Objective
Cortical lesions are common in multiple sclerosis (MS), but their visualization is challenging on conventional magnetic resonance imaging. The uniform image derived from magnetization prepared 2 rapid acquisition gradient echoes (MP2RAGEuni) detects cortical lesions with a similar rate as the criterion standard sequence, double inversion recovery. Fluid and white matter suppression (FLAWS) provides multiple reconstructed contrasts acquired during a single acquisition. These contrasts include FLAWS minimum image (FLAWSmin), which provides an exquisite sensitivity to the gray matter signal and therefore may facilitate cortical lesion identification, as well as high contrast FLAWS (FLAWShco), which gives a contrast that is similar to one of MP2RAGEuni. In this study, we compared the manual detection rate of cortical lesions on MP2RAGEuni, FLAWSmin, and FLAWShco in MS patients. Furthermore, we assessed whether the combined detection rate on FLAWSmin and FLAWShco was superior to MP2RAGEuni for cortical lesions identification. Last, we compared quantitative T1 maps (qT1) provided by both MP2RAGE and FLAWS in MS lesions.
Materials and Methods
We included 30 relapsing-remitting MS patients who underwent MP2RAGE and FLAWS magnetic resonance imaging with isotropic spatial resolution of 1 mm at 3 T. Cortical lesions were manually segmented by consensus of 3 trained raters and classified as intracortical or leukocortical lesions on (1) MP2RAGE uniform/flat images, (2) FLAWSmin, and (3) FLAWShco. In addition, segmented lesions on FLAWSmin and FLAWShco were merged to produce a union lesion map (FLAWSmin + hco). Number and volume of all cortical, intracortical, and leukocortical lesions were compared among MP2RAGEuni, FLAWSmin, and FLAWShco using Friedman test and between MP2RAGEuni and FLAWSmin + hco using Wilcoxon signed rank test. The FLAWS T1 maps were then compared with the reference MP2RAGE T1 maps using relative differences in percentage. In an exploratory analysis, individual cortical lesion counts of the 3 raters were compared, and interrater variability was quantified using Fleiss ϰ.
Results
In total, 633 segmentations were made on the 3 contrasts, corresponding to 355 cortical lesions. The median number and volume of single cortical, intracortical, and leukocortical lesions were comparable among MP2RAGEuni, FLAWSmin, and FLAWShco. In patients with cortical lesions (22/30), median cumulative lesion volume was larger on FLAWSmin (587 μL; IQR, 1405 μL) than on MP2RAGEuni (490 μL; IQR, 990 μL; P = 0.04), whereas there was no difference between FLAWSmin and FLAWShco, or FLAWShco and MP2RAGEuni. FLAWSmin + hco showed significantly greater numbers of cortical (median, 4.5; IQR, 15) and leukocortical (median, 3.5; IQR, 12) lesions than MP2RAGEuni (median, 3; IQR, 10; median, 2.5; IQR, 7; both P < 0.001). Interrater agreement was moderate on MP2RAGEuni (ϰ = 0.582) and FLAWShco (ϰ = 0.584), but substantial on FLAWSmin (ϰ = 0.614). qT1 in lesions was similar between MP2RAGE and FLAWS.
Conclusions
Cortical lesions identification in FLAWSmin and FLAWShco was comparable to MP2RAGEuni. The combination of FLAWSmin and FLAWShco allowed to identify a higher number of cortical lesions than MP2RAGEuni, whereas qT1 maps did not differ between the 2 acquisition schemes.
Key Words: cortical lesions, FLAWS, FLAWShco, FLAWSmin, multiple sclerosis, MRI, MP2RAGE, T1 mapping, 3 T
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system that leads to substantial disability.1 In patients with MS, cortical lesions are frequent,2,3 and baseline cortical lesion number and formation over time predict disability worsening and cognitive decline.4 In patients with clinically isolated syndrome, the presence of a single cortical lesion identifies patients that are at high risk of developing a clinically definite MS in the following years.5 As cortical lesions have not been found on magnetic resonance imaging (MRI) in many diseases mimicking MS, they may help to distinguish MS from neurologic conditions such as migraine or neuromyelitis optica.6 Because of this, cortical lesions have become an integral part of the current MS diagnostic criteria.7
Although MS white matter (WM) lesions are classically seen as periventricular, juxtacortical, infratentorial, or spinal T2 hyperintensities,8 gray matter (GM) lesions are barely visible on conventional 1.5 or 3.0 T MRI sequences.
Increasing the field strength improves the cortical lesion detection rate,9,10 but even on 7 T MR images, only a small proportion of lesions can be identified compared with histological postmortem specimen.11,12 Advanced 3 T MRI techniques such as double inversion recovery (DIR)13 or phase-sensitive inversion recovery (PSIR)14 have shown better results than conventional 3 T MRI to detect cortical lesions, with a higher pathological specificity, but still a poor overall maximum sensitivity of 23% and 24%, respectively.15 Furthermore, DIR was shown to be prone to flow-related artifacts13 and provided a low interrater accordance so that complete agreement between raters was only achieved in 19% of cortical lesions.16 To date, both PSIR and DIR are considered as criterion standard confirmatory sequences for cortical lesion detection in a clinical setting.6,17 Magnetization prepared 2 rapid acquisition gradient echo (MP2RAGE), a self bias-field corrected sequence for improved segmentation and T1 mapping, has shown equal18 or superior19 performance when compared with DIR.
MP2RAGE is a variant of the MPRAGE sequence where, after the inversion of the longitudinal magnetization, 2 images with 2 different inversion times TI1 and TI2 are acquired using rapid gradient echo readout trains.20 By optimizing the sequence parameters, a combination image is produced (MP2RAGEuni), which is free of proton density, T2* contrast, and reception bias-field. Recently, a different optimization of the MP2RAGE pulse sequence known as fluid and white matter suppression (FLAWS) has been proposed,21 which applies a different parameters optimization. The FLAWS sequence provides both WM- and cerebrospinal fluid (CSF)–suppressed 3D high spatial resolution contrasts simultaneously (FLAWS1, acquired at TI1, WM-suppressed; and FLAWS2, acquired at TI2, CSF-suppressed). Given that the 2 contrasts derive from the same acquisition, they naturally offer a near-perfect alignment. Hence, the signal intensities of FLAWS1 and FLAWS2 can be combined in a voxel-based manner to reconstruct multiple contrasts.
