Objective
The aim of this study was to evaluate whether using surface neuromuscular electrical stimulation (NMES) for paralyzed lower-limb muscles results in an increase in energy expenditure and whether the number of activated muscles and duty cycle affect the potential increase.
Design
This was a cross-sectional study.
Results
Energy expenditure during all NMES protocols was significantly higher than the condition without NMES (1.2 ± 0.2 kcal/min), with the highest increase (+51%; +0.7 kcal/min, 95% confidence interval, 0.3–1.2) for the protocol with more muscles activated and the duty cycle with a shorter rest period. A significant decrease in muscle contraction size during NMES was found with a longer stimulation time, more muscles activated, or the duty cycle with a shorter rest period.
Conclusion
Using NMES for paralyzed lower-limb muscles can significantly increase energy expenditure compared with sitting without NMES, with the highest increase for the protocol with more muscles activated and the duty cycle with a shorter rest period. Muscle fatigue occurred significantly with the more intense NMES protocols, which might cause a lower energy expenditure in a longer protocol. Future studies should further optimize the NMES parameters and investigate the long-term effects of NMES on weight management in people with SCI.
Key Words: Spinal Cord Injury, Neuromuscular Electrical Stimulation, Lower-Limb Muscles, Energy Expenditure
What Is Known
Neuromuscular electrical stimulation (NMES) could lead to a significant increase in energy expenditure for weight management in able-bodied people. Using NMES to activate paralyzed lower-limb muscles for increasing energy expenditure and subsequently weight management in people with spinal cord injury has not been fully investigated.
What Is New
Using NMES for paralyzed lower-limb muscles can significantly increase energy expenditure with the highest increase for the protocol with more muscles activated and the duty cycle with a shorter rest period (+51%).
Muscle fatigue occurred significantly with the more intense NMES protocols.
Future studies should further optimize the NMES parameters and investigate the long-term effects of NMES.
Obesity is a common secondary health complication in people with spinal cord injury (SCI), with about two of every three persons with SCI being obese and at risk for the metabolic consequences of obesity.1 Therefore, it is necessary to reach a healthy energy balance and prevent weight gain or achieve weight loss, respectively, in people with SCI.
Reducing energy intake and increasing energy expenditure or a combination are the possibilities of reaching a healthy energy balance. The SCI affects resting energy expenditure, which is markedly lower (14%–27%) in people with SCI compared with the able-bodied population.2 Resting energy expenditure is the greatest proportion (60%–80%) of the total daily energy expenditure especially in very sedentary individuals.3,4 Because resting energy expenditure is low in SCI, rather extreme dietary requirements, with very low energy intake, might cause a lower suboptimal protein and micronutrient intake, resulting in a higher risk of malnutrition and subsequent health complications.5 The other way to achieve a healthy energy balance, increasing energy expenditure, might therefore be more rational. By participating in physical exercises such as arm cranking, weight training, or wheelchair sports, people with SCI can gain muscle mass and strength and reduce fat mass.6,7 However, not all people with SCI are able or inclined to engage in physical exercise and it was also reported that the energy expenditure in response to exercises could be rather limited in people with SCI, especially in those with a high degree of paralysis,1,8 making it difficult to sufficiently increase energy expenditure using only the nonparalyzed muscles.
Activating the larger paralyzed muscles below the lesion using surface neuromuscular electrical stimulation (NMES) or functional electrical stimulation is a possible way to increase energy expenditure without many practical barriers and much discomfort. Although functional electrical stimulation–induced cycling and rowing have been reported to increase energy expenditure and muscle mass in the lower limbs in people with SCI,9,10 some drawbacks such as the adequate time for training responses to occur and the extra set-up time and professional assistance still limit its availability and accessibility in daily use.11,12 Compared with functional electrical stimulation, NMES is a different form of paralyzed muscle activation that could be less time-consuming and could even be applied during daily activities or during the night without the requirement of external equipment other than a portable stimulator and electrodes.11,13 If this form of NMES-induced contractions could lead to an increased energy expenditure, this method could be beneficial to long-term weight management in people with SCI.
Different stimulation parameters such as the target muscles and duty cycle can induce different effects.14 The lower-limb muscles, including gluteals, hamstrings, quadriceps, and calves, are naturally exposed to muscle atrophy owing to the loss of central activation and immobilization after SCI.15,16 Activating these large, clinically important muscles may result in a significant increase in energy expenditure.17 Another important stimulation parameter is duty cycle. Duty cycle describes the actual muscle activation and rest periods of an NMES program. Theoretically, duty cycle with a shorter rest period could induce more intense muscle contractions and subsequently contribute to a higher increase in energy expenditure.18 However, it could also lead to greater muscle fatigue, which reduces energy expenditure and the efficacy of NMES.18,19 It is still not clear what is the optimal stimulation-rest ratio to increase energy expenditure for people with SCI.
Lower-limb muscle fatigue can also influence the stimulation effects. With NMES, it was reported that selective recruitment of the large and fast motor units would result in a more rapid fatigue than with voluntary exercise because the axons of the larger motor units have less resistance to electrical current. Thus, the predominance activation of type II fibers in people with SCI during NMES could lead to greater muscle fatigue.14 Relevant factors such as training history, percentage of type II fibers, time since injury, and placement of electrodes could cause a different time to fatigue during NMES.14,18 For hamstrings and quadriceps, changes in muscle contraction size, measured by a muscle contraction sensor, can be an indicator of muscle fatigue.13 For gluteals, studies have shown that utilizing NMES to activate gluteals could result in a sizeable sitting pressure reduction below the ischial tuberosity and a redistribution of sitting pressure away from the ischial tuberosity area, which could contribute to a lower sitting pressure variance.19–23 Based on the rationale of sitting pressure relief by NMES, it is expected that when muscle fatigue occurs, muscle contractions will become less powerful and subsequently cause less reduction in sitting pressure variance.18,19 Because muscle fatigue can remarkably reduce the efficacy of NMES,14,24 it is important to detect possible muscle fatigue during NMES.
