional B cells have an upregulated IFNα signature that is associated with an abnormal ratio of IL-6 to IL-10 production, suggesting that these cells are driven towards a pro-inflammatory phenotype that hinders the immunoregulatory properties of the cells114.
The inflammatory T cell and B cell infiltrate within muscle biopsy samples (which is typically perivascular) correlates with interferon-driven MxA expression and drives the inflammatory domain score of a JDM muscle biopsy score48,103,111. This muscle biopsy score has prognostic value in predicting treatment and disease48,111. Tissue macrophages in JDM muscle are highly pro-inflammatory and secrete both cytokines and pro-inflammatory molecules, including calprotectin112. In an immunofluorescence analysis of muscle biopsy samples, the expression of IFNγ and HLA class II molecules was increased in patients with JDM not undergoing treatment compared with healthy individuals, and the type I and type II interferon scores were associated with muscle infiltration by endomysial and perimysial CD3+ cells, as well as with CD68+ cells, and perifascicular atrophy of the muscle106. Transcriptomic analyses suggest that skin lesions of patients with JDM contain higher numbers of macrophages and CD4+ memory T cells than non-lesional skin and share a similar gene expression pattern to skin lesions from patients with childhood-onset SLE115, including a prominent type I interferon signature. However, the factors most important in driving the type I interferon signature and immune cell dysregulation remain elusive. More work is needed to understand these mechanisms: high-resolution techniques (such as single-cell transcriptional analyses by RNA sequencing) for assessing skin, muscle and blood samples, as well as differential transcriptional expression in specific cell lineages, in parallel with functional studies in JDM, are ongoing and will generate important mechanistic insights into the interferon signature, its relation to other dysregulated pathways and how these processes are impacted by treatment or disease activity.
Neutrophils, NETs and mitochondrial dysfunction
Neutrophils, an essential component of the innate immune system, can produce neutrophil extracellular traps (NETs) that comprise DNA–histone complexes and other released proteins. The role of NETs is to help to capture, degrade and kill pathogens (such as bacteria)116. Various studies implicate dysregulated neutrophil pathways, including NET formation, in JDM. For example, a muscle biopsy analysis found increased amounts of NET remnants in patients with JDM compared with healthy individuals, which was more evident in patients with calcinosis117. In a concurrent study, the level of circulating NET complexes was also higher in patients with JDM than in healthy individuals118 and correlated with disease activity and the presence of anti-MDA5 antibodies, but conversely did not correlate with calcinosis117,118. In one of these studies, NETs were shown to contain mitochondrial DNA (mtDNA)117, which is notable as studies in SLE have shown that mitochondrial dysfunction leads to the extrusion of oxidized mtDNA in NETs, which in turn induces an type I interferon response119. Indeed, gene expression network analysis of muscle has implicated a role for mitochondrial dysfunction in JDM, and a recent study demonstrated that abnormal mitochondrial function in monocytes (including the presence of enlarged mitochondrial networks or ‘megamitochondria’) in patients with JDM leads to the production of oxidized mitochondrial DNA and drives further type I interferon production120,121. Furthermore, anti-mitochondrial autoantibodies are present in the serum of some patients (1% (4/371) of patients in one enzyme-linked immunosorbent assay (ELISA)-based analysis)122. This growing body of evidence supports the involvement of mitochondrial dysfunction in JDM pathogenesis and in type I interferon-mediated inflammation.
ER stress
JDM is characterized by an increased expression of MHC class I molecules on muscle fibres, which is thought to be driven by both type I and type II interferon signalling103,123. Accumulation of class I MHC proteins can result in ER stress and can lead to cell death124. ER stress might also synergise with factors secreted by infiltrating myeloid cells, such as myeloid-related protein 8 (MRP8), MRP14 and other endogenous TLR ligands, to further damage the muscle112. For example, in one study, concentrations of MRP8–MRP14 complexes were significantly increased in the serum of patients with JDM compared with age-matched healthy controls (P > 0.05); further analysis suggested that these inflammatory proteins were secreted by CD68+ myeloid cells and synergized with ER stress to promote the production of IL-6 and MCP1 in the muscle112. In a separate muscle biopsy analysis, the muscles of adults with IIM contained higher levels of proteins involved in the ER stress-induced-autophagy pathway (such as the ER chaperone protein glucose-regulated protein 78 (GRP78)) than muscles of individuals lacking any myopathic features, which correlated with levels of autophagy, muscle damage and disease activity125. These studies demonstrate that ER stress might have an important role in JIIM pathogenesis.
Diagnosis
The diagnosis of JIIM requires careful evaluation of a number of clinical features, supported by a combination of laboratory, radiological and histopathological investigations. A Single Hub and access point for Paediatric Rheumatology in Europe (SHARE) initiative-based consensus guideline has set out recommendations for the diagnosis of JIIM, including investigations to differentiate JIIM from other causes of muscle weakness, to confirm a diagnosis of JIIM and to determine the presence of organ involvement126. A similar process has been followed by the Paediatric Rheumatology Association of Japan and the Japan College of Rheumatology to produce a clinical practice guideline, recognizing that the frequency of complications and drug use differs among Europe, the USA and Japan127. Diagnostic testing has been discussed in detail elsewhere85, and therefore a full description of diagnostic work-up will not be repeated here but might include formal evaluation of muscle strength, detailed cutaneous assessment, testing for muscle enzymes or other parameters in the blood and performing pulmonary function tests, electrocardiography, echocardiography and radiological investigations. In this section we discuss notable changes in practice over the past decade, particularly relating to the role of MRI, muscle biopsy and MSAs85,126.
MRI is now favoured as a diagnostic tool, but muscle biopsy also remains important, particularly in the absence of skin rash or when presentation is atypical126. When performed, use of a standardized JDM biopsy score is helpful in quantifying the severity of histopathological abnormalities, and together with MSA status, might help to predict the disease course48,111,126.
A major advance over the last decade has been the routine use of MSAs to aid the diagnosis of JIIM, to help to define or predict disease phenotype and to develop a more personalized approach to management64,128,129 (although the absence of an MSA does not rule out JIIM5,128,130). A recent survey of members of the International Myositis Assessment and Clinical Studies (IMACS) group found that over 80% of participants reported that MSA testing increased their confidence in the diagnosis and information that they gave to patients on prognosis128. However, more than 90% of respondents expressed the need for more education on the interpretation of antibody results128. The MSA–MAA profile might influence the investigative screening or treatment decisions by indicating the risk of a chronic disease course or specific complications such as ILD or calcinosis (Table 1). Results of MSA–MAA testing can vary depending on which technique is used, with some techniques not reliably detecting certain MSAs, as described in Table 1. Measurement by immunoprecipitation is considered the gold standard, but is expensive and time consuming and additional testing is required to differentiate between the presence of anti-NXP2 antibodies and anti-MDA5 antibodies131. Other techniques used in practice include line blot, dot blot, commercial multiplex assays, ELISAs and gel precipitation. Line blot is a technique that is cheap and rapid to perform, but false positives can occur, and this technique does not reliably detect anti-TIF1 (ref. 130) antibodies, which is the most common MSA in JIIM130–132. ELISA is a reliable test for detecting anti-TIF1 antibodies and produces a fast and quantitative result, but multiple assays might be required to test for all MSAs. Some MSAs are cytoplasmic and therefore MSAs can still be present when an antinuclear antibody (ANA) test result is negative. The staining pattern seen on human epithelial type 2 (HEp-2) cells can be used, along with the clinical phenotype, to help ascertain if the results of MSA testing are correct131. False-positive results should be considered if more than one MSA is reported as positive, or if the MSA result does not fit with the HEp-2 staining pattern or expected clinical phenotype. Repeating a test using the same technique is rarely useful and in ambiguous cases a different testing technique or specialist laboratory is preferable. Further details on the expected HEp-2 cells staining pattern and challenges with MSA testing are summarized in Table 1.
Management
The treatment of JIIM needs to consider the disease severity of the patient, including the presence of systemic and/or organ involvement and the disease phenotype. As well as these features, the MSA–MAA profile can inform the management and treatment of the patient, given the associations of specific MSAs and MAAs with clinical phenotypes, prognosis and risks of complications (Fig. 2). Treatment decisions are best made in a specialist paediatric centre by a multidisciplinary team, owing to the rarity and heterogenicity of the diseases126,133. Consensus guidelines provide a framework for health care professionals on the basis of the best possible evidence available126,133. A 2022 evidence-based British Society for Rheumatology guideline for childhood and adult-onset myositis, and a previous European consensus recommendation for JIIM, emphasize the need for a safe and effective exercise programme and attention to psychological wellbeing in addition to drug therapies for the management of JIIM126,133. The Childhood Arthritis and Rheumatology research Alliance (CARRA) guideline provides Consensus Treatment Plans for different severity levels of juvenile myositis134–136. Treatments for JIIM have been well described in reviews elsewhere85,129,137,138. A suggested treatment algorithm based on the best available current evidence and integrating current recommendations from the various guidelines is shown in Fig. 3. In this section, we outline various drug-related and non-medication-related aspects in the management of JIIM.
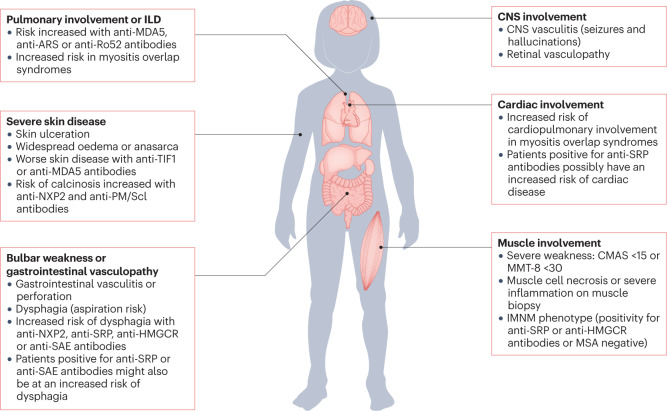
Fig. 2. Clinical features and autoantibody profile in JIIM indicative of severe disease and/or need for treatment escalation.
Owing to the rarity and heterogeneity of juvenile idiopathy inflammatory myopathy (JIIM), children and young people should be managed by a multidisciplinary team in a specialist centre. To predict the severity of the disease and the potential need for treatment escalation, many factors are considered, as illustrated, including the presence or absence of severe muscle weakness, dysphagia, ulcerative skin disease or major organ involvement. The myositis-specific autoantibody (MSA) and/or myositis-associated autoantibody (MAA) profile might predict the risk of JIIM-related complications, including major organ involvement. Some features associated with specific MSAs or MAAs are shown, but specific complications are not exclusive to patients with these MSA–MAA profiles and not all patients with a particular MSA–MAA profile will demonstrate these complications. CMAS, childhood myositis assessment scale; GI, gastrointestinal; ILD, interstitial lung disease; IMNM, immune-mediated necrotizing myopathy; MMT-8, manual muscle testing in eight muscle groups.