In this study, we used 2 reconstructed FLAWS contrasts: (1) FLAWSmin, which suppresses both CSF and WM signal and yields a GM-specific contrast22 that may facilitate cortical lesion identification; and (2) FLAWShco, which obtains a CSF-suppressed, MPRAGE-like, T1-weighted contrast23,24 that could be used similarly as one of the current criterion standards for cortical lesion detection, MPRAGE. The objective of this study was to compare the manual detection rate of cortical lesions when using MP2RAGEuni, FLAWSmin, and/or FLAWShco. Furthermore, we compared T1 relaxation times in cortical lesions and other brain regions of interest (ROIs) between MP2RAGE and FLAWS at 3 T, because both approaches provide the option to quantify tissue microstructural integrity.
MATERIALS AND METHODS
Study Design and Participants
This study was designed as a comparison of manual cortical lesion detection rate on 3 MRI contrasts (MP2RAGEuni and 2 reconstructed sets of FLAWS: FLAWSmin and FLAWShco; see Fig. 1) in patients with diagnosis of definite MS. The number and volume (cumulative lesion load per patient as well as lesion sizes) of cortical lesions served as primary outcomes. The null hypothesis was that there is no numerical difference in manual cortical lesion number and volume between MP2RAGE and FLAWS contrasts. Furthermore, T1 relaxation times of cortical lesions and specific normal-appearing ROIs (see below) as well as contrast-to-noise ratios (CNRs) were compared between MP2RAGE and FLAWS.
FIGURE 1.
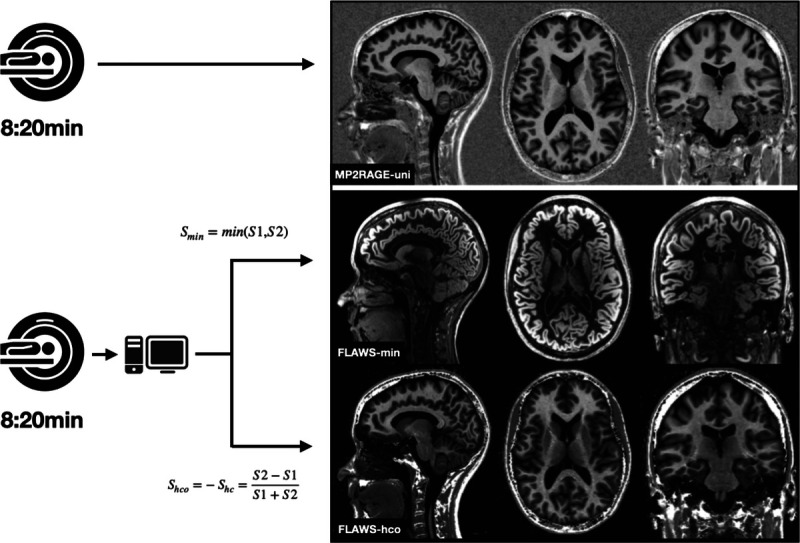
Acquisition procedure and final contrasts of MP2RAGEuni (top row), FLAWSmin (mid row), and FLAWShco (bottom row). Sagittal (left column), axial (mid column), and coronal (right column) view in an MS patient without cortical lesions. FLAWS indicates fluid and white matter suppression; MP2RAGE, magnetization prepared 2 rapid acquisition gradient echo.
Inclusion criteria were (1) relapsing remitting MS according to the McDonald criteria 2017,7 (2) 18 years of age or older, and (3) ability to give informed consent. Individuals with neurologic comorbidities, contraindications for MRI, and pregnant women were excluded. Images were acquired between November 2018 and December 2020. The study protocol was approved by the local ethics committee. All subjects gave written informed consent.
Magnetic Resonance Imaging Acquisition, Reconstruction, and Processing
All MRI scans were performed on a 3.0 T whole-body MR system (Magnetom Prisma; Siemens Healthineers, Erlangen, Germany) using a 64-channel head and neck RF coil for reception. All patients underwent MRI including (1) MP2RAGE uniform/flat images and (2) FLAWS alongside the conventional MRI protocol in 1 session. Acquisition time was 8:20 minutes for MP2RAGE and FLAWS each. No contrast agent was administered.
Both MP2RAGE and FLAWS used an isotropic 1 mm voxel size, anteroposterior phase encoding, and a symmetrical echo. Magnetic resonance imaging acquisition parameters of the sequences of interest are specified in Table 1. For each subject, a B1 map was obtained using the unbalanced steady-state free precession-based B1-TRAP approach25 (in-plane resolution, 4 × 4 mm; 15 slices of 5 mm thickness; slice gap, 5 mm; acquisition time, 2:09 minutes). MP2RAGEuni images were acquired based on 2 inversion times (see Table 1). Bloch equations were used to optimize the CNR and to minimize the effect of B1+ variations through space. The FLAWS used a TI1 of 449 milliseconds and a TI2 of 1270 milliseconds, resulting in 2 sets of images: FLAWS1 (acquired at TI1, WM-suppressed) and FLAWS2 (acquired at TI2, CSF-suppressed).26
TABLE 1.
Magnetic Resonance Imaging Sequence Parameters
| MP2RAGE | FLAWS | |
|---|---|---|
| Resolution, voxel size, mm3 | 1.0 × 1.0 × 1.0 | 1.0 × 1.0 × 1.0 |
| Orientation | Sagittal | Sagittal |
| Phase encoding | Anteroposterior | Anteroposterior |
| FoV, mm3 | 256 × 240 × 176 | 256 × 240 × 192 |
| TR/TE, ms | 5000/2.98 | 5000/2.19 |
| TI 1, ms | 700 | 449 |
| TI 2, ms | 2500 | 1270 |
| Flip angle 1, degrees | 4 | 5 |
| Flip angle 2, degrees | 5 | 6 |
FLAWS, fluid and white matter suppression; FoV, field of view; MP2RAGE, magnetization prepared 2 rapid acquisition gradient echo; TE, echo time; TI, 1 inversion time 1; TI, 2 inversion time 2; TR, repetition time.
