Abstract
Hearing impairment has been recently identified as a major modifiable risk factor for cognitive decline in later life and has been becoming of increasing scientific interest. Sensory and cognitive decline are connected by complex bottom-up and top-down processes, a sharp distinction between sensation, perception, and cognition is impossible. This review provides a comprehensive overview on the effects of healthy and pathological aging on auditory as well as cognitive functioning on speech perception and comprehension, as well as specific auditory deficits in the 2 most common neurodegenerative diseases in old age: Alzheimer disease and Parkinson syndrome. Hypotheses linking hearing loss to cognitive decline are discussed, and current knowledge on the effect of hearing rehabilitation on cognitive functioning is presented. This article provides an overview of the complex relationship between hearing and cognition in old age.
Key words: age related hearing loss, presbycusis, central auditory processing disorder, neurocognitive disorder, Alzheimer disease, Parkinson syndrome, hearing rehabilitation
1. Introduction
Successful communication in complex listening situations requires not only the detection of the target signal and the segregation of the scenario into different sound sources. The listener must also track who is speaking, grasp the meaning of the statement, memorize and compare it with already existing knowledge, suppress irrelevant interfering signals, formulate an own response in parallel and execute it at the right time. Longer conversations in groups require the integration of new information with already expressed contents of each speaker while again and again the attention switches between the persons involved.
This means that in order to assess and use the information contained in spoken language, a fluent and swiftly functioning integrative system of perceptual and cognitive processes is required. Both the auditory and cognitive systems are subject to typical aging processes; and with higher age, the incidence of neurodegenerative diseases increases, sometimes having a considerable influence on the ability to communicate. In recent years, hearing disorders have increasingly become the focus of scientific research as a potentially modifiable risk factor for neurocognitive impairment in an aging society. In this review, hypotheses on the causal relationship will be presented as well as specific auditory impairments in the context of the most common neurodegenerative disorders of the elderly. Finally the effect of hearing rehabilitation will be discussed.
2. Cognition and speech understanding
2.1 Definition and domains
Cognition (Latin: cognoscere =to recognize, to experience, to perceive) is a collective term for processes of reception, processing, storage, and retrieval of information as well as their results (knowledge, attitudes, beliefs, expectations). These processes can take place consciously, e. g. when solving tasks, or unconsciously, e. g. when forming opinions 1 . Human cognitive skills include, among others, processes of perception, attention, learning and memory, thinking, but also recognition of emotions, and control of one’s own behavior. The ability to use these skills to solve problems, adapt to new situations, and interact effectively with the environment is referred to in psychology as “intelligence” (Latin intelligentia =cognition, intellect). While Cattell’s intelligence model distinguished only between fluid intelligence (innate, experience-independent ability to reason and solve problems) and crystalline intelligence (predominantly culture-dependent ability to apply acquired knowledge), nowadays the Cattell-Horn-Carroll (CHC) model is considered the one that most comprehensively describes the structure of intelligence 2 . It includes 16 factors from the areas of acquired knowledge, thinking ability, processing speed, memory, sensory processing, psychomotor skills, and kinesthetics and serves as the basis for the most widely used intelligence tests.
For the diagnosis of neurocognitive disorders, the “Diagnostic and Statistical Manual of Mental Disorders – DSM-5” 3 defines 6 cognitive domains on which the diagnostic criteria are based and which can be assessed in standardized neuropsychological testing ( Table 1 ).
Table 1 Cognitive domains for the diagnosis of neurocognitive disorders in DSM-5 3
| Cognitive domain | Subdomains | |||||
|---|---|---|---|---|---|---|
| Complex attention | Permanent attention | Didived attention | Selective attention | Processing rate | ||
| Executive functions | Planning | Decision making | Working memory | Exploiting feedback/correting errors | Acting against habits/behavioral inhibition | Mental flexibility |
| Learning and memory | Immediate memory* | Short-term memory (including free recall, recall with cue stimuli, and recognition) | Ultra long-term memory (semantic and autobiographical) | Implicit (procedural) learning | ||
| Speech | Speech production (including naming, word finding, word fluency, grammar and syntax) | Speech comprehension | ||||
| Perceptive-motor | Visuo perception | Visuo construction | Perceptive-motor | Practice | Gnosis | |
| Social cognition | Recognizing emotions | Theory of mind (ability to observe the Erkennen von Emotionen Theory of Mind (ability to pay attention to another person's state of mind or experience) |
*is sometimes included in the term of working memory.
2.3 Normal cognitive aging
Cognitive processes are subject to chronological aging processes to varying degrees and are highly associated with the loss of everyday functioning, onset of dementia, and general mortality 4 5 . It is well known that basal, knowledge-independent “fluid” functions show a greater age decline than lifelong acquired “crystalline” knowledge, which can still show growth into old age 6 . A persons’ intelligence is seen as the result of function or knowledge build-up, loss, and compensation mechanisms. This means in order to maintain cognitive performance as fluid abilities are lost, we rely more and more on already established, automated crystalline processes to accomplish tasks 6 7 . Research continues to address the extent to which training can counteract functional loss and the importance of individual cognitive domains in this process. In a large cross-sectional study on 48,537 subjects and evaluation of normative values of standardized IQ and memory tests, Hartsthorne and Germine were able to show that there is greater heterogeneity with regard to the time of maximum functional capacity between the individual domains than previously assumed 8 : short-term memory and processing speed reach maximum values already in the teenage years, working memory peaks in young adulthood with the onset of decline in the 30ies. Peak performance in e. g. vocabulary and emotion recognition, on the other hand, is reached only in the middle age and maintained over a much longer period of several years. As an explanation for individual performance differences, however, non-specific age effects, such as a general slowing, must be considered in addition to these domain- and function-specific changes. Recent long-term studies indicate that approximately 30-50% of individual differences in age progression are due to a “general factor” 9 .
In addition to significantly reduced general processing speed and working memory compared to younger people, loss of executive functions and episodic memory occur in older age 10 11 . Morphologically, changes are seen in the middle temporal lobe (episodic memory) and the prefrontal/striatal system (executive functions) 12 . Neurodegenerative diseases such as Alzheimer’s disease or Parkinson’s syndrome affect these areas to varying degrees and lead to specific functional deficits.
2.4 Cognitive reserve
People of about the same age with similar central changes, e. g., in the context of a neurodegenerative disease but also in the course of normal aging, may nevertheless vary considerably in their clinical symptoms and cognitive performance. To explain this observation, the concept of cognitive reserve was introduced 13 . It refers to the ability to compensate for newly occurred damage and to maintain existing functions by using alternative neuronal networks 14 . Both congenital and acquired or environmental factors (e. g., intelligence, educational level, physical activity, recreational and social activities) play a role. Differences in cognitive reserve are also considered as an explanation for the individual impact of sensory impairment (e. g., hearing loss) in higher age 15 .
2.5 Information processing model and cognitive concepts in relation with hearing and speech comprehension
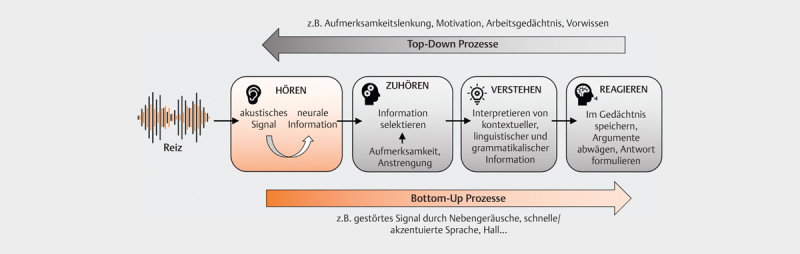
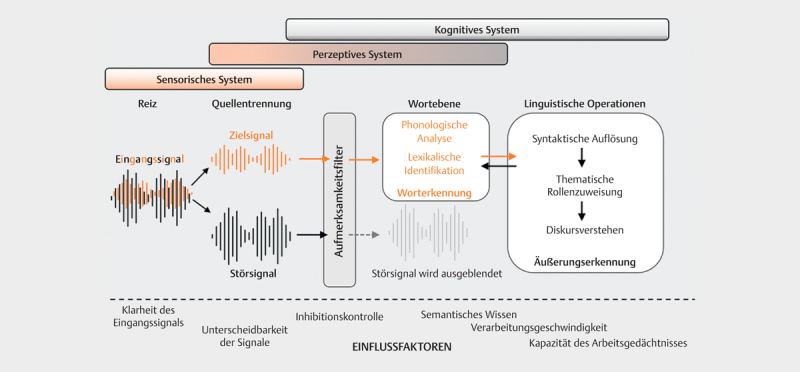
From a cognition-psychology perspective, spoken communication can be understood as a process of information processing: The incoming stimulus is perceived by the sensory system, processed, and finally leads to a reaction ( Fig 1 , adapted from 16 ). This complex process depends the properties of the incoming stimulus (bottom-up) and is also influenced by cognitive processes (top-down). In the theoretical model of Wingfield and Tun 17 ( Fig 2 ), the interactive roles of peripheral, central, cognitive, and linguistic factors to speech understanding are illustrated in more detail: In the periphery, the sensory system must receive the spectral and temporal cues of the speech signal and pass them on to the central auditory pathway for further processing with as little interference as possible. In the next stage of central auditory processing (perceptual system), binaural information is encoded in addition to spectral and temporal features of the speech signal (especially signal onset and duration). The so-called “object formation”, i. e. the ability to recognize a target signal and to follow it in the presence of competing background noise or speakers, also occurs at this level. This is followed by the linguistic operations of sound analysis and lexical recognition at the word level. Based on syntactic (position of a word in a sentence) and semantic (word meaning) prior knowledge, sentences are captured. The comparison with contextual information (speaker, situation, object, time, etc.) finally enables comprehension within the conversation 18 . The single processing steps are influenced by cognitive abilities or processes such as memory functions (prior knowledge, working memory) and general processing speed, attention, and executive functions (top-down). At the same time, the characteristics of the stimulus (e. g., speech rate, accent, type and number of noise sources, reverberation etc.) determine subsequent processing (bottom-up). Auditory and cognitive processes are so closely intertwined that a sharp separation of “peripheral” and “central” auditory functions does not adequately capture the complexity of speech processing 19 . The typical complaint of the elderly – to hear but to understand poorly – is merely a clinical symptom of normal age-related changes in all sections of this system from the periphery to the cortex, which may be additionally impaired by neurodegenerative diseases.
Fig 1.
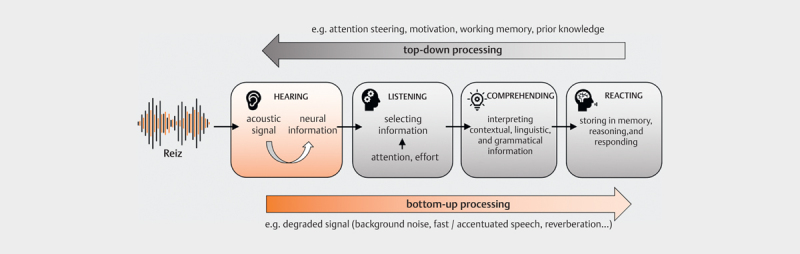
Generalized model for bottom-up and top-down processing of auditory information (adapted from [16]). The stimulus is first coded into neural information in the periphery, relevant information is selected and then interpreted in the next step. Finally, it is stored in memory while the answer is formulated at the same time. The quality and content of the stimulus influence further processing (bottom-up), information that has already been extracted or recorded content can lead to changes in the processing of subsequent stimuli (top-down).
Fig 2.
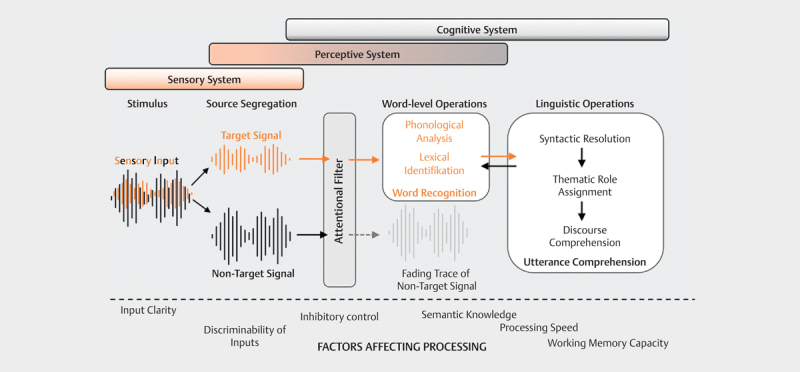
Wingfield and Tun's information processing model [17]. Sensory, perceptual and cognitive systems interact when processing auditory information. The mixed input signal must first be broken down into relevant (target signal) and irrelevant information (interfering signal). The attention filter determines the extent to which the individual signal components are further processed. Word recognition is first achieved via several intermediate steps, and finally, after further linguistic operations, discourse comprehension. The information processing process can be influenced at all levels by both cognitive and acoustic factors.
3. Age-related hearing loss
3.1 Prevalence and socio-economic consequences
In 2019, according to the WHO, about 1.5 billion people worldwide were affected by hearing loss 20 , and 430 million (about 5.5% of the world population) had at least moderate hearing impairment. The WHO expects this number to increase to 700 million people with moderate or higher levels of hearing loss in the better hearing ear by 2050, out of a projected total of 2.5 billion people affected. The individual development of hearing throughout the life span depends on various protective and damaging factors 21 . In addition to genetic, biological, and environmental influences, individual lifestyle (nicotine abuse, diet, noise exposure) also plays a role. Age-related hearing loss (ARHL) represents the greatest socio-economic burden over a lifetime due to its high prevalence in the population. According to current estimates, in 2019 approximately 42% of all people affected by hearing loss were at least 60 years old 20 , and the proportion of moderate or higher levels of hearing loss increases exponentially with higher age (prevalence at 60-69 years: 15.4%; more than 90 years: 58.2%). WHO estimates the annual costs due to hearing loss to be approximately $ 980 billion. In recent years, age-related hearing loss has been increasingly identified as a potential risk factor for neurocognitive disorders 22 23 24 25 . Positive effects of audiological rehabilitation with hearing aids for the course of these disorders 26 27 28 as well as health-related quality of life 29 are seen. Nevertheleess, in Europe, hearing aids are used by only about 33% of the approximately 57 million people with hearing loss in need of care, although they are widely available 20 30 .