For able-bodied individuals, studies have shown that NMES could lead to a significant increase in energy expenditure for weight management.25,26 However, using NMES to activate paralyzed lower-limb muscles for increasing energy expenditure and, subsequently, weight management in people with SCI has not been fully investigated.17,27 The purpose of this study, therefore, was to evaluate whether using NMES for paralyzed lower-limb muscles results in a significant increase in energy expenditure compared with a no NMES condition and how the number of activated muscles (gluteals, hamstrings, quadriceps, and calves vs. gluteals and hamstrings only) and duty cycle (1:4 s vs. 1:8 s) affect this increase.
METHODS
Participants
Nine men with an SCI for at least 6 mos and untrained for NMES participated in this study. Exclusion criteria were pressure sores, a flaccid paralysis, a known intolerance for NMES, a history of severe autonomic dysreflexia, or severe cognitive or communicative disorders. This study was approved by the Medical Ethical Committee of Vrije Universiteit Amsterdam Medical Center (NL22712.029.08) and Reade, center for rehabilitation and rheumatology. All participants signed an informed consent before the start of the experiments. This study conforms to all STROBE guidelines and reports the required information accordingly (see Supplementary Checklist, Supplemental Digital Content 1, http://links.lww.com/PHM/B869).
Design
There were five test conditions, including one without NMES and four with NMES. Energy expenditure without NMES was measured in a resting sitting position for 5 mins before NMES protocols started. Participants received NMES during four different 10-min protocols while sitting still on their wheelchair. The stimulated muscles were gluteals (Gl), hamstrings (Ham), quadriceps (Qua), and calves (Ca) vs. Gl and Ham only. The duty cycle was 1:4 s or 1:8 s. Energy expenditure was measured continuously during all conditions, that is, with and without NMES. The order in which the NMES protocols were provided was randomized by simple randomization to avoid the effect of muscle fatigue. Muscle fatigue was measured by the changes in the muscle contraction size and sitting pressure variance. During all measurements, participants were instructed to sit as still as possible and not to speak. The placement of the electrodes for NMES is shown in Figure 1.
FIGURE 1.
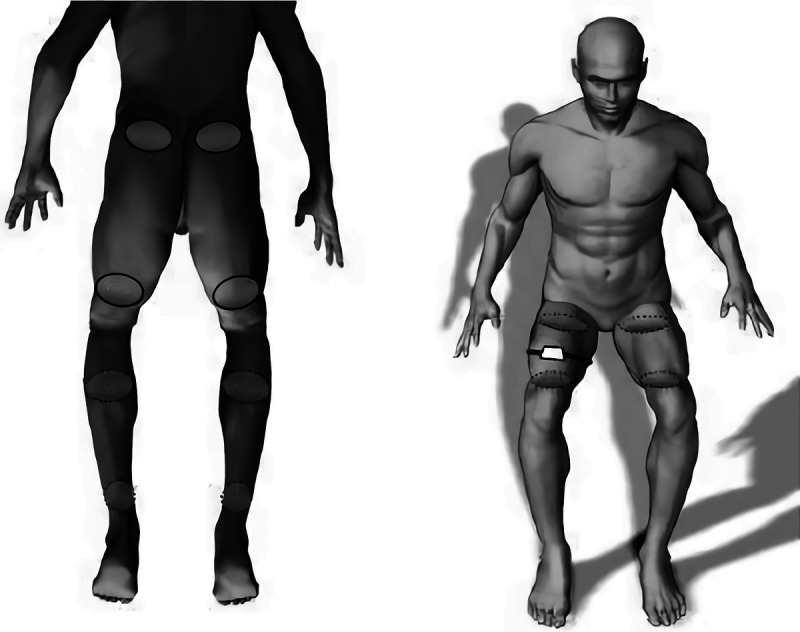
The placement of the electrodes for gluteals, hamstrings (electrodes with solid outline, left), calves (electrodes with dashed outline, left) stimulation and for quadriceps stimulation (electrodes with dashed outline, right) and the contraction sensor (right).
Electrical Stimulation
The muscles were electrically stimulated with a biphasic, squared, and balanced stimulation pattern provided by a portable eight-channel stimulator (NeuroPro, Berkelbike BV, Sint-Michielsgestel, the Netherlands). To induce a tetanic muscle contraction and activate a maximum number of muscle fibers with less chance of skin pain, a stimulation frequency of 70 Hz and a pulse duration of 0.5 msecs were applied.28,29 Before starting the actual NMES protocols, the stimulation current amplitude was determined for each participant and muscle group separately. Current amplitude (35–120 mA) was individually set to induce visible muscle contractions at comfortable levels, but low enough to prevent very strong contractions that could potentially disturb daily activity performance or even force the participant out of the wheelchair. In this way, a clinically relevant current level was achieved. To avoid unwanted movements (knee extension) during NMES, the feet were fixed to the footrests of the wheelchair with a Velcro strap. The duration of each NMES protocol was 10 mins, followed by a 10-min rest.