For the reconstruction of the FLAWS contrasts and computation of FLAWS T1 maps, the FLAWS-Tools open-source software was used (https://github.com/jerbeaumont/FLAWS-Tools).27 By using this tool, the total processing time, that is, the time to reconstruct 6 different FLAWS contrasts (including FLAWShco and FLAWSmin) and perform the T1 mapping with a B1+ correction, was 1:09 minutes on average (run on a Intel Core i7-67000K computer). MP2RAGEuni was processed and provided automatically by the scanner. FLAWSmin (GM-specific) was reconstructed in a voxel-wise manner23 by applying the equation
where S1 and S2 reflects the magnitude of the FLAWS1 and FLAWS2 signal, respectively. Similarly, FLAWShco (CSF-suppressed with low B1 sensitivity) was computed23 by applying the equation
| . |
The T1 maps of the MP2RAGE sequence were corrected for inhomogeneities as proposed by Marques and Gruetter.28 T1 maps of FLAWS were computed as described by Beaumont et al.27
Cortical Lesions Identification and Processing
Cortical lesions were segmented manually by 3 experienced raters (J.M., C.T., R.R.; 5, 6, and 5 years of experience in MS imaging, respectively) on MP2RAGEuni, FLAWSmin, and FLAWShco (Fig. 2) using ITK-SNAP.29 In a first step, raters segmented the cortical lesions individually on MP2RAGEuni images of every single patient, without consideration of any other MRI sequence. In a second step, a consensus for MP2RAGEuni was reached among the 3 raters. Subsequently, the same procedure was applied for FLAWSmin and FLAWShco (see Supplementary Fig. 1, http://links.lww.com/RLI/A688). Time between consensus reading sessions was >4 weeks. Consensus segmentation maps were used for the assessment of cortical lesion numbers and volumes, whereas individual segmentation maps of the raters were used for the analysis of interrater agreement. Raters were blinded to the clinical status of the patients at all times.
FIGURE 2.
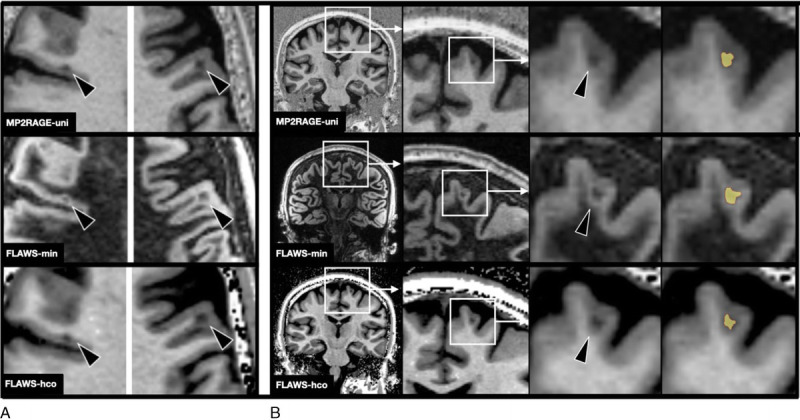
A, Example of 2 cortical lesions (arrowheads) on MP2RAGEuni (top row), FLAWSmin (mid row), and FLAWShco (bottom row). B, “Zoom in” on a cortical lesion on MP2RAGEuni (top row), FLAWSmin (mid row), and FLAWShco (bottom row) and its segmentation (in yellow). Note that the clear delineation on FLAWSmin allows a slightly larger segmentation of the cortical lesion. FLAWS indicates fluid and white matter suppression; MP2RAGE, magnetization prepared 2 rapid acquisition gradient echo.
Cortical lesions were defined as focal hypointensities relative to the adjacent normal-appearing GM, occupying at least 3 voxels13 on at least 2 orthogonal planes. Lesions that were entirely located within the cortical GM were subclassified as intracortical lesions; lesions that involved both the cortex and juxtacortical WM were scored as leukocortical lesions. On FLAWSmin, the hyperintense “halo” around the lesion (see Discussion) was not included to the lesions segmentation.
MP2RAGEuni images were patient-wise coregistered to the FLAWS space using advanced normalization tools.30 Subsequently, manually created lesion maps were aligned to the same space. Lesion counts and volumes were extracted from the lesion maps obtained by each reader and from the consensus union map using Python scripts. To assess the overlapping of lesions on different contrasts, segmentations were subsequently dilated by 3 voxels to mitigate residual coregistration imperfection between MP2RAGEuni and FLAWS. Lesions that overlapped by at least 3 voxels on all contrasts were defined as common cortical lesions. Lesions that were only segmented on 1 contrast (MP2RAGEuni only, FLAWSmin only, or FLAWShco only) were retrospectively assessed on the other contrasts and defined as retrospectively identified cortical lesion, if visible at the given locality.
To assess the combined detection rate of FLAWSmin and FLAWShco (FLAWSmin + hco) versus MP2RAGEuni, a union lesion mask of FLAWSmin and FLAWShco was produced by unifying lesions of consensus lesion maps on FLAWSmin and FLAWShco. Lesions that overlapped by at least 3 voxels on FLAWSmin and FLAWShco were counted as 1 lesion. For lesion volumes of FLAWSmin + hco, the sum of the lesion volumes of FLAWSmin and FLAWShco was used, by counting overlapping voxels only once.
Comparison of T1 Relaxation Times
T1 relaxation times (milliseconds) were assessed on MP2RAGE T1 and FLAWS T1 maps, in ROIs of healthy tissue and in cortical lesions. Normal-appearing WM, cortical GM, putamen, and caudate nucleus served as ROIs, as previously described.31 Regions of interest were manually segmented on the MP2RAGE and FLAWS T1 maps. For T1 values of cortical lesions, manual segmentation maps were rigidly coregistered to the according T1 maps (MP2RAGEuni lesion maps on MP2RAGE T1 map). For FLAWS T1 maps, the lesion map of FLAWShco was considered. For comparison of T1 values between MP2RAGE and FLAWS, the median T1 value of the ROI/lesion was used.
Contrast-to-Noise Ratio
To objectively compare the contrast of MP2RAGEuni and FLAWS, means and standard deviation of signal intensities were measured in ROIs of WM (splenium of the corpus callosum), GM (head of the caudate nucleus), and CSF (lateral ventricles). The CNR was calculated for WM/GM, WM/CFS, and GM/CSF as previously described21,23,27 on MP2RAGEuni and FLAWShco, before the B1+ correction.
Statistical Analysis
Continuous data are given as mean ± one standard deviation (SD) if normally distributed and median and interquartile range (IQR) if nonnormally distributed. Categorical data are summarized using cross tables providing counts and percentages. Data sets without any detected cortical lesions (n = 32/90) were included for analyses regarding the cortical lesion detection rate (lesion numbers), but excluded from the analyses comparing lesion volumes and T1 values.
Comparisons of lesion numbers and volumes of cortical lesions were performed using Friedman test. A Wilcoxon signed rank test was conducted to compare lesion counts and volumes on MP2RAGEuni and FLAWSmin + hco, and to assess CNR on MP2RAGE and FLAWShco. In case of significance, a post hoc Dunnett-Bonferroni test was used to correct for the comparisons of multiple groups. After that, a 2-tailed P < 0.05 was regarded as statistically significant.
The comparison of T1 relaxation times between MP2RAGE and FLAWS was described using relative differences in percentage.