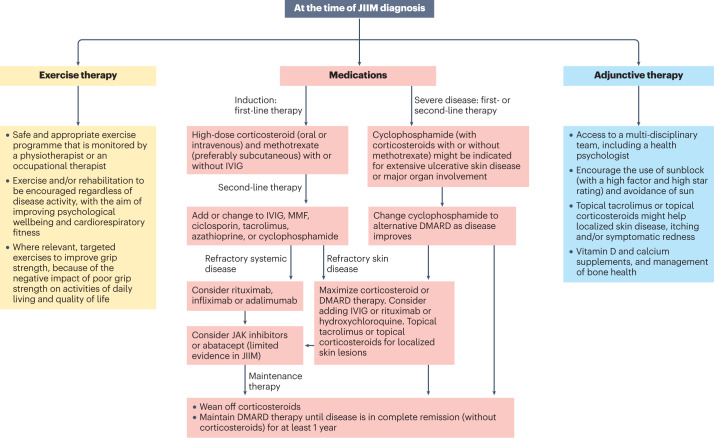
Fig. 3. Treatment algorithm for JIIM on the basis of current available evidence.
A treatment algorithm for juvenile idiopathic inflammatory myopathy (JIIM) is presented, based on evidence-informed consensus recommendations in UK and Europe126,133. Treatments need to be individualized and include consideration of the patient age, preferences for oral or parenteral administration of medications, severity of disease and response to treatment. No single approach will be right for every patient and clinicians need to use best judgement on the basis of evidence available. In most cases, with the exception of randomized controlled trials evaluating methotrexate versus ciclosporin, rituximab or exercise in myositis, evidence is limited to case series or cohort studies. More research is needed to compare the efficacy of second-line or third-line treatment options and determine the best treatment approach for myositis-related complications such as interstitial lung disease (ILD) or calcinosis. More evidence is also needed to determine the best treatment for refractory disease, which can be defined as myositis that responds inadequately to at least two immunosuppressant or immunomodulatory drugs given in their full dose for a minimum of 3 months, hindering weaning of corticosteroid. Patients with JIIM should have regular reviews that include measurement of muscle strength, assessment of skin disease and extra-muscular manifestations. Adherence to medication should be checked if patients fail to respond as expected. Treatment should be escalated if patients fail to respond adequately to treatment or are intolerant to the treatment. Exercise therapy and psychological support are important aspects to the management of JIIM in addition to medication. IVIG, intravenous immunoglobulin; JAK, janus kinase; MMF, mycophenolate mofetil.
Medication
A combination of high-dose corticosteroid in combination with methotrexate (15–20 mg/m2, maximum 40 mg/week) is the first-line induction treatment for most cases of JIIM126,133,139. Methotrexate is favoured over ciclosporin as it has a more favourable adverse effects profile; however, both medications, when used with prednisolone, were superior to prednisolone alone in a multi-centre randomized trial of 139 patients with new-onset JDM140. Clinicians have the choice of oral prednisolone (12 mg/kg/day with ceiling doses applied, typically capping at 60 mg/day) or intravenous methylprednisolone (10–30 mg/kg/day, maximum of 1 g/day)126,133,134,139. Intravenous administration might result in an increased therapeutic effect and less toxicity than with an oral corticosteroid and should be considered, especially when there are concerns about gastrointestinal absorption126,133. Intravenous methylprednisolone might have the additional benefit of reducing skin disease more rapidly than oral prednisolone141.
Evidence is lacking to determine the best second-line treatment when the combination of corticosteroids and methotrexate does not adequately control disease or patients are intolerant to methotrexate. Head-to-head comparison studies are needed. In the absence of current evidence, CARRA have developed a series of consensus treatment plans to limit treatment variation among patients and enable comparative effectiveness studies from registry data134–136,142. Some evidence, in the form of case series involving small to moderate numbers of patients, supports the use of mycophenolate mofetil (MMF) for the treatment of skin or muscle disease143–146. Evidence for the use of azathioprine comes from historical studies that included small numbers of patients, and although this drug can be used as an adjunctive treatment, it has become less favoured over the last two decades for the treatment of IIM in paediatric practice147,148. Some evidence is available on the use of tacrolimus to treat JIIM but is limited by the small number of patients involved149–151. Adult data suggest that tacrolimus or ciclosporin alongside corticosteroids should be considered for patients with myositis-associated ILD, and although these data are often extrapolated to JIIM, insufficient data are available to form evidence-based recommendations for this complication in childhood-onset disease133. Data from case series of adult and paediatric patients suggest that cyclophosphamide or rituximab could be considered when ILD is present and should be used early, potentially as part of an induction regime133. Although no standardized treatment guidelines are available on the management of ILD in adult patients with IIM, a summary of evidence and treatment approach has been presented in a review and, in the absence of evidence in JIIM, might provide useful guidance in the treatment approaches for childhood-onset disease152. In this review, the authors suggests that corticosteroids are used as the initial treatment for acute disease followed by MMF or azathioprine as first-line steroid-sparing agents. Tacrolimus is suggested as an appropriate second-line steroid-sparing agent for patients with disease that is refractory to MMF or azathioprine, or for select patients with severe disease. Cyclophosphamide is proposed as a third-line steroid-sparing agent. IVIG or rituximab are advocated as appropriate adjunctive agents in combination with traditional steroid-sparing agents for patients with refractory disease152.
Intravenous immunoglobulin (IVIG) might be a helpful adjunct for severe or refractory skin disease, muscle inflammation or dysphagia133. In a randomized placebo-controlled 16-week trial of IVIG in adult patients with dermatomyositis (the ProDERM trial), a higher proportion of patients in the IVIG treatment group reached the primary outcome of total improvement score (a composite measure of disease activity) of at least 20 (indicating at least minimal improvement) than in the placebo control group (P < 0.001)153. Although evidence in adult-onset disease includes randomized trials, evidence in JIIM is mostly limited to cohort studies or case series154–159. Interpreting observational evidence is challenging owing to the use of concomitant therapies, the variable doses or treatment courses of IVIG used and the small numbers of patients involved. In one notable study, the researchers applied bias reduction methods to assess the efficacy of IVIG in a retrospective cohort of 78 patients with JDM, demonstrating that IVIG was efficacious in controlling severe or refractory disease, particularly in those patients who had steroid-resistant disease158. Other immunomodulating drugs have also been reported to improve symptoms of dysphagia or improve objective measures of swallowing function133. Cyclophosphamide tends to be reserved for more severe or refractory disease in view of the toxicity of this drug, but might be considered in cases of major organ involvement, including ILD or ulcerative skin disease126,133,157,158,160,161. Despite a lack of evidence from randomized controlled trials, the use of IVIG or cyclophosphamide is supported by case reports, case series and analysis by marginal structural modelling157–160.
Evidence related to the treatment of skin manifestations in JIIM is limited, but IVIG or rituximab can be used to treat skin manifestations refractory to corticosteroid and DMARDs133,157,158. In the ProDERM trial, IVIG was efficacious in improving skin disease activity in patients with adult-onset dermatomyositis, as measured by the modified Cutaneous Dermatomyositis Disease Severity and Activity Index153. Despite the relative lack of evidence for use of hydroxychloroquine in JIIM, limited to case series with small numbers of patients, this drug is often used as an adjunctive treatment for skin disease and arthritis162–164. Hydroxychloroquine is included in the CARRA Consensus Treatment Plan for skin predominant disease142. However, in a prospective study of 184 children with JDM treated at a single children’s hospital, although hydroxychloroquine was often administered to those patients with higher skin activity scores, the drug did not lead to any statistically significant improvement in skin rash by the end of the observation period141. Topical tacrolimus (0.1%) or topical corticosteroids might help localized skin disease, particularly for symptomatic redness or itching126.
The use of biologics in JIIM has been summarized elsewhere in a systematic review165. Rituximab treatment for refractory muscle or skin disease is supported by one randomized controlled trial and various case series or cohort studies108,166–170. In the Rituximab in Myositis randomized controlled trial, despite failure to meet the primary or secondary endpoints, 83% of the patients met the definition of improvement108. Data were reported in aggregate but post hoc analyses suggested that patients with JIIM were more likely to respond to treatment than those patients with adult-onset myositis133,166. The presence of anti-Mi2 antibodies, anti-synthetase antibodies or other undefined autoantibodies were other predictors of a beneficial response, but anti-Mi2 antibodies and anti-synthetase antibodies are less common in JIIM than in adult disease165,166,168. In this trial, rituximab treatment also led to improvement in cutaneous disease168. Evidence in adult-onset myositis (that is, data from retrospective and prospective studies rather than randomized controlled trial data) suggests that rituximab might be helpful in IIM-related ILD, but more data are needed in JIIM64,133,152.
Data from case series and cohort studies suggest that TNF blockade by infliximab or adalimumab can be helpful for refractory muscle or skin disease, including calcinosis165,171–175. In an open-label 12-week trial of the TNF inhibitor etanercept, the drug showed no appreciable benefit in nine patients with refractory JDM174, whereas etanercept had a steroid-sparing effect in a randomized double-blind placebo-controlled 52-week trial involving 16 patients with adult-onset IIM176. In rare instances, TNF inhibitors have been reported to induce myositis or cause disease flares in adult patients with IIM177,178. Although TNF inhibitors, particularly adalimumab or infliximab, might be helpful in some patients with JIIM, evidence from a systemic review suggest that treatment with these drugs does not lead to complete remission and better treatments are needed165. Abatacept has demonstrated efficacy in a randomized controlled trial in adult onset myositis179 and in an open label therapeutic trial in JIIM180. Abatacept might be helpful for the treatment of resistant disease, including calcinosis179,181,182.
JAK–STAT inhibitors target the interferon pathway and show clear promise in the treatment of IIM-related muscle, skin and lung disease7,64,183. A number of reports have highlighted the potential safety and efficacy of JAK inhibitors (including tofacitinib and baricitinib) in treatment-resistant adult IIM7,184. In JIIM, various JAK inhibitors (including baricitinib, tofacitinib and ruxolitinib) have shown promise in numerous case reports and case series, predominantly involving patients with refractory muscle or skin disease that is unresponsive to alternative immunosuppressive treatment(s)8–10,185–187. These studies have been carefully reviewed in a systematic review elsewhere, which described 48 publications reporting 145 unique patients (including 61 cases of JIIM) with refractory disease at baseline and demonstrated that treatment with JAK inhibitors led to improvement in a wide range of manifestations, including of the skin, muscle and lungs7. As well as providing evidence on the clinical efficacy of JAK inhibition, these studies suggest that JAK inhibitors can modulate the disease at an immunopathogenic level, as demonstrated by the downregulation of interferon biomarkers, the type I interferon signature and STAT1 phosphorylation in T cells and monocytes to similar levels to that in healthy individuals8–11,186. These encouraging results suggest that JAK inhibition could be an effective, targeted treatment for JDM, and highlight the importance of confirming these findings in clinical trials7,183,188.