Energy Expenditure
The primary outcome in this study was the energy expenditure calculated from the oxygen consumption (VO2) and carbon dioxide production (VCO2) gathered with an on-line gas analysis system (Oxycon alpha, Mijnhardt BV, Bunnik, the Netherlands). Gas and volume calibration was performed before each measurement. All the measurements were done under the same conditions. The energy expenditure in kcal/min was calculated using the Weir equation.30
Energy expenditure (kcal/min) = 3.94 × VO2 + 1.11 × VCO2
With Matlab software (R2018b), mean energy expenditure was calculated for rest without NMES during the last 4 mins of the resting condition (the first minute was discarded to allow for determining energy expenditure in a stable state) and during the last 7 mins of each NMES protocol (the first 3 mins were discarded to allow for determining energy expenditure in a stable state).
Muscle Fatigue
When muscles fatigue, the muscle contractions in response to NMES become less powerful. A muscle contraction results in an increase in the muscle’s circumference compared with rest. Therefore, a system was developed to monitor the changes in the circumference of the upper leg to evaluate the degree of fatigue of the hamstrings and quadriceps during the NMES protocols. A Futek load cell (LSB200, 25 lb, JR S-beam load cell), measuring forces in one direction during rest and muscle contractions, was integrated into a Velcro strap with an elastic end and placed around the right upper leg (Fig. 1, right). When muscles contract because of NMES, the upper-leg muscle circumference increases as the muscles become shorter and thicker, and the elastic part will be more elongated, resulting in a force difference on the load cell. With muscle fatigue negatively affecting muscle contractions, a less pronounced change in muscle circumference and, subsequently, a smaller force difference will occur. The data were obtained with a data acquisition system of National Instruments (NI USB-6009 DAQ) and LabView software (Student Edition 8.2).
A second parameter to estimate fatigue of the gluteals and hamstrings was sitting pressure variance, measured using a pressure mapping system (mFLEX, Vista Medical, Canada). This system measures the pressure of an area of 533 × 533 mm with a total of 1024 (32 × 32) pressure sensors and was placed on the cushion of the participant’s wheelchair. During a nonactive period, most pressure will be located around the buttocks. When the gluteals and hamstrings contract, the total pressure will be more distributed to the surrounding area and will therefore result in a lower pressure variance during an activation period than in rest.19 Fatigue will make the muscle contractions less powerful, resulting in less pronounced changes of pressure distribution and therefore less decrease in pressure variance. This method was applied only for the protocols with gluteal and hamstring activation because activation of quadriceps and calves lifts the legs and has a disruptive effect on sitting pressure variance. With Matlab software, the changes in sitting pressure variance were analyzed.
The measurements of muscle contraction size and sitting pressure variance were started at least 1 min before the first NMES protocol started. The start and end time of each NMES protocol was recorded. The raw force data were filtered with a second-order Butterworth band-pass filter (0.05–0.8 Hz). Matlab software was used to further analyze the data. For muscle contraction size, all the peak and valley values of force in each protocol were determined at first. The peak values right before the valley values were then selected to calculate the force differences between them. The force differences were used to make a general fit exponential model with y = ae-bx + c-ed function to check the values at specific time. Subsequently, the force differences at the start (5 s) and at 3 and at 10 mins in each NMES protocol were determined. A higher force difference was associated with a larger change in muscle contraction size. Percentages of the difference at 3 and 10 mins relative to the start value were calculated to indicate the changes in muscle contraction size.
Similarly, all the peak and valley values of sitting pressure variance in each protocol were determined and the differences between them were used to make a general fit exponential model with y = ae-bx + c-ed function to check the values at specific time. Subsequently, these differences at the start (5 s) and at 3 and at 10 mins in each NMES protocol were determined. A higher difference was associated with a higher decrease in pressure variance. Percentages of the difference at 3 and 10 mins relative to the start value were calculated to indicate the changes in sitting pressure variance. Figure 2 shows the examples of the changes in muscle contraction size and sitting pressure variance during the NMES protocols.
FIGURE 2.
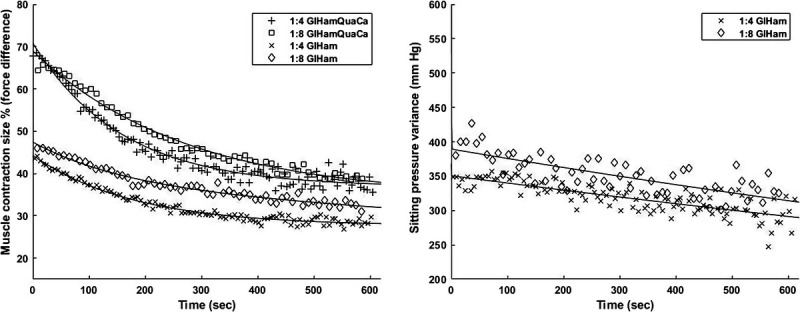
Examples of the changes in muscle contraction size (left, participant 4) and sitting pressure variance (right, participant 3) during NMES protocols. Lines are fit through the data by a general fit exponential model with y = ae-bx + c-ed function. Most of the participants showed similar trends as illustrated above.
Statistical Analysis
The normality of the data was checked by Shapiro-Wilk tests and Q-Q plots and all data were normally distributed (or approximately). Repeated-measures analysis of variance together with post hoc tests was used to check the systematic differences between (1) energy expenditure without and with NMES during all protocols; (2) energy expenditure with NMES in different protocols; (3) the muscle contraction size during all protocols and the interaction effects (2 × 2 × 2, stimulation time, muscles and duty cycle); and (4) the sitting pressure variance during the 1:4 s GlHam and 1:8 s GlHam protocols and the interaction effects (2 × 2, stimulation time, duty cycle). All statistical analyses were performed using SPSS software (version 25, IBM, Somers, NY). A P value <0.05 was considered significant.