In an exploratory analysis, individual cortical lesion counts of the 3 raters were compared, and interrater variability was quantified using Fleiss ϰ. Statistical analysis was conducted using IBM SPSS Statistics (version 25; IBM, Armonk, NY).
RESULTS
Patients characteristics are reported in Table 2.
TABLE 2.
Clinical Characteristics of MS Patients
| Females, n (Proportion) | 23/30 (76.7) |
|---|---|
| Disease course | |
| RRMS, n (proportion) | 23/30 (76.7) |
| SPMS, n (proportion) | 1/30 (3.3) |
| PPMS, n (proportion) | 6/30 (20) |
| Age, mean ± SD, y | 43.4 ± 13.6 |
| EDSS, median (range) | 2.0 (1.0–7.0) |
| Disease duration, median (interquartile range), y | 4.8 (14.0) |
EDSS, expanded disability status scale; PPMS, primary progressive multiple sclerosis; RRMS, relapsing remitting multiple sclerosis; SD, standard deviation; SPMS, secondary progressive multiple sclerosis.
Cortical Lesions Numbers
Lesion counts deriving from consensus lesion maps on MP2RAGEuni, FLAWSmin, and FLAWShco are summarized in Table 3. Cortical lesions were identified in 20/30 patients on MP2RAGE, 19/30 patients on FLAWSmin, and 19/30 patients on FLAWShco. In total, 633 segmentations were made on the 3 contrasts (109 intracortical, 524 leukocortical lesions), corresponding to 355 cortical lesions. Of the segmentations, 207 were made on MP2RAGE, 182 on FLAWSmin, and 244 on FLAWShco. The number of detected cortical lesions per patient was comparable among the 3 sequences (median MP2RAGEuni, 3.0; IQR, 10.0; FLAWSmin, 3.5; IQR, 9.0; FLAWShco, 3.0; IQR, 11.0; P > 0.05). The same was true for the subclasses of cortical lesions (leukocortical and intracortical lesions).
TABLE 3.
Cortical Lesion Number and Volumes Deriving From Consensus Segmentation Maps on the 3 Contrasts (MP2RAGE, FLAWSmin, FLAWShco) and the Merged Map (FLAWSmin + hco)
| MP2RAGEuni | FLAWSmin | FLAWShco | P (MP2RAGE vs FLAWSmin vs FLAWShco) | FLAWSmin + hco | P (FLAWSmin + hco vs MP2RAGE) | |
|---|---|---|---|---|---|---|
| No. CL, median (IQR), n | 3 (10) | 3.5 (9) | 3 (11) | 0.082 | 4.5 (15) | <0.001 |
| No. intracortical lesion, median (IQR) | 0 (2) | 0.5 (2) | 0 (1) | 0.241 | 1 (3) | 0.068 |
| No. leukocortical lesions, median (IQR) | 2.5 (7) | 2.0 (7) | 2.0 (9) | 0.011* | 3.5 (12) | <0.001 |
| Total cortical lesion volume, median (IQR), μL | 490 (990) | 587 (1405) | 611 (844) | 0.039† | 612 (1446) | <0.001 |
| Total intracortical lesion volume, median (IQR), μL | 58 (81) | 109 (151) | 52 (79) | 0.565 | 51 (133) | 0.037 |
| Total leukocortical lesion volume, median (IQR), μL | 471 (1012) | 433 (1365) | 593 (830) | 0.185 | 610 (922) | <0.001 |
| Volume per cortical lesion, median (IQR), μL | 47.7 (40) | 54.91 (42) | 53.4 (38) | 0.057 | 53.8 (40) | 0.027 |
| Volume per intracortical lesion, median (IQR), μL | 14.3 (8) | 22.1 (30) | 24.0 (14) | 0.565 | 18.5 (13) | 0.110 |
| Volume per leukocortical lesion, median (IQR), μL | 62.1 (56) | 77.8 (86) | 64.6 (52) | 0.05‡ | 71.5 (31) | 0.048 |
A P value less than 0.05 (typically ≤ 0.05) is statistically significant (boldface).
*After post hoc test of Dunnett-Bonferroni to correct for comparisons of multiple groups: MP2RAGE vs FLAWSmin, P = 1.0; MP2RAGE vs FLAWShco, P = 0.364; FLAWSmin vs FLAWShco, P = 0.099.
†After post hoc test of Dunnett-Bonferroni to correct for comparisons of multiple groups: MP2RAGE vs FLAWSmin, P = 0.04; MP2RAGE vs FLAWShco, P = 0.231; FLAWSmin vs FLAWShco, P = 1.0.
‡After post hoc test of Dunnett-Bonferroni to correct for comparisons of multiple groups: MP2RAGE vs FLAWSmin, P = 0.102; MP2RAGE vs FLAWShco, P = 1.0; FLAWSmin vs FLAWShco, P = 0.102.
CL, cortical lesion; FLAWS, fluid and white matter suppression; IQR, interquartile range; MP2RAGE, magnetization prepared 2 rapid acquisition gradient echo.
Cortical Lesions Volumes
In patients with cortical lesions (22/30), median cumulative lesion volume per patient was larger on FLAWSmin (587 μL; IQR, 1405 μL) than on MP2RAGEuni (490 μL; IQR, 990 μL; P = 0.04), whereas there was no difference between FLAWSmin and FLAWShco (611 μL; IQR, 844 μL), or FLAWShco and MP2RAGEuni. Cumulative lesion volume was comparable among the 3 contrasts when comparing leukocortical and intracortical lesions.
For the median volume per single cortical lesions, MP2RAGEuni (47.7 μL; IQR, 40 μL), FLAWSmin (54.9 μL; IQR, 41.8 μL), and FLAWShco (53.4 μL; IQR, 37.6 μL) showed similar results (P > 0.05). The same was true for the median volume of single intracortical lesions. Leukocortical lesions were numerically larger on FLAWSmin (median, 77.8 μL; IQR, 86.3 μL) than on MP2RAGEuni (62.1 μL; IQR, 56.4 μL) and FLAWShco (64.6 μL; IQR, 52.0 μL; P = 0.05), but this finding did not survive the correction for comparisons of multiple groups.