3.2 Age-related changes of the peripheral auditory system
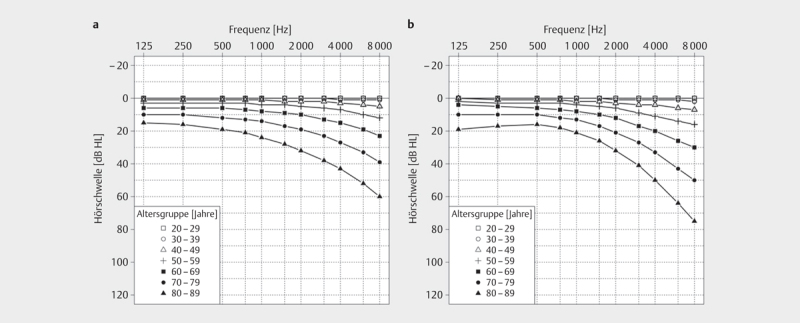
Age-related degenerative processes affect both outer and inner hair cells, supporting cells, stria vascularis, and spiral ganglion cells 31 32 33 34 35 36 . The pure-tone audiogram typically shows a loss of high frequency hearing 36 37 38 . For medical expert reports, DIN EN ISO 7029:2017 should be consulted, which allows estimation of normal hearing for ages between 20 and 80+ years 39 ( Fig 3 ). The current 3 rd version is based on data from healthy men and women published after 2000. Compared with previous versions, the average hearing loss is lower for all age groups, reflecting changes in living and working conditions.
Fig 3.
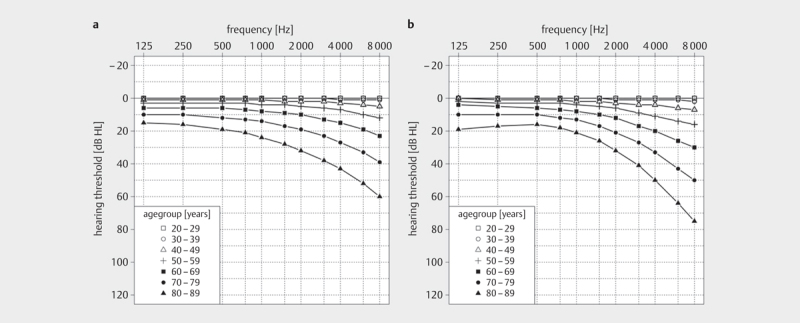
Average hearing threshold progression for men and women aged 20-80+ according to DIN EN ISO 7029:2017:06 (according to [39]). The 50th percentile of the respective age group is shown.
Based on experience from animal models regarding the underlying etiology, Dubno et al. 37 classified audiometric phenotypes of age-related hearing loss. A low-grade hearing loss up to 1 kHz and rather flat high-frequency hearing loss in indicative of metabolically-induced atrophy and degeneration of the stria vascularis, whereas a steeply declining hearing threshold between 2 and 8 kHz with normal low-frequency hearing indicates a sensory disorder (hair or supporting cell damage).
In the same study 37 , approximately 11% of pure-tone audiograms were classified as “older normal”, with an average hearing loss of no more than 20 dB HL in the high-frequency range. Nevertheless, elderlies with normal pure-tone audiograms also report hearing difficulties and tinnitus 40 41 . For this hidden hearing loss (HHL), different pathophysiological mechanisms have been discussed in recent years 42 43 44 . In addition to disturbances of the afferent synapse of the inner hair cells (cochlear synaptopathy 35 42 43 45 46 ), demyelination processes (temporary loss of cochlear Schwann cells 47 or in the context of demyelinating neuropathy 48 ), and persistent dysfunction of the outer hair cells 49 50 have been described. These changes lead to impaired transmission of temporal and spectral fine structure 51 , especially of rapid signal changes as well as signal duration. The phonetic contrasts necessary for accurate word recognition decrease, which manifests in reduced speech understanding, especially in noisy environments, even before high-frequency hearing loss is detected in the puretone audiogram.
Amplitude changes of wave I of the early auditory evoked potential elicited by suprathreshold stimulation 34 46 51 or an altered SP/AP amplitude ratio in electrocochleography 52 are discussed as electrophysiologic markers of the disturbed cochlear function.
Age-related changes of the central auditory system
3.2.1 Structural-morphological as well as neurochemical changes
Aging processes affect the entire central auditory pathway from the cochlear nucleus to the auditory cortex (see 53 54 for a comprehensive overview). Throughout the lifespan, the human cortex is subject to remodeling processes that become visible and measurable due to modern imaging techniques such as magnetic resonance imaging. MR spectroscopy also allows metabolic and neurochemical changes to be detected. In healthy adults, there is a general brain volume reduction with increasing age 55 56 57 . Volume changes in gray matter 58 59 60 and white matter 60 61 62 as well as cortex thickness 58 63 have been discribed. Regions particularly affected include the temporal lobe, hippocampus 60 64 65 and prefrontal cortex 59 61 66 67 . Lin et al. 68 demonstrated that hearing loss accelerates volume decline in both the total volume and the right temporal lobe. Further studies showed gray matter reductions beyond the age norm in the superior and medial temporal gyrus 69 and superior and medial frontal gyrus 69 70 71 , primary auditory cortex 72 73 , and occipital lobe and hypothalamus 70 . Diffusion-weighted MR images also showed changes in myelination, fiber density, and axonal parameters in the superior olive complex, lemniscus lateralis, and inferior colliculus 69 74 . MR spectroscopy has demonstrated dysfunction of GABAergic neurotransmission in the central auditory system of patients with presbycusis 54 75 76 .
This means that, on one hand, structural changes in the central auditory pathway already occur in the course of normal aging, which can have a negative effect on speech understanding; on the other hand, age-related hearing loss additionally leads to impairment of further areas in the association cortex 77 .
3.2.2 Changes of central-auditory processing and perception
Structural and neurochemical changes in the central hearing pathway lead to impaired encoding of temporal characteristics of speech. As part of normal aging processes, there are changes in neural timing and precision in speech processing 18 with implications for comprehension of speech both in quiet and in noise. In general, the ability to perceive rapid temporal changes in the speech signal decreases. That is, older people need larger differences or temporally longer features (voice onset time, vowel duration, pauses etc.) to distinguish individual speech sounds 78 . If the speech signal is additionally spectrally altered, these difficulties increase, as several studies with vocoded speech have shown 79 80 . This is particularly relevant with regard to cochlear implant fitting. Impaired neural encoding of signal onset is also thought to be the cause of greater difficulty for elders to understand speech with altered speed, stress, or rhythm. For example, research by Gordon-Salant et al. demonstrated that older normal-hearing subjects have significantly greater problems with understanding fast speakers or speech with a foreign accent 81 82 .
The ability to separate single speech streams, i. e. to follow a speaker in the presence of noise or competing speakers, also declines with age and has been demonstrated in a multitude of studies 83 84 85 86 . This has been attributed to impaired processing of temporal fine structure as well as perception of brief amplitude changes in the envelope of the speech signal (“listen to the dips”) 87 . In addition, age effects have been demonstrated in the binaural processing of speech signals 88 89 90 .
A comprehensive review of age-related electrophysiological changes in the central auditory pathway can be found in 91 . Early auditory evoked potentials, especially the so-called frequency following response (FFR) after stimulation with both tone and speech signals, objectify impaired temporal processing at the brainstem level. Depending on the experimental design, late auditory evoked potentials allow both the differential detection of disturbed temporal processing of auditory stimuli at the cortical level, independent of attention and cognition (N1-P2) and the assessment of cognitive processes if the potentials are measured in an event-related manner (P300, N200). Therefore, the latter can also be used to distinguish between normal aging processes, mild cognitive impairment, and Alzheimer’s dementia 92 .
3.2.3 Central presbycusis
In English-speaking countries, the described age-related disorders of central processing and perception of auditory information with age-appropriate pure-tone hearing threshold are summarized under the term of central auditory processing disorder (CAPD) or central presbycusis 93 94 . The disorder is considered to have multifactorial causes, correlations with age-related cognitive disorders are seen, clinically, a sharp separation between cognitive and auditory processing is hardly possible.
The German Society of Phoniatrics and Pediatric Audiology defines auditory processing and perception disorders in more detail: According to the current guideline, the diagnosis of auditory processing and perception disorders should only be made if, at age-appropriate pure-tone hearing thresholds, there are deficits in analysis, differentiation, and identification of time, frequency, and intensity changes of acoustic or auditory speech signals as well as processes of binaural interaction (e. g., for sound localization, lateralization, noise suppression, and summation) and dichotic processing that cannot be better explained by other disorders, such as attention deficits, general cognitive deficits, cross-modality mnestic disorders 95 . The deficits in the auditory domain must be significant compared to language-independent cognitive performance. At the same time, there is a high comorbidity to e. g. disorders of attention. Clinically, it must then be decided, taking into account all findings, which disorder is leading. With regard to differentiation from infantile auditory processing and perception disorder and in view of the usually modality-spanning aging processes, it seems to make sense to rather use the term of “central presbycusis” for disorders of central auditory processing newly occurring in older age.
3.3 Influence of cognitive processes on speech comprehension
In order to follow a conversation successfully and participate in it, listeners and speakers must not only perceive what is being said and understand the single words even under unfavorable complex conditions (background noise, reverberation, high speech rate, accent, etc.), but also grasp the content in context, compare it with their own prior knowledge, and formulate a response. On the cognitive level, this requires, among other things, keeping one’s attention on the target signal, storing it in working memory, and matching it with long-term memory – as quickly as possible in order to be able to follow the rest of the conversation. Working memory, executive functions, and processing speed are therefore seen as the most important cognitive factors for speech comprehension, especially in noise 96 , and a large number of studies have investigated them (e. g., executive functions and attention 97 98 , processing rate and working memory 87 ). The importance of auditory and cognitive factors and their interaction for the quality of speech comprehension has been increasingly taken into account in the last 10-20 years, so that the term of “cognitive hearing science” has been established 99 .
3.3.1 Inhibition control
In the information processing model by Wingfield and Tun 17 ( Fig 2 ), the “attention filter” symbolizes the ability to selectively follow a single signal in the presence of noise or competing speakers and thus to suppress further processing of the non-selected speech streams very early in the process. Disruption of inhibition control, e. g., in the course of normal aging, limits this ability and may thereby impair speech comprehension.
At the word level, perceived phonemes must be matched with the mental lexicon. The success of this lexical process depends on the frequency of occurrence of a word within a language as well as the number of words, with overlapping phonemes (neighborhood density). The Neighborhood Activation Model 100 theorizes that the more frequently a word occurs within a language (high frequency) and the fewer words with overlapping phonemes (low neighborhood density), the easier it is to recognize the word correctly. Accordingly, words with high neighborhood density have more competitors that must be suppressed by the listener to enable correct word retrieval. Research on the neighborhood density effect has shown that in older adults, there is a significant relationship between measures of inhibition control and speech comprehension in noise (e. g., 101 ). In addition, with increasing age, frequently occurring competing words are more intrusive, i. e. they are more often misidentified as a target signal 102 103 .
3.3.2 Working memory
In cognition psychology, working memory is understood as limited resource that allows information to be kept and processed in immediate memory 104 . In phonological analysis, working memory is considered to play a significant role as an interface to long-term memory. To explain why in some situations speech understanding is effortless while in others increased listening effort is required, Rönnberg et al. developed the Ease of Language Understanding (ELU) model (see 105 for a comprehensive review). The incoming multimodal signal is quickly and automatically matched (within 180-200 ms 16 ) with the mental lexicon. If a minimum number of matching phonological attributes is found, the implicit lexical process proceeds rapidly, and the signal is understood. If no match is found, semantic and episodic long-term memory must be explicitly accessed with the aid of working memory to enable language processing. If the input signal is difficult to understand – e. g., due to hearing impairment or unfavorable acoustic environment – it must be held longer in working memory and more cognitive resources must be expended to understand what is being said. Listening effort increases 106 . In particular, a significant dependence on working memory capacity has been shown for speech comprehension in noise, independent of age 107 108 .
3.3.3 Significance of the context
Phonological matching can be facilitated by the aid of contextual information, allowing partial compensation for the deficits caused by hearing impairment. Benichow et al. 109 , for example, demonstrated that although hearing loss had a significant effect on speech understanding in noise, it decreased with increasing probability of the target word to occur in the context of the sentence. At the same time, both age and cognitive performance (especially working memory as well as processing speed) were significant predictors of speech understanding independent of the amount of contextual information.
Increasing deficits in inhibition control with age may, in turn, contribute to wrongly identify acoustically unintelligible words as utterances that are probable within context 110 111 112 . A recent study by van Os et al. 113 revealed that older subjects are also able to rationally adjust their response behavior within a trial and, for example, rely more on the acoustic information than the context when the context offered is misleading.
3.3.4 Listening effort
If cognitive resources must be used to understand disturbed speech signal, they are lacking for other processes such as encoding what is heard into memory. The so-called “Framework for Understanding Effortful Listening” (FUEL) 114 describes successful speech comprehension as dependent on the quality of the acoustic stimulus, the demand of the task, and the listener’s motivation to exert the effort necessary to achieve it. Increased listening effort may not only deplete available cognitive resources more rapidly, but also reduce the listener’s motivation to exert that effort at all – even if the utterance itself was correctly understood.
4. Hearing disorders in frequent neurodegnerative diseases in higher ages
4.1 Neurocognitive disorders
Neurocognitive disorders (NCD) are disorders that are associated with a subjective or objective loss of previously existing cognitive abilities in at least one of the 6 cognitive domains of complex attention, executive function, learning and memory, language, perceptual-motor, social cognition (cf. Table 1) and do not only occur exclusively in the context of delirium or can be explained by another existing mental disorder (such as major depression, schizophrenia) 3 . The DSM-5 distinguished between mild (minor) and severe (major) forms, which are seen on a continuum of cognitive and functional impairment. In minor NCD, moderate cognitive performance impairments are present but do not affect the ability to perform activities of daily living independently, although greater effort or compensatory strategies may be required. In major NCD cognitive performance has significantly declined and impairs independence in performing activities of daily living. The impairment in everyday activities can me mild (only instrumental activities such as household, handling money), moderate (limitations in basic activities of daily life like eating, dressing), or severe (complete dependence). The major NCD is intended to replace the widely used, and sometimes stigmatizing, term of dementia. Specific pathophysiological processes are known for the majority of neurocognitive disorders, allowing further specification of both minor and major NCD ( Table 2 ).