RESULTS
Descriptives
Table 1 shows the descriptives of all participants. Because of measurement errors, the sitting pressure variance data of two participants were removed for analysis.
TABLE 1.
Descriptives of participants (n = 9)
| Participant | Age, y | Body Mass, kg | Height, m | Time Since Injury, yrs | Lesion Level | AIS | Current Amplitude | ||
|---|---|---|---|---|---|---|---|---|---|
| Gl Ham, mA | Qua, mA | Ca, mA | |||||||
| 1 | 58 | 86 | 1.85 | 1.5 | C3–4 | B | 120 | 100 | 100 |
| 2 | 31 | 63 | 1.75 | 9 | C5 | A | 90 | 80 | 90 |
| 3 | 49 | 63 | 1.87 | 22 | C5–6 | A | 60 | 90 | 50 |
| 4 | 24 | 80 | 1.91 | 8 | C6 | B | 75 | 90 | 70 |
| 5 | 41 | 81 | 1.70 | 14 | C6–7 | B | 40 | 50 | 35 |
| 6 | 31 | 70 | 1.80 | 5 | C7–8 | A | 60 | 60 | 40 |
| 7 | 28 | 92 | 1.86 | 4 | T3–4 | A | 70 | 70 | 65 |
| 8 | 66 | 120 | 1.73 | 4 | T9 | B | 50 | 40 | 45 |
| 9 | 29 | 73 | 1.84 | 11 | T11 | A | 45 | 60 | 60 |
| Mean (SD) | 40 (15) | 81 (18) | 1.81 (0.71) | 9 (6) | 68 (25) | 71 (20) | 62 (22) | ||
Lesion level: C, cervical; T, thoracic.
AIS indicates American Spinal Injury Association impairment scale; Gl, gluteals; Ham, hamstrings; Qua, quadriceps; Ca, calves.
Changes in Energy Expenditure Among Different Protocols
Energy expenditure (kcal/min) with or without NMES is shown in Figure 3. All NMES protocols resulted in a significant increase in energy expenditure (1:4 s GlHamQuaCa: +51%; 1:8 s GlHamQuaCa: +44%; 1:4 s GlHam: +36%; 1:8 s GlHam: +25%) compared with the energy expenditure without NMES (1.2 ± 0.2 kcal/min). Three participants showed a very positive increase in energy expenditure compared with other participants, with the highest increase (+106%, +171%, and +55%) during the protocol 1:4 s GlHamQuaCa (Fig. 4). When comparing between different protocols, the protocol 1:4 s GlHamQuaCa and 1:8 s GlHamQuaCa with more muscles activated showed a significantly higher increase in energy expenditure compared with the protocol 1:4 s GlHam (+11%) and 1:8 s GlHam (+15%), respectively. However, no significant difference was found between the protocol 1:8 s GlHamQuaCa and 1:4 s GlHam. Meanwhile, the protocol 1:4 s GlHam showed a significantly higher increase in energy expenditure than the protocol 1:8 s GlHam (+9%). Such difference was not found between the protocol 1:4 s GlHamQuaCa and 1:8 s GlHamQuaCa (Fig. 3).
FIGURE 3.
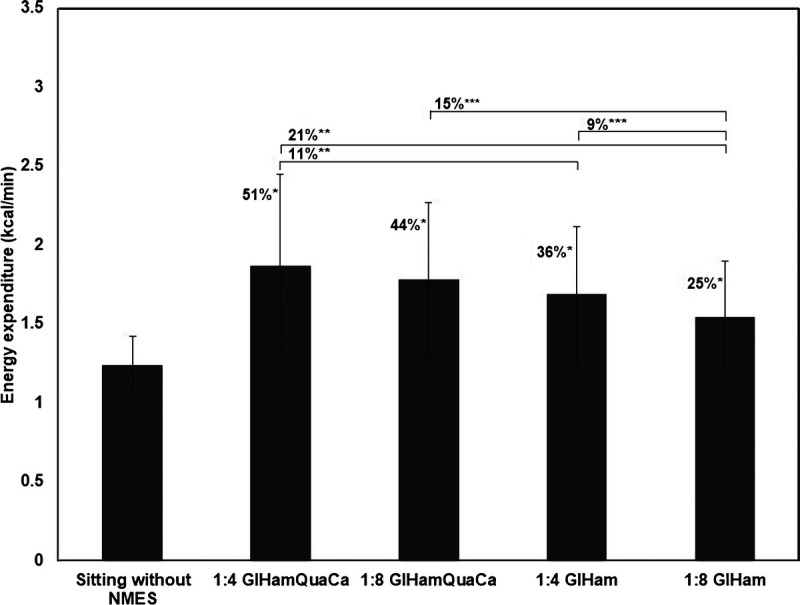
Mean energy expenditure (kcal/min) during sitting without NMES and during different NMES protocols (n = 9). *P < 0.05 compared with the mean energy expenditure during sitting without NMES; **P < 0.05 compared with the mean energy expenditure during the protocol 1:4 s GlHamQuaCa; ***P < 0.05 compared with the mean energy expenditure during the protocol 1:8 s GlHam.
FIGURE 4.