An important challenge in JIIM is the treatment of calcinosis. Some evidence is available on the use of DMARDs, medications that affect calcium and phosphorus metabolism, mechanical therapies and adjunctive therapies in the treatment of calcinosis in JIIM, as reviewed elsewhere189–191. However, the available evidence is limited and largely based on case reports or case series, cohort studies or limited controlled studies. A major unmet need exists for an improved understanding of calcinosis pathogenesis, for standardized tools to measure calcinosis and for efficacious treatment of this burdensome complication189,190. Consensus guidelines advocate for early aggressive treatment at disease onset to decrease the long-term risk of calcinosis, as well as consideration of an early increase in treatment of ongoing disease activity and intensifying immunosuppressive therapy in the presence of calcinosis126,133. Other than associations with some MSAs, as described above, evidence on risk factors for calcinosis is limited, but a single-centre retrospective study of 172 patients identified nailfold capillary abnormalities at baseline as a risk factor for calcinosis in univariate and multivariate analysis192. Some data are available on the histopathological and chemical composition of calcinosis, genetic and inflammatory markers in IIM-associated calcinosis and potential biomarkers of this complication, which have been reviewed in further detail elsewhere193.
Exercise
Cardiorespiratory fitness can be impaired in patients with JIIM during both inactive and active disease and in patients with both monocyclic and polycyclic disease courses owing to factors such as cardiovascular deconditioning and reduced thoracic compliance194–198. Studies, including a randomized controlled trial in children and adolescents with JDM, have demonstrated the safety and efficacy of exercise training programmes, including the positive effects of these programmes on health-related quality of life199–201. Hence, the management of JIIM should include a safe and appropriate exercise programme that is led and monitored by a specialist physiotherapist and/or occupational therapist126,133.
Some data are available on the efficacy of interventions to reduce fatigue in paediatric conditions such as JIIM, including land or aquatic-based exercise, medications and psychological interventions, which have been evaluated in a systematic review elsewhere202; however, in this study, the efficacy of current interventions to reduce fatigue could not be established owing to insufficient evidence. Fatigue is multi-dimensional and does not necessarily always correlate with disease activity and is instead strongly associated with biological, lifestyle, psychological and social factors202. Further multidimensional intervention studies are needed to identify the best management of this troublesome symptom.
Psychological support
JIIM has a notable impact on the emotional health of young people and their families203,204 Mental health issues, most commonly anxiety and depression, are reported frequently by children and young people with JIIM204,205. Psychological wellbeing, psychiatric comorbidities and health-related quality of life should be assessed using age-appropriate tools133. Access to mental health provision, ideally embedded within paediatric rheumatology services so that young people feel that counsellors understand their disease, is paramount204–206. Factors that impact negatively on the health-related quality of life of patients, including pain, muscle weakness, functional impairment or physical disability, poor sleep and fatigue, should be managed appropriately25,133,207,208.
Assessment of disease activity and treatment response
Disease activity should be measured in a quantifiable way in both clinical practice and clinical research studies to determine changes in disease activity over time and response to treatment. Tools to measure disease activity have been comprehensively reviewed by others129,209. The International Myositis and Clinical Studies group and Paediatric Rheumatology International Trials Organisation (PRINTO) have developed core sets to measure disease activity and damage, predominantly for use in research studies or clinical trials129,209. The ability to robustly define response to therapy is crucial for conducting clinical trials and has been addressed by the development of ACR–EULAR response criteria210,211. To define the optimal set of items collected in clinical practice to enable entry into research registries and comparison of data over time, an international collaboration has defined a consensus core dataset that is in use by several major registry studies15,212–214. Consensus recommendations advise the routine use of measures such as the Manual Muscle Testing in eight muscle groups (MMT-8) tool and the Childhood Myositis Assessment Scale (CMAS) to assess muscle strength and function126. Age-specific considerations need to be taken into account when using tools that measure muscle strength, function and quality of life133. For example, for the CMAS, results for head-lift, leg-lift and sit-up manoeuvres are dependent on the age of the patient and very young children should not be expected to achieve a score of 52, even when disease is inactive215,216. A shortened version of the MMT-8 tool that tests four (MMT-4) or six (MMT-6) muscle groups and a hybrid version that includes all eight items of the MMT-8 tool and three items from the CMAS have demonstrated good measurement properties and might be more suitable than MMT-8 or CMAS for routine clinical use217,218. More work is needed to define and reach consensus on the optimal tools for assessment of skin disease activity and measurement of quality of life in JDM129,214. Several tools are currently available, including the Cutaneous Assessment Tool, Disease Activity Score and Myositis Intention to Treat Activity Index, each of which correlates with the physicians’; skin Visual Analogue Scale219; furthermore, the Cutaneous Disease Area and Severity Index has been extensively used in studies of adult dermatomyositis and might be equally valuable for use in JDM129,209,220.
The importance of patient-reported outcome measures in outcome assessment within trials and in the clinic is becoming increasingly recognized. A study that included patients with JDM suggested that three tools from the Patient-Reported Outcomes Measurement Information System are an improvement over the previously widely-used Childhood Health Assessment Questionnaire (CHAQ) for capturing patient-reported outcomes221. Patient-Reported Outcomes Measurement Information System tools can be administered as fixed short forms or via computerized adaptive testing, the latter of which results in less pronounced floor and/or ceiling effects than fixed short forms222.
Conclusions
Our understanding and management of juvenile onset myositis has changed considerably in the past two decades, but numerous challenges remain (Box 1) and much work is still needed. A deeper appreciation of the underlying mechanisms that initiate and perpetuate inflammation of the blood vessels, muscles, skin and other organs, and how inflammatory mechanisms intersect with other aetiological pathways in JIIM, is needed. New insights are becoming available from studies at a single-cell level of gene expression, function and metabolic profiles. Further studies on the mechanisms of important patient-reported symptoms, such as fatigue, are also much needed. A vital aim is to ensure that novel data on underlying mechanisms are shared collaboratively and made accessible to drive the design of biomarker studies and enable validation studies and meta-analyses. The highly collaborative nature of myositis research, both basic and clinical, has enabled major progress thus far, and will support such platforms through which to generate evidence for new treatments, despite the rarity of JIIM (Box 1).
This strongly collaborative community, across paediatric, adolescent and adult myositis research, is reflected in the first ‘age-inclusive’ trial in myositis (the Rituximab in Myositis trial)108; such a design enables faster results for children and young people, rather than waiting for a ‘child-specific’ trial for drugs that have been granted a licence in adults. Progress has also been made using longitudinal observational data to support so-called ‘trial in silico’ analyses223; this approach will become more possible through widespread use of an agreed common clinical dataset214, which is embedded in clinical care and large research registries15,212,213. Translation of this core dataset for use in adolescent and adult care could facilitate evidence generation on long-term outcomes, which is currently lacking. Current long-term outcome data clearly indicate increased risks of cardiovascular or pulmonary disease in patients with IIMs compared with the general population. In the future, the integration of biomarker and pathogenesis data with long-term outcome data of those treated in the modern era will be critical for informing our patients and their families about comorbidities, outcomes and the chances of sustained remission.
Ultimately, a combination of a better understanding of disease mechanisms, biomarkers that accurately track disease activity, including subclinical disease, and definitions of outcomes that include the patient perspective will be needed to deliver a personalized approach to managing myositis in children, and in the young people and adults they become.
Box 1 Challenges in the management of JIIM.
JIIM as a group are rare conditions
Challenges
Randomized controlled trials (RCTs) are challenging
Within JIIM, rare sub-phenotypes or patients with severe disease are often excluded from clinical trials
Children and adolescents are often excluded from clinical trials of idiopathic inflammatory myopathies
Mitigated by
International collaboration through PRINTO and IMACS has led to successful RCTs and other important research studies
All clinical trials are advised to have a paediatric investigational protocol
Evidence is lacking
Challenges
Head-to-head comparison studies are needed to determine the best second-line treatment for JIIM
A paucity of evidence-based data is available for patients with refractory disease
Evidence is lacking to determine the best treatments for skin disease, calcinosis or disease involving vital organs
An unmet need exists to better understand the pathogenesis of calcinosis and define standardized assessment tools
The division of trials into ‘adult’ and ‘paediatric’ trials artificially dichotomizes the evidence base
Mitigated by
Evaluation of the disease course through practice and registry data, including the use of CARRA-developed consensus treatment plans
Collection of data and biospecimens within disease registries and the development of a consensus core dataset to enable uniform data collection and comparison across groups
Development of evidence-informed consensus guidelines, such as SHARE and/or BSR guidance on the management of IIM
Collaborative working of the PReS JDM working party, CARRA JDM working group, IMACS and iMYoS
Lost opportunity for long-term data collection
Challenges
Continuity of long-term data in research registries might be lost when young people transition to adult services
Ensuring continuity of data across the life course is crucial to better understanding the long-term risks of disease, such as the impact of disease on cardiovascular risk, fertility, mental health, education level and employment
Mitigated by
Research registries such as MYONET (formally Euromyositis) and some country-specific registries enable data collection across paediatric and adult registries
Attempts have been made to develop strategies to enable data sharing (while protecting data ownership and governance) but need to be developed further
BSR, British Society for Rheumatology; CARRA, Childhood Arthritis and Rheumatology Research Alliance; IMACS, International Myositis Assessment & Clinical Studies Group; IMyoS, The International Myositis Society; JIIM, juvenile idiopathic inflammatory myopathy; PRINTO, Paediatric Rheumatology International Trials Organisation; SHARE, Single Hub and Access Point for paediatric Rheumatology in Europe.
Acknowledgements
The authors would like to thank the members of the UK JDM Cohort and Biomarker Study (JDCBS) and the patients and families who contribute to it. The JDCBS is currently funded by grants from the Great Ormond Street Hospital (GOSH) Children’s Charity, National Institute for Health Research (NIHR) Biomedical Research Centre at GOSH, Cure JM, Myositis UK, Versus Arthritis and Remission Charity. M.G.L.W. is supported by grants from Cure JM, the NIHR Biomedical Research Centre at GOSH, and Versus Arthritis. The authors would also like to thank Dr S. Tansley for critical review of the manuscript. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author contributions
All authors researched data for the article, contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Rheumatology thanks L. Rider, who co-reviewed with M. Sherman; H. Kim; A. Huber; and K. Ardalan for their contribution to the peer review of this work.’
Competing interests
All authors declare that the UK JDM Cohort and Biomarker Study (JDCBS) is currently funded by grants from the Great Ormond Street Hospital (GOSH) Children’s Charity, the National Institute for Health Research (NIHR) Biomedical Research Centre at GOSH, Cure JM, Myositis UK, Versus Arthritis and Remission Charity. L.R.W. declares that she is a consultant for Pfizer Inc (with consulting fees going wholly to the University College London). The other authors all declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
British Society for Rheumatology: https://rheumatology.org.uk
Childhood Arthritis and Rheumatology Research Alliance: https://carragroup.org
International Myositis Assessment & Clinical Studies Group: https://www.niehs.nih.gov/research/resources/imacs/
Juvenile dermatomyositis cohort biomarker study and repository: https://juveniledermatomyositis.org.uk/study-tools/
Paediatric Rheumatology International Trials Organisation: https://printo.it
The International Myositis Society: https://imyos.org
These authors contributed equally: Liza McCann and Lucy R. Wedderburn.