Cortical Lesions in MP2RAGEuni only, FLAWSmin only, FLAWShco only, and Retrospectively Identified Cortical Lesion
Of 355 identified single cortical lesions, 100 were segmented on all sequences (common cortical lesions, 28.2%). A total of 175 lesions (49%) were segmented on 1 sequence only, hereof 43 (26%) MP2RAGEuni only, 75 (43%) FLAWSmin only, and 54 (31%) FLAWShco only. Of the 43 lesions that were seen on MP2RAGEuni only, 27 (62%) were retrospectively also visible on FLAWShco, and 23 (53%) also on FLAWSmin. Of the 75 lesions that were segmented on FLAWSmin only, 57 (76%) were retrospectively also detected on MP2RAGEuni and 51 (68%) also on FLAWShco. From the 54 lesions that were seen on FLAWShco only, 33 (61%) were also visible on MP2RAGEuni, and 31 (57%) also on FLAWSmin.
The number of lesions segmented both on MP2RAGEuni and FLAWShco (n = 54) was higher than the lesions seen on MP2RAGEuni and FLAWSmin (n = 10) or FLAWSmin and FLAWShco (n = 16).
Assessment of Combined FLAWSmin + hco Versus MP2RAGE
The merged map of FLAWSmin and FLAWShco (FLAWSmin + hco) yielded cortical lesions in 21/30 patients. Using this image contrast, 305 single lesions were identified, 243 leukocortical and 62 intracortical lesions. FLAWSmin + hco (median, 4.5; IQR, 15) showed significantly more cortical lesions per patient than MP2RAGEuni (median, 3.0; IQR, 10; P < 0.001). FLAWSmin + hco detected more leukocortical lesions per patient (median, 3.5; IQR, 12) than MP2RAGEuni (median, 2.5; IQR, 7; P < 0.001), whereas there was no difference regarding intracortical lesions.
FLAWSmin + hco showed larger cumulative volumes per patient than MP2RAGEuni (median, 612 μL; IQR, 1446 μL vs 490 μL; IQR, 990 μL; P < 0.001). This was also true for the 2 subdivisions of cortical lesions (leukocortical lesions: median, 610 μL; IQR, 922 μL vs 471 μL; IQR, 1012 μL; P < 0.001; intracortical lesions: median, 110 μL; IQR, 124 μL vs 58 μL; IQR, 81 μL; P = 0.037).
The median volume of single cortical lesions was larger on FLAWSmin + hco than on MP2RAGEuni (53.8 μL; IQR, 40 μL vs 47.7 μL; IQR, 40 μL; P = 0.027). The same was true for the subdivision of leukocortical lesions (71.5 μL; IQR, 31 μL vs 62.1 μL; IQR, 56 μL; P = 0.048), whereas there was no difference regarding median volume of intracortical lesions.
Interrater Variability
Interrater agreement of lesion counts was moderate for cortical lesions on MP2RAGEuni (ϰ = 0.582) and on FLAWShco (ϰ = 0.584) but substantial on FLAWSmin (ϰ = 0.614). For intracortical lesions, interrater agreement was moderate for MP2RAGEuni (ϰ = 0.541) but substantial for FLAWSmin (ϰ = 0.676) and FLAWShco (ϰ = 0.774). For leukocortical lesions, interrater agreement was moderate for all sequences (MP2RAGEuni ϰ = 0.518, FLAWSmin ϰ = 0.560, FLAWShco ϰ = 0.565).
T1 Mapping Analysis
Exemplary T1 maps from MP2RAGE and FLAWS and their relative difference in percentage are shown in Figure 3. In ROIs of healthy tissues (normal-appearing WM, cortical GM, putamen, and caudate nucleus), relative differences of T1 values between MP2RAGE and FLAWS were close to or below 5% for all ROIs (Table 4). The median T1 relaxation values in cortical lesions were approximately 1700 milliseconds on both MP2RAGE and FLAWS (Fig. 4). The same was true for the 2 subdivisions of leukocortical and intracortical lesions.
FIGURE 3.
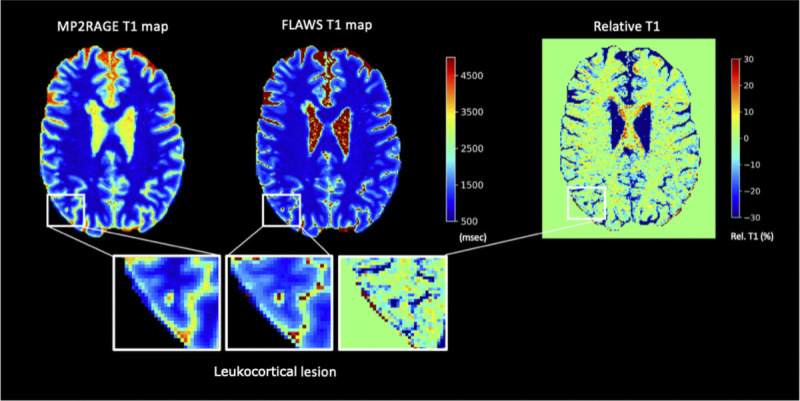
From left to right, MP2RAGE T1 map, FLAWS T1 map, and their relative difference in percentage. The “zoom in” shows a leukocortical lesion. FLAWS indicates fluid and white matter suppression; MP2RAGE, magnetization prepared 2 rapid acquisition gradient echo.
TABLE 4.
T1 Relaxation Times (Milliseconds) for the FLAWS and MP2RAGE T1 Maps Considering Areas of Healthy Tissue
| WM | Putamen | Caudate | Cortical GM | |
|---|---|---|---|---|
| MP2RAGE (n = 20) | 867 ± 31 | 1243 ± 39 | 1320 ± 34 | 1527 ± 28 |
| FLAWS (n = 20) | 823 ± 28 | 1245 ± 49 | 1315 ± 30 | 1543 ± 29 |
| Relative difference | 5.1% | 0.2% | 0.4% | 1.0% |
ROIs were manually segmented in the MP2RAGE T1 maps and FLAWS T1 maps separately.
FLAWS, fluid and white matter suppression; GM, gray matter; MP2RAGE, magnetization prepared 2 rapid acquisition gradient echo; ROI, region of interest; WM, white matter.
FIGURE 4.
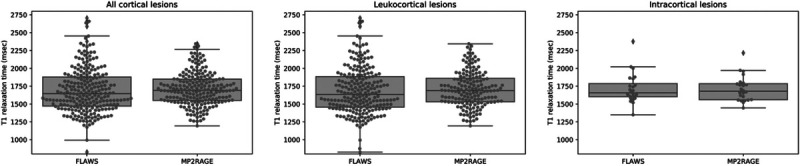
T1 relaxation times (milliseconds) for cortical lesions in the FLAWS T1 and MP2RAGE T1 maps. For the FLAWS T1 map, the cortical lesions segmented on FLAWShco were considered. FLAWS indicates fluid and white matter suppression; MP2RAGE, magnetization prepared 2 rapid acquisition gradient echo.