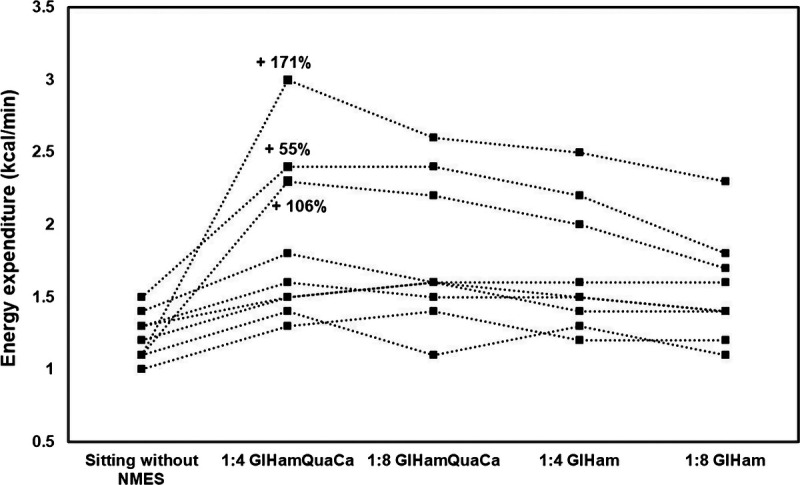
Individual differences in energy expenditure (kcal/min) during the different NMES protocols (n = 9). Percentages indicate the three participants who have very positive increases in energy expenditure during the protocol 1:4 s GlHamQuaCa.
The protocol with more muscles activated and the duty cycle with a shorter rest period (1:4 s GlHamQuaCa) showed the largest increase (+51%) in energy expenditure (+0.7 kcal/min, 95% confidence interval, 0.3–1.2), whereas the protocol with fewer muscles activated and the duty cycle with a longer rest period (1:8 s GlHam) showed the smallest increase (+25%) in energy expenditure (+0.4 kcal/min, 95% confidence interval, 0.1–0.6).
Muscle Fatigue
As shown in Table 2, a significantly larger decrease in the muscle contraction size was found with a longer stimulation time, more muscles activated, or the duty cycle with a shorter rest period, respectively (main effect). However, no interaction effect was found.
TABLE 2.
Mean of the muscle contraction size at 3 and 10 mins compared with the start value in different NMES protocols and the main and interaction effects of all the variables (n = 9)
| Protocols | Muscle Contraction Size (% of Start), Mean (SD) | P (Main Effect) | P (Interaction Effect) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ST | M | DC | ST × M | ST × DC | M × DC | ST × M × DC | |||
| 3 mins | 10 mins | ||||||||
| 1:4 s GlHam QuaCa | 58 (19) | 42 (19) | <0.001 | 0.04 | <0.001 | 0.58 | 0.62 | 0.51 | 0.86 |
| 1:8 s GlHam QuaCa | 76 (14) | 62 (16) | |||||||
| 1:4 s GlHam | 64 (21) | 46 (25) | |||||||
| 1:8 s GlHam | 86 (13) | 71 (22) | |||||||
Gl indicates gluteals; Ham, hamstrings; Qua, quadriceps; Ca, calves; ST, stimulation time; M, muscles; DC, duty cycle.
Table 3 shows the changes in the sitting pressure variance of gluteals and hamstrings at 3 and at 10 mins. A significantly larger decrease in the sitting pressure variance was found with a longer stimulation time but not with the duty cycle with a shorter rest period (main effect). No interaction effect was found.
TABLE 3.
Mean of the sitting pressure variance at 3 and 10 mins compared with the start value in different NMES protocols and the main and interaction effects of all the variables (n = 7)
| Protocols | Sitting Pressure Variance (% of Start), Mean (SD) | P (Main Effect) | P (Interaction Effect) | ||
|---|---|---|---|---|---|
| Stimulation Time | Duty Cycle | Stimulation Time × Duty Cycle | |||
| 3 mins | 10 mins | ||||
| 1:4 s GlHam | 93 (8) | 79 (22) | 0.03 | 0.64 | 0.64 |
| 1:8 s GlHam | 94 (6) | 82 (18) | |||
Gl indicates gluteals; Ham, hamstrings.
DISCUSSION
This study showed that using NMES for paralyzed lower-limb muscles in people with SCI resulted in a significant increase in energy expenditure compared with sitting without NMES. The protocol with more muscles activated and the duty cycle with a shorter rest period showed the largest increase in energy expenditure. Based on muscle contraction size and sitting pressure variance, muscle fatigue occurred significantly with the more intense protocols, which might be caused by a lower energy expenditure in a longer protocol.
In this study, the protocol 1:4 s GlHamQuaCa showed the largest increase in energy expenditure. Compared with a similar study, Woelfel et al.17 found a much larger increase in energy expenditure by applying NMES of quadriceps and hamstrings at low frequency (1 and 3 Hz) twitches with 50 and 100 mA current amplitude in 10 participants with motor complete SCI. Although that finding is in line with the studies that have recommended using a lower frequency while providing NMES in people with SCI,14,27 it is still somewhat surprising because the very low frequencies (1 and 3 Hz) only induced twitches without the recruitment of all the muscle fibers.17 One of the reasons could be, as reported by Petrie et al.,31 that some key genes associated with oxidative transcription showed a fivefold to sixfold increase during the lower-frequency (5 Hz) stimulation session compared with the higher-frequency (20 Hz) session in people with motor complete injury. Another reason might be that utilizing a low current frequency could attenuate the process of rapid muscle fatigue during NMES, which is the main obstacle that hinders the efficacy of NMES in people with SCI because the recruitment pattern of motor units by NMES would likely result in slightly greater fatigue than with voluntary contractions.14 Furthermore, because the recruitment of fast motor units would depend on the percentage of fast motor units in the muscles being stimulated,14 individuals with a different training history, percentage of type II fibers, or time since injury may have a different time to fatigue during NMES.14,32 A third reason could be the position and numbers of electrodes during NMES. Four electrodes per leg were adhered over the quadriceps and hamstrings in the study of Woelfel et al.,17 whereas in this study, two electrodes per leg were adhered over quadriceps and four electrodes per leg were adhered over gluteals and hamstrings together. This might cause different responses to NMES.14,25 Despite the fact that tetanic contractions were induced in this study, even in the most intense protocol, clear muscle contractions were found until the end. Thus, although muscle fatigue occurred in this study, the lower-limb muscles might still be further stimulated to gain more potential effects after 10 mins. In a recent study, Barton et al.33 tested the effects of 12 wks of daily gluteal and hamstring NMES in people with SCI; with 50 Hz frequency and 1:4 s duty cycle applied, participants were able to activate the lower-limb muscles with an adapted wearable clothing garment for 6 hrs per day without any adverse events and subsequently gained positive effects of increasing thigh circumference and improving the risk factors for developing pressure ulcers. Besides, a recent study showed that by using NMES-shorts to stimulate gluteals, hamstrings, and quadriceps with 35 Hz frequency and 1:4 s duty cycle, it was feasible to activate these muscles for 8 hrs overnight and it improved participants’ sleep quality with good usability.13 These advantages would likely increase participants’ adherence to the NMES regimen, which is important to gain and sustain adequate long-term benefits to weight management.