References
- 1.Lundberg IE, et al. European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol. 2017;69:2271–2282. doi: 10.1002/art.40320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel B, Khan N, Werth VP. Applicability of EULAR/ACR classification criteria for dermatomyositis to amyopathic disease. J. Am. Acad. Dermatol. 2018;79:77–83.e1. doi: 10.1016/j.jaad.2017.12.055. [DOI] [PubMed] [Google Scholar]
- 3.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N. Engl. J. Med. 1975;292:403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 4.Tansley SL, et al. Autoantibodies in juvenile-onset myositis: their diagnostic value and associated clinical phenotype in a large UK cohort. J. Autoimmun. 2017;84:55–64. doi: 10.1016/j.jaut.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rider LG, et al. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine. 2013;92:223–243. doi: 10.1097/MD.0b013e31829d08f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papadopoulou C, et al. The vasculopathy of juvenile dermatomyositis: endothelial injury, hypercoagulability, and increased arterial stiffness. Arthritis Rheumatol. 2021;73:1253–1266. doi: 10.1002/art.41639. [DOI] [PubMed] [Google Scholar]
- 7.Paik JJ, et al. Use of Janus kinase inhibitors in dermatomyositis: a systematic literature review. Clin. Exp. Rheumatol. 2023;41:348–358. doi: 10.55563/clinexprheumatol/hxin6o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabbagh S, et al. Treatment of anti-MDA5 autoantibody-positive juvenile dermatomyositis using tofacitinib. Brain. 2019;142:e59. doi: 10.1093/brain/awz293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aeschlimann FA, et al. A child with severe juvenile dermatomyositis treated with ruxolitinib. Brain. 2018;141:e80. doi: 10.1093/brain/awy255. [DOI] [PubMed] [Google Scholar]
- 10.Papadopoulou C, Hong Y, Omoyinmi E, Brogan PA, Eleftheriou D. Janus kinase 1/2 inhibition with baricitinib in the treatment of juvenile dermatomyositis. Brain. 2019;142:e8. doi: 10.1093/brain/awz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding Y, et al. Janus kinase inhibitor significantly improved rash and muscle strength in juvenile dermatomyositis. Ann. Rheum. Dis. 2021;80:543–545. doi: 10.1136/annrheumdis-2020-218582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tansley S, Wedderburn LR. Comparing and contrasting clinical and serological features of juvenile and adult-onset myositis: implications for pathogenesis and outcomes. Curr. Opin. Rheumatol. 2015;27:601–607. doi: 10.1097/BOR.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 13.Loarce-Martos J, et al. Clinical characteristics of juvenile idiopathic inflammatory myopathy and comparison with adult patients: analysis from a multicentric cohort in Spain. J. Clin. Rheumatol. 2022;28:e195–e202. doi: 10.1097/RHU.0000000000001696. [DOI] [PubMed] [Google Scholar]
- 14.Meyer A, et al. Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology. 2015;54:50–63. doi: 10.1093/rheumatology/keu289. [DOI] [PubMed] [Google Scholar]
- 15.Martin N, et al. A national registry for juvenile dermatomyositis and other paediatric idiopathic inflammatory myopathies: 10 years’ experience; the Juvenile Dermatomyositis National (UK and Ireland) Cohort biomarker study and repository for idiopathic inflammatory myopathies. Rheumatology. 2011;50:137–145. doi: 10.1093/rheumatology/keq261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravelli A, et al. Long-term outcome and prognostic factors of juvenile dermatomyositis: a multinational, multicenter study of 490 patients. Arthritis Care Res. 2010;62:63–72. doi: 10.1002/acr.20015. [DOI] [PubMed] [Google Scholar]
- 17.Huber AM, et al. Medium- and long-term functional outcomes in a multicenter cohort of children with juvenile dermatomyositis. Arthritis Rheum. 2000;43:541–549. doi: 10.1002/1529-0131(200003)43:3<541::AID-ANR9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 18.Okong’o LO, Esser M, Wilmshurst J, Scott C. Characteristics and outcome of children with juvenile dermatomyositis in Cape Town: a cross-sectional study. Pediatr. Rheumatol. Online J. 2016;14:60. doi: 10.1186/s12969-016-0118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanner H, Gran JT, Sjaastad I, Flato B. Cumulative organ damage and prognostic factors in juvenile dermatomyositis: a cross-sectional study median 16.8 years after symptom onset. Rheumatology. 2009;48:1541–1547. doi: 10.1093/rheumatology/kep302. [DOI] [PubMed] [Google Scholar]
- 20.Sharma A, Gupta A, Rawat A, Suri D, Singh S. Long-term outcome in children with juvenile dermatomyositis: a single-center study from North India. Int. J. Rheum. Dis. 2020;23:392–396. doi: 10.1111/1756-185X.13759. [DOI] [PubMed] [Google Scholar]
- 21.Huber AM, et al. Early illness features associated with mortality in the juvenile idiopathic inflammatory myopathies. Arthritis Care Res. 2014;66:732–740. doi: 10.1002/acr.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsaltskan V, et al. Long-term outcomes in juvenile myositis patients. Semin. Arthritis Rheum. 2020;50:149–155. doi: 10.1016/j.semarthrit.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rider LG, et al. Damage extent and predictors in adult and juvenile dermatomyositis and polymyositis as determined with the myositis damage index. Arthritis Rheum. 2009;60:3425–3435. doi: 10.1002/art.24904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathiesen P, et al. Long-term outcome in patients with juvenile dermatomyositis: a cross-sectional follow-up study. Scand. J. Rheumatol. 2012;41:50–58. doi: 10.3109/03009742.2011.608376. [DOI] [PubMed] [Google Scholar]
- 25.Apaz MT, et al. Health-related quality of life of patients with juvenile dermatomyositis: results from the Pediatric Rheumatology International Trials Organisation multinational quality of life cohort study. Arthritis Rheum. 2009;61:509–517. doi: 10.1002/art.24343. [DOI] [PubMed] [Google Scholar]
- 26.Nordal E, et al. Growth and puberty in juvenile dermatomyositis: a longitudinal cohort study. Arthritis Care Res. 2020;72:265–273. doi: 10.1002/acr.24065. [DOI] [PubMed] [Google Scholar]
- 27.Boros C, et al. Juvenile dermatomyositis: what comes next? Long-term outcomes in childhood myositis from a patient perspective. Pediatr. Rheumatol. Online J. 2022;20:102. doi: 10.1186/s12969-022-00754-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverberg JI, Kwa L, Kwa MC, Laumann AE, Ardalan K. Cardiovascular and cerebrovascular comorbidities of juvenile dermatomyositis in US children: an analysis of the national inpatient sample. Rheumatology. 2018;57:694–702. doi: 10.1093/rheumatology/kex465. [DOI] [PubMed] [Google Scholar]
- 29.Coyle K, et al. Metabolic abnormalities and cardiovascular risk factors in children with myositis. J. Pediatr. 2009;155:882–887. doi: 10.1016/j.jpeds.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz T, et al. In active juvenile dermatomyositis, elevated eotaxin and MCP-1 and cholesterol levels in the upper normal range are associated with cardiac dysfunction. Rheumatology. 2014;53:2214–2222. doi: 10.1093/rheumatology/keu256. [DOI] [PubMed] [Google Scholar]
- 31.Witczak BN, et al. Associations between cardiac and pulmonary involvement in patients with juvenile dermatomyositis — a cross-sectional study. Rheumatol. Int. 2022;42:1213–1220. doi: 10.1007/s00296-021-05071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah M, et al. The clinical phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine. 2013;92:25–41. doi: 10.1097/MD.0b013e31827f264d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walling HW, Gerami P, Sontheimer RD. Juvenile-onset clinically amyopathic dermatomyositis: an overview of recent progress in diagnosis and management. Paediatr. Drugs. 2010;12:23–34. doi: 10.2165/10899380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Mamyrova G, et al. Features distinguishing clinically amyopathic juvenile dermatomyositis from juvenile dermatomyositis. Rheumatology. 2018;57:1956–1963. doi: 10.1093/rheumatology/key190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCann LJ, Li CK, Varsani H, Wedderburn LR, Pilkington CA. Failure to over express MHC-CLASS-1 on muscle biopsy in a case of amyopathic juvenile dermatomyositis. Clin. Exp. Rheumatol. 2007;25:96–98. [PubMed] [Google Scholar]
- 36.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971–982. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 37.Kishi T, et al. Association of anti-3-Hydroxy-3-Methylglutaryl-Coenzyme A reductase autoantibodies with DRB1*07:01 and severe myositis in juvenile myositis patients. Arthritis Care Res. 2017;69:1088–1094. doi: 10.1002/acr.23113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Tansley SL. Juvenile dermatomyositis — clinical phenotypes. Curr. Rheumatol. Rep. 2019;21:74. doi: 10.1007/s11926-019-0871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orandi AB, et al. Assessment, classification and treatment of calcinosis as a complication of juvenile dermatomyositis: a survey of pediatric rheumatologists by the childhood arthritis and rheumatology research alliance (CARRA) Pediatr. Rheumatol. Online J. 2017;15:71. doi: 10.1186/s12969-017-0199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rider LG, Nistala K. The juvenile idiopathic inflammatory myopathies: pathogenesis, clinical and autoantibody phenotypes, and outcomes. J. Intern. Med. 2016;280:24–38. doi: 10.1111/joim.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwiatkowska D, Reich A. The significance of autoantibodies in juvenile dermatomyositis. Biomed. Res. Int. 2021;2021:5513544. doi: 10.1155/2021/5513544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Best M, et al. Use of anti-transcriptional intermediary factor-1 γ autoantibody in identifying adult dermatomyositis patients with cancer: a systematic review and meta-analysis. Acta Derm. Venereol. 2019;99:256–262. doi: 10.2340/00015555-3091. [DOI] [PubMed] [Google Scholar]
- 43.Oldroyd AGS, et al. A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology. 2021;60:2615–2628. doi: 10.1093/rheumatology/keab166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gunawardena H, et al. Autoantibodies to a 140-kd protein in juvenile dermatomyositis are associated with calcinosis. Arthritis Rheum. 2009;60:1807–1814. doi: 10.1002/art.24547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espada G, Maldonado Cocco JA, Fertig N, Oddis CV. Clinical and serologic characterization of an Argentine pediatric myositis cohort: identification of a novel autoantibody (anti-MJ) to a 142-kDa protein. J. Rheumatol. 2009;36:2547–2551. doi: 10.3899/jrheum.090461. [DOI] [PubMed] [Google Scholar]