Contrast-to-Noise Ratio
FLAWShco showed a significantly higher WM/GM contrast than MP2RAGEuni (P < 0.005). Contrast-to-noise ratio for GM/CSF was comparable between the 2 contrasts (see Supplementary Table 1, http://links.lww.com/RLI/A688).
DISCUSSION
Cortical lesion detection in MS is challenging, and various sequences and contrasts have been suggested to improve the cortical lesion detection rate such as DIR, PSIR, MP2RAGE, and high-spatial resolution MPRAGE.9,13,14,18 In this study, we used first FLAWS—a sequence that provides multiple 3D high-resolution contrasts in a single acquisition21,23,24—and demonstrated that FLAWSmin and FLAWShco are both equally effective as MP2RAGE to manually detect cortical lesions in MS patients. Moreover, our study provides evidence that the combination of these 2 FLAWS contrasts yields the highest number and the largest volumes of cortical lesions.
Achievement of higher cortical lesion detection rates using combinations of multiple MRI contrasts has previously been reported32,33 and is partly ascribed to the repetitive assessment of multiple MRIs of the same patient. However, compared with previous investigations, the present study suggests 2 major advantages of FLAWS. First, the approach of reconstructing multiple contrasts deriving from 1 session conserves acquisition time. Second, based on its reconstruction properties, FLAWS naturally provides a near-perfect alignment of the 2 contrasts, which is generally known to be favorable when analyzing small brain structures such as cortical lesions. The present study shows that FLAWS has similar characteristics to MP2RAGEuni (clinically compatible acquisition time, T1-weighted images with high WM-GM contrast, eg, FLAWShco, and T1 maps) but also the advantage to provide many more contrasts (FLAWSmin and FLAWShco, FLAWS1, FLAWS2, FLAWSuni, FLAWShco). Future studies should assess whether those contrasts may reveal specific properties of cortical and WM MS lesions.
Because of the way signal intensities are calculated in FLAWSmin (minimum signal intensity of FLAWS1 and FLAWS2 is used for each voxel), partial volume effects at interfaces between brain tissues with a high signal divergence (eg, brain vs CSF border; lesion vs nonlesion tissue) lead to hyperintense voxel signals at the respective border (Fig. 5). In cortical lesions, this generally results in a hyperintense “halo” around the lesions. We do not believe that this “halo” has any structural correlate and is rather caused by the aforementioned partial volume effect, only (it is therefore also not to confuse with so-called paramagnetic rim lesions34). However, this hyperintense “halo” considerably facilitates the delineation of the lesion against the surrounding cortex and/or WM (the same holds true for WM lesions, although those were not assessed in this study). The easier depiction of cortical lesions on FLAWSmin was shared by the subjective judgment of the raters and reflected in the higher interrater agreement between the raters on FLAWSmin compared with MP2RAGEuni and FLAWShco. In this study, we did not include the “halo” itself in the lesions segmentation. Still, the easier delineation may partly explain why leukocortical lesions were found to be larger on FLAWSmin than on MP2RAGEuni (Fig. 2B). In addition, the “halo” appearance with clear demarcation of lesion tissue against the cortical GM might help to distinguish cortical from juxtacortical lesions (see Supplementary Fig. 2, http://links.lww.com/RLI/A688).
FIGURE 5.
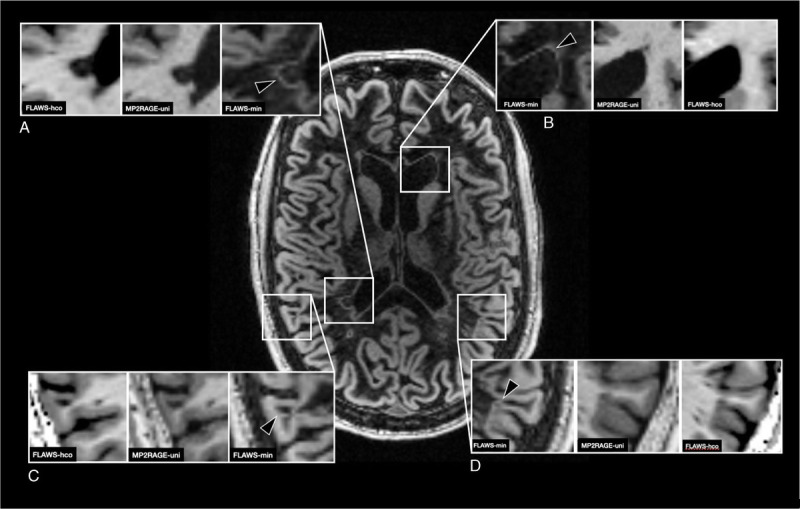
FLAWSmin of a patient with MS demonstrating the “halo” appearance. Partial volume effects at the border of tissues with high divergence of signal intensities lead to a hyperintense band at the respective border. MP2RAGEuni and FLAWShco are shown for reference. A, Hyperintense band (arrowhead) around a periventricular white matter lesion (not systematically assessed in this study). B, Hyperintense band (arrowhead) at the normal-appearing border between CSF and brain tissue. C, Hyperintense band (arrowhead “halo”) around a leukocortical lesion. D, Hyperintense band (arrowhead, “halo”) around an intracortical lesion. FLAWS indicates fluid and white matter suppression; MP2RAGE, magnetization prepared 2 rapid acquisition gradient echo.
The clear border between lesion and nonlesion tissue might also be from interest for future analyses of automated lesion detection, where a clear delineation may be particularly desirable. In fact, manually annotating cortical lesions in both FLAWSmin and FLAWShco, or analyzing both contrasts simultaneously, is more time-consuming compared with the evaluation of a single sequence such as the MP2RAGEuni. The characteristics of MS lesions in FLAWSmin and FLAWShco could therefore improve the sensitivity of recently proposed automated methods for cortical lesions detection/segmentation35 without any acquisition time increase.
The fact that only 28.2% of the lesions overlapped on all 3 contrasts and that a high proportion of the noncommon lesions were retrospectively also visible on the other contrasts (between 57% and 76%, depending on the contrasts combination) suggests a generally low sensitivity of manual cortical lesion detection, even when performed by 3 trained raters. Previous studies have also demonstrated a low sensitivity of MRI for cortical lesion detection compared with postmortem identification.11,15,36 This has been ascribed to their small size, the lack of inflammation compared with WM lesions, or partial volume effects from adjacent CSF and WM.2,11,36 Another factor that might have contributed to the low detection rate is that we purposely abstained from an additional consideration of cortical lesions on a T2-weighted image (eg, DIR), to render clearer the comparison between MP2RAGEuni and FLAWS.