Table 2 Specific etiology of neurocognitive disorder NCD) in DSM-5 3 .
| Minor/major NCD due to |
|---|
| Alzheimer’s disease |
| Fronto-temporal lobe degeneration |
| Lewy body dementia |
| Vascular disease |
| Cranio-cerebral trauma |
| Substance/drug consumption |
| HIV infection |
| Prion disease |
| Parkinson’s syndrome |
| Huntington disease |
| Other medical factors |
| Multiple etiologies |
| Not specified |
4.1.1 Socio-economic relevance
Neurocognitive disorders predominantly affect older age, so a global increase in the number of cases is expected with demographic change. Based on data from the Global Burden of Disease Study of 2019 115 , the number of dementia patients worldwide was estimated to 55.4 million in 2019, and projections expect and increase to 152.8 million affected people in 2050 116 . In some regions, however, decreases in new cases were observed: A recent analysis of the incidence rate over the last 25 years for Europe and North America showed a decrease in the incidence of dementia 13% per decade 252 . According to the German Alzheimer Society, approximately 1.8 million people in Germany were affected by dementia at the end of 2021, the vast majority (1.7 million) were over 65 years of age 117 , and women were twice as likely to develop the disease than men. The number of newly diagnosed cases in the 65+ age group was estimated at 430,000 117 . It is expected to increase to 2.8 million affected persons by 2050. At the same time, due to demographic change, the number of working-age individuals caring or paying for the care of dementia patients will decrease significantly 118 .
In view of this major social challenge, prevention is of particular importance. An expert consortium recently identified 12 potentially modifiable risk factors, which together explain almost 40% of all dementias ( Table 3 ). Hearing loss is the most important risk factor in middle age.
Table 3 Modifiable risk factors for the development of dementia according to 23
| Time | Risk factor | Relative risk | Attributable risk |
|---|---|---|---|
| Younger age (<45 years) | Education | 1.6 | 7.1% |
| Middle age (45-65 years) | Hearing loss | 1.9 | 8.2% |
| Cranio-cerebral trauma | 1.8 | 3.4% | |
| Hypertension | 1.6 | 1.9% | |
| Excessive alcohol consumption (>24g/d) | 1.2 | 0.8% | |
| Obesity (BMI ≥ 30) | 1.6 | 0.7% | |
| Higher age ( 65 Jahre) | Smoking | 1.6 | 5.2% |
| Depression | 1.9 | 3.9% | |
| Social isolation | 1.6 | 3.5% | |
| Physical inactivity | 1.4 | 1.6% | |
| Air pollution | 1.1 | 2.3% | |
| Diabetes | 1.5 | 1.1% |
*the attributable risk indicates the percentage by which one can reduce the incidence of disease if one completely eliminates the risk factor; BMI = Body-Mass-Index.
Societal changes such as improved education as well as adjustments in individual lifestyles could therefore contribute to a significant reduction in the risk of dementia and thus improve the quality of life in older age. For example, Norton et al. 119 estimated that even a prevalence reduction of 10-20% of each risk factor per decade could reduce the number of global Alzheimer patients by 8.8-16.2 million in 2050.
The national dementia strategy paper, adopted in 2020, seeks to address the increasing societal demands of dementia and aims to improve the lives and care of people with dementia in Germany. However, a concrete package of measures for the implementation of prevention strategies based on the above-mentioned risk factors is missing to date 120 .
4.2 Alzheimer’s disease
Alzheimer’s disease (AD) is the most common cause of major NCD, accounting for an estimated 2/3 of all cases 121 . It is a progressive neurodegenerative disease with characteristic biological changes, primarily associated with memory impairment, leading to dementia 121 . The biological feature is the increasing deposit of β-amyloid and tau proteins in the brain of affected individuals. Approximately, 95% of the cases occur sporadically and usually after the age of 65 years (“late onset Alzheimer’s disease”, LOAD), in less than 5% of the cases, the first symptoms appear before the age of 60 years (“early onset Alzheimer’s disease”, EOAD) 122 . The sporadic form usually progresses slowly over years to decades, whereas more rapid courses are often observed in EOAD. The most important genetic risk factor for the sporadic disease is the so-called ApoE-4 allele of the gene for apolipoprotein E, which is involved in lipid metabolism and plays a role in amyloid deposit. For the early onset of the disease, 3 genes (presenilin-1, presenilin-2, amyloid precursor protein) have been identified so far as risk factors 121 , which occur in a familial cluster in about 1% of all AD patients. In the course of the disease, β-amyloid accumulates between the nerve cells, initially in the form of oligomers, later as amyloid plaques, leading to a disturbance of nerve cell function and the associated development of clinical symptoms. Since about 20 years, subtypes of β-amyloid can be detected in CSF and used as biomarkers for AD (Aβ 42 and Aβ 42 /Aβ 40 ratio). In addition to extracellular amyloid deposits, intracellular deposits of defective tau proteins are typically found as neurofibrillary bundles or “tangles”. Total tau and phosphor-tau concentrations can be determined in the CSF. The first one indicates nonspecific nerve cell damage and may also be elevated in other neurodegenerative diseases or strokes. Phospho-tau (pTau), on the other hand, is significantly elevated exclusively in AD. The German S3 Dementia Guideline therefore recommends the combined measurement of Aβ 42 , total tau, and pTau to differentiate neurodegenerative and other causes in unclear dementias 123 .
The amyloid deposits can also be visualized by positron emission tomography (amyloid PET).
The leading clinical symptom is slowly progressive disturbances primarily of learning and memory, but also of attention as well as spatial and temporal orientation 121 122 . Radiologically, in addition to a general brain volume reduction, atrophy of the medial temporal lobe, especially the hippocampus, is typically found 124 . In approximately 10% of the cases, the disease manifests with atypical symptoms such as loss of visuospatial abilities (posterior parietal atrophy, Benson syndrome) 125 or as frontal or logopenic variants 126 127 , both of which resemble typical fronto-temporal dementias. Parieto-temporal metabolic disorders can be visualized by fluorodeoxyglucose PET (FDG-PET) and assist in confirming the diagnosis. Cognitive function loss is usually accompanied by neuropsychiatric symptoms, such as apathy, agitation, anxiety, sleep disturbances, and depression.
Alzheimer’s disease is nowadays understood as a continuum, as the biological processes begin years to decades before the onset of the first symptoms and result in cognitive changes as the disease progresses. Based on the biological markers, it is possible to identify patients as affected by Alzheimer’s disease already at the preclinical stage or at the stage of mild cognitive impairment (minor NCD or mild cognitive impairment, MCI).
4.2.1 Hearing loss and Alzheimer’s disease
Already in 1993, Sinha et al. 128 reported the involvement of the auditory system in Alzheimer’s disease. Amyloid plaques and intracellular neurofibrils were detected in the medial geniculate corpus and inferior colliculus, primary auditory cortex, and auditory association areas. A functional feature of temporo-parietal changes in AD is considered to be a disturbance in auditory scene analysis, i. e., the ability to identify auditory objects – e. g., a speaker – and to follow them even in the presence of noise 129 130 131 132 133 . For example, Goll et al. 129 demonstrated that Alzheimer patients were significantly worse at discriminating spectrally and temporally altered environmental sounds compared to healthy individuals with comparable peripheral auditory thresholds when non-verbal working memory was taken into account, while the ability to perceive pitch and timbre remained the same. Coeberg et al. 134 also found significantly more auditory agnosia for environmental sounds in patients with mild Alzheimer’s disease compared to healthy individuals, with 37% of patients showing impairment in recognition and 57% in naming test sounds. The mean hearing threshold of the patients affected by agnosia was significantly higher, independent of age. This means, peripheral hearing loss in combination with Alzheimer’s pathology increases the likelihood of the occurrence of further central auditory deficits (in this study, an odds ratio of 13.75 versus healthy subjects).
Already in 1986, Uhlmann et al. 135 described a correlation between peripheral hearing and significantly faster cognitive performance loss in AD. In a long-term study of 639 cognitively healthy individuals at study inclusion 136 , an increase in dementia risk of 20% was shown for each 10 dB increase in mean hearing threshold. Broken down by degree of hearing loss, the hazard ratios were 1.89 for low, 3.00 for moderate, and 4.94 for severe hearing loss. A meta-analysis of 33 studies confirmed the association of peripheral hearing and cognitive function 137 . The cognitive performance of patients with hearing loss was lower than that of hearing healthy individuals, regardless of whether the hearing loss was treated or not. Nevertheless, the difference between individuals with treated hearing loss and hearing healthy individuals was more than half. Hearing loss had a negative effect on all cognitive domains investigated (attention, processing speed, working memory, long-term memory, executive functions, semantic and lexical knowledge), but the effect size was small (accounting for 4-6% of variance).
A similar relationship has been shown for central hearing impairment. As early as 1996, Gates et al. 138 reported a 6-fold higher risk of dementia for patients with central hearing impairment, and further large longitudinal and cross-sectional studies came to similar conclusions 139 140 141 142 143 . Central hearing impairment in particular has therefore been discussed as a possible harbinger of later dementia 133 138 140 144 . A recent meta-analysis 145 concluded that although a number of subjective audiometric methods for assessing central auditory processing (including speech in noise, dichotic hearing/binaural processing, time-compressed speech) can discriminate well between normal cognitive aging and mild cognitive impairment or AD, a reliable differentiation between MCI and AD has not yet been possible. Moreover, whether in the preclinical phase of AD without cognitive impairment these investigations can contribute to an earlier diagnosis than by the currently known neurological and biological markers remains open 146 .
Auditory, event-related potentials could potentially close this gap. In a study of 26 patients with a positive family history of AD, it was shown that carriers of mutations in the presenilin-1 and APP genes already show significant changes in central auditory potentials even before cognitive deficits become clinically manifest 147 . The latency delay of late auditory-evoked potentials N100, P200, N200, and P300 demonstrated in this study was taken as an electrophysiological sign of slower cortical information processing. A later meta-analysis by Morrison et al. 92 , evaluating studies published between 2005 and 2017 on auditory-evoked potentials in patients over 60 years of age, concluded that P300 and N200 are appropriate electrophysiological markers for distinguishing normal cognitive aging, mild cognitive impairment, and AD.
4.3 Parkinson’s syndrome (PS)
Parkinson’s syndrome is the most common neurodegenerative disease after Alzheimer’s disease 148 149 . According to a recent epidemiological estimate based on health insurance data of 3.7 million insured persons, approximately 420,000 people in Germany were affected in 2015 150 , the standardized prevalence amounted to 511.4/100,000.
The incidence increases with higher age: while about 50/100,000 of the 65-year-old people are affected, about 400/100,000 patients are found in the age group of 85 years and older 151 . Due to demographic change, but also earlier detection, the number of people affected by PS in the EU is expected to increase to about 4.25 million by 2050 152 . Parkinson’s syndrome (PS) comprises an etiologically and phenotypically heterogeneous group of disorders. In addition to idiopathic Parkinson’s syndrome (IPS, about 75% of all cases), a distinction is made between genetic forms as well as Parkinson’s syndromes in the context of other neurodegenerative diseases (atypical PS, multisystem atrophy, Lewy body-type dementia, progressive supranuclear gaze palsy, corticobasal degeneration) and symptomatic (secondary) Parkinson’s syndrome (drug-induced, posttraumatic, toxic, metabolic, inflammatory, tumor-related) 153 154 155 156 . In addition to the cardinal motor symptoms (akinesia/bradykinesia, resting tremor, rigor, and postural instability), a wide variety of accompanying sensory, autonomic, psychological, and cognitive symptoms may occur and significantly impair quality of life 157 158 . Cognitive disorders mainly affect executive functions, such as planning, anticipatory thinking, working memory, and difficulties in switching attention between different tasks.
The incidence of so-called Parkinson’s dementia is estimated in international cross-sectional studies to be between 20-44%, which corresponds to a 3-6-fold higher risk of disease for Parkinson patients compared to non-affected individuals 159 160 . In a German cross-sectional study of 873 patients with idiopathic Parkinson’s syndrome, 28.6% of the patients met the diagnostic criteria for dementia according to DSM-5, with the frequency increasing significantly with higher age as well as disease stage 158 . The British CamPalGN study followed 142 patients newly diagnosed with IPS between 2000 and 2002 161 , 46% of this population developed dementia within the 10-year follow-up period, again including age at diagnosis and disease stage as significant prognostic factors.
4.3.1 Hearing loss and Parkinson’s syndrome
Hearing loss is discussed as another non-motor accompanying symptom of PS 162 163 164 165 166 . Several studies have shown that hearing impaired people suffer more frequently from PS 162 167 . In pure-tone audiometry, predominantly high-frequency losses 168 169 170 171 are found that exceed the extent of merely presbycusis 169 172 173 174 175 . A British case-control study of 55 patients with PS and early onset (≤55 years) found unilateral or bilateral hearing thresholds deviating from the age norm in 64.7% of patients and 28% of the age- and sex-matched control group 169 . No difference was found in brainstem audiometry between the two groups in this study, so the authors assumed pure cochlear involvement. The suggestion of dopamine-dependent cochlear dysfunction is supported by evidence of reduced DPOAE amplitudes that improved with levodopa substitution 172 ; in this study, DPOAE dysfunction correlated with the clinical severity of Parkinson’s syndrome. Another study group found additional significant lateral differences. Cochlear function measured by DPOAE and pure-tone audiometry was not only worse in Parkinson patients than in the control group of the same age, but also significantly more pronounced on the ipsilateral ear of motor symptoms 176 .
Beyond tone audiometric changes, difficulties in the perception of rhythms and tonal differences 177 178 have been reported.
A number of studies have demonstrated changes in the morphology, latency, and interpeak intervals of early auditory brainstem response (ABR) in PS patients 168 179 180 . Similarly, reduced amplitudes and prolonged latencies of vestibular evoked potentials (VEMP) were found 179 181 182 . The event-related potential P3 is suitable to detect stage and progression of Parkinson’s syndrome. The subject is offered sequences of repetitive standard stimuli that are rarely interrupted by a deviant stimulus (so-called oddball paradigm). The evoked potential (P300, P3a, P3b) is dependent on attention and working memory and therefore seems to be suitable to assess the impairment of executive functions in PS 183 184 185 186 187 . With increasing severity, there is a reduction in amplitude as well as prolongation of latency, so that patients with and without Parkinson’s dementia can be distinguished electrophysiologically 188 189 .
Although auditory stimuli and music are used for the treatment of Parkinson-related gait disorders and postural instability 190 191 192 , the importance of auditory rehabilitation for Parkinson patients is not discussed in therapy studies.
5. Correlation of hearing loss and cognitive impairment
The importance of cognitive processes for speech comprehension, especially in challenging listening situations, is well established. Age-related deficits lead to restrictions in communication ability, social isolation and, associated with this, to psychological stress and reduced quality of life. The question of a possible causal relationship between hearing loss and reduced cognitive abilities up to manifest dementia has increasingly become the focus of scientific research in recent years (see comprehensive reviews in e. g. 53 146 166 193 194 195 196 ). The analysis of already published study results is complicated by the great heterogeneity of the collected data, both in terms of audiological and cognitive parameters, as well as in terms of the studied groups, recorded influencing factors, and duration of follow-up.