- 46.Tansley SL, et al. Calcinosis in juvenile dermatomyositis is influenced by both anti-NXP2 autoantibody status and age at disease onset. Rheumatology. 2014;53:2204–2208. doi: 10.1093/rheumatology/keu259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogers A, Chung L, Li S, Casciola-Rosen L, Fiorentino DF. Cutaneous and systemic findings associated with nuclear matrix protein 2 antibodies in adult dermatomyositis patients. Arthritis Care Res. 2017;69:1909–1914. doi: 10.1002/acr.23210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deakin CT, et al. Muscle biopsy findings in combination with myositis-specific autoantibodies aid prediction of outcomes in juvenile dermatomyositis. Arthritis Rheumatol. 2016;68:2806–2816. doi: 10.1002/art.39753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamasaki Y, et al. Clinical impact of myositis-specific autoantibodies on long-term prognosis of juvenile idiopathic inflammatory myopathies: multicentre study. Rheumatology. 2021;60:4821–4831. doi: 10.1093/rheumatology/keab108. [DOI] [PubMed] [Google Scholar]
- 50.Mamyrova G, et al. Anti-MDA5 autoantibodies associated with juvenile dermatomyositis constitute a distinct phenotype in North America. Rheumatology. 2021;60:1839–49. doi: 10.1093/rheumatology/keaa429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobayashi N, et al. Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology. 2015;54:784–791. doi: 10.1093/rheumatology/keu385. [DOI] [PubMed] [Google Scholar]
- 52.Tansley SL, et al. Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res. Ther. 2014;16:R138. doi: 10.1186/ar4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J. Am. Acad. Dermatol. 2011;65:25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melki I, et al. Anti-MDA5 juvenile idiopathic inflammatory myopathy: a specific subgroup defined by differentially enhanced interferon-α signalling. Rheumatology. 2020;59:1927–1937. doi: 10.1093/rheumatology/kez525. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi I, et al. Anti-melanoma differentiation-associated gene 5 antibody is a diagnostic and predictive marker for interstitial lung diseases associated with juvenile dermatomyositis. J. Pediatr. 2011;158:675–677. doi: 10.1016/j.jpeds.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 56.Ueki M, et al. Myositis-specific autoantibodies in Japanese patients with juvenile idiopathic inflammatory myopathies. Mod. Rheumatol. 2019;29:351–356. doi: 10.1080/14397595.2018.1452353. [DOI] [PubMed] [Google Scholar]
- 57.Nombel A, Fabien N, Coutant F. Dermatomyositis with anti-MDA5 antibodies: bioclinical features, pathogenesis and emerging therapies. Front. Immunol. 2021;12:773352. doi: 10.3389/fimmu.2021.773352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yasin SA, et al. Histological heterogeneity in a large clinical cohort of juvenile idiopathic inflammatory myopathy: analysis by myositis autoantibody and pathological features. Neuropathol. Appl. Neurobiol. 2019;45:495–512. doi: 10.1111/nan.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caproni M, et al. Amyopathic dermatomyositis: a review by the Italian Group of Immunodermatology. Arch. Dermatol. 2002;138:23–27. doi: 10.1001/archderm.138.1.23. [DOI] [PubMed] [Google Scholar]
- 60.Betteridge ZE, et al. Clinical and human leucocyte antigen class II haplotype associations of autoantibodies to small ubiquitin-like modifier enzyme, a dermatomyositis-specific autoantigen target, in UK Caucasian adult-onset myositis. Ann. Rheum. Dis. 2009;68:1621–1625. doi: 10.1136/ard.2008.097162. [DOI] [PubMed] [Google Scholar]
- 61.Kishi T, et al. Anti-SAE autoantibody-positive Japanese patient with juvenile dermatomyositis complicated with interstitial lung disease — a case report. Pediatr. Rheumatol. Online J. 2021;19:34. doi: 10.1186/s12969-021-00532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abe S, et al. Clinically amyopathic dermatomyositis associated with anti-nuclear matrix protein 2 antibody. Rheumatol. Adv. Pract. 2021;5:rkab104. doi: 10.1093/rap/rkab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Damoiseaux J, Mammen AL, Piette Y, Benveniste O, Allenbach Y. 256th ENMC international workshop: myositis specific and associated autoantibodies (MSA-ab): Amsterdam, The Netherlands, 8–10 October 2021. Neuromuscul. Disord. 2022;32:594–608. doi: 10.1016/j.nmd.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 64.Lundberg IE, et al. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Prim. 2021;7:86. doi: 10.1038/s41572-021-00321-x. [DOI] [PubMed] [Google Scholar]
- 65.Martins P, et al. Clinical characterisation of a multicentre nationwide cohort of patients with antisynthetase syndrome. ARP Rheumatol. 2022;1:190–196. [PubMed] [Google Scholar]
- 66.Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-mediated necrotizing myopathy. Curr. Rheumatol. Rep. 2018;20:21. doi: 10.1007/s11926-018-0732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Binns EL, et al. Effective induction therapy for anti-SRP associated myositis in childhood: a small case series and review of the literature. Pediatr. Rheumatol. Online J. 2017;15:77. doi: 10.1186/s12969-017-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allenbach Y, Benveniste O. Peculiar clinicopathological features of immune-mediated necrotizing myopathies. Curr. Opin. Rheumatol. 2018;30:655–663. doi: 10.1097/BOR.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 69.Tansley SL, et al. Anti-HMGCR autoantibodies in Juvenile idiopathic inflammatory myopathies identify a rare but clinically important subset of patients. J. Rheumatol. 2017;44:488–492. doi: 10.3899/jrheum.160871. [DOI] [PubMed] [Google Scholar]
- 70.Mamyrova G, et al. Clinical and laboratory features distinguishing juvenile polymyositis and muscular dystrophy. Arthritis Care Res. 2013;65:1969–1975. doi: 10.1002/acr.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wedderburn LR, et al. HLA class II haplotype and autoantibody associations in children with juvenile dermatomyositis and juvenile dermatomyositis-scleroderma overlap. Rheumatology. 2007;46:1786–1791. doi: 10.1093/rheumatology/kem265. [DOI] [PubMed] [Google Scholar]
- 72.Khaosut P, Pilkington C, Wedderburn LR, Compeyrot-Lacassagne S. An international survey of developing classification criteria for juvenile dermatomyositis-scleroderma overlap. Rheumatology. 2019;58:2062–2064. doi: 10.1093/rheumatology/kez226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bitencourt N, Solow EB, Wright T, Bermas BL. Inflammatory myositis in systemic lupus erythematosus. Lupus. 2020;29:776–781. doi: 10.1177/0961203320918021. [DOI] [PubMed] [Google Scholar]
- 74.Sabbagh S, et al. Anti-Ro52 autoantibodies are associated with interstitial lung disease and more severe disease in patients with juvenile myositis. Ann. Rheum. Dis. 2019;78:988–995. doi: 10.1136/annrheumdis-2018-215004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Orandi AB, Eutsler E, Ferguson C, White AJ, Kitcharoensakkul M. Sarcoidosis presenting as granulomatous myositis in a 16-year-old adolescent. Pediatr. Rheumatol. Online J. 2016;14:59. doi: 10.1186/s12969-016-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang Y, et al. Muscle involvement in Polyarteritis nodosa: report of eight cases with characteristic contrast enhancement pattern on MRI. AJR Am. J. Roentgenol. 2016;206:378–384. doi: 10.2214/AJR.15.14774. [DOI] [PubMed] [Google Scholar]
- 77.Rama M, et al. A decision tree for the genetic diagnosis of deficiency of adenosine deaminase 2 (DADA2): a French reference centres experience. Eur. J. Hum. Genet. 2018;26:960–971. doi: 10.1038/s41431-018-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eleftheriou D, Brogan PA. Genetic interferonopathies: an overview. Best. Pract. Res. Clin. Rheumatol. 2017;31:441–459. doi: 10.1016/j.berh.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 79.Kim H, et al. Expression of interferon-regulated genes in juvenile dermatomyositis versus Mendelian autoinflammatory interferonopathies. Arthritis Res. Ther. 2020;22:69. doi: 10.1186/s13075-020-02160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujikawa K, Migita K, Tsukada T, Kawakami A, Eguchi K. Protracted febrile myalgia syndrome in a Japanese patient with fasciitis detected on MRI. Intern. Med. 2014;53:2817–2819. doi: 10.2169/internalmedicine.53.2871. [DOI] [PubMed] [Google Scholar]
- 81.Wedderburn LR, Rider LG. Juvenile dermatomyositis: new developments in pathogenesis, assessment and treatment. Best. Pract. Res. Clin. Rheumatol. 2009;23:665–678. doi: 10.1016/j.berh.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mamyrova G, Rider LG, Haagenson L, Wong S, Brown KE. Parvovirus B19 and onset of juvenile dermatomyositis. J. Am. Med. Assoc. 2005;294:2170–2171. doi: 10.1001/jama.294.17.2170. [DOI] [PubMed] [Google Scholar]
- 83.Pachman LM, et al. Lack of detection of enteroviral RNA or bacterial DNA in magnetic resonance imaging-directed muscle biopsies from twenty children with active untreated juvenile dermatomyositis. Arthritis Rheum. 1995;38:1513–1518. doi: 10.1002/art.1780381019. [DOI] [PubMed] [Google Scholar]
- 84.Pachman LM, et al. History of infection before the onset of juvenile dermatomyositis: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Research Registry. Arthritis Rheum. 2005;53:166–172. doi: 10.1002/art.21068. [DOI] [PubMed] [Google Scholar]
- 85.Pachman LM, Nolan BE, DeRanieri D, Khojah AM. Juvenile dermatomyositis: new clues to diagnosis and therapy. Curr. Treatm Opt. Rheumatol. 2021;7:39–62. doi: 10.1007/s40674-020-00168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okada Y, et al. Anti-NXP2 antibody-positive dermatomyositis developed after COVID-19 manifesting as type I interferonopathy. Rheumatology. 2022;61:e90–e92. doi: 10.1093/rheumatology/keab872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Islam B, Ahmed M, Islam Z, Begum SM. Severe acute myopathy following SARS-CoV-2 infection: a case report and review of recent literature. Skelet. Muscle. 2021;11:10. doi: 10.1186/s13395-021-00266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Okada S, et al. Global surface ultraviolet radiation intensity may modulate the clinical and immunologic expression of autoimmune muscle disease. Arthritis Rheum. 2003;48:2285–2293. doi: 10.1002/art.11090. [DOI] [PubMed] [Google Scholar]
- 89.Shah M, et al. Brief report: ultraviolet radiation exposure is associated with clinical and autoantibody phenotypes in juvenile myositis. Arthritis Rheum. 2013;65:1934–1941. doi: 10.1002/art.37985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neely J, Long CS, Sturrock H, Kim S, Childhood A. Rheumatology Research Alliance Registry I. Association of short-term ultraviolet radiation exposure and disease severity in juvenile dermatomyositis: results from the childhood arthritis and rheumatology research alliance legacy registry. Arthritis Care Res. 2019;71:1600–1605. doi: 10.1002/acr.23840. [DOI] [PubMed] [Google Scholar]
- 91.Orione MA, et al. Risk factors for juvenile dermatomyositis: exposure to tobacco and air pollutants during pregnancy. Arthritis Care Res. 2014;66:1571–1575. doi: 10.1002/acr.22358. [DOI] [PubMed] [Google Scholar]