Another parameter that might influence the increase in energy expenditure during NMES is the duty cycle. Although the 1:4 s duty cycle induced a greater decline in muscle contraction size, it resulted in a larger increase in energy expenditure compared with the 1:8 s duty cycle. Dreibati et al. reported that in able-bodied adults, increasing the rest time and decreasing the NMES frequency might be beneficial for clinical rehabilitation programs because it could attenuate muscle fatigue. However, that study did not compare different duty cycles.18 A previous study investigated the effects of duty cycle (1:1 s vs. 1:4 s) on the interface pressure distribution and subsequent muscle fatigue during NMES-induced gluteal and hamstring activation in people with SCI and concluded that the 1:4 s duty cycle was recommended because of the less fatiguing effect.19 That finding indicated the advantage of increasing the rest time during NMES. Based on the results of this study, although the 1:4 s duty cycle caused a significant decrease in muscle contraction size, the sitting pressure variance was not significantly different between the 1:4 s and 1:8 s duty cycles. As clear muscle contractions were found until the end of each protocol, the 1:4 s duty cycle could be more effective because it induced a higher increase in energy expenditure compared with the 1:8 s duty cycle. However, because a significant decrease in muscle contraction size might cause a lower energy expenditure, this finding needs to be confirmed in a longer NMES protocol.
Three participants showed very positive responses, whereas others only showed limited responses to NMES, which caused a rather lower increase in energy expenditure on a group level (Fig. 4). The reason for this finding might be associated with the stimulation intensity. As the current amplitude was individually set to induce visible muscle contractions at comfortable levels, the stimulation intensities of the three participants mentioned above were overall higher than the others. Time since injury might be another reason because three participants who showed limited responses to NMES had a longer duration of injury. It was reported that even oxidative slow-twitch fibers transitioned to a glycolytic fast-twitch phenotype, which indicated the decrements in muscle oxidative capacity and losses in fatigue resistance in people with chronic SCI.32 Thus, through the combined effects of prolonged muscle atrophy and muscle phenotypic shift, people with a longer duration of injury without exercise or training might show less responses to NMES.32 Lesion completeness could hypothetically be another reason to explain the lower increase in energy expenditure.14 Except for one participant, the other three participants with American Spinal Injury Association impairment scale B showed limited responses to NMES probably because they still had some sensory function, and thus, a high stimulation current amplitude could not be utilized. Besides, it should also be noted that NMES in this study was applied in NMES-untrained people, and with long-term training, the fatigue resistance of lower-limb muscles could be improved, which would increase the efficacy of NMES.34
To determine whether the increase in energy expenditure is helpful for weight management, an estimation of the energy excess was made using the results found in a previous study.35 It was reported that body mass increased on average by 1.36 kg each year after injury. Assuming that this gain is caused by an increase in fat mass, the surplus of energy intake is 12,240 kcal/yr or 34 kcal/day. Thus, theoretically, when extrapolating using the protocol 1:4 s GlHamQuaCa, 49 mins of daily stimulation could presumably reach that goal for weight management, which seems a feasible time to achieve. It should be noted, however, that this is an extrapolation based on the 10-min protocols, and longer protocols and long-term effects of NMES should be evaluated to determine a more accurate estimation of daily stimulation time. When extrapolating the estimated energy excess to the study of Woelfel et al., the efficacy (1 Hz: 1 hr/day for 203 days; 3 Hz: 20 min/day for 305 days) was higher than the estimation above in this study because they found a larger increase in energy expenditure in their protocols. However, their measurement protocols are shorter (6 mins), and it is unknown whether the status of oxygen consumption would be sustained for a longer period. When comparing with other exercise methods to increase energy expenditure for people with SCI, it seems obvious that arm cranking (32 W, 3.62–4.12 kcal/min), weight training (2.44–3.65 kcal/min), and functional electrical stimulated cycling (4.8 kcal/min) could induce higher levels of energy expenditure than the protocol 1:4 s GlHamQuaCa (1.9 kcal/min) in this study.8,12 This protocol, however, still has great potential for weight management because it is low risk, low cost, and feasible for at-home use without any transfer, and more importantly, it is not an exercise or training and could be used during other daily activities or even during the night without much interference.13 This can be very beneficial to long-term weight management in people with SCI.