Usually, the pure-tone hearing threshold is used for the assessment of (peripheral) hearing loss, but already here, there are differences in the grouping of the included subjects, depending on the method used to differentiate between subjects with and without hearing loss.
On the basis of 3 long-term studies 136 197 198 (at least 5 years of follow-up) of subjects without cognitive impairment with tone audiometrically determined hearing threshold, the Lancet Commission 24 25 calculated a relative risk of 1.9 for developing dementia in the presence of hearing impairment (defined as hearing loss greater than 25 dB HL in the pure-tone audiogram) in middle age (55 years and older) compared with normal hearing subjects. Hearing loss in middle age has been identified as the most important modifiable risk factor for developing dementia.
Few studies explicitly address the relationship between central auditory disorders and dementia or cognitive deficits in old age. A meta-analysis by Dryden et al. 199 identified 25 studies that investigated the relationship between cognitive performance and speech understanding in noise as a measure of central hearing impairment. For both the subset of studies that included only peripherally normal hearing subjects (16 articles) and studies that also included subjects with at most moderate hearing loss (up to 70 dB HL, 9 studies), the overall correlation (r=0.31 [normal hearing], r=0.32 [up to moderate hearing loss]) of cognitive function and speech understanding in noise was weak. Broken down by cognitive domains, the strongest correlation was seen for processing speed (r=0.39), followed by inhibition control (r=0.34), working memory (r=0.28), and episodic memory (r=0.26), whereas global measures of crystalline intelligence showed a significantly weaker correlation (r=0.18).
Wayne and Johnsrude 194 state that the use of global cognitive screening tests such as the Montreal Cognitive Assessment (MoCa 200 ), the Mini-Mental State Test (MMST 201 ), and the Modified Mini-Mental State Test (3MS 202 ) in normal aging individuals shows little variability, and thus may underestimate the impact of hearing loss on cognitive function.
At the same time, the presence of hearing impairment may interfere with the performance in cognitive tests and lead to an overestimation of the cognitive deficit present, especially when instructions are given verbally, as shown by several studies in normal-hearing, cognitively healthy subjects with simulated hearing loss 203 204 205 . Therefore, special versions of cognitive screening instruments for hearing-impaired people have been developed, which should be used preferentially in the future (refer to Völter et al. 206 for a comprehensive overview).
5.1 Explanatory models for the interaction of hearing and cognition
In order to explain the relationship between (age-related) hearing loss and cognitive decline, a number of models are discussed, which will be briefly described below. A comprehensive review is provided by Wayne and Johnsrude 194 .
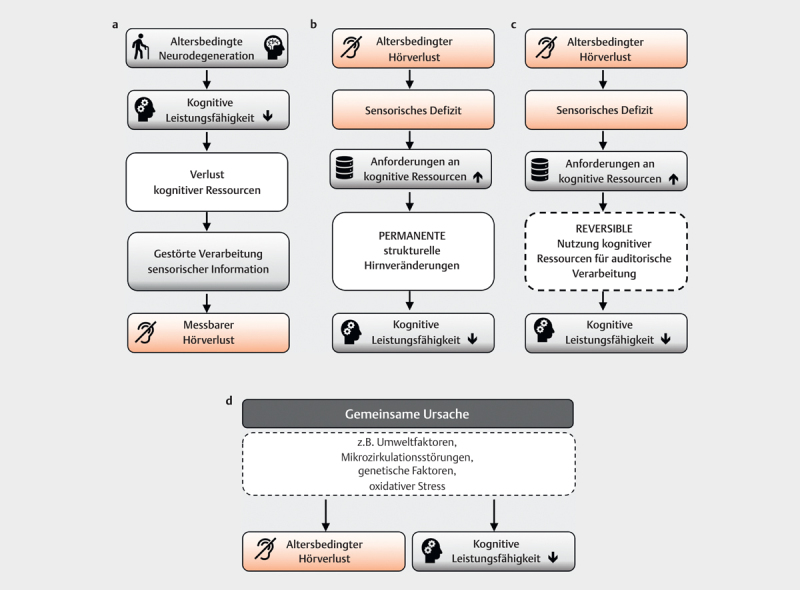
5.1.1 Model 1: Cognitive load on perception hypothesis
Declining cognitive capacity places increasing load on perception so that no longer sufficient resources are available for the processing of sensory information. This leads to an audiometrically measurable hearing impairment 207 208 . A study by Kiely et al. 209 seems to confirm this theory. After analyzing longitudinal data from a total of 4221 subjects, the authors concluded that, in addition to age and hypertension, a score of less than 24 on the Mini-Mental State Test was among the independent predictors of annual hearing threshold deterioration. Ex post, it remains unclear to what extent the hearing impairment itself affected the test result, because the test used was presented verbally ( Fig 4a ).
Fig 4.
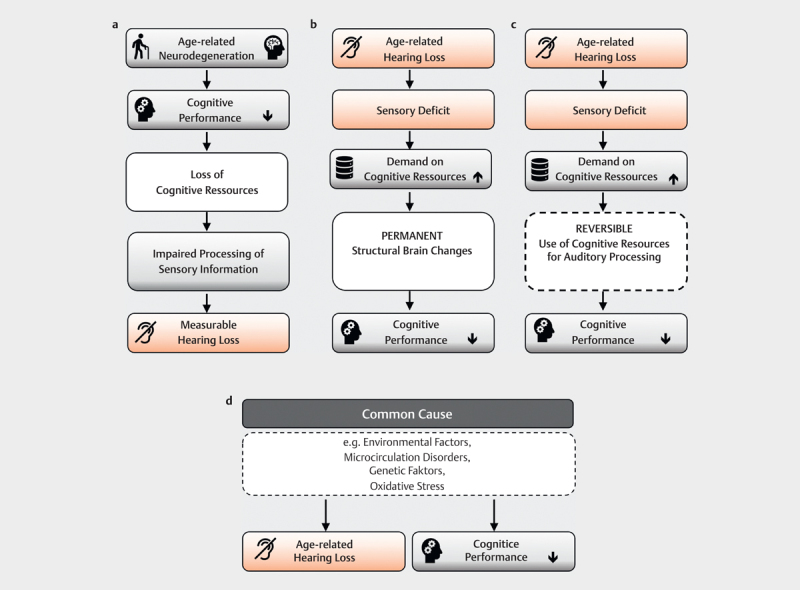
Explanatory models for the connection between age-related hearing loss and cognitive function loss: A) Cognitve load on perception hypothesis: Loss of cognitive function leads to a measurable hearing impairment via the disturbed processing of sensory information B) Information degradation hypothesis: Age-related hearing loss degrades the information available for further processing. Temporarily cognitive resources are used to compensate, which are then no longer available for other cognitive processes. This process is potentially reversible by providing hearing aids which improve the information available. C) Sensory deprivation hypothesis: The sensory deprivation associated with presbycusis leads to permanent structural brain changes and permanent loss of cognitive function D) Common cause hypothesis: Common endogenous and exogenous causes lead to both a loss of cognitive function and presbycusis.
5.1.2. Model 2: Information degradation hypothesis
This model assumes that reduced or impaired peripheral hearing triggers an upward cascade in which cognitive resources are applied to compensate for the hearing impairment, rendering them unavailable for other cognitive processes 207 210 . Evidence for this assumption is high; for example, several studies have shown that the ability to recall words or sentences deteriorates during a demanding perceptual experiment in elderly subjects 17 211 . The associated increased listening effort has negative effects on working memory and inhibition control 17 . The cognitive loss in this model is reversible – it is assumed that if peripheral input is improved, e. g., by compensating for hearing loss with hearing aids, at least partial recovery of cognitive performance is possible ( Fig 4b ).
5.1.3 Model 3: Sensory deprivation hypothesis
This model assumes that a lasting shift in resources to compensate for perceptual deficits leads to a permanent loss of cognitive function. Neuroplastic remodeling in central auditory areas and neurovascular and neurophysiological changes similar to those seen in dementia are postulated as possible mechanisms 106 212 213 214 . For congenital or early acquired hearing loss, the associated neuroplastic changes are already well established 215 216 , but cognitive performance is little affected 217 . Sensory deprivation alone is thus insufficient as an explanatory model for cognitive loss in old age ( Fig 4c ).
5.1.4 Model 4: Common cause hypothesis
General age-related neurodegeneration processes could have negative consequences for both cognitive performance and sensory perception 207 208 . For example, the decrease in processing speed is discussed as one such common factor 218 . In addition to genetic causes 219 , cerebrovascular disease 220 and general loss of physical functioning have been considered as possible mechanisms ( Fig 4d ).
5.1.5 Multifactorial model
None of the above assumptions alone can explain all observed changes in older age; a combination of several effects is most likely. Wayne and Johnsrude 194 therefore postulated a multifactorial model illustrating the interdependence of sensory and cognitive processes ( Fig 5 ).
Fig 5.
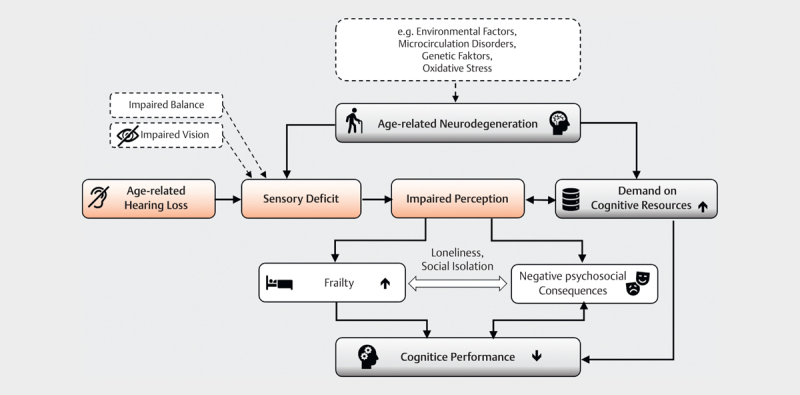
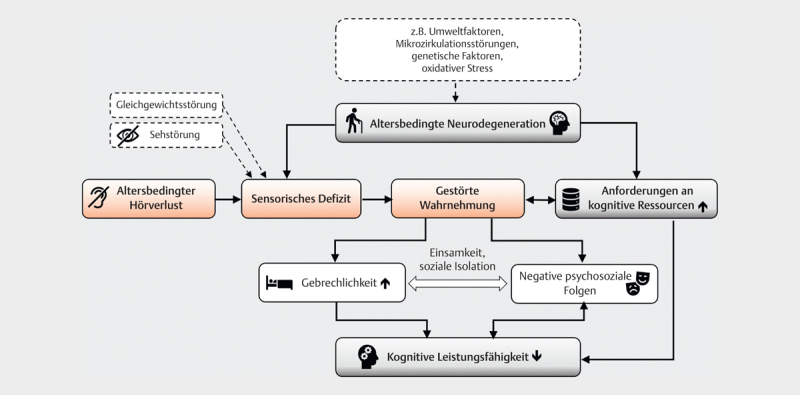
Multifactorial model of the connection between age-related hearing loss and cognitive function loss (adapted and expanded from [194]. Aging processes affect both the sensory and the cognitive system. Age-related hearing loss leads to a sensory deficit with impaired perception. Compensatory mechanisms increase access to cognitive resources which are already reduced by aging. The communication disorder resulting from the perceptual disorder promotes loneliness and social isolation, which has negative psychosocial consequences (e.g. depression) and potentially increases frailty. Cognitive performance decreases due to multiple loads.
Age-related neurodegenerative changes increase cognitive demands and, in combination with sensory deficits, lead to impaired perception. Compensation for perceptual deficits increases cognitive load, which can lead to declines in mental performance. Other sensory deficits (e. g., impaired vision or balance) amplify the impairment. The communication disorder caused by the hearing loss promotes social isolation and loneliness and with it depression and frailty – the latter being further risk factors for cognitive decline independent of hearing loss 53 221 .
6. Can treatment of hearing loss reduce cognitive impairment?
Due to the widespread availability of hearing aids, treatment of age-related hearing loss is perceived as an achievable target for dementia prevention. However, testing the effectiveness of such an intervention presents unique challenges. For example, in the context of an observational study, it is difficult to monitor the quality of hearing aid fitting as well as the duration of daily use. The latter is now facilitated by the possibility of data logging by the hearing aid. A recent study on datasets of more than 15,000 hearing aid users was able to objectify the considerable inter- but also intraindividual variance in daily hearing aid use 222 . At the same time, factors such as socio-economic status, education level, social environment, communication behavior, and access to health care play a role in both hearing aid use and risk of cognitive decline, making independent assessment of the impact of hearing rehabilitation difficult. Large epidemiologic aging studies in the past have partially included hearing threshold but not systematic hearing aid use (e. g., for the German-speaking countries 223 ).
A multicenter, randomized-controlled longitudinal intervention study initiated in 2018 in the USA including more than 800 70-84-year-old individuals without dementia with low to moderate hearing loss comparing the efficacy of hearing aid provision with health education alone with parallel collection of audiologic data as well as cognitive performance over a 3-year period (ACHIEVE study, 224 ) intends to address the issue, but completion is not expected until late 2022 at the earliest.
Regarding the different intervention options, currently most data are found on conventional hearing aid fitting, in recent years increasingly also on cochlear implantation.
6.1 Provision of hearing aids
The Lancet Commission 24 cites 3 recent studies to support the possible preventive effect of hearing aid use. A prospective study demonstrated a correlation between increased incidence of dementia in subjects with self-reported hearing loss within the 25-year observation period only if they did not use hearing aids 225 . The cross-sectional study of Ray et al. 226 also found cognitive deficits only in the subgroup of hearing impaired subjects who did not use their hearing aids, but the groups studied varied considerably in age and severity of hearing loss. The long-term study by Maharani et al. 227 found a slowing of age-related functional loss in episodic memory after the onset of hearing aid use.