- 92.Scalabrini JC, et al. Environmental factors associated with juvenile idiopathic inflammatory myopathy clinical and serologic phenotypes. Pediatr. Rheumatol. Online J. 2022;20:28. doi: 10.1186/s12969-022-00684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller FW, et al. Genome-wide association study identifies HLA 8.1 ancestral haplotype alleles as major genetic risk factors for myositis phenotypes. Genes Immun. 2015;16:470–480. doi: 10.1038/gene.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rothwell S, et al. Dense genotyping of immune-related loci in idiopathic inflammatory myopathies confirms HLA alleles as the strongest genetic risk factor and suggests different genetic background for major clinical subgroups. Ann. Rheum. Dis. 2016;75:1558–1566. doi: 10.1136/annrheumdis-2015-208119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deakin CT, et al. Association with HLA-DRβ1 position 37 distinguishes juvenile dermatomyositis from adult-onset myositis. Hum. Mol. Genet. 2022;31:2471–2481. doi: 10.1093/hmg/ddac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rothwell S, et al. Focused HLA analysis in Caucasians with myositis identifies significant associations with autoantibody subgroups. Ann. Rheum. Dis. 2019;78:996–1002. doi: 10.1136/annrheumdis-2019-215046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chinoy H, et al. The protein tyrosine phosphatase N22 gene is associated with juvenile and adult idiopathic inflammatory myopathy independent of the HLA 8.1 haplotype in British Caucasian patients. Arthritis Rheum. 2008;58:3247–3254. doi: 10.1002/art.23900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jani M, et al. Genotyping of immune-related genetic variants identifies TYK2 as a novel associated locus for idiopathic inflammatory myopathies. Ann. Rheum. Dis. 2014;73:1750–1752. doi: 10.1136/annrheumdis-2014-205440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McLellan K, Papadopoulou C. Update on biomarkers of vasculopathy in juvenile and adult myositis. Curr. Rheumatol. Rep. 2022;24:227–237. doi: 10.1007/s11926-022-01076-4. [DOI] [PubMed] [Google Scholar]
- 100.Kishi T, et al. Endothelial activation markers as disease activity and damage measures in juvenile dermatomyositis. J. Rheumatol. 2020;47:1011–1018. doi: 10.3899/jrheum.181275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khojah A, et al. Association of p155/140 autoantibody with loss of nailfold capillaries but not generalized lipodystrophy: a study of ninety-six children with juvenile dermatomyositis. Arthritis Care Res. 2022;74:1065–1069. doi: 10.1002/acr.24535. [DOI] [PubMed] [Google Scholar]
- 102.Bloom BJ, Miller LC, Blier PR. Soluble adhesion molecules in pediatric rheumatic diseases. J. Rheumatol. 2002;29:832–836. [PubMed] [Google Scholar]
- 103.Soponkanaporn S, et al. Expression of myxovirus-resistance protein A: a possible marker of muscle disease activity and autoantibody specificities in juvenile dermatomyositis. Neuropathol. Appl. Neurobiol. 2019;45:410–420. doi: 10.1111/nan.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodero MP, et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J. Exp. Med. 2017;214:1547–1555. doi: 10.1084/jem.20161451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roberson EDO, et al. Transcriptomes of peripheral blood mononuclear cells from juvenile dermatomyositis patients show elevated inflammation even when clinically inactive. Sci. Rep. 2022;12:275. doi: 10.1038/s41598-021-04302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moneta GM, et al. Muscle expression of type I and type II interferons is increased in juvenile dermatomyositis and related to clinical and histologic features. Arthritis Rheumatol. 2019;71:1011–1021. doi: 10.1002/art.40800. [DOI] [PubMed] [Google Scholar]
- 107.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 108.Oddis CV, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314–324. doi: 10.1002/art.37754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morita R, et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilkinson MGL, et al. Using peripheral blood immune signatures to stratify patients with adult and juvenile inflammatory myopathies. Rheumatology. 2020;59:194–204. doi: 10.1093/rheumatology/kez252. [DOI] [PubMed] [Google Scholar]
- 111.Varsani H, et al. Validation of a score tool for measurement of histological severity in juvenile dermatomyositis and association with clinical severity of disease. Ann. Rheum. Dis. 2015;74:204–210. doi: 10.1136/annrheumdis-2013-203396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nistala K, et al. Myeloid related protein induces muscle derived inflammatory mediators in juvenile dermatomyositis. Arthritis Res. Ther. 2013;15:R131. doi: 10.1186/ar4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sag E, et al. Inflammatory milieu of muscle biopsies in juvenile dermatomyositis. Rheumatol. Int. 2021;41:77–85. doi: 10.1007/s00296-020-04735-w. [DOI] [PubMed] [Google Scholar]
- 114.Piper CJM, et al. CD19+CD24hiCD38hi B cells are expanded in juvenile dermatomyositis and exhibit a pro-inflammatory phenotype after activation through toll-like receptor 7 and interferon-α. Front. Immunol. 2018;9:1372. doi: 10.3389/fimmu.2018.01372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Turnier JL, et al. Comparison of lesional juvenile myositis and lupus skin reveals overlapping yet unique disease pathophysiology. Arthritis Rheumatol. 2021;73:1062–1072. doi: 10.1002/art.41615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 117.Duvvuri B, et al. Neutrophil extracellular traps in tissue and periphery in juvenile dermatomyositis. Arthritis Rheumatol. 2020;72:348–358. doi: 10.1002/art.41078. [DOI] [PubMed] [Google Scholar]
- 118.Seto N, et al. Neutrophil dysregulation is pathogenic in idiopathic inflammatory myopathies. JCI Insight. 2020;5:e134189. doi: 10.1172/jci.insight.134189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Caielli S, et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 2016;213:697–713. doi: 10.1084/jem.20151876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhong D, et al. Co-expression network analysis reveals the pivotal role of mitochondrial dysfunction and interferon signature in juvenile dermatomyositis. PeerJ. 2020;8:e8611. doi: 10.7717/peerj.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilkinson MGL, et al. Role of CD14+ monocyte-derived oxidised mitochondrial DNA in the inflammatory interferon type 1 signature in juvenile dermatomyositis. Ann. Rheum. Dis. 2022 doi: 10.1136/ard-2022-223469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sabbagh SE, et al. Anti-mitochondrial autoantibodies are associated with cardiomyopathy, dysphagia, and features of more severe disease in adult-onset myositis. Clin. Rheumatol. 2021;40:4095–4100. doi: 10.1007/s10067-021-05730-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bhattarai S, et al. The immunoproteasomes are key to regulate myokines and MHC class I expression in idiopathic inflammatory myopathies. J. Autoimmun. 2016;75:118–129. doi: 10.1016/j.jaut.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 124.Nagaraju K, et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 2005;52:1824–1835. doi: 10.1002/art.21103. [DOI] [PubMed] [Google Scholar]
- 125.Ma X, et al. Endoplasmic reticulum stress is involved in muscular pathogenesis in idiopathic inflammatory myopathies. Front. Cell Dev. Biol. 2022;10:791986. doi: 10.3389/fcell.2022.791986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bellutti Enders F, et al. Consensus-based recommendations for the management of juvenile dermatomyositis. Ann. Rheum. Dis. 2017;76:329–340. doi: 10.1136/annrheumdis-2016-209247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kobayashi I, et al. Clinical practice guidance for juvenile dermatomyositis (JDM) 2018-update. Mod. Rheumatol. 2020;30:411–423. doi: 10.1080/14397595.2020.1718866. [DOI] [PubMed] [Google Scholar]
- 128.Tansley SL, et al. The promise, perceptions, and pitfalls of immunoassays for autoantibody testing in myositis. Arthritis Res. Ther. 2020;22:117. doi: 10.1186/s13075-020-02210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim H, Huber AM, Kim S. Updates on juvenile dermatomyositis from the last decade: classification to outcomes. Rheum. Dis. Clin. North Am. 2021;47:669–690. doi: 10.1016/j.rdc.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tansley SL, Li D, Betteridge ZE, McHugh NJ. The reliability of immunoassays to detect autoantibodies in patients with myositis is dependent on autoantibody specificity. Rheumatology. 2020;59:2109–2114. doi: 10.1093/rheumatology/keaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin. Rev. Allergy Immunol. 2017;52:1–19. doi: 10.1007/s12016-015-8510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Espinosa-Ortega F, et al. Comparison of autoantibody specificities tested by a line blot assay and immunoprecipitation-based algorithm in patients with idiopathic inflammatory myopathies. Ann. Rheum. Dis. 2019;78:858–860. doi: 10.1136/annrheumdis-2018-214690. [DOI] [PubMed] [Google Scholar]
- 133.Oldroyd AGS, et al. British Society for Rheumatology guideline on management of paediatric, adolescent and adult patients with idiopathic inflammatory myopathy. Rheumatology. 2022;61:1760–1768. doi: 10.1093/rheumatology/keac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huber AM, et al. Protocols for the initial treatment of moderately severe juvenile dermatomyositis: results of a Children’s Arthritis and Rheumatology Research Alliance Consensus Conference. Arthritis Care Res. 2010;62:219–225. doi: 10.1002/acr.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huber AM, et al. Consensus treatments for moderate juvenile dermatomyositis: beyond the first two months. Results of the second Childhood Arthritis and Rheumatology Research Alliance consensus conference. Arthritis Care Res. 2012;64:546–553. doi: 10.1002/acr.20695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huber AM, et al. Childhood Arthritis and Rheumatology Research Alliance Consensus Clinical Treatment Plans for juvenile dermatomyositis with persistent skin rash. J. Rheumatol. 2017;44:110–116. doi: 10.3899/jrheum.160688. [DOI] [PubMed] [Google Scholar]
- 137.McCann LJ, Livermore P, Wilkinson MGL, Wedderburn LR. Juvenile dermatomyositis. Where are we now? Clin. Exp. Rheumatol. 2022;40:394–403. doi: 10.55563/clinexprheumatol/56ilob. [DOI] [PubMed] [Google Scholar]
- 138.Wu Q, Wedderburn LR, McCann LJ. Juvenile dermatomyositis: latest advances. Best. Pract. Res. Clin. Rheumatol. 2017;31:535–557. doi: 10.1016/j.berh.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 139.Orandi AB, et al. Favorable outcomes with reduced steroid use in juvenile dermatomyositis. Pediatr. Rheumatol. Online J. 2021;19:127. doi: 10.1186/s12969-021-00615-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ruperto N, et al. Prednisone versus prednisone plus ciclosporin versus prednisone plus methotrexate in new-onset juvenile dermatomyositis: a randomised trial. Lancet. 2016;387:671–678. doi: 10.1016/S0140-6736(15)01021-1. [DOI] [PubMed] [Google Scholar]
- 141.Wang A, Morgan GA, Paller AS, Pachman LM. Skin disease is more recalcitrant than muscle disease: a long-term prospective study of 184 children with juvenile dermatomyositis. J. Am. Acad. Dermatol. 2021;84:1610–1618. doi: 10.1016/j.jaad.2020.12.032. [DOI] [PubMed] [Google Scholar]