A limitation of this study is the small sample size (n = 9). This could lead to a potential bias of the effects of NMES because three participants showed very positive results while others showed somewhat limited responses. Spasticity should also be considered because of its positive effects on muscle endurance and energy expenditure.36 Measuring spasticity by the modified Ashworth scale might better explain the variability in the effect of NMES on energy expenditure. Furthermore, the 10-min NMES protocols in this study were relatively short compared with previous studies.18,25 Considering that the lower-limb muscles were still capable of contracting in the last phase of each protocol, it would be helpful to investigate how the muscles would react and how the status of muscle fatigue would change during longer protocols. However, no validity and reliability studies exist regarding muscle contraction size and sitting pressure variance measurements, making it difficult to accurately estimate muscle fatigue during NMES.
Future studies with a larger sample size are needed to further improve the important parameters for NMES protocol, including current frequency and intensity, duty cycle, fatigue status, and duration of each session. If the sample size was large enough, some subgroups of people with SCI (eg, age, motor completeness, and time since injury) could be analyzed separately to determine the optimal NMES parameters for different subgroups. Measurements such as muscle volumes, perimeter, and length of limbs could give more insight into the optimal stimulation intensity of NMES for increasing energy expenditure in people with SCI. Besides, the training effects of NMES such as muscle hypertrophy and improved muscle fatigue resistance and how long-term NMES could contribute to weight management in people with SCI should be investigated.
CONCLUSIONS
Using NMES for paralyzed lower-limb muscles in people with SCI can significantly increase energy expenditure compared with sitting without NMES. Muscle fatigue occurred significantly with the more intense NMES protocols, which might cause a lower energy expenditure in a longer protocol. The protocol 1:4 s GlHamQuaCa showed the largest increase (+51%) in energy expenditure. Future studies should evaluate the effects of lower-limb NMES in a larger sample and further optimize NMES parameters (frequency, intensity, and duty cycle) to achieve a better efficacy as well as investigate the long-term effects of NMES on weight management in people with SCI.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the rehabilitation center Reade in Amsterdam which has contributed to the organization of measurements. They also thank China Scholarship Council (CSC), which has supported the PhD career of the corresponding author. The authors appreciate the cooperation of all the participants and the master’s students who performed the measurements.
Footnotes
Data availability: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions: YM: software, validation, formal analysis, data curation, writing–original draft, writing–review and editing, visualization. SdG: methodology, formal analysis, data curation, writing–review and editing, visualization, supervision. AV: software, validation, resources, investigation, formal analysis, writing–original draft, writing–review and editing. WH: software, validation, resources, investigation, formal analysis, writing–original draft, writing–review and editing. CAJS: writing–review and editing. JMS-S: writing–review and editing. PJMW: formal analysis, data curation, writing–review and editing, visualization, supervision. TWJJ: conceptualization, resources, formal analysis, data curation, writing–review and editing, visualization, supervision.
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ajpmr.com).
Contributor Information
Yiming Ma, Email: yiming.ma@vu.nl;y.ma@reade.nl.
Sonja de Groot, Email: s.d.groot@reade.nl.
Ad Vink, Email: vinkad@hotmail.com.
Wouter Harmsen, Email: w.harmsen@kennisinstituut.nl.
Christof A.J. Smit, Email: c.smit@tolbrug.nl.
Janneke M. Stolwijk-Swuste, Email: j.stolwijk@dehoogstraat.nl.
Peter J.M. Weijs, Email: p.j.m.weijs@hva.nl.
Thomas W.J. Janssen, Email: t.w.j2.janssen@vu.nl.
REFERENCES
- 1.Gater DR, Jr.: Obesity after spinal cord injury. Phys Med Rehabil Clin N Am 2007;18:333–51, vii [DOI] [PubMed] [Google Scholar]
- 2.Buchholz AC, Pencharz PB: Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care 2004;7:635–9 [DOI] [PubMed] [Google Scholar]
- 3.Farkas GJ, Pitot MA, Gater DR, Jr.: A systematic review of the accuracy of estimated and measured resting metabolic rate in chronic spinal cord injury. Int J Sport Nutr Exerc Metab 2019;29:548–58 [DOI] [PubMed] [Google Scholar]
- 4.Tremblay A, Despres JP, Bouchard C: The effects of exercise-training on energy balance and adipose tissue morphology and metabolism. Sports Med 1985;2:223–33 [DOI] [PubMed] [Google Scholar]
- 5.Chen Y Henson S Jackson AB, et al. : Obesity intervention in persons with spinal cord injury. Spinal Cord 2006;44:82–91 [DOI] [PubMed] [Google Scholar]
- 6.Gorla JI Costa e Silva Ade A Borges M, et al. : Impact of wheelchair rugby on body composition of subjects with tetraplegia: a pilot study. Arch Phys Med Rehabil 2016;97:92–6 [DOI] [PubMed] [Google Scholar]
- 7.van der Scheer JW Martin Ginis KA Ditor DS, et al. : Effects of exercise on fitness and health of adults with spinal cord injury: a systematic review. Neurology 2017;89:736–45 [DOI] [PubMed] [Google Scholar]
- 8.Collins EG Gater D Kiratli J, et al. : Energy cost of physical activities in persons with spinal cord injury. Med Sci Sports Exerc 2010;42:691–700 [DOI] [PubMed] [Google Scholar]