In a comprehensive systematic analysis of long-term studies published between 1990 and 2020 on the relationship between hearing aid use and cognitive function 228 , the authors concluded that to date, based on the current body of studies, no definitive conclusion on the preventive effect of hearing aid use can be drawn. The methodology of the existing studies is extremely heterogeneous, of particular importance is the generally short follow-up period with regard to the rather slow age-related cognitive function loss. In addition to the aforementioned study by Maharani et al. 227 , the authors were able to identify only 1 other study in which subjects were followed-up for at least 10 years, which did not reveal any differences between intervention group (with hearing aids) and control group for any cognitive measures 229 . In addition, a common problem in comparative studies was large hearing threshold differences between intervention and control groups. Furthermore, hearing aid compliance was poorly reported or not reported at all in 9/17 studies, leaving it unclear to what extent subjects used the hearing aid adequately. The greatest potential benefit of hearing aid provision appeared to be in the area of executive function – after all, 6/11 studies found improvement 228 . Two out of 4 studies found significant improvement with hearing aid use on screening tests (MMST). However, it was not reported whether the hearing impaired version was used, so it cannot be excluded that due to hearing impairment in baseline testing, cognitive function loss was overestimated and the improvement found by using the hearing aids was only due to a better understanding of the verbally presented tasks.
6.2 Cochlear implantation
It is well established that elderly patients with severe hearing loss or deafness benefit from cochlear implantation in terms of speech understanding and quality of life (e. g., 230 231 232 233 234 ). Compared to normal-hearing individuals, the incoming signal is already highly degraded by the signal processing of the cochlear implant, which requires a greater input of cognitive resources to understand speech in the first place. Assuming that aging processes of the central auditory pathway affect CI recipients to the same extent as normal-hearing individuals, older CI users are at an even greater disadvantage because impaired temporal processing further deteriorates the already degraded signal 235 . As in normal-hearing individuals, working memory function affects speech comprehension 236 237 , and linguistic context can be used to some extent to improve speech comprehension 238 .
In recent years, a number of studies have been published explicitly addressing the alteration of (global) cognitive functions by cochlear implantation 239 240 241 242 243 244 245 246 247 248 249 250 251 . Similar to studies on hearing aid users, the neurocognitive test batteries chosen varied widely, although tests suitable for hearing impaired people were increasingly used 244 245 246 247 248 251 252 . The follow-up period was relatively short (12 months) in most studies, probably because the long-term studies in questions were initiated only in recent years. Four research groups reported results after 18 251 , 24 246 , at least 25 242 , and 60 months 240 . Positive effects, especially on executive functions, were already reported within the short follow-up period. A limiting factor is the small number of cases – mostly<20 patients have been included 241 242 243 244 252 . The largest number of participants with simultaneous use of a neurocognitive test battery adapted for hearing impaired subjects has been studied so far by Völter et al. 246 247 248 . During a follow-up period of at least 24 months, 71 elderly CI patients (mean age at implantation 66.03 years) showed significant improvements in executive functions (attention, working memory, inhibition) already after 6 months compared to preoperative performance, and after 12 months, memory and word fluency had also significantly improved. After 24 months, there was an improvement in processing speed; inhibition control (flanker) was no longer significantly better, and there were no changes in mental flexibility throughout the study period. Preoperatively, the performance of 12 of the 71 subjects was below 68% confidence interval in 3 or more subtests; after 12 months, this was the case in only 3/71 subjects. By the end of the study, 5/71 subjects had deteriorated in more than 2 subtests. Cognitive performance had no significant effect on speech comprehension at rest.
A similar result was already reported by Mosnier et al. 239 in their investigation of 94 CI users aged 65-85 years: Of 37 subjects with preoperatively worse cognitive function, 81% improved within the first 12 months, and performance remained stable in 19%. Regarding dementia development, the follow-up study by the same research group is particularly interesting 240 . 80 subjects of the original 94 included were still alive 5 years after implantation, 70 of whom could be followed-up. Before cochlear implantation, 31 subjects had cognitive performance in the range of mild cognitive impairment. Of these, 32% recovered to normal function, 6% developed dementia, and 61% remained stable. Of the 38 subjects with preoperatively normal function, none developed dementia during the follow-up period, but in 32% of the cases, cognitive performance was in the range of mild cognitive impairment after 5 years. A correlation with the achieved speech comprehension could not be proven.
Overall, all studies published so far show a clear positive, at least stabilizing, mostly even improving effect of cochlear implantation.
7. Outlook
Sensory and cognitive deficits are closely linked via complex bottom-up and top-down processes. The consequences of both normal and pathological aging processes will inevitably pose major challenges to our society in the future. The realization that a number of risk factors can be modified already in young and middle ages offers opportunities for prevention. In particular, the consistent treatment of hearing loss must become an even greater focus of health education, also in view of the threat of social isolation and depression as further risk factors for cognitive decline, in order to increase the alarmingly low rate of care, even in industrialized countries. It is essential to take into account the special needs of the elderly, both with regard to the operation of hearing systems (fine motor requirements when changing batteries vs. using rechargeable batteries, simple operating structure/coupling with external systems) and the fitting process (possibly longer habituation phase, slower processing speed, lower differentiation acuity when comparing different settings). Appropriate compensation for the increased time required for consultation and repeated adjustment would increase the incentive for providers to devote the necessary attention to this patient group. The higher costs of care would be offset by a significantly improved quality of life and longer cognitive function preservation in the case of successful adaptation, which could lead to a reduction in the costs of care and thus to a reduction in the burden on society as a whole. To validate the success of the fitting, further long-term studies are required that record in detail both cognitive function and hearing performance as well as the type and extent of use of hearing systems and apply measurement methods that are methodologically adapted to possible cognitive and sensory deficits.
Footnotes
Interessenkonflikt Die Autorin gibt an, dass kein Interessenkonflikt besteht.
Literatur
- 1.Stangl, Werner. Online Lexikon für Psychologie und Pädagogik
- 2.Flanagan D P, Dixon S G. John Wiley & Sons, Ltd; 2014. The Cattell-Horn-Carroll Theory of Cognitive Abilities. In: Encyclopedia of Special Education. [Google Scholar]
- 3.American Psychiatric Association, Peter Falkai, Hans-Ulrich WittchenDiagnostisches und Statistisches Manual Psychischer Störungen DSM-5. 2. korrigierte Auflage 2018 Hogrefe; 2018 [Google Scholar]
- 4.Tucker-Drob E M. Neurocognitive functions and everyday functions change together in old age. Neuropsychology. 2011;25:368–377. doi: 10.1037/a0022348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tucker-Drob E M. Cognitive Aging and Dementia: A Life Span Perspective. Annu Rev. Dev Psychol. 2019;1:177–196. doi: 10.1146/annurev-devpsych-121318-085204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baltes P B. [Age and aging as incomplete architecture of human ontogenesis] Z Gerontol Geriatr. 1999;32:433–448. doi: 10.1007/s003910050141. [DOI] [PubMed] [Google Scholar]
- 7.Tucker-Drob E M, de la Fuente J, Köhncke Y et al. A strong dependency between changes in fluid and crystallized abilities in human cognitive aging. Sci Adv. 2022;8:eabj2422. doi: 10.1126/sciadv.abj2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartshorne J K, Germine L T. When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol Sci. 2015;26:433–443. doi: 10.1177/0956797614567339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tucker-Drob E M. Global and domain-specific changes in cognition throughout adulthood. Dev Psychol. 2011;47:331–343. doi: 10.1037/a0021361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckner R L. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Hedden T, Gabrieli JD E. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 12.Jagust W. Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron. 2013;77:219–234. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baltes P B, Dittmann-Kohli F, Kliegl R. Reserve capacity of the elderly in aging-sensitive tests of fluid intelligence: replication and extension. Psychol Aging. 1986;1:172–177. doi: 10.1037/0882-7974.1.2.172. [DOI] [PubMed] [Google Scholar]
- 14.Stern Y, Arenaza-Urquijo E M, Bartrés-Faz D et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement J Alzheimers Assoc. 2020;16:1305–1311. doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tucker A M, Stern Y. Cognitive reserve in aging. Curr Alzheimer Res. 2011;8:354–360. doi: 10.2174/156720511795745320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenfelt S, Rönnberg J. The signal-cognition interface: interactions between degraded auditory signals and cognitive processes. Scand J Psychol. 2009;50:385–393. doi: 10.1111/j.1467-9450.2009.00748.x. [DOI] [PubMed] [Google Scholar]
- 17.Wingfield A, Tun P A. Cognitive Supports and Cognitive Constraints on Comprehension of Spoken Language. J Am Acad Audiol. 2007;18:548–558. doi: 10.3766/jaaa.18.7.3. [DOI] [PubMed] [Google Scholar]
- 18.Gordon-Salant S, Shader M J, Wingfield A. Cham: Springer International Publishing; 2020. Age-Related Changes in Speech Understanding: Peripheral Versus Cognitive Influences. In: Helfer KS, Bartlett EL, Popper AN, et al., Hrsg. Aging and Hearing: Causes and Consequences; pp. 199–230. [Google Scholar]
- 19.Johnson JC S, Marshall C R, Weil R S et al. Hearing and dementia: from ears to brain. Brain J Neurol. 2021;144:391–401. doi: 10.1093/brain/awaa429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health OrganizationWorld report on hearing Geneva: World Health Organization; 2021 [Google Scholar]
- 21.Davis A, McMahon C M, Pichora-Fuller K M et al. Aging and Hearing Health: The Life-course Approach. The Gerontologist. 2016;56:S256–S267. doi: 10.1093/geront/gnw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin F R, Yaffe K, Xia J et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loughrey D G, Kelly M E, Kelley G A et al. Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-analysis. JAMA Otolaryngol-- Head Neck Surg. 2018;144:115–126. doi: 10.1001/jamaoto.2017.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livingston G, Huntley J, Sommerlad A et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet Lond Engl. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livingston G, Sommerlad A, Orgeta V et al. Dementia prevention, intervention, and care. Lancet Lond Engl. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 26.Rutherford B R, Brewster K, Golub J S et al. Sensation and Psychiatry: Linking Age-Related Hearing Loss to Late-Life Depression and Cognitive Decline. Am J Psychiatry. 2018;175:215–224. doi: 10.1176/appi.ajp.2017.17040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brewster K, Choi C J, He X et al. Hearing Rehabilitative Treatment for Older Adults With Comorbid Hearing Loss and Depression: Effects on Depressive Symptoms and Executive Function. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. 2022;30:448–458. doi: 10.1016/j.jagp.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brewster K K, Pavlicova M, Stein A et al. A pilot randomized controlled trial of hearing aids to improve mood and cognition in older adults. Int J Geriatr Psychiatry. 2020;35:842–850. doi: 10.1002/gps.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bigelow R T, Reed N S, Brewster K K et al. Association of Hearing Loss With Psychological Distress and Utilization of Mental Health Services Among Adults in the United States. JAMA Netw Open. 2020;3:e2010986. doi: 10.1001/jamanetworkopen.2020.10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orji A, Kamenov K, Dirac M et al. Global and regional needs, unmet needs and access to hearing aids. Int J Audiol. 2020;59:166–172. doi: 10.1080/14992027.2020.1721577. [DOI] [PubMed] [Google Scholar]
- 31.Liberman M C, Kujawa S G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear Res. 2017;349:138–147. doi: 10.1016/j.heares.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keithley E M. Pathology and mechanisms of cochlear aging. J Neurosci Res. 2020;98:1674–1684. doi: 10.1002/jnr.24439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frisina R D, Ding B, Zhu X et al. Age-related hearing loss: prevention of threshold declines, cell loss and apoptosis in spiral ganglion neurons. Aging. 2016;8:2081–2099. doi: 10.18632/aging.101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kujawa S G, Liberman M C. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330:191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu P Z, Liberman L D, Bennett K et al. Primary Neural Degeneration in the Human Cochlea: Evidence for Hidden Hearing Loss in the Aging Ear. Neuroscience. 2019;407:8–20. doi: 10.1016/j.neuroscience.2018.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gates G A, Mills J H. Presbycusis. The Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- 37.Dubno J R, Eckert M A, Lee F-S et al. Classifying human audiometric phenotypes of age-related hearing loss from animal models. J Assoc Res Otolaryngol JARO. 2013;14:687–701. doi: 10.1007/s10162-013-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer N, Weber B, Riechelmann H. [Presbycusis – Age Related Hearing Loss] Laryngorhinootologie. 2016;95:497–510. doi: 10.1055/s-0042-106918. [DOI] [PubMed] [Google Scholar]
- 39.Michel O. [DIN EN ISO 7029:2017-06 : The current DIN thresholds for evaluating normal hearing] HNO. 2021;69:1014–1018. doi: 10.1007/s00106-021-01111-3. [DOI] [PubMed] [Google Scholar]