- 142.Kim S, et al. Childhood Arthritis and Rheumatology Research Alliance consensus clinical treatment plans for juvenile dermatomyositis with skin predominant disease. Pediatr. Rheumatol. Online J. 2017;15:1. doi: 10.1186/s12969-016-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rouster-Stevens KA, Morgan GA, Wang D, Pachman LM. Mycophenolate mofetil: a possible therapeutic agent for children with juvenile dermatomyositis. Arthritis Care Res. 2010;62:1446–1451. doi: 10.1002/acr.20269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dagher R, et al. Mycophenolate mofetil in juvenile dermatomyositis: a case series. Rheumatol. Int. 2012;32:711–716. doi: 10.1007/s00296-010-1653-5. [DOI] [PubMed] [Google Scholar]
- 145.Cakan M, Karadag SG, Ayaz NA. Complete and sustained resolution of calcinosis universalis in a juvenile dermatomyositis case with mycophenolate mofetil. Turk. J. Pediatr. 2019;61:771–775. doi: 10.24953/turkjped.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 146.Varnier GC, et al. Experience with the use of mycophenolate mofetil in juvenile idiopathic inflammatory myopathies. Rheumatology. 2022;62(SI2):SI163–SI169. doi: 10.1093/rheumatology/keac404. [DOI] [PubMed] [Google Scholar]
- 147.Jacobs JC. Methotrexate and azathioprine treatment of childhood dermatomyositis. Pediatrics. 1977;59:212–218. doi: 10.1542/peds.59.2.212. [DOI] [PubMed] [Google Scholar]
- 148.Ng YT, Ouvrier RA, Wu T. Drug therapy in juvenile dermatomyositis: follow-up study. J. Child. Neurol. 1998;13:109–112. doi: 10.1177/088307389801300303. [DOI] [PubMed] [Google Scholar]
- 149.Hassan J, van der Net JJ, van Royen-Kerkhof A. Treatment of refractory juvenile dermatomyositis with tacrolimus. Clin. Rheumatol. 2008;27:1469–1471. doi: 10.1007/s10067-008-0973-2. [DOI] [PubMed] [Google Scholar]
- 150.Yamada A, Ohshima Y, Omata N, Yasutomi M, Mayumi M. Steroid-sparing effect of tacrolimus in a patient with juvenile dermatomyositis presenting poor bioavailability of cyclosporine A. Eur. J. Pediatr. 2004;163:561–562. doi: 10.1007/s00431-004-1497-7. [DOI] [PubMed] [Google Scholar]
- 151.Rao AP, Kolli V, Rajarathinam I, Raghuram J. Tacrolimus in refractory juvenile dermatomyositis: case report and review of literature. Indian J. Rheumatol. 2020;16:205–208. doi: 10.4103/injr.injr_128_20. [DOI] [Google Scholar]
- 152.Hallowell RW, Paik JJ. Myositis-associated interstitial lung disease: a comprehensive approach to diagnosis and management. Clin. Exp. Rheumatol. 2022;40:373–383. doi: 10.55563/clinexprheumatol/brvl1v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aggarwal R, et al. Trial of intravenous immune globulin in dermatomyositis. N. Engl. J. Med. 2022;387:1264–1278. doi: 10.1056/NEJMoa2117912. [DOI] [PubMed] [Google Scholar]
- 154.Dalakas MC, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N. Engl. J. Med. 1993;329:1993–2000. doi: 10.1056/NEJM199312303292704. [DOI] [PubMed] [Google Scholar]
- 155.Miyasaka N, et al. Effects of intravenous immunoglobulin therapy in Japanese patients with polymyositis and dermatomyositis resistant to corticosteroids: a randomized double-blind placebo-controlled trial. Mod. Rheumatol. 2012;22:382–393. doi: 10.3109/s10165-011-0534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aggarwal R, et al. Prospective, double-blind, randomized, placebo-controlled phase III study evaluating efficacy and safety of octagam 10% in patients with dermatomyositis (“ProDERM Study”) Medicine. 2021;100:e23677. doi: 10.1097/MD.0000000000023677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Doudouliaki T, Papadopoulou C, Deakin CT. Use of rescue therapy with IVIG or cyclophosphamide in juvenile myositis. Curr. Rheumatol. Rep. 2021;23:24. doi: 10.1007/s11926-021-00990-3. [DOI] [PubMed] [Google Scholar]
- 158.Lam CG, Manlhiot C, Pullenayegum EM, Feldman BM. Efficacy of intravenous Ig therapy in juvenile dermatomyositis. Ann. Rheum. Dis. 2011;70:2089–2094. doi: 10.1136/ard.2011.153718. [DOI] [PubMed] [Google Scholar]
- 159.Al-Mayouf SM, Laxer RM, Schneider R, Silverman ED, Feldman BM. Intravenous immunoglobulin therapy for juvenile dermatomyositis: efficacy and safety. J. Rheumatol. 2000;27:2498–2503. [PubMed] [Google Scholar]
- 160.Deakin CT, et al. Efficacy and safety of cyclophosphamide treatment in severe juvenile dermatomyositis shown by marginal structural modeling. Arthritis Rheumatol. 2018;70:785–793. doi: 10.1002/art.40418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Riley P, et al. Intravenous cyclophosphamide pulse therapy in juvenile dermatomyositis. A review of efficacy and safety. Rheumatology. 2004;43:491–496. doi: 10.1093/rheumatology/keh082. [DOI] [PubMed] [Google Scholar]
- 162.Kishi T, et al. Corticosteroid discontinuation, complete clinical response and remission in juvenile dermatomyositis. Rheumatology. 2021;60:2134–2145. doi: 10.1093/rheumatology/keaa371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ang GC, Werth VP. Combination antimalarials in the treatment of cutaneous dermatomyositis: a retrospective study. Arch. Dermatol. 2005;141:855–859. doi: 10.1001/archderm.141.7.855. [DOI] [PubMed] [Google Scholar]
- 164.Olson NY, Lindsley CB. Adjunctive use of hydroxychloroquine in childhood dermatomyositis. J. Rheumatol. 1989;16:1545–1547. [PubMed] [Google Scholar]
- 165.Marrani E, et al. A systematic review on biological therapies in juvenile idiopathic inflammatory myopathies: an evidence gap in precision medicine. Clin. Exp. Rheumatol. 2022;40:457–470. doi: 10.55563/clinexprheumatol/ltrj4l. [DOI] [PubMed] [Google Scholar]
- 166.Aggarwal R, et al. Predictors of clinical improvement in rituximab-treated refractory adult and juvenile dermatomyositis and adult polymyositis. Arthritis Rheumatol. 2014;66:740–749. doi: 10.1002/art.38270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vargas Lebron C, Ruiz Montesino MD, Moreira Navarrete V, Toyos Sainz de Miera FJ. Treatment with rituximab in juvenile dermatomyositis: effect on calcinosis. Reumatol. Clin. 2020;16:368–370. doi: 10.1016/j.reuma.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 168.Aggarwal R, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2017;56:247–254. doi: 10.1093/rheumatology/kew396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bader-Meunier B, et al. Safety and efficacy of rituximab in severe juvenile dermatomyositis: results from 9 patients from the French Autoimmunity and Rituximab registry. J. Rheumatol. 2011;38:1436–1440. doi: 10.3899/jrheum.101321. [DOI] [PubMed] [Google Scholar]
- 170.Cooper MA, et al. Rituximab for the treatment of juvenile dermatomyositis: a report of four pediatric patients. Arthritis Rheum. 2007;56:3107–3111. doi: 10.1002/art.22856. [DOI] [PubMed] [Google Scholar]
- 171.Campanilho-Marques R, et al. Retrospective analysis of infliximab and adalimumab treatment in a large cohort of juvenile dermatomyositis patients. Arthritis Res. Ther. 2020;22:79. doi: 10.1186/s13075-020-02164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Riley P, et al. Effectiveness of infliximab in the treatment of refractory juvenile dermatomyositis with calcinosis. Rheumatology. 2008;47:877–880. doi: 10.1093/rheumatology/ken074. [DOI] [PubMed] [Google Scholar]
- 173.Spencer CH, et al. Biologic therapies for refractory juvenile dermatomyositis: five years of experience of the Childhood Arthritis and Rheumatology Research Alliance in North America. Pediatr. Rheumatol. Online J. 2017;15:50. doi: 10.1186/s12969-017-0174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rouster-Stevens KA, Ferguson L, Morgan G, Huang CC, Pachman LM. Pilot study of etanercept in patients with refractory juvenile dermatomyositis. Arthritis Care Res. 2014;66:783–787. doi: 10.1002/acr.22198. [DOI] [PubMed] [Google Scholar]
- 175.Green KL, Twilt M, Southwood T. The role of etanercept in juvenile dermatomyositis (JDMS) in children. Pediatr. Rheumatol. Online J. 2014;12:P279. doi: 10.1186/1546-0096-12-S1-P279. [DOI] [Google Scholar]
- 176.Muscle Study Group. A randomized, pilot trial of etanercept in dermatomyositis. Ann. Neurol. 2011;70:427–436. doi: 10.1002/ana.22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zengin O, et al. Three cases of anti-TNF induced myositis and literature review. Rev. Bras. Reumatol. Engl. Ed. 2017;57:590–595. doi: 10.1016/j.rbr.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 178.Dastmalchi M, et al. A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Ann. Rheum. Dis. 2008;67:1670–1677. doi: 10.1136/ard.2007.077974. [DOI] [PubMed] [Google Scholar]
- 179.Tjarnlund A, et al. Abatacept in the treatment of adult dermatomyositis and polymyositis: a randomised, phase IIb treatment delayed-start trial. Ann. Rheum. Dis. 2018;77:55–62. doi: 10.1136/annrheumdis-2017-211751. [DOI] [PubMed] [Google Scholar]
- 180.Curiel RV, et al. Improvement in disease activity in refractory juvenile dermatomyositis following abatacept therapy. Arthritis Rheumatol. 2023 doi: 10.1002/art.42450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sukumaran S, Vijayan V. Abatacept in the treatment of juvenile dermatomyositis-associated calcifications in a 16-year-old girl. Case Rep. Rheumatol. 2020;2020:4073879. doi: 10.1155/2020/4073879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Arabshahi B, Silverman RA, Jones OY, Rider LG. Abatacept and sodium thiosulfate for treatment of recalcitrant juvenile dermatomyositis complicated by ulceration and calcinosis. J. Pediatr. 2012;160:520–522. doi: 10.1016/j.jpeds.2011.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ll Wilkinson MG, Deakin CT, Papadopoulou C, Eleftheriou D, Wedderburn LR. JAK inhibitors: a potential treatment for JDM in the context of the role of interferon-driven pathology. Pediatr. Rheumatol. Online J. 2021;19:146. doi: 10.1186/s12969-021-00637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen Z, Wang X, Ye S. Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease. N. Engl. J. Med. 2019;381:291–293. doi: 10.1056/NEJMc1900045. [DOI] [PubMed] [Google Scholar]
- 185.Le Voyer T, et al. JAK inhibitors are effective in a subset of patients with juvenile dermatomyositis: a monocentric retrospective study. Rheumatology. 2021;60:5801–5808. doi: 10.1093/rheumatology/keab116. [DOI] [PubMed] [Google Scholar]
- 186.Kim H, et al. Janus kinase (JAK) inhibition with baricitinib in refractory juvenile dermatomyositis. Ann. Rheum. Dis. 2021;80:406–408. doi: 10.1136/annrheumdis-2020-218690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yu Z, Wang L, Quan M, Zhang T, Song H. Successful management with Janus kinase inhibitor tofacitinib in refractory juvenile dermatomyositis: a pilot study and literature review. Rheumatology. 2021;60:1700–1707. doi: 10.1093/rheumatology/keaa558. [DOI] [PubMed] [Google Scholar]
- 188.Kim H. Updates on interferon in juvenile dermatomyositis: pathogenesis and therapy. Curr. Opin. Rheumatol. 2021;33:371–377. doi: 10.1097/BOR.0000000000000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kul Cinar O, Papadopoulou C, Pilkington CA. Treatment of calcinosis in juvenile dermatomyositis. Curr. Rheumatol. Rep. 2021;23:13. doi: 10.1007/s11926-020-00974-9. [DOI] [PubMed] [Google Scholar]