T1 relaxation times may be exploited to characterize lesions microstructural characteristics,37 and recent works have shown that those characteristics are related to patients clinical impairment38,39 and to specific histopathological MS lesion types.40 At 7 T, it has been shown that quantitative T1 mapping using MP2RAGE can be obtained with high spatial resolution.28 Recently, T1 mapping deriving from FLAWS at 7 T was shown to be similarly reliable.27
To date, only one study compared T1 values of MP2RAGE and FLAWS at 3 T in healthy individuals, showing remarkable consistency.26 In this work, we assessed T1 relaxation times provided by FLAWS and MP2RAGE in cortical lesions and ROIs of normal-appearing brain tissue of MS patients. Confirming the previous report in healthy subjects,26 we found very small differences between the maps provided by those sequences in normal-appearing brain tissue in MS patients (≤5%, Table 4). On the other hand, in cortical lesions, we showed first that MP2RAGE and FLAWS measured very similar T1 relaxation (mean qT1 ~ 1700 milliseconds, Fig. 5). This average value is slightly higher than the one previously described using MP2RAGE in cortical lesions in a small and homogeneous group of very early relapsing-remitting MS patients,18 possibly due to the more destructive characteristics of cortical lesions in the heterogeneous cohort of patients (including secondary and primary progressive patients) as the ones studied in this work. Also, these results should be interpreted with caution, given the challenges that arise when quantitatively assessing such small brain structures as cortical lesions.
In our study, the WM/GM contrast was higher on FLAWShco than MP2RAGEuni, which is in line with a previous study comparing the 3 contrasts (among others) at 7 T.27
This study has some limitations. We did not compare the results obtained with FLAWS to cortical lesion detection performed with DIR, PSIR, and MPRAGE, which are recommended as criterion standard confirmatory sequences in recent guidelines.6,17 However, we chose a comparator such as MP2RAGEuni, which has shown to identify a similar18 or higher19 number of cortical lesions as one of DIRs. MP2RAGEuni has the advantage not be susceptible to flow-related artifacts as DIR13 and PSIR.14 Also, 1-mm isotropic MP2RAGE may be acquired in shorter time (8:20 seconds) compared with DIR (12:02 minutes),18 which might render this sequence more applicable in daily clinical practice. Furthermore, compared with MPRAGE, MP2RAGE provides a contrast that is free of proton density, T2* contrast, and reception bias-field, which was shown to improve the identification of MS-related WM lesions.18 Still, it may be of interest to compare the performance of FLAWS to the current criterion standard sequences in future studies.
In this study, we decided not to acquire an additional T2-weighted contrast (eg, DIR) to enhance the sensitivity to cortical lesion detection, because this combination would not be realistic in clinical practice and because we aimed at gaining knowledge about the sensitivity of FLAWS alone. Another limitation of this study was the lack of postmortem validation of the cortical lesions detected by both FLAWS and MP2RAGEuni, hence we provided only a comparison of the sensitivity of those 2 acquisition methods to the number and volume of focal pathological cortical changes in MS patients in vivo. Future MRI histopathology studies should assess whether the additional lesions identified using the combined FLAWSmin + hco images are real or false-positive findings. In this study, we did not evaluate the correlation between cortical lesion number and clinical patient characteristics. Nevertheless, we provided first evidence that the contrasts provided by FLAWS deliver additional information compared with MP2RAGEuni, which significantly facilitates the detection and the quantification of the area of focal damage. Furthermore, we have supported with data that show that qT1 as provided by FLAWS and MP2RAGE yields similar results in lesions, hereby suggesting that both methods may be well used to quantify microstructural damage in lesioned cortical areas. Future work will aim at validating the specificity of FLAWS in a postmortem MRI setting and at assessing the utility of FLAWShco and FLAWSmin in comparison to other sequences such as DIR or PSIR, and in combination with T2-based sequences such as DIR or FLAIR.
Footnotes
Conflicts of interest and sources of funding: J.M., F.L.R., J.B., C.T., R.R., M.B.C., and G.G. have nothing to disclose. M.W. is partially funded by Biogen for the development of spinal cord magnetic resonance imaging for patients with spinal muscular atrophy. The University Hospital Basel, as the employer of C.G., has received the following fees, which were used exclusively for research support: (1) advisory board and consultancy fees from Actelion, Genzyme-Sanofi, Novartis, GeNeuro, and Roche; (2) speaker fees from Genzyme-Sanofi, Novartis, GeNeuro, and Roche; and (3) research support from Siemens, GeNeuro, and Roche.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.investigativeradiology.com).
Contributor Information
Jannis Müller, Email: jannis.mueller@usb.ch.
Francesco La Rosa, Email: francesco.larosa@epfl.ch.
Jeremy Beaumont, Email: jeremy.beaumont.2@gmail.com.
Charidimos Tsagkas, Email: charidimos.tsagkas@usb.ch.
Reza Rahmanzadeh, Email: reza.rahmanzadeh@unibas.ch.
Matthias Weigel, Email: matthias.weigel@usb.ch.
Meritxell Bach Cuadra, Email: meritxell.bachcuadra@unil.ch.
Giulio Gambarota, Email: gambarota@gmail.com.