- 40.Tremblay K L, Pinto A, Fischer M E et al. Self-Reported Hearing Difficulties Among Adults With Normal Audiograms: The Beaver Dam Offspring Study. Ear Hear. 2015;36:e290–e299. doi: 10.1097/AUD.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci Off J Soc Neurosci. 2011;31:13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bajin M D, Dahm V, Lin VY W. Hidden hearing loss: current concepts. Curr Opin Otolaryngol Head Neck Surg. 2022 doi: 10.1097/MOO.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 43.et al. Hidden Hearing Loss: A Disorder with Multiple Etiologies and Mechanisms. Cold Spring Harb Perspect Med. 2020;10:a035493. doi: 10.1101/cshperspect.a035493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pienkowski M. On the Etiology of Listening Difficulties in Noise Despite Clinically Normal Audiograms. Ear Hear. 2017;38:135–148. doi: 10.1097/AUD.0000000000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plack C J, Barker D, Prendergast G. Perceptual consequences of „hidden“ hearing loss. Trends Hear. 2014;18:2.331216514550621E15. doi: 10.1177/2331216514550621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parthasarathy A, Kujawa S G. Synaptopathy in the Aging Cochlea: Characterizing Early-Neural Deficits in Auditory Temporal Envelope Processing. J Neurosci Off J Soc Neurosci. 2018;38:7108–7119. doi: 10.1523/JNEUROSCI.3240-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wan G, Corfas G. Transient auditory nerve demyelination as a new mechanism for hidden hearing loss. Nat Commun. 2017;8:14487. doi: 10.1038/ncomms14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi J E, Seok J M, Ahn J et al. Hidden hearing loss in patients with Charcot-Marie-Tooth disease type 1A. Sci Rep. 2018;8:10335. doi: 10.1038/s41598-018-28501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mulders WHA M, Chin I L, Robertson D. Persistent hair cell malfunction contributes to hidden hearing loss. Hear Res. 2018;361:45–51. doi: 10.1016/j.heares.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Hoben R, Easow G, Pevzner S et al. Outer Hair Cell and Auditory Nerve Function in Speech Recognition in Quiet and in Background Noise. Front Neurosci. 2017;11:157. doi: 10.3389/fnins.2017.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sergeyenko Y, Lall K, Liberman M C et al. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci Off J Soc Neurosci. 2013;33:13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grant K J, Mepani A M, Wu P et al. Electrophysiological markers of cochlear function correlate with hearing-in-noise performance among audiometrically normal subjects. J Neurophysiol. 2020;124:418–431. doi: 10.1152/jn.00016.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jayakody DM P, Friedland P L, Martins R N et al. Impact of Aging on the Auditory System and Related Cognitive Functions: A Narrative Review. Front Neurosci. 2018;12:125. doi: 10.3389/fnins.2018.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ouda L, Profant O, Syka J. Age-related changes in the central auditory system. Cell Tissue Res. 2015;361:337–358. doi: 10.1007/s00441-014-2107-2. [DOI] [PubMed] [Google Scholar]
- 55.Hedman A M, van Haren NE M, Schnack H G et al. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp. 2012;33:1987–2002. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mori S, Onda K, Fujita S et al. Brain atrophy in middle age using magnetic resonance imaging scans from Japan’s health screening programme. Brain Commun. 2022;4:fcac211. doi: 10.1093/braincomms/fcac211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller K L, Alfaro-Almagro F, Bangerter N K et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19:1523–1536. doi: 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lemaitre H, Goldman A L, Sambataro F et al. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 2012;33:e1–e9. doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raz N, Gunning F M, Head D et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex N Y N 1991. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 60.Raz N, Rodrigue K M, Head D et al. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- 61.Raz N, Rodrigue K M, Kennedy K M et al. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- 62.Westlye L T, Walhovd K B, Dale A M et al. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex N Y N 1991. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- 63.Vidal-Pineiro D, Parker N, Shin J et al. Cellular correlates of cortical thinning throughout the lifespan. Sci Rep. 2020;10:21803. doi: 10.1038/s41598-020-78471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scahill R I, Frost C, Jenkins R et al. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- 65.Braak H, Thal D R, Ghebremedhin E et al. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 66.Pettemeridou E, Kallousia E, Constantinidou F. Regional Brain Volume, Brain Reserve and MMSE Performance in Healthy Aging From the NEUROAGE Cohort: Contributions of Sex, Education, and Depression Symptoms. Front Aging Neurosci. 2021;13:711301. doi: 10.3389/fnagi.2021.711301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalpouzos G, Persson J, Nyberg L. Local brain atrophy accounts for functional activity differences in normal aging. Neurobiol Aging. 2012;33:6230–6.23E15. doi: 10.1016/j.neurobiolaging.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 68.Lin F R, Ferrucci L, An Y et al. Association of hearing impairment with brain volume changes in older adults. NeuroImage. 2014;90:84–92. doi: 10.1016/j.neuroimage.2013.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Husain F T, Medina R E, Davis C W et al. Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Res. 2011;1369:74–88. doi: 10.1016/j.brainres.2010.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyen K, Langers DR M, de Kleine E et al. Gray matter in the brain: differences associated with tinnitus and hearing loss. Hear Res. 2013;295:67–78. doi: 10.1016/j.heares.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 71.Rosemann S, Thiel C M. Neuroanatomical changes associated with age-related hearing loss and listening effort. Brain Struct Funct. 2020;225:2689–2700. doi: 10.1007/s00429-020-02148-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peelle J E, Troiani V, Grossman M et al. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci Off J Soc Neurosci. 2011;31:12638–12643. doi: 10.1523/JNEUROSCI.2559-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eckert M A, Cute S L, Vaden K I et al. Auditory cortex signs of age-related hearing loss. J Assoc Res Otolaryngol JARO. 2012;13:703–713. doi: 10.1007/s10162-012-0332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang Y, Lee S-H, Lee Y-J et al. Auditory neural pathway evaluation on sensorineural hearing loss using diffusion tensor imaging. NeuroReport. 2004;15:1699–1703. doi: 10.1097/01.wnr.0000134584.10207.1a. [DOI] [PubMed] [Google Scholar]
- 75.Profant O, Balogová Z, Dezortová M et al. Metabolic changes in the auditory cortex in presbycusis demonstrated by MR spectroscopy. Exp Gerontol. 2013;48:795–800. doi: 10.1016/j.exger.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 76.Gao F, Wang G, Ma W et al. Decreased auditory GABA+concentrations in presbycusis demonstrated by edited magnetic resonance spectroscopy. NeuroImage. 2015;106:311–316. doi: 10.1016/j.neuroimage.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peelle J E, Wingfield A. The Neural Consequences of Age-Related Hearing Loss. Trends Neurosci. 2016;39:486–497. doi: 10.1016/j.tins.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gordon-Salant S, Yeni-Komshian G, Fitzgibbons P. The role of temporal cues in word identification by younger and older adults: effects of sentence context. J Acoust Soc Am. 2008;124:3249–3260. doi: 10.1121/1.2982409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schvartz K C, Chatterjee M, Gordon-Salant S. Recognition of spectrally degraded phonemes by younger, middle-aged, and older normal-hearing listeners. J Acoust Soc Am. 2008;124:3972–3988. doi: 10.1121/1.2997434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goupell M J, Gaskins C R, Shader M J et al. Age-Related Differences in the Processing of Temporal Envelope and Spectral Cues in a Speech Segment. Ear Hear. 2017;38:e335–e342. doi: 10.1097/AUD.0000000000000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gordon-Salant S, Yeni-Komshian G H, Fitzgibbons P J. Recognition of accented English in quiet by younger normal-hearing listeners and older listeners with normal-hearing and hearing loss. J Acoust Soc Am. 2010;128:444–455. doi: 10.1121/1.3397409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gordon-Salant S, Zion D J, Espy-Wilson C. Recognition of time-compressed speech does not predict recognition of natural fast-rate speech by older listeners. J Acoust Soc Am. 2014;136:EL268–EL274. doi: 10.1121/1.4895014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Helfer K S, Freyman R L. Aging and Speech-on-Speech Masking. Ear Hear. 2008;29:87–98. doi: 10.1097/AUD.0b013e31815d638b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dubno J R, Dirks D D, Morgan D E. Effects of age and mild hearing loss on speech recognition in noise. J Acoust Soc Am. 1984;76:87–96. doi: 10.1121/1.391011. [DOI] [PubMed] [Google Scholar]
- 85.Tun P A, Wingfield A. One voice too many: adult age differences in language processing with different types of distracting sounds. J Gerontol B Psychol Sci Soc Sci. 1999;54:P317–P327. doi: 10.1093/geronb/54b.5.p317. [DOI] [PubMed] [Google Scholar]
- 86.Pronk M, Deeg DJ H, Festen J M et al. Decline in older persons’ ability to recognize speech in noise: the influence of demographic, health-related, environmental, and cognitive factors. Ear Hear. 2013;34:722–732. doi: 10.1097/AUD.0b013e3182994eee. [DOI] [PubMed] [Google Scholar]
- 87.Füllgrabe C, Moore BC J, Stone M A. Age-group differences in speech identification despite matched audiometrically normal hearing: contributions from auditory temporal processing and cognition. Front Aging Neurosci. 2015;6:347. doi: 10.3389/fnagi.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gallun F J. Impaired Binaural Hearing in Adults: A Selected Review of the Literature. Front Neurosci. 2021;15:610957. doi: 10.3389/fnins.2021.610957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hommet C, Mondon K, Berrut G et al. Central auditory processing in aging: the dichotic listening paradigm. J Nutr Health Aging. 2010;14:751–756. doi: 10.1007/s12603-010-0097-7. [DOI] [PubMed] [Google Scholar]
- 90.Dillard L K, Fischer M E, Pinto A et al. Longitudinal Decline on the Dichotic Digits Test. Am J Audiol. 2020;29:862–872. doi: 10.1044/2020_AJA-20-00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harris K C. Cham: Springer International Publishing; 2020. The Aging Auditory System: Electrophysiology. In: Helfer KS, Bartlett EL, Popper AN, et al., Hrsg. Aging and Hearing: Causes and Consequences; pp. 117–141. [Google Scholar]
- 92.Morrison C, Rabipour S, Knoefel F et al. Auditory Event-related Potentials in Mild Cognitive Impairment and Alzheimer’s Disease. Curr Alzheimer Res. 2018;15:702–715. doi: 10.2174/1567205015666180123123209. [DOI] [PubMed] [Google Scholar]
- 93.Gates G A. Central presbycusis: an emerging view. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2012;147:1–2. doi: 10.1177/0194599812446282. [DOI] [PubMed] [Google Scholar]
- 94.Humes L E, Dubno J R, Gordon-Salant S et al. Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol. 2012;23:635–666. doi: 10.3766/jaaa.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF), Hrsg. S1-Leitlinie 2019 Auditive Verarbeitungs- und Wahrnehmungsstörungen (AVWS) Herausgegeben von der Deutschen Gesellschaft für Phoniatrie und Pädaudiologie
- 96.Schneider B A, Pichora-Fuller K, Daneman M. New York, NY: Springer; 2010. Effects of Senescent Changes in Audition and Cognition on Spoken Language Comprehension. In: Gordon-Salant S, Frisina RD, Popper AN, et al., Hrsg. The Aging Auditory System; pp. 167–210. [Google Scholar]
- 97.Janse E. A non-auditory measure of interference predicts distraction by competing speech in older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2012;19:741–758. doi: 10.1080/13825585.2011.652590. [DOI] [PubMed] [Google Scholar]
- 98.Ward K M, Shen J, Souza P E et al. Age-Related Differences in Listening Effort During Degraded Speech Recognition. Ear Hear. 2017;38:74–84. doi: 10.1097/AUD.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arlinger S, Lunner T, Lyxell B et al. The emergence of cognitive hearing science. Scand J Psychol. 2009;50:371–384. doi: 10.1111/j.1467-9450.2009.00753.x. [DOI] [PubMed] [Google Scholar]
- 100.Luce P A, Pisoni D B. Recognizing spoken words: the neighborhood activation model. Ear Hear. 1998;19:1–36. doi: 10.1097/00003446-199802000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taler V, Aaron G P, Steinmetz L G et al. Lexical neighborhood density effects on spoken word recognition and production in healthy aging. J Gerontol B Psychol Sci Soc Sci. 2010;65:551–560. doi: 10.1093/geronb/gbq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Helfer K S, Jesse A. Lexical influences on competing speech perception in younger, middle-aged, and older adults. J Acoust Soc Am. 2015;138:363–376. doi: 10.1121/1.4923155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jesse A, Helfer K S. Lexical Influences on Errors in Masked Speech Perception in Younger, Middle-Aged, and Older Adults. J Speech Lang Hear Res JSLHR. 2019;62:1152–1166. doi: 10.1044/2018_JSLHR-H-ASCC7-18-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baddeley A. Working memory: theories, models, and controversies. Annu Rev Psychol. 2012;63:1–29. doi: 10.1146/annurev-psych-120710-100422. [DOI] [PubMed] [Google Scholar]
- 105.Rönnberg J, Holmer E, Rudner M. Cognitive Hearing Science: Three Memory Systems, Two Approaches, and the Ease of Language Understanding Model. J Speech Lang Hear Res JSLHR. 2021;64:359–370. doi: 10.1044/2020_JSLHR-20-00007. [DOI] [PubMed] [Google Scholar]
- 106.Peelle J E. Listening Effort: How the Cognitive Consequences of Acoustic Challenge Are Reflected in Brain and Behavior. Ear Hear. 2018;39:204–214. doi: 10.1097/AUD.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rudner M, Rönnberg J, Lunner T. Working memory supports listening in noise for persons with hearing impairment. J Am Acad Audiol. 2011;22:156–167. doi: 10.3766/jaaa.22.3.4. [DOI] [PubMed] [Google Scholar]
- 108.Gordon-Salant S, Cole S S. Effects of Age and Working Memory Capacity on Speech Recognition Performance in Noise Among Listeners With Normal Hearing. Ear Hear. 2016;37:593–602. doi: 10.1097/AUD.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 109.Benichov J, Cox L C, Tun P A et al. Word recognition within a linguistic context: effects of age, hearing acuity, verbal ability, and cognitive function. Ear Hear. 2012;33:250–256. doi: 10.1097/AUD.0b013e31822f680f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rogers C S, Jacoby L L, Sommers M S. Frequent false hearing by older adults: the role of age differences in metacognition. Psychol Aging. 2012;27:33–45. doi: 10.1037/a0026231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rogers C S. Semantic priming, not repetition priming, is to blame for false hearing. Psychon Bull Rev. 2017;24:1194–1204. doi: 10.3758/s13423-016-1185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Failes E, Sommers M S, Jacoby L L. Blurring past and present: Using false memory to better understand false hearing in young and older adults. Mem Cognit. 2020;48:1403–1416. doi: 10.3758/s13421-020-01068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van Os M, Kray J, Demberg V. Mishearing as a Side Effect of Rational Language Comprehension in Noise. Front Psychol. 2021;12:679278. doi: 10.3389/fpsyg.2021.679278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pichora-Fuller M K, Kramer S E, Eckert M A et al. Hearing Impairment and Cognitive Energy: The Framework for Understanding Effortful Listening (FUEL) Ear Hear. 2016;37:5S. doi: 10.1097/AUD.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 115.Vos T, Lim S S, Abbafati C et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105–e125. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deutsche Alzheimer Gesellschaft e.V. Infoblatt 1: Die Häufigkeit von Demenzerkrankungen. . Im Internet:https://www.deutsche-alzheimer.de/publikationen/informationsblaetter;
- 118.Wancata J, Musalek M, Alexandrowicz R et al. Number of dementia sufferers in Europe between the years 2000 and 2050. Eur Psychiatry J Assoc Eur Psychiatr. 2003;18:306–313. doi: 10.1016/j.eurpsy.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 119.Norton S, Matthews F E, Barnes D E et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 120.Jessen F. Die Nationale Demenzstrategie. Fortschritte Neurol · Psychiatr. 2022;90:320–325. doi: 10.1055/a-1808-6459. [DOI] [PubMed] [Google Scholar]
- 121.Hans-Holger Bleß, Doron Benjamin SteinWeißbuch Versorgung der frühen Alzheimer Krankheit Springer; 2021 [Google Scholar]
- 122.Long J M, Holtzman D M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell. 2019;179:312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF), Hrsg. S3-Leitlinie „Demenzen“ (Langversion – Januar 2016)
- 124.Urbach H, Egger K.