- 190.Hoeltzel MF, Oberle EJ, Robinson AB, Agarwal A, Rider LG. The presentation, assessment, pathogenesis, and treatment of calcinosis in juvenile dermatomyositis. Curr. Rheumatol. Rep. 2014;16:467. doi: 10.1007/s11926-014-0467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Elahmar H, Feldman BM, Johnson SR. Management of calcinosis cutis in rheumatic diseases. J. Rheumatol. 2022;49:980–989. doi: 10.3899/jrheum.211393. [DOI] [PubMed] [Google Scholar]
- 192.Nozawa T, et al. Early abnormal nailfold capillary changes are predictive of calcinosis development in juvenile dermatomyositis. J. Rheumatol. 2022;49:1250–1255. doi: 10.3899/jrheum.220249. [DOI] [PubMed] [Google Scholar]
- 193.Chung MP, et al. Calcinosis biomarkers in adult and juvenile dermatomyositis. Autoimmun. Rev. 2020;19:102533. doi: 10.1016/j.autrev.2020.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Berntsen KS, et al. Cardiorespiratory fitness in long-term juvenile dermatomyositis: a controlled, cross-sectional study of active/inactive disease. Rheumatology. 2019;58:492–501. doi: 10.1093/rheumatology/key342. [DOI] [PubMed] [Google Scholar]
- 195.Blom KJ, et al. Trajectories of cardiorespiratory fitness in patients with juvenile dermatomyositis. Rheumatology. 2017;56:2204–2211. doi: 10.1093/rheumatology/kex366. [DOI] [PubMed] [Google Scholar]
- 196.Hicks JE, Drinkard B, Summers RM, Rider LG. Decreased aerobic capacity in children with juvenile dermatomyositis. Arthritis Rheum. 2002;47:118–123. doi: 10.1002/art.10237. [DOI] [PubMed] [Google Scholar]
- 197.Mathiesen PR, et al. Aerobic fitness after JDM — a long-term follow-up study. Rheumatology. 2013;52:287–295. doi: 10.1093/rheumatology/kes232. [DOI] [PubMed] [Google Scholar]
- 198.Takken T, Spermon N, Helders PJ, Prakken AB, Van Der Net J. Aerobic exercise capacity in patients with juvenile dermatomyositis. J. Rheumatol. 2003;30:1075–1080. [PubMed] [Google Scholar]
- 199.Habers GE, et al. Muscles in motion: a randomized controlled trial on the feasibility, safety and efficacy of an exercise training programme in children and adolescents with juvenile dermatomyositis. Rheumatology. 2016;55:1251–1262. doi: 10.1093/rheumatology/kew026. [DOI] [PubMed] [Google Scholar]
- 200.Riisager M, Mathiesen PR, Vissing J, Preisler N, Orngreen MC. Aerobic training in persons who have recovered from juvenile dermatomyositis. Neuromuscul. Disord. 2013;23:962–968. doi: 10.1016/j.nmd.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 201.Astley C, et al. Home-based exercise program for adolescents with juvenile dermatomyositis quarantined during COVID-19 pandemic: a mixed methods study. Pediatr. Rheumatol. Online J. 2021;19:159. doi: 10.1186/s12969-021-00646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kant-Smits K, Van Brussel M, Nijhof S, Van3 der Net J. Reducing fatigue in ped33iatric rheumatic conditions: a systematic review. Pediatr. Rheumatol. Online J. 2021;19:111. doi: 10.1186/s12969-021-00580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Livermore P, et al. Being on the juvenile dermatomyositis rollercoaster: a qualitative study. Pediatr. Rheumatol. Online J. 2019;17:30. doi: 10.1186/s12969-019-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ardalan K, et al. Parent perspectives on addressing emotional health for children and young adults with juvenile myositis. Arthritis Care Res. 2021;73:18–29. doi: 10.1002/acr.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fawole OA, et al. Engaging patients and parents to improve mental health intervention for youth with rheumatological disease. Pediatr. Rheumatol. Online J. 2021;19:19. doi: 10.1186/s12969-021-00503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Livermore P, et al. Mapping the current psychology provision for children and young people with juvenile dermatomyositis. Rheumatol. Adv. Pract. 2021;5:rkab062. doi: 10.1093/rap/rkab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Butbul Aviel Y, et al. Sleep and fatigue and the relationship to pain, disease activity and quality of life in juvenile idiopathic arthritis and juvenile dermatomyositis. Rheumatology. 2011;50:2051–2060. doi: 10.1093/rheumatology/ker256. [DOI] [PubMed] [Google Scholar]
- 208.Tollisen A, Sanner H, Flato B, Wahl AK. Quality of life in adults with juvenile-onset dermatomyositis: a case-control study. Arthritis Care Res. 2012;64:1020–1027. doi: 10.1002/acr.21637. [DOI] [PubMed] [Google Scholar]
- 209.Rider LG, et al. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: physician and patient/parent global activity, manual muscle testing (MMT), health assessment questionnaire (HAQ)/childhood health assessment questionnaire (C-HAQ), childhood myositis assessment scale (CMAS), myositis disease activity assessment tool (MDAAT), disease activity score (DAS), short form 36 (SF-36), child health questionnaire (CHQ), physician global damage, myositis damage index (MDI), quantitative muscle testing (QMT), myositis functional index-2 (FI-2), myositis activities profile (MAP), inclusion body myositis functional rating scale (IBMFRS), cutaneous dermatomyositis disease area and severity index (CDASI), cutaneous assessment tool (CAT), dermatomyositis skin severity index (DSSI), skindex, and dermatology life quality index (DLQI) Arthritis Care Res. 2011;63:S118–S157. doi: 10.1002/acr.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rider LG, et al. American College of Rheumatology/European League Against Rheumatism Criteria for Minimal, Moderate, and Major Clinical Response in Juvenile Dermatomyositis: an International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. 2017;69:911–923. doi: 10.1002/art.40060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Aggarwal R, et al. American College of Rheumatology/European League Against Rheumatism Criteria for Minimal, Moderate, and Major Clinical Response in Adult Dermatomyositis and Polymyositis: an International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. 2017;69:898–9130. doi: 10.1002/art.40064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu K, et al. Pilot study of the juvenile dermatomyositis consensus treatment plans: a CARRA registry study. J. Rheumatol. 2021;48:114–122. doi: 10.3899/jrheum.190494. [DOI] [PubMed] [Google Scholar]
- 213.Lilleker JB, et al. The EuroMyositis registry: an international collaborative tool to facilitate myositis research. Ann. Rheum. Dis. 2018;77:30–39. doi: 10.1136/annrheumdis-2017-211868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.McCann LJ, et al. Development of a consensus core dataset in juvenile dermatomyositis for clinical use to inform research. Ann. Rheum. Dis. 2018;77:241–250. doi: 10.1136/annrheumdis-2017-212141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rennebohm RM, et al. Normal scores for nine maneuvers of the Childhood Myositis Assessment Scale. Arthritis Rheum. 2004;51:365–370. doi: 10.1002/art.20397. [DOI] [PubMed] [Google Scholar]
- 216.Quinones R, et al. Lack of achievement of a full score on the childhood myositis assessment scale by healthy four-year-olds and those recovering from juvenile dermatomyositis. Arthritis Care Res. 2013;65:1697–1701. doi: 10.1002/acr.22041. [DOI] [PubMed] [Google Scholar]
- 217.Rosina S, et al. Development and testing of reduced versions of the manual muscle test-8 in juvenile dermatomyositis. J. Rheumatol. 2021;48:898–906. doi: 10.3899/jrheum.200543. [DOI] [PubMed] [Google Scholar]
- 218.Varnier GC, et al. Development and testing of a hybrid measure of muscle strength in juvenile dermatomyositis for use in routine care. Arthritis Care Res. 2018;70:1312–1319. doi: 10.1002/acr.23491. [DOI] [PubMed] [Google Scholar]
- 219.Campanilho-Marques R, et al. Comparison of the utility and validity of three scoring tools to measure skin involvement in patients with juvenile dermatomyositis. Arthritis Care Res. 2016;68:1514–1521. doi: 10.1002/acr.22867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ahmed S, Chen KL, Werth VP. The validity and utility of the cutaneous disease area and severity index (CDASI) as a clinical outcome instrument in dermatomyositis: a comprehensive review. Semin. Arthritis Rheum. 2020;50:458–462. doi: 10.1016/j.semarthrit.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Craig J, Feldman BM, Spiegel L, Dover S. Comparing the measurement properties and preferability of patient-reported outcome measures in pediatric rheumatology: PROMIS vs CHAQ. J. Rheumatol. 2021;48:1065–1072. doi: 10.3899/jrheum.200943. [DOI] [PubMed] [Google Scholar]
- 222.Patel RN, et al. Comparison of patient-reported outcomes measurement information system computerized adaptive testing versus fixed short forms in juvenile myositis. Arthritis Care Res. 2021;75:381–390. doi: 10.1002/acr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Deakin CT, et al. Identification and prediction of novel classes of long-term disease trajectories for patients with juvenile dermatomyositis using growth mixture models. Rheumatology. 2021;60:1891–1901. doi: 10.1093/rheumatology/keaa497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Aggarwal R, et al. Autoantibody levels in myositis patients correlate with clinical response during B cell depletion with rituximab. Rheumatology. 2016;55:991–999. doi: 10.1093/rheumatology/kev444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Reed AM, et al. Biologic predictors of clinical improvement in rituximab-treated refractory myositis. BMC Musculoskelet. Disord. 2015;16:257. doi: 10.1186/s12891-015-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tiniakou E, et al. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatology. 2017;56:787–794. doi: 10.1093/rheumatology/kew470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rice GI, et al. Assessment of type I interferon signaling in pediatric inflammatory disease. J. Clin. Immunol. 2017;37:123–132. doi: 10.1007/s10875-016-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lerkvaleekul B, et al. Siglec-1 expression on monocytes is associated with the interferon signature in juvenile dermatomyositis and can predict treatment response. Rheumatology. 2022;61:2144–2155. doi: 10.1093/rheumatology/keab601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Neely J, et al. Gene expression meta-analysis reveals concordance in gene activation, pathway, and cell-type enrichment in dermatomyositis target tissues. ACR Open Rheumatol. 2019;1:657–666. doi: 10.1002/acr2.11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bellutti Enders F, et al. Correlation of CXCL10, tumor necrosis factor receptor type II, and galectin 9 with disease activity in juvenile dermatomyositis. Arthritis Rheumatol. 2014;66:2281–2289. doi: 10.1002/art.38676. [DOI] [PubMed] [Google Scholar]
- 231.Wienke J, et al. Galectin-9 and CXCL10 as biomarkers for disease activity in juvenile dermatomyositis: a longitudinal cohort study and multicohort validation. Arthritis Rheumatol. 2019;71:1377–1390. doi: 10.1002/art.40881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wienke J, et al. Endothelial and inflammation biomarker profiles at diagnosis reflecting clinical heterogeneity and serving as a prognostic tool for treatment response in two independent cohorts of patients with juvenile dermatomyositis. Arthritis Rheumatol. 2020;72:1214–1226. doi: 10.1002/art.41236. [DOI] [PMC free article] [PubMed] [Google Scholar]