REFERENCES
- 1.GBD 2016 Multiple Sclerosis Collaborators . Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidd D Barkhof F McConnell R, et al. Cortical lesions in multiple sclerosis. Brain. 1999;122:17–26. [DOI] [PubMed] [Google Scholar]
- 3.Peterson JW Bo L Mork S, et al. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese M Poretto V Favaretto A, et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain. 2012;135(pt 10):2952–2961. [DOI] [PubMed] [Google Scholar]
- 5.Filippi M Rocca MA Calabrese M, et al. Intracortical lesions: relevance for new MRI diagnostic criteria for multiple sclerosis. Neurology. 2010;75:1988–1994. [DOI] [PubMed] [Google Scholar]
- 6.Filippi M Preziosa P Banwell BL, et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: practical guidelines. Brain. 2019;142:1858–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson AJ Banwell BL Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. [DOI] [PubMed] [Google Scholar]
- 8.Brownell B, Hughes JT. The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1962;25:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filippi M, Rocca MA. Cortical lesions on 7-T MRI in multiple sclerosis: a window into pathogenetic mechanisms? Radiology. 2019;291:750–751. [DOI] [PubMed] [Google Scholar]
- 10.Tallantyre EC Morgan PS Dixon JE, et al. 3 Tesla and 7 Tesla MRI of multiple sclerosis cortical lesions. J Magn Reson Imaging. 2010;32:971–977. [DOI] [PubMed] [Google Scholar]
- 11.Kilsdonk ID Jonkman LE Klaver R, et al. Increased cortical grey matter lesion detection in multiple sclerosis with 7 T MRI: a post-mortem verification study. Brain. 2016;139(pt 5):1472–1481. [DOI] [PubMed] [Google Scholar]
- 12.Beck ES Sati P Sethi V, et al. Improved visualization of cortical lesions in multiple sclerosis using 7T MP2RAGE. AJNR Am J Neuroradiol. 2018;39:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geurts JJ, Pouwels PJ, Uitdehaag BM. Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology. 2005;236:254–260. [DOI] [PubMed] [Google Scholar]
- 14.Harel A Ceccarelli A Farrell C, et al. Phase-sensitive inversion-recovery MRI improves longitudinal cortical lesion detection in progressive MS. PLoS One. 2016;11:e0152180. Published 2016 Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouman PM Steenwijk MD Pouwels PJW, et al. Histopathology-validated recommendations for cortical lesion imaging in multiple sclerosis. Brain. 2020;143:2988–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geurts JJ Roosendaal SD Calabrese M, et al. Consensus recommendations for MS cortical lesion scoring using double inversion recovery MRI. Neurology. 2011;76:418–424. [DOI] [PubMed] [Google Scholar]
- 17.Wattjes MP Ciccarelli O Reich DS, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20:653–670. [DOI] [PubMed] [Google Scholar]
- 18.Kober T Granziera C Ribes D, et al. MP2RAGE multiple sclerosis magnetic resonance imaging at 3 T. Invest Radiol. 2012;47:346–352. [DOI] [PubMed] [Google Scholar]
- 19.Beck ES Gai N Filippini S, et al. Inversion recovery susceptibility weighted imaging with enhanced T2 weighting at 3 T improves visualization of subpial cortical multiple sclerosis lesions. Invest Radiol. 2020;55:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marques JP Kober T Krueger G, et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2010;49:1271–1281. [DOI] [PubMed] [Google Scholar]
- 21.Tanner M Gambarota G Kober T, et al. Fluid and white matter suppression with the MP2RAGE sequence. J Magn Reson Imaging. 2012;35:1063–1070. [DOI] [PubMed] [Google Scholar]
- 22.Chen X Qian T Kober T, et al. Gray-matter-specific MR imaging improves the detection of epileptogenic zones in focal cortical dysplasia: a new sequence called fluid and white matter suppression (FLAWS). Neuroimage Clin. 2018;20:388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaumont J Saint-Jalmes H Acosta O, et al. Multi T1-weighted contrast MRI with fluid and white matter suppression at 1.5 T. Magn Reson Imaging. 2019;63:217–225. [DOI] [PubMed] [Google Scholar]
- 24.Beaumont J Saint-Jalmes H Acosta O, et al. High Contrast T1-Weigthed MRI with Fluid and White Matter Suppression Using Mp2Rage. Poster presented at: ISBI 2019 - 16th IEEE International Symposium on Biomedical Imaging; April 8–11, 2019; Venice, Italy. [Google Scholar]
- 25.Ganter C Settles M Dregely I, et al. B1+-mapping with the transient phase of unbalanced steady-state free precession. Magn Reson Med. 2013;70:1515–1523. [DOI] [PubMed] [Google Scholar]
- 26.Beaumont J Fripp J Raniga P, et al. Multi T1-weighted contrast imaging and T1 mapping with compressed sensing FLAWS at 3 T. bioRxiv. Preprint posted online December 21, 2021. doi: 10.1101/2021.12.18.473283. [DOI] [PubMed] [Google Scholar]
- 27.Beaumont J Gambarota G Saint-Jalmes H, et al. High-resolution multi-T1-weighted contrast and T1 mapping with low sensitivity using the fluid and white matter suppression (FLAWS) sequence at 7 T. Magn Reson Med. 2021;85:1364–1378. [DOI] [PubMed] [Google Scholar]
- 28.Marques JP, Gruetter R. New developments and applications of the MP2RAGE sequence—focusing the contrast and high spatial resolution R1 mapping. PLoS One. 2013;8:e69294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yushkevich PA Piven J Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. [DOI] [PubMed] [Google Scholar]
- 30.Avants BB, Tustison N, Song G. Advanced normalization tools (ANTs). Insight J. 2009;2:1–35. [Google Scholar]
- 31.Wright PJ Mougin OE Totman JJ, et al. Water proton T1 measurements in brain tissue at 7, 3, and 1.5 T using IR-EPI, IR-TSE, and MPRAGE: results and optimization. MAGMA. 2008;21(1–2):121–130. [DOI] [PubMed] [Google Scholar]
- 32.Nelson F Poonawalla AH Hou P, et al. Improved identification of intracortical lesions in multiple sclerosis with phase-sensitive inversion recovery in combination with fast double inversion recovery MR imaging. AJNR Am J Neuroradiol. 2007;28:1645–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Favaretto A Poggiali D Lazzarotto A, et al. The parallel analysis of phase sensitive inversion recovery (PSIR) and double inversion recovery (DIR) images significantly improves the detection of cortical lesions in multiple sclerosis (MS) since clinical onset. PLoS One. 2015;10:e0127805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maggi P Sati P Nair G, et al. Paramagnetic rim lesions are specific to multiple sclerosis: an international multicenter 3T MRI study. Ann Neurol. 2020;88:1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.La Rosa F Abdulkadir A Fartaria MJ, et al. Multiple sclerosis cortical and WM lesion segmentation at 3T MRI: a deep learning method based on FLAIR and MP2RAGE. Neuroimage Clin. 2020;27:102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seewann A, Kooi EJ, Roosendaal SD. Postmortem verification of MS cortical lesion detection with 3D DIR. Neurology. 2012;78:302–308. [DOI] [PubMed] [Google Scholar]
- 37.Granziera C Wuerfel J Barkhof F, et al. Quantitative magnetic resonance imaging towards clinical application in multiple sclerosis. Brain. 2021;144:1296–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnier G Roche A Romascano D, et al. Advanced MRI unravels the nature of tissue alterations in early multiple sclerosis. Ann Clin Transl Neurol. 2014;1:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonnier G Maréchal B Fartaria MJ, et al. The combined quantification and interpretation of multiple quantitative magnetic resonance imaging metrics enlightens longitudinal changes compatible with brain repair in relapsing-remitting multiple sclerosis patients. Front Neurol. 2017;8:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolb H Absinta M Beck ES, et al. 7T MRI differentiates remyelinated from demyelinated multiple sclerosis lesions. Ann Neurol. 2021;90:612–626. [DOI] [PMC free article] [PubMed] [Google Scholar]