- 125.Crutch S J, Lehmann M, Schott J M et al. Posterior cortical atrophy. Lancet Neurol. 2012;11:170–178. doi: 10.1016/S1474-4422(11)70289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ossenkoppele R, Pijnenburg YA L, Perry D C et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain J Neurol. 2015;138:2732–2749. doi: 10.1093/brain/awv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Warren J D, Fletcher P D, Golden H L. The paradox of syndromic diversity in Alzheimer disease. Nat Rev Neurol. 2012;8:451–464. doi: 10.1038/nrneurol.2012.135. [DOI] [PubMed] [Google Scholar]
- 128.Sinha U K, Hollen K M, Rodriguez R et al. Auditory system degeneration in Alzheimer’s disease. Neurology. 1993;43:779–785. doi: 10.1212/wnl.43.4.779. [DOI] [PubMed] [Google Scholar]
- 129.Goll J C, Kim L G, Hailstone J C et al. Auditory object cognition in dementia. Neuropsychologia. 2011;49:2755–2765. doi: 10.1016/j.neuropsychologia.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Golden H L, Agustus J L, Goll J C et al. Functional neuroanatomy of auditory scene analysis in Alzheimer’s disease. NeuroImage Clin. 2015;7:699–708. doi: 10.1016/j.nicl.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Golden H L, Agustus J L, Nicholas J M et al. Functional neuroanatomy of spatial sound processing in Alzheimer’s disease. Neurobiol Aging. 2016;39:154–164. doi: 10.1016/j.neurobiolaging.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goll J C, Kim L G, Ridgway G R et al. Impairments of auditory scene analysis in Alzheimer’s disease. Brain J Neurol. 2012;135:190–200. doi: 10.1093/brain/awr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Idrizbegovic E, Hederstierna C, Dahlquist M et al. Central auditory function in early Alzheimer’s disease and in mild cognitive impairment. Age Ageing. 2011;40:249–254. doi: 10.1093/ageing/afq168. [DOI] [PubMed] [Google Scholar]
- 134.Coebergh JA F, McDowell S. van Woerkom TCAM, et al. Auditory Agnosia for Environmental Sounds in Alzheimer’s Disease: Not Hearing and Not Listening? J Alzheimers Dis JAD. 2020;73:1407–1419. doi: 10.3233/JAD-190431. [DOI] [PubMed] [Google Scholar]
- 135.Uhlmann R F, Larson E B, Koepsell T D. Hearing impairment and cognitive decline in senile dementia of the Alzheimer’s type. J Am Geriatr Soc. 1986;34:207–210. doi: 10.1111/j.1532-5415.1986.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 136.Lin F R, Metter E J, O’Brien R J et al. Hearing loss and incident dementia. Arch Neurol. 2011;68:214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taljaard D S, Olaithe M, Brennan-Jones C G et al. The relationship between hearing impairment and cognitive function: a meta-analysis in adults. Clin Otolaryngol. 2016;41:718–729. doi: 10.1111/coa.12607. [DOI] [PubMed] [Google Scholar]
- 138.Gates G A, Cobb J L, Linn R T et al. Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Arch Otolaryngol Head Neck Surg. 1996;122:161–167. doi: 10.1001/archotol.1996.01890140047010. [DOI] [PubMed] [Google Scholar]
- 139.Gates G A, Beiser A, Rees T S et al. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J Am Geriatr Soc. 2002;50:482–488. doi: 10.1046/j.1532-5415.2002.50114.x. [DOI] [PubMed] [Google Scholar]
- 140.Gates G A, Anderson M L, McCurry S M et al. Central Auditory Dysfunction as a Harbinger of Alzheimer Dementia. Arch Otolaryngol Neck Surg. 2011;137:390–395. doi: 10.1001/archoto.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Quaranta N, Coppola F, Casulli M et al. The prevalence of peripheral and central hearing impairment and its relation to cognition in older adults. Audiol Neurootol. 2014;19:10–14. doi: 10.1159/000371597. [DOI] [PubMed] [Google Scholar]
- 142.Sardone R, Battista P, Donghia R et al. Age-Related Central Auditory Processing Disorder, MCI, and Dementia in an Older Population of Southern Italy. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2020;163:348–355. doi: 10.1177/0194599820913635. [DOI] [PubMed] [Google Scholar]
- 143.Mamo S K, Reed N S, Sharrett A R et al. Relationship Between Domain-Specific Cognitive Function and Speech-in-Noise Performance in Older Adults: The Atherosclerosis Risk in Communities Hearing Pilot Study. Am J Audiol. 2019;28:1006–1014. doi: 10.1044/2019_AJA-19-00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Iliadou V, Kaprinis S. Clinical psychoacoustics in Alzheimer’s disease central auditory processing disorders and speech deterioration. Ann Gen Hosp Psychiatry. 2003;2:12. doi: 10.1186/1475-2832-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tarawneh H Y, Menegola H K, Peou A et al. Central Auditory Functions of Alzheimer’s Disease and Its Preclinical Stages: A Systematic Review and Meta-Analysis. Cells. 2022;11:1007. doi: 10.3390/cells11061007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Powell D S, Oh E S, Reed N S et al. Hearing Loss and Cognition: What We Know and Where We Need to Go. Front Aging Neurosci. 2022:13. doi: 10.3389/fnagi.2021.769405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Golob E J, Ringman J M, Irimajiri R et al. Cortical event-related potentials in preclinical familial Alzheimer disease. Neurology. 2009;73:1649–1655. doi: 10.1212/WNL.0b013e3181c1de77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tönges L, Ehret R, Lorrain M et al. Epidemiologie der Parkinsonerkrankung und aktuelle ambulante Versorgungskonzepte in Deutschland. Fortschritte Neurol · Psychiatr. 2017;85:329–335. doi: 10.1055/s-0043-103275. [DOI] [PubMed] [Google Scholar]
- 149.de Lau LM L, Breteler MM B. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 150.Heinzel S, Berg D, Binder S et al. Do We Need to Rethink the Epidemiology and Healthcare Utilization of Parkinson’s Disease in Germany? Front Neurol. 2018;9:500. doi: 10.3389/fneur.2018.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pringsheim T, Jette N, Frolkis A et al. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord Off J Mov Disord Soc. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 152.Bach J-P, Ziegler U, Deuschl G et al. Projected numbers of people with movement disorders in the years 2030 and 2050. Mov Disord Off J Mov Disord Soc. 2011;26:2286–2290. doi: 10.1002/mds.23878. [DOI] [PubMed] [Google Scholar]
- 153.Poewe W, Seppi K, Tanner C M et al. Parkinson disease. Nat Rev Dis Primer. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 154.Antony PM A, Diederich N J, Krüger R et al. The hallmarks of Parkinson’s disease. FEBS J. 2013;280:5981–5993. doi: 10.1111/febs.12335. [DOI] [PubMed] [Google Scholar]
- 155.Kalia L V, Lang A E. Parkinson’s disease. Lancet Lond Engl. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 156.Williams-Gray C H, Worth P F. Parkinson’s disease. Medicine (Baltimore) 2016;44:542–546. doi: 10.1016/j.mpmed.2016.06.001. [DOI] [Google Scholar]
- 157.Chaudhuri K R, Healy D G, Schapira AH V et al. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 158.Riedel O, Klotsche J, Spottke A et al. Cognitive impairment in 873 patients with idiopathic Parkinson’s disease. Results from the German Study on Epidemiology of Parkinson’s Disease with Dementia (GEPAD) J Neurol. 2008;255:255–264. doi: 10.1007/s00415-008-0720-2. [DOI] [PubMed] [Google Scholar]
- 159.Aarsland D, Andersen K, Larsen J P et al. Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology. 2001;56:730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- 160.Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson’s disease in the United Kingdom. Mov Disord Off J Mov Disord Soc. 2004;19:1043–1049. doi: 10.1002/mds.20216. [DOI] [PubMed] [Google Scholar]
- 161.Williams-Gray C H, Mason S L, Evans J R et al. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry. 2013;84:1258–1264. doi: 10.1136/jnnp-2013-305277. [DOI] [PubMed] [Google Scholar]
- 162.Lai S-W, Liao K-F, Lin C-L et al. Hearing loss may be a non-motor feature of Parkinson’s disease in older people in Taiwan. Eur J Neurol. 2014;21:752–757. doi: 10.1111/ene.12378. [DOI] [PubMed] [Google Scholar]
- 163.Vitale C, Marcelli V, Allocca R et al. Hearing impairment in Parkinson’s disease: expanding the nonmotor phenotype. Mov Disord Off J Mov Disord Soc. 2012;27:1530–1535. doi: 10.1002/mds.25149. [DOI] [PubMed] [Google Scholar]
- 164.Vitale C, Marcelli V, Abate T et al. Speech discrimination is impaired in parkinsonian patients: Expanding the audiologic findings of Parkinson’s disease. Parkinsonism Relat Disord. 2016;22:S138–S143. doi: 10.1016/j.parkreldis.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 165.Jafari Z, Kolb B E, Mohajerani M H. Auditory Dysfunction in Parkinson’s Disease. Mov Disord Off J Mov Disord Soc. 2020;35:537–550. doi: 10.1002/mds.28000. [DOI] [PubMed] [Google Scholar]
- 166.Li S, Cheng C, Lu L et al. Hearing Loss in Neurological Disorders. Front Cell Dev Biol. 2021;9:716300. doi: 10.3389/fcell.2021.716300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Simonet C, Bestwick J, Jitlal M et al. Assessment of Risk Factors and Early Presentations of Parkinson Disease in Primary Care in a Diverse UK Population. JAMA Neurol. 2022;79:359–369. doi: 10.1001/jamaneurol.2022.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yýlmaz S, Karalý E, Tokmak A et al. Auditory evaluation in Parkinsonian patients. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol – Head Neck Surg. 2009;266:669–671. doi: 10.1007/s00405-009-0933-8. [DOI] [PubMed] [Google Scholar]
- 169.Shetty K, Krishnan S, Thulaseedharan J V et al. Asymptomatic Hearing Impairment Frequently Occurs in Early-Onset Parkinson’s Disease. J Mov Disord. 2019;12:84–90. doi: 10.14802/jmd.18048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Scarpa A, Cassandro C, Vitale C et al. A comparison of auditory and vestibular dysfunction in Parkinson’s disease and Multiple System Atrophy. Parkinsonism Relat Disord. 2020;71:51–57. doi: 10.1016/j.parkreldis.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 171.Leme M S, Sanches SG G, Carvallo RM M. Peripheral hearing in Parkinson’s disease: a systematic review. Int J Audiol. 2022:1–9. doi: 10.1080/14992027.2022.2109073. [DOI] [PubMed] [Google Scholar]
- 172.Pisani V, Sisto R, Moleti A et al. An investigation of hearing impairment in de-novo Parkinson’s disease patients: A preliminary study. Parkinsonism Relat Disord. 2015;21:987–991. doi: 10.1016/j.parkreldis.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 173.Seidel K, Mahlke J, Siswanto S et al. The brainstem pathologies of Parkinson’s disease and dementia with Lewy bodies. Brain Pathol Zurich Switz. 2015;25:121–135. doi: 10.1111/bpa.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Folmer R L, Vachhani J J, Theodoroff S M et al. Auditory Processing Abilities of Parkinson’s Disease Patients. BioMed Res Int. 2017;2017:2.618587E6. doi: 10.1155/2017/2618587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Neel A T. Effects of loud and amplified speech on sentence and word intelligibility in Parkinson disease. J Speech Lang Hear Res JSLHR. 2009;52:1021–1033. doi: 10.1044/1092-4388(2008/08-0119). [DOI] [PubMed] [Google Scholar]
- 176.Sisto R, Viziano A, Stefani A et al. Lateralization of cochlear dysfunction as a specific biomarker of Parkinson’s disease. Brain Commun. 2020;2:fcaa144. doi: 10.1093/braincomms/fcaa144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mollaei F, Shiller D M, Baum S R et al. The Relationship Between Speech Perceptual Discrimination and Speech Production in Parkinson’s Disease. J Speech Lang Hear Res JSLHR. 2019;62:4256–4268. doi: 10.1044/2019_JSLHR-S-18-0425. [DOI] [PubMed] [Google Scholar]
- 178.Cochen De Cock V, de Verbizier D, Picot M C et al. Rhythm disturbances as a potential early marker of Parkinson’s disease in idiopathic REM sleep behavior disorder. Ann Clin Transl Neurol. 2020;7:280–287. doi: 10.1002/acn3.50982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shalash A S, Hassan D M, Elrassas H H et al. Auditory- and Vestibular-Evoked Potentials Correlate with Motor and Non-Motor Features of Parkinson’s Disease. Front Neurol. 2017;8:55. doi: 10.3389/fneur.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu C, Zhang Y, Tang W et al. Evoked potential changes in patients with Parkinson’s disease. Brain Behav. 2017;7:e00703. doi: 10.1002/brb3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.de Natale E R, Ginatempo F, Paulus K S et al. Paired neurophysiological and clinical study of the brainstem at different stages of Parkinson’s Disease. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2015;126:1871–1878. doi: 10.1016/j.clinph.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 182.Pötter-Nerger M, Govender S, Deuschl G et al. Selective changes of ocular vestibular myogenic potentials in Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2015;30:584–589. doi: 10.1002/mds.26114. [DOI] [PubMed] [Google Scholar]
- 183.Heitland I, Kenemans J L, Oosting R S et al. Auditory event-related potentials (P3a, P3b) and genetic variants within the dopamine and serotonin system in healthy females. Behav Brain Res. 2013;249:55–64. doi: 10.1016/j.bbr.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 184.Pfabigan D M, Seidel E-M, Sladky R et al. P300 amplitude variation is related to ventral striatum BOLD response during gain and loss anticipation: an EEG and fMRI experiment. NeuroImage. 2014;96:12–21. doi: 10.1016/j.neuroimage.2014.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schomaker J, Berendse H W, Foncke EM J et al. Novelty processing and memory formation in Parkinson’s disease. Neuropsychologia. 2014;62:124–136. doi: 10.1016/j.neuropsychologia.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 186.Solís-Vivanco R, Rodríguez-Violante M, Rodríguez-Agudelo Y et al. The P3a wave: A reliable neurophysiological measure of Parkinson’s disease duration and severity. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2015;126:2142–2149. doi: 10.1016/j.clinph.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 187.Solís-Vivanco R, Rodríguez-Violante M, Cervantes-Arriaga A et al. Brain oscillations reveal impaired novelty detection from early stages of Parkinson’s disease. NeuroImage Clin. 2018;18:923–931. doi: 10.1016/j.nicl.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Matsui H, Nishinaka K, Oda M et al. Auditory event-related potentials in Parkinson’s disease: prominent correlation with attention. Parkinsonism Relat Disord. 2007;13:394–398. doi: 10.1016/j.parkreldis.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 189.Yilmaz F T, Özkaynak S S, Barçin E. Contribution of auditory P300 test to the diagnosis of mild cognitive impairment in Parkinson’s disease. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2017;38:2103–2109. doi: 10.1007/s10072-017-3106-3. [DOI] [PubMed] [Google Scholar]