- 9.Griffin L Decker MJ Hwang JY, et al. : Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 2009;19:614–22 [DOI] [PubMed] [Google Scholar]
- 10.Kim DI Park DS Lee BS, et al. : A six-week motor-driven functional electronic stimulation rowing program improves muscle strength and body composition in people with spinal cord injury: a pilot study. Spinal Cord 2014;52:621–4 [DOI] [PubMed] [Google Scholar]
- 11.Carty A McCormack K Coughlan GF, et al. : Increased aerobic fitness after neuromuscular electrical stimulation training in adults with spinal cord injury. Arch Phys Med Rehabil 2012;93:790–5 [DOI] [PubMed] [Google Scholar]
- 12.Perret C Berry H Hunt KJ, et al. : Feasibility of functional electrical stimulated cycling in subjects with spinal cord injury: an energetic assessment. J Rehabil Med 2010;42:873–5 [DOI] [PubMed] [Google Scholar]
- 13.Smit CAJ Berenpas F de Groot S, et al. : Feasibility of overnight electrical stimulation-induced muscle activation in people with a spinal cord injury. A pilot study. Spinal Cord Ser Cases 2020;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolbow DR, Holcomb WR, Gorgey AS: Improving the efficiency of electrical stimulation activities after spinal cord injury. Curr Phys Med Rehabil Rep 2014;2:169–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spungen AM Adkins RH Stewart CA, et al. : Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol (1985) 2003;95:2398–407 [DOI] [PubMed] [Google Scholar]
- 16.Gorgey AS, Dudley GA: Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 2007;45:304–9 [DOI] [PubMed] [Google Scholar]
- 17.Woelfel JR Kimball AL Yen CL, et al. : Low-force muscle activity regulates energy expenditure after spinal cord injury. Med Sci Sports Exerc 2017;49:870–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreibati B Lavet C Pinti A, et al. : Influence of electrical stimulation frequency on skeletal muscle force and fatigue. Ann Phys Rehabil Med 2010;53:266–71, 271–7 [DOI] [PubMed] [Google Scholar]
- 19.Smit CA Legemate KJ de Koning A, et al. : Prolonged electrical stimulation-induced gluteal and hamstring muscle activation and sitting pressure in spinal cord injury: effect of duty cycle. J Rehabil Res Dev 2013;50:1035–46 [DOI] [PubMed] [Google Scholar]
- 20.Levine S Kett R Cederna P, et al. : Electrical muscle stimulation for pressure variation at the seating interface. J Rehabil Res Dev 1989;26:1–8 [PubMed] [Google Scholar]
- 21.Levine SP Kett RL Cederna PS, et al. : Electric muscle stimulation for pressure sore prevention: tissue shape variation. Arch Phys Med Rehabil 1990;71:210–5 [PubMed] [Google Scholar]
- 22.Liu LQ Nicholson GP Knight SL, et al. : Pressure changes under the ischial tuberosities of seated individuals during sacral nerve root stimulation. J Rehabil Res Dev 2006;43:209–18 [DOI] [PubMed] [Google Scholar]
- 23.Smit CA Haverkamp GL de Groot S, et al. : Effects of electrical stimulation-induced gluteal versus gluteal and hamstring muscles activation on sitting pressure distribution in persons with a spinal cord injury. Spinal Cord 2012;50:590–4 [DOI] [PubMed] [Google Scholar]
- 24.Gorgey AS Black CD Elder CP, et al. : Effects of electrical stimulation parameters on fatigue in skeletal muscle. J Orthop Sports Phys Ther 2009;39:684–92 [DOI] [PubMed] [Google Scholar]
- 25.Hsu MJ, Wei SH, Chang YJ: Effect of neuromuscular electrical muscle stimulation on energy expenditure in healthy adults. Sensors (Basel) 2011;11:1932–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerjee P Caulfield B Crowe L, et al. : Prolonged electrical muscle stimulation exercise improves strength and aerobic capacity in healthy sedentary adults. J Appl Physiol (1985) 2005;99:2307–11 [DOI] [PubMed] [Google Scholar]
- 27.Gorgey AS Dolbow DR Dolbow JD, et al. : The effects of electrical stimulation on body composition and metabolic profile after spinal cord injury—part II. J Spinal Cord Med 2015;38:23–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hultman E Sjöholm H Jäderholm-Ek I, et al. : Evaluation of methods for electrical stimulation of human skeletal muscle in situ. Pflugers Arch 1983;398:139–41 [DOI] [PubMed] [Google Scholar]
- 29.Moreno-Aranda J, Seireg A: Electrical parameters for over-the-skin muscle stimulation. J Biomech 1981;14:579–85 [DOI] [PubMed] [Google Scholar]
- 30.Weir JB: New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition 1990;6:213–21 [PubMed] [Google Scholar]
- 31.Petrie M, Suneja M, Shields RK: Low-frequency stimulation regulates metabolic gene expression in paralyzed muscle. J Appl Physiol (1985) 2015;118:723–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shields RK: Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle. J Neurophysiol 1995;73:2195–206 [DOI] [PubMed] [Google Scholar]
- 33.Barton T Low DA Thijssen DH, et al. : Twelve-week daily gluteal and hamstring electrical stimulation improves vascular structure and function, limb volume, and sitting pressure in spinal cord injury: a pilot feasibility study. Am J Phys Med Rehabil 2022;101:913–9 [DOI] [PubMed] [Google Scholar]
- 34.Bogdanis GC: Effects of physical activity and inactivity on muscle fatigue. Front Physiol 2012;3:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Groot S Post MW Postma K, et al. : Prospective analysis of body mass index during and up to 5 years after discharge from inpatient spinal cord injury rehabilitation. J Rehabil Med 2010;42:922–8 [DOI] [PubMed] [Google Scholar]
- 36.D’Amico JM Condliffe EG Martins KJ, et al. : Recovery of neuronal and network excitability after spinal cord injury and implications for spasticity. Front Integr Neurosci 2014;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