- 190.Fan W, Li J, Wei W et al. Effects of rhythmic auditory stimulation on upper-limb movements in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2022;101:27–30. doi: 10.1016/j.parkreldis.2022.06.020. [DOI] [PubMed] [Google Scholar]
- 191.Trindade MF D, Viana R A. Effects of auditory or visual stimuli on gait in Parkinsonic patients: a systematic review. Porto Biomed J. 2021;6:e140. doi: 10.1097/j.pbj.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Koshimori Y, Thaut M H. Future perspectives on neural mechanisms underlying rhythm and music based neurorehabilitation in Parkinson’s disease. Ageing Res Rev. 2018;47:133–139. doi: 10.1016/j.arr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 193.Slade K, Plack C J, Nuttall H E. The Effects of Age-Related Hearing Loss on the Brain and Cognitive Function. Trends Neurosci. 2020;43:810–821. doi: 10.1016/j.tins.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 194.Wayne R V, Johnsrude I S. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev. 2015;23:154–166. doi: 10.1016/j.arr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 195.Uchida Y, Sugiura S, Nishita Y et al. Age-related hearing loss and cognitive decline — The potential mechanisms linking the two. Auris Nasus Larynx. 2019;46:1–9. doi: 10.1016/j.anl.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 196.Oluwole O G, James K, Yalcouye A et al. Hearing loss and brain disorders: A review of multiple pathologies. Open Med Wars Pol. 2022;17:61–69. doi: 10.1515/med-2021-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gallacher J, Ilubaera V, Ben-Shlomo Y et al. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79:1583–1590. doi: 10.1212/WNL.0b013e31826e263d. [DOI] [PubMed] [Google Scholar]
- 198.Deal J A, Betz J, Yaffe K et al. Hearing Impairment and Incident Dementia and Cognitive Decline in Older Adults: The Health ABC Study. J Gerontol A Biol Sci Med Sci. 2017;72:703–709. doi: 10.1093/gerona/glw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dryden A, Allen H A, Henshaw H et al. The Association Between Cognitive Performance and Speech-in-Noise Perception for Adult Listeners: A Systematic Literature Review and Meta-Analysis. Trends Hear. 2017;21:2.331216517744675E15. doi: 10.1177/2331216517744675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nasreddine Z S, Phillips N A, Bédirian V et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 201.Folstein M F, Folstein S E, McHugh P R. „Mini-mental state“. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 202.Teng E L, Chui H C. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 203.Jorgensen L E, Palmer C V, Pratt S et al. The Effect of Decreased Audibility on MMSE Performance: A Measure Commonly Used for Diagnosing Dementia. J Am Acad Audiol. 2016;27:311–323. doi: 10.3766/jaaa.15006. [DOI] [PubMed] [Google Scholar]
- 204.Dupuis K, Pichora-Fuller M K, Chasteen A L et al. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2015;22:413–437. doi: 10.1080/13825585.2014.968084. [DOI] [PubMed] [Google Scholar]
- 205.Wong C G, Rapport L J, Billings B A et al. Hearing loss and verbal memory assessment among older adults. Neuropsychology. 2019;33:47–59. doi: 10.1037/neu0000489. [DOI] [PubMed] [Google Scholar]
- 206.Völter C, Götze L, Bruene-Cohrs U et al. Hören und Kognition: neurokognitive Testbatterien in der HNO-Heilkunde. HNO. 2020;68:155–163. doi: 10.1007/s00106-019-00762-7. [DOI] [PubMed] [Google Scholar]
- 207.Speech understanding and agingWorking Group on Speech Understanding and Aging. Committee on Hearing, Bioacoustics, and Biomechanics, Commission on Behavioral and Social Sciences and Education, National Research Council J Acoust Soc Am 198883859–895. [PubMed] [Google Scholar]
- 208.Lindenberger U, Baltes P B. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 209.Kiely K M, Gopinath B, Mitchell P et al. Cognitive, health, and sociodemographic predictors of longitudinal decline in hearing acuity among older adults. J Gerontol A Biol Sci Med Sci. 2012;67:997–1003. doi: 10.1093/gerona/gls066. [DOI] [PubMed] [Google Scholar]
- 210.Pichora-Fuller M K. Cognitive aging and auditory information processing. Int J Audiol. 2003;42:2S26–32S26. [PubMed] [Google Scholar]
- 211.McCoy S L, Tun P A, Cox L C et al. Hearing loss and perceptual effort: downstream effects on older adults’ memory for speech. Q J Exp Psychol A. 2005;58:22–33. doi: 10.1080/02724980443000151. [DOI] [PubMed] [Google Scholar]
- 212.Wong PC M, Ettlinger M, Sheppard J P et al. Neuroanatomical characteristics and speech perception in noise in older adults. Ear Hear. 2010;31:471–479. doi: 10.1097/AUD.0b013e3181d709c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sheppard J P, Wang J-P, Wong PC M. Large-scale cortical functional organization and speech perception across the lifespan. PloS One. 2011;6:e16510. doi: 10.1371/journal.pone.0016510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Eckert M A, Vaden K I, Dubno J R. Age-Related Hearing Loss Associations With Changes in Brain Morphology. Trends Hear. 2019;23:2.331216519857267E15. doi: 10.1177/2331216519857267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kral A, Sharma A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 2012;35:111–122. doi: 10.1016/j.tins.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kral A. Auditory critical periods: a review from system’s perspective. Neuroscience. 2013;247:117–133. doi: 10.1016/j.neuroscience.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 217.Vernon M. Fifty Years of Research on the Intelligence of Deaf and Hard-of-Hearing Children: A Review of Literature and Discussion of Implications. J Deaf Stud Deaf Educ. 2005;10:225–231. doi: 10.1093/deafed/eni024. [DOI] [PubMed] [Google Scholar]
- 218.Salthouse T A. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 219.Lipnicki D M, Crawford J D, Dutta R et al. Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study. PLoS Med. 2017;14:e1002261. doi: 10.1371/journal.pmed.1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Laughlin G A, McEvoy L K, Barrett-Connor E et al. Fetuin-A, a new vascular biomarker of cognitive decline in older adults. Clin Endocrinol (Oxf) 2014;81:134–140. doi: 10.1111/cen.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jayakody DM P, Wishart J, Stegeman I et al. Is There an Association Between Untreated Hearing Loss and Psychosocial Outcomes? Front Aging Neurosci. 2022;14:868673. doi: 10.3389/fnagi.2022.868673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pasta A, Szatmari T-I, Christensen J H et al. Clustering Users Based on Hearing Aid Use: An Exploratory Analysis of Real-World Data. Front Digit Health. 2021;3:725130. doi: 10.3389/fdgth.2021.725130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lindenberger U, Ghisletta P. Cognitive and sensory declines in old age: gauging the evidence for a common cause. Psychol Aging. 2009;24:1–16. doi: 10.1037/a0014986. [DOI] [PubMed] [Google Scholar]
- 224.Deal J A, Goman A M, Albert M S et al. Hearing treatment for reducing cognitive decline: Design and methods of the Aging and Cognitive Health Evaluation in Elders randomized controlled trial. Alzheimers Dement N Y N. 2018;4:499–507. doi: 10.1016/j.trci.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Amieva H, Ouvrard C, Giulioli C et al. Self-Reported Hearing Loss, Hearing Aids, and Cognitive Decline in Elderly Adults: A 25-Year Study. J Am Geriatr Soc. 2015;63:2099–2104. doi: 10.1111/jgs.13649. [DOI] [PubMed] [Google Scholar]
- 226.Ray J, Popli G, Fell G. Association of Cognition and Age-Related Hearing Impairment in the English Longitudinal Study of Ageing. JAMA Otolaryngol-- Head Neck Surg. 2018;144:876–882. doi: 10.1001/jamaoto.2018.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Maharani A, Dawes P, Nazroo J et al. Longitudinal Relationship Between Hearing Aid Use and Cognitive Function in Older Americans. J Am Geriatr Soc. 2018;66:1130–1136. doi: 10.1111/jgs.15363. [DOI] [PubMed] [Google Scholar]
- 228.Sanders M E, Kant E, Smit A L et al. The effect of hearing aids on cognitive function: A systematic review. PloS One. 2021;16:e0261207. doi: 10.1371/journal.pone.0261207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dawes P, Cruickshanks K J, Fischer M E et al. Hearing-aid use and long-term health outcomes: Hearing handicap, mental health, social engagement, cognitive function, physical health, and mortality. Int J Audiol. 2015;54:838–844. doi: 10.3109/14992027.2015.1059503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Olze H, Knopke S, Gräbel S et al. Rapid Positive Influence of Cochlear Implantation on the Quality of Life in Adults 70 Years and Older. Audiol Neurootol. 2016;21:43–47. doi: 10.1159/000448354. [DOI] [PubMed] [Google Scholar]
- 231.Knopke S, Häussler S, Gräbel S et al. Age-Dependent Psychological Factors Influencing the Outcome of Cochlear Implantation in Elderly Patients. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2019;40:e441–e453. doi: 10.1097/MAO.0000000000002179. [DOI] [PubMed] [Google Scholar]
- 232.Shin Y J, Fraysse B, Deguine O et al. Benefits of cochlear implantation in elderly patients. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2000;122:602–606. doi: 10.1067/mhn.2000.98317. [DOI] [PubMed] [Google Scholar]
- 233.Pasanisi E, Bacciu A, Vincenti V et al. Speech recognition in elderly cochlear implant recipients. Clin Otolaryngol Allied Sci. 2003;28:154–157. doi: 10.1046/j.1365-2273.2003.00681.x. [DOI] [PubMed] [Google Scholar]
- 234.Vermeire K, Brokx JP L, Wuyts F L et al. Quality-of-life benefit from cochlear implantation in the elderly. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2005;26:188–195. doi: 10.1097/00129492-200503000-00010. [DOI] [PubMed] [Google Scholar]
- 235.Moberly A C, Lewis J H, Vasil K J et al. Bottom-Up Signal Quality Impacts the Role of Top-Down Cognitive-Linguistic Processing During Speech Recognition by Adults with Cochlear Implants. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2021;42:S33–S41. doi: 10.1097/MAO.0000000000003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tao D, Deng R, Jiang Y et al. Contribution of auditory working memory to speech understanding in mandarin-speaking cochlear implant users. PloS One. 2014;9:e99096. doi: 10.1371/journal.pone.0099096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Moberly A C, Houston D M, Harris M S et al. Verbal working memory and inhibition-concentration in adults with cochlear implants. Laryngoscope Investig Otolaryngol. 2017;2:254–261. doi: 10.1002/lio2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Winn M B. Rapid Release From Listening Effort Resulting From Semantic Context, and Effects of Spectral Degradation and Cochlear Implants. Trends Hear. 2016;20:2.331216516669723E15. doi: 10.1177/2331216516669723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mosnier I, Bebear J-P, Marx M et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol-- Head Neck Surg. 2015;141:442–450. doi: 10.1001/jamaoto.2015.129. [DOI] [PubMed] [Google Scholar]
- 240.Mosnier I, Vanier A, Bonnard D et al. Long-Term Cognitive Prognosis of Profoundly Deaf Older Adults After Hearing Rehabilitation Using Cochlear Implants. J Am Geriatr Soc. 2018;66:1553–1561. doi: 10.1111/jgs.15445. [DOI] [PubMed] [Google Scholar]
- 241.Castiglione A, Benatti A, Velardita C et al. Aging, Cognitive Decline and Hearing Loss: Effects of Auditory Rehabilitation and Training with Hearing Aids and Cochlear Implants on Cognitive Function and Depression among Older Adults. Audiol Neurootol. 2016;21:21–28. doi: 10.1159/000448350. [DOI] [PubMed] [Google Scholar]
- 242.Cosetti M K, Pinkston J B, Flores J M et al. Neurocognitive testing and cochlear implantation: insights into performance in older adults. Clin Interv Aging. 2016;11:603–613. doi: 10.2147/CIA.S100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sonnet M-H, Montaut-Verient B, Niemier J-Y et al. Cognitive Abilities and Quality of Life After Cochlear Implantation in the Elderly. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2017;38:e296–e301. doi: 10.1097/MAO.0000000000001503. [DOI] [PubMed] [Google Scholar]
- 244.Jayakody DM P, Friedland P L, Nel E et al. Impact of Cochlear Implantation on Cognitive Functions of Older Adults: Pilot Test Results. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2017;38:e289–e295. doi: 10.1097/MAO.0000000000001502. [DOI] [PubMed] [Google Scholar]
- 245.Mertens G, Andries E, Claes A J et al. Cognitive Improvement After Cochlear Implantation in Older Adults With Severe or Profound Hearing Impairment: A Prospective, Longitudinal, Controlled, Multicenter Study. Ear Hear. 2021;42:606–614. doi: 10.1097/AUD.0000000000000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Völter C, Götze L, Bajewski M et al. Cognition and Cognitive Reserve in Cochlear Implant Recipients. Front Aging Neurosci. 2022;14:838214. doi: 10.3389/fnagi.2022.838214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Völter C, Götze L, Haubitz I et al. Impact of Cochlear Implantation on Neurocognitive Subdomains in Adult Cochlear Implant Recipients. Audiol Neurootol. 2021;26:236–245. doi: 10.1159/000510855. [DOI] [PubMed] [Google Scholar]
- 248.Völter C, Götze L, Dazert S et al. Can cochlear implantation improve neurocognition in the aging population? Clin Interv Aging. 2018;13:701–712. doi: 10.2147/CIA.S160517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Huber M, Roesch S, Pletzer B et al. Can Cochlear Implantation in Older Adults Reverse Cognitive Decline Due to Hearing Loss? Ear Hear. 2021;42:1560–1576. doi: 10.1097/AUD.0000000000001049. [DOI] [PubMed] [Google Scholar]
- 250.Knopke S, Schubert A, Häussler S M et al. Improvement of Working Memory and Processing Speed in Patients over 70 with Bilateral Hearing Impairment Following Unilateral Cochlear Implantation. J Clin Med. 2021;10:3421. doi: 10.3390/jcm10153421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sarant J, Harris D, Busby P et al. The Effect of Cochlear Implants on Cognitive Function in Older Adults: Initial Baseline and 18-Month Follow Up Results for a Prospective International Longitudinal Study. Front Neurosci. 2019;13:789. doi: 10.3389/fnins.2019.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhan K Y, Lewis J H, Vasil K J et al. Cognitive Functions in Adults Receiving Cochlear Implants: Predictors of Speech Recognition and Changes After Implantation. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2020;41:e322–e329. doi: 10.1097/MAO.0000000000002544. [DOI] [PubMed] [Google Scholar]